- 1Surgery Department, Faculty of Veterinary Medicine and Animal Sciences, University of São Paulo, São Paulo, Brazil
- 2Department of Veterinary and Animal Sciences, Faculty of Health and Medical Sciences, University of Copenhagen, Frederiksberg, Denmark
- 3Department of Veterinary Medicine, Faculty of Animal Sciences and Food Engineering, University of São Paulo, Pirassununga, SP, Brazil
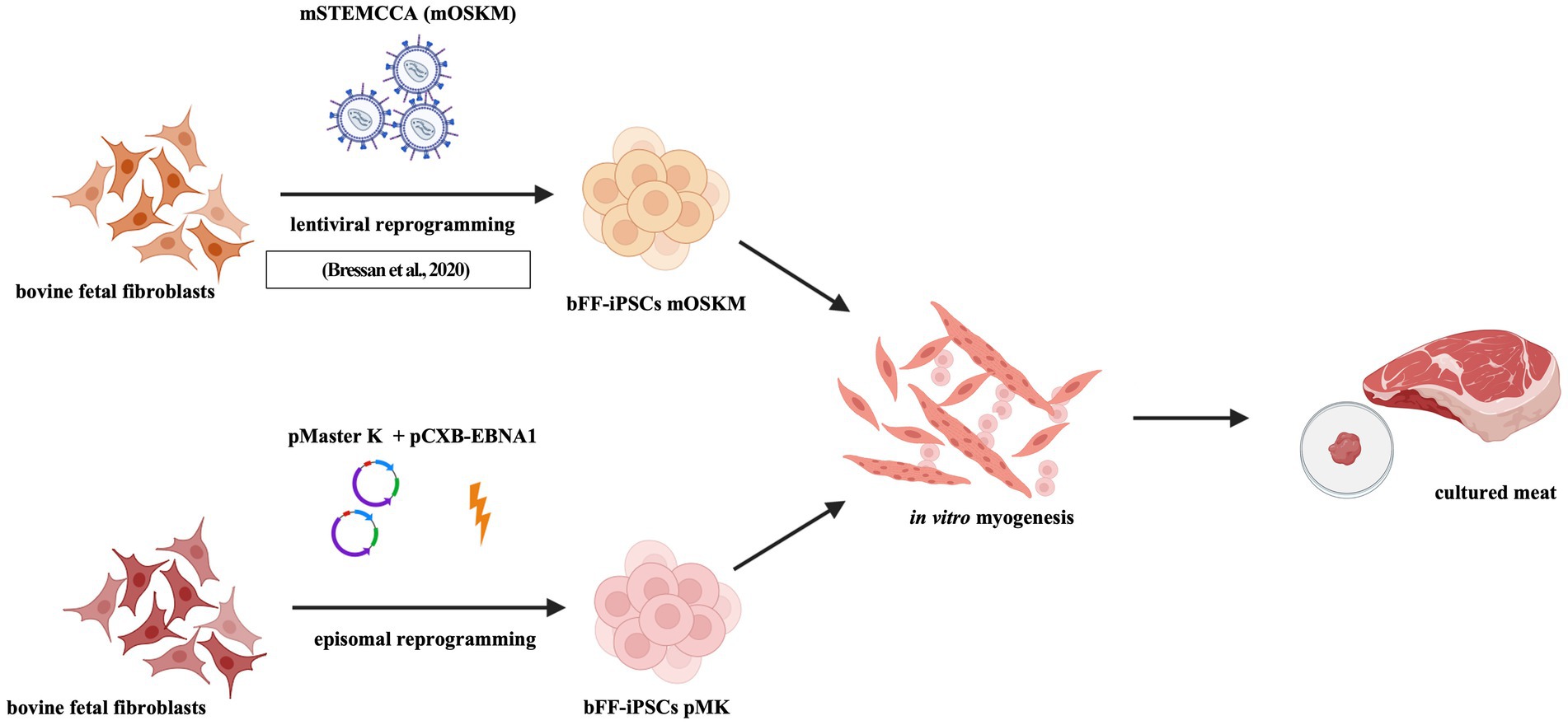
Introduction: Emerging biotechnologies are increasingly being explored for food production, including the development of cell-cultivated meat. Conventional approaches typically rely on satellite cell (SC) biopsies, which present challenges in scalability. Bovine induced pluripotent stem cells (biPSCs) represent a promising alternative due to their capacity for self-renewal and developmental plasticity.
Methods: This study utilized both lentiviral (integrating) and episomal (non-integrating) reprogramming strategies to generate biPSCs suitable for myogenic differentiation. Bovine fetal fibroblasts (bFFs) were reprogrammed using episomal vectors pMaster K and pCXB-EBNA1, leading to the emergence of putative iPSC colonies 13 days post-nucleofection. A clonal line, bFF-iPSCs pMK, was selected for further analysis.
Results: The bFF-iPSCs pMK line expressed key pluripotency markers including alkaline phosphatase (AP), OCT4, SOX2, and NANOG, and was stably maintained for over 33 passages, although episomal plasmids remained detectable. in vitro myogenic differentiation was assessed by comparing this line to a previously established lentiviral reprogrammed line (bFF-iPSCs mOSKM). Both lines exhibited downregulation of pluripotency markers and upregulation of the early myogenic marker PAX3. By day 30, the bFF-iPSCs pMK line formed elongated, multinucleated cells characteristic of myotubes and displayed a corresponding gene expression profile.
Discussion: These results provide new insights into bovine in vitro myogenesis and its application in cultured meat production. While promising, the study also highlights the difficulty in achieving complete myogenic differentiation, indicating a need for further optimization of differentiation protocols.
Highlights
•Bovine fetal fibroblasts were reprogrammed into pluripotency using episomal or lentiviral methods, and biPSCs were analyzed regarding myogenic differentiation potential.
1 Introduction
The advent of induced pluripotent stem cells (iPSCs) has significantly advanced the study of in vitro myogenesis (1–4). The ability to generate muscle cells in vitro is pivotal for alternative protein production, particularly as global demand for animal-derived products rises with population growth. As a result, there is a growing need for innovative solutions in the food system to revise current livestock systems or to produce animal proteins while minimizing environmental impact and mitigating the challenges of intensive animal farming (5, 6). In this context, in vitro technologies, including cultured meat (CM), also known as cell-based food, are presented as a promising alternative to traditional meat production (7–10).
One critical step in cultured meat production, among others, is the isolation and efficient differentiation of SCs into mature muscle fibers (10, 11). This differentiation depends on the timely activation of key lineage markers, such as MSGN1 and TBX6 in presomitic mesoderm, followed by expression of PAX3 and PAX7 in skeletal muscle progenitors (SCs), which subsequently differentiate into myoblasts through MYF5 and MYOD1 expression (12). However, challenges remain in CM production due to scalability issues, nutritional optimization, sensory properties, and consumer acceptance, despite significant investment in addressing these hurdles (13, 14, 64, 65). Current SC cultures have limited expansion potential, often requiring multiple tissue collections for large-scale production.
iPSC technology offers a solution by enabling the generation of large quantities of myogenic progenitors without the need for tissue sampling (3, 9, 15–18). The plasticity of iPSCs allows for in vitro myogenesis, as demonstrated in human models (1–3, 15, 16, 19), and muscle cell generation from human iPSCs has already been achieved within a month of differentiation, enabling large-scale production of myogenic progenitor cells (1, 15, 18).
Bovine iPSCs have also shown promise for generating myoblasts and myogenic progenitors. Although bovine embryonic stem cells (ESCs) have been used in short-term cultures to derive muscle cells (20, 21), iPSCs offer distinct advantages. They can be derived from abundant and easily accessible tissues, such as skin or blood, overcoming the limitations associated with ESCs, which require embryos that are more difficult to obtain and are often limited in supply (17). Induced reprogramming has been successfully performed in bovine cells, predominantly using lentiviral vectors (22–28) and lentiviral reprogramming methodology; nevertheless, no episomal protocol and no episomal reprogramming protocols have been reported for this species to date. The application of iPSC-derived myogenesis in large animal models, such as bovines, has the potential to advance alternative protein production technologies.
This study aims to generate SCs and myotubes from bovine iPSCs, providing novel insights into the myogenic differentiation process. The results not only enhance the understanding of myogenesis but also address critical challenges in cell culture techniques and alternative protein production.
2 Materials and methods
2.1 Bovine fetal fibroblasts
The bovine fetal fibroblasts (bFFs) were previously isolated and characterized (29). The cells were cultured in bFFs media, which was composed of DMEM/F12 (Gibco, cat. 31,331–028) supplemented with 10% FBS and 10 U/mL penicillin and streptomycin at 5% CO2 and 38.5°C.
2.2 Lentiviral-derived biPSCs
The bFF-iPSCs reprogrammed with a lentivirus containing the murine Stem Cell Cassette (STEMCCA) harboring the murine vesions of Oct4, Sox2, Klf4 and c-myc (mOSKM) was previously generated and characterized (23). The cells were cultured with KnockOut™ Dulbecco’s Modified Eagle Medium/Nutrient Mixture F-12 (KO DMEM/F12 from Gibco, cat. 12660012) supplemented with 20% KnockOut™ Serum Replacement (KSR from Gibco, cat. 10828028), 10 U/mL penicillin and streptomycin (Gibco, cat.15140122), 0.1 mM B-Mercaptoethanol (Sigma, cat. 31350-010), 1% Non-Essential Amino Acids (NEAA), 1% GlutaMAX (Gibco, cat. 35050061) and 10 ng/µl (bFGF from Peprotech, cat. 100-18B-250UG).
2.3 Episomal reprogramming and biPSCs culture
For cellular reprogramming of bovine fetal fibroblasts (bFFs) using episomal plasmids, 1×106 bFFs were nucleofected with 3 μg pMaster K (30) (Addgene, cat. 165,081) in association with 1 μg pCXB-EBNA1 (31) (Addgene, cat. 41,857) using the Nucleofector 4D Lonza and the P3 Primary Cell Solution Box (Lonza, cat. PBP3-02250), program CA-137. After the nucleofection, the cells were divided into two wells of six-well plates previously coated with Matrigel (Corning, cat. 354,277) and cultured with bFFs media.
