- 1Department of Chemistry, Boston College, Merkert Chemistry Center, Chestnut Hill, MA, United States
- 2School of Engineering, Macquarie University, Sydney, NSW, Australia
Photoelectrocatalytic (PEC) water splitting represents a highly ideal approach for the efficient conversion of solar energy into sustainable green hydrogen. Although tantalum nitride (Ta3N5) has emerged as a promising photoanode material, its performance is far below the theoretical limit. Among several photoelectrode design strategies, interfacial modification can be beneficial for suppressing interfacial charge recombination and promoting charge transfer process, which is a key focus in recent research. In the review, a brief overview of recent advances in interfacial modification strategies for Ta3N5 photoanodes and their influence on the structure-performance relationship are summarized, aiming at an in-depth understanding of the charge-transfer mechanism during PEC water oxidation, and providing insights into designing efficient and stable Ta3N5 photoanodes for solar-to-fuel conversion through photoelectrocatalysis.
1 Introduction
Photoelectrocatalytic (PEC) water splitting has emerged as an exceptionally promising approach for solar-to-fuel conversion, utilizing water as the sole feedstock to generate hydrogen through an environmentally benign and sustainable process that produces zero carbon emissions (Kim et al., 2019; Yu et al., 2021; Nishiyama et al., 2021). In contrast to photocatalytic powder systems, the PEC approach demonstrates enhanced charge separation efficiency, as the combination of irradiation and an external bias facilitates the directional separation and migration of photogenerated charge carriers (He et al., 2019). Moreover, the photovoltage produced under illumination in PEC system can compensate for the applied bias, thereby decreasing energy input requirements in electrocatalysis (EC) system. In addition, the reliance on cost-effective inorganic semiconductors gives PEC a significant economic advantage over hybrid Photovoltaic (PV)-EC approaches (Kim et al., 2019). Because of the complex four-hole process for evolving one oxygen molecule, PEC water oxidation is well recognized as the rate-determining step, therefore, efforts on the photoanode design are of significance (Ye et al., 2019; Seo et al., 2018; Minggu et al., 2010; Li Z. et al., 2013). However, most of the photoanode materials exhibit relatively low performance, far from their theoretical solar-to-hydrogen (STH) efficiencies, primarily due to the low charge separation ability and sluggish charge transport kinetics (Feng et al., 2020; Ning et al., 2019). Most importantly, serious charge recombination issues at the interfaces must be addressed to promote charge transfer kinetics for achieving efficient PEC systems (Haider et al., 2020; Zachaus et al., 2017).
A wide range of n-type semiconductor materials have been studied in the advancement of PEC water oxidation reaction (Wang S. et al., 2019). Titanium dioxide (TiO2) is the earliest material for PEC studies, however, it can only be activated by a small amount of ultraviolet light (<5%) due to its wide gap (3.0–3.2 eV), resulting in the limited theoretical STH efficiency within 1.5%–2.2% (Cho et al., 2013). As for tungsten trioxide (WO3), the sluggish charge transport kinetics can lead to serious hole-electron recombination, the low conduction band position can result in low photovoltage (Kim et al., 2013). Although bismuth vanadate (BiVO4) is a promising semiconductor photoanode to deliver photocurrent density of 5.8 mA cm-2 and applied bias photon-to-current efficiency (ABPE) of 2.7%, its charge transfer kinetics can be limited by short electron diffusion distance and its theoretical STH efficiency can be lower below 10% (9.1%) (Kim and Lee, 2019). Moreover, the low light absorption coefficient (103 cm-2), short hole diffusion length can result in poor STH efficiency (<1%) of hematite (ɑ-Fe2O3) despite its high theoretical STH efficiency (15.9%) (Wheeler et al., 2012). Tantalum nitride (Ta3N5) is a typical n-type semiconductor with a band gap of 2.1 eV and exhibits broad spectral absorption up to 590 nm (Seo et al., 2018). Moreover, its conduction and valence band positions straddle water reduction and oxidation potentials, respectively, of which this alignment fulfills fundamental thermodynamical requirements necessary for overall solar water splitting (Seo et al., 2018; Chun et al., 2003; Ishikawa et al., 2004; Yu et al., 2025). In theory, Ta3N5 can reach a maximum photocurrent density of 12.9 mA cm-2 under simulated AM 1.5G sunlight (1 sun), accompanied by a theoretical maximum STH of 15.9% (Seo et al., 2018). Till now, numerous efforts have been made to improve both the activity and durability of Ta3N5 photoanodes. For example, by using gradient Mg doping for band structure engineering, Li and coworkers constructed a In:GaN/Ta3N5/Mg:GaN heterojunction photoanode, and achieved an ABPE of nearly 3.5% (Fu et al., 2022). Apart from doping/defect control and heterojunction strategy to improve charge separation efficiency, interfacial modification also plays a crucial role in regulating charge transfer and transport behaviors (Ding et al., 2016; Chen et al., 2020; Xiao et al., 2025). As shown in Figure 1a, it has been demonstrated that controlling several interfaces of the semiconductor-electrolyte configuration, including cocatalysts and interfacial layers (such as hole storage layer), is an alternative to lower the reaction barriers and promote charge transfer. However, it still remains challenging to distinguish the functionalities of multilayers clearly in interfacial modification strategies. Consequently, the summary of these interfacial modification strategies and their effects on the OER performance is necessary and critical.
