- 1Sydney Medical School Nepean, The University of Sydney, Sydney, NSW, Australia
- 2Nepean Hospital, Nepean Blue Mountains Local Health District, Sydney, NSW, Australia
- 3Charles Perkins Centre, The University of Sydney, Sydney, NSW, Australia
- 4School of Life and Environmental Sciences, The University of Sydney, Sydney, NSW, Australia
- 5Sydney Precision Data Science Centre, The University of Sydney, Sydney, NSW, Australia
Background: Diet and nutrients are emerging key players in neurological disorders. Attention-Deficit/Hyperactivity Disorder (ADHD) is a major neurodevelopmental disorder on a global scale, affecting children and increasingly being recognized and diagnosed in adult populations. While its aetiology is unclear, it appears to involve a combination of genetic and environmental factors, particularly food environments including diets and nutrients. However, most studies so far have focused on the impacts of individual nutrients or dietary patterns on clinically established ADHD. In contrast, the interactive effects of diets and nutrients and their complex interplay with other factors like socioeconomic status on ADHD prevalence and incidence have so far been overlooked. Here, we aim to systematically interrogate the association between nutrient supply, reflecting the food exposure and dietary environment, socioeconomic status and ADHD disease prevalence and incidence at a global level over time.
Methods: ADHD disease burden data (incidence and prevalence), macronutrient supply and gross domestic product (GDP) were collated from more than 150 countries from 1990 to 2018 and analyzed with nutritional geometric framework generalized additive mixed models (GAMMs).
Results: Modeling results suggested the interactive effects of food environment and socioeconomic status on ADHD. Fat supply, especially plant-based fat supply, is associated with decreased ADHD prevalence and incidence. These associations were conserved across sexes and ages. They were not confounded by the total energy supply.
Conclusion: Globally, fat, particularly plant-based fat supply in food environment correlated with the reduction of ADHD prevalence and incidence, implying its potential protective effects. This is supported by previous reports about the amelioration of ADHD with ketogenic diets. Further in-depth studies are needed to elucidate the underlying mechanistic. This might potentially provide some evidence for future targeted dietary interventions for ADHD prevention.
Introduction
Diets and nutrients are emerging critical players in neurological pathologies. For example, we have recently shown that a high fat low carbohydrate dietary environment is linked to reduced disease prevalence and incidence of multiple sclerosis and epilepsy globally (1, 2). This prompts us to investigate the effects of diets and nutrients on other neurological disorders.
Attention-Deficit/Hyperactivity Disorder (ADHD) is a common major neurodevelopmental disorder (3, 4), with increasing cases and disease-related mortality globally, and accounting for close to 1% of the disability-adjusted life year (DALY) of global mental disorders (5). ADHD is most prevalent in childhood and exhibits a gender bias toward males (5). Globally, ADHD seems to be substantially impacting Australasia and North America (5). The aetiology and pathophysiology of ADHD are still unresolved but appears to involve both genetic and environmental factors (5, 6). In this context, diets and nutrients have been a focus of interest, but results of these studies are so far inconclusive. It is generally acknowledged that ADHD is related to unhealthy diets. For example, “Western diets” and saturated fat were linked to ADHD aggravation (7, 8), while Mediterranean diets and polyunsaturated fatty acids were reported to improve ADHD symptoms (6, 9–12). However, most studies have primarily focused on the effects of specific foods or diets for ADHD treatment, while overlooking the effects of macronutrients and the possible interactions among nutrients and the broader socioeconomic contexts. Furthermore, previous studies were mostly based on small-scale cohorts, and there is a lack of knowledge about the potential protective effects of dietary exposure and food environments on ADHD at a population level. Considering the emerging global burden of ADHD, insights at a global scale are of great research and clinical interests.
The term “global food and nutritional environment” encompasses the accessibility, quality, quantity, affordability, and source of nutrients and foods available for human consumption. It is emerging as a critical player in determining global health and diseases (13). Global nutrient supply data is one of the upstream determinants of population-wide dietary patterns, and ultimately, individual diets. National nutrient supply has been shown to correlate with average national diet (14, 15), and is thus widely accepted as proxy of the food and nutritional environment, presenting unprecedented opportunities to decipher how food environment might be linked to changes in the global ADHD disease burden. In this context, disentangling the intercorrelatedness of complex data with linear and non-linear effects is challenging, and requires a multi-dimensional approach (16, 17). We previously proposed adopting nutritional geometric framework (NGF) generalized additive mixed models (GAMMs) for such analyses (1, 2, 16–19). NGF is a multi-dimensional analytical tool to systematically elucidate the individual, additive, and interactive effects of various nutritional factors (e.g., 3 macronutrients, protein, carbohydrate and fat) on outcomes of interests, like ADHD prevalence and incidence.
Leveraging NGF GAMMs, we examined the associations between macronutrient supply and ADHD incidence and prevalence at a global scale, also distinguishing the effects of plant- versus animal-based macronutrients. Our analyses will help to direct future diet- and nutrient-targeted research for ADHD management.
