- 1College of Chinese Materia Medica, Guangdong Food and Drug Vocational College, Guangzhou, China
- 2School of Traditional Chinese Medicine, Shenyang Medical College, Shenyang, China
- 3College of Medical Technology, Ningbo College of Health Sciences, Ningbo, China
The gut microbiota is thought to be an important factor that influences brain processes and behaviors through the gut–brain axis. Pogostemon cablin is used in traditional Chinese medicine (TCM) to treat gastrointestinal symptoms. Patchouli essential oil (PCO), the main active agent in P. cablin, is used in aromatherapy for stress relief. The aim of our study was to investigate the effects of orally administered PCO on anxiety- and depressive-like behaviors and the gut microbiota. We constructed a rat model of chronic unpredictable mild stress (CUMS) and explored the anxiolytic- and antidepressant-like effects of PCO using the open field test (OFT) and forced swim test (FST). Changes in the abundance of the gut microbiota, short-chain fatty acids (SCFAs), and other related molecules were assessed to determine the role of the gut microbiota. Our results showed that CUMS induced an anxiety-like phenotype in the OFT, which was reversed by PCO, and that PCO also significantly mitigated the depression-like behaviors caused by CUMS in the FST. Furthermore, we found that PCO increased the relative abundances of several probiotics, including Bacteroides and Blautia, and decreased the relative abundances of Ruminococcus_1 and Ruminococcus_2, which were increased by CUMS. Regarding SCFAs, the metabolites of the gut microbiota, PCO increased the concentration of propionic acid and decreased that of caproic acid. Finally, PCO restored the serotonin (5-hydroxytryptamine, 5-HT) level in the hippocampus, which had been decreased by CUMS. The results of this study suggested that PCO can improve stress-related anxiety- and depression-like behaviors and might exert its effects on the central nervous system through interactions with the gut microbiota.
Introduction
Pogostemon cablin (Blanco) Benth. is a frequently utilized herbal medicinal product in traditional Chinese medicine (TCM). It is commonly used for treating gastrointestinal infectious disorders, including gastrointestinal cold, acute gastroenteritis, nausea, vomiting, and diarrhea. These conditions are believed to be linked to dampness and summer heat, as per the principles of TCM (1). P. cablin belongs to the Labiatae family, and patchouli essential oil (PCO) is considered its main effective component. Essential oils derived from plants are popular because of their positive emotional effects on calming nerves. PCO is also commonly used in aromatherapy for stress relief (2, 3). Stress is a significant contributing factor to the development of anxiety and depression, both of which are highly prevalent in the current world. However, initial treatment for these two disorders often results in adverse effects, such as loss of appetite, weight loss, drowsiness, dizziness, fatigue, and sexual dysfunction (4, 5). Therefore, the development of new psychopharmacological agents that have minimal adverse effects is urgently needed.
The importance of natural essential oils has increased due to their rich bioactivities and low risk of toxicity. Modern studies have revealed that PCO possesses antibacterial, antifungal, antioxidant, analgesic, and anti-inflammatory activities (2). However, to the best of our knowledge, the potential effects of PCO on anxiety and depression are still unknown. Accumulating evidence suggests a connection between the gut microbiota and the brain axis in terms of stress-related mental disorders, such as anxiety and depression (6). Research has shown that prebiotics have anxiolytic-like effects (7). Additionally, antidepressant treatments can impact the composition of the gut microbiota (8). Furthermore, essential oils can modulate the gut microbiota, thereby enhancing health and performance (9–12).
In this study, we established a rat model of chronic unpredictable mild stress (CUMS) and investigated the anxiolytic and antidepressant effects of PCO using the open field test (OFT) and the forced swim test (FST). To gain a deeper understanding of how PCO affects the central nervous system through modulation of the gut microbiota, we conducted 16S rRNA gene sequencing analysis. This allowed us to compare the differences in the taxonomic composition of the gut microbial community. Furthermore, the microbiota in the intestine plays a crucial role in metabolizing carbohydrates, resulting in the production of short-chain fatty acids (SCFAs). SCFAs have been found to be associated with the secretion of serotonin (5-hydroxytryptamine, 5-HT), and altered 5-HT has been implicated in the development of anxiety and depression (13). Furthermore, the gut microbiota can influence behaviors by regulating important molecules, including brain-derived neurotrophic factor (BDNF) and corticosterone (14). Therefore, we also examined the production of SCFAs and the levels of 5-HT, BDNF, and corticosterone. The results of this study may contribute to improving clinical applications and further pharmacological studies of PCO.
Materials and methods
Materials
Fluoxetine hydrochloride (Pathcon, France) was used as a reference drug. 5-HT and BDNF ELISA kits and a corticosterone immunoassay kit were purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, China). PCR amplicons purified from Agencourt AMPure Beads and a Quantifying PicoGreen dsDNA Assay Kit were purchased from Beckman Coulter (Indianapolis, United States) and Thermo Fisher Scientific (Carlsbad, United States), respectively. The Sequencing MiSeq Reagent Kit (v3) was purchased from Shanghai Personal Biotechnology Co., Ltd. (Shanghai, China). Saline (0.9%) was purchased from Qingdao Hope Bio-Technology Company (Qingdao, China). All other reagents and chemicals used were of analytical grade.
Preparation and GC-MS analysis of patchouli essential oil (PCO)
P. cablin was collected from the Medicinal Plant Garden of Guangdong Food and Drug Vocational College. The identity of the plant was confirmed with morphological evaluations and comparison to reference data. A voucher specimen of the plant (IBSC38pc15) was deposited in the Herbarium of Guangdong Food and Drug Vocational College for identification. The aerial part of P. cablin was dried in the shade and powdered. PCO was obtained by hydrodistillation for 3 h using a modified Clevenger system. The fraction of essential oil was separated by density, with a yield of 2.0% (g/g crude drug).
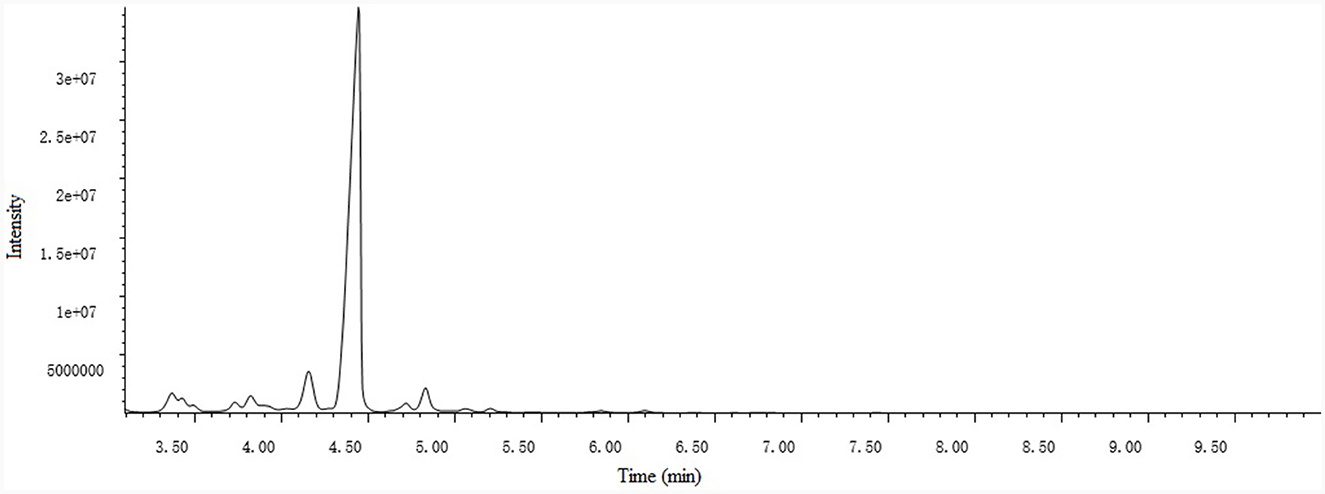
PCO was analyzed by GC-MS (Thermo Fisher) instrument using a TR column (30 m × 0.25 mm × 0.25 μm). Constant flow at 1 ml/min of carrier gas was used for sample analysis. The injector temperature of the instrument was programmed at 220°C. Oven temperature was started from 50°C to 250°C with ramp of 4°C/min. Sample was injected in a split mode (1:50) with injection volume of 1 μl. Temperature of the ion source was set at 220°C and transfer line temperature was at 300°C and ionization of the sample was performed in electron impact mode at an ionization voltage of 70 eV with mass range used from m/z 50 to 650 amu. The identification of the essential oil compounds was based on comparing mass spectra of compounds (NIST and WILEY libraty).
