- 1Department of General Surgery and Obesity and Metabolic Center, China-Japan Friendship Hospital, Beijing, China
- 2Medical College, Yanbian University, Yanbian, Jilin, China
- 3Graduate School, Beijing University of Chinese Medicine, Beijing, China
Background: The effects of resistant starch (RS) consumption on anthropometric and serum biomarkers in adults with metabolic syndrome (MetS)-related risks, each component of which similarly increases the incidence of cardiovascular disease, have yielded inconclusive results when compared to anticipated outcomes. The heterogenous effects of RS type, delivery mode, participant characteristics, intervention conditions, and the quality of study design on the observed outcomes are considered to be insufficiently understood.
Methods: A comprehensive search was conducted in five public databases and 30 previously published meta-analyses up to January 21, 2025, following the PRISMA guidelines. A total of 23 parallel or crossover randomized controlled trials were included for qualitative analysis via Cochrane Risk of Bias tool and the Jadad scale. Among, 19 studies were included for synthesizing effect sizes of changes in anthropometric parameters, glycemic and lipid profiles, inflammatory markers, and oxidative stress biomarkers using a random-effects model. Subgroup analysis was performed to explore contributes of heterogeneity. Sensitivity analysis and publication bias analysis were conducted.
Results: RS consumption was associated with significant reductions in hip circumference (MD = −1.83 cm; 95% CI: −2.03 to −1.64), total cholesterol (MD = −0.20 mmol/L; 95% CI: −0.32 to −0.08), low-density lipoprotein cholesterol (MD = −0.11 mmol/L; 95% CI: −0.18 to −0.04), and improved superoxide dismutase levels (SMD = 0.29; 95% CI: 0.08–0.51). Waist circumference, fasting insulin, HOMA-IR, and TNF-α were reduced by RS with high heterogeneity yet. High quality of study design, participants with younger age and overweight, a supplement as delivery, a dose of up to 30 g/day, and lasting over 8 weeks partly influenced the effects.
Conclusion: Steady effects of RS were observed on hip circumference, total cholesterol, low-density lipoprotein cholesterol, and superoxide dismutase in adults with MetS-related risks. For the intervention with RS, it is recommended that participants be younger and overweight, with a dosage of at least 30 g/day, and over a period of 8 weeks. Future studies should be designed with high methodological quality, with considerations of delivery mode, properties, as well as gut microbiome and metabolome.
Systematic review registration: https://www.crd.york.ac.uk/PROSPERO/view/CRD420251014654 CRD420251014654.
1 Introduction
Metabolic syndrome (MetS) constitutes a major global public health challenge in adults, feeding into various chronic diseases, especially cardiovascular disease (CVD) (1). The common components of MetS consist of visceral obesity, hyperglycemia, hypertension, low high-density lipoprotein cholesterol (HDL-C), raised triglycerides (TG), according to Adult Treatment Panel III and International Diabetes Federation guidelines (2). Excessive body mass index (BMI) is considered as another component based on American Association of Clinical Endocrinologists guideline (3). Either each component of MetS or the combination together serves as high risk factors for the development of CVD (4). A 13-year prospective study has reported that either MetS or its single components increased similar incidence of cardiovascular events by 20–60% (5). Inflammation and oxidative stress are involved in the development from MetS to CVD (6). Therefore, the prompt treatment and management of MetS-related risks is essential for preventing cardiovascular complications and alleviating metabolic burden.
Resistant starch (RS) is characterized as a type of starch that remains undigested in the small intestine and undergoes fermentation in the large intestine by producing short-chain fatty acids (SCFAs) (7). Evidence has shown RS can improve cardiometabolic outcomes and attenuates MetS risk-related diseases, such as overweight or obesity (8), prediabetes or type 2 diabetes mellitus (T2DM) (9), hyperlipidemia (10) and non-alcoholic fatty liver disease (NAFLD) (11) to varying degrees. RS improves cardiometabolic function including lowered body weight (BW), BMI, body dimensions and fat composition (12), stabled blood pressure (13), improved glycemic control and insulin sensitivity (14, 15), lowered lipid profiles (10, 16), and reduced inflammation and oxidative stress levels (17, 18). Therefore, RS is considered as a functional food with benefits for mitigating MetS-related risks. Due to its resistance to digestion in both native and modified forms, RS is categorized into five types, known as RS1 to RS5 (19). RS1 is a formation of natural starch granules that is encapsulated within indigestible plant structures, such as the cell wall or proteins, which physically hinder the interaction between RS1 and digestive enzymes. RS1 primarily exists in whole grains and beans that are not milled thoroughly. RS2 is a type of natural starch granules in raw potatoes or green bananas, with high content of amylose, high starch density and a unique crystalline form. The property of RS2 exhibits resistance to hydrolysis by digestive enzymes to some extent. RS3, a type of retrograded starch, is produced when the starch is heated to gelatinization and then undergoes a retrogradation process at a low temperature. The gelatinization-retrogradation cycle in starch creates a crystal structure that resisted digestion by enzymes. RS4 is resistant to digestive enzymes by chemically altering the functional groups or adding new functional groups of original starch, leading to the formation of carboxymethyl starch, starch ether, starch ester, and cross-linked starch. Combination of the extended branches of amylose with fatty acids generated RS5, a starch-FA complex, where the helical structure is hardly digested by amylase. These native and modified forms can affect functionality, digestibility and fermentability in a food product (20, 21). The previous review has taken insight into the impact of properties on physiological effects and mechanisms specific to each type of RS (22). In contrast, a recent review has qualitatively discussed that RS2 and RS3 are mostly examined their cardiometabolic effects in randomized controlled trials (RCTs), highlighting the inconclusive results (23). Overall, challenges still persist in understanding the functionality of RS and in accurately reporting its effects.
Due to the research gap between the expected functionality and actual effects of RS, this systematic review and meta-analysis on the effects of RS consumption on anthropometric and serum parameters in adults with MetS-related risks was necessary. Therefore, the review aimed to qualitatively and quantitatively assess the effects of RS consumption on cardiometabolic outcomes including anthropometric parameters, serum glycemic and lipid profiles, serum inflammatory factors, and serum oxidative stress biomarkers. Although RS effects on health are well-conceived, we assumed that studies were heterogeneous in terms of type of RS, delivery mode, participant characteristics, and dose and duration of intervention, which could influence the treatment outcomes. Hence, subgroup analysis on aforementioned factors contributing to heterogeneity was explored.
2 Materials and methods
2.1 Retrieve identification
This review was conducted in accordance with the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines. A comprehensive retrospective search was performed across five major literature databases—PubMed, Web of Science, Cochrane Library, Embase, and Scopus—covering publications up to January 21, 2025. The search strategy of five public databases was provided in Supplementary Table 1. In addition, all relevant systematic reviews containing applicable biomarkers or outcome indicators were manually screened to ensure that no eligible studies were omitted.
2.2 Literature screening and eligibility
Two independent investigators screened the literature and assessed study eligibility based on predefined inclusion and exclusion criteria. In cases of disagreement, a third investigator was consulted to reach a consensus. The initial screening was performed by reviewing titles and abstracts for references to treatment efficacy or biological effects. Studies were included if they met the following criteria:
1. published in English;
2. involved participants with MetS-related risks, including overweight, obesity, insulin resistance, MetS, prediabetes, T2DM, hyperlipidemia, or NAFLD;
3. employed a RCT assignment, either parallel or crossover;
4. enrolled adult participants;
5. included an intervention group receiving RS supplementation and a control group receiving either a placebo or standard starch;
6. had a minimum intervention duration of one week;
7. provided sufficient outcome data for effect size estimation, including anthropometric, glycemic, lipid, inflammatory, or oxidative stress markers.
Studies were excluded if they met any of the following criteria:
1. were non-original publications or duplicate reports;
2. used resistant dextrin instead of resistant starch as the intervention;
3. did not report the RS dosage clearly or reported the lifestyle recommendation;
4. the full text was not accessible.
2.3 Data extraction and characterization
Essential data were extracted from each included study, including the first author, year of publication, trial registration ID (if available), assignment model, study population, intervention details (RS type, dose and total intake), control substance characteristics (dose and total intake), intervention duration, and any reported washout period. Participant characteristics were recorded, including the total sample size, allocation to intervention and control groups, sex distribution, mean age, BW, and BMI. Effect sizes and safety outcomes were also collected. Effect sizes were categorized into five domains: (1) Anthropometric parameters, including BW, BMI, waist circumference (WC), hip circumference (HC), waist-to-hip ratio (WHR), fat mass (FM), body fat percentage, diastolic blood pressure (DBP), and systolic blood pressure (SBP); (2) Glycemic profiles, comprising fasting blood glucose (FBG), fasting insulin (FINS), glycated hemoglobin (HbA1c), homeostatic model assessment of insulin resistance (HOMA-IR), and beta-cell function (HOMA-β); (3) Lipid profiles, including TG, total cholesterol (TC), HDL-C, and low-density lipoprotein cholesterol (LDL-C); (4) Inflammatory markers, such as high-sensitivity C-reactive protein (hs-CRP), tumor necrosis factor-alpha (TNF-α), and interleukin-6 (IL-6); (5) Oxidative stress biomarkers, specifically malondialdehyde (MDA) and superoxide dismutase (SOD). Concerning their critical role in functionality, the parameters of intervention substances, including food source, purity, content analytical method, delivery mode, and feasibility, were also reported.
2.4 Quality assessment
Risk of bias was assessed using the Cochrane Risk of Bias (ROB) tool implemented in Review Manager version 5.3. The tool includes seven domains: (1) random sequence generation, (2) allocation concealment, (3) blinding of participants and personnel, (4) blinding of outcome assessment, (5) incomplete outcome data, (6) selective reporting, and (7) other sources of bias. The overall ROB judgment of each study was provided based on the previous domains. The Jadad scale was used to evaluate methodological quality. The scale awards up to five points based on the following criteria: (1) randomization (1 point for stating randomization, plus 1 additional point for appropriate randomization methods); (2) blinding (1 point for stating double-blinding, plus 1 additional point for appropriate blinding methods); and (3) description of withdrawals and dropouts (1 point for reporting the number and reasons for withdrawals).
2.5 Statistical analysis
Effect sizes were calculated by comparing the mean net changes between the intervention and control groups. The standard deviation (SD) of the net change was estimated using the following formula:
A correction coefficient (R) of 0.5 was assumed for the calculation of standard deviations. For studies that presented data using the median and interquartile range, established mathematical methods were employed to transform these values into the mean and SD (24, 25). If standard error (SE) was reported in some studies, the formula between SD and SE was used to convert to SD using the following formula:
Here, n meant the number of participants in the group. For studies presenting effect sizes in bar charts, mean values and SDs were extracted using ImageJ software. When outcomes were reported separately for subgroups (e.g., males and females), combined means and SDs were calculated using an online statistical tool.1 Each effect size was expressed as mean difference (MD) with corresponding 95% confidence intervals (CI). In cases where units could not be standardized across studies or when there were substantial differences in measurement scales, standard mean difference (SMD) with 95% CI were calculated. A random-effects model was employed for all meta-analyses to account for inter-study variability. Statistical heterogeneity was assessed using the I2 statistic, with values <50% considered low heterogeneity and ≥50% considered high. To explore potential sources of heterogeneity, subgroup analysis was conducted based on RS type (RS2 or RS3), delivery mode (supplement or food), dose (<30 g/day or ≥30 g/day) and duration (<8 weeks or ≥8 weeks) of intervention, geographic region (western developed countries or others), disease type (overweight/obesity, MetS, insulin resistance, prediabetes/T2DM, hyperlipidemia, or NAFLD), mean age (<45 years or ≥45 years), mean BMI (<30 kg/m2 or ≥30 kg/m2), overall ROB judgment (low, unclear or high), the score of the Jadad scale (low score of no more than 3 or high score of more than 3) and duration (<8 weeks or ≥8 weeks) of intervention, and assignment (crossover or parallel). Subgroup differences were assessed using Chi-square tests following subgroup meta-analysis. Sensitivity analysis was conducted within studies of non-high ROB judgment to investigate robustness of results. All meta-analyses were performed using Review Manager. Publication bias was assessed visually via funnel plots and statistically using Begg’s and Egger’s tests. If Egger’s test yielded a significant p value for a specific effect size, the Trim and Fill method was applied to estimate adjusted results. Publication bias was performed using Stata version 15.1. A p value < 0.05 was considered statistically significant in the present review.
3 Results
3.1 Search results
The study selection process was illustrated in the PRISMA flow diagram (Figure 1). A total of 5,893 records were identified through searches of public literature databases, and an additional 30 records were retrieved from previously published systematic reviews. After removing duplicates, 2,709 unique records remained. Two reviewers (RD and RZ) independently screened the records, and disagreements were resolved by a third reviewer (XM). Of the 2,709 records, 400 articles were deemed potentially eligible based on title and abstract screening. After full-text review, 377 articles were excluded for reasons detailed in Figure 1, resulting in 23 studies included for qualitative synthesis. Among these, 19 studies with extractable effect size data were further included in the quantitative synthesis (meta-analysis).
