Abstract
Background:
Serum uric acid (SUA), a byproduct of purine metabolism, exerts both antioxidant and pro-inflammatory effects, making its role in aging and chronic diseases a subject of ongoing debate. Despite this, the mechanisms by which SUA influences the aging process remain poorly understood.
Methods:
We analyzed data from the NHANES (1999–2010) and CHARLS (2011–2015) cohorts to investigate SUA’s impact on biological aging. Generalized linear regression models assessed SUA’s effect on biological aging markers [ΔKDM-BA, ΔPhenoAge, and allostatic load (AL)], while Cox regression models estimated its association with all-cause and premature mortality. Dose–response relationships between SUA levels and aging markers (ΔKDM-BA, ΔPhenoAge, and AL), as well as all-cause and premature mortality, were evaluated using restricted cubic splines (RCS).
Results:
In both cohorts, elevated SUA levels were significantly associated with accelerated aging. In the NHANES cohort, for each 1 mg/dL increase in SUA, ΔKDM-BA increased by 0.52 years (95% CI: 0.43–0.61, p < 0.0001), and AL increased by 0.38 (95% CI: 0.29–0.47, p < 0.0001). In the CHARLS cohort, SUA was similarly linked to an increase in ΔKDM-BA by 0.65 years (95% CI: 0.57–0.74, p < 0.0001) and AL by 0.15 (95% CI: 0.12–0.18, p < 0.0001). RCS analysis revealed a nonlinear association between SUA and ΔKDM-BA in NHANES, with a more pronounced acceleration of aging when SUA levels exceeded 4.16 mg/dL (nonlinear p < 0.0001). In CHARLS, SUA showed a nonlinear relationship with ΔKDM-BA (nonlinear p = 0.01). Additionally, in NHANES, SUA levels were associated with increased all-cause (HR: 1.04, 95% CI: 1.01–1.07, p = 0.01) and premature mortality (HR: 1.06, 95% CI: 1.00–1.13, p = 0.046). RCS analysis further demonstrated a U-shaped nonlinear relationship between SUA levels and both all-cause and premature mortality. In contrast, SUA did not show a significant association with mortality outcomes in the CHARLS cohort.
Conclusion:
Elevated SUA is associated with accelerated biological aging in both U.S. and Chinese populations, but its link to mortality was evident only in the NHANES cohort. These findings highlight SUA as a potential aging marker and call for further population-specific investigation.
Introduction
Aging represents a progressive decline in physiological integrity (1), increasing susceptibility to chronic diseases such as cardiovascular disease (CVD) (2), metabolic disorders (3), and neurodegenerative diseases (4). Although chronological age (CA) remains a strong predictor of health risk, it inadequately reflects inter-individual variability in the aging process. In contrast, biological age (BA)—derived from multi-system physiological biomarkers—offers a more dynamic and individualized assessment of aging (5). However, the metabolic determinants underlying biological aging are not yet fully understood.
Serum uric acid (SUA), the end product of purine metabolism, has emerged as a particularly contentious factor in aging research. On one hand, SUA functions as an evolutionarily conserved antioxidant capable of scavenging reactive oxygen species (ROS) (6). On the other, elevated SUA levels can activate the NLRP3 inflammasome (7), impair endothelial function, and are linked to hypertension (8), chronic kidney disease (CKD) (9), and cardiovascular events. This biological paradox has been reflected in epidemiologic studies, many of which describe a U-shaped association between SUA levels and mortality risk (10, 11). Nevertheless, the mechanisms driving this nonlinear relationship remain unclear. Additionally, prior research has predominantly focused on single aging biomarkers or ethnically homogeneous populations, limiting both mechanistic insight and generalizability.
To address these gaps, we conducted a comparative analysis leveraging data from two nationally representative cohorts: the National Health and Nutrition Examination Survey (NHANES, 1999–2010) in the United States and the China Health and Retirement Longitudinal Study (CHARLS, 2011–2015). We applied three complementary biological aging measures—Klemera–Doubal Method Biological Age (KDM-BA), Phenotypic Age (PhenoAge), and Allostatic Load (AL)—to evaluate the associations between SUA, biological aging, and mortality outcomes. This study aims to answer three key questions: (1) Does elevated SUA accelerate biological aging independent of traditional cardiometabolic risk factors? (2) What is the association between SUA and all-cause as well as premature mortality? (3) Do specific subgroups—such as women or individuals with CKD—exhibit heightened susceptibility to SUA-associated aging effects? By integrating multidimensional aging metrics across diverse populations, this research seeks to provide robust, population-based evidence to inform more targeted strategies for SUA monitoring and intervention in aging-related health.
Methods
Study population and data sources
This study is based on data from the NHANES and the CHARLS, two independent national cohorts. NHANES, organized by the National Center for Health Statistics (NCHS) under the Centers for Disease Control and Prevention (CDC), has been ongoing since the 1960s (12). It aims to collect data on health, nutrition, and lifestyle from a representative sample of the U.S. population. Detailed information on the survey and data collection methods is available on the official website.1 For this study, we used data from six NHANES cycles conducted between 1999 and 2010, comprising 62,160 participants. We excluded individuals under 20 years of age (n = 29,696), pregnant women (n = 1,299), those missing key biomarkers for KDM-BA or PhenoAge or SUA (n = 4,943), and those with incomplete mortality data as of December 31, 2019. After exclusions, 26,192 participants were included to assess the relationship between baseline SUA levels, accelerated aging, and all-cause mortality (Figure 1). All participants provided written informed consent and received ethical approval.
Figure 1

Flow diagram of participant selection.
CHARLS is a national longitudinal survey of Chinese adults aged 45 and older (13). This study used data from the 2011 baseline (Wave 1), which included 17,705 participants. Based on study requirements, we excluded individuals under 45 years old at baseline (n = 404), those with missing sex information (n = 12), participants missing key biomarkers for KDM-BA or AL or SUA (n = 10,935), and those lost to follow-up during the 2013 survey (n = 414). After exclusions, 5,940 participants were included to assess the relationship between baseline SUA levels, accelerated aging, and all-cause mortality (Figure 1). This project was approved by the Institutional Review Board at Peking University Medical School, and all participants provided written informed consent (IRB00001052-11014 and IRB00001052-11015).
BA measurement
To quantify aging, we utilized several previously published BA algorithms in both cohorts, including the KDM-BA, PhenoAge, and AL.
In the NHANES cohort, KDM-BA was calculated using the Klemera & Doubal method (14), which integrates eight biomarkers: hemoglobin A1c, systolic blood pressure (SBP), C-reactive protein, serum albumin, total cholesterol, alkaline phosphatase, serum creatinine, and blood urea nitrogen. PhenoAge, based on a multivariable analysis of mortality risk proposed by Levine et al. (15), uses nine clinical biomarkers—glucose, alkaline phosphatase, albumin, creatinine, C-reactive protein, white blood cell count, lymphocyte percentage, mean corpuscular volume, and red cell distribution width—to estimate an individual’s predicted age.
For the CHARLS cohort, KDM-BA was calculated using an adjusted algorithm validated for the Chinese population (16), which included total cholesterol, triglycerides, hemoglobin A1c, urea, creatinine, high-sensitivity C-reactive protein, platelet count, and SBP. Additionally, AL was used to assess cumulative physiological stress across multiple systems (17). This involved 14 biomarkers from various physiological systems, including systolic and diastolic blood pressure, body mass index (BMI), high-density lipoprotein (HDL), low-density lipoprotein (LDL), total cholesterol, triglycerides, HbA1c, fasting glucose, hemoglobin, C-reactive protein, creatinine, cystatin C, and blood urea nitrogen. Values exceeding established high-risk thresholds were assigned a score of 1, while lower values received a score of 0. The total AL score ranged from 0 to 14. KDM-BA and PhenoAge were computed using the R package BioAge2 (18). For each algorithm, aging acceleration was defined as ΔAge = BA − CA, where positive values indicate faster biological aging relative to chronological age. Detailed formulas and biomarker compositions for KDM-BA, PhenoAge, and AL are described in the Supplementary materials.