After 3 days, the media was changed to bovine pluripotent stem media: mTeSR™ 1 Basal Medium (Stem Cell Technologies, cat. 85,850) supplemented with 10 U/mL penicillin–streptomycin (Gibco, cat.15140122), 0.1 mM B-Mercaptoethanol (Sigma, cat. 31,350–010), 28.94 μg/mL ascorbic acid (Sigma, cat. A8960), 1 μM CHIR99021 (Sigma, cat. SML 1046), 20 ng/mL activin A (R&D system, cat. 11,348-AC), 5 μM XAV939 (Sellect Chem, cat. X3004), 0.3 μM WH-4-023 (SelleckChem, cat. S7565), and 10 ng/mL human FGF-basic. The media was changed every other day for up to 30 days at 5% CO2 and 38.5°C conditions. Once colonies were observed, they were manually replated on top of a monolayer of Mitomycin C-treated murine embryonic fibroblasts (MEFs, Sigma-Aldrich, cat. M4287). The media was changed daily, and the cells were cultured in 5% de CO2 at 38.5°C.
2.4 biPSCs differentiation into satellite cells—in vitro myogenesis
biPSCs were subjected to in vitro myogenesis differentiation using the commercially available Skeletal Muscle Induction Medium (ASMBIO, cat. SKM01), following the recommended protocol, previously validated for human PSCs differentiation (3, 19, 32). First, the biPSCs were dissociated into single cells and plated on six-well plates previously coated with Matrigel (Corning, cat. 354,277) and cultured with SKM01 media for 6 days. Then, the cells were snap-frozen and analyzed via Reverse Transcription quantitative Polymerase Chain Reaction (RT-qPCR). The media was changed every other day, and cells were cultured in 5% de CO2 at 38.5°C.
The bFF-iPSC pMK at passage 33 was submitted to an adapted protocol previously described for human iPSCs (1, 2, 15). In brief, the cells were pre-differentiated following the methodology I with SKM01 on Matrigel until day 8, after which the media was changed to DK-HIFL composed of DMEM media supplemented with 2 ng/mL insulin-like growth factor 1 (IGF-1, Peprotech, cat. 100–11), 10 ng/mL hepatocyte growth factor (HGF, Peprotech, cat. 315–23), 20 ng/mL bFGF (Peprotech), and 0.5 μM LDN193189 (Sigma, cat. SML0559). The media was changed daily until D10, when the cells were cultured in DK-I media (DMEM, 15% KSR (Gibco) supplemented with 2 ng/mL IGF1). After D12, the cells were cultured with DK-HI (DMEM/F12, 15% KSR, 2 ng/mL IGF1 and 10 ng/mL HGF) until D30. The media was changed every other day, and the cells were cultured in 5% de CO2 at 38.5°C.
2.5 Characterization of biPSCs and satellite cells
2.5.1 Alkaline phosphatase and immunocytochemistry of pluripotency markers
The detection of AP was performed with the Leukocyte Alkaline Phosphatase kit (Sigma, cat. 86R) following the manufacturer’s instructions and reported in different iPSC species (23, 33, 34). Immunocytochemistry (ICC) for pluripotency markers was performed via fixation of biPSCs with 4% Paraformaldehyde for 15 min, followed by permeabilization and blocking with 0.5% Triton-X and 5% Donkey Serum for 1 h at room temperature. Afterward, biPSCs were rinsed three times with 0.05% Triton-X in Phosphate-Buffered Saline (PBS) for 5 min (wash solution) followed by incubation with the primary antibodies OCT3/4 (1:200, Santa Cruz, SC-5279, Mouse), SOX2 (1:200, R&D, cat. AF2018, Goat), and NANOG (1:200, R&D, cat. AF1997, Goat) overnight at 4°C. The following day, biPSCs were rinsed in the wash solution and incubated with the respective secondary antibodies: donkey anti-Mouse (1:500, Alexa Fluor 594, Invitrogen, cat. A21203) or donkey anti-Goat (1:500, Alexa Fluor 594, Invitrogen, cat. A11058). The cell nuclei were labeled with DAPI (1:1000, ThermoScientific, cat. 62,248) and analyzed using the EVOS™ cell imaging system.
2.5.2 Plasmid detection analyses—genomic PCR and electrophoresis
DNA from bFFs-iPSC colonies generated through episomal reprogramming (pMaster K) was extracted using the DNeasy Blood & Tissue kit (QIAGEN, cat. 69,506), following the manufacturer’s instructions. DNA was analyzed and quantified using a spectrophotometer (NanoDrop Lite Plus Spectrophotometer, ThermoScientific, cat. NDLPLUSPRGL). To test for episomal plasmid detection, a PCR was performed using primers designed to amplify the oriP region (Supplementary Table 1) (30). For the reaction, 1 μL primer mix (forward and reverse at 100 uM), 7 μL SYBR Gold (Invitrogen, cat. S11494), 5 μL Nuclease-Free, water (Sigma, cat. WH502), and 100 ng/ul sample DNA were mixed. The amplification program was run in a thermocycler at 94°C for 10 min; 40 cycles at 94°C for 30 s, 60°C for 30 s, 72°C for 1 min, and a final extension at 72°C for 7 min. The PCR product was run in an electrophoresis gel composed of 1% TAE buffer (Thermo Scientific, cat. B49) and 2% UltraPure™ Agarose (Invitrogen, cat. 16,500,500), running at 100 mV for 40 min. The band was evaluated by photodocumentation.
2.5.3 Transcript quantification—RT-qPCR
RNA extraction was performed with the RNeasy Plus Mini kit (QIAGEN, cat. 74,134) following the manufacturer’s guide. The Ribonucleic Acid (RNA) samples were analyzed regarding quantity and quality using a spectrophotometer (NanoDrop Lite Plus Spectrophotometer, cat. NDLPLUSULCA). Reverse transcription of the extracted RNA was performed using the commercial GoScript™ Reverse Transcriptase (Promega, cat. A2801), according to the manufacturer’s instructions.
The target genes for the evaluation of pluripotency via RT-qPCR in biPSCs were endogenous OCT4, SOX2, and NANOG due to their importance in the reprogramming and maintenance of pluripotency, and PAX3, PAX7, and MYOG for differentiated cells due to their importance in the differentiation process from early mesoderm into striated myotubes and fibers. The GAPDH and PPIA genes were used as reference genes (ST1). PCR conditions for amplification were: 95°C for 15 min; 40 cycles of 95°C for 15 s, 60°C for 5 s, and 72°C for 30 s; and 72°C for 2 min; the melting curve was analyzed up to 90 cycles starting at 50°C with a 0.5°C increase. The samples were analyzed and compared based on the relative abundance of transcripts. The PowerUP SYBR Green reagent (Applied Biosystems, cat. A25778) was used in the reactions, and the primers were at 100 nM in standard thermocycling conditions (annealing temperature of 60°C for 45 cycles). Based on duplicates or triplicates, the relative abundance quantification was evaluated using the 2-ΔCT method.
2.5.4 Statistical analysis
Data obtained from the experimental procedures were analyzed using GraphPad Prism version 9.5.0 for Mac (GraphPad Software, San Diego, California United States).1 The analyses were performed using technical duplicates or triplicates (n = 2 or n = 3) for both bFF-iPSCs pMK episomal-derived and bFF-iPSCs mOSKM lentiviral-derived lines. The data were analyzed by one-way ANOVA followed by Tukey’s multiple comparison test or Welch’s t-test. A significance level of 5% (p < 0.05) was considered for all statistical analyses.
3 Results
3.1 Bovine fetal fibroblasts iPSCs reprogramming
bFFs in passage 6 underwent nucleofection with the plasmids pMaster K, which was associated with pCXB-EBNA1. Colony formation was observed after 13 days, and alkaline phosphatase (AP) activity was detected on day 18 when the last manual colony was replated (Figure 1).
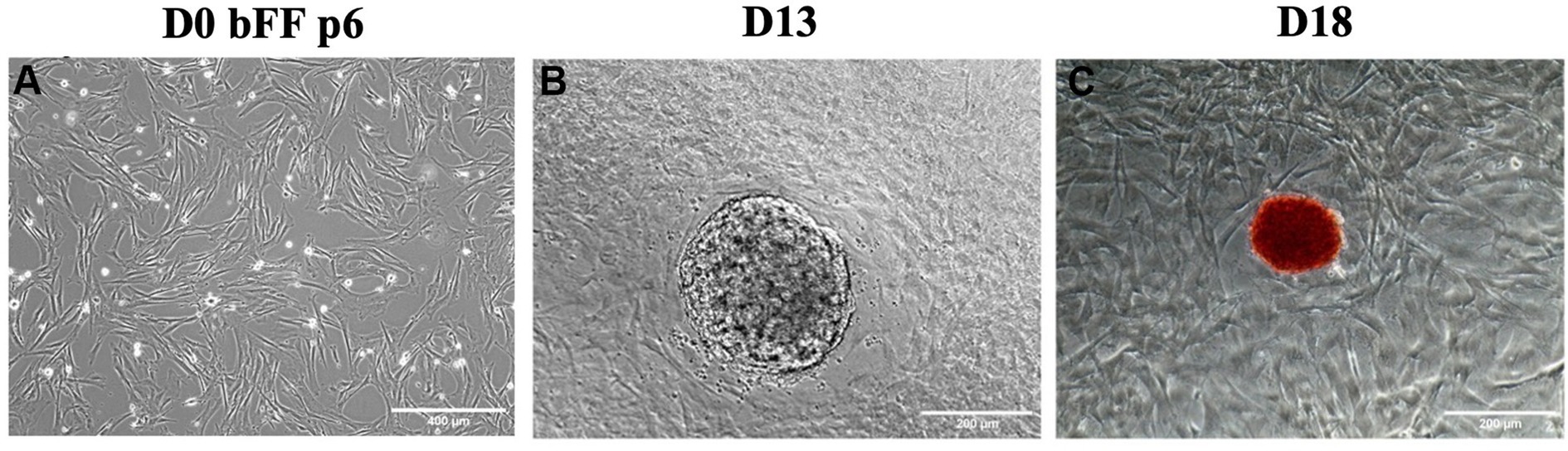
Figure 1. Morphological changes during episomal reprogramming of bFFs. (A) Day 0 (D0) of reprogramming protocol in bFFs at passage 6. (B) A representative colony was observed on day 13 (D13) after bFFs underwent nucleofection with pMaster K and EBNA1. (C) A representative AP-stained colony on day 18 (D18).
Fourteen colonies were manually selected and replated onto a monolayer of MEFs. The clonal line 12 was chosen based on its typical iPSC morphology, such as limited borders, dome-shape appearance, and faster proliferation, as well as the presence of transcripts and markers related to pluripotency. Pluripotency markers OCT4, SOX2, and NANOG were detected both at transcript and protein levels, indicating that the biPSCs had been successfully reprogrammed (Figure 2).
