Abstract
CO2 capture from post-combustion flue gas originating from coal or natural gas power plants, or even from the ambient atmosphere, is a promising strategy to reduce the atmospheric CO2 concentration and achieve global decarbonization goals. However, the co-existence of water vapor in these sources presents a significant challenge, as water often competes with CO2 for adsorption sites, thereby diminishing the performance of adsorbent materials. Selectively capturing CO2 in the presence of moisture is a key goal, as there is a growing demand for materials capable of selectively adsorbing CO2 under humid conditions. Among these, metal–organic frameworks (MOFs), a class of porous, highly tunable materials, have attracted extensive interest for gas capture, storage, and separation applications. The numerous combinations of secondary building units and organic linkers offer abundant opportunities for designing systems with enhanced CO2 selectivity. Interestingly, some recent studies have demonstrated that interactions between water and CO2 within the confined pore space of MOFs can enhance CO2 uptake, flipping the traditionally detrimental role of moisture into a beneficial one. These findings introduce a new paradigm: water-enhanced CO2 capture in MOFs. In this review, we summarize these recent discoveries, highlighting examples of MOFs that exhibit enhanced CO2 adsorption under humid conditions compared to dry conditions. We discuss the underlying mechanisms, design strategies, and structural features that enable this behavior. Finally, we offer a brief perspective on future directions for MOF development in the context of water-enhanced CO2 capture.
1 Introduction
The growing concentration of greenhouse gases, primarily carbon dioxide (CO2), in the atmosphere, has led to significant global warming and climate changes. Anthropogenic CO2 emissions are largely attributable to the increasing combustion of fossil fuels and various industrial processes designed to satisfy construction, energy, and manufacturing demands. Major contributors include coal- and gas-fired power plants, petrochemical facilities, hydrogen production via steam methane reforming followed by the water-gas shift reaction, and cement manufacturing using calcium carbonate as the primary raw material (Goel et al., 2016; Mukherjee et al., 2019; Dehimi et al., 2025; Park et al., 2021). To keep the atmospheric CO2 concentration from rising further, two primary strategies have been pursued. One focuses on developing alternative, clean energy sources that produce little to no CO2 (Davis et al., 2018). The other centers on the design of energy-efficient processes for CO2 capture, followed by either chemical conversion (Mukherjee et al., 2019; Wang et al., 2017; Sumida et al., 2012; Ran et al., 2018) or geological sequestration (Lin et al., 2024; Massarweh and Abushaikha, 2024; Spurin et al., 2025).
A variety of solid materials have been developed to achieve high CO2 uptake and high selectivity for CO2 over N2, including activated carbons (Jedli et al., 2024), zeolites (Mukherjee et al., 2019; Kumar et al., 2020), metal−organic frameworks (MOFs) (Ghanbari et al., 2020; Schoedel et al., 2016; Lin et al., 2021), polymers (Song et al., 2022), and metal oxides (Yong et al., 2002). Among these, MOFs stand out due to their diverse topologies, large pore volumes, and broadly tunable pore sizes, which can be adjusted by modifying metal nodes and/or organic linkers (Zhou et al., 2012; Horike et al., 2009; Alezi et al., 2016; Wang et al., 2016). Owing to their high surface area, large pore volume and particularly the presence of a high density of open metal sites (Britt et al., 2009), certain MOFs have demonstrated excellent CO2 uptake at room temperature. However, high CO2 capacity and selectivity in the presence of N2 are not enough, as CO2 typically coexists with other components, water vapor being one of the most challenging (Siegelman et al., 2019). Water and CO2 often target the same adsorption sites, with water typically binding more strongly, thus outcompeting CO2 and reducing uptake capacity. This competition becomes critical in applied CO2 capture scenarios. For instance, flue gas from natural gas combined cycle (NGCC) power plants contains approximately 75% N2, 4% CO2, 12% O2, and 9% H2O by volume (Siegelman et al., 2019; Zhang et al., 2020). At this concentration, water vapor can significantly impair CO2 capture by blocking adsorption sites, and must, therefore, be carefully considered in material design and application.
The earliest systems developed for selective CO2 capture in the presence of water were aqueous amine solutions, which rely on acid-base reactions to form carbamates (Li and Keeners, 2016). However, these liquid-phase systems suffer from several limitations, including low working capacities, high regeneration energies, and thermal instability (Zhao et al., 2012). To address these issues, researchers developed molecularly porous solid systems incorporating amine functionalities to enhance the selective adsorption of CO2 in the presence of water. These approaches include grafting amine groups onto porous materials, such as porous polymers, silica, alumina, and carbon (Filburn et al., 2005; Wurzbacher et al., 2011; Kuwahara et al., 2012; Chai et al., 2016; Li, et al., 2010), as well as functionalizing MOFs with diamine-containing molecules (Choe et al., 2019; Li et al., 2015; Kang et al., 2019; Choi et al., 2012; Demessence et al., 2009; McDonald et al., 2011; Liao et al., 2016; McDonald et al., 2012; Planas et al., 2013; McDonald et al., 2015; Siegelman et al., 2017; Milner et al., 2018; 2017). In diamine-appended MOFs, one end of the diamine molecule binds to an open metal site in the MOF, while the other end remains available for CO2 chemisorption. These diamine-functionalized MOFs introduce chemisorption sites, enhancing selectivity for CO2 over H2O. However, their overall CO2 uptake capacity and uptake kinetics may be affected, as the diamine molecules partially occupy the available pore volume.
Several studies have now demonstrated that interactions between water and CO2 within confined pore spaces can, in fact, enhance CO2 capture. These findings represent a new paradigm, revealing that certain MOFs can convert the traditionally negative impact of moisture into a beneficial factor for improving CO2 adsorption performance. This review highlights these key discoveries and examines the unique mechanisms underlying enhanced CO2 adsorption under humid conditions. We conclude with a brief perspective on future directions for MOF design and research in the field of water-enhanced CO2 capture.
