Abstract
Background:
Over the past 40 years, the global incidence of thyroid cancer has increased steadily. This study aimed to update the evaluation of thyroid cancer prevalence, incidence, mortality, and Disability–Adjusted Life Years (DALYs) rates from 1990 to 2021, with a focus on integrating prevalence data. Analyses were stratified by gender, age, and Socio–Demographic Index (SDI) at global, regional, and national levels.
Methods:
Data were obtained from the 2021 Global Burden of Disease, Injuries, and Risk Factors Study (GBD). The estimated annual percentage change (EAPC) was calculated to quantify temporal trends and evaluate age - standardized rates for prevalence (ASPR), incidence (ASIR), mortality (ASDR), and DALYs.
Results:
In 2021, the global thyroid cancer burden was substantial, with 1,987,148.5 cases. From 1990 to 2021, the ASPR increased from 14.9 (95% Uncertainty Interval [UI]: 14.1–16.0) to 23.1 (95% UI: 20.7–25.6) per 100,000 population, with an EAPC of 1.58 (95% UI: 1.44–1.73); the ASIR increased from 2.1 (95% UI: 2–2.2) to 2.9 (95% UI: 2.6–3.2) per 100,000 population, with an EAPC of 1.25 (95% UI: 1.14–1.37); the ASDR declined from 0.6 (95% UI: 0.5–0.6) to 0.5 (95% UI: 0.5–0.6) per 100,000 population, with an EAPC of −0.24 (95% UI: −0.24 – −0.21); the age - standardized DALY rate decreased from 15.2 (95% UI: 14.2–16.8) to 14.6 (95% UI: 12.8–16.1) per 100,000 population, with an EAPC of −0.14 (95% UI: −0.17 – –0.11). Western Sub–Saharan Africa had the lowest rates, while high–income North America had the highest ASPR and ASIR, and Andean Latin America had the highest ASDR. Higher SDI regions showed higher ASPR and ASIR, whereas lower SDI regions had higher ASDR. Saudi Arabia had the highest ASPR and ASIR, and Ethiopia had the highest ASDR and age–standardized DALY rate.
Conclusion:
From 1990 to 2021, the global health burden of thyroid cancer increased significantly, with marked geographical disparities. Prevention and control strategies should consider the unequal global distribution of the disease.
Introduction
In recent decades, thyroid cancer has emerged as a pressing global health concern, casting a wide-reaching shadow over public health (1). It affects individuals across all age spectrums, genders, and geographical boundaries (2). The disease’s impact is not only limited to the physical well-being of patients but also extends to their mental health, quality of life, and the economic burden on healthcare systems globally. From developed countries to developing nations, thyroid cancer cases are being diagnosed with increasing frequency, highlighting the need for a comprehensive understanding of its epidemiology (3).
Previous analyses using the Global Burden of Disease (GBD) database have provided valuable insights into the burden of thyroid cancer. The GBD study, which encompasses comprehensive epidemiological data from 204 countries and regions, has been instrumental in understanding the global trends of thyroid cancer incidence, prevalence, mortality, and disability-adjusted life years (DALYs) (4). For example, earlier GBD - based research has shown the increasing incidence of thyroid cancer in many parts of the world over the past few decades (5, 6). However, the complexity of the disease burden, especially in relation to factors such as the Socio - Demographic Index (SDI), age, and gender, still requires further in - depth exploration (7).
This current study builds upon previous GBD-related research and offers several distinct advantages. Firstly, it provides the most up-to-date assessment of thyroid cancer burden from 1990 to 2021, using the latest GBD 2021 results (8). This allows for a more accurate and timely understanding of the disease’s trends. Secondly, by stratifying the analysis by gender, 20 age groups, 21 GBD regions, 204 countries/territories, and 5 SDI categories, it offers a more comprehensive and detailed analysis of the factors associated with thyroid cancer burden (9). Thirdly, the study uses advanced statistical methods, such as the estimated annual percentage change (EAPC) and the Bayesian Age-Period-Cohort (BAPC) model (10, 11), to not only analyze the current situation but also predict future trends. This holistic approach can contribute to a more in - depth understanding of the epidemiology of thyroid cancer and provide a solid foundation for the development of targeted prevention and treatment strategies (12).
Methods
Data sources
The data in this study is derived from the GBD 2021 results (available at https://vizhub.healthdata.org/gbd-results/). The GBD 2021 study utilizes the latest epidemiological data and refined standardized methods to analyze age- and sex-specific mortality rates for 288 causes across 204 countries and regions, the prevalence and DALYs for 371 diseases and injuries, and the comparative risks for 88 risk factors (13). Detailed information on the study design and methodology of the GBD research has been extensively described in the existing GBD literature (4). Additionally, the Institutional Review Board at the University of Washington waived the informed consent requirement for accessing GBD data (14). All GBD 2021 analyses adhere to the guidelines for accurate and transparent health estimation reporting.
Estimation framework
The GBD study employs complex modeling techniques to estimate the burden of thyroid cancer. The DisMod-MR 2.1 (Disease Model-Bayesian Meta-Regression) tool was used to calculate incidence and prevalence. This Bayesian geospatial software integrates various disease parameters, epidemiological relationships, and geospatial data to generate reliable estimates. For mortality estimation, the Cause of Death Ensemble Modeling (CODEm) framework was applied. This method incorporates both vital registration and verbal autopsy data, including data with nonspecific codes. The data were rigorously adjusted to ensure accuracy prior to analysis. CODEm combines multiple models to estimate mortality rates with higher precision. These models were applied to the 2021 database to provide a comprehensive estimate of thyroid cancer burden. The method takes into account differences in the study design and methodology of multiple data sources, ensuring consistent and accurate estimates of thyroid cancer incidence, prevalence, and mortality. To calculate the DALYs attributable to thyroid cancer, we summarized two components: years lived with disability (YLD), which quantifies the burden of living with stroke-related impairments, and years of life lost (YLL), which measures the impact of premature death.
Sociodemographic index
SDI (Socio-Demographic Index) quantifies the level of development of a country or region using data on fertility rates, education levels, and per capita income. Ranging from 0 to 1, higher SDI values indicate faster socioeconomic development. SDI is known to be associated with disease incidence and mortality (4, 15). In this study, we categorized countries and regions into five SDI groups (low, low-middle, middle, middle-high, and high) to examine the relationship between the burden of thyroid cancer and socioeconomic development.
Statistical analyses
To assess the trends in age-standardized rates (ASR) of thyroid cancer incidence, mortality, DALY, and prevalence, the study employed the estimated annual percentage change (EAPC).
The ASR was computed per 100,000 individuals utilizing the subsequent formula:
(α i: the age-specific rate in ith the age group; w: the number of people in the corresponding ith age group among the standard population; A: the number of age groups).
Prior to applying linear regression, we systematically evaluated the appropriateness of modeling the natural logarithm of ASR (ln[ASR]) using both linear and nonlinear regression approaches. We calculated the estimated annual percentage change (EAPC) in ASR to evaluate the average changing trends over a specified time interval. To validate the linearity assumption, we performed the following analyses: 1. Visual inspection of scatter plots and residual plots to assess for non-linear patterns (e.g., curvature, heteroscedasticity). 2. Comparison of model fit using R2 values and Akaike Information Criterion (AIC) between the linear model (Y = α + βX + ε, where Y = ln[ASR] and X = calendar year) and a quadratic model (Y = α + β₁X + β₂X2 + ε) to capture potential non-linear trends. 3. Testing for autocorrelation in residuals using the Durbin-Watson test to ensure independence of observations.
Results of these analyses supported the linear model as the most appropriate: 1. The linear model exhibited higher R2 values compared to quadratic models (ΔR2 < 0.03 for all outcomes). 2. AIC values were lower for the linear model, indicating better goodness-of-fit. 3. Residual plots showed no systematic patterns, and the Durbin-Watson statistic (d ≈ 2) confirmed no significant autocorrelation.
Therefore, we assumed the natural logarithm of ASR fit the linear regressions model Y = α + βX + e, where Y refers to ln (ASR), and X is the calendar year:
We identified an ASR as indicative of an increasing or decreasing trend over time if both the EAPC and its 95% Confidence interval (CI) were above or below zero, respectively. In instances where the 95% CI encompassed zero, we deemed the change in ASR statistically insignificant.
Spearman correlation was used to assess the relationship between SDI and the age-standardized incidence rate of thyroid cancer. Bayesian age-period-cohort (BAPC) models, implemented with integrated nested Laplace approximation (INLA), were utilized to predict the future prevalence, incidence, mortality, and DALYs associated with thyroid cancer through 2040. Previous research has demonstrated that BAPC offers superior coverage and precision compared to alternative prediction methods (16–18). Our analysis was conducted using the ‘INLA’ and ‘BAPC’ R packages, in accordance with established protocols. Statistical analyses were performed using R software (version 4.4.2), with a significance threshold set at p < 0.05.
The detailed foundation and mathematical formulation of the BAPC model are provided in Supplementary Methods S1. Sensitivity analyses for the EAPC estimation, including overdispersion tests and linearity assessments, are described in Supplementary Methods S2.
Results
Global level
In 2021, the global burden of thyroid cancer remained substantial. There were a total of 1,987,148.5 cases (95% Uncertainty Interval [UI]: 1,776,275.3 - 2,198,245.2), representing an increase of 193.68% compared to 1990. Not only did the absolute number of cases increase significantly, but the age - standardized prevalence rate (ASPR) also rose from 14.9 cases per 100,000 population (95% UI: 14.1–16) in 1990 to 23.1 cases per 100,000 population (95% UI: 20.7–25.6) in 2021. The estimated annual percentage change (EAPC) of ASPR was 1.58 (95% CI: 1.44–1.73) (Table 1 and Figure 1). In 2021, the global incidence of thyroid cancer reached 249,538 cases (95% UI: 223,290.3–274,638.2), representing a 177.62% increase compared to 1990. The ASIR of thyroid cancer increased from 2 cases per 100,000 population (95% UI: 2–2.2) in 1990 to 2.9 cases per 100,000 population (95% UI: 2.6–3.2) in 2021. During the study period, The EAPC of ASIR was 1.25 (95% CI: 1.14–1.37), indicating a continuous upward trend in the incidence of thyroid cancer.(Table 1 and Figure 1). The mortality caused by thyroid cancer was estimated to be 44,798.5 (95% UI: 39,924.7 – 48,541). The ASDR was 0.5 per 100,000 population (95% UI: 0.5–0.6), and the EAPC was −0.24 (95% CI: −0.27 to −0.21, Table 1 and Figure 1). In 2021, the global disability - adjusted life years (DALYs) for thyroid cancer were 1,246,484.8 (95% UI: 1,094,415.6 - 1,375,852.5). The age - standardized DALY rate was 14.6 per 100,000 population (95% UI: 12.8–16.1), and the EAPC was - 0.14 (95% CI: –0.17 to – 0.11) (Table 1 and Figure 1).
