Abstract
Background and aim:
As a newly recommended healthy dietary blueprint, the EAT-Lancet diet emphasizes both environmental sustainability and human health. However, its impact on chronic liver diseases remains unclear. This study examined the influence of the EAT-Lancet diet on the risk of metabolic dysfunction-associated steatotic liver disease (MASLD) and other chronic liver diseases.
Methods:
Our study included 160,394 UK Biobank participants who completed 24-h dietary assessments between April 2009 and June 2012, from which EAT-Lancet diet scores were calculated. The Cox proportional hazards models were used to estimate hazard ratios (HRs) with 95% confidence intervals (CIs) for the primary outcome (MASLD) and secondary endpoints, including cirrhosis, liver cancer, and other liver diseases.
Results:
A total of 1,727 cases of MASLD, 602 cases of liver cirrhosis, 103 cases of liver cancer, and 2,053 cases of other liver diseases were identified over a median follow-up period of 13.3 years. Using the lowest tertile as the reference, the highest EAT-Lancet diet index group demonstrated a 33% reduction in MASLD incidence (HR:0.67, multivariate 95%CI: 0.55, 0.80). In several secondary outcome measures, similar associations were also observed. Furthermore, the risk of MASLD was lowest among individuals with both higher EAT-Lancet dietary scores and lower genetic risk (HR = 0.52; 95%CI: 0.36–0.74), although no significant interaction was detected between the two groups.
Conclusion:
Adherence to the EAT-Lancet diet is associated with a reduced risk of chronic liver disease, independent of genetic factors.
1 Introduction
Suboptimal dietary factors increase the population disease burden and reduce the quality of life. Promoting healthy dietary patterns may serve as an effective strategy for preventing chronic diseases and supporting environmental sustainability (1, 2). In recent years, the EAT-Lancet diet, serving as a blueprint for healthy eating, has garnered significant attention (3). This dietary framework, designed by the EAT-Lancet Commission in 2019, is adaptable to different cultures, supports sustainability, and aims to bring about global changes in food production and waste while improving human health. It is mostly plant-based, advocating for increased consumption of fruits, vegetables, legumes, whole grains, and nuts, while limiting the intake of animal-derived foods, added sugars, and saturated fats. This framework strives to harmonize the relationship between healthy eating and the ecological environment, promoting sustainable development for both human health and the Earth’s ecology (2, 4). Evaluating whether adherence to this dietary framework can reduce the incidence of chronic diseases represents a critical research direction in public health. Current studies have demonstrated that this dietary pattern exhibits a lower association with the risk of developing conditions such as heart failure, lung cancer, and type 2 diabetes (5–7).
Globally, 4% of annual deaths are attributed to chronic liver diseases, representing a significant health burden (8). The most common of them is metabolic dysfunction-associated steatotic liver disease (MASLD), which impacts over 24% of the global population. In Europe, approximately one-quarter of individuals are affected by MASLD (9, 10). MASLD is not only a leading cause of hepatic disorders such as cirrhosis and hepatocellular carcinoma (11), but is also closely associated with T2DM, hyperlipidemia, obesity, and metabolic syndrome. It is a major driving factor for chronic liver disease and imposes substantial economic burdens while significantly reducing health-related quality of life (12, 13). Currently, there are no effective drug treatments for MASLD. Adopting a healthy lifestyle and adhering to dietary patterns in accordance with recommended guidelines serve as effective tools for preventing its development (14). Several studies have demonstrated the beneficial effects of healthy dietary patterns on metabolic dysfunction-associated steatotic liver disease (MASLD) (15–19). However, insufficient evidence currently exists regarding the impact of the EAT-Lancet diet on MASLD.
As another important factor affecting the progression of MASLD, genetic factors have attracted increasing attention in recent years. Genetic factors determine approximately half of liver steatosis and also determine the risk of metabolic diseases and hepatic fibrosis (20). Polygenic risk scoring (PRS) provides enhanced risk stratification by aggregating multiple susceptibility loci, thereby outperforming single-nucleotide polymorphism approaches in predicting incident MASLD (21). However, the impact of its interaction with diet on MASLD has not been thoroughly studied.
Therefore, we conducted this study to assess the relationship between adherence to the EAT-Lancet reference diet and the risks of MASLD, cirrhosis, liver cancer, and other chronic liver conditions and investigate whether these associations are modified by genetic risk.
2 Methods
2.1 Study population
The UK Biobank served as the data source for the present study. Specifically, during the baseline assessment period (2006–2010), the cohort enrolled 500,000 participants aged 39–70 years. All participants completed standardized physical examinations at one of the 22 dedicated assessment centers across England, Scotland, and Wales, supplemented by a touchscreen questionnaire and structured interviews conducted by trained nurses. Written informed consent was obtained from all participants prior to data collection.
From an initial cohort of 502,371 individuals, the analytical cohort comprised 210,950 subjects with complete 24-h dietary recall data. The exclusion criteria included the following: (1) documented liver-related diseases or alcohol use (n = 509), (2) drug use disorders (n = 1,375), and (3) previous liver transplantation (n = 30) identified during baseline screening or historical records (Supplementary Table S3) (22, 23). The secondary exclusion criteria comprised the following: (1) MASLD (n = 195), (2) liver cirrhosis (n = 123), (3) liver cancer (n = 8, 4) other liver diseases (n = 463) detected at baseline assessment. Following rigorous adjustment for overlapping exclusions and missing covariate data, the final study population consisted of 160,394 eligible participants. Participants lacking genetic data (n = 2,982) were subsequently excluded from the PRS-diet interaction analysis, yielding a final analytical sample of 157,412 subjects for PRS–diet interaction modeling (Figure 1).
Figure 1

Flowchart of the study. MASLD, metabolic dysfunction-associated steatotic liver disease.
2.2 Dietary assessment
To quantify adherence to the EAT-Lancet diet, we developed a dietary score based on the methodology established by Knuppel et al., which represents one of the most widely applied healthy diet assessment tools. A binary scoring system was used to evaluate whether participants’ intake met the recommended upper and lower limits for each food category (24). The scoring system incorporated seven positively weighted components: whole grains, vegetables, fruits, legumes, nuts, fish, and unsaturated fats. In addition, seven inversely associated components were assessed: potatoes, dairy, eggs, pork, beef and lamb, poultry, and added sugars. Energy intake was standardized to 2,500 kcal/day for male participants and 2,000 kcal/day for female participants. The reference values for recommended intakes are assigned to each dietary component to determine the participants’ scores. A threshold value was set, and participants received 1 point if their score was below this value and 0 points otherwise. Higher cumulative scores reflected stronger alignment with healthier dietary patterns. The dietary index spanned a theoretical range of 0 (poorest adherence) to 14 (optimal adherence). The population distribution of dietary index scores is visualized in Supplementary Figure S1. For analytical purposes, scores were categorized into tertiles: lower adherence (0–10), moderate adherence (11), and higher adherence (12–14). More details on the calculations and food components are found in Supplementary Table S1.
2.3 PRS calculation
The PRS for MASLD is a weighted value obtained by weighting the number of risk alleles for each SNP. Our study combined 77 SNPs related to MASLD to determine the total PRS score. Specific details are provided in Supplementary Table S2. A higher score indicates a higher genetic susceptibility to MASLD. Participants who obtained scores were further stratified into three levels of genetic risk.
