- 1Department of Infectious Disease, Qingdao Municipal Hospital, Shandong University, Jinan, China
- 2Department of Clinical Nutrition, The Affiliated Hospital of Qingdao University, Qingdao, China
- 3Department of Gastroenterology, Qingdao Municipal Hospital, Shandong University, Jinan, China
- 4Clinical Research Center, Qingdao Municipal Hospital, Qingdao, China
- 5Department of Radiology, The Affiliated Hospital of Qingdao University, Qingdao, China
- 6Department of Liver Surgery and Transplantation, Liver Cancer Institute and Zhongshan Hospital, Fudan University, Shanghai, China
Background/objectives: Hepatic steatosis and fibrosis represent significant and growing global health burdens. There is an urgent need to seek strategies for early prevention and control of hepatic steatosis and fibrosis. This study attempted to comprehensively evaluate the relationship between dietary nutrient intake and the risk of hepatic steatosis and fibrosis to provide assistance for doctors in guiding the diet of the patients.
Methods: This observational study assembled 15,560 participants from the 2017–March 2020 cohorts of the National Health and Nutrition Examination Survey. 34 nutrient intake items were included. The liver ultrasound transient elastography was used to evaluate hepatic steatosis and hepatic fibrosis. Various variables, encompassing sociodemographic characteristics, and other potential confounders were considered to ensure the stability of the findings. Additionally, the analysis accounted for various covariates and employed restricted cubic spline analysis to examine potential nonlinear relationships. Weighted quantile sum (WQS) (mixed effect) models were used in the analysis.
Results: The negative correlations were found between low carbohydrate, vitamin C, pyridoxine, magnesium, iron and potassium intake with controlled attenuation parameter (CAP) after adjusting all the covariates and excluding non-linear correlations. Nonlinear correlation was found to exist between the consumption of energy, vitamin E, folate, sodium, alcohol, α-Linolenic acid and fish oil and hepatic steatosis (p < 0.05). The negative correlations were showed between low dietary fiber per energy and phosphorous intake with liver stiffness measurement (LSM) after adjusting all the covariates and excluding non-linear correlations (p < 0.05). High caffeine intake showed the positive correlation with LSM in Model3 after adjusting all covariates (p = 0.022). The majority of dietary nutrients intake were found to have nonlinear relationships with liver fibrosis.
Conclusion: Overall, many nutrient variables were newly identified associations with hepatic steatosis and fibrosis. Critical threshold intake levels were revealed that may elevate disease risk. These findings may help us better understand the complex relationship between diet and hepatic steatosis and fibrosis. Moreover, this data provides critical insights for establishing evidence-based clinical nutrition strategies to optimize the prevention and management of liver diseases.
1 Introduction
Chronic liver disease has emerged as a major public health burden worldwide, which can develop into cirrhosis and liver failure (1, 2), diminishing quality of life and even resulting in death (3, 4). Hepatic steatosis and fibrosis are common pathophysiological process during the development of liver diseases (5). Hepatic steatosis is a condition that occurs when excess fat builds up in the liver. It can be caused by a number of factors, including an imbalance of lipids, insulin resistance, and metabolic syndrome. Hepatic steatosis is increasingly common and represents a very frequent diagnosis in the medical field, which could contributes to the progression toward liver fibrosis (6). Despite the use of limited herbal medicines (e.g., silymarin, quercetin, hesperidin, and berberine) and natural compounds with fewer side effects in preventing and treating liver diseases, there is still a lack of effective treatment methods for hepatic steatosis and fibrosis (7–9).
Liver ultrasound transient elastography is widely used to provide objective measures for hepatic steatosis and fibrosis. By measuring the velocity of the mechanically generated shear wave through the liver, the transient elastography has been found to better diagnostic accuracy than other non-invasive diagnostic methods (10–12). Controlled attenuation parameter (CAP) and liver stiffness measurement (LSM) were obtained to assess hepatic steatosis and fibrosis (13).
Nutrition factors were revealed to have significant impacts on hepatic steatosis and fibrosis (14–17). However, there is a lack of large-scale cohort study research on the comprehensive analysis of the correlation between various nutrients and hepatic steatosis/fibrosis. The NHANES is the most in-depth survey that measures the health and nutrition of the US national population. Current research landscapes reveal persistent gaps in systematically elucidating the pan-nutrient hepatoprotective mechanisms through large-scale cohort studies, particularly regarding dose-dependent effects across liver disease spectra16. Notably, even for extensively investigated micronutrients (e.g., iron overload in steatosis, vitamin D pleiotropy), conflicting evidence persists about their therapeutic thresholds and pathophysiological duality (protective vs. pro-fibrotic roles). This survey collected data through interviews, standard exams, and biospecimen collection, providing a standardized environment for the health examinations (18). Liver ultrasound transient elastography was conducted for the first time from 2017 (19), while the latest updated data of the results is up to March 2020. We wanted to use the latest data from the cycles of the 2017–March 2020 to explore the associations between dietary nutrition consumption with hepatic steatosis and fibrosis. Therefore, in order to explore the relationship between nutrition and chronic liver disease, we conducted from the perspective of dietary nutrition intake.
2 Materials and methods
2.1 Study population
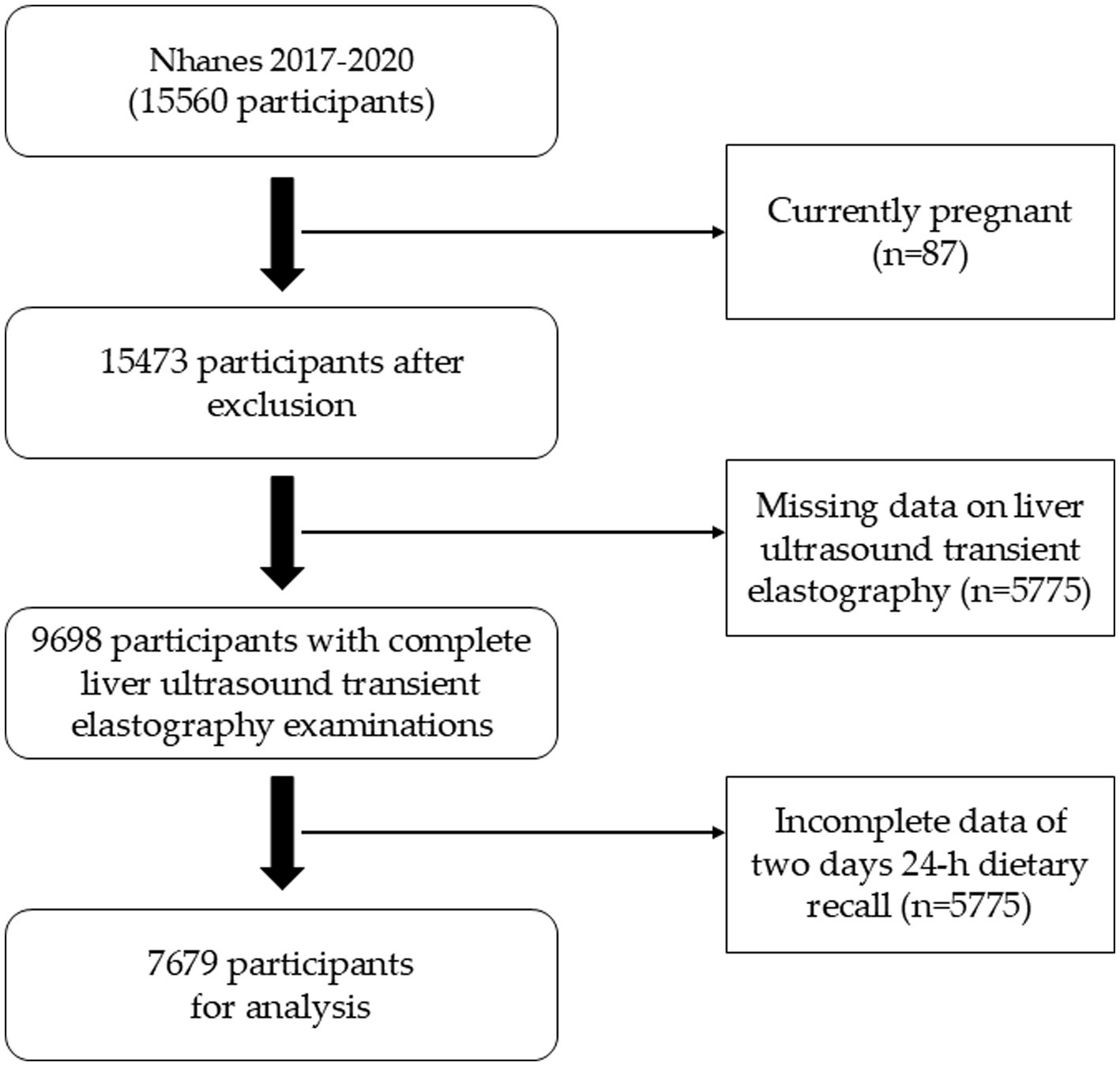
The databases were obtained from the NHANES website.1 We analyzed the data from the NHANES cycles of 2017–March 2020. A total of 15,560 participants were included. Those currently pregnant (n = 87) were excluded from the analysis. 5,862 participants without CAP or LSM measurement were excluded in the study. The participants lack of two-day dietary survey data were also excluded. Finally, our study enrolled 7,679 participants. Detailed study flow was shown as Figure 1.