Table 1
| Location | 1990 | 2021 | EAPC_95%CI | ||
|---|---|---|---|---|---|
| Number | ASR | Number | ASR | ||
| Prevalence | |||||
| Global | 676648.8 (636788.9–727722.8) | 14.9 (14.1–16) | 1987148.5 (1776275.3–2198245.2) | 23.1 (20.7–25.6) | 1.58 (1.44 to 1.73) |
| Andean Latin America | 2640.4 (2211.1–3138.8) | 9.8 (8.1–11.5) | 18047.3 (13837.2–22,793) | 28.1 (21.6–35.6) | 3.55 (3.34 to 3.77) |
| Australasia | 4599.9 (4076.9–5210.9) | 20.6 (18.3–23.3) | 16211.2 (13090.8–19620.8) | 38.9 (31.4–47.6) | 2.87 (2.29 to 3.47) |
| Caribbean | 3118.1 (2873.6–3378.8) | 10.6 (9.8–11.5) | 9357.3 (8102.5–10834.1) | 17.8 (15.4–20.6) | 1.86 (1.69 to 2.04) |
| Central Asia | 6945.8 (6347.8–7593.4) | 12.7 (11.6–13.9) | 13021.3 (11400.3–14782.4) | 13.4 (11.7–15.2) | 0.16 (−0.61 to 0.93) |
| Central Europe | 34174.4 (32484.3–36024.9) | 23.6 (22.4–24.8) | 37892.2 (34073.8–41467.7) | 21.9 (19.7–24) | −0.33 (−0.52 to −0.14) |
| Central Latin America | 11472.2 (11049.9–11916.4) | 10.2 (9.8–10.6) | 58,071 (51803–65647.6) | 21.8 (19.4–24.6) | 2.39 (2.28 to 2.5) |
| Central Sub-Saharan Africa | 870.5 (619–1301.5) | 2.6 (1.8–3.9) | 3,432 (2194.6–5420.7) | 4 (2.5–6.3) | 1.42 (1.14 to 1.7) |
| East Asia | 95529.4 (76898.9–113229.6) | 8.5 (6.9–10.1) | 411402.4 (334530–513557.1) | 20.5 (16.8–25.6) | 3.16 (3 to 3.33) |
| Eastern Europe | 50163.3 (47847.6–52997.6) | 18.9 (18.1–20) | 75906.4 (68504.6–84343.5) | 25.9 (23.3–28.8) | 1.47 (0.97 to 1.97) |
| Eastern Sub-Saharan Africa | 12,047 (9301.5–15328.6) | 9.4 (7.3–12) | 48,137 (34449.7–73226.7) | 15.4 (11.2–23.1) | 1.47 (1.29 to 1.66) |
| High SDI | 288686.4 (280129.1–297,109) | 28.5 (27.6–29.3) | 598,370 (566881.9–629719.5) | 38.2 (36.3–40.4) | 1.23 (0.93 to 1.53) |
| High-income Asia Pacific | 55344.6 (51670.9–60632.8) | 26.9 (25.1–29.6) | 110959.1 (98931.9–129334.7) | 37.1 (33.2–43.8) | 1.42 (0.91 to 1.93) |
| High-income North America | 100673.1 (97445.8–103,313) | 32.1 (31.1–32.9) | 237,732 (226609–247666.5) | 45.5 (43.6–47.3) | 1.23 (1.05 to 1.42) |
| High-middle SDI | 186,568 (174097.6–198522.8) | 17.6 (16.4–18.7) | 448569.8 (401405.4–510752.5) | 25.2 (22.6–28.8) | 1.4 (1.22 to 1.58) |
| Low SDI | 21971.9 (17309–27986.9) | 6.2 (4.9–7.9) | 92787.3 (71789.9–126,590) | 11.1 (8.7–14.9) | 1.87 (1.76 to 1.99) |
| Low-middle SDI | 56029.2 (47678.1–71121.3) | 6.3 (5.3–7.9) | 251682.8 (206843.3–308118.1) | 14 (11.5–16.9) | 2.67 (2.64 to 2.7) |
| Middle SDI | 122505.4 (108257.6–142672.6) | 8.6 (7.7–10.1) | 594136.2 (493696.7–673,492) | 21 (17.5–23.8) | 3.03 (2.95 to 3.12) |
| North Africa and Middle East | 31,166 (25920.4–41,990) | 13 (10.8–17.4) | 183491.2 (151632.4–216130.2) | 30.7 (25.4–36) | 3.16 (2.99 to 3.33) |
| Oceania | 308.3 (199–418.5) | 6.8 (4.5–9.3) | 960.2 (576–1382.1) | 8.6 (5.2–12.3) | 0.59 (0.48 to 0.7) |
| South Asia | 53580.2 (43818.6–70783.2) | 5.9 (4.9–7.8) | 282509.1 (227052.9–343508.3) | 15.3 (12.3–18.5) | 3.24 (3.16 to 3.33) |
| Southeast Asia | 45,506 (36198.2–51840.3) | 12.7 (10.3–14.5) | 206164.7 (161849.8–244210.6) | 26.9 (21.1–31.8) | 2.33 (2.23 to 2.43) |
| Southern Latin America | 6,690 (5949.1–7523.9) | 14.1 (12.6–15.9) | 15500.3 (13535.1–17754.5) | 19.7 (17.1–22.6) | 1.21 (1 to 1.42) |
| Southern Sub-Saharan Africa | 2655.2 (2243.8–3,153) | 6.9 (5.8–8.2) | 8000.6 (6622.7–9541.6) | 10.7 (8.9–12.7) | 1.8 (1.54 to 2.05) |
| Tropical Latin America | 9237.1 (8742.4–9743.8) | 7.8 (7.4–8.2) | 33082.7 (31066.3–34983.6) | 12.6 (11.8–13.3) | 1.32 (1.13 to 1.51) |
| Western Europe | 148253.3 (140945–155876.6) | 31.1 (29.5–32.7) | 211123.9 (193366–229245.3) | 32.7 (29.9–35.5) | 0.51 (0.09 to 0.94) |
| Western Sub-Saharan Africa | 1,674 (1212.8–2,106) | 1.2 (0.9–1.5) | 6146.3 (4496.6–8323.4) | 1.8 (1.3–2.3) | 1.09 (1 to 1.18) |
| Incidence | |||||
| Global | 89885.5 (84681.3–96998.8) | 2.1 (2–2.2) | 249,538 (223290.3–274638.2) | 2.9 (2.6–3.2) | 1.25 (1.14 to 1.37) |
| Andean Latin America | 422.2 (354–495.4) | 1.8 (1.5–2.1) | 2,424 (1907.2–3044.3) | 3.9 (3.1–4.8) | 2.6 (2.44 to 2.77) |
| Australasia | 585.9 (522.4–660.1) | 2.6 (2.3–2.9) | 1949.1 (1569.7–2343.2) | 4.6 (3.7–5.5) | 2.61 (2.06 to 3.16) |
| Caribbean | 442.8 (410.8–479.3) | 1.6 (1.5–1.7) | 1244.9 (1085.7–1427.4) | 2.4 (2.1–2.7) | 1.5 (1.32 to 1.69) |
| Central Asia | 914 (840.8–996.1) | 1.7 (1.6–1.9) | 1630.9 (1432.2–1845.3) | 1.7 (1.5–2) | 0.01 (−0.73 to 0.75) |
| Central Europe | 4654.9 (4428.6–4883.6) | 3.2 (3–3.4) | 4876.2 (4406.6–5323.5) | 2.7 (2.4–2.9) | −0.65 (−0.86 to −0.45) |
| Central Latin America | 1712.2 (1651.2–1775.4) | 1.7 (1.7–1.8) | 7752.6 (6907.4–8701.4) | 3 (2.6–3.3) | 1.66 (1.52 to 1.8) |
| Central Sub-Saharan Africa | 153.7 (111.5–225.9) | 0.6 (0.4–0.8) | 495.7 (319.2–770.7) | 0.7 (0.4–1.1) | 0.63 (0.42 to 0.83) |
| East Asia | 13203.4 (10809.5–15460.8) | 1.3 (1.1–1.5) | 50885.2 (41562–63161.9) | 2.5 (2.1–3.1) | 2.43 (2.26 to 2.6) |
| Eastern Europe | 6467.8 (6164.2–6,831) | 2.4 (2.3–2.6) | 9,617 (8698.1–10650.3) | 3.2 (2.9–3.5) | 1.27 (0.8 to 1.75) |
| Eastern Sub-Saharan Africa | 1908.4 (1516.6–2387.8) | 1.8 (1.5–2.2) | 6384.4 (4629.9–9478.7) | 2.4 (1.8–3.5) | 0.76 (0.6 to 0.92) |
| High SDI | 36533.2 (35292–37708.3) | 3.5 (3.4–3.7) | 72995.8 (68514–76746.9) | 4.5 (4.3–4.7) | 1.01 (0.75 to 1.28) |
| High-income Asia Pacific | 6950.1 (6496.3–7654.3) | 3.4 (3.2–3.8) | 14277.6 (12630.3–16476.5) | 4.4 (3.9–5.2) | 1.15 (0.68 to 1.61) |
| High-income North America | 12130.3 (11695.2–12,450) | 3.8 (3.7–3.9) | 28289.1 (26782.7–29,536) | 5.3 (5.1–5.5) | 1.15 (0.97 to 1.33) |
| High-middle SDI | 24410.6 (22753.3–25919.8) | 2.3 (2.2–2.5) | 55158.1 (49518.3–62,489) | 3.1 (2.8–3.5) | 1.05 (0.89 to 1.21) |
| Low SDI | 3431.3 (2759.4–4295.6) | 1.1 (0.9–1.4) | 12358.4 (9598.7–16514.5) | 1.7 (1.3–2.2) | 1.23 (1.12 to 1.34) |
| Low-middle SDI | 8233.9 (7035–10302.4) | 1 (0.9–1.3) | 33,464 (27896.3–40292.7) | 2 (1.7–2.3) | 2.09 (2.07 to 2.12) |
| Middle SDI | 17155.2 (15282.6–19997.3) | 1.4 (1.2–1.6) | 75356.9 (62756–84674.8) | 2.7 (2.3–3) | 2.37 (2.28 to 2.46) |
| North Africa and Middle East | 3,792 (3150.6–5143.1) | 1.7 (1.4–2.3) | 21222.4 (17602–24974.6) | 3.7 (3.1–4.3) | 2.89 (2.72 to 3.06) |
| Oceania | 44.6 (29.9–60.2) | 1.2 (0.8–1.6) | 131.1 (80.2–185.4) | 1.4 (0.9–1.9) | 0.33 (0.24 to 0.42) |
| South Asia | 7853.1 (6476.2–10284.5) | 1 (0.8–1.3) | 37335.6 (30262.7–44931.4) | 2.1 (1.7–2.6) | 2.54 (2.46 to 2.62) |
| Southeast Asia | 6440.6 (5205.8–7276.5) | 2 (1.7–2.3) | 26,559 (20898.8–31183.5) | 3.6 (2.9–4.2) | 1.82 (1.74 to 1.9) |
| Southern Latin America | 1000.1 (896.4–1118.9) | 2.1 (1.9–2.4) | 2046.9 (1787.2–2,334) | 2.5 (2.2–2.9) | 0.7 (0.48 to 0.92) |
| Southern Sub-Saharan Africa | 376.9 (317.8–447.9) | 1.1 (0.9–1.3) | 1116.8 (934.8–1315.9) | 1.6 (1.3–1.9) | 1.54 (1.3 to 1.79) |
| Tropical Latin America | 1371.6 (1299.4–1443.4) | 1.3 (1.2–1.3) | 4491.1 (4197.7–4757.9) | 1.7 (1.6–1.8) | 0.76 (0.6 to 0.91) |
| Western Europe | 19206.5 (18291.7–20159.2) | 3.9 (3.7–4.1) | 26004.5 (23787.6–28201.5) | 3.8 (3.5–4.2) | 0.28 (−0.1 to 0.67) |
| Western Sub-Saharan Africa | 254.3 (186.2–312.4) | 0.2 (0.2–0.3) | 803.8 (597.7–1068.9) | 0.3 (0.2–0.3) | 0.52 (0.45 to 0.58) |
| Deaths | |||||