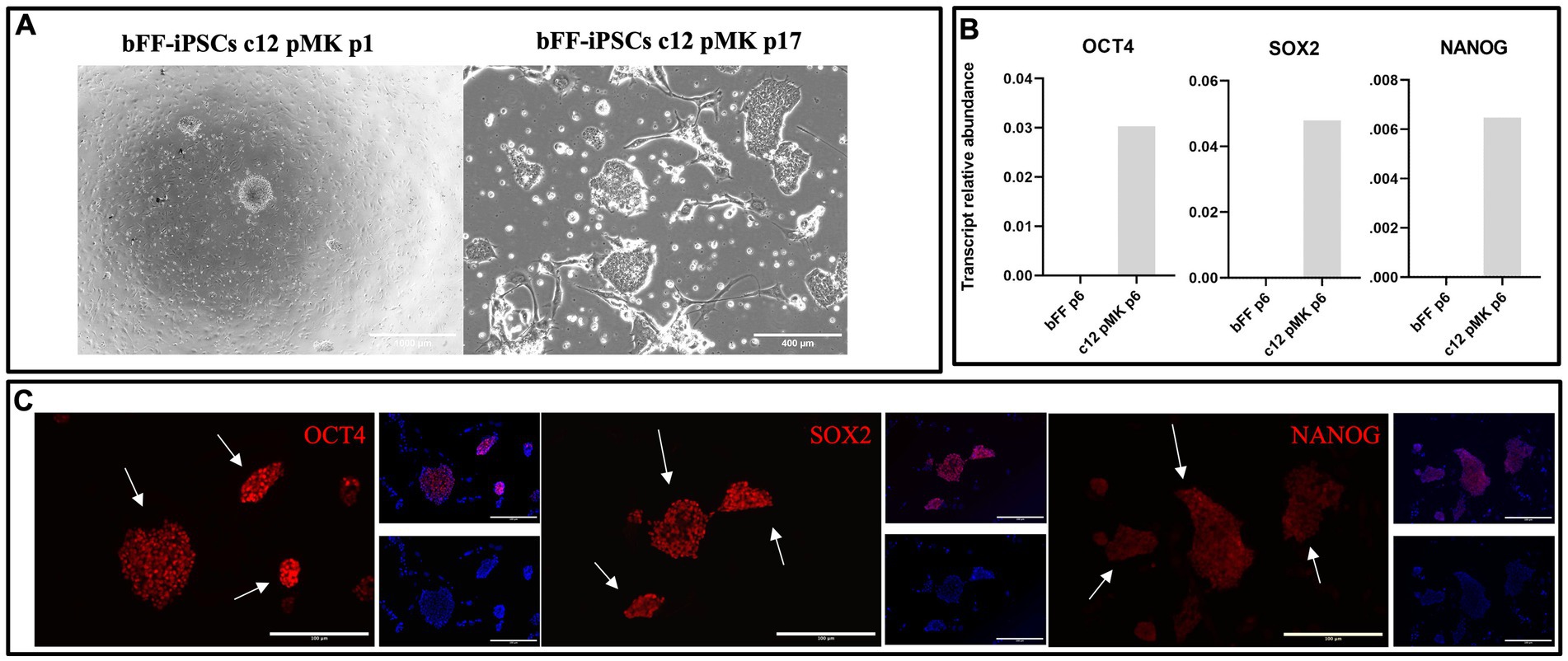
Figure 2. Characterization of clonal line bFF-iPSCs c12 pMK. (A) Clonal line bFF-iPSCs c12 pMK after manual picking at passage 1 and cultured until passage 17, displaying typical iPSCs morphology, with limited border and dome-shape appearance (Scale bar: 1000—bFF-iPSCs pMK c12 p1 or 400 μm—bFF-iPSCs pMK c12 p17). (B) A relative abundance of endogenous pluripotency transcripts (OCT4, SOX2, and NANOG) was detected in the clonal bFF-iPSCs c12 line at passage 6 but was absent in bFF at passage 6. (C) Immunocytochemistry (ICC) analysis confirmed the presence of pluripotency markers OCT4, SOX2, and NANOG in the clonal bFF-iPSCs pMK c12 line at passage 14 (Scale bar: 200 μm).
Based on these confirmed iPSC characteristics, clonal line 12 (bFF-iPSCs pMK) was selected for subsequent differentiation into satellite cells even though the presence of episomal plasmids was still detectable at passages 12 and 17 (Supplementary Figure 1).
3.2 Pluripotency profile between biPSCs generated from different cell lines and protocols
The relative abundance of endogenous pluripotency transcripts and the early muscle progenitor marker PAX3 was analyzed in both biPSC lines used for the myogenesis differentiation protocol (bFF-iPSCs mOSKM p22 and bFF-iPSCs pMK p5) (35, 36). PAX3 expression is upregulated during myogenic differentiation of iPSCs, serving as a key marker for early muscle progenitor cells. Its increased expression plays a crucial role in the transition from pluripotency to the myogenic lineage, promoting the proliferation and differentiation of muscle precursor cells (3, 15, 37). Prior to starting the differentiation, both biPSC lines were assessed for the expression of pluripotency markers OCT4, SOX2, and NANOG and the early myogenic marker PAX3 (Figure 3). All experiments were performed in technical replicates (n = 2).
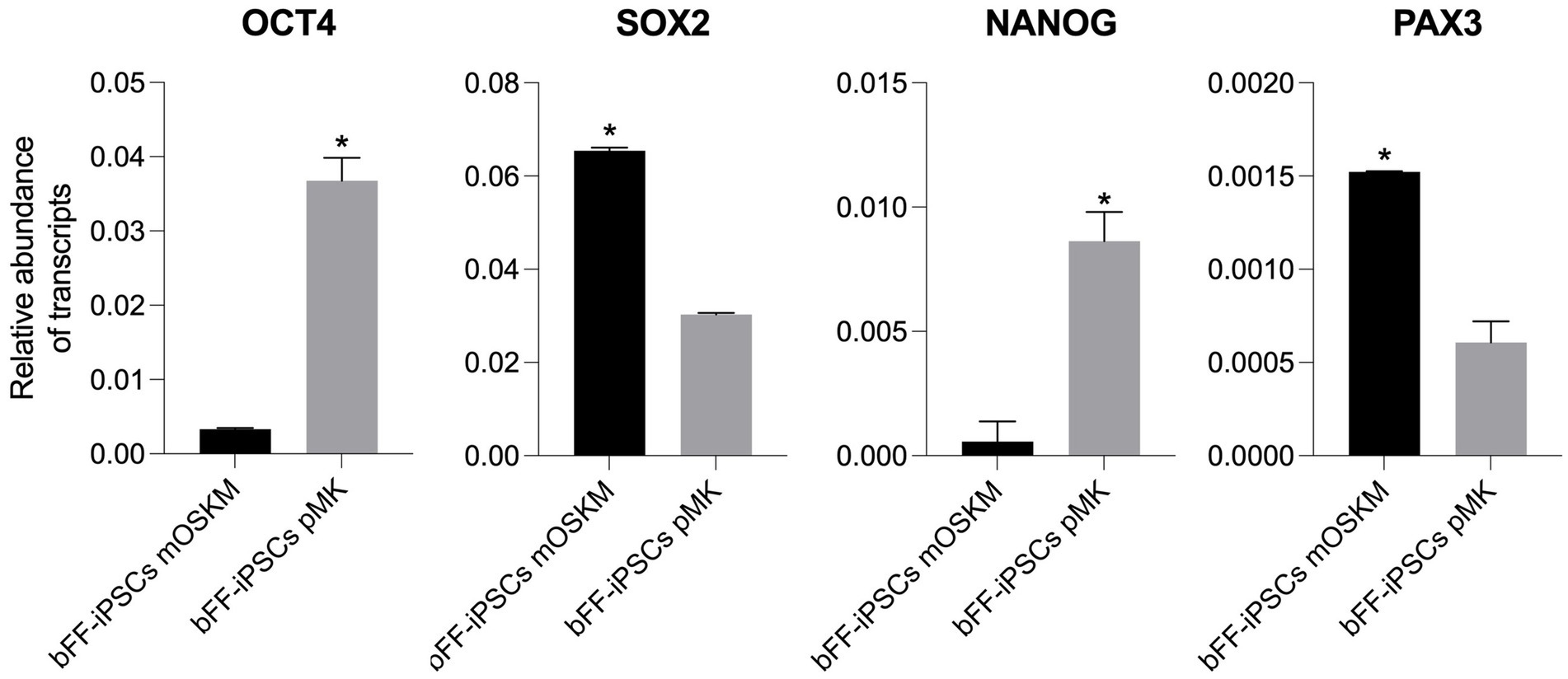
Figure 3. Comparison of pluripotency gene expression and early myogenic marker in bFF-iPSCs. Relative transcript abundance of pluripotency genes OCT4, SOX2, and NANOG, and the early myogenic marker PAX3, in two biPSC lines (bFF-iPSCs mOSKM and bFFs-iPSCs pMK). Endogenous OCT4 and NANOG were more abundant in bFF-iPSCs pMK, while SOX2 levels were comparable between the two iPSC lines. PAX3 expression was higher in bFF-iPSCs mOSKM. Experiments were conducted in technical duplicate, and results are presented as the mean ± standard deviation (SD) to reflect variability. Bars labeled with an asterisk indicate statistically significant differences between groups (p < 0.05).
3.3 biPSCs and myogenesis differentiation
The myogenesis differentiation of both biPSC lines was performed simultaneously. When differences in the results were observed, they were analyzed and discussed separately in this manuscript. Bovine primary satellite cells, donated by Aarhus University, were used as a control to evaluate the differentiation potential of the biPSCs.
3.3.1 Differentiation of bFF-iPSCs derived from mOSKM lentiviral reprogramming into SCs
Upon plating onto Matrigel with SKM01 media, the bFF-iPSCs mOSKM line displayed morphological changes, adopting either a fibroblast-like appearance or irregular cytoplasmic boundaries. By day 4, no biPSC colonies were present, indicating the onset of differentiation. By day 8, elongated cells emerged alongside smaller, round cells resembling satellite cells (SCs) (38) (Figure 4A).
RT-qPCR analysis revealed the presence of OCT4 and PAX3 transcripts, while SOX2, NANOG, and mOSKM were downregulated by day 8. Nevertheless, no PAX7 expression was observed, which was present in the SC control (Figure 4B).