2.2 Study variables
The outcome variables CAP and LSM were evaluated by liver ultrasonographic transient elastography using the FibroScan-equipped model 502 V2 Touch. CAP values ≥274 dB/m was considered indicative of non-steatosis NAFLD status, while CAP ≥302 dB/m was defined as severe steatosis (12, 13). Fibrosis grade was determined by LSM with values of 8.2, 9.7, and 13.6 kPa (13, 20). Serum specimens were taken and stored frozen to obtain biochemical evaluations, then shipped to the University of Minnesota, Minneapolis, MN, United States for further analysis.
The laboratory parameters encompassed plasma triglyceride (TG), total cholesterol (TC), fasting plasma glucose (FPG) and Glycohemoglobin A1c (HbA1c). Demographic data including age, gender, race, education, marital status, poverty income ratio (PIR), alcohol consumption, smoking habits, and medication usage, were collected through standardized questionnaires. Educational levels were categorized into three types: “less than high school,” “high school or equivalent,” and “higher than high school.” Marital status was categorized into three types: “never married,” “married/cohabiting,” or “separated/divorced/widowed.” PIR was classified as three categories: <1.30, 1.30–3.50, and > 3.50 (21). Alcohol consumption was delineated into three groups: individuals who never consumed alcohol, moderate drinkers (1–2 drinks per day for males, 1 drink per day for females), and heavy drinkers (≥3 drinks per day for males, ≥2 drinks per day for females) (21, 22). Smoking habits were categorized into three tiers: low (serum cotinine < 0.015 ng/mL), moderate (0.015–3 ng/mL), and high level (serum cotinine >3 ng/mL) (22, 23).
2.3 Dietary assessment
The dietary nutrients consumption was assessed using a 24-h dietary recall method conducted by trained interviewers, who are fluent in Spanish and English. Two dietary assessments were conducted for each participant, with a time interval of 3 to 10 days between them. The first assessment was conducted face-to-face, while the second assessment was conducted via telephone. The setting of the interview is a private room in the Mobile Examination Center (MEC). Each MEC dietary interview room contains a standard set of measuring guides. These tools are used to help the respondent report the volume and dimensions of the food items consumed. They are not intended to represent any one particular food, but rather are designed to help respondents estimate portion sizes. This set of measuring guides is designed specifically for use in the current NHANES setting with a target population of non-institutionalized U.S. civilians. The tools are helpful in portion size estimation for a wide variety of foods (24). We choose nutrients which had established cut points. 34 energy and nutrient intake items were estimated by 24-h dietary recall method. The normal range of each parameter is shown in Supplementary materials. These cut points were taken from a standard textbook, the Dietary Reference Intake (DRIs), published guidelines, and previous studies (25–32).
2.4 Statistical analyses
The demographic and abnormal nutrients intakes characteristics are presented as mean ± standard deviation (SD) for continuous variables and as frequency (%) for binary or categorical variables. All values of the data were weighted by weights provided by NHANES. Dietary nutrient consumptions were applied to natural log transformed to address the right-skewed distribution approximate a normal distribution. Descriptive statistics of outcome were presented according to the categories of CAP and LSM. Subgroup differences were explored by chi-square, ANOVA, or Kruskal–Wallis H-test as appropriate. Variables, with different distribution between groups column-stratified by outcomes variables, were further analyzed by multiple linear regression.
Multiple linear regression analysis was used to assess the associations between each dietary nutrient consumption and hepatic steatosis and liver cirrhosis. We formulated three distinct models were to assess the correlation between covariates and the outcomes. Model 1 remained unadjusted, while Model 2 included partial adjustments for age, race, gender, PIR, marital status, and educational level. Model 3 were adjusted for potential covariates including age, gender, race, marriage status, education level, PIR, smoking habits, drinking status, daily energy consumption, TC, TG, Glycohemoglobin and FPG. Energy and dietary fiber per energy intakes were not adjusted for energy intake. The analysis accounted for various covariates and employed restricted cubic spline analysis (RCS) to examine potential nonlinear relationships, when no linear association was found. Spearman’s correlation analysis was conducted to evaluate associations among dietary nutrients. All dietary nutrients were integrated into the weighted quantile sum (WQS) model to examine the collective impact of nutrient mixtures on hepatic steatosis and liver cirrhosis (33). All statistical analyses were performed using R version 4.4.1. A p-value below 0.05 was considered as statistically significant.
3 Results
3.1 Baseline characteristics
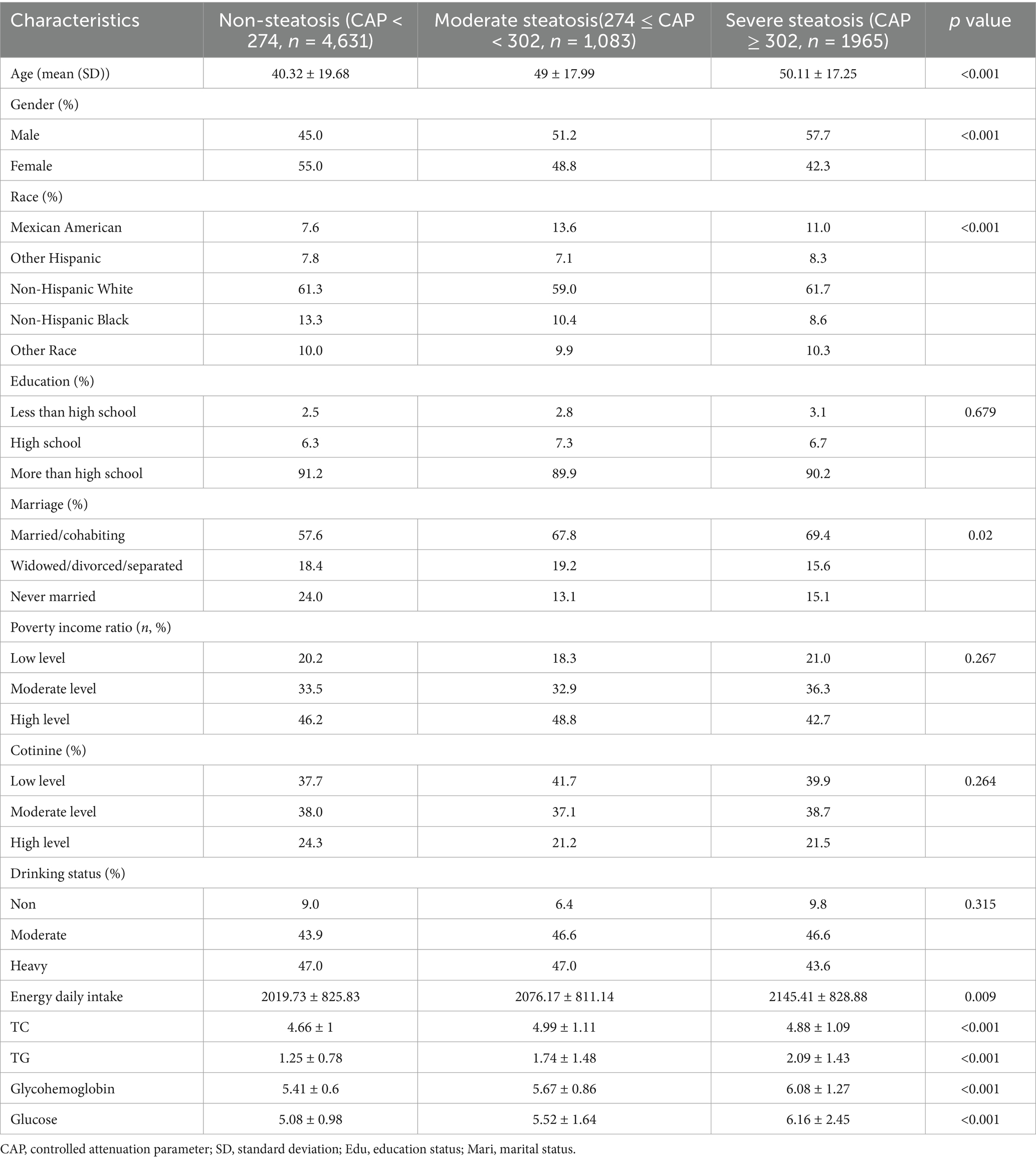
15,560 participants from 2017 to March 2020 were included in the study. 5,862 participates were removed participants for lacked of Liver ultrasound transient elastography results. Then, 2019 participates were excluded who did not complete the two-day 24-h dietary survey. Finally, a total of 7,679 subjects met the criteria for inclusion in this analysis (Figure 1). Table 1 showed the baseline characteristics of the participants column-stratified by CAP. Severe hepatic steatosis is more likely to be men, older, married/cohabiting and intake higher energy daily. As to race, Non-Hispanic White and Mexican Americans have a larger proportion. In addition, the non-steatosis group have lower TG, TC, FPG and glycohemoglobin.
As is shown in Table 2, the cirrhosis group is also more likely to be men and older. The differences in race and daily energy intake level are not as significant as in hepatic steatosis. The analysis of poverty income ratio showed severe liver fibrosis group is less likely to be high income group. The non-steatosis group more likely have lower TG, FPG and glycohemoglobin. The difference in TC is not as significant as for different hepatic steatosis groups.
