- 1College of Food Science and Technology, Yangzhou University, Yangzhou, Jiangsu, China
- 2Key Lab of Dairy Biotechnology and Safety Control, Yangzhou, Jiangsu, China
- 3Department of Public Health, Medical College, Yangzhou University, Yangzhou, Jiangsu, China
- 4Department of Animals Products Technology, Sindh Agriculture University Tandojam, Tando Jam, Pakistan
- 5College of Food Science and Technology, Huazhong Agricultural University, Wuhan, China
- 6Microelement Research Center, College of Resources and Environment, Huazhong Agricultural University, Wuhan, Hubei, China
Introduction: Fermented buffalo milk products from South Asia remain an underexplored source of microbial diversity with potential health-promoting benefits. This study investigates the probiotic and industrial suitability of lactic acid bacteria (LAB) and non-LAB isolates from traditional Pakistani dairy, addressing gaps in region-specific probiotic discovery.
Methods: Forty-seven bacterial isolates were obtained from fermented buffalo milk products (yogurt and cheese). Molecular identification (16S rRNA sequencing) classified isolates into LAB and non-LAB taxa. Probiotic potential was evaluated via in vitro assays for gastrointestinal stress tolerance (pH 2.0, 0.5% bile), antioxidant activity (DPPH scavenging), and industrial adaptability (growth at 4–45°C, 2–6% NaCl).
Results: Eight strains were prioritized, including Lactobacillus plantarum Y1, L. brevis Cc3, Streptococcus thermophilus Y6/Cc1/Cm5, and non-LAB Bacillus dendritiformis Y9. L. plantarum Y1 exhibited exceptional acid resistance (>5.0 log10 CFU/mL at pH 2.0) and bile tolerance (6.5 log10 CFU/mL). L. brevis Cc3 combined high bile resilience (6.0 log10 CFU/mL) with robust antioxidant activity (52% DPPH scavenging), while S. thermophilus Y6 showed 48% antioxidant capacity. Non-LAB isolates, particularly B. dendritiformis Y9, demonstrated unexpected bile stress survival (5.4–5.5 log10 CFU/mL). All strains grew under industrial conditions (4–45°C, 2–6% NaCl), except S. thermophilus Cc1, which was heat-sensitive above 40°C.
Conclusion: This study highlights South Asian buffalo milk as a reservoir of both conventional LAB and novel non-LAB strains with dual stress tolerance and antioxidant functionality. L. plantarum Y1 and L. brevis Cc3 emerge as prime candidates for developing culturally tailored functional foods to address regional nutritional challenges. The resilience of non-traditional isolates such as B. dendritiformis Y9 challenges existing probiotic taxonomical biases, suggesting broader microbial resources for gut-health innovations. These findings advocate for integrating regionally adapted probiotics into functional diets to enhance gastrointestinal health and oxidative stress mitigation in South Asian populations.
1 Introduction
Lactic acid bacteria (LAB) comprise a diverse group of Gram-positive, non-spore-forming, catalase-negative, and aerotolerant bacterial species that are commonly known for their rods or cocci-like shapes (1). They commonly reside in nutrient-rich environments, such as vegetables, meat, milk products, and various drinks. Even now, their primary function is as starter cultures in the food sector, where they have been fermenting foodstuffs and animal feed for ages (2). They are the essential key players in the production of dairy food products. According to a study (3, 4), they have a reputation for being able to ferment foods, which makes them healthier, improves their organoleptic qualities, and increases the abundance of nutrients. Another common use of LAB and Bifidobacterium is as a probiotic. Research on the potential health benefits of probiotics containing LAB, such as Lactobacillus and Pediococcus spp., has demonstrated their roles in gut microbiota modulation, immune enhancement, and pathogen inhibition (5). Probiotics are classified as a common category in the class of famous food supplements. These are functional foods due to their greater health advantages than conventional nutritional products (6). In the interim, numerous scientific studies have been conducted in recent decades to select LABs with distinct and specific functional properties as well as novel probiotic bacteria that are continuously isolated and identified (7).
Due to the rapid growth of the probiotic industry, there remains a strong demand for probiotics. However, potential probiotic strains must first demonstrate resilience against the harsh conditions imposed by the human body (8). Oxidative stress, a key contributor to many disorders, occurs when the balance between the body's antioxidant defenses and free radical production is disrupted (9). LABs are beneficial because they regulate the balance of the intestine, lower the blood cholesterol, reduce the risk of cancer, and revitalize the immune system, among other things (10). They have anti-oxidative effects as reported by (11) and many previously reported studies, e.g., bacterial strains, such as L. acidophilus, L. fermentum, and L. sake (12–14). Additionally, B. clausii has been used and proven effective in treating diarrhea in humans. These agents have demonstrated significant immune-modulatory properties in numerous in vitro and clinical studies (11, 15).
In the past 20 years, global milk production has nearly doubled, with buffalo milk experiencing an annual growth rate of approximately 2.5% higher than cow milk (16). India, Pakistan, and China are the largest producers of buffalo milk, with Italy being the highest producer in Europe and ranking sixth globally (17). In numerous developing countries, buffalo milk plays a crucial role in meeting the nutritional needs of humans (16). Buffalo milk is an appropriate raw ingredient for the production of several types of fermented dairy products, including cheeses and artisanal cheeses. Because its components are richer than those of cow milk, it is ideal for processing a broad range of dairy products that are liked by many cultures as their traditional food (18). Since limited studies have been done to explore the probiotic potential of Pakistani buffalo milk, this study is an attempt to fill the existing gap. Moreover, given the rising global demands for region-specific probiotics, this study highlights the Pakistani fermented buffalo milk as an under-explored source of stress-resilient and antioxidative microbes. By characterizing both traditional and non-conventional isolates, we focused to identify the candidates with dual probiotic-industrial potential, offering novel solutions for functional foods tailored to South Asian nutritional and oxidative stress challenges. This research set out to conclude the variety of beneficial microorganisms present in fermented buffalo milk products.
2 Materials and methods
2.1 Fermented milk samples collection and transportation
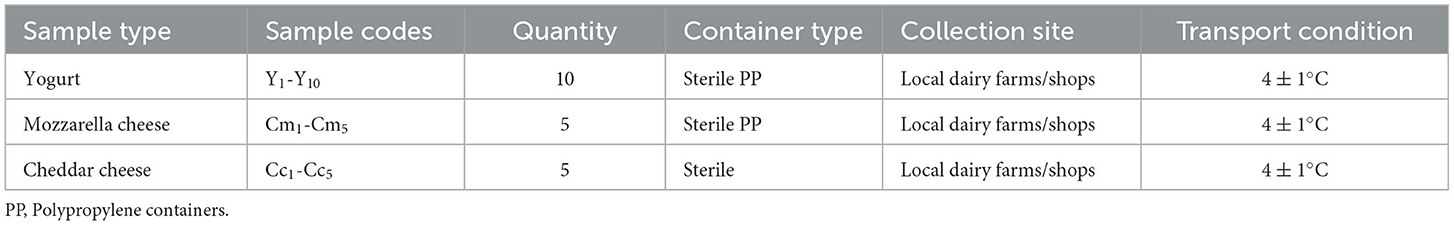
A total of 20 samples (n = 20) of buffalo fermented milk products were collected under hygienic conditions from the local dairy farm/shops of the district of Hyderabad, Pakistan. At the time of collection, all the samples were labeled properly, mentioned in Table 1, and brought under chilled conditions (4 ± 1°C) to the Department of Animal Products Technology, Sindh Agriculture University, Tandojam, and CVD Laboratory Tandojam, Pakistan. Bacterial isolation was done by growing them on de Man, Rogosa, and Sharpe (MRS) agar medium and subculturing to get pure cultures.
2.2 Isolation of lactic acid bacteria from fermented milk
Initially, 1 mL of fermented milk was mixed with 9 mL of sterile distilled water (DW) to create a 10−1 dilution. Serial dilutions were then prepared to achieve appropriate bacterial concentrations for isolation. Finally, 0.1 mL aliquots from the selected dilutions were spread onto MRS agar plates to culture LAB colonies. Within 48 h after the inoculation, the plates were placed in an incubator, and the temperature was set at 37°C. After LAB growth on MRS agar, distinctive colonies were streaked onto new MRS agar plates using sterile inoculating needles. The streaks were then re-cultured at 37°C for a period of 1 to 2 days to purify the colonies (19).
2.3 Identification and characterization of lactic acid bacteria
The LAB was identified from buffalo milk fermented products through morphological and biochemical analysis. For the characterization of the LAB, freshly grown cultures were studied according to the previously reported methods (20, 21) with slight modifications. Gram staining, motility, catalase, oxidase, Triple Sugar Iron Agar, Indole, Methyl red, and Voges–Proskauer's Test were conducted.
2.3.1 Gram staining
Gram staining was done using standard procedure, and Gram-positive bacilli/cocci and y shape were chosen for further characterization.
2.3.2 Motility test
One colony of bacteria was inserted into the sulfide, indole, and motility media in a test tube. The test tube was incubated for 48 h at 37°C. The observation of the motility test is the growth of bacteria on the media. The bacteria that only grow around the inserted location show a negative result, while the bacteria that grow on the media surface or spread in the media show a positive result.
2.3.3 Catalase test
The catalase test was conducted by adding a drop of a 3% solution of hydrogen peroxide to a glass slide on which a colony of bacteria was applied from a 24-h-old culture of each isolate (or directly on the Petri dish). NB: Catalase negative bacteria were subjected to further examination.
2.3.4 Oxidase test
The test was conducted by the Filter Paper Spot Method. Use a loop to pick a well-isolated colony from a fresh bacterial plate and rub it onto a small piece of filter paper. Place 1 or 2 drops of 1% Kovács oxidase reagent on the organism smear. Observe for color changes.
2.3.5 Voges–Proskauer's test
To the pre-sterilized glucose-phosphate broth tubes, test cultures were inoculated and incubated at 37°C for 48 h. After incubation, 10 drops of Barrit's reagent A were added and gently shaken, followed by the addition of Barrit's Reagent B. The development of pink color in the broth was taken as positive for the test.
2.3.6 Triple Sugar Iron agar
Triple Sugar Iron (TSI) agar was inoculated with a pure culture of the isolates by stabbing through the center of the medium to the bottom of the tube and then streaked to the surface of the slant. Finally, the tube was incubated at 37°C for 24 h. LAB isolates ferment three sugar units, such as glucose, sucrose, and lactose, within the TSI medium and produces acids, which are indicated by color changes to yellow.
2.3.7 Indole test
Inoculate the tube of tryptone broth with a small amount of a pure culture. Incubate at 35°C for 24 to 48 h. To test for indole production, add five drops of Kovács reagent directly to the tube. A positive indole test is indicated by the formation of a pink to red color (“cherry-red ring”) in the reagent layer on top of the medium within seconds of adding the reagent.
2.3.8 Methyl red test
Sterilized glucose-phosphate broth tubes were inoculated with the test culture and incubated at 30°C for 48 h. After incubation, five drops of methyl red indicator were added to each tube and gently shaken. Red color production was taken as positive, and yellow color production was taken as negative for the test.
2.4 Molecular identification of microbes
2.4.1 Extraction of total genomic DNA
Genomic DNA was extracted from 200 mg of bacterial biomass samples using the Qiagen AMP DNA Mini Extraction Kit following the manufacturer's instructions. The extraction process involved digestion of the sample with ATL Lysis Buffer and Proteinase K at 56°C for 1 h. Incubation with AL Buffer at 70°C for 10 min. DNA isolation using spin column centrifugation and subsequent washing with recommended buffers. Elution of purified DNA with TE buffer for downstream applications.
