- 1Department of Radiology, The Yancheng School of Clinical Medicine of Nanjing Medical University, Yancheng Third People's Hospital, Yancheng, China
- 2Department of Radiology, Binhai Maternal and Child Health Hospital, Yancheng, China
- 3Department of Neurology, The Yancheng School of Clinical Medicine of Nanjing Medical University, Yancheng Third People's Hospital, Yancheng, China
Background: Studies using voxel-based morphometry (VBM) have shown considerable variability in gray matter (GM) changes in anorexia nervosa (AN). However, it remains unclear whether these changes converge on common brain networks underlying the disorder.
Methods: A systematic review was conducted using the PubMed, Embase, and Web of Science databases to identify studies on whole-brain GM alterations in AN published up to October 10, 2024. The Human Connectome Project (HCP) dataset (n = 1,093) and functional connectivity network mapping (FCNM) approach to identify common brain networks associated with alterations in AN.
Results: A total of 26 studies involving 667 individuals with AN and 659 healthy controls (HC) were included in this study. Combining the HCP dataset and the FCNM technique, we demonstrated that the disrupted neural networks primarily involved the auditory network, ventral default mode network (DMN), dorsal DMN, and sensorimotor network (SMN). Subgroup analyses further revealed differences in the affected neural networks across specific subgroups, including females-only, adolescents, and adults.
Conclusion: The heterogeneous GM alterations in AN can be attributed to common abnormalities within the auditory network, DMN, and SMN. These disruptions are linked to distorted body image, impaired emotional regulation, and disrupted sensory-motor integration in AN. The FCNM technique provides a unified network-level understanding of the neurobiological mechanisms underlying AN, offering insights for targeted therapeutic strategies.
Introduction
Anorexia nervosa (AN) is a severe mental illness characterized by aberrant feeding behaviors, an intense desire for thinness, an inability to maintain a minimally normal weight, and obsessive concern with body image (1, 2). Recent global epidemiological reviews indicate that it primarily affects females, with a lifetime prevalence rate of up to 4% among women and approximately 0.3% among men (3). Excessive weight loss in AN can lead to widespread complications, including dysfunctions of the central nervous, cardiovascular, and gastrointestinal systems (4). The etiology of AN is complex, involving a combination of genetic, neurobiological, social-environmental, and psychological factors (1, 5). Clinically, the disorder entails severe medical complications, such as cardiovascular and endocrine dysfunction, and frequent psychiatric comorbidities including depression and anxiety, all of which contribute to its high mortality risk (6–8). This substantial public health burden highlights the need to clarify its underlying neural mechanisms. In the last 20 years, progress in magnetic resonance imaging (MRI) has opened up new opportunities for conducting thorough research on eating disorders (9). An increasing number of neuroimaging studies suggests that AN is associated with significant brain morphological and functional abnormalities (10–13). Despite these advancements, the pathophysiology of AN remains incompletely understood (10, 14, 15).
Voxel-based morphometry, a widely utilized automated technique for the analysis of gray and white matter alterations (16), is considered a key method for investigating the pathophysiological mechanisms of AN. Numerous neuroimaging studies using this technique have identified significant associations between brain GM abnormalities and AN (12, 13, 17, 18). The brain regions with reduced GM in individuals with AN primarily involve the anterior cingulate cortex (ACC), median cingulate cortex, posterior cingulate cortex, inferior frontal gyrus, frontal operculum, superior temporal gyrus, middle temporal gyrus, fusiform gyrus, inferior parietal cortex, occipital cortex, precentral gyrus, precuneus, cerebellum, striatum, and thalamus (11, 12, 18–24). While one recent study reported increased GM in specific brain regions, including the left orbitofrontal gyrus rectus, bilateral fusiform gyrus, bilateral hippocampus, right insula, and bilateral parahippocampal gyrus (25). Conversely, another study observed no significant reductions in GM (26). However, these inconsistent findings limit their utility for elucidating the neurobiological mechanisms of AN. Recent research has highlighted a strong correlation between the onset of AN and disruptions in brain networks, including basal ganglia network, sensorimotor network (SMN), Limbic network, visuospatial network, and default mode network (DMN) (21, 27–30). While coordinate-based meta-analysis (CBMA) has traditionally been used to consolidate diverse findings into common regions (31), accumulating evidence indicates that neuropsychiatric symptoms and diseases may be more precisely mapped to distinct brain networks than to isolated regional abnormalities (32–35). The significance of this network-based approach lies in the fundamental principle that focal structural lesions, such as GM alterations, are not functionally isolated; instead, they compromise the integrity and function of the entire large-scale network in which they are embedded (36, 37). Functional connectivity network mapping (FCNM) is a validated approach that integrates regions of interest with large-scale human connectome data to reveal the intricate relationships between different brain areas, facilitating a deeper understanding of how these connections influence cognitive processes and behaviors (38, 39). This technique can map heterogeneous neuroimaging findings to common neuroanatomical networks and identify disease-specific and symptom-specific brain networks (40). The FCNM method has effectively mapped various neurological and psychiatric symptoms to distinct brain networks (39, 41–46). Despite significant advancements in its application to other diseases, the FCNM approach has yet to be used to explore how focal GM alterations in AN impact brain function from a unified network-level perspective.
To address this question, we utilized the FCNM approach to identify brain networks implicated in the structural abnormalities of AN. A schematic representation of the study design and analysis pipeline is illustrated in Figure 1. We hypothesized that the heterogeneous alterations in GM across different brain regions in individuals with AN could be mapped onto common brain networks. The results hold the potential to reconcile the heterogeneous whole-brain GM changes reported in previous VBM studies through a network perspective.
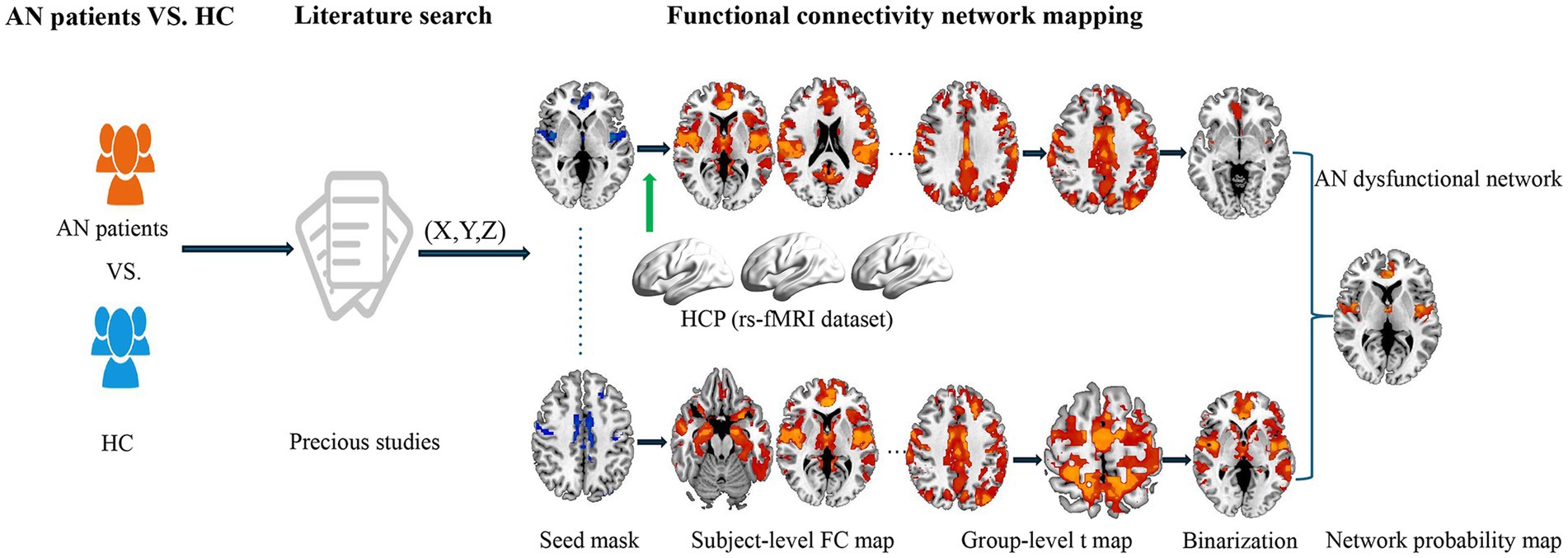
Figure 1. Study design and analysis pipeline. Our primary method entailed integrating published literature to determine the brain regions demonstrating alterations in GM between individuals with AN and HC. By integrating these impacted brain locations with large-scale human connectome data from HCP, we subsequently employed the FCNM approach to identify abnormal brain networks associated with AN. Specifically, spheres centered at each coordinate of a contrast were initially constructed and amalgamated to produce a contrast-specific integrated seed mask. Second, in accordance with the HCP dataset, we calculated a contrast seed-to-whole brain resting-state FC map for each participant. Third, the subject-level resting-state FC maps were subjected to a voxelwise one-sample t-test to determine brain regions functionally associated with each contrast seed. Fourth, the resulting group-level t maps were thresholded and binarized at p < 0.05 corrected for multiple comparisons using a voxel-level false discovery rate method. Finally, the binarized maps of GM contrasts were overlaid to produce a network probability map, which were thresholded at 50% to yield AN GM dysfunctional network. AN, anorexia nervosa; HC, health controls; GM, gray matter; HCP, Human Connectome Project; FCNM, functional connectivity network mapping; FC, functional connectivity; rs-fMRI, resting-state functional magnetic resonance imaging.
Methods
Literature search and selection
Following the PRISMA guidelines (47), we conducted a comprehensive and systematic search of the PubMed, Embase, and Web of Science databases to identify studies on GM changes in AN, published up to October 10, 2024. The search included keywords such as “voxel-based morphometry” OR “VBM” OR “gray matter” OR “gray matter” AND “anorexia nervosa” OR “eating disorders.” Additionally, a manual search of reference lists from relevant reviews and meta-analyses was performed to identify any potentially overlooked studies. The flow diagram in Supplementary Figure S1 details the study selection process.