3.2 Associations with CAP
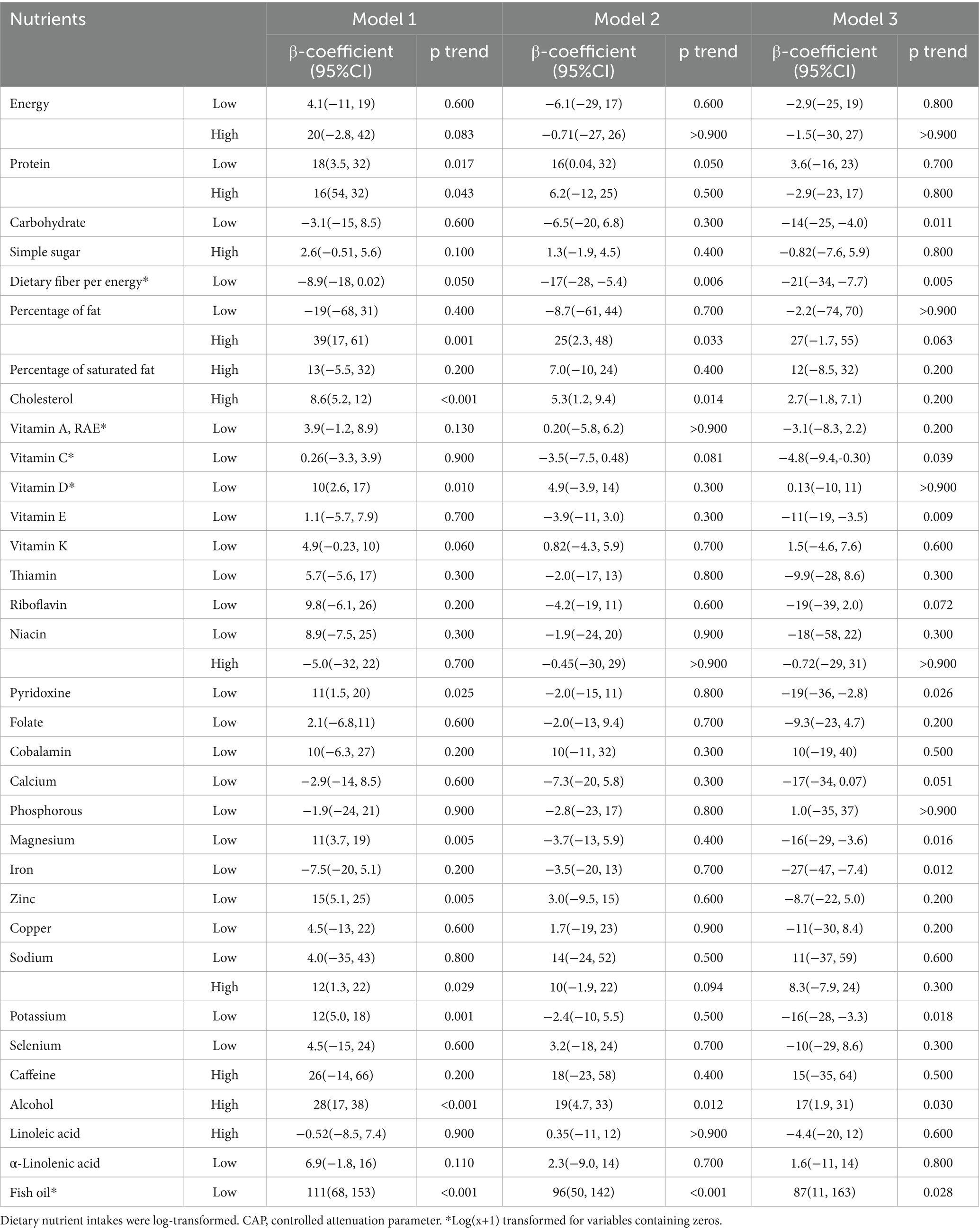
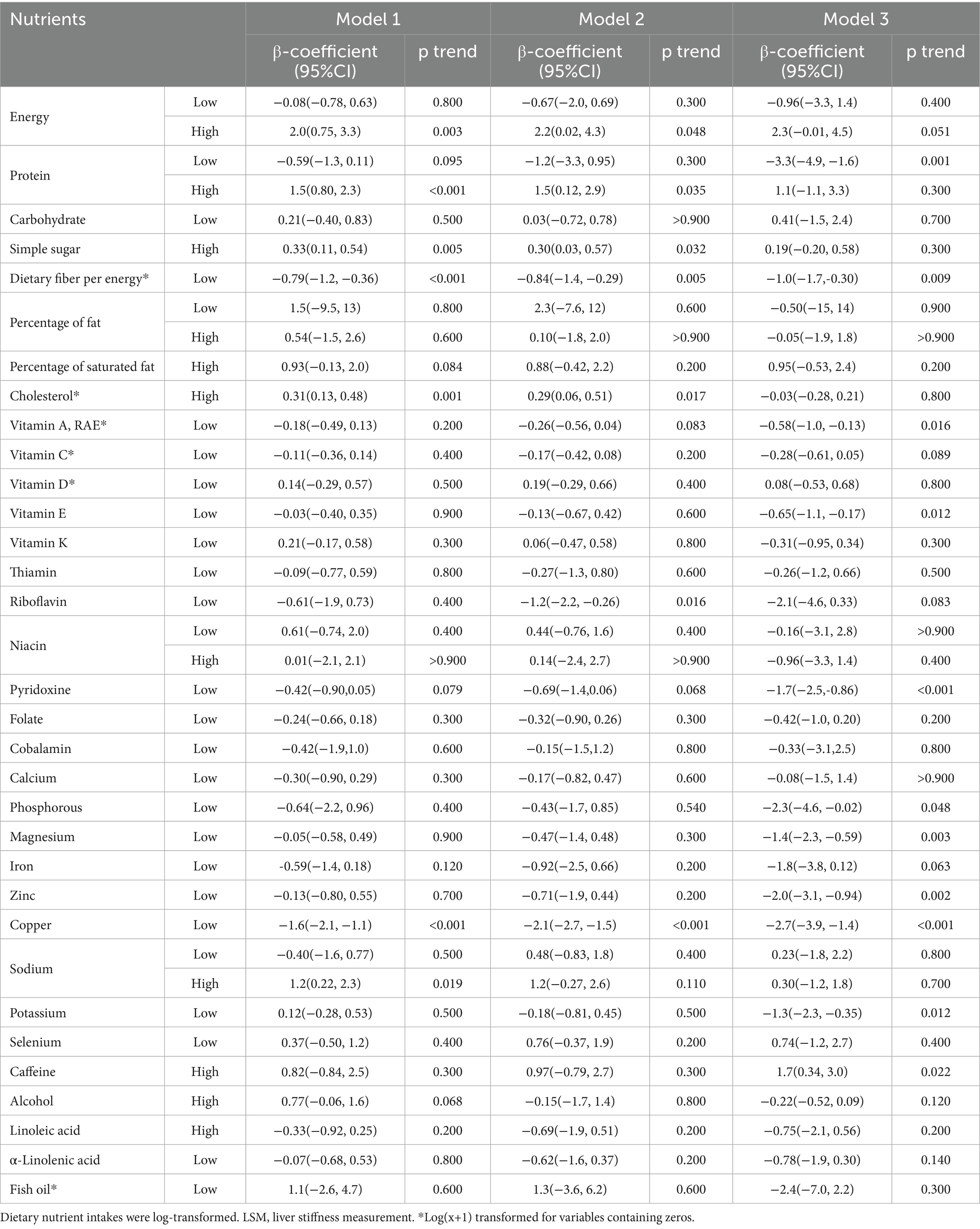
The participants column-stratified by CAP had different distribution of abnormal consumption of carbohydrate, dietary fiber per energy, cholesterol, thiamin, riboflavin, cobalamin, calcium, selenium and caffeine (Table 3). Then Table 4 presented the associations between daily nutrients consumption with CAP by a series of multiple linear regression models. The negative correlations were showed between low carbohydrate, low vitamin C, low vitamin E, pyridoxine, magnesium, iron and potassium intake with CAP only in Model 3 (p = 0.011, 0.039, 0.009, 0.024, 0.016, 0.012, and 0.018, respectively). Low dietary fiber per energy intake showed the negative correlation with CAP both in Model 2 and 3 (p = 0.006 and 0.005). High percentage of fat and high cholesterol intake showed the positive correlation with CAP both in Model 1 and 2 but not in Model 3 after adjusting all covariates (p = 0.001, 0.033, 0.063, <0.001, 0.014, and 0.200, respectively). Low vitamin D, low protein intake, high protein intake was only showed negative correlation with CAP only in Model 1 (p = 0.010, 0.017, and 0.043, respectively), the correlation is no longer significant after adjusting covariates. High sodium intake was only showed positive correlation with CAP only in Model 1 (p = 0.029). In Model 1, Model 2 and Model3, significant positive correlation was consistently evident between high alcohol intake and low fish oil with CAP (p < 0.001, 0.012, 0.030, <0.001, <0.001, and 0.028, respectively).
