- 1Anhui Provincial Key Laboratory of Livestock and Poultry Product Safety, Institute of Animal Husbandry and Veterinary Medicine, Anhui Academy of Agricultural Sciences, Hefei, China
- 2College of Animal Science, Anhui Science and Technology University, Chuzhou, China
- 3Department of Animal Nutrition and Feed Science, College of Animal Science and Technology, China Agricultural University, Beijing, China
Background: The benefits of Vitamin K (VK) in mitigating inflammation have been well-established, while the underlying mechanisms remain not yet fully elucidated. This study aimed to investigate its protective effects and underlying mechanisms in LPS-induced intestinal inflammation of piglets.
Methods: In a 21-day study, 24 weaned piglets were randomly assigned to 4 groups: CON group (basal diet), VK group (basal diet + 4.5 mg/kg VK3), LPS group (basal diet + LPS challenge), LPS+VK (basal diet + 4.5 mg/kg VK3 + LPS challenge). On day 21, LPS or saline was administered to the piglets by intraperitoneal injection. The IPEC-J2 cells were treated with or without VK and LPS for 24 h and analyzed with various assays.
Results: Morphological analysis revealed that VK significantly restored the decreased villus height and ratio of villus height to crypt depth induced by LPS. VK effectively upregulated tight junction proteins claudin-1 expression. Furthermore, VK notably suppressed the overexpression of TNF-α, IL-1β, IL-6, and IL-8, as well as the concentrations of diamine oxidase (DAO) and reactive oxygen (ROS), while restoring the reduced expression of glutathione peroxidase (GSH-Px) and superoxide dismutase (SOD). The combined analysis of transcriptome and proteome, as well as the Western blot verification, indicated that VK mainly mediates the restriction of the MAPK and PI3K-AKT signaling pathways. Moreover, VK relieved gut microbiota dysbiosis, such as decreasing in Spirochaetato.
Conclusion: These results indicated that VK alleviated intestinal inflammation in piglets by inhibiting the MAPK and PI3K-AKT signaling pathways as well as the regulation of in the gut microbiota.
1 Introduction
The intestine stands as the largest immune organ in the body, essential not only for digesting and absorbing nutrients but also for effectively defending against pathogenic microorganisms (1, 2). Weaned piglets are highly predisposed to intestinal dysfunction due to stressors including environmental/dietary changes, immature digestive tracts, and compromised immunity, leading to diarrhea, growth retardation, elevated morbidity and mortality, causing substantial economic losses in the swine industry (3, 4). Moreover, the shift toward antibiotic-free feed policies has exacerbated the challenge of intestinal immune dysregulation caused by uncontrolled pathogens, posing a serious threat to the sustainable development of swine farming (5). Thus, it is vital to develop safe and efficient strategies aimed at preserving intestinal integrity for animal health and the optimization of swine farming.
Recently, interest in natural nutritional supplements has grown due to their potential to support intestinal barrier function and alleviate symptoms of gut disorders (6, 7). Vitamin K (VK) is a crucial micronutrient made up of naphthalene quinone compounds with isoprene-type side chains. In addition to its roles in blood clotting and bone health (8–10). VK is increasingly gaining attention for its effects on intestinal health. It has been reported that VK can enhance the activity of antioxidant enzymes such as superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GSH-Px), glutathione S-transferase (GST), and glutathione reductase (GSR) in the liver, pancreas, and intestines of juvenile carp, thereby exerting its antioxidant function (11). Moreover, a deficiency of VK would aggravate DSS-induced colitis in mice, while supplementing with VK reduces pro-inflammatory cytokine production and improves both shortened colon length and weight loss (12). In vitro studies show that VK effectively reduces the activation of nuclear factor kappa B (NF-κB) and inhibits IKKα/β phosphorylation, thereby suppressing inflammation in human monocyte-macrophage THP-1 cells and mouse monocyte-macrophage cells induced by lipopolysaccharide (LPS) (13). Additionally, VK can inhibit inflammation by inhibiting the production of IL-6 in human macrophages and fibroblasts (14). Although these previous studies have provided evidence for the anti-inflammatory and antioxidant functions of VK, the impact of VK on the intestinal health of weaned piglets and its underlying mechanism remain unclear.
Therefore, this study attempted to investigate the protective regulatory role of VK and its mechanisms on the intestinal health by using the LPS-challenged model in vivo and in vitro, aiming to provide a theoretical basis for the potential administration of VK in regulating the intestinal health of weaned piglets.
2 Materials and methods
2.1 Animals and experiment design
This study was performed at Animal Experiment Base of Anhui Academy of Agricultural Sciences (Hefei, China). All animal experimental procedures were in accordance with the Chinese Guidelines for Animal Welfare and Experimental Protocols and were approved by the Anhui Academy of Agriculture Science Ethics Committee (AAAS2025-12).
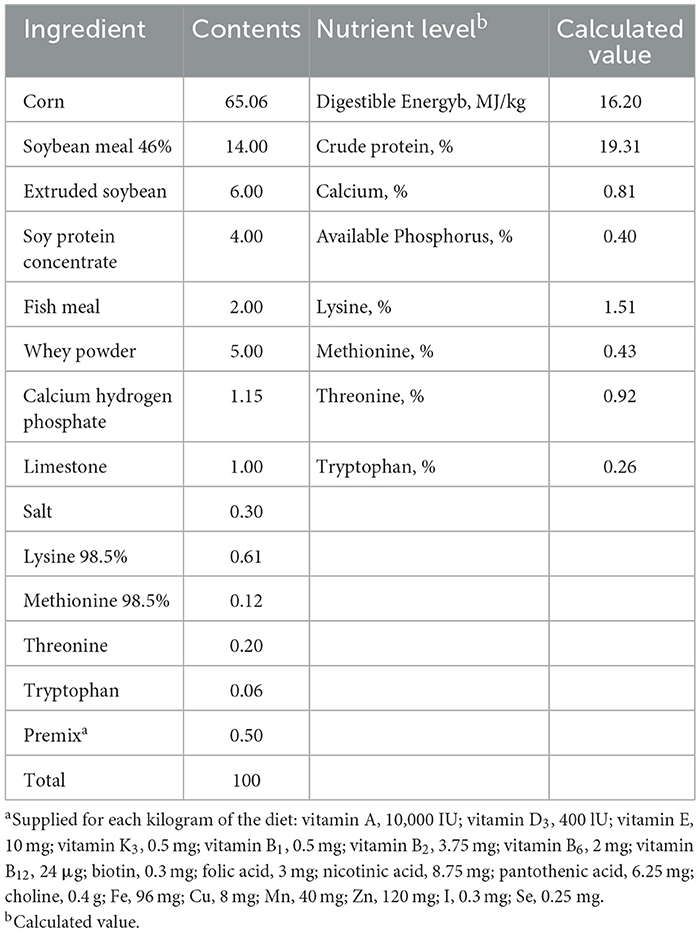
Twenty-four piglets (Duroc × Landrace × Yorkshire) aged 28 d with an average initial body weight of 7.54 ± 0.74 kg were randomly allocated into four dietary treatment groups (CON, VK, LPS, and LPS+VK groups) based on their body weight and gender, with six replicates per group and one piglet per replicate. Piglets in the CON and LPS groups were given a corn-soybean meal basal diet, and VK and LPS+VK group piglets were given a corn-soybean meal basal diet that was supplemented with 4.5 mg/kg VK3. The basal diet met the nutritional recommendations proposed by the National Research Council for piglets and nutrient levels are shown in Table 1 (15). On day 21, LPS and LPS+VK groups were injected intraperitoneally with LPS (100 μg/kg body weight; E. coli serotype 055:B5, Sigma) to mimic continued intestinal damage based on previous studies (16, 17). The piglets in the CON and VK groups were injected with the equal amount of saline. Each piglet was nursed in a single cage with a single-side feeder and nipple drinker. All the weaned piglets were housed in a room with the temperature (25–30 °C) and humidity (60% ± 10%). All piglets had unrestricted access to feed and water, and the experiment lasted for 21 day.
2.2 Growth performance and sample collection
Piglets were weighted individually on the first and last day of the experiment. Feed consumption was recorded for each piglet. Growth performance was evaluated based on the average daily gain (ADG), average daily feed intake (ADFI), and ADFI/ADG. Four hour after administering LPS or saline, blood was collected via anterior vena cava and serum was obtained through centrifugation at 3,000 × g and 4 °C for 15 min. Then, the serum samples were stored at −80 °C for subsequent analysis. After the collection of blood, all piglets were euthanized via an intravenous injection of sodium pentobarbital (50 mg/kg body weight) (18). A 2 cm jejunal segment was collected and stored in 4% paraformaldehyde for Hematoxylin-eosin (H&E) staining and immunocytochemistry. The jejunal mucosa and cecal chyme were collected, frozen with liquid nitrogen, and then stored at −80 °C for further analysis.
2.3 Cell culture and treatment
The IPEC-J2 cells (obtained from the China Center for Type Culture Collection, Wuhan, China) were cultured at 37 °C, 5% CO2 in DMEM/F12 (Thermo Scientific, Logan, UT, USA) supplemented with 10% FBS (Gibco, Grand Island, NY, USA) and 1% penicillin-streptomycin (Solarbio, Beijing, China), and the culture medium was replaced every 2 days. After the cell density reached about 80%, either VK (Sigma-Aldrich, MO, USA) or LPS (Sigma-Aldrich, MO, USA) treatment alone or combined treatment for 24 h is carried out. All experiments were conducted at least in triplicate on three separate batches.
