- 1Department of Nutrition and Food Hygiene, School of Public Health, Southwest Medical University, Luzhou, China
- 2Department of Clinical Nutrition, Chongqing General Hospital, Chongqing University, Chongqing, China
- 3School of Public Health, Southwest Medical University, Luzhou, China
- 4Department of Medical Cell Biology and Genetics, School of Basic Medical Science, Southwest Medical University, Luzhou, China
- 5Environmental Health Effects and Risk Assessment Key Laboratory of Luzhou, School of Public Health, Southwest Medical University, Luzhou, China
Background: Obesity is turning into a more critical problem for public health. Vitamin D3 (VD3) may be strongly linked to obesity.
Objectives: The study aims to examine the influence of VD3 on IL-27 levels and the molecular mechanism by which VD3 affects white fat beige through the IL-27/P38MAPK/PGC-1α pathway.
Methods: Firstly, a small sample population study was conducted to compare the disparities in serum 25(OH)D3 and IL-27 between individuals with obesity and healthy control groups. Secondly, twenty-four Wistar rats were separated into three groups: CON, HFD, and HFD + VD3 groups. Following 7 weeks of intervention, detection of biochemical indicators in serum by enzyme-linked immunosorbent assay (ELISA), mRNA, and protein expression of vitamin D receptor (VDR), IL-27R, P38MAPK, PGC-1α, and UCP-1 in inguinal adipose tissue by RT-qPCR and western blot. Finally, 3T3-L1 cells were induced into a hypertrophic adipose model, knock down IL-27 or PGC-1α using small interfering RNA, treated with 100 nM Calcitriol for 24 h, and divided into CON, PA, PA + 1,25(OH)2D3, PA + si IL-27, PA + si IL-27 + 1,25(OH)2D3, PA + si PGC-1α, and PA + si PGC-1α + 1,25(OH)2D3 groups. Detection of TC, TG, and IL-27 levels by ELISA, mRNA, and protein expression of VDR, IL-27R, P38MAPK, PGC-1α, UCP-1, and CD137 in cell supernatant by RT-qPCR and western blot.
Results: A correlation was identified between serum 25(OH)D3 and IL-27 in the population-based study. However, no statistically significant difference in serum 25(OH)D3 or IL-27 levels was observed between the observation group and the control group. After VD3 intervention, TC, TG, and the number of LDs were significantly reduced in both HFD rats and 3T3-L1 cells, and serum IL-6 and MCP-1 in HFD rats were decreased. Meanwhile, there was a significant increase in mRNA and protein expression for VDR, IL-27R, P38MAPK, and PGC-1α. The expressions of the UCP-1 protein and the CD137 mRNA dramatically increased. Knockdown of IL-27 eliminated the increasing effect of calcitriol on the expression of P38MAPK, PGC-1α, UCP-1, and CD137 in 3T3-L1 cells, and knockdown of PGC-1α eliminated the increasing effect of calcitriol on the expression of UCP-1 and CD137 in 3T3-L1 cells.
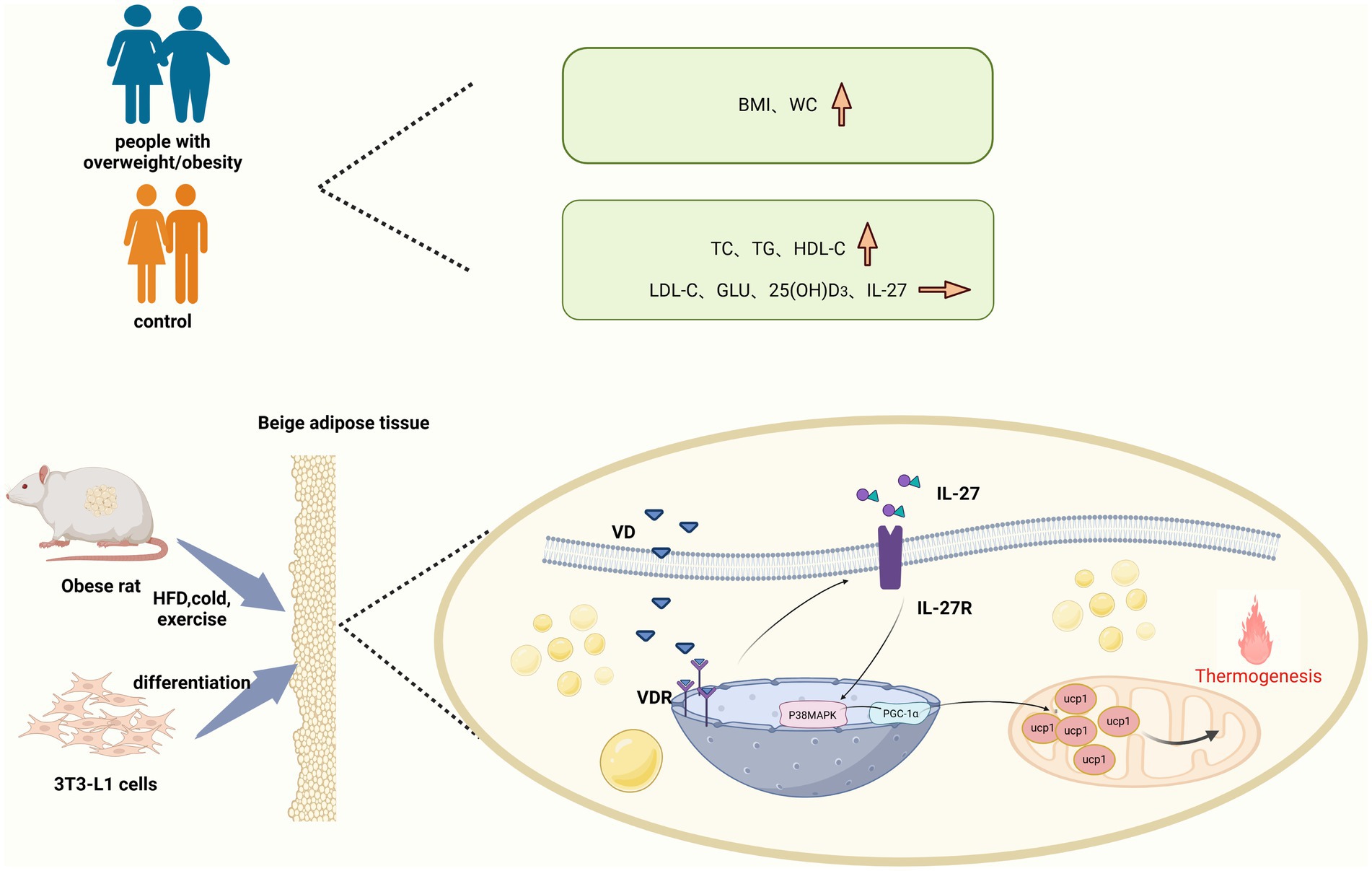
Conclusion: The study shows that VD3 may promote white fat beige through the IL-27/P38MAPK/PGC-1α pathway.
1 Introduction
Obesity is characterized by an excessive accumulation of body fat, particularly in specific regions. The imbalance between energy intake and expenditure in adipose tissue leads to weight gain, ultimately resulting in obesity. In recent decades, mammalian adipose tissue has been broadly classified into three categories: white adipose tissue (WAT), brown adipose tissue (BAT), and beige adipose tissue (1). White adipocytes function primarily as energy reservoirs, whereas brown adipocytes act as energy consumers. Certain progenitor cells and white adipocytes located in WAT can undergo a process known as “browning” when exposed to specific stimuli such as cold exposure, exercise, and pharmacological interventions (2, 3). Active beige adipose tissue is capable of generating substantial heat through the production of a key protein called mitochondrial uncoupling protein 1 (UCP-1) (4). Elevated levels of free fatty acids can enhance UCP-1 protein expression, prompting beige adipose tissue to metabolize a significant amount of fatty acids, thereby facilitating their clearance. Consequently, the activation of beige fat is anticipated to emerge as a novel therapeutic target for weight loss and the amelioration of related metabolic disorders and inflammatory responses (5).
The effect of nutrients on gene expression is termed nutrigenomics. This field concentrates on understanding how dietary components influence gene expression through epigenetic mechanisms. Nutrients found in the diet, such as polyphenols, fatty acids, and vitamins, can modulate epigenetic markers, including DNA methylation, histone modifications, and non-coding RNAs. These modifications subsequently impact gene expression related to glucose metabolism, lipid homeostasis, and inflammation. Nutrigenomics offers a scientific foundation for personalized nutritional interventions by examining the interactions between genes and nutrients, thereby playing a crucial role in managing chronic diseases, such as obesity and related metabolic syndromes (6).
Interleukin-27 (IL-27), a member of the IL-12 family, is an exogenous dimeric cytokine (7). IL-27 has a direct inhibitory effect on γ delta T17 cells, a class of innate immune cells that respond rapidly to inflammation. By activating the STAT1 pathway, IL-27 inhibits IL-17 production in γ delta T17 cells, thereby alleviating inflammation (8). A research team discovered that the overexpression of IL-27 promotes the expression of key thermogenic genes in brown adipose tissue, while simultaneously reducing chronic inflammation and macrophage infiltration within white adipose tissue (9). IL-27 and IL-27R interact to initiate the activation of the p38 MAPK signaling pathways (10). The p38MAPK pathway serves a crucial function in the differentiation of fat precursor cells. TAK-715 (p38 inhibitor) significantly inhibits adipocyte differentiation, reduces intracellular TG and lipid accumulation, and the p38MAPK pathway directly regulates adipogenesis (11). After IL-27 intervention, the key transcription activators PGC-1α and UCP-1, which control energy metabolism in beige adipocytes, were significantly upregulated (9). CD137 is one of the key biomarkers for the differentiation of beige adipocytes, and its expression changes are closely related to the process of beige (12, 13). UCP-1, PGC-1α, and CD137 expressions suggest the existence of beige adipose-like cells within white adipose tissue, which enable adaptive heat production and facilitate energy expenditure.
Furthermore, emerging evidence suggests that micronutrients may exert an effect on body weight regulation, adipocyte differentiation, and the transformation of white adipocytes into brown or beige adipocytes (14). Vitamin D3 (VD3) is a lipophilic hormone derived from cholesterol, with its main active form being 1,25(OH)2D3 (15). VD3 is not only involved in the regulation of calcium and phosphorus balance but is also closely related to inflammation, immune regulation, energy metabolism, and fat production (16). Vitamin D can directly inhibit inflammation in adipose tissue and reduce the expression of pro-inflammatory factors such as TNF-α and IL-6 (17). Vitamin D may partially mitigate the cancer-promoting effects of obesity by modulating mechanisms such as inflammation, insulin resistance, and oxidative stress (18). A large population study from China found that children and adolescents with vitamin D deficiency were significantly more susceptible to obesity and metabolically unhealthy obesity (19). A comprehensive meta-analysis demonstrated that individuals with obesity have a 35% greater likelihood of experiencing vitamin D3 deficiency when compared to individuals with normal weight, and a 24% higher risk when compared to those who are overweight (20). A wealth of evidence suggests that VD3 represents a viable target for food-based nutritional interventions (21–24). Chen et al. found that vitamin D3 supplementation has the potential to modulate mitochondrial function and improve tubulointerstitial fibrosis in diabetic rats (25). Results from Xiang’s team suggest that VD3 supplementation can alter the composition of the gut microbiota in mice and improve parameters of obesity caused by HFD (26).
Previous studies have reported that 1,25(OH)2D3 can impede lipid droplet (LD) fusion, promote LD decomposition, reduce LD volume, and inhibit lipogenesis in 3T3-L1 cells via the PPAR-α signaling pathway (27). However, the effects of vitamin D3 on white fat beige and the specific molecular mechanisms involved remain to be further explored. Therefore, this study aims to investigate the correlation between 25(OH)D3 and IL-27 in a small-sample population. Additionally, by constructing high-fat diet Wistar rat and 3T3-L1 mature adipocyte models, we explored the effects of VD3 on IL-27 levels and the specific mechanisms by which VD3 regulates energy metabolism and promotes white fat beige through the IL-27/P38MAPK/PGC-1α pathway.
