Abstract
Introduction:
Obesity is a globally prevalent metabolic disease, and high-calorie diets are major contributors to its development. Probiotic interventions can modulate the gut flora and alleviate systemic and low-grade inflammation, making them potential in-terventions for alleviating metabolic syndrome.
Methods:
This study explored the beneficial effects of the Limosilactobacillus reuteri HM108 strain derived from breast milk on obesity in high-fat diet-induced rats using a multi-gradient concentration in-tervention. Serum biochemical markers and inflammatory mediators were determined using enzyme-linked immunosorbent assay after 6-week intervention. Gut microbiota was assessed using 16S rRNA sequencing. The levels of short-chain fatty acid were detected using gas chromatography–mass spectrometry, fecal metabolites were ana-lysed using untargeted metabolomics, and the liver tissue was subjected to tran-scriptomics analysis.
Results and discussion:
The findings indicated that L. reuteri HM108 mitigated obesity, reduced blood lipids levels and immune factors, as well as altered the gut mi-crobiota composition, including reducing the Firmicutes/Bacteroidetes ratio. L. reuteri HM108 also inhibited the JAK-STAT signalling pathway. L. reuteri HM108 alleviates obesity caused by a high-fat diet in rats, offering a theoretical foun-dation and practical insights for utilizing this strain in obesity management.
1 Introduction
Obesity remains a serious global public health concern, linked to heightened risks of chronic conditions such as type 2 diabetes, hyperlipidaemia, cardiovascular disease, stroke, osteoarthritis, and certain cancers (1). The fundamental cause of obesity is an imbalance between energy intake and expenditure (2). To address obesity and its complications, numerous strategies have been devised, including exercise, surgery, and pharmacological interventions. However, dietary interventions remain the most common approach because of their affordability and low potential for adverse effects (3). Obesity in animal models induced by a chronic high-fat diet (HFD) has been extensively used to mimic human obesity (4). Consequently, various studies have investigated obesity risk factors and interventions, focusing on dietary strategies, identifying effective ingredients for obesity management, and exploring the underlying mechanisms.
Probiotics are increasingly recognised as a therapeutic strategy for obesity management (5), particularly strains of Lactobacillus and Bifidobacterium (6). Obese individuals exhibit distinct gut microbiome structures and functions compared to healthy individuals, with an increased Firmicutes/Bacteroidetes (F/B) ratio (7). This dysbiosis makes microbiome regulation a potential therapeutic target for obesity (8). Probiotic intake helps restore gut microbiota balance, modulate intestinal dysbiosis, and alleviate obesity-related symptoms (9). In addition, the gut microbiota plays a crucial role in nutrient acquisition and energy homeostasis (10). Certain genera within the Firmicutes and Bacteroidetes phyla ferment dietary fibre, producing short-chain fatty acids (SCFAs), such as butyrate, propionate, and acetate (11). These SCFAs contribute to host metabolism, immune modulation, and gut barrier integrity through receptor-mediated pathways (12), thereby exerting beneficial effects in obesity mitigation (13). Probiotics also influence weight regulation by modulating energy balance, enhancing satiety, strengthening the intestinal barrier, and impacting bile acid metabolism (14).
Limosilactobacillus reuteri, a versatile probiotic, colonises various host environments, including the human gut, skin, and breast milk (15). L. reuteri may counteract hyperlipidaemia and obesity by improving cholesterol and bile acid metabolism while reinforcing the intestinal barrier (16). However, probiotic effects vary among strains, even within the same species (17). For instance, L. reuteri ATCC PTA 4659 has been shown to reduce body weight in murine models, whereas strain L6798 induces weight gain (18). Further research is necessary to elucidate the specific mechanisms by which L. reuteri influences weight regulation.
In the current study, L. reuteri HM108, isolated from breast milk, demonstrated strong acid and bile salt tolerance along with hypoglycaemic capacity. This research used an HFD-induced obesity model in male, specific pathogen-free (SPF) Sprague–Dawley (SD) rats. By detecting blood lipid levels, histopathological changes, and inflammatory factors, the effects of this strain on HFD-induced inflammatory responses, intestinal microbiota composition, intestinal metabolites, and mRNA expression in liver tissues of model rats were evaluated. The findings offer fresh perspectives on the role of L. reuteri in obesity management and establish a theoretical foundation for developing functional foods. These insights pave the way for innovative approaches in dietary interventions aimed at improving gut health and metabolic conditions.
2 Materials and methods
2.1 Cultivation of Limosilactobacillus reuteri HM108
Limosilactobacillus reuteri HM108 is derived from healthy breast milk and is presently conserved at the China General Microbiological Culture Collection Center (CGMCC No. 26166). Limosilactobacillus reuteri HM108 was grown in de Man–Rogosa–Sharpe broth (Hopebio Company, Qingdao, China) at 37°C for 12 h and passaged twice to ensure viability and promote optimal growth. After being rinsed twice with sterile phosphate-buffered saline (PBS), the bacteria were adjusted to the target concentration for gavage in rats.
2.2 HFD-induced obesity rat model and probiotic intervention
All animal experiments were conducted in line with the Guidelines for the Care and Use of Laboratory Animals of Beijing Union University and received approval from the Animal Ethics Committee of Beijing Union University (JCZX11-2306-2, Beijing, China). Male SD rats aged 6 weeks of SPF grade were sourced from Vital River Laboratory Animal Technology Co., Ltd. (Beijing, China). A detailed outline of the experimental protocol is presented in Figure 1A.
Figure 1

L. reuteri HM108 ameliorates HFD-induced weight gain and fat accumulation in rats. (A) Experimental design process. (B) Final body weight. (C) Body weight changes during the experiment. (D) Body fat ratio. (E) Weight gain from initial to final period. (F) H&E staining of liver adipose tissue and adipocyte area fraction using Image-Pro Plus 6.0 to estimate adipocyte size percentage (scale bar, 100 μm). Data are presented as mean ± SEM. Line graphs were analysed using two-way ANOVA, and histograms were analysed using unpaired t-tests. #Significant difference between HFD and HFD-M groups; * significant difference between HFD and HFD-H groups, * or #, p < 0.05, ** or ##, p < 0.01, ****p < 0.0001. ND, normal diet; HFD, high-fat diet; HFD-L, high-fat + low-dose (2.5 × 108 CFU/rat); HFD-M, high-fat + medium-dose (5 × 108 CFU/rat); HFD-H, high-fat + high-dose (1.5 × 109 CFU/rat).
During the first week of acclimatisation, the animals were maintained at a constant temperature of 22 ± 2°C and a humidity level of 55 ± 10%. The animals were maintained on a normal diet (ND) under a 12-h light/dark regimen. Following this initial phase, 50 rats weighing 200 ± 20 g were divided randomly into five separate groups (n = 10): (1) ND; (2) HFD; (3) high-fat + low-dose (HFD-L, 2.5 × 108 CFU/rat); (4) high-fat + medium-dose (HFD-M, 5 × 108 CFU/rat); (5) high-fat + high-dose (HFD-H, 1.5 × 109 CFU/rat), and three intervention groups supplemented with different concentrations of L. reuteri HM108 in their diet simultaneously. The HFD consisted of 15% sucrose and 15% lard. Over the course of the 6-w experiment, the ND and HFD groups of rats received a daily dose of 2 mL of pure PBS solution via gavage. The remaining groups were administered 2 mL of freshly prepared PBS containing the specified concentrations of L. reuteri HM108.
Following gavage, faecal samples were collected following a 12-h fasting period. Blood was extracted via cardiac puncture under sodium pentobarbital-induced anaesthesia. After resting for 1 h at 25°C, the serum was separated by centrifugation. The rats were then dissected to collect liver tissue, which was promptly flash-frozen in liquid nitrogen and preserved at −80°C for future analysis.
