- 1School of Public Health, Chengdu Medical College, Chengdu, Sichuan, China
- 2Department of Clinical Nutrition, The General Hospital of Western Theater Command, Chengdu, Sichuan, China
- 3School of Public Health, Medical College of Soochow University, Suzhou, Jiangsu, China
- 4Department of Rehabilitation Medicine, The General Hospital of Western Theater Command, Chengdu, Sichuan, China
- 5Sichuan Provincial Key Laboratory of Philosophy and Social Sciences for Intelligent Medical Care and Elderly Health Management, Chengdu Medical College, Chengdu, Sichuan, China
Background: Diet is a recognized risk factor for cancer. Recently, the role of improving thyroid-related functions through diet has been questioned. This systematic review investigates the relationship between food groups/dietary patterns and thyroid cancer.
Methods: We conducted a systematic search of the literature through April 2025 in the PubMed, Scopus, Web of Science, and Embase database following PRISMA guidelines. ORs, HRs or RRs with 95% CIs were extracted as effect sizes and publication bias was assessed using funnel plots. Additionally, we conducted mendelian randomization (MR) analysis by selecting dietary factors (including nutrients) associated with thyroid cancer as exposure data to complement the results of meta-analysis.
Results: We collected data from 16 cohort and 21 case-control studies that met the collection criteria. Meta-analysis found that high consumption of fish and alcohol-containing beverages was associated with a reduced risk of thyroid cancer, whereas consumption of high amounts of refined cereal and nitrates increased thyroid cancer risk (P < 0.05). Our MR analysis data showed that some specific food items, especially seafood (like oily fish) might be the protective factors for thyroid cancer, which strengthen the previous meta-analysis results.
Conclusion: This comprehensive study investigated the relationships between dietary factors and thyroid cancer risk, synthesizing findings from a meta-analysis of observational studies and MR analysis to estimate causal associations. Consistently, both the meta-analysis and MR analysis revealed that consumption of certain types of fish may be linked to a decreased risk of thyroid cancer.
Systematic review registration: https://www.crd.york.ac.uk/PROSPERO/, identifier CRD420251101506.
1 Introduction
The incidence of thyroid cancer has continued to rise over the past few decades, and the burden of thyroid cancer is concentrated in women (1). According to the Global Cancer Incidence Statistics 2020, thyroid cancer is the ninth most prevalent cancer and is three times more prevalent in women than in men (2). A collaborative study analyzing data from 13 cancer registries in China, Japan, and Korea found that the age-specific incidence curves for Chinese and Korean individuals were characterized by an inverted U-shape (3). Dietary factors are considered putative risk factors for the development of cancer at different sites, while nutritional factors play an important role in the pathogenesis of different metabolic diseases. In recent years, many studies have attempted to establish a relationship between diet and thyroid cancer, but remain inconclusive. Dietary pattern appears to alter the risk of thyroid cancer. A diet low in starchy foods, products rich in salt, fat and sugar, and high in cruciferous/non-cruciferous vegetables, milk, dairy products and seafood can prevent thyroid cancer (4). One study showed a significant negative correlation between a dietary pattern rich in fruits and vegetables and thyroid cancer (5). However, another study revealed no significant association between vegetable/fruit intake and different types of thyroid cancer (6). Similarly, other studies did not present strong evidence for the association between dietary intake of selenium or other micronutrients and thyroid cancer risk (7). As for daily beverages such as alcohol, coffee and tea, one study demonstrated that the risk reduction associated with alcohol was eliminated after adjusting for smoking factors, and that thyroid cancer risk was not associated with coffee or tea consumption (8), but a large cohort demonstrated a potentially protective effect of alcohol consumption against thyroid cancer (9).
There has been a growing interest in the impact of dietary pattern or food intakes on thyroid cancer. However, in each clinical trial, various food items and different dietary patterns caused mixed effects. Meta-analysis needs to be updated for an overview of the current evidence on the association between diet factors (food groups and dietary patterns) and thyroid cancer. Besides, mendelian randomization (MR) uses genetic variants as instrumental variables (IVs) to estimate the causal effect of an exposure (diet) on an outcome (thyroid cancer) (10). The main objective of this meta-analysis is to outline the relationship between food groups [vegetables, fruits, meat, dairy products, tea, alcohol, seafood (fish, saltwater fish, shellfish and fresh-water fish)], dietary patterns and thyroid cancer, then MR was used to complement the observational findings. To our knowledge, this is among the first studies to synergistically combine meta-analysis and two-sample MR analysis, leveraging the complementary strengths of observational evidence (meta-analysis) and causal inference (MR). While meta-analysis provides a comprehensive overview of dietary associations, MR mitigates confounding and reverse causation, offering a robust framework to infer causality.
2 Materials and methods
2.1 Search strategy
This systematic review used the principles stated in the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA; Supplementary File 1) (11). The meta-analysis has been registered on PROSPERO (CRD420251101506). This screening process was independently completed by two reviewers (YD and JL). A systematic search was performed in MEDLINE (via PubMed), Scopus (via Elsevier), Web of Science-Science Citation Index and Social Sciences Citation Index (via Clarivate), and Embase (via Elsevier) on April 2025, with no restrictions regarding language or year of publication. The search included the following keywords: “’food groups” (vegetables, fruits, meat, dairy products, alcohol, starchy, fish, saltwater fish, fresh-water, fish, shellfish, nitrate, tea, seafood, seaweed) OR “dietary pattern” (healthy dietary pattern, unhealthy dietary pattern) AND “thyroid cancer” AND “incidence” OR “risk.” We also conducted manual searches of preprint platforms (medRxiv and Research Square) and other databases (CINAHL, China National Knowledge Internet Database, Wanfang database). We manually searched the gray literature through ClinicalTrials.gov, Cochrane Central Registry of Controlled Trials, and Web of Science: Conference Proceedings Citation Index-Science (via Wiley) to identify completed but unpublished studies that met our eligibility criteria for reducing publication bias. The authors were contacted to obtain missing data.
2.2 Eligibility criteria
We included the studies that met the criteria: (1) Article assessing the relationship between food groups or dietary patterns and thyroid cancer. (2) Thyroid cancer incidence as the outcome. (3) Case-control, cohort studies or randomized controlled trials. The following exclusion criteria were applied: (1) Studies on cell-level, animal or model studies. (2) Letters, case reports, conference reports, laboratory studies, editorials and any review.
2.3 Data extraction
A standardized data collection sheet was designed and created in Microsoft Excel. Two reviewers (YD and JL) performed the data extraction for all included articles. The detailed information collected from each included study was as follows: first author, year of publication, country, study design, sample sizes of cases/controls, duration of follow-up (this is appropriate for cohort studies), exposure intake of study/control groups, and potential adjustment factors (Table 1). In cases of missing information, the corresponding author was contacted via email to request the missing data.
2.4 Quality assessment
The quality of the included studies was assessed using the New Castries-Ottawa Scale (NOS). NOS evaluates the quality of the literature using the semi-quantitative principles, including selection, comparability, and exposure/outcome. Furthermore, study quality appraisal (out of 9 points) was divided into three groups: high-quality evidence (≥7 points), moderate-quality evidence (5–6 points), and low-quality evidence (4 points). If there was disagreement about the rating of a study, all authors researched the article and came to a consensus.
2.5 Statistical analysis for meta-analysis
Odds ratios (ORs), hazard ratios (HRs) or risk ratios (RRs), and 95% confidence intervals (CIs) were extracted and merged using STATA software (v.17.0) and Review Manager (v.5.4). Random-effects model was used for all studies. Quantitative analysis of inter-study heterogeneity was assessed using Cochran’s Q test and I2 statistic. P-values < 0.1 were considered indicative of heterogeneity for the chi-squared test. Heterogeneity was classified as low if I2 < 30%, moderate if I2 = 30%–60% and high if I2 > 60% (Supplementary Table 1). Publication bias was assessed through funnel plot, Begg’s rank correlation, and Egger’s weighted regression tests (Supplementary Table 2). When there was evidence of funnel plot asymmetry, potentially missing studies were imputed using the trim and fill method. Additional sensitivity analysis was performed using the Cochrane Handbook for Systematic Reviews of Interventions (V.6.1, 2020). For subgroup analysis, we regarded the lowest quantile in the study as low intake and the highest quantile as high intake, and conducted a pooled analysis of the relationship between high and low intake of various foods and thyroid cancer. We used Hill’s causal criteria to assess the possibility of a protective or enhanced causal relationship between high intake of various diets and thyroid cancer (Supplementary Table 3) (12).