2.4 Cell viability assay
The effect of VK and LPS on cell viability was assessed using the CCK-8 method. IPEC-J2 cells were cultured in 96-well plates for 24 h, then cultured with VK or LPS at varying levels for 24 h. Moreover, IPEC-J2 cells were treated using another protocol: the cells were inoculated with VK and LPS for 24 h. Then, 10 μL of CCK-8 reagent (Vazyme, Nanjing, China) was added to each well, incubation was continued for 1 h, and the absorbance values at 450 nm were read using a microplate reader (Bio-Rad, Hercules, CA, USA).
2.5 Intestinal morphology
The jejunal segments fixed 4% paraformaldehyde solution were dehydrated with a gradient concentration of ethanol, and embedded in paraffin blocks as previously described (16). The paraffin sections were 4 μm thick and then stained with hematoxylin and eosin. The villus height and crypt depth were measured using an automatic digital slice scanning and application system (VM1, Motic China Group Co., Ltd., China).
2.6 Serum analysis and cytokine measurement
Serum permeability indicators D-lactate, diamine oxidase (DAO), LPS, reactive oxygen (ROS), and cell supernatant cytokines (TNF-α, IL-1β, IL-6, IL-8) were determined using an ELISA kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China), according to the manufacturer's instructions.
2.7 Real-time PCR analysis
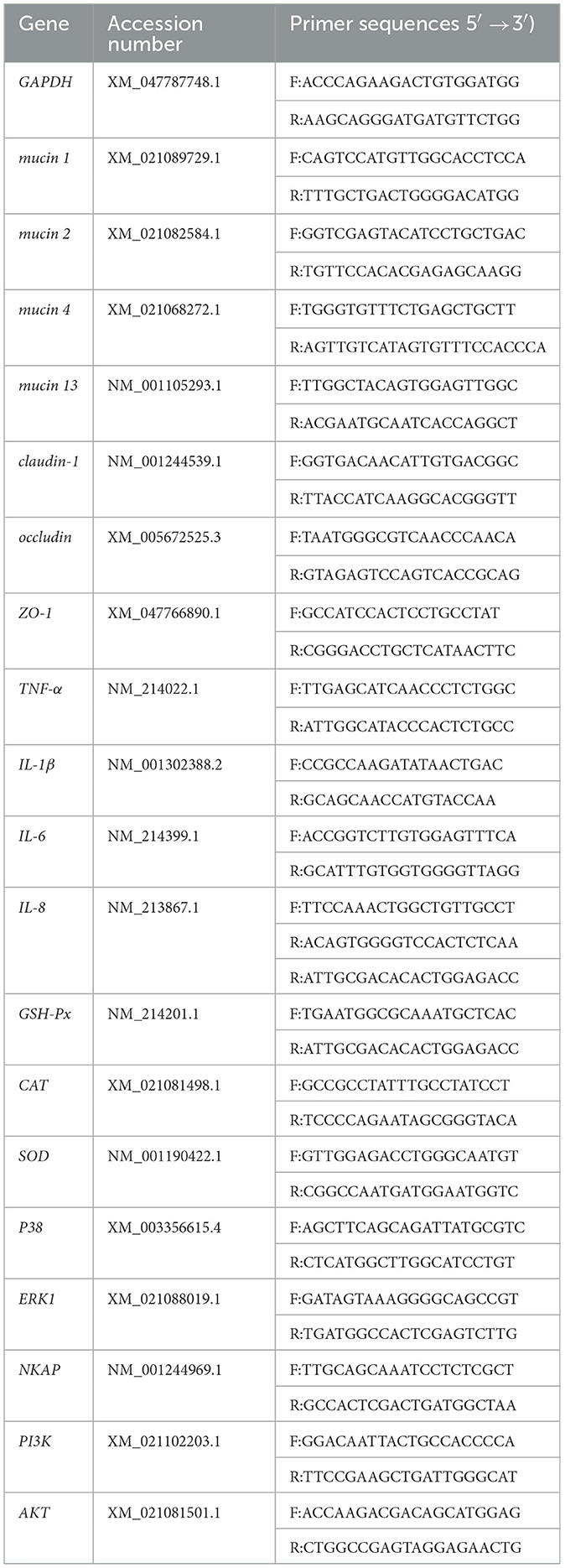
Total RNA was extracted from jejunal tissue or cells using Trizol Reagent following the manufacturer' instructions (Vazyme, Nanjing, China). The quality and concentration were determined using a Nanodrop 2000 (Thermo Fisher Scientific, Waltham, MA, USA). RNA-reverse transcription and quantitative PCR were conducted according to the previous study (12). The primer sequences are displayed in Table 2. GAPDH was used the reference gene.
2.8 Western blot analysis
Western blot analysis was performed as described previously (19). The primary antibodies were used as the following: occludin (proteintech, Cat No. 27260-1-AP), claudin-1 (proteintech, Cat No. 13050-1-AP), TNF-α (proteintech, Cat No. 60291-1-Ig), IL-1β (proteintech, Cat No. 16806-1-AP), IL-6 (proteintech, Cat No. 21865-1-AP), PI3K (ABclonal, Cat No. A4992), p-PI3K (ABclonal, Cat No. AP0427), AKT (proteintech, Cat No. 10176-2-AP), p-AKT (proteintech, Cat No. 66444-1), NF-κB p65 (CST, Cat No. 6956T), p-NF-κB p65 (CST, Cat No. 3033T), p38 (CST, Cat No. 8690T), p-p38 (CST, Cat No. 4511T), ERK (CST, Cat No. 4695T), p-ERK (CST, Cat No. 4370T), β-actin (proteintech, Cat No. 66009-1-Ig), GAPDH (proteintech, Cat No. 60004-1-Ig). Secondary antibodies were purchased from CST (Goat Anti-Mouse IgG, Cat No. 7074P2 and Goat Anti-rabbit IgG, Cat No. 91196S). The band gray value was analyzed by Image J, with β-actin or GAPDH as the internal reference.
2.9 Immunofluorescence staining
Tissue sections were deparaffinized, rehydrated, subjected to antigen retrieval, and subsequently blocked with 5% bovine serum albumin. Then, tissue sections were incubated with the primary antibody (ZO-1, occludin, claudin-1) at 4 °C overnight, followed by staining with Cy3 conjugated Goat Anti-Rabbit IgG (H + L) secondary antibody at room temperature for 50 min. Cell nucleus was stained with 4′,6-diamidino-2-phenylindole (DAPI), and the images were captured using a fluorescence microscope.
2.10 RNA-Seq analysis
Total RNA was extracted from the cells in the CON, LPS, and LPS+VK groups. The concentrations and quality of extracted RNA were checked by Nandrop 2000 and Agilent 2000 Bioanalyzer (Agilent, CA, USA). The obtained mRNA was reverse transcribed into double-stranded cDNA, purified for cDNA library construction, and finally sequenced by Guangzhou Gidi Bio-Tech Co., Ltd. (Guangzhou, China). Briefly, the raw sequencing data was filtered by adapter removal and low-quality filtering. The trimmed reads were aligned to the Sus scrofa reference genome using HISAT2, and then StringTie was used for reference genome-guided transcriptome assembly and gene expression quantification. Differentially expressed genes (DEGs) were identified using DEseq2 and genes with adjusted log2|fold-change| values and P < 0.05 were defined as DEGs. Based on GO and KEGG pathway categories, ClusterProfiler was used to conduct functional enrichment analysis for the significantly annotated DEGs and potential genes in identified modules. P < 0.05 was considered significant. Gene set enrichment analysis (GSEA) was conducted using the functions in the ClusterProfiler package, and the gene list was sorted by log2 fold-change.
2.11 Proteomic analysis
Protein extraction and digestion were done according to the previous study (20). LC-MS/MS analysis was conducted using a timsTOF Pro2 (Bruker Daltonics, MA, USA) coupled to UltiMate 3000 (Thermo Fisher Scientific, MA, USA). Approximately 200 ng of the sample was loaded onto a AUR3-15075C18 analytical column (IonOpticks, Fitzroy, Australia) and separated using a 60-min gradient at a column temperature of 50 °C. The column flow rate was controlled at 400 nL/min. The gradient started from 4% B phase (80% acetonitrile, 0.1% formic acid) and increased to 28% within 25 min, then rose to 44% within 10 min, increased to 90% within another 10 min, and remained at 90% for 7 min, with 4% returning to equilibrium after 8 min. The mass spectrometer uses the diaPASEF mode for DIA data acquisition, with the scanning range set from 349 to 1,229 m/z, and the isolation window width is set at 40 Da. During the PASEF MSMS scan process, the collision energy increases linearly with the ion mobility, rising from 59 eV (1/K0 = 1.6 Vs/cm2) to 20 eV (1/K0 = 0.6 Vs/cm2). The MS raw data for each sample was analyzed using Spectronaut18 default parameters for identification and quantitation analysis. Data analysis was performed on the online platform of Kidio Bio Cloud Platform (https://www.omicsmart.com/). Moreover, the integrated analysis of transcriptome and proteome was also performed on the online platform of Kidio Bio Cloud Platform.