2.3 Determination of biochemical and inflammatory factors
Enzyme-linked immunosorbent assay (ELISA) kits (Nanjing Jiancheng Institute of Biological Engineering, Nanjing, China) were used to measure various biomarkers, including blood lipid contents—total cholesterol (TC), triglycerides (TG), high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C)—as well as blood glucose, lipopolysaccharide (LPS), tumour necrosis factor-alpha (TNF-α), interleukin-1β (IL-1β), IL-10, and IL-6.
2.4 Histopathological analysis
Liver tissues were randomly selected, fixed in 10% formalin for 24 h, dehydrated, embedded in paraffin, sectioned into 5-μm slices, and stained with haematoxylin and eosin (H&E). Histological observations were performed using a light microscope, and the adipocyte area fraction was quantified (relative quantification) using Image-Pro Plus 6.0 (Media Cybernetics, Rockville, MD, United States).
2.5 16S rRNA measurement of intestinal microorganisms
The fresh, collected faeces were stored at −80°C. Gut microorganisms were measured and analysed by Shanghai Applied Protein Technology Co., Ltd. Briefly, the 16S rRNA V3–V4 region was amplified using primers 341F (CCTAYGGGRBGCASCAG) and 806R (TAATCTATGGGGNNCATAAGG). The Illumina Novaseq 6000 was used to conduct pyrosequencing of the polymerase chain reaction, followed by a rigorous quality assessment of the raw data, and merged paired-end reads.
The QIIME 2 package in R was used to select representative reads of each Amplicon Sequence Variant and perform alpha and beta diversity index analysis and visualisation. Finally, linear discriminant analysis effect size (LEfSe) was used to assess the importance of variations in species abundance and identify the different microbial biomarkers. The Kyoto Encyclopaedia of Genes and Genomes (KEGG) was used to predict the microflora metabolic function.
2.6 Gas chromatography-mass spectrometry analysis of SCFAs
The freshly-collected faeces were stored at −80°C, and SCFAs were measured and analysed by Shanghai Applied Protein Technology Co., Ltd. Briefly, the faeces were re-suspended in 50 μL of 20% phosphoric acid solution, with 4-methylpentanoic acid added as an internal standard. The mixtures were thoroughly agitated and then centrifuged, and the supernatant was carefully transferred into the gas chromatography-mass spectrometry (GC-MS) equipment (GC 7890 B, 5977 B, Agilent Technologies Co. Ltd., CA, United States) to determine SCFAs concentrations.
The samples were separated using a GC system equipped with an Agilent DB-FFAP capillary column (30 m × 250 μm, 0.25 μm). A 1-μL injection was performed using a 10:1 split ratio, with the column temperature initially set at 90°C. The temperature was increased to 160°C at a rate of 10°C/min, then to 240°C at 40°C/min, and held steady for 5 min. Helium was used as the carrier gas at a flow rate of 1.0 mL/min. MS followed, with inlet, ion source, and transmission line temperatures set at 250°C, 230°C, and 250°C, respectively, and a quadrupole temperature of 150°C. The electron ionisation source operated at 70 eV electron energy.
2.7 Untargeted faecal metabolism
The freshly collected faeces were stored at −80°C, and untargeted metabolomics were measured and analysed by Shanghai Applied Protein Technology Co., Ltd. Briefly, faecal samples were extracted with a cold methanol-acetonitrile-water (2:2:1, v/v), followed by vortexing and sonication. Finally, the supernatant was vacuum-dried, reconstituted in acetonitrile-water (1:1 v/v), centrifuged, and analysed using ultra-high-performance liquid chromatography coupled with a quadrupole time-of-flight system (UHPLC-QTOF) (Agilent 1290 Infinity/AB Sciex Triple TOF 6600).
The mobile phase consisted of two components: A (25 mM ammonium acetate/hydroxide in water) and B (acetonitrile). The gradient ran from 95% B (0.5 min) to 65% (6.5 min), then to 40% (1 min), held (1 min), returned to 95% (0.1 min), and re-equilibrated (3 min). Electrospray ionisation settings: ion source gas (Gas), curtain gas = 30, ion spray voltage = ±5,500 V. Primary m/z detection: 60–1,000 Da; secondary: 25–1,000 Da; collision energy = 35 ± 15 eV.
After sum-normalisation, the datasets were analysed using the ropls package in R and assessed using principal component analysis (PCA). The t-test was used to identify statistically significant differences between two independent sample groups. Significant differential metabolites were selected for KEGG pathway enrichment analysis.1
2.8 Liver tissue transcriptome analysis
The collected liver tissue samples were maintained at −80°C, and transcriptome was analysed by Shanghai Applied Protein Technology Co., Ltd. Briefly, liver RNA was quantified (A260/A280) using a Nanodrop ND-2000 system (Thermo Fisher Scientific, MA, United States). Sequencing was conducted using an Illumina Novaseq 6000. Using the HISAT2 package (version 2.2.1) in orientation mode, clean reads were individually aligned to the reference genome to acquire the mapped reads. The DESeq2 package (version 1.47.1) was analysed for differential expression genes (|log2 FC| >1 and padj <0.05). Finally, Gene Set Enrichment Analysis (GSEA) was conducted by calculating the enrichment scores, assessing their significance levels, and correcting for multiple hypothesis testing.
2.9 Statistical analysis
GraphPad Prism 10.1.2 was used for statistical analyses. A two-way analysis of variance followed by Dunnett’s test was used to analyse body weight. One-way analysis of variance (ANOVA) followed by Tukey’s test was used to analyse the differences in blood glucose and lipid content, inflammatory factors, proportion of SCFAs, and F/B ratio. Correlations between gut microbes and body fat ratio, blood lipid content, inflammatory factors, and differential metabolites were evaluated using Spearman’s correlation, with significance at p < 0.05.
3 Results
3.1 Limosilactobacillus reuteri HM108 mitigates weight gain in HFD rats
HFD feeding for 6 w substantially increased the total intake, total calories, weight gain, total body weight, body fat weight, body fat ratio, and feed intake of the rats (p < 0.05). In intervention groups with different doses of probiotics, there was no significant effect on the feed intake of rats, but the three parameters of body weight (p < 0.01), body fat weight, and body fat ratio (p < 0.05) showed dose-dependent improvements, indicating that supplementation with L. reuteri HM108 can significantly reduce high-fat diet induced weight gain and decrease body fat weight and body fat ratio (Figures 1B–E). Other indices were reduced to varying degrees, although not statistically (Supplementary Figures 1A–E).
Prolonged exposure to an HFD may result in hepatic lipid accumulation and a deterioration of liver function. Obesity elevates the risk of non-alcoholic fatty liver disease and associated conditions, which are characterised by enlarged lipid droplets and decreased interstitial spaces, which compress nearby cells (19). Analysis of the liver histopathological morphology using H&E staining indicates that adipose tissue stores excess energy by increasing the size and number of adipocytes (20). In the current study, L. reuteri HM108 inhibited HFD-driven adipocyte growth (Figure 1F).
3.2 Limosilactobacillus reuteri HM108 attenuates serum lipid content in HFD-fed rats
Changes in lipid content in rats are shown in Figures 2A–D. After 6 w of intervention, glucose content in the HFD group was similar to that in the other groups (Supplementary Figure 1F). However, TC, TG, and LDL-C levels were higher, and HDL-C levels were lower than those in the control and ND groups (p < 0.05). TG and TC levels were significantly reduced in the intervention group, indicating that L. reuteri HM108 can improve blood lipid levels.
