- 1School of Mathematics and Physics, University of Science and Technology Beijing, Beijing, China
- 2Center for Optical Imagery Analysis and Learning (OPTIMAL), Northwestern Polytechnical University, Xi’an, China
Hepatitis B virus (HBV) is a serious threat to human health as it can cause the chronic hepatitis B, and eventually liver cancer. It also has become one of the major threats to public health in the world. In this paper, considering the rationality of using standard incidence in Caputo-Fabrizio fractional order HBV infection model, we propose a model with standard incidence. The analysis of local stability about the equilibrium and the simulation of global stability are given. We also use the real data to estimate the parameters of this model. The simulation results can fit the data well. Moreover, we propose an optimal control model and give the optimal therapy strategy, which show that optimal therapy can reduce the cost and side effects while ensuring the therapeutic effect.
1 Introduction
Hepatitis B virus (HBV) is a partially double-stranded DNA virus and can cause liver diseases such as hepatitis, cirrhosis and liver cancer, and eventually death. When the virus invades the human body, it enters the liver cells by binding to the receptor. Then, HBV-DNA is transported to the nucleus of liver cells and converted into cccDNA under the action of host enzymes. Since cccDNA is not easily degraded, chronic hepatitis B is difficult to be cured [1, 2]. Despite the highly effective recombinant vaccine has been available from 1981 [3], and more and more population are actively vaccinated, the number of the HBV infected is still increasing. More than two billion people have been infected worldwide, and 350 million people are suffering from chronic hepatitis B [4]. HBV has become a major threat to global public health.
Mathematical models can be used to simulate and predict the process of biodynamics. Researchers analyzed the dynamics of the model from different aspects. An immune model was given by Su et al. [5] and the existence, uniqueness and global stability of the model were also analyzed. Anwarud [6] proposed a HBV model with the effect of vaccine and treatment and gave the optimal control strategy. Li et al. [7] analyzed the network vulnerability based on Markov criticality and applied it in lots of biosystem networks. Kalyan [8] considered the intracellular delay in the production of the infected hepatocytes and incorporated a time lag in his model. The spatial diffusion was also used by Khalid [9]. However, most of the papers are limited in the classical integer order.
Fractional calculus is an arbitrary order generalization of classical integral calculus which has the characters of memory, history and nonlocality. There are many definitions of fractional calculus. Among them, Caputo order is one of the widely used types. Kai Diethel [10] summarized the application of Caputo in various field and the numerical methods of fractional order. Devendra [11] derived analysis and numerical solutions of nonlinear fractional problems in nanofluid dynamics, nanoscale heat conduction and carbon nanotube current. Ivan [12] gave a fractional order Ebola epidemic model to study its outbreak. Min Ma [13] considered the diffusion issue in fractional order models and gave the numerical method. Hatıra [14] proposed HIV model with Caputo and constant proportional Caputo operators. Fatmawati [15] gave the Caputo order model of HIV transmission with awareness effect. To reduce the transmission of HIV, Caputo order model for the transmission of HIV epidemic with optimal control was proposed by Parvaiz [16]. The theory of Caputo fractional order in dynamic system has also been derived. The local stability was similar to that in integer order and derived by E. Ahmed [17]. The global stability of fractional order was also given by Boukhouima [18]. And other theorems [19] of the Caputo order systems have been derived and widely used in papers. However, the singular kernel of the Caputo fractional derivative causes a lot of difficult problems both in integral calculation and discretization.
To avoid the shortage of the singular kernel, some researchers have proposed new definitions of fractional order. These definitions include the Caputo-Fabrizio derivative defined by an exponential decay law [20] and Atangana–Baleanu derivative defined by a Mittag–Leffler law [21]. Although Caputo-Fabrizio order does not conform to the traditional fractional calculus [22], it is also used in lots of biodynamic system. Esmehan [23] gave a cancer-immune system and discussed the model by adding IL-12 cytokine and anti-PD-L1 inhibitor. Elvin [24] proposed and analyzed a Caputo–Fabrizio fractional model for the HIV/AIDS epidemic which includes an antiretroviral treatment compartment. Human liver model involving Caputo–Fabrizio derivative was also proposed by Dumitru [25]. Hakimeh [26] proposed a Caputo–Fabrizio model for hearing loss due to Mumps virus with optimal control. The basic theorems of this order have also been proved, including the existence and uniqueness of the solution and the local stability of the equilibrium point [27].
