- 1Center for Cognition and Brain Disorders, The Affiliated Hospital of Hangzhou Normal University, Hangzhou, Zhejiang, China
- 2Department of Psychology, University of Macau, Macau, Macao SAR, China
- 3The First Affiliated Hospital, College of Medicine, Zhejiang University, Hangzhou, China
Background: Obesity, particularly in high-risk groups for food addiction, adversely impacts the brain’s functional characteristics. However, its underlying neurobiological and molecular mechanisms remain elusive. The current study adopted a data-driven approach to investigate obesity-associated intrinsic functional architecture and neurotransmitter receptor patterns.
Methods: Resting-state fMRI data were acquired from 198 obese and 291 healthy weight individuals from the Human Connectome Project. Intrinsic connectivity contrast (ICC) and fractional amplitude of low-frequency fluctuations (fALFF) analyses were performed to identify the common altered brain regions and then seeds to whole brain functional connectivity (FC) analyses were conducted to determine obesity-related FC features. Additionally, the relationship between intrinsic functional characteristics and molecular imaging features was assessed to examine neurotransmitter-receptor distribution patterns underlying obesity.
Results: Obese individuals, compared to healthy weight individuals, showed aberrant ICC and fALFF in both the right dorsolateral prefrontal cortex (DLPFC) and left insula. For the FC results, the obese group displayed increased FC between the right DLPFC and precuneus, left insula and left inferior parietal lobule, right DLPFC as well as decreased FC between right DLPFC and left precentral, left postcentral gyrus, and bilateral paracentral lobule. Additionally, the fALFF alterations in insula/temploral pole and also the rDLPFC-PCL FC partially mediated the relationship between body mass index and the executive function. Furthermore, cross-modal correlation analyses indicated that ICC and fALFF alterations were related to noradrenaline transporter and dopamine receptor distributions, respectively.
Discussion: Together our findings suggested that obesity is associated with atypical neurotransmitter systems and dysfunctional architecture especially in the prefrontal cortex, insula, sensorimotor cortex, and default mode circuits. These may deepen our understanding the neurobiological basis of obesity and provide novel insights into neuroimaging-based treatment and intervention.
1 Introduction
Obesity is escalating to pandemic proportions globally. According to the World Health Organization (WHO), 43% of adults were overweight and 16% were obese in 20221, highlighting obesity as a significant global public health issue (1). Obesity has negative associations with physical and mental health that include increased risk for hypertension, diabetes, anxiety disorders, and depression (2–4). Mounting evidence suggests that obesity often impairs cognitive functions, such as executive control and memory (5–7). Given such evidence, it is imperative to deepen our understanding of the neurobiological mechanisms underlying obesity.
Emerging research indicates that obesity is associated with deficits in inhibitory control and reward processing, which are reflected in functional disturbances in corresponding brain areas (8–10). For example, obese and overweight individuals exhibit hyper-responsivity in the reward areas and hypo-responsivity in areas associated with inhibitory control when exposed to high-calorie food images, compared to healthy weight individuals (11). Furthermore, obesity is associated with dysfunction in the interoceptive system, implying that affected individuals have impaired perceptions of intersensory signals, such as hunger and satiety, which lead to increased food consumption (12–14). Despite these findings, further exploration is needed to elucidate the obesity-related variations in intrinsic functional connectivity when the brain is at rest.
In recent years, resting-state functional MRI (rs-fMRI) has emerged as a promising tool for assessing spontaneous neural activities and is widely accepted in both research and clinical settings (15, 16). Examples of related approaches include fractional amplitude of low-frequency fluctuation (fALFF) and intrinsic connectivity contrast (ICC) (17, 18). fALFF measures the magnitude of low-frequency oscillations at the regional level (18) and has been used to investigate neural underpinnings of obesity (19). For instance, recent research indicates that obese individuals exhibited elevated fALFF in the dorsolateral prefrontal cortex (DLPFC), insula, and precuneus (20), compared to healthy weight controls, suggesting aberrant spontaneous fluctuations in these regions during cognitive control and emotion processing. Other researchers (19, 21) have observed discordant patterns of spontaneous fluctuations between obese samples. Additionally, previous studies have identified contrasting patterns of intrinsic activity in the default mode network (DMN) between obese samples (22–24). These studies underscore considerable heterogeneity in the intrinsic brain functional organization in obese samples, which may be partially due to variations in regions of interest (ROIs) and relatively small sample sizes (10).
Of note, aberrations in regional spontaneous activity related to obesity do not provide insights about relationship of regional aberrations in spontaneous neural fluctuations with divergent connectivity. Consequently, alongside fALFF, the well-established exploratory analytical method of “intrinsic connectivity contrast” (ICC) analysis is recommended to address this gap. ICC quantifies the strength of global connectivity patterns between a voxel and the rest of the brain, based on network theory (17). This index requires no prior knowledge or assumptions (25) and has been extensively used to investigate functional brain changes in psychiatric and neurocognitive disorders (26–28). Prior research has demonstrated that individuals with nicotine dependence exhibit ICC alterations in the medial frontal cortex (mPFC), DLPFC, and supramarginal gyrus (29). Additionally, ICC in the nucleus accumbens and caudate has been found to accurately discriminate between cannabis-dependent individuals and controls (30). However, ICC has not yet been employed to investigate the intrinsic functional organization in obesity. Furthermore, recent evidence suggests that employing multilevel indices from different perspectives may enhance the ability to comprehensively uncover intrinsic brain dysfunction, thereby facilitating a more sensitive identification of regional abnormalities (31, 32). As such, in this study, we combined ICC and fALFF to explore alterations in intrinsic brain functions associated with obesity, offering a more comprehensive analysis approach.
In addition, obesity is closely linked to disruptions in neurotransmitter systems, such as dopaminergic system and noradrenaline system. Dopamine plays a significant role in modulating appetitive behaviors through brain regions involved in reward processing (33–35). Similarly, the noradrenaline system, widely distributed throughout the central nervous system, plays a crucial role in energy balance (36, 37). A positron emission tomography (PET) imaging study showed that noradrenaline transporter (NAT) availability in the DLPFC and hypothalamus strongly correlates with changes in body mass index (BMI) following gastric bypass surgery in morbidly obese individuals (38). Notably, brain function, as represented by resting-state functional connectivity (RSFC), may be coupled with neurotransmitter systems (39, 40). For instance, GABA levels in the primary motor cortex were negatively associated with the connectivity strength of the resting motor network (40). Nonetheless, there is a need to elucidate the nature of the association between neurotransmitter systems and spontaneous neural activity in obese individuals.
To address these gaps in the literature, we employed a data-driven, multi-algorithm approach that combines ICC and fALFF to explore intrinsic functional architecture alterations associated with obesity, based on a large sample from the Human Connectome Project (HCP). Participants were categorized into the obesity (OB) and healthy weight (HW) groups based on BMI. Brain regions exhibiting concurrent ICC and fALFF alterations were selected as ROIs to investigate FC patterns associated with obesity. To examine relationships between spontaneous brain activity, intrinsic functional characteristics, and neurotransmitters, we assessed obesity-related functional abnormalities linked to the dopaminergic system, serotonin system, and NAT using a novel cross-modal data analysis approach. Based on previous studies (8), we hypothesized that obese individuals, compared with healthy weight individuals, would exhibit intrinsic functional alterations in brain areas related to executive control, motivational reward, and self-reference. These regional alterations were expected to correlate with dopaminergic and noradrenaline system distribution.
2 Methods
2.1 Participants
We selected 489 participants (290 women) from the publicly available HCP database.2 The final sample was determined by the following criteria: (a) exclusion of subjects lacking T1 or 3T_RS-fMRI scans; (b) screened according to body weight criteria, specifically individuals with a BMI ranging from 20 to 24 and a BMI greater than 30; (c) exclusion of subjects with missing or corrupted REST1_LR data; (d) removal of subjects with excessive head movement (exceeding 2.5 mm or 2.5 degrees in max head motion). The final cohort of 489 participants was divided into two groups: an obesity group (BMI > 30) and a healthy weight group (BMI of 20 to 24). For additional details of HCP inclusion and exclusion criteria, please see Van Essen et al. (41). All participants provided informed consent, and the entire protocol received approval from the Institutional Review Board at Washington University School of Medicine. Group differences in age, BMI, and handedness were assessed for significance using independent-sample T-tests. Differences in the distribution of gender and ethnicity between groups were examined using chi-squared tests. All analyses were done using SPSS software with a threshold of p < 0.05.
2.2 Neuroimaging
2.2.1 MRI acquisition
The fMRI data were scanned at Washington University in St. Louis using a Siemens 3.0 T “Connectome Skyra” with a standard 32-channel receiver head coil. T1-weight structural images were acquired with the following parameters: Repetition Time (TR) = 2,400 ms, Echo Time (TE) = 2.14 ms, flip angle = 8°, Field-Of-View (FOV) = 224*224 mm, voxel size = 0.7 mm isotropic. Resting fMRI images were acquired using a gradient-echo-planar sequence with multiband factor 8, TR = 720 ms, TE = 33.1 ms, flip angle = 52°, FOV = 208*180 mm, Matrix = 104*90, voxel size = 2 mm isotropic.
The rs-fMRI data used in the current study was from the HCP database (that is “the REST1_LR run”), which lasted 15 min and included 1,200 frames. During the rsfMRI, participants were instructed to keep their eyes open and fixate on a projected bright crosshair on a dark background.
2.2.2 Image data preprocessing
All data were preprocessed using the minimal preprocessing pipeline and the details on data preprocessing can be found in (42). Structured artifacts within the time series were removed by independent component analysis (ICA) and FIX (FMRIB’s ICA-based X-noisifier) (43–45). Additional preprocessing was conducted using the CONN connectivity toolbox (v. 21a; 46). The following preprocessing steps were applied to the data: (a) spatial smoothing with a Gaussian kernel of full width at half maximum (FWHM) of 6 mm; (b) bandpass filtering between 0.01—0.1 Hz; (c) denoising via the CompCor algorithm (46) by regressing out the filtered white matter, cerebrospinal fluid (CSF), effect of the rest, and head-motion (12 variables from “Movement_Regressors_dt.txt” and their quadratic); (d) detrending to remove linear trends. Given the ongoing debate regarding the use of GSR in resting-state fMRI preprocessing (47)—particularly it may abolish or reverse important rsFC results (48)—we did not regress out the global signal in this study.
2.2.3 RS-fMRI data processing
The analysis flow is shown in Figure 1. All calculations were implemented using CONN software (49), with gender and age regressed as covariates.
