- 1Center for Endemic Disease Control, Chinese Center for Disease Control and Prevention, Harbin Medical University, Harbin, China
- 2Department of Preventive Medicine, Qiqihar Medical University, Qiqihar, China
- 3Institute of Cell Biotechnology, China and Russia Medical Research Center, Harbin Medical University, Harbin, China
- 4Harbin Municipal Center for Disease Control and Prevention, Harbin, China
Background: Moderate heavy metals can lead to the occurrence of liver injury, but the specific mechanism remains unclear.
Methods: This study, based on data from the National Health and Nutrition Examination Survey (NHANES), analyzed associations between 10 heavy metals and hepatic injury in 5,613 adults, with a focus on the mediating role of the Systemic Immune-Inflammation Index (SII). Partial correlation analysis, weighted linear regression, weighted quantile sum (WQS) regression, and mediation effect models were used in the study.
Results: SII showed significant negative correlations with hepatic fibrosis markers (FIB-4: r = −0.290; NFS: r = −0.382, both P < 0.001) but no association with hepatic steatosis indices. Arsenic (As), cobalt (Co), and cesium (Cs) were identified as critical metals linking fibrosis indicators and SII. As mediated its pro-fibrotic effects by completely suppressing SII (OR = 0.0220–0.0581), while Co promoted NFS risk through complete mediation by SII (Q2 vs. Q1 OR = 1.26). Conversely, Cs exhibited anti-fibrotic protectionvia complete positive mediation through SII.
Conclusion: The findings demonstrate that Heavy metals differentially regulate immune-inflammatory pathways to influence hepatic fibrosis progression, providing new evidence for the mechanisms of environmental exposure-induced hepatic injury.
Introduction
Heavy metal pollution is a major problem faced by every city. The heavy metal pollution index in Nigeria's drinking water was as high as 13,672.74, which was 136.72 times higher than the World Health Organization standard for drinking water (1). Heavy metal pollution is also a problem in Ecuador (2), China (3), Canada (4) and the United States (2–4), and has even been detected in the sparsely populated Antarctic (5). It may be produced by large-scale human activities such as the use of pesticides, internal combustion engines and automobiles, rapid industrialization, and imperfect environmental planning, and so on (6, 7). Heavy metal pollution has begun to attract the attention of all countries, but the management is still insufficient. After heavy metals enter the human body, the hepatic is the first to be affected, resulting in abnormal hepatic function and fibrosis (8–10). With the excessive accumulation of heavy metals, the human brain, lungs, and nerves are further damaged (11–15).What is more serious is that they also show carcinogenic effects, such as arsenic, chromium, lead etc. (16). However, the mechanism by which heavy metals cause hepatic damage has not yet been clarified.
The systemic immune-inflammation index (SII), as a new inflammatory biomarker, can reflect the systemic local immune response and systemic inflammation, and has shown its role in the pathogenesis and prognosis of a variety of diseases, such as coronary heart disease (17, 18), gastric cancer (19, 20), lung cancer (21–23) and so on. Some studies have pointed out that SII may be associated with the risk and severity of various hepatic diseases (24, 25). Alterations in inflammatory markers may result from the toxic effects of harmful metals. For instance, arsenic induces sustained immuno-inflammatory responses in the hepatic and kidneys (26), meanwhile, chronic cadmium (Cd) exposure triggers hepatic oxidative stress, endoplasmic reticulum stress, inflammatory reactions, and proliferation in aged female mice (27). Based on these studies, we hypothesize that SII may play a role in the relationship between heavy metals and hepatic injury. However, the specific role of SII in this regard has not been reported.
The development of hepatic injury has a certain process, from the ectopic accumulation of triglycerides in the cytoplasm of hepatic cells (i.e., hepatic steatosis), forming inflammation and hepatocyte damage [i.e., non-alcoholic steatohepatitis], to progressive fibrosis and development into hepatic cirrhosis, end-stage hepatic disease or hepatic cancer (28). Previously, the most common marker for diagnosing hepatic injury was alanine aminotransferase (ALT), but a single factor had the drawback of poor accuracy. Therefore, in this study, the Fatty Liver Index (FLI) (29), NAFLD hepatic fat score (LFS) (30), and Framingham Steatosis Index (FSI) were selected to reflect the level of hepatic steatosis. The degree of hepatic fibrosis was represented by the nonalcoholic fatty liver disease fibrosis score (NFS), an indicator of hepatic fibrosis (FIB-4). Therefore, we investigated the relationship between metal exposure and hepatic damage-related indicators (FLI, LFS, FSI, FIB-4 and NFS) using data from the National Health and Nutrition Survey (NHANES) of people aged 18–80. And we further studied the mediating role of SII in the relationship between heavy metals and Non-alcoholic Fatty Liver Disease (NAFLD).
Methods
NHANES is a program of studies designed to assess the health and nutritional status of adults and children in the United States, by using a hierarchical, multi-stage and probabilistic clustering design. Information will be used to assess the health promotion and disease prevention. The study protocol was approved by the National Center for Health Statistics Ethics Review Board, and all participants provided their informed consent.
Study design and participants
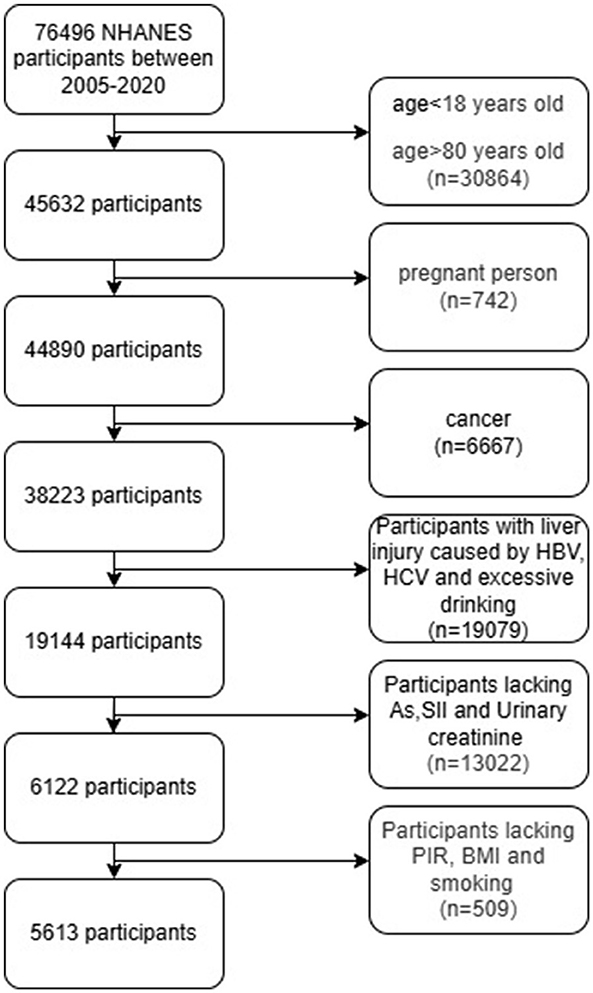
A total of 76,496 participants from five NHANES cycles in 2005–2006, 2007–2008, 2009–2010, 2011–2012, 2013–2014, 2015–2016 and 2017–2020 were included in this study. In NHANES, the heavy metal exposure of participants aged 18–80 was evaluated through urine metabolite analysis. We excluded hepatic injury caused by Hepatitis B Virus (HBV) and Hepatitis C Virus (HCV) (n = 859), and those who drank excessively or lacked drinking information (women drank ≥3 cups per day; Men who drink ≥4 cups of alcohol per day or 5 cups or more per month (n = 18,220), as well as pregnant women (n = 742) and cancer patients (n = 6,667), because these conditions can affect hepatic function indicators.
Ten heavy metals (Arsenic [As], Barium [Ba], Cobalt [Co], Cesium [Cs], Molybdenum [Mo], Lead [Pb], Antimony [Sb], Thallium [Tl], Tungsten [Tu] and Uranium [Ur] in urine were analyzed as the main indicators.
NHANES usually adopts stratified sampling or subsample analysis strategies and only conducts specific tests on some participants. Therefore, in this study, 12,829 samples with missing urinary arsenic data were deleted. The number of participants lacking the other nine heavy metals was relatively small. In this study, the median was used to complete the missing information. There were 2 participants with deficient urinary creatinine content. SII can reflect the local immune response and systemic inflammatory response of the human body. A total of 191 were removed due to the lack of SII.
Because the influence caused by confounding factors such as age, gender and poverty index (PIR) needs to be adjusted during the calculation process, participants with incomplete basic information were also deleted. Ultimately, there were a total of 5,613 participants with complete indicators of heavy metals, SII and urinary creatinine (Figure 1).
Evaluation of biomarkers related to hepatic steatosis and hepatic fibrosis
Biomarkers of hepatic steatosis
The FLI and LFS demonstrate high diagnostic accuracy as markers of hepatic injury, particularly in metabolically susceptible populations (31, 32). Due to this, FLI and LFS have been widely adopted in scientific research. In 2020, a research team led by Lars Lind compared four diagnostic markers for NAFLD (FLI, Hepatic Steatosis Index [HSI], Lipid Accumulation Product [LAP], and LFS) and found that FLI and LFS were the most suitable for assessing NAFLD. This is because FLI performs better in population-based settings, whereas LFS is optimal in high-risk cohorts (33).