3.3 Associations with LSM
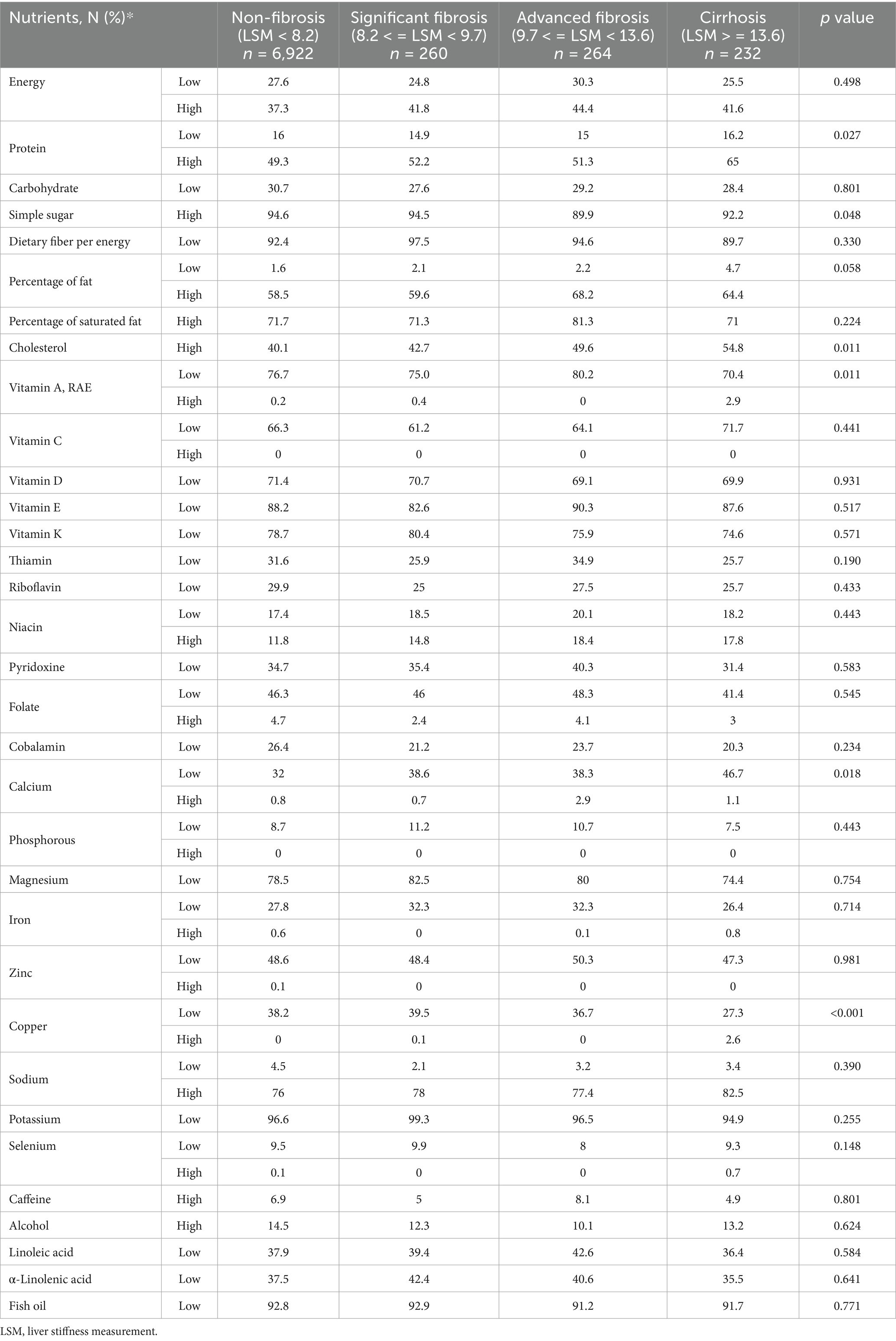
Table 5 revealed the outcomes derived from multiple logistic regression models scrutinizing the potential independent associations between 34 abnormal nutrients consumption and LSM. Protein, vitamin A, cholesterol, calcium and Copper’ abnormal consumption showed different distribution among participants groups column-stratified by LSM (Table 5). Table 6 presented the associations between protein per weight and cobalamin with LSM by a series of multiple linear regression models. The negative correlations were showed between low protein intake, low dietary fiber per energy, low vitamin A, low vitamin E, pyridoxine, phosphorous, magnesium, zinc, copper and potassium intake with LSM in Model 3 (p = 0.001, 0.009, 0.016, 0.012, <0.001, 0.048, 0.003, 0.002, 0.002, and 0.012, respectively). High caffeine intake showed the positive correlation with LSM in Model 3 after adjusting all covariates (p = 0.022).
3.4 Nonlinear relationships
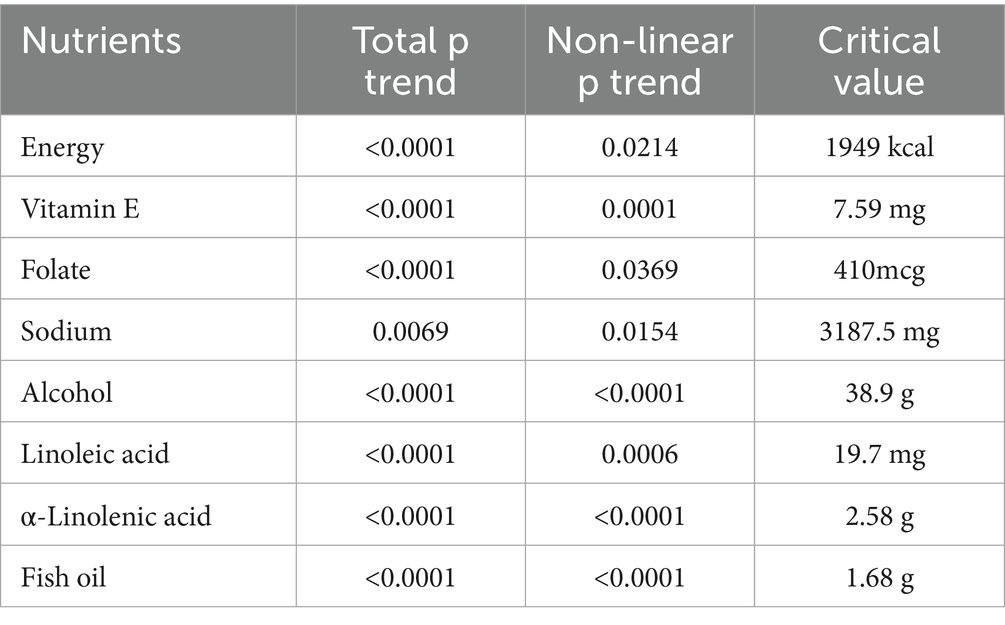
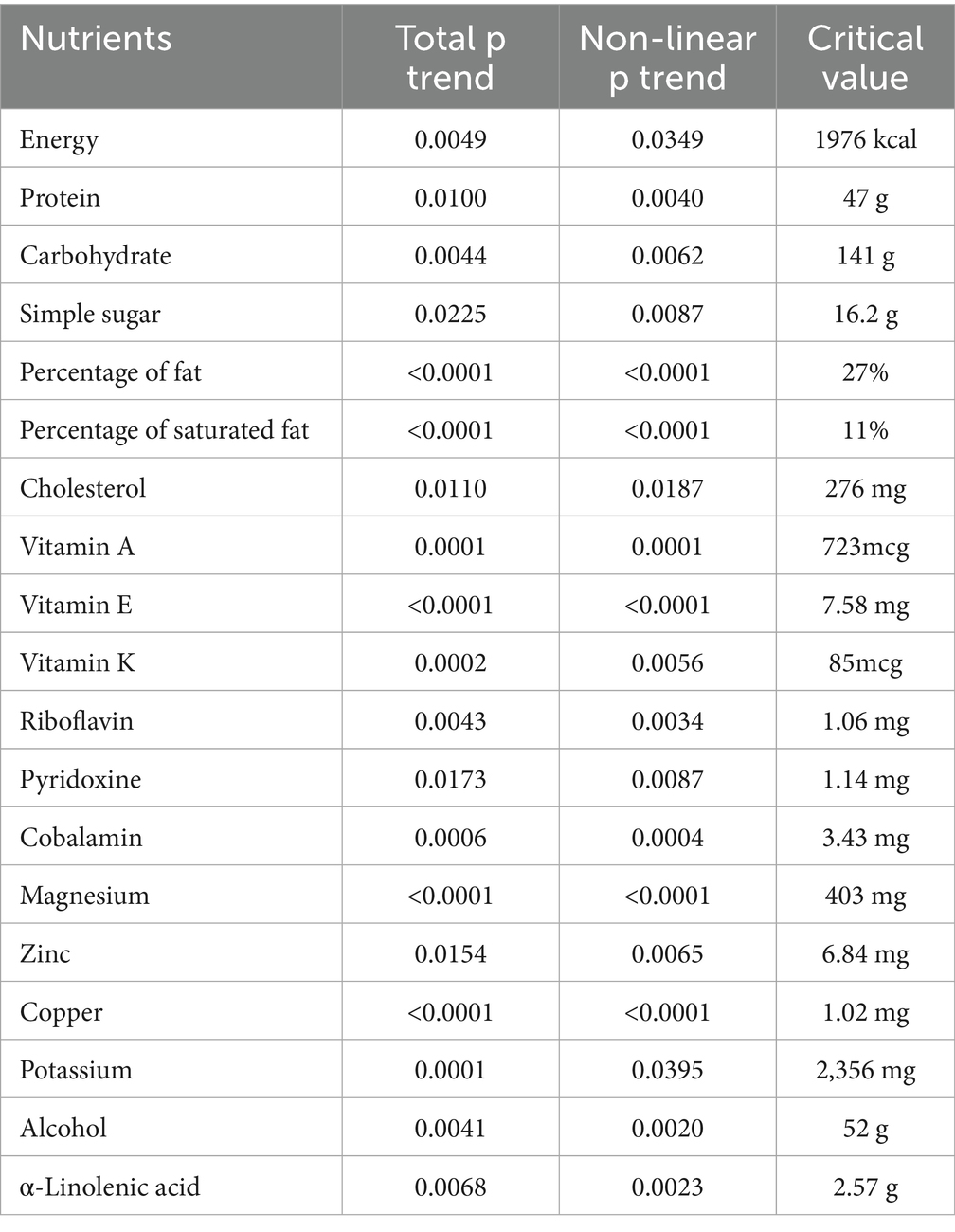
As is shown in Supplementary Figure 1, nonlinear relationships were found between energy, vitamin E, folate, sodium, alcohol, α-Linolenic acid and fish oil with hepatic steatosis in the fully adjusted model (p < 0.05). The associations of these nutrients with risk of hepatic steatosis changed at these points (energy: 1949 kcal, vitamin E: 7.59 mg, folate: 410mcg, sodium: 3187.5 mg, alcohol: 38.9 g, linoleic acid: 19.7 mg, α-linolenic acid: 2.58 g, fish oil: 1.68 g) (Table 7). The intake of most dietary nutrients, including energy, protein, carbohydrate, simple sugars, dietary fiber per energy, percentage of fat, percentage of saturated fat, cholesterol, vitamin A, vitamin E, vitamin K, pyridoxine, cobalamin, magnesium, zinc, copper, potassium, potassium, alcohol and α-Linolenic acid, were found to have nonlinear relationships with liver fibrosis (p < 0.05) (Supplementary Figure 1). The associations of these nutrients with increasing risk of liver cirrhosis changed at these points (energy: 1976 kcal, protein: 47 g, carbohydrate: 141 g, simple sugars: 16.2 g, percentage of fat: 27%; percentage of saturated fat: 11%; cholesterol: 276 mg; vitamin A: 723mcg, vitamin E: 7.58 mg, vitamin K: 85mcg, riboflavin: 1.06 mg, pyridoxine: 1.14 mg, cobalamin: 3.43 mg, magnesium: 403 mg, zinc: 6.84 mg, copper: 1.02 mg, potassium: 2356 mg, alcohol: 52 g, α-linolenic acid: 2.57 g) (Table 8).
