Abstract
The pathogenesis of functional dyspepsia (FD) is closely associated with intestinal microecological alterations. Dietary microorganisms, capable of modulating gut microecology and thereby influencing gastrointestinal function, are being explored as a promising therapeutic strategy against FD. However, the precise mechanisms underlying how dietary microbes exert beneficial effects through microecological modulation, along with therapeutic protocols, remain incompletely defined. This article systematically reviews the manifestations of intestinal microecological imbalance in FD and its proposed pathogenic mechanisms. We critically examine the role of dietary microorganisms in mitigating FD through microecological regulation, addressing their potential mechanisms of action and clinical impacts. Integrating advances in emerging diagnostic technologies, we further discuss feasible approaches and potential targets for personalized FD management. Current controversies and challenges within this research domain are analyzed, alongside perspectives for translating these findings into clinical practice. Collectively, this review aims to provide a comprehensive theoretical framework and inspire insights for both in-depth research and improved therapeutic strategies for FD.
1 Introduction
Functional dyspepsia (FD) is a prevalent functional gastrointestinal disorder, it represents significant global health burden characterized by chronic upper abdominal pain, epigastric burning, postprandial fullness, or early satiation (1). Notably, approximately 80% of patients presenting with dyspeptic symptoms receive an FD diagnosis following exclusion of organic gastrointestinal pathology, with epidemiological studies reporting a general population prevalence approaching 16% (1). Emerging evidence has established a pivotal role of gut microbial dysbiosis in FD pathogenesis (2, 3). Mechanistic studies have revealed that gut microbiota disorders are not only associated with dysregulated immune responses and impaired mucosal barrier function in the gastrointestinal tract, but one of their central roles is also manifested in the interference with bidirectional communication in the gut-brain axis (4, 5). This interference is manifested by the dysbiotic flora through a variety of pathways such as altering the signaling of the enteric nervous system, affecting vagal tone, stimulating the release of inflammatory factors, and interfering with the metabolism of neurotransmitters, which ultimately leads to an abnormal transmission of information between the gut and the brain, amplifying the perception of nociception and affecting gastrointestinal dynamics regulation (4). In light of these findings, strategies based on modulation of the gut microbiota, particularly dietary microbial interventions such as probiotic supplementation, are increasingly becoming a promising therapeutic approach in FD clinics.
Gut microecology comprises a diverse ecosystem of microorganisms including bacteria, viruses, fungi, protozoa, and bacteriophages, with bacterial populations constituting over 90% of the total microbial community (6). Predominant bacterial phyla include Firmicutes, Bacteroidetes, Proteobacteria, Actinobacteria, with Firmicutes and Bacteroidetes representing the most abundant groups (6–8). These microbial communities exert profound effects on human physiology through nutrient metabolism, vitamin biosynthesis, immunomodulation, and maintenance of intestinal epithelial barrier integrity (9).
Dietary microorganisms exhibit modulatory effects on gut microbiota composition and ameliorate the symptoms of FD through multiple pathways, including competitive exclusion, production of bioactive metabolites, and immunomodulation (10). Researches have established that probiotic supplementation, such as Lactobacillus gasseri LG21, Bacillus coagulans MY01 and Bacillus subtilis MY02, can restore microbial equilibrium, fortify intestinal barrier integrity, and enhance mucosal immunity—mechanisms that collectively mitigate intestinal inflammation and ameliorate FD-related symptoms (5, 11, 12). Consequently, targeted modulation of gut microbiota structure through dietary microorganisms represents a promising therapeutic approach. This strategy may offer novel intervention pathways for FD treatment.
Over the past 2 years, Tziatzios et al. and Shen et al. have systematically reviewed the mechanisms of action of probiotics in FD, respectively (13, 14). Tziatzios and colleagues conducted a comprehensive analysis of clinical trials investigating probiotic interventions for FD, concluding that current evidence remains insufficient to substantiate the clinical efficacy of probiotics in FD management (13). Concurrently, Shen’s team elucidated the mechanistic pathways through which probiotics may influence FD pathophysiology and symptom manifestation (14). Furthermore, Lacy et al. (15) have synthesized current therapeutic approaches for FD, including dietary modification, probiotic supplementation, antibiotic therapy, acid suppression, and neuromodulation. However, two key aspects remain unclear: the precise mechanisms by which dietary microorganisms modulate FD through gut microecological alterations, and the potential application of dietary microorganisms in FD diagnosis and treatment strategy.
This comprehensive review explores the impact and mechanisms of various dietary microorganisms on FD through their role in modulating the gut micro-ecological environment. It further investigates personalized treatment approaches and identifies potential therapeutic targets for FD, integrating advanced diagnostic technologies. We posit that systematic evaluation and clinical implementation of these multidisciplinary approaches will significantly enhance prognostic outcomes in FD management.
2 Epidemiologic characteristics of FD in association with intestinal microecology
2.1 Epidemiological characteristics of FD
FD exhibits a substantial global prevalence, with notable regional variations in its epidemiological profile. Among the general population, the overall prevalence of FD ranges from 10 to 20% (16). A multinational cross-sectional study conducted in the United States, Canada, and the United Kingdom revealed that the prevalence of FD meeting Rome IV diagnostic criteria averages approximately 10%, with country-specific rates of 12% in the United States, and 8% in both Canada and the United Kingdom (17). Clinically, FD manifests in distinct subtypes: postprandial distress syndrome (PDS), epigastric pain syndrome (EPS), and an overlapping subtype exhibiting features of both conditions. Epidemiological data indicate that PDS constitutes the predominant presentation, accounting for approximately 61% of cases, while EPS represents 18%, and the overlapping subtype comprises 21% of FD diagnoses. Evidence suggests these subtypes may demonstrate differential responses to therapeutic interventions, underscoring the importance of precise phenotyping in both clinical and research settings (17).
FD pathogenesis involves multiple etiological factors, notably psychological comorbidities, acute gastroenteritis, female sex, tobacco use, non-steroidal anti-inflammatory drug (NSAID) consumption, and Helicobacter pylori infection (1). Substantial clinical evidence demonstrates a significant association between H. pylori infection and FD symptom manifestation, with eradication therapy providing symptomatic relief in a subset of patients (18). Furthermore, psychiatric comorbidities including anxiety and depression constitute significant pathogenic contributors to FD. These psychological factors appear to modulate gastrointestinal sensory processing and motility through the gut-brain axis, ultimately influencing symptom perception and disease progression (19).
2.2 Association between FD and intestinal microecology in different populations
The relationship between FD and intestinal microecology exhibits significant heterogeneity across different populations. Age-related analyses reveal a progressive decline in beneficial intestinal bacteria among elderly populations, potentially elevating FD risk (20). This phenomenon may be attributed to age-associated physiological decline, including compromised intestinal barrier function, diminished microbial diversity, and impaired microbiota stability, collectively contributing to more pronounced FD symptoms and greater microecological dysbiosis in geriatric cohorts.
Gender-stratified analyses demonstrate a markedly higher FD prevalence in females compared to males, potentially mediated by hormonal fluctuations and psychological factors (1). Moreover, female FD patients tend to exhibit more significant intestinal microecological disturbances (1). Cross-ethnic and geographical comparisons indicate distinct patterns in FD prevalence and associated microbial characteristics. For instance, epidemiological data suggest stronger FD associations in Asian populations, while Western cohorts demonstrate differential microbiome-FD relationships, likely influenced by dietary patterns and lifestyle factors (18). Notably, patients with comorbid conditions such as diabetes mellitus and cardiovascular disease present with more complex FD pathogenesis. In these populations, metabolic dysregulation and pharmacological interventions may synergistically exacerbate FD risk while potentially confounding the interpretation of intestinal microecological imbalances (21, 22).
3 Pathogenesis of FD and interaction with intestinal microecology
The pathophysiological mechanisms underlying FD remain incompletely understood; emerging evidence suggests a predominant role of gut-brain axis dysregulation. The gut-brain axis constitutes a bidirectional neurohumoral communication network integrating neural, endocrine, and immune signaling pathways between the gastrointestinal tract and central nervous system (23). Current pathophysiological models implicate three principal components in FD pathogenesis: (1) psychological stress-mediated modulation of gut-brain signaling, (2) gut microbiota dysbiosis, and (3) immune system hyperreactivity (24, 25). These pathophysiological derangements manifest through several interrelated mechanisms: gastrointestinal motility abnormalities (particularly impaired gastric accommodation and delayed emptying), visceral hypersensitivity (with heightened duodenal sensitivity to acidic and lipid stimuli), intestinal barrier dysfunction, and low-grade mucosal inflammation (26–28). Crucially, the sustained low-grade inflammatory state and compromised epithelial barrier integrity collectively contribute to aberrant afferent signaling through both neural and humoral pathways, ultimately generating the characteristic symptom constellation of FD (29, 30).
3.1 Mechanisms of interaction between FD and intestinal microecology
An imbalance in gut microbiota may be one of the significant contributing factors to the development of FD. Comparative analyses reveal significant compositional differences between the gut microbiota of FD patients and healthy controls (31–33). These taxonomic alterations may contribute to disease progression through multifactorial mechanisms, including compromised intestinal barrier integrity, immune dysregulation, and disrupted neuro-endocrine signaling pathways (31, 34). Microbial profiling reveals significantly increased diversity in FD patients relative to healthy individuals (35). Notably, the microbiota of FD patients demonstrates marked reductions in beneficial bacterial genera (e.g., Bifidobacterium and Lactobacillus spp.), coupled with elevated levels of potentially pathogenic microorganisms (e.g., Escherichia coli and Enterococcus spp.) (36). Furthermore, metagenomic analyses have identified significant perturbations in the Firmicutes/Bacteroidetes ratio, a recognized microbial community stability index (37). The resultant microbial imbalance promotes the secretion of enterotoxins (including SEA/SEB), potentially inducing duodenal mucosal barrier dysfunction and localized immune-inflammatory responses (38). Additionally, microbial metabolites may directly stimulate intestinal afferent nerve terminals, contributing to visceral hypersensitivity and gastrointestinal motility abnormalities (19). An imbalanced gut microbiota can affect the production and metabolism of neurotransmitters such as serotonin, dopamine, etc. This can indirectly activate the vagus nerve and enteric nervous system, thereby altering signal transmission with the central nervous system, leading to symptoms like early satiety, postprandial bloating, anxiety, and depression in FD patients (38, 39).
On the other hand, alterations in the intestinal microenvironment of FD patients, including disturbances in gastric acid secretion and gastrointestinal motility, may significantly impact microbial survival and proliferation, thereby exacerbating dysbiosis. Mounting evidence indicates that delayed gastric emptying promotes prolonged food retention within the gastrointestinal tract, creating favorable conditions for bacterial overgrowth and subsequent microbial community structural modifications (26, 40). Furthermore, FD patients frequently exhibit anxiety, depression, and somatic symptoms. This activates the hypothalamic–pituitary–adrenal (HPA) axis and sympathetic nervous system (SNS), resulting in sustained elevation of cortisol levels. Increased cortisol suppresses intestinal tight junction proteins and activates the NF-kB inflammatory signaling pathway, thereby compromising the intestinal barrier (19, 41). Notably, stress reduces populations of beneficial bacteria (e.g., Lactobacillus, Bifidobacterium) while increasing pro-inflammatory bacteria (e.g., Proteobacteria) in FD patients (19). This shift alters gut-derived metabolites (including short-chain fatty acids, SCFAs, and gamma-aminobutyric acid, GABA), which in turn stimulates the vagus nerve to modulate the HPA axis, exacerbating core FD symptoms (41). Moreover, intestinal barrier impairment increases permeability, allowing bacterial products to enter systemic circulation. This activates body-wide immunoinflammatory and metabolic responses, exerting broader pathophysiological effects in FD (42). Future research should prioritize mechanistic studies to elucidate precise microbiota-host crosstalk in FD pathogenesis (Figure 1).
