- 1Department for Innovation in Biological, Agro-Food and Forest Systems (DIBAF), Università degli Studi della Tuscia, Viterbo, Italy
- 2ASST Fatebenefratelli Sacco Hospital, Milan, Italy
Objectives: Menopause marks the cessation of ovarian function, preceded by perimenopause, a transitional phase characterized by hormonal fluctuations and metabolic changes, including dyslipidemia. Therefore, a targeted nutritional approach is essential. In this retrospective, observational, pilot study, we evaluated the impact of a Mediterranean-based dietary regimen supplemented with specific natural compounds on lipid profiles and body composition in perimenopausal and menopausal women with hypercholesterolemia.
Methods: An individual dietary plan based on the Mediterranean diet, supplemented with a phytosterol-based formula containing bergamot, prickly pear extract, and vitamin B1, was recommended for each study participant. Additionally, omega-3 fatty acid supplementation was recommended due to its well-documented benefits in reducing cardiovascular disease risk factors, including elevated lipid levels. Lipid profile, body composition, and anthropometric values were recorded and carefully analyzed.
Results: Our findings indicated that this combined dietary approach significantly improved lipid profiles, as evidenced by reductions in total cholesterol, low-density lipoprotein cholesterol, and triglycerides and by an increment in high-density lipoprotein cholesterol values. Furthermore, the dietary plan positively impacted overall body composition and morphometric parameters.
Conclusion: These preliminary findings suggest that a personalized, nutritionally targeted approach may be an effective non-pharmacological strategy for managing cardiovascular and metabolic risk factors during the menopausal transition and postmenopausal period. Further large-scale, controlled studies are warranted to confirm these results and explore long-term outcomes.
1 Introduction
Menopause is clinically defined as the absence of menstrual periods for 12 consecutive months, with the cessation of ovarian function (1, 2). Menopause typically begins between the ages of 45 and 55, and it is preceded by perimenopause, a transitional phase during which the ovaries gradually reduce the production of key reproductive hormones, triggering symptoms that may persist in the menopausal period. Starting from the early stages of the menopausal transition, women frequently experience a variety of disorders, including weight gain, increased visceral fat, insulin resistance, dyslipidemia, and hypertension, which enhance the risk of cardiovascular disease (CVD) (1). Hyperlipidemia is a key factor associated with the menopausal transition and menopause, involving changes in the levels of apolipoproteins, low-density lipoproteins (LDLs), high-density lipoproteins (HDLs), and triglycerides (TGs) in the bloodstream (3). In particular, high TGs, total cholesterol (TC), LDL, and a high TC-to-HDL ratio could be observed. In this scenario, hormone therapy offers some long-term health benefits; however, it is not indicated for preventing CVD (2, 4). Therefore, a proper lifestyle is crucial, including enhancing nutrition (1). Indeed, a healthy dietary pattern can support the maintenance of a healthy body condition, alleviating menopause symptoms and improving overall health throughout life (5). In this respect, a recent study showed that natural oral supplements containing glucosinolates, phytosterols, and citrus flavonoids were more efficacious in reducing global, physical, and psychosocial menopausal symptoms in the short term than estrogen plus progestogen therapy (6). Additionally, supplementation with vitamin D, omega-3 fatty acids, antioxidants, and phytochemicals supports the effects of a healthy nutritional regimen (7). Thus, recent evidence supports the use of personalized nutritional approaches for alleviating menopausal symptoms (1). The Mediterranean diet (MD) and lifestyle, characterized by anti-inflammatory and antioxidant-rich foods, offer significant health benefits (8–10), and alleviate menopausal symptoms (11, 12). To some extent, the MD significantly impacts health as a medical prescription in obese menopausal women (11).
Mediterranean regions are particularly rich in foods that can be utilized as functional foods. This is the case of bergamot (Citrus bergamia Risso, family Rutaceae), an endemic plant native to the Calabrian region in southern Italy, that is rich in flavonoids and glycosides, which exhibit a wide range of pharmacological activities (13). Bergamot extract helps manage lipid levels (9, 11), and positively influences metabolic syndrome (14). Multiple studies indicate that the bergamot content of polyphenols, particularly flavonoids, is responsible for its beneficial effects on lipid profile (15–18). Noteworthy, a positive impact on lipid profile was reported in individuals after administering a nutraceutical compound based on a flavonoid complex from bergamot and opuntia (19). Opuntia ficus-indica (prickly pear, a member of the Cactaceae family), widely distributed in the Mediterranean region, is rich in pectins, mucilages, polyunsaturated fatty acids, and vitamins. Its supplementation has been shown to reduce CDV risk and positively affect blood lipids (20–22). Adherence to a dietary plan is a crucial issue in weight management and metabolic health (23). Albeit the adherence to the MD is decreasing due to the increased consumption of refined, processed food (24), we aimed to support the benefits of a specific dietary plan based on the Mediterranean pattern, in conjunction with specific supplements that potentially increase its efficacy, as a strategy to rebalance dyslipidemia during the menopausal transition and menopausal period. In particular, in this retrospective, observational, pilot study, we analyzed routinely collected data from a cohort of women in the perimenopausal and menopausal periods, who were affected by hypercholesterolemia, and followed a dietary regimen to improve their lipid profiles. Women were recommended to follow a personalized diet plan based on the MD concept, supplemented with a phytosterol-based formula containing bergamot, prickly pear extract, and vitamin B1. Since omega-3 fatty acids lessen CVD risk factors (25), diet supplementation with omega-3 fatty acids was also recommended.
During a few months of observation, individuals were monitored for lipidemic parameters, and in parallel, for anthropometric and body composition measurements. The data collection highlighted the positive effects of our dietary interventions in rebalancing the lipidemic profile and overall body size and composition, thus supporting the feasibility of larger-scale trials. Therefore, this pilot observational study was primarily intended to establish a basis for more comprehensive and rigorous future investigations.
2 Methods
2.1 Study design, participants, and ethics
This pilot study consisted of the observational retrospective analysis of data from a case series of 14 women, 9 menopausal and 5 perimenopausal, who attended the professional practice of a nutrition specialist and required dietary intervention due to dyslipidemia and weight management. The inclusion criteria were: TC > 200 mg/dL, LDL-C > 100 mg/dL, and a body mass index (BMI) between 18.5 and 39.9 kg/m2. The exclusion criteria included comorbidities, pharmacological treatment aimed at improving lipid profiles, and supplement intake (even if not explicitly targeting lipid levels). All participants provided written informed consent for the anonymous use of their data for research purposes at the end of the observational period. The study was conducted following the principles of the Declaration of Helsinki. In line with national regulations and due to the non-interventional, observational nature of the research involving fully anonymized data, ethical committee approval was not required. To note, the study did not collect or process data that could identify the participants or compromise their privacy.
