- 1Department of Rheumatology and Chinese Medicine, The 962nd Hospital of the Chinese PLA, Harbin, China
- 2Department of Rheumatology, People's Hospital Affiliated to Fujian University of Traditional Chinese Medicine, Fuzhou, China
Objective: Gout is a condition strongly associated with dietary patterns and elevated risk of cardiovascular disease (CVD) in affected individuals. Given the potential influence of dietary diversity on inflammatory responses, this study aimed to explore the association between the dietary inflammatory index (DII) and CVD prevalence in gout patients.
Methods: Data from gout patients in NHANES 2007–2018 were extracted for analysis. Correlation matrices were employed to examine the relationships among 28 dietary inflammation indices. Machine learning algorithms were utilized to identify key features for constructing a covariate subset for the final model, and Random Forest SHAP interpretations were applied to assess variable risk factors. The relationship between DII and CVD risk in gout patients was assessed using multi-model logistic regression. RCS were applied to evaluate the risk trend and to assess model discrimination, predictive probability, and clinical benefit using ROC, calibration curves, and DCA, respectively. Subgroup analysis was evaluated the heterogeneity in CVD across different populations.
Results: 1,437 gout patients met inclusion criteria were included in the study, with mean age of 60.84 years, consisting of 435 females (31.23%) and 1,002 males (68.77%), and an overall CVD prevalence of 32.92%. DII was linearly associated with CVD risk (P for overall = 0.002; P for nonlinear = 0.810). In the final model, DII was positively associated with CVD risk, showing 118% increased risk in Q4 compared to Q1 (OR: 2.18, 95%CI: 1.52–3.13, p < 0.001). The constructed model exhibited stability performance (AUC = 0.750, 95%CI: 0.722–0.775). Segmented subgroup analysis indicated that gout patients with high DII (> 1.934) had a increased risk of CVD (OR: 1.33, 95%CI: 0.06–1.65, p = 0.012), while those younger than 60 years had higher risk (OR: 2.19, 95%CI: 1.36–3.54, p = 0.001).
Conclusion: Higher DII was associated with increased prevalence of CVD in gout patients. Dietary modification may serve as an effective strategy for preventing disease progression and reducing CVD risk. Our findings support the clinical development of dietary and nutritional guidance programs.
Introduction
Gout is a group of disorders resulting from purine metabolism disturbances, as both metabolic and rheumatic diseases characterized by prolonged hyperuricemia and acute, self-limiting arthritic flare-ups as the primary clinical manifestations (1, 2). As the disease progresses, urate crystals continue to accumulate in the periarticular and subcutaneous tissues, as well as in the kidneys, leading to the formation of gout stones. In some cases, bone erosion and renal failure, advancing to refractory gout, which significantly impairs patients’ functional capacity and quality of life (3). From 1990 to 2019, the global number of gout patients increased from 22 million to 53 million. The age-standardized prevalence rate rose from 532.99 to 652.24 per 10,000 individuals, with the male-to-female prevalence ratio remaining at 3:1. The incidence of gout grew by 70.15% in male and 68.70% in female (4). A meta-analysis using Global Burden of Disease (GBD) data found that the all-cause treatment costs for employed, elderly, and refractory gout populations were $4,733, $16,925, and $18,362, respectively. These costs were positively correlated with blood uric acid levels and the frequency of acute gout episodes (5).
Previous epidemiological studies have shown that gout is associated with elevated risk of cardiovascular diseases (CVD), including coronary heart disease (CHD), myocardial infarction (MI), peripheral artery disease (PAD), congestive heart failure (CHF), and CVD mortality (6, 7). A large epidemiological study in Asia reported a 57% increase in the overall risk of CVD in patients with gout compared to the general population (8). The 2020 American College of Rheumatology (ACR) guidelines for gout management recommend screening for CVD comorbidities and updating the management of gout patients (9). Managing CVD complications in gout patients has become a significant public health challenge.
Gout is strongly associated with the consumption of rich foods and uncontrolled alcohol intake, while chronic low-grade inflammation from these dietary factors contributes to elevated blood uric acid levels and frequent gout flare-ups (10, 11). Diets such as the DASH diet, Mediterranean diet, and those rich in fiber and vegetables have been shown to reduce inflammation, which in turn lowers the prevalence of CVD (12, 13). Dietary inflammation may contribute significantly to various diseases, but fewer studies have explored how modifying dietary patterns can reduce the risk of CVD in gout patients. The dietary inflammation index (DII) is an innovative dietary tool designed to directly assess the inflammatory potential of the overall diet (14, 15). The DII evaluates the inflammatory potential of the diet based on 45 components and has been validated through various serum markers of inflammation, such as C-reactive protein (CRP) and interleukins (IL) (16). A review of the literature suggests that DII plays a critical role in regulating inflammation in metabolic diseases, neuropsychiatric disorders, respiratory conditions, and malignancy-associated chronic diseases (17–20). However, No clinical studies on the relationship between the DII and the risk of CVD in gout participants were found through searching the PubMed and Web of Science databases, and the extent to which DII influences the progression of CVD in this population remains unclear.
Highly representative sample was selected through a rigorous screening process using data from the National Health and Nutrition Examination Survey (NHANES) 2007–2018. Machine learning algorithms were employed to construct models for exploring and analyzing the potential association between DII and CVD risk in gout. This study aims to assist clinicians in more accurately assessing CVD risk in gout patients, identifying those at high risk, and providing a foundation for effective disease management strategies.
