- 1Department of Environmental Sciences, University of Haripur, Haripur, Pakistan
- 2Department of Environmental Sciences, COMSATS University Islamabad, Abbottabad, Pakistan
- 3Department of Civil Engineering, College of Engineering, University of Hail, Ha’il, Saudi Arabia
- 4“Ioan Haulica” Institute, Apollonia University, Iasi, Romania
- 5Department of Biology, Faculty of Biology, “Alexandru Ioan Cuza” University of Iasi, Iasi, Romania
- 6CENEMED Platform for Interdisciplinary Research, University of Medicine and Pharmacy “Grigore T. Pop”, Iasi, Romania
- 7“Olga Necrasov” Center, Depart. of Biomedical Research, Romanian Academy, Iasi, Romania
- 8Academy of Romanian Scientists, Bucuresti, Romania
- 9Department of Environmental Science, Abdul Wali Khan University Mardan, Mardan, Pakistan
Chromium (Cr(VI)) is often released from various industries in excess in developing countries, which constitutes non-compliance with environmental regulations. This metal is hazardous for the aquatic ecosystem and is responsible for toxicity, carcinogenicity, and mutagenicity in humans. Adsorption is an effective and relatively inexpensive approach for treating the excess Cr(VI) compared to conventional methods. Commercially available adsorbents cannot be considered economical yet for industrial applications, which has alternatively resulted in the use of natural adsorbents. The current study focuses on Cr(VI) removal using low-cost natural adsorbents and discusses the different conditions used for such treatments. Previous studies have shown the following order of average Cr(VI) removal using different adsorbents: leaves > bark > agriculture > dry shell > tea = fungi > yeast > algae > sawdust > bacteria. Moreover, acid modification has been reported to offer the best results. The adsorption data are best fitted to both the Langmuir and Freundlich isotherms. Hence, the abovementioned low-cost natural biomasses hold promising potential for Cr(VI) removal from wastewater, whose removal efficiency can be improved by adopting economical and effective pretreatment techniques. Literature also shows that leaves are more efficient and economical for Cr(VI) removal without pretreatment from among the various available bulk biomasses. The present review is expected to provide guidance for low-cost treatment of Cr(VI) at the industrial scale.
1 Introduction
The presence of toxic metals in industrial wastewater, marine water, and freshwater has become a global environmental issue (Ajmal et al., 2003; Kargar et al., 2011; Mzoughi and Chouba, 2011; Zou et al., 2019). Heavy metals poses serious threats to streams and lakes (Ghaderi et al., 2012; Alimohammad Kalhori et al., 2011; Ashraf et al., 2011; Hashem et al., 2017; Tripathi et al., 2024). Among the various heavy metals, Cr is often released into water bodies in the form of industrial wastewater. Over the past few decades, large quantities of wastes containing Cr have been directly discharged into the environment without any treatments (Qiang et al., 2011). The toxicity of Cr varies with its oxidation state. In natural water, Cr usually exists in two main oxidation states, namely, trivalent chromium (Cr(III)) and hexavalent chromium (Cr(VI)) (Sharma et al., 2022). Compared to Cr(III), Cr(VI) is 100–1000 times more toxic to organisms (Roşca et al., 2023; Chanda et al., 2024) because it has strong oxidizing ability and mobility; it is absorbed through the skin and also readily transported into the soil (Karthikeyan et al., 2005; Demirbas et al., 2008).
Cr(VI) is included in the priority list of hazardous substances of the Comprehensive Environmental Response Compensation and Liability Act (CERCLA) (Moussavi and Barikbin, 2010; Peng et al., 2019). Cr(VI) is used in various industries, and the major industrial sources of Cr(VI) in wastewater are tanning, metal processing, electroplating, wood preservative, pigment, paint, steel fabrication, textile, dyeing, and canning industries, as shown in Figure 1 (Demirbas et al., 2008; Moussavi and Barikbin, 2010; Singh et al., 2022). According to the US Environmental Protection Agency, the permissible limits for Cr(VI) are 0.1 and 0.05 mg L−1 in inland surface water and drinking water, respectively (Qiang et al., 2011; Brusseau and Artiola, 2019).
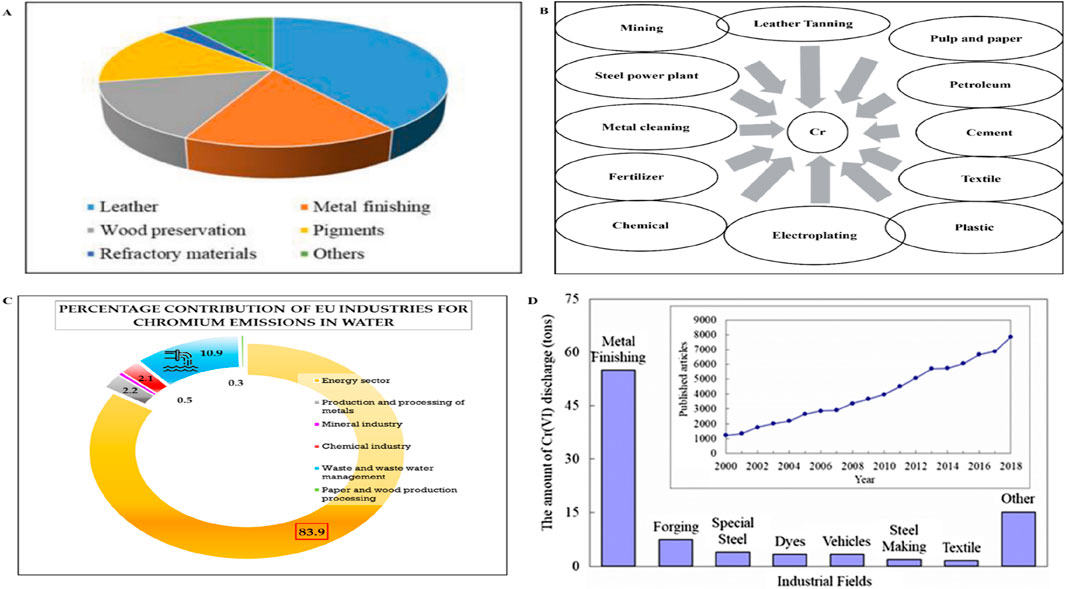
Figure 1. (A) Cr(VI) consumption in various industries (Kumar et al., 2024); (B) different industrial sources contributing to Cr(VI) pollution in water; (C) percentage contributions of EU industries to Cr in water (Tumolo et al., 2020); (D) Cr(VI) discharge from various industries (Xia et al., 2020).
It has been shown that excess intake of Cr by humans results in many diseases (Figure 2), such as damage of the digestive tract, lung cancer, gastrointestinal issues, central nervous system irritation, capillary damage, and hepatic and renal damage (Martins et al., 2006; Kiran et al., 2007; Wan Ngah and Hanafiah, 2008; Boyle et al., 2025). Different methods are available for the treatment of Cr(VI), such as ion exchange (Wang and Lin 2008; Yuan et al., 2008; Kim et al., 2008; Li et al., 2017), ultrafiltration (Muthumareeswaran et al., 2017), nanofiltration, microfiltration (Chen et al., 2010; Zolfaghari and Kargar, 2019), chemical precipitation (Peng et al., 2018), electrochemical reduction (Peng et al., 2019), solvent extraction, reverse osmosis (Çimen, 2015), cementation, electrodialysis, electrocoagulation (Muhammad et al., 2006; Ahmet and Tuzen, 2008; Tarun et al., 2008; Cristina et al., 2009; Dang et al., 2009; Sibel et al., 2009; Zainul et al., 2009; Suresh et al., 2010), and adsorption (Monser and Adhoum, 2002; Erdem et al., 2004; Cherdchoo et al., 2019; Sirusbakht et al., 2018; Haroon et al., 2017). These methods are effective for concentrations of 1–100 mg L−1; however, they involve high operating and capital costs (Liang et al., 2009). The waste generated after chemical precipitation also pose disposal pollution risks and hazards to the environment. Hence, efficient and cost-effective alternative methods need to be developed for scale-up applications (Oilgae, 2010).
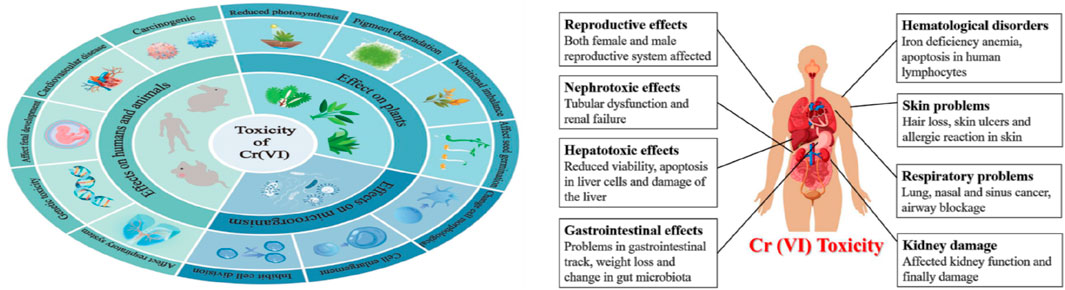
Figure 2. Toxicity of Cr(VI) on various living organisms (Zeng et al., 2025).
Metal removal using adsorbents has distinct advantages over other conventional methods. Adsorbents are usually inexpensive, efficient (Sheraz et al., 2024), and prone to producing lower quantities of sludge after treatment (Sud et al., 2008; Satyam and Patra, 2024). Moreover, another advantage of adsorbent-based methods is that the statutory discharge limits for industrial wastewater, including enhancement of their environmental responsibility and corporate social profiles, are relatively easier to meet. Various adsorbents can be used for metal removal, such as those based on nanoparticles (Rong et al., 2024; Wenjie et al., 2022; Tesnim et al., 2024), nanocomposites (Katiyar and Katiyar, 2024), chitosan-based composites (Zhou et al., 2025), CeO2-based functional materials (Zhang et al., 2025), and deep-eutectic-solvent-assisted functional materials (Nie et al., 2025). Many researchers have used agriculture feedstock for the treatment of heavy metal pollutants (Odoemelam et al., 2011; Gupta et al., 2009; Subbaiah et al., 2011; Opeolu, 2009), in addition to abundantly available organic materials. Agricultural or lignocellulosic products (Figure 3) have unique chemical compositions (Lee et al., 2014; Baruah et al., 2018) and relatively high numbers of surface functional groups (i.e., phenolic and carboxylic groups) that enable successful uptake of metals from wastes and are also useful for removing Cr(VI) from wastewater (Harmon et al., 2007; Opeolu et al., 2010). Chemical binding sites comprising carboxyl (COO), carbonyl (CO), hydroxyl (OH), amine (NH2), or amide (RC (O) NH2) groups are mostly present on biomass surfaces (Barriada et al., 2009). Polar-functional-group-containing compounds are also available in agricultural byproducts, such as alcohols (R-OH), aldehydes (HCOH), ketones (RCOR), carboxylates (RCOO), phenols (C6H5-OH), and ethers (R-O-R); these bind heavy metals through hydrogen replacement or complex formation by electron pair donation (Ofomaja and Ho, 2007). Different activated and non-activated bioadsorbents, including pine wood sawdust (Sirusbakht et al., 2018), Indian jujube (Ahmad et al., 2017), coffee pulp (Gómez Aguilar et al., 2019), coffee grounds (Cherdchoo et al., 2019), almond green hulls (Nasseh et al., 2016), groundnut shells (Bayuo et al., 2019), palm kernel shells (Razavi Mehr et al., 2019), Arachis hypogea (peanut) leaves (Ahmad et al., 2017), green moringa tea leaves (Timbo et al., 2017), Cassia fistula (Amaltas) leaves (Ahmad et al., 2017), Euclea schimperi leaves (Gebrehawaria et al., 2015), Acacia albida bark (Gebrehawaria et al., 2015), Eucalyptus camaldulensis sawdust (Haroon et al., 2016), sugarcane bagasse (Homagaia et al., 2010), white cedar sawdust (Haroon et al., 2017), and pea seed shells (Kebede et al., 2022), have been used as economical alternatives to expensive adsorbents.
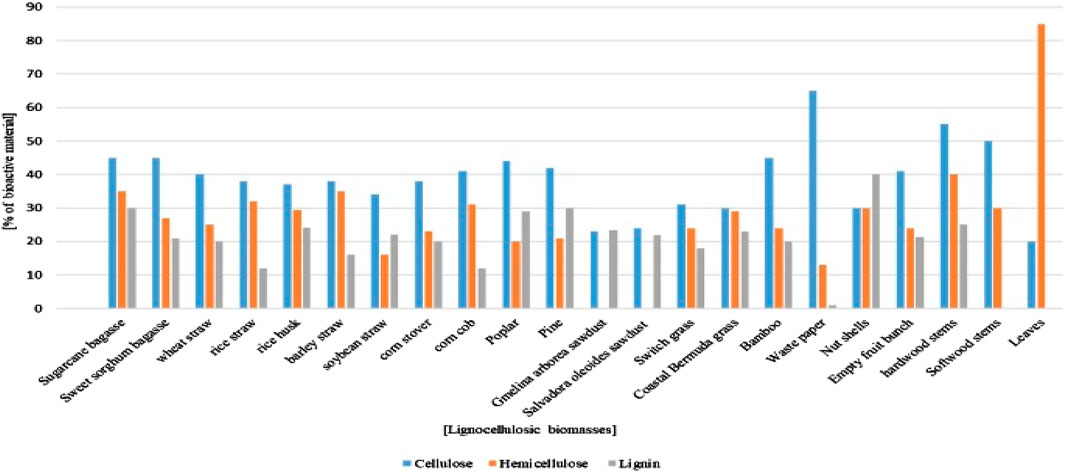
Figure 3. Percentage compositions of various lignocellulosic biomasses (Lee et al., 2014; Baruah et al., 2018).
Several agricultural byproducts and natural materials have been explored for Cr(VI) adsorption, but a critical gap remains in systematically comparing their effectiveness and evaluating the impacts of chemical modifications. The present review addresses this gap by offering detailed comparisons of a wide range of unmodified and chemically modified natural adsorbents for Cr(VI) removal. As a pioneering effort, we compile and analyze the Cr(VI) removal potentials of various natural biomasses in this study, including leaves, bark, shells, and agricultural residues, as well as rank them based on performance. Moreover, this review uniquely emphasizes the roles of chemical modifications, including acids, bases, and salts, in enhancing the adsorption capacities of various natural organic (lignocellulosic) materials. In highlighting these novel features, we not only combine various sources available in literature but also propose a framework for future directions for developing cost-effective, scalable, and sustainable treatment technologies using natural organic adsorbents.
2 Adsorption of Cr(VI) using various waste biomasses
2.1 Sawdust
Aspen sawdust is a lignocellulosic solid waste that is reported to be an effective adsorbent of Cr(VI) from industrial effluents. The relationship between the adsorption capacity and temperature reflects the endothermic nature of the adsorption process. The data display a good fit for the Freundlich isotherm rather than the Temkin and Langmuir isotherms, revealing the homogeneous and monolayer adsorption process. It was observed that Cr(VI) removal increased with the adsorbent dose (3 g) because of the availability of more adsorbent (sawdust) surface. The maximum removal of Cr(VI) was noted at a pH of 2, which is attributed to the large number of positive hydrogen ions on the adsorbent surface, resulting in more electrostatic attraction between the positive adsorbent and anionic Cr. The sawdust of other types of woods used in buildings and the furniture industry is available abundantly (Mosavian et al., 2012). Neem and mango sawdust showed 80% and 60% adsorption for Cr(VI) removal, respectively, at an acidic pH of 2; this is owed to the more positive charges in the aqueous media, which makes the adsorbent surface positive and results in the attraction of more negative ions of Cr toward the sawdust adsorbent surface. Cr(VI) removal using neem sawdust revealed the best fitting to the Langmuir isotherm and followed a pseudo-second-order kinetics (Vinodhini and Das, 2010), revealing the monolayer and homogeneous surface of the adsorbent as well as chemosorption nature of the adsorption process. Studies have shown that when the free ionic sites are occupied by anionic species like HCrO4−, CrO4−2, and Cr2O72-, the adsorption on the adsorbent surface decreases at pH values above 4 (Das et al., 2000; Dönmez and Aksu, 2002). This concept is further supported by the findings of Popuri (2007), who showed the competition between the oxyanion form of Cr and OH−, where the adsorption of Cr decreased at high pH. Politi and Sidiras (2012) observed 95.6% removal potential of Cr using pine sawdust at pH 1.2; they reported that the percentage removal increased linearly with the initial amount of Cr(VI) and decrease in pH.
In another study, neem sawdust was used for Cr(VI) treatment in a fixed bed column; here, maximum removal was observed at pH 2 due to protonation of the adsorbent surface and its electrostatic attraction to the anionic chromate ions. However, at higher pH, Cr(VI) removal decreased because of the competition between hydroxyl ions and oxyanions of Cr. The effects of co-ions on Cr(VI) removal were also studied, and it was found that cations (Na+ and Mg2+) did not affect adsorption, while anions (Cl−) interfered with adsorption by competing with the Cr species. The surface area of the neem sawdust was reported to be 3.76 m2 g−1; it was found that the adsorption data were well fitted to the bed depth service time (BDST) model and that the estimated Cr(VI) uptake capacity was 33,009.9 mg g−1; the column breakthrough time also decreased in the presence of co-ions (Vinodhini and Das, 2010).
White cedar sawdust has been reported to aid Cr(VI) removal by up to 62% under optimum conditions (Haroon et al., 2017). Ziziphus mauritiana (Indian jujube) sawdust also showed a monolayer adsorption capacity of 3.66 mg g−1 for Cr(VI) removal (Ilyas et al., 2014; Ahmad et al., 2017). Pine wood sawdust with a Brunauer–Emmett–Teller (BET) surface area of 0.434 m2 g−1 also revealed maximum Cr(VI) adsorption at acidic pH (Sirusbakht et al., 2018). E. camaldulensis sawdust (ECS) has been explored for Cr(VI) removal in both batch and column experiments. In the batch study, ECS showed a maximum uptake of 35.58 mg g−1 at pH = 2 through chemisorption, as confirmed by Cr(VI) desorption of only 15% with potassium hydroxide. The column study revealed that the experimental data best fitted the BDST model with an adsorption capacity of 2,443.636 mg L−1 at 60% breakthrough (Haroon et al., 2016). Local market-generated sawdust has also been shown to allow a maximum Cr(VI) removal of 96.4% (Erwa and Ibrahim, 2016). A comparison of all plant-derived biomasses used for Cr(VI) adsorption is given in Table 1.
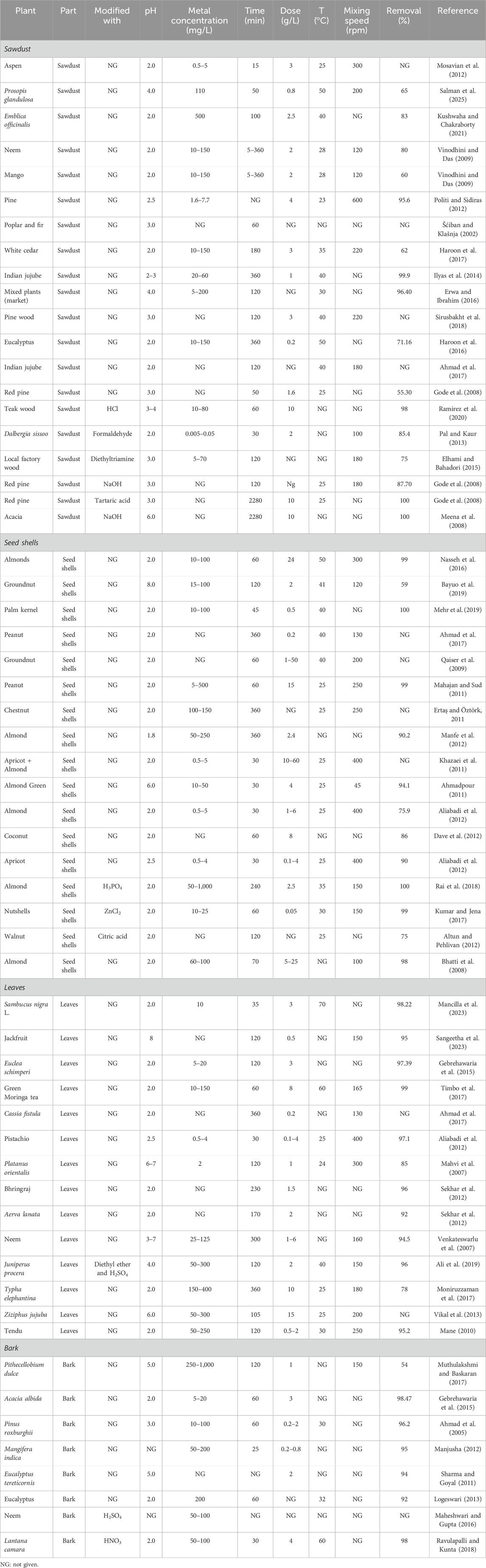
Table 1. Comparative analysis of the process variables for the adsorption of Cr (VI) using various plant-derived adsorbents.