All studies were included according to the following criteria: (1) published as an original article in a peer-reviewed English-language journal; (2) studies explored GM changes in individuals with AN compared to healthy controls; (3) voxel-wise analyses were conducted at the whole-brain level; (4) studies reported results in Talairach or Montreal Neurological Institute (MNI) space. For longitudinal studies, only baseline data were included in this study.
Exclusion criteria were as follows: (1) lacking a reported coordinate system; (2) only use of region of interest analysis; (3) all coordinates reported were located outside the gray matter mask; (4) absence of relevant comparisons between patients with AN and healthy controls; (5) studies involving animal experiments; (6) reviews or meta-analyses. Only the study with the largest sample size and most comprehensive information was included in the analysis to prevent data duplication from overlapping patient samples in multiple publications. The corresponding author of each study was contacted via email for any necessary additional information. Two investigators, Y. H. C. and W. S., conducted literature searches, selected relevant articles, and independently extracted data. Any discrepancies were discussed with another investigator (P. P. L) until they were resolved. Furthermore, we systematically evaluated the methodological quality of the neuroimaging protocols in all studies incorporated in the current analysis based on a 10-point checklist (48, 49) (Supplementary Table S1).
Functional MRI data acquisition and preprocessing
Data utilized in this study were extracted from the Human Connectome Project (HCP) 1,200 Subjects Release (S1200), encompassing imaging data of healthy adults aged 22–37 years. A total of 1,093 participants (594 female; mean age = 28.78 ± 3.69 years, SD) constituted the final study sample. Exclusion criteria utilized during HCP data collection encompassed MRI contraindications, existing psychiatric/neurological disorders, recent psychiatric medication intake, pregnancy, and a prior history of head trauma. Demographics of this cohort are detailed in Supplementary Table S2. All coordinates extracted from the previous studies were uniformly converted to the MNI standard space. If an original study reported coordinates in Talairach space, established conversion tools1 were used to transform them into MNI space.
High-resolution imaging data for the HCP dataset were acquired on a 3 T Siemens Trio MRI scanner, enabling detailed analyses (specific fMRI parameters are provided in Supplementary Table S3). Participants were excluded on account of poor scan quality, such as significant artifacts or incomplete brain coverage.
Subsequently, the resting-state fMRI data were preprocessed using SPM122 and DPABI3. Initial processing involved discarding the first 10 volumes from each participant’s scan to ensure signal stability and participant adaptation to the scanner environment. Subsequently, the remaining volumes underwent slice-timing correction to account for acquisition time differences between slices. Then, realignment was conducted to correct motion across time points, with head motion parameters calculated to determine translation and angular rotation for each volume. All participants met the predefined motion criteria (maximum translation < 2 mm, maximum rotation < 2°). We also computed framewise displacement to quantify inter-volume head motion. Subsequently, to mitigate the effects of physiological noise and motion artifacts, the following nuisance covariates were regressed from each participant’s preprocessed blood oxygenation level dependent time series: linear drift, the 24 motion parameters derived from the Friston model, spike volumes (FD > 0.5 mm), and the mean signals from global tissue, white matter, and cerebrospinal fluid. Global signal regression was included in the resting-state fMRI preprocessing pipeline, as this step has been shown to enhance system-specific correlations and improve functional connectivity estimation. Subsequently, the preprocessed functional data were bandpass filtered (0.01–0.1 Hz). Normalization involved first co-registering individual structural images to their corresponding mean functional image. These co-registered structural images then underwent segmentation and normalization to MNI space. Next, every filtered functional volume underwent spatial normalization to MNI space. The volumes were then resampled to 3-mm isotropic voxels. A Gaussian kernel with a full width at half maximum of 6 × 6 × 6 mm3 was applied for spatial smoothing of all data.
Functional connectivity network mapping (FCNM)
By utilizing the FCNM technique, we aimed to determine whether the diverse GM changes observed in AN are linked to a specific set of large-scale functional brain networks (35, 37, 40, 50–52). To create a contrast seed, 4-mm radius spheres were individually centered at each coordinate of a contrast and then amalgamated. We then performed seed-based functional connectivity (FC) analysis for each participant using the preprocessed HCP resting-state data. The calculation of Pearson correlation coefficients involved comparing the time series of the contrast seed with that of all other brain voxels, and subsequently Fisher-Z transformed to approximate a normal distribution, yielding individual FC maps. Third, the FC maps of 1,093 subjects were examined through a voxel-wise one-sample t-test to identify brain regions associated with each seed. Our analysis concentrated exclusively on positive FC, given the ongoing debate surrounding the interpretation of negative connectivity. Following thresholding, the group-level t maps were binarized at p < 0.05, with correction for multiple comparisons using a voxel-level false discovery rate method. Finally, binarized connectivity maps derived from each GM contrast were merged to form a network probability map, which was subsequently thresholded at 50% based on previous well-validated FCNM studies (38, 41) to outline the AN GM dysfunctional network.
Association with canonical brain networks
To enhance interpretability, we examined the spatial relationships between the dysfunctional AN brain networks and 14 established canonical brain networks. These networks include the auditory network (including bilateral superior temporal gyrus and right thalamus), basal ganglia network (including bilateral inferior frontal gyrus, bilateral caudate nucleus and bilateral putamen), language network (including left inferior frontal gyrus/Broca’s area, left middle temporal gyrus, and left supramarginal gyrus), SMN (including bilateral precentral gyrus, bilateral postcentral gyrus and bilateral supplementary motor area), primary visual network (including bilateral cuneus, bilateral lingual gyrus and pericalcarine cortex), dorsal DMN (including bilateral inferior parietal lobule, dorsomedial prefrontal cortex and bilateral posterior cingulate cortex), ventral DMN (including medial temporal lobe, hippocampus/parahippocampal gyrus and ventromedial prefrontal cortex), left executive control network (LECN)[including left dorsolateral prefrontal cortex, left superior parietal lobule and left supramarginal gyrus], right executive control network (RECN) [including right dorsolateral prefrontal cortex, right superior parietal lobule and right supramarginal gyrus], high visual network (including lateral occipital cortex, tempo-occipital junction and fusiform gyrus), visuospatial network (including superior parietal lobule, inferior parietal lobule and superior frontal sulcus), anterior salience network (including bilateral insula, bilateral ACC and bilateral middle frontal gyrus), posterior salience network (including bilateral posterior insula, bilateral posterior cingulate cortex and bilateral supramarginal gyrus), and the precuneus network [including precuneus, part of the parietal cortex and part of the posterior cingulate cortex (53)]. We quantified the spatial relationships by calculating the ratio of overlapping voxels between each dysfunctional AN network and its corresponding canonical network, relative to the total number of voxels within the canonical networks.
Subgroup analyses
We conducted subgroup analyses on the included samples, dividing participants into female-only, adolescent, and adult subgroups to explore potential differences in brain network abnormalities across these groups.
Results
Included studies and sample characteristics
A total of 842 candidate articles were initially identified and subjected to a thorough screening process. Ultimately, 26 studies including data from 667 individuals with AN and 659 healthy controls were included in the analysis (17, 24, 54–66). Subsequently, we conducted a planned subgroup analysis (female-only group, adolescent group, adult group). The female-only group comprised 598 women with AN and 593 healthy controls. The adolescent group included data from 215 individuals with AN and 189 healthy controls. The adult group consisted of 452 individuals with AN and 470 healthy controls. The sample and imaging characteristics of the studies included are outlined in Table 1.
Convergent aberrant FC associated with GM alterations in AN
In this study, by combining the FCNM approach and large-scale human brain connectome data obtained from the HCP, we identified that the convergent aberrant FC associated with GM alterations in AN involved widely distributed brain regions primarily including the bilateral superior temporal gyrus, right Rolandic operculum, right middle temporal gyrus, bilateral precuneus, bilateral ACC, and bilateral precentral gyrus (Figure 2 and Table 2). FCNM calculations were further conducted using spheres with 1-mm (Supplementary Figure S2) and 7-mm radii (Supplementary Figure S3), respectively, revealed similar brain regions to those obtained with a 4-mm radius sphere. Regarding canonical brain networks, the AN-associated network showed the highest overlap with the following networks: the auditory network includes brain regions such as the bilateral superior temporal gyrus and right Rolandic operculum, with an overlap proportion of 70.5%; the ventral DMN primarily includes the right middle temporal gyrus and bilateral precuneus, showing an overlap proportion of 19.2%. The dorsal DMN mainly involves the bilateral ACC, with an overlap proportion of 13.1%; and the SMN being primarily associated with the bilateral precentral gyrus, with an overlap proportion of 20.6% (Figure 3). The overlap proportions with other canonical brain networks were all below 10%. Then, replicating the FCNM procedure involved spheres with radii of 1-mm (Supplementary Figure S4) and 7-mm (Supplementary Figure S5), the resulting patterns of network overlap closely resembled those generated using the 4-mm radius sphere.
