- 1Department of Children’s Health Care Center, Beijing Children’s Hospital, Capital Medical University, National Center for Children’s Health, Beijing, China
- 2Novozymes Berlin GmbH, Berlin, Germany
- 3Chengdu Women's and Children's Central Hospital, School of Medicine, University of Electronic Science and Technology of China, Chengdu, China
- 4Baoding Children's Hospital, Baoding, China
- 5Xuzhou Children’s Hospital, Xuzhou, China
- 6Children’s Hospital of Hebei Province, Hebei, China
- 7Novonesis, Cork, Ireland
Introduction: Approximately one-third of the world’s children have elevated blood lead levels (BLLs), which may lead to often-irreversible decreased intelligence, behavioral difficulties, and learning problems. Identification and removal of the source of lead, along with good nutrition, are the only advocated initial management, with chelation therapy for higher threshold BLLs. Probiotics have shown promising beneficial effects pre-clinically. Here, we investigated the safety and efficacy of the probiotic Lactiplantibacillus plantarum DSM 33464 in children with elevated blood lead levels.
Methods: Children aged 3–12 years with elevated BLLs (>3.5 μg/dL) were enrolled in a randomized double-blind placebo-controlled multi-centered study and received either probiotic (1 × 109) colony-forming units (CFUs) or placebo (control group), along with a multivitamin/mineral supplement in both groups daily for 12 weeks.
Results: Overall, 66 children were randomized, 54 received intervention (probiotic; n = 30 and control; n = 24). The probiotic was well-tolerated. Probiotics, combined with a multivitamin/mineral supplement, significantly reduced the BLLs in these children within 12 weeks of supplementation by 40%, similar to that of the control group, which received a placebo plus multivitamin/mineral supplement. A larger reduction in urine lead levels at 8 weeks was observed in the probiotic group, along with a reduction of abdominal pain and psychosomatic feelings at week 12. No depletion of essential minerals was observed in any of the groups.
Conclusion: This study adds to previous findings suggesting that probiotic intervention may be a promising additional strategy to help reduce BLLs and their detrimental effects in children. Due to the preliminary nature of this study, larger studies investigating the effects of the strain alone, with a longer intervention period, are warranted to confirm the benefits observed.
Clinical trial registration: Clinicaltrials.gov, identifier NCT04891666.
1 Introduction
Lead exposure is a worldwide health threat with massive impacts but little awareness, with plenty of existing or even growing sources of exposure, especially in low- and middle-income countries (LMICs) (1, 2). The adverse effects of lead on health have been well documented (3), with cognitive deficits in children even at the lowest blood lead levels (BLLs) (≤5 μg/dL) being the most substantiated effects, where the decrements in intelligence quotient increase with BLLs (4) and are sustained over time.
At low to moderate levels of exposure, there are usually no symptoms, which can impede detection (2). There is no safe level of lead in blood; however, approximately one-third of children (800 million) globally have elevated BLLs at or above 5 μg/dL. In the US, the Center for Disease Control and Prevention has updated the blood lead reference value (BLRV) to 3.5 μg/dL to identify children <6 years with BLLs higher than majority of the children’s levels (5), while the BLRV for children in China remains at 10 μg/dL since 2006 (6).
In China, although the BLLs in children have declined fast over time since phasing out leaded gasoline from 2000, the average BLLs in children of 5.97 μg/dL are still higher than in the USA and Europe, with greater loss of disability adjusted life years than reported for other LMICs (7).
The largest source of lead exposure in China was water pollution (76.16%), followed by air and soil pollution (8), while the main dietary sources of lead were cereals (43.5%), vegetables (29.0%), and beverages and water (9.8%) (9). In addition to the respiratory tract, lead is predominantly absorbed via the gastrointestinal route of exposure, where children absorb approximately 50% of ingested lead after a meal and up to 100% on an empty stomach (10). Biomonitoring data from 2004 to 2014 showed an estimated daily lead exposure through diet in urban children (0–7 years) in China of 12.01 ± 6.27 μg/day (11). As children are more susceptible to lead than adults due to their developing systems, higher absorption, and behaviors that increase ingestion of surface dust (12, 13), solutions targeted at reducing lead absorption, in addition to strategies that prevent exposures, are necessary.
Among the dietary interventions studied for reducing the effects of lead exposure, probiotics have emerged in recent years as promising candidates (14, 15), as there is a mutual and bidirectional relationship between the gut microbiota and lead exposure. Lead exposure alters the microbiome composition and metabolomic profiles of the gut microbiota, which, in turn, is capable of metabolizing and limiting lead absorption, as well as promoting excretion (16–18).
The strain Lactiplantibacillus plantarum DSM 33464 (also known as L. plantarum CCFM 8610 or SmartGuard™), isolated from a Chinese plant, has shown strong ability to retain and sequester lead in simulated gastrointestinal condition, and has shown promising results of reduced dietary lead absorption in the gastrointestinal tract, leading to reduction in blood, urine, and organs such as kidney and liver, in mice models (unpublished reports: Jiangnan University, The protective effects of probiotics on lead detoxification in vivo, 2018; Nanna Ny Kristensen, Acute lead challenge model, 2019). Here, we report findings from a study that assessed the safety and tolerability of this strain and its efficacy in reducing BLLs in Chinese children aged 3–12 years with elevated BLLs.
2 Materials and methods
2.1 Lead binding assay and transmission electron microscopy of Lactiplantibacillus plantarum DSM 33464
Prior to the production of investigational products, the lead-binding capacity of the strain was investigated. First, 100 mg of freeze-dried powder was dissolved in 10 mL ultra-pure water, vortexed briefly, and allowed to rehydrate for 15 min following pH adjustment to 6. Lead-acetate solution (1.45 mM) was then incubated with the solution containing the rehydrated strain for 1 h at 37°C while shaking in a ThermoMixer comfort (150 rpm) (Eppendorf, Germany) to allow binding activity. After centrifugation (10 min, 4,500 g), the appropriate amount of pellet sample was placed in a fixative solution (2% paraformaldehyde + 2.5% glutaraldehyde) for 1 h and then rinsed with 0.1 M phosphate-buffered saline (PBS). The samples were further fixed in an osmium tetroxide solution (1% osmium tetroxide + 1.5% potassium ferrocyanide) for 1 h and dehydrated with a graded ethanol series (30, 50, 70, 80, 90, and 100% ethanol), followed by propylene oxide. After embedding in resin, the samples were further prepared into 70-nm sections by Leica EM UC7 (Leica Microsystems, Germany) for transmission electron microscopy observation (Hitachi H7650B, acceleration voltage 80 kV) (Hitachi High-Technologies Corporation, Japan).
2.2 Study design and participants
This randomized, double-blind, placebo-controlled, parallel-group, multi-center study was conducted from July 2021 to March 2023 at five clinical sites in China: Beijing Children’s Hospital/Capital Medical University, Baoding Children’s Hospital, Xuzhou Children’s Hospital, Chengdu Women’s and Children’s Central Hospital, and Children’s Hospital of Hebei Province (ClinicalTrials.gov registration: NCT04891666). The protocol was approved by the Ethics Committee of Beijing Children’s Hospital, Capital Medical University, on 21 September 2020, with three amendments: Version 1.1.0 (18 December 2020), Version 2.0.0 (6 August 2021), and Version 3.0.0 (21 December 2021). The study was conducted in accordance with the International Conference on Harmonization (ICH) Good Clinical Practice, as well as the Declaration of Helsinki on ethical principles for medical research involving human subjects (ICH 1996), and applicable regulatory requirements.
The study included five clinic visits: visit 1 screening (day–14 to day–2), visit 2 randomization (day–1), visit 3 follow-up (day 28 ± 3; week 4), visit 4 follow-up (day 56 ± 3; week 8), visit 5 (day 84 ± 3; week 12), and visit 6 follow-up phone calls 4 weeks post-intervention (day 112 ± 3; week 16). Healthy subjects who met the following selection criteria participated in this study: aged 3–12 years, with BLLs (3.5–24.9 μg/dL), subjects, their parents or legal guardians were able and willing to comply with research guidance, and subjects’ parents or legally acceptable representatives signed written informed consent. Eligible subjects were randomized into an experimental group (probiotic) or a control group (placebo), both of which received health education and a daily nutrition/multivitamin/mineral supplement from YingKangWei (Beijing Kangyuan Youte Medical Technology Co., Ltd., China) throughout the 12-week intervention period. Additional information on visit assessments and exclusion criteria is available in the Supplementary Material.
