- 1Department of Physical Education, Xidian University, Xi’an, Shaanxi, China
- 2Shaanxi Provincial Hospital of Chinese Medicine, Xi’an, Shaanxi, China
- 3Ersha Sports Training Center of Guangdong Province, Guangzhou, Guangdong, China
Autophagy, a regulated cellular process, serves as both a tumor suppressor and a survival mechanism for tumor cells under stress in cancer. Recent studies demonstrate that polyphenols, bioactive compounds present in plant-derived foods, and exercise, a potent physiological stimulus, can efficiently modulate autophagy in both cancer patients and healthy individuals. This review explores the synergistic effects of polyphenols and exercise in regulating autophagy through key molecular pathways, including AMPK/mTOR, PI3K/Akt, and SIRT1/FOXO. Polyphenols such as quercetin, resveratrol, and curcumin possess autophagy-inducing properties, which may enhance exercise-induced cellular adaptations, contribute to cancer prevention, and improve metabolic health. Moreover, regular physical activity promotes autophagic flux, reducing oxidative stress, inflammation, and apoptosis resistance—factors critical in cancer progression and overall health maintenance. The review highlights the potential of polyphenol-exercise synergy in modulating autophagy, which may result in innovative therapeutic approaches for cancer treatment and metabolic health.
1 Introduction
Organisms necessitate fitness, resilience, and adaptability for optimal health, while life sciences and medicine strive to enhance health and avert disease through systematic physical exercise (1). Exercise is essential in rehabilitation medicine for complete recovery from illnesses; however, the molecular mechanisms that facilitate health improvements are poorly understood, especially concerning its effects on cellular processes in different organ systems (2–4). Autophagy is a vital cellular process that breaks down and recycles intracellular components to maintain homeostasis (5–7). It encourages general health, disease prevention, differentiation, development, and survival. Given its critical role in cellular health, dysregulated autophagy is associated with several pathological conditions, including cancer, that indicate possible therapeutic applications by direct targeting of autophagy-related proteins (8, 9). Autophagy, an essential cellular protective mechanism, is being investigated as a potential treatment for cancer via pharmacological agents or dietary modifications.
Autophagy is pivotal in cancer, facilitating both the induction and suppression of tumor growth. It can inhibit inflammation, avert mutations, and forestall chronic tissue damage. Autophagy is crucial for the survival of tumor cells under conditions of cellular stress. Tumor cells lacking autophagy exhibit a survival disadvantage under metabolic stress. Oncogenic pathways enhance autophagy, elevating cellular energy expenditure and facilitating survival. Although autophagy is a non-pharmacological intervention induced by physical exercise, it is still unknown what molecular mechanisms govern autophagic flux and how they might be used to treat cancer (5, 10–12). Drugs that modulate autophagy, such as rapamycin, carbamazepine, cisplatin, and chloroquine, have received approval for human clinical application. Nonetheless, their specificity and organ/cellular selectivity constrain their applicability. Natural products, including polyphenols present in fruits, herbs, vegetables, tea, wine, and cereals, present a promising therapeutic approach for regulating autophagy. Despite their well-established antioxidative properties, current research has indicated other mechanisms through which polyphenols exhibit health benefits. The regulation of autophagy by polyphenols to benefit human health by exercise has become an important new area of research focus. The relationship between exercise and polyphenols in regulating autophagy for cancer recovery is still ambiguous, despite extensive research on their advantages (13, 14). This review analyzes the potential advantages of exercise and polyphenol-induced autophagy in enhancing well-being and preventing cancer. It investigates the molecular mechanisms, effects on particular tissues, and prospective future therapies utilizing polyphenols as chemoadjuvants.
2 Protein deficiencies in autophagy: mechanisms, diagnostics, and pathological implications
This section examines autophagy, encompassing chaperone-mediated, microautophagy, and macroautophagy, along with their mechanisms, diagnostic methods, and the effects of protein deletion on cellular homeostasis and human health (15). It underscores the significance of autophagic flux in preserving cellular homeostasis, averting disease, and offering therapeutic advantages, including the synthesis and lipidation of LC3 (16–18). Monitoring the accumulation of microtubule-associated protein 1A/1B, Beclin1, Atg7, and Light Chain 3-II, essential autophagy proteins, is a prevalent technique for assessing flux (19, 20). Deficiencies in proteins such as Atg7 can exacerbate neurodegenerative diseases like Alzheimer’s, promote muscle atrophy, osteoporosis, and cognitive deterioration, underscoring the necessity to comprehend their physiological implications. Deficiencies in autophagy-related 5 can result in heart failure and cardiomyopathy (21, 22). The balance of bone tissue is upset by optineurin deficiency, which results in osteoporosis. Disruption of autophagy-related genes can lead to neurodegeneration, musculoskeletal disorders, cardiovascular problems, and metabolic dysregulation (Table 1). To prevent and treat diseases, it is essential to have optimal autophagy function (23).
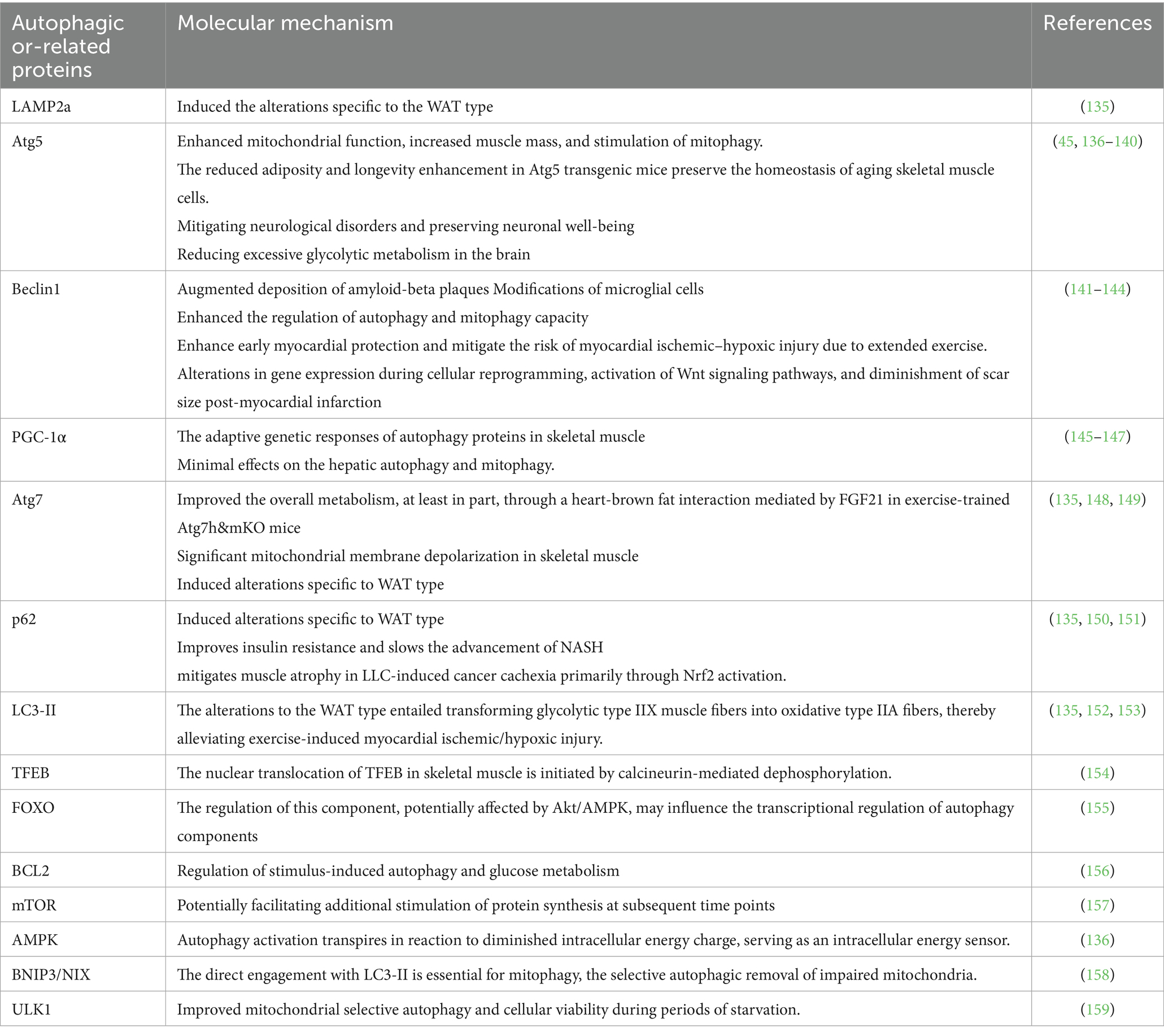
Table 1. The influence of exercise training on the alteration of crucial autophagic and associated proteins.
3 Exercise-induced autophagy’s signal transduction mechanisms
Exercise affects the body in two ways: through mechanical forces and biochemical pathways. This has an impact on autophagy in several systems. Mechanotransduction is an important part of the process. It involves the contraction of skeletal muscles, the compression of joints, and the shear stress of blood flow (24). Myokines, which have anti-inflammatory qualities, control metabolism, and affect general health, are also produced in greater quantities when one is physically active. The mechanisms linking exercise to autophagy are examined in this section (25) (Figure 1).
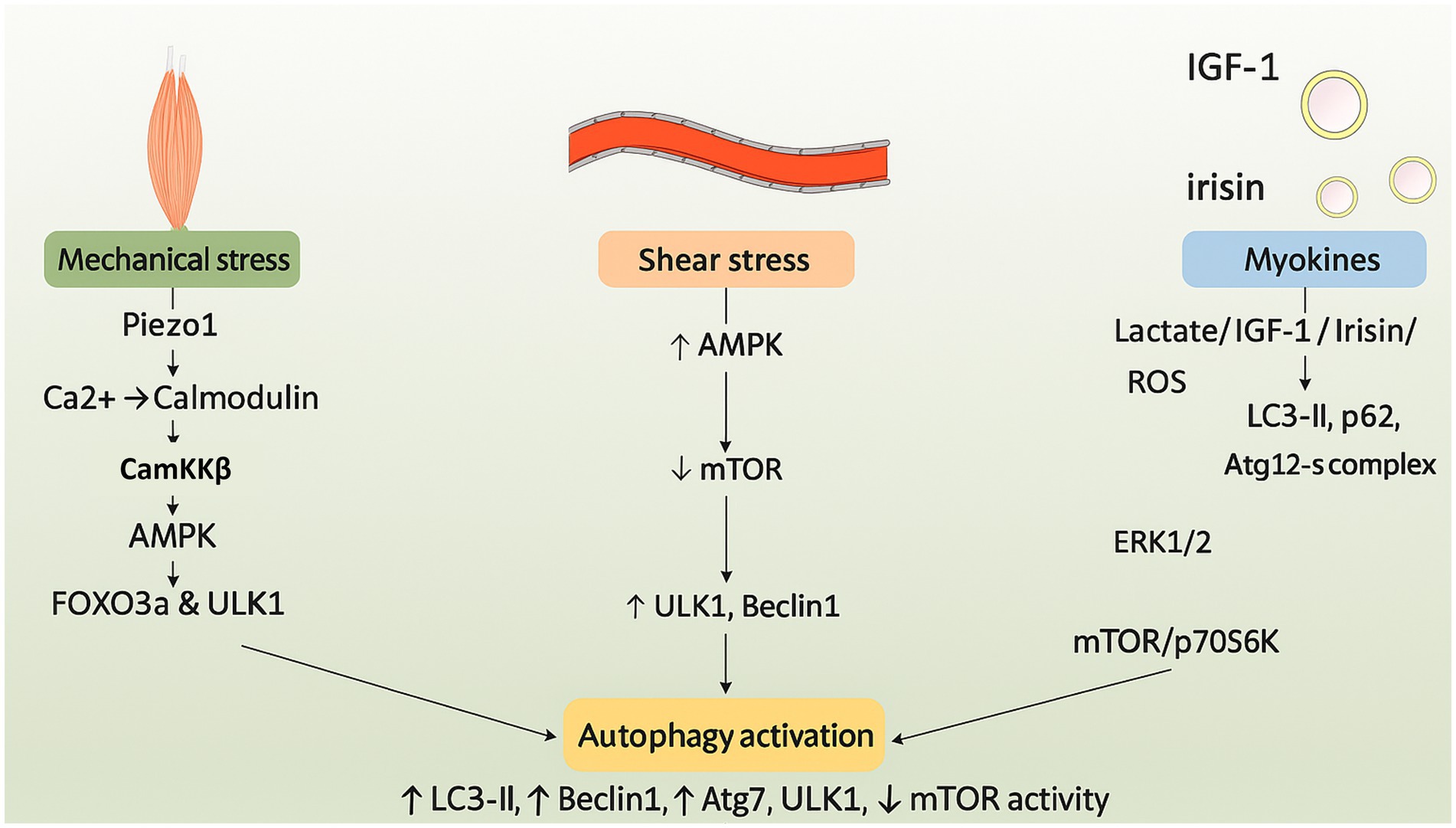
Figure 1. Schematic illustration of the molecular pathways through which exercise induces autophagy. Mechanotransduction and myokine signaling activate AMPK and downstream effectors such as CaMKKβ and FOXO3a. These events suppress mTORC1 and initiate autophagy through ULK1, Beclin1, and LC3 pathways.
3.1 Mechanochemical transduction
Mechanochemical transduction is the process by which external mechanical stimuli are transformed into bioelectric signals within cells. This mechanism influences exercise-induced autophagy through intracellular signaling pathways, cell membrane receptors, and ion channels (26, 27).
3.2 Poezol and AMPK: in-depth mechanical forces transmission
Physical activity stimulates mechanosensitive proteins in cells, converting mechanical signals into biochemical responses and altering cell membrane properties, facilitating touch, pain perception, and proprioception (28). Excessive mechanical stress elevates Piezo1 expression, impeding autophagy and hastening intervertebral disc degeneration, whereas AMPK activates calcium channels critical for exercise mechanotransduction (29). Calcium binding to calmodulin activates CaMKKb and AMPK, thereby initiating autophagy via FOXO3a and AMPK-ULK1 signaling pathways. AMPK inhibits mTORC1 and phosphorylates Beclin1, thereby diminishing inflammation, alleviating osteoarthritis, and preventing apoptosis (30). The AMPK-mTOR signaling pathway in the nervous system facilitates cardiac remodeling, cardiovascular function, brain adaptation, synaptic plasticity, exercise resilience, and mechanotransduction induced by exercise (31).