2 Water-enhanced CO2 capture
2.1 Dipole–quadrupole interaction
In a high-throughput screening study (Chanut et al., 2017), Chanut et al. investigated the effect of pre-equilibrated water on CO2 uptake in 45 MOFs using thermogravimetric analysis. The MOFs were grouped into various categories based on the extent to which pre-adsorbed H2O influenced CO2 uptake. One category, which included MIL-110(Al), MIL-163(Zr), Cu-HKUST-1 and UiO-66(Zr) (Table 1), exhibited a slight increase in CO2 uptake with a certain amount of pre-adsorbed water. For example, Cu-HKUST-1 showed an approximately 5 wt% increase in CO2 uptake in the presence of 2–4% relative humidity (RH). This observation is consistent with Yazaydin’s report that CO2 uptake and its selectivity over N2 increased in 4 wt% hydrated Cu-HKUST-1 due to the presence of water molecules coordinated to the framework open-metal sites (Yazaydın et al., 2009). This enhancement was initially predicted through molecular simulations and later validated by experiments. Detailed examination of interaction energies using grand canonical Monte Carlo simulations suggested that Coulombic interactions are responsible for the increased CO2 adsorption–specifically interactions between the quadrupole moment of CO2 and the electric field generated by water molecules bound to open metal sites. The LeVan group reported similar findings for Cu-HKUST-1 through volumetric measurements (Liu et al., 2010). Collectively, these results suggest an unexpected approach for enhancing CO2 capture in the presence of water. However, at high humidity levels, Cu-HKUST-1 undergoes structural degradation, which likely explains why enhanced CO2 uptake was not observed under conditions of high RH. Yu et al. (2016) investigated the effect of water on CO2 capture in an isostructural series of M-HKUST-1 frameworks (M = Zn, Co, Ni, and Mg) through simulation studies evaluating water coordination within the MOFs. Water-coordination enhanced CO2 uptake, similar to that observed in Cu-HKUST-1, was found for the Zn-, Co-, and Ni-based analogues. However, for Mg-HKUST-1, water coordination reduced CO2 adsorption at higher pressures.
TABLE 1
| MOF Name | MOFkey | MOFid |
|---|---|---|
| Cu-HKUST-1 | Cu.QMKYBPDZANOJGF.MOFkey-v1.tbo | [Cu][Cu].[O-]C(═O)c1cc(cc(c1)C(═O)[O-])C(═O)[O-] MOFid-v1.tbo.cat0 |
| UiO-66 | Zr.KKEYFWRCBNTPAC.MOFkey-v1.fcu | [O-]C(═O)c1ccc(cc1)C(═O)[O-].[O]12[Zr]34[OH]5[Zr]62[OH]2[Zr]71[OH]4[Zr]14[O]3[Zr]35[O]6[Zr]2([O]71)[OH]43 MOFid-v1.fcu.cat0 |
| MIL-100 | Cr.QMKYBPDZANOJGF.MOFkey-v1.moo | F[Cr][O]([Cr])[Cr].F[Cr][O]([Cr]F)[Cr].[Cr][O]([Cr])[Cr].[O-]C(═O)c1cc(cc(c1)C(═O)[O-])C(═O)[O-] MOFid-v1.moo.cat0 |
| Mg-MOF-74 | Mg.YXUXCIBWQAOXRL.MOFkey-v1.UNKNOWN | [Mg].[O-]C(═O)c1cc([O])c(cc1[O])C(═O)[O-] MOFid-v1.UNKNOWN.cat0 |
| MOF-808 | Zr.QMKYBPDZANOJGF.MOFkey-v1.spn | O[Zr]123([OH2])[OH]4[Zr]56([O]3[Zr]37([OH]2[Zr]28([O]1[Zr]14([O]6[Zr]([OH]53)([OH]21)([O]78)([OH2])O)([OH2])(O)O)[OH2])([OH2])(O)O)[OH2].[O–]C(O)c1cc(cc(c1)C(O)[O–])C(O)[O–] MOFid-v1.spn.cat0 |
| NOTT-400 | Sc.QURGMSIQFRADOZ.MOFkey-v1.UNKNOWN | [O-]C(=O)c1cc(cc(c1)C(=O)[O-])c1cc(cc(c1)C(=O)[O-])C(=O)[O-].[OH].[Sc] MOFid-v1.UNKNOWN.cat0 |
| Mg-CUK-1 | Mg.WAYLQVWVRREMCZ.MOFkey-v1.UNKNOWN | [Mg].[O-]C(=O)c1ccc(cc1)c1ccnc(c1)C(=O)[O-].[OH] MOFid-v1.UNKNOWN.cat0 |
MOFkey and MOFid codes for some MOFs mentioned in this paper (Bucior et al., 2019).
Another water-stable MOF, UiO-66, was reported by Hossain et al. to show a similar result, exhibiting a slight enhancement in CO2 adsorption at low water loading (1.5 mol/kg) under low CO2 partial pressure (below 5 kPa) at 25°C. This observation was based on experimental binary adsorption isotherms measured volumetrically using a mass balance approach (Hossain et al., 2019). However, increasing the co-adsorbed water loadings to 4.2 and 12 mol/kg led to reduced CO2 uptake (Figure 1 Left). Molecular simulations supported these findings and further revealed that the effect depends on the type of defect sites within the MOF: missing linker defects promoted the enhancement (Figure 1 Right), whereas missing cluster defects didn’t show this behavior. Expanding on this work, Hernandez et al. conducted computational studies on three amine-linker UiO-66 materials and found that water molecules bridge between metal-oxide clusters by occupying missing linker positions (Hernandez et al., 2021). These water bridges reduce the pore size in defect-laden MOFs and enhance CO2 adsorption in the presence of co-adsorbed water. Experimental binary isotherm data were consistent with these predictions. These studies underscore the importance of considering defect sites when evaluating CO2 capture performance in humid conditions.
FIGURE 1

Binary CO2/H2O adsorption isotherms for UiO-66 at various fixed water loadings, compared to the dry condition. (Left) Experimental results; (Right) Simulation studies. Reprinted with permission from Hossain et al. (2019). Copyright 2019, Elsevier Ltd.
2.2 H2O dissociation leading to new adsorption sites
MIL-100(Fe) was evaluated by Soubeyrand-Lenoir et al., who reported a five-fold increase in CO2 uptake (105 mg/g) at low pressure (200 mbar) under moderate humidity (40% RH) (Soubeyrand-Lenoir et al., 2012). They hypothesized that water molecules coordinate to the Lewis-acidic metal sites, forming water channels, while CO2 adsorption occurs in the center of these channels without carbonate formation. In addition to the water stability of the materials, its mesoporosity was highlighted as a key factor contributing to the observed enhancement, as it allows for the formation of microporous water pockets that can subsequently be filled with CO2 (see Figure 2).
