- Grupo de Química Orgánica Medicinal, Instituto de Quimica-Biologica, Facultad de Ciencias, Universidad de la República, Montevideo, Uruguay
Introduction
A phagolysosome is a cytoplasmic body formed through the fusion of a phagosome with a lysosome during the phagocytosis process (Alexander and Vickerman, 1975). The phagolysosome is characterized by an internal acidic environment (pH 4.5–5.0) and an internal temperature of 37°C. This internal acidic condition plays an important role in the intracellular destruction of pathogens via enzymatic hydrolytic degradation (Nguyen and Yates, 2021). This body is crucial for the survival of Leishmania parasites within the host cell (Zilberstein, 2021).
Leishmania is an intracellular parasite that cycles between the midgut of female sandfly vectors and phagolysosomes of mammalian hosts. The infection initiates with the transformation of the parasite found in the midgut of a sandfly into the flagellated promastigote form. Then the parasites are injected into the human skin during a sandfly blood meal and are rapidly phagocytosed by macrophages, which fuse with lysosomes to form phagolysosomes (Zilberstein, 2021). It is documented that the presence of a chemical component such as lipophosphoglycan (LPG) could be essential in the recognition of promastigote parasite by macrophage cells (Desjardins and Descoteaux, 1997; Moradin and Descoteaux, 2012). Once within the phagolysosome, promastigotes are differentiated to a smaller aflagellated intracellular amastigote form, which is favored by the extremely harsh environment inside the phagolysosome (Berman et al., 1979; Chang and Dwyer, 1976). The parasites at this stage survive and elude the host defense mechanism within the phagolysosome (Chang and Dwyer, 1976; Moradin and Descoteaux, 2012) and then proliferate by binary cell division and invade other macrophages or phagocytic (i.e. dendritic cells) or non-professional phagocytic (i.e. fibroblasts) cells. To elude the host immune defense, Leishmania parasites developed a mechanism directed to promote a shift in the macrophage polarization, from a defensive macrophage M1 to an attenuated macrophage M2 (Carneiro et al., 2021; Naderer and McConville, 2008; Tomiotto-Pellissier, et al., 2018), which allows their survival and proliferation inside phagolysosomes. Thus, the phagolysosome emerges as an attractive target for the development of leishmanicidal agents, and it is essential to design chemical structures that will be able to accumulate into the phagolysosome taking advantage of their internal acidic characteristic and highly lipophilic membrane. With this prelude in hand, the present article seeks to show the role of some physicochemical properties [e.g., ionization constant (pKa) and lipophilicity (log P)] to favor the accumulation of quinoline systems into the lysosome and to subsequently correlate these parameters with the in vitro leishmanicidal response against intracellular amastigotes.
Importance of basicity and lipophilicity in lysosome accumulation
From a physicochemical point of view, it is possible to predict the ability of a quinoline or any type of compound to accumulate into phagolysosomes and/or analogue organelles (e.g., lysosomes). Trapp et al. (2008) demonstrated, in general terms, the important role of basicity in the accumulation of molecules within the lysosome. They predicted the accumulation of organic compounds within the cell by studying the diffusion from the external solution to the cell organelle (e.g., cytosol, lysosome, or mitochondria) using the Fick–Nernst–Planck equation. In the present analysis, most of the studied compounds were based on quinolines including amodiaquine, chloroquine, quinine, mefloquine, primaquine, quinidine, and quinacrine. The rest of the tested compounds included cycloguanil, artemisinin, halofantrine, and pyrimethamine. The study shows that a high and selective accumulation in lysosomes was found for weak mono- and bivalent bases having intermediate to high values of log KOW. The authors proposed that the selective accumulation into lysosomes over other organelles (e.g., cytosol or mitochondria) can be mediated through an “ion trapping” mechanism, in which the protonation of the basic moiety captures the compounds, forming a more hydrophilic species whose outer diffusion is minimized. Physicochemical properties such as the ionization constant (pKa) and the equilibrium constant (log KOW) are key to understanding the accumulation via “ion trapping.” For monovalent weak bases, the optimal parameter for good lysosome accumulation consists of pKa and log KOW values ranging between 6 and 10 and between 0 and 3, respectively. An optimal accumulation was corresponded for bases with a pKa of 8. For bivalent bases, the optimal pKa2 (aliphatic amine) value ranged between 8 and 10 and the pKa1 value ranged between 4 and 8, whereas the optimal log KOW value ranged between 3 and 6. Neutral compounds (e.g., artemisinin) showed a negligible accumulation into lysosomes.
