Abstract
Purpose:
This study aimed to assess the effect of acupoint catgut embedding therapy (ACET) on anthropometric parameters and endocrine function in obese women through a systematic review and meta-analysis.
Methods:
A comprehensive search was conducted across international and Chinese databases [CNKI, Wanfang, Weipu, Sinomed, PubMed, Embase, Cochrane Library, Web of Science (WOS), and Scopus]. The search terms included “female,” “women,” “catgut implantation at acupoint,” “catgut embedding,” “acupoint embedding therapy,” “obesity,” “adiposity,” and “body weight,” etc. Studies included in this analysis were randomized controlled trials (RCTs) assessing the effects of ACET on obesity indicators such as body mass index (BMI), waist-to-hip ratio (WHR), lipid profiles such as triglycerides (TG), total cholesterol (TC), high-density lipoprotein (HDL), and low-density lipoprotein (LDL), and hormone levels such as luteinizing hormone (LH), follicle-stimulating hormone (FSH), testosterone (T), and estradiol (E2). We used ROB 2.0 to assess the risk of bias. Data was analyzed using weighted mean differences (WMD) and risk ratios (RR) to measure effect sizes, and heterogeneity was assessed using I2 statistics. Conduct sensitivity analysis, publication bias testing, and subgroup analysis on indicators with high heterogeneity to explore the sources of heterogeneity.
Results:
Twenty-three studies involving over 2,000 obese women were included. Risk of bias assessment revealed generally low bias in randomization and measurement domains, though selective reporting and missing data handling raised concerns in some studies. ACET significantly reduced BMI [−1.72 (95% CI: −2.13, −1.31)] and WHR [WMD −0.016 (95% CI: −0.034, 0.001)], with high heterogeneity in BMI analysis (I2 = 92.3%). Subgroup analyses suggested that heterogeneity decreased in different control groups and different treatment courses, such as diet guidance (I2 = 0.0%) and 12-week treatment duration (I2 = 32.9%). Publication bias assessments (Begg's and Egger's tests) indicated no significant bias for most indicators. However, the clinical efficacy rate showed potential publication bias upon trim-and-fill adjustment, though the effect remained significant. ACET significantly reduced TG and TC but not HDL, LDL, or insulin resistance. Hormonal changes included decreased LH and FSH and increased E2.
Conclusion:
Our meta-analysis demonstrates that ACET significantly improves anthropometric parameters and endocrine function in obese women, though it does not significantly impact lipid metabolism or insulin resistance. The therapy's influence on female hormones may contribute to its efficacy in obesity treatment, highlighting the need for further studies to explore long-term effects and mechanisms.
Systematic review registration:
PROSPERO, identifier: CRD42025640157.
1 Introduction
Obesity is a complex and multifactorial condition that continues to pose a significant public health challenge globally (1). Defined by a body mass index (BMI) of 30 kg/m2 or higher, obesity has seen alarming increases in prevalence over the past decades (2). As reported by the World Health Organization (WHO), obesity rates have tripled since 1975, with over 650 million adults classified as obese globally by 2021 (1, 3). This rise has directly contributed to the escalating burden of non-communicable diseases, including cardiovascular disease, type 2 diabetes, hypertension, and certain cancers, all of which are further straining healthcare systems worldwide (1, 3, 4). Particularly concerning is the disproportionate impact of obesity on women, as they are more susceptible to hormonal imbalances and metabolic disturbances that often accompany excess body fat (5). Women with obesity are at an increased risk for reproductive health problems, including polycystic ovary syndrome (PCOS), menstrual irregularities, and infertility (6). Furthermore, obesity is closely linked to mental health issues, such as depression and anxiety, which are frequently associated with body image concerns (7). Along with these concerns, obesity can disrupt metabolism, causing insulin resistance and unhealthy blood fat levels—both of which raise the risk of heart disease and type 2 diabetes (8).
Current strategies for managing obesity typically include lifestyle modifications, pharmacotherapy, and bariatric surgery (9). Lifestyle changes, such as dietary modifications and physical activity, remain the cornerstone of obesity treatment; however, these interventions often yield only modest and temporary results (10). Pharmacological therapies, including orlistat and GLP-1 receptor agonists, can assist in weight loss but usually come with side effects and limited long-term effectiveness (11). Bariatric surgery, such as gastric bypass and sleeve gastrectomy, offers more substantial and sustained weight loss but is invasive and carries surgical risks (12). Despite these existing treatments, maintaining weight loss remains a challenge for many, sparking greater interest in alternative therapies (13). Medications and surgeries may lead to fast results, but they frequently bring side effects ranging from stomach troubles to neurological complications.
Acupoint catgut embedding therapy (ACET) offers an alternative approach to managing obesity. Studies suggest that ACET, a specialized acupuncture technique, enhances insulin sensitivity, promotes satiety, and helps reduce food intake (14, 15). Recent randomized controlled trials (RCTs) have shown that patients with simple obesity who receive electroacupuncture combined with diet and exercise therapy experience greater weight loss compared to those who only receive diet and exercise (16). What's more, ACET appears to work particularly well for belly fat, with studies showing stronger results compared to other approaches (17). Another plus is that patients only need treatment every week or two, making it much easier to stick with than daily therapies, and this consistency likely boosts its effectiveness. Studies indicate ACET might help with several obesity-related problems women often face–like insulin resistance, cholesterol issues, and hormone fluctuations (18). That said, while early results look good, research findings have been mixed so far, and we still need more conclusive evidence. With studies showing mixed results, a meta-analysis gives us a way to thoroughly examine the research. By pooling data together, we can better identify trends, clarify whether ACET is truly effective against obesity, and evaluate how it impacts body composition and endocrine function.
2 Methods
This systematic review was conducted according to the PRISMA guidelines for systematic reviews and Meta-analyses (19). Additionally, it was registered with the international prospective register for reviews (PROSPERO), under registration number CRD42025640157.
2.1 Literature search strategy
We conducted a comprehensive literature search across Chinese and international databases to identify relevant studies for this meta-analysis. The search was carried out in eight databases: CNKI, Wanfang, Weipu (VIP), CBM, PubMed, Embase, Cochrane Library, Web of Science (WOS), and Scopus, with a cutoff date for publication up to October 1, 2024. The search strategy was designed to capture studies on the target population and intervention. For the population, the search terms included “female” and “women.” The intervention-related terms included “catgut implantation at acupoint,” “catgut embedding,” “catgut-implantation,” “catgut-point embedding,” and “acupoint embedding therapy.” In terms of outcomes, we used terms like “obesity,” “adiposity,” “body weight,” “abdominal obesity,” “central obesity,” and “visceral obesity.” The Complete Search Strategies were provided in Supplementary material 1.
2.2 Inclusion and exclusion criteria
Studies involving adult female participants aged between 18 and 60 years were included, diagnosed with obesity or overweight, defined by a BMI > 25 kg/m2. The intervention must involve acupoint catgut embedding therapy, which entails the implantation of absorbable surgical sutures (e.g., catgut or other absorbable threads) at specific acupoints. This may be combined with other acupuncture-related external therapies (such as needling, moxibustion, etc.). Only RCTs were included. Studies were required to report outcomes related to obesity or metabolic control. The primary outcome measures were BMI, waist-to-hip ratio (WHR), and clinical efficacy rate (the practical definition is the population with a weight loss of 3 kg or more). Secondary outcome measures included triglycerides (TG), total cholesterol (TC), high-density lipoprotein (HDL), low-density lipoprotein (LDL), homeostatic model assessment of insulin resistance (HOMA-IR), luteinizing hormone (LH), Testosterone (T), follicle-stimulating hormone (FSH), and estradiol (E2). Only studies published in English or Chinese were included to facilitate data extraction and analysis.
Exclusion criteria comprised studies focusing on non-female populations or participants under 18 years. Also excluded were studies that did not utilize acupoint embedding therapy or combined this with other treatments, such as pharmacological or surgical interventions. Non-randomized controlled trials, cohort studies, case-control studies, or those lacking a control group were excluded, as were studies with incomplete data or high risks of bias.
2.3 Data extraction and quality assessment
Data extraction was performed independently by two reviewers to ensure accuracy, with disagreements resolved by a third reviewer. We extracted study characteristics [author(s), year, country, study design, sample size, participant demographics, and intervention details], outcome measures (changes in BMI, waist-to-hip ratio, clinical efficacy rate, triglycerides, cholesterol, insulin resistance, and reproductive hormones), statistical data (means, standard deviations, effect sizes, and 95% confidence intervals), and adverse events. The quality of the studies was assessed using the Cochrane Risk of Bias 2.0 (ROB 2.0) tool, evaluating bias in five domains: (1) randomization, (2) intervention deviations, (3) missing outcome data, (4) outcome measurement, and (5) selective reporting. Each study had a low, high, or unclear risk of bias in each domain.