3.5 Spearman’s and WQS regression analysis
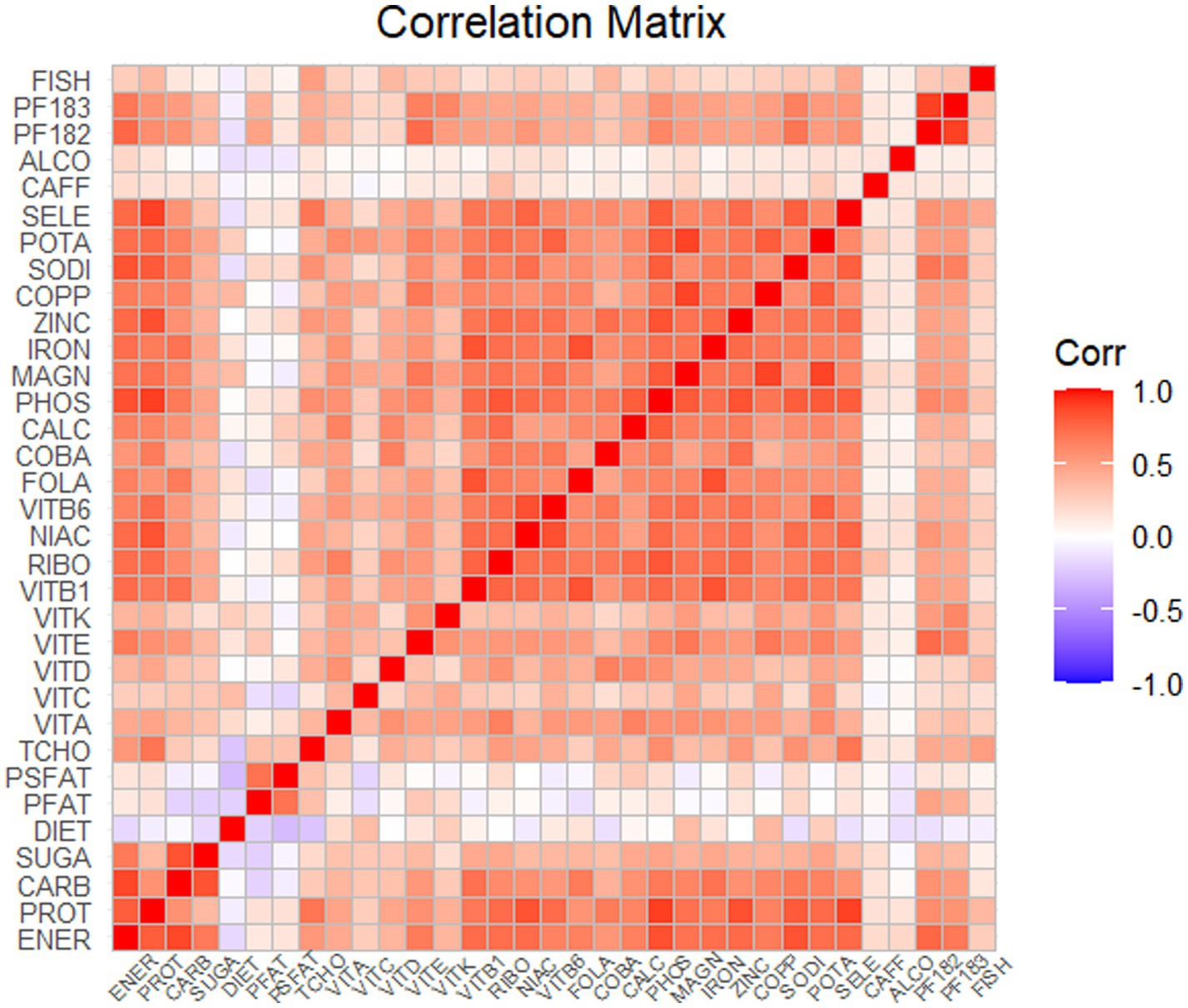
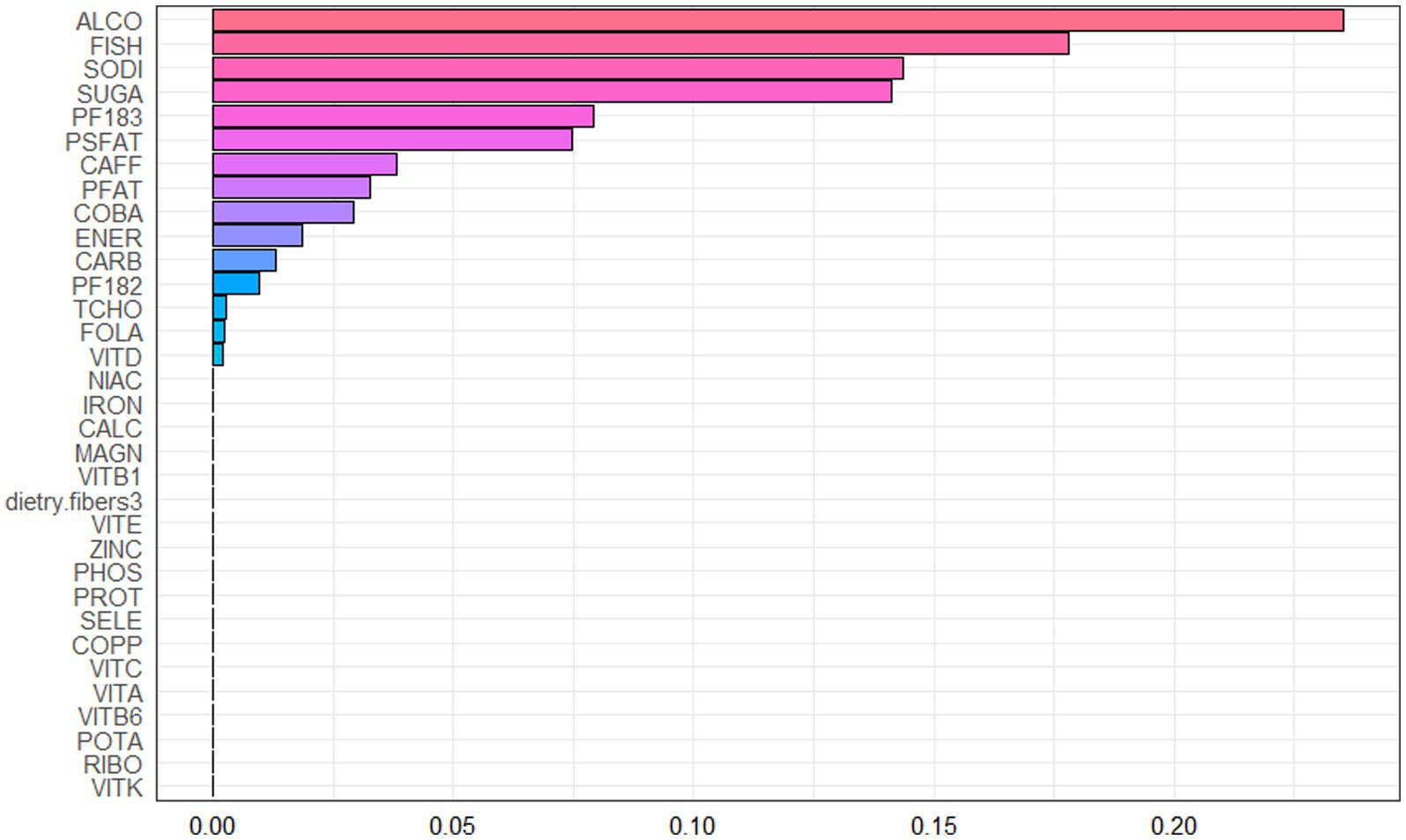
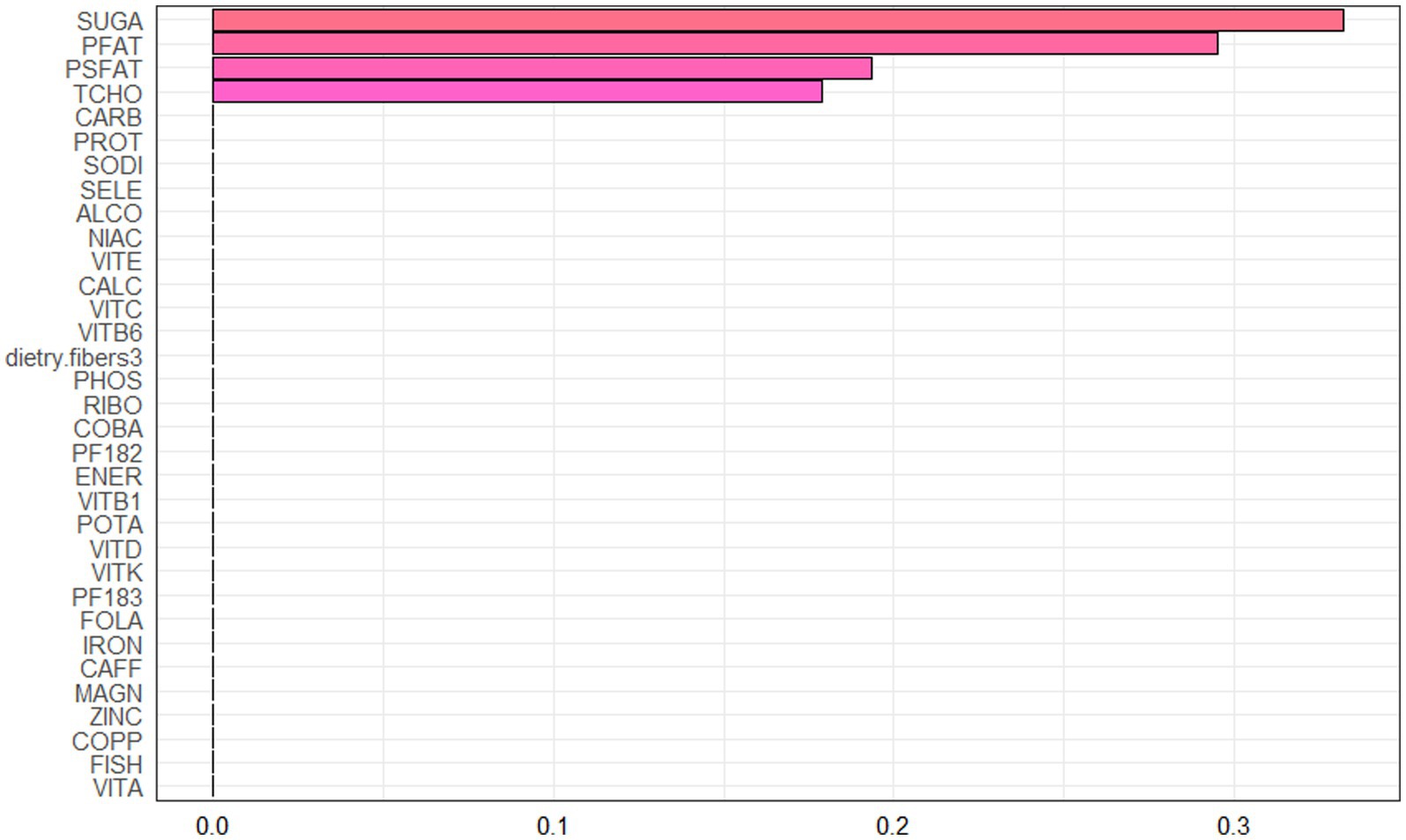
Spearman correlation analysis was used to analyze the association among the dietary nutrients. It revealed that significant positive associations among the majority of dietary nutrients (Figure 2, Supplementary Table 2). The WQS index of mixed dietary nutrients log-transformed intakes was positively associated with hepatic steatosis and liver fibrosis (β: 2.312, 95% CI: 0.897–2.578, p = 9.985e^−3; β: 0.143, 95% CI: 0.063–2.266, p = 0.024; respectively). The top four nutrients with higher weights associated with CAP in the WQS were: alcohol, fish oil, sodium and simple sugar. The top four nutrients with higher weights associated with LSM in the WQS were: simple sugar, percentage of fat, percentage of saturated fat and total cholesterol (Figures 3, 4).
4 Discussion
With the development of economy, the incidence of chronic liver disease has gradually increased and become a global health concern (34–37). The liver plays a major role in nutrient metabolism, including digestion, absorption, storage, synthesis and a series of physiological processes (38). The dysfunction of liver disrupt the homeostasis of nutrition metabolic homeostasis. Liver diseases include NAFLD, cirrhosis and liver failure could not only affect the metabolism of glucose and lipid, resulting in fat accumulation and protein deficiency, but also affect the metabolism a series of vitamins and microelements (39, 40).
The association between nutrition and liver diseases has been well recognized. This correlation exhibits bidirectional characteristics. On the one hand, recent studies have revealed that nutrition has a central prognostic and therapeutic role in the management of patients with liver disease (41). On the other hand, malnutrition and nutritional metabolic disorders is common in patients with liver disease and has a significant impact on disease progression and prognosis (42–44). Nutrition has been recognized as independent predictors of a higher rate of complications and lower survival in liver diseases (45–48).
Dietary nutrition is an important aspect of alimentology. Dietary patterns was revealed to have significant impacts on hepatic steatosis (17). As to liver cirrhosis, inadequate dietary intake seemed to be a self-dependent predictor of in-hospital mortality and affect progressive liver failure (49). The roles of various nutrients in this context have drawn attention. Current limited research findings indicate that the relationship between macronutrients and their effects remains controversial, particularly evident in the case of protein liver cirrhosis (14–17, 50, 51). As for micronutrients, the liver is important for their metabolism but the role of micronutrients in NAFLD remains less known (18, 33). Several micronutrients, such as zinc, copper, iron, selenium, magnesium, vitamins A, C, D, and E seems to have beneficial effects in NAFLD, however, the conclusions remain controversial and the appropriate dosage appears to be important but unclear (52, 53). Excess intake of some micronutrients such as iron and selenium may increase the severity of NAFLD as reported (54–56). Dissecting the specific contributions of micronutrients, however, remains challenging because human diets are complex and fail to replicate experimental dietary models (33). Exploring appropriate intake levels remains a critical research gap in current scientific investigations. In terms of liver fibrosis, zinc, vitamin D, vitamin E, copper and iron garnered research attention (57–61). Nevertheless, there is still a lack of comprehensive systematic research on the role of daily dietary nutrients in hepatic steatosis and liver fibrosis.