2.6 Data sources for MR analysis
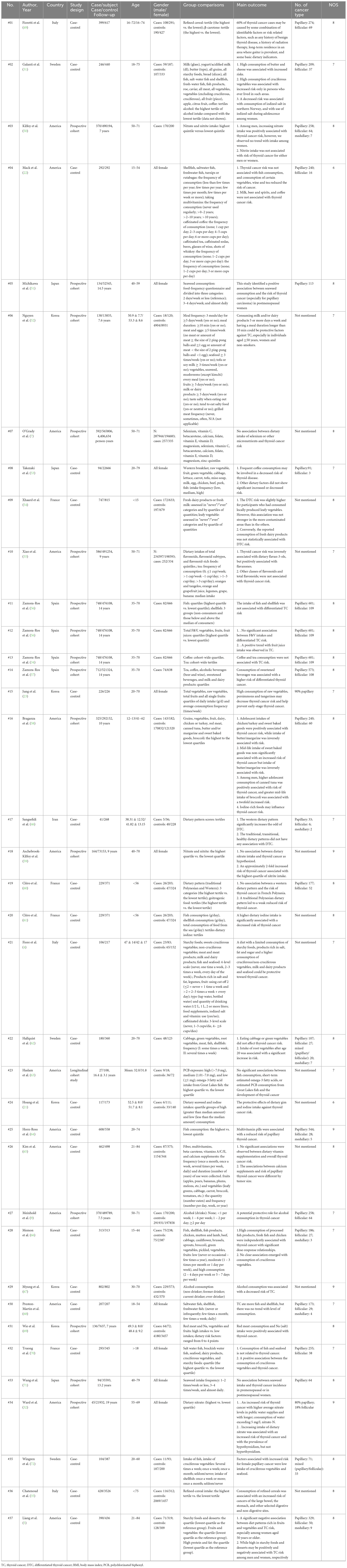
We selected 377 dietary factors (containing 37 nutrients) associated with thyroid cancer as exposure data and summary level data from the UK Biobank (as of August 1, 2024), including the major food groups of cereal, fruits and vegetables, meat and fish (shellfish), milk and eggs, and beverages (alcohol, tea, etc.). Thyroid cancer is a malignant tumor that originates from the follicular epithelial cells or parafollicular epithelial cells of the thyroid gland (13). Outcome data selected three thyroid malignancies from the FinnGen biological sample library, namely follicular adenocarcinoma of thyroid gland (FTC), malignant neoplasm of thyroid gland (MT), papillary adenocarcinoma of thyroid gland (PTC). Because of the re-analysis of previously summarized data, no additional ethical approval was required. Supplementary Tables 4, 5 provides a comprehensive description of the data sources for the respective result datasets. MR relies on three fundamental hypotheses: (1) The IVs hypothesis: The genetic variant selected as the IVs is unintentionally related to the exposure of interest in a casual manner. (2) The used genetic variants should not be associated with potential confounding variables in the exposure-outcome relationship. (3) The Pleiotropy Hypothesis: The genetic variant used as the IV is associated solely with the outcome via its effect on the exposure and no other biological pathways (Figure 1). A quality assessment was conducted based on adherence to the Strengthening the Reporting of Mendelian Randomization Studies (STROBE-MR) Guidelines (Supplementary File 2).
Figure 1. Flow diagram for identification of studies and three fundamental assumptions of a MR analysis.
2.7 SNP selection and strengthen IVs
We identified SNPs closely related to exposure and outcome, to ensure that the number of SNPs shared between exposure and outcome is sufficient, with genome-wide significance (P < 5 × 10–6). These selected SNPs are located in different gene regions and do not exhibit significant linkage disequilibrium (r2 < 0.001). Supplementary Table 6 contains additional detailed information related to the IVs used in our study. To minimize the impact of weak IVs on the causal analysis, F-statistic was used. The value greater than 10 indicates a low probability of weak instrument bias, indicating that the IVs possess sufficient strength to generate reliable and unbiased causal estimates in MR analysis. So we exclude all the tools where the F-statistic is less than 10 (e.g., 19 SNPs used for satsuma intake, all with F-statistics > 10). Evaluating of weak instrument bias helps ensure the validity and robustness of our findings related to the causal relationships between the exposure (dietary factors) and the outcomes (thyroid cancers).
2.8 Statistical analysis for MR analysis
The inverse variance weighting (IVW), MR-Egger, and weighted median (WM) methods were used to examine a causal association, with IVW being the primary analytical method. Horizontal pleiotropy is a main source of bias in MR, whereby genetic variants influence the exposure and outcome via two separate biological pathways. The IVW method can achieve unbiased causal estimates without horizontal pleiotropy where the variants affect the direction and outcome through pathways that are not on the causal pathway of interest. Diets affect disease only when two or more statistical methods are in the same direction. If only the IVW approach yields support for the influence of a factor, and the other approaches are consistent in the direction of the beta, based on previous research. We consider this factor to be a potential influencing factor. Several sensitivity analysis were conducted to obtain stable MR estimates. The IVW and MR-Egger were utilized to quantify the heterogeneity effect among genetic instruments. Cochran’s Q test assessed heterogeneity in the IVW model. Cochran’s Q test P < 0.05 indicates the presence of heterogeneity. Finally, the leave-one-out method was utilized to address sensitivity analysis. All analyses were performed in R software (version 4.2.1) using the TwoSampleMR package.
3 Results
3.1 Meta-analysis
3.1.1 Search results
The results of our initial screening by combining search records from all databases showed that 3 duplicate studies were removed. The 90 studies were screened from the titles and abstracts, and after careful evaluation of the full text, thirty-seven studies (including 16 cohort studies and 21 case-control studies) were ultimately included in the systematic review and meta-analysis (Figure 1).
3.1.2 Study characteristics and quality assessment
These studies evaluated the association of thyroid cancer risk with the risk of specific food groups such as vegetables (n = 7) and fruits (n = 8), meat (n = 5), refined grains (n = 2), all fish (n = 9), milk (n = 6), starchy foods (n = 4), freshwater fish (n = 4), alcohol (n = 6), shellfish (n = 8), saltwater fish (n = 4), seaweed (n = 3), coffee (n = 5), and tea (n = 4). Of all the included studies, most of them were conducted in the United States and Korea. The quality assessment scores for case-control and cohort studies ranged from 6 to 9 (Table 1).
3.1.3 Meta-analysis result of different food groups
3.1.3.1 Refined cereal and starchy foods consumption
Two case-control studies in the literature were used to examine the relationship between refined grain consumption and thyroid cancer risk. The pooled results indicated that high consumption of refined grains increased the risk of thyroid cancer with low heterogeneity (P < 0.00001, OR = 2.01, 95% CI = 1.60–2.53, I2 = 0%, Figure 2A and Supplementary Table 1). Subgroup analysis showed that consuming a smaller amount of refined grains still increased the risk of thyroid cancer (P < 0.00001, OR = 1.77, 95% CI = 1.45–2.15, I2 = 0%, Figure 2A and Supplementary Table 1). Four case-control studies examined the relationship between starchy food consumption and thyroid cancer risk. No statistical differences were observed in the pooled results (P = 0.09, OR = 1.22, 95% CI = 0.97–1.54, I2 = 0%, Figure 2A and Supplementary Table 1). After aggregating the results of consuming less starchy foods, there was still no statistical difference (P = 0.92, OR = 1.01, 95% CI = 0.77–1.33, I2 = 1%, Figure 2A and Supplementary Table 1).
