- 1School of Public Health, Capital Medical University, Beijing, China
- 2Department of Clinical Nutrition, Beijing Children's Hospital Affiliated to Capital Medical University, National Children's Medical Center, Beijing, China
- 3School of Public Health, Capital Medical University, Beijing, China
Background: The incidence rate of autism spectrum disorders (ASDs) has been rising; this study aimed to explore the relationship between nutritional intake and neurodevelopment in children with ASDs.
Methods: This study is a case–control cross-sectional comparison including 50 children with ASDs and 50 typically developing controls. A total of 500 children with neurodevelopmental disorders and 500 typically developing (TD) children were recruited based on clinical diagnosis to compare levels of inflammation. Among them, 50 children were diagnosed with ASDs based on DSM-5 criteria by experienced developmental behavioral pediatricians. A total of 50 typically developing children were matched with the 50 children with ASDs. Dietary surveys, Autism Diagnostic Observation Schedule (ADOS), and physical development evaluations were conducted on these 100 children.
Results: The children’s dietary inflammatory index (C-DII), systemic immune inflammatory index (SII), and systemic inflammatory response index (SIRI) in the ASD group were significantly higher compared to the TD group (p<0.05). A positive correlation was observed between C-DII and SII (r = 0.323, p = 0.022), as well as SIRI (r = 0.283, p = 0.046). The dietary diversity in the ASD group was lower than that of the TD group, with a higher prevalence of picky eating behaviors in the ASD group. After adjusting for demographic characteristics, the C-DII may be correlated with ASD [odds ratio (OR) = 1.842, 95% confidence interval (CI) = 1.114 ~ 3.046, p = 0.017].
Conclusion: Children with autism spectrum disorders (ASD) displayed higher C-DII scores. The results of this study suggest that there may be a correlation between C-DII and the neurodevelopment of children with ASD, not causality. Inflammatory dietary patterns may represent a modifiable factor linked to neurodevelopmental outcomes.
1 Introduction
Autism spectrum disorders, commonly referred to as autism, manifest in early childhood and are characterized by significant impairments in social communication and the presence of repetitive behaviors, which profoundly impact children’s social functioning and overall quality of life. In recent years, the incidence of ASDs has been on the rise, emerging as a global concern, with an estimated prevalence of approximately 7‰ among children in China (1). Notably, over 70% of children diagnosed with ASDs encounter nutritional challenges (2).
Current research suggests that the factors affecting children’s neurological development mainly include both genetic and environmental factors. Research indicates that the nutrients ingested can influence the development of the central nervous system and play an essential role in neurodevelopment (3). Nutritional factors, as modifiable lifestyle elements, suggest that feeding methods and the intake of various foods and nutrients may be associated with the neurodevelopment of children. A meta-analysis regarding the dose–response relationship between the duration of breastfeeding and the risk of ASDs has shown a significant association; as the duration of breastfeeding increases, the risk of developing ASDs decreases (4). A review focusing on micronutrients and brain development has elucidated that children who receive adequate nutrition are more likely to reach their developmental potential in cognitive, motor, and socioemotional domains, resulting in positive societal outcomes (5). It is arguable that overall dietary patterns, rather than single nutrients, exert a more comprehensive impact (6). Exploring a comprehensive dietary index can more accurately reflect the association between dietary quality and the neurodevelopment of children.
Furthermore, inflammation is increasingly acknowledged as a significant factor in central nervous system impairment in both developing children and adults (7). Inflammation is a ubiquitous underlying mechanism linking diet and cognitive functioning (8). The dietary inflammatory index (DII) has been established in nutritional research to characterize and quantify the overall inflammatory potential of dietary patterns (9). Current studies have observed that high DII scores were associated with lower overall cognitive function and a higher risk of mild cognitive impairment (MCI) (10–15). Evidence indicated that high DII scores and pro-inflammatory diets in children were closely related to common pediatric conditions that impact central nervous system functionality, such as obstructive sleep apnea–hypopnea syndrome (OSAHS) (16). The higher the children’s dietary inflammatory index (C-DII), the greater the impact on the severity of children’s OSAHS (16).
The systemic immune-inflammatory index (SII) and systemic inflammatory response index (SIRI) are emerging biomarkers that reflect chronic systemic inflammation. The SII highlights platelet-associated immune mechanisms, while the SIRI comprises monocyte counts, thereby improving its capacity to reflect immune regulatory processes (17). This offers a more comprehensive reflection of the potential systemic inflammatory status and immune responses pertinent to neurodevelopment than a single biomarker could achieve. Studies have shown that children with obesity, particularly those with metabolic syndrome, exhibit higher SII levels (18). Investigations have demonstrated that behaviors such as nutritional approaches and physical activity may modulate immune function, thereby impacting neurological development and systemic health (19, 20). These findings emphasize the impact of multiple factors, including nutritional intake, other behavioral patterns, and immune response, on neurodevelopmental outcomes. Diet-related inflammation may affect brain development through pathways such as cytokine activation, oxidative stress, alterations in gut microbiota, or changes in blood–brain barrier integrity (21). The comorbidities, sensory preferences, and feeding selectivity of ASDs also may have an impact on nutrition and inflammation (22). Therefore, it is necessary to explore the relationship between nutrition, inflammation, and ASDs.
Currently, there is a paucity of research on the relationship between C-DII and ASDs, and the association between C-DII and ASDs remains to be clarified. Is a higher C-DII associated with an increased risk of ASDs independent of confounding variables? The objective of this study was to explore whether there is a correlation between nutritional status and neurodevelopment of children with ASD. Investigating this area is crucial for improving neurodevelopmental outcomes through dietary interventions.
2 Methods
2.1 Study population
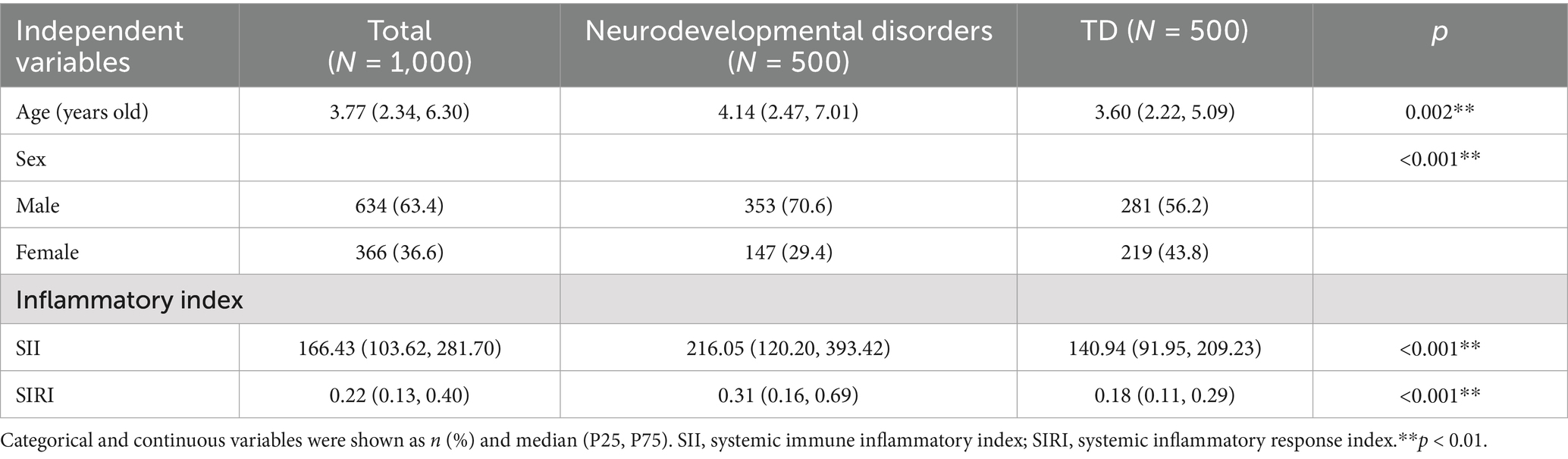
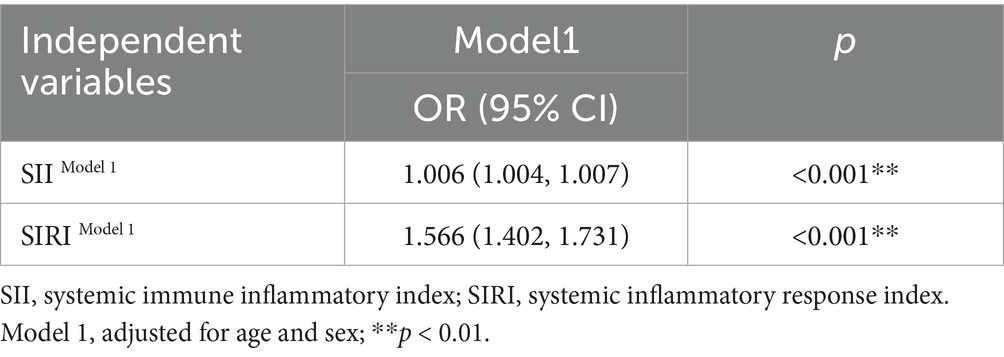
Previous studies have found that inflammatory markers may be elevated in individuals with neurodevelopmental disorders. We recruited 500 children with neurodevelopmental disorders and 500 typically developing (TD) children (aged 3.0 ± 1.70 years) from Beijing Children’s Hospital, affiliated with Capital Medical University, and a community in the Fengtai District of Beijing, to compare their levels of inflammation. All participants were enrolled based on their clinical diagnosis; the 500 TD children were selected from neurodevelopmentally typical children in outpatient and community settings. They received a clinical diagnosis and did not have ASDs or other neurodevelopmental disorders. By adjusting for covariates, including sex and age, in the logistic regression model, we aimed to observe the synergistic effect of SII and SIRI on neurodevelopment.