The FSI is another tool for determining NAFLD risk, incorporating age, body mass index (BMI), triglycerides (TG), aspartate aminotransferase (AST), alanine aminotransferase (ALT), diabetes status, and hypertension. A 2024 study reported that when used for NAFLD diagnosis, FSI exhibited discrimination and predictive performance with area under the curve (AUC) values of 0.8421 (95% CI: 0.8314–0.8527) and 0.7093 (95% CI: 0.6863–0.7322),
Among them, the diagnostic criteria for metabolic syndrome (MS) are that any three or more of the following adjustments are satisfied:
1. Fasting Blood Glucose (FBG) >100 mg/dL (5.6 mmol/L) or drug treatment for diabetes mellitus.
2. High-density lipoprotein cholesterol (HDL-C) <50 mg/dL in females <40 mg/dL in males or drug treatment for reduced HDL-C.
3. Plasma Triglyceride (TG) >150 mg/dL or drug treatment for raised TG.
4. Waist circumference >88 cm in women or >102 cm in men.
5. Blood pressure >130/85 mmHg or drug treatment for raised blood pressure
1. The diagnostic criteria for hypertension are: Systolic Blood Pressure (SBP)≥140 and/or Diastolic Blood Pressure (DBP)≥90, or having been diagnosed with hypertension by a doctor;
2. According to the latest prevention and treatment guidelines, the diagnostic criteria for hyperglycemia are that it can be diagnosed if any of the following conditions are met (34):
① FPG ≥7.0 mmol/L (126 mg/dL) (fasting means not having eaten for at least 8 h).
② The 2-h blood glucose level in the oral glucose tolerance test (OGTT) was ≥11.1 mmol/L (200 mg/dL).
③ Glycated hemoglobin (HbA1c) ≥6.5% (standardized detection methods should be adopted).
④ Random blood glucose ≥11.1 mmol/L (200 mg/dL) and accompanied by typical hyperglycemic symptoms (such as polydipsia, polyuria, and weight loss).
⑤ It had been confirmed as diabetes before the investigation.
Biomarkers of hepatic fibrosis
The EASL-EASD-EASO clinical practice guidelines recommend simple non-invasive scores such as the NFS and FIB-4 as part of the diagnostic protocol to exclude advanced fibrosis (35). A re-analysis of 13 studies showed that FIB-4 and NFS could stratify the risk of hepatic-related morbidity and mortality in patients, and their performance was comparable to that of hepatic biopsy (36).
Heavy metal exposure
Inductively coupled plasma mass spectrometry (ICP-MS) is a multielement analytical technique (37). The urine samples were collected and ICP-MS was used to detect 13 elements including As, Ba, Co, Cs, Mo, Pb, Sb, Tl, Tu, Ur, beryllium (Be), cadmium (Cd), and platinum (Pt) in the urine samples. Because Be, Cd, and Pt were missing more, the other 10 heavy metals were analyzed in this study. Urinary creatinine was used to standardize the content of heavy metals to control the bias caused by measurement error. Except for As, the missing values of the other 9 heavy metals were filled with the median, and logarithmic transformation was performed on all heavy metals for subsequent analysis.
SII
SII is a good and stable new inflammatory marker that can reflect the local immune response and systemic inflammatory response of the human body. SII was defined as platelet count (109/L) × neutrophil count (109/L)/lymphocyte count (109/L) (38). This comprehensive parameter, which combines peripheral platelet, neutrophils and lymphocytes, more comprehensively reflects the inflammatory state of the body compared with a single inflammatory index (39). Studies have now that SII was associated with a variety of diseases, such as testicular cancer (40), prostate cancer (41), coronary artery disease (42), COVID-19 (43). Since the data distribution of SII did not conform to the properties of a normal distribution, so ln (SII) was used for analysis in this study.
Covariates
Demographic variables, such as age (in years), gender, race, education years, marital status, poverty to income ratio, smoking, and drinking, were collected through interviewers assisted interviews. Gender, race/ethnicity, gender and smoking status were grouped by the respondents. Age was calculated in years and was divided into three groups (18–39, 40–59 and ≥60 years old) for subsequent analysis. This study divided the years of education into three groups: ≤ 12 years, 13–14 years, and ≥15 years. Socioeconomic status is a disease risk factor that is easily overlooked. Low household income is an indicator to measure socioeconomic status and may indicate a greater risk of diseases. The research participants were classified into low poverty (≤ 1), poverty (1–3), and high poverty (>3) based on PIR. The harm of smoking is obvious to all, but there are still people who smoke. In this study, the subjects were divided into smokers, quitters and never smokers for corrective analysis.
Statistical analysis
A total of 76,496 people from the NHANES dataset were collected. According to strict inclusion and exclusion criteria, a total of 5,613 people participated in the final calculation. Considering the complex, multi-stage and multi-level sampling methods used in the NHANES database, we included weighted variables in accordance with the instructions for use provided by the data providers. The weight for the period from 2005 to 2016 was WTSA2YR/6, and the weight for the period from 2017 to 2020 (~1.6 two-year periods) was WTSA2YR/1.6. Weighted non-parametric tests, t-tests, and analysis of variance were used to compare the differences in biomarkers of hepatic steatosis and hepatic fibrosis at different baseline levels. Weighted regression was used to analyze the correlation analysis of hepatic fibrosis indicators after overall and quartile grouping of heavy metals. Since multiple inter-group comparisons were required in the weighted regression of quartile grouping, the P-value was corrected by Bonferroni.
The effect of combined exposure to heavy metals on hepatic fibrosis was analyzed by WQS (44). The WQS regression model is usually expressed as: g(μ) = β0+β1(∑ωiqi)+z′ϕ. Among them: μ is the expected response; β0 is the intercept; qi is the quantile of the i-th exposed variable; wi is the weight of the i-th exposed variable (0 ≤ wi ≤ 1 and ∑wi = 1); β1 is the coefficient of the comprehensive index; z is the covariate vector; ϕ is the covariate coefficient vector. In this study, the WQS model was implemented in R using the “gWQS” package. We assumed that the effect of each exposure in the mixture in the study is in the positive direction. The parameters were set to gwqs(FIB4~ wqs, mix_name = name, data = data, q = 4, validation = 0.6, b = 100, b1_pos = TRUE, b_constr = FALSE, seed =1,003).
Restricted Cubic Splines (RCS) fits data through piecewise polynomials to automatically capture non-linear trends. There is no need to pre-assume the function form (such as linear or quadratic), and the relationship curves between variables and outcomes can be directly plotted (45). Therefore, in this study, the “rms” package of R software was further utilized. The effects of As, Co, and Cs on FIB-4, NFS, and SII were analyzed by weighted RCS.
Mediation analysis is often used to study how an independent variable (X) affects the dependent variable (Y) through a mediation variable (M). For this reason, mediation analysis was also used in this study, with the aim of exploring the mediating effect of SII in the correlation process among As, Co, Cs, and FIB-4, NFS, and it was achieved through the “mediation” package.
All data analyses were conducted using R version 4.4.3 (R Foundation for Statistical Computing, Vienna, Austria). A two-sided p-value was below 0.05. When Bonferroni correction was performed, the two-sided p-value was below 0.05/4, that is, the p-value was 0.0125.
