Abstract
Glucose oxidase (GOx), as a molecular recognition element of glucose biosensors, has high sensitivity and selectivity advantages. As a type of biosensor, the glucose oxidase electrode exhibits advantages such as ease of operation, high sensitivity, and strong specificity, promising broad application prospects in biomedical science, the food industry, and other fields. In recent years, with the advancement of nanotechnology, research efforts to enhance the performance of GOx biosensors have primarily focused on improving the conductive properties and specific surface area of nanomaterials, while neglecting the potential to modify the structure of the core component, GOx itself, to improve biosensor performance. Rapid modification of the GOx surface through chemical modification techniques yields a new modified enzyme (mGOx). Meanwhile, composite techniques involving carbon nanomaterials can be employed to further enhance sensor performance. This article reviews the construction methods and optimization strategies of glucose oxidase electrodes in recent years, along with research progress in their application in electrochemical sensing for glucose detection, and provides an outlook for future developments.
1 Necessity of glucose testing
With the rapid development of the social economy, people’s dietary structure has also changed, and carbohydrate-rich diets have become the norm and are increasing day by day. According to the International Diabetes Federation (IDF) in 2019, 463 million people worldwide are currently living with diabetes. China ranks first in the number of diabetic patients, with about 116.4 million people, 25% of the world’s share. The large diabetic community has attracted more and more social attention. People are starting to consciously avoid carbohydrate-rich foods, so foods with low-calorie or non-nutritive sweeteners and sugar-free products have become very popular. According to the GB28050-2011 National Food Safety Standard “General Principles for Nutrition Labeling of Prepackaged Foods”, “sugar-free or sugar-free” specifically refers to a sugar content of no more than 0.5 g per 100 g or 100 mL of solid or liquid food. The complex diversity of food samples makes it necessary to develop a highly sensitive, low-cost, and rapid glucose detection method. To help people understand the sugar content in food and to achieve the purpose of disease prevention (Zhang Y. et al., 2018; Wang et al., 2024). So far, a variety of glucose detection methods have been developed, including fluorescence, optical, sonic, thermal, electrochemical, and colorimetric methods have been used for the detection of glucose due to their simple operation, however, the sensitivity of this method is often poor and insufficient to quantify glucose in the sample, and to circumvent these problems, electrochemical methods have been extensively studied (Li et al., 2021; Ma et al., 2022; Sun et al., 2016). Although different glucose detection techniques have been reported, electrochemical sensors due to their high sensitivity, low cost, and simple instrumentation, it is still considered the most successful analytical tool for glucose detection (Han et al., 2021; Ma et al., 2021; Hu et al., 2024). Glucose electrochemical sensing detection has the advantages of high detection sensitivity, accuracy and rapidity, and low construction cost, which can not only detect human blood glucose levels but also can be used to detect trace amounts of glucose in food, medicine, and biological samples (Bai et al., 2020; Peng et al., 2019), Therefore, electrochemical sensing analysis occupies an important position in glucose detection. Meanwhile, in recent years, the improvement of sensor performance has become a research hotspot, especially in terms of sensitivity, selectivity and long-term stability. For example, Tong et al. (Tong et al., 2024) prepared nanocomposites (PGOx@M-Xene/CS) by efficient electrostatic assembly of GOx polygels (PGOx) onto MXene nanosheets, PGOx can enhance the stability of the enzyme, while MXene’s extensive large specific surface area reduces its influence on enzyme stability. The constructed glucose sensor had a linear range of 0.03–16.5 mM, with a sensitivity of 48.98 μA mM-1·cm-2, and the limit of detection was 3.1 μM. The current remained at 85.83% of the initial current value after 200 cycles. Ramachandran et al. (Ramachandran et al., 2022) effectively prepared hexagonal CoMn2O4 electrode material by simple hydrothermal technique using KOH as surfactant and the obtained sensor maintained 85% capacitance after 4,000 consecutive charge/discharge cycles with 81% maximum column efficiency.
2 Advances in electrochemical detection sensors for glucose
2.1 Glucose oxidase-based electrodes
The first enzyme electrodes and enzyme sensors were described by Clark and Updike in the 1960s (Hovancová et al., 2017). In their concept, glucose oxidase (GOx) could be trapped in a semi-dialysis membrane on an oxygen electrode, and glucose concentration could be determined indirectly by monitoring oxygen consumption. Five years later, Updike and Hicks proposed an innovative method for the preparation of glucose analysis enzyme electrodes, which was designed to achieve rapid and accurate determination of glucose concentration in the blood by firmly embedding glucose oxidase (GOx) in a polyacrylamide gel layer overlying an oxygen electrode (Wang P. et al., 2019). Since then, electrochemical glucose sensors have developed rapidly, and electrochemical enzyme electrode technology has become the main method for detecting glucose (Xia et al., 2022). Today, electrochemical glucose sensors have undergone four transformations in principle, forming four generations of sensors, the principle of which is shown in Figure (Figure 1).
FIGURE 1

Schematic diagram of electron transfer of fourth-generation electrochemical glucose sensor.
2.1.1 First-generation glucose biosensors
The first generation of glucose enzyme electrochemical sensors utilized molecular oxygen as an electron acceptor, and the electrochemical signal was transmitted by measuring the decreasing oxygen concentration or the released H2O2, but the detection method was greatly affected by dissolved oxygen, and the detection potential was too high, which made the detection results easily affected by other interfering substances (Wen et al., 2020; Bu et al., 2024). On the other hand, the H2O2 produced during the reaction accumulated, and the concentration increased, resulting in the loss of glucose oxidase activity (Qin et al., 2017).
In the 80s of the 20th century, a large number of studies focused on solving the problems of oxygen interference and redox interference (Zhang et al., 2019). There are many solutions to oxygen interference. Gough et al. (Gough et al., 1985) overcame oxygen interference by increasing oxygen/glucose permeability with the help of a mass migration limiting membrane. Wang et al. addressed oxygen restriction in glucose biosensors by using oxygen-rich Carbon Paste Electrodes (Wang and Lu, 2009). D’Costa et al. avoided oxygen demand deficiency by replacing GOx with glucose dehydrogenase, which does not require oxygen cofactors, and the solution to redox interference was mainly to reduce interference to the electrode surface by selective coating (D Costa et al., 1986). Zhang et al. found that a cellulose acetic-Nafion composite membrane can effectively eliminate electrochemical interference such as acetaminophen (Zhang et al., 1994). Millilista et al. immobilized GOx using an electrochemically synthesized polyphenylenediamine (PPD) membrane that selectively removed ascorbic acid interference (Malitesta et al., 1990; Rong et al., 2019).
2.1.2 Second-generation glucose biosensors
The second-generation biosensor overcame the limitations encountered by the first-generation biosensor. They used redox mediators instead of oxygen to transfer electrons from the enzyme to the surface of the working electrode. The resulting reducing medium is further oxidized on the electrode formation of oxidation mediators, thus the ampere signal was detected. Various organic/inorganic chemicals are used as electronic media, mainly ferrocene derivatives (Monkrathok et al., 2024), ferricyanide (Nikitina et al., 2023), quinones (Mtemeri and Hickey, 2023), transition metal complexes (Wijayanti et al., 2023), and phenothiazine (Teymourian et al., 2020). Campbell et al. realized the detection of glucose by covalently coupling glucose oxidase to a redox medium containing ferrocene, combined with intramolecular electron transfer and electron self-exchange (Campbell et al., 2017). Zhou et al. achieved the detection of glucose by incorporating ferrocene (FC) as an electronic medium and immobilizing glucose oxidase (Gox) on the gate electrode, and tests with simulated blood samples were performed, and the modified sensor showed a bilinear response in the range of 0.6–26.3 mM, whereas conventional sensors (e.g., PEDOT-based) have a narrower linear range (0.5 μM-0.1 mM) (Zhou et al., 2024). Donini et al. (Donini et al., 2020) fixed glucose oxidase to redox graphene and used it for the detection of glucose. Lin et al. (2019) used hydrophilic and positively charged alpha-poly-L-lysine (alpha PLL) as an embedded substrate to fix negatively charged glucose oxidase (GOx) and ferric cyanide (FIC) onto SPCE to construct a disposable second-generation glucose biosensor, and tested in real human serum samples, the sensitivity was significantly improved (from 117.4 to 212.1 nA/mM mm2), and the linear range and detection limit were also superior to the pre-improved sensor. The emergence of redox mediators solved the problem of the dependence of glucose sensors on oxygen, but the sensors still had limitations. The presence of redox agents increased the cost of sensors, and many redox agents were biotoxic, limiting the range of sensor applications. Therefore, it is hoped that a way to replace or eliminate redox mediators while maximizing the current intensity of the sensor (Ahmad et al., 2023). Therefore, in the third generation of electrochemical glucose sensors, the biocatalyst was directly bound to the electrode surface where the signal was converted and amplified, thus ensuring that the electron transfer between the biocatalyst and the contact surface of the conductive carrier was as efficient and fast as possible (Khan et al., 1996; Ravikumar et al., 2021).
2.1.3 Third-generation glucose biosensors
The third-generation glucose biosensor is not dependent on oxygen and redox mediators, and the goal is to transfer electrons directly from the redox site of GOx to the electrode in the absence of a medium. However, the redox center of GOx, flavin adenine dinucleotide (FAD), is deeply embedded in the three-dimensional macrostructure of the enzyme molecule, which acts as an inherent barrier to direct electron transfer between GOx and the electrode. A range of nanomaterials, such as conductive polymers, metal oxides, carbon nanotubes, and graphene, have been used as electrode materials for modified electrodes (Xu et al., 2017; Ma et al., 2019; Shen et al., 2019; Shah et al., 2024). The distance between the active center of the enzyme and the surface of the electrode is effectively reduced to create a suitable environment and promote the smooth progress of the direct electron transfer process between the enzyme and the electrode. Rafighi et al. (2016) synthesized a glucose biosensor using a hybrid of graphene and polyethyleneimine-gold nanoparticles as enzyme carriers, and tested in actual blood samples, the sensitivity of the sensor reached 9.3 μA·mM-1cm-2, superior to carbon quantum dots reported in the literature (6.1 μA mM-1). Tao et al. (Ming et al., 2024; Zhu et al., 2022)modified the sensor by electrochemically depositing gold nanoparticles to enhance its conductivity for better detection of glucose, tested in clinical serum samples, the improved sensor showed only a 13.1% decrease in sensitivity after 7 days in bovine serum compared to a 45.1% decrease in the uncoated version, demonstrating that the PU film significantly extends the service life. Chu et al. (2015) successfully prepared composite carriers by in situ electrodeposition of gold nanocubes on a thiol graphene film. These were uniformly distributed on the electrode surface and had a regular nanostructure. This glucose biosensor exhibits high sensitivity and selectivity, and increased sensitivity to 221.0 μA mM-1·cm-2, 4-7 times higher than similar sensors in the literature, and retained 79.3% sensitivity after 2 weeks of storage (Figure 2). However, the structure of the enzyme protein may change after fixation on the surface of the electrode, which will affect the enzyme activity and the stability of the electrode (Apaliya et al., 2017; Wang L. et al., 2019; Jing et al., 2020). Therefore, preliminary research on third-generation EBGS has focused on how to achieve DET, which mainly involves the following aspects: 1) adding an electron transfer domain to the enzyme itself, 2) modifying with conductive nanomaterials, and 3) modifying with other materials (Song et al., 2024).
FIGURE 2

The preparation scheme of Au nanocube/graphene composite film based biosensor. Due to the existence of -SH on graphene surface, oxidase can directly adhere on the film by the interaction produced by the -S-S- from protein and -SH from grapheneReproduced with permission from ref, (Chu et al., 2015). Copyright 2015, Elsevier Publication.
