Abstract
Objectives:
Sarcopenia is prevalent among individuals undergoing maintenance hemodialysis (MHD) and is influenced by sedentary lifestyles. Although leisure-time physical activities have been shown to prevent sarcopenia in patients undergoing MHD, the impact of nonleisure-time physical activities on sarcopenia has not yet been examined in prospective studies.
Methods:
This prospective cohort study, conducted in 2020 with a 12-month follow-up, included stable MHD patients without baseline sarcopenia. Sarcopenia was diagnosed according to the 2019 Asian Working Group for Sarcopenia criteria. Physical activity was assessed using the International Physical Activity Questionnaire. Additionally, demographic, dietary, nutritional, and laboratory data were collected. Modified Poisson regression analysis was employed to evaluate the impact of physical activity on the risk of developing sarcopenia.
Results:
Among the 196 MHD patients who completed the 1-year follow-up, 29 (14.8%) developed sarcopenia. The average total physical activity was 1,268 METs/week, with leisure-time activity averaging 300 METs/week and nonleisure-time activity averaging 724 METs/week. Adjusted analyses indicate that leisure-time physical activities do not significantly affect the risk of sarcopenia (RR = 0.920, 95% CI = 0.477–1.951; P > 0.05), whereas nonleisure-time physical activities are significantly associated with a reduced risk of sarcopenia (RR = 0.449, 95% CI = 0.248–0.814).
Conclusion:
Actively participating in physical activities (nonleisure-time physical activities) can reduce the incidence of sarcopenia in patients undergoing MHD. Promoting such activities may be an effective strategy to enhance physical fitness and mitigate sarcopenia risk among this population.
1 Introduction
Chronic kidney disease (CKD) has become a global public health issue, with a high prevalence rate of 9.1%, characterized by a progressive loss of kidney function (1). Maintenance hemodialysis (MHD) is a critical treatment for sustaining the lives of patients with CKD, accounting for 91% of all dialysis treatments (2). MHD replaces kidney function, enabling survival despite severe renal impairment. However, long-term MHD dependence leads to complications that affect health and quality of life (3, 4).
Sarcopenia, characterized by the progressive loss of skeletal muscle mass (SMM) and strength (5), is highly prevalent among patients on MHD, with rates ranging from 13.5% to 73.5% (6–9). Sarcopenia significantly impairs patients' physical functioning and independence, making it a critical concern in the management of CKD patients undergoing MHD. Furthermore, sarcopenia is associated with adverse clinical outcomes, including disability, reduced quality of life, and increased morbidity and mortality (9, 10). These outcomes not only affect patients' wellbeing but also impose substantial economic burdens on healthcare systems due to increased medical interventions and prolonged hospital stays (9). Several complex factors contribute to the development of sarcopenia in patients with MHD, including protein–energy wasting, hormonal imbalance, comorbidities, acid metabolism disturbance, inflammation, and a general lack of physical activity (11).
Physical activity encompasses any bodily movement produced by skeletal muscles that results in energy expenditure (12). It is categorized into leisure-time activities (such as aerobic and anaerobic exercises) and nonleisure-time activities (including household, occupational, and transport-related activities) (13). Physical activity may alleviate sarcopenia in patients undergoing MHD through various biological mechanisms, such as stimulating muscle protein synthesis, enhancing mitochondrial function, improving insulin sensitivity, reducing chronic inflammation, and supporting muscle regeneration (14, 15). While previous research has established a strong association between physical activity and sarcopenia (16, 17), most of the evidence focuses on leisure-time physical activities. In contrast, the clinical impact of non-leisure-time physical activity, which constitutes a significant portion of daily physical activity (18–22), remains underexplored, especially in populations with chronic diseases.
Additionally, patients on MHD often face challenges in participating in leisure-time physical activities due to dialysis-related weakness and fatigue, dialysis braking, concerns, and a lack of knowledge about such activities (23, 24). Decreased physical activity can trigger skeletal muscle atrophy, which further discourages patients on MHD from engaging in physical activities (25, 26). This creates a vicious cycle that ultimately exacerbates sarcopenia (25–27). Conversely, MHD patients may find it easier to adopt lifestyle-oriented nonleisure-time physical activities, thereby improving their physical activity levels, quality of life, and compliance (28, 29).
Therefore, this prospective cohort study aims to explore the relationship between various types of physical activity and the risk of sarcopenia in patients undergoing MHD. We hypothesize that increasing nonleisure-time physical activity levels may reduce the risk of sarcopenia, similar to leisure-time physical activity, in this population.
2 Methods
2.1 Study design and participants
This 1-year prospective cohort study builds on Ding et al.'s cross-sectional research on sarcopenia in patients undergoing MHD (8). Conducted from September 2020 to January 2022 at Hangzhou TCM Hospital, Zhejiang Chinese Medical University, China, the study included participants who were: (1) free of sarcopenia as per the Asian Working Group for Sarcopenia (AWGS); (2) age ≥18 years; (3) on hemodialysis for at least 3 months, undergoing dialysis three times weekly; and (4) capable of independent mobility. Exclusion criteria were: (1) trauma, severe infections, or malignant tumors within the past 3 months; (2) cognitive impairments; and (3) refusal to participate in the study or withdrawal during the study due to reasons such as lack of interest, time constraints, or limitations related to physical health.
2.2 Sarcopenia diagnosis and measurements
According to the diagnostic consensus of the AWGS (30), patients who meet the following criteria (1) and (2) and/or (3) are diagnosed with sarcopenia: (1) muscle mass loss defined as skeletal muscle index (SMI) < 7.00 kg/m2 for men and < 5.70 kg/m2 for women; (2) muscle strength reduction defined as handgrip strength (HGS) < 28 kg for men and < 18 kg for women; and (3) muscle dysfunction defined as 6-m gait speed (GS) < 1 m/s.
2.2.1 Bioimpedance measurements
Body composition was assessed using the Seca515 dual-energy electrical impedance analyzer (Seca GmbH & Co., Hamburg, Germany) 30 min post-dialysis, following bioelectrical impedance analysis (BIA) guidelines (31). Patients fasted for 2 h, emptied their bladders, removed metallic objects, wore lightweight clothing of known weight, and stood barefoot on the device. They held the handles with both hands, extended their arms, and entered personal information [name, age, sex, height, waist circumference (WC)] into the system. The Seca515 analyzer utilizes eight-electrode technology to perform segmented impedance measurements at a 100-volt current, relying on the device's proprietary algorithms for all calculations. SMM measurements were recorded at a frequency of 50 kHz, with results displayed in kilograms. Formulas used:
SMI (kg/m2) = SMM (kg)/height2 (m2) (32).
Body mass index (BMI): BMI (kg/m2) = weight (kg)/height2 (m2).
2.2.2 Muscle strength and function measurements
Prior to dialysis (after a short no-dialysis pause of one day), HGS was measured using a custom electronic meter (Guangdong Xiangshan Weighing Apparatus Group, China). Patients stood upright using their nonfistulated or dominant hand, fully extended elbow, exerted maximum force, rested for 3 min, and repeated the measurement. The higher of the two HGS values (kg) was recorded. GS was evaluated by having patients walk 6 m at their usual pace without assistance. After a 3-min rest, GS was measured again, and the average of the two GS values (m/s) was used (33). All measurements were conducted by the same evaluator during the same dialysis session as BIA.
2.3 Parameters of demographic and laboratory
Demographic and laboratory data were extracted from the electronic medical records, selecting routine fasting pre-dialysis laboratory test results within 4 weeks before and after the measurement. Variables included age, sex, duration of hemodialysis (dialytic age), primary disease, education level, comorbidities, blood urea nitrogen (BUN), serum calcium (Ca2+), glucose (GLU), intact parathyroid hormone (iPTH), phosphorus (P3−), total cholesterol (TCH), high-sensitivity C-reactive protein (hs-CRP), albumin (Alb), and hemoglobin (Hb).
2.4 Diet and nutritional status measurements
Dietary protein intake was estimated using the normalized protein equivalent of nitrogen appearance (nPNA) (34). Nutritional status was assessed with the Modified Quantitative Subjective Global Assessment (MQSGA) (35–37), evaluating weight changes, dietary intake, gastrointestinal symptoms, functional status, dialysis complications, subcutaneous fat loss, and muscle wasting over the past 6 months. Each category was scored from 1 (normal) to 5 (severe malnutrition), with total scores ranging from 7 to 35. Scores of 7–10 indicate normal nutrition, 11–20 indicate mild to moderate malnutrition, and 21–35 indicate severe malnutrition.