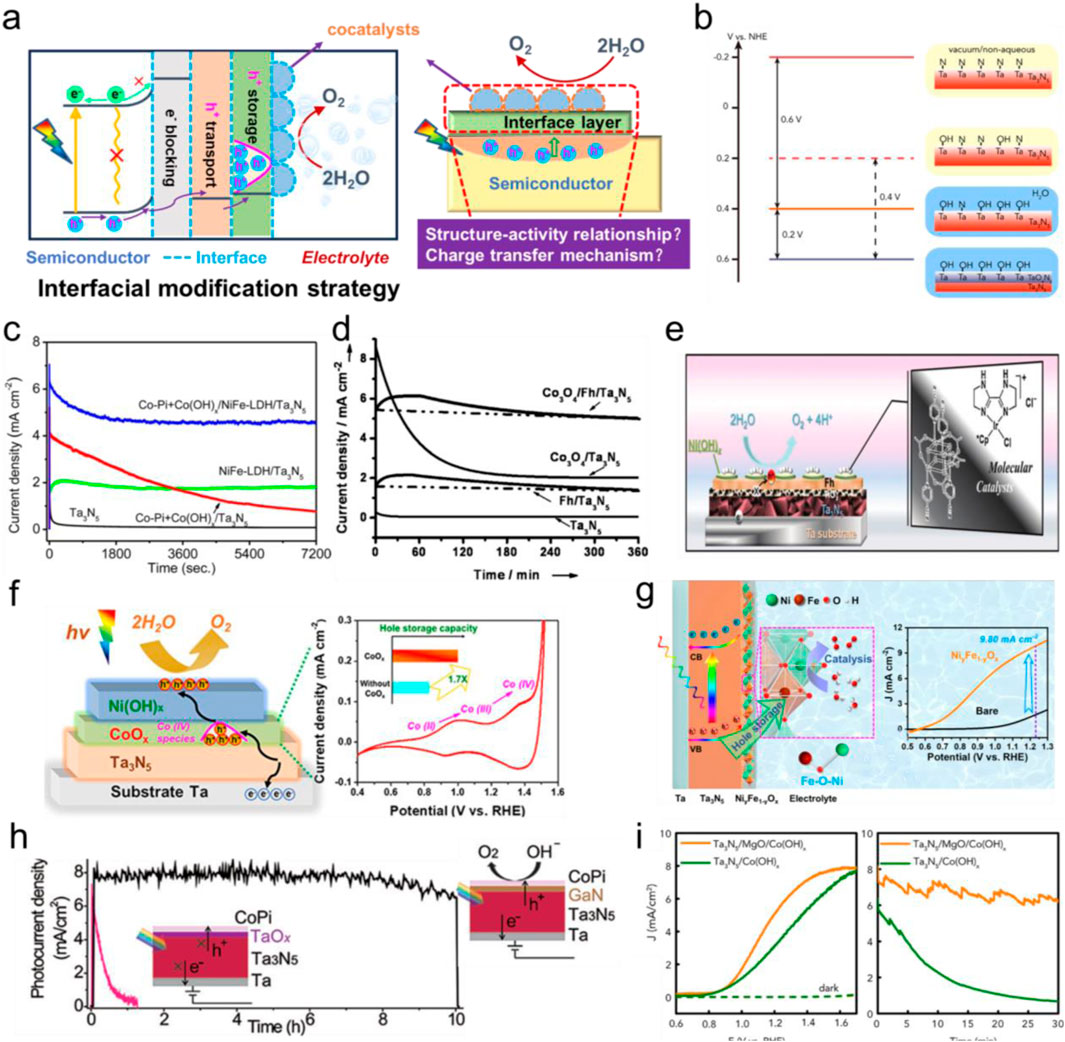
Figure 1. (a) The diagram of interfacial modification strategy and the related charge-transfer mechanism study. (b) The evolution of Ta3N5 surface energetics. Reprinted with permission from He et al. (2016). Copyright 2016, Elsevier Inc. (c) Steady-state photocurrent density versus time curves of Ta3N5 photoanodes with different cocatalysts loading. Reprinted with permission from Ref. (Wang L. et al., 2015). Copyright 2015, American chemical society. (d) Chronoamperometry measurements of the Ta3N5 photoanodes with decoration of Fh and Co3O4 at 1.23 V vs. RHE. Reprinted with permission from Liu et al. (2014). Copyright 2014, Wiley. (e) The schematic presentation of the integrated Ta3N5 photoanode system. Reprinted with permission from Liu et al. (2016). Copyright 2016, Royal society of chemistry. (f) Brief schematic diagram of interfacial energetics for the Ni(OH)x/CoOx/Ta3N5 photoanode in terms of PEC water oxidation. Reprinted with permission from Wang et al. (2021a). Copyright 2021, American Chemical Society. (g) Schematic illustrations of the charge transfer process and PEC water oxidation reaction for NiyFe1-yOx/Ta3N5 photoanodes and photocurrent density-potential curves. Reprinted with permission from Wang et al. (2023). Copyright 2023, American chemical society. (h) The stability measurement for the CoPi/GaN/Ta3N5 and CoPi/Ta3N5 photoanodes. Reprinted with permission from Zhong et al. (2017). Copyright 2017, Wiley. (i) Current-potential and stability curves of Co(OH)x/Ta3N5 photoanodes with and without MgO as a protection layer. Reprinted with permission from He et al. (2016). Copyright 2016, Elsevier Inc.
In this review, we summarize some recent advances in the design of Ta3N5 photoanodes for solar water oxidation through interfacial modification strategies. Then we uncover their charge transfer mechanisms based on an analysis of structure-activity relationship, which may open new avenues for the rational design of a highly efficient photoanode system.
2 Interfacial modification strategies on Ta3N5 photoanodes
2.1 Surface study of Ta3N5
Although Ta3N5 is a promising candidate for PEC water splitting, it suffers from severe photocorrosion, hindering it from practical applications (Ishikawa et al., 2004). Therefore, investigating the surface structure is helpful for comprehending the surface properties of Ta3N5 prior to discussing of interfacial modification. Li et al. observed a Ta3-xN5-yOy layer on the Ta3N5 surface, and found that its facile removal remarkably reduced charge recombination, resulting in an increased photocurrent (Li M. et al., 2013). Similarly, Nurlaela et al. found that the energetics at the Ta3N5/H2O interface were affected by the surface properties, while the introduction of metallic TaN can result in Fermi level pinning and the subsequent activity decrease (Nurlaela et al., 2014). When Liu et al. fabricated Ta3N5 electrode from NaTaO3 precursor, they found that mixed phases of TaN and TaO led to severe charge recombination (Liu et al., 2016). With regard to this, the introduction of mixed Ar and O2 during cooling stage of nitridation process can form a passivation layer. He et al. investigated the energetics of Ta3N5|H2O interface before and after the OER test, and found that the adsorption of H2O or hydroxyl species could induce positive shifts of band edge positions, which can explain the high turn-on potential (Von) and lower photovoltage (Figure 1b). (He et al., 2016) In addition, Chen et al. revealed that hydrophobic Ta3N5 surface after nitridation is not beneficial for water splitting, while impregnating the Ta2O5 powders in MgSO4 solutions could result in hydrophilic surface and more uniform deposition of CoOx as OER catalysts, while Mg doping was found to reduce surface defect densities, contributing to better charge separation efficiency (Chen et al., 2015). Moreover, some theoretical studies for Ta3N5/H2O were also conducted to get insights of the interfacial effects on performance. For instance, Watanabe et al. used density functional theory (DFT) to reveal a downward shift of overall band edge positions once the intermediates were absorbed on the surface of Ta3N5, a partial Fermi level pinning was observed due to charge redistribution (induced by surface states or absorbed species) (Watanabe et al., 2017). Fan et al. presented a theoretical study of Ta3N5 photoanodes, and found positive shift of band edge positions by 0.42 V when Ta3N5 was exposed to H2O, while the total shift can be reached 0.85 V once water dissociation occurred on the Ta3N5 surface (Fan et al., 2019).
These results demonstrate that the direct contact between Ta3N5 and electrolyte may be harmful for reaction activity, primarily due to surface oxidation and adverse interfacial energetics. Consequently, developing interfacial modification strategies is crucial for minimizing the surface oxidation and improving the performance of Ta3N5 photoanodes. Table 1 provides a summary of various modification methods (such as cocatalysts, hole-storage layers, physical protection layers) that have been employed to improve the performance of Ta3N5 photoanodes.
