- 1Department of Clinical Nutrition, Xinhua Hospital Affiliated to Shanghai Jiao Tong University School of Medicine, Shanghai, China
- 2School of Public Health and Primary Care, The Chinese University of Hong Kong, Shatin, Hong Kong SAR, China
- 3Shanghai Institute for Pediatric Research, Shanghai, China
Background: Hyperuricemia has been increasing among children with obesity in recent years. However, few studies in such a study group had explored the relationship between obesity-anthropometric indexes and hyperuricemia. This study aimed to examine the associations between hyperuricemia and different body components in children and adolescents with obesity, and further explore gender differences in these associations.
Methods: In this cross-sectional study, a total of 271 obese children and adolescents (153 boys and 118 girls) aged 6–17 years were recruited from Shanghai Xinhua Hospital. Data about basic information, anthropometric assessments, body composition, and laboratory tests of participants were collected.
Results: In this study, 73 boys (47.71%) and 57 girls (48.31%) were diagnosed to have hyperuricemia. The impacts of percentage of skeletal muscle (PSM) (OR = 1.221, P < 0.001) and skeletal muscle mass (SMM) (OR = 1.179, P < 0.001) on the risk of hyperuricemia was the largest, followed by hip circumference (HC) (OR = 1.109, P < 0.001), waist circumference (WC) (OR = 1.073, P < 0.001), and body fat mass (BFM) (OR = 1.056, P < 0.05) in whole sample, which was adjusted for age, gender and body mass index (BMI). After being stratified by gender, PSM (boys: OR = 1.309, P < 0.001) and SMM (boys: OR = 1.200, P < 0.001; girls: OR = 1.147, P < 0.05) were still the most predictors of hyperuricemia, followed by HC (boys: OR = 1.147, P < 0.001; girls: OR = 1.080, P < 0.05). WC showed a significant association with hyperuricemia only in boys (OR = 1.083, P < 0.05), while BFM showed no association with hyperuricemia in both gender groups after adjusting for age and BMI.
Conclusion: Our findings suggested that SMM was a stronger predictor of hyperuricemia than BFM in children and adolescents with obesity, especially in boys.
Introduction
The pace of the obese population has been increasing dramatically and has becoming a major public health problem. According to WHO, the prevalence of overweight and obesity increased from 4% to 18% worldwide among children and adolescents aged 5–17 years (1). The latest national estimates in China between 2015 and 2019 showed that the prevalence of overweight and obesity was 11.1% and 7.9% among 6–17-year Chinese children and adolescents, respectively (2). Obesity in childhood also raises many health concerns, such as the non-alcoholic fatty liver disease (3), type 2 diabetes mellitus (4), and cardiovascular disease (4, 5), which cause a huge burden for the health-care services and the whole society.
Serum uric acid (UA) is a weak organic acid with low solubility in water, which could be endogenously synthesized or derived from purines and their derivatives in the diet. During childhood and adolescence period, the UA level of individuals can be regulated by renal and endocrine function, diet and energy expenditure, and metabolism (6). Hyperuricemia (HUA) is caused by the imbalance of serum UA and is often accompanied by obesity and metabolic syndrome (7). In adults, children, and adolescents, HUA was found to be closely related to cardiovascular risk (8), hypertension (9), and insulin resistance (10). Therefore, it is important to further clarify the mechanism of obesity and HUA, which could help to subsequently design intervention to lower the risk of HUA and HUA-related diseases in children with obesity.
Previous studies have shown that HUA was closely associated with obesity in adults and children (10–12), but few studies focused on the associations between specific body components or anthropometric indices and the risk of HUA among the obese population. It was reported that several obesity measurements, such as body mass index (BMI), waist circumference (WC), and waist-to-height ratio (WHtR), were identified as predictors of HUA or gout in adults (13). Another obesity determiner is the U shape association between body fat percentage and prevalence of HUA among adolescent athletes (14). Despite the BMI being an overall evaluation of an individual’s body condition, it cannot differentiate the fat distribution from muscle mass, which means it cannot comprehensively assess the metabolic differences.
Some studies also suggested muscle mass and specific adiposity factors, such as WC, body fat mass (BFM), and visceral adipose tissue, were more clinically important predictors to evaluate an individual’s obesity condition and metabolic syndrome than BMI (15, 16). However, the relationships of these factors with the risk of HUA in obese children and adolescents have not been examined simultaneously to date. Therefore, identifying the associations between specific body components or anthropometric indices and HUA are particularly important for better designing interventions or treatments to ameliorate obesity and UA levels in children with obesity.
This study aimed to examine the relationship between different anthropometric indices or body components [BMI, WC, hip circumference (HC), skeletal muscle mass (SMM), and BFM] and the risk of HUA, as well as the magnitude of their effects on HUA in obese children and adolescents. Since gender differences in serum UA levels were found physiologically higher in men than in women among the children age group (17, 18), we also verified if the gender differences exist among these associations in this study. To our knowledge, this is the first epidemiologic study examining whether different body components are independently associated with HUA in children and adolescents with obesity.