Compared with the first and second generations of glucose biosensors, the third generation of glucose biosensors has achieved better results. However, its core principle is still controversial, and whether the DET that generates electrical signals is real is still doubtful. The redox peak may not come from the electron transfer of active enzymes but because the enzyme’s structure is destroyed, resulting in FAD exposure. Moreover, external environmental factors such as temperature, pH, and humidity may affect their dependence on enzyme activity. In addition, the performance of biosensors also depends on the thickness of the enzyme layer, which is high, resulting in signal attenuation or loss (Ou et al., 2021; Yin et al., 2023; Xu et al., 2016).
2.2 Glucose dehydrogenase-based electrodes
Glucose dehydrogenase (GDH), an NAD(P)+-independent oxidoreductase, has attracted a lot of attention in recent years for its application in glucose sensors. Different types of GDH play an important role in improving the sensitivity, selectivity, and stability of the sensor. Glucose dehydrogenase forms complexes with cofactors such as flavin adenine dinucleotide (FAD), nicotinamide adenine dinucleotide (NAD), or pyrroloquinoline quinone (PQQ) (Zhao et al., 2021). For example, the glucose sensor developed by Kim et al., which involves binding the enzyme to the anionic self-assembled monolayer on the electrode through electrostatic interactions, exhibits high sensitivity. (Kim et al., 2012) (Figure 3). Chen et al. (2022) developed an NAD (+)-dependent dehydrogenase/NPG/SPE biosensing platform for the electrochemical detection of glucose by modifying a screen-printed electrode (SPE) with NPG and NAD (+)-dependent dehydrogenase.
FIGURE 3

Schematic for binding of PQQ-GDH onto the SAMs of 11-MUA: (a) electrostatic and (b) covalent binding via EDC/NHS chemistry. Reproduced with permission from ref, (Kim et al., 2012). Copyright 2015, Springer Science Publication.
3 Non-enzymatic glucose sensor
Non-enzymatic glucose sensors are a technology that does not rely on an enzyme-catalyzed reaction to detect glucose concentration. The core principle of the non-enzymatic glucose sensor lies in the precise detection of glucose concentration through an electrochemical catalytic reaction. The detection system relies on the intrinsic catalytic activity of metal nanomaterials to realize the direct oxidation process of glucose molecules on the electrode surface in an alkaline medium. The catalytic unit is usually constructed with highly active metal nanoparticles, including two systems of noble metals (Au, Ag, Pt) and transition metals (Ni, Co, Cu) (Li H. et al., 2024). These nanostructures significantly enhance the charge transfer efficiency and catalytic site density of the sensor by increasing the effective reaction interface and optimizing the mass transfer channels. Although non-enzymatic sensors circumvent the problem of enzyme activity decay, their selectivity, environmental adaptability and long-term stability are still the main bottlenecks (Table.1). Therefore, glucose oxidase-based sensors are still in the main position for glucose detection.
TABLE 1
| Category | Core components | Detection mechanism | Sensitivity | Selectivity | Stability | Response time |
|---|---|---|---|---|---|---|
| Enzyme sensors | GOx/GDH | Enzymes catalyze glucose oxidation to produce a measurable electrical signal | High (detection limit as low as μm class) | Excellent (enzyme specifically recognizes glucose) | Low (easy inactivation of enzyme, limited by temperature, pH) | Faster (seconds) |
| Non-enzyme sensors | Metallic nanomaterials or carbon-based materials | Direct electrochemical catalysis of glucose oxidation, which generates an electric current signal through a reaction on the surface of a metal active site or carbon material | Higer (up to the nM level in some studies, but susceptible to interference in complex samples) | Poor (susceptible to interference from electroactive substances such as ascorbic acid, uric acid, etc., requires additional functionalization) | High (no biocomponents, resistant to high temperatures, wide pH range) | Faster (milliseconds as no enzyme catalytic step required) |
Comparison of enzyme versus non-enzyme sensors.
The fourth-generation glucose biosensor is a non-enzymatic glucose sensor that uses an artificial substance with enzyme-like catalytic properties to replace glucose oxidase and oxidize glucose directly on the surface of the electrode. Fourth-generation glucose sensors (FGGS) are designed to enhance glucose-sensing technology and reduce the number of intermediate stages equired for glucose measurement. Diagnostic efficiency and cost-effectiveness can be improved by using these sensors, which are fabricated using electrocatalytic copper nanostructures (Naikoo et al., 2022; Kilic et al., 2023). Ahmad et al. fabricated an electrochemical-based non-enzymatic glucose biosensor using engineered layered CuO nanoleaves, which shows high sensitivity (1467.32 μA/(mM cm2)), linear range (0.005–5.89 mM), and detection limit of 12 nM (S/N = 3) (Ahmad et al., 2021). The fourth generation has the advantage of using chemically derived materials, which is more suitable for large-scale production, and the chemically produced identification parts can exert better uniformity and reproducibility compared to enzymes prepared by biotechnology. On the other hand, this type of device can be affected (Pohanka, 2021). Therefore, the use of glucose oxidase electric sensors for glucose detection is still the mainstream.
4 Enhancement of glucose oxidase activity stability
In the development of glucose biosensors, the enhancement of glucose oxidase activity stability is the core technical difficulty. In recent years, the linkage strategies for Gox activity attenuation have focused on the following directions: nanomaterial encapsulation protection: encapsulating GOx by metal-organic frameworks (MOFs) or mesoporous silica to limit the enzyme molecular conformational changes. For example, Mao et al. (Mao et al., 2025) successfully synthesized glucose oxidase (GOx)@Zn-HHTP, which significantly improved the stability of the encapsulated GOx and was applied to construct an ECL glucose sensor with 8.5-fold increase in sensitivity and 25-fold decrease in detection limit, which was successfully applied to detect glucose in sweat. Covalent cross-linking enhancement: Glutaraldehyde or genetically engineered bifunctional cross-linkers (e.g., SpyCatcher/SpyTag) were used to enhance the enzyme-electrode interface binding. For example, Chmayssem et al. (2023) used a chitosan-based hydrogel to capture glucose oxidase (GOx) and crosslinked the entire substrate with glutaraldehyde, and the resulting biosensor was able to maintain its stability over 6 months of storage. Biomimetic polymer coatings: polydopamine (PDA) or amphoteric ionic polymers (e.g., poly (sulfobetaine)) were utilized to form antifouling coatings. For example, Chen et al. (2023) combined the good hydrophilicity and biocompatibility of PDA with the high loading properties and peroxidase-like activity of HKUST-1 to synthesize the PDA/HKUST-1/MWCNTs/GOx biosensor, which was used for glucose detection with a sensitivity of 178 μA mM-1cm-2, a linear range of 0.005 mM, and a limit of detection of 0.12 μ M. The initial current response value remained at 82.0% after 30 days (Figure 4).
FIGURE 4

Copper-based metal–organic frameworks (MOF) and multi-walled carbon nanotubes (HKUST-1-MWCNTs) composite were synthesized by one-step hydrothermal method, and PDA-enzyme-HKUST-1-MWCNTs composite was prepared by one-pot method for the construction of glucose biosensors. Reproduced with permission from ref, (Chen et al., 2023). Copyright 2022, Springer Science Publication.
5 Chemical modification of glucose oxidase
5.1 Introduction to glucose oxidase
In the presence of molecular oxygen, glucose oxidase catalyzes the oxidation of β-D-glucose to D-glucono-delta-lactone and hydrogen peroxide. The resulting D-glucono-delta-lactone is sequentially hydrolyzed to D-gluconate by lactonase, and the resulting hydrogen peroxide is hydrolyzed by catalase to oxygen and water (Bauer et al., 2022). GOx has a relative molecular mass of 130 × 103–175 × 103 and exists as a glycosylated homodimer, with each subunit noncovalently bound to one flavin adenine dinucleotide (FAD) molecule, and the two subunits are bound to each other by forces such as salt bridges and hydrogen bonds (Huang et al., 2022). In the catalytic process, GOx uses molecular oxygen as an electron acceptor and FAD coenzyme as an electron carrier to catalyze specifically the formation of D-glucose-delta-lactone and hydrogen peroxide (H2O2) from β-D-glucose (Wang et al., 2022). GOx is most active under weakly acidic conditions, and the pH range in which activity is stable is 3.5–7.5; other than that too much acid and too much alkali will inactivate the enzyme molecule. GOx is available from a variety of sources. Bacterial, fungal, herbal, and animal sources are the main ones. Fungi are considered to be the richest source and are widely used for industrial applications, A. niger, and P. glaucoma were the first identified sources of GOx isolates, and glucose oxidase isolated from Aspergillus niger is considered to be the most stable (Khatami et al., 2022; He et al., 2022; Guo et al., 2021; Shang et al., 2019). Glucose oxidase has been widely used in biomedical applications (Min et al., 2023), bio-Fenton oxidation (Vaidyanathan et al., 2023), feed field (Liang et al., 2023), textile bleaching (Tzanov et al., 2002), reducing wine processing (Pickering et al., 1998), food packaging deaerator (Ge et al., 2012), etc.
5.2 Methods of chemical modification of enzymes
Chemical protein modification provides a large toolbox for the study and modification of enzymes, which play key roles in many important biological events in organisms; in particular, by associating desired properties/functions (affinity probes, fluorophores, reactive tags, etc.) with naturally or synthetically modified amino acid residues, chemical protein modification provides a useful way to identify enzyme locations and elucidate enzyme functions (Yu et al., 2022; He et al., 2018; Yan et al., 2024).
Chemical modifications of enzymes can be divided into covalent modifications, non-covalent modifications, targeted modifications, and macromolecular coupling (Table.2). The specific modification mechanisms are shown in the following table (Table.3). Surface modification is carried out using functionalized small molecules bearing alcohol, aldehyde, carboxylic acid, or isothiocyanate groups, and these reactions are carried out on the enzyme surface with exposed functional groups to form covalent bonds (Giri et al., 2021; Noro et al., 2022). Polymer coupling is the most common strategy for macromolecular modification enzymes. Among macromolecules such as polyglycol, polypropylene, and dextran, polydiethanolization is the most studied method, such as covalent polyglycol bonding with partial molecules or macromolecules (Noro et al., 2022; Li T. et al., 2024; Zhang et al., 2020; Chen et al., 2024). Chemical modifications of enzymes can alter affinity, specificity, or stability, while selective modifications enable the labeling of enzymes, allowing insight into complex biological processes, significant improvements have been made in the field of chemical modification/capture strategies for proteomic analysis, these methods emerged to be able to analyze the activity of enzymes in the body, and the chemicals used are often site-specific variants of those chemicals that are more commonly used to modify proteins and enzymes (Diaz-Rodriguez and Davis, 2011; Ren et al., 2018).
TABLE 2
| Modification type | Mechanism of action | Technical examples | Impact on GOx performance |
|---|---|---|---|
| Covalent modification | Introduction of functional groups or molecules on the GOx surface through chemical bonding (e.g., amide bonds, thioether bonds) | Amino and carboxyl cross-linking (e.g., Ferrocene-GOx complex), acylation of lysine residues (citric anhydride modification) | Enhanced electron transfer efficiency, improved thermal stability, expanded pH tolerance range |
| Noncovalent modification | Functional materials based on physical adsorption or electrostatic loading | Porous silicate-loaded Gox, graphene/polyaniline complex embedding | Maintains enzyme activity, improves immobilization efficiency, reduces conformational changes |
| Directional modifier | Specific modifications targeting the GOx active center (FAD cofactor) | Epoxy acid modification of FAD with polyethyleneimine coupling and coenzyme analog replacement | Shortening of electron transfer pathways, enhancement of catalytic efficiency and coenzyme stability |
| Macromolecular coupling | Covalent binding of GOx via polymers (e.g., PEG, dextran) | PEGylation modification to improve enzyme solubility and dextran modification to enhance organic solvent tolerance | Extends enzyme life, improves biocompatibility, reduces non-specific adsorption |
Classification of chemical modifications of enzymes.