Figure 1
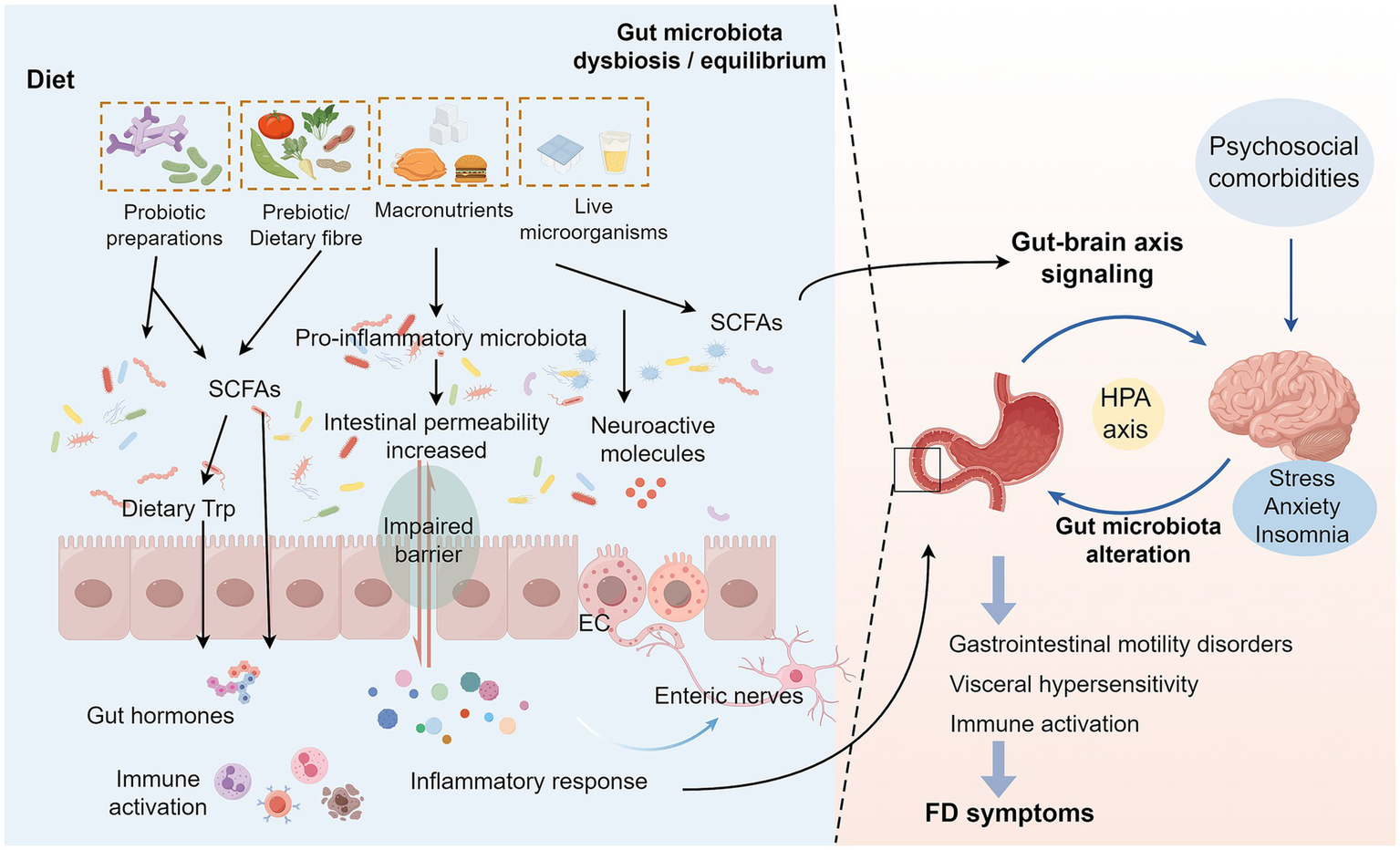
Interaction mechanisms between FD and intestinal microecology. Changes in the dietary structure of FD patients lead to a decrease in beneficial intestinal bacteria and an increase in pro-inflammatory bacteria, causing changes in SCFAs and other metabolites of the intestinal flora. On the one hand, these changes will affect the levels of various intestinal bioactive factors, resulting in impaired intestinal barrier function and increased permeability, which in turn activate the local inflammatory signaling pathway in the intestinal tract, allowing inflammatory factors and bacterial products to enter the blood circulation, and ultimately triggering systemic immune-inflammatory response and metabolic abnormalities, leading to abdominal pain, early satiety and other core symptoms of FD. On the other hand, an imbalanced intestinal microecology will interfere with neurotransmitter release, affecting the HPA axis function by stimulating the vagus nerve and enteric nervous system, leading to abnormal signaling interactions between the gut and the brain, which not only amplifies pain perception, but also disrupts gastrointestinal dynamics regulation (24). In addition, somatization symptoms such as anxiety and depression accompanying FD patients will also activate the HPA axis, further exacerbating intestinal flora dysbiosis and destroying the intestinal barrier, thus forming a vicious circle of mutual causation (19). Dietary microorganisms maintain intestinal homeostasis by regulating the balance of intestinal microecology and the production of SCFAs, repairing the intestinal barrier function, and regulating the immune response and metabolic status of the host (64, 70). FD, Functional dyspepsia; SCFAs, Short-chain fatty acids; EC, Enteroendocrine cells; HPA axis, Hypothalamic–Pituitary–Adrenal axis.
3.2 Intestinal microecological imbalance in FD
Intestinal microecological imbalance, via alterations in specific bacterial community structures and disruption of metabolic products, directly mediates the core symptomatic manifestations of FD. Changes in the gut microbiota correlate with the severity of FD symptoms (43). Research confirms that over-proliferation of Streptococcus in the duodenum exhibits a significantly positive correlation with postprandial fullness and early satiety (44). Its mechanism is linked to bacterial-mediated degradation of tight junction proteins, resulting in disruption of the intestinal mucosal barrier and activation of local immune-inflammatory responses, ultimately triggering epigastric burning pain and postprandial bloating (44). Concurrently, depletion of butyrate-producing bacteria (Butyricicoccus, Prevotella) leads to insufficient synthesis of SCFAs, impairing the repair capacity of intestinal epithelial cells and further exacerbating abdominal distension and discomfort (45). SCFAs serve not only as the primary energy source for intestinal epithelial cells but also play a crucial role in regulating intestinal barrier function and immune responses (46, 47).
Notably, reduced abundance of Veillonella is closely associated with delayed gastric emptying. Dysfunction in the metabolism of its products, such as hydrogen sulfide, can directly impact gastrointestinal motility, manifesting as food retention and nausea (44). Furthermore, translocation of oral-derived Neisseria to the duodenum exacerbates symptoms of PDS, specifically early satiety and epigastric pain, by inducing mucosal stress through enhanced protease activity (45). Simultaneously, fecal Butyricicoccus levels show a strong negative correlation with symptom severity, underscoring the driving role of microbial metabolic imbalance in persistent symptoms (45). Clinical studies further reveal that FD patients exhibit reduced concentrations of bile salts within the intestinal environment during fasting, coupled with increased expression of the vitamin D receptor (48). This impacts fat digestion and the secretion of intestinal hormones, further worsening dyspeptic symptoms in FD patients (48).
Collectively, the above evidence demonstrates that intestinal microecological imbalance can regulate the symptomatic expression of FD through multiple dimensions. This mechanism relates to intestinal microecological imbalance causing damage to the intestinal mucosal barrier, which then triggers chronic micro-inflammation and immune dysfunction (10, 49).
4 Dietary microbial modulation in the treatment of FD
Dietary microorganisms demonstrate considerable diversity and can be systematically classified into four primary categories: probiotics, prebiotics, live microorganisms, and macronutrient-associated microorganisms.
(1) Probiotics encompass multiple bacterial genera, including Lactobacillus, Bifidobacterium, and Bacillus. Specifically, lactic acid bacteria (e.g., Lactobacillus acidophilus and Lactobacillus plantarum) acidify the intestinal lumen through organic acid (particularly lactic acid) production, thereby inhibiting pathogenic bacterial growth while concurrently modulating intestinal immune responses (14). Bifidobacterium bifidum enhances intestinal barrier integrity, facilitates nutrient absorption, and maintains microbial homeostasis through immunomodulatory interactions (37). Bacillus spp. spores exhibit intestinal germination capacity, subsequently influencing microbiota composition and host immunity (50).
(2) Prebiotics represent selectively fermentable substrates (e.g., inulin, oligofructose) that preferentially stimulate growth of beneficial microbiota (particularly Bifidobacterium and Lactobacillus spp.), thereby indirectly modulating intestinal ecology (51).
(3) Live microorganisms predominantly present in fermented foods competitively exclude enteropathogens while stimulating SCFAs production and reinforcing intestinal barrier function (52).
(4) Macronutrients demonstrate significant microbiota-modulating effects: Carbohydrates (e.g., starch, glucose, fructose, lactose) undergo colonic microbial fermentation, serving as primary energy substrates for gut microbiota (53). Proteinaceous substrates elevate SCFAs concentrations while enhancing barrier function and immunological regulation (54–56). Dietary lipids exhibit differential effects: saturated fats promote Bilophila and Enterobacteriaceae proliferation with concomitant pro-inflammatory effects, whereas omega-3 polyunsaturated fatty acids (PUFAs) increase Bifidobacterium and Akkermansia abundance while attenuating inflammation and improving barrier integrity (57). These classifications and corresponding microecological impacts are systematically summarized in Table 1.