Figure 2
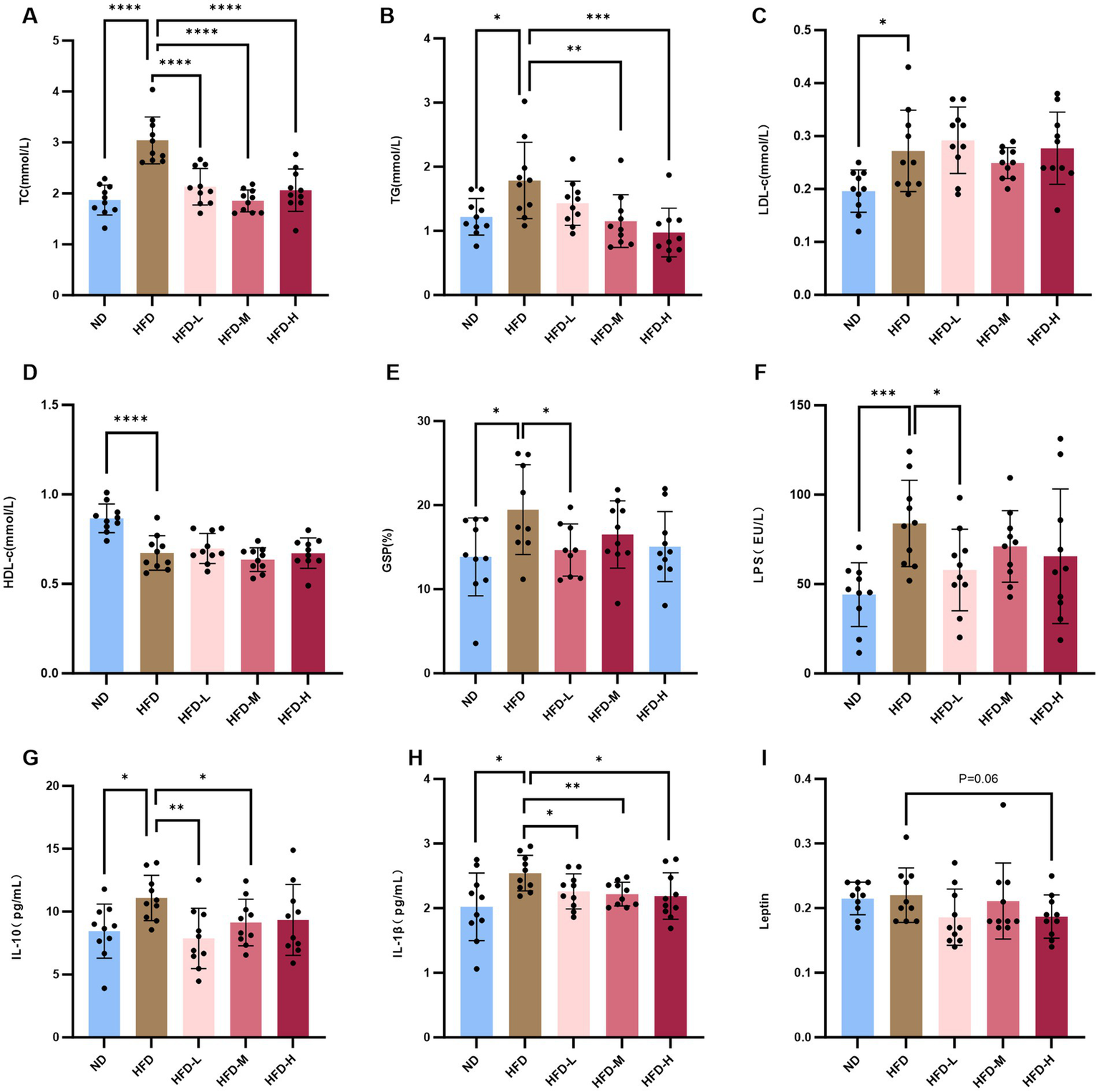
L. reuteri HM108 reduces serum lipid levels and inflammatory factors in HFD-fed rats. (A) Serum total cholesterol (TC) concentration. (B) Serum triglyceride (TG) concentration. (C) Serum low-density lipoprotein cholesterol (LDL-C) concentration. (D) Serum high-density lipoprotein cholesterol (HDL-C) concentration. (E) Serum glycated serum protein (GSP) concentration. (F) Serum lipopolysaccharide (LPS) concentration. (G) Serum interleukin-10 (IL-10) concentration. (H) Serum interleukin-1β (IL-1β) concentration. (I) Serum leptin concentration. Data are presented as mean ± SEM and analysed using unpaired t-tests. *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001. ND, normal diet; HFD, high-fat diet; HFD-L, high-fat + low-dose (2.5 × 108 CFU/rat); HFD-M, high-fat + medium-dose (5 × 108 CFU/rat); HFD-H, high-fat + high-dose (1.5 × 109 CFU/rat).
GSP reflects mean glycemia over the preceding 2–3 w and serves as a reliable indicator for short-term blood glucose monitoring. The GSP content in HFD rats was elevated compared to both the ND and the intervention groups with L. reuteri HM108, particularly the HFD-L group (Figure 2E).
These results indicate that L. reuteri HM108 alleviats obesity by reducing lipid and decreasing GSP contents in the blood of rats fed an HFD.
3.3 Limosilactobacillus reuteri HM108 reduces inflammatory factors in HFD-fed rats
HFD induces inflammation and dysregulation of adipocytokines as adipose tissue releases adipokines and biologically active molecules such as leptin, which affect systemic homeostasis. Most adipokines promote inflammation (21). Serum leptin, LPS, IL-6, IL-10, IL-1β, and TNF-α were quantified using ELISA (Supplementary Figures 1G,H). The findings indicated no marked alteration in TNF-α serum levels, whereas LPS, IL-6, IL-10, and IL-1β contents increased substantially in response to HFD. With L. reuteri HM108 treatment, the contents of LPS, IL-10, and IL-1β in HFD-induced rats were markedly reduced and restored to the contents of the ND group (Figures 2F–H). Leptin, an inflammatory factor that regulates body intake and energy expenditure, tended to decline (p = 0.06; Figure 2I).
The results suggest that L. reuteri HM108 can reduce chronic inflammation linked to obesity by decreasing serum inflammatory factor contents in a dose-dependent manner.
3.4 Effect of Limosilactobacillus reuteri HM108 on gut microbiota
To determine whether L. reuteri HM108 affected the gut microbiota and reduced obesity, 16S rRNA sequencing was used to investigate gut microbiota composition. The α diversity ACE index indicated that L. reuteri HM108 affected the diversity of intestinal microflora (Figure 3A), whereas the PCA of β diversity demonstrated no marked variation in gut microbiota across all HFD groups (Figure 3B). The relative abundance of the predominant intestinal flora was evaluated at the phylum level. All faecal samples were found to have a similar community structure; Firmicutes and Bacteroidetes were dominant, representing over 90% of the relative abundance. The F/B ratio rose to 3.46 ± 1.23 in obese rats on the HFD versus the ND group but dropped to 2.25 ± 0.94 in the intervention group with L. reuteri HM108 (Figures 3C,D).
Figure 3

Effect of L. reuteri HM108 on gut microbiota in rats analysed using 16S rRNA sequencing. (A) ACE index in α diversity analysis of gut microbiota. (B) UniFrac PCoA analysis in β diversity analysis of gut microbiota based on ASV data from five groups. (C) Relative abundance of the intestinal flora at the phylum level. (D) Firmicutes/Bacteroidetes (F/B) ratio in the five groups. (E) LEfSe evolutionary branching maps of enteric flora in the five groups. (F) Relative abundance of Firmicutes, Bacteroidetes, Desulfobacterota and Actinobacteriota in LEfSe analyses. *p < 0.05, **p < 0.01, ***p < 0.001. ND, normal diet; HFD, high-fat diet; HFD-L, high-fat + low-dose (2.5 × 108 CFU/rat); HFD-M, high-fat + medium-dose (5 × 108 CFU/rat); HFD-H, high-fat + high-dose (1.5 × 109 CFU/rat).