FIGURE 2

Schematic representation of the water channel formed in MIL-100(Fe) to enhance the CO2 adsorption (A) Adsorbed water molecules create pockets that can (B) adsorb CO2 molecules, which can in turn (C) displace some of the water molecules. Reprinted with permission from Soubeyrand-Lenoir et al. (2012). Copyright 2012, American chemical society.
Xian et al. further investigated this idea in MIL-100(Fe) using CO2 temperature programmed desorption (TPD) and in situ Fourier transform infrared spectroscopy (FTIR) (Xian et al., 2015). TPD measurements revealed two CO2 desorption peaks in the hydrated sample (50% RH), compared to only one in the dehydrated sample, indicating the creation of an additional adsorption site in the presence of water. The authors proposed that water molecules dissociate to form node hydroxyl groups, which serve as extra adsorption sites for CO2, thereby leading to an additional adsorption site to enhance the material’s uptake capacity. In situ FTIR results supported this conclusion by revealing faster CO2 adsorption under humid conditions, as evidenced by the more rapid appearance of CO2 stretching mode peaks (see peaks at 2,350, 3,600, and 3,700 cm−1 in Figure 3). The water dissociation hypothesis is worthy of further investigation.
FIGURE 3

In situ IR spectra of MIL-100(Fe) loaded with CO2 in the spectral region between 1,200 and 4,000 cm−1. (a) CO2 gas flow rate 40 mL/min, 298 K in dry condition. (b) CO2/H2O gas flow rate 40 mL/min, 298 K, 50% RH. Reprinted with permission from Xian et al. (2015). Copyright 2015, Elsevier Ltd.
2.3 Water nanopocket confinement effects
For MOFs containing bridging hydroxo ligands as components of nodes, computational studies have predicted that at low water loadings, H2O molecules can be efficiently packed through strong hydrogen bonding to the–OH groups (Liu et al., 2023; He et al., 2023). These well-ordered water molecules can, in turn, improve CO2 adsorption by forming favorable hydrogen bonds with CO2 within the microchannels. In other words, the hydroxo ligands act as directing agents for efficient water arrangement, and the pre-adsorbed water molecules introduce confinement effects that further promote CO2 uptake.
Building on this principle, the Ibarra group investigated a series of MOFs containing µ2/µ3-OH ligands, including NOTT-400 (Gonzalez et al., 2015), NOTT-401 (Lara-García et al., 2015; Sánchez-González et al., 2016), InOF-1 (Peralta et al., 2015), and Mg-CUK-1 (Sagastuy-Breña et al., 2018), and demonstrated that their CO2 capture capacities were enhanced to varying degrees under moderate humidity (RH < 40%) at 30°C. In a combined experimental and computational study, Breña et al. further reported humidity-enhanced CO2 adsorption in Mg-CUK-1, a framework featuring one-dimensional microporous channels (Sagastuy-Breña et al., 2018). Using static CO2 adsorption isotherms and thermogravimetric analysis under a constant CO2 flow (60 mL/min), they observed a maximum CO2 uptake of 8.5 wt% at 18% RH, compared to 4.6 wt% under dry conditions. However, beyond 20% RH, a rapid decline in CO2 adsorption was observed, with almost negligible CO2 uptake at 25% RH.
Chen et al. (2018) reported unusual moisture-enhanced CO2 adsorption in PCN-250(Fe3) and PCN-250(Fe2Co). These compounds are constructed from trimetallic-oxy clusters, i.e., Fe3(μ3-O)(CH3COO)6 or Fe2Co(μ3-O)(CH3COO)6, as nodes, and ABTC4- units as linkers (H4ABTC = 3,3′,5,5′-azobenzenetetracarboxylic acid). For PCN-250(Fe3), the uptake of CO2 increases by 54% under 50% RH, compared to dry conditions (from 1.18 to 1.82 mmol/g). PCN-250(Fe2Co) exhibited a 69% increase in CO2 uptake under the same conditions (from 1.32 to 2.23 mmol/g). Even at 90% RH, significant increases in CO2 adsorption were observed (44% for PCN-250(Fe3) and 70% for PCN-250(Fe2Co)) compared to their respective uptakes under dry conditions. Molecular simulations revealed that node-based, bridging oxo ions (μ3-O) act as directing agents for H2O adsorption. These water molecules, in turn, help position CO2 molecules closer to metal centers on the opposite side of the pore, enhancing CO2 adsorption via confinement effects. The CO2/MOF interaction is further strengthened by what the authors term a “plier effect”, where coordinated water molecules appear to “clamp” CO2 molecules onto open metal sites (see Figure 4), increasing the efficiency of adsorption under unsaturated conditions by maximizing the use of available adsorption sites. The plier effect, involving CO2, H2O, and the MOFs, enables effectively use of more of the candidate adsorption sites by CO2; in turn, the amount of CO2 adsorbed increases. The mechanism behind the enhanced CO2 uptake at 90% RH is less clear, as such high humidity would presumably saturate the pores with water, leaving little space for CO2.
FIGURE 4

Binding sites of CO2 in PCN-250(Fe2Co) structure (a) with and (b) without H2O. Reprinted with permission from Chen et al. (2018). Copyright 2018, American chemical society.
Shi et al. (2020) reported a series of metal-triazolate MOFs, constructed from ZnF rods and 1,2,4 triazolate linkers functionalized with various groups (e.g., -NH2 and -CH3), among which the MOF featuring a 3,5-diamino-1,2,4-triazolate linker (ZnF(daTZ)) exhibited a CO2/N2 thermodynamic adsorption selectivity of 120 at 298K and 0–101 kPa, and a CO2/H2O kinetic adsorption selectivity of 70 at 298K and 33% RH. DFT calculations revealed a 25%–30% increment in the heat of CO2 adsorption in the presence of co-adsorbed water, indicating stronger CO2 binding under humid conditions (see Figure 5). This enhancement was attributed to the preferential localization of water and CO2 molecules within the MOF framework, i.e., water molecules tended to occupy the corner sites, while CO2 molecules were primarily located at the center of the channels. However, this study does not fully elaborate on how this spatial distribution contributes to the enhanced CO2 adsorption under humid conditions. A similar spatial preference was reported in amine-functionalized UiO-66, where H2O and CO2 adsorbed at different sites (Hernandez et al., 2021). The authors proposed that water molecules formed hydrogen-bonded bridges between metal nodes by occupying missing linker positions, effectively reducing the pore size and enhancing CO2 adsorption.
