- 1Department of Digestion, Zhejiang Hospital, Hangzhou, Zhejiang, China
- 2Department of Clinical Nutrition, Zhejiang Hospital, Hangzhou, Zhejiang, China
Background: Although studies have reported the associations between certain dietary patterns and the risk of Parkinson’s disease, these findings are limited and inconclusive. Herein, we carried out a systematic review and meta-analysis of observational studies to search for the associations between a priori and a posteriori dietary patterns and the risk of developing Parkinson’s disease.
Methods: We systematically searched PubMed, Web of Science, Scopus, and China National Knowledge Infrastructure from database inception to January 2025 to clarify eligible observational studies investigating the links between whole dietary patterns and risk of Parkinson’s disease. Combined relative risks (RRs) and 95% confidence intervals (CIs) were calculated for the highest versus lowest categories of dietary patterns in relation to Parkinson’s disease risk. The Cochran’s Q test and I-squared (I2) statistic were used to assess statistical heterogeneity among the included studies.
Results: In total, 11 studies (five cohort, three case–control, and 3 cross-sectional studies) with 326,751 participants and 2,524 cases were included in this meta-analysis. The pooled analyses showed that adherence to the Mediterranean diet, healthy dietary index, and healthy dietary pattern were associated with a decreased risk of Parkinson’s disease (RR = 0.87; 95%CI: 0.78–0.97, p = 0.017; RR = 0.76; 95%CI: 0.65–0.91, p = 0.002; RR = 0.76; 95%CI: 0.62–0.93; p = 0.007, respectively). Additionally, the results showed that high adherence to the Western dietary pattern was associated with an increased risk of Parkinson’s disease (RR = 1.54; 95%CI: 1.10–2.15; p = 0.011).
Conclusion: Overall, our results demonstrate that adherence to the Mediterranean diet, a healthy dietary index, and a healthy dietary pattern were associated with a reduced risk of Parkinson’s disease, while the Western dietary pattern was linked to an increased risk of Parkinson’s disease. Further well-designed prospective studies and randomized controlled trials are required to confirm these findings.
Introduction
Parkinson’s disease is a common and progressive neurodegenerative disease, affecting approximately 6.1 million people worldwide (1). In the United Kingdom, the prevalence of Parkinson’s disease was estimated at 286.5 per 100,000 person-years in 2020 (2). Notably, studies have reported that the incidence of Parkinson’s disease increases with age, and is generally higher among White person/persons/people than among Black person/persons/people or Asians (3). In the United States, the economic burden of Parkinson’s disease is expected to increase from $52 billion in 2017 to $79 billion in 2037 (4). Currently, there is no curative therapy for Parkinson’s disease, so effective strategies for the prevention or delay of disease occurrence are needed (5). As far as we know, the exact cause of Parkinson’s disease remains uncertain, and it may involve the combined effects of various factors, including genetic predisposition, ageing and environmental exposures (6). Among environmental factors, dietary factors have been considered as the important modifiable factors for the prevention of Parkinson’s disease (7).
In past several decades, previous epidemiological studies have explored the potential correlations between intakes of individual foods, nutrients, or food groups and the risk of developing Parkinson’s disease, with inconsistent results (8–10). However, due to the complexity of the diet and potential interactions between food components (11), these studies showed a limited effect of diet on Parkinson’s disease. In reality, people usually eat foods that contain multiple combinations of foods and nutrients (12). As a result, dietary pattern analysis has emerged as a valuable approach and been widely used in nutritional epidemiology, taking into account the combined effects of foods and nutrients (13).
Currently, little is known about the role of whole dietary patterns in Parkinson’s disease risk. To date, limited observational studies have reported the associations between a priori and a posteriori dietary patterns and Parkinson’s disease risk (14–24). However, results from these previous studies have been inconsistent. Whilst some observational studies have shown the potential neuroprotective effect of adherence to healthy dietary patterns (e.g., Mediterranean diet and prudent pattern) on Parkinson’s disease (14–16, 18, 22), other studies did not find such association (17, 21, 23). For example, a case–control study reported an inverse association between higher Mediterranean-type diet adherence and Parkinson’s disease risk (OR = 0.86; 95%CI: 0.77–0.97) (15). By contrast, in a prospective population-based cohort Study, Strikwerda and colleagues found no significant association between adherence to the Mediterranean diet and Parkinson’s disease risk (HR = 0.80; 95%CI: 0.50–1.29) (20). Notably, a recent systematic review and meta-analysis of 12 observational studies found that high adherence to the Mediterranean diet was associated with a lower risk of Parkinson’s disease (25). But, in the aforementioned meta-analysis, the outcome of interests included Parkinson’s disease, prodromal Parkinson’s disease and two of included studies reported the impact of Mediterranean diet on prodromal Parkinson’s disease features. Additionally, Zhao et al.’s meta-analysis included a case–control study of healthy pattern (identified by factor analysis) and Parkinson’s disease (16) and thus had methodological limitations. Furthermore, to our knowledge, there are no systematic review and meta-analyses that have comprehensively assessed the associations between a posteriori and a priori dietary patterns and risk of Parkinson’s disease. Therefore, to determine the potential correlations between whole dietary patterns and risk of Parkinson’s disease, we conducted a systematic review and meta-analysis to summarize the available evidence from observational studies published up to January 2025.
Methods
Search strategy and selection criteria
A comprehensive literature search in four online electronic databases (PubMed, Web of Science, Scopus, and China National Knowledge Infrastructure) was performed for pertinent articles published up to January 2025, with the predefined search terms and keywords: (“dietary pattern” OR “eating pattern” OR “food pattern” OR “diet indices” OR “dietary score” OR “dietary quality” OR “dietary index” OR “diet”) AND (“Parkinson’s disease” OR “Parkinson disease” OR “Parkinsonism”). No publication date or language restrictions were applied during the search process. In addition, the reference lists of all eligible articles or reviews were manually searched to identify any additional relevant citations to ensure a comprehensive search. This meta-analysis was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) statement (26). The protocol for this systematic review and meta-analysis was developed, but not registered online in advance with the International Prospective Register of Systematic reviews (PROSPERO) database. The literature search was independently performed by two of the authors (R.Z. and L.S.). Any discrepancies in searching articles were resolved by consensus or by consulting a third author (N.L.). Details of search strategy have been shown in the Supplementary Table 1.
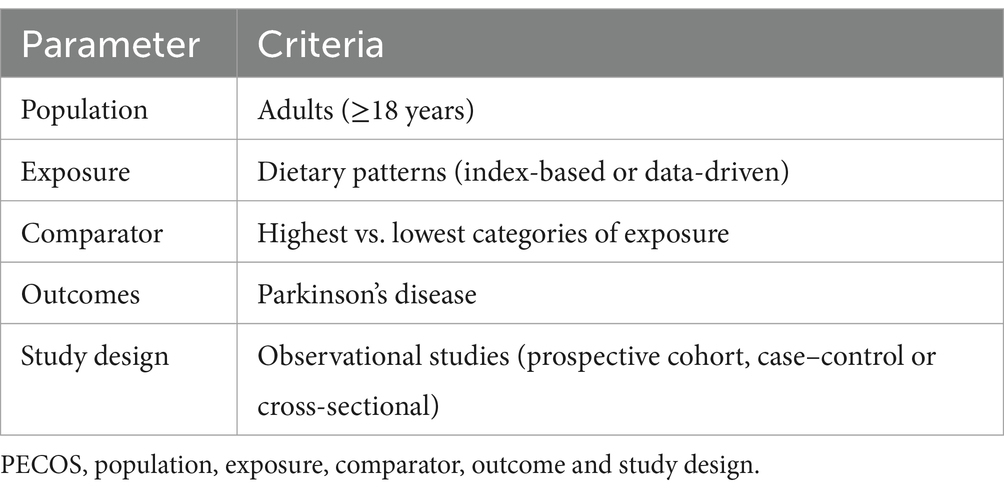
To be included in this review, the eligible articles met the following criteria: (1) observational research (e.g., cohort, case–control, cross-sectional study) performed in humans of any age; (2) studies exploring the correlations between whole dietary patterns and risk of Parkinson’s disease; (3) dietary patterns were identified using a posteriori methods (e.g., factor analysis or cluster analysis) or a priori methods; (4) provided risk estimates of ORs, RRs, HRs along with their corresponding 95%CIs; (5) Parkinson’s disease diagnosis was confirmed by medical records review or a neurologist; (6) if retrieved article lacked sufficient Parkinson’s disease-relevant data, the corresponding author of original study will be contacted for additional information by email.
Studies were excluded based on the following criteria: (1) unrelated articles, e.g., retrieved articles did not report the association between diet and risk of Parkinson’s disease; (2) non-observational studies, e.g., intervention studies, reviews, editorials, case reports and conference letters; (3) grey literature, which is generally not included in large electronic databases, such as PubMed, Web of Science, Scopus and China National Knowledge Infrastructure; (4) Parkinson’s syndrome instead of Parkinson’s disease; (5) studies not reporting HRs, RRs or ORs with 95% CIs. Two authors (R.Z. and L.S.) independently examined all the titles and abstracts, and obtained full texts of potentially relevant articles. Any disagreements about including eligible studies were addressed through discussion or, if need, in consultation with the third author (N.L.). Our selection criteria was based on the PECOS (e.g., participant, exposure, comparison, outcome, and study design) framework, which is shown in Table 1.
To minimize error, we ensured that dietary patterns chosen were similar in terms of the factor loads of foods consumed in those dietary patterns. For example, Mediterranean diet is characterized by high consumption of fruits, vegetables, nuts, legumes, whole grains and extra-virgin olive oil; moderate consumption of poultry, fish and alcohol; and low consumption of red and processed meats (15). Healthy dietary pattern is characterized by high consumption of fruit, vegetables, legumes, whole grains, poultry, and fish (14). Western dietary pattern is characterized by high consumption of red and/or processed meat, refined grains, French fries, sweets, desserts and high-fat dairy products, and low consumption of fruits and vegetables (14).