| Global | 21,893 (20437.5–24108.1) | 0.6 (0.5–0.6) | 44798.5 (39924.7–48,541) | 0.5 (0.5–0.6) | −0.24 (−0.27 to −0.21) |
| Andean Latin America | 182.3 (153.4–211.5) | 0.9 (0.8–1) | 641.2 (507.9–794.5) | 1.1 (0.9–1.4) | 0.66 (0.54 to 0.77) |
| Australasia | 97.6 (87.2–109.1) | 0.4 (0.4–0.5) | 199.1 (161.3–237.4) | 0.4 (0.3–0.4) | 0.14 (−0.13 to 0.41) |
| Caribbean | 142.5 (131.7–156) | 0.6 (0.5–0.6) | 321.3 (280.7–367.5) | 0.6 (0.5–0.7) | 0.42 (0.2 to 0.65) |
| Central Asia | 253.3 (235.5–274.3) | 0.5 (0.5–0.6) | 339.3 (301.7–378.5) | 0.4 (0.4–0.5) | −0.81 (−1.43 to −0.18) |
| Central Europe | 1266.4 (1213.2–1316.6) | 0.9 (0.8–0.9) | 985.7 (898–1064.6) | 0.4 (0.4–0.5) | −2.37 (−2.65 to −2.1) |
| Central Latin America | 635.6 (613.9–657.6) | 0.8 (0.8–0.8) | 1964.6 (1748.8–2165.4) | 0.8 (0.7–0.9) | −0.07 (−0.26 to 0.11) |
| Central Sub-Saharan Africa | 78.4 (57.7–112.8) | 0.4 (0.3–0.5) | 182.8 (118.4–284) | 0.3 (0.2–0.6) | −0.11 (−0.23 to 0.01) |
| East Asia | 3780.9 (3211.5–4380.1) | 0.5 (0.4–0.6) | 8063.8 (6456.3–9800.1) | 0.4 (0.3–0.5) | −0.65 (−0.74 to −0.55) |
| Eastern Europe | 1349.5 (1274–1423.5) | 0.5 (0.5–0.5) | 1650.5 (1508.2–1807.4) | 0.5 (0.4–0.5) | −0.25 (−0.6 to 0.09) |
| Eastern Sub-Saharan Africa | 838.7 (685.7–1015.1) | 1 (0.8–1.2) | 1800.9 (1337.6–2485.5) | 1 (0.7–1.3) | −0.18 (−0.29 to −0.07) |
| High SDI | 6504.9 (6098.3–6798.4) | 0.6 (0.5–0.6) | 9730.2 (8465.5–10437.2) | 0.4 (0.4–0.5) | −0.91 (−0.96 to −0.86) |
| High-income Asia Pacific | 1235.9 (1126.1–1404.5) | 0.6 (0.6–0.7) | 2843.9 (2308.9–3185.8) | 0.5 (0.4–0.6) | −0.82 (−0.98 to −0.65) |
| High-income North America | 1391.8 (1285–1450.1) | 0.4 (0.4–0.4) | 2765.7 (2473–2948.9) | 0.4 (0.4–0.4) | 0.15 (0.06 to 0.23) |
| High-middle SDI | 5609.4 (5200.9–5934.9) | 0.6 (0.5–0.6) | 8436.7 (7553.5–9304.5) | 0.4 (0.4–0.5) | −1.03 (−1.09 to −0.97) |
| Low SDI | 1454.5 (1199.2–1778.3) | 0.6 (0.5–0.7) | 3392.4 (2687.9–4328.7) | 0.6 (0.5–0.8) | 0.19 (0.11 to 0.28) |
| Low-middle SDI | 3015.5 (2624–3757.1) | 0.5 (0.4–0.6) | 8531.2 (7410.5–9771.6) | 0.6 (0.5–0.7) | 0.74 (0.71 to 0.78) |
| Middle SDI | 5275.6 (4815.1–6144.8) | 0.5 (0.5–0.6) | 14666.5 (12466.8–16170.9) | 0.6 (0.5–0.6) | 0.21 (0.16 to 0.26) |
| North Africa and Middle East | 657.9 (546.1–936.5) | 0.4 (0.3–0.6) | 1935.2 (1676.1–2236.4) | 0.4 (0.4–0.5) | 0.64 (0.49 to 0.79) |
| Oceania | 14.6 (10.3–19.3) | 0.6 (0.4–0.7) | 36.6 (23.3–50.4) | 0.5 (0.4–0.7) | −0.13 (−0.16 to −0.1) |
| South Asia | 2914.5 (2454.1–3700.4) | 0.5 (0.4–0.6) | 9323.8 (7794.2–10741.5) | 0.6 (0.5–0.7) | 0.92 (0.88 to 0.97) |
| Southeast Asia | 2008.1 (1698.1–2303.1) | 0.8 (0.7–1) | 5642.9 (4564.6–6450.7) | 0.9 (0.7–1) | 0.32 (0.24 to 0.4) |
| Southern Latin America | 359 (324.7–394.6) | 0.8 (0.7–0.9) | 485.2 (425.8–553.7) | 0.6 (0.5–0.6) | −1.01 (−1.26 to −0.77) |
| Southern Sub-Saharan Africa | 123.6 (102.4–148.3) | 0.5 (0.4–0.6) | 323.8 (264.5–372.4) | 0.6 (0.5–0.7) | 0.92 (0.65 to 1.18) |
| Tropical Latin America | 509 (480–537.2) | 0.6 (0.5–0.6) | 1252.8 (1142–1,332) | 0.5 (0.5–0.5) | −0.6 (−0.69 to −0.5) |
| Western Europe | 3,951 (3667.4–4173.8) | 0.7 (0.6–0.7) | 3829.2 (3318.9–4190.8) | 0.4 (0.3–0.4) | −1.7 (−1.77 to −1.64) |
| Western Sub-Saharan Africa | 102.7 (77.8–122.8) | 0.1 (0.1–0.1) | 210.4 (165.3–263.6) | 0.1 (0.1–0.1) | −0.51 (−0.59 to −0.43) |
| Disability-adjusted life years | |||||
| Global | 646740.5 (599118.8–717,357) | 15.2 (14.2–16.8) | 1246484.8 (1094415.6–1375852.5) | 14.6 (12.8–16.1) | −0.14 (−0.17 to −0.11) |
| Andean Latin America | 5273.8 (4419.1–6186.5) | 23 (19.3–27) | 16689.8 (13207.7–20766.9) | 27.5 (21.7–34.3) | 0.54 (0.43 to 0.65) |
| Australasia | 2,544 (2273.1–2867.8) | 11 (9.9–12.5) | 5041.1 (4103.8–6033.3) | 10.6 (8.7–12.8) | 0.51 (0.21 to 0.82) |
| Caribbean | 4116.6 (3769–4556.3) | 14.9 (13.7–16.5) | 8690.2 (7532.9–10099.5) | 16.3 (14.1–19) | 0.46 (0.25 to 0.68) |
| Central Asia | 8144.5 (7568–8817.1) | 15.9 (14.7–17.2) | 10272.9 (9055.5–11543.5) | 11.7 (10.3–13) | −1.19 (−1.81 to −0.57) |
| Central Europe | 34786.3 (33224.2–36474.4) | 23.5 (22.4–24.6) | 23433.7 (21250.4–25475.1) | 11.6 (10.5–12.7) | −2.47 (−2.76 to −2.17) |
| Central Latin America | 18293.1 (17666.1–18920.9) | 19.5 (18.9–20.2) | 52186.9 (47013.3–58083.4) | 20.4 (18.4–22.7) | 0 (−0.2 to 0.2) |
| Central Sub-Saharan Africa | 2545.3 (1889.3–3609.5) | 9.4 (6.9–13.7) | 5956.3 (3862.9–9187.8) | 8.9 (5.8–13.9) | −0.19 (−0.31 to −0.06) |
| East Asia | 116582.2 (98062.3–136720.9) | 12.2 (10.4–14.2) | 213609.2 (173066.2–262361.7) | 10.3 (8.3–12.5) | −0.56 (−0.67 to −0.46) |
| Eastern Europe | 37,930 (35923.3–40329.4) | 13.9 (13.1–14.7) | 42085.6 (38218.3–46388.2) | 12.9 (11.7–14.1) | −0.29 (−0.68 to 0.11) |
| Eastern Sub-Saharan Africa | 31,046 (25044.5–38047.3) | 30.1 (24.5–36.7) | 66490.9 (48520.2–94804.4) | 27.8 (20.6–38.4) | −0.43 (−0.55 to −0.32) |
| High SDI | 164381.5 (155870.6–173717.9) | 15.4 (14.6–16.3) | 220135.9 (201450.2–237834.8) | 11.8 (10.8–12.8) | −0.77 (−0.86 to −0.68) |
| High-income Asia Pacific | 30855.9 (28505.4–35131.3) | 15.4 (14.1–17.5) | 50783.2 (43704.3–57571.4) | 11.8 (10.5–13.7) | −0.75 (−0.99 to −0.5) |
| High-income North America | 37,316 (34968.1–39721.4) | 11.2 (10.5–12) | 70641.6 (65003.7–76456.6) | 12 (11–13) | 0.21 (0.11 to 0.31) |
| High-middle SDI | 158942.1 (146716.9–170152.9) | 15.6 (14.4–16.6) | 218961.3 (196634.7–244418.1) | 11.6 (10.4–13) | −0.99 (−1.05 to −0.92) |
| Low SDI | 52661.5 (43472.1–63947.7) | 17.8 (14.6–21.6) | 120143.3 (93672.8–157131.4) | 18 (14.2–23.1) | −0.05 (−0.12 to 0.02) |
| Low-middle SDI | 103647.8 (90342.8–129887.4) | 13.8 (12–17.1) | 269914.2 (229089.1–316826.3) | 16.8 (14.3–19.4) | 0.67 (0.63 to 0.7) |
| Middle SDI | 166,177 (149966.3–190691.4) | 14 (12.8–16.2) | 416217.6 (350751–460888.7) | 15.2 (12.8–16.8) | 0.28 (0.22 to 0.34) |
| North Africa and Middle East | 21944.4 (18189.4–30745.7) | 11 (9.1–15.5) | 65316.2 (55454.2–76496.3) | 12.7 (10.9–14.8) | 0.76 (0.62 to 0.9) |
| Oceania | 480.3 (324.9–641.5) | 13.9 (9.8–18.5) | 1173.6 (724.9–1641.6) | 13.5 (8.6–18.6) | −0.1 (−0.14 to −0.06) |
| South Asia | 105302.9 (88400.4–135793.4) | 14.2 (12–18.2) | 302256.8 (249827.8–356789.4) | 18.2 (15.1–21.4) | 0.85 (0.81 to 0.88) |
| Southeast Asia | 62694.8 (51465.3–70374.5) | 21.4 (17.9–24.3) | 164547.4 (130332.7–189174.4) | 23.6 (18.8–27) | 0.27 (0.21 to 0.34) |
| Southern Latin America | 9491.8 (8618.3–10469.6) | 20.3 (18.4–22.3) | 11959.8 (10465.3–13594.3) | 14.3 (12.5–16.2) | −1.02 (−1.28 to −0.75) |
| Southern Sub-Saharan Africa | 4035.3 (3398.8–4776.4) | 12.4 (10.3–14.8) | 10253.2 (8481–11,926) | 15.7 (12.8–18.1) | 1 (0.72 to 1.27) |
| Tropical Latin America | 15021.1 (14259–15822.7) | 14.8 (14–15.6) | 32680.5 (30547–34741.4) | 12.7 (11.8–13.5) | −0.65 (−0.75 to −0.55) |
| Western Europe | 94707.5 (89027.2–100543.1) | 17.6 (16.6–18.8) | 84464.5 (75823.1–92550.5) | 10.5 (9.4–11.5) | −1.5 (−1.64 to −1.36) |
| Western Sub-Saharan Africa | 3628.7 (2761.6–4379.4) | 3.2 (2.4–3.8) | 7951.2 (6109.3–10,289) | 2.8 (2.2–3.5) | −0.49 (−0.56 to −0.42) |
The prevalence, incidence, mortality and DALYs of thyroid cancer in 1990 and 2021, and changes from 1990 to 2021 at the global level and different regions.
