- State Key Laboratory of Chemical Safety, SINOPEC Research Institute of Safety Engineering Co., Ltd., Qingdao, Shandong, China
The dry reforming of methane converts methane and carbon dioxide into syngas (a mixture of H2 and CO), which can be utilized for synthesizing downstream chemical products. However, its high endothermicity necessitates elevated operating temperatures (∼900 °C), posing challenges in energy efficiency and catalyst stability. Microwave-assisted heating has emerged as a promising alternative to conventional thermal catalysis, offering potential for enhanced reaction rates, improved energy utilization, and catalyst reactivation. This review systematically examines the recent advancements in microwave-assisted dry reforming of methane. It begins with an analysis of the reaction thermodynamics and fundamentals of microwave heating, specifically addressing its mechanisms and advantages over conventional methods. The core of the review focuses on the rational design of catalysts tailored for effective microwave absorption and catalytic performance. A critical comparison of catalyst performance under microwave versus conventional heating is provided, highlighting the roles of microwave in boosting conversion, suppressing coke deposition, and enhancing catalyst longevity. Finally, the review discusses the persistent challenges in scaling this technology and proposes future research directions, particularly in catalyst and reactor design and process intensification. This work underscores the transformative potential of microwave catalysis to drive efficient and sustainable dry reforming of methane processes.
1 Introduction
Over the past decade, with the rapid growth of the world’s population, the consumption of resources has also shown a rapid increasing trend (Li, 2005). During the rapid industrialization process, the increasing energy demand has gradually changed the energy structure (Tanksale et al., 2010). At present, the world’s energy sources are still dominated by the three traditional fossil fuels: coal, oil and natural gas. The consumption of fossil energy generates a large amount of greenhouse gases and lead to serious environmental problems (Abdullah et al., 2017). Carbon dioxide (CO2) is one of the typical greenhouse gases and a major contributor to the greenhouse effect. But at the same time, it is also a natural source of carbon (Markewitz et al., 2012; Sokolov et al., 2012). In recent years, due to the abundant reserves of shale gas and the discovery of improved hydraulic fracturing technology, methane (CH4) has become a preferred energy source (Olah et al., 2013; Olah et al., 2015). However, a significant portion of natural gas, particularly associated gas from oil extraction, is inefficiently utilized because of technical, regulatory, or economic constraints. The World Bank’s Global Gas Flaring Reduction Partnership (GGFR) estimates that ∼143.4 billion cubic metres (b.c.m.) of associated petroleum gas (APG), equalling a sales value of US$16.5 billion, has been burned in 2021 (Elvidge et al., 2018; Liu et al., 2023). In this case, the reduction of carbon dioxide and methane emissions and their reuse are extremely urgent. Under the background of global warming, Carbon Capture Utilization and Storage (CCUS) is currently one of the most important technical options for achieving the low-carbon utilization of fossil energy (Conti et al., 2016). Within the framework of CCUS, the utilization methods of CO2 mainly include mineral carbonization, physical utilization, chemical utilization and biological utilization, etc. In recent years, oriented towards the production of valuable chemical products using carbon dioxide as raw material (Kondratenko et al., 2013), a large number of scientific research efforts have been dedicated to developing carbon dioxide reuse technologies, mainly including reforming reactions, hydrogen addition reactions, photoreduction reactions, electroreduction reactions, and copolymerization reactions (Huang et al., 2024; Saeidi and Amin, 2014; Wang et al., 2025; Yu et al., 2008).
Among the above-mentioned carbon dioxide reuse technologies, the methane-carbon dioxide reforming reaction provides an effective approach for converting carbon dioxide and methane and preparing syngas (H2 and CO). The syngas produced by the methane-carbon dioxide reforming reaction has a relatively low molar ratio of H2/CO (H2/CO = 1), and can be used as a general raw material for the synthesis of downstream high value-added chemical products (acetic acid (Schwab et al., 2015), dimethyl ether (Azizi et al., 2014), long-chain hydrocarbons (Gadalla and Bower, 1988; Mo et al., 2014), etc.). Therefore, the methane-carbon dioxide reforming reaction can not only convert and utilize carbon dioxide, but also provide a promising indirect utilization route of methane. This technology is of great significance for the efficient utilization of abundant natural gas resources, as well as for reducing carbon dioxide emissions and alleviating the greenhouse effect. Despite its economic and environmental potential, the dry reforming of methane (DRM) remains an immature industrial process, primarily due to challenges in catalyst development (Das et al., 2019; Park et al., 2019). The major issue is catalyst deactivation, which occurs through coke and carbon formation on the catalyst surface and through thermal sintering (Bradford and VANNICE, 1999; Mort et al., 2015; Pakhare and Spivey, 2014). Since the DRM reaction is highly endothermic and requires high reaction temperatures (above 700 °C), it accelerates both catalyst thermal sintering and the formation of local “cold spots” within the catalyst bed (Chen et al., 2012). Therefore, effective heat transfer control is crucial for the successful implementation of DRM.
Furthermore, the significant thermal energy demand of the high-temperature DRM reaction presents a major economic hurdle for standalone application. However, this challenge also unveils a significant opportunity for integration with other industrial processes. Many energy-intensive industries (e.g., metallurgy, cement production, chemical synthesis) generate vast amounts of high-grade waste heat, which often remains underutilized (Li, 2017). Coupling the endothermic DRM process with such sources of waste heat could dramatically improve its overall energy efficiency and economic attractiveness, transforming an industrial liability into a valuable resource for chemical production and emissions mitigation (Jastrząb, 2018; Liao and Horng, 2017; Tan et al., 2025). This synergy between waste heat recovery and catalytic CO2 utilization represents a promising direction for sustainable process intensification.
Compared to conventional heating, microwave heating offers several advantages, such as higher heating rates, better heating control, reduced equipment size, and significant time and energy savings (Bao et al., 2023; Jie et al., 2020; Jones et al., 2002; Motasemi, 2013). Microwave-assisted heating has been widely used to activate environmental catalytic reactions and catalytic deconstruction of plastic waste into hydrogen and high-value carbons (Bao et al., 2023; Jie et al., 2020). The application of microwave-assisted technology as a novel approach for DRM was first introduced by Shah and Gardner (Shah and Gardner, 2014). Microwave-assisted methane reforming has attracted significant attention over the past 2 decades due to its markedly higher conversion rates and yields, as well as reduced coke deposition, compared to conventional reforming systems (la Hoz et al., 2005; Jacob et al., 1995; Berlan, 1995). This technology is poised to play a key role in the near future by enabling the conversion of offshore and remote natural gas into liquid fuels via compact-to medium-scale reactors, using syngas (a mixture of H2 and CO) as a key intermediate (Hwang et al., 2013; Kim et al., 2014). There are presently several reviews available on the development of this field (Fidalgo et al., 2008; Nguyen Sunars et al., 2020; Palma et al., 2020; Zha et al., 2023). Nevertheless, the existing reviews have not exhaustively covered all emerging aspects and recent advances in this rapidly evolving field, such as new catalyst design systems and comparisons of energy consumption with conventional heating. Therefore, the aim of this paper is to provide a comprehensive review of catalysts and their catalytic performance in microwave-assisted DRM, with a particular focus on the challenges and opportunities associated with this process.
2 Overview
2.1 Brief history
As a reforming technology with commercial potential, DRM technology has become a research hotspot for researchers in various countries in recent years. In 1928, Fisher and Tropsch first proposed the concept of DRM (Fisher, 1928), with the aim of converting methane into syngas. Since then, related literature on DRM have been published one after another. It was not until the 1990s, affected by global climate warming, that carbon dioxide emission reduction became a common problem faced by the whole world. At this time, DRM technology gradually attracted widespread attention (Ashcroft et al., 1991; Gree et al., 1992).
DRM (Equation 1) is an extremely endothermic reaction:
In addition, the DRM is a complex reaction system (Table 1). Usually, in addition to the main reaction, there are also side reactions such as the revise water-gas shift reaction (RWGS), methane cracking reaction, and carbon monoxide disproportionation (Boudouard reaction) (Fan and Bhatia, 2009). It can be known from Equation 1 that DRM is a strong endothermic reaction in thermodynamics (∆H298K = 247 kJ/mol), and it requires a relatively high temperature to proceed. According to the thermodynamic calculation results, it is known that the minimum temperature required for the reforming reaction between methane and carbon dioxide is 640 °C, and increasing the temperature is conducive to the forward progress of the reaction (Wang, 1996). Usually, the use of effective catalysts can lower the reaction temperature. But even so, the high reaction temperatures mean that the catalyst needs to have good thermal stability, which is undoubtedly a strict requirement.

Table 1. Different reactions during DRM (Nikoo and Amin, 2011).
Furthermore, the reaction equilibrium of DRM for syngas production is usually affected by the concurrent reverse water gas shift (RWGS) reaction. This leads to the CO2 conversion rate usually being higher than the CH4 conversion rate at equilibrium, thereby resulting in the generated syngas H2/CO being lower than 1 (Gadalla and Bower, 1988). Apart from the influence of the side reaction RWGS, the formation of carbon deposits is the main cause of the inactivation of the catalyst during the DRM process. The formation of carbon deposits during the DRM reaction is mainly attributed to two side reactions: CH4 cracking and CO disproportionation (Jang et al., 2016). Although raising the temperature and increasing the amount of CO2 feed can reduce carbon deposits, both of these pose certain problems in industrial production. Excessively high reaction temperatures impose strict requirements on reforming equipment, while excessive CO2 feedstock gas will consume a large amount of heat. Therefore, from the perspective of industrial production, conducting the reforming reaction at a lower temperature and with a smaller stoichiometric ratio of CH4/CO2 is a more appropriate choice.
Although the DRM reaction has considerable potential for environmental friendliness, it is highly endothermic and carbon deposits form too rapidly during the reaction process, ultimately leading to the rapid deactivation of the catalyst (Figure 1). Therefore, the DRM reaction is not considered an industrially mature process.
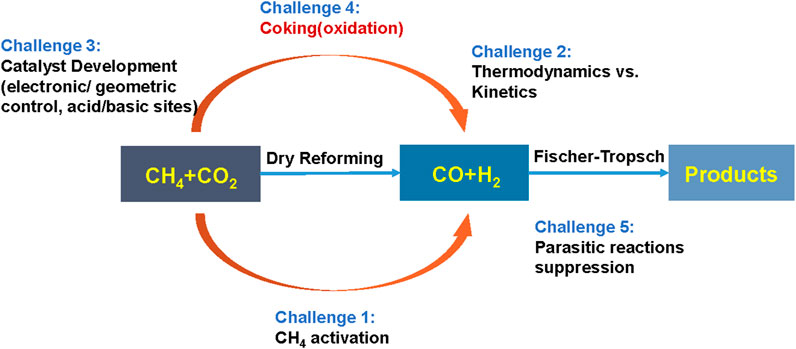
Figure 1. Challenges in DRM reaction. Adapted from Ref (Hussien and Polychronopoulou, 2022).
2.2 Thermodynamics
The DRM reaction is a strongly endothermic reaction. Since both CH4 and CO2 are very stable molecules, their dissociation energies are 435 (CH3-H) and 526 (CO-O) kJ/mol, respectively. Therefore, a relatively high temperature is required to achieve the equilibrium conversion to syngas. On the other hand, the reaction equilibrium is also affected by the reverse water-gas shift side reaction (RWGS, Equation 2).
This reaction leads to a CO2 conversion rate higher than the CH4 conversion rate at equilibrium. In practice, this is beneficial for the production of syngas with a H2/CO ratio of one or lower.
Apart from the RWGS reaction, in the DRM process, the formation of carbon deposits is also a major cause of catalyst deactivation. The carbon formed during the DRM process is mainly attributed to two reactions (Equations 3, 4):
Wang et al. reported that the decomposition of CH4 occurs above 557 °C, while the disproportionation reaction of CO occurs below 700 °C (Wang, 1996). To consider the conversion rates of CH4 and CO2 and the formation of carbon deposits, the optimal reaction temperature when the feed ratio CH4/CO2 = 1:1 is usually 870 °C–1,040 °C. In addition, some researchers have conducted systematic thermodynamic simulations on the effects of various experimental conditions such as reaction temperatures, CH4/CO2 feed ratio, reaction pressure, and additional oxidants on the DRM reaction, and have considered various reactions that lead to the formation of carbon deposits (Jang et al., 2016; Nikoo and Amin, 2011). They generally believe that the DRM reaction being carried out under high temperature and low-pressure conditions above 850 °C is necessary to achieve high conversion rates of CH4 and CO2.
3 Microwave heating technology
A new technique that received extensive attention in the last decades is based on using microwave energy potential for reforming of methane and carbon dioxide. In comparison to conventional heating methods, microwave applications for producing the required heat for reaction is more energy efficient and less expensive. Utilizing microwaves also provides more advantages than conventional heating including a rapid process heating, reduced processing time and work space, more accurate and uniform heating, and high quality (la Hoz et al., 2005; Caddick and Fitzmaurice, 2009; Ku et al., 2002; Gude et al., 2013).
