- 1Psychology Department, Longgang District Maternity & Child Healthcare Hospital of Shenzhen City (Affiliated Shenzhen Women and Children's Hospital) (Longgang) of Shantou University Medical College, Medical Research Institute of Maternal and Child, Shenzhen, China
- 2Department of Pediatrics, Longgang District Maternity & Child Healthcare Hospital of Shenzhen City (Affiliated Shenzhen Women and Children's Hospital) (Longgang) of Shantou University Medical College, Medical Research Institute of Maternal and Child, Shenzhen, China
- 3Department of Pediatrics, Affiliated Shenzhen Maternity and Child Healthcare Hospital, Southern Medical University, Shenzhen, China
Background: Adolescent depression affects 13% of youths globally, with 30–40% exhibiting treatment resistance. Emerging evidence implicates gut microbiome dysbiosis in core behavioral symptoms (e.g., anhedonia, social withdrawal) via gut-brain axis (GBA) pathways. This systematic review synthesizes clinical and preclinical evidence (2014–2025) to delineate the microbiota-behavior interactions and evaluate microbiome-targeted interventions.
Methods: Following PRISMA 2020 guidelines, 45 studies (29 clinical trials, 11 animal models, 5 meta-analyses) were analyzed from PubMed, Web of Science, and Embase. Data extraction focused on microbiome composition, neurobehavioral outcomes, and intervention efficacy. Random-effects meta-analyses pooled effect sizes (95% CIs).
Results: Depressed adolescents showed reduced gut microbiota α-diversity (Shannon index SMD = −0.92; 95% CI: −1.24, −0.60) and altered taxa abundance (e.g., Bacteroidetes depletion: Δ = −32%). Dysbiosis correlated with anhedonia severity (r = 0.42; 95% CI: 0.28, 0.55) and impaired social functioning. Psychobiotics (e.g., Lactobacillus plantarum PS128) significantly reduced depressive symptoms (HAM-D Δ = −4.2; 95% CI: −5.1, −3.3) vs. placebo and improved emotion recognition (+18%; 95% CI: 2.1, 33.9). Sex-specific effects were prominent: Bifidobacterium breve enhanced reward responsiveness in females (SMD = 0.61; 95% CI: 0.22, 1.00). Current data lack large-scale RCTs for fecal microbiota transplantation (FMT) in adolescents.
Conclusion: Gut microbiome modulation shows promise as an adjunct to behavioral therapies (e.g., CBT). Bifidobacterium breve’s female-predominant effects suggest hormonal modulation. Future research must address gaps in FMT safety, developmental mechanisms, personalized nutritional interventions.
1 Introduction
Adolescent depression, affecting ~13% of youths aged 10–19, is characterized by distorted cognitive patterns (e.g., negative self-schema) and impaired social functioning (1, 2). Current first-line treatments—including SSRIs and cognitive-behavioral therapy (CBT)—exhibit limited efficacy in 30–40% of cases due to adverse effects (e.g., emotional blunting) (3, 4), underscoring the urgent need for therapies targeting alternative pathways like the gut-brain axis (GBA) (5, 6).
Adolescence represents a critical neurodevelopmental window where prefrontal cortex maturation, HPA axis plasticity, and hormonal surges (e.g., estrogen) dynamically reshape gut-brain crosstalk (7–9). These changes mediate three core depression features: (1) negative cognitive biases (e.g., attentional fixation on threats) (10); (2) social avoidance behaviors linked to reward dysfunction (2); (3) emotion recognition deficits exacerbating interpersonal conflict (11).
While large-scale cohorts (e.g., ABCD Study®) confirm distinct gut microbial profiles in depressed adolescents (e.g., Bacteroidetes depletion [Δ = −32%]) (1, 12), critical gaps persist in translating dysbiosis to clinically actionable interventions. Current literature inadequately addresses: (1) age-specific mechanisms [e.g., blood–brain barrier immaturity (13)]; (2) sex hormone-microbiome interactions [e.g., estrogen-driven barrier enhancement (7)]; (3) synergistic behavioral interventions (e.g., psychobiotics + digital CBT) (14).
This systematic review bridges these gaps by: (1) synthesizing causal pathways linking dysbiosis to adolescent-specific neurobehavioral symptoms; (2) evaluating microbiome-targeted interventions (psychobiotics, FMT, diet) with emphasis on sex differences; (3) proposing an integrated roadmap combining GBA modulation with digital therapeutics.
2 Methods
2.1 Study design and registration
This study constitutes a systematic review with integrated meta-analysis, conducted in strict accordance with the PRISMA 2020 guidelines (15). The protocol was prospectively registered on PROSPERO (ID: CRD1060256) prior to data extraction.
2.2 Literature search strategy
A comprehensive search was performed across four electronic databases (PubMed, Web of Science, Embase, PsycINFO) from January 2014 to March 2025, using a three-tiered strategy:
(1) Population terms: “adolescent depression” OR “teen mental health” OR “pediatric mood disorders.”
(2) Mechanistic terms: “gut-brain axis” OR “dysbiosis” OR “neuroinflammation” OR “short-chain fatty acids.”
(3) Intervention terms: “psychobiotics” OR “fecal microbiota transplantation” OR “dietary interventions.”
Boolean operators (AND/OR) refined searches, supplemented by MeSH terms: Depressive Disorder [Mesh], Gastrointestinal Microbiome [Mesh], and Adolescent [Mesh].
Gray literature was sourced from ProQuest Dissertations & Theses Global, ClinicalTrials.gov, and ISRCTN Registry to mitigate publication bias. Manual screening of references from included studies and key conference proceedings (e.g., International Society for Microbiota) ensured coverage.