2.5 Physical activity evaluation
Physical activity levels were assessed using the International Physical Activity Questionnaire (IPAQ), a reliable and valid tool (38–40). The IPAQ measures nonleisure-time activities (occupation, housework, transportation) and leisure-time activities (aerobic, anaerobic) over the past week, quantified in metabolic equivalents (METs). Total energy expenditure was calculated based on the frequency, duration, and intensity of activities during seven consecutive days.
Participants were categorized into active or inactive groups according to the American College of Sports Medicine guidelines (41). The active group met at least one of the following criteria: (1) Vigorous-intensity activities ≥3 days/week, ≥20 min/day; (2) Moderate-intensity or walking activities ≥5 days/week, ≥30 min/day; (3) Any combination achieving ≥600 MET min/week. Participants not meeting these criteria were classified as inactive.
2.6 Management and follow-up
Participants underwent face-to-face assessments conducted by a single evaluator to ensure consistency. Biochemical indicators were measured at baseline, with laboratory tests performed in the early morning under fasting conditions using the automated biochemical analyzer (Mindray BS800) on samples from internal fistulas or catheters. Nutritional status, SMM, HGS, and GS were assessed at baseline and after one year. Physical activity was measured twice shortly after enrollment (weekly) and then monthly during the follow-up, with analysis based on the average of the first two IPAQ results. During the follow-up period, patients continued routine MHD treatments, were encouraged to maintain a healthy lifestyle, and did not receive additional interventions for preventing or treating sarcopenia.
2.7 Statistical analysis
Data were analyzed using IBM SPSS Statistics version 25.0 (IBM Corp., Armonk, NY, USA). Normally distributed variables are presented as mean ± standard deviation (SD), non-normally distributed variables as median (interquartile range, IQR), and categorical data as frequencies and percentages. Between-group comparisons for continuous variables used independent t-tests, chi-square tests for categorical variables, and Mann–Whitney U-tests for non-normal data. Sparse Principal Component Analysis (SPCA) was used for dimensionality reduction and feature extraction. A bi-plot was generated to visualize variable loadings and patient distribution. Pearson's correlation analysis was performed to evaluate the relationships between variables. A correlation heatmap was generated to visually represent the strength and direction of the associations. Using modified Poisson regression analysis to explore the relationship between physical activity and the risk of sarcopenia. Using METs on the x-axis and relative risk on the y-axis to illustrate the dose-response relationship between METs of physical activity and the risk of sarcopenia. A two-sided P < 0.05 was considered statistically significant.
3 Results
3.1 Participant characteristics
Of the 346 patients screened, 233 without sarcopenia were initially included (8). After excluding 37 patients due to hospital transfer, kidney transplantation, death, inability to perform body composition analysis, or cessation of hemodialysis within 1 year, 196 patients completed the study. Among them, 29 (14.8%) were diagnosed with sarcopenia (Figure 1). Baseline characteristics of the sarcopenia and nonsarcopenia groups are summarized in Table 1. Significant differences between the groups were found in age, sex, ALB, BMI, MQSGA, WC, HGS, and SMM (P < 0.05).
Figure 1
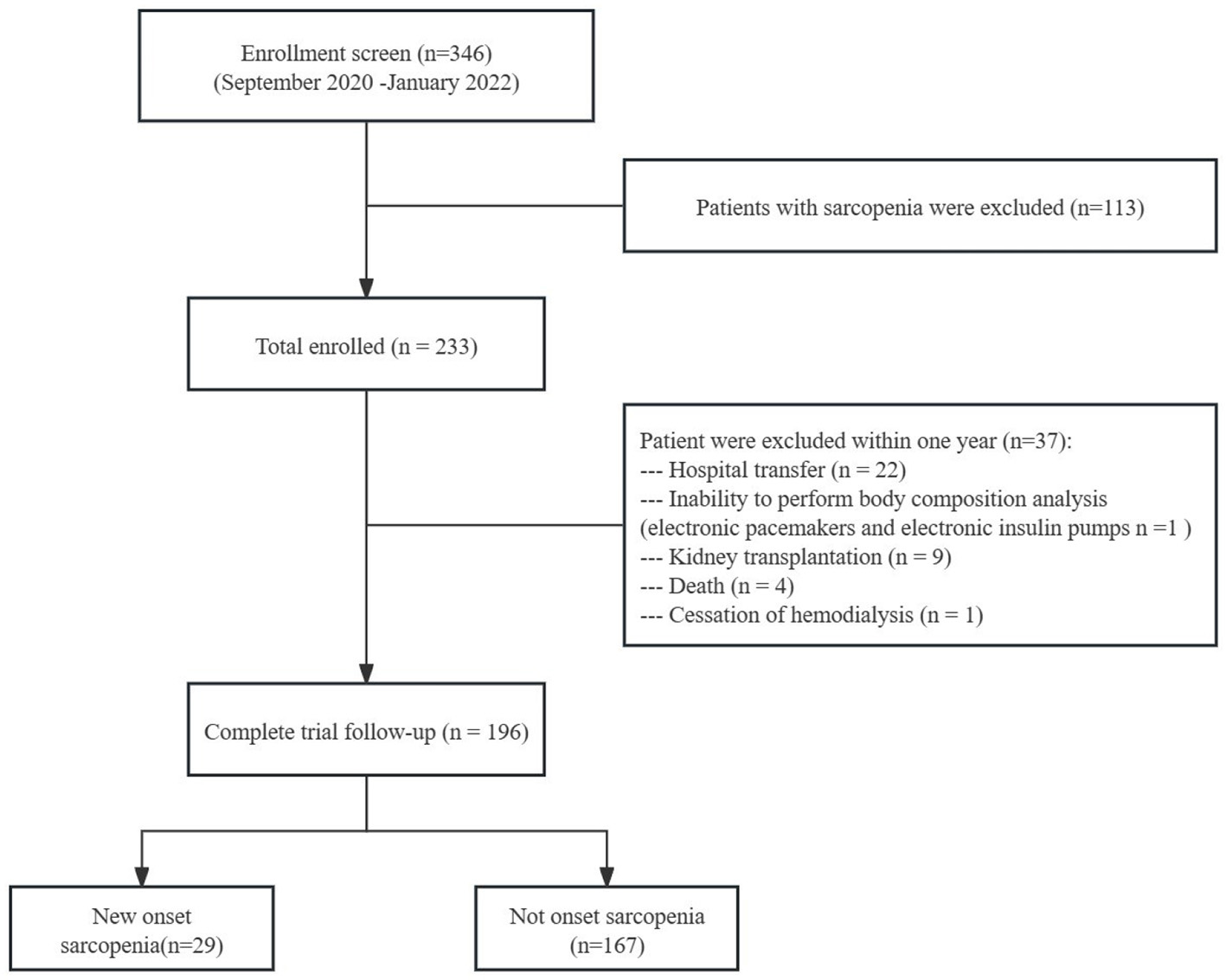
Flow chart of the study participants.
Table 1
| Parameters | Total | Sarcopenia | Nonsarcopenia | P |
|---|---|---|---|---|
| ( n = 196) | ( n = 29) | ( n = 167) | ||
| Age (y) | 53.57 ± 12.43 | 60.34 ± 10.35 | 52.40 ± 12.41 | 0.001 |
| Sex, n(%) | 0.003 | |||
| Men | 131 (66.8%) | 12 (41.4%) | 119 (71.3%) | |
| Women | 65 (33.2%) | 17 (58.6%) | 48 (28.7%) | |
| Dialysis vintage (mo) | 52 (21.25,110) | 97 (24,146.5) | 47 (21,105) | 0.148 |
| Protopathy, n(%) | 0.238 | |||
| Diabetic nephropathy | 46 (23.5%) | 4 (13.8%) | 42 (25.1%) | |
| Others | 150 (76.5%) | 25 (86.2%) | 125 (74.9%) | |
| Education level, n(%) | 0.422 | |||
| ≤ Middle school | 99 (50.5%) | 17 (58.6%) | 82 (49.1%) | |
| ≥High school | 97 (49.5%) | 12 (41.4%) | 85 (50.9%) | |
| BUN (mmol/L) | 22.19 ± 5.27 | 20.88 ± 4.38 | 22.42 ± 5.39 | 0.146 |
| TCH (mmol/L) | 3.85 (3.29, 4.53) | 4.12 (3.45, 4.75) | 3.82 (3.29, 4.49) | 0.287 |
| hs-CRP (g/L) | 2.20 (1.12, 4.53) | 1.90 (1.24, 3.25) | 2.21 (1.06, 4.89) | 0.419 |
| P3− (mmol/L) | 1.85 (1.58, 2.19) | 1.92 (1.64, 2.1) | 1.84 (1.58, 2.22) | 0.976 |
| Ca2+ (mmol/L) | 2.32 (2.19, 2.42) | 2.33 (2.2, 2.43) | 2.32 (2.19, 2.4) | 0.696 |
| ipTH (pg/ml) | 329.85 (197.88, 560.48) | 435.1 (219.65, 553.8) | 325.0 (187.8, 565.1) | 0.504 |
| GLU (mmol/L) | 5.84 (4.72, 7.92) | 6.57 (4.70, 7.98) | 5.59 (4.71, 7.78) | 0.836 |
| HB (g/L) | 113.34 ± 14.78 | 113.17 ± 14.55 | 113.37 ± 14.86 | 0.948 |
| ALB (g/L) | 39.6 (38.1, 41.58) | 38.7 (36.85, 40.45) | 39.8 (38.2, 42.1) | 0.009 |
| BMI (kg/m2) | 23.43 ± 3.69 | 20.25 ± 2.51 | 23.98 ± 3.59 | 0.001 |
| MQSGA (score) | 10 (10, 11.75) | 12 (10, 12) | 10 (9, 10) | 0.001 |
| nPNA (g/kg/d) | 1.10 ± 0.24 | 1.10 ± 0.22 | 1.10 ± 0.24 | 0.972 |
| WC (cm) | 0.86 ± 0.12 | 0.79 ± 0.11 | 0.87 ± 0.12 | 0.001 |
| Sedentary time (hours/week) | 56 (42, 56) | 56 (42, 56) | 56 (42, 56) | 0.907 |
| HGS (kg) | 31.06 ± 9.30 | 23.03 ± 6.90 | 32.45 ± 8.96 | 0.001 |
| SMM (kg) | 21.90 ± 4.84 | 17.12 ± 2.91 | 22.73 ± 4.63 | 0.001 |
General information of the sarcopenia and nonsarcopenia groups.
