- 1Department of Mathematics, School of Advanced Sciences, Vellore Institute of Technology, Vellore, India
- 2Department of Science and Humanities, KGiSL Institute of Technology, Coimbatore, India
Fractal molecular structures constitute a distinct category of systems characterized by self-similarity and hierarchical growth patterns, which can be effectively examined by graph-theoretical methods. This manuscript introduces a refined adaptation of Shannon’s entropy calculation method by evaluating the degree-based topological indices for fractal molecular structures, specifically Kekulene
1 Introduction
In chemical graph theory, a molecular graph is a representation of a molecule, where the edges signify chemical relationships between atoms and the vertices stand for atoms. Through the transformation of molecular structures into mathematical objects, this method makes the application of graph-theoretical concepts and methods easier. Topological indices are numerical values that represent different structural characteristics of the molecule and are derived from the molecular graph. Indices such as the Zagreb, Wiener, and Randić index provide information on reactivity, molecular stability, and other aspects of chemistry. Topological indices play a crucial role in cheminformatics by providing information on molecular size, branching, and connectivity. This information is useful in predicting biological activity, chemical behavior, and the design of novel compounds. In chemistry, fractal structures are useful because they facilitate the description and examination of intricate patterns and behaviors at different scales. Fractals have a wide range of applications, one of which is surface characterisation. Here, they are utilized to simulate surface imperfections and roughness at the atomic and molecular levels, which is essential for comprehending catalyst properties and reactions. Fractal analysis is useful in characterizing pore structures and their effects on adsorption, diffusion, and reactivity in porous materials, such as silica gels and zeolites. Additionally, fractals are essential to understanding reaction kinetics because they shed light on the uneven spatial distribution of reactive sites in heterogeneous catalysts, which affects reaction speeds and processes. Fractal principles are used in polymer chemistry to explain the features of fractal-like aggregates, network development, and the scaling behavior of polymer chains. Additionally, fractal models are employed to study diffusion processes in complex media, offering a more accurate depiction of how substances move through irregular structures. Overall, fractal structures offer a robust framework for exploring the complexities of various chemical systems (Weib et al., 2019). Computational studies on fractal chemical structures involve using computer-based methods to understand their structure, properties, interactions, and biological activities. Here are some possible computational studies and analysis on fractal based molecular structures that could be carried out to utilize the numerical invariants:
• Molecular Docking: Computational docking studies are commonly used to understand how fractal chemical architectures interact with biological targets such as enzymes, receptors, or proteins. By simulating the binding of fractal molecular structures to these targets, researchers can predict their potential biological activities and mechanisms of action.
• Quantum Chemical Calculations: Quantum chemical methods like density functional theory (DFT) or semi-empirical calculations are used to study the electronic structure, energy levels, and reactivity of fractal chemical architectures. These calculations provide insights into their stability, reactivity towards free radicals, and other chemical properties.
• Molecular Dynamics Simulations: Molecular dynamics (MD) simulations are used to study the dynamic behavior of fractal chemical architectures and their interactions with other chemical systems over time. These simulations can reveal information about the flexibility, conformational changes, and stability of fractal chemical complexes.
• Pharmacophore Modeling: Pharmacophore modeling involves identifying the essential structural features (pharmacophores) of certain fractal chemical architectures that are responsible for their reactivity. Computational methods can help in constructing pharmacophore models based on known bioactive fractal molecular structures and using them to design new compounds with desired properties.
• Virtual Screening: Virtual screening is a computational technique used to screen large libraries of compounds, including some chemical architectures, against specific biological targets. By using molecular docking or other methods, researchers can identify potential fractal chemical compounds with high binding affinity and selectivity for the target of interest.
• Network Pharmacology: Computational approaches such as network pharmacology can be applied to study the complex interactions between fractal structures, biological targets, and biological pathways. These studies can help in understanding the holistic effects of fractal molecular structures on biological systems and identifying synergistic interactions with other compounds.
• Quantitative Structure-Activity Relationship Studies: QSAR studies involve correlating the chemical structure of fractal chemical compounds with their biological activities using statistical and computational methods. These studies are useful for predicting the bioactivity of novel chemical derivatives and optimizing their structures for enhanced potency.
Studies on fractal supramolecular systems reveal that coordination-driven self-assembly is a key approach for designing supramolecular architectures, facilitating the evolution from simple two-dimensional macrocycles to intricate three-dimensional cage-like frameworks (Chichak et al., 2004; Hasenknopf et al., 1996; Olenyuk et al., 1999). Supramolecular chemistry, often characterized as “chemistry beyond the molecule,” is principally concerned with molecular recognition processes and the generation of higher-order assemblies mediated by non-covalent interactions. The reliance on such interactions allows supramolecular systems to function as modular entities, wherein discrete molecular units associate through well-defined binding motifs (Huang and Anslyn, 2015; Renai et al., 2025). These interactions are inherently multifaceted, encompassing both attractive and repulsive forces, most notably hydrogen bonding and metal–ligand coordination, which collectively underpin the stability and diversity of supramolecular assemblies (Roy et al., 2014).
According to a recent study the induced heat conduction within the polymers due to designing intermolecular interactions has been shown to be effective (Kim et al., 2015; Mehra et al., 2018a; Mehra et al., 2018b). Fine-tuning various non-covalent interactions in supramolecular structures could be another method for generating the best dissipating materials. In the development of supramolecular analysis, many hydrogen bonds are typically identified, which can operate as thermal highways for phonon transport. The capacity of supramolecular assembles to generate stable crystal structures is another important feature that qualifies them for thermal conduction. The thermal conduction properties of polymeric materials are strongly dependent on the morphology and dimensions of their crystalline domains (Mu et al., 2016). In supramolecular systems, the assembly of stable aggregates is frequently governed by non-covalent interactions involving complementary molecular motifs (Seto and Whitesides, 1990). Among various architectures, terpyridine-metal complex nanosheets in combination with Sierpinski triangle-type systems represent a particularly well-studied and versatile class of supramolecular assemblies.
