- 1Microbiome and Metabolism Research Unit, USDA-ARS, SEA, Little Rock, AR, United States
- 2Arkansas Children’s Nutrition Center, Little Rock, AR, United States
- 3Department of Agriculture, University of Arkansas at Pine Bluff, Pine Bluff, AR, United States
- 4Texas A&M AgriLife Institute for Advancing Health Through Agriculture, College Station, TX, United States
Fermented foods are a good source of nutrition, with microbiota and metabolites that can positively influence consumer health. With the increasingly negative health outcomes from using low-quality diets like processed diets, functional products like fermented foods are getting more attention than ever. All cultures of the world consume some kind of fermented foods. Extensive literature outlines positive health and clinical outcomes associated with fermented foods, yet most data are associative and lack longitudinal studies. This review explores the role of fermented foods during pregnancy and its subsequent impact on maternal and infant health, especially in the first 1,000 days of life. In this review, we have summarized the literature on fermented foods from preclinical and clinical studies that evaluated the impact of maternal consumption of fermented foods on mothers and offspring microbiota, immune system, and brain health outcomes. We also discussed existing knowledge gaps on maternal-child dyads and mechanistic studies needed to provide better scientific evidence to promote fermented foods consumption.
1 Overview
A broad range (~5,000 varieties) of traditionally produced fermented foods are consumed worldwide (1, 2). Though in recent years there has been a resurgence in the consumption of fermented foods due to proposed health benefits, advantages as a low-tech, resource-efficient processing, and concerns about food waste and environmental impact (3, 4), expansion in processing technologies, industrialization, and commercialization of food production has reduced the consumption of fermented foods overall, especially in Western countries (5). Many types of fermented foods are made and consumed, including dairy, plant, cereal, vegetable-based and alcoholic beverages (2, 6). Though alcoholic beverages fall under the larger umbrella of fermented foods, they are detrimental to maternal health. Given that the objective of our review is to explore the potential beneficial effects of maternal consumption of fermented foods, we exclude alcoholic beverages from our review.
During pregnancy, nutritional needs increase to support the growth and development of the fetus and to improve the tissue reserves and metabolic demands of the mother. These increased needs include energy, macronutrients, and specific micronutrients (7). Fermented foods can offer greater nutritional benefits than their unfermented counterparts as the microorganisms involved can break down complex compounds to produce multiple byproducts, such as vitamins and other micronutrients. For example, fermented grains are nutritionally superior to unfermented grains, as fermentation releases nutrients trapped within plant structures and cells (8). In addition, even after cooking, some nutrients trapped in food may remain inaccessible to the human digestive system (9). This can be countered by fermentation, which can break down the indigestible coatings and cell walls chemically and physically, thereby releasing essential nutrients. Cellulose, hemicellulose, and related polymers are indigestible by humans. During fermentation, enzymes from microorganisms can split complex carbohydrate molecules, breaking them down into more digestible simple sugars and sugar derivatives (10). Moreover, fermented dairy products serve as a source of probiotics, prebiotics, and bioactive compounds and are actively promoted as functional foods for their nutritional and therapeutic values (11). In addition to increasing nutrient accessibility, fermentation can also reduce the presence of antinutrients such as phytic acid, trypsin inhibitors and tannins, enhancing the bioavailability of essential minerals such as iron, protein, and simple sugars (12). Phytic acids can reduce the bioavailability of minerals and significantly impact pregnant women, lactating mothers, and infants, especially when large amounts of cereal-based foods are consumed (11, 13).
David Barker’s assertion that “Much of human development is completed during the first 1,000 days after conception” laid the foundation for a significant shift in our understanding of early life influences on long-term health (14). This concept evolved into the more comprehensive Developmental Origins of Health and Disease (DOHaD) theory (15, 16). “The first 1,000 days” are considered to impact a child’s health and future disease risk. In recent years, there has been a growing focus among policymakers on the critical importance of early childhood development. Specifically, two key periods have garnered significant attention: the “first 1,000 days” and the “0–3 years” age range. These timeframes are increasingly recognized as crucial windows of opportunity for positively shaping a child’s future health outcomes. Maternal nutrition, dietary behavior’s, and environment also play a vital role in shaping the first 1,000 days of life, resulting in “nutritional programming” (17). While micronutrient, mineral, and probiotic supplementation have recently gained increased attention in maternal nutrition, there is immense potential in focusing on traditional nutrient-dense foods to meet these dietary needs during pregnancy without increasing economic and accessibility burdens on the families. The maternal microbiome plays a critical role in modulating the infant gut microbiome, immune system and its subsequent role in the gut-brain axis, and fermented foods inherently act as probiotics. Given the critical role of nutrition during pregnancy, the influence of the maternal microbiome on infant development, and the promising potential of fermented foods to improve health outcomes, this review aims to highlight the existing literature on the role of fermented foods in modulating gut microbiome, immune function, and brain health. Further, this review evaluates the existing evidence and addresses gaps in the gut-immune-brain triad in mother and offspring with respect to maternal fermented foods intake. We also propose that the utilization of fermented foods during pregnancy may increase beneficial health outcomes in both mothers and infants, especially during the first 1,000 days of life.