Within the 500 children with neurodevelopmental disorders, 50 children were diagnosed with ASD through professional clinical neurodevelopmental assessments, with ages ranging from 1 to 3 years. We included these 50 children with ASD in the ASD group. From the 500 TD children, 50 TD were selected to be matched with the ASD group in a 1:1 ratio, as the TD group, based on sex and age. At the same time, dietary surveys, behavioral observation and evaluation, physical development measurements, and birth status such as delivery gestational week and history of birth asphyxia were conducted on these 100 research subjects. Before enrollment, the legal guardians of all eligible participants confirmed and provided informed consent. Children with other independent neurodevelopmental disorders, mental illnesses, or other health conditions were excluded from this study.
2.2 Dietary survey and assessment
The feeding behaviors and dietary surveys for children were designed with reference to other similar studies and the “Dietary Guidelines for Chinese Residents” (23). We utilized the standardized food frequency questionnaire (FFQ) to collect comprehensive dietary data. Trained interviewers conducted face-to-face surveys with the guardians of participants in community settings or outpatient clinics to collect dietary intake information when the children were 1.5 years old. The daily nutrient intake for children was calculated using the Chinese Food Composition Table. At the same time, the estimated average requirements (EER/EAR) were based on the Dietary Reference Intakes for Chinese (DRIs). The EAR represents the average nutrient requirement for individuals within specific sex, age, and physiological condition categories.
2.3 Calculation of DDS and C-DII
The dietary diversity scores (DDS) were calculated according to the Food and Agricultural Organization of the United Nations (FAO) guidelines titled “Guidelines for Measuring Household and Individual Dietary Diversity,” categorizing daily food intake into nine groups (24): (1) cereals, potatoes and mixed beans; (2) vegetables; (3) fruits; (4) meat and poultry; (5) eggs; (6) milk and dairy products; (7) soybeans and nuts; (8) aquatic product; (9) oil. A child must consume at least 10 g of a food from one distinct food group within the 24-h dietary record to earn one point, with a maximum score of 9. The scoring ranges from 1 to 9, where a score above 6 indicates no risk of nutrient deficiency. This information was collected through parental recollection of the food types consumed by their children in the past 24 h.
The dietary inflammatory index was used to assess the inflammatory potential of diet, according to Shivappa et al. (25). 25 types of food parameters were used to calculate the C-DII scores (26). Based on the mean daily global intake of 25 dietary components, and , from which the calibrated value (Z) of the average daily intake of a dietary component (x) per person was calculated as . The Z-score was converted to a percentile and multiplied by 2 minus 1 to obtain a symmetric distribution centered at 0, ranging from −1 to +1, which is denoted as p. The effect of the 25 dietary components on the inflammation-related indices was combined and converted into an “inflammatory effect score (denoted as e),” which has a lower limit of −1 and an upper limit of 1, with closer to −1 representing higher anti-inflammatory capacity and closer to 1 representing higher pro-inflammatory capacity. , where n is the number of dietary components included.
2.4 Neurodevelopmental assessment
All participants were consulted by at least one experienced developmental behavior pediatrician, according to DSM-5 diagnostic criteria (27). Neurodevelopmental scores were assessed using the Children’s Neuropsychological Development Scales and the Autism Diagnostic Observation Schedule (ADOS). The Children’s Neuropsychological Development Scales comprise five domains: gross motor skills, fine motor skills, adaptive behavior, speech, and social behavior. Intellectual development is represented by the Development Quotient (DQ), where DQ = Average Score of the five domains/actual chronological age×100. The ADOS includes social communication and repetitive behaviors.
2.5 Physical development measurement
Physical measurement indicators include weight, height, and body mass index (BMI). Weight in kilograms and height in centimeters were measured using calibrated electronic scales and height meters, ensuring the accuracy of both instruments before use and retaining results to one decimal place. BMI was calculated as weight (kg)/[height (m)]2. The World Health Organization (WHO) Anthro Plus and WHO Anthro software were employed to calculate the Height for Age Z-score (HAZ), Weight for Age Z-score (WAZ), and BMI for Age Z-score (BAZ). Based on BAZ, children’s nutritional status was categorized into severe obesity, obesity, overweight, normal, emaciated, and severely emaciated.
2.6 Laboratory measurements
Blood samples were collected from individuals who had fasted since 8 p.m. the previous night. The absolute counts of neutrophils, lymphocytes, monocytes, and platelets in peripheral blood were analyzed using an automatic analyzer. SII and SIRI were calculated based on the absolute platelet count in peripheral blood (P; ×109/L), the neutrophil count (N; ×109/L), the monocyte count (M; ×109/L), and the lymphocyte count (L; ×109/L), using the formulas SII=P × N/L and SIRI=N × M/L (28).
2.7 Statistical analysis
The statistical description and inference of the data were based on its characteristics, with suitable descriptive indices and hypothesis testing methods selected. Continuous variables were expressed as medians (interquartile ranges, IQR) when they were non-normally distributed; otherwise, a mean ± standard deviation (SD) was used in the normal distribution. For comparisons of non-normally distributed quantitative data between two groups, the Mann–Whitney U test was employed. Categorical data were expressed as counts and percentages (%), and comparisons between two groups of categorical data were performed using the chi-square test. Comparisons between groups were made using grouped t-tests, analysis of variance (ANOVA), or Wilcoxon rank-sum tests, depending on the distribution of the data. Multiple linear regression models were adjusted by confounding factors to assess the relationship between C-DII and the core symptoms of ASDs and neurodevelopmental domains. All p-values were False Discovery Rate (FDR) corrected using the Benjamini–Hochberg program to control false positive detection rates. All regression models have undergone hypothesis testing. No severe multicollinearity has been confirmed through variance inflation factor testing; the linear hypothesis between continuous independent variables and outcomes has been confirmed through component residual plots and polynomial term testing. A multivariate binary logistic regression model was used to evaluate the association of C-DII with ASDs. Statistical significance was set at a two-sided p < 0.05. Statistical Package for Social Sciences (SPSS) Statistics 26 (IBM Corporation, Armonk, NY, USA) was used to perform statistical analyses.