3.3 Piezo 1 and AMPK: inverse mechanical force transmission
Exercise-induced shear stress in atherosclerosis elevates Piezo1 expression, inhibits autophagy via YAP signaling activation, and facilitates nuclear translocation, underscoring the significance of physiological alterations in blood flow (32, 33). The cardiovascular system is protected and changes in brain tissue stiffness may be detected by exercise-induced Piezo1, which also improves vascular wall shear stress, blood flow optimization, Ca2 + signaling activation, and AMPK-dependent autophagy (34). In 5 FAD mice, piezo1 activation enhances neural plasticity, reduces the pathology of Alzheimer’s disease, and promotes autophagy by engulfing and breaking down amyloid-beta (35).
4 Bioactive compounds associated with physical activity
4.1 Myokines
Myokines, originating from skeletal muscle, are essential for the health benefits associated with physical exercise. They exert localized and pleiotropic effects. Inactivity diminishes myokine response, potentially associated with chronic diseases. Myokines improve health by secreting humoral factors that may facilitate the browning of adipose tissue (25). Myokines, generated during physical activity, are bioactive compounds such as IGF-1, VEGF, irisin, and lactate, which exert both local and systemic influences on organs including the heart, brain, and skeletal muscle. Exercise-induced metabolic adaptation, tissue repair, and general health depend on myokines, which can cross the blood–brain barrier and interact with particular tissue receptors (25, 36).
4.2 Growth factors
Exercise prompts muscle cells to generate growth factors such as IGF-1, which suppresses autophagy through the PI3K/Akt/FOXO and PI3K/Akt/mTOR paths, consequently enhancing the production of vital growth factors (37). Skeletal muscle adapts to lipids during fasting and exercise to maintain glycogen reserves and regulate blood glucose levels. AMPK is the primary sensor for these adaptations, converting information into SIRT1-mediated deacetylation of PGC-1α and FOXO transcriptional regulators. Insufficient AMPK activity undermines SIRT1-mediated responses, compromising PGC-1α deacetylation and mitochondrial gene expression. VEGF, an essential growth factor, facilitates angiogenesis and blood circulation in muscle tissue during exercise, thereby indirectly fostering the autophagic process by removing waste and dysfunctional cellular components, which are critical elements of autophagy (38).
4.3 Irisin
Irisin is a hormone-like myokine that is released into the bloodstream during physical activity after being cleaved from protein five, which contains the fibronectin type III domain. It modulates apoptosis, inflammation, and oxidative stress, and promotes autophagy, a process akin to hormonal function. Irisin increases the expression of LC3-II and p62 in the circulatory system, mitigating stress-induced myocardial hypertrophy (39). It facilitates autophagy in the musculoskeletal system via the Atg12-Atg5-Atg16L complex. By stimulating Wnt/β-catenin signaling, AMPK activity, and autophagy, it maintains skeletal integrity and reduces the buildup of β-amyloid proteins (40).
4.4 Lactate
The production of lactate, a necessary metabolite that regulates autophagy through signaling pathways including reactive oxygen species, ERK1/2, mTOR, and p70S6K, is stimulated by high-intensity exercise. Under the stress of exercise, lactate promotes Vps34 lactylation and aids in lysosomal degradation, maintaining muscle homeostasis. In diabetics, high-intensity interval training (HIIT) lowers blood glucose levels by stimulating autophagy and activating the ERK/Ribosomal protein S6 kinase, 90 kDa pathway (41). Studies indicate that polyphenols, especially quercetin, may mitigate post-exercise muscle damage, rendering them a beneficial nutritional approach for athletes. These compounds facilitate muscle recovery, improve exercise performance, and regulate inflammatory pathways, thereby enhancing mitochondrial function. They may further augment the advantages of lactate by facilitating autophagy (42).
5 Biological factors and various exercise parameters influencing autophagic responses
Exercise profoundly influences autophagy, a crucial modulator of the immune response, with its characteristics, intensity, and duration markedly affecting health and disease. Incorporating exercise regimens activates both aerobic and anaerobic systems; however, excessive training may result in maladaptive responses. Weight bias affects exercise identity, resulting in either adaptive or maladaptive behaviors. Individuals possessing a robust exercise identity and weight bias are more inclined to partake in maladaptive behaviors. Subsequent investigations ought to examine this correlation (43). The stages of life, sexual dimorphism, body composition, and muscle fiber types all have a significant impact on the autophagic response to physical exercise (44).
6 Different types of exercise in association with autophagy
Exercise, categorized as aerobic or anaerobic, encompasses metabolic processes such as oxidative metabolism and glycolytic pathways, with exercise-adapted molecules augmenting signaling pathways such as AMPK, PI3K/Akt, mTOR, Sirt1, and CaMKs, for autophagy (41, 42) (Figure 2).
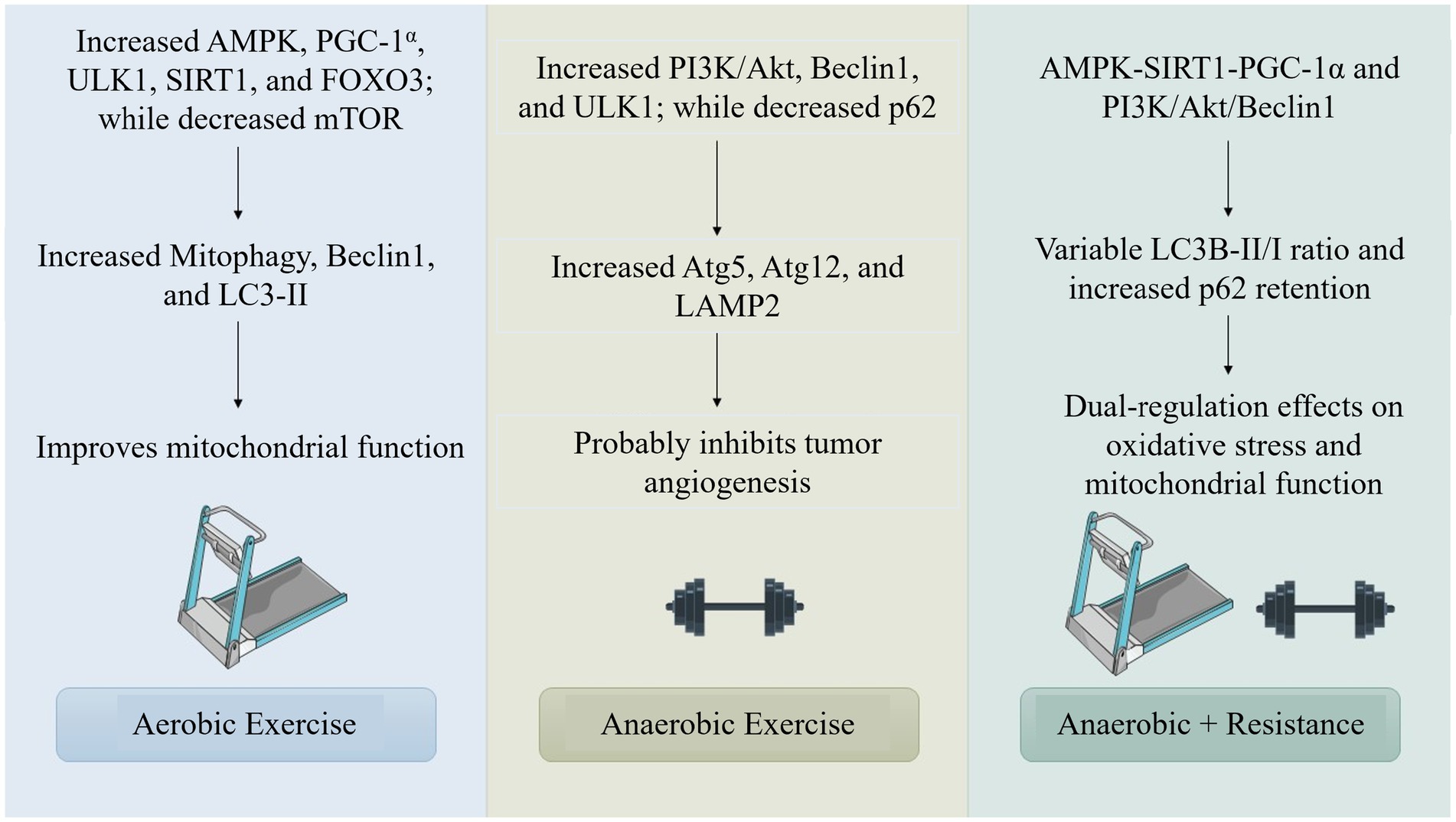
Figure 2. Comparison of molecular pathways activated by aerobic, anaerobic, and combined exercise modalities in regulating autophagy reveals diverse and complementary mechanistic roles. Aerobic exercise primarily stimulates the AMP-activated protein kinase (AMPK) pathway and its downstream effector peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α), which collectively enhance mitochondrial biogenesis and energy metabolism. This augments autophagic activity as a cellular adaptation to increased energetic demand. Mechanistically, AMPK activation inhibits the mechanistic target of rapamycin (mTOR), a central negative regulator of autophagy, thereby enabling the initiation of autophagic flux, particularly in skeletal muscle and cardiac tissues.
6.1 Combination exercise
The research indicates that the integration of aerobic and resistance training, a type of exercise, produces differing impacts on autophagy. A preclinical tumor study employed resistance training on a 1-meter ladder featuring rungs spaced 1.5 centimeters apart and inclined at an angle of 85 degrees (45). It has been shown that the training regimen, which consists of three sets of two repetitions on a treadmill for 25 min, is effective in inhibiting the growth of tumors by reducing autophagy, lowering the LC3B-II/I ratio, and maintaining high levels of p62 expression. Exercise evaluations and adrenergic stimulation do not significantly increase hepatic autophagy levels or autophagy responses, according to research on aged mice and clinical trials (46). According to the research, factors like type, intensity, and duration all influence how combined exercise affects autophagy, suggesting that longer or more intense exercise may be necessary to increase autophagic activity (47).
6.2 Anaerobic exercise
There is a lack of knowledge about how resistance training and other anaerobic processes affect autophagic regulation, as evidenced by the paucity of research on autophagy triggered by anaerobic exercise. Through pathways like PI3K/Akt, PINK1, Beclin1, and ULK1, resistance training increases autophagic activity (48).
6.3 Aerobic exercise
Aerobic exercise significantly influences cellular homeostasis and adaptation through the activation of autophagy (49). AMPK modulates cellular energy metabolism, which is associated with the progression of cancer cells. It functions as a tumor suppressor by inhibiting mTOR and promoting cellular autophagy. AMPK can enhance autophagy by activating ULK1 and suppressing the proliferation of breast cancer cells. Nonetheless, this may result in drug resistance during subsequent stages of the tumor. AMPK is also a key protein in the TME, having a bidirectional effect on tumor growth, promoting glucose metabolism and angiogenesis. Gene knockouts can impede tumor proliferation, particularly in preneoplastic lesions. Nevertheless, AMPK activity is diminished during energy sufficiency, influencing glycolysis and lipogenesis. Aerobic exercise markedly increases AMPK activity, facilitating angiogenesis and suppressing tumor cell metastasis. Liver kinase B1 (LKB1) inhibits mTOR by activating AMPK under low ATP conditions. The reduction of ATP during exercise increases the AMP/ATP ratio and activates AMPK through LKB1. In pathological conditions, hypoxia stimulation in the tumor microenvironment activates AMPK, thereby inhibiting angiogenic factors. Aerobic exercise intervention is thus advised during the early phase of tumorigenesis (Figure 3) (50, 51).
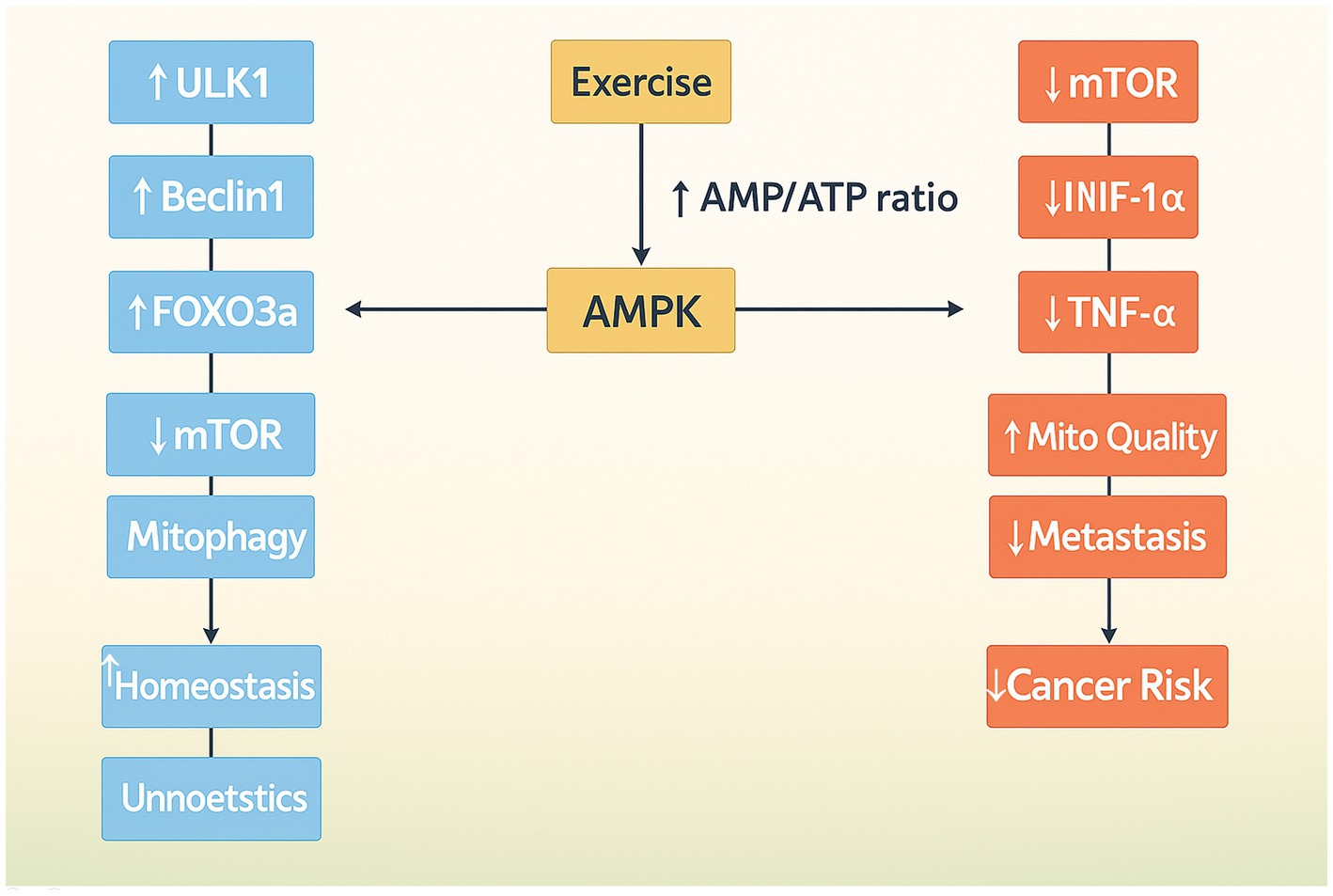
Figure 3. Illustration of AMPK as a central node in mediating both autophagy and anti-cancer effects during aerobic exercise. Through the inhibition of mTOR and regulation of glucose and oxygen homeostasis, AMPK contributes to metabolic reprogramming and tumor growth suppression.