Animals and treatment
Male Sprague–Dawley (SD) rats with body weights of 180~200 g were purchased and housed at room temperature (25 ± 2°C, 55 ± 10% relative humidity) on a 12:12 h light–dark cycle. The rats were allowed free access to regular laboratory rat chow and water for 1 week. Following 1 week of acclimation, the rats were randomized into 4 groups (n = 12): control, CUMS (chronic unpredictable mild stress), PCO (dissolved in 0.01% (v/v). Tween 80 in 0.9% saline; rats received 0.8 mL/kg essential oil orally once a day), and FLX (the rats were orally administered a dosage of 10 mg/kg fluoxetine hydrochloride once a day). The control group and CUMS group were given 0.9% saline (10 mL/kg). Except for those in the control group, the rats in the other groups were exposed to CUMS for 4 weeks according to previous methods (15). The stressors included 5-min swimming in cold water (4°C), 2-min electric foot shock (rats receive unavoidable 3 mA intensity electric foot shocks for a duration of 200 ms with a frequency of one shock per second), 2-min tail clamping, 3-h noise (100 dB), subject to a hot environment (45°C) for 5 min, 24-h water deprivation, 24-h food deprivation. The above stressors were imposed separately, and each rat received no more than one stressor simultaneously. If a rat received a stressor of water deprivation or food deprivation, no more stressor was imposed to this rat at the same day. The timeline of the experiment is shown in Figure 1A.
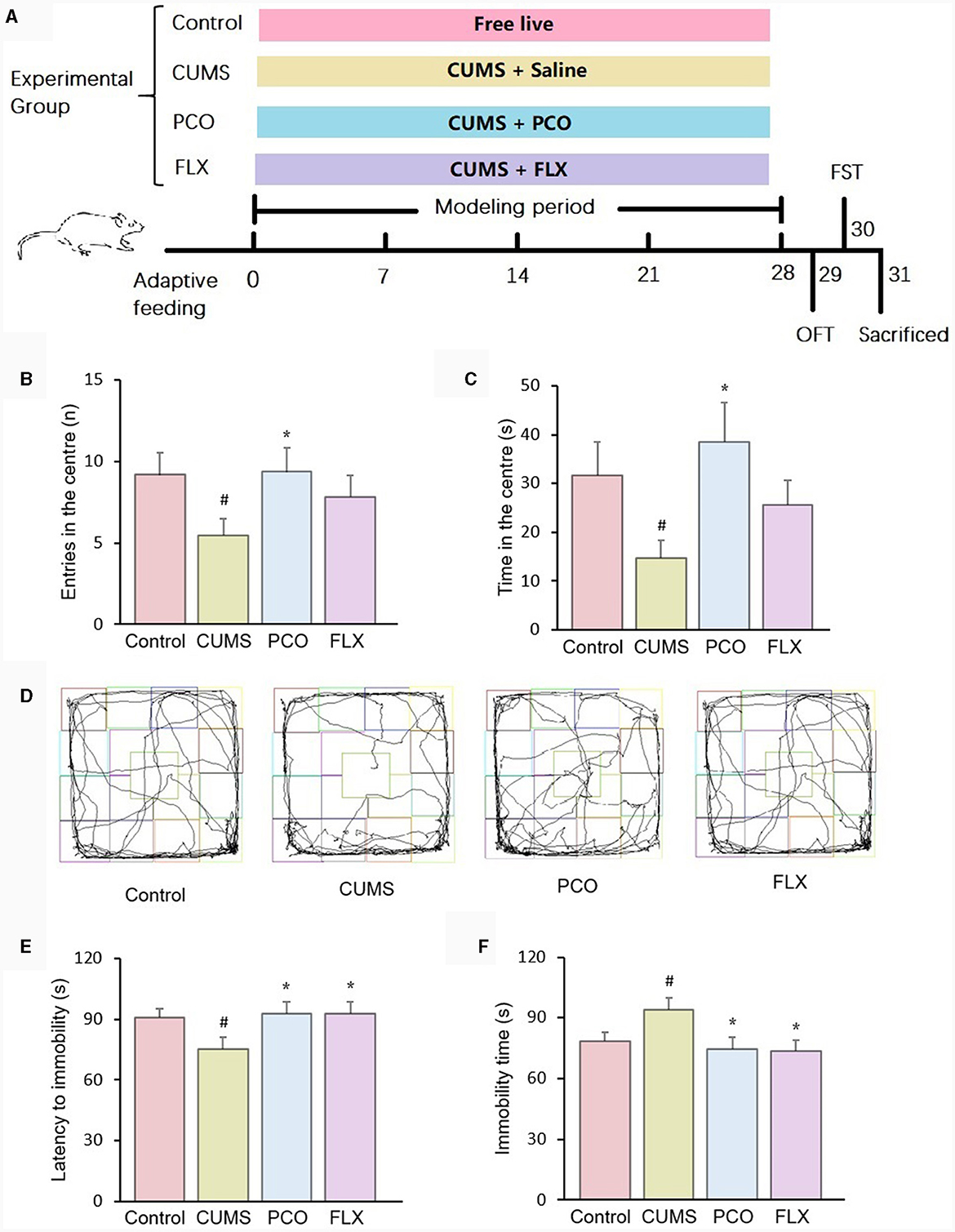
Figure 1. Effects of patchouli essential oil (PCO) on the behaviors of rats with chronic unpredictable mild stress (CUMS). (A) Schematic illustration of the animal experimental design. (B) Number of entries in the center in the open field test (OFT). (C) Time spent in the center in the OFT. (D) Representative track sheets of the OFT. (E) Latency immobility in the forced swim test (FST). (F) Immobility time in the FST. #P < 0.05 compared with the control group; *P < 0.05 compared with the CUMS group.
Open field test
Rat behavior was assessed for 5 min in an open field apparatus (122 × 122 × 45 cm), which was divided into 16 equal squares (30.5 × 30.5 cm). The rat was first placed in the center of the apparatus. The total duration (in seconds) and number of entries into the central zone were observed and counted. The apparatus was cleaned using 70% ethanol between tests (16).
Forced swim test
Briefly, rats were put in an open cylindrical container (diameter 35 cm and height 45 cm) filled to 30-cm height with water to swim for 6 min. The latency to immobility (in seconds) was noted in the first 2 min (maximum of 120 s). The total immobility time of the rats in the last 4 min was summed to reflect the depressive-like behavior of rats. Immobility status means the absence of movement, except for motion necessary to keep the heads above the water for breathing. The swimming water was changed after each test (17).
Sample collection and preparation
The animals were sacrificed by decapitation after the behavioral tests were completed. Feces from the colon were collected in sterile conical tubes for microbial community and SCFA analyses. The hippocampus was isolated and washed with 0.9% saline to measure the levels of 5-HT and BDNF. Blood was collected, treated with EDTA, and centrifuged, and the supernatant was used to measure the level of corticosterone. All the samples were kept at −80°C before assessment.
Analysis of intestinal microbial community diversity
The method used for this experiment has been previously reported (18). Briefly, microbial DNA was extracted from feces. We chose the V3-V4 16S rRNA region for generating amplicons and taxonomic analysis. PCR amplicons were purified, qualified, and paired-end sequenced. The sequences were subsequently grouped into operational taxonomic units (OTUs). The differences in the gut microbial taxonomic composition were compared using Metastats software. The sequencing data of this study was uploaded to the Global Pharmacopeia Herbal Genome Database (19) and could be accessed via http://www.gpgenome.com/species/673.