2.4 Statistical analysis methods
We used the weighted mean difference (WMD) for continuous outcomes and risk ratio (RR) for dichotomous outcomes as effect measures. The WMD was applied to compare changes in continuous variables such as BMI, WHR, and metabolic markers (e.g., triglycerides, cholesterol levels) between the intervention and control groups. For dichotomous outcomes, such as the clinical efficacy rate, the RR was used to compare the proportion of participants achieving significant improvements. A random-effects model was implemented when significant between-study heterogeneity was detected (I2 > 50%), while a fixed-effects model was adopted when heterogeneity was negligible. The heterogeneity of the studies was assessed using the I2 statistic, with values above 50% indicating substantial heterogeneity. If significant heterogeneity was observed, subgroup analyses based on factors such as study design, intervention duration, and participant characteristics were conducted to explore potential sources of variability. Additionally, sensitivity analyses were performed by excluding studies with a high risk of bias to assess their impact on the overall results. Publication bias was assessed using Egger's test and funnel plots. All statistical analyses were performed using STATA 15, and a P-value of < 0.05 was considered statistically significant.
3 Results
3.1 Characteristics of included studies
The study screening process involved a thorough examination of records from both international and Chinese databases. Initially, a total of 148 records were retrieved from international databases and 229 from Chinese databases. After removing duplicates, 185 records remained for further screening. A total of 106 records were screened after reading title and abstracts. Upon reviewing full text, 83 records were excluded. Eighty-three full-text articles were excluded for various reasons: 16 lacked RCT registration, 48 included males, 14 did not meet the PICO criteria, and five were not RCTs. Ultimately, 26 studies were included in the qualitative synthesis for the meta-analysis. The full-text articles of 23 studies were then assessed for eligibility (Figure 1).
Figure 1
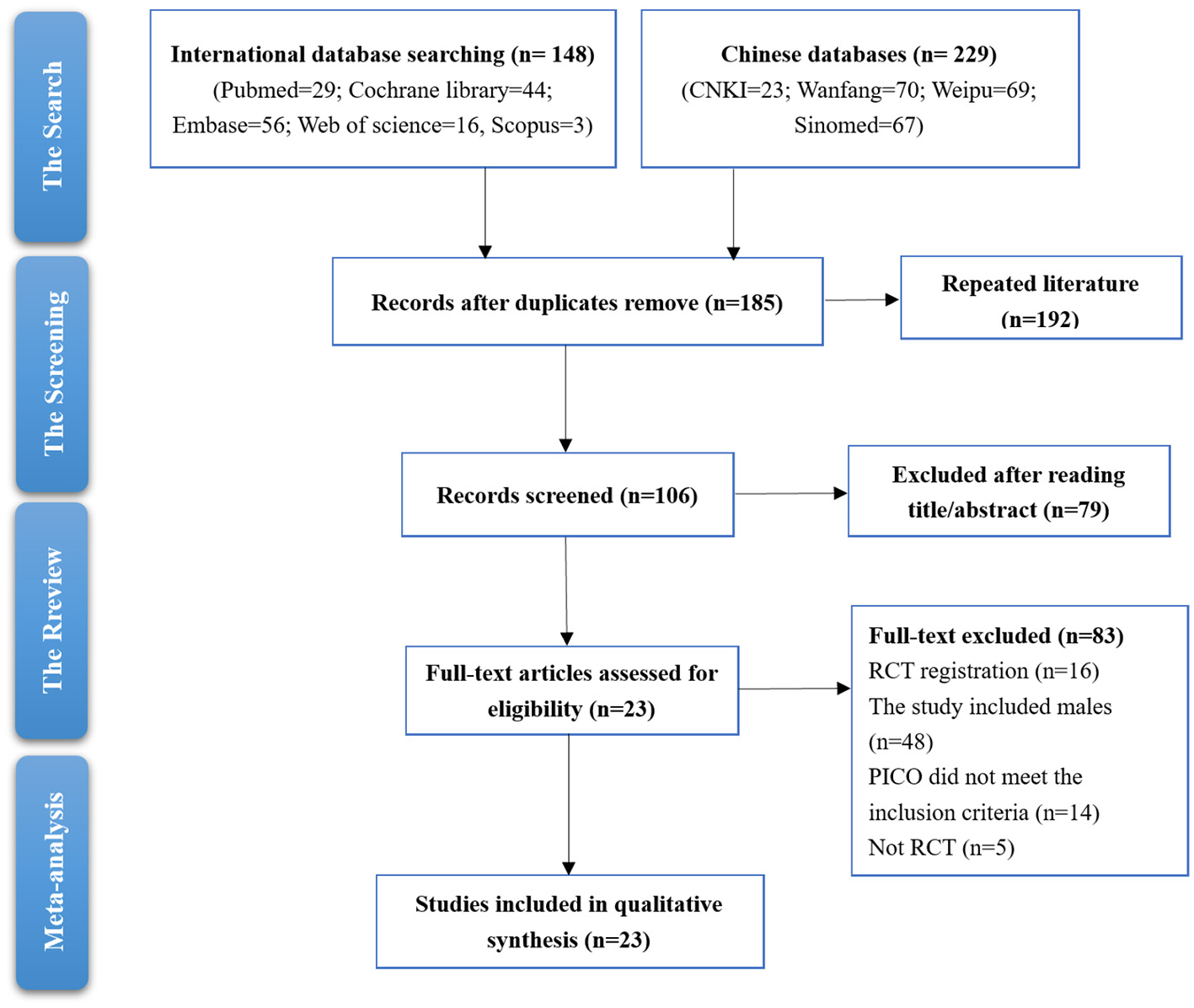
Studies screening process.
The characteristics of included studies are as follows. The sample sizes ranged from 40 to 300 participants, with an age range typically from 25 to 52 years. The BMI values at baseline ranged from ~26 to 38 kg/m2. The predominant treatment was catgut embedding therapy, which was often combined with additional measures such as dietary guidance, moxibustion, or acupuncture. The type of suture material used in these interventions varied, with most studies employing absorbable catgut (sizes 2/0 or 4-0). The duration of treatment weeks, with the frequency of intervention typically ranging from once every week to once every 2 weeks. Some studies utilized additional therapies, such as on or acupuncture, either alone or in combination with catgut embedding. The control groups included sham catgut embedding, placebo acupuncture, and other basic treatments. The primary outcomes measured across studies included BMI, waist circumference, waist-to-hip ratio, metabolic indicators (lipid profiles such as total cholesterol, triglycerides, HDL, LDL, and fasting blood glucose), and hormone levels (such as estradiol, luteinizing hormone, and follicle-stimulating hormone; Table 1).
