- 1Microbiology Laboratory, Department of Botany, Berhampur University, Berhampur, Odisha, India
- 2Bioenergy Lab, School of Biotechnology, Campus-11, KIIT Deemed-to-be-University, Bhubaneswar, Odisha, India
- 3School of Biotechnology, Campus-11, KIIT Deemed-to-be-University, Bhubaneswar, Odisha, India
- 4General Department of Medical Services, Ministry of Interior, Riyadh, Saudi Arabia
- 5Department of Pharmaceutical Chemistry, College of Pharmacy, King Saud University, Riyadh, Saudi Arabia
- 6Department of Chemistry, Government College of Engineering, Keonjhar, Odisha, India
Ethnopharmacological relevance: Therapeutic botanicals (plants and derivatives) are in use since antiquity for various health ailments. The ethnic community is the repository of the information, the multifactorial therapeutic applications of which may often need scientific validation. The spreading hogweed or Boerhaavia diffusa L., also known as Punarnava, is a reassuring medicinal herb with diverse pharmacological benefits. It is used in Ayurveda in Asia and Africa as a rejuvenator or “Rasayan” for its excellent antiaging and antioxidant properties.
Aim: The study aimed at compiling the state-of-art knowledge of the medicinal benefits of Boerhaavia diffusa L. and unraveling the unexplored commercially useful bioactive constituents by establishing their possible pharmacological benefits.
Methods: The data from published literature, confined to pharmacological manifestations of various phytocomponents of Boerhaavia diffusa L. or its parts like root, leaf and stem were extracted from scientific databases, Google, Science Direct, PubMed, etc. using its antifungal, antibacterial, anticancer, anti-inflammatory, antidiabetic, hepatoprotective, cardioprotective, renoprotective, antifertility benefits and molecular docking study as search strings and keywords. Further, the reported in silico studies for bioactivity and bioavailability are detailed.
Results: The botanicals possess numerous bioactive compounds, the most widely reported ones being phenolic (punarnavoside, trans-caftaric acid, boerhavic acid), rotenoid (boeravinones A-J), flavonoid (borhaavone, quercetin, kaempferol), isoflavonoid (2′-O-methyl abronisoflavone), alkaloid (punarnavine), steroid (boerhavisterol, β-Ecdysone), anthracenes and lignans (liriodendrin, syringaresinol mono-β-D-glucoside). Some of the reported reassuring benefits of their purified forms or even the crude extracts are antidiabetic, antimicrobial, anticancer, antioxidant, anti-inflammatory, hepatoprotective, renoprotective, cardioprotective, antifertility, etc.
Conclusion: The article provides an extensive study on such pharmacological utility to support the ethnomedicinal use of Boerhaavia diffusa L. and propose possible mechanism of the various bioactive compounds in optimising metabolic dysfunctions, healing and protecting vital body organs, often related to the magnificent antioxidant property of this ayurvedic panacea. Further, establishing specific roles of its yet-to-explore bioactive constituents for diverse pharmacological applications is suggested.
Highlights
1. Popular among ethnic communities as a leafy vegetable, every plant part has medicinal value
2. Plant parts have protective and therapeutic values especially towards critical internal organs
3. Being herbal derivatives, majority of the bioactive compounds are evidently less cytotoxic
4. Few derived bioactive compounds are commercially exploited; few others are unexplored yet
5. The prophylactic and therapeutic properties of bioactive extracts are detailed bioinformatically
6. Exploitation of the antimicrobial properties could protect humans against infectious diseases
Introduction
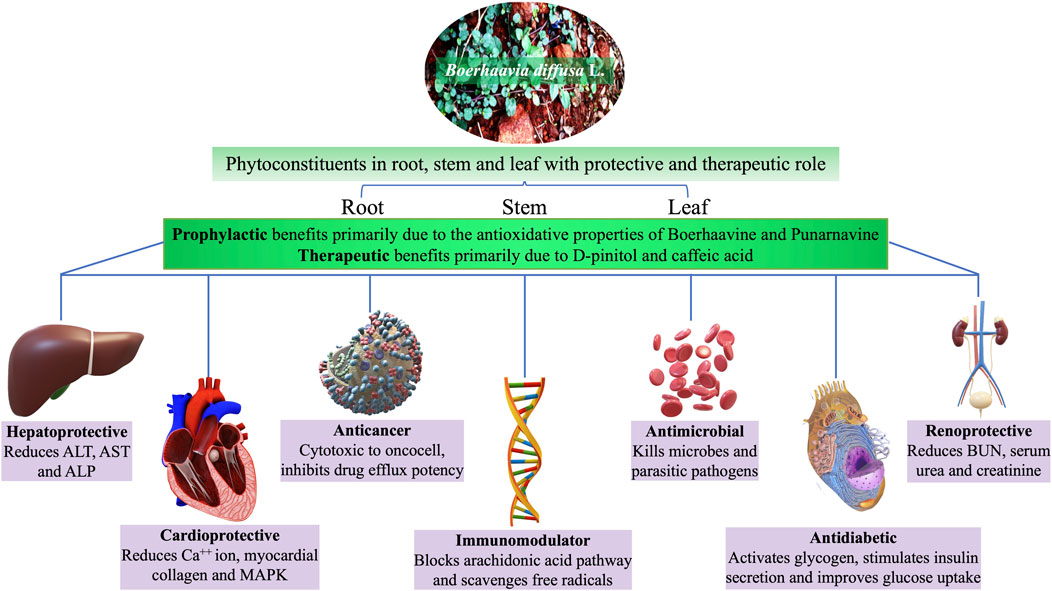
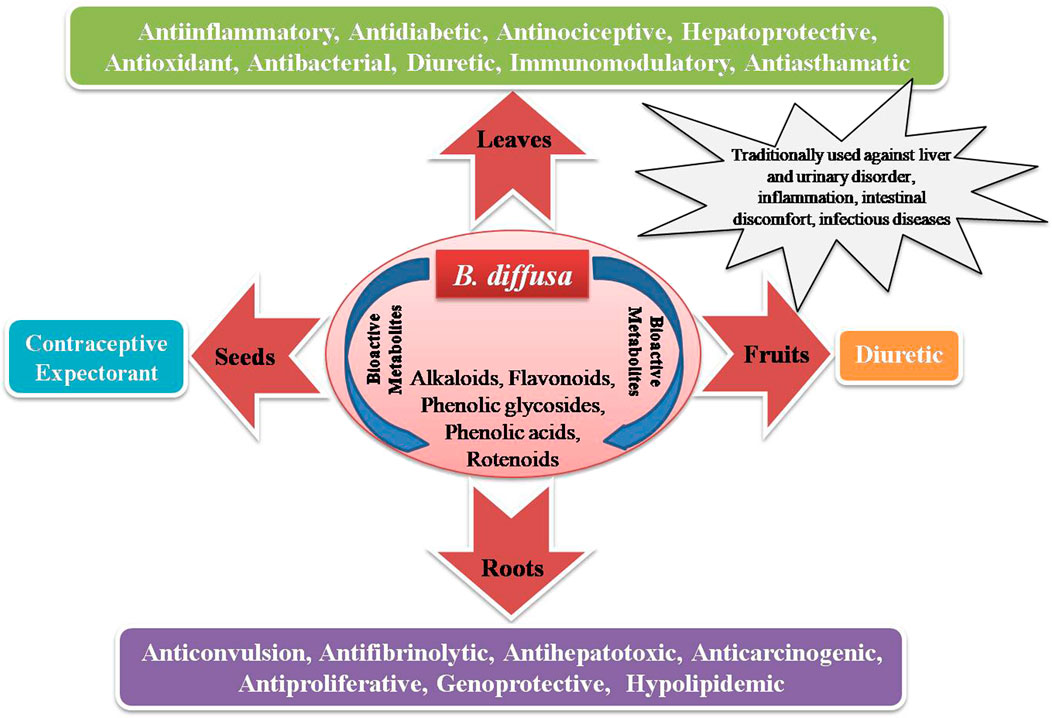
Boerhaavia diffusa L. (BD) of family Nyctaginaceae, commonly known as spreading hogweed, is a perennial, prostrate herb with pink flowers and sticky fruits (Figure 1). Popularly called Punarnava, it means renewer or rejuvenator of body because of its anti-aging property (Wahi et al., 1997). Its old root stock remains dormant during summer and regenerates during the rain. The various parts of Punarnava are rich in diverse bioactive compounds and are extensively used as a “rasayan” for its exceptional properties like immunity boosting, reestablishing youthfulness, and strengthening the body and mind (Samhita and Varanasi, 1998). Oxidative stress (due to an imbalance between the reactive oxygen species or oxidants production and their utilisation) often leads to diseases like dementia, Alzheimer’s disease, Parkinson’s disease, heart attack, myocardial infarction and other gerontological ailments (Demopoulos et al., 1980; Cadet, 1998). Promising antioxidant property of the BD parts makes it special for pharmacological applications (Das et al., 2022). Both the root and the shoot systems of this wonder plant are rich primarily in phenolics (punarnavoside, trans-caftaric acid and boerhavic acid), rotenoids (boeravinones A-J), flavonoids (borhaavone, quercetin, kaempferol), isoflavonoids (2′-O-methyl abronisoflavone), alkaloids (punarnavine), steroids (boerhavisterol, β-Ecdysone), anthracenes and lignans (liriodendrin, syringaresinol mono-β-D-glucoside) and many fatty acids and proteins contributing to its incredible biotherapeutic potential (Gaur et al., 2022). Its antidiabetic, antimicrobial and other similar bioactivities of BD is extensively studied, reviewed and are reported often.
Although, historically and ethnomedicinally the plant has been associated among especially the Asian and African ethnic population both as a food as well as an ayurvedic intervention, however the transition into the modern civilisation and the medical sciences is yet to realise its potential. The wellbeing benefits of BD are so much more everencompassing and reassuring that, considering it as a panacea against an array of modern day diseases shall not be an overstatement. In light of this, extensive research and both vertical (deeper insights into the heather to unknown medicinal benefits) as well as horizontal (spread of the medicinal benefits in the global population across ethnicity and race defying the geographical barriers) understandings shall benefit the humanity in general and the ethnic population in particular.
