- School of Public Health, Jilin University, Changchun, China
Aims: Cognitive impairment, frequently associated with neurodegenerative diseases such as Alzheimer's disease, may be associated with multiple factors including dietary fiber intake and inflammation. We aimed to explore the associations between reported dietary fiber intake, three novel inflammatory markers, and cognitive function.
Methods: This observational and exploratory cross-sectional study utilized the data from the 2011–2014 of the National Health and Nutrition Examination Survey (NHANES). Digit Symbol Substitution Test (DSST), Consortium to Establish a Registry for Alzheimer's Disease Word Learning (CERAD-WL), CERAD Delayed Recall (CERAD-DR), and Animal Fluency tests (AFT) were used to assess the cognitive function. Linear regression was conducted to explore the relationships between reported dietary fiber intake, three novel inflammatory markers [Albumin-to-alkaline phosphatase ratio (AAPR), Neutrophil-to-Albumin Ratio (NAR), and Systemic Inflammation Response Index (SIRI)] and cognitive function. Mediation analysis was performed to identify the mediating role of inflammatory markers in the relationship between reported dietary fiber intake and cognitive function.
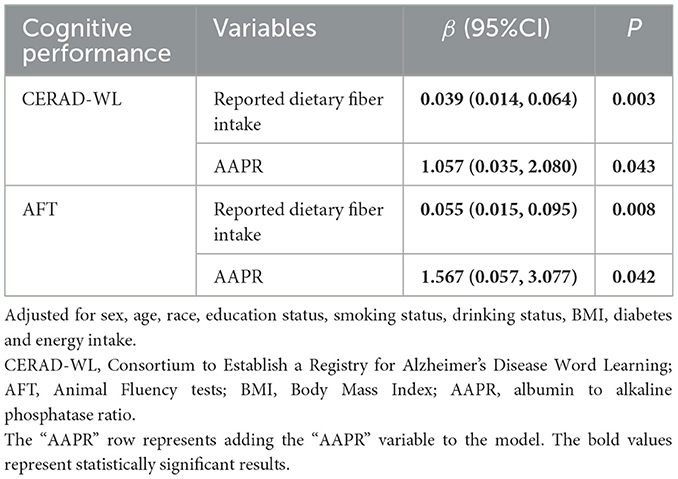
Results: The final analysis included 2,461 participants. Reported dietary fiber intake was associated with CERAD-WL (β = 0.042, 95% CI = 0.018 to 0.066), AFT (β = 0.060, 95% CI = 0.020 to 0.100) and inflammatory markers (AAPR: β = 0.003, 95% CI=0.002 to 0.004; NAR: β = −0.003, 95% CI = −0.006 to −0.001; SIRI: β = −0.008, 95% CI = −0.015 to −0.001). AAPR was positively associated with WL (β = 1.184, 95% CI = 0.165 to 2.204) and AFT (β = 1.747, 95% CI = 0.229 to 3.264). AAPR mediated the positive association between reported dietary fiber intake and AFT, with mediation proportion of 17.88%.
Conclusions: Reported dietary fiber intake, inflammatory markers, and cognitive function were pairwise associated. The AAPR played a mediating role in the association between reported dietary fiber intake and cognitive function.
1 Introduction
Cognitive impairment is an age-related state that occurs when various morphological, biochemical, metabolic, and circulatory changes caused by brain aging exceed a certain threshold (1). Cognitive impairment, the hallmark feature of dementia, is a severe neurological syndrome. Global projections indicate that 152.8 million people were affected by dementia worldwide in 2019 (2). Mild cognitive impairment (MCI) is often seen as an early indicator of Alzheimer's disease (AD) and is defined as a less severe stage of cognitive impairment, marked by slight alterations in memory and thinking skills (3). In older adults, dementia and cognitive impairment are the leading causes of long-term functional dependence and decline, leading to decreased quality of life, elevated healthcare expenses, and increased mortality rates (4). Cognitive impairment adversely influences an individual's capacity for daily living and imposes an economic burden on both society and families (5). Consequently, it is vital to recognize risk factors for cognitive impairment and inform the public about preventive measures to promote the cognitive health of older adults.
Previous studies have shown that cognitive impairment is associated with various factors such as dietary fiber intake, chronic low-grade inflammatory stress, sleep, and physical condition (6–9). Research has found higher dietary fiber intake is associated with improved specific components of cognitive function (10, 11). But some studies have also shown that dietary fiber intake is not associated with cognitive impairment in older adults (12). The effect of dietary fiber intake on cognitive performance in older adults is unclear, more research is needed to demonstrate this.
In addition to dietary factors, chronic inflammation in the body may cause morphological changes in the brain, damaging the normal function of specific areas of the brain, affecting cognitive function (13). The Albumin to Alkaline Phosphatase Ratio (AAPR), Neutrophil-Albumin Ratio (NAR), and Systemic Inflammatory Response Index (SIRI) are three novel inflammation-related biomarkers that reflect the systemic inflammatory state in the body and have been proven to be effective prognostic indicators for a range of diseases, particularly malignant tumors (14–16). The SIRI and AAPR were originally designed to predict the prognosis of digestive system cancer, and higher SIRI levels were associated with unfavorable prognostic outcomes (17, 18). Hooper et al. demonstrated that plasma alkaline phosphatase activity was significantly elevated in patients with AD and inversely correlated with cognitive function (19). In addition, some studies have confirmed that relatively low serum albumin concentration may be an independent risk factor for MCI in older adults (20). As a composite biomarker integrating both albumin and alkaline phosphatase, AAPR holds significant promise for assessing neuroinflammation-related cognitive decline. A study detected that NAR and SIRI levels were negatively related to the composite cognitive function score (Z-score) (21). NAR, as a comprehensive biomarker of neutrophils and albumin, represents the dual effects of neurotoxicity and protection in the acute phase, and can better reflect the degree of immune inflammation than a single indicator (22). Neutrophil count, lymphocyte count, and monocyte count are all closely related to immunity and inflammation. Given the critical role of inflammation in chronic diseases, hematologic markers readily available from routine blood tests were widely used to assess systemic inflammation (23). SIRI integrates the above three indicators, which can be used to distinguish the immune inflammatory response of three different pathways in vivo, and reflect the immune inflammatory state of the body more comprehensively (24). However, evidence on the relationships between inflammatory markers and cognitive function across various dimensions remains scarce. Recent evidence suggested that dietary fiber intake could also influence the systemic inflammation (25). A study by Saeed et al. identified that, in contrast to the high-fiber group, C-reactive protein (CRP) was higher in the low-fiber group (26). A cross-sectional study demonstrated that there was a significant negative correlation between dietary fiber intake and SIRI (27).