Figure 1
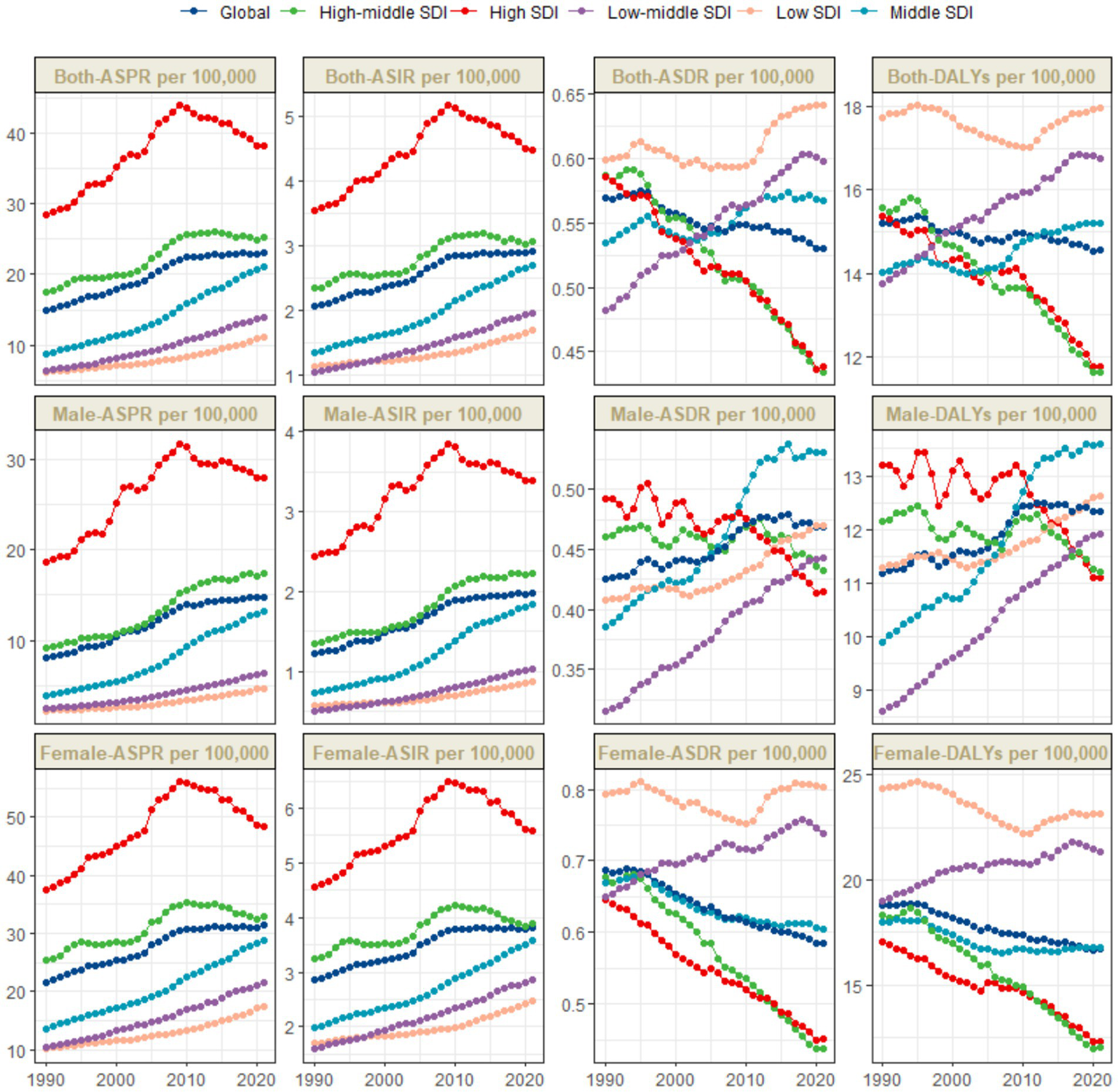
Trends in thyroid cancer prevalence, incidence, mortality and DALYs from 1990 to 2021.
Regional level
The global burden of thyroid cancer exhibits remarkable regional disparities, which are closely associated with the Socio–demographic Index (SDI) levels. The ASPR shows significant differences. The incidence is highest in high - SDI regions, reaching 38.2 per 100,000 population (95% UI: 36.3–40.4), while the lowest incidence is reported in low–SDI regions, at 11.1 per 100,000 population (95% UI: 8.7–14.9) (Table 1 and Figure 1).
The temporal trends of the ASPR and ASIR reveal distinct patterns across different SDI levels. Specifically, in high–SDI regions, there are two distinct phases. From 1990 to 2009, there was a marked upward trend, followed by a significant downward trend. The overall EAPC was 1.23 (95% CI: 0.93–1.53). In these regions, the ASPR gradually increased from 28.5 per 100,000 population (95% UI: 27.6–29.3) in 1990 to 44 per 100,000 population (95% UI: 42–46.3) in 2009, and then decreased to 38.2 per 100,000 population (95% UI: 36.3–40.4) in 2021. The ASIR increased from 3.5 per 100,000 population (95% UI: 3.4–3.7) in 1990 to 5.2 per 100,000 population (95% UI: 4.9–5.4) in 2009, and then declined to 4.5 per 100,000 population (95% UI: 4.3–4.7) in 2021 (Table 1 and Figure 1). In contrast, in other SDI regions, there has been a long–term upward trend. The middle - SDI regions had the highest growth rate, with an EAPC of 3.03 (95% CI: 2.95–3.12) (Table 1 and Figure 1).
In these regions, there is no significant difference in the ASDR. Among them, the low SDI, low - middle SDI, and middle SDI regions report relatively higher ASDRs, with the lowest SDI region having the highest. Meanwhile, the high SDI and upper–middle SDI regions have the lowest values for this indicator. Specifically, the ASDR in the low SDI region is 0.6 per 100,000 population (95% UI: 0.5–0.8), the ASDR in the low - middle SDI region is 0.6 per 100,000 population (95% UI: 0.5–0.7), and the ASDR in the middle SDI region is 0.6 per 100,000 population (95% UI: 0.5–0.6), while the ASIR in the high SDI and upper–middle SDI regions is 0.4 per 100,000 population (95% UI: 0.4–0.5) (Table 1 and Figures 1, 2C).
Figure 2
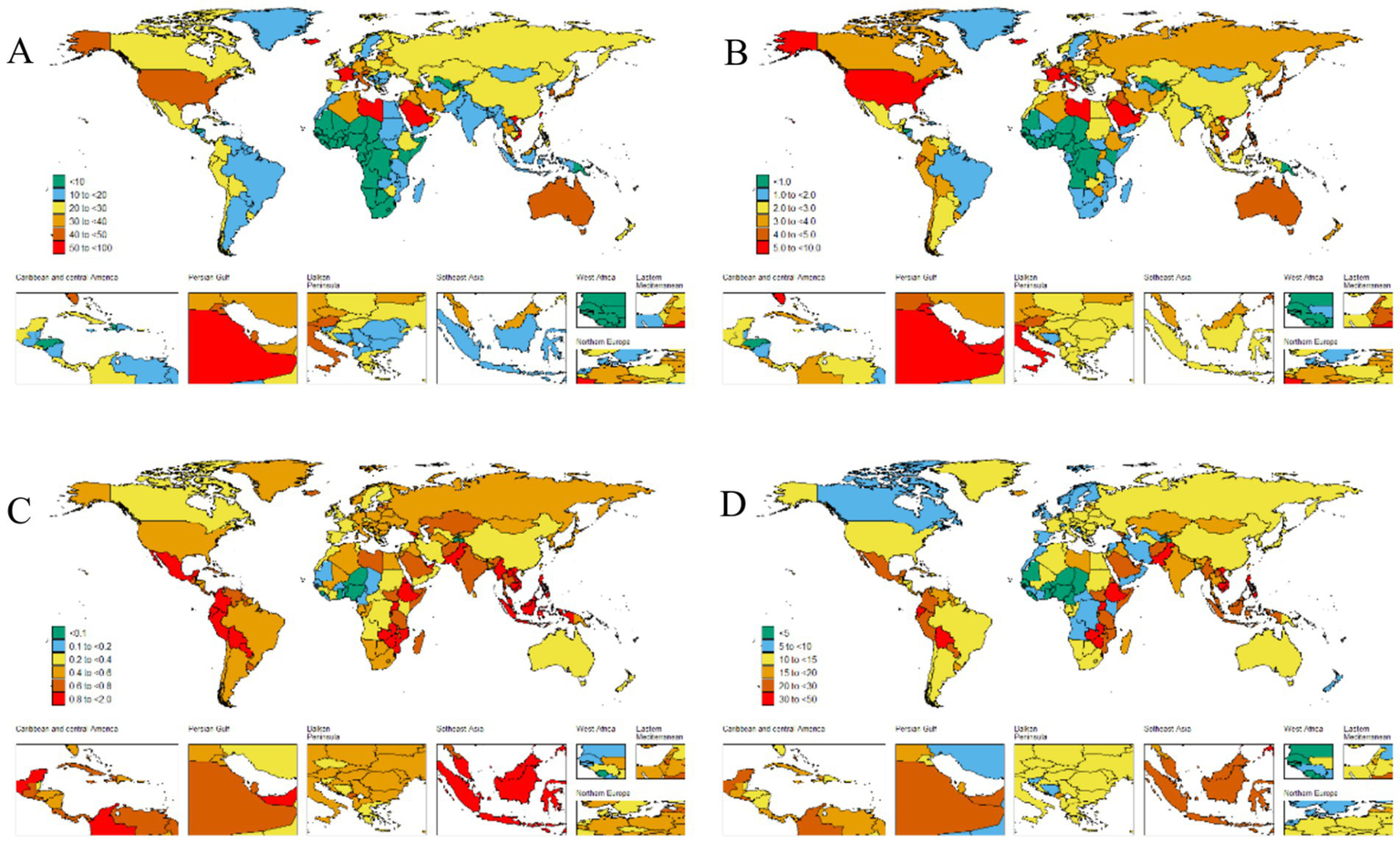
The global disease burden of thyroid cancer for both sexes in 204 countries and territories. (A) Prevalence rate. (B) Incidence rate. (C) Death rate. (D) DALYs rate.
The disease burden quantified by the age - standardized DALY rate further emphasizes these regional differences. The burden is highest in the low SDI region, with a DALY rate of 18 per 100,000 population (95% UI: 14.2–23.1), while it is lowest in the upper - middle SDI region, at 11.6 per 100,000 population (95% UI: 10.4–13) (Table 1 and Figures 1, 2D).
Our research findings indicate that the High-income North America bear the highest burden of thyroid cancer prevalence globally. Specifically, the ASPR in the High-income North America is the highest, reaching 45.5 per 100,000 population (95% UI: 43.6–47.3), followed closely by Australasia, with 38.9 per 100,000 population (95% UI: 31.4–47.6) (Table 1 and Figure 2A). The High-income Asia Pacific also shows a relatively high ASPR, with 37.1 per 100,000 population (95% UI: 33.2–43.8), ranking third among the studied regions.
Our analysis shows that the incidence of thyroid cancer in South America and Europe warrants high attention. The research results indicate that the Andean Latin America and Western Europe are among the regions with relatively high ASIR globally. Specifically, the ASIR in the regions along the Andean Latin America is 3.9 per 100,000 population (95% UI: 3.1–4.8), and the ASIR in Western Europe is 3.8 per 100,000 population (95% UI: 3.5–4.2). In contrast, the ASIR in the Western Sub-Saharan Africa is the lowest, at 0.3 per 100,000 population (95% UI: 0.2–0.3) (Table 1 and Figure 2B).
In addition, we found that the ASDR of thyroid cancer in the Eastern Sub-Saharan Africa was significantly higher, at 1 per 100,000 population (95% UI: 0.7–1.3). From 1990 to 2021, the thyroid cancer ASDR increased the most in the Southern Sub-Saharan Africa (EAPC 0.92, 95% CI: 0.65–1.18) and decreased the most in Central Europe (EAPC –2.37, 95% CI: −2.65 to −2.1) (Table 1). Conversely, the region with the highest age - standardized DALYs rate was the Eastern Sub-Saharan Africa, at 27.8 per 100,000 population (95% UI: 20.6–38.4). From 1990 to 2021, the age - standardized DALYs incidence of thyroid cancer increased the most in Southern Sub-Saharan Africa (EAPC 1, 95% CI: 0.72 to 1.27) and decreased the most in Central Europe (EAPC -2.47, 95% CI: −2.76 to −2.17) (Table 1).
At the regional level, we found an association between the SDI and the age–standardized DALYs rates of thyroid cancer from 1990 to 2021. The age–standardized DALYs rates decreased exponentially with the increase of the SDI, reaching a minimum of approximately 0.36, then increased to a maximum of 0.6, and then decreased again. From 1990 to 2021, the DALYs rates in the Eastern Sub-Saharan Africa, South Asia, Andean Latin America, and Central Latin America were higher than expected based on their SDI. In contrast, the burden in regions such as Central Sub - Saharan Africa, East Asia, North Africa and the Middle East, Tropical Latin America, High - income North America, Central Asia, and Central Latin America was lower than expected from 1990 to 2021 (Figure 3).
