Abstract
This study focuses on developing an electrochemical reaction to produce benzo[d]-1,3-dithiole-2-one (BDTO). This compound serves as a direct precursor for dibenzotetrathiafulvalenes (DBTTF), an important member of the charge transfer complex family. BDTO is synthesized in three steps (16%) starting from aniline. The key reaction is an anodically driven intramolecular cyclization (35%), involving a thiyl radical-cation intermediate formed from the oxidation of the S-aryl-O-ethyldithiocarbonate derivative. This derivative is obtained in good yields from its respective aromatic diazonium salt. This approach eliminates the need for advanced, costly intermediates and avoids long, complex synthetic routes previously used to produce BDTO, utilizing safer and cheaper reagents. This opens the door to generating DBTTF derivatives quickly.
1 Introduction
The tetrathiafulvalene (TTF) molecule and its derivatives have attracted the attention of scientists since the early 1970s due to their attractive properties as a conductive material (Andrieux et al., 1979; Melby et al., 1974; Yoneda et al., 1979). When it forms a charge transfer complex with the tetracyanoquinodimethane molecule (TTF-TCNQ) an organic metal is obtained since its conductivity reaches 104 S cm-1 at 59 K (Ferraris et al., 1973; Metz, 1973). The dibenzotetrathiafulvalene (DBTTF) family represents a great alternative to TTFs due to its ability to modulate redox potential by introducing functional groups in the benzyl ring. Their diverse applications are innumerable, and they play an important role as redox sites in different areas such as sensors, Balandier et al. (2007) starting materials for organic electronic systems, Gao et al. (2006) molecular redox switches, as a building block in supramolecular architectures, Jana et al. (2017) and many others. However, further development of these materials has been limited by a complicated or expensive synthesis route to prepare the final intermediaries of synthesis, such as benzo[d]-1,3-dithiole-2-ones (BDTO) (1) and benzo[d]-1,3-dithiole-2-thiones (BDTT) (2).
The final coupling from both to yield the symmetrical DBTTF ring is carried out using these intermediates in combination with P(OEt)3 as a coupling reagent (Scheme 1a); this final reaction has good yields (80%–90%) (Fanghänel et al., 1995; Inayoshi and Ono, 2000). Besides, one equivalent of BDTO (1) and one equivalent of BDTT (2) are needed to obtain asymmetric DBTTFs using the same reaction (Gao et al., 2006).
SCHEME 1

(a) Last step in DBTTFs preparation; (b) Synthetic routes for obtaining DBTO derivatives; (c) New synthetic route developed for the synthesis of DBTO (1).
Looking closer at the scarce synthetic routes of BDTO (Scheme 1b), Watson, and Col. (Sun et al., 1997). synthesized 1 derivatives from benzoquinone via 1,4-addition of dithiocarbamate anion to the 6-member ring followed by chemical oxidation. The obtained products contain the p-hydroquinone moiety in the benzene ring, limiting the reaction’s scope. Compound 1 can also be prepared using oxidative decomposition of 1,3-benzodithiol-2-alkyl derivatives (Aromdee et al., 1983) with moderated yields using Pb(OAc)4 or DDQ (Nakayama et al., 1977). BDTT has also been used as an intermediate to obtain BDTO via an atom replacement reaction using Hg(OAc)2/AcOH under chloroform reflux (de Mayo et al., 1979). The most used reaction to access this important heterocyclic ring requires 1,2-benzeneditiol, Takamasa and Takashi (2002) or 1,2-bis(S-benzylthio)-benzene derivatives, Loosli et al. (2005) and carbonylimidazole. All the routes described are long, use corrosive, toxic, and sensitive reagents (Gao et al., 2007) like carbonyl imidazole, prepared from phosgene and readily water reactive, and have a low atom economy (loss of two BnS moieties, for example). Due to the complexity of preparing BDTO derivatives, the most used routes for preparing DBTTFs involve BDTT intermediates (Inayoshi et al., 2016; Senga et al., 1997). Unfortunately, these are not readily prepared either.