Microwaves are electromagnetic waves whose wavelengths span 1 m–1 mm, corresponding to frequencies of 0.3–300 GHz. For practical heating purposes, the industrial, scientific and medical (ISM) bands—most commonly 0.915 GHz and 2.45 GHz—are employed to avoid interference with telecommunication services (Meredith, 1998). Whereas conventional heating relies on conduction, convection and radiation from a hot medium, microwave heating originates from the direct interaction of polar or conducting species with the oscillating electric (and, to a lesser extent, magnetic) field.
Currently, microwave heating has been widely applied in various fields, such as industrial wood drying, food processing, and rubber vulcanization, etc. Moreover, it has also been studied in areas like ceramic and polymer processing (Hoogenboom and Schubert, 2007; Komarneni et al., 1992; Wiesbrock et al., 2004), environmental applications (Jones et al., 2002; Verma and Samanta, 2018; Zhang and Hayward, 2006; Zlotorzy nski, 1995), biofuels and chemical production (Aravind et al., 2020; Hassan et al., 2020), as well as metallurgy and mineral processing (Jones et al., 2002; Kingman et al., 2004). It is worth noting that microwave heating technology has also been extensively studied in heterogeneous gas-phase catalysis (Durka et al., 2009; Zhang and Hayward, 2006), such as ammonia decomposition (Guler et al., 2017), methane decomposition (Domínguez et al., 2007; Zhang et al., 2003), hydrogen sulfide decomposition (Xu et al., 2017), nitrogen oxide and sulfur dioxide reduction (Peng et al., 2017; Zhang et al., 2001), CO2 reforming of CH4 (Lim and Chun, 2017; Zhang et al., 2003), and recently, alcohol steam reforming (Durka et al., 2011; Gündüz, 2015; Sarıyer et al., 2019). As for CO2 reforming of CH4, under microwave irradiation, CH4 and CO2 conversions regularly exceed thermodynamic equilibrium values predicted for the bulk temperature, while coke deposition is suppressed (Fidalgo et al., 2008; Fidalgo and Menéndez, 2012). The origin of this enhancement lies in the formation of microscopic “hot spots” on catalytic surfaces where local temperatures can be 100–300 K above the surrounding gas, accelerating both C–H and C=O bond scission (Fidalgo et al., 2008). Thus, microwave-assisted DRM offers a low-carbon, energy-efficient route to syngas (H2/CO) with markedly reduced external heat demand and a smaller reactor envelope compared to conventionally heated fixed-bed or fluidised-bed systems.
In fact, both components of the high-frequency electromagnetic radiation contribute to microwave heating (Figure 2). However, the dielectric heating caused by the electric field component is usually used to represent microwave heating, while the heating effect caused by the magnetic field component is rarely mentioned due to a lack of knowledge (Sun et al., 2016). When polarized molecules attempt to align with the high-frequency electric field by rotating, they collide with each other, resulting in dielectric heating. Therefore, the heating capacity of dielectric can be described by its dielectric loss tangent (tan δε), as shown in Equation 5:
The real (ε′) and imaginary (ε″) components of complex permittivity quantify, respectively, how readily microwaves penetrate a material and how efficiently that material converts the incident electromagnetic energy into heat (Jones et al., 2002; Menéndez et al., 2010). A widely cited criterion for effective microwave absorption is a loss tangent (tan δ = ε″/ε′) exceeding 0.1 (Rossi et al.). The dielectric response of a specific sample is governed by its anisotropy, homogeneity, surface roughness, and critically by temperature and excitation frequency (Janezic, 2001). Ignoring the effect of factors related to its nature and the applied frequency, the loss tangent of a sample generally increases with its temperature at a frequency of 900 MHz or 2.45 GHz (Grant et al., 1998; Zhang and Hayward, 2002).
At room temperature, many solid materials such as alumina and silica (Nightingale, 2001) are classified as ultra-low-loss dielectrics. However, once a threshold temperature is surpassed, their heating ability rises sharply, as illustrated in Table 2. Moderately lossy composites such as carbon-coated SiO2 (C–SiO2) or platinum-decorated carbon (Pt/C) exhibit an even more pronounced effect, with tan δ doubling above 800 °C relative to ambient conditions. Such behaviour renders them particularly attractive for microwave-assisted DRM, where the elevated reaction temperature itself reinforces microwave absorption and sustains the required thermal profile (Zhang et al., 2018a). Consequently, a precise knowledge of the temperature-dependent dielectric behaviour of both catalyst and support is indispensable for the rational design of microwave-assisted DRM systems.
In conductive materials, a high-frequency electric field induces an electric current, generating heat through collisions between charged particles and adjacent atoms or molecules (Meredith, 1998). While such conductive losses prevail in conductors, dielectric losses are dominant in lossy dielectrics like water, polar solvents, glass, and ceramics (Table 3). Nevertheless, in certain scenarios—such as microwave heating of electrolyte solutions (Horikoshi et al., 2012) or carbon-based materials (Menéndez et al., 2010)—both mechanisms contribute synergistically to heating within the high-frequency electric field, leading to an accelerated heating rate.
Existing experimental evidence indicates that microwave magnetic heating has advantages over electric heating in the heating of certain ferrites and conductive powders (such as Fe3O4, WC, Fe, and Co.) (Cheng and Agrawal, 2002; Cheng et al., 2001). Roy et al. reported that through a microwave-assisted synthesis method, new crystal structures of ferrites were prepared, including ZnFe2O4, NiFe2O4, BaFe12O19, CoFe2O4 and Fe3O4, etc (Roy et al., 2002). Moreover, it was found that the significant changes in the structural phase and magnetic properties of these ferrites were mainly attributed to the magnetic components. Collectively, existing studies confirm that magnetic losses play a substantial role in the microwave heating not only of magnetic materials, but also of conductors, semiconductors, and composites (Table 4) (Bhattacharya and Basak, 2016) (Cao et al., 2010; Haneishi et al., 2017; Rosa et al., 2016; Wen et al., 2011).
The magnetic heating ability of a material is described by its magnetic loss tangent (tan δμ) as given by Equation 6:
The real (μ’) and imaginary (μ’’) parts of permeability represent the amount of energy stored and lost, respectively (Durka et al., 2009; Sun et al., 2016).
Due to the fact that low-lossy or transparent materials cannot be rapidly heated to the high temperature required for efficient dry reforming of methane reactions. Currently, the gas reactants in DRM process and most common catalysts, such as nickel-based catalysts loaded on SiO2, Al2O3 or other mesoporous materials (Abdullah et al., 2017; Lovell et al., 2015; Wang et al., 2018), exhibit relatively low microwave absorption due to their low dielectric loss tangent (Fidalgo et al., 2011). Therefore, it is necessary to develop catalysts for the microwave-assisted DRM process.
4 Catalysts for microwave-assisted DRM
Since DRM reaction takes place under the presence of a catalyst, and gaseous substances themselves possess intrinsic dielectric properties that are not appreciably favouring the microwave interaction (Hamzehlouia et al., 2018). Therefore, the exploration and development of microwave-assisted DRM reactions have mainly focused on solid catalyst materials. While the active metal sites (e.g., Ni, Fe, Pt, Ru) remain crucial for the surface reactions (Abdullah et al., 2017), the catalyst must now also perform as an efficient microwave receptor. By converting the absorbed microwave radiation into thermal energy and thereby serving as a heat source, microwave-assisted DRM can be achieved (Fidalgo et al., 2008; Hamzehlouia et al., 2018). Therefore, the development of microwave-assisted DRM catalysts not only requires considering the catalyst's ability to resist carbon deposition and metal sintering, but also needs to examine the performance of microwave receptor in improving the efficiency of converting microwave radiation into heat sources (Table 5).

Table 5. Effects of process variables on the catalytic performance for microwave-assisted DRM reaction with different catalysts.
At the universal level, the combination of microwave technology with catalysts confers several key benefits:
1. Reduced Apparent Activation Energy: A consistent observation across numerous studies is the significant lowering of the apparent activation energy for DRM under microwave irradiation compared to conventional heating (Li et al., 2021; Li et al., 2024). This phenomenon, attributed to localized “hot spots” and potential non-thermal effects, enables high reactant conversions at markedly lower bulk gas temperatures, offering a profound energetic advantage.
2. Synergistic Coke Management: The most transformative advantage is the altered role of carbon deposits. While coke formation remains inevitable, under microwave heating, carbon acts as an excellent microwave receptor. Instead of solely causing deactivation, the coke generates intense local heating that vigorously drives the gasification reaction (C + CO2 → 2CO). This creates an in-situ self-cleaning mechanism, where the problematic byproduct is continuously consumed, thereby significantly enhancing catalyst longevity and operational stability (Che et al., 2004; Menéndez et al., 2010; Odedairo et al., 2016; Zhang et al., 2023a).
3. Direct and Volumetric Heating: Microwave energy is deposited directly within the catalyst bed, overcoming heat and mass transfer limitations associated with conventional external heating. This leads to rapid heating rates, elimination of reactor wall overheating, and superior overall energy efficiency, as energy is used to heat the catalyst itself rather than the entire reactor system (Fidalgo et al., 2008; Gangurde et al., 2018; Pham et al., 2020; Wang Sourav et al., 2023).
4.1 Metal-based catalysts
The quest is to combine high catalytic activity with strong microwave absorption. Most conventional metal oxide supports (e.g., γ-Al2O3, SiO2) are microwave-transparent with low dielectric loss, meaning they cannot directly couple with microwave energy to generate heat efficiently. In microwave-assisted DRM, catalysts utilizing such supports rely on several indirect heating strategies.
4.1.1 Heating via in-situ formed microwave receptors
During the DRM reaction, carbonaceous species (e.g., coke, carbon nanotubes, graphene) are inevitably deposited on the catalyst surface. While this coke deactivates catalysts under conventional heating, it becomes a crucial asset under microwave irradiation. Carbon is an excellent microwave receptor. Therefore, once a small amount of carbon is formed, it acts as a in-situ receptor, generating localized “hot spots” that provide the thermal energy required for the reaction to proceed on the adjacent metal sites supported on the oxide support. The catalytic performance then becomes coupled to the nature and amount of this carbon deposit.
Odedairo et al. investigated the structure of Ni/CeO2 catalyst with doping of Cr, Fe and Ta and the catalytic activity of the catalysts under microwave irradiation in dry reforming of methane was tested in a microwave reactor (Odedairo et al., 2016). The results show that the introduction of Cr and Ta to Ni/CeO2 can enhance the interaction between Ni and the support/promoter and inhibit the enlargement of NiO particles during the synthesis. The CH4 conversions in dry reforming on the catalysts follow the order: Ni/CeO2<2Fe-Ni<2Ta-Ni<2Cr-Ni. The superior performance of 2Ta-Ni and 2Cr-Ni may be attributed to the locally-heated Ni particles caused by the strong microwave absorption of the in situ grown graphene attached on them under microwave irradiation (Figure 3). The origin of this enhancement lies in the formation of microscopic “hot spots” on catalytic surfaces where local temperatures can be 100–300 K above the surrounding gas, accelerating both C–H and C=O bond scission (Fidalgo et al., 2008). Zhang et al. report efficient syngas production via methane reforming mediated by the microwave-triggered activation of Lanthanum nickelate (LNO) and carbon deposited on the catalysts could enhance the catalytic performance under microwave irradiation (Zhang et al., 2023a). It was observed that lanthanum oxycarbonates were active intermediates for CO2 activation and oxidation of carbonaceous intermediates from methane on the catalyst, thus improving catalytic activity and stability. This catalyst exhibited an advantage of process intensification and reaction stability in the microwave-assisted DRM process, in contrast to the conventional heating process.

Figure 3. TEM micrographs with different scales (a) 200 nm, (b) 100 nm, (c) 20 nm, (d,e) 5 nm of multiwalled carbon nanotubes/graphitic nanofiber (M/GNF) formed on Ni/CeO2 after 14 h. Adapted from Ref (Odedairo et al., 2016).
In fact, the structure of carbon formed during microwave-assisted DRM also has a substantial impact on the reforming activity. Odedairo et al. found that carbon structure and morphology vary among the Ni catalysts promoted by several metals. Transmission electron microscopy (TEM) results confirme the presence of multiwalled carbon nanotubes/graphitic nanofiber composite (M/GNF) on Ni/CeO2 (Figure 4), while multiwalled carbon nanotubes/layered graphene composite (M/GR) on 2Cr-Ni and multiwalled carbon nanotubes/cup-stacked CNT composite (M/CSCNT) on 2Fe-Ni. And Carboncoated Fe nanocapsules are observed on 2Fe-Ni, which presented excellent microwave absorption properties (Che et al., 2004; Odedairo et al., 2016). Previous studies have reported that graphene owns plentiful of sp2 π electrons, which makes it conducive to effectively absorbing microwaves and converting the subsequently absorbed microwaves into micro-plasmas or hotspots. Therefore, the generation of graphene during the DRM process is beneficial to the improvement of performance (Menéndez et al., 2010).