Table 1
| Microbial species | Genus/species | Typical strains | Functional role in intestinal |
|---|---|---|---|
| Probiotics | Lactobacillus rhamnosus | LGG | Enhance intestinal barrier function (129, 130) |
| Lactobacillus plantarum | L. plantarum L15; LR | Inhibit LPS-mediated NF-κB activation and improve intestinal flora dysbiosis (131, 132) | |
| Bifidobacterium lactis | B. lactis A6 | Regulate immune activation and increase SCFA production (133) | |
| Bifidobacterium longum | B. longum BB536 | Improve intestinal permeability and inhibit intestinal barrier damage (134) | |
| Lactobacillus casei | Lcr35 | Reduce inflammation scores and restore bacterial homeostasis (135) | |
| Lactobacillus delbrueckii | pExu:hsp65 | Reduce inflammatory infiltration and increase intestinal IgA levels (136) | |
| Bifidobacterium infantis | JYBR-190 | Protect intestinal mucosa from pathogen damage and enhance antimicrobial activity (137) | |
| Lactobacillus acidophilus | NCFM | Improve metabolic disorders (e.g., type 2 diabetes mellitus) and enhance host metabolic regulation (138, 139) | |
| Prebiotic-related microorganisms | Bifidobacterium spp. Bacteroides spp. | B. adolescentis B. breve B. xylanisolvens | Selectively utilizes FOS/GOS and promotes SCFA production, breaks down dietary fiber, and maintains intestinal homeostasis (140–143) |
| Live microorganisms (fermented foods) | Lactococcus spp. Weissella cibaria | L. lactis L. mesenteroides | Enhances host immunomodulation (144–146) |
| Macronutrient-related microorganisms | Prevotella spp. Clostridium spp. Ruminococcus spp. | Tryptophan | Catabolizes complex carbohydrates (e.g., cellulose, resistant starch) and regulates intestinal energy balance (147–149) |
| Others | Enterococcus faecium | C171 | Competitively inhibit pathogen colonization and modulate host immune response (150, 151) |
Classification of dietary microorganisms and their role in intestinal functions.
SCFA, Short-chain fatty acid; LPS, Lipopolysaccharides; NF-κB, Nuclear Factor kappa-B; FOS, Fructooligosaccharide; GOS, Galactooligosaccharides; LGG, Lactobacillus rhamnosus GG; L. plantarum L15, Lactobacillus plantarum L15; LR, Lactobacillus plantarum LR002; B. lactis A6, Bifidobacterium subsp. lactis A6; B. longum BB536, Bifidobacterium longum BB536; Lcr35, Lactobacillus casei variety rhamnosus; pExu:hsp65, Lactobacillus delbrueckii CIDCA 133; JYBR-190, Bifidobacterium lactis JYBR-190; NCFM, Lactobacillus acidophilus NCFM; C171, Enterococcus faecium C171.
4.1 Influence of dietary microorganisms on gut microecology and its health implications
Dietary microorganisms and their metabolic activities play a pivotal role in shaping gut microecology and influencing overall host health. This influence begins with the microbial community itself: specific dietary components (such as fiber and polyphenols) can significantly regulate the composition and functional activity of the gut microbiota. For instance, they promote the growth of beneficial bacteria (e.g., Bifidobacteria and Lactobacilli), suppress the proliferation of pathogens, and stimulate physiological processes like brown fat activation (58–60). Certain Lactobacilli can directly enhance intestinal barrier function, defending against pathogens and toxins (61, 62). The resulting changes in microbial structure profoundly affect key outputs of the gut microenvironment—particularly the levels and types of fermentation products such as SCFAs (63, 64). These dietary fiber-derived SCFAs (e.g., butyric acid, acetic acid, propionic acid) not only serve as an important source of energy for colonocytes and maintain a healthy intestinal pH (65, 66), but also act as important signaling molecules. They regulate host immune responses and intestinal epithelial integrity through G protein-coupled receptors (GPCRs) (63, 64). Furthermore, microbially derived secondary bile acids inhibit specific pathogens (e.g., Clostridia) and regulate lipid metabolism by activating the FXR receptor (67). The gut microbiota also influences host nutritional status through the synthesis of essential vitamins (e.g., vitamin K, folate, biotin, riboflavin). Conversely, imbalances (such as vitamin A deficiency) can feedback to affect microbial abundance (e.g., increased Bacteroides fragilis) and bile acid metabolism (68, 69).
The impact of dietary microbial metabolites extends far beyond the local environment of the gut. They can cross the intestinal barrier into the body circulation and systematically modulate the immune response and metabolic status of the host (64, 70). For example, dietary amino acid-derived metabolites can interact with various pattern recognition receptors [including Toll-like receptors, autoinducer-2 (AI-2), and NOD-like receptors (NLRs)] and activate signaling pathways, such as the aromatic hydrocarbon receptor (AhR) and serotonin/5-hydroxytryptophan (5-HT), which directly or indirectly shape the intestinal mucosal immunity and bacterial homeostasis, and jointly maintain intestinal homeostasis (70). Further, these microbial metabolic signals also form the basis of gut-brain axis communication. Substances produced by gut flora act on the central nervous system through neural, immune, and endocrine pathways, influencing brain function, behavior, and even mood regulation, and potentially playing a role in neurodevelopment and the pathology of certain neuropsychiatric disorders (71, 72).
Thus, dietary microbes profoundly influence the intestinal microecological environment and ultimately have a broad and far-reaching impact on host metabolic health, immune defense, and even neurological function through multilevel fine-tuned mechanisms including shaping colony structure, producing key metabolites, and mediating complex gut- neurological interactions (Figure 2). A deeper understanding of these interactions provides a key scientific basis for the development of diet-based intervention strategies to promote human health.
Figure 2

Mechanistic insights into dietary microorganism-mediated modulation of intestinal microecology. Dietary microorganisms influence intestinal microecology in four main ways: (1) Dietary fiber and polyphenols promote the proliferation of beneficial bacteria (e.g., Bifidobacterium and Lactobacillus) and inhibit pathogenic bacteria by regulating the composition of the intestinal flora, and at the same time enhance the intestinal barrier function, promote the metabolism of secondary bile acids, and assist in the synthesis of a variety of vitamins. (2) Fermented dietary fiber produces metabolites, such as SCFAs, to maintain the intestinal environment in a homeostatic state, increase the integrity of intestinal tightly linked proteins, promote the recovery of intestinal barrier function, and influence systemic immune regulation through GPCRs. (3) SCFAs exert both direct and indirect immunomodulatory effects on intestinal mucosal immunity and inflammatory responses through their interactions with host pattern recognition receptors, including TLRs and NLRs. Following their absorption, these microbial metabolites enter the systemic circulation, thereby influencing systemic immune responses and metabolic status. (4) Dietary microorganisms and metabolites such as SCFAs interact through the gut-brain axis to influence brain function with the help of the neuro-immune-endocrine network. SCFAs, Short-chain fatty acids; TLRs, Toll-like receptors; NLRs, NOD-like receptors; GPCRs, G protein-coupled receptors.
4.2 Efficacy of dietary microbial interventions for FD
Dietary microorganisms have shown potential in the treatment of FD, with mechanisms of action involving repair of the intestinal mucosal barrier (e.g., probiotics improve permeability and protect the mucosa through enhancement of intercellular junctional proteins) (73), immunomodulation (e.g., modulation of immune cell activity and cytokine secretion to attenuate inflammatory responses) (14), and synergization of the neuroendocrine system (e.g., influencing gastrointestinal hormone secretion, kinetic-sensory function to alleviate symptoms) (37). As representatives of beneficial active microorganisms, specific probiotic strains (e.g., Bifidobacterium, Lactobacillus acidophilus, Probiotics Bacillus) have been widely demonstrated to alleviate the symptoms of FD due to their ability to regulate the microecological balance of the intestinal tract (3, 12, 74). For example, clinical studies have shown that a combination preparation containing Bacillus coagulans MY01 and Bacillus subtilis MY02 significantly improved abdominal pain and fullness in patients, and the mechanism may involve its regulation of Th17 immune signaling-mediated flora modulation (12); whereas a probiotic combination containing four strains of Lactobacillus rhamnosus LR04, etc. was effective in reducing symptoms such as bloating and nausea, especially in patients with the PDS subtype associated with gastrointestinal dynamics sensitivity, and its mechanism of action may be closely related to the regulation of flora composition and reduction of visceral hypersensitivity (74). In addition, Lactobacillus reuteri DSM 17938 strain has shown specific effects on infantile abdominal pain (75, 76). As a complement to the probiotic strategy, the prebiotic kelp polysaccharide (Laminarin) corrects FD-associated flora imbalances (e.g., an imbalance in the ratio of phylum Anabaena to phylum Thicket) and ameliorates symptoms by modulating corticosterone hormone levels and inhibiting 5-HT3 receptor overexpression (37). Equally noteworthy is dietary intervention; although the low-FODMAP (fermentable oligo-, di-, mono-saccharides and polyols) diet has been less studied in FD than in irritable bowel syndrome (77, 78), this strategy has also been shown to be effective in relieving symptoms such as bloating and abdominal pain in FD patients by reducing intestinal gas production and optimizing gastrointestinal function (79, 80). It should be noted that the efficacy of existing studies on probiotics, prebiotics, and specific dietary patterns varies somewhat, which may be influenced by a variety of factors such as strain selection, dosage, treatment duration, and individual patient differences.
4.3 Long-term impact of dietary microbial regulation on intestinal microecology
The long-term impact of dietary microbial modulation on the intestinal microecology of patients with FD centers on three aspects: repair of the mucosal barrier, remodeling of the flora structure and function, and modulation of the neuroimmunity. Significant microecological imbalances are present in patients with FD, including barrier disruption due to aberrant expression of duodenal tight junction proteins, and dysregulation of the gastric and duodenal bacterial flora (e.g., increased Streptococcus and decreased Prevotella), >60% concomitant small intestinal bacterial overgrowth, and diminished anti-inflammatory and barrier repair functions due to inadequate synthesis of SCFAs (10, 73, 81, 82). Long-term probiotic interventions (e.g., supplementation with LG21 strains over 8–12 weeks) ameliorate this imbalance through multiple mechanisms. First, for barrier repair, up-regulation of tight junction proteins (ZO-1, Claudin-8) reduces intestinal permeability and decreases antigen penetration (11, 81–83). Second, for flora optimization, the abundance of Lactobacillus, Bifidobacterium, and SCFA-producing bacteria (e.g., Ruminococcaceae, Prevotella) was elevated by inhibiting pathogenic bacterial colonization, and the intestinal epithelium was supplied with energy by increasing SCFAs such as butyric acid (51, 84, 85). Third, neuroimmunomodulation promotes anti-inflammatory factors and modulates the brain-gut axis via neurotransmitters (GABA, serotonin) to alleviate visceral hypersensitivity (82). In addition, studies have shown that specific strains such as L. gasseri OLL2716 significantly reduced bile reflux and repaired the mucosa in non-H. pylori-infected FD patients, with a symptom elimination rate of 35.3% in the 12-week intervention (17.3% in the placebo group) (11), while multi-strain combinations (e.g., Lactobacillus+Bifidobacterium) resulted in >70% relief of abdominal distension and abdominal pain through synergistic potentiation (74, 84, 86).
Long-term stability and key influences of the intervention need to be focused on: continued supplementation for >6 months maintains colony alpha diversity and abundance of SCFA producers (Prevotella, etc.), and enhances colony structure against fluctuations (85, 87). However, host age (older adults cause resistance to colonization due to increased Bacteroidetes) (88, 89), medication history (antibiotics/PPIs diminish effectiveness) (90), and dietary patterns (response rates are lower in those high in animal protein than in those high in fiber) (91) significantly influence individual efficacy. Nevertheless, excessive or inappropriate microbial interventions may potentially disrupt microbial equilibrium or promote antimicrobial resistance, necessitating careful protocol design and longitudinal monitoring of therapeutic outcomes.