Table 1
| Study | Characteristics of obese women | Intervention group | Control group | Outcome |
|---|---|---|---|---|
| Chen 2018 (20) | 90 cases. age from 39 to 43 years and had an average BMI of 30 kg/m2 | Catgut embedding. Chromic catgut (UNIK surgical sutures MFG.CO.) size 0. 6 weeks | Sham catgut embedding. 6 weeks | BMI, WHR, TG, TC, HDL, LDL, AE |
| Chu 2014 (48) | 50 cases. IC: 29 ± 5 years, 25.78 ± 4.81 kg/m2; CG: 27 ± 5 years, 26.88 ± 4.53 kg/m2 | Catgut embedding. No. 0 medical catgut. 2 months | Electroacupuncture. 0.30 mm × 40 cm 75 mm filiform needle. 2 months | BMI, WHR, clinical efficacy |
| Deng 2021 (49) | 60 cases. IG: 49.5 ± 2.5 years, 31.05 ± 1.15 kg/m2; CG: 50.2 ± 2.8 years, 31.08 ± 1.17 kg/m2 | Catgut embedding+ Dietary guidance. Surgical suture (polyglycolic acid PGA suture 4-0). 3 months | Dietary guidance. 3 months | BMI, E2, LH, FSH |
| Du 2011 (50) | 64 cases. IG: 50.3 ± 3.0 years, 30.61 ± 3.32 kg/m2; CG: 49.8 ± 3.2 years, 30.38 ± 2.97 kg/m2 | Catgut embedding+ Dietary guidance. Medical catgut. 12 weeks | Dietary guidance. 12 weeks | BMI, clinical efficacy |
| Jessica 2014 (51) | 99 cases. 35.9 ± 7.8 years. Mean BMI was between 33.4 ± 1.3 kg/m2 and 38.6 ± 2.6 kg/m2 | Catgut embedding+ Moxibustion. Chromic catgut strand 00. 6 weeks | Sham acupuncture. 6 weeks | BMI, WHR, TC, HOMA-IR |
| Jin 2024 (23) | 82 cases. IG: 26.3 (23.2, 27.4) kg/m2, CG: 25.5 (23.95, 27.58) kg/m2 | Catgut embedding. Absorbable surgical suture (4–0 RA-1012). 8 weeks | The placebo embedding needle. 8 weeks | BMI, WHR, TG, TC, HDL, LDL, HOMA-IR |
| Qin 2016 (21) | 41 cases. IG: 28.33 ± 5.12 years; 27.44 ± 2.35 kg/m2; CG: 28.77 ± 4.77 years, 27.06 ± 2.01 kg/m2 | Catgut embedding 3-0, 3 months | Kidney-supplementing and blood-quickening decoction. 3 months | BMI, WHR, TG, TC, HDL, LDL, HOMA-IR, clinical efficacy, AE |
| Fu 2024 (52) | 90 cases. IG: 31.41 ± 2.56 years; 26.35 ± 1.0 kg/m2; CG: 27.33 ± 2.41 years, 26.52 ± 1.12 kg/m2 | Catgut embedding+ IVF-ET. Medical absorbable protein thread | IVF-ET | LH, T, FSH, TC, TG |
| Han 2022 (53) | 68 cases. IG: 42.5 ± 10.3 years; CG: 39.2 ± 10.7 years | Catgut embedding. No. 2 absorbable surgical suture. 8 weeks | Sham catgut embedding. 8 weeks | BMI |
| Jin 2023 (54) | 90 cases. IG: 27.58 ± 2.46 years; CG: 27.62 ± 2.47 years | Catgut embedding+ ACU. Medical “4-0” absorbable surgical suture+ 1.5~ 2.0 inch filiform needle. 8 weeks | Catgut embedding. Medical “4-0” absorbable surgical suture. 8 weeks | Clinical efficacy, T, LH, FSH, AE |
| Lai 2015 (55) | 40 cases. IG: 37.34 years; 28.21 ± 2.84 kg/m2; CG: 36.13 years, 28.31 ± 2.73 kg/m2 | Catgut embedding+ ACU. “4-0” sterile absorbable catgut + 0.3 × 40 mm disposable acupuncture needle. 8 weeks | Catgut embedding. “4-0” sterile absorbable catgut. 8 weeks | BMI, clinical efficacy |
| Liu 2013 (56) | 60 cases. 27.15 years; IG: 29.3 ± 3.7 kg/m2; CG: 29.2 ± 3.2 kg/m2 | Catgut embedding. The No. 2-0 medical catgut. 3 months | Electroacupuncture. 3 months | BMI, WHR, clinical efficacy |
| Liu 2023 (57) | 60 cases. IG: 26.8 ± 4.01 years; 27.58 ± 30.31 kg/m2; CG: 27.45 ± 4.02 years, 27.96(26.79, 30.12) kg/m2 | Catgut embedding. Line 4-0. 3 months | Needle knife. 3 months | BMI, WHR, clinical efficacy |
| Lu 2020 (58) | 106 cases. IG: 52.0 ± 4.1 years; 30.17 ± 2.24 kg/m2; CG: 51.2 ± 4.6 years, 30.21 ± 2.19 kg/m2 | Catgut embedding+ Basic treatment. Absorbable gut No. 4-0. 12 weeks | Basic treatment. Mirtazapine tablet+ Nilestriol tablets. 12 weeks | BMI, E2, FSH, LH |
| Song 2018 (22) | 300 cases. IG: 33.2 ± 2.6 years; 28.8 ± 2.9 kg/m2; CG: 33.5 ± 2.5 years, 28.7 ± 2.5 kg/m2 | Catgut embedding+ Massage. Absorbable medical catgut (size 4-0). 8 weeks | Massage. 8 weeks | BMI, WHR, TG, TC, clinical efficacy, AE |
| Wang 2022 (39) | 98 cases. IG: 52.61 ± 3.06 years; 30.66 ± 1.59 kg/m2; CG: 52.64 ± 3.02 years, 30.72 ± 1.58 kg/m2 | Catgut embedding+ Basic treatment. Absorbable medical catgut (Specification: 2/0). 12 weeks | Basic treatment. Targeted exercise and diet adjustment. 12 weeks | BMI, WHR, TG, TC, LDL, clinical efficacy |
| Wang 2014 (40) | 240 cases. IG: 36.7 ± 10.1 years; 33.21 ± 3.17 kg/m2; CG: 37.7 ± 9.2 years, 32.74 ± 2.65 kg/m2 | Catgut embedding+ Aerobic exercise. Catgut. 3 months | Aerobic exercise. 3 months | Clinical efficacy |
| Wang 2013 (41) | 90 cases. IG: 39.1 ± 11.8 years; 28.226 ± 2.63 kg/m2; CG: 37.9 ± 11.5 years, 28.226 ± 2.63 kg/m2 | Catgut embedding +Cupping. No. 0-1 medical catgut. 3 months | Acupuncture. 3 months | BMI, clinical efficacy |
| Xu 2021 (42) | 70 cases. IG: 42.48 ± 2.69 years; 24.25 ± 4.62 kg/m2; CG: 42.51 ± 2.64 years, 25.42 ± 4.58 kg/m2 | Catgut embedding. Protein molecular line | Electroacupuncture | BMI, WHR |
| Xue 2017 (43) | 57 cases. IG: 34 ± 9.61 years; 27.23 ± 2.71 kg/m2; CG: 38 ± 12.48 years, 26.43 ± 2.35 kg/m2 | Catgut embedding + Cupping. Absorbable surgical thread for hand surgery+ No. 2 cupping. 3 months | Catgut embedding. Cupping. Absorbable surgical thread for hand surgery. 3 months | BMI, clinical efficacy |
| Yang 2023 (44) | 134 cases. IG: 29.85 ± 5.64 years; 29.41 ± 2.81 kg/m2; CG: 30.31 ± 5.94 years, 29.57 ± 3.09 kg/m2 | Catgut embedding+ Embedding beans. The Complete shell-free vaccinium seeds. 3 months | Catgut embedding. 3 months | HOMA-IR |
| Yu 2017 (45) | 140 cases. IG: 51.07 ± 4.96 years; 30.38 ± 3.21 kg/m2; CG: 50.38 ± 5.42 years, 30.21 ± 3.34 kg/m2 | Catgut embedding+ Basic treatment. 2/0 absorbable catgut (manufactured by B. Braun Melsungen AG). 2/0 absorbable catgut (manufactured by B. Braun Melsungen AG). 12 weeks | Basic treatment. Exercise therapy+ Diet control+ Mirtazapine tablets (Huayu Pharmaceutical Co., Ltd., National Medicine Approval No. H20041656, specification 30 mg)+ Nilestriol tablets (Beijing Sihuan Pharmaceutical Co., Ltd., National Medicine Approval No. H11020124, specification 2 mg). 6 months | BMI, WHR, E2, LH, FSH |
| Zhang 2017 (46) | 71 cases. IG: 34 ± 6 years; 27.73 ± 1.52 kg/m2; CG: 32 ± 5 years, 22.43 ± 1.56 kg/m2 | Catgut embedding+ Basic treatment. 4-0 suture thread (lot number: thread VCP422). 20 weeks | Basic treatment. Routine postpartum nutrition counseling guidance. 20 weeks | BMI, WHR |
Basic characteristics of included studies.
BMI, body mass index; WHR, waist-to-hip ratio; TG, triglycerides; TC, triglycerides; HDL, high-density lipoprotein; LDL, low-density lipoprotein; HOMA-IR, homeostasis model assessment of insulin resistance; LH, luteinizing hormone; FSH, follicle-stimulating hormone; T, testosterone; E2, estradiol; AE, adverse events.
3.2 Bias of risk
The majority of studies showed low risk of bias in most domains, particularly for the randomization process, where most studies adhered to appropriate randomization methods, ensuring unbiased selection of participants. Additionally, most studies employed reliable and valid measurement methods. The missing data were not adequately addressed or imputed, which may have impacted the robustness of the findings. Finally, the “Selection of the reported result” domain showed mixed results, with some studies exhibiting a high risk of bias due to selective reporting of outcomes. In these cases, certain results may have been emphasized over others, potentially skewing the overall findings of the meta-analysis (Figure 2).