2.4.2 PCR amplification
Polymerase Chain Reaction (PCR) amplification of the extracted DNA was performed using the PCR Dream Taq Green Master Mix (Thermo Scientific), with universal bacterial 16S rRNA primers 27F (5′-AGAGTTTGATCMTGGCTCAG-3′) and 1492R (5′-TACGGYTACCTTGTTACGACTT-3′). The reaction was set up according to the manufacturer's protocol, with the following conditions optimized for target amplification: Initial denaturation at 95°C, followed by 35 cycles of denaturation, annealing, and extension, with a final extension step at 72°C. Specific cycling parameters were adjusted according to the target DNA fragment.
2.4.3 Agarose gel electrophoresis
Agarose gel electrophoresis was used to analyze the PCR products: A 1.5% agarose gel was prepared in 50X Tris-Acetate-EDTA (TAE) buffer. PCR products and the 100 bp DNA Ladder (Thermo Scientific) were loaded into the gel wells. Electrophoresis was conducted at 110 V for 30 min. DNA bands were visualized under UV light to confirm the size and integrity of the amplified products.
2.4.4 PCR product verification
PCR amplification of the extracted DNA was performed using the PCR Dream Taq Green Master Mix (Thermo Scientific). The reaction was set up according to the manufacturer's protocol, with optimized conditions for target amplification.
2.4.5 Gene sequencing
The purified PCR products were subjected to Sanger sequencing, which was performed by APICAL SCIENTIFIC SDN. BHD. Sequencing was carried out using standard protocols to confirm the identity and accuracy of the amplified DNA fragments.
2.5 Stress tolerance
The selected identified isolates from dairy products were further subjected to tests evaluating their tolerance to different pH levels, bile salt concentrations, and temperature ranges, following the probiotic activity assessment method reported by Menconi et al. (22).
2.5.1 Tolerance to acidic pH values
Strains were grown in MRS broth at 37°C overnight, 0.1 mL aliquots of each active culture were adjusted to pH 5.0, 3.0, and 2.0 with 5 N HCl and incubated at 37°C for 3 h. Samples were taken every hour for 3 h, and the viable number of bacteria was enumerated by pour plate counts of all samples using 10-fold serial dilutions prepared in 0.1% peptone water. Simultaneously, bacterial growth was monitored by measuring absorbance with a spectrophotometer (Nova Spec II, Pharmacia) at 600 nm. All the experiments were replicated thrice.
2.5.2 Bile tolerance
Strains were cultured overnight in MRS broth at 37°C. A saturated bile solution was prepared separately by dissolving powdered bile extract (Oxoid). The pH was maintained at 6.5 ± 0.2 using 1N HCl/NaOH during bile exposure. Bile solution was then sterilized by a 4-micron filter and was added to two of the cultures to achieve a final concentration of 0.3% and the second culture with 0% bile served as a control sample. The cultures were incubated at 37°C for 3 h and then every hour for 3 h. Viable counts of Lactobacillus strains were determined by pour plate counts of all the samples using 10-fold serial dilutions prepared in 0.1% peptone water. Simultaneously, bacterial growth was monitored by measuring absorbance with a spectrophotometer (Nova Spec II, Pharmacia) at 600 nm. All the experiments were replicated thrice.
2.5.3 Resistance to temperature and sodium chloride
A basal MRS medium was utilized in these in vitro studies to cultivate the bacterial isolates. An overnight culture of each isolate served as the inoculum, with the cells being centrifuged and resuspended in 0.9% sterile saline. A 100-μL aliquot of the suspension was then inoculated into 10 mL of MRS broth in each test tube. Two incubation time points (2 h and 4 h) were evaluated for each of the variables: temperature and sodium chloride (NaCl) concentration. The temperatures tested were 5°C, 10°C, 15°C, and 45°C, and the NaCl concentrations tested were 2%, 4%, and 6.5% (w/v). The tubes were incubated with reciprocal shaking at the specified test temperatures and NaCl concentrations. At each time point, a sample from each tube was streaked onto MRS agar to assess the presence or absence of growth, which was used to confirm the viability of the strains. The turbidity of each tube was also recorded as an indicator of growth or no growth. Each treatment was tested in triplicate.
2.6 DPPH free radical scavenging ability
Free radical assays using 2,2-diphenyl-1-picrylhydrazyl hydrate (DPPH) (Sigma-Aldrich, St. Louis, MO, USA) was used for the evaluation of the radical-removing capabilities of LAB strains after vigorous mixing of 800 μL of a recently made 0.2 mM DPPH solution in 80% methanol with 400 μL of either intact cells or colony forming cells for 30 s (23–25). After this, it was kept at room temperature in the absence of light for 30 min, and the scavenging activity (%) was calculated from the absorbance at 517 nm (A517) relative to the methanol blank. Uninoculated MRS broth and PBS served as the control samples for the colony-forming cells and intact cell DPPH scavenging assays, and the percentage change in the scavenging activity was calculated using the following equation:
2.7 Statistical analysis
The results of the statistical analysis were performed using Statistix 8.1 software. Data are reported as the mean ± standard deviation (SD). The data were subjected to a one-way ANOVA followed by LSD multiple comparison tests. Lowercase letters above the bars indicate a significant difference (P < 0.05).
3 Results
3.1 Isolation, morpho-physiological, and biochemical characterization of bacterial strains
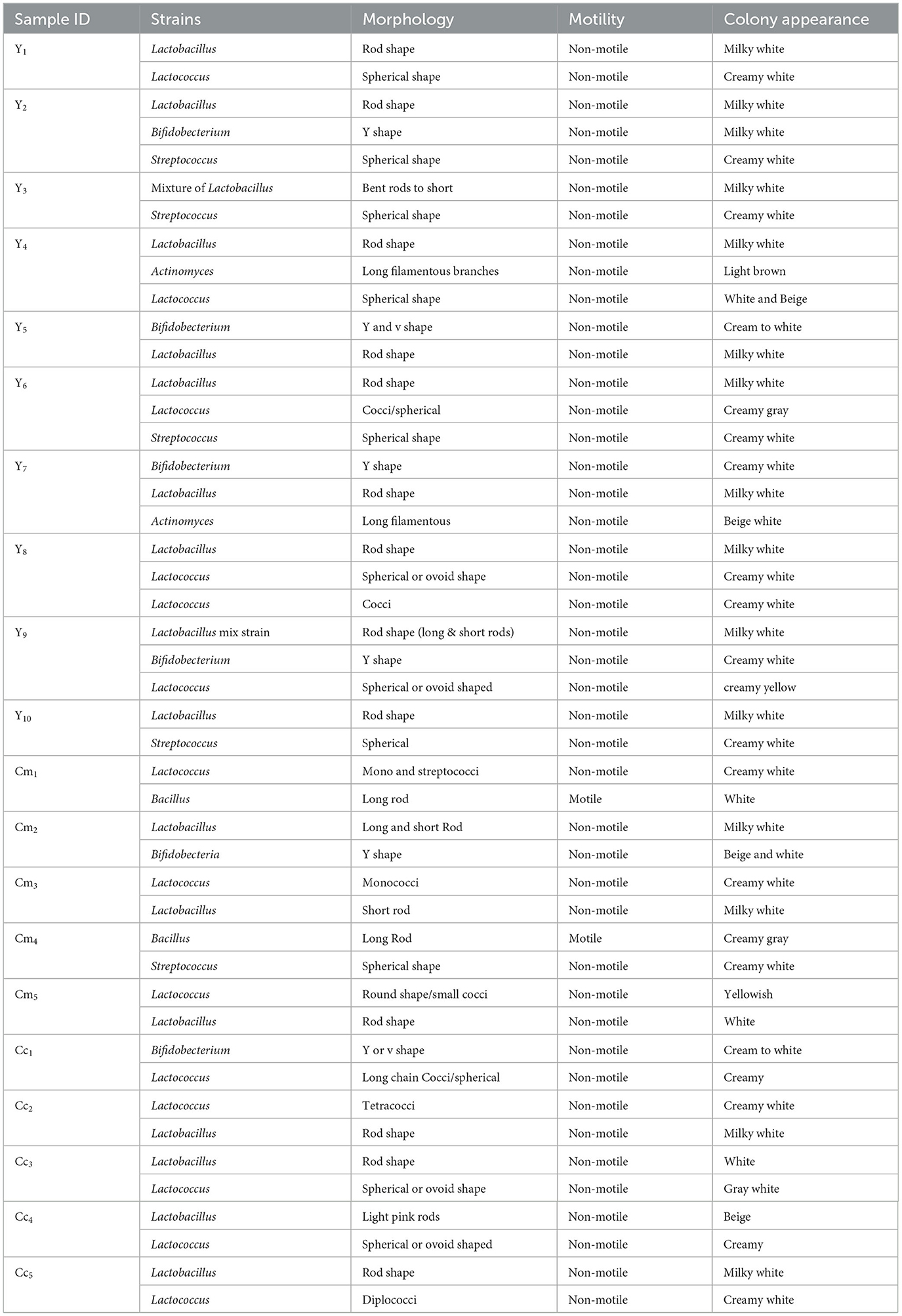
A comprehensive isolation study identified 47 bacterial strains from traditional fermented dairy products, with 27 strains originating from yogurt samples and 20 strains from artisanal cheeses. Isolates were cultured on MRS agar plates to examine their morphological features. The findings of the motility test, Gram staining, and colony characteristics analysis are shown in Table 2. Staining revealed a positive character for all of the isolates, which produced a purple or violet color. The isolates obtained from MRS plates were rods, cocci, and v/y-shaped. Based on the observed shape, isolates were long, short, and bent rods, pink rods, cocci, tetra cocci, spherical, oval shapes, Y or V shapes, and also long filamentous forms presented in physiological test tables. Finding out if bacteria are motile or not and whether they have flagella that helps them move is the goal of the motility test. Motility testing of all isolates showed negative results except for Cm1 and Cm6. Colonies isolated from fermented dairy products were milky white, creamy, beige, yellowish, and light brown, as presented in Table 2.