Many papers study HBV with mathematic models. The earliest study can be traced back to the three-state model proposed by Novak et al. [28] and the analysis of the model and basic reproduction number were given. With the development of HBV, researchers have found that the bilinear incidence can not describe the real infection within body well. So Min et al. [29] modified βxv to
So far, almost no paper considered the Caputo-Fabrizio order model with standard incidence which is more reasonable. And Caputo-Fabrizio order also has the character of memory and can effectively avoid the singularity of Caputo order. Besides, there are seldom papers considered parameter estimation based on the real data and optimal control in Caputo Fabrizio order HBV infection models. Based on the above discussion, we first propose a Caputo-Fabrizio fractional order model with standard incidence. Then, we use the clinical data to estimate the parameters of our model. Finally, we propose an optimal control model based on the treatment for chronic hepatitis B and give the optimal therapy strategy. Using the actual data to estimate the parameters of the HBV model can make our model have medical significance. And the optimal treatment strategy can reduce the cost and side effects while ensuring the therapeutic effect.
2 Model Formulation
In general, the process of hepatitis B infection [34] mainly includes susceptible hepatocytes, infected hepatocytes, and hepatitis B virus. Uninfected hepatocytes are produced at a certain rate. HBV particles can invade hepatocytes through interaction with the receptors on the liver surface. They reproduce new viruses by various functions and release them into the bloodstream with the lysis of infected cells. The hepatitis B virus genes can naturally be lost which lead to a certain proportion of the infected cells transforming into uninfected hepatocytes. At the same time, liver cells and viruses will gradually die out. The simplified process is shown in Figure 1. And based on this process, we first give the Caputo-Fabrizio model based on the standard incidence as follows:
Here
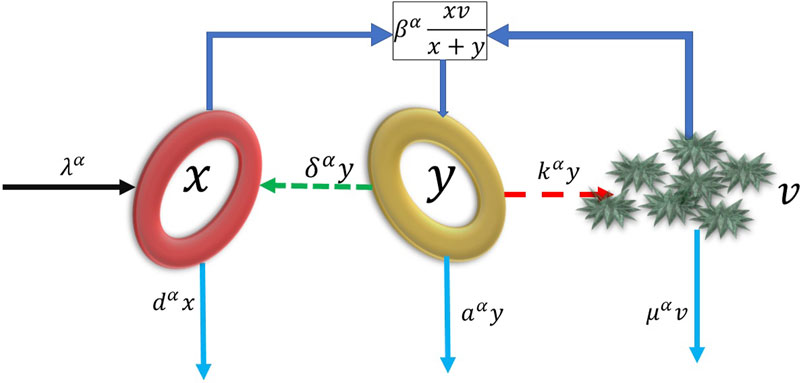
FIGURE 1. Diagram for basic virus dynamics model. x, y, v represent the healthy hepatocytes, infected hepatocytes and hepatitis B virus respectively. The healthy hepatocytes are produced at the rate of λα and die at dα. At the same time, it can be invaded by hepatitis B virus and become infected hepatocytes at the rate of
Assume that λ = λα, d = dα, β = βα, δ = δα, a = aα, k = kα, μ = μα, model 1) will be transformed to model (2). And the meaning of parameters in our framework is given in Table 1.
Since our model is a fractional order model, CF represents the Caputo-Fabrizio order, and here we will give some basic definition of Caputo-Fabrizio fractional calculus in Definition 1 and 2.
Let
Definition 1 ([21]) The definition of Caputo-Fabrizio order differential was later modified by Losada and Nieto as
Definition 2. ([21]) Let
Remark 1. In Definition 1, the definition of Eq. 4 is the mean of
3 Stability Analysis
In biology models, the stable analysis of the system can help us to understand the process of the biology phenomenon. The following two basic lemmas [35] can be used for stability analysis. Lemma 1 give the characteristic equation form of Caputo-Fabrizio order linear system. Lemma two give the criterion of local stability.
