- 1Department and Graduate Institute of Pharmacology, National Taiwan University, Taipei, Taiwan
- 2Office of Postgraduate Studies, UCSI University, Kuala Lumpur, Malaysia
- 3College of Health Sciences, Chang-Jung Christian University, Tainan, Taiwan
- 4Graduate Institute of Brain and Mind Sciences, College of Medicine, National Taiwan University, Taipei, Taiwan
- 5Graduate Institute of Anatomy and Cell Biology, College of Medicine, National Taiwan University, Taipei, Taiwan
- 6Department of Physiology, College of Medicine, Taipei Medical University, Taipei, Taiwan
- 7Institute of Biotechnology and Pharmaceutical Research, National Health Research Institutes, Miaoli, Taiwan
- 8School of Pharmacy, College of Pharmacy, China Medical University, Taichung, Taiwan
- 9Graduate Institute of Acupuncture Science, China Medical University, Taichung, Taiwan
Background: Obesity is associated with cognitive function impairment. We previously found that male, but not female, mice have poorer performance in learning and memory tasks and impaired hippocampal synaptic plasticity after long-term high-fat diet (HFD) consumption, compared to regular chow-fed counterparts. To elucidate the potential morphological mechanism(s), here we further performed morphometric analysis of hippocampal dendritic morphology and complexity in HFD and control groups of both sexes.
Methods: C57BL/6 J mice with both sexes were fed HFD (45% kcal% fat) after weaning for 12 months. Age-matched control mice were fed regular chows (13.5 kcal% fat). Morphometric analysis of Golgi-stained dendrites in hippocampal slices was performed to compare the dendritic morphology and complexity of CA1 pyramidal neurons between HFD and control groups in male and female mice.
Results: Compared with the control group, HFD-fed male mice showed lower dendritic spine density in both apical and basal dendrites, and lesser dendritic complexity in basal dendrites, which was indicated by fewer bifurcation nodes, terminal endings and dendritic segments, and shorter total dendritic length. However, in female mice, HFD did not affect dendritic spine density and induced subtle changes in dendritic complexity. Nevertheless, in control groups, male mice inherently had higher dendritic spine density and more dendritic complexity than females.
Conclusion: The present study provides the structural evidence, including the reduction of dendritic complexity and spine density, for HFD-induced male-specific functional impairments in hippocampal synaptic plasticity and memory performance.
Introduction
Hippocampus is an evolutionarily conserved brain region crucial for navigation and episodic memory in mammals (1, 2). Morphological changes in this region are often associated with memory-loss conditions such as dementia and Alzheimer’s disease (AD) (3, 4). Especially in AD patients, obesity is one of the risk factors that negatively impact the hippocampal-dependent memory (5, 6). A neuroimaging study has shown that overweight adults, compared to normal controls, have a higher degree of hippocampal atrophy (7), a condition highly associated with memory impairment (8). This association was, surprisingly, also observed in children with obesity (9). Although the manifestation of memory loss usually commences in older adults, numerous studies have revealed detrimental changes in earlier life. The impact of juvenile obesity-induced memory impairment is across the lifespan (10, 11) and highly correlated with daily diet (12–14).
High-fat diet (HFD)-induced obesity models in rodents can recapitulate large parts of the pathogenesis of human obesity (15), compared to the genetically-manipulated obesity model, like ob-ob mice. In HDF-induced obesity models, impairment of hippocampal-dependent memory has been reported in juvenile/adolescent (16), middle-aged (17), and aged (18) rodents. Especially, in our previous study, we found a sex difference in the negative impact of HFD on the learning and memory performance of mice (17). Mice fed HFD after weaning until 1-year-old (HFD-mice), beside exhibited significant obesity-related metabolic changes, such as elevation of plasma glucose, cholesterol, insulin, leptin and adiponectin, also had poorer performance in hippocampus-dependent learning and memory tasks and impaired hippocampal synaptic plasticity, long-term potentiation (LTP), in the male, but not female, group (17). Our findings were in-line with the recent report (19) that male mice are more vulnerable than female juvenile mice in HFD-induced reduction of hippocampus-dependent aversive memory. Such interplay between obesity and sex has also been reported in AD patients (20).
Morphological changes in hippocampal dendritic spines are a histological biomarker of synaptic plasticity (21), which is crucial in memory formation and consolidation (22). For example, hippocampal CA1 dendritic spine density is directly proportional to the memory capacity of mice (23) and rats (24). The dendritic spine number and length of hippocampal CA1 neurons were decreased in an AD mouse model (25). However, so far, there is no morphological analysis for the impact of long-term HFD on hippocampal dendritic morphology between sexes, while the available literature revolves around the hippocampal dendritic spine density affected by obesogenic diet in male rodents (26–28). Therefore, in this study, we investigated whether long-term HFD induced changes, with relation to sex differences, in the dendritic morphology of hippocampal CA1 pyramidal neurons using morphometric analyses.
Materials and methods
All experimental protocols were conducted in adherence to the ethical guidelines approved by the Institutional Animal Care and Use Committees in College of Medicine, National Taiwan University and in National Health Research Institute, Taiwan.
Animals
HFD-induced obese C57BL/6 J mice and normal diet-fed mice in both sexes were raised as reported previously (17). The mice were purchased from the National Laboratory Animal Center, Taiwan. Briefly, both male and female mice were randomly divided into HFD and normal diet groups. Mice in HFD and normal diet groups were fed rodent chows consisting of 45% (research diets Inc., NJ, United States, product ID: D12451) and 13.5% (LabDiet®, PA, United States. product ID: 5010) kcal% fat, respectively, after weaning (3–4 weeks old), for 12 months. The body weight was measured individually twice and once a week, respectively, before and after 12 weeks of age. Mice were housed in groups of 5 per cage under controlled temperature and 12:12 h light–dark cycle with abovementioned diets and water ad libidum
Golgi-Cox impregnation
After deeply anesthetized with 5% isoflurane, mice were sacrificed by intracardiac perfusion with 4% paraformaldehyde. The brains were isolated and post-fixed for later morphological studies. Brain sections of 200 μm-thick containing the hippocampus were sliced horizontally with Microslicer (DTK-1000, D. S. K., Japan) and collected.
The Golgi-Cox impregnation method was used for visualizing dendritic structures as reported previously (29) with modifications. Briefly, hippocampal slices were immersed into an impregnation solution, which was prepared by mixing solution A (1.0 g potassium dichromate and 1.0 g mercuric chloride in 85 mL water) and solution B (0.8 g potassium chromate and 0.5 g sodium tungstate in 20 mL water), at room temperature for 2 weeks. Sections were then collected and reacted with 15% ammonium hydroxide for 2 min and rinsed thoroughly in distilled water. Subsequently, sections were placed in diluted rapid fixer solution (1:5; Ilford, Marly, Switzerland) for 10 min and rinsed thoroughly in distilled water. Finally, all sections were mounted with a glycerol-based mounting medium.
Morphometric analysis
Dendritic spines
The dendritic spines from both basal and apical dendrites of Golgi-Cox-impregnated hippocampal CA1 pyramidal neurons were captured with an Olympus light microscope (Olympus BX51, Tokyo, Japan) under a 60 × lens, and their numbers were counted using the ImageJ software (NIH, MA, United States). For basal dendrites, we counted spine numbers in the dendritic segments of orders over three. Since the spine density is usually low and varied in the primary and secondary orders, we chose segments over order 3 to reduce the intra-group variation (30). For apical dendrites, spine numbers beyond the distance of 150–200 μm apart from the soma were counted, as the spine density in the proximal segments (beyond 50 μm from the soma) is usually low and varied (31).