Recent studies have highlighted the ability of dietary interventions to influence inflammatory pathways associated with cognitive decline (28). For instance, high-fiber diets were associated with lower concentrations of pro-inflammatory cytokines, which were recognized for their role in neuroinflammation and consequent cognitive decline (29). Moreover, the Mediterranean diet, rich in dietary fiber, has demonstrated protective effects against cognitive decline, potentially through its anti-inflammatory properties (30). These findings underscore the importance of considering the impact of diet and inflammation on cognitive health when investigating older adults.
However, few studies have concentrated on the relationships between dietary fiber intake, inflammatory markers and cognitive function. Given the existing evidence regarding the potential advantages of fiber intake in improving inflammation levels and cognitive function, along with the influence of inflammation on cognitive performance, we hypothesized that the positive association between reported dietary fiber intake and cognitive function might be mediated through its anti-inflammatory effects. The objective of this research was to examine associations between reported dietary fiber intake, three inflammatory markers (AAPR, NAR and SIRI) and cognitive function and to determine the mediating role of inflammatory markers in the association of reported dietary fiber intake and cognitive function.
2 Materials and methods
2.1 Study population
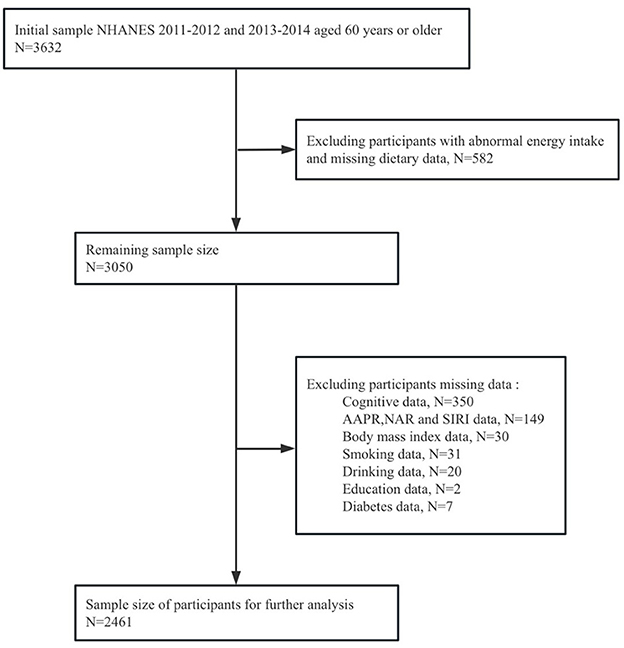
This cross-sectional study utilized data from the National Health and Nutrition Examination Survey (NHANES), a national survey employing a complex, multistage, and probabilistic sampling design to evaluate the health status of U.S. adults. This program consists of five main data sets collected through interviews and physical examinations, including demographic information, diet, medical assessment, laboratory results and questionnaire data. Each participant completed the questionnaire and underwent examinations at the mobile examination center (MEC) over the course of 1 year, in order to obtain important research variables. The study included 3,632 adults aged 60 and above in NHANES from 2011 to 2014. Additionally, considering the complex sampling design and sample weights of NHANES, we removed missing values. 564 participants were excluded due to incomplete or unreliable 24-h recall data. Individuals who had extreme total energy intakes of < 500 or > 5,000 kcal/day for women (n = 13), and < 500 or > 8,000 kcal/day for men (n = 5) (31, 32) were further omitted. Participants with missing data on cognitive measures (n = 350), AAPR, NAR and SIRI (n = 149), and other covariates (n = 90) were excluded. Ultimately, a total of 2,461 participants were incorporated into this study, as detailed in Figure 1.
The NCHS ethics review board approved the NHANES study protocols, and all participants signed informed consent forms.
2.2 Reported dietary fiber intake
Participants underwent two interviews recalling dietary intake across the past 24-h, the initial one took place face-to-face at a mobile test center, while 3 to 10 days later, the second occurred over the phone. If dietary recall data for 2 days were available, the average was calculated. Otherwise, a single dietary recall was used (33).
2.3 Cognitive function
This study assessed cognitive performance by four cognitive tests: Digit Symbol Substitution Test (DSST), Consortium to Establish a Registry for Alzheimer's Disease Word Learning (CERAD-WL), CERAD Delayed Recall (CERAD-DR) and Animal Fluency tests (AFT) (34). The DSST was a paper-and-pencil cognitive test presented on a sheet of paper, which could be used to assess the reaction speed, long-term attention, and working memory of the subjects (35). The CERAD-WL requires participants to recall as many words as possible after reading 10 unrelated words aloud in various orders, for a total of 30 points. The CERAD-DR was performed after the AFT and DSST. Participants were asked to recall words from the CERAD-WL test, which was used to assess transient and delayed learning abilities. AFT was used to assess verbal fluency, in which participants were required to name as many animals as possible within 1 min, without any prompts. A Z-score was employed as a standardized measure of total cognitive performance to eliminate discrepancies in individual cognitive scores in this investigation. The Z-score was calculated as Z = (x-m)/σ, where x was the raw score, m was the population mean, and σ was the population SD. Composite Z-scores were created by summing the Z-scores of these four individual tests (DSST, AFT, CERAD-WL, CERAD-DR) (36). Z scores < -1 were characterized as “cognitive impairment” (37).
2.4 Definition of inflammatory markers (AAPR, NAR and SIRI)
Peripheral blood samples from NHANES participants were analyzed at a mobile Examination center (MEC) using a Beckman Coulter HMX hematology analyzer. Lymphocyte, neutrophil, and monocyte counts were measured by complete blood count. The Beckman UniCel®DxC800 Synchron analyzer was employed to assess serum ALP levels and serum albumin levels. Based on peripheral blood cell counts, AAPR, NAR, and SIRI are calculated as follows. AAPR = albumin (g/L)/alkaline phosphatase (U/L) (38), NAR = neutrophil count (109/L)/albumin (g/dL) (39), SIRI = monocyte count (109/L) × neutrophils count (109/L)/lymphocyte count (109/L) (40). The specific measures and detailed information can be found in the NHANES Laboratory/Medical Technician Procedures Manual (41).