Mortality outcome determination
In NHANES, mortality data through December 31, 2019, were obtained from the National Death Index, matched with death certificate records. The primary outcomes of interest were all-cause mortality and premature mortality, defined as death before age 70, based on global life expectancy in 2010 (19). In CHARLS, all-cause mortality was confirmed during the 2013 follow-up, with exact death dates recorded to calculate the interval between baseline birth dates and death dates. Premature mortality was also defined as death before age 70.
Covariates
In the NHANES cohort, covariates included sex, race, age, marital status, BMI (<25 or ≥25) (20), education level (categorized as below high school or high school and above, based on whether participants had completed 12th grade or obtained a high school diploma), family income-to-poverty ratio (PIR: <1, 1–3, >3), smoking status (never or current smoker), and drinking habits (never, low-to-moderate, heavy), as well as disease information for hypertension, diabetes, hyperlipidemia, atherosclerotic cardiovascular disease (ASCVD), and CKD. In the CHARLS cohort, covariates included sex, age, residential area (urban or rural), geographical location (south or north), education level, marital status, drinking frequency (none, less than once a month, or once a month or more), BMI (<24 or ≥24) (21), smoking status (never or current smoker), and diagnoses for diabetes, hypertension, hyperlipidemia, CVD, and CKD. In both studies, hyperuricemia was defined as SUA levels ≥420 μmol/L (7 mg/dL) for male and ≥360 μmol/L (6 mg/dL) for female (10). Hypertension was defined as average SBP ≥ 140 mmHg, average diastolic blood pressure (DBP) ≥ 90 mmHg, or confirmation via physician diagnosis or recorded antihypertensive medication use. Diabetes was defined based on the following criteria: self-reported diagnosis, HbA1c > 6.5%, fasting glucose ≥7.0 mmol/L, random glucose ≥11.1 mmol/L, or 2-h oral glucose tolerance test (OGTT) glucose ≥11.1 mmol/L, or the use of diabetes medications or insulin (22). Hyperlipidemia was defined as triglycerides ≥150 mg/dL, total cholesterol ≥200 mg/dL, LDL-C ≥ 130 mg/dL, or HDL-C ≤ 40 mg/dL for men and ≤50 mg/dL for women; participants reporting the use of cholesterol-lowering medications were also considered to have hyperlipidemia (23). ASCVD was diagnosed if participants reported being informed by a doctor or healthcare professional that they had coronary artery disease, angina, heart attack, or stroke. The diagnostic criteria for CVD in CHARLS are based on two survey questions: “Have you been told by a doctor that you have been diagnosed with heart disease (including angina, heart attack, heart failure, coronary heart disease, or other heart problems)?” and “Have you been told by a doctor that you have been diagnosed with a stroke?.” CKD was defined by estimated glomerular filtration rate (eGFR) < 59 mL/min/1.73 m2 or albumin-to-creatinine ratio (ACR) > 30 mg/g (24).
Sensitivity analysis
For the NHANES cohort, we included participants from the 1999–2002 cycles (n = 2,525), which uniquely provide access to DNA methylation data (Figure 1). This enabled the calculation of epigenetic biological age using two established biomarkers: Levine’s DNA methylation–based PhenoAge (DNAmPhenoAge) (15) and Horvath’s DNA methylation-predicted mortality (GrimAge) (25). These measures were derived from DNA methylation-based telomere length (DNAmTL) or quantitative polymerase chain reaction–based telomere length (qPCRTL) data and allowed for a sensitivity analysis incorporating epigenetic markers of aging. In the CHARLS cohort, we used data from the 2015 follow-up survey (n = 10,327) to replicate the primary analysis (Figure 1). This wave was chosen for its superior data completeness and a longer observational interval compared to the 2013 survey, thereby enhancing the robustness of aging-related inferences.
Statistical analysis
In the NHANES cohort analysis, sampling weights, stratification, and clustering were incorporated into all analyses. Data are presented as weighted mean ± standard error (SE) for continuous variables and as unweighted frequencies with weighted percentages for categorical variables. In the CHARLS cohort, unweighted analyses were performed. Continuous variables are presented as mean ± standard deviation (SD), and categorical variables as frequencies with percentages. Generalized linear regression models were used to evaluate the relationship between baseline SUA and biological aging (ΔKDM-BA, ΔPhenoAge, AL). Cox regression models were employed to estimate the hazard ratios (HR) and 95% confidence intervals (CI) for the association between baseline SUA and all-cause mortality and premature mortality, adjusting for multiple covariates in different models. To explore the dose–response relationship between SUA and outcomes and visualize it, restricted cubic splines (RCS) were used, with percentiles set at the 10th, 50th, and 90th percentiles (26). Kaplan–Meier survival curves were used to visualize survival rates, and log-rank tests were conducted to compare survival differences between groups. Stratified and interaction analyses were performed for subgroups such as age, sex, BMI, smoking, drinking, diabetes, hypertension, hyperlipidemia, and CKD to examine their modifying effects on the main outcomes. All statistical analyses were performed using R software (version 4.4.2), with two-sided p < 0.05 considered statistically significant.
Results
Baseline characteristics
In the NHANES cohort (n = 26,192), the mean age was 46.85 ± 0.22 years, with females comprising 50.72%. The mean SUA level was 5.43 ± 0.01 mg/dL, with approximately 20.6% (n = 5,402) meeting the criteria for hyperuricemia. Individuals with hyperuricemia tended to be male, older, and former smokers, with higher rates of BMI, HbA1c, and various chronic diseases (hypertension, diabetes, hyperlipidemia, ASCVD, CKD) (Table 1). In the CHARLS cohort (n = 5,940), the mean age was 60.72 ± 9.84 years, with females comprising 53.41%. The mean SUA level was 4.44 ± 1.25 mg/dL, with approximately 5.4% (n = 323) meeting the criteria for hyperuricemia. In this cohort, individuals with hyperuricemia were also typically older, had a higher proportion of males, lived more often in urban areas, and frequently had comorbidities such as hypertension, diabetes, hyperlipidemia, ASCVD, or CKD (Table 2).
