- Department of Critical Care Medicine, The First Hospital of Jilin University, Changchun, China
Background: To identify appropriate nutritional support strategies for critically ill patients with different levels of nutritional risk.
Methods: A systematic search of PubMed, MEDLINE, Cochrane Library, and Embase was conducted from database inception to 19 May 2025, which included critically ill patients classified into high risk (5–9) and low risk (0–4) groups based on the modified Nutrition Risk in the Critically Ill (mNUTRIC) score. Data on study characteristics, patient demographics, and nutritional support details were extracted. The primary outcome was all-cause mortality following nutritional support stratified by nutritional risk among critically ill patients. A meta-regression analysis was performed to assess the influence of covariates on effect sizes and to identify potential sources of heterogeneity. Trial sequential analysis (TSA) was conducted to evaluate the robustness and reliability of the pooled effect estimates.
Results: Eleven eligible trials, comprising a total of 7,442 participants, were included in this systematic review. The meta-analysis demonstrated that high nutritional risk was significantly associated with increased mortality (OR: 2.26, 95% CI: 1.80–2.83, p < 0.0001). Adequate energy intake was associated with a significantly lower 28-day mortality among patients at high nutritional risk (OR: 0.60, 95% CI: 0.38–0.94, p = 0.03). However, in randomized controlled trials, adequate energy support did not reduce 28-day mortality (OR: 1.09, 95% CI: 0.74–1.60) or 90-day mortality (OR: 1.03, 95% CI: 0.87–1.23) in high-risk patients.
Conclusion: The mNUTRIC score is a validated prognostic tool in critically ill patients, but its effectiveness in guiding energy support remains limited.
Systematic Review registration: https://www.crd.york.ac.uk/PROSPERO/view/CRD42020188064, Identifier: CRD42020188064.
1 Introduction
Critically ill patients who remain in the intensive care units (ICU) for more than 48 h are at a high risk of malnutrition (1), which is independently associated with poor outcomes such as prolonged ICU and hospitalization, prolonged mechanical ventilation, and higher incidences of infectious complications (2). Therefore, optimizing nutritional support during ICU care is essential for enhancing long term outcomes and reducing the risk of patients becoming adversely affected by critical illness (3, 4).
Prospective observational studies on nutrition practices in ICU worldwide have demonstrated that sufficient nutritional support can improve patient prognosis (5–8). However, several other studies have indicated that nutritional support provided during the acute phase had minimal impact on clinical outcomes and may even be harmful to critically ill patients (9–13). One possible explanation for these inconsistency among findings is the heterogeneity among patients from different studies (5). Heyland et al. (14) developed the Nutrition Risk in the Critically Ill (NUTRIC) score, which incorporates the severity of illness into its calculation and aims to quantify the risk of malnutrition and identify patients who would benefit from adequate nutritional support. Since quantifying interleukin-6 in typical clinical settings is difficult, a modified NUTRIC (mNUTRIC) score has been devised to eliminate interleukin-6 levels while preserving other relevant clinical indicators (15).
Previous research suggests that the mNUTRIC score might serve as an indicator for determining the need for intensive nutritional support in these patients (16–18) and as a predictor of mortality (19–21). However, it is unclear whether patients with higher nutritional risk actually derive greater survival benefit from adequate energy and protein intake.
The objective of this meta-analysis is to determine whether adequate energy and protein nutritional support is associated with reduced mortality in critically ill patients stratified by nutritional risk and to evaluate the prognostic value of the mNUTRIC score in this context.
2 Methods
2.1 Search strategy
This systematic review and meta-analysis was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines. The study protocol was registered with PROSPERO database (CRD42020188064). Two reviewers conducted independent searches of PubMed, MEDLINE, Cochrane Library, and Embase databases from their inception to 19 May 2025. The search included the terms ‘nutritional support,’ ‘nutrition therapy,’ ‘nutritional risk,’ ‘NUTRIC score,’ ‘mNUTRIC score,’ ‘critical care,’ ‘intensive care,’ and ‘critically ill.’ Only articles published in English were included.
2.2 Study selection
Eligible studies included randomized controlled trials (RCTs), cohort studies, post-hoc analysis, and both prospective and retrospective observational studies. Research focusing on critically ill patients, specifically those categorized by the mNUTRIC scores, were prioritized for inclusion. Patients were categorized into high risk (5–9) and low risk (0–4) groups based on their mNUTRIC score.
2.3 Inclusion and exclusion criteria
The inclusion criteria were as follows: (1) clinical trials; (2) studies involving critically ill patients classified according to the mNUTRIC score; (3) studies evaluating nutritional support administered through enteral and/or parenteral nutrition; (4) comparisons between patients receiving adequate versus inadequate energy and/or protein intake; and (5) studies reporting mortality as an outcome measure. The primary outcome was overall mortality, including 28-day, 30-day, 60-day, and 90-day mortality. The exclusion criteria were as follows: (1) studies that did not report or classify patients according to the mNUTRIC score; (2) studies lacking a clear definition or comparison of adequate versus inadequate energy and/or protein intake; (3) studies with insufficient data to extract or calculate effect estimates; (4) studies enrolling non-critically ill populations or employing other nutritional risk tools instead of the mNUTRIC score; and (5) reviews, editorials, case reports, conference abstracts, or non-English publications.
2.4 Data extraction
Two reviewers independently extracted data from all eligible studies using a predefined spreadsheet. Extracted variables included study design, inclusion criteria, baseline patient characteristics, definitions of adequate nutritional support, and mortality outcomes. Discrepancies were addressed via consensus.
2.5 Quality assessment
The risk of bias in RCTs was assessed using the Cochrane Risk of Bias Tool, which evaluates domains including selection, performance, detection, attrition, and reporting biases. The Newcastle-Ottawa Scale was utilized for observational studies, emphasizing selection, comparability, and outcome assessment. Due to the limited number of included studies, the calculation of inter-rater reliability (Cohen’s κ) would be statistically unstable and was not performed. Any discrepancies between the two reviewers were resolved through discussion.