This article attempts to compile the advances on the therapeutic role of BD for multiple human health benefits including in metabolic disorders, vital organ protection, antimicrobial, anticancer, anti-inflammatory and healing properties against various diseases especially in the last decade (2013–2023), to validate and support its ethnomedicinal utility. It attempts also to reorients and synthesise all its pharmacological aspects, the possible relationship between phytocompounds, the bioactivities, and the probable mechanism of action in blood sugar management. BD is an incredible herbal remedy containing an array of pharmaceutically active chemical components.
BD has free radicals scavenging and glutathione content increasing antioxidants that help treat hepatitis and hepatic cirrhosis. BD leaf is a source of significant quantity of flavonoid glycoside moiety with high eupalitin-3-O-D-galactopyranoside (yellow powder crystal) yield. Silymarin, a flavonoid glycoside structurally similar to eupalitin-3-O-D-galactopyranoside was the only known natural stuff used for hepatoprotection till late. Human hepatoma cell lines are proposed as an alternative to human hepatocytes for in vitro modeling of normal liver cells. HepG2 hepatoma cell line is widely used in liver function, metabolism and drug toxicity research. The biochemical and morphological properties of these cells being similar to normal hepatocytes, they are used in studies that determine the hepatoprotective properties of medicinal plants.
Materials and methods
Relevant research and review articles were considered, and the mentioned aspects were collected and compiled. Extensive literature search on the medicinal, antimicrobial and pharmacological properties of the whole plant or the parts of BD was conducted using specialised and dedicated search engines and websites like Google Scholar, PubMed, ScienceDirect, EBSCO, Sci-Hub, SciFinder, etc. Major thrust was on the literature that covered the antibacterial, antifungal, anticancer, anti-inflammatory, hepatoprotective, cardioprotective, renoprotective, antidiabetic, antifertility characteristics of BD or its parts (like root, leave, stem, etc.) and molecular docking study as keywords.
Ethnomedicinal usage
With cooling effect and bitter taste, BD is used since ages to treat various ailments. It rejuvenates the whole body enhancing the vigour and vitality upon its routine use justifying its Indian name Punarnava. It has emetic, expectorant, diaphoretic, stomachic, laxative and diuretic properties (Nadkarni, 1976). Due to its multifarious therapeutic potential against abdominal pain, diarrhoea, epilepsy, dysentery, urinary and kidney complications, jaundice, anemia, pneumonia, splenomegaly, etc., it is extensively used globally as a rejuvenator in various medicinal formulations, especially in Asia, Africa and Latin America. It is a well-known tonic, blood purifier and a uterine bleeding preventer to check postpartum heamorrhage. Also, it is useful in wound healing and skin ailments like itching and eczema (Agrawal et al., 2011). It improves digestion, maintains healthy body mass index, prevents anaemia, hernia and respiratory distress. Its root extract is a potential kidney, heart and liver stimulant. It protects dilapidated kidneys in the diabetic. Due to its diuretic, renoprotective and laxative properties, it is useful in treating asthma, constipation, cough and detoxifying body. It is reported to be used against dropsy, ascites, gonorrohea, swelling of legs, intestinal worm infestation, jaundice and other hepatic complications. It relieves from inflammation and joint pains, boosts immunity and strengthens the lungs. Its root paste may be used as a miraculous dressing for ulcers and swellings. It is very much beneficial in nerve flaws and paralysis, in treating fever and a loss of appetite (Bhowmik et al., 2012). In case of ascites, liver cirrhosis is normally followed by congestive heart failure. Here herbal diuretics are preferred to correct abnormal fluid dynamics in the body. Having anti-inflammatory property, the whole leaf or its formulation is taken orally or applied locally for wound-healing or to detoxify from scorpion sting and snake bites (Mishra et al., 2014).
Pharmacological properties of BD