Data extraction
For each eligible article, two independent authors (R.Z. and L.S.) extracted the following data using a standardized form: first author’s name, publication year, study design, country where the study was performed, number of participants, number of Parkinson’s disease cases, mean age or age range (year), dietary assessment method, reported risk estimates (HRs/ORs/RRs) and the corresponding 95%CIs and confounding factors that were adjusted for in the multivariate analyses. Any disagreements about data extraction were resolved through consensus or discussion with a third author (N.L).
Quality assessment of included studies
For non-randomized studies, the quality of eligible study was evaluated using the Newcastle-Ottawa Scale (NOS) (27). This scale assigns 0 ~ 9 “stars” to each study based on three aspects: selection of participants (maximum of 4 stars), comparability of study groups (maximum of 2 stars), and ascertainment of outcomes of interests (maximum of 3 stars). Studies with NOS scores ≥ 7 points were deemed to be of high quality (28).
Data synthesis and statistical analysis
In this study, the reported ORs from case–control and cross-sectional studies were converted to RRs using the following formula: RR = OR/[(1-P0) + (P0*OR)], where P0 represents the incidence of the outcome of interest in the non-exposed group (29). In addition, HRs were considered as approximations of RRs. Statistical heterogeneity among the studies was evaluated using the Cochran’s Q test and I-squared (I2) statistics. If p values of Cochran’s Q-test ≤0.10 or I2 ≥ 50% indicated substantial heterogeneity among studies, and a random-effects model (DerSimonnian and Laird method) was used to pool the RRs and 95%CIs of the highest versus the lowest categories of priori and posteriori dietary patterns in relation to Parkinson’s disease. Otherwise, a fixed-effect model is adopted (30). To explore the possible sources of observed heterogeneity across studies, we performed subgroup analyses by study design (cohort or case–control/cross-sectional studies), study area (Western countries or other countries), mean age (≥50y or <50y), sample size (≥5,000 or <5,000), study quality (≥7 or <7) and dietary assessment method (FFQ or other/24 h dietary recall). A sensitivity analysis was also performed by removing one study at a time, and to test if the observed associations were robust or sensitive to the influence of each individual study. Publication bias was assessed through the visual inspection of the funnel plots and quantified by Begg’s and Egger’s tests (31). If the results showed evidence of publication bias, we used the trim and fill methods to adjust the asymmetry of the funnel plot by inferring the potentially missing studies (32). All statistical analyses were performed using STATA, version 12.0 (StataCorp, College Station, TX, USA). A 2-tailed p-value less than 0.05 was considered statistically significant.
Results
Search results
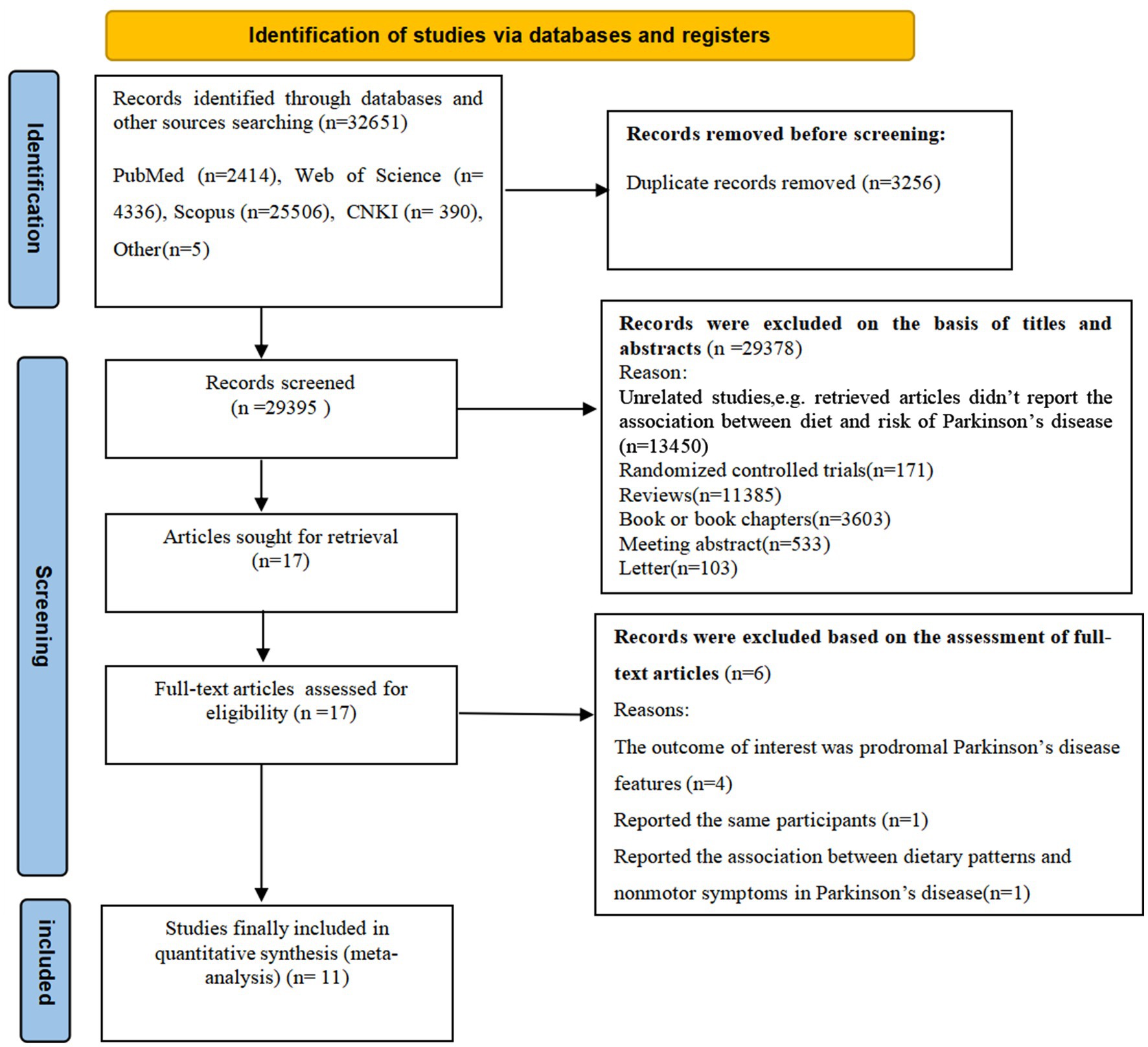
Figure 1 illustrates the PRISMA flow chart of literature search process. Our searches initially generated 32,651 articles from four databases and other sources. After removing 3,256 duplicates, 29,395 records remained. Subsequently, 29,378 articles were removed based on the review of titles and abstracts of retrieved articles and irrelevant articles. The remaining 17 full-text articles were reviewed in details, and 6 articles were excluded for the following reasons: the outcome of interest was prodromal Parkinson’s disease features (n = 4); reported the same participants (n = 1); reported the association between dietary patterns and nonmotor symptoms (n = 1). Accordingly, 11 studies met the eligibility criteria and were finally included in this meta-analysis.
Study characteristics
The characteristics of included studies were detailed in Table 2. Eleven studies, including 5 prospective cohort (14, 17, 19, 20, 24), 3 case–control (15, 16, 22) and 3 cross-sectional studies (18, 21, 23), with a total of 326,751 participants and 2,524 cases were included in this systematic review and meta-analysis. These included studies were published between 2007 and 2024. Sample size varied from 170 to 131,368. The age of participants ranged from ages 18 to above. Among these published studies, four studies were carried out in the United States (14, 15, 18, 23), two in Iran (21, 22), one in Japan (16), one in Sweden (17), one in Netherlands (20), one in Finland (24), and one study in United Kingdom (19). The majority of the included studies used FFQ to measure dietary intake (14, 15, 17, 20–23), two studies used dietary questionnaire (16, 19), one used dietary history interview (24), and one used 24-h dietary recalls (18). Finally, according to NOS tool, eight studies were considered to be of high quality (14–20, 22, 24), and the remaining two articles were identified as medium-quality (21, 23).