Materials and methods
Data collection and processing
Data collation and processing were adapted from our previous studies (17, 19) (Supplementary material). ADHD data was obtained from the Global Burden of Disease Study (GBD), in which ADHD is defined as an externalising disorder, incorporating disability from persistent inattention and/or hyperactivity-impulsivity. DSMIV-TR (314.0, 314.01) and ICD-10 (F90) diagnostic criteria were used. Macronutrient supply data were collected from the Food and Agriculture Organization Corporate Statistical Database (FAOSTAT, www.fao.org/faostat/en/#home). For protein and fat, their supply data (g/capita/day) were directly obtained from FAOSTAT and converted to supply data of caloric value (kcal/capita/day). For carbohydrate, supply data was calculated by subtracting protein, fat and alcohol supply quantities from the reported total caloric supply (kcal/capita/day), as previously described (13). For plant- versus animal-based nutrients comparison, the corresponding data were obtained from FAOSTAT directly based on FAOSTAT’s classifications. Gross domestic product (GDP) data was from the Maddison project (20).
In brief, analyses were based on global data from 1990 to 2018, which were first filtered for countries or time points with no data record. Where ADHD, macronutrient supply, or GDP data were missing for a given (country, time point) combination, it would be excluded. Resulting data, covering more than 150 countries globally were analyzed with R (Supplementary Figure S1).
Generalized additive mixed models (GAMMs)
Details of GAMMs were described in (1, 2, 17, 19) Supplementary material. GAMM is a type of multiple regression with similar assumptions to generalized linear models (21–23) and was used to examine the changes in ADHD prevalence and incidence over time and to evaluate the impacts from macronutrient supply. GAMMs account for the nonlinear terms as nonparametric smoothed functions, often in a form of spline. GAMMs provide a flexible manner to estimate the nonlinear associations, which are commonly found in nutritional studies and are gradually receiving more interests, given their implication in both health and diseases (1, 2, 16). Notably, GAMMs also account for a series of confounders, such as GDP in this case, which reflects broader national and socioeconomic development and can strongly influence diet and nutrient availability.
All modeling was run with the mgcv package (v1.9–1) (24, 25) in R v4.4.1. They considered the country that the data were based on as a random effect, partly accounting for some inter-country differences in environmental and genetic factors. Models with several variables (e.g., macronutrient supplies, GDP and time) and their different combinations, as well as a null model where only the random effect from the country is considered, are compared. Following our prior works (1, 2, 13, 17, 19), models with multiple variables consider all combinations of the individual, additive and interactive effects among parameters such as macronutrient supply, year and GDP data. The gamma parameter, stipulating the smoothing degrees of the modeled effects was defined as log(n)/2, where n is the number of combinations for countries and years with available data. A Gaussian family with log-link function was used for modeling.
Modeling results were compared using Akaike information criterion (AIC) and the models with the lowest AICs were selected. To examine if our models might be overfitted, latent scale intraclass correlations (ICCs) were computed. ICCs reflect the degree of variance explained by the countries’ random effects. In this context, when comparing two models, if including more parameters leads to a decrease in ICC, this suggests that the parameters help to account for part of the inter-country variance, indicating that their inclusions are meaningful.
GAMMs rely on visual depiction for the interpretation of the smooth terms, because it is not possible to quantify the fitted effects as effect sizes. Selected models were visualized by plotting their predicted values based on the corresponding variables (e.g., macronutrient supplies, etc.) as response surface heatmaps over the nutrient space. Within the modeling surfaces, red reflects higher, while blue indicates lower values. Along the black contour lines, the modeling values are unchanged and the numbers on the lines note the magnitude of the parameters. Visualization was done utilizing ggplot2 (v3.5.2), Cairo (v1.6–2), gridExtra (v2.3) packages in R.
Results
Global age-standardized ADHD prevalence and incidence mildly decreased over time (Figure 1A), accompanied by increases in global macronutrient supplies and GDP (Figure 1B). These parameters are interconnected, posing challenges to delineating their detailed associations (Figures 1C–E). Hence, GAMMs, a multi-dimensional modeling tool for global health analysis (13, 17), were deployed to interrogate simultaneously the potential complex interactive effects among various parameters and their links to ADHD. A model considering the interactions between macronutrient supplies and GDP, adding the time effects, was favored (Supplementary material), suggesting the interactive effects of macronutrients and socioeconomic status on ADHD. This model also had a lower latent scale ICC relative to the model only considering the effects of time, GDP, and the countries’ random effects, implying that inclusion of macronutrient parameters helps to explain some of the inter-country differences, justifying their inclusions in our modeling.