The fractal geometric structures of terpyridine and kekulene molecules, such as the terpyridine complex Sierpinski triangle fractal and the kekulene fractal, have been examined in this study. Fractal structures are essentially complex geometric forms that exhibit self-similarity, or patterns that repeat at different scales. These forms are recognized for their intricate, frequently indefinitely detailed patterns that can be found in both mathematical constructions and natural environments. Fractals differ from conventional geometric shapes having whole number dimensions in that they have fractional or non-integer dimensions. Because of this property, fractals can effectively simulate and depict the asymmetrical, fragmented forms that can be found in the natural world, such as mountain ranges, coastlines, cloud formations, and tree branches. Iterative procedures are used to create fractals, where simple rules are repeatedly applied to create the overall complex structure. This iterative nature results in infinite detail, with each smaller part mirroring the larger structure. Fractals are useful in many branches of science and the arts because of their recursive and self-similar characteristics. They are employed in computer graphics to produce lifelike textures and sceneries. They aid in the understanding of complicated, dynamic processes in the study of chaotic systems. Therefore, fractal structures offer deep insights into the patterns and complexities of the world around us and constitute an intriguing intersection of mathematics, nature, and art. In order to improve our comprehension of chemical systems, mathematical chemistry is an interdisciplinary field that integrates chemical study and mathematical ideas and methods. This area of study models molecular structures, investigates reaction mechanisms, and predicts the features of novel compounds using a range of mathematical techniques, such as graph theory, linear algebra, and computational algorithms. Topological indices, commonly referred to as molecular descriptors, provide evidence linking important physicochemical and biological processes to molecular structure. These descriptors are a component of a theoretical instrument that characterizes the molecular structures. Topological indices represent the underlying connections in molecular networks as structure-based invariants associated with molecular graphs. In QSAR and QSPR