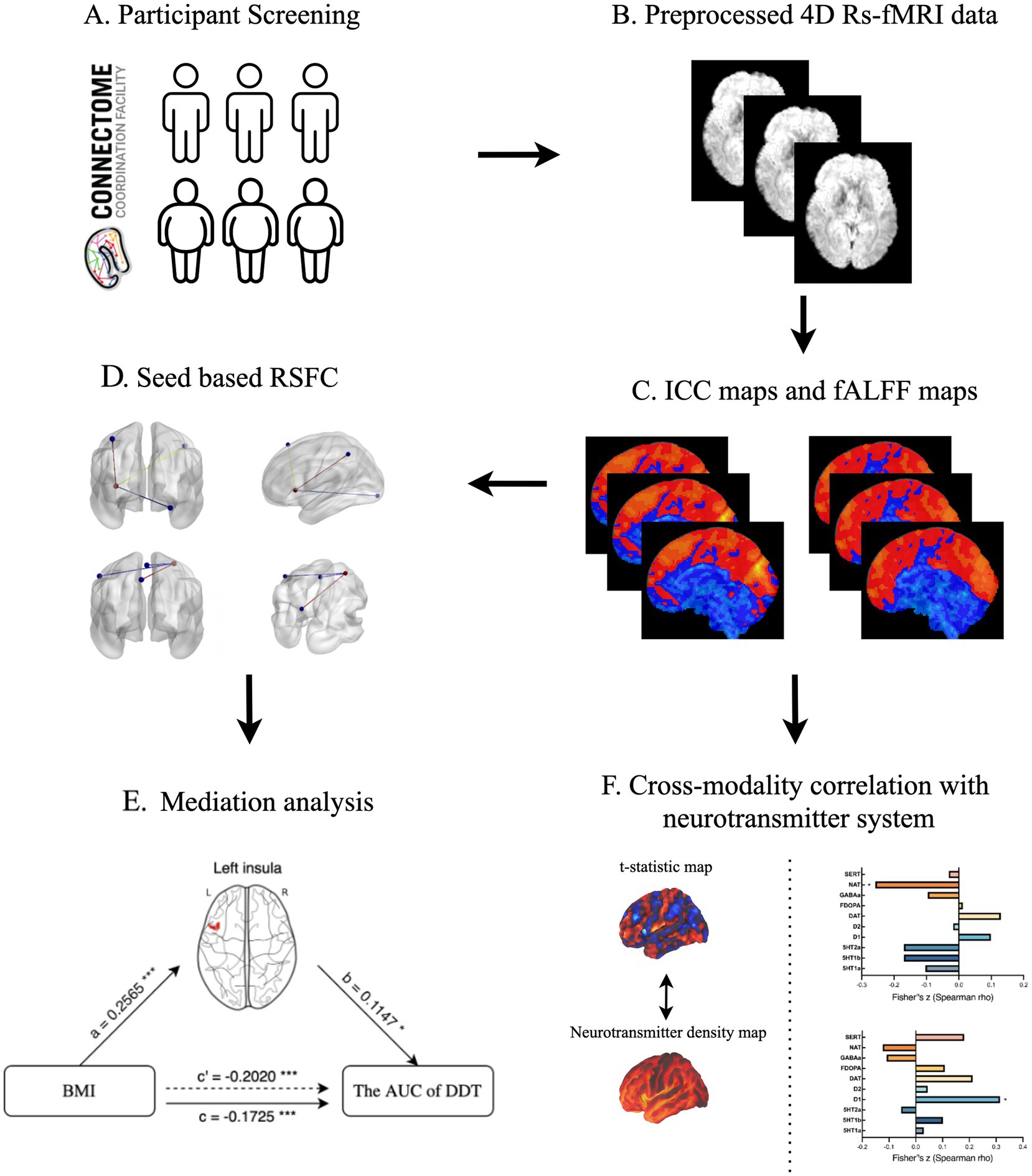
Figure 1. The schematic description presenting a step-by-step process of statistical analysis. (A) Participant selection; (B) Image data preprocessing; (C) ICC and fALFF analysis; (D) Seed-based RSFC analysis; (E) Mediation analysis; (F) Cross-modality correlation analysis. ICC, intrinsic connectivity contrast; fALFF, fractional amplitude of low frequency fluctuation; RSFC, resting-state functional connectivity; BMI, body mass index; AUC, area under curve; DDT, Delay Discounting Task.
2.2.3.1 Fractional amplitude of low-frequency fluctuation
The calculation of fALFF aligns with the methods proposed by Zou et al. (18). The power spectrum was obtained by transforming the time series data for each voxel into the frequency domain using a fast Fourier transform algorithm. Subsequently, the square root of the power spectrum was calculated and the amplitude of low-frequency fluctuation (ALFF) was determined by averaging the square roots across the 0.01–0.1 Hz range for each voxel (50). Additionally, fALFF was calculated by computing the ratio of the power spectrum within the low-frequency range (0.01–0.1 Hz) to that of the entire frequency spectrum. For analyses, fALFF values were z-transformed.
2.2.3.2 Intrinsic connectivity contrast
Voxel-wise global connectivity was assessed through ICC, a completely data-driven metric that does not require a preset threshold. ICC represents an estimate of the association strength between the time series of a specific voxel and all other voxels in the brain, with higher values indicating stronger connectivity. Specifically, we calculated the root mean square (RMS) of each voxel’s connections with other voxels throughout the brain based on blood oxygenation level-dependent (BOLD) time series (17). ICC values were then z-transformed for analysis.
2.2.3.3 Seed-based functional connectivity (RSFC)
To further elucidate specific networks underlying observed group differences in global connectivity, a subsequent seed-to-voxel FC analysis was conducted. Seed regions were determined as spheres with a radius of 6 mm, according to the overlay brain regions of significant group difference from ICC and fALFF (17). Correlation coefficients were computed between the mean BOLD signal time series of each seed and all other brain voxels, yielding individual voxel-wise FC maps via a weighted general linear model (weighted-GLM). To normalize FC maps, correlation coefficients (r values) were transformed into Z-scores using Fisher’s r-to-z transformation.
2.2.3.4 Threshold
For fALFF and ICC, obesity versus control group differences were assessed using an independent-sample T test with a conservative threshold of voxel-level p < 0.001 and cluster-level PFWE < 0.05, corrected using family-wise error (FWE). To better explore obesity—related differences in intrinsic functional architecture, we applied a relatively lenient false discovery rate (FDR) correction for FC (voxel-level p < 0.001 and cluster-level PFDR < 0.05).
2.3 Cross-modality correlation analysis
To investigate the relationship between receptor systems activity and spontaneous brain activity alterations, we utilized voxel-wise non-threshold T statistic maps of ICC and fALFF to assess Spearman correlations with PET-and SPECT-derived maps in JuSpace3 (51). Significant results were defined as p < 0.05 (adjusted for spatial correlation; N = 10,000 permutations; FDR corrected). In this study, we included 10 maps from JuSpace that represent different types of neurotransmitters for the analysis. Detailed PET and SPECT map selections are available in the Supplementary materials.
2.4 Mediation analysis
To further explore the association between altered brain functionality and behavioral performance in obese individuals, we utilized performance scores from executive function tasks such as the Dimensional Change Card Sort Task (DCCS, “CardSort_AgeAdj”) and Flanker Test (“Flanker_AgeAdj”), as well as impulsivity measures from the Delay Discounting Task (DDT, mean of the area under curve (AUC) variables for the $200 and the $40,000 delayed reward conditions) (52), sourced from the HCP database. With the DDT, a smaller AUC is indicative of greater impulsivity.
To elucidate the potential mediating role of the brain’s functional alterations on the relationship between BMI and behavioral data, the mediation analysis was performed with the PROCESS macro in SPSS 26.0.4 Similar to the prior studies (53, 54), we used the bootstrapping method (5,000 steps) to assess the significance of the mediation effect. Mediation effects were deemed statistically significant when the bootstrapped 95% confidence intervals (CI) did not encompass zero.
We conducted a power analysis using the online tool Monte Carlo Power Analysis for Indirect Effects of Mediation Models [(55), https://schoemanna.shinyapps.io/mc_power_med/] to ensure the adequacy of our sample size. The results indicated a power of 1 for a sample size of 198, assuming path correlations of 0.65 (a and b) and 0.60 (c’), with standard deviations of 1 for all paths.
3 Results
3.1 Demographic characteristics
The detailed information of the final sample is shown in Supplementary Table S1. There were no group differences in age, sex, handedness or ethnicity between the obese and healthy weight groups. There were significant differences in scores of DCCS and AUC of DDT between the two groups. Mediating effects of FC and fALFF on associations between BMI and cognitive task performance based on these measures were assessed in subsequent analyses.
3.2 Group differences in the intrinsic connectivity contrast
Compared to the healthy weight group, the obese group exhibited significantly increased ICC in the left insula, and right DLPFC (Table 1; Figure 2A). Furthermore, brain regions with decreased ICC in the obese included the right inferior occipital gyrus (IOG) and right supramarginal gyrus compared to the healthy weight group (Table 1; Figure 2A).
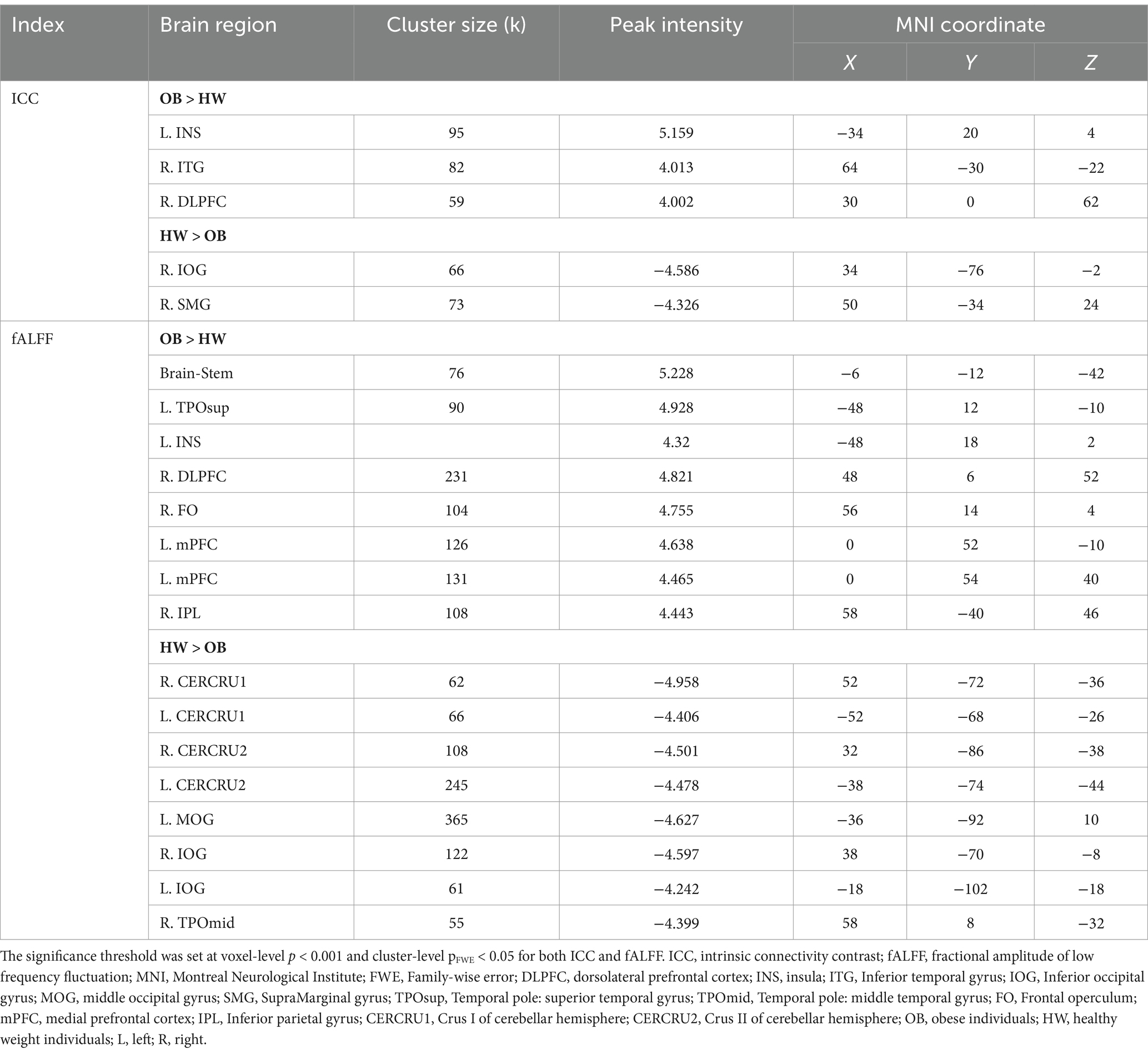
Table 1. Significant group differences in ICC and fALFF between obese individuals and healthy weight individuals.