6.3.1 Running
Running is a prevalent form of exercise in clinical training and autophagy research, utilizing treadmill models in preclinical studies. This form of exercise improves the AMP/ATP ratio, thereby activating P2X7 receptors and inducing AMPK activation. AMPK activates ULK1 kinase, thereby initiating autophagy, through the inhibition of mTOR activity (52). Running influences NAD + and AMPK concentrations, activating Sirt1, which promotes autophagy via FOXO3 and BNIP3, consequently impacting downstream targets. AMPK augments PGC-1a activity, which in turn elevates PINK1 and Parkin levels in mitophagy regulators, thereby facilitating mitochondrial quality control via autophagy and improving mitochondrial function (53, 54).
6.3.2 Swimming
AMPK, Akt, mTOR, FABP1, Beclin1, HSP70, and microRNAs are among the key signaling pathways that swimming affects to affect autophagic activity. AMPK activation promotes autophagy by elevating PGC-1a and FOXO3a levels, whereas swimming inhibits FABP1, thereby activating autophagy (55). The exercise-autophagy axis encompasses micro-RNAs that regulate autophagy proteins such as ULK1 and Beclin1 (56).
6.3.3 Cycling
AMPK activation, mTOR inhibition, and ULK1 stimulation are some of the molecular pathways through which cycling triggers autophagy. Its importance in cellular homeostasis and stress response is highlighted by the possibility that it may also promote autophagy by activating Tumor Protein 53 (p53) (57, 58).
6.3.4 Exercise programs: acute and chronic
Chronic exercise, also known as long-term exercise, includes planned, repetitive, and sustained physical activity over a longer period, while acute exercise is short-term, fleeting physical activity that does not involve regular or prolonged training (59). Acute exercise is brief, inducing immediate physiological stress, whereas chronic exercise persists for several weeks and results in temporary stress. Extended physical activity results in enduring physiological adaptations such as enhanced muscular strength and improved cardiorespiratory endurance (60, 61). The effects of autophagy differ based on exercise duration, with acute and chronic regimens yielding distinct outcomes in various tissues (Table 2). Acute exercise may either augment or diminish autophagy, while chronic exercise predominantly enhances it in particular tissues. Research indicates that chronic exercise enhances autophagy in rodent cardiac muscle, cycling promotes it in skeletal muscle, while treadmill exercise diminishes it in the liver (62). Chronic exercise consistently enhances autophagy in essential tissues such as skeletal muscle, cardiac muscle, and brain tissue, as demonstrated by increased autophagy in murine models. Autophagy is an essential regulator of central nervous system aging and neurodegeneration, maintaining neuronal health and survival by transporting organelles and toxic substances to lysosomes. Autophagic responses to exercise differ based on intensity, duration, and tissue-specific adaptability, with intense, brief exertions stimulating autophagy as a result of cellular stress. Prolonged exercise causes long-lasting changes, including increased mitochondrial biogenesis and cellular robustness, which lead to autophagic activation that continuously removes damaged organelles and maintains homeostasis. Particular tissue responses signify unique metabolic demands and molecular pathways, exemplified by the differing autophagic adaptation mechanisms of cardiac and skeletal muscles (63). Cardiomyocytes, as terminally differentiated cells, are essential for blood circulation and demand substantial energy, rendering them highly reliant on mitochondrial function for sustained contractile activities. In the heart, mitophagy is crucial because it removes damaged mitochondria, preserving regular energy metabolism and cellular respiration (64, 65). This is especially true for cardiomyocytes, which have high energy needs and mitochondrial activity. Autophagy is vital for protein metabolism and energy provision in skeletal muscle, which is essential for daily activities and movement, as it is closely linked to the health and function of cardiomyocytes (66). Exercise increases the body’s metabolic capacity, and autophagy is crucial for removing aged proteins and mitochondria, which promotes the growth of new mitochondria and muscle mass. Research has shown that chronic exercise is more important than acute exercise because it consistently increases autophagy (67).
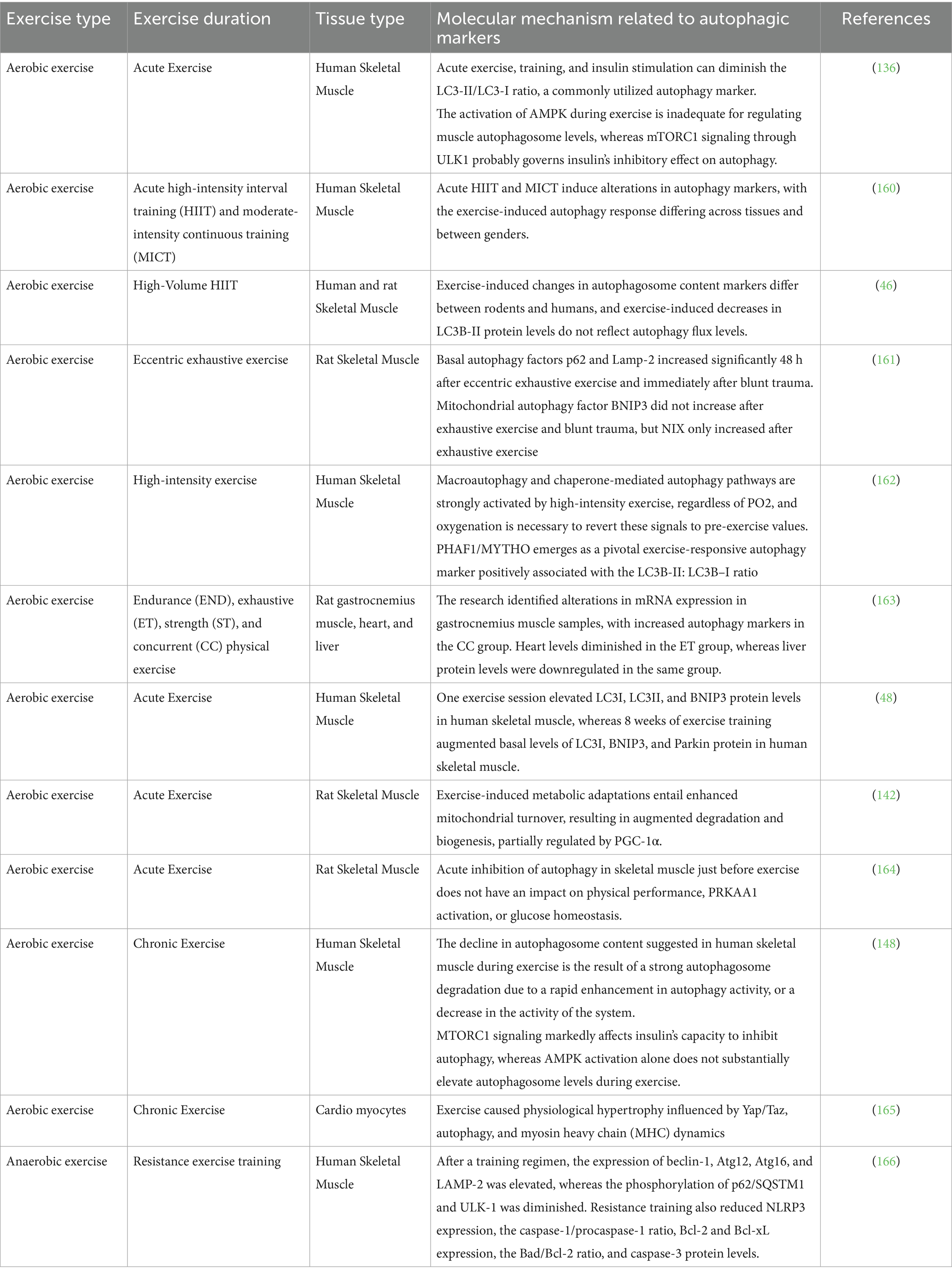
Table 2. The molecular difference of autophagic biomarkers based on exercise type and duration, in various tissues.
7 Insufficient autophagic response training
Muscle injury resulting from vigorous physical activity can impede daily functions. Overtraining may increase autophagy levels, which may be related to this damage and may be caused by the FOXO3/GABA type A receptor-associated protein-like 1 (GABARAPL1) signaling pathway (68). Increased skeletal muscle growth is linked to higher levels of Atrogin-1 and Muscle RING-finger protein-1 (MuRF1), two important factors that control muscle atrophy. Excessive exercise can also cause cardiac remodeling to shift from adaptive hypertrophy to harmful pathological alterations (69). Overexertion influences autophagic flux in skeletal muscle, but not in cardiac or hepatic tissue, underscoring the dual function of autophagy in overtraining and tissue-specific reactions (70).
8 Effects of sex metamorphosis, the composition of the body, life cycle, and muscle fiber type
8.1 Sexual metamorphosis
The hepatic mitochondrial adaptability and skeletal muscle performance are significantly impacted by sexual metamorphosis. This is particularly true in the liver, where autophagy is triggered by the mitochondrial biogenesis (PGC-1a) and mitophagy (BNIP3) pathways, sexual metamorphosis has a significant impact on exercise-induced autophagy, affecting skeletal muscle function and hepatic mitochondrial adaptability (71). Compared to male mice, female mice have lower autophagic flux but higher mitochondrial content and levels of autophagy-related proteins. Sex-specific variations in autophagy and adaptive responses are revealed by the requirement for daily physical activity in male mice to attain a similar mitochondrial phenotype. In skeletal muscle, the basal autophagic flux is higher in females, whereas in young male mice, exercise specifically promotes autophagy. Male skeletal muscle also exhibits increased nuclear localization of TFEB, an essential transcription factor (72).
8.2 Muscle fiber types
Skeletal muscle fiber types differ in their structural characteristics and metabolic capacity, which affects the autophagic response to exercise. A greater variety of fiber types, such as Type I, IIA, and IIX myosin heavy chain subtypes, are found in rodent skeletal muscle. While IIX and IIB fibers maximize anaerobic metabolism and rapid contraction, type I fibers improve endurance and energy efficiency. Intermediate fibers, or type IIA fibers, contract at moderate rates and possess oxidative and glycolytic characteristics. Different muscle fibers exhibit different patterns of autophagy; slow-twitch muscles spare slow-twitch muscles, while fast-twitch muscles, such as the gastrocnemius, are affected by starvation-induced autophagy (73). Autophagy is more strongly induced in oxidative soleus muscles by endurance training. In skeletal muscle, prolonged exercise promotes the shift to slow-twitch fiber predominance, which in turn increases autophagic capacity. According to research, autophagy is only found in oxidative soleus (SOL) muscles and not in glycolytic extensor digitorum longus (EDL) muscles, as indicated by LC3-II expression (74). Exercise may cause changes in the type of muscle fibers because it stimulates autophagy in oxidative muscle fibers. In oxidative muscles, endurance exercise promotes autophagic repair processes, which may protect them from excessive catabolism (75).
8.3 Life cycle
Exercise-induced autophagy exhibits considerable variation across different life stages, especially in younger individuals, facilitating swift cellular repair, metabolic adaptation, and enhanced physiological resilience, although it remains robust in older individuals. Research conducted by Zhou, Luo, and Yao showed that older adults exhibit a diminished autophagic response attributable to the age-related decline of autophagy-associated pathways. The skeletal muscle of older mice has substantially fewer autophagy-related genes, such as Beclin-1, Atg14, and LC3, than that of younger mice (76). Exercise partially reinstates autophagic activity in aged skeletal muscle, improving muscle function and bone mass. Exercise-induced autophagy facilitates osteogenic differentiation in older individuals, whereas younger animals preserve cardiac homeostasis despite heightened myocardial apoptosis and fibrosis (77). Even in older adults, exercise can reverse age-related changes by boosting basal autophagy capacity, restoring autophagic activity, and improving cardiac function (78).
8.4 The composition of the body
Exercise diminishes body fat, enhances glucose and lipid metabolism, and mitigates obesity by decreasing autophagy. Obesity results in compromised cellular autophagy, characterized by elevated levels of Atg5, LC3-I, and LC3-II mRNA in visceral adipose tissue, and a positive correlation with BMI. Obesity induced by a high-fat diet in mice leads to reduced autophagy activity in skeletal muscle relative to control mice. Obese mice exhibit heightened early autophagy stages in white adipose tissue, facilitating the development of autophagosomes. Obesity can induce alterations in energy metabolism within adipose tissue (79, 80). Autophagy modulates adipocyte functionality and energy equilibrium, as evidenced by knockout mice exhibiting alterations in lipid metabolism, including diminished adipose tissue, reduced adipocyte size, and weight loss. Exercise promotes autophagy in subcutaneous adipose tissue and inhibits it in perirenal adipose tissue, whereas obesity diminishes autophagy by decreasing lysosomal quantity, acidity, and fusion. Impairments in autophagic function, a hormone predominantly synthesized by white adipose tissue and governed by the obesity gene, may obstruct the body’s capacity to sustain cellular homeostasis amid metabolic stress (81, 82). Obesity frequently results in leptin resistance, marked by increased blood leptin concentrations and diminished responsiveness, especially in the hypothalamus, which is essential for energy regulation. Due to a lack of autophagy and poor energy regulation, leptin resistance causes obesity and metabolic diseases. Although autophagic function and leptin sensitivity can be improved by physical activity, there is no concrete proof that exercise-induced autophagy and increased leptin sensitivity are related. In certain tissues, obesity modifies systemic autophagy, whereas exercise promotes autophagy, maintaining cellular homeostasis and increasing energy expenditure. Further investigation is required to comprehend the influence of body fat composition on the autophagic response to exercise (13).