2.6 Definition of adequate energy and protein intake
Adequate nutritional support was defined according to the criteria used in the included studies. Specifically, adequate energy intake referred to 65–100% of the prescribed energy target, while adequate protein intake was defined as 1.0–1.5 g/kg per day or at least two-thirds of the prescribed protein target.
2.7 Statistical analysis
All statistical analyses were performed using R software (version 4.2.2, R Foundation for Statistical Computing, Vienna, Austria). The primary outcome was mortality, which were statistically represented by odds ratios (ORs) with 95% confidence intervals (CIs). A random-effects model was used for studies with significant heterogeneity (p < 0.10, I2 > 50%).
A leave-one-out sensitivity analysis was performed using the metainf function from the meta package in R. Each study was sequentially excluded from the meta-analysis to assess its influence on the overall odds ratio (OR), and the robustness of the pooled effect was evaluated accordingly.
Trial sequential analysis (TSA) was conducted using TSA software (v0.9.5.10, Copenhagen Trial Unit) with a two-sided α of 5 and 80% statistical power. The required information size (RIS) was estimated assuming a 24% relative risk reduction for 28-day mortality and 22.5% reduction for 90-day mortality, applying the O’Brien-Fleming α-spending function and variance-based heterogeneity correction under a random-effects model. TSA was performed to control the risk of random errors due to repeated significance testing and to assess whether the cumulative evidence was sufficient to support firm and reliable conclusions.
3 Result
3.1 Study characteristics and quality assessment
A total of 332 articles were initially identified. After screening the titles and abstracts, 34 studies were deemed potentially eligible. The full text articles of these 34 studies were reviewed individually, and 11 studies (22–32) were deemed eligible for further analysis. Study Selection Flow Diagram is shown in Figure 1. Among the included studies, 11 studies focused on energy administration, of which 4 (22, 25, 27, 28) investigated both energy and protein intake. The 11 eligible trials included a total of 7,442 participants, and the key characteristics of these studies are summarized in Table 1.
3.2 Quality assessment
The observational studies, assessed using the Newcastle–Ottawa Scale, were found to have a moderate risk of bias, with all studies rated as moderate quality (5*). The RCTs were evaluated using the Cochrane Risk of Bias tool and also showed a moderate risk of bias, primarily due to concerns related to the blinding of participants, personnel, and outcome assessment (Supplementary Table S1; Supplementary Figure S1).
3.3 Mortality associated with different nutrition risk in critically ill patients
To compare mortality between patients with high and low nutritional risk, a meta-analysis was performed by pooling data from studies that reported mortality outcomes stratified by nutritional risk (22–28, 30–32). The results demonstrated that high nutritional risk was significantly associated with increased 28-day mortality (OR: 2.26, 95% CI: 1.80–2.83, p < 0.00001, Figure 2). Sensitivity analyses, conducted by excluding each study in turn, showed consistent results, indicating the robustness of the findings (Supplementary Figure S2).
3.4 Mortality associated with energy adequacy in critically ill patients
A total of 4,205 patients were identified as high nutritional risk patients based on the mNUTRIC score. The meta-analysis suggested that adequate energy intake might be associated with a reduction in 28-day mortality among patients at high nutritional risk (OR: 0.60, 95% CI: 0.38–0.94, p = 0.03) and that moderate heterogeneity was observed among the included studies (I2 = 53%, Figure 3).
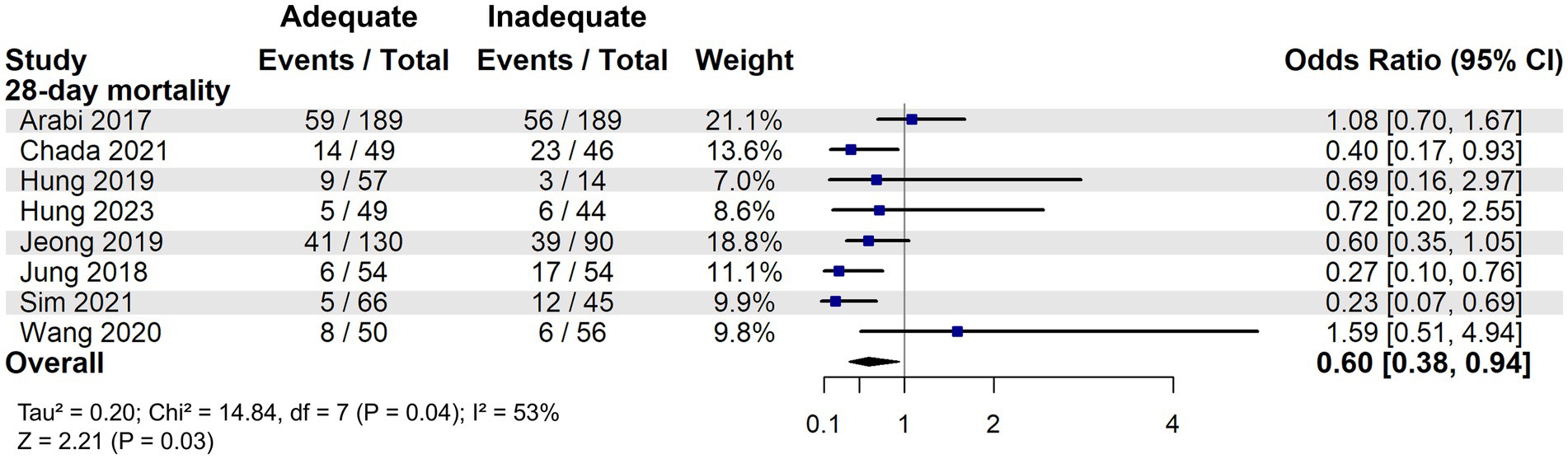
Figure 3. 28-day mortality associated with energy adequacy in critically ill patients with high nutritional risk.