2 Methodology
2.1 Objectives and review design
We evaluate the current knowledge of fermented food’s impact on maternal and infant health. We relate maternal fermented food intake with alteration in the mother’s and offspring’s gut microbiome and subsequent effects on the infant’s immune functions and brain health. For this review, we searched literature databases using the following terms: “fermented foods/gut microbiota,” “infant microbiome/fermented foods,” “fermented foods/maternal outcomes,” “pregnancy complications/fermented foods,” “infant brain/fermented food,” “fermented foods/immune functions” in “humans,” “mice,” “porcine,” and “primate” in different combinations, focusing on studies published from the year 2000 until December 2024. This comprehensive literature search was conducted across multiple academic databases, including PubMed, Google Scholar, Web of Science, Scopus, Cochrane, and Science Direct. Both observational and experimental study designs were included in this review. Since the first 1,000 days are considered the “Golden Opportunity” (18, 19) to stimulate a child’s development and growth, we limit our inclusions to infant health during the first 1,000 days (<3 years) alone. We also limit our literature search to whole fermented foods, not the individual components within fermented foods, such as metabolites or nutrients. Further, we discussed the existing knowledge gap on the role of fermented foods in maternal-child health. This review addresses the research question, “Can incorporating fermented foods in the maternal diet produce positive health outcomes in mothers and infants?.” With this review, our objective is not to narrate the existing literature in the adult population but to contribute to a better understanding of the areas within maternal fermented food intake and infant gut, immune and brain outcomes where there is a knowledge gap.
2.1.1 Inclusion criteria
Studies published in peer-reviewed journals, cohort studies in humans reporting consumption of traditional, homemade, or commercially available fermented foods during pregnancy using food-frequency questionnaires including 24-h dietary recall and self-reported formats; intervention studies in humans where pregnant women were fed specific fermented foods; clinical studies examining the effects of maternal fermented food consumption on offspring’s health; animal studies involving intervention with specific fermented foods during pregnancy, and studies exploring the outcomes based on the transition from normal diet to maternal fermented food diet.
2.1.2 Exclusion criteria
Studies focusing on fermented foods in addition with any probiotic, micronutrients or mineral supplementation, with exercise, and vaccine as these factors could confound the specific nutritional, immunological and microbial effects of fermented foods, influence biomarkers such as serum insulin affecting gestational diabetes independently of diet, or interfere with the immunomodulatory effects; intervention studies in humans on the effect of fermented foods without any direct relationship to pregnancy or maternal diet; animal studies examining fermented foods outside the context of maternal nutrition or offspring health; longitudinal studies focusing on maternal consumption of fermented foods and health outcomes in children extending beyond 3 years of the child’s age; review articles, opinion pieces, or non-empirical publications; studies with significant methodological flaws or insufficient data reporting.
3 Role of fermented foods in ameliorating pregnancy-related complications
Nutritional inadequacy in the form of malnutrition can lead to pregnancy-related complications such as infertility, gestational diabetes, maternal hypertension, preterm birth, and asthma (20), so it is not surprising that most maternal-related deaths occur in regions and groups facing increased food insecurity (21, 22). With inconclusive and contradictory evidence from supplementation studies during pregnancy (23, 24), the ideal scenario would be to strengthen nutritional intake through foods. Fermented foods, rich in bioavailable nutrients and probiotics, could offer a practical dietary strategy to address these deficiencies, especially in low-income and middle-income countries. Fermented foods such as miso soup, yogurt, cheese and fermented soybeans have been shown to reduce the risk of pre-term birth (25). In this regard, it is essential to explore the role of fermented foods in ameliorating pregnancy-related complications. Enterobacterial microbiota differences found between groups of pregnant women consuming fermented foods and control groups, respectively, were hypothesized to reduce pre-term birth cases, by reducing pro-inflammatory Enterobacteriaceae associated with infection or immune dysregulation (25). In another study, Mexican women who reported consuming yogurt during the last 3 months of pregnancy had a decreased risk for pre-term birth, suggesting that prenatal yogurt consumption may reduce the risk of pre-term birth among non-overweight pregnant women. The authors suggest this effect could be due to probiotic strains in yogurt with anti-inflammatory properties (26). However, this was a questionnaire-based prospective study and entirely observational thus, observations should be interpreted cautiously.
Though the global prevalence of anxiety and depression during pregnancy varies between studies, especially across developing and developed countries (27), it is now widely accepted that maternal anxiety, stress and depression can have a lasting effect on both the mother and the baby (28). In addition, the impact of prenatal stress is further exacerbated by nutrient restriction (29). A high-quality maternal diet, as defined by the presence of fruits, vegetables, fishes and whole grains, has been shown to lower the risk for prenatal depressive symptoms as compared to the consumption of other food groups such as refined grains, fast foods, and energy drinks (30, 31). Only a few studies have explored the consumption of fermented foods in maternal depressive symptoms and stress. In a Japanese cohort, consumption of seaweed, yogurt, tofu, fermented soybeans and miso soup was found to be negatively associated with depression during pregnancy, which could mitigate the impact of maternal stress on the offspring (32). On the contrary, a Japanese cohort of around 9,030 pregnant women showed no association between maternal fermented food diet and psychological distress (33). Also, the mechanisms behind these effects have not been identified.