Result
NAFLD correlation index distribution among different participant feature groups
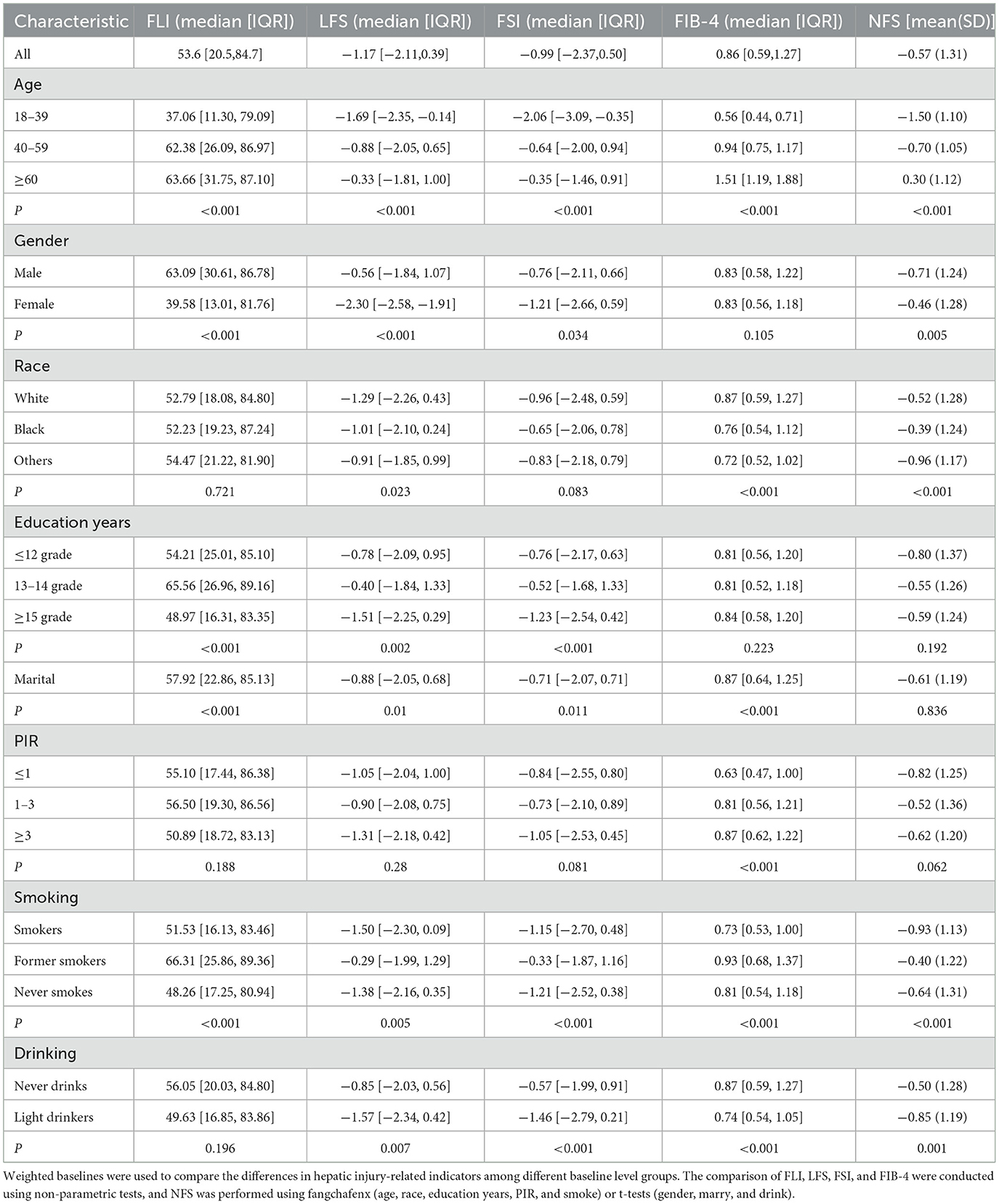
Among 5,613 adults, based on the demographics of the study, Weighted analysis was performed to compare Hepatic steatosis related biomarkers (FLI, LFS, FSI) and Hepatic fibrosis related biomarkers (FIB-4, NFS) in Table 1.
Of all the participants, the older the subjects had higher level of NAFLD related index including Hepatic steatosis-related biomarkers (FLI, LFS, FSI), and Hepatic fibrosis-related biomarkers (FIB-4, NFS), P < 0.001. The median level of Hepatic steatosis related biomarkers in males was significantly higher than that in females, while that in NFS was significantly lower than that in females (P < 0.01). LFS of Black people was higher than that of White people (P = 0.023), however, the conclusions of biomarkers for hepatic fibrosis were inconsistent among different races. Compared with White people, Black people had lower FIB-4 and higher NFS, with P < 0.001. Comparing the five indexes in the population with different years of education years, the result showed that there was more significant difference in the relevant indexes of Hepatic steatosis level, indicating that people with years of education ≥15 years have lower hepatic steatosis score, P < 0.05. PIR only shows its role in FIB-4 score. The results showed that compared with poor people, FIB-4 index of relatively rich people also increased, P < 0.001, which may be related to abnormal hepatic metabolism due to excess nutrition. In the comparison of groups with different smoking conditions, it was found that the group who quit smoking had the highest NAFLD-related index, while the group who did not smoke had the lowest level of most indicators (P < 0.05). However, the five indexes of light drinkers were significantly lower than those of those who did not drink at all, P < 0.05.
Partial correlation analysis of heavy metals, SII and NAFLD correlation index
In order to accurately compare the correlation between heavy metals, SII and NAFLD correlation index, we used partial correlation analysis to calculate this, and 8 covariables including age, gender, race, education years, marry, PIR, smoke and drink were controlled. The results were shown as a heat map in Figure 2. Generally speaking, most of the 10 heavy metals were positively correlated with each other. SII appeared to be more closely associated with hepatic fibrosis, with FBI-4 (r = −0.290, P < 0.001) and NFS (r = −0.382, P < 0.001), but not with steatosis. There was no positive correlation between heavy metals and hepatic steatosis index FLI, LFS, and FSI, indicating that Co, Pb, Ur were positively correlated with FIB-4, and the correlation coefficients were r = 0.084(P = 0.036), r = 0.121(P = 0.002), r = 0.131(P = 0.001), respectively. Among them, this might be related to heavy metal poisoning after which the induced oxidative stress response may preferentially trigger hepatic fibrosis, It was not associated with steatosis (44). Comparing the correlation between heavy metals and SII, the results showed that Co (r = 0.083, P = 0.037) and Tu (r = 0.080, P = 0.044) were significantly positively correlated with SII.
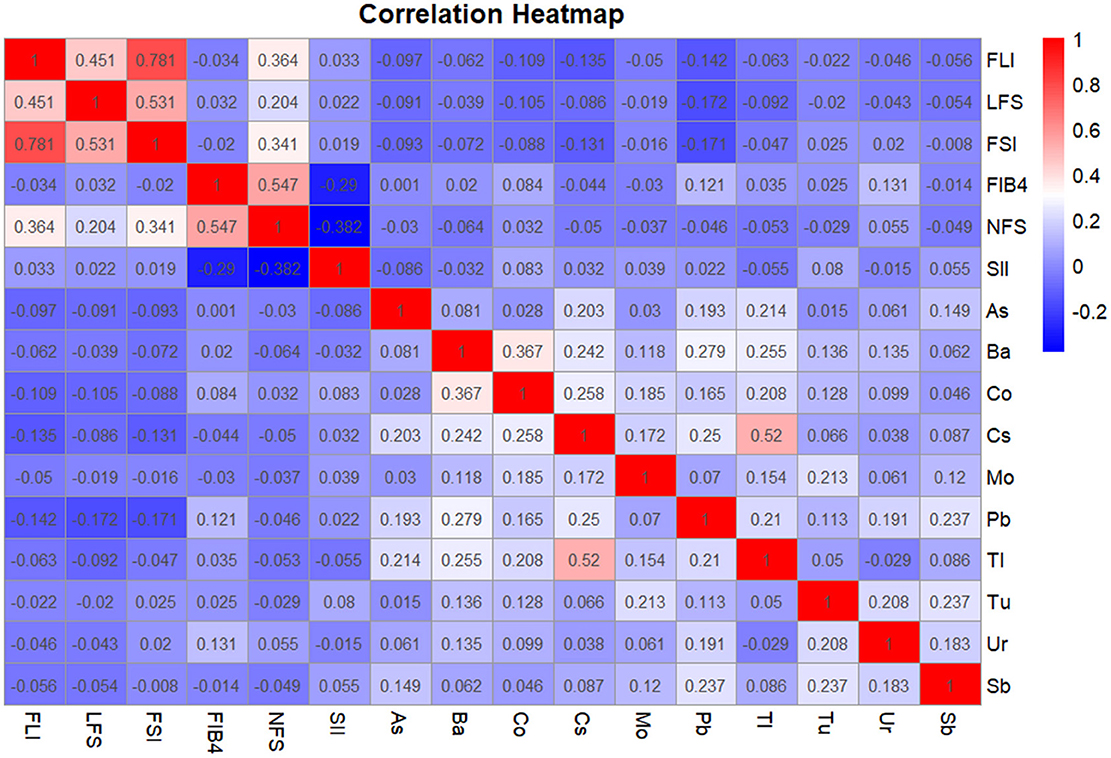
Figure 2. Partial correlation analysis of heavy metals, SII, and NAFLD correlation index. The covariates included sex, age after grouping, race, marriage, education years, smoking, Alcohol drinking, and PIR. The correlation coefficients are shown in color. Blue is negatively correlated red is a positive correlation. The darker the color, the stronger the correlation. When conducting the overall analysis of heavy metals, logarithmic transformed values are all adopted.
Associations of FIB-4, NFS, and SII with heavy metals by GLM models
Since SII showed no significant correlation with hepatic steatosis indices (FLI, LFS, FSI), further analysis was performed only on hepatic fibrosis indices (FIB-4, NFS). The results were illustrated in Figure 3. Analyzing each heavy metal's overall association with SII revealed that As (OR = 0.96, 95% CI = [0.95–0.97], P < 0.001) and Cs (OR = 0.97, 95% CI = [0.94, 1.00], P = 0.039) showed significant negative correlations, while Sb (OR = 1.02, 95% CI = [1.00, 1.05], P = 0.016) exhibited a positive correlation. When taking Q1 as the reference and comparing the correlations between Q2, Q3, Q4 and SII, As (Q4: OR = 0.95, 95% CI = [0.94–0.97], P < 0.001) and Co (Q2: OR = 0.98, 95% CI = [0.96–0.99], P = 0.011; Q3: OR = 0.98, 95% CI = [0.96–0.99], P = 0.009). The negative correlation with SII was highlighted (P < 0.0125). This may be related to the fact that arsenic-induced oxidative stress can damage immune cells and promote the proliferation of T lymphocytes, thereby reducing SII (46).