2 Materials and methods
2.1 Population trial design
In the Introduction of the Recommended Opinions on Chinese Adult Body Mass Index Classification (28), the proposed body mass index (BMI) standards for Chinese individuals are as follows: “healthy weight” is defined as a BMI of 18.5 kg/m2 ≤ BMI < 24 kg/m2; “Overweight” is classified as a BMI of 24 kg/m2 ≤ BMI < 28 kg/m2; and “Obese” is defined as a BMI of 28 kg/m2 or greater. For the observation group (people with overweight and obesity), the inclusion criteria were as follows: ① age ranging from 18 to 45 years and ② BMI ≥ 24 kg/m2. The control group inclusion criteria were: ① age ranging from 18 to 45 years and ② BMI ranging from 18.5 kg/m2 to < 24 kg/m2. Participants were excluded if they met any of the following conditions: individuals with diabetes; patients who had taken diet pills within the past 3 months; individuals who had received vitamin D supplementation or complex preparations within the past 3 months; individuals with osteoporosis and autoimmune diseases. Previous studies have indicated that the difference of ±s of serum 25(OH)D3 level between healthy people and people with overweight and obesity was 6.4 ± 8.71. The minimum sample size required for each group was 31 cases by means of comparison of two independent samples using PASS 15.0 software. Eventually, the trial included 64 individuals, where 33 were designated for the observation group and 31 for the control group.
Participants were recruited from a local university through posters and online platforms. Following an overnight fast of 8 hours, physiological parameters were assessed, and approximately 3–5 mL of blood was drawn from the median cubital vein of the volunteers for serological analysis. This case–control study received ethical approval from the Research Ethics Committee of Southwest Medical University, and written informed consent was obtained from each participant (Reference Number: SWMUIRBKS-202307-0007).
2.2 Human serological test
Following an overnight fast of at least 8 hours, all participants underwent a comprehensive medical evaluation in the morning. A trained nurse measured and documented their age, height, weight, BMI, and waist circumference. Fasting venous blood samples were collected, and serum was separated through centrifugation. Enzyme-linked immunosorbent assay (ELISA) was utilized to analyze the serum levels of 25(OH)D3, IL-27, TG, TC, HDL-C, LDL-C, and GLU. The kits were sourced from Mlbio (Mlbio, China) and Jiancheng (Jiancheng Bio, China).
2.3 Animals
Five-week-old male Wistar rats (140-150 g) were bought from Dossy Experimental Animal Co., Ltd. (Chengdu, Sichuan, China). The rats were maintained in a controlled environment at a temperature of 23 ± 1°C and a humidity of 50 ± 10%, with a 12-h light/dark cycle, permitting ad libitum access to food and water. A total of 24 rats were randomly assigned to two groups: the CON group (n = 8), which received standard feed for 14 weeks, and the high-fat feed group (n = 16), which was administered high-fat feed for 14 weeks. At week 7, 16 rats in the HFD group were weighing 20% higher than the mean weight in the NCD group and randomly partitioned into the HFD group (n = 8) and the HFD + VD group (n = 8) for a 7-week intervention. In the HFD + VD group, cholecalciferol (1,25(OH)D3; Sigma, Germany) was solubilized in maize oil (Aladdin, China) at a concentration of 2.5 mg/mL, administered intragastrically at a dosage of 12.5 mg/kg (29, 30). During the experiment, the rats’ food intake was recorded every day, and their weight was measured and recorded every week. At the end of the 14th week, the rats were anesthetized with pentobarbital sodium (45 mg·kg−1·BW) and killed. Blood was collected from the heart and centrifuged at 4000 rpm for 15 min. Then, serum samples were extracted for further examination. Inguinal adipose tissue was removed from each animal, weighed immediately, and stored at −80°C. The Lee’s index was calculated as follows: [(weight)⅓/body length ×1,000]. All sampling processes were carried out in an SPF environment. All animal research procedures strictly adhere to the 1996 National Institutes of Health Guidelines for the Care and Use of Laboratory Animals. The experimental protocols conducted in this study have received approval from the Ethics Committee of Southwest Medical University, in accordance with established guidelines (Approval No. 2020612; Date of approval: August 17, 2020).
2.4 Cell culture
3T3-L1 adipocytes were cultured in DMEM supplemented with 10% fetal bovine serum (FBS; Cellmax, Australia). The cells were maintained in a standard cell culture incubator at 37°C with 5% CO2. To induce differentiation in the 3T3-L1 cells, the “cocktail method” was employed. The cells were seeded into 6-well plates and allowed to reach confluency. Two days after reaching confluency, the growth media was supplemented with 10 μg/mL insulin (Sigma-Aldrich, Germany), 1 μmol/L dexamethasone (Sigma-Aldric, Germany), and 0.5 mmol/L 1-methyl-3-isobutyl-xanthine (Sigma-Aldrich, Germany). The differentiation process continued for 2 days. From Days 4 to 7, the cells were maintained in growth media supplemented with 10 μg/mL insulin. Subsequently, the mature adipocytes were cultured in regular growth media. Following 8–10 days of culture, the cells reached a mature adipocyte state. Building upon this foundation, a model of hypertrophic adipocytes was established by intervening with 300 μM palmitic acid (PA; Pythonbio, China) for 24 h. After this intervention, the research group further intervened with 100 nM calcitriol (MCE, USA) for an additional 24 h (27).
2.5 Small-interfering RNA (siRNA) transfection
RNA oligonucleotides targeting IL-27 (palindromic sequence: CAAUCAGGUGUCAUCCCAA) and PGC-1α (palindromic sequence: GUAGCGACCAAUCGGAAAUTT) were procured from TSINGKE (Beijing, China) and GenePharma (Shanghai, China) to facilitate the silencing of the target proteins. Negative control siRNAs, which exhibited no homology with the mouse genome, were also obtained from TSINGKE and GenePharma. The 3T3-L1 cells were transfected with siRNA utilizing Lipofectamine 2000 (Invitrogen, USA). Following the establishment of the cells as a model for hypertrophic adipocytes, the aforementioned siRNA (2.5 μg) and 5 μL of Lipofectamine 2000 reagent were diluted in 250 μL of Opti-MEM medium (BasalMedia, China) and incubated separately for 5 min at room temperature. After this incubation period, equal volumes of the diluted siRNA and Lipofectamine 2000 reagent were gently mixed and allowed to incubate for an additional 15 min at room temperature to form siRNA-lipid complexes. For transfection, these siRNA-lipid complexes were subsequently incubated with the 3T3-L1 cells in 6-well culture plates for 24 h. Following transfection, the cells were treated with 100 nM calcitriol (MCE, USA) for 24 h and subsequently harvested.
2.6 ORO staining of frozen section and 3T3-L1 cells
A cryotome was used to cut 22 μm sections of frozen adipose tissue, which were then immersed in phosphate buffer solution (PBS) for 10 s, followed by three consecutive rinses in distilled water, each lasting 2 s. The samples were subsequently soaked in 60% isopropyl alcohol for 5 min and rinsed. They were then dipped in oil red O staining solution for 10 min, followed by a brief wash in distilled water for 2 s, and finally encased in glycerin gelatin. Images were captured using a microscope (Motic, China).
After being washed twice with phosphate-buffered saline (PBS; Solarbio, China), 3T3-L1 cells were fixed in 4% paraformaldehyde for 30 min. Following fixation, the cells were washed with a detergent for 30 s and then incubated with a refined Oil Red O (ORO) working solution (Beyotime, China).
2.7 Biochemical analysis
The serum levels of TG, TC, HDL-C, LDL-C, and GLU were measured using an automatic biochemical analyzer (Olympus, Japan).
2.8 ELISA analysis
The serum levels of TNF-α (NeoBioscience, China), MCP-1 (NeoBioscience, China), IL-6 (NeoBioscience, China), IL-27 (Mlbio, China), and 25(OH)D3 (Elabscience, China) were quantified using ELISA kits according to the manufacturer’s instructions. Homogenize the inguinal adipose tissue in working fluid, 12,000 × g, Centrifuge for 10 min, remove the supernatant, and repeat this process twice. The TG content and TC content of adipose tissue were tested according to the production manual. TC and TG in the cell lysate were detected. After VD3 intervention, the cell supernatant was centrifuged, and the levels of IL-27 (Mlbio, China) were detected through ELISA.
2.9 Western blot analysis
Total protein was extracted from inguinal adipose tissue and 3T3-L1 cells, and the concentration was measured using the bicinchoninic acid (BCA) method (Solarbio, China). Protein was separated by 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis (Solarbio, China) and transferred to polyvinylidene fluoride (PVDF) membrane (Millipore, USA). The membranes were blocked with 5% skim milk (Solarbio, China) containing 0.1% Tween® 20 (Solarbio, China) for 2 h. After washing with 0.1% tris buffer saline 20 (TBST), incubate the membrane with specific antibodies overnight. On the second day after TBST washing, incubate with antibody (CST, USA) conjugated with rabbit immunoglobulin G (IgG) and horseradish peroxidase (HRP) at a dilution of 1:2000 for 1 h. Protein expression was detected using the GelView system (BLT, China), and immune response analysis was performed using ImageJ (ImageJ 1.45 s, USA). The specific antibodies were against vitamin D receptor (VDR) (Abcam, UK), IL-27R (ABclonal, Chinese), P38MAPK (CST, USA), PGC-1α (Affinity, China), and UCP-1 (Abcam, UK). β-actin (CST, USA) and GAPDH (CST, USA) were used as internal references.
2.10 RT-qPCR
Total RNA was isolated from frozen inguinal adipose tissue (rats) or 3T3-L1 cells using RNAiso Plus (Takara, Japan). The purity and concentration of total RNA were measured using an Ultra-Micro Nucleic Acid Assay (ThermoFisher, USA) and PrimeScript RT kit (Takara, Japan) to reverse transcribe the total RNA into cDNA. Purified TB green fluorescent dye mixture (Takara, Japan) was used to conduct RT qPCR analysis in a real-time fluorescence quantitative PCR instrument (qTOWER3G, Analytical Jena) according to the protocol. The relative expression levels of the genes were calculated with the 2−ΔΔCt method, using β-actin as an endogenous reference. The sequences of the primers were listed in Supplementary Table 1.
2.11 Statistical analysis
Statistical analyses were performed with SPSS 26.0 (SPSS Inc., USA) and GraphPad 10 (GraphPad Software, USA). Data are shown as the mean ±SEM. Univariate analysis of variance (ANOVA) was used to evaluate the differences among the three groups. When p < 0.05, the Dunnett multiple comparison method was used to compare the differences between the two groups. The correlation between human serum 25(OH)D3 and IL-27 was evaluated using the Spearman correlation method. Statistical significance was set at p < 0.05 (two-tailed).
3 Results
3.1 The fundamental information and biochemical parameters of the participants
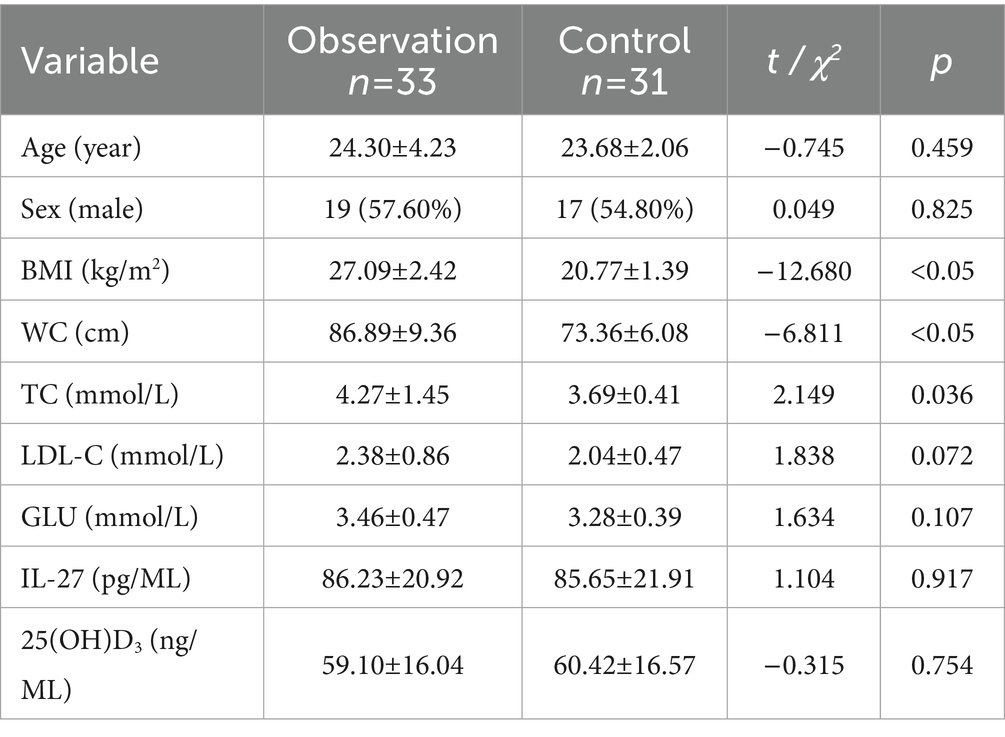
Listed in Table 1 are the fundamental information and biochemical parameters of the participants. Compared with the control group, the BMI, WC, TC, and TG of the participants in the observation group were significantly increased, and HDL-C was significantly decreased (Table 1; Figures 1A–C). The two groups showed no disparity in serum LDL-C and GLU (Table 1; Figures 1D–E).