LEfSe analysis was used to identify the microbiota altered by L. reuteri HM108 in HFD rats (Figures 3E,F). Limosilactobacillus reuteri HM108 increased the abundance of Bacteroides, Prevotellaceae, Eubacterium, Anaerovoracaceae, Holdemanella, Enterococcaceae, Jeotgalicoccus, and Ruminococcaceae while reducing the abundance of harmful bacteria such as Lachnospiraceae, Clostridia_UCG-014, Butyricimonas, Staphylococcaceae, and Rothia. The abundance of Lactobacillus showed no marked variation across the groups.
Functional changes in the gut microbiota with L. reuteri HM108 intervention were predicted using the KEGG database (Supplementary Figure 2I). Compared to the ND group, HFD consumption enriched the metabolism-related KEGG pathways, including the pentose phosphate pathway, ABC transporters, pyruvate metabolism, TCA cycle, PPAR signalling pathway, LPS biosynthesis, carbohydrate digestion, and absorption, all of which were reversed by L. reuteri HM108. Moreover, L. reuteri HM108 substantially enhanced the synthesis of several small molecules and metabolic pathways, including amino acid and polyamine metabolism; these small-molecule metabolites act as growth signals and nutrients for microbes and the host gut epithelium (22).
These results suggest that L. reuteri HM108 can alter the gut flora composition of HFD rats to promote the expansion of bacteria that are beneficial in the context of obesity.
3.5 Effects of Limosilactobacillus reuteri HM108 on SCFAs
SCFAs are key regulators of lipid metabolism. Propionic, acetic, and butyric acids account for >95% of intestinal SCFAs (23). GC-MS analysis of rat faeces indicated that the HFD group reduced most SCFAs (p < 0.05), except for butyric acid (p = 0.15), compared to the ND group, suggesting that HFD inhibited SCFAs production. Limosilactobacillus reuteri HM108 tended to increase butyric acid contents in rat faeces (p = 0.097) (Supplementary Figures 2A–H). These results suggest that HFD inhibits SCFAs production, which can be reversed by L. reuteri HM108.
3.6 Effects of Limosilactobacillus reuteri HM108 on faecal metabolites
To assess the metabolic alterations caused by L. reuteri HM108 in HFD-induced rats, faecal samples were subjected to untargeted metabolomic profiling using UHPLC-Q-TOF MS. After combining the positive and negative ions, PCA indicated that the HFD diet altered metabolites and was screened for differential metabolites (|log2FC| >1 and p < 0.05) (Figure 4A). Compared to the HFD, 61 differential metabolites were present in the HFD-L group, 27 in the HFD-M group, and 73 in the HFD-H group. Lipids and lipid molecules with substantial changes in abundance across the five groups, including sn-glycero-3-phosphocholine, butyric acid, hetisine, and linoleic acid, were analysed using heat maps (Figure 4B).
Figure 4
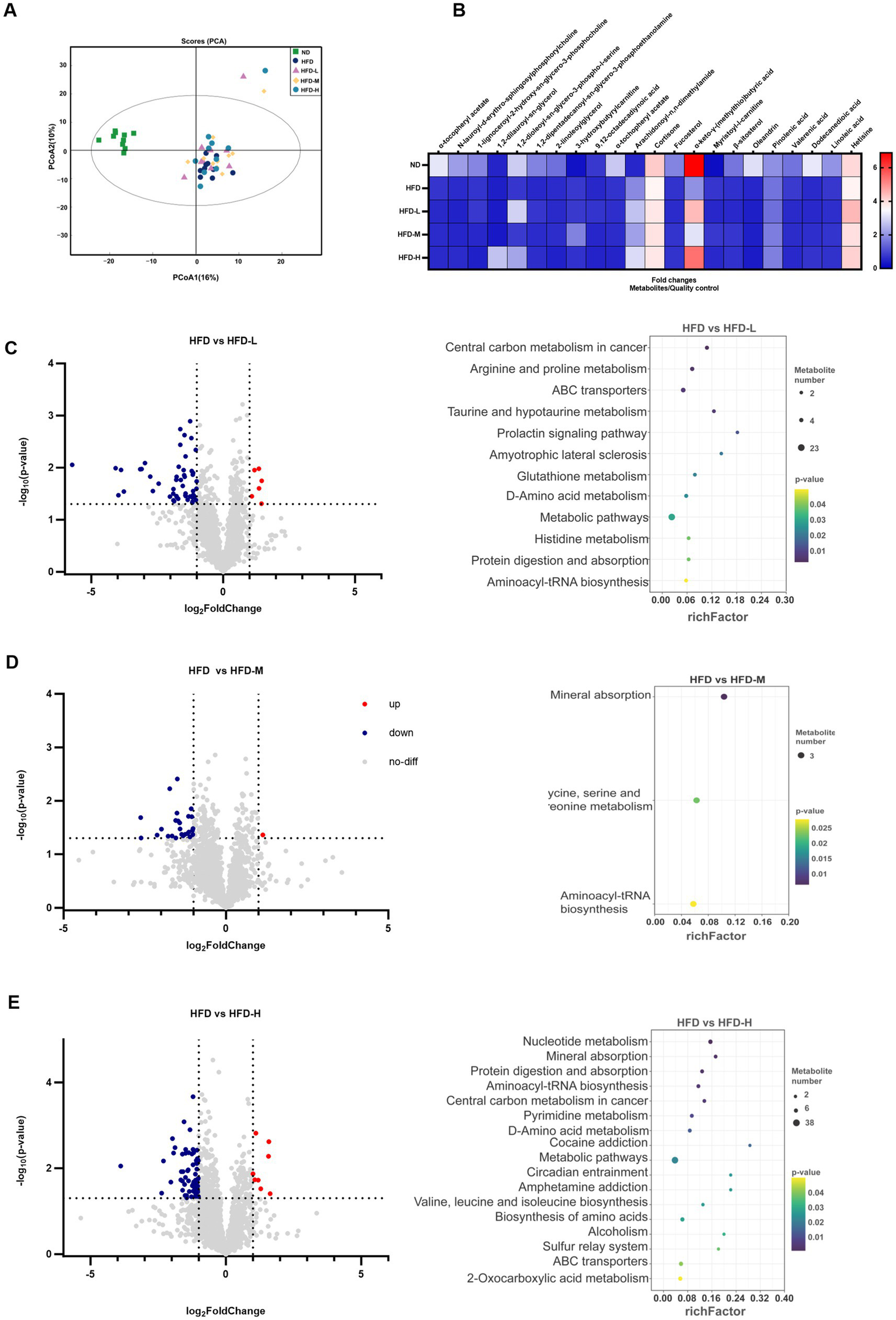
Effects of L. reuteri HM108 on faecal metabolites in HFD-fed rats. (A) Principal component analysis (PCA). (B) Heatmap of significant changes in faecal metabolites. (C) Differential metabolites and KEGG pathway prediction for HFD and HFD-L groups. (D) Differential metabolites and KEGG pathway prediction for HFD and HFD-M groups. (E) Differential metabolites and KEGG pathway prediction for HFD and HFD-H groups. ND, normal diet; HFD, high-fat diet; HFD-L, high-fat + low-dose (2.5 × 108 CFU/rat); HFD-M, high-fat + medium-dose (5 × 108 CFU/rat); HFD-H, high-fat + high-dose (1.5 × 109 CFU/rat).
PICRUSt2 was used to forecast the gut microbiota’s KEGG function before and after the L. reuteri HM108 intervention (Figures 4C–E). Relative to the HFD group, the HFD-L group exhibited 11 pathways associated with obesity: arginine and proline metabolism, ABC transporters, taurine and hypotaurine metabolism, metabolic pathways, glutathione metabolism, d-amino acid metabolism, histidine metabolism, protein digestion and absorption; and aminoacyl-tRNA biosynthesis. The HFD-M group also changed mineral absorption and glycine, serine, and threonine metabolism. The HFD-H group increased nucleotide and pyrimidine metabolism and valine, leucine, and isoleucine biosynthesis.