ALB, albumin; BMI, body mass index; BUN, blood urea nitrogen; Ca2+, serum calcium; GLU, glucose; HB, hemoglobin; HGS, handgrip strength; hs-CRP, high-sensitivity C-reactive protein; ipTH, parathyroid hormone; MQSGA, modified quantitative subjective global assessment; nPNA, normalized protein equivalent of nitrogen appearance; P3−, serum phosphorus; SMM, skeletal muscle mass; TCH, total cholesterol; WC, waist circumference. Two independent-sample t-test, Mann–Whitney U-test, and chi-square test were used in normally distributed quantitative data, non-normally distributed quantitative data, and qualitative data, respectively.
3.2 Physical activity evaluation
The total physical activity was 1,268 (687, 1,981) METs/week. The leisure-time and nonleisure-time physical activities were 300 (37, 740) and 724 (369, 1,338) METs/week, respectively. Nonsarcopenia patients exhibit higher physical activity levels than sarcopenia patients across all categories measured. They are more active in total physical activity (P = 0.041), leisure-time physical activity (P = 0.826), and nonleisure time physical activity (P = 0.073). However, there was no statistically significant difference in leisure-time physical activity and nonleisure-time physical activity between the two groups (Figure 2).
Figure 2
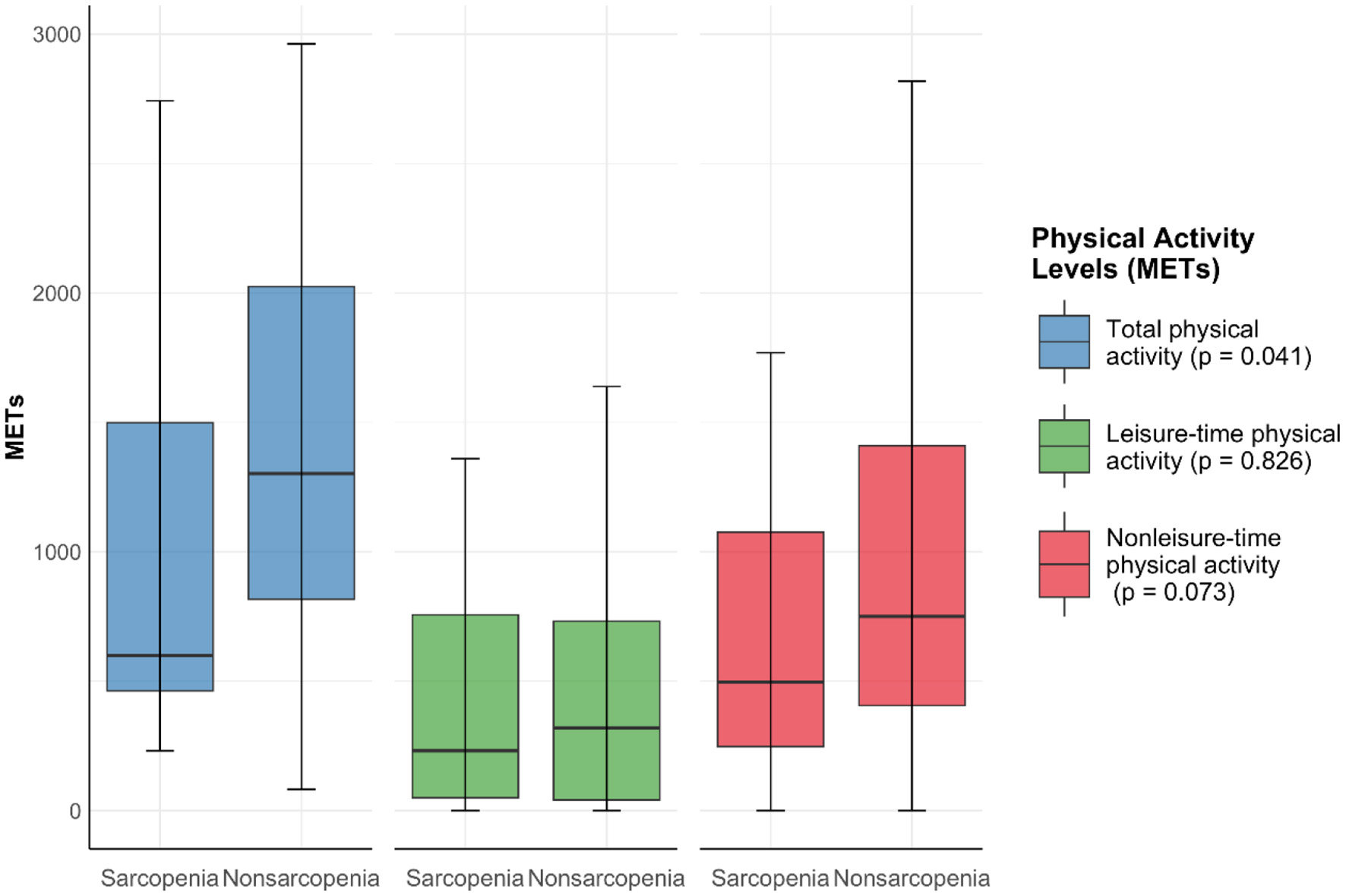
Physical activity levels between sarcopenia group and nonsarcopenia group. Data expressed as median ± range.
3.3 Sparse principal component analysis of sarcopenia-related factors
Figure 3 presents the SPCA bi-lot, where blue and red dots represent individuals with and without sarcopenia, respectively, and ellipses indicate the 95% confidence interval. The first two principal components (PC1: 16.1%, PC2: 12.3%) explain 28.4% of the total variance. Nonleisure-time physical activity, HGS, SMM, BMI, WC, and ALB align with PC1, suggesting their importance in distinguishing sarcopenia status. Age, Protopathy, nPAN, and Sex align with PC2, indicating their influence on individual distribution. Sarcopenia cases cluster in the upper-right region, associated with Age and disease-related factors, while non-sarcopenia cases concentrate in the lower-left, linked to higher muscle mass and nonleisure-time physical activity.