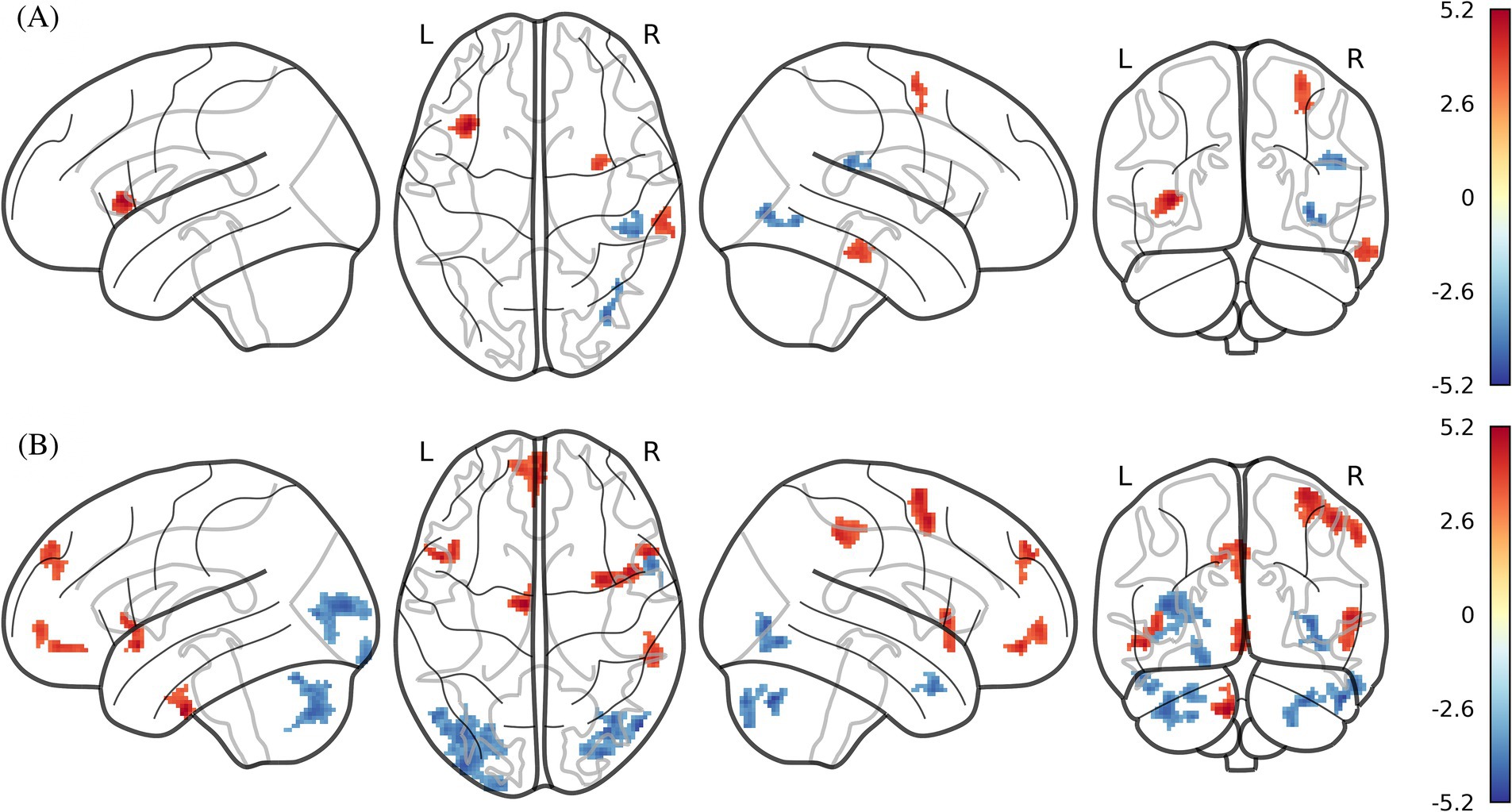
Figure 2. ICC and fALFF maps of statistically significant differences by two-sample t-test between obese individuals and healthy weight individuals (PFWE < 0.05 at the cluster-level with a cluster-defining voxel-wise statistical threshold of p < 0.001 uncorrected). (A) ICC; (B) fALFF. ICC, intrinsic connectivity contrast; fALFF, fractional amplitude of low frequency fluctuation. Color bar indicates the T score.
3.3 Group differences in fractional amplitude of low-frequency fluctuation
The obese group showed significantly increased fALFF in the right DLPFC, left mPFC, right inferior parietal lobule (IPL), left temporal pole/insula, and brain stem compared to the healthy weight group (Table 1; Figure 2B). Furthermore, fALFF was significantly lower in the bilateral cerebellum crus (I and II), bilateral IOG, right middle occipital gyrus (MOG), and right temporal pole of the obese group versus healthy weight group (Table 1; Figure 2B).
3.4 Seed-based RSFC
Using the right DLPFC, right IOG, and left insula as seeds, we tested differences in seed-based FC between the two groups. As for the right DLPFC seed, obese group versus healthy weight group had a stronger FC in the left precuneus (k = 194) and a lower FC in the left postcentral gyrus extending to the precentral gyrus (k = 714), as well as the bilateral paracentral lobule (PCL, k = 546) (Table 2; Figure 3). The analysis based on the left insula seed showed significantly enhanced FC in the left IPL extending to the angular gyrus (AG, k = 713), right DLPFC (k = 487), and right lingual gyrus (k = 100) for the obese group versus healthy weight group (Table 2; Figure 3). Finally, right IOG seed exhibited comparatively decreased connectivity with the occipital cortex (i.e., bilateral IOG and MOG, kR = 2,553, kL = 1943) in the obese group (Table 2).
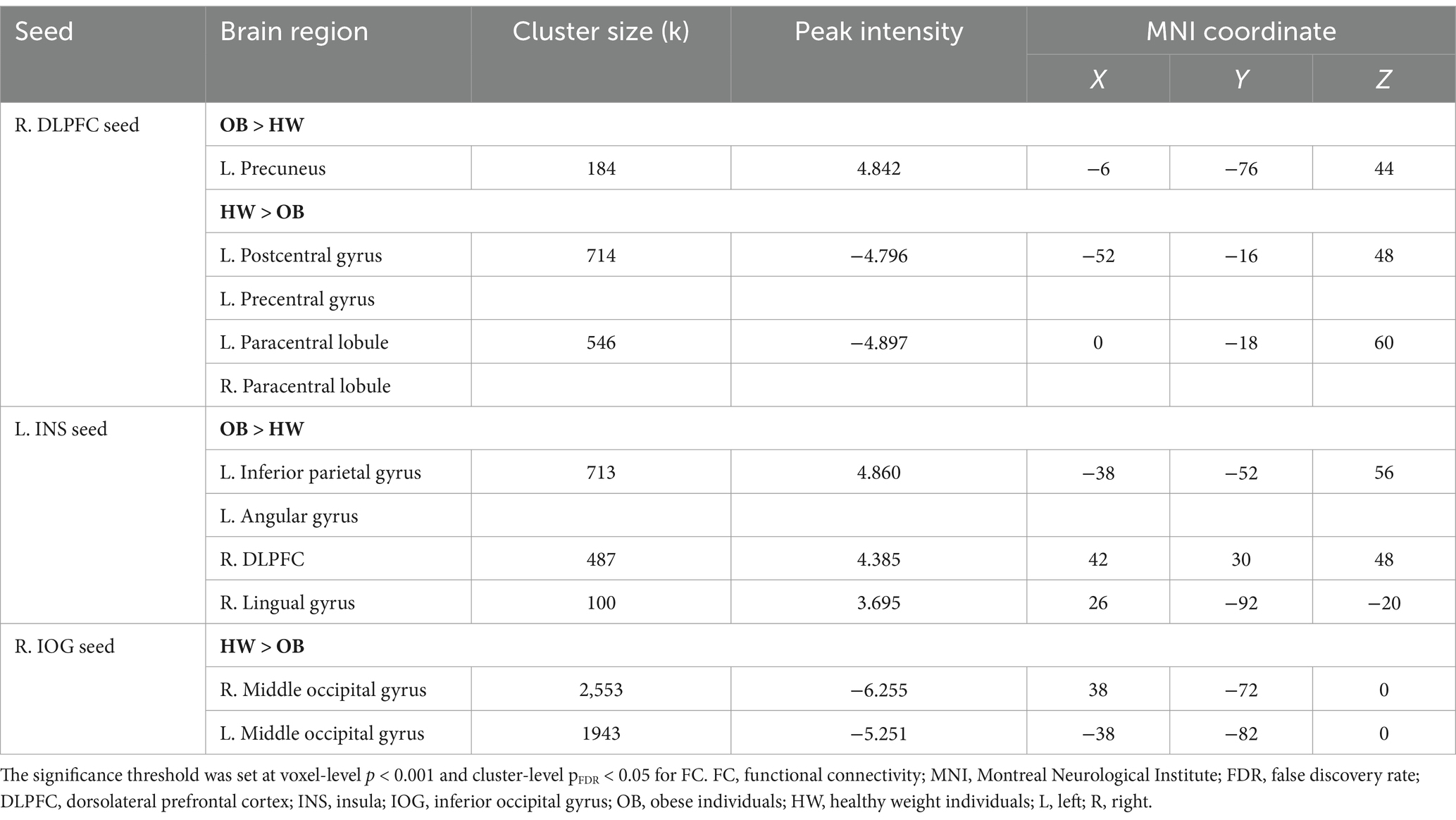
Table 2. Significant group differences in seed-based functional connectivity between obese individuals and healthy weight individuals.