Over the last decade, interest in electrosynthesis has increased due to its ability to access unusual functionalities and reactivity, as well as its role in promoting greener and more sustainable synthesis (Cembellín and Batanero, 2021; Frontana-Uribe et al., 2010; Pollok and Waldvogel, 2020). This method also enables the generation of in situ reactive intermediates useful in synthetic protocols. Our work focuses on developing a new synthetic route to obtain benzo[d]-1,3-dithiolen-2-one (BDTO) in a simple, fast, and direct manner through electrochemical activation of O-ethyl-S-phenyldithiocarbonate, which is derived from the corresponding benzene diazonium salt (Scheme 1c). This article presents our approach and initial findings in an ambitious program aimed at creating a broad range of DBTO products, leveraging the fact that aniline derivatives are widely substituted, affordable, and commercially available precursors for organic synthesis, using green chemistry techniques with significant time and cost savings.
2 Materials and methods
2.1 General
Commercially purchased reagents were used as starting materials without any additional purification steps. The supporting electrolyte salts were dried in the oven for at least one night before use. Anhydrous-grade solvents were used for cyclic voltammetry and preparative electrolysis experiments. For purification by column chromatography (CC), silica gel (70–230 mesh) and technical grade solvents, but previously distilled, were used. TLC analysis was carried out using Merck TLC Silica gel 80 F254 aluminum sheets.
Melting points were not corrected and carried out on a Fisher-Scientific 12–144 melting point apparatus. NMR spectra were recorded on Bruker (300 MHz) using TMS as an internal reference for 1H (0.0 ppm) and CDCl3 for 13C (77.16 ppm). All the prepared and isolated compounds are known and fit with the reported physical and spectroscopic description. Voltammetric studies were carried out using a PGSTAT204 Potentiostat and a conventional glass cell of 10 mL. Reference electrode: Ag/Ag+ (filled with AgNO3 0.01 M in CH3CN). Working electrode: glassy carbon disk (diameter: 3 mm). Counter electrode: platinum wire (99.95% purity).
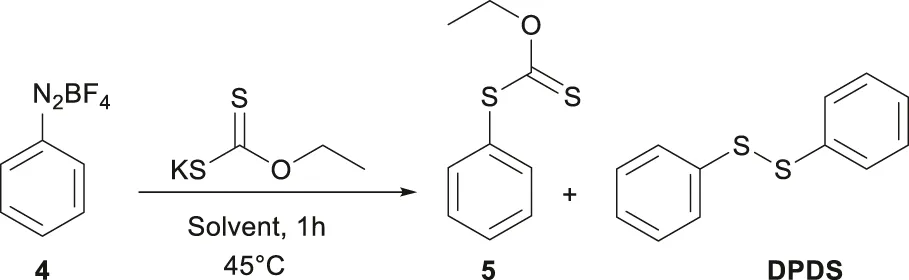
2.2 Synthesis of the O-ethyl-S-phenyldithiocarbonate (5)
O-ethyl-S-phenyldithiocarbonate (5) was prepared in two steps using a variation of the Leuckart methodology, 4; Leuckart and Prakt (1889), Cox et al. (1960) via diazonium tetrafluoroborate salt (4). The crystalline solid obtained can be stored in the dark at low temperatures to prevent decomposition. In a typical experiment, 0.5 mL (1 equiv.) of aniline was reacted with tetrafluoroboric acid (2.1 equiv.) added dropwise under constant magnetic stirring, forming a white precipitate corresponding to the anilinium salt. The temperature was maintained at 0 °C and kept under a nitrogen atmosphere. A cold solution (0 °C) of sodium nitrite (1.1 equiv.) in water was slowly added and stirred for 40 min. The precipitate was rapidly recrystallized from an acetone-ether mixture, yielding the product 4 in 83%.
For the second step, several solvents with different polarities were tested as reaction media (Table 1). The reaction cell temperature was set at 45 °C, and 460 mg (2.87 mmol, 1.1 equivalents) of potassium O-ethylxanthogenate was dissolved in 6 mL of the respective solvent. In another flask, 100 mg of 4 (0.52 mmol, 1 equivalent) was dissolved in the same volume of solvent. The solution containing compound 4 was added dropwise to the reaction cell and stirred for 1 hour. The reaction produced two major products (5 and DPDS), which were purified by chromatography (Hexane/AcOEt Mixtures).