Regarding the lipophilicity parameter, Marceau et al. (2012) identified the correlation between the log P value and the optimal accumulation into lysosomes. Based on a series of cationic triethylamine derivatives including triethanolamine, procainamide, triethylamine, lidocaine, imatinib, chloroquine, astemizole, quinacrine, dronedarone, and amiodarone, which displayed pKa values ranging between 8 and 10, compounds with a log P value ranging between 1 and 4 showed the highest accumulation in lysosomes, whereas a decrease in lysosome accumulation was found with the increase in log P for values higher than 4. Thus, the optimal combination and appropriate control of these two variables could be pivotal in the rational design of leishmanicidal agents, whose goal is to guarantee quinoline accumulation within the phagolysosome. Furthermore, the incorporation of the lipophilic group, which provides a general log P ∼ 1–4 to the molecule, seeks to facilitate the penetration of the quinoline drug through the lipophilic phagolysosome membrane, whereas the incorporation of a basic moiety, which provides pKa ∼ 4–10 (pKa1 and pKa2 correspond to the quinolinic and alkyl amine chain) to the molecule, seeks to guarantee the accumulation inside the lysosome through an “ion trapping” mechanism that involves the capture of the molecule by generating a polar and cationic form through the protonation of the basic moieties in an acidic environment, as depicted in Scheme 1.
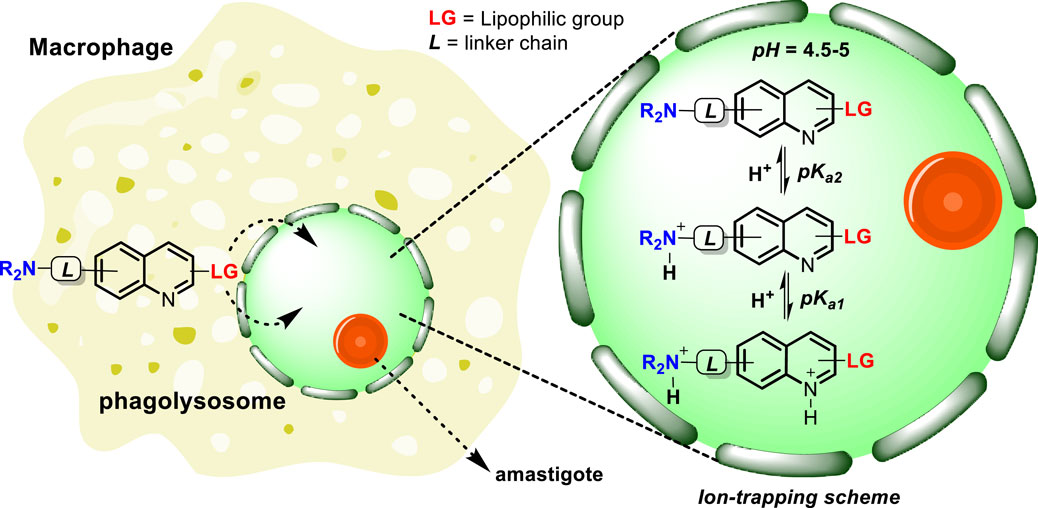
Scheme 1. Tentative mechanism of accumulation of quinolines inside the phagolysosome via lipophilic penetration and the subsequent ion trapping mechanism through the protonation of basic moieties in an acidic environment.