Dendrites
The morphology of Golgi-Cox-impregnated pyramidal neurons in the CA1 region was examined under a 20 × lens of the Olympus BX51 light microscope. The image stacks were captured with 1.5 μm Z-axis interval under the control of the Stereo Investigator system (MicroBrightField, MBF Bioscience, VT, United States). The morphology of CA1 pyramidal neurons with basal and apical dendrites was reconstructed by the Neurolucida software (MBF Bioscience). Morphometric analyses of basal dendrites of CA1 pyramidal neurons were performed according to the anatomical definition (32). The dendrites derived directly from the soma are the first-order branches or primary dendrites. The daughter branches arising from that are second-order branches, and so on. The highest branching order was determined by the maximum order of the selected neurons. The point at which a dendrite gives rise to two daughter branches was defined as a bifurcation node. The termination of a dendrite was counted as a terminal ending. The portion between two bifurcation nodes was defined as a segment. The Neurolucida Explorer program was used to count the numbers of primary dendrites, bifurcation nodes, terminal endings, and the highest orders of dendritic branching of the collected CA1 neurons in various groups. Due to the steric distribution of lengthy apical dendrites, it was technically challenging to obtain sufficient CA1 pyramidal neurons with complete apical dendrites. We only performed morphometric analysis for basal dendrites.
Statistical analysis
Data are expressed as mean ± SEM. For body weight comparisons (Figure 1), we employed two-way ANOVA with repeated measures over time followed by Bonferroni’s post-hoc test. For dendritic spine density (Figure 2), the data was analyzed with two-way ANOVA over diet and sex factors, followed by Bonferroni’s post-hoc test, and passed the Shapiro–Wilk normality test. For morphological analyses of dendrites (Figure 3), the data underwent logarithmic transformations then analyzed with two-way ANOVA over diet and sex factors, followed by Bonferroni’s post-hoc test, and passed the Shapiro–Wilk normality test. Spearman’s test was used to test for heteroscedasticity in these datasets. p < 0.05 was considered to be significant.
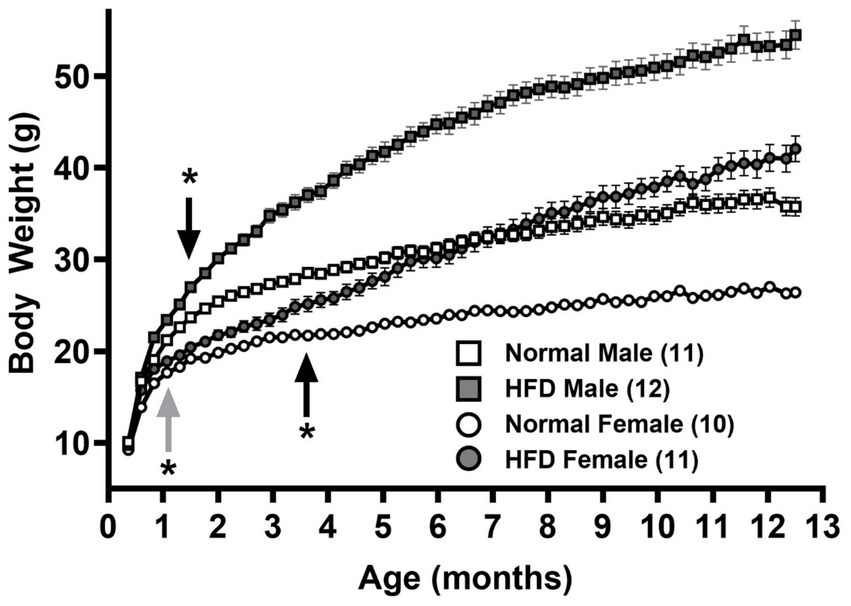
Figure 1. Growth curves of male and female mice fed normal chow and high-fat diet (HFD), respectively. Body weights of male (squares) and female (circles) mice fed normal chow (empty symbols) or HFD (filled symbols) after weaning (P21) for 1 year were measured twice and once a week, respectively, before and after 12 weeks of age. Data are mean ± SEM *p < 0.05 vs. the sex-specific control group (black arrows) or vs. the control male group (gray arrow) from the indicated ages (Two-way ANOVA followed by Bonferroni’s post-hoc test.) The numbers in parentheses are the numbers of animals in each group.
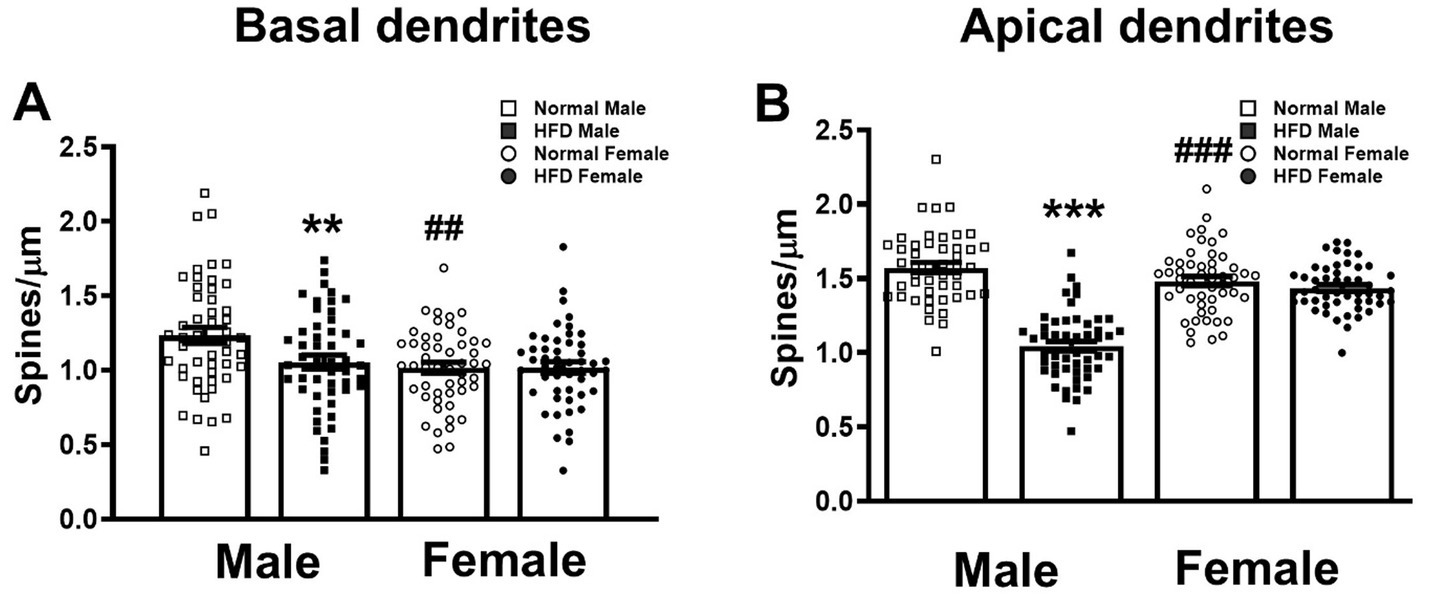
Figure 2. Effects of HFD on the spine density of basal and apical dendrites of hippocampal CA1 neurons. The dendritic spines of basal (A) and apical (B) dendrites of CA1 pyramidal neurons in both sexes of HFD-fed mice (HFD♂ and HFD♀) and normal control mice (NC♂ and NC♀). Dendritic spines from dendritic segments collected from each group were analyzed by Image J. Data are the mean ± SEM. n: the number of dendritic segments. **p < 0.01, ***p < 0.001 vs. the sex-specific control group, ##p < 0.01, ###p < 0.001 vs. the control male group, two-way ANOVA followed by Bonferroni’s post-hoc test.