3.2 Biochemical characterization of LAB isolates
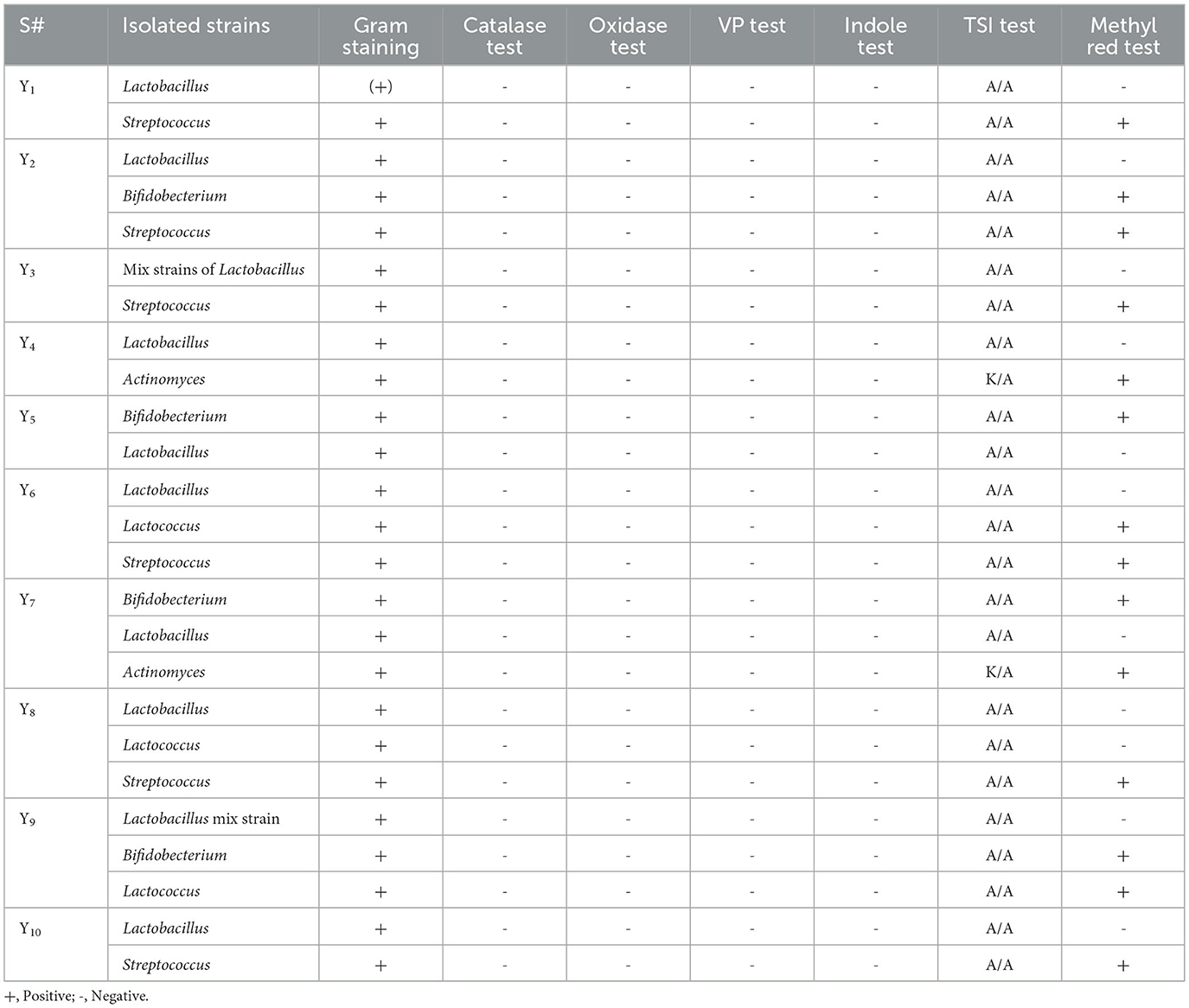
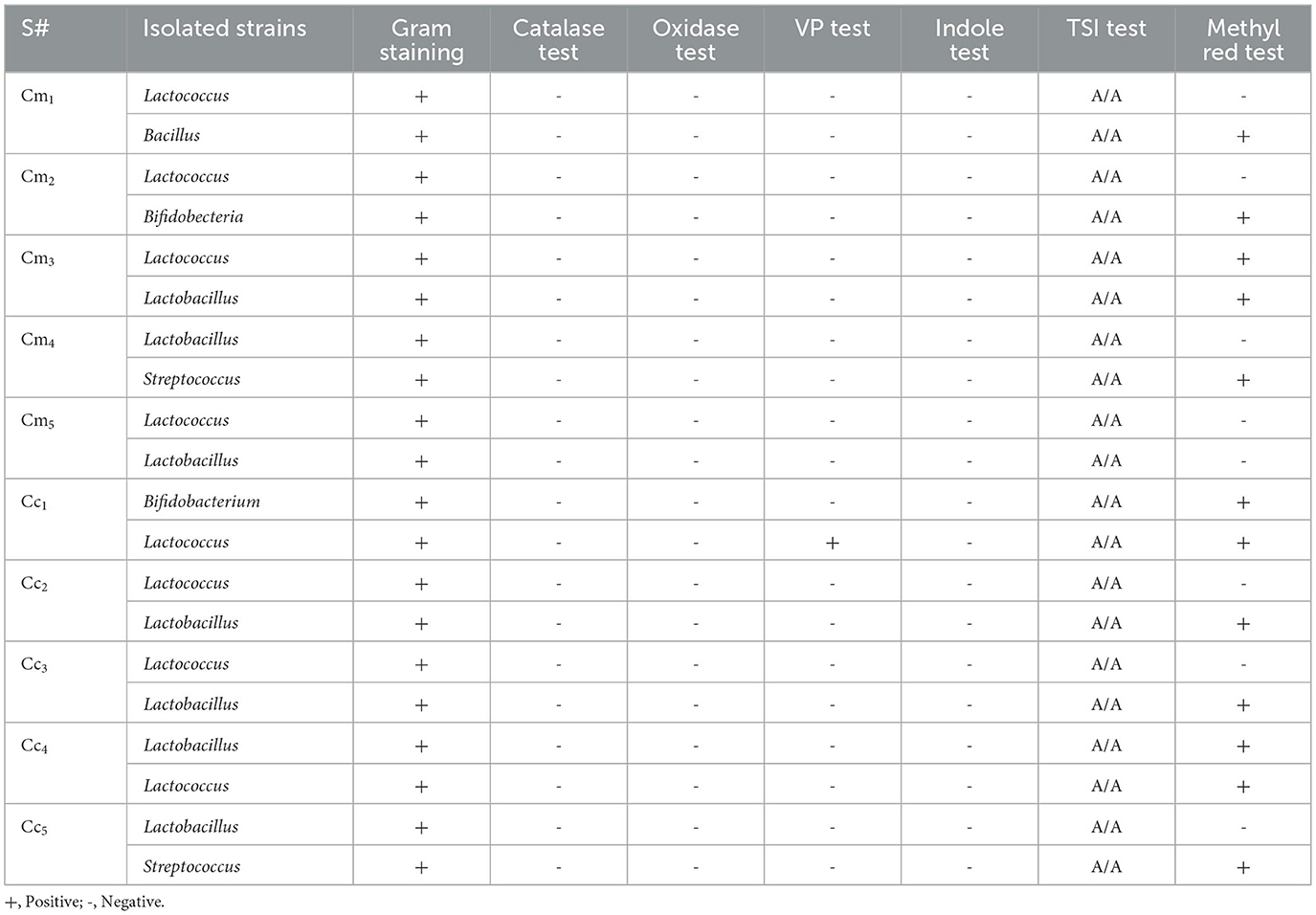
To identify the specific strain of LAB, a series of biochemical tests was conducted, including Gram staining, the assessment of catalase and oxidase, methyl red, triple sugar iron, gas generation, and fermentation. These are the most common tests used to identify lactic acid bacteria. Surprisingly, no single strain of yogurt and cheese samples was found to be positive for the catalase and oxidase, as presented in Tables 3, 4. The VP test checks the production of acetyl methyl carbinol by any microbe through glucose fermentation. Most of the isolates showed negative results on the VP test. At the same time, some isolated strains of some samples, such as Y4, Y6, and Cc1, showed positive results on the VP test in Tables 3, 4. Except for one strain, every single isolate fermented glucose, lactose, and sucrose, turning yellow upon the slant/butt, and every single strain tested negative for hydrogen sulfide formation of Y4, and Y7 showed K/A (alkaline over acid) on TSI that means only glucose was metabolized (Tables 3, 4). Glucose fermentation to pyruvic acid and subsequent oxidation to other acids (e.g., lactic, acetic, and formic acids) leads to a reduction in pH; this is the basis of the methyl red test. The subsequent reddening of the methyl red indicator shows a favorable response. Some isolated strain from samples Y1, Y2, Y3, Y4, Y6, Y7, Y9, Y10, Cm1, Cm3, Cm 4, Cc1, Cc 2, Cc 4, and Cc5 tested positive for methyl red test. The remaining isolates tested negative for the methyl red test presented in Tables 3, 4. The isolates under study did not possess the characteristic of producing indole from tryptophan by “tryptophanase.” Therefore, the addition of Kovac's reagent did not lead to the formation of a deep red color on the surface layer; it remained yellow as it was initially. It means that all isolated strains showed negative results on the indole test (Tables 3, 4).
3.3 Species identification
The characteristics of the bacterial isolates from the study were examined for their biochemical identification of species. Out of these, eight strains underwent species identification through sequencing in the Gene-Bank database. A phylogenetic tree was developed after the identification (Figure 1), which revealed the connections of isolate Y1 to the genus Lactobacillus with 98.7% similarity to L. plantarum MT597700.1 strain. Strain Y6 had associations with the genus Lactococcus, with 98.9% similarity to the S. thermophilous MT473585.1 strain. Y9 had a similarity to genus Bacillus and showed 94.9% similarity to the B. dedritiformis OX216966.1 strain, while Cc1 had connections with the S. thermophilus strain MT544739.1. Cm1 was revealed to be related to the genus Bacillus with 98.9% similarity to B. subtilis CP120681.1 strain. Cc3 isolated bacterial strain was matched with the lactobacillus strain L. brevis OQ848053.1. The complete phylogenetic tree of all the studied strains is shown in Figure 1.
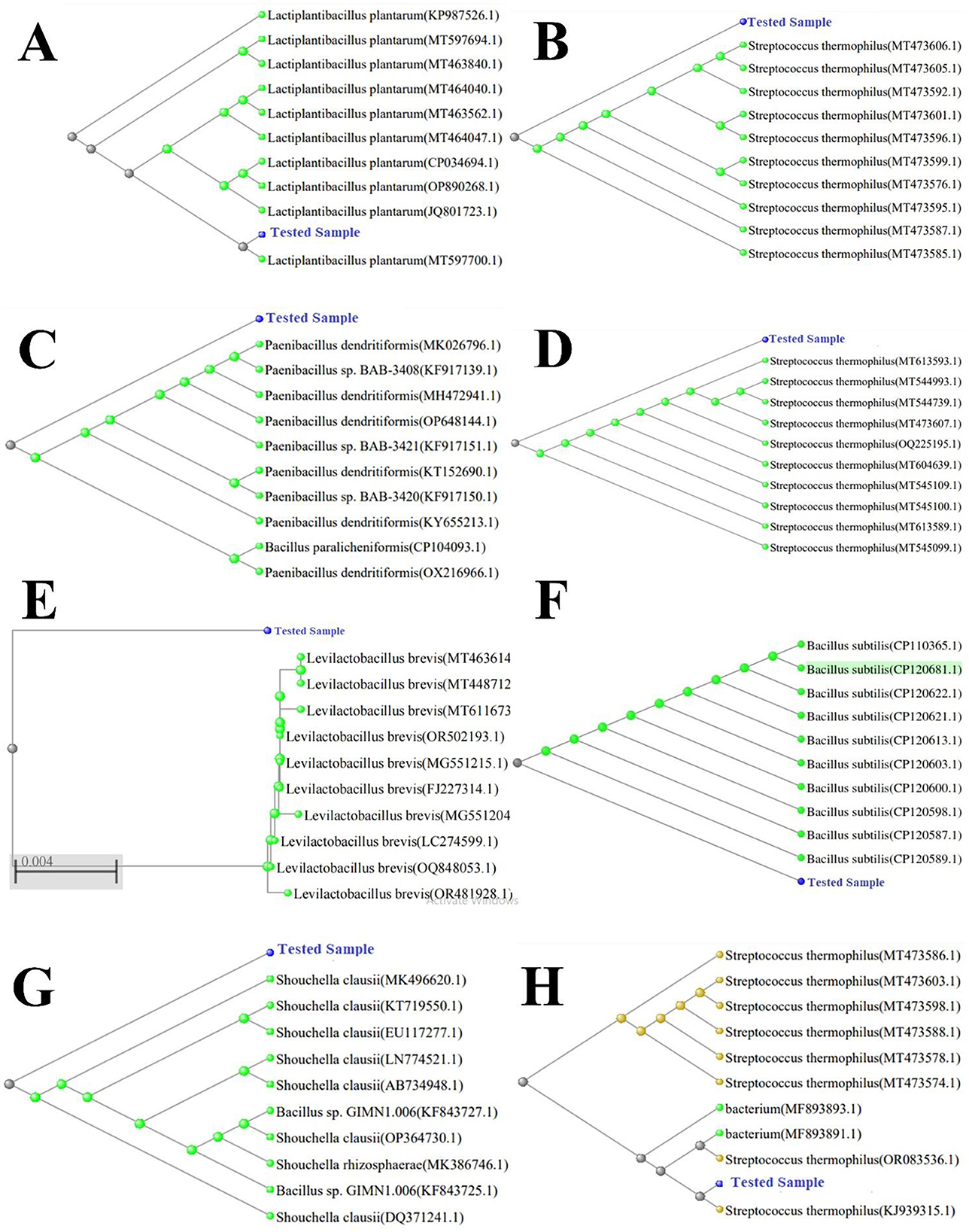
Figure 1. Phylogenetic tree with bootstrap values. Tree was constructed using Neighbor-Joining (NJ) method. (A) L. plantarum (accession PP565108), (B) S. thermophilus (accession PP565114), (C) B. dendritiformis (accession PP565143), (D) S. thermophilus (accession PP565141), (E) L. brevis (accession PP549910), (F) B. subtilis (accession PP89025), (G) S. clausii (accession PP565148), and (H) S. thermophilus (accession PP565129).