2.12 Gut microbiota analysis
16S rRNA gene sequencing and bioinformatics analysis were performed as described previously (21). Bacterial V3-V4 region primer 341F (5′-CCTACGGGNGGCWGCAG-3′) and 806R (5′-GGACTACHVGGGTWTCTAAT-3′) were used for the PCR amplification of the target region.
2.13 Statistical analysis
All data in this study were statistically analyzed by using the SPSS software (Version 29.0), while graphs were generated using GraphPad Prism 9 software (GraphPad Software, La Jolla, USA). Data analysis employed the single-factor variance method, and pairwise comparisons were conducted using the t-test. Data are presented as the mean ± standard error of the mean (SEM) and P < 0.05 was considered statistically significant.
3 Results
3.1 VK ameliorated intestinal barrier injury in LPS-induced intestinal injury of piglets
There were no differences in ADG, ADFI, and F/G of piglets among the four groups (Table 3). The results of the jejunal morphology showed that the LPS group exhibited lower villus height and ratio of villus height to crypt depth compared to the CON group, whereas VK supplementation alleviated these changes (P < 0.05; Figures 1A, B). LPS adminstration significantly increased the serum LPS concentration in piglets (Figure 1C). The concentration of DAO, which was substantially increased by LPS administration, was notably restored following VK supplementation (P < 0.05; Supplementary Figure S1A). Compared with the CON group, LPS administration significantly the protein expression of claudin-1 and occludin (P < 0.05). Compared with the LPS group, the LPS+VK group exhibited a significant increase in occludin and claduin-1 protein expression (P < 0.05; Figures 1D, E, Supplementary Figure S1B). Besides, the results of gene expression showed that LPS administration decreased occludin, claudin-1, and mucin 13 gene expression (P < 0.05). In comparison to the LPS group, VK supplementation significantly increased occludin, claudin-1, and mucin 13 gene expression (P < 0.05; Supplementary Figure S1C). These results were mirrored at the IPEC-J2 cells, where similar changes were observed in LPS-challenged IPEC-J2 cells (Supplementary Figure S2D).
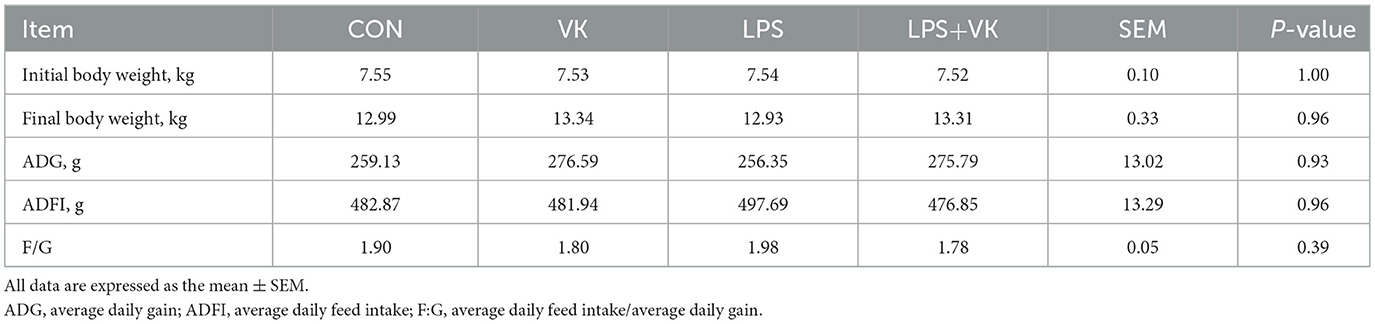
Table 3. Effects of VK supplementation on growth performance in LPS-induced intestinal injury of piglets.
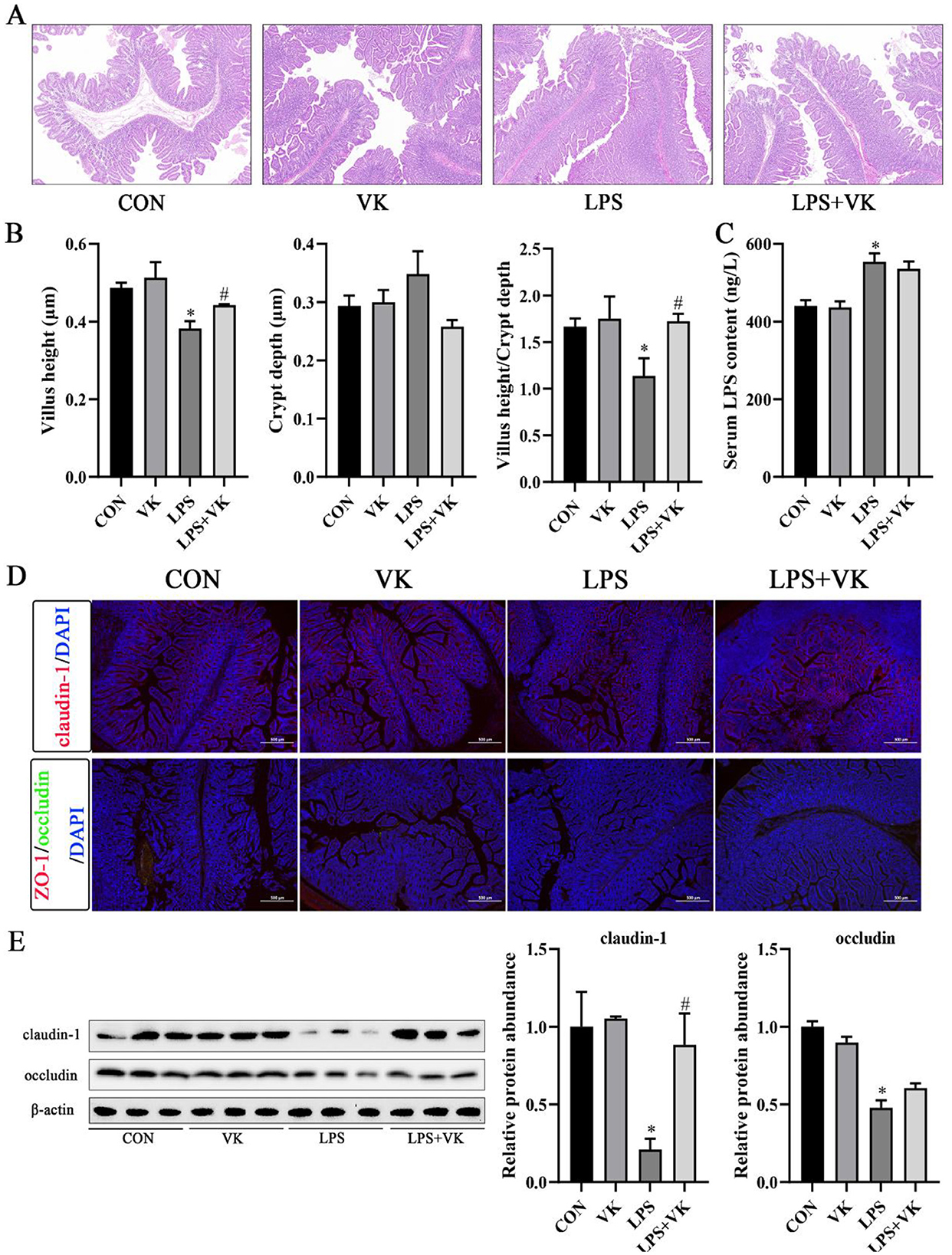
Figure 1. Effects of VK supplementation on intestinal morphology and tight junctions in LPS-induced intestinal injury of piglets. (A) HE staining of piglet jejunum. Scale bar, 200 μm; (B) Jejunal villus height, jejunal crypt depth, and ratio of villus height to crypt depth in piglets; (C) Serum LPS concentration in piglets; (D) Immunofluorescence detected the expression of claudin-1, ZO-1, and occludin in piglets (scale bar represents 500 μm); (E) Relative protein levels of claudin-1 and occludin in the jejunum. All data are expressed as the mean ± SEM. *P < 0.05 as compared to the CON group, #P < 0.05 as compared to the LPS group.
3.2 VK ameliorated inflammatory factors expression in LPS-induced intestinal injury of piglets
As shown in Figure 2A, LPS significantly promoted the protein expression of the inflammatory indicators TNF-α and IL-6 in the jejunum (P < 0.05). Supplemented with VK, however, significantly decreased the protein expression of TNF-α, IL-1β, and IL-6 (P < 0.05). Additionally, LPS-induced damage resulted in a noteworthy increase in the mRNA of TNF-α, IL-6, and IL-8 (P < 0.05). However, VK supplementation effectively restored TNF-α and IL-8 expression (P < 0.05; Figure 2B). Furthermore, these results were verified in LPS-challenged IPEC-J2 cells (Figure 2C). The concentration of serum ROS which was substantially increased by LPS administration, was notably restored following VK supplementation. Moreover, LPS administration reduced the mRNA levels of GSH-Px and SOD in the jejunum, while VK supplementation reversed these changes (P < 0.05; Figure 2D).