Analysis of short-chain fatty acids
This experiment was performed as described previously (20). The concentrations of SCFAs were measured by a GC-2010 gas chromatograph fitted with a DB-FFAP column (30 m × 0.25 mm × 0.25 μm; Agilent, United States). Standard solutions of acetic acid, propionic acid, butyric acid, valeric acid, and caproic acid were prepared at 1, 0.4, 0.2, 0.1, 0.05, 0.01 and 0.005 mg/ml. Each fecal sample (0.2 g) was blended in 0.8 ml of deionized water by vortexing and subsequently centrifuged at 5,000 rpm and 4°C for 20 min. A total of 450 μl of the supernatant was added to 50 μl of 50% H2SO4, vortexed and centrifuged at 12,000 rpm for 10 min, after which the mixture was incubated at 4°C for 30 min. The supernatant was collected for GC analysis.
Measurement of the 5-HT and BDNF levels in the hippocampus
The hippocampal tissue was homogenized in 0.9% saline and centrifuged. The supernatant was collected and used for the determination of 5-HT and BDNF levels (21). The procedures were performed according to the manufacturer's instructions. Briefly, the competitive enzyme immunoassay technique was used to determine the level of 5-HT. Standards or samples were added to a microplate precoated with ST/5-HT antigen, and then, a biotin-conjugated antibody specific for ST/5-HT was added. After that, HRP-conjugated streptavidin was added to form an immune complex, and the chromogenic substrate TMB was subsequently added to produce a discernible color. The solid-phase sandwich ELISA was used to measure the amount of BDNF. Samples or standards were added to a microplate precoated with BDNF antibody. Then, HRP-conjugated streptavidin was added, and the samples were incubated and washed. Finally, the chromogenic substrate TMB was added to react with the enzyme-antibody-target complex to produce a measurable signal. The concentrations of 5-HT or BDNF in the samples were then determined by comparing the OD to the standard curve.
Measurement of plasma corticosterone levels
The plasma corticosterone concentrations were analyzed using an immunoassay kit according to the manufacturer's protocols (22). In brief, standards or samples were added to a microplate precoated with rat corticosterone antigen. After incubation, biotin-conjugated anti-rat corticosterone antibody was added, the mixture was combined with HRP-conjugated streptavidin to form an immune complex, incubated, washed, and added to the chromogenic substrate TMB to produce a color for determination.
Statistical analysis
Statistical analysis was performed using GraphPad Prism 8 (version 8.2.1 (441), ©1992–2019 GraphPad Software, Inc.). The data are expressed as the mean ± standard error of the mean. One-way ANOVA was applied to determine significant differences. P < 0.05 was considered to indicate statistical significance.
Results
Chemical analysis of PCO
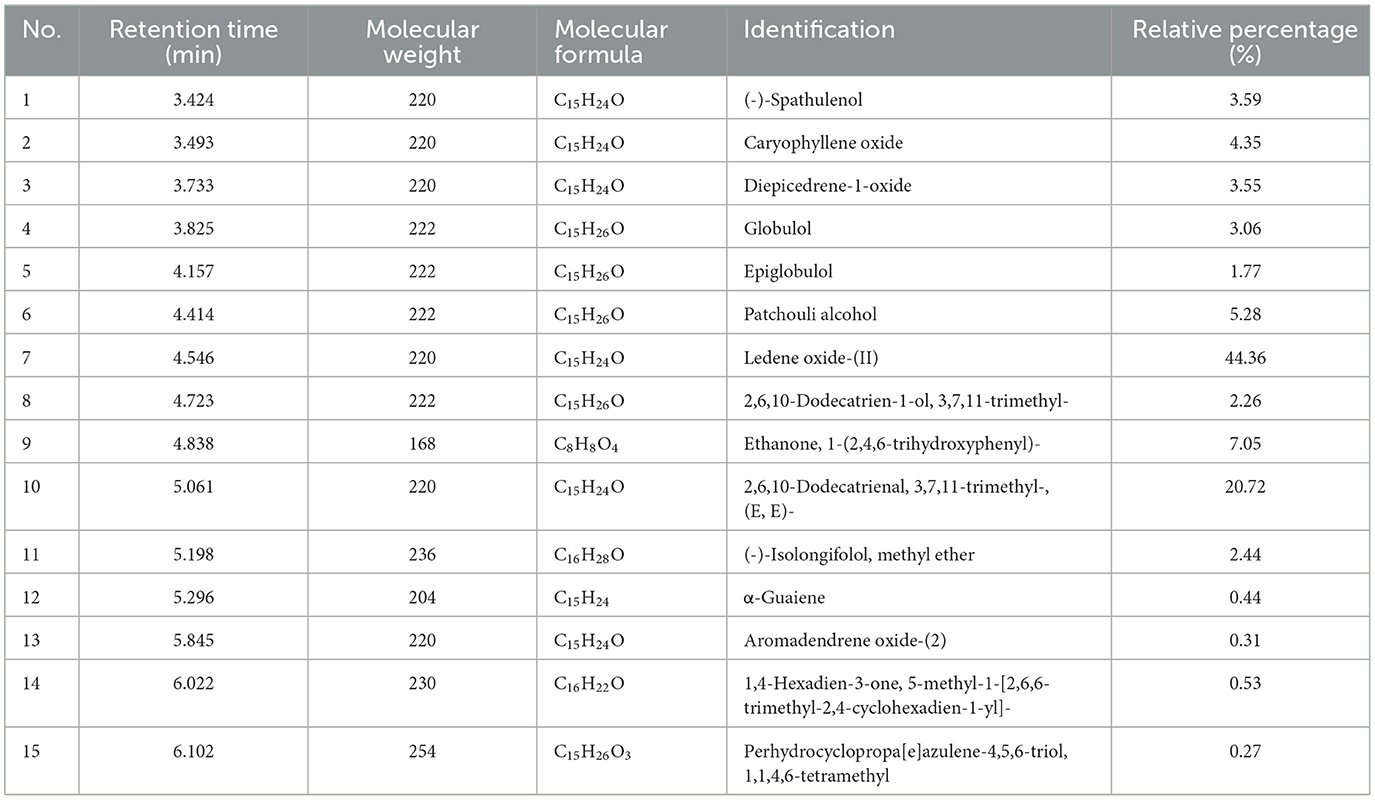
A total of 15 compounds were identified by GC-MS analysis in PCO. Figure 2 shows the total ions current (TIC) diagram. Among them, four main compounds, Ledene oxide-(II) (44.36%), 2,6,10-Dodecatrienal, 3,7,11-trimethyl-, (E, E)- (20.72%), Ethanone, 1-(2,4,6-trihydroxyphenyl)- (7.05%) and Patchouli alcohol (5.28%), together account for 77.42% of the total relative peak area (Table 1).
PCO alleviated anxiety- and depressive-like behaviors caused by CUMS
In the OFT, the administration of PCO reversed both the decreased number of entries and time spent in the center zone in CUMS rats. These indicators are associated with anxiety-related behaviors (Figures 1B, C). Figure 1D shows representative track sheets from the OFT. The FST data revealed that after 4 weeks of CUMS, there was a significant decrease in the latency to immobility and an increase in the immobility time compared to those in the control group. These findings suggest that the rats exhibited a depressive-like state. PCO treatment significantly reversed these two changes, indicating its antidepressant-like effect (Figures 1E, F).
PCO increased the abundance of beneficial microbes and reversed intestinal flora disorder caused by CUMS
16S rRNA sequencing analysis yielded a total of 4,539,206 raw reads. We identified 9 phyla, 14 classes, 18 orders, 35 families, 119 genera, and 118 species in the four groups that were considered qualified. The PCO group exhibited significant decreases in the Chao1 index and Shannon index of alpha diversity (P < 0.001, P < 0.01) compared to those in the CUMS group (Figures 3A, B). Additionally, beta diversity-based principal component analysis (PCA) demonstrated that the microbial community composition in the PCO group differed from that in the CUMS group, as indicated by the clustering of the samples in the plots (Figure 3C).