TABLE 3
| Modification type | Mechanism explanation | |
|---|---|---|
| Covalent modification | Hydrophobic modification: forming a rigid hydrophobic core, reducing conformational fluctuations caused by water molecule intrusion, and improving thermal stability | Hydrophilic modification: Enhance the dispersion of the enzyme in the aqueous phase to reduce aggregation inactivation |
| Noncovalent modification | Conductive network enhancement: Utilizing the high conductivity of carbon nanomaterials (e.g., graphene, carbon nanotubes) to construct a continuous electron transfer pathway and reduce interfacial resistance | Micropores (<2 nm) in porous materials (e.g., hollow carbon spheres) limit the conformational changes of the enzyme molecule and reduce the inactivation caused by thermal movement |
| Directional modifier | Direct electron transfer by anchoring the electron mediator to the vicinity of the FAD coenzyme through chemical modification (e.g., epoxy acid coupling) or genetic engineering (e.g., introduction of cysteine tags) | Introduction of hydrophilic groups (e.g., carboxymethyl) at the substrate entrance to lower the substrate binding energy barrier and enhance catalytic efficiency |
| Macromolecular coupling | PEGylation modification reduces hydrophobic interactions between enzyme molecules through spatial site resistance | Dextran coupling forms a hydrophilic protective layer, preventing protease from approaching the enzyme molecule, extending the lifetime from 1 week to 6 months |
Mechanistic explanation of chemical modification of enzymes.
5.3 Research progress on glucose oxidase modification
The specific recognition of glucose by glucose oxidase makes glucose oxidase biosensors the most common electrochemical biosensors for glucose detection. However, the current sources and types of glucose oxidase are limited, and rapid molecular modification of the enzyme, such as chemical modification techniques, is needed to alter its activity and selectivity. In recent years, the surface modification of glucose oxidase has attracted the attention of researchers (Table.4) (Liu et al., 2024). Back in 1991, Kunugi et al. (1992) introduced a ferrocenyl group on glucose oxidase by combining the carboxyl group on ferrocene acid with the amino group on glucose oxidase to form a modified enzyme, the modified enzyme can be detached from oxygen for electron transfer with the electrode. Halalipour et al. (2020) attached phenyl derivatives to the carboxyl side chain or amino side chain of glucose oxidase (Gox) and modified Gox with hydrophobic aniline and benzoate, respectively, which showed that aniline-modified Gox had the highest catalytic efficiency, followed by benzoate-modified Gox, and the natural Gox performed the worst. It is demonstrated that hydrophobic modification increases Gox activity and is more resistant to high temperatures, at 80°C and 240 MPa, the rate of inactivation of aniline-modified GOx was 3.7-fold lower than that of the natural enzyme and 2.8-fold lower than that of the benzoic acid-modified enzyme. Hosseinkhani et al. (Hosseinkhani et al., 2004; Shi et al., 2019; Jiang et al., 2020) used citric anhydride to modify the lysine residues of glucose oxidase chemically, the pH tolerance of the modified enzyme was enhanced, and UV and fluorescence spectra also indicated that the chemical modification resulted in more exposure to hydrophilic residues. In addition, Liu et al. (Liu et al., 2013) also utilized the carboxyl group on 1-pyrenebutyric acid to covalently bind to the amino group on the surface of glucose oxidase, so that the enzyme surface carries a pyrenyl group with a conjugated structure, which was then loaded on graphene and assembled into a glucose oxidase electrode, which greatly improved the detection range of glucose concentration, the linear detection range of the modified sensor was 0.2–40 mM with a detection limit of 0.154 mM (S/N = 3), which greatly improved the detection range of glucose concentration, and was tested in real human serum samples, which greatly improved the detection range of glucose concentration, with a 5.1% decrease in activity in the first week, and still retaining 82.2% activity after 4 weeks. In addition to the surface modifications of the Gox, Zappelli et al. (Zappelli et al., 1978; Hou et al., 2017) modified FAD with epoxy acid and subsequently coupled it with polyethyleneimine to form a macromolecular FAD, which showed a 12-fold increase in stability compared to unmodified FAD in a circulating system with alanine as substrate. Fornerod et al (2023) used porous aluminosilicates and silicates for surface modification of Gox loading, the glucose detection sensitivity of the amino-modified electrode was 0.26 μA/mM (0–14 mM), which was higher than that of the unmodified electrode of 0.16 μA/mM (0–8 mM), and the detection limit of the modified electrode was 1.4 mM lower than that of the unmodified electrode (3.6 mM) (Figure 5). Lv et al. (2021) modified Gox with amines to obtain enzymes with higher enzymatic activity and to give the sensor a higher peak current intensity, the electron transfer rate constant of the amine-modified sensor, 2.54 s-1, was 1.39 times higher than that of the unmodified sensor (1.83 s-1). It can be seen that chemical modification can quickly and inexpensively change the properties of glucose oxidase, and glucose oxidase will be more and more widely used.
TABLE 4
| Modification type | Functionalization strategy | Performance enhancement | Application scenarios | References |
|---|---|---|---|---|
| Covalent modification | Ferrocen covalently binds to the amino group on the surface of Gox to form an electron-mediated complex | Electron transfer efficiency increased by 2.5-fold, oxygen dependence reduced by 80% | Medical diagnostics (blood glucose monitoring) | Kunugi et al. (1992) |
| Covalent modification | Aniline hydrophobically modifies Gox carboxyl/amino group to form a rigid hydrophobic core | Catalytic efficiency increased to 1.8 times that of natural enzyme, activity retention increased from 40% to 85% at 70°C | Food testing (high-fat samples) | Halalipour et al. (2020) |
| Covalent modification | Modification of Gox lysine residue by citric anhydride to enhance hydrophilicity | Expanded pH tolerance from 4.0-7.5 to 3.0-9.0 and increased activity retention from 40% to 90% in acidic environment | Industrial catalysis (bioreactors) | Hosseinkhani et al. (2004) |
| Covalent modification | Pyrene moiety covalently bound to Gox surface via carboxyl-amino group and complexed with graphene | Linear range of detection extended from 0.1 to 10 mM to 0.01–50 mM, sensitivity up to 98.7 μA mM-1·cm-2 | Highly sensitive biosensing | Liu et al. (2013) |
| Directed modification | FAD coenzyme modified by epoxy acid coupled with polyethyleneimine to form stable coenzyme-polymer complexes | 12-fold increase in coenzyme half-life and 3.5-fold increase in current response strength | Long-term stability sensor | Zappelli et al. (1978) |
| Non-covalent modification | Aminoated porous silicate loaded with Gox, immobilization of enzyme molecules by electrostatic adsorption | Aminoated porous silicate loaded with Gox, immobilization of enzyme molecules by electrostatic adsorption | Complex sample detection (blood) | Fornerod et al. (2023) |
| Macromolecular coupling | PEGylated modification of Gox surface amino groups to form a hydrophilic protective layer | Enzyme activity retention in organic solvents increased from 30% to 75%, extending the lifetime to 6 months | Industrial enzyme-catalyzed reactions | Kajiwara et al. (2019) |
Chemical modification of glucose oxidase.
FIGURE 5
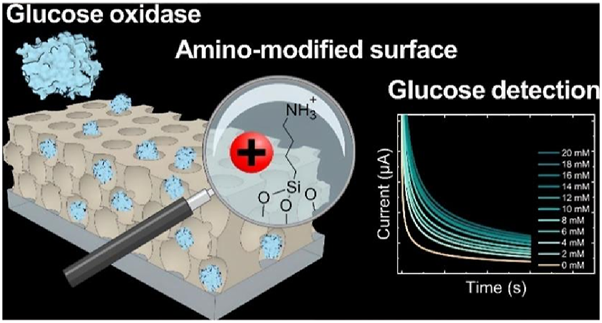
Poly (isoprene)-block-poly (ethylene oxide) (PI-b-PEO) micelles were co-assembled with aluminum silicate nanoparticles in solution and then spin-coated onto the working electrode to form a thin film. The hybrid coating was then calcined to condense the inorganic nanoparticles into a continuous matrix and remove the block copolymer (BCP) micelles, resulting in pores. Reproduced with permission from ref, (Fornerod et al., 2023). Copyright 2023, ACS Publication.
6 Multifunctional applications of carbon nanomaterials for glucose sensing
Since the birth of the first glucose oxidase electrode in 1962, people have been trying to find suitable materials to modify glucose oxidase on the electrode surface and maximize enzyme activity. Carbon nanomaterials have attracted great research interest due to their unique size, strength, electrical and surface area properties, and good biocompatibility and stability (Zeng et al., 2016). Therefore, carbon nanomaterials have become popular materials in the field of catalysis and sensing (Table 5). Currently people apply carbon nanomaterials to electrochemical sensors, which not only simplifies the size of the sensor, but improves the sensitivity as well as the stability of the electrode, but also greatly shortens the response time so that the electrochemical sensor is toward the direction of practical, miniaturization, and multifunctional development (Table 6) (Kaçar et al., 2014; Bi et al., 2018; Dong et al., 2024).
TABLE 5
| Material classes | Structural properties | Functional advantages | Typical study cases |
|---|---|---|---|
| One-dimensional materials | |||
| Carbon nanotubes (CNTs) | Tubular structure、sp2 hybridization、high L/D ratio (L/D > 106) | High electrical conductivity (104 S/cm)、strong adsorption capacity、 large specific surface area (∼500 m2/g) | (Hyun et al., 2015): CNTs immobilized GOx, sensitivity 53.5 μA mM-1·cm -2 with 86% retention of 2-week activity |
| Carbon nanofibers (CNFs) | Fibrous porous structure、 50–200 nm diameter | High mechanical strength (∼3 GPa)、 3D interconnected pores (pore sizes 2–50 nm) | (Zhang et al., 2018b): detection limits as low as 0.015 mM, recoveries in the range of 101.0%–104.8% |
| Two-dimensional materials | |||
| Graphene (GR) | Single atomic layer honeycomb structure, theoretical specific surface area 2,630 m2/g | Ultra-high carrier mobility (2 × 105 cm2/V·s), abundant edge active sites | (Wu et al., 2022): Pt/GO composite electrode sensitivity 11.64 mA mM-1, response time <3 s |
| Graphene oxide (GO) | Layered structure modified with oxygen-containing functional groups | Good hydrophilicity, easy functionalization (e.g., -NH2, -COOH modification) | (Pakapongpan and Poo-Arporn, 2017): Fe3O4/GO self-assembled system, detection limit 0.1 μM |
| Graphyne (GDY) | sp-sp2 hybridized network, intrinsic pores (∼0.5 nm) | Excellent porosity, high density of catalytically active sites (∼1015 sites/cm2) | (Liu et al., 2019): Fe-GDY/GOx electrodes in the range of 5–160 μM glucose concentration, R2 = 0.998 |
| three-dimensional material | |||
| Carbon aerogel (CA) | Three-dimensional porous network, density 0.1–0.5 g/cm3 | Ultra-low density, high electrical conductivity (∼10 S/cm), compression resilience >90% | (Yu et al., 2015): ZrP-CA/GOx Linear calibration in the range of 0.12–2.0 mM, sensitivity 5.56 μA mM-1·cm-2 |
| zero-dimensional material | |||
| Carbon quantum dots (CQD) | Particle size <10 nm, surface rich in -OH/-COOH groups | Tunable fluorescence properties, excellent biocompatibility, easy surface functionalization | (Hu et al., 2020): CdTe QDs/CQDs Concentration detection range from 0 mM to 13 mM, detection limit 0.223 mM |
| Nanodiamonds (NCD) | Diamond cores (∼5 nm) + surface sp2 carbon layer | Chemically inert, high hardness, surface functionalization (e.g., -NH2 modification) | (Zhao et al., 2006): N-NCD/Gox has a wide linear calibration range of 10 μM–15 mM and a low detection limit of 5 μM |
Classification of carbon nanomaterials in glucose sensors.