Materials and Methods
Study Design and Participants
We conducted a retrospective study among children and adolescents, aged 6–17 years, with obesity who paid visits to the outpatient of Clinical Nutrition Department of Xinhua Hospital (Shanghai, China) from September 2012 to December 2019. The inclusion criteria for this study were the following: (a) children and adolescents aged 6–17 years old; (b) children and adolescents with obesity, wherein their BMI reached or exceeded the 95th percentile (P95) of children at the same age and gender according to the WHO standards (19). Participants with the following conditions were excluded: (a) currently receiving treatment for UA; (b) severe systemic, liver, and renal disorders; (c) taking medications that would impact obesity, such as stimulants or psychotropic drugs; (d) obesity caused by endocrine or genetic metabolic diseases. This study was approved by the Ethics Committee of Xinhua Hospital, School of Medicine, Shanghai Jiao Tong University (No. XHEC-D-2021-177).
Data Collections
Anthropometric Assessments
Anthropometric measurements, including the height, body weight, WC, and HC of subjects were obtained. A vertical measuring board (Seca 264, Seca, Germany) was used to measure the height of subjects with no shoes at the floor level. Subjects were weighed on a hospital scale with indoor clothing and without shoes. WC and HC measurements were taken using a tape measure by a professional, and subjects were required to stand naturally and look straight ahead. For the WC measurement, the tape was wrapped around the waist horizontally from the midpoint between the bottom of the lower edge of the ribs and the highest point of the anterior superior iliac crest. As for measuring HC, the professional chose the most prominent part of the buttocks and then wrapped it horizontally around the buttocks using the same tape. All measurements were recorded to the nearest 0.1 cm or kg.
Body mass index, waist-to-hip ratio (WHR), and WHtR were calculated as follows: BMI was calculated as the weight in kilograms, divided by the height in meters squared (kg/m2); the WHR was calculated by dividing the WC (cm) by the HC (cm); the WHtR was calculated by dividing the WC (cm) by the height (cm).
The BFM and SMM of subjects were measured using bioelectrical impedance analyses (BIA) by whole-body impedance (InBody720, Biospace Inc., South Korea). The percentage of body fat (PBF) and percentage of skeletal muscle (PSM) were calculated as follows: PBF (%) = (BFM in kg)/(weight in kg); PSM (%) = (SMM in kg)/(weight in kg).
Laboratory Test
Blood samples were collected in the morning by experienced nurses in Xinhua Hospital with the participants in fasting state and sent to the Clinical Laboratory Center for laboratory test of serum UA. The UA values (μmol/L) were detected by the uricase method using a Hitachi 7600 automatic biochemical analyzer (Hitachi, Tokyo, Japan).
The diagnosing criteria of HUA used the reference value for serum UA in Kubota’s study (18) since UA values are highly dependent upon age. The reference cut-off levels are as follows: 5.5 mg/dL for 4–6 years, 5.9 mg/dL for 7–9 years, 6.1 mg/dL for 10–12 years in both genders, and 7 mg/dL (men) and 6.2 mg/dL (women) for 13–15 years. Furthermore, HUA was defined as above the mean UA values + 2 SD in each age group (16). For better comparison, we converted the UA values in this study with μmol/L to mg/dL using a conversion rate of 16.81 (1 mg/dl = 59.48 μmol/L).
Statistical Analysis
Data were analyzed using SPSS version 22 software (SPSS Inc., Chicago, IL, United States). Continuous variables were presented as mean ± SD if they were normally distributed; otherwise, median (interquartile ranges) were used. For the categorical variables, they were presented as numbers and percentages (%). To compare the difference between gender, as well as between HUA and non-HUA, independent two-tailed t-tests were performed for normally distributed continuous data, Wilcoxon signed-rank tests were performed for non-normally distributed continuous variables, while the Chi-square test was used for categorical variables. Binary logistic regression models were used to evaluate the impacts of different body component variables on the risk of HUA. Model 1 was an unadjusted regression; Model 2 was a regression with age and sex-adjusted; Model 3 was a regression with BMI adjusted building upon model 2. All P-values were calculated by two-sided tests, and the significance level for each test was set at P < 0.05.
