- 1Department of Pharmaceutical Chemistry, College of Pharmacy, Prince Sattam Bin Abdulaziz University, Al-Kharj, Saudi Arabia
- 2Department of Chemical Engineering, Faculty of Engineering and Technology Marwadi University, Rajkot, Gujarat, India
- 3Department of Chemistry and Biochemistry, School of Sciences, JAIN (Deemed to be University), Bangalore, Karnataka, India
- 4Department of medical analysis, Medical laboratory technique college, the Islamic University, Najaf, Iraq
- 5Department of medical analysis, Medical laboratory technique college, the Islamic University of Al Diwaniyah, Al Diwaniyah, Iraq
- 6Department of medical analysis, Medical laboratory technique college, the Islamic University of Babylon, Babylon, Iraq
Today, wastewater treatment is essential and inevitable due to the water crisis caused by climate change and population growth. Although numerous methods and synthetic compounds have been successful in practical and laboratory applications, developing novel multifunctional compounds remains of interest to scientists. For this purpose, a new nanofiber containing Cobalt-MOF (metal-organic framework), polyvinyl alcohol (PVA), and polyvinyl pyrrolidone (PVP), which are known environmentally friendly polymers, was synthesized. After characterization and structure determination, the nanofiber was investigated for its ability to adsorb Congo Red dye and inhibit known microbial species in wastewater. This study showed that 91% of the 400 mg/L Congo red solution was absorbed by 0.06 g/L of the synthesized Cobalt-MOF/PVA-PVP Nanofibers at 1 h. Additionally, seven well-known strains (including Aeromonas hydrophila, Legionella pneumophila, Mycobacterium tuberculosis, Salmonella enterica, Pseudomonas aeruginosa, Escherichia coli, and Giardia lamblia) in wastewater were inhibited (with MIC of 8 μg/mL to 64 μg/mL) to the extent that some antibiotics could not affect them. This performance of the newly synthesized nanofiber can be attributed to its physical, chemical, and structural characteristics, such as compounds with biological properties present in its structure, as well as its high specific surface area. Therefore, researching and synthesizing similar compounds using the method presented in this study can lead to their development and application in wastewater treatment processes.
1 Introduction
Pathogenic microbial agents such as Aeromonas hydrophila, Legionella pneumophila, Mycobacterium tuberculosis, Salmonella enterica, Pseudomonas aeruginosa, Escherichia coli, and Giardia lamblia can cause dangerous and sometimes fatal infections in humans. These microbia are predominantly found in sewage systems (LeChevallier et al., 2024). Due to population growth, climate change, and the scarcity of freshwater resources, there is an increasing need for adequate water purification methods for various applications, including industry, agriculture, and irrigation (Gude, 2017; Khondoker et al., 2023). Therefore, it is essential to remove pathogenic microbial and hazardous chemical pollutants from wastewater (Baig et al., 2021).
In addition to microbial pathogens, wastewater often contains chemical and industrial contaminants. One well-known hazardous chemical found in urban and industrial wastewater is dyes (Karri et al., 2021; Waheed et al., 2021). Congo Red, a highly water-soluble dye classified as a diazo dye, is considered a carcinogenic compound due to the diazo group in its structure (Siddiqui et al., 2023). Historically, Congo Red has been widely used in the textile industry and is currently employed in medical diagnostics, including amyloidosis (Samsami et al., 2020; Liang et al., 2022).
Although numerous synthetic compounds, such as nanoparticles, have been reported as antimicrobial agents or adsorbents for removing chemical pollutants, a compound that possesses both capabilities are particularly valuable (Rani et al., 2021; Ribeiro et al., 2022). Nanotechnology has emerged as a promising field for developing compounds with excellent antimicrobial properties (Hetta et al., 2023). Among these compounds are nanometal oxides, nanocomplexes, nanopolymers, metal-organic frameworks (MOFs), and nanofibers (Tang et al., 2021; Behyar et al., 2023).
Nanofibers are an important class of nanocomposites that not only have applications in pollutant absorption but also play significant roles in the medical industry as wound dressings and medical bandages (Homaeigohar and Boccaccini, 2020; El-Aswar et al., 2022; Parham et al., 2022). In synthesizing nanofibers, environmentally friendly polymers such as polyvinyl alcohol (PVA) and polyvinylpyrrolidone (PVP) are primarily used (Wu et al., 2022). Compounds with biological properties, such as plant extracts or metal-organic frameworks (MOFs), can be incorporated into nanofibers to enhance their activity (Kiadeh et al., 2021; Sharifi and Bahrami, 2024). This technique allows for the transfer of biological activity into a fibrous structure with a high specific surface area and high compressive and flexural strength (Zhou et al., 2020).
MOFs have attracted considerable attention from scientists due to their remarkable structures characterized by porosity and specific active surface areas (Zhang et al., 2022). Some reports indicate their antimicrobial and pollutant-absorbing activities (Adegoke et al., 2020; Pettinari et al., 2021). The choice of multidentate ligands and metals used in synthesis significantly influences their properties (Younis et al., 2021; Moharramnejad et al., 2023). Cobalt is one metal frequently reported in various MOFs due to its beneficial characteristics. Among the beneficial characteristics of cobalt MOF are its significant antibacterial, anticancer, and catalytic properties (Fan and Tahir, 2022; Li et al., 2022).
2,2′-Bipyridine-4,4′-dicarboxylic acid, which features two pyridine rings and two carboxylic acid groups, has been identified in several studies as an important ligand for producing MOFs (Li et al., 2023; Ren et al., 2024). Pyridine itself possesses numerous biological properties; thus, the presence of two pyridine units in 2,2′-bipyridine-4,4′-dicarboxylic acid enhances the bioactivity of compounds containing it (Allaka and Katari, 2023; Varshney and Mishra, 2023).
Given the importance of wastewater treatment processes for removing microbial pathogens and chemical pollutants, this study aims to synthesize a compound that combines these two functionalities. We utilized a mixture of polyvinyl alcohol (PVA) and polyvinylpyrrolidone (PVP) as environmentally friendly polymers, along with a metal-organic framework (MOF) containing 2,2′-bipyridine-4,4′-dicarboxylic acid, both of which are known for their antimicrobial and absorbent properties, to create a new type of nanofiber. We hypothesize that this newly synthesized fiber will exhibit a high capacity for absorbing Congo Red dye while effectively inhibiting and removing some of the most prevalent microbial agents found in wastewater, potentially rivaling some commercially available antibiotics.
2 Experimental
2.1 Materials and devices
Cobalt (II) nitrate hexahydrate (Merck) and 2,2′-bipyridine-4,4′-dicarboxylic acid (Sigma-Aldrich) were used in the synthesis of metal-organic frameworks (MOFs) via Ningbo Scientz Biotechnology Laboratory ultrasonic cleaner. Polyvinylpyrrolidone (130,000 g/mol, Sigma-Aldrich), polyvinyl alcohol (85,000 g/mol, Sigma-Aldrich), and acetic acid (Merck) were employed in the synthesis of nanofibers using an NE100 Single Nozzle electrospinning machine.
Congo Red (Merck) and a Labtronics L2T90 UV-Visible spectrophotometer were utilized in the absorption process.