Figure 3
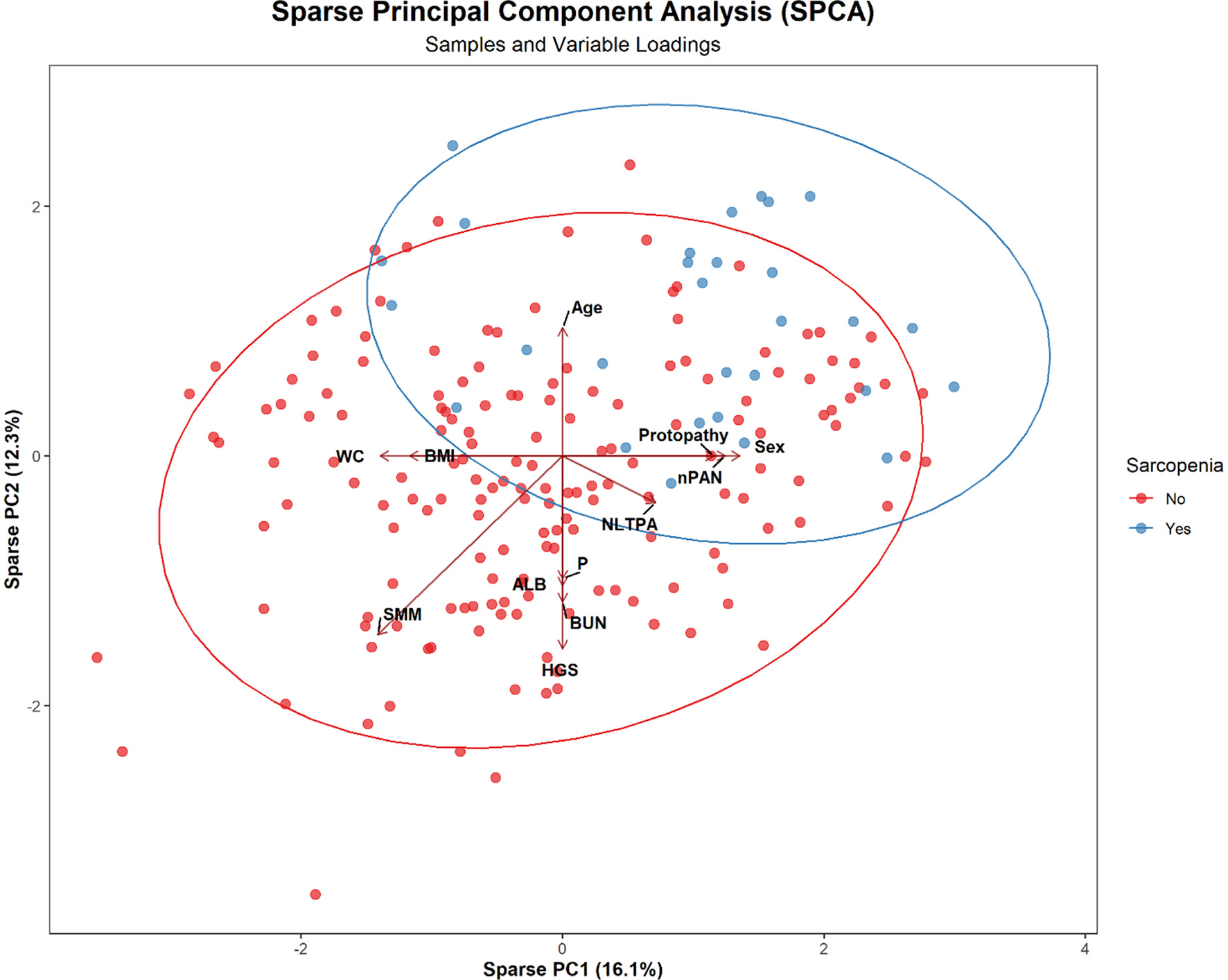
SPCA Bi-plot showing the distribution of sarcopenia and non-sarcopenia patients with key variable loadings.
3.4 Relationship between physical activity types and sarcopenia risk
Among 196 adults, 67 (34.2%) and 112 (57.1%) actively participated in leisure-time and nonleisure-time physical activities, respectively (Table 2). Figure 4 presents a correlation heatmap illustrating the relationships between physical activity, demographic characteristics, nutritional indicators, biochemical markers, and muscle mass parameters in MHD patients. Using the inactive group as the control group for comparison, after adjusting for age, sex, BMI, ALB, MQSGA, WC, HGS, and SMM, active participation in both leisure-time and nonleisure-time physical activities negatively correlated with sarcopenia risk; however, only nonleisure-time physical activity was significantly associated with sarcopenia risk (P < 0.05). After further adjustment for age, sex, BMI, ALB, protopathy, nPAN, P, BUN, WC, HGS, and SMM, nonleisure-time physical activity was still negatively correlated with sarcopenia risk (nonleisure-time physical activity: RR = 0.449, 95% CI = 0.248–0.814, P < 0.05) (Table 3). Using nonleisure-time physical activity level as the X-axis and RR as the Y-axis, RR shows a decreasing trend as nonleisure-time physical activity increases (Figure 5).
Table 2
| Parameters | LTPA | NLTPA | ||||
|---|---|---|---|---|---|---|
| Inactive | Active | P | Inactive | Active | P | |
| ( n = 129) | ( n = 67) | ( n = 84) | ( n = 112) | |||
| Age (y) | 53.0 ± 12.76 | 54.67 ± 11.78 | 0.373 | 54.63 ± 12.09 | 52.78 ± 12.67 | 0.303 |
| Sex, n(%) | 0.751 | 0.001 | ||||
| Men | 85 (65.9%) | 46 (68.7%) | 68 (81.0%) | 63 (56.3%) | ||
| Women | 44 (34.1%) | 21 (31.3%) | 16 (19.0%) | 49 (43.8%) | ||
| Dialysis vintage (mo) | 49 (21.5, 121.5) | 56 (21, 105) | 0.987 | 38 (16, 118.5) | 56 (26.5, 107.75) | 0.159 |
| Protopathy, n(%) | 0.216 | 0.017 | ||||
| Diabetic nephropathy | 34 (26.4%) | 12 (17.9%) | 27 (32.1%) | 19 (17.0%) | ||
| Others | 95 (73.6%) | 55 (82.1%) | 57 (67.9%) | 93 (83.0%) | ||
| Educational level, n(%) | 0.765 | 0.043 | ||||
| ≤ Middle school | 64 (49.6%) | 35 (52.2%) | 35 (41.7%) | 64 (57.1%) | ||
| ≥High school | 65 (50.4%) | 32 (47.8%) | 49 (58.3%) | 48 (42.9%) | ||
| BUN (mmol/L) | 21.81 ± 5.21 | 22.93 ± 5.34 | 0.156 | 22.22 ± 5.54 | 22.17 ± 5.08 | 0.946 |
| TCH (mmol/L) | 3.85 (3.30, 4.50) | 3.9 (3.29, 4.66) | 0.728 | 3.80 (3.31, 4.38) | 3.91 (3.27, 4.67) | 0.354 |
| hs-CRP (g/L) | 2.34 (1.17, 4.50) | 1.83 (0.98, 4.89) | 0.305 | 2.49 (1.23, 5.34) | 2.00 (0.99, 4.15) | 0.265 |
| P3− (mmol/L) | 1.78 (1.57, 2.19) | 1.91 (1.61, 2.22) | 0.335 | 1.85 (1.59, 2.22) | 1.85 (1.58, 2.15) | 0.942 |
| Ca2+ (mmol/L) | 2.29 ± 0.17 | 2.34 ± 0.18 | 0.097 | 2.29 ± 0.19 | 2.32 ± 0.16 | 0.158 |
| ipTH (pg/ml) | 275.1 (165.45, 559.75) | 396.5 (227.3, 562.6) | 0.058 | 376.2 (208.13, 544.8) | 318.95 (172.8, 564.45) | 0.613 |
| GLU (mmol/L) | 6.29 (4.81, 8.07) | 5.32 (4.55, 7.45) | 0.055 | 6.53 (4.79, 9.1) | 5.66 (4.65, 7.45) | 0.070 |
| HB (g/L) | 112.14 ± 15.53 | 115.64 ± 13.00 | 0.116 | 113.42 ± 16.79 | 113.28 ± 13.15 | 0.948 |
| ALB (g/L) | 39.4 (37.5, 41.4) | 40.2 (38.4, 42.2) | 0.034 | 39.1 (37.35, 41.35) | 40.15 (38.4, 42) | 0.012 |
| BMI (kg/m2) | 23.52 (20.8, 25.72) | 22.85 (20.36, 24.92) | 0.286 | 23.76 (20.78, 26.10) | 23.15 (20.44, 25.16) | 0.191 |
| MQSGA (score) | 10 (10, 12) | 10 (9, 11) | 0.371 | 10 (10, 12) | 10 (10, 11) | 0.637 |
| WC (cm) | 0.87 ± 0.12 | 0.85 ± 0.12 | 0.189 | 0.89 ± 0.11 | 0.84 ± 0.12 | 0.004 |
| LTPA | 0.650 | |||||
| Inactive | / | / | 57 (67.9%) | 72 (64.3%) | ||
| Active | / | / | 27 (32.1%) | 40 (35.7%) | ||
| NLTPA | 0.650 | |||||
| Inactive | 57 (44.2%) | 27 (40.3%) | / | / | ||
| Active | 72 (55.8%) | 40 (59.7%) | / | / | ||
Characteristics of the participants in the LTPA and NLTPA groups.