3 Results
3.1 Demographic characteristics and blood inflammation index of participants
The comparison of characteristics between 500 children with neurodevelopmental disorders and 500 TD children is shown in Table 1. Among the 1,000 participants, 63.4% were male. The SII and SIRI of children with neurodevelopmental disorders were higher than those of the TD children (p < 0.001). Table 2 shows the synergistic effect of SII and SIRI on neurodevelopment using a binary logistic regression model.
3.2 Demographic characteristics, C-DII, SII, and SIRI of ASDs and TD
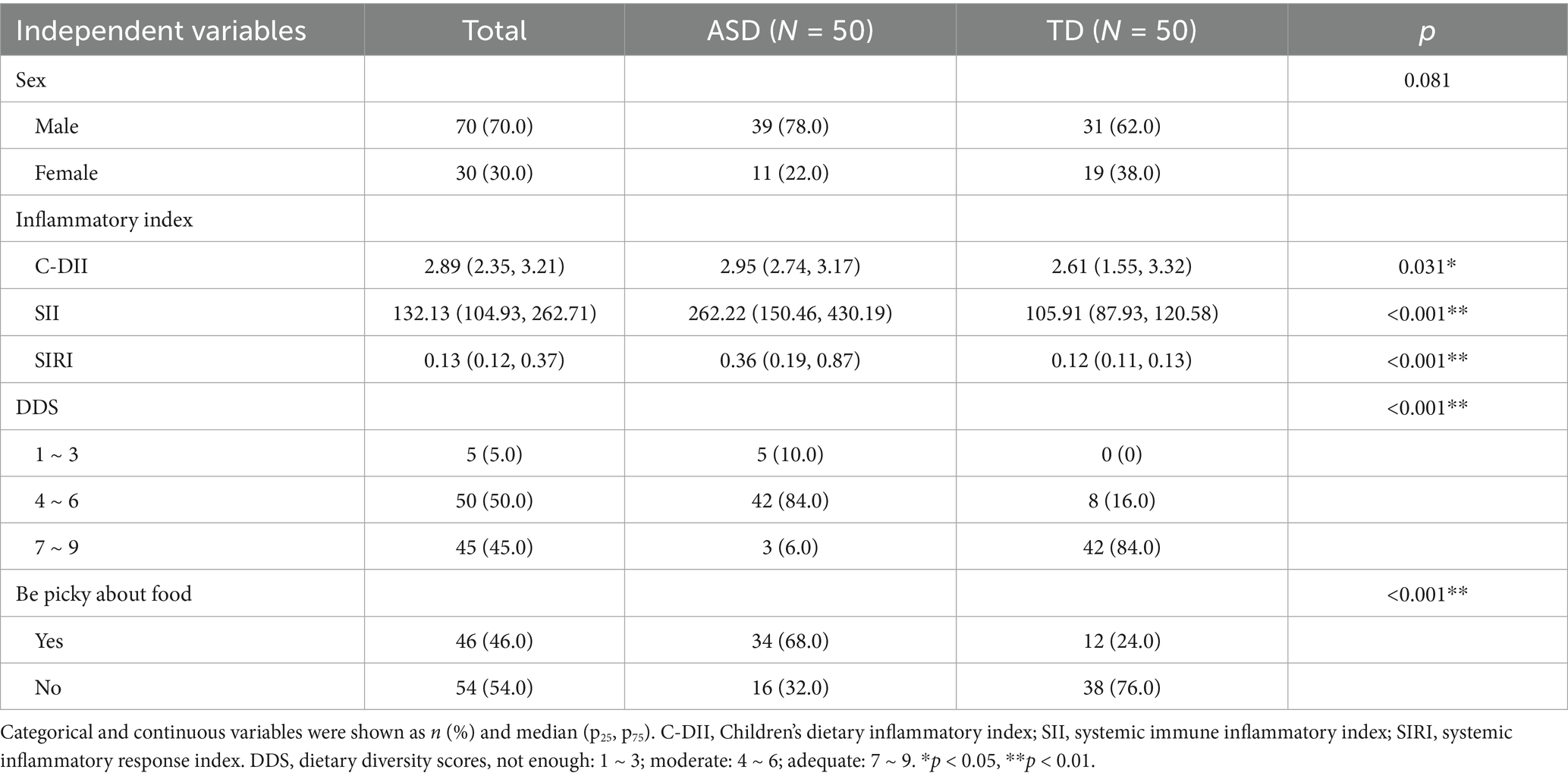
The general conditions, C-DII, SII, and SIRI of 50 children with ASDs and 50 TD children were compared in Table 3 and Supplementary Table S1. Of these participants, 70% were male, and there were no significant differences between the two groups regarding sex, age, or introduction of complementary feeding, HAZ, WAZ, or BAZ. However, there were differences in feeding patterns between the two groups (p < 0.05). The interquartile range (IQR) of the C-DII score was 2.89 (2.35, 3.21), with a range from −0.710 to 4.063. The SIRI was 0.13 (0.12, 0.37), with a range of 0.1 to 6.89. The SII was 132.13 (104.93, 262.71), with a range of 54.63 to 1411.36. The C-DII (p = 0.031), SIRI (p < 0.001), and SII (p < 0.001) of the ASD group were significantly higher than those of the TD group.
3.3 Correlation between SII, SIRI, and C-DII
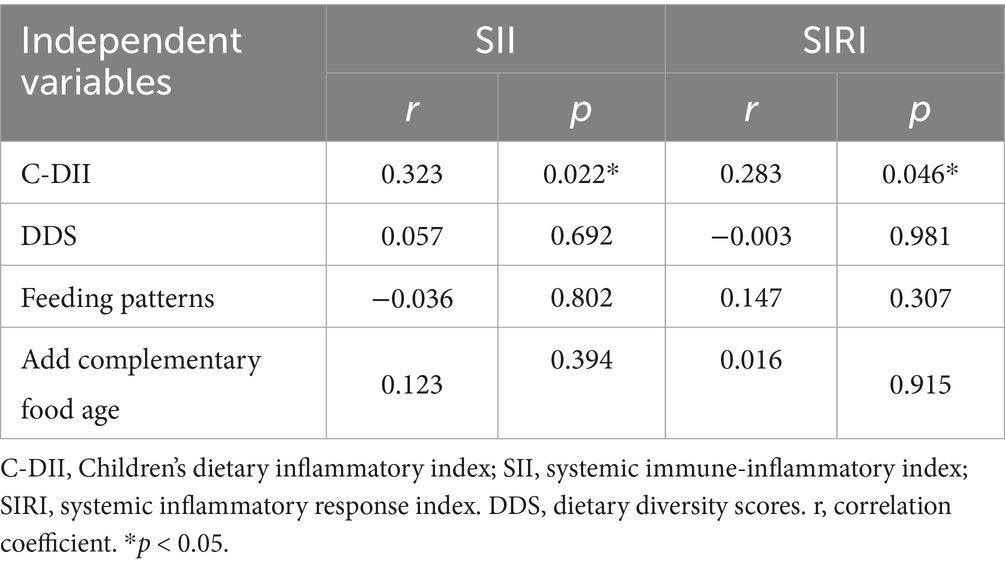
Using Pearson correlation analysis in 50 children with ASDs, this study demonstrated a positive correlation between C-DII and SII (r = 0.323, p = 0.022), as well as a positive correlation between C-DII and SIRI (r = 0.283, p = 0.046) (Table 4). There was no correlation between SII/SIRI and DDS, feeding patterns, and the timing of complementary food addition.
3.4 Comparison of dietary situation in the ASD and TD groups
The dietary diversity of the ASD group was lower than that of the TD group (p < 0.001), and the phenomenon of picky eating in the ASD group was significantly higher than in the TD group (p < 0.001). In the ASD group, 68% of the children were picky eaters, compared to 24% in the TD group. There was no difference in appetite loss or poor feeding interaction between the two groups (Table 3).