TABLE 1
|
|||
|---|---|---|---|
| Entry | Solvent | 5 yield (%) | DPDS yield (%) |
| 1 | H2O | 55 | 4 |
| 2 | H2O/ACN | 30 | 25 |
| 3 | ACN | 20 | 30 |
| 4 | DMSO | 12 | 36 |
| 5 | THF | 3 | 41 |
Synthesis of O-ethyl-S-phenyldithiocarbonate 5 in different solvents.
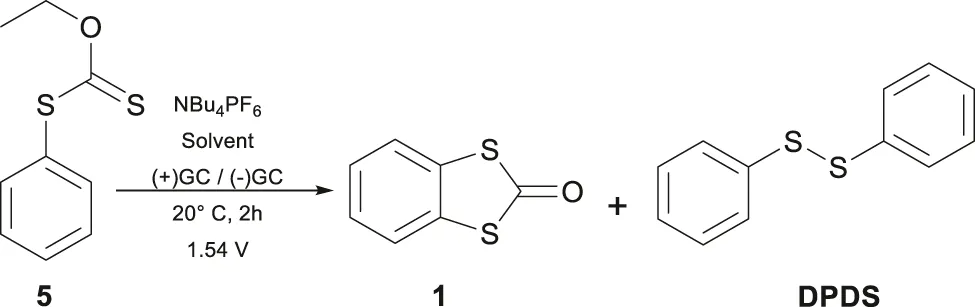
2.3 General preparative electrolysis of O-ethyl-S-phenyldithiocarbonate (5), preparation of BDTO
Preparative electrolysis was performed potentiostatically at 1.55 V with 100 mg of compound 5 in an H-type cell at 20 °C. Two glassy carbon plates (4.2 cm2) served as the working and counter electrodes, with an Ag/Ag+ reference electrode and 0.1 mol L-1 NBu4PF6 in 5 mL of ACN. Each reaction involved degassing both sides with N2 for 15 min before initiating the current; the cell was kept under an inert atmosphere. After electrolysis, solvents were removed under vacuum using rotary evaporation, and products were separated by chromatography on a silica gel column (230–400 mesh) with a Hexane–AcOEt mixture. For the other experiments described in Table 2, only the solvent was changed to 25% CH2Cl2/75% ACN and 25% HFIP/75% ACN.
TABLE 2
|
||||
|---|---|---|---|---|
| Entry | Solvent | E(V) | BDTO 1 (%) | DPDS (%) |
| 1 | ACN | 1.55 | 30 | 15 |
| 2 | ACN | 1.50 | 20 | 25 |
| 3 | ACN | 1.60 | 22 | 30 |
| 4 | ACN/CH2Cl2 | 1.55 | 10 | 23 |
| 5 | HFIP/ACN | 1.55 | 35 | 10 |
| 6 | HFIP/ACN | 1.60 | 25 | 27 |
Electrosynthesis of compound BDTO one in different solvents.
3 Results and discussion
The key intermediate O-ethyl-S-phenyldithiocarbonate (5) was obtained by reacting benzene diazonium tetrafluoroborate salt (4) with potassium O-ethylxanthogenate; the one-pot reaction was not efficient, and it was necessary to purify 4 to obtain good yields. It was reported that the diazonium tetrafluoroborate salt is more stable than other anions, (Firth and Fairlamb, 2020), and other counter ions were not prepared. The reaction was solvent-dependent, but using H2O, the best yield was obtained (Table 1). Interestingly, with more polar solvents, the yield of the target compound increased and the secondary compound DPDS decreased; in low-polarity solvents, this tendency is reversed. A plausible explanation for the presence of DPDS is shown in the (Supplementary Scheme S1). Neutral free radicals are generally considered unaffected by the solvent. However, in cases such as thiyl radicals, the solvent can significantly alter radical reactivity and influence the reaction outcome. Therefore, water promotes the formation of 5 via an ionic mechanism and reduces homolytic fragmentation that triggers radical reactions and the formation of DPDS. Ito and colleagues (Ito and Matsuda, 1982; Ito and Matsuda 1984) showed that thiyl radical intermediates are highly stable, and the recombination rate constant decreases as solvent polarity increases, supporting our observations.