All participants were of the same ethnicity and resided in the same Mediterranean area. A preliminary clinical interview was conducted to assess dietary habits and lifestyle, allowing the formulation of personalized nutritional plans tailored to individual needs. Nearly identical questions were administered to all participants by the nutritionist and systematically assessed at the initial evaluation and each subsequent follow-up.
This is an observational, open-label study, with no randomization of participants.
2.2 Data collection
Data collection took place between 2023 and 2024, beginning with the recruitment phase and followed by monthly check-ins. The duration of follow-up varied between individuals, ranging from a minimum of 2 months to a maximum of 7 months, depending on each participant’s clinical progress. This follow-up period variation reflects the frequency of parameters that require follow-up outside the nutritionist’s area of expertise (e.g., blood tests), as indicated by the attending physician, based on each participant’s progress. Furthermore, the notable improvements in lipid profiles, observed as early as two months after the initial assessment, support the rationale behind this specific aspect of the study.
Anthropometric measurements and body composition analyses and basal metabolic rate were performed using bioelectrical impedance analysis (Akern srl, Italy). The level of physical activity was estimated during the initial interview by determining the Metabolic Equivalent of Task (MET) value, a physiological measure that expresses the intensity of physical activities (26, 27). Menopausal women were found to be sedentary, while perimenopausal women were engaging in light physical activity. The metabolic rate and MET value were used to calculate the daily energy requirement for each participant. Biochemical parameters, including TC, HDL-C, LDL-C, and TG, were obtained from routine blood tests carried out independently by each participant at certified laboratories, based on recommendations provided by their general practitioners.
2.3 Dietary regimen
Dietary schemes were developed using commercial software (Progeo Medical® Software, Italy), and based on the 2018 revision of the Guidelines for a Healthy Diet, which is known for its effectiveness in managing lipid profiles and weight (28, 29), developed by the Council for Agricultural Research and Economics Analysis (CREA) - an agency that operates under the supervision of the Italian Ministry of Agriculture, Food Sovereignty and Forests. It is recommended to consume five balanced meals daily: breakfast, lunch, dinner, and two snacks. Suggested foods should be indicated by their raw weights and net of waste, and possible substitutions should be specified. Extra-virgin olive oil should be considered as a condiment fat. Furthermore, fats should be 25%–35% of total calories, with saturated fats <7% of total calories, polyunsaturated fats up to 10% of total calories, monounsaturated fats up to 20% of total calories, and cholesterol <200 mg/day. Based on these general recommendations and women’s lifestyle, age, and the level of physical activity, the nutritionist recommends a personalized dietary plan with an average daily caloric intake of 1,378 kcal for menopausal women and 1,479 kcal for those in perimenopause. In particular, a 500-kilocalorie (kcal) deficit was applied from the estimated daily energy requirement, allowing for a well-tolerated and healthy body weight loss (30). Specifically, macronutrient intake is structured as follows: 1.5 grams of protein per kilogram of lean body mass, 0.6 grams of fat per kilogram of total body weight, and carbohydrates are calculated based on the remaining caloric needs, with a daily intake of 20–30 grams of fiber.
During the observational period participants intake two tablets of an omega-3 supplement containing EPA (300 mg), DHA (200 mg), and vitamin E (2.5 mg) after the three main meals (breakfast, lunch and dinner) and one tablet of a phytosterol-based supplement containing phytosterols (400 mg), bergamot (200 mg), prickly pear (150 mg), and vitamin B1 (12.5 mg) per tablet after dinner. Adherence to the diet and supplement intake was self-reported.
2.4 Statistics
The sample size of the experimental group was evaluated using G*Power 3 software (31). The power analysis indicated that a sample size of 13 items would provide 80% power to detect a substantial effect size (Cohen’s d = 0.85) at an alpha level of 0.05. In our study, a high effect size is expected based on the characteristics of typical blood tests and anthropometric measures, which are highly standardized, reliable, and subject to minor periodic variations. Therefore, the sample size of this study is adequately powered to detect the effect, although it is at the limit of the recommended size. Raw data were analyzed by comparing the final follow-up (tf) available and initial evaluation (t0) values of each participant, for each parameter under investigation, using the paired t-test (considering a p-value ≤ 0.05 as statistically significant). The 95% confidence intervals (95% CI) were set for all estimates. Data from different individuals were then normalized and averaged in the same graph using GraphPad Prism 6 software (GraphPad Software, San Diego, CA, USA). Results were expressed as the mean ± SEM of the indicated n-values.
3 Results
3.1 Characterization of participants and personalized dietary plan approach
In this study, we examined data collected from a group of women in the menopausal (mean age 60.1 ± 2.7 years; Supplementary Table 1) or perimenopausal (mean age 52.8 ± 1.6) phase with dyslipidemia who followed a dietary plan and supplementation as part of routine nutritional care. The key markers, including TC, LDL-C, HDL-C, and TG values, used to assess the lipid profile, were recorded during a follow-up period based on the results of serum analyses prescribed by the primary care physician. Therefore, the tf for these values corresponds to a time window ranging from 2 to 7 months.
At t0, the average BMI of menopausal and perimenopausal participants was 26.8 ± 3.7 kg/m2 and 27.8 ± 3.7 kg/m2, respectively (Supplementary Table 1). Therefore, most participants started with a BMI in the overweight or obesity range (32).
The women’s lipid profile (Supplementary Table 1) revealed elevated TC and LDL-C levels and low HDL-C levels. In addition, 22% of menopausal women displayed elevated plasma TG levels.
Participants’ daily diet was modified according to the MD pattern, and the nutritionist incorporated omega-3 and phytosterol-based supplements into the routine plan. Results at tf reflected a positive and promising response to dietary intervention and showed improved cholesterol profiles in all participants in the study (Supplementary Table 2). Statistical analysis of pooled data revealed a significant reduction in TC (Figures 1A, B) and in the LDL-C levels (Figures 1C, D), and a significant increase in HDL-C levels (Figures 1E, F) in both the two menopause phases. The HDL-C increment, up to 65% in menopausal women and up to 58% in perimenopausal women (Supplementary Table 2), appeared particularly relevant because the threshold of 50 mg/dl is considered a diagnostic marker for metabolic syndrome and a risk factor for CVD. Based on this evidence, the TC/HDL-C ratio and plasma TG levels were further analyzed (Supplementary Table 3). All participants at t0 had a TC/HDL-C ratio above or close to 4.5, possibly associated with an elevated CVD risk. By the tf, most participants had lowered their TC/HDL-C ratios to values below 4.0, achieving the target for primary prevention. Statistical analysis confirmed a significant and robust reduction in the TC/HDL-C ratio in menopausal and perimenopausal women (Figures 2A, B, respectively). Furthermore, a significant decrease in TG levels in the menopausal group (Figure 2C) was revealed. On the contrary, no difference was achieved in perimenopausal participants (Figure 2D), probably due to data variability within this group.