FIGURE 5
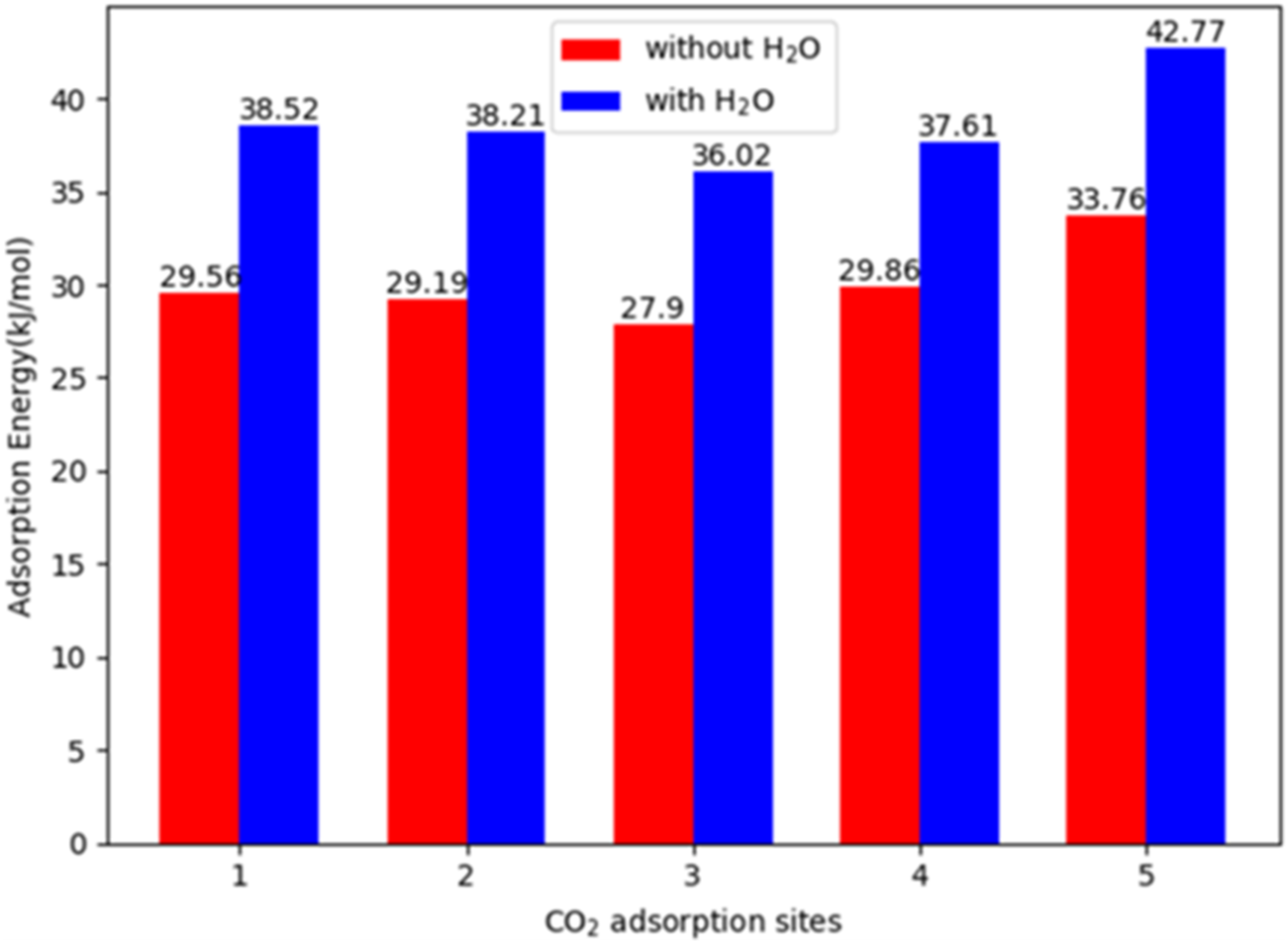
Comparison between CO2 adsorption energies without H2O and with H2O for five distinct CO2 adsorption sites in ZnF(daTZ). Reprinted with permission from Shi et al. (2020). Copyright 2020, American chemical society.
2.4 Ammonium carbamate, carbamic acid and bicarbonate formation
Functionalizing MOFs with diamines allows one amine group to coordinate to an open metal site, while the other points into the pore to interact with CO2 molecules. For instance, diamine-modified MOF-74 materials have displayed selective CO2 adsorption over water due to the formation of carbamate species, particularly at low CO2 concentrations (Milner et al., 2018). However, carbamate formation typically requires two amine groups to react with one single CO2 molecule, which limits the overall CO2 uptake capacity.
The impact of RH on the performance of amine-appended MOFs remains relatively underexplored, with few studies reporting CO2 uptake across a broad range humidity levels. Holmes et al. (2023) investigated this effect on (2-ampd)2Mg2(dobpdc) MOF (2-ampd is 2-(aminomethyl)piperidine) using both gravimetric and breakthrough adsorption techniques. Their findings identified three distinct RH regions based on the influence of water on CO2 uptake: competitive adsorption from 0%–20% RH, enhanced adsorption between 20%–40% RH, and hindered adsorption due to pore saturation at RH levels above 40%, see Figure 6. A significant enhancement in CO2 uptake at 1 bar and 40°C was observed at 30% RH (5.7 ± 0.2 mmol/g), compared to 3.35 mmol/g under dry conditions, see Figure 6. This increase was attributed to a mixed adsorption mechanism, wherein CO2 binds to both primary and secondary amines in 2-ampd, forming ammonium carbamate and carbamic acid, respectively. This mechanism was supported by TPD data showing co-desorption of water and CO2, as well as DRIFTS measurements revealing the loss of N-H stretching from secondary amines and the emergence of O-H stretching in humid samples.
FIGURE 6

Three distinct RH regions for CO2 uptake by (2-ampd)2Mg2(dobpdc): competitive adsorption from 0%–20% RH, enhanced adsorption between 20%–40% RH, and hindered adsorption at RH levels above 40% at 1 bar and 40°C. Reprinted with permission from Holmes et al. (2023). Copyright 2023, Elsevier Ltd.