The cyclic voltammetry of O-ethyl-S-phenyldithiocarbonate (5) in the range of −3 V–2 V (Figure 1) showed a well-defined oxidation signal at 1.54 V and 1.84 V, both irreversible. Likewise, two signals are observed at −2.17 V and −2.6 V in the reduction zone, exhibiting irreversible behavior. The anodic signals did not alter with the presence of 1,1,1,3,3,3-hexafluoro-2-propanol (HFIP) as cosolvent (see Supplementary Material); HFIP can stabilize cationic reactive species and produce positive changes in the reactivity (Yoshida et al., 2003). It was hypothesized that the radical cation obtained by the first anodic electron transfer could trigger an intramolecular ring closure to yield the BDTO compound (Scheme 2a). Thus, preparative electrolysis was carried out potentiostatically at 1.55 V (Table 2).
FIGURE 1

CV of 5 (0.5 mmol L−1) in ACN, 0.1 mol L−1 NBu4PF6, v = 100 mV s−1, WE: Glassy Carbon, RE: Ag/Ag+, CE: platinum wire.
SCHEME 2

Plausible mechanism for the preparation of BDTO, route a) in blue arrows, and for the preparation of DPDS, route b) in green arrows.
The potentiostatic electrolysis at 1.55 V resulted in multiple products; however, two were found after column separation in a significant proportion, one being the desired product BDTO (1) (White crystals from hexane/EtOAc m.p. uncorr. 77 °C-78 °C, lit.15, 78 °C-78.5 °C; 1H NMR (see Supplementary Material), agrees with the reported spectra, He et al., 2025; Smith et al., 1989) and a subproduct, the compound DPDS, yielding 30% and 15%, respectively. As in our study, it is proposed that the radical cation intermediate electrochemically produced favors the reaction generally obtained at temperatures greater than 300 °C. The use of HFIP (Ramos-Villaseñor et al., 2020) as cosolvent with ACN (25:75) to generate microdomains that stabilize the cation radical, (Fioroni et al., 2001; Yoshida et al., 2003), just slightly increases the obtained yield to 35%, and the presence of DPDS is still detected. These results imply that these two main reactions compete after forming the radical cation intermediate 5•+ (Scheme 2). Other experimental conditions attempted did not give a better yield of 1.
To increase the yield, other electrolysis modes could be used, for example, rapid alternating polarity, pulsated alternating electrolysis, which have shown the possibility of avoiding by-products and controlling the reactivity pathway of electrogenerated intermediates (Atkins and Lennox, 2024; Rodrigo et al., 2021). The scalability of this reaction can be envisaged using electrochemical flow reactors with the most promising mode of electrolysis; this technique is well known in industrial processes and has been successfully used in the pharmaceutical industry (Gabriele, 2025).
Scheme 2 depicts the two possible routes to the observed products. On the one hand (Route a), the intramolecular cyclization, which follows a second electron transfer and ethylene extrusion to yield BDTO (1), and, on the other (Route b), the elimination of ethylene from intermediate 5•+ and proton transfer to the thiocarbonyl group through a six-member ring intermediate to produce the radical cation 6. Its decomposition generates carbonyl sulfide and a phenylthiyl radical, which produces the obtained DPDS. This last pathway can be seen as a Chugaev-like elimination reaction, but interestingly favored at room temperature. Classical Chugaev reaction generally occurs at 150 °–200 °C (Tschugaeff and Dtsch, 1900). The fact that this reaction operates at 20 °C can be explained by a process activated by the electron transfer, which produces an activated species similar to the Newman-Kwart reaction, electrochemically favored recently described by Francke et al., (Roesel et al., 2020), which also occurs at room temperature. Investigations into this new reaction for constructing the benzo[d]-1,3-dithiolen-2-one (BDTO) are ongoing in our lab to increase yield and clarify the scope, and the results will be reported in due time.