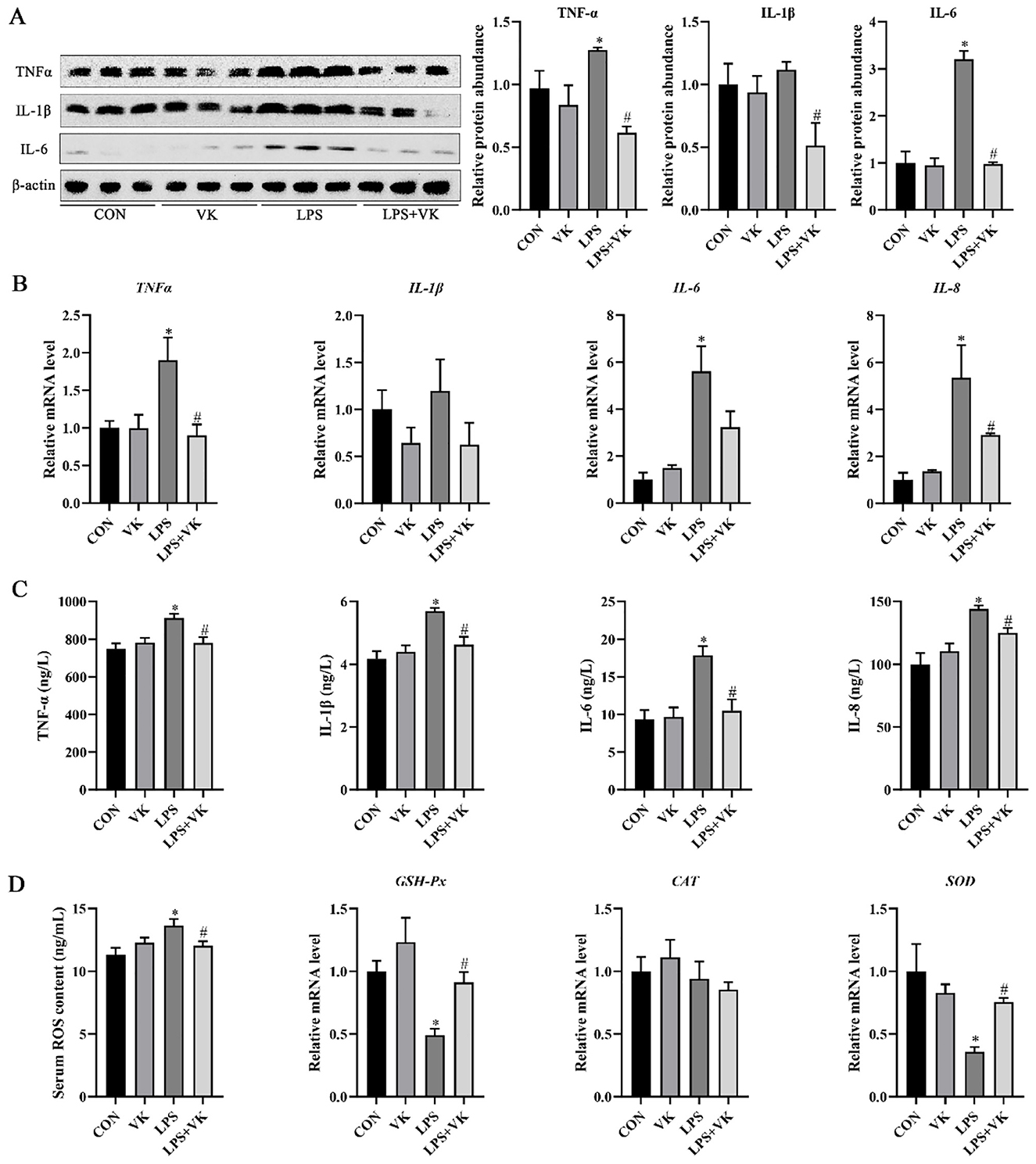
Figure 2. Effects of VK supplementation on inflammatory markers in LPS-induced intestinal injury of piglets and LPS-challenged IPEC-J2 cells. (A) Relative protein levels of TNF-α, IL-1β, and IL-6 in the jejunum; (B) Relative gene expression levels of TNF-α, IL-1β, IL-6, and IL-8 in the jejunum; (C) levels of TNF-α, IL-1β, IL-6, and IL-8 in LPS-challenged IPEC-J2 cells; (D) Serum ROS concentration and relative gene expression levels of GSH-Px, CAT, and SOD. All data are expressed as the mean ± SEM. *P < 0.05 as compared to the CON group, #P < 0.05 as compared to the LPS group.
3.3 Transcriptomic and proteomic analysis of the intestinal protective effects of VK
To further reveal the potential protective mechanism of VK on intestinal inflammation, transcriptomic and proteomic analysis were employed to examine the KEGG and GO enrichment pathways. One hundred fifty-nine DEGs were analyzed between the CON and LPS groups, with 97 genes upregulated and 62 genes downregulated. In contrast, 1,390 DEGs screened between the LPS and LPS+VK groups, comprising 87 upregulated and 1,303 downregulated genes (Figures 3A, B). The KEGG pathway enrichment analysis showed that DEGs in the CON vs. LPS and LPS vs. LPS+VK comparisons were associated with key signaling pathways (Figure 3C). Notably, 15 pathways were prominently annotated in the LPS group compared with the CON group, including the IL-17, TNF, and NF-κB signaling pathways. Similarly, the LPS+VK group exhibited enrichment in several of these same pathways. Moreover, GO enrichment analysis of DEGs revealed their biological significance, highlighting multiple functional categories (Supplementary Figures S3A, B).
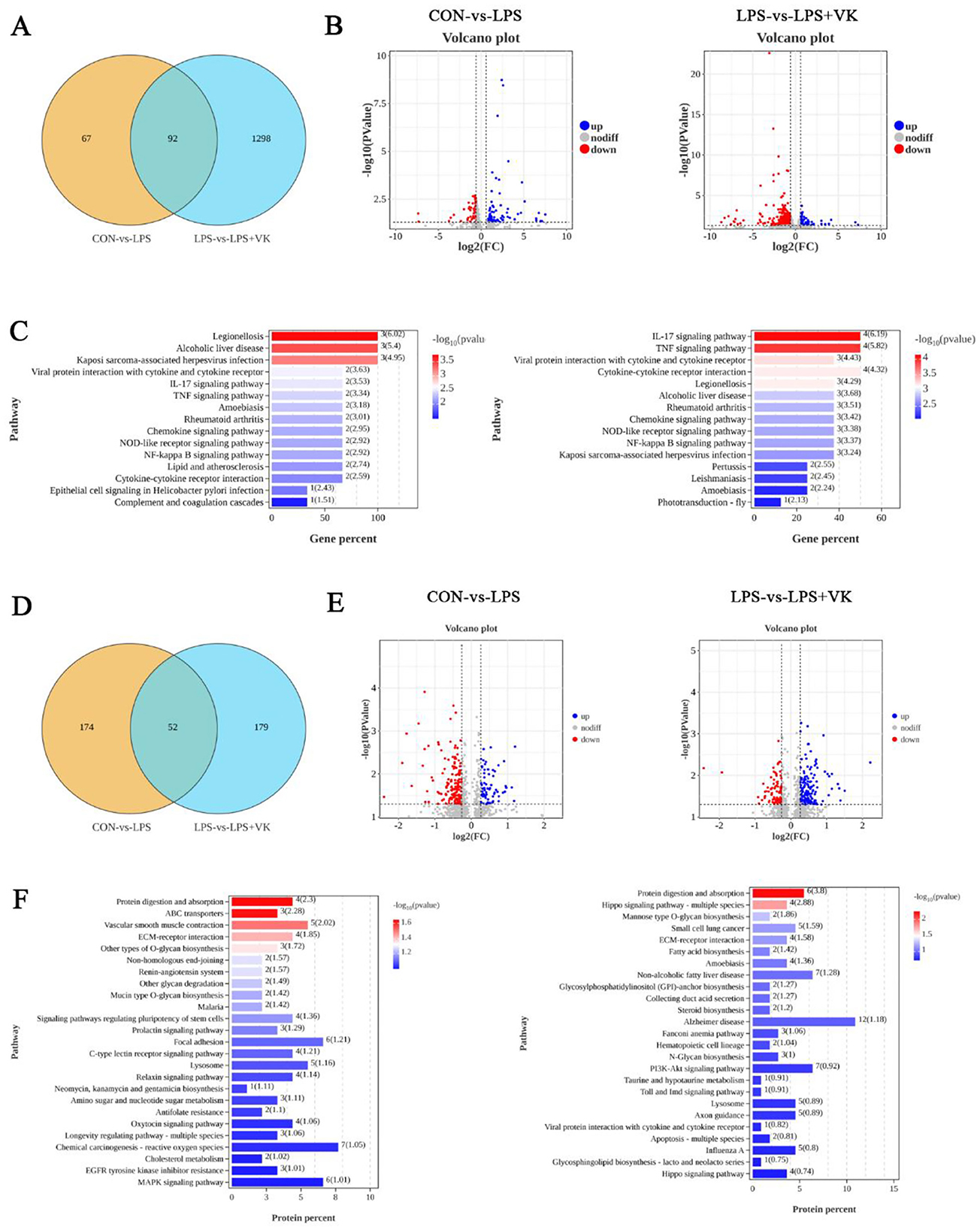
Figure 3. Transcriptomic data and proteomic data in LPS-challenged IPEC-J2 cells. (A) Venn diagram of transcriptomic data; (B) Volcano plot of gene expression from transcriptomic data; (C) KEGG enrichment of DEGs in various pathways between the CON and LPS groups, as well as between the LPS and LPS+VK groups from the transcriptomic data; (D) Venn diagram of proteomic data; (E) Volcano plot of protein expression from proteomic data; (F) KEGG enrichment of DEGs in various pathways between the CON and LPS groups, as well as between the LPS and LPS+VK groups from the proteomic data.