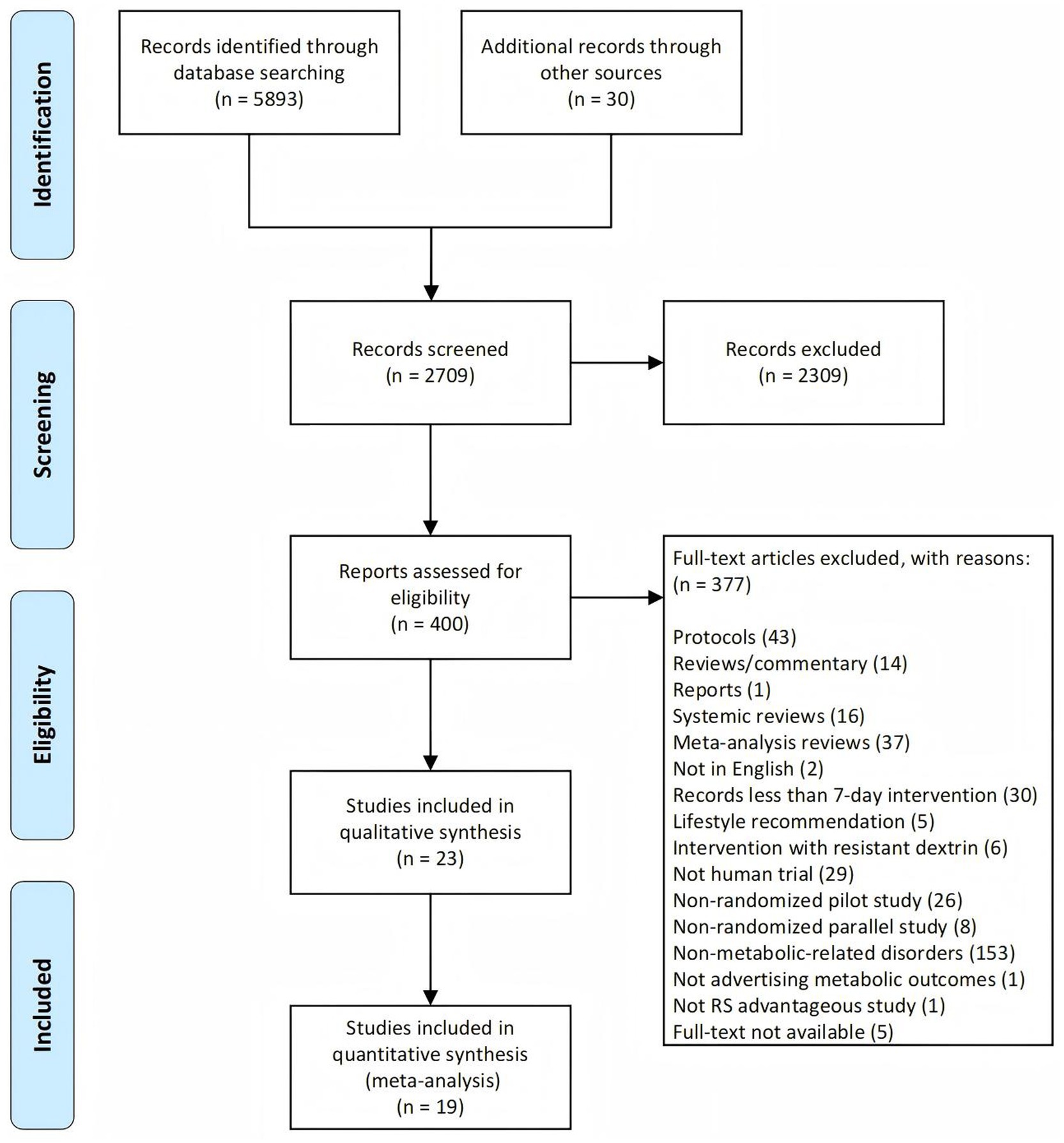
Figure 1. PRISMA flowchart illustrating the study selection process. PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
3.2 Study characteristics
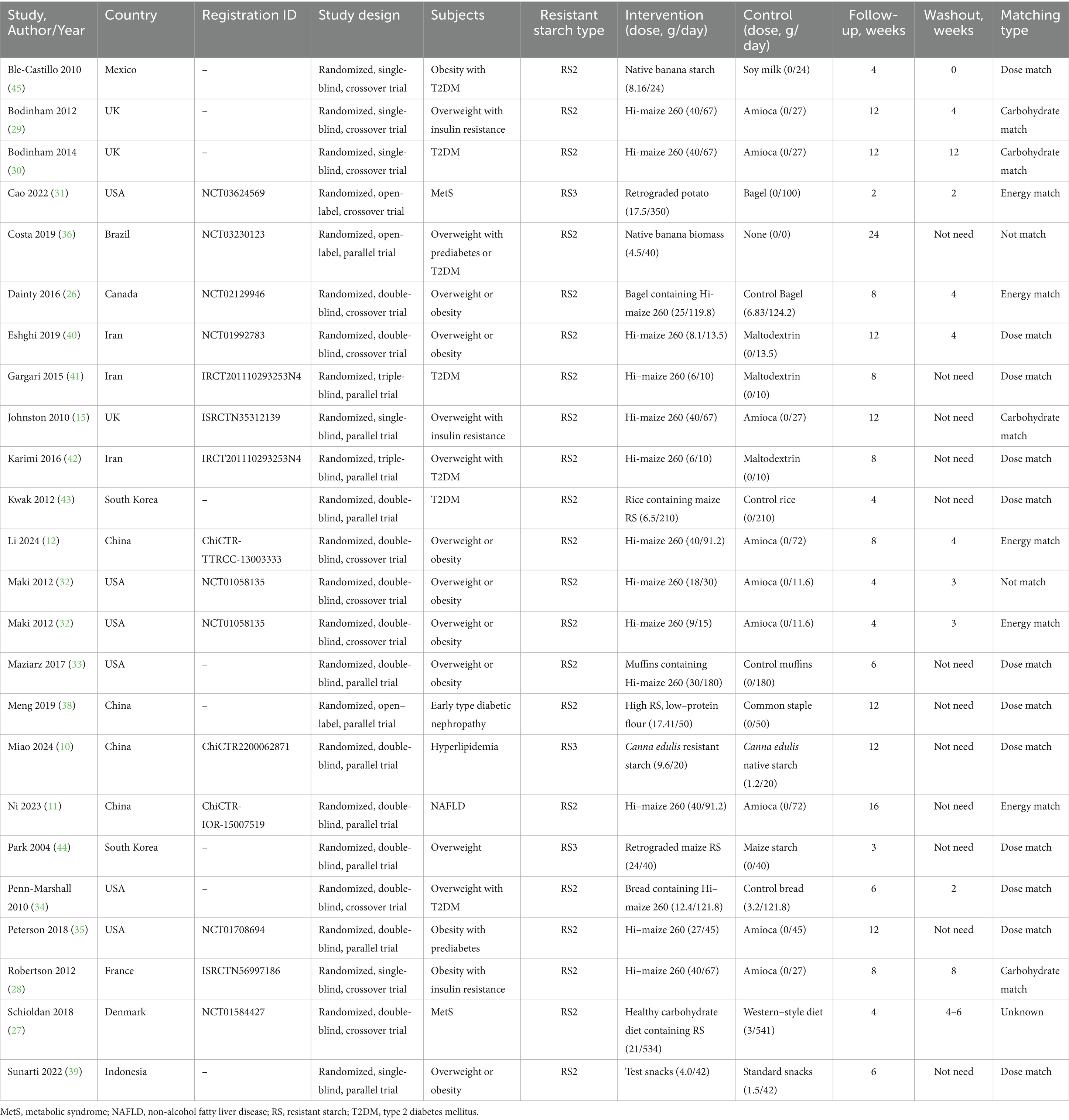
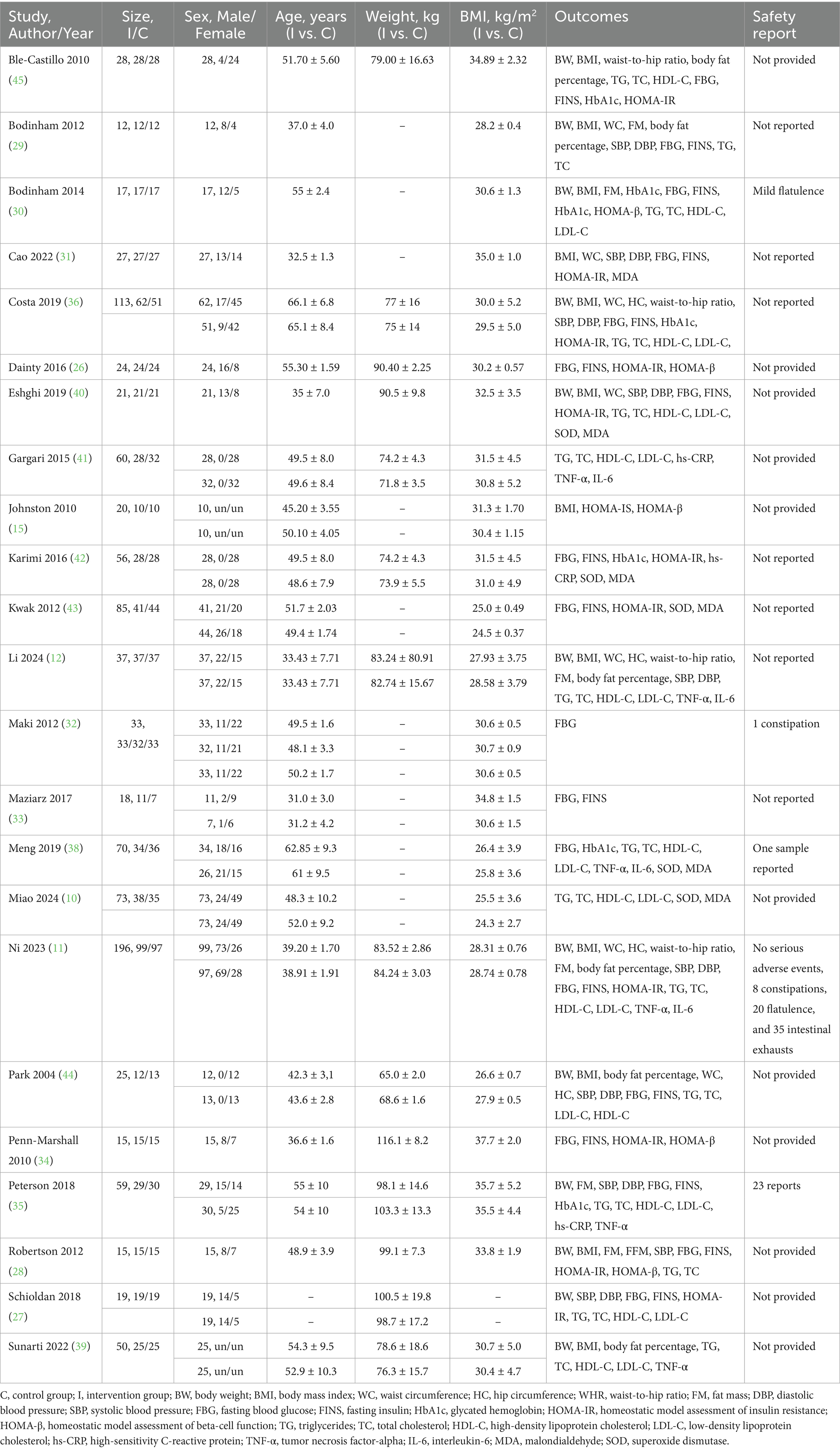
Table 1 summarized the characteristics of the 23 studies included in this review, published over a 20-year period from 2004 to 2024. Of these, 11 were crossover randomized controlled trials, and 12 were parallel-group trials. Geographically, 11 studies were conducted in Western developed countries: one in Canada (26), one in Denmark (27), one in France (28), three in the United Kingdom (15, 29, 30), and five in the United States (31–35). The remaining 12 studies were conducted in other regions: one in Brazil (36), four in China (10, 11, 37, 38), one in Indonesia (39), three in Iran (40–42), two in South Korea (43, 44), and one in Mexico (45). The included studies covered five categories of metabolic conditions: seven focused on overweight or obesity, six on MetS, eight on T2DM, one on hyperlipidemia, and one on NAFLD. RS doses ranged from 6 to 40 g/day, with intervention durations varying from 4 to 16 weeks. In Maki’s study, the data from higher-dose and lower-dose groups were reported separately; therefore, these two groups were considered as distinct trials in the subsequent analysis. The matching criteria for RS and control interventions were categorized as follows: 12 studies used dose-matched controls, four used carbohydrate-matched controls, five used energy-matched controls, two were unmatched, and one lacked matching details.
A total of 1,073 participants were included across the studies, comprising 430 males and 545 females, with sex-specific data unavailable in two trials. The participants were aged between 31 and 66.1 years, with body weight ranging from 71.8 to 116.1 kg and BMI values between 24.5 and 37.7 kg/m2. Effect sizes and safety outcomes are summarized in Table 2. Notably, two studies were identified as having the same registration ID (41, 42). Consequently, to avoid duplication, only one effect size from each study was selected for analysis.