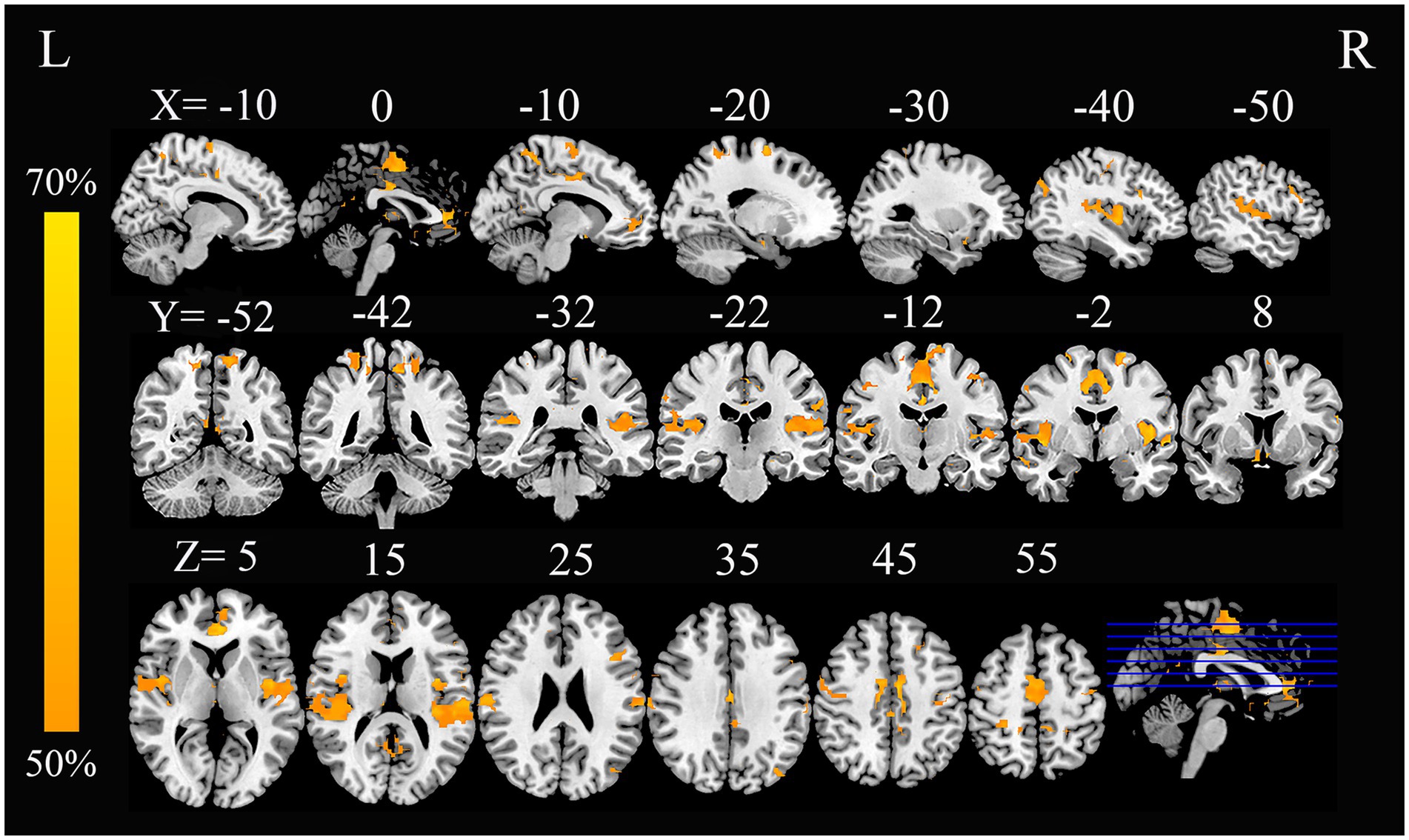
Figure 2. FC overlap maps based on 4-mm radius sphere in AN. Dysfunctional brain networks are shown as FC probability maps thresholded at 50%, showing brain regions functionally connected to more than 50% of the contrast seeds. AN, anorexia nervosa; FC, functional connectivity; L, left; R, right.
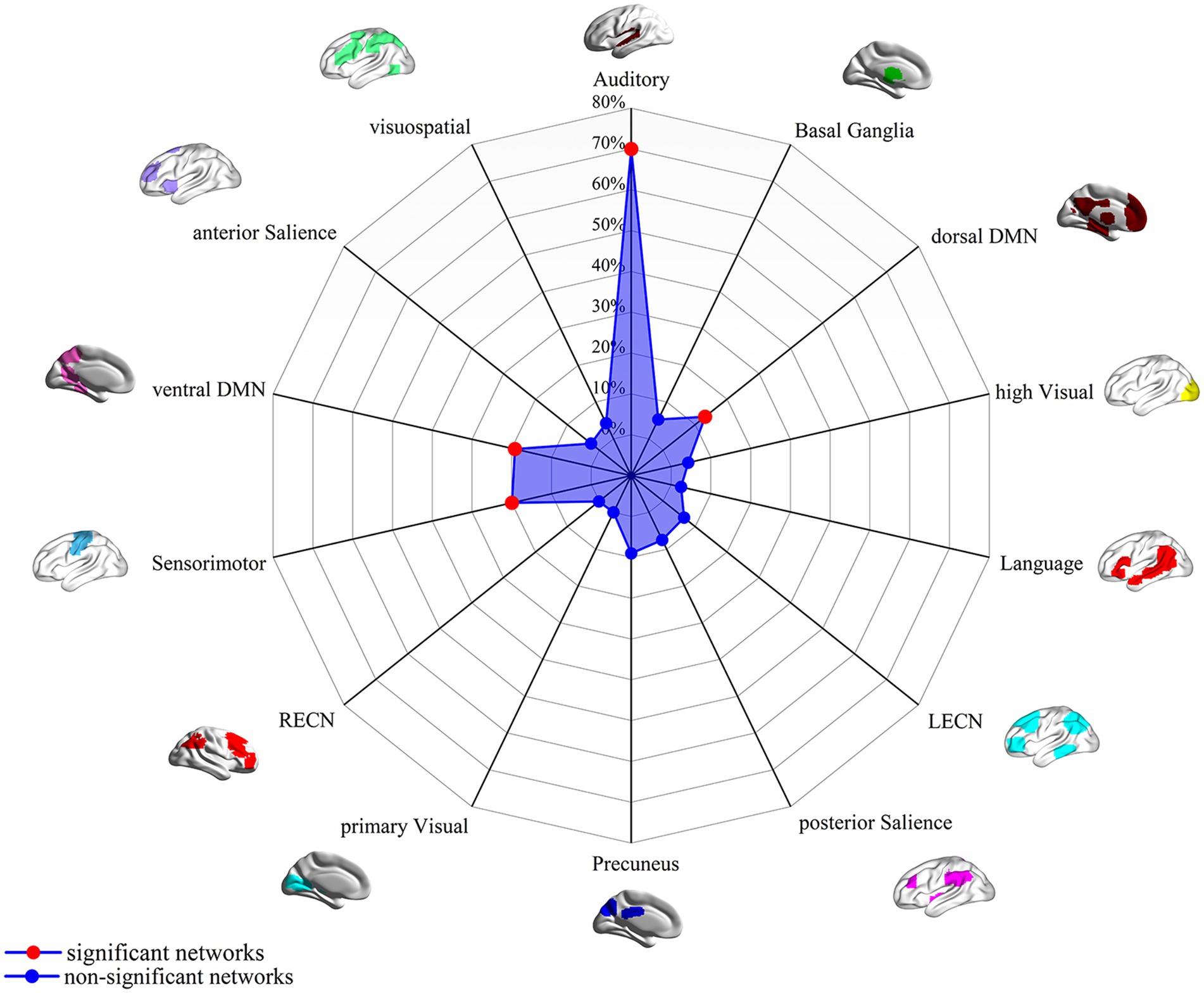
Figure 3. FC overlap maps based on 4-mm radius sphere in association with canonical brain networks. Polar plots illustrate the proportion of overlapping voxels between each AN dysfunctional network and a canonical network to all voxels within the corresponding canonical network. Note: The red dot represents brain dysfunction networks, defined as significant networks, exhibiting ≥ 10% overlap with canonical networks, whereas the blue dot represents non-significant networks with <10% overlap. DMN, default mode network; FC, functional connectivity; LECN, left executive control network; RECN, right executive control network.
Subgroup analyses
In the female-only group, the primary abnormal networks identified were the auditory network (22%), dorsal DMN (28%), and ventral DMN (12%) (Supplementary Figure S6). The adolescent group showed a broader distribution of abnormal networks, with predominant involvement of the auditory network (77%), LECN (79%), ventral DMN (92%), SMN (93%), precuneus network (90%), primary visual network (75%), and visuospatial network (49%) (Supplementary Figure S7). In the adult group, predominant abnormalities were found in the auditory network (92%), posterior salience network (60%), SMN (35%), and basal ganglia network (32%) (Supplementary Figure S8).
Discussion
To the best of our knowledge, this study is the first to integrate the FCNM approach with large-scale resting-state fMRI data from the HCP, ultimately mapping GM changes in AN onto specific brain functional networks. Using the FCNM approach, we analyzed data from 26 VBM studies examining whole-brain GM alterations including a total of 667 individuals with AN and 659 healthy controls. The FCNM approach identified specific brain functional networks associated with GM alterations in AN, including the auditory network (e.g., bilateral superior temporal gyrus and right Rolandic operculum), the ventral and dorsal DMN (e.g., bilateral precuneus, bilateral ACC, and right middle temporal gyrus), and the sensorimotor network (SMN) (e.g., bilateral precentral gyrus). Network overlap was calculated using a 4-mm radius sphere, with validations using 1-mm and 7-mm sphere radii yielding robust and replicable results. Subgroup analyses showed that gender appeared to modulate the involvement of the SMN. Significant differences were also observed in the ECN, visual network, and salience network between adolescents and adults with AN. Notably, our findings offer a compelling bridge between neurobiology and the broader biopsychosocial framework of AN, suggesting the structural network vulnerabilities we identified serve as a crucial neural substrate upon which psychological traits and sociocultural pressures act to entrench the disorder.
Auditory network alterations in AN
Our study found that GM alterations associated with AN spatially map onto the auditory network. The auditory network, as defined by the 14 canonical brain networks, encompasses the primary and secondary auditory cortices, superior temporal gyri, Rolandic operculum, and prefrontal cortex, is vital for processing environmental stimuli (53, 67). Auditory cortex dysfunction, characterized by altered FC, is implicated in body image distortion and interoceptive deficits in AN (68, 69). This dysfunction may reflect impaired integration of visual, auditory, and tactile stimuli, crucial for accurate body perception (70, 71). Reduced activity in the primary auditory cortex and insula is linked to abnormal bodily state perception (68). Furthermore, reduced left superior temporal gyrus volume in adolescents with AN correlates with weight and shape concerns (72, 73). Experiments manipulating footstep frequencies (high and low) further indicate that auditory signals, processed by the auditory cortex, may influence perceptions of body weight (69). Overall, the auditory cortex is crucial for multimodal sensory integration, and its functional abnormalities may distort the processing of auditory signals in AN patients, further exacerbating distortions in body image and weight perception (74, 75).