To further explore heterogeneity, a multivariate metaregression analysis was performed in high nutritional risk patients. BMI and APACHE II score were initially identified as covariates potentially associated with the effect size (p < 0.1) and were included in the final model. This model significantly explained the heterogeneity (R2 = 100%, I2 = 0%; p = 0.0066). Among these, only BMI remained a significant moderator (β = 0.13, 95% CI: 0.02–0.24; p = 0.02). A potential interaction between BMI and the effect of adequate energy intake was also observed: patients with BMI < 27 kg/m2 appeared to benefit from adequate energy support, whereas those with BMI ≥ 27 kg/m2 showed no clear 28-day survival advantage (Figure 4). In addition, the meta-analysis of the effect of adequate energy support on 60-day mortality among high-risk patients is shown in Supplementary Figure S3.
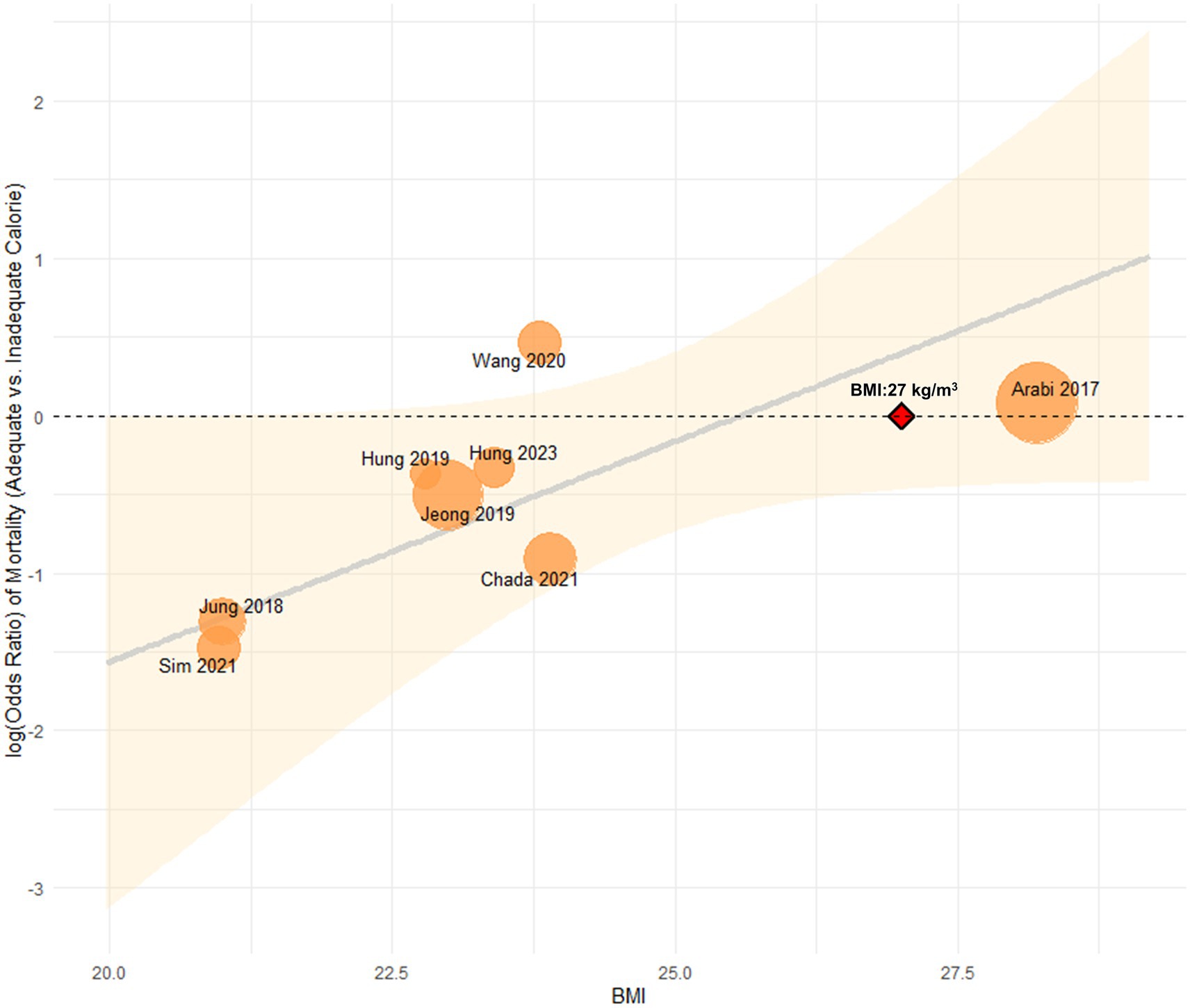
Figure 4. Association between BMI and the effect of energy intake on 28-day mortality in high nutritional risk.
However, when considering only RCTs, including two secondary analyses of RCTs [Arabi et al. (23) and Casaer et al. (29)], adequate energy support did not confer a survival benefit. This was consistent across both the 28-day mortality group (OR: 1.09, 95% CI: 0.74–1.60, Figure 5) and the 90-day mortality group (OR: 1.03, 95% CI: 0.87–1.23, Figure 6), suggesting no significant reduction in mortality at either time point.