Results
Characteristics of Subjects
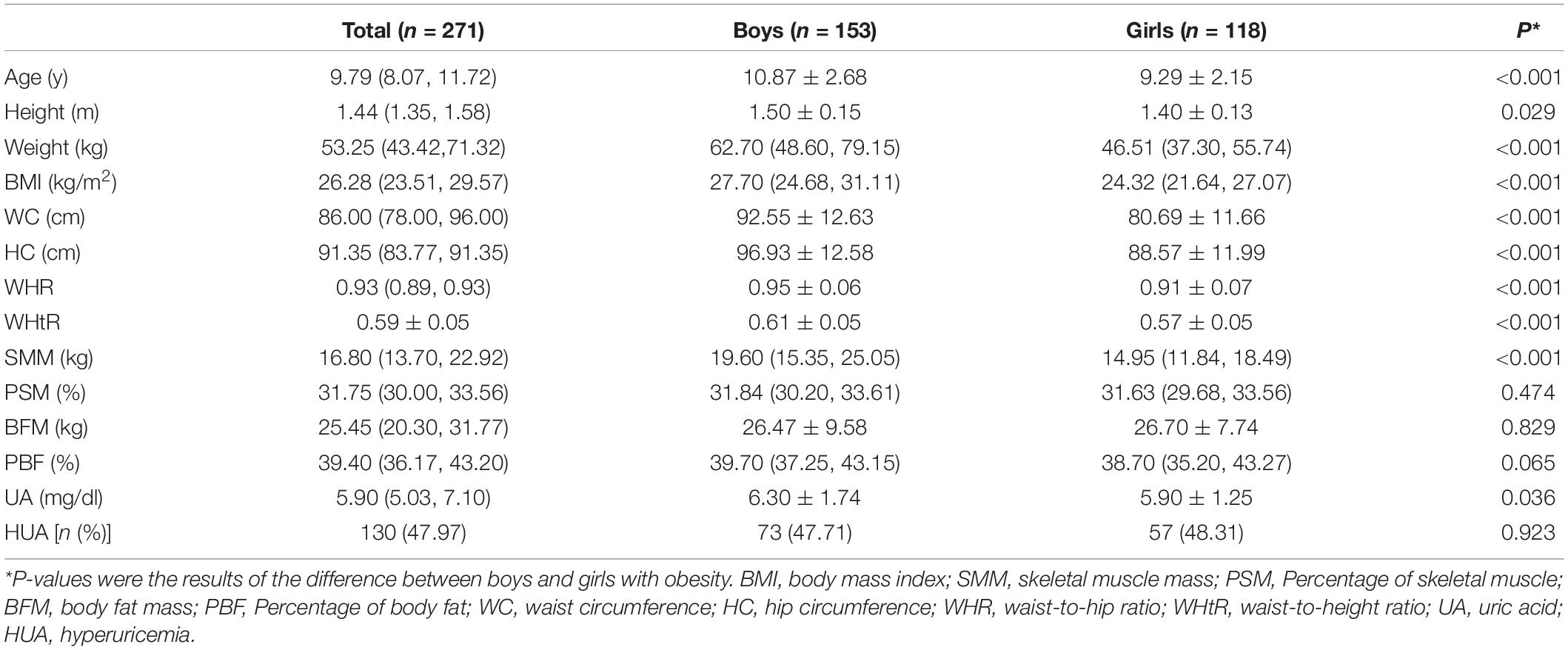
A total of 271 subjects consisted of 153 boys and 118 girls were included in this study. The total mean age was 9.79 years (10.87 years in boys and 9.29 years in girls). As presented in Table 1, boys had higher height, weight, WC, HC, BMI, WHR, and WHtR in comparison with girls (P < 0.05). Also, boys had higher SMM and UA levels than girls (P < 0.05) when it comes to body components and serum UA level, while no significant differences between PSM, BFM, and HUA were found between the two groups.
Comparison Between Hyperuricemia and Non-Hyperuricemia Groups
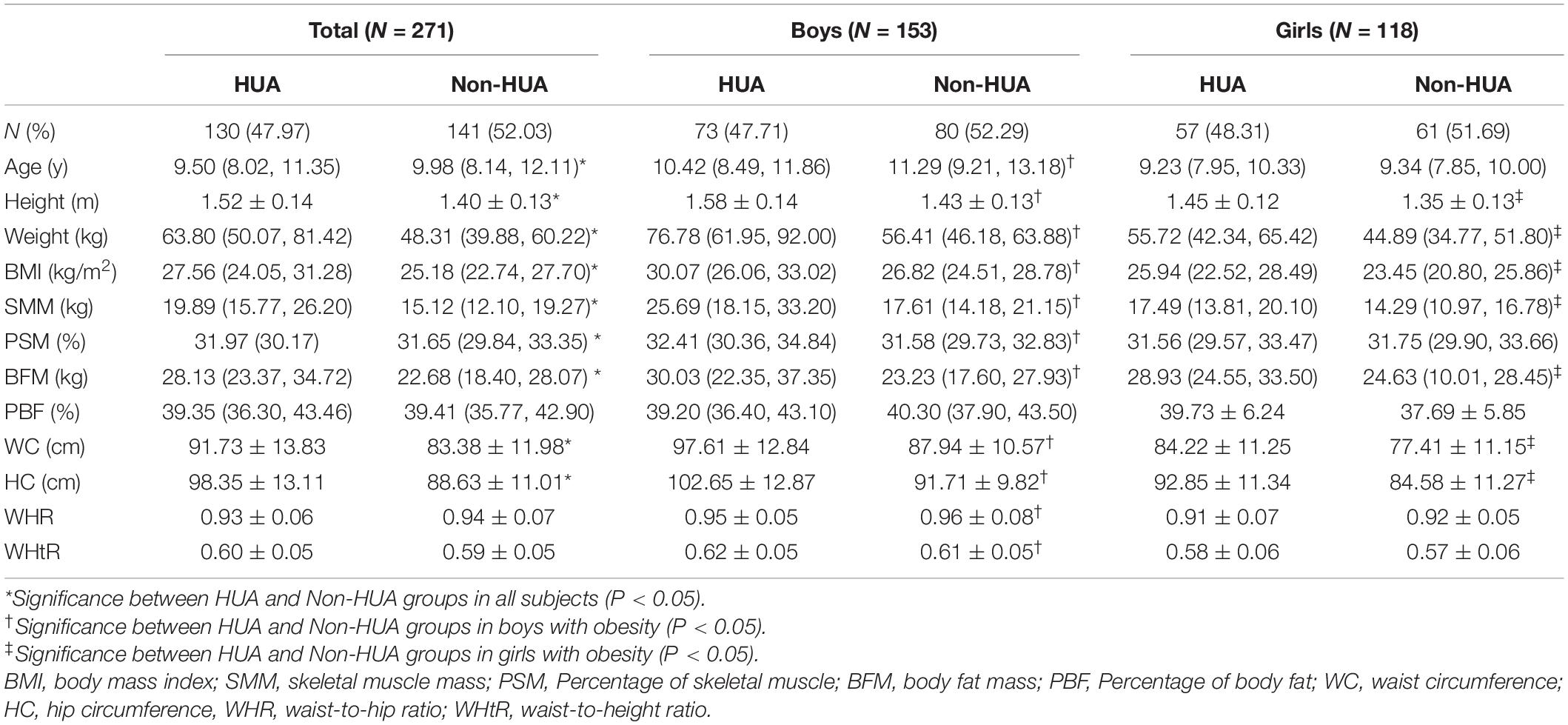
In 271 subjects, 130 of them (47.97%) were diagnosed to be HUA, among which 73 were boys while 57 were girls. As shown in Table 2, the average age in the HUA group was lower than non-HUA group, but other variables, including height, weight, BMI, SMM, PSM, BFM, WC, and HC, in the HUA group