ALB, albumin; BMI, body mass index; BUN, blood urea nitrogen; Ca2+, serum calcium; GLU, glucose; HB, hemoglobin; hs-CRP, high-sensitivity C-reactive protein; ipTH, parathyroid hormone; LTPA, leisure-time physical activity; MQSGA, modified quantitative subjective global assessment; NLTPA, nonleisure-time physical activity; P3−, serum phosphorus; TCH, total cholesterol; WC, waist circumference. Two independent-sample t-test, Mann–Whitney U-test, and chi-square test were used in normally distributed quantitative data, non-normally distributed quantitative data, and qualitative data, respectively.
Figure 4
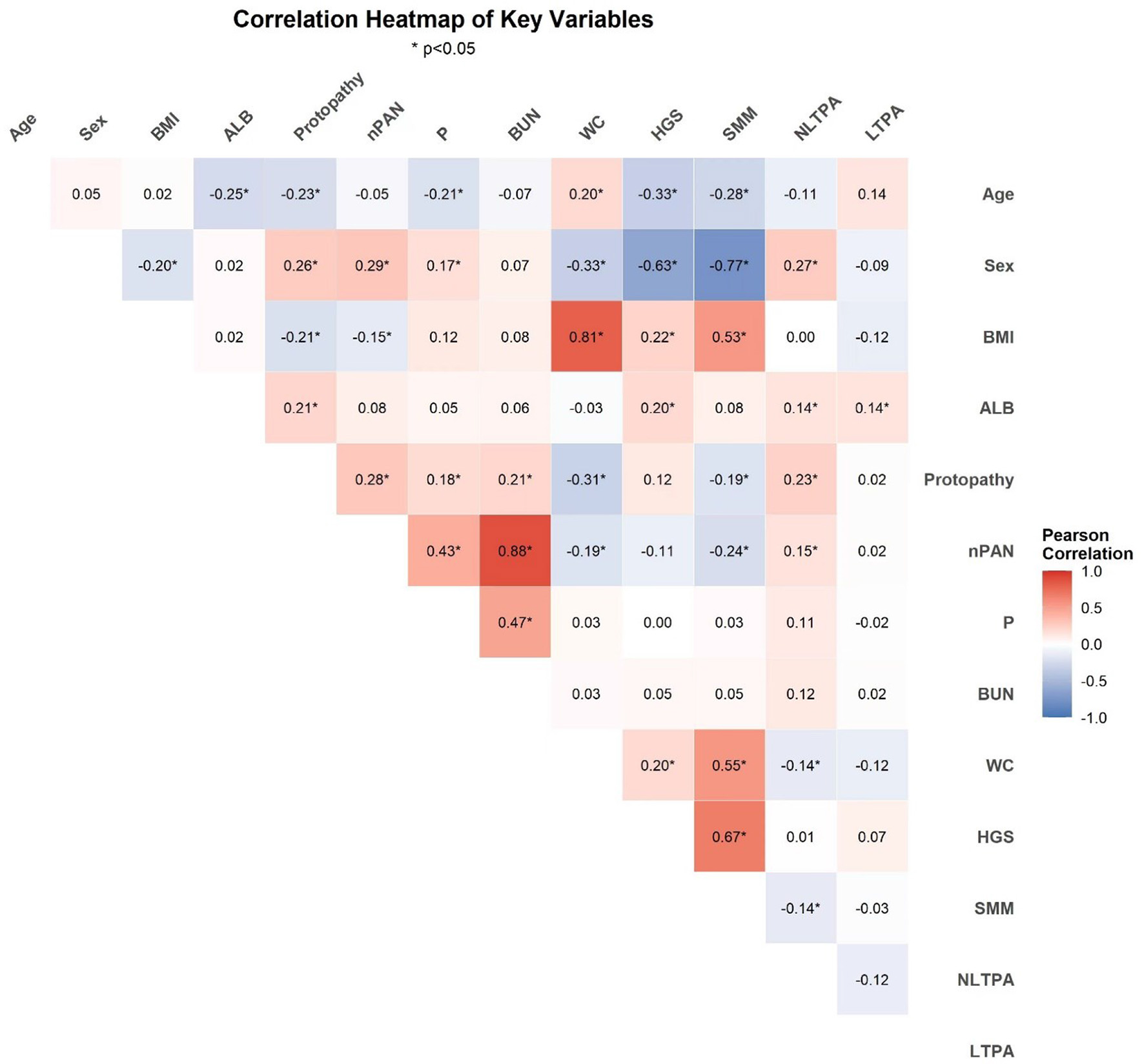
Correlation heatmap of physical activity, demographics, nutritional indicators, biochemical markers, and muscle mass parameters in MHD patients (*P < 0.05).
Table 3
| Physical activity | Incident cases/total (n) | RRa (95%CI) | RRb (95%CI) | RRc (95%CI) |
|---|---|---|---|---|
| LTPA | ||||
| Inactive | 20/129 | 1 | 1 | 1 |
| Active | 9/67 | 0.866 (0.418, 1.797) | 0.963 (0.477, 1.942) | 0.964 (0.477, 1.951) |
| P | 0.700 | 0.916 | 0.920 | |
| NLTPA | ||||
| Inactive | 18/84 | 1 | 1 | 1 |
| Active | 11/112 | 0.458 (0.229, 0.918) | 0.478 (0.272, 0.841) | 0.449 (0.248, 0.814) |
| P | 0.028 | 0.010 | 0.008 | |
Relationship between physical activity types and sarcopenia risk.
LTPA, leisure-time physical activity; NLTPA, nonleisure-time physical activity.
Model a: uncorrected. Model b: adjusted for covariates such as age, sex, BMI, ALB, MQSGA, WC, HGS, and SMM. Model c: adjusted for covariates such as age, sex, BMI, ALB, protopathy, nPAN, P, BUN, WC, HGS, and SMM.
Figure 5
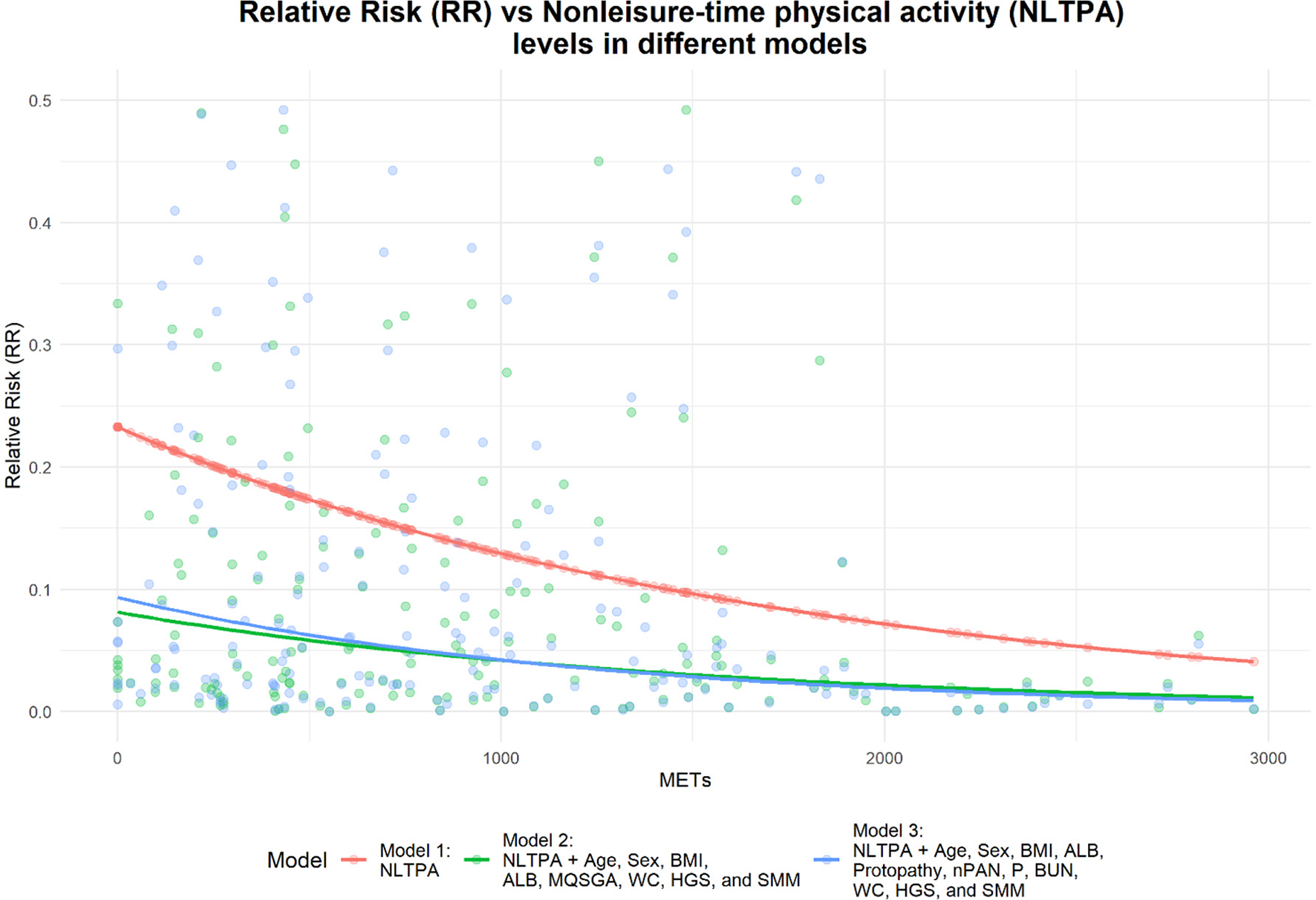
Relationship between nonleisure-time physical activity levels and sarcopenia risk.