Figure 3
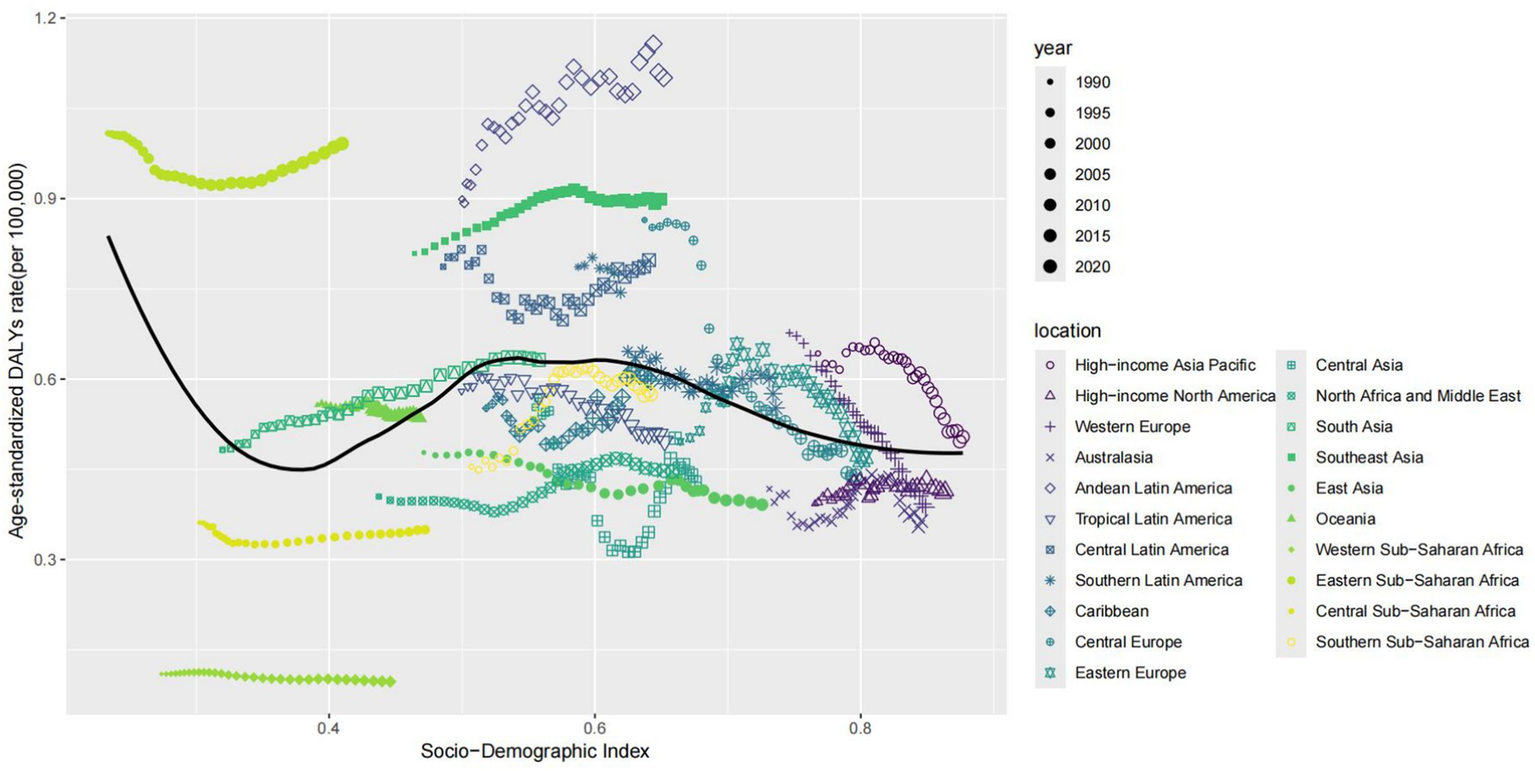
Association between age-standardized thyroid cancer DALYs rate and socio-demographic index in 21 regions.
It is also noteworthy that in regions with a higher SDI, the proportion of thyroid cancer cases among the elderly is lower. In 1990 and 2021, the proportion of thyroid cancer cases among the young is higher in regions with a higher SDI. From 1990 to 2021, the incidence of thyroid cancer first increased gradually with age, particularly among the young population, and then decreased gradually. Across all SDI regions, the incidence of thyroid cancer in the young population is significantly higher than that in the elderly (Figures 4, 5).
Figure 4

The age-specific numbers and ASIRs of thyroid cancer by SDI regions in 2021.
Figure 5
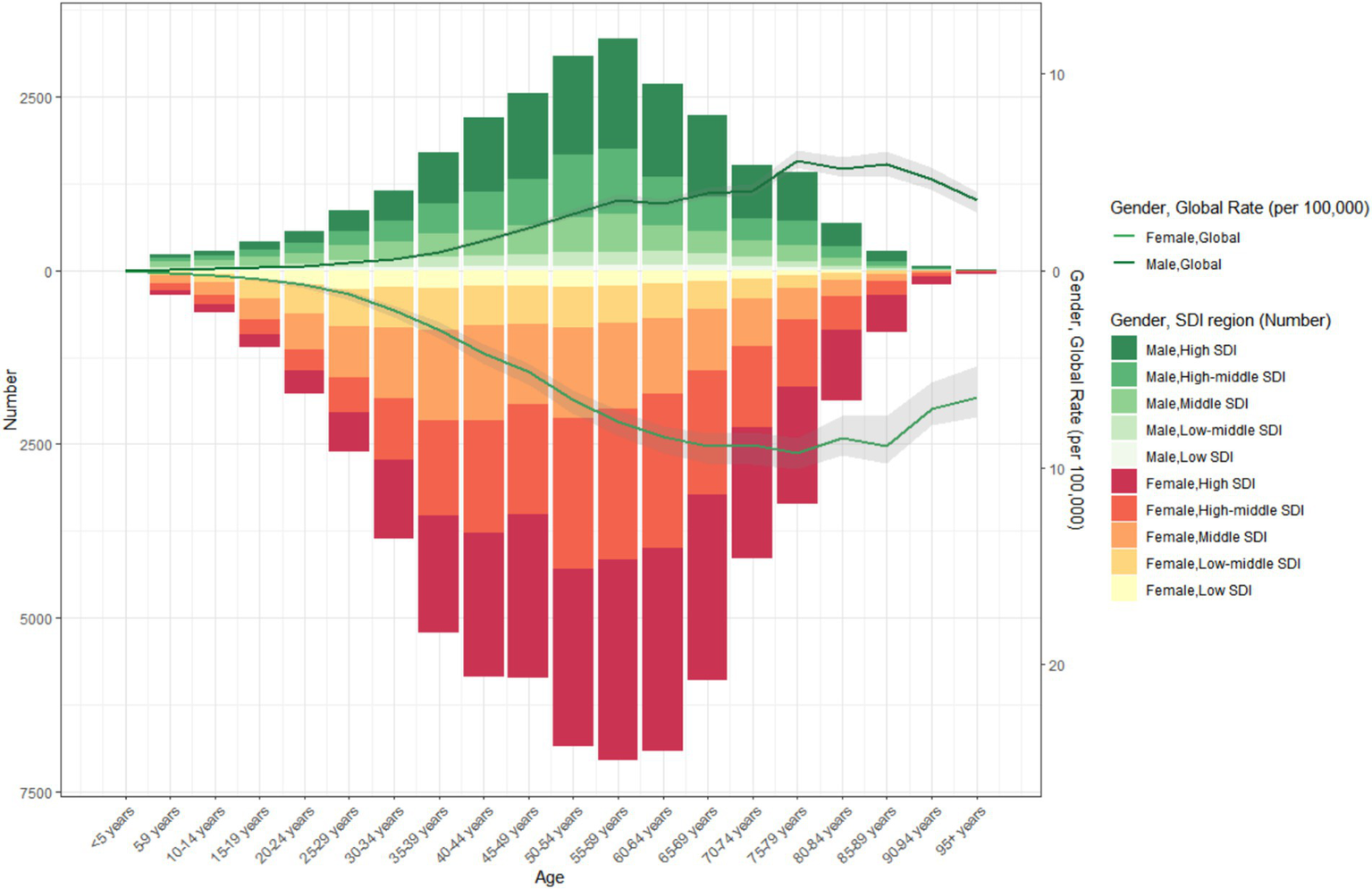
The age-specific numbers and ASIRs of thyroid cancer by SDI regions in 1990.
National level
The ASPR of thyroid cancer varies from approximately 0.1 to 60.1 per 100,000 population. Among all countries, Saudi Arabia (60.1 per 100,000 population; 95% UI: 44.7–79.4), France (58.8 per 100,000 population; 95% UI: 48.4–70.9), Iceland (55.2 per 100,000 population; 95% UI: 45–68.6), and Libya (51.1 per 100,000 population; 95% UI: 35.5–70.9) exhibited the highest ASPRs (Figure 2A and Supplementary Table S1). The most significant increase in the ASPR of thyroid cancer was observed in Cabo Verde (EAPC: 6.05, 95% CI 5.07–7.04) (Supplementary Table S1).
The country–specific distribution of the ASIR is presented in Figure 2B and Supplementary Table S2. Saudi Arabia had the highest ASIR of thyroid cancer (7.1 cases per 100,000 population; 95% UI: 5.4–9.3), while Tajikistan had the lowest (0 cases per 100,000 population; 95% UI: 0–0).
Analyzing the data on the ASDR and DALYs caused by thyroid cancer in 2021, we observed a significant consistency among the most severely affected countries. Ethiopia, Bolivia (Plurinational State of), and Zimbabwe stood out in both indicators. Ethiopia led in both categories, with the highest ASDR (1.6 per 100,000 population; 95% UI: 1.1–2.4) and the highest DALY s (44.2 per 100,000 population; 95% UI: 29.8–67.4). Zimbabwe ranked third in ASDR (1.4 per 100,000 population; 95% UI: 1–1.8) and second in DALYs (38.9 per 100,000 population; 95% UI: 27.4–50.7), while the Bolivia (Plurinational State of) ranked second in ASDR (1.6 per 100,000 population; 95% UI: 1–2.2) and third in DALYs (38.4 per 100,000 population; 95%: 25.3–53.3) (Figure 2D and Supplementary Tables S3, S4). Poland (EAPC –3.52, 95% CI –4.09 to −2.94), Kazakhstan (EAPC –2.69, 95% CI –3.27 to −2.1), and Cyprus (EAPC -2.63, 95% CI –2.78 to −2.48) had the largest decreases in the age–standardized DALYs numbers due to thyroid cancer (Supplementary Table S4).
Between 1990 and 2021, the global prevalence of thyroid cancer showed an upward trend. Among them, the increase in the number of prevalence cases in Saudi Arabia was the most remarkable, with a staggering 1,401% increase, while that in Poland decreased by 7% (Figure 6A). Similarly, the United Arab Emirates had the highest increase in the number of incidence cases, rising by 1,317%, while Poland and Hungary decreased by 11 and 12%, respectively, (Figure 6B). In terms of the number of death cases, Ecuador had the largest increase, rising by 481%, while Hungary decreased by 40% (Figure 6C). Finally, Ecuador also witnessed the most significant increase in the number of DALYs, with a 410% increase, while Hungary decreased by 40% (Figure 6D).
Figure 6

Change cases of thyroid cancer for both sexes in 204 countries and territories. (A) Change prevalence cases. (B) Change incidence cases. (C) Change deaths cases. (D) Change DALYs.
Age and gender patterns
In 2021, globally, the ASPR of thyroid cancer gradually increased with age, peaked at the age of 55–59 years, and then gradually decreased. At all age groups, the ASPR in females was consistently higher than that in males (Figure 7 and Supplementary Figure S1). Similarly, the trend of the ASIR of global thyroid cancer in 2021 was similar to that of the ASPR (Supplementary Figure S2).
Figure 7

Age-standardized prevalence rates of thyroid cancer by sex, age group, and socio-demographic index, 1990 and 2021.
Compared with 1990, the ASDR of thyroid cancer in 2021 showed no significant changes in both males and females. In the population under 50 years old, both men and women had an extremely low ASDR for thyroid cancer, which strongly indicates that thyroid cancer has a favorable prognosis in young people. However, in the population over 50 years old, the ASDR gradually increased with age, and the ASDR in females was slightly higher than that in males (Supplementary Figure S3). The DALYs also indicated that, compared with 1990, there was a slight upward trend in 2021, and the age - standardized DALYs rates in females was still higher than that in males (Supplementary Figure S4).