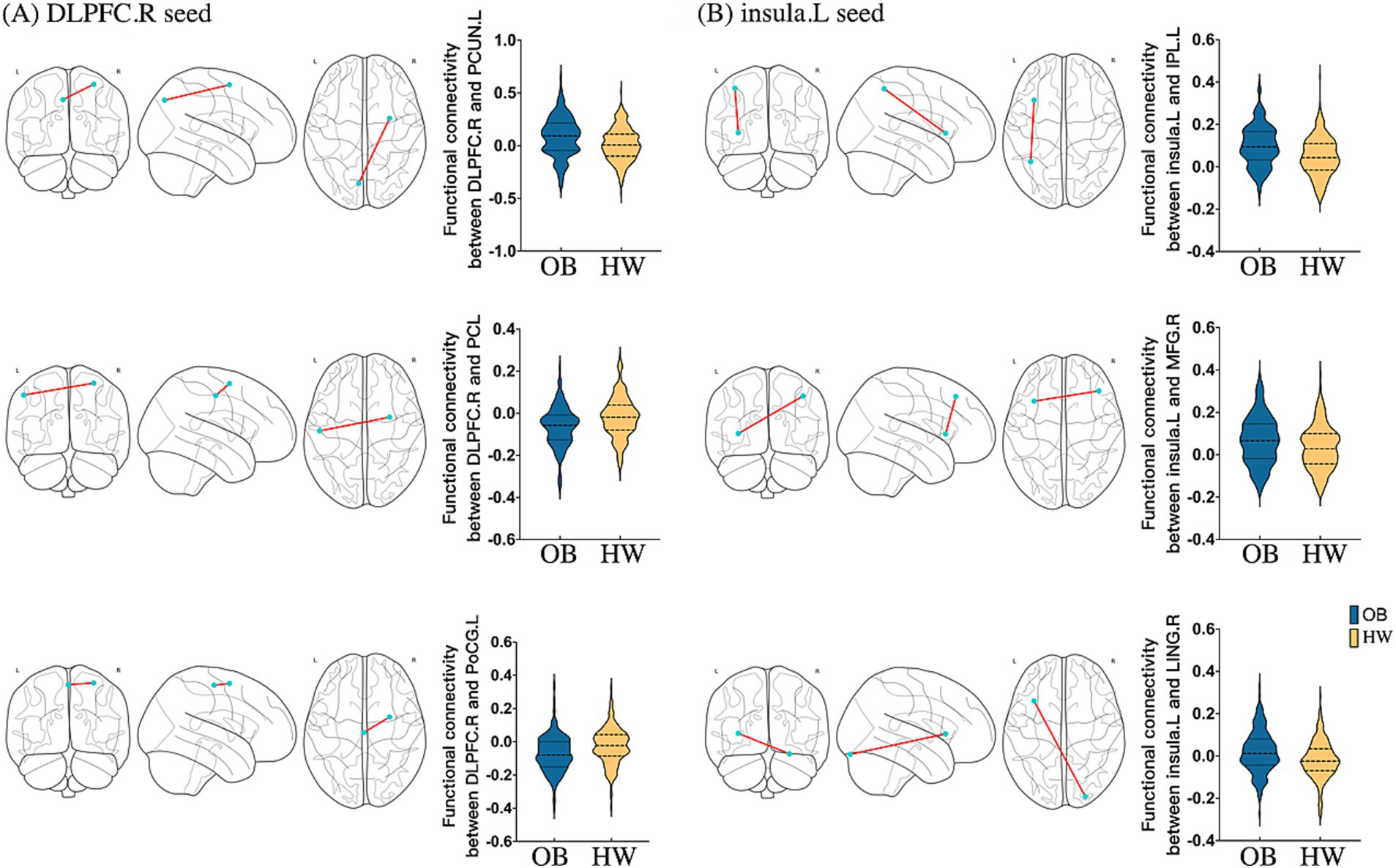
Figure 3. Seed-based FC maps of statistically significant differences by two-sample t-test between obese individuals and healthy weight individuals (PFDR < 0.05 at the cluster-level). (A) RSFC based on the right DLPFC; (B) RSFC based on the left insula. DLPFC, dorsolateral prefrontal cortex; INS, insula; PCL, paracentral lobule; PoCG, postcentral gyrus; PCUN, precuneus; IPL, inferior parietal lobule; MFG, middle frontal gyrus; LING, lingual gyrus; R, right; L, left; RSFC, resting-state functional connectivity; OB, obeses individuals; HW, healthy weight individuals.
3.5 Relationships between neuronal and neurotransmitter systems
ICC changes between the two groups were significantly correlated with NAT (exact p = 0.004, PFDR = 0.039) (Figure 4). In addition, changes in fALFF were spatially correlated with dopamine receptor (D1) (exact p < 0.001, PFDR = 0.008) (Figure 4).
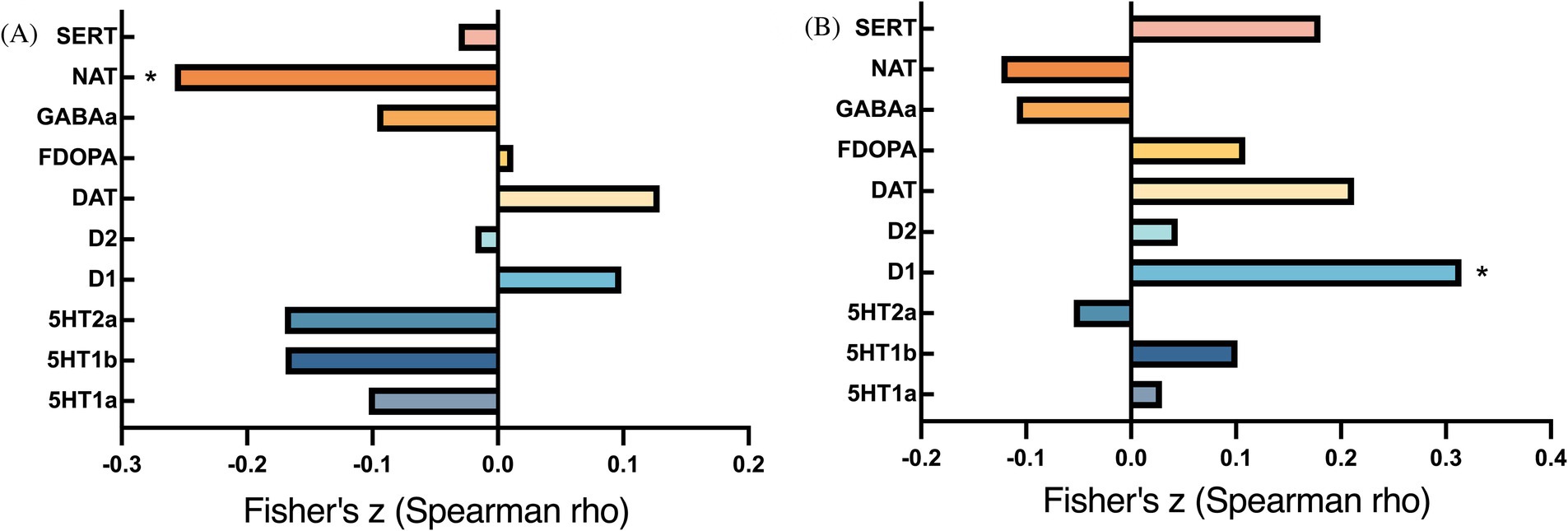
Figure 4. Bar plots of cross-modal correlations between receptor systems and ICC/fALFF components. (A) Correlations between ICC and receptor systems; (B) Correlations between fALFF and receptor systems. p < 0.05, false discovery rate corrected.
3.6 Mediation analysis results
For obese individuals, rDLPFC-PCL FC partially mediated the relationship between BMI and the DCCS scores. Additionally, fALFF values for the left insula, bilateral cerebellum crus 2, and right temporal pole each partially mediated the relationship between BMI and the area under curve (AUC) results for the DDT. For healthy weight individuals, no significant results were found in Mediation analyses. Detailed mediation analysis results are presented in Supplementary materials.
4 Discussion
In the current study, we examined the alterations of intrinsic functional architecture in obese group versus non-obese groups using a data-driven multiple-algorithm analysis combining ICC and fALFF with seed-based RSFC. Obese individuals, compared to healthy weight individuals, showed significant differences in the right DLPFC and left insula both in the intrinsic spontaneous activity and global connectivity index (i.e., fALFF and ICC). Seed-based FC analysis showed enhanced connectivity between the right DLPFC and the left precuneus, accompanied by reduced connectivity between the DLPFC and the sensorimotor cortex in the obese group versus healthy weight group. Additionally, using a seed placed in the left insula, the obese group exhibited significantly higher FC with the left IPL extending to the AG, right DLPFC, and lingual gyrus. Crucially, changes in ICC and fALFF correlated, respectively, with NAT and dopaminergic (D1) system. Mediation analyses revealed that fALFF in the left insula and the DLPFC-PCL FC partially mediated relations between BMI and impulsivity as well as cognitive flexibility. Taken together, these findings provide new neuroimaging evidence for possible neurophysiological mechanisms underlying obesity.
4.1 Alterations of the prefrontal control cortex and insula
In the current study, obese individuals exhibited increased spontaneous fluctuations in the right DLPFC. The DLPFC, in conjunction with the executive control network (ECN), has been implicated in the top-down control of appetitive processes and self-regulation of overeating (56, 57). Supporting this finding, recent studies have also demonstrated elevated spontaneous fluctuations in the DLPFC among overweight individuals (20, 118). Concurrently, structural imaging studies have observed an association between obesity and smaller DLPFC volumes (58) that are also negatively correlated with disinhibited eating (59). Dovetailing with spontaneous fluctuation data, obese individuals also had elevated global connectivity (i.e., ICC) in the right DLPFC which is posited to play a pivotal area in regulating obesogenic eating behaviors (60). Specifically, recent work has demonstrated that the suppression of food cravings in response to palatable foods activates the DLPFC (61), with the degree of activation correlating with subsequent weight loss success in a dietary interventions program (62–64). In addition, the neuromodulation and neurofeedback training targeting the DLPFC can reduce food craving and promote top-down regulation of appetite in obese individuals (65, 66).
In the subsequent FC analysis, the obese group showed comparatively lower FC between the right DLPFC and the sensorimotor network (i.e., left postcentral gyrus, left precentral gyrus, and bilateral PCL), which is regarded as a central hub and “engine of desire” connecting the body and brain in the neural vulnerability model of overeating (67). Importantly, we also found right DLPFC-PCL FC mediated a negative relationship between BMI and cognitive flexibility performance. Although obese participants may have attempted weight control, they also hold positive implicit attitudes and automatic reward tendencies toward palatable foods (68, 69). As such, these individuals may experience stronger conflicts between weight control goals and hedonic eating impulses than non-obese peers do (70). In line with these perspectives, obese people may expend more cognitive effort and resources to suppress their impulses as reflected by the increment of spontaneous activity in DLPFC (12).