Didas et al. (2014) reported that co-adsorption of CO2 and water on mesoporous silica with low amine surface coverage leads to bicarbonate formation. Recently, Lyu et al. (2022) and Chen et al. (2024) both demonstrated that bicarbonate formation within MOFs can enhance CO2 uptake. In their studies, they developed amine-functionalized MOF-808 materials: one featuring amino acids coordinated to Zr ions (MOF-808-AAs), first reported by Lyu et al. (2022), and the other incorporating polyamines covalently attached to a chloro-functionalized framework (MOF-808-PAs), reported by Chen et al. (2024), see Figure 7 (top). Both series exhibited improved CO2 capture performance under humid conditions for direct air capture of CO2, where the CO2 concentration is approximately 420 ppm in the atmosphere. At 50% RH, the l-lysine- and tris(3-aminopropyl)amine-functionalized variants exhibited remarkable uptakes of 1.205 and 0.872 mmol/g at 400 ppm CO2 and 25°C corresponding to 97% and 75% increases compared to the dry uptakes, respectively. The detailed sorption study using solid-state NMR revealed that for the wet conditions, MOF-808-Lys exhibited a single signal at 167 ppm under 50% RH attributed to ammonium bicarbonate formation. The presence of water enhanced the amine utilization efficiency by forming bicarbonate species (see Figure 7 (bottom)), resulting in increased CO2 uptake compared to the dry conditions.
FIGURE 7

(Top) Two series of amine-functionalized MOF-808: MOF-808-AAs and MOF-808-PAs. (Bottom) Proposed chemisorption mechanism of amines with CO2 under dry and wet conditions. Reprinted with permission from Chen et al. (2024). Copyright 2024, American chemical society.
3 Conclusion
The presence of water vapor, long considered a challenge in CO2 capture, is now recognized as a potential partner in enhancing CO2 adsorption performance in certain MOFs. A growing body of experimental and computational studies has revealed several mechanisms through which water can improve CO2 uptake, including dipole–quadrupole interactions, water-assisted formation of new adsorption sites, confinement effects via water nanopockets, and ammonium carbamate, carbamic acid and bicarbonate formation at reactive amine sites. These mechanisms, observed in a diverse range of MOFs, such as HKUST-1, MIL-100(Fe), PCN-250, and MOF-808, demonstrate that well-designed frameworks can convert water from a disruptive presence into a cooperative one. While many of these enhancements occur under low or moderate humidity, challenges remain in boosting performance under high relative humidity, where water saturation can reduce pore accessibility. Continued exploration of MOF structures, functional groups, and water-CO2 interactions will be key to designing next-generation materials for practical CO2 capture, especially in humid environments such as flue gas streams and ambient air. The development of MOFs exhibiting high CO2 selectivity, capacity, and stability under humid conditions represents a promising path forward in advancing scalable carbon capture technologies.
4 Perspectives
The emerging understanding of water-enhanced CO2 capture in MOFs presents an exciting opportunity to rethink the role of moisture in gas capture. While water vapor has traditionally been viewed as a challenge, competing with CO2 for adsorption sites and destabilizing frameworks, recent findings demonstrate that, under specific structural and chemical conditions, water can become a cooperative agent that enhances CO2 uptake. Mechanisms such as dipole–quadrupole interactions, molecular confinement, water-induced site activation, and bicarbonate formation at amine-functionalized sites have all been shown to improve performance in humid environments. The simple dipole–quadrupole interaction model between water and CO2 molecules suggests that positively charged adsorption sites formed via water coordination can promote CO2 uptake. If a framework offers an environment that facilitates the creation of such sites, enhanced CO2 adsorption can be achieved. Rational incorporation of hydrophilic functional groups, such as μ2/μ3-OH bridges and open metal sites, can facilitate structured water adsorption, promoting CO2 capture. Additionally, frameworks with hierarchical pore structures may provide the spatial freedom to accommodate water and CO2 without compromising access to active sites. The “plier effect” and the formation of new reactive sites from water dissociation further suggest that cooperative interactions can be engineered to improve performance under humid conditions.
Despite these advances, significant knowledge gaps remain. For instance, systematic studies of water-enhanced CO2 capture across a range of relative humidities are lacking. Likewise, the effect of varying CO2 concentrations, such as those relevant to direct air capture, has not been thoroughly explored in humid conditions. Advanced in situ techniques, such as solid-state NMR, IR spectroscopy, and X-ray scattering, coupled with multiscale computational modeling, will be vital for unraveling these complex interactions at the molecular level. Furthermore, future research should include a focus on bridging the gap between fundamental discovery and practical deployment. This focus would include improving the stability and regenerability of MOFs under cyclic operation, scaling up synthesis methods, and integrating these materials into realistic gas separation processes, such as post-combustion carbon capture and direct air capture.
Statements
Author contributions
CC: Writing – original draft. JC: Writing – original draft. TG: Writing – review and editing. RS: Writing – review and editing. JH: Writing – review and editing. JL: Funding acquisition, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. RS and JH gratefully acknowledge research support from the National Science Foundation under grant no. 2119433. TG gratefully acknowledges research support from the National Science Foundation under grant no. 2119033. JL gratefully acknowledges a startup research grant from the College of Science, Rochester Institute of Technology.