Table 1
| NHANES | Total (n = 26,192) | Without hyperuricemia (n = 20,790) | With hyperuricemia (n = 5,402) | p value |
|---|---|---|---|---|
| Age, years, mean (SE) | 46.85 ± 0.22 | 45.86 ± 0.21 | 50.92 ± 0.35 | < 0.0001 |
| Sex, n (%) | < 0.0001 | |||
| Female | 12,977 (50.72) | 10,615 (52.88) | 2,362 (41.87) | |
| Male | 13,215 (49.28) | 10,175 (47.12) | 3,040 (58.13) | |
| Ethnicity, n (%) | < 0.0001 | |||
| White | 13,208 (72.04) | 10,352 (71.75) | 2,856 (73.24) | |
| Black | 4,885 (10.14) | 3,641 (9.79) | 1,244 (11.59) | |
| Mexican | 5,335 (7.70) | 4,557 (8.25) | 778 (5.44) | |
| Other | 2,764 (10.12) | 2,240 (10.21) | 524 (9.74) | |
| Marital status, n (%) | < 0.0001 | |||
| Married/live with partner | 15,776 (63.48) | 12,681 (65.28) | 3,095 (62.26) | |
| Never married | 4,132 (16.31) | 3,397 (16.86) | 735 (15.61) | |
| Widowed/divorced/separated | 5,874 (18.34) | 4,387 (17.85) | 1,487 (22.13) | |
| Education, n (%) | 0.04 | |||
| High school and above | 18,156 (80.48) | 14,430 (80.82) | 3,726 (79.53) | |
| Below high school | 7,997 (19.41) | 6,328 (19.18) | 1,669 (20.47) | |
| Alcohol user, n (%) | 0.15 | |||
| Heavy | 4,932 (20.00) | 3,918 (20.94) | 1,014 (21.74) | |
| Low-to-moderate | 16,339 (63.78) | 12,943 (67.63) | 3,396 (65.91) | |
| Never | 3,434 (11.00) | 2,712 (11.42) | 722 (12.36) | |
| Smoke, n (%) | < 0.0001 | |||
| Former | 6,854 (25.13) | 5,076 (23.71) | 1778 (31.01) | |
| Never | 13,460 (51.27) | 10,879 (51.89) | 2,581 (48.88) | |
| Now | 5,853 (23.54) | 4,814 (24.39) | 1,039 (20.11) | |
| BMI, kg/m2, mean (SE) | 28.29 ± 0.08 | 27.50 ± 0.07 | 31.59 ± 0.15 | < 0.0001 |
| Uric acid, mg/dl, mean (SE) | 5.43 ± 0.01 | 4.94 ± 0.01 | 7.43 ± 0.02 | < 0.0001 |
| HbA1c, %, mean (SE) | 5.51 ± 0.01 | 5.49 ± 0.01 | 5.62 ± 0.01 | < 0.0001 |
| KDM-BA, years, mean (SE) | 46.32 ± 0.23 | 44.72 ± 0.22 | 52.85 ± 0.36 | < 0.0001 |
| ΔKDM-BA, years, mean (SE) | −0.54 ± 0.10 | −1.14 ± 0.10 | 1.93 ± 0.16 | < 0.0001 |
| PhenoAge, years, mean (SE) | 42.21 ± 0.25 | 40.66 ± 0.24 | 48.57 ± 0.37 | < 0.0001 |
| ΔPhenoAge, years, mean (SE) | −4.64 ± 0.08 | −5.20 ± 0.08 | −2.35 ± 0.12 | < 0.0001 |
| Hypertension, n (%) | 11,071(36.00) | 7,740(31.33) | 3,331(55.16) | < 0.0001 |
| Diabetes, n (%) | 4,180 (11.18) | 2,934 (9.63) | 1,246 (17.56) | < 0.0001 |
| Hyperlipidemia, n (%) | 19,400 (72.93) | 14,878 (70.21) | 4,522 (84.08) | < 0.0001 |
| ASCVD, n (%) | 2,822 (8.03) | 1872 (6.65) | 950 (13.67) | < 0.0001 |
| CKD, n (%) | 4,911 (13.84) | 3,105 (11.02) | 1806 (25.96) | < 0.0001 |
| Death during follow-up, n (%) | 5,907 (16.25) | 4,110 (14.13) | 1797 (24.92) | < 0.0001 |
| Premature death during follow-up, n (%) | 1,313 (4.93) | 947 (31.94) | 366 (26.68) | 0.002 |
Baseline characteristics of NHANES participants.
Data are presented as weighted mean ± standard error (SE) for continuous variables and as unweighted frequencies with weighted percentages for categorical variables. p-values were calculated using survey-weighted t-tests for continuous variables and Chi-square tests for categorical variables to compare characteristics between groups. BMI, body mass index; KDM-BA, Klemera–Doubal Method Biological Age; CKD, chronic kidney disease; ASCVD, atherosclerotic cardiovascular disease.
Table 2
| CHARLS | Total (n = 5,940) | Without hyperuricemia (n = 5,617) | With hyperuricemia (n = 323) | p value |
|---|---|---|---|---|
| Age, years, mean (SD) | 60.72 ± 9.84 | 60.56 ± 9.80 | 63.34 ± 10.09 | <0.0001 |
| Sex, n (%) | <0.01 | |||
| Female | 3,148 (53.00) | 3,000 (53.41) | 148 (45.82) | |
| Male | 2,792 (47.00) | 2,617 (46.59) | 175 (54.18) | |
| Marital status, n (%) | 0.23 | |||
| Married | 4,919 (82.81) | 4,654 (82.86) | 265 (82.04) | |
| Never married | 43 (0.72) | 43(0.77) | ||
| Widowed/divorced/separated | 978 (16.46) | 920 (16.38) | 58 (17.96) | |
| Education, n (%) | 0.92 | |||
| High school and above | 452 (7.62) | 429 (7.64) | 23 (7.12) | |
| Below high school | 5,486 (92.39) | 5,186 (92.36) | 300 (92.88) | |
| Residence place, n (%) | 0.02 | |||
| Rural | 3,892 (65.52) | 3,701 (65.89) | 191 (59.13) | |
| Urban | 2048 (34.48) | 1916 (34.11) | 132 (40.87) | |
| Drink frequency last year, n (%) | 0.07 | |||
| >1/month | 1,484 (24.98) | 1,389 (24.73) | 95 (29.41) | |
| <1/month | 446 (7.51) | 429 (7.64) | 17 (5.26) | |
| No | 4,010 (67.51) | 3,799 (67.63) | 211 (65.33) | |
| Smoke status, n (%) | 0.21 | |||
| Never | 3,591 (60.46) | 3,407 (60.67) | 184 (56.97) | |
| Smoke | 2,348 (39.54) | 2,209 (39.33) | 139 (43.03) | |
| BMI, kg/m2, mean (SD) | 23.42 ± 3.92 | 23.37 ± 3.90 | 24.38 ± 4.21 | <0.0001 |
| Uric acid, mg/dl, mean (SD) | 4.44 ± 1.25 | 4.27 ± 1.05 | 7.36 ± 0.96 | <0.0001 |
| HbA1c, %, mean (SD) | 5.29 ± 0.81 | 5.29 ± 0.82 | 5.30 ± 0.62 | 0.73 |
| KDM-BA, years, mean (SD) | 60.18 ± 10.57 | 59.84 ± 10.48 | 66.08 ± 10.36 | <0.0001 |
| ΔKDM-BA, years, mean (SD) | −0.53 ± 4.27 | −0.72 ± 4.16 | 2.73 ± 4.68 | <0.0001 |
| Allostatic load, score, mean (SD) | 2.52 ± 1.75 | 2.44 ± 1.71 | 3.86 ± 1.91 | <0.0001 |
| Hypertension, n (%) | 2,553 (42.98) | 2,358 (41.98) | 195 (60.37) | <0.0001 |
| Diabetes, n (%) | 937 (15.77) | 860 (15.31) | 77 (23.84) | <0.0001 |
| Hyperlipidemia, n (%) | 1,405 (23.65) | 1,263 (22.49) | 142 (43.96) | <0.0001 |
| CVD, n (%) | 826 (13.94) | 768 (13.71) | 58 (18.01) | 0.04 |
| CKD, n (%) | 153 (2.58) | 96 (1.71) | 57 (17.65) | <0.0001 |
| Death during follow-up, n (%) | 97 (1.63) | 92 (1.64) | 5 (1.55) | 1.00 |
| Premature death during follow-up, n (%) | 33 (0.56) | 31 (0.55) | 2 (0.62) | 0.95 |
Baseline characteristics of CHARLS participants.
Data are presented as mean ± standard deviation (SD) for continuous variables and as frequencies with percentages for categorical variables. p-values were calculated using independent-samples t-tests for continuous variables and Chi-square tests for categorical variables to compare characteristics between groups. BMI, body mass index; KDM-BA, Klemera–Doubal Method Biological Age; CKD, chronic kidney disease; CVD, cardiovascular disease.
Association between SUA and accelerated aging
In both cohorts, baseline SUA levels were significantly positively correlated with multiple markers of accelerated aging. In the NHANES cohort, for each 1 mg/dL increase in SUA, the difference between ΔKDM-BA increased by 1.22 years (95% CI: 1.14–1.29, p < 0.0001), and the difference between ΔPhenoAge increased by 0.99 years (95% CI: 0.92–1.07, p < 0.0001). In the CHARLS cohort, for every 1 mg/dL increase in SUA, the difference between ΔKDM-BA increased by 1.25 years (95% CI: 1.17–1.33, p < 0.0001) in the crude model. Similarly, in the crude model, each 1 mg/dL increase in SUA was associated with an increase of 0.34 in AL (95% CI: 0.30–0.37, p < 0.0001). After comprehensive adjustment for confounding factors, this association weakened but remained statistically significant (Table 3). In multivariable regression, while the effect sizes for ΔKDM-BA and AL decreased slightly after stepwise adjustments, they still indicated a stable positive association between elevated SUA levels, accelerated aging, and increased multi-system stress load.