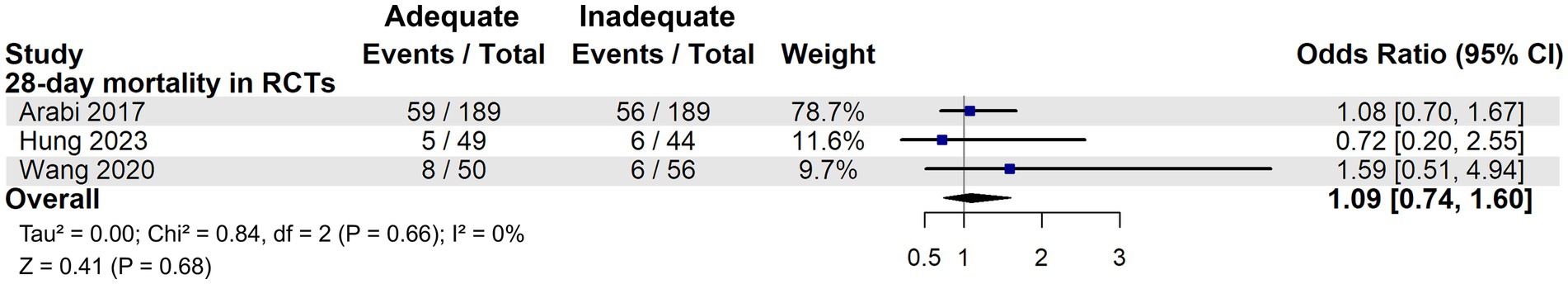
Figure 5. 28-day mortality in critically ill patients with high nutritional risk: results from RCTs on energy adequacy.
![Forest plot showing 90-day mortality in randomized controlled trials (RCTs). Two studies, Arabi 2017 and Casear 2024, report odds ratios with 95% confidence intervals: 1.19 [0.79, 1.79] and 1.00 [0.82, 1.22], respectively. The overall odds ratio is 1.03 [0.87, 1.23]. Statistical heterogeneity is low with I² = 0%.](https://www.frontiersin.org/files/Articles/1667389/fnut-12-1667389-HTML/image_m/fnut-12-1667389-g006.jpg)
Figure 6. 90-day mortality in critically ill patients with high nutritional risk: results from RCTs on energy adequacy.
TSA was performed separately for the 28-day and 90-day mortality subgroups, and no evidence was found to support a survival benefit from adequate energy intake. Notably, in the 90-day mortality group, the Z-curve crossed both the futility area and the required information size (RIS) line, suggesting that further increases in sample size are unlikely to demonstrate 90-day mortality benefit from adequate energy support in patients at high nutritional risk (Figure 7).
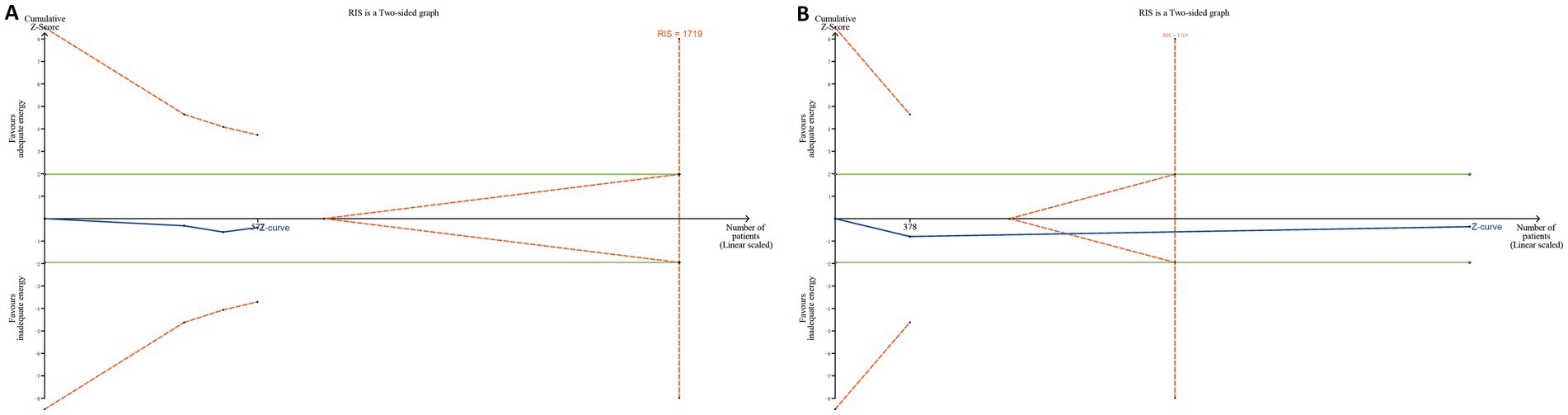
Figure 7. Trial sequential analyses in RCTs. (A) For the 28-day mortality group, the cumulative Z-curve did not cross either the conventional significance boundary (green dashed line) or the trial sequential monitoring boundary for benefit (red dashed line), nor did it reach the required information size (RIS) line. (B) For the 90-day mortality group, the Z-curve entered the futility area and crossed the RIS line, but it did not cross the conventional significance boundary.
Although two studies (26, 28) suggested a potential mortality benefit of adequate energy support in low nutritional risk patients, our meta-analysis found no statistically significant difference compared to inadequate support in 28-day mortality (OR: 0.82, 95% CI: 0.43–1.56, p = 0.54, Figure 8). In the RCTs, adequate energy intake did not reduce 28-day mortality (OR: 1.05, 95% CI: 0.66–1.67, p = 0.84, Supplementary Figure S4) or 90-day mortality (OR: 1.08, 95% CI: 0.75–1.54, p = 0.68, Supplementary Figure S5).