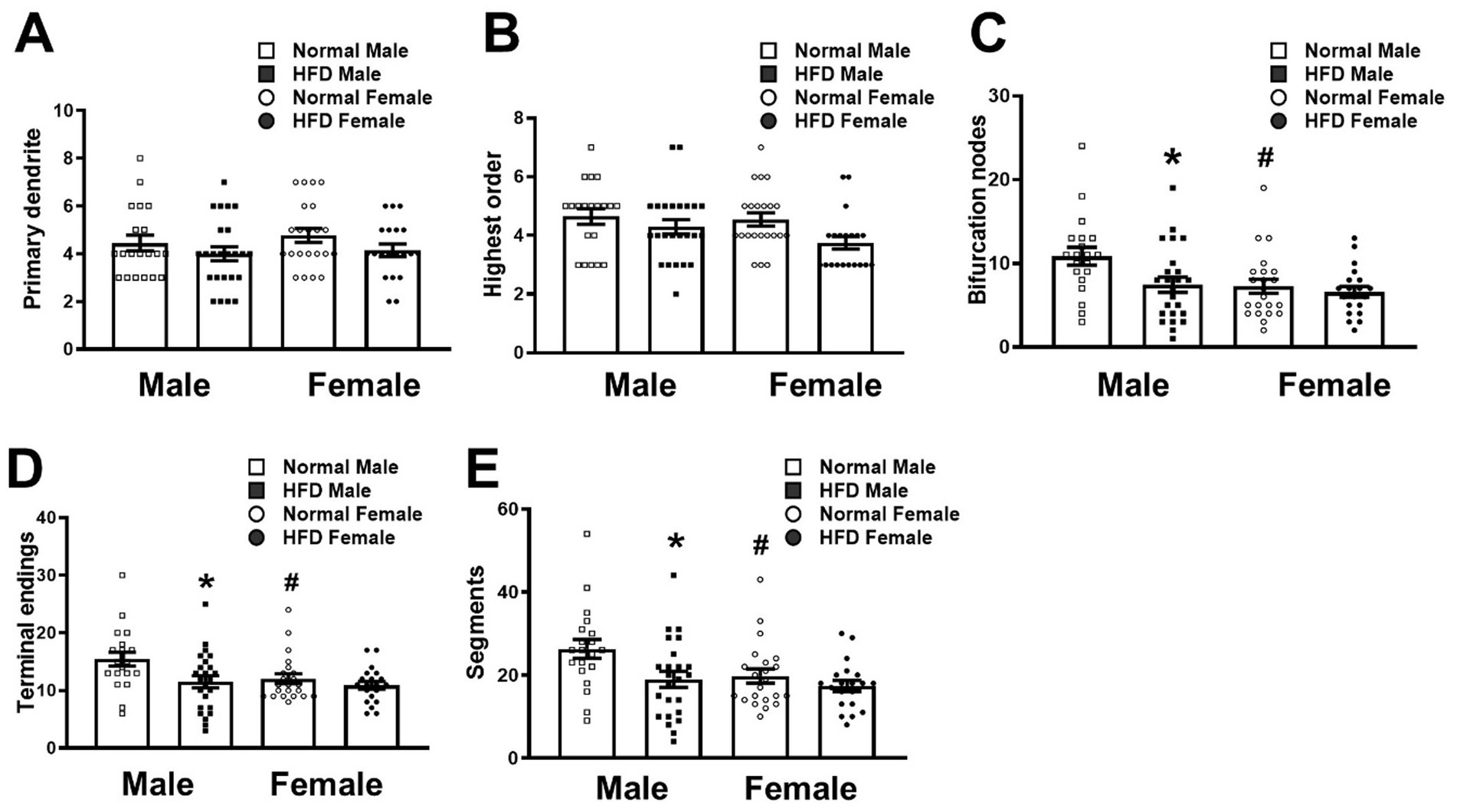
Figure 3. Morphometric analysis of the branching pattern of basal dendrites of CA1 pyramidal neurons in different groups. The number of primary dendrites (A), the highest dendritic branching order (B), the number of bifurcation nodes, terminal endings (D) and dendritic segments (E) in the basal dendrites of CA1 pyramidal neurons collected from HFD-fed mice (HFD♂, n = 24 neurons from 3 animals; HFD♀, n = 20 neurons from 6 animals) and normal control mice (NC♂, n = 20 neurons from 3 animals; NC♀, n = 22 neurons from 5 animals) were analyzed by the Neurolucida Explorer software. Data are the mean ± SEM. *p < 0.05 vs. the sex-specific control group. #p < 0.05 vs. the control male group, two-way ANOVA followed by Bonferroni’s post-hoc test.
Results
Body weight
Figure 1 shows the growth curves of normal diet control-male (empty squares), normal diet control-female (empty circles), HFD-male (filled squares) and HFD-female (filled circles) groups of mice. A two-way ANOVA with repeated measures over Age shows main effects of Age [F(52, 2080) = 841.5, p < 0.001, η2p = 0.955], Group [F(3, 40) = 115.0, p < 0.001, η2p = 0.958] and a significant interaction between Age and Group [F(156, 2080) = 47.90, p < 0.001, η2p = 0.782]. Post hoc analyses with the Bonferroni’s test show that in normal diet control groups, male mice grew significantly heavier than females starting at 32-day old (upward gray arrow, Figure 1, p = 0.032), and throughout the feeding duration till 1-year-old (p < 0.001, main Group effect). On the other hand, consistent with our previous study (17), HFD groups gained significantly more weight than control groups in both sexes. Compared with control groups of the same sex, HFD-male mice significantly gained more weights starting at 43-day old (downward black arrow, Figure 1, p = 0.041), in contrast with HFD-females at 109-day old (upward black arrow, Figure 1, p = 0.042).
Effects of HFD on the spine density of hippocampal CA1 apical and basal dendrites
Figure 4 shows the morphology of Neurolucida-reconstructed CA1 pyramidal neurons with well-impregnated apical and basal dendrites in hippocampal sections taken from control-male, HFD-male, control-female and HFD-female groups of mice, respectively. Representative micrograms of dendritic spines on basal dendrites (upper panel) and apical dendrites (lower panel) from four groups were also shown.
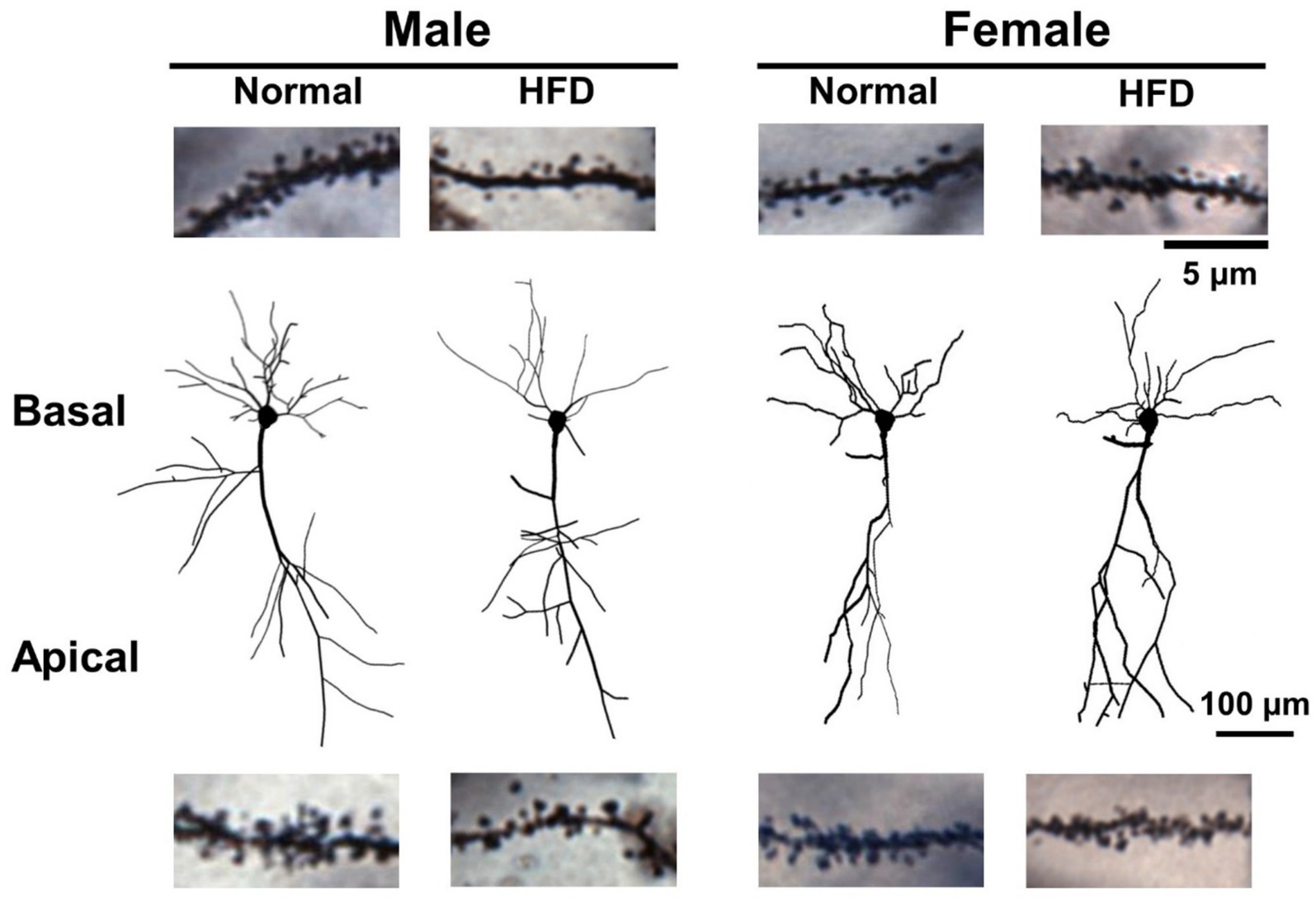
Figure 4. The dendritic morphology and dendritic spines of hippocampal CA1 pyramidal neurons of control males, control females, HFD-males and HFD-females. The Neurolucida-reconstructed morphology of Golgi-Cox impregnated CA1 pyramidal neurons with well impregnated dendrites collected from four groups of mice. The dendritic spines were omitted in this illustration. Representative photographs of basal (upper) and apical (lower) dendritic segments containing dendritic spines were collected from CA1 neurons of different groups. Scale bar: 100 μm for CA1 neurons and 5 μm for dendritic segments.