The primary objective was to evaluate the efficacy of L. plantarum DSM 33464 (probiotic) in children with elevated BLLs. The secondary objective was to evaluate the safety of L. plantarum DSM 33464 in children with elevated BLLs.
2.3 Investigational products (IPs) and blinding
The experimental group intervention consisted of L. plantarum DSM 33464 (probiotic) at a dose of 1 × 109 colony forming units (CFUs) and dextrin in one sachet (2 g) daily, and the multivitamin/mineral supplement YingKangWei (Beijing Kangyuan Youte Medical Technology Co., Ltd., China) (vitamin B1: 1.5 mg; vitamin B2: 0.8 mg; vitamin B3: 5 mg; vitamin B5: 1 mg; vitamin B6: 0.5 mg; vitamin B7: 10 μg; vitamin B9: 80 μg; vitamin B12: 1 μg; vitamin A: 600 IU; vitamin D: 200 IU; iron pyrophosphate: 5 mg; calcium: 180 mg; zinc gluconate: 4 mg; maltodextrin: 1.4 g) one sachet daily for 12 weeks. The control group received one sachet of placebo (dextrin only, 2 g) and the supplement YingKangWei one sachet daily for 12 weeks. Subjects were instructed to take one sachet of IPs (probiotic or placebo) orally 0.5 h before having a meal, by dissolving into a moderate amount of lukewarm water, once daily, and one sachet of YingKangWei orally, by dissolving into moderate amount of warm water or food, with dosing interval of at least 4 h from IP intake. An IP dispensing and return log were used to account for all IPs dispensed and returned.
Permuted block randomization was used to randomize eligible children into two arms with a 1:1 ratio. The subjects, investigators, and all clinical personnel were blinded to intervention arms until the database was locked. No emergency arose throughout the study that necessitated unblinding. The IPs had the same visual appearance to avoid compromising the study blinding. Additional information is available in the Supplementary Material.
2.4 Endpoints
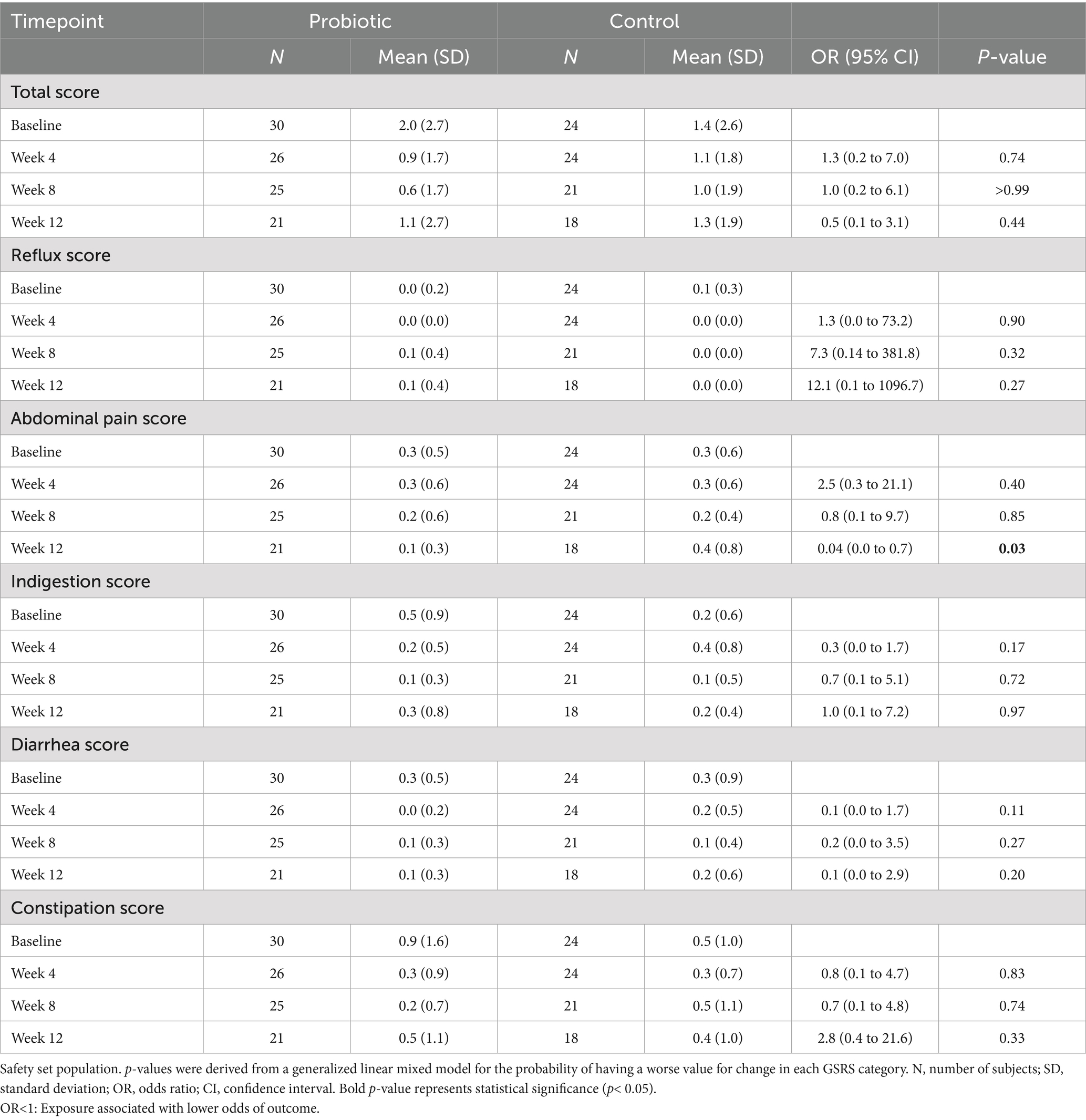
The primary endpoint of the study was the difference in change from baseline BLLs between the probiotic group and the control group at week 12. Secondary endpoints included the differences in change from baseline BLLs between groups at weeks 4 and 8; the differences in change from baseline urine lead levels (ULLs) between groups at weeks 4, 8, and 12; the differences in change from baseline improvement of common trace elements (calcium, zinc, copper, magnesium, iron) levels in the blood between groups at weeks 4, 8, and 12. Exploratory endpoints included the changes in total scores and subscores of the 48-item Conners’ Parent Rating Scale (CPRS) (19) that measures children’s emotional and behavioral attitude by including five subscales assessing conduct problem, learning problem, anxiety, impulsive/hyperactive behavior and psychosomatic feelings and Gastrointestinal Symptoms Rating Scale (GSRS) (20, 21) which measures 15 gastrointestinal symptom items by a 4-point scale rated from 0 to 3 (with higher score representing worse symptom), resulting in a total score and five subscore categories: reflux, abdominal pain, indigestion, diarrhea, and constipation; from baseline to week 12 between groups.
2.5 Safety assessment
Safety assessments included adverse events (evaluated and graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events v5.0) throughout the study period (visit 1–visit 6), vital signs (temperature, pulse, systolic blood pressure, and diastolic blood pressure), physical examination, 12-lead electrocardiogram, clinical laboratory examination (routine blood test, blood biochemistry) and GSRS. Vital signs, physical examination, and clinical laboratory examination were measured at visits 1, 3, 4, and 5, while a 12-lead electrocardiogram was measured at visit 1 and after completion of intervention at visit 5. GSRS was assessed at visits 2 to 5.