Mueller-Hinton broth (Thermo Scientific), Mueller-Hinton Agar (Thermo Scientific), microbial strains obtained from the American Type Culture Collection (ATCC), and Labtronics L2T90 UV-Visible spectrophotometer (To prepare desired concentration of microbial suspension) was used in antimicrobial evaluations.
2.2 Synthesis method
2.2.1 Synthesis of cobalt-MOF/PVA-PVP nanofibers
10 mmol Cobalt (II) nitrate hexahydrate and 10 mmol 2,2′-bipyridine-4,4′-dicarboxylic acid were added to 100 mL of double-distilled water and stirred until a homogeneous solution was obtained. The mixture was then subjected to ultrasonication at 300 W and 25°C. After synthesis, the metal-organic framework (MOF) compound was separated using nanofiltration and washed three times with ethanol, followed by three washes with double-distilled water. Finally, the compound was dried in an oven at 100°C for 1 hour under vacuum (Ahmad et al., 2022; Ramírez-Coronel et al., 2022).
A 20 mL solution of polyvinyl alcohol (PVA) and polyvinylpyrrolidone (PVP) was prepared in a 1:1 ratio using acetic acid, resulting in a concentration of 0.004%. Separately, a solution containing 0.01 mg of the metal-organic framework (MOF) was prepared by dispersing it in 25 mL of deionized water. The two solutions were then combined and stirred for 20 min at 80°C. Electrospinning were performed with a flow rate of 0.4 mL/h, a needle-to-collector distance of 22 cm, and an applied voltage of 28 kV. Finally, Cobalt-MOF/PVA-PVP Nanofibers was synthesized after the evaporation of the solvents (deionized water and acetic acid) at ambient temperature (Sargazi et al., 2019; Azizabadi et al., 2021; Moghaddam-manesh et al., 2022).
2.2.2 Characterization
FT-IR spectra, SEM images, CHNO elemental analysis, XRD patterns, nitrogen adsorption/desorption isotherms, TGA curves, compressive strength, and flexural strength were used to identify and confirm the structure of the products. These analyses were obtained using equipment 1, 2, 3, 4, 5, 6, 7, and 8, respectively.
2.3 Congo red adsorption method
To determine the percentage of Congo red absorption, a specified quantity of V/BP-MOF was introduced into a Congo red solution in deionized water and mixed thoroughly at 200 rpm. The adsorption process was evaluated under various conditions, including adsorbent concentration, pH level, temperature, and duration of the adsorption. Subsequently, the absorbance was measured at 497 nm using a spectrophotometer (Moghaddam-Manesh et al., 2024).
2.4 Antimicrobial evaluation method
Clinical and Laboratory Standards Institute (CLSI) methods were used to investigate the antimicrobial activity against several microbial strains present in wastewater, such as Aeromonas hydrophila, Legionella pneumophila, Mycobacterium tuberculosis, Salmonella enterica, Pseudomonas aeruginosa, Escherichia coli, and Giardia lamblia. The strains were prepared at a concentration of 1 × 105 CFU/mL in Mueller Hinton broth. This study investigated the Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC) using microdilution and kill assays (Moghaddam-Manesh et al., 2020; Ramírez-Coronel et al., 2022).
3 Result and discussion
3.1 Synthesis and characterization of cobalt-MOF/PVA-PVP nanofibers
A new cobalt-MOF/PVA-PVP nanofibers was produced by electrospinning a mixture of PVP and PVA polymers.
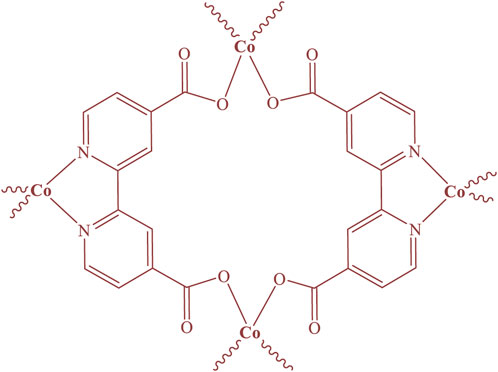
Cobalt-MOF was synthesized from the reaction of Cobalt (II) nitrate hexahydrate and 2,2′-bipyridine-4,4′-dicarboxylic acid using ultrasonication. The structure of the synthesized MOF was proposed in Figure 1.
After confirming its structure, the electrospinning process was carried out, and the desired product (cobalt-MOF/PVA-PVP nanofibers) was synthesized and characterized by the necessary analyses.
The optimal conditions for nanofiber production, including the solvent ratio, the amount of metal-organic framework (MOF), temperature, flow rate, needle-to-collector distance, and applied voltage, were determined based on previous reports (Sargazi et al., 2019; Azizabadi et al., 2021).
For comparison, the analyses of cobalt-MOF and cobalt-MOF/PVA-PVP nanofibers are presented.
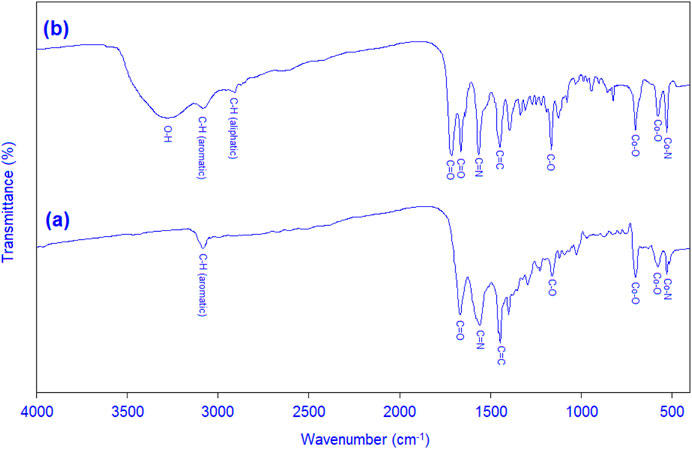
FT-IR spectrum of cobalt-MOF (a) and cobalt-MOF/PVA-PVP (b) presented in Figure 2. In FT-IR spectrum of cobalt-MOF (a), cobalt-oxygen in the region of 560 cm-1, and 690 cm-1 (due to Co-ligand) (Ramírez-Coronel et al., 2022; Almeleebia et al., 2024), cobalt -nitrogen in the region of 530 cm-1 (due to Co-ligand) (Nayak et al., 2003), carbon-hydrogen aromatic in the region of 3,050 cm-1 (due to ligand), carbon = oxygen in the region of 1,650–1700 cm-1 (due to ligand), carbon = nitrogen in the region of 1,530 cm-1 (due to ligand), carbon = carbon aromatic in the region of 1,450 cm-1 (due to ligand), and carbon-oxygen in the region of 1,140 cm-1 (due to ligand) can be mentioned.
In FT-IR spectrum of cobalt-MOF/PVA-PVP (b), cobalt-oxygen in the region of 560 cm-1, and 690 cm-1 (due to Co-ligand) (Ramírez-Coronel et al., 2022; Almeleebia et al., 2024), cobalt -nitrogen in the region of 530 cm-1 (due to Co-ligand) (Nayak et al., 2003), oxygen-hydrogen in the region of 3,300 cm-1 (due to PVA), carbon-hydrogen aromatic in the region of 3,050 cm-1 (due to ligand), carbon-hydrogen aliphatic in the region of 2,900 cm-1 (due to PVA and PVP), two carbon = oxygen in the region of 1,650–1700 cm-1 (due to PVP and ligand), carbon = nitrogen in the region of 1,530 cm-1 (due to ligand), carbon = carbon aromatic in the region of 1,450 cm-1 (due to ligand), and carbon-oxygen in the region of 1,140 cm-1 (due to ligand) can be mentioned.