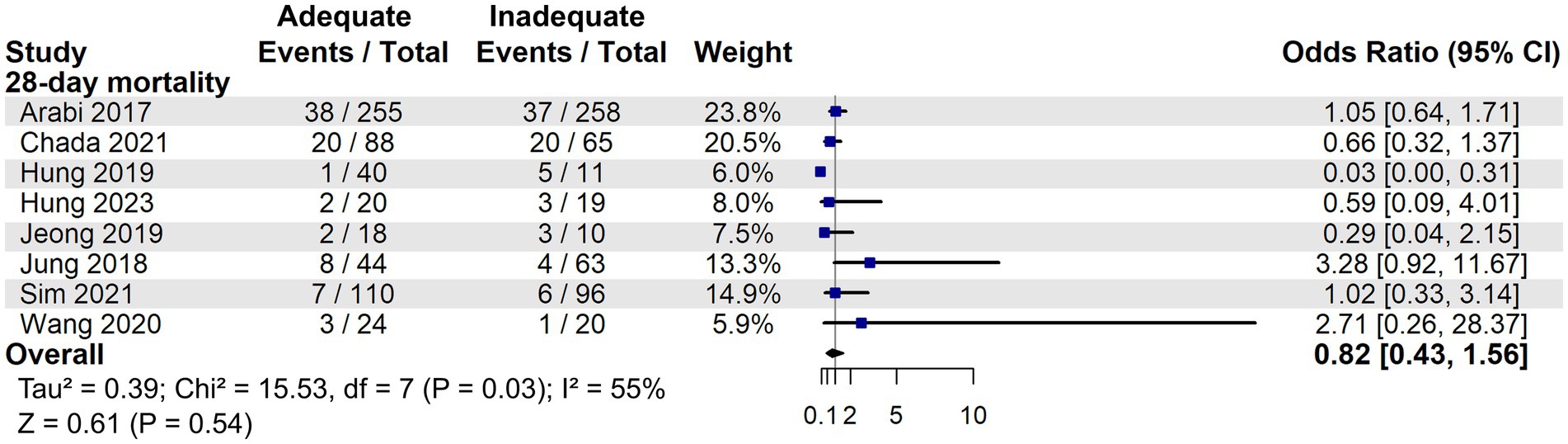
Figure 8. 28-day mortality associated with energy adequacy in critically ill patients with low nutritional risk.
3.5 Mortality associated with protein adequacy in critically ill patients
Among the 11 included studies, 4 investigated whether adequate protein intake is associated with a reduction in mortality rates among critically ill patients (22, 25, 27, 28). One suggested that adequate protein intake may reduce mortality in critically ill patients (28). However, meta-analysis demonstrated that adequate protein intake (≥1 g/kg/day) compared with relatively inadequate intake (<1 g/kg/day) did not significantly reduce mortality, regardless of whether the nutritional risk of critically ill patients is high (OR: 0.70, 95% CI: 0.38–1.30, p = 0.26, Supplementary Figure S6) or low (OR: 0.62, 95%CI: 0.09–4.13, p = 0.63, Supplementary Figure S7).
4 Discussion
This meta-analysis examined whether the mNUTRIC score is associated with mortality and whether it can serve as a tool for guiding nutritional support in critically ill patients. In patients with high nutritional risk, adequate energy support might contribute to reduced mortality. However, this association was not observed in the subgroup analysis limited to RCTs. Moreover, TSA analyses based on RCTs for both 28-day and 90-day mortality showed no trend toward a survival benefit from adequate energy support in high nutritional risk patients. In the 90-day analysis, the Z-curve crossed both futility boundary and RIS, suggesting that further RCTs are unlikely to demonstrate a significant survival benefit.
One possible explanation is that, in observational studies, patients with more severe illness may have inherently lower energy intake due to clinical limitations. As a result, higher mortality in the inadequate intake group may reflect underlying disease severity rather than the effect of insufficient nutrition itself. In contrast, the RCTs, through randomized allocation of nutrition strategies, minimized confounding and provided more reliable evidence. Therefore, the findings from the RCTs are considered more robust. Overall, while the mNUTRIC score remains a useful tool for risk stratification, its utility in guiding individualized energy provision strategies appears limited.
The results of our study were in agreement with those reported in large-scale RCTs, which have shown that early full nutritional support does not improve outcomes in critically ill patients (12, 33–37). For example, the EPaNIC trial found that harm was more related to the dose of nutrition rather than the route of administration. Similarly, the CALORIES and NUTRIREA-2 trials reported no mortality difference between enteral and parenteral nutrition. The NUTRIREA-3 trial, which allowed both routes, further showed worse outcomes with early high-dose feeding compared with permissive underfeeding.
Although the mNUTRIC score is widely recognized as a reliable tool for assessing risk of mortality (19–21), it does not appear to be a reliable indicator for guiding nutritional support strategies. Some studies, including the PermiT trial, suggest that it may not effectively identify patients at risk of malnutrition or those who are likely to benefit from nutritional support (23). Moreover, EFFORT trial similarly reached a comparable conclusion about mNUTRIC score (7). Therefore, nutritional support strategies may require greater emphasis on indicators that reflect the severity of illness rather than relying only on composite measures such as the mNUTRIC score based on our research.
Moreover, this study found a potential interaction between BMI and adequate energy intake: patients with BMI < 27 kg/m2 benefited from sufficient energy support, while those with BMI ≥ 27 kg/m2 showed no significant 28-day survival advantage. This finding is consistent with a previous study, which reported that increased calorie intake was associated with lower mortality in patients with BMI < 25 kg/m2 and ≥35 kg/m2, but conferred no benefit to those with BMI 25 kg/m2 to <35 kg/m2 (5). However, it should be noted that BMI has several limitations as a nutritional screening tool and requires careful consideration during application (38). Given the small number of available studies, this subgroup finding should be considered hypothesis-generating and requires prospective validation in future research.
Our study has several strengths and limitations. A notable strength is that we applied meta-regression techniques to explore potential sources of heterogeneity among the included studies, providing deeper insight into variability in treatment effects. Another strength of our study is the stratification of patients according to the mNUTRIC score, which allowed us to investigate the potential heterogeneity in the effect of early adequate nutritional support across different levels of nutritional risk in critically ill patients. Nonetheless, limitations include the following: (1) the limited number of high-quality studies, which may have impacted the stability of our conclusions; (2) the lack of consideration for the potential need to restrict energy intake within the first 72 h of the acute phase, as most included studies focused on feeding strategies over the first week; (3) the analysis of protein adequacy may be limited in statistical power, and therefore, the conclusions should be interpreted with caution; and (4) the conclusions may not reflect a limitation of the mNUTRIC score itself, but rather the discrepancy between identifying nutritional risk and ensuring the actual delivery of adequate nutritional support in clinical practice. Previous evidence suggests that mNUTRIC-based screening alone may not confer clinical benefit unless coupled with comprehensive multidisciplinary nutritional assessment and proactive interventions. Therefore, further high-quality studies are warranted to determine how best to integrate nutritional risk stratification with timely and adequate nutritional therapy to improve outcomes, so additional high quality studies are warranted to elucidate the appropriate timing, amount, and duration of early nutritional support in critically ill patients.