Two hundred twenty-six proteins were analyzed between the CON and LPS groups, with 71 proteins upregulated and 155 proteins downregulated. In contrast, 231 proteins screened between the LPS and LPS+VK groups, comprising 148 upregulated and 83 downregulated proteins (Figures 3D, E). The KEGG pathway enrichment analysis indicated that 25 pathways were prominently annotated in the LPS group compared with the CON group, including the MAPK signaling pathway. However, the LPS+VK group exhibited enrichment in the PI3K-AKT signaling pathway (Figure 3F). The enrichment of DEGs and proteins underscored the complexity of the protective mechanisms in which VK mitigated intestinal inflammation induced by LPS.
3.4 VK ameliorated intestinal inflammation by inhibiting MAPK and PI3K-AKT signaling pathways
Transcriptomic and proteomic combined analysis highlighted the role of the MAPK and PI3K-AKT signaling pathways in VK alleviating LPS-induced intestinal inflammation (Figure 4A). To verify this hypothesis, we analyzed the expression levels of key proteins in the MAPK and PI3K-AKT pathways. Results showed that LPS administration led to an obvious increase in the mRNA levels of p-38, ERK-1, NKAP, PI3K, and AKT (Supplementary Figure S4), as well as the phosphorylation levels of p-p38, p-ERK, p-NF-κB p65, p-PI3K, and p-AKT, while VK supplementation significantly reversed these changes (Figures 4B, C). Moreover, the levels of nonphosphorylated proteins of p38, ERK, NF-κB p65, PI3K, and AKT remained largely unchanged. These findings demonstrated that the underlying mechanism of VK against LPS-induced intestinal dysfunction was primarily mediated through attenuation of the excessive activation of the MAPK and PI3K-AKT signaling pathways.
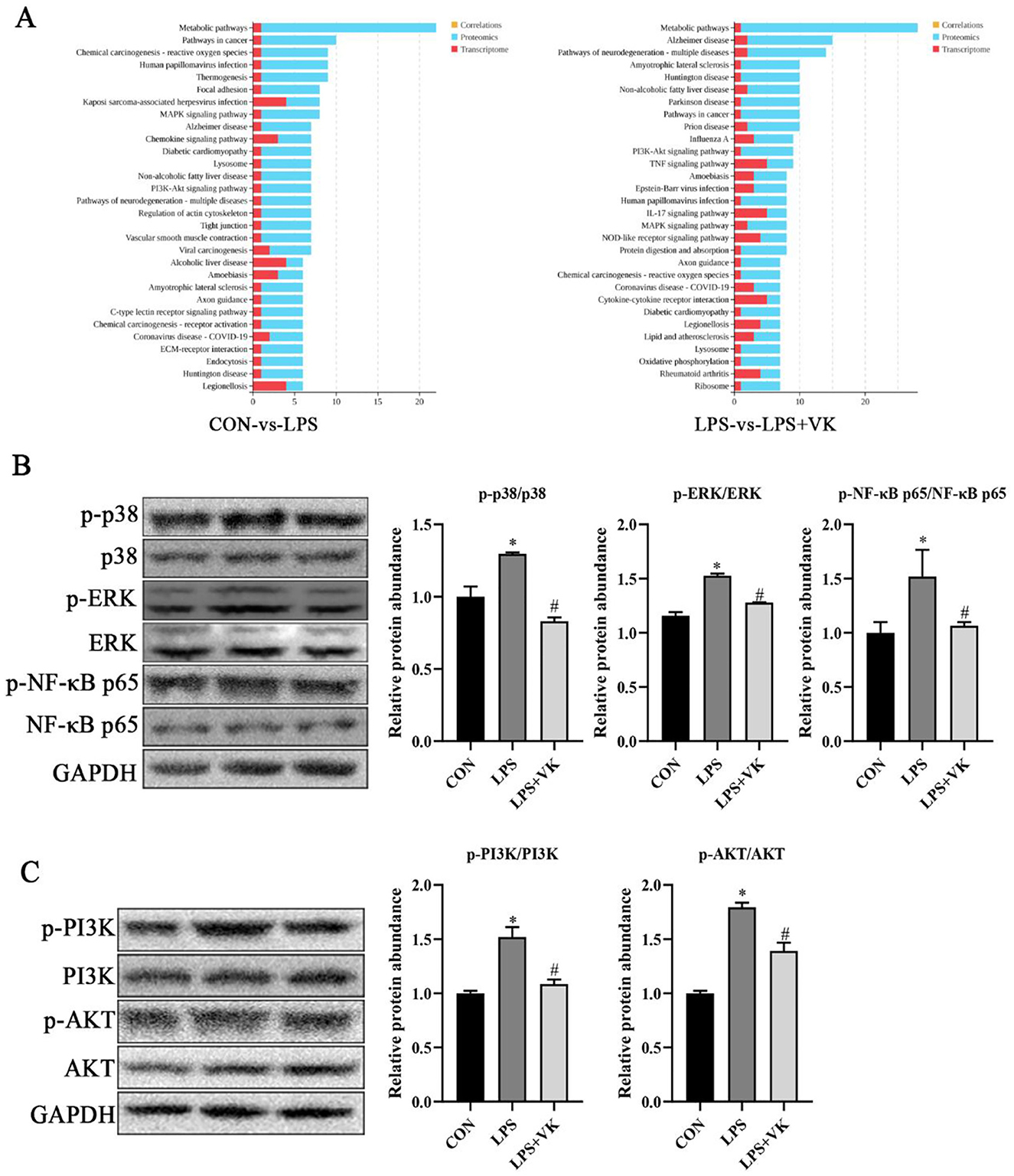
Figure 4. Effects of VK supplementation on related pathways in LPS-challenged IPEC-J2 cells. (A) KEGG enrichment of DEGs and proteins in various pathways between the CON and LPS groups, as well as between the LPS and LPS+VK groups from transcriptome and proteome combined analysis; (B) Relative protein levels of p-p38, p38, p-ERK, ERK, p-NF-κB p65, and NF-κB p65; (C) Relative protein levels of p-PI3K, PI3K, p-AKT, and AKT. All data are expressed as the mean ± SEM. *P < 0.05 as compared to the CON group, #P < 0.05 as compared to the LPS group.
3.5 VK protected against disruption of gut microbiota in LPS-induced intestinal injury of piglets
The effects of VK on gut microbiota was investigated by using 16S rRNA sequencing. As shown in Figure 5A, various α-diversity indices reflected species richness and evenness across the groups. Supplemented with VK decreased the ACE and Chao1 indexes compared with the LPS group (P < 0.05). PCoA analysis showed that a distinct separation among the LPS and LPS+VK groups (Figure 5B). Figure 5C shows the relative abundance of the intestinal flora at the phylum level. Compared with the LPS group, Spirochaetato showed a significant decrease in the LPS+VK group (P < 0.05). Furthermore, VK also influenced the intestinal flora at the genus levels (Figure 5D), increasing Faecalibacterium, Phascolarctobacterium, Limosilactobacillus, and Lactobacillus while reducing Treponema, Macellibacteroides, Anaerocolumna, Anaerocella, Quinella, and Anaerosporobacter levels (P < 0.05).