4 Conclusion
The developed approach introduces a new method to directly obtain the BDTO ring from aniline and serves as a formal synthesis of DBTTF. After three steps, BDTO was synthesized with an overall yield of 16%. Electrochemical analysis showed that phenylxanthate is electroactive, displaying irreversible signals during both oxidation (1.5 V) and reduction (−2.4 V). The electrochemical cyclization of O-ethyl-S-phenyldithiocarbonate (5), a key step in this synthesis, produced the BDTO ring with moderate yields of up to 35% (using HFIP/ACN). However, a competing process between electrochemical cyclization and an intramolecular hydrogen transfer, which results in diphenyl disulfide, limited the yield. It is also worth noting that the hazards and drawbacks of using aryldiazonium salts to produce aryl xanthate 5 can now be avoided through an alternative photoactivated route recently published by Wang. (Zhang et al., 2022). The method developed involves a two-step reaction, similar to the diazonium salts method. First, preparation of the aryldibenzothiophenium salt from the corresponding aryl compound using dibenzothiopheneoxide, trifluoroacetic acid, and boron trifluoride etherate. The yields of most compounds reported are good to very good. The second step is the photoactivation of the electron donor-acceptor complex formed by the aryldibenzothiophenium salt and the ethylxanthogenate under 390 nm radiation (purple LED) to produce the aryl xanthate. This method is robust, working with a large variety of functional groups and not requiring the presence of a functional group or protecting groups, as it involves C-H functionalization of the aromatic ring. Following this methodology, the key intermediate, the aryl xanthate 5, is produced in good yield without the explosion risk associated with the work of large amounts of aryl diazonium salts. Thus, the production of BDTO can be achieved in an orthogonal way, because a large group of functional groups is tolerated during the preparation of the heterocyclic ring. This makes the electrochemical method an attractive way to generate DBTO and, subsequently, the DBTTF system.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
ÁT-A: Data curation, Investigation, Methodology, Writing – original draft. FC-A: Data curation, Investigation, Writing – original draft. BL-M: Supervision, Validation, Writing – review and editing. BF-U: Conceptualization, Funding acquisition, Resources, Supervision, Writing – review and editing, Validation.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. BAFU acknowledges the support of CONAHCYT project A1-S-18230 and the project PAPIIT-DGAPA UNAM IV 200222. AVTA thanks CONACHYT for the scholarship received (CVU- 1177986).
Acknowledgments
We thank María Citlalit Martínez Soto, Nievez Zavala, Lizbeth Triana and Alejandra Nuñez for the technical support.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fchem.2025.1666772/full#supplementary-material
References
1
Andrieux A. Schulz H. J. Jerome D. Bechgaard K. (1979). Conductivity of the one-dimensional conductor tetrathiafulvalene-tetracyanoquinodimethane (TTF-TCNQ) near commensurability. Phys. Rev. Lett.43, 227–230. 10.1103/physrevlett.43.227
2
Aromdee C. Cole E. R. Crank G. (1983). Oxidations with lead tetraacetate. IV. Oxidations of 1,3-benzodithioles. Aust. J. Chem.36, 2499. 10.1071/ch9832499
3