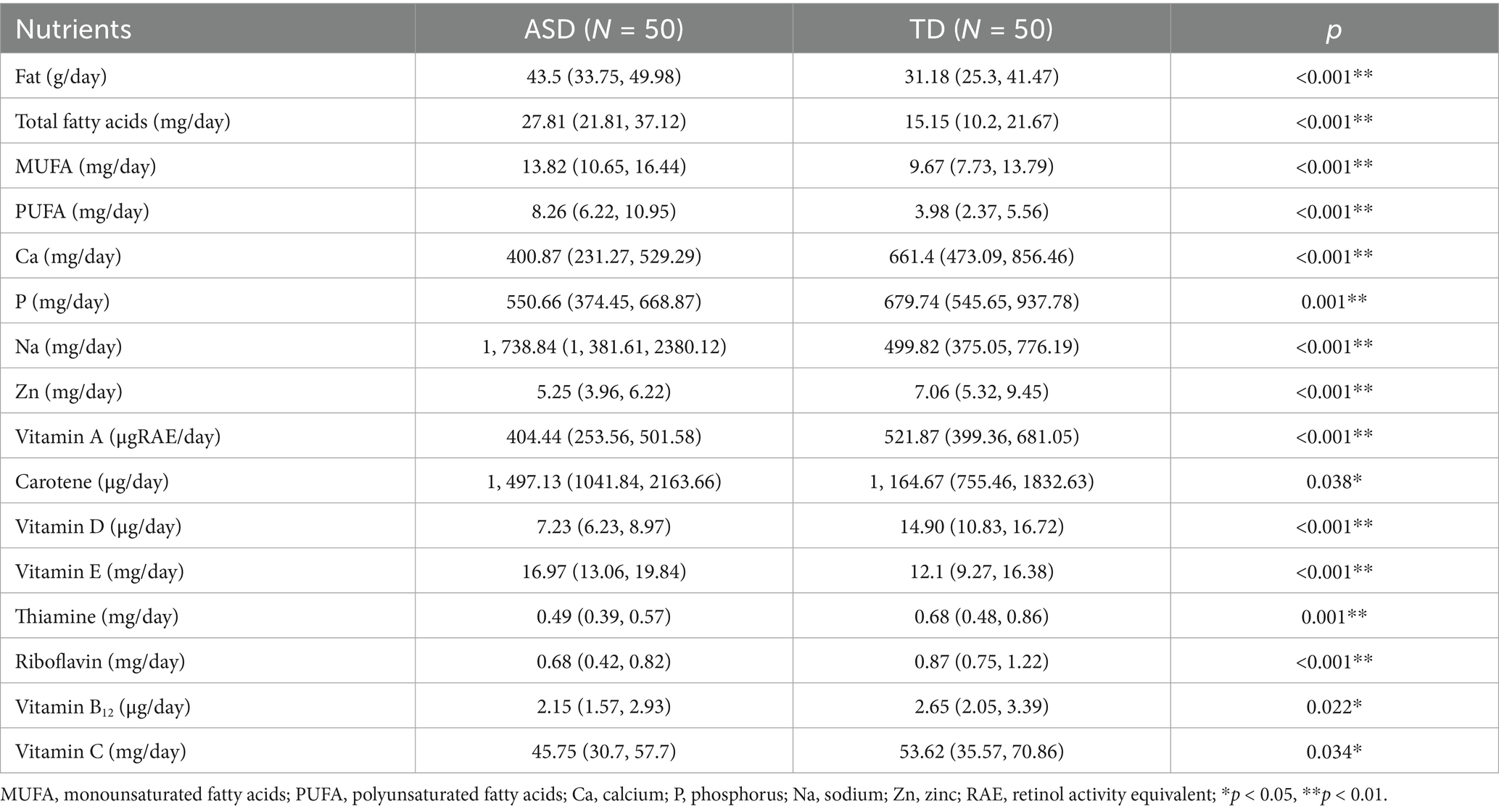
3.5 Comparison of dietary energy and nutrient intake between ASD and TD groups
There were statistically significant differences in the intake of fat, total fatty acids, calcium, phosphorus, sodium, zinc, total vitamin A, vitamin C, vitamin D, thiamine, riboflavin, vitamin B12, and carotene among the ASD group. The intake of fat, total fatty acids, and sodium in the ASD group was higher than that in the TD group. In comparison, the intake of calcium, phosphorus, zinc, total vitamin A, vitamin C, vitamin D, thiamine, riboflavin, and vitamin B12 was significantly lower than in the TD group (Table 5). Table 5 shows the nutrients with statistically significant differences in intake between the two groups.
3.6 Comparison of nutrient intake with EAR between ASD and TD groups
The average daily intake of carbohydrates, vitamin B1, vitamin B2, vitamin C, vitamin D, vitamin B12, and calcium per person in the ASD group was lower than that of the TD group. The proportion of boys in the ASD group whose protein intake reached EAR was higher than that of the TD group, while the proportion of girls in the ASD group whose protein intake reached EAR was lower than that of the TD group. The proportion of boys in the ASD group who reached the EAR for vitamin A intake was lower than that of the TD group, and the proportion of girls in the ASD group who reached the EAR for vitamin A intake was also lower than that of the TD group. The proportion of the ASD group with phosphorus intake reaching EAR was higher than that of the TD group. The proportion of zinc intake reaching EAR in the ASD group was roughly equal to that of the TD group (Supplementary Table S2).
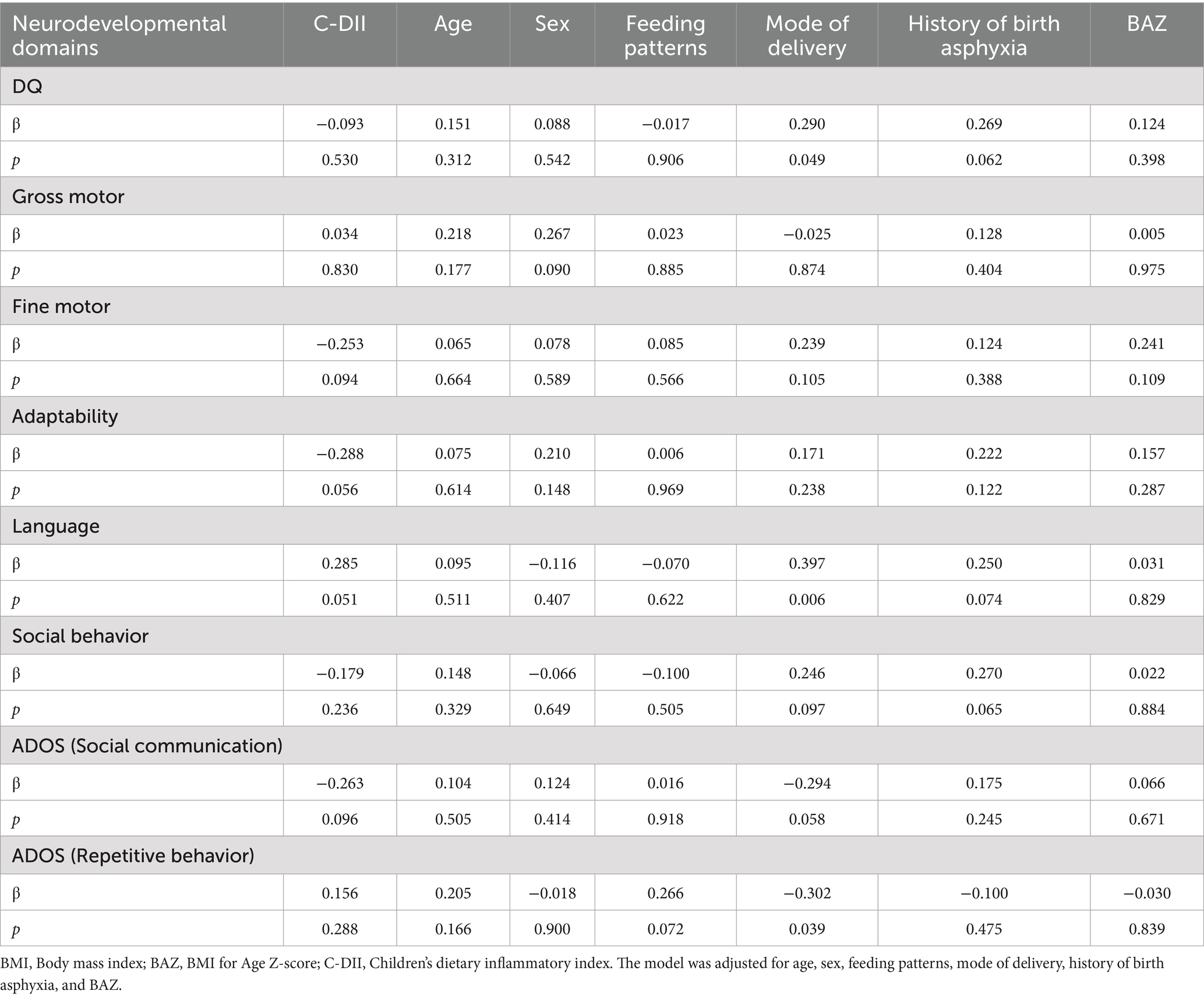
3.7 Correlation between C-DII and neurodevelopmental domains in ASDs
The correlation between C-DII and various neurodevelopmental attribute scores was explored through multiple linear regression (Table 6). After adjusting for age, sex, feeding patterns, gestational week at delivery, history of birth asphyxia, and BAZ, we did not find a significant correlation between C-DII and the scores in the five domains of the Children’s Neuropsychological Development Scales, as well as the ADOS scores.