2.4 Outcome ascertainment
The primary outcome, MASLD, was defined according to the expert panel consensus statement using ICD-10 codes K76.0 (non-alcoholic fatty liver disease) and K75.8 (other specified inflammatory liver diseases) (22). It was identified based on hospital admission records and death registries. Secondary outcomes included liver cirrhosis, liver cancer, and other liver diseases (25). The ICD-10 codes used to ascertain these outcomes are detailed in Supplementary Table S4. The complete dates of inpatient data are as follows: October 2022 for England, August 2022 for Scotland, and May 2022 for Wales. Follow-up duration was censored at the earliest occurrence of (1) primary endpoint diagnosis, (2) loss-to-follow-up, (3) death, or (4) the study termination date (31 October 2022).
2.5 Assessment of covariates
Details regarding age and sex (male or female) were determined from self-reports. Covariates for sociodemographic factors and lifestyle were collected at baseline, including education (college or university degree), A levels/AS levels or equivalent, O levels/GCSEs or equivalent, CSEs or equivalent, NVQ or HND or HNC or equivalent, other professional qualifications (nursing, teaching, and none of the above), and income (<18,000, 18,000–30,999, 31,000–51,999, 52,000–100,000, and >£100,000 £/year). Using the Townsend score, the Townsend deprivation index is calculated from the residential postcode. Physical activity was grouped into low, moderate, or high. Smoking status and drinking status were categorized as current, former, or never. The body mass index (BMI) is calculated by dividing weight in kilograms by the square of height in meters (<25, ≥25 kg/m2). Information on whether participants had CVD, cancer, or diabetes was also collected.
2.6 Statistical analysis
Participant characteristics were summarized as mean ± standard deviation for continuous variables and frequency (percentage) for categorical variables. Multivariable-adjusted Cox regression models were implemented to estimate hazard ratios (HRs) with 95% confidence intervals (CIs) for associations between EAT-Lancet diet adherence and incident: (1) MASLD, (2) liver cirrhosis, (3) liver cancer, and (4) other liver diseases. The proportional hazards assumption was validated through Schoenfeld residuals (α = 0.05). Model 1 was adjusted to account for age and gender factors. On this basis, Model 2 further incorporated covariates including BMI, ethnicity, education qualifications, household income, smoking status, drinking status, physical activity, the Townsend deprivation index, and whether they suffer from CVD, cancer, and diabetes. Non-linear associations were characterized using restricted cubic splines with four knots placed at quintiles of the EAT-Lancet diet score distribution, implemented within the Cox proportional hazards framework. The joint effects of genetic predisposition (PRS tertiles) and dietary patterns (diet score tertiles) were assessed by creating a 3 × 3 matrix (9 combinations), using the highest-risk stratum (upper PRS tertile + lower diet score tertile) as the reference category. In a multivariable-adjusted model, hazard ratios (HRs) and 95% confidence intervals (CIs) were used to calculate the incidence of MASLD. Additive interaction was quantified using relative excess risk due to interaction (RERI) with delta method-derived confidence intervals, while multiplicative interaction was assessed through likelihood ratio tests comparing models with/without cross-product terms. Additionally, we performed subgroup analyses that were stratified by age (<65 and ≥65 years old), sex (female and male), smoking/drinking status (never and former/current), education (college or university and above, and other), and diabetes (no and yes).
We finally conducted two sensitivity analyses. First, we excluded participants who had been diagnosed with liver cirrhosis, liver cancer, MASLD, or other liver diseases within the first 2 or 4 years of the trial to evaluate the strength of the connection. Second, we attempted to account for missing covariates by performing multiple imputations.
A two-tailed test and a p-value of < 0.05 were considered significant. All analyses were performed using R software version 4.3.3.
3 Results
3.1 Baseline characteristics
Baseline characteristics are shown in Table 1. Among the 160,394 participants at baseline, the mean (SD) age was 56.2 years (7.97), and 83,870 (52.3%) were female. Overall, higher scores were associated with participants who are female, non-smokers or non-drinkers, have more free time for physical exercise, have higher education levels, and have a lower BMI.
Table 1
| Variables | EAT-Lancet diet index | |||
|---|---|---|---|---|
| Low (N = 88,687) | Moderate (N = 52,422) | High (N = 19,285) | Total (N = 160,394) | |
| Age | ||||
| Baseline age, years, mean (SD) | 55.8 (8.05) | 56.7 (7.88) | 56.5 (7.79) | 56.2 (7.97) |
| Sex | ||||
| Female | 41,732 (47.1%) | 29,836 (56.9%) | 12,302 (63.8%) | 83,870 (52.3%) |
| Male | 46,955 (52.9%) | 22,586 (43.1%) | 6,983 (36.2%) | 76,524 (47.7%) |
| Ethnicity | ||||
| White | 85,011 (95.9%) | 50,378 (96.1%) | 18,326 (95.0%) | 153,715 (95.8%) |
| Non-white | 3,676 (4.1%) | 2,044 (3.9%) | 959 (5.0%) | 6,679 (4.2%) |
| Smoking status | ||||
| Never smoker | 48,445 (54.6%) | 30,734 (58.6%) | 11,369 (59.0%) | 90,548 (56.5%) |
| Previous smoker | 32,063 (36.2%) | 18,434 (35.2%) | 6,876 (35.7%) | 57,373 (35.8%) |
| Current smoker | 8,179 (9.2%) | 3,254 (6.2%) | 1,040 (5.4%) | 12,473 (7.8%) |
| Drinking status | ||||
| Never drinker | 2,465 (2.8%) | 1,512 (2.9%) | 662 (3.4%) | 4,639 (2.9%) |
| Previous drinker | 2,450 (2.8%) | 1,425 (2.7%) | 635 (3.3%) | 4,510 (2.8%) |
| Current drinker | 83,772 (94.5%) | 49,485 (94.4%) | 17,988 (93.3%) | 151,245 (94.3%) |
| Physical activity | ||||
| Low | 17,930 (20.2%) | 8,806 (16.8%) | 2,725 (14.1%) | 29,461 (18.4%) |
| Moderate | 37,388 (42.2%) | 22,671 (43.2%) | 8,207 (42.6%) | 68,266 (42.6%) |
| High | 33,369 (37.6%) | 20,945 (40.0%) | 8,353 (43.3%) | 62,667 (39.1%) |
| Household income | ||||
| Less than 18,000 | 13,271 (15.0%) | 7,357 (14.0%) | 2,714 (14.1%) | 23,342 (14.6%) |
| 18,000–30,999 | 20,911 (23.6%) | 12,622 (24.1%) | 4,497 (23.3%) | 38,030 (23.7%) |
| 31,000–51,999 | 25,417 (28.7%) | 15,018 (28.6%) | 5,485 (28.4%) | 45,920 (28.6%) |
| 52,000–100,000 | 22,325 (25.2%) | 13,377 (25.5%) | 4,990 (25.9%) | 40,692 (25.4%) |
| Greater than 100,000 | 6,763 (7.6%) | 4,048 (7.7%) | 1,599 (8.3%) | 12,410 (7.7%) |
| Education level | ||||
| College or university degree | 37,908 (42.7%) | 25,373 (48.4%) | 10,656 (55.3%) | 73,937 (46.1%) |
| A levels/AS levels or equivalent | 12,035 (13.6%) | 6,927 (13.2%) | 2,502 (13.0%) | 21,464 (13.4%) |
| O levels/GCSEs or equivalent | 18,630 (21.0%) | 9,935 (19.0%) | 3,134 (16.3%) | 31,699 (19.8%) |
| CSEs or equivalent | 4,029 (4.5%) | 1,761 (3.4%) | 444 (2.3%) | 6,234 (3.9%) |
| NVQ or HND or HNC or equivalent | 5,282 (6.0%) | 2,517 (4.8%) | 767 (4.0%) | 8,566 (5.3%) |
| Other professional qualifications e.g., nursing, teaching | 4,082 (4.6%) | 2,604 (5.0%) | 858 (4.4%) | 7,544 (4.7%) |
| None of the above | 6,721 (7.6%) | 3,305 (6.3%) | 924 (4.8%) | 10,950 (6.8%) |
| BMI (kg/m2) | ||||
| <25 | 29,469 (33.2%) | 21,417 (40.9%) | 9,688 (50.2%) | 60,574 (37.8%) |
| ≥25 | 59,218 (66.8%) | 31,005 (59.1%) | 9,597 (49.8%) | 99,820 (62.2%) |
| Townsend deprivation index, mean (SD) | −1.56 (2.89) | −1.70 (2.80) | −1.49 (2.87) | −1.60 (2.86) |
| CVD | ||||
| No | 64,867 (73.1%) | 39,386 (75.1%) | 15,117 (78.4%) | 119,370 (74.4%) |
| Yes | 23,820 (26.9%) | 13,036 (24.9%) | 4,168 (21.6%) | 41,024 (25.6%) |
| Cancer | ||||
| No | 82,305 (92.8%) | 48,345 (92.2%) | 17,703 (91.8%) | 148,353 (92.5%) |
| Yes | 6,382 (7.2%) | 4,077 (7.8%) | 1,582 (8.2%) | 12,041 (7.5%) |
| Diabetes | ||||
| No | 84,718 (95.5%) | 50,457 (96.3%) | 18,721 (97.1%) | 153,896 (95.9%) |
| Yes | 3,969 (4.5%) | 1,965 (3.7%) | 564 (2.9%) | 6,498 (4.1%) |
Baseline characteristics of 160,394 participants by the EAT-Lancet diet index.