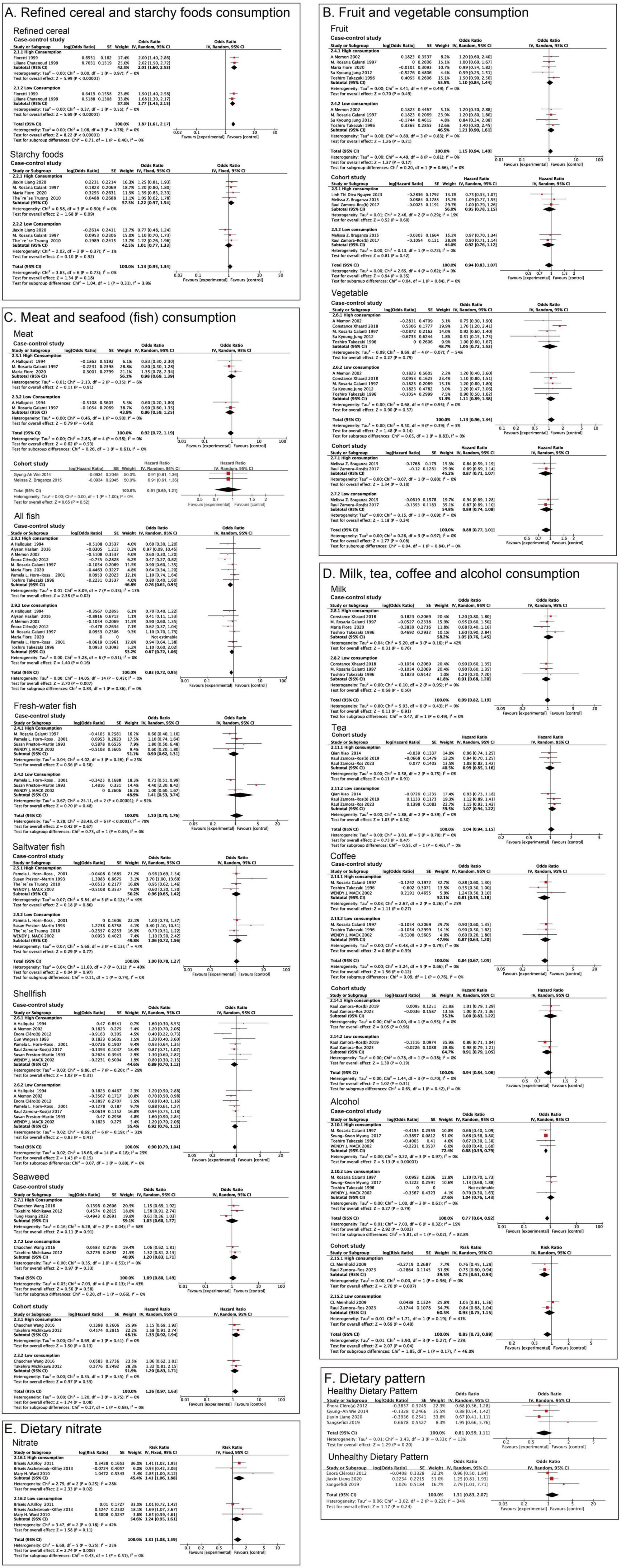
Figure 2. Meta-analysis for associations of the food groups and dietary pattern with thyroid cancer risk. (A) Refined cereal and Starchy foods; (B) Fruit and Vegetable; (C) Meat, All fish, Fresh-water fish, Saltwater fish, Shellfish and Seaweed; (D) Milk, Tea, Coffee and Alcohol; (E) Nitrate; (F) Healthy Dietary Pattern and Unhealthy Dietary Pattern.
3.1.3.2 Fruit and vegetable consumption
Three cohort studies and five case-control studies assessed fruit consumption and thyroid cancer risk. There was no significant association between more fruit consumption and thyroid cancer in the case-control (P = 0.49, OR = 1.10, 95% CI = 0.84–1.44, I2 = 0%) and cohort studies (P = 0.60, HR = 0.95, 95% CI = 0.78–1.15, I2 = 19%, Figure 2B and Supplementary Table 1). Similarly, whether in case-control studies (P = 0.21, OR = 1.21, 95% CI = 0.90–1.61, I2 = 0%) or cohort studies, consuming smaller amounts of fruit is also not associated with thyroid cancer (P = 0.42, HR = 0.92, 95% CI = 0.76–1.12, I2 = 0%, Figure 2B and Supplementary Table 1). There were no statistically significant differences between vegetables and thyroid cancer (case-control: P = 0.79, OR = 1.05, 95% CI = 0.72–1.53, I2 = 54%; cohort: P = 0.18, RR = 0.87, 95% CI = 0.71–1.07, I2 = 0%, Figure 2B and Supplementary Table 1). After subgroup analysis, a lower intake of vegetables was also not associated with the risk of thyroid cancer (case-control: P = 0.37, OR = 1.11, 95% CI = 0.89–1.38, I2 = 0%; cohort: P = 0.24, RR = 0.89, 95% CI = 0.74–1.08, I2 = 0%, Figure 2B and Supplementary Table 1).
3.1.3.3 Meat and seafood (fish) consumption
Two cohort and three case-control studies explored the relationship between total meat consumption and thyroid cancer risk. The pooled results showed that high meat intake was not statistically different from the risk of thyroid cancer (case-control: P = 0.91, OR = 0.98, 95% CI = 0.69–1.39, I2 = 6%; cohort: P = 0.52, HR = 0.91, 95% CI = 0.69–1.21, I2 = 0%, Figure 2C and Supplementary Table 1). In case-control studies, there was no statistically significant difference between low meat intake and the risk of thyroid cancer. Cohort studies did not present data on low intake (case-control: P = 0.43, OR = 0.86, 95% CI = 0.59–1.25, I2 = 0%, Figure 2C and Supplementary Table 1).
All fish consumption was associated with reduced risk of thyroid cancer risk (P = 0.02, OR = 0.76, 95% CI = 0.61–0.95, I2 = 13%, Figure 2C and Supplementary Table 1). In the analysis of all high-consumption and low-consumption subgroups of fish, since Maria Fiore 2020 did not provide data on low-consumption, the remaining data combined showed that all fish were not associated with thyroid cancer at lower intake levels (P = 0.16, OR = 0.87, 95% CI = 0.72–1.06, I2 = 0%, Figure 2C). However, no statistically significant differences in outcomes were observed in high consumption of freshwater fish (P = 0.58, OR = 0.90, 95% CI = 0.62–1.31, I2 = 25%), saltwater fish consumption (P = 0.86, OR = 0.96, 95% CI = 0.65–1.42, I2 = 49%), shellfish consumption (P = 0.31, OR = 0.89, 95% CI = 0.70–1.12, I2 = 29%), seaweed consumption (case-control: P = 0.91, OR = 1.03, 95% CI = 0.60–1.77, I2 = 68%; cohort: P = 0.13, HR = 1.33, 95% CI = 0.92–1.94, I2 = 0%, Figure 2C and Supplementary Table 1). Subgroup analysis showed that low-consumption freshwater fish (P = 0.48, OR = 1.41, 95% CI = 0.53–3.74, I2 = 92%), saltwater fish (P = 0.77, OR = 1.06, 95% CI = 0.72–1.56, I2 = 47%), and shellfish (P = 0.41, OR = 0.92, 95% CI = 0.76–1.12, I2 = 31%), seaweed consumption (case-control: P = 0.33, OR = 1.20, 95% CI = 0.83–1.71, I2 = 0%; Cohort: P = 0.33, HR = 1.20, 95% CI = 0.83–1.71, I2 = 0%). There is no statistically significant difference in the results of Figure 2C and Supplementary Table 1. However, when high and low consumption volumes are combined, there is a certain degree of heterogeneity (fresh-water fish: I2 = 79%; saltwater fish: I2 = 40%; shellfish: I2 = 25%).
3.1.3.4 Milk, tea, coffee and alcohol consumption
There were no statistical differences in consumption of milk (P = 0.76, OR = 1.05, 95% CI = 0.76–1.45, I2 = 42%), tea (P = 0.91, HR = 0.99, 95% CI = 0.85–1.16, I2 = 0%), coffee (Case-control: P = 0.27, OR = 0.81, 95% CI = 0.55–1.18, I2 = 25%; Cohort: P = 0.96, HR = 1.0, 95% CI = 0.83–1.22, I2 = 0%, Figure 2D and Supplementary Table 1). Drinking less milk (P = 0.50, OR = 0.91, 95% CI = 0.68–1.20, I2 = 0%), tea (P = 0.30, HR = 1.07, 95% CI = 0.94–1.22, I2 = 0%), and coffee (Case-control: P = 0.39, OR = 0.87, 95% CI = 0.63–1.20, I2 = 0%; Cohort: P = 0.19, HR = 0.91, 95% CI = 0.79–1.05, I2 = 0%, Figure 2D and Supplementary Table 1) also showed no statistical difference. A total of four case-control studies and two cohort studies examined the relationship between total alcohol consumption and thyroid cancer risk. The pooled results of both the case-control studies and the cohort studies indicated that alcohol was a protective factor against thyroid cancer and reduced the risk of thyroid cancer (Case-control: P < 0.00001, OR = 0.68, 95% CI = 0.59–0.79, I2 = 0%, Cohort: P = 0.007, RR = 0.75, 95% CI = 0.61–0.93, I2 = 0%, Figure 2D and Supplementary Table 1). However, subgroup analyses showed that a small amount of alcohol intake was not associated with thyroid cancer, whether in case-control studies or cohort studies (Case-control: P = 0.79, OR = 1.04, 95% CI = 0.76–1.43, I2 = 0%, Cohort: P = 0.49, RR = 0.93, 95% CI = 0.75–1.15, I2 = 41%, Figure 2D and Supplementary Table 1).