Thus, we conducted the study by analyzing the latest NHANES cycle. We used liver ultrasonographic transient elastography to monitor noninvasive hepatic steatosis and liver fibrosis. Daily dietary nutrient intake data were obtained through a standardized 24-h dietary survey to explore the associations between comprehensive dietary nutrients consumption and hepatic steatosis and liver fibrosis. Our study had discovered a correlation between some previously overlooked nutrients and NAFLD, such as pyridoxine, magnesium, potassium intake, linoleic acid,α-Linolenic acid. Nonlinear correlation was found to exist between the consumption of energy, vitamin E, folate, sodium, alcohol, α-Linolenic acid and fish oil and hepatic steatosis. High caffeine intake surprisingly showed the positive correlation with LSM in Model 3 after adjusting all covariates. We identified that intakes of many nutrients had significant nonlinear associations with hepatic fibrosis. The correlation between dietary nutrients and disease showed actually more complex relationship. RCS curves helped to reveal the recommended nutritional intake threshold to prevent hepatic steatosis. Energy less than 1,949 kcal, vitamin E higher than 7.59 mg, folate higher than 410mcg, sodium lower than 3187.5 mg, alcohol lower than 38.9 g, fish oil higher than 1.68 g 1 day, etc. may be the recommended consumption for fatty liver. It can be seen that these doses are not exactly the same as our daily RNI or UI values, which may provide the evidence to build a precise diseases-oriented nutrition strategy. The consumption such as fish oil’s benefits can only obtain when reaching a certain intake level, which exceed the daily intake level. Additional supplements may be necessary based on the evidence. As to liver fibrosis, enough nutrients intake was important, for example, protein intake higher than 47 g/d and carbohydrate higher than 141 g. In addition, micronutrients such as vitamin A, vitamin E, magnesium, zinc, copper also played their roles. RCS curves also revealed the recommended nutritional intake threshold as mentioned in the article. In contrast to previous studies, our research has identified that the majority of nutrient intake still follows a threshold effect. For certain nutrients previously considered of critical importance, such as vitamin D, alterations in daily intake do not appear to effectively reduce disease risks. These findings have provided us with significant insights.
Several limitations should be acknowledged in our study. This article involved a wide range of dietary nutrients providing guidance on dietary nutrients for hepatic steatosis and liver cirrhosis. However, it is limited by its excessive coverage, then many questions need further explore refined to each specific nutrient. Moreover, nutrition and liver damage, who is the cause? Who is the outcome? A positive or negative correlation is not enough. The status of severe steatosis and fibrosis may affect the dietary habit through affecting digestive function and feeding center. This is another issue worth exploring and considering. Large-scale prospective cohort studies, statistical analysis such as Mendelian analysis or more complex mathematical modeling methods are needed to help us further explore the relationship between nutrients and hepatic steatosis and liver fibrosis. By precise dietary adjustment, we may obtain more health benefits on our liver health.