Study population
Data from the NHANES, a multi-institutional series designed to assess the health and nutritional status of both adults and children in the U.S. The survey protocol was approved by the Institutional Review Board (IRB) of the National Center for Health Statistics (NCHS). All participants provided written informed consent, ensuring the study’s ethical compliance. Data for this study were obtained from the official NHANES website, ensuring data transparency and accessibility.
Data from 6 NHANES cycles conducted between 2007 to 2018 were included in this study, which initially screened 59,842 participants. Strict exclusion criteria were applied to maintain the rigor of the study design. A total of 44,562 ineligible participants were excluded, including those younger than 20 years (n = 25,072), pregnant female (n = 372), participants missing gout information (n = 32,740), missing CVD information (n = 34), and missing DII information (n = 185). After applying these exclusions, 1,437 eligible participants remained in the study. Figure 1 illustrates the participant screening process.
Definition of gout
The diagnosis of gout was based on a self-reported physician’s diagnosis, with participants being asked, “doctor ever told you that you have gout?” If a participant answered “yes,” they were considered to have the condition (21).
Definition of cardiovascular disease
The diagnosis of CVD was established through self-reported physician diagnoses obtained via an individual interview using a standardized medical condition questionnaire. CVD was defined as self-reported physician diagnoses of CHF, CHD, angina, MI, or stroke. If a participant answered “yes,” they were considered to have the condition (22).
Definition of dietary inflammation index
45 specific foods and nutrients were associated with various inflammatory or anti-inflammatory biomarkers, and the inflammatory potential of each dietary component was scored based on biomarkers, including CRP, tumor necrosis factor (TNF)-α, IL-1β, IL-4, IL-6, and IL-10. A score of +1 was assigned to dietary components that significantly increased inflammatory biomarkers, and a score of −1 was assigned to those that decreased them (15, 16). The global means and standard deviations of 45 food parameters were calculated using data from 11 countries (23, 24).
The NHANES collected dietary information via 24 h dietary recall interviews conducted at mobile examination center (MEC). Two 24 h dietary recall interviews were used to calculate DII for each participant. However, due to missing nutrient data in the NHANES dietary database (25), 28 food parameters were included in this cross-sectional study to calculate DII. The parameters analyzed included energy, protein, carbohydrates, dietary fiber, total fat, saturated fat, monounsaturated fatty acids, polyunsaturated fatty acids, cholesterol, vitamin (Vit) A, β-carotene, thiamine, riboflavin, niacin, VitB6, VitB12, VitC, VitD, VitE, magnesium, iron, zinc, selenium, caffeine, alcohol, and n-3 and n-6 fatty acids.
Covariates in NHANES
Covariates for this cross-sectional study included demographic data from NHANES 2007–2018, physical examination results, laboratory examinations, and questionnaire data. These covariates included information on age, gender, body mass index (BMI), blood pressure, smoke, drink, sleep disorder, 12 self-reported comorbidities, and 21 laboratory examinations. Drink status was defined as “had drink more than 12 times in the past year” or “drink more than once a month,” and smoke status as “had smoke more than 100 cigarettes in lifetime” or “current smoke.” Hypertension was defined as “systolic blood pressure average (SBP Avg) ≥ 135 mmHg, diastolic blood pressure average (DBP Avg) ≥ 85 mmHg, ever been told by a doctor or other health professional that had hypertension, or taking prescription for hypertension”.
According to World Health Organization (WHO) criteria, participants with fasting blood glucose ≥ 126 mg/dL, 2 h blood glucose ≥ 200 mg/dL on the oral glucose tolerance test (OGTT), or glycosylated hemoglobin (HbA1c) ≥ 6.5% were defined as having diabetes (26). Chronic kidney disease (CKD) was defined according to KDIGO criteria: eGFR < 60 mL/min; or a total urine protein (UPRO) /creatinine ratio > 30 mg/g when eGFR ≥ 60 mL/min. Participants met one of these criteria were defined as CKD (27). Asthma, chronic obstructive pulmonary disease (COPD), kidney stones, and cancer were assessed based on self-reported health data with the question, “Doctor told you had asthma, COPD, kidney stones, cancer, or malignancy.” Blood and urine specimens were collected at the MEC and sent to a standardized laboratory for testing.
Statistical analysis
The baseline study population across 6 cycles was weighted according to the statistical methods recommended by NHANES analysis guidelines. Missing data were imputed using simple interpolation, with no more than 20% of model variables missing. In the descriptive analysis, continuous variables were expressed as means with standard error (SE), while categorical variables were presented as frequencies. The Chi-square test was used for categorical data, and the t-test was applied to continuous variables for group comparison.
Correlation matrix was plotted to show the relationship among the 28 DIIs. Important features were selected using the Boruta and Random Forest algorithms to construct a subset of covariates for the final model, and risk measures of the assessment variables were interpreted using Random Forest SHAP. Gout patients were grouped according to DII quartiles for logistic regression analysis. RCS was applied to validate the risk trend, and discrimination, predictive probability, and clinical benefit were assessed using ROC, calibration curves, and DCA, respectively. Segmented subgroup analysis was performed to assess the heterogeneity of CVD occurrence across different populations.
Statistical analysis and data visualization were conducted using SPSS 27.0.1, R-studio 4.4.2 and DCPM 6.03.1. p value < 0.05 was considered statistically significant.