5 Gut microecology diagnostic techniques in FD
5.1 Gut microecological assessment to assist in the diagnosis of FD
Gut microecological assessment has important potential in the diagnosis of FD. With the help of 16S rRNA sequencing, macrogenomics and metabolomics, the composition, diversity, functional activity and changes in their metabolite profiles of the intestinal microbiota can be systematically analyzed to gain insights into the development of FD (35, 92). Specifically, 16S rRNA sequencing is used to analyze the species and abundance of the flora, macrogenomics reveals the gene functions and metabolic pathways of microorganisms, and metabolomics monitors the dynamic changes of microbial metabolites (35, 92). The application of these techniques has clearly revealed that the duodenal microbiota of FD patients at the genus level is characterized by a specific flora structure and associated microbial functions (35), and these findings provide new perspectives for understanding the pathogenesis of FD. In addition, indicators of intestinal barrier function (e.g., serum connexin, fecal calreticulin) can indirectly reflect the health status of the intestinal microecology and provide valuable additional information for FD diagnosis (73). Current studies consistently show that intestinal microecological imbalance in FD patients often coexists with impaired intestinal barrier function. Of interest, restoring microbial balance through interventions such as probiotics and prebiotics has been shown to promote the production of SCFAs, improve the diversity and stability of the bacterial flora, and ultimately enhance intestinal barrier function (93, 94).
5.2 Novel gut microbial detection technologies in FD
Recent advances in gut microbial detection technologies have significantly expanded research and diagnostic capabilities for FD. Emerging artificial intelligence (AI)-based analytical approaches enable rapid and precise evaluation of extensive gut microbiome datasets, facilitating identification of specific microbial signatures associated with FD pathogenesis. Application of AI algorithms to comparative analyses of gut microbiota from FD patients versus healthy controls has revealed distinct microbial consortium patterns that correlate strongly with disease progression. These microbial biomarkers show potential for early diagnostic applications and longitudinal disease monitoring (95).
High-resolution microendoscopy (HRME) represents an innovative imaging modality that provides real-time, cellular-level visualization of intestinal mucosa. This technique enables detection of subtle architectural alterations in mucosal microstructure resulting from gut microbial dysbiosis. In FD investigations, HRME has demonstrated significant abnormalities in duodenal mucosal epithelial cell morphology and spatial organization, which may reflect inflammatory processes and compromised barrier function secondary to microbial imbalance (96). Endomicroscopic analyses have further revealed a higher prevalence of gastric metaplasia in the duodenal bulb among FD patients compared to asymptomatic controls (97), suggesting potential pathophysiological relevance and future therapeutic targeting.
In addition, Spectroscopic methodologies, including elastic scattering spectroscopy and angle-resolved low-coherence interferometry, offer additional diagnostic precision by characterizing tissue optical properties to evaluate mucosal microstructure and biochemical composition. These non-invasive techniques enhance detection of early tissue-level changes induced by microbial dysregulation, providing valuable complementary data for FD diagnosis (98).
5.3 Biomarkers of FD and intestinal microecology
The identification of FD-related intestinal microecological biomarkers is significant in enabling early diagnosis, monitoring the condition and guiding treatment. Current research has identified several promising candidate biomarkers. At the microbial level, decreased abundance of specific gut bacteria (e.g., Bifidobacterium bifidum, Lactobacillus lactis) as well as an increase in harmful bacteria, such as Escherichia coli, are associated with the pathogenesis of FD, and the pattern of their imbalance can serve as an important indicator of microecological homeostasis (36, 45). Among the microbial metabolites, SCFAs, especially the decreased levels of butyric acid due to the reduction of butyric acid-producing bacteria, are of great interest (99). Decreased levels of SCFAs have been shown to affect intestinal barrier function and immune regulation, and have been associated with the symptomatic manifestations of FD (66). In addition, indicators reflecting local and systemic immune activation status in the gut have potential. For example, FD patients are seen to have increased infiltration of immune cells such as mast cells and eosinophils in the intestinal mucosa, and elevated serum levels of pro-inflammatory cytokines (e.g., interleukin-6, tumor necrosis factor-α) (5, 73). Meanwhile, the low-grade inflammation at the duodenal site and the abnormal alteration of tight junction protein expression further corroborate the central role of immune dysregulation of the intestinal microenvironment in the pathophysiologic process of FD (100).
Currently, most candidate biomarkers in the field of FD are still in the exploratory phase of research. To advance their clinical use, future rigorous work is needed for validation: this includes assessing their diagnostic sensitivity and specificity in large, independent and representative cohorts of FD patients and control populations. Further long-term follow-up studies are needed to clarify whether dynamic changes in these markers are associated with fluctuations in FD symptoms, disease progression or remission, and the efficacy of different interventions (e.g., probiotics, prebiotics, or dietary modifications). To overcome the limitations of single-marker performance, improve overall accuracy and lay the foundation for precision medicine, it is necessary to integrate multidimensional data from the microbiome, metabolome (e.g., SCFAs, etc.), immunome, and host genome, and to combine them with exhaustive clinical phenotypic and lifestyle information to construct diagnostic, typing, or prognostic models by using advanced methods such as machine learning. These integrated approaches are expected to yield reliable performance data and enable clinical translation, enhancing predictive power through complementary information. However, the high financial and time costs involved in these ambitious research programs, as well as the high complexity of integrating, processing, and analyzing data from multiple sources, pose potentially significant obstacles.
5.4 Personalized dietary microbial regulation programs for patients with FD
The management of patients with FD is increasingly focused on precision nutritional interventions based on the gut microbiota. Given the complex interactions between gut microbes and their hosts, as well as the significant individual differences in microecological profiles, symptom manifestations, and therapeutic responses (101, 102), the design of personalized dietary microbial modulation protocols is key. The basis of this lies in the accurate assessment of the patient’s intestinal microecology (e.g., 16S rRNA sequencing and macro-genomics to reveal the imbalance in flora composition, diversity, and function), and the in-depth integration of individualized factors such as symptomatic characteristics, dietary patterns, lifestyle habits, and comorbidities (36). For example, for specific flora imbalances (e.g., Bifidobacteria deficiency), the corresponding probiotic preparation can be supplemented (13). Specific to symptoms, patients with dyskinesia who are predominantly postprandial fullness and early satiety may use probiotics that improve gut motility, like Lactobacillus rhamnosus GG (LGG) which promotes mucin production by modulating the 5-HT4 receptor and flora to alleviate constipation (103), or probiotic combinations (Lacticaseibacillus paracasei JY062 and Lactobacillus gasseri JM1) restores motility by bi-directional modulation of pro-motility factors (gastric motility, gastrin, 5-hydroxytryptamine) and inhibitory factors (peptide YY, nitric oxide) (104). At the same time, the choice of intervention modalities needs to take into account dietary interactions with flora and hormones, e.g., low FODMAP diets and specific dietary patterns (e.g., polyphenol-rich anti-inflammatory diets) can attenuate associated symptoms or inflammation by modulating flora and immune responses (60, 105). For special groups (e.g., FD patients with comorbid diabetes), additional assessment of the impact of interventions on glycemia is required.
In order to consolidate efficacy and reduce the risk of relapse, personalized regimens need to focus on their long-term effects and be clinically stratified: e.g., specific single strains (e.g., L. plantarum) are preferred for the PDS type, whereas prokinetic drugs may be used in combination with the predominantly EPS type (74). The use of enteric capsule technology is essential to ensure effective colonization of key areas of the probiotic (e.g., duodenum) (106). The use of a synbiotic strategy (probiotics in combination with specific prebiotics such as inulin) significantly increases the production of SCFAs and significantly reduces relapse rates (107). Long-lasting colonization techniques and customized strain combinations based on host gut type need to be developed in the future to enhance the efficacy of the intervention (91, 108). In clinical practice, a phased intervention is recommended: an initial phase with a high-dose multi-strain combination (e.g., Lactobacillus gasseri + Bifidobacterium lactis) for rapid symptomatic improvement, and a maintenance phase with a low-FODMAP diet and prebiotics to stabilize the flora (74, 86). This precise protocol, which combines assessment, individualized analysis of intervention elements, and multi-level implementation strategies, is expected to significantly optimize the overall treatment outcome of FD and improve patients’ quality of life (4, 109, 110).
In addition, fecal transplantation (FMT) technology has also demonstrated potential for the treatment of FD by correcting host intestinal dysbiosis. The core technology of FMT, in particular optimized washout microbiota transplantation (WMT), involves the introduction of functioning microbial communities into the patient through the upper gastrointestinal tract (e.g., naso-duodenal tube) or lower gastrointestinal tract (e.g., colonoscopy) routes by means of a rigorously screened, healthy donor fecal flora, aiming at reestablishing a healthy microecological balance in the patient. The aim is to re-establish a healthy micro-ecological balance. Clinical studies have shown that more than 62.5% of FD patients experience symptomatic improvement after three FMTs, with a significant improvement in quality of life (111). Randomized controlled trials further confirmed that the symptom disappearance rate and total relief rate of patients in the WMT group were significantly better than those in the conventional probiotic group (112). This may be related to the increased abundance of Lactobacillus and Bacteroides and decreased Streptococcus spp. in patients after FMT, as well as increased levels of metabolites related to digestion and mucosal repair (e.g., arginine, N-acetylglutamic acid) (111). The short-term effects of FMT are clear and the safety is relatively controllable, but the sample sizes of the studies are small, the long-term efficacy data are insufficient, and individualized regimens are missing. Addressing the maintenance of long-term efficacy, exploring individualized protocols, and improving the convenience of the technology are key to promoting its clinical application.
6 Points of controversy and future prospects
6.1 Controversies and challenges of dietary microbial modulation in FD
The application of dietary microbial modulation in FD presents substantial controversies and unresolved challenges. First, while certain studies demonstrate symptom amelioration through probiotic interventions, considerable heterogeneity exists among clinical outcomes, with multiple trials failing to establish statistically significant efficacy (113). This variability likely stems from confounding factors including strain selection, dosage regimens, treatment duration, and interindividual differences in microbiome composition. Particularly, baseline microbiota profiles and comorbid conditions may further influence therapeutic response (64). Consequently, precision microbiota-based interventions necessitate comprehensive characterization of individual microbial ecosystems (114).
Second, FD represents a complex gut-brain axis disorder with incompletely elucidated pathophysiological mechanisms, resulting in the absence of consensus regarding optimal microbial modulation strategies (115). Critical knowledge gaps persist concerning selection criteria for specific microbial taxa, optimal combinatorial approaches, and evidence-based dosing protocols. Furthermore, longitudinal safety assessments remain imperative, particularly regarding potential iatrogenic dysbiosis and antimicrobial resistance development (4, 80, 116). Of particular clinical relevance is the established role of psychological factors in FD pathogenesis; however, mechanistic understanding of microbiota-psychology crosstalk remains limited, presenting a fundamental barrier to developing integrated biopsychosocial treatment paradigms (19).