Figure 2
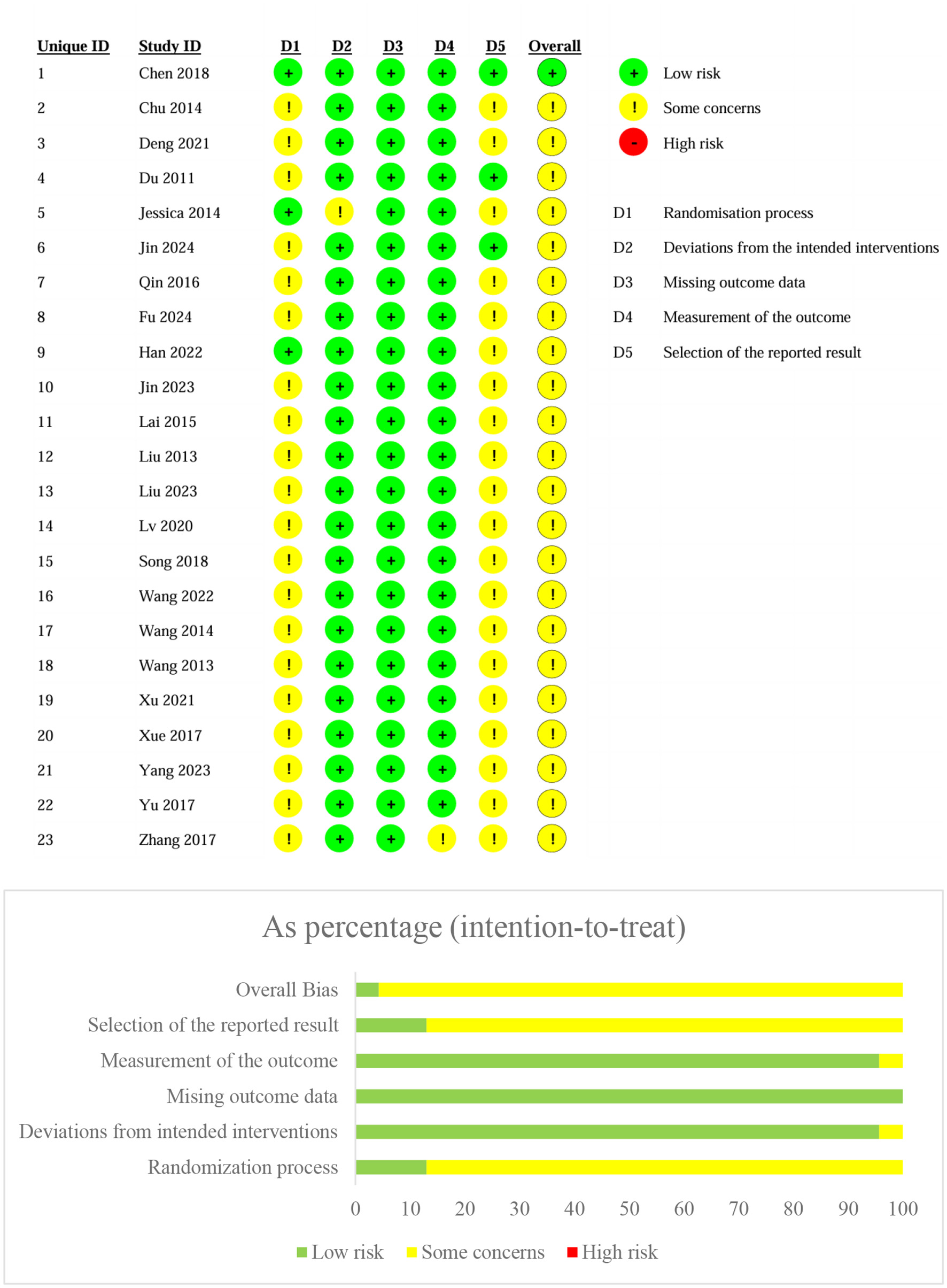
Bias of risk assessment.
3.3 Meta-analysis results
3.3.1 Body mass index (BMI) and waist-to-hip ratio (WHR)
A total of 19 studies reported BMI results. The meta-analysis of BMI changes demonstrated a significant reduction following acupoint embedding therapy, with a WMD of −1.72 (95% CI: −2.13, −1.31; Figure 3), indicating a positive therapeutic effect. However, the analysis revealed high heterogeneity (I2 = 92.3%). Begg's Test (P = 0.944) and Egger's Test (P = 0.996) indicated no significant publication bias, and the funnel plot appeared relatively symmetrical (Figure 4). Sensitivity analysis confirmed that no single study had a substantial influence on the overall effect, ensuring the robustness of the results. A total of 10 studies reported WHR results. The meta-analysis for WHR showed significant effect of acupoint embedding therapy, with a WMD of −0.016 (95% CI: −0.034, 0.001; Figure 3), and moderate heterogeneity (I2 = 66.6%). Both Begg's Test (P = 0.602) and Egger's Test (P = 0.211) suggested no significant publication bias, with sensitivity analysis confirming the stability of the results (Figure 3).
Figure 3

Meta analysis of BMI and WHR.
Figure 4
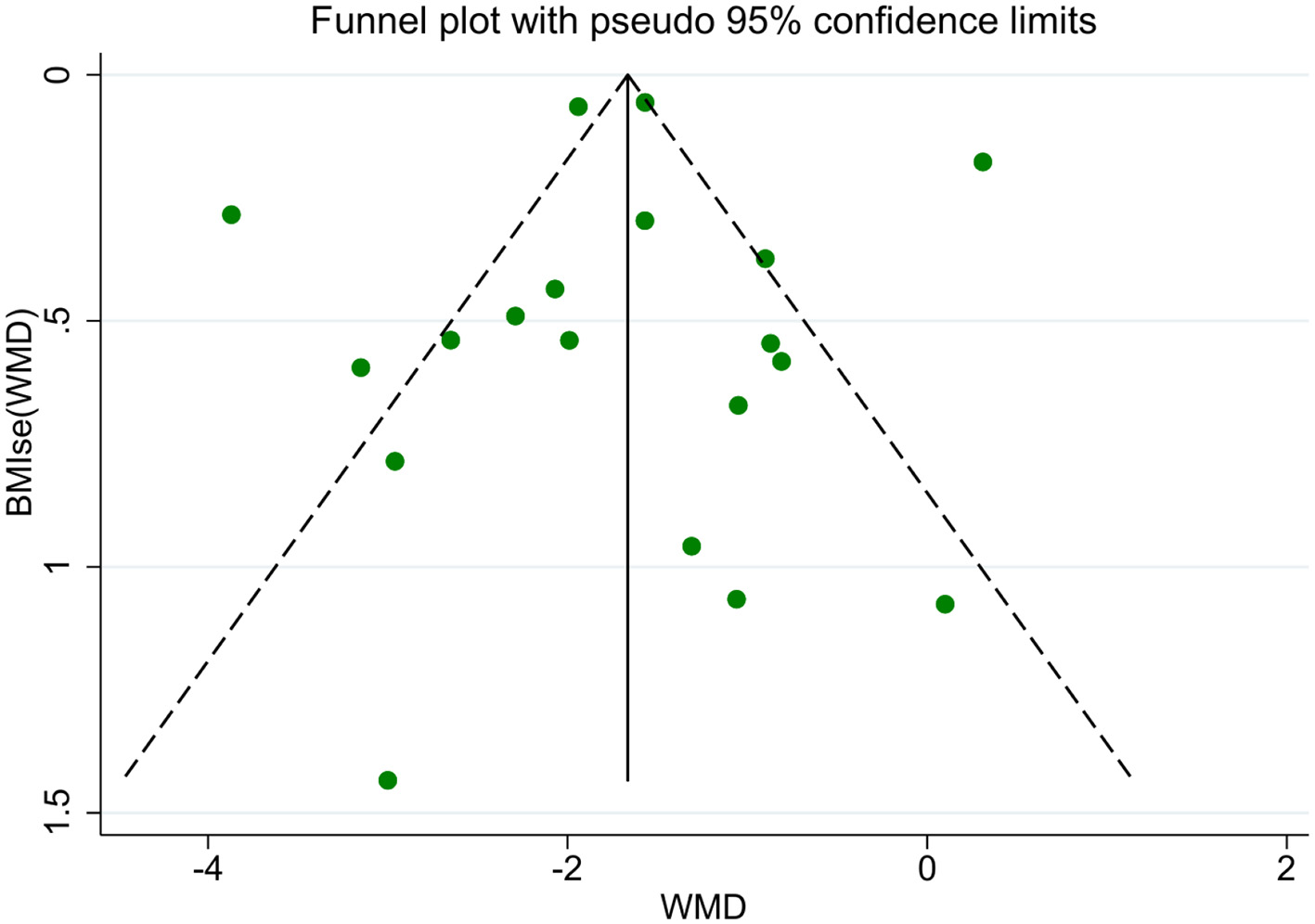
BMI funnel plot.
3.3.2 Clinical efficacy rate
A total of 10 studies reported clinical efficacy rate. The meta-analysis yielded an overall significant effect of acupoint embedding therapy. The random effects model estimated the pooled RR at 1.265 (95% CI: 1.077, 1.487; Figure 5), suggesting a positive effect. The heterogeneity was moderate (I2 = 88.0%, P = 0.000), which indicates variability across the included studies. Begg's test (P = 0.529) did not reveal significant bias, and Egger's test indicated a potential bias (P = 0.039). The trim-and-fill analysis suggested potential publication bias, with five studies imputed on the left side of the funnel plot. The adjusted fixed-effect estimate increased from 1.159 (95% CI: 1.112–1.205) to 2.911 (2.792–3.035), while the random-effects estimate rose from 1.287 (1.136–1.438) to 2.942 (2.518–3.439). Significant heterogeneity persisted (Q = 151.000, P < 0.001), with between-study variance increasing from 0.046 to 0.079. These results indicate that the original analysis likely underestimated the true effect size due to missing studies. Despite this adjustment, the effect remained statistically significant, though the wider confidence intervals reflect greater uncertainty after accounting for potential publication bias. Sensitivity analysis indicated that no individual study had a substantial impact on the pooled estimate.