2.2 Tea waste
Dave et al. (2012) showed the Cr(VI) removal potential of tea waste in a batch experiment; here, the equilibrium was established at 60 min when using 8 g of the adsorbent, and the highest uptake capacity was reported as 34.25 mg g−1 at a low pH of 2. Furthermore, the data were best fitted to the Lagergren equation. Dizadji et al. (2011) also reported the use of brewed tea waste for adsorption of Cr(VI), where the metal uptake was reportedly maximum at a pH of 2 (adsorption capacity = 19 mg g−1); this confirmed the electrostatic attraction mechanism for Cr(VI) adsorption. The experimental data followed pseudo-second-order kinetics with R2 > 0.99, showing chemisorption and formation of strong covalent bonds between the adsorbent and adsorbate. Attia (2012) compared decolorized cooking tea waste (CTW) with commercially available activated carbon (AC) for Cr(VI) treatment in batch experiments. Both adsorbents were found to be highly dependent on the pH, with the maximum adsorption (85.6 mg g−1 for AC and 89.24 mg g−1 for CTW) occurring at a low pH of 2.0. At a pH of 12, the percentage efficiencies decreased to 18 and 11 mg g−1 for AC and CTW, respectively, because of competition between the hydroxyl and chromate anions. Equilibrium was established at 3 h, and the data were better fitted to the Freundlich adsorption model than Langmuir, with R2 values of 0.974 and 0.991 for CTW and AC, respectively. Ion diffusion was the dominant mechanism in the adsorption process. At 20°C, 30°C, and 40°C, Cr(VI) removal by AC were 74%, 73%, and 72.9% and that by CTW were 81.2%, 74.4%, and 73%, respectively. At an adsorbent dose of 0.9 g L−1 and 50 mg L−1of Cr(VI), the Cr(VI) treatment efficiency with AC was 99.4% and that with CTW was 99.5%. The percentage efficiencies of Cr(VI) with AC and CTW reduced from 90.6% to 50.4% and 94.8% to 53.6%, respectively, when Cr(VI) increased from 25 to 300 mg L−1; however, the metal uptake capacities of AC and CTW increased from 4.5 to 30.26 mg g−1 and from 4.74 to 32.16 mg g−1, respectively. The efficiencies decreased owing to less diffusion of ions. Both adsorbents followed pseudo-second-order kinetics, where CTW was more efficient than AC (Attia, 2012). Ahalya et al. (2010) investigated the potential of coffee husk as an alternative material for Cr(VI) adsorption; the experimental findings were fitted well to both the Langmuir and Freundlich isotherms; the Cr(VI) uptake capacity calculated for the Langmuir model was 44.95 mg g−1. Infrared spectral studies confirmed that the hydroxyl and carboxyl functional groups on the surface of coffee husk participated in the adsorption of Cr(VI) (Yadav et al., 2013).
Coffee pulp (Castilla variety) was also investigated and showed up to 87.94% removal of Cr(VI), where the point of zero charge (PZC) was found to be 3.95. The experimental data confirmed monolayer adsorption of the adsorbate. Fourier-transform infrared (FTIR) spectroscopy confirmed the presence of OH, CH, CH2, C=O, and C-O-C bonds associated with the adsorption mechanism. Coffee pulp was thus concluded to be an economical solution for wastewater treatment because of its low cost of implementation and maintenance without sludge production (Gómez Aguilar et al., 2019). Coffee grounds and mixed tea waste were also explored as alternatives for sequestration of Cr(VI); here, FTIR studies revealed the involvement of carbon and oxygen functional groups in both adsorbents. The maximum adsorption capacities of coffee grounds and mixed tea waste were 87.72 mg g−1 and 94.34 mg g−1, respectively. Both biomasses can be reused up to four times with more than 50% reusable efficiency. The efficiency of mixed tea waste was also assessed in a packed reactor, which revealed a breakthrough time of 30 min for 100 mg L–1 of Cr(VI) (Cherdchoo et al., 2019).
2.3 Dry shells
Groundnuts are a type of agricultural product that is often available in bulk. Here, only the seeds are used, while the shells are wasted. The potential of groundnut shells to remove Cr(VI) has been studied by various researchers. Qaiser et al. (2009) used groundnut hulls for Cr(VI) adsorption and reported a maximum uptake capacity of 30.21 mg g−1. According to Mahajan and Sud (2011), A. hypogea shells removed almost 95% of Cr(VI) from synthetic wastewater at pH = 2; their experimental results fitted the Freundlich and Langmuir models well and followed pseudo-second-order kinetics. Ertaş and Öztürk (2011) investigated the potential of chestnut shells for removing Cr(VI) from synthetic solutions and reported an adsorption capacity of 9.47 mg g−1; here, the Langmuir isotherm was noted to be a better fit than the Freundlich isotherm. Cellulose, hemicellulose, and lignin are the important components of various plant and fruit shells that are responsible for Cr(VI) adsorption. The Cr(VI) removal potential of Prunus amygdalus (almond) shell from polluted wastewater was explored in a batch experiment (Manfe et al., 2012); this study showed that increasing the Cr(VI) solution concentration from 50 to 250 mg L−1 decreased the percentage removal from 80.1% to 47.1% and that increasing the adsorbent quantity from 0.8 to 2.4 g L−1 increased the percentage adsorption from 55.1% to 90.2%. Kali et al. (2025) observed in the case of almond shells that the maximum adsorption of Cr(VI) occurred at pH = 2 because of protonation of the shell surface (Figure 4), which indicated electrostatic attraction between the chromium anions and almond shell. Later, the electron–donor functional groups present on the almond shell surface reduced Cr(VI) to Cr(III).
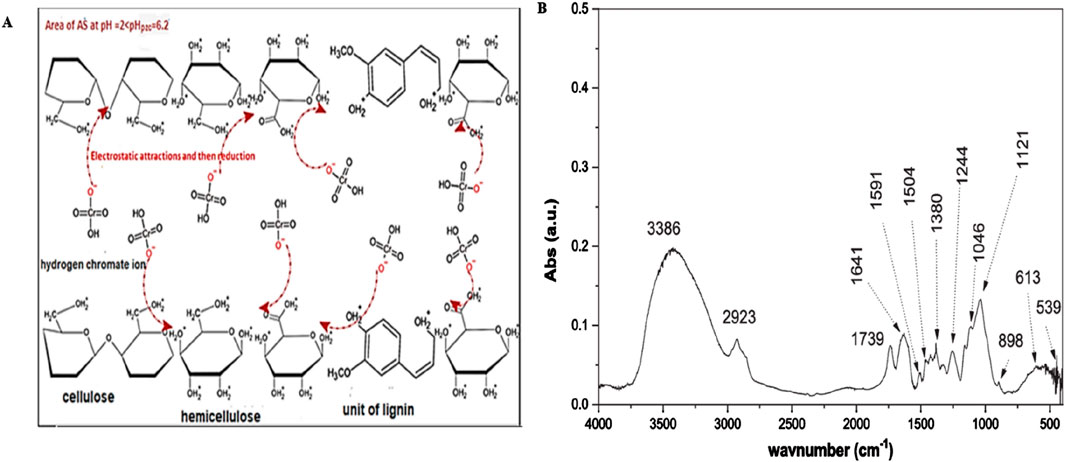
Figure 4. Schematic showing the (A) removal mechanism of Cr (VI) and (B) Fourier-transform infrared spectrum of almond shell (Kali et al., 2025).
Wahiba et al. (2025) used pinecone powder (PCP) for Cr(VI) adsorption and noted that Cr(VI) was removed through the binding of anionic HCrO4− to the positively charged PCP surface as well as surface complexation. In an acidic medium, Cr(VI) ions are reduced to Cr(III) ions by the electron–donor groups on the adsorbent surface, where the acidic pH provides a large number of H+ sites for the reduction of Cr(VI). Khazaei et al. (2011) noted that Cr(VI) removal increased directly with the adsorbent dose when using almond and apricot shells as the adsorbents because of the increased availability of binding sites on the biomass surface. The results of batch experiments revealed that the maximum adsorption occurred at a low pH of 2. Specifically, at this pH, the Cr compounds form more HCrO4−, which are strongly attracted to the active sites. Aliabadi et al. (2012) also reported the use of almond shells for Cr(VI) treatment in a batch reactor; they recorded a high uptake at pH 2.0 and 30 min of contact time. The data were well fitted to the Freundlich isotherm and followed second-order rate kinetics. The adsorbent active sites acquired positive charges because of the excess hydrogen ions at pH 2.0, leading to strong attractions between HCrO−4 and the positively charged sites.
Almond nut shells showed 90% removal at a pH of 1.8, while almond green hulls offer the best removal of 99.6% at pH = 2 and 94% at pH = 6. This reduction in adsorption is because of the lower electrostatic attraction between the adsorbent and adsorbate. Different functional groups like amine, carboxyl, and hydroxyl groups are present on the surface of almond green hulls, which are responsible for Cr(VI) removal (Sahranavard et al., 2011). Almond green hull powder showed a maximum adsorption capacity of 10.123 mg g−1 at a pH of 2. The Dubinin–Raduskevich (D-R) model revealed that Cr(VI) adsorption was physical in nature as the value of E is less than 8 kJ mol–1 (Nasseh et al., 2016). Groundnut shells also showed maximum adsorption at optimum time, adsorbent dosage, pH, and temperature values of 120 min, 2 g L–1, 8, and 41.5°C, respectively. Among the four tested isotherm models, the adsorption data were best fitted to the Temkin model. The FTIR spectra revealed that –OH and –C–O groups were involved in the adsorption on groundnut shells (Bayuo et al., 2019). The adsorption potential of palm kernel shell was also investigated, and it was observed that the adsorption increased with decrease in pH and that the maximum adsorption was attained at a pH of 2. The BET surface area was reported to be 121.82 m2 g−1, and the adsorption data were well fitted to the Freundlich isotherm with a maximum adsorption capacity of 125 mg g−1 (Mehr et al., 2019). A. hypogea shells also revealed a monolayer adsorption capacity of 4.32 mg g−1, where the surface area and pore volume were found to be 1.78 m2 g−1 and 0.003 cm3 g−1, respectively (Ahmad et al., 2017).
2.4 Leaves
The leaves of various trees constitute the bulk of plant waste that can be used for the removal of different contaminants. The leaves of the mulberry, acacia, poplar, and other trees contain cellulose and hemicelluloses that are effective for adsorbing different pollutants from wastewater. Aliabadi et al. (2012) developed adsorbents from the leaves of the apricot and pistachio trees and tested their potential for percentage of Cr(VI) adsorption; this study revealed maximum Cr(VI) adsorption potentials of 97.1 mg g−1 at a pH of 2.0 for pistachio and 90.1 mg g−1 for apricot tree leaves in approximately 30 min. At acidic pH, the H+ ions present on the surface of the adsorbent increase, resulting in strong attractive forces between the positively charged adsorbent surface and chromate ions. At pH greater than 6.0, there is competition between the chromate and OH− anions for adsorption on the adsorbent surface, which the hydroxyl ions tend to dominate, thus decreasing adsorption. The experimental data were fitted well to the Freundlich equation compared to the Temkin and Langmuir equations. Sekhar et al. (2012) reported the Cr(VI) removal potentials of the leaf powders of Bhringraj, Aerva lanata, and Trileafa portulacastrum as 96.0%, 92.0%, and 84.0%, respectively, where the equilibrium was established in 3.5 h. Lignocellulosic materials like leaves have −OH/−COOH surface functional groups whose dissociations depend on the pH. Their surfaces are negatively charged at high pH and attract cations, whereas the surfaces are positively charged at low pH due to protonation and show affinities toward weak anions. Moringa leaf powder was shown to remove Cr(VI) by up to 90% at an equilibrium time of 90 min (Madhuranthakam et al., 2021). Aegle marmelos leaves also showed almost 86.6% removal of Cr(VI) at a pH of 2 (Mathai et al., 2022). Reddy et al. (2012) explored the potentials of the leaves and ashes of Tephrosia purpurea and Solanum nigrum for Cr(VI) removal from a synthetic solution in a batch experiment at low pH. Here, the physical activation was achieved at 105°C in an oven, following which 84.0% and 88.0% extractions of Cr(VI) were observed with the leaf powder and ash of T. purpurea, respectively, along with 100% removal with both the leaf powder and ash of S. nigrum. Saueprasearsit (2011) investigated durian peel for Cr(VI) removal and concluded that the optimum conditions for maximum Cr removal (10.67 mg g−1) were pH of 2, Cr(VI) solution concentration of 75 mg L−1, and contact time of 30 min. The Langmuir model and second-order kinetics were best fitted to the experimental results when desorption was carried out using 1M of HCl. Gopalakrishnan et al. (2013) reported the use of neem (Azadirachta indica) leaf powder for Cr(VI) adsorption; their data best followed the Freundlich adsorption isotherm, and the equilibrium time was 3 h. Neem leaves contain polar groups like –NH2, –COOH, and –OH, which act as adsorption sites for the adsorbate. Haleem and Abdulgafoor (2010) analyzed the utility of date palm fibers (leaf) for Cr(VI) adsorption in terms of different parameters like the metal solution concentration, pH, sorbent amount, and contact time; they found that the process was pH-dependent and that the data were best fitted to the Langmuir isotherm with a correlation coefficient of 0.908. Their results showed 98.7% efficiency for Cr(VI) removal using 5 g of the biomass, i.e., date palm leaves.
Pine leaves, pine needles, and pine sawdust have also been used for Cr treatment in batch and column studies (Aliabadi et al., 2006; Hadjmohammadi et al., 2011) (Figure 5). Powdered rubber leaf was also explored for Cr(VI) removal potential in batch and fixed column studies. The important functional groups involved in adsorption were amines, alkenes, carboxyl, and aromatic acids. The exhausted adsorbent was regenerated using 0.5 N NaOH, and the percentage removal of Cr(VI) by the regenerated rubber leaf powder dropped to 91.57% compared to 97.0% with the fresh adsorbent. The regenerated adsorbent could be used up to three times, but the adsorption capacity reduced to 79.4% at the end of the third cycle. The column study revealed that as the flow rate increased from 5 to 20 mL min−1, the percentage removal decreased from 100% to 26.89%; this is attributed to the low retention time of the adsorbate with the adsorbent. The experimental data were best fitted to the Yoon–Nelson model than the Thomas model (Nag et al., 2016).
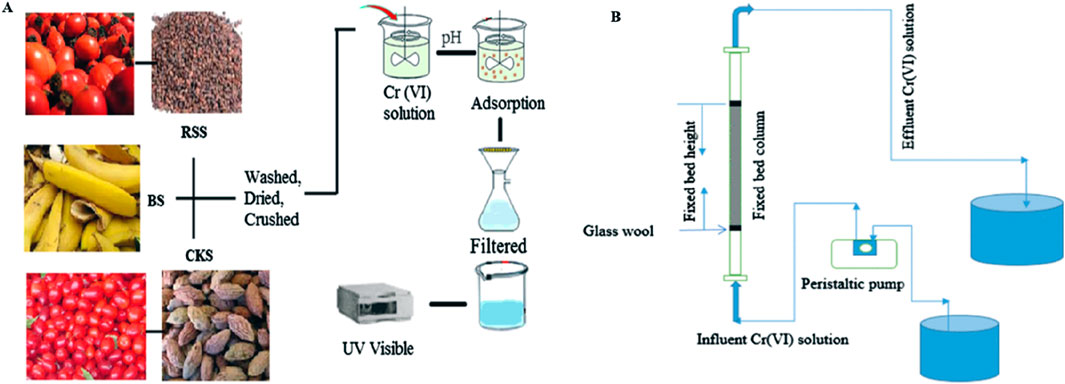
Figure 5. (A) Batch and (B) column experiment setups for the adsorption studies (Parlayici and Pehlivan, 2019; Abdu et al., 2024).
Both raw and sulfuric-acid-modified leaves of Ruellia patula Jacq have been evaluated for their removal efficiencies of Cr(VI); here, the Langmuir adsorption capacity was higher for the modified adsorbent (62.50 mg g−1) than the raw form (37.03 mg g−1). Desorption was carried out using sodium hydroxide and offered the best results for the first three cycles (Saranya et al., 2017). Okra leaves have also been investigated for Cr(VI) removal, where the maximum adsorption capacity of 81.94 mg g−1 was observed at pH = 2. From a sample of industrial wastewater, okra leaves were noted to adsorb approximately 92.15% of Cr. A regeneration study was also conducted using different chemicals, which showed that 96% of the adsorbent ions could be recovered using up to 5 mL of 1M HCl solution. An interference study revealed that in the presence of Na2C2O4, Cr(VI) adsorption increased by up to 6%. FTIR studies have specified the involvement of hydroxyl, oxime, and carboxylic acid groups in adsorption (Khaskheli et al., 2016). Waste material like the leaves of green tea have also shown the highest percentage removal (99%) of Cr(VI). A desorption study showed that 0.1 N HCl had the best performance among four different solutions. Jeyaseelan and Gupta (2016) showed through an interference study that Cd and Zn allow increased adsorption of Cr(VI) owing to their increased surface areas, whereas Cu, Ni, and Fe decrease Cr(VI) adsorption through competition among the various ions.
E. schimperi leaves showed a maximum adsorption of 97.39% at an optimum pH of 2, where the Langmuir isotherm best described the adsorption of Cr(VI) (Gebrehawaria et al., 2015). Green moringa tea leaves were used to accomplish a Cr(VI) uptake capacity of 33.9 mg g−1 at a pH of 2 with an initial adsorbate concentration of 100 mg L−1; here, the equilibrium data were found to be best fitted to the Freundlich isotherm (Timbo et al., 2017). C. fistula leaves were found to have a maximum monolayer adsorption of 4.48 mg g−1 at an equilibrium time of 360 min, where the surface area of the leaves was 1.09 m2 g−1 (Ahmad et al., 2017). FTIR spectrum of the Typha elephantina Roxb (Hogla) leaves indicates that stretching of the OH, O=C=O, C–H, and C=C bonds are responsible for Cr(VI) removal; treatment of industrial effluents with hogla leaves was reported to decrease adsorption from 78.7% to 44.8% owing to the presence of other contaminants (dyes and metal ions) in addition to Cr(VI) in the effluent (Moniruzzaman et al., 2017).
2.5 Agricultural waste
Naiya et al. (2011) investigated different low-cost agricultural wastes like rice straw, bran, and husk for Cr(VI) adsorption. Characterization of the raw and Cr(VI)-loaded adsorbents by FTIR spectroscopy revealed the presence of hydroxyl, alkene, aromatic, nitro, silicon oxide, and carboxylate anion groups on the adsorbents that were responsible for the uptake of metals from wastewater. Biomass-based natural waste materials follow the complex process of Cr(VI) adsorption through several mechanisms, including electrostatic attraction, reduction, and ion exchange (Assefa et al., 2024). Agricultural waste like crop residues undergo ion exchange through the interactions of various functional groups (amine, hydroxyl, and carboxyl) on the adsorbent surface with the Cr(VI) anions. The solution pH plays a critical role in the exchange of these functional group cations with Cr(VI) anions (Njoya et al., 2021). The surfaces of organic waste become more attractive to Cr(VI) anions at acidic pH values owing to protonation, as observed by Gning et al. (2024); however, deprotonation of the adsorbent functional groups occurs at basic or higher pH values, resulting in reduced ion exchange (Xie et al., 2021).