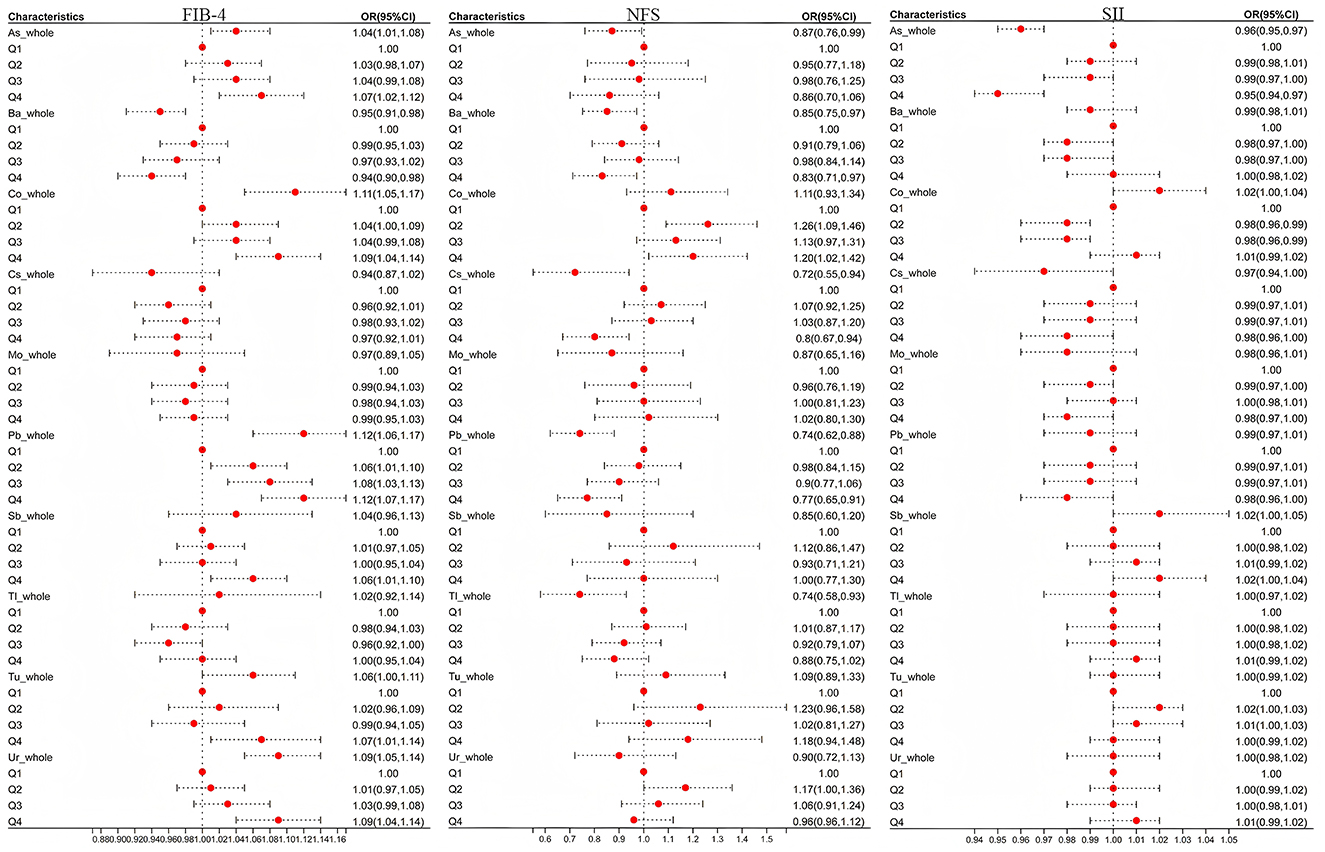
Figure 3. The influence of heavy metal exposure on FIB-4,NFS, and SII. OR was the odds ratio calculated by weighted linear regression after adjusting for age, sex, race, education years, marry, PIR, smoke, and drink. When conducting the overall analysis of heavy metals, logarithmic transformed values are all adopted, When comparing the correlation between the overall level and hepatic fibrotic markers, P < 0.05 was taken as the criterion; Taking Q1 as the reference, when comparing the associations of Q2, Q3, and Q4 with hepatic fibrous markers, correction was made according to Bonferroni, with P < 0.0125(0.05/4) as the standard.
Subsequent focus on As, Co, Cs, and Sb in FIB-4/NFS analyses demonstrated that at overall levels, As (OR = 1.04, 95% CI = [1.01, 1.08], P = 0.012) and Co (OR = 1.11, 95% CI = [1.05, 1.17], P < 0.001) were positively associated with FIB-4. Quartile regression maintained these positive associations (PAs_Q4 = 0.002, PCo_Q4 <0.001). Conversely, in NFS analyses, As and Cs showed negative correlations at overall levels, while quartile analysis revealed Co's positive association (PCo_Q2 = 0.002 <0.0125) and Cs's persistent negative correlation (PCs_Q4 = 0.007 <0.0125) with NFS.
WQS regression model to assess the association of heavy metal co-exposure with FIB-4, NFS, and SII
After exposure to heavy metals, it often showed the combined effect of multiple metals rather than a single metal acting alone. After adjusting for covariates, the combined effect of heavy metals was significantly positively correlated with FIB-4 and SII (PFIB − 4 <0.001, PSII <0.001), but not correlated with NFS (Table 2).
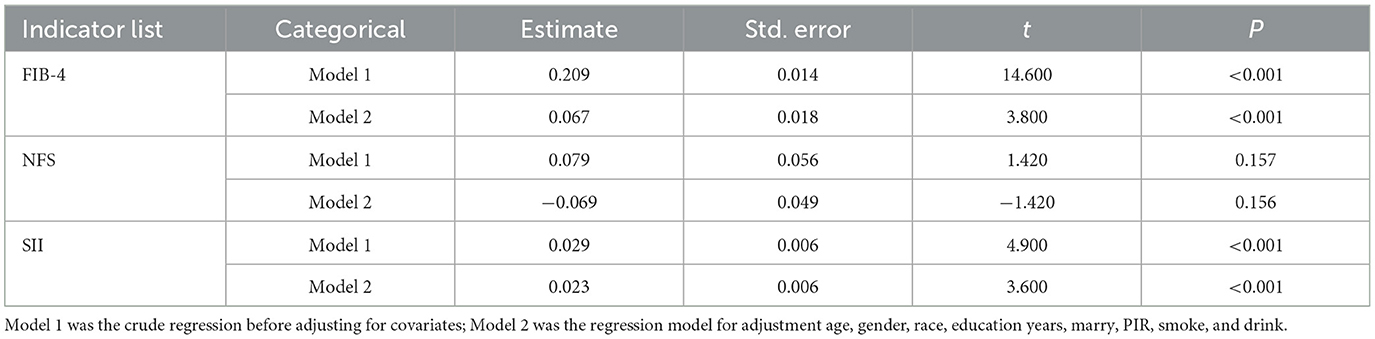
Table 2. The association between WQS index of combined heavy metal exposure and FIB-4, NFS, and SII.
In Figure 4, the weights of each metal in the combined exposure were presented. The results showed that Co has the greatest impact on FIB-4, NFS, and SII.
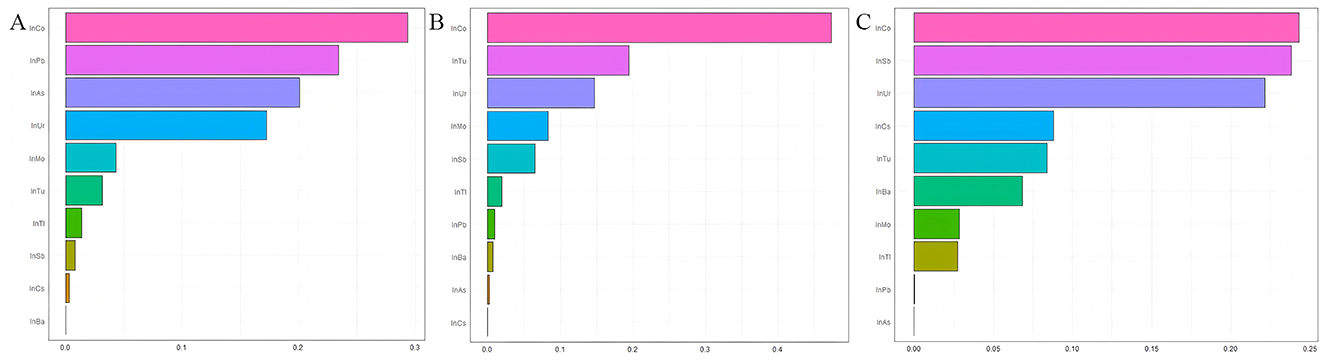
Figure 4. Estimated weights assigned to each exposure based on WQS regression modeled in the positive direction with respect to the FIB-4, NFS, and SII. (A) Estimated weights assigned for FIB-4; (B) Estimated weights assigned for FIB-4; (C) Estimated weights assigned for FIB-4; Models were adjusted for age, gender, race, education years, marry, PIR, smoke and drink.
Restricted Cubic Spline analysis was conducted to explore the relationship between As, Co, Ur, and FIB-4, NFS
Based on the results of weighted linear regression and WQS, the associations of As, Co, and Cs with FIB-4 and NFS were screened out. Further RCS analysis was conducted, and the results was shown in the Figure 5. The overall analysis showed that there was a statistically significant association between As and FIB-4, P = 0.011, and the non-linear relationship holds (P = 0.146 > 0.05), indicating that high-concentration exposure to As was a risk factor for hepatic fibrosis.