Lipophilic and basic groups and the leishmanicidal response in quinolines
The present section seeks to correlate the role of lipophilicity and basicity with leishmanicidal activity based on the leishmanicidal response derived from an in vitro intracellular amastigote model. The analysis focused on the recent reviews published by Romero and Delgado (2025a); Del Carpio et al. (2025); Avanzo et al. (2025); Loiseau et al. (2022), which fully recompiles the leishmanicidal potential of 4-aminoquinolines, metal based-quinolines, antimalarial quinolines, and 2-substituted quinolines, respectively. The pKa and log P values were estimated using ChemDraw software (ChemDraw Professional, 2016) and the SwissADME platform (Daina et al., 2017), respectively. Relevant cases are shown in Figure 1. Regarding the dibasic 4-aminoquinolines (Romero and Delgado, 2025a), it should be noted that most of the potent and selective 4-aminoquinolines (e.g., compounds 1–6) are characterized by incorporating in their structures either a basic group (e.g., tertiary dialkylamine or N-heteroarene) or a lipophilic group (e.g., aryl or alkyl chain) that disclose appropriate or acceptable log P and pKa parameters. For example, the most selective compound (compound 1), which displayed an IC50 value of 0.023 µM against the amastigote of Leishmania donovani and a selectivity index (S.I.) of 1,739, showed an optimal pKa2 value of 8.32 and a discretely high log P value of 5.29. Compound 2, which displayed a high anti-amastigote response against L. donovani (IC50 = 0.36 µM) and selectivity (S.I. > 1,111), showed an optimal log P of 4.10 and an appropriate pKa2 of 3.98. Meanwhile, compound 3, which presented appropriate values of log P (4.15) and pKa2 (9.78), exhibited a high leishmanicidal response and selectivity against amastigote models of Leishmania amazonensis (IC50 = 0.34 µM; S.I. = 145) and Leishmania infantum (IC50 = 0.18 µM; S.I. = 392). Moreover, compound 4 showed moderate potency (IC50 = 5.48 µM) and selectivity (S.I. = 41) against the amastigote of L. amazonensis, which could be correlated more with its lower lipophilicity (log P = 3.31) than with its basicity because it presented an optimal pKa2 (6.61). Compound 5, a quinoline-based metallic complex, was considered the most potent due to its excellent in vitro (IC50 = 0.5 vs. L. donovani amastigote) and in vivo responses (Del Carpio et al., 2025), with appropriate log P and pKa2 values of 4.10 and 3.68, respectively. Finally, of the analyzed 4-aminoquinolines (1–6), compound 6 (log P = 6.58; pKa2 = 9.17) was highly potent but highly toxic, which could be associated with its extremely high lipophilicity. In general, based on examples of 4-aminoquinolines, it has been consistently documented that the inclusion of either several basic or extended lipophilic moieties into the quinoline structure compromises the selectivity and leishmanicidal potency of the 4-aminoquinolines (Romero and Delgado, 2025a). Furthermore, it shows that the most active and selective leishmanicidal dibasic 4-aminoquinolines are characterized as having a log P ranging between 4 and 5.3 and a pKa2 ranging between 4 and 9.