Cr(VI) can be reduced to Cr(III) by direct or indirect reduction, as shown in Figure 6. When the pH is low, direct reduction of Cr(VI) to Cr(III) is observed because of its higher reduction potential than the functional groups present on the adsorbent surface, which results in bond formation of Cr(III) with the adsorbent functional groups. On the other hand, three steps are involved in indirect reduction. First, oxoanions of Cr(VI) are adsorbed on the protonated functional groups like amino and carboxyl groups; second, Cr(VI) is reduced to Cr(III) with the help of the electron-donating functional groups on the adsorbent surface; third, Cr(III) forms complexes with the adsorbent functional groups or is repulsed by the electron-rich groups on the adsorbent surface and is released into the solution (Basnet et al., 2022). Under acidic conditions, adsorption coupled with reduction was observed for Cr(VI) in the presence of Prosopis cineraria leaf powder (Soleimani et al., 2024).

Figure 6. Possible mechanisms of Cr(VI) adsorption (Basnet et al., 2022).
Rice husk has been employed to remove Cr(VI) and has shown almost 78.6% removal efficiency at a pH of 5.2 (Khalil et al., 2021). Maize biomass can also be used as an adsorbent; maize as a grain is used as food, whereas the other parts of the plant are used as fodder for livestock. The tassels and cobs of maize are abundantly available in developing countries and are economical alternatives for Cr adsorption. Murugesan et al. (2012) tested the potentials of acid-modified corn cob (AMCAC) and unmodified corn cob (UMCAC) for Cr(VI) adsorption. At lower metal-ion concentrations, 96% adsorption was observed, which is attributed to the interactions between all metallic ions and the active sites of the adsorbent. At higher concentrations of the metal ions, adsorption decreases as the metals compete for available active sites on the biomass surface, resulting in saturation of the binding sites; in this case, 95% removal was observed at the optimum time of 60 min. High amounts of the adsorbent (25–100 mg) can increase the removal of Cr ions from 26.22% to 90.06% because of the increased number of exchangeable binding sites. Here, the optimum adsorbent dose was reported to be 100 mg; the equilibrium data were best fitted to the Redlich–Peterson model and followed pseudo-second-order kinetics. The maximum metal uptake capacities were 22.82 mg g−1 and 54.11 mg g−1 for UMCAC and AMCAC, respectively. Hydroxyl, aldehyde, and halide groups are present on the biomass surface and are responsible for Cr(VI) adsorption (Murugesan et al., 2012). Mahajan and Sud (2013) studied three different forms of Acacia saligna pods (lignocellulosic nitrogenous waste), namely A. saligna pods natural (ASPN), A. saligna pods carbon (ASPC) in the AC form, and A. saligna pods beads (ASPBs) impregnated in hydrated beads form, for the batch treatment of Cr(VI). Here, the FTIR spectrum reveals that various functional moieties like C-H, hydroxyl, –C=O, and –OCH3 groups are responsible for Cr(VI) adsorption on A. saligna pods. The data follow second-order kinetics with good correlation coefficients, and the greatest removal was achieved at a low pH of 2. The efficiency of removal was observed in the following order: ASPC (97%) > ASPBs (94%) > ASPN (92%). The equilibrium condition was attained at 30 min for ASPC as well as 60 min for ASPBs and ASPN. Thus, it was concluded that ASPBs possessed more removal efficiency than natural forms of the pods (Mahajan and Sud, 2013). Singha et al. (2011) explored the Cr batch removal efficiencies of naturally available adsorbents, including rice straw, rice bran, hyacinth roots, neem bark, and neem leaves. The FTIR spectra revealed the presence of various functional moieties like hydroxyl, aromatic, alkene, silicon oxide, carboxylate, nitro, and sulfonic acid groups, which were responsible for Cr(VI) adsorption. The maximum adsorption was attained at 10 g L−1 of the adsorbent amount in 3–6 h. The experimental data were best fitted to the Langmuir isotherm, and the sorption energy was obtained from the D-R model, which showed the involvement of a chemisorption-based adsorption process (Singha et al., 2011). Manjusha (2012) examined the utility of papaya peel powder and observed higher Cr(VI) removal at lower initial metal-ion concentrations in wastewater.
The novel adsorbent Artemisia absinthium showed that Cr(VI) treatment efficiency decreases in the presence of an electrolyte such as 0.1 N potassium nitrate; the monolayer capacity was reportedly 46.99 mg g−1. According to the intraparticle diffusion model, Cr(VI) adsorption occurs in three stages; further, approximately 93% of Cr(VI) can be recovered from the column using 0.01 N sodium hydroxide (Rao et al., 2015). A previous report showed that approximately 1 g of Caryota urens inflorescence waste as an adsorbent biomass can treat approximately 175 mL of Cr(VI) solution (Rangabhashiyam and Selvaraju, 2015). Ash gourd peel waste having a BET surface area of 0.4854 m2 g−1 was found to be effective for Cr(VI) adsorption (18.7 mg g−1); according to the BDST, the uptake capacity was found to be 128.98 mg g−1 (Sreenivas et al., 2014).
Grass pea is a common food legume and is an agricultural waste. Chakravarty et al. (2013) investigated the use of Lathyrus sativus husk (grass pea or chickling vetch) for Cr(VI) adsorption; the results of this study displayed that the optimum pH was 2.0 and optimum time was 90 min. The FTIR data reflected the presence of different functional groups like -NH2, -COOH, -OH, and -PO43−, which were responsible for Cr adsorption through complexation and ion exchange mechanisms. According to Zeng et al. (2025), Cr(VI) adsorption on carbon-based organic adsorbents occurs through various mechanisms, such as electrostatic interactions, surface complexation, and ion exchange. In particular, ion exchange and electrostatic interaction mechanisms were reported to be dominant in acidic pH along with carbon-based adsorbents (Sinha et al., 2022). Logeswari et al. (2013) investigated low-cost biomaterials like eucalyptus bark (EB) and rice husk for Cr(VI) removal, where the maximum uptake of Cr(VI) was observed to be 45 mg g-1 at a low pH of 2 and an equilibrium time of 2 h. The maximum removal efficiency of 92% was achieved with EB, while rice husk showed an efficiency of only 26%; this difference in the percentage removal efficiencies of different adsorbents is attributable to the different functional groups present on the adsorbents for binding Cr(VI) (Logeswari et al., 2013). The seeds of Phyllanthus acidus (gooseberry) enabled 96% Cr(VI) removal under an acidic pH of 2 and equilibrium time of 1 h; the adsorption was confirmed to be monolayer and had a Langmuir R2 of 0.992 (Aravind et al., 2015).
2.6 Bark
Sharma and Goyal (2011) showed that the bark of Eucalyptus tereticornis had removal efficiencies of 70% and 94% for Cr(VI) present in tannery effluents and chrome-plating effluent, respectively. Manjusha (2012) also reported the use of the ecofriendly material Mangifera indica bark dust for Cr(VI) removal in a batch system, where the optimum adsorbent dosage was 0.8 g L−1 and optimum time was 25 min; the Cr(VI) removal was observed to follow a smooth curve, indicating monolayer coverage. The bark of Pithecellobium dulce was investigated for Cr(VI) removal, and it was found that maximum Cr(VI) removal was obtained at a pH of 5 with an adsorbent dose of 1 g L−1 (Muthulakshmi and Baskaran, 2017). A. albida bark was shown to have a Cr(VI) removal efficiency of up to 98.47% at an optimum pH of 2; here, the equilibrium data followed the Langmuir isotherm and pseudo-second-order kinetics, and the FTIR spectrum revealed the presence of polar functional groups on the adsorbent surface like hydroxyl, amide, and amine groups for Cr(VI) adsorption (Gebrehawaria et al., 2015). The optimum conditions for Cr(VI) removal using a fixed-bed column containing sago bark (Metroxylon sagu) were a flow rate of 2 mL min−1 and bed depth of 9 cm; it was also found that used sago bark could be regenerated using 0.01 M of nitric acid (Fauzia et al., 2021).
2.7 Microbial biomasses
2.7.1 Algae
Raw and chemically modified Spirulina platensis were reported to have adsorption capacities of 79.6 and 158.7 mg g−1, respectively, in a batch reaction. A continuous column study with the modified adsorbent confirmed maximum adsorption capacity at a flow rate of 50 mL h−1 and an initial metal concentration of 200 mg L−1. Both the raw and modified algae could be regenerated by up to 89.6% and 94.3%, respectively (Bayramoglu et al., 2016). Blue–green marine algae have been reported to have a maximum adsorption capacity of 37.426 mg g−1 under optimum time and agitation speed conditions (Ramadoss and Subramaniam, 2018). Sargassum myriocystum is a marine brown alga that has been explored for Cr(VI) removal potential; it has been shown to have sulfate groups on the surface along with hydroxyl, carboxylic, aldehyde, and carbonyl groups that are involved in Cr(VI) adsorption. Economic analysis of treatment with S. myriocystum revealed 60% lower cost for chromium effluents compared to activated charcoal treatment (Jayakumar et al., 2015). Pseudopediastrum boryanum var. longicorne also displayed maximum removal at an adsorbent dose of 2 g L−1 and initial concentration of 10 mg L−1 (Sutkowy and Kłosowski, 2018). Three types of algae were evaluated for Cr(VI) removal, and the results showed that Cladophora glomerata was a better adsorbent than Enteromorpha intestinalis and Microspora amoena; increase in the adsorbent dose beyond 1 g resulted in decreased metal removal, which may be attributed to large block formation of the adsorbent particles resulting in lower available surface area for Cr(VI) removal (Al-Homaidan et al., 2018).
Pithophora oedogonia contains carbonyl, carboxyl, and hydroxyl functional groups that are responsible for 90% Cr(VI) removal at pH = 4 (Suleman et al., 2017). Chlorella vulgaris green micro algae was explored to be an economical and efficient adsorbent for Cr(VI) adsorption of up to 99.75% (Indhumathi et al., 2014). The economical green algae Ulva fasciata sp. showed efficient removal (77%) of Cr(VI) at pH = 6 (Prasanna Kumar et al., 2017). Polysiphonia urceolata and Chondrus ocellatus were found have maximum uptake capacities of 170.6 mg g−1 and 113.4 mg g−1, respectively, at optimum conditions and obeyed pseudo-second-order kinetics reflecting a chemisorption mechanism; the results also revealed the involvement of carbonyl, hydroxyl, and amino groups in Cr(VI) adsorption (Li et al., 2015). Valli et al. (2013) explored the efficiency of green algae biomass for Cr(VI) adsorption in a batch experiment and reported 75% removal. Kurniasih et al. (2013) investigated the efficiency of immobilized algal bloom biomass for Cr(VI) removal and observed the highest percentage removal when the algal bloom biomass was treated with 0.1 N HCl; here, the maximum uptake capacity was reported to be 11.494 mg g−1.
Esmaeili et al. (2010) studied and compared the Cr(VI) removal efficiencies of dried (BD) Sargassum spp. (brown marine algae) and its AC form; the optimum pH and equilibrium time were found to be 2.0 and 120 min, respectively, while the maximum adsorption capacities was observed to be 3.69 mg g−1 for the BD and 6.877 mg g−1 for the AC forms. Sujin Jeba Kumar et al. (2013) examined the immobilized cells of a microalgae (Isochrysis galbana) for Cr(VI) adsorption; they reported a maximum uptake capacity of 29.21 mg g−1 at pH = 4 and temperature of 35°C, where the experimental data were well fitted to the Langmuir model. Table 2 presents a comparison of different algal biomasses used as adsorbents for Cr(VI) removal.
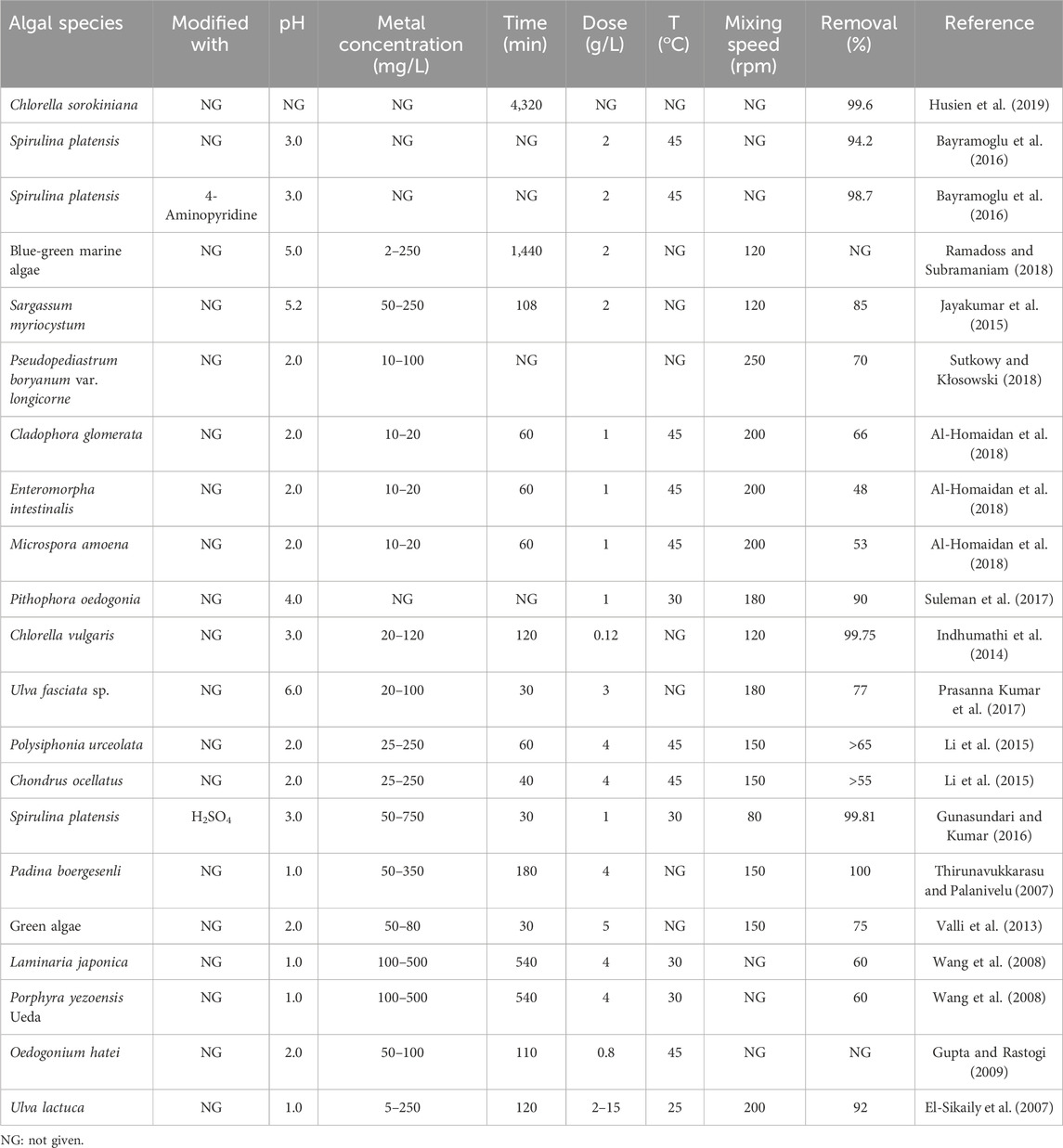
Table 2. Comparative analysis of the process variables for the adsorption of Cr(VI) using algal biomass adsorbents.
2.7.2 Bacteria
Deepali (2011) explored Cr(VI) treatment using three bacterial strains, namely Pseudomonas putida, Pseudomonas aeruginosa, and Bacillus sp. At an initial Cr(VI) concentration of 10 mg L−1, the maximum Cr(VI) removal was observed to be 75% by Bacillus sp., while P. aeruginosa showed 69.70% removal at a concentration of 40 mg L−1. However, over a duration of 96 h, P. putida showed maximum removal of 90.88% at an initial Cr(VI) concentration of 10 mg L−1; this bacterium also showed maximum Cr(VI) removal at lower initial concentrations than the other two bacteria (Deepali, 2011). Karthikeyan and Mythili (2011) investigated the potential of Bacillus sp. and Staphylococcus sp. for the adsorption of Cr(VI); for Bacillus sp., the optimum temperature and pH were found to be 37°C and 7, respectively; however, for Staphylococcus sp., the optimum temperature and pH were found to be 37°C and 8, respectively. Table 3 presents a comparison of various bacterial biomasses used as adsorbents for Cr(VI) removal.
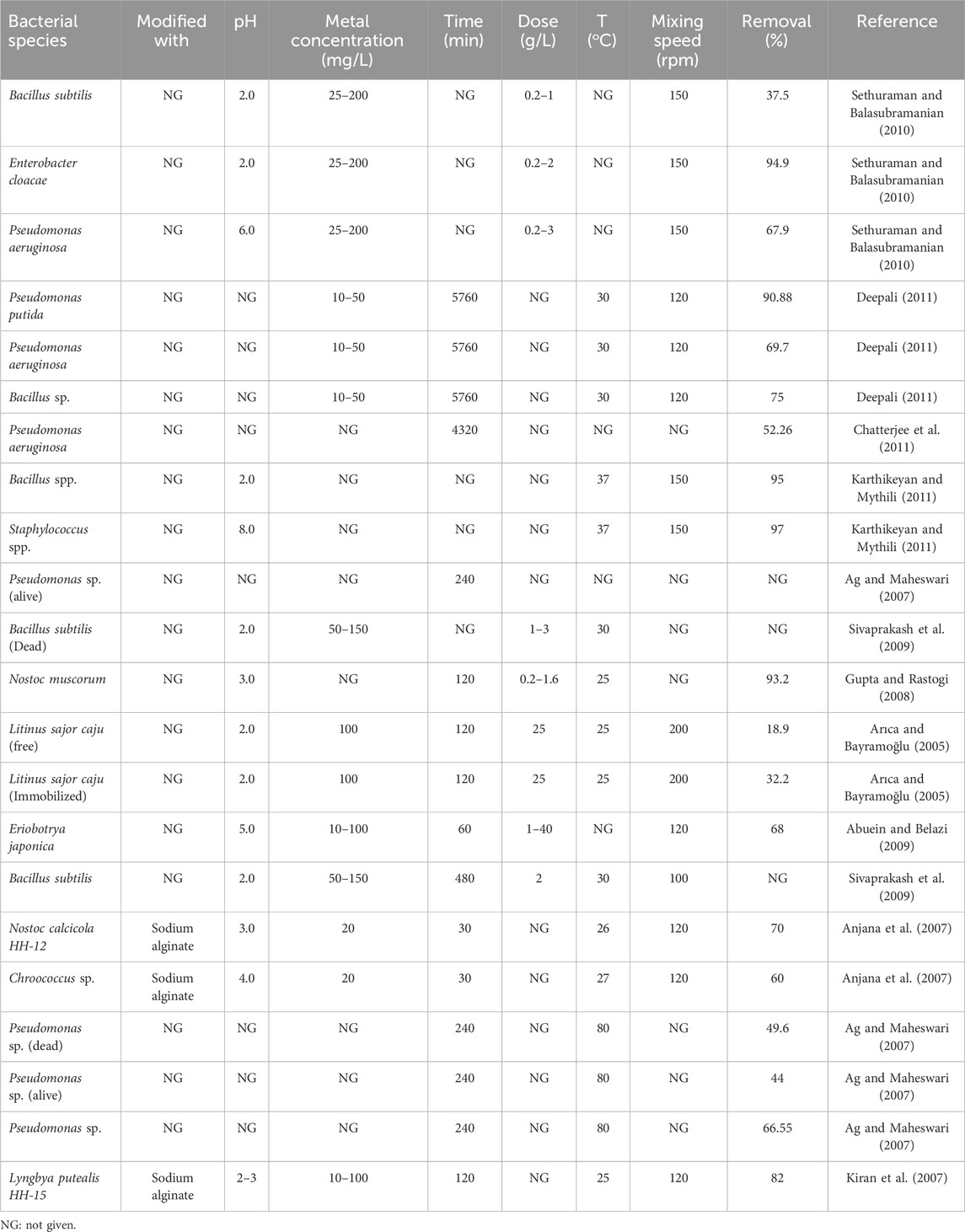
Table 3. Comparative analysis of the process variables for the adsorption of Cr(VI) using bacterial biomass adsorbents.