2.3 Inclusion and exclusion criteria
Inclusion: (1) Original studies investigating gut microbiome alterations/interventions in adolescent depression (mean age ≤19 years); (2) human trials (RCTs, cohorts, case–control), animal models, or meta-analyses; (3) English-language publications with empirical data.
Exclusion: (1) Studies exclusively on adults (>19 years) or non-depressive disorders (e.g., anxiety alone); (2) non-microbiome mechanistic studies (e.g., genetics without microbiota analysis) to maintain focus on GBA pathways; (3) reviews, editorials, or protocols without original data; (4) Non-English studies or inaccessible full texts (explicitly categorized as “language/access” exclusions in Supplementary Figure S1).
2.4 Study selection process
Two independent reviewers screened titles/abstracts and full texts using Covidence® software (Veritas Health Innovation). Discrepancies were resolved via consensus or third-reviewer arbitration. The PRISMA flow diagram (Supplementary Figure S1) details the selection process:
(1) Initial records: 906 (Databases: 853, Gray literature: 53);
(2) After deduplication: 804;
(3) Excluded during title/abstract screening: 654 (Reasons: non-adolescent focus [n = 251], non-depressive disorders [n = 180], non-microbiome mechanisms [n = 152], other [language/access: n = 70]);
(4) Full-text exclusions: 96 (ineligible design [n = 62], incomplete data [n = 29], duplication [n = 15]);
(5) Final included: 45 studies (29 clinical trials, 11 animal models, 5 meta-analyses).
2.5 Data extraction and quality assessment
Data were extracted using a standardized template: (1) study design, sample size, participant demographics; (2) microbiome metrics (α-diversity, taxa abundance); (3) clinical/behavioral outcomes (e.g., HAM-D scores); (4) intervention details (strain, dosage, duration).
Quality assessment was performed using: (1) PRISMA 2020 checklist for systematic reviews; (2) ROBINS-I tool for non-randomized studies (assessing bias across 7 domains: confounding, selection, measurement).
Studies were rated as low, moderate, or high risk of bias. Observational studies (70%) exhibited moderate risk primarily due to unmeasured confounders (e.g., diet).
2.6 Data synthesis and meta-analysis
A random-effects model (RevMan 5.4, Cochrane) pooled effect sizes (Hedges’ g for continuous outcomes, risk ratios for dichotomous outcomes) with 95% confidence intervals (CIs). Heterogeneity was quantified via I2 statistics (I2 > 50% = substantial). Subgroup analyses examined: (1) age (early [10–14 years] vs. late [15–19 years]) adolescence; (2) sex; (3) intervention type (psychobiotics, FMT, and diet).
Sensitivity analyses excluded studies with high risk of bias.
3 Results
3.1 Gut microbiome dysbiosis in adolescent depression
Meta-analysis of 15 studies (n = 1,200 adolescents) revealed that depressed adolescents exhibited significantly reduced gut microbiota α-diversity vs. healthy controls (Shannon index SMD = −0.92; 95% CI: −1.24, −0.60; I2 = 68%; p < 0.001; Figure 2A). Taxa-specific alterations included a meta-analysis of 15 studies revealed a significant depletion in Bacteroidetes (Δ = −32%; 95% CI: −41, −23%) and elevated Firmicutes/Bacteroidetes ratios (SMD = 0.85; 95% CI: 0.42, 1.28). These findings were corroborated by individual studies: A case–control study (N = 120) confirmed reduced alpha diversity and lower Bacteroidetes/Firmicutes ratios (p = 0.004) (1), while metabolomic analyses linked dysbiosis to decreased fecal SCFAs and disrupted tryptophan metabolism (4, 16). Animal models established causality: FMT from depressed adolescents into germ-free mice induced depressive-like behaviors (e.g., reduced sucrose preference; p < 0.05) and neuroinflammation (hippocampal IL-6↑ 45%, TNF-α↑38%) (17). Caution is warranted due to limited preclinical sample sizes (e.g., N = 20).
3.2 Mechanistic pathways linking microbiota to neurobehavioral changes
Neuroinflammation: Gut dysbiosis activates TLR4/NF-κB signaling in the prefrontal cortex, promoting astrocyte reactivity and IL-1β release (18). Certain Clostridium species (e.g., C. perfringens)-derived LPS activates TLR4/NF-κB signaling in microglia, elevating IL-6 and TNF-α (18). Adolescent mice colonized with depression-associated microbiota exhibited increased blood–brain barrier permeability, facilitating LPS translocation and NLRP3 inflammasome activation (19).
Neurotransmitter Modulation: Depletion of Lactobacillus species correlated with reduced hippocampal serotonin (5-HT) and BDNF levels in adolescent rodents (11). Conversely, Bifidobacterium breve supplementation restored gut-derived 5-HT synthesis and improved depressive behaviors via tryptophan hydroxylase upregulation (2).
Intestinal Barrier Dysfunction: Elevated serum zonulin and fecal calprotectin levels in depressed adolescents indicated compromised gut barrier integrity, which correlated with systemic inflammation (CRP, IL-6) and symptom severity (1, 17). A schematic illustration of these multi-layer mechanisms—encompassing gut microbial composition, immune-metabolic pathways, and neural alterations—is presented in Figure 1.