The properties of included studies were documented in Supplementary Table 2. Among these, the most of 20 studies using RS2, and remaining three using RS3. In terms of the sources of RS, 16 studies identified maize as the primary source, two studies focused on banana, one study on potato, one on canna edulis, one on both maize and potato, and one study utilized multiple sources. RS substances had a purity of 34–34.8% when delivered as a powdered supplement in 15 studies, and 3.1–21.2% (26) when delivered as food matrices, including diet, rice, bread, bagel, and snack, in eight studies. The studies utilized Hi-maize 260, whose purity was confirmed by AOAC method 991.43. Besides, two studies employed AOAC 2002.02, two studies utilized the Goni method, one study used a total dietary fiber determination kit, and the remaining three studies did not specify the method used.
3.3 Data synthesis and quality assessment
Four studies were excluded from the meta-analysis due to the absence of net changes of effect sizes. In Maki’s study, effect sizes for the higher-dose and lower-dose groups were calculated separately. Each effect size included in the meta-analysis was derived from a minimum of three studies. Most of effect sizes were pooled as MDs, while some effect sizes, such as HOMA-β, hs-CRP, TNF-α, SOD, and MDA, were pooled as SMDs.
The results of quality assessment using Cochrane ROB tool were presented in Figure 2 and Supplementary Table 3. All included studies were judged as low risk for random sequence generation. Allocation concealment was assessed as low risk in five studies, while one study was judged as high due to lack of concealment; the remaining 16 studies did not report this information. Blinding of participants and personnel was considered adequate in 20 studies, with three studies rated as high risk in this domain. Regarding blinding of outcome assessment, eight studies were rated as low risk, while 15 studies did not provide sufficient details. For incomplete outcome data, 21 studies demonstrated as low risk, whereas two studies were rated as high due to dropout rates exceeding 20%. Selective reporting and other potential sources of bias were also assessed, and all 23 studies were considered low risk in both categories. For overall ROB judgment, three studies were judged as low risk, 15 were as unclear, and five were as high. The results of the Jadad scale assessments are provided in Supplementary Table 4. In brief, seven studies scored 2 points, two studies scored 3 points, 11 studies scored 4 points, and four studies achieved the maximum score of 5.
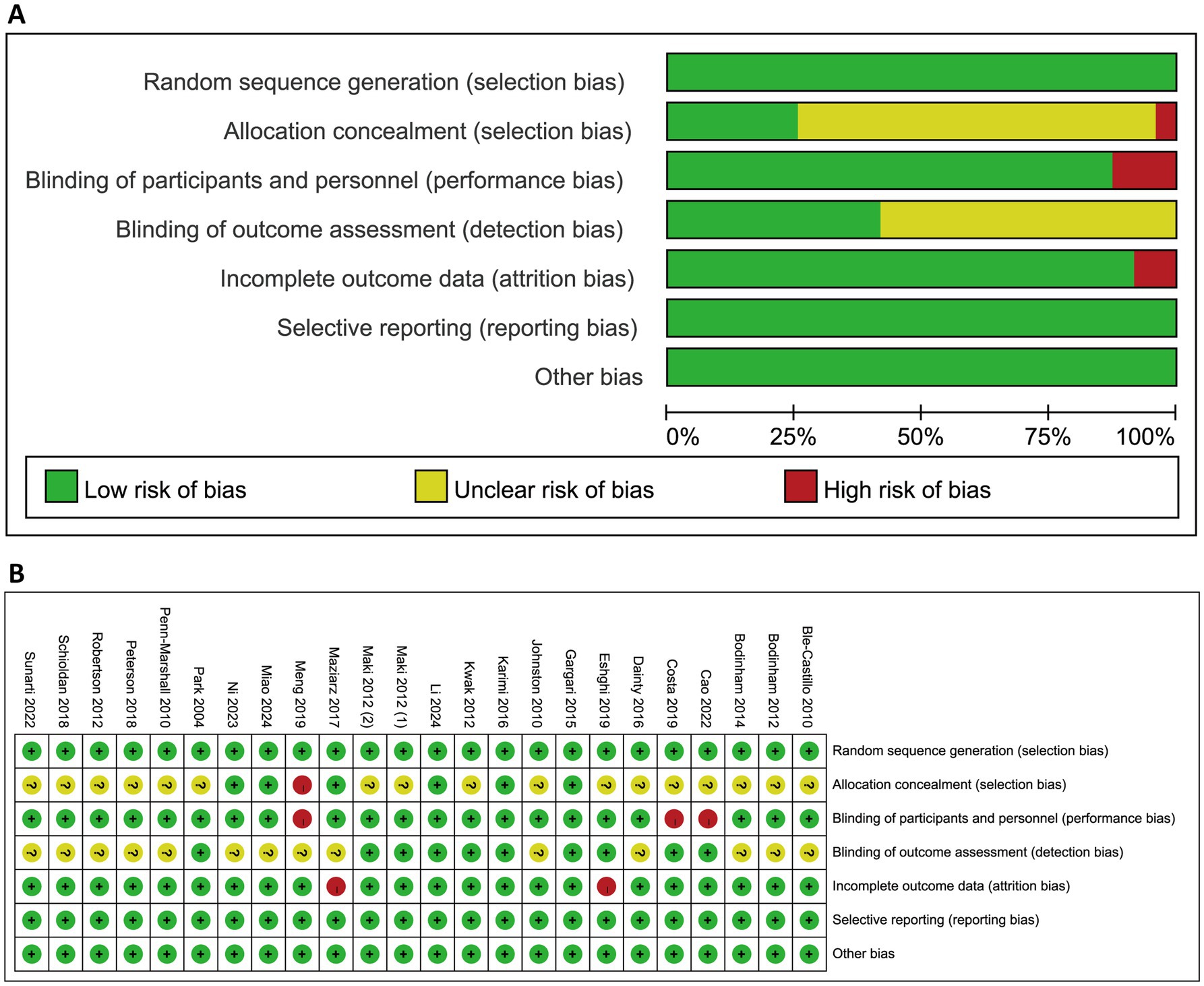
Figure 2. Quality assessment for the included studies using Cochrane risk of bias tool. (A) Proportions of studies exhibiting different levels of bias across the seven assessed domains. (B) Risk of bias judgment for each study. Symbols indicated the use of bias-reducing measures: “+” denotes low risk (i.e., bias-minimizing method used), “−” denotes high risk (i.e., method not used), and “?” indicates unclear risk (i.e., insufficient information).
3.4 Anthropometric parameters
Nine anthropometric outcomes were evaluated across the included studies (Figure 3). Nine trials assessed the effects of RS consumption on BW and BMI, with no statistically significant differences observed (BW: MD = −1.33 kg, 95% CI: −3.37 to 0.71, p = 0.20, I2 = 96%; BMI: MD = −0.52 kg/m2, 95% CI: −1.12 to 0.08, p = 0.09, I2 = 92%). In terms of body shape, RS intake was associated with a significant reduction in WC (MD = −2.58 cm, 95% CI: −4.71 to −0.45, p = 0.02, I2 = 52%; six trials) and HC (MD = −1.83 cm, 95% CI: −2.03 to −1.64, p < 0.0001, I2 = 0%; four trials). The change in waist-to-hip ratio, based on four trials, was minimal and not statistically significant (MD = −0.01, 95% CI: −0.03 to 0.00, p = 0.08, I2 = 48%). Regarding fat-related outcomes, RS consumption did not significantly affect FM (MD = −1.55 kg, 95% CI: −3.80 to 0.71, p = 0.18, I2 = 97%) or body fat percentage (MD = −1.07, 95% CI: −2.51 to 0.36, p = 0.14, I2 = 93%). For blood pressure parameters, no significant effects were observed. SBP showed negligible change (MD = −0.06 mmHg, 95% CI: −3.05 to 2.93, p = 0.97, I2 = 69%), while DBP exhibited a non-significant trend toward reduction (MD = −1.47 mmHg, 95% CI: −3.40 to 0.47, p = 0.14, I2 = 67%).
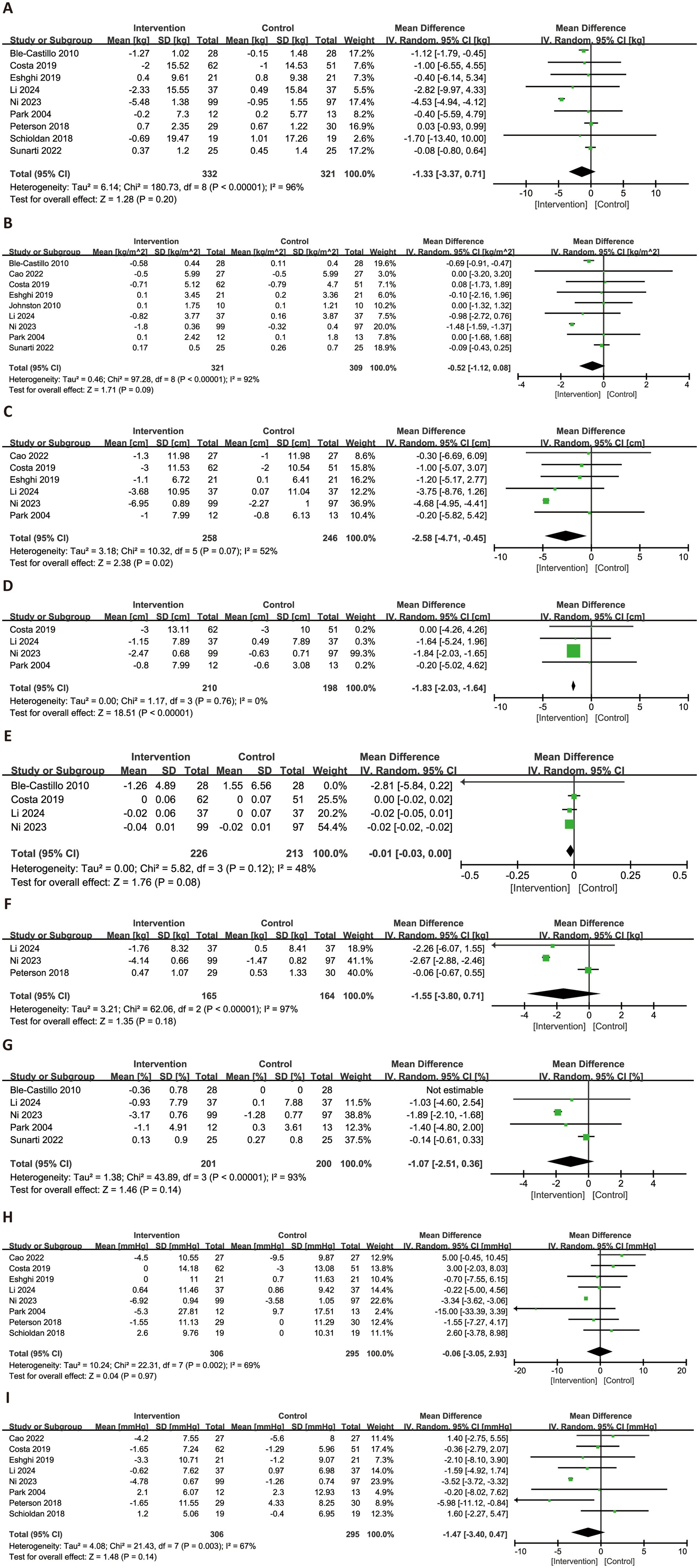
Figure 3. Forest plots illustrating the effects of RS versus control on anthropometric parameters. Outcomes included: BW (A), BMI (B), WC (C), HC (D), waist-to-hip ratio (E), FM (F), body fat percentage (G), SBP (H), and DBP (I). BW, body weight; BMI, body mass index; WC, waist circumference; HC, hip circumference; WHR, waist-to-hip ratio; FM, fat mass; DBP, diastolic blood pressure; SBP, systolic blood pressure.
3.5 Glycemic profiles
Figure 4 illustrated the effects of RS consumption on glycemic profiles. A total of 16 trials evaluated FBG, with no statistically significant effect observed (MD = 0.02 mmol/L, 95% CI: −0.05 to 0.09, p = 0.57), and low heterogeneity (I2 = 34%). A meta-analysis of 12 trials demonstrated a significant reduction in FINS levels (MD = −2.39 μU/mL, 95% CI: −3.47 to −1.30, p < 0.0001), although substantial heterogeneity was present (I2 = 82%). For HbA1c, a significant reduction was observed with low heterogeneity (MD = −0.14, 95% CI: −0.24 to −0.04, p = 0.008, I2 = 3%). HOMA-IR, based on 10 trials, also showed a significant improvement (MD = −0.58, 95% CI: −0.91 to −0.24, p = 0.0008), though heterogeneity was high (I2 = 63%). In contrast, three trials reporting on HOMA-β were included in a separate meta-analysis, which showed a non-significant reduction (SMD = −1.08, 95% CI: −2.76 to 0.60, p = 0.21) and high heterogeneity (I2 = 92%).