9 Autophagy induced by exercise in maintaining health and recovering from disease
In systemic diseases where autophagy is often impaired, exercise is a rehabilitative therapy that enhances the body’s ability to break down damaged cellular components. Exercise promotes autophagy, which improves cellular homeostasis and disease resistance while preventing relapse. This process aids in preserving organ health, mitigating aging, and fostering longevity. Exercise promotes autophagy, modifies pathological processes, and restores normal organ functions in the neurological and hormonal systems, heart and blood Systems, digestive, and musculoskeletal, exercise promotes healing and maintains health (56). Exercise improves disease management and health promotion by stimulating autophagy to modulate essential signaling pathways such as BMP, FOXO, NLRP3, IGF, HIPPO, ERK, and NFjB. This mechanism modulates glycolipid metabolism, diminishing cellular senescence, oxidative stress, apoptosis, and inflammation (83).
9.1 System of musculoskeletal
Exercise improves muscle and bone health by increasing fiber strength, promoting bone formation, and decreasing musculoskeletal injuries. Autophagy mitigates mitochondrial dysfunction, enhances antioxidant defense mechanisms, and stimulates downstream signaling pathways (84). Exercise-induced autophagy also controls important pathways for muscle growth and degeneration. The activation of the PI3K/Akt pathway results in an elevation of anti-apoptotic proteins and a reduction of pro-apoptotic factors, concurrently inhibiting the ubiquitin ligases MuRF-1 and MAFbx. This indirectly inhibits the HIPPO pathway, mitigating muscle dysfunction linked to sarcopenia. Through the inhibition of protein degradation via the Ubiquitin-Proteasome System, the modulation of IGF-mTOR signaling and the NFjB inflammatory pathways, and the upregulation of autophagy-related molecules like Beclin-1 and Atg7, exercise reduces growth inhibitor activity. By triggering the Wnt/β-catenin signaling pathway, activating autophagy-related complexes, encouraging osteogenic differentiation and bone formation, and preventing protein accumulation, exercise helps prevent aging-induced sarcopenia (85–87). This maintains chondrocyte homeostasis and prevents misfolded proteins. Exercise improves osteopenia and cartilage degradation in arthritis by boosting the NLRP3 signaling pathway, which in turn reduces pyrophosphate toxicity and degrades inflammatory vesicles. Initial exercise treatments are beneficial for musculoskeletal conditions because they reverse muscle atrophy, restore function, and improve tissue health while controlling molecular pathways that promote strength and recovery (88).
9.2 The neurological system
While autophagy helps maintain neuronal homeostasis by removing dysfunctional mitochondria and amyloid-beta, exercise enhances cognitive abilities and lowers neurological disorders by encouraging synaptogenesis, axonogenesis, neuroplasticity, and neuronal proliferation. Exercise-induced autophagy enhances neuronal health by preventing the accumulation of neurotoxic aggregates, optimizing mitochondrial function, diminishing oxidative stress, and maintaining neuronal integrity via essential signaling pathways. Neurodegenerative diseases such as Parkinson’s and Alzheimer’s may be mitigated by stimulating the Akt-FOXO-mTOR pathway and Beclin1-dependent autophagy, an essential regulator of cellular catabolism. Autophagy reduces neuronal damage, improves neuronal plasticity, and minimizes neurodegeneration by eliminating neurotoxic aggregates such as amyloid-beta peptides and alpha-synuclein. In Alzheimer’s disease, exercise-induced autophagy triggers the Beclin1-dependent pathway, which raises Neuregulin 1 expression and triggers the Akt-FOXO-mTOR signaling cascade (89). Physical activity enhances ERK-RSK-cAMP-response element binding protein activity enhances antioxidant defenses, mitigates neuronal degeneration, and facilitates autophagy, rendering it essential for Parkinson’s disease, Alzheimer’s disease, and cognitive rehabilitation. Exercise improves neuroplasticity, corrects deficiencies in synaptic and axonal transport, and enhances learning and cognitive abilities. It promotes neuroprotection through autophagy, regulating stress responses and enhancing angiogenesis in ischemic injuries. In reaction to injury, p62 amplifies the ERK pathway, Atg3 alleviates stress, and the PI3K-Akt–mTOR axis is stimulated, consequently reducing neuronal necrosis, strengthening defense mechanisms, and promoting tissue recovery (90, 91).
9.3 Heart and blood systems
Exercise stimulates autophagy to sustain energy balance and degrade damaged proteins, thereby decreasing cardiomyocyte apoptosis and reestablishing cellular homeostasis. Mitophagy induced by exercise enhances mitochondrial quality and cardioprotection, while autophagic markers such as Beclin1 and LC3-II are elevated in myocardial ischemia and hypoxic injury cases, encouraging the breakdown of organelles and metabolic waste products. By enhancing mitochondrial ATP-sensitive K + channels, lowering ischemic injury, and promoting autophagy via the AMPK-ULK1 pathway, exercise inhibits cardiomyocyte apoptosis and lowers endoplasmic reticulum stress. Exercise increases mitochondrial oxidative capacity and ATP synthesis boosts mVps34 activity, and stimulates autophagy, all of which improve myocardial function and prevent heart failure (92, 93). These enhancements reduce cardiac stress and facilitate the heart in fulfilling its energy requirements. In patients with atherosclerosis, swimming decreases inflammatory cytokines such as MMP-9, IL-6, and sICAM-1-1, enhances autophagy by activating LC3 and Beclin1, and slows the development of aortic plaque. Myocardial metabolic function and mitochondrial biogenesis are improved by exercise training, which also improves heart and blood health. It improves cardiac output, circulation, and myocardial architecture while also increasing membrane permeability. The cardiovascular system is protected by exercise-induced autophagy, which improves mitochondrial function and breaks down malfunctioning components to aid in recovery during stress and illness (94–96).
9.4 Hormonal system
Myocardial metabolic function and mitochondrial biogenesis are improved by exercise training, which also improves cardiovascular health. It improves cardiac output, circulation, and myocardial architecture while also increasing membrane permeability. The cardiovascular system is protected by exercise-induced autophagy, which improves mitochondrial function and breaks down malfunctioning components to aid in recovery during stress and illness. Exercise-induced autophagy is essential for metabolic regulation and lipid clearance, supporting the health of the endocrine system, particularly in metabolic diseases such as non-alcoholic fatty liver disease (97, 98). Additionally, it promotes hepatocyte autophagy, which lowers the buildup of cholesterol and triglycerides. Myelin and C1q/TNF-related protein 5 are two components that control this process. By boosting Atg7 expression, encouraging autophagy elongation, and lowering endoplasmic reticulum stress, exercise can lessen hepatic steatosis in fatty liver conditions. Additionally, it alters the Akt signaling pathway, which triggers autophagy and inhibits the release of TNF-α. This lowers MIF levels and lessens the metabolic alterations linked to obesity. Autophagy brought on by exercise decreases the buildup of fat in the liver by triggering the AMPK pathway, which regulates glucose metabolism and disorders (99–101). Physical activity in individuals with diabetes stimulates AMPK, PGC-1a, and BNIP3 expression, reduces endoplasmic reticulum stress, and promotes autophagic function, thereby alleviating renal damage in patients with chronic kidney disease (102).
9.5 Malignancy and aging
In aged organisms, exercise-induced autophagy improves tissue vitality and durability by preventing cell death and lipofuscin accumulation. Through AMPK-dependent autophagy, exercise extends lifespan by modifying the FOXO/Eukaryotic signaling pathway during senescence. Exercise suppresses apoptosis, increases autophagy, lowers antiapoptotic B-cell lymphoma-extra-large proteins, and delays cellular senescence (103). Additionally, it reduces the activity of cancer cells by removing oncogenic molecules, reactive oxygen species, misfolded proteins, and damaged mitochondria. Through the preservation of cellular homeostasis, inhibition of tumor growth, and enhancement of cell viability, exercise-induced autophagy promotes cancer recovery. Research indicates that it prolongs the life of colon cancer mice by decreasing the expression of Atrogin-1 and MuRF-1 (103).
10 Natural polyphenols combined with exercise in cancer
Natural polyphenols, plant-derived organic compounds, have been investigated for their potential health benefits, including protection against diabetes, cardiovascular diseases, oxidative stress, neurodegenerative disorders, and aging. They can suppress cancer by modifying signaling pathways, inducing apoptosis, and obstructing cell cycle processes, ultimately eradicating cancer cells. Polyphenols regulate enzymes implicated in tumor cell growth and proliferation. They additionally impede angiogenesis, avert metastasis, and engage with DNA (104). Flavonoids, phenolic acids, and tannins are essential phenolic compounds that confer health benefits by modulating inflammatory and oxidative pathways and eradicating cancer cells. Numerous athletes endorse dietary supplements to enhance physical performance during training, with research indicating the advantageous effects of specific compounds such as quercetin, resveratrol, and polyphenolic compounds derived from grape extract or beetroot juice. Exercise and antioxidant supplements may synergistically influence cancer development and progression through their antioxidant effects, thereby enhancing treatment efficacy (105).
10.1 Saffron
Saffron, obtained from the stigmas of the Crocus sativus L. plant, serves as a herbal remedy, coloring agent, and flavoring agent, and has demonstrated efficacy in addressing various health concerns (106).
The research indicates that high-intensity interval training (HIIT) and saffron aqueous extract may lower breast cancer risk by increasing Sirtuin-1 and p53 expression in tumor tissue. The study demonstrated that HIIT and SAE can reduce tumor volume and increase the expression of anti-and pro-apoptotic proteins in mice with 4T1 breast cancer (107). Nonetheless, these treatments failed to augment apoptotic induction, despite facilitating the apoptotic pathway. Its immunomodulatory properties are facilitated by multiple mechanisms, including the modulation of innate and adaptive immunity components. The pharmacological effects of saffron are chiefly attributed to crocin and crocetin, which can influence the MAPK and NF-κB signaling pathways. It regulates the expression of genes that encode pro-inflammatory cytokines, inducible enzymes, adhesion molecules, chemokines, and acute-phase proteins. These factors are essential in regulating inflammatory processes within the immune system. Consequently, saffron and its constituents may be regarded as a promising immunoregulatory agent for the treatment of immune disorders. A study conducted by Mirzaei and associates revealed that the application of saffron, honey, and rose water over 4 weeks diminished fatigue in 75 breast cancer patients. The Jollab group exhibited a marked decrease in the Visual Analogue Fatigue Scale (VAFS), Fatigue Severity Scale (FSS), and both physical and cognitive subscales of the Cancer Fatigue Scale (CFS) in comparison to the placebo group. Nonetheless, the scores on the affective subscale exhibited no significant alteration post-intervention in either group. This indicates that saffron may serve as a potential remedy for cancer-related fatigue in women diagnosed with breast cancer (108). The analyzed articles indicate that although saffron and exercise may positively influence breast cancer cells, their combination might be less effective or yield paradoxical effects. Additional in vitro studies may elucidate these effects and propel the investigation forward.
10.2 Curcumin
Curcumin, a plant comprising 120 species, is recognized for its therapeutic properties, which include antiproliferative, anti-thrombotic, antitumor, anti-inflammatory, antihepatotoxic, diuretic, hypotensive, antimicrobial, antioxidant, and antityrosinase effects (109).
Guo et al. discovered that the conjunction of curcumin treatment and swimming exercise markedly diminished breast cancer by influencing signaling pathways such as IL-17, calcium, PI3K-Akt, and Wnt (110). The combined effects of curcumin and exercise also influenced amino sugar and nucleotide sugar metabolism (111). The research indicates that endurance training combined with curcumin may improve tumor suppression. Research indicates that aerobic exercise and curcumin do not substantially mitigate oxidative stress in cancerous mice; however, they do significantly affect gene expression in mice with breast cancer (Figure 4) (111).
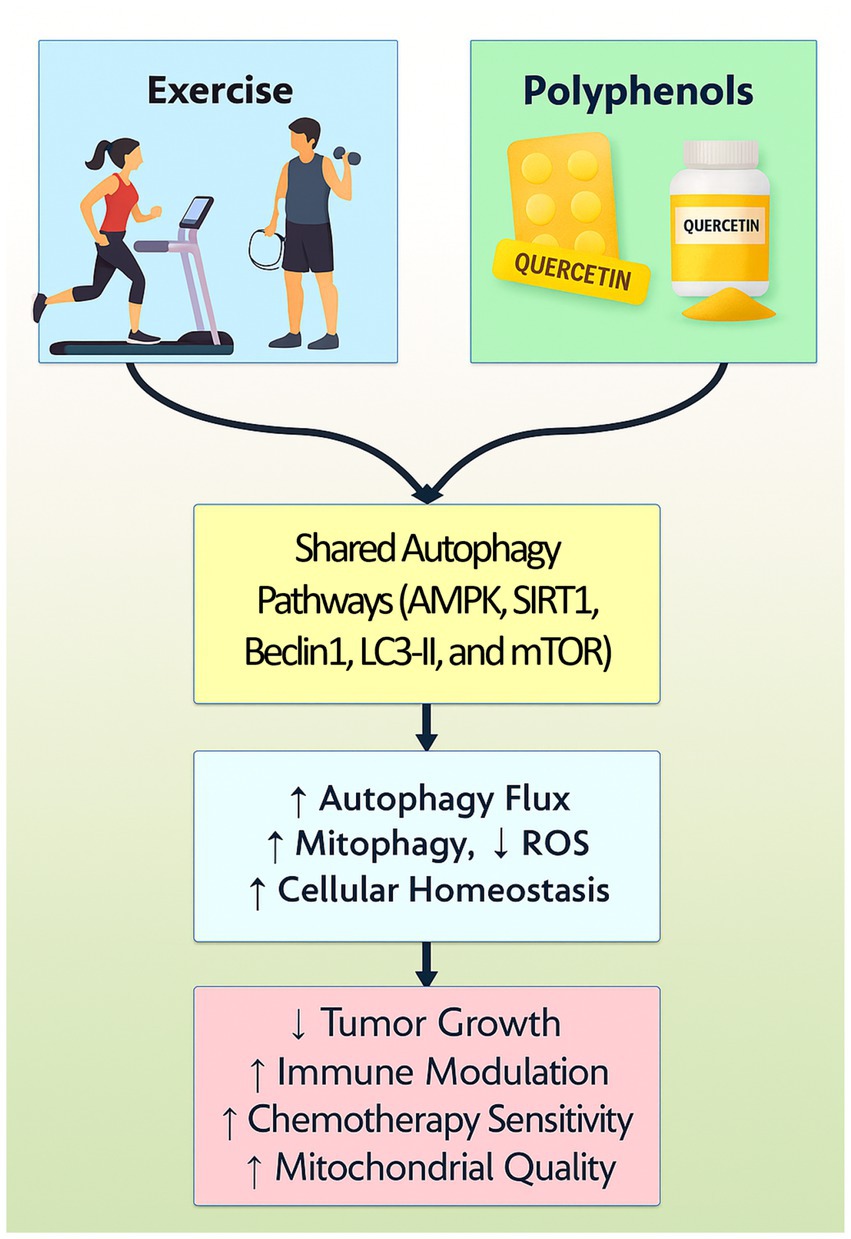
Figure 4. A graphical representation of the synergy between polyphenols and exercise in regulating autophagy elegantly illustrates how these two distinct but complementary stimuli converge on critical intracellular signaling networks that maintain cellular homeostasis and exert therapeutic effects. Both polyphenols and exercise activate AMP-activated protein kinase (AMPK), a master energy sensor that promotes autophagy initiation by inhibiting the mechanistic target of rapamycin (mTOR) pathway, a central negative regulator of autophagy. Concurrently, Sirtuin 1 (SIRT1), a NAD+-dependent deacetylase modulated by both interventions, enhances autophagic flux by deacetylating key transcription factors such as FOXO and autophagy-related proteins, thus facilitating tumor cells quality control.