Conflict of interest
RS and JH have a financial interest in Numat, a company that is commercializing MOFs. TG has a financial interest in Atoco Inc., which is seeking to commercialize related technologies.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
Alezi D. Spanopoulos I. Tsangarakis C. Shkurenko A. Adil K. Belmabkhout Y. et al (2016). Reticular chemistry at its best: directed assembly of hexagonal building units into the awaited metal-organic framework with the intricate polybenzene topology, pbz-MOF. J. Am. Chem. Soc.138, 12767–12770. 10.1021/jacs.6b08176
2
Britt D. Furukawa H. Wang B. Glover T. G. Yaghi O. M. (2009). Highly efficient separation of carbon dioxide by a metal-organic framework replete with open metal sites. Proc. Natl. Acad. Sci.106, 20637–20640. 10.1073/pnas.0909718106
3
Bucior B. J. Rosen A. S. Haranczyk M. Yao Z. Ziebel M. E. Farha O. K. et al (2019). Identification schemes for metal-organic frameworks to enable rapid search and cheminformatics analysis. Cryst. Growth Des.19, 6682–6697. 10.1021/acs.cgd.9b01050
4
Chai S.-H. Liu Z.-M. Huang K. Tan S. Dai S. (2016). Amine functionalization of microsized and nanosized mesoporous carbons for carbon dioxide capture. Ind. Eng. Chem. Res.55, 7355–7361. 10.1021/acs.iecr.6b00823
5
Chanut N. Bourrelly S. Kuchta B. Serre C. Chang J.-S. Wright P. A. et al (2017). Screening the effect of water vapour on gas adsorption performance: application to CO2 capture from flue gas in metal–organic frameworks. ChemSusChem10, 1543–1553. 10.1002/cssc.201601816
6
Chen O.I.-F. Liu C.-H. Wang K. Borrego-Marin E. Li H. Alawadhi A. H. et al (2024). Water-enhanced direct air capture of carbon dioxide in metal–organic frameworks. J. Am. Chem. Soc.146, 2835–2844. 10.1021/jacs.3c14125
7
Chen Y. Qiao Z. Huang J. Wu H. Xiao J. Xia Q. et al (2018). Unusual moisture-enhanced CO2 capture within microporous PCN-250 frameworks. ACS Appl. Mater. Interfaces10, 38638–38647. 10.1021/acsami.8b14400
8
Choe J. H. Kang D. W. Kang M. Kim H. Park J. R. Kim D. W. et al (2019). Revealing an unusual temperature-dependent CO2 adsorption trend and selective CO2 uptake over water vapors in a polyamine-appended metal–organic framework. Mater. Chem. Front.3, 2759–2767. 10.1039/c9qm00581a
9
Choi S. Watanabe T. Bae T.-H. Sholl D. S. Jones C. W. (2012). Modification of the Mg/DOBDC MOF with amines to enhance CO2 adsorption from ultradilute gases. J. Phys. Chem. Lett.3, 1136–1141. 10.1021/jz300328j
10
Davis S. J. Lewis N. S. Shaner M. Aggarwal S. Arent D. Azevedo I. et al (2018). Net-zero emissions energy systems. Science360, eaas9793. 10.1126/science.aas9793
11
Dehimi L. Alioui O. Benguerba Y. Yadav K. K. Bhutto J. K. Fallatah A. M. et al (2025). Hydrogen production by the water gas shift reaction: a comprehensive review on catalysts, kinetics, and reaction mechanism. Fuel Process. Technol.267, 108163. 10.1016/j.fuproc.2024.108163
12
Demessence A. D’Alessandro D. M. Foo M. L. Long J. R. (2009). Strong CO2 binding in a water-stable, triazolate-bridged metal−organic framework functionalized with ethylenediamine. J. Am. Chem. Soc.131, 8784–8786. 10.1021/ja903411w
13
Didas S. A. Sakwa-Novak M. A. Foo G. S. Sievers C. Jones C. W. (2014). Effect of amine surface coverage on the co-adsorption of CO2 and water: spectral deconvolution of adsorbed species. J. Phys. Chem. Lett.5, 4194–4200. 10.1021/jz502032c
14
Filburn T. Helble J. J. Weiss R. A. (2005). Development of supported ethanolamines and modified ethanolamines for CO2 capture. Ind. Eng. Chem. Res.44, 1542–1546. 10.1021/ie0495527
15
Ghanbari T. Abnisa F. Daud W. M. A. W. (2020). A review on production of metal organic frameworks (MOF) for CO2 adsorption. Sci. Total Environ.707, 135090. 10.1016/j.scitotenv.2019.135090
16
Goel C. Bhunia H. Pramod B. K. (2016). Novel nitrogen enriched porous carbon adsorbents for CO2 capture: breakthrough adsorption study. J. Environ. Chem. Eng.4, 346–356. 10.1016/j.jece.2015.11.017
17
Gonzalez M. R. González-Estefan J. H. Lara-García H. A. Sánchez-Camacho P. Basaldella E. I. Pfeiffer H. et al (2015). Separation of CO2 from CH4 and CO2 capture in the presence of water vapour in NOTT-400. New J. Chem.39, 2400–2403. 10.1039/C4NJ01933D
18
He M. Zhao H. Yang X. Jia J. Liu X. Qu Z. et al (2023). Reconsideration about the competitive adsorption of H2O and CO2 on carbon surfaces: the influence of oxygen functional groups. J. Environ. Chem. Eng.11, 111288. 10.1016/j.jece.2023.111288
19
Hernandez A. F. Impastato R. K. Hossain M. I. Rabideau B. D. Glover T. G. (2021). Water bridges substitute for defects in amine-functionalized UiO-66, boosting CO2 adsorption. Langmuir37, 10439–10449. 10.1021/acs.langmuir.1c01149
20
Holmes H. E. Ghosh S. Li C. Kalyanaraman J. Realff M. J. Weston S. C. et al (2023). Optimum relative humidity enhances CO2 uptake in diamine-appended M2 (dobpdc). Chem. Eng. J.477, 147119. 10.1016/j.cej.2023.147119
21
Horike S. Shimomura S. Kitagawa S. (2009). Soft porous crystals. Nat. Chem.1, 695–704. 10.1038/nchem.444
22
Hossain M. I. Cunningham J. D. Becker T. M. Grabicka B. E. Walton K. S. Rabideau B. D. et al (2019). Impact of MOF defects on the binary adsorption of CO2 and water in UiO-66. Chem. Eng. Sci.203, 346–357. 10.1016/j.ces.2019.03.053
23