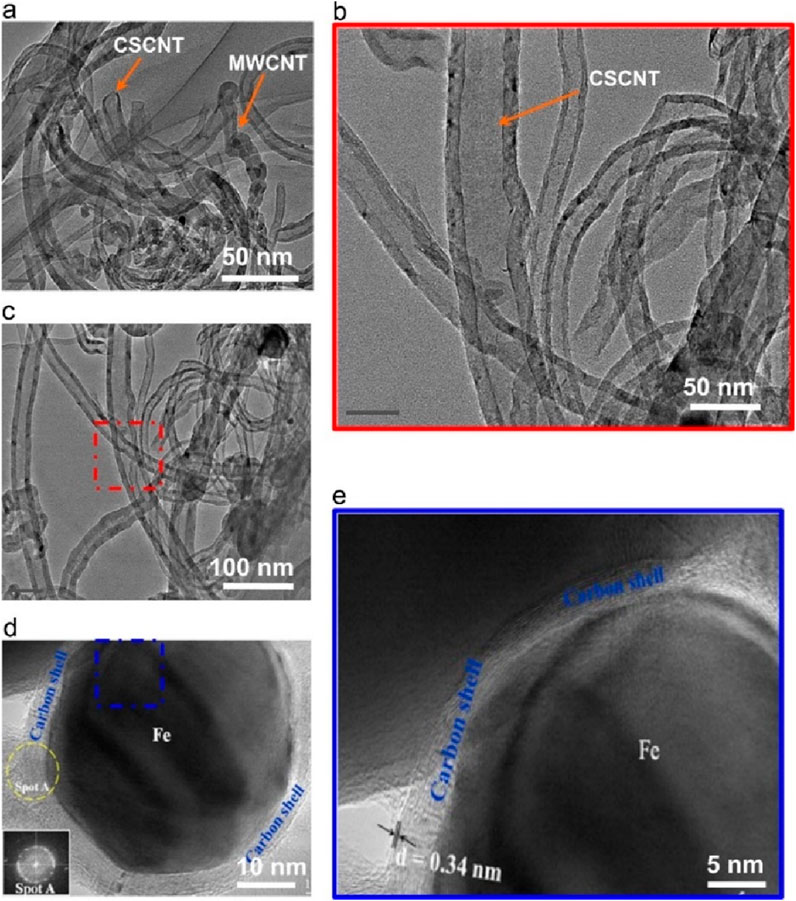
Figure 4. TEM micrographs with different scales (a,b) 50 nm, (c) 100 nm, (d) 10 nm, (e) 5 nm of multiwalled carbon nanotubes/cup-stacked CNT (M/CSCNT) formed on 2Fe-Ni after 14 h. Adapted from Ref (Odedairo et al., 2016).
Kinetic analysis and empirical observations reveal that microwave-assisted DRM substantially reduces the activation energy required for the reaction, significantly enhancing the conversion rates of CH4 and CO2 into syngas (Li et al., 2024). Li et al. reported that compared to conventional heating dry reforming of methane (CH-DRM) processes, CsRu/CeO2 achieved higher methane (84.6%) and carbon dioxide (85.7%) conversion rates at a lower temperature of 500 °C in microwave-assisted DRM, significantly reducing energy consumption (Li et al., 2024). The significant decrease in activation energy for the microwave-assisted DRM process can be attributed to microwave-specific interactions with the CsRu/CeO2 catalyst (Figure 5). Microwave irradiation creates localized “hot spots” and enhances the electromagnetic field at the catalyst surface, likely reducing the energy barrier for CH4 and CO2 molecules to react (Li et al., 2021). This effect is further enhanced by structural and electronic modifications of the catalyst induced by microwave irradiation, promoting more efficient reactant conversion at lower temperatures.

Figure 5. Activation energy fitting of CsRu/CeO2 under conventional heating (black) and microwave heating (red) conditions. Adapted from Ref (Li et al., 2024).
4.1.2 Heating via magnetic metal components
The introduction of other metals can also lead to an improvement in the microwave-assisted DRM performance. Some active metals or promoters (e.g., Fe, Co., Ni) possess magnetic properties. Under microwave irradiation, these components can generate heat through magnetic loss mechanisms, effectively turning the metal nanoparticles into localized heaters. This heat is then transferred to the oxide support and the reaction site. Previous research revealed the crucial role played by Fe species in imparting microwave receptivity to the alloy catalysts, thereby significantly impacting DRM activity. Olowoyo et al. explored the effects of both microwave power and the temperature-responsive behavior of the catalysts, as well as delving into the influence of the active component content (Ni and Fe) and space velocity on DRM reactions (Olowoyo and Zheng, 2024). And found that the 25Ni40Fe/MgAl2O4 catalyst has a uniform distribution of Ni and Fe, the important role of iron incorporation in the structure of catalyst to increase its microwave absorption capability. (Figure 6) Zhang et al. evaluated the factors influencing the catalytic performance of Al2O3-SiC supported catalysts in the microwave-assisted DRM, including the catalyst carrier, microwave power, active component content, and space velocity (Zhang et al., 2018b). They found that an increase in the content of active Fe (4-12 wt%) could rapidly enhance the catalytic activity. However, when the Fe content reached 16 wt%, the catalytic activity decreased. When the Fe content reached the optimal value (12 wt%), the conversion rates of CO2 and CH4 reached approximately 92% and 93% respectively. When the content of active Fe was lower than 12 wt%, the active Fe was insufficient to be evenly distributed on the entire surface of the carrier, thus unable to provide sufficient active sites for the microwave-assisted DRM reaction, while a content of iron higher than 12 wt% was prone to cause metal sintering at high temperatures.

Figure 6. The temperature- dependent variation of (a) ε′(dielectric constant), (b) ε″ (dielectric loss factor), and (c) loss tangent for the Ni/MgAl2O4, 25Ni30Fe/MgAl2O4, and 25Ni40Fe/MgAl2O4 catalysts. Adapted from Ref (Olowoyo and Zheng, 2024).
The magnetic properties of Co. particles led to a higher microwave absorption ability and exhibited better microwave-assisted DRM activity. Nguyen et al. reports on M-Mo bimetallic catalysts (M = Co. or Cu) supported on TiO2 for DRM under microwave irradiation (Nguyen Pham et al., 2020). Experimental results displayed outstanding activity of such M-Mo/TiO2 catalysts, on which high reaction efficiency of methane reforming can be sustained at a much lower microwave-assisted power of 100 W compared to literature results of 200 W. For DRM, about 81% CH4 and 86% CO2 were converted to syngas with a H2/CO ratio of 0.9 over the CoMo1 catalyst while the CuMo1 catalyst translated 76% CH4 and 62% CO2 into syngas with a H2/CO ratio of 0.8. The reason behind the excellent performance of the Co-Mo/TiO2 catalyst is the good exposure of the well-defined hexagonal microwave-assisted receptor. Meanwhile, the formation of a high dielectric layer of MoO2 surrounding active Cu0 can promote the microwave-assisted absorption of the Cu-Mo/TiO2 catalyst and thereby enhance its catalytic performance. The Co-Mo catalyst exhibited better activity than the Cu-Mo samples given that the magnetic properties of Co. particles led to a higher microwave-assisted absorption ability. Both M-Mo/TiO2 catalysts exhibited brilliant stability under microwave-assisted irradiation.
4.1.3 Defect-driven microwave absorption in specialized oxides
Furthermore, materials like Ce-Zr oxides can exhibit unique microwave-specific reaction pathways. Previous study revealed that Zr addition to ceria helps form Ce3+-VO centers and suggesting the crucial role of these sites for the microwave-assisted DRM reaction. Wang et al. reported that the Ce3+-VO pairs are reaction active sites and also the microwave absorbing sites, improving microwave heating and efficiency and propose an oxidation/reduction cycle (Figure 7) where a turnover invokes a Mars-van-Krevelen (MvK)-type chemical looping (Wang Sourav et al., 2023). The first half of the cycle is initiated by converting CH4 and OL to CO and H2, forming an oxygen vacancy. The vacancy allows an adjacent lattice oxygen to hop in, rotating the imbalanced Ce3+-VO dipole in the EM field and adsorbing energy to elevate the energy states at the nanoscale. This local excitation promotes additional conversion of CH4 and further reduction of the catalyst, creating a “reductive propagation” process. In the second half cycle, CO2 converts into a second CO molecule on the VO site and re-oxidizes the crystal. It effectively quenches the local energy states and creates a continuous single-bed periodic chemical loop. The reaction is periodically excited and quenched at the reactive centers without elevating the bulk measured temperature.

Figure 7. Schematic illustration of the oxidation/reduction Cycle. Adapted from Ref (Wang Sourav et al., 2023).
4.2 Non-metallic catalysts
In addition to metals, carbon materials also discharge in the microwave field (Li Tao et al., 2020). When the carbon material is irradiated by microwave, the delocalized π electrons move freely in a relatively wide area. The kinetic energy of some electrons may increase, causing them to jump out of the material, which is thought to be a spark or arc. But at the micro-level, these hot spots are plasma (Menéndez et al., 2010). Compared with microwave-induced metal discharge, induced carbon material discharge has unique advantages in that it can generate plasma while maintaining intrinsic catalysis, and the material preparation cost is lower. Zhang et al. reported that the different carbon materials had different discharge intensities, but they all produced star-shaped sparks and particles with cis-fiber structure and tips placed in opposition to each other were easier to discharge in microwave electric fields (Figure 8) (Zhang Zhang et al., 2023). In addition, multi-point and frequent discharges are beneficial to improve the reforming effect. Based on similar catalyst structures, the enhancement of the discharge fluctuation improved the reaction effect more than that of increasing the temperature. And they revealed the mechanism of microwave discharge promoting the reaction process can be attributed to the synergistic effect of surface thermal catalysis and microwave plasma catalysis.

Figure 8. (a,b) Animated demonstration of charge accumulation and discharge evolution, (c–f) apparent phenomena of coconut shell activated carbon discharge with different particle sizes (20 s). Adapted from Ref (Zhang Zhang et al., 2023).
During microwave discharge, approximately 10% of the electron energy is utilized for electron excitation, while the remainder is allocated to vibrational excitation (Snoeckx and Bogaerts, 2017). As a result, vibrationally excited molecules play a critical role in plasma-catalysis reactions. Due to the relatively low electron energy in microwave discharge plasma, there is insufficient energy (>7 eV) to dissociate CO2 molecules directly into CO and O via electron collisions (Snoeckx and Bogaerts, 2017). In contrast, vibrationally excited CO2* species can persist for 10–100 μs and participate in chemical reactions (Liu and Chen, 2020). Similarly, microwave discharge generates a substantial amount of vibrationally excited CH4*, which also contributes to the reaction. Given that the C–H bond energy is lower than that of the C=O bond, CH4 fragmentation via electron collisions occurs more readily compared to CO2 (Khoja et al., 2019; Snoeckx et al., 2013). These collisions result in the formation of CHx radicals and atomic hydrogen. Zhang et al. detected C and CH* peaks in the microwave discharge spectrum of a carbon catalyst, confirming that discharge excited CH4 and led to electron collision dissociation producing CHx radicals, thereby facilitating the reaction process (Zhang Zhang et al., 2023). With the assistance of plasma, the adsorption and dissociation of CO2 and CH4 molecules on the catalyst surface are enhanced. Vibrational activation significantly increases the adsorption and dissociation probabilities of CO2* (Jiang and Guo, 2016) and CH4* (Fleming Crim, 2008), rendering them highly reactive. Meanwhile, CHx radicals can also adsorb onto the catalyst surface and act as reactive intermediates in subsequent surface reactions (Nozaki and Okazaki, 2004), further promoting conversion.
Li et al. reported that bio-char catalytic activity could be affected by raw material for char preparation, since it was greatly connected to the remained metal after char preparation (Li Chen et al., 2018). In addition, original bio-char could only maintain its catalytic effect at an acceptable level in 70 min, due to an unavoidable carbon gasification reaction. Nevertheless, carbon gasification meanwhile generated part of CO production and it was a contributor to total syngas production. Tan et al. investigated the effect of activated carbon as catalyst on methane dry reforming and found that the wood-derived activated carbon exhibits better catalytic effect on methane reforming (Tan et al., 2019). Furthermore, they also calculated the migration amount of carbon and obtained carbon migration rate reduces with the increase of microwave power or decrease of CH4/CO2 ratio.
Fidalgo et al. had synthesized various carbon materials with different textural and surface properties to investigate the influence of various carbon materials on microwave-assisted DRM performance and found that the dry reforming of CH4 occurs mainly in micropores and that, in addition to large micropore volume, the carbon material used as catalyst needs to show a good CO2 reactivity (Fidalgo et al., 2010). Thus, carbon materials with a low CO2 reactivity are not good catalysts for dry reforming reaction. Furthermore, the oxidized activated carbons are not the best catalysts for dry reforming, since the presence of oxygen surface groups reduces the catalytic activity of the carbon material dramatically, especially under microwave heating (Figure 9). The low conversions over oxidized carbons achieved in the microwave oven were due to their reduced CO2 reactivity and to the difficulty involved in heating them up.