TABLE 6
|
Material classes |
Long-term stability | Anti-interference performance |
|---|---|---|
| One-dimensional materials | ||
| Carbon nanotubes (CNTs) | Literature sensor retains 86% of initial activity after 14 days of continuous use in a simulated serum environment (25°C, pH = 7.4) | π-π conjugate shielding: the sp2 hybridized surface of single-walled carbon nanotubes forms a π-π stacking with the tryptophan residues of GOx, which preferentially adsorbs glucose molecules (hydrophobicity), while repelling hydrophilic interferences such as uric acid, ascorbic acid, and others |
| Carbon nanofibers (CNFs) | The sensor in the literature retained 71.9% activity after 30 days | 3D mesh filtration: sub-micron pores formed by interwoven fibers block macromolecular interferents through size exclusion effect, while allowing glucose to diffuse freely |
| Two-dimensional materials | ||
| Graphene (GR) | The sensor in the literature showed only a 5% decrease in sensitivity after 30 repetitions in tomato juice, indicating excellent short-term stability | Surface charge repulsion: negatively charged surfaces inhibit negatively charged interferents through electrostatic repulsion |
| Graphene oxide (GO) | The current response of the sensor in the literature remained 95.6% of the initial value after 1 month of storage at 4°C | Surface charge repulsion and negatively charged surfaces suppress negatively charged interferences through electrostatic repulsion |
| Graphyne (GDY) | High short-term stability of the literature sensor | Catalytic site specificity, graphyne modification site preferentially catalyzes glucose oxidation |
| Three-dimensional material | ||
| Carbon aerogel (CA) | After 90 scans of the sensor in the literature, the peak current decreased by less than 8%, indicating good stability | Hierarchical pore design: macroporous-mesoporous-microporous three-stage structure (pore size distribution of 50 μm-2 nm) enables the mass transfer rate of interferents (e.g., ascorbic acid diffusion coefficient of 1.2 × 10−5 cm2/s) to be only 1/3 of that of glucose by the difference in the diffusion paths. only 1/3 of that of glucose |
| zero-dimensional material | ||
| Carbon quantum dots (CQD) | Fluorescent sensors in the literature show <10% signal attenuation after 28 days of storage in urine samples | Pore size sieving effect to physically block large interfering molecules |
| Nanodiamonds (NCD) | Sensors in the literature retain 75% of initial sensitivity after 1 month of storage | Chemically inert surfaces to reduce non-specific adsorption Surface modifications, such as boron or nitrogen doping to improve conductivity and grafting of selective membranes (e.g., polymers) to block interfering substances |
Mechanism of the effect of different carbon nanomaterials on sensor performance.
6.1 One-dimensional materials: carbon nanotubes and nanofibers
Carbon nanotubes are allotropes of carbon, which are part of the fullerene family of structures and are small in diameter (nanoscale) and length (micrometer) (Wang et al., 2021; Sridharan et al., 2022). Typical carbon nanotubes are tubular carbon atom systems composed of hexagonal carbon atoms, which have special properties due to their symmetrical structure. Their behavior depends entirely on their spiral nature, and because of this, they play the role of semiconductors or metals (Soni et al., 2020). Carbon nanotubes (CNTs) are highly regarded members of the synthetic carbon allotrope due to their unique arrangement of carbon atoms, sp2 hybridization, and cylindrical structure arranged between C-C distances of 1.42 Å and 3.4 Å layers, which make them different from other nanocarriers (Prajapati et al., 2022). According to their number of layers, they are divided into (a) single-walled carbon nanotubes (SWCNTs): graphite sheets of single-atom thickness bent into cylinders; (b) Multi-walled carbon nanotubes (MWCNTs): several layers of graphite sheets arranged concentrically (Bin et al., 2022). Single-walled carbon nanotubes (SWCNTs) have a simple chemical structure and clean surface properties, which give them a high degree of chemical stability. In contrast, multi-walled carbon nanotubes (MWCNTs) have a more complex wall layer, resulting in a complex and variable surface structure, which can adsorb and bind a large number of surface functional groups. This structural complexity provides significant advantages for MWCNTs in a wide range of applications (Han et al., 2024; Xia et al., 2023; Su et al., 2023) (Figure 6).
FIGURE 6

The role of carbon nanotubes in electrochemical sensors.
Carbon nanotubes were introduced by Sumio Iijima in 1991 (Sumio, 1991) and formally described and named, its unique tubular nanostructure (L/D ratio may be as high as 132 million:1), superior strength, and significant physicochemical properties quickly caught the attention of researchers (Upadhyay et al., 2011; Zeng et al., 2022). In addition, CNTs have excellent electrical conductivity, strong adsorption capacity, high sensitivity, good biocompatibility, and excellent chemical stability, making them ideal nanomaterials for the preparation of biosensors (Ma et al., 2019; Wang et al., 2021; Wang et al., 2009). Kyuhwan et al. discovered that carbon nanotubes (CNTs) as covalently immobilized materials for Gox can effectively maintain Gox based on activity and stability, the biosensor prepared based on this was used to detect glucose, and the sensitivity reached 53.5 μA·mM-1cm-2, which remained 86% active after 2 weeks, compared to the DTSSP modified gold electrode (0.026 s-1) and glassy carbon electrode (0.2 s-1), this electrode achieved an electron transfer rate of 1.14 s-1, a 5.7∼44-fold improvement (Hyun et al., 2015). Yin et al. (2024) used a glucose monitoring skin patch prepared from a hollow syringe modified with glucose oxidase (GOD) and carbon nanotubes (CNTs) as an electrochemical sensor for glucose monitoring and an integrated circuit for signal processing and transmission, and displaying real-time blood glucose levels on a smartphone via Bluetooth, which continuously measures glucose in real time in live animals with micromolar sensitivity and a lifetime of more than 14 days of useful life.
Carbon nanofibers (CNFs), with their excellent electrical conductivity and remarkable specific surface area, have attracted much attention in nanotechnology and materials science in recent years. Its excellent electron mobility ensures that electrons can be transferred at the electrode interfaces in an efficient and low-resistance manner, thus realizing an efficient electrical signal transduction mechanism. In addition, the nanoscale structure of CNFs provides many active sites, further facilitating rapid electron transfer and reaction kinetics. For example, Zhang et al. (2018a) modified GOx electrodes by combining manganese dioxide nanoparticles and carbon nanofiber nanocomposites and completed practical application validation by spiking samples of urine in order to obtain sensors with detection limits as low as 0.015 mM, recoveries in the range of 101.0%–104.8%, and retention of 71.9% of activity after 30 days, and the sensitivity of the MnO2- CNFs-modified sensor was 5.4 times more sensitive to H2O2 (33.1 μA/mM) than the MnO2-modified electrode only (6.1 μA/mM) (Figure 7).
FIGURE 7

(A) Schematic illustration for the synthesis of MnO2–CNFs nanocomposites. the MnO2–CNFs conjugates were prepared via the interaction of the carboxyl group with Mn2+ for subsequent on-spot chemical deposition of MnO2 onto CNFs. (B) Schematic representation of the mechanism of electrocatalysis of glucose catalyzed by glucose oxidase. Reproduced with permission from ref, (Zhang et al., 2018b). Copyright 2018, Springer Science Publication.
6.2 Two-dimensional materials: graphene, graphene oxide and graphyne
Following CNTs, scientists Geim and Novoselov prepared a new carbon nanomaterial, graphene (GR) in 2004 (Morales-Narvaez et al., 2017; Sun and Joshi, 2010; Wu et al., 2017). Graphene biosensors have been vigorously developed in the past decade due to their small size, unique conductive and optical properties (such as fluorescence quenching and conductivity), and good biocompatibility that meet the high efficiency and diversity requirements of biosensors (Zhu et al., 2017; Zhou et al., 2016; Zhao et al., 2022). Pakapongpan and Poo-Arporn, (2017); Wu et al. (2017) prepared a GOx biosensor by self-assembling glucose oxidase (GOx) on covalently modified magnetic nanoparticles (Fe3O4 NPs). The graphene material facilitated the electron transfer between the enzyme and the electrode surface, and the biosensor showed a fast amperometric response to glucose (3 s), a linear range from 0.05 to 1 mM, a low detection limit of 0.1 μM, significantly lower than that of GNs/ZnO/SPE (70 μM), and high sensitivity (5.9 μA mM-1), and the current response of the sensor remained 95.6% of the initial value after 1 month’s storage at 4°C. Wu et al. (2022) developed a microelectrode glucose biosensor based on 3D hybridized nanoporous platinum/graphene oxide nanostructures for rapid glucose detection in tomato and cucumber fruits, which achieved high glucose detection sensitivity (11.64 muA calibrated in glucose standard solution), low detection limit (13 mumol/L) and fast response time (95% steady-state response within 3 s). Liu et al. (2019) immobilized ferrous ions and glucose oxidase on GDY sheets and presented GDY-based composites with dual enzyme activity. Rat serum was used as a test sample and the electrodes obtained were superior to V2O5 nanowires (10–2000 µM) and Cu-Ag/GO composites (1–30 µM), with R2 = 0.998, and 0.89 µM for Fe-GDY/GOx, which is significantly lower than Fe/CeO2 NPs (3.41 µM) and H2TCPP/Fe2O3 NPs (2.54 µM) (Figure 8). Antonova et al. (2024) developed a soft microfluidic glucose sensor catalyzed and mediated by bimetallic palladium and platinum supported on reduced graphene oxide with 1,10-phenanthroline-5,6-dione. The sensor demonstrated a linear amperometric response to glucose within the range of 50–900 μM at an applied potential of 0.2 V, exhibiting a detection limit of 37 μM and a sensitivity of 30 μA cm-2 mM-1. The wearable sensor prototype enables convenient non-invasive measurement of exercise-induced sweat glucose levels for personalized diabetes monitoring.
FIGURE 8

Schematic representation of the mechanism for immobilizing ferrous ions and glucose oxidase on Graphdiyne. Reproduced with permission from ref, (Liu et al., 2019). Copyright 2018, ACS Publication.
6.3 Three-dimensional materials: hollow carbon spheres and carbon aerogels
Carbon aerogels (CAs) are mesoporous materials with abundant porosity and high specific surface area that are suitable for many practical applications. In addition, CAs have been found to have excellent biocompatibility due to their three-dimensional (3D) structural network, good electrical conductivity, exceptional chemical and environmental stability, and strong adhesion capabilities, which are ideal for the development of next-generation catalyst materials (Thirumalraj et al., 2018). Yu et al. (2015) reported a zirconium phosphate-carbon aerogel (ZrP-CA) composite material, where the ZrP-CA/GOx configuration exhibited a linear calibration range of 0.12–2.0 mM for glucose detection, surpassing conventional GOD/In2O3–chitosan (0.005–1.3 mM) and GOD–graphene–CdS (0.025–1.19 mM) systems. The sensitivity reached 5.56 μA mM-1cm-2, demonstrating a enhancement compared to traditional ZrP-based sensors (0.41 μA mM-1cm-2).
6.4 Zero-dimensional materials: carbon quantum dots and nanodiamonds
Carbon quantum dots (CDs) are a class of fluorescent carbon-based nanoparticles with a particle size of <10 nm, and the abundant oxygen-containing functional groups (-OH, -COOH) on their surfaces provide multiple anchor sites for Gox immobilization. Compared with conventional materials, the advantages of CDs are reflected in the excitation wavelength-dependent fluorescence property of CDs that can work synergistically with electrochemical signals, and the cytotoxicity of CDs (IC50 > 500 μg/mL) is significantly lower than that of CNTs (IC50 ≈ 50 μg/mL) for implantable sensors. Hu et al. (Hu et al., 2020) developed a sensitive fluorescent microfluidic sensor based on carbon quantum dots (CQDs), cadmium telluride quantum dots (CdTe QDs) aerogel, and glucose oxidase (GOx), with all experimental validations performed using human urine specimens for glucose detection. The sensor demonstrated exceptional storage stability, retaining stable fluorescence signal (R/G ratio) and colorimetric response after 30-day storage at −20°C. It achieved a broad glucose detection range from 0 to 13 mM with a detection limit of 0.223 mM (S/N = 3). Zhao et al. (2006) developed an electrochemically pretreated glucose biosensor based on non-doped nanocrystalline diamond (N-NCD)-modified gold electrodes for selective glucose detection, achieving a broad linear calibration range from 10 μM to 15 mM with a low detection limit of 5 μM (S/N = 3), which significantly outperforms conventional sensors. The biosensor retains 75% of its initial sensitivity after 30 days of storage.