were all higher in HUA than non-HUA group (P < 0.05). In addition, the WHR was lower while the WHtR was higher in the HUA group than in the non-HUA group, wherein this significance only existed in boys with obesity (P < 0.05).
Body Component Parameters and the Risk of Hyperuricemia
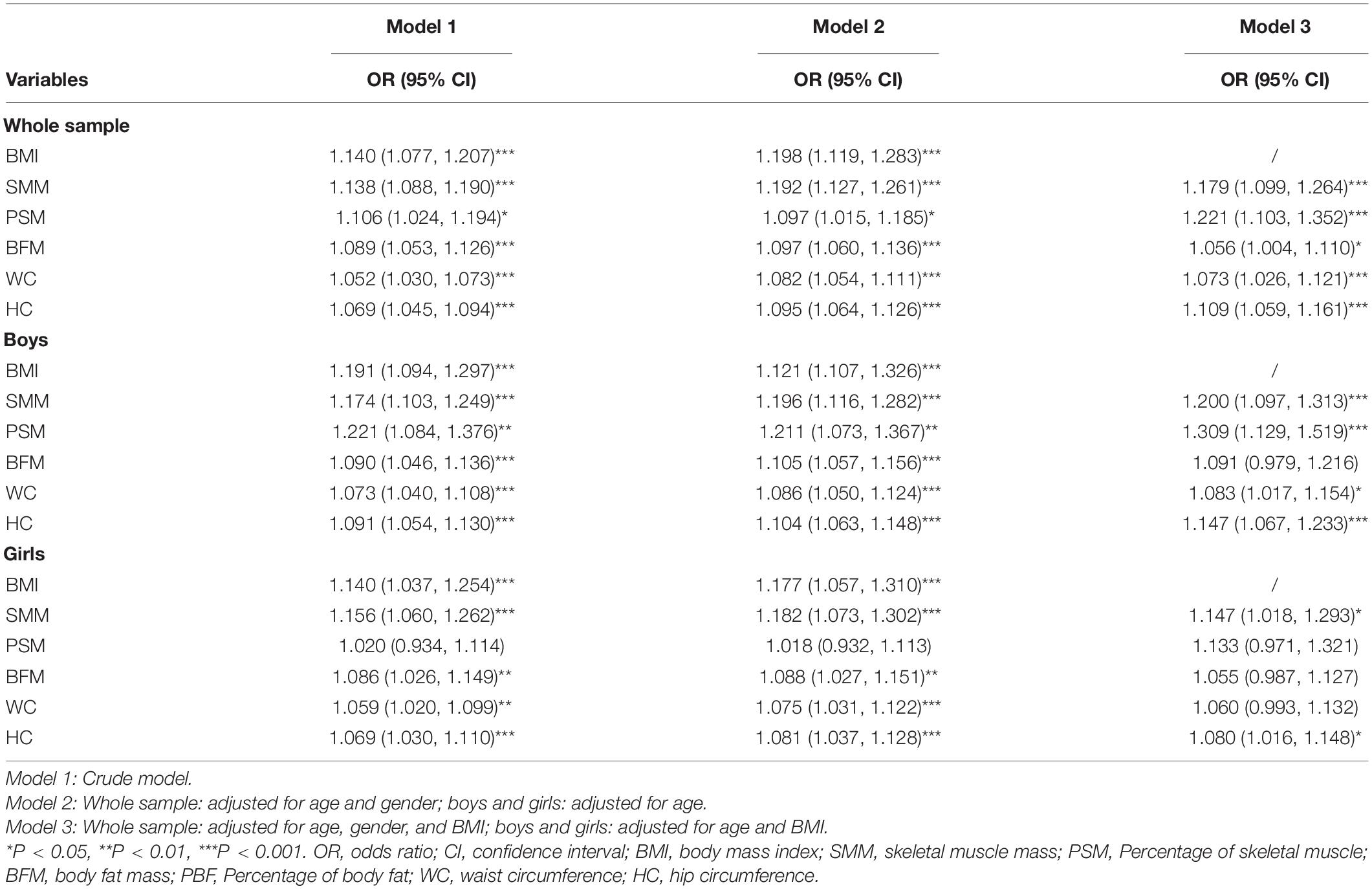
Binary logistic regression results were shown in Table 3, BMI [odds ratio (OR) = 1.198, 95% CI: 1.119–1.283], SMM, PSM, BFM, WC, and HC were positively associated with the risk of HUA after adjusting for age and gender among all participants (Model 2, all P < 0.001). Also, the SMM, PSM, BFM, WC, and HC were still positively associated with the risk of HUA after adjusting for age, gender and BMI in Model 3 (all P < 0.05), with the extent of PSM (OR = 1.221, 95% CI: 1.103, 1.352) > SMM (OR = 1.179, 95% CI: 1.099–1.264) > HC (OR = 1.109, 95% CI: 1.059 to 1.161) > WC (OR = 1.073, 95% CI: 1.026–1.121) > BFM (OR = 1.056, 95% CI: 1.004–1.11).