2.5 Covariates
We adjusted covariates factors that might be associated with reported dietary fiber intake and cognitive function (including age, sex, race, education status, body mass index (BMI), smoking status, drinking status, energy intake (42) and history of diabetes). Smoking status was assessed as non-smokers (had smoked < 100 cigarettes in their entire life), former smoker (had smoked ≥ 100 cigarettes in their entire life and answered negatively to the question, “Do you smoke now?”), or current smoker (≥ 100 cigarettes in their entire life and answered affirmatively to the question, “Do you smoke now?”). Smoking status was categorized into “never smokers” and “ever smokers” by combining former smokers and current smokers (43). Drinking status was classified into never drinkers (no alcohol consumption throughout lifetime or in the past 12 months), former drinkers (prior alcohol consumption but no intake in the past 12 months), and current drinkers (consumption of ≥12 alcoholic drinks in the past year or in their lifetimes and at least one drinking day reported in the past year). The drinking status was later categorized as “never drinker” and “ever drinker” by combing former and current drinkers (44). BMI was categorized into three categories: underweight or healthy weight (BMI < 25.0 kg/m2), overweight (25.0 ≤ BMI < 30.0 kg/m2), and obese (≥ 30.0 kg/m2). Energy intake was categorized into three categories: low intake (500 to 1,600 per day for women, 500 to 2,000 calories per day for men), adequate intake (1,600 to 2,400 calories per day for women, 2,000 to 3,000 calories per day for men) and high intake (>2,400 calories per day for women and 3,000 calories per day for men) (45).
2.6 Statistical analysis
According to the analysis guidelines of NHANES, weighting was performed using strata, PSU and weight. The weight used was WTDRD1 or WTDR2D. All the statistical analysis was conducted under the instruction of NHANES complex sample weight. In this study, IBM SPSS24.0 software and R4.3.2 were used to analyze the data. Continuous and categorical data were characterized using medians (IQRs) or numbers (weighted proportions), respectively. Differences between groups in continuous and categorical variables were assessed by the Wilcoxon rank-sum test and chi-square test, respectively. Linear regression was conducted to explore the relationships between reported dietary fiber intake, inflammatory markers and cognitive function. The results were presented as regression coefficients (β) and 95% confidence intervals (CI). Model 1 was adjusted for sex, age, race, and education status, and model 2 further adjusted for BMI, smoking status, drinking status, energy intake and history of diabetes based on model 1. The multicollinearity of the independent variables was tested by calculating the variance inflation factor (VIF). The VIF values of all independent variables were < 5, indicating that there was no significant multicollinearity problem in the model. Finally, mediation analysis was performed by “mediation” packages to explore the mediating role of inflammatory markers in the relationship between reported dietary fiber intake and cognitive function. The bootstrap method was used to obtain the CI of the indirect effect. The proportion of the mediated effect was calculated using the following formula: (mediated effect/total effect) × 100%. Statistical significance was determined when the two-tailed P-value < 0.05. No multiple comparison correction was performed.
3 Results
3.1 General characteristics
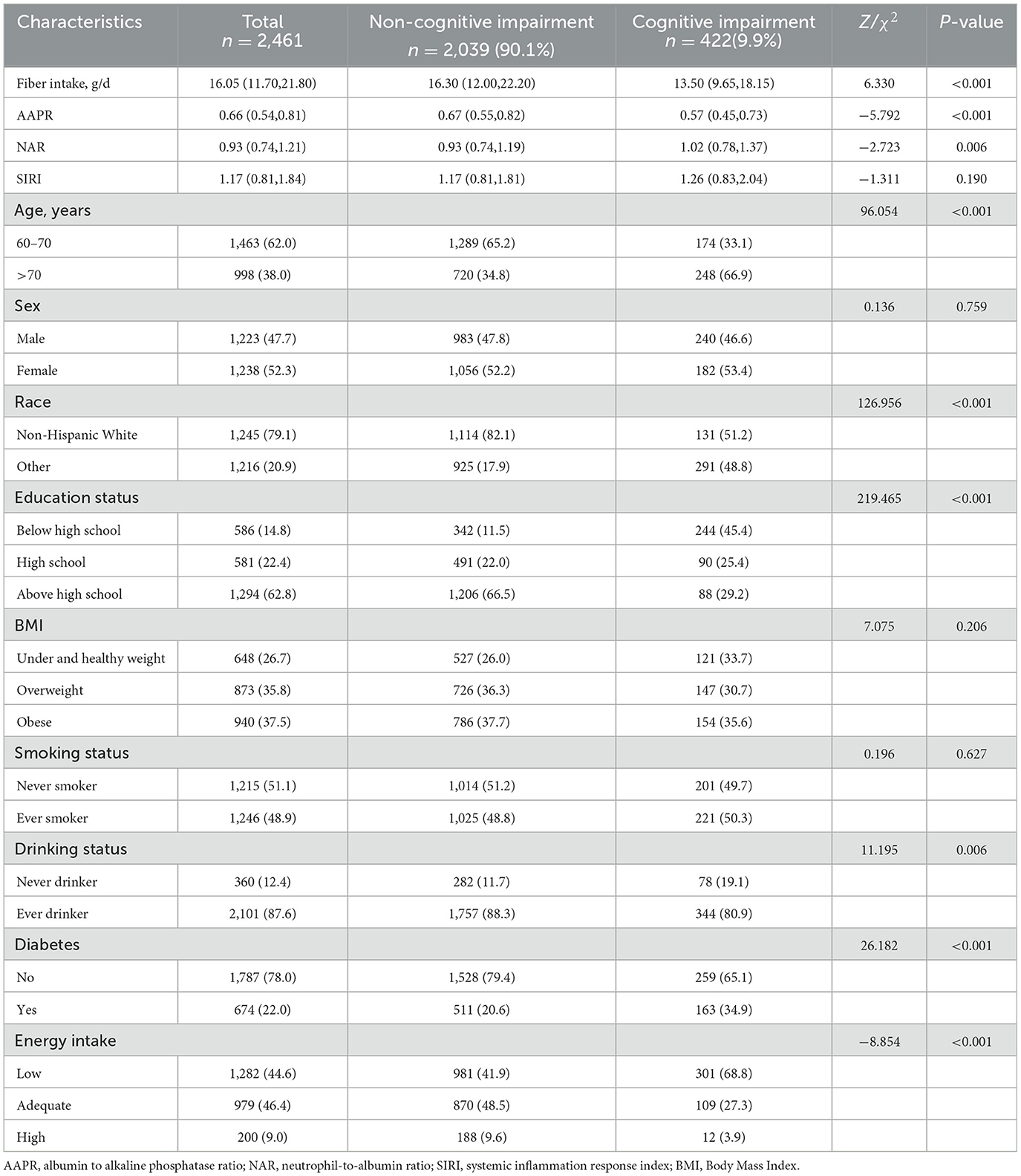
Among the 2,461 participants involved in this study, 422 (9.9%) were assigned to the cognitively impaired group. As shown in Table 1, reported fiber intake and AAPR in cognitive impairment group were lower than that in non-cognitive impairment group, while NAR was higher in cognitive impairment group. SIRI had no differences between participants with and without cognitive impairment. Meanwhile, there were differences in age, race, education status, drinking status, history of diabetes and energy intake (P < 0.05).