Jedli H. Almonnef M. Rabhi R. Mbarek M. Abdessalem J. Slimi K. (2024). Activated carbon as an adsorbent for CO2 capture: adsorption, kinetics, and RSM modeling. ACS Omega9, 2080–2087. 10.1021/acsomega.3c02476
24
Kang M. Kim J. E. Kang D. W. Lee H. Y. Moon D. Hong C. S. (2019). A diamine-grafted metal–organic framework with outstanding CO2 capture properties and a facile coating approach for imparting exceptional moisture stability. J. Mater. Chem. A7, 8177–8183. 10.1039/C8TA07965J
25
Kumar S. Srivastava R. Koh J. (2020). Utilization of zeolites as CO2 capturing agents: advances and future perspectives. J. CO2Util.41, 101251. 10.1016/j.jcou.2020.101251
26
Kuwahara Y. Kang D.-Y. Copeland J. R. Brunelli N. A. Didas S. A. Bollini P. et al (2012). Dramatic enhancement of CO2 uptake by poly(ethyleneimine) using zirconosilicate supports. J. Am. Chem. Soc.134, 10757–10760. 10.1021/ja303136e
27
Lara-García H. A. Gonzalez M. R. González-Estefan J. H. Sánchez-Camacho P. Lima E. Ibarra I. A. (2015). Removal of CO2 from CH4 and CO2 capture in the presence of H2O vapour in NOTT-401. Inorg. Chem. Front.2, 442–447. 10.1039/C5QI00049A
28
Li L.-J. Liao P.-Q. He C.-T. Wei Y.-S. Zhou H.-L. Lin J.-M. et al (2015). Grafting alkylamine in UiO-66 by charge-assisted coordination bonds for carbon dioxide capture from high-humidity flue gas. J. Mater. Chem. A3, 21849–21855. 10.1039/C5TA05997F
29
Li T. Keeners T. C. (2016). A review: desorption of CO2 from rich solutions in chemical absorption processes. Int. J. Greenh. Gas. Control51, 290–304. 10.1016/j.ijggc.2016.05.030
30
Li W. Choi S. Drese J. H. Hornbostel M. Krishnan G. Eisenberger P. M. et al (2010). Steam-stripping for regeneration of supported amine-based CO2 adsorbents. ChemSusChem3, 899–903. 10.1002/cssc.201000131
31
Liao P.-Q. Chen X.-W. Liu S.-Y. Li X.-Y. Xu Y.-T. Tang M. et al (2016). Putting an ultrahigh concentration of amine groups into a metal–organic framework for CO2 capture at low pressures. Chem. Sci.7, 6528–6533. 10.1039/C6SC00836D
32
Lin J. Nguyen T. T. T. Vaidhyanathan R. Burner J. Taylor J. M. Durekova H. et al (2021). A scalable metal-organic framework as a durable physisorbent for carbon dioxide capture. Science374, 1464–1469. 10.1126/science.abi7281
33
Lin Z. Kuang Y. Li W. Zheng Y. (2024). Research status and prospects of CO2 geological sequestration technology from onshore to offshore: a review. Earth Sci. Rev.258, 104928. 10.1016/j.earscirev.2024.104928
34
Liu J. Prelesnik J. L. Patel R. Kramar B. V. Wang R. Malliakas C. D. et al (2023). A nanocavitation approach to understanding water capture, water release, and framework physical stability in hierarchically porous MOFs. J. Am. Chem. Soc.145, 27975–27983. 10.1021/jacs.3c07624
35
Liu J. Wang Y. Benin A. I. Jakubczak P. Willis R. R. LeVan M. D. (2010). CO2/H2O adsorption equilibrium and rates on metal−organic frameworks: HKUST-1 and Ni/DOBDC. Langmuir26, 14301–14307. 10.1021/la102359q
36
Lyu H. Chen O. L. Hanikel N. Hossain M. I. Flaig R. W. Pei X. et al (2022). Carbon dioxide capture chemistry of amino acid functionalized metal-organic frameworks in humid flue gas. J. Am. Chem. Soc.144, 2387–2396. 10.1021/jacs.1c13368
37
Massarweh O. Abushaikha A. S. (2024). CO2 sequestration in subsurface geological formations: a review of trapping mechanisms and monitoring techniques. Earth Sci. Rev.253, 104793. 10.1016/j.earscirev.2024.104793
38
McDonald T. M. D’Alessandro D. M. Krishna R. Long J. R. (2011). Enhanced carbon dioxide capture upon incorporation of N,N′-dimethylethylenediamine in the metal–organic framework CuBTTri. Chem. Sci.2, 2022–2028. 10.1039/C1SC00354B
39
McDonald T. M. Lee W. R. Mason J. A. Wiers B. M. Hong C. S. Long J. R. (2012). Capture of carbon dioxide from air and flue gas in the alkylamine-appended metal–organic framework mmen-Mg2(dobpdc). J. Am. Chem. Soc.134, 7056–7065. 10.1021/ja300034j
40
McDonald T. M. Mason J. A. Kong X. Bloch E. D. Gygi D. Dani A. et al (2015). Cooperative insertion of CO2 in diamine-appended metal-organic frameworks. Nature519, 303–308. 10.1038/nature14327
41
Milner P. J. Martell J. D. Siegelman R. L. Gygi D. Weston S. C. Long J. R. (2018). Overcoming double-step CO2 adsorption and minimizing water co-adsorption in bulky diamine-appended variants of Mg2(dobpdc). Chem. Sci.9, 160–174. 10.1039/C7SC04266C
42
Milner P. J. Siegelman R. L. Forse A. C. Gonzalez M. I. Runčevski T. Martell J. D. et al (2017). A diaminopropane-appended metal–organic framework enabling efficient CO2 capture from coal flue gas via a mixed adsorption mechanism. J. Am. Chem. Soc.139, 13541–13553. 10.1021/jacs.7b07612
43
Mukherjee A. Okolie J. A. Abdelrasoul A. Niu C. Dalai A. K. (2019). Review of post combustion carbon dioxide capture technologies using activated carbon. J. Environ. Sci.83, 46–63. 10.1016/j.jes.2019.03.014
44
Park S. Ahn Y. Lee S. Choi J. (2021). Calcium carbonate synthesis from waste concrete for carbon dioxide capture: from laboratory to pilot scale. J. Hazard. Mater.403, 123862. 10.1016/j.jhazmat.2020.123862
45
Peralta R. A. Alcántar-Vázquez B. Sánchez-Serratos M. González-Zamora E. Ibarra I. A. (2015). Carbon dioxide capture in the presence of water vapour in InOF-1. Inorg. Chem. Front.2, 898–903. 10.1039/C5QI00077G
46
Planas N. Dzubak A. L. Poloni R. Lin L.-C. McManus A. McDonald T. M. et al (2013). The mechanism of carbon dioxide adsorption in an alkylamine-functionalized metal–organic framework. J. Am. Chem. Soc.135, 7402–7405. 10.1021/ja4004766