Aerobic exercise training diminished high-sensitivity C-reactive protein and PTX3, concurrently lowering body fat percentage and BMI, independent of curcumin supplementation. An 8-week exercise program incorporating curcumin may reduce inflammatory markers (112). Research by Moghiseh et al. demonstrates that aerobic exercise and curcumin nano micelles can mitigate the impact of doxorubicin on cardiac tissues in breast cancer patients. Aerobic exercise diminishes the expression of CAS3, CAS9, and BAX genes, whereas curcumin supplementation enhances BCL2 gene expression (113). The combination of curcumin and physical activity diminishes tumor proliferation and decreases Il4 and Stat-6 gene expression. A five-week endurance training regimen combined with curcumin is more efficacious in cancer treatment than non-pharmacological methods alone. The protective effects of curcumin against chemotherapy-induced side effects in breast cancer patients have been confirmed (114). Hemati and colleagues’ clinical trials validated curcumin’s protective properties against chemotherapy’s adverse effects in breast cancer patients. Curcumin supplementation was found to be advantageous in countering tamoxifen-induced non-alcoholic fatty liver disease, indicating its potential as a preventive adjunct to tamoxifen therapy (115).
10.3 Quercetin
Quercetin, a natural compound, possesses potential therapeutic applications for conditions such as diabetes, gouty arthritis, allergies, hyperuricemia, obesity, and cancer by inhibiting cancer progression, enhancing cell membrane integrity, and modulating autophagy (116). Quercetin influences multiple signaling pathways related to cell proliferation, survival, and apoptosis, including VEGF, NF-κB, and Akt/mTOR. Studies indicate that aerobic exercise and quercetin supplementation can diminish TIE-2 and VEGF-A expression in breast cancer models. This indicates that the combination may inhibit tumor angiogenesis (117). A study demonstrated that physical exercise markedly diminished tumor progression in a murine model, yielding a 75% reduction in the placebo group and a 40% reduction in the quercetin group. Additional research is required to substantiate these findings (118). Additional research is required to thoroughly understand the synergistic effects of quercetin and exercise training in cancer, encompassing optimal combinations, mechanisms, and long-term advantages. Quercetin and exercise training may substantially diminish tumor size, improve survival rates, and elevate the quality of life for cancer patients. However, the research indicates that the effects of quercetin on human subjects remain unverified, necessitating additional investigation for clinical application. The authors propose that the integration of quercetin with various training modalities may yield greater benefits or efficacy (118).
10.4 Daidzein
Daidzein, an isoflavone present in soy, along with exercise training, may effectively combat breast cancer by enhancing natural killer (NK) cell activity and inducing apoptosis in cancer cells. When integrated, these interventions can efficiently activate NK cells and trigger apoptosis. A study by Wang et al. revealed that consistent exercise and daidzein can markedly inhibit breast cancer proliferation in BALB/c mice. The combination also increased epinephrine and IL-6 levels, enhancing natural killer cell activity and inducing apoptosis in cancer cells (119). The study indicates that this combination could serve as an effective approach to breast cancer prevention and treatment; however, additional research is required (120).
10.5 Gallic acid and kaempferol
The research indicated that chemotherapy diminished JAG1 gene expression, whereas supplementation with Gallic acid and Kaempferol, along with aerobic exercise, significantly lowered its expression in both breast cancer and cancer-chemotherapy cohorts. The expression levels of BDNF and NGF genes were elevated in the cancer group receiving chemotherapy combined with supplements and chemotherapy combined with aerobic exercise, with BDNF and NGF genes exhibiting a significant increase relative to other groups. They concluded aerobic exercises and supplements declined the side effects of paclitaxel and improved the neurogenesis (121). Endurance training diminishes tumor growth and development by lowering the expression of genes such as HIF1α and VEGFα, whereas gallic acid and kaempferol regulate these genes and affect other cancer-associated genes (121). Owing to uncertainties, the routine clinical application of Gallic acid and Kaempferol is not advised, and their prospective role in clinical contexts remains conjectural (122).
10.6 Green tea
Catechins can mitigate muscle damage and oxidative stress in senescence-accelerated mice. Green tea extract augments physical performance in both animals and humans by enhancing endurance and lipid metabolism. Research indicates that EGCG can stimulate fat oxidation genes in the muscle mitochondria of mice subjected to a high-fat diet (123). Concentrated green tea supplements rich in catechins and caffeine can elevate daily energy expenditure in humans. These findings indicate that green tea extract may alleviate the impact of exercise on muscle health. Green tea extract has been shown to enhance fat oxidation and insulin sensitivity during moderate exercise (124). Nonetheless, short-term EGCG supplementation can result in elevated levels in adults. A controlled experiment demonstrated no notable effect on lipid and energy metabolism, inflammatory markers, or oxidative stress indicators. A study revealed no significant alterations in biomarkers following 640 mg of green tea catechins, indicating an inadequate dosage for alleviating oxidative stress and muscle damage; however, augmented aerobic exercise and green tea extract may impede prostate cancer (125). The research included cancer-afflicted rats and a healthy control cohort. A study indicates that green tea extract markedly diminishes prostate cancer risk in rats by reestablishing the equilibrium between pro-oxidants and antioxidants, involving rats subjected to HEGT treatment alone or in conjunction with aerobic exercise (126). Research indicated that aerobic exercise and green tea extract can diminish levels of cyclooxygenase-2 (COX-2), NF-kB, and P53 in the prostates of rats. The rats were categorized into six groups: healthy, cancer, low to moderate-intensity exercise, green tea extract, cancer training with green tea extract, and sham (127). Post-mortem analysis of prostate tissues revealed elevated NF-kB levels in the CCt group and diminished p53 levels in the CTr, CEx, and CTr + CEx groups, indicating that a regular intake of green tea may contribute to the reduction of these levels (128).
Physical activity is associated with cancer, as angiogenesis influences blood vessel formation. The combination of exercise training and plant-derived phytochemicals may aid in cancer prevention. Moderate aerobic exercise is more efficacious in suppressing angiogenesis markers in tumor tissue. MMPs, including MMP-9 and MMP-2, play a role in cancer cell invasion, tumor growth, and the facilitation of metastasis. Endothelial cells can selectively express and activate matrix metalloproteinases (MMPs), initiating angiogenesis and the angiogenic switch, highlighting the potential of combining exercise with plant-derived phytochemicals in cancer treatment (129). The study by Khosravi et al. investigated the effects of aerobic exercise and green tea extract on MMP-2/−9 and VEGF levels in both healthy rats and prostate cancer patients. The results indicated no significant differences in MMP-2, MMP-9, or VEGF levels between the healthy and cancer groups. The study indicates that additional research should concentrate on the regulation of tumor dissemination and angiogenesis concerning physical activity and antioxidant use (130).
10.7 Resveratrol
Resveratrol, a naturally occurring polyphenolic compound, is an antitoxin produced by plants in reaction to external stimuli. It is found in grapes, mulberries, cranberries, and peanuts, and has received significant attention for its cancer-preventive and anti-cancer properties in recent years. Research demonstrates that resveratrol can induce autophagic cell death through the Ca2+/AMPK-mTOR signaling pathway, leading to the death of human non-small cell lung cancer cells (A549). It can also induce apoptosis in human ovarian cancer (OVCAR-3) cells, an effect that is reduced by the autophagy inhibitor chloroquine. Resveratrol can induce autophagy in SKOV3 human ovarian cancer cells and inhibit apoptosis. However, in conjunction with the autophagy inhibitor 3-methyladenine (3-MA), resveratrol markedly increases cell apoptosis by inhibiting autophagy, suggesting that autophagy induced by resveratrol may protect SKOV3 cells. Resveratrol can stimulate autophagy and apoptosis in cisplatin-resistant human oral cancer CAR cells, whereas 3-MA obstructs autophagosome fusion and enhances CAR cell viability (131–133). Resveratrol supplementation modulates inflammation, metabolism, glucose and lipid metabolism, and muscle atrophy by enhancing AMPK activity, decreasing protein degradation, and inhibiting NF-κB; however, its effects in vivo remain contentious. Recently, the research demonstrated that resistance training and resveratrol supplementation significantly diminish tumor volume via mTORC1 and AMPK signaling pathways, leading to decreased phosphorylation and activation of factors and carcinogenic markers (134). Additional research is required to enhance confidence. A study demonstrated that RSV synergistically amplified the anticancer effects of DTX in prostate carcinoma LNCaP cells, resulting in heightened apoptosis and necroptosis. This indicates RSV as a potential adjuvant for DTX therapy in prostate carcinoma.it has been also determined that resveratrol, diminished A549 cell viability in a concentration-dependent manner and exhibited a synergistic effect with cisplatin and carboplatin, potentially facilitating apoptosis via autophagy and elevating reactive oxygen species levels (133).
11 Conclusion and future perspectives
The interplay between polyphenols and exercise in modulating autophagy presents a promising, non-invasive approach to improving health outcomes in both cancer patients and healthy individuals. By targeting key molecular pathways such as AMPK/mTOR, PI3K/Akt, and SIRT1/FOXO, polyphenols and exercise work synergistically to regulate cellular homeostasis, reduce oxidative stress, and modulate inflammatory responses. This review highlights the emerging evidence that polyphenol supplementation, combined with regular physical activity, can enhance autophagic flux, ultimately contributing to cancer prevention, improved treatment responses, and overall metabolic health. However, despite promising preclinical and clinical studies, several knowledge gaps remain. Future research should focus on a few approaches. Determining the optimal doses and bioavailability-enhancing strategies for polyphenols to maximize their autophagy-inducing effects in cancer patients and healthy populations. Also, the role of individual genetic and epigenetic variations in autophagic responses to polyphenols and exercise should be investigated to develop personalized therapeutic strategies. In addition, well-designed, large-scale clinical trials are needed to validate the efficacy of combined polyphenol-exercise interventions in cancer prevention and treatment. Further elucidating the precise molecular mechanisms by which polyphenols and exercise modulate autophagy across different cancer types and physiological states. Furthermore, exploring the long-term health benefits and potential risks of sustained polyphenol intake alongside exercise, particularly in aging populations and cancer survivors. In conclusion, integrating polyphenols with exercise holds significant potential for enhancing autophagy and promoting cellular resilience. With further research and clinical validation, this dual approach may offer an effective, accessible, and non-toxic strategy for cancer management and health optimization.