Obese participants also showed aberrant alterations in the left insula both in spontaneous fluctuations and global connectivity. The insula is involved in the integrative processing of food craving, gustatory perception, and hedonic consumption (71–73) and has been identified as a high expression region of the obesity-susceptibility genes (74). In addition, as an important node of the salience network (SN), the insula also plays a crucial part in interoceptive awareness, conscious urges to food-seeking and the homeostasis regulation of hunger and satiety (75, 76). Consistent with our results, obese and overweight people had increased low-frequency fluctuation in the insula (20, 77). These findings also converge with evidence that obese individuals display higher activation of the insula in the reward processing (119). Prior research has also linked higher BMI with an augmented activation in the insula and frontal operculum during attention reallocation to appetizing food images (73). Moreover, under a pleasure mindset, obese individuals have been found to select larger food portions than the healthy weight individuals do, as reflected by heightened activation of the frontal operculum (120). Consequently, these findings suggest obese individuals more readily shift attention toward appetizing food cues and show augmented hedonic enjoyment, characterized by heightened spontaneous fluctuation and global connectivity in the insula and frontal operculum.
4.2 Aberrational spontaneous activity of brain regions in DMN
We also found that obese individuals have a higher fALFF value in the left mPFC and right IPL, known as important hubs of the DMN, implicated in self-reflection, decision-making, information integration, and episodic memory retrieval (78–80). Atypical activation of mPFC implicated in food anticipation, prediction error, and intertemporal choice has been observed in obesity and binge-eating disorder (81–84). A recent meta-analysis also reported consistently lower gray matter volume in the mPFC in obese samples (85). In addition, IPL engagement in priority coding, attentional shifting, and body-image representation (86) has been found to play a role in the alterations of spontaneous fluctuation and functional interaction of obese and binge eating disorder samples (87, 88). Moreover, obese individuals show greater default mode circuit activity under food cue-reactivity and resting state conditions (24, 89, 90). On the other hand, previous studies have demonstrated that weight loss interventions are associated with a reduction in intrinsic activity and local function synchrony in DMN (91, 92) that are accompanied by greater fat mass loss (92). Our findings of a stronger functional connection between the DMN and SN in obese individuals align with those of Lee et al. (93) who found enhanced RSFC between the DMN and SN is associated with body image distortions in individuals with eating disorders. Together, aberrations of intrinsic functional patterns in default mode circuits observed in obese individuals may reflect deficits in forming a healthy and comprehensive self-representation and ability in making sensible decisions, which would contribute to the loss of control during eating and abandonment of longer-term goals related to weight control in daily life (94, 95).
4.3 Aberrational FC based on the DLPFC and insula
Notably, FC analyses showed enhanced FC between (1) right DLPFC and left precuneus; (2) left insula and DMN (IPL/AG) in obese individuals; Moreover, we found enhanced FC between SN (insula) and ECN (DLPFC) in the obese group. The triple network model proposes that disrupted organization and functioning of the ECN, SN and DMN might be prominent features of various psychiatric and addictive disorders (96, 97). Deficits in functional interactions between these networks have been demonstrated in addiction and disordered eating based on resting-state imaging (97–99). Our results complement those of Boehm et al. (100) who found increased functional coupling between the SN and DMN among individuals with eating disorders. Functional interactions between the SN and DMN are also associated with self-control, which might modulate overeating and hedonic consumption of palatable food (101). In addition, a recent electroencephalographic (EEG) study observed increased delta connectivity between SN and ECN in problematic cannabis users that was accompanied by distributed patterns of excessive cannabis usage (102). According to the triple-network model, maladaptive patterns of dysfunctional connectivity in the ECN, SN, and DMN may manifest as disturbances in the capacity to integrate information from internally focused processing and externally focused processing, resulting in out of control eating and hedonic overconsumption among obese individuals.
4.4 Altered spontaneous activity of the cerebellum
There is increasing evidence that the cerebellum is involved in various higher-order cognitive processes (103), including impulse control (104), reward anticipation, and decision-making. The dysregulation of these functions is closely associated with addictive behaviors. Recent rsfMRI studies have demonstrated decreased intrinsic activity (e.g., ALFF and ReHo) in the cerebellum among obese individuals and smokers (105, 106), and stronger cravings (107). Zhu et al. (108) has proposed that activated cerebellar regions play a key role in integrating sensory, visceral, and affective signals related to appetite, taste, and olfaction during feeding or feeding control. This aligns with our findings that obese individuals exhibit decreased fALFF in the bilateral cerebellum compared to non-obese controls, suggesting a potential disruption in these processes in obesity. Impulsivity, as a risk factor for obesity, is associated with disinhibited eating (109) and atypical BOLD activation of the cerebellum for food odors among impulsive children compared to controls (110). A recent meta-analysis has also highlighted the cerebellum’s role in appetite control and behavioral regulation, with structural abnormalities observed in obesity (111). In line with these findings, we identified cerebellum crus2 fALFF as a partial mediator of the relationship between BMI and impulsivity. Overall, our results highlighting possible cerebellum involvement in impulsive control deficits and reward processing among obese individuals provide foundations for further related investigations.
4.5 Association between the brain and neurotransmitter systems
The disturbance of intrinsic functional architecture in obesity was also associated with noradrenaline and dopaminergic (D1) neurotransmitter systems activity. These pathways play key roles in the onset of impulsivity and overeating for obesity and metabolic syndrome (112). In an animal study, D1 receptors gene expression were associated with weight gain in overeating and proneness-to-obesity (113). In addition, prior research has shown that noradrenaline availability is related to subjective feelings of hunger in humans (38) while baseline noradrenaline levels predict the efficacy of subsequent weight loss (114). In obesity treatment, noradrenaline reuptake inhibitors have emerged as targets for anti-obesity interventions (115). Notably, our findings suggest that the distinct associations of ICC and fALFF with neurotransmitter systems may stem from their representation of different aspects of intrinsic brain function. In line with previous findings, the current study suggested that obesity-related dysfunctions may be associated with abnormalities in the dopaminergic and noradrenaline systems (116, 117).
4.6 Strengths and limitations of the research
The main strengths of this study included its relatively large sample size compared to numerous related studies, a methodological approach that facilitated the assessment of not only regional aberrations in spontaneous neural fluctuations but also FC and neurotransmitter involvement in obesity, and evaluation of neural influences that may partially explain why higher BMI levels are associated with behavioral performance deficits related to impulsivity as well as cognitive flexibility. Strengths aside, findings should be interpreted in light of the following limitations: First, because this study was cross-sectional, future research is needed to track dynamic changes that correspond with BMI alteration. Second, because sample was relatively young (ages 22–36), extensions are needed in samples with a broader age range as well as those within particular developmental stages (e.g., adolescents, older adults). Third, associations between spontaneous brain activity alterations and neurotransmitter receptor activity should be regarded as indirect evidence. Future research should employ simultaneous PET and MRI probes to obtain more direct evidence. Finally, because we focused on resting-state fMRI. Future research should also consider structural bases of functional abnormalities in obese individuals.
5 Conclusion
In summary, through ICC, fALFF, and FC analyses, the current study investigated links between obesity and intrinsic functional alterations using resting-state fMRI. Compared to non-obese peers, obese individuals showed dysfunctional spontaneous activity in the prefrontal cortex, insula, sensorimotor cortex, and default mode circuits. In addition, we observed functional interaction disturbances between key hubs in the three-network-model including the SN, ECN, and DMN among obese individuals. Finally, aberrations of intrinsic functional architecture were related to dopaminergic and noradrenaline neurotransmitter systems. The integration of neuroimaging and molecular perspectives might help characterize the neurophysiological mechanisms underlying obesity, potentially facilitating the development of more effective clinical interventions that could decrease its prevalence.
Data availability statement
Publicly available datasets were analyzed in this study. This data can be found here: the Human Connectome Project (http://www.humanconnectome.org/).
Ethics statement
The studies involving humans were approved by Institutional Review Board at Washington University School of Medicine. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
TW: Data curation, Formal analysis, Visualization, Writing – original draft, Writing – review & editing. TJ: Writing – review & editing. ML: Writing – review & editing. HL: Writing – review & editing. JY: Formal analysis, Writing – review & editing. QZ: Conceptualization, Formal analysis, Methodology, Writing – review & editing. SC: Conceptualization, Formal analysis, Funding acquisition, Methodology, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by National Natural Science Foundation of China Grants (32100888 and 32200904), Zhejiang Natural Science Foundation (LQ21C090007), and Medical and Health Technology Project of Zhejiang Provincial Health Commission (2021ky247).