Lemma 1. Let
Lemma 2. Suppose that the matrix
Theorem 1. The disease-free equilibriumE0is locally asymptotically stable ifR0 < 1. IfR0 > 1, it is unstable.Proof. The Jacobian matrix at E0 is given:
From Lemma 1, the characteristic equation of system (2) is
Here
Theorem 2.IfR0 > 1, the endemic equilibriumE1is asymptotically stable.
Proof. The Jacobian matrix at E1 is given as
From Lemma 1, the characteristic equation of system (2) is (I − (1 − α)J2) −αJ2 = 0 which can be rewritten as a1s3 + a2s2 + a3s1 + a4 = 0. Let
As 0 < α < 1, thus a1, a2, a3, a4 > 0. Set
As 0 < α < 1, a2a3 − a1a4 > 0 and
4 Simulation and Parameter Estimation
First we use the predictor-corrector Adams-Bashforth-Moulton (ABM) technique of the Caputo-Fabrizio equation to carry out the simulation. we will give the simulation results of equilibria E0 and E1. And the initial values are given in Table. 2.
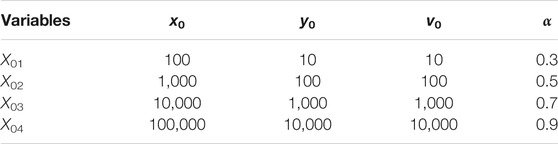
TABLE 2. The initial values of the simulation of E0 and E1. Here x0, y0, v0 are the initial values of the healthy, infected hepatocyte and HBV.
For the disease-free equilibrium E0, we select the parameters in Table. 3 which is satisfied the condition in Theorem 1. The simulation results are shown in Figure 2. As we have proved the local stability of the equilibrium in Section 3 and Figure 2 can also validates this proof. Here x can approach to 500 which is
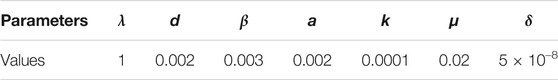
TABLE 3. The parameters using in the simulation of disease-free equilibrium E0. The meaning of the parameters are in Table. 1. Here the basic reproduction number R0 = 0.0075 < 1.
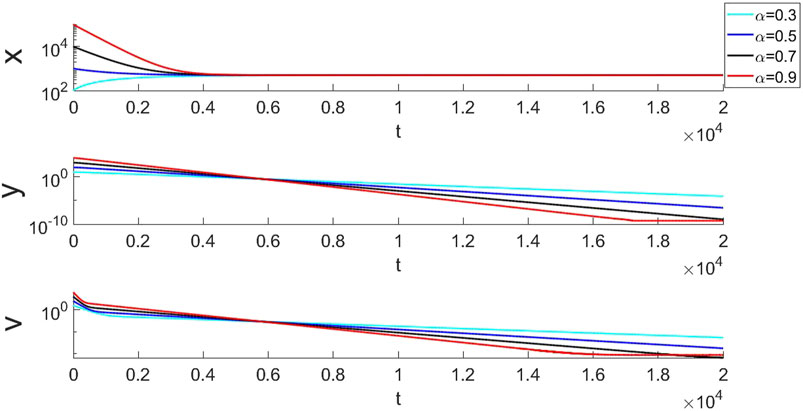
FIGURE 2. The simulation results of E0 with four different set of initial values. Under different fractional orders and initial values, x can always approach to 500 with different initial values. y and v approaches to 0.
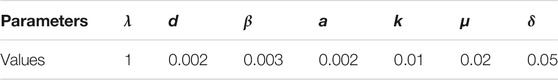
TABLE 4. The parameters using in the simulation of endemic equilibrium E1. The basic reproducion number R0 = 2 > 1.