3.4 Bile salt stress tolerance potential of isolates
The gastrointestinal stress tolerance and functional antioxidant capacity of isolated probiotic strains were systematically evaluated through a series of in vitro assays simulating physiological challenges. As shown in Figures 2–4 and Table 4, the isolates exhibited significant strain-dependent variations in their ability to withstand bile salts, acidic pH, temperature fluctuations, osmotic stress, and their free radical scavenging potential. The bile tolerance results demonstrated a concentration and time-dependent decline in the viability of all tested bacterial strains (Figure 2). At 0.1% bile concentration, bacterial survival remained relatively high after 3 h of exposure. L. plantarum exhibited the highest bile resistance, maintaining optical densities corresponding to viable counts between 6.4 and 6.5 log10 CFU/mL, while S. clausii showed the lowest tolerance, with final counts ranging from 3.7 to 3.9 log10 CFU/mL. At 0.3% bile, a moderate but noticeable reduction in viability was observed across all strains. L. plantarum continued to display superior tolerance, retaining viable counts of 5.7–5.8 log10 CFU/mL, followed by B. subtilis at 5.4–5.5 log10 CFU/mL. In contrast, S. clausii exhibited a further decline in viability, with counts ranging between 4.0 and 4.2 log10 CFU/mL. At the highest concentration, 0.5% bile, the inter-strain variability became most evident. L. plantarum maintained strong resistance, sustaining viable counts between 5.9 and 6.0 log10 CFU/mL even after 3 h. Conversely, S. clausii experienced a significant drop in viability, decreasing to 4.2 to 4.4 log10 CFU/mL, which reflects a reduction of approximately 2.2 log units from its initial value.
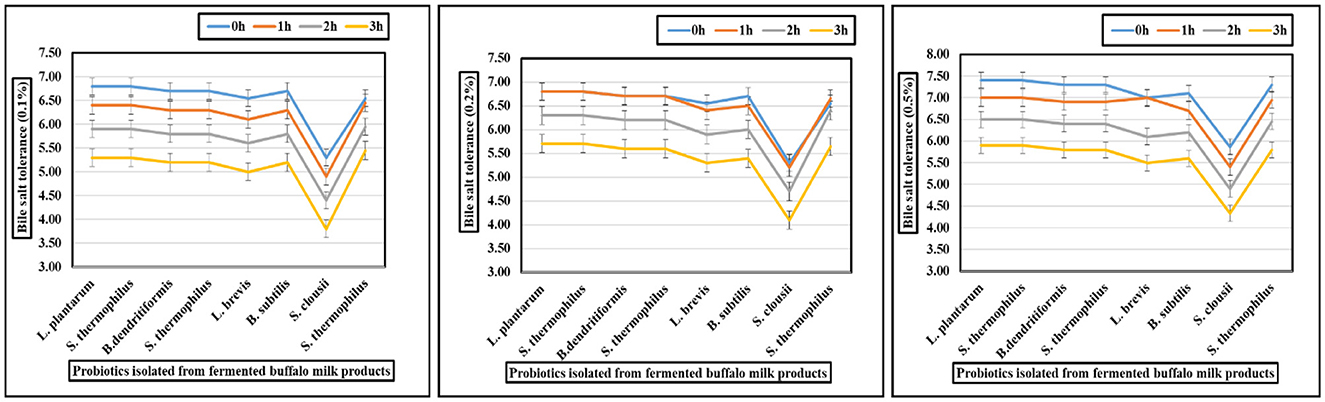
Figure 2. Graphs showing bile (0.1, 0.2, and 0.5%) tolerance of probiotic isolated from fermented buffalo milk products.
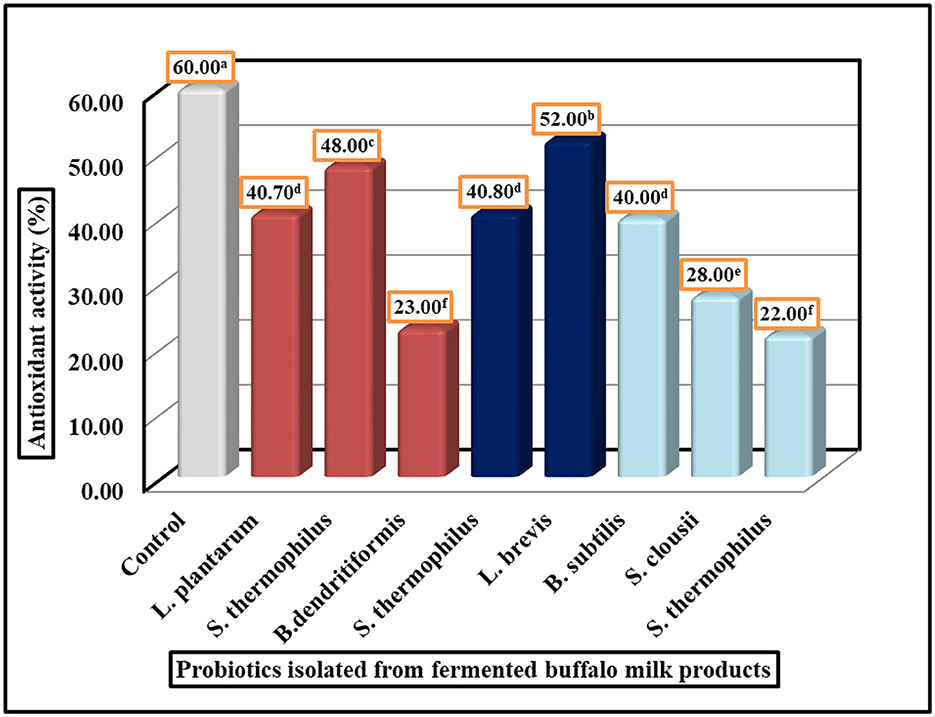
Figure 4. Antioxidant activity of probiotic isolated strains by DPPH assay. LSD All-Pairwise Comparisons Test (0.05) = 05, SE ± 0.80.
3.5 Low pH stress tolerance potential of isolates
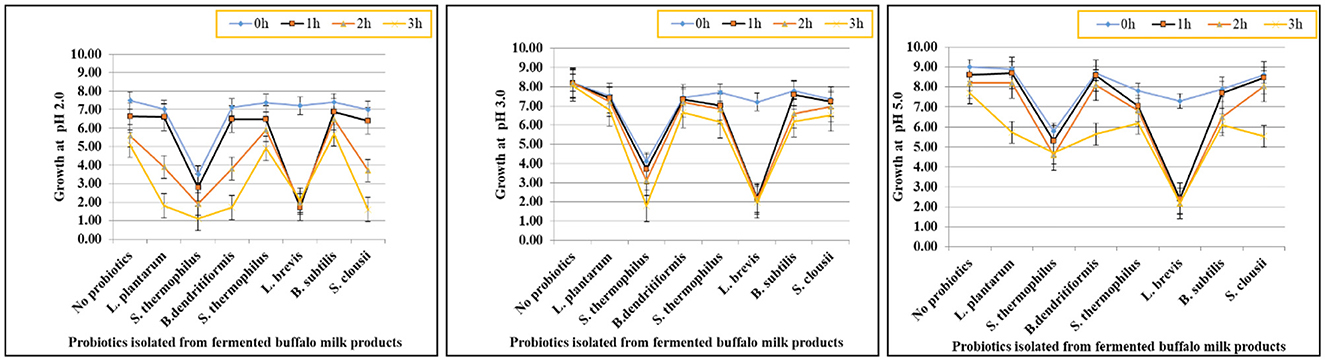
The acid tolerance of eight probiotic strains was assessed at pH 2.0, 3.0, and 5.0 over a 3-h period. L. plantarum, S. thermophilus (Strain 1), and B. dendritiformis showed the highest acid tolerance, retaining viability above 5.0 log10 CFU/mL at pH 2.0 after 3 h, with survival rates between 66.8% and 69.2%. At pH 5.0, all three exceeded 7.6 log10 CFU/mL, maintaining survival rates above 83%. L. brevis and S. thermophilus (Strain 2) showed moderate tolerance, dropping to 1.6–1.9 log10 CFU/mL at pH 2.0 (survival: 22–27%), but remained viable at pH 5.0 (final counts: 5.3–5.9 log10 CFU/mL). B. subtilis, S. clausii, and S. thermophilus (Strain 3) exhibited low acid tolerance, with survival below 2.2 log10 CFU/mL at pH 2.0 and reduced growth even at pH 5.0 (final counts: 2.1–4.7 log10 CFU/mL). Survival rates were as low as 21–31% at pH 2.0. Statistical analysis (one-way ANOVA, p < 0.05) confirmed significant differences in acid survival across strains and pH levels. L. plantarum, S. thermophilus (Strain 1), and B. dendritiformis were identified as the most acid-resistant strains. These findings are presented visually in Figure 3, which illustrates the differential survival patterns across pH conditions and time points.