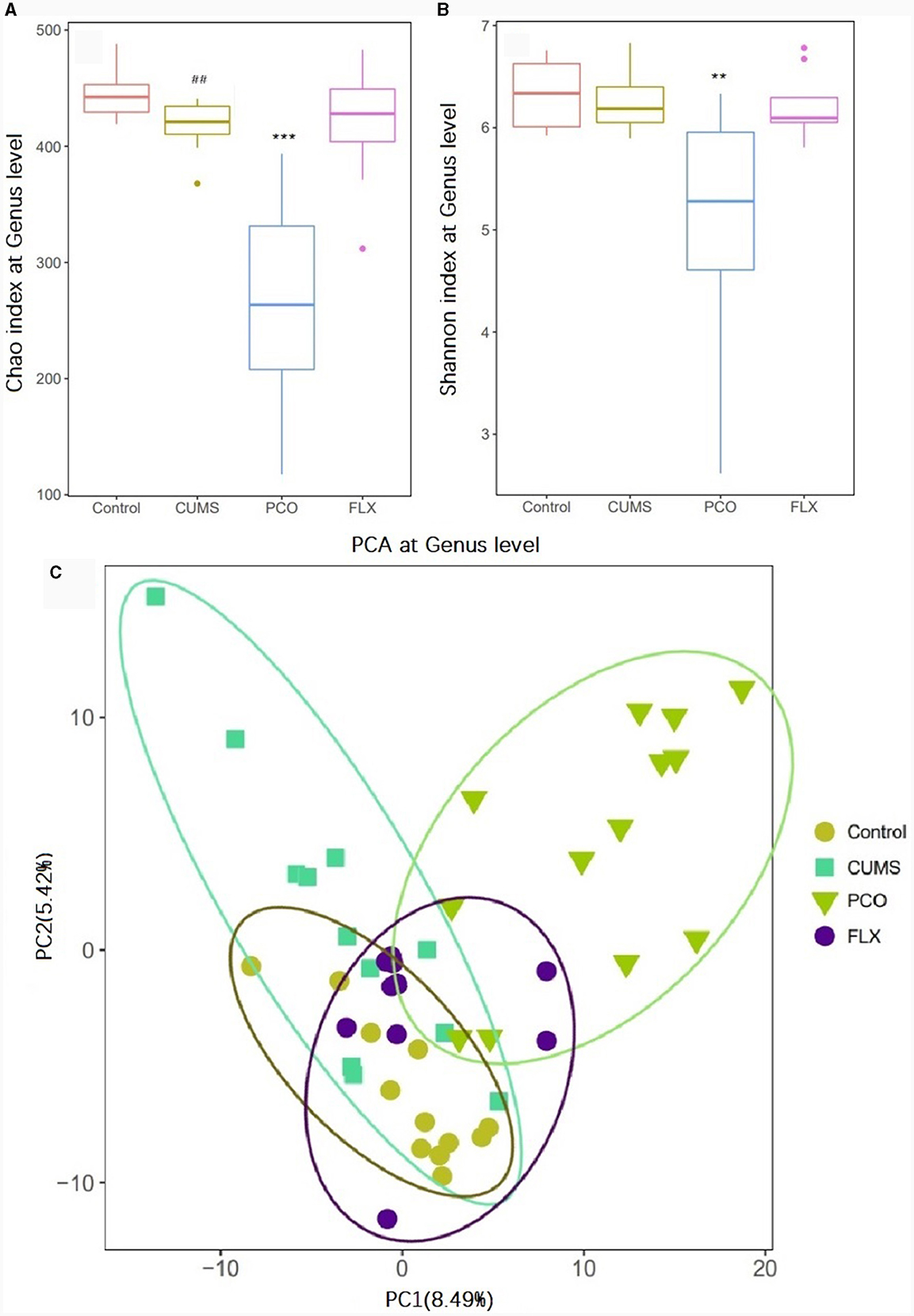
Figure 3. Effects of patchouli essential oil (PCO) on the α diversity (A, B) and β diversity (C) of the gut microbiota at the genus level. ##P < 0.01 compared with the control group; **P < 0.01 and ***P < 0.001 compared with the chronic unpredictable mild stress (CUMS) group.
Metastats analysis revealed that at the phylum level, the dominant microbial taxa were Firmicutes and Bacteroidetes (Figure 4A). Additionally, Lactobacillus was identified as the most abundant microbial taxon at the genus level (Figure 4B).
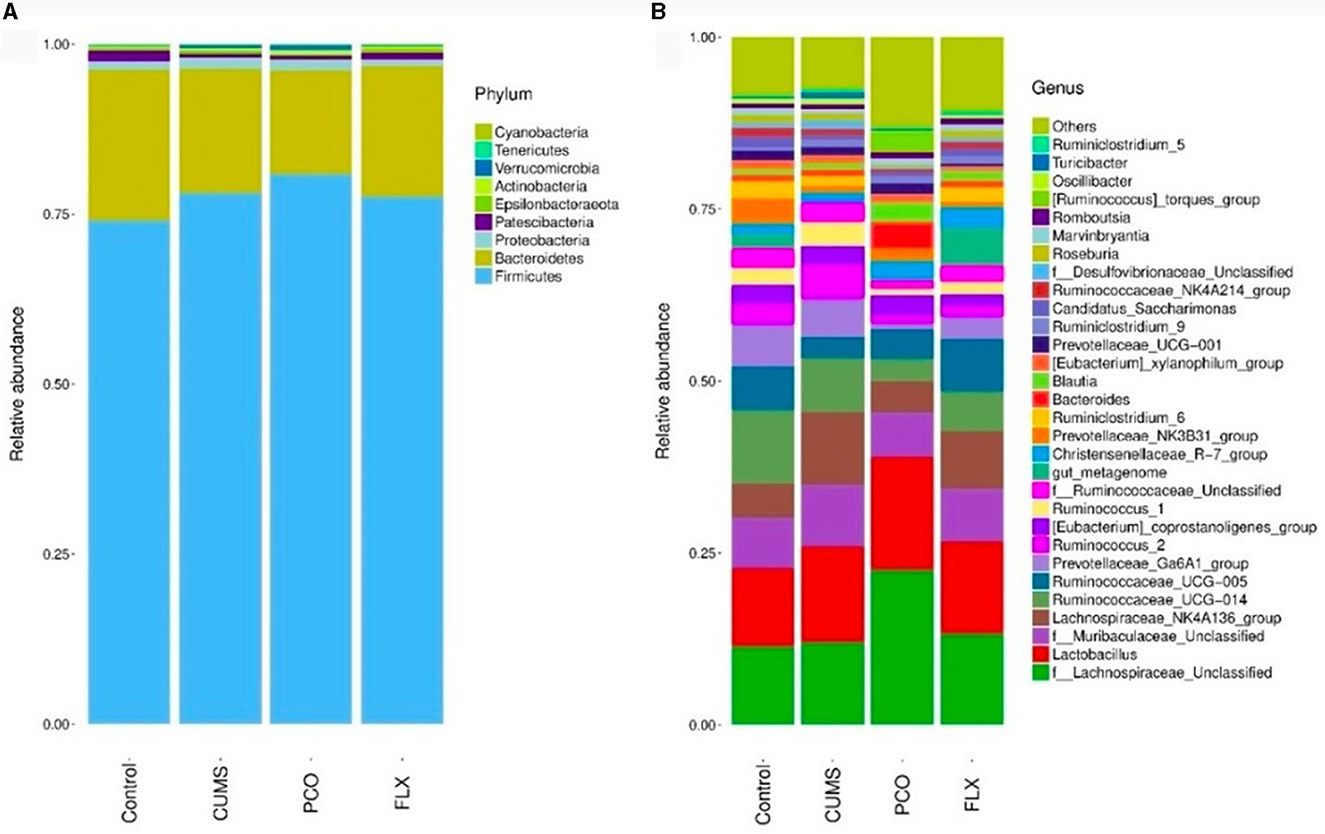
Figure 4. Changes in bacterial taxa caused by patchouli essential oil (PCO). (A) Phylum level. (B) Genus level.