From the striking differences between FT-IR spectra of cobalt-MOF (a) and cobalt-MOF/PVA-PVP nanofibers (b) are the presence of several carbons = oxygen peaks, carbon-hydrogen aliphatic in the region of 2,900 cm-1, and oxygen-hydrogen in the region of 3,200 cm-1 in FT-IR spectrum of cobalt-MOF/PVA-PVP nanofibers.
Therefore, the binding and presence of cobalt-MOF in the final product can be confirmed.
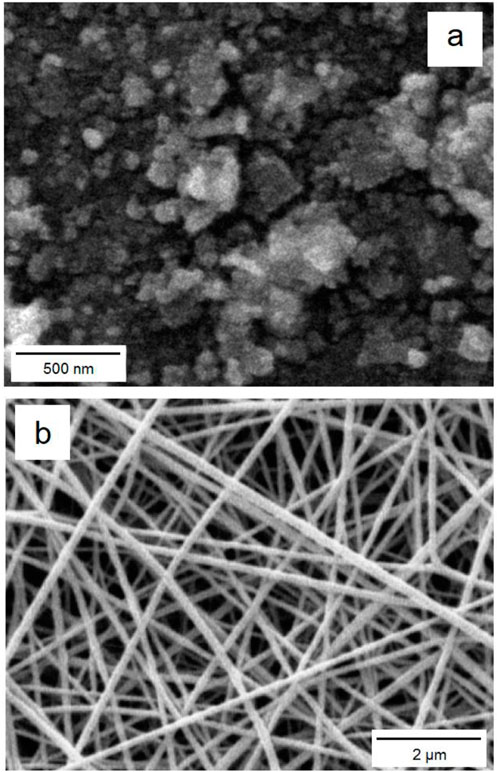
The SEM images (Figure 3) of cobalt-MOF (a) and cobalt-MOF/PVA-PVP nanofibers (b) proved their nanosized as well as their uniform morphology.
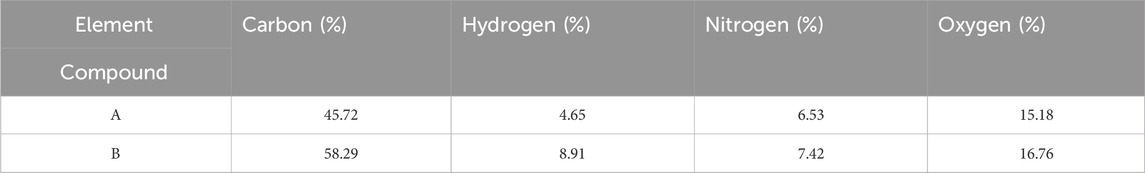
Table 1 presents the results of CHNO elemental analysis for cobalt-MOF (a) and cobalt-MOF/PVA-PVP nanofibers (b). The percentages of elements, particularly hydrogen and carbon, in cobalt-MOF/PVA-PVP nanofibers have increased compared to those in cobalt-MOF. From the results, it can be concluded that other hydrocarbon groups are attached to cobalt-MOF, which can be used to prove the connection of PVA and PVP in the final product.
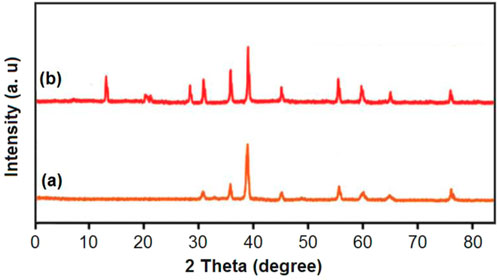
The peaks of the cobalt cubic structure corresponding to the planes 220, 311, 222, 400, 422, 511, 440, and 533 were observed at near 31°, 37°, 39°, 45°, 56°, 60°, 65°, and 77° (Lendzion-Bielun et al., 2013; Zawrah et al., 2016; Almeleebia et al., 2024) in the XRD (Figure 4) patterns of cobalt-MOF (a), and cobalt-MOF/PVA-PVP nanofibers (b). As can be seen in the XRD pattern of the final product, other peaks also appear, which are related to PVA (20°,29°) (Rajan et al., 2022) and PVP (12°, 21°) (Lv et al., 2019; Hu et al., 2021). Therefore, the presence of cobalt-MOF, PVA and PVP in the cobalt-MOF/PVA-PVP nanofibers can be proven.
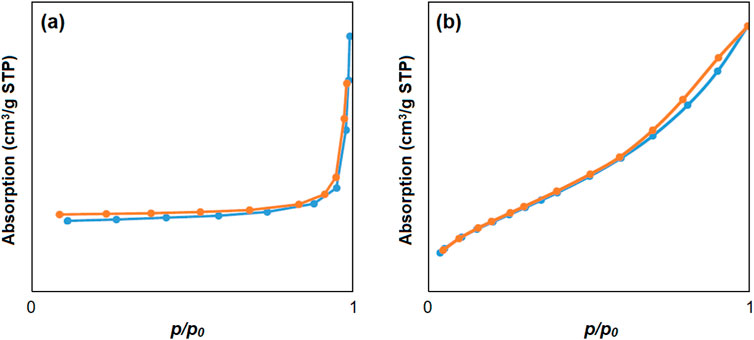
The specific active surface area for cobalt-MOF (a), cobalt-MOF/PVA-PVP nanofibers (b) was calculated as 1742 m2/g and 2,415 m2/g, respectively, based on their nitrogen absorption/desorption behavior as shown in Figure 5. The nitrogen absorption/desorption of cobalt-MOFs is similar to type III and cobalt-MOF/PVA-PVP nanofibers is similar to type IV (Al-Ghouti and Da’ana, 2020). The pore volume of cobalt-MOFs and cobalt-MOF/PVA-PVP nanofibers were obtained as 0.004 cm3/g and obtained 0.007 cm3/g, respectively. Therefore, cobalt-MOF/PVA-PVP nanofibers exhibits the behavior of mesoporous materials and has higher porosity and specific surface area (Al-Ghouti and Da’ana, 2020). The placement of the cobalt-MOF in the nanofiber substrate through hydrogen bonding with PVA and PVP can be considered the reason for the high specific surface area of the Cobalt-MOF/PVA-PVP nanofibers compared to Cobalt-MOF. According to previous studies, with increasing specific surface area, the contact of the compound with the factors or activity being studied increases. For example, with increasing specific surface area in the study of antimicrobial activity, the contact surface of the compound with microbial strains increases. Or in the study of dye adsorption activity, with increasing specific surface area, the contact of the compound with dyes increases. Therefore, higher properties of the compound are observed (Ghnim et al., 2025; Lyu et al., 2024; Mamman et al., 2024).