3.1.3.5 Nitrate intake consumption
High consumption of dietary nitrate intake increased thyroid cancer risk and some heterogeneity was observed (P = 0.02, RR = 1.41, 95% CI = 1.06–1.88, I2 = 28%, Figure 2E and Supplementary Table 1). The results of subgroup analysis showed that consuming a small amount of nitrate was not associated with thyroid cancer and was no longer a risk factor for thyroid cancer (P = 0.11, RR = 1.24, 95% CI = 0.95–1.61, I2 = 42%, Figure 2E and Supplementary Table 1).
3.1.3.6 Dietary pattern
Healthy dietary patterns (Balanced diets, traditional Bosnian diets) may reduce thyroid cancer risk, and unhealthy dietary patterns (Meat and western dietary patterns) may elevate thyroid cancer risk, but neither is significantly different (Healthy diet: P = 0.20, OR = 0.81, 95% CI = 0.59–1.11, I2 = 13%, Unhealthy diet: P = 0.24, OR = 1.31, 95% CI = 0.83–2.07, I2 = 34%, Figure 2F and Supplementary Table 1).
3.1.4 Public bias
This meta-analysis showed no publication bias for studies with the following correlations, and the asymmetry of the funnel plot was confirmed by Egger and Begg tests (Supplementary Figure 1 and Supplementary Table 2).
3.2 MR analysis
The baseline characteristics of the 345,313 (FTC), 347,429 (MT) and 346,859 (PTC) eligible participants were shown in Table 1. Figures 3A, B illustrate the beta values for various food intakes or nutrients in three types of thyroid cancer MR analysis with IVW methods. A positive beta value indicates a significant association between the exposure and outcome, with higher values representing a greater effect size. Figure 3C shows the estimated causal effects of different foods/nutrients on thyroid cancer, as well as a forest plot of the estimated values for each outcome using different MR methods (see detailed results in Supplementary Tables 7, 8).
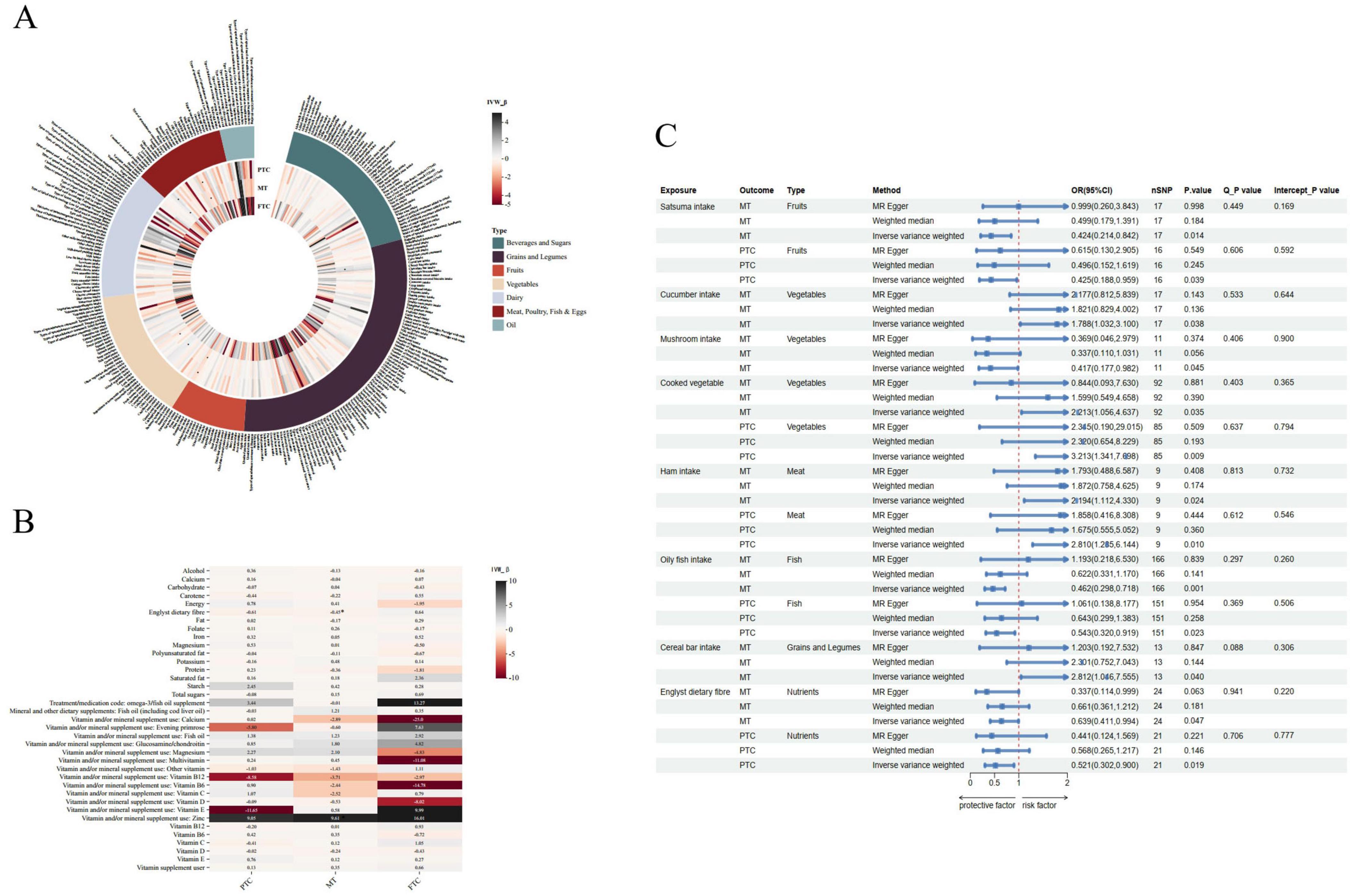
Figure 3. Mendelian randomization (MR) analysis for the causal associations of dietary factors and thyroid cancer risk. (A) The heatmap shows the beta values of the IVW method, *P < 0.05. (B) Causal risk between food and three types of thyroid cancer was estimated using conventional IVW MR analysis, MR-Egger, and weighted median MR. (C) Forest plot and sensitivity analysis of MR analysis showing the effect of food on the risk of thyroid cancer.
3.2.1 Cereal
Cereal intake and thyroid cancer studies found that cereal bars showed a positive causal relationship with MT (OR: 2.812, 95% CI: 1.046–7.555, P = 0.040).
3.2.2 Fruit and vegetable
In a study examining the relationship between fruit intake and thyroid cancer, the results showed a statistically significant correlation between satsuma consumption and an decreased risk of MT and PTC. The OR for satsuma intake in IVW analysis was 0.424 (95% CI: 0.214–0.842; P = 0.014) in MT, and was 0.425 (95% CI: 0.188–0.959; P = 0.039) in PTC. Regarding vegetable intake and thyroid cancer, cucumber consumption demonstrated a positive causal relationship with MT, as seen in the IVW analysis with an OR of 1.788 (95% CI: 1.032–3.100; P = 0.038). Additionally, cooked vegetable intake showed a positive causal relationship in MT (OR: 2.213, 95% CI: 1.056–4.637; P = 0.035) and PTC (OR: 3.213, 95% CI: 1.341–7.698; P = 0.009). In contrast, mushroom intake demonstrated a negative causal relationship with MT, as seen in the IVW analysis with an OR of 0.417 (95% CI: 0.177–0.982; P = 0.045).