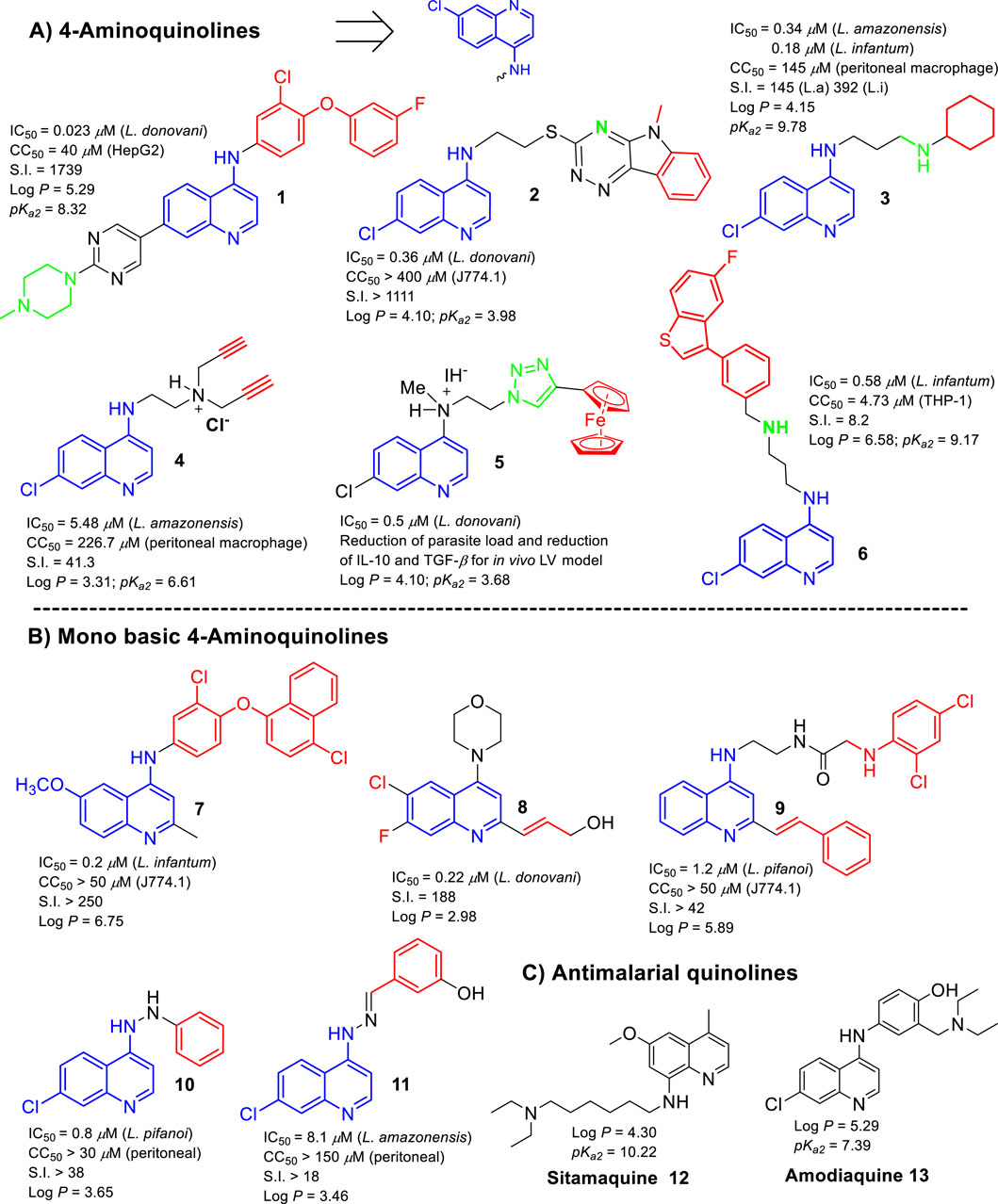
Figure 1. Structures of the most promising leishmanicidal quinoline compounds. (A) 4-Aminoquinolines; (B) monobasic 4-aminoquinolines; (C) antimalarial quinolines.
Regarding the monobasic 4-aminoquinolines (with a pKa1 ∼4 by quinolinic nitrogen), some compounds (e.g., compounds 7, 8, 9, 10, and 11) exhibited good anti-amastigote effects in a similar range to that of the most active 4-aminoquinolines mentioned above, but they were less selective. This indicates that the selectivity of these compounds appears to have a correlation with its lipophilic characteristic. For example, the most active monobasic quinoline (compound 7), which showed an excellent potency against L. infantum (IC50 = 0.20 µM) and a good selectivity (S.I. ≥ 250), presented a high log P value of 6.75. The second most selective compound of this group (compound 8) (S.I. = 188; IC50 = 0.22 µM vs. L. donovani) (Loiseau, et al., 2022) presented a log P value of 2.98. In this case, the inclusion of the halogens (F and Cl) and the morpholine moiety in the quinoline core could be essential to conveniently modulate the log P and pKa magnitudes for favoring the accumulation inside the macrophage phagolysosome in the infected model. Another potent (IC50 = 1.20 µM vs. Leishmania pifanoi) and selective (S.I. > 42) compound (compound 9) exhibited a high log P of 5.89. Meanwhile, compounds 10 (log P = 3.46) and 11 (log P = 3.65), which showed a log P < 4, exhibited more limited leishmanicidal activities and selectivities.
Finally, analyzing the leishmanicidal response of antimalarial quinolines (Avanzo et al., 2025), lipophilic amodiaquine (AQ), sitamaquine (SQ), mefloquine, and tafenoquine bear a second basic group and display the highest anti-amastigote response against Leishmania spp. However, the high cytotoxicity of mefloquine and tafenoquine reflects the importance of the selection of the type of lipophilic chain and its location on the quinoline ring. Furthermore, SQ (log P = 4.30; pKa2 = 10.22) and AQ (log P = 5.29; pKa2 = 7.39) were considered the most selective and potent antimalarial quinolines against the intracellular amastigote, which could be associated with their optimal pKa2 and log P values. Further reports have demonstrated that SQ is able to accumulate in membranous organelles such as acidocalcisomes (López-Martín et al., 2008) and parasite mitochondria (Vercesi and Docampo, 1992; Vercesi et al., 2000). This last finding supports that the internalization of the SQ into these organelles could be favored by their appropriate pKa2 and log P parameters.