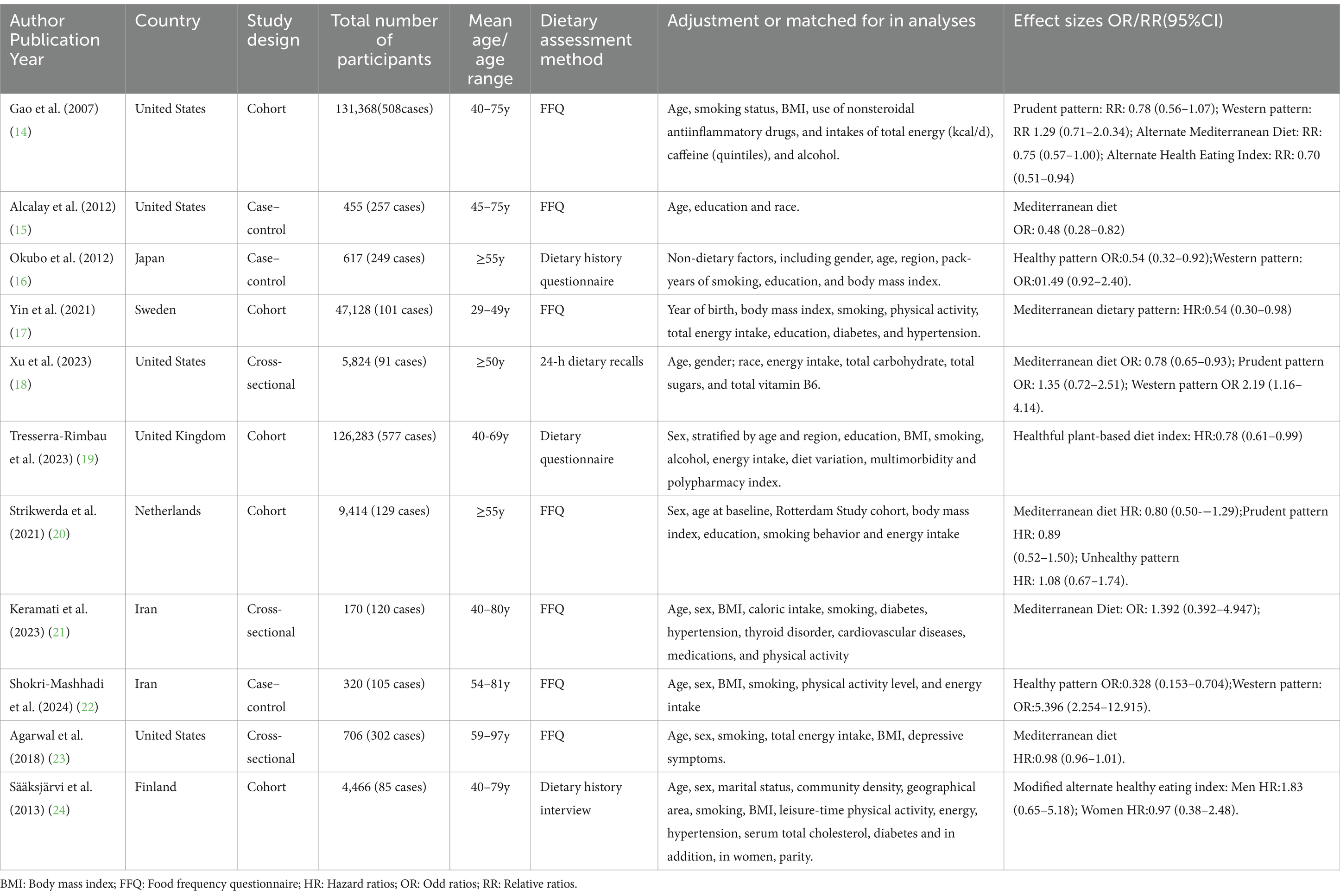
Table 2. Characteristics of included studies on the associations between a priori and a posteriori dietary patterns and Parkinson’s disease risk.
Priori dietary patterns and Parkinson’s disease
Mediterranean diet and Parkinson’s disease
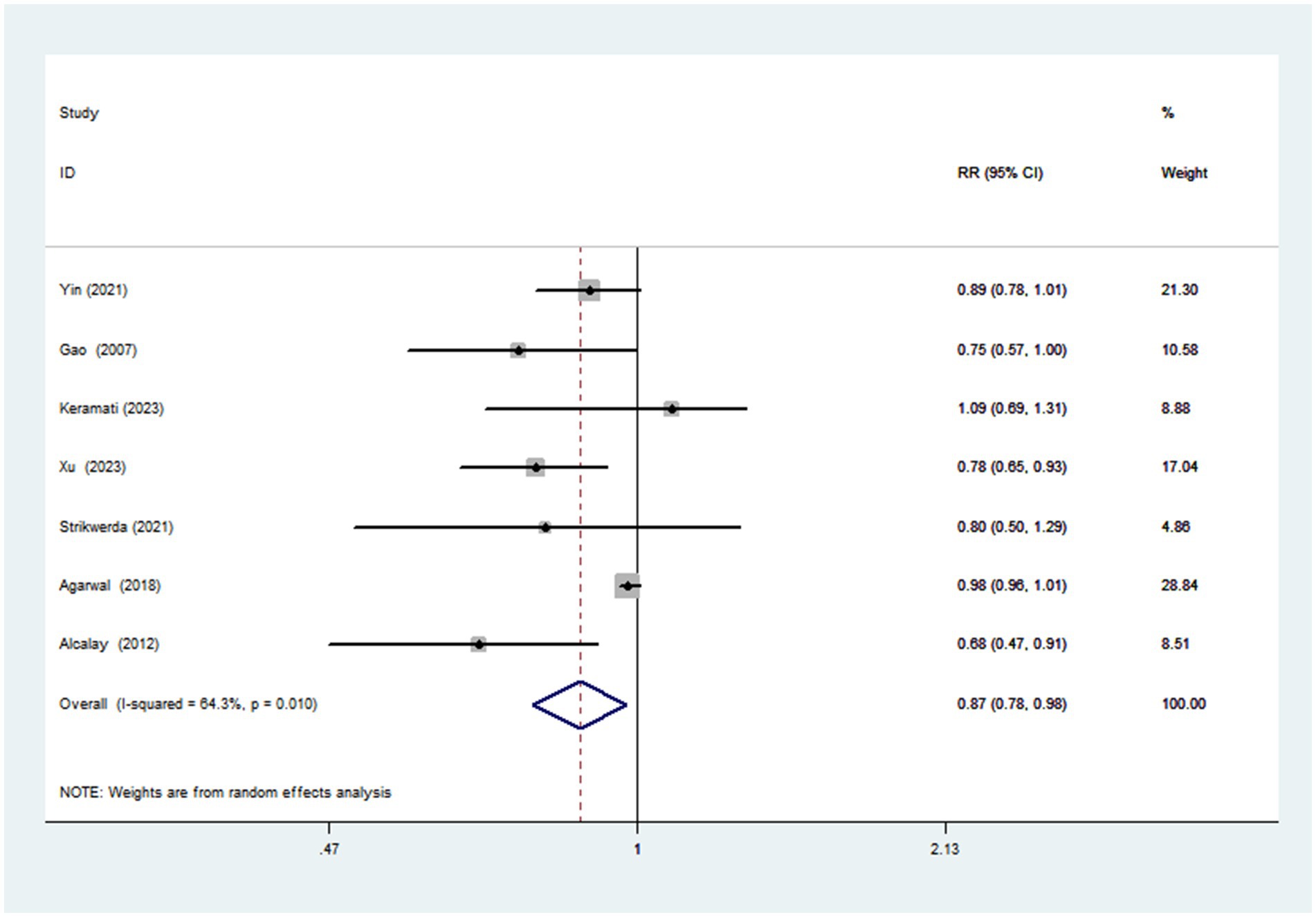
Seven articles with a total of 195,065 participants and 1,508 cases evaluated the association between adherence to the Mediterranean diet and risk of Parkinson’s disease. Figure 2 showed the evidence of a reduced risk of Parkinson’s diseases in the highest compared with lowest categories of Mediterranean diet (RR = 0.87; 95%CI: 0.78–0.97, p = 0.017). Substantial heterogeneity was observed in the included studies (I2 = 64.3%, p = 0.010), and we used a random-effects model to calculate the pooled RRs. To explore the reasons for substantial heterogeneity across studies, we carried out subgroup analyses according to study design, study area, mean age, sample size, study quality and dietary assessment method. The results showed that study design, sample size and study quality might contribute to significant heterogeneity (Table 3).
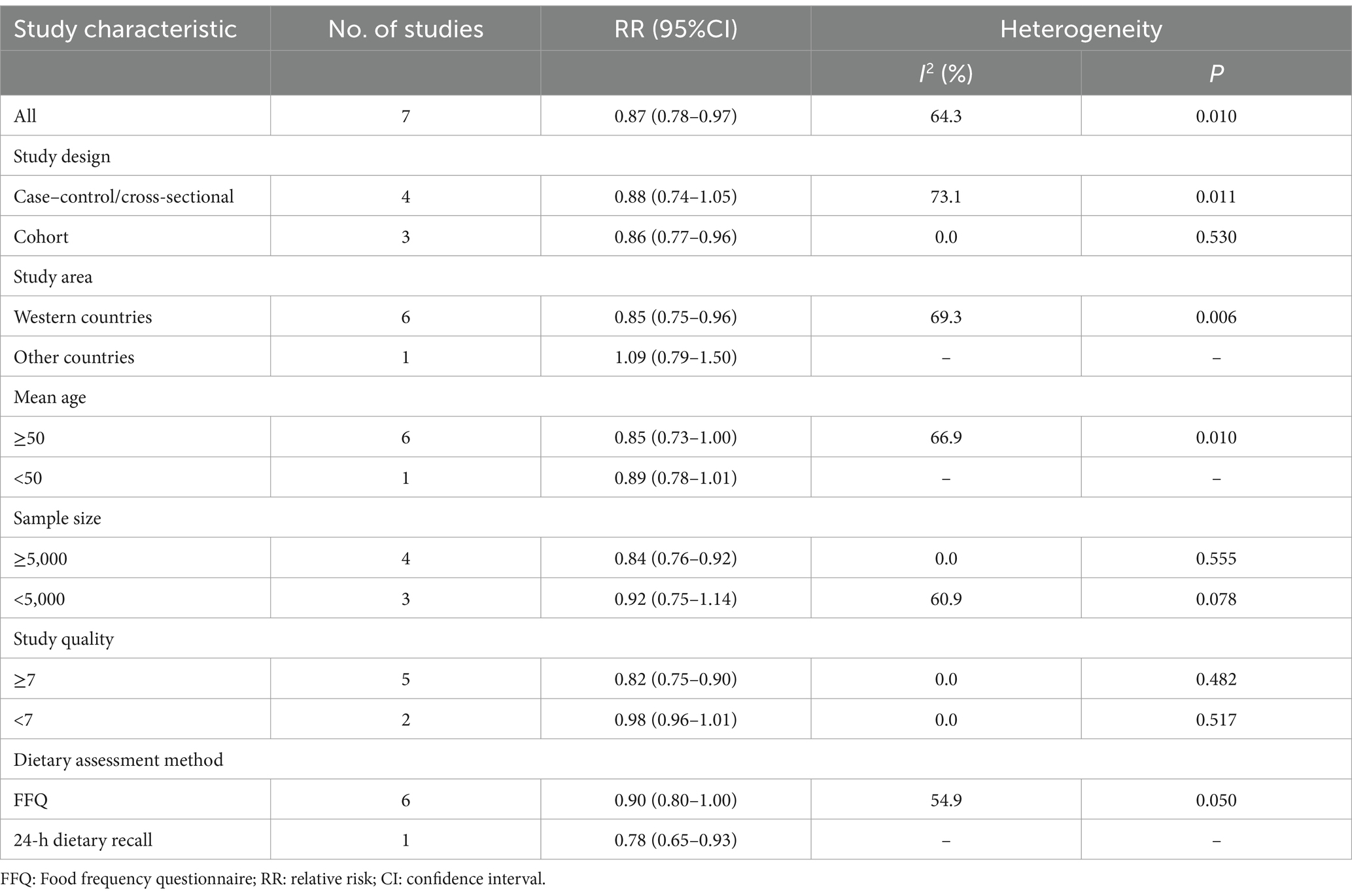
Table 3. Subgroup analyses for the association between adherence to the Mediterranean diet and Parkinson’s disease risk.