Although there were no significant differences between the control group and the CUMS group, the PCO group exhibited greater relative abundances of Bacteroides (Figure 5B) and Blautia (Figure 5C) than did the CUMS group. The relative abundance of Lactobacillus (Figure 5A), which was the most abundant genus identified, was not significantly increased by PCO (P>0.05). CUMS changed the gut microbial communities to have significantly greater relative abundances of Ruminococcus_1 (Figure 5D) and Ruminococcus_2 (Figure 5E). The PCO group presented lower relative abundances of Ruminococcus_1, Ruminococcus_2, and Oscillibacter (Figure 5F) than the CUMS group.
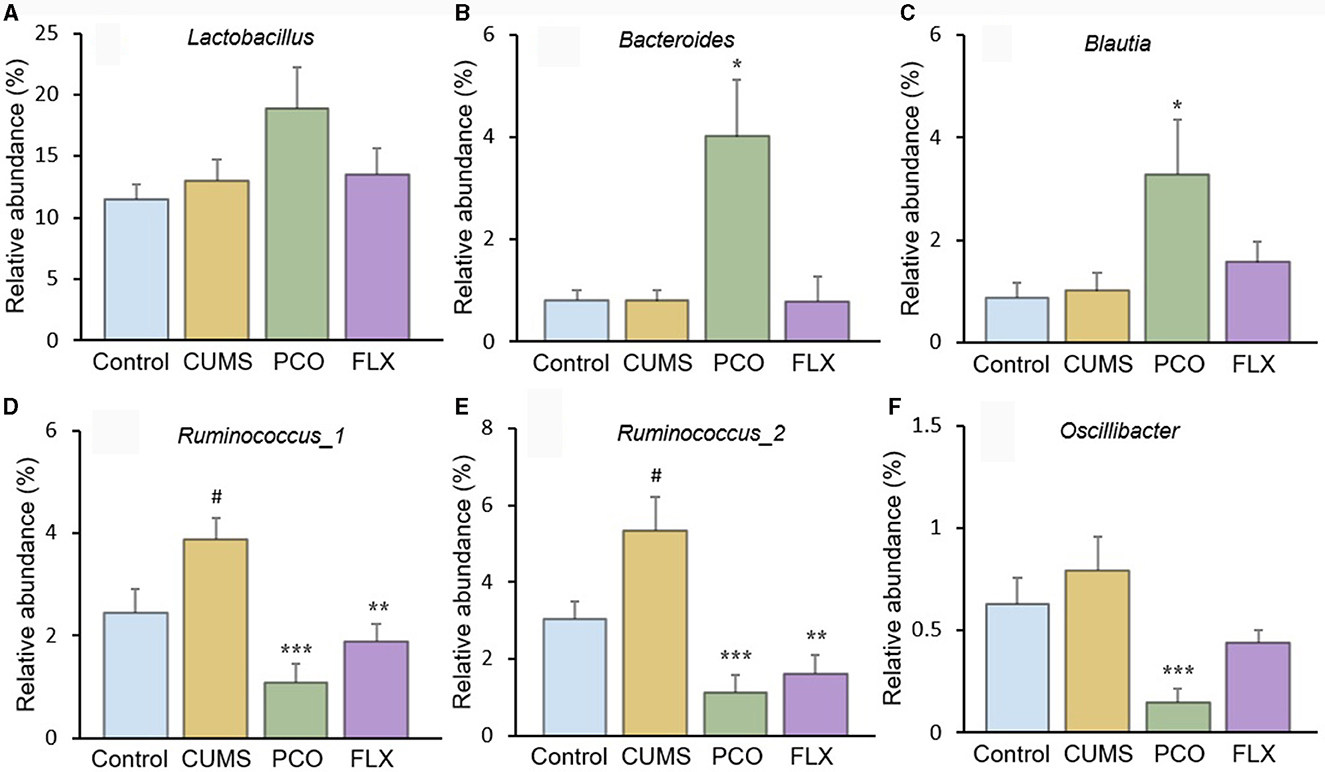
Figure 5. Relative abundances of specific genera affected by patchouli essential oil (PCO). (A) Lactobacillus, (B) Bacteroides, (C) Blautia, (D) Ruminococcus_1, (E) Ruminococcus_2, and (F) Oscillibacter. #P < 0.05 compared with the control group; *P < 0.05, **P < 0.01 and ***P < 0.001 compared with the chronic unpredictable mild stress (CUMS) group.
PCO recovered SCFA disorder caused by CUMS
The changes in SCFA levels in the rat fecal samples are illustrated in Figure 6. The levels of acetic acid and butyric acid remained unchanged by CUMS or PCO. However, compared with that in the control group, the concentration of propionic acid in the CUMS group decreased, while the concentration of caproic acid increased. In contrast, compared with the CUMS group, PCO significantly increased the level of propionic acid and decreased the level of caproic acid to the point where it was undetectable. CUMS had no effect on valeric acid, but PCO significantly decreased its level compared to that in the CUMS group.
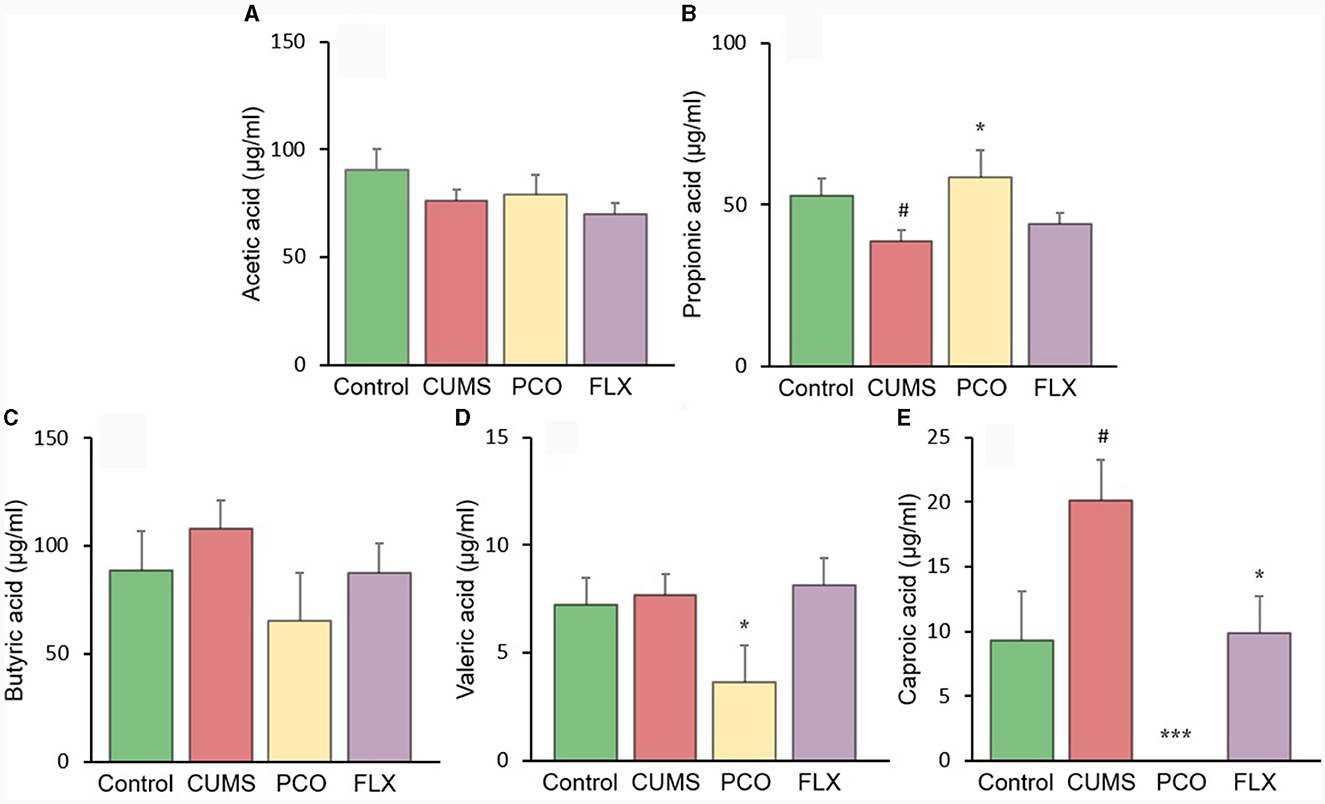
Figure 6. Changes in short-chain fatty acid (SCFA) levels in fecal samples. (A) acetic acid, (B) propionic acid, (C) butyric acid, (D) valeric acid, and (E) caproic acid. #P < 0.05 compared with the control group; *P < 0.05 and ***P < 0.001 compared with the chronic unpredictable mild stress (CUMS) group.