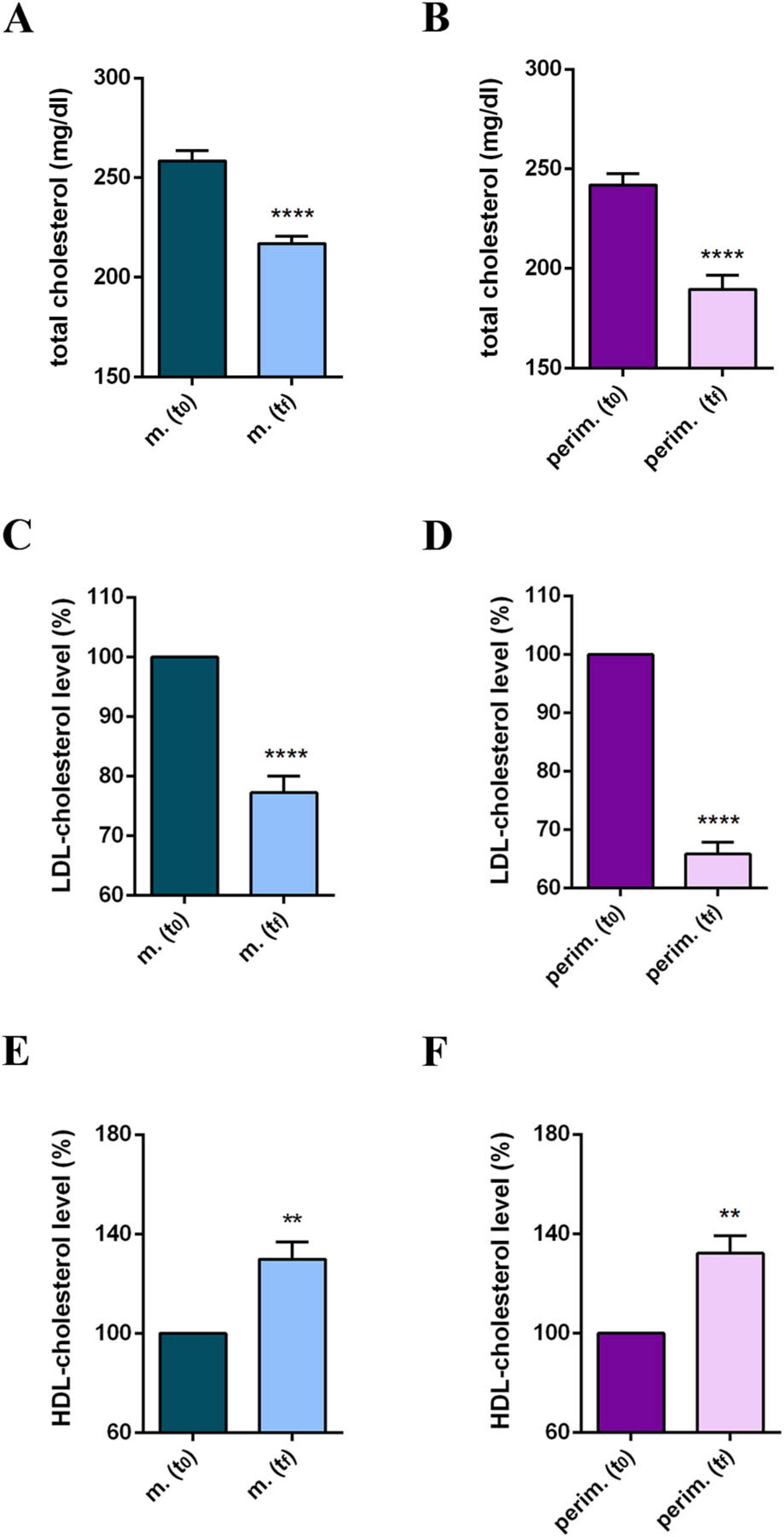
Figure 1. Cholesterol profile of m. (menopausal; n = 9) and perim. (perimenopausal; n = 5) groups at t0 (initial evaluation) and tf (final follow-up). Total cholesterol (mg/dl) levels of m. (A) and perim. (B) groups. LDL (low-density lipoprotein)-cholesterol levels in m. (C) and perim. (D) groups. HDL (high-density lipoprotein)-cholesterol levels in m. (E) and perim. (F) groups expressed as the percentage of the t0 group. Raw data were analyzed using the paired t-test (considering a p-value ≤ 0.05 as statistically significant). (A,B) 95% CI = [–49,55 to –33,34] and [–60,07 to –44,33], respectively. (C,D) 95% CI = [–54,55 to –29,41] and [–72,26 to –52,06], respectively. (E,F) 95% CI = [7,257 to 21,41] and [7,622 to 21,18], respectively. **p < 0.01 and ****p < 0.0001 vs t0.