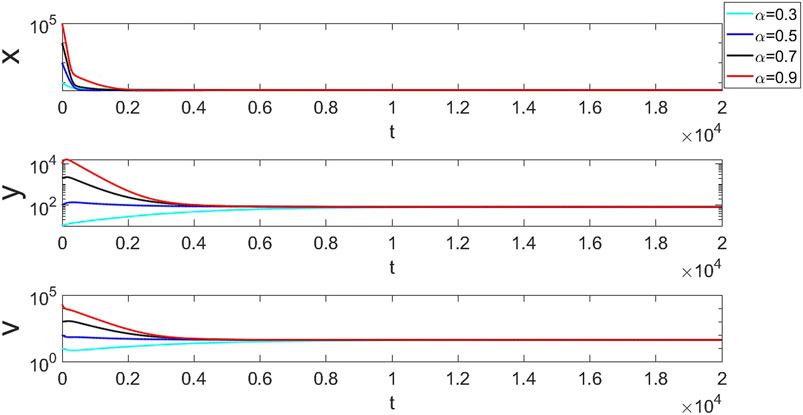
FIGURE 3. The simulation results of the endemic equilibrium E1. In this simulation, x can always approach to 41.667. y, v are 81.3 and 41.667 respectively. This is consistent with our calculation of the value of the equilibrium point.
Then, we use the real data in Table 5 [37] to estimate the parameters of our model. The estimation range of parameters are

TABLE 5. The number of hepatitis B virus and the corresponding measurement time during the treatment of hepatitis B patients. The number of HBV here is shown in log format.

TABLE 6. The estimated parameters by the least square method with using the read data of HBV virus and date. The parameters estimated here are in the normal range.
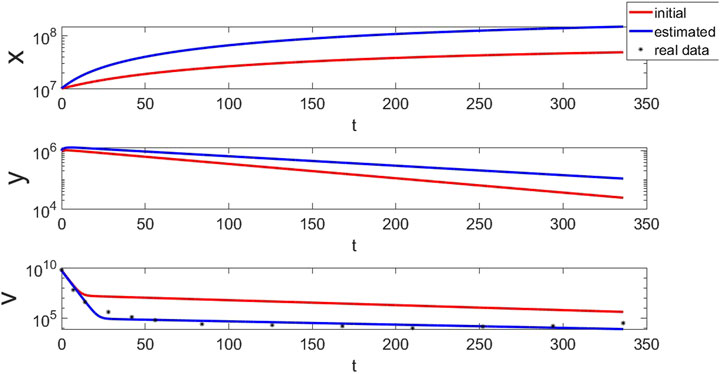
FIGURE 4. The simulation results using the estimated parameters in Table. 5. Although the simulation results of the initial parameters are quite different from the real data, it can fit real data well with the estimated parameters.
5 Optimal Control Problem
In paper [38], a combined treatment of HBV infection was designed with Entecavir (ETV) and Chinese herbal Tiaogan Jianpi Jiedu granules (TGJPJD). ETV can be used to reduce the replication of hepatitis B virus [39]. TGJPJD can kill hepatitis B virus by activating immunity. And based on the combined therapy of HBV, we propose our control model.
The parameters have the same meaning as given in Table. 1 u1(t) (0 < u1(t) < 1) and u2(t) (0 < u2(t) < 1) are the control variables and represent the drug effect of ETV and TGJPJD respectively. In order to achieve the therapeutic effect with lower the cost and side effects, we give the following objective function. Here a2, a3, b2, b3, c1, c2 represent the corresponding weight of each variable.
The Hamilton function of
Optimization process has many applications in dynamic systems [40–42]. In our paper, the optimal control problem is a process in which we find the best u(t) to minimize the performance index functional J(t). By using Pontryagin’s minimum principlex [43], we can transform the original problem into a constrained functional and find its theoretical optimization. And the optimal control therapy our model based the above theorem which can make J(u) minimum are given as follows:
Using the parameters as we estimated in Section 5, we give the simulation results in Figure 5 and Figure 6. From the simulation in Figure 5 and Figure 6, we can see that the dosage of the two drugs should be kept at the maximum at the initial stage, but the dosage of TGJPJD can be gradually reduced at about 138 days and will last 12 days. The dosage of ETV could be reduced at about 150 days, but the reduction of ETV would result in the rebound of virus, it is necessary to further strengthen the dosage of TGJPJD. So the dose of TGJPJD will increase at about 150 days and then reduce. Finally, it will be the lowest dose at about 174 days. With the enhancement of the immunity by TGJPJD, the two drugs can be maintained at a lower level to achieve better therapeutic effect.