3.2.3 Meat, fish and shellfish
In meat intake and thyroid cancer, ham consumption showed a positive causal relationship with MT (OR: 2.194; 95% CI: 1.112–4.330; P = 0.024) and PTC (OR: 2.810, 95% CI: 1.285–6.144; P = 0.010). In fish or shellfish intake and thyroid cancer, oily fish consumption demonstrated a significant negative causal relationship with MT (OR: 0.462, 95% CI: 0.298–0.718, P = 0.001). Oily fish also showed consistent results in PTC (OR: 0.543, 95% CI: 0.320–0.919, P = 0.023), while no association has been found between shellfish consumption and thyroid cancer.
3.2.4 Englyst dietary fiber
We identified Englyst dietary fiber as nutrients of interest. The study found that Englyst dietary fiber intake was negatively associated with MT (OR: 0.639, 95% CI: 0.411–0.994; P = 0.047) with no heterogeneity or horizontal pleiotropy. Similarly, Englyst dietary fiber showed a negative causal relationship with PTC (OR: 0.521, 95% CI: 0.302–0.900; P = 0.019).
3.2.5 Sensitivity analysis
The sensitivity analysis findings are presented in Figure 3C. Cochran’s Q test shows that there is no heterogeneity in our results. The findings of the MR-Egger intercept test revealed no horizontal pleiotropy (P all > 0.05). The results were not significantly changed before or after MR-PRESSO correction for outliers. The Steiger directionality test also shows that there is no reverse causality in our analyses, as detailed in Supplementary Table 9. Supplementary Figures 2, 3 illustrate the scatter plots and forest plots of the MR analysis. The leave-one-out analyses indicated that no individual SNP had a significant impact on the MR estimate results, as the OR values were all on the one side of the zero-line. The comparison of results from the meta-analysis and Mendelian randomization (MR) analyses has been included in Supplementary Table 10.
4 Discussion
This comprehensive study investigated the relationships between dietary factors and thyroid cancer risk, firstly provided comparisons between observational associations by meta-analysis and genetically estimated causality by MR analyses. The meta-analysis is based on case-control studies and cohort studies examining the relationship between specific food groups and dietary patterns and the risk of thyroid cancer. MR analysis further revealed the potential causal effects of specific foods.
When assessing the causal relationship between thyroid cancer risk and dietary factors, this study emphasized temporality as a necessary condition for causal inference based on the Hill criterion. All the included studies conformed to the temporality of the Hill causal relationship. Meta-analysis shows that the intake of refined cereal (OR = 2.01) and nitrates (OR = 1.41) is significantly positively correlated with the risk of thyroid cancer. The mechanism involves hyperinsulinemia and elevated insulin-like growth factor I caused by refined carbohydrates (14), and nitrate is converted into carcinogenic N-nitroso compounds in the body and competitively inhibits iodine absorption (15–17). Conversely, high consumption of fish (OR = 0.91) and alcohol (OR = 0.68) showed protective effects, which might be related to the alleviation of thyroid-related dysfunction by omega-3 polyunsaturated fatty acids in fish and the influence of alcohol on the hypothalamic-pituitary-thyroid axis function (18–20). Among them, the intake of fish also presented a dose-response relationship. The correlations of the other factors were not significant. Consistency assessment indicates that there are inconsistencies among some studies regarding the relationship between nitrate removal and thyroid cancer, while other significant factors all meet the Hill consistency criteria. For the experimental evidence in Hill causality, since randomized controlled trials were not included in this meta-analysis, the NOS scale was used to evaluate the quality of the studies. Except for Hoang et al., 2022 (21) and Mack et al., 2002 (22), the scores of other articles were all 7 points or above, which belonged to high-quality studies.
According to common perception, consuming fruits and vegetables is believed to have a protective effect against cancer. A previous study suggested that high consumption of raw vegetables, persimmons, and oranges may lower the risk of developing thyroid cancer and even help prevent early-stage thyroid cancer (23). Consumption of oranges was consistent with our MR findings, this may be because citrus fruits are a good source of bioactive compounds such as flavonoids, vitamin C, carotenoids, limonene and citric acid. Citrus flavonoids are known to have strong anti-proliferative activity in human cancer cells (24). The β-glucans and polysaccharides in mushrooms can activate immune cells, inhibit tumor growth, and show anticancer potential by regulating a single molecule in specific signaling pathways, or by having multiple targets in the same or different signaling pathways (including PI3K/Akt, Wnt/β-catenin and MAPK pathways) (25). Cooked vegetables seem to demonstrate hazardous effects on thyroid cancer. Overcooking may destroy antioxidant components such as vitamin C, while the reduced saponins in cruciferous vegetables (which are goitrogenic factors) still pose potential interference with iodine metabolism through thiocyanate accumulation. These compounds competitively inhibit thyroid iodine uptake and exhibit strong heat resistance (26, 27). However, current MR analysis did not account for iodine intake, potentially amplifying the risks associated with cooked vegetables. Cucumber has been shown to have a risk effect on thyroid cancer, but the existing literature does not support this conclusion. We consider that it is related to the cooking and eating methods of cucumber (raw with skin or peeled and cooked). For example, pickled cucumber contains a large amount of salt and nitrites, which may promote the enlargement of thyroid hormones or form carcinogenic nitrosamines, and indirectly increase the risk of thyroid cancer (28). While research on the relationship between meat intake and thyroid cancer risk is limited, and in their study, Tavani et al. identified red meat intake as an important factor in the nutritional etiology of cancer in humans (29). Only ham was associated with the risk of thyroid cancer in MR. And Ham intake data was from questionnaires “How many slices of ham, Parma ham, salami, pastrami, cured meats did you have?” Ham appears to be a risk factor, potentially due to the high nitrate content in ham. Another study of dietary patterns and differentiated thyroid cancer found that a diet of low-fat meats may be an important protective factor and thus further breakdown of meat types or different types of meat processing needs to be studied (30).
A previous study stated that higher intake of milk and dairy products can prevent thyroid cancer (4). While our analysis did not find a clear relationship between milk intake and thyroid cancer. Another case-control study in Sweden and Norway also found no association between milk consumption and thyroid cancer (31).
Nitrates inhibit the uptake of iodide by the thyroid gland, decreasing levels of T3 and T4, which increase thyroid-stimulating hormones (16). Some studies have observed that increased dietary nitrate intake is associated with an increased risk of thyroid cancer (32), and our findings also show that high nitrate intake is a risk factor for thyroid cancer. Polyphenols such as flavonoids and phenolic acids, which are abundant in tea, may play a role in thyroid cancer by regulating enzymatic activities and signaling pathways related to cell proliferation, differentiation, apoptosis, and inflammation (33). The EPIC study found no association between tea intake and thyroid cancer risk (34), which is consistent with our findings.
Previous researches found an increased risk of thyroid cancer has been shown to be associated with excessive intake of starch-rich foods such as refined grains, pasta or rice, bread, pastries and potatoes (35, 36). In terms of plasma glucose and insulin responses, GI and GL are indicators of physiological responses to different foods and are highly correlated with high intake of refined carbohydrates (37). High dietary levels of GI and GL are associated with thyroid cancer risk, while high total energy may increase the risk of differentiated thyroid cancer (38). This is similar to our results, but we did not find significant differences in the pooled starchy foods, which may be due to their different foods and therefore different levels of GI after starch digestion. Caffeine in coffee increases intracellular levels of cyclic adenosine monophosphate, which has an inhibitory effect on tumor growth (39). Meanwhile, chlorogenic acid in coffee is an antioxidant, and its activity is thought to play a role in preventing cancer (40). However, we did not find a link between coffee and thyroid cancer, suggesting that more prospective studies may be needed to examine the impact of coffee consumption on thyroid cancer risk, in line with previous conclusions (41).
Since different foods are often consumed in combination and interact with each other in complex situations in the daily diet, a more comprehensive dietary pattern provides a constructive tool for assessing the impact of diet on health (42). This meta-analysis harmonized balanced diets, traditional Bosnian diets and healthy diets as healthy dietary patterns. Meat, high fat intake and Western dietary patterns were harmonized as unhealthy dietary patterns. Previous studies have shown that a healthy dietary pattern may reduce the risk of cardiovascular disease and total mortality (43), and it has also been associated with a lower risk of several cancers (44, 45). In contrast, unhealthy dietary patterns such as the Western dietary pattern have been associated with an increased chance of developing differentiated thyroid cancer (46). However, the pooled results of this meta-analysis showed that there was no statistically significant difference between the different dietary patterns and the risk of thyroid cancer, which may be related to the small number of included literature and some heterogeneity, and further studies are recommended to provide more conclusive evidence about the association between dietary patterns and thyroid cancer.