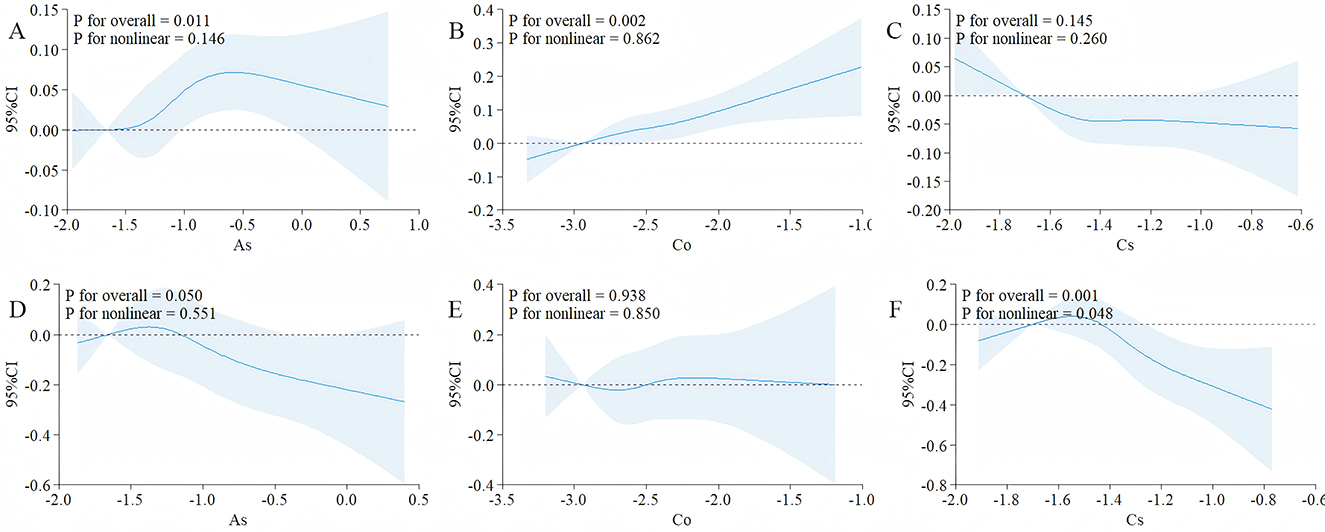
Figure 5. After adjusting for covariates, the correlations between As, Co and Cs with FIB-4 and NFS were evaluated using RCS. (A) The correlations between As and FIB-4; (B) the correlations between Co and FIB-4; (C) the correlations between Cs and FIB-4; (D) the correlations between As and NFS; (E) the correlations between Co and NFS; (F) the correlations between Cs and NFS; The covariates included sex, age after grouping, race, marriage, education years, smoking, Alcohol drinking; The solid black lines correspond to the central estimates, and the gray-shaded regions indicate the 95% confidence intervals; When conducting the overall analysis of heavy metals, logarithmic transformed values are all adopted.
A significant association was observed between cobalt (Co) and the FIB-4 index (Poverall = 0.002), with the relationship best described by a linear model (Pnon − linear = 0.862). Similarly, cesium (Cs) showed a significant association with the NFS score (Poverall = 0.001), and the relationship also followed a linear trend (Pnon − linear = 0.048).
The mediation analysis of SII on the relationship between As, Co Cs, and FIB-4, NFS
The result of the mediating effect is presented in Figure 6. After adjusting for confounding factors, SII showed a mediating role in the associations among As, Co, Cs, and FIB-4, and the β-values of its mediating effect were 0.0220 (95% CI: 0.0119, 0.0300) and −0.0430 (95% CI:), respectively. −0.0617, −0.0200) and −0.0349 (95% CI: −0.0612, −0.0100) (Figures 6A–C). The mediating effect ratios were 86.73% and 34.11%, respectively, indicating that SII successively explained 86.73% and 34.11% of the effects of As and Cs on fibrosis. Meanwhile, SII inhibited the positive effect of Co on FIB-4 (49.46%) and NFS (40.54%). If SII was ignored, the effect of Co would be underestimated.
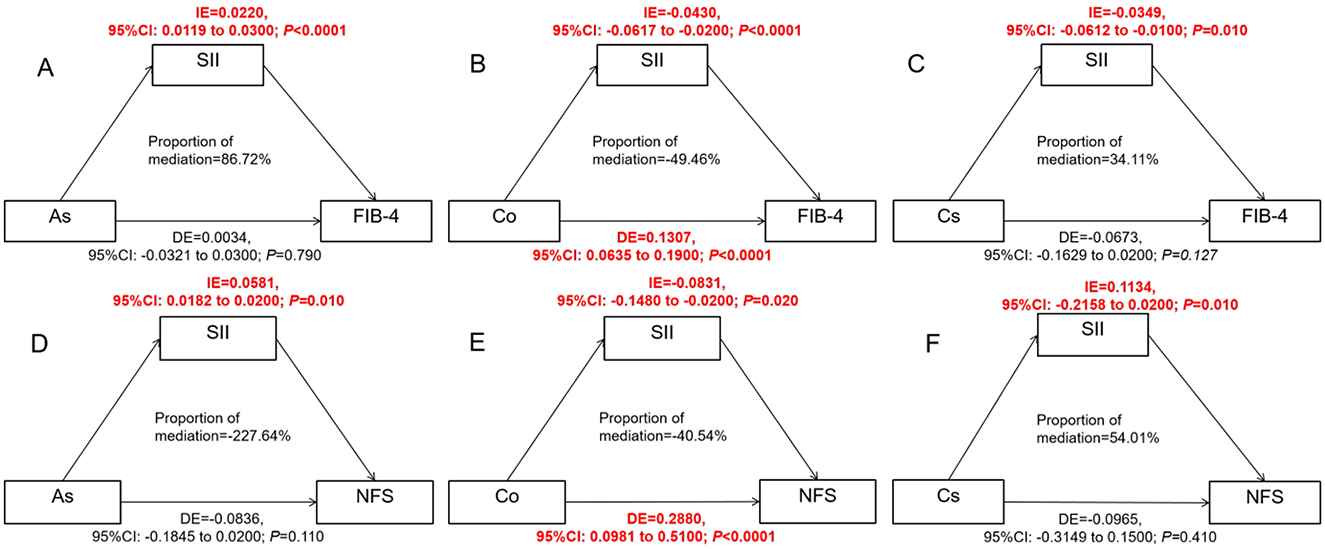
Figure 6. Estimate the association ratio among SII-mediated As, Co, Cs, and FIB-4, NFS. (A–C) were for analyzing the effects of As, Co and Cs on FIB-4 respectively; (D–F) were to analyze the influences of As, Co and Cs on NFS respectively. The model has been adjusted according to age, gender, race, education years, marry, PIR, smoke and drink; SII, As, Cs, and Co have all undergone logarithmic transformation; The mediation effect is calculated using the “Mediation” package. When conducting the overall analysis of heavy metals, logarithmic transformed values are all adopted.
After covariate adjustment, SII mediated the associations between As, Co, Cs and NFS, and the β values of its mediating effect were 0.0581 (95% CI: 0.0182, 0.0200) and −0.0831 (95% CI: −0.1480, −0.0200) respectively and 0.1134 (95% CI: −0.2158, 0.0200) (Figures 6D–F).
Discussion
To our knowledge, this is the first study to evaluate the mediating role of SII in heavy metal-induced non-alcoholic hepatic injury. With global industrialization, toxic pollutants (e.g., heavy metals) are increasingly released into the environment, accumulating in air, soil, drinking water, and food, ultimately leading to human exposure and tissue damage (47, 48). Existing epidemiological and experimental studies support the role of toxic metal exposure in NAFLD (49), primarily through mechanisms such as oxidative stress, endoplasmic reticulum (ER) stress, pyroptosis, ferroptosis, and dysregulated autophagy (50).
In this study involving 5,613 participants, SII was mainly significantly negative associated with the indicators FIB-4 and NFS representing hepatic fibrosis, rather than hepatocellular steatosis, and although As (arsenic) and Co reduced SII, they ultimately promoted the formation of hepatic fibrosis. Cs was shown to inhibit the occurrence of hepatic fibrosis by reducing SII.
Regarding the association between SII and hepatic fibrosis, the current research results are not completely consistent. This might be caused by the differences in the selected research population. Ma et al. found that a higher SII had a higher positive correlation with the risk of FIB-4 [OR (95% CI): 5.69 (2.17–14.90), p < 0.001] (51). However, an analysis of adults with NHANES published in 2022 did not find an association between SII and hepatic fibrosis (52). The results published in 2024 showed that SII was significantly negatively correlated with hepatic fibrosis in the entire population and the diabetic population (53, 54), and the conclusion of this study is consistent with this. The inconsistent research results may, on the one hand, stem from the included population. In this study, the focus was on the population exposed to heavy metals. Heavy metal exposure causes apoptosis of immune cells through pathways such as oxidative stress (55) and DeoxyriboNucleic Acid (DNA) methylation (56), thereby reducing the level of SII. On the other hand, with the increase of heavy metal exposure, the disease progresses to the stage of hepatic fibrosis, and the blood content in the body significantly decreases, mainly lymphocytes, thereby significantly reducing the SII level and presenting a negative correlation phenomenon (53).