Different BD parts like root, leaf, flower, fruit and seed are frequently employed to treat various ailments, either on their own or as a bioactive component in medicinal formulations (Rao, 2016). Distinct BD parts have diverse phytochemical compositions with diversified therapeutic benefits. Thoroughly analysed literature from databases agrees that BD and its parts pose numerous bioactive large variety (quality) and amount (quantity) of phytocompounds (Figure 1). The leaves are utilised as a leafy vegetable by Odias in India owing to the superior nutraceutical aspects, high on protein, fatty acid, vitamin C and B complex and calcium. Figure 1 highlights the active substances in BD leaves and the notable bioactivities. Owing to its numerous bioactivities including as antibacterial, antidiabetic, antioxidant, anticancer, hepatoprotective, renoprotective, cardioprotective, diuretic, anti-inflammatory, and immunomodulatory characteristics, in-depth research has been performed on BD (Figure 2).
Several studies conducted globally on this wonder herb correlated the diverse phytocompounds and the bioactive potentials. All the reports are classified under five categories of bioactivity, i.e., metabolic disorders, antimicrobial activities, anti-inflammatory and healing properties, anticancer property and major organ protective role of different compounds extracted from BD of its parts for better understanding and ease of citation. The extracts and isolated phytocompounds of BD studied in vivo, ex vivo and in vitro in the last decade between 2013 and 2023 are compiled and presented in Table 1 through 5 (Table 1: the prophylactic role; Table 2: antibacterial, antihelminthic and antimalarial activities; Table 3: anti-inflammatory, anticataract, wound and gastric ulcer healing properties; Table 4: anticancer and antiproliferative activities; and Table 5: organ protective role).
Protective role against metabolic disorders
Globally, 422 million people suffered from diabetes as per the global reports on diabetes by the World Health Organisation (WHO), 2023. Three out of every four diabetic belonged to younger age groups primarily in the under-developed and developing economies greatly impairing their productivity and productive years of life. Nearly 1.5 million diabetic die each year due to hyperglycaemia related complications. The diabetic is also prone to infectious diseases like TB (tuberculosis), malaria and HIV/AIDS. As the cases are rising alarmingly, diabetes is becoming a global challenge. With increasing number of diabetics in the present era majorly due to sedentary lifestyle, anomalous food habits, stress, etc., India is becoming the “diabetic hub”.
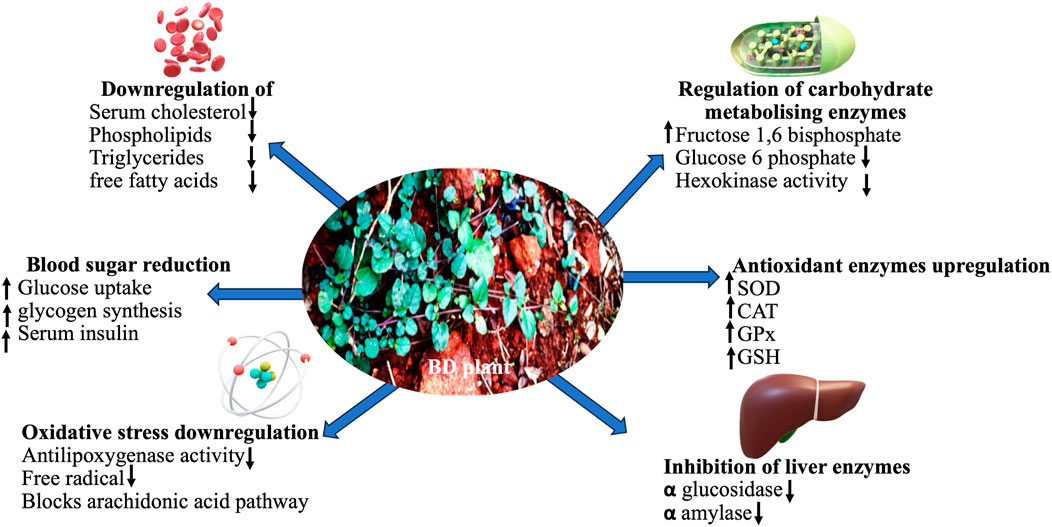
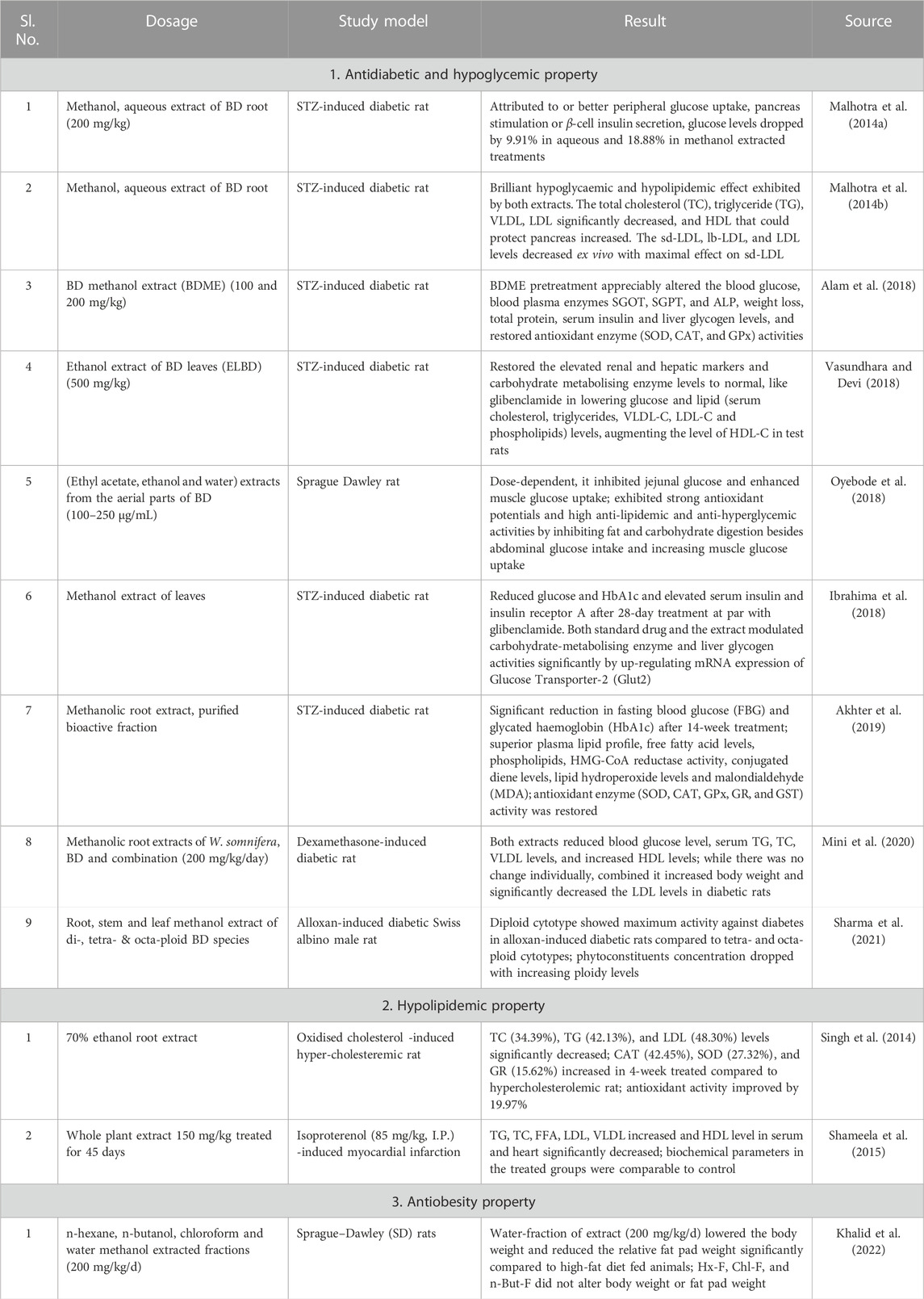
The compared antidiabetic potential of root, stem and leaf extracts of BD to standard over-the-counter drugs in streptozotocin-induced diabetic animal models significantly reduced blood glucose level, downregulated oxidative stress markers and showed free radicals scavenging ability (Montefusco-Pereira et al., 2013; Alam et al., 2018; Vasundhara and Devi, 2018). Ethanol-based leaf-extract (500 mg/kg, 45 days) altered carbohydrate metabolising enzymes considerably to near normal in Streptozotocin (STZ) (60 mg/kg I.P.) mediated diabetic rat (Vasundhara and Devi, 2018), validating its antidiabetic effect. Significantly reduced liver glycogen content in diabetic rat was restored with leaf-extract treatment, mediated through inhibited jejunal glucose uptake and augmented glucose uptake by skeletal muscle (Oyebode et al., 2018). Increased liver enzymes (ALP, SGPT, and SGOT) levels in plasma upon being released from liver cytosol into the bloodstream in STZ-induced diabetic rat confirmed liver necrosis. The methanol extract completely reversed this clinical condition in test rats. Elevated glucose levels in diabetic rats due to oxidative stress that inactivated antioxidant enzymes like SOD, CAT, and GPx possibly through glycation was stabilised by administering methanol-based BD extract (Alam et al., 2018). Elevated renal and hepatic marker levels in STZ-induced diabetic rat was normalised after treating with ethanol-based leaf-extract of BD (Vasundhara and Devi, 2018). Most of the therapeutic manifestations of BD are attributed to its incredible antioxidant properties (Das et al., 2022). Metabolic disorders often increase due to an imbalance in oxidative status. A compilation of the prophylactic role of BD reported in the last decade is presented in Table 1. The reported possible modes of action of it as an antidiabetic as proposed by various researchers are compiled in Figure 3.
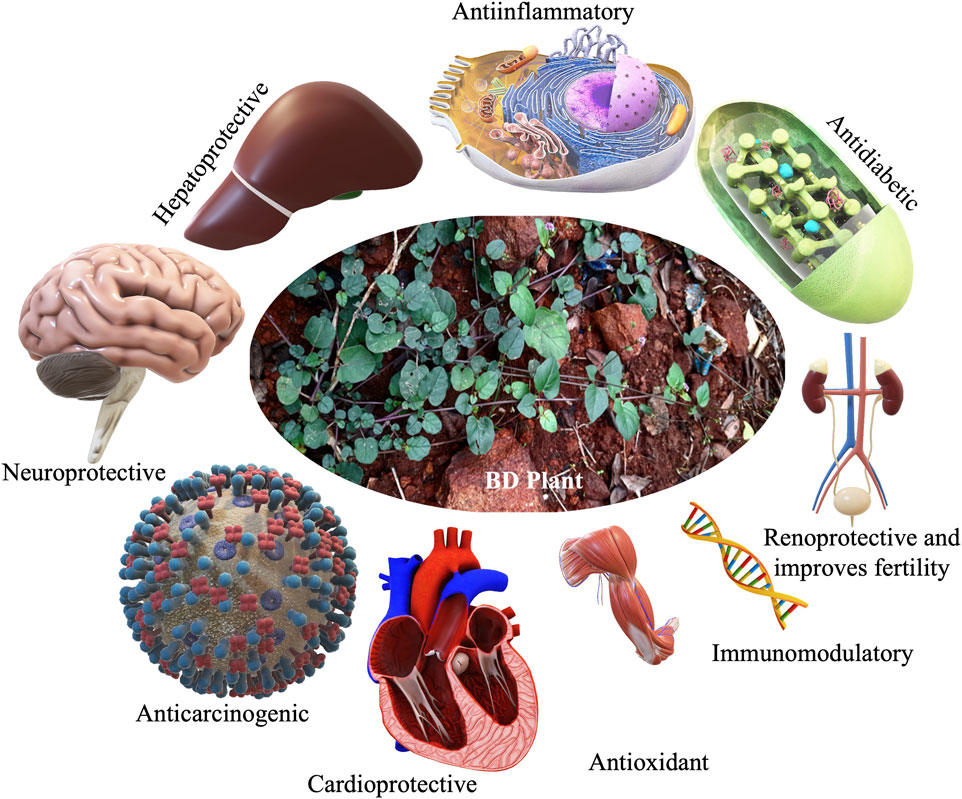
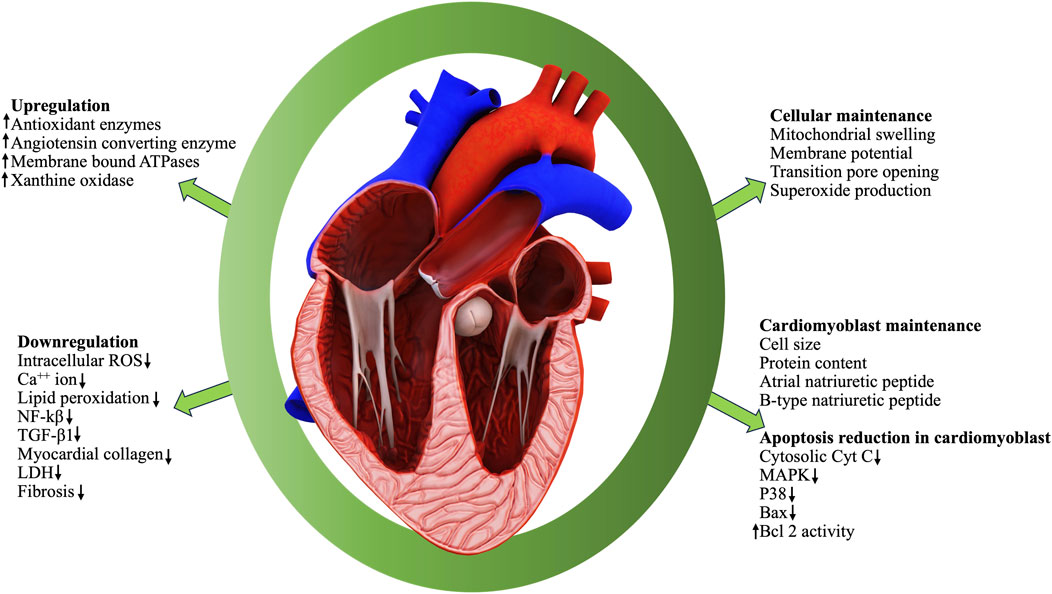
FIGURE 3. A schematic presentation of the various medicinal benefits of BD on human tissues and organs.