Atkins A. P. Lennox A. J. J. (2024). Application of pulsed electrolysis in organic electrosynthesis. Curr. Opin. Electrochem.44, 101441. 10.1016/j.coelec.2024.101441
4
Balandier J. Y. Belyasmine A. Sallé M. (2007). Eur. J. Org. Chem.2008, 269. 10.1002/ejoc.200700705
5
Cembellín S. Batanero B. (2021). Chem. Rec.21, 1. 10.1002/tcr.202100128
6
Cox J. R. Jr Gladys C. L. Field L. Pearson D. E. (1960). General Formation of Aryl Dithiolcarbonates and Ethyl Ethylxanthate in the Leuckart Thiophenol Synthesis1. J. Org. Chem.25, 1083–1092. 10.1021/jo01077a004
7
de Mayo P. Weedon A. C. Wong G. S. (1979). Low-temperature photochemical matrix aposynthesis of an oxathiete and its valence tautomerism with the corresponding.alpha.-ketothione. J. Org. Chem.44, 1977–1981. 10.1021/jo01326a019
8
Fanghänel E. Wegner R. Beye N. Peters K. Müllen K. Prakt J. (1995). Chem.337, 299–306. 10.1002/prac.19953370161
9
Ferraris J. Cowan D. O. Walatka V. Perlstein J. H. (1973). Electron transfer in a new highly conducting donor-acceptor complex. J. Am. Chem. Soc.95, 948–949. 10.1021/ja00784a066
10
Fioroni M. Burger K. Mark A. E. Roccatano D. (2001). Model of 1,1,1,3,3,3-Hexafluoro-propan-2-ol for molecular dynamics simulations. J. Phys. Chem. B105 (44), 10967–10975. 10.1021/jp012476q
11
Firth J. D. Fairlamb I. J. S. (2020). A Need for Caution in the Preparation and Application of Synthetically Versatile Aryl Diazonium Tetrafluoroborate Salts. Org. Lett.22, 7057–7059. 10.1021/acs.orglett.0c02685
12
Frontana-Uribe B. A. Little R. D. Ibanez J. G. Palma A. Vasquez-Medrano R. (2010). Organic electrosynthesis: a promising green methodology in organic chemistry. Green Chem.12, 2099. 10.1039/c0gc00382d
13
Gabriele L. (2025). The role of synthetic organic electrochemistry in the technological revolution of pharmaceutical industry. Chimia79, 417–423. 10.2533/chimia.2025.417
14
Gao X. Wu W. Liu Y. Qiu W. Sun X. Yu G. et al (2006). A facile synthesis of linear benzene-fused bis(tetrathiafulvalene) compounds and their application for organic field-effect transistors. Chem. Commun.26, 2750–2752. 10.1039/b603632e
15
Gao X. Wang Y. Yang X. Liu Y. Qiu W. Wu W. et al (2007). Dibenzotetrathiafulvalene bisimides: new building blocks for organic electronic materials**. Adv. Mater.19 (19), 3037–3042. 10.1002/adma.200700007
16
He B.-Q. Zhao L. Zhang J. Bao W.-H. Yang M. Wu X. (2025). Alcohol activation by benzodithiolylium for deoxygenative alkylation driven by photocatalytic energy transfer. Angew. chem. int. Ed. 64, e202423795. 10.1002/anie.202423795
17
Inayoshi T. Ono I. (2000). Syntheses, properties and crystal structures of new symmetrical DBTTF derivatives: TMO-DBTTF and BMDO-DBTTF. Synth. Met.110, 153–163. 10.1016/s0379-6779(99)00293-3
18
Inayoshi T. Sanada M. Matsumoto S. (2016). DBTTF derivative with bis(propylenedioxy) (BPDO) groups: synthesis, electrochemical properties, conductivity, and crystal and band structures of salts. Synth. Met.220, 174–186. 10.1016/j.synthmet.2016.06.005
19
Ito O. Matsuda M. (1982). J. Am. Chem. Soc.104, 568. 10.1021/ja00366a033
20
Ito O. Matsuda M. (1984). Solvent effect on rates of free-radical reactions. 2. Addition of the p-(dimethylamino)benzenethiyl radical to.alpha.-methylstyrene. J. Phys. Chem.88, 1002–1005. 10.1021/j150649a031
21
Jana A. Ishida M. Park J. S. Bähring S. Jeppesen J. O. Sessler J. L. (2017). Tetrathiafulvalene- (TTF-) derived oligopyrrolic macrocycles. Chem. Rev.117, 2641–2710. 10.1021/acs.chemrev.6b00375
22
Leuckart R. Prakt J. (1889). Chem.41, 179.