2.7.3 Fungi
Abdel-Razek (2011) studied the efficiency of Ca-alginate (CA) beads as well as dead and immobilized alive Cunninghamella elegans beads for Cr(VI) adsorption; here, the dead beads displayed greater Cr(VI) removal efficiency than the alive and CA beads. Different fungal species obtained from contaminated samples of tannery industrial wastewater in a study area in India (Nagalkeni, Chennai) were investigated for their Cr(VI) removal potential; it was observed that at an optimum pH of 3 and adsorbent dose of 6 g, Aspergillus niger enabled maximum Cr(VI) removal than Aspergillus fumigatus and Aspergillus flavus (Shankar et al., 2014). A. fumigatus Fresenius obtained from Sukinda (India) mine water was proven to be a good adsorbent as it removed 97% Cr(VI) at an optimum pH of 5.5 and optimum time of 120 h; the FTIR spectrum confirmed the presence of hydroxyl, amine, amide, and carboxylate groups on the cell wall of the fungus; the exhausted adsorbent could also be reportedly regenerated by shaking with 0.5 M of HCl at 35°C for 2 h (Dhal and Pandey, 2018).
The adsorption capacities of both active and inactive Pleurotus ostreatus were evaluated, which revealed that inactive P. ostreatus allowed 100% removal of 50 mg L−1 of Cr(VI) at an optimum time of 22 min while active P. ostreatus enabled 100% removal of 25 mg L−1 of Cr(VI) at 360 h (da Rocha Ferreira et al., 2019). As a novel adsorbent, A. niger spores were tested for Cr(VI) adsorption capacity in a batch reactor, which revealed a maximum uptake of 97.1 mg g−1 at optimum conditions of temperature (40°C), dose, pH (2), and initial concentration. Interference studies of Cr(VI) with Na, K, Ca, and Mg ions showed that there was very little effect on the adsorption of Cr(VI) in the presence of these interfering ions. The proposed adsorption mechanism involved reduction of Cr(VI) to Cr(III), following which Cr(III) attached to the surfaces of the spores through electrostatic forces, complex formation, or redox reaction. The FTIR spectrum reflected that the nucleic acids and proteins of the adsorbent participated in the binding of Cr(VI) to the adsorbent surface (Ren et al., 2018).
Artist’s bracket fungus was explored for its Cr(VI) adsorption potential at various batch parameter values of the initial metal concentration, dose, and pH, which showed that the PZC value of the fungus was 3 (Pourkarim et al., 2017). A. niger was found to have an adsorption capacity of 11.792 mg g–1 at pH = 2 and follow pseudo-second-order kinetics; here, approximately 94% of the Cr(VI) was removed, and the adsorbent could be regenerated using 0.5 M EDTA; further, amines of the adsorbent cell wall were found to be involved in Cr(VI) adsorption (Mondal et al., 2017). Different species of fungi were isolated from the contaminated soil of a tannery in India, and the results revealed a maximum removal efficiency of 96.3% of Cr(VI) by A. niger compared to other fungal species (Sivakumar, 2016). Majumder et al. (2017) reported a maximum uptake capacity of 100.69 mg g−1 for Arthrinium malaysianum, where the used adsorbent can be regenerated by up to 41.17% using sodium hydroxide solution. Table 4 presents a comparison of various fungal biomasses used as adsorbents for Cr(VI) removal.
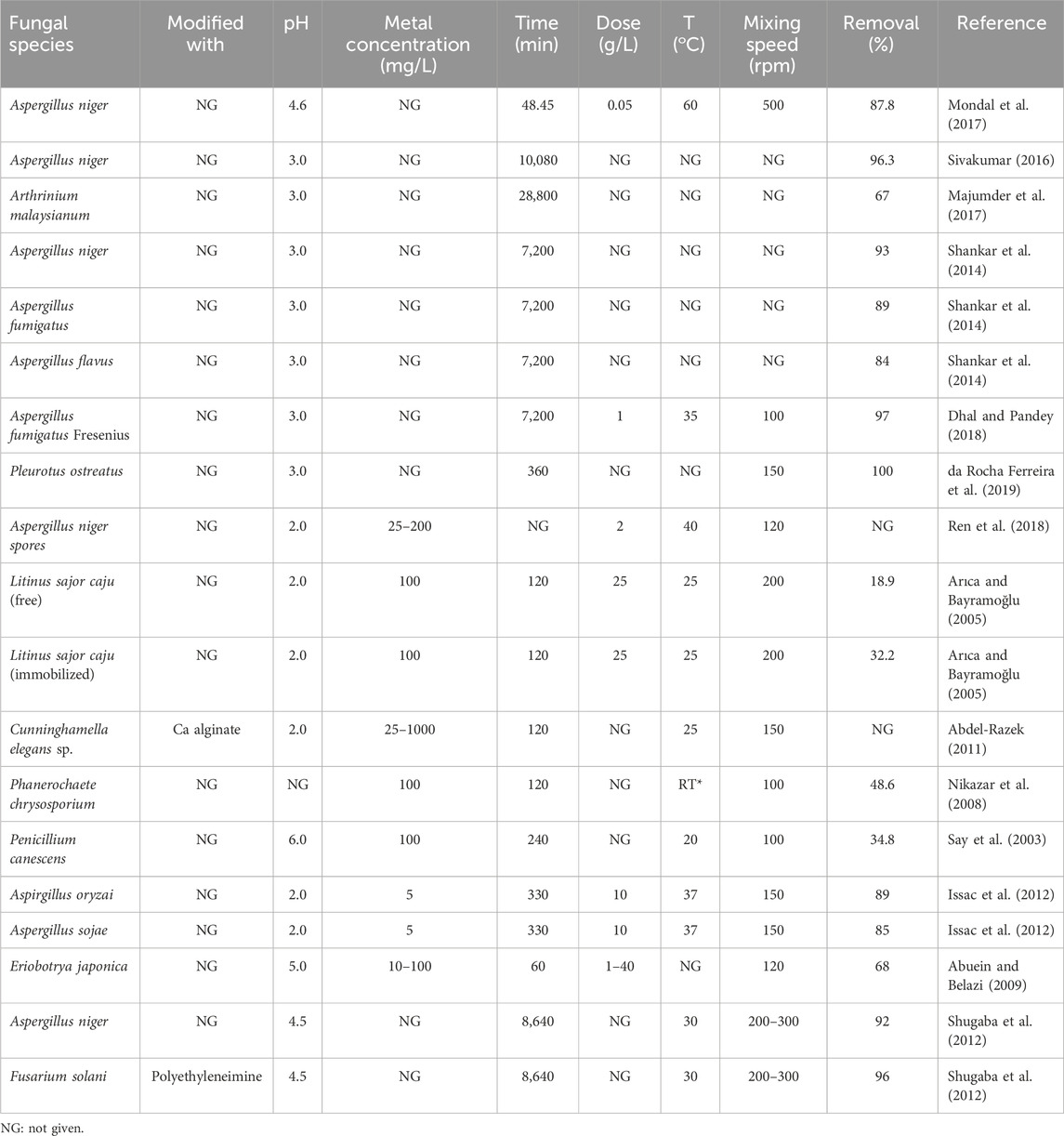
Table 4. Comparative analysis of the process variables for the adsorption of Cr(VI) using fungal biomass adsorbents.
2.7.4 Yeast
Yeast is a eukaryotic organism that is usually unicellular; the cytoplasm present in the living yeast cell is involved in interactions with metal ions, so yeast was tested for Cr(IV) removal potential. The carboxymethyl cellulose present in Lentinus sajor caju was evaluated for Cr(VI) treatment, which showed a removal capacity of 32 mg g−1 at a pH of 2, temperature of 25°C, and initial metal solution concentration of 100 mg L−1. Numerous functional groups are present of the cell wall of mycelia (COOH, =NH, -NH2, -OH, and -SH), which indicate the interactions of the adsorbent surface with metal ions (Arıca and Bayramoğlu, 2005). The biosorption capacity of Kodamaea transpacifica was found to be higher than those of Kazachstania yasuniensis and Saturnispora quitensis because of its higher surface area of 1,588.27 m2 L−1 (Campaña-Pérez et al., 2019). Table 5 presents a comparison of various types of yeasts used as adsorbents for Cr(VI) removal.
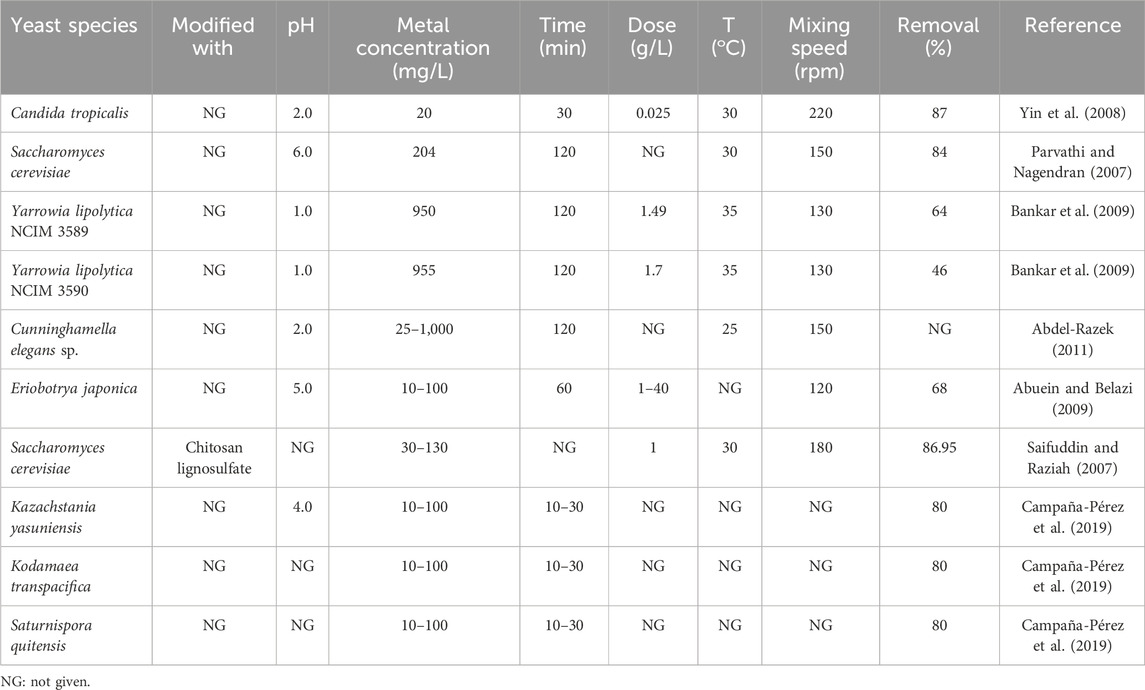
Table 5. Comparative analysis of the process variables for the adsorption of Cr(VI) using yeast-based adsorbents.
3 Development of AC from waste biomasses
Pal and Kaur (2013) investigated the effects of different particle sizes (0.3–1.0 mm) of physically activated sawdust of Dalbergia sissoo (ASD) for Cr(VI) adsorption in a batch experiment. They noted that the Cr(VI) removal improved from 66% to 85.4% with increase in adsorbent amount from 0.2 to 1.2 g per 100 mL for ASD particles of size 0.3 mm. Moreover, improved adsorption from 61% to 81.2% was observed with a particle size of 1.0 mm. However, when the metal concentration was increased over 5–50 mg L−1, the efficiency of Cr removal reduced from 87% to 73% for ASD of 0.3 mm size and from 83% to 70% for ASD of 1.0 mm size. Maximum adsorption was achieved at a pH of 2.0, at which point the adsorptive surface became more positively charged and showed greater attraction to anionic species of Cr(VI). At high pH, lower adsorption was observed owing to reduced attractive forces between the anionic species of Cr and adsorbent surface. Equilibrium was established within 60 min, and there was no further adsorption thereafter because of non-availability of vacant sites. The data were best fitted to the Freundlich and Langmuir isotherms (Pal and Kaur, 2013). Physically activated Ziziphus spina-christi leaves showed a maximum Cr(VI) removal efficiency of 97.22% at optimum time, pH, initial metal concentration, adsorbent dose, and temperature conditions. The adsorption data were fitted well to the Langmuir isotherm, reflecting a maximum adsorption capacity of 13.81 mg g−1. The thermodynamic parameters also revealed the endothermic nature of Cr(VI) adsorption (Abshirini et al., 2018). Physically AC of rice straw showed a maximum Cr(VI) uptake of 90% at pH = 8. At optimum time, pH, and temperature, the rice straw AC displayed a maximum adsorption capacity of 3.5 mg g−1 (Kumar et al., 2017). Teak wood sawdust modified using HCl was also shown to enable maximum Cr(VI) adsorption (Jha, 2016).
H2SO4-activated cashew nut was used for Cr(VI) removal in a column study, which showed the best results at a bed height of 10 cm (Yahya et al., 2020). HCl-activated M. indica leaves showed a maximum adsorption capacity of 78.96 mg g−1 (Pathania et al., 2020). Vikal et al. (2013) investigated activated Ziziphus jujuba leaf powder (local name Bordi), which showed maximum Cr(VI) removal at an adsorbent dose of 15 g within 105 min. Physical activation of the adsorbent was performed at 80°C for 48 h, and FTIR spectroscopy confirmed that the hydroxyl, aliphatic alkane, and O-C stretching of the ether groups were responsible for adsorption. The experimental data were fitted well to both the Langmuir and Freundlich models (Vikal et al., 2013). Sawdust modified with diethylenetriamine also revealed 75% adsorption of Cr(VI) at an optimum adsorbent dosage of 2 g L−1 (Elhami and Bahadori, 2015). Chemically (phosphoric acid) activated ECS also showed 87% removal of Cr(VI) at a pH of 3 (Haroon et al., 2020). Spruce sawdust treated with diethylene glycol and sulfuric acid revealed a maximum adsorption capacity of 318.3 mg g−1 for Cr(VI) (Politi and Sidiras, 2020). Teakwood sawdust modified with zinc chloride showed an adsorption capacity of 72.46 mg g−1 for Cr(VI) (Ramirez et al., 2020). Leucaena leucocephala activated with orthophosphoric acid showed a maximum Cr(VI) uptake of 13.85 mg g−1 at pH = 4; its PZC was revealed to be 5.42 (Malwade et al., 2016). Chemically activated almond shell (with 40% H3PO4) was also found to have a high adsorption capacity of 202.34 mg g−1 under optimum batch experiments; the BET surface area of the activated almond shells was reported as 1,223.4 m2 g−1 (Rai et al., 2018). Nut shells activated with ZnCl2 were found to have a BET surface area of 2,869 m2 g−1, and the maximum adsorption was observed at pH = 2; the experimental data were fitted well to the Langmuir model with an adsorption capacity of 46.21 mg g−1 (Kumar and Jena, 2017). The adsorption potentials of acid-activated Juniperus procera leaves for Pb(II) and Cr(VI) were investigated in a batch experiment, which showed maximum removal efficiencies of 98% and 96% at optimum pH values of 4.6 and 4, respectively. The activated J. procera leaves could be reused up to three times, and the data were best fitted to pseudo-first-order kinetics (Ali et al., 2019). Corn cob is an abundant agricultural waste that is mostly burned or discarded. Corn cob activated with a mixture of ZnCl2 and NH4Cl solutions was explored for Cr(VI) adsorption in a batch experiment, which revealed a BET surface area of 924.9 m2 g−1; however, the iodine adsorption value of corn cob AC was 1,188 mg g−1, and a lower/acidic pH was found to enhance adsorption owing to protonation of the adsorbent surface (Tang et al., 2016).
Sulfuric-acid-activated rice husk showed maximum removal of the adsorbate at a contact time of 120 min and pH of 2. Low desorption results, i.e., 0.1%–9%, revealed strong bonding between the adsorbent and adsorbate as well as the chemisorption nature of the activated adsorbent; the BET surface area was reported to be 58.54 m2 g−1 (Khan et al., 2016). Sodium-hydroxide-activated carbon prepared from L. leucocephala seed pod showed a Langmuir adsorption capacity of 26.94 mg g−1 under optimum conditions. The data were best fitted to pseudo-second-order kinetics, indicating chemical adsorption. Furthermore, the desorption efficiency decreased from 63.42% to 47.56% from the first to third cycles as the metal uptake capacity decreased with adsorbent reuse (Yusuff, 2019). Cicer arietinum (chickpea) husk was chemically activated using KOH and K2CO3 and tested for removal of Pb, Cu, and hexavalent Cr; the results showed better porosity formation upon impregnation with KOH (50 wt%), with a BET surface area and total pore volume of 2,082 m2 g−1 and 1.07 cm3 g−1, respectively. The maximum adsorption capacities were in the order of Pb(II) > Cr(VI) > Cu(II), i.e., corresponding to 135.8, 59.6, and 56.2 mg g−1, and the thermodynamic parameters indicate an endothermic adsorption process (Özsin et al., 2019). Great millet husk was also shown to have an adsorption capacity of 22.21 mg g−1 for Cr(VI) removal after chemical modification with sulfuric acid (Prajapati et al., 2022); the metal uptake potential of sulfuric-acid-activated neem bark was tested in a column experiment for hexavalent Cr. When the adsorbent mass was increased from 25 to 175 g at a constant metal ion concentration and flow rate, the breakthrough time was noted to increase from 9.25 to 111.66 h. Activated neem adsorbent has been shown to have excellent effects on a treated mixture of metals (hexavalent Cr, Cu, and Zn) from wastewater; it was revealed that 40% Cr(VI) could be recovered by shaking the exhausted adsorbent with distilled water at 90°C for 2 h, and the data were best fitted to the Yoon–Nelson kinetic model (Maheshwari and Gupta, 2016). Lantana camara plant activated with nitric acid displayed a maximum adsorption capacity of 26.25 mg g−1 at optimum time, initial concentration, temperature, and pH conditions; the adsorption of Cr(VI) was tested in the presence of interference ions to assess the utility of the activated adsorbent in real industrial effluent applications, and it was observed that the adsorption of Cr(VI) was slightly affected by tenfold excess of co-anions like NO3−, Cl−, and CO32–; however, very little effect was noted in the case of SO42– and PO43– (89.0%), while no interference was observed in the case of co-cations like Zn+2 (Ravulapalli and Kunta, 2018).
Sulfuric-acid-modified holly sawdust was studied for Cr(VI) treatment in a batch experiment (Siboni et al., 2011); it was observed that the percentage removal of Cr(VI) increased from 34.65% to 99.99% with increasing adsorbent amounts from 2 to 10 g L–1. Increasing the initial concentration of the metal ions in the range of 20–100 mg L–1 reduced the efficiency from 99.37% to 40.24%. Moreover, the Cr(VI) removal efficiency decreased from 99.67% to 29.78% with increase in pH from 2 to 12. At low or acidic pH, the adsorbent surface acquires positive charges and Cr(VI) generally exists in the form of HCrO4−. However, the HCrO4− changes to CrO42− and Cr2O72− at high pH, where the competition between CrO42− and OH− anions also decreases. Equilibrium was attained within 90 min, and the Langmuir model was observed to define the experimental results better than the Freundlich isotherm, with the adsorbent following pseudo-second-order kinetics. The maximum uptake was reported as 18.86 mg g−1 at a high pH of 7.0. Two different forms of sawdust, namely, powder sawdust activated with acid (ASDP) and bead-form sawdust with surface modification (SDCCB), were investigated for the treatment of Cr(VI) in a batch study (Begum and Alhaji, 2013). Here, oxidizing agents like H2SO4, HNO3, and H2O2 were successively used for chemical activation of the sawdust. The highest Cr(VI) uptake capacities for ASDP and SDCCB were 45.5 and 125 mg g−1, respectively. The maximum contact times were approximately 120 min for the ASDP and 150 min for the SDCCB. The order of isotherm fitness was Freundlich > Langmuir > Temkin (based on R2 values), while pseudo-second-order kinetics was found to best fit the data. It was concluded through the thermodynamic parameters that the adsorption was endothermic, spontaneous, and non-specific chemisorption for both adsorbents (Begum and Alhaji, 2013). The Cr(VI) removal efficiencies of boiled sawdust, formaldehyde-treated sawdust, and sawdust carbon were examined in a batch reactor (Subhan and Pardeep, 2011), which showed that the adsorption process was favored at low a pH of 2.0 when using biomass doses of approximately 4 g L−1 and 25°C temperature. The percentage removal efficiency of Cr(VI) was in the following order: sawdust carbon > formaldehyde-treated sawdust > boiled sawdust.