Conclusion
In summary, the present opinion article introduces the importance of the phagolysosome and its mechanism to favor its drug accumulation as pivotal concepts in the design of potent and selective leishmanicidal agents, which are applicable to the design of not only quinolines but also other types of leishmanicidal compounds. Based on the reported cases, it can be inferred that the high anti-amastigote response and selectivity of the quinoline compounds could be favored by their appropriate log P and pKa parameters, which seeks to facilitate their transmembrane penetration and lysosome accumulation via “ion trapping.” It should be noted that the dibasic quinolines tend to generate more active and selective leishmanicidal compounds than monobasic quinolines and even more than tri- or polybasic quinolines. Within the dibasic quinolines, mainly those based on 4-aminoquinolines, the most potent and selective compounds are characterized as having pKa2 ranging between 6 and 9 and log P values ranging from 4 to 6. Meanwhile, the most promising monobasic quinolines presented, in general, log P values higher than 3 and lower than 6.5, with some exceptions, such as compound 8, in which incorporation of halogen and morpholine moieties could be key to conveniently modulate the lipophilicity and basicity of the quinoline for good penetration and accumulation inside the phagolysosome.
In general, the ionization and lipophilic parameter requirements to achieve a good leishmanicidal response in the quinoline compounds are in good concordance with Trapp’s and Marceau’s findings, where a compound with a higher lipophilicity (log P ranging from 4 to 6) than expected for typical lysosome accumulation (log P ranging between 1 and 4) is still considered for lysosomal targeting. Probably, the combination of both factors under appropriate magnitudes could be highly required for the design of potent and selective leishmanicidal agents for macrophage phagolysosome targeting. However, there is an urgent need for further studies either to demonstrate its effective accumulation into the macrophage phagolysosome or to elucidate the most appropriate parameter magnitudes to achieve a good phagolysosome accumulation and control of the selectivity in the design of quinoline-based drugs and other types of leishmanicidal agents based on a screening study.
Author contributions
AR: Writing - review and editing, Conceptualization, Supervision, Formal Analysis, Methodology, Project administration, Software, Writing - original draft, Investigation, Visualization, Resources, Data curation, Funding acquisition, Validation.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by PEDECIBA (Programa de Desarrollo de las Ciencias Básicas) under Despegue-Científico 2023 funds. AHR thanks to Sistema Nacional de Investigadores (SNI) for grant SNI_2023_1_1013178.
Conflict of interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Alexander, J., and Vickerman, K. (1975). Fusion of host cell secondary lysosomes with the parasitophorous vacuoles of leishmania Mexicana-Inlected macrophages. J. Protozool. 22, 502–508. doi:10.1111/j.1550-7408.1975.tb05219.x
Avanzo, R. E., Garcia-Linares, G., Rogriguez, N., and Romero, A. H. (2025). A comprenhensive revision on the use of quinoline antimalarial drugs as leishmanicidal agents. Front. Chem. 13, 1608340. doi:10.3389/fchem.2025.1608340
Berman, J. D., Wyler, D. J., and Dwyer, D. M. (1979). Multiplication of leishmania in human macrophages in vitro. Infect. Immun. 26, 375–379. doi:10.1128/iai.26.1.375-379.1979
Carneiro, M. B., Vaz, L. G., Afonso, L. C. C., Horta, M. F., and Vieira, L. Q. (2021). Regulation of macrophage subsets and cytokine production in leishmaniasis. Cytokine 147, 155309. doi:10.1016/j.cyto.2020.155309