6.2 Future directions and technological breakthroughs in intestinal microecology research
In the future, intestinal microecology research will develop in the direction of deeper and more precise. In terms of technology, the integration of multi-omics technologies will become an important trend. By combining multiple technologies such as macrogenomics, transcriptomics, proteomics and metabolomics, the multilevel and dynamic interaction network between gut microbes and hosts will be systematically analyzed. This integrated analysis will profoundly reveal the functional mapping of gut microecology and its mechanism of action in health and disease states (117–119). Critically, the research will focus on the complex interactions between the gut microbiota and the host immune and neuro-endocrine systems. The “complexity” of this interplay is reflected in the fact that, on the one hand, the gut flora acts as a key signaling molecule through its metabolites (e.g., short-chain fatty acids, tryptophan, neurotransmitter precursors, etc.), and at the same time, acts on the immune cells of intestinal mucosa, cells of the enteric nervous system, and cells of the enteroendocrine system, which together maintain the function of intestinal barriers, regulate the level of local and systemic inflammation levels, influence neural signaling (e.g., via vagal pathways) and secretion of key hormones (e.g., cortisol) (38, 70, 120, 121).
On the other hand, host status (e.g., dietary structure, stress level, etc.) profoundly affects the gut microenvironment and neuroendocrine activity, which in turn regulates the composition and function of the microbiota; at the same time, cytokines produced by the host immune system as well as neuroendocrine signals (e.g., neuropeptides and stress hormones) can in turn shape the microbial community structure and activity, forming a closed-loop regulatory signaling network (19, 122). An in-depth study of the fine-tuned operation of this interplay network in different individuals will provide a key breakthrough in elucidating the pathogenesis of such diseases. Therefore, future studies will not only require the use of more powerful integrated multi-omics analyses to paint a panoramic picture, but advances in single-cell analysis technologies will also facilitate the acquisition of multidimensional information at the single-cell level (123, 124), precisely resolving the heterogeneity of different cell types and their specific roles in the microbiota-host interactions network (125). Based on the results of these insightful understandings, the ultimate goal is to develop novel diagnostic markers and design precise and personalized intervention strategies (e.g., probiotics or dietary therapies targeting microbial metabolism, immunomodulation, or gut-brain axis signaling, etc.) to target the dysfunctional mechanisms of the aforementioned interactions, opening new pathways for the prevention and treatment of FD.
6.3 Clinical translational prospects of FD and intestinal microecology research
Research on FD and intestinal microecology holds significant translational potential for clinical applications. First, investigations into gut microecology-derived biomarkers may enable early and precise diagnosis of FD. The identification of disease-associated microbial signatures, including specific gut microorganisms and their metabolic byproducts, could facilitate the development of rapid, accurate diagnostic tools (36, 49, 126). Such advancements would permit timely clinical intervention, potentially improving patient outcomes.
Second, dietary microbial modulation and microecological interventions represent promising therapeutic approaches for FD management. Empirical evidence demonstrates that targeted dietary fiber supplementation can elicit symptom-specific therapeutic effects through functional modulation of intestinal microbiota (127). Furthermore, microecological therapies, including probiotics and synbiotics, have exhibited clinical potential in FD treatment, likely mediated through modification of microbial metabolic functions (126, 128).
Emerging mechanistic insights into the FD-microbiota interaction are informing the development of next-generation probiotics, prebiotics, and personalized microecological treatment regimens. These innovations promise to deliver more precise, safer, and efficacious therapeutic options for FD patients. When integrated with novel diagnostic technologies—including artificial intelligence-assisted analysis and high-resolution imaging—these approaches enable comprehensive patient assessment and real-time treatment monitoring, thereby optimizing therapeutic outcomes.
Notably, findings from intestinal microecology research may have broader implications for managing other functional gastrointestinal disorders. This research trajectory may accelerate progress in clinical gastroenterology, potentially enhancing patient quality of life while alleviating the socioeconomic burden of gastrointestinal diseases.
Statements
Author contributions
QH: Writing – original draft. FY: Writing – original draft. FL: Investigation, Supervision, Writing – review & editing, Conceptualization. CM: Writing – review & editing, Conceptualization, Supervision, Funding acquisition, Project administration.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was supported by the Traditional Chinese Medicine Research Project of Wuhan Municipal Health Commission (No. WZ22A06).
Acknowledgments
The figures were created using www.figdraw.com. The authors would like to express their gratitude to all the staff of this tool. The authors express gratitude to Deepseek V3 (version 3.0, Deepseek Inc.) for providing AI-powered language editing services, including grammar correction and stylistic improvements. This tool was exclusively utilized for textual re-finement and did not participate in data interpretation or scientific conclusions. The authors have reviewed and edited the output and take full responsibility for the content of this publication.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that Gen AI was used in the creation of this manuscript. The authors used Deepseek V3 (version 3.0, Deepseek Inc.) for providing AI-powered language editing services, including grammar correction and stylistic improvements. This tool was exclusively utilized for textual re-finement and did not participate in data interpretation or scientific conclusions.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1.
FordACMahadevaSCarboneMFLacyBETalleyNJ. Functional Dyspepsia. Lancet (London, England). (2020) 396:1689–702. doi: 10.1016/s0140-6736(20)30469-4
2.
FarcasRAGradSGradCDumitrașcuDL. Microbiota and digestive metabolites alterations in Functional Dyspepsia. J Gastrointest Liver Dis. (2024) 33:102–6. doi: 10.15403/jgld-5024
3.
ZhouLZengYZhangHMaY. The role of gastrointestinal microbiota in Functional Dyspepsia: a review. Front Physiol. (2022) 13:910568. doi: 10.3389/fphys.2022.910568
4.
RuppSKStengelA. Bi-directionality of the microbiota-gut-brain Axis in patients with Functional Dyspepsia: relevance of psychotherapy and probiotics. Front Neurosci. (2022) 16:844564. doi: 10.3389/fnins.2022.844564
5.
CeulemansMWautersLVanuytselT. Targeting the altered duodenal microenvironment in Functional Dyspepsia. Curr Opin Pharmacol. (2023) 70:102363. doi: 10.1016/j.coph.2023.102363
6.
SenderRFuchsSMiloR. Revised estimates for the number of human and Bacteria cells in the body. PLoS Biol. (2016) 14:e1002533. doi: 10.1371/journal.pbio.1002533
7.
JandhyalaSMTalukdarRSubramanyamCVuyyuruHSasikalaMNageshwarRD. Role of the normal gut microbiota. World J Gastroenterol. (2015) 21:8787–803. doi: 10.3748/wjg.v21.i29.8787
8.
DonaldsonGPLeeSMMazmanianSK. Gut biogeography of the bacterial microbiota. Nat Rev Microbiol. (2016) 14:20–32. doi: 10.1038/nrmicro3552
9.
Van HulMCaniPDPetitfilsCDe VosWMTilgHEl-OmarEM. What defines a healthy gut microbiome?Gut. (2024) 73:1893–908. doi: 10.1136/gutjnl-2024-333378
10.
BrownGHoedtECKeelySShahAWalkerMMHoltmannGet al. Role of the duodenal microbiota in Functional Dyspepsia. Neurogastroenterol Motil. (2022) 34:e14372. doi: 10.1111/nmo.14372
11.
OhtsuTTakagiAUemuraNInoueKSekinoHKawashimaAet al. The ameliorating effect of Lactobacillus Gasseri Oll2716 on Functional Dyspepsia in Helicobacter Pylori-uninfected individuals: a randomized controlled study. Digestion. (2017) 96:92–102. doi: 10.1159/000479000
12.
WautersLSlaetsHDe PaepeKCeulemansMWetzelsSGeboersKet al. Efficacy and safety of spore-forming probiotics in the treatment of Functional Dyspepsia: a pilot randomised, double-blind, placebo-controlled trial. Lancet Gastroenterol Hepatol. (2021) 6:784–92. doi: 10.1016/s2468-1253(21)00226-0
13.
TziatziosGGkolfakisPLeiteGMathurRDamorakiGGiamarellos-BourboulisEJet al. Probiotics in Functional Dyspepsia. Microorganisms. (2023) 11:351:2. doi: 10.3390/microorganisms11020351
14.
ShenXXieALiZJiangCWuJLiMet al. Research Progress for probiotics regulating intestinal Flora to improve Functional Dyspepsia: a review. Foods (Basel, Switzerland). (2024) 13:151. doi: 10.3390/foods13010151
15.
LacyBEChaseRCCangemiDJ. The treatment of Functional Dyspepsia: present and future. Expert Rev Gastroenterol Hepatol. (2023) 17:9–20. doi: 10.1080/17474124.2023.2162877
16.
MadischAAndresenVEnckPLabenzJFrielingTSchemannM. The diagnosis and treatment of Functional Dyspepsia. Deutsches Arzteblatt Int. (2018) 115:222–32. doi: 10.3238/arztebl.2018.0222
17.
AzizIPalssonOSTörnblomHSperberADWhiteheadWESimrénM. Epidemiology, clinical characteristics, and associations for symptom-based Rome iv Functional Dyspepsia in adults in the USA, Canada, and the Uk: a Cross-sectional population-based study. Lancet Gastroenterol Hepatol. (2018) 3:252–62. doi: 10.1016/s2468-1253(18)30003-7
18.
SuzukiHMoayyediP. Helicobacter Pylori infection in functional dyspepsia. Nat Rev Gastroenterol Hepatol. (2013) 10:168–74. doi: 10.1038/nrgastro.2013.9
19.
Van OudenhoveLAzizQ. The role of psychosocial factors and psychiatric disorders in functional dyspepsia. Nat Rev Gastroenterol Hepatol. (2013) 10:158–67. doi: 10.1038/nrgastro.2013.10
20.
MulderMRadjabzadehDKiefte-de JongJCUitterlindenAGKraaijRStrickerBHet al. Long-term effects of antimicrobial drugs on the Composition of the human gut microbiota. Gut Microbes. (2020) 12:1795492. doi: 10.1080/19490976.2020.1791677
21.
YafarovaAADementevaEVZlobovskayaOASheptulinaAFLopatukhinaEVTimofeevYSet al. Gut microbiota and metabolic alterations associated with heart failure and coronary artery disease. Int J Mol Sci. (2024) 25:11295. doi: 10.3390/ijms252011295
22.
HamjaneNMechitaMBNouroutiNGBarakatA. Gut microbiota Dysbiosis -associated obesity and its involvement in cardiovascular diseases and type 2 diabetes. A systematic review. Microvasc Res. (2024) 151:104601. doi: 10.1016/j.mvr.2023.104601
23.
GrundyDAl-ChaerEDAzizQCollinsSMKeMTachéYet al. Fundamentals of Neurogastroenterology: basic science. Gastroenterology. (2006) 130:1391–411. doi: 10.1053/j.gastro.2005.11.060
24.
KraimiNRossTPujoJDe PalmaG. The gut microbiome in disorders of gut-brain interaction. Gut Microbes. (2024) 16:2360233. doi: 10.1080/19490976.2024.2360233
25.
De PalmaGCollinsSMBercikP. The microbiota-gut-brain Axis in Functional gastrointestinal disorders. Gut Microbes. (2014) 5:419–29. doi: 10.4161/gmic.29417
26.
VanheelHFarréR. Changes in gastrointestinal tract function and structure in Functional Dyspepsia. Nat Rev Gastroenterol Hepatol. (2013) 10:142–9. doi: 10.1038/nrgastro.2012.255
27.
TalleyNJ. Functional Dyspepsia: new insights into pathogenesis and therapy. Korean J Intern Med. (2016) 31:444–56. doi: 10.3904/kjim.2016.091
28.