Results
Baseline characteristics of study population
The baseline characteristics of the study population, which included 1,437 gout participants with mean age of 60.84 years, 435 females (31.23%) and 1,002 males (68.77%) were presented in Table 1. The overall prevalence of CVD was 32.92%, with 191 (9.54%) cases of CHF, 192 (12.64%) cases of CHD, 118 (7.75%) cases of Angina, 204 (11.45%) cases of MI, and 148 (7.44%) cases of Stroke. CVD participants were older than those without CVD (67.24 vs. 58.49, p < 0.001). The prevalence of hypertension, diabetes, asthma, COPD, CKD, and kidney stones was also higher among CVD participants. The prevalence of kidney stones was also higher in participants with CVD.
21 laboratory examinations were included in the study. CVD participants exhibited higher DII (1.96 vs. 1.46, p < 0.001), UPRO, Scr, BUN, GLB, GLU, HbA1c, and K, and lower PLT, RBC, Hb, ALB, eGFR, ALT, TC, TG, and LDL compared to participants without CVD. The CVD prevalence of the Quartitles of DII were presented in Supplementary Table 2. Relative Q4 compared to Q1 showed a higher prevalence of CVD (34.56 vs. 18.69%, p < 0.001), CHF (15.10 vs. 4.87%, p < 0.001), and stroke (11.59 vs. 3.15%, p = 0.005).
Correlation analysis of 28 dietary inflammation indices
The correlation matrix and coefficients for the 28 dietary inflammation indices are presented in Figure 2 and Supplementary Table 3. In the analysis of the 28 dietary inflammation indices in 1,437 gout patients, we found that energy, protein, carbohydrates, total fat, saturated fat, cholesterol, VitB12, and iron were associated with pro-inflammatory diets. In contrast, dietary fiber, monounsaturated fatty acids, polyunsaturated fatty acids, VitA, β-carotene, thiamine, riboflavin, niacin, VitB6, folic acid, VitC, VitD, VitE, magnesium, zinc, selenium, caffeine, alcohol, n-3 and n-6 fatty acids showed negative correlations of varying strengths.
Feature selection of dietary inflammation indices with cardiovascular disease
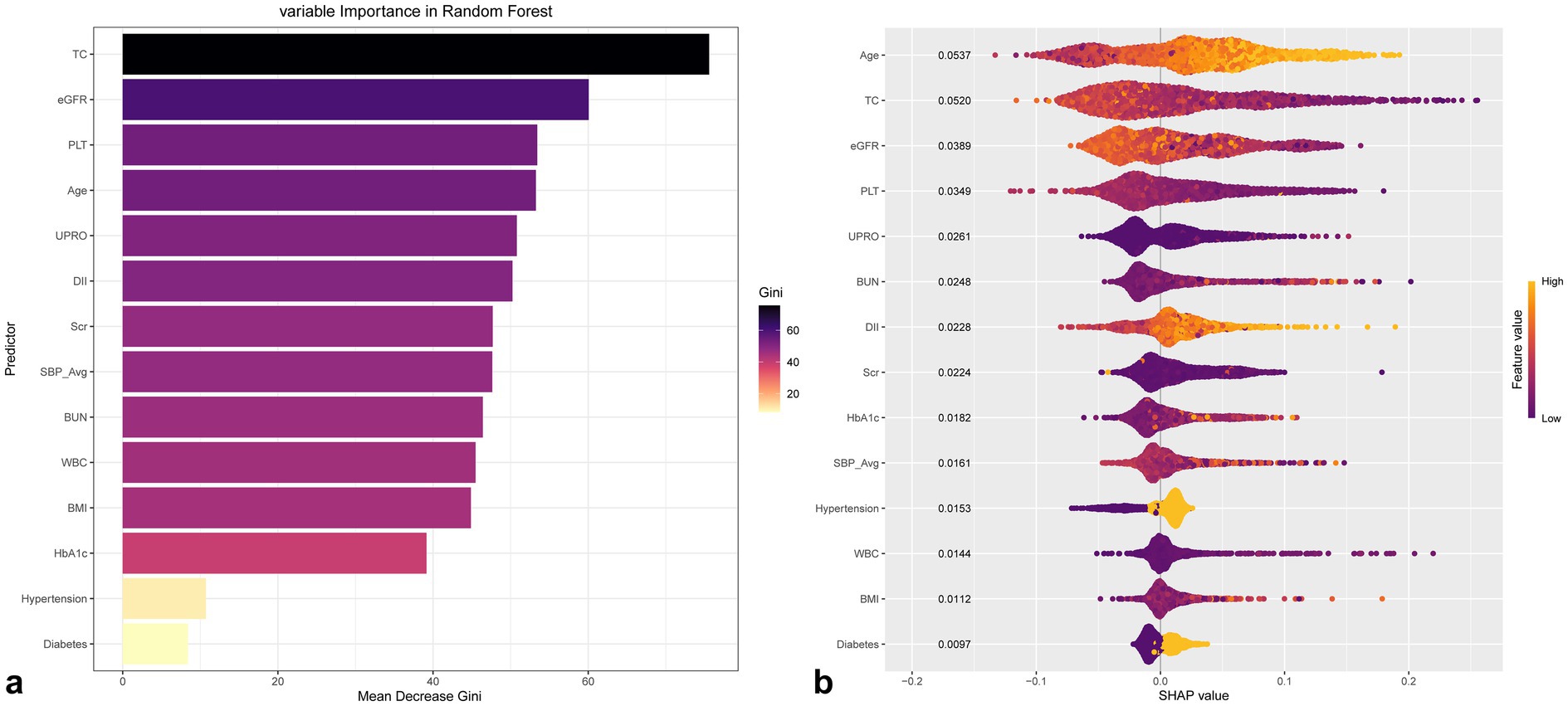
3 logistic regression models were constructed in our study to explore the association between DII and the prevalence of CVD. The adjusted covariates in Model 3 were selected through Boruta and Random Forest algorithm (Figure 3; Supplementary Figures 1–5; Supplementary Table 4). The screening covariates were as follows:
CVD: Age, Hypertension, Diabetes, BMI, eGFR, BUN, Scr, HbA1c, PLT, UPRO, WBC, SBP Avg, TC
CHF: Age, eGFR, BUN, Scr, UPRO, PLT, HbA1c, COPD, GLU, CKD, K, TC, SBP Avg
CHD: Age, eGFR, BUN, Scr, TC, Hb, UPRO, HDL-C, CKD, ALT, RBC, GLU, K, PLT
Angina: Age, CKD, BMI, eGFR, BUN, Scr, HbA1c, Hb, RBC, UPRO, DBP Avg, WBC, TC, PLT, HDL-C, SBP Avg
MI: Age, CKD, eGFR, BUN, Scr, TC, Hb, RBC, PLT, HDL-C, UPRO, DBP Avg, SBP Avg
Stroke: Age, CKD, eGFR, BUN, Scr, RBC, UPRO, ALT, Hb, PLT, GLU, TC, SBP Avg

Figure 3. Ranking of variable importance based on Boruta (a) and Random Forest (b) algorithms of CVD in gout.
Multi-model analysis of cardiovascular disease and subtypes
The results of univariate and multivariate logistic regression analyses for CVD and its four subtypes were presented in Table 2. Model 1 represents an unadjusted analysis, Model 2 is adjusted for sex and age, and Model 3 is adjusted for a subset of variables selected by eigenvalues. Random Forest variable importance ranking and SHAP values are also used to explain the contribution of each variable to the model outcomes, with the influence of the eigenvalues illustrated in Figure 4.
As the DII increased from Q1 to Q4, we observed a positive correlation between higher DII scores and a higher risk of CVD, CHF, CHD, MI, and stroke in both Model 1 and Model 2. In Model 2 and Model 3, higher DII was significantly associated with increased risk of CVD, CHF, MI, and stroke.