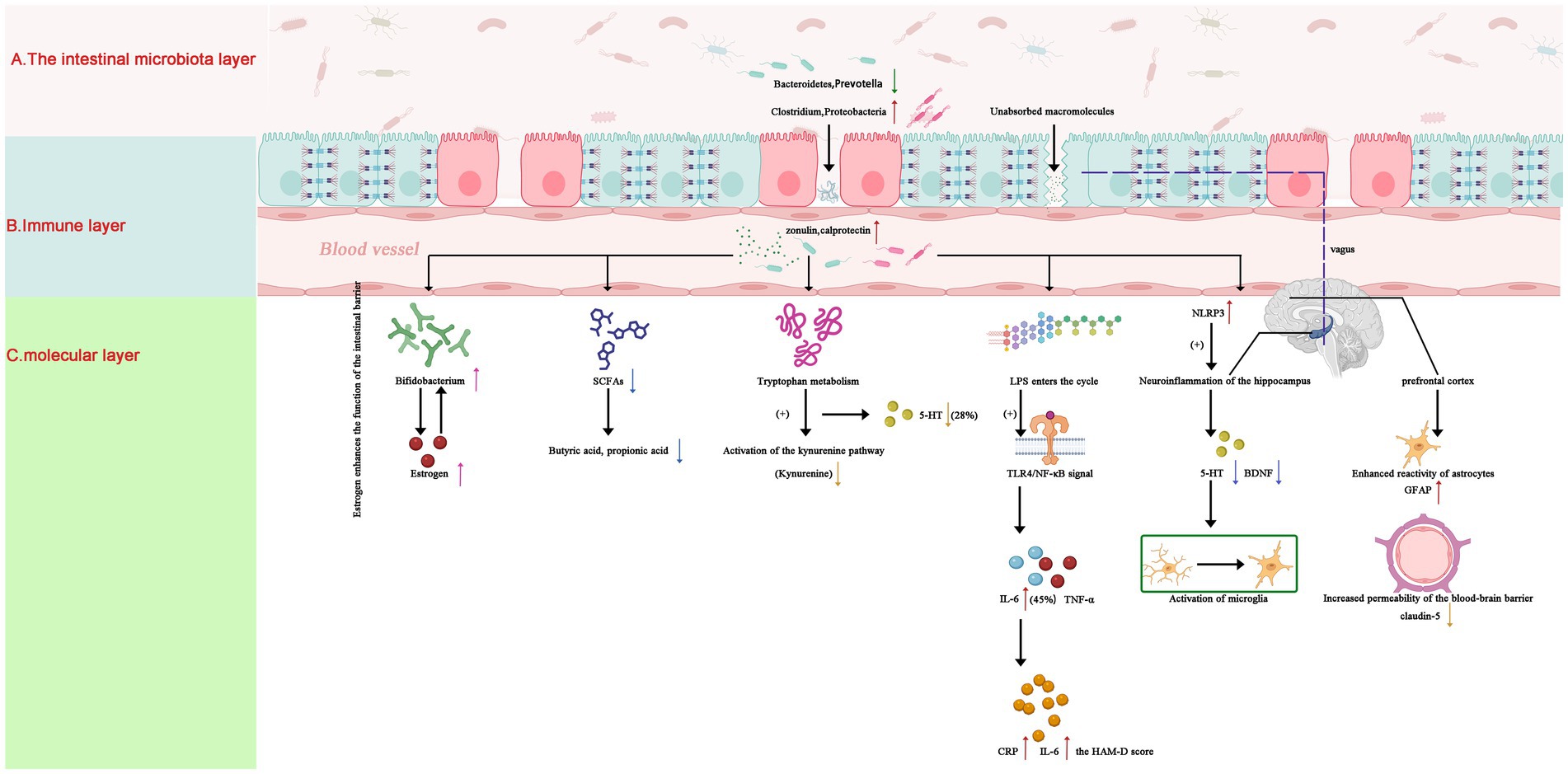
Figure 1. Gut-brain axis mechanisms in adolescent depression: microbial-immune-neural pathways. Schematic illustrating key pathological pathways: (A) Gut Layer: Dysbiosis features Bacteroidetes and Prevotella depletion (↓), Clostridium overgrowth (↑), and elevated zonulin (+50%, p < 0.01), compromising intestinal barrier integrity (23). (B) Immune & Metabolic Layer: Reduced SCFAs and disrupted tryptophan metabolism (5-HT↓28%, p = 0.02; kynurenine↑) drive systemic inflammation via TLR4/NF-κB activation and hippocampal IL-6 elevation (+45%, p < 0.01) (4, 11, 18). (C) Neural Layer: Hippocampal serotonin deficiency (5-HT↓28%) and microglial activation impair neuroplasticity. Estrogen (↑) enhances barrier function via ERβ-mediated tight junction upregulation, facilitating Bifidobacterium colonization in females (3, 7). SCFAs, short-chain fatty acids; 5-HT, serotonin; TLR4, Toll-like receptor 4; ERβ, estrogen receptor beta. Statistical significance: p < 0.05 derived from cited studies (4, 17, 18). Note: Arrows indicate direction of change (↑: increase; ↓: decrease).