47
Ran J. Jaroniec M. Qiao S.-Z. (2018). Cocatalysts in semiconductor-based photocatalytic CO2 reduction: achievements, challenges, and opportunities. Adv. Mater.30, 1704649. 10.1002/adma.201704649
48
Sagastuy-Breña M. Mileo P. G. M. Sánchez-González E. Reynolds J. E. Jurado-Vázquez T. Balmaseda J. et al (2018). Humidity-induced CO2 capture enhancement in Mg-CUK-1. Dalton Trans.47, 15827–15834. 10.1039/C8DT03365J
49
Sánchez-González E. Álvarez J. R. Peralta R. A. Campos-Reales-Pineda A. Tejeda-Cruz A. Lima E. et al (2016). Water adsorption properties of NOTT-401 and CO2 capture under humid conditions. ACS Omega1, 305–310. 10.1021/acsomega.6b00102
50
Schoedel A. Ji Z. Yaghi O. M. (2016). The role of metal–organic frameworks in a carbon-neutral energy cycle. Nat. Energy1, 16034. 10.1038/nenergy.2016.34
51
Shi Z. Tao Y. Wu J. Zhang C. He H. Long L. et al (2020). Robust metal–triazolate frameworks for CO2 capture from flue gas. J. Am. Chem. Soc.142, 2750–2754. 10.1021/jacs.9b12879
52
Siegelman R. L. McDonald T. M. Gonzalez M. I. Martell J. D. Milner P. J. Mason J. A. et al (2017). Controlling cooperative CO2 adsorption in diamine-appended Mg2(dobpdc) metal–organic frameworks. J. Am. Chem. Soc.139, 10526–10538. 10.1021/jacs.7b05858
53
Siegelman R. L. Milner P. J. Forse A. C. Lee J.-H. Colwell K. A. Neaton J. B. et al (2019). Water enables efficient CO2 capture from natural gas flue emissions in an oxidation-resistant diamine-appended metal–organic framework. J. Am. Chem. Soc.141, 13171–13186. 10.1021/jacs.9b05567
54
Song K. S. Fritz P. W. Coskun A. (2022). Porous organic polymers for CO2 capture, separation and conversion. Chem. Soc. Rev.51, 9831–9852. 10.1039/D2CS00727D
55
Soubeyrand-Lenoir E. Vagner C. Yoon J. W. Bazin P. Ragon F. Hwang Y. K. et al (2012). How water fosters a remarkable 5-fold increase in low-pressure CO2 uptake within mesoporous MIL-100(Fe). J. Am. Chem. Soc.134, 10174–10181. 10.1021/ja302787x
56
Spurin C. Callas C. Darraj N. Rucker M. Benson S. (2025). The importance and challenges associated with multi-scale heterogeneity for geological storage. InterPore J.2, IPJ260225–2. 10.69631/ipj.v2i1nr76
57
Sumida K. Rogow D. L. Mason J. A. McDonald T. M. Bloch E. D. Herm Z. R. et al (2012). Carbon dioxide capture in metal–organic frameworks. Chem. Rev.112, 724–781. 10.1021/cr2003272
58
Wang T. C. Vermeulen N. A. Kim I. S. Martinson A. B. F. Stoddart J. F. Hupp J. T. et al (2016). Scalable synthesis and post-modification of a mesoporous metal-organic framework called NU-1000. Nat. Protoc.11, 149–162. 10.1038/nprot.2016.001
59
Wang Y. Zhao L. Otto A. Robinius M. Stolten D. (2017). A review of post-combustion CO2 capture technologies from coal-fired power plants. Energy Procedia114, 650–665. 10.1016/j.egypro.2017.03.1209
60
Wurzbacher J. A. Gebald C. Steinfeld A. (2011). Separation of CO2 from air by temperature-vacuum swing adsorption using diamine-functionalized silica gel. Energy Environ. Sci.4, 3584–3592. 10.1039/C1EE01681D
61
Xian S. Peng J. Zhang Z. Xia Q. Wang H. Li Z. (2015). Highly enhanced and weakened adsorption properties of two MOFs by water vapor for separation of CO2/CH4 and CO2/N2 binary mixtures. Chem. Eng. J.270, 385–392. 10.1016/j.cej.2015.02.041
62
Yazaydın A. Ö. Benin A. I. Faheem S. A. Jakubczak P. Low J. J. Willis R. R. et al (2009). Enhanced CO2 adsorption in metal-organic frameworks via occupation of open-metal sites by coordinated water molecules. Chem. Mater.21, 1425–1430. 10.1021/cm900049x
63
Yong Z. Mata V. Rodrigues A. E. (2002). Adsorption of carbon dioxide at high temperature—a review. Sep. Purif. Technol.26, 195–205. 10.1016/S1383-5866(01)00165-4
64
Yu J. Wu Y. Balbuena P. B. (2016). Response of metal sites toward water effects on postcombustion CO2 capture in metal–organic frameworks. ACS Sustain. Chem. Eng.4, 2387–2394. 10.1021/acssuschemeng.6b00080
65
Zhang W. Sun C. Snape C. E. Sun X. Liu H. (2020). Cyclic performance evaluation of a polyethylenimine/silica adsorbent with steam regeneration using simulated NGCC flue gas and actual flue gas of a gas-fired boiler in a bubbling fluidized bed reactor. Int. J. Greenh. Gas. Control95, 102975. 10.1016/j.ijggc.2020.102975
66
Zhao B. Su Y. Tao W. Li L. Peng Y. (2012). Post-combustion CO2 capture by aqueous ammonia: a state-of-the-art review. Int. J. Greenh. Gas. Control9, 355–371. 10.1016/j.ijggc.2012.05.006
67
Zhou H.-C. Long J. R. Yaghi O. M. (2012). Introduction to metal–organic frameworks. Chem. Rev.112, 673–674. 10.1021/cr300014x
Summary
Keywords
CO2 capture, metal-organic framework, nanoporous material, water, humidity
Citation
Cammarere C, Cortés J, Glover TG, Snurr RQ, Hupp JT and Liu J (2025) Water-enhanced CO2 capture in metal–organic frameworks. Front. Chem. 13:1634637. doi: 10.3389/fchem.2025.1634637
Received
24 May 2025
Accepted
18 June 2025
Published
08 July 2025
Volume
13 - 2025
Edited by
Sonja Grubisic, University of Belgrade, Serbia
Reviewed by
Miljan Dragan Dasic, University of Belgrade, Serbia
Nicolina Pop, Politehnica University of Timișoara, Romania
Updates
Copyright
© 2025 Cammarere, Cortés, Glover, Snurr, Hupp and Liu.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jian Liu, kjlsch@rit.edu
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.