investigations, topological indices are frequently employed as an effective tool. Their application in these areas has led to increased attention and recognition in recent years. In this work we have computed the general mathematical expression using various degree-based topological indices for the kekulene fractal and terpyridine complex Sierpinski triangle fractal. Also, along with that we have calculated the information entropy, namely Shannon entropy for these two fractal geometry structures (Ma et al., 2006; Ma et al., 2007; Ida et al., 2008; Yan et al., 2009; Gao and Yan, 2018; Bauer et al., 2011; Payamyar et al., 2014).
2 Materials and methods
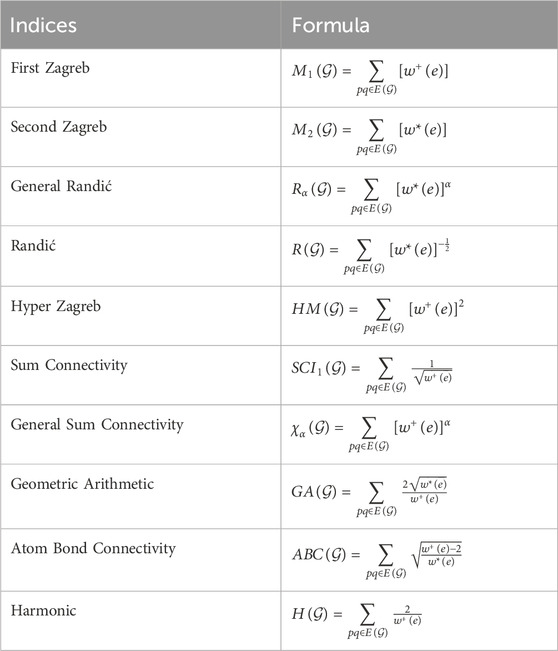
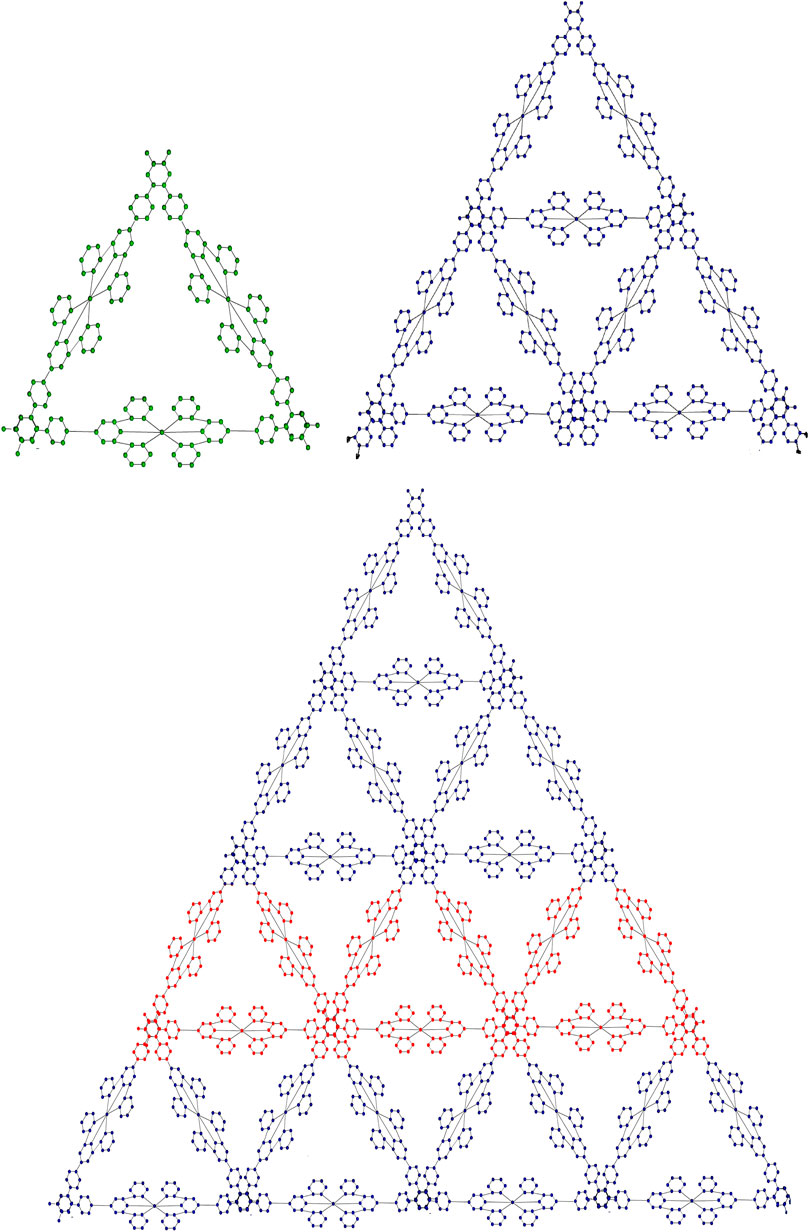
2.1 Sierpinski triangle system
Honoring Polish mathematician Waclaw Sierpinski, the Sierpinski triangle, also referred to as the Sierpinski gasket is a fractal structure. An equilateral triangle is created by recursively splitting it into smaller equilateral triangles, taking off the center triangle at each stage. This results in the formation of a self-similar pattern that is structurally stable at all magnification levels. The Sierpinski triangle fractal illustrates infinite complexity in a finite space and serves as an excellent example of recursion theory. Its uses in a variety of domains, including as computer graphics, antenna design, and the investigation of natural phenomena like snowflakes and crystal formation, demonstrate the fusion of mathematics and environment. Each fractal molecule can be regarded as a crystalline complex consisting of a finite set of atoms (vertices) and hydrogen bonds (edges), arising from the coordination of terpyridine (TPY) ligands with metal centers such as Fe or Co in a threefold arrangement. From a graph-theoretical perspective, atoms correspond to vertices while hydrogen bonds are represented as edges. The resulting fractal molecular complexes incorporate regular cyclic substructures of length six
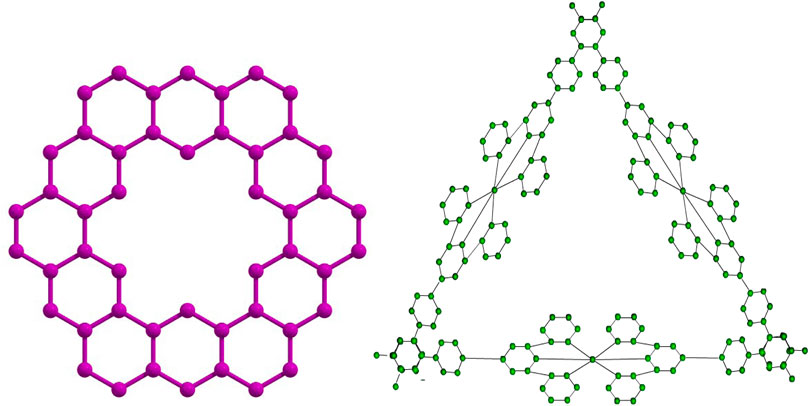
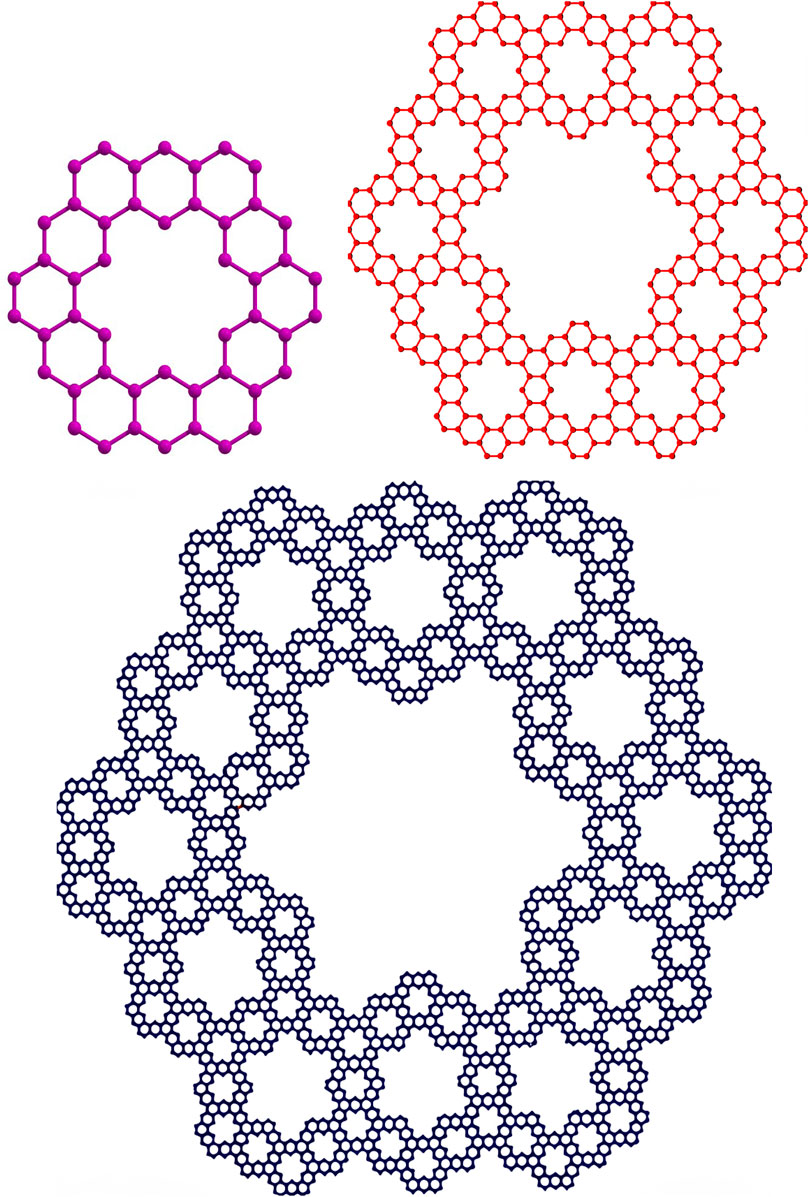
2.2 Kekulene ring
Kekulene is an intriguing polycyclic aromatic hydrocarbon that is distinguished by its distinct and extremely symmetrical structure, which is made up of twelve benzene rings united in a circular pattern. Accordingly, it is categorized as a circulene having the molecular formula
2.3 Terpyridine system