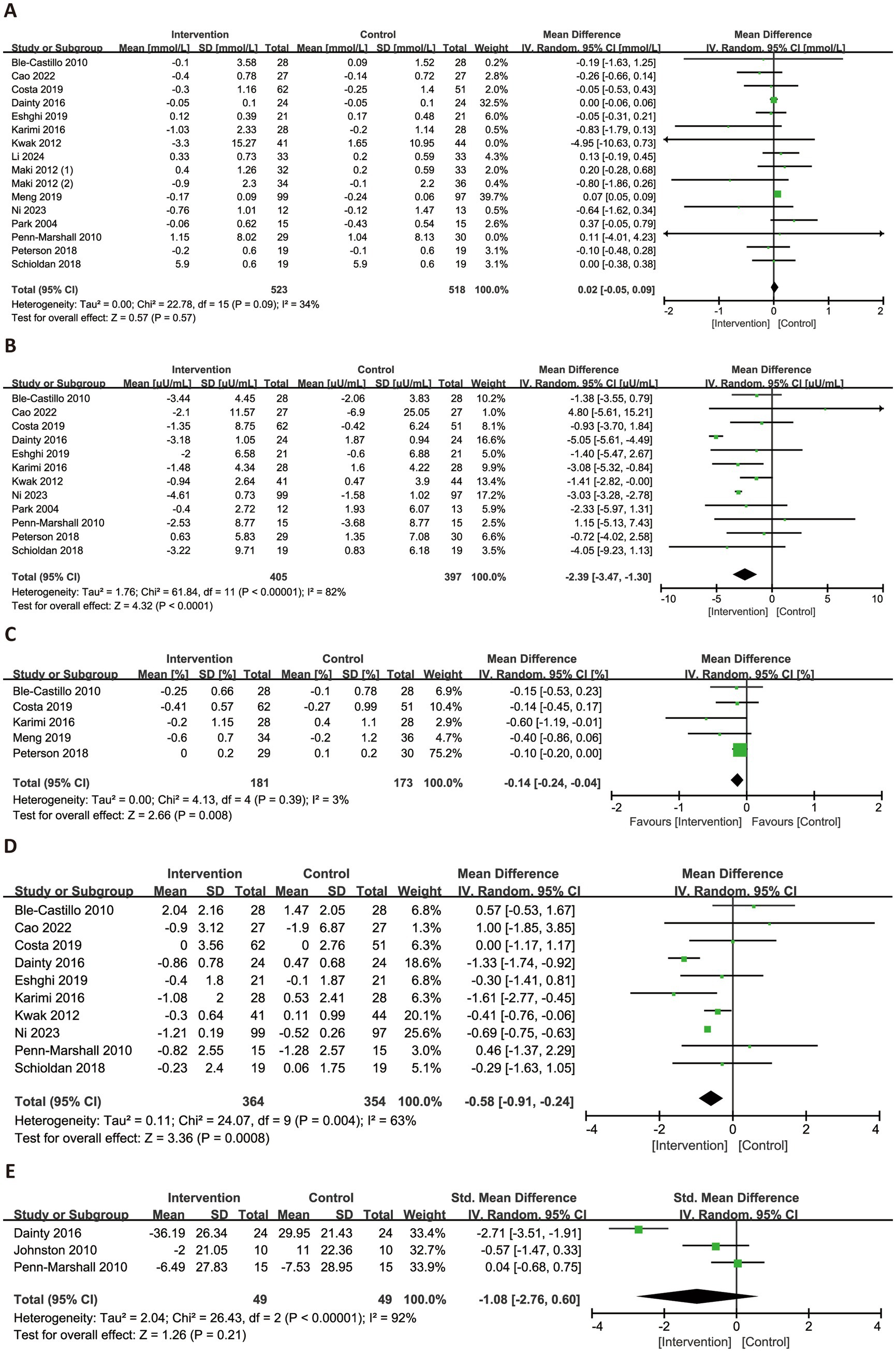
Figure 4. Forest plots illustrating the effects of RS versus control on glycemic profiles. Outcomes included: FBG (A), FINS (B), HbA1c (C), HOMA-IR (D), and HOMA-β (E). FBG, fasting blood glucose; FINS, fasting insulin; HbA1c, glycated hemoglobin; HOMA-IR, homeostatic model assessment of insulin resistance; HOMA-β, homeostatic model assessment of beta-cell function.
3.6 Lipid profiles
Figure 5 presented the forest plots for the effects of RS consumption on four lipid profile parameters. Across 12 trials, RS intake was not associated with a significant reduction in TG (MD = −0.11 mmol/L, 95% CI: −0.33 to 0.11, p = 0.33), with considerable heterogeneity observed (I2 = 88%). However, RS supplementation significantly reduced TC (MD = −0.20 mmol/L, 95% CI: −0.32 to −0.08, p = 0.001, I2 = 34%) and LDL-C (MD = −0.11 mmol/L, 95% CI: −0.18 to −0.04, p = 0.003, I2 = 12%), both with low heterogeneity. HDL-C levels were not significantly affected by RS intake (MD = −0.02 mmol/L, 95% CI: −0.02 to 0.07, p = 0.29), and substantial heterogeneity was noted (I2 = 66%).
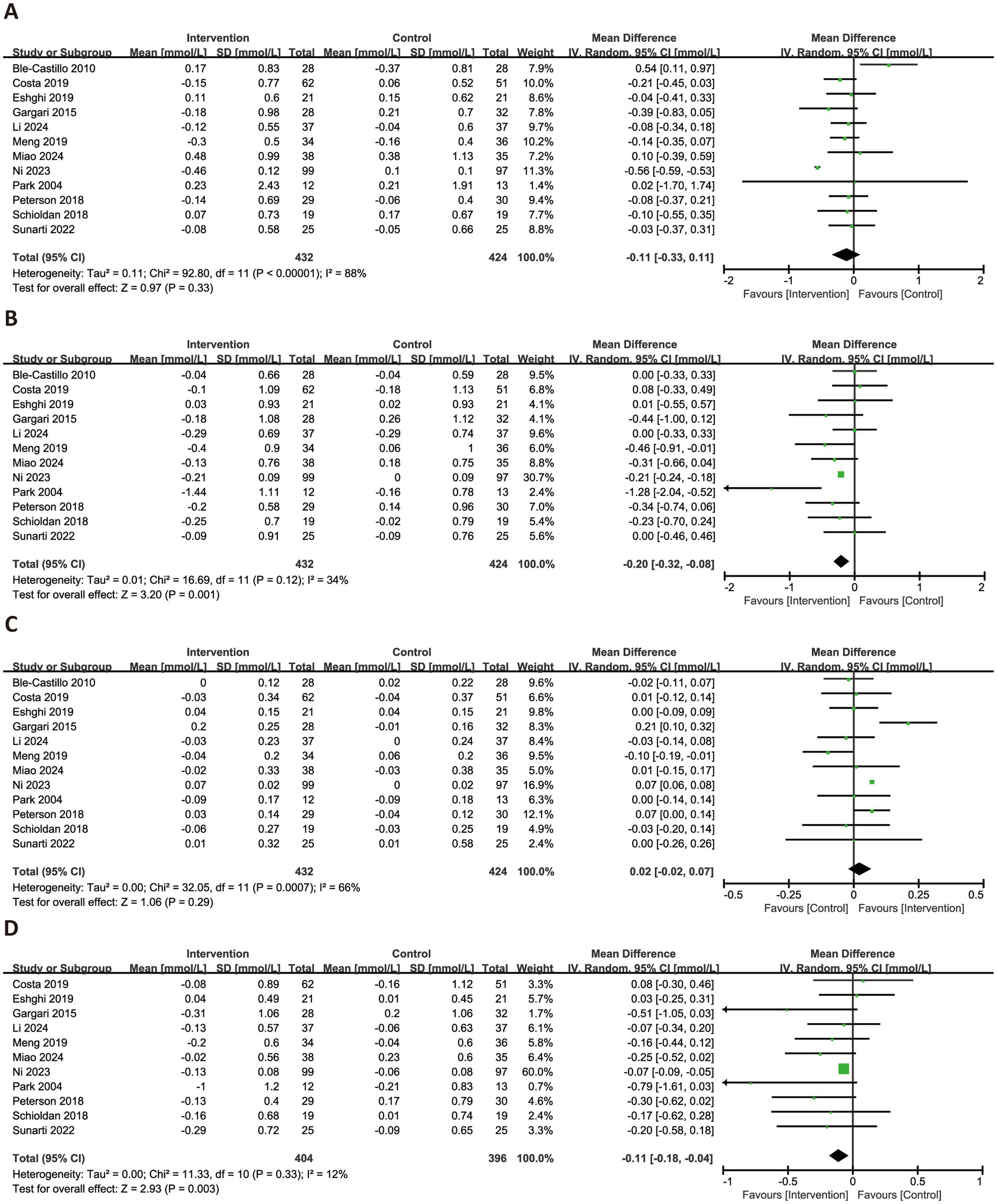
Figure 5. Forest plots illustrating the effects of RS versus control on lipid profiles. Outcomes included: TG (A), TC (B), HDL-C (C), and LDL-C (D). TG, triglycerides; TC, total cholesterol; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol.
3.7 Inflammatory factors
Figure 6 illustrated the effects of RS consumption on three commonly reported inflammatory markers. Based on three trials, RS intake did not significantly reduce levels of hs-CRP (SMD = −0.23, 95% CI: −0.52 to 0.07, p = 0.14), with no observed heterogeneity (I2 = 0%). For TNF-α, a significant reduction was observed across six trials (SMD = −0.52, 95% CI: −1.00 to −0.05, p = 0.03), although heterogeneity was substantial (I2 = 84%). Regarding IL-6, four trials were synthesized, and the pooled analysis revealed no significant effect (MD = −0.12 pg./mL, 95% CI: −0.50 to 0.26, p = 0.54), with high heterogeneity (I2 = 65%).
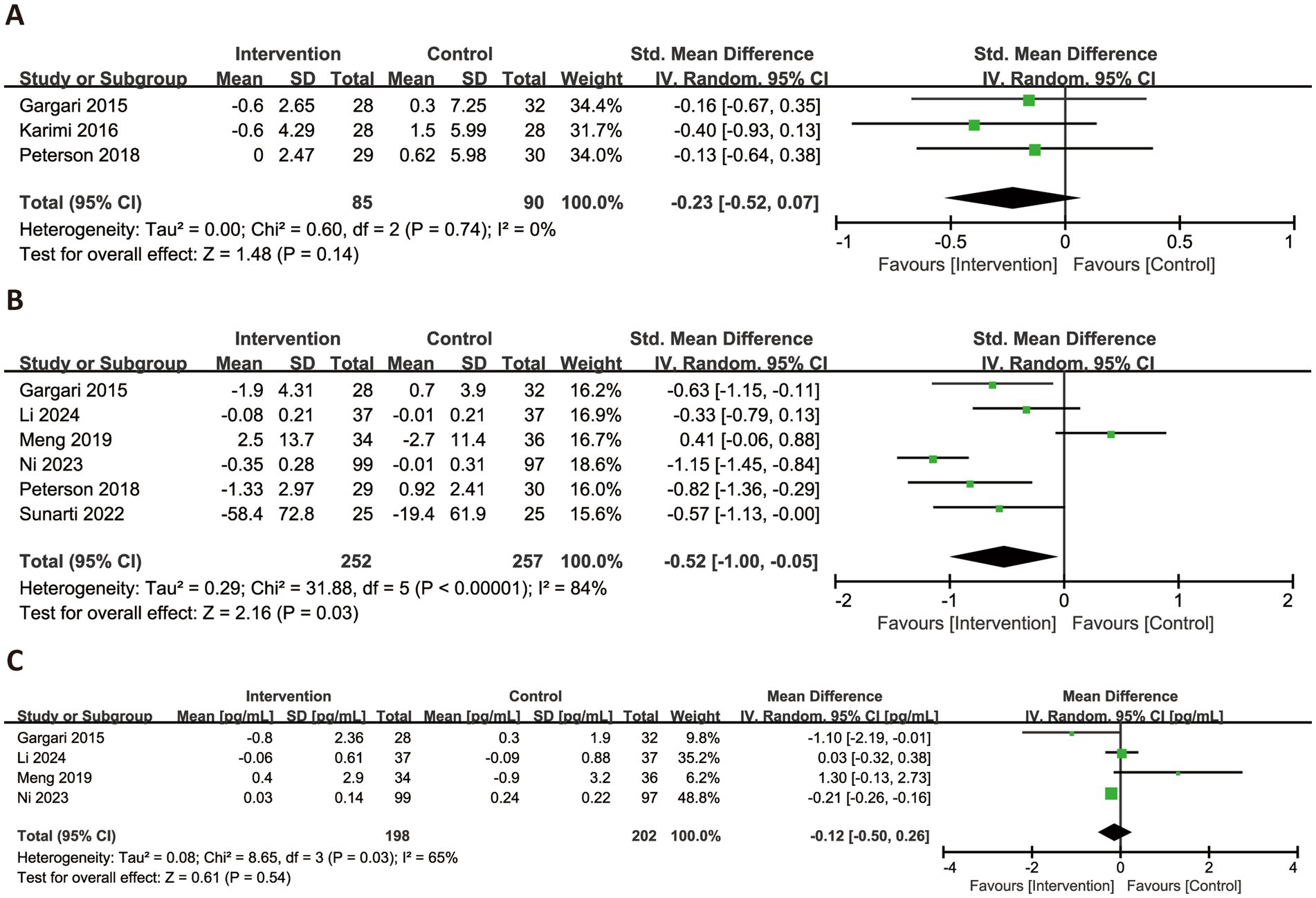
Figure 6. Forest plots illustrating the effects of RS versus control on inflammatory factors. Outcomes included: hs-CRP (A), TNF-α (B), and IL-6 (C). hs-CRP, high-sensitivity C-reactive protein; TNF-α, tumor necrosis factor-alpha; IL-6, interleukin-6.