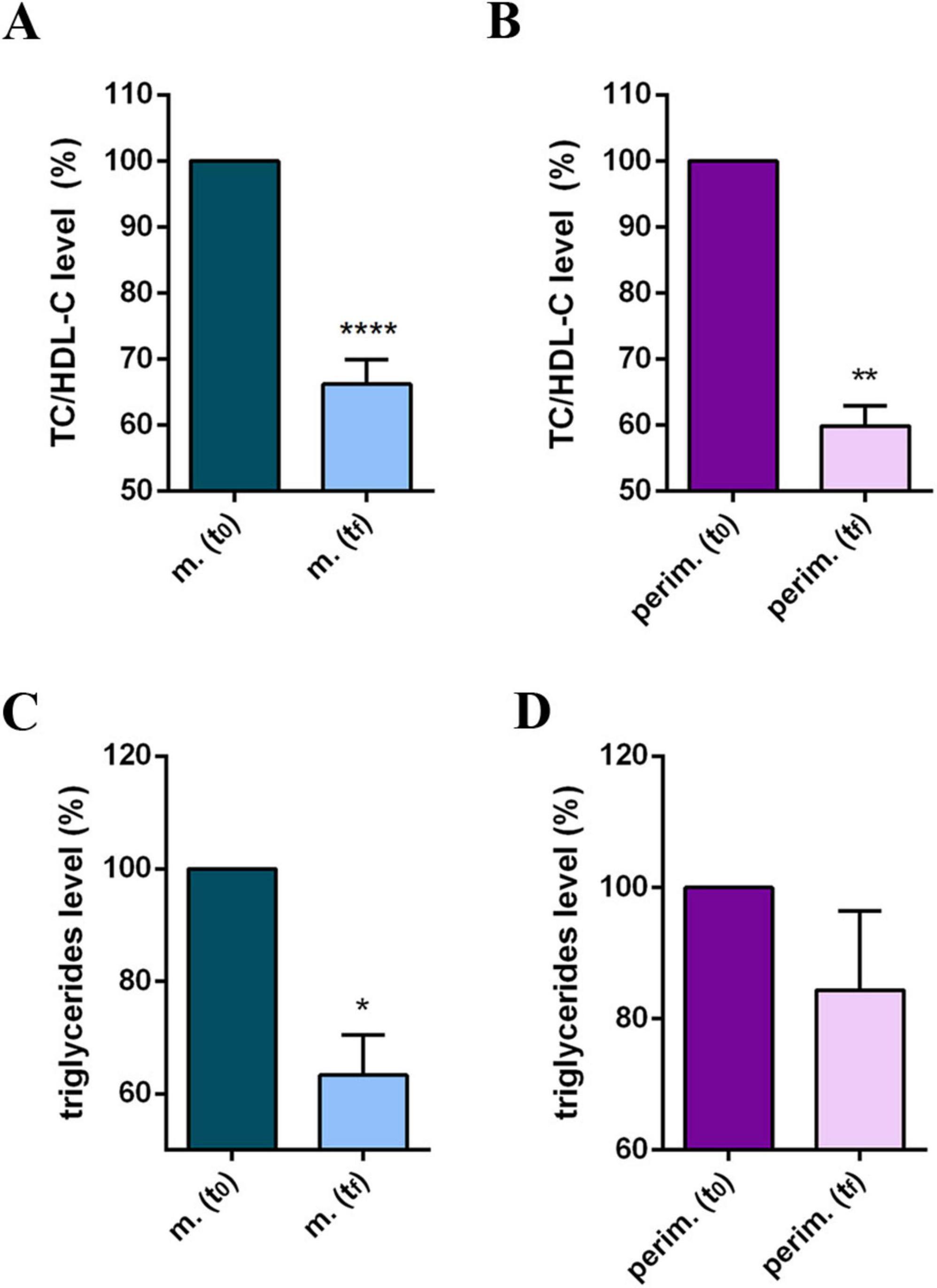
Figure 2. Lipid profile of m. (menopausal) and perim. (perimenopausal) groups at t0 (initial evaluation) and tf (final follow-up). TC (total cholesterol)/HDL-C (high-density lipoprotein-cholesterol) ratio of m. (A) and perim. (B) groups. Triglyceride levels in m. (C) and perim. (D) groups. Raw data were analyzed using the paired t-test (considering a p-value ≤ 0.05 as statistically significant). (A,B) 95% CI = [–2,31 to –1,26] and [–2,94 to –1,40], respectively. (C,D) 95% CI = [–100,7 to –10,61] and [–56,09 to 22,89], respectively. *p < 0.05, **p < 0.01, ****p < 0.0001 vs t0.
3.2 Anthropometric and body composition benefits following a personalized dietary plan and supplementation
The benefits of the dietary interventions were then assessed by anthropometric and body composition measurements and analysis (Supplementary Table 4).
The observed progress in BMI values is noteworthy, although not all women reached the healthy range of values. Additionally, a consistent fat mass (FM) reduction of up to 28% in menopausal and 32% in perimenopausal women was observed. Interestingly, FM reduction was also observed in a woman who failed to lose weight and had an overweight BMI. Statistical analysis confirmed a significant body weight (BW; Figures 3A, B), BMI (Figures 3C, D), and FM decrement (Figures 3E, F) in the two menopausal phases.
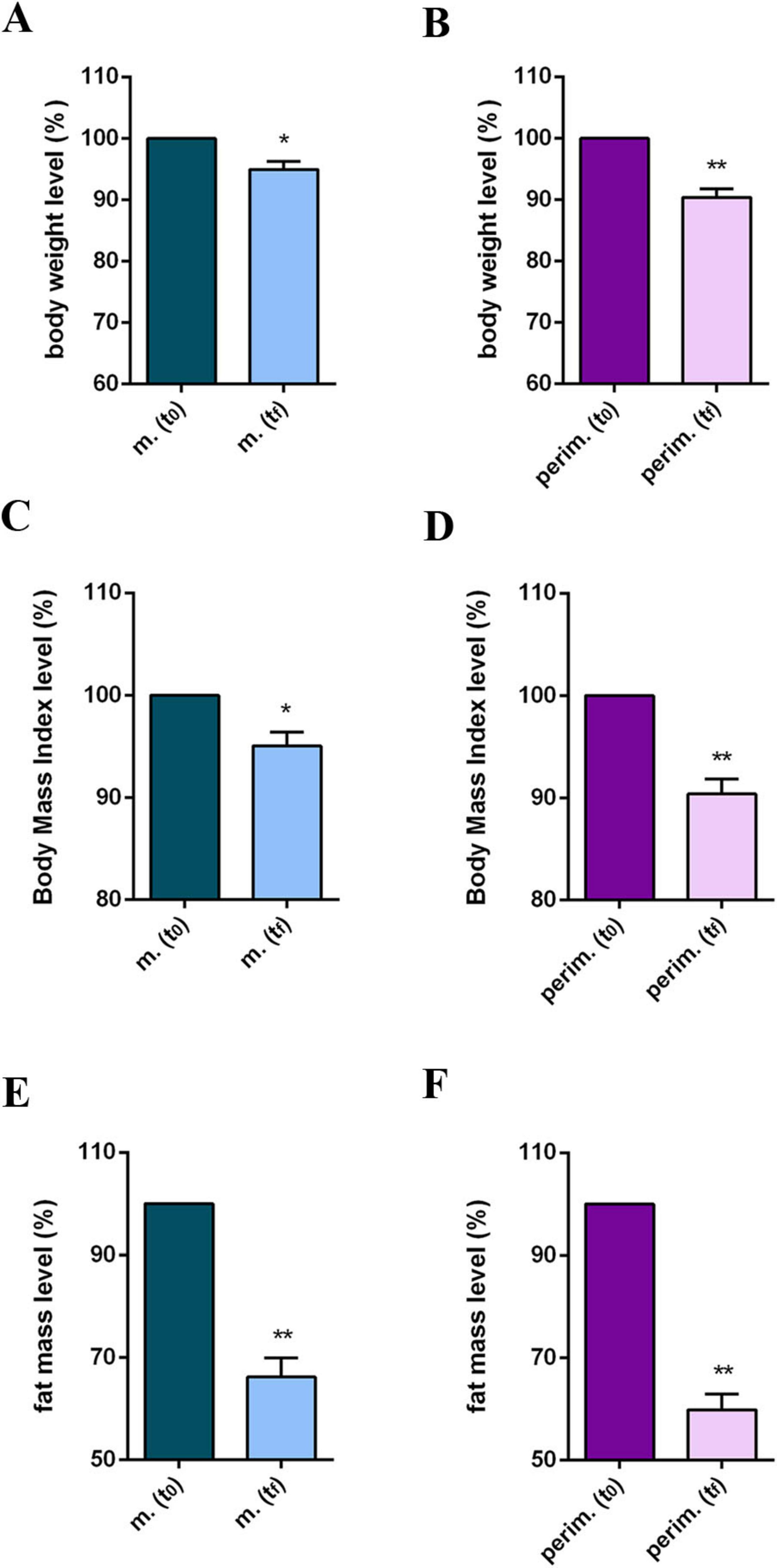
Figure 3. Anthropometric and body composition levels of m. (menopausal) and perim. (perimenopausal) groups at t0 (initial evaluation) and tf (final follow-up). Body weight levels of m. (A) and perim. (B) groups. Body mass index levels in m. (C) and perim. (D) groups. Fat mass levels in m. (E) and perim. (F) groups. Raw data were analyzed using the paired t-test (considering a p-value ≤ 0.05 as statistically significant). (A, B) 95% CI = [–6,71 to –0,27] and [–11,19 to –3,49], respectively. (C,D) 95% CI = [–2,59 to –0,39] and [–4,23 to –1,29], respectively. (E,F) 95% CI = [–6,55 to –2,00] and [–10,39 to –3,89], respectively. *p < 0.05, **p < 0.01 vs t0.