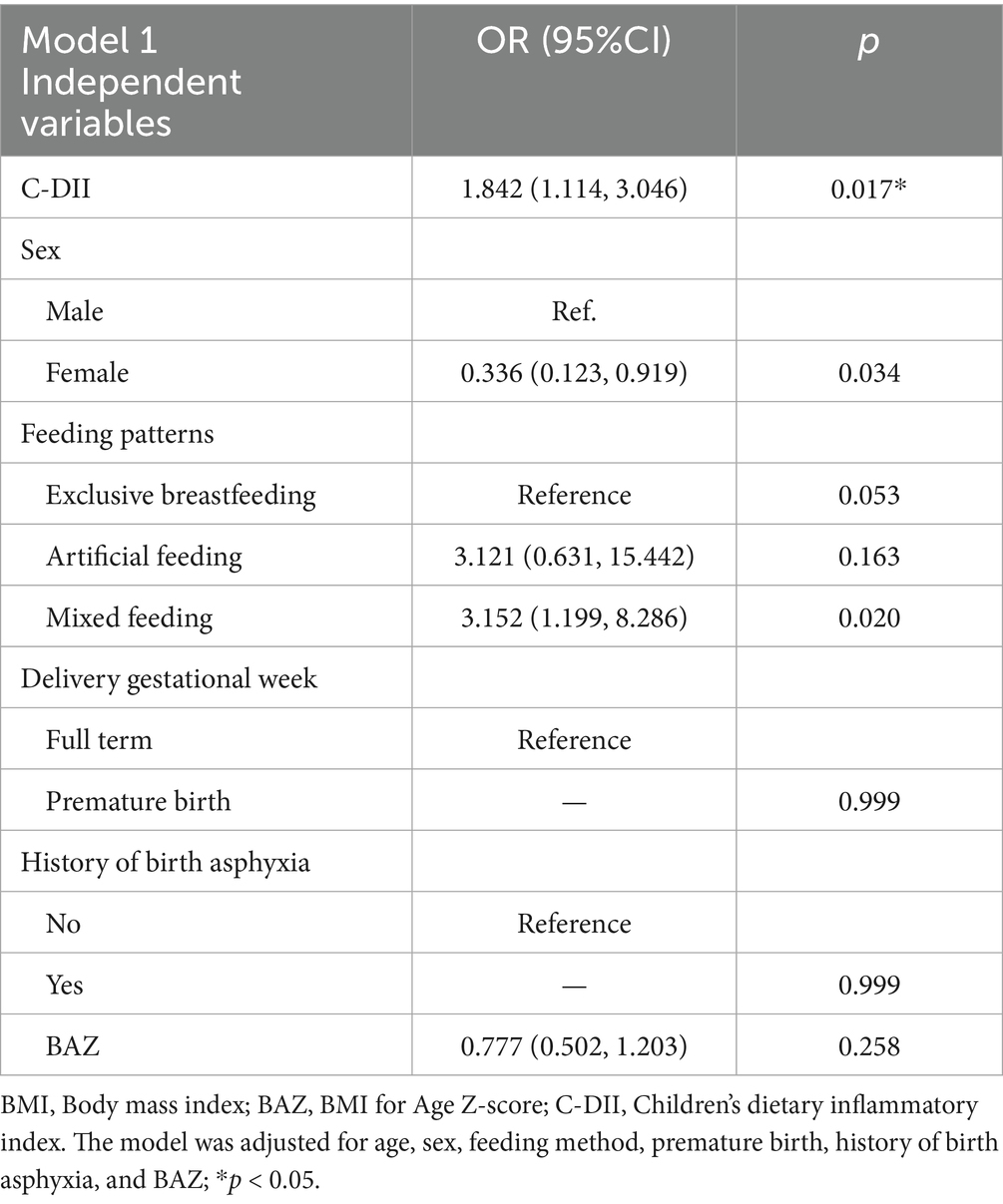
3.8 Correlation between C-DII and ASD
A generalized linear model, specifically a binary logistic regression model, was used to explore the correlation between C-DII and neurodevelopment (Table 7). After adjusting for age, sex, feeding patterns, picky eating, premature birth, history of birth asphyxia, and BAZ, the C-DII may be correlated with ASD [odds ratio (OR) = 1.842, 95% confidence interval (CI) = 1.114 ~ 3.046, p = 0.017].
4 Discussion
NDDs represent a group of chronic developmental brain function disorders with a high prevalence, typically emerging during early developmental stages, often in childhood (29). The symptoms and functional impairments associated with NDDs may persist into adolescence and adulthood, underscoring the necessity for a life-cycle approach to diagnosis and management (30). NDDs exhibit a higher prevalence in boys, with a male-to-female ratio of approximately 4:1 among individuals with ASDs in this study, aligning with findings from another study (31). We found significant differences in the blood inflammation index between children with neurodevelopmental disorders and their typically developing peers across a sample of 1,000. Considering the importance of the inflammation index for cognitive development (10–15), we designed a matched case–control cross-sectional comparison with a 1:1 ratio. By analyzing whether there were differences in C-DII between children with ASDs and TD children, as well as the correlation between C-DII and blood inflammation index, we aimed to explore the relationship between nutritional intake, dietary inflammatory potential, and neurodevelopmental status in children with ASD, and to provide novel insights and methodologies for the identification of early intervention strategies.
C-DII has emerged as an objective indicator for assessing the inflammatory potential in children, facilitating the development of personalized intervention strategies aligned with individual dietary objectives to mitigate the risk of inflammation and associated health complications. This study indicated that the ASD group had higher C-DII, SII, and SIRI values. Additionally, C-DII was positively correlated with SII and SIRI. An increase in C-DII or adherence to a pro-inflammatory dietary pattern may be associated with poorer neurodevelopmental outcomes. This study suggested a possible link between a pro-inflammatory diet and neurodevelopmental features, not causality. This investigation’s results on food diversity in children with autism indicated that their food diversity was significantly lower than that of the TD group; 68% of children with ASDs were picky eaters. DDS serves as a reliable metric for assessing micronutrient deficiencies in children (32). Children with a higher DDS are generally associated with increased micronutrient intake (33). The ASD group exhibited significantly lower intakes of calcium, phosphorus, zinc, total vitamin A, vitamin D, thiamine, riboflavin, vitamin B12, and vitamin C compared to the TD group. The proportion of average daily nutrient intake meeting the EAR for calcium, vitamin D, thiamine, riboflavin, vitamin B12, and vitamin C was lower in the ASD group than in the TD group; 50% of children in the ASD group failed to meet the average calcium requirement, and only 30% achieved the average vitamin D requirement. This phenomenon may be linked to the food choices of children with ASDs, influenced by factors such as food type, texture, and appearance, leading to low food diversity or nutrient deficiencies.