3.6 Temperature and salt tolerance potential of isolates
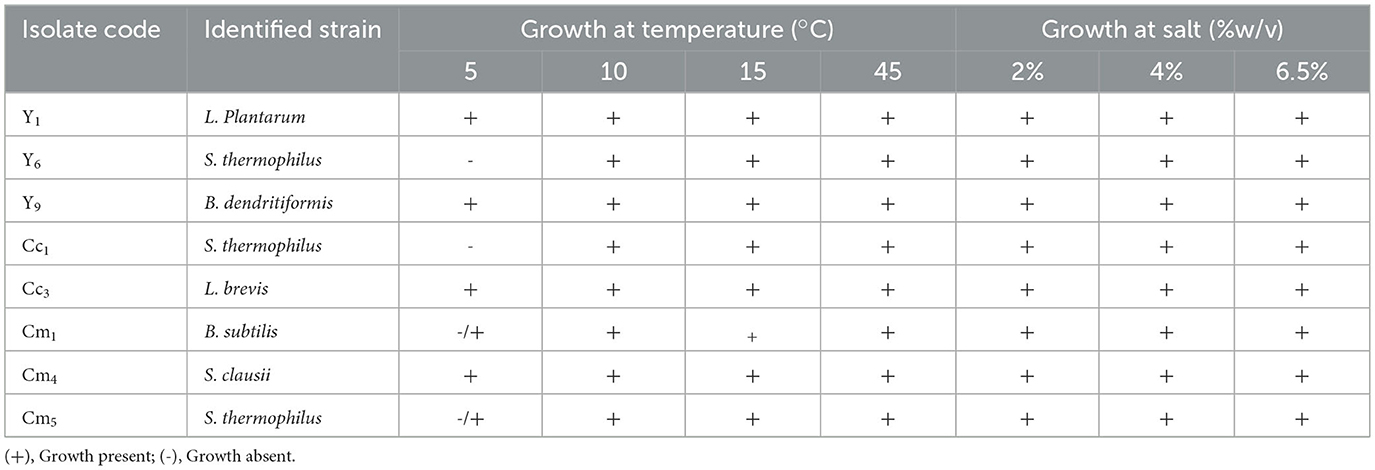
The tested isolates Y1, Y9, Cc3, and Cm4 showed growth at temperature range 5, 10, 15, and 45°C, while Y6, Cc1, isolated strain S. thermophilus, and Cm1 isolated strains B. subtilis could grow at 10 to 45°C but showed partial growth at 5°C. The ability to thrive in high conditions is a good characteristic of lactic acid production, which decreases the chances of contamination by other microorganisms (Table 5). The ability to grow in different salt conditions is also an in vitro test guideline to evaluate when selecting potential probiotic bacteria. The selected identified strains were determined for salt tolerance. All isolates can grow in a 2, 4, and 6.5% salt range. The results indicated positive growth rates for all six isolated strains mentioned in Table 5. The isolated strains could resist a 2–6.5% w/v concentration of NaCl in MRS broth.
3.7 DPPH free radical scavenging ability of selected isolates
The antioxidant activity of probiotic strains was evaluated using the DPPH radical scavenging assay. The results demonstrate significant variation in antioxidant potential among the tested strains, with values ranging from 22% to 52% inhibition (Figure 4). Strain 5 (L. brevis) exhibited the highest antioxidant activity at 52%, followed by Strain 2 (S. thermophilus) at 48% and Strain 4 (S. thermophilus) at 40.80%. In contrast, Strain 3 (B. dendritiformis) showed the lowest activity at 22%, with Strain 6 (B. subtilis) and Strain 8 (S. clausii) also displaying relatively weak antioxidant capacity at 23% and 28%, respectively. The remaining strains demonstrated intermediate levels of antioxidant activity. Statistical analysis confirmed the reliability of these measurements, with a standard error of ± 0.80 and an LSD All-Pairwise Comparisons Test indicating meaningful differences between most strains. These findings reveal that certain probiotic isolates, particularly Lactobacillus brevis and specific S. thermophilus strains, possess substantial antioxidant properties that may be valuable for developing functional foods or dietary supplements aimed at oxidative stress reduction. The results provide a basis for selecting optimal strains for further investigation and potential commercial applications where antioxidant activity is desired.
4 Discussion
4.1 Morphological and physiological characteristics of the isolates
The LAB strains were isolated from the collected samples of yogurt and other fermented dairy products. Isolated from the materials, 47 strains were further examined for microscopic, physiological, and biochemical traits before their molecular characterization. A total of 20 bacterial strains (rod-shaped, cocci, and long filaments) were identified, with the majority of the bacterial colonies being pure white. This is due to the fact that color is contingent upon the isolate's origin (26–28). Furthermore, it has also been reported that the morphology of LAB colonies isolated from buffalo milk is oval to round-shaped with a yellow zone around the colony (29, 30). In this study we found four shapes of the bacteria and the first group comprises 20 isolates, the second group comprises 19 isolates in the form of cocci, diplococci, and streptococcus, third group consisted of six strains in the shape of “v” and “y-shaped” bacteria, and the fourth group contained only one bacterial strain with long filamentous shape (Tables 2, 3). Many studies, including (31), have characterized LAB isolates from traditional fermented mare milk, and our results align with these prior reports. A study by (28) reported that LAB collected from yogurt were Gram-positive. Moreover, another study (32) also found that LAB from buffalo milk curd belonged to Lactobacillus sp. and Streptococcus spp. Similarly, the study by (33) reported similar results, indicating that approximately 68% of the LAB isolates from fresh and fermented milk were Gram-positive. It has also been reported previously that 84 out of the 96 LAB from bovine milk were found as Gram-positive, with shapes ranging from round to rod-shaped (34, 35). Thakur et al. also reported similar results after analyzing samples of dairy and non-dairy products (36). This finding implies that flagella are absent from all LAB isolates. The lactic acid bacteria isolates from bovine milk curd are non-motile (28). The genus Lactobacillus is characterized by non-motile lactic acid bacteria (37).
4.2 Biochemical characterization of LAB isolates
4.2.1 Catalase and oxidase test
Lactic acid bacterial cells isolated from fermented dairy products have been the subject of several reports about their distinctive catalase test. All of the isolates tested negative for catalase and oxidase in our study. It has been found that specific Lactobacillus isolates, including Lactobacillus delbrueckii ssp. Bulgaricus, isolated from yogurt and other medicines and probiotics also exhibited no oxidase and catalase production (36), with only negative reaction observed in Lactobacillus isolates during experiments (38, 39). Buffalo milk microbial diversity was also investigated. Isolates were Gram-positive, oxidase-negative, catalase-negative, and characterized by tiny, localized colonies, all of which are hallmarks of LAB (40). Further identification of Lactobacilli species in local raw milk revealed that the catalase test was negative for all species (41). Similar catalase oxidase-negative reactions have been reported previously; they also reported that LAB isolates from dadih were non-motile, catalase, and oxidase-negative (42, 43). In addition, the study by (44) reported that the lactic acid bacteria in dadih exhibit negative catalase and oxidase activity. A further investigation found that 52 out of 70 lactic acid bacterial isolates in bovine milk from the North Sumatera River did not contain catalase (29). Additionally, it has also been previously reported that 99% of the LAB isolated from the milk of buffalo were catalase-negative (29, 45).
4.2.2 Voges–Proskauer's test
The presence or absence of acetyl methyl carbinol during glucose fermentation is shown by the VP test. The VP test came out negative for the majority of the isolates. These results agreed with those found by (46), who reported that L. plantarum from kimchi-fermented products exhibited an adverse reaction. Lactobacillus isolates from yogurt exhibited an adverse reaction, as reported by (47). In the Voges Proskauer test reaction conducted by (48), the prevalent characteristics of Lactobacillus species yielded negative results. The current investigation results are comparable to those of (49), who reported that the Voges–Proskauer test did not induce any color changes in LAB strains. Similarly, Ngene et al. (50) noted that the Voges–Proskauer's test yielded negative results for the L. lactis, L. brevis, L. fermentum, L. casei, and L. plantarum strains in yogurt. The single isolated isolates, including Y4, Y6, Y7, and Cc1, exhibited positive results on the VP test in our research. An additional study indicated that certain LAB isolates exhibited positive and negative reactions (51).
4.2.3 TSI test
The outcomes of this investigation on TSI were favorable. Fermented milk carbohydrate fermentation patterns have been used by certain studies to identify Lactobacillus species. Findings were the same when analyzed by (52), who characterized LABs and bifidobacterium from yogurt. Other studies, including (53), also reported comparable TSI results for LAB isolates, such as Lactococcus lactis subsp. lactis, L. acidophilus, L. plantarum, L. fermentum, and L. lactis. Manolopoulou et al. (54) found 20 distinct strains of Lactobacillus delbrueckii ssp. bulgaricus in the fermented fructose, glucose, galactose, lactose, and sucrose extracts from typical Feta cheese (54). Certain researchers have identified Lactobacillus species in fermented milk through carbohydrate fermentation patterns. Six different species of lactic acid bacteria have been isolated from dadih, an Indonesian food made from buffalo milk: L. lactis, L. mesenteroides, L. plantarum, L. casei, and L. paracasei (55). Similarly, six different species of lactic acid bacteria, L. bulgaricus, L. plantarum, L. lactis, L. acidophilus, L. brevis, and L. rhamnosus, were isolated from buffalo milk in India. Carbohydrate fermentation patterns were utilized in both cases to identify Lactobacillus species in fermented milk (56, 57).
4.2.4 Methyl red and indole test
Similar results to our study regarding positive MR tests were also previously reported by (58), who found that LAB in the fermented products showed a positive reaction. The methyl red test was negative for those isolates (59). Traditional fermented foods, including dosa butter, appam batter, buttermilk, yogurt, and cabbage, were found to contain Lactobacillus bacteria that showed a negative response (60). These current results are in line with the reported work of (61), who observed that L. acidophilus from the yogurt samples showed a negative reaction. It was determined that certain LAB isolates from fermented foods exhibited positive reactions to the methyl red test, while others exhibited negative reactions (1). According to (62), adverse reactions were exhibited by L. bulgaricus and L. fermentum from curd and milk. An additional study revealed that L. lactis, L. brevis, L. fermentum, L. casei, and L. plantarum from yogurt exhibited a negative reaction. The isolates under investigation did not exhibit the ability to synthesize indole (50). The same results were documented by (63). Additionally, LAB isolated from regional cow milk kefir and found to be indole-negative have been described (64).
4.3 Phylogenic tree
Recent studies have used 16S rDNA phylogenetic analysis to isolate lactic acid bacteria (LAB) with probiotic potential from fermented foods (1, 65). Our 16S rRNA gene analysis similarly identified Lactobacillus strains phylogenetically related to L. fermentum, L. acidophilus, L. casei, and L. buchneri. Other studies have likewise characterized Lactobacillus spp. with probiotic traits via 16S rRNA sequencing (58, 66–70). Coulibaly et al. (70) further applied 16S rDNA sequencing to compare 12 LAB strains, including Pediococcus (P. acidilactici and P. pentosaceus) and Lactobacillus. These strains were selected based on probiotic functionality, stability, and safety criteria (70). The Bacillaceae family (Firmicutes) comprises mesophilic, Gram-positive bacteria (71). Notably, B. dendritiformis strains exhibit gastrointestinal adaptability, a key probiotic trait. In this study, we successfully isolated B. dendritiformis as a candidate probiotic.
4.4 Stress tolerance potential
4.4.1 Bile salt and acid tolerance
This study evaluated the bile salt and acid tolerance of eight probiotic strains isolated from fermented buffalo milk products, including Lactobacillus plantarum, Streptococcus thermophilus, Bacillus dendritiformis, Lactobacillus brevis, Bacillus subtilis, and Souchella clausii. B. subtilis and B. dendritiformis showed the highest bile salt tolerance (5.4–6.0 log10 CFU/mL at 0.5% bile), while L. plantarum demonstrated superior resistance (5.9–6.5 log10 CFU/mL) and S. clausii showed moderate survival (4.2–4.4 log10 CFU/mL). S. thermophilus exhibited the lowest bile tolerance due to its lack of bile salt hydrolase activity (72). In terms of acid tolerance, L. plantarum, S. thermophilus (Strain 1), and B. dendritiformis displayed the greatest acid resilience (>5.0 log10 CFU/mL at pH 2.0), while B. subtilis and S. clausii showed low tolerance (< 2.2 log10 CFU/mL), supported by bile salt hydrolase production and spore formation (37). L. brevis and S. thermophilus showed moderate acid tolerance, while B. dendritiformis and S. thermophilus exhibited the lowest acid resistance, particularly at pH 2.0 (73). Overall, L. plantarum, B. dendritiformis, and S. thermophilus (Strain 1) were most promising for gastrointestinal survival, showing dual bile/acid tolerance.