Healthy dietary index and Parkinson’s disease
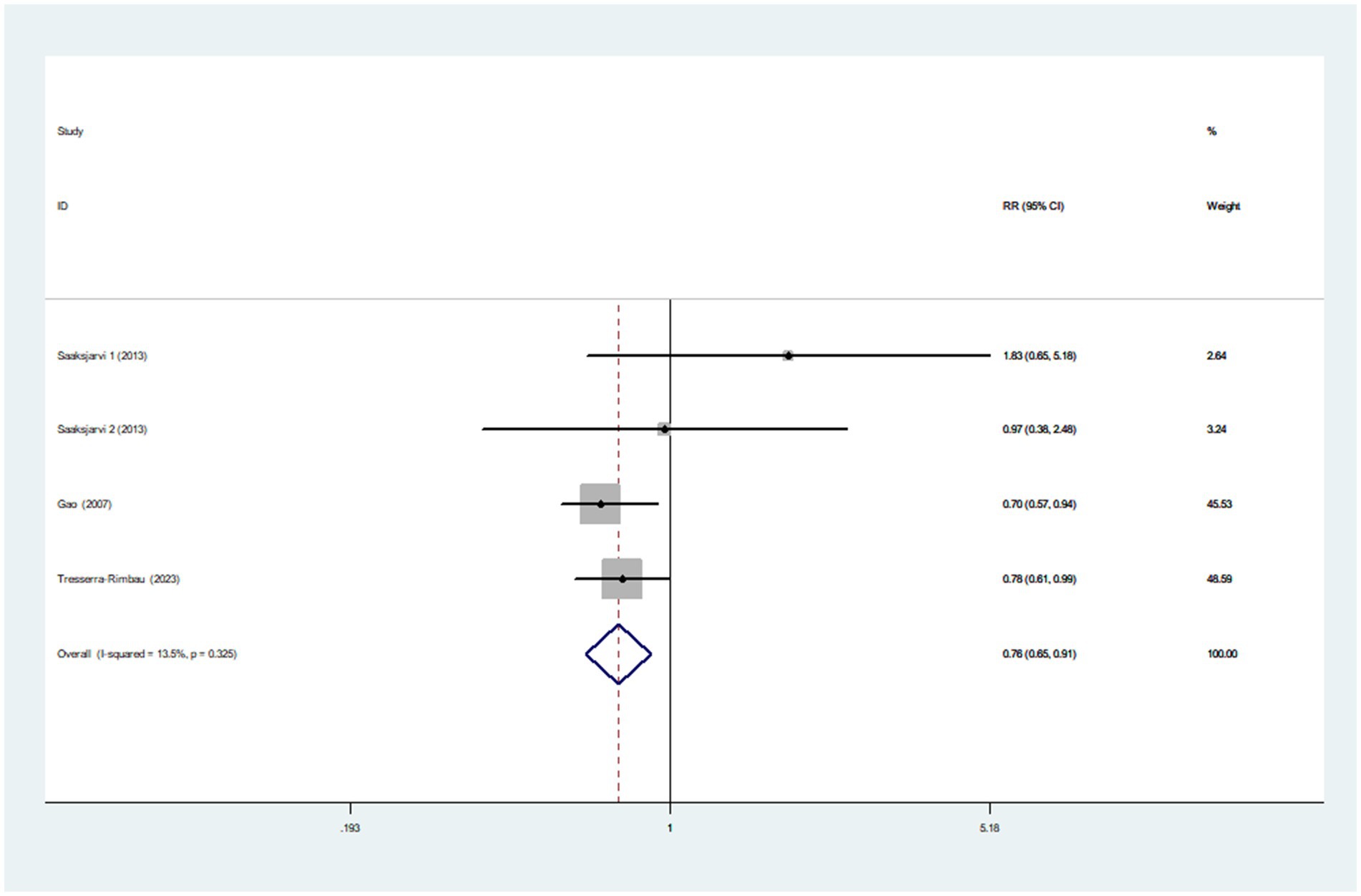
Three articles incorporating four studies, were included in the analysis of healthy dietary index and the risk of Parkinson’s disease. Figure 3 showed that the highest category of healthy dietary index had a reduced risk of Parkinson’s disease (RR = 0.76; 95%CI: 0.65–0.91, p = 0.002) than the lowest category. Low heterogeneity between studies was found (I2 = 13.5%, p = 0.325). Therefore, the effect size was assessed using a fixed-effects model.
Posteriori dietary patterns and Parkinson’s disease
Healthy dietary pattern and Parkinson’s disease
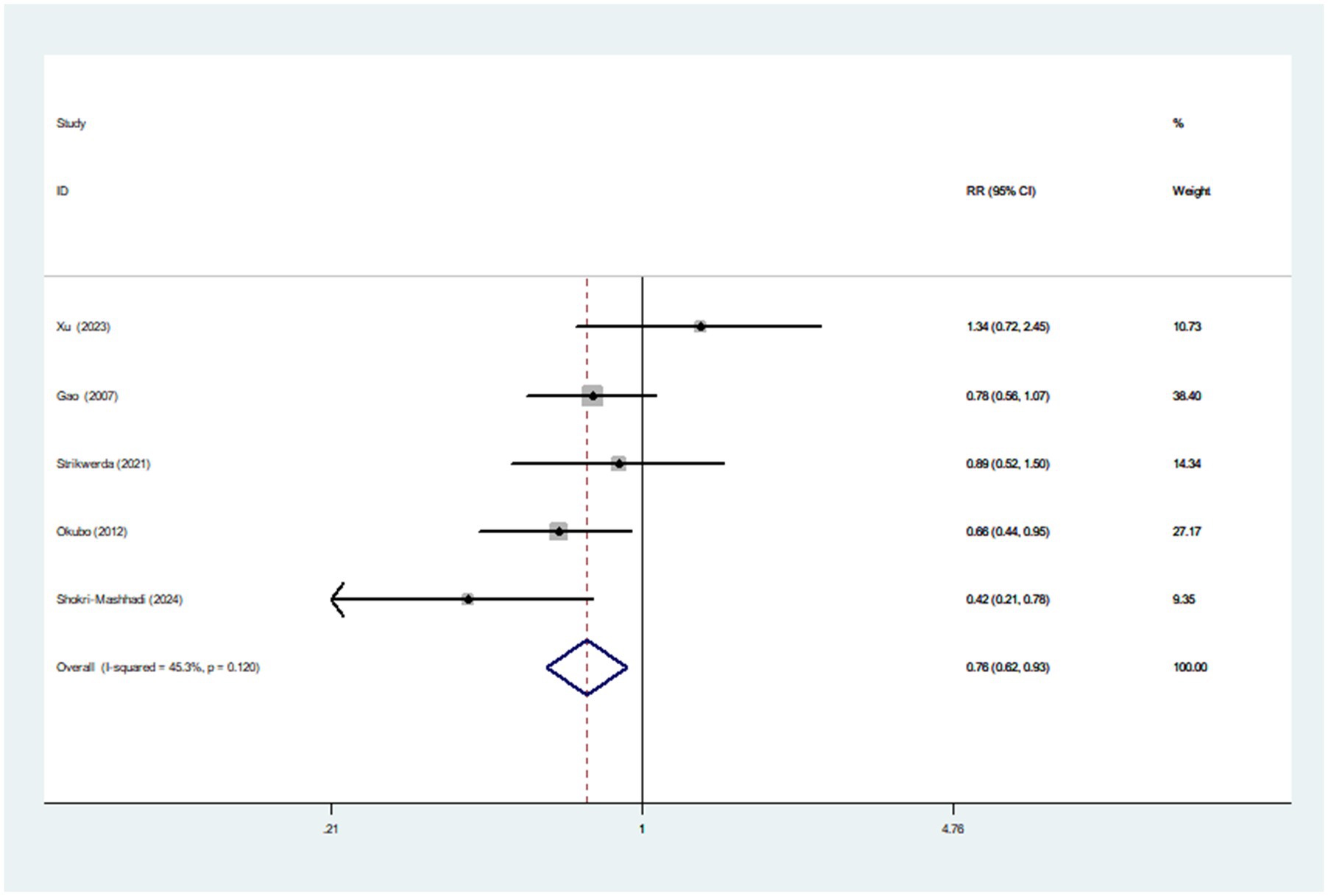
Five articles involving 1,082 cases and 147,543 participants, were included in this meta-analysis. Figure 4 showed that the highest category of healthy dietary pattern had a 24% reduced risk of Parkinson’s disease than the lowest category (RR = 0.76; 95%CI: 0.62–0.93; p = 0.007). The moderate heterogeneity across studies was found (I2 = 45.3%, p = 0.120), and a fixed-effects model was used for data analysis. To further detect the probable sources of moderate heterogeneity, we carried out subgroup analyses depending on study design, study area, sample size and dietary assessment method. The results of subgroup analyses showed that study area and sample size might be potential source of moderate heterogeneity in included studies (Table 4).