2.6 Statistical analyses
Due to the exploratory nature of the study, the sample size (n = 124) was not based on formal statistical considerations. Based on the intent-to-treat principle, all randomized subjects who received at least one intervention dose were included in the Full Analysis Set (FAS), which was used to analyze the demographic and baseline characteristics and efficacy assessment. Safety analysis was based on the Safety Set (SS), that is, all subjects who received at least one intervention dose and had available safety assessment data after baseline. A linear mixed-effects model for repeated measures (MMRMs) was applied to primary and secondary endpoints. Two-tailed tests with a specified type I error rate of 0.05 were used. The significance level was set to 0.05. The least squares means (LSM), standard error (SE), LSM difference (LSMD), and 95% two-sided confidence intervals (CIs) and p-values were presented. Sensitivity analyses were performed based on the per-protocol set (PPS) and analysis of covariance (ANCOVA) method. Post hoc analysis of GSRS used the MMRM model, while Analysis of Covariance (ANCOVA) was used for the CPRS questionnaire. The analyses were performed using Statistical Analysis System (SAS version 9.4). The graphs were constructed using GraphPad Prism version 10.2.2 (GraphPad Software, San Diego, CA, USA). The results presented focus on the FAS population. Additional information is available in the Supplementary Material.
3 Results
3.1 Lactiplantibacillus plantarum DSM 33464 binding lead
The strain used in this trial was shown to have the ability to bind and retain lead in vitro, as shown by transmission electron microscopy (Figure 1A) compared to the control (Figure 1B).
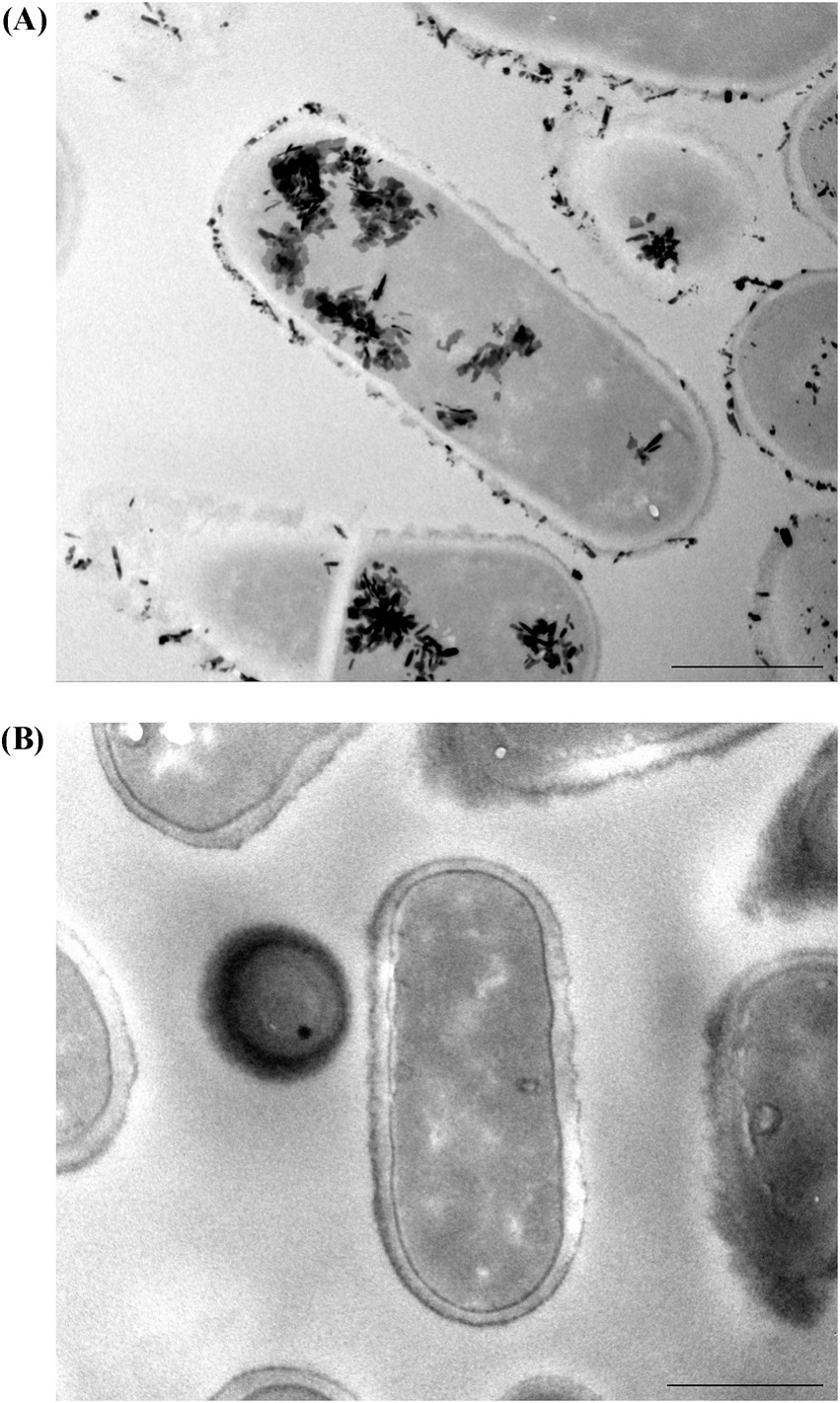
Figure 1. TEM images show (A) in vitro lead binding and sequestration of L. plantarum DSM 33464 and (B) L. plantarum DSM 33464 in PBS without lead incubation as a control. Scale bar = 500 nm.
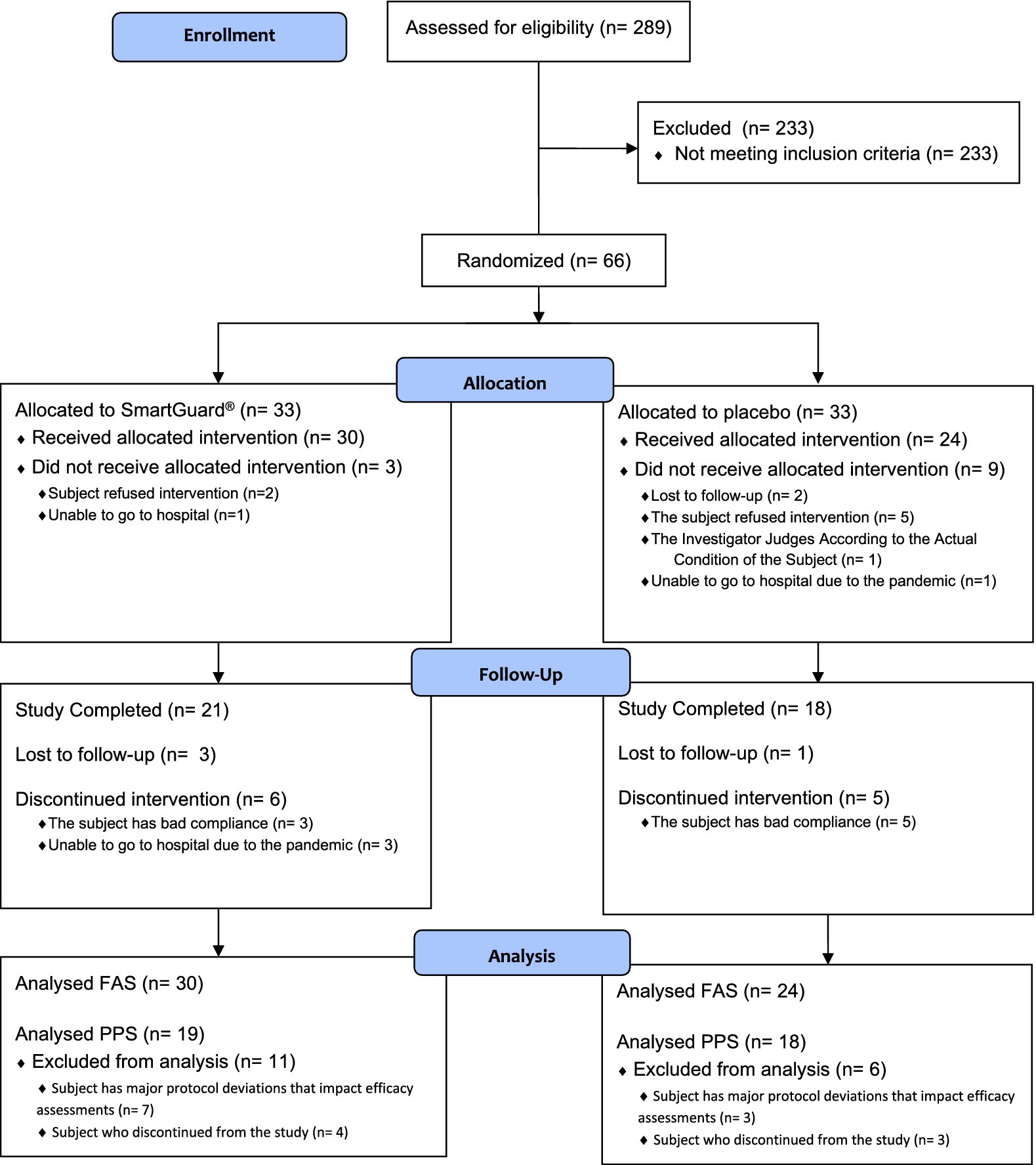
3.2 Study population
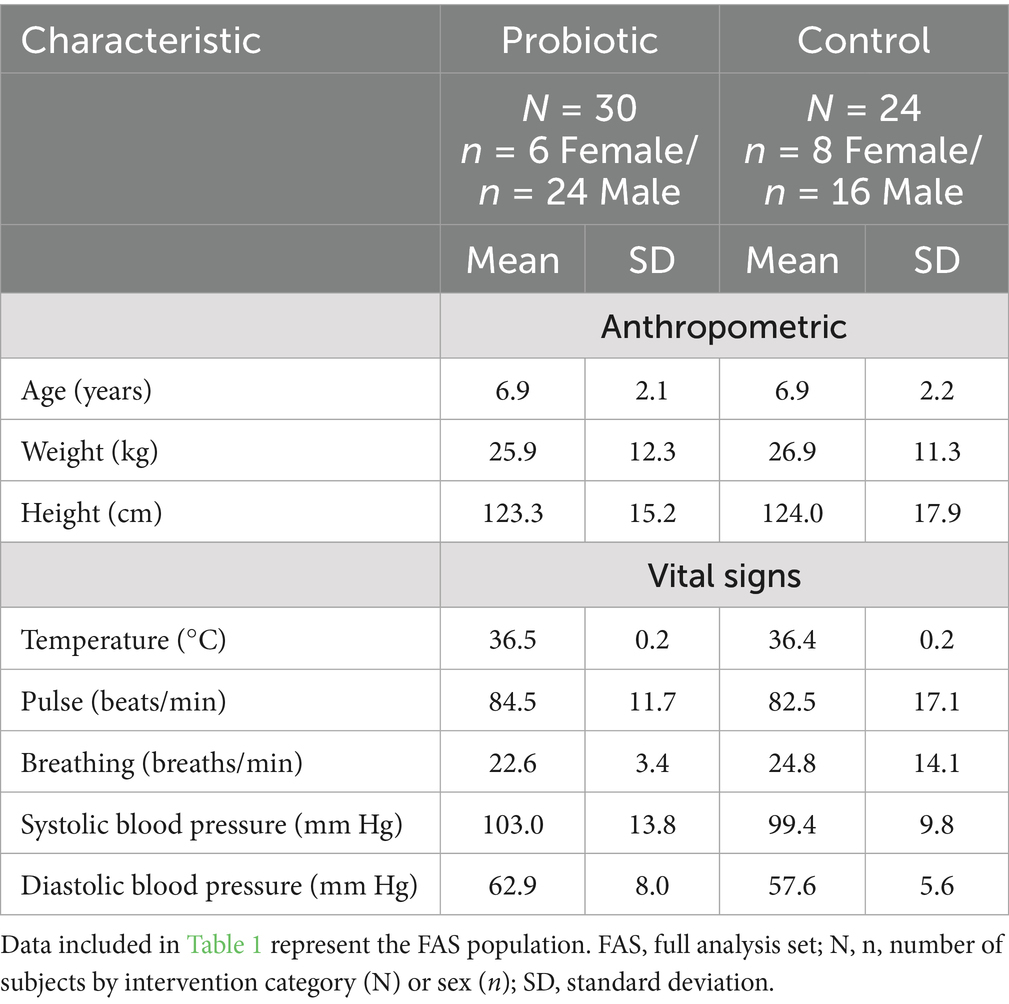
Sixty-six children were randomized (n = 33 per arm) (Figure 2). Fifty-four (81.8%) children received at least one intervention and were included in the FAS and SS (probiotic; n = 30 and control; n = 24). Only 37 (56.1%) children who completed the study and had no major protocol deviations that impacted efficacy assessments were included in the PPS (probiotic; n = 19 and control; n = 18). The majority (94.4%) of