23
Loosli C. Jia C. Liu S. X. Haas M. Dias M. Levillain E. et al (2005). Synthesis and electrochemical and photophysical studies of tetrathiafulvalene-annulated phthalocyanines. J. Org. Chem.70, 4988–4992. 10.1021/jo0501801
24
Melby L. R. Hartzler H. D. Sheppard W. A. (1974). Improved synthesis of tetrathiafulvalene. J. Org. Chem.39, 2456–2458. 10.1021/jo00930a043
25
Metz W. D. (1973). Organic crystals: hints of extraordinary conductivity. Sci.180, 1041–1042. 10.1126/science.180.4090.1041
26
Nakayama J. Ishihara M. Hoshino M. (1977). Chem. Lett.1, 77. 10.1246/cl.1977.77
27
Pollok D. Waldvogel S. R. (2020). Electro-organic synthesis – a 21stcentury technique. Chem. Sci.11, 12386–12400. 10.1039/d0sc01848a
28
Ramos-Villaseñor J. M. Rodríguez-Cárdenas E. Díaz C. E. B. Frontana-Uribe B. A. (2020). Review—use of 1,1,1,3,3,3–hexafluoro–2–propanol (HFIP) Co-Solvent mixtures in organic electrosynthesis. J. Electrochem. Soc.167, 155509. 10.1149/1945-7111/abb83c
29
Rodrigo S. Gunasekera D. Mahajan J. P. Luo L. (2021). Alternating current electrolysis for organic synthesis. Curr. Opin. Electrochem.28, 100712. 10.1016/j.coelec.2021.100712
30
Roesel A. F. Ugandi M. Huyen N. T. T. Májek M. Broese T. Roemelt M. et al (2020). Electrochemically catalyzed newman–kwart rearrangement: mechanism, structure–reactivity relationship, and parallels to photoredox catalysis. J. Org. Chem.85, 8029–8044. 10.1021/acs.joc.0c00831
31
Senga T. Kamoshida K. Kushch L. A. Saito G. Inayoshi T. Ono I. (1997). Molecular crystals and liquid crystals science and technology section A: molecular crystals and. Liq. Cryst.296, 97–143. 10.1080/10587259708032316
32
Smith K. Lindsay C. M. Pritchard G. J. (1989). Directed lithiation of arenethiols. J. Am. Chem. Soc.111, 665–669. 10.1021/ja00184a040
33
Sun D. Krawiec M. Watson W. H. (1997). Benzoquinone derived 1,3-dithiole-2-ones and thiones. J. Chem. Crystallogr.27, 515–526. 10.1007/bf02576442
34
Takamasa K. Takashi M. (2002). N. Toshio JP pat, 2002226463A.
35
Tschugaeff L. Dtsch B. (1900). Chem. Ges.33, 3118.
36
Yoneda S. Kawase T. Yasuda Y. Yoshida Z. (1979). New syntheses of tetrathiafulvalene. J. Org. Chem.44, 1728–1729. 10.1021/jo01324a038
37
Yoshida K. Yamaguchi T. Adachi T. Otomo T. Matsuo D. Takamuku T. et al (2003). Structure and dynamics of hexafluoroisopropanol-water mixtures by x-ray diffraction, small-angle neutron scattering, NMR spectroscopy, and mass spectrometry. J. Chem. Phys.119 (12), 6132–6142. 10.1063/1.1602070
38
Zhang M. Wang B. Cao Y. Liu Y. Wang Z. Wang Q. (2022). Visible-light-driven synthesis of aryl xanthates and aryl dithiocarbamates via an electron donor–acceptor complex. Org. Lett.24, 8895–8900. 10.1021/acs.orglett.2c03736
Summary
Keywords
DBTTF, benzo[d]-1,3-dithiole-2-ones (BDTO), electrosynthesis, electrochemical cyclization, O-ethyl-S-phenyldithiocarbonate
Citation
Terán-Alcocer ÁV, Cifuentes-Ajuchan FMJ, López-Mayorga BJ and Frontana-Uribe BA (2025) Formal synthesis of dibenzotetrathiafulvalene (DBTTF), through practical electrochemical preparation of benzo[d]-1,3-dithiole-2-one (BDTO). Front. Chem. 13:1666772. doi: 10.3389/fchem.2025.1666772
Received
15 July 2025
Accepted
18 August 2025
Published
04 September 2025
Volume
13 - 2025
Edited by
Yan He, Henan Normal University, China
Reviewed by
Sagnik Sengupta, University of Texas Southwestern Medical Center, United States
N. Vijaya Ganesh, LGC Standards, United States
Updates
Copyright
© 2025 Terán-Alcocer, Cifuentes-Ajuchan, López-Mayorga and Frontana-Uribe.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bernardo A. Frontana-Uribe, bafrontu@unam.mx
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.