Our MR results indicated that oily fish consumption both decrease the risk of developing thyroid cancer. Prior research has shown that moderate fish consumption does not significantly elevate the risk of thyroid cancer, and may even have a beneficial effect in areas with iodine deficiency (47), which is relatively consistent with our findings. In the study of thyroid cancer in women, Mack et al. found that shellfish and saltwater fish reduced risk independent of the risk of fish consumption, but our meta-study found no significant relationship between high shellfish and saltwater fish consumption and thyroid cancer risk (22). One possible explanation is that iodine deficiency or excess may exist in the study area. In areas of severe iodine deficiency, a high intake of fish had a protective effect, whereas in areas of adequate iodine intake there was no effect (48).
This meta-analysis has some limitations, firstly the small number of studies pooled and combined in some food groups does not accurately reflect the true picture. Secondly, there was some variation in consumption categories among the included studies, and different exposure assessment methods can cause some bias. The data on fish research was included after a comprehensive search of published literature. However, only two studies included more than 1,000 people, while the rest were small-scale research results. More large-scale studies are needed in the future to confirm our findings. However, there are some advantages, firstly, we observed inconsistent results across different study designs, so different study designs were pooled and aggregated separately. Second, the literature search was conducted independently by other authors to minimize errors. We employed the MR design to reduce the risks of confounding and reverse causation, which are significant limitations of observational studies. However, there are some limitations associated with MR as well. Although we selected the largest sample size and the most recent GWAS datasets available for our MR analysis, the sample size and number of events in our study were relatively small compared to those in population-based studies. Due to the generally low heritability of dietary exposure, which may limit statistical efficacy, future studies need to combine high-precision metabolomics or proteomics data to improve the explanatory power of instrumental variables. This study mainly explores the long-term average effect of dietary exposure. In the future, potential threshold effects can be explored by stratified MR analysis (e.g., stratified by intake) or non-linear MR methods (e.g., quadratic model), which require a larger sample size to support. Additionally, our analysis was performed on populations with specific ancestries, which may introduce ascertainment bias. Consequently, these findings may not accurately reflect the broader population. Furthermore, the lack of demographic and detailed clinical information in the GWAS database hindered our ability to conduct subgroup analysis, limiting the depth of our insights.
5 Conclusion
This study conducted a meta-analysis of case-control and cohort studies to summarize the evidence for the association between specific food groups, dietary patterns, and thyroid cancer risk. We also conducted an MR analysis to investigate the relationship between specific foods and thyroid cancer. Both meta-analysis and MR analysis found that fish was associated with a reduced risk of thyroid cancer.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary material.
Author contributions
CK: Conceptualization, Funding acquisition, Writing – original draft. YD: Data curation, Formal analysis, Writing – original draft. JL: Formal analysis, Investigation, Writing – original draft. YY: Investigation, Methodology, Writing – original draft. JpL: Investigation, Methodology, Validation, Visualization, Writing – original draft. MZ: Methodology, Validation, Visualization, Writing – original draft. JS: Software, Validation, Visualization, Writing – original draft. NL: Conceptualization, Supervision, Writing – review & editing. XM: Conceptualization, Writing – review & editing. XP: Conceptualization, Funding acquisition, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was supported by the Department of Science and Technology of Sichuan Province (No. 2024ZYD0193), Joint Foundation of Chengdu Medical College and Jianyang Center for Disease Control and Prevention (No. 2022LHJY01), The Project of Chengdu Pidu District People’s Hospital (No. 23LHPDZYB22), and the Open Fund of Sichuan Provincial Key Laboratory of Philosophy and Social Sciences for Intelligent Medical Care and Elderly Health Management (No. ZHYYZKYB2401).
Acknowledgments
Chao Kang and Xiaoli Peng are the guarantors of this work and had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2025.1664129/full#supplementary-material
Supplementary Figure 1 | Funnel plot detailing publication bias in the case-control and cohort studies reporting.
Supplementary Figure 2 | The scatter plot for MR analysis of the causal relationship between different dietary factors SNPs and three types of thyroid cancer.
Supplementary Figure 3 | Results of leave-one-out sensitivity analysis.
References
1. Bao W, Zi H, Yuan Q, Li L, Deng T. Global burden of thyroid cancer and its attributable risk factors in 204 countries and territories from 1990 to 2019. Thorac Cancer. (2021) 12:2494–503. doi: 10.1111/1759-7714.14099
2. Sung H, Ferlay J, Siegel R, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 Countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
3. Lin Y, Wu Y. Trends in incidence and overdiagnosis of thyroid cancer in China, Japan, and South Korea. Cancer Sci. (2023) 114:4052–62. doi: 10.1111/cas.15909
4. Fiore M, Cristaldi A, Okatyeva V, Lo Bianco S, Oliveri Conti G, Zuccarello P, et al. Dietary habits and thyroid cancer risk: a hospital-based case-control study in Sicily (South Italy). Food Chem Toxicol. (2020) 146:111778. doi: 10.1016/j.fct.2020.111778
5. Liang J, Zhao N, Zhu C, Ni X, Ko J, Huang H, et al. Dietary patterns and thyroid cancer risk: a population-based case-control study. Am J Transl Res. (2020) 12:180–90.
6. Zamora-Ros R, Béraud V, Franceschi S, Cayssials V, Tsilidis K, Boutron-Ruault M, et al. Consumption of fruits, vegetables and fruit juices and differentiated thyroid carcinoma risk in the European Prospective Investigation into Cancer and Nutrition (EPIC) study. Int J Cancer. (2018) 142:449–59. doi: 10.1002/ijc.30880
7. O’Grady T, Kitahara C, DiRienzo A, Gates M. The association between selenium and other micronutrients and thyroid cancer incidence in the NIH-AARP Diet and Health Study. PLoS One. (2014) 9:e110886. doi: 10.1371/journal.pone.0110886
8. Mack W, Preston-Martin S, Dal Maso L, Galanti R, Xiang M, Franceschi S, et al. A pooled analysis of case-control studies of thyroid cancer: cigarette smoking and consumption of alcohol, coffee, and tea. Cancer Causes Control. (2003) 14:773–85. doi: 10.1023/a:1026349702909