Evidence suggests that C-DII may act as a mediator for the influence of physiological inflammatory biomarkers on cognitive development, particularly through its anti-inflammatory components. Additionally, our previous research has demonstrated a positive correlation between DII and blood inflammation (10, 11). A high consumption of pro-inflammatory foods such as sweets, desserts, and chips has been linked to increased systemic inflammation and cognitive decline (34). Previous investigations into DII have predominantly focused on adult populations (35–39), yielding inconsistent results. This study focused on Chinese children, providing reference points for the prevention and prognosis of ASDs in Chinese children. Children with ASDs exhibited similar dietary preferences, demonstrating a strong preference for carbohydrates, snacks, and processed foods such as sweets and fried foods, while rejecting green leafy vegetables. This pattern aligns with findings from other studies (40–44), which report that severe nutrient deficiencies in children with ASDs may result from selective eating behaviors, resistance to specific tastes or colors, feeding behavior problems (e.g., distractibility during meals and refusal to eat), and dietary habits influenced by varying degrees of gastrointestinal symptoms. The comparison of nutrient intake between the two groups was consistent with findings from previous studies (45–50). Studies have shown that deficiency in certain micronutrients, such as zinc and vitamin B12, may lead to neurodevelopmental disorders (51). We did not observe significant deficiencies in the three macronutrients or severe growth and developmental issues among children with ASDs. This may be attributed to their preference for staple foods, sweets, and fried foods, which ensured adequate intake of macronutrients, particularly excess fat. Nevertheless, deficiencies in micronutrients and essential minerals warrant attention. Early screening and intervention may help reduce ASD-related malnutrition and improve the dietary quality in these ASD patients.
Mechanistically, a pro-inflammatory diet may serve as a stimulus that elevates inflammatory markers and augments the inflammatory response, potentially leading to brain dysfunction and the development of neurodegenerative disease (52). Evidence indicates that increased oxidative stress and altered apoptosis contribute to the pathogenesis of neurodegenerative diseases (53). Free radicals are involved in the progression of cognitive deficits by interrupting synaptic transmission, impairing mitochondrial function, promoting neuroinflammation, and interfering with axonal transport, all of which can lead to neuronal loss in neurodegenerative diseases (54). Furthermore, dietary patterns can regulate intestinal immunity through gut microbiota. Adherence to a pro-inflammatory diet may disrupt the balance of gut microbiota, thereby affecting the gut-brain axis, contributing to immune dysregulation, and influencing neural development (55, 56). Anti-inflammatory diets may serve as non-pharmacological interventions to mitigate immune-related dysfunction in individuals with ASD (57, 58). Modulating dietary inflammation could complement pharmacologic or behavioral interventions. Inflammation and nutrition jointly influence both metabolic and neurodevelopmental pathways, suggesting a shared etiological risk factor underlying metabolic and neuropsychiatric disorders (59). This study targets metabolic and inflammatory pathways, highlighting the convergence between nutritional and molecular strategies for early intervention.
This study has several limitations. First, the cross-sectional design precludes any causal inference, and the possibility of reverse causality cannot be ruled out; that is, ASD-related behavioral patterns, such as picky eating and restricted food preferences, may themselves lead to pro-inflammatory dietary profiles. Second, the absence of validated dietary assessment tools means that the FFQ and 24-h recall methods relied largely on parental recall, introducing potential recall and reporting biases—for example, parents may forget certain snacks or inaccurately estimate weights and portions of food their children consumed. In this study, an artificial food mold, a booklet containing food quality estimation pictures, and a placemat marked with grids were used to facilitate portion estimation (60). Multiple imputation was used to address missing data, generating several complete imputation datasets through statistical modeling. Third, this study did not include socioeconomic and clinical variables. However, all participants were recruited from the same geographical region and city, which, to some extent, reduces the possibility of significant socioeconomic disparities caused by differences due to residence. Nonetheless, systematically collecting socioeconomic data in future research will be crucial. Fourth, a small sample size limits statistical power; the negative results we observed should be interpreted cautiously, as they may exist type II errors. These findings necessitate validation through subsequent large-sample, multi-center, prospective studies that further explore the underlying mechanisms. This study may help provide a basis for exploring early intervention approaches, such as lifestyle and dietary programs, aimed at improving ASD outcomes through dietary and inflammatory pathways, and may inform similar approaches in pediatric ASD research (61).
5 Conclusion
Children with ASDs showed higher dietary inflammatory scores and lower dietary diversity, suggesting a possible link between a pro-inflammatory diet and neurodevelopmental features. The C-DII may serve as a modifiable correlate of neurodevelopmental differences in ASDs. Future research should focus on longitudinal or interventional studies to determine whether dietary changes affect inflammation and neurodevelopmental outcomes. These findings highlight the potential clinical relevance of monitoring diet and inflammation status in children with ASDs.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
The study was conducted in accordance with the Declaration of Helsinki and the ethics committee of the institutional research committee and the protocol was approved by the Medical Ethics Committee of Capital Medical University (Z2022SY069) on August 30, 2022. Informed consent was obtained from all subjects involved in the study. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
Author contributions
JS: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Visualization, Writing – original draft, Writing – review & editing. JY: Investigation, Writing – original draft. WW: Investigation, Writing – original draft. WY: Investigation, Writing – original draft. WZ: Investigation, Writing – original draft. JZ: Investigation, Writing – original draft. HD: Investigation, Writing – original draft. YX: Conceptualization, Funding acquisition, Project administration, Resources, Supervision, Validation, Writing – review & editing. HH: Conceptualization, Funding acquisition, Project administration, Resources, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was funded by the Capital Medical University Long-Term Program (CXZDS2024) and Science and Technology Innovation Development Project, Beijing Children’s Hospital, Capital Medical University, National Center for Children’s Health (number: YN2020408).