children in FAS had slightly elevated BBL at baseline [median (interquartile range): 4.2 (1.3) μg/dL]. Table 1 summarizes the children’s baseline demographic and physiological characteristics. The data collected showed that those randomized to each group were similar on all demographic and physiological measures (Table 1). Questionnaire on lead exposure sources showed no major differences in both groups (Supplementary Table 1). Interestingly, 53.7% of children overall frequently put things in their mouths or might eat non-food items. The overall IP compliance in the SS was high (probiotic: 90.0%; control: 82.6% subjects with relative dose intensity ≥80% and ≤120%).
3.3 Efficacy endpoints
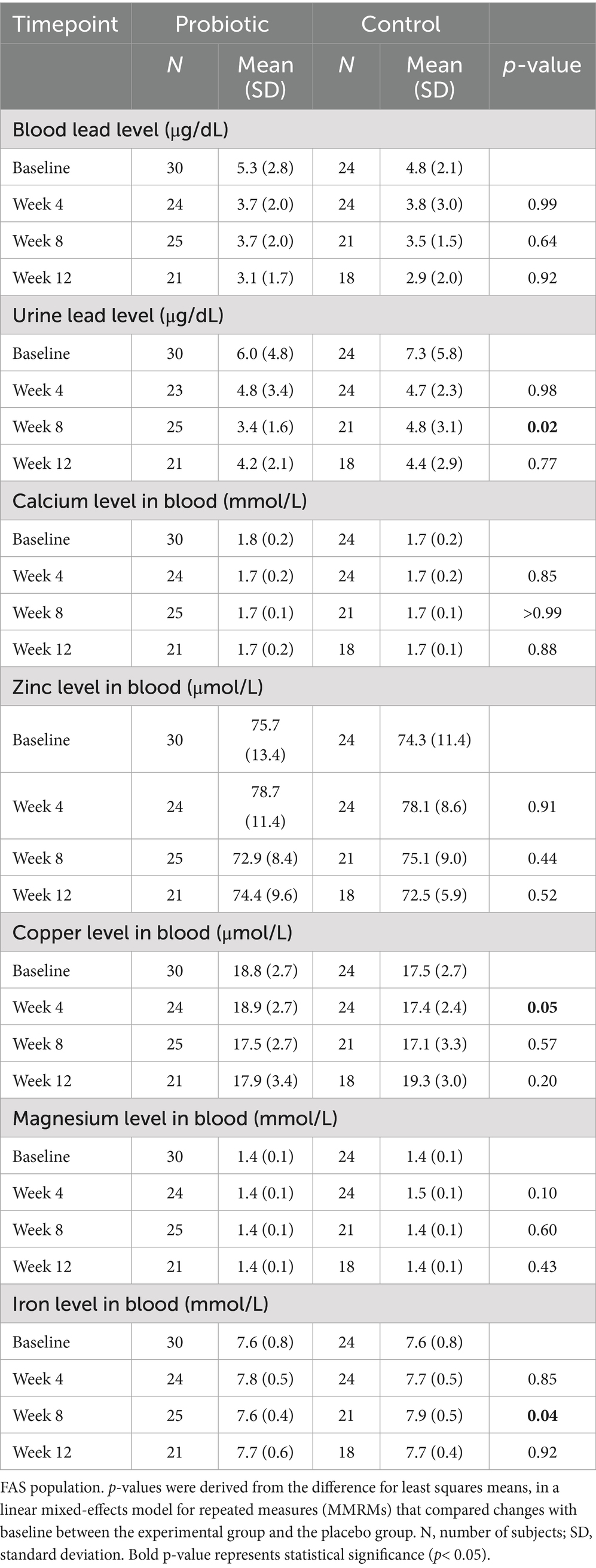
Blood lead levels reduced from baseline to the end of supplementation in both groups by approximately 40% from 5.3 to 3.1 μg/dL in the probiotic and from 4.8 to 2.9 μg/dL in the placebo group, respectively (both p < 0.001; Table 2). Probiotic had no further effect on the change in BLLs from baseline to week 4 (p = 0.99), week 8 (p = 0.64), or week 12 (p = 0.92) over that of control [LSMD (95% CI): 0.04 (-10.2, 10.3); -2.2 (-11.5, 7.1); and -0.6 (-11.9, 10.8) for each timepoint respectively] (Figure 3). The results from PPS were consistent with those from FAS [p-values for LSMD >0.05 for all timepoints]. Urine lead levels were also reduced from baseline in both groups throughout the study (Figure 4). At week 8, the ULL LSM (SE) for probiotic was -30.0 (4.6) while control was -13.2 (5.0) [LSMD (95% CI): -16.8 (-30.4, -3.2) μg/L] (p = 0.02) (Table 2). There was no statistical difference between groups for change from baseline ULLs at weeks 4 and 12. The blood levels of all essential trace elements (calcium, zinc, copper, magnesium, and iron) at week 4, 8, and 12 stayed within normal physiological ranges, without any deficiencies reported. At the end of the intervention, there was no statistically significant difference between groups for all essential elements. The GSRS total score improved for both groups from baseline to week 12, with the abdominal pain score reduced significantly in the probiotic group compared to the control at week 12 (p = 0.03) (Table 3). CPRS total score and all subscores improved for both groups from baseline to week 12, with improvement in psychosomatic feelings (p = 0.01) and learning problems (p = 0.06) in the probiotic group more pronounced than in the control group at week 12 (Table 4; Figure 5).