SD, standard deviation; BMI, body mass index; CVD, cardiovascular disease; NVQ, National Vocational Qualification; HND, Higher National Diploma; HNC, Higher National Certificate; GCSEs, General Certificate of Secondary Education; CSEs, Certificate of Secondary Education.
3.2 EAT-Lancet diet and incidence of chronic liver diseases
During a median follow-up period of 13.3 years, a total of 1,727 MASLD cases were reported. In age-sex adjusted models, participants in the moderate (HR = 0.68, 95%CI 0.62–0.75) and high (HR = 0.53, 95%CI 0.45–0.62) EAT-Lancet diet adherence tertiles demonstrated significantly lower MASLD risk compared to the lowest tertile (p < 0.001 for both). In the multivariate-adjusted Cox proportional hazards model, the hazard ratio (HR) was 0.67 (95%CI: 0.55–0.80; p < 0.001) for participants in the highest EAT-Lancet diet index group compared to the lowest group. Furthermore, each 3-point increase in the EAT-Lancet diet score was associated with a 29% reduction in the risk of MASLD (with HRs 0.71 [95%CI, 0.58–0.89]) (Table 2).
Table 2
| Cases/person-years | Model 1 | Model 2 | |||
|---|---|---|---|---|---|
| HR (95% CI) | p-value | HR (95% CI) | p-value | ||
| MASLD | |||||
| tertile1 | 1,570/2,745,613 | 1.00 (Ref.) | 1.00 (Ref.) | ||
| tertile2 | 639/2,745,516 | 0.68 (0.62, 0.75) | <0.001 | 0.81 (0.73, 0.91) | <0.001 |
| tertile3 | 178/2,745,353 | 0.53 (0.45, 0.62) | <0.001 | 0.67 (0.55, 0.80) | <0.001 |
| Continuous (per 3 unit) | 0.54 (0.49, 0.60) | <0.001 | 0.71 (0.58, 0.89) | 0.002 | |
| Liver cirrhosis | |||||
| tertile1 | 546/2,752,353 | 1.00 (Ref.) | 1.00 (Ref.) | ||
| tertile2 | 232/2,752,256 | 0.74 (0.63, 0.86) | <0.001 | 0.87 (0.72, 1.04) | 0.118 |
| tertile3 | 55/2,752,093 | 0.51 (0.39, 0.68) | <0.001 | 0.55 (0.39, 0.78) | <0.001 |
| Continuous (per 3 unit) | 0.57 (0.48, 0.68) | <0.001 | 0.71 (0.57, 0.88) | 0.002 | |
| Liver cancer | |||||
| tertile1 | 92/2,754,781 | 1.00 (Ref.) | 1.00 (Ref.) | ||
| tertile2 | 37/2,754,685 | 0.69 (0.47, 1.01) | 0.057 | 0.74 (0.47, 1.14) | 0.173 |
| tertile3 | 3/2,754,521 | 0.17 (0.05, 0.53) | 0.002 | 0.17 (0.04, 0.70) | 0.014 |
| Continuous (per 3 unit) | 0.41 (0.26, 0.64) | <0.001 | 0.48 (0.29, 0.80) | 0.005 | |
| Other liver diseases | |||||
| tertile1 | 1,644/2,745,329 | 1.00 (Ref.) | 1.00 (Ref.) | ||
| tertile2 | 858/2,745,232 | 0.85 (0.79, 0.93) | <0.001 | 0.94 (0.85, 1.04) | 0.218 |
| tertile3 | 285/2,745,069 | 0.80 (0.71, 0.91) | <0.001 | 0.85 (0.73, 0.99) | 0.038 |
| Continuous (per 3 unit) | 0.76 (0.68, 0.84) | <0.001 | 0.85 (0.76, 0.96) | 0.009 | |
Hazard ratios (95% confidence intervals) of MASLD, cirrhosis, liver cancer, and other liver diseases according to the tertiles of the EAT-Lancet diet index.
Model 1 was adjusted for age and sex.
Model 2 was adjusted for age, sex, ethnicity, Townsend deprivation index, educational level, BMI, physical activity, drinking status, smoking status, household income, CVD, cancer, and diabetes.
Bold value indicates Levels of significance: p < 0.05 (Cox regression model).
MASLD, metabolic dysfunction-associated steatotic liver; HR, hazard ratio; CI, confidence interval; CVD, cardiovascular disease.
For other chronic liver diseases, in the fully adjusted model, the highest EAT-Lancet diet adherence group exhibited a 45% reduction in cirrhosis risk (HR = 0.55, 95% CI 0.39–0.78), an 83% reduction in liver cancer risk (HR = 0.17, 95% CI 0.04–0.70), and a 15% reduction in other liver disease risks (HR = 0.85, 95% CI 0.73–0.99). Additionally, the hazard ratios (HRs) for cirrhosis, liver cancer, and other liver diseases were 0.71 (0.57–0.88), 0.48 (0.29–0.80), and 0.85 (0.76–0.96) per 3-point increase.
When examining the dose–response relationship, we observed a linear deviation in the association between the risk of MASLD and the EAT-Lancet diet (Figure 2, p-non-linearity > 0.05). Similar findings were also observed in liver cancer, cirrhosis, and other liver diseases (p for overall < 0.05, p-non-linearity > 0.05) (Supplementary Figures S2–S4).