3.2 Correlations of reported dietary fiber intake with cognitive function and inflammatory markers
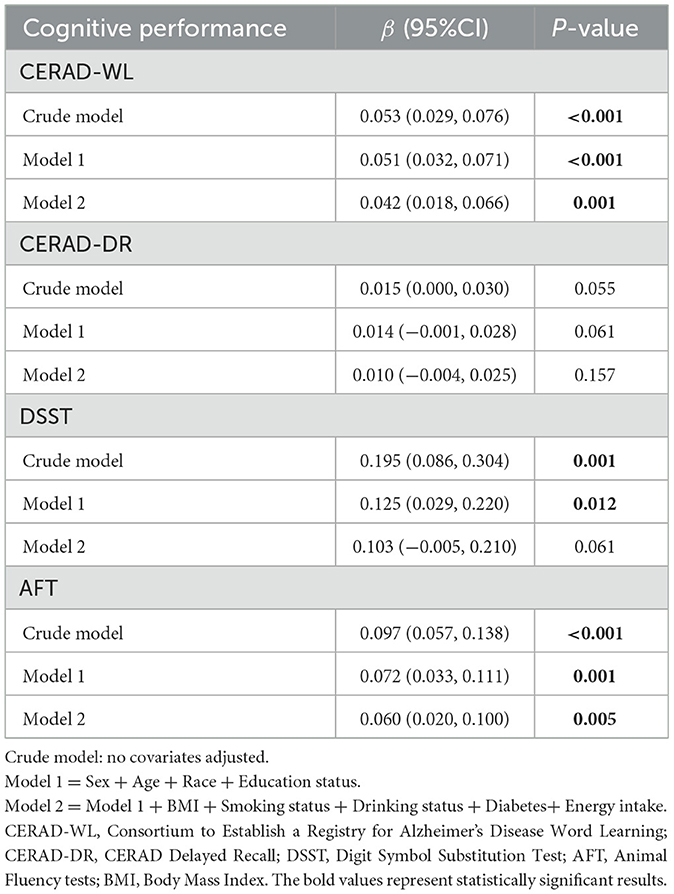
As shown in Table 2, higher reported dietary fiber intake was associated with higher cognitive function scores of CERAD-WL, DSST and AFT in the crude model. Reported dietary fiber intake remained a significant association with CERAD-WL (β = 0.042, 95% CI = 0.018 to 0.066) and AFT (β = 0.060, 95% CI = 0.020 to 0.100) in the fully adjusted model, which implied that increasing reported dietary fiber intake by 1 g/day increased CERAD-WL and AFT scores by 0.042 and 0.060 points, respectively.
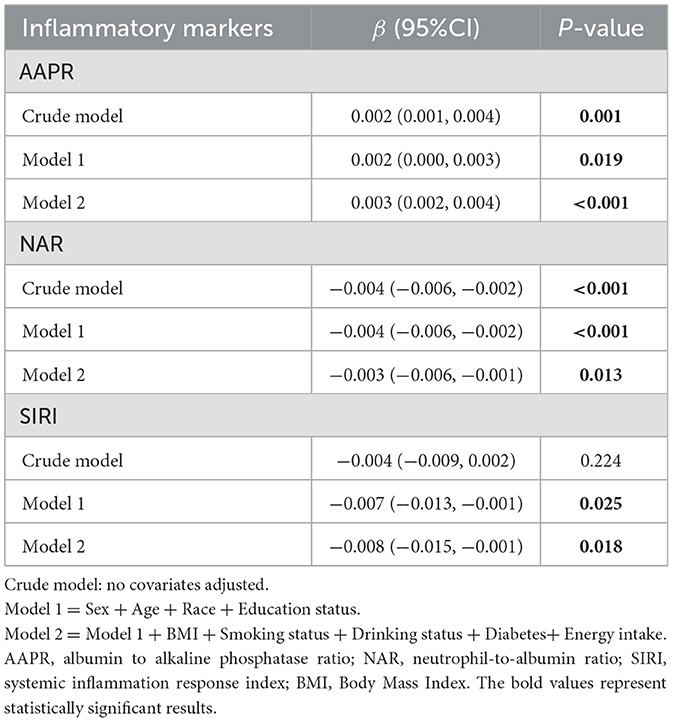
Reported dietary fiber intake was associated with AAPR and NAR in the crude model. Similarly, reported dietary fiber intake was significantly associated with AAPR (β = 0.003, 95% CI = 0.002 to 0.004), NAR (β = −0.003, 95% CI = −0.006 to −0.001), and SIRI (β = −0.008, 95% CI = −0.015 to −0.001) in the fully adjusted model (Table 3), which implied that increasing reported dietary fiber intake by 1 g/day increased AAPR by 0.003 and decreased NAR and SIRI by 0.003 and 0.008, respectively.
3.3 Associations between inflammatory markers and cognitive function
As shown in Figure 2, in model 2, AAPR was significantly positively associated with CERAD-WL (β = 1.184, 95% CI = 0.165 to 2.204) and AFT (β = 1.747, 95% CI = 0.229 to 3.264). NAR was significantly negatively associated with DSST (β = −2.930, 95% CI = −5.257 to −0.604), and SIRI was negatively associated with CERAD-DR (β = −0.183, 95% CI = −0.292 to −0.075).
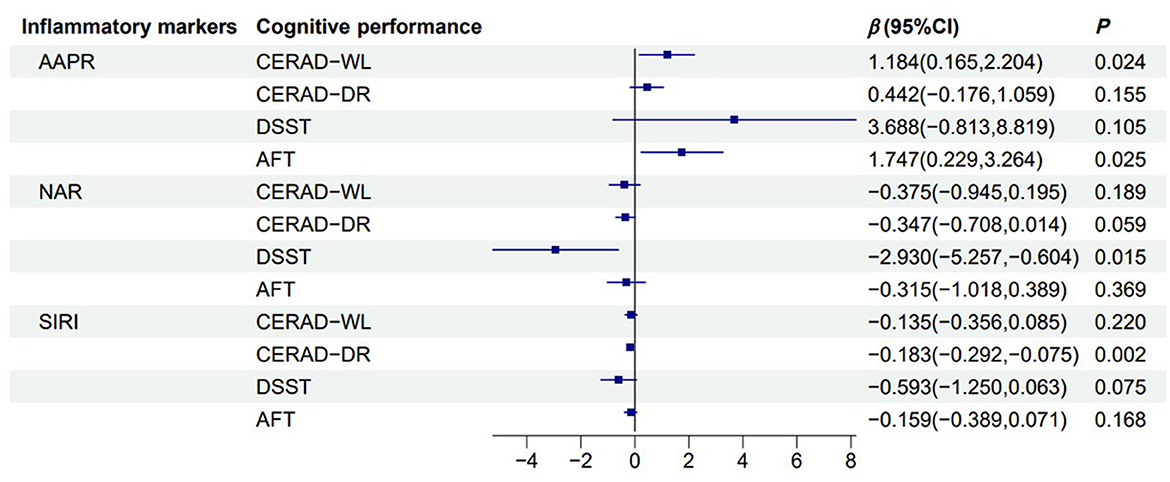
Figure 2. Forest plot of the association between inflammatory markers and cognitive function. Linear regression model illustrating the association between reported dietary fiber intake (g) and cognitive function across multiple cognitive assessments. The black solid line represents 0, the blue solid line represents the 95% confidence interval, and the blue square represents the estimated value (β). Adjusted for sex, age, race, education status, smoking status, drinking status, BMI, diabetes, energy intake. CERAD-WL, Consortium to Establish a Registry for Alzheimer's Disease Word Learning; CERAD-DR, CERAD Delayed Recall; DSST, Digit Symbol Substitution Test; AFT, Animal Fluency tests; AAPR, albumin to alkaline phosphatase ratio.