Table 3
| NHANES | ΔKDM-BA | P | ΔPhenotypic age | p |
|---|---|---|---|---|
| Crude model | 1.22 (1.14,1.29) | <0.0001 | 0.99 (0.92,1.07) | <0.0001 |
| Model 1 | 1.15 (1.05, 1.24) | <0.0001 | 0.85 (0.76, 0.94) | <0.0001 |
| Model 2 | 0.93 (0.82, 1.03) | <0.0001 | 0.63 (0.53, 0.72) | <0.0001 |
| Model 3 | 0.52 (0.43, 0.61) | <0.0001 | 0.38 (0.29, 0.47) | <0.0001 |
| CHARLS | ΔKDM-BA | P | Allostatic load | P |
|---|---|---|---|---|
| Crude model | 1.25 (1.17,1.33) | <0.0001 | 0.34 (0.30,0.37) | <0.0001 |
| Model 1 | 1.10 (1.01, 1.19) | <0.0001 | 0.42 (0.38, 0.46) | <0.0001 |
| Model 2 | 1.00 (0.91, 1.09) | <0.0001 | 0.33 (0.30, 0.37) | <0.0001 |
| Model 3 | 0.65 (0.57, 0.74) | <0.0001 | 0.15 (0.12,0.18) | <0.0001 |
Association between SUA and accelerated aging.
For NHANES: model 1: age, ethnicity, sex, education, marital status, poverty; model 2: age, ethnicity, sex, education, marital status, poverty, BMI, alcohol user, smoke; model 3: age, ethnicity, sex, education, marital status, poverty, BMI, alcohol user, smoke, diabetes, hypertension, hyperlipidemia, atherosclerotic cardiovascular disease, chronic kidney disease. For CHARLS: model 1: sex, age, residence place, south/north, education, marital status; model 2: sex, age, residence place, south/north, education, marital status, drink frequency, smoke status, BMI; model 3: sex, age, residence place, south/north, education, marital status, drink frequency, smoke status, BMI, diabetes, hypertension, hyperlipidemia, cardiovascular disease, chronic kidney disease.
Nonlinear relationship between SUA and accelerated aging
Using RCS to assess the dose–response relationship between SUA and accelerated aging, we found a significant nonlinear association with ΔPhenoAge in the NHANES cohort (nonlinear-p < 0.0001), with accelerated aging effects becoming more pronounced after SUA levels exceeded approximately 4.16 mg/dL. SUA was also nonlinearly associated with ΔKDM-BA (nonlinear-p = 0.02) (Figures 2A,B). In the CHARLS cohort, SUA was nonlinearly associated with ΔKDM-BA (nonlinear-p = 0.01) and linearly associated with AL (nonlinear-p = 0.31) (Figures 2C,D). The overall trend showed that higher SUA levels were associated with a shift toward accelerated aging markers.
Figure 2

The RCS analysis between serum uric acid and accelerated aging. (A) NHANES - ΔKlemera-Doubal Biological Age, (B) NHANES - Δphenotypic age, (C) CHARLS - ΔKlemera-Doubal Biological Age, (C) CHARLS - allostatic load. For NHANES, the model was adjusted for age, ethnicity, sex, education, marital status, poverty, BMI, alcohol user, smoke, diabetes, hypertension, hyperlipidemia, atherosclerotic cardiovascular disease, chronic kidney disease. For CHARLS, the model was adjusted for sex, age, residence place, south/north, education, marital status, drink frequency, smoke status, BMI, diabetes, hypertension, hyperlipidemia, cardiovascular disease, chronic kidney disease.
Subgroup analysis
After stratification by age, sex, BMI, smoking, drinking, diabetes, hypertension, hyperlipidemia, CKD, and other factors in both NHANES and CHARLS, the impact of SUA on accelerated aging markers remained consistent across most subgroups (Figures 3A–D). Some subgroups exhibited statistical interactions, suggesting that SUA had a more significant effect on accelerated aging in older adults, females and CKD patients.
Figure 3

Subgroup analysis of the association between serum uric acid and accelerated aging. (A) NHANES - ΔKlemera-Doubal Biological Age, (B) NHANES - Δphenotypic age, (C) CHARLS - ΔKlemera-Doubal Biological Age, (D) CHARLS - allostatic load. For NHANES, the model was adjusted for age, ethnicity, sex, education, marital status, poverty, BMI, alcohol user, smoke, diabetes, hypertension, hyperlipidemia, atherosclerotic cardiovascular disease, chronic kidney disease. For CHARLS, the model was adjusted for sex, age, residence place, south/north, education, marital status, drink frequency, smoke status, BMI, diabetes, hypertension, hyperlipidemia, cardiovascular disease, chronic kidney disease.
SUA and risk of all-cause and premature mortality
During long-term follow-up in NHANES (mean duration: 12.9 years), a total of 5,907 deaths were recorded, including 1,313 premature deaths. Cox regression analysis showed a statistically significant positive association between elevated SUA and both all-cause mortality and premature mortality. In the fully adjusted model, each 1 mg/dL increase in SUA was associated with a HR for all-cause mortality of 1.04 (95% CI: 1.01–1.07, p = 0.01), and an HR for premature mortality of 1.06 (95% CI: 1.00–1.13, p = 0.046) (Table 4). The restricted cubic spline results indicated a U-shaped nonlinear relationship between SUA and mortality risk (Figures 4A,B), and Kaplan–Meier survival analysis showed a higher cumulative mortality rate in individuals with hyperuricemia (Figures 4C,D). Subgroup analysis trends were consistent with the main results, with SUA having a greater impact on mortality or premature mortality in individuals with chronic diseases, although no significant interaction was observed (Figures 4E,F).
Table 4
| Death | p | Premature death | p | |
|---|---|---|---|---|
| NHANES | ||||
| Crude model | 1.23 (1.20,1.26) | <0.0001 | 1.202 (1.150,1.256) | <0.0001 |
| Model 1 | 1.10 (1.07, 1.13) | <0.0001 | 1.074 (1.002,1.151) | 0.044 |
| Model 2 | 1.12 (1.09, 1.16) | <0.0001 | 1.090 (1.016,1.170) | 0.016 |
| Model 3 | 1.04 (1.01, 1.07) | 0.01 | 1.064 (1.001,1.132) | 0.046 |
| CHARLS | ||||
| Crude model | 1.08 (0.93,1.26) | 0.30 | 1.07 (0.82,1.39) | 0.62 |
| Model 1 | 0.91 (0.77, 1.08) | 0.28 | 0.94 (0.69, 1.28) | 0.70 |
| Model 2 | 0.92 (0.77,1.10) | 0.36 | 0.93 (0.68, 1.27) | 0.64 |
| Model 3 | 0.90 (0.75,1.08) | 0.25 | 0.93 (0.68, 1.27) | 0.64 |
Association between SUA and risk of all-cause and premature mortality.
For NHANES: model 1: age, ethnicity, sex, education, marital status, poverty; model 2: age, ethnicity, sex, education, marital status, poverty, BMI, alcohol user, smoke; model 3: age, ethnicity, sex, education, marital status, poverty, BMI, alcohol user, smoke, diabetes, hypertension, hyperlipidemia, atherosclerotic cardiovascular disease, chronic kidney disease. For CHARLS: model 1: sex, age, residence place, south/north, education, marital status; model 2: sex, age, residence place, south/north, education, marital status, drink frequency, smoke status, BMI; model 3: sex, age, residence place, south/north, education, marital status, drink frequency, smoke status, BMI, diabetes, hypertension, hyperlipidemia, cardiovascular disease, chronic kidney disease.