Author contributions
WL: Writing – review & editing, Writing – original draft, Validation, Conceptualization, Visualization, Methodology, Data curation, Investigation. MH: Supervision, Writing – review & editing, Conceptualization, Investigation, Methodology, Writing – original draft, Validation, Visualization. FC: Data curation, Investigation, Writing – original draft, Conceptualization, Methodology, Writing – review & editing, Visualization, Validation. SJ: Writing – original draft, Project administration, Visualization, Investigation, Writing – review & editing, Conceptualization, Methodology, Validation.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study supported by “the Fundamental Research Funds for the Central Universities” No: ZYTS25277.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Nieman, DC, and Wentz, LM. The compelling link between physical activity and the body's defense system. J Sport Health Sci. (2019) 8:201–17. doi: 10.1016/j.jshs.2018.09.009
2. Neufer, PD, Bamman, MM, Muoio, DM, Bouchard, C, Cooper, DM, Goodpaster, BH, et al. Understanding the cellular and molecular mechanisms of physical activity-induced health benefits. Cell Metab. (2015) 22:4–11. doi: 10.1016/j.cmet.2015.05.011
3. Li, W, Chen, L, Mohammad Sajadi, S, Baghaei, S, and Salahshour, S. The impact of acute and chronic aerobic and resistance exercise on stem cell mobilization: a review of effects in healthy and diseased individuals across different age groups. Reg Therapy. (2024) 27:464–81. doi: 10.1016/j.reth.2024.04.013
4. Docherty, S, Harley, R, McAuley, JJ, Crowe, LAN, Pedret, C, Kirwan, PD, et al. The effect of exercise on cytokines: implications for musculoskeletal health: a narrative review. BMC Sports Sci Med Rehabil. (2022) 14:5. doi: 10.1186/s13102-022-00397-2
5. Gómez-Virgilio, L, Silva-Lucero, MD, Flores-Morelos, DS, Gallardo-Nieto, J, Lopez-Toledo, G, Abarca-Fernandez, AM, et al. Autophagy: a key regulator of homeostasis and disease: an overview of molecular mechanisms and modulators. Cells. (2022) 11:2262. doi: 10.3390/cells11152262
6. Chun, Y, and Kim, J. Autophagy: an essential degradation program for cellular homeostasis and life. Cells. (2018) 7:278. doi: 10.3390/cells7120278
7. Ryter, SW, Cloonan, SM, and Choi, AMK. Autophagy: a critical regulator of cellular metabolism and homeostasis. Mol Cell. (2013) 36:7–16. doi: 10.1007/s10059-013-0140-8
8. Guo, F, Liu, X, Cai, H, and Le, W. Autophagy in neurodegenerative diseases: pathogenesis and therapy. Brain Pathol. (2018) 28:3–13. doi: 10.1111/bpa.12545
9. Sehrawat, A, Mishra, J, Mastana, SS, Navik, U, Bhatti, GK, Reddy, PH, et al. Dysregulated autophagy: a key player in the pathophysiology of type 2 diabetes and its complications. Biochim Biophys Acta (BBA) - Mol Basis Dis. (2023) 1869:166666. doi: 10.1016/j.bbadis.2023.166666
10. Ortega, MA, Fraile-Martinez, O, de Leon-Oliva, D, Boaru, DL, Lopez-Gonzalez, L, García-Montero, C, et al. Autophagy in its (proper) context: molecular basis, biological relevance, pharmacological modulation, and lifestyle medicine. Int J Biol Sci. (2024) 20:2532–54. doi: 10.7150/ijbs.95122
11. Kwon, I, Song, W, Jang, Y, Choi, MD, Vinci, DM, and Lee, Y. Elevation of hepatic autophagy and antioxidative capacity by endurance exercise is associated with suppression of apoptosis in mice. Ann Hepatol. (2020) 19:69–78. doi: 10.1016/j.aohep.2019.08.010
12. Jiang, B, Zhou, X, Yang, T, Wang, L, Feng, L, Wang, Z, et al. The role of autophagy in cardiovascular disease: cross-interference of signaling pathways and underlying therapeutic targets. Front Cardiovasc Med. (2023) 10:10. doi: 10.3389/fcvm.2023.1088575
13. Halling, JF, and Pilegaard, H. Autophagy-dependent beneficial effects of exercise. Cold Spring Harb Perspect Med. (2017) 7:9777. doi: 10.1101/cshperspect.a029777
14. Wu, NN, Tian, H, Chen, P, Wang, D, Ren, J, and Zhang, Y. Physical exercise and selective autophagy: benefit and risk on cardiovascular health. Cells. (2019) 8:1436. doi: 10.3390/cells8111436
15. Dashti, Z, Yousefi, Z, Kiani, P, Taghizadeh, M, Maleki, MH, Borji, M, et al. Autophagy and the unfolded protein response shape the non-alcoholic fatty liver landscape: decoding the labyrinth. Metabolism. (2024) 154:155811. doi: 10.1016/j.metabol.2024.155811
16. Parzych, KR, and Klionsky, DJ. An overview of autophagy: morphology, mechanism, and regulation. Antioxid Redox Signal. (2014) 20:460–73. doi: 10.1089/ars.2013.5371
17. Gallagher, LE, Williamson, LE, and Chan, EYW. Advances in autophagy regulatory mechanisms. Cells. (2016) 5:24. doi: 10.3390/cells5020024
18. Kiani, P, Khodadadi, ES, Nikdasti, A, Yarahmadi, S, Gheibi, M, Yousefi, Z, et al. Autophagy and the peroxisome proliferator-activated receptor signaling pathway: a molecular ballet in lipid metabolism and homeostasis. Mol Cell Biochem. (2025) 480:3477–99. doi: 10.1007/s11010-025-05207-0
19. Murugan, S, and Amaravadi, RK. Methods for studying autophagy within the tumor microenvironment. Adv Exp Med Biol. (2016) 899:145–66. doi: 10.1007/978-3-319-26666-4_9
20. Mawed, SA, Zhang, J, Ren, F, He, Y, and Mei, J. Atg 7 and beclin 1 are essential for energy metabolism and survival during the larval-to-juvenile transition stage of zebrafish. Aquac Fish. (2022) 7:359–72. doi: 10.1016/j.aaf.2021.01.002
21. Collier, JJ, Suomi, F, Oláhová, M, McWilliams, TG, and Taylor, RW. Emerging roles of ATG7 in human health and disease. EMBO Mol Med. (2021) 13:e14824. doi: 10.15252/emmm.202114824
22. Miceli, C, Leri, M, Stefani, M, and Bucciantini, M. Autophagy-related proteins: potential diagnostic and prognostic biomarkers of aging-related diseases. Ageing Res Rev. (2023) 89:101967. doi: 10.1016/j.arr.2023.101967
23. Yin, X, Zhou, C, Li, J, Liu, R, Shi, B, Yuan, Q, et al. Autophagy in bone homeostasis and the onset of osteoporosis. Bone Res. (2019) 7:28. doi: 10.1038/s41413-019-0058-7
24. Chen, J, Zhou, R, Feng, Y, and Cheng, L. Molecular mechanisms of exercise contributing to tissue regeneration. Signal Transduct Target Ther. (2022) 7:383. doi: 10.1038/s41392-022-01233-2
25. Leal, LG, Lopes, MA, and Batista, ML Jr. Physical exercise-induced Myokines and muscle-adipose tissue crosstalk: a review of current knowledge and the implications for health and metabolic diseases. Front Physiol. (2018) 9:1307. doi: 10.3389/fphys.2018.01307
26. Marshall, KL, and Lumpkin, EA. The molecular basis of mechanosensory transduction. Adv Exp Med Biol. (2012) 739:142–55. doi: 10.1007/978-1-4614-1704-0_9
27. Cox, CD, Bavi, N, and Martinac, B. Biophysical principles of Ion-Channel-mediated Mechanosensory transduction. Cell Rep. (2019) 29:1–12. doi: 10.1016/j.celrep.2019.08.075
28. Delmas, P, Parpaite, T, and Coste, B. PIEZO channels and newcomers in the mammalian mechanosensitive ion channel family. Neuron. (2022) 110:2713–27. doi: 10.1016/j.neuron.2022.07.001
29. Shi, S, Kang, XJ, Zhou, Z, He, ZM, Zheng, S, and He, SS. Excessive mechanical stress-induced intervertebral disc degeneration is related to Piezo 1 overexpression triggering the imbalance of autophagy/apoptosis in human nucleus pulpous. Arthritis Res Ther. (2022) 24:119. doi: 10.1186/s13075-022-02804-y
30. Yang, M, Pi, H, Li, M, Xu, S, Zhang, L, Xie, J, et al. From the cover: autophagy induction contributes to cadmium toxicity in mesenchymal stem cells via AMPK/FOXO3a/BECN1 signaling. Toxicol Sci. (2016) 154:101–14. doi: 10.1093/toxsci/kfw144
31. Wang, J, Huang, Y, Wang, Z, Liu, J, Liu, Z, Yang, J, et al. The mTOR signaling pathway: key regulator and therapeutic target for heart disease. Biomedicine. (2025) 13:397. doi: 10.3390/biomedicines13020397
32. Mao, J, Yang, R, Yuan, P, Wu, F, Wei, Y, Nie, Y, et al. Different stimuli induce endothelial dysfunction and promote atherosclerosis through the Piezo 1/YAP signaling axis. Arch Biochem Biophys. (2023) 747:109755. doi: 10.1016/j.abb.2023.109755
33. Zhou, J, Li, YS, and Chien, S. Shear stress-initiated signaling and its regulation of endothelial function. Arterioscler Thromb Vasc Biol. (2014) 34:2191–8. doi: 10.1161/ATVBAHA.114.303422
34. Huang, J, Zhang, K, Du, R, Liu, W, Zhang, H, Tian, T, et al. The Janus-faced role of Piezo 1 in cardiovascular health under mechanical stimulation. Genes Dis. (2023) 10:1956–68. doi: 10.1016/j.gendis.2022.08.015
35. Chu, F, Tan, R, Wang, X, Zhou, X, Ma, R, Ma, X, et al. Transcranial magneto-acoustic stimulation attenuates synaptic plasticity impairment through the activation of Piezo 1 in Alzheimer's disease mouse model. Research (Wash D C). (2023) 6:0130. doi: 10.34133/research.0130
36. Arjunan, A, and Song, J. Pharmacological and physiological roles of adipokines and myokines in metabolic-related dementia. Biomed Pharmacother. (2023) 163:114847. doi: 10.1016/j.biopha.2023.114847
37. Stitt, TN, Drujan, D, Clarke, BA, Panaro, F, Timofeyva, Y, Kline, WO, et al. The IGF-1/PI3K/Akt pathway prevents expression of muscle atrophy-induced ubiquitin ligases by inhibiting FOXO transcription factors. Mol Cell. (2004) 14:395–403. doi: 10.1016/S1097-2765(04)00211-4
38. Wagner, PD. The critical role of VEGF in skeletal muscle angiogenesis and blood flow. Biochem Soc Trans. (2011) 39:1556–9. doi: 10.1042/BST20110646
39. Ning, K, Wang, Z, and Zhang, XA. Exercise-induced modulation of myokine irisin in bone and cartilage tissue-positive effects on osteoarthritis: a narrative review. Front Aging Neurosci. (2022) 14:934406. doi: 10.3389/fnagi.2022.934406
40. Pesce, M, Ballerini, P, Paolucci, T, Puca, I, Farzaei, MH, and Patruno, A. Irisin and autophagy: first update. Int J Mol Sci. (2020) 21:7587. doi: 10.3390/ijms21207587
41. Nikooie, R, Moflehi, D, and Zand, S. Lactate regulates autophagy through ROS-mediated activation of ERK1/2/m-TOR/p-70S6K pathway in skeletal muscle. J Cell Commun Signal. (2021) 15:107–23. doi: 10.1007/s12079-020-00599-8
42. Volpe-Fix, AR, de França, E, Silvestre, JC, and Thomatieli-Santos, RV. The use of some polyphenols in the modulation of muscle damage and inflammation induced by physical exercise: a review. Foods. (2023) 12:916. doi: 10.3390/foods12050916
43. Palermo, M, Staples, C, and Rancourt, D. Examining the impact of weight bias on the association between exercise identity and maladaptive exercise behaviors. Eat Behav. (2021) 41:101503. doi: 10.1016/j.eatbeh.2021.101503
44. Scheffer, DDL, and Latini, A. Exercise-induced immune system response: anti-inflammatory status on peripheral and central organs. Biochim Biophys Acta Mol basis Dis. (2020) 1866:165823. doi: 10.1016/j.bbadis.2020.165823
45. Wang, C, Liang, J, Ren, Y, Huang, J, Jin, B, Wang, G, et al. A preclinical systematic review of the effects of chronic exercise on autophagy-related proteins in aging skeletal muscle. Front Physiol. (2022) 13:930185. doi: 10.3389/fphys.2022.930185
46. Botella, J, Jamnick, NA, Granata, C, Genders, AJ, Perri, E, Jabar, T, et al. Exercise and training regulation of autophagy markers in human and rat skeletal muscle. Int J Mol Sci. (2022) 23:2619. doi: 10.3390/ijms23052619
47. Tarawan, VM, Gunadi, JW, Setiawan, LR, Goenawan, H, Meilina, DE, et al. Alteration of autophagy gene expression by different intensity of exercise in gastrocnemius and soleus muscles of Wistar rats. J Sports Sci Med. (2019) 18:146–54.
48. Mejías-Peña, Y, Estébanez, B, Rodriguez-Miguelez, P, Fernandez-Gonzalo, R, Almar, M, de Paz, JA, et al. Impact of resistance training on the autophagy-inflammation-apoptosis crosstalk in elderly subjects. Aging (Albany NY). (2017) 9:408–18. doi: 10.18632/aging.101167
49. Rocchi, A, and He, C. Activating autophagy by aerobic exercise in mice. J Vis Exp. (2017). doi: 10.3791/55099
50. Keerthana, CK, Rayginia, TP, Shifana, SC, Anto, NP, Kalimuthu, K, Isakov, N, et al. The role of AMPK in cancer metabolism and its impact on the immunomodulation of the tumor microenvironment. Front Immunol. (2023) 14:1114582. doi: 10.3389/fimmu.2023.1114582
51. Pang, H, and Badehnoosh, B. Synergistic strength: unleashing exercise and polyphenols against breast cancer. Cancer Cell Int. (2025) 25:144. doi: 10.1186/s12935-025-03767-1
52. Wang, P, Li, CG, Zhou, X, Cui, D, Ouyang, T, Chen, W, et al. A single bout of exhaustive treadmill exercise increased AMPK activation associated with enhanced autophagy in mice skeletal muscle. Clin Exp Pharmacol Physiol. (2022) 49:536–43. doi: 10.1111/1440-1681.13632
53. Cantó, C, Gerhart-Hines, Z, Feige, JN, Lagouge, M, Noriega, L, Milne, JC, et al. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature. (2009) 458:1056–60. doi: 10.1038/nature07813
54. Mishra, J, Bhatti, GK, Sehrawat, A, Singh, C, Singh, A, Reddy, AP, et al. Modulating autophagy and mitophagy as a promising therapeutic approach in neurodegenerative disorders. Life Sci. (2022) 311:121153. doi: 10.1016/j.lfs.2022.121153
55. Rovira, M, Arrey, G, and Planas, JV. Exercise-induced hypertrophic and oxidative signaling pathways and Myokine expression in fast muscle of adult zebrafish. Front Physiol. (2017) 8:1063. doi: 10.3389/fphys.2017.01063
56. Zhou, X-H, Luo, Y-X, and Yao, X-Q. Exercise-driven cellular autophagy: a bridge to systematic wellness. J Adv Res. (2025). doi: 10.1016/j.jare.2024.12.036
57. Alers, S, Löffler, AS, Wesselborg, S, and Stork, B. Role of AMPK-mTOR-Ulk 1/2 in the regulation of autophagy: cross talk, shortcuts, and feedbacks. Mol Cell Biol. (2012) 32:2–11. doi: 10.1128/MCB.06159-11
58. Kim, J, Kundu, M, Viollet, B, and Guan, KL. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk 1. Nat Cell Biol. (2011) 13:132–41. doi: 10.1038/ncb2152
59. Whyte, JJ, and Laughlin, MH. The effects of acute and chronic exercise on the vasculature. Acta Physiol (Oxford). (2010) 199:441–50. doi: 10.1111/j.1748-1716.2010.02127.x
60. Basso, JC, and Suzuki, WA. The effects of acute exercise on mood, cognition, neurophysiology, and neurochemical pathways: a review. Brain Plast. (2017) 2:127–52. doi: 10.3233/BPL-160040
61. Skouras, AZ, Antonakis-Karamintzas, D, Tsantes, AG, Triantafyllou, A, Papagiannis, G, Tsolakis, C, et al. The acute and chronic effects of resistance and aerobic exercise in hemostatic balance: a brief review. Sports. (2023) 11:74. doi: 10.3390/sports11040074
62. Wang, C, Kirberger, M, and Chen, N. Acute and chronic exercise on autophagy In: N Chen, editor. Exercise, autophagy and chronic diseases. Singapore: Springer Singapore (2021). 29–46.