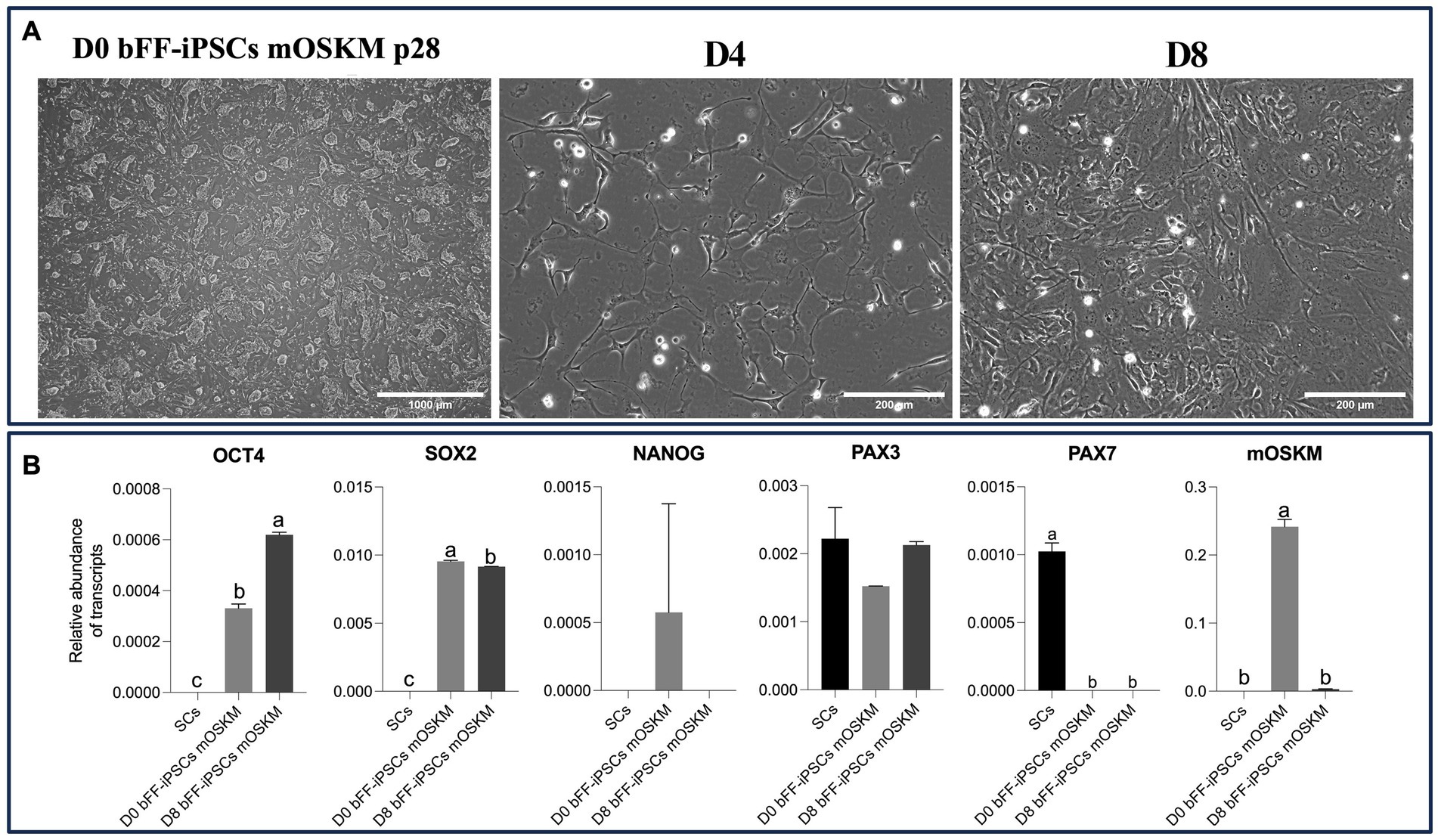
Figure 4. Characterization of biPSC differentiation capacity into the myogenic lineage. (A) Morphological changes of bFF-iPSCs mOSKM at passage 28 after 8 days of culture in SKM01 media (scale bar: 1000 μm at D0; 200 μm at D4, D8). By day 4, no colonies were observed, and cells began to elongate, forming small clusters. By day 8, an increased number of elongated, fibroblast-like cells were evident. (B) Relative transcript abundance of pluripotency and myogenesis-related genes in biPSCs, differentiated cells, and bovine primary satellite cells. Pluripotency markers (OCT4, SOX2, and NANOG) were highly expressed in D0 bFF-iPSCs mOSKM, with levels decreasing following differentiation. The myogenic marker PAX3 was more abundant in bovine primary satellite cells (SCs ctrl). mOSKM was detected exclusively in D0 bFF-iPSCs mOSKM, and PAX7 was specific to SCs ctrl. Technical duplicates were performed for each time point, and the results are presented as the mean ± SD to ensure accurate representation. Bars labeled with different letters are different from each other (p < 0.05).
Initial results showed that the bFF-iPSCs mOSKM appeared to start in vitro differentiation into SCs; however, 8 days in culture were not enough to further progress in the differentiation process. Therefore, a “long-term” condition was implemented using SKM01 media for 18 days on Matrigel. Throughout this longer culture period, the cells underwent morphological changes similar to those observed in previous reports (19). However, by the end of the protocol, most cells exhibited a fibroblast-like morphology (Figure 5A). Additionally, during the initial days of the differentiation protocol, OCT4 and SOX2 expression were clearly detected, suggesting that the pluripotent state was being maintained. Subsequently, both endogenous and mOSKM abundance were diminished over the course of the protocol. Although PAX3 was initially detected at high levels, its expression unexpectedly decreased after day 4, and no PAX7 expression was detected (Figure 5B).
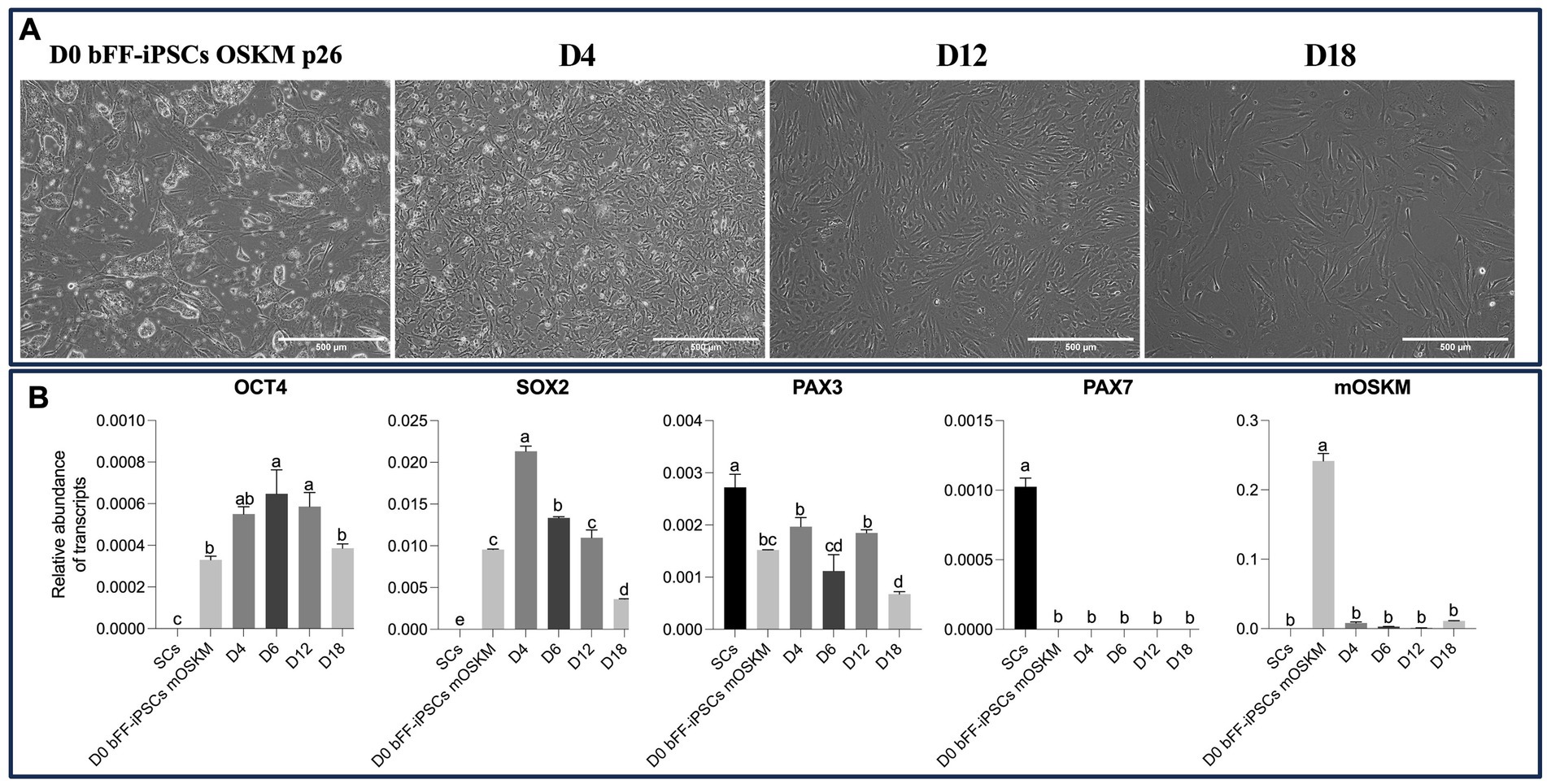
Figure 5. Impact of long-term culture on pluripotent and myogenic markers in bFF-iPSCs mOSKM. (A) Morphological changes of bFF-iPSCs mOSKM at passage 26 following 18 days of culture in SKM01 media. By day 4, cells began to adopt a fibroblast-like morphology. By days 12 and 18, cells maintained a fusiform shape and showed increased elongation (scale bar: 1000 μm at D0 and 500 μm at D4, D12, and D18). (B) Relative transcript abundance of pluripotency (OCT4 and SOX2) and early myogenic genes (PAX3 and PAX7) at different time points of differentiation (D0, D4, D12, and D18), compared to bovine primary satellite cells (SCs-Ctrl). OCT4 and SOX2 expressions decreased over time, while PAX3 levels progressively increased. However, no PAX7 expression was detected during differentiation, and mOSKM expression decreased throughout the protocol. Technical duplicates were performed for each time point, and the results are presented as the mean ± SD to ensure accurate representation. Bars labeled with different letters are different from each other (p < 0.05).
3.3.2 Differentiation of bFF-iPSCs derived from pMK episomal reprogramming into SCs
After being plated onto Matrigel with SKM01 media, the bFF-iPSCs pMK line rapidly began to change its morphology. No biPSC colonies were observed, and a few cells exhibited a fibroblast-like morphology, while others appeared more round and smaller, resembling SCs (38). This morphology was maintained until day 8 (Figure 6A). As expected, the abundance of OCT4 and SOX2 transcripts was found to be lower, while the PAX3 transcript was increased at D8. However, no PAX7 transcript was detected in the biPSCs or in D8 (Figure 6B).