3.4 Mediating effect of inflammatory markers on reported dietary fiber intake and cognitive function
As shown in Table 4, the effects of reported dietary fiber intake on WL and AFT were reduced after adjusting for inflammatory markers. AAPR mediated the association between reported dietary fiber intake and AFT (proportion of medaiiton: 17.88%; indirect effect = 0.0123; 95% CI = 0.0002 to 0.0100; Figure 3). However, no mediating effect of AAPR was observed in the association between reported dietary fiber intake and CERAD-WL (Supplementary Figure 1).
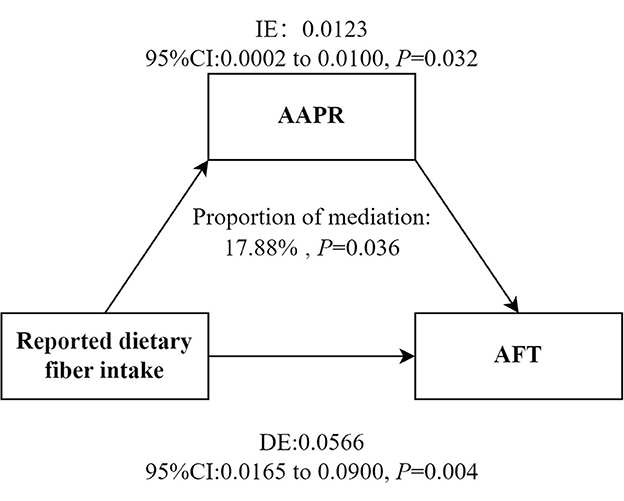
Figure 3. Mediation models. Figure demonstrating mediation models with independent variables of reported dietary fiber intake, mediators of AAPR and dependent variable of AFT. IE, Indirect effect; DE, Direct effect. Adjusted for sex, age, race, education status, smoking status, drinking status, BMI, diabetes and energy intake. AFT, Animal Fluency tests; AAPR, albumin to alkaline phosphatase ratio.
4 Discussion
This study demonstrated the association between higher reported dietary fiber intake and better cognitive performance assessed by CERAD-WL and AFT, and the association between higher AAPR and better cognitive performance measured by CERAD-WL and AFT also. In addition, AAPR played a mediating role in the relationship between reported dietary fiber intake and cognitive function, with a mediation proportion of 17.88%.
He et al. analyzed 2,713 older adults and found that dietary fiber had a significant positive correlation with AFT in the fourth quarter (42). Previous studies have demonstrated that fiber intake was positively associated with AFT and WL (46). These findings are consistent with our results, demonstrating that higher fiber intake is associated with better performance on both CERAD-WL and AFT. However, the relationship between fiber intake and DSST remains controversial. A study demonstrated a significant association between dietary fiber and the DSST in older adults, revealing an approximate linear dose-response relationship between dietary fiber intake and DSST (10). However, some previous studies found no association between dietary fiber intake and DSST (11), which was similar to our finding. More persuasive studies are needed to further explore the relationship of fiber with DSST. Future studies should employ longitudinal designs to clarify the temporal relationship between dietary fiber intake and specific cognitive domains, particularly DSST. The potential mechanisms underlying the observed association between dietary fiber consumption and lower cognitive decline have not been fully elucidated. The gut microbiome significantly affects brain function via the gut-brain axis (47). After reaching the colon, dietary fiber is fermented and metabolized by bacteria to produce short-chain fatty acids (SCFA) (48), exerting their metabolic benefits through gut-cranial neural circuits (49). SCFAs such as acetate, propionate, and butyrate regulate neuroinflammation and synaptic growth, survival, and differentiation (50). These interactions underscore the significant function of dietary fiber not just in digestive health but also in sustaining cognitive vitality through complex biological pathways.
Our findings suggested that dietary fiber may beneficially influence inflammatory level, while higher inflammation levels showed a strong association with poorer cognitive function. Dietary fiber plays an anti-inflammatory role by changing intestinal flora and producing short-chain fatty acids (51). Another study showed that dietary fiber could directly attenuate the production of inflammatory cytokines by dendritic cells co-cultured with intestinal epithelial supernatants through interaction with Toll-like receptors (52). Chronic inflammation is a key pathogenetic element that leads to the progression of AD and cognitive impairment. Chronic inflammation in the body may cause morphological changes in the brain and impair the normal function of specific brain regions (13). In addition, inflammation also disrupts the integrity of the blood-brain barrier (53), allowing inflammatory markers to penetrate and irritate nerves, resulting in neurodegeneration. Our findings revealed that AAPR partly mediated the association between fiber intake and cognitive function among older adults, accounting for 17.88% of the total effect. AAPR can be utilized to forecast the prognosis of cancer, such as lung cancer (54) and cholangiocarcinoma (55). Higher AAPR reflects elevated albumin and reduced ALP, potentially benefiting cognition through dual pathways: albumin maintains blood volume, regulates osmotic pressure, transports nutrients and metabolites, and acts as an antioxidant, all of which are beneficial to brain cells (56), while lower ALP may indicate reduced vascular inflammation. Maintaining a low level of ALP may exert a protective effect on cognitive function by inhibiting neuroinflammatory responses, without causing significant harm (57). High alkaline phosphatase (58) and low albumin were associated with high levels of inflammation (59). Approximately one-fifth of the dietary fiber-cognitive benefits may be achieved through this inflammatory pathway, while the remaining 80% of the effects may be mediated by other mechanisms such as the gut microbiota/short-chain fatty acids. Moreover, foods rich in fiber are usually rich in anti-inflammatory components such as whole grains, providing a theoretical basis for their substantive mediating role (60). It is suggested that decreased AAPR indicates the existence of different degrees of malnutrition and inflammation (61). Therefore, according to the Dietary Guidelines for Americans (2020–2025), for older adults whose fiber intake is below the recommended levels (< 22 grams per day for women and < 28 grams per day for men), increasing the fiber intake in their diet to meet these standards may help reduce systemic inflammation and may also lower the risk of cognitive decline.