PCO restored 5-HT dysfunction caused by CUMS but not the changes in BDNF or corticosterone
CUMS resulted in a significant reduction in 5-HT levels in the hippocampus, which was significantly restored by PCO or FLX treatment (Figure 7A). Similarly, BDNF levels were significantly lower in the CUMS group than in the control group; however, PCO did not significantly affect BDNF levels, whereas FLX increased BDNF concentrations in the hippocampus (Figure 7B). Additionally, CUMS significantly increased the basal corticosterone concentration, which was attenuated by FLX treatment (Figure 7C). PCO did not influence the corticosterone concentration. A schema diagram demonstrating the hypothetical mechanisms of anxiolytic- and antidepressant-like effects of PCO is shown in Figure 8.
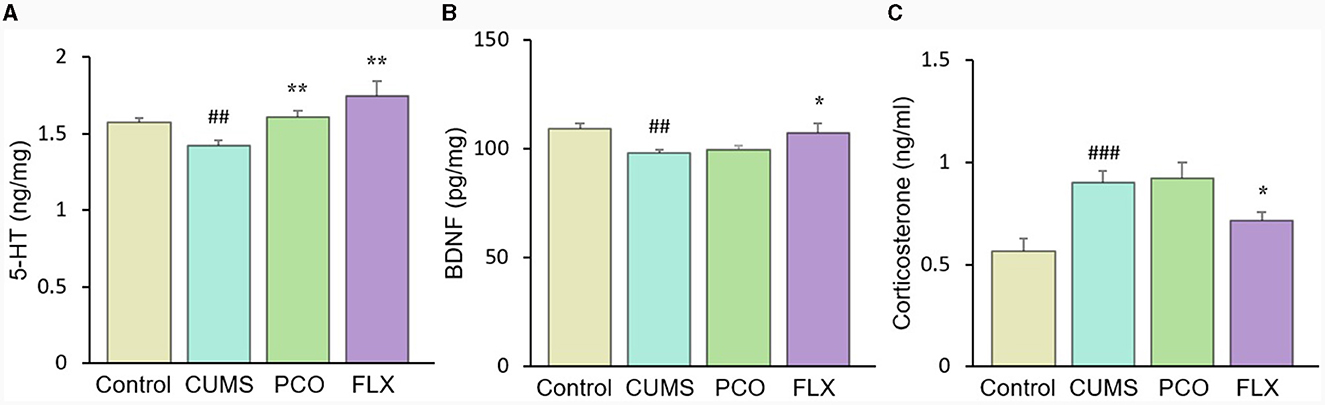
Figure 7. Changes in (A) 5-HT and (B) brain-derived neurotrophic factor (BDNF) levels in the hippocampus and (C) corticosterone levels in plasma. ##P < 0.01 and ###P < 0.001 compared with the control group; *P < 0.05 and **P < 0.01 compared with the chronic unpredictable mild stress (CUMS) group.
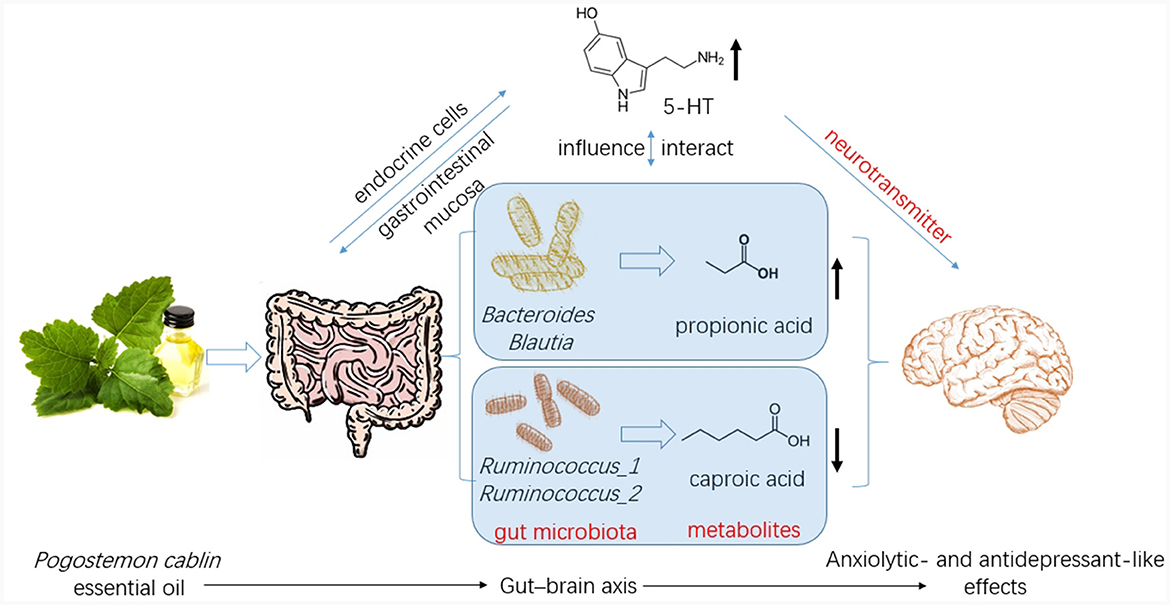
Figure 8. Patchouli essential oil (PCO) improves stress-related anxiety- and depression-like behaviors through gut-brain axis.
Discussion
Depression is a chronic and debilitating disorder that is receiving increasing attention for its association with long-term stress (23). The CUMS model, a classical model of depression, is considered to be more similar to the pathogenesis of human depression. Anxiety, on the other hand, is a normal response to threats or psychological stress (24). CUMS can also be used to study anxiolytic activity, as it induces the development of anxious behaviors in animals (25–29). We provide new evidence that PCO (the intervention studied here) can prevent the development of anxiety- and depressive-like behaviors induced by CUMS (the stressor). Furthermore, our findings suggest that the effects of PCO may be attributed to its ability to modulate the gut microbiota.
In this study, we identified several changes that occurred in the gut microenvironment. While CUMS did not have any significant effects, we observed that the consumption of PCO had a positive impact on the relative abundances of Bacteroides and Blautia, which are known as probiotics. Additionally, we found that the relative abundance of Lactobacillus was greater after the administration of PCO (P>0.05). The results of this study are consistent with previous findings suggesting that PCO has a prebiotic-like effect (30). Prebiotics play a significant role in the development and management of anxiety and depression (31). Recent reports have shown that oral treatment with Bacteroides can reverse anxiety-like behaviors in mice, as well as correct gut permeability and abnormal intestinal cytokine profiles (32). Strains of Blautia and Lactobacillus have been found to attenuate anxiety- and depressive-like behaviors (33, 34). Short-chain fatty acids (SCFAs) play crucial roles in both digestion and central functions and also have significant effects on various behaviors (35). Species of Bacteroides and Blautia are primary producers of acetic acid and propionic acid in the human gut. Acetic acid is believed to have the ability to prevent gut infections and maintain the integrity of the gut barrier (36). Propionic acid has also been shown to play a role in maintaining proper intestinal permeability (37). Propionic acid levels are significantly decreased in depressed animals (38). Here, we found a similar result, which was reversed by PCO. Our results demonstrated that PCO might exert anxiolytic- and antidepressant-like effects by modulating probiotic bacteria, including Bacteroides, Blautia, and even Lactobacillus species.