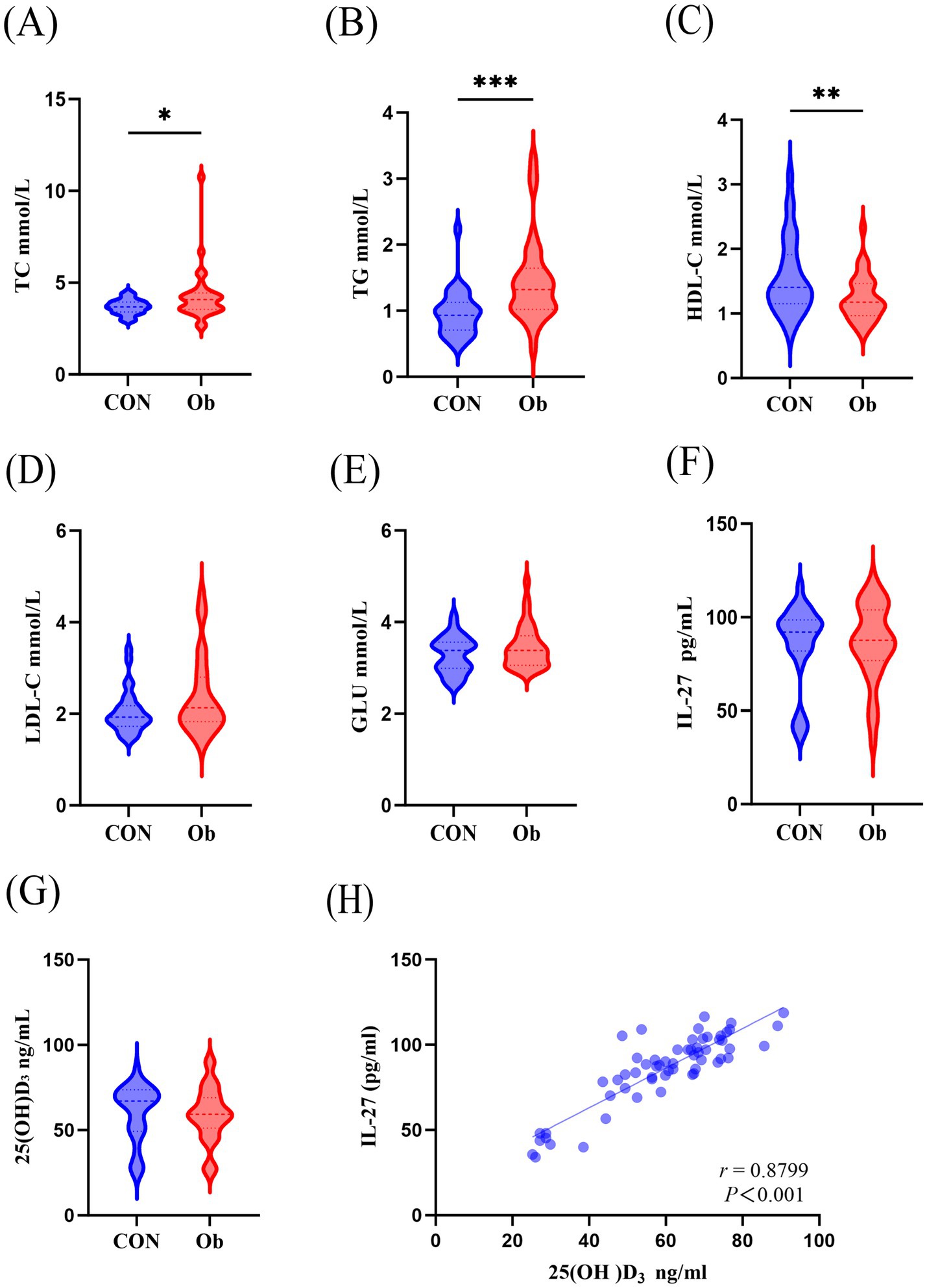
Figure 1. Comparison of serum biochemical indicators between the observation group (people with overwight/obese) and the control group. (A) Serum TC, Total cholesterol, (B) TG, Triglyceride, (C) HDL-C, High density lipoprotein cholesterol, (D) LDL-C, Low density lipoprotein cholesterol, (E) GLU, Glucose, (F) IL-27, Interleukin-27, (G) 25(OH)D3 levels. (H) Correlation analysis of serum 25(OH)D3 and IL-27. Data were shown as the mean ±SEM. (n = 64) *p < 0.05, **p < 0.01, ***p < 0.001.
3.2 IL-27 is positively correlated with 25(OH)D3
There were no changes in serum 25(OH)D3 and IL-27 levels between the two groups (Figures 1F,G), but interestingly, a positive correlation was observed between serum 25(OH)D3 and IL-27 levels (Figure 1H).
3.3 VD3 reduces fat accumulation and inflammatory secretion in vivo
The results showed that compared to the CON rats, the levels of large round transparent LD, TC, and TG in the inguinal adipose tissue of rats induced by HFD were significantly increased. Following intervention with VD3, the size of LDs in the adipose tissue was reduced, and there was a notable decrease in TC and TG levels (Figures 2A,B,G,H). Additionally, HFD rats exhibited significantly increased energy intake and Lee’s index compared to CON rats; however, after VD3 treatment, energy intake was substantially reduced. Notably, there were no significant changes observed in body weight, Lee’s index, or iWAT following VD3 supplementation (Figures 2C–F). Furthermore, serum TC, TG, GLU, IL-6, and TNF-α of rats in the HFD group were not obviously changed, while LDL-C and MCP-1 were sharply improved. Conversely, the serum HDL-C, 25(OH)D3, and IL-27 were remarkably reduced (Figures 3A–J). Following VD3 intervention, serum GLU, IL-6, MCP-1, and 25(OH)D3 were significantly decreased (Figures 3A–I), while the serum IL-27 level of HFD rats was significantly increased (Figure 3J).
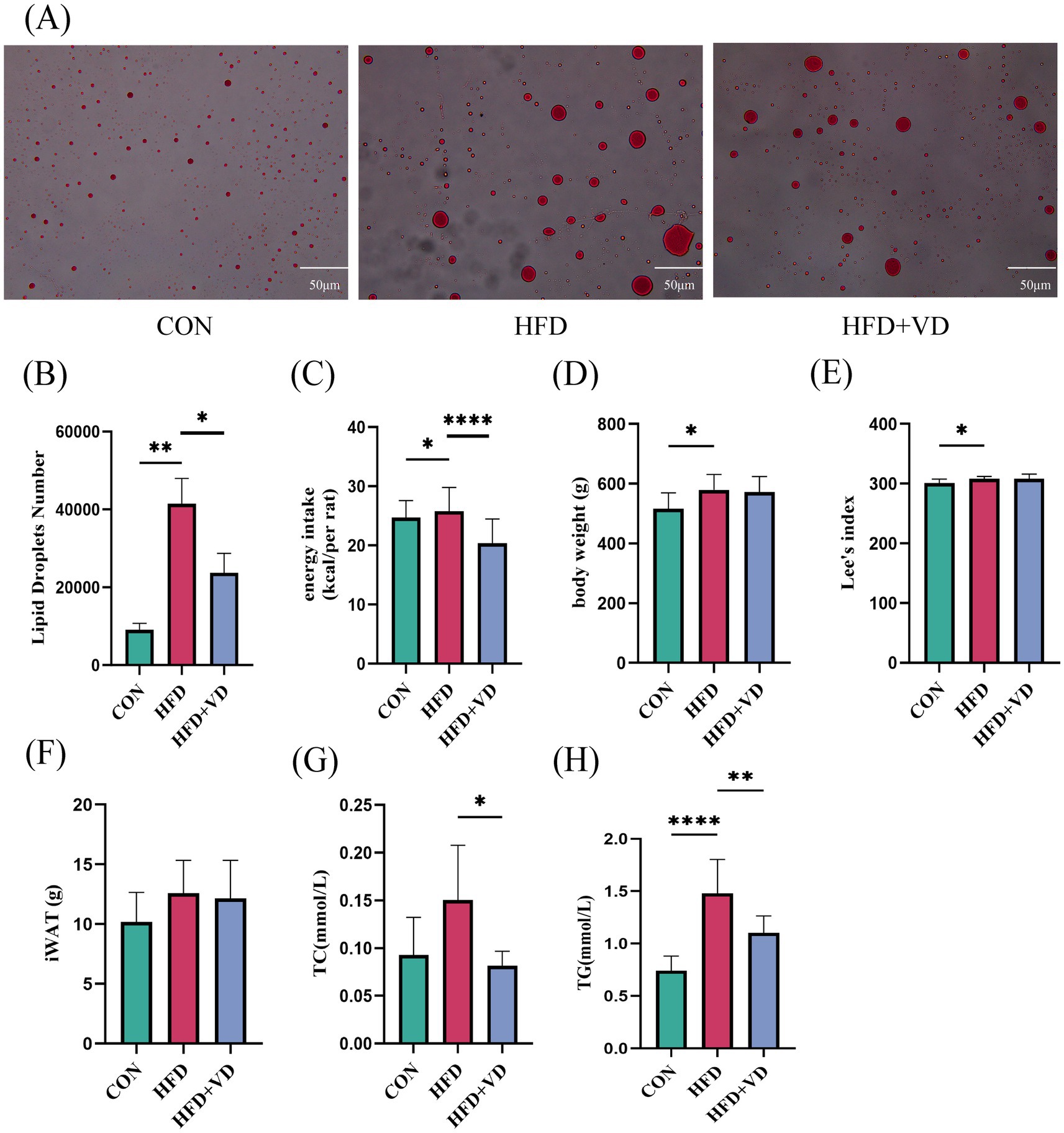
Figure 2. Vitamin D3 intervention improved lipid deposition in rats on a high fat diet. (A) Oil red O staining of white adipose tissue in the groin (400 × magnification). (B) Number of LDs. (C) Energy intake. (D) Body weight. (E) Lee’s index, (F) Inguinal adipose tissue. (G) TC, (H) TG levels in inguinal adipose tissue. Data were shown as the mean ±SEM. *p < 0.05, **p < 0.01, ****p < 0.0001.