Figure 5
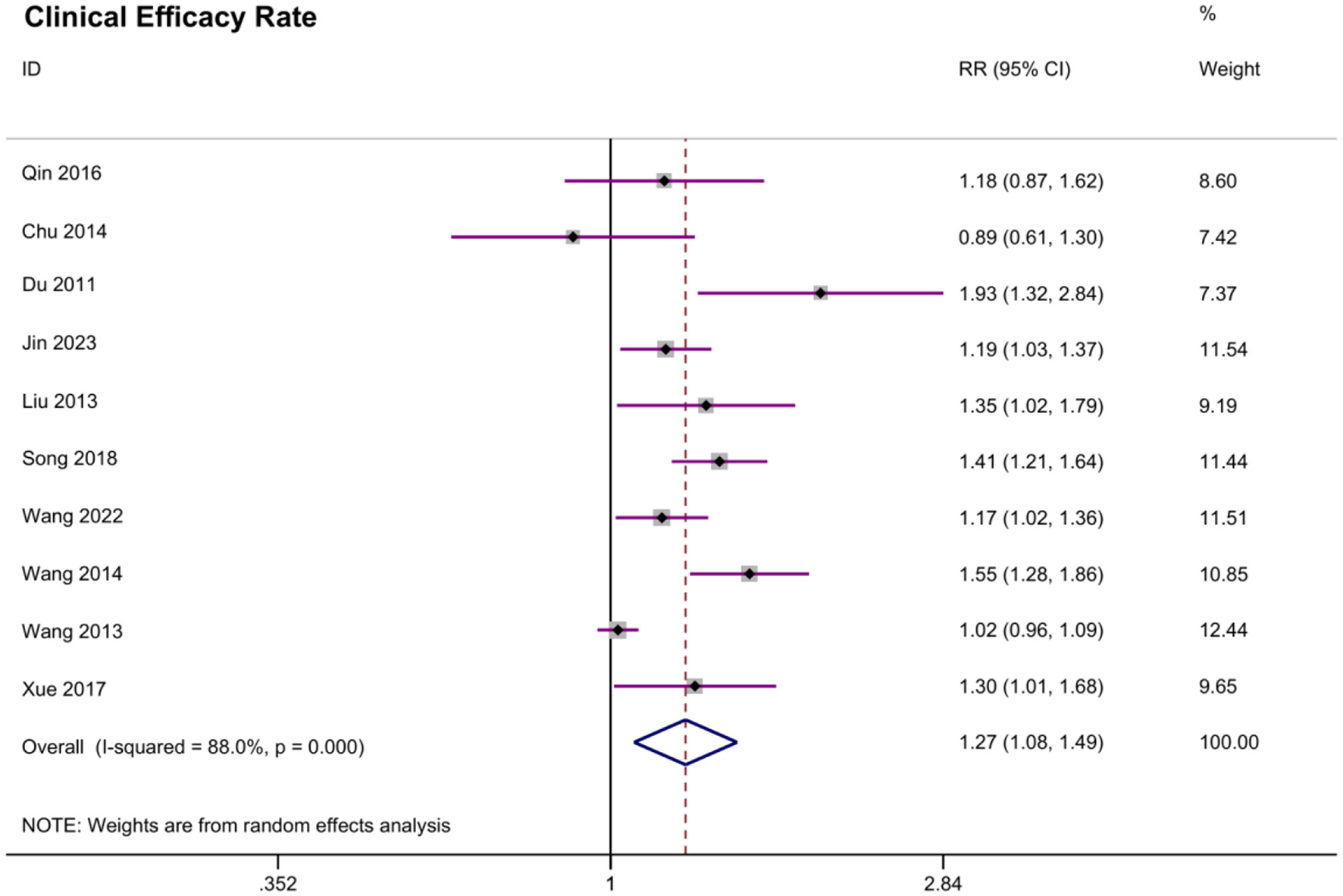
Meta analysis of clinical efficacy rate.
3.3.3 Blood lipid indicators and HOMA-IR
A total of eight studies reported triglycerides (TG) results, seven studies reported total cholesterol (TC) results, three studies reported high density lipoprotein (HDL), and four studies reported low density lipoprotein (LDL) results. Three studies reported the HOMA-IR result. The meta-analysis for TG, TC, HDL, LDL, and HOMA-IR revealed no significant effects of acupoint embedding therapy on these outcomes. For TG, the WMD was −0.241 (95% CI: −0.43, −0.051; P = 0.013), and for TC, the WMD was −0.521 (95% CI: −0.912, −0.131; P = 0.009), both of which showed statistical significance. Similarly, the WMD for HDL was −0.024 (95% CI: −0.227, 0.18) (P = 0.821) and for LDL was −0.425 (95% CI: −1.006, 0.157; P = 0.152), both of which were also not significant. The meta-analysis of HOMA-IR indicated a reduction in insulin resistance with a WMD of −0.484 (95% CI: −1.111, 0.143; P = 0.13), but this also did not reach statistical significance. Heterogeneity across the studies was moderate to high for all indicators, with I2 values ranging from 56.9 to 84.8%. Publication bias was assessed using Begg's Test and Egger's Test, which showed no significant bias for any of the outcomes (P > 0.05). Sensitivity analysis confirmed the stability of the results, with no individual study significantly influencing the overall outcomes.
3.3.4 Hormonal indicators
A total of five studies reported luteinizing hormone (LH) results, five studies reported follicle stumbling hormone (FSH) results, and two studies reported testosterone (T) results, three studies reported estradiol (E2). The meta-analysis for LH showed a moderate reduction following acupoint embedding therapy, with a WMD of −6.508 (95% CI: −9.772, −3.295; P < 0.001), and statistically significant effect was found. The heterogeneity was high (I2 = 95.8%), and Begg's Test (P = 0.806) and Egger's Test (P = 0.426) indicated there was no publication bias. Sensitivity analysis confirmed the robustness of the results. For FSH, a significant decrease was observed, with a WMD of −2.919 (95% CI: −4.963, −0.876; P = 0.005), and high heterogeneity (I2 = 97.8%). Begg's Test (P = 0.806) and Egger's Test (P = 0.064) showed no significant publication bias. Similarly, T showed no significant reduction, with a WMD of −0.647 (95% CI: −1.4, 0.105; P = 0.092). In contrast, for E2, the WMD was 7.131 (95% CI: 3.506, 10.756; P < 0.001), indicating a slight increase, and this was statistically significant. High heterogeneity (I2 = 88.3%) was present, and no publication bias was detected (Begg's Test P = 1.000; Egger's Test P = 0.19). Sensitivity analyses confirmed the stability of the findings for all hormones.
3.3.5 Adverse events
The meta-analysis of adverse events included three studies with a total of 470 participants. The pooled risk ratio (RR) was 0.787 (95% CI: 0.554–1.116, P = 0.179), indicating no statistically significant difference in adverse events between the experimental and control groups. Heterogeneity was low (I2 = 0.0%, P = 0.423), supporting the use of a fixed-effects model. In Chen et al. (20), transient local pain, bruising, and itching occurred in both catgut embedding and sham groups. Qin et al. (21) reported decreased appetite (24/57) and mild implantation-site reactions (5/57), all resolving with conservative management. Song et al. (22) observed comparable low incidences of fever, bruising, and induration (11.0 vs. 10.0%, P = 0.947) between experimental and control groups.
3.3.6 Subgroup analysis
In the subgroup analysis of main outcome BMI, we explored the sources of heterogeneity across the studies. In the original meta-analysis of BMI in nineteen studies, the heterogeneity was high, with an I2 value of 92.3%. However, when conducting the subgroup analysis, the sources of heterogeneity were explored, and a reduction in heterogeneity was observed across various subgroups. Specifically, for the sham embedding group, the I2 value decreased to 76.3%, indicating a moderate reduction in heterogeneity. The diet guidance group showed no heterogeneity (I2 = 0.0%). In the drug group, the heterogeneity decreased to 51.7%, reflecting a moderate reduction compared to the original analysis. On the other hand, the other acu-techniques group had a moderate I2 of 58.2% (Table 2).
Table 2
| Subgroup | Number of studies (n) | 95% CI | P value | I 2 |
|---|---|---|---|---|
| Different control groups | ||||
| Sham embedding group | 4 | −1.533 (−2.353, −0.712) | < 0.05 | 76.3% |
| Diet guidance | 5 | −1.936 (−2.058, −1.815) | < 0.05 | 0.0% |
| Drug group | 4 | −1.968 (−2.832, −1.104) | < 0.05 | 51.7% |
| Other acu-techniques | 3 | −0.246 (−1.188, 0.695) | 0.608 | 58.2% |
| Different intervention frequencies | ||||
| Once every week | 5 | −0.785 (−1.96, 0.39) | 0.19 | 86.4% |
| Once every 2 weeks | 12 | −2.178 (−2.555, −1.800) | < 0.05 | 87.7% |
| Different treatment courses | ||||
| 6 weeks | 3 | −1.195 (−2.256, −0.135) | 0.027 | 66.5% |
| 8 weeks | 3 | −1.379 (−2.915, 0.157) | 0.078 | 98.2% |
| 12 weeks | 9 | −1.868 (−2.217, −1.519) | < 0.05 | 32.9% |
| Different types of wires | ||||
| Synthetic absorbable suture | 5 | −1.948 (−2.543, −1.352) | < 0.05 | 61.0% |
| Catgut | 11 | −1.606 (−2.425, −0.787) | < 0.05 | 95.3% |
| Different age groups | ||||
| < 30 years | 7 | −2.173 (−3.915, −0.431) | < 0.05 | 96.7% |
| 30–50 years | 6 | −0.886 (−1.390, −0.383) | < 0.05 | 0% |
| >50 years | 4 | −1.932 (−2.054, −1.810) | < 0.05 | 0% |
BMI subgroup analysis results.