First, we compared the dendritic spine density of basal and apical dendrites among four groups. The detailed statistical parameters of datasets in Figures 2, 3 are listed in Supplementary Table S1. Two-way ANOVA followed by Bonferroni’s post-hoc test shows that, in basal dendrites (Figure 4 upper panel), HFD-males had significantly lower dendritic spine density than control-males (open square bar vs. filled square bar, Figure 2A, P = 0.009, Bonferroni’s test). However, there was no significant difference in the basal spine density between HFD-female and control-female groups (Figure 4 upper panel; filled circle bar vs. open circle bar, Figure 2A, P = 0.999). Interestingly, in normal-diet control groups, males had significantly higher basal spine density than females control group (Figure 4 upper panel; open square bar vs. open circle bar, Figure 2A, P = 0.002, Bonferroni’s test). Similar trend was also observed in the apical dendrites (Figure 4 lower panel; Figure 2B). HFD significantly decreased the apical dendritic spine density in male mice (p < 0.001), but not in female mice (p > 0.999), as compared with the control group with the same-sex (Figure 2B, two-way ANOVA followed by Bonferroni’s post-hoc test). Interestingly, compared with females, control males also had a significantly higher spine density on apical dendrites (Figure 4 lower panel; Figure 2B open square bar vs. open circle bar, p < 0.001, Bonferroni’s test), as observed on basal dendrites.
Effects of HFD on the morphology of basal dendrites of hippocampal CA1 neurons
Next, we compared the complexity of basal dendrites of hippocampal CA1 neurons in four groups of mice. Morphological analyses on the dendritic branching pattern using the Neurolucida Explorer software (Figure 3) showed that the HFD-male group (filled square bars) had significantly fewer bifurcation nodes (Figure 3C, P = 0.017), terminal endings (Figure 3D, P = 0.035) and dendritic segments (Figure 3E, P = 0.034, two-way ANOVA with Bonferroni’s post-hoc test) than the control male group (open square bars). Nevertheless, both groups had similar numbers of primary dendrites (Figure 3A, P > 0.999) and the highest branching order (Figure 3B, P = 0.583, Bonferonni’s post-hoc test). On the other hand, HFD did not induce a significant change in the branching pattern of basal dendrites in female mice (open circle bars vs. filled circle bars, Figure 3). Interestingly, as compared with the control male group, the basal dendrites of CA1 neurons in control females had fewer bifurcation nodes (Figure 3C, P = 0.018), terminal endings (Figure 3D, P = 0.05) and dendritic segments (Figure 3E, P = 0.045, Bonferroni’s post-hoc test).
These results suggest that middle age male mice have more dendritic arbors of CA1 pyramidal neurons than age-matched females, and that chronic HFD reduces the complexity of basal dendritic of CA1 pyramidal neurons preferentially in male mice, but not in females.
Discussion
In continuation with our previous study that revealed a sex difference in the role of chronic HFD in memory impairment of middle-aged mice (17), the present study further disclosed its possible underlying morphological evidence in hippocampal CA1 pyramidal neurons. The complexity of dendritic arbors determines the number and distribution of receptive synaptic contacts (33, 34). In hippocampal CA1 pyramidal neurons, dendritic spines are the primary postsynaptic sites for excitatory neurotransmission (35, 36). The spine number and morphology of CA1 dendritic spines are vulnerable to the experience of the subject (35, 37, 38), and their changes are associated with memory encoding and retrieval (39, 40). Reduced dendritic spines and arbors in the hippocampal neurons have been reported in animals with memory impairment (34, 36, 41) and in patients with mild cognitive impairment and AD (34, 41–43). Therefore, in the present study, the dendritic spine density and dendritic arbors of hippocampal CA1 pyramidal neurons were chosen as the structural base for the functional implications of HFD. As summarized in Table 1, there was inherent sex difference in dendritic structures of CA1 pyramidal neurons in 1 year-old normal chow-fed mice. More importantly, our results demonstrated, for the first time, that 1 year of HFD feeding in male, but not female, mice after weaning leads to impairments of dendritic spine density and complexity in hippocampal CA1 pyramidal neurons.
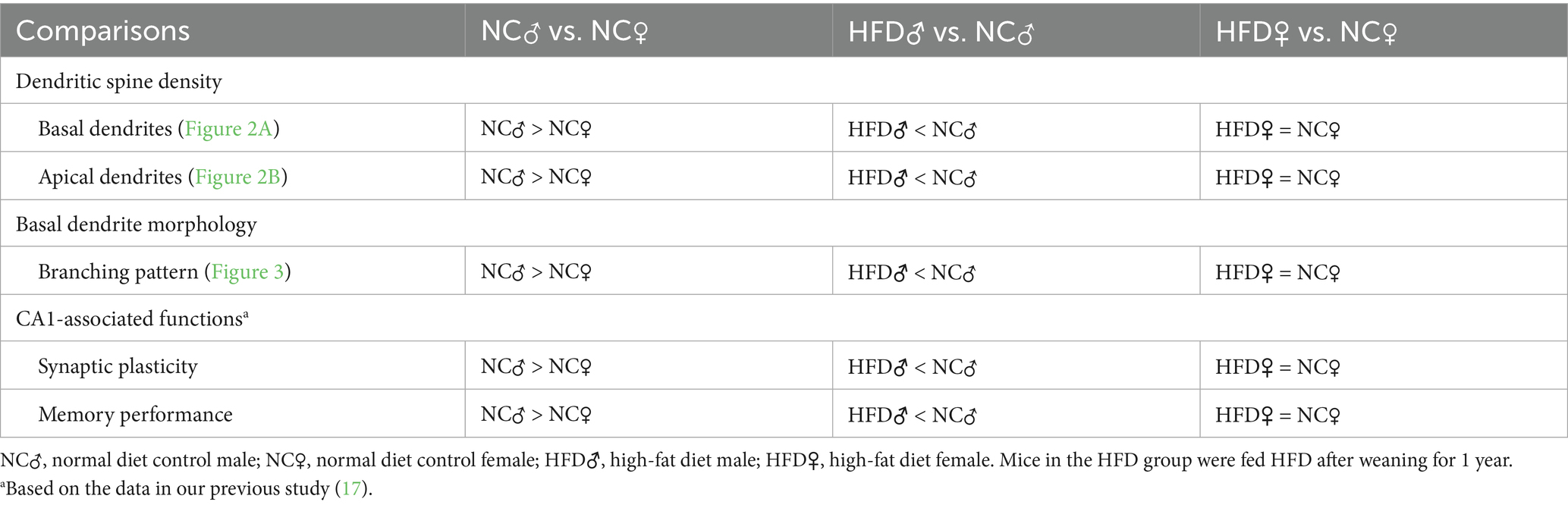
Table 1. Comparisons of hippocampal CA1 dendritic morphology, synaptic plasticity and functions between different sexes and diets.