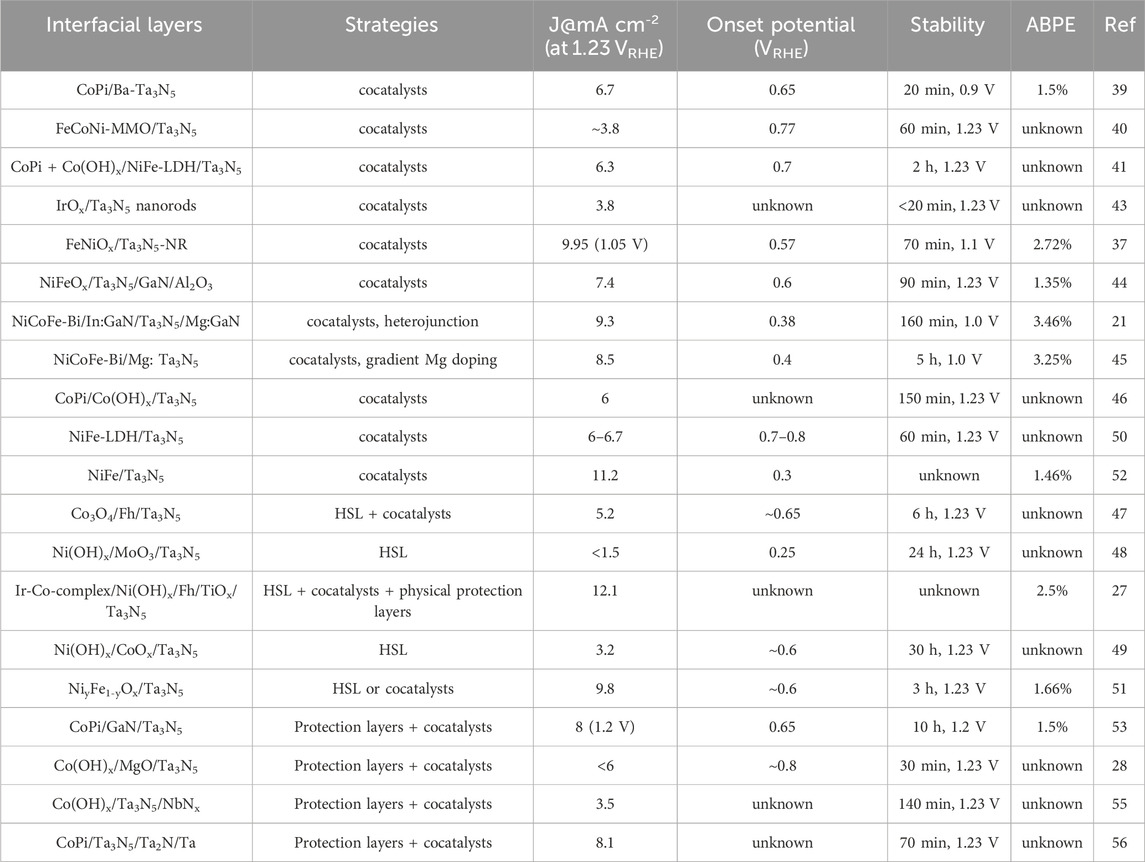
Table 1. Some representative interfacial modification materials for enhancing the performance of Ta3N5 photoanodes.
2.2 Cocatalysts
A wide range of OER catalysts have been applied for improving the activity of photoanodes, including IrOx (Wang Z. et al., 2015), CoPi (Wang L. et al., 2019; Wang et al., 2022), CoOx (Lee et al., 2019; Liao et al., 2012), and Fe-Co-Ni based (oxy-)hydroxides (Pihosh et al., 2020; Zhang et al., 2022). In general, the decoration of cocatalysts on the surface of photoanodes were proved to be significantly reduce the OER energy barrier, allowing photogenerated holes to be kinetically favored toward OER. Meanwhile, it has been reported that cocatalysts loading can efficiently passivate surface states and suppress interfacial recombination centers as well as provide more active sites to enhance the photocurrent densities (Yu et al., 2025; Liao et al., 2012). In general, the strategy is always combined with doping method to enhance performance, in some cases multiple layers of cocatalysts are also applied. For instance, Li et al. found that Ba doping could suppress the formation of less conductive Ta5N6 phase within Ta3N5 photoanode and shift flat-band potential negatively. With the addition of CoPi as the cocatalysts, a photocurrent density of 6.7 mA cm-2 was reached at 1.23 V (Li et al., 2013c). Haleem et al. synthesized FeNiCoOx as the cocatalysts on Ta3N5 via photo-assisted electrodeposition and exhibited stable photocurrent densities around 4 mA cm-2 at 1.23 V for 2 h (Abdel Haleem et al., 2017). Wang et al. obtained a solar photocurrent of 6.3 mA cm-2 at 1.23 V for Ta3N5 nanorod photoanode by the sequential modifications of NiFe-layered double hydroxide (LDH), Co(OH)x and CoPi as the cocatalysts (Wang L. et al., 2015). Moreover, a steady photocurrent of ∼5 mA cm-2 was remained at 1.23 V for at least 2 h of irradiation (Figure 1c). Despite being as an efficient OER catalysts, IrOx modified Ta3N5 photoanodes have been displayed to exhibit rapid decay of OER performance (Yokoyama et al., 2011; Li et al., 2013d). This is primarily due to incomplete coverage of IrOx on Ta3N5 interface and the subsequent Ta3N5 surface oxidation, where Fermi level pinning could be formed to undermine the charge separation efficiency. Pihosh et al. fabricated highly-conductive polycrystalline Ta3N5-nanorods for advantageous light harvesting, FeNiOx cocatalyst loading can result in efficient charge separation and a completely saturated photocurrent density of 9.95 mA cm-2 at 1.05 V (Pihosh et al., 2020). Later, the same group deposited an ultrathin NiFeOx electrocatalyst layer on semitransparent Ta3N5 photoanode and dramatically improved the stability and generated a photocurrent density of 7.4 mA cm-2 at 1.23 V (Higashi et al., 2022). Furthermore, this photoanode was coupled with CuInSe2 to form PEC-PV tandem cells and achieved an initial STH efficiency of 9%. Moreover, combined with band structure engineering of Ta3N5 photoanodes or heterojunction construction, the decoration of NiCoFe-Bi cocatalysts can deliver exceptional ABPE values toward solar water oxidation (Fu et al., 2022; Xiao et al., 2020). He et al. deposited CoPi and Co(OH)2 nanosheet cocatalysts on the Ta3N5 surface and the PEC performance was found to be dramatically enhanced (He et al., 2017). Systematical studies indicated that light-induced O· radicals could lead to the formation of Ta-O-Co bonds between Co(OH)2 and Ta3N5, thereby largely suppressing Fermi-level pinning, reducing charge recombination and accelerating charge transfer efficiency.
Nevertheless, it is significant to recognize that both the kinetics and energetics at the electrode/electrolyte surface can be regulated upon cocatalysts loading, as the consequence of the decrease of charge extraction barrier. Moreover, sometimes the cocatalyst decoration can also passivate surface defects and decrease the possibility of charge recombination. Therefore, the charge transfer process can be promoted, both the photocurrent density and durability can be improved.
2.3 Hole-storage layers
The concept of “hole-storage-layer” (HSL) was first proposed by Liu et al. when they used ferrihydrite (Fh) to protect Ta3N5 from photocorrosion (Liu et al., 2014). As displayed in Figure 1d, the decoration of Fh could promptly extract and store photogenerated holes from Ta3N5, thereby prolonging OER durability (at 1.23 V) for 6 h without obvious photocurrent degradation. Although Co3O4 nanoparticles cocatalysts impressively accelerate charge transfer kinetics and improve activity, Ta3N5 was not effectively protected from photocorrosion, highlighting the vital role of HSL in electrode design. Later, Ni(OH)x/MoO3 bilayer was designed as HSL for remaining the photocurrent density stable over 24 h, benefiting from the high hole mobility and efficient hole extraction contributed from this HSL (Liu et al., 2015). The most impressive demonstration for HSL was reported by Liu et al., in 2016, they prepared an integrated Ta3N5 photoanode for delivering a photocurrent density of 12.1 mA cm-2 (approaching the theoretical value of 12.9 mA cm-2) and an ABPE of 2.5%, where the Ni(OH)x and Fh were combined as HSLs, the surface of Ta3N5 was passivated via Ar/O2 gas, along with TiOx as an electron blocking layer and Co-Ir molecular complexes as the OER cocatalysts (Figure 1e). (Liu et al., 2016) Intensity-modulated photocurrent spectroscopy (IMPS) was utilized for revealing the role of HSL, showing that the modification of HSLs only suppressed interfacial charge recombination but not influenced the charge transfer kinetics. Recently, Wang et al. used Ni(OH)x/CoOx as the HSL for improving photostability of Ta3N5 for 30 h, while the reversible formation of Co(IV) species within the ultrathin CoOx layer was considered to be the main cause for facilitating hole extraction toward Ni(OH)x, as illustrated in Figure 1f (Wang et al., 2021a).