Acknowledgments
Data were provided by the Human Connectome Project, WU-Minn Consortium (Principal Investigators: David Van Essen and Kamil Ugurbil; 1U54MH091657); funded by the 16 NIH Institutes and Centers that support the NIH Blueprint for Neuroscience Research; and by the McDonnell Center for Systems Neuroscience at Washington University. The authors are deeply appreciative to the Human Connectome Project for open access to its data.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2025.1559325/full#supplementary-material
Footnotes
1. ^https://www.who.int/zh/news-room/fact-sheets/detail/obesity-and-overweight
2. ^https://www.humanconnectome.org/study/hcp-young-adult
References
1. Kachur, S, Lavie, CJ, de Schutter, A, Milani, RV, and Ventura, HO. Obesity and cardiovascular diseases. Minerva Med. (2017) 108:212–28. doi: 10.23736/S0026-4806.17.05022-4
2. Beltrán-Garrayo, L, Solar, M, Blanco, M, Graell, M, and Sepúlveda, AR. Examining associations between obesity and mental health disorders from childhood to adolescence: a case-control prospective study. Psychiatry Res. (2023) 326:115296. doi: 10.1016/j.psychres.2023.115296
3. Corbin, LJ, Richmond, RC, Wade, KH, Burgess, S, Bowden, J, Smith, GD, et al. BMI as a modifiable risk factor for type 2 diabetes: refining and understanding causal estimates using Mendelian randomization. Diabetes. (2016) 65:3002–7. doi: 10.2337/db16-0418
4. Gelber, RP, Gaziano, JM, Manson, JE, Buring, JE, and Sesso, HD. A prospective study of body mass index and the risk of developing hypertension in men. Am J Hypertens. (2007) 20:370–7. doi: 10.1016/j.amjhyper.2006.10.011
5. Elias, MF, Elias, PK, Sullivan, LM, Wolf, PA, and D’Agostino, RB. Lower cognitive function in the presence of obesity and hypertension: the Framingham heart study. Int J Obes Relat Metab Disord. (2003) 27:260–8. doi: 10.1038/sj.ijo.802225
6. Shi, Y, Yu, H, Di, S, and Ma, C. Body mass index and academic achievement among Chinese secondary school students: the mediating effect of inhibitory control and the moderating effect of social support. Front Psychol. (2022) 13:835171. doi: 10.3389/fpsyg.2022.835171
7. Volkow, ND, Wang, G-J, Telang, F, Fowler, JS, Goldstein, RZ, Alia-Klein, N, et al. Inverse association between BMI and prefrontal metabolic activity in healthy adults. Obesity (Silver Spring). (2009) 17:60–5. doi: 10.1038/oby.2008.469
8. Donofry, SD, Stillman, CM, and Erickson, KI. A review of the relationship between eating behavior, obesity and functional brain network organization. Soc Cogn Affect Neurosci. (2020) 15:1157–81. doi: 10.1093/scan/nsz085
9. Li, G, Hu, Y, Zhang, W, Wang, J, Ji, W, Manza, P, et al. Brain functional and structural magnetic resonance imaging of obesity and weight loss interventions. Mol Psychiatry. (2023) 28:1466–79. doi: 10.1038/s41380-023-02025-y
10. Parsons, N, Steward, T, Clohesy, R, Almgren, H, and Duehlmeyer, L. A systematic review of resting-state functional connectivity in obesity: refining current neurobiological frameworks and methodological considerations moving forward. Rev Endocr Metab Disord. (2022) 23:861–79. doi: 10.1007/s11154-021-09665-x
11. Meng, X, Huang, D, Ao, H, Wang, X, and Gao, X. Food cue recruits increased reward processing and decreased inhibitory control processing in the obese/overweight: an activation likelihood estimation meta-analysis of fMRI studies. Obes Res Clin Pract. (2020) 14:127–35. doi: 10.1016/j.orcp.2020.02.004
12. Brooks, SJ, Cedernaes, J, and Schiöth, HB. Increased prefrontal and Parahippocampal activation with reduced dorsolateral prefrontal and insular cortex activation to food images in obesity: a Meta-analysis of fMRI studies. PLoS One. (2013) 8:e60393. doi: 10.1371/journal.pone.0060393
13. Mata, F, Verdejo-Roman, J, Soriano-Mas, C, and Verdejo-Garcia, A. Insula tuning towards external eating versus interoceptive input in adolescents with overweight and obesity. Appetite. (2015) 93:24–30. doi: 10.1016/j.appet.2015.03.024
14. Tomasi, D, Wang, G-J, Wang, R, Backus, W, Geliebter, A, Telang, F, et al. Association of Body Mass and Brain Activation during gastric distention: implications for obesity. PLoS One. (2009) 4:e6847. doi: 10.1371/journal.pone.0006847
15. Biswal, B, Yetkin, FZ, Haughton, VM, and Hyde, JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. (1995) 34:537–41. doi: 10.1002/mrm.1910340409
16. Wang, J, Zhang, J-R, Zang, Y-F, and Wu, T. Consistent decreased activity in the putamen in Parkinson’s disease: a meta-analysis and an independent validation of resting-state fMRI. Gigascience. (2018) 7:giy 071. doi: 10.1093/gigascience/giy071
17. Martuzzi, R, Ramani, R, Qiu, M, Shen, X, Papademetris, X, and Constable, RT. A whole-brain voxel based measure of intrinsic connectivity contrast reveals local changes in tissue connectivity with anesthetic without a priori assumptions on thresholds or regions of interest. Neuroimage. (2011) 58:1044–50. doi: 10.1016/j.neuroimage.2011.06.075
18. Zou, Q-H, Zhu, C-Z, Yang, Y, Zuo, X-N, Long, X-Y, Cao, Q-J, et al. An improved approach to detection of amplitude of low-frequency fluctuation (ALFF) for resting-state fMRI: fractional ALFF. J Neurosci Methods. (2008) 172:137–41. doi: 10.1016/j.jneumeth.2008.04.012
19. Zhang, Y, Ji, G, Li, G, Hu, Y, Liu, L, Jin, Q, et al. Ghrelin reductions following bariatric surgery were associated with decreased resting state activity in the hippocampus. Int J Obes. (2019) 43:842–51. doi: 10.1038/s41366-018-0126-x
20. Gao, X, Zhang, M, Yang, Z, Niu, X, Zhou, B, Chen, J, et al. Nicotine addiction and overweight affect intrinsic neural activity and neurotransmitter activity: a FMRI study of interaction effects. Psychiatry Clin Neurosci. (2022) 77:178–85. doi: 10.1111/pcn.13516
21. Han, X-D, Zhang, H-W, Xu, T, Liu, L, Cai, H-T, Liu, Z-Q, et al. How impulsiveness influences obesity: the mediating effect of resting-state brain activity in the dl PFC. Front Psych. (2022) 13:873953. doi: 10.3389/fpsyt.2022.873953
22. Beyer, F, Kharabian Masouleh, S, Huntenburg, JM, Lampe, L, Luck, T, Riedel-Heller, SG, et al. Higher body mass index is associated with reduced posterior default mode connectivity in older adults. Hum Brain Mapp. (2017) 38:3502–15. doi: 10.1002/hbm.23605
23. Ding, Y, Ji, G, Li, G, Zhang, W, Hu, Y, Liu, L, et al. Altered interactions among resting state networks in obese individuals. Obesity (Silver Spring). (2020) 28:601–8. doi: 10.1002/oby.22731
24. Kullmann, S, Heni, M, Veit, R, Ketterer, C, Schick, F, Häring, H-U, et al. The obese brain: association of body mass index and insulin sensitivity with resting state network functional connectivity. Hum Brain Mapp. (2012) 33:1052–61. doi: 10.1002/hbm.21268
25. Walpola, IC, Nest, T, Roseman, L, Erritzoe, D, Feilding, A, Nutt, DJ, et al. Altered insula connectivity under MDMA. Neuropsychopharmacology. (2017) 42:2152–62. doi: 10.1038/npp.2017.35
26. Antypa, D, Simos, NJ, Kavroulakis, E, Bertsias, G, Fanouriakis, A, Sidiropoulos, P, et al. Anxiety and depression severity in neuropsychiatric SLE are associated with perfusion and functional connectivity changes of the frontolimbic neural circuit: a resting-state f (unctional) MRI study. Lupus Sci Med. (2021) 8:e000473. doi: 10.1136/lupus-2020-000473
27. Huang, H, Rong, B, Chen, C, Wan, Q, Liu, Z, Zhou, Y, et al. Common and distinct functional connectivity of the orbitofrontal cortex in depression and schizophrenia. Brain Sci. (2023) 13:997. doi: 10.3390/brainsci13070997
28. Xu, X, Dai, J, Chen, Y, Liu, C, Xin, F, Zhou, X, et al. Intrinsic connectivity of the prefrontal cortex and striato-limbic system respectively differentiate major depressive from generalized anxiety disorder. Neuropsychopharmacology. (2021) 46:791–8. doi: 10.1038/s41386-020-00868-5
29. Zhou, Y, Xue, T, Cheng, Y, Wang, J, Dong, F, Jia, S, et al. The changes of intrinsic connectivity contrast in young smokers. Addict Biol. (2023) 28:e13347. doi: 10.1111/adb.13347
30. Zhou, F, Zimmermann, K, Xin, F, Scheele, D, Dau, W, Banger, M, et al. Shifted balance of dorsal versus ventral striatal communication with frontal reward and regulatory regions in cannabis-dependent males. Hum Brain Mapp. (2018) 39:5062–73. doi: 10.1002/hbm.24345
31. Zhang, Z, Xu, Q, Liao, W, Wang, Z, Li, Q, Yang, F, et al. Pathological uncoupling between amplitude and connectivity of brain fluctuations in epilepsy. Hum Brain Mapp. (2015) 36:2756–66. doi: 10.1002/hbm.22805
32. Zuo, X-N, and Xing, X-X. Test-retest reliabilities of resting-state FMRI measurements in human brain functional connectomics: a systems neuroscience perspective. Neurosci Biobehav Rev. (2014) 45:100–18. doi: 10.1016/j.neubiorev.2014.05.009
33. Avena, NM, Rada, P, and Hoebel, BG. Underweight rats have enhanced dopamine release and blunted acetylcholine response in the nucleus accumbens while bingeing on sucrose. Neuroscience. (2008) 156:865–71. doi: 10.1016/j.neuroscience.2008.08.017
34. Baldo, BA, and Kelley, AE. Discrete neurochemical coding of distinguishable motivational processes: insights from nucleus accumbens control of feeding. Psychopharmacology. (2007) 191:439–59. doi: 10.1007/s00213-007-0741-z
35. Volkow, ND, Wang, G-J, and Baler, RD. Reward, dopamine and the control of food intake: implications for obesity. Trends Cogn Sci. (2011) 15:37–46. doi: 10.1016/j.tics.2010.11.001
36. Pruccoli, J, Parmeggiani, A, Cordelli, DM, and Lanari, M. The role of the noradrenergic system in eating disorders: a systematic review. Int J Mol Sci. (2021) 22:11086. doi: 10.3390/ijms222011086
37. Wellman, PJ. Norepinephrine and the control of food intake. Nutrition. (2000) 16:837–42. doi: 10.1016/s0899-9007(00)00415-9
38. Soeder, JM, Luthardt, J, Rullmann, M, Becker, GA, Hankir, MK, Patt, M, et al. Central noradrenergic neurotransmission and weight loss 6 months after gastric bypass surgery in patients with severe obesity. Obes Surg. (2021) 31:4868–76. doi: 10.1007/s11695-021-05657-7