Among specific gender groups, the regression results showed that the BMI (OR = 1.121, 95% CI: 1.107–1.326), SMM, PSM, BFM, WC, and HC were all positively associated with the risk of HUA after adjusting for age in boys with obesity (all P < 0.001). On the other hand, after adjusting for both age and BMI in Model 3, the PSM (OR = 1.309, 95% CI: 1.129, 1.519), SMM (OR = 1.2, 95% CI: 1.097–1.313), HC (OR = 1.147, 95% CI: 1.067–1.233), and WC (OR = 1.083, 95% CI: 1.017–1.154) were still positively associated with the risk of HUA in boys (P < 0.05).
Among girls with obesity, the BMI (OR = 1.117, 95% CI: 1.057–1.31), SMM, BFM, WC, and HC were also positively associated with the risk of HUA after adjusting for age (all P < 0.01). Whereas after adjusting for age and BMI, only the SMM (OR = 1.147, 95% CI: 1.018–1.293) and HC (OR = 1.08, 95% CI: 1.016–1.148) were still positively associated with the risk of HUA (P < 0.05) in the said study group.
Discussion
In this study, we found that the risk of HUA was positively associated with PSM, SMM, HC, WC, and BFM in obese children after controlling for age, gender, and BMI. When we analyzed the data by gender, we found that PSM, SMM, HC, and WC were still positively associated with HUA in boys, while SMM and HC were also still positively associated with HUA in girls, after adjusting for age and BMI. Specifically, the impacts of SMM (especially PSM) and HC on the risk of HUA were the largest and second in children and adolescents with obesity, in which these results were consistent after stratified by gender.
Obesity, generally defined as abnormal or excessive fat accumulation, has an adverse impact on an individual’s health (20). In our study, the proportion of obese children with HUA was 47.97% (47.71% in boys and 48.31% in girls), which was higher than some previous studies which documented around 20% HUA among obese children (7). BMI increased overall 19.8% risk of HUA in children and adolescents with obesity in this study. The relationship between obesity and HUA was interactional and complex. For example, HUA can be caused by obesity through increasing the UA synthesis and inhibiting its excretion, but it consequently results to a high level of UA which also develops obesity by accelerating body fat accumulation (especially visceral fat) (21). BMI reflects the overall body condition and is shown to be positively associated with UA levels or the risk of HUA in previous studies (22, 23). In their discussions about this finding, the author usually mentioned the effects of SMM and BFM on UA, since BMI could provide potential estimates of body fat and muscle. Thus, these findings and discussions also prompted us to further clarify the associations between HUA and some body components, such as BFM and SMM.
Our findings showed that BFM and WC were positively associated with the risk of HUA in children with obesity after adjusting for age, gender, and BMI, which was consistent with some previous studies (24, 25). The mechanisms of the association between BFM and HUA have been discussed and about the following aspects: firstly, with the accumulation of body fat, the levels of several inflammation markers, such as interleukin (IL)-6 and tumor necrosis factor α (TNF-α), have increased, which were positively related to serum UA levels in the obese population (26, 27). In addition, excess adipose tissue would be a major contributor to insulin resistance (IR) or peripheral IR (28, 29), which has been confirmed as a key risk of HUA, since IR was found to have an ability to (1) directly affect the reabsorption of UA and ultimately lead to HUA (30) or (2) indirectly promote lipolysis to increase the production of nicotinamide adenine dinucleotide phosphate (an important source of UA) (31, 32). In this study, increased WC led to a higher risk of HUA than the BFM in total participants. However, stratified analysis by gender revealed that only WC still showed positively related to HUA in boys, and neither BFM nor WC showed an association with HUA in girls with obesity. As a marker of abdominal fat accumulation and visceral adiposity in children and adolescents (33, 34), WC showed a greater association with HUA than BFM (a relative overall indicator of body fat). Such findings further elucidated visceral adipose accumulation was more closely related to HUA in children and adolescents, especially in boys, which was consistent with previous studies in adults (23–25).