4 Discussion
This prospective cohort study investigated the relationship between different types of physical activity and the risk of sarcopenia in patients undergoing MHD. After adjusting for factors such as age, sex, BMI, ALB, protopathy, nPAN, P, BUN, WC, HGS, and SMM, we found that nonleisure-time physical activity was significantly negatively associated with sarcopenia risk. These findings suggest that actively engaging in nonleisure-time physical activities can effectively reduce the risk of sarcopenia in MHD patients.
In our study, 14.8% of the participants developed sarcopenia over one year, a rate notably higher than the 5% to 10% typically observed in healthy older populations (42, 43). Previous studies have reported varying incidence rates of sarcopenia in MHD patients, ranging from 17.8% to 24.9% (44, 45), underscoring the elevated risk in this population. This increased susceptibility may be attributed to multiple factors, including reduced physical activity, selective muscle structural changes, decreased muscle strength, and significant muscle atrophy (46, 47). Additionally, MHD patients often experience protein–energy wasting, hormonal imbalances, disturbances in acid metabolism, and chronic inflammation (11), all of which contribute to the heightened risk of sarcopenia.
In our study, nonleisure-time physical activity emerged as the primary contributor to total daily physical activity. This aligns with a prospective survey of 150 MHD patients using the IPAQ, which also found that their physical activities were predominantly nonleisure-time activities (19). Unlike in Western countries, where leisure-time physical activities are more common (48, 49), Chinese MHD patients tend to rely more on nonleisure-time activities, such as household chores and daily mobility, as their primary sources of physical activity, which are part of their daily routine (18). Household responsibilities and daily tasks are often considered a core part of life in China. As a result, patients' physical activity is more likely to be derived from these tasks rather than from structured exercise or fitness activities aimed at maintaining health in this population. Notably, MHD patients who actively engaged in nonleisure-time physical activities had a 55.1% reduction in sarcopenia risk in our results. To the best of our knowledge, this is the first study to examine the relationship between sarcopenia and nonleisure-time physical activity specifically in MHD patients. Studies involving healthy individuals have demonstrated that nonleisure-time physical activities are beneficial for maintaining muscle mass and strength. Park et al. (50) found that nonleisure-time physical activities, such as regular farming, may help increase step counts in older individuals, thereby delaying the onset of sarcopenia. Moreover, Pan et al. (51) identified positive correlations between household physical activity and both muscle mass and strength, as well as between transport-related physical activity and muscle weight. These findings support the notion that both leisure-time and nonleisure-time physical activities can mitigate sarcopenia risk by enhancing muscle strength and mass. As few studies have specifically investigated the mechanisms by which nonleisure-time physical activity improves sarcopenia, its effects may be similar to the well-documented biological mechanisms of aerobic exercise in alleviating sarcopenia in MHD patients. These include stimulating muscle protein synthesis, reducing chronic inflammation (e.g., lowering TNF-α and IL-6 levels), improving mitochondrial function, enhancing hormonal regulation (e.g., increased IGF-1 and insulin sensitivity), and preserving neuromuscular function (52–54). Additionally, improved circulation from regular movement facilitates oxygen and nutrient delivery to muscles, counteracting atrophy (55, 56). Taken together, these mechanisms underscore the importance of exploring the potential impact of nonleisure-time physical activity on sarcopenia.
In contrast, while structured leisure-time physical activities have been shown to improve muscle mass and strength in both healthy individuals and patients with chronic diseases (57–62), our study did not find a significant relationship between leisure-time physical activity and sarcopenia risk. Our participants exhibited relatively low levels of leisure-time physical activity (231 METs/week in the sarcopenia group vs. 320 METs/week in the nonsarcopenia group). Although leisure-time physical activity was slightly higher in the nonsarcopenia group, the difference was not statistically significant, possibly due to the small sample size. Furthermore, implementing and maintaining structured leisure-time physical activities consistently in clinical settings remains challenging. Only 34.2% of patients in our study actively participated in leisure-time physical activities, and a previous large-scale survey indicated that over one-third1 of MHD patients had little or no participation in these activities, which is insufficient to confer health benefits (63). Additionally, in a study by Manfredini et al. (28), only 69% of patients on dialysis completed a 6-month leisure-time physical activity training program. From a practical and physiological perspective, nonleisure-time physical activities, as an integral part of daily life, are more frequent and regular, do not require additional time or resource allocation, and are easier to sustain over the long term, significantly increasing the overall energy expenditure of patients. In contrast, due to the lack of professional guidance, exercise-related knowledge, and limitations imposed by the disease itself, MHD patients often face challenges in maintaining participation in leisure-time physical activities, which may reduce their effectiveness in preventing sarcopenia (24, 64–66). Therefore, increasing nonleisure-time physical activities may serve as a more appropriate alternative for MHD patients. In clinical practice, while promoting structured physical activity programs, it is also important to evaluate and encourage nonleisure-time physical activities. Nephrologists and physical therapists should tailor physical activity intervention strategies to this population, encouraging increased daily activity through tasks such as carrying small objects, sweeping, window cleaning, and other household chores, as well as engaging in moderate-intensity physical activities related to transportation, such as walking (67). Individuals with chronic diseases, provided they have no contraindications, should maximize physical activity and minimize sedentary behavior to achieve optimal health benefits (68). This study follows the guidelines of the American College of Sports Medicine, setting 600 METs per week as the threshold for group classification (41). We recommend that patients engage in at least 600 METs of nonleisure-time physical activity per week to confer protective effects against sarcopenia. However, since evidence on nonleisure-time physical activity remains relatively limited, future research should explore the minimum METs/week threshold for sarcopenia prevention and the potential biological mechanisms linking nonleisure-time physical activity and sarcopenia, providing stronger evidence to support its feasibility and ultimately benefiting more patients with low levels of physical activity.
This study has several limitations. First, the IPAQ is a self-reported and recall-based questionnaire to evaluate physical activity; however, it can distinguish between leisure and nonleisure-time physical activities compared with accelerometers or pedometers. Second, the data were collected from a single center with a limited number of cases and a short follow-up period, which may not adequately represent the entire population undergoing MHD. Therefore, extensive multicenter studies with larger sample sizes and longer follow-up periods are warranted to further investigate the relationship between physical activity types and sarcopenia risk in MHD patients. Finally, while our study did not explore the impact of dietary composition on sarcopenia, future research should further investigate the role of nutritional components and dietary patterns in sarcopenia prevention and management in MHD patients to enhance clinical guidance.
5 Conclusion
The incidence of sarcopenia is notably high among patients undergoing MHD. Most of these patients engage in nonleisure-time physical activities. Actively participating in such activities can significantly reduce the risk of sarcopenia. Furthermore, nonleisure-time physical activities are often easier to implement and more readily accepted by MHD patients, making them an effective strategy to enhance the physical fitness and overall health of individuals undergoing MHD.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Ethics Committee of Hangzhou TCM Hospital Affiliated to Zhejiang Chinese Medical University (No. 2020KY116). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
LC: Conceptualization, Writing – original draft, Writing – review & editing. YZ: Conceptualization, Formal analysis, Methodology, Writing – original draft, Writing – review & editing. YD: Investigation, Writing – original draft. ZL: Formal analysis, Writing – original draft. HZ: Conceptualization, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
We would like to express our sincere thanks to Dr. Jian'ao Chen from Hangzhou TCM Hospital Affiliated to Zhejiang Chinese Medical University and Dr. Wen Gao from the School of Nursing at Hangzhou Normal University for their assistance with this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1.
Global, Regional, and National Burden of Chronic Kidney Disease . Global, regional, and national burden of chronic kidney disease, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. (2020) 395:709–33. 10.1016/S0140-6736(20)30045-3
2.
Zhang L Zhao MH Zuo L Wang Y Yu F Zhang H et al . China Kidney Disease Network (CK-NET) 2015 annual data report. Kidney Int Suppl. (2019) 9:e1–e81. 10.1016/j.kisu.2018.11.001
3.