39. Kringelbach, ML, Cruzat, J, Cabral, J, Knudsen, GM, Carhart-Harris, R, Whybrow, PC, et al. Dynamic coupling of whole-brain neuronal and neurotransmitter systems. Proc Natl Acad Sci USA. (2020) 117:9566–76. doi: 10.1073/pnas.1921475117
40. Stagg, CJ, Bachtiar, V, Amadi, U, Gudberg, CA, Ilie, AS, Sampaio-Baptista, C, et al. Local GABA concentration is related to network-level resting functional connectivity. eLife. (2014) 3:e01465. doi: 10.7554/eLife.01465
41. Van Essen, DC, Smith, SM, Barch, DM, Behrens, TEJ, Yacoub, E, and Ugurbil, K. The WU-Minn human connectome project: an overview. Neuroimage. (2013) 80:62–79. doi: 10.1016/j.neuroimage.2013.05.041
42. Glasser, MF, Sotiropoulos, SN, Wilson, JA, Coalson, TS, Fischl, B, Andersson, JL, et al. The minimal preprocessing pipelines for the human connectome project. Neuroimage. (2013) 80:105–24. doi: 10.1016/j.neuroimage.2013.04.127
43. Griffanti, L, Salimi-Khorshidi, G, Beckmann, CF, Auerbach, EJ, Douaud, G, Sexton, CE, et al. ICA-based artefact removal and accelerated fMRI acquisition for improved resting state network imaging. Neuroimage. (2014) 95:232–47. doi: 10.1016/j.neuroimage.2014.03.034
44. Salimi-Khorshidi, G, Douaud, G, Beckmann, CF, Glasser, MF, Griffanti, L, and Smith, SM. Automatic denoising of functional MRI data: combining independent component analysis and hierarchical fusion of classifiers. Neuroimage. (2014) 90:449–68. doi: 10.1016/j.neuroimage.2013.11.046
45. Smith, SM, Beckmann, CF, Andersson, J, Auerbach, EJ, Bijsterbosch, J, Douaud, G, et al. Resting-state fMRI in the human connectome project. Neuroimage. (2013) 80:144–68. doi: 10.1016/j.neuroimage.2013.05.039
46. Behzadi, Y, Restom, K, Liau, J, and Liu, TT. A component based noise correction method (comp Cor) for BOLD and perfusion based fMRI. Neuroimage. (2007) 37:90–101. doi: 10.1016/j.neuroimage.2007.04.042
47. Murphy, K, and Fox, MD. Towards a consensus regarding global signal regression for resting state functional connectivity MRI. Neuroimage. (2017) 154:169–73. doi: 10.1016/j.neuroimage.2016.11.052
48. Scalabrini, A, Vai, B, Poletti, S, Damiani, S, Mucci, C, Colombo, C, et al. All roads lead to the default-mode network—global source of DMN abnormalities in major depressive disorder. Neuropsychopharmacology. (2020) 45:2058–69. doi: 10.1038/s41386-020-0785-x
49. Whitfield-Gabrieli, S, and Nieto-Castanon, A. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect. (2012) 2:125–41. doi: 10.1089/brain.2012.0073
50. Zang, Y-F, He, Y, Zhu, C-Z, Cao, Q-J, Sui, M-Q, Liang, M, et al. Altered baseline brain activity in children with ADHD revealed by resting-state functional MRI. Brain Dev. (2007) 29:83–91. doi: 10.1016/j.braindev.2006.07.002
51. Dukart, J, Holiga, S, Rullmann, M, Lanzenberger, R, Hawkins, PCT, Mehta, MA, et al. JuSpace: a tool for spatial correlation analyses of magnetic resonance imaging data with nuclear imaging derived neurotransmitter maps. Hum Brain Mapp. (2021) 42:555–66. doi: 10.1002/hbm.25244
52. Agarwal, K, Demiral, SB, Manza, P, Volkow, ND, and Joseph, PV. Relationship between BMI and alcohol consumption levels in decision making. Int J Obes. (2021) 45:2455–63. doi: 10.1038/s41366-021-00919-x
53. Liu, Y, Zhou, F, Zhang, R, and Feng, T. The Para-hippocampal–medial frontal gyrus functional connectivity mediates the relationship between dispositional optimism and procrastination. Behav Brain Res. (2023) 448:114463. doi: 10.1016/j.bbr.2023.114463
54. Wang, J, Li, G, Ji, G, Hu, Y, Zhang, W, Ji, W, et al. Habenula volume and functional connectivity changes following laparoscopic sleeve gastrectomy for obesity treatment. Biol Psychiatry. (2023) 95:916–25. doi: 10.1016/j.biopsych.2023.07.009
55. Schoemann, AM, Miller, P, Pornprasertmanit, S, and Wu, W. Using Monte Carlo simulations to determine power and sample size for planned missing designs. Int J Behav Dev. (2014) 38:471–9. doi: 10.1177/0165025413515169
56. Alonso-Alonso, M, and Pascual-Leone, A. The right brain hypothesis for obesity. JAMA. (2007) 297:1819–22. doi: 10.1001/jama.297.16.1819
57. Garavan, H, Ross, TJ, and Stein, EA. Right hemispheric dominance of inhibitory control: an event-related functional MRI study. Proc Natl Acad Sci USA. (1999) 96:8301–6. doi: 10.1073/pnas.96.14.8301
58. Zhang, M, Gao, X, Yang, Z, Niu, X, Wang, W, Han, S, et al. Integrative brain structural and molecular analyses of interaction between tobacco use disorder and overweight among male adults. J Neurosci Res. (2023) 101:232–44. doi: 10.1002/jnr.25141
59. Yao, L, Li, W, Dai, Z, and Dong, C. Eating behavior associated with gray matter volume alternations: a voxel based morphometry study. Appetite. (2016) 96:572–9. doi: 10.1016/j.appet.2015.10.017
60. Lowe, CJ, Reichelt, AC, and Hall, PA. The prefrontal cortex and obesity: a health neuroscience perspective. Trends Cogn Sci. (2019) 23:349–61. doi: 10.1016/j.tics.2019.01.005
61. Siep, N, Roefs, A, Roebroeck, A, Havermans, R, Bonte, M, and Jansen, A. Fighting food temptations: the modulating effects of short-term cognitive reappraisal, suppression and up-regulation on mesocorticolimbic activity related to appetitive motivation. Neuroimage. (2012) 60:213–20. doi: 10.1016/j.neuroimage.2011.12.067
62. Szabo-Reed, AN, Martin, LE, Hu, J, Yeh, H-W, Powell, J, Lepping, RJ, et al. Modeling interactions between brain function, diet adherence behaviors, and weight loss success. Obes Sci Pract. (2020) 6:282–92. doi: 10.1002/osp4.403
63. Weygandt, M, Mai, K, Dommes, E, Leupelt, V, Hackmack, K, Kahnt, T, et al. The role of neural impulse control mechanisms for dietary success in obesity. Neuroimage. (2013) 83:669–78. doi: 10.1016/j.neuroimage.2013.07.028
64. Weygandt, M, Mai, K, Dommes, E, Ritter, K, Leupelt, V, Spranger, J, et al. Impulse control in the dorsolateral prefrontal cortex counteracts post-diet weight regain in obesity. Neuroimage. (2015) 109:318–27. doi: 10.1016/j.neuroimage.2014.12.073
65. Ljubisavljevic, M, Maxood, K, Bjekic, J, Oommen, J, and Nagelkerke, N. Long-term effects of repeated prefrontal cortex transcranial direct current stimulation (tDCS) on food craving in Normal and overweight young adults. Brain Stimul. (2016) 9:826–33. doi: 10.1016/j.brs.2016.07.002
66. Spetter, MS, Malekshahi, R, Birbaumer, N, Lührs, M, van der Veer, AH, Scheffler, K, et al. Volitional regulation of brain responses to food stimuli in overweight and obese subjects: a real-time fMRI feedback study. Appetite. (2017) 112:188–95. doi: 10.1016/j.appet.2017.01.032
67. Jack Rejeski, W, Laurienti, PJ, Bahrami, M, Fanning, J, Simpson, SL, and Burdette, JH. Aging and neural vulnerabilities in overeating: a conceptual overview and model to guide treatment. PCN Rep. (2022) 1:e39. doi: 10.1002/pcn5.39
68. Czyzewska, M, Graham, R, and Ceballos, NA. Explicit and implicit attitudes to food. In: Preedy VR, Watson RR, and Martin CR, editors. Handbook of behavior, food and nutrition. New York, NY: Springer. (2011) 673–92.
69. Kemps, E, and Tiggemann, M. Approach bias for food cues in obese individuals. Psychol Health. (2015) 30:370–80. doi: 10.1080/08870446.2014.974605
70. Stroebe, W. The goal conflict model: a theory of the hedonic regulation of eating behavior. Curr Opin Behav Sci. (2022) 48:101203. doi: 10.1016/j.cobeha.2022.101203
71. Craig, ADB. How do you feel--now? The anterior insula and human awareness. Nat Rev Neurosci. (2009) 10:59–70. doi: 10.1038/nrn2555
72. Elliott, R, Friston, KJ, and Dolan, RJ. Dissociable neural responses in human reward systems. J Neurosci. (2000) 20:6159–65. doi: 10.1523/JNEUROSCI.20-16-06159.2000
73. Yokum, S, Ng, J, and Stice, E. Attentional bias to food images associated with elevated weight and future weight gain: an fMRI study. Obesity (Silver Spring). (2011) 19:1775–83. doi: 10.1038/oby.2011.168
74. Ndiaye, FK, Huyvaert, M, Ortalli, A, Canouil, M, Lecoeur, C, Verbanck, M, et al. The expression of genes in top obesity-associated loci is enriched in insula and substantia nigra brain regions involved in addiction and reward. Int J Obes. (2020) 44:539–43. doi: 10.1038/s41366-019-0428-7
75. Carnell, S, Gibson, C, Benson, L, Ochner, CN, and Geliebter, A. Neuroimaging and obesity: current knowledge and future directions. Obes Rev. (2012) 13:43–56. doi: 10.1111/j.1467-789X.2011.00927.x
76. Frank, S, Kullmann, S, and Veit, R. Food related processes in the insular cortex. Front Hum Neurosci. (2013) 7:499. doi: 10.3389/fnhum.2013.00499
77. Hogenkamp, PS, Zhou, W, Dahlberg, LS, Stark, J, Larsen, AL, Olivo, G, et al. Higher resting-state activity in reward-related brain circuits in obese versus normal-weight females independent of food intake. Int J Obes. (2016) 40:1687–92. doi: 10.1038/ijo.2016.105
78. Andrews-Hanna, JR, Reidler, JS, Huang, C, and Buckner, RL. Evidence for the default network’s role in spontaneous cognition. J Neurophysiol. (2010) 104:322–35. doi: 10.1152/jn.00830.2009
79. DeWitt, SJ, Ketcherside, A, McQueeny, TM, Dunlop, JP, and Filbey, FM. The hyper-sentient addict: an exteroception model of addiction. Am J Drug Alcohol Abuse. (2015) 41:374–81. doi: 10.3109/00952990.2015.1049701
80. Zhang, R, and Volkow, ND. Brain default-mode network dysfunction in addiction. Neuroimage. (2019) 200:313–31. doi: 10.1016/j.neuroimage.2019.06.036
81. Adise, S, Geier, CF, Roberts, NJ, White, CN, and Keller, KL. Is brain response to food rewards related to overeating? A test of the reward surfeit model of overeating in children. Appetite. (2018) 128:167–79. doi: 10.1016/j.appet.2018.06.014
82. Balodis, IM, Grilo, CM, Kober, H, Worhunsky, PD, White, MA, Stevens, MC, et al. A pilot study linking reduced fronto-striatal recruitment during reward processing to persistent bingeing following treatment for binge-eating disorder. Int J Eat Disord. (2014) 47:376–84. doi: 10.1002/eat.22204