Future projections of the global burden of thyroid cancer
It is projected that from 2021 to 2040, the global burden of thyroid cancer will continue to change, with varying trends across different indicators. The total ASPR of thyroid cancer in both men and women globally is expected to increase, rising from approximately 23 per 100,000 population in 2021 to around 25 per 100,000 population in 2040, representing an increase of approximately 9% over two decades (Figure 8). The prevalence in men is predicted to remain relatively stable, increasing slightly from about 15 per 100,000 population in 2021 to 16 per 100,000 population in 2040. In contrast, the number of female patients is expected to experience a more significant increase, rising from approximately 32 per 100,000 population in 2021 to around 35 per 100,000 population in 2040, indicating a steeper upward trend.
Figure 8
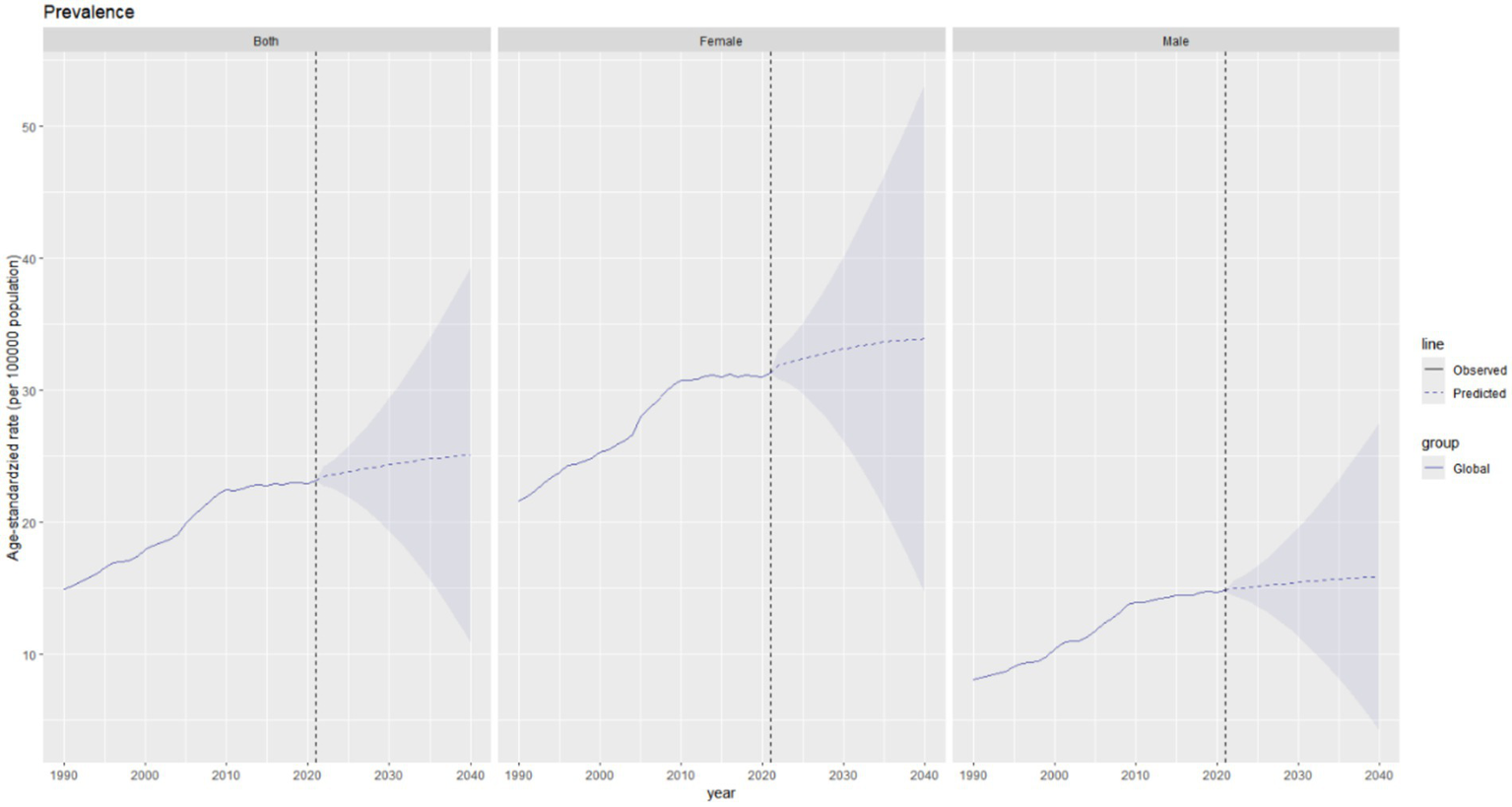
Future projections of the global burden of thyroid cancer prevalence.
The global incidence of thyroid cancer shows a significant upward trend in the combined data of both sexes. The ASIR is expected to increase from approximately 2.9 per 100,000 population in 2021 to around 3.3 per 100,000 population in 2040. The upward trend of the incidence rate in females is predicted to be more pronounced than that in males (Figure 9).
Figure 9
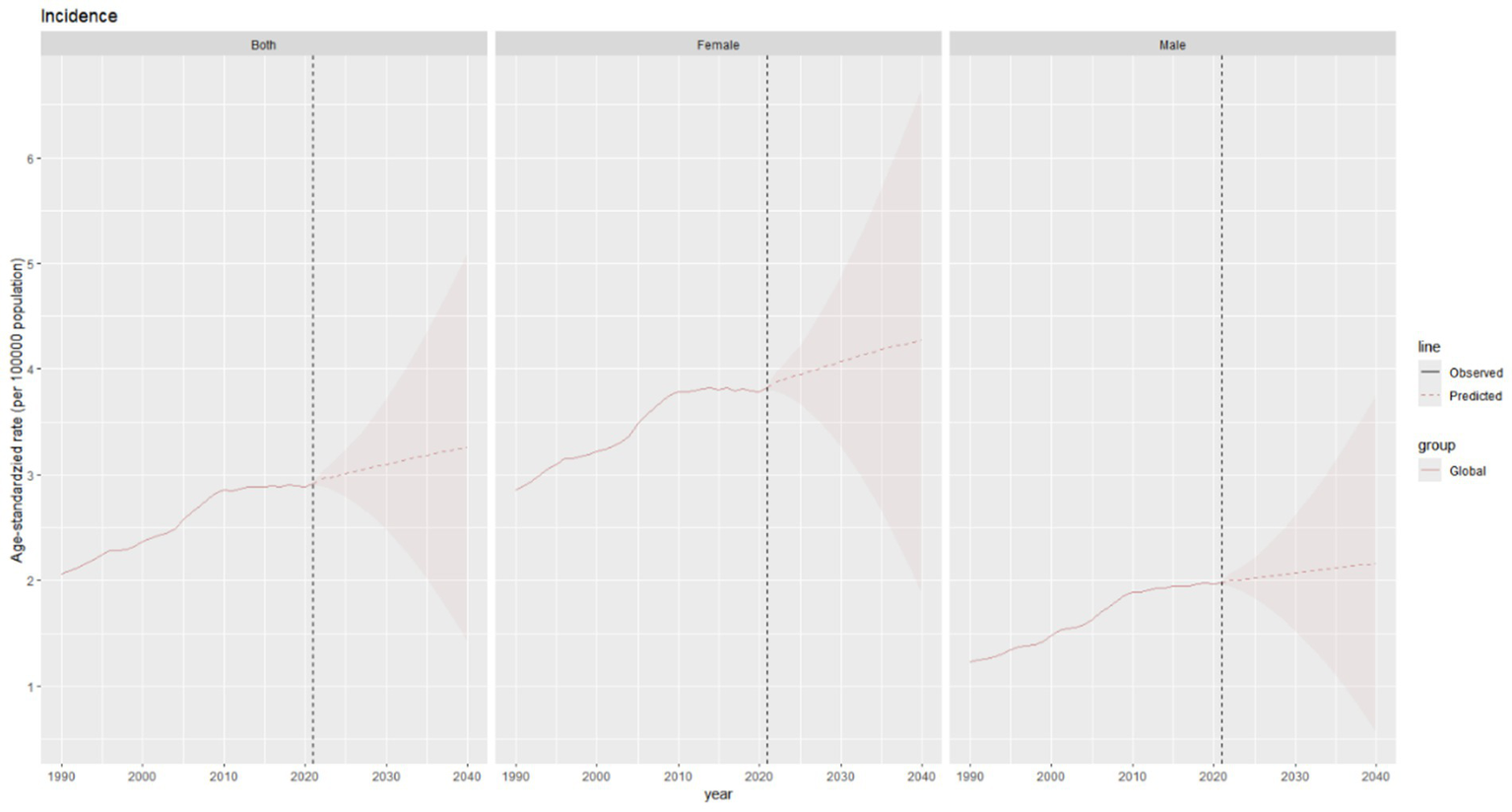
Future projections of the global burden of thyroid cancer incidence.
The total ASDR of thyroid cancer is expected to decline slightly, from approximately 0.54 per 100,000 population in 2021 to around 0.49 per 100,000 population in 2040 (Figure 10).
Figure 10
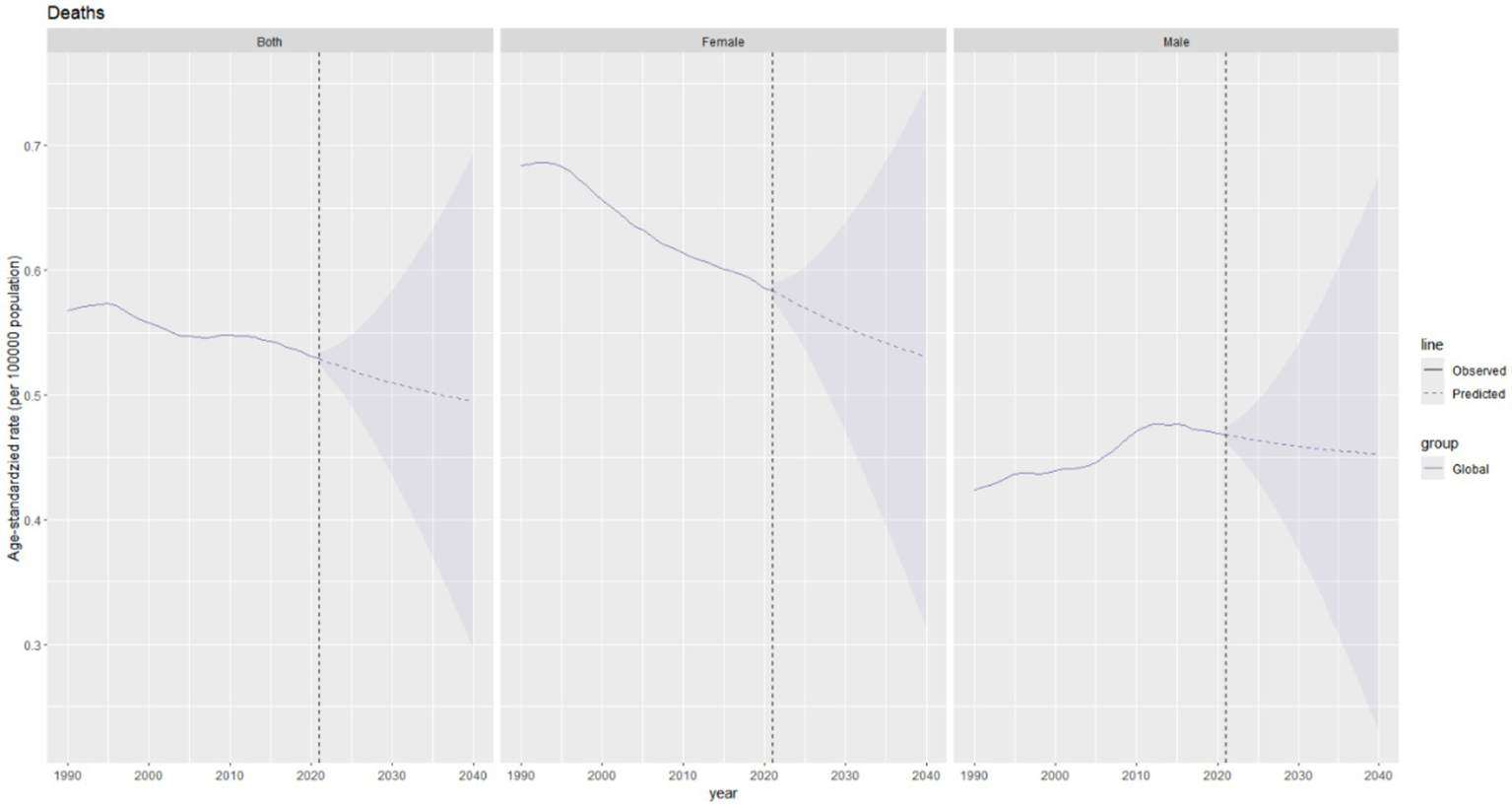
Future projections of the global burden of thyroid cancer deaths.