For the intervention frequency subgroups, the once every week group exhibited high heterogeneity (I2 = 86.4%), while the once every 2 weeks group had very high heterogeneity (I2 = 87.7%), indicating that different frequencies may not be the source of differences between studies. In terms of treatment duration, the heterogeneity in the 6-week group decreased to 66.5%, and in the 12-week group, it was reduced significantly to 32.9%. However, the 8-week group had very high heterogeneity (I2 = 98.2%). Finally, regarding wire types, the synthetic absorbable suture group had moderate heterogeneity (I2 = 61.0%), while the catgut group exhibited very high heterogeneity (I2 = 95.3%; Table 2).
Subgroup analysis by age revealed that the heterogeneity of intervention effects varied markedly across age groups. Participants under 30 years showed the largest BMI reduction but also exhibited substantial heterogeneity (I2 = 96.7%), indicating considerable variability in response within this group. In contrast, both the 30–50 years and >50 years groups demonstrated consistent effects with no observed heterogeneity (I2 = 0%), suggesting that age may be an important source of heterogeneity in treatment outcomes (Table 2).
4 Discussion
4.1 Main findings of the research
The most robust finding of our meta-analysis was the significant reduction in BMI among patients receiving acupoint embedding therapy. As shown in the BMI meta-analysis forest plot (Figure 3), the pooled weighted mean difference (WMD) in BMI was ~-1.72 (95% CI: −2.13 to −1.31), indicating a clinically meaningful weight reduction. This finding is consistent with results from previous studies on acupoint embedding for weight management (15, 17). However, high between-study heterogeneity was observed (I2 ≈ 92.3% for the BMI outcome, Figure 3), suggesting considerable variability in study populations, intervention protocols, or control treatments. The WHR reduction, though modest (WMD: −0.016), as depicted in Figure 3, further supports a potential role in central obesity management. Interestingly, the clinical efficacy rate was significant, but Egger's test (P = 0.039) and trim-and-fill analysis suggested possible publication bias, with an adjusted effect size increasing to RR = 2.942 (2.518–3.439). As Figure 5 illustrates, acupoint embedding continued to outperform the controls in terms of clinical efficacy even when potential publication bias was accounted for. This consistency reinforces the conclusion that the therapy's beneficial effect is genuine, despite the presence of some publication bias. Our meta-analysis demonstrated that acupoint embedding therapy produced a significant reduction in TG and TC, while changes in HDL, LDL and HOMA-IR did not reach statistical significance. These findings suggest that while acupoint embedding may favorably modulate overall lipid burden, its effects on lipoprotein subfractions and insulin resistance remain inconclusive. Early mechanism studies have shown that ACET may improve metabolic function by modulating key pathways (AMPK, PPAR-γ, mTOR) to enhance fat oxidation, glucose metabolism, and insulin sensitivity (23–25). Hormonal outcomes exhibited mixed effects: LH and FSH decreased significantly, while E2 increased. These findings align with prior evidence that acupoint stimulation may modulate hypothalamic-pituitary-gonadal axis activity, potentially benefiting conditions like polycystic ovary syndrome (PCOS) (26, 27). These therapies appear to regulate leptin activity and lipid profiles while promoting energy homeostasis through endocrine and circulatory effects (28, 29).
The pooled adverse event analysis showed no significant difference between acupoint embedding and control groups. Reported events (e.g., local pain, bruising, mild inflammation) were transient and manageable, supporting the therapy's safety. The comparable AE rates between active and sham procedures suggest that minor reactions may stem from needle insertion rather than the embedding material itself.
Subgroup analyses (Table 2) provided further insight into the sources of heterogeneity and the conditions under which acupoint embedding is most effective. As shown in Table 2, heterogeneity dropped to I2 = 0.0% among trials that provided uniform dietary guidance to all groups, indicating that standardizing co-interventions can markedly improve consistency across studies. Similarly, studies using synthetic absorbable sutures demonstrated lower heterogeneity (I2 = 61.0%), suggesting that embedding material may affect outcome stability. When compared to dietary guidance or medication alone, embedding therapy yielded a substantial and clinically meaningful reduction in BMI (mean difference: −1.936 and −1.968, respectively), with diet guidance as the control showing both strong effect size and no heterogeneity, underscoring the importance of standardized comparator arms. Further, analysis of intervention duration revealed that a 12-week treatment course was associated with the largest BMI reduction and relatively low heterogeneity (I2 = 32.9%), highlighting the benefit of extended therapy. Notably, the < 30 years group showed the greatest BMI reduction (−2.173), suggesting that younger women may be more responsive to embedding therapy. However, this subgroup had substantial heterogeneity (I2 = 96.7%), possibly reflecting variability in baseline metabolism, lifestyle, or adherence. In contrast, the >50 years group also achieved significant BMI reduction (−1.932) with complete consistency (I2 = 0%), indicating stable benefits among older women. The 30–50 years group had a smaller effect (−0.886) but similarly low heterogeneity. These trends may reflect that younger women benefit from higher metabolic rates (30), while older women may be more motivated or compliant (31), especially in the presence of comorbidities. Women aged 30–50 may experience more competing lifestyle pressures or metabolic plateaus, potentially limiting the intervention's impact (32).
In our included studies, acupoint selection for catgut embedding in obesity predominantly follows traditional acupuncture principles. Most protocols select 8–10 points per session, mainly targeting meridian points of the stomach, Ren Meridian, and Spleen Meridian. Commonly used acupoints are Tianshu (ST25), Zhongwan (CV12), Fenglong (ST40), Zusanli (ST36), and Guanyuan (CV4). Acupoint selection plays a crucial role in determining the therapeutic efficacy of ACET for women obesity. Studies show that more individualized or syndrome-based acupoint combinations can enhance outcomes. For instance, specialized abdominal point prescriptions has demonstrated superior effects on waist and abdominal circumference reduction compared to routine point selection (33, 34). Embedding at Jiaji (EX-B2) points, which are adjacent to spinal nerve branches, has shown greater improvements in BMI and body weight, likely through modulating visceral and autonomic functions (35).
4.2 Comparison with other systematic review and meta-analyses
Compared with other studies, Chen et al.'s (36) study performed a network meta-analysis of various acupuncture techniques targeting obesity with insulin resistance. Jiali et al. (37) directly compared ACET with manual acupuncture in simple obesity. Wujie et al. (15) combined acupuncture and embedding for abdominal obesity without isolating embedding effects; and Yue et al. (38) compared verum vs. sham ACET without examining hormonal changes. Our findings uniquely demonstrate that ACET not only reduces body weight and waist-hip ratio but also significantly alters luteinizing hormone, follicle-stimulating hormone, and estradiol levels, underscoring potential endocrine mechanisms in obesity management.
4.3 Limitations of the research
Despite the promising results, several limitations must be considered when interpreting these findings. One major limitation is the variation in study quality across the included research. Some studies (22, 39–46) showed concerns in areas such as randomization processes and incomplete reporting of outcomes, which may introduce bias and reduce the reliability of the conclusions. Additionally, there is substantial heterogeneity between the studies, particularly in terms of intervention protocols (e.g., catgut material, needle insertion depth, treatment frequency) and outcome measures (e.g., BMI, WC, metabolic markers). This variability makes it difficult to draw uniform conclusions about the optimal parameters for catgut embedding therapy.
4.4 Implications for future research
Future studies should standardize intervention protocols, including acupoint selection, and employ larger, multicenter, and longitudinal designs to strengthen statistical power and clarify long-term effects of catgut embedding for obesity. Mechanistic research should further explore how embedding at different sites influences neuroendocrine and metabolic pathways, focusing on hormones such as leptin, adiponectin, and insulin, as well as inflammatory and oxidative stress markers (47). Subgroup analysis suggest that personalized acupoint selection, tailored to age and metabolic status, may further optimize clinical outcomes. Refining protocols and integrating patient characteristics are essential for advancing the field.
5 Conclusion
Acupoint embedding therapy significantly reduced BMI and WHR and improved overall clinical efficacy in obese women, with additional benefits on triglycerides, total cholesterol and key reproductive hormones (LH, FSH, and E2). Effects on HDL, LDL, HOMA-IR, and testosterone were inconclusive. Treatment was well-tolerated, with adverse events comparable to controls. High heterogeneity, variable protocols and limited sample sizes warrant larger, standardized trials with longer follow-up to confirm these findings and elucidate underlying mechanisms.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Author contributions
SL: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. HZ: Conceptualization, Data curation, Funding acquisition, Methodology, Resources, Software, Writing – original draft. MZ: Formal analysis, Funding acquisition, Resources, Writing – original draft. LLo: Conceptualization, Formal analysis, Investigation, Writing – original draft. LLu: Formal analysis, Software, Supervision, Writing – original draft. SJ: Data curation, Investigation, Resources, Writing – original draft.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2025.1583556/full#supplementary-material
References
1.