Inherent sex difference in the dendritic spine density and morphology of hippocampal CA1 neurons of middle-aged mice
As summarized in Table 1, there was sex difference in the number of dendritic spines in both basal and apical dendrites as well as the arborization of basal dendrites of hippocampal CA1 neurons. Compared with female mice in the control group, males had a higher spine density in both basal and apical dendrites of hippocampal CA1 neurons (Figure 2). Besides, morphometric analysis also showed that the control male group had higher dendritic complexity in basal dendrites of hippocampal CA1 neurons (Figure 3). Taken together with our previous findings that control male mice, at the same age as employed here, exhibited better hippocampus-dependent memory and synaptic plasticity than age-matched control females (17), it can be speculated that higher inherent CA1 dendritic spine density in both basal and apical dendrites, and probably higher basal CA1 dendritic complexity may contribute to the inherent sex difference in hippocampus-dependent memory and synaptic plasticity in normal middle-aged mice. This observation is in-line with previous literature regarding the presence of sex difference in hippocampal-dependent memory processing in rodents (27, 44–47).
The inherent sex difference in the dendritic spine density and morphology of hippocampal CA1 neurons could largely be attributed to complex interactions of sex hormones, particularly estrogen and testosterone, which both promote the hippocampal dendritic spine density in the respective sex (48, 49). The plasma testosterone levels of male C57BL/6 mice did not drop drastically between ages 4 and 12 months (50). However, the plasma estrogen level decreased drastically in a similar age comparison (80), probably due to the gradual regression of ovarian function in female mice at 12 months.
HFD reduces the dendritic spine density and arborization of hippocampal CA1 neurons in male, but not female, mice
The impact of HFD on the dendritic spine density of hippocampal CA1 pyramidal neurons was similar between basal and apical dendrites (Figure 2). Interestingly, these changes were only observed in male but not female mice (Table 1). Similarly, HFD-induced reduction of dendritic complexity was also observed in male but not in female mice (Table 1). These male-specific changes in hippocampal dendritic morphology after HFD are coincident with our previous finding in HFD-induced decline in the performance of hippocampal CA1-dependent memory tasks and hippocampal synaptic plasticity (17). It is therefore suggested that the loss of dendritic spines in both apical and basal dendrites and the impaired basal dendritic complexity of hippocampal CA1 neurons contribute to the male-specific change in memory performance affected by HFD.
In the present study, we could not obtain sufficient CA1 neurons with thoroughly impregnated apical dendrites for analysis due to the limitation that apical dendrites were mostly truncated during sample preparation. Nonetheless, several studies have reported that the morphometric complexity of both apical and basal dendrites of hippocampal CA1 neurons was simultaneously impacted under various conditions, such as chronic stress (51), radiation (52), and chemotherapy (53). It remains to be further elucidated whether long-term HFD may affect the morphometric complexity of CA1 apical dendrites in a similar trend to basal dendrites.
HFD induces male-specific changes in hippocampal CA1 dendritic morphology, synaptic plasticity and memory performance
It has been reported that the hippocampus-dependent associative and spatial learning is associated with an increased dendritic spine density specifically in the basal, but not apical, dendrites of CA1 neurons (39, 40). Basal dendrites of CA1 neurons receive fewer inhibitory inputs from GABA interneurons than apical dendrites (54). and thus exhibit a lower threshold to induce LTP (55). Thus, basal dendrites may have a higher capacity for synaptic plasticity, the essence of memory formation (56). Nonetheless, apical dendritic spines of CA1 pyramidal cells are also crucial in memory consolidation and retrieval via LTP formation (57).
Here, we found that the spine density of both basal and apical dendrites of hippocampal CA1 neurons was decreased by chronic HFD for 12 months, which also impaired CA1 LTP and hippocampal-dependent memory performance, specifically in male mice (17). Thus, both basal and apical dendritic spine loss in male mice may contribute to their inferior performance in hippocampus-dependent memory tasks. Interestingly, previous studies showed that HFD administration in mice after weaning until 8–12 week-old enhanced LTP (58) and dendritic spine turnover (59) at hippocampal CA1 synapses, while disrupted LTP in younger (6–7 month-old) (60) and older (12 month-old) (17) mice. It would be interesting to study the gradual time-progression effect of HFD from 1 to 12 months of administration or longer on hippocampal morphology and electrophysiological properties in mice of both sexes in the future.
Possible factors contributing to HFD-induced CA1 dendritic morphological changes in different sexes
Female gonadal hormones play an important role in modulating the plasticity of dendritic spines in the central nervous system (49), particularly in the hippocampus (61, 62). Li et al. (63) has shown that hippocampal-dependent memory and dendritic spines were reduced in ovariectomized female mice in a manner improved by estrogen. Luine and Frankfurt (64) also found reduced dendritic spines on both CA1 apical and basal dendrites of ovariectomized female rats, while estrogen restored the spines only on basal, but not on apical, dendrites. Thus, it can be postulated that the surge of gonadal hormones during the estrus cycle may provide neuroprotection against HFD-induced morphological impacts in female mice. However, in female mice, there was a fluctuation in hippocampal dendritic spine density between diestrus and proestrus phases (65). In the present study, the estrus staging of the tested female mice was not measured. Given that female mice are predominantly acyclic at the age of 13 months (66–68), the impact of the estrus cycle and estrogen level is expected to be minimal. Nonetheless, the influence of the estrus cycle in these cohorts of female mice still cannot be ruled out and thus poses a limitation of the current study, which requires further studies in the future.
Neuro-inflammation due to diet-induced obesity can be induced in several brain regions, including the hippocampus, and thus results in cognitive deficit in mice (26, 69). This is due to obesity-induced systemic inflammation that leads to increased infiltration of peripheral immune cells through the blood–brain barrier, elevated pro-inflammatory cytokines, and activation of microglia in the hippocampus (70). Microglial activation induced by dietary obesity can lead to reduced hippocampal dendritic spine density, due to “synaptic stripping” (26), i.e., an internalization of synaptic terminals induced by microglia (71). It was reported that the dendrites of CA1 neurons in male obese mice fed by HFD for 3 months suffered from the same fate, although females were not examined (72). However, 13-week-old male mice have been reported to inherently have more and larger microglia cells in the hippocampus than age-matched female mice (73). Besides, the number of microglia in the mouse hippocampus does not change with age (74). It remains to be further elucidated whether the inherently more hippocampal microglial cells in male mice may result in more microglia activation and synaptic stripping after neuroinflammation due to chronic HFD-induced obesity.
Moreover, current trends indicate that the microbiota-gut-brain axis may influence cognitive functions. Long-term consumption of a high-fat diet, recognized for its impact on cognitive function (17, 75), can also alter microbiota composition (76). However, a previous preclinical study indicates that, irrespective of microbiota composition, a high-fat diet will inevitably lead to obesity (77). Nevertheless, it remained unclear if the cognitive functions, particularly those reliant on the hippocampus, are influenced by the composition of the microbiota. HFD has been demonstrated to affect the microbiome in a sex-dependent manner (78), similar to HFD-induced obesity (17, 75). Although many studies indicate estrogen-dependent variations in HFD-altered gut microbiota (78, 79), no research has directly compared the microbiota compositions of males and females in relation to hippocampus-dependent cognitive function. This may necessitate additional research on the potential sexual dimorphism in the microbiome alterations related to HFD-induced obesity and cognitive impairment.
Conclusion
The present study provides the morphological evidence showing the sex difference in CA1 neurons that could be linked to the impairment of memory performance in chronic HFD-induced obese mice. It is suggested that the deficits in the synaptic plasticity and learning and memory performance in obese male, but not female, mice are associated with the morphological changes, including the reductions in dendritic arbors and spine density, of hippocampal CA1 pyramidal neurons.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the corresponding author based on reasonable request.