Figure 4

Association between serum uric acid and the risk of all-cause and premature mortality. Panel (A,B) show the results of RCS analysis depicting the relationship between serum uric acid levels and all-cause mortality and premature mortality, respectively. Kaplan–Meier survival curves are presented for all-cause mortality (C) and premature mortality (D). Panel (E,F) display subgroup analyses evaluating the association between SUA levels and all-cause mortality and premature mortality, respectively. The model was adjusted for age, ethnicity, sex, education, marital status, poverty, BMI, alcohol user, smoke, diabetes, hypertension, hyperlipidemia, atherosclerotic cardiovascular disease, chronic kidney disease.
In the short-term follow-up in CHARLS (mean duration: 2 years), 97 deaths and 33 premature deaths were observed. Cox regression analysis did not find a significant association between elevated SUA and all-cause or premature mortality (Table 4). In various adjusted models, the HR for all-cause mortality did not reach statistical significance. Specifically, in the crude model, each 1 mg/dL increase in SUA was associated with an HR for all-cause mortality of 1.08 (95% CI: 0.93–1.26, p = 0.30). After adjustment for demographic factors (age, sex, etc.), the HR decreased to 0.91 (95% CI: 0.77–1.08, p = 0.28). Further adjustment for lifestyle factors (BMI, smoking, drinking) yielded an HR of 0.92 (95% CI: 0.77–1.10, p = 0.36). In the fully adjusted model (including diabetes, hypertension, CVD, etc.), the association between SUA levels and all-cause mortality was not significant (HR = 0.90, 95% CI: 0.75–1.08, p = 0.25). Similarly, no significant association between SUA levels and premature mortality was observed in all models.
Sensitivity analysis
In NHANES, a subset of participants from the 1999–2002 period was included, and DNA methylation-derived BA was recalculated (Figures 5A–F). A sensitivity analysis was conducted by including the 2015 follow-up population in CHARLS, and the results were consistent with the main analysis (Figures 6A–F). The association between SUA and aging processes remained consistent with the primary analysis, supporting the robustness of the findings.
Figure 5
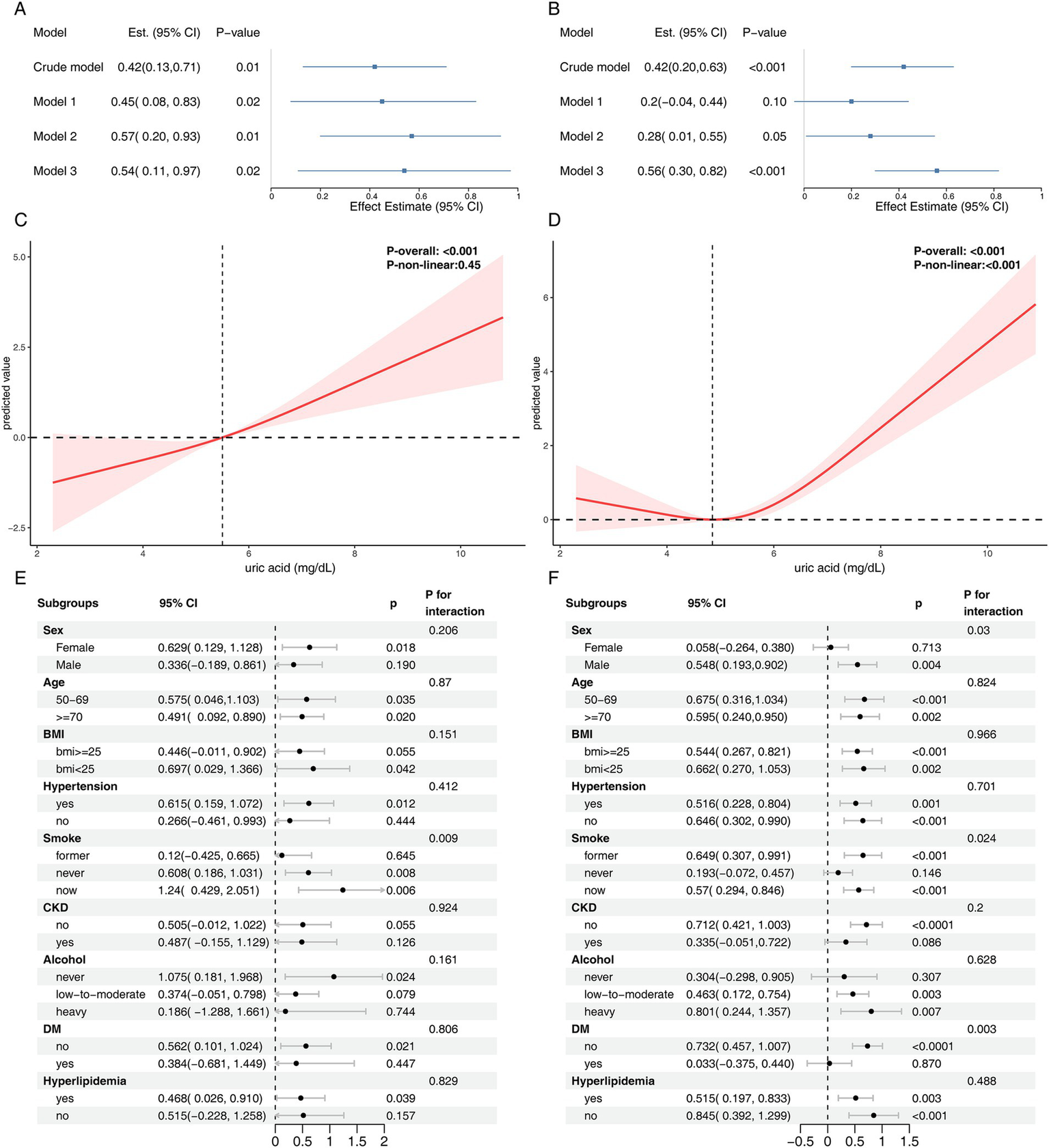
Sensitivity analysis results for the NHANES cohort. Panels (A,B) present the association between serum uric acid levels and ΔDNA methylation–based PhenoAge and ΔGrimAge. Panels (C,D) display the results of RCS analysis examining the relationship between serum uric acid and ΔDNA methylation–based PhenoAge and ΔGrimAge, respectively. Subgroup analyses of the association between serum uric acid levels and ΔDNA methylation–based PhenoAge and ΔGrimAge are shown in panel (E,F). Model 1: age, ethnicity, sex, education, marital status, poverty; Model 2: age, ethnicity, sex, education, marital status, poverty, BMI, alcohol user, smoke; Model 3: age, ethnicity, sex, education, marital status, poverty, BMI, alcohol user, smoke, diabetes, hypertension, hyperlipidemia, atherosclerotic cardiovascular disease, chronic kidney disease.
Figure 6

Sensitivity analysis results for the CHARLS cohort. Panels (A,B) present the association between serum uric acid levels and Klemera-Doubal Biological Age and allostatic load, respectively, in the CHARLS cohort. Panels (C,D) display the results of RCS analysis examining the relationship between serum uric acid and Klemera-Doubal Biological Age and allostatic load, respectively. Subgroup analyses of the association between serum uric acid levels and (E) Klemera-Doubal Biological Age and (F) allostatic load are shown. Model 1: sex, age, place of residence, south/north, education, marital status; Model 2: sex, age, place of residence, south/north, education, marital status, drink frequency, smoking status, BMI; Model 3: sex, age, place of residence, south/north, education, marital status, drink frequency, smoking status, BMI, diabetes, hypertension, hyperlipidemia, cardiovascular disease, chronic kidney disease.
Discussion
Uric acid, a product of purine metabolism, has long been a subject of debate regarding its role in aging and chronic disease. Initially recognized for its antioxidant properties (27), SUA was believed to counteract oxidative stress and free radical damage, thus potentially protecting against aging and cancer (28). However, increasing evidence now suggests that elevated SUA levels may contribute to oxidative stress (29, 30), leading to cellular damage and accelerating aging. Hyperuricemia has also been strongly associated with chronic low-grade inflammation, with studies indicating that SUA can induce an inflammatory state by promoting the release of pro-inflammatory cytokines such as IL-6 and TNF-α (31). Additionally, hyperuricemia contributes to endothelial dysfunction (32), increases arterial stiffness (33), and heightens the risk of hypertension and CVD. Furthermore, elevated SUA levels are thought to impact telomere length and mitochondrial function, thus accelerating the aging process at the molecular level (34, 35).