These results suggest that L. reuteri HM108 can alter the metabolites in faeces and affect pathways associated with obesity, such as energy metabolism, protein synthesis, and amino acids, thereby alleviating obesity in rats.
3.7 Effects of Limosilactobacillus reuteri HM108 on the liver transcriptome
After identifying the obesity-reducing phenotype of L. reuteri HM108, transcriptome analysis was conducted on liver tissues from HFD and HFD-H rats to elucidate the association between changes in gene expression related to liver tissue and obesity resulting from the HFD. A total of 81 differentially expressed genes were identified, with 33 upregulated and 48 downregulated genes. The upregulated genes included Map3k5, Reg3b, G6pc, Ndst2, Flrt1, Reg3g, Plcd3, and Ndufa1; downregulated genes included Rgs3, Dact1, Hmgcr, Socs3, Cyp7a1, Foxo3, Lpin1, Cish, Gck, and Insig1 (Figure 5A). KEGG pathway enrichment analysis results are shown in Supplementary Table S1.
Figure 5
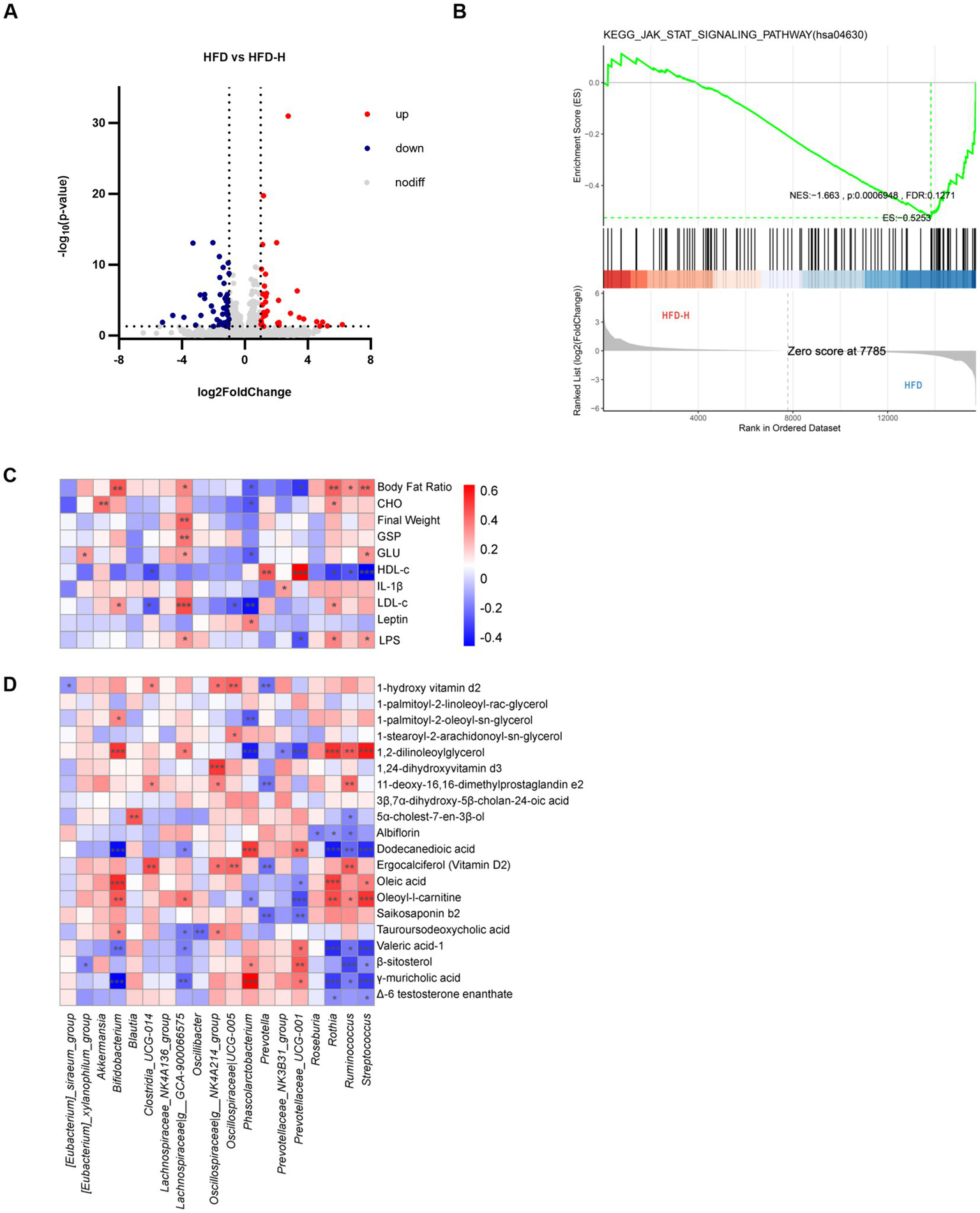
Effects of L. reuteri HM108 on liver transcription in HFD-fed rats and correlation analysis of gut microbiota with phenotype and metabolome. (A) Volcano plot of differentially expressed genes in HFD and HFD-H groups. (B) GSEA analysis between HFD and HFD-H groups. (C) Spearman correlation analysis of phenotypes with gut microbiota. (D) Spearman correlation analysis between gut microbiota and differential metabolites. *p < 0.05, **p < 0.01, and ***p < 0.001. HFD, high-fat diet; HFD-H, high-fat + high-dose (1.5 × 109 CFU/rat).
GSEA was examined using actual overall trends rather than specific differential gene thresholds. In this research, compared to the HFD group, the expression of JAK-STAT signalling pathway in liver tissue decreased in the intervention group (Figure 5B). The main genes involved in this pathway were Myc (ENSRNOG00000004500), Cish (ENSRNOG00000029543), and Osmr (ENSRNOG00000033192).
In this study, compared to the HFD group, the expression of JAK-STAT signalling pathway in liver tissue decreased in the intervention group of Lactobacillus rhamnosus HM108.
The results indicate that intervention with L. reuteri HM108 can effectively inhibit the JAK-STAT signalling pathway, with the important downregulation of Myc, Cish, and Osmr genes.
3.8 Associations between phenotype, microbiome, and metabolome
Obesity and metabolic disorders induced by an HFD largely stem from a gut microbiota imbalance. To study the effect of L. reuteri HM108 on obesity via the gut microbiota, 20 gut microbiota with marked changes in genus-level abundance before and after the intervention were selected for Spearman’s correlation analysis (Figure 5C). Although Lachnospiraceae are SCFAs producers (24), they are more abundant in obese and diabetic mice and humans (25). The correlation analysis indicated that Lachnospiraceae_GCA-900066575 exhibited a positive association with several markers, including body fat ratio, body weight, GSP, glucose, low-density lipoprotein, and LPS. This suggests that it plays a key role in the development of obesity.
Limosilactobacillus reuteri HM108 affected the composition of gut microbes and metabolite profiles. Therefore, Spearman’s correlation analysis was conducted for these 20 gut microorganisms and 20 significantly different metabolites associated with obesity (Figure 5D). The important intestinal bacterial genus, Prevotellaceae_UCG-001 was positively correlated with valeric acid-1, β-sitosterol, and γ-muricholic acid and negatively correlated with oleic acid, oleoyl-l-carnitine, and saikosaponin b2. Prevotella was correlated with 1-hydroxyvitamin D, 11-deoxy-16,16-dimethylprostaglandin e2, ergocalciferol (vitamin D2), and saikosaponin b2.