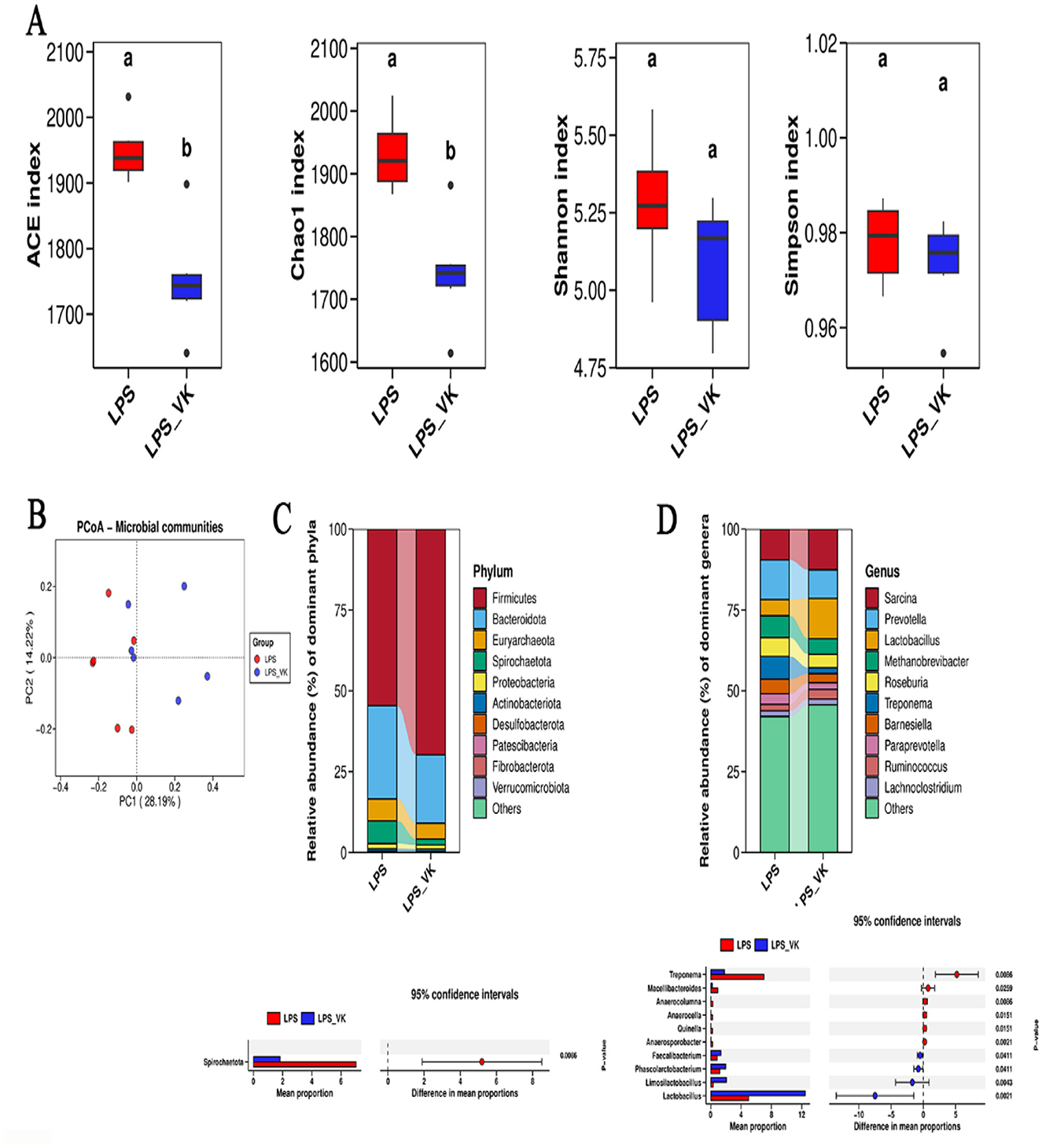
Figure 5. Effects of VK supplementation on gut microflora in LPS-induced intestinal injury of piglets. (A) Alpha diversity index of ACE, Chao 1, Shannon, and Simpson; (B) Principal component analysis; (C) Abundance of the intestinal microbiota at the phylum levels; (D) Abundance of the intestinal microbiota at the genus levels. All data are expressed as the mean ± SEM. a, bvalues with different superscripts mean significant difference (P < 0.05).
4 Discussion
Early weaning induces weaning stress in piglets, leading to gut microbiota dysbiosis and mucosal barrier dysfunction, which manifests as reduced physical condition, severe diarrhea, compromised immunity, and systemic inflammatory response (22, 23). Therefore, reducing intestinal mucosal barrier damage and inflammation while maintaining a healthy gut microbiota during the weaning period is crucial for ensuring swine production, animal welfare, and consumer safety. LPS, also as known endotoxin, binds to the TLR4 receptor on host cells to initiate intracellular signaling pathways, leading to the production and release of inflammatory cytokines, and is commonly administered via intraperitoneal injection to establish intestinal injury models in weaning piglets (24–26). VK, a naphthoquinone compound with an isoprene-type side chain, plays a critical role in regulating the immune responses, oxidative stress, and intestinal health, among other functions (11, 12, 27). However, its potential mechanism of operation on intestinal health still remains unknown. Therefore, we established the LPS-induced piglet and cell models to explore the protective effects of VK on relieving inflammatory response.
Consistent with the previous study (28), our results indicated that LPS disrupted the morphological structure of the piglet jejunum, mainly manifested as a decrease in villus length and the ratio of villus height to crypt depth. Serum D-lactate and DAO can be used as indicators of intestinal integrity, as they are usually present in extremely low concentrations in the bloodstream, but their levels will significantly increase when the mucosa is damaged (29). The present study showed that the LPS challenge led to a significant increase in LPS and DAO levels. These biological phenotypic phenomena demonstrated that we have successfully established an experimental model of intestinal inflammation in piglets (30). However, VK supplementation effectively maintained jejunal villi morphology and reduced the DAO level in LPS-challenged piglets, which indicated that VK may have protective effects against intestinal barrier injury in response to LPS stimulation. Tight junction proteins, such as occludin, claudin-1, and ZO-1, prevent intestinal inflammation by separating the internal and external environments and blocking the passage of potentially harmful substances (31, 32). VK supplementation restored the LPS-induced reduction in these tight junction proteins, thus improving the intestinal barrier function in piglets. Consistent with this, our earlier research also confirmed that VK supplementation elevated occludin, claudin-1, and ZO-1 expression in colitis mice (12). Taken together, VK supplementation has a protective effect on the intestinal integrity and barrier function of piglets.
Although environmental factors, feed, bacteria, and viruses induce intestinal inflammation through distinct mechanisms, a shared feature is that once initiated, the inflammation becomes amplified, resulting in the production and release of large amounts of inflammatory cytokines that cause severe damage to the intestinal tract (33). As it has been known, the TNF-α, IL-1β, IL-6, and IL-8 are typical pro-inflammatory cytokines (34). In the present study, the LPS administration promoted the expression and secretion of pro-inflammatory cytokines such as TNF-α, IL-1β, IL-6, and IL-8 in both the jejunum and IPEC-J2 cells compared to the CON group. In contrast, VK supplementation reduced the expression of these pro-inflammatory cytokines, thereby improving the intestinal inflammation in piglets. In agreement with the present study, Wang et al. (12) and Shiraishi et al. (35) demonstrated that VK exerts a protective effect against DSS colitis by down-regulating TNF-α, IL-1β, and IL-6 and up-regulating IL-10. Furthermore, oxidative stress plays a crucial role in damaging the intestinal barrier function and causing intestinal diseases (36). In this study, VK supplementation enhanced the antioxidant capacity of LPS-challenged piglet by reducing the ROS concentration and increasing the mRNA expression of GSH-Px and SOD. These results aligned with the previous study that mitigated oxidative stress by enhancing antioxidant capacity in the intestine of juvenile Jian carp (11).
Transcriptomic analysis is employed to explore gene expression differences under specific conditions through high-throughput sequencing technology, thereby identifying key regulatory genes, revealing their functional mechanisms, and elucidating the molecular regulatory networks of biological processes (37). The proteomic analysis systematically examines protein dynamics in biological samples, revealing the mechanisms underlying life activities (38). In this study, VK modulated DEGs and proteins through intricate physiological and biochemical processes. KEGG enrichment results of the combined analysis of transcriptome and proteomic analysis indicated that the DEGs and proteins in the VK-treated group were predominantly associated with some crucial pathways, such as MAPK and PI3K-AKT. Previous study has demonstrated that VK has the potential to alleviate LPS-induced inflammation by inhibiting NF-κB and IKKα/β phosphorylation (13). Additionally, VK has been proven to suppress pyroptosis by restraining the NF-κB and JNK pathways in THP-1 Cells (39). MAPK is an upstream target of the NF-κB signaling pathway, and its key components include ERK and p38 (40). Zhang et al. proved that low-molecular-weight chitosan attenuated LPS-induced inflammation by suppressing the NF-κB pathway in IPEC-J2 cells (41). Furthermore, chalcone could ameliorated DSS-induced colitis by inhibiting ERK and p38 phosphorylation levels in mice (42). Consistent with these findings, key proteins within the MAPK pathway, including p-p38, p-ERK, and p-NF-κB p65, were obviously elevated by LPS, but VK supplementation effectively inhibited their phosphorylation levels in the present study. A recent study has revealed that the activation of the PI3K-Akt signaling pathway is involved in the development process of ulcerative colitis (43). Furthermore, another study has found that inhibiting the phosphorylation of proteins in the PI3K-AKT signaling pathway can produce a significant anti-inflammatory effect in the piglets (19). These evidences suggest that inhibiting the PI3K-Akt signaling pathway can be regarded as a novel therapeutic strategy for intestinal inflammation. In the present study, LPS administration increased the mRNA levels of PI3K and AKT, as well as the protein levels of p-PI3K and p-AKT, while VK supplementation effectively inhibited their expression. Collectively, the anti-inflammatory role of VK was mainly attributed to the modulation in the excessive activation of the MAPK and PI3K-AKT signaling pathways induced by LPS.