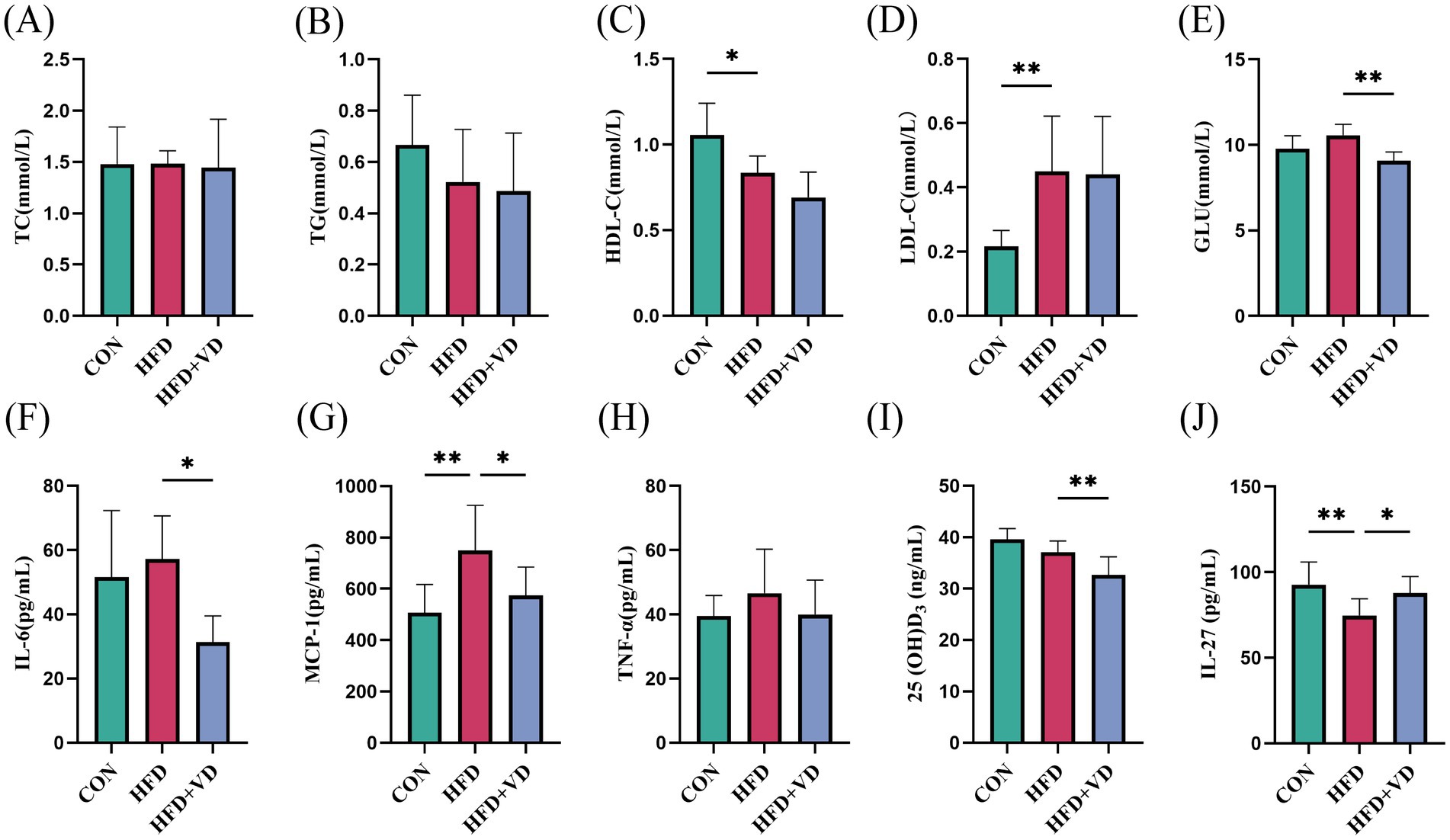
Figure 3. Effects of Vitamin D3 on levels of serum lipids, inflammatory factors, 25(OH)D3 and IL-27 in high-fat feeding rats. (A) Serum TC, (B) TG, (C) HDL-C, (D) LDL-C, (E) GLU, (F) IL-6, (G) MCP-1, (H) TNF-α, (I) 25(OH)D3,(J) IL-27 levels. Data were shown as the mean ±SEM. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
3.4 VD3 promotes white fat beige in HFD rats
The experimental results indicated that, compared to the CON rats, the protein expressions of VDR, IL-27R, and P38MAPK, as well as the mRNA expressions of IL-27R and PGC-1α, were significantly decreased in the inguinal adipose tissue of HFD rats. In contrast, the protein expressions of PGC-1α and UCP-1, along with the mRNA expressions of VDR and P38MAPK, did not show significant changes (Figures 4A–K). Following VD3 intervention, there was a substantial improvement in the protein expressions of VDR, IL-27R, P38MAPK, and UCP-1, and in the mRNA expressions of VDR, IL-27R, P38MAPK, and PGC-1α; additionally, the protein expression of PGC-1α exhibited an upward trend (Figures 4A–K). Therefore, it is suggested that VD3 can affect the IL-27/P38MAPK/ PGC-1α pathway, which may enhance lipid deposition in serum and inguinal fat of high-fat diet rats and promote white fat beige.
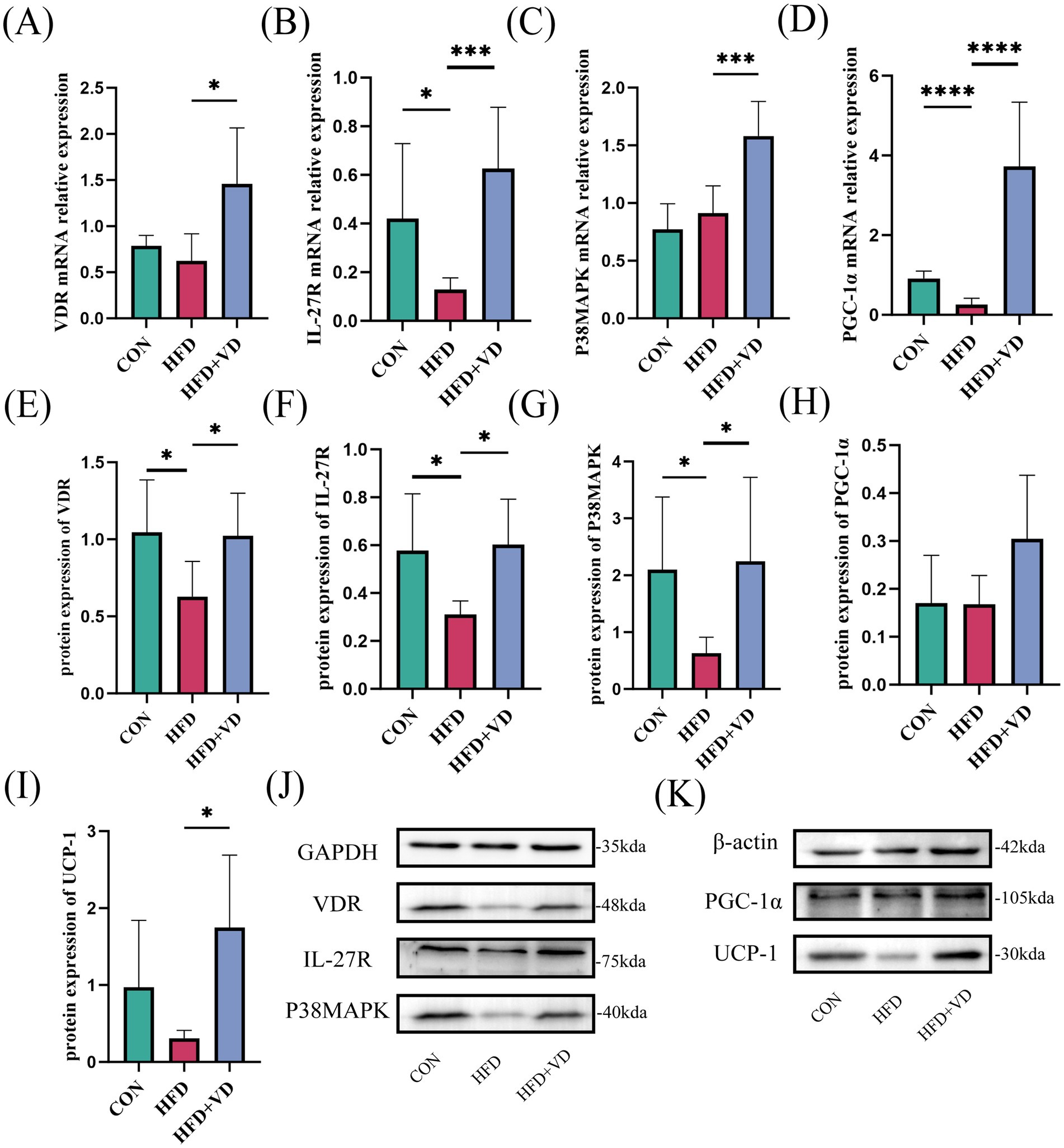
Figure 4. Effects of vitamin D3 on mRNA and protein levels of IL-27/P38MAPK/PGC-1α pathway in high-fat diet rats. (A–D) The mRNA expression of VDR, IL - 27R, P38MAPK, and PGC-1α were detected by qPCR, (E–K) The protein expression of VDR, IL-27R, P38MAPK, PGC-1α, and UCP-1 were detected by western blot, Data were shown as the mean ±SEM. *p < 0.05, ***p < 0.001, ****p < 0.0001.
3.5 Calcitriol reduces lipid deposition in vitro
To investigate the impact of calcitriol on lipid accumulation, an in vitro model of hypertrophic adipocytes was developed. Hypertrophic adipocytes were treated with calcitriol at a concentration of 100 nM. The results indicated that, compared to the control group of mature adipocytes, PA induced a significant accumulation of LD, intracellular TG, and intracellular TC in mature adipocytes. Furthermore, the level of IL-27 was significantly lower in the PA group compared to the CON group. Treatment with 100 nM calcitriol significantly inhibited PA-induced lipid accumulation; the number of LDs in hypertrophic adipocytes was significantly decreased, and the levels of TC and TG were significantly decreased, while the level of IL-27 was significantly increased (Figures 5A–E).
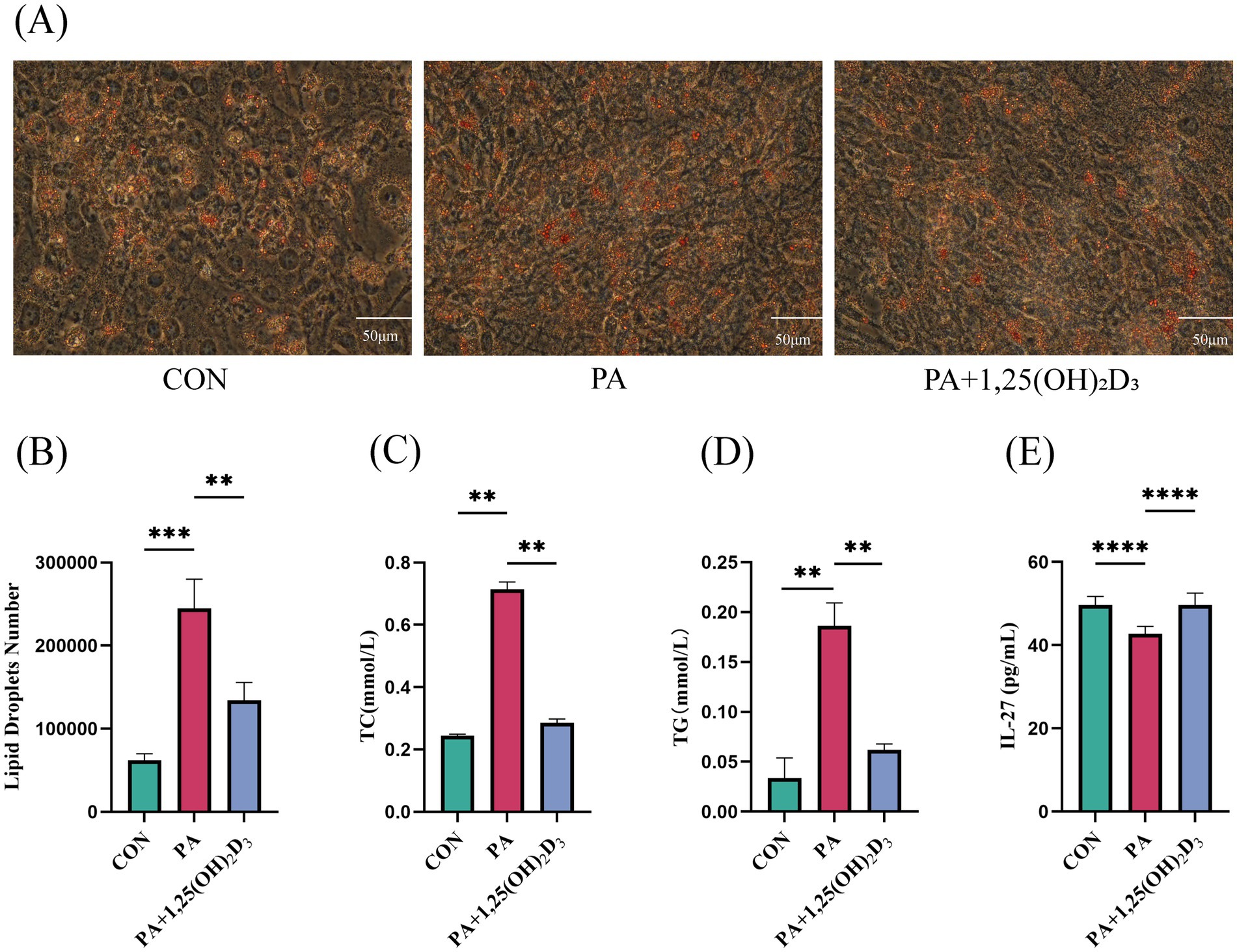
Figure 5. Calcitriol reduces lipid deposition and increases the levels of IL-27 in 3T3-L1 cell. (A) Intracellular lipid accumulation was detected by Oil red O staining method using an inverted microscope (400 × magnification). (B) Number of LDs. (C) Content of TC, (D) TG,(E) IL-27 in 3T3-L1 cells. Data were shown as the mean ±SEM. *p < 0.05, **p < 0.01, ***p < 0.001.