4 Discussion
Globally, obesity presents a major health crisis and is increasingly regarded as a non-communicable pandemic (26). Obesity exacerbates cardiovascular disease progression and mortality beyond its association with risk factors such as dyslipidaemia, type 2 diabetes, and hypertension (27). However, behavioural and pharmacological therapies yield only 3–10% body weight loss, and maintaining this reduction is challenging (28). Numerous studies have shown that probiotic strains, either alone or in combination with other compounds, can exert anti-obesity effects by regulating gut microbiota, enhancing insulin sensitivity, and modulating appetite (29).
A calorie-dense, fatty, and sugary diet with low fibre content is a primary driver of weight gain. The rodent obesity model closely reflects human physiology and weight regulation (30). In the current study, a high-sugar and HFD containing 15% sucrose and 15% lard was used to induce obesity in male SPF rats and investigate the ameliorative effects of different doses of L. reuteri HM108 via gavage. The results showed that L. reuteri HM108 reduced body weight gain, inhibited adipocyte enlargement, and regulated blood lipid content in a dose-dependent manner.
An HFD induces chronic inflammation in obese individuals (31). Obesity disrupts gut microbiota balance, increases intestinal permeability, and facilitates the translocation of metabolic waste and microbial toxins into the bloodstream. This process stimulates cytokine release, contributing to chronic inflammation and insulin resistance (32). In the current study, HFD-induced obesity in rats was accompanied by the upregulation of inflammatory factors, whereas L. reuteri HM108 substantially reduced serum LPS and IL-1β levels.
HFD-induced obesity and associated metabolic disorders are characterised by gut flora dysbiosis, which affects nutrition, digestion, and energy metabolism (33). The primary gastrointestinal bacterial phyla were Firmicutes and Bacteroidetes; the F/B ratio serves as a key biomarker of malnutrition and metabolism-related disorders. In the current study, HFD-induced gut flora imbalance was manifested by an increase in Firmicutes, a decrease in Bacteroidetes, and a marked elevation in the F/B ratio, consistent with previous findings (34). Limosilactobacillus reuteri HM108 increased the prevalence of Bacteroidetes while reducing Firmicutes, leading to a marked decrease in the F/B ratio in HFD-fed rats. This shift substantially altered gut microbiota composition. Limosilactobacillus reuteri HM108 also increased the abundance of Bacteroides, Prevotellaceae, Eubacterium, Anaerovoracaceae, g-Holdemanella, Enterococcaceae, Jeotgalicoccus, and Ruminococcaceae. Previous studies have shown that Bacteroides (35), Lachnospiraceae (36), Prevotellaceae (37), and Ruminiclostridium (38) are negatively correlated with body weight, lipid metabolism, obesity, and related diseases. Holdemanella biformis has been shown to improve glucose tolerance in obese mice (39). Limosilactobacillus reuteri HM108 reduced the abundance of Lachnospiraceae, Clostridia_UCG-014, Butyricimonas, Staphylococcaceae, Rothia, and other harmful bacteria. Clostridia_UCG-014 has been positively correlated with blood glucose concentration and is more abundant in diabetic and obese mice (40). The primary functions of these gut microbiota include regulating polysaccharide biodegradation, SCFAs production, specific LPS enrichment, and the synthesis of vitamins and essential amino acids. These processes influence energy uptake, hunger signals, fat accumulation, systemic inflammation, and circadian rhythms, thereby alleviating obesity (39). Therefore, L. reuteri HM108 positively impacts diet-induced gut microbiota dysbiosis.
Alterations in gut microbiota composition influence SCFAs levels, as most SCFAs originate from the microbial fermentation of indigestible dietary components (40). Bacteroides, enhanced by L. reuteri HM108, reduce inflammation through propionate production (41). Lactobacillaceae, Ruminococcaceae, and Lachnospiraceae hydrolyse polysaccharides to produce butyrate and other SCFAs (42). Jeotgalicoccus is positively correlated with acetate, propionate, and butyrate concentrations (43). Eubacterium not only produces SCFAs but also regulates bile acid metabolism to alleviate obesity (44). These SCFAs play key roles in maintaining intestinal integrity, cellular function, and immune responses, potentially influencing the development of cardiovascular and metabolic diseases associated with high-fat or high-sugar intake (45).
Gut microbiota produces SCFAs and other metabolites that act as messengers between microbes and their hosts. In this study, L. reuteri HM108 intervention substantially ameliorated HFD-induced lipid metabolism disorders in obese rats. The following metabolites were involved: phosphatidylcholine and phosphatidylethanolamine, which regulate hepatic insulin signalling (46); linoleic acid, which influences adipocyte and skeletal muscle metabolism (47); 7α-hydroxy-4-cholesten-3-one, lithocholic acid, and taurine, which are intermediates in cholesterol metabolism via bile acids (48); and other substances that may improve HDL cholesterol levels, such as 1-hydroxy vitamin D2, L-ascorbic acid, cedrol (49), and betaine. Additionally, L. reuteri HM108 modulated the amino acid metabolic pathways that influence fatty acid synthesis in vivo more efficiently than glucose (50).
Probiotics influence signalling pathways that regulate gene expression, impacting energy metabolism and inflammation. Transcriptome analysis indicated that L. reuteri HM108 strongly inhibited the JAK-STAT signalling pathway, which regulates multiple downstream biological processes, including adipogenesis, inflammation, and apoptosis (51). Aberrant activation of this pathway leads to dysregulation of hepatic gluconeogenesis, hepatic steatosis, and insulin resistance (52, 53). Mice fed an HFD exhibit markedly increased fat deposition when deficient in the socs3 gene, whereas JAK pathway inhibition attenuates HFD-induced obesity (54). Inhibiting the JAK/STAT signalling pathway not only alleviates HFD-induced obesity but also improves the chronic inflammatory state of adipose tissue in obese individuals. Cytokines released during obesity can activate JAK 1/2, which in turn phosphorylate STAT proteins, a pathway persistently activated in various tumour cells (55). Blocking JAK-STAT signalling substantially reduces intestinal inflammation and improves barrier function in HFD-fed mice (53).
Limosilactobacillus reuteri HM108, especially at high doses, inhibited weight gain and reduced adipose tissue enlargement in HFD-fed rats. The findings indicate that L. reuteri HM108 dose-dependently reduces HFD-induced obesity-related phenotypes in rats. However, additional clinical studies are required to validate the efficacy of L. reuteri against HFD-induced obesity in humans, alongside a more comprehensive investigation of its underlying molecular mechanisms.
5 Conclusion
Limosilactobacillus reuteri HM108 dose-dependently attenuated body weight and adipocyte hypertrophy, reduced inflammatory factors and blood lipid content in HFD-fed rats, and alleviated HFD-induced obesity through modifications in the intestinal flora, including lowering the F/B ratio and altering metabolite profiles. The JAK-STAT signalling pathway was inhibited. The results offer a theoretical foundation and practical insights for utilising L. reuteri HM108 in interventions for HFD-induced obesity.
Statements
Data availability statement
The original contributions presented in the study are publicly available. This data can be found in the Mendeley Data repository: 10.17632/3jfj6d4t2g.1.
Ethics statement
The animal study was approved by Animal Ethics Committee of Beijing Union University. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
MT: Methodology, Investigation, Conceptualization, Writing – review & editing. XL: Writing – review & editing, Investigation, Supervision, Conceptualization, Methodology. JR: Writing – original draft, Data curation. CY: Writing – original draft, Data curation. LL: Data curation, Writing – original draft. XL: Writing – original draft, Visualization. XY: Writing – original draft, Visualization. JZ: Supervision, Writing – review & editing. BL: Writing – review & editing, Supervision. WQ: Supervision, Writing – review & editing. LC: Conceptualization, Writing – review & editing, Methodology, Supervision, Funding acquisition.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was funded by the National Natural Science Foundation of China (Grant No. 32072191), Special Funds for Guiding Local Scientific and Technological Development by the Central Government (Grant No. GuikeZY22096025), Beijing Science and Technology Plan (Grant No. Z221100006422012), Beijing Capital Agribusiness & Foods Group Science and Technology Project.