It is worth noting that cocatalysts and HSLs often exhibit synergistic effects, as demonstrated by Ni-Fe oxide/hydroxides decorated Ta3N5 photoanodes. By using electrodeposition method, Fang et al. prepared NiFe-LDH and Ni0.9Fe0.1OOH as cocatalysts, dramatically improving the PEC performance of Ta3N5 photoanodes (Fang et al., 2018). Domen et al. deposited FeNiOx cocatalysts on Ta3N5 nanorod photoanodes and achieved an exceptional photocurrent density of 9.95 mA cm-2 at 1.05 V, accompanied with ABPE of 2.72% (Pihosh et al., 2020). Despite these advancements, the charge transfer mechanism of Ni-FeOx/Ta3N5 photoanodes for PEC water oxidation remains unclear. With this regard, Wang et al. prepared Ni-Fe oxyhydroxides (NiyFe1-yOx) with different Ni and Fe loading ratios for modifying Ta3N5 photoanodes and elucidated the reaction mechanism (Wang et al., 2023). As shown in Figure 1g, it was found that a photocurrent density of 9.8 mA cm-2 was reached at 1.23 V upon loading Ni0.5Fe0.5Ox, while the ABPE value could be increased from 0.1% to 1.66%. They revealed the role of NiyFe1-yOx as both the cocatalysts and HSL. Ni species primarily store holes from Ta3N5, while the Fe-O-Ni species are responsible for promptly extracting and transferring the stored holes to electrode/electrolyte interface, the synergetic effects can result in effective charge transfer efficiencies for OER. Similarly, Dong et al. revealed that Fe catalysts within NiFe-catalysts effectively promoted charge separation and hole transfer of Ta3N5 photoanode, while Ni catalyst nanolayers provided catalytic active-sites for PEC water splitting (Dong et al., 2023).
After the full comprehension, it is hard to distinguish cocatalysts from HSLs in photoanode modification over solar water oxidation. Both the cocatalysts and HSLs can facilitate charge extraction, separation, transport and injection, except that cocatalysts seem to demonstrate more obvious advantages over HSLs in charge injection at electrode/electrolyte interface. On the other hand, the decoration of HSLs can generally protect Ta3N5 from photocorrosion and improve the stability, while sometimes cocatalyst nanoparticles can’t be covered completely on Ta3N5 surface to get rid of photoanode degradation during long-term water splitting operation, although photocurrent density can be enhanced. Taking Fe-Co-Ni based (oxy-)hydroxide cocatalysts as the example, there are several complex steps including charge extraction, hole-storage processes (in the form of high-valence species) and hole-injection/release for water oxidation. Overall, from the aspect of interfacial modification, it must be more significant to concentrate on energy diagram matching and material choices for rationally designing highly efficient photoanode system.
2.4 Physical protection layers
Apart from cocatalysts and HSLs, transparent oxide/nitride films were widely used as the protection layers for enhancing the stability of photoelectrodes. Moreover, the activity could be also boosted with the assistence of cocatalysts. For example, Zhong et al. grew a ∼50 nm GaN layer on Ta3N5 by nitriding the deposited GaOx, with the further loading of CoPi cocatalyst, the GaN protected Ta3N5 photoanode exhibited stable photocurrent densities over 8 mA cm-2 for 10 h (Figure 1h). (Zhong et al., 2017) By comparison, it was found that the CoPi/Ta3N5 photoanode showed a negligible photocurrent density within 1 h, emphasizing the critical role of GaN in preventing the penetration of electrolytes into the Ta3N5 surface. Zhang et al. found that the deposited TiO2 on the Ta3N5 surface significantly removed surface states, reduced charge recombination and promoted charge separation, the onset potential was hence shifted negatively (Zhang et al., 2016). When He et al. introduced a compact MgO layer between Ta3N5 and outmost Co(OH)x cocatalyst via atom layer deposition (ALD) method, they found that MgO could not only effectively separate Ta3N5 from electrolytes or active oxygen species, but also improve the attachment of cocatalysts on Ta3N5 surface (He et al., 2016). Therefore, the fill factor of J-V curve for Ta3N5 photoanode was improved, implying the promotion of charge separation. Moreover, the stability of PEC water oxidation system was obviously enhanced (Figure 1i). Considering the high Schottky barriers formed between Ta3N5 film and Ta substrate to hinder electron transfer, Wang et al. deposited several different back contact layers such as NbNx, TiNx and CdS and found that the Ta3N5/NbNx/Ta photoanode yielded highest photocurrent density, demonstrating the effective promotion of electron transfer from Ta3N5 to the substrate (Wang et al., 2016). The same group later found that the presence of Ta2N interlayer can facilitate electron transfer between Ta3N5 and Ta substrate, thereby delivering an improved photocurrent density of 8.1 mA cm-2 at 1.23 V with the additional assistance of CoPi cocatalysts (Nurlaela et al., 2020).
It should be mentioned that both the decoration of cocatalysts and HSLs can be employed to provide additional driving force for charge separation and extraction within the photoanodes over solar water oxidation, while the primary functionality of the physical protection layers focuses on the inhibition of electron-hole recombination, promotion of electron transfer to substrate as well as the interfacial passivation effects.
3 Conclusion and outlook
As mentioned above, great efforts have been made to develop efficient and stable Ta3N5 photoanodes by interfacial modification strategies such as cocatalysts loading, HSL decoration and the introduction of physical protection layer. It is worth noting that these methods have been always combined through rational interfacial modification engineering to promote charge separation, boost reaction kinetics and address the instability issues. Currently, the achievable photocurrent density of Ta3N5 photoanode reaches 12.1 mA cm-2 (Liu et al., 2016), very close to the theoretical value of 12.9 mA cm-2. However, the onset potential is 0.38 V, far from the theoretical value (<0 V), and the recorded ABPE reported is only 3.46% (Fu et al., 2022), posing a significant bottleneck for the practical application of Ta3N5 photoelectrode material. Therefore, although interfacial modification strategies have been developed for many years, several challengs still remains unresolved. (i) The structure properties of most of the interfacial modification layers lack in-depth research and charge transfer pathway under operando condition still remain unclear. (ii) There is a necessity to develop more interfacial materials with superior charge mobility properties and suitable energy positions. (iii) Defects and the internal electric field of Ta3N5 material could be further optimized to secure a more efficient charge separation efficiency. (iv) More in-depth studies of synergetic effects between interfacial modification and other techniques are required to design improved structures and systems. Accordingly, we will conduct the discussion as follows:
First, although numerous interfacial layers have been developed to significantly enhance the performance of Ta3N5 photoanode, few researches have focused on the effect of the phase/structure of interfacial layers on OER activity. For instance, by carefully regulating the phase structures of FeOOH, it was found that highly crystalline β-FeOOH and mixed-phase FeOOH(α+β) cocatalysts could achieve higher photocurrent densities for BiVO4 photoanodes than those of amorphous FeOOH and single phase FeOOH, respectively, primarily due to optimized crystalline structure and abundant oxygen vacancies (Zhang et al., 2018; Wang et al., 2025). By dehydrating the Fh via a careful calculation, the gradual weakening of the hole-storage capability of Fh/Ta3N5 photoanodes was observed, primarily caused by the irreversible loss of crystal water and mutation of coordination symmetry of [FeO6] hydration units (Wang et al., 2021b). As a consequence, the hydration structure of Fh was identified to be responsible for hole-storage ability of Fh/Ta3N5 photoanodes (Figure 2a). Moreover, the application of some (quasi-)operando spectroscopies (such as FT-IR and Raman) are recommended to detect interfacial structure evolutions during PEC water oxidation for revealing deeper insights into the charge transfer mechanism (Pan et al., 2023; Liu et al., 2025). In a word, the careful regulation of interfacial structures of the interfacial layers and the in-depth understanding of charge transfer pathway will be beneficial for the rational design of photoanodes in the near future.