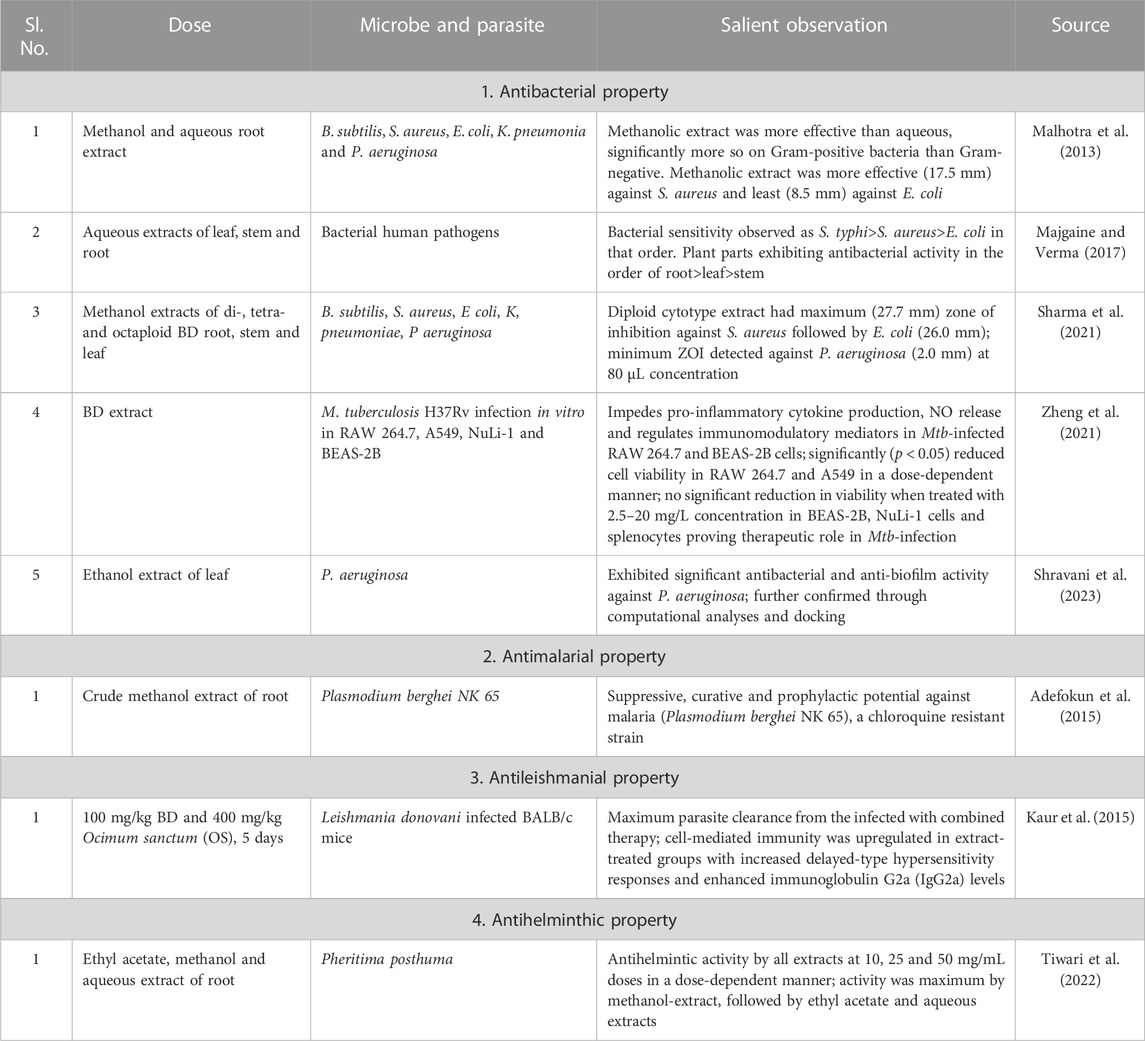
Antimicrobial activities of BD
The extracts of root, stem and leaf parts of BD have demonstrated their antimicrobial properties. Higher (200 and 100 mg/mL) doses of root extract inhibited S. aureus better with a 12–16 mm zone of inhibition, whereas the leaf extract showed similar result of 11–13 mm zone of inhibition even at lower (50 and 25 mg/mL) doses. While root extract was effective against S. typhi also, none was effective against E. coli (Majgaine and Verma, 2017), contradicting the findings of few other researchers. The antiplasmodial effectiveness was examined on Plasmodium berghei NK 65, a chloroquine-resistant strain, using suppressive, curative and preventive malaria male and female albino mice models. The extract exhibited the best antipyretic effect at 125 mg/kg by the third day in suppressive models confirming its antimalarial activity. The extract reduced the plasma Ca2+ levels better at 500 mg/kg than the nifedipine (positive control) treated at 1.043 vs. 1.35 mmol/L. Its efficacy in reducing malaria-induced pyrexia by blocking Ca2+ channel in the erythrocytes validates its ethnic use as an antimalarial (Adefokun et al., 2015). The evaluated antileishmanial activity of BD and OS on L. donovani infected BALB/c mice model had the maximum parasite clearance at 5-day BD:OS:100:400 mg/kg combination therapy. It also upregulated cell-mediated immunity with increased delayed hypersensitivity and enhanced immunoglobulin G2a (IgG2a) production. The extract normalised abnormally high serum urea, blood urea nitrogen (BUN), creatinine, glutamic oxaloacetate transaminase and glutamic pyruvate transaminase levels (Kaur et al., 2015). Reports on antibacterial, antihelminthic and antimalarial activities of BD published between 2013 and 2023 are compiled and presented in Table 2.
Anti-inflammatory, anti-cataract and wound-healing properties of BD
Methanol extract of BD leaf reportedly had promising antioxidant and anti-inflammatory activity due to high polyphenolics content. Inflammatory mediators like leukotrienes (LTs) are generated in body via 5-LOX pathway. Polyphenolics, flavonoids, saponin, etc. As antioxidants reportedly exhibited antilipoxygenase activities possibly by blocking arachidonic acid pathway and serving as free radicals scavengers (Ibrahima et al., 2018). BD is reported to have significant anti-inflammatory, anticataract, wound and gastric ulcer healing traits (Table 3). Antioxidant defense mechanism by reducing ROS production and inflammation is schematically presented in Figure 4.
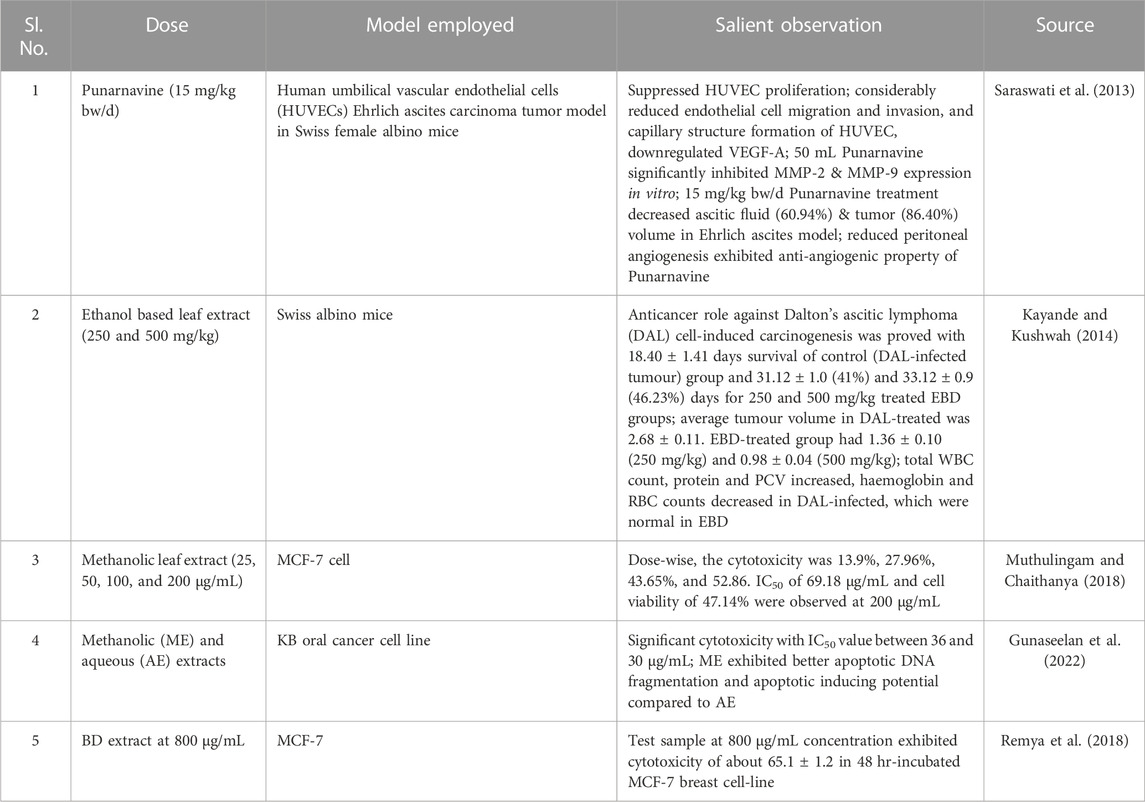
Anticancer and anti-proliferative properties of BD
The oncoprotective and chemopreventive properties of phytocompounds from BD are reported by many, often due to the antioxidant properties. Alcohol and water-based extracts could contain several biocompounds like punarnavine, punarnavosides, rotenoids, reducing sugars, starch, and lignans, liriodendrin, syrigaresinol and several boeravinones (boeravinone A–J) (Das et al., 2022). Boeravinones G and H reportedly inhibited drug efflux potency of BCRP/ABCG2, the breast cancer resistance protein, a multidrug transporter that induces chemotherapy resistance in cancer cell (Ahmed-Belkacem et al., 2007). As BD reportedly controls unregulated cell growth through S phase inhibition and apoptosis, its cytotoxic activity was effective against many tested cancer cell lines proving its claimed anticancer activity. Relevant reports published between 2013 and 2023 are presented in Table 4.
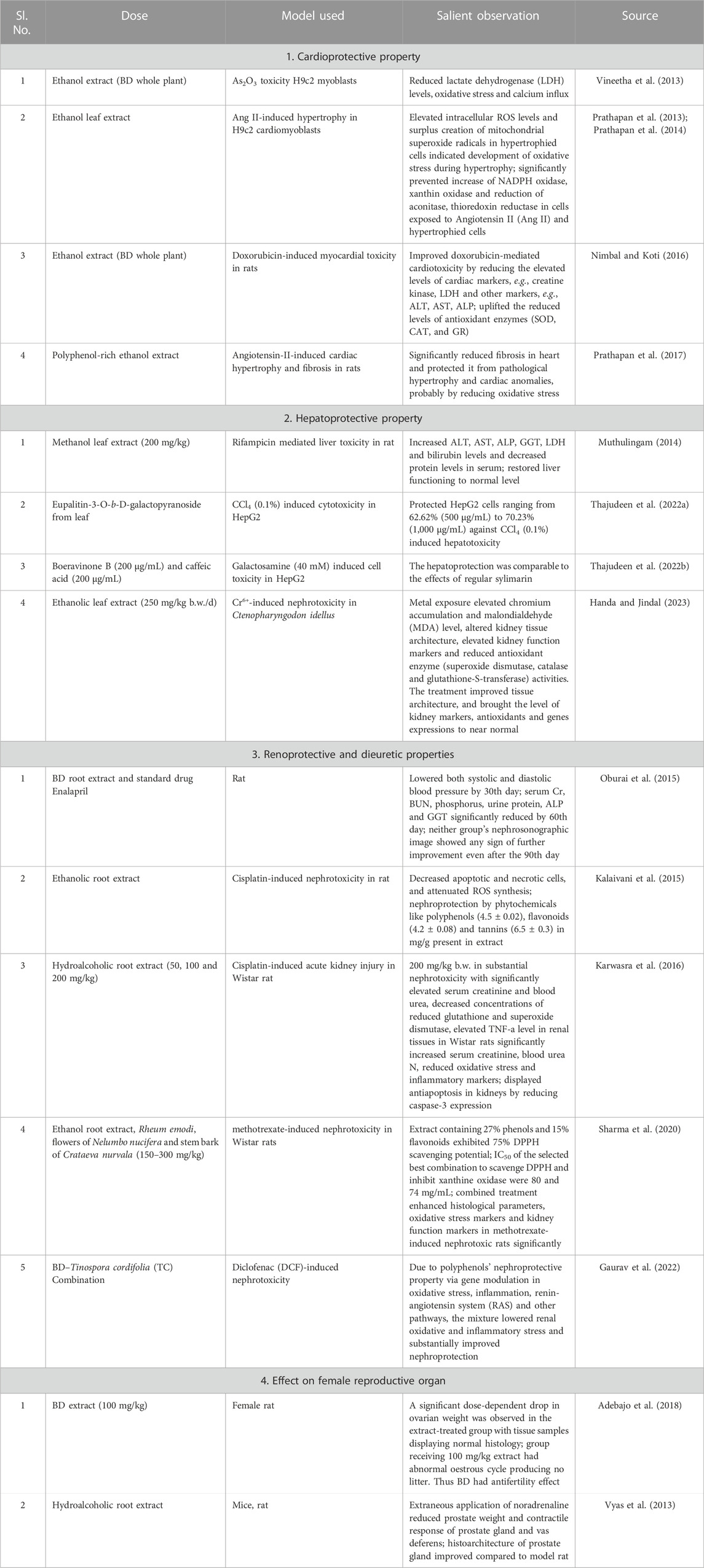
Organ protective role of BD
The whole BD is popular among ethnic community for its excellent healing properties against diseases which is time-and-again validated through scientific investigations for the proclaimed ethnopharmacological properties. Vital organs of human body such as heart (Figure 5), liver and kidney are protected by the phytocomponents safeguarding them. The various reports pertinent to the major organ protective roles of BD published in the last decade are compiled and presented in Table 5.