Figure 5 shows that DII was linearly associated with the risk of CVD in gout patients (P for overall = 0.002; P for nonlinear = 0.810). The risk of CVD increased when the DII exceeded the median value (Median = 1.934), with significant positive correlation. Additionally, the area under the curve (AUC) for CVD was significantly higher in Model 3 (AUC = 0.750, 95% CI: 0.722–0.775) compared to Model 1 (Delong test: Z = 10.04, p < 0.001; Bootstrap Delong test: Z = 9.22, p < 0.001) and Model 2 (Delong test: Z = 6.28, p < 0.001; Bootstrap Delong test: Z = 6.00, p < 0.001). Subtypes of CVD (CHF, CHD, Angina, MI, and Stroke) multi-model analysis presents in Supplementary Figures 6–10.

Figure 5. Performance evaluation of multi-model incorporating confounders with RCS (a), ROC (b), DCA (c) and calibration curve (d) of CVD in gout.
Segmented subgroup analysis
Segmented subgroup analysis of 1,437 gout patients were divided into 2 groups, based on the DII (Median = 1.934), revealed a higher risk of CVD in those with DII > 1.934 (OR = 1.33, 95% CI: 1.06–1.65, p = 0.012). Among gout patients aged ≤ 60 years with high DII, the risk of CVD was significantly elevated (OR = 2.19, 95% CI: 1.36–3.54, p = 0.001), with significant interaction effects (P for interaction = 0.012). The prevalence of CVD was slightly higher in females with high DII (38.06% vs. 36.26%) compared to males (OR = 1.46, 95% CI: 0.95–2.25), but this difference was not statistically significant (p = 0.087). Gout patients with hypertension and CKD also showed an increased risk of CVD with high DII, although no significant interaction was found (see Table 3).
Discussion
The association between DII and the occurrence of CVD in patients with gout was explored. Key adjustment variables were screened using machine learning algorithms, revealing a significant positive association between DII and the prevalence of CVD in patients with gout, characterized by a significant linear relationship. Evaluation of the model performance indicated that the fully adjusted model exhibited higher accuracy and discriminative ability. Differences in the risk of CVD in gout patients were observed based on gender and age, but the results remained stability.
DII is an important indicator of dietary regulation of body inflammation, reflects the impact of food components on inflammatory cells, the regulation of oxidative stress, and inflammation mediated by intestinal flora (28, 29). Following the absorption of food components such as cholesterol and fatty acids through the digestive tract, macrophages are activated to release TNF-α and IL-6. Concurrently, macrophages phagocytose saturated fat particles, activating intracellular inflammatory signaling pathways, including the NF-κB pathway, which initiates gene transcription, leading to the massive expression of pro-inflammatory cytokines and triggering inflammatory responses (30, 32). Diets containing saturated fats, high levels of alcohol, and caffeine alter the composition and function of the inherently colonized intestinal flora, increasing Enterobacteriaceae and anaerobic bacteria. This results in decreased integrity of the intestinal epithelium, increased intestinal permeability, and enhanced translocation of bacterial endotoxins into the bloodstream, thereby activating the immune response and producing chronic, low-grade inflammation (33). The rational regulation of pro-inflammatory foods and dietary structure interventions warrants further in-depth research and exploration.