Figure 2. (a) Basic structural motif of Fh, PEC water oxidation performance changes of Fh/Ta3N5 photoanodes with sequential loss of crystal water within Fh, and the corresponding diagram of charge-transfer and hole-storage active sites. Reprinted with permission from Wang et al. (2021b). Copyright 2021, Wiley. (b) Energy band diagrams energy of TiN, Ta3N5, and CPF-TTB and schematic illustration of the NiFeOx/CPF-TTB/Ta3N5/TiN photoanode during PEC water oxidation, and photocurrent density-potential curves of the above photoanodes. Reprinted with permission from Yang et al. (2024). Copyright 2024, Wiley. (c) ABPE curves of the Ta3N5-based photoanodes, with NiCoFe-Bi as the cocatalysts. Reprinted with permission from Fu et al. (2022). Copyright 2022, Springer Nature. (d) Schematic diagram and working principle of tandem device comprised of serially connected semi-transparent Ta3N5 photoanode with dual-CuInSe2 photovoltaic cells and Pt/Ni electrode, and the J-V curves of dual-CuInSe2 cells and Ta3N5 photoanode. Reprinted with permission from Pihosh et al. (2023). Copyright 2023, Wiley.
Second, learned from interface energetical engineering strategy in solar cells, designing interfacial layers with high hole mobility/transport properties between cocatalysts and Ta3N5 photoanode is also critical, of which the work function matching should be also considered. Recently, Yang et al. elaborately introduced a conjugated polythiophene framework (CPF-TTB) as the hole-selective layer between Ta3N5 and the outmost NiFeOx cocatalyst, the enhanced hole extraction enabled the NiFeOx/CPF-TTB/Ta3N5/TiN photoanode to generate a remarkable photocurrent density of 9.12 mA cm-2 for water oxidation at 1.23 V (Figure 2b). (Yang et al., 2024) Moreover, the energy band diagrams of substrate TiN, Ta3N5 and CPF-TTB were determined to be advantageous for efficient charge extraction from Ta3N5. Specially, the presence of CPT-TTB could restrain charge recombination and expedite hole transport from Ta3N5 to NiFeOx by the formation of energetically favorable type II heterojunction. This example provides a novel perspective in the design of interfacial layer by considering energy band diagram, and hole transport through organic-inorganic hybrid method.
Third, the rational design of highly efficient Ta3N5 photoanode requires not only the interfacial layer with excellent activity and highly matched energetics between multiple layers, but also the optimized Ta3N5 electrode with effective charge separation and transport properties. Xiao et al. used gradient Mg doping in Ta3N5 to induce a gradient of band edge energetics for greatly enhancing charge separation efficiency. In addition, defect-related recombination could be significantly suppressed due to the passivation effect of Mg dopants on deep-level defects. As a consequence, the Mg-doped Ta3N5 photoanode delivered a low onset potential of 0.4 V with the assistence of Ni-Co-Fe-Bi cocatalyst, accompanied with ABPE of 3.25% (Xiao et al., 2020). Later, the same group designed the In:GaN/Ta3N5/Mg:GaN heterojunction photoanode and achieved a record ABPE of 3.46% for Ta3N5-based photoanodes(Figure 2c), this excellent performance was attributed to the enhanced bulk carrier separation capability and better injection efficiency at the photoanode/electrolyte interface (Fu et al., 2022). These results highlight the effectiveness of proper interface engineering for achieving an efficient PEC water splitting system.
Finally, achieving the unassisted PEC water splitting with sunlight as the sole energy input and no external bias, is the ultimate goal for sustainable solar-to-hydrogen energy conversion. However, it remains challenging to achieve this goal using a single Ta3N5 photoanode. A more practical approach involves constructing tandem cells, either combining a Ta3N5 photoanode with a photocathode or integrating it with a photovoltaic(PV) cell (Higashi et al., 2022; Higashi et al., 2019; Pihosh et al., 2023). Domen and coworkers prepared transparent Ta3N5-NRs/Ta3N5-TF/GaN/Al2O3 photoanodes that could deliver the photocurrent density of 10.8 mA cm-2 at 1.23 V (Pihosh et al., 2023). Subsequently, these Ta3N5 photoanodes were connected in series and driven by a dual-CuInSe2 solar cell to achieve a matching photocurrent density of 9.5 mA cm-2 with an operating voltage of 1.16 V (Figure 2d).
Typically speaking, an efficient interfacial engineering may meet several criteria: (1) Low-defect interfaces of semiconductors and lattice-matched semiconductor/interfacial layers to minimize defects, (2) suitable energy level alignment for ensuring favorable charge transfer, (3) compatible fabrication processes for integrated photoanodes. These considerations can be beneficial for screening the optimized interfacial materials for the rational design of highly efficient water splitting systems. When effectively implemented, the interfacial engineering strategies (discussed above) can efficiently improve the PEC performance of Ta3N5 photoanodes by addressing a series of challenges associated with the interfacial defects and charge extraction barriers.
Meanwhile, in the past few years, some advanced (quasi-)operando techniques (such as Raman, FT-IR, XAS) have been widely used to grasp charge transfer pathway, in combination with DFT theoretical calculations. For instance, Ismail et al. employed operando XAS to investigate interfacial dynamics at the NiFeOOH/ɑ-Fe2O3 interface and found that the formation of FeOOH plays a critical role in the surface passivation and hole extraction of ɑ-Fe2O3 (Ismail et al., 2021). When Pan et al. decorated ZnCoFe polyphthalocyanine on BiVO4 to form core-shell photoanode, they found that the interfacial charge transfer can be facilitated by lowering the Fe d band center and orbital spin (Pan et al., 2023). Based on quasi-operando Raman measurements and DFT calculation, they revealed that the promotion of *OOH desorption is the potential limiting step for modulating the catalytic activity. Recently, Liu et al. found that the accumulated high-density holes on ɑ-Fe2O3 surface could form adjacent FeV = O intermediates that effectively activate surface-adsorbed H2O molecules via the hydrogen-bonding effect, as revealed by operando Raman measurements and ab initio molecular dynamics simulations (Liu et al., 2025). Therefore, one potential research technical route may manifest: (1) Carefully regulating phase structure or bonding characteristics of interfacial layers modified photoanodes, (2) investigating the changes of PEC water oxidation activity and charge-transfer kinetics with the structural changes of interfacial layers, (3) exploring the real-time evolution of interfacial layers during PEC water oxidation by using operando techniques and theoretical calculations. This assumption must be one of the research directions regarding in-depth understanding of charge-transfer mechanism.