9. Meinhold C, Park Y, Stolzenberg-Solomon R, Hollenbeck A, Schatzkin A, Berrington de Gonzalez A. Alcohol intake and risk of thyroid cancer in the NIH-AARP Diet and Health Study. Br J Cancer. (2009) 101:1630–4. doi: 10.1038/sj.bjc.6605337
10. Fang J, Song K, Zhang D, Liang Y, Zhao H, Jin J, et al. Coffee intake and risk of diabetic nephropathy: a Mendelian randomization study. Front Endocrinol. (2023) 14:1169933. doi: 10.3389/fendo.2023.1169933
11. Liberati A, Altman D, Tetzlaff J, Mulrow C, Gøtzsche P, Ioannidis J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. (2009) 339:b2700. doi: 10.1136/bmj.b2700
12. Hill A. The environment and disease: association or causation? Proc R Soc Med. (1965) 58:295–300. doi: 10.1177/003591576505800503
13. Baloch Z, Asa S, Barletta J, Ghossein R, Juhlin C, Jung C, et al. Overview of the 2022 WHO Classification of Thyroid Neoplasms. Endocr Pathol. (2022) 33:27–63. doi: 10.1007/s12022-022-09707-3
14. La Vecchia C, Braga C, Negri E, Franceschi S, Russo A, Conti E, et al. Intake of selected micronutrients and risk of colorectal cancer. Int J Cancer. (1997) 73:525–30. doi: 10.1002/(sici)1097-0215(19971114)73:43.0.co;2-8
15. Grosse Y, Baan R, Straif K, Secretan B, El Ghissassi F, Cogliano V. Carcinogenicity of nitrate, nitrite, and cyanobacterial peptide toxins. Lancet Oncol. (2006) 7:628–9. doi: 10.1016/s1470-2045(06)70789-6
16. Tonacchera M, Pinchera A, Dimida A, Ferrarini E, Agretti P, Vitti P, et al. Relative potencies and additivity of perchlorate, thiocyanate, nitrate, and iodide on the inhibition of radioactive iodide uptake by the human sodium iodide symporter. Thyroid. (2004) 14:1012–9. doi: 10.1089/thy.2004.14.1012
17. De Groef B, Decallonne B, Van der Geyten S, Darras V, Bouillon R. Perchlorate versus other environmental sodium/iodide symporter inhibitors: potential thyroid-related health effects. Eur J Endocrinol. (2006) 155:17–25. doi: 10.1530/eje.1.02190
18. Pal A, Mohan V, Modi D, Sinha R, Rastogi L, Kumar P, et al. Iodine plus n-3 fatty acid supplementation augments rescue of postnatal neuronal abnormalities in iodine-deficient rat cerebellum. Br J Nutr. (2013) 110:659–70. doi: 10.1017/s0007114512005569
19. Awumey E, Paton D, Pehowich D. Thyroid status and dietary fatty acids affect beta-adrenoceptor agonist stimulation of tension development in rat myocardium. J Auton Pharmacol. (1995) 15:73–84. doi: 10.1111/j.1474-8673.1995.tb00293.x
20. Zoeller R, Fletcher D, Simonyl A, Rudeen P. Chronic ethanol treatment reduces the responsiveness of the hypothalamic-pituitary-thyroid axis to central stimulation. Alcohol Clin Exp Res. (1996) 20:954–60. doi: 10.1111/j.1530-0277.1996.tb05277.x
21. Hoang T, Lee E, Lee J, Hwangbo Y, Kim J. Seaweed and iodine intakes and SLC5A5 rs77277498 in relation to thyroid cancer. Endocrinol Metab. (2022) 37:513–23. doi: 10.3803/EnM.2021.1306
22. Mack W, Preston-Martin S, Bernstein L, Qian D. Lifestyle and other risk factors for thyroid cancer in Los Angeles County females. Ann Epidemiol. (2002) 12:395–401. doi: 10.1016/s1047-2797(01)00281-2
23. Jung S, Kim K, Tae K, Kong G, Kim M. The effect of raw vegetable and fruit intake on thyroid cancer risk among women: a case-control study in South Korea. Br J Nutr. (2013) 109:118–28. doi: 10.1017/S0007114512000591
24. Manthey J, Guthrie N. Antiproliferative activities of citrus flavonoids against six human cancer cell lines. J Agric Food Chem. (2002) 50:5837–43. doi: 10.1021/jf020121d
25. Park H. Current uses of mushrooms in cancer treatment and their anticancer mechanisms. Int J Mol Sci. (2022) 23:10502. doi: 10.3390/ijms231810502
26. Serrano-Nascimento C, Nunes M. Perchlorate, nitrate, and thiocyanate: environmental relevant NIS-inhibitors pollutants and their impact on thyroid function and human health. Front Endocrinol. (2022) 13:995503. doi: 10.3389/fendo.2022.995503
27. Zhang Y, Makaza N, Jiang C, Wu Y, Nishanbaev S, Zou L, et al. Supplementation of cooked broccoli with exogenous moringa myrosinase enhanced isothiocyanate formation. Food Chem. (2022) 395:133651. doi: 10.1016/j.foodchem.2022.133651
28. Said Abasse K, Essien E, Abbas M, Yu X, Xie W, Sun J, et al. Association between dietary nitrate, nitrite intake, and site-specific cancer risk: a systematic review and meta-analysis. Nutrients. (2022) 14:666. doi: 10.3390/nu14030666
29. Tavani A, La Vecchia C, Gallus S, Lagiou P, Trichopoulos D, Levi F, et al. Red meat intake and cancer risk: a study in Italy. Int. J. Cancer. (2000) 86:425–8. doi: 10.1002/(sici)1097-0215(20000501)86:33.0.co;2-s
30. Przybylik-Mazurek E, Hubalewska-Dydejczyk A, Kuźniarz-Rymarz S, Kieć-Klimczak M, Skalniak A, Sowa-Staszczak A, et al. Dietary patterns as risk factors of differentiated thyroid carcinoma. Postepy Hig Med Dosw. (2012) 66:11–5. doi: 10.5604/17322693.974647
31. Galanti M, Hansson L, Bergström R, Wolk A, Hjartåker A, Lund E, et al. Diet and the risk of papillary and follicular thyroid carcinoma: a population-based case-control study in Sweden and Norway. Cancer Causes Control. (1997) 8:205–14. doi: 10.1023/a:1018424430711
32. Ward M, Kilfoy B, Weyer P, Anderson K, Folsom A, Cerhan J. Nitrate intake and the risk of thyroid cancer and thyroid disease. Epidemiology. (2010) 21:389–95. doi: 10.1097/EDE.0b013e3181d6201d
33. Gonçalves C, de Freitas M, Ferreira A. Flavonoids, thyroid iodide uptake and thyroid cancer-a review. Int J Mol Sci. (2017) 18:1247. doi: 10.3390/ijms18061247
34. Zamora-Ros R, Alghamdi M, Cayssials V, Franceschi S, Almquist M, Hennings J, et al. Coffee and tea drinking in relation to the risk of differentiated thyroid carcinoma: results from the European Prospective Investigation into Cancer and Nutrition (EPIC) study. Eur J Nutr. (2019) 58:3303–12. doi: 10.1007/s00394-018-1874-z
35. Chatenoud L, La Vecchia C, Franceschi S, Tavani A, Jacobs D, Parpinel M, et al. Refined-cereal intake and risk of selected cancers in Italy. Am J Clin Nutr. (1999) 70:1107–10. doi: 10.1093/ajcn/70.6.1107
36. Franceschi S, Levi F, Negri E, Fassina A, La Vecchia C. Diet and thyroid cancer: a pooled analysis of four European case-control studies. Int J Cancer. (1991) 48:395–8. doi: 10.1002/ijc.2910480315
37. Gnagnarella P, Gandini S, La Vecchia C, Maisonneuve P. Glycemic index, glycemic load, and cancer risk: a meta-analysis. Am J Clin Nutr. (2008) 87:1793–801. doi: 10.1093/ajcn/87.6.1793
38. Zamora-Ros R, Rinaldi S, Tsilidis K, Weiderpass E, Boutron-Ruault M, Rostgaard-Hansen A, et al. Energy and macronutrient intake and risk of differentiated thyroid carcinoma in the European Prospective Investigation into Cancer and Nutrition study. Int J Cancer. (2016) 138:65–73. doi: 10.1002/ijc.29693
39. Linos A, Linos D, Vgotza N, Souvatzoglou A, Koutras D. Does coffee consumption protect against thyroid disease? Acta Chir Scand. (1989) 155:317–20.