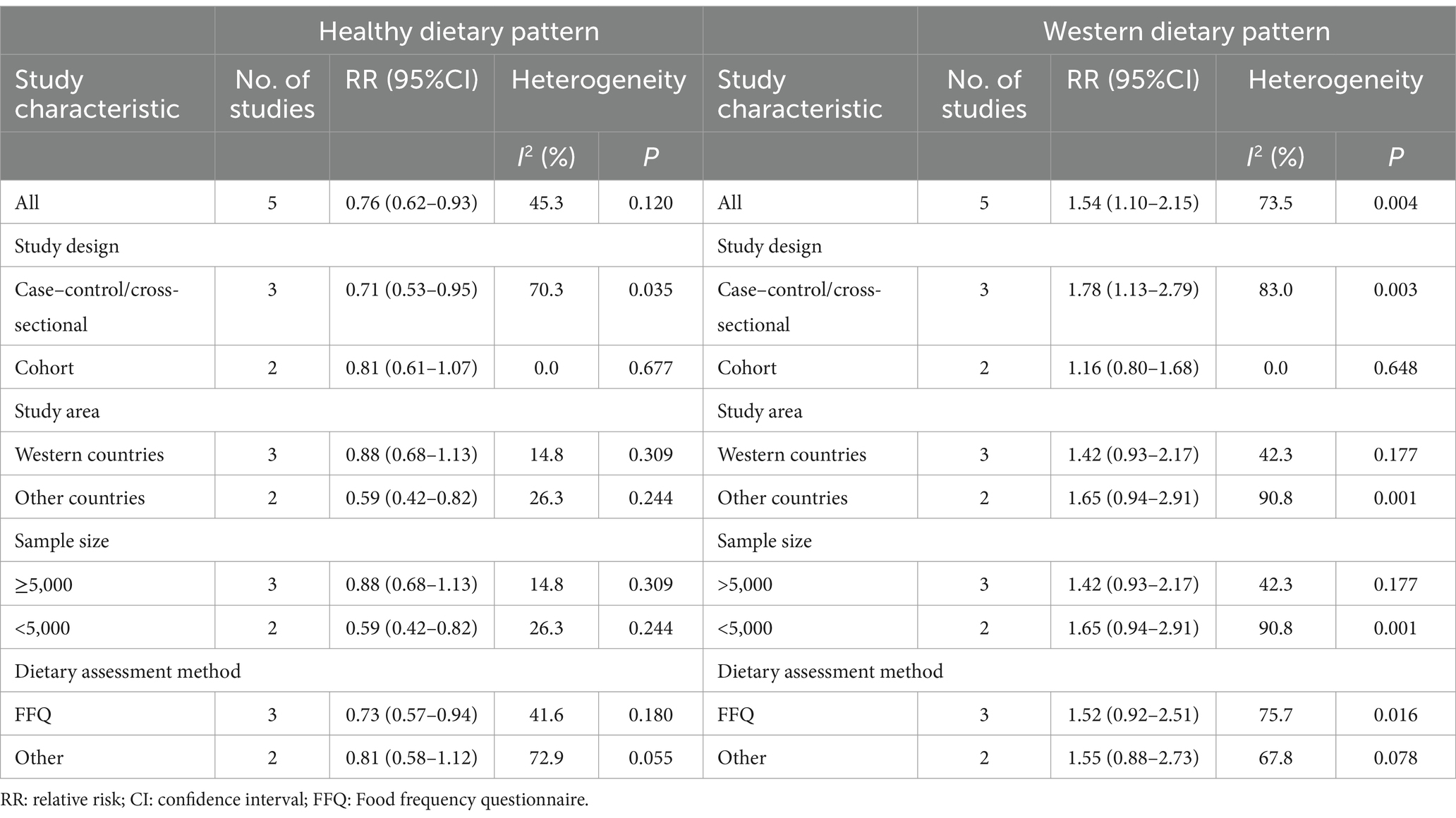
Table 4. Subgroup analyses for the association between posteriori dietary patterns and Parkinson’s disease risk.
Western dietary pattern and Parkinson’s disease
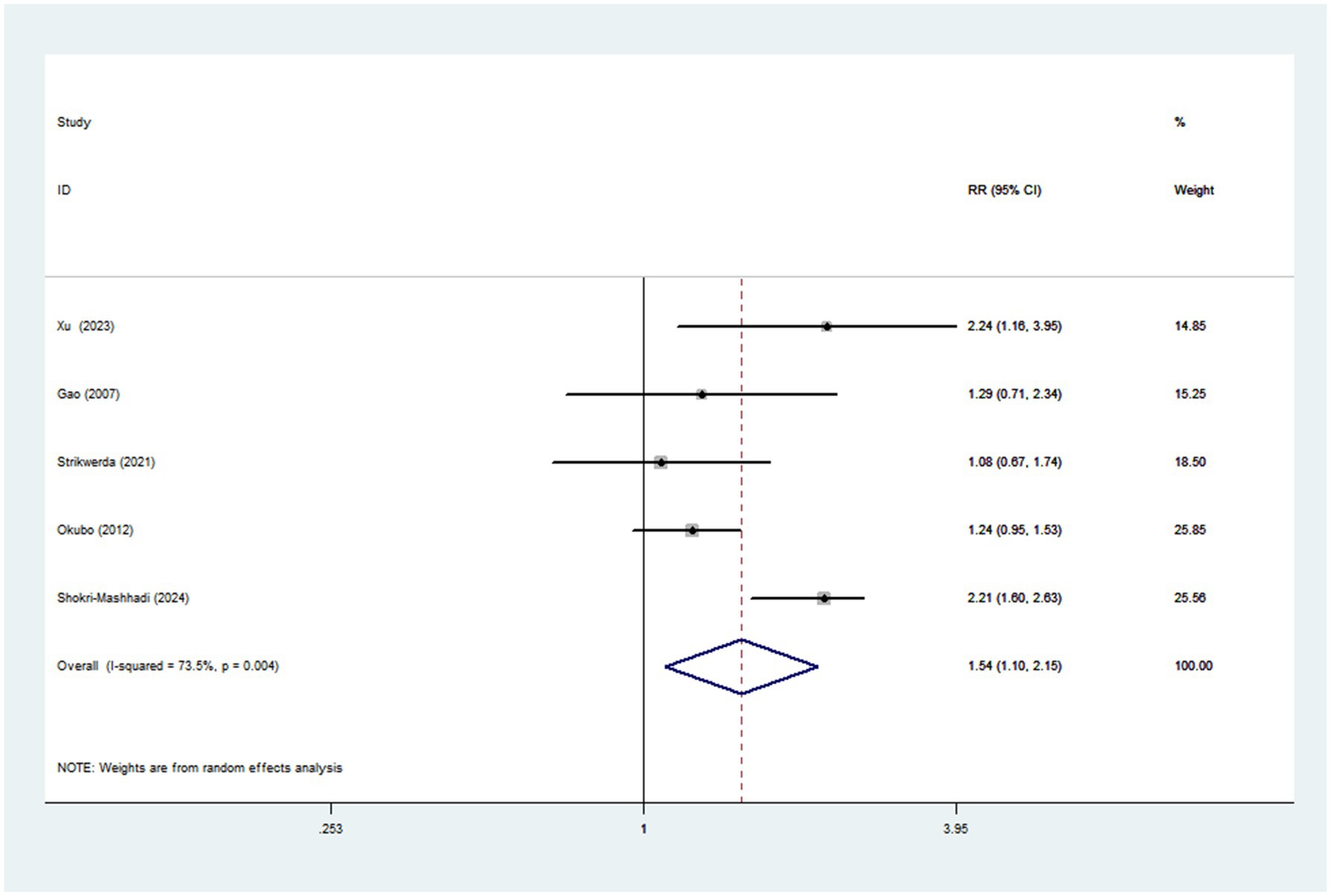
Pooled results from five articles identified the Western dietary pattern. Figure 5 showed the obvious evidence of an increased risk of Parkinson’s disease in the highest compared with lowest categories of Western dietary pattern (RR = 1.54; 95%CI: 1.10–2.15; p = 0.011). There was significant heterogeneity in the included studies (p = 0.004; I2 = 73.5%), and thus a random-effects model was used to calculate the combined RR. In our analyses, subgroup analyses were carried out based on study design, study area, sample size and dietary assessment method. The results showed that study design might explain, to some extent, the significant heterogeneity across studies (Table 4).
Quality assessment
The quality of included studies using NOS criteria is shown in Table 5. Based on NOS tool, eight article receiving a score of seven or higher, were considered to be of high quality (14–20, 22, 24). Additionally, the remaining two articles were identified as medium-quality (21, 23).