3.6 Calcitriol promotes the beige of 3T3-L1 cells
Rt-qPCR and western blot were used to detect the expression levels of IL-27/P38MAPK/PGC-1α pathway-related mRNA and protein. In comparison to the control group, the mRNA and protein expressions of VDR, IL-27R, and the mRNA expression of CD137 in the PA group remained unchanged (Figures 6A,B,E–G). Simultaneously, the mRNA expression of P38MAPK was significantly reduced, while its protein expression did not exhibit significant changes (Figures 6C,H). Additionally, both mRNA and protein expressions of PGC-1α were significantly decreased (Figures 6D,I). A decreasing trend was observed in the protein expression of UCP-1 (Figures 6J,L). Following treatment with 100 nM 1,25(OH)2D3 for 24 h, the mRNA expression levels of VDR, IL-27R, P38MAPK, PGC-1α, and CD137 were significantly elevated (Figures 6A–E). Simultaneously, the protein expression levels of VDR, IL-27R, P38MAPK, PGC-1α, and UCP-1 also exhibited significant increases (Figures 6F–L).
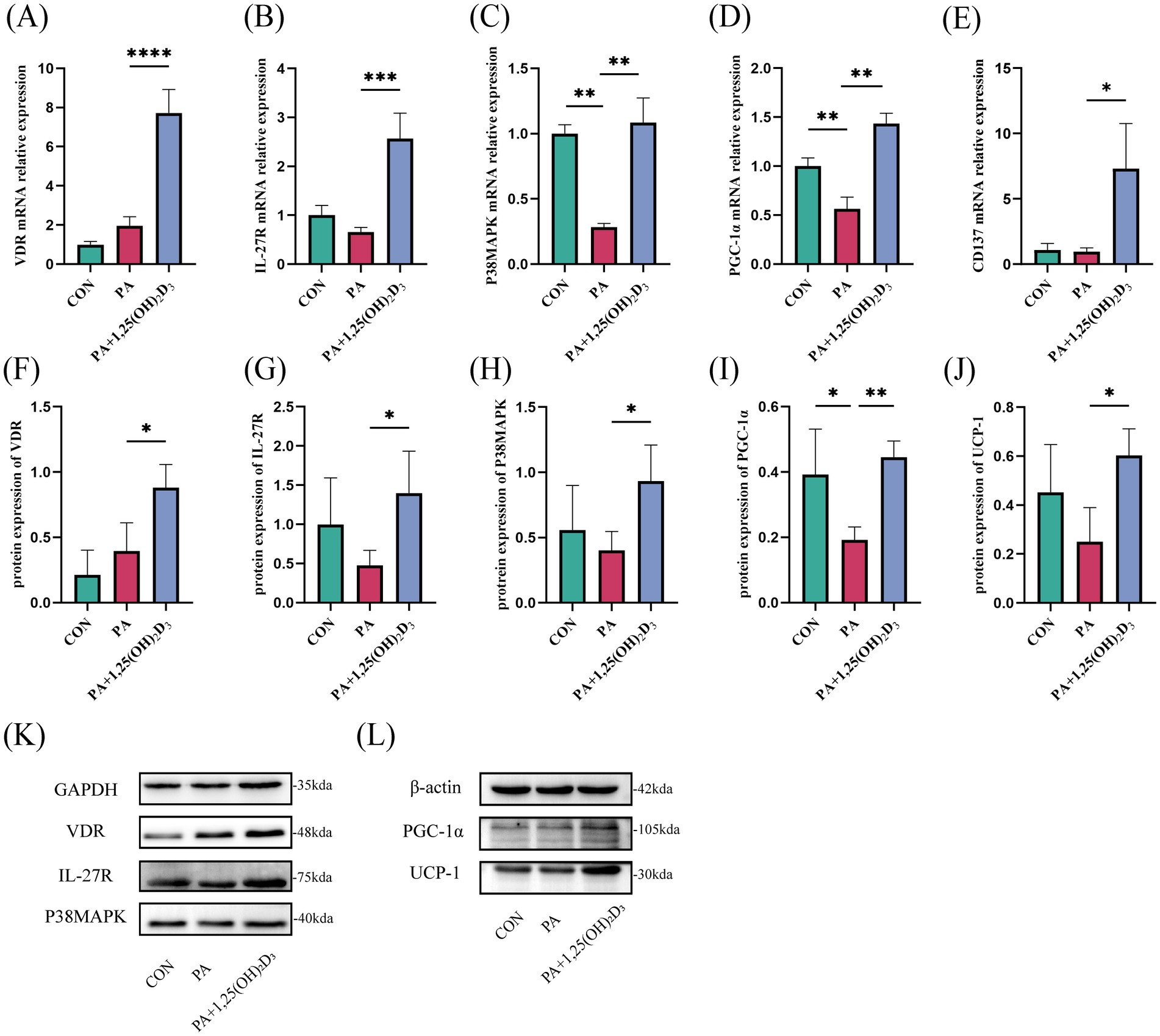
Figure 6. Effects of vitamin D3 on the IL-27/P38MAPK/PGC-1α pathway at mRNA and protein levels in 3T3-L1 cells. (A–E) The mRNA expression of VDR,IL-27R,P38MAPK,PGC-1α, and CD137 were detected by qPCR. (F–L) The protein expression of VDR, IL-27R, P38MAPK, PGC-1α and UCP-1 were detected by western blot, Data were shown as the mean ±SEM. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
3.7 IL-27 siRNA and PGC-1α siRNA reverse the amelioration of calcitriol in 3T3-L1 cells
IL-27 siRNA and PGC-1α siRNA were utilized to further explore whether vitamin D3 promotes browning of hypertrophic adipocytes by affecting the IL-27/P38MAPK/PGC-1α pathway. Initially, following IL-27 siRNA transfection, the mRNA and protein expressions of VDR in the PA + si IL-27 group were not significantly changed compared to the PA group (Figures 7A,G,L). Conversely, the mRNA and protein expression of IL-27R, P38MAPK, PGC-1α, UCP-1, and the mRNA expression of CD137 were significantly decreased (Figures 7B–F,L,M). After treatment with 100 nM 1,25(OH)2D3 for 24 h, the mRNA and protein expressions of VDR were markedly increased (Figures 7A,G,L). However, the mRNA and protein expression of IL-27R, P38MAPK, PGC-1α, UCP-1, and the mRNA expression of CD137 remained unchanged (Figures 7B–F,H).
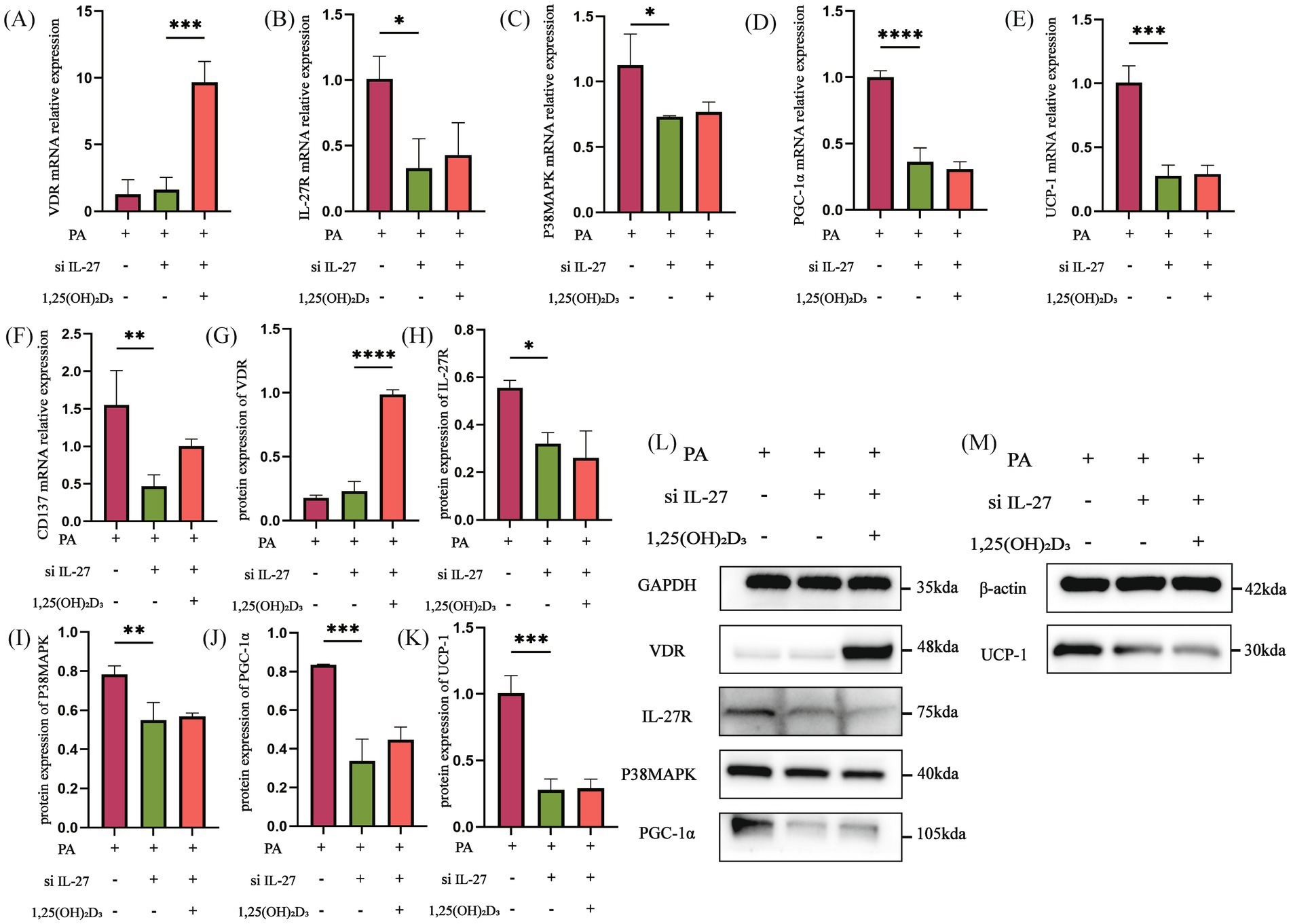
Figure 7. IL-27 siRNA inhibited the effect of Vitamin D3. (A–F) The mRNA expression of VDR, IL-27R, P38MAPK, PGC-1α, UCP-1 and CD137 were detected by qPCR. (G–M) The protein expression of VDR, IL-27R, P38MAPK, PGC-1α, and UCP-1 were detected by western blot, Data were shown as the mean ±SEM. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
Then, following PGC-1α siRNA transfection, the mRNA and protein expressions of VDR, IL-27R, and P38MAPK in the PA + si PGC-1α group did not show significant changes compared to the PA group (Figures 8A–C,G). However, the mRNA and protein expressions of PGC-1α, UCP-1, and the mRNA expression of CD137 were significantly decreased (Figures 8D–F,J). After treatment with 100 nM 1,25(OH)2D3 for 24 h, both the mRNA and protein expressions of VDR and IL-27R were significantly increased (Figures 8A,B,G,H,L). The mRNA expression of P38MAPK was significantly increased, whereas its protein expression did not exhibit significant changes (Figures 8C,I,L). Additionally, the mRNA and protein expressions of PGC-1α, UCP-1 and the mRNA expression of CD137 were not significantly changed (Figures 8D–F,J,K,M). Following IL-27/PGC-1α siRNA transfection, the activity of the IL-27/P38MAPK/PGC-1α pathway was decreased.