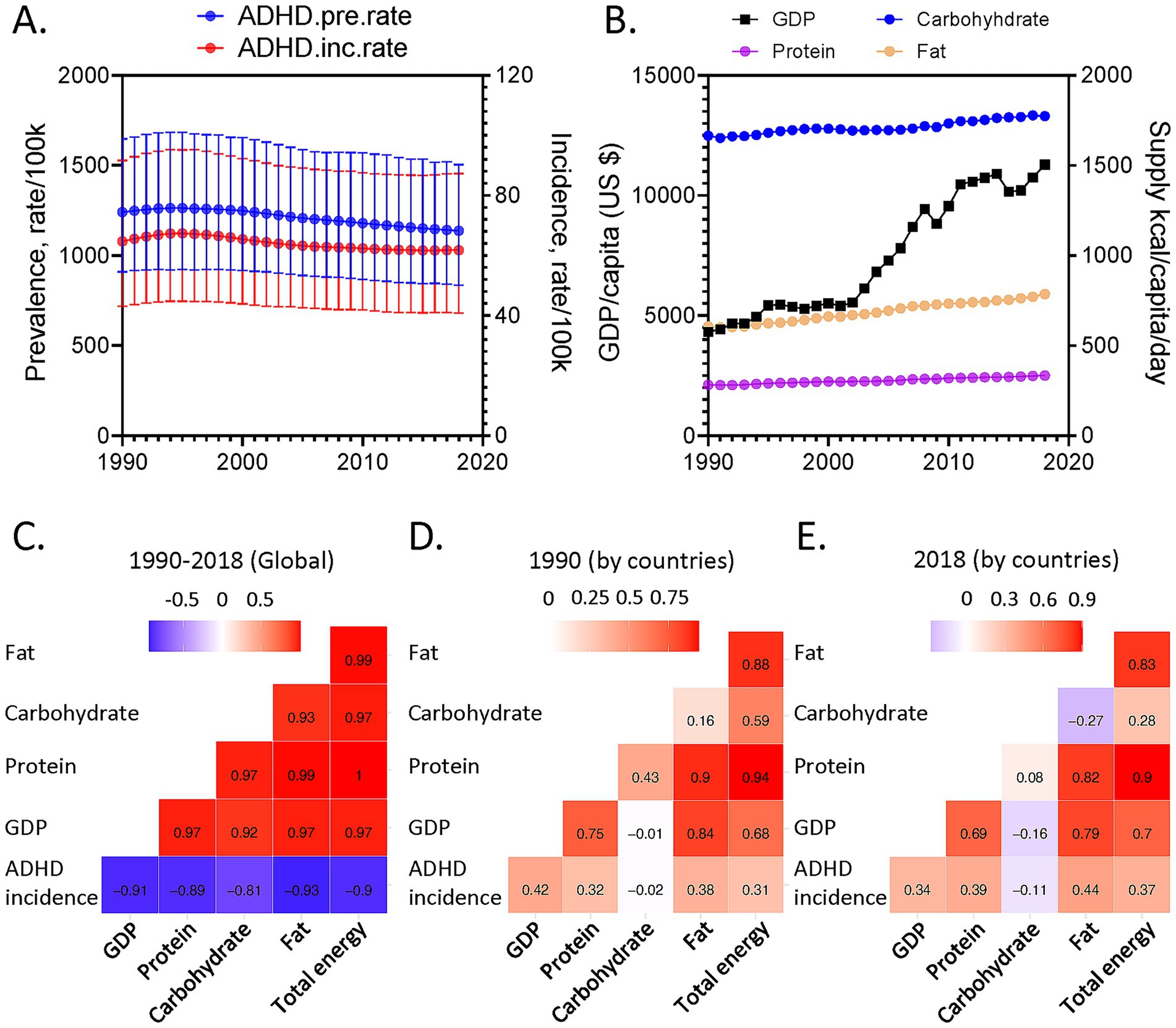
Figure 1. (A) Global age-standardized Attention-Deficit/Hyperactivity Disorder (ADHD) prevalence (blue) and incidence rate (red) of both sexes as functions of year. (B) Global GDP per capita (in US dollars, black) and supplies of carbohydrate (blue), protein (purple) and fat (brown) as functions of year. (C–E) Correlations for global variables from 1990 to 2018 (C) and for variables among different countries in 1990 (D) and 2018 (E). Correlation coefficients are shown.
Results for 2018 was shown as representative (Figure 2A), the most recent year with relatively comprehensive data coverage for more than 150 countries globally. Protein supply minimally influenced ADHD. In contrast, increasing fat supply correlated with lower ADHD incidence (Supplementary Tables S1, S2). The purple vector is an isocaloric line, along which the total energy supply is constant, and fat is isocalorically substituted for carbohydrate. Increasing fat:carbohydrate ratio is associated with lowered ADHD incidence. Similar patterns were consistently found for ADHD prevalence (Figure 2B; Supplementary Tables S3, S4) and across genders and ages (Supplementary Figures S2A–C). The red radial is a food rail. Along this vector, total energy supply differs, while fat:carbohydrate ratio is constant. Changing total energy supply only mildly impacted ADHD incidence. Importantly, sensitivity tests were performed, where (1) countries with the highest (Australia, Supplementary Figure S3A) or lowest (United Arab Emirates, Supplementary Figure S3B) ADHD incidences were excluded; (2) half of the countries from the dataset were randomly excluded (Supplementary Figure S4). They retrieved similar modeling results to our original findings, suggesting the robustness of our analyses.