4.4.2 Temperature and NaCl tolerance
For LAB fermentation to work properly, temperature is also crucial. Yogurt is best fermented at 42°C and stored at 4°C, so it is important that the probiotic strain you choose can adjust to these temperatures (74). As shown in Table 4, the isolated strains showed positive growth at 10–45 °C, but only S. thermophilus (PP565141) showed negative growth at 50°C. The detected strains were able to adjust effectively to the temperature of fermented foods and dairy, as the growth was still excellent. In (75), a total of 14 samples of fermented milk and products were studied, and it was observed that pH values were approximately 4.0. Since temperature is a critical prerequisite for bacterial development, the fact that LAB were able to live in the specified temperature range (25–40°C) suggests that they may be able to survive in the human gut temperature. The only exception to this is L. cremoris, whose growth was halted at 0°C. To mimic the typical temperature, range of the human body, a certain range was determined. Since the growth/viability of probiotics during storage and usage is a crucial component in determining their functioning, this element is very significant in evaluating the efficacy of probiotics (76). In addition, the fact that all of the isolates were able to withstand concentrations of NaCl ranging from 4% to 6% further suggests that they may be able to withstand the severe circumstances and bile salt found in the colon (77). The strain's survival under harsh conditions suggests strong potential for probiotic applications. According to (78), not only LAB in fermented food, but some species of Bacillus also have potential probiotics. Despite B. clausii's long history of usage, the literature more effectively promotes the therapeutic characteristics of other probiotics, such as Lactobacillus sp. and Bifidobacterium sp. (79–81). Among the four selected strains, all of them could survive well at 0.8% bile salts, three of them could tolerate up to 1.8% bile salts (82).
4.5 Scavenging of DPPH radicals
Because of its ease of use, rapidity, sensitivity, and reproducibility, the DPPH radical scavenging technique is widely employed to evaluate antioxidant activity (23–25, 83). The basic idea behind the test is to convert an ethanolic DPPH solution to the non-radical form DPPH-H in the presence of an antioxidant that donates hydrogen. Diphenyl picryl hydrazine, a stable radical, may be transformed from purple to yellow by the antioxidant. The following figure serves as an illustration of this. The tested isolates (Streptococcus spp., Bacillus spp., and Lactobacillus spp.) showed DPPH scavenging ranging from 22% to 52% (Figure 4), with L. brevis Cc3 exhibiting the highest activity (52%), which agrees with the value given by (84). Similarly, the study by (85) recorded DPPH levels of ~50% for 11 different strains of Lactobacillus. At 8 log CFU/mL, B. subtilis P223 showed 41.52% antioxidant activity in the DPPH experiment, while B. subtilis ATCC 6633 showed 22.45%. B. subtilis s DPPH scavenging (22.45%) aligned with B. subtilis ATCC 6633 (86), suggesting conserved antioxidant mechanisms among Bacillus strains. Our research showed that L. plantarum has a 40.70% antioxidant activity. Ten strains of L. plantarum were previously tested for resistance to hydrogen peroxide and hydroxyl and 2,2-diphenyl-1-picrylhydrazyl (DPPH) free radicals in vitro. The strains were selected from traditional fermented foods in China. At 10 CFU/ml, L. plantarum C88 showed the strongest DPPH and hydroxyl radical scavenging capabilities, with inhibition rates of 53.15% and 44.31%, respectively. L. plantarum Y1's activity (40.70%) resembled that of L. plantarum C88 (53.15%) from fermented dairy (87), highlighting cross-regional consistency in Lactobacillus antioxidants. The free radical 2,2-diphenyl-1-picrylhydrazyl (DPPH) was scavenged by the isolates in the in vitro antioxidant tests. L. plantarum IH16L had the greatest hydroxyl radical scavenging activity (82.25 ± 1.60%), compared to the other isolates, whereas L. plantarum IH26L had the lowest (35.60 ± 4.50%). Between 7.22 ± 0.04% and 21.63 ± 1.32%, the microorganisms exhibited superoxide radical scavenging capabilities. These findings suggest a correlation between DPPH and hydroxyl radical scavenging, as seen in L. plantarum IH16L (82.25% hydroxyl scavenging) (88). The traditional yogurt starter is S. thermophilus. When tested in vitro, ST-EPS2 outperformed the other compounds in terms of antioxidant activity. Enhanced free radical scavenging activities (ABTS, DPPH, and ) were seen in the barley fluid fermented with S. thermophilus 7G10, which aligns with our results (89). Nevertheless, the juice or products' antioxidant activity can be enhanced by altering the form and constituents of phenolic compounds by LAB fermentation (90). Clinical evidence establishes S. clausii as an effective antidiarrheal probiotic across age groups. Our isolate S. clausii Cm4 demonstrated significant antioxidant capacity (28% DPPH scavenging), comparable to literature reports for strain CSI08, which shows immunomodulation via U937 macrophage activation and cyto-protection in C. elegans oxidative stress models. These findings align with our observations, reinforcing its dual role in antioxidative and immunomodulatory functions. These documented strain-specific properties validate S. clausii as a therapeutically promising probiotic with dual antioxidant-immunomodulatory activity (11). A total of 27 studies found evidence of B. dendritiformis's possible effects. A study by (91) demonstrated that the levels of glutathione s-transferase (GST) and glutathione reductase (GR) were enhanced when B. subtilis was used in conjunction with fish. An additional investigation demonstrated that dietary supplementation with B. dedritiformis Dahb1 could enhance the innate immune system, which reduces the oxidative stress associated with the deposition of ammonia in tissues and blood (92).
5 Conclusion
This study highlights Pakistani fermented buffalo milk as a rich source of diverse probiotics, including both lactic acid bacteria (Lactobacillus and Streptococcus) and hardy non-LAB strains (Bacillus spp.). Among the notable discoveries, L. plantarum Y1 demonstrates remarkable survival in harsh gastrointestinal conditions, with a strong tolerance to pH 2.0 and 0.5% bile, indicating a high potential for gut colonization. Another standout strain, L. brevis Cc3, exhibits a rare combination of bile resistance and significant antioxidant activity, scavenging 52% of DPPH radicals. All tested strains display adaptability to industrial conditions, thriving across a wide temperature range (4–45°C) and tolerating NaCl, though S. thermophilus s heat sensitivity underscores the need for tailored processing methods. The findings suggest these probiotics, especially the multifunctional L. brevis Cc3 holds great promise for developing regionally appropriate functional foods in South Asia. However, further clinical studies are needed to confirm their immunomodulatory properties and safety for human consumption.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/supplementary material.
Author contributions
GS: Writing – original draft, Conceptualization, Data curation, Methodology. BR: Writing – review & editing. GK: Writing – review & editing, Supervision, Conceptualization. HQ: Writing – review & editing. MA: Formal analysis, Writing – review & editing. MQ: Writing – review & editing, Methodology. RG: Funding acquisition, Writing – review & editing, Supervision, Writing – original draft. XC: Writing – review & editing, Conceptualization, Supervision.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The authors extend their appreciation to the Yangzhou Key R&D Programme (Social Development) Project (NO. YZ2023062), Jiangsu Yangzhou University Postgraduate Science and Technology Innovation (NO.X20220891), and Sichuan Province Science and Technology Plan “Unveiling the List and Leading the Way” Project (2023YFN0101).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Mulaw G, Sisay Tessema T, Muleta D, Tesfaye A. In vitro evaluation of probiotic properties of lactic acid bacteria isolated from some traditionally fermented Ethiopian food products. Int J Microbiol. (2019) 2019:7179514. doi: 10.1101/574194
2. Boonmee M, Leksawasdi N, Bridge W, Rogers PL. Batch and continuous culture of Lactococcus lactis NZ133: experimental data and model development. Biochem Eng J. (2003) 14:127–35. doi: 10.1016/S1369-703X(02)00171-7
3. Sharma R, Sanodiya BS, Bagrodia D, Pandey M, Sharma A, Bisen PS. Efficacy and potential of lactic acid bacteria modulating human health. Int J Pharma Bio Sci. (2012) 3:935–48.
4. Steele J, Broadbent J, Kok J. Perspectives on the contribution of lactic acid bacteria to cheese flavor development. Curr Opin Biotechnol. (2013) 24:135–41. doi: 10.1016/j.copbio.2012.12.001
5. Chowdhury A. Hossain Md. N, Mostazir NJ, Fakruddin Md, Md. Billah M, et al. Screening of Lactobacillus spp. from Buffalo Yoghurt for Probiotic and Antibacterial Activity. J Bacteriol Parasitol. (2012) 3:156. doi: 10.4172/2155-9597.1000156
6. Tavakoli M, Hamidi-Esfahani Z, Hejazi MA, Azizi MH, Abbasi S. Characterization of probiotic abilities of Lactobacilli isolated from Iranian Koozeh traditional cheese. Polish J Food Nutr Sci. (2017) 67:3. doi: 10.1515/pjfns-2016-0003
7. de Melo Pereira GV, de Oliveira Coelho B, Júnior AIM, Thomaz-Soccol V, Soccol CR. How to select a probiotic? A review and update of methods and criteria. Biotechnol Adv. (2018) 36:2060–76. doi: 10.1016/j.biotechadv.2018.09.003
8. Lebeer S, Vanderleyden J, De Keersmaecker SCJ. Genes and molecules of lactobacilli supporting probiotic action. Microbiol Mol Biol Rev. (2008) 72:728–64. doi: 10.1128/MMBR.00017-08
9. Sharifi-Rad M, Anil Kumar N V, Zucca P, Varoni EM, Dini L, Panzarini E, et al. Lifestyle, oxidative stress, and antioxidants: back and forth in the pathophysiology of chronic diseases. Front Physiol. (2020) 11:694. doi: 10.3389/fphys.2020.00694
10. Saleem GN, Gu R, Qu H, Bahar Khaskheli G, Rashid Rajput I, Qasim M, et al. Therapeutic potential of popular fermented dairy products and its benefits on human health. Front Nutr. (2024) 11:1328620. doi: 10.3389/fnut.2024.1328620
11. Khokhlova E, Colom J, Simon A, Mazhar S, García-Lainez G, Llopis S, et al. Immunomodulatory and antioxidant properties of a novel potential probiotic Bacillus clausii CSI08. Microorganisms. (2023) 11:240. doi: 10.3390/microorganisms11020240
12. Amanatidou A, Smid EJ, Bennik MHJ, Gorris LGM. Antioxidative properties of Lactobacillus sake upon exposure to elevated oxygen concentrations. FEMS Microbiol Lett. (2001) 203:87–94. doi: 10.1111/j.1574-6968.2001.tb10825.x
13. Kullisaar T, Zilmer M, Mikelsaar M, Vihalemm T, Annuk H, Kairane C, et al. Two antioxidative lactobacilli strains as promising probiotics. Int J Food Microbiol. (2002) 72:215–24. doi: 10.1016/S0168-1605(01)00674-2
14. Lin M-Y, Chang F-J. Antioxidative effect of intestinal bacteria Bifidobacterium longum ATCC 15708 and Lactobacillus acidophilus ATCC 4356. Dig Dis Sci. (2000) 45:1617–22.