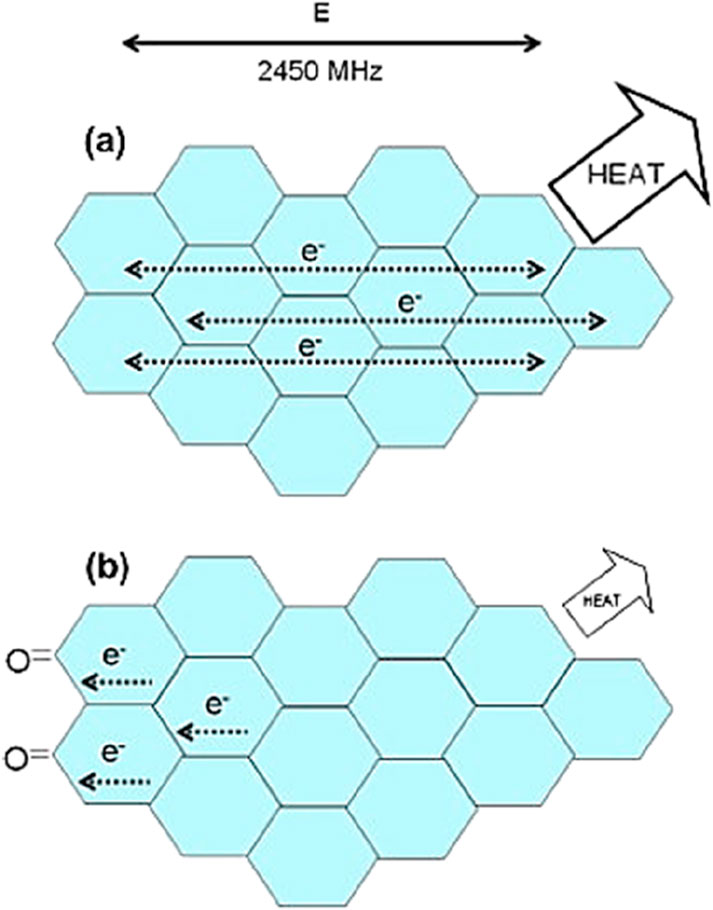
Figure 9. Schematic representation of some of the effects involved in the microwave heating of carbons: (a) When microwave heating is caused by the Maxwell-Wagner effect (Interfacial polarization), the delocalized π-electrons try to couple the changes of phase of the electric component of the electromagnetic field dissipating heat; (b) Hypothesis: oxygen-containing surface groups are electron-withdrawing, limiting the mobility of some of the π-electrons of the basal planes and therefore restricting the heat released. Adapted from Ref (Fidalgo et al., 2010).
4.3 Carbon-supported metal composite catalysts
The synergistic integration of carbon materials with active metals represents a cornerstone in the development of advanced catalysts for microwave-assisted DRM. This architecture is highly effective, leveraging the complementary properties of each component: the metal nanoparticles provide catalytic activity for CH4 and CO2 activation, while the carbon support acts as an efficient microwave receptor, enabling rapid and localized heating. This synergy is crucial for achieving high catalytic activity, stability, and product yields under the extreme conditions typical of DRM.
Zhang et al. prepared the activated carbon (AC)-supported Ni catalysts modified with Mg, Ca, La, Ce, and the Ni-Ce/AC catalyst was demonstrated the optimum catalyst for microwave-assisted DRM process (Figure 10) (Zhang et al., 2025). By converting microwave energy attenuation into heat and plasma, the locally-formed high-energy active sites composed of adjacent Ni, CeO2 and AC support of the Ni-Ce/AC catalyst could contribute to achieving the effective and localized activation of CH4 and CO2 molecules, thus leading to the enhancement of the reforming activity and the reduction of the loss of AC support due to CO2 gasification. Moreover, microwave heating method could avoid the excessive consumption of the AC support by increasing the graphitization degree of AC support and keeping a dynamic equilibrium state of the CH4 decomposition and CO2 gasification reaction, therefore contributing to the enhanced stability of the AC-based catalysts.

Figure 10. (a) Effect of (a) different promoters and (b–d) microwave power on reactant conversion rates and H2/CO ratio during microwave-assisted DRM process, (CH4: CO2: N2 = 1: 1: 6, Gas flowrate = 160 mL/min). Adapted from Ref (Zhang et al., 2025).
The surface engineering on the defects of the carbon-supported metal composite catalysts could strongly promote the electron transfer and improved the microwave absorption and conversion capacity and catalytic performance. Zhang et al. prepared the double-hierarchical Ni@C-NCNTs with different loading amount of defective N-doped carbon nanotubes (NCNTs) on the Ni@C microstructures, which significantly improved the microwave absorption and conversion capacity and catalytic performance due to the synergistic effect of multiple loss mechanism of the incident microwave and the mitigation of active metal sintering (Zhang et al., 2023c). Furthermore, Zhang et al. found the activation of lanthanum nickelates triggered by microwaves leads to the formation of lanthanum oxycarbonates, which were active intermediates for CO2 activation and oxidation of carbonaceous intermediates from methane on the Ni-La/AC catalyst, thus improving catalytic activity and stability (Zhang et al., 2023a).
Li et al. prepared the Ni/bio-char catalysts with varying Ni loading of 0–20 wt% to study the synergetic influence on their catalytic performance for microwave-assisted DRM at temperature of 800 °C (Li et al., 2017). The addition of Ni (from 0 to 20 wt%) could significantly enhance the bio-char’s catalytic performance and the optimal value was achieved at 10% Ni/bio-char respecting reactant conversions and catalytic stability. With the employment of Ni loading at 10 wt% and below, the equilibrium between carbon consumption and carbon deposition rate could be achieved, and hence, recovery of active sites led to higher CH4 conversion and better stability (Li et al., 2017). While the Ni/bio-char with Ni loading beyond 10 wt% experienced severe deactivation probably due to the active site blockage induced by metal sinterization and deposition of carbonaceous species, as evidenced by the post-reaction Fourier transform infrared spectroscopy and scanning electron microscopy measurements. It is worth noting that the conversion of CO2 was always greater than CH4 and the decrease in CO2 conversion was insignificant with growing Ni loading. This behaviour was possibly due to the gasification of carbon presented in bio-char and the reverse water–gas shift. From this study, Li et al. found that in the process of the carbon removal with CO2, the activeness of carbon from bio-char was much higher than carbon deposits in accordance with Fidalgo et al. (Fidalgo et al., 2008).
Apart from transition metal-based catalyst, Li et al. had prepared several alkaline metal-based catalysts and assessed their catalytic performance for microwave-assisted DRM (Li Chen et al., 2018; Li Yang et al., 2018). Interestingly, the advantages of employing alkaline metal were pronounced to the improvement in CO2 conversion, indicating the enhancement in gasification of carbonaceous deposits possibly owing to the extent of alkalinity of catalyst. In comparison with original bio-char, the embedment of K and Na improved the conversion of CO2 by 10.8% and 12.1%, respectively, while the embedment of Mg and Ca unexpectedly promote the rate of CH4 decomposition. Considering the economic feasibility, applicability and availability, Li et al. continuously study the microwave-assisted DRM process over the Fe-rich biomass-derived chars (Li et al., 2019). They found that Fe addition appreciably enhances the activity of methane reforming compared to original char. However, at Fe content beyond 10%, a drop in CH4 conversion was observed owing to the metal sintering at higher temperature. Conversely, the CO2 conversion was unaffected over different Fe content bio chars. This behaviour was attributed to the acceleration of both gasification of carbon and reverse water–gas shift contributing to the high consumption of CO2 reactant.
Above all, these findings provide new insights for the synergistic effect of microwave with carbon-supported metal composite catalysts for syngas production.
5 Catalyst performance comparison
The comparative assessment on catalyst performance of conventional and microwave heating in DRM is seldom reviewed. It is evidently confirmed that microwave heating excelled in CH4-CO2 conversion performance compared to conventional furnace heating in terms of energy consumption and heating rates (Fidalgo et al., 2008; Gangurde et al., 2018). Hence, the microwave energy directly transfers to the microwave receptor/catalysts and enhances the internal temperature with precisely uniform distribution, eluding the energy wastage by heating the environs and reactor wall. However, a comprehensive comparison study of catalyst performance between conventional and microwave heating is yet to be done (Pham et al., 2020). Wang et al. were the first to measure core bed temperatures at high temperatures under microwave heating by using the fiber Bragg grating (FBG) temperature sensor, unachievable by pyrometers and IR cameras, enables a direct comparison with the conventional heating (Wang Sourav et al., 2023). And found that the reaction temperature under microwave heating reaches a steady state within 4 min, compared to ∼1.5 h in conventional heating. Importantly, microwave heating consumes less energy than conventional heating at these similar conversions: ∼150× lower (using 10 °C/min ramping rate in conventional heating) during temperature ramping and ∼10× lower at steady state, due to rapid and selective heating on the microwave heating susceptible catalyst. For the reactivity of ceria zirconia catalysts under microwave heating and conventional heating, the CH4 consumption rate and H2 selectivity increase with increasing temperature and are superior in microwave heating to conventional heating (Figure 11). The higher activity under microwave heating suggests that the density or turnover frequency of the active sites under microwave heating is higher.
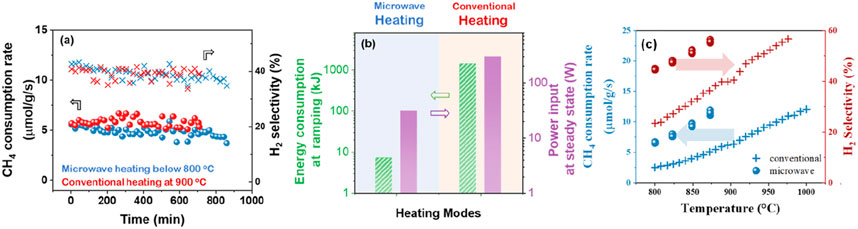
Figure 11. (a) Time-on-stream DRM reaction on ceria-zirconia catalyst in conventional heating and microwave heating at similar conversions. Reaction conditions: 100 cc/min total flow of 5% CH4 and 10% CO2 in N2. 250 mg catalyst loading, 900 °C in conventional heating, and ∼800 °C in microwave heating. (b) Energy consumption during temperature ramping and steady-state under conventional heating and microwave heating at similar conversions shown in (a). Effect of temperature on the DRM reaction on the ceria-zirconia catalyst under microwave heating and conventional heating. (c) CH4 consumption rate (blue) and hydrogen selectivity (red) under microwave heating (●) and conventional heating (+). Adapted from Ref (Wang Sourav et al., 2023).
Sharifvaghefi et al. synthesized Ni-MgO/AC catalysts and compare the conversion, selectivity, thermal efficiency under convention heating and microwave heating (Sharifvaghefi et al., 2019). And found that the conversions of CO2 and CH4 were always higher under microwave conditions with an almost constant difference with conventional heating (Figure 12). In contrast to conventional heating, microwave heating can generate multiple hot spots at the catalyst surface, where local reaction temperatures are higher than the overall reaction temperature. In addition, with conventional heating, the H2/CO ratio was lower than the equilibrium values. In contrast, the H2/CO ratio was higher than the equilibrium value in the higher temperature range under microwave heating and it can be explained by the creation of hot spots, which is similar to that from the thermal temperature effect.
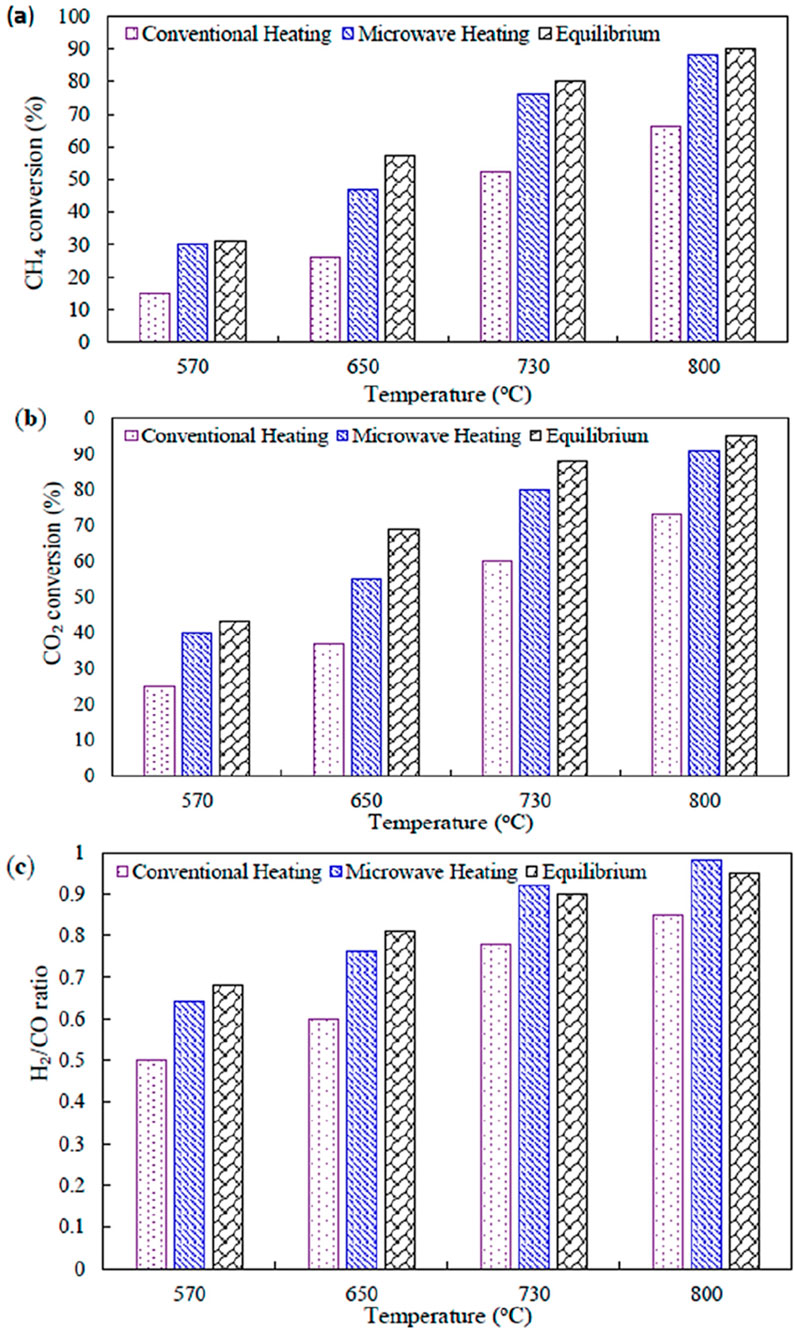
Figure 12. Comparison of microwave and conventional heating and equilibrium conditions for (a) CO2 and (b) CH4 conversions and (c) product selectivity as a function of temperature over Ni/MgO/AC catalyst and gas hourly space velocity (GHSV) of 33,000 mL/g cat. h. Adapted from Ref (Sharifvaghefi et al., 2019).