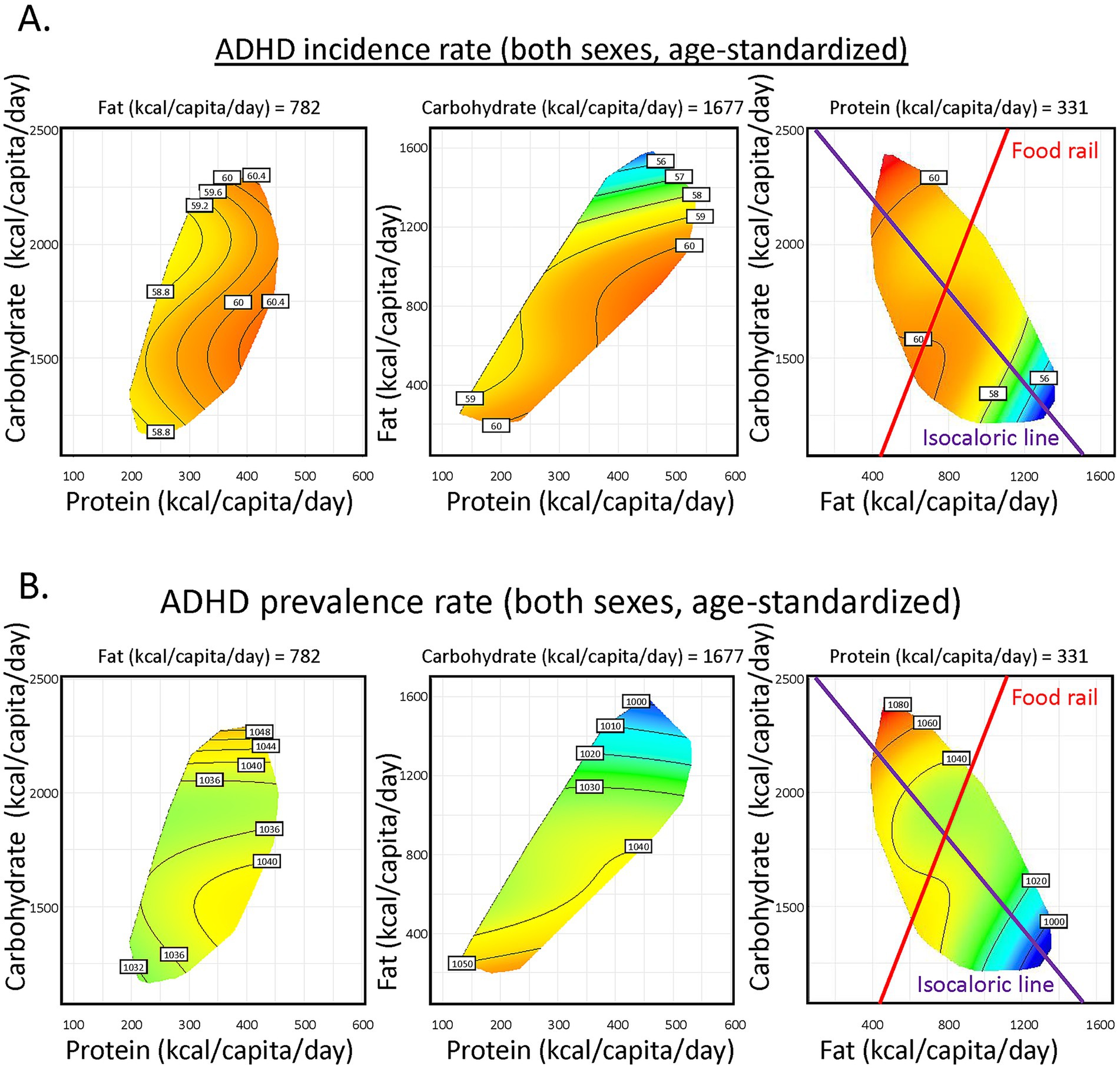
Figure 2. Global association of macronutrient supplies and Attention-Deficit/Hyperactivity Disorder (ADHD) incidence and prevalence. (A) Predicted effects of macronutrient supply on age-standardized ADHD incidence rate of both sexes. The left panel shows the effect of carbohydrate and protein supply, the middle shows fat and protein and the right shows carbohydrate and fat supplies. In each panel, the third macronutrient is set at median level (value shown). Surface colors reflect the modeled results, with red indicating higher ADHD incidence and blue indicating lower. (B) Predicted effects of macronutrient supply on age-standardized ADHD prevalence rate of both sexes (See Supplementary material for statistics and interpretation).