Ethics statement
The animal study was approved by Institutional Animal Care and Use Committees in College of Medicine, National Taiwan University and in National Health Research Institute, Taiwan. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
S-FT: Formal analysis, Writing – original draft, Methodology, Investigation. ML: Validation, Writing – review & editing, Formal analysis, Visualization, Writing – original draft. L-JL: Data curation, Resources, Methodology, Software, Validation, Formal analysis, Writing – review & editing. L-LH: Investigation, Formal analysis, Data curation, Resources, Writing – original draft. C-PC: Methodology, Investigation, Project administration, Writing – review & editing, Data curation. H-JL: Project administration, Methodology, Investigation, Writing – original draft. C-TC: Supervision, Writing – review & editing, Methodology, Investigation, Project administration, Resources. L-CC: Investigation, Validation, Funding acquisition, Resources, Conceptualization, Writing – review & editing, Supervision.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by grants from the Ministry of Science and Technology Council, Taiwan (NSTC 114-2320-B-002-009, NSTC 114-2320-B-002-028, and NSTC 113-2320-B-002-001), National Health Research Institutes, Taiwan (NHRI-EX113-11114NI), UCSI University Research Excellence and Innovation Grant (REIG-OPS-2025/043).
Acknowledgments
We thank the imaging core at First Core Labs, National Taiwan University College of Medicine, for the technical support.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that Gen AI was used in the creation of this manuscript. Generative AI was used to improve the grammar and language of the manuscript only. After using this tool/service, the author(s) reviewed and edited the content as needed and take(s) full responsibility for the content of the publication.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2025.1687060/full#supplementary-material
References
1. Lee, SM, Shin, J, and Lee, I. Significance of visual scene-based learning in the hippocampal systems across mammalian species. Hippocampus. (2023) 33:505–21. doi: 10.1002/hipo.23483
2. Thome, A, Marrone, DF, Ellmore, TM, Chawla, MK, Lipa, P, Ramirez-Amaya, V, et al. Evidence for an evolutionarily conserved memory coding scheme in the mammalian Hippocampus. J Neurosci. (2017) 37:2795–801. doi: 10.1523/JNEUROSCI.3057-16.2017
3. Burgess, N, Maguire, EA, and O'Keefe, J. The human hippocampus and spatial and episodic memory. Neuron. (2002) 35:625–41. doi: 10.1016/s0896-6273(02)00830-9
4. Tudor, A, Vasile, AI, Trifu, SC, and Cristea, MB. Morphological classification and changes in dementia (review). Exp Ther Med. (2022) 23:33. doi: 10.3892/etm.2021.10955
5. Del Olmo, N, and Ruiz-Gayo, M. Influence of high-fat diets consumed during the juvenile period on hippocampal morphology and function. Front Cell Neurosci. (2018) 12:439. doi: 10.3389/fncel.2018.00439
6. Patel, V, and Edison, P. Cardiometabolic risk factors and neurodegeneration: a review of the mechanisms underlying diabetes, obesity and hypertension in Alzheimer's disease. J Neurol Neurosurg Psychiatry. (2024) 95:581–9. doi: 10.1136/jnnp-2023-332661
7. Cherbuin, N, Sargent-Cox, K, Easteal, S, Sachdev, P, and Anstey, KJ. Hippocampal atrophy is associated with subjective memory decline: the PATH through life study. Am J Geriatr Psychiatry. (2015) 23:446–55. doi: 10.1016/j.jagp.2014.07.009
8. van de Pol, LA, Hensel, A, van der Flier, WM, Visser, PJ, Pijnenburg, YA, Barkhof, F, et al. Hippocampal atrophy on MRI in frontotemporal lobar degeneration and Alzheimer's disease. J Neurol Neurosurg Psychiatry. (2006) 77:439–42. doi: 10.1136/jnnp.2005.075341
9. Mestre, ZL, Bischoff-Grethe, A, Eichen, DM, Wierenga, CE, Strong, D, and Boutelle, KN. Hippocampal atrophy and altered brain responses to pleasant tastes among obese compared with healthy weight children. Int J Obes. (2017) 41:1496–502. doi: 10.1038/ijo.2017.130
10. Chiesa, ST, Rader, L, Garfield, V, Foote, I, Suri, S, Davey Smith, G, et al. Childhood adiposity underlies numerous adult brain traits commonly attributed to midlife obesity. Brain. (2025) 148:133–42. doi: 10.1093/brain/awae198
11. Wang, C, Chan, JS, Ren, L, and Yan, JH. Obesity reduces cognitive and motor functions across the lifespan. Neural Plast. (2016) 2016:2473081. doi: 10.1155/2016/2473081
12. Chuang, YF, An, Y, Bilgel, M, Wong, DF, Troncoso, JC, O'Brien, RJ, et al. Midlife adiposity predicts earlier onset of Alzheimer's dementia, neuropathology and presymptomatic cerebral amyloid accumulation. Mol Psychiatry. (2016) 21:910–5. doi: 10.1038/mp.2015.129
13. Francis, H, and Stevenson, R. The longer-term impacts of Western diet on human cognition and the brain. Appetite. (2013) 63:119–28. doi: 10.1016/j.appet.2012.12.018
14. Jensen, DEA, Ebmeier, KP, Akbaraly, T, Jansen, MG, Singh-Manoux, A, Kivimaki, M, et al. Association of Diet and Waist-to-hip Ratio with Brain Connectivity and memory in aging. JAMA Netw Open. (2025) 8:e250171. doi: 10.1001/jamanetworkopen.2025.0171
15. Kleinert, M, Clemmensen, C, Hofmann, SM, Moore, MC, Renner, S, Woods, SC, et al. Animal models of obesity and diabetes mellitus. Nat Rev Endocrinol. (2018) 14:140–62. doi: 10.1038/nrendo.2017.161
16. Khazen, T, Hatoum, OA, Ferreira, G, and Maroun, M. Acute exposure to a high-fat diet in juvenile male rats disrupts hippocampal-dependent memory and plasticity through glucocorticoids. Sci Rep. (2019) 9:12270. doi: 10.1038/s41598-019-48800-2
17. Hwang, LL, Wang, CH, Li, TL, Chang, SD, Lin, LC, Chen, CP, et al. Sex differences in high-fat diet-induced obesity, metabolic alterations and learning, and synaptic plasticity deficits in mice. Obesity. (2010) 18:463–9. doi: 10.1038/oby.2009.273
18. Spencer, SJ, D'Angelo, H, Soch, A, Watkins, LR, Maier, SF, and Barrientos, RM. High-fat diet and aging interact to produce neuroinflammation and impair hippocampal- and amygdalar-dependent memory. Neurobiol Aging. (2017) 58:88–101. doi: 10.1016/j.neurobiolaging.2017.06.014
19. N'Diaye, M, Ducourneau, EG, Bakoyiannis, I, Potier, M, Lafenetre, P, and Ferreira, G. Obesogenic diet induces sex-specific alterations of contextual fear memory and associated hippocampal activity in mice. Cereb Cortex. (2024) 34. doi: 10.1093/cercor/bhae254
20. Moser, VA, and Pike, CJ. Obesity and sex interact in the regulation of Alzheimer's disease. Neurosci Biobehav Rev. (2016) 67:102–18. doi: 10.1016/j.neubiorev.2015.08.021
21. Yuste, R, and Bonhoeffer, T. Morphological changes in dendritic spines associated with long-term synaptic plasticity. Annu Rev Neurosci. (2001) 24:1071–89. doi: 10.1146/annurev.neuro.24.1.1071
22. Kasai, H, Fukuda, M, Watanabe, S, Hayashi-Takagi, A, and Noguchi, J. Structural dynamics of dendritic spines in memory and cognition. Trends Neurosci. (2010) 33:121–9. doi: 10.1016/j.tins.2010.01.001