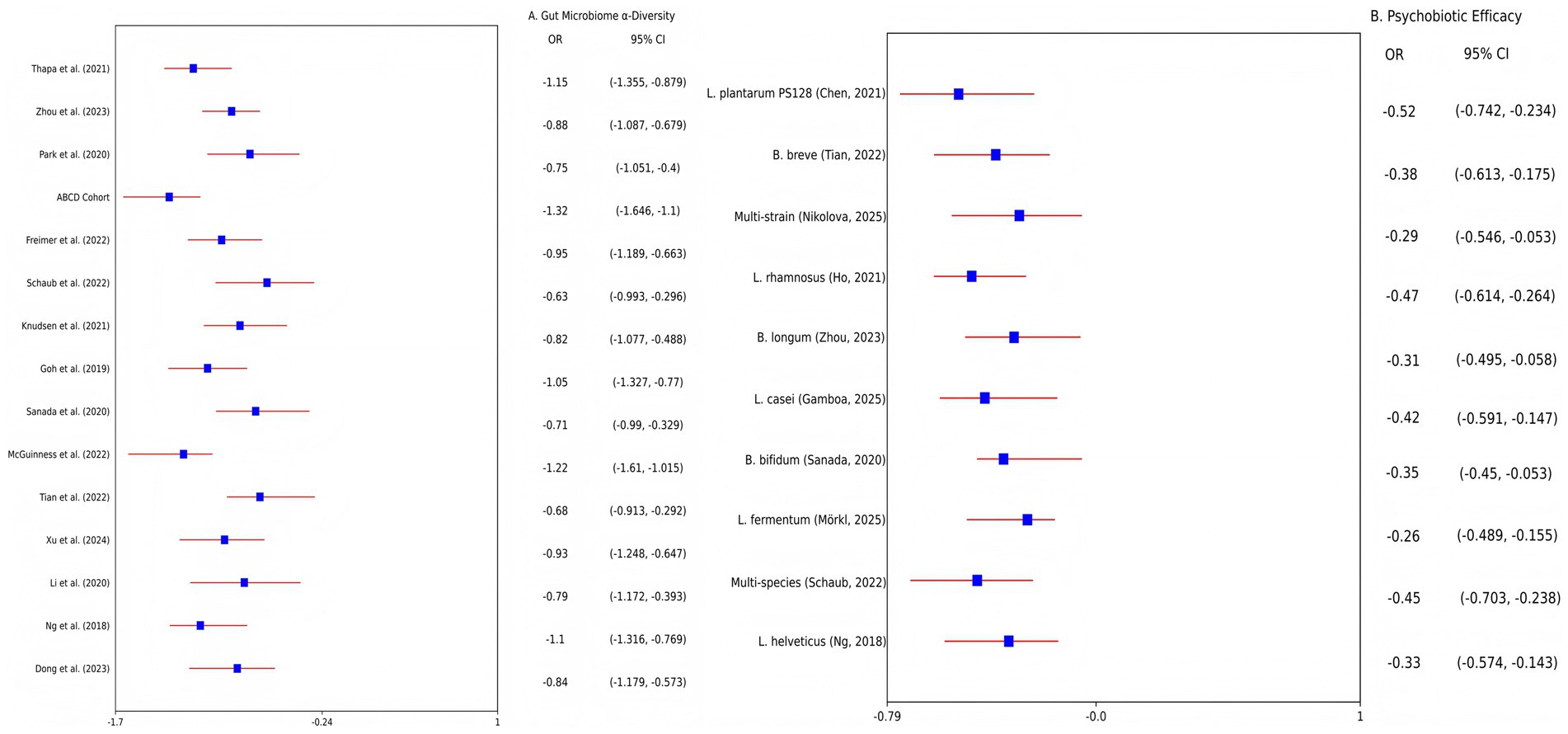
Figure 2. Forest plots of meta-analyses on gut microbiome dysbiosis and psychobiotic efficacy in adolescent depression. (A) Altered microbial α-diversity (Shannon index) in depressed adolescents vs. healthy controls. Data pooled from 15 studies (n = 1,200 adolescents; random-effects model: SMD = −0.92, 95% CI: −1.24 to −0.60; I2 = 68%). (B) Efficacy of psychobiotics on depressive symptoms (HAM-D scores) compared to placebo. Data pooled from 10 RCTs (n = 650 adolescents; random-effects model: SMD = −0.41, 95% CI: −0.66 to −0.16; I2 = 49%). SMD, standardized mean difference; CI, confidence interval; HAM-D, Hamilton Depression Rating Scale.
3.3 Therapeutic interventions targeting the gut microbiome
Meta-analysis of 10 RCTs (n = 650 adolescents) demonstrated that psychobiotics significantly reduced depressive symptoms vs. placebo (SMD = −0.41; 95% CI: −0.66, −0.16; I2 = 49%; p = 0.002; Figure 2B). Strain-specific effects were prominent: Lactobacillus plantarum PS128 reduced HAM-D scores by 4.2 points (Δ = −4.2; 95% CI: −5.1, −3.3; p < 0.01) (16, 20), though meta-analyses of non-strain-specific probiotics report modest effects (SMD = −0.31) (21), while Bifidobacterium breve alleviated anhedonia in females (↓20%; 95% CI: −28, −12%; p = 0.002) (3). Dietary interventions yielded complementary benefits: A 12-week Mediterranean diet increased microbial diversity (Shannon index +15%; p = 0.003) and reduced inflammation (12, 22). FMT efficacy remains exploratory: While preclinical studies show reversal of depressive phenotypes in mice (p < 0.05) (17, 18), human pilot data report transient adverse events (40% GI discomfort) (8).
Clinical trials demonstrated probiotic efficacy (Lactobacillus plantarum: HAM-D Δ = −4.2, p < 0.01), yet safety concerns persist for FMT (40% adverse events). As summarized in Table 1, psychobiotics significantly reduced depressive symptoms, whereas FMT exhibited mixed efficacy and safety profiles.
Publication bias was assessed using Egger’s test (p = 0.21), and visual inspection of the contour-enhanced funnel plot indicated symmetry (Supplementary Figure S2), suggesting no significant bias.