G. Morgan and F. H. Burstall originally synthesized terpyridine, a heterocyclic molecule generated from pyridine, in 1932 by oxidatively combining pyridines. Terpyridine (2,2′; 6′,2″-terpyridine, often abbreviated as Terpy or Tpy) is referred to be a tridentate ligand because it binds at three meridional locations to produce two neighboring 5-membered
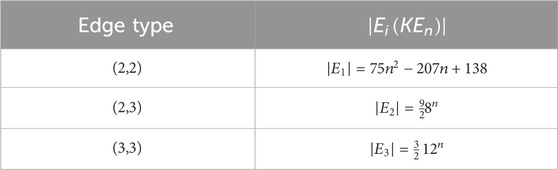
2.4 Degree-based topological indices
In the context of QSPR analysis, the application of complex chemical compounds has been accompanied by a notable growth in the development and utilization of topological indices, which serve as effective tools for correlating and classifying the physicochemical properties of molecules. Among their various applications, QSPR and QSAR studies are the most prominent (Gute et al., 1999; Hayat et al., 2019; Renai and Roy, 2023; Viswanadhan et al., 2001). Topological indices, being structure-based invariants of molecular graphs, capture the intrinsic connectivity patterns of molecular networks and, as such, have attracted considerable attention in recent years for their utility in QSPR models (Balasubramanian, 2022; Balasubramanian et el., 2018; Balasubramanian, 2021; Jyothish and Roy, 2024; Jyothish and Roy, 2025; Kalaam and Greeni, 2025). When combined with entropy-based descriptors, these indices provide a powerful framework for QSPR and QSAR analyses. Notably, in several classes of natural chemical systems, entropy-related quantities exhibit direct correlations with fullerene properties (Gutman and Tosovic, 2013). Based by these insights, we extend our study to the computation of entropy measures for fractal molecular complexes to explore their potential applications.
To establish a mathematical framework, we introduce certain notations that will be employed throughout the paper. Let
1.
2.
2.5 Entropy
According to thermodynamics the term entropy represent a disorder of a system considered. More precisely, depending on the rise in temperature of a matter leads to the deformation of its present state to another state. The highest entropy value we obtained the more energy loss could be predicted. Claude Shannon created Shannon’s information entropy in 1948 to quantify the uncertainty or unpredictability in a piece of data. It measures the typical amount of data produced by a data source. Shannon’s entropy determines the expected information value for each possible outcome for a random variable based on its probability.