15. Maity C, Gupta AK. Therapeutic efficacy of probiotic Alkalihalobacillus clausii 088AE in antibiotic-associated diarrhea: a randomized controlled trial. Heliyon. (2021) 7:e07993. doi: 10.1016/j.heliyon.2021.e07993
16. Abd El-Salam MH, El-Shibiny S. A comprehensive review on the composition and properties of buffalo milk. Dairy Sci Technol. (2011) 91:663–99. doi: 10.1007/s13594-011-0029-2
17. Cesarani A, Biffani S, Garcia A, Lourenco D, Bertolini G, Neglia G, et al. Genomic investigation of milk production in Italian buffalo. Ital J Anim Sci. (2021) 20:539–47. doi: 10.1080/1828051X.2021.1902404
18. Licitra G, Caccamo M, Lortal S. Artisanal products made with raw milk. In: Raw milk. London: Elsevier (2019). p. 175–221.
19. Ismail YS, Yulvizar C, Mazhitov B. Characterization of lactic acid bacteria from local cow s milk kefir. In: IOP Conference Series: Earth and Environmental Science. Bristol: IOP Publishing (2018). p. 12019 doi: 10.1088/1755-1315/130/1/012019
20. Idoui T, Boudjerda J, Leghouchi E, Karam N-E. Lactic acid bacteria from “Sheep's Dhan”, a traditional butter: Isolation, identification and major technological traits. Grasas y aceites. (2009) 60:177–83. doi: 10.3989/gya.085408
21. Maqsood S, Hasan F, Masud T. Characterization of lactic acid bacteria isolated from indigenous dahi samples for potential source of starter culture. African J Biotechnol. (2013) 12:1172. doi: 10.5897/AJB09.1172
22. Menconi A, Kallapura G, Latorre JD, Morgan MJ, Pumford NR, Hargis BM, et al. Identification and characterization of lactic acid bacteria in a commercial probiotic culture. Biosci Microbiota, Food Heal. (2014) 33:25–30. doi: 10.12938/bmfh.33.25
23. Karaçelik AA, Seker ME, Karaköse M. Determination of antioxidant activity of different extracts from bark of Pinus spp. grown in Giresun (Turkey) province–phenolic analysis by RP-HPLC-DAD. Kahramanmaraş Sütçü Imam Üniversitesi Tarim ve Doga Derg. (2022) 25:10–8. doi: 10.18016/ksutarimdoga.vi.875313
24. Karaçelik AA, Türkuçar SA, Karaköse M. Phytochemical composition and biological activities of Angelica sylvestris L. var stenoptera Avé-Lall ex Boiss: an endangered medicinal Plant of Northeast Turkey. Chem Biodivers. (2022) 19:e202200552. doi: 10.1002/cbdv.202200552
25. Efe D, Karaköse M, Karaçelik AA, Ertan B, Seker ME. GC-MS analyses and bioactivities of essential oil obtained from the roots of Chrysopogon zizanioides (L) roberty cultivated in Giresun, Turkey. Turkish J Chem. (2021) 45:1543–50. doi: 10.3906/kim-2009-64
26. Hayakawa K. Classification and Actions of Food Microorganisms-With Particular Reference to Fermented Foods and Lactic Acid Bacteria. London: Elsevier Applied Science (1992).
27. Nur F, Hafsan H, Wahdiniar A. Isolasi bakteri asam laktat berpotensi probiotik pada dangke, makanan tradisional dari susu kerbau di Curio Kabupaten Enrekang. Biog J Ilm Biol. (2015) 3:60–5. doi: 10.24252/bio.v3i1.568
28. Sunaryanto R, Marwoto B. Isolasi, identifikasi, dan karakterisasi bakteri asam laktat dari dadih susu kerbau. J Sains dan Teknol Indones. (2013) 14:228–33. doi: 10.29122/jsti.v14i3.931
29. Rizqiati H, Sumantri C, Noor RR, Damayanthi E, Rianti EI. Isolation and Identification of Indigenous Lactic Acid Bacteria from North Sumatra River Buffalo Milk. Bogor: Faculty of Animal Science, IPB University (2015).
30. Rizqiati H. Isolation and Identification of Lactic Acid Bacteria from Pampangan Buffalo milk of South Sumatera Indonesia. Bristol: IOP Publishing (2021).
31. Shi T, Nishiyama K, Nakamata K, Aryantini NPD, Mikumo D, Oda Y, et al. Isolation of potential probiotic Lactobacillus rhamnosus strains from traditional fermented mare milk produced in Sumbawa Island of Indonesia. Biosci Biotechnol Biochem. (2012) 76:1897–903. doi: 10.1271/bbb.120385
32. Dekumpitiya N, Gamlakshe D, Abeygunawardena SI, Jayaratne D. Identification of the microbial consortium in Sri Lankan buffalo milk curd and their growth in the presence of prebiotics. J Food Sci Technol Nepal. (2016) 9:20–30. doi: 10.3126/jfstn.v9i0.12579
33. Bin Masalam MS, Bahieldin A, Alharbi MG, Al-Masaudi S, Al-Jaouni SK, Harakeh SM, et al. Isolation, molecular characterization and probiotic potential of lactic acid bacteria in Saudi raw and fermented milk. Evidence-Based Complement Altern Med. (2018) 2018:7970463. doi: 10.1155/2018/7970463
34. Adjoudj F, Guessas B, Spano G, Capozzi V, Kihal M. Phenotypic and genotypic characterization of Lactobacilli and Pediococ-ci isolated from traditional Algerian fermentation products. South Asian J Exp Biol. (2020) 10:95–103. doi: 10.38150/sajeb.10(2).p95-103
35. Forouhandeh H, Zununi Vahed S, Hejazi MS, Nahaei MR, Akbari Dibavar M. Isolation and Phenotypic characterization of Lactobacillus species from various dairy products. Curr Res Bacteriol. (2010) 3:84–8. doi: 10.3923/crb.2010.84.88
36. Thakur M, Deshpande HW, Bhate MA. Isolation and identification of lactic acid bacteria and their exploration in non-dairy probiotic drink. Int J Curr Microbiol Appl Sci. (2017) 6:1023–30. doi: 10.20546/ijcmas.2017.604.127
37. Pot B, Felis GE, Bruyne K De, Tsakalidou E, Papadimitriou K, Leisner J, et al. The genus Lactobacillus. Lact acid Bact Biodivers Taxon. (2014) 2014:249–353. doi: 10.1002/9781118655252.ch19
38. Moukala MB, Kayath CA, Ahombo G, Dangui NPM, Kinavouidi DJK, Mouélé ECN, et al. Giving more benefits to biosurfactants secreted by lactic acid bacteria isolated from plantain wine by using multiplex PCR identification. Adv Microbiol. (2019) 9:917–30. doi: 10.4236/aim.2019.911058
39. Saif F. Efficacy of Lactic Acid Bacteria isolated from some fruits and vegetables. Egypt J Microbiol. (2016) 51:13–28. doi: 10.21608/ejm.2016.1091
40. Ejaz A, Khan ZI, Ahmad K, Muhammad FG, Akhtar S. Hussain MI. Appraising growth, daily intake, health risk index, and pollution load of Zn in wheat (Triticum aestivum L) grown in soil differentially spiked with zinc. Environ Sci Pollut Res. (2022) 29:34685–700. doi: 10.1007/s11356-021-18130-w
41. Hussain W, Asghar A, Afzal MU, Qureshi TM, Shabbir S, A. comprehensive overview of probiotic and antimicrobial attributes of lactic acid bacteria commonly employed in the dairy industry. Food Sci Appl Microbiol Reports. (2023) 2:93–105.
42. Retnaningrum E, Yossi T, Nur'azizah R, Sapalina F, Kulla PDK. Characterization of a bacteriocin as biopreservative synthesized by indigenous lactic acid bacteria from dadih soya traditional product used in West Sumatra, Indonesia. Biodiversitas J Biol Divers. (2020) 21:d210933. doi: 10.13057/biodiv/d210933
43. Saral Ö, Baltaş N, Karaköse M. An inhibition potential on some metabolic enzymes (urease and xanthine oxidase), essential oil contents and antioxidant effect of Sideritis lanata L. Chem Pap. (2024) 78:8211–7. doi: 10.1007/s11696-024-03661-6
44. Siburian IRR, Lister INE, Ginting CN, Fachrial E. Molecular identification, characterization, and antimicrobial activity of isolated lactic acid bacteria from dali ni horbo. In: IOP Conference Series: Earth and Environmental Science. Bristol: IOP Publishing (2021). p. 12017 doi: 10.1088/1755-1315/713/1/012017
45. Harmoko D, Ardyati T, Jatmiko YD. Isolation and screening of lactic acid bacteria from sumbawa buffalo milk (Bubalus bubalis) as Potential Starter Cultures. J Exp Life Sci. (2022) 12:88–97. doi: 10.21776/ub.jels.2022.012.03.03
46. Khan I, Kang SC. Probiotic potential of nutritionally improved Lactobacillus plantarum DGK-17 isolated from Kimchi–A traditional Korean fermented food. Food Control. (2016) 60:88–94. doi: 10.1016/j.foodcont.2015.07.010
47. Hoque MZ, Akter F, Hossain KM, Rahman MSM, Billah MM, Islam KMD. Isolation, Identification and Analysis of Probiotic Properties of Lactobacillus spp. from Selective Regional Yoghurts. Nairobi: Academic Journals (2010).
48. Yasmine S, Eddine SD, Miloud H, Mebrouk K. Characterization of dominant cultivable lactic acid bacteria isolated from west Algerian raw camel's milk and assessment of their technological properties. Int J Biosci. (2019) 15:400−11.
49. Rhaiem N, Chahboun N, Inekach S, Ouhssine M. Identification and characterization of lactic acid bacteria isolated from cow milk and olives brine. J Mater Environ Sci. (2016) 7:1504–9.
50. Ngene AC, Onwuakor CE, Aguiyi JC, Ifeanyi VO, Ohaegbu CG, Okwuchukwu CP, et al. Screening of some lactic acid bacteria isolated from selected Nigerian fermented foods for vitamin production. Adv Microbiol. (2019) 9:943–55. doi: 10.4236/aim.2019.911060
51. Roy A, Rai C. Isolation and characterization of lactic acid bacteria with probiotic potential from pickles. Biosci Discov. (2017) 8:866–75.
52. Sharifi Yazdi MK, Davoodabadi A, Khesht Zarin HR, Tajabadi Ebrahimi M, Soltan Dallal MM. Characterisation and probiotic potential of lactic acid bacteria isolated from Iranian traditional yogurts. Ital J Anim Sci. (2017) 16:185–8. doi: 10.1080/1828051X.2016.1222888
53. Mahato S, Shahani AK. Identifying the diversity of dominant LABs from fermented dairy products dahi and yoghurt in eastern region of Nepal. J Food Sci Technol Nepal. (2019) 11:60–4. doi: 10.3126/jfstn.v11i0.29699
54. Manolopoulou E, Sarantinopoulos P, Zoidou E, Aktypis A, Moschopoulou E, Kandarakis IG, et al. Evolution of microbial populations during traditional Feta cheese manufacture and ripening. Int J Food Microbiol. (2003) 82:153–61. doi: 10.1016/S0168-1605(02)00258-1
55. Surono IS. Probiotik susu fermentasi dan kesehatan. Jakarta: YAPMMI (Yayasan Pengusaha Makanan dan Minuman Indonesia) (2004).