3.8 Oxidative stress biomarkers
Figure 7 presented the changes in MDA and SOD levels across all subjects. Based on six trials, RS consumption did not significantly reduce MDA levels (SMD = −0.30, 95% CI: −0.65 to 0.05, p = 0.09), with high heterogeneity (I2 = 65%). In contrast, data from five trials showed a significant improvement in SOD activity following RS intake (SMD = 0.29, 95% CI: 0.08–0.51, p = 0.008), with no observed heterogeneity (I2 = 0%).

Figure 7. Forest plots illustrating the effects of RS versus control on oxidative stress biomarkers. Outcomes included: MDA (A) and SOD (B). MDA, malondialdehyde; SOD, superoxide dismutase.
3.9 Subgroup analysis
3.9.1 Subgroup analysis on anthropometric parameters
The findings from the subgroup analysis of anthropometric parameters were detailed in Supplementary Table 5. In the case of BW, a significant reduction was observed in the subgroup with NAFLD, the subgroup where the mean participant age was below 45 years, the subgroup where the mean BMI was less than 30 kg/m2, and the cohort with a higher dosage of 30 g/day or more. A more significant decrease in BMI was demonstrated in the subgroup with the high score of the Jadad scale, the subgroup with prediabetes or T2DM and the subgroup with NAFLD. A significant reduction in WC was observed in the subgroup consuming 30 g/day or more and the subgroup with NAFLD.
Regarding body shape, the subgroup from outside western developed countries, with a mean age under 45 years old as well as a mean BMI below 30 kg/m2, and the subgroup with NAFLD observed a significant decrease in FM. There was a significant reduction in body fat percentage in the subgroup that achieved a high score on the Jadad scale, with a mean participant age of less than 45 years and a mean BMI of less than 30 kg/m2, the subgroup where RS was administered as a supplement, there was a reduction in body fat percentage, and the subgroup with NAFLD.
In terms of blood pressure, a significant reduction in SBP level was observed in the NAFLD subgroup, the subgroup using RS as a supplement, and the subgroup receiving at least 30 g/day of RS. DBP level was significantly decreased in the NAFLD subgroup, the subgroup using RS as a supplement, the subgroup taking a dose of 30 g/day or more, and those with intervention lasting 8 weeks or longer.
3.9.2 Subgroup analysis on glycemic profiles
The findings from the subgroup analysis of glycemic profiles were summarized in Supplementary Table 6. For FINS, significant reductions were observed across different disease subgroups. Specifically, the intake of RS resulted in a statistically significant reduction in FINS levels among participants with overweight or obesity, those with prediabetes or T2DM, and those with NAFLD. RS significantly enhanced HOMA-IR in the subgroup characterized by a high score and the subgroup with NAFLD. A substantial reduction of HOMA-β was reported in overweight or obese participants.
3.9.3 Subgroup analysis on lipid profiles
The findings from the subgroup analysis of lipid profiles were detailed in Supplementary Table 7. A notable decrease in serum TG levels was observed among participants with NAFLD. Regarding HDL-C, a modest yet statistically significant increase was observed in the subgroup that attained a high score on the Jadad scale as well as in the subgroup comprising participants with a mean age below than 45 years.
3.9.4 Subgroup analysis on inflammatory factors
The findings from subgroup analysis of inflammatory markers were presented in Supplementary Table 8. RS consumption notably reduced TNF-α levels across various subgroups: low ROB, unclear ROB, overweight or obese participants and NAFLD. No significant differences were observed for IL-6 levels across any subgroups.
3.9.5 Subgroup analysis on oxidative stress biomarkers
The subgroup analysis primarily focused on MDA in the evaluation of oxidative stress biomarkers, as detailed in Supplementary Table 9. RS consumption notably reduced TNF-α levels across various subgroups: low ROB, unclear ROB, high score of the Jadad scale, other regions outside western developed countries, hyperlipidemia, and a mean age 45 years or more as well as parallel assignment.
3.10 Sensitivity analysis and publication bias
Sensitivity analysis indicated that the results were robust in the effect sizes, including HC, TC, LDL-C and SOD. Significant differences yet high heterogeneity persisted in the effect sizes, including WC, FINS, HOMA-IR, and TNF-α. The effect sizes of HDL-C and MDA newly demonstrated a significant enhancement after excluding the studies judges with a high ROB (Supplementary Table 10).
Publication bias was assessed using Begg’s and Egger’s tests, with results summarized in Supplementary Table 11. Begg’s test indicated potential publication bias for HbA1c (PBegg = 0.027). Egger’s test detected evidence of publication bias for WC (PEgger = 0.007), DBP (PEgger = 0.046), FBG (PEgger = 0.033), and TG (PEgger < 0.001). Corresponding funnel plots and bias assessments for these five outcomes were presented in Supplementary Figures 1–5. The Trim and Fill methods were applied to WC, DBP, FBG, and TG to adjust for potential bias. For WC, inclusion of three imputed studies yielded a non-significant change in the overall effect size (MD = −4.159 cm, 95% CI: −6.085 to −2.233, p < 0.001). For FBG, the adjusted meta-analysis remained robust, with no additional studies imputed and no change in significance (MD = 0.019 mmol/L, 95% CI: −0.055 to 0.094, p = 0.608). However, significant corrected effect sizes were observed after adjustment in DBP (MD = −2.981 mmHg, 95% CI: −4.825 to −1.136, p = 0.002; 3 imputed studies) and TG (MD = −0.466 mmol/L, 95% CI: −0.657 to −0.276, p < 0.001; 7 imputed studies).
4 Discussion
This present systematic review and meta-analysis was the first to assess the effects of RS consumption on anthropometric parameters and serum biomarkers in the adults with MetS-related risks. A total of 23 RCTs were subjected to quality assessment, while 20 trials were incorporated into for meta-analysis. Our findings revealed the effects of RS consumption on HC, TC, LDL-C and SOD with low heterogeneity. The low heterogeneity was attributed to several factors such as the high quality of study design, participants with younger age and overweight, a supplement as delivery, a dose of up to 30 g/day, and lasting over 8 weeks.
4.1 Effect sizes of anthropometric and serum parameters
The present review showed RS lowered WC and HC by 2.58 cm and 1.83 cm, respectively. Between the two, WC serves as an indicator of adipose distribution, and abdominal obesity, as determined by WC, is one component of MetS (46). The prior review consistently demonstrated the effect of nondigestible fermentable carbohydrates intake on WC (47). The decrease in WC indirectly indicated a reduction in central adiposity, a pattern of fat distribution, similarly observed in murine models administered with RS (48).
In the current review, neither SBP nor DBP was reduced by RS consumption. However, the findings indicated a reduced impact of RS on DBP following the application of the simulated method. To the best of our knowledge, there is a lack of research investigating the effect of RS on hypertension trials or systematic reviews related to blood pressure. A prior review regarding on the effect of fermentable carbohydrates intake on SBP in adults with overweight and obesity (47). The study on individuals with NAFLD revealed the a significant reduction in SBP and DBP following the consumption of RS for 3 months (11). Another study demonstrated a lessened result of DBP after consuming RS for 12 weeks (35). A possible explanation was that protein intake, compared to carbohydrate intake, was consistently associated with beneficial effect on blood pressure (49).
The meta-analysis showed that consumption of RS improved insulin sensitivity, as indicated by measures such as FINS, HOMA-IR, and HbA1c. Elevated blood glucose levels prompts the secretion of insulin by pancreatic islet beta cells. Insulin resistance is characterized by compensatory hyperinsulinemia. Previous systematic reviews have supported our findings regarding the reduction of FINS level through RS consumption in populations with overweight, obesity, MetS, prediabetes, and T2DM (8, 16, 50). HOMA-IR was used to assess the insulin resistance. Two reviews documented the same results of HOMA-IR (14, 16). The anticipated reduction in FBG levels due to RS intake was not observed. Previous studies demonstrated that RS reduced glucose levels during the postprandial period rather than during fasting (51–53). As for HbA1c, it is conventionally employed as a mean indicator of blood glucose levels to assess glycemic control. We found that HbA1c was lowered by 0.14% after RS consumption. The result was consistent with the preliminary review concerning overweight, obesity, and MetS (8, 16).
The current review identified a reduction in TC and LDL-C concentrations by 0.20 mmol/L and 0.11 mmol/L, respectively, after RS consumption. The effects of RS intake on two biomarkers were consistent across healthy individuals, as well as patients with overweight, obesity and MetS in the previous reviews (16, 47, 54). In a double-blind parallel RCT conducted by Ni et al. (11), it was reported that the intake of RS for 4 months improved TG and HDL-C concentrations. Another study published by Gargari et al. (41) showed that the intake of RS for 8 weeks increased HDL-C levels.
Three inflammatory indicators, including TNF-α, IL-6, and hs-CRP, were reported to be associated with component score of MetS, according to the previous review (55). In the current review, it was demonstrated that the intake of RS led to a reduction in TNF-α levels, while no significant effect was observed on IL-6 levels. These findings are consistent with those reported in previous systematic reviews (16). However, two reviews, which included both healthy subjects and those with diseases and the intervention of RS and resistant dextrin, reported improvements in two biomarkers (56, 57). Previous reviews noted the minimal impact of RS consumption on CRP levels (16–18, 56, 57). Some report indicated hs-CRP is more precise and suitable for detecting elevated cardiometabolic risks than the conventional measurement (58). In the present review, we investigated the impact of RS on hs-CRP levels; however, the finding indicated little effect of RS with low heterogeneity.
As for oxidative stress biomarkers, the concentration of SOD increased, while MDA levels remained unchanged following the consumption of RS. A prior review has provided evidence of improvements in the two biomarkers within the included trials, following interventions with RS and resistant dextrin, in the context of diseases involving chronic kidney disease (17), while another review demonstrated the negative effects of RS intake on the two biomarkers (57). SOD is an enzyme that protects by eliminating superoxide free radicals and their metabolic byproducts. The SOD gene has been reported to be associated with various metabolic disorders (59–61). MDA is produced as a result of lipid peroxidation during metabolic stress. Glucolipotoxicity linked to MetS results in higher levels of plasma MDA (62). Both two reviews reported an enhanced serum level of total antioxidant capacity (TAC), although they exhibited high heterogeneity, including overlapping trials (17, 57). TAC serves as a measure reflecting overall antioxidant buffering power in the body. Conversely, a well-established study indicated that plasma TAC was unable to predict components of cardiometabolic risk, possibly due to the activation of compensatory mechanisms under physiological conditions (63).
Four studies were excluded from the meta-analysis. Of these, three did not provide baseline information, and one concentrated on postprandial changes in serum glucose and insulin. Despite the lack of baseline data, the study by Bodinham et al. (29) demonstrated a reduction in FINS, while Bodinham et al. (30) reported a decrease in TNF-α. Additionally, Robertson et al. (28) observed reductions in FBG, FINS, and HOMA-IR. In contrast, Maziarz et al. (33) found no significant between-group differences in biomarkers.
4.2 Potential factors of heterogeneity
The classification of RS types was considered to introduce bias, as various in vitro and animal studies have demonstrated that different RS types exhibit variations in digestibility, fermentation rates, and SCFA profiles (21, 64, 65). Only RS2 and RS3 were included in the present meta-analysis, and most (16 of 20) of the trials used RS2. Two records from a well-established study investigating the effect of RS4-enriched flour on metabolic risks were identified, and a significant reduction of TC was observed (66, 67). However, these records were excluded from the current review due to the consumption being ad libitum without a specified dosage, and the inclusion of both healthy individuals and those with MetS. In the present review, subgroup analysis revealed there were no differences between RS2 and RS3 across any parameters. Pugh et al. (68) identified a statistically significant effect of RS2 on FINS level in comparison to RS1. However, the content of RS was not reported in RS1 subgroup, making it difficult determine the differences among RS type. Furthermore, Yuan et al. (54) indicated that RS2, rather than RS3, was associated with the regulation of serum TC and TG, regardless of participants’ health status. We considered there was substantial heterogeneity within the subgroups of two RS types, suggesting that other factors influenced the RS effects.
Food matrix has been considered as a critical contributor to heterogeneity, since starch processing changes not only properties, such as digestibility, gelatinization, retrogradation, crystallinity, amylose to amylopectin ratio, but also bioavailability in recent reports (69–71). Most (13 of 20) of trials encompassed in the current review administered RS in the form of a powdered supplement, thereby preserving the integrity of the heat-labile RS. Conversely, seven studies incorporated RS into food matrices that were subsequently subjected to cooking or baking processes, likely leading to a diminishment in RS content. Moreover, the incorporation of RS flour into food matrices, resulted in a reduced RS content, thereby leading to a decreased actual intake. Thus, the delivery method can affect RS intake and potentially alter outcomes. In the subgroup receiving the supplement delivery, a higher content ranging from 34 to 60% and a dose between 6 g and 40 g were achieved, compared to the subgroup utilizing food matrices, which exhibited a content range of 3.1–21.2% and a dose of 4–30.9 g. Most of RS-rich powders using in the included studies were commercially manufactured Hi-maize 260. In the subgroup receiving RS as a supplement, body fat percentage, SBP, and DBP significantly decreased, but these effects were absent when RS was part of food matrices. Wei et al. (57) reported the supplement form influenced the RS effects on inflammation biomarkers. Unfortunately, due to the limited information provided in the included studies, details regarding the properties of intervention, such as amylose to amylopectin ratio or crystallinity, were not available within the context of the articles.