The male-to-female prevalence ratio of gout was 2.2:1, and the overall prevalence of CVD was 32.92%, with male-to-female prevalence ratio of approximately 2.3:1. A study conducted at the University of Glasgow, United Kingdom, enrolled 152,663 patients with gout, of whom 120,324 (78.8%) were male. This study found that the risk of CVD was increased by 58% among gout patients compared to the healthcare group (HR 1.88, 95% CI: 1.75–2.02), and 58% increase in the risk of CVD was observed in female with gout (HR = 1.88, 95% CI: 1.75–2.02), despite the higher proportion of male in the cohort (34). A study from the University of Nottingham, UK, including 4,398 patients (66.9% male), found that the incidence of CVD was significantly higher within 30 days of the first diagnosis of gout (HR = 1.55, 95% CI: 1.33–1.83). However, female had a higher incidence of CVD after the diagnosis of gout compared to male, although the statistical difference was not significant (35). These studies and our data were consistent.
DII and the risk of CVD development were positively and linearly correlated. A review of the literature indicates that DII is positively associated with the development of diabetes, hypertension, and hyperuricemia, which are potential risk factors for CVD (17, 36, 37). Covariates screened by machine learning, including age, hypertension, diabetes, BMI, eGFR, BUN, Scr, HbA1c, and UPRO, were identified as correlates of gout comorbidities and their occurrence and progression (38, 39). A study from Peking University in China, involving 7,880 participants from the 2009 China Health and Nutrition Survey (CHNS), found that higher DII was associated with increased risk of hyperuricemia, with 31% reduction in risk in the lowest DII group compared to the highest (40). A study from Zhejiang University of Traditional Chinese Medicine, involving 5,006 participants with CVD, found that as the DII increased, the risk of diabetes and hypertension also increased significantly. Cox proportional hazards modeling revealed that participants in higher DII quartiles exhibited higher CVD mortality (HR = 1.34, 95% CI: 1.21–1.61), showing a positive and linear correlation (41). A Jilin University study, involving 3,930 participants with hyperuricemia, found a positive correlation between the highest quartile of DII levels and the incidence of hyperuricemia (OR = 1.34, 95% CI: 1.13–1.57). With median follow-up of 136 months, 892 deaths were documented, of which 254 were attributed to CVD. Kaplan–Meier curves indicated a 50% higher CVD mortality in participants with higher DII levels (HR = 1.50, 95% CI: 1.00–2.26) (42). A KoGES cohort study of 162,773 healthy participants, with average follow-up of 7.4 years between 2004 and 2013, found that 1,111 participants developed CVD, including 578 males (52.03%) and 533 females (47.97%). Higher DII was associated with increased mortality in males (HR = 1.43, 95% CI: 1.04–1.96) and females (HR = 1.19, 95% CI: 0.85–1.67), with increased risk of developing CVD (43). Although these studies did not directly establish a positive association between DII and the occurrence of CVD in gout patients, they all confirmed the positive association between DII and gout comorbidities and potential risk factors for CVD, offering valuable insights.
We found that the risk of CVD was higher in young and middle-aged gout patients with elevated DII compared to older patients in our subgroup analysis. Gout typically manifests at a younger age in the affected population. Studies have found that the prevalence of gout in males is 2.9 times higher than in females across all age groups, peaking at 7.3 times between the ages of 30 to 34 years (44). Some studies have shown that individuals with gout under the age of 45 have the highest risk of developing CVD after follow-up (HR = 2.22, 95% CI: 1.92–2.57), with excess risk observed across all 12 CVD subtypes investigated (35). Patients diagnosed with gout at or before the age of 40 typically exhibit CVD risk factors, a higher proportion of gout-related family history, lower success in achieving target UA levels, a poorer response to uric acid-lowering medications, and an increased risk of recurrent gout and CVD compared to older patients managed in routine clinical practice (45, 46). Aging and dietary status contribute to differences in dietary inflammatory indices, with reduced physical activity in older adults leading to physiological anorexia and lower intake of food groups (47). A study from the Federal University of Maranhão, Brazil, involving 34,003 healthy participants, found that the DII index was significantly higher in adolescents and adults compared to older adults (1.42 vs. 0.61, p < 0.001) (48).
Our study was based on the analysis of U.S. national data with a large, representative sample size, the screening of covariates through machine learning algorithms, and multiple modeling and subgroup analyses. We adhered strictly to the STROBE statement, ensuring the stabilition and reliability of the results. However, the cross-sectional nature of the study presented limitations, including the lack of regular laboratory examinations reviews for the participants and the absence of follow-up on disease progression, as well as the absence of stratified analysis of different types of drug interventions introduced to the participant population, which precluded establishing causality. DII as a complex dietary inflammation-weighting algorithm, may be influenced by confounding factors such as food and water quality. Future clinical patient-based longitudinal studies and randomized controlled trials will further clarify the association between the DII and the development of CVD in gout patients, providing stronger evidence for its use in predicting the risk of CVD in this population.