Figure 2
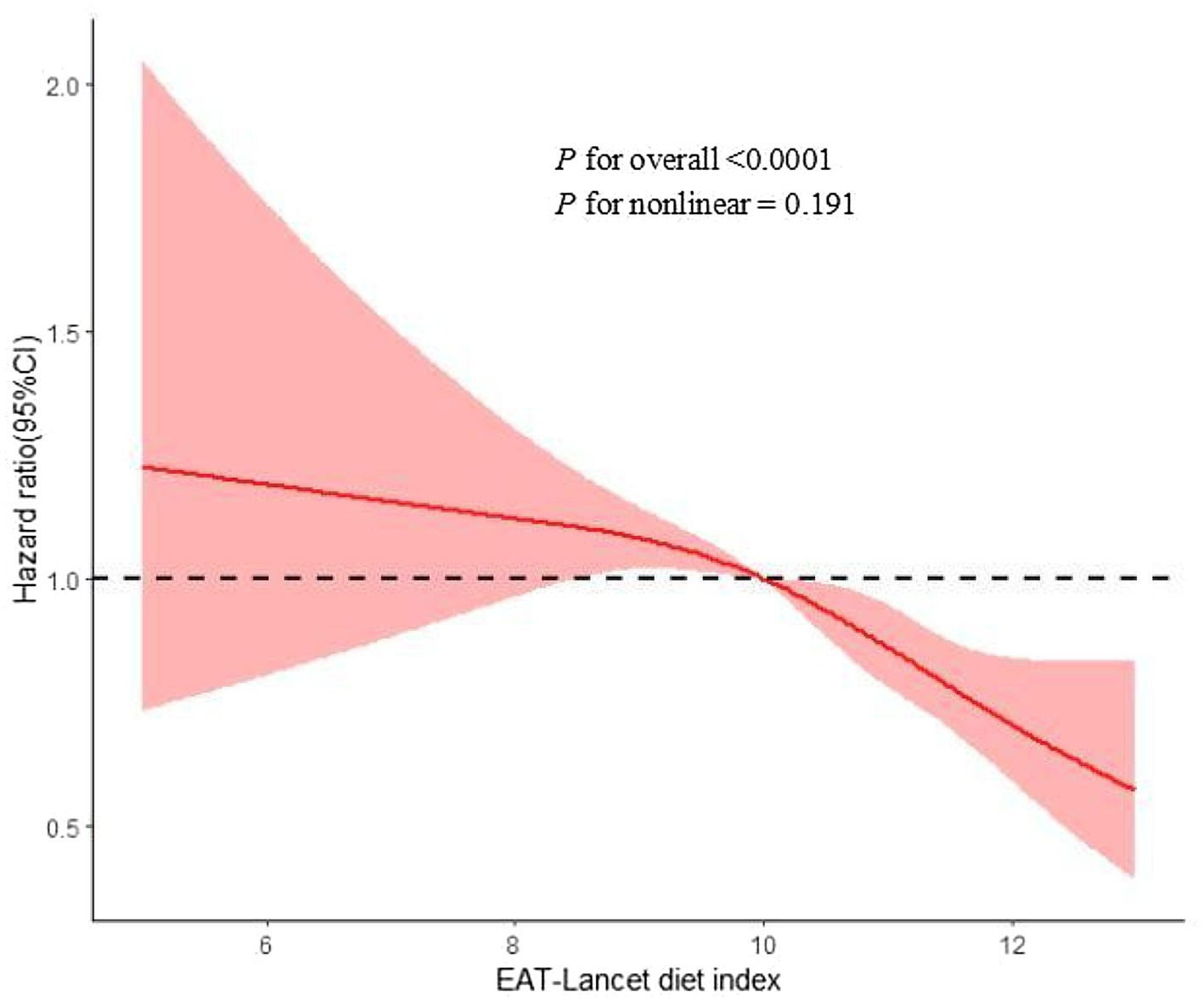
Restrict cubic spline for associations of the EAT-Lancet diet index with MASLD risk. Adjusted for age, sex, ethnicity, Townsend deprivation index, educational level, BMI, physical activity, drinking status, smoking status, household income, CVD, cancer, and diabetes. BMI, body mass index; CI, confidence interval; HR, hazard ratio; MASLD, metabolic dysfunction-associated steatotic liver; CVD, cardiovascular disease.
3.3 The interaction of the EAT-Lancet diet and PRS on MASLD
As detailed in Supplementary Table S5, participants in the highest PRS tertile exhibited 24% elevated risk of MASLD compared to the lowest tertile (HR = 1.24, 95%CI 1.10–1.40). Figure 3 illustrates the interactions between the genetic component and adherence to the EAT-Lancet diet. As genetic risk increases, the risk of MASLD onset decreases with higher EAT-Lancet diet scores. The protective effect was most pronounced in the low PRS/high diet adherence group (HR = 0.52, 95% CI 0.36–0.74, compared to the high PRS/low adherence group). However, neither additive nor multiplicative effects were observed (Table 3).
Figure 3
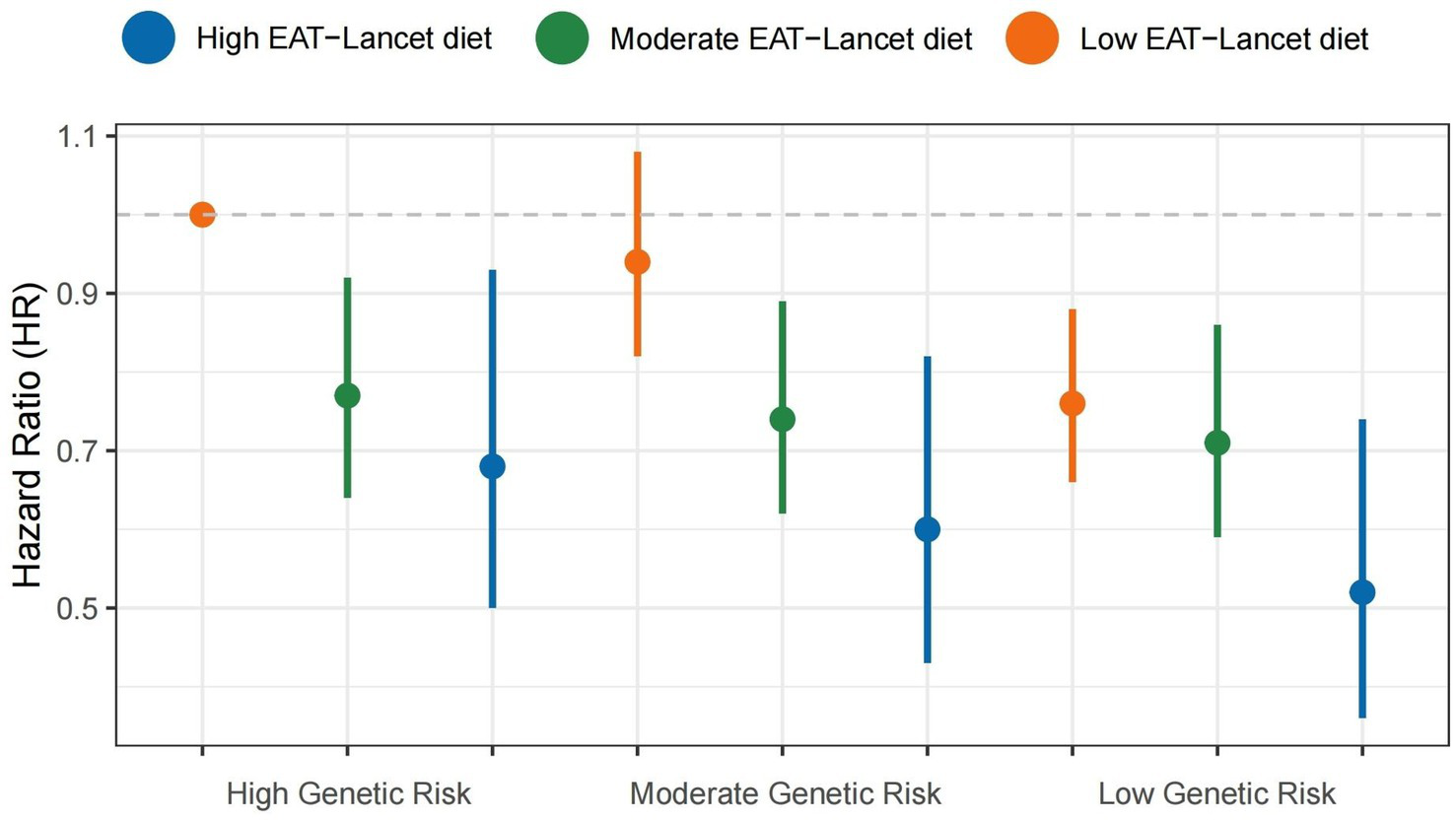
Combined effects of the EAT-Lancet diet index, genetic risk, and the risk of MASLD. MASLD, metabolic dysfunction-associated steatotic liver disease.