5 Conclusion
Our findings indicate that the mNUTRIC score possesses prognostic value for predicting mortality in critically ill patients; however, its role in guiding energy provision strategies remains limited.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
LB: Conceptualization, Writing – original draft. YW: Conceptualization, Writing – review & editing. YL: Methodology, Writing – original draft. DZ: Writing – original draft. HL: Conceptualization, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Natural Science Foundation of Jilin Province, China (Grant No. YDZJ202201ZYTS015).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2025.1667389/full#supplementary-material
References
1. Singer, P, Blaser, AR, Berger, MM, Calder, PC, Casaer, M, Hiesmayr, M, et al. ESPEN practical and partially revised guideline: clinical nutrition in the intensive care unit. Clin Nutr. (2023) 42:1671–89. doi: 10.1016/j.clnu.2023.07.011
2. Lew, CCH, Yandell, R, Fraser, RJ, Chua, AP, Chong, MFF, and Miller, M. Association between malnutrition and clinical outcomes in the intensive care unit: a systematic review. JPEN J Parenter Enteral Nutr. (2017) 41:744–58. doi: 10.1177/0148607115625638
3. van Zanten, ARH, De Waele, E, and Wischmeyer, PE. Nutrition therapy and critical illness: practical guidance for the ICU, post-ICU, and long-term convalescence phases. Crit Care. (2019) 23:1–10. doi: 10.1186/s13054-019-2657-5
4. Schuetz, P, Fehr, R, Baechli, V, Geiser, M, Deiss, M, Gomes, F, et al. Individualised nutritional support in medical inpatients at nutritional risk: a randomised clinical trial. Lancet. (2019) 393:2312–21. doi: 10.1016/S0140-6736(18)32776-4
5. Alberda, C, Gramlich, L, Jones, N, Jeejeebhoy, K, Day, AG, Dhaliwal, R, et al. The relationship between nutritional intake and clinical outcomes in critically ill patients: results of an international multicenter observational study. Intensive Care Med. (2009) 35:1728–37. doi: 10.1007/s00134-009-1567-4
6. Wang, C-Y, Fu, P-K, Huang, C-T, Chen, C-H, Lee, B-J, and Huang, Y-C. Targeted energy intake is the important determinant of clinical outcomes in medical critically ill patients with high nutrition risk. Nutrients. (2018) 10:1731. doi: 10.3390/nu10111731
7. Heyland, DK, Patel, J, Bear, D, Sacks, G, Nixdorf, H, Dolan, J, et al. The effect of higher protein dosing in critically ill patients: a multicenter registry-based randomized trial: the EFFORT trial. J Parenter Enter Nutr. (2019) 43:326–34. doi: 10.1002/jpen.1449
8. Villet, S, Chiolero, RL, Bollmann, MD, Revelly, JP, Cayeux, RNM, Delarue, J, et al. Negative impact of hypocaloric feeding and energy balance on clinical outcome in ICU patients. Clin Nutr. (2005) 24:502–9. doi: 10.1016/j.clnu.2005.03.006
9. Bally, MR, Yildirim, PZB, Bounoure, L, Gloy, VL, Mueller, B, Briel, M, et al. Nutritional support and outcomes in malnourished medical inpatients: a systematic review and meta-analysis. JAMA Intern Med. (2016) 176:43–53. doi: 10.1001/jamainternmed.2015.6587
10. de Man, AM, Gunst, J, and Reintam Blaser, A. Nutrition in the intensive care unit: from the acute phase to beyond. Intensive Care Med. (2024) 50:1035–48. doi: 10.1007/s00134-024-07458-9
11. Pardo, E, Lescot, T, Preiser, JC, Massanet, P, Pons, A, Jaber, S, et al. Association between early nutrition support and 28-day mortality in critically ill patients: the FRANS prospective nutrition cohort study. Crit Care. (2023) 27:7. doi: 10.1186/s13054-022-04298-1
12. Rice, TW, Wheeler, AP, Thompson, BT, Steingrub, J, Hite, RD, Moss, M, et al. Initial trophic vs full enteral feeding in patients with acute lung injury: the EDEN randomized trial. JAMA. (2012) 307:795–803. doi: 10.1001/jama.2012.137
13. Deane, AM, Little, L, Bellomo, R, Chapman, MJ, Davies, AR, Ferrie, S, et al. Outcomes six months after delivering 100% or 70% of enteral calorie requirements during critical illness (TARGET). A randomized controlled trial. Am J Respir Crit Care Med. (2020) 201:814–22. doi: 10.1164/rccm.201909-1810OC