Altun and Pehlivan (2012) investigated the efficiency of chemically modified walnut shell (WNS) (Juglans regia) for removal of Cr(VI) from water in a batch experiment; here, approximately 10 g of WNS was chemically modified with 10 g of citric acid per 50 mL of water and heated for 4 h at 120°C, which showed that functional groups like alcohols, carbonyls, carboxyls, and phenols were associated with Cr(VI) adsorption. The equilibrium was attained at 120 min and the optimum pH range was between 2 and 3. The adsorption increased directly with temperature because of more chemical interactions between the adsorbent and adsorbate. The maximum Cr(VI) uptake capacities were 0.596 and 0.154 mmol g−1 for CA-WNS and untreated WNS, respectively. The process of adsorption was endothermic and spontaneous (Altun and Pehlivan, 2012). Demirbas et al. (2004) revealed the Cr(VI) removal potential of sulfuric-acid-modified cornelian cherry, apricot, and almond shell from solution. Various environmental parameters like the particle size (0.63–1.60 mm), pH (1–4), and initial Cr(VI) concentration (20–300 mg L−1) were optimized in a batch experiment, and the adsorption process was dependent on pH. The equilibrium contact time was 72 h, and the optimum pH was 1. The experimental data were fitted better to second-order kinetics than first-order kinetics in terms of the diffusion model (Demirbas et al., 2004). Attia et al. (2010) conducted a comparative study on sulfuric-acid-modified olive stones and AC to assess their removal potentials for solutions containing various Cr(VI) concentrations (4–50 mg L−1) in a batch experiment. The highest removal was reported at the lowest pH of 1.5, and the FTIR spectrum revealed that the negatively charged groups were neutralized to form binding sites for Cr. The adsorption data were suitably fitted to the first-order kinetic and Langmuir models. The AC from olive stones was more efficient than acid-treated commercial AC for Cr(VI) removal (Attia et al., 2010).
Swietenia mahagoni shell modified by sulfuric acid and orthophosphoric acid was found to be an excellent adsorbent for the treatment of Cr(VI) in a column. The Cr(VI) adsorption capacities of sulfuric-acid- and orthophosphoric-acid-modified S. mahagoni shells from the BDST model were found to be 1,989.4 mg L−1 and 2,785.2 mg L−1, respectively (Rangabhashiyam et al., 2016). Mane (2010) revealed the potentials of chemically (sulfuric acid) activated tendu (Diospyros melanoxylon) leaf refuse (TLR) and commercial activated carbon (CA-CAC) for the treatment of Cr(VI) from solution. The optimum pH was reported as 2, at which point neutralization of negative charges occurred on the adsorbent surface due to excess hydrogen ions, resulting in diffusion or adsorption of the hydrogen chromate ions (HCrO4−). At pH between 1.0 and 4.0, hydrogen chromate ions are the dominant form of Cr(VI). Another reason for maximum removal at low/acidic pH is the oxidation of Cr2O72– to Cr3+, which is easily replaced by the positive species owing to its small size. The equilibrium time attained for CA-CAC was 120 min and that for CA-TLR was 30 min. Thus, it was found that CA-TLR is more efficient for Cr(VI) removal than CA-CAC (Mane, 2010). Pandhram and Nimbalkar (2013) explored the efficiency of low-cost activated neem leaves for Cr(VI) removal from industrial effluents; here, the adsorbent was physically activated for 3 h in a furnace at 250°C. In a batch experiment at a pH of 4, the maximum removal observed was 67.5%. At a solution concentration of 30 mg/100 mL, the maximum percentage removal efficiency of Cr(VI) was 98%. At an adsorbent dose of 8 mg/100 mL, the removal efficiency was observed to be maximum at 85% (Pandhram and Nimbalkar, 2013).
The Cr(VI) percentage removal efficiencies of boiled rice husk, formaldehyde-treated rice husk, and rice husk carbon from wastewater were examined in a batch study. The adsorption process was favored at pH = 2 when using an adsorbent dose of 4 g L−1 at 25°C. The Cr(VI) removal efficiency was in the order of rice husk carbon > formaldehyde-treated rice husk > boiled rice husk (Subhan and Pardeep, 2011). Ahmed et al. (2012) investigated physically activated rice husk (at 700°C for 1 h) for sequestering Cr from a synthetic aqueous solution using particles of sizes 50 and 100 mesh (150 µm) in batch experiments. The maximum adsorption of 95.2% was attained at an optimum time of 150 min, a Cr(VI) concentration of 20 mg L−1, a low pH of 2, and an adsorbent dose of 5 g L−1 (Ahmed et al., 2012). Strychnos potatorum seeds were modified using sulfuric acid to investigate their efficiency of Cr(VI) removal in both batch and column reactors; here, the maximum Langmuir uptake capacity was reported as 202.7 mg g−1, and the column data best fitted the Thomas model (Anbalagan et al., 2016).
Grafted peels of banana displayed 96% Cr(VI) removal at optimum pH and dose of 3 and 4 g L−1, respectively (Ali et al., 2016). Pomegranate seeds were also used for Cr(VI) removal (Ghaneian et al., 2017). At an acidic pH of 2, tea husk showed a maximum Cr(VI) removal of 98.5% (Saed, 2016). Sulfuric-acid-activated neem bark was assessed in a column study for Cr(VI) removal, and the result showed increase in breakthrough time from 9.25 to 111.66 h with increase in adsorbent dose (25–175 g). However, the breakthrough time is achieved earlier in multisolutes containing Cu and Zn. The experimental data were explained well by the Yoon–Nelson model. The saturation loading capacity of the column was reported to be 27.5 mg g-1, which is very close to the Cr(VI) uptake capacity of 26.95 mg g-1 in the batch study (Maheshwari and Gupta, 2016). Stems of the plant Ocimum tenuiflorum were activated using sulfuric acid and investigated for Cr(VI) removal in a batch system, where the maximum uptake capacity was found to be 95.9 mg g-1. The adsorption mechanism was also evaluated, which showed interparticle diffusion at higher Cr(VI) concentrations and film diffusion at lower Cr(VI) concentrations (Suganya et al., 2017). Sulfuric-acid-modified S. platensis with a BET surface area of 765.1 m2 g–1 was proven to be a good adsorbent and remove up to 55.252% of Cr(VI) even in the presence of sulfate ions; the used adsorbent could be recovered up to 94.48% with sodium hydroxide (Gunasundari and Kumar, 2016).
4 Comparisons of adsorbents and chemical modifications for Cr(VI) removal
Previous reports in literature have revealed the following order for average Cr(VI) removal (Figure 7) when using different biomasses without any modifications or activation: leaves > bark > agricultural waste > dry shells > tea = fungi > yeast > algae > sawdust > bacteria. The adsorption data of different types of leaves are best fitted to both the Langmuir and Freundlich isotherm models, reflecting single-layer physisorption as well as multilayer chemisorption. Leaf modifications do not increase the maximum adsorption efficiency, so it is economical to use leaves that are available in bulk refuse without prior treatment with chemicals in various industries, especially in developing countries. Studies also show that adsorption depends on the solution pH, functional groups on the adsorbent surface, particle size of the adsorbent, and metal-ion concentration.
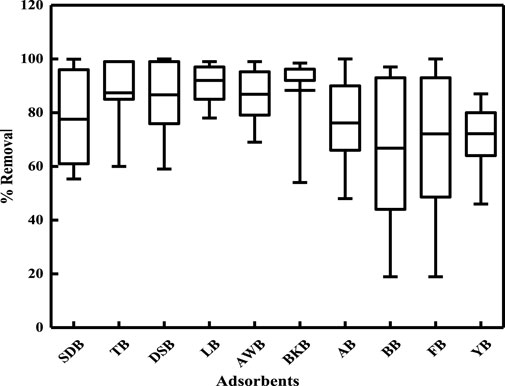
Figure 7. Box-and-whisker plot showing percentage removal of Cr(VI) by various natural biomasses. SDB: sawdust biomass, TB: tea biomass, DSB: dry shell biomass, LB: leaf biomass, AWB: agricultural waste biomass, BKB: bark biomass, AB: algal biomass, BB: bacterial biomass, FB: fungal biomass, YB: yeast biomass.
The physical and/or chemical modifications enhanced Cr(VI) removal with almost all natural lignocellulosic (organic) adsorbents. The major mechanisms driving metal-ion adsorption are electrostatic interactions, surface complexation, and ion exchange. Modification of the adsorbents with acids produced the best results for Cr(VI) removal, followed by modifications with salts and bases (Figure 8). Modification with an acid results in positive charges on the adsorbent surface (Abegunde et al., 2020). Modification of agricultural or organic materials with acids through the wet oxidation process causes dissolution of the constituents or removal of mineral impurities along with an increase in oxygen content and a decrease in pore tortuosity. Modification of organic adsorbents with various acids increases the reactivity of the functional groups and different surface properties of the adsorbents, such as the surface area and porosity, thereby increasing adsorption. Acid modification can also increase the hydrolysis of cellulose, which results in a more reactive adsorbent compared to the untreated adsorbent (Liu et al., 2020). Modification of adsorbents with acids also reduces the mineral content of the modified adsorbent, which in turn results in increased percentage removal of Cr(VI) from water because of the decrease in competition between the active sites of the adsorbent and cations. Sulfuric acid is the most widely used substance for modification and increases the removal efficiency by 80%–100% in all the reviewed adsorbents. This is because of the presence of -SO3 groups on the adsorbent that increases the percentage removal of Cr(VI) through covalent bonding along with increases in the surface area and porosity of the adsorbent (Goswami and Phukan, 2017). Phosphoric-acid- and tartaric-acid-based modifications are also reported in literature, and these enhance the porosities and surface areas of natural adsorbents. Modifications of adsorbents with sulfuric acid, phosphoric acid, and nitric acid increase both the surface areas and surface functional groups (Lesaoana et al., 2019). Modification with phosphoric acid has been reported to form different phosphorus groups on the adsorbent surface (PO and POOH), which then react with Cr(VI) and result in the formation of metal complexes (Kainth et al., 2024). However, in the case of modification using nitric acid, there is enhancement of the nitrate groups along with increased pore volume on the adsorbent surface, which results in more adsorption of Cr(VI). Sodium hydroxide as a modification source for biomass showed poor results because of fewer positive charges, as in the case of acid modification. The optimum pH was found to be 2 in most of the studies with both raw and modified adsorbents. In the case of treated biomass, the Langmuir model was found to be the best fit most often, indicating the availability of more surface sites for Cr(VI) removal. It is important to note that the different chemical modifications summarized in Figure 8 are generally applicable for all lignocellulosic (organic) biomasses because of their similar structural compositions; further, the best modification (chemical) may differ based on specific biomass properties along with the experimental conditions noted in the various studies.
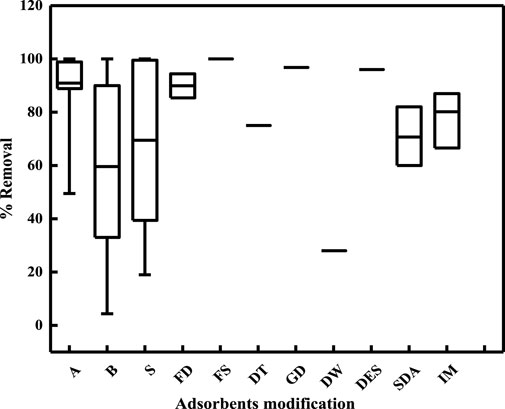
Figure 8. Box-and-whisker plot showing percentage removal of Cr(VI) by modifications of natural adsorbents. A: acid, B: base, S: salt, FD: formaldehyde, FS: formaldehyde with sulfuric acid, DT: diethylenetriamine, GD: glutaraldehyde, DW: distilled water, DES: diethyl ether and sulfuric acid, SDA: sodium alginate, IM: immobilized.
In the box-and-whisker plots shown in Figures 7 and 8, the rectangles (boxes) represent the interquartile range (IQR; range between the 25th and 75th percentiles) while the lines (whiskers) represent the spread of the data, which extend to the minimum and maximum values (excluding outliers). The central line within each box indicates the median value.
5 Future directions
In most of the reported works, researchers have focused on batch experimentation; however, it is important to work on column designs using natural adsorbents for field applications. Hence, further exploration is needed for the development of novel and efficient natural adsorbents from natural materials, such as functionalized adsorbents, nanocomposites, engineered biochars, and hybrid materials. More studies are needed on the treatment of real industrial wastewater and effluents containing multiple pollutants as there is less available work on this area given that most of the literature is on synthetic solutions of single adsorbates. In most cases, the Freundlich and Langmuir isotherms were used for the analyses while there are very few reported cases of using other models like the Elovich, D-R, and Temkin isotherms; this area also needs to be explored further for better understanding of the nature of adsorption processes. Exploration of the microscopic mechanisms of adsorption is also needed using advanced techniques like X-ray photoelectron spectroscopy and density functional theory simulations. The development of cost-effective methods to produce AC from locally available biomasses is the most desired need for field applications. Hence, more research efforts are required for optimizing methods for economical development of AC from various available waste biomasses. Mixtures or compositions with varying ratios of the activated and non-activated adsorbents should also be evaluated for pollutant removal. Low regeneration has been observed with most of the adsorbents; therefore, further desorption/regeneration processes should be studied using different variables like pH, temperature, rotation speed, chemicals, etc. for renewal of exhausted adsorbents. Another area for future development is the exploration of hybrid or synergetic treatment approaches in which adsorption is combined with other technologies like biological treatments, membranes, electrochemical reduction, photocatalysis, and advanced oxidation processes. There is also a need to carry out lifecycle assessments of the Cr adsorption processes. Lastly, we note the importance of conducting cost-benefit analyses to better understand the practical applications of the adsorbents.
6 Conclusion
The bulk of organic biomass is often treated as waste and refuse all around the world. The present review on these waste biomasses reveals their effectiveness as low-cost adsorbents for the removal of Cr(VI) from wastewater. Our survey indicates the following order of utility of various biomasses for Cr(VI) removal: leaves > bark > agricultural waste > dry shells > tea = fungi > yeast > algae > sawdust > bacteria. Among the various types of bulk biomasses available, leaves have been proven to be the most efficient and economical sources for Cr(VI) removal that do not require any prior treatment. The optimum pH for the treatment was found to be 2 for both raw and modified adsorbents. The major mechanisms driving metal ion adsorption are electrostatic interactions, surface complexation, and ion exchange. Both the Langmuir and Freundlich isotherms show best fits to the adsorption data in most cases, which is indicative of both physisorption and chemisorption mechanisms. Previous literature also indicates that the adsorption depends on the solution pH, surface functional groups on the adsorbent, particle size of the adsorbent, and metal ion concentration in the solution. Lastly, we recommend that the commercial application of these adsorbents be enforced and a standardized practice be established for the treatment of wastewater at the industrial level.
Author contributions
HH: Methodology, Formal Analysis, Writing – original draft. TB: Writing – review and editing, Project administration. JS: Methodology, Investigation, Writing – review and editing. AC: Writing – review and editing, Resources. LR: Data curation, Writing – review and editing. VB: Conceptualization, Writing – review and editing, Methodology. HB: Writing – review and editing, Visualization. MB: Project administration, Writing – review and editing, Supervision, Funding acquisition.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The authors wish to thank the Higher Education Commission of Pakistan for providing funding for the research project (no. 20-1915/R&D/10/5253).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abdel-Razek, A. (2011). Removal of chromium ions from liquid waste solutions using immobilized Cunninghamella elegans. Nat. Sci. 9 (7).
Abdu, K. Y., Endris, Y. A., and Shah, M. A. (2024). Chromium removal using a basalt rock adsorbent in a packed bed column for efficient environmental protection. ACS Omega 10 (1), 727–739. doi:10.1021/acsomega.4c07650
Abegunde, S. M., Idowu, K. S., Adejuwon, O. M., and Adeyemi-Adejolu, T. (2020). A review on the influence of chemical modification on the performance of adsorbents. Resour. Environ. Sustain. 1, 100001. doi:10.1016/j.resenv.2020.100001
Abshirini, Y., Foroutan, R., and Esmaeili, H. (2018). Cr (VI) removal from aqueous solution using activated carbon prepared from Ziziphus spina-christi leaf. Mater. Res. Express 6, 045607. doi:10.1088/2053-1591/aafb45
Abuein, M., and Belazi, A. (2009). Biosorption of chromium (VI) and mercury (II) from aqueous solutions by Eriobotrya japonica (Rosaceae). Asian J. Chem. 21 (3), 1707–1712.
Ag, M., and Maheswari, S. (2007). Uptake of hexavalent chromium from aqueous solution employing live, dead and immobilized bacterial biomass. J. Appl. Sci. Environ. Manage. 11 (4), 71–75.
Ahalya, N., Kanamadi, R., and Ramachandra, T. (2010). Removal of hexavalent chromium using coffee husk. Int. J. Environ. Pollut. 43 (1), 106–116. doi:10.1504/ijep.2010.035917
Ahmad, A., Ghazi, Z. A., Saeed, M., Ilyas, M., Ahmad, R., Muqsit Khattak, A., et al. (2017). A comparative study of the removal of Cr (VI) from synthetic solution using natural biosorbents. New J. Chem. 41 (19), 10799–10807. doi:10.1039/c7nj02026k
Ahmad, R., Rao, R. A. K., and Masood, M. M. (2005). Removal and recovery of Cr (VI) from synthetic and industrial wastewater using bark of Pinus roxburghii as an adsorbent. Water Qual. Res. J. Can. 40 (4), 462–468. doi:10.2166/wqrj.2005.049
Ahmadpour, A. (2011). Biosorption of hexavalent chromium ions from aqueous solutions using almond green hull as a low-cost biosorbent. Eur. J. Sci. Res. 58.
Ahmed, I., Attar, S., and Parande, M. (2012). Removal of hexavalent chromium (Cr (VI)) from industrial wastewater by using biomass adsorbent (rice husk carbone). Int. J. Adv. Eng. Res. Stud. 1 (2), 92–94.
Ahmet, S., and Tuzen, M. (2008). Biosorption of total chromium from aqueous solution by red algae Ceramium virgatum: equilibrium, kinetic and thermodynamic studies. J. Hazard. Mater. 160, 349–355. doi:10.1016/j.jhazmat.2008.03.005
Ajmal, M., Ali Khan Rao, R., Anwar, S., Ahmad, J., and Ahmad, R. (2003). Adsorption studies on rice husk: removal and recovery of Cd (II) from wastewater. Biores. Technol. 86 (2), 147–149. doi:10.1016/s0960-8524(02)00159-1
Al-Homaidan, A. A., Al-Qahtani, H. S., Al-Ghanayem, A. A., Ameen, F., and Ibraheem, I. B. (2018). Potential use of green algae as a biosorbent for hexavalent chromium removal from aqueous solutions. Saudi J. Biol. Sci. 25 (8), 1733–1738. doi:10.1016/j.sjbs.2018.07.011
Ali, A., Saeed, K., and Mabood, F. (2016). Removal of chromium (VI) from aqueous medium using chemically modified banana peels as efficient low-cost adsorbent. Alexandria Eng. J. 55 (3), 2933–2942. doi:10.1016/j.aej.2016.05.011
Ali, I. H., Al Mesfer, M. K., Khan, M. I., Danish, M., and Alghamdi, M. M. (2019). Exploring adsorption process of lead (II) and chromium (VI) ions from aqueous solutions on acid activated carbon prepared from Juniperus procera leaves. Processes 7 (4), 217. doi:10.3390/pr7040217
Aliabadi, M., Khazaei, I., Fakhraee, H., and Mousavian, M. T. H. (2012). Hexavalent chromium removal from aqueous solutions by using low-cost biological wastes: equilibrium and kinetic studies. Int. J. Environ. Sci. Technol. 9 (2), 319–326. doi:10.1007/s13762-012-0045-7
Aliabadi, M., Morshedzadeh, K., and Soheyli, H. (2006). Removal of hexavalent chromium from aqueous solution by lignocellulosic solid wastes. Int. J. Environ. Sci. Technol. 3 (3), 321–325. doi:10.1007/bf03325940
Alimohammad Kalhori, A., Jafari, H. R., Yavari, A. R., Prohić, E., and Ahmadzadeh Kokya, T. (2011). Evaluation of anthropogenic impacts on soil and regolith materials based on BCR sequential extraction analysis. Int. J. Environ. Res. 6 (1), 185–194.