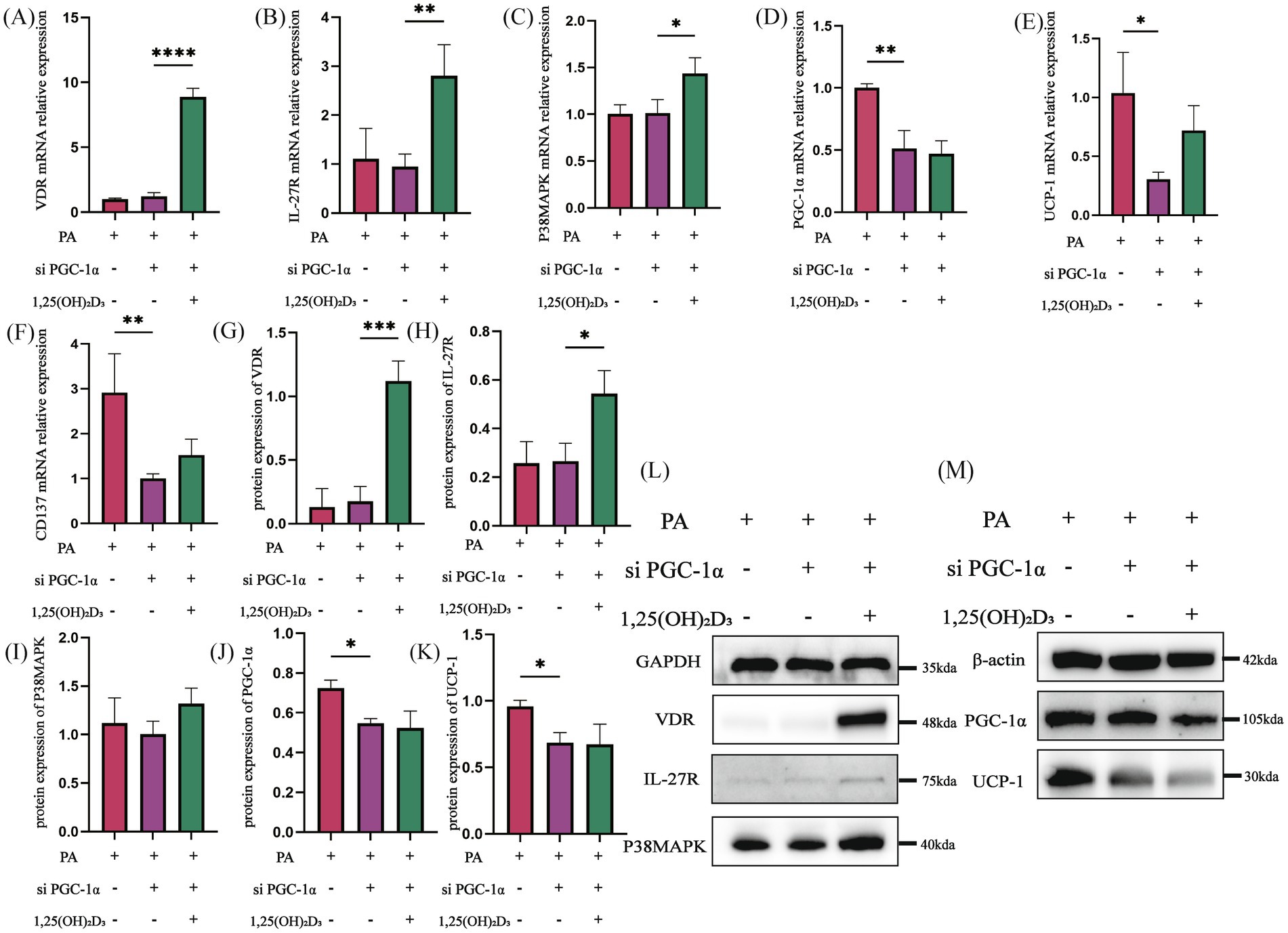
Figure 8. PGC-1α siRNA inhibited the effect of Vitamin D3. (A–F) The mRNA expression of VDR, IL-27R, P38MAPK, PGC-1α, UCP-1 and CD137 were detected by qPCR. (G–M) The protein expression of VDR, IL-27R, P38MAPK, PGC-1α, and UCP-1 were detected by western blot, Data were shown as the mean ±SEM. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
4 Discussion
Overweight and obesity have become a global epidemic, with prevalence rates demonstrating a sustained upward trajectory worldwide (31). Currently, promoting white fat beige is considered one of the feasible strategies for obesity prevention. Accumulating studies have increasingly stressed the critical role of VD3 in the pathogenesis of obesity (32). Previous research has also found that vitamin D3 can enhance lipolysis and inhibit lipogenesis (27). This study reveals the significant and essential role of VD3 in ameliorating lipid deposition and explores its effects on the IL-27/P38MAPK/PGC-1α pathway.
The level of IL-27 may be associated with VD3. Following VD3 supplementation, both IL-27 mRNA expression and plasma levels of IL-27 were observed to increase (33). The intervention with VD3 and/or RSV alleviated metabolic abnormalities induced by type 2 diabetes mellitus (T2DM), resulting in elevated levels of the anti-inflammatory factor IL-27 and reduced levels of the pro-inflammatory factor IL-23 in the hippocampus of rats (34). Additionally, the expression of IL-27 in the spinal cord of experimental autoimmune encephalomyelitis (EAE) mice may be enhanced by vitamin D (35). Consistent with these findings, our study revealed that after VD3 intervention, the levels of IL-27, along with the mRNA and protein expressions of IL-27R, significantly increased in HFD rats and 3T3-L1 cells. Notably, when IL-27 was knocked down in 3T3-L1 cells, the mRNA and protein expressions of IL-27R did not show significant changes following VD3 intervention.
Prior research has demonstrated that multiple signaling pathways are involved in white fat beige. Following the activation of β3-adrenergic receptors, the cAMP-PKA (protein kinase A) signaling pathway is triggered, leading to the activation of downstream P38 kinase and ERK1/2. This process promotes the transformation of white adipocytes into beige adipocytes and enhances their thermogenic capacity (36). Additionally, some bioactive substances promote white fat beige by activating the PI3K-ERα signaling pathway (37). Naringenin has been shown to reduce lipid accumulation via the AMPK pathway (38). Furthermore, astragalin and rutin modulate PPAR-γ and AMPK-mediated signaling pathways to alleviate obesity disorders (39). In studies involving 3T3-L1 adipocytes and mice fed a high-fat diet deficient in vitamin D, vitamin D3 was found to inhibit the activation of the PI3K/Akt/mTOR signaling pathway, thereby suppressing the expression of browning markers in white adipocytes, such as PPAR-γ, PGC-1α, and UCP-1 (40). The synergistic effect of these signaling pathways plays a crucial role in regulating energy metabolism and lipid storage in adipocytes, potentially contributing to obesity prevention.
A positive correlation between 25(OH)2D3 and IL-27 was observed in both the observation and control groups. The IL-27/P38MAPK/PGC-1α pathway is closely associated with inflammation and white fat beige. Meanwhile, calcitriol can inhibit the p38 MAPK signaling pathway by up-regulating MAPK phosphatase-5 (MKP-5), thereby reducing the production of pro-inflammatory cytokines such as IL-6 and TNF (41). One study demonstrated that the IL-27/P38MAPK/PGC-1α pathway could promote thermogenesis and energy expenditure of adipocytes (9). Therefore, this study hypothesizes that the molecular mechanism by which VD3 promotes white fat beige may be through this pathway. Subsequently, the mRNA and protein expression levels of the IL-27/P38MAPK/PGC-1α pathway, as well as its upstream and downstream genes, were assessed using 3T3-L1 cells and rat inguinal adipose tissue.
In vivo experiments demonstrated that VD3 intervention significantly upregulated the expression of VDR, IL-27R, and P38MAPK at both mRNA and protein levels compared to the HFD group. Additionally, PGC-1α mRNA expression showed an increase; however, no significant difference in protein expression was observed between the groups. The results may be attributed to several factors: First, mRNA stability is inherently low, leading to rapid degradation that hinders effective protein synthesis. Second, the process of mRNA-to-protein translation is inherently limited. Finally, the transcription and translation of mRNA are tightly coupled processes; if this coupling is disrupted, mRNA levels may increase while protein levels remain unchanged. Notably, UCP-1 protein expression in inguinal adipose tissue also significantly increased following VD3 intervention, with similar results observed in mast cells. The intervention of 1,25(OH)2D3 in 3T3-L1 cells enhanced both mRNA and protein expression of VDR, IL-27R, P38MAPK, and PGC-1α compared to the PA group. Surprisingly, UCP-1 protein levels and CD137 mRNA levels in mast cells were significantly elevated after VD3 intervention. However, both IL-27 siRNA and PGC-1α siRNA inhibited the beneficial effect of VD3 in 3T3-L1 cells. Therefore, the relationship between VD3 and IL-27 may involve VD3 binding to VDR upon entering the body, which stimulates the secretion of IL-27. Consequently, P38MAPK/PGC-1α, as downstream targets of IL-27, are mobilized to further enhance the levels of PGC-1α, UCP-1, and CD137, which serve as markers for beige fat (Figure 9). This suggests that VD3 intervention may stimulate the generation of the thermogenic gene UCP-1 and CD137 via the IL-27/P38MAPK/PGC-1α pathway, thereby increasing adaptive thermogenesis, promoting white fat beige, and reducing lipid accumulation.
However, in this small sample population study, no significant differences were observed in the serum 25(OH)D3 levels and serum IL-27 levels between the two groups. The results may be attributed to the following points: First, vitamin D is primarily synthesized in the skin upon exposure to ultraviolet rays (42). An intervention trial indicated that serum 25(OH)D3 levels increased from 36.4 nmol/L in winter to 49.8 nmol/L in summer, subsequently decreasing to 39.6 nmol/L in winter when relying solely on natural sunlight exposure (43). The recruitment, sampling, and testing of participants in this study were conducted exclusively in summer, a season characterized by relatively abundant ultraviolet rays, during which the levels of vitamin D in the human body are typically elevated and stable (44). Secondly, an individual’s daily activity level and clothing choices can significantly impact sunlight exposure, thereby influencing the levels of vitamin D in the body (45). This study did not collect or analyze information regarding the participants’ outdoor exercise duration, frequency, or sun protection habits. Previous population-based case–control studies have reported that the observation group exhibited increased serum IL-27 levels compared to the normal control group, which contradicts the findings of this study. Several factors may contribute to these discrepancies. Firstly, the participants in this study were primarily college students and a few teachers, representing a specific subgroup characterized by a higher prevalence of simple obesity and fewer comorbidities. Secondly, the participants in this study had a BMI of 27.09 kg/m2, which is significantly lower than the BMI of 38.92 kg/m2 reported in another study (9). Higher BMI is often associated with greater adiposity and metabolic dysfunction, making it more likely to observe significant differences between groups with a larger BMI disparity (46, 47). The lack of difference in 25(OH)D3 between the two groups may also be attributed to smaller differences in BMI (48). This study conducted a correlation analysis between 25(OH)D3 and IL-27 levels in all participants, which revealed a positive correlation.
The level of serum 25(OH)D3 was reduced after VD3 intervention in HFD rats. This reduction may be attributed to corn oil, the lipid carrier for vitamin D supplements. In the study conducted by Du et al., it was observed that the serum 25(OH)D3 level in the HFD + oil group was significantly lower than that in the HFD group. Conversely, the serum 25(OH)D3 level in the HFD + VD group was significantly higher than that in the HFD + oil group when vitamin D was dissolved in corn oil and administered via gavage (49). Furthermore, a randomized controlled trial demonstrated that the choice of lipid carriers for vitamin D supplementation influences serum 25(OH)D3 levels (50). Vitamin D3, derived from dietary sources and from 7-dehydrocholesterol (7DHC) present in the skin, is first hydroxylated to 25(OH)D3 in the liver. This compound is then converted to 1,25(OH)2D3 via the enzyme CYP27B1 in the kidneys. Corn oil, which is abundant in vitamin E, serves as an antioxidant. Additionally, it has been shown that rosemarinic acid (RA), another antioxidant, specifically enhances the enzymatic activity of peroxidase 1 (PRDX1), thus alleviating nonalcoholic steatohepatitis (NASH) (51). The higher levels of unsaturated fatty acids in corn oil may interact with vitamin D metabolism, potentially influencing metabolic processes in the body (52). These factors may indirectly hinder the conversion of vitamin D3 in the liver, ultimately leading to lower serum 25(OH)D3 levels.
In a previous study, it was demonstrated that VD3 intervention resulted in significantly higher levels of inflammatory cytokines, such as IL-6, MCP-1, and TNF-α, compared to the control group (27). In in vivo experiments, VD3 intervention significantly reversed the HFD-induced upregulation of serum inflammatory cytokines IL-6 and MCP-1. However, there was no significant improvement in TNF-α levels, which is inconsistent with the findings of Cordeiro et al. (53). This discrepancy may be attributed to the use of different rat models; Cordeiro et al. employed an experimental model that more closely resembles the obesogenic conditions found in humans (53). Obesity is associated with the accumulation of inflammatory immune cells in white adipose tissue, and white adipose beige was suppressed when the body was in an inflammatory state (54, 55). The findings suggest that VD3 may promote white fat beige by decreasing the expression of inflammatory factors within adipocytes.