40. Nkondjock A. Coffee consumption and the risk of cancer: an overview. Cancer Lett. (2009) 277:121–5. doi: 10.1016/j.canlet.2008.08.022
41. Michikawa T, Inoue M, Shimazu T, Sasazuki S, Iwasaki M, Sawada N, et al. Green tea and coffee consumption and its association with thyroid cancer risk: a population-based cohort study in Japan. Cancer Causes Control. (2011) 22:985–93. doi: 10.1007/s10552-011-9771-2
42. Tucker K. Dietary patterns, approaches, and multicultural perspective. Appl Physiol Nutr Metab. (2010) 35:211–8. doi: 10.1139/H10-010
43. Heidemann C, Schulze M, Franco O, van Dam R, Mantzoros C, Hu F. Dietary patterns and risk of mortality from cardiovascular disease, cancer, and all causes in a prospective cohort of women. Circulation. (2008) 118:230–7. doi: 10.1161/CIRCULATIONAHA.108.771881
44. Bertuccio P, Rosato V, Andreano A, Ferraroni M, Decarli A, Edefonti V, et al. Dietary patterns and gastric cancer risk: a systematic review and meta-analysis. Ann Oncol. (2013) 24:1450–8. doi: 10.1093/annonc/mdt108
45. Brennan S, Cantwell M, Cardwell C, Velentzis L, Woodside J. Dietary patterns and breast cancer risk: a systematic review and meta-analysis. Am J Clin Nutr. (2010) 91:1294–302. doi: 10.3945/ajcn.2009.28796
46. Sangsefidi Z, Ghafouri-Taleghani F, Zakavi S, Norouzy A, Kashanifar R, Pourbaferani R, et al. Major dietary patterns and differentiated thyroid cancer. Clin Nutr ESPEN. (2019) 33:195–201. doi: 10.1016/j.clnesp.2019.05.015
47. Bosetti C, Kolonel L, Negri E, Ron E, Franceschi S, Dal Maso L, et al. A pooled analysis of case-control studies of thyroid cancer. VI. Fish and shellfish consumption. Cancer Causes Control. (2001) 12:375–82. doi: 10.1023/a:1011267123398
48. Andersson M, Karumbunathan V, Zimmermann M. Global iodine status in 2011 and trends over the past decade. J Nutr. (2012) 142:744–50. doi: 10.3945/jn.111.149393
49. Fioretti F, Tavani A, Gallus S, Franceschi S, Negri E, La Vecchia C. Case-control study of thyroid cancer in Northern Italy: attributable risk. Int J Epidemiol. (1999) 28:626–30. doi: 10.1093/ije/28.4.626
50. Kilfoy B, Zhang Y, Park Y, Holford T, Schatzkin A, Hollenbeck A, et al. Dietary nitrate and nitrite and the risk of thyroid cancer in the NIH-AARP Diet and Health Study. Int J Cancer. (2011) 129:160–72. doi: 10.1002/ijc.25650
51. Michikawa T, Inoue M, Shimazu T, Sawada N, Iwasaki M, Sasazuki S, et al. Seaweed consumption and the risk of thyroid cancer in women: the Japan Public Health Center-based Prospective Study. Eur J Cancer Prev. (2012) 21:254–60. doi: 10.1097/CEJ.0b013e32834a8042
52. Nguyen L, Gunathilake M, Lee J, Kim J. Association between dietary habits and incident thyroid cancer: a prospective cohort study. Front Nutr. (2023) 10:1104925. doi: 10.3389/fnut.2023.1104925
53. Takezaki T, Hirose K, Inoue M, Hamajima N, Kuroishi T, Nakamura S, et al. Risk factors of thyroid cancer among women in Tokai, Japan. J Epidemiol. (1996) 6:140–7. doi: 10.2188/jea.6.140
54. Xhaard C, Rubino C, Souchard V, Maillard S, Ren Y, Borson-Chazot F, et al. Dietary habits during the 2 months following the Chernobyl accident and differentiated thyroid cancer risk in a population-based case-control study. Cancer Epidemiol. (2018) 52:142–7. doi: 10.1016/j.canep.2017.12.015
55. Xiao Q, Park Y, Hollenbeck A, Kitahara C. Dietary flavonoid intake and thyroid cancer risk in the NIH-AARP diet and health study. Cancer Epidemiol Biomarkers Prev. (2014) 23:1102–8. doi: 10.1158/1055-9965.EPI-13-1150
56. Zamora-Ros R, Castañeda J, Rinaldi S, Cayssials V, Slimani N, Weiderpass E, et al. Consumption of fish is not associated with risk of differentiated thyroid carcinoma in the european prospective investigation into cancer and nutrition (EPIC) Study. J Nutr. (2017) 147:1366–73. doi: 10.3945/jn.117.247874
57. Zamora-Ros R, Cayssials V, Clèries R, Torrents M, Byrnes G, Weiderpass E, et al. Sweetened beverages are associated with a higher risk of differentiated thyroid cancer in the EPIC cohort: a dietary pattern approach. Eur J Nutr. (2023) 62:105–14. doi: 10.1007/s00394-022-02953-5
58. Braganza M, Potischman N, Park Y, Thompson F, Hollenbeck A, Kitahara C. Adolescent and mid-life diet and subsequent risk of thyroid cancer in the NIH-AARP diet and health study. Int J Cancer. (2015) 137:2413–23. doi: 10.1002/ijc.29600
59. Aschebrook-Kilfoy B, Shu X, Gao Y, Ji B, Yang G, Li H, et al. Thyroid cancer risk and dietary nitrate and nitrite intake in the Shanghai women’s health study. Int J Cancer. (2013) 132:897–904. doi: 10.1002/ijc.27659
60. Cléro É, Doyon F, Chungue V, Rachédi F, Boissin J, Sebbag J, et al. Dietary patterns, goitrogenic food, and thyroid cancer: a case-control study in French Polynesia. Nutr Cancer. (2012) 64:929–36. doi: 10.1080/01635581.2012.713538
61. Cléro É, Doyon F, Chungue V, Rachédi F, Boissin J, Sebbag J, et al. Dietary iodine and thyroid cancer risk in French Polynesia: a case-control study. Thyroid. (2012) 22:422–9. doi: 10.1089/thy.2011.0173
62. Hallquist A, Hardell L, Degerman A, Boquist L. Thyroid cancer: reproductive factors, previous diseases, drug intake, family history and diet. A case-control study. Eur J Cancer Prev. (1994) 3:481–8.
63. Haslam A, Robb S, Bonner M, Lindblad W, Allegra J, Shen Y, et al. Polychlorinated biphenyls and omega-3 fatty acid exposure from fish consumption, and thyroid cancer among New York anglers. J Environ Sci. (2016) 41:270–7. doi: 10.1016/j.jes.2015.05.004
64. Horn-Ross P, Morris J, Lee M, West D, Whittemore A, McDougall I, et al. Iodine and thyroid cancer risk among women in a multiethnic population: the Bay Area Thyroid Cancer Study. Cancer Epidemiol Biomarkers Prev. (2001) 10:979–85.
65. Kim C, Huang H, Zhao N, Lerro C, Dai M, Chen Y, et al. Use of dietary vitamin supplements and risk of thyroid cancer: a population-based case-control study in connecticut. Int J Vitam Nutr Res. (2016) 86:189–97. doi: 10.1024/0300-9831/a000403
66. Memon A, Varghese A, Suresh A. Benign thyroid disease and dietary factors in thyroid cancer: a case-control study in Kuwait. Br J Cancer. (2002) 86:1745–50. doi: 10.1038/sj.bjc.6600303
67. Myung S, Lee C, Lee J, Kim J, Kim H. Risk factors for thyroid cancer: a hospital-based case-control study in Korean adults. Cancer Res Treat. (2017) 49:70–8. doi: 10.4143/crt.2015.310
68. Preston-Martin S, Jin F, Duda M, Mack W. A case-control study of thyroid cancer in women under age 55 in Shanghai (People’s Republic of China). Cancer Causes Control. (1993) 4(5):431–40. doi: 10.1007/BF00050862
69. Wie G, Cho Y, Kang H, Ryu K, Yoo M, Kim Y, et al. Red meat consumption is associated with an increased overall cancer risk: a prospective cohort study in Korea. Br J Nutr. (2014) 112:238–47. doi: 10.1017/S0007114514000683
70. Truong T, Baron-Dubourdieu D, Rougier Y, Guénel P. Role of dietary iodine and cruciferous vegetables in thyroid cancer: a countrywide case-control study in New Caledonia. Cancer Causes Control. (2010) 21:1183–92. doi: 10.1007/s10552-010-9545-2
71. Wang C, Yatsuya H, Li Y, Ota A, Tamakoshi K, Fujino Y, et al. Prospective study of seaweed consumption and thyroid cancer incidence in women: the Japan collaborative cohort study. Eur J Cancer Prev. (2016) 25:239–45. doi: 10.1097/CEJ.0000000000000168
Keywords: food groups, dietary pattern, thyroid cancer, meta-analysis, mendelian randomization analysis
Citation: Kang C, Du Y, Li J, Yang Y, Li J, Zhou M, Shi J, Lin N, Ma X and Peng X (2025) Dissecting the causal association of diet with thyroid cancer: a systematic review with meta-analysis and mendelian randomization analysis. Front. Nutr. 12:1664129. doi: 10.3389/fnut.2025.1664129
Received: 11 July 2025; Accepted: 01 September 2025;
Published: 17 September 2025.
Edited by:
Elma Izze da Silva Magalhães, Federal University of Rio Grande do Sul, BrazilReviewed by:
Van Cuong Nguyen, Hanyang University, Republic of KoreaSara Jambarsang, Shahid Sadoughi University of Medical Sciences and Health Services, Iran
Copyright © 2025 Kang, Du, Li, Yang, Li, Zhou, Shi, Lin, Ma and Peng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ning Lin, aGVsZW5tZWRpY0B5ZWFoLm5ldA==; Xin Ma, MTM4ODA0MjIzMzZAMTYzLmNvbQ==; Xiaoli Peng, cGVuZ3hpYW9saUBjbWMuZWR1LmNu
†These authors have contributed equally to this work
 Chao Kang
Chao Kang Yongyao Du1†
Yongyao Du1†