63. Bloemberg, D, McDonald, E, Dulay, D, and Quadrilatero, J. Autophagy is altered in skeletal and cardiac muscle of spontaneously hypertensive rats. Acta Physiol (Oxford). (2014) 210:381–91. doi: 10.1111/apha.12178
64. Lin, R, and Kerkelä, R. Regulatory mechanisms of mitochondrial function and cardiac aging. Int J Mol Sci. (2020) 21:1359. doi: 10.3390/ijms21041359
65. Zong, Y, Li, H, Liao, P, Chen, L, Pan, Y, Zheng, Y, et al. Mitochondrial dysfunction: mechanisms and advances in therapy. Signal Transduct Target Ther. (2024) 9:124. doi: 10.1038/s41392-024-01839-8
66. Vásquez-Trincado, C, García-Carvajal, I, Pennanen, C, Parra, V, Hill, JA, Rothermel, BA, et al. Mitochondrial dynamics, mitophagy and cardiovascular disease. J Physiol. (2016) 594:509–25. doi: 10.1113/JP271301
67. Vainshtein, A, and Hood, DA. The regulation of autophagy during exercise in skeletal muscle. J Appl Physiol. (2016) 120:664–73. doi: 10.1152/japplphysiol.00550.2015
68. Grumati, P, Coletto, L, Schiavinato, A, Castagnaro, S, Bertaggia, E, Sandri, M, et al. Physical exercise stimulates autophagy in normal skeletal muscles but is detrimental for collagen VI-deficient muscles. Autophagy. (2011) 7:1415–23. doi: 10.4161/auto.7.12.17877
69. Bodine, SC, and Baehr, LM. Skeletal muscle atrophy and the E3 ubiquitin ligases MuRF1 and MAFbx/atrogin-1. Am J Physiol Endocrinol Metab. (2014) 307:E469–84. doi: 10.1152/ajpendo.00204.2014
70. da Rocha, AL, Pinto, AP, Morais, GP, Marafon, BB, Rovina, RL, Veras, ASC, et al. Moderate, but not excessive, training attenuates autophagy machinery in metabolic tissues. Int J Mol Sci. (2020) 21:8416. doi: 10.3390/ijms21228416
71. Christensen, NM, Ringholm, S, Buch, BT, Gudiksen, A, Halling, JF, and Pilegaard, H. Muscle PGC-1α modulates hepatic mitophagy regulation during aging. Exp Gerontol. (2023) 172:112046. doi: 10.1016/j.exger.2022.112046
72. Triolo, M, Oliveira, AN, Kumari, R, and Hood, DA. The influence of age, sex, and exercise on autophagy, mitophagy, and lysosome biogenesis in skeletal muscle. Skelet Muscle. (2022) 12:13. doi: 10.1186/s13395-022-00296-7
73. Talbot, J, and Maves, L. Skeletal muscle fiber type: using insights from muscle developmental biology to dissect targets for susceptibility and resistance to muscle disease. Wiley Interdiscip Rev Dev Biol. (2016) 5:518–34. doi: 10.1002/wdev.230
74. Kwon, I, Kim, KS, and Lee, Y. Relationships between endurance exercise training-induced muscle fiber-type shifting and autophagy in slow-and fast-twitch skeletal muscles of mice. Phys Act Nutr. (2024) 28:23–34. doi: 10.20463/pan.2024.0013
75. Ferraro, E, Giammarioli, AM, Chiandotto, S, Spoletini, I, and Rosano, G. Exercise-induced skeletal muscle remodeling and metabolic adaptation: redox signaling and role of autophagy. Antioxid Redox Signal. (2014) 21:154–76. doi: 10.1089/ars.2013.5773
76. Sanchez, AM, Bernardi, H, Py, G, and Candau, RB. Autophagy is essential to support skeletal muscle plasticity in response to endurance exercise. Am J Phys Regul Integr Comp Phys. (2014) 307:R956–69. doi: 10.1152/ajpregu.00187.2014
77. Xie, G, Jin, H, Mikhail, H, Pavel, V, Yang, G, Ji, B, et al. Autophagy in sarcopenia: possible mechanisms and novel therapies. Biomed Pharmacother. (2023) 165:115147. doi: 10.1016/j.biopha.2023.115147
78. Andreotti, DZ, Silva, JDN, Matumoto, AM, Orellana, AM, de Mello, PS, and Kawamoto, EM. Effects of physical exercise on autophagy and apoptosis in aged brain: human and animal studies. Front Nutr. (2020) 7:94. doi: 10.3389/fnut.2020.00094
79. Jakubek, P, Pakula, B, Rossmeisl, M, Pinton, P, Rimessi, A, and Wieckowski, MR. Autophagy alterations in obesity, type 2 diabetes, and metabolic dysfunction-associated steatotic liver disease: the evidence from human studies. Intern Emerg Med. (2024) 19:1473–91. doi: 10.1007/s11739-024-03700-w
80. Khan, F, Khan, H, Khan, A, Yamasaki, M, Moustaid-Moussa, N, Al-Harrasi, A, et al. Autophagy in adipogenesis: molecular mechanisms and regulation by bioactive compounds. Biomed Pharmacother. (2022) 155:113715. doi: 10.1016/j.biopha.2022.113715
81. Zhang, Y, Goldman, S, Baerga, R, Zhao, Y, Komatsu, M, and Jin, S. Adipose-specific deletion of autophagy-related gene 7 (atg 7) in mice reveals a role in adipogenesis. Proc Natl Acad Sci USA. (2009) 106:19860–5. doi: 10.1073/pnas.0906048106
82. Lahiri, V, Hawkins, WD, and Klionsky, DJ. Watch what you (self-) eat: Autophagic mechanisms that modulate metabolism. Cell Metab. (2019) 29:803–26. doi: 10.1016/j.cmet.2019.03.003
83. Pahlavani, HA. Exercise-induced signaling pathways to counteracting cardiac apoptotic processes. Front Cell Dev Biol. (2022) 10:950927. doi: 10.3389/fcell.2022.950927
84. Hong, AR, and Kim, SW. Effects of resistance exercise on bone health. Endocrinol Metab (Seoul). (2018) 33:435–44. doi: 10.3803/EnM.2018.33.4.435
85. Huang, L, Li, M, Deng, C, Qiu, J, Wang, K, Chang, M, et al. Potential therapeutic strategies for skeletal muscle atrophy. Antioxidants. (2023) 12:44. doi: 10.3390/antiox12010044
86. Lenk, K, Schuler, G, and Adams, V. Skeletal muscle wasting in cachexia and sarcopenia: molecular pathophysiology and impact of exercise training. J Cachexia Sarcopenia Muscle. (2010) 1:9–21. doi: 10.1007/s13539-010-0007-1
87. Vainshtein, A, and Sandri, M. Signaling pathways that control muscle mass. Int J Mol Sci. (2020) 21:4759. doi: 10.3390/ijms21134759
88. Fujii, Y, Liu, L, Yagasaki, L, Inotsume, M, Chiba, T, and Asahara, H. Cartilage homeostasis and osteoarthritis. Int J Mol Sci. (2022) 23:6316. doi: 10.3390/ijms23116316
89. Parihar, MS, and Brewer, GJ. Amyloid-β as a modulator of synaptic plasticity. J Alzheimer's Dis. (2010) 22:741–63. doi: 10.3233/JAD-2010-101020
90. Hirata, M, Sakuma, K, Okajima, S, Fujiwara, H, Inashima, S, Yasuhara, M, et al. Increased expression of neuregulin-1 in differentiating muscle satellite cells and in motoneurons during muscle regeneration. Acta Neuropathol. (2007) 113:451–9. doi: 10.1007/s00401-007-0198-5
91. Gupta, R, Ambasta, RK, and Pravir, K. Autophagy and apoptosis cascade: which is more prominent in neuronal death? Cell Mol Life Sci. (2021) 78:8001–47. doi: 10.1007/s00018-021-04004-4
92. Li, J, Xie, Y, Zheng, S, He, H, Wang, Z, Li, X, et al. Targeting autophagy in diabetic cardiomyopathy: from molecular mechanisms to pharmacotherapy. Biomed Pharmacother. (2024) 175:116790. doi: 10.1016/j.biopha.2024.116790
93. Titus, AS, Sung, E-A, Zablocki, D, and Sadoshima, J. Mitophagy for cardioprotection. Basic Res Cardiol. (2023) 118:42. doi: 10.1007/s00395-023-01009-x
94. Yamamoto, E, Lai, ZF, Yamashita, T, Tanaka, T, Kataoka, K, Tokutomi, Y, et al. Enhancement of cardiac oxidative stress by tachycardia and its critical role in cardiac hypertrophy and fibrosis. J Hypertens. (2006) 24:2057–69. doi: 10.1097/01.hjh.0000244956.47114.c1
95. Dudley, P. Exercise and physical activity in the prevention and treatment of atherosclerotic cardiovascular disease. Arterioscler Thromb Vasc Biol. (2003) 23:1319–21. doi: 10.1161/01.ATV.0000087143.33998.F2Ñ40
96. Li, Y, Sun, D, Zheng, Y, and Cheng, Y. Swimming exercise activates aortic autophagy and limits atherosclerosis in Apo E(−/−) mice. Obes Res Clin Pract. (2020) 14:264–70. doi: 10.1016/j.orcp.2020.04.008
97. Zhang, H, Zhang, Y, Zhang, J, and Jia, D. Exercise alleviates cardiovascular diseases by improving mitochondrial homeostasis. J Am Heart Assoc. (2024) 13:e036555. doi: 10.1161/JAHA.124.036555
98. Zhang, F, Lin, JJ, Tian, HN, and Wang, J. Effect of exercise on improving myocardial mitochondrial function in decreasing diabetic cardiomyopathy. Exp Physiol. (2024) 109:190–201. doi: 10.1113/EP091309
99. Shah, IA, Ishaq, S, Lee, S-D, and Wu, B-T. Effects of exercise training on cardiac mitochondrial functions in diabetic heart: a systematic review. Int J Mol Sci. (2025) 26:8. doi: 10.3390/ijms26010008
100. Fulghum, K, and Hill, BG. Metabolic mechanisms of exercise-induced cardiac remodeling. Front Cardiovasc Med. (2018) 5:127. doi: 10.3389/fcvm.2018.00127
101. Li, K, Wan, B, Li, S, Chen, Z, Jia, H, Song, Y, et al. Mitochondrial dysfunction in cardiovascular disease: towards exercise regulation of mitochondrial function. Front Physiol. (2023) 14:1063556. doi: 10.3389/fphys.2023.1063556
102. Lim, AY, Chen, Y-C, Hsu, C-C, Fu, T-C, and Wang, J-S. The effects of exercise training on mitochondrial function in cardiovascular diseases: a systematic review and Meta-analysis. Int J Mol Sci. (2022) 23:12559. doi: 10.3390/ijms232012559
103. Zeng, Z, Liang, J, Wu, L, Zhang, H, Lv, J, and Chen, N. Exercise-induced autophagy suppresses sarcopenia through Akt/mTOR and Akt/fox O3a signal pathways and AMPK-mediated mitochondrial quality control. Front Physiol. (2020) 11:583478. doi: 10.3389/fphys.2020.583478
104. Iqbal, I, Wilairatana, P, Saqib, F, Nasir, B, Wahid, M, Latif, MF, et al. Plant polyphenols and their potential benefits on cardiovascular health: a review. Molecules. (2023) 28:6403. doi: 10.3390/molecules28176403
105. Zahra, M, Abrahamse, H, and George, BP. Flavonoids: antioxidant powerhouses and their role in nanomedicine. Antioxidants (Basel). (2024) 13:13. doi: 10.3390/antiox13080922
106. Shahi, T, Assadpour, E, and Jafari, SM. Main chemical compounds and pharmacological activities of stigmas and tepals of ‘red gold’; saffron. Trends Food Sci Technol. (2016) 58:69–78. doi: 10.1016/j.tifs.2016.10.010
107. Nezamdoost, Z, Saghebjoo, M, Hoshyar, R, Hedayati, M, and Keska, A. High-intensity training and saffron: effects on breast cancer-related gene expression. Med Sci Sports Exerc. (2020) 52:1470–6. doi: 10.1249/MSS.0000000000002274
108. Mirzaei, H, Gharehgozlou, R, Heydarirad, G, Fahimi, S, Ghafari, S, Mosavat, SH, et al. Efficacy and safety of Jollab (a saffron-based beverage) on Cancer-related fatigue in breast Cancer patients: a double-blind randomized clinical trial. Complement Med Res. (2022) 29:437–45. doi: 10.1159/000525775
109. Sharifi-Rad, J, Rayess, YE, Rizk, AA, Sadaka, C, Zgheib, R, Zam, W, et al. Turmeric and its major compound curcumin on health: bioactive effects and safety profiles for food, pharmaceutical, biotechnological and medicinal applications. Front Pharmacol. (2020) 11:01021. doi: 10.3389/fphar.2020.01021
110. Guo, J, Li, Z, Yao, Y, Fang, L, Yu, M, and Wang, Z. Curcumin in the treatment of inflammation and oxidative stress responses in traumatic brain injury: a systematic review and meta-analysis. Front Neurol. (2024) 15:1380353. doi: 10.3389/fneur.2024.1380353
111. Guo, Y, Su, J, Jiang, S, Xu, Y, Dou, B, Li, T, et al. Transcriptomics and metabonomics study on the effect of exercise combined with curcumin supplementation on breast cancer in mice. Heliyon. (2024) 10:e28807. doi: 10.1016/j.heliyon.2024.e28807
112. Daryanoosh, F, Zolfaghari, M, Hashemi, SM, Jahromi, MK, Jalili, A, Khazaei, H, et al. Synergistic effect of exercise training and curcumin supplementation on inflammation indices in overweight breast-cancer patients after adjuvant chemotherapy and/or radiation therapy: a randomized controlled trial study. Sport Sci Health. (2025) 21:251–9. doi: 10.1007/s11332-024-01252-2
113. Mirzayan Shanjani, S, and Benaifar, AA. The effect of aerobic exercise training and consumption of curcumin nano micelles on the expression level of CASP3, CASP9, Bax and BCL2 genes on cardiac tissues of Balb/C mice with induced breast cancer treated with doxorubicin. Iran J Breast Dis. (2023) 16:67–83. doi: 10.30699/ijbd.16.2.67
114. Branch, T. (2020). Synergistic effect of endurance training combined with curcumin on intratumoral expression of interleukin-4 (Il4) and Stat-6 in female mice with breast cancer.