Since waist circumference (WC) is a key parameter for diagnosing metabolic syndrome, and it is particularly problematic for women in the menopausal phase, we analyzed WC measurements and waist-to-hip ratio (WHR) values (Supplementary Table 5). Additionally, the BCM, as a metabolically active mass index, was also evaluated. Most women achieved a WC below 88 cm, the threshold for diagnosing metabolic syndrome, and a slight decrease in WHR. Additionally, a positive increase in BCM values was observed in nearly all women, higher than 10% in some cases, and slightly positive in others. The statistical analysis of WC (Figures 4A, B) and WHR (Figures 4C, D) parameters confirmed a positive progress, although menopausal women had a less robust reduction than perimenopausal women. No statistically significant differences were obtained in pooled data for BCM at tf (Figures 4E, F).
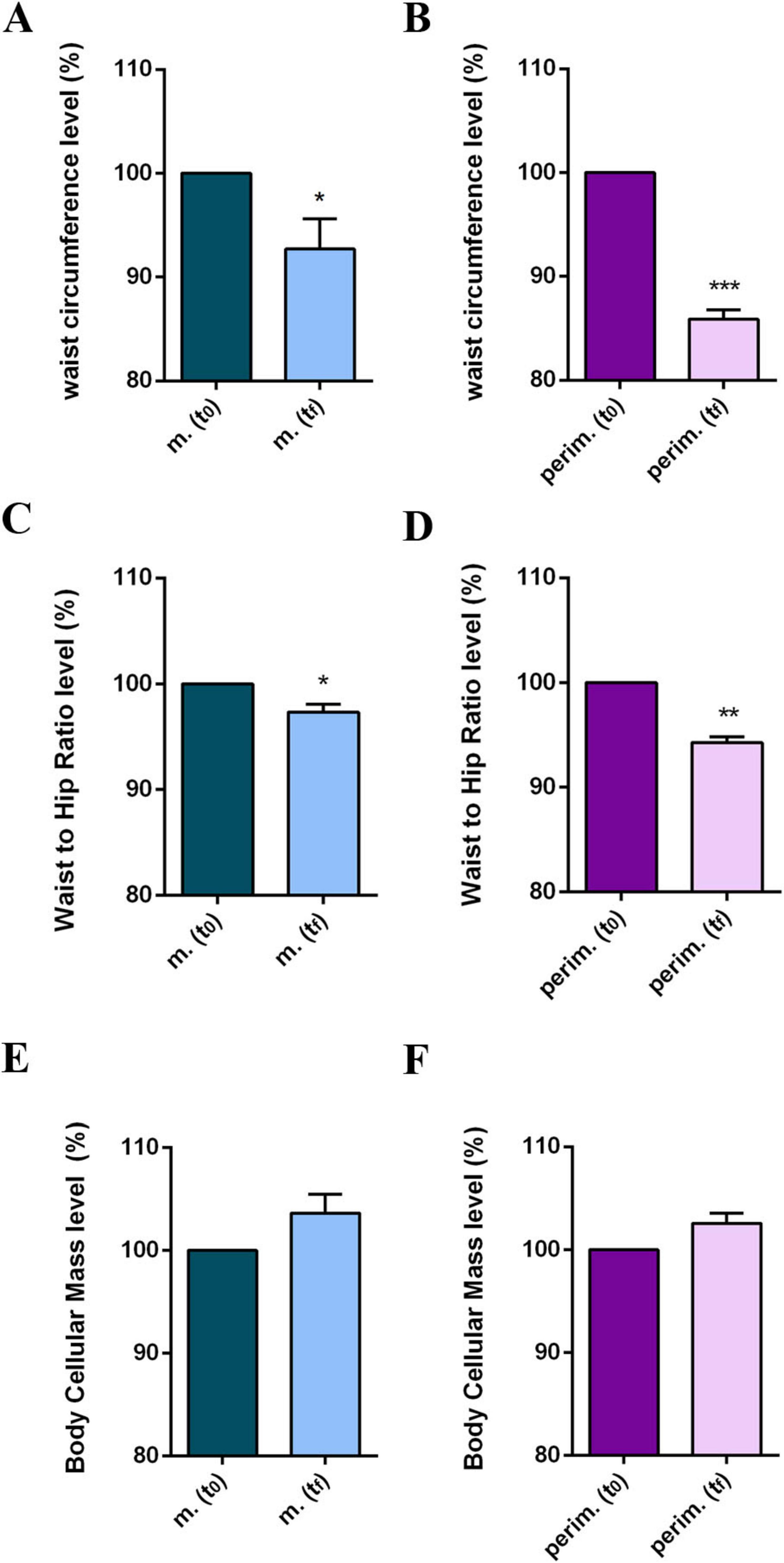
Figure 4. Anthropometric and body composition levels of m. (menopausal) and perim. (perimenopausal) groups at t0 (initial evaluation) and tf (final follow-up). Waist circumference of m. (A) and perim. (B) Groups. Waist to hip ratio in m. (C) and perim. (D) groups. Body cellular mass in m. (E) and perim. (F) groups. Raw data were analyzed using the paired t-test (considering a p-value ≤ 0.05 as statistically significant). (A,B) 95% CI = [–12,93 to –0,40] and [–16,19 to –8,61], respectively. (C,D) 95% CI = [–0,038 to –0,007] and [–0,063 to –0,029], respectively. (E,F) 95% CI = [–0,145 to 1,900] and [–0,088 to 1,408], respectively. *p < 0.05, **p < 0.01, ***p < 0.001 vs t0.