In this study, As (arsenic) was negatively correlated with SII, that is, higher As levels were associated with lower SII values. The weighted group regression analysis for FIB-4 indicated that low-dose As did not induce hepatic fibrosis. However, when the body was exposed to high-dose As, hepatic fibrosis became obvious, and SII played a major positive mediating role in this process. This indicates that low doses of arsenic are harmless to physical health, while high concentrations of arsenic exposure cause hepatic damage. Based on the conclusion that low doses was harmless, arsenic preparations (As) could be used in disease treatment, such as the treatment of acute myeloid leukemia (57). This phenomenon could be attributed to the reduced expression of arsenic transporter aquaporin-9 (AQP9), which led to a decrease in intracellular arsenic accumulation and reduced the sensitivity of these cells to arsenic trioxide (ATO) treatment (58). These findings were consistent with the anti-tumor and immunomodulatory effects of ATO in autoimmune diseases, and excessive use of ATO could lead to hepatotoxicity, nephrotoxicity and cardiotoxicity (59).
In 2017, Gao et al. discovered that ATO reduces the percentage of myeloid-derived suppressor cells (MDSCs) in the spleen, weakening their immunosuppressive effect on T cells and thereby promoting T-cell proliferation and immunoregulation (60). Additionally, As suppresses bone marrow hematopoiesis by reducing GATA-2 DNA-binding activity, inhibiting the proliferation and differentiation of neutrophil precursor cells (61). In summary, arsenic exposure promotes neutrophil apoptosis while enhancing lymphocyte proliferation, ultimately leading to the negative correlation between As and SII. When As accumulates beyond a certain threshold, it decreases the activity of antioxidant enzymes, significantly increasing oxidative stress, inflammation, DNA damage, and apoptosis, contributing to the development of various diseases, including hepatic injury (62, 63).
Cobalt(Co) was also a relatively important heavy metal in this study, due to its dual characteristics. It is not only an indispensable trace element in the human body, but also can cause damage to the body when excessive. Co has become increasingly prevalent in daily life with the development of new electric vehicles, as a cathode material for batteries (64). In this study, Co was identified through WQS (weighted quantile sum) analysis. Correlation analysis revealed significant positive associations between Co and both FIB-4 and SII. Further mediation analysis demonstrated that SII exerted a partial negative mediating effect in the relationship between Co and FIB-4/NFS. Co can induce inflammatory responses in vivo through oxidative stress. Additionally, it promotes a HIF-1α-dependent metabolic shift from oxidative phosphorylation to glycolysis in macrophages, which plays a crucial role in activating inflammatory responses (65), thereby disrupting normal immune function (66). High concentrations of Co may lead to excessive inflammatory responses, endocrine disruption, adverse developmental effects, and even mortality (67), findings that are consistent with the results of this study (68).
Initially, cesium (Cs) attracted attention due to its radioactive properties (69). Recent studies had demonstrated that its isotope, cesium-131 (131Cs), could be encapsulated in seeds or microspheres and implanted into tumors (e.g., prostate cancer), delivering localized high-dose radiation while minimizing damage to surrounding healthy tissues, thus enabling its application in treating brain, prostate, and head and neck cancers (70–72). However, research on the association between Cs and hepatic fibrosis remains limited. In 2020, Ziasmin Khatun et al. co-cultured NIH/3T3 mouse fibroblasts with metal chlorides (Li, Na, K, Rb, and Cs) and found that Cs suppressed fibroblast proliferation and migration (73). The cellular effects of Cs might be related to intracellular metabolism. Given its similar ionic radius to potassium (K+), Cs could permeate potassium channels—sometimes even with higher selectivity than K+ itself (74), which potentially disrupting normal cellular metabolism by interfering with potassium uptake (75, 76). In our study, Cs exhibited a negative correlation with both the systemic immune-inflammation index (SII) and hepatic fibrosis. Notably, SII demonstrated a positive mediating effect in their association, which aligns with the aforementioned findings. These results suggested that Cs may influence fibrotic processes through immunometabolic pathways, warranting further investigation into its mechanistic role in hepatic fibrosis.
This study possesses several notable strengths. First, the data were derived from the National Health and Nutrition Examination Survey (NHANES) conducted by the Centers for Disease Control and Prevention (CDC), representing the highest quality of experimental results. Second, to our knowledge, this is the first study to comprehensively analyze the associations between heavy metal exposure and multiple NAFLD-related indicators of hepatic steatosis (including FLI, LFS, and FSI) as well as hepatic fibrosis markers (FIB-4 and NFS). Furthermore, we employed mediation analysis to evaluate the potential mediating role of SII in these relationships. However, several limitations should be acknowledged. The cross-sectional design of our study precludes the establishment of causal relationships between the examined variables. Future longitudinal studies are warranted to validate these observations and elucidate the underlying mechanisms.
Conclusions
In this study, there was a significant correlation of As and Co between heavy metals and hepatic fibrosis indicators FIB-4 and NFS, and SII played a mediating role in this association. Cs was significantly negatively correlated with SII and hepatic fibrosis indicators, and SII played a positive mediating role in this association.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
This study used publicly available data from NHANES. CDC/NCHS Ethics Review Board (ERB) approved the NHANES study and gave approval for public communication. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
NW: Data curation, Formal analysis, Software, Writing – original draft, Writing – review & editing. XL: Methodology, Supervision, Writing – original draft. RH: Data curation, Formal analysis, Writing – original draft. XZ: Software, Writing – original draft. ML: Data curation, Software, Writing – original draft. SN: Formal analysis, Writing – review & editing. KW: Conceptualization, Funding acquisition, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by grants from the National Natural Science Foundation of China (No. 81773367).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Bawa-Allah KA. Assessment of heavy metal pollution in Nigerian surface freshwaters and sediment: a meta-analysis using ecological and human health risk indices. J Contam Hydrol. (2023) 256:104199. doi: 10.1016/j.jconhyd.2023.104199
2. Jiménez-Oyola S, Valverde-Armas PE, Romero-Crespo P, Capa D, Valdivieso A, Coronel-León J, et al. Heavy metal(loid)s contamination in water and sediments in a mining area in Ecuador: a comprehensive assessment for drinking water quality and human health risk. Environ Geochem Health. (2023) 45:4929–49. doi: 10.1007/s10653-023-01546-3
3. Xiang L, Jiang S, Cheng H, Wang Y, Liu Q, Yin Y, et al. Heavy metal concentration profiles and pollution assessment in the Jiangsu offshore area, China. Mar Pollut Bull. (2023) 193:115187. doi: 10.1016/j.marpolbul.2023.115187
4. Kay ML, Jasiak I, Klemt WH, Wiklund JA, Faber JA, MacDonald LA, et al. Paleolimnological evaluation of metal(loid) enrichment from oil sands and gold mining operations in northwestern Canada. Environ Res. (2023) 216:114439. doi: 10.1016/j.envres.2022.114439
5. Darham S, Zakaria NN, Zulkharnain A, Sabri S, Khalil KA, Merican F, et al. Antarctic heavy metal pollution and remediation efforts: state of the art of research and scientific publications. Braz J Microbiol. (2023) 54:2011–26. doi: 10.1007/s42770-023-00949-9
6. Dutta S, Gorain B, Choudhury H, Roychoudhury S, Sengupta P. Environmental and occupational exposure of metals and female reproductive health. Environ Sci Pollut Res Int. (2022) 29:62067–92. doi: 10.1007/s11356-021-16581-9