This study has several notable advantages. Firstly, it was the first study exploring the mediating effect of AAPR on the relationship between dietary fiber intake and cognitive function. Secondly, our study was based on NHANES, a survey that represents the national population. However, there are some limitations in the present study. Firstly, as a cross-sectional study, causation cannot be inferred and there might be a reverse causal relationship. The effect values for some of the associations were relatively small. Future large-scale longitudinal or interventional studies, particularly randomized controlled trials (RCTs), are required to establish causation for the observed associations and to explore their underlying mechanisms. Secondly, the study population was located in the U.S., and the results may not be generalizable to other populations with different dietary habits, genetic backgrounds, or healthcare systems. Thirdly, although we adjusted for key confounders, residual confounding from unmeasured factors may still influence the observed associations. Future research should strive to collect more detailed data on these factors to better control these potential residual confounders. In addition, the assessment of reported dietary fiber intake relied on 24-h recall interviews, which may have been affected by recall bias. Finally, supplements were not considered for this study, which may result in inaccurate calculations of dietary fiber intake.
5 Conclusion
Reported dietary fiber intake, inflammatory markers, and cognitive function were pairwise associated. The AAPR played a mediating role in the association between reported dietary fiber intake and cognitive function.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by National Center for Health Statistics (NCHS) Ethics Review Board (ERB). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
KY: Writing – original draft, Writing – review & editing, Investigation, Formal analysis, Conceptualization. XW: Writing – review & editing, Writing – original draft, Formal analysis, Investigation. FW: Conceptualization, Writing – review & editing, Investigation. BC: Investigation, Writing – review & editing. ZZ: Writing – review & editing. JT: Writing – review & editing. JZ: Writing – review & editing. BL: Writing – review & editing, Supervision, Conceptualization.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
The authors thank the staff and participants of the National Health and Nutrition Examination Survey.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2025.1638315/full#supplementary-material
References
1. Borrás Blasco C, Viña Ribes J. Neurofisiología Y envejecimiento. Concepto y bases fisiopatológicas del deterioro cognitivo. Rev Esp Geriatr Gerontol. (2016) 51:3–6. doi: 10.1016/S0211-139X(16)30136-6
2. Estimation of the global prevalence of dementia in 2019 and forecasted prevalence in 2050: an analysis for the global burden of disease study 2019. Lancet Public Health. (2022) 7:e105–e25. doi: 10.1016/S2468-2667(21)00249-8
3. Mian M, Tahiri J, Eldin R, Altabaa M, Sehar U, Reddy PH. Overlooked cases of mild cognitive impairment: implications to early alzheimer's disease. Ageing Res Rev. (2024) 98:102335. doi: 10.1016/j.arr.2024.102335
4. Agüero-Torres H, Fratiglioni L, Guo Z, Viitanen M, von Strauss E, Winblad B. Dementia is the major cause of functional dependence in the elderly: 3-year follow-up data from a population-based study. Am J Public Health. (1998) 88:1452–6. doi: 10.2105/AJPH.88.10.1452
5. Hsiao YH, Chang CH, Gean PW. Impact of social relationships on alzheimer's memory impairment: mechanistic studies. J Biomed Sci. (2018) 25:3. doi: 10.1186/s12929-018-0404-x
6. Okura M, Ogita M, Arai H. Self-reported cognitive frailty predicts adverse health outcomes for community-dwelling older adults based on an analysis of sex and age. J Nutr Health Aging. (2019) 23:654–64. doi: 10.1007/s12603-019-1217-7
7. Sattler C, Toro P, Schönknecht P, Schröder J. Cognitive activity, education and socioeconomic status as preventive factors for mild cognitive impairment and alzheimer's disease. Psychiatry Res. (2012) 196:90–5. doi: 10.1016/j.psychres.2011.11.012
8. Tan BL, Norhaizan ME. Effect of high-fat diets on oxidative stress, cellular inflammatory response and cognitive function. Nutrients. (2019) 11:2579. doi: 10.3390/nu11112579
9. Wang Y-Y, Zhang M, Wang X-X, Liu S, Ding H. Correlates of cognitive impairment in the elderly in China: a cross-sectional study. Front Public Health. (2022) 10:973661. doi: 10.3389/fpubh.2022.973661
10. Prokopidis K, Giannos P, Ispoglou T, Witard OC, Isanejad M. Dietary fiber intake is associated with cognitive function in older adults: data from the national health and nutrition examination survey. Am J Med. (2022) 135:e257–e62. doi: 10.1016/j.amjmed.2022.03.022
11. Li F, Chen H, Mao N, Liu H. Dietary fiber intake and cognitive impairment in older patients with chronic kidney disease in the United States: a cross-sectional study. PLoS ONE. (2023) 18:e0291690. doi: 10.1371/journal.pone.0291690
12. Unión-Caballero A, Meroño T, Andrés-Lacueva C, Hidalgo-Liberona N, Rabassa M, Bandinelli S, et al. Apolipoprotein E gene variants shape the association between dietary fibre intake and cognitive decline risk in community-dwelling older adults. Age Ageing. (2023) 52:afac329. doi: 10.1093/ageing/afac329
13. Naomi R, Teoh SH, Embong H, Balan SS, Othman F, Bahari H, et al. The role of oxidative stress and inflammation in obesity and its impact on cognitive impairments-a narrative review. Antioxidants. (2023) 12:1071. doi: 10.3390/antiox12051071
14. Balcioglu YH, Kirlioglu SS. C-Reactive protein/albumin and neutrophil/albumin ratios as novel inflammatory markers in patients with schizophrenia. Psychiatry Investig. (2020) 17:902–10. doi: 10.30773/pi.2020.0185
15. Wang RH, Wen WX, Jiang ZP, Du ZP, Ma ZH, Lu AL, et al. The clinical value of neutrophil-to-lymphocyte ratio (Nlr), systemic immune-inflammation index (Sii), platelet-to-lymphocyte ratio (Plr) and systemic inflammation response index (Siri) for predicting the occurrence and severity of pneumonia in patients with intracerebral hemorrhage. Front Immunol. (2023) 14:1115031. doi: 10.3389/fimmu.2023.1115031