Altun, T., and Pehlivan, E. (2012). Removal of Cr (VI) from aqueous solutions by modified walnut shells. Food Chem 132 (2), 693–700. doi:10.1016/j.foodchem.2011.10.099
Anbalagan, K., Kumar, P. S., and Karthikeyan, R. (2016). Adsorption of toxic Cr (VI) ions from aqueous solution by sulphuric acid modified Strychnos potatorum seeds in batch and column studies. Desalin. Water Treat. 57 (27), 12585–12607. doi:10.1080/19443994.2015.1049965
Anjana, K., Kaushik, A., Kiran, B., and Nisha, R. (2007). Biosorption of Cr (VI) by immobilized biomass of two indigenous strains of cyanobacteria isolated from metal contaminated soil. J. Hazard. Mater. 148 (1), 383–386. doi:10.1016/j.jhazmat.2007.02.051
Aravind, J., Sudha, G., Kanmani, P., Devisri, A. J., Dhivyalakshmi, S., and Raghavprasad, M. (2015). Equilibrium and kinetic study on chromium (VI) removal from simulated waste water using gooseberry seeds as a novel biosorbent. Glob. J. Environ. Sci. Manage. 1 (3), 233–244.
Arıca, M. Y., and Bayramoğlu, G. (2005). Cr (VI) biosorption from aqueous solutions using free and immobilized biomass of Lentinus sajor-caju: preparation and kinetic characterization. Colloids Surfaces A Physicochem. Eng. Asp. 253 (1-3), 203–211. doi:10.1016/j.colsurfa.2004.11.012
Ashraf, M., Maah, M., and Yusoff, I. (2011). Bioaccumulation of heavy metals in fish species collected from former tin mining catchment. Int. J. Environ. Res. 6 (1), 209–218.
Assefa, H., Singh, S., Olu, F. E., Dhanjal, D. S., Mani, D., Khan, N. A., et al. (2024). Advances in adsorption technologies for hexavalent chromium removal: mechanisms, materials, and optimization strategies. Desalination Water Treat. 319, 100576. doi:10.1016/j.dwt.2024.100576
Attia, A. A., Khedr, S. A., and Elkholy, S. A. (2010). Adsorption of chromium ion (VI) by acid activated carbon. Braz. J. Chem. Eng. 27 (1), 183–193. doi:10.1590/s0104-66322010000100016
Attia, H. G. (2012). A Comparison between cooking tea-waste and commercial activated carbon for removal of chromium from artificial wastewater. J. Eng. Dev. 16 (1).
Bankar, A. V., Kumar, A. R., and Zinjarde, S. S. (2009). Environmental and industrial applications of Yarrowia lipolytica. Appl. Microbiol. Biotechnol. 84 (5), 847–865. doi:10.1007/s00253-009-2156-8
Barriada, J. L., Caridad, S., Lodeiro, P., Herrero, R., and de Vicente, M. E. S. (2009). Physicochemical characterisation of the ubiquitous bracken fern as useful biomaterial for pre-concentration of heavy metals. Biores. Technol. 100, 1561–1567. doi:10.1016/j.biortech.2008.09.027
Baruah, J., Nath, B. K., Sharma, R., Kumar, S., Deka, R. C., Baruah, D. C., et al. (2018). Recent trends in the pretreatment of lignocellulosic biomass for value-added products. Front. Energy Res. 6, 141. doi:10.3389/fenrg.2018.00141
Basnet, P., Gyawali, D., Nath Ghimire, K., and Paudyal, H. (2022). An assessment of the lignocellulose-based biosorbents in removing Cr (VI) from contaminated water: a critical review. Results Chem. 4, 100406. doi:10.1016/j.rechem.2022.100406
Bayramoglu, G., Akbulut, A., and Arica, M. Y. (2016). Aminopyridine modified Spirulina platensis biomass for chromium (VI) adsorption in aqueous solution. Water Sci. Technol. 74 (4), 914–926. doi:10.2166/wst.2016.281
Bayuo, J., Pelig-Ba, K. B., and Abukari, M. A. (2019). Adsorptive removal of chromium (VI) from aqueous solution unto groundnut shell. Appl. Water Sci. 9 (4), 107. doi:10.1007/s13201-019-0987-8
Begum, K. M. T. M., and Alhaji, N. (2013). Adsorption efficiency of sawdust in activated and surface-modified forms-A comparative study. Chemical Science Transactions, 2(4), 1364–1369.
Bhatti, I., Qureshi, K., Kazi, R. A., and Ansari, A. K. (2008). Preparation and characterisation of chemically activated almond shells by optimization of adsorption parameters for removal of chromium VI from aqueous solutions. Int. J. Chem. Biomol. Eng. 1 (3).
Boyle, O., Xiao, B., and Mangwandi, C. (2025). Valorization of banana peel waste into advanced adsorbent beads for the removal of emerging pollutants from wastewater. Materials 18 (5), 1084. doi:10.3390/ma18051084
Brusseau, M., and Artiola, J. (2019). “Chemical contaminants,” in Environmental and pollution science (Elsevier), 175–190.
Campaña-Pérez, J. F., Portero Barahona, P., Martín-Ramos, P., and Carvajal Barriga, E. J. (2019). Ecuadorian yeast species as microbial particles for Cr (VI) biosorption. Environ. Sci. Pollut. Res., 1–11. doi:10.1007/s11356-019-06035-8
Chakravarty, R., Khan, M. M. R., Das, A. R., and Guha, A. K. (2013). Biosorptive removal of chromium by husk of Lathyrus sativus: Evaluation of the binding mechanism, kinetic and equilibrium study. Engineering in Life Sciences, 13(3), 312–322. doi:10.1002/elsc.201200044
Chanda, R. K., Jahid, T., Hassan, M. N., Yeasmin, T., and Biswas, B. K. (2024). Cauliflower stem-derived biochar for effective adsorption and reduction of hexavalent chromium in synthetic wastewater: a sustainable approach. Environ. Adv. 15, 100458. doi:10.1016/j.envadv.2023.100458
Chatterjee, S., Ghosh, I., and Mukherjea, K. K. (2011). Uptake and removal of toxic Cr (VI) by Pseudomonas aeruginosa: physico-chemical and biological evaluation. Curr. Sci. 101 (5), 00113891.
Chen, D., Zhang, J., and Chen, J. (2010). Adsorption of methyl tert-butyl ether using granular activated carbon: equilibrium and kinetic analysis. Int. J. Environ. Sci. Technol. 7 (2), 235–242. doi:10.1007/bf03326133
Cherdchoo, W., Nithettham, S., and Charoenpanich, J. (2019). Removal of Cr (VI) from synthetic wastewater by adsorption onto coffee ground and mixed waste tea. Chemosphere 221, 758–767. doi:10.1016/j.chemosphere.2019.01.100
Çimen, A. (2015). Removal of chromium from wastewater by reverse osmosis. Russ. J. Phys. Chem. A 89 (7), 1238–1243. doi:10.1134/s0036024415070055
Cristina, Q., Zelia, R., Bruna, F., Hugo, F., Teresa, T., and Tavares, T. (2009). Biosorptive performance of an Escherichia coli biofilm supported on zeolite naY for the removal of Cr (VI), Cd (II), Fe (III) and Ni(II). Chem. Eng. J. 152, 110–115. doi:10.1016/j.cej.2009.03.039
Dang, V., Doan, H., Dang-Vu, T., and Lohi, A. (2009). Equilibrium and kinetics of biosorption of cadmium (II) and copper (II) ions by wheat straw. Biores. Technol. 100 (1), 211–219. doi:10.1016/j.biortech.2008.05.031
da Rocha Ferreira, G. L., Vendruscolo, F., and Antoniosi Filho, N. R. (2019). Biosorption of hexavalent chromium by Pleurotus ostreatus. Heliyon 5 (3), e01450. doi:10.1016/j.heliyon.2019.e01450
Das, D. D., Mahapatra, R., Pradhan, J., Das, S. N., and Thakur, R. S. (2000). Removal of Cr (VI) from aqueous solution using activated cow dung carbon. J. Colloid Interface Sci. 232 (2), 235–240. doi:10.1006/jcis.2000.7141
Dave, P. N., Pandey, N., and Thomas, H. (2012). Adsorption of Cr (VI) from aqueous solutions on tea waste and coconut husk. Indian J. Chem. Technol. 19 (2), 111–117.
Deepali, P. (2011). Bioremediation of chromium (vi) from textile industry’s effluent and contaminated soil using Pseudomonas putida. Iran. J. Energy Environ. 2 (1), 24–31.
Demirbas, E., Kobya, M., and Konukmanc, A. E. S. (2008). Error analysis of equilibrium studies for the almond shell activated carbon adsorption of Cr (VI) from aqueous solutions. J. Hazard. Mater. 154, 787–794. doi:10.1016/j.jhazmat.2007.10.094
Demirbas, E., Kobya, M., Senturk, E., and Ozkan, T. (2004). Adsorption kinetics for the removal of chromium(VI) from aqueous solutions on the activated carbons prepared from agricultural wastes. Water SA 30 (4), 533–540. doi:10.4314/wsa.v30i4.5106
Dhal, B., and Pandey, B. D. (2018). Mechanism elucidation and adsorbent characterization for removal of Cr (VI) by native fungal adsorbent. Sustain. Environ. Res. 28 (6), 289–297. doi:10.1016/j.serj.2018.05.002
Dizadji, N., Abootalebi Anaraki, N., and Nouri, N. (2011). Adsorption of chromium and copper in aqueous solutions using tea residue. Int. J. Environ. Sci. Technol. 8 (3), 631–638. doi:10.1007/BF03326248
Dönmez, G., and Aksu, Z. (2002). Removal of chromium(VI) from saline wastewaters by Dunaliella species. Process Biochem. 38 (5), 751–762. doi:10.1016/s0032-9592(02)00204-2
Elhami, S., and Bahadori, A. (2015). “Using modified sawdust for removal of chromium (VI) from aqueous solutions,” in Biological forum. Research Trend.
El-Sikaily, A., Nemr, A. E., Khaled, A., and Abdelwehab, O. (2007). Removal of toxic chromium from wastewater using green alga Ulva lactuca and its activated carbon. J. Hazard. Mater. 148 (1), 216–228. doi:10.1016/j.jhazmat.2007.01.146
Erdem, E., Karapinar, N., and Donat, R. (2004). The removal of heavy metal cations by natural zeolites. J. Colloid Interface Sci. 280 (2), 309–314. doi:10.1016/j.jcis.2004.08.028
Ertaş, R., and Öztürk, N. (2013). Removal of Cr (VI) from aqueous solution onto chestnut shell: application of full factorial design and equilibrium studies. Desalination and Water Treatment, 51(13-15), 2909-2914. doi:10.1080/19443994.2012.748296
Erwa, I. Y., and Ibrahim, A. A. (2016). Removal of chromium (VI) ions from aqueous solution using wood sawdust as adsorbent. J. Sci. Technol. 17 (2).
Esmaeili, A., Ghasemi, S., and Rustaiyan, A. (2010). Removal of hexavalent chromium using activated carbons derived from marine algae gracilaria and sargassum sp. J. Mar. Sci. Technol 18 (4), 587–592. doi:10.51400/2709-6998.1922
Fauzia, S., Aziz, H., Dahlan, D., and Zein, R. (2021). Modelling for removal of Cr (VI) and Pb (II) using sago bark (Metroxylon sagu) by fixed-bed column method. Egypt. J. Chem. 64 (8), 3981–3989. doi:10.21608/ejchem.2020.20172.2212
Gebrehawaria, G., Hussen, A., and Rao, V. (2015). Removal of hexavalent chromium from aqueous solutions using barks of Acacia albida and leaves of Euclea schimperi. Int. J. Environ. Sci. Technol. 12 (5), 1569–1580. doi:10.1007/s13762-014-0530-2
Ghaneian, M. T., Bhatnagar, A., Ehrampoush, M. H., Amrollahi, M., Jamshidi, B., Dehvari, M., et al. (2017). Biosorption of hexavalent chromium from aqueous solution onto pomegranate seeds: kinetic modeling studies. International journal of environmental science and technology, 14, 331–340. doi:10.1007/s13762-014-0530-2
Ghaderi, A. A., Abduli, M. A., Karbassi, A. R., Nasrabadi, T., and Khajeh, M. (2012). Evaluating the effects of fertilizers on bioavailable metallic pollution of soils, case study of sistan farms, Iran. Int. J. Environ. Res. 6 (2), 565–570.
Gning, A. S., Gaye, C., Kama, A. B., Diaw, P. A., Thiare, D. D., and Fall, M. (2024). Hexavalent chromium Cr (VI) removal from water by mango kernel powder. J. Mater. Sci. Chem. Eng. 12 (1), 84–103. doi:10.4236/msce.2024.121007
Gode, F., Atalay, E. D., and Pehlivan, E. (2008). Removal of Cr (VI) from aqueous solutions using modified red pine sawdust. J. Hazard. Mater. 152 (3), 1201–1207. doi:10.1016/j.jhazmat.2007.07.104
Gómez Aguilar, D. L., Rodríguez Miranda, J. P., Esteban Muñoz, J. A., and Betancur P., J. F. (2019). Coffee pulp: a sustainable alternative removal of Cr (VI) in wastewaters. Processes 7 (7), 403. doi:10.3390/pr7070403
Gopalakrishnan, S., Kannadasan, T., Velmurugan, S., Muthu, S., and Vinoth Kumar, P. (2013). Biosorption of chromium (VI) from industrial effluent using neem leaf adsorbent. Res. J. Chem. Sci. 3 (4), 48–53.
Goswami, M., and Phukan, P. (2017). Enhanced adsorption of cationic dyes using sulfonic acid modified activated carbon. J. Environ. Chem. Eng. 5 (4), 3508–3517. doi:10.1016/j.jece.2017.07.016
Gunasundari, E., and Kumar, P. S. (2016). Higher adsorption capacity of Spirulina platensis alga for Cr (VI) ions removal: parameter optimisation, equilibrium, kinetic and thermodynamic predictions. IET Nanobiotechnol. 11 (3), 317–328. doi:10.1049/iet-nbt.2016.0121
Gupta, S., Kumar, D., and Gaur, J. P. (2009). Kinetic and isotherm modeling of lead (II) sorption onto some waste plant materials. Chem. Eng. J. 148, 226–233. doi:10.1016/j.cej.2008.08.019
Gupta, V., and Rastogi, A. (2008). Sorption and desorption studies of chromium(VI) from nonviable cyanobacterium Nostoc muscorum biomass. J. Hazard. Mater. 154 (1), 347–354. doi:10.1016/j.jhazmat.2007.10.032
Gupta, V., and Rastogi, A. (2009). Biosorption of hexavalent chromium by raw and acid-treated green alga Oedogonium hatei from aqueous solutions. J. Hazard. Mater. 163 (1), 396–402. doi:10.1016/j.jhazmat.2008.06.104
Hadjmohammadi, M. R., Salary, M., and Biparva, P. (2011). Removal of Cr (VI) from aqueous solution using pine needles powder as a biosorbent. J. Appl. Sci. Environ. Sanitation 6 (1), 1–13.
Haleem, A. M., and Abdulgafoor, E. A. (2010). The biosorption of Cr (VI) from aqueous solution using date palm fibers (leef). Al-Khawarizmi Eng. J. 6 (4), 31–36.
Harmon, G., Patrick, R., and Spittler, T. (2007). Removal of heavy metals from polluted waters using lignocellulosic agricultural waste products. Ind. Biotechnol. 3 (4), 366–374. doi:10.1089/ind.2007.3.366
Haroon, H., Ashfaq, T., Gardazi, S. M. H., Sherazi, T. A., Ali, M., Rashid, N., et al. (2016). Equilibrium kinetic and thermodynamic studies of Cr (VI) adsorption onto a novel adsorbent of Eucalyptus camaldulensis waste: batch and column reactors. Kor. J. Chem. Eng. 33 (10), 2898–2907. doi:10.1007/s11814-016-0160-0
Haroon, H., Gardazi, S. M. H., Butt, T. A., Pervez, A., Mahmood, Q., and Bilal, M. (2017). Novel lignocellulosic wastes for comparative adsorption of Cr (VI): equilibrium kinetics and thermodynamic studies. Pol. J. Chem. Technol. 19 (2), 6–15. doi:10.1515/pjct-2017-0021
Haroon, H., Shah, J. A., Khan, M. S., Alam, T., Khan, R., Asad, S. A., et al. (2020). Activated carbon from a specific plant precursor biomass for hazardous Cr (VI) adsorption and recovery studies in batch and column reactors: isotherm and kinetic modeling. J. Water Process Eng. 38, 101577. doi:10.1016/j.jwpe.2020.101577
Hashem, M. A., Nur-A-Tomal, M. S., Mondal, N. R., and Rahman, M. A. (2017). Hair burning and liming in tanneries is a source of pollution by arsenic, lead, zinc, manganese and iron. Environ. Chem. Lett. 15 (3), 501–506. doi:10.1007/s10311-017-0634-2
Homagaia, P. L., Ghimirea, K. N., and Inoueb, K. (2010). Adsorption behavior of heavy metals onto chemically modified sugarcane bagasse. Biores. Technol. 101 (6), 2067–2069. doi:10.1016/j.biortech.2009.11.073
Husien, S., Labena, A., El-Belely, E. F., Mahmoud, H. M., and Hamouda, A. S. (2019). Absorption of hexavalent chromium by green micro algae Chlorella sorokiniana: live planktonic cells. Water Pract. Technol. 14, 515–529. doi:10.2166/wpt.2019.034
Ilyas, M., Ahmad, A., Ghazi, Z. A., Sohail, M., Arif, M., and Khan, Z. U. (2014). Investigation of the activity of sawdust as biosorbent towards Cr (VI) removal from waste water: kinetic an thermodynamic studies. Int. J. Environ. Sci. 3, 5–12.
Indhumathi, P., Syed Shabudeen, P.S., Shoba, U.S., and Saraswathy, C.P. (2014). The removal of chromium from aqueous solution by using green micro algae. J. Chem. Pharm. Res. 6 (6), 799–808.
Jayakumar, R., Rajasimman, M., and Karthikeyan, C. (2015). Sorption and desorption of hexavalent chromium using a novel brown marine algae Sargassum myriocystum. Kor. J. Chem. Eng. 32 (10), 2031–2046. doi:10.1007/s11814-015-0036-8
Jeyaseelan, C., and Gupta, A. (2016). Green tea leaves as a natural adsorbent for the removal of Cr VI from aqueous solutions. Air Soil Water Res. 9, ASWR.S35227. ASWR. S35227. doi:10.4137/aswr.s35227
Jha, B. (2016). Chromium removal from aqueous medium using modified sawdust J. Nat. Sci. Res. 6(11): p. 47–50.
Kainth, S., Sharma, P., and Pandey, O. P. (2024). Green sorbents from agricultural wastes: a review of sustainable adsorption materials. Appl. Surf. Sci. Adv. 19, 100562. doi:10.1016/j.apsadv.2023.100562
Kali, A., Dehmani, Y., Amar, A., Loulidi, I., El-kordy, A., Jabri, M., et al. (2025). Almond shells based-lignocellulosic waste materials for a high removal efficacy of chromium (VI) ions from waste water. Interactions 246 (1), 44. doi:10.1007/s10751-025-02264-1
Kargar, M., Khorasani, N. A., Karamir, M., Rafiee, G. H., and Naseh, R. (2011). An investigation on as, Cd, Mo and Cu contents of soils surrounding the meyduk tailings dam. Int. J. Environ. Res. 6 (1), 173–184.
Karthikeyan, B., and Mythili, K. (2011). Bioremediation of Cr (VI) from Tannery effluent using Bacillus spp and Staphylococcus spp. Int. J. Multidiscip. Res. J. 1 (6).