Intervention of VD3 in these rats reduced energy intake, without substantial fluctuations in body weight. The study conducted by Zhang et al. (56) supports the notion that substantial changes in body weight may not occur solely with VD3 intervention. Supports the notion that significant changes in body weight may not occur with only VD3 intervention. While VD3 alone may have no significant impact on weight, it may still play a vital role in modulating various metabolic pathways that contribute to obesity development (57). By targeting these pathways and influencing obesity-related biomarkers, VD3 intervention could potentially contribute to obesity reduction (58, 59). These findings are consistent with previously reported studies suggesting that VD3 intervention effectively mitigates obesity (60, 61).
The level of HDL-C was also reduced after VD3 intervention in HFD rats. Previous studies have reported similar findings, indicating that serum HDL-C levels decrease after VD3 intervention in mice (26). A meta-analysis also revealed that VD supplementation did not significantly improve HDL-C levels in patients with NAFLD (62). The formation of new HDL-C involves the secretion of apolipoprotein A-I by the liver and small intestine (63). HDL-C particles not only transport cholesterol from various cells but also incorporate proteins, hormones, and vitamins (64). In the context of a diet high in fats and cholesterol, mice may develop NAFLD (49). During this condition, lipids accumulate within the liver, leading to impaired lipid metabolism regulation (65). Following VD3 intervention, there is an accelerated process of HDL-C transport, while the secretion of HDL-C by the liver is diminished (66). This reduction in liver HDL-C secretion may contribute to the abnormal decrease in HDL-C levels observed after VD3 intervention.
This study has several limitations. Firstly, the population only has cross-sectional data. Vitamin D3 exhibits significant potential in obesity prevention; nonetheless, its efficacy warrants additional assessment in forthcoming clinical research. Secondly, the in vivo experiments were restricted to male rats; future research should include female subjects. Thirdly, IL-27 siRNA and PGC-1α siRNA were used in this study, whereas gene overexpression and silencing methods in vitro and in vivo are needed in future research. Finally, VD3 may also reduce lipid accumulation and promote energy expenditure through pathways other than the IL-27 pathway. Therefore, whether VD3 is a mediator or a cause of obesity treatment needs to be further investigated.
5 Conclusion
In conclusion, VD3 promotes white fat beige by elevating IL-27 levels, an effect potentially linked to the IL-27/P38MAPK/PGC-1α pathway.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by the Research Ethics Committee in the Department of Southwest Medical University (Reference Number: SWMUIRBKS-202307-0007). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. The animal study was approved by the Ethics Committee of Southwest Medical University (Approval No. 2020612; Date of approval: August 17, 2020). The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
YQZ: Data curation, Investigation, Methodology, Software, Conceptualization, Writing – review & editing, Writing – original draft, Formal analysis, Validation. JS: Validation, Data curation, Methodology, Writing – review & editing, Investigation, Writing – original draft, Software, Formal analysis. YYZ: Investigation, Writing – review & editing, Data curation, Writing – original draft. XW: Supervision, Writing – review & editing, Writing – original draft. ZH: Writing – original draft, Investigation, Writing – review & editing. XL: Investigation, Writing – review & editing, Writing – original draft. YZ: Writing – review & editing, Supervision, Project administration, Methodology, Writing – original draft, Visualization, Formal analysis, Data curation, Conceptualization, Resources. LM: Conceptualization, Funding acquisition, Writing – original draft, Resources, Writing – review & editing, Project administration, Supervision, Methodology, Visualization, Data curation, Formal analysis.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Sichuan Science and Technology Program (2024NSFSC0581), the Youth Science Foundation Project of Southwest Medical University (2023QN057), and the College Student’s Innovation and Entrepreneurship Training Program (202510632070).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that Generative AI was used in the creation of this manuscript. DeepSeek was used by the authors to improve language while preparing this work, but it was not involved in designing the research. Following the use of this tool, the authors examined and adjusted the content as necessary and accept full accountability for the publication’s content.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2025.1661072/full#supplementary-material
References
1. Sakers, A, De Siqueira, MK, Seale, P, and Villanueva, CJ. Adipose-tissue plasticity in health and disease. Cell. (2022) 185:419–46. doi: 10.1016/j.cell.2021.12.016
2. Li, B-Y, Peng, W-Q, Liu, Y, Guo, L, and Tang, Q-Q. HIGD1A links SIRT1 activity to adipose browning by inhibiting the ROS/DNA damage pathway. Cell Rep. (2023) 42:112731. doi: 10.1016/j.celrep.2023.112731
3. Wang, Q, Li, H, Tajima, K, Verkerke, ARP, Taxin, ZH, Hou, Z, et al. Post-translational control of beige fat biogenesis by PRDM16 stabilization. Nature. (2022) 609:151–8. doi: 10.1038/s41586-022-05067-4
4. Vargas-Castillo, A, Sun, Y, Smythers, AL, Grauvogel, L, Dumesic, PA, Emont, MP, et al. Development of a functional beige fat cell line uncovers independent subclasses of cells expressing UCP1 and the futile creatine cycle. Cell Metab. (2024) 36:2146–2155.e5. doi: 10.1016/j.cmet.2024.07.002
5. Kononova, YA, Tuchina, TP, and Babenko, AY. Brown and Beige adipose tissue: one or different targets for treatment of obesity and obesity-related metabolic disorders? Int J Mol Sci. (2024) 25:13295. doi: 10.3390/ijms252413295
6. Sharma, P, and Dwivedi, S. Nutrigenomics and Nutrigenetics: New insight in disease prevention and cure. Indian J Clin Biochem. (2017) 32:371–3. doi: 10.1007/s12291-017-0699-5
7. Imamichi, T, Bai, X-F, Robinson, C, and Gee, K. Editorial: IL-27 in health and disease. Front Immunol. (2023) 14:1191228. doi: 10.3389/fimmu.2023.1191228
8. Zhang, M, Li, D, Zhu, J, Xia, X, Zhang, H, Wu, J, et al. IL-27 disturbs lipid metabolism and restrains mitochondrial activity to inhibit γδ T17 cell-mediated skin inflammation. Cell Death Dis. (2024) 15:491. doi: 10.1038/s41419-024-06887-0
9. Wang, Q, Li, D, Cao, G, Shi, Q, Zhu, J, Zhang, M, et al. IL-27 signalling promotes adipocyte thermogenesis and energy expenditure. Nature. (2021) 600:314–8. doi: 10.1038/s41586-021-04127-5
10. Shahi, A, Afzali, S, Salehi, S, Aslani, S, Mahmoudi, M, Jamshidi, A, et al. IL-27 and autoimmune rheumatologic diseases: the good, the bad, and the ugly. Int Immunopharmacol. (2020) 84:106538. doi: 10.1016/j.intimp.2020.106538
11. Perumal, NL, Mufida, A, Yadav, AK, Son, D-G, Ryoo, Y-W, Kim, S-A, et al. Suppression of lipid accumulation in the differentiation of 3T3-L1 Preadipocytes and human adipose stem cells into adipocytes by TAK-715, a specific inhibitor of p38 MAPK. Life. (2023) 13:412. doi: 10.3390/life13020412
12. Cheung, WW, Hao, S, Zheng, R, Wang, Z, Gonzalez, A, Zhou, P, et al. Targeting interleukin-1 for reversing fat browning and muscle wasting in infantile nephropathic cystinosis. J Cachexia Sarcopenia Muscle. (2021) 12:1296–311. doi: 10.1002/jcsm.12744
13. Zuo, L, Geng, Z, Song, X, Li, J, Wang, Y, Zhang, X, et al. Browning of mesenteric white adipose tissue in Crohn’s disease: a New pathological change and therapeutic target. J Crohns Colitis. (2023) 17:1179–92. doi: 10.1093/ecco-jcc/jjad046
14. Yang, Y, Xu, H, Zhang, Y, Chen, L, Tian, C, Huang, B, et al. Associations of dietary antioxidant micronutrients with the prevalence of obesity in adults. Front Nutr. (2023) 10:1098761. doi: 10.3389/fnut.2023.1098761
15. Huang, H-Y, Lin, T-W, Hong, Z-X, and Lim, L-M. Vitamin D and diabetic kidney disease. Int J Mol Sci. (2023) 24:3751. doi: 10.3390/ijms24043751
16. Bennour, I, Haroun, N, Sicard, F, Mounien, L, and Landrier, J-F. Recent insights into vitamin D, adipocyte, and adipose tissue biology. Obes Rev. (2022) 23:e13453. doi: 10.1111/obr.13453
17. Lontchi-Yimagou, E, Kang, S, Goyal, A, Zhang, K, You, JY, Carey, M, et al. Insulin-sensitizing effects of vitamin D repletion mediated by adipocyte vitamin D receptor: studies in humans and mice. Mol Metab. (2020) 42:101095. doi: 10.1016/j.molmet.2020.101095
18. Martinez, P, and Grant, WB. Vitamin D: what role in obesity-related cancer? Semin Cancer Biol. (2025) 112:135–49. doi: 10.1016/j.semcancer.2025.03.007
19. Liu, F, Li, Y, Liang, C, Kong, B, Zhang, Q, Yin, X, et al. The associations of vitamin D status and lifestyle behaviors with general obesity and metabolically unhealthy obesity in Chinese children and adolescents. Nutrients. (2025) 17:666. doi: 10.3390/nu17040666
20. Pereira-Santos, M, Costa, PRF, Assis, AMO, Santos, C, and Santos, DB. Obesity and vitamin D deficiency: a systematic review and meta-analysis. Obes Rev. (2015) 16:341–9. doi: 10.1111/obr.12239
21. de Siqueira, EA, Magalhães, EP, de Assis, ALC, Sampaio, TL, Lima, DB, Marinho, MM, et al. 1α,25-Dihydroxyvitamin D3 (VD3) shows a neuroprotective action against rotenone toxicity on PC12 cells: an in vitro model of Parkinson’s disease. Neurochem Res. (2023) 48:250–62. doi: 10.1007/s11064-022-03735-5
22. Huang, J-R, Song, J-R, Cai, W-S, Shao, Z-W, Zhou, D-Y, and Song, L. Enhancing vitamin D3 bioaccessibility: unveiling hydrophobic interactions in soybean protein isolate and vitamin D3 binding via an infant in vitro digestion model. Food Chem. (2024) 451:139507. doi: 10.1016/j.foodchem.2024.139507
23. Gao, R, Xu, S, Chen, C, Liu, D, He, Y, Zang, Y, et al. Impact of 1,25-dihydroxyvitamin D3 PLGA-nanoparticles/chitosan hydrogel on osteoimmunomodulation. Int J Biol Macromol. (2023) 247:125624. doi: 10.1016/j.ijbiomac.2023.125624
24. Chen, M, He, Y, Hu, X, Dong, X, Yan, Z, Zhao, Q, et al. Vitamin D3 attenuates SARS-CoV-2 nucleocapsid protein-caused hyperinflammation by inactivating the NLRP3 inflammasome through the VDR-BRCC3 signaling pathway in vitro and in vivo. MedComm. (2023) 4:e318. doi: 10.1002/mco2.318
25. Chen, H, Zhang, H, Li, A, Liu, Y, Liu, Y, Zhang, W, et al. VDR regulates mitochondrial function as a protective mechanism against renal tubular cell injury in diabetic rats. Redox Biol. (2024) 70:103062. doi: 10.1016/j.redox.2024.103062