115. Hemati, S, Mehrabinejad, F, Elhaie, M, and Najafizade, N. Curcumin supplementation as a preventive strategy against tamoxifen-induced nonalcoholic fatty liver disease in ER+ breast Cancer patients: a triple-blind randomized placebo-controlled trial. J Diet Suppl. (2025) 22:274–83. doi: 10.1080/19390211.2025.2465412
116. Aghababaei, F, and Hadidi, M. Recent advances in potential health benefits of quercetin. Pharmaceuticals (Basel). (2023) 16:1020. doi: 10.3390/ph16071020
117. Wang, Y. The interplay of exercise and polyphenols in cancer treatment: a focus on oxidative stress and antioxidant mechanisms. Phytother Res. (2024) 38:3459–88. doi: 10.1002/ptr.8215
118. Sadighparvar, S, Darband, SG, Yousefi, B, Kaviani, M, Ghaderi-Pakdel, F, Mihanfar, A, et al. Combination of quercetin and exercise training attenuates depression in rats with 1, 2-dimethylhydrazine-induced colorectal cancer: possible involvement of inflammation and BDNF signalling. Exp Physiol. (2020) 105:1598–609. doi: 10.1113/EP088605
119. Wang, B, Xu, H, Hu, X, Ma, W, Zhang, J, Li, Y, et al. Synergetic inhibition of daidzein and regular exercise on breast cancer in bearing-4T1 mice by regulating NK cells and apoptosis pathway. Life Sci. (2020) 245:117387. doi: 10.1016/j.lfs.2020.117387
120. Sauter, ER. Breast Cancer prevention: current approaches and future directions. Eur J Breast Health. (2018) 14:64–71. doi: 10.5152/ejbh.2018.3978
121. Malekpoor, Z, Taghian, F, and Jalali Dehkordi, K. The effect of endurance training and consumption of gallic acid and kaempferol on the expression of ARC, VEGFα, HIF1α and CREB1 genes in mice with breast cancer treated with paclitaxel. J Breast Dis. (2024) 17:22–40. doi: 10.61186/ijbd.17.1.22
122. Huang, H-L, Lin, C-C, Jeng, K-CG, Yao, P-W, Chuang, L-T, Kuo, S-L, et al. Fresh green tea and gallic acid ameliorate oxidative stress in kainic acid-induced status epilepticus. J Agric Food Chem. (2012) 60:2328–36. doi: 10.1021/jf203709q
123. Hodgson, AB, Randell, RK, and Jeukendrup, AE. The effect of green tea extract on fat oxidation at rest and during exercise: evidence of efficacy and proposed mechanisms. Adv Nutr. (2013) 4:129–40. doi: 10.3945/an.112.003269
124. Hodgson, AB, Randell, RK, Boon, N, Garczarek, U, Mela, DJ, Jeukendrup, AE, et al. Metabolic response to green tea extract during rest and moderate-intensity exercise. J Nutr Biochem. (2013) 24:325–34. doi: 10.1016/j.jnutbio.2012.06.017
125. Rasaei, N, Asbaghi, O, Samadi, M, Setayesh, L, Bagheri, R, Gholami, F, et al. Effect of green tea supplementation on antioxidant status in adults: a systematic review and Meta-analysis of randomized clinical trials. Antioxidants. (2021) 10:1731. doi: 10.3390/antiox10111731
126. Bosland, MC, Horton, L, and Condon, MS. Effects of green tea on prostate carcinogenesis in rat models and a human prostate cancer xenograft model. Prostate. (2022) 82:1117–24. doi: 10.1002/pros.24364
127. Saedmocheshi, S, Saghebjoo, M, Vahabzadeh, Z, and Sheikholeslami-Vatani, D. Aerobic training and green tea extract protect against N-methyl-N-nitrosourea-induced prostate Cancer. Med Sci Sports Exerc. (2019) 51:2210–6. doi: 10.1249/MSS.0000000000002054
128. Gesztes, WR, Lap, CJ, Rajendran, R, Dalivand, MM, Diao, G, Liu, S, et al. Investigating intensity and percentage of p 53 nuclear expression in prostate cancer: findings from a cohort of U.S. military veterans. Cancers (Basel). (2025) 17:1004. doi: 10.3390/cancers17061004
129. Liu, B, Tian, H, and Momeni, MR. The interplay of exercise and green tea: a new road in cancer therapy. Cancer Cell Int. (2025) 25:6. doi: 10.1186/s12935-024-03632-7
130. Khosravi, A, Saghebjoo, M, and Vahabzadeh, Z. The effects of eight weeks of aerobic training and green tea extract consumption on some angiogenesis and metastasis markers in prostate cancer-induced rats. Middle East J Rehabilit Health Stud. (2020) 7:99183. doi: 10.5812/mejrh.99183
131. Koushki, M, Amiri-Dashatan, N, Ahmadi, N, Abbaszadeh, HA, and Rezaei-Tavirani, M. Resveratrol: a miraculous natural compound for diseases treatment. Food Sci Nutr. (2018) 6:2473–90. doi: 10.1002/fsn3.855
132. Li, J, Fan, Y, Zhang, Y, Liu, Y, Yu, Y, and Ma, M. Resveratrol induces autophagy and apoptosis in non-small-cell lung cancer cells by activating the NGFR-AMPK-mTOR pathway. Nutrients. (2022) 14:2413. doi: 10.3390/nu14122413
133. Wang, H, Peng, Y, Wang, J, Gu, A, Li, Q, Mao, D, et al. Effect of autophagy on the resveratrol-induced apoptosis of ovarian cancer SKOV3 cells. J Cell Biochem. (2019) 120:7788–93. doi: 10.1002/jcb.28053
134. Kulkarni, SS, and Cantó, C. The molecular targets of resveratrol. Biochim Biophys Acta (BBA) - Mol Basis Dis. (2015) 1852:1114–23. doi: 10.1016/j.bbadis.2014.10.005
135. Tanaka, G, Kato, H, and Izawa, T. Endurance exercise training induces fat depot-specific differences in basal autophagic activity. Biochem Biophys Res Commun. (2015) 466:512–7. doi: 10.1016/j.bbrc.2015.09.061
136. Fritzen, AM, Madsen, AB, Kleinert, M, Treebak, JT, Lundsgaard, AM, Jensen, TE, et al. Regulation of autophagy in human skeletal muscle: effects of exercise, exercise training and insulin stimulation. J Physiol. (2016) 594:745–61. doi: 10.1113/JP271405
137. Chun, SK, Lee, S, Yang, MJ, Leeuwenburgh, C, and Kim, JS. Exercise-induced autophagy in fatty liver disease. Exerc Sport Sci Rev. (2017) 45:181–6. doi: 10.1249/JES.0000000000000116
138. Pyo, J-O, Yoo, S-M, Ahn, H-H, Nah, J, Hong, S-H, Kam, T-I, et al. Overexpression of Atg 5 in mice activates autophagy and extends lifespan. Nat Commun. (2013) 4:2300. doi: 10.1038/ncomms3300
139. Kim, M, Sandford, E, Gatica, D, Qiu, Y, Liu, X, Zheng, Y, et al. Mutation in ATG5 reduces autophagy and leads to ataxia with developmental delay. eLife. (2016) 5:e12245. doi: 10.7554/eLife.12245
140. Tutas, J, Tolve, M, Özer-Yildiz, E, Ickert, L, Klein, I, Hosseini, M, et al. Autophagy regulator ATG5 preserves cerebellar function by safeguarding its glycolytic activity. bioRxiv. (2024):2024.01.27.577372. doi: 10.1038/s42255-024-01196-4
141. Masiero, E, Agatea, L, Mammucari, C, Blaauw, B, Loro, E, Komatsu, M, et al. Autophagy is required to maintain muscle mass. Cell Metab. (2009) 10:507–15. doi: 10.1016/j.cmet.2009.10.008
142. Brandt, N, Gunnarsson, TP, Bangsbo, J, and Pilegaard, H. Exercise and exercise training-induced increase in autophagy markers in human skeletal muscle. Phys Rep. (2018) 6:e13651. doi: 10.14814/phy2.13651
143. Huang, Y, Liu, HT, Yuan, Y, Guo, YP, Wan, DF, and Pan, SS. Exercise preconditioning increases Beclin 1 and induces autophagy to promote early myocardial protection via intermittent myocardial ischemia-hypoxia. Int Heart J. (2021) 62:407–15. doi: 10.1536/ihj.20-597
144. Wang, L, Ma, H, Huang, P, Xie, Y, Near, D, Wang, H, et al. Down-regulation of Beclin 1 promotes direct cardiac reprogramming. Sci Transl Med. (2020) 12:12. doi: 10.1126/scitranslmed.aay7856
145. Vainshtein, A, Desjardins, EM, Armani, A, Sandri, M, and Hood, DA. PGC-1α modulates denervation-induced mitophagy in skeletal muscle. Skelet Muscle. (2015) 5:1–17. doi: 10.1186/s13395-015-0033-y
146. Vainshtein, A, Tryon, LD, Pauly, M, and Hood, DA. Role of PGC-1α during acute exercise-induced autophagy and mitophagy in skeletal muscle. Am J Phys Cell Phys. (2015) 308:C710–9. doi: 10.1152/ajpcell.00380.2014
147. Dethlefsen, MM, Kristensen, CM, Tøndering, AS, Lassen, SB, Ringholm, S, and Pilegaard, H. Impact of liver PGC-1α on exercise and exercise training-induced regulation of hepatic autophagy and mitophagy in mice on HFF. Phys Rep. (2018) 6:e13731. doi: 10.14814/phy2.13731
148. Lo Verso, F, Carnio, S, Vainshtein, A, and Sandri, M. Autophagy is not required to sustain exercise and PRKAA1/AMPK activity but is important to prevent mitochondrial damage during physical activity. Autophagy. (2014) 10:1883–94. doi: 10.4161/auto.32154
149. Yan, Z, Kronemberger, A, Blomme, J, Call, JA, Caster, HM, Pereira, RO, et al. Exercise leads to unfavourable cardiac remodelling and enhanced metabolic homeostasis in obese mice with cardiac and skeletal muscle autophagy deficiency. Sci Rep. (2017) 7:7894. doi: 10.1038/s41598-017-08480-2
150. Miura, I, Okada, K, Ishii, A, Warabi, E, Watahiki, T, To, K, et al. P 62/Sqstm 1 rescue in muscle retards the progression of steatohepatitis in p 62/Sqstm 1-null mice fed a high-fat diet. Front Physiol. (2022) 13:993995. doi: 10.3389/fphys.2022.993995
151. Yamada, M, Warabi, E, Oishi, H, Lira, VA, and Okutsu, M. Muscle p 62 stimulates the expression of antioxidant proteins alleviating cancer cachexia. FASEB J. (2023) 37:e23156. doi: 10.1096/fj.202300349R
152. Tam, B, Pei, X, Yu, A, Sin, T, Leung, K, Au, K, et al. Autophagic adaptation is associated with exercise-induced fibre-type shifting in skeletal muscle. Acta Physiol. (2015) 214:221–36. doi: 10.1111/apha.12503
153. Guo, YP, Pan, SS, Chen, TR, Huang, Y, Wan, DF, and Tong, YS. Exercise preconditioning promotes myocardial GLUT4 translocation and induces autophagy to alleviate exhaustive exercise-induced myocardial injury in rats. J Mol Histol. (2023) 54:453–72. doi: 10.1007/s10735-023-10152-7
154. Medina, DL, Di Paola, S, Peluso, I, Armani, A, De Stefani, D, Venditti, R, et al. Lysosomal calcium signalling regulates autophagy through calcineurin and TFEB. Nat Cell Biol. (2015) 17:288–99. doi: 10.1038/ncb3114
155. Wang, P, Li, CG, Qi, Z, Cui, D, and Ding, S. Acute exercise induced mitochondrial H2O2 production in mouse skeletal muscle: association with p66Shc and FOXO3a signaling and antioxidant enzymes. Oxidative Med Cell Longev. (2015) 2015:536456. doi: 10.1155/2015/536456
156. He, C, Bassik, MC, Moresi, V, Sun, K, Wei, Y, Zou, Z, et al. Exercise-induced BCL2-regulated autophagy is required for muscle glucose homeostasis. Nature. (2012) 481:511–5. doi: 10.1038/nature10758
157. Ogasawara, R, Fujita, S, Hornberger, TA, Kitaoka, Y, Makanae, Y, Nakazato, K, et al. The role of mTOR signalling in the regulation of skeletal muscle mass in a rodent model of resistance exercise. Sci Rep. (2016) 6:31142. doi: 10.1038/srep31142
158. Youle, RJ, and Narendra, DP. Mechanisms of mitophagy. Nat Rev Mol Cell Biol. (2011) 12:9–14. doi: 10.1038/nrm3028
159. Zhao, M, and Klionsky, DJ. AMPK-dependent phosphorylation of ULK1 induces autophagy. Cell Metab. (2011) 13:119–20. doi: 10.1016/j.cmet.2011.01.009
160. Escobar, KA, Welch, AM, Wells, A, Fennel, Z, Nava, R, Li, Z, et al. Autophagy response to acute high-intensity interval training and moderate-intensity continuous training is dissimilar in skeletal muscle and peripheral blood mononuclear cells and is influenced by sex. Human Nutrition & Metabolism. (2021) 23:200118. doi: 10.1016/j.hnm.2020.200118
161. Pan, T, Jiao, J, Ye, L, Tong, X, Wang, Q, and Ji, M. Changes related to muscle autophagy after exhaustive exercise and blunt trauma. Rev Bras Med Esporte. (2023) 30:e2023_0218. doi: 10.1590/1517-8692202430022023_0218i
162. Martinez-Canton, M, Galvan-Alvarez, V, Gallego-Selles, A, Gelabert-Rebato, M, Garcia-Gonzalez, E, Gonzalez-Henriquez, JJ, et al. Activation of macroautophagy and chaperone-mediated autophagy in human skeletal muscle by high-intensity exercise in normoxia and hypoxia and after recovery with or without post-exercise ischemia. Free Radic Biol Med. (2024) 222:607–24. doi: 10.1016/j.freeradbiomed.2024.07.012
163. Pinto, AP, da Rocha, AL, Marafon, BB, Rovina, RL, Muñoz, VR, da Silva, LE, et al. Impact of different physical exercises on the expression of autophagy markers in mice. Int J Mol Sci. (2021) 22:2635. doi: 10.3390/ijms22052635
164. Halling, JF, Ringholm, S, Nielsen, MM, Overby, P, and Pilegaard, H. PGC-1α promotes exercise-induced autophagy in mouse skeletal muscle. Phys Rep. (2016) 4:e12698. doi: 10.14814/phy2.12698
165. Sanchez, AM. Autophagy regulation in human skeletal muscle during exercise. J Physiol. (2016) 594:5053–4. doi: 10.1113/JP272993
Keywords: polyphenols, exercise, autophagy, cancer, oxidative stress, metabolic health
Citation: Liu W, Hu M, Cao F and Jin S (2025) Polyphenols and exercise in autophagy regulation: potential benefits for cancer management and healthspan. Front. Nutr. 12:1618813. doi: 10.3389/fnut.2025.1618813
Edited by:
Krista Austin, Performance and Nutrition Coaching, United StatesReviewed by:
Oliver Dean John, Universiti Malaysia Sabah, MalaysiaYubing Wang, Qilu Normal University, China
Manuel Humberto Chairez-Ramirez, Durango Institute of Technology, Mexico
Copyright © 2025 Liu, Hu, Cao and Jin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: MengDi Hu, aG1kODhzQDE2My5jb20=
 Wei Liu1
Wei Liu1 MengDi Hu
MengDi Hu