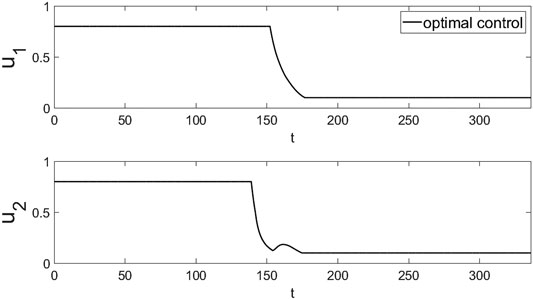
FIGURE 5. The optimal control results of the two drugs. Here u1(t) is the drug dosage of ETV and u2(t) is TGJPJD. And u1 reduce at 152 days and will last until 177 days. u2 will first reduce at about 138 days until 150 days. Then, due to the rebound of the virus, its dosage will increase in about 152 days and then decrease.
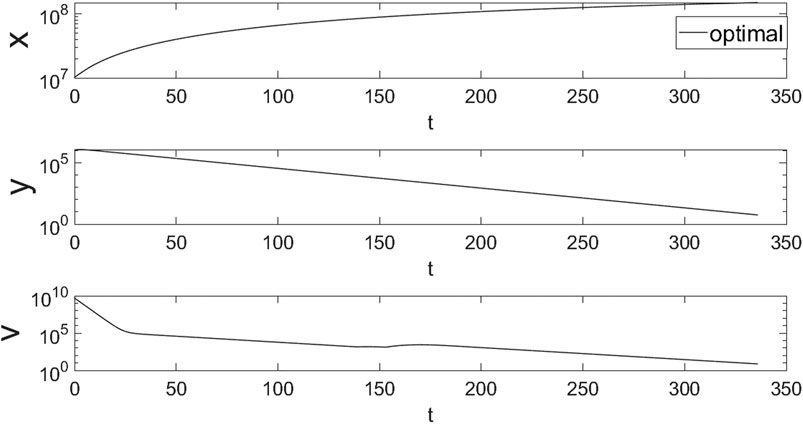
FIGURE 6. The simulation results of the hepatocytes and hepatitis B virus under the optimal control. Both y and v will finally reduce to a satisfying results. And v will have a rebound due to the decrease of ETV dose at about 150 days.
In Figure 6, the healthy hepatocytes will always keep increasing and then begin to stabilize. And the infected cells will reduce due to the drugs and the reduce of virus. hepatitis B virus will first reduce at about 150 days. And then due to the decrease of the ETV, the number of viruses showed a rebound trend. However, by adjusting the dosage of TGJPJD granule, the final number of virus can be reduced to less than 500, reaching the standard of cure.
In order to show the advantages of optimal control of therapeutic strategies, we also compare the objective function values of optimal as we give above with the maximizing drug dosage (u1 = u2 = 0.9) and minimizing drug dosage (u1 = u2 = 0.1), and show them in Table. 7. The results show that with the optimal therapy, the cost can be minimized, and the corresponding treatment effect can also be satisfying.

TABLE 7. The comparasion of objective function J with different drug dosage. The maximum dosage is (u1 = u2 = 0.9) and the minimum dosage is (u1 = u2 = 0.1).
6 Conclusion
In this paper, a Caputo-Fabrizio fractional HBV model with standard incidence were proposed. The local stability of the equilibria were analyzed and global stability were simulated. At the same time, parameters were estimated based on the real data. With the estimated parameters, our model can simulate the data well. Finally, based on clinic combination therapy of HBV with ETV and TGJPJD, we proposed an optimal control model and gave the optimal therapy, which showed therapeutic effect can be achieved with lower drug dosage and side effects. However, cellular immunity and humoral immunity are not carefully considered in our model. In the optimal control model, the metabolism of drugs in the liver is not analyzed, and HBV replication in cells is not reflected. For our future work, we will apply the latest therapeutic regimens for chronic hepatitis B in our model and analyze it according to the corresponding clinical data. The metabolism of drugs should also be considered. Besides, we will consider the effect of cellular immunity and humoral immunity which is essential.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author Contributions
All authors conceived the study and performed the analyses. LY wrote the code. LY and YS wrote the article. All authors reviewed and revised the article.