Mohajan D Mohajan HK . Obesity and its related diseases: a new escalating alarming in global health. J Innov Med Res. (2023) 2:12–23. 10.56397/JIMR/2023.03.04
2.
Blüher M . Obesity: global epidemiology and pathogenesis. Nat Rev Endocrinol. (2019) 15:288–98. 10.1038/s41574-019-0176-8
3.
Ahmed B Konje J C . The epidemiology of obesity in reproduction. Best Pract Res Clin Obstet Gynaecol. (2023) 89:102342. 10.1016/j.bpobgyn.2023.102342
4.
Szymonik A . Obesity as a threat to health security. DSD. (2023) 9:151–63. 10.34739/dsd.2023.01.12
5.
Michalakis K Mintziori G Kaprara A Tarlatzis BC Goulis DG . The complex interaction between obesity, metabolic syndrome and reproductive axis: a narrative review. Metabolism. (2013) 62:457–78. 10.1016/j.metabol.2012.08.012
6.
Pasquali R Gambineri A Pagotto U . The impact of obesity on reproduction in women with polycystic ovary syndrome. BJOG. (2006) 113:1148–59. 10.1111/j.1471-0528.2006.00990.x
7.
Matos MIR Aranha LS Faria AN Ferreira SRG Bacaltchuck J Zanella MT . Binge eating disorder, anxiety, depression and body image in grade III obesity patients. Braz J Psychiatry. (2002) 24:165–9. 10.1590/S1516-44462002000400004
8.
Alam S Aijaz M . Complications of cardiovascular disease: the impact of diabetes, dyslipidemia, and metabolic disorders. World J Pharm Res. (2024) 13:321–56. 10.20959/wjpr202421-34375
9.
Kushner RF . Weight loss strategies for treatment of obesity: lifestyle management and pharmacotherapy. Prog Cardiovasc Dis. (2018) 61:246–52. 10.1016/j.pcad.2018.06.001
10.
Siwiec J Spiołek O Wasowicz A Sztyler-Krakowska M Teofilak M Smyl N et al . The growing obesity epidemic: integrating lifestyle, pharmacological, and surgical interventions for effective management. J Educ Health Sport. (2025) 77:56962–56962. 10.12775/JEHS.2025.77.56962
11.
Tak YJ Lee SY . Long-term efficacy and safety of anti-obesity treatment: where do we stand?Curr Obes Rep. (2021) 10:14–30. 10.1007/s13679-020-00422-w
12.
Aderinto N Olatunji G Kokori E Olaniyi P Isarinade T Yusuf IA . Recent advances in bariatric surgery: a narrative review of weight loss procedures. Ann Med Surg. (2023) 85:6091–104. 10.1097/MS9.0000000000001472
13.
Lua PL Roslim NA Ahmad A Mansor M Aung MMT Hamzah F . Complementary and alternative therapies for weight loss: a narrative review. J Evid Based Integr Med. (2021) 26:2515690X211043738. 10.1177/2515690X211043738
14.
Bing-guo X Hui W Shu-lan W Li-juan D . Effects of acupoint thread-embedding therapy on serum apelin and GLP-1 in type 2 diabetes mellitus patients with obesity due to dampness-heat encumbering spleen. J Acupunct Tuina Sci. (2021) 19:123–8. 10.1007/s11726-021-1235-y
15.
Wujie YE Jingyu X Zekai YU Xingang HU Yan Z . Systematic review and meta-analysis of acupuncture and acupoint catgut embedding for the treatment of abdominal obesity. J Tradit Chin Med. (2022) 42:848. 10.19852/j.cnki.jtcm.2022.06.002
16.
Lam TF Lyu Z Wu X Wong YP Cao P Wong EY et al . Electro-acupuncture for central obesity: a patient-assessor blinded, randomized sham-controlled clinical trial. BMC Complement Med Ther. (2024) 24:62. 10.1186/s12906-024-04340-5
17.
Sheng J Jin X Zhu J Chen Y Liu X . The effectiveness of acupoint catgut embedding therapy for abdominal obesity: a systematic review and meta-analysis. Evid Based Complement Alter Med. (2019) 2019:9714313. 10.1155/2019/9714313
18.
Niu S Ren L . Treatment of obesity by acupuncture combined with medicine based on pathophysiological mechanism: a review. Medicine. (2023) 102:e36071. 10.1097/MD.0000000000036071
19.
Salameh JP Bossuyt PM McGrath TA Thombs BD Hyde CJ Macaskill P et al . Preferred reporting items for systematic review and Meta-analysis of diagnostic test accuracy studies (PRISMA-DTA): explanation, elaboration, and checklist. BMJ. (2020) 370:m2632. 10.1136/bmj.m2632
20.
Chen IJ Yeh YH Hsu CH . Therapeutic effect of acupoint catgut embedding in abdominally obese women: a randomized, double-blind, placebo-controlled study. J Women Health. (2018) 27:782–90. 10.1089/jwh.2017.6542
21.
Qin W Zhao K Yang H . Effect of acupoint catgut embedding therapy combined with Chinese medicine for nourishing the kidneys and promoting blood circulation and improving blood glucose and lipid levels as well as the pregnancy rate in obese PCOS patients with infertility. Exp Ther Med. (2016) 12:2909–14. 10.3892/etm.2016.3715
22.
Song Y Wang L Ma Z . Clinical study on the combination of acupoint catgut embedding and massage therapy in the treatment of female abdominal obesity with spleen deficiency and dampness syndrome. J Tradit Chin Med Pharmacol. (2018) 40:1150–3. 10.3760/cma.j.issn.1673-4246.2018.12.011
23.
Jin Y Huang Y Zhu J Liao D Zeng S Jin X . Acupoint catgut embedding regulates community structure of intestinal flora in central obesity during perimenopause. Women Health. (2024) 64:857–69. 10.1080/03630242.2024.2422876
24.
Xiong Y Wang X Gong M Ji Q Li Y Hu A et al . Acupoints catgut embedding recovers leptin resistance via improving autophagy progress mediated by AMPK-mTOR signaling in obese mice. Heliyon. (2024) 10:e29094. 10.1016/j.heliyon.2024.e29094
25.
Garcia-Vivas JM Galaviz-Hernandez C Fernandez-Retana J Pedroza-Torres A Perez-Plasencia C Lopez-Camarillo C et al . Transcriptomic profiling of adipose tissue in obese women in response to acupuncture catgut embedding therapy with moxibustion. J Alternat Complement Med. (2016) 22:658–68. 10.1089/acm.2015.0200
26.
de Oliveira NM Machado J Lopes L Criado MB . A review on acupuncture efficiency in human polycystic ovary/ovarian syndrome. J Pharmacopunct. (2023) 26:105. 10.3831/KPI.2023.26.2.105
27.
Aquino CI Nori SL . Complementary therapy in polycystic ovary syndrome. Translat Med Unisa. (2014) 9:56. Available online at: https://pmc.ncbi.nlm.nih.gov/articles/PMC4012377/
28.
Wang L Yu CC Li J Tian Q Du YJ . Mechanism of action of acupuncture in obesity: a perspective from the hypothalamus. Front Endocrinol. (2021) 12:632324. 10.3389/fendo.2021.632324
29.
Landgraaf RG Bloem MN Fumagalli M Benninga MA de Lorijn F Nieuwdorp M . Acupuncture as multi-targeted therapy for the multifactorial disease obesity: a complex neuro-endocrine-immune interplay. Front Endocrinol. (2023) 14:1236370. 10.3389/fendo.2023.1236370
30.
Mathot KJ Dingemanse NJ Nakagawa S . The covariance between metabolic rate and behaviour varies across behaviours and thermal types: meta-analytic insights. Biol Rev. (2019) 94:1056–74. 10.1111/brv.12491
31.
Regwelski M Lange E Głabska D Guzek D . Analysis of the influence of age, BMI, and WHtR on body mass acceptance, attitudes, and motivation towards body mass reduction in overweight and obese Caucasian women. Nutrients. (2019) 11:542. 10.3390/nu11030542
32.
Tauqeer Z Gomez G Stanford FC . Obesity in women: insights for the clinician. J Women Health. (2018) 27:444–57. 10.1089/jwh.2016.6196
33.
Gu T Wang D Wang RH . Clinical observation of Bo's abdominal acupuncture catgut embedding therapy for simple abdominal obesity in young adults. World Late Med Inform. (2019) 19:176–7. 10.19613/j.cnki.1671-3141.2019.08.084
34.
Dong X Li L Zhang L . Clinical observation of “Bietong matching” acupoint catgut embedding therapy for simple obesity. Henan Tradit Chin Med. (2021) 41:1583–6. 10.16367/j.issn.1003-5028.2021.10.0357
35.