The entropy formula produces a value that is often expressed in bits by adding the products of the probability of each result and its logarithm. Since it establishes the bounds of data compression and the effectiveness of communication networks, this idea is crucial to information theory. Shannon’s information entropy is important in information theory and other areas because of a number of important features. Firstly, since entropy is always positive, there can never be less than zero uncertainty or information content. Second, the highest amount of uncertainty is shown by a uniform distribution, where all outcomes have an equal probability of occurring. This is where entropy reaches its maximum. Thirdly, since entropy is cumulative for independent events, the combined entropy of independent events is equal to the sum of the entropies of each individual event. Because of these characteristics, Shannon’s entropy is an important metric in many fields. Its significance arises from its capacity to establish boundaries for data compressibility and communication system efficiency, acting as a standard for lossless data compression. Entropy is also useful for assessing the information content and unpredictability of cryptographic systems, which helps to guarantee data security. Shannon’s entropy is crucial for data transmission, storage, and many other applications that call for effective information processing and management because it offer a mathematical foundation for comprehending information and uncertainty. Shannon’s entropy is an important tool in data transmission, cryptography, chemical graph theory, and other domains where an understanding of information amount is essential. It does this by evaluating the unpredictability or information content in a dataset. In this study, Shannon’s probabilistic function is employed to calculate entropy measures derived from topological indices across different values of
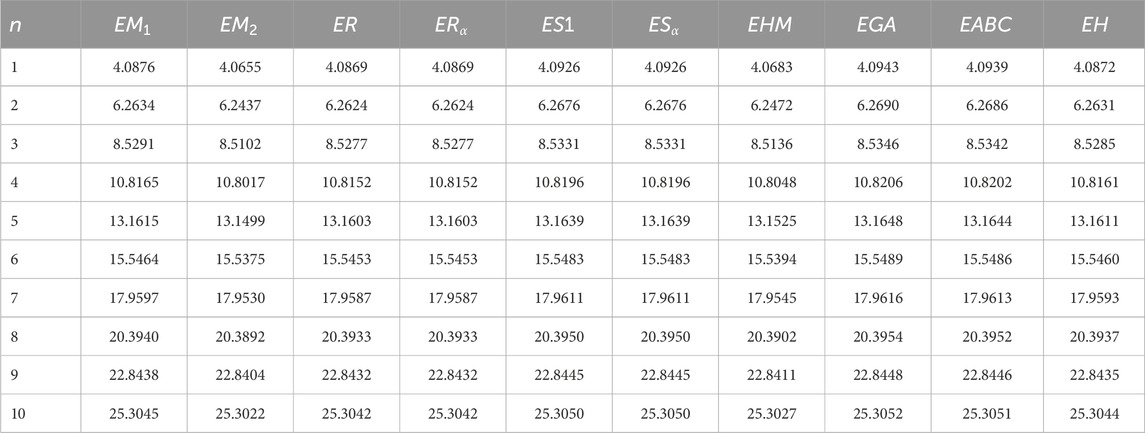
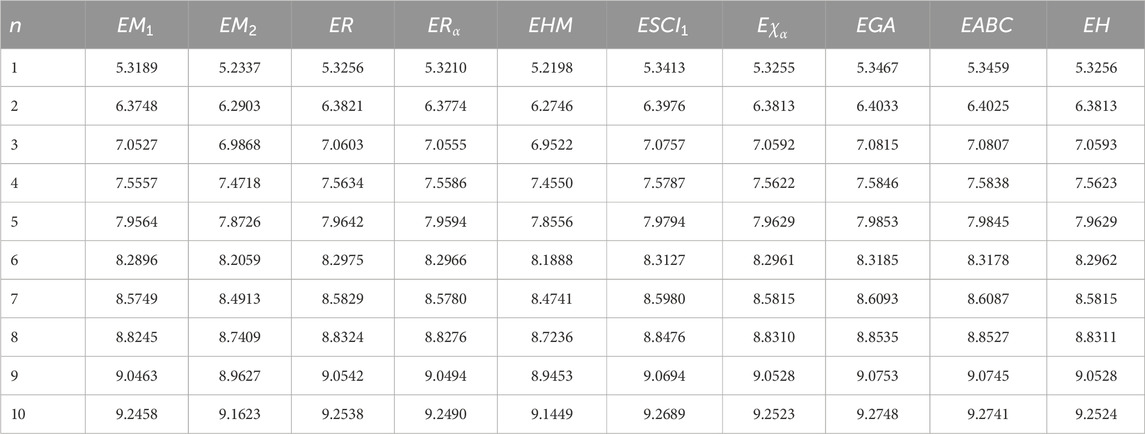
The descriptors summarized in Table 1 find wide-ranging uses in thermodynamics, chemistry, QSPR/QSAR analyses, as well as related scientific domains. For instance, the atom–bond connectivity index has been shown to exhibit strong correlations (Gutman and Tosovic, 2013). To further capture the structural complexity of molecular graphs, the concept of graph entropy was introduced (Mowshowitz and Dehmer, 2012). Although originally formulated to address challenges in communication and information transmission, graph entropy has since found significant applications in diverse fields, including engineering systems, biological networks, and physical dissipative structures (Sabirov and Shepelevich, 2021). Graph entropies are broadly categorized into two classes: probabilistic and deterministic. Intrinsic measures partition the graph into substructures with identical topological characteristics and then define a probability distribution over these components. Extrinsic measures, by contrast, assign probability distributions directly to graph elements, such as vertices or edges. By applying an entropy function to these distributions, one obtains quantitative values that reflect graph complexity in probabilistic terms (Mowshowitz and Dehmer, 2012).
Among the various formulations of probabilistic entropy, Shannon’s method remains the most widely applied. In this approach, probability operators are assigned to each information unit, denoted as
When adapted to chemical graphs, this formulation is modified to characterize molecular topology. Specifically, the edges of the chemical graph are regarded as the fundamental elements, and probability values are assigned to them through appropriate topological descriptors. Accordingly, the entropy associated with a given topological index D, is expressed as follows (Kazemi, 2016; Mowshowitz and Dehmer, 2012; Sabirov and Shepelevich, 2021).
All calculations of topological indices and entropy values were performed using custom-developed codes in MATLAB software. The molecular graphs of Kekulene and terpyridine-based Sierpinski triangle systems were represented through their adjacency matrices, and degree distributions were derived accordingly. Edge partitions were systematically obtained and used as inputs for computing degree-based topological indices such as Zagreb, Randic, Hyper-Zagreb, and ABC indices. Shannon entropy values were then calculated numerically by applying the defined probability functions to these descriptors.
3 Results
3.1 Computation of degree based topological indices of fractal molecular structures
• Based on the expressions and edge partitions provided in Tables 1–3, the topological indices of the fractal molecular compounds are computed and tabulated. Then the corresponding entropy measures are further computed and illustrated through diagrams to analyze and compare the trends associated with different topological descriptors.
• Graphical representations of the two fractal molecular compounds under consideration are depicted in Figures 2, 3.