Bioinformatics approaches to decode the benefits
Crude BD extract is highly effective and traditionally used against mastitis caused by S. aureus. The antimastitis activity of the phytochemicals in BD was examined in silico by Sruthy et al. (2018) through docking analysis. Based on ADMET properties, 20 BD phytochemicals were selected for docking. Boeravinone A, B, C, D, E, and F had good docking scores and interacted better with the active sites of the target receptor protein of S. aureus. This study helped design novel drugs to treat mastitis. Similarly, Kaviya et al. (2022) analysed the hot and cold solvent extracted BD phytochemicals from leaves, stems and roots, and found that ethanolic root extract demonstrated clear zones of inhibition for P. aeruginosa (8 mm) and S. aureus (20 mm) at 200 µg concentration. Molecular docking of 2-(1,2 dihydroxyethyl)-5-{[2,5,7,8-tetramethyl-2-(4,8,12-trimethyltridecyl)-3,4-dihydrochromen-6-yl]oxy}oxolane-3,4-diol exhibited minimal binding score against P. aeruginosa. Patients with nephrotic syndrome are resistant to steroid and immunosuppressive treatments. Many natural compounds are traditionally used to treat chronic diseases. Sahu et al. (2022a) screened the potency of 66 bioactive compounds phytochemicals in BD against mutant nephrin protein, against wild and mutant Ig4 domain of nephrin protein through AutoDock raccoon. Simulated molecular dynamics in 100 ns trajectory of Ig4 domain of nephrin protein with Boeravinone M and Boeravinone E showed that the latter’s binding increased the compactness, effectively modulating the stability and function. Sahu et al. (2022b) extensively investigated the anticancer properties of BD phytochemicals Boeravinone A, B, C, D, E, F, G, H, I, and J. While analysing binding efficacy and free energy landscape of both the native and mutant CDK2AP1 protein with these by molecular docking, Boeravinone J showed the best binding affinity (−7.9 kcal/mol) towards native CDKAP1. MD simulation for 100 ns suggested that H23R mutant-Boeravinone J complex had the minimum structural changes with less conformational mobility compared to C105A mutant. Such study can help develop Boeravinone E therapeutics.
The in vitro molecular docking analysis by JunaBeegum et al. (2017) to determine the potent inhibitory effect of recombinant NS3 genotype 3b catalytic activity of ethanolic extract of BD found that most flavonoids were deeply bound in HCV NS3 protease and interacted with the catalytic triad. The lead molecules liriodendrin, 3,5,6-tri-hydroxyl-4-methoxy flavone7-β-D glucoside, astragalin, caffeoyl tartaric acid, quercetin, 3,3,5,7-tetra-hydroxyl-4-methoxy flavones, boerhavin A, 4,25-secoobscurinervan and 3,5,7,2,5-penta-hydroxyl flavones had anti HCV activity. With −10.72 kcal/mol energy score, liriodendrin was the best interacting compound. Especially, the flavonoids and triterpenoids phytochemicals direct inhibited HCV NS3 protease. They found BD as a potential natural non-toxic anti-HCV agent that inhibited NS3 protease and reduced viral counts inside hepatocytes.
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) responsible for the ongoing COVID-19 pandemic affected over 110 million people worldwide (https://covid19.who.int/), with no clearly targeted efficient therapeutic yet. Finding a suitable docking ligand to inhibit the function of the responsible protein groups is continuously being researched (Abdalla et al., 2021; Mohapatra et al., 2021a; Mohapatra et al., 2021b; Mohapatra et al., 2022; Mohapatra et al., 2023). 3CL-like protease (3CLpro) is a non-structural protein involved in post-translational modification in SARS-CoV-2, and is vital to viral replication (https://www.news-medical.net/news/20210328/Boerhavia-diffusa-possess-potential-therapeutic-properties-against-COVID-19.aspx). Thus, 3CLpro could be a preferred drug target.
Surya and Praveen (2021) examined 2-3-4 β-Ecdysone, Bioquercetin, Boeravinone J, Biorobin, Boerhavisterol, Liriodendrin, Kaempferol, Quercetin and Trans-caftaric acid through molecular docking as the major active BD phytochemicals. Biorobin (−8.17 kcal/mol), Bioquercetin (−7.97 kcal/mol) and Boerhavisterol (−6.77 kcal/mol) had low binding energies as ligands against SAR-CoV-2 main protease, and favoured efficient docking and protease inhibition. The drug-likeness property of Bioquercetin, Biorobin, and Boeravisterol was ADME analysed, and Boeravisterol was the most suitable that obeyed the Lipinsky’s rule. Comparative docking of these carried out also against MERS Mpro showed that the obtained binding energies were unfavourable.
Toxicity studies and clinical trials
Because of the age-old tried and tested use with no known side-effect, traditional herbs and their products are often considered as safe as ethnomedicines. However, few toxicity studies on BD extracts have been reported to empirically verify and validate this perception. Karwasra et al. (2016) observed no adverse effect level in BD root extract even at 1,000 mg/kg in Wistar rats. Orisakwe et al. (2003) studied the acute and subchronic toxicity of BD leaf at 500, 1,000, and 2,000 mg/kg aqueous extract in albino mice and rats, and the lethal dose (LD50) for both was more than 2,000 mg/kg (p.o.). With increased food and fluid intake, the rats treated with the extract had a progressive body weight gain, significantly higher than control. Also, there was no significantly comparable change in absolute and relative weights of the vital organs, proving its safety and efficacy.
Adults and children suffering from helminth infection became worm-free within 5 days of oral administration of dried BD root powder (Singh and Udupa, 1972). A clinical trial of BD extract as a chemotherapy adjuvant conducted on 50 patients freshly diagnosed as having pulmonary tuberculosis recovered faster than the control. 80% of BD-treated patients were relieved from cough in 4 weeks as compared to 52% from among the control. Likewise, 88% of the patients were afebrile in 6 weeks as compared to 60% among the control, with the similar afebrile state setting in by the 8th week in the control. The BD treated group had a higher average weight gain, and a considerably faster sputum conversion rate (Kant et al., 2001). Aqueous plant extract could significantly reduce osmotic fragility of erythrocyte in polycystic end-stage renal disease (ESRD) by altering the composition of erythrocyte membrane or affecting the intracellular sodium and improving the oxidative stress (Sathyapriya et al., 2009). The haematological, biochemical and urine parameters of patients with Type-I or Type-II diabetic nephropathy (DM) with >500 mg proteinuria daily and >5 mg/dL serum Cr were examined every month for 6 months in a clinical trial after dividing them into two groups (14 patients of 40–70 years with 60.78 ± 6.37 years mean age in group I and 11 of 45–73 years with 60.27 ± 9.00 years mean age in group II). All patients were having edema, anorexia, weakness and vomiting symptoms. While Ramipril (a standard drug) reduced 24-h urine protein excretion significantly, six-month BD treatment decreased it only to a statistically insignificant level (Singh et al., 2010).
Discussion
By categorising the collected relevant literature and conducting bibliometric analyses, it was observed that the maximum number of literature (15) were related to organ protective role of BD, followed by the literature related to metabolic disorders (12), anti-inflammatory property (9), antimicrobial and antiparasitic (8) and anticancer (5) potentials. This provides a strong reason to consider the plant and it extracts as a panacea to treat numerous physiological, pathological and lifestyle related illnesses.
Numerous crude extraction, purification and optimisation techniques for scale-up have been suggested in recent reports. Chromatographic technique is advantageous in evaluating retinoid and phenolic acids due to the ease of sample preparation, chemicals optimisation and the scope to simultaneously compare several samples. Chromatogram comparison allows detect similarities and differences between the various bioactive extracts under investigation even at the minutest level. Eupalitin-3-O-D-galactopyranoside has powerful hepatoprotective properties against carbon tetrachloride-induced toxicity. It could be crystallised from sedimented residue, making it a simple, quick and the most economical commercially viable scale-up technique. This novel approach was used to isolate Eupalitin-3-O-β-D-galactopyranoside with up to 0.1578% w/w yield. Obtaining Eupalitin-3-O-β-D-galactopyranoside from hydro-alcoholic extract of BD employing high-performance thin layer chromatography (HPTLC) technique through a single solvent system (toluene:acetone:water:5:15:1) was optimised, validated and quantified.