Epidemiological studies have demonstrated that SUA levels are significantly correlated with several aging-related diseases, including CVD (36–38), CKD (39, 40), diabetes (41, 42), and cognitive decline (43). This study extends these findings by systematically evaluating the impact of SUA on various aging markers and mortality outcomes in two large cohorts. Consistent with prior research, we found a strong association between elevated SUA levels and accelerated biological aging (35, 43, 44). Notably, our study employed three complementary aging assessment models—KDM-BA, PhenoAge, and AL—which further confirmed that this association remained stable after adjusting for a variety of covariates. Moreover, the RCS analysis revealed a nonlinear relationship between SUA and biological aging, indicating that there is no clear “safe” threshold for SUA levels with respect to biological aging. This result was validated in two distinct national cohorts, providing robust evidence for SUA’s role in aging.
Subgroup analyses revealed that the impact of SUA on accelerated biological aging was more pronounced in women and patients with CKD. This suggests that SUA may influence the aging process through different biological mechanisms across various populations. Estrogen has been shown to lower SUA levels by inhibiting the URAT1 transporter (45), but this protective effect is lost in postmenopausal women, who are at a higher risk for gout compared to premenopausal women (46). Previous studies have indicated that hyperuricemia accelerates CKD progression through activation of the renin-angiotensin-aldosterone system (RAAS), inhibition of nitric oxide (NO) synthesis, and induction of chronic inflammation (47). Our findings align with these studies, as we observed the most significant effect of SUA on biological aging in CKD patients, with a clear interaction with all-cause mortality. This may be due to urate-induced vascular damage, oxidative stress, and immune activation. While urate-lowering therapy may help slow CKD progression, direct evidence supporting its clinical benefits for aging and mortality is lacking. Future studies should explore the underlying mechanisms and potential interventions.
The relationship between hyperuricemia, urate-lowering therapy, and mortality risk remains a topic of contention. Many observational studies have suggested a U-shaped curve between SUA levels and mortality risk (48–50), and our findings in the NHANES cohort are consistent with these studies, showing a U-shaped relationship between SUA levels and both all-cause and premature mortality. However, this relationship was not observed in the CHARLS cohort, likely due to its shorter follow-up duration (mean: 2.0 years) compared to NHANES (mean: 12.9 years), which may have reduced the statistical power to detect significant mortality associations. Notably, the average SUA in the CHARLS cohort (4.44 mg/dL) was significantly lower than in the NHANES cohort (5.43 mg/dL), and the prevalence of hyperuricemia was also much lower (5.4% vs. 20.6%). This discrepancy may be attributed to racial differences or dietary patterns, such as low-purine diets (51), though the lack of dietary data in CHARLS prevents further investigation. Previous studies have shown that gout can lead to premature death (52), but the specific impact remains unclear. This study is the first to demonstrate a significant association between hyperuricemia and premature mortality. After adjusting for confounders, the association between SUA levels and premature mortality showed a U-shaped nonlinear relationship. Janis Timsans and colleagues suggested that hyperuricemia without renal dysfunction (metabolic hyperuricemia) may increase the risk of premature death through the induction of CVD (53), a finding that is consistent with our subgroup analysis. We observed that SUA had a more pronounced effect on premature death in individuals with hypertension, hyperlipidemia, ASCVD, and CKD, emphasizing the importance of managing SUA levels in cardiovascular, renal, and metabolic diseases.
SUA levels also reflect nutritional status (54), and some studies have found that sarcopenia and weight loss are more common in patients with low SUA levels (55). Differences in body composition may help explain the association between low SUA levels and higher mortality (56). Studies conducted on hemodialysis populations found that patients with lower SUA levels, along with a low lean tissue index and high geriatric nutrition risk index, exhibited greater mortality risk. This suggests that better nutritional status, rather than elevated SUA levels, may help reduce mortality risk (57). Furthermore, the effect of ULT on mortality risk remains debated. The CARES trial showed that febuxostat treatment in gout patients with CVD resulted in significantly higher all-cause (HR = 1.22) and cardiovascular (HR = 1.34) mortality rates compared to allopurinol, with the difference being more pronounced in patients with lower baseline SUA levels (58). This suggests that excessively lowering SUA may increase mortality risk, potentially due to the loss of SUA’s antioxidant effects, acute inflammation from urate crystal dissolution, and vascular dysfunction (59). Further research is needed to clarify the causal relationship between uric acid-lowering therapy and mortality.
This study advances our understanding of SUA’s role in biological aging through several methodological improvements. By using data from two nationally representative cohorts (NHANES and CHARLS), we provide the first multi-country evidence that elevated SUA levels are consistently associated with accelerated aging across various biomarkers, including Klemera-Doubal BA, PhenoAge, and AL. These findings were robust even after adjusting for various demographic, behavioral, and confounding factors. Furthermore, restricted cubic spline analysis further described the nonlinear relationship between SUA and mortality risk in the NHANES cohort. However, this study has several limitations. First, as an observational study, it cannot establish causality, and residual confounding from unmeasured factors (e.g., dietary patterns, genetic polymorphisms in urate transporters) may still be present despite comprehensive adjustments. Second, the statistical power of the CHARLS cohort is limited due to fewer death events (n = 97) and a shorter follow-up period, which may have weakened our ability to detect significant mortality associations. Third, reliance on a single SUA measurement may obscure the cumulative effects of chronic hyperuricemia, as longitudinal SUA trajectories are more strongly associated with clinical outcomes than static assessments. Fourth, while the use of standardized aging biomarkers enhances comparability, differences in biomarker measurement protocols across cohorts (e.g., CRP measurement methods) may introduce heterogeneity in BA measurements. Finally, although we applied a unified definition of premature mortality across cohorts to facilitate comparison, differences in life expectancy between the U.S. and China may affect its clinical relevance (19, 60, 61). Future studies should consider cohort-specific thresholds to better reflect population-level aging and mortality risk.
Conclusion
Based on data from the NHANES and CHARLS cohorts, we found that elevated SUA levels were significantly associated with accelerated biological aging in both populations. In the NHANES cohort, higher SUA levels were also linked to an increased risk of all-cause and premature mortality, with a U-shaped nonlinear relationship. However, this association was not observed in the CHARLS cohort, suggesting potential population-specific differences. These findings underscore the role of SUA as a potential contributor to aging and mortality risk, highlighting the need for further research to clarify the causal relationship and evaluate the long-term benefits and risks of uric acid-lowering strategies.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
This project was approved by the Institutional Review Board at Peking University Medical School, and all participants provided written informed consent (IRB00001052-11014 and IRB00001052-11015).
Author contributions
CZ: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. LZ: Conceptualization, Formal analysis, Methodology, Software, Supervision, Validation, Writing – original draft, Writing – review & editing. YL: Data curation, Validation, Writing – original draft. L-qS: Formal analysis, Methodology, Writing – original draft. X-rL: Supervision, Validation, Writing – original draft. YW: Data curation, Formal analysis, Project administration, Writing – review & editing. WS: Conceptualization, Funding acquisition, Investigation, Project administration, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was funded by Beijing University of Chinese Medicine Young Teachers Program (Grant No. Y2023B08). Weiwei Sun obtained the fundings.
Acknowledgments
We extend our appreciation to all the researchers who generously shared the data utilized in this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Author disclaimer
The views expressed in the paper are those of the authors and not necessarily of the listed funders.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2025.1569798/full#supplementary-material
References
1.
LiYTianXLuoJBaoTWangSWuX. Molecular mechanisms of aging and anti-aging strategies. Cell Commun Signal. (2024) 22:285. doi: 10.1186/s12964-024-01663-1
2.