The age–standardized DALY rate of thyroid cancer is projected to decrease slightly in the combined data of both genders, declining marginally from 14.6 per 100,000 population in 2021 to approximately 14.2 per 100,000 population in 2040. The DALY rates in both males and females show a downward trend (Figure 11).
Figure 11
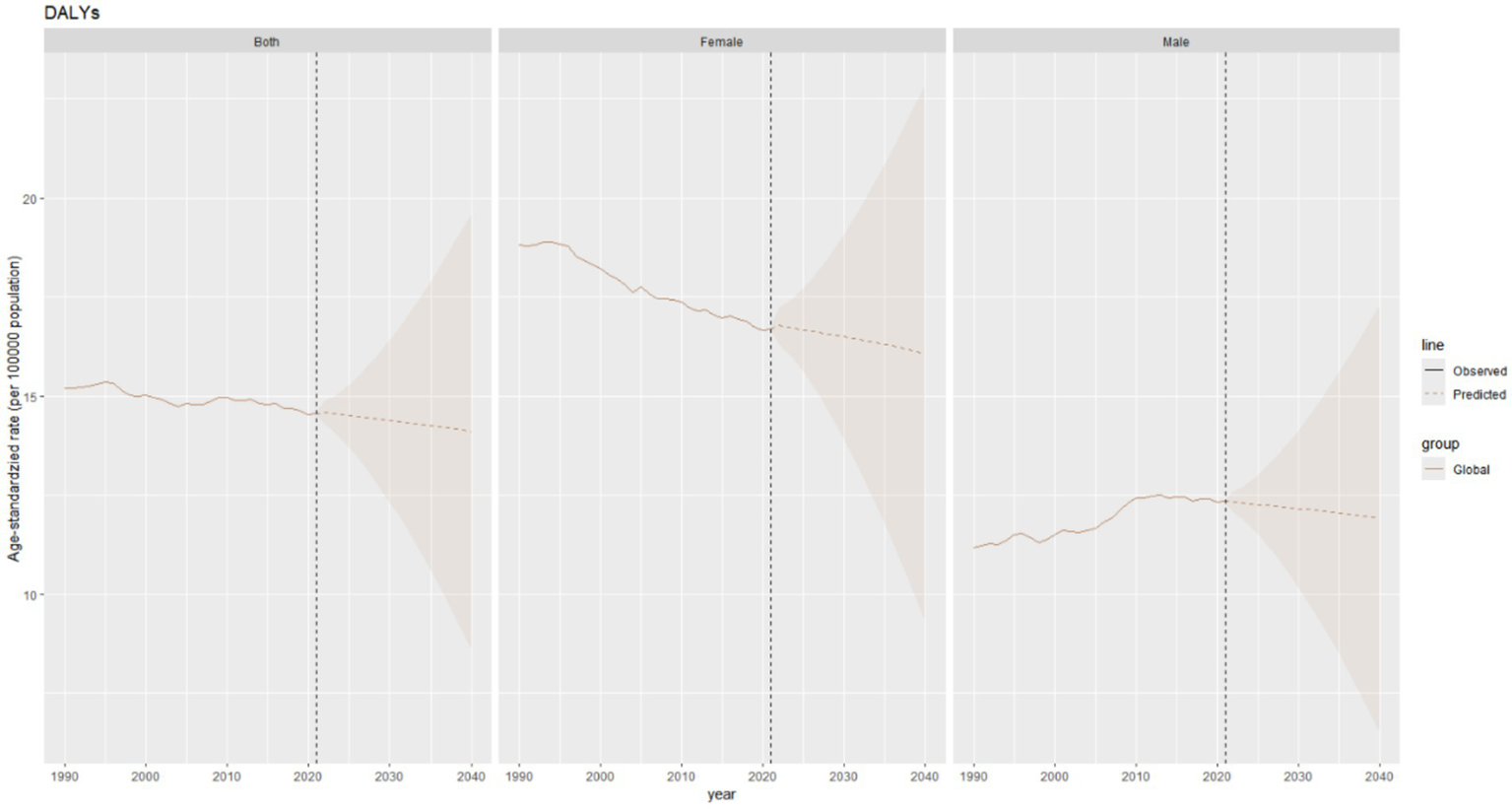
Future forecasts of global burden of thyroid cancer DALYs.
Discussion
This study comprehensively evaluated the global burden of thyroid cancer from 1990 to 2021, clearly presenting trends in prevalence, incidence, mortality, and DALYs. Globally, the burden of thyroid cancer is remarkable, with distinct differences in regions, age groups, and genders.
Temporal trends in thyroid cancer ASPR and ASIR varied by SDI, reflecting distinct epidemiological transition stages. High-SDI regions showed a biphasic pattern: an initial upward trend followed by a decline, suggesting effective prevention/management interventions. In contrast, lower-SDI regions exhibited sustained increases, with middle-SDI areas showing the steepest rise. This trend may reflect the complex interplay of multiple factors. These include advancements in medical examination techniques, which enhance diagnostic capabilities and lead to a higher detection rate. The proactive participation in physical check-ups and cancer screenings, as well as the extension of life expectancy, expand the high-risk population. Additionally, there is an increasing exposure to risk factors associated with rapid urbanization and lifestyle changes. In modern life, increased stress, poor dietary habits, weight gain, and changes in hormone levels (such as estrogen levels) may all be related to the increased burden of thyroid cancer (19–22).
The simultaneous analysis of ASPR and ASIR provides complementary insights. ASIR, as a marker of new cases, highlights etiological drivers and intervention efficacy; for example, the post-2009 decline in ASIR in high-SDI regions suggests successful implementation of prevention strategies targeting modifiable risks (e.g., radiation exposure) and overdiagnosis reduction (23, 24). Conversely, ASPR, which integrates incidence and survival, is critical for healthcare resource planning. The plateau in ASPR in high-SDI regions may indicate a steady state of prevalent cases managed through long-term follow-up systems, whereas the continuous rise in ASPR in middle-SDI regions underscores the urgent need for expanded clinical capacity to address growing patient populations. In low-SDI regions, the discrepancy between rising ASIR and stable ASPR may reflect limited access to diagnosis and treatment, leading to shorter survival and lower prevalence despite increasing incidence. Together, these metrics reveal that high-SDI regions have transitioned from a phase of diagnostic expansion to one of quality improvement, while lower-SDI regions remain challenged by both emerging risk factors and inadequate healthcare infrastructure.
Compared with previous research based on the GBD database, this study not only updates the time span to 2021 but also adopts a more comprehensive analysis dimension. Although previous studies have indicated an upward trend in the incidence of thyroid cancer (25), this study further reveals unique change patterns in different SDI regions. In high-SDI regions, the incidence rate shows a downward trend after an initial increase, which may be attributed to effective prevention and management strategies (26, 27). In other SDI regions, the incidence rate continues to rise, indicating that these regions still face challenges in the prevention and control of thyroid cancer (5). Meanwhile, the detailed analysis of age and gender patterns in this study complements the deficiencies of previous research, clarifying the differences in the burden of thyroid cancer among different age groups and genders (28, 29). We have found that women are more likely to develop thyroid cancer, and the incidence rate in women is increasing at a faster pace. Previous studies have also shown that the thyroid cancer incidence rate in women is three times higher than that in men (30). This is largely explained by other factors commonly present in women, such as the expression of estrogen receptors (ER) in thyroid cancer tissues. Estrogen itself, or its metabolites, particularly the enhanced 2-hydroxylation reaction, may be related to the development of thyroid cancer (31, 32). Some more consistent epidemiological findings—such as autoimmune diseases (33), infertility and the use of fertility drugs (34, 35), recent pregnancy, irregular menstrual cycles (36), and hysterectomy or surgical menopause (37, 38)—may provide etiological clues. Such findings warrant further exploration.
Notably, the updated GBD 2021 results reveal critical divergences from prior GBD assessments. Compared to the GBD 2017 estimates (39), the global age-standardized incidence rate (ASIR) of thyroid cancer in 2021 (6.9 per 100,000) reflects a 24.1% increase from 2017 (5.56 per 100,000), yet this growth rate has markedly decelerated (annualized increase: 1.2% during 2017–2021 vs. 2.8% during 1990–2017). Regionally, high-SDI areas exhibited contrasting trends: the ASIR in North America declined by 7.3% between 2017 and 2021 (from 15.2 to 14.1 per 100,000), whereas GBD 2017 projected continued growth (40). Conversely, low-middle-SDI regions demonstrated accelerated incidence growth (3.5% annual increase post-2017 vs. 2.1% historically), exceeding prior predictions. Mortality patterns also diverged; the 2021 global death rate (0.5 per 100,000) showed a 12% reduction from GBD 2017 estimates (0.57 per 100,000) (41), largely driven by improved survival in high-SDI countries. These discrepancies may stem from methodological refinements in GBD 2021, including enhanced cancer registry coverage (now incorporating 48% more population-based registries in low-income regions) (8) and recalibrated DisMod-MR 2.1 models addressing overdiagnosis bias (42).
In addition, we applied advanced statistical methods to predict future trends in thyroid cancer. Over the next 20 years, the burden of thyroid cancer will exhibit a complex “two increases and two decreases” pattern. Specifically, the ASPR is expected to increase by 9%, the ASIR will rise by 13.8%, the ASDR will decrease by 9.3%, and the age - standardized DALY rate will decline by 2.7%. Policies should focus on three key areas: precise prevention and control, standardized diagnosis and treatment, and survival care. Particular attention must be given to the needs of women and long-term cancer survivors to ensure the health benefits of the entire population as the disease burden rises.
Given the ongoing global increase in the burden of thyroid cancer and the significant regional heterogeneity (with high-SDI regions facing the risk of diagnosing clinically irrelevant thyroid carcinomas, while low-SDI regions struggle with higher mortality and disease burden due to limited diagnostic and treatment resources), public health interventions must adopt a dual-track approach. In high-resource regions, risk-based screening and active monitoring strategies should be implemented, such as adhering to American Thyroid Association (ATA) guidelines to limit imaging screening and monitor low-risk microcarcinomas, in order to curb the detection of indolent tumors. In resource-limited areas, priority should be given to improving access to basic diagnosis and treatment, such as strengthening palpation skills at the grassroots level, promoting ultrasound-guided fine needle aspiration techniques, and ensuring coverage of surgery and radioactive iodine therapy, to reduce preventable deaths in high-burden regions like East Africa. At the global level, cancer registration systems need to be enhanced to track disease patterns, iodine nutrition management and radiation protection research should be strengthened, and long-term health management for high-risk female populations should be prioritized, in order to achieve precise prevention and control of the disease burden.
The strength of this study lies in the utilization of the latest GBD 2021 data, ensuring the timeliness and accuracy of the research results. Through multi - dimensional stratified analysis, the relationships between the burden of thyroid cancer and gender, age, region, and SDI are comprehensively explored, providing rich information for a deep understanding of the epidemiological characteristics of the disease. In addition, the application of advanced statistical methods to predict future trends provides a basis for formulating long - term prevention and control strategies. However, the study also has certain limitations. Since the data are sourced from the GBD database, they may be affected by errors in the data collection and collation process. Moreover, although the associations between multiple factors and the burden of thyroid cancer have been analyzed, some potential factors, such as specific gene mutations and exposure to environmental pollutants, have not been explored in depth for their impacts on the disease.
In conclusion, this study provides a comprehensive analysis of the global burden of thyroid cancer from 1990 to 2021, revealing temporal trends, geographical differences, and sex - related patterns. These findings deepen our understanding of the disease’s epidemiology and lay a foundation for targeted public health interventions.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The institutional review board granted an exemption for this study, as it utilized publicly accessible data that contained no confidential or personally identifiable patient information.