Ruminococcus is common in the human gut microbiota. Antidepressants reduce the abundance of Ruminococcus, which is causally related to their antidepressant effects (39). Our results indicated that both PCO and FLX decreased the levels of Ruminococcus-1 and Ruminococcus-2, which were significantly increased by CUMS. Therefore, the present study suggested that Ruminococcus-1 and Ruminococcus-2 may play roles in depressive behaviors, which aligns with the findings of previous research indicating a change in the relative abundance of Ruminococcus. The Ruminococcus bacterium strain is highly prolific in its ability to produce caproic acid (40). Caproic acid also partly contributes to depressive phenotypes through the gut–brain axis (41). Valeric acid may play a role in the pathological process of depression due to its resemblance to GABA (42). Caproic acid levels were significantly increased in the CUMS group but not detectable in the PCO group. Additionally, the valeric acid concentration was lower in the PCO group than in the CUMS group. Taken together, these findings suggest that Ruminococcus and its metabolite SCFAs, especially caproic acid, may be important segments of PCO-related gut microbiota-brain interactions. The genus Oscillibacter is also significantly associated with depression (42). The main metabolic end product of the Oscillibacter type strain is valeric acid (42). Our results suggest that Oscillibacter, like Ruminococcus, may be the bacteria responsible for mediating the antidepressant-like effect of PCO.
Brain 5-HT deficiency is considered to be a significant pathogenic mechanism in depression (41). The neurotransmitter 5-HT also plays significant roles in stress and anxiety (36). The restored level of 5-HT in the hippocampus of rats exposed to CUMS and treated with PCO may explain the anxiolytic- and antidepressant-like effects of PCO. The majority of human 5-HT is synthesized by specific gut endocrine cells. Additionally, the presence of certain gut bacteria can influence the biosynthesis of 5-HT (43). Furthermore, 5-HT can influence the maintenance of the gastrointestinal mucosa (36). Gut-derived fatty acids can cross the blood–brain barrier (BBB), interact with synaptic neurotransmitters, and influence behaviors (32). The results here confirmed that the central nervous system effects of PCO are associated with interactions with the gut microbiota.
Conclusion
In the present study, the impact of PCO on stress-sensitive behaviors was investigated using a rat model of CUMS. Our results suggest that PCO may have anxiolytic and antidepressant effects. These effects may be mediated by modulation of the gut microbiota and SCFAs, which in turn affect 5-HT levels in the brain via the gut–brain axis. These findings have important implications for the potential therapeutic use of PCO in the fields of medicine and health.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The animal study was approved by the Ethics Committee of Guangdong Provincial Hospital of Chinese Medicine. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
PO: Funding acquisition, Methodology, Resources, Writing—original draft. DK: Conceptualization, Funding acquisition, Supervision, Writing—original draft, Writing—review & editing. WY: Data curation, Formal analysis, Funding acquisition, Methodology, Validation, Writing—review & editing. XS: Methodology, Project administration, Writing—review & editing. XM: Investigation, Methodology, Project administration, Supervision, Writing—review & editing. YL: Formal analysis, Methodology, Writing—review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was funded by the Ningbo Natural Science Foundation (2019A610372), the Medical and Health Science and Technology Project of Zhejiang Province (2019RC270), the Key-Field Project of Colleges and Universities of Guangdong Province (Biomedicine and Health) from Department of Education of Guangdong Province (2021ZDZX2083), and the Natural Science Research Project of Guangdong Food and Drug Vocational College (2020ZR05).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Zhao M, Chen Y, Wang C, Xiao W, Chen S, Zhang S, et al. Systems pharmacology dissection of multi-scale mechanisms of action of Huo-Xiang-Zheng-Qi formula for the treatment of gastrointestinal diseases. Front Pharmacol. (2019) 9:1448. doi: 10.3389/fphar.2018.01448
2. Swamy MK, Sinniah UR. A comprehensive review on the phytochemical constituents and pharmacological activities of Pogostemon cablin Benth: an aromatic medicinal plant of industrial importance. Molecules. (2015) 20:8521–47. doi: 10.3390/molecules20058521
3. Akhila A, Tewari R. Chemistry of patchouli oil: a review. Curr Res Med Aromat Plants. (1984) 6:38–54.
4. Doukkali Z, Taghzouti K, Bouidida EL, Nadjmouddine M, Cherrah Y, Alaoui K. Evaluation of anxiolytic activity of methanolic extract of Urtica urens in a mice model. Behav Brain Funct. (2015) 11:19. doi: 10.1186/s12993-015-0063-y
5. Deng XY, Xue JS Li HY, Ma ZQ, Fu Q, Qu R, et al. Geraniol produces antidepressant-like effects in a chronic unpredictable mild stress mice model. Physiol Behav. (2015) 152:264–71. doi: 10.1016/j.physbeh.2015.10.008
6. Clapp M, Aurora N, Herrera L, Bhatia M, Wilen E, Wakefield S. Gut microbiota's effect on mental health: the gut-brain axis. Clin Pract. (2017) 7:987. doi: 10.4081/cp.2017.987
7. Burokas A, Arboleya S, Moloney RD, Peterson VL, Murphy K, Clarke G, et al. Targeting the microbiota-gut-brain axis: prebiotics have anxiolytic and antidepressant-like effects and reverse the impact of chronic stress in mice. Biol Psychiatry. (2017) 82:472–87. doi: 10.1016/j.biopsych.2016.12.031
8. Kelly JR, Borre Y, O'Brien C, Patterson E, Aidy SE, Deane J, et al. Transferring the blues: depression-associated gut microbiota induces neurobehavioural changes in the rat. J Psychiatr Res. (2016) 82:109–18. doi: 10.1016/j.jpsychires.2016.07.019
9. Hall HN, Wilkinson DJ, Le Bon M. Oregano essential oil improves piglet health and performance through maternal feeding and is associated with changes in the gut microbiota. Anim Microbiome. (2021) 3:2. doi: 10.1186/s42523-020-00064-2
10. Sánchez-Quintero MJ, Delgado J, Medina-Vera D, Becerra-Muñoz VM, Queipo-Ortuño MI, Estévez M, et al. Beneficial effects of essential oils from the mediterranean diet on gut microbiota and their metabolites in ischemic heart disease and type-2 diabetes mellitus. Nutrients. (2022) 14:4650. doi: 10.3390/nu14214650
11. Huang YJ, Tsai MS, Panyod S, Liu PY, Lu KH, Weng CY, et al. Garlic essential oil ameliorates depression-like behaviors in unpredictable chronic mild stress by modulating the brain NLRP3 inflammasome pathway and influencing the gut barrier and microbiota. Food Funct. (2023) 14:6998–7010. doi: 10.1039/D3FO00270E
12. Li D, Wu H, Dou H, Guo L, Huang W. Microcapsule of sweet orange essential oil changes gut microbiota in diet-induced obese rats. Biochem Biophys Res Commun. (2018) 505:991–5. doi: 10.1016/j.bbrc.2018.10.035
13. Evans JM, Morris LS, Marchesi JR. The gut microbiome: the role of a virtual organ in the endocrinology of the host. J Endocrinol. (2013) 218:R37–47. doi: 10.1530/JOE-13-0131
14. Bibbò S, Fusco S, Ianiro G, Settanni CR, Ferrarese D, Grassi C, et al. Gut microbiota in anxiety and depression: pathogenesis and therapeutics. Front Gastroenterol. (2022) 1:1019578. doi: 10.3389/fgstr.2022.1019578