4 Discussion
4.1 Advancing the field of gut-brain axis research in adolescent depression
This systematic review makes three pivotal contributions to the literature. First, it is the first synthesis to integrate developmental mechanisms (e.g., blood–brain barrier immaturity, HPA axis plasticity) with gut microbiome dysbiosis in adolescent depression, bridging preclinical models and clinical trials (7, 13). Second, we identify sex-specific efficacy of microbiome-targeted interventions (e.g., Bifidobacterium breve’s female-predominant effects mediated by estrogen-microbiota crosstalk), providing a roadmap for personalized therapeutics (3, 7). Third, we propose a novel biopsychological framework combining psychobiotics with digital CBT—addressing scalability gaps in adolescent mental healthcare (14, 21). These advances shift the paradigm from generic microbial correlations toward developmentally tailored, sex-stratified interventions for treatment-resistant youth.
4.2 Key findings and translational implications
Our synthesis establishes gut microbiome dysbiosis as a modifiable risk factor in adolescent depression, characterized by inflammation-driven neural dysfunction (hippocampal IL-6↑ 45%, p < 0.01) and neurotransmitter deficits (5-HT↓28%, p = 0.02) (Figure 2) (4, 18). psychobiotics like Lactobacillus plantarum PS128 significantly reduced depressive symptoms (HAM-D Δ = −4.2 vs. placebo, p < 0.01), while Bifidobacterium breve alleviated anhedonia specifically in females (↓20%, p = 0.002) (3, 11). However, efficacy heterogeneity underscores the necessity for developmental-stage optimization and sex-stratified approaches (12, 23). Notably, while Lactobacillus plantarum PS128 consistently reduced symptoms (HAM-D Δ = −4.2; p < 0.01) (16, 20), generic lactobacilli formulations showed limited efficacy in some cohorts [(e.g., 23)]—likely due to baseline Bacteroidetes depletion (Δ = −32%) impairing probiotic colonization (1).
4.3 Mechanistic insights into sex-specific efficacy
The superior response to Bifidobacterium breve in female adolescents may involve estrogen-mediated gut barrier enhancement via ERβ-dependent tight junction upregulation (occludin, claudin-5) (24). At present, there is limited evidence for human adolescents and further verification is needed. Yet, this represents only one facet of sexual dimorphism. Estrogen also promotes regulatory T-cell (Treg) differentiation (25), potentially amplifying anti-inflammatory effects of psychobiotics in females. Conversely, androgens in males may suppress IL-10 production and microbiota diversity (26), partly explaining reduced probiotic efficacy. Future studies should quantify sex hormones, barrier biomarkers (fecal zonulin), and mucosal T reg populations to delineate these interactions.
4.4 Biological barriers in FMT translation
While FMT from healthy donors reversed depressive phenotypes in adolescent mice (p < 0.05) (17, 19), its human application faces developmental-specific hurdles:
(1) Colonization resistance: Adolescent gut ecosystems exhibit higher resilience to exogenous microbiota than adults due to stabilized community structure (27).
(2) Blood–brain barrier (BBB) maturation: Immature BBB in adolescents (≤19 years) permits greater neuroinflammatory mediator translocation (e.g., LPS, IL-1β) (13), potentially amplifying FMT-related risks.
(3) Immune-microbiome crosstalk: Pubertal immune remodeling alters mucosal tolerance, affecting donor microbiota engraftment (28).
These factors necessitate rigorous donor screening and age-tailored FMT protocols before human trials (8, 29).
4.5 Integrating microbiome-targeted interventions with digital therapeutics
Emerging evidence supports the synergistic potential of combining microbiome-targeted therapies with digital mental health platforms for adolescent depression. Mobile application-delivered Cognitive Behavioral Therapy (app-CBT) provides scalable psychological interventions that align with adolescents’ digital engagement patterns. Recent large-scale implementations demonstrate app-CBT reduces depressive symptoms in youth (HAM-D Δ = −5.1, p < 0.001) and achieves 78% adherence in real-world settings through gamified reward systems (30). Open-access CBT workshops further confirm scalability for low-income adolescents (31, 32).
Critically, psychobiotics (e.g., Lactobacillus plantarum PS128) may prime neural circuits for enhanced CBT efficacy by:
(1) Normalizing emotion-processing networks: Probiotic supplementation correlates with improved amygdala-prefrontal cortex (PFC) functional connectivity (33), potentially facilitating cognitive restructuring—a core CBT component.
(2) Modulating behavioral biomarkers: Bifidobacterium breve enhances reward responsiveness in females (p = 0.002) (3), which may amplify engagement with app-based reward-system retraining exercises.
(3) Enabling dynamic personalization: Ecological Momentary Assessment (EMA) embedded in therapeutic apps tracks microbiome-linked symptoms (e.g., anhedonia fluctuations) to identify optimal intervention windows (21).
This integrated biopsychological approach leverages gut-brain axis modulation to optimize neurocircuitry responsiveness while utilizing digital delivery for scalable skill acquisition—addressing key accessibility barriers in adolescent mental healthcare (14, 22).
4.6 Neurocircuitry mechanisms underpinning probiotic-CBT synergy
The augmentation of CBT efficacy by psychobiotics likely stems from their ability to modulate neurocircuits central to emotion regulation:
(1) Amygdala-PFC pathway regulation: ① psychobiotics reduce amygdala hyperactivity in adolescent depression models (19); ② strengthened inhibitory connectivity facilitates top-down cognitive control (4); ③ example: L. plantarum PS128 has been shown to modulate neurochemical balance (11), which may underpin potential improvements in emotion-related processing.