In addition, the integration of photoanodes with other research fields, such as thermal catalysis and organic synthesis, to advance PEC water splitting. Thermal catalysis is known to rely on elevated temperatures, once combined the photoanodes with thermal catalysts, both solar energy and thermal energy can be utilized to improve water splitting conversion efficiency without external bias. On the other hand, as Ta3N5 is a promising alternative for overall water splitting, considering that water oxidation half reaction is the rate-determining step, we consider to replace it with organic synthesis to obtain high-value chemicals. Consequently, combining the hydrogen production on the cathode with organic molecule activation on the anode may make a difference. This highlights some extensive study potentials of photoanodes with some hybrid catalytic systems.
In summary, this mini-review provides a comprehensive overview of representative advancements in interfacial modification strategies for Ta3N5 photoanodes, highlighting the crucial role of interfacial structure engineering in promoting charge separation, transfer and enhancement of PEC performance. We also provide suggestions for achieving good performance through interfacial modifications of Ta3N5 photoanodes. An in-depth understanding for the physical and chemical properties of interfacial layers and the structure-activity relationship can bring enlightenment for electrode design. High-performance photoanodes can be fabricated through developing interfacial materials with high charge mobility and suitable energetic matching, defect control and gradient doping of Ta3N5, as well as integrating with PV-PEC coupling systems, etc. We believe that the combination of interfacial modification and other strategies will be effective in achieving a high-performance Ta3N5 photoanode system for solar-to-fuel conversion and other energy, catalytic applications in the near future.
Author contributions
PW: Writing – review and editing, Supervision, Conceptualization, Investigation, Writing – original draft. AT: Project administration, Writing – review and editing, Validation. LC: Writing – review and editing, Validation. DZ: Supervision, Project administration, Writing – review and editing, Funding acquisition, Visualization.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was financially supported by the funding support from Boston College and Macquarie University Research Fellowships.
Acknowledgments
The authors would like to acknowledge financial support from Boston College and Macquarie University.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abdel Haleem, A., Majumder, S., Perumandla, N., Zahran, Z., and Naruta, Y. (2017). Enhanced performance of pristine Ta3N5 photoanodes for solar water splitting by modification with Fe-Ni-Co mixed-metal oxide cocatalysts. J. Phys. Chem. C 121 (37), 20093–20100. doi:10.1021/acs.jpcc.7b04403
Chen, S., Shen, S., Liu, G., Qi, Y., Zhang, F., and Li, C. (2015). Interface engineering of a CoOx/Ta3N5 photocatalyst for unprecedented water oxidation performance under visible-light-irradiation. Angew. Chem. Int. Ed. 54 (10), 3047–3051. doi:10.1002/anie.201409906
Chen, X., Shen, X., Shen, S., Reese, M. O., and Hu, S. (2020). Stable CdTe photoanodes with energetics matching those of a coating intermediate band. ACS Energy Lett. 5, 1865–1871. doi:10.1021/acsenergylett.0c00603
Cho, I. S., Lee, C. H., Feng, Y., Logar, M., Rao, P. M., Cai, L., et al. (2013). Codoping titanium dioxide nanowires with tungsten and carbon for enhanced photoelectrochemical performance. Nat. Commun. 4, 1723. doi:10.1038/ncomms2729
Chun, W., Ishikawa, A., Fujisawa, H., Takata, T., Kondo, J., Hara, M., et al. (2003). Conduction and valence band positions of Ta2O5, TaON, and Ta3N5 by UPS and electrochemical methods. J. Phys. Chem. B 107 (8), 1798–1803. doi:10.1021/jp027593f
Ding, C., Shi, J., Wang, Z., and Li, C. (2016). Photoelectrocatalytic water splitting: significance of cocatalysts, electrolyte, and interfaces. ACS Catal. 7 (1), 675–688. doi:10.1021/acscatal.6b03107
Dong, C., Zhang, X., Ding, Y., Zhang, Y., and Bi, Y. (2023). Unveiling the high activity origin of NiFe catalysts decorated Ta3N5 photoanodes for oxygen evolution reaction. Appl. Catal. B Environ. 338, 123055–123063. doi:10.1016/j.apcatb.2023.123055
Fan, G., Fang, T., Wang, X., Zhu, Y., Fu, H., Feng, J., et al. (2019). Interfacial effects on the band edges of Ta3N5 photoanodes in an aqueous environment: a theoretical view. iScience 13, 432–439. doi:10.1016/j.isci.2019.02.024
Fang, T., Huang, H., Feng, J., Hu, Y., Guo, Y., Zhang, S., et al. (2018). Exploring facile strategies for high-oxidation-state metal nitride synthesis: carbonate-assisted one-step synthesis of Ta3N5 films for solar water splitting. Sci. Bull. 63 (21), 1404–1410. doi:10.1016/j.scib.2018.10.005
Feng, S., Wang, T., Liu, B., Hu, C., Li, L., Zhao, Z., et al. (2020). Enriched surface oxygen vacancies of photoanodes by photoetching with enhanced charge separation. Angew. Chem. Int. Ed. 59 (5), 2044–2048. doi:10.1002/anie.201913295
Fu, J., Fan, Z., Nakabayashi, M., Ju, H., Pastukhova, N., Xiao, Y., et al. (2022). Interface engineering of Ta3N5 thin film photoanode for highly efficient photoelectrochemical water splitting. Nat. Commun. 13 (1), 729–737. doi:10.1038/s41467-022-28415-4
Haider, Z., Yim, H., Lee, H., and Kim, H. (2020). Surface and bulk modification for advanced electrode design in photoelectrochemical water splitting. Int. J. Hydrogen Energy 45 (10), 5793–5815. doi:10.1016/j.ijhydene.2019.08.114
He, Y., Hamann, T., and Wang, D. (2019). Thin film photoelectrodes for solar water splitting. Chem. Soc. Rev. 48 (7), 2182–2215. doi:10.1039/c8cs00868j
He, Y., Ma, P., Zhu, S., Liu, M., Dong, Q., Espano, J., et al. (2017). Photo-induced performance enhancement of tantalum nitride for solar water oxidation. Joule 1 (4), 831–842. doi:10.1016/j.joule.2017.09.005
He, Y., Thorne, J. E., Wu, C. H., Ma, P., Du, C., Dong, Q., et al. (2016). What limits the performance of Ta3N5 for solar water splitting? Chem 1 (4), 640–655. doi:10.1016/j.chempr.2016.09.006
Higashi, T., Nishiyama, H., Nandal, V., Pihosh, Y., Kawase, Y., Shoji, R., et al. (2022). Design of semitransparent tantalum nitride photoanode for efficient and durable solar water splitting. Energy Environ. Sci. 15 (11), 4761–4775. doi:10.1039/d2ee02090d