The superior temporal gyrus, including Heschl’s gyrus (the core of the primary auditory cortex), plays a key role in spectro-temporal analysis, phonological processing, and integrating cues for speech comprehension (76). It also contributes to processing social and emotional cues, which are often impaired in AN (77, 78). Studies show altered superior temporal gyrus activation in AN during exposure to food and body-image stimuli, with both hyper-activation and hypo-activation reported (79, 80). Decreased superior temporal gyrus activation when viewing food images may reflect diminished emotional engagement or reward processing (81, 82). The Rolandic operculum plays a key role in motor control related to oral and pharyngeal functions, taste perception, somatosensory processing, and multimodal integration (83). Given its involvement in taste perception and oral motor functions, the Rolandic operculum likely influences eating habits through its interactions with neural networks regulated by the hypothalamus (84). In individuals with AN, reduced GMV in the left Rolandic operculum has been linked to distortions in self-perception and social cognition (24).
DMN alterations in AN
The DMN is traditionally categorized into the ventral DMN and the dorsal DMN, distinguished by anatomical and functional characteristics (53, 85). Key DMN regions include the precuneus, medial prefrontal cortex, posterior cingulate cortex, angular gyrus, hippocampus, and temporal and parietal areas (86). Our study found that the dysfunctional ventral DMN (middle temporal gyrus and precuneus) and dorsal DMN (ACC) were linked to GMV alterations in AN. The dorsal DMN plays a crucial role in assessing the emotional significance of envisioned scenarios, thereby impacting emotional processing and the development of prospective strategies (87), while the ventral DMN is mainly involved in the constructive process of imagination, assisting in combining memory fragments to create vivid and detailed mental images (85). DMN aberrations in AN, particularly affecting the precuneus, posterior cingulate cortex, and medial prefrontal cortex, are associated with distorted self-perception and intensified body image concerns (29, 88, 89). Additionally, increased DMN connectivity has been associated with heightened rumination on weight and body shape, suggesting that AN patients may experience intensified self-focused thoughts that perpetuate body image distortions (90, 91). Importantly, nutritional and psychological treatments have been shown to modulate abnormal DMN connectivity (30, 92), potentially restoring network function to support positive self-perception and body image, and reducing weight and body preoccupation in AN.
The middle temporal gyrus is essential for semantic cognition as it integrates information from the anterior temporal lobe and links it to goal-directed cognitive processes (93, 94). Studies have revealed structural and functional modifications in the middle temporal gyrus in patients with AN, which are closely related to symptoms including emotional dysregulation and body weight control (95–97). For instance, AN patients often experience a decrease in GMV in the middle temporal gyrus, which is associated with symptoms of body dissatisfaction and distorted self-image (19, 98). Functional MRI studies indicate modified activation in the middle temporal gyrus during tasks related to body, food, and cognitive processing, which could potentially worsen symptoms of AN (98, 99). Furthermore, reduced activation in the middle temporal gyrus is linked to impaired emotional recognition and theory of mind, impacting social interactions and self-perception in those with AN (100, 101).
The precuneus, part of the posterior DMN, is associated with self-referential processes and body awareness, functions that are often disrupted in AN (98). Structural changes like cortical thinning and GMV have been observed, sometimes reversing with weight restoration (102, 103). Altered precuneus resting-state FC, particularly with the ACC, correlates with body image concerns, emotional regulation difficulties, and distorted body perception (104–106). The ACC is essential for integrating emotion, motivation, and cognitive control to support goal-directed behavior, decision-making, and adaptive responses to rewards and punishments (107). The ACC plays a significant role in the pathophysiology of AN, with studies demonstrating both structural and functional alterations that relate to core symptoms of the disorder (10, 77). For instance, research has shown consistent gray matter reduction in the ACC of AN patients, even after weight recovery, suggesting long-lasting structural deficits associated with the severity of the disorder (63, 108). Resting-state studies further reveal disrupted synchrony between the ACC and regions like the precuneus, which correlates with concerns about body shape, highlighting a network-level dysfunction underlying the obsessive focus on body image (106).
SMN alterations in AN
The SMN, as defined by the 14 canonical brain networks, encompasses the precentral gyrus, postcentral gyrus, and supplementary motor area, plays a crucial role in integrating and processing sensory and motor information within the brain (109–111). We determined that the altered SMN associated with GMV changes in AN involved the precentral gyrus. Research indicates that AN patients exhibit abnormal connectivity within the SMN, especially in areas related to the integration of somatosensory and visuospatial information (29, 102). The dysfunction of the SMN in AN is linked to impaired body image perception and sensory processing, which are central to the disorder’s pathology (68, 112). The biological underpinnings of body image distortions in AN patients involve impaired sensorimotor integration and disrupted FC (21, 113).
The precentral gyrus is essential for voluntary body movement control, motor planning, and coordination (114, 115). Neuroimaging has shown altered FC involving the precentral gyrus in AN patients, often associated with behaviors regarding food intake and body image (21, 116). Moreover, studies have found decreased GMV in the precentral gyrus of AN patients, a structural alteration that might underpin difficulties in flexible motor responses and contribute to the disorder persistent behavioral patterns (11, 117). These findings highlight the precentral gyrus role in the neurobiological mechanisms underlying AN and suggest that both functional and structural disruptions in this area may reinforce the motor control and body perception challenges characteristic of the disorder (88, 118).
Subgroup analyses in AN
When considering the female-only subgroups (24 studies), patients with AN primarily exhibited significant alterations in the auditory network, dorsal DMN, and ventral DMN, with comparatively lesser involvement observed for the SMN. Research has demonstrated anatomical and functional differences in the sensorimotor cortex between males and females, especially in the precentral and postcentral gyri (110). In females, these regions are generally more involved in emotional processing and bodily self-awareness, whereas males show a stronger association with motor control functions (119). A typical symptom of AN is an intense preoccupation with weight and body image (120). Women are typically more vulnerable than men to the sociocultural pressures that promote the thin ideal, which leads to a more pronounced body image distortion (121). Given the close connection between the SMN and bodily self-awareness, female patients may experience more significant impairments in network functionality because of enhanced body image distortion (122). Additionally, among AN patients, emotional disturbances such as anxiety and depression are more prevalent in females and are closely linked to dysfunctions in the sensorimotor network (123).
Our study reveals that the ECN, visual network, and salience network are influenced by age in patients with AN. Research indicates that the development of the prefrontal cortex, essential for executive functions, continues during adolescence, possibly resulting in weaker executive control, emotional regulation, and social skills in adolescents compared to adults (27, 124). In adolescents, the still-developing prefrontal cortex may relate to increased impulsivity and emotional instability when faced with emotional and social challenges that require self-regulation (125). Furthermore, the salience network and visual network exhibit different patterns of activity in adolescent and adult AN patient. The salience network in adolescents shows greater plasticity and heightened responsiveness to environmental stimuli (126). In contrast, the functioning of these networks in adult AN patients may be more stable (127).
Limitations
There are several limitations to this study. First, the analysis relies on coordinate-based data extracted from published studies, which offers a summary statistic (peak location) and inherently lacks spatial information in comparison to analyses using full statistical maps or individual participant data (40, 42, 128). This coordinate-based approach is correlational and does not have the capacity to determine causality between the identified network and AN-related GM alterations. Second, although the FCNM approach has effectively mapped neuropsychiatric symptoms and diseases onto common brain networks, we utilized resting-state fMRI data from a large cohort of healthy adults provided by the HCP. It may be more suitable to employ data that aligns more closely with the demographic and clinical profiles of the patients included in the analyzed studies. Third, due to the limited number of previous studies, network localization analyses were not conducted separately for different stages of AN (acute, chronic, and recovered). Future studies should address this by performing stage-specific network localization analyses to enhance result precision. Fourth, since many individuals with AN are also treated with antidepressants, it creates a challenge in distinguishing whether the brain network abnormalities observed are a result of AN itself or are influenced by the medications. This is a common issue in clinical research, as medications may alter brain function, potentially confounding the results. It highlights the need for more controlled studies that separate the effects of AN from those of psychotropic treatments. Fourth, while the FCNM approach is effective for mapping GM abnormalities to specific brain networks, it is constrained by the spatial resolution and methodological limitations of neuroimaging techniques. This limitation may lead to an underestimation of network-level abnormalities as subtle changes in smaller or overlapping network regions may not be fully captured. Additionally, the current study combined coordinates representing both increases and decreases in GM, which might obscure network specificities related to the direction of change. Future research should focus on validating these findings in clinical cohorts, exploring the longitudinal trajectories of brain network alterations, and assessing their potential as biomarkers for treatment response and prognosis. By combining advanced imaging techniques with network analysis methods, a more comprehensive understanding of the complex neurobiological mechanisms underlying AN can be achieved, providing new scientific evidence for targeted therapeutic interventions.
Conclusion
In conclusion, this study integrated the FCNM approach and large-scale human brain connectome data obtained from the HCP, revealing that the heterogeneous GM abnormalities in AN converge onto specific brain networks. Our findings indicated that the aberrant brain networks linked to AN predominantly implicate the auditory network (bilateral superior temporal gyrus and right Rolandic operculum), the ventral DMN (right middle temporal gyrus and bilateral precuneus), the dorsal DMN (bilateral ACC), and the SMN (bilateral precentral gyrus). Furthermore, network abnormalities in AN are influenced to some extent by both gender and age. These disruptions in brain networks are associated with distorted body image perception, impaired emotional regulation, and disrupted sensory and motor integration in individuals with AN. Network localization offers a comprehensive and unified framework that may help address concerns regarding the reproducibility of GM findings across VBM studies in AN. These findings could enhance our comprehension of the pathological mechanisms that underlie AN from a network perspective.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Author contributions
HY: Conceptualization, Supervision, Validation, Visualization, Writing – original draft. SW: Conceptualization, Formal analysis, Supervision, Visualization, Writing – original draft. HL: Formal analysis, Investigation, Methodology, Visualization, Writing – review & editing. SG: Conceptualization, Formal analysis, Methodology, Visualization, Writing – review & editing. FZ: Formal analysis, Investigation, Methodology, Visualization, Writing – review & editing. HW: Investigation, Methodology, Validation, Visualization, Writing – original draft. ZD: Formal analysis, Investigation, Methodology, Visualization, Writing – original draft, Writing – review & editing. PP: Formal analysis, Investigation, Methodology, Visualization, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Jiangsu Commission of Health (LKZ2023019 and ZD2022009), Yancheng Science and Technology Bureau (YCBK2024018), and Yancheng Commission of Health (YCBK202216).