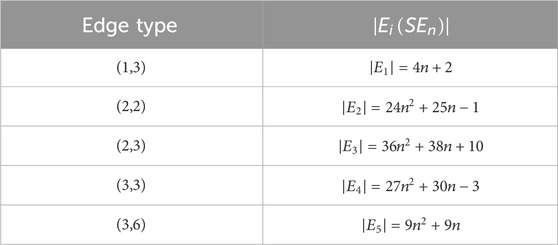
• The edge partitions of
Theorem 3.1. Let
Proof. The edges of molecular graph are partitioned according to the degrees of their terminal vertices. Using the edge partitions listed in Table 2 together with the index formulations provided in Table 1, the corresponding topological indices are computed.
Theorem 3.2. Let
Proof. The edges are partitioned according to the degrees of their incident vertices. Utilizing the edge partitions provided in Table 3, together with the index equations summarized in Table 1, the corresponding topological indices are calculated.
4 Discussion
4.1 Computational strategies for computing topological indices based entropy measures and numerical values
• Based on the computed degree-based topological descriptors, the entropy values are obtained using Shannon’s method by defining an appropriate probability function.
• Shannon’s entropy equation is applied to evaluate these measures, with the procedure illustrated through the calculation of entropy values for the fractal structures using the Hyper Zagreb index.
• Let
Through the substitution of the edge partitions provided in Table 2 into the respective formulations, we obtain the following,
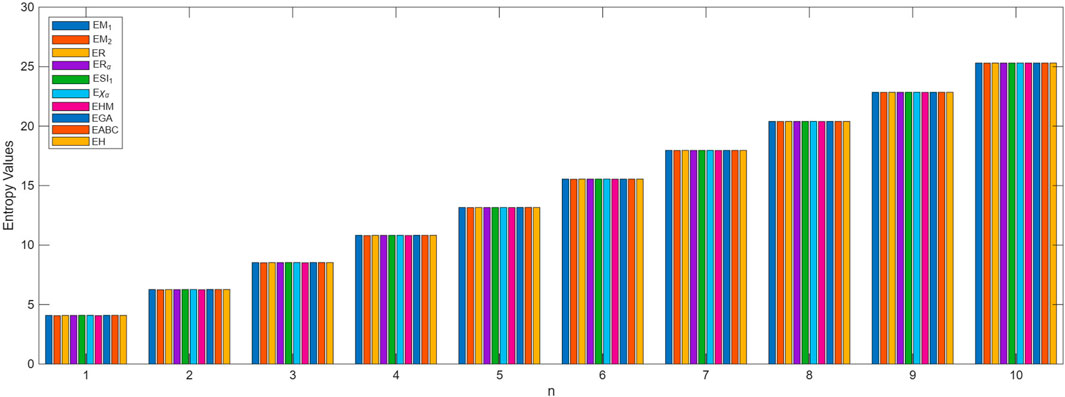
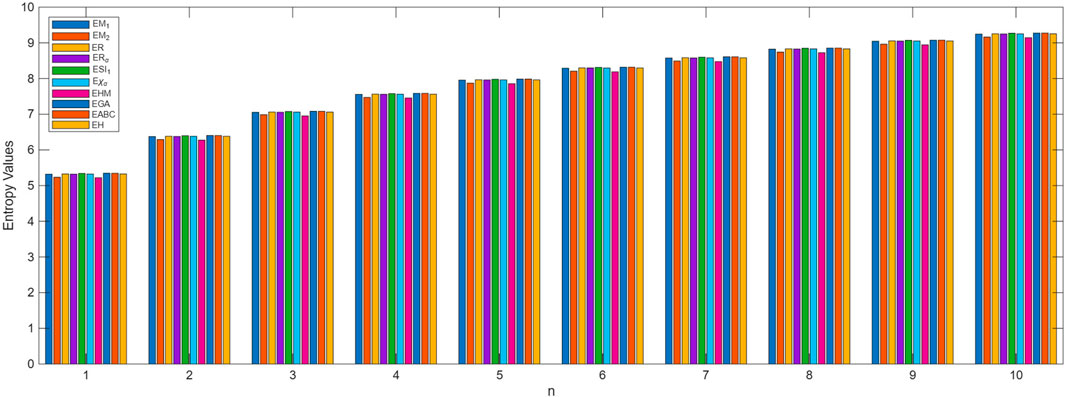
The process of deriving a comprehensive entropy expression using Shannon’s approach would require a substantial explanation if formulated as theorems. However, individuals can effectively apply the outlined strategy for entropy computation across various topological indices for chemical structures. The subsequent discussion delves into numerical entropy values (refer Tables 4, 5), and graphical representations (see Figures 4, 5) of both fractal compound structures, as detailed.
This study demonstrates that entropy, based on degree-based topological descriptors, acts as a sensitive measure of structural complexity in fractal molecular systems. This method, compared to traditional entropy estimates reliant on electron density distributions, just rely on the fundamental graph structure, making it computationally efficient and broadly accessible. The consistent increase in entropy observed in successive generations of fractal designs emphasizes the hierarchical evolution and enhanced connectedness inherent to these systems. These developments are particularly relevant for QSPR and QSAR research, where entropy functions as an interpretable descriptor connecting molecule structure to physical properties.