Funding
This work is jointly supported by the Fundamental Research Funds for the Central Universities of China, grants 2020XD-A01-1, National Natural Science Foundation of China 71871233) and Beijing Natural Science Foundation (9182015), 2015 National traditional Medicine Clinical Research Base Business Construction Special Topics (JDZX2015299).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Le Mire MF, Miller DS, Foster WK, Burrell CJ, Jilbert AR. Covalently Closed Circular Dna Is the Predominant Form of Duck Hepatitis B Virus Dna that Persists Following Transient Infection. J Virol (2005) 79:12242–52. doi:10.1128/jvi.79.19.12242-12252.2005
2. Hoofnagle JH, Doo E, Liang TJ, Fleischer R, Lok AS. Management of Hepatitis B: Summary of a Clinical Research Workshop. Hepatology (2007) 45:1056–75. doi:10.1002/hep.21627
3. Huzair F, Sturdy S. Biotechnology and the Transformation of Vaccine Innovation: The Case of the Hepatitis B Vaccines 1968-2000. Stud Hist Philos Sci C Stud Hist Philos Biol Biomed Sci (2017) 64:11–21. doi:10.1016/j.shpsc.2017.05.004
4. Who . Hepatitis B virues, WHO. Available at: https://www.who.int/news-room/fact-sheets/detail/hepatitis-b and accessed date: 27 July 2021.
5. Su Y, Sun D, Zhao L. Global Analysis of a Humoral and Cellular Immunity Virus Dynamics Model with the Beddington-Deangelis Incidence Rate. Math Meth Appl Sci (2015) 38:2984–93. doi:10.1002/mma.3274
6. Din A, Li Y, Liu Q. Viral Dynamics and Control of Hepatitis B Virus (Hbv) Using an Epidemic Model. Alexandria Eng J (2020) 59:667–79. doi:10.1016/j.aej.2020.01.034
7. Li H-J, Wang L, Bu Z, Cao J, Shi Y. Measuring the Network Vulnerability Based on Markov Criticality. ACM Trans Knowl Discov Data 16 (2021). p. 1–24. doi:10.1145/3464390
8. Manna K, Chakrabarty SP. Chronic Hepatitis B Infection and Hbv Dna-Containing Capsids: Modeling and Analysis. Commun Nonlinear Sci Numer Simulation (2015) 22:383–95. doi:10.1016/j.cnsns.2014.08.036
9. Hattaf K, Yousfi N. A Generalized Hbv Model with Diffusion and Two Delays. Comput Math Appl (2015) 69:31–40. doi:10.1016/j.camwa.2014.11.010
10. Diethelm K. The Analysis of Fractional Differential Equations: An Application-Oriented Exposition Using Differential Operators of Caputo Type. Lecture Notes Math (2010) 2004:85–132. doi:10.1007/978-3-642-14574-2
11. Kumar D, Singh J, Baleanu D. Numerical Computation of a Fractional Model of Differential-Difference Equation. J Comput Nonlinear Dyn (2016) 11. doi:10.1115/1.4033899
12. Area I, Batarfi H, Losada J, Nieto JJ, Shammakh W, Torres Á. On a Fractional Order Ebola Epidemic Model. Adv Differ Equ, (2015) 2015 1–12. doi:10.1186/s13662-015-0613-5
13. Ma M, Baleanu D, Gasimov YS, Yang XJ. New Results for Multidimensional Diffusion Equations in Fractal Dimensional Space. Rom J Phys (2016) 61:784–794.