Acknowledgments
We would like to thank the team of JiaJia Zhu from The First Affiliated Hospital of Anhui Medical University for providing the technical support.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that Gen AI was used in the creation of this manuscript. Declaration of generative AI and AI-assisted technologies in the writing process. During the preparation of this work, the authors used Gemini 2.5 Pro in order to improve the readability and language of the manuscript. After using this tool/service, the authors reviewed and edited the content as needed and takes full responsibility for the content of the published article.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2025.1667729/full#supplementary-material
Footnotes
References
1. Roubalová, R, Procházková, P, Papežová, H, Smitka, K, Bilej, M, and Tlaskalová-Hogenová, H. Anorexia nervosa: Gut microbiota-immune-brain interactions. Clin Nutr. (2020) 39:676–84. doi: 10.1016/j.clnu.2019.03.023
2. Galmiche, M, Achamrah, N, Déchelotte, P, Ribet, D, and Breton, J. Role of microbiota-gut-brain axis dysfunctions induced by infections in the onset of anorexia nervosa. Nutr Rev. (2022) 80:381–91. doi: 10.1093/nutrit/nuab030
3. van Eeden, AE, van Hoeken, D, and Hoek, HW. Incidence, prevalence and mortality of anorexia nervosa and bulimia nervosa. Curr Opin Psychiatry. (2021) 34:515–24. doi: 10.1097/YCO.0000000000000739
4. Cost, J, Krantz, MJ, and Mehler, PS. Medical complications of anorexia nervosa. Cleve Clin J Med. (2020) 87:361–6. doi: 10.3949/ccjm.87a.19084
5. Zhang, S, Wang, W, Su, X, Kemp, GJ, Yang, X, Su, J, et al. Psychoradiological investigations of gray matter alterations in patients with anorexia nervosa. Transl Psychiatry. (2018) 8:277. doi: 10.1038/s41398-018-0323-3
6. Pruccoli, J, Pelusi, M, Romagnoli, G, Malaspina, E, Moscano, F, and Parmeggiani, A. Timing of psychopharmacological and nutritional interventions in the inpatient treatment of anorexia nervosa: an observational study. Brain Sci. (2021) 11:1242. doi: 10.3390/brainsci11091242
7. Puckett, L, Grayeb, D, Khatri, V, Cass, K, and Mehler, P. A comprehensive review of complications and new findings associated with anorexia nervosa. J Clin Med. (2021) 10:2555. doi: 10.3390/jcm10122555
8. Marucci, S, Ragione, LD, De Iaco, G, Mococci, T, Vicini, M, Guastamacchia, E, et al. Anorexia nervosa and comorbid psychopathology. Endocr Metab Immune Disord Drug Targets. (2018) 18:316–24. doi: 10.2174/1871530318666180213111637
9. Walton, E, Bernardoni, F, Batury, VL, Bahnsen, K, Larivière, S, Abbate-Daga, G, et al. Brain structure in acutely underweight and partially weight-restored individuals with anorexia nervosa: a coordinated analysis by the ENIGMA eating disorders working group. Biol Psychiatry. (2022) 92:730–8. doi: 10.1016/j.biopsych.2022.04.022
10. Su, T, Gong, J, Tang, G, Qiu, S, Chen, P, Chen, G, et al. Structural and functional brain alterations in anorexia nervosa: a multimodal meta-analysis of neuroimaging studies. Hum Brain Mapp. (2021) 42:5154–69. doi: 10.1002/hbm.25602
11. Tose, K, Takamura, T, Isobe, M, Hirano, Y, Sato, Y, Kodama, N, et al. Systematic reduction of gray matter volume in anorexia nervosa, but relative enlargement with clinical symptoms in the prefrontal and posterior insular cortices: a multicenter neuroimaging study. Mol Psychiatry. (2024) 29:891–901. doi: 10.1038/s41380-023-02378-4
12. Mishima, R, Isobe, M, Noda, T, Tose, K, Kawabata, M, Noma, S, et al. Structural brain changes in severe and enduring anorexia nervosa: a multimodal magnetic resonance imaging study of gray matter volume, cortical thickness, and white matter integrity. Psychiatry Res Neuroimaging. (2021) 318:111393. doi: 10.1016/j.pscychresns.2021.111393
13. Oliva, R, Baiano, M, Salvo, P, Cereser, L, Castiello, U, and Begliomini, C. Metacognition in individuals recovered from anorexia nervosa: a voxel-based morphometry study. Psychiatry Res Neuroimaging. (2020) 304:111138. doi: 10.1016/j.pscychresns.2020.111138
14. Salehi, MA, Nilsson, IA, Figueira, J, Thornton, LM, Abdulkarim, I, Pålsson, E, et al. Serum profiling of anorexia nervosa: a (1) H NMR-based metabolomics study. Eur Neuropsychopharmacol. (2021) 49:1–10. doi: 10.1016/j.euroneuro.2021.02.015
15. Butler, MJ, Perrini, AA, and Eckel, LA. The role of the gut microbiome, immunity, and Neuroinflammation in the pathophysiology of eating disorders. Nutrients. (2021) 13:500. doi: 10.3390/nu13020500
16. Lin, J, Xu, X, Hou, Y, Yang, J, and Shang, H. Voxel-based Meta-analysis of gray matter abnormalities in multiple system atrophy. Front Aging Neurosci. (2020) 12:591666. doi: 10.3389/fnagi.2020.591666
17. Björnsdotter, M, Davidovic, M, Karjalainen, L, Starck, G, Olausson, H, and Wentz, E. Grey matter correlates of autistic traits in women with anorexia nervosa. J Psychiatry Neurosci. (2018) 43:79–86. doi: 10.1503/jpn.170072
18. Martin Monzon, B, Henderson, LA, Madden, S, Macefield, VG, Touyz, S, Kohn, MR, et al. Grey matter volume in adolescents with anorexia nervosa and associated eating disorder symptoms. Eur J Neurosci. (2017) 46:2297–307. doi: 10.1111/ejn.13659
19. Kohmura, K, Adachi, Y, Tanaka, S, Katayama, H, Imaeda, M, Kawano, N, et al. Regional decrease in gray matter volume is related to body dissatisfaction in anorexia nervosa. Psychiatry Res Neuroimaging. (2017) 267:51–8. doi: 10.1016/j.pscychresns.2017.07.004
20. van Opstal, AM, Westerink Am, AM, Teeuwisse Wm, WM, van der Geest, MA, van Furth, EF, and van der Grond, J. Hypothalamic BOLD response to glucose intake and hypothalamic volume are similar in anorexia nervosa and healthy control subjects. Front Neurosci. (2015) 9:159. doi: 10.3389/fnins.2015.00159
21. Gupta, A, Bhatt, RR, Rivera-Cancel, A, Makkar, R, Kragel, PA, Rodriguez, T, et al. Complex functional brain network properties in anorexia nervosa. J Eat Disord. (2022) 10:13. doi: 10.1186/s40337-022-00534-9
22. Joos, A, Klöppel, S, Hartmann, A, Glauche, V, Tüscher, O, Perlov, E, et al. Voxel-based morphometry in eating disorders: correlation of psychopathology with grey matter volume. Psychiatry Res. (2010) 182:146–51. doi: 10.1016/j.pscychresns.2010.02.004
23. Suchan, B, Busch, M, Schulte, D, Grönemeyer, D, Herpertz, S, and Vocks, S. Reduction of gray matter density in the extrastriate body area in women with anorexia nervosa. Behav Brain Res. (2010) 206:63–7. doi: 10.1016/j.bbr.2009.08.035
24. Phillipou, A, Rossell, SL, Gurvich, C, Castle, DJ, Abel, LA, Nibbs, RG, et al. Differences in regional grey matter volumes in currently ill patients with anorexia nervosa. Eur J Neurosci. (2018) 47:177–83. doi: 10.1111/ejn.13793
25. Frank, GK, Shott, ME, Hagman, JO, and Yang, TT. Localized brain volume and white matter integrity alterations in adolescent anorexia nervosa. J Am Acad Child Adolesc Psychiatry. (2013) 52:1066–75.e5. doi: 10.1016/j.jaac.2013.07.007
26. Olivo, G, Solstrand Dahlberg, L, Wiemerslage, L, Swenne, I, Zhukovsky, C, Salonen-Ros, H, et al. Atypical anorexia nervosa is not related to brain structural changes in newly diagnosed adolescent patients. Int J Eat Disord. (2018) 51:39–45. doi: 10.1002/eat.22805
27. Favaro, A, Santonastaso, P, Manara, R, Bosello, R, Bommarito, G, Tenconi, E, et al. Disruption of visuospatial and somatosensory functional connectivity in anorexia nervosa. Biol Psychiatry. (2012) 72:864–70. doi: 10.1016/j.biopsych.2012.04.025
28. Collantoni, E, Alberti, F, Meregalli, V, Meneguzzo, P, Tenconi, E, and Favaro, A. Brain networks in eating disorders: a systematic review of graph theory studies. Eat Weight Disord. (2022) 27:69–83. doi: 10.1007/s40519-021-01172-x
29. McFadden, KL, Tregellas, JR, Shott, ME, and Frank, GK. Reduced salience and default mode network activity in women with anorexia nervosa. J Psychiatry Neurosci. (2014) 39:178–88. doi: 10.1503/jpn.130046
30. Via, E, Calvo, A, de la Serna, E, Blázquez, A, Lázaro, L, Andrés-Perpiñá, S, et al. Longitudinal study in adolescent anorexia nervosa: evaluation of cortico-striatal and default mode network resting-state brain circuits. Eur Child Adolesc Psychiatry. (2023) 32:513–26. doi: 10.1007/s00787-021-01880-w
31. Müller, VI, Cieslik, EC, Laird, AR, Fox, PT, Radua, J, Mataix-Cols, D, et al. Ten simple rules for neuroimaging meta-analysis. Neurosci Biobehav Rev. (2018) 84:151–61. doi: 10.1016/j.neubiorev.2017.11.012