Higashi, T., Nishiyama, H., Suzuki, Y., Sasaki, Y., Hisatomi, T., Katayama, M., et al. (2019). Transparent Ta3N5 photoanodes for efficient oxygen evolution toward the development of tandem cells. Angew. Chem. Int. Ed. 58 (8), 2300–2304. doi:10.1002/anie.201812081
Ishikawa, A., Takata, T., Kondo, J., Hara, M., and Domen, K. (2004). Electrochemical behavior of thin Ta3N5 semiconductor film. J. Phys. Chem. B 108 (30), 11049–11053. doi:10.1021/jp048802u
Ismail, A., Garcia-Torregrosa, I., Vollenbroek, J., Folkertsma, L., Bomer, J., Haarman, T., et al. (2021). Detection of spontaneous FeOOH formation at the hematite/Ni(Fe)OOH interface during photoelectrochemical water splitting by operando X-ray absorption spectroscopy. ACS Catal. 11 (19), 12324–12335. doi:10.1021/acscatal.1c02566
Kim, J., Hansora, D., Sharma, P., Jang, J., and Lee, J. (2019). Toward practical solar hydrogen production-an artificial photosynthetic leaf-to-farm challenge. Chem. Soc. Rev. 48 (7), 1908–1971. doi:10.1039/c8cs00699g
Kim, J., and Lee, J. (2019). Elaborately Modified BiVO4 photoanodes for solar water splitting. Adv. Mater. 31 (20), e1806938. doi:10.1002/adma.201806938
Kim, W., Tachikawa, T., Monllor-Satoca, D., Kim, H.-i., Majima, T., and Choi, W. (2013). Promoting water photooxidation on transparent WO3 thin films using an alumina overlayer. Energy Environ. Sci. 6 (12), 3732–3739. doi:10.1039/c3ee42151a
Lee, S., Lee, T., Kim, C., Choi, M., Park, H., Choi, S., et al. (2019). Amorphous cobalt oxide nanowalls as catalyst and protection layers on n-type silicon for efficient photoelectrochemical water oxidation. ACS Catal. 10 (1), 420–429. doi:10.1021/acscatal.9b03899
Li, M., Luo, W., Cao, D., Zhao, X., Li, Z., Yu, T., et al. (2013b). A co-catalyst-loaded Ta3N5 photoanode with a high solar photocurrent for water splitting upon facile removal of the surface layer. Angew. Chem. Int. Ed. 52 (42), 11016–11020. doi:10.1002/anie.201305350
Li, Y., Takata, T., Cha, D., Takanabe, K., Minegishi, T., Kubota, J., et al. (2013d). Vertically aligned Ta3N5 nanorod arrays for solar-driven photoelectrochemical water splitting. Adv. Mater. 25 (1), 125–131. doi:10.1002/adma.201202582
Li, Y., Zhang, L., Torres-Pardo, A., Gonzalez-Calbet, J., Ma, Y., Oleynikov, P., et al. (2013c). Cobalt phosphate-modified barium-doped tantalum nitride nanorod photoanode with 1.5% solar energy conversion efficiency. Nat. Commun. 4, 2566. doi:10.1038/ncomms3566
Li, Z., Luo, W., Zhang, M., Feng, J., and Zou, Z. (2013a). Photoelectrochemical cells for solar hydrogen production: current state of promising photoelectrodes, methods to improve their properties, and outlook. Energy Environ. Sci. 6 (2), 347–370. doi:10.1039/c2ee22618a
Liao, M., Feng, J., Luo, W., Wang, Z., Zhang, J., Li, Z., et al. (2012). Co3O4 nanoparticles as robust water oxidation catalysts towards remarkably enhanced photostability of a Ta3N5 photoanode. Adv. Funct. Mater. 22 (14), 3066–3074. doi:10.1002/adfm.201102966
Liu, G., Fu, P., Zhou, L., Yan, P., Ding, C., Shi, J., et al. (2015). Efficient hole extraction from a hole-storage-layer-stabilized tantalum nitride photoanode for solar water splitting. Chem. Eur. J. 21 (27), 9624–9628. doi:10.1002/chem.201500745
Liu, G., Shi, J., Zhang, F., Chen, Z., Han, J., Ding, C., et al. (2014). A tantalum nitride photoanode modified with a hole-storage layer for highly stable solar water splitting. Angew. Chem. Int. Ed. 53 (28), 7295–7299. doi:10.1002/anie.201404697
Liu, G., Ye, S., Yan, P., Xiong, F., Fu, P., Wang, Z., et al. (2016). Enabling an integrated tantalum nitride photoanode to approach the theoretical photocurrent limit for solar water splitting. Energy Environ. Sci. 9 (4), 1327–1334. doi:10.1039/c5ee03802b
Liu, S., Dang, K., Wu, L., Bai, S., Zhang, Y., and Zhao, J. (2025). Nearly barrierless four-hole water oxidation catalysis on semiconductor photoanodes with high density of accumulated surface holes. J. Am. Chem. Soc. 147 (5), 4520–4530. doi:10.1021/jacs.4c16443
Minggu, L., Wan Daud, W., and Kassim, M. (2010). An overview of photocells and photoreactors for photoelectrochemical water splitting. Int. J. Hydrogen Energy 35 (11), 5233–5244. doi:10.1016/j.ijhydene.2010.02.133
Ning, X., Lu, B., Zhang, Z., Du, P., Ren, H., Shan, D., et al. (2019). An efficient strategy for boosting photogenerated charge separation by using porphyrins as interfacial charge mediators. Angew. Chem. Int. Ed. 58 (47), 16800–16805. doi:10.1002/anie.201908833
Nishiyama, H., Yamada, T., Nakabayashi, M., Maehara, Y., Yamaguchi, M., Kuromiya, Y., et al. (2021). Photocatalytic solar hydrogen production from water on a 100-m2 scale. Nature 598 (7880), 304–307. doi:10.1038/s41586-021-03907-3
Nurlaela, E., Nakabayashi, M., Kobayashi, Y., Shibata, N., Yamada, T., and Domen, K. (2020). Plasma-enhanced chemical vapor deposition Ta3N5 synthesis leading to high current density during PEC oxygen evolution. Sustain. Energy Fuels 4 (5), 2293–2300. doi:10.1039/c9se01319a
Nurlaela, E., Ould-Chikh, S., Harb, M., del Gobbo, S., Aouine, M., Puzenat, E., et al. (2014). Critical role of the semiconductor-electrolyte interface in photocatalytic performance for water-splitting reactions using Ta3N5 particles. Chem. Mater. 26 (16), 4812–4825. doi:10.1021/cm502015q
Pan, J., Wang, B., Shen, S., Chen, L., and Yin, S. (2023). Introducing bidirectional axial coordination into BiVO4@metal phthalocyanine core-shell photoanodes for efficient water oxidation. Angew. Chem. Int. Ed. 62 (38), e202307246. doi:10.1002/anie.202307246
Pihosh, Y., Minegishi, T., Nandal, V., Higashi, T., Katayama, M., Yamada, T., et al. (2020). Ta3N5-nanorods enabling highly efficient water oxidation via advantageous light harvesting and charge collection. Energy Environ. Sci. 13 (5), 1519–1530. doi:10.1039/d0ee00220h
Pihosh, Y., Nandal, V., Higashi, T., Shoji, R., Bekarevich, R., Nishiyama, H., et al. (2023). Tantalum nitride-enabled solar water splitting with efficiency above 10%. Adv. Energy Mater. 13 (36), 2301327–2301338. doi:10.1002/aenm.202301327
Seo, J., Nishiyama, H., Yamada, T., and Domen, K. (2018). Visible-light-responsive photoanodes for highly active, stable water oxidation. Angew. Chem. Int. Ed. 57 (28), 8396–8415. doi:10.1002/anie.201710873