For children and adolescents with simple obesity, it was less noticed that the muscle mass also increased greatly as a fat mass did (35). The first study about the association between muscle mass and UA levels in 823 Brazilian children and adolescents, which was published in 2020 and showed that a positive association between muscle mass and UA levels in both boys and girls, and muscle mass has explained the respective 43 and 7.7% of the variability of UA levels (17). In our study, it was worth noting that PSM and SMM were found to be the most powerful predictors to the risk of HUA in children with obesity, even after being stratified by gender. As known, UA is an end-product of purine catabolism from endogenous (nucleic acids and internal pool of purines, accounting for approximately 80%) and exogenous sources (dietary purines, accounting for 20%) (36) of the body. Furthermore, muscle mass is considered as the largest source of purine in the body (37). During the process of its growth, muscle cells release plenty of nucleic acids and purines due to the depletion of themselves or metabolism of adenosine triphosphate, which in turn results in increased production of UA (36, 38). Therefore, it is reasonable to attribute an increased serum UA level to an increase in muscle mass. On the other hand, increased levels of intramyocellular lipids could also elevate IR from skeletal muscles (39). Thus, we speculated that the IR may be further enhanced and the risk of HUA might increase among children with high muscle and high fat, which needs more empirical evidence to support. In our study, PSM and SMM were the most powerful predictor of the risk of HUA, followed by HC, and these magnitudes were greater in boys than in girls, which also suggested the role of muscle played in the increase of UA levels. In this study, the PSM, SMM, and HC were significantly greater in boys than girls. Larger HC usually reflects higher muscle mass (40, 41), which might be a potential explanation of the magnitude of HC on HUA higher than WC. These findings also provided evidence about the effect of muscle on HUA.
To our best knowledge, this is the first study that showed that SMM had a stronger association with the risk of HUA than BFM in obese children. Considering the gender differences in body compositions and UA levels, boys and girls were also stratified in the analysis to ensure the reliability of the results. The results among these study groups were mostly consistent to support muscle as the most powerful predictor to HUA in our study, especially in boys with greater muscle mass. However, several limitations also need to be mentioned. Firstly, due to the cross-sectional design of this study, our findings cannot be regarded as causal inferences between different body components and UA levels or HUA. Longitudinal studies are warranted to examine their relationships. Secondly, as mentioned before, part of UA can also be catabolically produced from exogenous sources (e.g., dietary purines), which means lack of dietary data about protein intake and analysis about its impact on HUA could also be a limitation. Despite this, the body composition can be regarded as an indirect reflection of dietary and nutritional status.
To be concluded, not only the BMI, higher PSM, SMM, HC, WC, and BFM were found to be positively associated with a higher risk of HUA independently in children with obesity. PSM and SMM were stronger predictors than BFM in HUA in obese children, especially in boys. The relationships between obesity and HUA are more complex than it appears. Thus, in clinical practice, more attention should be paid only not to BFM and BMI, but also to muscle mass in children with obesity (especially in boys) in order to reduce the risk of HUA and potential related metabolic diseases. Moreover, further prospective studies are warranted to clarify whether a reduction of muscle mass and fat mass could reduce UA levels, and the underlying mechanisms of the relationships among them still need more evidence.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by the Ethic Committee of Xinhua Hospital, School of Medicine, Shanghai Jiao Tong University. Written informed consent from the participants’ legal guardian/next of kin was not required to participate in this study in accordance with the national legislation and the institutional requirements.
Author Contributions
LX, YN, PM, WC, and YF conceptualized and designed the study, drafted the initial manuscript, and reviewed and revised the manuscript. XZ, XLZ, YN, and QT designed the data collection instruments, collected data, carried out the initial analyses, and reviewed and revised the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank the participants and the parents of the participants.