14. Heyland, DK, Dhaliwal, R, Jiang, X, and Day, AG. Identifying critically ill patients who benefit the most from nutrition therapy: the development and initial validation of a novel risk assessment tool. Crit Care. (2011) 15:1–11. doi: 10.1186/cc10546
15. Rahman, A, Hasan, RM, Agarwala, R, Martin, C, Day, AG, and Heyland, DK. Identifying critically-ill patients who will benefit most from nutritional therapy: further validation of the "modified NUTRIC" nutritional risk assessment tool. Clin Nutr. (2016) 35:158–62. doi: 10.1016/j.clnu.2015.01.015
16. Compher, C, Chittams, J, Sammarco, T, Higashibeppu, N, Higashiguchi, T, and Heyland, DK. Greater nutrient intake is associated with lower mortality in western and eastern critically ill patients with low BMI: a multicenter, multinational observational study. J Parenter Enter Nutr. (2019) 43:63–9. doi: 10.1002/jpen.1180
17. Park, S, Park, SH, Kim, Y, Lee, GH, Kim, H-s, Lim, SY, et al. Optimal nutritional support strategy based on the association between modified NUTRIC score and 28-day mortality in critically ill patients: a prospective study. Nutrients. (2023) 15:2465. doi: 10.3390/nu15112465
18. Zhang, P, He, Z, Yu, G, Peng, D, Feng, Y, Ling, J, et al. The modified NUTRIC score can be used for nutritional risk assessment as well as prognosis prediction in critically ill COVID-19 patients. Clin Nutr. (2021) 40:534–41. doi: 10.1016/j.clnu.2020.05.051
19. Brascher, J, Peres, W, and Padilha, P. Use of the modified “nutrition risk in the critically ill” score and its association with the death of critically ill patients. Clin Nutr ESPEN. (2020) 35:162–6. doi: 10.1016/j.clnesp.2019.10.005
20. Canales, C, Elsayes, A, Yeh, DD, Belcher, D, Nakayama, A, McCarthy, CM, et al. Nutrition risk in critically ill versus the nutritional risk screening 2002: are they comparable for assessing risk of malnutrition in critically ill patients? JPEN J Parenter Enteral Nutr. (2019) 43:81–7. doi: 10.1002/jpen.1181
21. Mahmoodpoor, A, Sanaie, S, Sarfaraz, T, Shadvar, K, Fattahi, V, Hamishekar, H, et al. Prognostic values of modified NUTRIC score to assess outcomes in critically ill patients admitted to the intensive care units: prospective observational study. BMC Anesthesiol. (2023) 23:131. doi: 10.1186/s12871-023-02086-0
22. Lee, Z-Y, Airini, IN, and Barakatun-Nisak, M-Y. Relationship of energy and protein adequacy with 60-day mortality in mechanically ventilated critically ill patients: a prospective observational study. Clin Nutr. (2018) 37:1264–70. doi: 10.1016/j.clnu.2017.05.013
23. Arabi, YM, Aldawood, AS, Al-Dorzi, HM, Tamim, HM, Haddad, SH, Jones, G, et al. Permissive underfeeding or standard enteral feeding in high- and low-nutritional-risk critically ill adults. Post hoc analysis of the PermiT trial. Am J Respir Crit Care Med. (2017) 195:652–62. doi: 10.1164/rccm.201605-1012OC
24. Chada, RR, Chidrawar, S, Goud, BA, Maska, A, Medanki, R, and Nagalla, B. Association between nutrition delivery, modified nutrition risk in critically iII score, and 28-day mortality. Nutr Clin Pract. (2021) 36:1020–33. doi: 10.1002/ncp.10673
25. Hung, K-Y, Chen, T-H, Lee, Y-F, and Fang, W-F. Using body composition analysis for improved nutritional intervention in septic patients: a prospective interventional study. Nutrients. (2023) 15:3814. doi: 10.3390/nu15173814
26. Hung, K-Y, Chen, Y-M, Wang, C-C, Wang, Y-H, Lin, C-Y, Chang, Y-T, et al. Insufficient nutrition and mortality risk in septic patients admitted to ICU with a focus on immune dysfunction. Nutrients. (2019) 11:367. doi: 10.3390/nu11020367
27. Jeong, DH, Hong, S-B, Lim, C-M, Koh, Y, Seo, J, Kim, Y, et al. Relationship between nutrition intake and 28-day mortality using modified NUTRIC score in patients with sepsis. Nutrients. (2019) 11:1906. doi: 10.3390/nu11081906