Effects of fat were further investigated, by sub-setting total fat into plant- (x-axis) and animal-based (y-axis) fat supplies based on the classifications from FAOSTAT, while combining carbohydrate and protein supplies (Figure 3; Supplementary Tables S5, S6). We showed the effects of plant and animal fats, assuming low, medium and high carbohydrate and protein supplies. Increasing plant-based fat supply associated with decreased ADHD incidence while animal-based fat exhibited limited effects. Along the purple isocaloric line, substituting animal-based fat with plant-based fat while holding total energy content constant is associated with reduced ADHD incidence rate. Surprisingly, for proteins of different sources, plant-based protein supply was linked to increased ADHD incidence (Supplementary Figure S4).
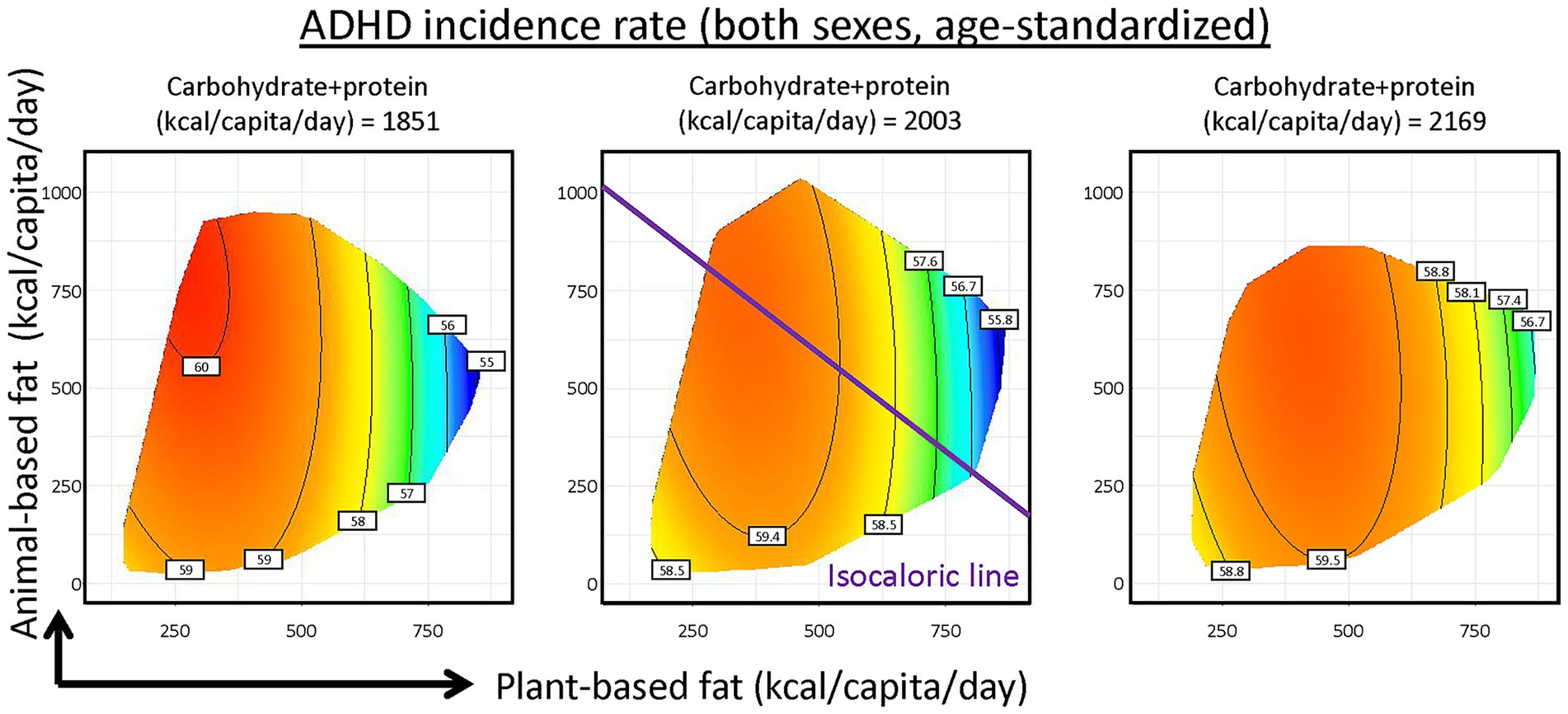
Figure 3. Predicted effects of carbohydrate and protein, animal- and plant-based fat supplies on Attention-Deficit/Hyperactivity Disorder (ADHD) incidence. Mode results were plotted in 2-dimensional nutritional space with plant-based fat supply as x-axis and animal-based fat supply as y-axis, with supply of carbohydrate and protein held at 25, 50 and 75% quantiles of global data (See Supplementary material for statistics and interpretation).