PhungNTalleyNJ. Functional Dyspepsia: new insights into the pathophysiology. J Gastroenterol Hepatol. (1998) 13:S246–s51. doi: 10.1111/j.1440-1746.1998.tb01886.x
29.
BurnsGCarrollGMatheAHorvatJFosterPWalkerMMet al. Evidence for local and systemic immune activation in Functional Dyspepsia and the irritable bowel syndrome: a systematic review. Am J Gastroenterol. (2019) 114:429–36. doi: 10.1038/s41395-018-0377-0
30.
WautersLTalleyNJWalkerMMTackJVanuytselT. Novel concepts in the pathophysiology and treatment of Functional Dyspepsia. Gut. (2020) 69:591–600. doi: 10.1136/gutjnl-2019-318536
31.
ÖhmanLTörnblomHSimrénM. Crosstalk at the mucosal border: importance of the gut microenvironment in Ibs. Nat Rev Gastroenterol Hepatol. (2015) 12:36–49. doi: 10.1038/nrgastro.2014.200
32.
HoltmannGJFordACTalleyNJ. Pathophysiology of irritable bowel syndrome. Lancet Gastroenterol Hepatol. (2016) 1:133–46. doi: 10.1016/s2468-1253(16)30023-1
33.
TziatziosGStylianakisEDamorakiGGkolfakisPLeiteGMathurRet al. Third generation sequencing analysis detects significant differences in duodenal microbiome Composition between Functional Dyspepsia patients and control subjects. Neurogastroenterol Motil. (2025) 37:e14955. doi: 10.1111/nmo.14955
34.
GhoshSWhitleyCSHaribabuBJalaVR. Regulation of intestinal barrier function by microbial metabolites. Cell Mol Gastroenterol Hepatol. (2021) 11:1463–82. doi: 10.1016/j.jcmgh.2021.02.007
35.
ZhengYFLiangSPZhongZSZhangWWuYYLiuJBet al. Duodenal microbiota makes an important impact in Functional Dyspepsia. Microb Pathog. (2022) 162:105297. doi: 10.1016/j.micpath.2021.105297
36.
BarbaraGFeinle-BissetCGhoshalUCQuigleyEMSantosJVannerSet al. The intestinal microenvironment and Functional gastrointestinal disorders. Gastroenterology. (2016) 150:1305–1318.e8. doi: 10.1053/j.gastro.2016.02.028
37.
LiuTAsifIMLiuLZhangMLiBWangL. Laminarin ameliorates Iodoacetamide-induced Functional Dyspepsia via modulation of 5-Ht(3) receptors and the gut microbiota. Int J Biol Macromol. (2024) 268:131640. doi: 10.1016/j.ijbiomac.2024.131640
38.
ZhangXChenLZhangTGaboRWangQZhongZet al. Duodenal microbiota Dysbiosis in Functional Dyspepsia and its potential role of the duodenal microbiota in gut-brain Axis interaction: a systematic review. Front Microbiol. (2024) 15:1409280. doi: 10.3389/fmicb.2024.1409280
39.
ZhuFTuHChenT. The microbiota-gut-brain Axis in depression: the potential pathophysiological mechanisms and microbiota combined Antidepression effect. Nutrients. (2022) 14:2081. doi: 10.3390/nu14102081
40.
GarciaMAAguilaEJDimaculanganMRCuaIH. Delayed gastric emptying and microorganisms in tetrads. Clin Endosc. (2025) 58:144–6. doi: 10.5946/ce.2024.137
41.
MarwahaKCainRAsmisKCzaplinskiKHollandNMayerDCGet al. Exploring the complex relationship between psychosocial stress and the gut microbiome: implications for inflammation and immune modulation. J Appl Physiol (Bethesda, Md: 1985). (2025) 138:518–35. doi: 10.1152/japplphysiol.00652.2024
42.
KabatAMSrinivasanNMaloyKJ. Modulation of immune development and function by intestinal microbiota. Trends Immunol. (2014) 35:507–17. doi: 10.1016/j.it.2014.07.010
43.
TziatziosGGkolfakisPPapanikolaouISMathurRPimentelMGiamarellos-BourboulisEJet al. Gut microbiota dysbiosis in functional dyspepsia. Microorganisms. (2020) 8:691. doi: 10.3390/microorganisms8050691
44.
ShanahanERKangSStaudacherHShahADoABurnsGet al. Alterations to the duodenal microbiota are linked to gastric emptying and symptoms in Functional Dyspepsia. Gut. (2023) 72:929–38. doi: 10.1136/gutjnl-2021-326158
45.
KimSHChoiYOhJLimEYLeeJESongEJet al. Associations among the duodenal ecosystem, gut microbiota, and nutrient intake in Functional Dyspepsia. Gut Liver. (2024) 18:621–31. doi: 10.5009/gnl230130
46.
LiuPWangYYangGZhangQMengLXinYet al. The role of short-chain fatty acids in intestinal barrier function, inflammation, oxidative stress, and colonic carcinogenesis. Pharmacol Res. (2021) 165:105420. doi: 10.1016/j.phrs.2021.105420
47.
MaJPiaoXMahfuzSLongSWangJ. The interaction among gut microbes, the intestinal barrier and short chain fatty acids. Anim Nutr (Zhongguo xu mu shou yi xue hui). (2022) 9:159–74. doi: 10.1016/j.aninu.2021.09.012
48.
BeeckmansDRiethorstDAugustijnsPVanuytselTFarréRTackJet al. Altered duodenal bile salt concentration and receptor expression in Functional Dyspepsia. United European Gastroenterol J. (2018) 6:1347–55. doi: 10.1177/2050640618799120
49.
JiSYouYPengBZhongTKuangYLiSet al. Multi-omics analysis reveals the metabolic regulators of duodenal low-grade inflammation in a Functional Dyspepsia model. Front Immunol. (2022) 13:944591. doi: 10.3389/fimmu.2022.944591
50.
MulliePVankrunkelsvenP. Spore-forming probiotics for Functional Dyspepsia. Lancet Gastroenterol Hepatol. (2021) 6:982–3. doi: 10.1016/s2468-1253(21)00340-x
51.
LiuHLiaoCWuLTangJChenJLeiCet al. Ecological dynamics of the gut microbiome in response to dietary Fiber. ISME J. (2022) 16:2040–55. doi: 10.1038/s41396-022-01253-4
52.
MukherjeeABreselgeSDimidiEMarcoMLCotterPD. Fermented foods and gastrointestinal health: underlying mechanisms. Nat Rev Gastroenterol Hepatol. (2024) 21:248–66. doi: 10.1038/s41575-023-00869-x
53.
SchneiderEO'RiordanKJClarkeGCryanJF. Feeding gut microbes to nourish the brain: unravelling the diet-microbiota-gut-brain Axis. Nat Metab. (2024) 6:1454–78. doi: 10.1038/s42255-024-01108-6
54.
ConlonMABirdAR. The impact of diet and lifestyle on gut microbiota and human health. Nutrients. (2014) 7:17–44. doi: 10.3390/nu7010017
55.
SheridanPOMartinJCLawleyTDBrowneHPHarrisHMBBernalier-DonadilleAet al. Polysaccharide utilization loci and nutritional specialization in a dominant Group of Butyrate-Producing Human Colonic Firmicutes. Microb Genom. (2016) 2:e000043. doi: 10.1099/mgen.0.000043
56.
MoszakMSzulińskaMBogdańskiP. You are what You eat-the relationship between diet, microbiota, and metabolic disorders-a review. Nutrients. (2020) 12:1096. doi: 10.3390/nu12041096
57.
BarberTMValsamakisGMastorakosGHansonPKyrouIRandevaHSet al. Dietary influences on the microbiota-gut-brain axis. Int J Mol Sci. (2021) 22:3502. doi: 10.3390/ijms22073502
58.
AzadMAKSarkerMLiTYinJ. Probiotic species in the modulation of gut microbiota: An overview. Biomed Res Int. (2018) 2018:1–8. doi: 10.1155/2018/9478630
59.
ZhangXShaoJCuiQNiWYangYYanB. Bioactivities of dietary polyphenols and their effects on intestinal microbiota. Mini Rev Med Chem. (2023) 23:361–77. doi: 10.2174/1389557522666220811123115
60.
WangZZengMWangZQinFWangYChenJet al. Food Phenolics stimulate adipocyte Browning via regulating gut microecology. Crit Rev Food Sci Nutr. (2023) 63:4026–52. doi: 10.1080/10408398.2021.1997905
61.
LeeJYTsolisRMBäumlerAJ. The microbiome and gut homeostasis. Science (New York, NY). (2022) 377:eabp9960. doi: 10.1126/science.abp9960
62.
ShangZPaiLPatilS. Unveiling the dynamics of gut microbial interactions: a review of dietary impact and precision nutrition in gastrointestinal health. Front Nutr. (2024) 11:1395664. doi: 10.3389/fnut.2024.1395664
63.
KasubuchiMHasegawaSHiramatsuTIchimuraAKimuraI. Dietary gut microbial metabolites, short-chain fatty acids, and host metabolic regulation. Nutrients. (2015) 7:2839–49. doi: 10.3390/nu7042839
64.
Murga-GarridoSMHongQCrossTLHutchisonERHanJThomasSPet al. Gut microbiome variation modulates the effects of dietary Fiber on host metabolism. Microbiome. (2021) 9:117. doi: 10.1186/s40168-021-01061-6
65.
ZhangYJLiSGanRYZhouTXuDPLiHB. Impacts of gut Bacteria on human health and diseases. Int J Mol Sci. (2015) 16:7493–519. doi: 10.3390/ijms16047493
66.
HanXMaYDingSFangJLiuG. Regulation of dietary Fiber on intestinal microorganisms and its effects on animal health. Anim Nutr (Zhongguo xu mu shou yi xue hui). (2023) 14:356–69. doi: 10.1016/j.aninu.2023.06.004
67.
KangJDMyersCJHarrisSCKakiyamaGLeeIKYunBSet al. Bile acid 7α-Dehydroxylating gut Bacteria secrete antibiotics that inhibit Clostridium Difficile: role of secondary bile acids. Cell Chem Biol. (2019) 26:27–34.e4. doi: 10.1016/j.chembiol.2018.10.003
68.
MorowitzMJCarlisleEMAlverdyJC. Contributions of intestinal Bacteria to nutrition and metabolism in the critically ill. Surg Clin North Am. (2011) 91:771–85, viii. doi: 10.1016/j.suc.2011.05.001
69.
HibberdMCWuMRodionovDALiXChengJGriffinNWet al. The effects of micronutrient deficiencies on bacterial species from the human gut microbiota. Sci Transl Med. (2017) 9:eaal4069. doi: 10.1126/scitranslmed.aal4069
70.
MaNMaX. Dietary amino acids and the gut-microbiome-immune Axis: physiological metabolism and therapeutic prospects. Compr Rev Food Sci Food Saf. (2019) 18:221–42. doi: 10.1111/1541-4337.12401
71.
CeppaFManciniATuohyK. Current evidence linking diet to gut microbiota and brain development and function. Int J Food Sci Nutr. (2019) 70:1–19. doi: 10.1080/09637486.2018.1462309
72.