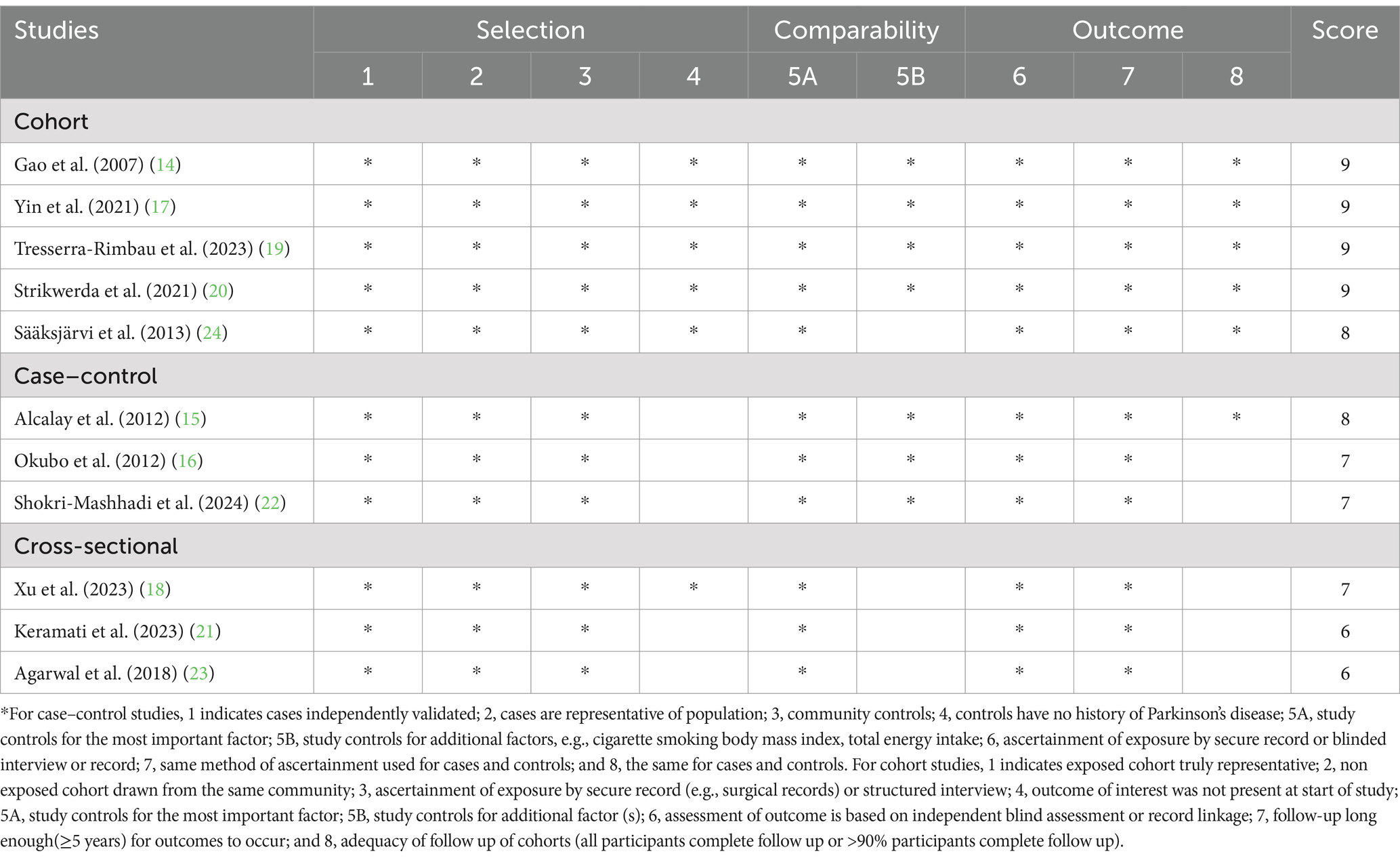
Table 5. Priori and posteriori dietary patterns and risk of Parkinson’s disease: Assessment of Study Quality.
Publication bias and sensitivity analyses
Visual inspection of the funnel plots indicated no evidence of asymmetry (Supplementary Figures 1–4). Similarly, Egger’s and Begg’s tests for publication bias were not statistically significant (highest compared with lowest category of Mediterranean diet: Begg’s test, p = 0.764; Egger’s test, p = 0.043; Healthy dietary index: Begg’s test, p = 0.308; Egger’s test, p = 0.161; Healthy dietary pattern: Begg’s test, p = 1.000; Egger’s test, p = 0.943; Western pattern: Begg’s test, p = 0.462; Egger’ s test, p = 0.860). Based on the results of sensitivity analyses, no significant changes in pooled RRs were found after eliminating any single or a few studies of both a priori and a posteriori dietary patterns in respect to risk of Parkinson’s disease (Supplementary Figures 5–10).
Discussion
The existing literature regarding the associations between dietary patterns and risk of Parkinson’s disease are limited and controversial. As far as we are aware, this is the latest and most comprehensive systematic review and meta-analysis evaluating the associations between a priori and a posteriori dietary patterns and Parkinson’ s disease. This meta-analysis demonstrated that adherence to the Mediterranean diet, healthy dietary index and healthy dietary pattern were associated with a reduced risk of Parkinson’s disease, whereas the Western dietary pattern was linked to an increased risk of Parkinson’s disease. Given the significant heterogeneity observed in this meta-analysis, these results should be interpreted with caution. Collectively, our findings provide substantive evidence for the significant associations between dietary patterns and Parkinson’s disease risk, and support the adoption of whole dietary patterns for the prevention of Parkinson’s disease.
Comparison with epidemiological studies
Over the past few decades, Parkinson’s disease has become the second most common neurodegenerative disease worldwide, and its incidence has steadily increased (6, 33). Additionally, studies have shown that the economic burden of Parkinson’s disease in the United States is expected to increase from $52 billion in 2017 to $79 billion in 2037 (4). Considering the tremendous cost on public health, it is essential to explore the modifiable dietary factors for the prevention of Parkinson’s disease. Previous studies have shown the associations between whole dietary patterns (e.g., Mediterranean diet, the Alternative healthy eating index, and prudent pattern) and Parkinson’s disease risk (14–24). Nevertheless, it was noteworthy that these studies yielded inconsistent results. In the Health Professionals Follow-Up Study and the Nurses’ Health Study, Gao et al. found that higher adherence to the alternate Mediterranean diet score was inversely associated with Parkinson’s disease risk (HR = 0.75; 95%CI: 0.57–1.00) (14). However, at variance with the aforementioned study, Strikwerda and colleagues observed no significant association between adherence to the Mediterranean diet and Parkinson’s disease risk in the prospective population-based cohort study (HR = 0.80; 95%CI: 0.50–1.29) (20). The reasons for the inconsistent results may be attributed to differences in study design, sample size, study population, and dietary assessment method. In this study, we found that higher adherence to the Mediterranean diet, healthy dietary index and healthy dietary pattern were associated with a lower risk of Parkinson’s disease. Similar to our meta-analysis, a recent systematic review and meta-analysis conducted in 2024 demonstrated a significant negative correlation between adherence to Mediterranean diet and Parkinson’s disease (25). Notably, in Zhao’s meta-analysis, the outcome of interests included Parkinson’s disease, prodromal Parkinson’s disease and prodromal Parkinson’s disease features. Moreover, a hospital-based case–control study in Japan that reported an association between healthy pattern and Parkinson’s disease (16) has also been included in their analyses. Furthermore, the aforementioned meta-analysis only reported an association between Mediterranean diet adherence and Parkinson’s disease, and did not report the associations between a posteriori dietary patterns and risk of Parkinson’s disease. In this context, we performed this systematic review and meta-analysis of observational studies to determine the role of a priori and a posteriori dietary patterns in Parkinson’s disease.
While epidemiological evidence regarding the links between the Mediterranean diet, healthy dietary index, and healthy dietary pattern and Parkinson’s disease risk remains inconclusive, a number of possible mechanisms have been proposed to explain the observed associations. First, it is well-known that the Mediterranean diet, healthy dietary index and healthy dietary pattern emphasize a high consumption of vegetables, fruits, nuts, legumes and whole grains. The protective effect of vegetables and fruits on Parkinson’s disease may be related to high concentration of antioxidants. Earlier studies have shown an inverse association between Parkinson’s disease risk and intake of antioxidants, such as vitamin C, vitamin E and carotenoids (34). In parallel, a recent systematic review and dose–response meta-analysis of observational studies also showed that higher intake of antioxidant-rich foods was associated with a lower risk of Parkinson’s disease (35). Second, all three dietary patterns mentioned above are considered as plant-based diets. Previous studies have shown that plant-based diets can reduce inflammation (22), which is an important mechanistic link in neurodegenerative diseases, including Parkinson’s disease (36). Third, legumes that contain procyanidin (an important food component in a healthy diet), are high in magnesium and have a low glycemic index, which is linked to the reduced oxidative stress (37). Indeed, neuroinflammation caused by oxidative stress is known to be implicated in the pathogenesis of Parkinson’s disease (38). Fourth, vegetables, fruits and whole grains are rich in dietary fiber, which may mitigate gut dysbiosis by favoring beneficial gut bacteria (39). Studies have shown that gut dysbiosis, an alteration of the gut microbiome, has been suggested as a mechanism by which neuroinflammation leads to Parkinson’s disease (40). Also, dietary fiber produces short-chain fatty acids, which play an anti-inflammatory role and delay the progression of Parkinson’s disease (41). As already discussed above, these mechanisms could together explain the favorable associations observed between adherence to the Mediterranean diet, healthy dietary index, and healthy dietary pattern and risk of Parkinson’s disease.