Discussion
This represents the first study to comprehensively examine global associations between macronutrient supply, as a proxy for food environments, and the incidence and prevalence of ADHD. Our state-of-the-art GAMM analyses provide evidence indicating potential protective effects in ADHD from fat, particularly plant-based fat, with their increasing supplies associated with reduced ADHD incidence and prevalence. This might inform future dietary interventions using plant-based fat for ADHD prevention and/or treatment.
Compared with previous dietary studies on ADHD, our analysis explored its associations with dietary macronutrient supplies and shed light on the potential protective effects of food environment in ADHD. Harnessing unprecedented nutrient supply and global epidemiological data, our study also exceeded any previous similar research regarding sample sizes and the time scales coverage. Our observations applied to both genders and across different age groups, underscoring their robustness. Moreover, we adapted the cutting-edge GAMM approach, thoroughly dissecting the individual, additive and interactive effects of various factors like different macronutrient supplies. This powerful tool might pave the way for future studies related to food environments.
There are still some limitations in our study to be addressed. For example, the diagnostic criteria for ADHD are evolving (26, 27), probably due to the fact that the detailed pathogenesis mechanisms of ADHD are still unresolved but likely to be multifaceted (5, 6). Therefore, in addition to the adjustments included in our present modeling, investigations into other aspects, particularly other environmental factors such as environmental toxins like lead, would be of interests (28). Changes in ADHD diagnostic criteria and a series of other factors, such as fluctuations in ADHD symptoms, differences in health infrastructure, and variations in ADHD reporting compliance, might together contribute to the underestimation of ADHD disease burden in GBD (5). Further validating analyses are needed when methodological issues are addressed in future GBD editions. Similarly, the spatial or temporal autocorrelations within the global data would warrant further explorations. Additionally, more granular global nutrient supply data are not available to allow comparison of simple versus complex carbohydrates, saturated versus non-saturated fats, or associations with supplies of micronutrients like iron, zinc and vitamins, requiring future investigation. Although nutrient supply data provide a proxy for the food environment and dietary exposure, further validation with cohort- or population-based longitudinal studies with more detailed dietary and food intake data, and ADHD data like disease biomarkers, is needed.
Of note, despite the clear patterns we observed, they are correlative and should be interpreted with caution. The causal links between dietary macronutrients and ADHD remains to be further validated and characterized. Previously, systemic investigations on the effects of dietary macronutrients on ADHD prevention and/or treatment are lacking. The most relevant dietary interventions to our results include high fat very low carbohydrate ketogenic diets (29, 30), Mediterranean diets (31, 32), and Mediterranean-DASH Intervention for Neurodegenerative Delay (MIND) diets (33). These have been shown to confer benefits toward ADHD in animal models and small-scale clinical cohorts, but they did not specifically investigate the impacts from macronutrients and their interactions. The fact that plant-based but not animal-based fat was linked to reduced ADHD prevalence and incidence in our analysis, is also in accord with prior studies demonstrating the benefits of vegetable-enriched diets for existing ADHD (12). Similarly, omega-3 polyunsaturated fatty acids, a major component of plant-based fats, have been reported as co-adjuvants for ADHD treatment (34). Nevertheless, further research is required to demonstrate the potential causality, delineate the mechanisms, as well as to interrogate whether plant-based fats potentially exert preventive effects in utero, during early infancy and/or directly modify ADHD.
Collectively, our multi-dimensional ecological analyses using NGF GAMM has revealed associations between nutrient supplies and ADHD, highlighting the correlations between the supplies of fat, particularly plant-based fat, and decreased ADHD incidence and prevalence. These findings may potentially provide guide toward future studies to investigate causality and, ultimately, interventions for ADHD management through food and dietary modifications.
Data availability statement
Publicly available datasets were analyzed in this study. This data can be found at: https://www.healthdata.org/research-analysis/gbd, https://www.fao.org/faostat/en/#data/SUA.
Author contributions
DN: Writing – review & editing, Writing – original draft. AS: Writing – original draft, Writing – review & editing. DR: Writing – review & editing. SS: Writing – review & editing. RN: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This project is supported by the Norman Ernest Bequest Fund.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2025.1658228/full#supplementary-material
Abbreviations
ADHD, Attention-Deficit/Hyperactivity Disorder; GDP, gross domestic product; NGF, nutritional geometric framework; GAMMs, generalized additive mixed models; GBD, Global Burden of Disease; FAOSTAT, Food and Agriculture Organization Corporate Statistical Database; AIC, Akaike information criterion.