Due to altered lifestyle, less physical exercise, work stress, increasing physical stress, junk food preference and rising childhood obesity, the number of diabetics is in the rise at an alarming rate in recent time worldwide including India. To address such metabolic disorders, nutraceuticals including herbal therapeutants like BD as dietary supplement could be suggested. BD reportedly has excellent antidiabetic activity that regulates and optimises biochemical factors in diabetic test models as listed in Table 1. Such wonder manifestation of BD is attributable to a probable mode of action of the phytoconstituents to ameliorate diabetes as proposed in Figure 3. More focused clinical trials and evidence-based intensive investigations to validate its efficacy are needed. Further, the multifarious other benefits extended by BD makes it a genuinely wonder herb that needs it due place in the society for sustainable human wellbeing.
Although several studies validate the traditional use of BD, but most of them lack in deciphering the mechanism of action of the underlying bioactive therapeutic phytocompounds in preventing or protecting against infectious diseases. Investigations confirming its molecular-level action against various diseases, the pharmacology and the toxicology of unexplored secondary metabolites if any could be undertaken.
Conclusion and future prospects
BD reportedly has multiple and multifunctional pharmacological traits including antifungal, anticancer, antibacterial, antidiabetic, antiparasitic, cardioprotective, hepatoprotective, renoprotective, anti-inflammatory and antifertility characteristics as opined and concluded by numerous investigators. Though the reports on the pharmacological properties of BD are many, the molecular action of the therapeutic phytocompounds in preventing or protecting against infectious diseases, for instance, most of them are inconclusive. Although there are multiple reports on antimicrobial activities yet the molecular mechanisms of specific interaction between the purified compounds and the microbial effector proteins/toxins or their interference with molecular pathogenesis are limited. Thus, clinical trials and advance studies targeting the understanding of the mechanisms of action at the molecular level can be further undertaken to decipher it, especially of the isolated unexplored secondary metabolites in BD. Additionally, investigations to understand prophylactic and therapeutic roles of these phytomolecules at the molecular level using appropriate test models need to be prioritised.
Author contributions
SD: Conceptualization, Writing–original draft. PS: Writing–original draft. SA: Writing–original draft. BK: Writing–original draft. WO: Writing–original draft. AO: Writing–original draft. SM: Supervision, Writing–review and editing. RM: Writing–review and editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
The authors extend their appreciation to the Researchers Supporting Project number (RSPD2023R620), King Saud University, Riyadh, Saudi Arabia. All the authors are thankful to their respective institutes of affiliation for the infrastructure and administrative support. All the authors express their sincere gratitude to SM, Associate Professor, Department of Biotechnology, KIIT Deemed-to-be University, Bhubaneswar for his valuable suggestions and constant supervision during the preparation of this review article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abdalla, M., Mohapatra, R. K., Sarangi, A. K., Mohapatra, P. K., Eltayb, W. A., Alam, M., et al. (2021). In silico studies on phytochemicals to combat the emerging COVID-19 infection. J. Saudi Chem. Soc. 25 (12), 101367. doi:10.1016/j.jscs.2021.101367
Adebajo, A. O., Isah, K. P., and Ajayi, A. J. (2018). Studies on some effect of the aqueous extract of the leaves of Boerhavia diffusa on the ovaries. J. Horm. Dis. Endocr. Res. 1, 102.
Adefokun, D. I., Iwalewa, E. O., Omisore, N. O., Obuotor, E., and Idowu, I. J. (2015). The Antimalarial effect and mechanism of action of methanolic root extract of Boerhaavia diffusa in mice. Br. J Pharm. Res 8 (2), 1–14. doi:10.9734/bjpr/2015/13606
Agrawal, B., Das, S., and Pandey, A. (2011). Boerhaavia diffusa Linn.: a review on its phytochemical and pharmacological profile. Asian J. Appl. Sci. 4, 663–684. doi:10.3923/ajaps.2011.663.684
Ahmed-Belkacem, A., Macalou, S., Borrelli, F., Capasso, R., Fattorusso, E., Taglialatela-Scafati, O., et al. (2007). Nonprenylated rotenoids, a new class of potent breast cancer resistance protein inhibitors. J. Med. Chem. 50 (8), 1933–1938. doi:10.1021/jm061450q
Akhter, F., Alvi, S. S., Ahmad, P., Iqbal, D., Alshehri, B. M., and Khan, M. S. (2019). Therapeutic efficacy of Boerhaavia diffusa (Linn.) root methanolic extract in attenuating streptozotocin--induced diabetes, diabetes-linked hyperlipidemia and oxidative-stress in rats. Biomed. Res. Ther. 6 (7), 3293–3306. doi:10.15419/bmrat.v6i7.556
Alam, P., Shahzad, N., Gupta, A. K., Mahfoz, A. M., Bamagous, G. A., Al-Ghamdi, S. S., et al. (2018). Anti-diabetic effect of Boerhavia diffusa L. root extract via free radical scavenging and antioxidant mechanism. Toxicol. Env. Health Sci. 10 (3), 220–227. doi:10.1007/s13530-018-0367-z
Bairwa, K., and Jachak, S. M. (2015). Anti-inflammatory potential of a lipid-based formulation of a rotenoid-rich fraction prepared from Boerhavia diffusa. Pharm. Biol. 53 (8), 1231–1238. doi:10.3109/13880209.2014.971382
Bhowmik, D., Sampath, K. P., Srivastava, S., Paswan, S., and Dutta, A. S. (2012). Traditional Indian herbs Punarnava and its medicinal importance. J. Pharmacogn. Phytochem. 1, 52–57.
Cadet, J. L. (1998). Free radical mechanisms in the central nervous system: an overview. Int. J. Neurosci. 40, 13–18. doi:10.3109/00207458808985722
Dapurkar, V. K., Sahu, G. K., Sharma, H., Meshram, S., and Rai, G. (2013). Anti-arthritic activity of roots extract of Boerhaavia diffusa in adjuvant -induced arthritis rats. Sch. Acad. J. Pharm. 2 (2), 107–109.
Das, S., Sahoo, B. M., and Bhattamisra, S. K. (2022). Multifunctional role of phytochemicals derived from Boerhaavia diffusa L. in human health, ailments and therapy. Curr. Nutr. Food Sci. 18 (6), 574–588. doi:10.2174/1573401318666220308141939
Demopoulos, H. B., Flamm, E. S., Pietronegro, D. D., and Seligman, M. L. (1980). The free radical pathology and the microcirculation in the major central nervous system disorders. Acta Physiol. Stand. Suppl. 492, 91–119.
Gaur, P. K., Rastogi, S., and Lata, K. (2022). Correlation between phytocompounds and pharmacological activities of Boerhavia diffusa Linn with traditional-ethnopharmacological insights. Phytomed Plus 2 (2), 100260. doi:10.1016/j.phyplu.2022.100260
Gaurav, G., Khan, M. U., Basist, P., Zahiruddin, S., Ibrahim, M., Parveen, R., et al. (2022). Nephroprotective potential of Boerhaavia diffusa and Tinospora cordifolia herbal combination against diclofenac induced nephrotoxicity. South Afr. J. Bot. 151, 238–247. doi:10.1016/j.sajb.2022.01.038
Gharate, M., and Kasture, V. (2013). Evaluation of anti-inflammatory, analgesic, antipyretic and antiulcer activity of Punarnavasava: an Ayurvedic formulation of Boerhavia diffusa. Orient Pharm. Exp. Med. 13, 121–126.
Gunaseelan, D., Ali, M. S., Albert, A., Prabhakaran, R., Beno, D. L., and Nagarethinam, B. (2022). Biochemical and molecular anticancer approaches for Boerhaavia diffusa root extracts in oral cancer. J. Can. Res. Ther. 18, S244–S252. doi:10.4103/jcrt.jcrt_932_20
Handa, K., and Jindal, R. (2023). Mitigating the nephrotoxic impact of hexavalent chromium in Ctenopharyngodon idellus (grass carp) with Boerhavia diffusa (punarnava) leaf extract. Environ. Sci. Pollut. Res. Int. 30 (14), 42399–42415. doi:10.1007/s11356-022-24931-4
Ibrahima, M. S., Eugène, A. S., Justin, B. G., Madjid, A. A., Machioud, S. M., Rodrigue, A., et al. (2018). The effect of methanolic leaf extract of Boerhavia diffusa Linn. (Nictaginaceae) on the activities of antidiabetic, anti-inflammatory and antioxidant enzymes in experimental diabetes. J. Pharm. Res. Int. 8, 1–25. doi:10.9734/jpri/2018/45640
JunaBeegum, G. R., Revikumar, A., SuharaBeevy, S., and Sugunan, V. S. (2017). Identification of novel inhibitors of HCV NS3 protease genotype 3 subtype B through molecular docking studies of phytochemicals from Boerhavia diffusa L. Med. Chem. Res. 26, 327–334. doi:10.1007/s00044-016-1745-1
Juneja, K., Mishra, R., Chauhan, S., Gupta, S., Roy, P., and Sircar, D. (2020). Metabolite profiling and wound-healing activity of Boerhavia diffusa leaf extracts using in vitro and in vivo models. J. Trad. Complement. Med. 10 (1), 52–59. doi:10.1016/j.jtcme.2019.02.002
Kalaivani, M. K., Soundararajan, P., Vasanthi, H. R., and Arociaswamy, S. (2015). In-vitro nephroprotective role of ethanolic root extract of Boerhaavia diffusa against cisplatin--induced nephrotoxicity. Int. J. Phytomed 7 (4), 388–395.
Kant, S., Agnihotri, M. S., and Dixit, K. S. (2001). Clinical evaluation of Boerhaavia diffusa as an adjuvant in the treatment of pulmonary tuberculosis. Phytomedica 2 (1-2), 89–94.
Karwasra, R., Kalra, P., Nag, T. C., Gupta, Y. K., Singh, S., and Panwar, A. (2016). Safety assessment and attenuation of cisplatin -induced nephrotoxicity by tuberous roots of Boerhaavia diffusa. Regul. Toxicol. Pharmacol. 81, 341–352. doi:10.1016/j.yrtph.2016.09.020
Kaur, S., Bhardwaj, K., and Sachdeva, H. (2015). Antileishmanial efficacy of Boerhaavia diffusa L. and Ocimum sanctum L. against experimental visceral leishmaniasis. Ind. J. Exp. Biol. 53 (8), 522–529.