LiuXCaoSZhangX. Modulation of gut microbiota-brain Axis by probiotics, prebiotics, and diet. J Agric Food Chem. (2015) 63:7885–95. doi: 10.1021/acs.jafc.5b02404
73.
VanheelHVicarioMVanuytselTVan OudenhoveLMartinezCKeitaÅVet al. Impaired duodenal mucosal integrity and low-grade inflammation in Functional Dyspepsia. Gut. (2014) 63:262–71. doi: 10.1136/gutjnl-2012-303857
74.
DragoLMeroniGPistoneDPasqualeLMilazzoGMonicaFet al. Evaluation of Main Functional Dyspepsia symptoms after probiotic Administration in Patients Receiving Conventional Pharmacological Therapies. J Int Med Res. (2021) 49:300060520982657. doi: 10.1177/0300060520982657
75.
WadhwaAKesaveluDKumarKChatterjeePJogPGopalanSet al. Role of Lactobacillus Reuteri&Nbsp;Dsm 17938 on crying time reduction in infantile colic and its impact on maternal depression: a real-life clinic-based study. Clin Pract. (2022) 12:37–45. doi: 10.3390/clinpract12010005
76.
XuMWangJWangNSunFWangLLiuXH. The efficacy and safety of the probiotic bacterium Lactobacillus Reuteri Dsm 17938 for infantile colic: a Meta-analysis of randomized controlled trials. PLoS One. (2015) 10:e0141445. doi: 10.1371/journal.pone.0141445
77.
ManningLPBiesiekierskiJR. Use of dietary interventions for Functional gastrointestinal disorders. Curr Opin Pharmacol. (2018) 43:132–8. doi: 10.1016/j.coph.2018.09.003
78.
ZhangHSuQ. Low-Fodmap diet for irritable bowel syndrome: insights from microbiome. Nutrients. (2025) 17:544. doi: 10.3390/nu17030544
79.
GoyalONohriaSBattaSDhaliwalAGoyalPSoodA. Low fermentable oligosaccharides, disaccharides, monosaccharides, and polyols diet versus traditional dietary advice for Functional Dyspepsia: a randomized controlled trial. J Gastroenterol Hepatol. (2022) 37:301–9. doi: 10.1111/jgh.15694
80.
StaudacherHMNevinANDuffCKendallBJHoltmannGJ. Epigastric symptom response to low Fodmap dietary advice compared with standard dietetic advice in individuals with Functional Dyspepsia. Neurogastroenterol Motil. (2021) 33:e14148. doi: 10.1111/nmo.14148
81.
HoriKMatsumotoTMiwaH. Analysis of the gastrointestinal symptoms of uninvestigated Dyspepsia and irritable bowel syndrome. Gut Liver. (2009) 3:192–6. doi: 10.5009/gnl.2009.3.3.192
82.
SánchezBDelgadoSBlanco-MíguezALourençoAGueimondeMMargollesA. Probiotics, gut microbiota, and their influence on host health and disease. Mol Nutr Food Res. (2017) 61:1600240. doi: 10.1002/mnfr.201600240
83.
MestreLCarrillo-SalinasFJFeliúAMechaMAlonsoGEspejoCet al. How Oral probiotics affect the severity of an experimental model of progressive multiple sclerosis? Bringing commensal Bacteria into the neurodegenerative process. Gut Microbes. (2020) 12:1813532. doi: 10.1080/19490976.2020.1813532
84.
Soheilian KhorzoghiMRostami-NejadMYadegarADabiriHHadadiARodrigoL. Impact of probiotics on gut microbiota Composition and clinical symptoms of coeliac disease patients following gluten-free diet. Contemp Clin Trials Commun. (2023) 35:101201. doi: 10.1016/j.conctc.2023.101201
85.
ZhangJZhaoJJinHLvRShiHDeGet al. Probiotics maintain the intestinal microbiome homeostasis of the sailors during a Long Sea voyage. Gut Microbes. (2020) 11:930–43. doi: 10.1080/19490976.2020.1722054
86.
ZhengYXuLZhangSLiuYNiJXiaoG. Effect of a probiotic formula on gastrointestinal health, immune responses and metabolic health in adults with Functional constipation or Functional diarrhea. Front Nutr. (2023) 10:1196625. doi: 10.3389/fnut.2023.1196625
87.
ParkSYKimYHKimSJHanJH. Impact of long-term supplementation with probiotics on gut microbiota and growth performance in post-weaned piglets. Animals. (2024) 14:1652. doi: 10.3390/ani14111652
88.
OdamakiTKatoKSugaharaHHashikuraNTakahashiSXiaoJZet al. Age-related changes in gut microbiota Composition from newborn to centenarian: a Cross-sectional study. BMC Microbiol. (2016) 16:90. doi: 10.1186/s12866-016-0708-5
89.
ClaessonMJCusackSO'SullivanOGreene-DinizRde WeerdHFlanneryEet al. Composition, variability, and temporal stability of the intestinal microbiota of the elderly. Proc Natl Acad Sci USA. (2011) 108 Suppl 1:4586–91. doi: 10.1073/pnas.1000097107
90.
DethlefsenLRelmanDA. Incomplete recovery and individualized responses of the human distal gut microbiota to repeated antibiotic perturbation. Proc Natl Acad Sci USA. (2011) 108:4554–61. doi: 10.1073/pnas.1000087107
91.
WuGDChenJHoffmannCBittingerKChenYYKeilbaughSAet al. Linking Long-term dietary patterns with gut microbial Enterotypes. Science (New York, NY). (2011) 334:105–8. doi: 10.1126/science.1208344
92.
ShahATalleyNJHoltmannG. Current and future approaches for diagnosing small intestinal Dysbiosis in patients with symptoms of Functional Dyspepsia. Front Neurosci. (2022) 16:830356. doi: 10.3389/fnins.2022.830356
93.
Pérez-ReytorDPueblaCKarahanianEGarcíaK. Use of short-chain fatty acids for the recovery of the intestinal epithelial barrier affected by bacterial toxins. Front Physiol. (2021) 12:650313. doi: 10.3389/fphys.2021.650313
94.
Markowiak-KopećPŚliżewskaK. The effect of probiotics on the production of short-chain fatty acids by human intestinal microbiome. Nutrients. (2020) 12:1107. doi: 10.3390/nu12041107
95.
Le BerreCSandbornWJAridhiSDevignesMDFournierLSmaïl-TabboneMet al. Application of artificial intelligence to gastroenterology and hepatology. Gastroenterology. (2020) 158:76–94.e2. doi: 10.1053/j.gastro.2019.08.058
96.
LouieJSRichards-KortumRAnandasabapathyS. Applications and advancements in the use of high-resolution microendoscopy for detection of gastrointestinal neoplasia. Clin Gastroenterol Hepatol. (2014) 12:1789–92. doi: 10.1016/j.cgh.2014.08.004
97.
JiRYuTGuXMZuoXLAnKZhouCJet al. Gastric metaplasia of the duodenum: in vivo diagnosis by Endomicroscopy and its relationship with Functional Dyspepsia. J Gastroenterol Hepatol. (2011) 26:73–7. doi: 10.1111/j.1440-1746.2010.06479.x
98.
RoyHKBackmanV. Spectroscopic applications in gastrointestinal endoscopy. Clin Gastroenterol Hepatol. (2012) 10:1335–41. doi: 10.1016/j.cgh.2012.10.002
99.
LuoLHuMLiYChenYZhangSChenJet al. Association between metabolic profile and Microbiomic changes in rats with Functional Dyspepsia. RSC Adv. (2018) 8:20166–81. doi: 10.1039/c8ra01432a
100.
TakiMOshimaTLiMSeiHTozawaKTomitaTet al. Duodenal low-grade inflammation and expression of tight junction proteins in Functional Dyspepsia. Neurogastroenterol Motil. (2019) 31:e13576. doi: 10.1111/nmo.13576
101.
De FilippisFVitaglionePCuomoRBerni CananiRErcoliniD. Dietary interventions to modulate the gut microbiome-how far away are we from precision medicine. Inflamm Bowel Dis. (2018) 24:2142–54. doi: 10.1093/ibd/izy080
102.
VandeputteD. Personalized nutrition through the gut microbiota: current insights and future perspectives. Nutr Rev. (2020) 78:66–74. doi: 10.1093/nutrit/nuaa098
103.
GuYQinXZhouGWangCMuCLiuXet al. Lactobacillus Rhamnosus gg supernatant promotes intestinal mucin production through regulating 5-Ht4r and gut microbiota. Food Funct. (2022) 13:12144–55. doi: 10.1039/d2fo01900k
104.
ChengSLiHDingYHuoJZhengYJiangYet al. The probiotic combination of Lacticaseibacillus paracasei Jy062 and Lactobacillus Gasseri Jm1 alleviates gastrointestinal motility disorder via improving gut microbiota. Nutrients. (2023) 15:839. doi: 10.3390/nu15040839
105.
El-SalhyMHatlebakkJGHauskenT. Diet in irritable bowel syndrome (Ibs): interaction with gut microbiota and gut hormones. Nutrients. (2019) 11:1824. doi: 10.3390/nu11081824
106.
Corona-HernandezRIÁlvarez-ParrillaELizardi-MendozaJIslas-RubioARde la RosaLAWall-MedranoA. Structural stability and viability of microencapsulated probiotic Bacteria: a review. Compr Rev Food Sci Food Saf. (2013) 12:614–28. doi: 10.1111/1541-4337.12030
107.
DuysburghCVelumaniDGargVCheongJWYMarzoratiM. Combined supplementation of inulin and Bacillus Coagulans Lactospore demonstrates Synbiotic potential in the mucosal simulator of the human intestinal microbial ecosystem (M-Shime(®)) model. J Diet Suppl. (2024) 21:737–55. doi: 10.1080/19390211.2024.2380262
108.
ZmoraNZilberman-SchapiraGSuezJMorUDori-BachashMBashiardesSet al. Personalized gut mucosal colonization resistance to empiric probiotics is associated with unique host and microbiome features. Cell. (2018) 174:1388–405.e21. doi: 10.1016/j.cell.2018.08.041
109.
MurtazaNCuívPÓMorrisonM. Diet and the microbiome. Gastroenterol Clin N Am. (2017) 46:49–60. doi: 10.1016/j.gtc.2016.09.005
110.
AbeltinoAHatemDSerantoniCRienteADe GiulioMMDe SpiritoMet al. Unraveling the gut microbiota: implications for precision nutrition and personalized medicine. Nutrients. (2024) 16:3806. doi: 10.3390/nu16223806
111.
XieLXuCFanYLiYWangYZhangXet al. Effect of fecal microbiota transplantation in patients with slow transit constipation and the relative mechanisms based on the protein digestion and absorption pathway. J Transl Med. (2021) 19:490. doi: 10.1186/s12967-021-03152-2
112.
YeXYChenJYWuLHLuoDPYeXHWuLQet al. Washed microbiota transplantation improves symptoms and intestinal barrier function in patients with Functional bowel disorders: a propensity-score matching analysis. BMC Gastroenterol. (2024) 24:45. doi: 10.1186/s12876-024-03131-z
113.
PittayanonRYuanYBollegalaNPKhannaRLeontiadisGIMoayyediP. Prokinetics for functional dyspepsia. Cochrane Database Syst Rev. (2018) 10:CD009431. doi: 10.1002/14651858.CD009431.pub3
114.