5 Conclusion
This study investigated the utilization of Shannon entropy in fractal chemical structures by the application of vertex degree-based topological descriptors. The proposed framework combines graph-theoretical invariants with information-theoretic measures, providing a new approach to defining the structural complexity of self-similar molecular architectures. The entropy patterns seen over many fractal generations indicate that structural development is associated with significant alterations in information content, thereby establishing a quantifiable connection between topology and complexity. This entropy-based approach is not merely of theoretical significance; it provides a basis for predictive modeling in QSPR/QSAR research. This technique may be beneficial in supramolecular chemistry, and the creation of sophisticated functional materials that often exhibit fractal-like structures. The current study focuses on degree-based descriptors; however, the methodology can be extended to include additional structural parameters and different entropy measures, thereby facilitating future research on the relationship between molecular topology, information theory, and physicochemical properties.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
SR: Supervision, Writing – original draft, Writing – review and editing, Validation. PN: Software, Conceptualization, Investigation, Writing – review and editing, Validation, Formal Analysis, Writing – original draft, Visualization, Methodology. KJ: Writing – original draft, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The authors wish to express their sincere gratitude to Vellore Institute of Technology, Vellore, for providing financial support.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Balasubramanian, K. (2018). Mathematical and computational techniques for drug discovery: promises and developments. Curr. Top. Med. Chem. 18 (32), 2774–2799. doi:10.2174/1568026619666190208164005
Balasubramanian, K. (2021). Combinatorics, big data, neural network and AI for medicinal chemistry and drug administration. Lett. Drug. Des. Discov. 18 (10), 943–948. doi:10.2174/1570180818666210719130052
Balasubramanian, K. (2022). Computational and artificial intelligence techniques for drug discovery and administration. Compr. Ph., 553–616. doi:10.1016/B978-0-12-820472-6.00015-3
Bauer, T., Zheng, Z., Renn, A., Enning, R., Stemmer, A., Sakamoto, J., et al. (2011). Synthesis of free-standing, monolayered organometallic sheets at the air/water interface. Angew. Chem. Int. Ed. Engl. 50 (34), 7879–7884. doi:10.1002/anie.201100669
Chichak, K. S., Cantrill, S. J., Pease, A. R., Chiu, S. H., Cave, G. W. V., Atwood, J. L., et al. (2004). Molecular borromean rings. Science. 304, 1308–1312. doi:10.1126/science.1096914
Dlubak, B., Marti, M. B, Deranlot, C., Servet, B., Xavier, S., Mattana, R., et al. (2012). Highly efficient spin transport in epitaxial graphene on SiC. Nature Phys. 8, 557–561. doi:10.1038/nphys2331
Gao, R., and Yan, D. (2018). Fast formation of single-unit-cell-thick and defect-rich layered double hydroxide nanosheets with highly enhanced oxygen evolution reaction for water splitting. Nano. Res. 11, 1883–1894. doi:10.1007/s12274-017-1806-x
Gute, B., Grunwald, G., and Basak, S. C. (1999). Prediction of the deral penetration of polycyclic aromatic hydrocarbons (PAHs): a hierarchical qsar approach. Sar. QSAR Environ. Res. 10, 1–15. doi:10.1080/10629369908039162
Gutman, I., and Tosovic, J. (2013). Testing the quality of molecular structure descriptors: vertex-degree-based topological indices. J. Serbian Chem. Soc. 78 (6), 805–810. doi:10.2298/jsc121002134g
Hasenknopf, B., Lehn, J. M., Kneisel, B. O., Baum, G., and Fenske, D. (1996). Self assembly of a circular double helicate. Angew. Chem. Int. Ed. 35, 1838–1840. doi:10.1002/anie.199618381
Hayat, S., Imran, M., and Liu, J. B. (2019). Correlation between the estrada index and π-electronic energies for benzenoid hydrocarbons with applications to boron Nanotubes. Int. J. Quantum Chem. 23 119, e26016. doi:10.1002/qua.26016
Huang, F., and Anslyn, E. V. (2015). Introduction: supramolecular chemistry. Chem. Rev. 115, 6999–7000. doi:10.1021/acs.chemrev.5b00352
Ida, S., Shiga, D., Koinuma, M., and Matsumoto, Y. (2008). Synthesis of hexagonal nickel hydroxide nanosheets by exfoliation of layered nickel hydroxide intercalated with dodecyl sulfate ions. J. Am. Chem. Soc. 130, 14038–14039. doi:10.1021/ja804397n
Jyothish, K., and Roy, S. (2024). Topological characterization of [n]-triangulenes through degree-based molecular descriptors with the prediction to π-electron energy. Phys. Scr. 100 (1), 015266. doi:10.1088/1402-4896/ad9cd1
Jyothish, K., and Roy, S. (2025). Molecular characterization of coronene fractals with predictive analysis of spectral and energetic properties through entropy measures. Chem. Eng. Sci., 122266. doi:10.1016/j.ces.2025.122266
Kalaam, A. R. A., and Greeni, A. B. (2025). Two-dimensional coronene fractals: modified reverse degree indices, comparative analysis of information entropy and predictive modeling of spectral properties. Front. Chem. 13, 1588942. doi:10.3389/fchem.2025.1588942
Kazemi, R. (2016). Entropy of weighted graphs with the degree-based topological indices as weights. MATCH Commun. Math. Comput. Chem. 76, 69–80.