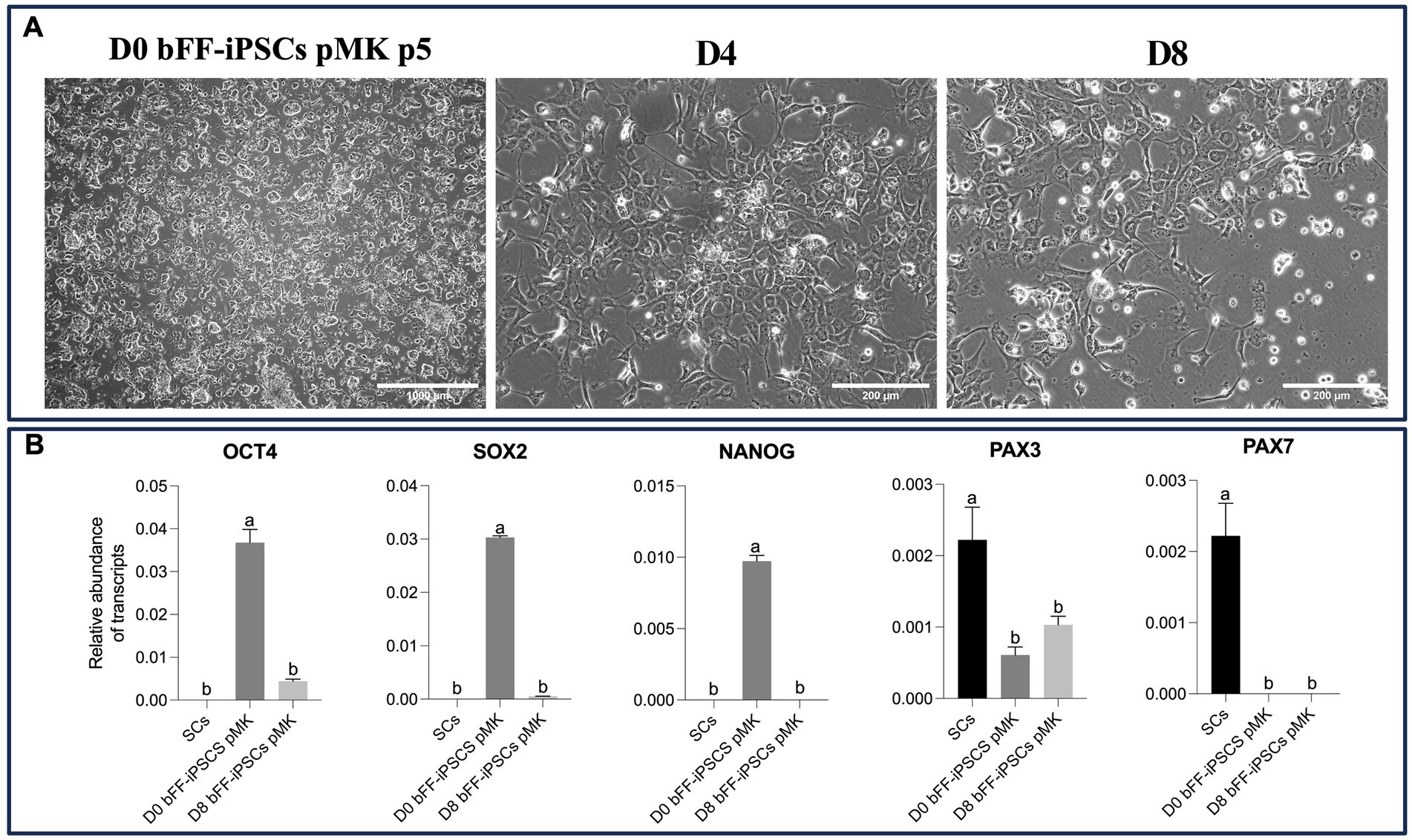
Figure 6. Impact of long-term culture on pluripotent and myogenic markers in bFF-iPSCs pMK (A) Morphological changes of bFF-iPSCs pMK at passage 5 following 8 days of culture in SKM01 media. At day 0, cells exhibited a compact, colony-like morphology characteristic of pluripotent stem cells. By days 4 and 8, cells displayed elongated edges, with some maintaining a large nucleus-cytoplasm ratio (scale bar: 1000 μm at D0 and 200 μm at D4, D8). (B) Relative transcript abundance of pluripotency markers (OCT4 and SOX2) and early myogenic markers (PAX3 and PAX7) in bFF-iPSCs pMK at different time points (D0, D4, and D8), compared to bovine primary satellite cells (SCs-Ctrl). As expected, pluripotency genes (OCT4 and SOX2) were downregulated, and early myogenic marker PAX3 increased, indicating progression toward differentiation. However, no PAX7 expression was detected. Technical duplicates were performed for each time point, and the results are presented as the mean ± SD to ensure accurate representation. Bars labeled with different letters are different from each other (p < 0.05).
Based on these findings, a long-term culture protocol was established using SKM01 media on Matrigel for 24 days. Throughout the culture period, the cells underwent the expected morphological changes. However, by the end of the protocol, most cells exhibited fibroblast-like morphology (Figure 7A). OCT4 expression was detected throughout the protocol, although it decreased following differentiation. Similarly, SOX2 expression was reduced over the 24-day differentiation period. PAX3 expression initially increased but began to decrease after day 8. PAX7 was undetectable in all biPSCs but was present in the primary satellite cells (Figure 7B).
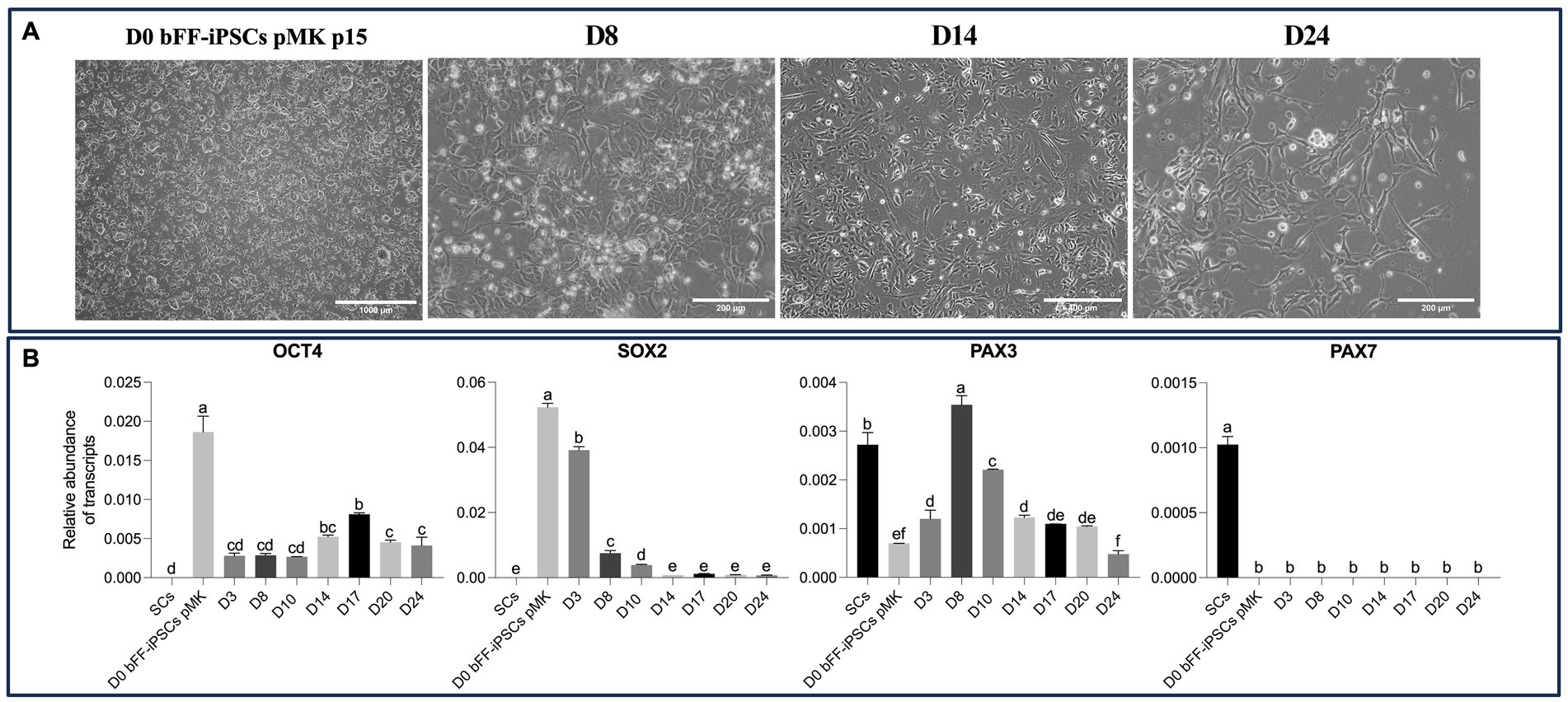
Figure 7. Morphological and gene expression changes during long-term differentiation of bFF-iPSCs pMK. (A) bFF-iPSCs pMK at passage 15 and their morphology differentiation after the long-term cultured with SKM01 media for 24 days. At D0, cells exhibit a compact and colony-like morphology. By day 8, cells began to display a more dispersed and elongated morphology. By day 14, further elongation was observed, with some cells adopting a spindle-shaped appearance. By day 24, an increased number of elongated cells was present (Scale bar: 1000 μm at D0, 400 μm at D8 and D14, and 400 μm at D24). (B) Relative transcript abundance of pluripotency markers (OCT4 and SOX2) and early myogenic markers (PAX3 and PAX7) in bFF-iPSCs pMK at different time points (D0, D8, D14, D17, and D24), compared to bovine primary satellite cells (SCs-Ctrl). A progressive downregulation of pluripotency genes (OCT4 and SOX2) was observed, along with a fluctuating expression pattern of PAX3, which stabilized after day 14. No PAX7 expression was detected. Technical duplicates were performed for each time point, and the results are presented as the mean ± SD to ensure accurate representation. Bars labeled with different letters are different from each other (p < 0.05).
In both attempts to differentiate bFF-iPSCs pMK into SCs, the cells seemed to initiate differentiation with increased levels of PAX3 and a lower abundance of the pluripotent transcripts. However, after day 8, PAX3 expression was progressively reduced and expression of the pluripotency marker OCT4 was increased.