23. Attardo, A, Fitzgerald, JE, and Schnitzer, MJ. Impermanence of dendritic spines in live adult CA1 hippocampus. Nature. (2015) 523:592–6. doi: 10.1038/nature14467
24. Mahmmoud, RR, Sase, S, Aher, YD, Sase, A, Groger, M, Mokhtar, M, et al. Spatial and working memory is linked to spine density and mushroom spines. PLoS One. (2015) 10:e0139739. doi: 10.1371/journal.pone.0139739
25. Perez-Cruz, C, Nolte, MW, van Gaalen, MM, Rustay, NR, Termont, A, Tanghe, A, et al. Reduced spine density in specific regions of CA1 pyramidal neurons in two transgenic mouse models of Alzheimer's disease. J Neurosci. (2011) 31:3926–34. doi: 10.1523/JNEUROSCI.6142-10.2011
26. Hao, S, Dey, A, Yu, X, and Stranahan, AM. Dietary obesity reversibly induces synaptic stripping by microglia and impairs hippocampal plasticity. Brain Behav Immun. (2016) 51:230–9. doi: 10.1016/j.bbi.2015.08.023
27. Stranahan, AM, Norman, ED, Lee, K, Cutler, RG, Telljohann, RS, Egan, JM, et al. Diet-induced insulin resistance impairs hippocampal synaptic plasticity and cognition in middle-aged rats. Hippocampus. (2008) 18:1085–8. doi: 10.1002/hipo.20470
28. Valladolid-Acebes, I, Fole, A, Martin, M, Morales, L, Cano, MV, Ruiz-Gayo, M, et al. Spatial memory impairment and changes in hippocampal morphology are triggered by high-fat diets in adolescent mice. Is there a role of leptin? Neurobiol Learn Mem. (2013) 106:18–25. doi: 10.1016/j.nlm.2013.06.012
29. Lee, LJ, Lo, FS, and Erzurumlu, RS. NMDA receptor-dependent regulation of axonal and dendritic branching. J Neurosci. (2005) 25:2304–11. doi: 10.1523/JNEUROSCI.4902-04.2005
30. Bolsius, YG, Meerlo, P, Kas, MJ, Abel, T, and Havekes, R. Sleep deprivation reduces the density of individual spine subtypes in a branch-specific fashion in CA1 neurons. J Sleep Res. (2022) 31:e13438. doi: 10.1111/jsr.13438
31. Pillai, AG, de Jong, D, Kanatsou, S, Krugers, H, Knapman, A, Heinzmann, JM, et al. Dendritic morphology of hippocampal and amygdalar neurons in adolescent mice is resilient to genetic differences in stress reactivity. PLoS One. (2012) 7:e38971. doi: 10.1371/journal.pone.0038971
32. Bannister, NJ, and Larkman, AU. Dendritic morphology of CA1 pyramidal neurones from the rat hippocampus: I. branching patterns. J Comp Neurol. (1995) 360:150–60. doi: 10.1002/cne.903600111
33. Kirchner, JH, Euler, L, Fritz, I, Ferreira Castro, A, and Gjorgjieva, J. Dendritic growth and synaptic organization from activity-independent cues and local activity-dependent plasticity. eLife. (2025) 12:RP87527. doi: 10.7554/eLife.87527
34. Koleske, AJ. Molecular mechanisms of dendrite stability. Nat Rev Neurosci. (2013) 14:536–50. doi: 10.1038/nrn3486
35. von Bohlen Und Halbach, O. Structure and function of dendritic spines within the hippocampus. Ann Anat. (2009) 191:518–31. doi: 10.1016/j.aanat.2009.08.006
36. Yasmin, F, Marwick, KFM, Hunter, DW, Nawaz, S, Marshall, GF, Booker, SA, et al. Absence of GluN2A in hippocampal CA1 neurons leads to altered dendritic structure and reduced frequency of miniature excitatory synaptic events. Brain Commun. (2025) 7:fcaf124. doi: 10.1093/braincomms/fcaf124
37. Fu, M, and Zuo, Y. Experience-dependent structural plasticity in the cortex. Trends Neurosci. (2011) 34:177–87. doi: 10.1016/j.tins.2011.02.001
38. Jung, CK, and Herms, J. Structural dynamics of dendritic spines are influenced by an environmental enrichment: an in vivo imaging study. Cereb Cortex. (2014) 24:377–84. doi: 10.1093/cercor/bhs317
39. Leuner, B, Falduto, J, and Shors, TJ. Associative memory formation increases the observation of dendritic spines in the hippocampus. J Neurosci. (2003) 23:659–65. doi: 10.1523/JNEUROSCI.23-02-00659.2003
40. Moser, MB, Trommald, M, and Andersen, P. An increase in dendritic spine density on hippocampal CA1 pyramidal cells following spatial learning in adult rats suggests the formation of new synapses. Proc Natl Acad Sci USA. (1994) 91:12673–5. doi: 10.1073/pnas.91.26.12673
41. Dorostkar, MM, Zou, C, Blazquez-Llorca, L, and Herms, J. Analyzing dendritic spine pathology in Alzheimer's disease: problems and opportunities. Acta Neuropathol. (2015) 130:1–19. doi: 10.1007/s00401-015-1449-5
42. Counts, SE, He, B, Nadeem, M, Wuu, J, Scheff, SW, and Mufson, EJ. Hippocampal drebrin loss in mild cognitive impairment. Neurodegener Dis. (2012) 10:216–9. doi: 10.1159/000333122
43. Scheff, SW, Price, DA, Schmitt, FA, DeKosky, ST, and Mufson, EJ. Synaptic alterations in CA1 in mild Alzheimer disease and mild cognitive impairment. Neurology. (2007) 68:1501–8. doi: 10.1212/01.wnl.0000260698.46517.8f
44. Le, AA, Lauterborn, JC, Jia, Y, Cox, CD, Lynch, G, and Gall, CM. Metabotropic NMDAR signaling contributes to sex differences in synaptic plasticity and episodic memory. J Neurosci. (2024) 44:e0438242024. doi: 10.1523/JNEUROSCI.0438-24.2024
45. Le, AA, Lauterborn, JC, Jia, Y, Wang, W, Cox, CD, Gall, CM, et al. Prepubescent female rodents have enhanced hippocampal LTP and learning relative to males, reversing in adulthood as inhibition increases. Nat Neurosci. (2022) 25:180–90. doi: 10.1038/s41593-021-01001-5
46. Le, AA, Palmer, LC, Chavez, J, Gall, CM, and Lynch, G. Sex differences in the context dependency of episodic memory. Front Behav Neurosci. (2024) 18:1349053. doi: 10.3389/fnbeh.2024.1349053
47. Torromino, G, Loffredo, V, Cavezza, D, Sonsini, G, Esposito, F, Crevenna, AH, et al. Thalamo-hippocampal pathway regulates incidental memory capacity in mice. Nat Commun. (2022) 13:4194. doi: 10.1038/s41467-022-31781-8
48. Guo, G, Kang, L, Geng, D, Han, S, Li, S, Du, J, et al. Testosterone modulates structural synaptic plasticity of primary cultured hippocampal neurons through ERK - CREB signalling pathways. Mol Cell Endocrinol. (2020) 503:110671. doi: 10.1016/j.mce.2019.110671
49. Woolley, CS, and McEwen, BS. Roles of estradiol and progesterone in regulation of hippocampal dendritic spine density during the estrous cycle in the rat. J Comp Neurol. (1993) 336:293–306. doi: 10.1002/cne.903360210
50. Eleftheriou, BE, and Lucas, LA. Age-related changes in testes, seminal vesicles and plasma testosterone levels in male mice. Gerontologia. (1974) 20:231–8. doi: 10.1159/000212019
51. Ivy, AS, Rex, CS, Chen, Y, Dube, C, Maras, PM, Grigoriadis, DE, et al. Hippocampal dysfunction and cognitive impairments provoked by chronic early-life stress involve excessive activation of CRH receptors. J Neurosci. (2010) 30:13005–15. doi: 10.1523/JNEUROSCI.1784-10.2010
52. Banerjee, S, Alexander, T, Majumdar, D, Groves, T, Kiffer, F, Wang, J, et al. Loss of C/EBPdelta exacerbates radiation-induced cognitive decline in aged mice due to impaired oxidative stress response. Int J Mol Sci. (2019) 20:885. doi: 10.3390/ijms20040885
53. Groves, TR, Farris, R, Anderson, JE, Alexander, TC, Kiffer, F, Carter, G, et al. 5-fluorouracil chemotherapy upregulates cytokines and alters hippocampal dendritic complexity in aged mice. Behav Brain Res. (2017) 316:215–24. doi: 10.1016/j.bbr.2016.08.039