Gut microbiota are crucial for maintaining health and play a significant role in the pathogenesis of gastrointestinal diseases, making them an integral component of the host organism (44). A stable gut microbiota serves as a vital barrier against pathogen invasion and disruption of this balance can lead to various diseases, such as infectious diarrhea, inflammatory bowel disease, irritable bowel syndrome, etc. (45). Spirochaetota can cause both acute and chronic inflammation through mechanisms such as the direct release of virulence factors, the deposition of immune complexes, and molecular mimicry, which affects multiple organ systems (46, 47). VK supplementation reduced the abundance of Spirochaetota in the LPS-induced piglets. Faecalibacterium, Phascolarctobacterium, Limosilactobacillus, and Lactobacillus are beneficial bacteria in the gut. Faecalibacterium is linked to various health tissues, including colorectal cancer, dermatitis, and depression (48). Phascolarctobacterium promotes the production of short-chain fatty acids, modulating the innate immune system in mice and thereby alleviating obesity and metabolic disorders (49). Limosilactobacillus and Lactobacillus are almost naturally present in the intestines of all vertebrates and mammals, and it can regulate the body's immunity (50, 51). LPS-induced inflammation in this study resulted in a reduction of these bacteria, which was successfully mitigated by VK supplementation, preserving these beneficial bacteria. Treponema, a harmful bacteria in the gut, has been reported to be associated with the occurrence of periodontal disease (52). VK supplementation effectively regulated the abundance of Treponema, thus promoting the microbial balance within the gut.
5 Conclusion
In conclusion, VK effectively suppressed LPS-induced intestinal inflammation, restored intestinal integrity, and enhanced barrier function. The anti-inflammatory mechanisms of VK were associated with the inhibition of MAPK and PI3K-AKT signaling pathways as well as the modulation in the gut microbiota. These findings highlight the potential of VK as a natural feed additive to alleviate intestinal dysfunction.
Data availability statement
The original contributions presented in the study are publicly available. This data can be found here: https://www.ncbi.nlm.nih.gov/sra/PRJNA1348319.
Ethics statement
The animal study was approved by Anhui Academy of Agriculture Science Ethics Committee (AAAS2025-12). The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
HW: Writing – original draft, Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation. RM: Investigation, Methodology, Project administration, Resources, Writing – review & editing. RQ: Methodology, Project administration, Resources, Writing – review & editing. ZL: Methodology, Validation, Visualization, Writing – review & editing. YL: Conceptualization, Project administration, Writing – review & editing. XW: Project administration, Writing – review & editing. CZ: Conceptualization, Writing – review & editing. QM: Conceptualization, Methodology, Supervision, Writing – review & editing. KZ: Conceptualization, Methodology, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was funded by Anhui Postdoctoral Scientific Research Program Foundation (2025C1110).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2025.1704168/full#supplementary-material
References
1. Vancamelbeke M, Vermeire S. The intestinal barrier: a fundamental role in health and disease. Expert Rev Gastroenterol Hepatol. (2017) 11:821–34. doi: 10.1080/17474124.2017.1343143
2. Liu G, Liu X, Wang F, Jia G, Zhao H, Chen X, et al. Effects of dietary glutamine supplementation on the modulation of microbiota and Th17/Treg immune response signaling pathway in piglets after lipopolysaccharide challenge. J Nutr. (2024) 154:1711–21. doi: 10.1016/j.tjnut.2024.02.014
3. Mooyottu S, Muyyarikkandy MS, Yousefi F, Li G, Sahin O, Burrough E, et al. Fecal microbiota transplantation modulates jejunal host-microbiota interface in weanling piglets. Microbiome. (2025) 13:45. doi: 10.1186/s40168-025-02042-9
4. Xu J, Qiao H, Gan L, Wang P, Zhao Y, Lei Z, et al. Impacts of zinc caproate supplementation on growth performance, intestinal health, anti-inflammatory activity, and Zn homeostasis in weaned piglets challenged with Escherichia coli K88. J Anim Sci Biotechnol. (2025) 16:44. doi: 10.1186/s40104-025-01172-2
5. Xu J, Andrani M, Kjærup RB, Dalgaard TS, Eriksen C, Laustsen AH, et al. In-feed provision of binding proteins sustains piglet gut health and mitigates ETEC-induced post-weaning diarrhea. J Anim Sci Biotechnol. (2025) 16:78. doi: 10.1186/s40104-025-01209-6
6. Han M, Song P, Huang C, Rezaei A, Farrar S, Brown MA, et al. Dietary grape seed proanthocyanidins (GSPs) improve weaned intestinal microbiota and mucosal barrier using a piglet model. Oncotarget. (2016) 7:80313–26. doi: 10.18632/oncotarget.13450
7. Zhao L, Geng T, Sun K, Su S, Zhao Y, Bao N, et al. Proteomic analysis reveals the molecular mechanism of Hippophae rhamnoides polysaccharide intervention in LPS-induced inflammation of IPEC-J2 cells in piglets. Int J Biol Macromol. (2020) 164:3294–304. doi: 10.1016/j.ijbiomac.2020.08.235
8. Wang H, Zhang N, Li L, Yang P, Ma Y. Menaquinone 4 reduces bone loss in ovariectomized mice through dual regulation of bone remodeling. Nutrients. (2021) 13:2570. doi: 10.3390/nu13082570
9. Wang H, Li L, Zhang N, Ma Y. Vitamin K2 improves osteogenic differentiation by inhibiting STAT1 via the Bcl-6 and IL-6/JAK in C3H10 T1/2 Clone 8 cells. Nutrients. (2022) 14:2934. doi: 10.3390/nu14142934
10. Mishima E, Wahida A, Seibt T, Conrad M. Diverse biological functions of vitamin K: from coagulation to ferroptosis. Nat Metab. (2023) 5:924–32. doi: 10.1038/s42255-023-00821-y
11. Yuan J, Feng L, Jiang WD, Liu Y, Jiang J, Li SH, et al. Effects of dietary vitamin K levels on growth performance, enzyme activities and antioxidant status in the hepatopancreas and intestine of juvenile Jian carp (Cyprinus carpio var. Jian). Aquac Nutr. (2014) 22:352–66. doi: 10.1111/anu.12264
12. Wang H, Liu Z, Zhan K, Ma Q, Xu L, Li Y. et al. Vitamin K2 alleviates dextran sulfate sodium-induced colitis via inflammatory responses, gut barrier integrity, and the gut microbiota in mice. Int J Biol Macromol. (2024) 280:136091. doi: 10.1016/j.ijbiomac.2024.136091
13. Ohsaki Y, Shirakawa H, Miura A, Giriwono PE, Sato S, Ohashi A, et al. Vitamin K suppresses the lipopolysaccharide-induced expression of inflammatory cytokines in cultured macrophage-like cells via the inhibition of the activation of nuclear factor κB through the repression of IKKα/β phosphorylation. J Nutr Biochem. (2010) 21:1120–6. doi: 10.1016/j.jnutbio.2009.09.011
14. Reddi K, Henderson B, Meghji S, Wilson M, Poole S, Hopper C, et al. Interleukin 6 production by lipopolysaccharide-stimulated human fibroblasts is potently inhibited by naphthoquinone (vitamin K) compounds. Cytokine. (1995) 7:287–90. doi: 10.1006/cyto.1995.0034
15. National Research Council. Nutrient Requirements of Swine: Eleventh revised Edition. Washington: The National Academies Press (2012).