To determine whether the protocol could be improved, a combination of protocols was implemented, as described below. In summary, SKM01 media was utilized until day 8, after which it was replaced with the media previously reported by Chal et al. (15).
By Day 30, various cell types were observed. Among these various cells, cells presenting putative myotubes with elongated morphology and multinuclear features were detected (Figure 8A). Concomitantly, expression of OCT4 and SOX2 was remarkably reduced, while PAX3 expression was maintained at comparable levels to the primary bovine satellite cell control sample. PAX7 expression, however, was still not detectable. Interestingly, MYOG expression was strongly induced in biPSCs differentiation cultures after day 21 (Figure 8B). MYOG is typically found in myocytes and myotubes and stimulates further differentiation of SCs into these cell lineages (15, 39).
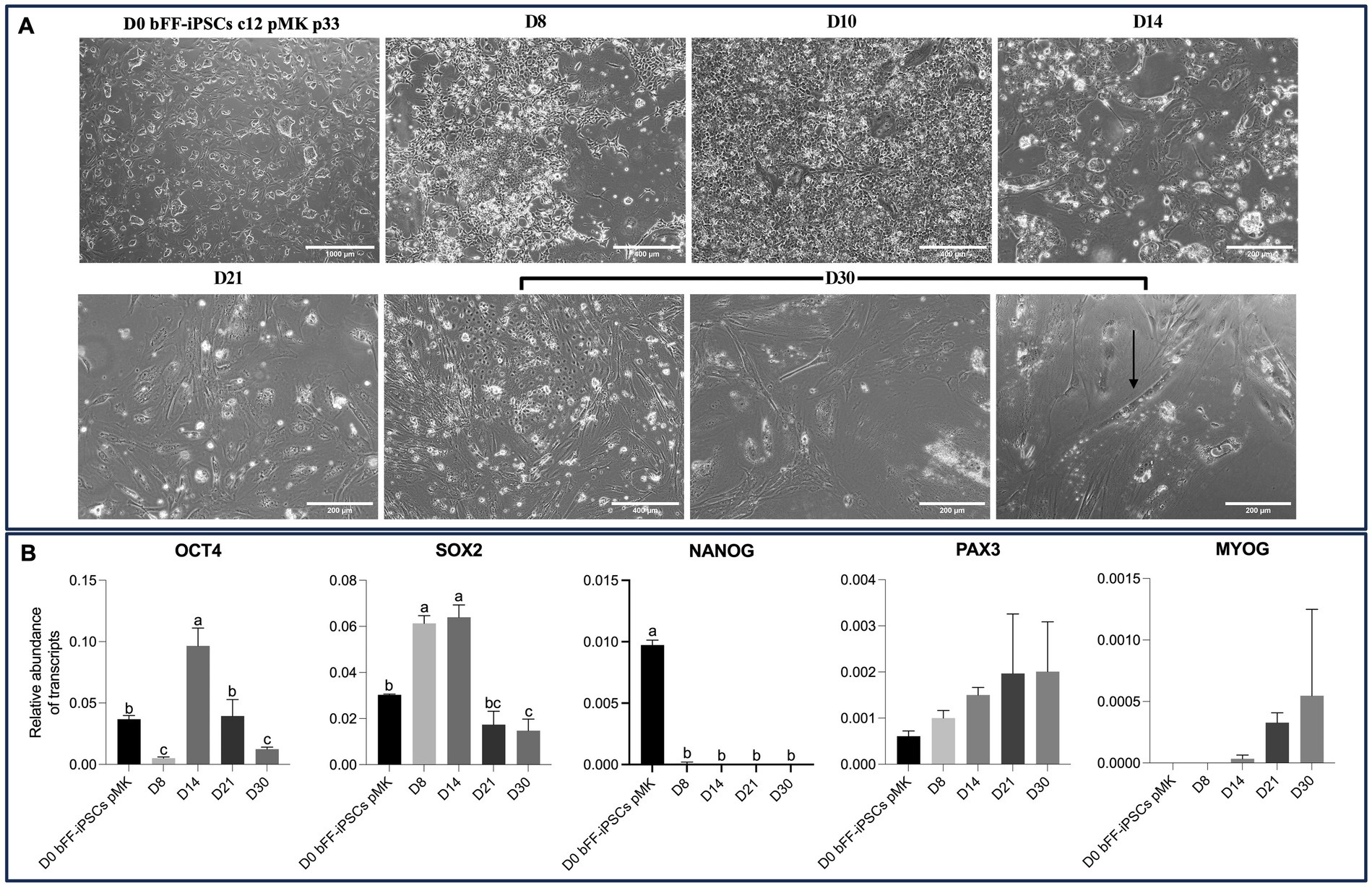
Figure 8. Extended culture effects on morphology and gene expression of bFF-iPSCs pMK. (A) Morphological changes of bFF-iPSCs pMK at passage 33 following 30 days of differentiation, showing characteristics indicative of myogenesis. By day 30, various cell types were observed, including cells with elongated, multinucleated morphology typical of myotubes (arrow) (scale bar: 1000 μm at D0, 400 μm at D8, D10, and D30-1 or 200 μm at D14, D21, D30-2, and D30-3). The arrow highlights a multinucleated cell at day 30. (B) Transcript levels of pluripotency markers (OCT4 and SOX2) and myogenesis markers (PAX3, PAX7, and MYOG) at different time points during the differentiation protocol. OCT4 and SOX2 expressions were significantly reduced by day 30, while PAX3 expression remained elevated, comparable to the primary bovine satellite cell control. MYOG expression was strongly induced after day 21. Notably, PAX7 expression was undetectable at all time points. Technical triplicates were performed for each time point, and the results are presented as the mean ± SD to ensure accurate representation. Bars labeled with different letters are different from each other (p < 0.05).
4 Discussion
The bFFs were successfully reprogrammed after nucleofection of episomal plasmids, and the clonal line bFF-iPSCs pMK demonstrated rapid proliferation, formed colonies with well-defined borders, large nuclei, exhibited AP activity, and transcripts and markers associated with pluripotency. Nevertheless, the bFF-iPSCs pMK line at passage 17 showed the presence of the episomal plasmids, which could indicate that either the episomal plasmids were not lost during cell divisions or that they may have integrated into the cell’s genome, similar to all bovine iPSCs generated to date (22, 23, 25, 40–44).
Herein, bovine iPSCs generated via episomal methodology were maintained in the media optimized by Zhao et al. (45), where biPSCs generated through PiggyBac Dox-inducible transposon and isolated bovine ESCs were successfully maintained in vitro for 63 passages. Additional supplementation was carried out using 10 ng/μl bFGF, which was found to be critical for self-renewal and proliferation through MEK/ERK and PI3K signaling pathways and helped to prevent differentiation (46). A lower concentration of antibiotics was used, and no Leukemia Inhibitory Factor (LIF) was added to the media once previous studies demonstrated that bFGF is more efficient in promoting the acquisition of pluripotency (22).
Differentiation of pluripotent stem cells into muscle cells through in vitro myogenesis has been well studied in humans, while it remains not fully elucidated in large animals such as pigs and cattle. Studies have reported that the cells need to undergo morphological changes and exhibit a higher abundance of PAX3, which plays a crucial role during early myogenesis (3, 35, 47). PAX7 expression is commonly used to identify SCs, which are essential for tissue regeneration and development (11, 15, 35, 38, 48). In our study, both PAX3 and PAX7 primers were validated using SCs as controls (11).
The biPSCs lines described herein showed to be suitable for the myogenesis protocol. In the initial attempt to differentiate both lines, a commercial protocol was employed, and the cells were maintained in culture for 8 days. By day 8, the bFF-iPSCs mOSKM lentiviral-derived line exhibited OCT4 and PAX3 expressions, lower levels of SOX2, and importantly, silencing of mOSKM; however, no PAX7 transcripts were detected. After initial differentiation of the bFF-iPSCs pMK episomal-derived line, all pluripotent transcripts were detected in a lower magnitude, as expected. Previous reports using human PSCs by day 10 using this commercial media (19) indicated approximately only 20% of the population being satellite cells expressing the PAX7 marker (49).
During the extended culture period employed herein, both cell lines initially exhibited higher levels of PAX3 and reduced abundance of pluripotent transcripts, as expected (19, 32). Interestingly, PAX7 was not detected at any of the time points analyzed, which may suggest that the long-term culture with SKM01 media did not sufficiently induce further differentiation, or else, the exact window of PAX7 expression was not evaluated.
Hence, we next used a combined protocol with the use of SKM01 media for initial differentiation until day 8, when the bFF-iPSCs pMK episomal-derived line exhibited a higher relative abundance of PAX3 transcripts. This was followed by replacing the culture media with the DK-HIFL media, as described by Chal et al. (15), which was used after the emergence of a higher abundance of the PAX3 transcripts in the cell lines population.
The DK-HIFL media is supplemented with LDN-193189, hepatocyte growth factor (HGF), insulin growth factor 1 (IGF-1), and basic fibroblast growth factor (bFGF), all of which play an important role in myogenesis differentiation. LDN-193189 inhibits BMP signaling, a crucial function considering BMP’s established role in promoting the development of lateral plate mesoderm derivatives, including hematopoietic and cardiovascular cell types, both in vivo and in vitro (50–52). Furthermore, HGF, supplemented in the media, is produced by the lateral plate mesoderm and is essential for proper myoblast migration (53–55). The insulin (IGF-1) signaling, in collaboration with Wnt signaling, has been shown to promote myogenesis and myoblast fusion (56, 57). Moreover, bFGF signaling plays a role in promoting myoblast proliferation while impeding differentiation (58). Additionally, the modulation achieved in the pathways IGF-1, MAPK/Erk, TGF-β, and Wnt/β-catenin with the different cytokines used has shown to be of great importance during the human or mouse myogenesis (59–62); however, the details of these pathways in bovine remain not fully elucidated.
During the culture period, the cells exhibited a higher abundance of OCT4 and SOX2, followed by a subsequent decrease until the end of the protocol. PAX3 was found in higher levels and maintained its abundance throughout the culture period, in contrast to the results observed when using only the SKM01 media. No expression of PAX7 was detected; however, the MYOG expression was found to be increased after day 21, correlating with the morphological changes observed in elongated cells with multinuclear characteristics typical of myotubes. These results are supported by the study of Zhu et al. (63) in porcine. Additionally, morphologies of other cell types could be observed, as described by Chal et al. (15) and Wada et al. (18).