Chang, K. P., and Dwyer, D. M. (1976). Multiplication of a human parasite (Leishmania donovani) in phagolysosomes of hamster macrophages in vitro. Science 193, 678–680. doi:10.1126/science.948742
Daina, A., Michielin, O., and Zoete, V. (2017). SwissADME: a free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 7, 42717. doi:10.1038/srep42717
Del Carpio, E., Hernández, L., Lubes, V., Jourdan, F., Cerecetto, H., Scalese, G., et al. (2025). Metal complexes based on quinoline for development of leishmanicidal agents: structure and mechanism of action. Front. Chem. 13, 1586044. doi:10.3389/fchem.2025.1586044
Desjardins, M., and Descoteaux, A. (1997). Inhibition of phagolysosomal biogenesis by the leishmania lipophosphoglycan. J. Exp. Med. 185, 2061–2068. doi:10.1084/jem.185.12.2061
Loiseau, P. M., Balaraman, K., Frézard, F., Figadère, B., Durand, R., Frézard, F., et al. (2022). The potential of 2-Substituted quinolines as antileishmanial drug candidates. Molecules 27, 2313. doi:10.3390/molecules27072313
López-Martín, C., Pérez-Victoria, J. M., Carvalho, L., Castanys, S., and Gamarro, F. (2008). Sitamaquine sensitivity in leishmania species is not mediated by drug accumulation in acidocal cisomes. Antimicr. Agent. Chemother. 52, 4030–4036. doi:10.1128/aac.00964-08
Marceau, F., Bawolak, M. T., Lodge, R., Bouthillier, J., Gagné-Henley, A., Gaudreault, R. C., et al. (2012). Cation trapping by cellular acidic compartments: beyond the concept of lysosomotropic drugs. Toxicol. Appl. Pharmacol. 259, 1–12. doi:10.1016/j.taap.2011.12.004
Moradin, N., and Descoteaux, A. (2012). Leishmania promastigotes: building a safe niche within macrophages. Front. Cell Infect. Microbiol. 2, 121. doi:10.3389/fcimb.2012.00121
Naderer, T., and McConville, M. J. (2008). The Leishmania–macrophage interaction: a metabolic perspective. Cel. Microbiol. 10, 301–308. doi:10.1111/j.1462-5822.2007.01096.x
Nguyen, J. A., and Yates, R. M. (2021). Better together: current insights into phagosome-lysosome fusion. Front. Immunol. 12, 636078. doi:10.3389/fimmu.2021.636078
Romero, A. H., and Delgado, F. (2025a). 4-Aminoquinoline as a privileged scaffold for the design of leishmanicidal agents: structure-property relationships and key biological targets. Front. Chem. 12, 1527946. doi:10.3389/fchem.2024.1527946
Tomiotto-Pellissier, F., Bortoleti, B., Assolini, J. P., Gonçalves, M. D., Carloto, A. C. M., Miranda-Sapla, M. M., et al. (2018). Macrophage polarization in leishmaniasis: broadening Horizons. Front. Immunol. 9, 2529. doi:10.3389/fimmu.2018.02529
Trapp, S., Rosania, G. S., Horobin, R. W., and Kornhuber, J. (2008). Quantitative modeling of selective lysosomal targeting for drug design. Eur. Biophys. J. 37, 1317–1328. doi:10.1007/s00249-008-0338-4
Vercesi, A., and Docampo, R. (1992). Ca2+ transport by digitonin-permeabilized leishmania donovani. Effects of Ca2+, pentamidine and WR-6026 on mitochondrial membrane potential in situ. Biochem. J. 284, 463–467. doi:10.1042/bj2840463
Vercesi, A., Rodrigues, C., Castisti, R., and Docampo, R. (2000). Presence of a Na+/H+ exchanger in acidocalcisomes of Leishmania donovani and their alkalization by anti-leishmanial drugs. FEBS Lett. 473, 203–206. doi:10.1016/s0014-5793(00)01531-3
Keywords: phagolysosome, Leishmania, log P, ionization constant (pKa), quinolines
Citation: Romero AH (2025) Are basic and lipophilic chain groups highly required in leishmanicidal quinolines to favor the phagolysosome accumulation?. Front. Chem. 13:1655979. doi: 10.3389/fchem.2025.1655979
Received: 29 June 2025; Accepted: 29 July 2025;
Published: 21 August 2025.
Edited by:
Waquar Ahsan, Jazan University, Saudi ArabiaReviewed by:
Ayoub El-Mrabet, Sidi Mohamed Ben Abdellah University, MoroccoCopyright © 2025 Romero. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Angel H. Romero, YW5nZWwudWN2LnVzYkBnbWFpbC5jb20=
†ORCID: Angel H. Romero, orcid.org/0000-0001-8747-5153
 Angel H. Romero
Angel H. Romero