Kim, G. H., Lee, D., Shanker, A., Shao, L., Kwon, M. S., Gidley, D., et al. (2015). High thermal conductivity in amorphous polymer blends by engineered interchain interactions. Nat. Mater. 14, 295–300. doi:10.1038/nmat4141
Ma, R. L., Li, L., Iyi, N., and Sasaki, T. (2006). Exfoliating layered double hydroxides in formamide: a method to obtain positively charged nanosheets. J. Mater. Chem. 16, 3809. doi:10.1039/b605422f
Ma, R., Liu, Z., Takada, K., Iyi, N., Bando, Y., Sasaki, T., et al. (2007). Synthesis and exfoliation of Co2+-Fe3+ layered double hydroxides: an innovative topochemical approach. J. Am. Chem. Soc. 129 (16), 5257–5263. doi:10.1021/ja0693035
Mehra, N., Mu, L., Ji, T., Yang, X., Kong, J., Gu, J., et al. (2018a). Thermal transport in polymeric materials and across composite interfaces. Appl. Mater. Today 12, 92–130. doi:10.1016/j.apmt.2018.04.004
Mehra, N., Kashfipour, M. A., and Zhu, J. F. (2018b). Filler free technology for enhanced thermally conductive optically transparent polymeric materials using low thermally conductive organic linkers. Mater. Today 13, 207–216. doi:10.1016/j.apmt.2018.09.007
Mowshowitz, A., and Dehmer, M. (2012). Entropy and the complexity of graphs revisited. Entropy 14 (3), 559–570. doi:10.3390/e14030559
Mu, L., Ji, T., Chen, L., Mehra, N., Shi, Y., and Zhu, J. (2016). Paving the thermal highway with self-organized nanocrystals in transparent polymer composites. ACS Appl. Mater. Interfaces 8, 29080–29087. doi:10.1021/acsami.6b10451
Noah, P., and Roy, S. (2022). On degree based topological indices of self-assembled tetraphenylethylene and terpyridine rosettes. Int. J. Quantum Chem. 122, 20. doi:10.1002/qua.26973
Novoselov, K. S., Geim, A. K., Morozov, S. V., Jiang, D., Zhang, Y., Dubonos, S. V., et al. (2004). Electric field effect in atomically thin carbon films. Science 306 (5696), 666–669. doi:10.1126/science.1102896
Olenyuk, B., Whiteford, J. A., and Stang, A. P. J. (1999). Self assembly of nanoscale cuboctahedra by coordination chemistry. Nature 398, 1999 796–799. doi:10.1038/19740
Payamyar, P., Kaja, K., Ruiz-Vargas, C., Stemmer, A., Murray, D. J., Johnson, C. J., et al. (2014). Synthesis of a covalent monolayer sheet by photochemical anthracene dimerization at the air/water interface and its mechanical characterization by AFM indentation. Adv. Mater. 26 (13), 2052–2058. doi:10.1002/adma.201304705
Renai, P. N. A. D., and Roy, S. (2023). On computation of degree based entropy measures for terpyridine complex nanosheet. Int. J. Quantum Chem. 123, e27190. doi:10.1002/qua.27190
Renai, P. N. A. D., Roy, S., and Husin, M. N. (2025). On shannon's strategy of computing degree based entropy measures for melamine cyanuric acid molecular structure. Malays. J. Math ematical Sci. 19 (1), 241–267. doi:10.47836/mjms.19.1.13
Roy, B., Bairi, P., and Nandi, A. K. (2014). Supramolecular assembly of melamine and its derivatives: nanostructures to functional materials. RCS Adv. 4, 2014 1708–1734. doi:10.1039/c3ra44524k
Sabirov, D. S., and Shepelevich, I. S. (2021). Information entropy in chemistry: an overview. Entropy 23 (10), 1240. doi:10.3390/e23101240
Sakamoto, R., Yi, S., and Nisihihara, H. (2015). An electrochromic bis(terpyridine)metal complex nanosheet. J. Am. Chem. Soc. 137, 14. doi:10.1021/ja510788b
Seto, C. T., and Whitesides, G. M. (1990). Self-assembly based on the cyanuric acid-melamine lattice. J. Am. Chem. Soc. 12, 6409–6411. doi:10.1021/ja00173a046
Viswanadhan, V. N., Mueller, G. A., Basak, S. C., and Weinstein, J. N. (2001). Comparison of a neural net-based QSAR algorithm (PCANN) with hologram-and multiple linear regression-based QSAR approaches: application to 1,4Dihydropyridine-based calcium channel antagonists. J. Chem. Inf. Comput. Sci. 41, 505–511. doi:10.1021/ci000072+
Weib, C., Heb, Y., Shia, X., and Songa, Z. (2019). Terpyridine-metal complexes; Applications in catalysis and supramolecular chemistry. Coord. Chem. Rev. 15, 385 1–19. doi:10.1016/j.ccr.2019.01.005
Yan, D. L., Han, M., Ma, J., Li, J., Evans, D. G., et al. (2009). Ordered poly(p-phenylene)/layered double hydroxide ultrathin films with blue luminescence by layer-by-layer assembly. Angew. Chem. Int. Ed. 48, 3073–3076. doi:10.1002/anie.200900178
Keywords: degree-based entropies, topological indices, kekulene, terpyridine complex, fractal molecular structures
Citation: Roy S, Noah Antony Daniel Renai P and Jyothish K (2025) On computation of information entropy measures for certain fractal molecular architectures. Front. Chem. 13:1677841. doi: 10.3389/fchem.2025.1677841
Received: 01 August 2025; Accepted: 27 August 2025;
Published: 15 October 2025.
Edited by:
Pratim Kumar Chattaraj, Birla Institute of Technology, IndiaReviewed by:
Shubin Liu, University of North Carolina at Chapel Hill, United StatesDongbo Zhao, Yunnan University, China
Copyright © 2025 Roy, Noah Antony Daniel Renai and Jyothish. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: S. Roy, cm95LnNhbnRpYWdvQHZpdC5hYy5pbg==
 S. Roy
S. Roy P. Noah Antony Daniel Renai
P. Noah Antony Daniel Renai K. Jyothish
K. Jyothish