5 Conclusion
In this study, we successfully generated biPSCs from fetal fibroblasts and utilized two biPSC lines to further investigate in vitro myogenesis. The optimization of a standardized human protocol using commercial media initially induced the differentiation of bFF-iPSC lines, with elevated PAX3 levels observed until day 8. Subsequently, the combination of commercial and lab-made media in long-term culture maintained PAX3 abundance and facilitated the formation of multinucleated, elongated cells with MYOG transcripts persisting until the protocol’s conclusion. This achievement in the bovine model holds significant promise for advancing cultured meat production. In conclusion, ur findings mark progress in successfully deriving episomal bovine iPSCs and establishing a preliminary protocol for bovine in vitro myogenesis. These milestones facilitate translational studies in veterinary and pave the way for the development of innovative technologies in tissue engineering, such as alternative animal protein production. As the global demand for alternative protein sources grows, CM, produced through cellular agriculture and tissue engineering principles, presents a promising approach. As CM continues to evolve, addressing these challenges will be critical for realizing its commercial potential, offering a broad impact across the biotechnology and food sectors.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
Protocols were approved by the Ethics Committee on Animal Use of the School of Veterinary Medicine and Animal Science University of São Paulo, Brazil (protocol number 8077020516) and by the Ethics Committee on the Use of Animals of the Faculty of Animal Science and Food Engineering, University of São Paulo, Brazil (protocol number 3526250717). The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
KR: Conceptualization, Formal analysis, Methodology, Validation, Writing – original draft. MW: Formal analysis, Methodology, Writing – review & editing. FB: Conceptualization, Funding acquisition, Writing – review & editing. KF: Conceptualization, Funding acquisition, Resources, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was supported by the São Paulo Research Foundation (FAPESP - grant numbers: 2022/14534-6; 2020/15122-8; 2015/26818-5) and CNPq (grant number: 408899/2023-9), as well as the Danish Research Council (CRISPR-cow, grant number: 3105-00068B).
Acknowledgments
We thank the laboratory staff from laboratory Diseases Stem Cells Model and Embryology, and Jette Feveile Young from Aarhus University for the cDNA from primary satellite cells.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2025.1562981/full#supplementary-material
Footnotes
References
1. Chal, J, Oginuma, M, Al Tanoury, Z, Gobert, B, Sumara, O, Hick, A, et al. Differentiation of pluripotent stem cells to muscle fiber to model Duchenne muscular dystrophy. Nat Biotechnol. (2015) 33:962–9. doi: 10.1038/nbt.3297
2. Chal, J, and Pourquié, O. Making muscle: Skeletal myogenesis in vivo and in vitro. Development. (2017) 144:2104–22. doi: 10.1242/dev.151035
3. Guo, D, Daman, K, Chen, JJC, Shi, M-J, Yan, J, Matijasevic, Z, et al. iMyoblasts for ex vivo and in vivo investigations of human myogenesis and disease modeling. eLife. (2022) 11:e70341. doi: 10.7554/eLife.70341
4. Yamanaka, S. Pluripotent stem cell-based cell therapy—promise and challenges. Cell Stem Cell. (2020) 27:523–31. doi: 10.1016/j.stem.2020.09.014
5. Herrero, M, Thornton, PK, Mason-D’Croz, D, Palmer, J, Benton, TG, Bodirsky, BL, et al. Innovation can accelerate the transition towards a sustainable food system. Nat Food. (2020) 1:266–72. doi: 10.1038/s43016-020-0074-1
6. Springmann, M, Clark, M, Mason-D’Croz, D, Wiebe, K, Bodirsky, BL, Lassaletta, L, et al. Options for keeping the food system within environmental limits. Nature. (2018) 562:519–25. doi: 10.1038/s41586-018-0594-0
8. Kang, D-H, Louis, F, Liu, H, Shimoda, H, Nishiyama, Y, Nozawa, H, et al. Engineered whole cut meat-like tissue by the assembly of cell fibers using tendon-gel integrated bioprinting. Nat Commun. (2021) 12:5059. doi: 10.1038/s41467-021-25236-9
9. Martins, B, Bister, A, Dohmen, RGJ, Gouveia, MA, Hueber, R, Melzener, L, et al. Advances and challenges in cell biology for cultured meat. Annual Rev Animal Biosci. (2025). doi: 10.1146/annurev-animal-021022
10. Messmer, T, Klevernic, I, Furquim, C, Ovchinnikova, E, Dogan, A, Cruz, H, et al. A serum-free media formulation for cultured meat production supports bovine satellite cell differentiation in the absence of serum starvation. Nat Food. (2022) 3:74–85. doi: 10.1038/s43016-021-00419-1
11. Skrivergaard, S, Rasmussen, MK, Therkildsen, M, and Young, JF. Bovine satellite cells isolated after 2 and 5 days of tissue storage maintain the proliferative and myogenic capacity needed for cultured meat production. Int J Mol Sci. (2021) 22, 2–7. doi: 10.3390/ijms22168376
12. Iberite, F, Gruppioni, E, and Ricotti, L. Skeletal muscle differentiation of human iPSCs meets bioengineering strategies: perspectives and challenges. NPJ Regen Med. (2022) 7:23. doi: 10.1038/s41536-022-00216-9
13. Hocquette, J-F, Chriki, S, Fournier, D, and Ellies-Oury, M-P. Review: will “cultured meat” transform our food system towards more sustainability? Animal. (2025) 19:101145. doi: 10.1016/j.animal.2024.101145
14. Tavan, M, Smith, NW, McNabb, WC, and Wood, P. Reassessing the sustainability promise of cultured meat: a critical review with new data perspectives. Crit Rev Food Sci Nutr. (2025) 2:1–9. doi: 10.1080/10408398.2025.2461262
15. Chal, J, Al Tanoury, Z, Hestin, M, Gobert, B, Aivio, S, Hick, A, et al. Generation of human muscle fibers and satellite-like cells from human pluripotent stem cells in vitro. Nat Protoc. (2016) 11:1833–50. doi: 10.1038/nprot.2016.110
16. Lisowska, M, Rowińska, M, Suszyńska, A, Bearzi, C, Łaczmańska, I, Hanusek, J, et al. Human iPSC-derived muscle cells as a new model for investigation of EDMD1 pathogenesis. Int J Mol Sci. (2025) 26, 2–7. doi: 10.3390/ijms26041539
17. Nunes, OBDS, Buranello, TW, Farias, FDA, Rosero, J, Recchia, K, and Bressan, FF. Can cell-cultured meat from stem cells pave the way for sustainable alternative protein? Curr Res Food Sci. (2025) 10:100979. doi: 10.1016/j.crfs.2025.100979
18. Wada, E, Susumu, N, Okuzaki, Y, Hotta, A, Sakurai, H, and Hayashi, YK. Optimized simple culture protocol for inducing mature myotubes from MYOD1-overexpressed human iPS cells. Sci Rep. (2024) 14:28783. doi: 10.1038/s41598-024-79745-w
19. Bruge, C, Geoffroy, M, Benabides, M, Pellier, E, Gicquel, E, Dhiab, J, et al. Skeletal muscle cells derived from induced pluripotent stem cells: a platform for limb girdle muscular dystrophies. Biomedicine. (2022) 10, 2. doi: 10.3390/biomedicines10061428
20. Bogliotti, YS, Wu, J, Vilarino, M, Okamura, D, Soto, DA, Zhong, C, et al. Efficient derivation of stable primed pluripotent embryonic stem cells from bovine blastocysts. Proc Natl Acad Sci USA. (2018) 115:2090–5. doi: 10.1073/pnas.1716161115
21. Shirasawa, A, Hayashi, M, Shono, M, Ideta, A, Yoshino, T, and Hayashi, K. Efficient derivation of embryonic stem cells and primordial germ cell-like cells in cattle. J Reprod Dev. (2024) 70:82–95. doi: 10.1262/jrd.2023-087
22. Botigelli, RC, Pieri, NCG, Bessi, BW, Machado, LS, Bridi, A, de Souza, AF, et al. Acquisition and maintenance of pluripotency are influenced by fibroblast growth factor, leukemia inhibitory factor, and 2i in bovine-induced pluripotent stem cells. Front Cell Dev Biol. (2022) 10, 2–7. doi: 10.3389/fcell.2022.938709
23. Bressan, FF, Bassanezze, V, de Figueiredo Pessôa, LV, Sacramento, CB, Malta, TM, Kashima, S, et al. Generation of induced pluripotent stem cells from large domestic animals. Stem Cell Res Ther. (2020) 11:247. doi: 10.1186/s13287-020-01716-5
24. Canizo, JR, Vazquez Echegaray, C, Klisch, D, Aller, JF, Paz, DA, Alberio, RH, et al. Exogenous human OKSM factors maintain pluripotency gene expression of bovine and porcine iPS-like cells obtained with STEMCCA delivery system. BMC Res Notes. (2018) 11:509–8. doi: 10.1186/s13104-018-3627-8
25. Han, X, Han, J, Ding, F, Cao, S, Lim, SS, Dai, Y, et al. Generation of induced pluripotent stem cells from bovine embryonic fibroblast cells. Cell Res. (2011) 21:1509–12. doi: 10.1038/cr.2011.125
26. Pillai, VV, Kei, TG, Reddy, SE, Das, M, Abratte, C, Cheong, SH, et al. Induced pluripotent stem cell generation from bovine somatic cells indicates unmet needs for pluripotency sustenance. Anim Sci J. (2019) 90:1149–60. doi: 10.1111/asj.13272
27. Pillai, VV, Koganti, PP, Kei, TG, Gurung, S, Butler, WR, and Selvaraj, V. Efficient induction and sustenance of pluripotent stem cells from bovine somatic cells. Biol Open. (2021) 10, 2. doi: 10.1242/bio.058756
28. Recchia, K, Pessôa, LVDF, Pieri, NCG, Pires, PRL, and Bressan, FF. Influence of cell type in in vitro induced reprogramming in cattle. Lifestyles. (2022) 12, 2. doi: 10.3390/life12081139