Acknowledgments
The authors sincerely thank Shanghai Applied Protein Technology Co., Ltd. for their expert support and state-of-the-art resources, which were instrumental in generating the essential data for this study.
Conflict of interest
MT, XL, JR, CY, LL, XL, XY, JZ, BL, WQ, and LC were employed by Beijing Sanyuan Foods Co., Ltd.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2025.1597334/full#supplementary-material
Footnotes
References
1.
ZhangNWangQLinFZhengBHuangYYangYet al. Neoagarotetraose alleviates high fat diet induced obesity via white adipocytes browning and regulation of gut microbiota. Carbohydr Polym. (2022) 296:119903. doi: 10.1016/j.carbpol.2022.119903
2.
ZhangHChenSYangLZhangSQinLJiangH. Distinct gut microbiota and arachidonic acid metabolism in obesity-prone and obesity-resistant mice with a high-fat diet. Nutrients. (2024) 16:1579. doi: 10.3390/nu16111579
3.
KeWFlayKJHuangXHuXChenFLiCet al. Polysaccharides from Platycodon grandiflorus attenuates high-fat diet induced obesity in mice through targeting gut microbiota. Biomed Pharmacother. (2023) 166:115318. doi: 10.1016/j.biopha.2023.115318
4.
QinQShouJLiMGuMMengZXuPet al. Stk24 protects against obesity-associated metabolic disorders by disrupting the NLRP3 inflammasome. Cell Rep. (2021) 35:109161. doi: 10.1016/j.celrep.2021.109161
5.
KoberASahaSAyyashMNamaiFNishiyamaKYodaKet al. Insights into the anti-adipogenic and anti-inflammatory potentialities of probiotics against obesity. Nutrients. (2024) 16:1373. doi: 10.3390/nu16091373
6.
van ZylWFDeaneSMDicksLMT. Molecular insights into probiotic mechanisms of action employed against intestinal pathogenic bacteria. Gut Microbes. (2020) 12:1831339. doi: 10.1080/19490976.2020.1831339
7.
Chavoya-GuardadoMAVasquez-GaribayEMRuiz-QuezadaSLRamírez-CorderoMILarrosa-HaroACastro-AlbarranJ. Firmicutes, Bacteroidetes and Actinobacteria in human milk and maternal adiposity. Nutrients. (2022) 14:2887. doi: 10.3390/nu14142887
8.
GreenMAroraKPrakashS. Microbial medicine: prebiotic and probiotic functional foods to target obesity and metabolic syndrome. Int J Mol Sci. (2020) 21:2890. doi: 10.3390/ijms21082890
9.
CaiYLiuPZhouXYuanJChenQ. Probiotics therapy show significant improvement in obesity and neurobehavioral disorders symptoms. Front Cell Infect Microbiol. (2023) 13:1178399. doi: 10.3389/fcimb.2023.1178399
10.
WeiBZhangBDuAQZhouZYLuDZZhuZHet al. Saccharina japonica fucan suppresses high fat diet-induced obesity and enriches fucoidan-degrading gut bacteria. Carbohydr Polym. (2022) 290:119411. doi: 10.1016/j.carbpol.2022.119411
11.
CaniPD. Targeting gut microbiota with a complex mix of dietary fibers improves metabolic diseases. Kidney Int. (2019) 95:14–6. doi: 10.1016/j.kint.2018.11.012
12.
HaysKEPfaffingerJMRyznarR. The interplay between gut microbiota, short-chain fatty acids, and implications for host health and disease. Gut Microbes. (2024) 16:2393270. doi: 10.1080/19490976.2024.2393270
13.
LiuYGaoYMaFSunMMuGTuoY. The ameliorative effect of Lactobacillus plantarum Y44 oral administration on inflammation and lipid metabolism in obese mice fed with a high fat diet. Food Funct. (2020) 11:5024–39. doi: 10.1039/d0fo00439a
14.
CerdóTGarcía-SantosJABermúdezMGCampoyC. The role of probiotics and prebiotics in the prevention and treatment of obesity. Nutrients. (2019) 11:635. doi: 10.3390/nu11030635
15.
MuQTavellaVJLuoXM. Role of Lactobacillus reuteri in human health and diseases. Front Microbiol. (2018) 9. doi: 10.3389/fmicb.2018.00757
16.
LiCSuZChenZCaoJLiuXXuF. Lactobacillus reuteri strain 8008 attenuated the aggravation of depressive-like behavior induced by CUMS in high-fat diet-fed mice through regulating the gut microbiota. Front Pharmacol. (2023) 14:1149185. doi: 10.3389/fphar.2023.1149185
17.
YangBZhengFStantonCRossRPZhaoJZhangHet al. Lactobacillus reuteri Fynlj109l1 attenuating metabolic syndrome in mice via gut microbiota modulation and alleviating inflammation. Foods. (2021) 10:2081. doi: 10.3390/foods10092081
18.
FåkFBäckhedF. Lactobacillus reuteri prevents diet-induced obesity, but not atherosclerosis, in a strain dependent fashion in Apoe−/− mice. PLoS One. (2012) 7:e46837. doi: 10.1371/journal.pone.0046837
19.
HongYSongGFengXNiuJWangLYangCet al. The probiotic Kluyveromyces lactis JSA 18 alleviates obesity and hyperlipidemia in high-fat diet C57BL/6J mice. Foods. (2024) 13:1124. doi: 10.3390/foods13071124
20.
JiTFangBWuFLiuYChengLLiYet al. Diet change improves obesity and lipid deposition in high-fat diet-induced mice. Nutrients. (2023) 15:4978. doi: 10.3390/nu15234978
21.
OhashiKShibataRMuroharaTOuchiN. Role of anti-inflammatory adipokines in obesity-related diseases. Trends Endocrinol Metab. (2014) 25:348–55. doi: 10.1016/j.tem.2014.03.009
22.
WangPGaoJKeWWangJLiDLiuRet al. Resveratrol reduces obesity in high-fat diet-fed mice via modulating the composition and metabolic function of the gut microbiota. Free Radic Biol Med. (2020) 156:83–98. doi: 10.1016/j.freeradbiomed.2020.04.013
23.
CongJZhouPZhangR. Intestinal microbiota-derived short chain fatty acids in host health and disease. Nutrients. (2022) 14:1977. doi: 10.3390/nu14091977
24.
VaccaMCelanoGCalabreseFMPortincasaPGobbettiMDe AngelisM. The controversial role of human gut Lachnospiraceae. Microorganisms. (2020) 8:573. doi: 10.3390/microorganisms8040573
25.
TakeuchiTKameyamaKMiyauchiENakanishiYKanayaTFujiiTet al. Fatty acid overproduction by gut commensal microbiota exacerbates obesity. Cell Metab. (2023) 35:361–75.e9. doi: 10.1016/j.cmet.2022.12.013
26.
BlüherM. Obesity: global epidemiology and pathogenesis. Nat Rev Endocrinol. (2019) 15:288–98. doi: 10.1038/s41574-019-0176-8
27.
PichéMETchernofADesprésJP. Obesity phenotypes, diabetes, and cardiovascular diseases. Circ Res. (2020) 126:1477–500. doi: 10.1161/circresaha.120.316101
28.
BlüherM. Metabolically healthy obesity. Endocr Rev. (2020) 41:3. doi: 10.1210/endrev/bnaa004
29.
AbenavoliLScarpelliniEColicaCBoccutoLSalehiBSharifi-RadJet al. Gut microbiota and obesity: a role for probiotics. Nutrients. (2019) 11:2690. doi: 10.3390/nu11112690
30.
SpeakmanJR. Use of high-fat diets to study rodent obesity as a model of human obesity. Int J Obes. (2019) 43:1491–2. doi: 10.1038/s41366-019-0363-7
31.