TriposkiadisFXanthopoulosAButlerJ. Cardiovascular aging and heart failure: JACC review topic of the week. J Am Coll Cardiol. (2019) 74:804–13. doi: 10.1016/j.jacc.2019.06.053
3.
SpinelliRParrilloLLongoMFloresePDesiderioAZatteraleFet al. Molecular basis of ageing in chronic metabolic diseases. J Endocrinol Investig. (2020) 43:1373–89. doi: 10.1007/s40618-020-01255-z
4.
KritsilisMVRizouSKoutsoudakiPNEvangelouKGorgoulisVGPapadopoulosD. Ageing, cellular senescence and neurodegenerative disease. Int J Mol Sci. (2018) 19:2937. doi: 10.3390/ijms19102937
5.
ZhangBTrappAKerepesiCGladyshevVN. Emerging rejuvenation strategies-reducing the biological age. Aging Cell. (2022) 21:e13538. doi: 10.1111/acel.13538
6.
TanPKFarrarJEGaucherEAMinerJN. Coevolution of URAT1 and Uricase during primate evolution: implications for serum urate homeostasis and gout. Mol Biol Evol. (2016) 33:2193–200. doi: 10.1093/molbev/msw116
7.
BragaTTForniMFCorrea-CostaMRamosRNBarbutoJABrancoPet al. Soluble uric acid activates the NLRP3 Inflammasome. Sci Rep. (2017) 7:39884. doi: 10.1038/srep39884
8.
SochaMWMalinowskiBPukODubielMWicińskiM. The NLRP3 Inflammasome role in the pathogenesis of pregnancy induced hypertension and preeclampsia. Cells. (2020) 9:1642. doi: 10.3390/cells9071642
9.
WuMMaYChenXLiangNQuSChenH. Hyperuricemia causes kidney damage by promoting autophagy and NLRP3-mediated inflammation in rats with urate oxidase deficiency. Dis Model Mech. (2021) 14:dmm048041. doi: 10.1242/dmm.048041
10.
ChoSKChangYKimIRyuS. U-shaped association between serum uric acid level and risk of mortality: a cohort study. Arthritis Rheumatol. (2018) 70:1122–32. doi: 10.1002/art.40472
11.
HuangYLiJSunFZhouHJiangHChenL. U-shaped relationship between uric acid levels and all-cause mortality in patients with hypertension. Sci Rep. (2025) 15:97. doi: 10.1038/s41598-024-83831-4
12.
JohnsonCLPaulose-RamROgdenCLCarrollMDKruszon-MoranDDohrmannSMet al. National health and nutrition examination survey: analytic guidelines, 1999-2010. Vital Health Stat. (2013) 2:1–24.
13.
ZhaoYHuYSmithJPStraussJYangG. Cohort profile: the China health and retirement longitudinal study (CHARLS). Int J Epidemiol. (2014) 43:61–8. doi: 10.1093/ije/dys203
14.
KlemeraPDoubalS. A new approach to the concept and computation of biological age. Mech Ageing Dev. (2006) 127:240–8. doi: 10.1016/j.mad.2005.10.004
15.
LevineMELuATQuachAChenBHAssimesTLBandinelliSet al. An epigenetic biomarker of aging for lifespan and healthspan. Aging (Albany NY). (2018) 10:573–91. doi: 10.18632/aging.101414
16.
LiuZ. Development and validation of 2 composite aging measures using routine clinical biomarkers in the Chinese population: analyses from 2 prospective cohort studies. J Gerontol A Biol Sci Med Sci. (2021) 76:1627–32. doi: 10.1093/gerona/glaa238
17.
YeXZhuDDingRHeP. Association of life-course socioeconomic status with allostatic load in Chinese middle-aged and older adults. Geriatr Gerontol Int. (2022) 22:425–32. doi: 10.1111/ggi.14373
18.
KwonDBelskyDW. A toolkit for quantification of biological age from blood chemistry and organ function test data: BioAge. Geroscience. (2021) 43:2795–808. doi: 10.1007/s11357-021-00480-5
19.
Norheim OFJhaPAdmasuKGodalTHumRJKrukMEet al. Avoiding 40% of the premature deaths in each country, 2010-30: review of national mortality trends to help quantify the UN sustainable development goal for health. Lancet. (2015) 385:239–52. doi: 10.1016/S0140-6736(14)61591-9
20.
WHO consultation. Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser. (2000) 894:1–253.
21.
ZhouB-FCooperative Meta-analysis Group of the Working Group on obesity in China. Predictive values of body mass index and waist circumference for risk factors of certain related diseases in Chinese adults--study on optimal cut-off points of body mass index and waist circumference in Chinese adults. Biomed Environ Sci. (2002) 15:83–96. PMID:
22.
American Diabetes Association Professional Practice Committee. 2. Diagnosis and classification of diabetes: standards of Care in Diabetes-2024. Diabetes Care. (2024) 47:S20–42. doi: 10.2337/dc24-S002
23.
National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III) final report. Circulation. (2002) 106:3143–421.
24.
RovinBHAdlerSGBarrattJBridouxFBurdgeKAChanTMet al. KDIGO 2021 clinical practice guideline for the Management of Glomerular Diseases. Kidney Int. (2021) 100:S1–S276. doi: 10.1016/j.kint.2021.05.021
25.
LuATQuachAWilsonJGReinerAPAvivARajKet al. DNA methylation GrimAge strongly predicts lifespan and healthspan. Aging (Albany NY). (2019) 11:303–27. doi: 10.18632/aging.101684
26.
DesquilbetLMariottiF. Dose-response analyses using restricted cubic spline functions in public health research. Stat Med. (2010) 29:1037–57. doi: 10.1002/sim.3841
27.
NietoFJIribarrenCGrossMDComstockGWCutlerRG. Uric acid and serum antioxidant capacity: a reaction to atherosclerosis?Atherosclerosis. (2000) 148:131–9. doi: 10.1016/s0021-9150(99)00214-2
28.
AmesBNCathcartRSchwiersEHochsteinP. Uric acid provides an antioxidant defense in humans against oxidant- and radical-caused aging and cancer: a hypothesis. Proc Natl Acad Sci USA. (1981) 78:6858–62. doi: 10.1073/pnas.78.11.6858
29.
Zamudio-CuevasYMartínez-FloresKFernández-TorresJLoissell-BaltazarYAMedina-LunaDLópez-MacayAet al. Monosodium urate crystals induce oxidative stress in human synoviocytes. Arthritis Res Ther. (2016) 18:117. doi: 10.1186/s13075-016-1012-3
30.
ZhangMCuiRZhouYMaYJinYGouXet al. Uric acid accumulation in the kidney triggers mast cell degranulation and aggravates renal oxidative stress. Toxicology. (2023) 483:153387. doi: 10.1016/j.tox.2022.153387
31.
Luis-RodríguezDDonate-CorreaJMartín-NúñezEFerriCTaguaVGPérez CastroAet al. Serum urate is related to subclinical inflammation in asymptomatic hyperuricaemia. Rheumatology (Oxford). (2021) 60:371–9. doi: 10.1093/rheumatology/keaa425
32.
OtaniNToyodaSSakumaMHayashiKOuchiMFujitaTet al. Effects of uric acid on vascular endothelial function from bedside to bench. Hypertens Res. (2018) 41:923–31. doi: 10.1038/s41440-018-0095-4
33.
CanepaMViazziFStraitJBAmeriPPontremoliRBrunelliCet al. Longitudinal association between serum uric acid and arterial stiffness. Hypertension. (2017) 69:228–35. doi: 10.1161/HYPERTENSIONAHA.116.08114
34.
YuJLiuHHeSLiPMaCPingFet al. Negative Association of Serum URIC acid with peripheral blood cellular aging markers. J Nutr Health Aging. (2019) 23:547–51. doi: 10.1007/s12603-019-1200-3
35.
LvZCuiJZhangJ. Associations between serum urate and telomere length and inflammation markers: Evidence from UK biobank cohort. Front Immunol. (2022) 13:1065739. doi: 10.3389/fimmu.2022.1065739
36.