Table 3
| EAT-Lancet diet index | PRS* | p for interaction++ | |||
|---|---|---|---|---|---|
| Moderate | Low | ||||
| RERI+ | AP+ | RERI | AP | ||
| Moderate | −0.03 (−0.19, 0.24) | 0.04 (−0.25, 0.32) | 0.18 (−0.02, 0.38) | 0.26 (−0.02, 0.53) | 0.625 |
| High | −0.03 (−0.33, 0.27) | −0.05 (−0.56, 0.47) | 0.07 (−0.22, 0.37) | 0.14 (−0.40, 0.69) | |
Combined effects of the EAT-Lancet diet, PRS, and the risk of MASLD.
AP, attributable proportion due to the interaction; CI, confidence interval; PRS, polygenic risk score; RERI, relative excess risk due to the interaction; CVD, cardiovascular disease.
Adjusted for age, sex, ethnicity, Townsend deprivation index, educational level, BMI, physical activity, drinking status, smoking status, household income, CVD, cancer, and diabetes.
*Defined by PRS: low (lowest tertiles), intermediate (second tertiles), and high (highest tertiles).
+To estimate the RERI and AP, the low EAT-Lancet diet index and the highest genetic risk (high PRS) groups were the reference categories.
++Likelihood tests were applied to test the significance of the interaction term by comparing the model with and without the interaction term.
3.4 Additional analyses
Figure 4 displays the stratified analysis results. The risk of MASLD was not substantially impacted by variables, including age, gender, BMI, smoking or drinking, household income, education level, or diabetes (p for all interactions > 0.05). Supplementary Figures S5–S7 display a stratified analysis involving liver cirrhosis, liver cancer, and other liver diseases. We observed a significant interaction between the EAT-Lancet diet and liver cancer, as well as other liver diseases, among individuals of different genders (p for interaction < 0.05).
Figure 4
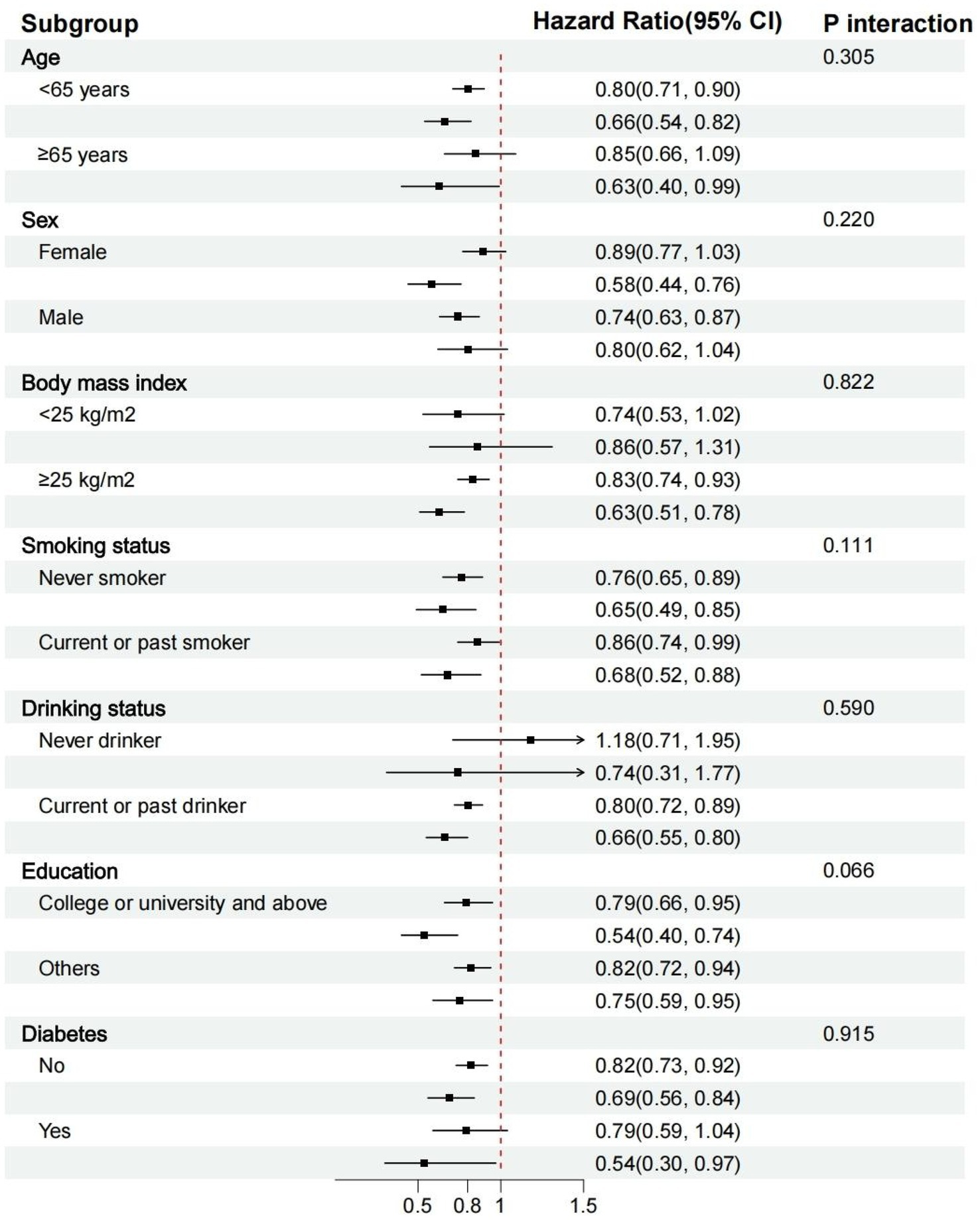
Stratified analyses of the association between the EAT-Lancet diet index and the risk of MASLD. MASLD, metabolic dysfunction-associated steatotic liver diseases.
In sensitivity analysis, we excluded cases that occurred within the first 2 or 4 years, as shown in Supplementary Table S6. The results of multiple interpolations of covariates are shown in Supplementary Table S7.
4 Discussion
In this prospective study based on the UK Biobank, we observed that the EAT-Lancet diet index demonstrated significant protective effects against chronic liver disease. Compared to the lowest adherence group, individuals in the highest adherence group exhibited a 33% reduced risk of developing MASLD. Consistent results were obtained for secondary outcomes. Furthermore, the protective association persisted across genetic risk strata, supporting the EAT-Lancet diet as an evidence-based dietary recommendation for hepatic health promotion.