16. Zhang X, Xin Y, Chen Y, Zhou X. Prognostic effect of albumin-to-alkaline phosphatase ratio on patients with hepatocellular carcinoma: a systematic review and meta-analysis. Sci Rep. (2023) 13:1808. doi: 10.1038/s41598-023-28889-2
17. Qi Q, Zhuang L, Shen Y, Geng Y, Yu S, Chen H, et al. A novel systemic inflammation response index (SIRI) for predicting the survival of patients with pancreatic cancer after chemotherapy. Cancer. (2016) 122:2158–67. doi: 10.1002/cncr.30057
18. Xiong JP, Long JY, Xu WY, Bian J, Huang HC, Bai Y, et al. Albumin-to-alkaline phosphatase ratio: a novel prognostic index of overall survival in cholangiocarcinoma patients after surgery. World J Gastrointest Oncol. (2019) 11:39–47. doi: 10.4251/wjgo.v11.i1.39
19. Hooper NM, Kellett KAB, Vardy ERLC, Cocklin SL, Smith AD, Williams J. P2-205: plasma alkaline phosphatase inversely correlates with cognitive function in alzheimer's disease. Alzheimers Dement. (2010) 6:S374-S. doi: 10.1016/j.jalz.2010.05.1254
20. Wang L, Wang F, Liu J, Zhang Q, Lei P. Inverse relationship between baseline serum albumin levels and risk of mild cognitive impairment in elderly: a seven-year retrospective cohort study. Tohoku J Exp Med. (2018) 246:51–7. doi: 10.1620/tjem.246.51
21. Li W, Li S, Shang Y, Zhuang W, Yan G, Chen Z, et al. Associations between dietary and blood inflammatory indices and their effects on cognitive function in elderly Americans. Front Neurosci. (2023) 17:1117056. doi: 10.3389/fnins.2023.1117056
22. Mousa N, Salah M, Elbaz S, Elmetwalli A, Elhammady A, Abdelkader E, et al. Neutrophil percentage-to-albumin ratio is a new diagnostic marker for spontaneous bacterial peritonitis: a prospective multicenter study. Gut Pathog. (2024) 16:18. doi: 10.1186/s13099-024-00610-2
23. Wang X, Wen Q, Li Y, Zhu H, Zhang F, Li S, et al. Systemic inflammation markers (Sii and Siri) as predictors of cognitive performance: evidence from nhanes 2011–2014. Front Neurol. (2025) 16:1527302. doi: 10.3389/fneur.2025.1527302
24. Jiang P, Chen J, Li J. Association of the systemic immune-inflammatory index and systemic inflammatory response index with all-cause and cardiovascular mortality in individuals with metabolic inflammatory syndrome. Eur J Med Res. (2025) 30:444. doi: 10.1186/s40001-025-02611-6
25. Galland L. Diet and inflammation. Nutr Clin Pract. (2010) 25:634–40. doi: 10.1177/0884533610385703
26. Saeed MA, Gribben KC, Alam M, Lyden ER, Hanson CK, LeVan TD. Association of dietary fiber on asthma, respiratory symptoms, and inflammation in the adult national health and nutrition examination survey population. Ann Am Thorac Soc. (2020) 17:1062–8. doi: 10.1513/AnnalsATS.201910-776OC
27. Qi X, Li Y, Fang C, Jia Y, Chen M, Chen X, et al. The associations between dietary fibers intake and systemic immune and inflammatory biomarkers, a multi-cycle study of nhanes 2015–2020. Front Nutr. (2023) 10:1216445. doi: 10.3389/fnut.2023.1242115
28. Shivappa N, Steck SE, Hurley TG, Hussey JR, Hébert JR. Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr. (2014) 17:1689–96. doi: 10.1017/S1368980013002115
29. Marx W, Lane M, Hockey M, Aslam H, Berk M, Walder K, et al. Diet and depression: exploring the biological mechanisms of action. Mol Psychiatry. (2021) 26:134–50. doi: 10.1038/s41380-020-00925-x
30. Guasch-Ferré M, Willett WC. The mediterranean diet and health: a comprehensive overview. J Intern Med. (2021) 290:549–66. doi: 10.1111/joim.13333
31. Dong X, Li S, Chen J, Li Y, Wu Y, Zhang D. Association of dietary Ω-3 and Ω-6 fatty acids intake with cognitive performance in older adults: national health and nutrition examination survey (Nhanes) 2011-2014. Nutr J. (2020) 19:25. doi: 10.1186/s12937-020-00547-7
32. Liang X, Lu H, Lin P, Huang X. Association between dietary fiber to carbohydrate ratio and risk of dental caries in diabetic patients: an analysis of the national health and nutrition examination survey 2015-2020. Front Nutr. (2024) 11:1440306. doi: 10.3389/fnut.2024.1440306
33. Zhang H, Wei X, Pan J, Chen X, Sun X. Anemia and frailty in the aging population: implications of dietary fiber intake (findings of the us nhanes from 2007–2018). BMC Geriatr. (2023) 23:634. doi: 10.1186/s12877-023-04352-9
34. Song W, Feng Y, Gong Z, Tian C. The association between dietary inflammatory index and cognitive performance in older adults aged 60 years and older. Front Nutr. (2022) 9:748000. doi: 10.3389/fnut.2022.748000
35. Ge S, Dong F, Tian C, Yang C-H, Liu M, Wei J. Serum soluble alpha-klotho klotho and cognitive functioning in older adults aged 60 and 79: an analysis of cross-sectional data of the national health and nutrition examination survey 2011 to 2014. BMC Geriatr. (2024) 24:245. doi: 10.1186/s12877-024-04661-7
36. Lv JJ, Li XY, Wang JB, Yang XT, Yin MY, Yang CH. Association of dietary live microbe intake with various cognitive domains in us adults aged 60 years or older. Sci Rep. (2024) 14:5714. doi: 10.1038/s41598-024-51520-x
37. Li W, Li S, Zhuang W, Shang Y, Yan G, Lu J, et al. Non-linear relationship between dietary vitamin e intake and cognitive performance in older adults. Public Health. (2023) 219:10–7. doi: 10.1016/j.puhe.2023.03.012
38. Zhang T, Liu Y, Tian T. Predicting pathological complete response after neoadjuvant chemotherapy in breast cancer by clinicopathological indicators and ultrasound parameters using a nomogram. Sci Rep. (2024) 14:16348. doi: 10.1038/s41598-024-64766-2
39. Xie H, Wei L, Liu M, Liang Y, Yuan G, Gao S, et al. Neutrophil-albumin ratio as a biomarker for postoperative complications and long-term prognosis in patients with colorectal cancer undergoing surgical treatment. Front Nutr. (2022) 9:976216. doi: 10.3389/fnut.2022.976216
40. Guo M, Zhu C. Serum neurofilament light chain, markers of systemic inflammation and clinically relevant depressive symptoms in us adults. J Affect Disord. (2024) 363:572–8. doi: 10.1016/j.jad.2024.07.146
41. 2011–2012 Laboratory Data-Continuous Nhanes. Available online at: https://wwwn.cdc.gov/nchs/nhanes/search/datapage.aspx?Component=Laboratory&CycleBeginYear=2011 (Accessed September 20, 2024).