We compare the effects of different carbon nanomaterials on glucose sensors (Table 7) and add the mechanisms by which they increase the electron transfer rate of the sensors (Table 8).
TABLE 7
| Material | Core advantage | Sensitivity | Selectivity | Stability | Cost |
|---|---|---|---|---|---|
| Carbon nanotubes | High electrical conductivity, mechanical strength, wide detection range | High | Medium | Medium | Medium |
| Graphene | Ultra-high electron transfer rate, large specific surface area | Very high | High | High | High |
| Graphene oxide | Easy surface modification, synergistic effects | High | Extremely high | High | Medium |
| Carbon quantum dots | Fluorescence properties, low detection limit | Extremely high | High | High | Low |
| Carbon aerogel | 3D porous structure, high loading capacity | Medium | Medium | Medium | High |
| Nanodiamond | Chemical stability, biocompatibility | Medium | High | Extremely high | Extremely high |
| Graphdiyne | High electrical conductivity and unique pore structure | Extremely high | Extremely high | Medium | High |
| Carbon nanofibers | 3D network structure, easy surface functionalization | Medium | Medium | High | Medium |
Comparison of different carbon nanomaterials for glucose sensor applications.
TABLE 8
| Material | Influence mechanism | Main limitations | |
|---|---|---|---|
| Carbon nanotubes | One-dimensional conductive channels: π-π conjugation effect of tubular structure provides direct electron transfer pathway, lower interfacial resistance | High aspect ratio: enhanced contact area with enzymes, promotes direct electron transfer (DET) for glucose oxidase (GOx) | Poor dispersibility, potential biotoxicity |
| Graphene | Ultra-high electrical conductivity: 2D honeycomb structure of sp2 hybridized carbon atoms creates continuous electron channels, high electron mobility, significantly shortens electron transfer path | Large specific surface area (2,630 m2/g): exposes more active sites, enhances loading efficiency of enzymes or catalysts, promotes interfacial charge transfer | Reduced number of active sites due to interlayer stacking |
| Graphene oxide | Surface functional groups: oxygen-containing groups (-OH, -COOH) enhance immobilization of biomolecules (e.g., enzymes) but block conductivity | Reduction treatment (rGO): restoration of part of the sp2 structure by thermal or chemical reduction, significant increase in electrical conductivity (close to 80% of graphene) | Reduction treatment is required to restore conductivity |
| Carbon aerogel | 3D porous network: high porosity (>90%) shortens ion diffusion paths, facilitates electrolyte penetration, and reduces charge transfer impedance | Conductive backbone: graphene or carbon nanotubes reinforced 3D structure provides continuous electron transport paths | Complex preparation process |
| Carbon quantum dots | Quantum size effect: small size (<10 nm) provides high surface activity but low conductivity, need to be compounded with conductive substrates (e.g., GO, CNTs) | Fluorescence-electrochemical synergy: enhanced catalytic efficiency through light-induced electron transfer | Need to compound with conductive substrate to compensate for conductivity |
Effect of different carbon nanomaterials on the electron transfer rate of glucose sensors.
7 Synergistic chemical modification of carbon nanomaterials and enzymes with glucose sensors
The high conductivity of carbon nanomaterials provides a fast channel for electron transfer between the enzyme and the electrode; their large specific surface area also provides abundant sites for enzyme immobilization, while chemical modification can shorten the electron transfer path and further reduce the interfacial resistance; by regulating the enzyme microenvironment, conformational changes can be reduced, which improves the catalytic efficacy and thermal stability of the enzyme. The adsorption properties of carbon nanomaterials synergize with the selective screen of chemical modification, which can effectively shield interferences such as ascorbic acid and uric acid and improve the specificity of the sensor (Table 9).
TABLE 9
| Carbon nanomaterials | Enzyme modification strategies | Performance enhancement | References |
|---|---|---|---|
| Graphene | Covalent modification of Gox by pyrene moiety | Extended detection range to 0.01–50 mM, 3-fold increase in sensitivity | Liu et al. (2013) |
| Multi-walled carbon nanotubes | Covalent immobilization of Gox by amino groups | Sensitivity 53.5 μA-mM-1-cm-2, 2-week activity retention 86% | Hyun et al. (2015) |
| Carbon quantum dots | Electrostatic adsorption of Gox | Detection limit 0.223 mM, significant biocompatibility | Hu et al. (2020) |
| Graphyne | Fe2+ co-loading with Gox | Linear range 5–160 μM, R2 = 0.998 | Liu et al. (2019) |
Comparison of typical cases of synergies.
8 Significance and challenges of glucose oxidase electrode modification
Glucose oxidase electrochemical sensors have attracted much attention because of their ability to detect glucose specifically, but maintaining the activity of the enzyme is also a challenge. By introducing materials such as carbon nanotubes, graphene, and metal nanoparticles with high specific surface area, good electrical conductivity, and catalytic activity on the electrode surface and by chemically modifying the enzyme, the efficiency of electron transfer between the enzyme and the electrode can be significantly improved, thereby increasing the sensitivity of the sensor. These modified materials not only provide more attachment sites for GOx, but also enhance the interaction between the enzyme and the electrode, making the oxidation of glucose more efficient. For sensor performance, future research should focus on optimizing sensor sensitivity, improving selectivity and interference immunity, and increasing long-term stability and commercialization potential.
In recent years, some enzyme-modified nanomaterial sensors have entered the commercialization stage, e.g., FreeStyle Libre (Abbott) has successfully occupied a significant position in the global market of glucose detection. However, the following key issues still need to be addressed in order to realize their large-scale production: reproducibility of material synthesis (e.g., the diameter and chirality control of single-walled carbon nanotubes (SWCNTs) still relies on a complex gas-phase deposition process, resulting in large performance variations between batches), compatibility of enzyme immobilization processes (existing enzyme modification technologies (e.g., covalent modification, macromolecular coupling) are susceptible to environmental fluctuations in continuous production), device miniaturization and integration (lab lab labs are susceptible to environmental fluctuations), and the use of enzyme-enabled devices.), miniaturization and integration of equipment (laboratory sensors rely on bulky electrochemical workstations, while commercial equipment requires integrated signal processing modules).
9 Outlook for future applications of the glucose oxidase electrode
Most of the current research is still based on standard solutions or simulated samples, and the validation of real blood samples needs to be further optimized for immunity (e.g., ascorbic acid, uric acid, etc.) and long-term stability. Future studies need to focus on the validation of sensor performance in real complex samples (e.g., whole blood, food extracts) and the development of portable devices in combination with miniaturization techniques to meet the needs of clinical diagnosis and immediate testing (POCT). At the same time, there are many issues to be faced to realize the utility of glucose oxidase electrodes as follows. Existing carbon nanomaterials (e.g., graphene, carbon nanotubes) may trigger an inflammatory response upon long-term skin contact, so encapsulating materials that are flexible, breathable, and biologically inert, such as polyurethane-nanofibrillar cellulose composite membranes, need to be developed. At the same time, surface functionalization techniques (e.g., PEG modification) are used to reduce the immunogenicity of the materials, thus further improving their biocompatibility. Second, wearable devices need to adapt to sweat pH fluctuations, mechanical deformation and temperature changes to ensure sensor stability in dynamic environments. Meanwhile, continuous glucose monitoring requires the integration of multiple sensors (e.g., pH, temperature compensation modules), but the energy consumption of nanomaterial devices limits miniaturization, so multimodal data synchronization needs to be optimized to balance energy consumption.
Statements
Author contributions
GG: Conceptualization, Funding acquisition, Writing – review and editing. QL: Writing – review and editing. ZYa: Writing – review and editing. WP: Writing – original draft. KF: Writing – review and editing. ZYu: Writing – review and editing. LZ: Writing – review and editing. NX: Writing – review and editing. ZX: Writing – review and editing. LQ: Writing – review and editing. ZB: Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was financially supported by the Scientific Research Startup Funding Project for Introduced Talents of Wuhu Institute of Technology (No. wzyrc202403), the Wuhu Science and Technology Innovation Strategy Research Special Project (No. 2024swwzx07), the Wuhu Institute of Technology Food Nutrition and Health Innovation Team (No. 2023jxtd06), the “Double-Teacher and Dual-Ability” Teacher Team Construction Project of Anhui Provincial Department of Education (No. [2024]117).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
Ahmad N. Naeem M. Ali H. Alabbosh K. F. Hussain H. Khan I. et al (2023). From challenges to solutions: the impact of melatonin on abiotic stress synergies in horticultural plants via redox regulation and epigenetic signaling. Sci. Hortic.321, 112369. 10.1016/j.scienta.2023.112369
2
Ahmad R. Khan M. Mishra P. Jahan N. Ahsan M. A. Ahmad I. et al (2021). Engineered hierarchical CuO nanoleaves based electrochemical nonenzymatic biosensor for glucose detection. J. Electrochem. Soc.168 (1), 017501. 10.1149/1945-7111/abd515
3
Antonova I. V. Ivanov A. I. Shavelkina M. B. Poteryayev D. A. Buzmakova A. A. Soots R. A. (2024). Engineering of graphene-based composites with hexagonal boron nitride and PEDOT:PSS for sensing applications. Phys. Chem. Chem. Phys.26 (9), 7844–7854. 10.1039/d3cp05953g