14. Günerhan H, Dutta H, Dokuyucu MA, Adel W. Analysis of a Fractional Hiv Model with Caputo and Constant Proportional Caputo Operators. Chaos, Solitons & Fractals (2020) 139:110053. doi:10.1016/j.chaos.2020.110053
15. Fatmawati , Khan MA, Odinsyah HP. Fractional Model of Hiv Transmission with Awareness Effect. Chaos, Solitons & Fractals (2020) 138:109967. doi:10.1016/j.chaos.2020.109967
16. Naik PA, Zu J, Owolabi KM. Global Dynamics of a Fractional Order Model for the Transmission of Hiv Epidemic with Optimal Control. Chaos, Solitons & Fractals (2020) 138:109826. doi:10.1016/j.chaos.2020.109826
17. Ahmed E, El-Sayed AMA, El-Saka HAA. On Some Routh-Hurwitz Conditions for Fractional Order Differential Equations and Their Applications in Lorenz, Rössler, Chua and Chen Systems. Phys Lett A (2006) 358:1–4. doi:10.1016/j.physleta.2006.04.087
18. Boukhouima A, Hattaf K, Yousfi N. Dynamics of a Fractional Order Hiv Infection Model with Specific Functional Response and Cure Rate. Int J Differential Equations (2017) 2017:1–8. doi:10.1155/2017/8372140
19. Odibat ZM, Shawagfeh NT. Generalized Taylor's Formula. Appl Math Comput (2007) 186:286–93. doi:10.1016/j.amc.2006.07.102
20. Caputo M, Fabrizio M. A New Definition of Fractional Derivative without Singular Kernel. Prog Fractional Differ Appl (2015) 1:487–497. doi:10.12785/pfda/010201
21. Losada J, Nieto JJ. Properties of a New Fractional Derivative without Singular Kernel. Prog Fractional Differ Appl (2015) 1:87–92. doi:10.12785/pfda/010202 Available at https://www.who.int/news-room/fact-sheets/detail/hepatitis-b” [Accessed July 27, 2021]
22. Ortigueira MD, Machado JT. A Critical Analysis of the Caputo–Fabrizio Operator. Commun Nonlinear Sci Numer Simulation 59 (2018). doi:10.1016/j.cnsns.2017.12.001
23. Uçar E, Özdemir N. A Fractional Model of Cancer-Immune System with Caputo and Caputo–Fabrizio Derivatives. Eur Phys J Plus (2021) 136:1–17. doi:10.1140/epjp/s13360-020-00966-9
24. Moore EJ, Sirisubtawee S, Koonprasert S. A Caputo–Fabrizio Fractional Differential Equation Model for Hiv/aids with Treatment Compartment. Adv Difference Equations (2019) 2019. doi:10.1186/s13662-019-2138-9
25. Baleanu D, Jajarmi A, Mohammadi H, Rezapour S. A New Study on the Mathematical Modelling of Human Liver with Caputo–Fabrizio Fractional Derivative. Chaos, Solitons and Fractals (2020) 134:109705. doi:10.1016/j.chaos.2020.109705
26. Mohammadi H, Kumar S, Rezapour S, Etemad S. A Theoretical Study of the Caputo–Fabrizio Fractional Modeling for Hearing Loss Due to Mumps Virus with Optimal Control. Chaos, Solitons and Fractals (2021) 144:110668. doi:10.1016/j.chaos.2021.110668
27. Sene N. Stability Analysis of the Fractional Differential Equations with the Caputo-Fabrizio Fractional Derivative. J Fractional Calculus Appl (2020) 1:160–72.