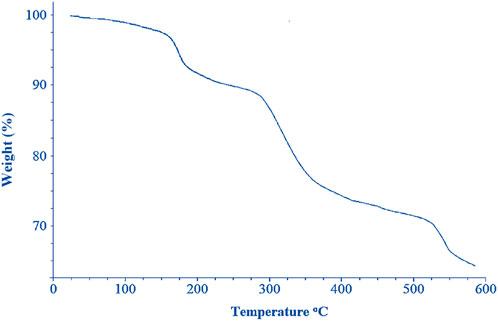
The TGA curve (Figure 6) of cobalt-MOF/PVA-PVP nanofibers showed four weight loss at 147°C, 184°C, 320°C, and 519 °C. The weight loss near 320°C and 519°C can be attributed to the decomposition of the 2,2′-bipyridine-4,4′-dicarboxylic acid and the destruction of the complex network. The other two significant weight losses in the final product at temperatures 147°C and 184°C, respectively, can be attributed to the decomposition of PVP and PVA.
Therefore, using the results obtained from the analyses, the structure of Figure 7 can be predicted for the cobalt-MOF/PVA-PVP nanofibers.
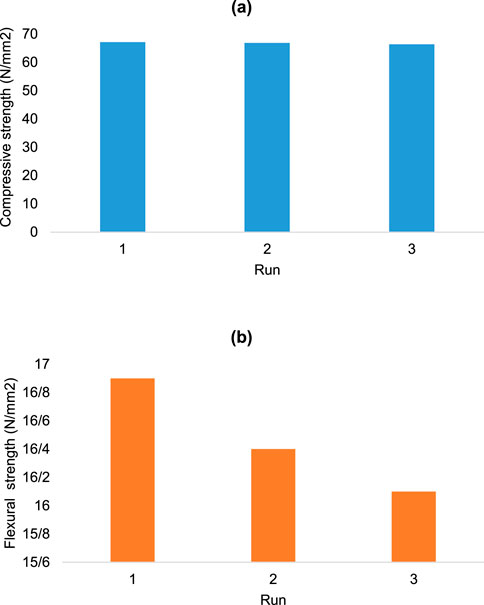
Compressive strength and flexural strength measurements (Figure 8) were the final analyses performed on the final product. The compressive strength (a) of the final product was 66.8 N/mm2 (n = 3) ± SD,) and the flexural strength (b) was 16.5 N/mm2 (n = 3) ± SD).
The compressive strength and flexural strength values of the final product were higher than those of some previously reported polymer blends that used PVA or PVP (Asiri et al., 2023; Khursheed et al., 2023). Therefore, it can be concluded that the mix of these two polymer blends resulted in increased compressive strength and flexural strength.
The results of the final product analysis proved that the nanofiber produced in this study is nano-sized, has a relatively high specific surface area and thermal stability, and has acceptable compressive strength and flexural strength. Therefore, the methods used in its production are acceptable.
The acceptable characteristics of the final product indicate its effectiveness in absorbing and inhibiting pathogenic strains. Therefore, the absorption properties of Congo red, one of the most important chemical agents in wastewater, as well as the ability to inhibit certain known pathogenic strains, were further tested on the final product.
3.2 Congo red adsorption of cobalt-MOF/PVA-PVP nanofibers
The high specific surface area, which increases the surface area of the MOFs, can be introduced as one of the capabilities of these compounds in the adsorption process. In addition, the product structure and ability to create interactions are among the most important factors in the absorption process (Du et al., 2021; Huang et al., 2021). Based on previous studies, hydrogen bonding is an important factor in the adsorption process of Congo Red.
It was proven that the synthesized nanofiber in this study has a high specific surface area. Therefore, its performance in the Congo Red adsorption process was evaluated.
Several factors are effective in the adsorption process, the most important of which are the concentration of the adsorbed substance, the concentration of the adsorbent, the pH, the temperature, and the time of the adsorption process (Soliman and Moustafa, 2020). All of these factors were tested in the study of the adsorption of Congo Red by the cobalt-MOF/PVA-PVP nanofibers.
The percentage of Congo red adsorption was obtained in accordance with the equation described in Equation 1.
I: Initial concentration of Congo red (mg/L). R: Residual concentration of Congo red (mg/L).
Equation 1 Calculation of absorption (%).
For comparison, all tests were also performed on cobalt-MOF.
In the first step of investigating the adsorption of Congo Red by the final product, a fixed concentration of the final product was selected and tested on different concentrations of Congo Red. Factors such as pH, temperature, and time of the adsorption process were also kept constant.
For this purpose, 0.05 g/L of cobalt-MOF/PVA-PVP nanofibers were placed in solutions of 50, 100, 200, 400, 800, and 1,600 mg/L of Congo Red solution at pH seven and ambient temperature, and the adsorption process was studied for 1 h.
The results were completely selective, and it was determined that as the concentration of the Congo Red solution increased, the percentage of absorption decreased. So 95%, 82%, 76%, 59%, 41%, and 24% absorption was observed in Congo Red solution of 50, 100, 200, 400, 800, and 1,600 mg/L, respectively (Figures 9a).
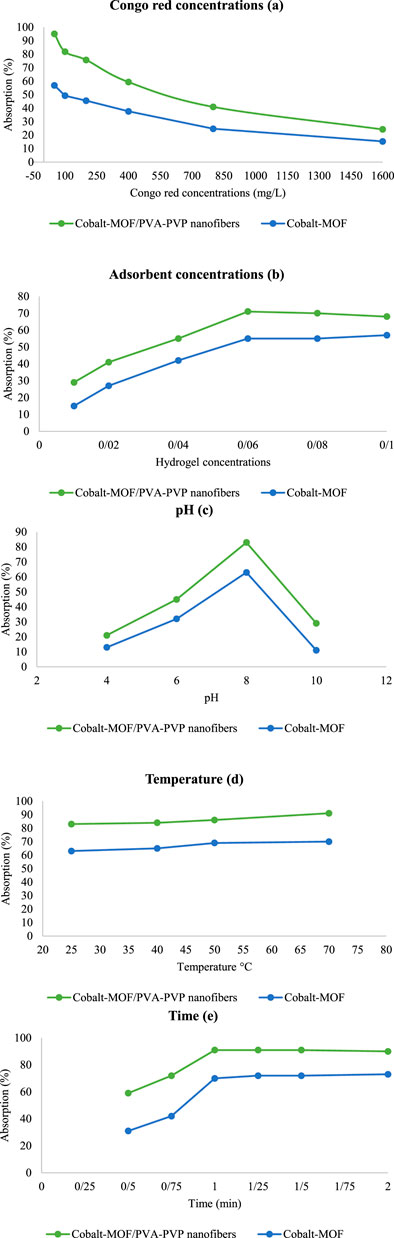
Figure 9. Congo Red absorption results in different conditions [Congo red concentrations (a) Adsorbent concentrations (b) pH (c) Temperature (d) Time (e)].
The decrease in the adsorption percentage can be attributed to the saturation of the active adsorption sites of the adsorbent. One of these concentrations was designated as a fixed concentration, while other factors were tested and evaluated based on it. For this purpose, a concentration of 400 mg/L was chosen.
In evaluating the adsorbent concentration, concentrations of 0.01, 0.02, 0.04, 0.06, 0.08, and 0.1 g/L cobalt-MOF/PVA-PVP nanofibers were tested under the constant conditions of the previous step to absorb 400 mg/L of Congo Red solution.
The results obtained at concentrations of 0.01, 0.02, 0.04, 0.06, 0.08 and 0.1 g/L showed 29%, 41%, 55%, 71%, 70% and 68% absorption, respectively (Figures 9b).