As reported in Figure 5, Western dietary pattern was associated with an increased risk of Parkinson’s disease. Our results were in agreement with previous studies (22), which suggested that adherence to western dietary pattern could increase the risk of Parkinson’s disease. To our knowledge, there are several plausible explanations associating Western dietary pattern with increased risk of Parkinson’s disease. First, previous research has suggested that consumption of sweeteners and processed foods may lead to changes in the composition and function of the gut microbiota, which may contribute to the development of inflammation (42). As mentioned previously, inflammation is an important mechanistic link in the development of Parkinson’s disease (36). Moreover, high intake of sugar has been shown to increase insulin resistance, a potential risk factor for Parkinson’s disease (43). Second, Western dietary pattern was characterized by low intake of dietary fiber. Evidence suggests that low dietary fiber intake favors the growth of Gram-negative bacterial, which can lead to neurodegenerative diseases (44). Third, red meat often contains high content of iron. A previous meta-analysis showed that higher intake of iron was associated with an increased risk of Parkinson’s disease in Western population (45). Additionally, Jiang and colleagues found that dietary iron intake was positively associated with insulin resistance (46), an important risk factor for Parkinson’s disease (43). Fourth, high-fat dairy products, French fries, desserts and red meat are the main food components of Western dietary pattern, containing high amounts of saturated fat. Epidemiological studies have shown that high intake of saturated fatty acids was associated with increased risk of Parkinson’s disease (47).
In our analyses, the results showed substantial heterogeneity in the associations between adherence to the Mediterranean diet (I2 = 64.3%, p = 0.010) and Western dietary pattern (p = 0.004; I2 = 73.5%) and risk of Parkinson’s disease. Heterogeneity between studies has been common in previous meta-analyses (11), but there is a need to explore potential sources of substantial heterogeneity. In this study, we carried out subgroup analyses by study design, study area, mean age, sample size, study quality and dietary assessment method. For the Mediterranean diet, the results showed that differences in study design, sample size and study quality might contribute to substantial heterogeneity. When subgroup analyses were performed for study design, sample size and study quality separately, heterogeneity decreased from 64.3 to 0.0%. For Western dietary pattern, the results showed that difference in study design might partly explain the significant heterogeneity. Similarly, when subgroup analysis was performed for study design, heterogeneity decreased from 73.5 to 0.0%. Although significant heterogeneity cannot be fully explained by differences in the above variables, there are several possible explanations for significant heterogeneity. First, recall bias resulting from dietary assessment methods in the eligible studies, including FFQs and dietary questionnaire, should be noted. Second, because all included studies were observational, the results may have been affected by residual or unmeasured factors. Third, the included studies used inconsistent adjusted variables, which could explain the significant heterogeneity. Fourth, given the differences in the definition of Mediterranean diet in different countries, dietary habits may lead to significant heterogeneity. Fifth, our analyses did not use the pre-registering search methods, which may have led to bias. Finally, significant heterogeneity still persisted in the subgroup analyses, suggesting the presence of other unmeasured confounding factors.
Strengths and limitations
There are numerous strengths in this study. First, this is the latest and most comprehensive systematic review and meta-analysis evaluating the associations between a priori and a posteriori dietary patterns and Parkinson’ s disease. Compared with previous meta-analyses, we performed a rigorous article screening and excluded several articles on dietary patterns and prodromal Parkinson’s disease. Additionally, our findings provide some evidence for the associations between whole dietary patterns and Parkinson’s disease risk, and emphasize the importance of adherence to the healthy dietary patterns for the prevention of Parkinson’ s disease. Second, Parkinson’ s disease cases have been identified by hospital records or a neurologist, avoiding misdiagnosis bias. Third, subgroup and sensitivity analyses were carried out to explore the possible sources of heterogeneity between studies, improving the accuracy of the results. Fourth, the funnel plots and Begg’s and Egger’s tests did not show any significant asymmetry, suggesting a low risk of publication bias. Despite these strengths, some limitations should be considered when interpreting the results of our meta-analysis. First of all, the observational design of all eligible studies precludes us from establishing a causal association between a priori and a posteriori dietary patterns and risk of Parkinson’ s disease. Second, the large majority of included studies used FFQs to collect dietary data, which may lead to the under- or over- estimation of healthy foods. Third, although all included studies attempted to control or adjust for various potentially confounding variables, the possibility of residual confounding cannot be excluded. Fourth, we could not perform a dose–response analysis, due to insufficient data reported in the included studies. Fifth, the protocol of this study was not registered online in advance with PROSPERO database, but we strictly followed the PRISMA guidelines to reduce selection bias. Additionally, since we did not search for grey literature or unpublished studies, selective reporting bias could not be ruled out in this study. Sixth, only three studies reported the association between healthy dietary index and Parkinson’s disease, preventing us from performing a subgroup analysis. Finally, significant heterogeneity was observed in our analyses. Even though we performed subgroup and sensitivity analyses to explore the potential sources of heterogeneity, we could not ascertain and explain the sources of inter-study heterogeneity sufficiently.
Conclusion
In conclusion, the results of this study showed that adherence to the Mediterranean diet, a healthy dietary index and a healthy dietary pattern were associated with a reduced risk of Parkinson’s disease, whereas the Western dietary pattern was linked to an increased risk of Parkinson’s disease. Our findings add the current evidence and emphasize the importance of adopting healthy patterns, such as prudent pattern and Mediterranean diet for the prevention of Parkinson’s disease. As epidemiological evidence on this topic is limited, additional prospective studies and randomized controlled trials are urgently required to corroborate these findings.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary material.
Author contributions
RZ: Writing – original draft, Formal analysis, Conceptualization, Methodology. LS: Methodology, Formal analysis, Writing – review & editing, Validation. QZ: Supervision, Funding acquisition, Methodology, Validation, Writing – review & editing. NL: Writing – review & editing, Resources, Writing – original draft, Conceptualization, Data curation, Supervision, Validation.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was funded by the National Natural Science Foundation of China (grant number: 82004040). The funders had no role in planning the study design or in the collection, analysis, or interpretation of data, the writing of the report, or the decision to submit the article for publication.
Acknowledgments
We are grateful to Shu for his important contribution to data analysis in this study. Furthermore, we are also grateful to Zhu for providing additional data and financial support for this meta-analysis.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2025.1600955/full#supplementary-material
Abbreviations
AICR, American Institute for Cancer Research; BMI, Body mass index; CNKI, China National Knowledge Infrastructure; CIs, Confidence intervals; DASH, Dietary Approaches to Stop Hypertension; FFQ, Food frequency questionnaire; HRs, Hazards ratios; ORs, Odds ratios; RR, Relative risks; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; WHO, World Health Organization.
References
1. GBD 2016 Parkinson's Disease Collaborators. Global, regional, and national burden of Parkinson's disease, 1990-2016: a systematic analysis for the global burden of disease study 2016. Lancet Neurol. (2018) 17:939–53. doi: 10.1016/S1474-4422(18)30295-3
2. Parkinson’s-UK. The incidence and prevalence of Parkinson’s in the UK. Results from the clinical practice research datalink. (2022). Available online at: https://www.parkinsons.org.uk/sites/default/files/2018-01/CS2960%20Incidence%20and%20prevalence%20report%20branding%20summary%20report.pdf (Accessed January 15, 2025).