Morfin JA Fluck RJ Weinhandl ED Kansal S McCullough PA Komenda P . Intensive hemodialysis treatment complications and tolerability. Am J Kidney Dis. (2016) 68:S43–s50. 10.1053/j.ajkd.2016.05.021
4.
Wang R Tang C Chen X Zhu C Feng W Li P et al . Poor sleep and reduced quality of life were associated with symptom distress in patients receiving maintenance hemodialysis. Health Qual Life Outcomes. (2016) 14:125. 10.1186/s12955-016-0531-6
5.
Cruz-Jentoft AJ Baeyens JP Bauer JM Boirie Y Cederholm T Landi F et al . Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on Sarcopenia in Older People. Age Ageing. (2010) 39:412–23. 10.1093/ageing/afq034
6.
Ren H Gong D Jia F Xu B Liu Z . Sarcopenia in patients undergoing maintenance hemodialysis: incidence rate, risk factors and its effect on survival risk. Renal Fail. (2016) 38:364–71. 10.3109/0886022X.2015.1132173
7.
Lamarca F Carrero JJ Rodrigues JC Bigogno FG Fetter RL Avesani CM . Prevalence of sarcopenia in elderly maintenance hemodialysis patients: the impact of different diagnostic criteria. J Nutri Health Aging. (2014) 18:710–7. 10.1007/s12603-014-0505-5
8.
Ding Y Chang L Zhang H Wang S . Predictive value of phase angle in sarcopenia in patients on maintenance hemodialysis. Nutrition. (2022) 94:111527. 10.1016/j.nut.2021.111527
9.
Lin YL Liou HH Wang CH Lai YH Kuo CH Chen SY et al . Impact of sarcopenia and its diagnostic criteria on hospitalization and mortality in chronic hemodialysis patients: A 3-year longitudinal study. J Formos Med Assoc. (2020) 119:1219–29. 10.1016/j.jfma.2019.10.020
10.
Nishi H Takemura K Higashihara T Inagi R . Uremic sarcopenia: clinical evidence and basic experimental approach. Nutrients. (2020) 12:1814. 10.3390/nu12061814
11.
Watanabe H Enoki Y Maruyama T . Sarcopenia in chronic kidney disease: factors, mechanisms, therapeutic interventions. Biol Pharm Bull. (2019) 42:1437–45. 10.1248/bpb.b19-00513
12.
Caspersen CJ Powell KE Christenson GM . Physical activity exercise, and physical fitness: definitions and distinctions for health-related research. Public Health Rep. (1985) 100:126–31.
13.
Kandula NR Lauderdale DS . Leisure time non-leisure time, and occupational physical activity in Asian Americans. Ann Epidemiol. (2005) 15:257–65. 10.1016/j.annepidem.2004.06.006
14.
Wang J Jia D Zhang Z Wang D . Exerkines and sarcopenia: unveiling the mechanism behind exercise-induced mitochondrial homeostasis. Metabolites. (2025) 15:59. 10.3390/metabo15010059
15.
Montero-Fernández N Serra-Rexach JA . Role of exercise on sarcopenia in the elderly. Eur J Phys Rehabil Med. (2013) 49:131–43.
16.
Lu L Mao L Feng Y Ainsworth BE Liu Y Chen N . Effects of different exercise training modes on muscle strength and physical performance in older people with sarcopenia: a systematic review and meta-analysis, BMC Geriat. (2021) 21:708. 10.1186/s12877-021-02642-8
17.
Deutz NE Bauer JM Barazzoni R Biolo G Boirie Y Bosy-Westphal A et al . Protein intake and exercise for optimal muscle function with aging: recommendations from the ESPEN Expert Group. Clin Nutr. (2014) 33:929–36. 10.1016/j.clnu.2014.04.007
18.
Wang Q Zhang X Fang L Guan Q Gao L Li Q . Physical activity patterns and risk of type 2 diabetes and metabolic syndrome in middle-aged and elderly Northern Chinese Adults. J Diabet Res. (2018) 2018:7198274. 10.1155/2018/7198274
19.
Chen J Liu L Chen J Jiang W Wu B Zhu J et al . Physical activity and posttraumatic growth in patients receiving maintenance hemodialysis: a prospective study. J Health Psychol. (2021) 26:2896–907. 10.1177/1359105320937056
20.
Li M Li L Fan X . Patients having haemodialysis: physical activity and associated factors. J Adv Nurs. (2010) 66:1338–45. 10.1111/j.1365-2648.2010.05283.x
21.
Arrieta A Russell LB . Effects of leisure and non-leisure physical activity on mortality in US adults over two decades. Ann Epidemiol. (2008) 18:889–95. 10.1016/j.annepidem.2008.09.007
22.
Csizmadi I Lo Siou G Friedenreich CM Owen N Robson PJ . Hours spent energy expended in physical activity domains: results from the Tomorrow Project cohort in Alberta, Canada. Int J Behav Nutr Phys Act. (2011) 8:110. 10.1186/1479-5868-8-110
23.
Li T Lv A Xu N Huang M Su Y Zhang B et al . Barriers facilitators to exercise in haemodialysis patients: A systematic review of qualitative studies. J Adv Nurs. (2021) 77:4679–92. 10.1111/jan.14960
24.
Painter P Clark L Olausson J . Physical function and physical activity assessment and promotion in the hemodialysis clinic: a qualitative study. Am J Kidney Dis. (2014) 64:425–33. 10.1053/j.ajkd.2014.01.433
25.
Michou V Kouidi E Liakopoulos V Dounousi E Deligiannis A . Attitudes of hemodialysis patients, medical and nursing staff towards patients' physical activity. Int Urol Nephrol. (2019) 51:1249–60. 10.1007/s11255-019-02179-1
26.
Bossola M Pellu V Di Stasio E Tazza L Giungi S Nebiolo PE . Self-reported physical activity in patients on chronic hemodialysis: correlates and barriers. Blood Purificat. (2014) 38:24–9. 10.1159/000363599
27.
Mori K . Maintenance of skeletal muscle to counteract sarcopenia in patients with advanced chronic kidney disease and especially those undergoing hemodialysis. Nutrients. (2021) 13:1538. 10.3390/nu13051538
28.
Manfredini F Mallamaci F D'Arrigo G Baggetta R Bolignano D Torino C et al . Exercise in patients on dialysis: a multicenter, randomized clinical trial. J Am Soc Nephrol: JASN. (2017) 28:1259–68. 10.1681/ASN.2016030378
29.
Baggetta R D'Arrigo G Torino C ElHafeez SA Manfredini F Mallamaci F et al . Effect of a home based, low intensity, physical exercise program in older adults dialysis patients: a secondary analysis of the EXCITE trial. BMC Geriat. (2018) 18:248. 10.1186/s12877-018-0938-5
30.
Chen LK Woo J Assantachai P Auyeung TW Chou MY Iijima K et al . Asian working group for sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J Am Med Dir Assoc. (2020) 21:300–7.e302. 10.1016/j.jamda.2019.12.012
31.
Kyle UG Bosaeus I De Lorenzo AD Deurenberg P Elia M Gómez JM et al . Bioelectrical impedance analysis–part I: review of principles and methods. Clin Nutr. (2004) 23:1226–43. 10.1016/j.clnu.2004.06.004
32.
Janssen I Baumgartner RN Ross R Rosenberg IH Roubenoff R . Skeletal muscle cutpoints associated with elevated physical disability risk in older men and women. Am J Epidemiol. (2004) 159:413–21. 10.1093/aje/kwh058
33.
Dent E Morley JE Cruz-Jentoft AJ Arai H Kritchevsky SB Guralnik J et al . International clinical practice guidelines for sarcopenia (ICFSR): screening, diagnosis and management. J Nutr Health Aging. (2018) 22:1148–61. 10.1007/s12603-018-1139-9
34.
National Kidney Foundation . K/DOQI clinical practice guidelines for nutrition in chronic renal failure. Am J Kidney Dis. (2000) 35:S1–S140. 10.1053/ajkd.2001.20748
35.
Hou Y Li X Hong D Zou H Yang L Chen Y et al . Comparison of different assessments for evaluating malnutrition in Chinese patients with end-stage renal disease with maintenance hemodialysis. Nutr Res. (2012) 32:266–71. 10.1016/j.nutres.2012.02.006
36.
Chen J Peng H Yuan Z Zhang K Xiao L Huang J et al . Combination with anthropometric measurements and MQSGA to assess nutritional status in Chinese hemodialysis population. Int J Med Sci. (2013) 10:974–80. 10.7150/ijms.5811
37.
Jiang J Ni L Ren W Zhou X Su K Wang L et al . Nutritional status in short daily hemodialysis versus conventional hemodialysis patients in China. Int Urol Nephrol. (2018) 50:755–62. 10.1007/s11255-018-1804-2
38.