Although diet is recognized as a critical pillar in mitigating the development of chronic liver diseases, longitudinal studies investigating the association between the EAT-Lancet diet and the risk of MASLD remain limited. One study investigating this association yielded results consistent with our findings: Adherence in the highest quartile was associated with a 27% reduced risk of MASLD (26). However, no studies have yet explored its potential impact on other chronic liver diseases. A number of previous studies have pointed out that plant foods such as vegetables and beans are protective factors for MASLD (27). A cohort study from Tianjin found that compliance with animal-based or sugar-rich dietary patterns was positively associated with MASLD, whereas vegetable-rich dietary patterns showed no significant association with MASLD risk (28). Similarly, a UK Biobank study suggested that plant-based diets were associated with a 22 and 26% reduction in MASLD risk, respectively (16, 29). A randomized controlled trial has shown that adhering to a green-MED diet primarily composed of plant-based foods can halve the prevalence of MASLD (30). Our study corroborates this finding, indicating a positive relationship between the MASLD and the EAT-Lancet diet.
Genetic factors serve as another identified pathogenic risk, yet the interaction between these factors and diet has not yielded meaningful results in existing studies. The reason for this lack of interaction may be that the PRS constructed using a limited number of SNPs cannot accurately represent genetic risk. Constructing a more comprehensive PRS that covers a wider range of pathways and functions may better explain disease risk (31, 32). Although we used a larger number of SNPs (77) to construct the PRS compared to previous studies that included only five SNPs, no significant interaction was observed (33). Therefore, it is necessary to integrate more loci in the future to improve the prediction of individual genetic risk for MASLD. With the ongoing expansion of genome-wide association study (GWAS) sample sizes and advances in polygenic risk score (PRS) construction methods, future PRS models are expected to exhibit greater predictive accuracy and resolution. Reassessing the interactions between the EAT-Lancet dietary pattern and genetic susceptibility using these refined tools will be an important area for future investigation (34, 35). In our study, we found that both PRS and the EAT-Lancet diet can independently predict MASLD. This finding implies that individuals with different genetic risks should all pay attention to the quality of their diet. From the standpoint of public health, this dietary pattern is beneficial for patients with MASLD, regardless of genetic risk.
We first explored the connection between the EAT-Lancet diet and other chronic liver diseases (except for MASLD). Previous studies concentrated on the relationship between diet and liver-related diseases. For instance, Brazilian patients with liver cirrhosis consume more grains, rice, beans, and yogurt in their diet compared to American patients. This dietary pattern is linked to greater gut microbiota diversity and demonstrates a decreased hospitalization rate (36). A prospective study conducted as part of another Women’s Health Initiative found a negative correlation between adherence to a dietary pattern for reducing diabetes risk and the risk of liver cancer, as well as mortality from chronic liver disease. This dietary pattern aimed at reducing diabetes risk involves decreasing the total intake of red and processed meat, foods with a high glycemic index, sugar-sweetened beverages (SSBs), and dietary trans fats, while increasing the intake of cereal fiber, coffee, nuts, and polyunsaturated fatty acids (37). Furthermore, some studies have also found that higher baseline intakes of breakfast cereals, tea, fruits, and dietary fiber, coupled with lower intakes of red meat and processed meat, can reduce the risk of liver cirrhosis, liver cancer, and other related conditions (38).
The development of MASLD involves a wide range of pathophysiological mechanisms, starting with hepatocellular death, followed by inflammation and compensatory proliferation, and ultimately developing into different stages of liver fibrosis, cirrhosis, and hepatocellular carcinoma (39, 40). Currently, the biological mechanisms linking the EAT-Lancet diet and chronic liver diseases remain unclear, but diet also serves as a risk factor contributing to the burden of chronic liver diseases (41). High-fat and high-fructose diets can disturb the gut microbiota, inducing hepatic steatosis and inflammation and promoting tumorigenesis (41, 42). Insulin resistance is also a key triggering factor (43). Studies have shown that macronutrients such as saturated fatty acids (SFAs), trans fats, monosaccharides (sucrose and fructose), and animal proteins can regulate the accumulation of triglycerides and antioxidant activity in the liver, thereby affecting its sensitivity (44, 45). Additionally, necroptosis or pyroptosis of hepatocytes also drives the progression of MASLD (46). Similarly, certain biomarkers also play a profound mediating role in the relationship between healthy dietary patterns and disease risk (47, 48). Diet-associated proteins (e.g., FSTL3, STC1, and CD302 antigen) significantly mediate risk associations for various chronic disorders, including cardiovascular diseases and chronic respiratory diseases (49). Meanwhile, metabolic biomarkers related to the EAT-Lancet diet (specifically, triglycerides in HDL) exhibit a significant positive correlation with MASLD incidence, suggesting their potential involvement in mediating dietary influences on MASLD risk (33). After validation, these biomarkers may serve as comprehensive health indicators to compensate for biases inherent in conventional dietary assessments (47).
Our study’s strengths include its prospective design and large sample size. Our study has certain limitations as well. First, in large-scale population studies, the precise calculation of individual dietary intake is challenging. Dietary assessment relies on 24-h recall methods, which may be subject to recall bias and fail to adequately reflect long-term dietary habits. However, the dietary assessment metrics used in our primary analysis have been demonstrated to correlate with repeatedly measured dietary indices (50). Second, over half of the participants were eliminated due to incomplete 24-h dietary recall questionnaires, but this discrepancy can be clinically negligible (51). Third, currently, there is no unified standard for quantifying adherence to the EAT-Lancet diet. In addition to the methodology used in our study, the Stubbendorff scoring system is also commonly utilized. However, the Stubbendorff scoring method may not comprehensively account for the balance between health and environmental impacts (52). Nevertheless, both scoring systems demonstrated good adherence within the cohort. Some researchers have raised concerns that the Knuppel method may not serve as an optimal indicator for assessing adherence. However, Knuppel et al. clarified that their analysis was adjusted for energy intake, thereby enabling valid comparisons between participants with different adherence scores at equivalent energy intake levels (53). Fourth, although most of the confounding factors have been controlled, there are still some potential confounding factors. Finally, the majority of participants were white, which limited our study’s ability to accurately reflect the entire population.
5 Conclusion
Our study found that adherence to the EAT-Lancet diet is associated with a reduced risk of chronic liver diseases. Following this sustainable diet may attenuate the development of multiple chronic liver conditions, thereby substantiating the EAT-Lancet Commission’s global health recommendations.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors without undue reservation.
Author contributions
S-YW: Conceptualization, Data curation, Methodology, Software, Writing – original draft, Writing – review & editing. Y-CB: Conceptualization, Data curation, Methodology, Software, Writing – original draft, Writing – review & editing. Z-YL: Data curation, Methodology, Writing – review & editing. X-YH: Conceptualization, Writing – review & editing. Y-YN: Conceptualization, Writing – review & editing. JH: Conceptualization, Data curation, Writing – review & editing. J-XZ: Conceptualization, Data curation, Writing – review & editing. Y-JZ: Conceptualization, Funding acquisition, Supervision, Validation, Writing – review & editing. Z-LY: Conceptualization, Funding acquisition, Supervision, Validation, Writing – review & editing. H-YL: Conceptualization, Funding acquisition, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. Support for this effort came from the Scientific and Technological Research Program of Henan Province (No. 242102311127) and the Joint Fund of Henan Province Science and Technology R&D Program (No. 235200810020).
Acknowledgments
We acknowledge all contributors to the completion of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2025.1589424/full#supplementary-material
Abbreviations
MASLD, metabolic dysfunction-associated steatotic liver disease; T2DM, type 2 diabetes mellitus; PRS, polygenic risk score; SNP, single-nucleotide polymorphism; BMI, body mass index; CVD, cardiovascular disease; HR, hazard ratio; CI, confidence interval; RCS, restricted cubic splines.