PerticoneMMaioRShehajEGigliottiSCaroleoBSuraciEet al. Sex-related differences for uric acid in the prediction of cardiovascular events in essential hypertension. A population prospective study. Cardiovasc Diabetol. (2023) 22:298. doi: 10.1186/s12933-023-02006-z
37.
CuiKSongYYinDSongWWangHZhuCet al. Uric acid levels, number of standard modifiable cardiovascular risk factors, and prognosis in patients with coronary artery disease: a large cohort study in Asia. J Am Heart Assoc. (2023) 12:e030625. doi: 10.1161/JAHA.123.030625
38.
KuwabaraMHisatomeINiwaKHaraSRoncal-JimenezCABjornstadPet al. Uric acid is a strong risk marker for developing hypertension from prehypertension: a 5-year Japanese cohort study. Hypertension. (2018) 71:78–86. doi: 10.1161/HYPERTENSIONAHA.117.10370
39.
SilvaNRGonçalvesCETGonçalvesDLNCottaRMMda SilvaLS. Association of uric acid and uric acid to creatinine ratio with chronic kidney disease in hypertensive patients. BMC Nephrol. (2021) 22:311. doi: 10.1186/s12882-021-02521-9
40.
GonçalvesDLNMoreiraTRda SilvaLS. A systematic review and meta-analysis of the association between uric acid levels and chronic kidney disease. Sci Rep. (2022) 12:6251. doi: 10.1038/s41598-022-10118-x
41.
van der SchaftNBrahimajAWenK-XFrancoOHDehghanA. The association between serum uric acid and the incidence of prediabetes and type 2 diabetes mellitus: the Rotterdam study. PLoS One. (2017) 12:e0179482. doi: 10.1371/journal.pone.0179482
42.
TianXWangAZuoYChenSZhangLZhaoYet al. Time course of serum uric acid accumulation and the risk of diabetes mellitus. Nutr Diabetes. (2022) 12:1. doi: 10.1038/s41387-021-00179-8
43.
KueiderAMAnYTanakaTKitner-TrioloMHStudenskiSFerrucciLet al. Sex-dependent associations of serum uric acid with brain function during aging. J Alzheimers Dis. (2017) 60:699–706. doi: 10.3233/JAD-170287
44.
BulutOTembaGSKoekenVACMMoorlagSJCFMde BreeLCJMouritsVPet al. Common and distinct metabolomic markers related to immune aging in Western European and east African populations. Mech Ageing Dev. (2024) 218:111916. doi: 10.1016/j.mad.2024.111916
45.
LiuLZhaoTShanLCaoLZhuXXueY. Estradiol regulates intestinal ABCG2 to promote urate excretion via the PI3K/Akt pathway. Nutr Metab (Lond). (2021) 18:63. doi: 10.1186/s12986-021-00583-y
46.
HakAECurhanGCGrodsteinFChoiHK. Menopause, postmenopausal hormone use and risk of incident gout. Ann Rheum Dis. (2010) 69:1305–9. doi: 10.1136/ard.2009.109884
47.
PonticelliCPodestàMAMoroniG. Hyperuricemia as a trigger of immune response in hypertension and chronic kidney disease. Kidney Int. (2020) 98:1149–59. doi: 10.1016/j.kint.2020.05.056
48.
ZhengYOuJHuangDZhouZDongXChenJet al. The U-shaped relationship between serum uric acid and Long-term all-cause mortality in coronary artery disease patients: a cohort study of 33,034 patients. Front Cardiovasc Med. (2022) 9:858889. doi: 10.3389/fcvm.2022.858889
49.
TsengW-CChenY-TOuS-MShihC-JTarngD-C. Taiwan geriatric kidney disease (TGKD) research group. U-shaped association between serum uric acid levels with cardiovascular and all-cause mortality in the elderly: the role of malnourishment. J Am Heart Assoc. (2018) 7:e007523. doi: 10.1161/JAHA.117.007523
50.
OddenMCAmaduA-RSmitELoLPeraltaCA. Uric acid levels, kidney function, and cardiovascular mortality in US adults: National Health and nutrition examination survey (NHANES) 1988-1994 and 1999-2002. Am J Kidney Dis. (2014) 64:550–7. doi: 10.1053/j.ajkd.2014.04.024
51.
BeydounMAFanelli-KuczmarskiMTCanasJ-ABeydounHAEvansMKZondermanAB. Dietary factors are associated with serum uric acid trajectory differentially by race among urban adults. Br J Nutr. (2018) 120:935–45. doi: 10.1017/S0007114518002118
52.
FisherMCRaiSKLuNZhangYChoiHK. The unclosing premature mortality gap in gout: a general population-based study. Ann Rheum Dis. (2017) 76:1289–94. doi: 10.1136/annrheumdis-2016-210588
53.
TimsansJKerolaAMRantalaihoVMHakkarainenKNKautiainenHJKauppiMJ. “Metabolic” type of hyperuricemia increases mortality mainly by leading to premature death from cardiovascular disease. Mayo Clin Proc. (2024) 99:1835–7. doi: 10.1016/j.mayocp.2024.07.011
54.
ParkCObiYStrejaERheeCMCatabayCJVaziriNDet al. Serum uric acid, protein intake and mortality in hemodialysis patients. Nephrol Dial Transplant. (2017) 32:gfw419–gfw1757. doi: 10.1093/ndt/gfw419
55.
Molino-LovaRSofiFPasquiniGVannettiFDel RySVassalleCet al. Higher uric acid serum levels are associated with better muscle function in the oldest old: results from the Mugello study. Eur J Intern Med. (2017) 41:39–43. doi: 10.1016/j.ejim.2017.03.014
56.
BakerJFWeberDRNeogiTGeorgeMDLongJHelgetLNet al. Associations between low serum urate, body composition, and mortality. Arthritis Rheumatol. (2023) 75:133–40. doi: 10.1002/art.42301
57.
GanWZhuFFangXWangWShaoDMaoHet al. Association between serum uric acid and all-cause and cardiovascular-related mortality in hemodialysis patients. Front Nutr. (2024) 11:1499438. doi: 10.3389/fnut.2024.1499438
58.
WhiteWBSaagKGBeckerMABorerJSGorelickPBWheltonAet al. Cardiovascular safety of Febuxostat or allopurinol in patients with gout. N Engl J Med. (2018) 378:1200–10. doi: 10.1056/NEJMoa1710895
59.
Perez-GomezMVBartschL-ACastillo-RodriguezEFernandez-PradoRKanbayMOrtizA. Potential dangers of serum urate-lowering therapy. Am J Med. (2019) 132:457–67. doi: 10.1016/j.amjmed.2018.12.010
60.
ChenYFreedmanNDRodriquezEJShielsMSNapolesAMWithrowDRet al. Trends in premature deaths among adults in the United States and Latin America. JAMA Netw Open. (2020) 3:e1921085. doi: 10.1001/jamanetworkopen.2019.21085
61.
ChristopoulosKEleftheriouK. Premature mortality in the US: a convergence study. Soc Sci Med. (2020) 258:113141. doi: 10.1016/j.socscimed.2020.113141
Summary
Keywords
serum uric acid, aging, mortality risk, CHARLS, NHANES
Citation
Zhao C, Zhao L, Liu Y, Sun L-q, Li X-r, Wang Y and Sun W (2025) The impact of serum uric acid on biological aging and mortality risk: insights from the NHANES and CHARLS cohorts. Front. Nutr. 12:1569798. doi: 10.3389/fnut.2025.1569798
Received
01 February 2025
Accepted
07 April 2025
Published
22 April 2025
Volume
12 - 2025
Edited by
Zhiping Niu, Fudan University, China
Reviewed by
Danrong Zhong, University Medical Center Groningen, Netherlands
Zhe Yang, Zhejiang University School of Medicine, China
Updates
Copyright
© 2025 Zhao, Zhao, Liu, Sun, Li, Wang and Sun.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Weiwei Sun, sunweitcm@163.com
†These authors have contributed equally to this work and share first authorship
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.