Conclusion
Our study suggests a positive, linear association between DII and CVD, as well as its subtypes in gout patients. The predictive performance for CVD and its subtypes was superior in a fully adjusted logistic regression model constructed with machine learning to select variables. Gout patients with high DII and younger age had increased risk of CVD. Future large-scale longitudinal cohort studies are needed to investigate and validate whether a causal relationship exists between DII and CVD, including its subtypes, in gout patients or other populations. The results of this study provide clinicians with both a theoretical and data-driven foundation for the early identification, prevention, and management of CVD in gout patients.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary material.
Ethics statement
The studies involving humans were approved by the NCHS Ethics Review Board approved all techniques used in this study that involved using materials, data, or human subjects in accordance with the declaration of Helsinki. With written informed consent, the patients/participants gave their approval to be included in this research. Our research was granted an exemption from ethical review by the Medical Ethics Committee of the 962nd Hospital of the PLA. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
QZ: Writing – original draft, Writing – review & editing. X-bL: Writing – review & editing, Data curation, Investigation, Writing – original draft. C-qL: Writing – review & editing, Data curation, Investigation, Writing – original draft. W-zZ: Writing – review & editing, Data curation, Investigation, Writing – original draft. Y-gW: Writing – review & editing, Data curation, Investigation, Writing – original draft. W-zD: Writing – review & editing, Writing – original draft. X-hY: Writing – review & editing, Project administration, Writing – original draft.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. Research was funded by Fujian Provincial Senior Talent Training Program on Western Medicine Doctors Learning from Traditional Chinese Medicine no. 1969 (2024).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2025.1591472/full#supplementary-material
References
1. Dalbeth, N, Choi, HK, Joosten, LAB, Khanna, PP, Matsuo, H, Perez-Ruiz, F, et al. Gout. Nat Rev Dis Primers. (2019) 5:69. doi: 10.1038/s41572-019-0115-y
2. Vargas-Santos, AB, and Neogi, T. Management of Gout and Hyperuricemia in CKD. Am J Kidney Dis. (2017) 70:422–39. doi: 10.1053/j.ajkd.2017.01.055
3. Khanna, P, Johnson, RJ, Marder, B, LaMoreaux, B, and Kumar, A. Systemic urate deposition: an unrecognized complication of gout? J Clin Med. (2020) 9:3204. doi: 10.3390/jcm9103204
4. He, Q, Mok, TN, Sin, TH, Yin, J, Li, S, Yin, Y, et al. Global, regional, and National Prevalence of gout from 1990 to 2019: age-period-cohort analysis with future burden prediction. JMIR Public Health Surveill. (2023) 9:e45943. doi: 10.2196/45943
5. Rai, SK, Burns, LC, De Vera, MA, Haji, A, Giustini, D, and Choi, HK. The economic burden of gout: a systematic review. Semin Arthritis Rheum. (2015) 45:75–80. doi: 10.1016/j.semarthrit.2015.02.004
6. Clarson, LE, Hider, SL, Belcher, J, Heneghan, C, Roddy, E, and Mallen, CD. Increased risk of vascular disease associated with gout: a retrospective, matched cohort study in the UK clinical practice research datalink. Ann Rheum Dis. (2015) 74:642–7. doi: 10.1136/annrheumdis-2014-205252
7. Zhu, J, Zeng, Y, Zhang, H, Qu, Y, Ying, Z, Sun, Y, et al. The association of hyperuricemia and gout with the risk of cardiovascular diseases: a cohort and mendelian randomization study in UK biobank. Front Med. (2022) 8:817150. doi: 10.3389/fmed.2021.817150
8. FitzGerald, JD, Dalbeth, N, Mikuls, T, Brignardello-Petersen, R, Guyatt, G, Abeles, AM, et al. 2020 American College of Rheumatology guideline for the management of gout. Arthritis Care Res (Hoboken). (2020) 72:744–60. doi: 10.1002/acr.24180
9. Kang, HS, Lee, NE, Yoo, DM, Han, KM, Hong, JY, Choi, HG, et al. An elevated likelihood of stroke, ischemic heart disease, or heart failure in individuals with gout: a longitudinal follow-up study utilizing the National Health Information database in Korea. Front Endocrinol (Lausanne). (2023) 14:1195888. doi: 10.3389/fendo.2023.1195888
10. Zhang, Y, Chen, S, Yuan, M, Xu, Y, and Xu, H. Gout and diet: a comprehensive review of mechanisms and management. Nutrients. (2022) 14:3525. doi: 10.3390/nu14173525
11. Zhang, Y, Song, J, Lai, Y, Li, A, Zhang, Y, Zhou, H, et al. Association between the dietary inflammatory index and gout in the National Health and nutrition examination survey 2007-2018. Heliyon. (2023) 9:e22930. doi: 10.1016/j.heliyon.2023.e22930