Acknowledgments
We are extremely grateful to all the families who took part in this study. We would also like to thank the doctors, midwives, and nurses involved in our study for their patient management.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2025.1705682/full#supplementary-material
References
1. Zhou, H, Xu, X, Yan, W, Zou, X, Wu, L, Luo, X, et al. Prevalence of autism Spectrum disorder in China: a Nationwide multi-center population-based study among children aged 6 to 12 years. Neurosci Bull. (2020) 36:961–71. doi: 10.1007/s12264-020-00530-6
2. Developmental Behavioral Group of the Pediatric Branch of the Chinese Medical Association. Identification and management principles for common comorbid issues in children with autism spectrum disorder. Chin J Pediatr. (2018) 56:174–8. doi: 10.3760/cma.j.issn.0578-1310.2018.03.004
3. Jiyoung, SK, and Claire, BL. Diet, gut microbiota composition and feeding behavior. Physiol Behav. (2018) 192:177–81. doi: 10.1016/j.physbeh.2018.03.026
4. Dong, M, Yan, Z, Wu, H, and Wu, D. A meta-analysis of the dose-response relationship between the duration of breastfeeding and the risk of autism spectrum disorder. Evid Based Nurs. (2023) 9:2702–9. doi: 10.12102/j.issn.2095-8668.2023.15.006
5. Davide, M, and Angelo, P. Micronutrients and brain development. Curr Nutr Rep. (2019) 8:99–107. doi: 10.1007/s13668-019-0268-z
6. Tapsell, LC, Neale, EP, Satija, A, and Hu, FB. Foods, nutrients, and dietary patterns: interconnections and implications for dietary guidelines. Adv Nutr. (2016) 7:445–54. doi: 10.3945/an.115.011718
7. Catherine, MP, Chen, L, Barbara, H, Jonathan, YB, Nicholas, CH, Liesbeth, D, et al. Dietary inflammatory index and non-communicable disease risk: a narrative review. Nutrients. (2019) 11:1873. doi: 10.3390/nu11081873
8. Kesse-Guyot, E, Assmann, KE, Andreeva, VA, Touvier, M, Neufcourt, L, Shivappa, N, et al. Long-term association between the dietary inflammatory index and cognitive functioning: findings from the SU.VI.MAX study. Eur J Nutr. (2017) 56:1647–55. doi: 10.1007/s00394-016-1211-3
9. Nitin, S, James, RH, Ernst, RR, Marc, LD, Michel, L, Evi, D, et al. Associations between dietary inflammatory index and inflammatory markers in the Asklepios study. Br J Nutr. (2015) 113:665–71. doi: 10.1017/S000711451400395X
10. Wang, X, Li, T, Li, H, Li, D, Wang, X, Zhao, A, et al. Association of Dietary Inflammatory Potential with blood inflammation: the prospective markers on mild cognitive impairment. Nutrients. (2022) 14:2417. doi: 10.3390/nu14122417
11. Li, T. The role of dietary and blood inflammation on the relation of diabetes and cognition in Chinese elderly people. Glob Transit. (2022) 4:58–67. doi: 10.1016/j.glt.2022.11.002
12. Dayeon, S, Simona, CK, Mi, HK, Kyung, WL, Soe, YC, Nitin, S, et al. Inflammatory potential of diet is associated with cognitive function in an older adult Korean population. Nutrition. (2018) 55-56:56–62. doi: 10.1016/j.nut.2018.02.026
13. Michael, JH, Susan, JT, Sarah, AM, and Catherine, MM. Dietary patterns and associations with biomarkers of inflammation in adults: a systematic review of observational studies. Nutr J. (2021) 20:24. doi: 10.1186/s12937-021-00674-9
14. Liselot, K, Caue, ER, and Krasimira, A. Effects of dietary patterns on biomarkers of inflammation and immune responses: a systematic review and meta-analysis of randomized controlled trials. Adv Nutr. (2022) 13:101–15. doi: 10.1093/advances/nmab086
15. Emily, F, Nitin, S, Joshua, RM, James, RH, Michael, DW, and Paul, DL. Dietary inflammatory index and memory function: population-based national sample of elderly Americans. Br J Nutr. (2018) 119:552–8. doi: 10.1017/S0007114517003804
16. Long, Z, Li, H, Wen, C, Mo, X, Xie, X, Mo, Y, et al. Effect of dietary inflammation index on the condition of OSAHS in children. Chinese J Integrative Otolaryngol. (2022) 30:136–139+95. doi: 10.16542/j.cnki.issn.1007-4856.2022.02.013
17. Huang, Y, Yin, X, and Li, Z. Impact of systemic immune inflammation index and systemic inflammation response index on all-cause and cardiovascular mortality in cardiovascular-kidney-metabolic syndrome. Eur J Med Res. (2025) 30:645. doi: 10.1186/s40001-025-02929-1
18. Delia-Maria, N, Andrei-Ioan, M, Alexandra-Cristina, S, Niculina, M, Iulius, J, Giorgiana-Flavia, B, et al. Assessing the relationship between systemic immune-inflammation index and metabolic syndrome in children with obesity. Int J Mol Sci. (2023) 24:8414. doi: 10.3390/ijms24098414
19. Hughes, HK, Mills, KE, Rose, D, and Ashwood, P. Immune dysfunction and autoimmunity as pathological mechanisms in autism spectrum disorders. Front Cell Neurosci. (2018) 12:405. doi: 10.3389/fncel.2018.00405
20. Toscano, CVA, Barros, L, Lima, AB, Nunes, T, Carvalho, HM, and Gaspar, JM. Neuroinflammation in autism spectrum dis-orders: exercise as a "pharmacological" tool. Neurosci Biobehav Rev. (2021) 129:63–74. doi: 10.1016/j.neubiorev.2021.07.023
21. Mou, Y, Du, Y, Zhou, L, Yue, J, Hu, X, Liu, Y, et al. Gut microbiota interact with the brain through systemic chronic inflammation: implications on Neuroinflammation, neurodegeneration, and aging. Front Immunol. (2022) 13:796288. doi: 10.3389/fimmu.2022.796288
22. Barlattani, T, D'Amelio, C, Cavatassi, A, De Luca, D, Di Stefano, R, di Berardo, A, et al. Autism spectrum disorders and psychiatric comorbidities: a narrative review. J Psychopathol. (2023) 29:3–24. doi: 10.36148/2284-0249-N281
23. Chinese Nutrition Society. Dietary guidelines for Chinese residents (2022). Beijing: People's Health Publishing House (2022).
24. Gina K,, Terri B,, and Marie CD, Nutrition D. Guidelines for measuring household and individual dietary diversity. Rome, Italy: FAO (2011).
25. Nitin, S, Michael, DW, Thomas, GH, and James, RH. Association between the dietary inflammatory index (DII) and telomere length and C-reactive protein from the National Health and nutrition examination Survey-1999-2002. Mol Nutr Food Res. (2017) 61:10. doi: 10.1002/mnfr.201600630
26. Samira, K, Michael, DW, Andrew, O, Christian, RA, Nitin, S, Thomas, GH, et al. Design, development and construct validation of the children's dietary inflammatory index. Nutrients. (2018) 10:993. doi: 10.3390/nu10080993
27. American Psychiatric Association, DSM-5 Task Force. Diagnostic and statistical manual of mental disorders: DSM-5™. 5th ed. American: American Psychiatric Publishing, Inc (2013).
28. Ho, JY, Jae, HS, and Dong, HL. Systemic inflammation response index and systemic immune-inflammation index are associated with clinical outcomes in patients treated with mechanical thrombectomy for large artery occlusion. World Neurosurg. (2021) 153:282–9. doi: 10.1016/j.wneu.2021.06.113
29. Anita, T, Miriam, C, and Michael, R. Neurodevelopmental disorders. Lancet Psychiatry. (2017) 4:339–46. doi: 10.1016/S2215-0366(16)30376-5
30. Antolini, G, and Colizzi, M. Where do neurodevelopmental disorders go? Casting the eye away from childhood towards adulthood. Healthcare (Basel). (2023) 11:1015. doi: 10.3390/healthcare11071015
31. Zhang, H, Xue, M, Wang, B, Zhang, C, and Li, S. Advances in early genetic diagnosis and treatment research of autism spectrum disorder. Chin J Psychiatry. (2022) 55:232–7. doi: 10.3760/cma.j.cn113661-20211123-00339
32. Zhao, W, Yu, K, Tan, S, Zheng, Y, Zhao, A, Wang, P, et al. Dietary diversity scores: an indicator of micronutrient inadequacy instead of obesity for Chinese children. BMC Public Health. (2017) 17:440. doi: 10.1186/s12889-017-4381-x