29. Bressan, FF, dos Santos Miranda, M, Perecin, F, De Bem, TH, Pereira, FTV, Russo-Carbolante, EM, et al. Improved production of genetically modified fetuses with homogeneous transgene expression after transgene integration site analysis and Recloning in cattle. Cell Rep. (2011) 13:29–36. doi: 10.1089/cell.2010.0022
30. Yoshimatsu, S, Qian, E, Sato, T, Yamamoto, M, Ishikawa, M, and Okano, H. Establishing an induced pluripotent stem cell line from neonatal common marmoset fibroblasts by an all-in-one episomal vector approach. Stem Cell Res. (2021) 53:102380. doi: 10.1016/j.scr.2021.102380
31. Okita, K, Yamakawa, T, Matsumura, Y, Sato, Y, Amano, N, Watanabe, A, et al. An efficient nonviral method to generate integration-free human-induced pluripotent stem cells from cord blood and peripheral blood cells. Stem Cells. (2013) 31:458–66. doi: 10.1002/stem.1293
32. Vu Hong, A, Bourg, N, Sanatine, P, Poupiot, J, Charton, K, Gicquel, E, et al. Dlk1-Dio3 cluster miRNAs regulate mitochondrial functions in the dystrophic muscle in Duchenne muscular dystrophy. Life Sci Alliance. (2023) 6, 3. doi: 10.26508/lsa.202201506
33. Pessoa, LVF, Pires, PRL, Collado, M, Pieri, NCG, Recchia, K, Souza, AF, et al. Generation and miRNA characterization of equine induced pluripotent stem cells derived from fetal and adult multipotent tissues. Stem Cells Int. (2019) 2019:1–15. doi: 10.1155/2019/1393791
34. Recchia, K, Machado, LS, Botigelli, RC, Pieri, NCG, Barbosa, G, de Castro, RVG, et al. In vitro induced pluripotency from urine-derived cells in porcine. World J Stem Cells. (2022) 14:231–44. doi: 10.4252/wjsc.v14.i3.231
35. Buckingham, M, and Relaix, F. PAX3 and PAX7 as upstream regulators of myogenesis. Semin Cell Dev Biol. (2015) 44:115–25. doi: 10.1016/j.semcdb.2015.09.017
36. Williams, BA, and Ordahl, CP. Pax-3 expression in segmental mesoderm marks early stages in myogenic cell specification. Development. (1994) 120:785–96. doi: 10.1242/dev.120.4.785
37. Belay, E, Mátrai, J, Acosta-Sanchez, A, Ma, L, Quattrocelli, M, Mátés, L, et al. Novel hyperactive transposons for genetic modification of induced pluripotent and adult stem cells: a nonviral paradigm for coaxed differentiation. Stem Cells. (2010) 28:1760–71. doi: 10.1002/stem.501
38. Dumont, NA, Bentzinger, CF, Sincennes, MC, and Rudnicki, MA. Satellite cells and skeletal muscle regeneration. Compr Physiol. (2015) 5:1027–59. doi: 10.1002/cphy.c140068
39. Tong, HL, Jiang, RY, Zhang, WW, and Yan, YQ. MiR-2425-5p targets RAD9A and MYOG to regulate the proliferation and differentiation of bovine skeletal muscle-derived satellite cells. Sci Rep. (2017) 7:418. doi: 10.1038/s41598-017-00470-8
40. Cao, H, Yang, P, Pu, Y, Sun, X, Yin, H, Zhang, Y, et al. Characterization of bovine induced pluripotent stem cells by lentiviral transduction of reprogramming factor fusion proteins. Int J Biol Sci. (2012) 8:498–511. doi: 10.7150/ijbs.3723
41. Jiang, Y, An, XL, Yu, H, Cai, NN, Zhai, YH, Li, Q, et al. Transcriptome profile of bovine iPSCs derived from Sertoli cells. Theriogenology. (2020) 146:120–32. doi: 10.1016/j.theriogenology.2019.11.022
42. Jiang, Y, Cai, N-N, An, X-L, Zhu, W-Q, Yang, R, Tang, B, et al. Naïve-like conversion of bovine induced pluripotent stem cells from Sertoli cells. Theriogenology. (2023) 196:68–78. doi: 10.1016/j.theriogenology.2022.10.043
43. Su, Y, Wang, L, Fan, Z, Liu, Y, Zhu, J, Kaback, D, et al. Establishment of bovine-induced pluripotent stem cells. Int J Mol Sci. (2021) 22, 6. doi: 10.3390/ijms221910489
44. Sumer, H, Liu, J, Malaver-Ortega, LF, Lim, ML, Khodadadi, K, and Verma, PJ. NANOG is a key factor for induction of pluripotency in bovine adult fibroblasts1. J Anim Sci. (2011) 89:2708–16. doi: 10.2527/jas.2010-3666
45. Zhao, L, Gao, X, Zheng, Y, Wang, Z, Zhao, G, Ren, J, et al. Establishment of bovine expanded potential stem cells. Proc Natl Acad Sci. (2021) 118:e2018505118. doi: 10.1073/pnas.2018505118
46. Haghighi, F, Dahlmann, J, Nakhaei-Rad, S, Lang, A, Kutschka, I, Zenker, M, et al. BFGF-mediated pluripotency maintenance in human induced pluripotent stem cells is associated with NRAS-MAPK signaling 06 biological sciences 0601 biochemistry and cell biology. Cell Commun Signaling. (2018) 16, 7. doi: 10.1186/s12964-018-0307-1
47. Ridgeway, AG, and Skerjanc, IS. Pax3 is essential for skeletal Myogenesis and the expression of Six1 and Eya2. J Biol Chem. (2001) 276:19033–9. doi: 10.1074/jbc.M011491200
48. Stout, AJ, Mirliani, AB, Rittenberg, ML, Shub, M, White, EC, Yuen, JSK, et al. Simple and effective serum-free medium for sustained expansion of bovine satellite cells for cell cultured meat. Commun Biol. (2022) 5, 7. doi: 10.1038/s42003-022-03423-8
49. Tanoury, Z, Rao, J, Tassy, O, Gobert, B, and Gapon, S. Differentiation of the human PAX7-positive myogenic precursors/satellite cell lineage in vitro. Development. (2020) 147, 7. doi: 10.1242/dev.187344
50. Adelman, CA, Chattopadhyay, S, and Bieker, JJ. The BMP/BMPR/Smad pathway directs expression of the erythroid-specific EKLF and GATA1 transcription factors during embryoid body differentiation in serum-free media. Development. (2002) 129:539–49. doi: 10.1242/dev.129.2.539
51. Murry, CE, and Keller, G. Differentiation of embryonic stem cells to clinically relevant populations: lessons from embryonic development. Cell. (2008) 132:661–80. doi: 10.1016/j.cell.2008.02.008
52. Orlova, VV, de Sousa, C, Lopes, S, and Valdimarsdottir, G. BMP-SMAD signaling: from pluripotent stem cells to cardiovascular commitment. Cytokine Growth Factor Rev. (2016) 27:55–63. doi: 10.1016/j.cytogfr.2015.11.007
53. Andermarcher, E, Surani, MA, and Gherardi, E. Co-expression of the HGFISF and c-met genes during early mouse embryogenesis precedes reciprocal expression in adjacent tissues during organogenesis. Dev Genet. (1996) 18:254–66.
54. Bladt, F, Riethmacher, D, Isenmann, S, Aguzzi, A, and Birchmeier, C. Essential role for the c-met receptor in the migration of myogenic precursor cells into the limb bud. Nature. (1995) 376:768–71. doi: 10.1038/376768a0
55. Takayama, H, La Rochelle, WJ, Anver, M, Bockman, DE, and Merlino, G. Scatter factor/hepatocyte growth factor as a regulator of skeletal muscle and neural crest development. Proc Natl Acad Sci. (1996) 93:5866–71. doi: 10.1073/pnas.93.12.5866
56. Chargé, SBP, and Rudnicki, MA. Cellular and molecular regulation of muscle regeneration. Physiol Rev. (2004) 84:209–38. doi: 10.1152/physrev.00019.2003
57. van der Velden, JLJ, Langen, RCJ, Kelders, MCJM, Wouters, EFM, Janssen-Heininger, YMW, and Schols, AMWJ. Inhibition of glycogen synthase kinase-3β activity is sufficient to stimulate myogenic differentiation. Am J Phys Cell Phys. (2006) 290:C453–62. doi: 10.1152/ajpcell.00068.2005
58. Itoh, N, Mima, T, and Mikawa, T. Loss of fibroblast growth factor receptors is necessary for terminal differentiation of embryonic limb muscle. Development. (1996) 122:291–300. doi: 10.1242/dev.122.1.291
59. Hernández-Hernández, JM, García-González, EG, Brun, CE, and Rudnicki, MA. The myogenic regulatory factors, determinants of muscle development, cell identity and regeneration. Semin Cell Dev Biol. (2017) 72:10–8. doi: 10.1016/j.semcdb.2017.11.010
60. Koch, AJ, and Holaska, JM. Emerin in health and disease. Semin Cell Dev Biol. (2014) 29:95–106. doi: 10.1016/j.semcdb.2013.12.008
61. Koch, AJ, and Holaska, JM. Loss of Emerin alters myogenic signaling and miRNA expression in mouse myogenic progenitors. PLoS One. (2012) 7:e37262. doi: 10.1371/journal.pone.0037262
62. Xi, H, Langerman, J, Sabri, S, Chien, P, Young, CS, Younesi, S, et al. A human skeletal muscle atlas identifies the trajectories of stem and progenitor cells across development and from human pluripotent stem cells. Cell Stem Cell. (2020) 27:158–176.e10. doi: 10.1016/j.stem.2020.04.017
63. Zhu, G, Gao, D, Li, L, Yao, Y, Wang, Y, Zhi, M, et al. Generation of three-dimensional meat-like tissue from stable pig epiblast stem cells. Nat Commun. (2023) 14:8163. doi: 10.1038/s41467-023-44001-8
64. Riquelme-Guzmán, C, Stout, AJ, Kaplan, DL, and Flack, JE. Unlocking the potential of cultivated meat through cell line engineering. iScience. (2024) 27:110877. doi: 10.1016/j.isci.2024.110877
Keywords: bovine, episomal reprogramming, lentiviral reprogramming, pluripotent stem cell, in vitro myogenesis, myotube, cultured meat
Citation: Recchia K, Wathikthinnakon M, Bressan FF and Freude K (2025) Generation of bovine iPSCs from fetal fibroblasts for in vitro myogenesis and cultured meat. Front. Nutr. 12:1562981. doi: 10.3389/fnut.2025.1562981
Edited by:
Jean-Francois Hocquette, INRA UMR1213 Unité Mixte de Recherche sur les Herbivores (UMRH), FranceReviewed by:
Armel Hervé Nwabo Kamdje, University of Garoua, CameroonSishir Kamalapuram, Consultant, Hyderabad, India
Copyright © 2025 Recchia, Wathikthinnakon, Bressan and Freude. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kristine Freude, a2tmQHN1bmQua3UuZGs=
 Kaiana Recchia
Kaiana Recchia Methi Wathikthinnakon2
Methi Wathikthinnakon2 Fabiana Fernandes Bressan
Fabiana Fernandes Bressan Kristine Freude
Kristine Freude