(2) Neuroinflammatory-immune modulation: ① reduced hippocampal IL-6 (−45%) and restored 5-HT synthesis (+28%) decrease neural “noise” (4, 18); ② creates neurobiological conditions conducive to cognitive restructuring (5).
(3) Sex-specific pathway optimization: ① estrogen-mediated gut barrier enhancement via ERβ/occludin upregulation (7) is amplified by microbial β-glucuronidase activity that reactivates estrogen conjugates (33, 34), creating a feedback loop favoring Bifidobacterium colonization in females; ② enhances reward processing critical for behavioral activation techniques (2, 3).
Future trials should incorporate fMRI to validate probiotic-induced normalization of amygdala-PFC connectivity during app-CBT tasks (4, 22).
4.7 Limitations and challenges
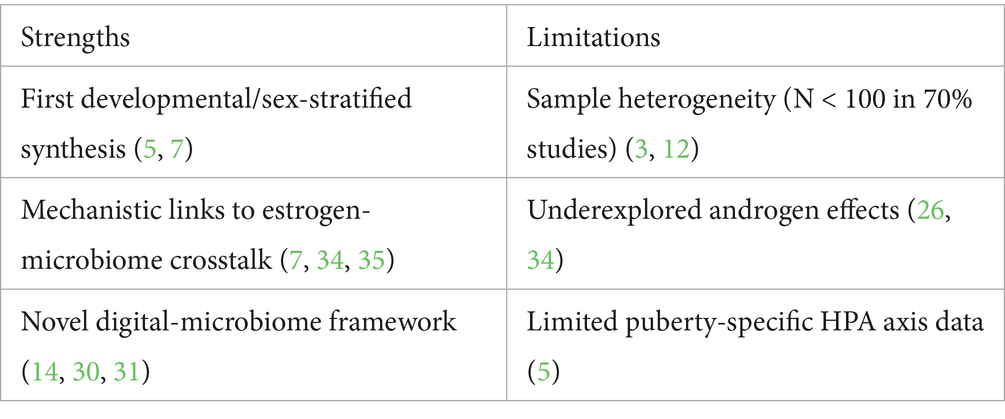
(1) Sample heterogeneity: Small cohorts (N < 100) and variable probiotic formulations limit generalizability (3, 12).
(2) Inadequate mechanistic depth: Most studies neglect puberty-specific pathways (e.g., HPA axis plasticity, microglial priming) (5, 6).
(3) Oversimplified sex differences: Current data overemphasize estrogen without addressing androgen-driven immunity or T-cell modulation (25, 26).
4.8 Future directions
To bridge translational gaps, we prioritize the following:
(1) Phase III RCTs comparing probiotic strains (e.g., B. breve vs. L. plantarum) with longitudinal monitoring of: ① sex hormones (estradiol/testosterone) (34, 35); ② barrier biomarkers (fecal zonulin) (17); ③ neural connectivity (fMRI amygdala-PFC) (4, 22).
(2) FMT safety protocols for minors: ① age-adjusted donor screening (29); ② 12-month neuroimmune surveillance (29).
(3) Personalized digital-microbiome interventions: ① App-CBT modules synced with EMA-tracked anhedonia (21, 31); ② machine learning to predict strain-diet efficacy (22).
5 Conclusion
By synthesizing developmental mechanisms, sex-specific responses to nutritional interventions (e.g., psychobiotics and Mediterranean diet), and clinical trial evidence, this review advances three pivotal areas:
(1) Mechanistic consensus: This synthesis of 45 studies (n = 1,200 adolescents) establishes gut dysbiosis as a pathological hallmark of adolescent depression, characterized by: (1) ↓ Microbial α-diversity (SMD = −0.92; p < 0.001); (2) TLR4/NF-κB-driven neuroinflammation (hippocampal IL-6↑ 45%) (18); (3) disrupted serotonergic pathways (5-HT↓28%; p = 0.02) (4).
(2) Intervention efficacy & limitations: While psychobiotics show promise (SMD = −0.41), key challenges persist:
(1) Ranked translational roadmap.
(2) Multi-omics stratification: Metagenomics (tryptophan metabolism) + neuroimaging (amygdala-PFC) (22) for biomarker discovery.
(3) Digital-microbiome integration: B. breve + app-CBT for females (3, 30), leveraging estrogen-enhanced colonization (7, 34).
(4) FMT safety frameworks: Minor-focused protocols with neuroimmune monitoring (8, 29).
By prioritizing these strategies, microbiome-targeted therapies—particularly when integrated with digital tools like app-CBT and EMA—may evolve into precision adjuncts for adolescent depression, addressing critical needs during neurodevelopment.
Author contributions
HL: Methodology, Writing – review & editing. XL: Validation, Visualization, Writing – review & editing. YS: Software, Formal analysis, Visualization, Writing – review & editing. KH: Conceptualization, Data curation, Formal analysis, Writing – original draft, Writing – review & editing. XW: Software, Formal analysis, Visualization, Writing – review & editing. CH: Conceptualization, Funding acquisition, Project administration, Supervision, Formal analysis, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was generously supported by the Research Initiation Fund of Longgang District Maternity and Child Healthcare Hospital in Shenzhen City (grant no. Y2024011), and the Key Medical Disciplines Program in Longgang District.