15. Willner P. Validity, reliability and utility of the chronic mild stress model of depression: a 10-year review and evaluation. Psychopharmacology. (1997) 134:319–29. doi: 10.1007/s002130050456
16. Kraeuter AK, Guest PC, Sarnyai Z. The open field test for measuring locomotor activity and anxiety-like behavior. Methods Mol Biol. (2019) 1916:99–103. doi: 10.1007/978-1-4939-8994-2_9
17. Porsolt RD, Anton G, Blavet N, Jalfre M. Behavioural despair in rats: a new model sensitive to antidepressant treatments. Eur J Pharmacol. (1978) 47:379–91. doi: 10.1016/0014-2999(78)90118-8
18. Li Y, Peng Y, Ma P, Yang H, Xiong H, Wang M, et al. Antidepressant-like effects of Cistanche tubulosa extract on chronic unpredictable stress rats through restoration of gut microbiota homeostasis. Front Pharmacol. (2018) 9:967. doi: 10.3389/fphar.2018.00967
19. Liao B, Hu H, Xiao S, Zhou G, Sun W, Chu Y, et al. Global Pharmacopoeia Genome Database is an integrated and mineable genomic database for traditional medicines derived from eight international pharmacopoeias. Sci China Life Sci. (2022) 65:809–17. doi: 10.1007/s11427-021-1968-7
20. Wang R, Peng Y, Meng H, Li X. Protective effect of polysaccharides fractions from Sijunzi decoction in reserpine-induced spleen deficiency rats. RSC Adv. (2016) 6:60657–65. doi: 10.1039/C6RA06361F
21. Hou T, Li X, Peng C. Borneol enhances the antidepressant effects of asiaticoside by promoting its distribution into the brain. Neurosci Lett. (2017) 646:56–61. doi: 10.1016/j.neulet.2017.02.068
22. Clarke G, Grenham S, Scully P, Fitzgerald P, Moloney RD, Shanahan F, et al. The microbiome-gut-brain axis during early life regulates the hippocampal serotonergic system in a sex-dependent manner. Mol Psychiatry. (2013) 18:666–73. doi: 10.1038/mp.2012.77
23. Yang L, Zhao Y, Wang Y, Liu L, Zhang X, Li B, et al. The effects of psychological stress on depression. Curr Neuropharmacol. (2015) 13:494–504. doi: 10.2174/1570159X1304150831150507
24. De Rooij SR, Schene AH, Phillips DI, Roseboom TJ. Depression and anxiety: Associations with biological and perceived stress reactivity to a psychological stress protocol in a middle-aged population. Psychoneuroendocrinology. (2010) 35:866–77. doi: 10.1016/j.psyneuen.2009.11.011
25. Dubey VK, Ansari F, Vohora D, Khanam R. Possible involvement of corticosterone and serotonin in antidepressant and antianxiety effects of chromium picolinate in chronic unpredictable mild stress induced depression and anxiety in rats. J Trace Elem Med Biol. (2015) 29:222–6. doi: 10.1016/j.jtemb.2014.06.014
26. Hao Z, Wang W, Guo R, Liu H. Faecalibacterium prausnitzii (ATCC 27766) has preventive and therapeutic effects on chronic unpredictable mild stress-induced depression-like and anxiety-like behavior in rats. Psychoneuroendocrinology. (2019) 104:132–42. doi: 10.1016/j.psyneuen.2019.02.025
27. Huang HJ, Zhu XC, Han QQ, Wang YL, Yue N, Wang J, et al. Ghrelin alleviates anxiety-and depression-like behaviors induced by chronic unpredictable mild stress in rodents. Behav Brain Res. (2017) 326:33–43. doi: 10.1016/j.bbr.2017.02.040
28. Li N, Wang Q, Wang Y, Sun A, Lin Y, Jin Y, et al. Fecal microbiota transplantation from chronic unpredictable mild stress mice donors affects anxiety-like and depression-like behavior in recipient mice via the gut microbiota-inflammation-brain axis. Stress. (2019) 22:592–602. doi: 10.1080/10253890.2019.1617267
29. Xia J, Lu Z, Feng S, Yang J, Ji M. Different effects of immune stimulation on chronic unpredictable mild stress-induced anxiety-and depression-like behaviors depending on timing of stimulation. Int Immunopharmacol. (2018) 58:48–56. doi: 10.1016/j.intimp.2018.03.010
30. Leong W, Huang G, Khan I, Xia W, Li Y, Liu Y, et al. Patchouli essential oil and its derived compounds revealed prebiotic-like effects in C57BL/6J mice. Front Pharmacol. (2019) 10:1229. doi: 10.3389/fphar.2019.01229
31. Paiva IHR, Duarte-Silva E, Peixoto CA. The role of prebiotics in cognition, anxiety, and depression. Eur Neuropsychopharmacol. (2020) 34:1–18. doi: 10.1016/j.euroneuro.2020.03.006
32. Hsiao EY, McBride SW, Hsien S, Sharon G, Hyde ER, McCue T, et al. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell. (2013) 155:1451–63. doi: 10.1016/j.cell.2013.11.024
33. Sen P, Sherwin E, Sandhu K, Bastiaanssen TFS, Moloney GM, Golubeva A, et al. The live biotherapeutic Blautia stercoris MRx0006 attenuates social deficits, repetitive behaviour, and anxiety-like behaviour in a mouse model relevant to autism. Brain Behav Immun. (2022) 106:115–26. doi: 10.1016/j.bbi.2022.08.007
34. Un-Nisa A, Khan A, Zakria M, Siraj S, Ullah S, Tipu MK, et al. Updates on the role of probiotics against different health issues: focus on Lactobacillus. Int J Mol Sci. (2022) 24:142. doi: 10.3390/ijms24010142
35. Caspani G, Kennedy S, Foster JA, Swann J. Gut microbial metabolites in depression: understanding the biochemical mechanisms. Microb Cell. (2019) 6:454–81. doi: 10.15698/mic2019.10.693
36. Carhart-Harris RL, Nutt DJ. Serotonin and brain function: a tale of two receptors. J Psychopharmacol. (2017) 31:1091–120. doi: 10.1177/0269881117725915
37. Szczesniak O, Hestad KA, Hanssen JF, Rudi K. Isovaleric acid in stool correlates with human depression. Nutr Neurosci. (2016) 19:279–83. doi: 10.1179/1476830515Y.0000000007
38. Wu M, Tian T, Mao Q, Zou T, Zhou CJ, Xie J, et al. Associations between disordered gut microbiota and changes of neurotransmitters and short-chain fatty acids in depressed mice. Transl Psychiatry. (2020) 10:350. doi: 10.1038/s41398-020-01038-3
39. Lukić I, Getselter D, Ziv O, Oron O, Reuveni E, Koren O, et al. Antidepressants affect gut microbiota and Ruminococcus flavefaciens is able to abolish their effects on depressive-like behavior. Transl Psychiatry. (2019) 9:133. doi: 10.1038/s41398-019-0466-x
40. Lu S, Jin H, Wang Y, Tao Y. Genome-wide transcriptomic analysis of n-caproic acid production in Ruminococcaceae bacterium CPB6 with Lactate supplementation. J Microbiol Biotechnol. (2021) 31:1533–44. doi: 10.4014/jmb.2107.07009
41. Capuco A, Urits I, Hasoon J, Chun R, Gerald B, Wang JK, et al. Current perspectives on gut microbiome dysbiosis and depression. Adv Ther. (2020) 37:1328–46. doi: 10.1007/s12325-020-01272-7
42. Naseribafrouei A, Hestad K, Avershina E, Sekelja M, Linløkken A, Wilson R, et al. Correlation between the human fecal microbiota and depression. Neurogastroenterol Motil. (2014) 26:1155–62. doi: 10.1111/nmo.12378
Keywords: Pogostemon cablin, essential oil, microbiota, gut–brain axis, chronic unpredictable mild stress
Citation: Ouyang P, Kang D, You W, Shen X, Mo X and Liu Y (2024) Pogostemon cablin essential oil affects anxiety- and depressive-like behaviors and the gut microbiota in chronic unpredictable mild stress model rats. Front. Nutr. 11:1303002. doi: 10.3389/fnut.2024.1303002
Received: 27 September 2023; Accepted: 29 January 2024;
Published: 14 February 2024.
Edited by:
Kuo Zhang, Shenyang Pharmaceutical University, ChinaReviewed by:
Khairan Khairan, Syiah Kuala University, IndonesiaMei Bai, South China Agricultural University, China
Kaijian Hou, Shantou University, China
Copyright © 2024 Ouyang, Kang, You, Shen, Mo and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dali Kang, ZGxrYW5nNTAwQHN5bWMuZWR1LmNu
 Puyue Ouyang1
Puyue Ouyang1 Dali Kang
Dali Kang Xiaozhong Shen
Xiaozhong Shen