28. Nowak MA, Bangham CR. Population Dynamics of Immune Responses to Persistent Viruses. Science (1996) 272:74. doi:10.1126/science.272.5258.74
29. Min L, Su Y, Kuang Y. Mathematical Analysis of a Basic Virus Infection Model with Application to Hbv Infection. Rocky Mountain J Math (2008) 38:1573–85. doi:10.1216/rmj-2008-38-5-1573
30. Ji Y, Min L, Ye Y. Global Analysis of a Viral Infection Model with Application to Hbv Infection. J Biol Syst (2010) 18:325–37. doi:10.1142/S0218339010003299
31. Salman S, Yousef A. On a Fractional-Order Model for Hbv Infection with Cure of Infected Cells. J Egypt Math Soc (2017) 25:445–451. doi:10.1016/j.joems.2017.06.003
32. Ahmad S, ur Rahman M, Arfan M. On the Analysis of Semi-analytical Solutions of Hepatitis B Epidemic Model under the Caputo-Fabrizio Operator. Chaos, Solitons and Fractals (2021) 146:110892. doi:10.1016/j.chaos.2021.110892
33. Gao F, Li X, Li W, Zhou X. Stability Analysis of a Fractional-Order Novel Hepatitis B Virus Model with Immune Delay Based on Caputo-Fabrizio Derivative. Chaos, Solitons and Fractals (2021) 142. doi:10.1016/j.chaos.2020.110436
34. Iannacone M, Guidotti L. Immunobiology and Pathogenesis of Hepatitis B Virus Infection. Nat Rev Immunol (2021):1–14. doi:10.1038/s41577-021-00549-4
35. Li H, Cheng J, Li HB, Zhong SM. Stability Analysis of a Fractional-Order Linear System Described by the Caputo-Fabrizio Derivative. Mathematics (2019) 7. doi:10.3390/math7020200
36. Dreessche P, Watmough J. Reproduction Numbers and Sub-threshold Endemic Equilibria for Compartmental Models of Disease Transmission. Math Biosciences (2002) 180:29–48. doi:10.1016/S0025-5564(02)00108-6
37. Young P, Woo J. The Adefovir-Lamivndine Combination Therapy Effectively Suppresses the Emergence of Geotypic Resistance to Adefovir in Lamivudine Resistant Hepatitis B. Hepatol Int (2009) 3:70–85. doi:10.1007/s12072-008-9120-z
38. Li X, Zhou D, Chi X, Li Q, Wang L, Lu B, et al. Entecavir Combining Chinese Herbal Medicine for Hbeag-Positive Chronic Hepatitis B Patients: a Randomized, Controlled Trial. Hepatol Int (2020) 14:985–996. doi:10.1007/s12072-020-10097-z
39. Park JY, Kim CW, Bae SH. Entecavir Plus Tenofovir Combination Therapy in Patients with Multidrug‐resistant Chronic Hepatitis B: Results of a Multicentre, Prospective Study [J]. Liver Int (2016) 36(8):1108–1115.
40. Li H, Bu Z, Wang Z, Cao J. Dynamical Clustering in Electronic Commerce Systems via Optimization and Leadership Expansion. IEEE Trans Ind Inform (2020) 16:5327–34. doi:10.1109/TII.2019.2960835
41. Li H, Wang Z, Pei J, Cao J, Shi Y. Optimal Estimation of Low Rank Factors via Feature Level Data Fusion of Multiplex Signal Systems. IEEE Trans Knowledge Data Eng (2020) 1–24. doi:10.1109/TKDE.2020.3015914
42. Brokate M. Pontryagin’s Principle for Control Problems in Age-dependent Population Dynamics. J Math Biol (1985) 23:75–101. doi:10.1007/bf00276559
Keywords: caputo-fabrizio, optimal control, parameter estimation, HBV model, stability
Citation: Yang L, Su Y, Yang X and Wang Z (2021) The Optimal Control Strategy of Virus Transmission Based on Caputo-Fabrizio Order. Front. Phys. 9:731972. doi: 10.3389/fphy.2021.731972
Received: 28 June 2021; Accepted: 12 August 2021;
Published: 03 September 2021.
Edited by:
Hui-Jia Li, Beijing University of Posts and Telecommunications (BUPT), ChinaReviewed by:
Wen-Xuan Wang, Beijing University of Posts and Telecommunications (BUPT), ChinaLing-Yun Wu, Academy of Mathematics and Systems Science (CAS), China
Copyright © 2021 Yang, Su, Yang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yongmei Su, c3V5bTcxQHVzdGIuZWR1LmNu
 Liangli Yang
Liangli Yang Yongmei Su1*
Yongmei Su1*