The highest percentage of adsorption was observed at a concentration of 0.06 g/L and was therefore used as the optimal concentration in other experiments. The reason for the constant and reduced adsorption at concentrations higher than 0.06 g/L can be attributed to the aggregation of nanoparticles and, as a result, their accumulation and unavailability of active adsorption sites.
Strong and mildly acidic (4 and 6) and alkaline (8 and 10) pHs were another factor that was tested and evaluated in the process of absorbing a concentration of 400 g of Congo Red by 0.06 nanoparticles under constant temperature and time conditions according to the previous steps.
According to the results, the highest absorption occurred in mild alkaline conditions, with 83% observed at pH 8. At pHs 4, 6, and 10, the absorption was 21%, 45%, and 29%, respectively (Figures 9c).
At strongly acidic and alkaline pH levels, hydrolysis of the metal-ligand bond in the final product MOF leads to its degradation, resulting in reduced adsorption (Pramanik et al., 2023). At mildly acidic pH, the adsorption process is diminished due to the protonation of the nitrogen atoms in Congo Red and the oxygen atoms in the adsorbent, which reduces hydrogen bonding. In a mild alkaline environment, OH groups interact with the carbonyl groups of the adsorbent, increasing in the concentration of negative charge on the oxygen of the carbonyl group, ultimately leading to increased hydrogen bonding with Congo Red and increased adsorption (Guo and Bulin, 2021; Moghaddam-Manesh et al., 2024).
As the temperature increased from 25°C to 70°C, the absorption rate increased. At temperatures of 25, 40, 50, and 70°C, the absorption was 83%, 84%, 86%, and 91%, respectively (Figures 9d). This can be attributed to the increase in molecular movement (Wang et al., 2020).
In temperature tests, adsorption was also investigated at a concentration of 400 mg/L Congo Red solution by 0.06 g/L nanoparticles at pH eight for 1 h.
In the last tests, the adsorption process time was investigated under the above constant conditions. The adsorption process was tested at times of 0.5, 0.75, 1, 1.25, 1.5 and 2 h (Figures 9e). The highest adsorption occurred at 1 h, and its stability at 1, 1.25, 1.5, and 2 h can be attributed to the saturation of the active sites of the adsorbent.
Figure 10 illustrates the process of Congo Red absorption by the final product.
As shown in the figure, the presence of multiple active sites in the final product, along with Congo Red, contributes to a high percentage of absorption.
As mentioned before, the high specific surface area of the final product, along with its increased contact surface with Congo Red, is also very effective in this regard.
3.3 Antimicrobial activity of cobalt-MOF/PVA-PVP nanofibers
The antimicrobial activity of specific Cobalt-MOF/PVA-PVP Nanofibers was evaluated against various microbial strains present in wastewater, including strains Aeromonas hydrophila (ATCC 7966), Legionella pneumophila (ATCC 33152), Mycobacterium tuberculosis (ATCC 27294), Salmonella enterica (ATCC 35664), Pseudomonas aeruginosa (ATCC 15442), Escherichia coli (ATCC 25922), and Giardia lamblia (ATCC 50803). The concentration of the nanofibers used in these studies ranged from 1 to 1,024 μg/mL, with a two-fold increase in concentration (e.g., 1, 2, 4, 8, 16, 32, up to 1,024). For comparison, biological evaluations were conducted on the synthesized Cobalt-MOF, PVA-PVP nanofibers without Cobalt-MOF, and amikacin.
The most significant result obtained from the study was the effectiveness of the nanofibers against all microbial strains tested. The Minimum Inhibitory Concentration (MIC) values for Aeromonas hydrophila, Legionella pneumophila, Mycobacterium tuberculosis, Salmonella enterica, Pseudomonas aeruginosa, Escherichia coli, and Giardia lamblia were determined to be 32 μg/mL, 32 μg/mL, 16 μg/mL, 8 μg/mL, 16 μg/mL, 32 μg/mL and 16 μg/mL, respectively.
The Minimum Bactericidal Concentration (MBC) values for Aeromonas hydrophila, Legionella pneumophila, Mycobacterium tuberculosis, Salmonella enterica, Pseudomonas aeruginosa, Escherichia coli, and Giardia lamblia were determined to be 32 μg/mL, 64 μg/mL, 32 μg/mL, 16 μg/mL, 16 μg/mL, 64 μg/mL and 32 μg/mL, respectively.
In Figure 11, as an example, the MBC image obtained from Cobalt-MOF/PVA-PVP Nanofibers (c) against Escherichia coli (ATCC 25922) is shown.
According to previous reports, cobalt and 2,2′-bipyridine-4,4′-dicarboxylic acid have strong antimicrobial properties. Combining them and producing Cobalt-MOF and Cobalt-MOF/PVA-PVP nanofibers containing them can lead to the production of compounds with unique antimicrobial properties (Mahadevi et al., 2022; Ameen and Majrashi, 2023). As the results proved, the effectiveness of Cobalt-MOF/PVA-PVP nanofibers was higher than that of Cobalt-MOF. In this regard, it can be pointed out that the specific surface area of Cobalt-MOF/PVA-PVP nanofibers is higher than that of Cobalt-MOF and as mentioned in Section 3.1. The increase in specific surface area leads to more contact of the studied microbial strains with the Cobalt-MOF/PVA-PVP nanofibers and consequently more contact of cobalt and 2,2′-bipyridine-4,4′-dicarboxylic acid (which have antimicrobial properties) (Ghnim et al., 2025; Lyu et al., 2024; Mamman et al., 2024).
The high specific surface area of the nanofibers is also crucial. This characteristic leads to increased contact with microbial strains and enhances the effectiveness of the Cobalt-MOF/PVA-PVP nanofibers compared to Cobalt-MOF.
Another notable finding was that the antimicrobial properties of the nanofibers were superior to those of amikacin (a well-known antibiotic). As shown in Table 2 of the results, amikacin had no effect on strains Legionella pneumophila, and Giardia lamblia, indicating microbial resistance. In contrast, the nanofibers demonstrated promising efficacy and adhesion.
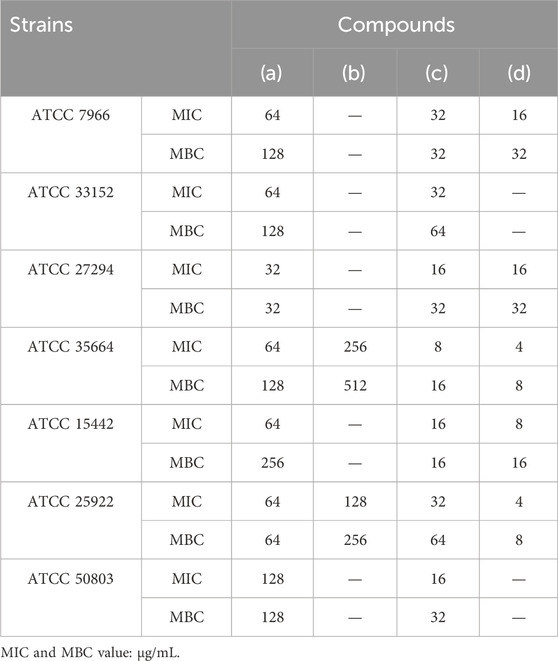
Table 2. Antimicrobial activity results [Cobalt-MOF (a); PVA-PVP Nanofibers (b); Cobalt-MOF/PVA-PVP Nanofibers (c); Amikacin (d)].