12. Yokose, C, McCormick, N, Lu, N, Joshi, AD, Curhan, G, and Choi, HK. Adherence to 2020 to 2025 dietary guidelines for Americans and the risk of new-onset female gout. JAMA Intern Med. (2022) 182:254–64. doi: 10.1001/jamainternmed.2021.7419
13. Fung, TT, Chiuve, SE, McCullough, ML, Rexrode, KM, Logroscino, G, and Hu, FB. Adherence to a DASH-style diet and risk of coronary heart disease and stroke in women. Arch Intern Med. (2008) 168:713–20. doi: 10.1001/archinte.168.7.713
14. Meadows, RJ, Paskett, ED, Bower, JK, Kaye, GL, Lemeshow, S, and Harris, RE. Socio-demographic differences in the dietary inflammatory index from National Health and nutrition examination survey 2005-2018: a comparison of multiple imputation versus complete case analysis. Public Health Nutr. (2024) 27:e184. doi: 10.1017/S1368980024001800
15. Marx, W, Veronese, N, Kelly, JT, Smith, L, Hockey, M, Collins, S, et al. The dietary inflammatory index and human health: an umbrella review of Meta-analyses of observational studies. Adv Nutr. (2021) 12:1681–90. doi: 10.1093/advances/nmab037
16. Shivappa, N, Steck, SE, Hurley, TG, Hussey, JR, and Hébert, JR. Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr. (2014) 17:1689–96. doi: 10.1017/S1368980013002115
17. Wang, L, Liu, T, Zhang, Q, Wang, L, Zhou, Q, Wang, J, et al. Correlation between dietary inflammation and mortality among hyperlipidemics. Lipids Health Dis. (2023) 22:206. doi: 10.1186/s12944-023-01975-0
18. Jayedi, A, Emadi, A, and Shab-Bidar, S. Dietary inflammatory index and site-specific Cancer risk: a systematic review and dose-response Meta-analysis. Adv Nutr. (2018) 9:388–403. doi: 10.1093/advances/nmy015
19. Zhu, M, Wang, X, Peng, Z, Yan, W, Deng, Q, Li, M, et al. The role of the estimated glomerular filtration rate and body roundness index in the risk assessment of uric acid-lowering therapy-resistant gout in U.S. adults: evidence from the national health and nutrition examination survey (2007-2018). Ren Fail. (2025) 47:2441398. doi: 10.1080/0886022X.2024.2441398
20. Dang, K, Wang, X, Hu, J, Zhang, Y, Cheng, L, Qi, X, et al. The association between triglyceride-glucose index and its combination with obesity indicators and cardiovascular disease: NHANES 2003-2018. Cardiovasc Diabetol. (2024) 23:8. doi: 10.1186/s12933-023-02115-9
21. Cavicchia, PP, Steck, SE, Hurley, TG, Hussey, JR, Ma, Y, Ockene, IS, et al. A new dietary inflammatory index predicts interval changes in serum high-sensitivity C-reactive protein. J Nutr. (2009) 139:2365–72. doi: 10.3945/jn.109.114025
22. Phillips, CM, Chen, LW, Heude, B, Bernard, JY, Harvey, NC, Duijts, L, et al. Dietary inflammatory index and non-communicable disease risk: a narrative review. Nutrients. (2019) 11:1873. doi: 10.3390/nu11081873
23. Zhao, Q, Xu, Y, Li, X, and Chen, X. L-shaped association of dietary inflammatory index (DII) and chronic diarrhea: results from NHANES 2005-2010. BMC Public Health. (2025) 25:81. doi: 10.1186/s12889-025-21292-8
24. Zaccara, TA, Paganoti, CF, Mikami, FCF, Francisco, RPV, and Costara,. Who criteria for diabetes in pregnancy: a retrospective cohort. BMC Pregnancy Childbirth. (2022) 22:385. doi: 10.1186/s12884-022-04708-w
25. Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2024 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int. (2024) 105:S117–314. doi: 10.1016/j.kint.2023.10.018
26. Tabung, FK, Smith-Warner, SA, Chavarro, JE, Wu, K, Fuchs, CS, Hu, FB, et al. Development and validation of an empirical dietary inflammatory index. J Nutr. (2016) 146:1560–70. doi: 10.3945/jn.115.228718
27. Aleksandrova, K, Koelman, L, and Rodrigues, CE. Dietary patterns and biomarkers of oxidative stress and inflammation: a systematic review of observational and intervention studies. Redox Biol. (2021) 42:101869. doi: 10.1016/j.redox.2021.101869
28. Miller, ER, Appel, LJ, and Risby, TH. Effect of dietary patterns on measures of lipid peroxidation: results from a randomized clinical trial. Circulation. (1998) 98:2390–5. doi: 10.1161/01.cir.98.22.2390
29. Harte, AL, Tripathi, G, Piya, MK, Barber, TM, Clapham, JC, Al-Daghri, N, et al. NFκB as a potent regulator of inflammation in human adipose tissue, influenced by depot, adiposity, T2DM status, and TNFα. Obesity (Silver Spring). (2013) 21:2322–30. doi: 10.1002/oby.20336
30. Panizza, C, Wilkens, L, Shvetsov, Y, Maskarinec, G, Park, SY, Shepherd, J, et al. Associations of the dietary inflammatory index with total adiposity and ectopic fat and the mediating effect of the gut microbiota. Curr Dev Nutr. (2021) 5:1173. doi: 10.1093/cdn/nzab054_028
31. Ferguson, LD, Molenberghs, G, Verbeke, G, Rahimi, K, Rao, S, McInnes, IB, et al. Gout and incidence of 12 cardiovascular diseases: a case-control study including 152 663 individuals with gout and 709 981 matched controls. Lancet Rheumatol. (2024) 6:e156–67. doi: 10.1016/S2665-9913(23)00338-7