32. Fox, MD, Snyder, AZ, Vincent, JL, Corbetta, M, Van Essen, DC, and Raichle, ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci USA. (2005) 102:9673–8. doi: 10.1073/pnas.0504136102
33. Fox, MD. Mapping symptoms to brain networks with the human connectome. N Engl J Med. (2018) 379:2237–45. doi: 10.1056/NEJMra1706158
34. Boes, AD, Prasad, S, Liu, H, Liu, Q, Pascual-Leone, A, Caviness, VS, et al. Network localization of neurological symptoms from focal brain lesions. Brain. (2015) 138:3061–75. doi: 10.1093/brain/awv228
35. Tetreault, AM, Phan, T, Orlando, D, Lyu, I, Kang, H, Landman, B, et al. Network localization of clinical, cognitive, and neuropsychiatric symptoms in Alzheimer's disease. Brain. (2020) 143:1249–60. doi: 10.1093/brain/awaa058
36. Joutsa, J, Corp, DT, and Fox, MD. Lesion network mapping for symptom localization: recent developments and future directions. Curr Opin Neurol. (2022) 35:453–9. doi: 10.1097/WCO.0000000000001085
37. Burke, MJ, Joutsa, J, Cohen, AL, Soussand, L, Cooke, D, Burstein, R, et al. Mapping migraine to a common brain network. Brain. (2020) 143:541–53. doi: 10.1093/brain/awz405
38. Zhang, X, Xu, R, Ma, H, Qian, Y, and Zhu, J. Brain structural and functional damage network localization of suicide. Biol Psychiatry. (2024) 95:1091–9. doi: 10.1016/j.biopsych.2024.01.003
39. Kletenik, I, Gaudet, K, Prasad, S, Cohen, AL, and Fox, MD. Network localization of awareness in visual and motor Anosognosia. Ann Neurol. (2023) 94:434–41. doi: 10.1002/ana.26709
40. Darby, RR, Joutsa, J, and Fox, MD. Network localization of heterogeneous neuroimaging findings. Brain. (2019) 142:70–9. doi: 10.1093/brain/awy292
41. Mo, F, Zhao, H, Li, Y, Cai, H, Song, Y, Wang, R, et al. Network localization of state and trait of auditory verbal hallucinations in schizophrenia. Schizophr Bull. (2024) 50:1326–36. doi: 10.1093/schbul/sbae020
42. Wang, Y, Yang, Y, Xu, W, Yao, X, Xie, X, Zhang, L, et al. Heterogeneous brain abnormalities in schizophrenia converge on a common network associated with symptom remission. Schizophr Bull. (2024) 50:545–56. doi: 10.1093/schbul/sbae003
43. Ding, L, Liu, H, Jing, J, Jiang, Y, Meng, X, Chen, Y, et al. Lesion network mapping for neurological deficit in acute ischemic stroke. Ann Neurol. (2023) 94:572–84. doi: 10.1002/ana.26721
44. Younger, E, Ellis, EG, Parsons, N, Pantano, P, Tommasin, S, Caeyenberghs, K, et al. Mapping essential tremor to a common brain network using functional connectivity analysis. Neurology. (2023) 101:e1483–94. doi: 10.1212/WNL.0000000000207701
45. Yang, H, Gu, S, Sun, H, Zhang, F, Dai, Z, and Pan, P. Neural network localization in Parkinson's disease with impulse control disorders. Front Aging Neurosci. (2025) 17:1549589. doi: 10.3389/fnagi.2025.1549589
46. Song, Y, Yang, H, Gu, S, Zhu, Y, Dai, Z, Pan, P, et al. Network localization of regional homogeneity alterations in Parkinson's disease. Front Aging Neurosci. (2025) 17:1607691. doi: 10.3389/fnagi.2025.1607691
47. Moher, D, Liberati, A, Tetzlaff, J, and Altman, DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. (2009) 339:b2535–5. doi: 10.1136/bmj.b2535
48. Sheng, L, Zhao, P, Ma, H, Qi, L, Yi, Z, Shi, Y, et al. Grey matter alterations in restless legs syndrome: a coordinate-based meta-analysis. J Sleep Res. (2021) 30:e13298. doi: 10.1111/jsr.13298
49. Luo, R, Pan, P, Xu, Y, and Chen, L. No reliable gray matter changes in essential tremor. Neurol Sci. (2019) 40:2051–63. doi: 10.1007/s10072-019-03933-0
50. Schaper, F, Nordberg, J, Cohen, AL, Lin, C, Hsu, J, Horn, A, et al. Mapping lesion-related epilepsy to a human brain network. JAMA Neurol. (2023) 80:891–902. doi: 10.1001/jamaneurol.2023.1988
51. Xu, R, Zhang, X, Zhou, S, Guo, L, Mo, F, Ma, H, et al. Brain structural damage networks at different stages of schizophrenia. Psychol Med. (2024) 1–11. doi: 10.1017/S0033291724003088
52. Lan, H, Liu, W, Zuo, C, Chen, L, Wang, S, Luo, C, et al. Heterogeneous brain abnormalities in subjective cognitive decline converge on a common network and their transcriptional signature. Alzheimers Dement. (2025) 21:e70073. doi: 10.1002/alz.70073
53. Shirer, WR, Ryali, S, Rykhlevskaia, E, Menon, V, and Greicius, MD. Decoding subject-driven cognitive states with whole-brain connectivity patterns. Cereb Cortex. (2012) 22:158–65. doi: 10.1093/cercor/bhr099
54. Fonville, L, Giampietro, V, Williams, SC, Simmons, A, and Tchanturia, K. Alterations in brain structure in adults with anorexia nervosa and the impact of illness duration. Psychol Med. (2014) 44:1965–75. doi: 10.1017/S0033291713002389
55. Frank, GK, Shott, ME, Hagman, JO, and Mittal, VA. Alterations in brain structures related to taste reward circuitry in ill and recovered anorexia nervosa and in bulimia nervosa. Am J Psychiatry. (2013) 170:1152–60. doi: 10.1176/appi.ajp.2013.12101294
56. Fujisawa, TX, Yatsuga, C, Mabe, H, Yamada, E, Masuda, M, and Tomoda, A. Anorexia nervosa during adolescence is associated with decreased gray matter volume in the inferior frontal gyrus. PLoS One. (2015) 10:e0128548. doi: 10.1371/journal.pone.0128548
57. Lenhart, L, Gander, M, Steiger, R, Dabkowska-Mika, A, Mangesius, S, Haid-Stecher, N, et al. Attachment status is associated with grey matter recovery in adolescent anorexia nervosa: findings from a longitudinal study. Eur J Neurosci. (2022) 55:1373–87. doi: 10.1111/ejn.15614
58. D'Agata, F, Caroppo, P, Amianto, F, Spalatro, A, Caglio, MM, Bergui, M, et al. Brain correlates of alexithymia in eating disorders: a voxel-based morphometry study. Psychiatry Clin Neurosci. (2015) 69:708–16. doi: 10.1111/pcn.12318
59. Amianto, F, Caroppo, P, D'Agata, F, Spalatro, A, Lavagnino, L, Caglio, M, et al. Brain volumetric abnormalities in patients with anorexia and bulimia nervosa: a voxel-based morphometry study. Psychiatry Res. (2013) 213:210–6. doi: 10.1016/j.pscychresns.2013.03.010
60. Castro-Fornieles, J, Bargalló, N, Lázaro, L, Andrés, S, Falcon, C, Plana, MT, et al. A cross-sectional and follow-up voxel-based morphometric MRI study in adolescent anorexia nervosa. J Psychiatr Res. (2009) 43:331–40. doi: 10.1016/j.jpsychires.2008.03.013
61. Bomba, M, Riva, A, Morzenti, S, Grimaldi, M, Neri, F, and Nacinovich, R. Global and regional brain volumes normalization in weight-recovered adolescents with anorexia nervosa: preliminary findings of a longitudinal voxel-based morphometry study. Neuropsychiatr Dis Treat. (2015) 11:637–45. doi: 10.2147/NDT.S73239
62. Gaudio, S, Nocchi, F, Franchin, T, Genovese, E, Cannatà, V, Longo, D, et al. Gray matter decrease distribution in the early stages of anorexia nervosa restrictive type in adolescents. Psychiatry Res. (2011) 191:24–30. doi: 10.1016/j.pscychresns.2010.06.007
63. Mühlau, M, Gaser, C, Ilg, R, Conrad, B, Leibl, C, Cebulla, MH, et al. Gray matter decrease of the anterior cingulate cortex in anorexia nervosa. Am J Psychiatry. (2007) 164:1850–7. doi: 10.1176/appi.ajp.2007.06111861
64. Friederich, HC, Walther, S, Bendszus, M, Biller, A, Thomann, P, Zeigermann, S, et al. Grey matter abnormalities within cortico-limbic-striatal circuits in acute and weight-restored anorexia nervosa patients. NeuroImage. (2012) 59:1106–13. doi: 10.1016/j.neuroimage.2011.09.042
65. Joos, A, Hartmann, A, Glauche, V, Perlov, E, Unterbrink, T, Saum, B, et al. Grey matter deficit in long-term recovered anorexia nervosa patients. Eur Eat Disord Rev. (2011) 19:59–63. doi: 10.1002/erv.1060
66. Brooks, SJ, Barker, GJ, O’Daly, OG, Brammer, M, Williams, SC, Benedict, C, et al. Restraint of appetite and reduced regional brain volumes in anorexia nervosa: a voxel-based morphometric study. BMC Psychiatry. (2011) 11:179. doi: 10.1186/1471-244X-11-179
67. Hackett, TA. Information flow in the auditory cortical network. Hear Res. (2011) 271:133–46. doi: 10.1016/j.heares.2010.01.011
68. Scaife, JC, Godier, LR, Filippini, N, Harmer, CJ, and Park, RJ. Reduced resting-state functional connectivity in current and recovered restrictive anorexia nervosa. Front Psych. (2017) 8:30. doi: 10.3389/fpsyt.2017.00030
69. Tajadura-Jiménez, A, Crucianelli, L, Zheng, R, Cheng, C, Ley-Flores, J, Borda-Más, M, et al. Body weight distortions in an auditory-driven body illusion in subclinical and clinical eating disorders. Sci Rep. (2022) 12:20031. doi: 10.1038/s41598-022-24452-7
70. King, AJ, and Walker, KM. Integrating information from different senses in the auditory cortex. Biol Cybern. (2012) 106:617–25. doi: 10.1007/s00422-012-0502-x