References
2. Pan XF, Wang L, Pan A. Epidemiology and determinants of obesity in China. Lancet Diabetes Endocrinol. (2021) 9:373–92. doi: 10.1016/S2213-8587(21)00045-0
3. Selvakumar PKC, Kabbany MN, Nobili V, Alkhouri N. Nonalcoholic fatty liver disease in children: hepatic and extrahepatic complications. Pediatr Clin. (2017) 64:659–75. doi: 10.1016/j.pcl.2017.01.008
4. Bendor CD, Aya B, Orit PH, Arnon A, Gilad T. Cardiovascular morbidity, diabetes and cancer risk among children and adolescents with severe obesity. Cardiovasc Diabetol. (2020) 19:79. doi: 10.1186/s12933-020-01052-1
5. Choukem SP, Tochie JN, Sibetcheu AT, Nansseu JR, Hamilton-Shield JP. Overweight/obesity and associated cardiovascular risk factors in sub-Saharan African children and adolescents: a scoping review. Int J Pediatr Endocrinol. (2020) 2020:6. doi: 10.1186/s13633-020-0076-7
6. Wilcox WD. Abnormal serum uric acid levels in children. J Pediatr. (1996) 128:731–41. doi: 10.1016/S0022-3476(96)70322-0
7. Tang L, Kubota M, Nagai A, Mamemoto K, Tokuda M. Hyperuricemia in obese children and adolescents: the relationship with metabolic syndrome. Pediatr Rep. (2010) 2:38–41. doi: 10.4081/pr.2010.e12
8. Lurbe E, Torro MI, Alvarez-Pitti J, Redon J, Borghi C, Redon P. Uric acid is linked to cardiometabolic risk factors in overweight and obese youths. J Hypertens. (2018) 36:1840–6. doi: 10.1097/HJH.0000000000001814
9. Orlando A, Cazzaniga E, Giussani M, Palestini P, Genovesi S. Hypertension in children: role of obesity, simple carbohydrates, and uric acid. Front Public Health. (2018) 6:129. doi: 10.3389/fpubh.2018.00129
10. Gil-Campos M, Aguilera C, Cañete R, Gil A. Uric acid is associated with features of insulin resistance syndrome in obese children at prepubertal stage. Nutr Hosp. (2009) 24:607–13. doi: 10.3305/nh.2009.24.5.4491
11. Krzystek-Korpacka M, Patryn E, Kustrzeba-Wojcicka I, Chrzanowska J, Gamian A, Noczynska A. Gender-specific association of serum uric acid with metabolic syndrome and its components in juvenile obesity. Clin Chem Lab Med. (2011) 49:129–36. doi: 10.1515/CCLM.2011.011
12. Kuwahara E, Murakami Y, Okamura T, Komatsu H, Nakazawa A, Ushiku H, et al. Increased childhood BMI is associated with young adult serum uric acid levels: a linkage study from Japan. Pediatr Res. (2017) 81:293–8. doi: 10.1038/pr.2016.213
13. Zhang N, Chang Y, Guo X, Chen Y, Ye N, Sun YA. body shape index and body roundness index: two new body indices for detecting association between obesity and hyperuricemia in rural area of China. Eur J Intern Med. (2016) 29:32–6. doi: 10.1016/j.ejim.2016.01.019
14. Kuo KL, Chen HM, Hsiao SH, Chu D, Huang SJ, Huang KC, et al. The relationship between anthropometric factors and hyperuricemia in adolescent athletes. Obes Res Clin Pract. (2021) 15:375–80.
15. Kim S, Cho B, Lee H, Choi K, Hwang SS, Kim D, et al. Distribution of abdominal visceral and subcutaneous adipose tissue and metabolic syndrome in a Korean population. Diabetes Care. (2011) 34:504–6. doi: 10.2337/dc10-1364
16. Hamdy O, Porramatikul S, Al-Ozairi E. Metabolic obesity: the paradox between visceral and subcutaneous fat. Curr Diabetes Rev. (2006) 2:367–73. doi: 10.2174/1573399810602040367
17. Alvim RO, Siqueira JH, Zaniqueli D, Dutra DM, Oliosa PR, Mill JG. Influence of muscle mass on the serum uric acid levels in children and adolescents. Nutr Metab Cardiovasc Dis. (2020) 30:300–5. doi: 10.1016/j.numecd.2019.08.019
18. Kubota M, Nagai A, Tang L, Tokuda M. Investigation on hyperuricemia in children with obesity or various pediatric disorders. Nucleosides Nucleotides Nucleic Acids. (2011) 30:1051–9. doi: 10.1080/15257770.2011.597370
19. Cole TJ, Bellizzi MC, Flegal KM, Dietz WH. Establishing a standard definition for child overweight and obesity worldwide: international survey. BMJ. (2000) 320:1240–3. doi: 10.1136/bmj.320.7244.1240
21. Han T, Meng X, Shan R, Zi T, Li Y, Ma H, et al. Temporal relationship between hyperuricemia and obesity, and its association with future risk of type 2 diabetes. Int J Obes. (2018) 42:1336–44. doi: 10.1038/s41366-018-0074-5
22. Johnson RJ, Nakagawa T, Sanchez-Lozada LG, Shafiu M, Sundaram S, Le M, et al. Sugar, uric acid, and the etiology of diabetes and obesity. Diabetes. (2013) 62:3307–15. doi: 10.2337/db12-1814
23. Matsuura F, Yamashita S, Nakamura T, Nishida M, Nozaki S, Funahashi T, et al. Effect of visceral fat accumulation on uric acid metabolism in male obese subjects: visceral fat obesity is linked more closely to overproduction of uric acid than subcutaneous fat obesity. Metabolism. (1998) 47:929–33. doi: 10.1016/S0026-0495(98)90346-8
24. Hikita M, Ohno I, Mori Y, Ichida K, Yokose T, Hosoya T. Relationship between hyperuricemia and body fat distribution. Intern Med. (2007) 46:1353–8. doi: 10.2169/internalmedicine.46.0045