26. Xiang, L, Du, T, Zhang, J, Zhang, Y, Zhou, Y, Zhao, Y, et al. Vitamin D3 supplementation shapes the composition of gut microbiota and improves some obesity parameters induced by high-fat diet in mice. Eur J Nutr. (2024) 63:155–72. doi: 10.1007/s00394-023-03246-1
27. Xiang, W, Cheng, S, Zhou, Y, and Ma, L. Effects of 1,25(OH)2 D3 on lipid droplet growth in adipocytes. Biofactors. (2020) 46:943–54. doi: 10.1002/biof.1610
28. Zhou, B-FCooperative Meta-Analysis Group of the Working Group on Obesity in China. Predictive values of body mass index and waist circumference for risk factors of certain related diseases in Chinese adults-study on optimal cut-off points of body mass index and waist circumference in Chinese adults. Biomed Environ Sci. (2002) 15:83–96. doi: 10.1046/j.1440–6047.11.s8.9.x
29. Li, N, Yao, M, Liu, J, Zhu, Z, Lam, T-L, Zhang, P, et al. Vitamin d promotes remyelination by suppressing c-Myc and inducing oligodendrocyte precursor cell differentiation after traumatic spinal cord injury. Int J Biol Sci. (2022) 18:5391–404. doi: 10.7150/ijbs.73673
30. Alrefaie, Z, Awad, H, Alsolami, K, and Hamed, EA. Uncoupling proteins: are they involved in vitamin D3 protective effect against high-fat diet-induced cardiac apoptosis in rats? Arch Physiol Biochem. (2022) 128:438–46. doi: 10.1080/13813455.2019.1690526
31. GBD 2021 Adult BMI Collaborators. Global, regional, and national prevalence of adult overweight and obesity, 1990-2021, with forecasts to 2050: a forecasting study for the global burden of disease study 2021. Lancet. (2025) 405:813–38. doi: 10.1016/S0140-6736(25)00355-1
32. Fernández-Arroyo, S, Hernández-Aguilera, A, de Vries, MA, Burggraaf, B, van der Zwan, E, Pouw, N, et al. Effect of vitamin D3 on the postprandial lipid profile in obese patients: a non-targeted lipidomics study. Nutrients. (2019) 11:1194. doi: 10.3390/nu11051194
33. Hashemi, R, Hosseini-Asl, SS, Arefhosseini, SR, and Morshedi, M. The impact of vitamin D3 intake on inflammatory markers in multiple sclerosis patients and their first-degree relatives. PLoS One. (2020) 15:e0231145. doi: 10.1371/journal.pone.0231145
34. Muneeb, M, Mansou, SM, Saleh, S, and Mohammed, RA. Vitamin D and rosuvastatin alleviate type-II diabetes-induced cognitive dysfunction by modulating neuroinflammation and canonical/noncanonical Wnt/β-catenin signaling. PLoS One. (2022) 17:e0277457. doi: 10.1371/journal.pone.0277457
35. Mohammadi-Kordkhayli, M, Ahangar-Parvin, R, Azizi, SV, Nemati, M, Shamsizadeh, A, Khaksari, M, et al. Vitamin D modulates the expression of IL-27 and IL-33 in the central nervous system in experimental autoimmune encephalomyelitis (EAE). Iran J Immunol. (2015) 12:35–49. doi: 10.22034/iji.2015.16733
36. Zhang, N, Liu, J, Wang, M, Guo, X, Fan, B, and Wang, F. Potato protease inhibitor II prevents obesity by inducing browning of white adipose tissue in mice via β3 adrenergic receptor signaling pathway. Phytother Res. (2022) 36:3885–99. doi: 10.1002/ptr.7451
37. Sreekumar, S, and Kiran, MS. Combinatorial effect of Apigenin-resveratrol on white adipocyte plasticity and trans-differentiation for activating lipid metabolism. Biofactors. (2025) 51:e2111. doi: 10.1002/biof.2111
38. Cai, X, Wang, S, Wang, H, Liu, S, Liu, G, Chen, H, et al. Naringenin inhibits lipid accumulation by activating the AMPK pathway in vivo and vitro. Food Sci Human Wellness. (2023) 12:1174–83. doi: 10.1016/j.fshw.2022.10.043
39. Ganjayi, MS, Sankaran, KR, Meriga, B, Bhatia, R, Sharma, S, and Kondepudi, KK. Astragalin and rutin restore gut microbiota dysbiosis, alleviate obesity and insulin resistance in high-fat diet-fed C57BL/6J mice. Food Sci Human Wellness. (2024) 13:3256–65. doi: 10.26599/FSHW.2023.9250012
40. Zhao, Y, and Qin, R. Vitamin D3 affects browning of white adipocytes by regulating autophagy via PI3K/Akt/mTOR/p53 signaling in vitro and in vivo. Apoptosis. (2022) 27:992–1003. doi: 10.1007/s10495-022-01765-6
41. Dwivedi, S, Singh, V, Sen, A, Yadav, D, Agrawal, R, Kishore, S, et al. Vitamin D in disease prevention and cure-part I: an update on molecular mechanism and significance on human health. Indian J Clin Biochem. (2025) 40:339–81. doi: 10.1007/s12291-024-01251-7
42. Wacker, M, and Holick, MF. Sunlight and vitamin D: a global perspective for health. Dermatoendocrinol. (2013) 5:51–108. doi: 10.4161/derm.24494
43. Maboshe, W, Macdonald, HM, Wassall, H, Fraser, WD, Tang, JCY, Fielding, S, et al. Low-dose vitamin D3 supplementation does not affect natural regulatory T cell population but attenuates seasonal changes in T cell-produced IFN-γ: results from the D-SIRe2 randomized controlled trial. Front Immunol. (2021) 12:623087. doi: 10.3389/fimmu.2021.623087
44. Nakajima, H, Sakamoto, Y, Honda, Y, Sasaki, T, Igeta, Y, Ogishima, D, et al. Estimation of the vitamin D (VD) status of pregnant Japanese women based on food intake and VD synthesis by solar UV-B radiation using a questionnaire and UV-B observations. J Steroid Biochem Mol Biol. (2023) 229:106272. doi: 10.1016/j.jsbmb.2023.106272
45. Holick, MF. Sunlight, UV radiation, vitamin D, and skin cancer: how much sunlight do we need? Adv Exp Med Biol. (2020) 1268:19–36. doi: 10.1007/978-3-030-46227-7_2
46. Tobias, DK, Luttmann-Gibson, H, Mora, S, Danik, J, Bubes, V, Copeland, T, et al. Association of body weight with response to vitamin D supplementation and metabolism. JAMA Netw Open. (2023) 6:e2250681. doi: 10.1001/jamanetworkopen.2022.50681
47. Chen, K, Shen, Z, Gu, W, Lyu, Z, Qi, X, Mu, Y, et al. Prevalence of obesity and associated complications in China: a cross-sectional, real-world study in 15.8 million adults. Diabetes Obes Metab. (2023) 25:3390–9. doi: 10.1111/dom.15238
48. van den Heuvel, EG, Lips, P, Schoonmade, LJ, Lanham-New, SA, and van Schoor, NM. Comparison of the effect of daily vitamin D2 and vitamin D3 supplementation on serum 25-hydroxyvitamin D concentration (total 25(OH)D, 25(OH)D2, and 25(OH)D3) and importance of body mass index: a systematic review and meta-analysis. Adv Nutr. (2024) 15:100133. doi: 10.1016/j.advnut.2023.09.016
49. Du, T, Xiang, L, Zhang, J, Yang, C, Zhao, W, Li, J, et al. Vitamin D improves hepatic steatosis in NAFLD via regulation of fatty acid uptake and β-oxidation. Front Endocrinol. (2023) 14:1138078. doi: 10.3389/fendo.2023.1138078
50. McCourt, AF, Mulrooney, SL, O’Neill, GJ, O’Riordan, ED, and O’Sullivan, AM. Serum 25-hydroxyvitamin D response to vitamin D supplementation using different lipid delivery systems in middle-aged and older adults: a randomised controlled trial. Br J Nutr. (2023) 130:1548–57. doi: 10.1017/S0007114523000636
51. Yin, W, Xu, H, Bai, Z, Wu, Y, Zhang, Y, Liu, R, et al. Inhibited peroxidase activity of peroxiredoxin 1 by palmitic acid exacerbates nonalcoholic steatohepatitis in male mice. Nat Commun. (2025) 16:598. doi: 10.1038/s41467-025-55939-2
52. Hahn, J, Cook, NR, Alexander, EK, Friedman, S, Walter, J, Bubes, V, et al. Vitamin D and marine omega 3 fatty acid supplementation and incident autoimmune disease: VITAL randomized controlled trial. BMJ. (2022) 376:e066452. doi: 10.1136/bmj-2021-066452
53. Cordeiro, MM, Biscaia, PB, Brunoski, J, Ribeiro, RA, Franco, GCN, and Scomparin, DX. Vitamin D supplementation decreases visceral adiposity and normalizes leptinemia and circulating TNF-α levels in western diet-fed obese rats. Life Sci. (2021) 278:119550. doi: 10.1016/j.lfs.2021.119550
54. DHA alleviated hepatic and adipose inflammation with increased adipocyte browning in high-fat diet-induced obese mice. J Nutr Biochem. (2023) 122:109457. doi: 10.1016/j.jnutbio.2023.109457
55. Zhang, W, Liu, S, Kong, L, Wu, S, Zhong, Z, Yu, L, et al. Lipopolysaccharide-induced persistent inflammation ameliorates fat accumulation by promoting adipose browning in vitro and in vivo. Int J Biol Macromol. (2023) 252:126511. doi: 10.1016/j.ijbiomac.2023.126511
56. Zhang, J, Zhang, Y, Zhou, Y, Zhao, W, Li, J, Yang, D, et al. Effect of vitamin D3 on lipid droplet growth in adipocytes of mice with HFD-induced obesity. Food Sci Nutr. (2023) 11:6686–97. doi: 10.1002/fsn3.3618
57. Cordeiro, A, Pereira, SE, Saboya, CJ, and Ramalho, A. Vitamin D supplementation and its relationship with loss of visceral adiposity. Obes Surg. (2022) 32:3419–25. doi: 10.1007/s11695-022-06239-x
58. Li, P, Li, K, Yuan, W, Xu, Y, Li, P, Wu, R, et al. 1α,25(OH)2D3 ameliorates insulin resistance by alleviating γδ T cell inflammation via enhancing fructose-1,6-bisphosphatase 1 expression. Theranostics. (2023) 13:5290–304. doi: 10.7150/thno.84645
59. Liang, Y, Jiang, X, Zhao, X, Tang, T, Fan, X, Wang, R, et al. Vitamin D alleviates HFD-induced hepatic fibrosis by inhibiting DNMT1 to affect the TGFβ1/Smad3 pathway. iScience. (2024) 27:111262. doi: 10.1016/j.isci.2024.111262
60. Chen, H, Zhang, Y, Miao, Y, Song, H, Tang, L, Liu, W, et al. Vitamin D inhibits ferroptosis and mitigates the kidney injury of prediabetic mice by activating the klotho/p53 signaling pathway. Apoptosis. (2024) 29:1780–92. doi: 10.1007/s10495-024-01955-4
61. Lu, S, and Cao, Z-B. Interplay between vitamin D and adipose tissue: implications for Adipogenesis and adipose tissue function. Nutrients. (2023) 15:4832. doi: 10.3390/nu15224832
62. Guo, X-F, Wang, C, Yang, T, Li, S, Li, K-L, and Li, D. Vitamin D and non-alcoholic fatty liver disease: a meta-analysis of randomized controlled trials. Food Funct. (2020) 11:7389–99. doi: 10.1039/d0fo01095b
63. Endo, Y, Fujita, M, and Ikewaki, K. HDL functions-current status and future perspectives. Biomolecules. (2023) 13:105. doi: 10.3390/biom13010105
64. Li, K, Xie, X, and Guo, Y. HDL and therapy. Adv Exp Med Biol. (2022) 1377:171–87. doi: 10.1007/978-981-19-1592-5_14
65. Ouyang, S, Zhuo, S, Yang, M, Zhu, T, Yu, S, Li, Y, et al. Glycerol kinase drives hepatic de novo lipogenesis and triglyceride synthesis in nonalcoholic fatty liver by activating SREBP-1c transcription, upregulating DGAT1/2 expression, and promoting glycerol metabolism. Adv Sci. (2024) 11:e2401311. doi: 10.1002/advs.202401311
Keywords: vitamin D3, white fat beige, obesity, IL-27, PGC-1α, UCP-1
Citation: Zhou Y, Shu J, Zhao Y, Wu X, He Z, Lyu X, Zhou Y and Ma L (2025) Vitamin D3 promotes white fat beige through IL-27/P38MAPK/PGC-1α pathway. Front. Nutr. 12:1661072. doi: 10.3389/fnut.2025.1661072
Edited by:
Tim Lambers, FrieslandCampina, NetherlandsReviewed by:
Sarbjeet Makkar, University of Michigan, United StatesShailendra Dwivedi, All India Institute of Medical Sciences Gorakhpur, India
Copyright © 2025 Zhou, Shu, Zhao, Wu, He, Lyu, Zhou and Ma. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yong Zhou, emhvdXlfeGpAc3dtdS5lZHUuY24=; Ling Ma, eGptbEBzd211LmVkdS5jbg==
†These authors have contributed equally to this work
 Yanqiu Zhou1,2†
Yanqiu Zhou1,2† Junfang Shu
Junfang Shu