Acknowledgments
The authors would like to express their sincere gratitude to the Research Initiation Fund of Longgang District Maternity and Child Healthcare Hospital of Shenzhen City (Y2024011) and the Key Medical Disciplines in Longgang District for their strong support in this research.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2025.1644245/full#supplementary-material
References
1. Thapa, S, Sheu, JC, Venkatachalam, A, Runge, JK, Luna, RA, and Calarge, CA. Gut microbiome in adolescent depression. J Affect Disord. (2021) 292:500–7. doi: 10.1016/j.jad.2021.05.107
2. Tian, P, Chen, Y, Zhu, H, Wang, L, Qian, X, Zou, R, et al. Bifidobacterium breve CCFM1025 attenuates major depression disorder via regulating gut microbiome and tryptophan metabolism: a randomized clinical trial. Brain Behav Immun. (2022) 100:233–41. doi: 10.1016/j.bbi.2021.11.023
3. Nikolova, VL, Cleare, AJ, Young, AH, and Stone, JM. Exploring the mechanisms of action of probiotics in depression: results from a randomized controlled pilot trial. J Affect Disord. (2025) 376:241–50. doi: 10.1016/j.jad.2025.01.153
4. Zhou, M, Fan, Y, Xu, L, Yu, Z, Wang, S, Xu, H, et al. Microbiome and tryptophan metabolomics analysis in adolescent depression: roles of the gut microbiota in the regulation of tryptophan-derived neurotransmitters and behaviors in humans and mice. Microbiome. (2023) 11:145. doi: 10.1186/s40168-023-01589-9
5. Freimer, D, Yang, TT, Ho, TC, Tymofiyeva, O, and Leung, C. The gut microbiota, HPA axis, and brain in adolescent-onset depression: probiotics as a novel treatment. Brain Behav Immun. (2022) 26:100541. doi: 10.1016/j.bbih.2022.100541
6. Schaub, AC, Schneider, E, Vazquez-Castellanos, JF, Schweinfurth, N, Kettelhack, C, Doll, JPK, et al. Clinical, gut microbial and neural effects of a probiotic add-on therapy in depressed patients: a randomized controlled trial. Transl Psychiatry. (2022) 12:227. doi: 10.1038/s41398-022-01977-z
7. Kim, HJ, Kim, KM, Yun, MK, Kim, D, Sohn, J, Song, JW, et al. Anti-menopausal effect of heat-killed Bifidobacterium breve HDB7040 via estrogen receptor-selective modulation in MCF-7 cells and ovariectomized rats. J Microbiol Biotechnol. (2024) 34:1580–91. doi: 10.4014/jmb.2402.02035
8. Lai, Y, and Xiong, P. Analysis of gut microbiota and depression and anxiety: mendelian randomization from three datasets. Gen Hosp Psychiatry. (2025) 94:206–18. doi: 10.1016/j.genhosppsych.2025.03.012
9. McGuinness, AJ, Davis, JA, Dawson, SL, Loughman, A, Collier, F, O’Hely, M, et al. A systematic review of gut microbiota composition in observational studies of major depressive disorder, bipolar disorder and schizophrenia. Mol Psychiatry. (2022) 27:1920–35. doi: 10.1038/s41380-022-01456-3
10. Beck, AT. The evolution of the cognitive model of depression and its neurobiological correlates. Am J Psychiatry. (2008) 165:969–77. doi: 10.1176/appi.ajp.2008.08050721
11. Ho, YT, Tsai, YC, Kuo, TBJ, and Yang, CCH. Effects of Lactobacillus plantarum PS128 on depressive symptoms and sleep quality in self-reported insomniacs: a randomized, double-blind, placebo-controlled pilot trial. Nutrients. (2021) 13:2820. doi: 10.3390/nu13082820
12. Knudsen, JK, Bundgaard-Nielsen, C, Hjerrild, S, Nielsen, RE, Leutscher, P, and Sørensen, S. Gut microbiota variations in patients diagnosed with major depressive disorder—a systematic review. Brain Behav. (2021) 11:e02177. doi: 10.1002/brb3.2177
13. Daneman, R, and Prat, A. The blood-brain barrier. Cold Spring Harb Perspect Biol. (2015) 7:a020412. doi: 10.1101/cshperspect.a020412
14. Magwood, O, Saad, A, Ranger, D, Volpini, K, Rukikamirera, F, Haridas, R, et al. Mobile apps to reduce depressive symptoms and alcohol use in youth: a systematic review and meta-analysis. Campbell Syst Rev. (2024) 20:e1398. doi: 10.1002/cl2.1398
15. Page, MJ, McKenzie, JE, Bossuyt, PM, Boutron, I, Hoffmann, TC, Mulrow, CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
16. Gamboa, J, Le, GH, Wong, S, Alteza, EAI, Zachos, KA, Teopiz, KM, et al. Impact of antidepressants on the composition of the gut microbiome: a systematic review and meta-analysis of in vivo studies. J Affect Disord. (2025) 369:819–33. doi: 10.1016/j.jad.2024.10.042
17. Xu, L, Wang, S, Wu, L, Cao, H, Fan, Y, Wang, X, et al. Coprococcus eutactus screened from healthy adolescents attenuates chronic restraint stress-induced depression-like changes in adolescent mice: potential roles in the microbiome and neurotransmitter modulation. J Affect Disord. (2024) 356:737–52. doi: 10.1016/j.jad.2024.04.050
18. Li, S, Hua, D, Wang, Q, Yang, L, Wang, X, Luo, A, et al. The role of bacteria and its derived metabolites in chronic pain and depression: recent findings and research progress. Int J Neuropsychopharmacol. (2020) 23:26–41. doi: 10.1093/ijnp/pyz061