5 Conclusion
The study identified various dietary nutrients associated with hepatic steatosis and liver fibrosis. Nonlinear correlations were observed between the consumption of energy, vitamin E, folate, sodium, alcohol, α-linolenic acid, fish oil, and hepatic steatosis. Similarly, liver fibrosis showed nonlinear relationships with the intake of multiple nutrients, including energy, protein, carbohydrates, simple sugars, dietary fiber per energy, fat percentage, saturated fat percentage, cholesterol, vitamins (A, E, K, pyridoxine, cobalamin), and minerals (magnesium, zinc, copper, potassium). Critical threshold intake levels in associated nutrients were revealed that may elevate disease risk. The impact of abnormal nutrient consumption appeared to be more pronounced in hepatic steatosis. These findings may help us better understand the complex relationship between diet and hepatic steatosis and fibrosis. Moreover, this data provides critical insights for establishing evidence-based clinical nutrition strategies to optimize the prevention and management of liver diseases. While recommended nutrient intake for liver conditions was identified, further research is needed to explore the effects of nutritional interventions and clarify causal relationships in chronic liver diseases.
Data availability statement
Publicly available datasets were analyzed in this study. This data can be found here: the databases were obtained from the NHANES website (https://www.cdc.gov/nchs/nhanes/index.htm).
Author contributions
QS: Conceptualization, Data curation, Project administration, Software, Validation, Writing – original draft. SL: Conceptualization, Project administration, Software, Writing – original draft. HY: Writing – original draft, Validation. HS: Validation, Writing – original draft. YX: Conceptualization, Resources, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by Grants of National Natural Science Foundation of China (32171277).
Acknowledgments
We are grateful to all NHANES participants and all NHANES researchers for making this data publicly available.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2025.1510860/full#supplementary-material
Footnotes
References
1. Wong, WA-O, Emdin, C, Bick, AG, Zekavat, SA-O, Niroula, A, Pirruccello, JA-O, et al. Clonal haematopoiesis and risk of chronic liver disease. Nature. (2023) 616:747–54. doi: 10.1038/s41586-023-05857-4
2. Estes, C, Razavi, HA-O, Loomba, R, Younossi, ZA-OX, and Sanyal, AJ. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology. (2018) 67:123–33. doi: 10.1002/hep.29466
3. Kim, DA-O, Wijarnpreecha, KA-O, Cholankeril, GA-O, and Ahmed, AA-O. Steatotic liver disease-associated all-cause/cause-specific mortality in the United States. Aliment Pharmacol Ther. (2024) 60:33–42. doi: 10.1111/apt.18011
4. Ye, Y, Wang, H, Gao, J, and Kostallari, E. Editorial: chronic liver disease: new targets and new mechanisms. Front Mol Biosci. (2022) 9:963630. doi: 10.3389/fmolb.2022.963630
5. Kisseleva, T, and Brenner, D. Molecular and cellular mechanisms of liver fibrosis and its regression. Nat Rev Gastroenterol Hepatol. (2021) 18:151–66. doi: 10.1038/s41575-020-00372-7
6. Tarantino, G, and Citro, V. What are the common downstream molecular events between alcoholic and nonalcoholic fatty liver? Lipids Health Dis. (2024) 23:41. doi: 10.1186/s12944-024-02031-1
7. Yan, J, Nie, Y, Luo, M, Chen, Z, and He, B. Natural compounds: a potential treatment for alcoholic liver disease? Front Pharmacol. (2021) 12:694475. doi: 10.3389/fphar.2021.694475
8. Nucera, S, Bulotta, RM, Ruga, S, Caminiti, R, Serra, M, Bava, R, et al. Natural products for the treatment of non-alcoholic fatty liver disease: a comprehensive review. Sci Pharm. (2023) 91:53. doi: 10.3390/scipharm91040053
9. The Lancet Gastroenterology Hepatology. The problem of antimicrobial resistance in chronic liver disease. Lancet Gastroenterol Hepatol. (2022) 7:495. doi: 10.1016/S2468-1253(22)00130-3
10. Castera, L, Friedrich-Rust, M, and Loomba, R. Noninvasive assessment of liver disease in patients with nonalcoholic fatty liver disease. Gastroenterology. (2019) 156:1264–1281.e4. doi: 10.1053/j.gastro.2018.12.036
11. Boursier, J, and Tsochatzis, EA. Case-finding strategies in non-alcoholic fatty liver disease. JHEP Rep. (2020) 3:100219. doi: 10.1016/j.jhepr.2020.100219
12. Eddowes, PJ, Sasso, M, Allison, M, Tsochatzis, E, Anstee, QM, Sheridan, D, et al. Accuracy of fibroscan controlled attenuation parameter and liver stiffness measurement in assessing steatosis and fibrosis in patients with nonalcoholic fatty liver disease. Gastroenterology. (2019) 156:1717–30. doi: 10.1053/j.gastro.2019.01.042
13. Xie, R, Xiao, M, Li, L, Ma, N, Liu, M, Huang, X, et al. Association between sii and hepatic steatosis and liver fibrosis: a population-based study. Front Immunol. (2022) 13:925690. doi: 10.3389/fimmu.2022.925690
14. Yu, J, Chen, Y, Li, D, Zhang, L, Zhang, Y, Zhang, J, et al. Specific macronutrient clusters associated with lower mortality risk: evidence from NHANES 1999–2018. J Adv Res. (2025) S2090-1232(25)00117-1. doi: 10.1016/j.jare.2025.02.019
15. Qiu, H, and Schlegel, V. Impact of nutrient overload on metabolic homeostasis. Nutr Rev. (2018) 76:693–707. doi: 10.1093/nutrit/nuy023
16. Solon-Biet, SM, McMahon, AC, Ballard, JWO, Ruohonen, K, Wu, LE, Cogger, VC, et al. The ratio of macronutrients, not caloric intake, dictates cardiometabolic health, aging, and longevity in ad libitum-fed mice. Cell Metab. (2020) 31:654. doi: 10.1016/j.cmet.2020.01.010
17. Mao, TA-O, Sun, YA-O, Xu, XA-O, and He, KA-O. Overview and prospect of NAFLD: significant roles of nutrients and dietary patterns in its progression or prevention. Hepatol Commun. (2023) 7:e0234. doi: 10.1097/HC9.0000000000000234
18. CDC National Center for Health Statistics (Nchs) of the Centers for Disease Control and Prevention (Cdc). Available online at: https://www.cdc.gov/nchs/nhanes/ (Accessed September 18, 2024).
19. Fan, HA-OX, Xu, C, Li, W, Huang, Y, Hua, R, Xiong, Y, et al. Ideal cardiovascular health metrics are associated with reduced severity of hepatic steatosis and liver fibrosis detected by transient elastography. Nutrients. (2022) 14:5344. doi: 10.3390/nu14245344
20. Ciardullo, S, and Perseghin, G. Statin use is associated with lower prevalence of advanced liver fibrosis in patients with type 2 diabetes. Metabolism. (2021) 121:154752. doi: 10.1016/j.metabol.2021.154752
21. Tuma, PA. Dietary guidelines 2020-2025: update on academy efforts. J Acad Nutr Diet. (2019) 119:672–4. doi: 10.1016/j.jand.2018.05.007
22. Cai, J, Chen, D, Luo, W, Xu, F, Feng, X, Zhang, L, et al. The association between diverse serum folate with MAFLD and liver fibrosis based on NHANES 2017–2020. Front Nutr. (2024) 11:1366843. doi: 10.3389/fnut.2024.1366843
23. Reja, D, Makar, M, Visaria, A, Karanfilian, B, and Rustgi, V. Blood Lead level is associated with advanced liver fibrosis in patients with non-alcoholic fatty liver disease: a nationwide survey (Nhanes 2011-2016). Ann Hepatol. (2020) 19:404–10. doi: 10.1016/j.aohep.2020.03.006
24. Li, J, Huang, J, Lv, Y, and Ji, H. Association between dietary intakes of b vitamins and nonalcoholic fatty liver disease in postmenopausal women: a cross-sectional study. Front Nutr. (2023) 10:1272321. doi: 10.3389/fnut.2023.1272321
25. Jayanama, K, Theou, O, Blodgett, JM, Cahill, L, and Rockwood, K. Frailty, nutrition-related parameters, and mortality across the adult age spectrum. BMC Med. (2018) 16:188. doi: 10.1186/s12916-018-1176-6
26. Aparicio-Ugarriza, R, Fau-Palacios, G, Palacios, G, Fau-Alder, M, Alder, M, Fau-González-Gross, M, et al. A review of the cut-off points for the diagnosis of vitamin b12 deficiency in the general population. Clin Chem Lab Med. (2015) 53:1149–59. doi: 10.1515/cclm-2014-0784
27. Institute of Medicine (US) Standing Committee on the Scientific Evaluation of Dietary Reference Intakes. Dietary Reference Intakes for Calcium, Phosphorus, Magnesium, Vitamin D, and Fluoride. National Academies Press (US). (1997).
28. Institute of Medicine (US) Standing Committee on the Scientific Evaluation of Dietary Reference Intakes and its Panel on Folate, Other B Vitamins, and Choline. Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline. National Academies Press (US). (1998).