71. Basura, GJ, Koehler, SD, and Shore, SE. Multi-sensory integration in brainstem and auditory cortex. Brain Res. (2012) 1485:95–107. doi: 10.1016/j.brainres.2012.08.037
72. Fuglset, TS, Endestad, T, Hilland, E, Bang, L, Tamnes, CK, Landrø, NI, et al. Brain volumes and regional cortical thickness in young females with anorexia nervosa. BMC Psychiatry. (2016) 16:404. doi: 10.1186/s12888-016-1126-9
73. Solstrand Dahlberg, L, Wiemerslage, L, Swenne, I, Larsen, A, Stark, J, Rask-Andersen, M, et al. Adolescents newly diagnosed with eating disorders have structural differences in brain regions linked with eating disorder symptoms. Nord J Psychiatry. (2017) 71:188–96. doi: 10.1080/08039488.2016.1250948
74. Gaudio, S, Brooks, SJ, and Riva, G. Nonvisual multisensory impairment of body perception in anorexia nervosa: a systematic review of neuropsychological studies. PLoS One. (2014) 9:e110087. doi: 10.1371/journal.pone.0110087
75. Blomberg, M, Schlegel, K, Stoll, L, Febry, H, Wünsch-Leiteritz, W, Leiteritz, A, et al. Reduced emotion recognition from nonverbal cues in anorexia nervosa. Eur Eat Disord Rev. (2021) 29:868–78. doi: 10.1002/erv.2860
76. Henderson, D, Bichoutar, I, Moxham, B, Faidherbe, V, Plaisant, O, and Guédon, A. Descriptive and functional anatomy of the Heschl gyrus: historical review, manual labelling and current perspectives. Surg Radiol Anat. (2023) 45:337–50. doi: 10.1007/s00276-023-03114-x
77. Chen, X, Ai, C, Liu, Z, and Wang, G. Neuroimaging studies of resting-state functional magnetic resonance imaging in eating disorders. BMC Med Imaging. (2024) 24:265. doi: 10.1186/s12880-024-01432-z
78. McAdams, CJ, Efseroff, B, McCoy, J, Ford, L, and Timko, CA. Social processing in eating disorders: neuroimaging paradigms and research domain organizational constructs. Curr Psychiatry Rep. (2022) 24:777–88. doi: 10.1007/s11920-022-01395-4
79. Gaudio, S, Carducci, F, Piervincenzi, C, Olivo, G, and Schiöth, HB. Altered thalamo–cortical and occipital–parietal– temporal–frontal white matter connections in patients with anorexia and bulimia nervosa: a systematic review of diffusion tensor imaging studies. J Psychiatry Neurosci. (2019) 44:324–39. doi: 10.1503/jpn.180121
80. Alfano, V, Mele, G, Cotugno, A, and Longarzo, M. Multimodal neuroimaging in anorexia nervosa. J Neurosci Res. (2020) 98:2178–207. doi: 10.1002/jnr.24674
81. van Kuyck, K, Gérard, N, Van Laere, K, Casteels, C, Pieters, G, Gabriëls, L, et al. Towards a neurocircuitry in anorexia nervosa: evidence from functional neuroimaging studies. J Psychiatr Res. (2009) 43:1133–45. doi: 10.1016/j.jpsychires.2009.04.005
82. Bronleigh, M, Baumann, O, and Stapleton, P. Neural correlates associated with processing food stimuli in anorexia nervosa and bulimia nervosa: an activation likelihood estimation meta-analysis of fMRI studies. Eat Weight Disord. (2022) 27:2309–20. doi: 10.1007/s40519-022-01390-x
83. Mălîia, MD, Donos, C, Barborica, A, Popa, I, Ciurea, J, Cinatti, S, et al. Functional mapping and effective connectivity of the human operculum. Cortex. (2018) 109:303–21. doi: 10.1016/j.cortex.2018.08.024
84. Roger, C, Lasbleiz, A, Guye, M, Dutour, A, Gaborit, B, and Ranjeva, JP. The role of the human hypothalamus in food intake networks: an MRI perspective. Front Nutr. (2021) 8:760914. doi: 10.3389/fnut.2021.760914
85. Lee, S, Parthasarathi, T, and Kable, JW. The ventral and dorsal default mode networks are Dissociably modulated by the vividness and valence of imagined events. J Neurosci. (2021) 41:5243–50. doi: 10.1523/JNEUROSCI.1273-20.2021
86. Buckner, RL, Andrews-Hanna, JR, and Schacter, DL. The brain's default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. (2008) 1124:1–38. doi: 10.1196/annals.1440.011
87. Andrews-Hanna, JR, Reidler, JS, Sepulcre, J, Poulin, R, and Buckner, RL. Functional-anatomic fractionation of the brain's default network. Neuron. (2010) 65:550–62. doi: 10.1016/j.neuron.2010.02.005
88. Geisler, D, Borchardt, V, Lord, AR, Boehm, I, Ritschel, F, Zwipp, J, et al. Abnormal functional global and local brain connectivity in female patients with anorexia nervosa. J Psychiatry Neurosci. (2016) 41:6–15. doi: 10.1503/jpn.140310
89. E, V, X, G, I, S, L, F, Bj, H, Cg, D, et al. Self and other body perception in anorexia nervosa: the role of posterior DMN nodes. World J Biol Psychiatry. (2018) 19:210–24. doi: 10.1080/15622975.2016.1249951
90. Cowdrey, FA, Park, RJ, Harmer, CJ, and McCabe, C. Increased neural processing of rewarding and aversive food stimuli in recovered anorexia nervosa. Biol Psychiatry. (2011) 70:736–43. doi: 10.1016/j.biopsych.2011.05.028
91. Cowdrey, FA, Filippini, N, Park, RJ, Smith, SM, and McCabe, C. Increased resting state functional connectivity in the default mode network in recovered anorexia nervosa. Hum Brain Mapp. (2014) 35:483–91. doi: 10.1002/hbm.22202
92. Gondo, M, Kawai, K, Moriguchi, Y, Hiwatashi, A, Takakura, S, Yoshihara, K, et al. Effects of integrated hospital treatment on the default mode, salience, and frontal-parietal networks in anorexia nervosa: a longitudinal resting-state functional magnetic resonance imaging study. PLoS One. (2023) 18:e0283318. doi: 10.1371/journal.pone.0283318
93. Davey, J, Thompson, HE, Hallam, G, Karapanagiotidis, T, Murphy, C, De Caso, I, et al. Exploring the role of the posterior middle temporal gyrus in semantic cognition: integration of anterior temporal lobe with executive processes. NeuroImage. (2016) 137:165–77. doi: 10.1016/j.neuroimage.2016.05.051
94. Kuroki, N, Shenton, ME, Salisbury, DF, Hirayasu, Y, Onitsuka, T, Ersner-Hershfield, H, et al. Middle and inferior temporal gyrus gray matter volume abnormalities in first-episode schizophrenia: an MRI study. Am J Psychiatry. (2006) 163:2103–10. doi: 10.1176/ajp.2006.163.12.2103
95. Fuglset, TS, Landrø, NI, Reas, DL, and Rø, Ø. Functional brain alterations in anorexia nervosa: a scoping review. J Eat Disord. (2016) 4:32. doi: 10.1186/s40337-016-0118-y
96. Kodama, N, Moriguchi, Y, Takeda, A, Maeda, M, Ando, T, Kikuchi, H, et al. Neural correlates of body comparison and weight estimation in weight-recovered anorexia nervosa: a functional magnetic resonance imaging study. Biopsychosoc Med. (2018) 12:15. doi: 10.1186/s13030-018-0134-z
97. Seiger, R, Reggente, N, Majid, DS, Ly, R, Tadayonnejad, R, Strober, M, et al. Neural representations of anxiety in adolescents with anorexia nervosa: a multivariate approach. Transl Psychiatry. (2023) 13:283. doi: 10.1038/s41398-023-02581-5
98. Zhong, S, Su, T, Gong, J, Huang, L, and Wang, Y. Brain functional alterations in patients with anorexia nervosa: a meta-analysis of task-based functional MRI studies. Psychiatry Res. (2023) 327:115358. doi: 10.1016/j.psychres.2023.115358
99. Lyall, AE, Breithaupt, L, Ji, C, Haidar, A, Kotler, E, Becker, KR, et al. Lower region-specific gray matter volume in females with atypical anorexia nervosa and anorexia nervosa. Int J Eat Disord. (2024) 57:951–66. doi: 10.1002/eat.24168
100. Phillipou, A, Abel, LA, Castle, DJ, Hughes, ME, Gurvich, C, Nibbs, RG, et al. Self perception and facial emotion perception of others in anorexia nervosa. Front Psychol. (2015) 6:1181. doi: 10.3389/fpsyg.2015.01181
101. Ruan, VA, Hartz, A, Hueck, M, Dahmen, B, von Polier, G, Herpertz-Dahlmann, B, et al. Neural mechanisms underlying social recognition and theory of mind in adolescent patients with bulimia nervosa and transdiagnostic comparison with anorexia nervosa. Eur Eat Disord Rev. (2022) 30:486–500. doi: 10.1002/erv.2911
102. de la Cruz, F, Schumann, A, Suttkus, S, Helbing, N, Zopf, R, and Bär, KJ. Cortical thinning and associated connectivity changes in patients with anorexia nervosa. Transl Psychiatry. (2021) 11:95. doi: 10.1038/s41398-021-01237-6
103. Yue, L, Wang, Y, Kaye, WH, Kang, Q, Huang, JB, Cheung, EFC, et al. Structural alterations in the caudate nucleus and precuneus in un-medicated anorexia nervosa patients. Psychiatry Res Neuroimaging. (2018) 281:12–8. doi: 10.1016/j.pscychresns.2018.08.009
104. Kappou, K, Ntougia, M, Kourtesi, A, Panagouli, E, Vlachopapadopoulou, E, Michalacos, S, et al. Neuroimaging findings in adolescents and young adults with anorexia nervosa: a systematic review. Children. (2021) 8:137. doi: 10.3390/children8020137