4 Discussion
4.1 Mediterranean diet and supplementation improve lipid profile in perimenopausal and menopausal women
The findings of this preliminary study, conducted on a convenience sample of peri- and menopausal women, highlight how a personalized dietary intervention based on the MD, supplemented with phytosterols and omega-3 fatty acids, suggests potential for improving the lipid profile, including total TC, LDL-C, HDL-C, and TG levels. Our findings support previous positive claims about the MD, which modulates metabolic and molecular mechanisms that promote health, reducing lipid levels, protecting against oxidative stress, inflammation, and the risk of developing multiple chronic diseases (33, 34). The MD includes fruits, whole grains, legumes, minimally processed plant foods, and low saturated fatty acids. Dried fruits are an excellent source of omega-6 and omega-3 fatty acids, which can improve the lipid profile and reduce the risk of atherosclerosis. In menopausal women, supplementation with omega-3 fatty acids significantly reduced TG levels and slightly elevated HDL-C and LDL-C levels without affecting TC values (35). Our results demonstrate that supplementation with phytosterol and omega-3 fatty acids may contribute to the modulation of the lipid profile, including the TC/HDL-C ratio, in menopausal and perimenopausal women. On the contrary, we also observed a decrease in LDL values, probably due to the diet regimen and the supplementation of phytosterols, thus supporting the efficacy of this nutritional approach. Surprisingly, we observed a considerable increase in HDL-cholesterol. Possibly, among the complexities of the HDL-cholesterol balance, a personalized approach, adherence to the Mediterranean diet style, a correct balance of nutrients, and the use of supplements could play a role. Since adherence to a dietary plan is a critical factor in its success (23), the Mediterranean diet is a suitable choice, as it has been shown to promote long-term adherence and a balanced intake of nutrients (36).
4.2 Mediterranean diet and supplementation improve anthropometric and body composition in perimenopausal and menopausal women
Our results showed a reduction in body FM but a modest decrease in BW and BMI. Since BMI does not differentiate between lean and FM and may not be as accurate across different age groups, WC may be a more effective tool for identifying individuals at higher risk of cardiovascular diseases or adverse health outcomes (37). Additionally, it has been reported that reducing visceral fat can be achieved through lifestyle modifications, even without significant BW loss (38). Therefore, it is rational that WC reduction might be a more desirable therapeutic goal than simple weight loss. Our results support this claim, demonstrating a significant WC and WHR decrease. In this context, the MD dietary pattern combined with physical activity has shown promising results in managing obesity and menopausal symptoms (39), promoting weight loss maintenance (40, 41), and preventing weight gain, even without energy restriction (34). In menopausal women, maintaining a balanced diet and engaging in moderate physical activity are crucial for overall health. Recent observations suggest that strict adherence to the MD enables menopausal women to improve body composition and reduce FM while preserving muscle mass (42). Our data confirms a positive trend in metabolically active mass, thereby supporting the benefits of the MD and lifestyle.
Collectively, the data from this study highlight the importance of proper nutritional management during the period leading up to menopause and during the early stages of menopause. The MD dietary and supplement approach suggests potential for improving body composition, anthropometric parameters, and lipid profile when combined with specific hypolipidemic nutraceuticals derived from Mediterranean plant extracts, even in the short term. Symptoms during the perimenopausal and menopausal phases could become disabling disorders and possibly deteriorate health; therefore, the period of menopausal transition could be an optimal window to promote long-term healthiness. Omega-3 fatty acid intakes produce a promising positive impact on several menopause symptoms (43). Moreover, emerging evidence suggests that combining natural bioactive compounds targeting complementary pathways, particularly those containing phytosterols, may offer an effective alternative for managing obesity and improving cardiometabolic health in menopausal women (44). Thus, supporting the general benefit of the supplement approach used in this study.
Considering the significance of all these aspects for women during the menopausal period, these preliminary observations deserve further investigation and a deeper analysis. Although the sample size represents a weakness point of this pilot study, a priori power analysis and statistical analysis of the data obtained support the feasibility of our approach and the strength of the results obtained. At the same time, the primary aim of this pilot observational study was to lay the ground for more comprehensive future investigations. Therefore, to improve the validity and reliability of the results, future studies should consider a larger sample size and account for ethnic and cultural variability. Furthermore, future investigations should acknowledge the methodological limitations inherent in the non-standardized interviews conducted in this observational study. Although comparable questions were administered to all participants, this represents a methodological limitation that has to be considered.
During the observational period, adherence to the diet and supplement intake was self-reported and not supervised. Although it is typical in observational studies, this limitation needs to be considered in future deeper analyses. To note that participants were informed about the chance to use their outcomes for research purposes after the conclusion of the study, thereby minimizing the risk of reporting bias. Nevertheless, despite the limitations of this observational study, the approach described here could play a key role in reducing the risk of cardiovascular and metabolic diseases. It encourages natural supplements to manage dyslipidemia when conventional medical therapies are not yet necessary in perimenopausal and menopausal women. Thus, it offers valuable insights for managing mild hypercholesterolemia/hyperlipidemia in the proximity of the first period of menopause.
Data availability statement
The original contributions presented in this study are included in this article/Supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
Ethical approval was not required for the study involving humans in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and the institutional requirements.