33. Meng, L, Wang, Y, Li, T, Carolien, AL, Zhang, Y, and Ignatius, MS. Dietary diversity and food variety in Chinese children aged 3-17 years: are they negatively associated with dietary micronutrient inadequacy? Nutrients. (2018) 10:1674. doi: 10.3390/nu10111674
34. Catherine, MM, and Sarah, AM. Dietary patterns and successful ageing: a systematic review. Eur J Nutr. (2016) 55:423–50. doi: 10.1007/s00394-015-1123-7
35. Hye-Young, K, Jeonghee, L, and Jeongseon, K. Association between dietary inflammatory index and metabolic syndrome in the general Korean population. Nutrients. (2018) 10:648. doi: 10.3390/nu10050648
36. Claudia, MC, Jorge, EC, Mara, CR, and Robinson, R. Dietary inflammatory index and cardiometabolic risk parameters in overweight and sedentary subjects. Int J Environ Res Public Health. (2017) 14:1104. doi: 10.3390/ijerph14101104
37. Carolina, AC, Antônio, AM, Maria, CA, Poliana, CF, Marco, AB, Heloisa, B, et al. The dietary inflammatory index and insulin resistance or metabolic syndrome in young adults. Nutrition. (2019) 58:187–93. doi: 10.1016/j.nut.2018.07.014
38. Alexis, S, Michael, DW, Marta, M, Nitin, S, Katarzyna, Z, Thomas, GH, et al. Association between the dietary inflammatory index, waist-to-hip ratio and metabolic syndrome. Nutr Res. (2016) 36:1298–303. doi: 10.1016/j.nutres.2016.04.004
39. Leila, N, Zeinab, N, Nitin, S, and James, RH. The association between dietary inflammatory index and metabolic syndrome components in Iranian adults. Prim Care Diabetes. (2018) 12:467–72. doi: 10.1016/j.pcd.2018.07.008
40. Ahearn, WH, Castine, T, Nault, K, and Green, G. An assessment of food acceptance in children with autism or pervasive developmental disorder-not otherwise specified. J Autism Dev Disord. (2001) 31:505–11. doi: 10.1023/a:1012221026124
41. Kimberly, AS, Keith, W, and Angela, FS. A comparison of eating behaviors between children with and without autism. J Autism Dev Disord. (2004) 34:433–8. doi: 10.1023/b:jadd.0000037419.78531.86
42. Liem, TC, Linda, GB, Aviva, M, Sarah, P, Sharon, AC, and Carol, C. Sensory sensitivity and food selectivity in children with autism spectrum disorder. J Autism Dev Disord. (2018) 48:583–91. doi: 10.1007/s10803-017-3340-9
43. Heewon, LG, and Hsu-Min, C. Brief report: mealtime behaviors of Chinese American children with autism spectrum disorder. J Autism Dev Disord. (2017) 47:892–7. doi: 10.1007/s10803-016-2993-0
44. Barbara, OM, Courtney, M, Saul, K, and William, GS. Gastrointestinal symptoms in autism spectrum disorder: a meta-analysis. Pediatrics. (2014) 133:872–83. doi: 10.1542/peds.2013-3995
45. Guo, M, Zhu, J, Yang, T, Lai, X, Lei, Y, Chen, J, et al. Vitamin a and vitamin D deficiencies exacerbate symptoms in children with autism spectrum disorders. Nutr Neurosci. (2019) 22:637–47. doi: 10.1080/1028415X.2017.1423268
46. Li, Y, Yang, T, Liu, J, Liu, X, Chen, J, and Li, T. Nutritional status survey of children with autism spectrum disorder aged 3-7 years at a special education institution in Chongqing. J Xi'an Jiaotong Univ. (2019) 40:629–34. doi: 10.7652/jdyxb201904027
47. Li, T, and Tan, M. Monitoring and intervention principles for micronutrient deficiencies in children with autism spectrum disorder. Chin J Child Health Care. (2021) 29:1–4. doi: 10.11852/zgetbjzz2020-1787
48. Liu, X, Liu, J, Xiong, X, Yang, T, Hou, N, Liang, X, et al. Correlation between nutrition and symptoms: nutritional survey of children with autism Spectrum disorder in Chongqing, China. Nutrients. (2016) 8:294. doi: 10.3390/nu8050294
49. Shi, R, Han, Y, Gao, L, Wang, G, and Zhang, X. Dietary nutritional status of children with autism spectrum disorder in Tianjin. Chinese School Health. (2021) 42:1318–22. doi: 10.16835/j.cnki.1000-9817.2021.09.010
50. Prahbhjot, M, Lolam, V, Bhavneet, B, and Pratibha, S. Feeding problems and nutrient intake in children with and without autism: a comparative study. Indian J Pediatr. (2017) 84:283–8. doi: 10.1007/s12098-016-2285-x
51. Michael, KG, Sara, ER, and Sarah, EC. Nutritional influences on brain development. Acta paediatrica (Oslo, Norway: 1992). (2018) 107:1310–21. doi: 10.1111/apa.14287
52. Tan, BL, and Norhaizan, ME. Effect of high-fat diets on oxidative stress, cellular inflammatory response and cognitive function. Nutrients. (2019) 11:2579. doi: 10.3390/nu11112579
53. Bhat, AH, Dar, KB, Anees, S, Zargar, MA, Masood, A, Sofi, MA, et al. Oxidative stress, mitochondrial dysfunction and neurodegenerative diseases; a mechanistic insight. Biomed Pharmacother. (2015) 74:101–10. doi: 10.1016/j.biopha.2015.07.025
54. Swerdlow, RH, Burns, JM, and Khan, SM. The Alzheimer's disease mitochondrial cascade hypothesis: progress and perspectives. Biochim Biophys Acta. (2014) 1842:1219–31. doi: 10.1016/j.bbadis.2013.09.010
55. Thomas, MB, Georgios, V, George, M, Petra, H, Ioannis, K, Harpal, SR, et al. Dietary influences on the microbiota-gut-brain axis. Int J Mol Sci. (2021) 22:3502. doi: 10.3390/ijms22073502
56. Anastasia, IP, Smaro, P, Erifili, H, Julia, MS, Pio, C, and Theoharis, CT. Gut-microbiota-brain axis and its effect on neuropsychiatric disorders with suspected immune dysregulation. Clin Ther. (2015) 37:984–95. doi: 10.1016/j.clinthera.2015.04.002
57. Jyonouchi, H. Autism spectrum disorder and a possible role of anti-inflammatory treatments: experience in the pediatric allergy/immunology clinic. Front Psych. (2024) 15:1333717. doi: 10.3389/fpsyt.2024.1333717
58. Naranjo-Galvis, CA, Trejos-Gallego, DM, Correa-Salazar, C, Triviño-Valencia, J, Valencia-Buitrago, M, Ruiz-Pulecio, AF, et al. Anti-inflammatory diet and probiotic supplementation as strategies to modulate immune dysregulation in autism Spectrum disorder. Nutrients. (2025) 17:2664. doi: 10.3390/nu17162664
59. Hassamal, S. Chronic stress, neuroinflammation, and depression: an overview of pathophysiological mechanisms and emerging anti-inflammatories. Front Psych. (2023) 14:1130989. doi: 10.3389/fpsyt.2023.1130989
60. Wang, Z, Sun, Z, and Zhong, C. Retrospective dietary survey assisted reference to the development of food atlas maternal and child nutrition branch of Chinese nutrition society Collection of materials for the seminar on Internet technology application for dietary evaluation of women and children. Department of Nutrition and Food Hygiene, School of Public Health, Nanjing Medical University (2016). 5 p.
61. Luciano, M, Sampogna, G, D'Ambrosio, E, Rampino, A, Amore, M, Calcagno, P, et al. One-year efficacy of a lifestyle behavioural intervention on physical and mental health in people with severe mental disorders: results from a randomized controlled trial. Eur Arch Psychiatry Clin Neurosci. (2024) 274:903–15. doi: 10.1007/s00406-023-01684-w
Keywords: nutrition, neurodevelopment, autism spectrum disorder, dietary patterns, inflammation
Citation: Shen J, You J, Wang W, Yang W, Zhao W, Zhao J, Ding H, Xi Y and Huang H (2025) Imbalanced nutrition and increased dietary inflammatory index in children with autism spectrum disorder: associations with neurodevelopmental disorders. Front. Nutr. 12:1705682. doi: 10.3389/fnut.2025.1705682
Edited by:
Patrick Noël Pallier, Queen Mary University of London, United KingdomReviewed by:
Francesca Pacitti, University of L'Aquila, ItalyFrancesco Di Giacomo Barbagallo, University of Catania, Italy
Emanuela Bianciardi, University of Rome Tor Vergata, Italy
Copyright © 2025 Shen, You, Wang, Yang, Zhao, Zhao, Ding, Xi and Huang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hongmei Huang, aGhtMTVAdHNpbmdodWEub3JnLmNu; Yuandi Xi, eGlhb2VyNzExQDE2My5jb20=
 Jing Shen1,2
Jing Shen1,2 Yuandi Xi
Yuandi Xi