4
Apaliya M. T. Zhang H. Yang Q. Zheng X. Zhao L. Kwaw E. et al (2017). Hanseniaspora uvarum enhanced with trehalose induced defense-related enzyme activities and relative genes expression levels against Aspergillus tubingensis in table grapes. Postharvest Biol. Technol.132, 162–170. 10.1016/j.postharvbio.2017.06.008
5
Bai J. Zhu Y. Li J. Zhang Y. Dong Y. Xiao X. (2020). Effects of bitter melon saponin on the glucose and lipid metabolism in HepG2 cell and C. elegans. J. Food Qual.2020, 1–9. 10.1155/2020/8860356
6
Bauer J. A. Zámocká M. Majtán J. Bauerová-Hlinková V. (2022). Glucose oxidase, an enzyme “ferrari”: its structure, function, production and properties in the light of various industrial and biotechnological applications. Biomolecules12 (3), 472. 10.3390/biom12030472
7
Bi J. Li Y. Wang H. Song Y. Cong S. Yu C. et al (2018). Presence and formation mechanism of foodborne carbonaceous nanostructures from roasted pike eel (Muraenesox cinereus). J. Agric. Food Chem.66 (11), 2862–2869. 10.1021/acs.jafc.7b02303
8
Bin Z. Feng L. Yan Y. (2022). Biomimetic metalloporphyrin oxidase modified carbon nanotubes for highly sensitive and stable quantification of anti-oxidants tert-butylhydroquinone in plant oil. Food Chem., 388. 10.1016/j.foodchem.2022.132898
9
Bu Q. Yu F. Cai J. Bai J. Xu J. Wang H. et al (2024). Preparation of sugarcane bagasse-derived Co/Ni/N/MPC nanocomposites and its application in H2O2 detection. Industrial Crops Prod.211, 118218. 10.1016/j.indcrop.2024.118218
10
Campbell A. S. Islam M. F. Russell A. J. (2017). Intramolecular electron transfer through poly-ferrocenyl glucose oxidase conjugates to carbon electrodes: 1. Sensor sensitivity, selectivity and longevity. Electrochimica Acta248, 578–584. 10.1016/j.electacta.2017.07.150
11
Chen S. Y. Shang K. Gao X. Wang X. (2017). The development of NAD+-dependent dehydrogenase screen-printed biosensor based on enzyme and nanoporous gold co-catalytic strategy. Biosens. Bioelectron.211, 578–584. 10.1016/j.bios.2022.114376
12
Chen C. P. Xu H. Zhan Q. Zhang Y. Wang B. Chen C. et al (2023). Preparation of novel HKUST-1-glucose oxidase composites and their application in biosensing. Microchim. Acta190 (1), 10. 10.1007/s00604-022-05563-4
13
Chen Z. Chen J. Ni D. Xu W. Zhang W. Mu W. (2024). Microbial dextran-hydrolyzing enzyme: properties, structural features, and versatile applications. Food Chem.437, 137951. 10.1016/j.foodchem.2023.137951
14
Chmayssem A. Shalayel I. Marinesco S. Zebda A. (2023). Investigation of GOx stability in a chitosan matrix: applications for enzymatic electrodes. Sensors23 (1), 465. 10.3390/s23010465
15
Chu Z. Liu Y. Xu Y. Shi L. Peng J. Jin W. (2015). In-situ fabrication of well-distributed gold nanocubes on thiol graphene as a third-generation biosensor for ultrasensitive glucose detection. Electrochimica Acta176, 162–171. 10.1016/j.electacta.2015.06.123
16
D Costa E. J. Higgins I. J. Turner A. P. F. (1986). Quinoprotein glucose dehydrogenase and its application in an amperometric glucose sensor. Biosensors2 (2), 71–87. 10.1016/0265-928x(86)80011-6
17
Diaz-Rodriguez A. Davis B. G. (2011). Chemical modification in the creation of novel biocatalysts. Curr. Opin. Chem. Biol.15 (2), 211–219. 10.1016/j.cbpa.2010.12.002
18
Dong X. Huang A. He L. Cai C. You T. (2024). Recent advances in foodborne pathogen detection using photoelectrochemical biosensors: from photoactive material to sensing strategy. Front. Sustain. Food Syst.8. 10.3389/fsufs.2024.1432555
19
Donini C. A. Silva M. K. L. Bronzato G. R. Leão A. L. Cesarino I. (2020). Evaluation of a biosensor based on reduced graphene oxide and glucose oxidase enzyme on the monitoring of second-generation ethanol production. J. Solid State Electrochem.24 (8), 2011–2018. 10.1007/s10008-019-04471-7
20
Fornerod M. J. J. Alvarez-Fernandez A. Michalska M. Papakonstantinou I. Guldin S. (2023). Glucose oxidase loading in ordered porous aluminosilicates: exploring the potential of surface modification for electrochemical glucose sensing. Chem. Mater.35 (18), 7577–7587. 10.1021/acs.chemmater.3c01202
21
Ge L. Zhao Y. s. Mo T. Li J. r. Li P. (2012). Immobilization of glucose oxidase in electrospun nanofibrous membranes for food preservation. Food control.26 (1), 188–193. 10.1016/j.foodcont.2012.01.022
22
Giri P. Pagar A. D. Patil M. D. Yun H. (2021). Chemical modification of enzymes to improve biocatalytic performance. Biotechnol. Adv.53, 107868. 10.1016/j.biotechadv.2021.107868
23
Gough D. A. Lucisano J. Y. Tse P. H. S. (1985). Two-dimensional enzyme electrode sensor for glucose. Anal. Chem.57 (12), 2351–2357. 10.1021/ac00289a042
24
Guo Z. Wang M. Barimah A. O. Chen Q. Li H. Shi J. El-Seedi H. R. et al (2021). Label-free surface enhanced Raman scattering spectroscopy for discrimination and detection of dominant apple spoilage fungus. Int. J. Food Microbiol., 338. 10.1016/j.ijfoodmicro.2020.108990
25
Halalipour A. Duff M. R. Jr. Howell E. E. Reyes-De-Corcuera J. I. (2020). Catalytic activity and stabilization of phenyl-modified glucose oxidase at high hydrostatic pressure. Enzyme Microb. Technol.137, 109538. 10.1016/j.enzmictec.2020.109538
26
Han E. Li L. Gao T. Pan Y. Cai J. (2024). Nitrite determination in food using electrochemical sensor based on self-assembled MWCNTs/AuNPs/poly-melamine nanocomposite. Food Chem.437, 137773. 10.1016/j.foodchem.2023.137773
27
Han J. Feng H. Wu J. Li Y. Zhou Y. Wang L. et al (2021). Construction of multienzyme Co-immobilized hybrid nanoflowers for an efficient conversion of cellulose into glucose in a cascade reaction. J. Agric. Food Chem.69 (28), 7910–7921. 10.1021/acs.jafc.1c02056
28
He P. Hassan M. M. Tang F. Jiang H. Chen M. Liu R. et al (2022). Total fungi counts and metabolic dynamics of volatile organic compounds in paddy contaminated by Aspergillus Niger during storage employing gas chromatography-ion mobility spectrometry. Food Anal. Methods15 (6), 1638–1651. 10.1007/s12161-021-02186-y
29
He W.-S. Zhu H. Chen Z.-Y. (2018). Plant sterols: chemical and enzymatic structural modifications and effects on their cholesterol-lowering activity. J. Agric. Food Chem.66 (12), 3047–3062. 10.1021/acs.jafc.8b00059
30
Hosseinkhani S. Ranjbar B. Naderi-Manesh H. Nemat-Gorgani M. (2004). Chemical modification of glucose oxidase: possible formation of molten globule-like intermediate structure. FEBS Lett.561 (1-3), 213–216. 10.1016/s0014-5793(04)00134-6
31
Hou F. Ding W. Qu W. Oladejo A. O. Xiong F. Zhang W. et al (2017). Alkali solution extraction of rice residue protein isolates: influence of alkali concentration on protein functional, structural properties and lysinoalanine formation. Food Chem.218, 207–215. 10.1016/j.foodchem.2016.09.064
32
Hovancová J. Šišoláková I. Oriňaková R. Oriňak A. (2017). Nanomaterial-based electrochemical sensors for detection of glucose and insulin. J. Solid State Electrochem.21 (8), 2147–2166. 10.1007/s10008-017-3544-0
33
Hu T. Xu K. Qiu S. Han Y. Chen J. Xu J. et al (2020). Colorimetric detection of urine glucose using a C/CdTe QDs-GOx aerogel based on a microfluidic assay sensor. J. Mater. Chem. B8 (32), 7160–7165. 10.1039/d0tb00328j
34
Hu Y. Wang X. Li W. Lai Y. Chen Y. Wei Z. et al (2024). Metal-organic frameworks and related materials for nonenzymatic electrochemical glucose sensors. Int. J. Electrochem. Sci.19 (2), 100466. 10.1016/j.ijoes.2024.100466
35
Huang F. Wang X. Cao Y. (2022). Mechanism and research progress of glucose oxidase in animal feed. Chin. J. Animal Nutr.34 (9), 5500–5515. 10.3969/j.issn.1006-267x.2022.09.006
36
Hyun K. Han S. W. Koh W. G. Kwon Y. (2015). Direct electrochemistry of glucose oxidase immobilized on carbon nanotube for improving glucose sensing. Int. J. Hydrogen Energy40 (5), 2199–2206. 10.1016/j.ijhydene.2014.12.019
37
Jiang C. Wang X. Hou B. Hao C. Li X. Wu J. (2020). Construction of a lignosulfonate-lysine hydrogel for the adsorption of heavy metal ions. J. Agric. Food Chem.68 (10), 3050–3060. 10.1021/acs.jafc.9b07540
38
Jing Y. Zhang Y. Han I. Wang P. Mei Q. Huang Y. (2020). Effects of different straw biochars on soil organic carbon, nitrogen, available phosphorus, and enzyme activity in paddy soil. Sci. Rep.10 (1), 8837. 10.1038/s41598-020-65796-2
39
Kaçar C. Dalkiran B. Erden P. E. Kiliç E. (2014). An amperometric hydrogen peroxide biosensor based on Co3O4 nanoparticles and multiwalled carbon nanotube modified glassy carbon electrode. Appl. Surf. Sci.311, 139–146. 10.1016/j.apsusc.2014.05.028
40
Kajiwara S. Komatsu K. Yamada R. Matsumoto T. Yasuda M. Ogino H. (2019). Improvement of the organic solvent stability of a commercial lipase by chemical modification with dextran. Biochem. Eng. J.142, 1–6. 10.1016/j.bej.2018.11.003
41
Khan G. F. Ohwa M. Wernet W. (1996). Design of a stable charge transfer complex electrode for a third-generation amperometric glucose sensor. Anal. Chem.68, 2939–2945. 10.1021/ac9510393
42
Khatami S. H. Vakili O. Ahmadi N. Soltani Fard E. Mousavi P. Khalvati B. et al (2022). Glucose oxidase: applications, sources, and recombinant production. Biotechnol. Appl. Biochem.69 (3), 939–950. 10.1002/bab.2165
43
Kilic N. M. Singh S. Keles G. Cinti S. Kurbanoglu S. Odaci D. (2023). Novel approaches to enzyme-based electrochemical nanobiosensors. Biosensors-Basel13 (6), 622. 10.3390/bios13060622
44
Kim Y.-P. Park S. J. Lee D. Kim H. S. (2012). Electrochemical glucose biosensor by electrostatic binding of PQQ-glucose dehydrogenase onto self-assembled monolayers on gold. J. Appl. Electrochem.42 (6), 383–390. 10.1007/s10800-012-0409-1
45
Kunugi S. Murakami Y. Ikeda K. Itoh N. (1992). Chemical modification of proteins under high pressure. Preparation of ferrocene-attached bovine serum albumin and glucose oxidase. J. Biol. Chem.14 (4), 210–214. 10.1016/s0141-8130(05)80029-7
46
Li H. Xiao N. Jiang M. Long J. Li Z. Zhu Z. (2024a). Advances of transition metal-based electrochemical non-enzymatic glucose sensors for glucose analysis: a review. Crit. Rev. Anal. Chem., 1–37. 10.1080/10408347.2024.2339955
47
Li T. Cheng C. Liu J. (2024b). Chemical and enzyme-mediated chemical reactions for studying nucleic acids and their modifications. Chembiochem25 (15), e202400220. 10.1002/cbic.202400220
48
Li X. Li C. Zhang S. Cui C. Li J. Gao Q. (2021). Simple and fast colorimetric and electrochemical methods for the ultrasensitive detection of glucose. Anal. Bioanal. Chem.413 (23), 5725–5731. 10.1007/s00216-021-03547-6