References
1.
KimokotiRWMillenBE. Nutrition for the prevention of chronic diseases. Med Clin North Am. (2016) 100:1185–98. doi: 10.1016/j.mcna.2016.06.003
2.
WillettWRockströmJLokenBSpringmannMLangTVermeulenSet al. Food in the Anthropocene: the EAT-lancet commission on healthy diets from sustainable food systems. Lancet. (2019) 393:447–92. doi: 10.1016/s0140-6736(18)31788-4
3.
TullochAITBorthwickFBoguevaDEltholthMGrechAEdgarDet al. How the EAT-lancet commission on food in the Anthropocene influenced discourse and research on food systems: a systematic review covering the first 2 years post-publication. Lancet Glob Health. (2023) 11:e1125–36. doi: 10.1016/s2214-109x(23)00212-7
4.
LawrenceMABakerPIPulkerCEPollardCM. Sustainable, resilient food systems for healthy diets: the transformation agenda. Public Health Nutr. (2019) 22:2916–20. doi: 10.1017/s1368980019003112
5.
ZhangSStubbendorffAOlssonKEricsonUNiuKQiLet al. Adherence to the EAT-lancet diet, genetic susceptibility, and risk of type 2 diabetes in Swedish adults. Metabolism. (2023) 141:155401. doi: 10.1016/j.metabol.2023.155401
6.
ZhangSMarkenIStubbendorffAEricsonUQiLSonestedtEet al. The EAT-lancet diet index, plasma proteins, and risk of heart failure in a population-based cohort. JACC Heart Fail. (2024) 12:1197–208. doi: 10.1016/j.jchf.2024.02.017
7.
XiaoYPengLXuZTangYHeHGuHet al. Association between adherence to eat-lancet diet and incidence and mortality of lung cancer: a prospective cohort study. Cancer Sci. (2023) 114:4433–44. doi: 10.1111/cas.15941
8.
DevarbhaviHAsraniSKArabJPNarteyYAPoseEKamathPS. Global burden of liver disease: 2023 update. J Hepatol. (2023) 79:516–37. doi: 10.1016/j.jhep.2023.03.017
9.
YounossiZAnsteeQMMariettiMHardyTHenryLEslamMet al. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. (2018) 15:11–20. doi: 10.1038/nrgastro.2017.109
10.
YounossiZMKoenigABAbdelatifDFazelYHenryLWymerM. Global epidemiology of nonalcoholic fatty liver disease-meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. (2016) 64:73–84. doi: 10.1002/hep.28431
11.
PowellEEWongVWRinellaM. Non-alcoholic fatty liver disease. Lancet. (2021) 397:2212–24. doi: 10.1016/s0140-6736(20)32511-3
12.
RiaziKAzhariHCharetteJHUnderwoodFEKingJAAfsharEEet al. The prevalence and incidence of NAFLD worldwide: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. (2022) 7:851–61. doi: 10.1016/s2468-1253(22)00165-0
13.
YounossiZM. Non-alcoholic fatty liver disease - a global public health perspective. J Hepatol. (2019) 70:531–44. doi: 10.1016/j.jhep.2018.10.033
14.
WangPSongMEliassenAHWangMFungTTClintonSKet al. Optimal dietary patterns for prevention of chronic disease. Nat Med. (2023) 29:719–28. doi: 10.1038/s41591-023-02235-5
15.
Zelber-SagiSRatziuVOrenR. Nutrition and physical activity in NAFLD: an overview of the epidemiological evidence. World J Gastroenterol. (2011) 17:3377–89. doi: 10.3748/wjg.v17.i29.3377
16.
LvYRongSDengYBaoWXiaYChenL. Plant-based diets, genetic predisposition and risk of non-alcoholic fatty liver disease. BMC Med. (2023) 21:351. doi: 10.1186/s12916-023-03028-w
17.
Petermann-RochaFWirthMDBoonporJParra-SotoSZhouZMathersJCet al. Associations between an inflammatory diet index and severe non-alcoholic fatty liver disease: a prospective study of 171, 544 UK biobank participants. BMC Med. (2023) 21:123. doi: 10.1186/s12916-023-02793-y
18.
Petermann-RochaFCarrasco-MarinFBoonporJParra-SotoSShannonOMalcomsonFet al. Association of five diet scores with severe NAFLD incidence: a prospective study from UK biobank. Diabetes Obes Metab. (2024) 26:860–70. doi: 10.1111/dom.15378
19.
GBD 2021 Risk Factors Collaborators. Global burden and strength of evidence for 88 risk factors in 204 countries and 811 subnational locations, 1990-2021: a systematic analysis for the global burden of disease study 2021. Lancet. (2024) 403:2162–203. doi: 10.1016/s0140-6736(24)00933-4
20.
EslamMValentiLRomeoS. Genetics and epigenetics of NAFLD and NASH: clinical impact. J Hepatol. (2018) 68:268–79. doi: 10.1016/j.jhep.2017.09.003
21.
SunYYuanSChenXSunJKallaRYuLet al. The contribution of genetic risk and lifestyle factors in the development of adult-onset inflammatory bowel disease: a prospective cohort study. Am J Gastroenterol. (2023) 118:511–22. doi: 10.14309/ajg.0000000000002180
22.
HagströmHAdamsLAAllenAMByrneCDChangYGrønbaekHet al. Administrative coding in electronic health care record-based research of NAFLD: an expert panel consensus statement. Hepatology. (2021) 74:474–82. doi: 10.1002/hep.31726
23.
LonardoAMantovaniAPettaSCarraroAByrneCDTargherG. Metabolic mechanisms for and treatment of NAFLD or NASH occurring after liver transplantation. Nat Rev Endocrinol. (2022) 18:638–50. doi: 10.1038/s41574-022-00711-5
24.
KnuppelAPapierKKeyTJTravisRC. EAT-lancet score and major health outcomes: the EPIC-Oxford study. Lancet. (2019) 394:213–4. doi: 10.1016/s0140-6736(19)31236-x
25.
ZhongQZhouRHuangYNHuangRDLiFRChenHWet al. Frailty and risk of metabolic dysfunction-associated steatotic liver disease and other chronic liver diseases. J Hepatol. (2024) 82:427–37. doi: 10.1016/j.jhep.2024.08.024
26.
ZhangSYanYZengXFGuYWuHZhangQet al. Associations of the EAT-lancet reference diet with metabolic dysfunction-associated steatotic liver disease and its severity: a multicohort study. Hepatology. (2024) 81:1583–94. doi: 10.1097/hep.0000000000001039
27.
UllahRRaufNNabiGUllahHShenYZhouYDet al. Role of nutrition in the pathogenesis and prevention of non-alcoholic fatty liver disease: recent updates. Int J Biol Sci. (2019) 15:265–76. doi: 10.7150/ijbs.30121
28.
ZhangSGuYBianSGórskaMJZhangQLiuLet al. Dietary patterns and risk of non-alcoholic fatty liver disease in adults: a prospective cohort study. Clin Nutr. (2021) 40:5373–82. doi: 10.1016/j.clnu.2021.08.021
29.
SatijaABhupathirajuSNRimmEBSpiegelmanDChiuveSEBorgiLet al. Plant-based dietary patterns and incidence of type 2 diabetes in US men and women: results from three prospective cohort studies. PLoS Med. (2016) 13:e1002039. doi: 10.1371/journal.pmed.1002039
30.