Kaviya, M., Balasubramanian, B., Bharathi, K., Malaisamy, A., Al-Dhabi, N. A., Mariadhas, V. A., et al. (2022). Evaluation of nutritional substances and investigation of antioxidant and antimicrobial potentials of Boerhavia diffusa with in silico molecular docking. Molecules 27, 1280. doi:10.3390/molecules27041280
Kayande, N., and Kushwah, P. (2014). In vitro and in vivo evaluation of anticancer activity of Boerhaavia diffusa Linn. Exp. Anim. 2, 133–137. Pharma Tutor.
Khalid, M., Alqarni, M. H., Shoaib, A., Wahab, S., Foudah, A. I., Aljarba, T. M., et al. (2022). Anti-Obesity action of Boerhavia diffusa in rats against high-Fat diet-induced obesity by blocking the cannabinoid receptors. Plants 11, 1158. doi:10.3390/plants11091158
Lakshmi, S. V. V. N. S. M., Reddy, A. S., Ganapaty, S., Ganga Rao, B., and Ramesh, A. (2017). Anticataract potential of Boerhavia diffusa root on galactose -induced cataractogenesis. Ind. J. Exp. Biol. 55, 838–844.
Majgaine, S., and Verma, D. L. (2017). Antibacterial activity of Boerhaavia diffusa L. (Punarnava) on certain bacteria. IOSR J. Pharm. 7, 1–3.
Malhotra, D., Ishaq, F., and Khan, A. (2014a). Antihyperglycemic activity of Boerhaavia diffusa in streptozotocin -induced diabetic rats. Int. J. Chem. Anal. Sci. 5 (1), 21–23.
Malhotra, D., Ishaq, F., and Khan, A. (2014b). Effect of B. diffusa root extracts on ex vivo and Cu++-mediated in vitro susceptibility of LDL, sd-LDL and lb-LDL to oxidation in absence or presence of glucose in diabetic-hyperlipidemic rats. J. Pharm. Res. 8 (1), 7–11.
Malhotra, D., Khan, A., and Ishaq, F. (2013). Phytochemical screening and antibacterial effect of root extract of Boerhaavia diffusa L. (Family Nyctaginaceae). J. Appl. Nat. Sci. 5 (1), 221–225. doi:10.31018/jans.v5i1.310
Mini, M. S., Krishnan, S. A., and Sridevi, K. (2020). Evaluation of antidiabetic and antihyperlipidemic activity of methanolic root extracts of Withania somnifera, Boerhaavia diffusa and their combination in dexamethasone-induced diabetic rats. Int. J. Pharm. Sci. Res. 11 (11), 5733–5740.
Mishra, S., Aeri, V., Gaur, P. K., and Jachak, S. M. (2014). Phytochemical, therapeutic, and ethnopharmacological overview for a traditionally important herb: Boerhavia diffusa Linn. Bio Med. Res. Int. 2014, 1–19. doi:10.1155/2014/808302
Mohapatra, R. K., Azam, M., Mohapatra, P. K., Sarangi, A. K., Abdalla, M., Perekhoda, L., et al. (2022). Computational studies on potential new anti-Covid-19 agents with a multi-target mode of action. J. King Saud Univ. – Sci. 34, 102086. doi:10.1016/j.jksus.2022.102086
Mohapatra, R. K., Dhama, K., El–Arabey, A. A., Sarangi, A. K., Tiwari, R., Emran, T. B., et al. (2021b). Repurposing benzimidazole and benzothiazole derivatives as potential inhibitors of SARS-CoV-2: DFT, QSAR, molecular docking, molecular dynamics simulation, and in-silico pharmacokinetic and toxicity studies. J. King Saud Univ. – Sci. 33 (8), 101637. doi:10.1016/j.jksus.2021.101637
Mohapatra, R. K., Mahal, A., Ansari, A., Kumar, M., Guru, J. P., Sarangi, A. K., et al. (2023). Comparison of the binding energies of approved mpox drugs and phytochemicals through molecular docking, molecular dynamics simulation, and ADMET studies: an in silico approach. J. Biosaf. Biosecurity 5, 118–132. doi:10.1016/j.jobb.2023.09.001
Mohapatra, R. K., Perekhoda, L., Azam, M., Suleiman, M., Sarangi, A. K., Semenets, A., et al. (2021a). Computational investigations of three main drugs and their comparison with synthesized compounds as potent inhibitors of SARS-CoV-2 main protease (Mpro): DFT, QSAR, molecular docking, and in silico toxicity analysis. J. King Saud Univ. – Sci. 33, 101315. doi:10.1016/j.jksus.2020.101315
Montefusco-Pereira, C. V., de Carvalho, M. L., de Araujo Boleti, A. P., Teixeira, L. S., Matos, H. R., and Lima, E. S. (2013). Antioxidant, anti-inflammatory, and hypoglycemic effects of the leaf extract from Passiflora nitida Kunth. Appl. Biochem. Biotechnol. 170, 1367–1378. doi:10.1007/s12010-013-0271-6
Muthulingam, M. (2014). Antihepatotoxic role of Boerhaavia diffusa (Linn.) against antituberculosis drug rifampicin -induced hepatotoxicity in male albino Wistar rats. J. Pharm. Res. 8, 1226–1232.
Muthulingam, M., and Chaithanya, K. K. (2018). In vitro anticancer activity of methanolic leaf extract of Boerhaavia diffusa Linn. against MCF-7 cell line. Drug Invent. Today 10 (2), 3107–3111.
Nadkarni, A. K. (1976). Indian materia medica, 1. Bombay, Maharashtra, India: AK Nadkarni, Popular Prakashan Pvt. Ltd., 203–205.
Nimbal, S. K., and Koti, B. C. (2016). Cardioprotective effect of Boerhaavia diffusa against doxorubicin -induced myocardial toxicity in albino rats. Sch. Acad. J. Biosci. 4, 171–178.
Oburai, N. L., Rao, V., and Bonath, R. B. N. (2015). Comparative clinical evaluation of Boerhavia diffusa root extract with standard Enalapril treatment in Canine chronic renal failure. J. Ayurveda Integr. Med. 6 (3), 150–157. doi:10.4103/0975-9476.166390
Orisakwe, O. E., Afonne, O. J., Chude, M. A., Obi, E., and Dioka, C. E. (2003). Sub-chronic toxicity studies of the aqueous extract of Boerhavia diffusa leaves. J. Health Sci. 49 (6), 444–447. doi:10.1248/jhs.49.444
Oyebode, O. A., Erukainure, O. L., Chukwuma, C. I., Ibeji, C. U., Koorbanally, N. A., and Islam, S. (2018). Boerhaavia diffusa inhibits key enzymes linked to type 2 diabetes in vitro and in silico; and modulates abdominal glucose absorption and muscle glucose uptake ex vivo. Biomed. Pharmacother. 106, 1116–1125. doi:10.1016/j.biopha.2018.07.053
Parmar, D., Jain, N. K., and Tomar, V. (2018). Anti-arthritic evaluation of different extracts of Boerhaavia diffusa Linn. in FCA -induced arthritis in rats. J. Drug Deliv. Ther. 8, 388–393. doi:10.22270/jddt.v8i5-s.1922
Prathapan, A., Mathews, V., Varghese, S., Abhilash, P., Salin, R., Anil, K., et al. (2017). Polyphenol rich ethanolic extract from Boerhavia diffusa L. mitigates angiotensin II -induced cardiac hypertrophy and fibrosis in rats. Biomed. Pharmacother. 87, 427–436. doi:10.1016/j.biopha.2016.12.114
Prathapan, A., Vineetha, V. P., Abhilash, P. A., and Raghu, K. G. (2013). Boerhaavia diffusa L. attenuates angiotensin II--induced hypertrophy in H9c2 cardiac myoblast cells via modulating oxidative stress and down-regulating NF-κβ and transforming growth factor β1. Br. J Nutr 110 (7), 1201–1210. doi:10.1017/s0007114513000561
Prathapan, A., Vineetha, V. P., and Raghu, K. G. (2014). Protective effect of Boerhaavia diffusa L. against mitochondrial dysfunction in angiotensin II -induced hypertrophy in H9c2 cardiomyoblast cells. PLoS One 9 (4), e96220. doi:10.1371/journal.pone.0096220
Rao, P. P. (2016). Ophthalmic uses of Boerhaavia diffusa L. (Punarnava)-Review. Int. J. Herb. Med. 4 (2), 05–09.
Remya, M. J., Shahul, H. A., and Sujathan, K. (2018). Cytotoxicity of punarnava (Boerhaavia diffusa L.) in breast cell line. Int. J. Ayurveda Pharma Res. 6 (6), 1–5.
Sahu, S. N., Satpathy, S. S., Mohanty, C., and Pattanayak, S. K. (2022b). Computational study to evaluate the potency of phytochemicals in Boerhavia diffusa and the impact of point mutation on cyclin-dependent kinase 2-associated protein 1. J. Biomol. Struct. Dyn. 40 (18), 8587–8601. doi:10.1080/07391102.2021.1914169
Sahu, S. N., Satpathy, S. S., Pattnaik, S., Mohanty, C., and Pattanayak, S. K. (2022a). Boerhavia diffusa plant extract can be a new potent therapeutics against mutant nephrin protein responsible for type1 nephrotic syndrome: insight into hydrate-ligand docking interactions and molecular dynamics simulation study. J. Indian Chem. Soc. 99 (10), 100669. doi:10.1016/j.jics.2022.100669
Samhita, C., and Varanasi, C. O. (1998). Section 6 chikitsasthanam. Chapter 1, Qtr 1, 3–4. Shloka 7-8.