Author contributions
The institutional review board granted an exemption for this study, as it utilized publicly accessible data that contained no confidential or personally identifiable patient information.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Henan Provincial Science and Technology Research and Development Program (Grant No. 242102311102), the Henan Provincial Natural Science Foundation for Young Scholars (Grant No. 222300420334) and the Henan Province Medical Science and Technology Project (Joint Construction, Grant No. LHGJ20240267).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2025.1613737/full#supplementary-material
References
1.
LyuZZhangYShengCHuangYZhangQChenK. Global burden of thyroid cancer in 2022: incidence and mortality estimates from GLOBOCAN. Chin Med J. (2024) 137:2567–76. doi: 10.1097/cm9.0000000000003284
2.
HuangJNgaiCHDengYPunCNLokVZhangLet al. Incidence and mortality of thyroid cancer in 50 countries: a joinpoint regression analysis of global trends. Endocrine. (2023) 80:355–65. doi: 10.1007/s12020-022-03274-7
3.
SungHFerlayJSiegelRLLaversanneMSoerjomataramIJemalAet al. Global Cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
4.
GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: a systematic analysis for the global burden of disease study 2019. Lancet. (2020) 396:1204–22. doi: 10.1016/s0140-6736(20)30925-9
5.
Miranda-FilhoALortet-TieulentJBrayFCaoBFranceschiSVaccarellaSet al. Thyroid cancer incidence trends by histology in 25 countries: a population-based study. Lancet Diabetes Endocrinol. (2021) 9:225–34. doi: 10.1016/s2213-8587(21)00027-9
6.
KitaharaCMSosaJA. The changing incidence of thyroid cancer. Nat Rev Endocrinol. (2016) 12:646–53. doi: 10.1038/nrendo.2016.110
7.
VaccarellaSFranceschiSBrayFWildCPPlummerMDal MasoL. Worldwide thyroid-Cancer epidemic? The increasing impact of Overdiagnosis. N Engl J Med. (2016) 375:614–7. doi: 10.1056/NEJMp1604412
8.
GBD 2021 Risk Factors Collaborators. Global burden and strength of evidence for 88 risk factors in 204 countries and 811 subnational locations, 1990-2021: a systematic analysis for the global burden of disease study 2021. Lancet. (2024) 403:2162–203. doi: 10.1016/s0140-6736(24)00933-4
9.
LiYPiaoJLiM. Secular trends in the epidemiologic patterns of thyroid cancer in China over three decades: an updated systematic analysis of global burden of disease study 2019 data. Front Endocrinol. (2021) 12:12707233. doi: 10.3389/fendo.2021.707233
10.
DuZChenWXiaQShiOChenQ. Trends and projections of kidney cancer incidence at the global and national levels, 1990-2030: a Bayesian age-period-cohort modeling study. Biomark Res. (2020) 8:16. doi: 10.1186/s40364-020-00195-3
11.
CleggLXHankeyBFTiwariRFeuerEJEdwardsBK. Estimating average annual per cent change in trend analysis. Stat Med. (2009) 28:3670–82. doi: 10.1002/sim.3733
12.
PizzatoMLiMVignatJLaversanneMSinghDLa VecchiaCet al. The epidemiological landscape of thyroid cancer worldwide: GLOBOCAN estimates for incidence and mortality rates in 2020. Lancet Diabetes Endocrinol. (2022) 10:264–72. doi: 10.1016/s2213-8587(22)00035-3
13.
GBD 2021 Diseases and Injuries Collaborators. Global incidence, prevalence, years lived with disability (YLDs), disability-adjusted life-years (DALYs), and healthy life expectancy (HALE) for 371 diseases and injuries in 204 countries and territories and 811 subnational locations, 1990-2021: a systematic analysis for the global burden of disease study 2021. Lancet. (2024) 403:2133–61. doi: 10.1016/s0140-6736(24)00757-8
14.
LiXYKongXMYangCHChengZFLvJJGuoHet al. Global, regional, and national burden of ischemic stroke, 1990-2021: an analysis of data from the global burden of disease study 2021. EClinicalMedicine. (2024) 75:102758. doi: 10.1016/j.eclinm.2024.102758
15.
GBD 2019 Stroke Collaborators. Global, regional, and national burden of stroke and its risk factors, 1990-2019: a systematic analysis for the global burden of disease study 2019. Lancet Neurol. (2021) 20:795–820. doi: 10.1016/s1474-4422(21)00252-0
16.
KnollMFurkelJDebusJAbdollahiAKarchAStockC. An R package for an integrated evaluation of statistical approaches to cancer incidence projection. BMC Med Res Methodol. (2020) 20:257. doi: 10.1186/s12874-020-01133-5
17.
LiuNYangDWWuYXXueWQLiDHZhangJBet al. Burden, trends, and risk factors for breast cancer in China from 1990 to 2019 and its predictions until 2034: an up-to-date overview and comparison with those in Japan and South Korea. BMC Cancer. (2022) 22:826. doi: 10.1186/s12885-022-09923-4
18.
WuBLiYShiBZhangXLaiYCuiFet al. Temporal trends of breast cancer burden in the Western Pacific region from 1990 to 2044: implications from the global burden of disease study 2019. J Adv Res. (2024) 59:59189-199. doi: 10.1016/j.jare.2023.07.003
19.
LudgateMEMasettiGSoaresP. The relationship between the gut microbiota and thyroid disorders. Nat Rev Endocrinol. (2024) 20:511–25. doi: 10.1038/s41574-024-01003-w
20.
KimJGosnellJERomanSA. Geographic influences in the global rise of thyroid cancer. Nat Rev Endocrinol. (2020) 16:17–29. doi: 10.1038/s41574-019-0263-x
21.
SeibCDSosaJA. Evolving understanding of the epidemiology of thyroid cancer. Endocrinol Metab Clin N Am. (2019) 48:23–35. doi: 10.1016/j.ecl.2018.10.002
22.
BonjocKJYoungHWarnerSGernonTMaghamiEChaudhryA. Thyroid cancer diagnosis in the era of precision imaging. J Thorac Dis. (2020) 12:5128–39. doi: 10.21037/jtd.2019.08.37
23.
CaoWQinKLiFChenW. Socioeconomic inequalities in cancer incidence and mortality: an analysis of GLOBOCAN 2022. Chin Med J. (2024) 137:1407–13. doi: 10.1097/cm9.0000000000003140
24.
LiMDal MasoLPizzatoMVaccarellaS. Evolving epidemiological patterns of thyroid cancer and estimates of overdiagnosis in 2013-17 in 63 countries worldwide: a population-based study. Lancet Diabetes Endocrinol. (2024) 12:824–36. doi: 10.1016/s2213-8587(24)00223-7
25.
LimHDevesaSSSosaJACheckDKitaharaCM. Trends in thyroid Cancer incidence and mortality in the United States, 1974-2013. JAMA. (2017) 317:1338–48. doi: 10.1001/jama.2017.2719
26.
DaviesLWelchHG. Increasing incidence of thyroid cancer in the United States, 1973-2002. JAMA. (2006) 295:2164–7. doi: 10.1001/jama.295.18.2164
27.
HaugenBRAlexanderEKBibleKCDohertyGMMandelSJNikiforovYEet al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid Cancer: the American Thyroid Association guidelines task force on thyroid nodules and differentiated thyroid Cancer. Thyroid. (2016) 26:1–133. doi: 10.1089/thy.2015.0020
28.
PaciniFSchlumbergerMDralleHEliseiRSmitJWWiersingaW. European consensus for the management of patients with differentiated thyroid carcinoma of the follicular epithelium. Eur J Endocrinol. (2006) 154:787–803. doi: 10.1530/eje.1.02158
29.
CooperDSDohertyGMHaugenBRKloosRTLeeSLMandelSJet al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. (2009) 19:1167–214. doi: 10.1089/thy.2009.0110
30.
KitaharaCMSchneiderAB. Epidemiology of thyroid Cancer. Cancer Epidemiol Biomarkers Prev. (2022) 31:1284–97. doi: 10.1158/1055-9965.Epi-21-1440
31.
LiuJXuTMaLChangW. Signal pathway of estrogen and estrogen receptor in the development of thyroid cancer. Front Oncol. (2021):11593479. doi: 10.3389/fonc.2021.593479
32.
ZengQChenGGVlantisACvan HasseltCA. Oestrogen mediates the growth of human thyroid carcinoma cells via an oestrogen receptor-ERK pathway. Cell Prolif. (2007) 40:921–35. doi: 10.1111/j.1365-2184.2007.00471.x
33.
TroisiRBjørgeTGisslerMGrotmolTKitaharaCMMyrtveit SaetherSMet al. The role of pregnancy, perinatal factors and hormones in maternal cancer risk: a review of the evidence. J Intern Med. (2018) 283:430–45. doi: 10.1111/joim.12747
34.
Zamora-RosRRinaldiSBiessyCTjønnelandAHalkjaerJFournierAet al. Reproductive and menstrual factors and risk of differentiated thyroid carcinoma: the EPIC study. Int J Cancer. (2015) 136:1218–27. doi: 10.1002/ijc.29067
35.
NegriEDal MasoLRonELa VecchiaCMarkSDPreston-MartinSet al. A pooled analysis of case-control studies of thyroid cancer. Part II. Menstrual and reproductive factors. Cancer Causes Control. (1999) 10:143–55. doi: 10.1023/a:1008880429862
36.
Horn-RossPLCancholaAJMaHReynoldsPBernsteinL. Hormonal factors and the risk of papillary thyroid cancer in the California teachers study cohort. Cancer Epidemiol Biomarkers Prev. (2011) 20:1751–9. doi: 10.1158/1055-9965.Epi-11-0381
37.
ShinSSawadaNSaitoEYamajiTIwasakiMShimazuTet al. Menstrual and reproductive factors in the risk of thyroid cancer in Japanese women: the Japan public health center-based prospective study. Eur J Cancer Prev. (2018) 27:361–9. doi: 10.1097/cej.0000000000000338
38.
RahmanSTPandeyaNNealeREMcLeodDSABaadePDYoulPHet al. Risk of thyroid cancer following hysterectomy. Cancer Epidemiol. (2021) 72:101931. doi: 10.1016/j.canep.2021.101931
39.
AzadnajafabadSSaeedi MoghaddamSMohammadiERezaeiNGhasemiEFattahiNet al. Global, regional, and national burden and quality of care index (QCI) of thyroid cancer: a systematic analysis of the global burden of disease study 1990-2017. Cancer Med. (2021) 10:2496–508. doi: 10.1002/cam4.3823
40.
WangCWuZLeiLDongXCaoWLuoZet al. Geographic disparities in trends of thyroid cancer incidence and mortality from 1990 to 2019 and a projection to 2030 across income-classified countries and territories. J Glob Health. (2023):1304108. doi: 10.7189/jogh.13.04108
41.
CuiYMubarikSLiRNawsherwanYuC. Trend dynamics of thyroid cancer incidence among China and the U.S. adult population from 1990 to 2017: a joinpoint and age-period-cohort analysis. BMC Public Health. (2021) 21:624. doi: 10.1186/s12889-021-10635-w
42.
The global epidemiology and health burden of the autism spectrum: findings from the global burden of disease study 2021. Lancet Psychiatry. (2025) 12:111–21. doi: 10.1016/s2215-0366(24)00363-8
Summary
Keywords
thyroid cancer, global burden of disease, disability-adjusted life years, incidence, prevalence
Citation
Zhao Z, Fan Y, Sun P, Zhang S, Xu M, Li J and Xu P (2025) Temporal trends and geographic disparities in thyroid cancer burden: a global analysis from 1990 to 2021. Front. Nutr. 12:1613737. doi: 10.3389/fnut.2025.1613737
Received
17 April 2025
Accepted
30 June 2025
Published
16 July 2025
Volume
12 - 2025
Edited by
Erivelto Martinho Volpi, Hospital Alemão Oswaldo Cruz, Brazil
Reviewed by
Aqeeb Ur Rehman, University of Alabama at Birmingham, United States
Sashiprabha Nawaratne, Government of Sri Lanka, Sri Lanka
Alina Ghazou, Jordan University of Science and Technology, Jordan
Updates
Copyright
© 2025 Zhao, Fan, Sun, Zhang, Xu, Li and Xu.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Pengfei Xu, xpfdoctor@126.com; Jianhua Li, lijianhuadoctor@163.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.