3. Van Den Eeden, SK, Tanner, CM, Bernstein, AL, Fross, RD, Leimpeter, A, Bloch, DA, et al. Incidence of Parkinson's disease: variation by age, gender, and race/ethnicity. Am J Epidemiol. (2003) 157:1015–22. doi: 10.1093/aje/kwg068
4. Yang, W, Hamilton, JL, Kopil, C, Beck, JC, Tanner, CM, Albin, RL, et al. Current and projected future economic burden of Parkinson's disease in the U.S. NPJ Parkinsons Dis. (2020) 6:15. doi: 10.1038/s41531-020-0117-1
5. Emamzadeh, FN, and Surguchov, A. Parkinson's disease: biomarkers, treatment, and risk factors. Front Neurosci. (2018) 12:612. doi: 10.3389/fnins.2018.00612
6. Tanner, CM, and Ostrem, JL. Parkinson's disease. N Engl J Med. (2024) 391:442–52. doi: 10.1056/NEJMra2401857
7. Knight, E, Geetha, T, Burnett, D, and Babu, JR. The role of diet and dietary patterns in Parkinson's disease. Nutrients. (2022) 14:4472. doi: 10.3390/nu14214472
8. Chen, H, O'Reilly, E, McCullough, ML, Rodriguez, C, Schwarzschild, MA, Calle, EE, et al. Consumption of dairy products and risk of Parkinson's disease. Am J Epidemiol. (2007) 165:998–1006. doi: 10.1093/aje/kwk089
9. Chen, H, Zhang, SM, Schwarzschild, MA, Hernán, MA, Logroscino, G, Willett, WC, et al. Folate intake and risk of Parkinson's disease. Am J Epidemiol. (2004) 160:368–75. doi: 10.1093/aje/kwh213
10. Jiang, W, Ju, C, Jiang, H, and Zhang, D. Dairy foods intake and risk of Parkinson's disease: a dose-response meta-analysis of prospective cohort studies. Eur J Epidemiol. (2014) 29:613–9. doi: 10.1007/s10654-014-9921-4
11. He, LQ, Wu, XH, Huang, YQ, Zhang, XY, and Shu, L. Dietary patterns and chronic kidney disease risk: a systematic review and updated meta-analysis of observational studies. Nutr J. (2021) 20:4. doi: 10.1186/s12937-020-00661-6
12. Zhang, XY, Shu, L, Si, CJ, Yu, XL, Liao, D, Gao, W, et al. Dietary patterns, alcohol consumption and risk of coronary heart disease in adults: a Meta-analysis. Nutrients. (2015) 7:6582–605. doi: 10.3390/nu7085300
13. Hu, FB. Dietary pattern analysis: a new direction in nutritional epidemiology. Curr Opin Lipidol. (2002) 13:3–9. doi: 10.1097/00041433-200202000-00002
14. Gao, X, Chen, H, Fung, TT, Logroscino, G, Schwarzschild, MA, Hu, FB, et al. Prospective study of dietary pattern and risk of Parkinson disease. Am J Clin Nutr. (2007) 86:1486–94. doi: 10.1093/ajcn/86.5.1486
15. Alcalay, RN, Gu, Y, Mejia-Santana, H, Cote, L, Marder, KS, and Scarmeas, N. The association between Mediterranean diet adherence and Parkinson's disease. Mov Disord. (2012) 27:771–4. doi: 10.1002/mds.24918
16. Okubo, H, Miyake, Y, Sasaki, S, Murakami, K, Tanaka, K, Fukushima, W, et al. Dietary patterns and risk of Parkinson's disease: a case-control study in Japan. Eur J Neurol. (2012) 19:681–8. doi: 10.1111/j.1468-1331.2011.03600.x
17. Yin, W, Löf, M, Pedersen, NL, Sandin, S, and Fang, F. Mediterranean dietary pattern at middle age and risk of Parkinson's disease: a Swedish cohort study. Mov Disord. (2021) 36:255–60. doi: 10.1002/mds.28314
18. Xu, S, Li, W, and Di, Q. Association of Dietary Patterns with Parkinson's disease: a cross-sectional study based on the United States National Health and nutritional examination survey database. Eur Neurol. (2023) 86:63–72. doi: 10.1159/000527537
19. Tresserra-Rimbau, A, Thompson, AS, Bondonno, N, Jennings, A, Kühn, T, and Cassidy, A. Plant-based dietary patterns and Parkinson's disease: a prospective analysis of the UK biobank. Mov Disord. (2023) 38:1994–2004. doi: 10.1002/mds.29580
20. Strikwerda, AJ, Dommershuijsen, LJ, Ikram, MK, and Voortman, T. Diet quality and risk of Parkinson's disease: the Rotterdam study. Nutrients. (2021) 13:3970. doi: 10.3390/nu13113970
21. Keramati, M, Kheirouri, S, and Etemadifar, M. Dietary approach to stop hypertension (DASH), but not Mediterranean and MIND, dietary pattern protects against Parkinson's disease. Food Sci Nutr. (2023) 12:943–51. doi: 10.1002/fsn3.3809
22. Shokri-Mashhadi, N, Ghiasvand, R, Feizi, A, Ebrahimi-Monfared, M, Vahid, F, and Banijamali, A. Association between major dietary patterns and Parkinson's disease risk: a case-control study. Neurol Sci. (2024) 45:2003–10. doi: 10.1007/s10072-023-07204-x
23. Agarwal, P, Wang, Y, Buchman, AS, Holland, TM, Bennett, DA, and Morris, MC. MIND diet associated with reduced incidence and delayed progression of parkinsonism in old age. J Nutr Health Aging. (2018) 22:1211–5. doi: 10.1007/s12603-018-1094-5
24. Sääksjärvi, K, Knekt, P, Lundqvist, A, Männistö, S, Heliövaara, M, Rissanen, H, et al. A cohort study on diet and the risk of Parkinson's disease: the role of food groups and diet quality. Br J Nutr. (2013) 109:329–37. doi: 10.1017/S0007114512000955
25. Zhao, J, Peng, Y, Lin, Z, and Gong, Y. Association between Mediterranean diet adherence and Parkinson's disease: a systematic review and meta-analysis. J Nutr Health Aging. (2024) 29:100451. doi: 10.1016/j.jnha.2024.100451
26. Moher, D, Liberati, A, Tetzlaff, J, and Altman, DGPRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. (2009) 151:264–9. doi: 10.7326/0003-4819-151-4-200908180-00135
27. Stang, A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. (2010) 25:603–5. doi: 10.1007/s10654-010-9491-z
28. Zheng, PF, Shu, L, Si, CJ, Zhang, XY, Yu, XL, and Gao, W. Dietary patterns and chronic obstructive pulmonary disease: a Meta-analysis. COPD. (2016) 13:515–22. doi: 10.3109/15412555.2015.1098606
29. Grant, RL. Converting an odds ratio to a range of plausible relative risks for better communication of research findings. BMJ. (2014) 348:f7450. doi: 10.1136/bmj.f7450
30. Higgins, JP, Thompson, SG, Deeks, JJ, and Altman, DG. Measuring inconsistency in meta-analyses. BMJ. (2003) 327:557–60. doi: 10.1136/bmj.327.7414.557
31. Begg, CB, and Mazumdar, M. Operating characteristics of a rank correlation test for publication bias. Biometrics. (1994) 50:1088–101. doi: 10.2307/2533446
32. Duval, S, and Tweedie, R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. (2000) 56:455–63. doi: 10.1111/j.0006-341x.2000.00455.x
33. Greenland, S. Quantitative methods in the review of epidemiologic literature. Epidemiol Rev. (1987) 9:1–30. doi: 10.1093/oxfordjournals.epirev.a036298
34. Etminan, M, Gill, SS, and Samii, A. Intake of vitamin E, vitamin C, and carotenoids and the risk of Parkinson's disease: a meta-analysis. Lancet Neurol. (2005) 4:362–5. doi: 10.1016/S1474-4422(05)70097-1
35. Talebi, S, Ghoreishy, SM, Jayedi, A, Travica, N, and Mohammadi, H. Dietary antioxidants and risk of Parkinson's disease: a systematic review and dose-response Meta-analysis of observational studies. Adv Nutr. (2022) 13:1493–504. doi: 10.1093/advances/nmac001
36. Dexter, DT, and Jenner, P. Parkinson disease: from pathology to molecular disease mechanisms. Free Radic Biol Med. (2013) 62:132–44. doi: 10.1016/j.freeradbiomed.2013.01.018
37. Mirmiran, P, Hosseinpour-Niazi, S, and Azizi, F. Therapeutic lifestyle change diet enriched in legumes reduces oxidative stress in overweight type 2 diabetic patients: a crossover randomised clinical trial. Eur J Clin Nutr. (2018) 72:174–6. doi: 10.1038/ejcn.2017.113
38. Aborode, AT, Pustake, M, Awuah, WA, Alwerdani, M, Shah, P, Yarlagadda, R, et al. Targeting oxidative stress mechanisms to treat Alzheimer's and Parkinson's disease: a critical review. Oxidative Med Cell Longev. (2022) 2022:7934442. doi: 10.1155/2022/7934442
39. Solch, RJ, Aigbogun, JO, Voyiadjis, AG, Talkington, GM, Darensbourg, RM, O'Connell, S, et al. Mediterranean diet adherence, gut microbiota, and Alzheimer's or Parkinson's disease risk: a systematic review. J Neurol Sci. (2022) 434:120166. doi: 10.1016/j.jns.2022.120166
40. Yemula, N, Dietrich, C, Dostal, V, and Hornberger, M. Parkinson's disease and the gut: symptoms, nutrition, and microbiota. J Parkinsons Dis. (2021) 11:1491–505. doi: 10.3233/JPD-212707
41. Dalile, B, Van Oudenhove, L, Vervliet, B, and Verbeke, K. The role of short-chain fatty acids in microbiota-gut-brain communication. Nat Rev Gastroenterol Hepatol. (2019) 16:461–78. doi: 10.1038/s41575-019-0157-3
42. Ruiz-Ojeda, FJ, Plaza-Díaz, J, Sáez-Lara, MJ, and Gil, A. Effects of sweeteners on the gut microbiota: a review of experimental studies and clinical trials. Adv Nutr. (2019) 10:S31–48. doi: 10.1093/advances/nmy037
43. Hogg, E, Athreya, K, Basile, C, Tan, EE, Kaminski, J, and Tagliati, M. High prevalence of undiagnosed insulin resistance in non-diabetic subjects with Parkinson's disease. J Parkinsons Dis. (2018) 8:259–65. doi: 10.3233/JPD-181305
44. Jackson, A, Forsyth, CB, Shaikh, M, Voigt, RM, Engen, PA, Ramirez, V, et al. Diet in Parkinson's disease: critical role for the microbiome. Front Neurol. (2019) 10:1245. doi: 10.3389/fneur.2019.01245
45. Cheng, P, Yu, J, Huang, W, Bai, S, Zhu, X, Qi, Z, et al. Dietary intake of iron, zinc, copper, and risk of Parkinson's disease: a meta-analysis. Neurol Sci. (2015) 36:2269–75. doi: 10.1007/s10072-015-2349-0
46. Jiang, R, Ma, J, Ascherio, A, Stampfer, MJ, Willett, WC, and Hu, FB. Dietary iron intake and blood donations in relation to risk of type 2 diabetes in men: a prospective cohort study. Am J Clin Nutr. (2004) 79:70–5. doi: 10.1093/ajcn/79.1.70
Keywords: Parkinson’s disease, dietary patterns, systematic review, meta-analysis, observational studies
Citation: Zhang R, Shu L, Zhu Q and Li N (2025) Adherence to a priori and a posteriori dietary patterns and risk of Parkinson’s disease: a systematic review and meta-analysis of observational studies. Front. Nutr. 12:1600955. doi: 10.3389/fnut.2025.1600955
Edited by:
Andrei Surguchov, University of Kansas Medical Center, United StatesReviewed by:
Louise Hartley, RTI Health Solutions, United KingdomIrina G. Sourgoutcheva, University of Kansas Medical Center, United States
Copyright © 2025 Zhang, Shu, Zhu and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nan Li, bGluYW4yMDE2MDRAMTYzLmNvbQ==
 Rong Zhang1
Rong Zhang1 Long Shu
Long Shu Qin Zhu
Qin Zhu Nan Li
Nan Li