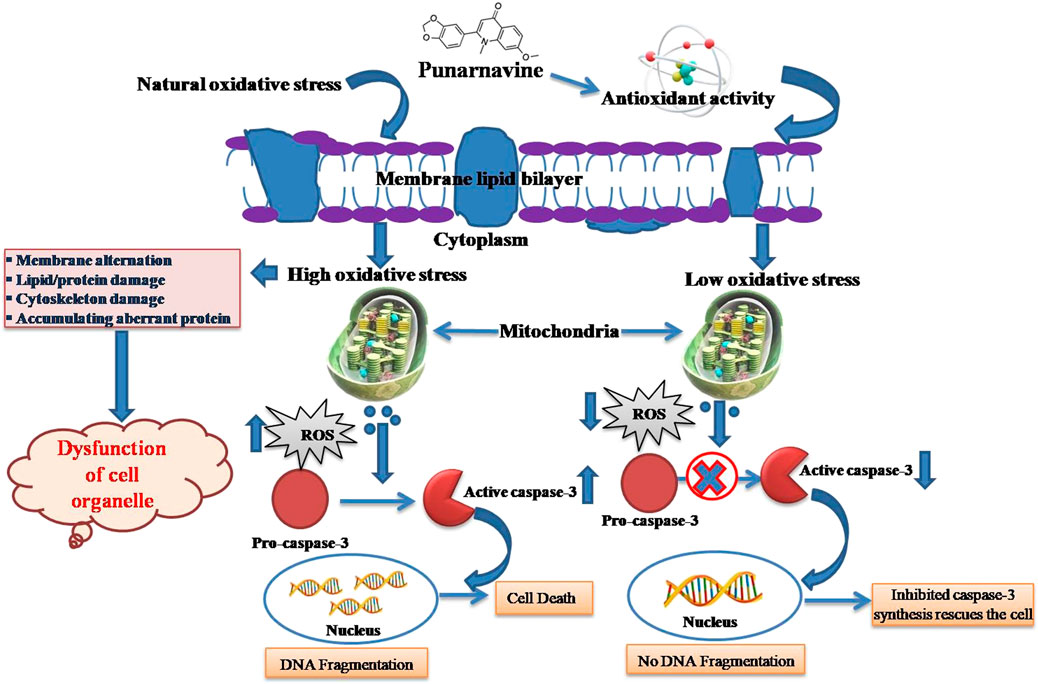
Saraswati, S., Alhaider, A. A., and Agrawal, S. S. (2013). Punarnavine, an alkaloid from Boerhaavia diffusa exhibits anti-angiogenic activity via downregulation of VEGF in vitro and in vivo. Chemico-Biological Interact. 206 (2), 204–213. doi:10.1016/j.cbi.2013.09.007
Sathyapriya, K., Vijayachandrika, V., and Parameswari, C. S. (2009). Antioxidant status in polycystic end-staged renal diseased patients and antihemolytic effect of Boerhaavia diffusa. Ind. J. Biochem. Biophy 46 (3), 269–272.
Shameela, S., Shamshad, S., Priyadarsini, A. I., Paul, M. J., and Devi, K. L. (2015). Hypolipidemic and antiinflammatory activity of Boerhaavia diffusa in isoproterenol-induced myocardial infarcted rats. Int. J. Pharm. Bio Sci. 6 (2), 1–6. doi:10.2174/1874325001509010001
Sharma, N., Singh, B., Bhatia, A., Gupta, R. C., and Wani, M. S. (2021). Morphological and cytogenetic analysis of different cytotypes of Boerhaavia diffusa L. and their evaluation for biological activity. Adv. Tradit. Med. (ADTM) 21, 791–803. doi:10.1007/s13596-020-00518-7
Sharma, S., Baboota, S., Amin, S., and Mir, S. R. (2020). Ameliorative effect of a standardized polyherbal combination in methotrexate induced nephrotoxicity in the rat. Pharm. Biol. 58, 184–199. doi:10.1080/13880209.2020.1717549
Shravani, V., Girija, A. S., Himabindu, S., Krishnan, M., and Babu, S. (2023). Anti-quorum sensing activity of Boerhavia diffusa against Pseudomonas aeruginosa PAO1. Bioinformation 19 (3), 310–318. doi:10.6026/97320630019310
Shubha, G., and Govindaraju, B. (2013). Antiinflammatory and analgesic activity of Boerhaavia diffusa L. Int. Res. J. Pharm. App Sci. 3 (1), 131–135.
Singh, P. K., Ojha, S. K., Mishra, S., Kumar, S., Khan, A., and Chauhan, S. K. (2014). Biotherapeutic activity of Boerhaavia diffusa against oxidized cholesterol -induced lipid peroxidation and anti-oxidant status in hypercholesterolemic rats. Int. J. Appl. Sci. Biotechnol. 2 (1), 69–74. doi:10.3126/ijasbt.v2i1.9483
Singh, R. G., Kumar, G., Singh, S. K., Tripathi, Y. B., and Singh, R. H. (2010). Evaluation of antiproteinuric and renoprotective effect of Punarnava (Boerhavia diffusa Linn.) in diabetic nephropathy. J. Res. Educ. Ind. Med. 16 (1-2), 45–48.
Singh, R. H., and Udupa, K. N. (1972). Studies on the Indian indigenous drug punarnava (Boerhaaviadiffusa Linn.). Part IV: preliminary controlled clinical trial in nephrotic syndrome. J. Res. Ind. Med. 7, 28–33.
Sruthy, B., Latha, M. S., and Anand, S. P. (2018). Identification of antimastits componenets in boerhavia diffusa as an inhibitor of staphalococus aureus monofunctional glycosyltransferase, causing bovine mastitis (an in silico approach). Orient. J. Chem. 34 (2), 1114–1119. doi:10.13005/ojc/340264
Sudhamadhuri, A., and Kalasker, V. (2014). Evaluation of anti-inflammatory effect of aqueous extract of Boerhaavia diffusa leaves in rats. Int. J. Res. Health Sci. 2, 517–521.
Surya, U. R., and Praveen, N. (2021). A molecular docking study of SARS-CoV-2 main protease against phytochemicals of Boerhavia diffusa Linn. for novel COVID-19 drug discovery. Virus Dis. 32 (1), 46–54. doi:10.1007/s13337-021-00683-6
Thajudeen, K. Y., Alsayari, A., Najib Ullah, S. N. M., Salam, S., Elayadeth-Meethal, M., and Uoorakkottil, I. (2022a). Validation, optimization and hepatoprotective effects of Boeravinone B and caffeic acid compounds from Boerhavia diffusa Linn. Separations 9, 177. doi:10.3390/separations9070177
Thajudeen, K. Y., Asiri, Y. I., Salam, S., Thorakkattil, S. A., Rahamathulla, M., and Uoorakkottil, I. A. (2022b). Box–behnken extraction design and hepatoprotective effect of isolated eupalitin-3-O-b-D-Galactopyranoside from Boerhavia diffusa linn. Molecules 27, 6444. doi:10.3390/molecules27196444
Tiwari, S., Dixena, S., Jain, M., Tiwari, N., and Jain, V. (2022). Pharmacognostical, phytochemical and in-vitro anthelmintic activity of Cassia roxburghii seed and Boerhaavia diffusa root against Pheritima posthuma model. J. Drug Deliv. Ther. 12 (6), 96–101. doi:10.22270/jddt.v12i6-s.5711
Vasundhara, C. C. S., and Devi, S. G. (2018). Effect of ethanolic extract of leaves of Boerhavia diffusa on carbohydrate metabolising enzymes, renal and hepatic markers in streptozotocin -induced diabetic rats. Int. J. Pharm. Clin. Res. 10 (2), 43–47.
Vineetha, V. P., Prathapan, A., Soumya, R. S., and Raghu, K. G. (2013). Arsenic trioxide toxicity in H9c2 myoblasts-damage to cell organelles and possible amelioration with Boerhavia diffusa. Cardiovasc. Toxicol. 13, 123–137. doi:10.1007/s12012-012-9191-x
Vyas, B. A., Desai, N. Y., Patel, P. K., Joshi, S. V., and Shah, D. R. (2013). Effect of Boerhaavia diffusa in experimental prostatic hyperplasia in rats. Ind. J. Pharmacol. 45, 264–269. doi:10.4103/0253-7613.111946
Wahi, A. K., Agrawal, V. K., and Gupta, R. C. (1997). Phytochemicals and pharmacological studies on Boerhaavia diffusa Linn. (Punarnava) alkaloids. Natl. Acad. Sci. Lett. 20, 9–10.
Zheng, Q., Ren, H., and Yang, S. (2021). Boerhaavia diffusa extract acts as a specific antituberculosis agent in vitro against Mycobacterium tuberculosis H37Rv Infection. Dokl. Biochem. Biophys. 499 (1), 266–272. doi:10.1134/s160767292104013x
Glossary
Keywords: antidiabetic, antimicrobial, anticancer, Boerhaavia diffusa, flavonoids, phenolics, punarnavine, bioactivity
Citation: Das S, Singh PK, Ameeruddin S, Kumar Bindhani B, Obaidullah WJ, Obaidullah AJ, Mishra S and Mohapatra RK (2023) Ethnomedicinal values of Boerhaavia diffusa L. as a panacea against multiple human ailments: a state of art review. Front. Chem. 11:1297300. doi: 10.3389/fchem.2023.1297300
Received: 19 September 2023; Accepted: 25 October 2023;
Published: 14 November 2023.
Edited by:
Abdul Ajees Abdul Salam, Manipal Academy of Higher Education, IndiaReviewed by:
Thiyagarajan S., Institute of Bioinformatics and Applied Biotechnology, IndiaAjmer Singh Grewal, Guru Gobind Singh College of Pharmacy, India
Copyright © 2023 Das, Singh, Ameeruddin, Kumar Bindhani, Obaidullah, Obaidullah, Mishra and Mohapatra. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sarita Das, c2FyaXRhZGFzN0B5YWhvby5jb20=; Snehasish Mishra, c25laGFzaXNoLm1pc2hyYUBnbWFpbC5jb20=; Ranjan K. Mohapatra, cmFuamFua19tb2hhcGF0cmFAeWFob28uY29t
†These authors have contributed equally to this work and share first authorship
‡ORCID: Sarita Das, https://orcid.org/0000-0003-2153-2551; Snehasish Mishra, https://orcid.org/0000-0002-3896-5831
 Sarita Das1*†‡
Sarita Das1*†‡ Puneet K. Singh
Puneet K. Singh Ahmad J. Obaidullah
Ahmad J. Obaidullah Snehasish Mishra
Snehasish Mishra Ranjan K. Mohapatra
Ranjan K. Mohapatra