Subgroup analysis on the dose and the duration of intervention was conducted. The result showed that consuming 30 g/day of RS improved anthropometric parameters, such as BW, WC, body fat percentage, SBP and DBP, significantly. Johnston et al. (15) reported that the RS supplement was integrated into the daily food twice per day. Li et al. (12) proposed that the RS supplement be administered as a powder dissolved in 300 mL of water. Meanwhile, Maziarz et al. (33) ensured an adequate RS content by incorporating RS powder into muffins. Two reviews set the dose cut-off at 20 g/day (18, 54), but only one reported its role of the mediator in RS effect on TC level. We considered the limited role of the dosage appeared in previous reviews, as it did not meet the recommended dietary fiber intake (72). As for the duration, according to published reviews, RS has been demonstrated to reduce serum inflammation biomarkers for a loner duration (56, 57). Here, we found the subgroup with over 8 weeks of intervention showed greater reductions in body fat percentage and DBP compared to those with less than 8 weeks.
We conducted subgroup analysis on participants characteristics, such as region, disease, mean age and mean BMI. The world’s division into western and eastern regions influenced the RS effects on glycemic, lipid and inflammatory profiles, as noted in two previous reviews (16, 57). We found that the RS consumption significantly decreased the levels of FM and MDA in the participants from outside western developed region. The RS effects in the current review were partly influenced by the disease profile. The result was cautiously presented, as only single trial with disease, such as hyperlipidemia, or NAFLD, was included in the review. In the younger subgroup, RS consumption significantly affected BW, FM, body fat percentage, and HDL-C, while it reduced MDA level in the middle and elder subgroup. Previous reviews also noted significant RS effects on glycemic, lipid, and inflammatory biomarkers in the younger subgroup (16, 57). RS consumption led to reductions in BW, FM and body fat percentage was decreased among overweight participants. We concerned RS consumption, as the supplement intervention, exhibited greater sensitivity in younger individuals and those with a lower BMI.
Subgroup analysis on study design based on Cochrane ROB tool, the score of the Jadad scale, and assignment, was explored as well. The levels of TNF-α and MDA were significantly improved by RS intake in the subgroups of trials judged as low or unclear ROB, while the parameters of BMI, body fat percentage, HOMA-IR, HDL-C, and MDA were enhanced in the subgroups of trials achieved a higher score of the Jadad scale. Both quality assessment tools were employed in systematic reviews. The first tool was evaluated across a broad range of aspects, whereas the second scale emphasized rigorous randomization and blinding methods. The results of the subgroup analysis indicated the necessity for a high-quality study design to research on the clinical functionalities of RS. Differences of assignment was only influenced the results of MDA. RS intervention is highly susceptible to carryover effects due to microbiota adaptation. Therefore, we recommend that a longer washout period be implemented in crossover trials.
4.3 Biological mechanisms
In relation to mechanisms of regulating anthropometric and serum biomarkers, the functional benefits of RS consumption can be attributed to either its physical–chemical structure or its biological impact.
Furthermore, the application of RS enhances the water absorption capacity of doughs and diminishes the viscoelastic properties of foods (73), thereby potentially augmenting satiety and decreasing the intake of digestible carbohydrates. RS-containing diets notably decreased energy intake and BW in vivo (74). In human, the preliminary review gave inconclusive evidence that RS had effects on appetite, hunger, food intake, satiety (75). The malabsorption of RS in the small intestine leads to a reduction in the glycemic index following ingestion, resulting in minimal fluctuations in postprandial blood glucose levels, which effectively enhances glucose tolerance (76). RS3 can be fermented in the colon and may bind bile salts, reducing bile acid reabsorption in the ileum, stimulating hepatic bile acid production, and increasing cholesterol use (77).
The mechanism of RS consumption may be partially attributed to the indirect effects mediated by the gut microbiome and the production of SCFAs. Indigestible RS are fermented by the predominant beneficial gut microbiota, such as Bifidobacterium, Lactobacilli, Ruminococcus, Roseburia, Eubacterium, Faecalibacterium, and Clostridium among others (78–80). Bifidobacterium, Faecalibacterium and Ruminococcus were typically increased after RS2, RS3, and RS4 supplementations, respectively, according to published trials (10, 37, 67). Fermentation of RS generates SCFAs including acetate, propionate, butyrate (81). RS can reduce appetite by enhancing the secretion of satiety-associated hormones like glucagon-like peptide-1 (GLP-1) and peptide YY (PYY) by SCFAs targeting G protein-coupled receptors (GPRs) (82). It was found that muffins with RS content enhanced satiety, prolonged digestion period, and benefited to weight loss (33). Replacing carbohydrates with RS led to a notable increase in postprandial fat oxidation (83). Additionally, the fermented SCFAs shifted metabolic pathway from lipogenesis to fat oxidation by activating peroxisome proliferator-activated receptor-γ (84). These findings suggest that RS may contribute to reducing fat accumulation.
Fermented SCFAs could reduce the gastric motility and intestinal glucose transport capacity by stimulating PYY and GLP-1, respectively (85, 86). The Dose-dependent Kudzu RS enhanced insulin sensitivity and reinstated the hepatic expression of insulin receptor substrate 1 and glucose transporter 4, both of which contributed to insulin efficacy and glucose homeostasis, in T2DM mice (87). Serum SCFAs have been shown to improve insulin sensitivity by interacting with G protein-coupled receptors (GPRs) [61]. Butyrate has been shown to mitigate adipocyte inflammation by inhibiting the NOD-like receptor thermal protein domain associated protein 3 pathway [71]. Bacterial toxins, particularly lipopolysaccharides (LPS), which are integral components of Gram-negative bacteria, are primary contributors to the cause of low-grade chronic systemic inflammation (88). Gut microbiota-derived LPS affect intestinal permeability and tight junction (TJ) proteins by activating toll-like receptor 4-dependent pathways in intestine (89). Compromised intestinal permeability led to LPS translocation into the blood. Dietary RS addition increased propionate and butyrate and ameliorated LPS-induced inflammation during LPS challenge in vivo (90). Both SCFAs improve the intestinal barrier function by upregulating TJ proteins in epithelial intestine cells (91, 92). LPS induces inflammatory cytokines secretion, like TNF-α, by mediating inflammatory host cells, such as macrophage (93). Chronic inflammation induces lipid peroxidation through inhibition of catalase and glutamine peroxidase activities (94). SCFAs play protective role in inflammatory cytokines and oxidative stress (94, 95). However, there have been no reports demonstrating that SCFAs fermented by RS modulate inflammation and oxidative stress. The probable mechanism is that the fermentation of RS reduces the diversity and expression of hazard gut microbiota (7).
Dietary RS mediates bile acid (BA) metabolism and promotes GLP-1 secretion by targeting Takeda GPR 5 (96). Lei et al. demonstrated lotus seed RS lowered serum lipid by promoting BAs excretion and regulating gut microbiome (97). Included trials reported distinct changes of BA metabolism after RS2 and RS3 (10–12).
4.4 Strengths and limitations
We implemented strict eligibility criteria for including RCTs, standardized algorithms and uniformity in the units before calculating effect sizes. The heterogeneity was evaluated from the variety of aspects, such as RS type, intervention settings, participant characteristics, and study design. We employed a variety of measurement tools for qualitive assessments, sensitivity analysis and publication bias.
However, our study still had several limitations. Additional detailed parameters of RS, such as crystallinity, amylose to amylopectin ratio, gelatinization, and retrogradation, were not collected, even though these factors potentially influenced the observed effects. It was hard to draw a solid conclusion due to the absence of proposed mechanisms in the majority of the included studies. The present review focused on the limited scope of adults excluding children or adolescents. We included the participants with metabolic risks, including overweight, obesity, MetS, prediabetes, T2DM, hyperlipidemia, and NAFLD. While these conditions exhibited similarities in terms of pathogenesis and symptoms, it was important to acknowledge the distinctions that existed between them.
5 Conclusion
In conclusion, this meta-analysis demonstrated the beneficial effects of RS on HC, TC, LDL-C, and SOD in the management of adults with embolic syndrome-related risks. The inconclusive evidence showed the effects of RS on WC, FINS, HOMA-IR, TNF-α, and HDL-C and MDA. A dose of at least 30 g/day and duration over 8 weeks partly help with a positive observation of RS. The participants with younger age and overweight, and the high-quality study design are recommended. Future studies are warranted with the assessments of the properties, delivery mode, gut microbial composition, and metabolome, for further insights.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Author contributions
XL: Writing – original draft, Writing – review & editing, Supervision. ZL: Writing – review & editing. DZ: Investigation, Writing – review & editing. RD: Formal analysis, Writing – review & editing. RZ: Formal analysis, Visualization, Writing – review & editing. CL: Methodology, Writing – review & editing. HM: Funding acquisition, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study received supportive funding from the National High Level Hospital Clinical Research Funding, No. 2023-NHLHCRF-YYPP-TS-02; Beijing Clinical Research Program for the Construction of Research-Oriented Wards, with grant number 2022-YJXBF-03-02; National Natural Science Foundation of China No. 82470857; Beijing Natural Science Foundation No. 7242125.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2025.1655664/full#supplementary-material
Footnotes
References
1. Saklayen, MG. The global epidemic of the metabolic syndrome. Curr Hypertens Rep. (2018) 20:12. doi: 10.1007/s11906-018-0812-z
2. Alberti, KG, Zimmet, P, and Shaw, J. Metabolic syndrome--a new world-wide definition. A consensus statement from the international diabetes federation. Diabet Med. (2006) 23:469–80. doi: 10.1111/j.1464-5491.2006.01858.x
3. Bloomgarden, ZT. American Association of Clinical Endocrinologists (AACE) consensus conference on the insulin resistance syndrome: 25-26 August 2002, Washington, DC. Diabetes Care. (2003) 26:933–9. doi: 10.2337/diacare.26.3.933
4. Tune, JD, Goodwill, AG, Sassoon, DJ, and Mather, KJ. Cardiovascular consequences of metabolic syndrome. Transl Res. (2017) 183:57–70. doi: 10.1016/j.trsl.2017.01.001
5. Guembe, MJ, Fernandez-Lazaro, CI, Sayon-Orea, C, Toledo, E, Moreno-Iribas, C, Cosials, JB, et al. Risk for cardiovascular disease associated with metabolic syndrome and its components: a 13-year prospective study in the RIVANA cohort. Cardiovasc Diabetol. (2020) 19:195. doi: 10.1186/s12933-020-01166-6