16. Xun W, Ji M, Ma Z, Deng T, Yang W, Hou G, et al. Dietary emodin alleviates lipopolysaccharide-induced intestinal mucosal barrier injury by regulating gut microbiota in piglets. Anim Nutr. (2023) 14:152–62. doi: 10.1016/j.aninu.2023.05.004
17. Cui C, Wei Y, Wang Y, Ma W, Zheng X, Wang J, et al. Dietary supplementation of benzoic acid and essential oils combination enhances intestinal resilience against LPS stimulation in weaned piglets. J Anim Sci Biotechnol. (2024) 15:4. doi: 10.1186/s40104-023-00958-6
18. Zhang Y, Deng ZX, He ML, Pastor JJ, Tedo G, Liu JX, et al. Olive oil cake extract stabilizes the physiological condition of lipopolysaccharide-challenged piglets by reducing oxidative stress and inflammatory responses and modulating the ileal microbiome. Food Funct. (2021) 12:10171–83. doi: 10.1039/D0FO03012K
19. Chen Y, Luo C, Zhan Z, Liu S, Teng C, Mao R, et al. Limosilactobacillus reuteri SXDT-32-derived shikimic acid protects against colonic inflammation in piglets by inhibiting the PI3K-Akt pathway. J Anim Sci Biotechnol. (2025) 16:84. doi: 10.1186/s40104-025-01221-w
20. Li B, Zheng S, Yin S, Chen J, He Y, Yao J, et al. Integrated transcriptome and proteome analyses of β-Conglycinin-induced intestinal damage in piglets. J Agric Food Chem. (2024) 72:6601–12. doi: 10.1021/acs.jafc.3c06329
21. Zhang Y, Duan X, Wassie T, Wang HH Li T, Xie C, et al. Enteromorpha prolifera polysaccharide-zinc complex modulates the immune response and alleviates LPS-induced intestinal inflammation via inhibiting the TLR4/NF-κB signaling pathway. Food Funct. (2022) 13:52–63. doi: 10.1039/D1FO02171K
22. Campbell JM, Crenshaw JD, Polo J. The biological stress of early weaned piglets. J Anim Sci Biotechnol. (2013) 4:19. doi: 10.1186/2049-1891-4-19
23. Moeser AJ, Pohl CS, Rajput M. Weaning stress and gastrointestinal barrier development: implications for lifelong gut health in pigs. Anim Nutr. (2017) 3:313–21. doi: 10.1016/j.aninu.2017.06.003
24. Hu R, Wu S, Li B, Tan J, Yan J, Wang Y, et al. Dietary ferulic acid and vanillic acid on inflammation, gut barrier function and growth performance in lipopolysaccharide-challenged piglets. Anim Nutr. (2022) 8:144–52. doi: 10.1016/j.aninu.2021.06.009
25. He J, Huang C, Kong L, Huang Y, Ou Z, Yang M, et al. Betulinic acid mitigates lipopolysaccharide-induced intestinal injury of weaned piglets through modulation of the mitochondrial quality control. Int Immunopharmacol. (2025) 148:114097. doi: 10.1016/j.intimp.2025.114097
26. Hu P, Zhao F, Wang J, Zhu W. Lactoferrin attenuates lipopolysaccharide-stimulated inflammatory responses and barrier impairment through the modulation of NF-κB/MAPK/Nrf2 pathways in IPEC-J2 cells. Food Funct. (2020) 11:8516–26. doi: 10.1039/D0FO01570A
27. Xie Y, Li S, Wu D, Wang Y, Chen J, Duan L, et al. Vitamin K: infection, inflammation, and auto-immunity. J Inflamm Res. (2024) 17:1147–60. doi: 10.2147/JIR.S445806
28. Liu Y, Xu Q, Wang Y, Liang T, Li X, Wang D, et al. Necroptosis is active and contributes to intestinal injury in a piglet model with lipopolysaccharide challenge. Cell Death Dis. (2021) 12:62. doi: 10.1038/s41419-020-03365-1
29. Wen X, Wan F, Wu Y, Liu L, Liu Y, Zhong R, et al. Caffeic acid supplementation ameliorates intestinal injury by modulating intestinal microbiota in LPS-challenged piglets. Food Funct. (2023) 14:7705–17. doi: 10.1039/D3FO02286B
30. Li J, Feng S, Pi Y, Jiang X, Li X, Zhou Z, et al. Limosilactobacillus johnsoni and Limosilactobacillus mucosae and their extracellular vesicles alleviate gut inflammatory injury by mediating macrophage polarization in a lipopolysaccharide-challenged piglet model. J Nutr. (2023) 153:2497–511. doi: 10.1016/j.tjnut.2023.06.009
31. Groschwitz KR, Hogan SP. Intestinal barrier function: molecular regulation and disease pathogenesis. J Allergy Clin Immunol. (2009) 124:3–20. doi: 10.1016/j.jaci.2009.05.038
32. Slifer ZM, Blikslager AT. The integral role of tight junction proteins in the repair of injured intestinal epithelium. Int J Mol Sci. (2020) 21:972. doi: 10.3390/ijms21030972
33. Yang Q, Wang Y, Jia A, Wang Y, Bi Y, Liu G. The crosstalk between gut bacteria and host immunity in intestinal inflammation. J Cell Physiol. (2021) 236:2239–54. doi: 10.1002/jcp.30024
34. Wu C, Xu Q, Wang R, Qin L, Peng X, Hu L, et al. Effects of dietary β-glucan supplementation on growth performance and immunological and metabolic parameters of weaned pigs administered with Escherichia coli lipopolysaccharide. Food Funct. (2018) 9:3338–43. doi: 10.1039/C7FO01980G
35. Shiraishi E, Iijima H, Shinzaki S, Nakajima S, Inoue T, Hiyama S, et al. Vitamin K deficiency leads to exacerbation of murine dextran sulfate sodium-induced colitis. J Gastroenterol. (2016) 51:346–56. doi: 10.1007/s00535-015-1112-x
36. Bhattacharyya A, Chattopadhyay R, Mitra S, Crowe SE. Oxidative stress: an essential factor in the pathogenesis of gastrointestinal mucosal diseases. Physiol Rev. (2014) 94:329–54. doi: 10.1152/physrev.00040.2012
37. Lowe R, Shirley N, Bleackley M, Dolan S, Shafee T. Transcriptomics technologies. PLoS Comput Biol. (2017) 13:e1005457. doi: 10.1371/journal.pcbi.1005457
38. Rozanova S, Barkovits K, Nikolov M, Schmidt C, Urlaub H, Marcus K. Quantitative mass spectrometry-based proteomics: an overview. Methods Mol Biol. (2021) 2228:85–116. doi: 10.1007/978-1-0716-1024-4_8
39. Zhao C, Zhou Z, Wu X, Wang Y, Zuo L, Zheng R, et al. Vitamin K3 suppresses pyroptosis in THP-1 cells through inhibition of NF-κB and JNK signaling pathways. Biol Pharm Bull. (2023) 46:52–60. doi: 10.1248/bpb.b22-00522
40. Lv Y, Qian Y, Ou-Yang A, Fu L. Hydroxysafflor yellow A attenuates neuron damage by suppressing the lipopolysaccharide-induced TLR4 pathway in activated microglial cells. Cell Mol Neurobiol. (2016) 36:1241–56. doi: 10.1007/s10571-015-0322-3
41. Zhang J, Wan J, Chen D, Yu B, He J. Low-molecular-weight chitosan attenuates lipopolysaccharide-induced inflammation in IPEC-J2 cells by inhibiting the nuclear factor-κB signalling pathway. Molecules. (2021) 26:569. doi: 10.3390/molecules26030569
42. Choi YH, Bae JK, Chae HS, Choi YO, Nhoek P, Choi JS, et al. Isoliquiritigenin ameliorates dextran sulfate sodium-induced colitis through the inhibition of MAPK pathway. Int Immunopharmacol. (2016) 31:223–32. doi: 10.1016/j.intimp.2015.12.024
43. Zhang X, Zhang F, Li Y, Fan N, Zhao K, Zhang A, et al. Blockade of PI3K/AKT signaling pathway by Astragaloside IV attenuates ulcerative colitis via improving the intestinal epithelial barrier. J Transl Med. (2024) 22:406. doi: 10.1186/s12967-024-05168-w
44. Adak A, Khan MR. An insight into gut microbiota and its functionalities. Cell Mol Life Sci. (2019) 76:473–93. doi: 10.1007/s00018-018-2943-4
45. Fehily SR, Basnayake C, Wright EK, Kamm MA. The gut microbiota and gut disease. Intern Med J. (2021) 51:1594–604. doi: 10.1111/imj.15520
46. Zhang Y, Deng J, Chen T, Liu S, Tang Y, Zhao JR, et al. Formononetin alleviates no reflow after myocardial ischemia-reperfusion via modulation of gut microbiota to inhibit inflammation. Life Sci. (2024) 358:123110. doi: 10.1016/j.lfs.2024.123110
47. Chen M, Xie W, Zhou S, Ma N, Wang Y, Huang J, et al. A high-concentrate diet induces colonic inflammation and barrier damage in Hu sheep. J Dairy Sci. (2023) 106:9644–62. doi: 10.3168/jds.2023-23359
48. Martín R, Rios-Covian D, Huillet E, Auger S, Khazaal S, Bermúdez-Humarán LG, et al. Faecalibacterium: a bacterial genus with promising human health applications. FEMS Microbiol Rev. (2023) 47:fuad039. doi: 10.1093/femsre/fuad039
49. Liébana-García R, López-Almela I, Olivares M, Romaní-Pérez M, Manghi P, Torres-Mayo A, et al. Gut commensal Phascolarctobacterium faecium retunes innate immunity to mitigate obesity and metabolic disease in mice. Nat Microbiol. (2025) 10:1310–22. doi: 10.1038/s41564-025-01989-7
50. Luo Z, Chen A, Xie A, Liu X, Jiang S. Yu R. Limosilactobacillus reuteri in immunomodulation: molecular mechanisms and potential applications. Front Immunol. (2023) 14:1228754. doi: 10.3389/fimmu.2023.1228754
51. Shah AB, Baiseitova A, Zahoor M, Ahmad I, Ikram M, Bakhsh A, et al. Probiotic significance of Lactobacillus strains: a comprehensive review on health impacts, research gaps, and future prospects. Gut Microbes. (2024) 16:2431643. doi: 10.1080/19490976.2024.2431643
Keywords: Vitamin K, intestinal inflammation, tight junction protein, piglets, IPEC-J2 cells
Citation: Wang H, Ma R, Qi R, Liu Z, Li Y, Wu X, Zhao C, Ma Q and Zhan K (2025) Vitamin K protects against lipopolysaccharide-induced intestinal inflammation in piglets by inhibiting the MAPK and PI3K-AKT pathways. Front. Nutr. 12:1704168. doi: 10.3389/fnut.2025.1704168
Received: 12 September 2025; Accepted: 20 October 2025;
Published: 17 November 2025.
Edited by:
Yang Yuhui, Henan University of Technology, ChinaReviewed by:
Shenglan Hu, Guangdong Academy of Agricultural Sciences, ChinaShuiping Liu, Hunan Agricultural University, China
Copyright © 2025 Wang, Ma, Qi, Liu, Li, Wu, Zhao, Ma and Zhan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qiugang Ma, bWFxaXVnYW5nQGNhdS5lZHUuY24=; Kai Zhan, emhhbmthaTYzM0AxMjYuY29t
 Huakai Wang
Huakai Wang Ruiyu Ma1
Ruiyu Ma1 Xudong Wu
Xudong Wu Qiugang Ma
Qiugang Ma Kai Zhan
Kai Zhan