Table 6 shows the comparison of reported DRM catalysts under microwave-assisted system as well as conventional heating system. It can be seen that the conversion of CH4 and CO2 in the microwave-assisted heating system are generally higher than those in the conventional heating system in most catalysts, indicating that microwave heating promotes the catalytic performance of DRM.

Table 6. Comparison of reported DRM catalysts under microwave -assisted system as well as conventional heating system.
6 Current challenges and future perspective
Dry reforming of methane (DRM) holds significant environmental and energy sustainability implications as it converts greenhouse gases (CH4/CO2) into syngas (H2/CO), simultaneously addressing carbon neutrality objectives (Figure 13). The resultant syngas serves as a versatile feedstock for Fischer-Tropsch synthesis of energy-dense chemicals and hydrogenation processes for crude oil upgrading and methanol production, effectively reducing reliance on conventional fossil resources. However, technical challenges persist in mitigating Ni nanoparticle sintering and carbon deposition under high-temperature operating conditions (Bian et al., 2017). Moreover, the operating conditions (temperature, pressure, reactant ratio) may also lead to catalyst deactivation. For instance, an unbalanced reactant ratio or excessive steam content can promote carbon formation, accelerate catalyst deactivation, and cause the active sites of the catalyst to be blocked by carbon deposits, thereby inhibiting the adsorption of reactants. It leads to the reduction of catalytic activity and selectivity (Bian et al., 2017). Therefore, in order to solve this problem, researchers have studied the structural engineering of catalysts, such as developing structural catalysts with mixed metal oxides, zeolites, supported metals, etc., to improve their stability and anti-carbon ability (Kawi et al., 2015).
Because the DRM process involves competitive reactions, such as the reforming of methane and the decomposition of carbon dioxide. Therefore, a thorough understanding of the kinetics and mechanism of the reaction is necessary to achieve selective control of the reaction path, maximize the selectivity of the desired product, and minimize by-products as much as possible. By studying the reaction intermediate and reaction pathways to optimize the process conditions, the overall efficiency of the DRM process can be improved (Han et al., 2020). Furthermore, due to the high dissociation energy of CH4 and CO2, they require a very high temperature for activation, and this reaction condition may lead to the deactivation of the catalyst (Jang et al., 2019). However, the current research lacks comprehensive thermodynamic or kinetic models as well as experimental data, which further hinders the progress in this field (Foppa et al., 2017). In addition, the carbon dioxide source of DRM usually comes from industrial flue gas, which contains impurities such as sulfur and may poison the catalyst, causing it to deactivate. Therefore, efficient carbon dioxide capture technologies are also needed to ensure high-quality raw material sources for the DRM process (Al-Mamoori et al., 2017).
In response to the challenges faced by DRM in conventional heating system, the microwave-assisted DRM has improved both in terms of energy consumption and catalytic performance, and has given rise to promising results, thus deserved to be investigated deeply. However, there are still challenges in its industrial application, such as the penetration depth (Dp), at which microwave power decays to one/e of its surface value, imposes a strict constraint on reactor geometry. This limits the viable diameter of a fixed-bed reactor, as microwaves cannot uniformly heat large-scale beds, leading to significant temperature gradients and loss of process efficiency. Large multimode cavities require careful design to ensure even electric field distribution. Inconsistent coupling can cause arcing, localized overheating, and reactor damage. Preventing microwave leakage in large systems requires robust shielding and interlocking. Power scaling to megawatt levels also demands high-efficiency magnetrons and waveguides, which increase operational costs. The following are outlooks of microwave-assisted DRM:
1. Due to the high magnetic loss tangent value of Ni-based catalysts and their excellent catalytic performance in dry reforming of methane, more thoroughly research on them is necessary. Although metals are classified as microwave reflectors, as long as their particles are fine enough and the fineness is less than their penetration depth, they can be effectively heated by microwaves. Since the surface area of most microwave receptors is low, it is necessary to increase the number of fine nickel particles coated on microporous or mesoporous materials as much as possible, or by mixing high-loss materials (such as silicon carbide) with nickel-based catalysts to enhance its microwave absorption capacity (Shi et al., 2022; Shi et al., 2025).
2. A thorough understanding of the kinetics and mechanism of the reaction is necessary to achieve selective control of the reaction path, maximize the selectivity of the desired product, and minimize by-products as much as possible. By studying the reaction intermediate and reaction pathways to optimize the process conditions, the overall efficiency of the DRM process can be improved. There is a need to develop the thermodynamic equilibrium models which take into account the formation of hotspots or microplasmas to provide improved estimates of overall efficiencies (Li et al., 2021; Li et al., 2022; Marin et al., 2021; Rao et al., 2023; Shi et al., 2021; Shi et al., 2023; Wang Pu et al., 2023; Yabe et al., 2019; Zhao et al., 2022).
3. Integrating multiscale modeling approaches: Density functional theory (DFT) for mechanistic elucidation and catalyst interface optimization (Alotaibi et al., 2023; Khan et al., 2023; Yoon et al., 2022), machine learning algorithms for predictive catalyst screening and reaction parameter optimization (Artrith et al., 2020) and ASPEN for compare mass and energy balance of the conventional dry reforming of methane and microwave-assisted DRM (Almaraz et al., 2025). This theoretical framework enables rational catalyst design while minimizing empirical trial-and-error approaches, significantly accelerating developmental timelines. Particular emphasis should be placed on establishing structure-activity relationships for confined systems under non-equilibrium reaction conditions.
4. The industrial-scale implementation of microwave-assisted DRM necessitates sophisticated engineering solutions to preserve catalytic integrity (activity/stability/selectivity) alongside comprehensive techno-economic analyses encompassing capital/operational expenditures for commercial feasibility. Furthermore, multi-process integration strategies demonstrate enhanced system versatility through synergistic combination with complementary technologies. Furthermore, microwave-assisted DRM can also be integrated with steam reforming, Sabatier process, Fischer-Tropsch synthesis, CH4 synthesis and other processes, thereby obtaining a more flexible and universal production system. This system can simultaneously enhance carbon utilization efficiency, reduce greenhouse gas emissions, and prepare carbon-value-added chemicals. In addition, microwave-assisted DRM can also be integrated with renewable energy sources such as solar and wind power to enhance its sustainability and economic feasibility.
5. Novel reactor designs may be needed, moving away from traditional fixed beds toward fluidized beds, monolithic reactors, or sequential small-scale reactor arrays can mitigate penetration depth issues and improve heating uniformity. Multiphysics simulations combining electromagnetics, thermodynamics, and reaction kinetics can guide reactor optimization. Real-time adaptive control systems powered by AI may dynamically adjust power and gas flow to maintain stability.
6. A microwave-plasma hybrid system could uniquely address overcoming penetration depth limitations. A localized plasma torch, ignited and sustained by microwaves, acts as an intense, point-source of energy and active species. An array of such plasma sources could be strategically deployed within a large-scale reactor, effectively bypassing the penetration depth problem and ensuring uniform activation across the reactor volume. Furthermore, this synergy potentially leading to dramatic improvements in energy efficiency and providing novel pathways to suppress carbon deposition.
7. Shifting from continuous-wave to pulsed microwave operation opens new dimensions for probing reaction mechanisms and enhancing control. Applying short, high-power microwave pulses (µs to ms duration) allows for the decoupling of thermal and non-thermal effects. Time-resolved in situ characterization (e.g., transient DRIFTS, mass spectrometry) during and after a pulse can capture short-lived intermediate species and surface changes. This approach is powerful for unraveling the intrinsic reaction pathways and quantifying the specific role of the microwave field in lowering activation barriers. And pulsed operation enables sophisticated dynamic control strategies. By tailoring pulse frequency, duration, and power, it is possible to manage the rate of energy delivery to the catalyst surface. This can prevent localized overheating, allow for surface relaxation, and enable in-situ regeneration cycles to periodically remove carbon, thereby significantly enhancing catalyst longevity and process stability.
7 Conclusion
This review underscores the profound environmental and energetic significance of microwave-assisted DRM as a transformative technology for sustainable syngas production. By simultaneously utilizing two potent greenhouse gases, CH4 and CO2, microwave-assisted DRM directly contributes to carbon neutrality goals and offers a versatile route for producing value-added fuels and chemicals, thereby reducing dependence on conventional fossil resources. The transition from conventional thermal heating to microwave irradiation represents a paradigm shift in catalytic reaction engineering. This review has elaborated on the unique mechanisms through which microwaves enhance the DRM process. These features collectively lead to marked improvements in energy efficiency, catalytic activity, and stability.
Despite these promising advantages, the industrial implementation of microwave-assisted DRM faces multidisciplinary challenges encompassing reactor design, catalyst development, process scaling, and system integration. Overcoming limitations such as microwave penetration depth, electric field distribution, and the design of cost-effective high-power systems requires continued innovation and interdisciplinary collaboration. Looking forward, microwave-assisted DRM is poised to play a pivotal role in future sustainable chemical processes, especially when integrated with renewable energy sources and carbon capture technologies. By enabling more efficient conversion of greenhouse gases into synthetic fuels and chemicals, microwave-assisted DRM holds the potential to close the carbon cycle and facilitate a transition toward a circular economy. This review not only summarizes current advancements but also provides a roadmap for future research, emphasizing the need for integrated multiscale modeling, advanced reactor design, and hybrid process systems to realize the full potential of microwave catalysis in industrial applications.
Author contributions
SK: Investigation, Visualization, Writing – original draft, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the SINOPEC science and technology project (324012).
Acknowledgments
AcknowledgementsThis work was supported by the SINOPEC science and technology project (324012). The author is grateful to the editor and the reviewers.