References
1. Ni, D, Senior, A, Raubenheimer, D, Simpson, SJ, and Nanan, R. High-fat and low-carbohydrate dietary environments are linked to reduced idiopathic epilepsy incidence and prevalence. Ann Clin Transl Neurol. (2025) 12:1077–81. doi: 10.1002/acn3.70017
2. Ni, D, Tan, J, Reyes, J, Senior, AM, Andrews, C, Taitz, J, et al. High fat low carbohydrate diet is linked to CNS autoimmunity protection. Adv Sci (Weinh). (2025) 12:e2412236. doi: 10.1002/advs.202412236
3. Salari, N, Ghasemi, H, Abdoli, N, Rahmani, A, Shiri, MH, Hashemian, AH, et al. The global prevalence of ADHD in children and adolescents: a systematic review and meta-analysis. Ital J Pediatr. (2023) 49:48. doi: 10.1186/s13052-023-01456-1
4. Polanczyk, G, de Lima, MS, Horta, BL, Biederman, J, and Rohde, LA. The worldwide prevalence of ADHD: a systematic review and metaregression analysis. Am J Psychiatry. (2007) 164:942–8. doi: 10.1176/ajp.2007.164.6.942
5. Cortese, S, Song, M, Farhat, LC, Yon, DK, Lee, SW, Kim, MS, et al. Incidence, prevalence, and global burden of ADHD from 1990 to 2019 across 204 countries: data, with critical re-analysis, from the global burden of disease study. Mol Psychiatry. (2023) 28:4823–30. doi: 10.1038/s41380-023-02228-3
6. Faraone, SV, Bellgrove, MA, Brikell, I, Cortese, S, Hartman, CA, Hollis, C, et al. Attention-deficit/hyperactivity disorder. Nat Rev Dis Primers. (2024) 10:11. doi: 10.1038/s41572-024-00495-0
7. Colter, AL, Cutler, C, and Meckling, KA. Fatty acid status and behavioural symptoms of attention deficit hyperactivity disorder in adolescents: a case-control study. Nutr J. (2008) 7:8. doi: 10.1186/1475-2891-7-8
8. Morandini, HAE, Watson, P, Stewart, RM, Wong, JWY, Rao, P, and Zepf, FD. Implication of saturated fats in the aetiology of childhood attention deficit/hyperactivity disorder - a narrative review. Clin Nutr ESPEN. (2022) 52:78–85. doi: 10.1016/j.clnesp.2022.10.004
9. Del-Ponte, B, Quinte, GC, Cruz, S, Grellert, M, and Santos, IS. Dietary patterns and attention deficit/hyperactivity disorder (ADHD): a systematic review and meta-analysis. J Affect Disord. (2019) 252:160–73. doi: 10.1016/j.jad.2019.04.061
10. Martin, SM I, Sanz Rojo, S, Gonzalez Cosano, L, Conty De La Campa, R, Garicano Vilar, E, and Blumenfeld Olivares, JA. Impulsiveness in children with attention-deficit/hyperactivity disorder after an 8-week intervention with the Mediterranean diet and/or omega-3 fatty acids: a randomised clinical trial. Neurologia (Engl Ed). (2022) 37:513–23. doi: 10.1016/j.nrleng.2019.09.009
11. Sinn, N, and Bryan, J. Effect of supplementation with polyunsaturated fatty acids and micronutrients on learning and behavior problems associated with child ADHD. J Dev Behav Pediatr. (2007) 28:82–91. doi: 10.1097/01.DBP.0000267558.88457.a5
12. Pinto, S, Correia-de-Sa, T, Sampaio-Maia, B, Vasconcelos, C, Moreira, P, and Ferreira-Gomes, J. Eating patterns and dietary interventions in ADHD: a narrative review. Nutrients. (2022) 14:14. doi: 10.3390/nu14204332
13. Andrews, CJ, Raubenheimer, D, Simpson, SJ, and Senior, AM. Associations between national plant-based vs animal-based protein supplies and age-specific mortality in human populations. Nat Commun. (2025) 16:3431. doi: 10.1038/s41467-025-58475-1
14. Remans, R, Wood, SA, Saha, N, Anderman, TL, and DeFries, RS. Measuring nutritional diversity of national food supplies. Glob Food Secur. (2014) 3:174–82. doi: 10.1016/j.gfs.2014.07.001
15. Del Gobbo, LC, Khatibzadeh, S, Imamura, F, Micha, R, Shi, P, Smith, M, et al. Assessing global dietary habits: a comparison of national estimates from the FAO and the global dietary database. Am J Clin Nutr. (2015) 101:1038–46. doi: 10.3945/ajcn.114.087403
16. Senior, AM, Legault, V, Lavoie, FB, Presse, N, Gaudreau, P, Turcot, V, et al. Multidimensional associations between nutrient intake and healthy ageing in humans. BMC Biol. (2022) 20:196. doi: 10.1186/s12915-022-01395-z
17. Senior, AM, Nakagawa, S, Raubenheimer, D, and Simpson, SJ. Global associations between macronutrient supply and age-specific mortality. Proc Natl Acad Sci USA. (2020) 117:30824–35. doi: 10.1073/pnas.2015058117
18. Senior, AM, Raubenheimer, D, Couteur, DGL, and Simpson, SJ. The geometric framework for nutrition and its application to rodent models. Annu Rev Anim Biosci. (2024) 13:389–410. doi: 10.1146/annurev-animal-111523-102327
19. Ni, D, Senior, AM, Raubenheimer, D, Simpson, SJ, Macia, L, and Nanan, R. Global associations of macronutrient supply and asthma disease burden. Allergy. (2024) 79:1989–91. doi: 10.1111/all.16067
20. Inklaar, R, de Jong, H, Bolt, J, and Van Zanden, JL“Rebasing ‘Maddison’: new income comparisons and the shape of long-run economic development” in GGDC Research Memorandum (Groningen Growth and Development Center), (2018). Vol. GD174.