Karthikeyan, T., Rajgopal, S., and Miranda, L. R. (2005). Chromium(VI) adsorption from aqueous solution by sawdust activated carbon. J. Hazard. Mater 124, 192–199. doi:10.1016/j.jhazmat.2005.05.003
Katiyar, S., and Katiyar, R. (2024). A comprehensive review on synthesis and application of nanocomposites for adsorption of chromium: status and future prospective. Appl. Water Sci. 14 (1), 11. doi:10.1007/s13201-023-02062-6
Kebede, A., Kedir, K., Melak, F., and Asere, T. G. (2022). Removal of Cr (VI) from aqueous solutions using biowastes: tella residue and pea (pisum sativum) seed shell. Sci. World J. 2022, 1–12. doi:10.1155/2022/7554133
Khalil, U., Shakoor, M. B., Ali, S., Ahmad, S. R., Rizwan, M., Alsahli, A. A., et al. (2021). Selective removal of hexavalent chromium from wastewater by rice husk: kinetic, isotherm and spectroscopic investigation. Water 13 (3), 263. doi:10.3390/w13030263
Khan, T., Isa, M. H., Ul Mustafa, M. R., Yeek-Chia, H., Baloo, L., Binti Abd Manan, T. S., et al. (2016). Cr (VI) adsorption from aqueous solution by an agricultural waste based carbon. RSC Adv. 6 (61), 56365–56374. doi:10.1039/c6ra05618k
Khaskheli, M. I., Memon, S. Q., Chandio, Z. A., Jatoi, W. B., Mahar, M. T., and Khokhar, F. M. (2016). Okra leaves—agricultural waste for the removal of Cr (III) and Cr (VI) from contaminated water. Am. J. Anal. Chem. 7 (04), 395–409. doi:10.4236/ajac.2016.74037
Khazaei, I., Aliabadi, M., and Mosavian, H. H. (2011). Use of agricultural waste for removal of Cr (VI) from aqueous solution. Iran. J. Chem. Eng. 8 (4).
Kim, H., Baek, K., Kim, B. K., Shin, H. J., and Yang, J. W. (2008). Removal characteristics of metal cations and their mixtures using micellar-enhanced ultrafiltration. Kor. J. Chem. Eng. 25 (2), 253–258. doi:10.1007/s11814-008-0045-y
Kiran, B., Kaushik, A., and Kaushik, C. (2007). Biosorption of Cr(VI) by native isolate of Lyngbya putealis (HH-15) in the presence of salts. J. Hazard. Mater. 141 (3), 662–667. doi:10.1016/j.jhazmat.2006.07.026
Kumar, A., and Jena, H. M. (2017). Adsorption of Cr (VI) from aqueous phase by high surface area activated carbon prepared by chemical activation with ZnCl2. Process Saf. Environ. Prot. 109, 63–71. doi:10.1016/j.psep.2017.03.032
Kumar, M., and Saini, H. S. (2024). Novel approaches for sustainable management of chromium contaminated wastewater.
Kumar, R., Arya, D. K., Singh, N., and Vats, H. K. (2017). Removal of Cr (VI) using low cost activated carbon developed by agricultural waste. IOSR J. Appl. Chem. 10 (1), 76–79. doi:10.9790/5736-1001017679
Kurniasih, A. H., Sulaeman, A., and Kardena, E. (2013). Biosorption of Chromium (VI) using immobilized algal-bloom biomass: Kinetics and Equilibrium studies. International Journal of Environmental and Resource, 2, 24-31.
Kushwaha, P., and Chakraborty, S. (2021). Removal of Cr VI from synthetic wastewater by low cost adsorbent developed from amla wood sawdust (emblica officinalis). Int. J. Eng. Res. Technol. 10, 246–248.
Lee, H., Hamid, S. B. A., and Zain, S. (2014). Conversion of lignocellulosic biomass to nanocellulose: structure and chemical process. Sci. World J. 2014 (1), 1–20. doi:10.1155/2014/631013
Lesaoana, M., Mlaba, R., Mtunzi, F., Klink, M., Ejidike, P., and Pakade, V. (2019). Influence of inorganic acid modification on Cr (VI) adsorption performance and the physicochemical properties of activated carbon. South Afr. J. Chem. Eng. 28, 8–18. doi:10.1016/j.sajce.2019.01.001
Li, L.-L., Feng, X. Q., Han, R. P., Zang, S. Q., and Yang, G. (2017). Cr VI removal via anion exchange on a silver-triazolate MOF. J. Hazard. Mater. 321, 622–628. doi:10.1016/j.jhazmat.2016.09.029
Li, Y. X., Wang, Y., and Zhao, F. J. (2015). Kinetic and equilibrium studies of chromium (VI) biosorption by spent macroalgae Polysiphonia urceolata and Chondrus ocellatus. Biotechnol. Biotechnol. Equip. 29 (3), 498–505. doi:10.1080/13102818.2015.1011374
Liang, S., Guo, X. Y., Fend, N. C., and Tian, Q. H. (2009). Application of orange peel xanthate for the adsorption of Pb2+ from aqueous solutions. J. Hazard. Mater. 170 (1), 425–429. doi:10.1016/j.jhazmat.2009.04.078
Liu, G., Liao, L., Dai, Z., Qi, Q., Wu, J., Ma, L. Q., et al. (2020). Organic adsorbents modified with citric acid and Fe3O4 enhance the removal of Cd and Pb in contaminated solutions. Chem. Eng. J. 395, 125108. doi:10.1016/j.cej.2020.125108
Logeswari, A., Mano, S., Xavier, A. M., Thirumarimurugan, M., and Kannadasan, T. (2013). Removal of chromium from synthetic tannery effluent by using bioadsorbents. IOSR J. Environ. Sci. Toxicol. Food. Technol. 3 (1), 72–76.
Madhuranthakam, C. M. R., Thomas, A., Akhter, Z., Fernandes, S. Q., and Elkamel, A. (2021). Removal of chromium (VI) from contaminated water using untreated moringa leaves as biosorbent. Pollutants 1 (1), 51–64. doi:10.3390/pollutants1010005
Mahajan, G., and Sud, D. (2011). Kinetics and equilibrium studies of cr (vi) metal ion remediation by arachis hypogea shells: a green approach. BioResources 6 (3), 3324–3338. doi:10.15376/biores.6.3.3324-3338
Mahajan, G., and Sud, D. (2013). Bioremediation of cr (vi) metal ion from aqueous solutions using modified lignocellulosic agricultural waste biomass. Int. J. Adv. Technol. Eng. Res. 3 (2).
Maheshwari, U., and Gupta, S. (2016). Removal of Cr (VI) from wastewater using activated neem bark in a fixed-bed column: interference of other ions and kinetic modelling studies. Desalin. Water Treat. 57 (18), 8514–8525. doi:10.1080/19443994.2015.1030709
Mahvi, A. H., Nabizadeh, R., Gholami, F., and Khairi, A. (2007). Adsorption of chromium from wastewater by platanus orientalis leaves. Iran. J. Environ. Health Sci. Eng. 4 (3).
Majumder, R., Sheikh, L., Naskar, A., Vineeta, , Mukherjee, M., and Tripathy, S. (2017). Depletion of Cr (VI) from aqueous solution by heat dried biomass of a newly isolated fungus Arthrinium malaysianum: a mechanistic approach. Sci. Rep. 7 (1), 11254. doi:10.1038/s41598-017-10160-0
Malwade, K., Lataye, D., Mhaisalkar, V., Kurwadkar, S., and Ramirez, D. (2016). Adsorption of hexavalent chromium onto activated carbon derived from Leucaena leucocephala waste sawdust: kinetics, equilibrium and thermodynamics. Int. J. Environ. Sci. Technol. 13 (9), 2107–2116. doi:10.1007/s13762-016-1042-z
Mancilla, H. B., Cerrón, M. R., Aroni, P. G., Paucar, J. E. P., Tovar, C. T., Jindal, M. K., et al. (2023). Effective removal of Cr (VI) ions using low-cost biomass leaves (Sambucus nigra L.) in aqueous solution. Environ. Sci. Pollut. Res. 30 (49), 106982–106995. doi:10.1007/s11356-022-24064-8
Mane, P. C., Bhosle, A. B., Deshmukh, P. D., and Jangam, C. M. (2010). Chromium Adsorption onto activated carbon Derived from tendu (Diospyros melanoxylon) leaf refuse: Influence of metal/Carbon ratio, time and pH. Adv. Appl. Sci. Res. 1 (3), 212–221.
Manfe, M. M., Attar, S. J., Parande, M., and Topare, N. S. (2012). Treatment of Cr(VI) contaminated waste water using biosorbent prunus amygdalus(almond) nut shell carbon. Int. J. Chem. Sci. 10 (2), 609–618.
Manjusha, A., Gandhi, N., and Sirisha, D. (2012). Removal of Chromium (VI) from paint manufacturing industry waste water by using Papaya peel powder. Int. J. Pharma World Res. 3(2).
Martins, B. L., Cruz, C. C. V., Luna, A. S., and Henriques, C. A. (2006). Sorption and desorption of Pb2+ ions by dead sargassum sp biomass. Biochem. Eng. J. 27, 310–314. doi:10.1016/j.bej.2005.08.007
Mathai, R. V., Mitra, J. C., Sar, S. K., and Jindal, M. K. (2022). Adsorption of Chromium (VI) from aqueous phase using Aegle marmelos leaves: kinetics, isotherm and thermodynamic studies. Chem. Data Collect. 39, 100871. doi:10.1016/j.cdc.2022.100871
Meena, A. K., Kadirvelu, K., Mishra, G., Rajagopal, C., and Nagar, P. (2008). Adsorptive removal of heavy metals from aqueous solution by treated sawdust (Acacia arabica). J. Hazard. Mater. 150 (3), 604–611. doi:10.1016/j.jhazmat.2007.05.030
Mehr, M. R., Fekri, M. H., Omidali, F., Eftekhari, N., and Akbari-Adergani, B. (2019). Removal of Chromium (VI) from Wastewater by Palm Kernel Shell-based on a Green Method. Journal of Chemical Health Risks, 9 (1). doi:10.22034/jchr.2019.584177.1012
Mondal, N. K., Samanta, A., Dutta, S., and Chattoraj, S. (2017). Optimization of Cr (VI) biosorption onto Aspergillus Niger using 3-level Box-Behnken design: equilibrium, kinetic, thermodynamic and regeneration studies. J. Genet. Eng. Biotechnol. 15 (1), 151–160. doi:10.1016/j.jgeb.2017.01.006
Moniruzzaman, M., Rahman, M., Aktar, S., and Khan, M. (2017). Equilibrium and kinetic parameters determination of Cr (VI) adsorption by hogla leaves (Typha elephantina Roxb.). Int. J. Waste Resour. 7 (301), 2. doi:10.4172/2252-5211.1000301
Monser, L., and Adhoum, N. (2002). Modified activated carbon for the removal of copper, zinc, chromium and cyanide from wastewater. Sep. Purif. Technol. 26, 137–146. doi:10.1016/s1383-5866(01)00155-1
Mosavian, M. H., Khazaei, I., and Aliabadi, M. (2012). Use of sawdust of aspen tree for the removal of chromium VI from aqueous solution. Iran. J. Earth Sci. 4.
Moussavi, G., and Barikbin, B. (2010). Biosorption of chromium VI from industrial wastewater onto pistachio hull waste biomass. Chem. Eng. J. 162 (3), 893–900. doi:10.1016/j.cej.2010.06.032
Muhammad, N., Mahmood, A., Shahid, S. A., Shah, S. S., Khalid, A. M., and McKay, G. (2006). Sorption of lead from aqueous solution by chemically modified carbon adsorbents. J. Hazard. Mater 138, 604–613. doi:10.1016/j.jhazmat.2006.05.098
Murugesan, A., Vidhyadevi, T., Kirupha, S. D., Ravikumar, L., and Sivanesan, S. (2012). Removal of chromium (VI) from aqueous solution using chemically modified corncorb-activated carbon: equilibrium and kinetic studies. Environ. Prog. Sustain. Energy 32, 673–680. doi:10.1002/ep.11684
Muthulakshmi, A., and Baskaran, R. (2017). Adsorption of hexavalent chromium in industrial water using Pithecellobium dulce bark. Res. J. Pharm. Biol. Chem. 8 (1), 1450–1456.
Muthumareeswaran, M., Alhoshan, M., and Agarwal, G. P. (2017). Ultrafiltration membrane for effective removal of chromium ions from potable water. Sci. Rep. 7, 41423. doi:10.1038/srep41423
Mzoughi, N., and Chouba, L. (2011). Heavy metals and PAH assessment based on mussel caging in the North coast of Tunisia (Mediterranean Sea). Int. J. Environ. Res. 6 (1), 109–118.
Nag, S., Mondal, A., Mishra, U., Bar, N., and Das, S. K. (2016). Removal of chromium (VI) from aqueous solutions using rubber leaf powder: batch and column studies. Desalin. Water Treat. 57 (36), 1–16. doi:10.1080/19443994.2015.1083893
Naiya, T. K., Singha, B., and Das, S. K. (2011). FT-IR study for the Cr (VI) removal from aqueous solution using rice waste. Int. Proc. Chem. Biol. Environ. Eng. 10, 114.
Nasseh, N., Taghavi, L., Barikbin, B., and Harifi-Mood, A. R. (2016). The removal of Cr (VI) from aqueous solution by almond green hull waste material: kinetic and equilibrium studies. J. Water Reuse Desalin. 7 (4), 449–460. doi:10.2166/wrd.2016.047
Nie, Y., Zhou, Y., Zhang, Y., Sun, D., Wu, D., Ban, L., et al. (2025). Sustainable synthesis of functional materials assisted by deep eutectic solvents for biomedical, environmental, and energy applications. Adv. Funct. Mater. 35, 2418957. doi:10.1002/adfm.202418957
Nikazar, M., Davarpanah, L., and Vahabzadeh, F. (2008). Biosorption of aqueous chromium (VI) by living mycelium of Phanerochaete chrysosporium. Chem. Eng. Trans. 14, 475–480.
Njoya, O., Zhao, S., Kong, X., Shen, J., Kang, J., Wang, B., et al. (2021). Efficiency and potential mechanism of complete Cr (VI) removal in the presence of oxalate by catalytic reduction coupled with membrane filtration. Sep. Purif. Technol. 275, 118915. doi:10.1016/j.seppur.2021.118915
Odoemelam, S. A., Iroh, C. U., and Igwe, J. C. (2011). Copper (II), cadmium (II) and lead (II) adsorption kinetics from aqueous metal solutions using chemically modified and unmodified cocoa pod husk (theobroma cacao) waste biomass. Res. J. Appl. Sci. 6, 44–52. doi:10.3923/rjasci.2011.44.52
Ofomaja, A. E., and Ho, Y. (2007). Effect of pH on cadmium biosorption by coconut copra meal. J. Hazard. Mater 139, 356–362. doi:10.1016/j.jhazmat.2006.06.039
Opeolu, B. (2009). Utilization of maize (Zea mays) cob as an adsorbent for lead (II) removal from aqueous solutions and industrial effluents. Afr. J. Biotechnol. 8 (8).
Opeolu, B. O., Bamgbose, O., Arowolo, T. A., and Adetunji, M. T (2010). Utilization of biomaterials as adsorbents for heavy metals’ removal from aqueous matrices. Sci. Res. Essays 5 (14), 1780–1787.
Özsin, G., Kılıç, M., Apaydın-Varol, E., and Pütün, A. E. (2019). Chemically activated carbon production from agricultural waste of chickpea and its application for heavy metal adsorption: equilibrium, kinetic, and thermodynamic studies. Appl. Water Sci. 9 (3), 56. doi:10.1007/s13201-019-0942-8
Pal, J., and Kaur, M. (2013). Adsorption of hexavalent chromium from textile industry effluent by sawdust of Dalbergia sissoo. J. Environ. Sci. Sustain. 1 (1), 34–40.
Pandhram, P., and Nimbalkar, S. (2013). Adsorption of chromium from industrial waste water by using neem leaves as a low cost adsorbent. Int. J. Chem. Phys. Sci. 2 (Special Issue).
Parlayici, Ş., and Pehlivan, E. (2019). Comparative study of Cr (VI) removal by bio-waste adsorbents: equilibrium, kinetics, and thermodynamic. J. Anal. Sci. Technol. 10 (1), 15–18. doi:10.1186/s40543-019-0175-3
Parvathi, K., and Nagendran, R. (2007). Biosorption of chromium from effluent generated in chrome-electroplating unit using Saccharomyces cerevisiae. Sep. Sci. Technol. 42 (3), 625–638. doi:10.1080/01496390601070158
Pathania, D., Sharma, A., and Srivastava, A. (2020). Modelling studies for remediation of Cr (VI) from wastewater by activated Mangifera indica bark. Curr. Res. Green Sustain. Chem. 3, 100034. doi:10.1016/j.crgsc.2020.100034
Peng, H., Guo, J., Li, B., Liu, Z., and Tao, C. (2018). High-efficient recovery of chromium VI with lead sulfate. J. Taiwan Inst. Chem. Eng. 85, 149–154. doi:10.1016/j.jtice.2018.01.028
Peng, H., Leng, Y., and Guo, J. (2019). Electrochemical removal of chromium (VI) from wastewater. Appl. Sci. 9 (6), 1156. doi:10.3390/app9061156
Politi, D., and Sidiras, D. (2012). Chromium (VI) purification using pine sawdust in batch systems. AIP Conf. Proc., 1079–1082. doi:10.1063/1.4772113
Politi, D., and Sidiras, D. (2020). Modified spruce sawdust for sorption of hexavalent chromium in batch systems and fixed-bed columns. Molecules 25 (21), 5156. doi:10.3390/molecules25215156
Pourkarim, S., Ostovar, F., Mahdavianpour, M., and Moslemzadeh, M. (2017). Adsorption of chromium (VI) from aqueous solution by Artist’s Bracket fungi. Sep. Sci. Technol. 52 (10), 1733–1741. doi:10.1080/01496395.2017.1299179
Prajapati, A. K., Verma, P., Singh, S., and Mondal, M. K. (2022). Adsorption-desorption surface bindings, kinetics, and mass transfer behavior of thermally and chemically treated great millet husk towards Cr (VI) removal from synthetic wastewater. Adsorpt. Sci. Technol. 2022. doi:10.1155/2022/3956977
Prasanna Kumar, P., Prasanna Kumar, Y., and Venkateswara Rao, B. (2017). Removal and equilibrium studies of Cr (VI) using Ulva fasciata sp. (marine algae) and optimization of Cr(VI) removal by response surface methodology. Int. J. Appl. Environ. Sci. 12 (8), 1479–1496.
Qaiser, S., Saleemi, A. R., and Umar, M. (2009). Biosorption of lead (II) and chromium (VI) on groundnut hull: equilibrium, kinetics and thermodynamics study. Electron. J. Biotechnol. 12 (4), 3–4. doi:10.2225/vol12-issue4-fulltext-6
Qiang, W., Jiaoyan, S., and Mengzhu, S. (2011). Characteristic of adsorption, desorption and oxidation of Cr (III) on birnessite. Energy Procedia 5, 1104–1108. doi:10.1016/j.egypro.2011.03.194
Rai, M., Giri, B. S., Nath, Y., Bajaj, H., Soni, S., Singh, R. P., et al. (2018). Adsorption of hexavalent chromium from aqueous solution by activated carbon prepared from almond shell: kinetics, equilibrium and thermodynamics study. J. Water Supply. Res. Technol. Aqua 67 (8), 724–737. doi:10.2166/aqua.2018.047
Ramadoss, R., and Subramaniam, D. (2018). Adsorption of chromium using blue green algae-modeling and application of various isotherms. Int. J. Chem. Technol. 10, 1–22.
Ramirez, A., Ocampo, R., Giraldo, S., Padilla, E., Flórez, E., and Acelas, N. (2020). Removal of Cr (VI) from an aqueous solution using an activated carbon obtained from teakwood sawdust: kinetics, equilibrium, and density functional theory calculations. J. Environ. Chem. Eng. 8 (2), 103702. doi:10.1016/j.jece.2020.103702
Rangabhashiyam, S., and Selvaraju, N. (2015). Evaluation of the biosorption potential of a novel Caryota urens inflorescence waste biomass for the removal of hexavalent chromium from aqueous solutions. Journal of the Taiwan Institute of Chemical Engineers, 47, 59-70.
Rangabhashiyam, S., Suganya, E., and Selvaraju, N. (2016). Packed bed column investigation on hexavalent chromium adsorption using activated carbon prepared from Swietenia Mahogani fruit shells. Desalin. Water Treat. 57 (28), 13048–13055. doi:10.1080/19443994.2015.1055519
Rao, R. A. K., Ikram, S., and Uddin, M. K. (2015). Removal of Cr (VI) from aqueous solution on seeds of Artemisia absinthium (novel plant material). Desalin. Water Treat. 54 (12), 3358–3371. doi:10.1080/19443994.2014.908147
Ravulapalli, S., and Kunta, R. (2018). Enhanced removal of chromium (VI) from wastewater using active carbon derived from Lantana camara plant as adsorbent. Water Sci. Technol. 78 (6), 1377–1389. doi:10.2166/wst.2018.413
Reddy, R. K., Rao, N. N. M., Krishna, J. S., and Ravindhranath, K. (2012). Removal of chromium (VI) from polluted waters: a biological approach. J. Pharm. Biomed. Sci. (JPBMS) 17 (17).