In general, substances with antimicrobial activity and a high specific surface area in the structure of the Cobalt-MOF/PVA-PVP Nanofibers are important factors contributing to their high antimicrobial properties. The study underscores the potential of combining Cobalt-MOF with PVA-PVP to enhance antimicrobial effectiveness against resistant microbial strains.
4 Conclusion
Considering the importance of wastewater treatment and the elimination of pathogenic microbial agents and chemical contaminants, this study synthesized a new fiber containing Cobalt, 2,2′-bipyridine-4,4′-dicarboxylic, polyvinyl alcohol and, polyvinylpyrrolidone (Cobalt-MOF/PVA-PVP Nanofibers). The structure and characterization of the Cobalt-MOF/PVA-PVP Nanofibers were precisely confirmed using FT-IR spectra, SEM images, CHNO elemental analysis, XRD patterns, nitrogen adsorption/desorption isotherms, TGA curves, compressive strength, and flexural strength. In line with the research objectives, the effects of adsorption, particularly concerning Congo Red as a significant contaminant in wastewater, as well as the inhibition of microbial strains such as Aeromonas hydrophila, Legionella pneumophila, Mycobacterium tuberculosis, Salmonella enterica, Pseudomonas aeruginosa, Escherichia coli, and Giardia lamblia, were investigated using the Cobalt-MOF/PVA-PVP Nanofibers. The results demonstrated that the Cobalt-MOF/PVA-PVP nanofibers produced in this study possess biological properties and a high specific surface area of 2,415 m2/g, both of which are crucial for effective adsorption 91% of a 400 mg/L Congo Red solution was absorbed by 0.06 g/L of Cobalt-MOF/PVA-PVP nanofibers within 1 h and for the inhibition of microbial strains in wastewater, with a minimum inhibitory concentration (MIC) ranging from 8 μg/mL to 64 μg/mL. The main novelty of this study is to produce novel Cobalt-MOF/PVA-PVP Nanofibers with potential applications in removing chemical and microbial contaminants from wastewater. This indicates a strong capability for removing chemical and microbial contaminants from wastewater. Therefore, it is recommended that further studies focus on similar compounds.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
SM: Methodology, Writing – original draft. DS: Writing – review and editing. AA: Data curation, Writing – original draft. RR: Writing – original draft. AH: Visualization, Writing – review and editing. TA: Formal Analysis, Writing – original draft.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The authors extend their appreciation to Prince Sattam bin Abdulaziz University for funding this research work through the project number (PSAU/2024/03/32097).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Adegoke, K. A., Agboola, O. S., Ogunmodede, J., Araoye, A. O., and Bello, O. S. (2020). Metal-organic frameworks as adsorbents for sequestering organic pollutants from wastewater. Mater. Chem. Phys. 253, 123246. doi:10.1016/j.matchemphys.2020.123246
Ahmad, I., Jasim, S. A., Yasin, G., Al-Qargholi, B., and Hammid, A. T. (2022). Synthesis and characterization of new 1, 4-dihydropyran derivatives by novel Ta-MOF nanostructures as reusable nanocatalyst with antimicrobial activity. Front. Chem. 10, 967111. doi:10.3389/fchem.2022.967111
Al-Ghouti, M. A., and Da'ana, D. A. (2020). Guidelines for the use and interpretation of adsorption isotherm models: a review. J. Hazard. Mater. 393, 122383. doi:10.1016/j.jhazmat.2020.122383
Allaka, T. R., and Katari, N. K. (2023). “Synthesis of pyridine derivatives for diverse biological activity profiles: a review,” in Recent developments in the synthesis and applications of pyridines, 605–625.
Almeleebia, T. M., Jasim Naser, M., Saeed, S. M., Abid, M. M., Altimari, U. S., Shaghnab, M. L., et al. (2024). Multi-component synthesis and invitro biological assessment of novel pyrrole derivatives and pyrano [2, 3-c] pyrazole derivatives using Co3O4 nanoparticles as recyclable nanocatalyst. Front. Mater. 11, 1354560. doi:10.3389/fmats.2024.1354560
Ameen, F., and Majrashi, N. (2023). Recent trends in the use of cobalt ferrite nanoparticles as an antimicrobial agent for disability infections: a review. Inorg. Chem. Commun. 156, 111187. doi:10.1016/j.inoche.2023.111187
Asiri, M., Jawad Bahraluloom, Y., Abdullateef Alzubaidi, M., Mourad Mohammed, I., Suliman, M., Ramzy Muhammad, E., et al. (2023). Synthesis of Cu/Co-hybrid MOF as a multifunctional porous compound in catalytic applications, synthesis of new nanofibers, and antimicrobial and cytotoxicity agents. Front. Mater. 10, 1214426. doi:10.3389/fmats.2023.1214426
Azizabadi, O., Akbarzadeh, F., Sargazi, G., and Chauhan, N. P. S. (2021). Preparation of a novel Ti-metal organic framework porous nanofiber polymer as an efficient dental nano-coating: physicochemical and mechanical properties. Polymer-Plastics Technol. Mater. 60, 734–743. doi:10.1080/25740881.2020.1844231
Baig, U., Faizan, M., and Sajid, M. (2021). Effective removal of hazardous pollutants from water and deactivation of water-borne pathogens using multifunctional synthetic adsorbent materials: a review. J. Clean. Prod. 302, 126735. doi:10.1016/j.jclepro.2021.126735
Behyar, M. B., Hasanzadeh, M., Seidi, F., and Shadjou, N. (2023). Sensing of amino acids: critical role of nanomaterials for the efficient biomedical analysis. Microchem. J. 188, 108452. doi:10.1016/j.microc.2023.108452
Du, C., Zhang, Z., Yu, G., Wu, H., Chen, H., Zhou, L., et al. (2021). A review of metal organic framework (MOFs)-based materials for antibiotics removal via adsorption and photocatalysis. Chemosphere 272, 129501. doi:10.1016/j.chemosphere.2020.129501
El-Aswar, E. I., Ramadan, H., Elkik, H., and Taha, A. G. (2022). A comprehensive review on preparation, functionalization and recent applications of nanofiber membranes in wastewater treatment. J. Environ. Manag. 301, 113908. doi:10.1016/j.jenvman.2021.113908
Fan, W. K., and Tahir, M. (2022). Recent advances on cobalt metal organic frameworks (MOFs) for photocatalytic CO2 reduction to renewable energy and fuels: a review on current progress and future directions. Energy Convers. Manag. 253, 115180. doi:10.1016/j.enconman.2021.115180
Ghnim, Z. S., Adhab, A. H., Altalbawy, F., Salih Mahdi, M., Salah Mansoor, A., Radi, U. K., et al. (2025). Novel Pectin/Chitosan Mo-MOF Hydrogel for dye adsorption and pathogenic bacteria inhibition. Front. Mater. 12, 1535825. doi:10.3389/fmats.2025.1535825
Gude, V. G. (2017). Desalination and water reuse to address global water scarcity. Rev. Environ. Sci. Bio/Technology 16, 591–609. doi:10.1007/s11157-017-9449-7
Guo, T., and Bulin, C. (2021). Facile preparation of MgO/graphene oxide nanocomposite for efficient removal of aqueous Congo red: adsorption performance and interaction mechanism. Res. Chem. Intermed. 47, 945–971. doi:10.1007/s11164-020-04310-9
Hetta, H. F., Ramadan, Y. N., Al-Harbi, A. I., A. Ahmed, E., Battah, B., Abd Ellah, N. H., et al. (2023). Nanotechnology as a promising approach to combat multidrug resistant bacteria: a comprehensive review and future perspectives. Biomedicines 11, 413. doi:10.3390/biomedicines11020413
Homaeigohar, S., and Boccaccini, A. R. (2020). Antibacterial biohybrid nanofibers for wound dressings. Acta biomater. 107, 25–49. doi:10.1016/j.actbio.2020.02.022
Hu, X., He, J., Zhu, L., Machmudah, S., Kanda, H., Goto, M., et al. (2021). Synthesis of hollow PVP/Ag nanoparticle composite fibers via electrospinning under a Dense CO2 environment. Polymers 14, 89. doi:10.3390/polym14010089
Huang, L., Shen, R., and Shuai, Q. (2021). Adsorptive removal of pharmaceuticals from water using metal-organic frameworks: a review. J. Environ. Manag. 277, 111389. doi:10.1016/j.jenvman.2020.111389
Karri, R. R., Ravindran, G., and Dehghani, M. H. (2021). “Wastewater—sources, toxicity, and their consequences to human health,” in Soft computing techniques in solid waste and wastewater management (Elsevier), 3–33.