49
Liang Z. Q. Yan Y. Zhang W. Luo H. Yao B. Huang H. et al (2023). Review of glucose oxidase as a feed additive: production, engineering, applications, growth-promoting mechanisms, and outlook. Crit. Rev. Biotechnol.43 (5), 698–715. 10.1080/07388551.2022.2057275
50
Lin M.-J. Wu C.-C. Chang K.-S. (2019). Effect of poly-l-lysine polycation on the glucose oxidase/ferricyanide composite-based second-generation blood glucose sensors. Sensors19 (6), 1448. 10.3390/s19061448
51
Liu G. Liu Z. Sun Y. Sun M. Duan J. Tian Y. et al (2024). Cascade amplifying electrochemical bioanalysis for zearalenone detection in agricultural products: utilizing a glucose-fenton-HQ system on bimetallic-ZIF@CNP nanocomposites. Foods13 (19), 3192. 10.3390/foods13193192
52
Liu J. Kong N. Li A. Luo X. Cui L. Wang R. et al (2013). Graphene bridged enzyme electrodes for glucose biosensing application. Analyst138 (9), 2567–2575. 10.1039/c3an36929c
53
Liu J. M. Shen X. Baimanov D. Wang L. Xiao Y. Liu H. et al (2019). Immobilized ferrous ion and glucose oxidase on graphdiyne and its application on one-step glucose detection. Acs Appl. Mater. and Interfaces11 (3), 2647–2654. 10.1021/acsami.8b03118
54
Lv C. Yang X. Wang Z. Ying M. Han Q. Li S. (2021). Enhanced performance of bioelectrodes made with amination-modified glucose oxidase immobilized on carboxyl-functionalized ordered mesoporous carbon. Nanomaterials11 (11), 3086. 10.3390/nano11113086
55
Ma S. Pan L. g. You T. Wang K. (2021). g-C3N4/Fe3O4 nanocomposites as adsorbents analyzed by UPLC-MS/MS for highly sensitive simultaneous determination of 27 mycotoxins in maize: aiming at increasing purification efficiency and reducing time. J. Agric. Food Chem.69 (16), 4874–4882. 10.1021/acs.jafc.1c00141
56
Ma S. Wang M. You T. Wang K. (2019). Using magnetic multiwalled carbon nanotubes as modified QuEChERS adsorbent for simultaneous determination of multiple mycotoxins in grains by UPLC-MS/MS. J. Agric. Food Chem.67 (28), 8035–8044. 10.1021/acs.jafc.9b00090
57
Ma X. Huang W. Song Y. Han J. Wu J. Wang L. et al (2022). Novel recyclable UCST-type immobilized glucose isomerase biocatalyst with excellent performance for isomerization of glucose to fructose. J. Agric. Food Chem.70 (43), 13959–13968. 10.1021/acs.jafc.2c05667
58
Malitesta C. Palmisano F. Torsi L. Zambonin P. G. (1990). Glucose fast-response amperometric sensor based on glucose oxidase immobilized in an electropolymerized poly(o-phenylenediamine) film. Anal. Chem.62 (24), 2735–2740. 10.1021/ac00223a016
59
Mao M. F. Limin L. Chuanhui H. Zhenyu L. Weijia W. (2025). Highly sensitive electrochemiluminescence glucose sensor under alkaline conditions based on glucose oxidase@conductive metal-organic framework nanocapsules. Sensors Actuators B-Chemical, 434. 10.1016/j.snb.2025.137595
60
Min S. Y. Yu Q. Ye J. Hao P. Ning J. Hu Z. et al (2023). Nanomaterials with glucose oxidase-mimicking activity for biomedical applications. Molecules28 (12), 4615. 10.3390/molecules28124615
61
Ming T. Lan T. Yu M. Duan X. Cheng S. Wang H. et al (2024). A novel electrochemical microneedle sensor for highly sensitive real time monitoring of glucose. Microchem. J., 207. 10.1016/j.microc.2024.112021
62
Monkrathok J. Janphuang P. Suphachiaraphan S. Kampaengsri S. Kamkaew A. Chansaenpak K. et al (2024). Enhancing glucose biosensing with graphene oxide and ferrocene-modified linear poly(ethylenimine). Biosensors14 (4), 161. 10.3390/bios14040161
63
Morales-Narvaez E. Baptista‐Pires L. Zamora‐Gálvez A. Merkoçi A. (2017). Graphene-based biosensors: going simple. Adv. Mater29 (7). 10.1002/adma.201604905
64
Mtemeri L. Hickey D. P. (2023). Model-driven design of redox mediators: quantifying the impact of quinone structure on bioelectrocatalytic activity with glucose oxidase. J. Phys. Chem. B127 (36), 7685–7693. 10.1021/acs.jpcb.3c03740
65
Naikoo G. A. Awan T. Salim H. Arshad F. Hassan I. U. Pedram M. Z. et al (2022). Fourth-generation glucose sensors composed of copper nanostructures for diabetes management: a critical review. Bioeng. and Transl. Med.7 (1), e10248. 10.1002/btm2.10248
66
Nikitina V. N. Karastsialiova A. R. Karyakin A. A. (2023). Glucose test strips with the largest linear range made via single step modification by glucose oxidase-hexacyanoferrate-chitosan mixture. Biosens. Bioelectron.220, 114851. 10.1016/j.bios.2022.114851
67
Noro J. Cavaco-Paulo A. Silva C. (2022). Chemical modification of lipases: a powerful tool for activity improvement. Biotechnol. J.17 (8), e2100523. 10.1002/biot.202100523
68
Ou L. Liu G. Xia N. (2021). Research progress and application prospects of electrochemical glucose sensors. Int. J. Electrochem. Sci.16 (6), 210633. 10.20964/2021.06.42
69
Pakapongpan S. Poo-Arporn R. P. (2017). Self-assembly of glucose oxidase on reduced graphene oxide-magnetic nanoparticles nanocomposite-based direct electrochemistry for reagentless glucose biosensor. Mater Sci. Eng. C Mater Biol. Appl.76, 398–405. 10.1016/j.msec.2017.03.031
70
Peng Y. Sun Q. Park Y. (2019). Chicoric acid promotes glucose uptake and Akt phosphorylation via AMP-activated protein kinase α-dependent pathway. J. Funct. Foods59, 8–15. 10.1016/j.jff.2019.05.020
71
Pickering G. J. Heatherbell D. A. Barnes M. F. (1998). Optimising glucose conversion in the production of reduced alcohol wine using glucose oxidase. Food Res. Int.31 (10), 685–692. 10.1016/s0963-9969(99)00046-0
72
Pohanka M. (2021). Glucose electrochemical biosensors: the past and current trends. Int. J. Electrochem. Sci.16 (7), 210719. 10.20964/2021.07.52
73
Prajapati S. K. Malaiya A. Kesharwani P. Soni D. Jain A. (2022). Biomedical applications and toxicities of carbon nanotubes. Drug Chem. Toxicol.45 (1), 435–450. 10.1080/01480545.2019.1709492
74
Qin C. Guo W. Liu Y. Liu Z. Qiu J. Peng J. (2017). A novel electrochemical sensor based on graphene oxide decorated with silver nanoparticles–molecular imprinted polymers for determination of sunset yellow in soft drinks. Food Anal. Methods10 (7), 2293–2301. 10.1007/s12161-016-0753-6
75
Rafighi P. Tavahodi M. Haghighi B. (2016). Fabrication of a third-generation glucose biosensor using graphene-polyethyleneimine-gold nanoparticles hybrid. Sensors Actuators B Chem.232, 454–461. 10.1016/j.snb.2016.03.147
76
Ramachandran T. Mourad A. I. Raji R. K. Krishnapriya R. Cherupurakal N. Subhan A. et al (2022). KOH mediated hydrothermally synthesized hexagonal‐CoMn2O4 for energy storage supercapacitor applications. Int. J. Energy Res.46 (12), 16823–16838. 10.1002/er.8350
77
Ravikumar Y. Ponpandian L. N. Zhang G. Yun J. Qi X. (2021). Harnessing -arabinose isomerase for biological production of -tagatose: recent advances and its applications. Trends Food Sci. and Technol.107, 16–30. 10.1016/j.tifs.2020.11.020
78
Ren X. Liang Q. Zhang X. Hou T. Li S. Ma H. (2018). Stability and antioxidant activities of corn protein hydrolysates under simulated gastrointestinal digestion. Cereal Chem.95 (6), 760–769. 10.1002/cche.10092
79
Rong J. Zhou Z. Wang Y. Han J. Li C. Zhang W. et al (2019). Immobilization of horseradish peroxidase on multi-armed magnetic graphene oxide composite: improvement of loading amount and catalytic activity. Food Technol. Biotechnol.57 (2), 260–271. 10.17113/ftb.57.02.19.5832
80
Shah S. S. Niaz F. Ehsan M. A. Das H. T. Younas M. Khan A. S. et al (2024). Advanced strategies in electrode engineering and nanomaterial modifications for supercapacitor performance enhancement: a comprehensive review. J. Energy Storage79, 110152. 10.1016/j.est.2023.110152
81
Shang L. Bai X. Chen C. Liu L. Li M. Xia X. et al (2019). Isolation and identification of a Bacillus megaterium strain with ochratoxin A removal ability and antifungal activity. Food control.106, 106743. 10.1016/j.foodcont.2019.106743
82
Shen L. Zhou X. Zhang C. Yin H. Wang A. Wang C. (2019). Functional characterization of bimetallic CuPdxnanoparticles in hydrothermal conversion of glycerol to lactic acid. J. Food Biochem.43 (8), e12931. 10.1111/jfbc.12931
83
Shi T. Yuan L. Mu J. Gao R. (2019). The effect of Arginine, Lysine and Histidine in the myosin secondary structure by circular dichroism and Raman spectroscopy. Cyta-Journal Food17 (1), 656–660. 10.1080/19476337.2019.1635648
84
Song C. Guo J. Wang Y. Xiang H. Yang Y. (2024). Electrochemical glucose sensors: classification, catalyst innovation, and sampling mode evolution. Biotechnol. J.19 (10), e202400349. 10.1002/biot.202400349
85
Soni S. K. Thomas B. Kar V. R. (2020). A comprehensive review on CNTs and CNT-reinforced composites: syntheses, characteristics and applications. Mater. Today Commun.25, 101546. 10.1016/j.mtcomm.2020.101546
86
Sridharan R. Monisha B. Kumar P. S. Gayathri K. V. (2022). Carbon nanomaterials and its applications in pharmaceuticals: a brief review. Chemosphere294, 133731. 10.1016/j.chemosphere.2022.133731
87
Su X. et al (2023). An advanced ratiometric molecularly imprinted sensor based on metal ion reoxidation for indirect and ultrasensitive glyphosate detection in fruit. Food Chem., 429. 10.1016/j.foodchem.2023.136927
88
Sumio I. a. (1991). Helical microtubules of graphitic carbon. Nature354 (6348), 56–58. 10.1038/354056a0
89
Sun B. Joshi R. (2010). Formulation of 2D graphene deformation based on chiral‐tube base vectors. J. Nanomater.2010 (1). 10.1155/2010/402591
90
Sun J. Zhou X. Mao H. Wu X. Zhang X. Gao H. et al (2016). Identification of pesticide residue level in lettuce based on hyperspectra and chlorophyll fluorescence spectra. Int. J. Agric. Biol. Eng.9 (6), 231–239. 10.3965/j.ijabe.20160906.2519
91
Tatsumi H. Osaku N. (2011). Sensitive electrochemical detection of the hydroxyl radical using enzyme-catalyzed redox cycling. Anal. Sci.27 (11), 1065–1067. 10.2116/analsci.27.1065
92
Teymourian H. Barfidokht A. Wang J. (2020). Electrochemical glucose sensors in diabetes management: an updated review (2010–2020). Chem. Soc. Rev.49 (21), 7671–7709. 10.1039/d0cs00304b
93
Thirumalraj B. Rajkumar C. Chen S. M. Veerakumar P. Perumal P. Liu S. B. (2018). Carbon aerogel supported palladium-ruthenium nanoparticles for electrochemical sensing and catalytic reduction of food dye. Sensors Actuators B-Chemical257, 48–59. 10.1016/j.snb.2017.10.112
94
Tong X. L. Jiang L. Ao Q. Lv X. Song Y. Tang J. (2024). “Highly stable glucose oxidase polynanogel@MXene/chitosan electrochemical biosensor based on a multi-stable interface structure for glucose detection,”, 248. Biosens. and Bioelectron. 10.1016/j.bios.2023.115942