Wang, C., Hisatomi, T., Minegishi, T., Nakabayashi, M., Shibata, N., Katayama, M., et al. (2016). Thin film transfer for the fabrication of tantalum nitride photoelectrodes with controllable layered structures for water splitting. Chem. Sci. 7 (9), 5821–5826. doi:10.1039/c6sc01763k
Wang, L., Dionigi, F., Nguyen, N., Kirchgeorg, R., Gliech, M., Grigorescu, S., et al. (2015b). Tantalum nitride nanorod arrays: introducing Ni-Fe layered double hydroxides as a cocatalyst strongly stabilizing photoanodes in water splitting. Chem. Mater. 27 (7), 2360–2366. doi:10.1021/cm503887t
Wang, L., Zhang, T., Su, J., and Guo, L. (2019b). Room-temperature photodeposition of conformal transition metal based cocatalysts on BiVO4 for enhanced photoelectrochemical water splitting. Nano Res. 13 (1), 231–237. doi:10.1007/s12274-019-2605-3
Wang, P., Ding, C., Deng, Y., Chi, H., Zheng, H., Liu, L., et al. (2023). Simultaneous improvement in hole storage and interfacial catalysis over Ni-Fe oxyhydroxide-modified tantalum nitride photoanodes. ACS Catal. 13 (4), 2647–2656. doi:10.1021/acscatal.2c06365
Wang, P., Ding, C., Li, D., Cao, Y., Li, Z., Wang, X., et al. (2022). Coupling effect between hole storage and interfacial charge transfer over ultrathin CoPi-modified hematite photoanodes. Dalton Trans. 51 (24), 9247–9255. doi:10.1039/d2dt00765g
Wang, P., Fu, P., Ma, J., Gao, Y., Li, Z., Wang, H., et al. (2021a). Ultrathin cobalt oxide interlayer facilitated hole storage for sustained water oxidation over composited tantalum nitride photoanodes. ACS Catal. 11 (20), 12736–12744. doi:10.1021/acscatal.1c03298
Wang, P., Li, D., Chi, H., Zhao, Y., Wang, J., Li, D., et al. (2021b). Unveiling the hydration structure of ferrihydrite for hole storage in photoelectrochemical water oxidation. Angew. Chem. Int. Ed. 60 (12), 6691–6698. doi:10.1002/anie.202014871
Wang, S., Jiao, M., Jian, J., Li, F., Zhang, Z., Wang, J., et al. (2025). Proton-acceptor interfered hydrolysis enabling highly stable FeOOH(α+β) cocatalysts for efficient photoelectrochemical water oxidation. Appl. Catal. B Environ. Energy 366, 125026–125036. doi:10.1016/j.apcatb.2025.125026
Wang, S., Liu, G., and Wang, L. (2019a). Crystal facet engineering of photoelectrodes for photoelectrochemical water splitting. Chem. Rev. 119 (8), 5192–5247. doi:10.1021/acs.chemrev.8b00584
Wang, Z., Liu, G., Ding, C., Chen, Z., Zhang, F., Shi, J., et al. (2015a). Synergetic effect of conjugated Ni(OH)2/IrO2 cocatalyst on titanium-doped hematite photoanode for solar water splitting. J. Phys. Chem. C 119 (34), 19607–19612. doi:10.1021/acs.jpcc.5b04892
Watanabe, E., Ushiyama, H., and Yamashita, K. (2017). First-principles study of the band diagrams and Schottky-type barrier heights of aqueous Ta3N5 interfaces. ACS Appl. Mater. Interfaces 9 (11), 9559–9566. doi:10.1021/acsami.6b12261
Wheeler, D., Wang, G., Ling, Y., Li, Y., and Zhang, J. (2012). Nanostructured hematite: synthesis, characterization, charge carrier dynamics, and photoelectrochemical properties. Energy Environ. Sci. 5, 6682–6702. doi:10.1039/c2ee00001f
Xiao, Y., Feng, C., Fu, J., Wang, F., Li, C., Kunzelmann, V., et al. (2020). Band structure engineering and defect control of Ta3N5 for efficient photoelectrochemical water oxidation. Nat. Catal. 3 (11), 932–940. doi:10.1038/s41929-020-00522-9
Xiao, Y., Fu, J., Pihosh, Y., Karmakar, K., Zhang, B., Domen, K., et al. (2025). Interface engineering for photoelectrochemical oxygen evolution reaction. Chem. Soc. Rev. 54 (3), 1268–1317. doi:10.1039/d4cs00309h
Yang, J., Kwon, H., Ji, S., Kim, J., Lee, S., Lee, T., et al. (2024). Conjugated polythiophene frameworks as a hole-selective layer on Ta3N5 photoanode for high-performance solar water oxidation. Adv. Funct. Mater. 34 (34), 2400806–2400818. doi:10.1002/adfm.202400806
Ye, S., Ding, C., Liu, M., Wang, A., Huang, Q., and Li, C. (2019). Water oxidation catalysts for artificial photosynthesis. Adv. Mater. 31 (50), e1902069. doi:10.1002/adma.201902069
Yokoyama, D., Hashiguchi, H., Maeda, K., Minegishi, T., Takata, T., Abe, R., et al. (2011). Ta3N5 photoanodes for water splitting prepared by sputtering. Thin Solid Films 519 (7), 2087–2092. doi:10.1016/j.tsf.2010.10.055
Yu, W., Feng, C., Li, R., Zhang, B., and Li, Y. (2025). Recent advances in tantalum nitride for photoelectrochemical water splitting. Chin. J. Catal. 68, 51–82. doi:10.1016/s1872-2067(24)60165-8
Yu, Z., Duan, Y., Feng, X., Yu, X., Gao, M., and Yu, S. (2021). Clean and affordable hydrogen fuel from alkaline water splitting: past, recent progress, and future prospects. Adv. Mater. 33 (31), e2007100. doi:10.1002/adma.202007100
Zachaus, C., Abdi, F., Peter, L., and van de Krol, R. (2017). Photocurrent of BiVO4 is limited by surface recombination, not surface catalysis. Chem. Sci. 8 (5), 3712–3719. doi:10.1039/c7sc00363c
Zhang, B., Wang, L., Zhang, Y., Ding, Y., and Bi, Y. (2018). Ultrathin FeOOH nanolayers with abundant oxygen vacancies on BiVO4 photoanodes for efficient water oxidation. Angew. Chem. Int. Ed. 57 (8), 2248–2252. doi:10.1002/anie.201712499
Zhang, P., Wang, T., and Gong, J. (2016). Passivation of surface states by ALD-grown TiO2 overlayers on Ta3N5 anodes for photoelectrochemical water oxidation. Chem. Commun. 52 (57), 8806–8809. doi:10.1039/c6cc03411j
Zhang, Z., Huang, X., Zhang, B., and Bi, Y. (2022). High-performance and stable BiVO4 photoanodes for solar water splitting via phosphorus-oxygen bonded FeNi catalysts. Energy Environ. Sci. 15 (7), 2867–2873. doi:10.1039/d2ee00936f
Keywords: tantalum nitride, interfacial modification, cocatalysts, hole storage, photoelectrocatalysis, water oxidation
Citation: Wang P, Than Oo AM, Chen L and Zhang D (2025) Recent advances of interfacial modification over tantalum nitride photoanodes for solar water oxidation: a mini review. Front. Chem. 13:1600959. doi: 10.3389/fchem.2025.1600959
Received: 27 March 2025; Accepted: 23 April 2025;
Published: 09 May 2025.
Edited by:
Zheng Li, University of Calgary, CanadaReviewed by:
Munkhbayar Batmunkh, Griffith University, AustraliaZhiliang Wang, The University of Queensland, Australia
Jiu Wang, University of British Columbia, Canada
Copyright © 2025 Wang, Than Oo, Chen and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Pengpeng Wang, cHB3YW5nMjAyMkBzaW5hbm8uYWMuY24=; Doudou Zhang, ZG91ZG91LnpoYW5nQG1xLmVkdS5hdQ==
 Pengpeng Wang
Pengpeng Wang Aman Maung Than Oo2
Aman Maung Than Oo2 Doudou Zhang
Doudou Zhang