Conflict of interest
Author SK was employed by SINOPEC Research Institute of Safety Engineering Co., Ltd. The author declares that this study received funding from SINOPEC. The funder had the following involvement in the study: the decision to submit it for publication.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abdullah, A. G., Abd Ghani, N. A., and Vo, D. V. N. (2017). Recent advances in dry reforming of methane over Ni-based catalysts. J. Clean. Prod. 162, 170–185. doi:10.1016/j.jclepro.2017.05.176
Almaraz, R., Riley, J., and Palanki, S. (2025). Technoeconomic analysis of microwave-assisted dry reforming integrated with chemical looping for production of methanol. Industrial Eng. Chem. Res. 64, 12074–12086. doi:10.1021/acs.iecr.5c00543
Alotaibi, B., Berrouk, A. S., and Saeed, M. (2023). Optimization of yield and conversion rates in methane dry reforming using artificial neural networks and the multiobjective genetic algorithm. Industrial Eng. Chem. Res. 62, 17084–17099. doi:10.1021/acs.iecr.3c01813
Al-Mamoori Krishnamurthy, R., Rownaghi, A. A., and Rezaei, F. (2017). Carbon capture and utilization update. Energy Technol. 5, 834–849. doi:10.1002/ente.201600747
Aravind, K., Kumar, P. S., and Siddarth, N. (2020). Conversion of green algal biomass into bioenergy by pyrolysis. A review. Environ. Chem. Lett. 18, 829–849. doi:10.1007/s10311-020-00990-2
Artrith, L., Lin, Z., and Chen, J. G. (2020). Predicting the activity and selectivity of bimetallic metal catalysts for ethanol reforming using machine learning. ACS Catal. 10, 9438–9444. doi:10.1021/acscatal.0c02089
Ashcroft, C., Cheetham, A. K., Green, M. L. H., and Vernon, P. D. F. (1991). Partial oxidation of methane to synthesis gas using carbon dioxide. Lett. Nat. 352, 225–226. doi:10.1038/352225a0
Azizi, R., Rezaeimanesh, M., Tohidian, T., and Rahimpour, M. R. (2014). Dimethyl ether: a review of technologies and production challenges. Chem. Eng. Process. Process Intensif. 82, 150–172. doi:10.1016/j.cep.2014.06.007Tohidian
Bao, S.-L., Serrano-Lotina, A., Niu, M., Portela, R., Li, Y., Lim, K. H., et al. (2023). Microwave-associated chemistry in environmental catalysis for air pollution remediation: a review. Chem. Eng. J. 466, 142902. doi:10.1016/j.cej.2023.142902
Berlan, J. (1995). Microwaves in chemistry: another way of heating reaction mixtures. Radiat. Phys. Chem. 45, 581–589. doi:10.1016/0969-806x(94)00072-r
Bermudez, F., Fidalgo, B., Arenillas, A., and Menendez, J. A. (2012). Mixtures of steel-making slag and carbons as catalyst for microwave-assisted dry reforming of CH4. Chin. J. Catal. 33, 1115–1118. doi:10.1016/s1872-2067(11)60386-0
Bhattacharya, B., and Basak, T. (2016). A review on the susceptor assisted microwave processing of materials. Energy 97, 306–338. doi:10.1016/j.energy.2015.11.034
Bian, D., Das, S., Wai, M. H., Hongmanorom, P., and Kawi, S. (2017). A review on bimetallic nickel-based catalysts for CO2 reforming of methane. ChemPhysChem 18, 3117–3134. doi:10.1002/cphc.201700529
Bradford, V., and Vannice, M. A. (1999). CO2 reforming of CH4. Catal. Rev. 41, 1–42. doi:10.1081/cr-100101948
Caddick, F., and Fitzmaurice, R. (2009). Microwave enhanced synthesis. Tetrahedron 65, 3325–3355. doi:10.1016/j.tet.2009.01.105
Cao, Y., Yoshikawa, N., and Taniguchi, S. (2010). Microwave heating behaviors of Si substrate materials in a single-mode cavity. Mater. Chem. Phys. 124, 900–903. doi:10.1016/j.matchemphys.2010.08.004
Che, P., Peng, L., Duan, X., Chen, Q., and Liang, X. (2004). Microwave absorption enhancement and complex permittivity and permeability of Fe encapsulated within carbon nanotubes. Adv. Mater. 16, 401–405. doi:10.1002/adma.200306460
Chen, S., Sheng, W., Cao, F., and Lu, Y. (2012). Microfibrous entrapment of Ni/Al2O3 for dry reforming of methane: heat/mass transfer enhancement towards carbon resistance and conversion promotion. Int. J. Hydrogen Energy 37, 18021–18030. doi:10.1016/j.ijhydene.2012.09.080
Cheng, R., and Agrawal, D. (2002). Radically different effects on materials by separated microwave electric and magnetic fields. Mater. Res. Innovations 5, 170–177. doi:10.1007/s10019-002-8642-6
Cheng, R., Roy, R., and Agrawal, D. (2001). Experimental proof of major role of magnetic field losses in microwave heating of metal and metallic composites. J. Mater. Sci. Lett. 20, 1561–1563. doi:10.1023/a:1017900214477
Conti, J., Holtberg, P., Diefenderfer, J., LaRose, A., Turnure, J. T., and Westfall, L. (2016). International energy outlook 2016 with projections to 2040. Washington, DC United States: USDOE Energy Information Administration EIA.
Das, S., Shah, M., Gupta, R. K., and Bordoloi, A. (2019). Enhanced dry methane reforming over Ru decorated mesoporous silica and its kinetic study. J. CO2 Util. 29, 240–253. doi:10.1016/j.jcou.2018.12.016
Domínguez, F., Fernández, Y., Fidalgo, B., Pis, J. J., and Menéndez, J. A. (2007). Biogas to syngas by microwave-assisted dry reforming in the presence of char. Energy Fuels 21, 2066–2071. doi:10.1021/ef070101j
Durka, V. G., Van Gerven, T., and Stankiewicz, A. (2009). Microwaves in heterogeneous gas-phase catalysis: experimental and numerical approaches. Chem. Eng. Technol. 32, 1301–1312. doi:10.1002/ceat.200900207
Durka, S., Gerven, V., Van Gerven, T., and Stankiewicz, A. I. (2011). Microwave-activated methanol steam reforming for hydrogen production. Int. J. Hydrogen Energy 36, 12843–12852. doi:10.1016/j.ijhydene.2011.07.009
Elvidge, B., Bazilian, M. D., Zhizhin, M., Ghosh, T., Baugh, K., and Hsu, F. C. (2018). The potential role of natural gas flaring in meeting greenhouse gas mitigation targets. Energy strategy Rev. 20, 156–162. doi:10.1016/j.esr.2017.12.012
Fan, A., and Bhatia, S. (2009). Catalytic technology for carbon dioxide reforming of methane to synthesis gas. ChemCatChem 1, 192–208. doi:10.1002/cctc.200900025
Fidalgo, M., and Menéndez, J. (2012). Study of energy consumption in a laboratory pilot plant for the microwave-assisted CO2 reforming of CH4. Fuel Process. Technol. 95, 55–61. doi:10.1016/j.fuproc.2011.11.012
Fidalgo, D., Dominguez, A., Pis, J., and Menendez, J. (2008). Microwave-assisted dry reforming of methane. Int. J. Hydrogen Energy 33, 4337–4344. doi:10.1016/j.ijhydene.2008.05.056
Fidalgo, A., Arenillas, A., and Menéndez, J. (2010). Influence of porosity and surface groups on the catalytic activity of carbon materials for the microwave-assisted CO2 reforming of CH4. Fuel 89, 4002–4007. doi:10.1016/j.fuel.2010.06.015
Fidalgo, A., Arenillas, A., and Menéndez, J. (2011). Mixtures of carbon and Ni/Al2O3 as catalysts for the microwave-assisted CO2 reforming of CH4. Fuel Process. Technol. 92, 1531–1536. doi:10.1016/j.fuproc.2011.03.015
Fleming Crim, F. (2008). Chemical dynamics of vibrationally excited molecules: controlling reactions in gases and on surfaces, Proc. Natl. Acad. Sci. U. S. A. 105 (35), 12654–12661. doi:10.1073/pnas.0803010105
Foppa, M., Margossian, T., Kim, S. M., Müller, C., Copéret, C., Larmier, K., et al. (2017). Contrasting the role of Ni/Al2O3 interfaces in water–gas shift and dry reforming of methane. J. Am. Chem. Soc. 139, 17128–17139. doi:10.1021/jacs.7b08984
Gadalla, B., and Bower, B. (1988). The role of catalyst support on the activity of nickel for reforming methane with CO2. Chem. Eng. Sci. 43, 3049–3062. doi:10.1016/0009-2509(88)80058-7
Gangurde, S., Sturm, G. S. J., Devadiga, T. J., Stankiewicz, A. I., and Stefanidis, G. D. (2017). Complexity and challenges in noncontact high temperature measurements in microwave-assisted catalytic reactors. Industrial Eng. Chem. Res. 56, 13379–13391. doi:10.1021/acs.iecr.7b02091
Gangurde, L. S., Sturm, G. S. J., Valero-Romero, M. J., Mallada, R., Santamaria, J., and Stankiewicz, A. I. (2018). Synthesis, characterization, and application of ruthenium-doped SrTiO3 perovskite catalysts for microwave-assisted methane dry reforming. Chem. Eng. Processing-Process Intensif. 127, 178–190. doi:10.1016/j.cep.2018.03.024
Grant, H., Gabriel, S., H. Grant, E., H. Grant, E., S. J. Halstead, B., and Michael P. Mingos, D. (1998). Dielectric parameters relevant to microwave dielectric heating. Chem. Soc. Rev. 27, 213–224. doi:10.1039/a827213z
Green, C., and Green, M. (1992). Partial oxidation of methane to synthesis gas, and carbon dioxide as an oxidising agent for methane conversion. Catal. Today 13, 417–426. doi:10.1016/0920-5861(92)80167-l
Gude, P., Patil, P., Martinez-Guerra, E., Deng, S., and Nirmalakhandan, N. (2013). Microwave energy potential for biodiesel production. Sustain. Chem. Process. 1, 5. doi:10.1186/2043-7129-1-5
Guler, D., Dogu, T., and Varisli, D. (2017). Hydrogen production over molybdenum loaded mesoporous carbon catalysts in microwave heated reactor system. Appl. Catal. B Environ. 219, 173–182. doi:10.1016/j.apcatb.2017.07.043
Gündüz, D. (2015). Hydrogen by steam reforming of ethanol over Co–Mg incorporated novel mesoporous alumina catalysts in tubular and microwave reactors. Appl. Catal. B Environ. 168, 497–508. doi:10.1016/j.apcatb.2015.01.006
Hamzehlouia, J., Jaffer, S. A., and Chaouki, J. (2018). Microwave heating-assisted catalytic dry reforming of methane to syngas. Sci. Rep. 8, 8940. doi:10.1038/s41598-018-27381-6
Han, L., Liang, Y., Qin, L., Wang, Y., Wang, H., Yu, F., et al. (2020). The reaction mechanism and its kinetic model of CO2 reforming with CH4 over Ni-Mg 15@HC catalyst. Catal. Lett. 150, 1479–1488. doi:10.1007/s10562-019-03052-7
Haneishi, T., Maitani, M. M., Suzuki, E., Fujii, S., and Wada, Y. (2017). Electromagnetic and heat-transfer simulation of the catalytic dehydrogenation of ethylbenzene under microwave irradiation. Industrial Eng. Chem. Res. 56, 7685–7692. doi:10.1021/acs.iecr.7b01413
Hassan, J., Hitam, C. N. C., Vo, D. V. N., and Nabgan, W. (2020). Biofuels and renewable chemicals production by catalytic pyrolysis of cellulose: a review. Environ. Chem. Lett. 18, 1625–1648. doi:10.1007/s10311-020-01040-7
He, Li, Li, M., Li, W. C., Xu, W., Wang, Y., Wang, Y. B., et al. (2021). Robust and coke-free Ni catalyst stabilized by 1–2 nm-thick multielement oxide for methane dry reforming. ACS Catal. 11, 12409–12416. doi:10.1021/acscatal.1c02995
Hoogenboom, S., and Schubert, U. S. (2007). Microwave-assisted polymer synthesis: recent developments in a rapidly expanding field of research. Macromol. Rapid Commun. 28, 368–386. doi:10.1002/marc.200600749
Horikoshi, S., Sumi, T., and Serpone, N. (2012). Unusual effect of the magnetic field component of the microwave radiation on aqueous electrolyte solutions. J. Microw. Power Electromagn. Energy 46, 215–228. doi:10.1080/08327823.2012.11689838
Huang, Z., Zhou, T., Zhang, J., Zhang, Y., Wu, Y., Wang, Y., et al. (2024). Competing CO and HCOOH pathways in CO2 electroreduction. ChemCatChem 16, e202400504. doi:10.1002/cctc.202400504
Hussien, P., and Polychronopoulou, K. (2022). A review on the different aspects and challenges of the dry reforming of methane (DRM) reaction. Nanomaterials 12, 3400. doi:10.3390/nano12193400
Hwang, Ku, Roh, M. I., and Lee, K. Y. (2013). Optimal design of liquefaction cycles of liquefied natural gas floating, production, storage, and offloading unit considering optimal synthesis. Industrial Eng. Chem. Res. 52, 5341–5356. doi:10.1021/ie301913b
Jacob, C., Chia, L. H. L., and Boey, F. Y. C. (1995). Thermal and non-thermal interaction of microwave radiation with materials. J. Mater. Sci. 30, 5321–5327. doi:10.1007/bf00351541
Janezic, P. (2001). Blendell. Dielectric and conductor-loss characterization and measurements on electronic packaging materials. NIST Tech. note, 1520.