6. Silveira Rossi, JL, Barbalho, SM, Reverete de Araujo, R, Bechara, MD, Sloan, KP, and Sloan, LA. Metabolic syndrome and cardiovascular diseases: going beyond traditional risk factors. Diabetes Metab Res Rev. (2022) 38:e3502. doi: 10.1002/dmrr.3502
7. Chen, Z, Liang, N, Zhang, H, Li, H, Guo, J, Zhang, Y, et al. Resistant starch and the gut microbiome: exploring beneficial interactions and dietary impacts. Food Chem X. (2024) 21:101118. doi: 10.1016/j.fochx.2024.101118
8. Wang, Y, Chen, J, Song, YH, Zhao, R, Xia, L, Chen, Y, et al. Effects of the resistant starch on glucose, insulin, insulin resistance, and lipid parameters in overweight or obese adults: a systematic review and meta-analysis. Nutr Diabetes. (2019) 9:19. doi: 10.1038/s41387-019-0086-9
9. Rashed, AA, Saparuddin, F, Rathi, DG, Nasir, NNM, and Lokman, EF. Effects of resistant starch interventions on metabolic biomarkers in pre-diabetes and diabetes adults. Front Nutr. (2021) 8:793414. doi: 10.3389/fnut.2021.793414
10. Miao, T, Zhang, X, Zhang, C, Wu, J, Zhu, Y, Xiao, M, et al. Type 3 resistant starch from Canna edulis reduce lipid levels in patients with mild hyperlipidemia through altering gut microbiome: a double- blind randomized controlled trial. Pharmacol Res. (2024) 205:107232. doi: 10.1016/j.phrs.2024.107232
11. Ni, Y, Qian, L, Siliceo, SL, Long, X, Nychas, E, Liu, Y, et al. Resistant starch decreases intrahepatic triglycerides in patients with NAFLD via gut microbiome alterations. Cell Metab. (2023) 35:1530–47.e8. doi: 10.1016/j.cmet.2023.08.002
12. Li, H, Zhang, L, Li, J, Wu, Q, Qian, L, He, J, et al. Resistant starch intake facilitates weight loss in humans by reshaping the gut microbiota. Nat Metab. (2024) 6:578–97. doi: 10.1038/s42255-024-00988-y
13. Jama, HA, Rhys-Jones, D, Nakai, M, Yao, CK, Climie, RE, Sata, Y, et al. Prebiotic intervention with HAMSAB in untreated essential hypertensive patients assessed in a phase II randomized trial. Nat Cardiovasc Res. (2023) 2:35–43. doi: 10.1038/s44161-022-00197-4
14. Xiong, K, Wang, J, Kang, T, Xu, F, and Ma, A. Effects of resistant starch on glycaemic control: a systematic review and meta-analysis. Br J Nutr. (2021) 125:1260–9. doi: 10.1017/S0007114520003700
15. Johnston, KL, Thomas, EL, Bell, JD, Frost, GS, and Robertson, MD. Resistant starch improves insulin sensitivity in metabolic syndrome. Diabet Med. (2010) 27:391–7. doi: 10.1111/j.1464-5491.2010.02923.x
16. Halajzadeh, J, Milajerdi, A, Reiner, Ž, Amirani, E, Kolahdooz, F, Barekat, M, et al. Effects of resistant starch on glycemic control, serum lipoproteins and systemic inflammation in patients with metabolic syndrome and related disorders: a systematic review and meta-analysis of randomized controlled clinical trials. Crit Rev Food Sci Nutr. (2020) 60:3172–84. doi: 10.1080/10408398.2019.1680950
17. Lu, J, Ma, B, Qiu, X, Sun, Z, and Xiong, K. Effects of resistant starch supplementation on oxidative stress and inflammation biomarkers: a systematic review and meta-analysis of randomized controlled trials. Asia Pac J Clin Nutr. (2021) 30:614–23. doi: 10.6133/apjcn.202112_30(4).0008
18. Haghighatdoost, F, Gholami, A, and Hariri, M. Effect of resistant starch type 2 on inflammatory mediators: a systematic review and meta-analysis of randomized controlled trials. Complement Ther Med. (2021) 56:102597. doi: 10.1016/j.ctim.2020.102597
19. Wang, Z, Wang, S, Xu, Q, Kong, Q, Li, F, Lu, L, et al. Synthesis and functions of resistant starch. Adv Nutr. (2023) 14:1131–44. doi: 10.1016/j.advnut.2023.06.001
20. Arif AI, MS, Hussain, R, Noor, M, Khalid, S, Basharat, N, Raza, H, et al. A comprehensive review on resistant starch, its types, sources, application and health benefits. Pure Appl Biol. (2025) 14:531–42. doi: 10.19045/bspab.2025.140052
21. Tekin, T, and Dincer, E. Effect of resistant starch types as a prebiotic. Appl Microbiol Biotechnol. (2023) 107:491–515. doi: 10.1007/s00253-022-12325-y
22. Das, M, Santra, S, Chakraborty, M, Rajan, N, Sarvanabhupathy, S, Anusha,, et al. Resistant starch: insights into better health and metabolism. Biocatal Agric Biotechnol. (2024) 59:103275. doi: 10.1016/j.bcab.2024.103275
23. Maiya, M, Adorno, A, Toulabi, SB, Tucker, WJ, and Patterson, MA. Resistant starch improves cardiometabolic disease outcomes: a narrative review of randomized trials. Nutr Res. (2023) 114:20–40. doi: 10.1016/j.nutres.2023.04.001
24. Luo, D, Wan, X, Liu, J, and Tong, T. Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat Methods Med Res. (2018) 27:1785–805. doi: 10.1177/0962280216669183
25. Wan, X, Wang, W, Liu, J, and Tong, T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. (2014) 14:135. doi: 10.1186/1471-2288-14-135
26. Dainty, SA, Klingel, SL, Pilkey, SE, McDonald, E, McKeown, B, Emes, MJ, et al. Resistant starch bagels reduce fasting and postprandial insulin in adults at risk of type 2 diabetes. J Nutr. (2016) 146:2252–9. doi: 10.3945/jn.116.239418
27. Schioldan, AG, Gregersen, S, Hald, S, Bjørnshave, A, Bohl, M, Hartmann, B, et al. Effects of a diet rich in arabinoxylan and resistant starch compared with a diet rich in refined carbohydrates on postprandial metabolism and features of the metabolic syndrome. Eur J Nutr. (2018) 57:795–807. doi: 10.1007/s00394-016-1369-8
28. Robertson, MD, Wright, JW, Loizon, E, Debard, C, Vidal, H, Shojaee-Moradie, F, et al. Insulin-sensitizing effects on muscle and adipose tissue after dietary fiber intake in men and women with metabolic syndrome. J Clin Endocrinol Metab. (2012) 97:3326–32. doi: 10.1210/jc.2012-1513
29. Bodinham, CL, Smith, L, Wright, J, Frost, GS, and Robertson, MD. Dietary fibre improves first-phase insulin secretion in overweight individuals. PLoS One. (2012) 7:e40834. doi: 10.1371/journal.pone.0040834
30. Bodinham, CL, Smith, L, Thomas, EL, Bell, JD, Swann, JR, Costabile, A, et al. Efficacy of increased resistant starch consumption in human type 2 diabetes. Endocr Connect. (2014) 3:75–84. doi: 10.1530/EC-14-0036
31. Cao, S, Shaw, EL, Quarles, WR, Sasaki, GY, Dey, P, Hodges, JK, et al. Daily inclusion of resistant starch-containing potatoes in a dietary guidelines for Americans dietary pattern does not adversely affect cardiometabolic risk or intestinal permeability in adults with metabolic syndrome: a randomized controlled trial. Nutrients. (2022) 14:1545. doi: 10.3390/nu14081545
32. Maki, KC, Pelkman, CL, Finocchiaro, ET, Kelley, KM, Lawless, AL, Schild, AL, et al. Resistant starch from high-amylose maize increases insulin sensitivity in overweight and obese men. J Nutr. (2012) 142:717–23. doi: 10.3945/jn.111.152975
33. Maziarz, MP, Preisendanz, S, Juma, S, Imrhan, V, Prasad, C, and Vijayagopal, P. Resistant starch lowers postprandial glucose and leptin in overweight adults consuming a moderate-to-high-fat diet: a randomized-controlled trial. Nutr J. (2017) 16:14. doi: 10.1186/s12937-017-0235-8
34. Penn-Marshall, M, Holtzman, GI, and Barbeau, WE. African Americans may have to consume more than 12 grams a day of resistant starch to lower their risk for type 2 diabetes. J Med Food. (2010) 13:999–1004. doi: 10.1089/jmf.2009.0195
35. Peterson, CM, Beyl, RA, Marlatt, KL, Martin, CK, Aryana, KJ, Marco, ML, et al. Effect of 12 wk of resistant starch supplementation on cardiometabolic risk factors in adults with prediabetes: a randomized controlled trial. Am J Clin Nutr. (2018) 108:492–501. doi: 10.1093/ajcn/nqy121
36. Costa, ES, França, CN, Fonseca, FAH, Kato, JT, Bianco, HT, Freitas, TT, et al. Beneficial effects of green banana biomass consumption in patients with pre-diabetes and type 2 diabetes: a randomised controlled trial. Br J Nutr. (2019) 121:1365–75. doi: 10.1017/S0007114519000576
37. Li, Y, Xia, D, Chen, J, Zhang, X, Wang, H, Huang, L, et al. Dietary fibers with different viscosity regulate lipid metabolism via ampk pathway: roles of gut microbiota and short-chain fatty acid. Poult Sci. (2022) 101:101742. doi: 10.1016/j.psj.2022.101742
38. Meng, Y, Bai, H, Yu, QT, Yan, J, Zhao, LL, Wang, SJ, et al. High-resistant starch, low-protein flour intervention on patients with early type 2 diabetic nephropathy: a randomized trial. J Ren Nutr. (2019) 29:386–93. doi: 10.1053/j.jrn.2018.12.005
39. Sunarti, S, Rubi, DS, Pramana, AAC, Huriyati, E, and Santoso, U. The benefits of high-resistant starch and beta-carotene snack in ameliorating atherogenic index and inflammation in obesity. Open Access Maced J Med Sci. (2022) 10:1767–73. doi: 10.3889/oamjms.2022.9302
40. Eshghi, F, Bakhshimoghaddam, F, Rasmi, Y, and Alizadeh, M. Effects of resistant starch supplementation on glucose metabolism, lipid profile, lipid peroxidation marker, and oxidative stress in overweight and obese adults: randomized, double-blind, crossover trial. Clin Nutr Res. (2019) 8:318–28. doi: 10.7762/cnr.2019.8.4.318
41. Gargari, BP, Namazi, N, Khalili, M, Sarmadi, B, Jafarabadi, MA, and Dehghan, P. Is there any place for resistant starch, as alimentary prebiotic, for patients with type 2 diabetes? Complement Ther Med. (2015) 23:810–5. doi: 10.1016/j.ctim.2015.09.005
42. Karimi, P, Farhangi, MA, Sarmadi, B, Gargari, BP, Javid, AZ, Pouraghaei, M, et al. The therapeutic potential of resistant starch in modulation of insulin resistance, endotoxemia, oxidative stress and antioxidant biomarkers in women with type 2 diabetes: a randomized controlled clinical trial. Ann Nutr Metab. (2016) 68:85–93. doi: 10.1159/000441683
43. Kwak, JH, Paik, JK, Kim, HI, Kim, OY, Shin, DY, Kim, HJ, et al. Dietary treatment with rice containing resistant starch improves markers of endothelial function with reduction of postprandial blood glucose and oxidative stress in patients with prediabetes or newly diagnosed type 2 diabetes. Atherosclerosis. (2012) 224:457–64. doi: 10.1016/j.atherosclerosis.2012.08.003
44. Park, OJ, Kang, NE, Chang, MJ, and Kim, WK. Resistant starch supplementation influences blood lipid concentrations and glucose control in overweight subjects. J Nutr Sci Vitaminol. (2004) 50:93–9. doi: 10.3177/jnsv.50.93
45. Ble-Castillo, JL, Aparicio-Trápala, MA, Francisco-Luria, MU, Córdova-Uscanga, R, Rodríguez-Hernández, A, Méndez, JD, et al. Effects of native banana starch supplementation on body weight and insulin sensitivity in obese type 2 diabetics. Int J Environ Res Public Health. (2010) 7:1953–62. doi: 10.3390/ijerph7051953
46. Eckel, RH, and Cornier, MA. Update on the NCEP ATP-III emerging cardiometabolic risk factors. BMC Med. (2014) 12:115. doi: 10.1186/1741-7015-12-115
47. Xu, B, Cao, J, Fu, J, Li, Z, Jin, M, Wang, X, et al. The effects of nondigestible fermentable carbohydrates on adults with overweight or obesity: a meta-analysis of randomized controlled trials. Nutr Rev. (2022) 80:165–77. doi: 10.1093/nutrit/nuab018
48. So, PW, Yu, WS, Kuo, YT, Wasserfall, C, Goldstone, AP, Bell, JD, et al. Impact of resistant starch on body fat patterning and central appetite regulation. PLoS One. (2007) 2:e1309. doi: 10.1371/journal.pone.0001309
49. Lee, YP, Puddey, IB, and Hodgson, JM. Protein, fibre and blood pressure: potential benefit of legumes. Clin Exp Pharmacol Physiol. (2008) 35:473–6. doi: 10.1111/j.1440-1681.2008.04899.x
50. Gao, C, Rao, M, Huang, W, Wan, Q, Yan, P, Long, Y, et al. Resistant starch ameliorated insulin resistant in patients of type 2 diabetes with obesity: a systematic review and meta-analysis. Lipids Health Dis. (2019) 18:205. doi: 10.1186/s12944-019-1127-z
51. Tan, LL, Duan, WQ, Chen, MX, Mei, Y, Qi, XY, and Zhang, Y. Naturally cultured high resistant starch rice improved postprandial glucose levels in patients with type 2 diabetes: a randomized, double-blinded, controlled trial. Front Nutr. (2022) 9:1019868. doi: 10.3389/fnut.2022.1019868
52. Patterson, MA, Fong, JN, Maiya, M, Kung, S, Sarkissian, A, Nashef, N, et al. Chilled potatoes decrease postprandial glucose, insulin, and glucose-dependent insulinotropic peptide compared to boiled potatoes in females with elevated fasting glucose and insulin. Nutrients. (2019) 11:2066. doi: 10.3390/nu11092066