Khondoker, M., Mandal, S., Gurav, R., and Hwang, S. (2023). Freshwater shortage, salinity increase, and global food production: a need for sustainable irrigation water desalination—a scoping review. Earth 4, 223–240. doi:10.3390/earth4020012
Khursheed, M., Hadi, K. M., and Faraj, M. (2023). Methanol extract of Iraqi Kurdistan Region Daphne mucronata as a potent source of antioxidant, antimicrobial, and anticancer agents for the synthesis of novel and bioactive polyvinylpyrrolidone nanofibers. Front. Chem. 1, 2296–2646. doi:10.3389/fchem.2023.1287870
Kiadeh, S. Z. H., Ghaee, A., Farokhi, M., Nourmohammadi, J., Bahi, A., and Ko, F. K. (2021). Electrospun pectin/modified copper-based metal–organic framework (MOF) nanofibers as a drug delivery system. Int. J. Biol. Macromol. 173, 351–365. doi:10.1016/j.ijbiomac.2021.01.058
Lechevallier, M. W., Prosser, T., and Stevens, M. (2024). Opportunistic pathogens in drinking water distribution systems—a review. Microorganisms 12, 916. doi:10.3390/microorganisms12050916
Lendzion-Bielun, Z., Narkiewicz, U., and Arabczyk, W. (2013). Cobalt-based catalysts for ammonia decomposition. Materials 6, 2400–2409. doi:10.3390/ma6062400
Li, B., Wang, Y.-F., Zhang, L., and Xu, H.-Y. (2022). Enhancement strategies for efficient activation of persulfate by heterogeneous cobalt-containing catalysts: a review. Chemosphere 291, 132954. doi:10.1016/j.chemosphere.2021.132954
Li, Q., Wu, Z.-Q., Li, D., Liu, T.-H., Yin, H.-Y., Cai, X.-B., et al. (2023). A Tb 3+-anchored Zr (iv)-bipyridine MOF to promote photo-induced electron transfer and simultaneously enhance photoluminescence ability and photocatalytic reduction efficiency towards Cr 2 O 7 2. J. Mater. Chem. A 11, 2957–2968. doi:10.1039/d2ta07769h
Liang, C., Qin, S., Ai, H., Li, S., and Du, K. (2022). Novel amyloid-like porous lysozyme skeletons as “green” superadsorbent presenting ultrahigh capacity and rapid sequestration towards hazardous Congo red. Chem. Eng. J. 441, 136005. doi:10.1016/j.cej.2022.136005
Lv, J., Gu, W., Cui, X., Dai, S., Zhang, B., and Ji, G. (2019). Nanofiber network with adjustable nanostructure controlled by PVP content for an excellent microwave absorption. Sci. Rep. 9, 4271. doi:10.1038/s41598-019-38899-8
Lyu, H., Lim, J. Y., Zhang, Q., Senadheera, S. S., Zhang, C., Huang, Q., et al. (2024). Conversion of organic solid waste into energy and functional materials using biochar catalyst: bibliometric analysis, research progress, and directions. Appl. Catal. B Environ. 340, 123223. doi:10.1016/j.apcatb.2023.123223
Mahadevi, P., Sumathi, S., Metha, A., and Singh, J. (2022). Synthesis, spectral, antioxidant, in vitro cytotoxicity activity and thermal analysis of Schiff base metal complexes with 2, 2′-Bipyridine-4, 4′-dicarboxylic acid as co-ligand. J. Mol. Struct. 1268, 133669. doi:10.1016/j.molstruc.2022.133669
Mamman, S., Abdullahi, S. S. A., Birniwa, A. H., Opaluwa, O. D., Mohammad, R. E. A., Okiemute, O., et al. (2024). Influence of adsorption parameters on phenolic compounds removal from aqueous solutions: a mini review. Desalination Water Treat. 320, 100631. doi:10.1016/j.dwt.2024.100631
Moghaddam-Manesh, M., Darvishi, R., and Moshkriz, A. (2024). Innovative high-performance antimicrobial agent and dye adsorbent based on magnetic/copper nanoparticles. J. Polym. Environ. 32, 5231–5253. doi:10.1007/s10924-024-03289-3
Moghaddam-Manesh, M., Ghazanfari, D., Sheikhhosseini, E., and Akhgar, M. (2020). Synthesis of bioactive magnetic nanoparticles spiro [indoline-3, 4′-[1, 3] dithiine]@ Ni (NO3) 2 supported on Fe3O4@ SiO2@ CPS as reusable nanocatalyst for the synthesis of functionalized 3, 4-dihydro-2H-pyran. Appl. Organomet. Chem. 34, e5543. doi:10.1002/aoc.5543
Moghaddam-Manesh, M., Sargazi, G., Roohani, M., Zanjani, N. G., Khaleghi, M., and Hosseinzadegan, S. (2022). Synthesis of PVA/Fe3O4@ SiO2@ CPS@ SID@ Ni as novel magnetic fibrous composite polymer nanostructures and evaluation of anti-cancer and antimicrobial activity. Polym. Bull. 80, 11919–11930. doi:10.1007/s00289-022-04584-6
Moharramnejad, M., Ehsani, A., Shahi, M., Gharanli, S., Saremi, H., Malekshah, R. E., et al. (2023). MOF as nanoscale drug delivery devices: synthesis and recent progress in biomedical applications. J. Drug Deliv. Sci. Technol. 81, 104285. doi:10.1016/j.jddst.2023.104285
Nayak, S., Das, P., and Sahoo, K. (2003). Synthesis and characterization of some cobalt (III) complexes containing heterocyclic nitrogen donor ligands. name Chem. Pap. 57, 91.