7. Navarrete-Meneses MDP, Salas-Labadía C, Gómez-Chávez F, Pérez-Vera P. Environmental Pollution and Risk of Childhood Cancer: a Scoping Review of Evidence from the Last Decade. Int J Mol Sci. (2024) 25:3284. doi: 10.3390/ijms25063284
8. Teschke R, Xuan TD. Heavy metals, halogenated hydrocarbons, phthalates, glyphosate, cordycepin, alcohol, drugs, and herbs, assessed for liver injury and mechanistic steps. Front Biosci (Landmark Ed). (2022) 27:314. doi: 10.31083/j.fbl2711314
9. Ren C, Ren L, Yan J, Bai Z, Zhang L, Zhang H, et al. Cadmium causes hepatopathy by changing the status of DNA methylation in the metabolic pathway. Toxicol Lett. (2021) 340:101–13. doi: 10.1016/j.toxlet.2020.12.009
10. Ma G, Yan X, Wang C, Ran X, Liang Z, Chen X, et al. Mechanism of arsenic-induced liver injury in rats revealed by metabolomics and ionomics based approach. Ecotoxicol Environ Saf. (2025) 293:118038. doi: 10.1016/j.ecoenv.2025.118038
11. Porru S, Esplugues A, Llop S, Delgado-Saborit JM. The effects of heavy metal exposure on brain and gut microbiota: a systematic review of animal studies. Environ Pollut. (2024) 348:123732. doi: 10.1016/j.envpol.2024.123732
12. Jomova K, Alomar SY, Nepovimova E, Kuca K, Valko M. Heavy metals: toxicity and human health effects. Arch Toxicol. (2025) 99:153–209. doi: 10.1007/s00204-024-03903-2
13. Hama Aziz KH, Mustafa FS, Omer KM, Hama S, Hamarawf RF, Rahman KO. Heavy metal pollution in the aquatic environment: efficient and low-cost removal approaches to eliminate their toxicity: a review. RSC Adv. (2023) 13:17595–610. doi: 10.1039/D3RA00723E
14. Yin G, Xin M, Zhao S, Zhao M, Xu J, Chen X, et al. Heavy metals and elderly kidney health: a multidimensional study through Enviro-target Mendelian Randomization. Ecotoxicol Environ Saf. (2024) 281:116659. doi: 10.1016/j.ecoenv.2024.116659
15. Smyth D, Kramarz C, Carr AS, Rossor AM, Lunn MP. Toxic neuropathies: a practical approach. Pract Neurol. (2023) 23:120–30. doi: 10.1136/pn-2022-003444
16. Speer RM, Zhou X, Volk LB, Liu KJ, Hudson LG. Arsenic and cancer: evidence and mechanisms. Adv Pharmacol. (2023) 96:151–202. doi: 10.1016/bs.apha.2022.08.001
17. Yang YL, Wu CH, Hsu PF, Chen SC, Huang SS, Chan WL, et al. (2020). Systemic immune-inflammation index (SII) predicted clinical outcome in patients with coronary artery disease. Eur J Clin Invest. 50:e13230. doi: 10.1111/eci.13230
18. Candemir M, Kiziltunç E, Nurkoç S, Sahinarslan A. Relationship Between Systemic Immune-Inflammation Index (SII) and the severity of stable coronary artery disease. Angiology. (2021) 72:757–581. doi: 10.1177/0003319720987743
19. Yang X, Wu C. Systemic immune inflammation index and gastric cancer prognosis: a systematic review and meta-analysis. Exp Ther Med. (2024) 27:122. doi: 10.3892/etm.2024.12410
20. Uzunoglu H, Kaya S. Does systemic immune inflammation index have predictive value in gastric cancer prognosis? North Clin Istanb. (2023) 10:24–32. doi: 10.14744/nci.2021.71324
21. Ye C, Yuan L, Wu K, Shen B, Zhu C. Association between systemic immune-inflammation index and chronic obstructive pulmonary disease: a population-based study. BMC Pulm Med. (2023) 23:295. doi: 10.1186/s12890-023-02583-5
22. Li C, Wu J, Jiang L, Zhang L, Huang J, Tian Y, et al. The predictive value of inflammatory biomarkers for major pathological response in non-small cell lung cancer patients receiving neoadjuvant chemoimmunotherapy and its association with the immune-related tumor microenvironment: a multi-center study. Cancer Immunol Immunother. (2023) 72:783–94. doi: 10.1007/s00262-022-03262-w
23. Jiang R, Li P, Shen W, Deng H, Qin C, Qiu X, et al. The predictive value of the preoperative systemic immune-inflammation index in the occurrence of postoperative pneumonia in non-small cell lung cancer: a retrospective study based on 1486 cases. Thorac Cancer. (2023) 14:30–5. doi: 10.1111/1759-7714.14691
24. Liu K, Tang S, Liu C, Ma J, Cao X, Yang X, et al. Systemic immune-inflammatory biomarkers (SII, NLR, PLR and LMR) linked to non-alcoholic fatty liver disease risk. Front Immunol. (2024) 15:1337241. doi: 10.3389/fimmu.2024.1337241
25. Wang G, Zhao Y, Li Z, Li D, Zhao F, Hao J, et al. Association between novel inflammatory markers and non-alcoholic fatty liver disease: a cross-sectional study. Eur J Gastroenterol Hepatol. (2024) 36:203–9. doi: 10.1097/MEG.0000000000002686
26. Duan X, Xu G, Li J, Yan N, Li X, Liu X, et al. Arsenic Induces Continuous Inflammation and Regulates Th1/Th2/Th17/Treg Balance in Liver and Kidney In Vivo. Mediators Inflamm. (2022) 2022:8414047. doi: 10.1155/2022/8414047
27. Zhang J, Wang Y, Fu L, Wang B, Ji YL, Wang H, et al. Chronic cadmium exposure induced hepatic cellular stress and inflammation in aged female mice. J Appl Toxicol. (2019) 39:498–509. doi: 10.1002/jat.3742
28. Peiseler M, Schwabe R, Hampe J, Kubes P, Heikenwälder M, Tacke F. Immune mechanisms linking metabolic injury to inflammation and fibrosis in fatty liver disease - novel insights into cellular communication circuits. J Hepatol. (2022) 77:1136–60. doi: 10.1016/j.jhep.2022.06.012
29. Ito H, Kimura T, Takuro S, Higashitani M, Yamamoto K, Kobayashi D. Liver injury indicators and subsequent cancer development among non-fatty liver population. Cancer Med. (2023) 12:12173–86. doi: 10.1002/cam4.5910
30. Thomson ES, Oommen AT, Pillai G, Oomen AT, Sheejamol VS. Comparison of non-invasive liver fat scoring systems as markers of metabolic dysfunction-associated liver disease. Cureus. (2024) 16:e72222 doi: 10.7759/cureus.72222
31. Polyzos SA, Goulis DG, Kountouras J, Mintziori G, Chatzis P, Papadakis E, et al. Non-alcoholic fatty liver disease in women with polycystic ovary syndrome: assessment of non-invasive indices predicting hepatic steatosis and fibrosis. Hormones (Athens). (2014) 13:519–31. doi: 10.14310/horm.2002.1493
32. Di Stasi V, Maseroli E, Rastrelli G, Scavello I, Cipriani S, Todisco T, et al. SHBG as a Marker of NAFLD and Metabolic Impairments in Women Referred for Oligomenorrhea and/or Hirsutism and in Women With Sexual Dysfunction. Front Endocrinol. (2021) 12:641446. doi: 10.3389/fendo.2021.641446
34. American Diabetes Association. Diagnosis and classification of diabetes: standards of care in diabetes-2024. Diabetes Care. (2024) 47:S20–S42. doi: 10.2337/dc24-S002
35. European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO) (2016). EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. 64:1388–402. doi: 10.1016/j.jhep.2015.11.004
36. Lee J, Vali Y, Boursier J, Spijker R, Anstee QM, Bossuyt PM, et al. Prognostic accuracy of FIB-4, NAFLD fibrosis score and APRI for NAFLD-related events: a systematic review. Liver Int. (2021) 41:261–70. doi: 10.1111/liv.14669
37. Tang C, Wang Y, Hong H. Unraveling the link between heavy metals, perfluoroalkyl substances and depression: insights from epidemiological and bioinformatics strategies. Ecotoxicol Environ Saf . (2024) 279:116482. doi: 10.1016/j.ecoenv.2024.116482
38. Chen S, Xiao J, Cai W, Lu X, Liu C, Dong Y, et al. Association of the systemic immune-inflammation index with anemia: a population-based study. Front Immunol. (2024) 15:1391573. doi: 10.3389/fimmu.2024.1391573
39. Guo J, Xu R, Liu R, Lai W, Hu C, He H, et al. Association between the systemic immune inflammation index and periodontitis: a cross-sectional study. J Transl Med. (2024) 22:96. doi: 10.1186/s12967-024-04888-3
40. Salazar-Valdivia FE, Valdez-Cornejo VA, Ulloque-Badaracco JR, Hernandez-Bustamante EA, Alarcón-Braga EA, Mosquera-Rojas MD, et al. Systemic immune-inflammation index and mortality in testicular cancer: a systematic review and meta-analysis. Diagnostics. (2023) 13:843. doi: 10.3390/diagnostics13050843
41. Meng L, Yang Y, Hu X, Zhang R, Li X. Prognostic value of the pretreatment systemic immune-inflammation index in patients with prostate cancer: a systematic review and meta-analysis. J Transl Med. (2023) 21:79. doi: 10.1186/s12967-023-03924-y
42. Zhao Z, Zhang X, Sun T, Huang X, Ma M, Yang S, et al. Prognostic value of systemic immune-inflammation index in CAD patients: systematic review and meta-analyses. Eur J Clin Invest. (2024) 54:e14100. doi: 10.1111/eci.14100
43. Karimi A, Shobeiri P, Kulasinghe A, Rezaei N. Novel systemic inflammation markers to predict COVID-19 prognosis. Front Immunol. (2021) 12:741061. doi: 10.3389/fimmu.2021.741061