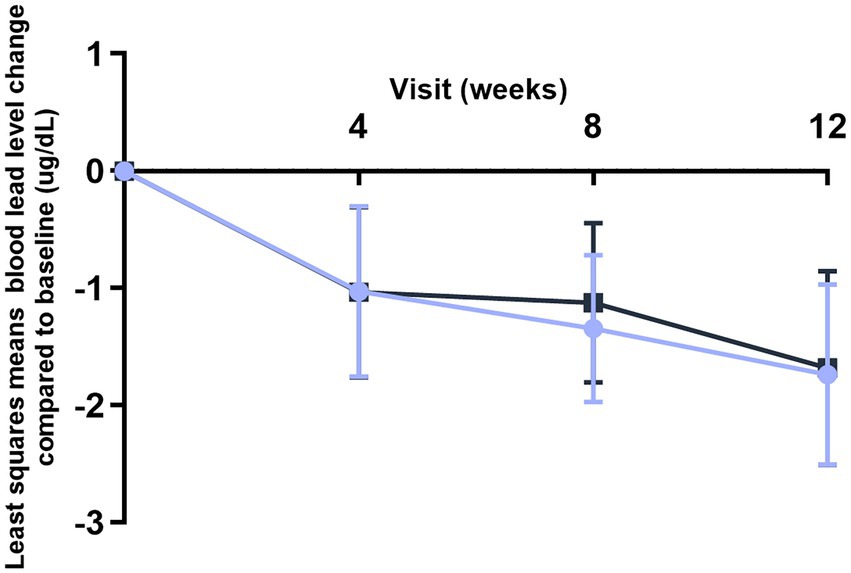
Figure 3. Improvement of blood lead levels (BLLs) over time (FAS population). Values plotted are the least squares means of the changes at weeks 4, 8, and 12 compared to baseline, with 95% confidence intervals. Control.
L. plantarum DSM 33464.
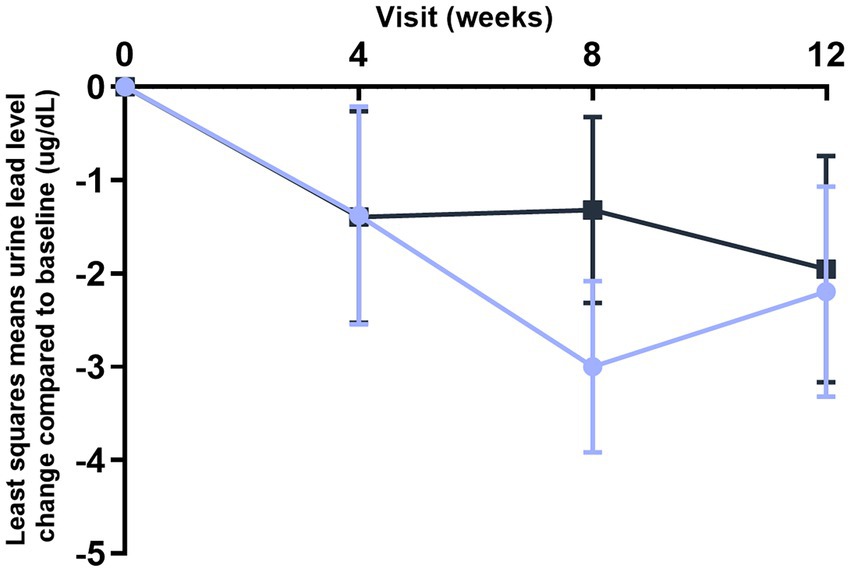
Figure 4. improvement of urine lead levels (ULLs) over time (FAS population). Values plotted are the least squares means of the changes at week 4, 8, and 12 compared to baseline, with 95% confidence intervals. Control.
L. plantarum DSM 33464.
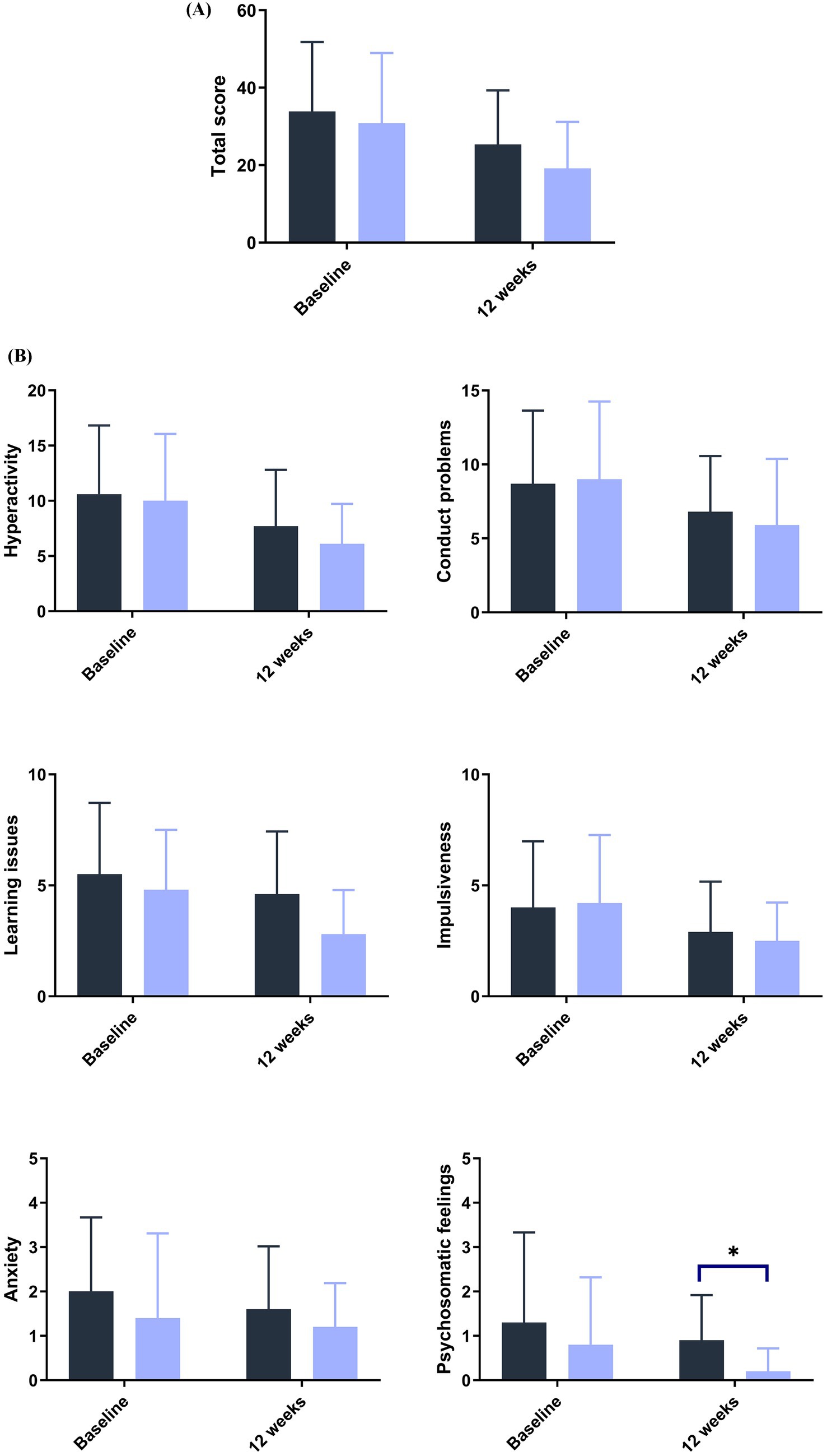
Figure 5. Improvement in emotional behavior as measured by the Conner’s scale score at baseline and 12 weeks (A) total and (B) subscores. ANCOVA. Post hoc analysis. Control.