Jang, J., Jeong, D. W., Shim, J. O., Kim, H. M., Roh, H. S., Son, I. H., et al. (2016). Combined steam and carbon dioxide reforming of methane and side reactions: Thermodynamic equilibrium analysis and experimental application. Appl. energy 173, 80–91. doi:10.1016/j.apenergy.2016.04.006
Jang, S., Shim, J. O., Kim, H. M., Yoo, S. Y., and Roh, H. S. (2019). A review on dry reforming of methane in aspect of catalytic properties. Catal. Today 324, 15–26. doi:10.1016/j.cattod.2018.07.032
Jastrząb (2018) “Utilization of heat from High Temperature Reactors (HTR) for dry reforming of methane,” in E3S Web of Conferences. Paris: EDP Sciences.01016
Jiang, G., and Guo, H. (2016). Communication: enhanced dissociative chemisorption of CO2 via vibrational excitation. J. Chem. Phys. 144, 091101. doi:10.1063/1.4943002
Jie, Li, Li, W., Slocombe, D., Gao, Y., Banerjee, I., Gonzalez-Cortes, S., et al. (2020). Microwave-initiated catalytic deconstruction of plastic waste into hydrogen and high-value carbons. Nat. Catal. 3, 902–912. doi:10.1038/s41929-020-00518-5
Jones, L., Lelyveld, T., Mavrofidis, S., Kingman, S., and Miles, N. (2002). Microwave heating applications in environmental engineering—a review. Resour. Conserv. Recycl. 34, 75–90. doi:10.1016/s0921-3449(01)00088-x
Kawi, K., Kathiraser, Y., Ni, J., Oemar, U., Li, Z., and Saw, E. T. (2015). Progress in synthesis of highly active and stable nickel-based catalysts for carbon dioxide reforming of methane. ChemSusChem 8, 3556–3575. doi:10.1002/cssc.201500390
Khan, C., Challiwala, M. S., Prakash, A. V., and Elbashir, N. O. (2023). Conceptual modeling of a reactor bed of a nickel-copper bi-metallic catalyst for dry reforming of methane. Chem. Eng. Sci. 267, 118315. doi:10.1016/j.ces.2022.118315
Khoja, T., Tahir, M., and Amin, N. A. S. (2019). Recent developments in non-thermal catalytic DBD plasma reactor for dry reforming of methane. Energy Convers. Manag. 183, 529–560. doi:10.1016/j.enconman.2018.12.112
Kim, Y., Yang, D. R., Moon, D. J., and Ahn, B. S. (2014). The process design and simulation for the methanol production on the FPSO (floating production, storage and off-loading) system. Chem. Eng. Res. Des. 92, 931–940. doi:10.1016/j.cherd.2013.08.009
Kingman, J., Jackson, K., Bradshaw, S., Rowson, N., and Greenwood, R. (2004). An investigation into the influence of microwave treatment on mineral ore comminution. Powder Technol. 146, 176–184. doi:10.1016/j.powtec.2004.08.006
Komarneni, R., Roy, R., and Li, Q. (1992). Microwave-hydrothermal synthesis of ceramic powders. Mater. Res. Bull. 27, 1393–1405. doi:10.1016/0025-5408(92)90004-j
Kondratenko, M., Mul, G., Baltrusaitis, J., Larrazábal, G. O., and Pérez-Ramírez, J. (2013). Status and perspectives of CO2 conversion into fuels and chemicals by catalytic, photocatalytic and electrocatalytic processes. Energy Environ. Sci. 6, 3112–3135. doi:10.1039/c3ee41272e
Ku, S., Taube, A., and Ball, J. (2002). Productivity improvement through the use of industrial microwave technologies. Comput. Industrial Eng. Chem. Res. 42, 281–290. doi:10.1016/s0360-8352(02)00026-8
Kustov, T., Tarasov, A. L., Tkachenko, O. P., and Kapustin, G. I. (2017). Nickel–alumina catalysts in the reaction of carbon dioxide re-forming of methane under thermal and microwave heating. Industrial Eng. Chem. Res. 56, 13034–13039. doi:10.1021/acs.iecr.7b01254
la Hoz, De, Díaz-Ortiz, A., and Moreno, A. (2005). Microwaves in organic synthesis. Thermal and non-thermal microwave effects. Chem. Soc. Rev. 34, 164–178. doi:10.1039/b411438h
Li, X. (2005). Diversification and localization of energy systems for sustainable development and energy security. Energy policy 33, 2237–2243. doi:10.1016/j.enpol.2004.05.002
Li, P. (2017). Thermodynamic analysis of waste heat recovery of molten blast furnace slag. Int. J. Hydrogen Energy 42, 9688–9695. doi:10.1016/j.ijhydene.2016.12.135
Li, J., Jiang, X., Wang, H., Wang, J., Song, Z., Zhao, X., et al. (2017). Methane dry and mixed reforming on the mixture of bio-char and nickel-based catalyst with microwave assistance. J. Anal. Appl. Pyrolysis 125, 318–327. doi:10.1016/j.jaap.2017.03.009
Li, C., Chen, J., Yan, K., Qin, X., Feng, T., Wang, J., et al. (2018a). Methane dry reforming with microwave heating over carbon-based catalyst obtained by agriculture residues pyrolysis. J. CO2 Util. 28, 41–49. doi:10.1016/j.jcou.2018.09.010
Li, Y., Yang, Z., Chen, J., Qin, X., Jiang, X., Wang, F., et al. (2018b). Performance of bio-char and energy analysis on CH4 combined reforming by CO2 and H2O into syngas production with assistance of microwave. Fuel 215, 655–664. doi:10.1016/j.fuel.2017.11.107
Li, Y., Yan, K., Chen, J., Feng, T., Wang, F., Wang, J., et al. (2019). Fe-rich biomass derived char for microwave-assisted methane reforming with carbon dioxide. Sci. Total Environ. 657, 1357–1367. doi:10.1016/j.scitotenv.2018.12.097
Li, T., Tao, J., Yan, B., Cheng, K., Chen, G., and Hu, J. (2020a). Microwave reforming with char-supported Nickel-Cerium catalysts: a potential approach for thorough conversion of biomass tar model compound. Appl. energy 261, 114375. doi:10.1016/j.apenergy.2019.114375
Li, C., Chen, J., Zhang, Q., Yang, Z., Sun, Y., and Zou, G. (2020b). Methane dry reforming over activated carbon supported Ni-catalysts prepared by solid phase synthesis. J. Clean. Prod. 274, 122256. doi:10.1016/j.jclepro.2020.122256
Li, Xu, Xu, W., Deng, J., and Zhou, J. (2021). Coke-resistant Ni–Co/ZrO2–CaO-based microwave catalyst for highly effective dry reforming of methane by microwave catalysis. Industrial Eng. Chem. Res. 60, 17458–17468. doi:10.1021/acs.iecr.1c03164
Li, G., Gao, Y., Chen, J., and Jia, H. (2022). An in situ defect engineering approach for light-driven methane dry reforming over atomically distributed nickel. Cell Rep. Phys. Sci. 3, 101127. doi:10.1016/j.xcrp.2022.101127
Li, L., Luo, C., Robinson, B., Hu, J., Yang, J., and Wang, Y. (2024). Production of syngas at lower temperatures through microwave-enhanced dry reforming of methane. Int. J. Hydrogen Energy 81, 187–192. doi:10.1016/j.ijhydene.2024.07.254
Liao, H., and Horng, R. F. (2017). Experimental study of syngas production from methane dry reforming with heat recovery strategy. Int. J. Hydrogen Energy 42, 25213–25224. doi:10.1016/j.ijhydene.2017.01.238
Lim, C., and Chun, Y. N. (2017). Biogas to syngas by microwave-assisted reforming in the presence of char. Energy Fuels 31, 13761–13768. doi:10.1021/acs.energyfuels.7b02799
Liu, W., and Chen, J. G. (2020). Review of plasma-assisted catalysis for selective generation of oxygenates from CO2 and CH4. ACS Catal. 10, 2855–2871. doi:10.1021/acscatal.9b04811
Liu, Pu, Pu, Y., Hu, X., Dong, Y., Wu, W., Hu, C., et al. (2023). Global declines of offshore gas flaring inadequate to meet the 2030 goal. Nat. Sustain. 6, 1095–1102. doi:10.1038/s41893-023-01125-5
Lovell, S., Scott, J., and Amal, R. (2015). Ni-SiO2 catalysts for the carbon dioxide reforming of methane: varying support properties by flame spray pyrolysis. Molecules 20, 4594–4609. doi:10.3390/molecules20034594
Marinho, T., Toniolo, F. S., Noronha, F. B., Epron, F., Duprez, D., and Bion, N. (2021). Highly active and stable Ni dispersed on mesoporous CeO2-Al2O3 catalysts for production of syngas by dry reforming of methane. Appl. Catal. B Environ. 281, 119459. doi:10.1016/j.apcatb.2020.119459
Marin, C. M., Popczun, E. J., Nguyen-Phan, T.-D., Tafen, D. N., Alfonso, D., and Waluyo, I. (2021). Designing perovskite catalysts for controlled active-site exsolution in the microwave dry reforming of methane. Appl. Catal. B Environ. 284, 119711. doi:10.1016/j.apcatb.2020.119711
Markewitz, K., Kuckshinrichs, W., Leitner, W., Linssen, J., Zapp, P., Bongartz, R., et al. (2012). Worldwide innovations in the development of carbon capture technologies and the utilization of CO2. Energy Environ. Sci. 5, 7281–7305. doi:10.1039/c2ee03403dLeitner
Menéndez, A., Arenillas, A., Fidalgo, B., Fernández, Y., Zubizarreta, L., Calvo, E., et al. (2010). Microwave heating processes involving carbon materials. Fuel Process. Technol. 91, 1–8. doi:10.1016/j.fuproc.2009.08.021
Meredith, E. (1998). Handbook of industrial microwave heating. London: IET, The Institution of Electrical Engineers.
Mo, L., Leong, K. K. M., and Kawi, S. (2014). A highly dispersed and anti-coking Ni–La2O3/SiO2 catalyst for syngas production from dry carbon dioxide reforming of methane. Catal. Sci. Technol. 4, 2107–2114. doi:10.1039/c3cy00869j
Mortensen, D., and Dybkjær, I. (2015). Industrial scale experience on steam reforming of CO2-rich gas. Appl. Catal. A General 495, 141–151. doi:10.1016/j.apcata.2015.02.022
Motasemi, A. (2013). A review on the microwave-assisted pyrolysis technique. Renew. Sustain. Energy Rev. 28, 317–330. doi:10.1016/j.rser.2013.08.008
Nguyen, S., Sunarso, J., Pham, G. H., Phan, C., and Liu, S. (2020a). Microwave-assisted catalytic methane reforming: a review. Appl. Catal. A General 599, 117620. doi:10.1016/j.apcata.2020.117620
Nguyen, P., Pham, G. H., Tade, M., Phan, C., Vagnoni, R., and Liu, S. (2020b). Microwave-assisted dry and bi-reforming of methane over M–Mo/TiO2 (M= Co, Cu) bimetallic catalysts. Energy Fuels 34, 7284–7294. doi:10.1021/acs.energyfuels.0c00757
Nightingale (2001). Interfacial phenomena in microwave sintering. Ionics 7, 327–331. doi:10.1007/bf02373566
Nikoo, A., and Amin, N. (2011). Thermodynamic analysis of carbon dioxide reforming of methane in view of solid carbon formation. Fuel Process. Technol. 92, 678–691. doi:10.1016/j.fuproc.2010.11.027
Nozaki, H., and Okazaki, K. (2004). Partial oxidation of methane using a microscale non-equilibrium plasma reactor. Catal. Today 98, 607–616. doi:10.1016/j.cattod.2004.09.053
Odedairo, Ma, Ma, J., Chen, J., Wang, S., and Zhu, Z. (2016). Influences of doping Cr/Fe/Ta on the performance of Ni/CeO2 catalyst under microwave irradiation in dry reforming of CH4. J. Solid State Chem. 233, 166–177. doi:10.1016/j.jssc.2015.10.025
Olah, G., Czaun, M., and Prakash, G. K. S. (2013). Bi-reforming of methane from any source with steam and carbon dioxide exclusively to metgas (CO–2H2) for methanol and hydrocarbon synthesis. J. Am. Chem. Soc. 135, 648–650. doi:10.1021/ja311796n
Olah, G., Czaun, M., Mathew, T., May, R. B., and Prakash, G. K. S. (2015). Single step bi-reforming and oxidative bi-reforming of methane (natural gas) with steam and carbon dioxide to metgas (CO-2H2) for methanol synthesis: self-sufficient effective and exclusive oxygenation of methane to methanol with oxygen. J. Am. Chem. Soc. 137, 8720–8729. doi:10.1021/jacs.5b02029
Olowoyo, S., and Zheng, Y. (2024). Syngas production via microwave-assisted Dry Reforming of Methane over NiFe/MgAl2O4 alloy catalyst. Chem. Eng. Processing-Process Intensif. 203, 109899. doi:10.1016/j.cep.2024.109899
Pakhare, S., and Spivey, J. (2014). A review of dry (CO2) reforming of methane over noble metal catalysts. Chem. Soc. Rev. 43, 7813–7837. doi:10.1039/c3cs60395d
Palma, B., Barba, D., Cortese, M., Martino, M., Renda, S., and Meloni, E. (2020). Microwaves and heterogeneous catalysis: a review on selected catalytic processes. Catalysts 10, 246. doi:10.3390/catal10020246
Park, H., Heo, I., and Chang, T. S. (2019). Dry reforming of methane over Ni-substituted CaZrNiOx catalyst prepared by the homogeneous deposition method. Catal. Commun. 120, 1–5. doi:10.1016/j.catcom.2018.11.006