Ren, B., Zhang, Q., Zhang, X., Zhao, L., and Li, H. (2018). Biosorption of Cr (vi) from aqueous solution using dormant spores of Aspergillus Niger. RSC Adv. 8 (67), 38157–38165. doi:10.1039/c8ra07084a
Reya Issac, I., Rajamehala, M., Lakshmi Prabha, M., and Emilin Renitta, R. (2012). Comparitive study on biosorption of hexavalent chromium using Aspergillus oryzae NCIM 637 and Aspergillus sojae NCIM 1198 from electroplating effluent. Int. J. ChemTech Res. 4 (4).
Rong, K., Wang, J., Li, X., Zhang, Z., Yang, ·., Shan, ·., et al. (2024). Removal of Cr (VI) by iron nanoparticles synthesized by a novel green method using Yali pear peels extracts: optimization, reactivity, and mechanism. Biomass Convers. Biorefinery 14 (3), 4355–4368. doi:10.1007/s13399-022-02464-7
Roşca, M., Silva, B., Tavares, T., and Gavrilescu, M. (2023). Biosorption of hexavalent chromium by Bacillus megaterium and rhodotorula sp. inactivated biomass. Processes 11 (1), 179. doi:10.3390/pr11010179
Saed, U. A. (2016). Batch and continuous adsorption of chromium (VI) from aqueous solution using alhagi forks and tea husk. ALNAHRAIN J. Eng. Sci. 19 (1), 98–106.
Sahranavard, M., Ahmadpour, A., and Doosti, M. R. (2011). Biosorption of hexavalent chromium ions from aqueous solutions using almond green hull as a low-cost biosorbent. Eur. J. Sci. Res. 58 (3), 392–400.
Saifuddin, N., and Raziah, A. (2007). Removal of heavy metals from industrial effluent using Saccharomyces cerevisiae (Baker’s yeast) immobilized in chitosan/lignosulphonate matrix. J. Appl. Sci. Res. 3 (12), 2091–2099.
Salman, S. M., Razzaq, S., Adnan, M., Rozina, , Sultana, S., Kamal, A., et al. (2025). Green adsorbents for water remediation: removal of Cr (vi) and Ni (ii) using Prosopis glandulosa sawdust and biochar. Green Process. Synthesis 14 (1), 20240197. doi:10.1515/gps-2024-0197
Sangeetha, A., Rabitha, R., Sivasree, B., Nivedha, B., Stanlin, J.S., Arun, C., et al. (2023). Adsorbent potential of the leaf powder of Artocarpus heterophyllus lam (jackfruit) in efficiently removing hexavalent chromium from landfill leachate. Glob. NEST J. 25 (9), 88–96.
Saranya, N., Nakkeeran, E., Shrihari, S., and Selvaraju, N. (2017). Equilibrium and kinetic studies of hexavalent chromium removal using a novel biosorbent: Ruellia patula Jacq. Arabian J. Sci. Eng. 42 (4), 1545–1557. doi:10.1007/s13369-017-2416-3
Satyam, S., and Patra, S. (2024). Innovations and challenges in adsorption-based wastewater remediation: a comprehensive review. Heliyon 10, e29573. doi:10.1016/j.heliyon.2024.e29573
Saueprasearsit, P. (2011). “Adsorption of chromium (Cr? 6) using durian peel,” in International conference on biotechnology and environment management IPCBEE.
Say, R., Yilmaz, N., and Denizli, A. (2003). Removal of chromium (VI) ions from synthetic solutions by the fungus Penicillium canescens. Eur. J. Mineral Process. Environ. Prot. 3 (1).
Šćiban, M. B., and Klašnja, M. T. (2002). The kinetics of chromium (VI) adsorption from water on some natural materials. Acta Period. Technol. (33), 101–108. doi:10.2298/apt0233101s
Sekhar, K., Vishnu Babu, R., and RohiniT, R. K. (2012). New bio-sorbents in the control of chromium (VI) pollution in wastewaters. Int. J. Appl. Biol. Pharm. Technol. 3 (2), 115–125.
Sethuraman, P., and Balasubramanian, N. (2010). Removal of Cr (VI) from aqueous solution using Bacillus subtilis, Pseudomonas aeruginosa and Enterobacter cloacae. Int. J. Eng. Sci. Technol. 2, 1811–1825.
Shankar, D., Sivakumar, D., and Yuvashree, R. (2014). Chromium (VI) removal from tannery industry wastewater using fungi species. Pollut. Res. 33, 505–510.
Sharma, I., and Goyal, D. (2011). Chromium removal from industrial effluent by Eucalyptus tereticornis bark. Asian J. Exp. Sci. 25 (1), 29–35.
Sharma, P., Singh, S. P., Parakh, S. K., and Tong, Y. W. (2022). Health hazards of hexavalent chromium (Cr (VI)) and its microbial reduction. Bioengineered 13 (3), 4923–4938. doi:10.1080/21655979.2022.2037273
Sheraz, N., Shah, A., Haleem, A., and Iftikhar, F. J. (2024). Comprehensive assessment of carbon-biomaterial-and inorganic-based adsorbents for the removal of the most hazardous heavy metal ions from wastewater. RSC Adv. 14 (16), 11284–11310. doi:10.1039/d4ra00976b
Shugaba, A. B. F., Kolo, B. G., Nok, A. J., Ameh, D. A., and Lori, J. A. (2012). Uptake and reduction of hexavalent chromium by Aspergillus Niger and Aspergillus parasiticus. J. Pet. Environ. Biotechnol. 3 (3), 119. doi:10.4172/2157-7463.1000119
Sibel, T., Yasemin, Y., and Tevfik, G. (2009). Removal of chromium (VI) ions from aqueous solutions by using Turkish montmorillonite clay: effect of activation and modification. Desalination 244, 97–108. doi:10.1016/j.desal.2008.04.040
Siboni, M. S., Azizian, S., Lee, S., and Kim, W. (2011). The removal of hexavalent chromium from aqueous solutions using modified holly sawdust: equilibrium and kinetics studies. Environ. Eng. Res. 16 (2), 55–60. doi:10.4491/eer.2011.16.2.55
Singh, V., Singh, N., Verma, M., Kamal, R., Tiwari, R., Sanjay Chivate, M., et al. (2022). Hexavalent-chromium-induced oxidative stress and the protective role of antioxidants against cellular toxicity. Antioxidants 11 (12), 2375. doi:10.3390/antiox11122375
Singha, B., Naiya, T. K., Bhattacharya, A. k., and Das, S. K. (2011). Cr (VI) ions removal from aqueous solutions using natural adsorbents—FTIR studies. J. Environ. Prot. 2 (6), 729–735. doi:10.4236/jep.2011.26084
Sinha, R., Kumar, R., Sharma, P., Kant, N., Shang, J., and Aminabhavi, T. M. (2022). Removal of hexavalent chromium via biochar-based adsorbents: state-of-the-art, challenges, and future perspectives. J. Environ. Manage. 317, 115356. doi:10.1016/j.jenvman.2022.115356
Sirusbakht, S., Vafajoo, L., Soltani, S., and Habibi, S. (2018). Sawdust bio sorption of chromium (VI) ions from aqueous solutions. Chem. Eng. Trans. 70, 1147–1152. doi:10.3303/CET1870192
Sivakumar, D. (2016). Biosorption of hexavalent chromium in a tannery industry wastewater using fungi species. Glob. J. Environ. Sci. Manage. 2 (2), 105–124. doi:10.7508/gjesm.2016.02.002
Sivaprakash, A., Aravindhan, R., Rao, J. R., and Nair, B. U (2009). Kinetics and equilibrium studies on the biosorption of hexavalent chromium from aqueous solutions using Bacillus subtilis biomass. Appl. Ecol. Environ. Res. 7 (1), 45–57. doi:10.15666/aeer/0701_045057
Soleimani, M., Jamali, H. A., Mousazadehgavan, M., and Ghanbari, R. (2024). Chromium adsorption using powdered leaves of Prosopis cineraria: kinetic, isotherm, and optimization by response surface methodology. Desalin. Water Treat. 317, 100286. doi:10.1016/j.dwt.2024.100286
Sreenivas, K., Inarkar, M., Gokhale, S., and Lele, S. (2014). Re-utilization of ash gourd (Benincasa hispida) peel waste for chromium (VI) biosorption: equilibrium and column studies. J. Environ. Chem. Eng. 2 (1), 455–462. doi:10.1016/j.jece.2014.01.017
Subbaiah, M. V., Yuvaraja, G., Vijaya, Y., and Krishnaiah, A. (2011). Equilibrium, kinetic and thermodynamic studies on biosorption of Pb(II) and Cd(II) from aqueous solution by fungus (Trametes versicolor) biomass. J. Taiwan Inst. Chem. Eng. 42, 965–971. doi:10.1016/j.jtice.2011.04.007
Subhan, M., and Pardeep, D. (2011). Study to adsorbent of rice husk & saw dust (agriculture waste & timber waste). Int. J. Res. Sci. Technol. 1 (3).
Sud, D., Mahajan, G., and Kaur, M. (2008). Agricultural waste material as potential adsorbent for sequestering heavy metal ions from aqueous solutions–a review. Biores. Technol. 99 (14), 6017–6027. doi:10.1016/j.biortech.2007.11.064
Suganya, R. S., Venugopal, T., and Kannan, K. (2017). Adsorption of hexavalent chromium from aqeous solution onto chemically activated Ocimum tenuiflorum stem-A kinetic, linear equilibrium and thermodynamical studies. Asian J. Res. Soc. Sci. Humanit. 7 (1), 208–237. doi:10.5958/2249-7315.2017.00016.8
Sujin Jeba Kumar, T., Balavigneswaran, C. K., Arun, V. M., and Srinivasa Kumar, K. P. (2013). Biosorption of lead(II) and chromium(VI) by immobilized cells of microalga Isochrysis galbana. J. Algal Biomass Utln. 4 (4), 42–50.
Suleman, S., Noman, S., Faizan, N., and Arooj, M. (2017). Thermodynamic and kinetic investigations for biosorption of chromium (VI) with green algae (pithophora oedogonia). J. Bioremediat. Biodegrad. 8 (6).
Suresh, S., Srivastava, V., and Mishra, I. (2010). Isotherm, thermodynamics, desorption, and disposal study for the adsorption of catechol and resorcinol onto granular activated carbon. J. Chem. Eng. Data 56 (4), 811–818. doi:10.1021/je100303x
Sutkowy, M., and Kłosowski, G. (2018). Use of the coenobial green algae Pseudopediastrum boryanum Chlorophyceae to remove hexavalent chromium from contaminated aquatic ecosystems and industrial wastewaters. Water 10 (6), 712. doi:10.3390/w10060712
Tang, S., Chen, Y., Xie, R., Jiang, W., and Jiang, Y. (2016). Preparation of activated carbon from corn cob and its adsorption behavior on Cr VI removal. Water Sci. Technol. 73 (11), 2654–2661. doi:10.2166/wst.2016.120
Tarun, K., Chowdhury, P., Bhattacharya, A. K., and Das, S. K. (2008). Saw dust and neem bark as low-cost natural biosorbent for adsorptive removal of Zn(II) and Cd(II) ions from aqueous solutions. Chem. Eng. J. 148, 68–79. doi:10.1016/j.cej.2008.08.002
Tesnim, D., Hédi, B. A., Ridha, D., and Cid-Samamed, A. (2024). Green low-cost synthesis of zero-valent iron nanoparticles from Palm Petiole Extract for Cr VI removal from water. Environ. Sci. Pollut. Res. 31 (31), 44272–44288. doi:10.1007/s11356-024-34092-1
Thirunavukkarasu, E., and Palanivelu, K. (2007). Biosorption of Cr (VI) from plating effluent using marine algal mass. Indian J. Biotechnol. 6 (3), 359–364.
Timbo, C. C., Kandawa-Schulz, M., Amuanyena, M., and Kwaambwa, H. M. (2017). Adsorptive removal from aqueous solution of Cr (VI) by green moringa tea leaves biomass. J. Encapsulation Adsorpt. Sci. 7 (02), 108–119. doi:10.4236/jeas.2017.72008
Tripathi, M., Pathak, S., Singh, R., Singh, P., Singh, P. K., Shukla, A. K., et al. (2024). A comprehensive review of lab-scale studies on removing hexavalent chromium from aqueous solutions by using unmodified and modified waste biomass as adsorbents. Toxics 12 (9), 657. doi:10.3390/toxics12090657
Tumolo, M., Ancona, V., De Paola, D., Losacco, D., Campanale, C., Massarelli, C., et al. (2020). Chromium pollution in European water, sources, health risk, and remediation strategies: an overview. Int. J. Environ. Res. Public Health 17 (15), 5438. doi:10.3390/ijerph17155438
Valli, V. G., Rao, G., Sridevi, V., and Keerthi, K. (2013). Optimization of process parameters for biosorption of chromium using green algae. Parameters 2(6).
Venkateswarlu, P., Ratnam, M. V., Rao, D. S., and Rao, M. V. (2007). Removal of chromium from an aqueous solution using Azadirachta indica neem leaf powder as an adsorbent. Int. J. Phys. Sci 2 (8), 188–195.
Vikal, G., Sumit, L. M., and Mahendra, B. (2013). Biosorption of Cr (VI) by activated Ziziphus Jujuba leaf powder. Res. J. Chem. Environ. 17, 5.
Vinodhini, V., and Das, N. (2009). Biowaste materials as sorbents to remove chromium (VI) from aqueous environment-a comparative study. ARPN J. Agric. Biol. Sci. 4 (6), 19–25.
Vinodhini, V., and Das, N. (2010). Relevant approach to assess the performance of sawdust as adsorbent of chromium VI ions from aqueous solutions. Int. J. Environ. Sci. Tech. 7 (1), 85–92. doi:10.1007/bf03326120
Wahiba, M., Souad, B., Mouna, C., Djellabi, R., Nabila, B., Djilani, C., et al. (2025). Adsorption performance and mechanistic pathways of raw powdered pine cone toward the removal of dye and Cr VI. Int. J. Chem. Kinet. 57, 263–283. doi:10.1002/kin.21774
Wang, L-H., and Lin, C-I. (2008). Adsorption of chromium (III) ion from aqueous solution using rice hull ash. J. Chin. Inst. Chem. Eng. 39 (4), 367–373. doi:10.1016/j.jcice.2008.02.004
Wang, X. S., Li, Z. Z., and Sun, C. (2008). Removal of Cr (VI) from aqueous solutions by low-cost biosorbents: marine macroalgae and agricultural by-products. J. Hazard. Mater. 153 (3), 1176–1184. doi:10.1016/j.jhazmat.2007.09.079
Wan Ngah, W., and Hanafiah, M. (2008). Removal of heavy metal ions from wastewater by chemically modified plant wastes as adsorbents: a review. Biores. Technol. 99 (10), 3935–3948. doi:10.1016/j.biortech.2007.06.011
Wenjie, O., Ahmed, W., Xiuxian, F., Lu, W., Jiannan, L., Jie, Y., et al. (2022). The adsorption potential of Cr from water by ZnO nanoparticles synthesized by Azolla pinnata. Bioinorg. Chem. Appl. 2022 (1), 6209013. doi:10.1155/2022/6209013
Xia, S., Song, Z., Jeyakumar, P., Bolan, N., and Wang, H. (2020). Characteristics and applications of biochar for remediating Cr (VI)-contaminated soils and wastewater. Environ. Geochem. Health 42, 1543–1567. doi:10.1007/s10653-019-00445-w
Xie, H., Wan, Y., Chen, H., Xiong, G., Wang, L., Xu, Q., et al. (2021). Cr (VI) adsorption from aqueous solution by UiO-66 modified corncob. Sustainability 13 (23), 12962. doi:10.3390/su132312962
Yadav, S., Srivastava, V., Banerjee, S., Weng, C. H., and Sharma, Y. C. (2013). Adsorption characteristics of modified sand for the removal of hexavalent chromium ions from aqueous solutions: kinetic, thermodynamic and equilibrium studies. Catena 100, 120–127. doi:10.1016/j.catena.2012.08.002
Yahya, M., Aliyu, A. S., Obayomi, K. S., Olugbenga, A. G., and Abdullahi, U. B. (2020). Column adsorption study for the removal of chromium and manganese ions from electroplating wastewater using cashew nutshell adsorbent. Cogent Eng. 7 (1), 1748470. doi:10.1080/23311916.2020.1748470
Yin, H., He, B., Peng, H., Ye, J., Yang, F., and Zhang, N. (2008). Removal of Cr VI and Ni II from aqueous solution by fused yeast: study of cations release and biosorption mechanism. J. Hazard. Mater. 158 (2), 568–576. doi:10.1016/j.jhazmat.2008.01.113
Yuan, X. Z., Meng, Y., Zeng, G. M., Fang, Y., and Shi, J. G. (2008). Evaluation of tea derived biosurfactant on removing heavy metal ions from dilute wastewater by ion flotation. Colloids Surf. A Physicochem. Eng. Asp. 317, 256–261. doi:10.1016/j.colsurfa.2007.10.024
Yusuff, A. S. (2019). Adsorption of hexavalent chromium from aqueous solution by Leucaena leucocephala seed pod activated carbon: equilibrium, kinetic and thermodynamic studies. Arab. J. Basic Appl. Sci. 26 (1), 89–102. doi:10.1080/25765299.2019.1567656
Zainul, A. Z., Marlini, S., Nufadilah, M., and Wan, A. A. (2009). Chromium (VI) removal from aqueous solution by untreated rubber wood saw dust. Desalination 244, 109–122. doi:10.1016/j.desal.2008.05.018
Zeng, W., Luo, Y., Wang, C., Liu, Z., Xue, M., and Xie, X. (2025). A review on recent advances of adsorption and photoreduction of Cr (VI). Environ. Pollut. Bioavailab. 37 (1), 2493057. doi:10.1080/26395940.2025.2493057
Zhang, Y., Zhou, Y., Sun, D., Nie, Y., Wu, D., Ban, L., et al. (2025). CeO2-based functional materials: advancing photo and electro-driven catalysis for environmental remediation and energy conversion. Coord. Chem. Rev. 527, 216395. doi:10.1016/j.ccr.2024.216395
Zhou, Y., Zhang, Y., Nie, Y., Sun, D., Wu, D., Ban, L., et al. (2025). Recent advances and perspectives in functional chitosan-based composites for environmental remediation, energy, and biomedical applications. Prog. Mater. Sci. 152, 101460. doi:10.1016/j.pmatsci.2025.101460
Zolfaghari, G., and Kargar, M. (2019). Nanofiltration and microfiltration for the removal of chromium, total dissolved solids, and sulfate from water. MethodsX 6, 549–557. doi:10.1016/j.mex.2019.03.012
Keywords: adsorption, adsorbents, activation, batch experiments, chromium (VI)
Citation: Haroon H, Butt TA, Shah JA, Ciobica A, Romila LE, Burlui V, Bibi H and Bilal M (2025) Valorization of natural adsorbents for removing chromium (VI) from industrial wastewater: a review. Front. Chem. 13:1608863. doi: 10.3389/fchem.2025.1608863
Received: 09 April 2025; Accepted: 09 May 2025;
Published: 12 June 2025.
Edited by:
Tamer S. Saleh, National Research Centre, EgyptReviewed by:
Heng Zhang, Guizhou University, ChinaAlessandro Piovano, Polytechnic University of Turin, Italy
Copyright © 2025 Haroon, Butt, Shah, Ciobica, Romila, Burlui, Bibi and Bilal. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hajira Haroon, aGFqaXJhQHVvaC5lZHUucGs=; Alin Ciobica, YWxpbi5jaW9iaWNhQHVhaWMucm8=
 Hajira Haroon
Hajira Haroon Tayyab Ashfaq Butt3
Tayyab Ashfaq Butt3 Alin Ciobica
Alin Ciobica