Parham, S., Kharazi, A. Z., Bakhsheshi-Rad, H. R., Kharaziha, M., Ismail, A. F., Sharif, S., et al. (2022). Antimicrobial synthetic and natural polymeric nanofibers as wound dressing: a review. Adv. Eng. Mater. 24, 2101460. doi:10.1002/adem.202101460
Pettinari, C., Pettinari, R., Di Nicola, C., Tombesi, A., Scuri, S., and Marchetti, F. (2021). Antimicrobial MOFs. Coord. Chem. Rev. 446, 214121. doi:10.1016/j.ccr.2021.214121
Pramanik, B., Sahoo, R., and Das, M. C. (2023). pH-stable MOFs: design principles and applications. Coord. Chem. Rev. 493, 215301. doi:10.1016/j.ccr.2023.215301
Rajan, S., Marimuthu, K., Ayyanar, C. B., and Hoque, M. E. (2022). Development and in-vitro characterization of HAP blended PVA/PEG bio-membrane. J. Mater. Res. Technol. 18, 4956–4964. doi:10.1016/j.jmrt.2022.04.130
Ramírez-Coronel, A. A., Mezan, S. O., Patra, I., Sivaraman, R., Riadi, Y., Khakberdiev, S., et al. (2022). A green chemistry approach for oxidation of alcohols using novel bioactive cobalt composite immobilized on polysulfone fibrous network nanoparticles as a catalyst. Front. Chem. 10, 1015515. doi:10.3389/fchem.2022.1015515
Rani, M., Yadav, J., Chaudhary, S., Shanker, U., and Shanker, U. (2021). An updated review on synthetic approaches of green nanomaterials and their application for removal of water pollutants: current challenges, assessment and future perspectives. J. Environ. Chem. Eng. 9, 106763. doi:10.1016/j.jece.2021.106763
Ren, X. Y., Chen, F. Y., Zhang, C. H., Liang, Z. G., Yu, X. Y., Han, S. D., et al. (2024). Regulating the topologies and photoresponsive properties of lanthanum-organic frameworks. Chemistry–A Eur. J. 30, e202402581. doi:10.1002/chem.202402581
Ribeiro, A. I., Dias, A. M., and Zille, A. (2022). Synergistic effects between metal nanoparticles and commercial antimicrobial agents: a review. ACS Appl. Nano Mater. 5, 3030–3064. doi:10.1021/acsanm.1c03891
Samsami, S., Mohamadizaniani, M., Sarrafzadeh, M.-H., Rene, E. R., and Firoozbahr, M. (2020). Recent advances in the treatment of dye-containing wastewater from textile industries: overview and perspectives. Process Saf. Environ. Prot. 143, 138–163. doi:10.1016/j.psep.2020.05.034
Sargazi, G., Ebrahimi, A. K., Afzali, D., Badoei-Dalfard, A., Malekabadi, S., and Karami, Z. (2019). Fabrication of PVA/ZnO fibrous composite polymer as a novel sorbent for arsenic removal: design and a systematic study. Polym. Bull. 76, 5661–5682. doi:10.1007/s00289-019-02677-3
Sharifi, M., and Bahrami, S. H. (2024). Review on application of herbal extracts in biomacromolecules-based nanofibers as wound dressings and skin tissue engineering. Int. J. Biol. Macromol. 277, 133666. doi:10.1016/j.ijbiomac.2024.133666
Siddiqui, S. I., Allehyani, E. S., Al-Harbi, S. A., Hasan, Z., Abomuti, M. A., Rajor, H. K., et al. (2023). Investigation of Congo red toxicity towards different living organisms: a review. Processes 11, 807. doi:10.3390/pr11030807
Soliman, N. K., and Moustafa, A. (2020). Industrial solid waste for heavy metals adsorption features and challenges; a review. J. Mater. Res. Technol. 9, 10235–10253. doi:10.1016/j.jmrt.2020.07.045
Tang, Z., Zhao, P., Wang, H., Liu, Y., and Bu, W. (2021). Biomedicine meets Fenton chemistry. Chem. Rev. 121, 1981–2019. doi:10.1021/acs.chemrev.0c00977
Varshney, S., and Mishra, N. (2023). “Pyridine-based polymers and derivatives: synthesis and applications,” in Recent developments in the synthesis and applications of pyridines (Elsevier), 43–69.
Waheed, A., Baig, N., Ullah, N., and Falath, W. (2021). Removal of hazardous dyes, toxic metal ions and organic pollutants from wastewater by using porous hyper-cross-linked polymeric materials: a review of recent advances. J. Environ. Manag. 287, 112360. doi:10.1016/j.jenvman.2021.112360
Wang, H., Warren, M., Jagiello, J., Jensen, S., Ghose, S. K., Tan, K., et al. (2020). Crystallizing atomic xenon in a flexible MOF to probe and understand its temperature-dependent breathing behavior and unusual gas adsorption phenomenon. J. Am. Chem. Soc. 142, 20088–20097. doi:10.1021/jacs.0c09475
Wu, S., Li, K., Shi, W., and Cai, J. (2022). Preparation and performance evaluation of chitosan/polyvinylpyrrolidone/polyvinyl alcohol electrospun nanofiber membrane for heavy metal ions and organic pollutants removal. Int. J. Biol. Macromol. 210, 76–84. doi:10.1016/j.ijbiomac.2022.05.017
Younis, S. A., Bhardwaj, N., Bhardwaj, S. K., Kim, K.-H., and Deep, A. (2021). Rare earth metal–organic frameworks (RE-MOFs): synthesis, properties, and biomedical applications. Coord. Chem. Rev. 429, 213620. doi:10.1016/j.ccr.2020.213620
Zawrah, M., El-Okr, M., Ashery, A., and Abou Hammad, A. (2016). Characterization of sol-gel fabricated cobalt ferrite CoFe2O4 nanoparticles. Middle East J. Appl. Sci. 6, 362–366.
Zhang, W., Taheri-Ledari, R., Saeidirad, M., Qazi, F. S., Kashtiaray, A., Ganjali, F., et al. (2022). Regulation of porosity in MOFs: a review on tunable scaffolds and related effects and advances in different applications. J. Environ. Chem. Eng. 10, 108836. doi:10.1016/j.jece.2022.108836
Keywords: cobalt-metal organic framework, poly vinyl alcohol, poly vinyl pyrrolidone, wastewater contaminants, Congo red, antimicrobial activity
Citation: M. Alqahtani S, Sur D, Altharawi A, Roopashree R, Hussein Zwamel A and Aldakhil T (2025) Sustainable innovative nanofibers containing Cobalt-MOF: a dual-action solution for microbial and chemical wastewater contamination. Front. Chem. 13:1584064. doi: 10.3389/fchem.2025.1584064
Received: 26 February 2025; Accepted: 23 April 2025;
Published: 08 May 2025.
Edited by:
Mijanur Rahaman Molla, University of Calcutta, IndiaReviewed by:
Shivshankar R. Mane, University of California, Santa Barbara, United StatesNabendu B. Pramanik, Institute of Chemical Technology, India
Copyright © 2025 M. Alqahtani, Sur, Altharawi, Roopashree, Hussein Zwamel and Aldakhil. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Safar M. Alqahtani, c2FmYXIuYWxxYWh0YW5pQHBzYXUuZWR1LnNh
 Safar M. Alqahtani
Safar M. Alqahtani Dharmesh Sur2
Dharmesh Sur2 Ali Altharawi
Ali Altharawi