25. Wang JY, Chen YL, Hsu CH, Tang SH, Wu CZ, Pei D. Predictive value of serum uric acid levels for the diagnosis of metabolic syndrome in adolescents. J Pediatr. (2012) 161:753–6. doi: 10.1016/j.jpeds.2012.03.036
26. Cardoso AS, Gonzaga NC, Medeiros CC, de Carvalho DF. Association of uric acid levels with components of metabolic syndrome and non-alcoholic fatty liver disease in overweight or obese children and adolescents. J Pediatr. (2013) 89:412–8. doi: 10.1016/j.jped.2012.12.008
27. Ruggiero C, Cherubini A, Ble A, Bos AJ, Maggio M, Dixit VD, et al. Uric acid and inflammatory markers. Eur Heart J. (2006) 27:1174–81. doi: 10.1093/eurheartj/ehi879
28. Kim K, Park SM. Association of muscle mass and fat mass with insulin resistance and the prevalence of metabolic syndrome in Korean adults: a cross-sectional study. Sci Rep. (2018) 8:2703. doi: 10.1038/s41598-018-21168-5
29. Garg A. Regional adiposity and insulin resistance. J Clin Endocrinol Metab. (2004) 89:4206–10. doi: 10.1210/jc.2004-0631
30. Facchini F, Chen YDI, Hollenbeck CB, Reaven GM. Relationship between resistance to insulin-mediated glucose uptake, urinary uric acid clearance, and plasma uric acid concentration. JAMA. (1991) 266:3008–11. doi: 10.1001/jama.266.21.3008
31. Clausen JO, Borch-Johnsen K, Ibsen H, Pedersen O. Analysis of the relationship between fasting serum uric acid and the insulin sensitivity index in a population-based sample of 380 young healthy Caucasians. Eur J Endocrinol. (1998) 138:63–9. doi: 10.1530/eje.0.1380063
32. Vuorinen-Markkola H, Yki-Järvinen H. Hyperuricemia and insulin resistance. J Clin Endocrinol Metab. (1994) 78:25–9. doi: 10.1210/jcem.78.1.8288709
33. Brambilla P, Bedogni G, Moreno L, Goran M, Gutin B, Fox K, et al. Crossvalidation of anthropometry against magnetic resonance imaging for the assessment of visceral and subcutaneous adipose tissue in children. Int J Obes. (2006) 30:23–30. doi: 10.1038/sj.ijo.0803163
34. McCarthy HD. Body fat measurements in children as predictors for the metabolic syndrome: focus on waist circumference. Proc Nutr Soc. (2006) 65:385–92. doi: 10.1017/S0029665106005143
35. Rauch R, Veilleux L, Rauch F, Bock D, Welisch E, Filler G, et al. Muscle force and power in obese and overweight children. J Musculoskelet Neuronal Interact. (2012) 12:80–3. doi: 10.1016/j.humov.2012.02.007
36. Maiuolo J, Oppedisano F, Gratteri S, Muscoli C, Mollace V. Regulation of uric acid metabolism and excretion. Int J Cardiol. (2016) 213:8–14. doi: 10.1016/j.ijcard.2015.08.109
37. Lowenstein J. Ammonia production in muscle and other tissues: the purine nucleotide cycle. Physiol Rev. (1972) 52:382–414. doi: 10.1152/physrev.1972.52.2.382
38. Hammouda O, Chtourou H, Chaouachi A, Chahed H, Ferchichi S, Kallel C, et al. Effect of short-term maximal exercise on biochemical markers of muscle damage, total antioxidant status, and homocysteine levels in football players. Asian J Sports Med. (2012) 3:239. doi: 10.5812/asjsm.34544
39. Sinha R, Dufour S, Petersen KF, LeBon V, Enoksson S, Ma YZ, et al. Assessment of skeletal muscle triglyceride content by 1H nuclear magnetic resonance spectroscopy in lean and obese adolescents: relationships to insulin sensitivity, total body fat, and central adiposity. Diabetes. (2002) 51:1022–7. doi: 10.2337/diabetes.51.4.1022
40. Seidell J, Han T, Feskens EJ, Lean M. Narrow hips and broad waist circumferences independently contribute to increased risk of non-insulin-dependent diabetes mellitus. J Intern Med. (1997) 242:401–6. doi: 10.1046/j.1365-2796.1997.00235.x
Keywords: hyperuricemia, obesity, skeletal muscle mass (SMM), body fat mass (BFM), children
Citation: Xie L, Mo PKH, Tang Q, Zhao X, Zhao X, Cai W, Feng Y and Niu Y (2022) Skeletal Muscle Mass Has Stronger Association With the Risk of Hyperuricemia Than Body Fat Mass in Obese Children and Adolescents. Front. Nutr. 9:792234. doi: 10.3389/fnut.2022.792234
Received: 01 November 2021; Accepted: 25 February 2022;
Published: 28 March 2022.
Edited by:
Caterina Conte, Università telematica San Raffaele, ItalyReviewed by:
Masahide Hamaguchi, Kyoto Prefectural University of Medicine, JapanNeha Bakshi, University of Delhi, India
Copyright © 2022 Xie, Mo, Tang, Zhao, Zhao, Cai, Feng and Niu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wei Cai, Y2FpdzE5NzhAMTYzLmNvbQ==; Yi Feng, ZmVuZ3lpQHhpbmh1YW1lZC5jb20uY24=; Yang Niu, bml1eWFuZ0B4aW5odWFtZWQuY29tLmNu
†These authors have contributed equally to this work
 Luyao Xie
Luyao Xie Phoenix K. H. Mo
Phoenix K. H. Mo Qingya Tang1†
Qingya Tang1† Xuan Zhao
Xuan Zhao Xuelin Zhao
Xuelin Zhao Wei Cai
Wei Cai Yang Niu
Yang Niu