Hallal PC Victora CG . Reliability validity of the International Physical Activity Questionnaire (IPAQ). Med Sci Sports Exerc. (2004) 36:556. 10.1249/01.MSS.0000117161.66394.07
39.
Lou X He Q . Validity and reliability of the international physical activity questionnaire in Chinese hemodialysis patients: a multicenter study in China. Med Sci Monit Int Med J Exp Clin Res. (2019) 25:9402–8. 10.12659/MSM.920900
40.
Craig CL Marshall AL Sjöström M Bauman AE Booth ML Ainsworth BE et al . International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. (2003) 35:1381–95. 10.1249/01.MSS.0000078924.61453.FB
41.
Pate RR Pratt M Blair SN Haskell WL Macera CA Bouchard C et al . Physical activity and public health. A recommendation from the Centers for Disease Control and Prevention and the American College of Sports Medicine. JAMA. (1995) 273:402–7. 10.1001/jama.1995.03520290054029
42.
Yu R Wong M Leung J Lee J Auyeung TW Woo J . Incidence, reversibility, risk factors and the protective effect of high body mass index against sarcopenia in community-dwelling older Chinese adults. Geriat Gerontol Int. (2014) 14:15–28. 10.1111/ggi.12220
43.
Sallfeldt ES Mallmin H Karlsson MK Mellström D Hailer NP Ribom EL . Sarcopenia prevalence incidence in older men - a MrOs Sweden study. Geriat Nurs. (2023) 50:102–8. 10.1016/j.gerinurse.2023.01.003
44.
Yang Y Da J Yuan J Zha Y . One-year change in sarcopenia was associated with cognitive impairment among haemodialysis patients. J Cachexia, Sarcopenia Muscle. (2023) 14:2264–74. 10.1002/jcsm.13311
45.
Du X Chen G Zhang H Liu Y Gu F Wang Y et al . Development of a practical screening tool to predict sarcopenia in patients on maintenance hemodialysis. Med Sci Monit. (2022) 28:e937504. 10.12659/MSM.937504
46.
Fahal IH . Uraemic sarcopenia: aetiology and implications. Nephrol Dial Transplant. (2014) 29:1655–65. 10.1093/ndt/gft070
47.
Johansen KL Shubert T Doyle J Soher B Sakkas GK Kent-Braun JA . Muscle atrophy in patients receiving hemodialysis: effects on muscle strength, muscle quality, physical function. Kidney Int. (2003) 63:291–7. 10.1046/j.1523-1755.2003.00704.x
48.
Bhatnagar P Townsend N Shaw A Foster C . The physical activity profiles of South Asian ethnic groups in England. J Epidemiol Community Health. (2016) 70:602–8. 10.1136/jech-2015-206455
49.
Wen M Li L Su D . Physical activity and mortality among middle-aged and older adults in the United States. J Phys Act Health. (2014) 11:303–12. 10.1123/jpah.2011-0281
50.
Park H Park S Shephard RJ Aoyagi Y . Yearlong physical activity and sarcopenia in older adults: the Nakanojo Study. Eur J Appl Physiol. (2010) 109:953–61. 10.1007/s00421-010-1424-8
51.
Pan L Wu M Wen QR Lyu J Guo Y Pei P Du HD et al . The correlation of physical activity and sedentary leisure time with low muscle mass, strength, and quality in Chinese adults. Zhonghua Liu Xing Bing Xue Za Zhi. (2022) 43:162–8. 10.3760/cma.j.cn112338-20210402-00273
52.
Distefano G Goodpaster BH . Effects of exercise and aging on skeletal muscle. Cold Spring Harb Perspect Med. (2018) 8:a029785. 10.1101/cshperspect.a029785
53.
Marzetti E Calvani R Tosato M Cesari M Di Bari M Cherubini A et al . Physical activity and exercise as countermeasures to physical frailty and sarcopenia. Aging Clin Exp Res. (2017) 29:35–42. 10.1007/s40520-016-0705-4
54.
Nascimento CM Ingles M Salvador-Pascual A Cominetti MR Gomez-Cabrera MC Viña J . Sarcopenia, frailty and their prevention by exercise. Free Radic Biol Med. (2019) 132:42–9. 10.1016/j.freeradbiomed.2018.08.035
55.
Bosaeus I Rothenberg E . Nutrition and physical activity for the prevention and treatment of age-related sarcopenia. Proc Nutr Soc. (2016) 75:174–80. 10.1017/S002966511500422X
56.
Landi F Marzetti E Martone AM Bernabei R Onder G . Exercise as a remedy for sarcopenia. Curr Opini Clini Nutr Metabol Care. (2014) 17:25–31. 10.1097/MCO.0000000000000018
57.
Bakaloudi DR Siargkas A Poulia KA Dounousi E Chourdakis M . The effect of exercise on nutritional status and body composition in hemodialysis: a systematic review. Nutrients. (2020) 12:3071. 10.3390/nu12103071
58.
Noor H Reid J Slee A . Resistance exercise and nutritional interventions for augmenting sarcopenia outcomes in chronic kidney disease: a narrative review. J Cachexia Sarcopenia Muscle. (2021) 12:1621–40. 10.1002/jcsm.12791
59.
Beaudart C Dawson A Shaw SC Harvey NC Kanis JA Binkley N et al . Nutrition and physical activity in the prevention and treatment of sarcopenia: systematic review. Osteoporosis Int. (2017) 28:1817–33. 10.1007/s00198-017-3980-9
60.
Lee SY Tung HH Liu CY Chen LK . Physical activity and sarcopenia in the geriatric population: a systematic review. J Am Med Dir Assoc. (2018) 19:378–83. 10.1016/j.jamda.2018.02.003
61.
Shen Y Shi Q Nong K Li S Yue J Huang J et al . Exercise for sarcopenia in older people: A systematic review and network meta-analysis. J Cachexia, Sarcopenia Muscle. (2023) 14:1199–211. 10.1002/jcsm.13225
62.
Jang MK Park C Tussing-Humphreys L Fernhall B Phillips S Doorenbos AZ . The effectiveness of sarcopenia interventions for cancer patients receiving chemotherapy: a systematic review and meta-analysis. Cancer Nurs. (2023) 46:E81–e90. 10.1097/NCC.0000000000000957
63.
O'Hare AM Tawney K Bacchetti P Johansen KL . Decreased survival among sedentary patients undergoing dialysis: results from the dialysis morbidity and mortality study wave 2. Am J Kidney Dis. (2003) 41:447–54. 10.1053/ajkd.2003.50055
64.
Huang M Lv A Yang F Tang Y Li Y Hua Y et al . Impacts of cognition of exercise on physical activity participation in hemodialysis patients. Seminars Dialysis. (2023) 36:366–73. 10.1111/sdi.13138
65.
Ghafourifard M Mehrizade B Hassankhani H Heidari M . Hemodialysis patients perceived exercise benefits and barriers: the association with health-related quality of life. BMC Nephrol. (2021) 22:94. 10.1186/s12882-021-02292-3
66.
Sutherland S Penfold R Doherty A Milne Z Dawes H Pugh C et al . A cross-sectional study exploring levels of physical activity and motivators and barriers towards physical activity in haemodialysis patients to inform intervention development. Disab Rehabilitat. (2021) 43:1675–81. 10.1080/09638288.2019.1672214
67.
Ainsworth BE Haskell WL Whitt MC Irwin ML Swartz AM Strath SJ et al . Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc. (2000) 32:S498–504. 10.1097/00005768-200009001-00009
68.
Bull FC Al-Ansari SS Biddle S Borodulin K Buman MP Cardon G et al . World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br J Sports Med. (2020) 54:1451–62. 10.1136/bjsports-2020-102955
Summary
Keywords
maintenance hemodialysis, sarcopenia, physical activity, nonleisure-time physical activity, leisure-time physical activity
Citation
Chang L, Zheng Y, Ding Y, Long Z and Zhang H (2025) Nonleisure-time physical activity as a protective factor against sarcopenia in hemodialysis patients: a prospective cohort study. Front. Nutr. 12:1517429. doi: 10.3389/fnut.2025.1517429
Received
26 October 2024
Accepted
11 March 2025
Published
26 March 2025
Volume
12 - 2025
Edited by
Heitor S. Ribeiro, University of Brasilia, Brazil
Reviewed by
Thiago Santos Rosa, Catholic University of Brasilia (UCB), Brazil
Hugo Luca Corrêa, Catholic University of Brasilia (UCB), Brazil
Damiano Zemp, Ente Ospedaliero Cantonale (EOC), Switzerland
Updates
Copyright
© 2025 Chang, Zheng, Ding, Long and Zhang.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hongmei Zhang hzzhanghongmei@163.com
†These authors have contributed equally to this work
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.