32. Cipolletta, E, Nakafero, G, Richette, P, Avery, AJ, Mamas, MA, Tata, LJ, et al. Short-term risk of cardiovascular events in people newly diagnosed with gout. Arthritis Rheumatol. (2025) 77:202–11. doi: 10.1002/art.42986
33. Zhou, N, Xie, ZP, Liu, Q, Xu, Y, Dai, SC, Lu, J, et al. The dietary inflammatory index and its association with the prevalence of hypertension: a cross-sectional study. Front Immunol. (2023) 13:1097228. doi: 10.3389/fimmu.2022.1097228
34. He, Q, Zheng, Q, Diao, H, Li, M, Zhu, Q, Fang, F, et al. The role of body mass index on the association between the energy-adjusted dietary inflammatory index and hyperuricemia: a mediation analysis based on NHANES (2007-2016). Int J Obes. (2024) 48:339–45. doi: 10.1038/s41366-023-01418-x
35. Dumusc, A, and So, A. Advances in treatment of hyperuricemia and gout. Ther Umsch. (2024) 81:168–71. doi: 10.23785/TU.2024.05.006
36. Dang, W, Zhao, L, Wang, J, Xu, D, Liu, J, and You, L. Association between serum uric acid levels of patients with gout and their complications and examination fees: a cross-sectional analysis. Int J Rheum Dis. (2023) 26:673–81. doi: 10.1111/1756-185X.14609
37. Ye, C, Huang, X, Wang, R, Halimulati, M, Aihemaitijiang, S, and Zhang, Z. Dietary inflammatory index and the risk of hyperuricemia: a cross-sectional study in Chinese adult residents. Nutrients. (2021) 13:4504. doi: 10.3390/nu13124504
38. Yang, M, Miao, S, Hu, W, and Yan, J. Association between the dietary inflammatory index and all-cause and cardiovascular mortality in patients with atherosclerotic cardiovascular disease. Nutr Metab Cardiovasc Dis. (2024) 34:1046–53. doi: 10.1016/j.numecd.2023.11.015
39. Huang, J, Zhang, Y, Li, J, Li, H, Wei, Y, and Sun, M. Association of dietary inflammatory index with all-cause and cardiovascular disease mortality in hyperuricemia population: a cohort study from NHANES 2001 to 2010. Medicine (Baltimore). (2023) 102:e36300. doi: 10.1097/MD.0000000000036300
40. Khan, I, Kwon, M, Shivappa, N, Hébert, JR, and Kim, MK. Positive Association of Dietary Inflammatory Index with incidence of cardiovascular disease: findings from a Korean population-based prospective study. Nutrients. (2020) 12:588. doi: 10.3390/nu12020588
41. Kuo, CF, Grainge, MJ, See, LC, Yu, KH, Luo, SF, Zhang, W, et al. Epidemiology and management of gout in Taiwan: a nationwide population study. Arthritis Res Ther. (2015) 17:13. doi: 10.1186/s13075-015-0522-8
42. Li, Y, Piranavan, P, Sundaresan, D, and Yood, R. Clinical characteristics of early-onset gout in outpatient setting. ACR Open Rheumatol. (2019) 1:397–402. doi: 10.1002/acr2.11057
43. Chuang, TJ, Wang, YH, Wei, JC, and Yeh, CJ. Anti-gout medications and risk of cardiovascular disease: a nested case-control study. Front Med Lausanne. (2021) 8:739680. doi: 10.3389/fmed.2021.739680
44. Pourmontaseri, H, and Khanmohammadi, S. Demographic risk factors of pro-inflammatory diet: a narrative review. Front Nutr. (2024) 11:1448806. doi: 10.3389/fnut.2024.1448806
45. Pereira, NO, Carvalho, CA, Sperandio, N, Marques, KDS, Viola, PCAF, Shivappa, N, et al. Factors associated with the inflammatory potential of the Brazilian population's diet. Br J Nutr. (2021) 126:285–94. doi: 10.1017/S0007114520004079
46. Tolkien, K, Bradburn, S, and Murgatroyd, C. An anti-inflammatory diet as a potential intervention for depressive disorders: a systematic review and meta-analysis. Clin Nutr. (2019) 38:2045–52. doi: 10.1016/j.clnu.2018.11.007
47. McDiarmid, KP, Wood, LG, Upham, JW, MacDonald-Wicks, LK, Shivappa, N, Hebert, JR, et al. The impact of meal dietary inflammatory index on exercise-induced changes in airway inflammation in adults with asthma. Nutrients. (2022) 14:4392. doi: 10.3390/nu14204392
Keywords: gout, dietary inflammation index, hyperuricemia, cardiovascular disease, machine learning
Citation: Zhang Q, Lyu X-b, Liu C-q, Zhang W-z, Wang Y-g, Deng W-z and Yu X-h (2025) A cross-sectional exploration of the dietary inflammation index association with cardiovascular disease in gout: application of machine learning algorithms. Front. Nutr. 12:1591472. doi: 10.3389/fnut.2025.1591472
Edited by:
Xiao Li, Affiliated Hospital of Shandong University of Traditional Chinese Medicine, ChinaReviewed by:
Jan Kubicek, VSB-Technical University of Ostrava, CzechiaDorota Formanowicz, Poznan University of Medical Sciences, Poland
Laxmikant Borse, Sandip Institute of Pharmaceutical Sciences, India
Zhengqi Liu, Shenzhen University, China
Copyright © 2025 Zhang, Lyu, Liu, Zhang, Wang, Deng and Yu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wei-zhe Deng, ZGVuZy13ekAxNjMuY29t; Xuan-hua Yu, eXV4dWFuaHVhQGZqdGNtLmVkdS5jbg==
 Qiang Zhang
Qiang Zhang Xue-bing Lyu2
Xue-bing Lyu2