Author contributions
FC: Conceptualization, Writing – review & editing, Methodology, Data curation, Writing – original draft, Investigation, Formal analysis. EA: Formal analysis, Investigation, Writing – review & editing, Writing – original draft, Data curation, Conceptualization. KB: Writing – review & editing, Writing – original draft. SD: Writing – review & editing, Writing – original draft. DB: Writing – review & editing, Writing – original draft. DC: Writing – original draft, Formal analysis, Data curation, Conceptualization, Writing – review & editing. EC: Data curation, Conceptualization, Writing – review & editing, Writing – original draft, Formal analysis.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2025.1645102/full#supplementary-material
References
1. Davis S, Pinkerton J, Santoro N, Simoncini T. Menopause-biology, consequences, supportive care, and therapeutic options. Cell. (2023) 186:4038–58. doi: 10.1016/j.cell.2023.08.016
2. Peeples L. The new science of menopause: these emerging therapies could change women’s health. Nature. (2025) 637:782–4. doi: 10.1038/d41586-025-00069-4
3. Torosyan N, Visrodia P, Torbati T, Minissian M, Shufelt C. Dyslipidemia in midlife women: approach and considerations during the menopausal transition. Maturitas. (2022) 166:14–20. doi: 10.1016/j.maturitas.2022.08.001
4. Crandall C, Mehta J, Manson J. Management of menopausal symptoms: a review. JAMA. (2023) 329:405–20. doi: 10.1001/jama.2022.24140
5. Yelland S, Steenson S, Creedon A, Stanner S. The role of diet in managing menopausal symptoms: a narrative review. Nutr Bull. (2023) 48:43–65. doi: 10.1111/nbu.12607
6. Villar-López M, Soto-Becerra P, Chedraui P, Osorio-Manyari J, Al-Kassab-Córdova A, Osorio-Manyari A, et al. Short-term effects and safety of a natural oral supplement containing glucosinolates, phytosterols, and citrus flavonoids compared with hormone treatment for the management of postmenopausal symptomatic women: a pilot single-center randomized phase 2 clinical trial. Menopause. (2023) 30:1230–40. doi: 10.1097/GME.0000000000002268
7. Ko S, Kim H. Menopause-associated lipid metabolic disorders and foods beneficial for postmenopausal women. Nutrients. (2020) 12:202. doi: 10.3390/nu12010202
8. Kushkestani M, Moghadassi M, Sidossis L. Mediterranean lifestyle: more than a diet, a way of living (and thriving). Endocr Metab Immune Disord Drug Targets. (2024) 24:1785–93. doi: 10.2174/0118715303279769240215105510
9. Mach F, Baigent C, Catapano A, Koskinas K, Casula M, Badimon L, et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. (2020) 41:111–88. doi: 10.1093/eurheartj/ehz455
10. Guasch-Ferré M, Willett W. The mediterranean diet and health: a comprehensive overview. J Intern Med. (2021) 290:549–66. doi: 10.1111/joim.13333
11. Barrea L, Pugliese G, Laudisio D, Colao A, Savastano S, Muscogiuri G. Mediterranean diet as medical prescription in menopausal women with obesity: a practical guide for nutritionists. Crit Rev Food Sci Nutr. (2021) 61:1201–11. doi: 10.1080/10408398.2020.1755220
12. Silva T, Oppermann K, Reis F, Spritzer P. Nutrition in menopausal women: a narrative review. Nutrients. (2021) 13:2149. doi: 10.3390/nu13072149
13. Adorisio S, Muscari I, Fierabracci A, Thi Thuy T, Marchetti M, Ayroldi E, et al. Biological effects of bergamot and its potential therapeutic use as an anti-inflammatory, antioxidant, and anticancer agent. Pharm Biol. (2023) 61:639–46. doi: 10.1080/13880209.2023.2197010
14. Patti A, Al-Rasadi K, Giglio R, Nikolic D, Mannina C, Castellino G, et al. Natural approaches in metabolic syndrome management. Arch Med Sci. (2018) 14:422–41. doi: 10.5114/aoms.2017.68717
15. Palacio T, Siqueira J, de Paula B, Rego R, Vieira T, Baron G, et al. Bergamot (Citrus bergamia) leaf extract improves metabolic, antioxidant and anti-inflammatory activity in skeletal muscles in a metabolic syndrome experimental model. Int J Food Sci Nutr. (2023) 74:64–71. doi: 10.1080/09637486.2022.2154328
16. Giglio R, Patti A, Nikolic D, Li Volti G, Al-Rasadi K, Katsiki N, et al. The effect of bergamot on dyslipidemia. Phytomedicine. (2016) 23:1175–81. doi: 10.1016/j.phymed.2015.12.005
17. Nakandakare-Maia E, Siqueira J, Ferron A, Vieira T, Palacio T, Grandini N, et al. Treatment with bergamot (Citrus bergamia) leaves extract attenuates leptin resistance in obese rats. Mol Cell Endocrinol. (2023) 566-567:566-67:111908. doi: 10.1016/j.mce.2023.111908
18. Pierdomenico M, Cicero A, Veronesi M, Fogacci F, Riccioni C, Benassi B. Effect of Citrus bergamia extract on lipid profile: a combined in vitro and human study. Phytother Res. (2023) 37:4185–95. doi: 10.1002/ptr.7897
19. Fogacci F, Di Micoli V, Veronesi M, Cicero A. Comparative effect of a nutraceutical compound based on a flavonoid complex from bergamot on plasma lipids, glucose metabolism, and liver enzymes: a 3-arm, double-blind, placebo-controlled, randomized clinical trial. Arch Med Sci. (2023) 19:1180–5. doi: 10.5114/aoms/152791
20. Giglio R, Carruba G, Cicero A, Banach M, Patti A, Nikolic D, et al. Pasta supplemented with opuntia ficus-indica extract improves metabolic parameters and reduces atherogenic small dense low-density lipoproteins in patients with risk factors for the metabolic syndrome: a four-week intervention study. Metabolites. (2020) 10:428. doi: 10.3390/metabo10110428
21. Gouws C, Mortazavi R, Mellor D, McKune A, Naumovski N. The effects of Prickly Pear fruit and cladode (Opuntia spp.) consumption on blood lipids: a systematic review. Complement Ther Med. (2020) 50:102384. doi: 10.1016/j.ctim.2020.102384
22. Silva M, Albuquerque T, Pereira P, Ramalho R, Vicente F, Oliveira M, et al. Opuntia ficus-indica (L.) Mill.: a multi-benefit potential to be exploited. Molecules. (2021) 26:951. doi: 10.3390/molecules26040951
23. Thom G, Lean M. Is there an optimal diet for weight management and metabolic health? Gastroenterology. (2017) 152:1739–51. doi: 10.1053/j.gastro.2017.01.056
24. Sam-Yellowe T. Nutritional barriers to the adherence to the mediterranean diet in non-mediterranean populations. Foods. (2024) 13:1750. doi: 10.3390/foods13111750
25. Tutor A, O’Keefe E, Lavie C, Elagizi A, Milani R, O’Keefe J. Omega-3 fatty acids in primary and secondary prevention of cardiovascular diseases. Prog Cardiovasc Dis. (2024) 84:19–26. doi: 10.1016/j.pcad.2024.03.009
26. Herrmann S, Willis E, Ainsworth B, Barreira T, Hastert M, Kracht C, et al. 2024 adult compendium of physical activities: a third update of the energy costs of human activities. J Sport Health Sci. (2024) 13:6–12. doi: 10.1016/j.jshs.2023.10.010
27. Bull F, Al-Ansari S, Biddle S, Borodulin K, Buman M, Cardon G, et al. World health organization 2020 guidelines on physical activity and sedentary behaviour. Br J Sports Med. (2020) 54:1451–62. doi: 10.1136/bjsports-2020-102955
28. Rossi L, Berni Canani S, Censi L, Gennaro L, Leclercq C, Scognamiglio U, et al. The 2018 revision of Italian dietary guidelines: development process, novelties, main recommendations, and policy implications. Front Nutr. (2022) 9:861526. doi: 10.3389/fnut.2022.861526
29. CREA. Centro di Ricerca Alimenti e la Nutrizione. Linee Guida per Una Sana Alimentazione. (2018). Available online at: https://www.crea.gov.it/en/web/alimenti-e-nutrizione/-/linee-guida-per-una-sana-alimentazione-2018 (accessed May 14, 2025).