95
Tzanov T. Costa S. A. Gübitz G. M. Cavaco-Paulo A. (2002). Hydrogen peroxide generation with immobilized glucose oxidase for textile bleaching. J. Biotechnol.93, 87–94. 10.1016/s0168-1656(01)00386-8
96
Upadhyay A. K. Chen S. M. Ting T. W. Peng Y. Y. (2011). A biosensor for hydrogen peroxide based on single walled carbon nanotube and metal oxide modified indium tin oxide electrode. Int. J. Electrochem. Sci.6 (8), 3466–3482. 10.1016/s1452-3981(23)18265-4
97
Vaidyanathan V. K. Alanazi A. K. Kumar P. S. Rajendran D. S. Chidambaram A. Venkataraman S. et al (2023). Cost-effective, scalable production of glucose oxidase using Casuarina equisetifolia biomass and its application in the bio-Fenton oxidation process for the removal of trace organic contaminants from wastewater. Bioresour. Technol., 377. 10.1016/j.biortech.2023.128958
98
Wang F. Chen X. Wang Y. Li X. Wan M. Zhang G. et al (2022). Insights into the structures, inhibitors, and improvement strategies of glucose oxidase. Int. J. Mol. Sci.23 (17), 9841. 10.3390/ijms23179841
99
Wang F. Zhu Y. Qian L. Yin Y. Yuan Z. Dai Y. et al (2024). Lamellar Ti 3 C 2 MXene composite decorated with platinum-doped MoS 2 nanosheets as electrochemical sensing functional platform for highly sensitive analysis of organophosphorus pesticides. Food Chem.459, 140379. 10.1016/j.foodchem.2024.140379
100
Wang J. a.F. L. Lu F. (2009). Oxygen-rich oxidase enzyme electrodes for operation in oxygen-free solutions. J. Am. Chem. Soc.120 (5), 1048–1050. 10.1021/ja972759p
101
Wang L. Li W. Liu Y. Zhi W. Han J. Wang Y. et al (2019b). Green separation of bromelain in food sample with high retention of enzyme activity using recyclable aqueous two-phase system containing a new synthesized thermo-responsive copolymer and salt. Food Chem.282, 48–57. 10.1016/j.foodchem.2019.01.005
102
Wang P. Hassan M. M. Guo Z. Zhang Z. Z. Chen Q. (2019a). Fabricating an acetylcholinesterase modulated UCNPs-Cu2+fluorescence Biosensor for ultrasensitive Detection of organophosphorus pesticides-Diazinon in food. J. Agric. Food Chem.67 (14), 4071–4079. 10.1021/acs.jafc.8b07201
103
Wang X. Li Q. Xie J. Jin Z. Wang J. Li Y. et al (2009). Fabrication of ultralong and electrically uniform single-walled carbon nanotubes on clean substrates. Nano Lett.9 (9), 3137–3141. 10.1021/nl901260b
104
Wang X. Xu Y. Li Y. Li Y. Li Z. Zhang W. et al (2021). Rapid detection of cadmium ions in meat by a multi-walled carbon nanotubes enhanced metal-organic framework modified electrochemical sensor. Food Chem.357, 129762. 10.1016/j.foodchem.2021.129762
105
Wen C. Zhang J. Feng Y. Duan Y. Ma H. Zhang H. (2020). Purification and identification of novel antioxidant peptides from watermelon seed protein hydrolysates and their cytoprotective effects on H2O2-induced oxidative stress. Food Chem.327, 127059. 10.1016/j.foodchem.2020.127059
106
Wijayanti S. D. Schachinger F. Ludwig R. Haltrich D. (2023). Electrochemical and biosensing properties of an FAD-dependent glucose dehydrogenase from Trichoderma virens. Bioelectrochemistry153, 108480. 10.1016/j.bioelechem.2023.108480
107
Wu B. Xu H. Shi Y. Yao Z. Yu J. Zhou H. et al (2022). Microelectrode glucose biosensor based on nanoporous platinum/graphene oxide nanostructure for rapid glucose detection of tomato and cucumber fruits. Food Qual. Saf.6. 10.1093/fqsafe/fyab030
108
Wu S. Duan N. Qiu Y. Li J. Wang Z. (2017). Colorimetric aptasensor for the detection of Salmonella enterica serovar typhimurium using ZnFe2O4-reduced graphene oxide nanostructures as an effective peroxidase mimetics. Int. J. Food Microbiol.261, 42–48. 10.1016/j.ijfoodmicro.2017.09.002
109
Xia J. Feng L. Lishi Y. Hongbo S. Jingya Q. Bin Z. (2023). Simultaneous determination of tert-butylhydroquinone, butylated hydroxyanisole and phenol in plant oil by metalloporphyrin-based covalent organic framework electrochemical sensor. J. Food Compos. Analysis122, 105486. 10.1016/j.jfca.2023.105486
110
Xia J. Zou B. Liu F. Wang P. Yan Y. (2022). Sensitive glucose biosensor based on cyclodextrin modified carbon nanotubes for detecting glucose in honey. J. Food Compos. Analysis105, 104221. 10.1016/j.jfca.2021.104221
111
Xu Y. Zhang W. Shi J. Zou X. Li Y. Haroon Elrasheid T. et al (2017). Electrodeposition of gold nanoparticles and reduced graphene oxide on an electrode for fast and sensitive determination of methylmercury in fish. Food Chem.237, 423–430. 10.1016/j.foodchem.2017.05.096
112
Xu Y. Zhang W. Shi J. Zou X. Li Z. Zhu Y. (2016). Microfabricated interdigitated Au electrode for voltammetric determination of lead and cadmium in Chinese mitten crab (Eriocheir sinensis). Food Chem.201, 190–196. 10.1016/j.foodchem.2016.01.078
113
Yan M. Chen Y. Feng Y. Saeed M. Fang Z. Zhen W. et al (2024). Perspective on agricultural industrialization: modification strategies for enhancing the catalytic capacity of keratinase. J. Agric. Food Chem.72 (23), 13537–13551. 10.1021/acs.jafc.4c03025
114
Yin L. Jayan H. Cai J. El-Seedi H. R. Guo Z. Zou X. (2023). Development of a sensitive SERS method for label-free detection of hexavalent chromium in tea using carbimazole redox reaction. Foods12 (14), 2673. 10.3390/foods12142673
115
Yin S. J. et al (2024). A long lifetime and highly sensitive wearable microneedle sensor for the continuous real-time monitoring of glucose in interstitial fluid. Amsterdam, NetherlandsBiosensors and Bioelectronics, 244.
116
Yu H. B. Feng J. Zhong F. Wu Y. (2022). Chemical modification for the “Off-/On” regulation of enzyme activity. Macromol. Rapid Commun.43 (18), e2200195. 10.1002/marc.202200195
117
Yu Z. H. Kou Y. Dai Y. Wang X. Wei H. Xia D. (2015). Direct electrochemistry of glucose oxidase on a three-dimensional porous zirconium phosphate-carbon aerogel composite. Electrocatalysis6 (4), 341–347. 10.1007/s12678-015-0249-y
118
Zappelli P. Pappa R. Rossodivita A. Re L. (1978). Carboxylic and polyethyleneimine-bound FAD derivatives. Synthesis, coenzymic properties, conformational and enzyme-coenzyme interaction studies. IEEE89 (2), 491–499. 10.1111/j.1432-1033.1978.tb12553.x
119
Zeng K. Chen B. Li Y. Meng H. Wu Q. Yang J. et al (2022). Gold nanoparticle-carbon nanotube nanohybrids with peroxidase-like activity for the highly-sensitive immunoassay of kanamycin in milk. Int. J. Food Sci. Technol.57 (9), 6028–6037. 10.1111/ijfs.15955
120
Zeng K. Wei W. Jiang L. Zhu F. Du D. (2016). Use of carbon nanotubes as a solid support to establish quantitative (centrifugation) and qualitative (filtration) immunoassays to detect gentamicin contamination in commercial milk. J. Agric. Food Chem.64 (41), 7874–7881. 10.1021/acs.jafc.6b03332
121
Zhang C. Shi X. Yu F. Quan Y. (2020). Preparation of dummy molecularly imprinted polymers based on dextran-modified magnetic nanoparticles Fe3O4 for the selective detection of acrylamide in potato chips. Food Chem.317, 126431. 10.1016/j.foodchem.2020.126431
122
Zhang D. Wang Y. Sun X. Liu Y. Zhou Y. Shin H. et al (2019). Voltammetric, spectroscopic, and cellular characterization of redox functionality of eckol and phlorofucofuroeckol-A: a comparative study. J. Food Biochem.43 (7), e12845. 10.1111/jfbc.12845
123
Zhang L. Chen Q. Han X. Zhang Q. (2018b). MnO2Nanoparticles and carbon nanofibers Nanocomposites with high sensing performance toward glucose. J. Clust. Sci.29 (6), 1089–1098. 10.1007/s10876-018-1421-3
124
Zhang Y. Cui Y. Hong X. Du D. (2018a). Using of tyramine signal amplification to improve the sensitivity of ELISA for aflatoxin B1 in edible oil samples. Food Anal. Methods11 (9), 2553–2560. 10.1007/s12161-018-1235-9
125
Zhang Y. Hu Y. Wilson G. S. Moatti-Sirat D. Poitout V. Reach G. (1994). Elimination of the acetaminophen interference in an implantable glucose sensor. Anal. Chem.66 (7), 1183–1188. 10.1021/ac00079a038
126
Zhao S. Guo D. Zhu Q. Dou W. Guan W. (2021). Display of microbial glucose dehydrogenase and cholesterol oxidase on the yeast cell surface for the detection of blood biochemical parameters. Biosensors-Basel11 (1), 13. 10.3390/bios11010013
127
Zhao W. Xu J. J. Qiu Q. Q. Chen H. Y. (2006). Nanocrystalline diamond modified gold electrode for glucose biosensing. Biosens. and Bioelectron.22 (5), 649–655. 10.1016/j.bios.2006.01.026
128
Zhao Y. Ma Y. Zhou R. He Y. Wu Y. Yi Y. et al (2022). Highly sensitive electrochemical detection of paraoxon ethyl in water and fruit samples based on defect-engineered graphene nanoribbons modified electrode. J. Food Meas. Charact.16 (4), 2596–2603. 10.1007/s11694-022-01366-6
129
Zhou G. Cao Z. Liu Y. Zheng H. Xu K. (2024). Highly sensitive and stable glucose sensing using N-type conducting polymer based organic electrochemical transistor. J. Electroanal. Chem.952, 117961. 10.1016/j.jelechem.2023.117961
130
Zhou W. Li C. Sun C. Yang X. (2016). Simultaneously determination of trace Cd2+ and Pb2+ based on L-cysteine/graphene modified glassy carbon electrode. Food Chem.192, 351–357. 10.1016/j.foodchem.2015.07.042
131
Zhu C. Du D. Lin Y. (2017). Graphene-like 2D nanomaterial-based biointerfaces for biosensing applications. Biosens. Bioelectron.89 (Pt 1), 43–55. 10.1016/j.bios.2016.06.045
132
Zhu L. Dong X. X. Gao C. B. Gai Z. He Y. X. Qian Z. J. et al (2022). Development of a highly sensitive and selective electrochemical immunosensor for controlling of rhodamine B abuse in food samples. Food control.133, 108662. 10.1016/j.foodcont.2021.108662
Summary
Keywords
glucose oxidase, electrochemical sensing, carbon nanomaterials, glucose detection, chemical modification
Citation
Guoqiang G, Liang Q, Yani Z, Pengyun W, Fanzhuo K, Yuyang Z, Zhiyuan L, Xing N, Xue Z, Qiongya L and Bin Z (2025) Recent advances in glucose monitoring utilizing oxidase electrochemical biosensors integrating carbon-based nanomaterials and smart enzyme design. Front. Chem. 13:1591302. doi: 10.3389/fchem.2025.1591302
Received
12 March 2025
Accepted
09 April 2025
Published
28 April 2025
Volume
13 - 2025
Edited by
Shuai Wang, Qilu University of Technology, China
Reviewed by
Yi Hu, Hong Kong Polytechnic University, Hong Kong SAR, China
Tholkappiyan Ramachandran, Khalifa University, United Arab Emirates
Updates
Copyright
© 2025 Guoqiang, Liang, Yani, Pengyun, Fanzhuo, Yuyang, Zhiyuan, Xing, Xue, Qiongya and Bin.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zou Bin, binzou2009@ujs.edu.cn
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.