21. Diederich, A. Generalized additive models.: an introduction with. J Math Psychol. (2007) 51:339–9.
22. Verbeke, T. Generalized additive models: an introduction with R. J Roy Stat Soc A. (2007) 170:262. doi: 10.1111/j.1467-985X.2006.00455_15.x
23. Wood, SN, Goude, Y, and Shaw, S. Generalized additive models for large data sets. J Royal Statis. Soc. Series C. (2015) 64:139–55. doi: 10.1111/rssc.12068
24. Wood, SN. Fast stable restricted maximum likelihood and marginal likelihood estimation of semiparametric generalized linear models. J R Stat Soc Series B Stat Methodol. (2011) 73:3–36. doi: 10.1111/j.1467-9868.2010.00749.x
25. Wood, SN. Generalized additive models: An introduction with R Chapman and Hall/CRC, New York (2017). Available online at: https://www.taylorfrancis.com/books/mono/10.1201/9781315370279/generalized-additive-models-simon-wood
26. Rigler, T, Manor, I, Kalansky, A, Shorer, Z, Noyman, I, and Sadaka, Y. New DSM-5 criteria for ADHD - does it matter? Compr Psychiatry. (2016) 68:56–9. doi: 10.1016/j.comppsych.2016.03.008
27. Sibley, MH, and Kuriyan, AB. DSM-5 changes enhance parent identification of symptoms in adolescents with ADHD. Psychiatry Res. (2016) 242:180–5. doi: 10.1016/j.psychres.2016.05.036
28. Moore, S, Paalanen, L, Melymuk, L, Katsonouri, A, Kolossa-Gehring, M, and Tolonen, H. The association between ADHD and environmental chemicals-a scoping review. Int J Environ Res Public Health. (2022) 19:19. doi: 10.3390/ijerph19052849
29. Liu, Y, Yang, C, Meng, Y, Dang, Y, and Yang, L. Ketogenic diet ameliorates attention deficit hyperactivity disorder in rats via regulating gut microbiota. PLoS One. (2023) 18:e0289133. doi: 10.1371/journal.pone.0289133
30. Garner, S, Davies, E, Barkus, E, and Kraeuter, AK. Ketogenic diet has a positive association with mental and emotional well-being in the general population. Nutrition. (2024) 124:112420. doi: 10.1016/j.nut.2024.112420
31. Darabi, Z, Vasmehjani, AA, Darand, M, Sangouni, AA, and Hosseinzadeh, M. Adherence to Mediterranean diet and attention-deficit/hyperactivity disorder in children: a case control study. Clin Nutr ESPEN. (2022) 47:346–50. doi: 10.1016/j.clnesp.2021.11.014
32. Rios-Hernandez, A, Alda, JA, Farran-Codina, A, Ferreira-Garcia, E, and Izquierdo-Pulido, M. The Mediterranean diet and ADHD in children and adolescents. Pediatrics. (2017) 139:139. doi: 10.1542/peds.2016-2027
33. Bayranj, Z, Fotros, D, Sohouli, MH, Rohani, P, Eslahi, M, Ferdosi, S, et al. The relation between MIND diet with odds of attention-deficit/hyperactivity disorder in Iranian children: a case-control study. Child Neuropsychol. (2025) 31:331–45. doi: 10.1080/09297049.2024.2375493
Keywords: Attention-Deficit/Hyperactivity Disorder (ADHD), fat, plant-based fat, nutrient, diet, food environment
Citation: Ni D, Senior A, Raubenheimer D, Simpson SJ and Nanan R (2025) Food environment with high plant-based fat supply is associated with Attention-Deficit/Hyperactivity Disorder (ADHD) protection: a global study with more than 150 countries. Front. Nutr. 12:1658228. doi: 10.3389/fnut.2025.1658228
Edited by:
Patrick Noël Pallier, Queen Mary University of London, United KingdomReviewed by:
Hugo Morandini, Child and Adolescent Health Service, AustraliaAbhishek Das, International Crops Research Institute for the Semi-Arid Tropics (ICRISAT), India
Copyright © 2025 Ni, Senior, Raubenheimer, Simpson and Nanan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ralph Nanan, cmFscGgubmFuYW5Ac3lkbmV5LmVkdS5hdQ==
 Duan Ni
Duan Ni Alistair Senior
Alistair Senior David Raubenheimer
David Raubenheimer Stephen J. Simpson
Stephen J. Simpson Ralph Nanan
Ralph Nanan