L. plantarum DSM 44646. *p< 0.05.
3.4 Safety endpoints
The incidence and severity of adverse events were similar in both groups (Supplementary Table 2). There were no treatment-related adverse events (TRAEs), no treatment-emergent adverse events (TEAEs) leading to the investigational product being permanently discontinued or study discontinuation, and no grade 4 or above TEAEs. TEAEs are detailed in Supplementary Table 3.
Routine blood test and blood biochemistry revealed no major findings. No major differences were noted between the groups for all vital signs (Supplementary Table 4).
4 Discussion
Prior to clinical intervention, we confirmed the ability of live L. plantarum DSM 33464 to bind and retain lead via electron microscopy after a lead-binding assay. The results gave reassurance that the freeze-drying process did not impair the strain’s capacity to bind lead. We then examined the safety and efficacy of L. plantarum DSM 33464 in children with elevated BLLs in a randomized, placebo-controlled multi-center study with a 12-week intervention period. For the primary outcome, we found no statistically significant difference between intervention groups, although both groups showed a reduction of BLLs by 40% compared to baseline. For secondary outcomes, probiotics reduced ULLs beyond the control at week 8, while other timepoints showed no statistically significant difference. Overall, the strain was safe and well-tolerated, did not deplete minerals, and significantly improved the abdominal pain score and psychosomatic feelings compared to the control at week 12.
With a median BLL of <5 μg/dL, the children were characterized by having slightly elevated BLLs rather than hyperlipemia according to the Chinese guidelines (22). There are recommendations from the World Health Organization for the management of lead exposure for BLLs ≥ 5 μg/dL, including nutritional supplementation with calcium and iron in case of deficiency, but not for BLLs <5 μg/dL (23). Even so, at this low level, children, especially those younger than 5 years, are most susceptible to the often-irreversible adverse effects of lead, including behavioral and learning problems, lower intelligence quotient, hyperactivity, and impaired growth (2).
Our study is the first to demonstrate BLL reduction with probiotic intervention in addition to multivitamin/mineral supplements in children with elevated BLLs. Two studies have already investigated the effects of probiotics in lead-exposed subjects; one with yogurt containing 1010 CFUs of Lacticaseibacillus rhamnosus GR-1 that showed no improvement in BLLs in children and pregnant women in Tanzania (24), while another found beneficial effects when L. plantarum CCFM8661 with prebiotic was given to lead-exposed adults, by modulating the gut microbiota, and both the metabolism of the host and gut microbiota (25).
A major limitation of our study is that both groups were given multivitamin/mineral supplements including calcium, iron, zinc, and several vitamins on top of the probiotic product or placebo, which may have complemented the effects of the probiotic group. Iron, zinc, calcium, and vitamin C levels and supplementation can influence the gastrointestinal absorption and distribution of lead (26, 27). Diets low in calcium have been reported to enhance lead accumulation (28) while iron-deficient children have higher gastrointestinal absorption of lead (29). Although no specific history of mineral or vitamin deficiency was present in the majority of the children enrolled, this supplementation is a standard care for children with high BLLs at the study site in Beijing, and therefore given to all participating children. There is insufficient evidence on the effects of calcium or iron supplementation on BLLs and clinical outcomes (30), but the observations of significant BLL reduction with calcium supplementation in children and pregnant women with slightly elevated BLLs (31, 32) indicate that the standard care administered likely contributed to the positive outcomes in both groups. Therefore, the lack of a difference in BLLs between the two groups may be attributed to the additional supplementation of multivitamins and minerals given to all children. Furthermore, as the measure of change from baseline BLLs is dependent on the baseline values, the high baseline variability (see Table 2) may have contributed to the lack of difference observed between groups. The other limitations include the effects of the coronavirus disease 2019 (COVID-19) pandemic, such as frequent or constant lockdowns and increased hand-washing, which could have reduced exposure and contributed to improved BLLs and other outcomes measured. The pandemic also delayed and reduced the number of children enrolled (from 124 to 66) and affected protocol compliance. The planned intervention period was also halved from 24 weeks due to the reluctance to participate during this challenging period.
We found a significantly larger reduction in ULLs with probiotics compared to the control at week 8, and a trend at week 12 that was no longer statistically significant, possibly due to the reduced samples available and large inter-individual variation (33). Urine lead, which reflects recent absorption (33), has been used for monitoring only to some extent (34, 35) as >99% of lead in blood is found in erythrocytes, while urine lead originates from plasma (36). As urine and feces are the two main pathways of lead excretion from the body, a decrease in urine lead excretion could reflect reduced absorption in the gastrointestinal tract and/or more excretion through the fecal route, in parallel with reduced BLLs observed. Fecal lead was not studied as the analytical methods are not validated nor available in the central laboratory. The ULLs in our study were not adjusted for diuresis using creatinine, which would have made it more reliable, as creatinine-corrected urine may account for variations by normalizing individual differences in hydration and kidney function, reflecting the lead burden in the body more accurately (37, 38). Therefore, creatinine excretion correction for ULLs is recommended for future studies (39).
There are multiple mechanisms described for how probiotics may remove heavy metals (40), while L. plantarum DSM 33464 showed, in addition to heavy metal binding and sequestration ability, strengthening of the gut barrier (41, 42). In addition, the regulation of bile acid enterohepatic circulation, which is related to gut microbiota remodeling, has been found in cadmium-exposed adults after 8 weeks of supplementation with the strain, resulting in significantly reduced blood cadmium levels compared to placebo (26). This strain did not show any TRAEs in adults when previously studied in four studies with different indications (26, 43–45). We have now confirmed that it is also well-tolerated in children.
The positive effects of the probiotic group over the control group on abdominal pain and psychosomatic feelings are encouraging, even with complementary effects from multivitamin/mineral supplementation, and potential reduced exposure due to the pandemic and education. Although few children reported only minor gastrointestinal symptoms at baseline, they improved over time in the probiotic group. These results are in line with previously demonstrated symptoms’ improvements in irritable bowel syndrome patients after 8 weeks’ intervention with the strain (43). The observed improvement in psychosomatic symptoms could be plausibly linked to reduced abdominal pain.
5 Conclusion
L. plantarum DSM 33464, combined with a multivitamin/mineral supplement, reduced BLLs in children with elevated BLLs within 12 weeks, and improved associated abdominal pain and psychosomatic feelings; therefore, it is a promising, safe intervention for children with slightly elevated BLLs. Larger studies with the strain alone, featuring a longer intervention period, and more thorough lead monitoring are warranted to confirm the effects of the strain.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors upon reasonable request.