44. Ma N, Bansal MB, Chu J, Woodward M, Branch AD. Heavy metals are liver fibrosis risk factors in people without traditional liver disease etiologies. J Environ Sci. (2025) 155:329–42. doi: 10.1016/j.jes.2024.08.027
45. Tan Y, Fu Y, Yao H, Wu X, Yang Z, Zeng H, et al. Relationship between phthalates exposures and hyperuricemia in U.S. general population, a multi-cycle study of NHANES 2007-2016. Sci Total Environ. (2023) 859:160208. doi: 10.1016/j.scitotenv.2022.160208
46. Lee CH, Liao WT, Yu HS. Aberrant immune responses in arsenical skin cancers. Kaohsiung J Med Sci. (2011) 29:396–401. doi: 10.1016/j.kjms.2011.05.007
47. Clemens S, Ma JF. Toxic heavy metal and metalloid accumulation in crop plants and foods. Annu Rev Plant Biol. (2016) 67:489–512. doi: 10.1146/annurev-arplant-043015-112301
48. Chen L, Zhao Y, Liu F, Chen H, Tan T, Yao P, et al. Biological aging mediates the associations between urinary metals and osteoarthritis among U.S. adults. BMC Med. (2022) 20:207. doi: 10.1186/s12916-022-02403-3
49. Renu K, Chakraborty R, Myakala H, Koti R, Famurewa AC, Madhyastha H, et al. Molecular mechanism of heavy metals (Lead, Chromium, Arsenic, Mercury, Nickel and Cadmium) - induced hepatotoxicity—a review. Chemosphere. (2021) 271:129735. doi: 10.1016/j.chemosphere.2021.129735
50. Tinkov AA, Aschner M, Santamaria A, Bogdanov AR, Tizabi Y, Virgolini MB, et al. Dissecting the role of cadmium, lead, arsenic, and mercury in non-alcoholic fatty liver disease and non-alcoholic steatohepatitis. Environ Res. (2023) 238:117134. doi: 10.1016/j.envres.2023.117134
51. Ma Y, Wang J, Du L, Tang H. Association between the systemic immune-inflammation index and the outcome of liver fibrosis in patients with chronic hepatitis C. Front Med. (2024) 11:1486503. doi: 10.3389/fmed.2024.1486503
52. Xie R, Xiao M, Li L, Ma N, Liu M, Huang X, et al. Association between SII and hepatic steatosis and liver fibrosis: a population-based study. Front Immunol. (2022) 13:925690. doi: 10.3389/fimmu.2022.925690
53. Duan S, Tu Z, Duan L, Tu R. Differential effects of systemic immune inflammation indices on hepatic steatosis and hepatic fibrosis: evidence from NHANES 1999-2018. BMC Gastroenterol. (2024) 24:463. doi: 10.1186/s12876-024-03557-5
54. Chen G, Fan L, Yang T, Xu T, Wang Z, Wang Y, et al. Prognostic nutritional index (PNI) and risk of non-alcoholic fatty liver disease and advanced liver fibrosis in US adults: evidence from NHANES 2017-2020. Heliyon. (2024) 10:e25660. doi: 10.1016/j.heliyon.2024.e25660
55. Jomova K, Jenisova Z, Feszterova M, Baros S, Liska J, Hudecova D, et al. Arsenic: toxicity, oxidative stress and human disease. J Appl Toxicol. (2011) 31:95–107. doi: 10.1002/jat.1649
56. Reichard JF, Puga A. Effects of arsenic exposure on DNA methylation and epigenetic gene regulation. Epigenomics. (2010) 2:87–104. doi: 10.2217/epi.09.45
57. Ma YF, Lu Y, Wu Q, Lou YJ, Yang M, Xu JY, et al. Oral arsenic and retinoic acid for high-risk acute promyelocytic leukemia. J Hematol Oncol. (2022) 15:148. doi: 10.1186/s13045-022-01368-3
58. Chau D, Ng K, Chan TS, Cheng YY, Fong B, Tam S, et al. Azacytidine sensitizes acute myeloid leukemia cells to arsenic trioxide by up-regulating the arsenic transporter aquaglyceroporin 9. J Hematol Oncol. (2015) 8:46. doi: 10.1186/s13045-015-0143-3
59. Chêne C, Rongvaux-Gaïda D, Thomas M, Rieger F, Nicco C, Batteux F. Optimal combination of arsenic trioxide and copper ions to prevent autoimmunity in a murine HOCl-induced model of systemic sclerosis. Front Immunol. (2023) 14:1149869. doi: 10.3389/fimmu.2023.1149869
60. Zhong L, Xu F, Chen F. Arsenic trioxide induces the apoptosis and decreases NF-κB expression in lymphoma cell lines. Oncol Lett. (2018) 16:6267–74. doi: 10.3892/ol.2018.9424
61. Medina S, Zhang H, Santos-Medina LV, Wan G, Bolt AM, Zhou X, et al. Arsenic impairs the lineage commitment of hematopoietic progenitor cells through the attenuation of GATA-2 DNA binding activity. Toxicol Appl Pharmacol. (2022) 452:116193. doi: 10.1016/j.taap.2022.116193
62. Unsal V, Cicek M, Aktepe N, Oner E. Morin attenuates arsenic-induced toxicity in 3T3 embryonic fibroblast cells by suppressing oxidative stress, inflammation, and apoptosis: in vitro and silico evaluations. Toxicol Res. (2024) 13:tfae113. doi: 10.1093/toxres/tfae113
63. Li, Z, Li, H, Wang, D, Peng, X, Syed, B. M, Liu, Q. S-glutathionylation in hepatocytes is involved in arsenic-induced liver fibrosis through activation of the NLRP3 inflammasome, an effect alleviated by NAC. Sci Total Environ. (2024) 947:174534. doi: 10.1016/j.scitotenv.2024.174534
64. Annu, Park SS, Alam MN, Yewale M, Shin DK. Unraveling the electrochemical insights of cobalt oxide/conducting polymer hybrid materials for supercapacitor, battery, and supercapattery applications. Polymers. (2024) 16:2907. doi: 10.3390/polym16202907
65. Salloum Z, Lehoux EA, Harper ME, Catelas I. Effects of cobalt and chromium ions on glycolytic flux and the stabilization of hypoxia-inducible factor-1α in macrophages in vitro. J Orthop Res. (2021) 39:112–20. doi: 10.1002/jor.24758
66. Yan N, Zhou H, Jin P, Li T, Liu Q, Ning H, et al. A multifunctional cobalt-containing implant for treating biofilm infections and promoting osteointegration in infected bone defects through macrophage-mediated immunomodulation. Adv Sci. (2025) 12:e2409200. doi: 10.1002/advs.202409200
67. Liu G, Wang X, Zhou X, Zhang L, Mi J, Shan Z, et al. Modulating the cobalt dose range to manipulate multisystem cooperation in bone environment: a strategy to resolve the controversies about cobalt use for orthopedic applications. Theranostics. (2020) 10:1074–89. doi: 10.7150/thno.37931
68. Deng Y, Li G, Xie L, Li X, Wu Y, Zheng J, et al. Associations of occupational exposure to micro-LiNiCoMnO2 particles with systemic inflammation and cardiac dysfunction in cathode material production for lithium batteries. Environ Pollut. (2024) 359:124694. doi: 10.1016/j.envpol.2024.124694
69. Qin YC, Tang LY, Su Y, Chen LJ, Su FX, Lin Y, et al. Association of urinary cesium with breast cancer risk. Asian Pac J Cancer Prev. (2014) 15:9785–90. doi: 10.7314/APJCP.2014.15.22.9785
70. Luginbuhl A, Calder A, Kutler D, Zender C, Wise-Draper T, Patel J, et al. Multi-institutional study validates safety of intraoperative cesium-131 brachytherapy for treatment of recurrent head and neck cancer. Front Oncol. (2021) 11:786216. doi: 10.3389/fonc.2021.786216
71. Greenwald J, Taube S, Yondorf MZ, Smith A, Sabbas A, Wernicke AG. Placement of 131Cs permanent brachytherapy seeds in a large combined cavity of two resected brain metastases in one setting: case report and technical note. J Contemp Brachytherapy. (2019) 11:356–60. doi: 10.5114/jcb.2019.87230
72. Agarwal A, Pinto J, Renslo B, Bar-Ad V, Taleei R, Luginbuhl A. Feasibility of collagen matrix tiles with cesium-131 brachytherapy for use in the treatment of head and neck cancer. Brachytherapy. (2023) 22:120–4. doi: 10.1016/j.brachy.2022.09.160
73. Khatun Z, Nishimura N, Kobayashi D, Hazama A. Cesium suppresses fibroblast proliferation and migration. Fukushima J Med Sci. (2020) 66:97–102. doi: 10.5387/fms.2020-08
74. Lam YL, Zeng W, Sauer DB, Jiang Y. The conserved potassium channel filter can have distinct ion binding profiles: structural analysis of rubidium, cesium, and barium binding in NaK2K. J Gen Physiol. (2014) 144:181–92. doi: 10.1085/jgp.201411191
75. Palmer BF, Clegg DJ. Physiology and pathophysiology of potassium homeostasis. Adv Physiol Educ. (2016) 40:480–90. doi: 10.1152/advan.00121.2016
Keywords: hepatic steatosis, hepatic fibrosis, systemic immune verification index, heavy metal, mediating effect
Citation: Wang N, Li X, He R, Zheng X, Li M, Nian S and Wang K (2025) Mediating role of systemic immune-inflammation index between heavy metal exposure and hepatic steatosis/hepatic fibrosis: evidence from NHANES 2005–2020. Front. Nutr. 12:1566345. doi: 10.3389/fnut.2025.1566345
Received: 27 February 2025; Accepted: 28 April 2025;
Published: 21 May 2025.
Edited by:
Mostafa Gouda, National Research Centre, EgyptReviewed by:
Nam-Eun Kim, Seoul National University, Republic of KoreaYanwu Nie, Nanchang University, China
Copyright © 2025 Wang, Li, He, Zheng, Li, Nian and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kewei Wang, a2V3ZWl3YW5nMDcxOEAxNjMuY29t
 Ningning Wang1,2,3
Ningning Wang1,2,3 Kewei Wang
Kewei Wang