83. Kishinevsky, FI, Cox, JE, Murdaugh, DL, Stoeckel, LE, Cook, EW, and Weller, RE. fMRI reactivity on a delay discounting task predicts weight gain in obese women. Appetite. (2012) 58:582–92. doi: 10.1016/j.appet.2011.11.029
84. Kube, J, Mathar, D, Horstmann, A, Kotz, SA, Villringer, A, and Neumann, J. Altered monetary loss processing and reinforcement-based learning in individuals with obesity. Brain Imaging Behav. (2018) 12:1431–49. doi: 10.1007/s11682-017-9786-8
85. García-García, I, Michaud, A, Dadar, M, Zeighami, Y, Neseliler, S, Collins, DL, et al. Neuroanatomical differences in obesity: meta-analytic findings and their validation in an independent dataset. Int J Obes. (2019) 43:943–51. doi: 10.1038/s41366-018-0164-4
86. Naito, E, Morita, T, and Amemiya, K. Body representations in the human brain revealed by kinesthetic illusions and their essential contributions to motor control and corporeal awareness. Neurosci Res. (2016) 104:16–30. doi: 10.1016/j.neures.2015.10.013
87. Wang, J-N, Tang, L-R, Li, W-H, Zhang, X-Y, Shao, X, Wu, P-P, et al. Regional neural activity abnormalities and whole-brain functional connectivity reorganization in bulimia nervosa: evidence from resting-state fMRI. Front Neurosci. (2022) 16:858717. doi: 10.3389/fnins.2022.858717
88. Zhang, P, Liu, Y, Lv, H, Li, M, Yu, F, Wang, Z, et al. Integration of neural reward processing and appetite-related signaling in obese females: evidence from resting-state fMRI. J Magn Reson Imaging. (2019) 50:541–51. doi: 10.1002/jmri.26576
89. Frank, S, Wilms, B, Veit, R, Ernst, B, Thurnheer, M, Kullmann, S, et al. Altered brain activity in severely obese women may recover after roux-en Y gastric bypass surgery. Int J Obes. (2014) 38:341–8. doi: 10.1038/ijo.2013.60
90. Tregellas, JR, Wylie, KP, Rojas, DC, Tanabe, J, Martin, J, Kronberg, E, et al. Altered default network activity in obesity. Obesity (Silver Spring). (2011) 19:2316–21. doi: 10.1038/oby.2011.119
91. Li, G, Ji, G, Hu, Y, Xu, M, Jin, Q, Liu, L, et al. Bariatric surgery in obese patients reduced resting connectivity of brain regions involved with self-referential processing. Hum Brain Mapp. (2018) 39:4755–65. doi: 10.1002/hbm.24320
92. McFadden, KL, Cornier, M-A, Melanson, EL, Bechtell, JL, and Tregellas, JR. Effects of exercise on resting-state default mode and salience network activity in overweight/obese adults. Neuroreport. (2013) 24:866–71. doi: 10.1097/WNR.0000000000000013
93. Lee, S, Ran Kim, K, Ku, J, Lee, J-H, Namkoong, K, and Jung, Y-C. Resting-state synchrony between anterior cingulate cortex and precuneus relates to body shape concern in anorexia nervosa and bulimia nervosa. Psychiatry Res. (2014) 221:43–8. doi: 10.1016/j.pscychresns.2013.11.004
94. Hays, NP, and Roberts, SB. Aspects of eating behaviors “disinhibition” and “restraint” are related to weight gain and BMI in women. Obesity (Silver Spring). (2008) 16:52–8. doi: 10.1038/oby.2007.12
95. Maayan, L, Hoogendoorn, C, Sweat, V, and Convit, A. Disinhibited eating in obese adolescents is associated with orbitofrontal volume reductions and executive dysfunction. Obesity (Silver Spring). (2011) 19:1382–7. doi: 10.1038/oby.2011.15
96. Menon, V. Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cogn Sci. (2011) 15:483–506. doi: 10.1016/j.tics.2011.08.003
97. Sutherland, MT, McHugh, MJ, Pariyadath, V, and Stein, EA. Resting state functional connectivity in addiction: lessons learned and a road ahead. Neuroimage. (2012) 62:2281–95. doi: 10.1016/j.neuroimage.2012.01.117
98. Lerman, C, Gu, H, Loughead, J, Ruparel, K, Yang, Y, and Stein, EA. Large-scale brain network coupling predicts acute nicotine abstinence effects on craving and cognitive function. JAMA Psychiatry. (2014) 71:523–30. doi: 10.1001/jamapsychiatry.2013.4091
99. Zhai, T, Gu, H, Salmeron, BJ, Stein, EA, and Yang, Y. Disrupted dynamic interactions between large-scale brain networks in cocaine users are associated with dependence severity. Biol Psychiatry Cogn Neurosci Neuroimaging. (2023) 8:672–9. doi: 10.1016/j.bpsc.2022.08.010
100. Boehm, I, Geisler, D, King, JA, Ritschel, F, Seidel, M, Deza Araujo, Y, et al. Increased resting state functional connectivity in the fronto-parietal and default mode network in anorexia nervosa. Front Behav Neurosci. (2014) 8:346. doi: 10.3389/fnbeh.2014.00346
101. Li, Q, Xiang, G, Song, S, Li, Y, Du, X, Liu, X, et al. Sex difference in neural substrates underlying the association between trait self-control and overeating in the COVID-19 pandemic. Neuropsychologia. (2021) 163:108083. doi: 10.1016/j.neuropsychologia.2021.108083
102. Imperatori, C, Massullo, C, Carbone, GA, Panno, A, Giacchini, M, Capriotti, C, et al. Increased resting state triple network functional connectivity in undergraduate problematic Cannabis users: a preliminary EEG coherence study. Brain Sci. (2020) 10:136. doi: 10.3390/brainsci10030136
103. Buckner, RL. The cerebellum and cognitive function: 25 years of insight from anatomy and neuroimaging. Neuron. (2013) 80:807–15. doi: 10.1016/j.neuron.2013.10.044
104. Miquel, M, Nicola, SM, Gil-Miravet, I, Guarque-Chabrera, J, and Sanchez-Hernandez, A. A working hypothesis for the role of the cerebellum in impulsivity and compulsivity. Front Behav Neurosci. (2019) 13:99. doi: 10.3389/fnbeh.2019.00099
105. Xu, Y-L, Wang, X-Y, Chen, J, Kang, M, Wang, Y-X, Zhang, L-J, et al. Altered spontaneous brain activity patterns of Meibomian gland dysfunction in severely obese population measured using the fractional amplitude of low-frequency fluctuations. Front Psych. (2022) 13:914039. doi: 10.3389/fpsyt.2022.914039
106. Zhang, M, Gao, X, Yang, Z, Niu, X, Chen, J, Wei, Y, et al. Weight status modulated brain regional homogeneity in Long-term male smokers. Front Psych. (2022) 13:857479. doi: 10.3389/fpsyt.2022.857479
107. Wen, Z, Han, X, Wang, Y, Ding, W, Sun, Y, Kang, Y, et al. Sex-dependent alterations of regional homogeneity in cigarette smokers. Front Psych. (2022) 13:874893. doi: 10.3389/fpsyt.2022.874893
108. Zhu, J-N, and Wang, J-J. The cerebellum in feeding control: possible function and mechanism. Cell Mol Neurobiol. (2008) 28:469–78. doi: 10.1007/s10571-007-9236-z
109. Gerlach, G, Herpertz, S, and Loeber, S. Personality traits and obesity: a systematic review. Obes Rev. (2015) 16:32–63. doi: 10.1111/obr.12235
110. de Celis-Alonso, B, Hidalgo-Tobón, SS, Barragán-Pérez, E, Castro-Sierra, E, Dies-Suárez, P, Garcia, J, et al. Different food odors control brain connectivity in impulsive children. CNS Neurol Disord Drug Targets. (2019) 18:63–77. doi: 10.2174/1871527317666181105105113
111. Sader, M, Waiter, GD, and Williams, JHG. The cerebellum plays more than one role in the dysregulation of appetite: review of structural evidence from typical and eating disorder populations. Brain Behav. (2023) 13:e3286. doi: 10.1002/brb3.3286
112. Esler, M, Rumantir, M, Kaye, D, and Lambert, G. The sympathetic neurobiology of essential hypertension: disparate influences of obesity, stress, and noradrenaline transporter dysfunction?*. Am J Hypertens. (2001) 14:S139–46. doi: 10.1016/S0895-7061(01)02081-7
113. Alsiö, J, Olszewski, PK, Norbäck, AH, Gunnarsson, ZEA, Levine, AS, Pickering, C, et al. Dopamine D1 receptor gene expression decreases in the nucleus accumbens upon long-term exposure to palatable food and differs depending on diet-induced obesity phenotype in rats. Neuroscience. (2010) 171:779–87. doi: 10.1016/j.neuroscience.2010.09.046
114. Vettermann, FJ, Rullmann, M, Becker, GA, Luthardt, J, Zientek, F, Patt, M, et al. Noradrenaline transporter availability on [11C] MRB PET predicts weight loss success in highly obese adults. Eur J Nucl Med Mol Imaging. (2018) 45:1618–25. doi: 10.1007/s00259-018-4002-7
115. Scheen, AJ. Sibutramine on Cardiovascular Outcome. Diabetes Care. (2011) 34:S114–9. doi: 10.2337/dc11-s205
116. Small, DM. Dopamine adaptations as a common pathway for neurocognitive impairment in diabetes and obesity: a neuropsychological perspective. Front Neurosci. (2017) 11:134. doi: 10.3389/fnins.2017.00134
117. Hesse, S, Rullmann, M, Zientek, F, Schewe, D, Becker, G-A, Patt, M, et al. Noradrenergic control of neurobehavior in human binge-eating disorder and obesity (NOBEAD): a smartphone-supported behavioral emotion regulation intervention study protocol integrating molecular brain imaging. Int J Eat Disord. (2024) 57:206–20. doi: 10.1002/eat.24080
118. Gao, X, Zhang, M, Yang, Z, Niu, X, Chen, J, Zhou, B, et al. Explore the effects of overweight and smoking on spontaneous brain activity: independent and reverse. Front Neurosci. (2022) 16:944768. doi: 10.3389/fnins.2022.944768
119. Richter, M, Widera, S, Malz, F, Goltermann, J, Steinmann, L, Kraus, A, et al. Higher body weight-dependent neural activation during reward processing. Brain Imaging Behav. (2023) 17:414–24. doi: 10.1007/s11682-023-00769-3
Keywords: obesity, resting-state fMRI, intrinsic connectivity contrast, fractional amplitude of low-frequency fluctuation, functional connectivity
Citation: Wang T, Jackson T, Lock M, Li H, Yan J, Zhuang Q and Chen S (2025) Obesity-related alterations of intrinsic functional architecture: a resting-state fMRI study based on the human connectome project. Front. Nutr. 12:1559325. doi: 10.3389/fnut.2025.1559325
Edited by:
Adrian Soto-Mota, National Institute of Medical Sciences and Nutrition Salvador Zubirán, MexicoReviewed by:
Federico d’Oleire Uquillas, Princeton University, United StatesBlanca Lizarbe, Autonomous University of Madrid, Spain
Yuko Nakamura, University of Tokyo, Japan
Copyright © 2025 Wang, Jackson, Lock, Li, Yan, Zhuang and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shuaiyu Chen, Y2hlbnNodWFpeXVAaHpudS5lZHUuY24=; Qian Zhuang, cWlhbnpodWFuZy51ZXN0Y0BvdXRsb29rLmNvbQ==
 Tongtong Wang1
Tongtong Wang1 Todd Jackson
Todd Jackson Hui Li
Hui Li Jin Yan
Jin Yan Shuaiyu Chen
Shuaiyu Chen