Yaskolka MeirARinottETsabanGZelichaHKaplanARosenPet al. Effect of green-Mediterranean diet on intrahepatic fat: the DIRECT PLUS randomised controlled trial. Gut. (2021) 70:2085–95. doi: 10.1136/gutjnl-2020-323106
31.
ZhuangPLiuXLiYWanXWuYWuFet al. Effect of diet quality and genetic predisposition on hemoglobin a (1c) and type 2 diabetes risk: gene-diet interaction analysis of 357, 419 individuals. Diabetes Care. (2021) 44:2470–9. doi: 10.2337/dc21-1051
32.
VujkovicMKeatonJMLynchJAMillerDRZhouJTcheandjieuCet al. Discovery of 318 new risk loci for type 2 diabetes and related vascular outcomes among 1.4 million participants in a multi-ancestry meta-analysis. Nat Genet. (2020) 52:680–91. doi: 10.1038/s41588-020-0637-y
33.
WuHWeiJWangSChenLZhangJWangNet al. Dietary pattern modifies the risk of MASLD through metabolomic signature. JHEP Rep. (2024) 6:101133. doi: 10.1016/j.jhepr.2024.101133
34.
LiRNLiQMLiangSXHongCZhangRFWangJRet al. Association between red blood cell indices and non-alcoholic fatty liver disease: prospective study and two-sample Mendelian randomization analysis based on large cohorts. Ann Hepatol. (2025) 30:101775. doi: 10.1016/j.aohep.2025.101775
35.
YangQLiMChenPDouNLiuMLuPet al. Systematic evaluation of the impact of a wide range of dietary habits on myocardial infarction: a two-sample Mendelian randomization analysis. J Am Heart Assoc. (2025) 14:e035936. doi: 10.1161/jaha.124.035936
36.
Álvares-da-SilvaMROliveiraCPFaganALongoLThoenRUYoshimura ZitelliPMet al. Interaction of microbiome, diet, and hospitalizations between Brazilian and American patients with cirrhosis. Clin Gastroenterol Hepatol. (2022) 20:930–40. doi: 10.1016/j.cgh.2021.03.045
37.
ChenYZhaoLJungSYPichardoMSLopez-PentecostMRohanTEet al. Diabetes risk reduction diet and risk of liver cancer and chronic liver disease mortality: a prospective cohort study. J Intern Med. (2024) 296:410–21. doi: 10.1111/joim.20007
38.
GuoWGeXLuJXuXGaoJWangQet al. Diet and risk of non-alcoholic fatty liver disease, cirrhosis, and liver cancer: a large prospective cohort study in UK biobank. Nutrients. (2022) 14:5335. doi: 10.3390/nu14245335
39.
LueddeTKaplowitzNSchwabeRF. Cell death and cell death responses in liver disease: mechanisms and clinical relevance. Gastroenterology. (2014) 147:765–83.e4. doi: 10.1053/j.gastro.2014.07.018
40.
PerdomoCMFrühbeckGEscaladaJ. Impact of nutritional changes on nonalcoholic fatty liver disease. Nutrients. (2019) 11:677. doi: 10.3390/nu11030677
41.
AdolphTETilgH. Western diets and chronic diseases. Nat Med. (2024) 30:2133–47. doi: 10.1038/s41591-024-03165-6
42.
TilgHAdolphTEDudekMKnolleP. Non-alcoholic fatty liver disease: the interplay between metabolism, microbes and immunity. Nat Metab. (2021) 3:1596–607. doi: 10.1038/s42255-021-00501-9
43.
Romero-GómezMZelber-SagiSTrenellM. Treatment of NAFLD with diet, physical activity and exercise. J Hepatol. (2017) 67:829–46. doi: 10.1016/j.jhep.2017.05.016
44.
MussoGGambinoRDe MichieliFCassaderMRizzettoMDurazzoMet al. Dietary habits and their relations to insulin resistance and postprandial lipemia in nonalcoholic steatohepatitis. Hepatology. (2003) 37:909–16. doi: 10.1053/jhep.2003.50132
45.
BernáGRomero-GomezM. The role of nutrition in non-alcoholic fatty liver disease: pathophysiology and management. Liver Int. (2020) 40:102–8. doi: 10.1111/liv.14360
46.
GautheronJGoresGJRodriguesCMP. Lytic cell death in metabolic liver disease. J Hepatol. (2020) 73:394–408. doi: 10.1016/j.jhep.2020.04.001
47.
WilliamsSAKivimakiMLangenbergCHingoraniADCasasJPBouchardCet al. Plasma protein patterns as comprehensive indicators of health. Nat Med. (2019) 25:1851–7. doi: 10.1038/s41591-019-0665-2
48.
ShoaieSGhaffariPKovatcheva-DatcharyPMardinogluASenPPujos-GuillotEet al. Quantifying diet-induced metabolic changes of the human gut microbiome. Cell Metab. (2015) 22:320–31. doi: 10.1016/j.cmet.2015.07.001
49.
ZhuKLiRYaoPYuHPanAMansonJEet al. Proteomic signatures of healthy dietary patterns are associated with lower risks of major chronic diseases and mortality. Nat Food. (2025) 6:47–57. doi: 10.1038/s43016-024-01059-x
50.
HeianzaYZhouTSunDHuFBQiL. Healthful plant-based dietary patterns, genetic risk of obesity, and cardiovascular risk in the UK biobank study. Clin Nutr. (2021) 40:4694–701. doi: 10.1016/j.clnu.2021.06.018
51.
LuXWuLShaoLFanYPeiYLuXet al. Adherence to the EAT-lancet diet and incident depression and anxiety. Nat Commun. (2024) 15:5599. doi: 10.1038/s41467-024-49653-8
52.
StubbendorffASternDEricsonUSonestedtEHallströmEBornéYet al. A systematic evaluation of seven different scores representing the EAT-lancet reference diet and mortality, stroke, and greenhouse gas emissions in three cohorts. Lancet Planet Health. (2024) 8:e391–401. doi: 10.1016/s2542-5196(24)00094-9
53.
HarcombeZ. This is not the EAT-lancet diet. Lancet. (2020) 395:271–2. doi: 10.1016/s0140-6736(19)32551-6
Summary
Keywords
EAT-Lancet diet, polygenic risk score, metabolic dysfunction-associated steatotic liver disease, chronic liver disease, gene-diet interaction
Citation
Wu S-Y, Bo Y-C, Li Z-Y, Hu X-Y, Ning Y-Y, Huang J, Zhang J-X, Zhu Y-J, Yu Z-L and Liu H-Y (2025) EAT-Lancet diet and risk of metabolic dysfunction-associated steatotic liver disease and other liver chronic diseases: a large prospective cohort study in the UK Biobank. Front. Nutr. 12:1589424. doi: 10.3389/fnut.2025.1589424
Received
07 March 2025
Accepted
14 July 2025
Published
31 July 2025
Volume
12 - 2025
Edited by
Arpita Mukhopadhyay, St. John’s Research Institute, India
Reviewed by
Anna Maria Giudetti, University of Salento, Italy
Aquartuti Tri Darmayanti, School of Health Sciences Mamba’ul Ulum, Indonesia
Updates
Copyright
© 2025 Wu, Bo, Li, Hu, Ning, Huang, Zhang, Zhu, Yu and Liu.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hong-Yan Liu, liuhongyanqhhy@126.com; Zeng-Li Yu, zly@zzu.edu.cn; Yong-Jian Zhu, zhu412825@126.com
†These authors have contributed equally to this work and share first authorship
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.