30. Finkler E, Heymsfield S, St-Onge M. Rate of weight loss can be predicted by patient characteristics and intervention strategies. J Acad Nutr Diet. (2012) 112:75–80. doi: 10.1016/j.jada.2011.08.034
31. Faul F, Erdfelder E, Lang A, Buchner AG. *Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. (2007) 39:175–91. doi: 10.3758/bf03193146
32. World Health Organization. Regional Office for Europe. WHO European Regional Obesity Report 2022. Geneva: World Health Organization (2022).
33. Tosti V, Bertozzi B, Fontana L. Health benefits of the mediterranean diet: metabolic and molecular mechanisms. J Gerontol A Biol Sci Med Sci. (2018) 73:318–26. doi: 10.1093/gerona/glx227
34. Muscogiuri G, Verde L, Sulu C, Katsiki N, Hassapidou M, Frias-Toral E, et al. Mediterranean diet and obesity-related disorders: what is the evidence? Curr Obes Rep. (2022) 11:287–304. doi: 10.1007/s13679-022-00481-1
35. Wang J, Gaman M, Albadawi N, Salem A, Kord-Varkaneh H, Okunade K, et al. Does omega-3 fatty acid supplementation have favorable effects on the lipid profile in postmenopausal women? A systematic review and dose-response meta-analysis of randomized controlled trials. Clin Ther. (2023) 45:e74–87. doi: 10.1016/j.clinthera.2022.12.009
36. Galasso M, Verde L, Barrea L, Savastano S, Colao A, Frühbeck G, et al. The impact of different nutritional approaches on body composition in people living with obesity. Curr Obes Rep. (2025) 14:45. doi: 10.1007/s13679-025-00636-w
37. Yoo J, Han K, Jung J, Hur Y, Kim Y, Kim E, et al. Body mass index, waist circumference and cardiovascular diseases in transitional ages (40 and 66 years). J Cachexia Sarcopenia Muscle. (2023) 14:369–81. doi: 10.1002/jcsm.13138
38. Ross R, Neeland I, Yamashita S, Shai I, Seidell J, Magni P, et al. Waist circumference as a vital sign in clinical practice: a consensus statement from the IAS and ICCR working group on visceral obesity. Nat Rev Endocrinol. (2020) 16:177–89. doi: 10.1038/s41574-019-0310-7
39. Pugliese G, Barrea L, Laudisio D, Aprano S, Castellucci B, Framondi L, et al. Mediterranean diet as tool to manage obesity in menopause: a narrative review. Nutrition. (2020) 79-80:110991. doi: 10.1016/j.nut.2020.110991
40. Poulimeneas D, Anastasiou C, Santos I, Hill J, Panagiotakos D, Yannakoulia M. Exploring the relationship between the mediterranean diet and weight loss maintenance: the MedWeight study. Br J Nutr. (2020) 124:874–80. doi: 10.1017/S0007114520001798
41. Dominguez L, Veronese N, Di Bella G, Cusumano C, Parisi A, Tagliaferri F, et al. Mediterranean diet in the management and prevention of obesity. Exp Gerontol. (2023) 174:112121. doi: 10.1016/j.exger.2023.112121
42. Lombardo M, Perrone M, Guseva E, Aulisa G, Padua E, Bellia C, et al. Losing weight after menopause with minimal aerobic training and mediterranean diet. Nutrients. (2020) 12:2471. doi: 10.3390/nu12082471
43. Minihane A. Omega-3 fatty acids, brain health and the menopause. Post Reprod Health. (2025) 31:97–104. doi: 10.1177/20533691251341701
Keywords: menopause, dyslipidemia, cholesterol, Mediterranean diet, supplement
Citation: Cannella F, Algaria EA, Brunetti K, Del Quondam S, Bottan D, Cervia D and Catalani E (2025) Synergistic effects of Mediterranean diet combined with phytosterol-based supplements and omega-3 fatty acids on lipid profiles: a pilot study in menopausal women. Front. Nutr. 12:1645102. doi: 10.3389/fnut.2025.1645102
Received: 11 June 2025; Accepted: 01 September 2025;
Published: 11 September 2025.
Edited by:
Eva Szabo, University of Pécs, HungaryReviewed by:
Zakira Naureen, University of Nizwa, OmanRicardo Santos, University of Porto, Portugal
Copyright © 2025 Cannella, Algaria, Brunetti, Del Quondam, Bottan, Cervia and Catalani. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Elisabetta Catalani, ZWNhdGFsYW5pQHVuaXR1cy5pdA==
 Fabiana Cannella1
Fabiana Cannella1 Elisa Assunta Algaria
Elisa Assunta Algaria Davide Cervia
Davide Cervia Elisabetta Catalani
Elisabetta Catalani