Wang Y Li J Qin H Liu J Wang G Wang B et al . Clinical efficacy of catgut embedding at Jiaji points for simple obesity. J Liaoning Univ Tradit Chin Med. (2020) 22:72–5. 10.13194/j.issn.1673-842x.2020.04.018
36.
Chen J Gu Y Yin L He M Liu N Lu Y et al . Network meta-analysis of curative efficacy of different acupuncture methods on obesity combined with insulin resistance. Front Endocrinol. (2022) 13:968481. 10.3389/fendo.2022.968481
37.
Jiali W Lily L Zhechao L Jianping L Mei H . Acupoint catgut embedding versus acupuncture for simple obesity: a systematic review and meta-analysis of randomized controlled trials. J Tradit Chin Med. (2022) 42:839–47. 10.19852/j.cnki.jtcm.2022.06.001
38.
Yue JH Li XL Zhang YY Yang GH Mah JZ Li A et al . Comparing verum and sham acupoint catgut embedding for adults with obesity: a systematic review and meta-analysis of randomized clinical trials. Medicine. (2024) 103:e36653. 10.1097/MD.0000000000036653
39.
Wang M . The evaluation of the effect of acupoint catgut embedding combined with exercise and dietary adjustments in treating obesity in menopausal women. East Med Cuisine. (2022) 7:164–6.
40.
Wang W Wang Y Sun D . Observation on the treatment of female simple obesity with acupoint catgut embedding combined with aerobic exercise in 120 cases. Zhejiang J Tradit Chin Med. (2014) 49:913. 10.13633/j.cnki.zjtcm.2014.12.038
41.
Wang X Yan L Liu G Wei Y . Observation on the therapeutic effect of cupping combined with acupoint catgut embedding for female obesity. Liaoning J Tradit Chin Med. (2013) 40:1902–4. 10.13192/j.issn.1000-1719.2013.09.059
42.
Xu J . The effects of acupoint catgut embedding and electroacupuncture therapy on weight and anthropometric parameters in female obese patients. Trace Elem Health Res. (2021) 38:42–3. Available online at: https://kns.cnki.net/kcms2/article/abstract?v=GkDm8A2i92VIxedKhisdmzM0sulehhlx87rhrqdh26yw0eUnXwG3TGIaY1jG-lz3S4BmuOrG3IDYEkCqHdXNv2Zu6dlq4sD3wEV6eKvlUvpMStbgebUWMXTTVur_EkuDjVeN5hKIIfrIDxOxc85cIqtHi5rW9uxB7gnUpz79wfQJ5RAthwdqSQ==&uniplatform=NZKPT&language=CHS
43.
Xue D Cai J Zhang X Fu J . Clinical efficacy of Yu-Mu point combination embedding and umbilical cupping for female spleen deficiency and dampness syndrome obesity. Hebei J Tradit Chin Med. (2017) 39:1383–6.
44.
Yang X Wang M Wang X Ma J Zhao X Li X . The effect of acupoint catgut embedding combined with auricular catgut embedding on obesity-related PCOS infertility and its impact on gut microbiota and glucose-lipid metabolism. Chin J Sex Sci. (2023) 32:72–6. 10.3969/j.issn.1672-1993.2023.08.019
45.
Yu H Zhang D . The efficacy of acupoint catgut embedding in treating liver qi stagnation type obesity in menopausal women and its mechanism on serum reproductive hormones. J Clin Acupunct. (2017) 33:39–43. 10.3969/j.issn.1005-0779.2017.11.011
46.
Zhang H Ma X Jiang C Shi R . Clinical study on the effect of acupoint catgut embedding on postpartum weight retention in women. Chin Acupunct. (2017) 37:725–8. 10.13703/j.0255-2930.2017.07.011
47.
Kazemi AH Adel-Mehraban MS Jamali Dastjerdi M Alipour R . A comprehensive practical review of acupoint embedding as a semi-permanent acupuncture: a mini review. Medicine. (2024) 103:e38314. 10.1097/MD.0000000000038314
48.
Chu X Xu SF . The effects of acupoint catgut embedding and electroacupuncture therapy on weight and anthropometric parameters in female obese patients. Shanghai Acupunct J. (2014) 33:636–7. 10.13460/j.issn.1005-0957.2014.07.0636
49.
Deng L Ma X Huang D Lin Y . Clinical observation of acupoint catgut embedding in the treatment of female obesity complicated with menopausal syndrome. Famous Doctors. (2021). 11:20–1. Available online at: https://kns.cnki.net/kcms2/article/abstract?v=GkDm8A2i92V41xkDqL57c8oGSBIZar-EheBhxL9RykgfOyDwqgeDPFMl0L1qJrT-pQQEZqUotDABuql7_pdNZ27z-qmrKFvnjNCec_U4ansXuimxX-msOfNo0WS1iGwwks3HsogoON-cBAfqV4v-6kJiBblLSGf8LktoG4AsxxAhNeU3FxnODw==&uniplatform=NZKPT&language=CHS
50.
Du GS . Therapeutic observation on catgut embedding therapy for menopausal obesity. World J Acupunct Moxibust. (2011) 21:5–9. Available online at: https://kns.cnki.net/kcms2/article/abstract?v=GkDm8A2i92WObYezt_c4zy6NMkGJlsqm1mUe3YCkhGIICmUuanRybDpO8dpbM7WozpR7ikqJ0B7q_xq3796b_ivP5tArWM5qeylPRBq2T-oau1ut2z8FcPxcAsTt5KZeDSLbWdsOjpn1mpwwtoAm5HV5dhvC46e13dtSQgJnMuPCqJBkRnog0Q==&uniplatform=NZKPT&language=CHS
51.
Garcia-Vivas JM Galaviz-Hernandez C Becerril-Chavez F Lozano-Rodriguez F Zamorano-Carrillo A Lopez-Camarillo C et al . Acupoint catgut embedding therapy with moxibustion reduces the risk of diabetes in obese women. J Res Med Sci. (2014) 19:610. Available online at: https://pmc.ncbi.nlm.nih.gov/articles/PMC4214018/
52.
Fu H Du H Song X Ma J Lu N . The effect of eight acupoints around the umbilicus embedding on the IVF-ET treatment outcomes in obese polycystic ovary syndrome (PCOS) infertility patients. Shandong J Tradit Chin Med. (2024) 43:53–8. 10.16295/j.cnki.0257-358X.2024.01.011
53.
Han G Ni G Sun J Pei L Chen L Li Z et al . The short-term efficacy and safety of embedding at different acupoint levels under B ultrasound in female abdominal obesity and bowel movement function. Acupunct Res. (2020) 1:53–8. 10.13702/j.1000-0607.201042
54.
Jin P Jiang Y Wang F Fang X . Mechanisms of regulating sex hormones with Zhoutian hot needle therapy combined with acupoint catgut embedding for the treatment of female simple obesity. Guangming Tradit Chin Med. (2023) 38:1336–8. 10.3969/j.issn.1003-8914.2023.07.038
55.
Lai M Zhu Y . Clinical observation on the efficacy of Jiaji acupoint in acupoint embedding for weight loss. Pop Sci Technol. (2015) 7:84–5,88. 10.3969/j.issn.1008-1151.2015.07.028
56.
Liu X Liu Y Li X Liang R Liu R Pan Z . Study on acupoint catgut embedding combined with acupuncture in treating obesity-related PCOS infertility with intrauterine insemination. Chin J Eugen Genet. (2013) 21:97–9. 10.13404/j.cnki.cjbhh.2013.01.025
57.
Liu X Zhou F Ji L . Acupoint catgut embedding combined with blade needle therapy in the treatment of female spleen deficiency and dampness obstructing type simple obesity: a study of 30 cases. Hunan J Tradit Chin Med. (2023) 39:75–8. 10.16808/j.cnki.issn1003-7705.2023.05.018
58.
Lu J . The effect of acupoint catgut embedding on obesity in menopausal women and its impact on serum sex hormone levels and quality of life. Chin J Mater Child Health. (2020) 35:1873–5. 10.19829/j.zgfybj.issn.1001-4411.2020.10.035
Summary
Keywords
acupoint embedding therapy, obesity, women, BMI, lipid profile, hormone, meta-analysis, systematic review
Citation
Li S, Zhou H, Zhou M, Lou L, Luo L and Ji S (2025) The effects of acupoint catgut embedding therapy on anthropometric parameters and endocrine function in obese women: a systematic review and meta-analysis. Front. Nutr. 12:1583556. doi: 10.3389/fnut.2025.1583556
Received
05 March 2025
Accepted
12 June 2025
Published
01 July 2025
Volume
12 - 2025
Edited by
Parth Shah, Amgen, United States
Reviewed by
Asmaa ShamsEldeen, Cairo University, Egypt
Reihane Alipour, Shahid Beheshti University of Medical Sciences, Iran
Updates
Copyright
© 2025 Li, Zhou, Zhou, Lou, Luo and Ji.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shuangqiu Li 13478328652@163.comHongfei Zhou hf-zhou0817@163.com
†These authors have contributed equally to this work and share first authorship
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.