Ethics statement
The studies involving humans were approved by the Ethics Committee of Beijing Children’s Hospital, Capital Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
Author contributions
WJ: Investigation, Writing – review & editing, Supervision. DS: Writing – original draft, Data curation, Project administration, Writing – review & editing. LZ: Supervision, Writing – review & editing, Investigation. JL: Writing – review & editing, Investigation, Supervision. JG: Writing – review & editing, Investigation, Supervision. XW: Writing – review & editing, Investigation, Supervision. CH: Conceptualization, Writing – review & editing, Funding acquisition, Methodology. AL: Investigation, Methodology, Supervision, Writing – review & editing. H-TT: Data curation, Project administration, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by the Novozymes A/S, part of Novonesis Group.
Acknowledgments
The authors would like to acknowledge the role of Clinical Service Center Co., Ltd., Suzhou, China, in conducting the study and thank Wenqian Gu for the support in the sponsor’s study project management and Jouni Junnila for further statistical support. Furthermore, we thank Zhang Ying, Wu Xin, and Zhao Qiang from Novonesis, China Research Center for the lead binding study, and Chenguang Zhao, Yanfei Hu, Yan Yang, and Qian Li from the Cell Biology Facility, Center of Biomedical Analysis of Tsinghua University, for their technical assistance with TEM. We wish to extend our gratitude to all the study participants and their families.
Conflict of interest
SmartGuard™ is a trademark of Novozymes A/S, part of Novonesis Group. DS, CH, H-TT are employees of Novonesis.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2025.1641839/full#supplementary-material
Abbreviations
ANCOVA, Analysis of covariance; BLLs, Blood lead levels; BLRV, Blood lead reference value; CIs, Confidence intervals; CPRS, Conners’ Parent Rating Scale; FAS, Full analysis set; GSRS, Gastrointestinal Symptoms Rating Scale; IPs, Investigational products; LSM, least squares means; LSMD, Least squares mean difference; LMICs, Low-income and middle-income countries; MMRM, Mixed-effects model for repeated measures; PPS, Per-protocol set; SE, Standard error; SS, Safety set; TEAEs, Treatment-emergent adverse events; TRAEs, Treatment-related adverse events; ULLs, Urine lead levels.
References
1. Kordas, K, Ravenscroft, J, Cao, Y, and McLean, E. Lead exposure in low and middle-income countries: perspectives and lessons on patterns, injustices, economics, and politics. Int J Environ Res Public Health. (2018) 15:2351. doi: 10.3390/ijerph15112351
2. UNICEFEarth, P. The toxic truth: children’s exposure to lead pollution undermines a generation of future potential (2020). Available online at: https://www.unicef.org/reports/toxic-truth-childrens-exposure-to-lead-pollution-2020 UNICEF; Pure Earth.
3. Wani, AL, Ara, A, and Usmani, JA. Lead toxicity: a review. Interdiscip Toxicol. (2015) 8:55–64. doi: 10.1515/intox-2015-0009
4. Canfield, RL, Henderson, CR, Cory-Slechta, DA, Cox, C, Jusko, TA, and Lanphear, BP. Intellectual impairment in children with blood lead concentrations below 10 microg per deciliter. N Engl J Med. (2003) 348:1517–26. doi: 10.1056/NEJMoa022848
5. (CDC) USCfDCaP. CDC updates blood Lead reference value (2024). U.S. Centers for Disease Control and Prevention (CDC). Available online at: https://www.cdc.gov/lead-prevention/php/news-features/updates-blood-lead-reference-value.html
6. Li, T. Time for a change in blood lead reference value for Chinese children. Chemosphere. (2021) 267:128868. doi: 10.1016/j.chemosphere.2020.128868
7. Dong, J, and Li, X. Lead pollution-related health of children in China: disparity, challenge, and policy. Sci Total Environ. (2023) 882:163383. doi: 10.1016/j.scitotenv.2023.163383
8. Zhao, X, Li, Z, Wang, D, Li, J, Zou, B, Tao, Y, et al. Assessment of residents' total environmental exposure to heavy metals in China. Sci Rep. (2019) 9:16386. doi: 10.1038/s41598-019-52649-w
9. Zhao, X, Shao, Y, Ma, L, Shang, X, Zhao, Y, and Wu, Y. Exposure to lead and cadmium in the sixth total diet study - China, 2016-2019. China CDC Wkly. (2022) 4:176–9. doi: 10.46234/ccdcw2022.045
10. Agency for Toxic Substances and Disease Registry (US). Toxicological Profile for Lead Atlanta, GA (2020). Agency for Toxic Substances and Disease Registry (US).
11. Zhong, B, Giubilato, E, Critto, A, Wang, L, Marcomini, A, and Zhang, J. Probabilistic modeling of aggregate lead exposure in children of urban China using an adapted IEUBK model. Sci Total Environ. (2017) 584–585:259–67. doi: 10.1016/j.scitotenv.2016.11.164
12. Landrigan, PJ, Kimmel, CA, Correa, A, and Eskenazi, B. Children's health and the environment: public health issues and challenges for risk assessment. Environ Health Perspect. (2004) 112:257–65. doi: 10.1289/ehp.6115
13. Moya, J, Bearer, CF, and Etzel, RA. Children's behavior and physiology and how it affects exposure to environmental contaminants. Pediatrics. (2004) 113:996–1006. doi: 10.1542/peds.113.S3.996
14. Bhattacharya, S. Probiotics against alleviation of lead toxicity: recent advances. Interdiscip Toxicol. (2019) 12:89–92. doi: 10.2478/intox-2019-0010
15. Chen, Z, Leng, X, Zhou, F, Shen, W, Zhang, H, Yu, Q, et al. Screening and identification of probiotic lactobacilli from the infant gut microbiota to alleviate lead toxicity. Probiotics Antimicrob Proteins. (2023) 15:821–31. doi: 10.1007/s12602-021-09895-0
16. Li, X, Brejnrod, AD, Ernst, M, Rykær, M, Herschend, J, Olsen, NMC, et al. Heavy metal exposure causes changes in the metabolic health-associated gut microbiome and metabolites. Environ Int. (2019) 126:454–67. doi: 10.1016/j.envint.2019.02.048
17. Eggers, S, Safdar, N, Kates, A, Sethi, AK, Peppard, PE, Kanarek, MS, et al. Urinary lead level and colonization by antibiotic resistant bacteria: evidence from a population-based study. Environ Epidemiol. (2021) 5:e175. doi: 10.1097/EE9.0000000000000175
18. Eggers, S, Safdar, N, Sethi, AK, Suen, G, Peppard, PE, Kates, AE, et al. Urinary lead concentration and composition of the adult gut microbiota in a cross-sectional population-based sample. Environ Int. (2019) 133:105122. doi: 10.1016/j.envint.2019.105122
19. Goyette, CH, Conners, CK, and Ulrich, RF. Normative data on revised Conners parent and teacher rating scales. J Abnorm Child Psychol. (1978) 6:221–36. doi: 10.1007/BF00919127
20. Svedlund, J, Sjödin, I, and Dotevall, G. GSRS--a clinical rating scale for gastrointestinal symptoms in patients with irritable bowel syndrome and peptic ulcer disease. Dig Dis Sci. (1988) 33:129–34.
21. Qin, Y, Zhao, T, Liu, F, Wang, X, Cao, X, Sun, M, et al. Research of the measurement properties of the Chinese version of the gastrointestinal symptom rating scale for patients with gastrointestinal diseases. Chin Gen Pract. (2023) 26:2277–85. doi: 10.12114/j.issn.1007-9572.2022.0820
22. China NHCotPsRo. The children Lead acidosis and Lead poisoning prevention guide and children’s blood Lead and Lead poisoning classification principles.: National Health Commission of the people’s republic of China. (2006) Available online at: http://www.nhc.gov.cn/wjw/gfxwj/201304/a02eab3564bd46ae8c75645631aafa6d.shtml.
23. World Health Organization. WHO guideline for clinical management of exposure to lead. Geneva: World Health Organization (2021).