29. Kromhout, D, Giltay, E, Geleijnse, JM, and Geleijnse, JM. N-3 fatty acids and cardiovascular events after myocardial infarction. N Engl J Med. (2010) 363:2015–26. doi: 10.1056/NEJMoa1003603
30. Genuth, S, Alberti, KG, Bennett, P, Buse, J, Defronzo, R, Kahn, R, et al. Follow-up report on the diagnosis of diabetes mellitus: the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care. (2003) 26:3160–7. doi: 10.2337/diacare.26.11.3160
31. National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third report of the national cholesterol education program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III) final report. Circulation. (2002) 106:3143–421. doi: 10.1161/circ.106.25.3143
32. Trumbo, P, Yates, AA, Schlicker, S, and Poos, M. Dri, dietary reference intakes for vitamin a, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. J Am Diet Assoc. (2001) 101:294–301. doi: 10.1016/S0002-8223(01)00078-5
33. Rives, C, Fougerat, A, Ellero-Simatos, SA-O, Loiseau, NA-O, Guillou, H, Gamet-Payrastre, L, et al. Oxidative stress in Nafld: role of nutrients and food contaminants. Biomol Ther. (2020) 10:1702. doi: 10.3390/biom10121702
34. GBD 2017 Cirrhosis Collaborators. The global, regional, and national burden of cirrhosis by cause in 195 countries and territories, 1990-2017: a systematic analysis for the global burden of disease study 2017. Lancet Gastroenterol Hepatol. (2020) 5:245–66. doi: 10.1016/S2468-1253(19)30349-8
35. Riazi, K, Azhari, H, Charette, JH, Underwood, FE, King, JA, Afshar, EE, et al. The prevalence and incidence of NAFLD worldwide: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. (2022) 7:851–61. doi: 10.1016/S2468-1253(22)00165-0
36. Younossi, ZM, Golabi, P, de Avila, L, Paik, JM, Srishord, M, Fukui, N, et al. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: a systematic review and meta-analysis. J Hepatol. (2019) 71:793–801. doi: 10.1016/j.jhep.2019.06.021
37. Ajmera, V, Cepin, S, Tesfai, K, Hofflich, H, Cadman, K, Lopez, S, et al. A prospective study on the prevalence of NAFLD, advanced fibrosis, cirrhosis and hepatocellular carcinoma in people with type 2 diabetes. J Hepatol. (2023) 78:471–8. doi: 10.1016/j.jhep.2022.11.010
38. Puri, P, Dhiman, RK, Taneja, S, Tandon, P, Merli, M, Anand, AC, et al. Nutrition in chronic liver disease: consensus statement of the indian national association for study of the liver. J Clin Exp Hepatol. (2021) 11:97–143. doi: 10.1016/j.jceh.2020.09.003
39. Liu, Y, Dou, X, Zhou, WY, Ding, M, Liu, L, Du, RQ, et al. Hepatic small ubiquitin-related modifier (sumo)-specific protease 2 controls systemic metabolism through Sumoylation-dependent regulation of liver-adipose tissue crosstalk. Hepatology. (2021) 74:1864–83. doi: 10.1002/hep.31881
40. Zhou, Y, Lin, X, Yin, S, Zhu, L, Yang, Y, Li, Y, et al. Emerging trends and hot spots in hepatic glycolipid metabolism research from 2002 to 2021: a bibliometric analysis. Front Nutr. (2022) 9:933211. doi: 10.3389/fnut.2022.933211
41. Bischoff, SC, Bernal, W, Dasarathy, S, Merli, M, Plank, LD, Schütz, T, et al. ESPEN practical guideline: clinical nutrition in liver disease. Clin Nutr. (2020) 39:3533–62. doi: 10.1016/j.clnu.2020.09.001
42. Müller, MJ, Böttcher, J, Selberg, O, Weselmann, S, Böker, KH, Schwarze, M, et al. Hypermetabolism in clinically stable patients with liver cirrhosis. Am J Clin Nutr. (1999) 69:1194–201. doi: 10.1093/ajcn/69.6.1194
43. Owen, OE, Trapp, VE, Reichard, GA, Mozzoli, MA, Moctezuma, J, Paul, P, et al. Nature and quantity of fuels consumed in patients with alcoholic cirrhosis. J Clin Invest. (1983) 72:1821–32. doi: 10.1172/JCI111142
44. Ferreira, S, Marroni, CA, Stein, JT, Rayn, R, Henz, AC, Schmidt, NP, et al. Assessment of resting energy expenditure in patients with cirrhosis. World J Hepatol. (2022) 14:802–11. doi: 10.4254/wjh.v14.i4.802
45. European Association for the Study of the Liver. EASL clinical practice guidelines on nutrition in chronic liver disease. J Hepatol. (2019) 70:172–93. doi: 10.1016/j.jhep.2018.06.024
46. Huisman, EJ, Trip, EJ, Siersema, PD, van Hoek, B, and van Erpecum, KJ. Protein energy malnutrition predicts complications in liver cirrhosis. Eur J Gastroenterol Hepatol. (2011) 23:982–9. doi: 10.1097/MEG.0b013e32834aa4bb
47. Gunsar, F, Raimondo, ML, Jones, S, Terreni, N, Wong, C, Patch, D, et al. Nutritional status and prognosis in cirrhotic patients. Aliment Pharmacol Ther. (2006) 24:563–72. doi: 10.1111/j.1365-2036.2006.03003.x
48. Montano-Loza, AJ, Meza-Junco, J, Prado, CM, Lieffers, JR, Baracos, VE, Bain, VG, et al. Muscle wasting is associated with mortality in patients with cirrhosis. Clin Gastroenterol Hepatol. (2012) 10:e1:166–73. doi: 10.1016/j.cgh.2011.08.028
49. Campillo, B, Richardet, JP, Scherman, E, and Bories, PN. Evaluation of nutritional practice in hospitalized cirrhotic patients: results of a prospective study. Nutrition. (2003) 19:515–21. doi: 10.1016/s0899-9007(02)01071-7
50. De Chiara, F, Ureta Checcllo, C, and Ramón Azcón, J. High protein diet and metabolic plasticity in non-alcoholic fatty liver disease: myths and truths. Nutrients. (2019) 11:2985. doi: 10.3390/nu11122985
51. Liao, Y, Chen, Q, Liu, L, Huang, H, Sun, J, Bai, X, et al. Amino acid is a major carbon source for hepatic lipogenesis. Cell Metab. (2024) 36:2437–48.e8. doi: 10.1016/j.cmet.2024.10.001
52. Pickett-Blakely, O, Young, K, and Carr, RM. Micronutrients in nonalcoholic fatty liver disease pathogenesis. Cell Mol Gastroenterol Hepatol. (2018) 6:451–62. doi: 10.1016/j.jcmgh.2018.07.004
53. Berná, G, and Romero-Gomez, M. The role of nutrition in non-alcoholic fatty liver disease: pathophysiology and management. Liver Int. (2020) 40:102–8. doi: 10.1111/liv.14360
54. Valenti, L, Fracanzani, A, Bugianesi, E, Bugianesi, E, Dongiovanni, P, Dongiovanni, P, et al. HFE genotype, parenchymal iron accumulation, and liver fibrosis in patients with nonalcoholic fatty liver disease. Gastroenterology. (2010) 138:905–12. doi: 10.1053/j.gastro.2009.11.013
55. Nelson, JE, Wilson, L, Brunt, EM, Yeh, MM, Kleiner, DE, Unalp-Arida, A, et al. Relationship between the pattern of hepatic iron deposition and histological severity in nonalcoholic fatty liver disease. Hepatology. (2011) 53:448–57. doi: 10.1002/hep.24038
56. Britton, LJ, Subramaniam, VN, and Crawford, DH. Iron and non-alcoholic fatty liver disease. World J Gastroenterol. (2016) 22:8112–22. doi: 10.3748/wjg.v22.i36.8112
57. Kennedy, M, Failla, ML, Ml, F, Smith, JC, and Smith, JC. Influence of genetic obesity on tissue concentrations of zinc, copper, manganese and iron in mice. J Nutr. (1986) 116:1432–41. doi: 10.1093/jn/116.8.1432
58. Jayawardena, R, Ranasinghe, P, Malkanthi, R, Malkanthi, R, Constantine, G, Constantine, G, et al. Effects of zinc supplementation on diabetes mellitus: a systematic review and meta-analysis. Diabetol Metab Syndr. (2012) 4:13. doi: 10.1186/1758-5996-4-1359
59. Parola, M, Leonarduzzi, G, Biasi, F, Albano, E, Biocca, ME, and Poli, G. Vitamin E dietary supplementation protects against carbon tetrachloride-induced chronic liver damage and cirrhosis. Hepatology. (1992) 16:1014–21. doi: 10.1002/hep.1840160426
60. Al-Othman, AA, Rosenstein, F, Fau-Lei, KY, and Lei, KY. Copper deficiency increases in vivo hepatic synthesis of fatty acids, triacylglycerols, and phospholipids in rats. Proc Soc Exp Biol Med. (1993) 204:97–103. doi: 10.3181/00379727-204-43640
Keywords: daily nutrients consumption, hepatic steatosis, fibrosis, National Health and Nutrition Examination Survey, chronic liver disease
Citation: Sheng Q, Liu S, Yu H, Shi H and Xin Y (2025) The association of dietary nutrients consumption with hepatic steatosis and fibrosis from NHANES 2017–2020. Front. Nutr. 12:1510860. doi: 10.3389/fnut.2025.1510860
Edited by:
Weimin Guo, Boston University, United StatesReviewed by:
Giovanni Tarantino, University of Naples Federico II, ItalyHarsh Kishore, Dayanand Medical College & Hospital, India
Copyright © 2025 Sheng, Liu, Yu, Shi and Xin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yongning Xin, eGlueW9uZ25pbmdAMTYzLmNvbQ==
 Qi Sheng
Qi Sheng Shousheng Liu
Shousheng Liu Haiyang Yu
Haiyang Yu Huanchen Shi6
Huanchen Shi6 Yongning Xin
Yongning Xin