28. Im, KM, and Kim, EY. Reducing in-hospital and 60-Day mortality in critically ill patients after surgery with strict nutritional supplementation: a prospective, single-Labeled, randomized controlled trial. Nutrients. (2023) 15:4684. doi: 10.3390/nu15214684
29. Casaer, MP, Stragier, H, Hermans, G, Hendrickx, A, Wouters, PJ, Dubois, J, et al. Impact of withholding early parenteral nutrition on 2-year mortality and functional outcome in critically ill adults. Intensive Care Med. (2024) 50:1593–602. doi: 10.1007/s00134-024-07546-w
30. Sim, J, Hong, J, Na, EM, Doo, S, and Jung, YT. Early supplemental parenteral nutrition is associated with reduced mortality in critically ill surgical patients with high nutritional risk. Clin Nutr. (2021) 40:5678–83. doi: 10.1016/j.clnu.2021.10.008
31. Wang, C-Y, Fu, P-K, Chao, W-C, Wang, W-N, Chen, C-H, and Huang, Y-C. Full versus trophic feeds in critically ill adults with high and low nutritional risk scores: a randomized controlled trial. Nutrients. (2020) 12:3518. doi: 10.3390/nu12113518
32. Jung, YT, Park, JY, Jeon, J, Kim, MJ, Lee, SH, and Lee, JG. Association of inadequate caloric supplementation with 30-day mortality in critically ill postoperative patients with high modified NUTRIC score. Nutrients. (2018) 10:1589. doi: 10.3390/nu10111589
33. Casaer, MP, Mesotten, D, Hermans, G, Wouters, PJ, Schetz, M, Meyfroidt, G, et al. Early versus late parenteral nutrition in critically ill adults. N Engl J Med. (2011) 365:506–17. doi: 10.1056/NEJMoa1102662
34. Harvey, SE, Segaran, E, and Leonard, R. Trial of the route of early nutritional support in critically ill adults. N Engl J Med. (2015) 372:488–9. doi: 10.1056/NEJMc1414479
35. Reignier, J, Boisramé-Helms, J, Brisard, L, Lascarrou, JB, Ait Hssain, A, Anguel, N, et al. Enteral versus parenteral early nutrition in ventilated adults with shock: a randomised, controlled, multicentre, open-label, parallel-group study (NUTRIREA-2). Lancet. (2018) 391:133–43. doi: 10.1016/S0140-6736(17)32146-3
36. Chapman, M, Peake, SL, Bellomo, R, Davies, A, Deane, A, Horowitz, M, et al. Energy-dense versus routine enteral nutrition in the critically ill. N Engl J Med. (2018) 379:1823–34. doi: 10.1056/NEJMoa1811687
37. Reignier, J, Plantefeve, G, Mira, JP, Argaud, L, Asfar, P, Aissaoui, N, et al. Low versus standard calorie and protein feeding in ventilated adults with shock: a randomised, controlled, multicentre, open-label, parallel-group trial (NUTRIREA-3). Lancet Respir Med. (2023) 11:602–12. doi: 10.1016/S2213-2600(23)00092-9
Keywords: nutritional support, critically ill patients, mNUTRIC score, energy intake, protein intake
Citation: Bao L, Wang Y, Li Y, Zhang D and Li H (2025) Impact of nutritional support on mortality among critically ill patients with different nutritional risks: a systematic review with meta-analysis. Front. Nutr. 12:1667389. doi: 10.3389/fnut.2025.1667389
Edited by:
Alin Horatiu Nedelcu, Grigore T. Popa University of Medicine and Pharmacy, RomaniaReviewed by:
Qin Zhang, Shanghai Jiao Tong University, ChinaKyoung Moo Im, The Catholic University, Republic of Korea
Copyright © 2025 Bao, Wang, Li, Zhang and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hongxiang Li, bGlfaHhAamx1LmVkdS5jbg==
 Lingling Bao
Lingling Bao Youquan Wang
Youquan Wang Hongxiang Li
Hongxiang Li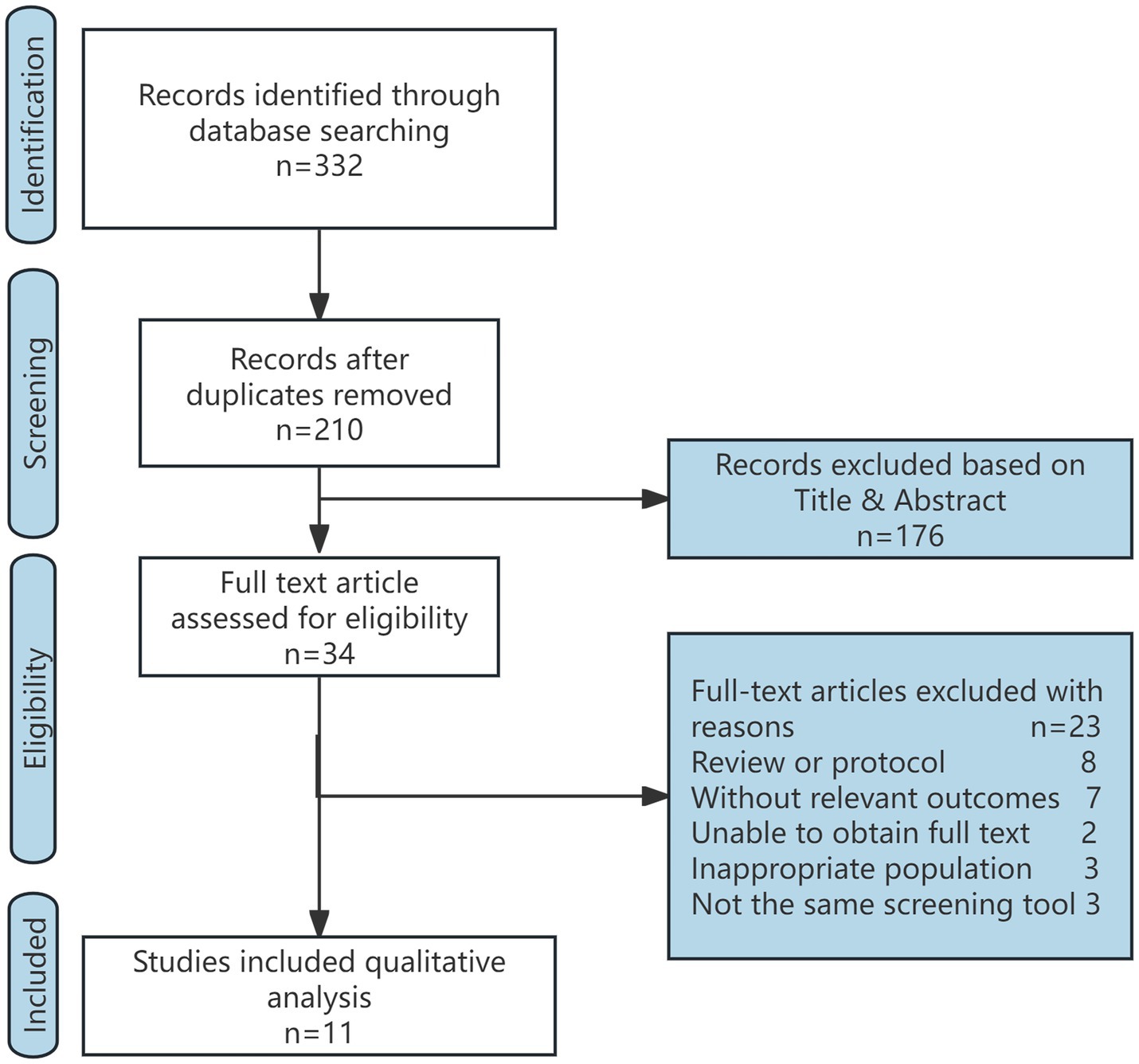
![Forest plot showing meta-analysis of different studies on 28-day mortality in high-risk and low-risk groups. It lists study names, events, totals, weights, and odds ratios with 95% confidence intervals. Overall odds ratio is 2.26 [1.80, 2.83]. Heterogeneity is quantified with Tau-squared, Chi-squared, degrees of freedom, and I-squared. Statistical significance is indicated (P < 0.00001).](https://www.frontiersin.org/files/Articles/1667389/fnut-12-1667389-HTML/image_m/fnut-12-1667389-g002.jpg)