105. Esposito, R, Cieri, F, di Giannantonio, M, and Tartaro, A. The role of body image and self-perception in anorexia nervosa: the neuroimaging perspective. J Neuropsychol. (2018) 12:41–52. doi: 10.1111/jnp.12106
106. Lee, S, Ran Kim, K, Ku, J, Lee, JH, Namkoong, K, and Jung, YC. Resting-state synchrony between anterior cingulate cortex and precuneus relates to body shape concern in anorexia nervosa and bulimia nervosa. Psychiatry Res. (2014) 221:43–8. doi: 10.1016/j.pscychresns.2013.11.004
107. Rolls, ET. Emotion, motivation, decision-making, the orbitofrontal cortex, anterior cingulate cortex, and the amygdala. Brain Struct Funct. (2023) 228:1201–57. doi: 10.1007/s00429-023-02644-9
108. Gaudio, S, Piervincenzi, C, Beomonte Zobel, B, Romana Montecchi, F, Riva, G, Carducci, F, et al. Altered resting state functional connectivity of anterior cingulate cortex in drug naïve adolescents at the earliest stages of anorexia nervosa. Sci Rep. (2015) 5:10818. doi: 10.1038/srep10818
109. Chenji, S, Jha, S, Lee, D, Brown, M, Seres, P, Mah, D, et al. Investigating default mode and sensorimotor network connectivity in amyotrophic lateral sclerosis. PLoS One. (2016) 11:e0157443. doi: 10.1371/journal.pone.0157443
110. Han, Z, Liu, T, Shi, Z, Zhang, J, Suo, D, Wang, L, et al. Investigating the heterogeneity within the somatosensory-motor network and its relationship with the attention and default systems. PNAS Nexus. (2023) 2:pgad 276. doi: 10.1093/pnasnexus/pgad276
111. Edwards, LL, King, EM, Buetefisch, CM, and Borich, MR. Putting the “sensory” into sensorimotor control: the role of sensorimotor integration in goal-directed hand movements after stroke. Front Integr Neurosci. (2019) 13:16. doi: 10.3389/fnint.2019.00016
112. Olivo, G, Swenne, I, Zhukovsky, C, Tuunainen, AK, Salonen-Ros, H, Larsson, EM, et al. Reduced resting-state connectivity in areas involved in processing of face-related social cues in female adolescents with atypical anorexia nervosa. Transl Psychiatry. (2018) 8:275. doi: 10.1038/s41398-018-0333-1
113. Lotter, LD, von Polier, G, Offermann, J, Buettgen, K, Stanetzky, L, Eickhoff, SB, et al. Recovery-associated resting-state activity and connectivity alterations in anorexia nervosa. Biol Psychiatry Cogn Neurosci Neuroimaging. (2021) 6:1023–33. doi: 10.1016/j.bpsc.2021.03.006
114. Jensen, MA, Huang, H, Valencia, GO, Klassen, BT, van den Boom, MA, Kaufmann, TJ, et al. A motor association area in the depths of the central sulcus. Nat Neurosci. (2023) 26:1165–9. doi: 10.1038/s41593-023-01346-z
115. Silva, AB, Liu, JR, Zhao, L, Levy, DF, Scott, TL, and Chang, EF. A neurosurgical functional dissection of the middle precentral gyrus during speech production. J Neurosci. (2022) 42:8416–26. doi: 10.1523/JNEUROSCI.1614-22.2022
116. Scaife, JC, Godier, LR, Reinecke, A, Harmer, CJ, and Park, RJ. Differential activation of the frontal pole to high vs. low calorie foods: the neural basis of food preference in anorexia nervosa? Psychiatry Res Neuroimaging. (2016) 258:44–53. doi: 10.1016/j.pscychresns.2016.10.004
117. Sato, Y, Saito, N, Utsumi, A, Aizawa, E, Shoji, T, Izumiyama, M, et al. Neural basis of impaired cognitive flexibility in patients with anorexia nervosa. PLoS One. (2013) 8:e61108. doi: 10.1371/journal.pone.0061108
118. Schultz, CC, Wagner, G, de la Cruz, F, Berger, S, Reichenbach, JR, Sauer, H, et al. Evidence for alterations of cortical folding in anorexia nervosa. Eur Arch Psychiatry Clin Neurosci. (2017) 267:41–9. doi: 10.1007/s00406-015-0666-1
119. Zhang, X, Liang, M, Qin, W, Wan, B, Yu, C, and Ming, D. Gender differences are encoded differently in the structure and function of the human brain revealed by multimodal MRI. Front Hum Neurosci. (2020) 14:244. doi: 10.3389/fnhum.2020.00244
120. Todisco, P, De Mico, A, and Meneguzzo, P. Vitamin D status and behavioral impulsivity in anorexia nervosa: insights from a longitudinal study. Nutrients. (2024) 16:2523. doi: 10.3390/nu16152523
121. Grabe, S, Ward, LM, and Hyde, JS. The role of the media in body image concerns among women: a meta-analysis of experimental and correlational studies. Psychol Bull. (2008) 134:460–76. doi: 10.1037/0033-2909.134.3.460
122. Gaudio, S, and Quattrocchi, CC. Neural basis of a multidimensional model of body image distortion in anorexia nervosa. Neurosci Biobehav Rev. (2012) 36:1839–47. doi: 10.1016/j.neubiorev.2012.05.003
123. Kaye, WH, Fudge, JL, and Paulus, M. New insights into symptoms and neurocircuit function of anorexia nervosa. Nat Rev Neurosci. (2009) 10:573–84. doi: 10.1038/nrn2682
124. Dow-Edwards, D, Mac Master, FP, Peterson, BS, Niesink, R, Andersen, S, and Braams, BR. Experience during adolescence shapes brain development: from synapses and networks to normal and pathological behavior. Neurotoxicol Teratol. (2019) 76:106834. doi: 10.1016/j.ntt.2019.106834
125. Hare, TA, Tottenham, N, Galvan, A, Voss, HU, Glover, GH, and Casey, BJ. Biological substrates of emotional reactivity and regulation in adolescence during an emotional go-nogo task. Biol Psychiatry. (2008) 63:927–34. doi: 10.1016/j.biopsych.2008.03.015
126. Uddin, LQ. Salience processing and insular cortical function and dysfunction. Nat Rev Neurosci. (2015) 16:55–61. doi: 10.1038/nrn3857
127. Boehm, I, Geisler, D, King, JA, Ritschel, F, Seidel, M, Deza Araujo, Y, et al. Increased resting state functional connectivity in the fronto-parietal and default mode network in anorexia nervosa. Front Behav Neurosci. (2014) 8:346. doi: 10.3389/fnbeh.2014.00346
128. Cheng, Y, Cai, H, Liu, S, Yang, Y, Pan, S, Zhang, Y, et al. Brain network localization of gray matter atrophy and neurocognitive and social cognitive dysfunction in schizophrenia. Biol Psychiatry. (2025) 97:148–56. doi: 10.1016/j.biopsych.2024.07.021
129. Boghi, A, Sterpone, S, Sales, S, D’Agata, F, Bradac, GB, Zullo, G, et al. In vivo evidence of global and focal brain alterations in anorexia nervosa. Psychiatry Res. (2011) 192:154–9. doi: 10.1016/j.pscychresns.2010.12.008
130. Mainz, V, Schulte-Rüther, M, Fink, GR, Herpertz-Dahlmann, B, and Konrad, K. Structural brain abnormalities in adolescent anorexia nervosa before and after weight recovery and associated hormonal changes. Psychosom Med. (2012) 74:574–82. doi: 10.1097/PSY.0b013e31824ef10e
131. Bär, KJ, de la Cruz, F, Berger, S, Schultz, CC, and Wagner, G. Structural and functional differences in the cingulate cortex relate to disease severity in anorexia nervosa. J Psychiatry Neurosci. (2015) 40:269–79.
132. Nickel, K, Joos, A, Tebartz van Elst, L, Matthis, J, Holovics, L, Endres, D, et al. Recovery of cortical volume and thickness after remission from acute anorexia nervosa. Int J Eat Disord. (2018) 51:1056–69. doi: 10.1002/eat.22918
Glossary
ACC - anterior cingulate cortex
AN - anorexia nervosa
CBMA - coordinate-based meta-analysis
DBM - deformation-based morphometry
DMN - default mode network
FA - flip angle
FC - functional connectivity
FCNM - functional connectivity network mapping
FDR - false discovery rate
fMRI - functional magnetic resonance imaging
FOV - field of view
FWE - family-wise error
FSL - FMRIB Software Library
FWHM - Full Width at Half Maximum
GRE-EPI - gradient-recalled echo-Planar Imaging
GM - gray matter
GMV - gray matter volume
HC - healthy controls
HCP - Human Connectome Project
LECN - left executive control network
MRI - magnetic resonance imaging
MNI - Montreal Neurological Institute
NA - not applicable
ROI - region of interest
RECN - right executive control network
SMN - sensorimotor network
SPM - Statistical Parametric Mapping
TE - echo time
TFCE - Threshold-Free Cluster Enhancement
TR - repetition time
VBM - Voxel-based morphometry.
Keywords: anorexia nervosa, voxel-based morphometry, gray matter, network localization, functional connectivity network mapping
Citation: Yang H, Wang S, Li H, Gu S, Zhang F, Wang H, Dai Z and Pan P (2025) Mapping gray matter changes in anorexia nervosa: a functional connectivity network approach. Front. Nutr. 12:1667729. doi: 10.3389/fnut.2025.1667729
Edited by:
Romate John, Central University of Karnataka, IndiaCopyright © 2025 Yang, Wang, Li, Gu, Zhang, Wang, Dai and Pan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: PingLei Pan, cGFucGluZ2xlaUAxNjMuY29t; ZhenYu Dai, eWNzeWR6eUAxNjMuY29t
†These authors have contributed equally to this work
 HuCheng Yang
HuCheng Yang Shu Wang1†
Shu Wang1† HuaLiang Li
HuaLiang Li SiYu Gu
SiYu Gu HongHui Wang
HongHui Wang ZhenYu Dai
ZhenYu Dai PingLei Pan
PingLei Pan