24. Bisanz, JE, Enos, MK, Mwanga, JR, Changalucha, J, Burton, JP, Gloor, GB, et al. Randomized open-label pilot study of the influence of probiotics and the gut microbiome on toxic metal levels in Tanzanian pregnant women and school children. MBio. (2014) 5:e01580–14. doi: 10.1128/mBio.01580-14
25. Chen, F, Zhu, J, Yu, L, Zhang, Q, Guo, M, Tian, F, et al. Effect of Lactiplantibacillus plantarum CCFM8661 on serum metabolites and gut microbiota in a lead-exposed population. Int J Biol Macromol. (2024) 261:129815. doi: 10.1016/j.ijbiomac.2024.129815
26. Zhai, Q, Liu, Y, Wang, C, Zhao, J, Zhang, H, Tian, F, et al. Increased cadmium excretion due to oral administration of Lactobacillus plantarum strains by regulating enterohepatic circulation in mice. J Agric Food Chem. (2019) 67:3956–65. doi: 10.1021/acs.jafc.9b01004
27. Dawson, EB, Evans, DR, Harris, WA, Teter, MC, and McGanity, WJ. The effect of ascorbic acid supplementation on the blood lead levels of smokers. J Am Coll Nutr. (1999) 18:166–70. doi: 10.1080/07315724.1999.10718845
28. Goyer, RA. Nutrition and metal toxicity. Am J Clin Nutr. (1995) 61:646S–50S. doi: 10.1093/ajcn/61.3.646S
29. Barton, JC, Conrad, ME, Nuby, S, and Harrison, L. Effects of iron on the absorption and retention of lead. J Lab Clin Med. (1978) 92:536–47.
30. Cantor, AG, Hendrickson, R, Blazina, I, Griffin, J, Grusing, S, and McDonagh, MS. Screening for elevated blood lead levels in children: a systematic review for the U.S. preventive services task force. (2019). Preventive Services Task Force [Internet]. Rockville (MD): Agency for Healthcare Research and Quality (US).
31. Syofyan, SS, Wahyuni, AS, Rusmil, K, and Lelo, A. The effects of calcium supplementation on blood lead levels and short-term memory of chronically exposed children: a clinical trial study. Open Access Maced J Med Sci. (2020) 8:1144–51. doi: 10.3889/oamjms.2020.3285
32. Ettinger, AS, Lamadrid-Figueroa, H, Téllez-Rojo, MM, Mercado-García, A, Peterson, KE, Schwartz, J, et al. Effect of calcium supplementation on blood lead levels in pregnancy: a randomized placebo-controlled trial. Environ Health Perspect. (2009) 117:26–31. doi: 10.1289/ehp.11868
33. Olympio, KPK, Salles, FJ, Akiba, N, and Luz, MS. Biomarkers of Lead exposure: platforms and analysis In: VB Patel, VR Preedy, and R Rajendram, editors. Biomarkers in toxicology. Cham: Springer International Publishing (2022). 1–25.
34. Bergdahl, IA, Schütz, A, Gerhardsson, L, Jensen, A, and Skerfving, S. Lead concentrations in human plasma, urine and whole blood. Scand J Work Environ Health. (1997) 23:359–63. doi: 10.5271/sjweh.232
35. de Barbanson, B. Biological monitoring of urinary lead: preshift and postshift sampling detects efficiently recent lead exposure and signals the need to review and possibly improve controls at work. Toxicol Lett. (2020) 331:53–6. doi: 10.1016/j.toxlet.2020.05.037
36. Tsaih, SW, Schwartz, J, Lee, ML, Amarasiriwardena, C, Aro, A, Sparrow, D, et al. The independent contribution of bone and erythrocyte lead to urinary lead among middle-aged and elderly men: the normative aging study. Environ Health Perspect. (1999) 107:391–6. doi: 10.1289/ehp.99107391
37. Sallsten, G, Ellingsen, DG, Berlinger, B, Weinbruch, S, and Barregard, L. Variability of lead in urine and blood in healthy individuals. Environ Res. (2022) 212:113412. doi: 10.1016/j.envres.2022.113412
38. Sommar, JN, Hedmer, M, Lundh, T, Nilsson, L, Skerfving, S, and Bergdahl, IA. Investigation of lead concentrations in whole blood, plasma and urine as biomarkers for biological monitoring of lead exposure. J Expo Sci Environ Epidemiol. (2014) 24:51–7. doi: 10.1038/jes.2013.4
39. Barbosa, F, Tanus-Santos, JE, Gerlach, RF, and Parsons, PJ. A critical review of biomarkers used for monitoring human exposure to lead: advantages, limitations, and future needs. Environ Health Perspect. (2005) 113:1669–74. doi: 10.1289/ehp.7917
40. Mirza Alizadeh, A, Hosseini, H, Mollakhalili Meybodi, N, Hashempour-Baltork, F, Alizadeh-Sani, M, Tajdar-Oranj, B, et al. Mitigation of potentially toxic elements in food products by probiotic bacteria: a comprehensive review. Food Res Int. (2022) 152:110324. doi: 10.1016/j.foodres.2021.110324
41. Zhai, Q, Yin, R, Yu, L, Wang, G, Tian, F, Yu, R, et al. Screening of lactic acid bacteria with potential protective effects against cadmium toxicity. Food Control. (2015) 54:23–30. doi: 10.1016/j.foodcont.2015.01.037
42. Zhai, Q, Tian, F, Zhao, J, Zhang, H, Narbad, A, and Chen, W. Oral administration of probiotics inhibits absorption of the heavy metal cadmium by protecting the intestinal barrier. Appl Environ Microbiol. (2016) 82:4429–40. doi: 10.1128/AEM.00695-16
43. Liu, Y, Yu, X, Yu, L, Tian, F, Zhao, J, Zhang, H, et al. Lactobacillus plantarum CCFM8610 alleviates irritable bowel syndrome and prevents gut microbiota dysbiosis: a randomized, double-blind, placebo-controlled, pilot clinical trial. Engineering. (2021) 7:376–85. doi: 10.1016/j.eng.2020.06.026
44. Fang, Z, Lu, W, Zhao, J, Zhang, H, Qian, L, Wang, Q, et al. Probiotics modulate the gut microbiota composition and immune responses in patients with atopic dermatitis: a pilot study. Eur J Nutr. (2020) 59:2119–30. doi: 10.1007/s00394-019-02061-x
45. Wang, S, Zhang, M, Yu, L, Tian, F, Lu, W, Wang, G, et al. Evaluation of the potential protective effects of Lactobacillus strains against Helicobacter pylori infection: a randomized, double-blinded, placebo-controlled trial. Can J Infect Dis Med Microbiol. (2022) 2022:1–13. doi: 10.1155/2022/6432750
Keywords: probiotic, children, blood lead levels, abdominal pain, developmental health, multivitamin and mineral supplement
Citation: Ji W, Saulnier DM, Zhang L, Liu J, Gao J, Wang X, Holz C, Liang A and Tina H-TT (2025) Effects of Lactiplantibacillus plantarum DSM 33464 in children with elevated blood lead levels: a randomized, double-blind, placebo-controlled study. Front. Nutr. 12:1641839. doi: 10.3389/fnut.2025.1641839
Edited by:
Francesco Savino, University Hospital of the City of Health and Science of Turin, ItalyReviewed by:
Awatif Abid Al-Judaibi, Jeddah University, Saudi ArabiaChand Ram, National Dairy Research Institute (ICAR), India
Copyright © 2025 Ji, Saulnier, Zhang, Liu, Gao, Wang, Holz, Liang and Tan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Delphine Marie Anne Saulnier, ZHNuQG5vdm9uZXNpcy5jb20=
†These authors share first authorship
DM Saulnier, orcid.org/0000-0002-3539-7699
C Holz, orcid.org/0009-0002-5166-2250
H-T T Tan's, orcid.org/0000-0002-8289-7573
 Wenjing Ji
Wenjing Ji Delphine Marie Saulnier2*†
Delphine Marie Saulnier2*† Xia Wang
Xia Wang Hern-Tze Tina Tan
Hern-Tze Tina Tan