54. Arai, A, Black, J, and Lynch, G. Origins of the variations in long-term potentiation between synapses in the basal versus apical dendrites of hippocampal neurons. Hippocampus. (1994) 4:1–9. doi: 10.1002/hipo.450040103
55. Kaibara, T, and Leung, LS. Basal versus apical dendritic long-term potentiation of commissural afferents to hippocampal CA1: a current-source density study. J Neurosci. (1993) 13:2391–404. doi: 10.1523/JNEUROSCI.13-06-02391.1993
56. Neves, G, Cooke, SF, and Bliss, TV. Synaptic plasticity, memory and the hippocampus: a neural network approach to causality. Nat Rev Neurosci. (2008) 9:65–75. doi: 10.1038/nrn2303
57. Turner, RW, and Richardson, TL. Apical dendritic depolarizations and field interactions evoked by stimulation of afferent inputs to rat hippocampal CA1 pyramidal cells. Neuroscience. (1991) 42:125–35. doi: 10.1016/0306-4522(91)90153-f
58. Vouimba, RM, Bakoyiannis, I, Ducourneau, EG, Maroun, M, and Ferreira, G. Bidirectional modulation of hippocampal and amygdala synaptic plasticity by post-weaning obesogenic diet intake in male rats: influence of the duration of diet exposure. Hippocampus. (2021) 31:117–21. doi: 10.1002/hipo.23278
59. Chakraborty, P, Dromard, Y, Andre, EM, Dedin, M, Arango-Lievano, M, Raner, A, et al. Meal scheduling corrects obesogenic diet induced-uncoupling of cortico-hippocampal activities supporting memory. EBioMedicine. (2025) 117:105783. doi: 10.1016/j.ebiom.2025.105783
60. Davis, JA, Paul, JR, McMeekin, LJ, Nason, SR, Antipenko, JP, Yates, SD, et al. High-fat and high-sucrose diets impair time-of-day differences in spatial working memory of male mice. Obesity. (2020) 28:2347–56. doi: 10.1002/oby.22983
61. Frick, KM, and Kim, J. Mechanisms underlying the rapid effects of estradiol and progesterone on hippocampal memory consolidation in female rodents. Horm Behav. (2018) 104:100–10. doi: 10.1016/j.yhbeh.2018.04.013
62. Frick, KM, Kim, J, and Koss, WA. Estradiol and hippocampal memory in female and male rodents. Curr Opin Behav Sci. (2018) 23:65–74. doi: 10.1016/j.cobeha.2018.03.011
63. Li, C, Brake, WG, Romeo, RD, Dunlop, JC, Gordon, M, Buzescu, R, et al. Estrogen alters hippocampal dendritic spine shape and enhances synaptic protein immunoreactivity and spatial memory in female mice. Proc Natl Acad Sci USA. (2004) 101:2185–90. doi: 10.1073/pnas.0307313101
64. Luine, V, and Frankfurt, M. Interactions between estradiol, BDNF and dendritic spines in promoting memory. Neuroscience. (2013) 239:34–45. doi: 10.1016/j.neuroscience.2012.10.019
65. Rocks, D, Cham, H, and Kundakovic, M. Why the estrous cycle matters for neuroscience. Biol Sex Differ. (2022) 13:62. doi: 10.1186/s13293-022-00466-8
66. Felicio, LS, Nelson, JF, and Finch, CE. Longitudinal studies of estrous cyclicity in aging C57BL/6J mice: II. Cessation of cyclicity and the duration of persistent vaginal cornification. Biol Reprod. (1984) 31:446–53. doi: 10.1095/biolreprod31.3.446
67. Habermehl, TL, Underwood, KB, Welch, KD, Gawrys, SP, Parkinson, KC, Schneider, A, et al. Aging-associated changes in motor function are ovarian somatic tissue-dependent, but germ cell and estradiol independent in post-reproductive female mice exposed to young ovarian tissue. Geroscience. (2022) 44:2157–69. doi: 10.1007/s11357-022-00549-9
68. Nelson, JF, Felicio, LS, Randall, PK, Sims, C, and Finch, CE. A longitudinal study of estrous cyclicity in aging C57BL/6J mice: I. Cycle frequency, length and vaginal cytology. Biol Reprod. (1982) 27:327–39. doi: 10.1095/biolreprod27.2.327
69. Miller, AA, and Spencer, SJ. Obesity and neuroinflammation: a pathway to cognitive impairment. Brain Behav Immun. (2014) 42:10–21. doi: 10.1016/j.bbi.2014.04.001
70. Guillemot-Legris, O, and Muccioli, GG. Obesity-induced Neuroinflammation: beyond the hypothalamus. Trends Neurosci. (2017) 40:237–53. doi: 10.1016/j.tins.2017.02.005
71. Kettenmann, H, Kirchhoff, F, and Verkhratsky, A. Microglia: new roles for the synaptic stripper. Neuron. (2013) 77:10–8. doi: 10.1016/j.neuron.2012.12.023
72. Cope, EC, LaMarca, EA, Monari, PK, Olson, LB, Martinez, S, Zych, AD, et al. Microglia play an active role in obesity-associated cognitive decline. J Neurosci. (2018) 38:8889–904. doi: 10.1523/JNEUROSCI.0789-18.2018
73. Guneykaya, D, Ivanov, A, Hernandez, DP, Haage, V, Wojtas, B, Meyer, N, et al. Transcriptional and translational differences of microglia from male and female brains. Cell Rep. (2018) 24:2773–2783.e6. doi: 10.1016/j.celrep.2018.08.001
74. Long, JM, Kalehua, AN, Muth, NJ, Calhoun, ME, Jucker, M, Hengemihle, JM, et al. Stereological analysis of astrocyte and microglia in aging mouse hippocampus. Neurobiol Aging. (1998) 19:497–503. doi: 10.1016/s0197-4580(98)00088-8
75. Sanz-Martos, AB, Roca, M, Plaza, A, Merino, B, Ruiz-Gayo, M, and Olmo, ND. Long-term saturated fat-enriched diets impair hippocampal learning and memory processes in a sex-dependent manner. Neuropharmacology. (2024) 259:110108. doi: 10.1016/j.neuropharm.2024.110108
76. Imdad, S, So, B, Jang, J, Park, J, Lee, SJ, Kim, JH, et al. Temporal variations in the gut microbial diversity in response to high-fat diet and exercise. Sci Rep. (2024) 14:3282. doi: 10.1038/s41598-024-52852-4
77. Rabot, S, Membrez, M, Blancher, F, Berger, B, Moine, D, Krause, L, et al. High fat diet drives obesity regardless the composition of gut microbiota in mice. Sci Rep. (2016) 6:32484. doi: 10.1038/srep32484
78. Hases, L, Stepanauskaite, L, Birgersson, M, Brusselaers, N, Schuppe-Koistinen, I, Archer, A, et al. High-fat diet and estrogen modulate the gut microbiota in a sex-dependent manner in mice. Commun Biol. (2023) 6:20. doi: 10.1038/s42003-022-04406-5
79. Acharya, KD, Graham, M, Raman, H, Parakoyi, AER, Corcoran, A, Belete, M, et al. Estradiol-mediated protection against high-fat diet induced anxiety and obesity is associated with changes in the gut microbiota in female mice. Sci Rep. (2023) 13:4776. doi: 10.1038/s41598-023-31783-6
Keywords: high-fat diet, sex difference, dendritic spine density, dendritic arbor, hippocampal CA1 neurons
Citation: Teng S-F, Lee MT, Lee L-J, Hwang L-L, Chen C-P, Lee H-J, Chen C-T and Chiou L-C (2025) High-fat diet impairs the dendritic morphology of hippocampal CA1 pyramidal neurons in male but not female mice. Front. Nutr. 12:1687060. doi: 10.3389/fnut.2025.1687060
Edited by:
Freddy Jeanneteau, INSERM U1191 Institut de Génomique Fonctionnelle (IGF), FranceReviewed by:
Marie-Pierre Moisan, INRAE Nouvelle-Aquitaine Bordeaux, FranceGuillaume Ferreira, INRA Centre Bordeaux-Aquitaine, France
Prabahan Chakraborty, SRM Institute of Science and Technology (Deemed to be University) Research Kattankulathur, India
Copyright © 2025 Teng, Lee, Lee, Hwang, Chen, Lee, Chen and Chiou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lih-Chu Chiou, bGNjaGlvdUBudHUuZWR1LnR3
†These authors have contributed equally to this work
 Shu-Fang Teng1†
Shu-Fang Teng1† Ming Tatt Lee
Ming Tatt Lee Li-Jen Lee
Li-Jen Lee Ling-Ling Hwang
Ling-Ling Hwang Lih-Chu Chiou
Lih-Chu Chiou