56. Singh GP, Sharma RR. Dominating species of Lactobacilli and Leuconostocs present among the lactic acid bacteria of milk of different cattles. Asian J Exp Sci. (2009) 23:173−9.
57. Shafakatullah N, Chandra M. Screening of raw buffalo's milk from Karnataka for potential probiotic strains. Res J Rec Sci. (2014) 3:2502.
58. Sharma A, Lavania M, Singh R, Lal B. Identification and probiotic potential of lactic acid bacteria from camel milk. Saudi J Biol Sci. (2021) 28:1622–32. doi: 10.1016/j.sjbs.2020.11.062
59. Bansal S, Singh A, Mangal M, Sharma SK. Isolation and characterization of lactic acid bacteria from fermented foods. Vegetos. (2013) 26:325–30. doi: 10.5958/j.2229-4473.26.2.092
60. Jagadeeswari S, Vidya P, Kumar DJM, Balakumaran MD. Isolation and Characterization of Bacteriocin Producing Lactobacillus sp. from Traditional Fermented Foods. Faisalabad: Maxwell Scientific Publications (2010).
61. Kavitha S, Kumar SA, Yogalakshmi KN, Kaliappan S, Banu JR. Effect of enzyme secreting bacterial pretreatment on enhancement of aerobic digestion potential of waste activated sludge interceded through EDTA. Bioresour Technol. (2013) 150:210–9. doi: 10.1016/j.biortech.2013.10.021
62. Bamgbose T, Sinha S, Abdullahi IO, Inabo HI, Bello M, Kori LD, et al. Identification of predominant lactic acid bacteria associated with kunun-zaki and kindirmo a traditional fermented food of Nigeria. Curr Top Lact Acid Bact Probiotics. (2022) 8:17–31. doi: 10.35732/ctlabp.2022.8.1.17
63. Chris B, Paul N, Anthony PW. Food Microbiology and Laboratory Practices. Milton Keynes: Open University Press (2006).
64. Abdullahi AA. Production of Bacteriocin by Lactic Acid Bacteria Isolated From Cheese to Enchance the Shelf Life of Millet Dough Ball (FURA). Minna: Federal University of Technology (2021).
65. Tserovska L, Stefanova S, Yordanova T. Identification of Lactic Acid Bacteria Isolated from Katyk, Goat's Milk and Cheese. London: Taylor & Francis (2002).
66. Shokryazdan P, Sieo CC, Kalavathy R, Liang JB, Alitheen NB, Faseleh Jahromi M, et al. Probiotic potential of Lactobacillus strains with antimicrobial activity against some human pathogenic strains. Biomed Res Int. (2014) 2014:927268. doi: 10.1155/2014/927268
67. Angelescu I-R, Zamfir M, Stancu M-M, Grosu-Tudor S-S. Identification and probiotic properties of lactobacilli isolated from two different fermented beverages. Ann Microbiol. (2019) 69:1557–65. doi: 10.1007/s13213-019-01540-0
68. Cho IJ, Lee NK, Hahm YT. Characterization of Lactobacillus spp. isolated from the feces of breast-feeding piglets. J Biosci Bioeng. (2009) 108:194–8. doi: 10.1016/j.jbiosc.2009.03.015
69. Kim S, Lee JY, Jeong Y, Kang C-H. Antioxidant activity and probiotic properties of lactic acid bacteria. Fermentation. (2022) 8:29. doi: 10.3390/fermentation8010029
70. Coulibaly WH, Kouadio NR, Camara F, Digu, t ă C, Matei F. Functional properties of lactic acid bacteria isolated from Tilapia (Oreochromis niloticus) in Ivory Coast. BMC Microbiol. (2023) 23:152. doi: 10.1186/s12866-023-02899-6
71. Makowski K, Leszczewicz M, Broncel N, Lipińska-Zubrycka L, Głebski A, Komorowski P, et al. Izolacija, biokemijske značajke i identifikacija termotolerantnih i celulolitičkih bakterija Paenibacillus lactis i Bacillus licheniformis. Food Technol Biotechnol. (2021) 59:325–36. doi: 10.17113/ftb.59.03.21.7096
72. Cutting SM. Bacillus probiotics. Food Microbiol. (2011) 28:214–20. doi: 10.1016/j.fm.2010.03.007
73. Hamdaoui N, Azghar A, Benkirane C, Bouaamali H, Mohamed M, Ou-yahia D, et al. Probiotic properties, antioxidant potential, bile salts tolerance and antibiotic susceptibility assessment of Streptococcus thermophilus isolates palest. Med Pharm J. (2024) 9:6. doi: 10.59049/2790-0231.1223
74. Lopes RP, Mota MJ, Sousa S, Gomes AM, Delgadillo I, Saraiva JA. Combined effect of pressure and temperature for yogurt production. Food Res Int. (2019) 122:222–9. doi: 10.1016/j.foodres.2019.04.010
75. Gueimonde M, Delgado S, Mayo B, Ruas-Madiedo P, Margolles A, de los Reyes-Gavilán CG. Viability and diversity of probiotic Lactobacillus and Bifidobacterium populations included in commercial fermented milks. Food Res Int. (2004) 37:839–50. doi: 10.1016/j.foodres.2004.04.006
76. Saarela M, Mogensen G, Fonden R, Mättö J, Mattila-Sandholm T. Probiotic bacteria: safety, functional and technological properties. J Biotechnol. (2000) 84:197–215. doi: 10.1016/S0168-1656(00)00375-8
77. Yateem A, Balba MT, Al-Surrayai T, Al-Mutairi B, Al-Daher R. Isolation of Lactic Acid Bacteria with Probiotic Potential from Camel Milk. New York, NY: Academic Journals Inc. (2008).
78. Nuraida L. A review: Health promoting lactic acid bacteria in traditional Indonesian fermented foods. Food Sci Hum Wellness. (2015) 4:47–55. doi: 10.1016/j.fshw.2015.06.001
79. Ghelardi E, Abreu y Abreu AT, Marzet CB, Álvarez Calatayud G, Perez III M, Moschione Castro AP. Current progress and future perspectives on the use of Bacillus clausii. Microorganisms. (2022) 10:1246. doi: 10.3390/microorganisms10061246
80. Hoyles L, Honda H, Logan NA, Halket G, La Ragione RM, McCartney AL. Recognition of greater diversity of Bacillus species and related bacteria in human faeces. Res Microbiol. (2012) 163:3–13. doi: 10.1016/j.resmic.2011.10.004
81. Lopetuso LR, Scaldaferri F, Franceschi F, Gasbarrini A. Bacillus clausii and gut homeostasis: state of the art and future perspectives. Expert Rev Gastroenterol Hepatol. (2016) 10:943–8. doi: 10.1080/17474124.2016.1200465
82. Liu WJ, Chen YF, Kwok LY Li MH, Sun T, Sun CL, Wang XN, et al. Preliminary selection for potential probiotic Bifidobacterium isolated from subjects of different Chinese ethnic groups and evaluation of their fermentation and storage characteristics in bovine milk. J Dairy Sci. (2013) 96:6807–17. doi: 10.3168/jds.2013-6582
83. Milardović S, Iveković D, Grabarić BS. A novel amperometric method for antioxidant activity determination using DPPH free radical. Bioelectrochemistry. (2006) 68:175–80. doi: 10.1016/j.bioelechem.2005.06.005
84. Duz M, Dogan YN, Dogan I. Antioxidant activitiy of Lactobacillus plantarum, Lactobacillus sake and Lactobacillus curvatus strains isolated from fermented Turkish Sucuk. An Acad Bras Cienc. (2020) 92:105. doi: 10.1590/0001-3765202020200105
85. Ji K, Jang NY, Kim YT. Isolation of lactic acid bacteria showing antioxidative and probiotic activities from kimchi and infant feces. J Microbiol Biotechnol. (2015) 25:1568–77. doi: 10.4014/jmb.1501.01077
86. Jeong S, Jung J-H, Jung K-W, Ryu S, Lim S. From microbes to molecules: a review of microbial-driven antioxidant peptide generation. World J Microbiol Biotechnol. (2024) 40:29. doi: 10.1007/s11274-023-03826-7
87. Li S, Zhao Y, Zhang L, Zhang X, Huang L, Li D, et al. Antioxidant activity of Lactobacillus plantarum strains isolated from traditional Chinese fermented foods. Food Chem. (2012) 135:1914–9. doi: 10.1016/j.foodchem.2012.06.048
88. Andrew M, Jayaraman G. Structural features of microbial exopolysaccharides in relation to their antioxidant activity. Carbohydr Res. (2020) 487:107881. doi: 10.1016/j.carres.2019.107881
89. Mao B, Guo W, Chen M, Tang X, Zhang Q, Zhao J, et al. Effects of Streptococcus thermophilus fermentation on the flavors and antioxidant properties of barley juice. Fermentation. (2023) 9:623. doi: 10.3390/fermentation9070623
90. Zhang J, Wang P, Tan C, Zhao Y, Zhu Y, Bai J, et al. Effects of L. plantarum dy-1 fermentation time on the characteristic structure and antioxidant activity of barley β-glucan in vitro. Curr Res Food Sci. (2022) 5:125–30. doi: 10.1016/j.crfs.2021.12.005
91. Salehi M, Bagheri D, Sotoudeh E, Ghasemi A, Mozanzadeh MT. The combined effects of propionic acid and a mixture of Bacillus spp. probiotic in a plant protein–rich diet on growth, digestive enzyme activities, antioxidant capacity, and immune-related genes mRNA transcript abundance in lates calcarifer fry probiotics. Antimicrob Proteins. (2023) 15:655–67. doi: 10.1007/s12602-021-09902-4
92. Gopi N, Iswarya A, Vijayakumar S, Jayanthi S, Nor SAM, Velusamy P, et al. Protective effects of dietary supplementation of probiotic Bacillus licheniformis Dahb1 against ammonia induced immunotoxicity and oxidative stress in Oreochromis mossambicus. Comp Biochem Physiol Part C Toxicol Pharmacol. (2022) 259:109379. doi: 10.1016/j.cbpc.2022.109379
Keywords: buffalo milk, fermented products, probiotic bacteria, stress tolerance, antioxidant activity
Citation: Saleem G, Rao B, Khaskheli GB, Qu H, Ahamed MS, Qasim M, Gu R and Chen X (2025) Antioxidant and stress-adaptive properties of putative probiotic bacteria in Pakistani fermented buffalo milk. Front. Nutr. 12:1619212. doi: 10.3389/fnut.2025.1619212
Received: 27 April 2025; Accepted: 07 July 2025;
Published: 25 August 2025.
Edited by:
Tanaji Kudre, Central Food Technological Research Institute (CSIR), IndiaReviewed by:
Mustafa Karaköse, Giresun University, TürkiyeShiqi Li, Northwest A&F University, China
Copyright © 2025 Saleem, Rao, Khaskheli, Qu, Ahamed, Qasim, Gu and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ruixia Gu, Z3VydWl4aWE5NjNAMTYzLmNvbQ==; Xia Chen, Y2hlbnhpQHl6dS5lZHUuY24=
 Gulnaz Saleem
Gulnaz Saleem Bisma Rao3
Bisma Rao3 Gul Bahar Khaskheli
Gul Bahar Khaskheli Muhammad Qasim
Muhammad Qasim