OlofssonLEBäckhedF. The metabolic role and therapeutic potential of the microbiome. Endocr Rev. (2022) 43:907–26. doi: 10.1210/endrev/bnac004
32.
NakanishiTFukuiHWangXNishiumiSYokotaHMakizakiYet al. Effect of a high-fat diet on the small-intestinal environment and mucosal integrity in the gut-liver axis. Cells. (2021) 10:3168. doi: 10.3390/cells10113168
33.
AmabebeERobertFOAgbalalahTOrubuESF. Microbial dysbiosis-induced obesity: role of gut microbiota in homoeostasis of energy metabolism. Br J Nutr. (2020) 123:1127–37. doi: 10.1017/s0007114520000380
34.
StojanovSBerlecAŠtrukeljB. The influence of probiotics on the Firmicutes/Bacteroidetes ratio in the treatment of obesity and inflammatory bowel disease. Microorganisms. (2020) 8:1715. doi: 10.3390/microorganisms8111715
35.
LiHWangXKTangMLeiLLiJRSunHet al. Bacteroides thetaiotaomicron ameliorates mouse hepatic steatosis through regulating gut microbial composition, gut-liver folate and unsaturated fatty acids metabolism. Gut Microbes. (2024) 16:2304159. doi: 10.1080/19490976.2024.2304159
36.
XiangXZhouXWangWZhouYZhouXDengSet al. Effect of Antarctic krill phospholipid (Kopl) on high fat diet-induced obesity in mice. Food Res Int. (2021) 148:110456. doi: 10.1016/j.foodres.2021.110456
37.
LiQLiuWFengYHouHZhangZYuQet al. Radix Puerariae thomsonii polysaccharide (RPP) improves inflammation and lipid peroxidation in alcohol and high-fat diet mice by regulating gut microbiota. Int J Biol Macromol. (2022) 209:858–70. doi: 10.1016/j.ijbiomac.2022.04.067
38.
LiuFTangXMaoBZhangQZhaoJCuiSet al. Ethanol extract of licorice alleviates HFD-induced liver fat accumulation in association with modulation of gut microbiota and intestinal metabolites in obesity mice. Nutrients. (2022) 14:4180. doi: 10.3390/nu14194180
39.
LiuBNLiuXTLiangZHWangJH. Gut microbiota in obesity. World J Gastroenterol. (2021) 27:3837–50. doi: 10.3748/wjg.v27.i25.3837
40.
CanforaEEJockenJWBlaakEE. Short-chain fatty acids in control of body weight and insulin sensitivity. Nat Rev Endocrinol. (2015) 11:577–91. doi: 10.1038/nrendo.2015.128
41.
PriceCEVallsRARamseyARLoevenNAJonesJTBarrackKEet al. Intestinal bacteroides modulates inflammation, systemic cytokines, and microbial ecology via propionate in a mouse model of cystic fibrosis. mBio. (2024) 15:e0314423. doi: 10.1128/mbio.03144-23
42.
FuscoWLorenzoMBCintoniMPorcariSRinninellaEKaitsasFet al. Short-chain fatty-acid-producing bacteria: key components of the human gut microbiota. Nutrients. (2023) 15:2211. doi: 10.3390/nu15092211
43.
PeiLLiuWLiuLWangXJiangLChenZet al. Morel (Morchella spp.) intake alters gut microbial community and short-chain fatty acid profiles in mice. Front Nutr. (2023) 10:1237237. doi: 10.3389/fnut.2023.1237237
44.
MukherjeeALordanCRossRPCotterPD. Gut microbes from the phylogenetically diverse genus Eubacterium and their various contributions to gut health. Gut Microbes. (2020) 12:1802866. doi: 10.1080/19490976.2020.1802866
45.
KayeDMShihataWAJamaHATsyganovKZiemannMKiriazisHet al. Deficiency of prebiotic fiber and insufficient signaling through gut metabolite-sensing receptors leads to cardiovascular disease. Circulation. (2020) 141:1393–403. doi: 10.1161/circulationaha.119.043081
46.
van der VeenJNLingrellSMcCloskeyNLeBlondNDGalleguillosDZhaoYYet al. A role for phosphatidylcholine and phosphatidylethanolamine in hepatic insulin signaling. FASEB J. (2019) 33:5045–57. doi: 10.1096/fj.201802117R
47.
BasakSDuttaroyAK. Conjugated linoleic acid and its beneficial effects in obesity, cardiovascular disease, and cancer. Nutrients. (2020) 12:1913. doi: 10.3390/nu12071913
48.
WeiWLyuXMarkhardALFuSMardjukiRECavanaghPEet al. PTER is a N-acetyltaurine hydrolase that regulates feeding and obesity. Nature. (2024) 633:182–8. doi: 10.1038/s41586-024-07801-6
49.
ZhaoYLiMGuoJFangJGengRWangYet al. Cedrol, a major component of cedarwood oil, ameliorates high-fat diet-induced obesity in mice. Mol Nutr Food Res. (2023) 67:e2200665. doi: 10.1002/mnfr.202200665
50.
LiaoYChenQLiuLHuangHSunJBaiXet al. Amino acid is a major carbon source for hepatic lipogenesis. Cell Metab. (2024) 36:2437–48.e8. doi: 10.1016/j.cmet.2024.10.001
51.
HuXLiJFuMZhaoXWangW. The JAK/STAT signaling pathway: from bench to clinic. Signal Transduct Target Ther. (2021) 6:402. doi: 10.1038/s41392-021-00791-1
52.
DodingtonDWDesaiHRWooM. JAK/STAT—emerging players in metabolism. Trends Endocrinol Metab. (2018) 29:55–65. doi: 10.1016/j.tem.2017.11.001
53.
ZongXZhangHZhuLDeehanECFuJWangYet al. Auricularia auricula polysaccharides attenuate obesity in mice through gut commensal Papillibacter cinnamivorans. J Adv Res. (2023) 52:203–18. doi: 10.1016/j.jare.2023.08.003
54.
LiuSJiangWLiuCGuoSWangHChangX. Chinese chestnut shell polyphenol extract regulates the JAK2/STAT3 pathway to alleviate high-fat diet-induced, leptin-resistant obesity in mice. Food Funct. (2023) 14:4807–23. doi: 10.1039/d3fo00604b
55.
YangMWuSCaiWMingXZhouYChenX. Hypoxia-induced MIF induces dysregulation of lipid metabolism in Hep2 laryngocarcinoma through the IL-6/JAK-STAT pathway. Lipids Health Dis. (2022) 21:82. doi: 10.1186/s12944-022-01693-z
Summary
Keywords
Limosilactobacillus reuteri, obesity, high-fat diet, inflammation, gut microbiota, untargeted metabolomics, transcriptomics
Citation
Tang M, Li X, Ren J, Yao C, Liu L, Li X, Yuan X, Zhao J, Liu B, Qiao W and Chen L (2025) Limosilactobacillus reuteri HM108 alleviates obesity in rats fed a high-fat diet by modulating the gut microbiota, metabolites, and inhibiting the JAK-STAT signalling pathway. Front. Nutr. 12:1597334. doi: 10.3389/fnut.2025.1597334
Received
21 March 2025
Accepted
09 June 2025
Published
25 June 2025
Volume
12 - 2025
Edited by
Omar Guzmán Quevedo, Higher Technological Institute of Tacambaro, Mexico
Reviewed by
Orsolya Inczefi, University of Szeged, Hungary
Eréndira Valencia Avilés, Universidad Michoacana de San Nicolás de Hidalgo, Mexico
Updates
Copyright
© 2025 Tang, Li, Ren, Yao, Liu, Li, Yuan, Zhao, Liu, Qiao and Chen.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lijun Chen, chenlijun@sanyuan.com.cn
†These authors have contributed equally to this work and share first authorship
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.