Peng, Z., Zhou, J., Xu, W., You, Z., Long, W., Xiang, M., et al. (2017). Microwave irradiation-selective catalytic reduction of NO to N2 by activated carbon at low temperature. Energy Fuels 31, 7344–7351. doi:10.1021/acs.energyfuels.7b01102
Pham, Ro, Ro, K. S., Chen, L., Mahajan, D., Siang, T. J., Ashik, U. P. M., et al. (2020). Microwave-assisted dry reforming of methane for syngas production: a review. Environ. Chem. Lett. 18, 1987–2019. doi:10.1007/s10311-020-01055-0
Rao, W., Wang, K., Cao, Y., Feng, Y., Huang, Z., Chen, Y., et al. (2023). Light-reinforced key intermediate for anticoking to boost highly durable methane dry reforming over single atom Ni active sites on CeO2. J. Am. Chem. Soc. 145, 24625–24635. doi:10.1021/jacs.3c07077
Rosa, V., Veronesi, P., Casagrande, A., and Leonelli, C. (2016). Microwave ignition of the combustion synthesis of aluminides and field-related effects. J. Alloys Compd. 657, 59–67. doi:10.1016/j.jallcom.2015.10.044
Rossi, F., Faria, M. G., Pereira, M. S., and Ataíde, C. H. (2017). Kinetics of microwave heating and drying of drilling fluids and drill cuttings. Dry. Technol. 35, 1130–1140. doi:10.1080/07373937.2016.1233425
Roy, P., Grimes, C., Cheng, J., and Agrawal, D. (2002). Major phase transformations and magnetic property changes caused by electromagnetic fields at microwave frequencies. J. Mater. Res. 17, 3008–3011. doi:10.1557/jmr.2002.0437
Saeidi, A., and Amin, N. A. S. (2014). Rahimpour. Hydrogenation of CO2 to value-added products—a review and potential future developments. J. CO2 Util. 5, 66–81. doi:10.1016/j.jcou.2013.12.005
Sarıyer, B., Bozdağ, A. A., Sezgi, N. A., and Doğu, T. (2019). Performance comparison of microwave and conventionally heated reactors for sorption enhanced reforming of ethanol over Ni impregnated SBA-15. Chem. Eng. J. 377, 120138. doi:10.1016/j.cej.2018.10.075
Schwab, M., Schunk, S. A., Behrens, A., and Schödel, N. (2015). Dry reforming and reverse water gas shift: alternatives for syngas production? Chem. Ing. Tech. 87, 347–353. doi:10.1002/cite.201400111
Shah, G., and Gardner, T. H. (2014). Dry reforming of hydrocarbon feedstocks. Catal. Rev. 56, 476–536. doi:10.1080/01614940.2014.946848
Sharifvaghefi, S., Shirani, B., Eic, M., and Zheng, Y. (2019). Application of microwave in hydrogen production from methane dry reforming: comparison between the conventional and microwave-assisted catalytic reforming on improving the energy efficiency. Catalysts 9, 618. doi:10.3390/catal9070618
Shi, W., Ge, X., Deng, S., Chen, B., and Shen, J. (2021). A review of different catalytic systems for dry reforming of methane: conventional catalysis-alone and plasma-catalytic system. J. CO2 Util. 46, 101462. doi:10.1016/j.jcou.2021.101462
Shi, H., Han, K., and Wang, F. (2022). Wang. Ni–Cu alloy nanoparticles confined by physical encapsulation with SiO2 and chemical metal–support interaction with CeO2 for methane dry reforming. Inorg. Chem. 61, 15619–15628. doi:10.1021/acs.inorgchem.2c02466
Shi, W., Wang, L., Wu, M., and Wang, F. (2023). Order-of-magnitude increase in rate of methane dry reforming over Ni/CeO2-SiC catalysts by microwave catalysis. Appl. Catal. B Environ. 337, 122927. doi:10.1016/j.apcatb.2023.122927
Shi, T., Tian, X., Deng, Z., and Wang, F. (2025). Microwave catalytic dry reforming of methane over Ni/SiC catalysts for efficient syngas production. Fuel 388, 134574. doi:10.1016/j.fuel.2025.134574
Snoeckx, B., and Bogaerts, A. (2017). Plasma technology–a novel solution for CO2 conversion? Chem. Soc. Rev. 46, 5805–5863. doi:10.1039/c6cs00066e
Snoeckx, A., Aerts, R., Tu, X., and Bogaerts, A. (2013). Plasma-based dry reforming: a computational study ranging from the nanoseconds to seconds time scale. J. Phys. Chem. C 117, 4957–4970. doi:10.1021/jp311912b
Sokolov, K., Pohl, M. M., Barkschat, A., and Rodemerck, U. (2012). Stable low-temperature dry reforming of methane over mesoporous La2O3-ZrO2 supported Ni catalyst. Appl. Catal. B Environ. 113, 19–30. doi:10.1016/j.apcatb.2011.09.035
Sun, W., Wang, W., and Yue, Q. (2016). Review on microwave-Matter interaction fundamentals and efficient microwave-associated heating strategies. Materials 9, 231. doi:10.3390/ma9040231
Tan, W., Wang, S., Li, L., Meng, B., Chen, J., Yang, Z., et al. (2019). Application of microwave heating for methane dry reforming catalyzed by activated carbon. Chem. Eng. Processing-Process Intensif. 145, 107662. doi:10.1016/j.cep.2019.107662
Tan, Li, Li, X., Wang, Y., Ji, X., Zou, L., Yan, H., et al. (2025). From waste to syngas: integrated numerical insights into methane conversion and crystallization via slag waste heat. Int. J. Heat Mass Transf. 251, 127387. doi:10.1016/j.ijheatmasstransfer.2025.127387
Tanksale, B., Beltramini, J. N., and Lu, G. M. (2010). A review of catalytic hydrogen production processes from biomass. Renew. Sustain. Energy Rev. 14, 166–182. doi:10.1016/j.rser.2009.08.010
Verma, S., and Samanta, S. K. (2018). Microwave-enhanced advanced oxidation processes for the degradation of dyes in water. Environ. Chem. Lett. 16, 969–1007. doi:10.1007/s10311-018-0739-2
Wang, Lu (1996). Carbon dioxide reforming of methane to Produce synthesis gas over metal-supported Catalysts: state of the art. Energy Fuels 10, 896–904. doi:10.1021/ef950227t
Wang, Y., Yao, L., Wang, S., Mao, D., and Hu, C. (2018). Low-temperature catalytic CO2 dry reforming of methane on Ni-based catalysts: a review. Fuel Process. Technol. 169, 199–206. doi:10.1016/j.fuproc.2017.10.007
Wang, S., Sourav, S., Yu, K., Kwak, Y., Zheng, W., and Vlachos, D. G. (2023a). Green syngas production by microwave-assisted dry reforming of methane on doped ceria catalysts. ACS Sustain. Chem. and Eng. 11, 13353–13362. doi:10.1021/acssuschemeng.3c02693
Wang, Pu, Pu, Z., Shi, Y., Wu, M., Shi, W., and Wang, F. (2023b). Photothermal methane dry reforming catalyzed by multifunctional (Ni–Cu/CeO2)@ SiO2 catalyst. ACS Sustain. Chem. Eng. 11, 17384–17399. doi:10.1021/acssuschemeng.3c05248
Wang, Z., Zhou, F., Lv, B., Yu, J., Zhang, J., Zhang, Y., et al. (2025). Unveiling pH-dependent mechanistic shifts in Cu2O-catalyzed nitrate electroreduction for selective ammonia synthesis. Adv. Sustain. Syst., e00211. doi:10.1002/adsu.202500211
Wen, Z., Zhang, F., and Liu, Z. (2011). Investigation on microwave absorption properties for multiwalled carbon nanotubes/Fe/Co/Ni nanopowders as lightweight absorbers. J. Phys. Chem. C 115, 14025–14030. doi:10.1021/jp202078p
Westphal, S. (2025). Dielectric constant and loss data, air Force materials laboratory. Air Force Syst. Command1972.
Wiesbrock, H., Hoogenboom, R., and Schubert, U. S. (2004). Microwave-assisted polymer synthesis: state-of-the-art and future perspectives. Macromol. Rapid Commun. 25, 1739–1764. doi:10.1002/marc.200400313
Xu, W., Hu, X., Xiang, M., Luo, M., Peng, R., and Lan, L. (2017). Highly effective direct decomposition of H2S into H2 and S by microwave catalysis over CoS-MoS2/γ-Al2O3 microwave catalysts. Chem. Eng. J. 326, 1020–1029. doi:10.1016/j.cej.2017.06.027
Yabe, Y., Yamada, K., Murakami, K., Toko, K., Ito, K., Higo, T., et al. (2019). Role of electric field and surface protonics on low-temperature catalytic dry reforming of methane. ACS Sustain. Chem. Eng. 7, 5690–5697. doi:10.1021/acssuschemeng.8b04727
Yoon, Y., You, H. M., Kim, H. J., Curnan, M. T., Kim, K., and Han, J. W. (2022). Computational catalyst design for dry reforming of methane: a review. Energy Fuels 36, 9844–9865. doi:10.1021/acs.energyfuels.2c01776
Yu, C., Curcic, I., Gabriel, J., and Tsang, S. (2008). Recent advances in CO2 capture and utilization. ChemSusChem 1, 893–899. doi:10.1002/cssc.200800169
Zhao, W., Wang, D., Zhang, L., He, M., Ma, W., and Zhao, S. (2023). Microwave-enhanced hydrogen production: a review. RSC Adv. 13, 15261–15273. doi:10.1039/d3ra01898a
Zhang, H., and Hayward, D. O. (2002). Mingos. Dielectric properties of MoS2 and Pt catalysts: effects of temperature and microwave frequency. Catal. Lett. 84, 225–233. doi:10.1023/a:1021488222243
Zhang, H., and Hayward, D. O. (2006). Applications of microwave dielectric heating in environment-related heterogeneous gas-phase catalytic systems. Inorganica Chim. Acta 359, 3421–3433. doi:10.1016/j.ica.2006.01.037
Zhang, H., Hayward, D. O., Lee, C., and Mingos, D. P. (2001). Microwave assisted catalytic reduction of sulfur dioxide with methane over MoS2 catalysts. Appl. Catal. B Environ. 33, 137–148. doi:10.1016/s0926-3373(01)00171-0
Zhang, L., Lee, C. S. M., Mingos, D. M. P., and Hayward, D. O. (2003). Carbon dioxide reforming of methane with Pt catalysts using microwave dielectric heating. Catal. Lett. 88, 129–139. doi:10.1023/a:1024049403422
Zhang, S., Song, Z., Zhu, J., Liu, L., Sun, J., Zhao, X., et al. (2018a). Process of CH4-CO2 reforming over Fe/SiC catalyst under microwave irradiation. Sci. Total Environ. 639, 1148–1155. doi:10.1016/j.scitotenv.2018.04.364
Zhang, S., Song, Z., Zhu, J., Sun, J., Zhao, X., Mao, Y., et al. (2018b). Factors influencing CH4CO2 reforming reaction over Fe catalyst supported on foam ceramics under microwave irradiation. Int. J. Hydrogen Energy 43, 9495–9502. doi:10.1016/j.ijhydene.2018.03.171
Zhang, Z., Zhang, X., Song, Z., Chen, H., Zhao, X., Sun, J., et al. (2022). Fe/HZSM-5 synergizes with biomass pyrolysis carbon to reform CH4–CO2 to syngas in microwave field. Int. J. Hydrogen Energy 47, 11153–11163. doi:10.1016/j.ijhydene.2022.01.158
Zhang, G., Gao, Y., Mao, Y., Wang, W., Sun, J., Song, Z., et al. (2023a). Enhanced dry reforming of methane by microwave-mediated confined catalysis over Ni-La/AC catalyst. Chem. Eng. J. 451, 138616. doi:10.1016/j.cej.2022.138616
Zhang, Z., Zhang, X., Song, Z., Li, X., Zhao, X., Sun, J., et al. (2023b). Promotion of microwave discharge over carbon catalysts for CO2 reforming of CH4 to syngas. Fuel 331, 125914. doi:10.1016/j.fuel.2022.125914
Zhang, G., Gao, Y., Mao, Y., Jin, Y., Wang, W., Sun, J., et al. (2023c). Hierarchical core–shell Ni@ C-NCNTs nanocomposites tailored for microwave-induced dry reforming of methane process. J. Mater. Chem. A 11, 21908–21926. doi:10.1039/d3ta05379b
Zhang, G., Gao, Y., Wang, W., Song, Z., and Mao, Y. (2025). Effect of promoters on the syngas production in the microwave-enhanced methane dry reforming over Ni-x/AC (x= Mg, Ca, La, Ce) catalysts. J. CO2 Util. 95, 103064. doi:10.1016/j.jcou.2025.103064
Zhao, G., Guo, X., Shi, R., Waterhouse, G. I. N., Zhang, X., Dai, Q., et al. (2022). NiFe nanoalloys derived from layered double hydroxides for photothermal synergistic reforming of CH4 with CO2. Adv. Funct. Mater. 32, 2204056. doi:10.1002/adfm.202204056
Keywords: dry reforming of methane, syngas, catalysts, microwave-assisted, sintering
Citation: Kuang S (2025) Recent advances in microwave-assisted dry reforming of methane: catalyst, performance, and fundamentals. Front. Chem. 13:1681282. doi: 10.3389/fchem.2025.1681282
Received: 07 August 2025; Accepted: 01 September 2025;
Published: 17 September 2025.
Edited by:
Xiulin Yang, Guangxi Normal University, ChinaCopyright © 2025 Kuang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Siyu Kuang, a3VhbmdzeTExMjZAb3V0bG9vay5jb20=
 Siyu Kuang
Siyu Kuang