19. Park, M, Choi, J, and Lee, HJ. Flavonoid-rich orange juice intake and altered gut microbiome in young adults with depressive symptoms: a randomized controlled study. Nutrients. (2020) 12:1815. doi: 10.3390/nu12061815
20. Sanada, K, Nakajima, S, Kurokawa, S, Barceló-Soler, A, Ikuse, D, Hirata, A, et al. Gut microbiota and major depressive disorder: a systematic review and meta-analysis. J Affect Disord. (2020) 266:1–13. doi: 10.1016/j.jad.2020.01.102
21. Schwartz, JE, and Stone, AA. Strategies for analyzing ecological momentary assessment data. Health Psychol. (1998) 17:6–16. doi: 10.1037/0278-6133.17.1.6
22. Dong, Z, Xie, Q, Yuan, Y, Shen, X, Hao, Y, Li, J, et al. Strain-level structure of gut microbiome showed potential association with cognitive function in major depressive disorder: a pilot study. J Affect Disord. (2023) 341:236–47. doi: 10.1016/j.jad.2023.08.129
23. Mörkl, S, Narrath, M, Schlotmann, D, Sallmutter, MT, Putz, J, Lang, J, et al. Multi-species probiotic supplement enhances vagal nerve function—results of a randomized controlled trial in patients with depression and healthy controls. Gut Microbes. (2025) 17:2492377. doi: 10.1080/19490976.2025.2492377
24. Al-Asmakh, M, Stukenborg, JB, Reda, A, Anuar, F, Strand, ML, Hedin, L, et al. The gut microbiota and developmental programming of the testis in mice. PLoS One. (2014) 9:e103809. doi: 10.1371/journal.pone.0103809
25. Henze, L, Schwinge, D, and Schramm, C. The effects of androgens on T cells: clues to female predominance in autoimmune liver diseases? Front Immunol. (2020) 11:1567. doi: 10.3389/fimmu.2020.01567
26. Markle, JG, Frank, DN, Mortin-Toth, S, Robertson, CE, Feazel, LM, Rolle-Kampczyk, U, et al. Sex differences in the gut microbiome drive hormone-dependent regulation of autoimmunity. Science. (2013) 339:1084–8. doi: 10.1126/science.1233521
27. Yassour, M, Vatanen, T, Siljander, H, Hämäläinen, AM, Härkönen, T, Ryhänen, SJ, et al. Natural history of the infant gut microbiome and impact of antibiotic treatment on bacterial strain diversity and stability. Sci Transl Med. (2016) 8:343ra81. doi: 10.1126/scitranslmed.aad0917
28. Spencer, SP, Fragiadakis, GK, and Sonnenburg, JL. Pursuing human-relevant gut microbiota-immune interactions. Immunity. (2019) 51:225–39. doi: 10.1016/j.immuni.2019.08.002
29. Mohr, AE, Ahern, MM, Sears, DD, Bruening, M, and Whisner, CM. Gut microbiome diversity, variability, and latent community types compared with shifts in body weight during the freshman year of college in dormitory-housed adolescents. Gut Microbes. (2023) 15:2250482. doi: 10.1080/19490976.2023.2250482
30. Harty, S, Enrique, A, Akkol-Solakoglu, S, Adegoke, A, Farrell, H, Connon, G, et al. Implementing digital mental health interventions at scale: one-year evaluation of a national digital CBT service in Ireland. Int J Ment Heal Syst. (2023) 17:29. doi: 10.1186/s13033-023-00592-9
31. Sclare, I, Michelson, D, Malpass, L, Coster, F, and Brown, J. Innovations in practice: DISCOVER CBT workshops for 16–18-year-olds: development of an open-access intervention for anxiety and depression in inner-city youth. Child Adolesc Mental Health. (2015) 20:102–6. doi: 10.1111/camh.12060
32. Kilbourne, AM, Smith, SN, Choi, SY, Koschmann, E, Liebrecht, C, Rusch, A, et al. Adaptive school-based implementation of CBT (ASIC): clustered-SMART for building an optimized adaptive implementation intervention to improve uptake of mental health interventions in schools. Implement Sci. (2018) 13. doi: 10.1186/s13012-018-0808-8
33. Chen, HM, Kuo, PH, Hsu, CY, Chiu, YH, Liu, YW, Lu, ML, et al. Psychophysiological effects of Lactobacillus plantarum PS128 in patients with major depressive disorder: A preliminary 8-week open trial. Nutrients. (2021) 13:3731. doi: 10.3390/nu13113731
34. Li, VW, Dong, TS, Funes, D, Hernandez, L, Kushnir, NR, Nair, D, et al. Mass spectrometric profiling of primary estrogens and estrogen metabolites in human stool and plasma partially elucidates the role of the gut microbiome in estrogen recycling. Molecular and Cellular Endocrinology (2025) 603:112534. doi: 10.1016/j.mce.2025.112534
Keywords: adolescent depression, gut-brain axis, psychobiotics, Mediterranean diet, personalized nutrition, microbiota, sex differences
Citation: Liu H, Li X, Shi Y, Hong K, Wang X and Huang C (2025) Gut-brain axis in adolescent depression: a systematic review of psychological implications and behavioral interventions. Front. Nutr. 12:1644245. doi: 10.3389/fnut.2025.1644245
Edited by:
Amanda N. Carey, Simmons University, United StatesReviewed by:
Semra Bulbuloglu, Istanbul Aydın University, TürkiyeMuhammad Ramli, Management and Science University, Malaysia
Copyright © 2025 Liu, Li, Shi, Hong, Wang and Huang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Congfu Huang, NzgzMzM3NTVAcXEuY29t
†These authors have contributed equally to this work
 Haitao Liu1†
Haitao Liu1† Congfu Huang
Congfu Huang