42. He Q, An L, Yue Y, Cui C, Wang C, Xu H, et al. Non-linear association between dietary fiber intake and cognitive function mediated by vitamin E: a cross-sectional study in older adults. Front Nutr. (2025) 12:1611162. doi: 10.3389/fnut.2025.1611162
43. Ba DM, Gao X, Al-Shaar L, Muscat JE, Chinchilli VM, Beelman RB, et al. Mushroom intake and depression: a population-based study using data from the US national health and nutrition examination survey (Nhanes), 2005–2016. J Affect Disord. (2021) 294:686–92. doi: 10.1016/j.jad.2021.07.080
44. Butler L, Popkin BM, Poti JM. Associations of alcoholic beverage consumption with dietary intake, waist circumference and BMI in US adults, Nhanes 2003–2012. J Acad Nutr Diet. (2018) 118:409–20.e3. doi: 10.1016/j.jand.2017.09.030
45. US Department of Health and Human Services. Dietary Guidelines for Americans, 2020–2025. (2020). Available online at: https://health.gov/our-work/nutrition-physical-activity/dietary-guidelines (Accessed July 22, 2024).
46. Sun W, Li S, Chen C, Lu Z, Zhang D. Dietary fiber intake is positively related with cognitive function in US older adults. J Funct Foods. (2022) 90:104986. doi: 10.1016/j.jff.2022.104986
47. Kim CS. Roles of diet-associated gut microbial metabolites on brain health: cell-to-cell interactions between gut bacteria and the central nervous system. Adv Nutr. (2024) 15:100136. doi: 10.1016/j.advnut.2023.10.008
48. Gubert C, Kong G, Costello C, Adams CD, Masson BA, Qin W, et al. Dietary fibre confers therapeutic effects in a preclinical model of huntington's disease. Brain Behav Immun. (2024) 116:404–18. doi: 10.1016/j.bbi.2023.12.023
49. De Vadder F, Kovatcheva-Datchary P, Goncalves D, Vinera J, Zitoun C, Duchampt A, et al. Microbiota-generated metabolites promote metabolic benefits via gut-brain neural circuits. Cell. (2014) 156:84–96. doi: 10.1016/j.cell.2013.12.016
50. Silva YP, Bernardi A, Frozza RL. The role of short-chain fatty acids from gut microbiota in gut-brain communication. Front Endocrinol. (2020) 11:25. doi: 10.3389/fendo.2020.00025
51. Niero M, Bartoli G, De Colle P, Scarcella M, Zanetti M. Impact of dietary fiber on inflammation and insulin resistance in older patients: a narrative review. Nutrients. (2023) 15:2365. doi: 10.3390/nu15102365
52. Bermudez-Brito M, Sahasrabudhe NM, Rösch C, Schols HA, Faas MM, de Vos P. The impact of dietary fibers on dendritic cell responses in vitro is dependent on the differential effects of the fibers on intestinal epithelial cells. Mol Nutr Food Res. (2015) 59:698–710. doi: 10.1002/mnfr.201400811
53. Medina-Rodriguez EM, Beurel E. Blood brain barrier and inflammation in depression. Neurobiol Dis. (2022) 175:105926. doi: 10.1016/j.nbd.2022.105926
54. Yang Y, Wang Y, Li X, Xie X. Clinical role of pretreatment albumin-to-alkaline phosphatase ratio in lung cancer: a meta-analysis. Sci Rep. (2024) 14:1166. doi: 10.1038/s41598-024-51844-8
55. Wang S, Liu M, Xiang L, Qiu H, Cheng L, Huang Z, et al. tumour burden score combined with albumin-to-alkaline phosphatase ratio predicts prognosis in patients with intrahepatic cholangiocarcinoma. J Cell Mol Med. (2024) 28:e18530. doi: 10.1111/jcmm.18530
56. Wigner P, Czarny P, Galecki P, Su K-P, Sliwinski T. The molecular aspects of oxidative & nitrosative stress and the tryptophan catabolites pathway (trycats) as potential causes of depression. Psychiatry Res. (2018) 262:566–74. doi: 10.1016/j.psychres.2017.09.045
57. Hanley AJ, Williams K, Festa A, Wagenknecht LE, D'Agostino RB Jr, Haffner SM. Liver markers and development of the metabolic syndrome: the insulin resistance atherosclerosis study. Diabetes. (2005) 54:3140–7. doi: 10.2337/diabetes.54.11.3140
58. Seo MS, Shim JY, Lee YJ. Relationship between serum alkaline phosphatase level, c-reactive protein and leukocyte counts in adults aged 60 years or older. Scand J Clin Lab Invest. (2019) 79:233–7. doi: 10.1080/00365513.2019.1585567
59. Don BR, Kaysen G. Serum albumin: relationship to inflammation and nutrition. Semin Dial. (2004) 17:432–7. doi: 10.1111/j.0894-0959.2004.17603.x
60. Milesi G, Rangan A, Grafenauer S. Whole grain consumption and inflammatory markers: a systematic literature review of randomized control trials. Nutrients. (2022) 14:374. doi: 10.3390/nu14020374
Keywords: cognitive function, dietary fiber intake, inflammatory markers, NHANES, mediation analysis
Citation: Yan K, Wang X, Wang F, Chen B, Zong Z, Tian J, Zhao J and Li B (2025) The association between dietary fiber intake and cognitive function: mediating role of inflammatory markers. Front. Nutr. 12:1638315. doi: 10.3389/fnut.2025.1638315
Received: 30 May 2025; Accepted: 02 September 2025;
Published: 29 September 2025.
Edited by:
Eddy Roccati, University of Tasmania, AustraliaReviewed by:
Carlos Miguel Rios-González, Ministerio de Salud Pública y Bienestar Social, ParaguayJames J. R. Brady, University of Tasmania, Australia
Copyright © 2025 Yan, Wang, Wang, Chen, Zong, Tian, Zhao and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bo Li, bGlfYm9Aamx1LmVkdS5jbg==
†These authors have contributed equally to this work and share first authorship
 Kaiyun Yan†
Kaiyun Yan† Bo Li
Bo Li