53. García-Rodríguez, CE, Mesa, MD, Olza, J, Buccianti, G, Pérez, M, Moreno-Torres, R, et al. Postprandial glucose, insulin and gastrointestinal hormones in healthy and diabetic subjects fed a fructose-free and resistant starch type IV-enriched enteral formula. Eur J Nutr. (2013) 52:1569–78. doi: 10.1007/s00394-012-0462-x
54. Yuan, HC, Meng, Y, Bai, H, Shen, DQ, Wan, BC, and Chen, LY. Meta-analysis indicates that resistant starch lowers serum total cholesterol and low-density cholesterol. Nutr Res. (2018) 54:1–11. doi: 10.1016/j.nutres.2018.02.008
55. Bae, YJ, Kim, SH, Chung, JH, Song, SW, Kim, KS, Kim, MK, et al. Evaluation of adiposity-related biomarkers as metabolic syndrome indicators. Clin Nutr Res. (2013) 2:91–9. doi: 10.7762/cnr.2013.2.2.91
56. Vahdat, M, Hosseini, SA, Khalatbari Mohseni, G, Heshmati, J, and Rahimlou, M. Effects of resistant starch interventions on circulating inflammatory biomarkers: a systematic review and meta-analysis of randomized controlled trials. Nutr J. (2020) 19:33. doi: 10.1186/s12937-020-00548-6
57. Wei, Y, Zhang, X, Meng, Y, Wang, Q, Xu, H, and Chen, L. The effects of resistant starch on biomarkers of inflammation and oxidative stress: a systematic review and meta-analysis. Nutr Cancer. (2022) 74:2337–50. doi: 10.1080/01635581.2021.2019284
58. Castro, AR, Silva, SO, and Soares, SC. The use of high sensitivity C-reactive protein in cardiovascular disease detection. J Pharm Pharm Sci. (2018) 21:496–503. doi: 10.18433/jpps29872
59. Lee, SJ, and Choi, MG. Association of manganese superoxide dismutase gene polymorphism (V16A) with diabetic macular edema in Korean type 2 diabetic patients. Metabolism. (2006) 55:1681–8. doi: 10.1016/j.metabol.2006.08.011
60. Kangas-Kontio, T, Vavuli, S, Kakko, SJ, Penna, J, Savolainen, ER, Savolainen, MJ, et al. Polymorphism of the manganese superoxide dismutase gene but not of vascular endothelial growth factor gene is a risk factor for diabetic retinopathy. Br J Ophthalmol. (2009) 93:1401–6. doi: 10.1136/bjo.2009.159012
61. Namikawa, C, Shu-Ping, Z, Vyselaar, JR, Nozaki, Y, Nemoto, Y, Ono, M, et al. Polymorphisms of microsomal triglyceride transfer protein gene and manganese superoxide dismutase gene in non-alcoholic steatohepatitis. J Hepatol. (2004) 40:781–6. doi: 10.1016/j.jhep.2004.01.028
62. Moreto, F, de Oliveira, EP, Manda, RM, and Burini, RC. The higher plasma malondialdehyde concentrations are determined by metabolic syndrome-related glucolipotoxicity. Oxidative Med Cell Longev. (2014) 2014:505368. doi: 10.1155/2014/505368
63. Costa, JO, Vásquez, CMP, Santana, GJ, Silva, NJ, Braz, JM, Jesus, AMR, et al. Plasma total antioxidant capacity and cardiometabolic risk in non-obese and clinically healthy young adults. Arq Bras Cardiol. (2017) 109:01. doi: 10.5935/abc.20170095
64. Liu, S, Reimer, M, and Ai, Y. In vitro digestibility of different types of resistant starches under high-temperature cooking conditions. Food Hydrocoll. (2020) 107:105927. doi: 10.1016/j.foodhyd.2020.105927
65. Liang, D, Li, N, Dai, X, Zhang, H, and Hu, H. Effects of different types of potato resistant starches on intestinal microbiota and short-chain fatty acids under in vitro fermentation. Int J Food Sci Technol. (2021) 56:2432–42. doi: 10.1111/ijfs.14873
66. Nichenametla, SN, Weidauer, LA, Wey, HE, Beare, TM, Specker, BL, and Dey, M. Resistant starch type 4-enriched diet lowered blood cholesterols and improved body composition in a double blind controlled cross-over intervention. Mol Nutr Food Res. (2014) 58:1365–9. doi: 10.1002/mnfr.201300829
67. Upadhyaya, B, McCormack, L, Fardin-Kia, AR, Juenemann, R, Nichenametla, S, Clapper, J, et al. Impact of dietary resistant starch type 4 on human gut microbiota and immunometabolic functions. Sci Rep. (2016) 6:28797. doi: 10.1038/srep28797
68. Pugh, JE, Cai, M, Altieri, N, and Frost, G. A comparison of the effects of resistant starch types on glycemic response in individuals with type 2 diabetes or prediabetes: a systematic review and meta-analysis. Front Nutr. (2023) 10:1118229. doi: 10.3389/fnut.2023.1118229
69. Singh, J, Dartois, A, and Kaur, L. Starch digestibility in food matrix: a review. Trends Food Sci Technol. (2010) 21:168–80. doi: 10.1016/j.tifs.2009.12.001
70. Aguilera, JM. Food matrices as delivery units of nutrients in processed foods. J Food Sci. (2025) 90:e70049. doi: 10.1111/1750-3841.70049
71. Miao, M, and Hamaker, BR. Food matrix effects for modulating starch bioavailability. Annu Rev Food Sci Technol. (2021) 12:169–91. doi: 10.1146/annurev-food-070620-013937
72. Bazzano, LA, Green, T, Harrison, TN, and Reynolds, K. Dietary approaches to prevent hypertension. Curr Hypertens Rep. (2013) 15:694–702. doi: 10.1007/s11906-013-0390-z
73. Li, P-H, Wang, C-W, Lu, W-C, Chan, Y-J, and Wang, C-CR. Effect of resistant starch sources on the physical properties of dough and on the eating quality and glycemic index of salted noodles. Foods. (2022) 11:814. doi: 10.3390/foods11060814
74. Belobrajdic, DP, King, RA, Christophersen, CT, and Bird, AR. Dietary resistant starch dose-dependently reduces adiposity in obesity-prone and obesity-resistant male rats. Nutr Metab (Lond). (2012) 9:93. doi: 10.1186/1743-7075-9-93
75. Guo, J, Brown, PR, Tan, L, and Kong, L. Effect of resistant starch consumption on appetite and satiety: a review. J Agric Food Res. (2023) 12:100564. doi: 10.1016/j.jafr.2023.100564
76. Arias-Córdova, Y, Ble-Castillo, JL, García-Vázquez, C, Olvera-Hernández, V, Ramos-García, M, Navarrete-Cortes, A, et al. Resistant starch consumption effects on glycemic control and glycemic variability in patients with type 2 diabetes: a randomized crossover study. Nutrients. (2021) 13:4052. doi: 10.3390/nu13114052
77. Dongowski, G, Jacobasch, G, and Schmiedl, D. Structural stability and prebiotic properties of resistant starch type 3 increase bile acid turnover and lower secondary bile acid formation. J Agric Food Chem. (2005) 53:9257–67. doi: 10.1021/jf0507792
78. Metzler-Zebeli, BU, Canibe, N, Montagne, L, Freire, J, Bosi, P, Prates, JAM, et al. Resistant starch reduces large intestinal pH and promotes fecal lactobacilli and bifidobacteria in pigs. Animal. (2019) 13:64–73. doi: 10.1017/S1751731118001003
79. Thompson, MS, Yan, HT, Saari, N, and Sarbini, SR. A review: resistant starch, a promising prebiotic for obesity and weight management. Food Biosci. (2022) 50:101965. doi: 10.1016/j.fbio.2022.101965
80. Xu, M, Wang, S, Zou, J, Qin, X, Lv, Q, and Li, B. Effects of Lactobacillus plantarum fermentation on the structure and digestion of resistant starch type 3 and properties of fermented starch in the simulated digestion system. Carbohydr Polym. (2025) 353:123264. doi: 10.1016/j.carbpol.2025.123264
81. Sobh, M, Montroy, J, Daham, Z, Sibbald, S, Lalu, M, Stintzi, A, et al. Tolerability and SCFA production after resistant starch supplementation in humans: a systematic review of randomized controlled studies. Am J Clin Nutr. (2022) 115:608–18. doi: 10.1093/ajcn/nqab402
82. Zhou, J, Martin, RJ, Tulley, RT, Raggio, AM, KL, MC, Shen, L, et al. Dietary resistant starch upregulates total GLP-1 and PYY in a sustained day-long manner through fermentation in rodents. Am J Physiol Endocrinol Metab. (2008) 295:E1160–6. doi: 10.1152/ajpendo.90637.2008
83. Higgins, JA, Higbee, DR, Donahoo, WT, Brown, IL, Bell, ML, and Bessesen, DH. Resistant starch consumption promotes lipid oxidation. Nutr Metab (Lond). (2004) 1:8. doi: 10.1186/1743-7075-1-8
84. den Besten, G, Bleeker, A, Gerding, A, van Eunen, K, Havinga, R, van Dijk, TH, et al. Short-chain fatty acids protect against high-fat diet-induced obesity via a PPARγ-dependent switch from lipogenesis to fat oxidation. Diabetes. (2015) 64:2398–408. doi: 10.2337/db14-1213
85. Cuche, G, Cuber, JC, and Malbert, CH. Ileal short-chain fatty acids inhibit gastric motility by a humoral pathway. Am J Physiol Gastrointest Liver Physiol. (2000) 279:G925–30. doi: 10.1152/ajpgi.2000.279.5.G925
86. Massimino, SP, McBurney, MI, Field, CJ, Thomson, AB, Keelan, M, Hayek, MG, et al. Fermentable dietary fiber increases GLP-1 secretion and improves glucose homeostasis despite increased intestinal glucose transport capacity in healthy dogs. J Nutr. (1998) 128:1786–93. doi: 10.1093/jn/128.10.1786
87. Song, X, Dong, H, Zang, Z, Wu, W, Zhu, W, Zhang, H, et al. Kudzu resistant starch: an effective regulator of type 2 diabetes mellitus. Oxidative Med Cell Longev. (2021) 2021:4448048. doi: 10.1155/2021/4448048
88. Anhê, FF, Barra, NG, Cavallari, JF, Henriksbo, BD, and Schertzer, JD. Metabolic endotoxemia is dictated by the type of lipopolysaccharide. Cell Rep. (2021) 36:109691. doi: 10.1016/j.celrep.2021.109691
89. Nighot, M, Al-Sadi, R, Guo, S, Rawat, M, Nighot, P, Watterson, MD, et al. Lipopolysaccharide-induced increase in intestinal epithelial tight permeability is mediated by toll-like receptor 4/myeloid differentiation primary response 88 (MyD88) activation of myosin light chain kinase expression. Am J Pathol. (2017) 187:2698–710. doi: 10.1016/j.ajpath.2017.08.005
90. Qin, S, Bai, W, Applegate, TJ, Zhang, K, Tian, G, Ding, X, et al. Dietary resistant starch ameliorating lipopolysaccharide-induced inflammation in meat ducks associated with the alteration in gut microbiome and glucagon-like peptide 1 signaling. J Animal Sci Biotechnol. (2022) 13:91. doi: 10.1186/s40104-022-00735-x
91. Isayama, K, Rini, DM, Yamamoto, Y, and Suzuki, T. Propionate regulates tight junction barrier by increasing endothelial-cell selective adhesion molecule in human intestinal Caco-2 cells. Exp Cell Res. (2023) 425:113528. doi: 10.1016/j.yexcr.2023.113528
92. Miao, W, Wu, X, Wang, K, Wang, W, Wang, Y, Li, Z, et al. Sodium butyrate promotes reassembly of tight junctions in Caco-2 monolayers involving inhibition of MLCK/MLC2 pathway and phosphorylation of PKCβ2. Int J Mol Sci. (2016) 17:1696. doi: 10.3390/ijms17101696
93. Schilling, E, Weiss, R, Grahnert, A, Bitar, M, Sack, U, and Hauschildt, S. Molecular mechanism of LPS-induced TNF-α biosynthesis in polarized human macrophages. Mol Immunol. (2018) 93:206–15. doi: 10.1016/j.molimm.2017.11.026
94. Ferrer, M, Buey, B, Grasa, L, Mesonero, JE, and Latorre, E. Protective role of short-chain fatty acids on intestinal oxidative stress induced by TNF-α. Cell Stress Chaperones. (2024) 29:769–76. doi: 10.1016/j.cstres.2024.11.002
95. Liu, T, Li, J, Liu, Y, Xiao, N, Suo, H, Xie, K, et al. Short-chain fatty acids suppress lipopolysaccharide-induced production of nitric oxide and proinflammatory cytokines through inhibition of NF-κB pathway in RAW264.7 cells. Inflammation. (2012) 35:1676–84. doi: 10.1007/s10753-012-9484-z
96. Reuter, MA, Tucker, M, Marfori, Z, Shishani, R, Bustamante, JM, Moreno, R, et al. Dietary resistant starch supplementation increases gut luminal deoxycholic acid abundance in mice. Gut Microbes. (2024) 16:2315632. doi: 10.1080/19490976.2024.2315632
Keywords: resistant starch, effects, metabolic syndrome-related risks, systematic review, meta-analysis
Citation: Lin X, Li Z, Zheng D, Du R, Zhong R, Lin C and Meng H (2025) Effects of resistant starch consumption on anthropometric and serum parameters in adults with metabolic syndrome-related risks: a systematic review and meta-analysis. Front. Nutr. 12:1655664. doi: 10.3389/fnut.2025.1655664
Edited by:
Omar Guzmán Quevedo, Higher Technological Institute of Tacambaro, MexicoReviewed by:
Emmanouella Magriplis, Agricultural University of Athens, GreeceYingshuang Lu, Nankai University, China
Copyright © 2025 Lin, Li, Zheng, Du, Zhong, Lin and Meng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Changqing Lin, bGNxMDYwOEAxNjMuY29t; Hua Meng, bWVuZ2h1YWRlQGhvdG1haWwuY29t
 Ximing Lin
Ximing Lin Zaizhen Li2
Zaizhen Li2 Ruikang Zhong
Ruikang Zhong Hua Meng
Hua Meng