Ecklu-MensahGGilbertJDevkotaS. Dietary selection pressures and their impact on the gut microbiome. Cell Mol Gastroenterol Hepatol. (2022) 13:7–18. doi: 10.1016/j.jcmgh.2021.07.009
115.
OlsonCGTraversPLacyBE. Current opinion: Functional Dyspepsia. Curr Opin Gastroenterol. (2024) 40:470–6. doi: 10.1097/mog.0000000000001045
116.
DingCYangDMaJJinMShenZShiDet al. Effects of free antibiotic resistance genes in the environment on intestinal microecology of mice. Ecotoxicol Environ Saf. (2020) 204:111119. doi: 10.1016/j.ecoenv.2020.111119
117.
ManesNPShulzhenkoNNuccioAGAzeemSMorgunANita-LazarA. Multi-omics comparative analysis reveals multiple layers of host signaling pathway regulation by the gut microbiota. mSystems. (2017) 2:e00107–17. doi: 10.1128/mSystems.00107-17
118.
SuFSuMWeiWWuJChenLSunXet al. Integrating multi-omics data to reveal the host-microbiota Interactome in inflammatory bowel disease. Gut Microbes. (2025) 17:2476570. doi: 10.1080/19490976.2025.2476570
119.
MottaweaWYousufBSultanSAhmedTYeoJHüttmannNet al. Multi-level analysis of gut microbiome extracellular vesicles-host interaction reveals a connection to gut-brain Axis signaling. Microbiol Spectr. (2025) 13:e0136824. doi: 10.1128/spectrum.01368-24
120.
FarziAFröhlichEEHolzerP. Gut microbiota and the neuroendocrine system. Neurotherapeutics. (2018) 15:5–22. doi: 10.1007/s13311-017-0600-5
121.
ShiNLiNDuanXNiuH. Interaction between the gut microbiome and mucosal immune system. Mil Med Res. (2017) 4:14. doi: 10.1186/s40779-017-0122-9
122.
PersonHKeeferL. Psychological comorbidity in gastrointestinal diseases: update on the brain-gut-microbiome Axis. Prog Neuro-Psychopharmacol Biol Psychiatry. (2021) 107:110209. doi: 10.1016/j.pnpbp.2020.110209
123.
WuXYangXDaiYZhaoZZhuJGuoHet al. Single-cell sequencing to multi-omics: technologies and applications. Biomark Res. (2024) 12:110. doi: 10.1186/s40364-024-00643-4
124.
KimYJTakahashiK. Emerging Technologies of Single-Cell Multi-Omics. Haematologica. (2025) 110:1269–77. doi: 10.3324/haematol.2022.282557
125.
ChappellLRussellAJCVoetT. Single-cell (multi)omics technologies. Annu Rev Genomics Hum Genet. (2018) 19:15–41. doi: 10.1146/annurev-genom-091416-035324
126.
MarsRATFrithMKashyapPC. Functional gastrointestinal disorders and the microbiome-what is the best strategy for moving microbiome-based therapies for Functional gastrointestinal disorders into the clinic?Gastroenterology. (2021) 160:538–55. doi: 10.1053/j.gastro.2020.10.058
127.
SoDGibsonPRMuirJGYaoCK. Dietary Fibres and Ibs: translating Functional characteristics to clinical value in the era of personalised medicine. Gut. (2021) 70:2383–94. doi: 10.1136/gutjnl-2021-324891
128.
SimrénMBarbaraGFlintHJSpiegelBMSpillerRCVannerSet al. Intestinal microbiota in Functional bowel disorders: a Rome foundation report. Gut. (2013) 62:159–76. doi: 10.1136/gutjnl-2012-302167
129.
von OssowskiISatokariRReunanenJLebeerSDe KeersmaeckerSCVanderleydenJet al. Functional characterization of a mucus-specific Lpxtg surface Adhesin from probiotic Lactobacillus Rhamnosus gg. Appl Environ Microbiol. (2011) 77:4465–72. doi: 10.1128/aem.02497-10
130.
ShawkyLMAbo El WafaSMBeheryMBahrMHAbu AlnasrMTMorsiAA. Lactobacillus Rhamnosus gg and tannic acid synergistically promote the gut barrier integrity in a rat model of experimental diarrhea via selective immunomodulatory cytokine targeting. Mol Nutr Food Res. (2024) 68:e2400295. doi: 10.1002/mnfr.202400295
131.
YuPKeCGuoJZhangXLiB. Lactobacillus Plantarum L15 alleviates colitis by inhibiting Lps-mediated Nf-Κb activation and ameliorates Dss-induced gut microbiota Dysbiosis. Front Immunol. (2020) 11:575173. doi: 10.3389/fimmu.2020.575173
132.
ZangRZhouRLiYWuHLuLXuH. The probiotic Lactobacillus Plantarum alleviates colitis by modulating gut microflora to activate Pparγ and inhibit Mapks/Nf-Κb. Eur J Nutr. (2024) 64:32. doi: 10.1007/s00394-024-03520-w
133.
ChenMLiYZhaiZWangHLinYChangFet al. Bifidobacterium Animalis Subsp. Lactis A6 ameliorates bone and muscle loss via modulating gut microbiota Composition and enhancing butyrate production. Bone Res. (2025) 13:28. doi: 10.1038/s41413-024-00381-1
134.
ToscanoMDe GrandiRStronatiLDe VecchiEDragoL. Effect of Lactobacillus Rhamnosus Hn001 and Bifidobacterium Longum Bb536 on the healthy gut microbiota Composition at Phyla and species level: a preliminary study. World J Gastroenterol. (2017) 23:2696–704. doi: 10.3748/wjg.v23.i15.2696
135.
YeungCYChiauJCChengMLChanWTChangSWJiangCBet al. Immune modulation effects of Lactobacillus Casei variety Rhamnosus on enterocytes and intestinal stem cells in a 5-Fu-induced mucositis mouse model. Gastroenterol Res Pract. (2021) 2021:3068393. doi: 10.1155/2021/3068393
136.
BarrosoFALde JesusLCLde CastroCPBatistaVLFerreiraÊFernandesRSet al. Intake of Lactobacillus Delbrueckii (Pexu:Hsp65) prevents the inflammation and the disorganization of the intestinal mucosa in a mouse model of mucositis. Microorganisms. (2021) 9:107. doi: 10.3390/microorganisms9010107
137.
YangLChenYBaiQChenXShaoYWangRet al. Protective effect of Bifidobacterium Lactis Jybr-190 on intestinal mucosal damage in chicks infected with Salmonella Pullorum. Front Vet Sci. (2022) 9:879805. doi: 10.3389/fvets.2022.879805
138.
SanchezMDarimontCDrapeauVEmady-AzarSLepageMRezzonicoEet al. Effect of Lactobacillus Rhamnosus Cgmcc1.3724 supplementation on weight loss and maintenance in obese men and women. Br J Nutr. (2014) 111:1507–19. doi: 10.1017/s0007114513003875
139.
AndreasenASLarsenNPedersen-SkovsgaardTBergRMMøllerKSvendsenKDet al. Effects of Lactobacillus Acidophilus Ncfm on insulin sensitivity and the systemic inflammatory response in human subjects. Br J Nutr. (2010) 104:1831–8. doi: 10.1017/s0007114510002874
140.
CroninPJoyceSAO'ToolePWO'ConnorEM. Dietary fibre modulates the gut microbiota. Nutrients. (2021) 13:1655. doi: 10.3390/nu13051655
141.
ChenWTanDYangZTangJBaiWTianL. Fermentation patterns of prebiotics Fructooligosaccharides-Scfa esters inoculated with fecal microbiota from ulcerative colitis patients. Food Chem Toxicol. (2023) 180:114009. doi: 10.1016/j.fct.2023.114009
142.
SlavinJ. Fiber and prebiotics: mechanisms and health benefits. Nutrients. (2013) 5:1417–35. Epub 2013/04/24. doi: 10.3390/nu5041417
143.
YangQLiangQBalakrishnanBBelobrajdicDPFengQJZhangW. Role of dietary nutrients in the modulation of gut microbiota: a narrative review. Nutrients. (2020) 12:381. doi: 10.3390/nu12020381
144.
RezacSKokCRHeermannMHutkinsR. Fermented foods as a dietary source of live organisms. Front Microbiol. (2018) 9:1785. doi: 10.3389/fmicb.2018.01785
145.
PadhiSSarkarPSahooDRaiAK. Potential of fermented foods and their metabolites in improving gut microbiota function and lowering gastrointestinal inflammation. J Sci Food Agric. (2024) 105:4058–69. doi: 10.1002/jsfa.13313
146.
PyoYKwonKHJungYJ. Probiotic functions in fermented foods: anti-viral, immunomodulatory, and anti-cancer benefits. Foods (Basel, Switzerland). (2024) 13:2386. doi: 10.3390/foods13152386
147.
LangJMPanCCantorRMTangWHWGarcia-GarciaJCKurtzIet al. Impact of individual traits, saturated fat, and protein source on the gut microbiome. mBio. (2018) 9:e01604-18. doi: 10.1128/mBio.01604-18
148.
ZhangZTunHMLiRGonzalezBJMKeenesHCNyachotiCMet al. Impact of xylanases on gut microbiota of growing pigs fed corn- or wheat-based diets. Anim Nutr (Zhongguo xu mu shou yi xue hui). (2018) 4:339–50. doi: 10.1016/j.aninu.2018.06.007
149.
HuangZWellsJMFoglianoVCapuanoE. Microbial tryptophan catabolism as an actionable target via diet-microbiome interactions. Crit Rev Food Sci Nutr. (2024) 65:3650–64. doi: 10.1080/10408398.2024.2369947
150.
MiJHeTHuXWangZWangTQiXet al. Enterococcus Faecium C171: modulating the immune response to acute lethal viral challenge. Int J Antimicrob Agents. (2023) 62:106969. doi: 10.1016/j.ijantimicag.2023.106969
151.
XuLWuYYangXPangXWuYLiXet al. The Fe-S cluster biosynthesis in Enterococcus Faecium is essential for anaerobic growth and gastrointestinal colonization. Gut Microbes. (2024) 16:2359665. doi: 10.1080/19490976.2024.2359665
Summary
Keywords
gut microbiota, probiotics, prebiotics, dietary microbiota, functional dyspepsia
Citation
Huang Q, You F, Liang F and Ma C (2025) Dietary microbes and functional dyspepsia: modulating the gut microecology for therapeutic benefit. Front. Nutr. 12:1625987. doi: 10.3389/fnut.2025.1625987
Received
09 May 2025
Accepted
08 August 2025
Published
19 August 2025
Volume
12 - 2025
Edited by
Bei Gao, Nanjing University of Information Science and Technology, China
Reviewed by
Florinda Jimenez Vega, Universidad Autónoma de Ciudad Juárez, Mexico
Catia Sternini, University of California, Los Angeles, United States
Updates
Copyright
© 2025 Huang, You, Liang and Ma.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fengxia Liang, fxliang5@hotmail.com; Chaoyang Ma, chaoyangm2023@163.com
†These authors have contributed equally to this work
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.