Gestational diabetes (GDM) is another widely prevalent pregnancy complication. According to the Centers for Disease Control and Prevention (CDC), the percentage of mothers with gestational diabetes in the United States increased from 6% in 2016 to 8.3% in 2021 (34). Recent clinical studies have shown substituting higher complex carbohydrates for simple carbohydrates can help control maternal glycemia and reduce postprandial glucose. In a randomized crossover study, two distinct diets, one rich in complex carbohydrates with less fat, and the other lower in carbohydrates with more fat were evaluated by continuous glucose monitoring (CGM) over 72 h in 16 women with gestational diabetes mellitus (GDM). The results indicated that the diet higher in complex carbohydrates and lower in fat maintained blood glucose levels below established treatment goals while also reducing postprandial free fatty acid concentrations (35). Fermented foods derived from grains, legumes, or starchy vegetables can be sources of complex carbohydrates. Further, the ingestion of fermented foods, especially plant-based, can increase the digestibility of complex carbohydrates by degrading starch into more digestible oligosaccharides (36). For example, sourdough-leavened breads are more digestible than conventionally made breads due to the pre-breakdown of gluten by lactic acid bacteria (LAB) through proteolysis during fermentation (37). Among pregnant women, soy-bean oligosaccharides were found to alleviate insulin resistance among those with gestational diabetes (38), and several studies have suggested fermented food supplementation can reduce the risk of diabetes through antioxidant and anti-inflammatory properties (39). In a study involving 70 pregnant women of singleton pregnancy, subjects who consumed probiotic yogurt had higher levels of erythrocyte glutathione reductase (GR), an enzyme linked with insulin sensitivity, than those who consumed conventional yogurt (40). Another study reported that daily consumption of probiotic yogurt by pregnant women for 9 weeks stabilized serum insulin levels, suggesting potential prevention of pregnancy-induced insulin resistance (41). A study comparing patients with gestational diabetes mellitus and healthy pregnant women found that consumption of white wheat bread resulted in 45.5% higher insulin secretion and a 9.6% increase in first-hour postprandial blood glucose levels compared to sourdough whole grain bread in both groups (42). Among women with gestational diabetes, probiotic yogurt containing the probiotic strains Lactobacillus acidophillus and Bifidobacterium animalis (formerly known as Bifidobacterium lactis), reduced the risk of gestational diabetes (41, 43–45).
Gestational hypertension is considered a risk factor for preeclampsia, preterm birth, and low birth weight. Dietary strategies, including consumption of fermented foods to prevent hypertension, have yielded positive results. Fermentation of milk by lactic acid bacteria can produce various bioactive peptides with hypotensive properties (46). These peptides maintain their activity throughout the digestive process and can be absorbed into different organs and tissues via the bloodstream. One group of these peptides is angiotensin-converting enzyme inhibitory peptides (ACE-I), which has garnered significant interest for their potential use in managing hypertension. Both clinical and animal studies have demonstrated the blood pressure-lowering effects of certain fermented milk products containing these peptides (47). Among yeasts, two strains, namely Pichia kudriavzevii KL84A and Kluyveromyces marxianus KL26A from Kumis, a traditional fermented Colombian milk, showed the presence of these ACEI peptides (48). In one study, lactic acid bacteria with high ACEI activity, Lactiplantibacillus plantarum B (formerly known as Lactobacillus plantarum), Lactobacillus gasseri A, Lactiplantibacillus plantarum R5 (LPR5), and Lactiplantibacillus plantarum R7 (LPR7), showed antihypertensive effect in rat models with pregnancy-induced hypertension (49). In general, though large cohort studies and countable intervention studies have found a link between maternal fermented foods consumption and reduced risk of pregnancy complications, more in-depth clinical studies with fermented foods intervention among pregnant women would inform preventive strategies for reducing pregnancy-related complications.
4 Can maternal consumption of fermented foods modulate the gut microbiome of mothers and offspring?
Human gut microbiota has gained much attention due to increasing evidence of compositional and structural changes of gut microbiota playing a vital role in various aspects of human health, including immune, metabolic, and neuro-behavioral traits (50). Multiple factors, such as environment, genetics, infection, and mode of delivery, can influence gut microbiome composition. However, diet is also one of the most important variables in modulating gut microbiota composition throughout life (51).
Fermented foods can modulate the gut microbiota across the human lifespan due to the microbial strains present in the fermented foods (Table 1). Published literature suggests that one of the reasons for the beneficial health effects of fermented foods could be attributed to the promotion of probiotic strains and reduction in “pathobionts”—microorganisms that are non-pathogenic under ideal gut conditions but can turn pathogenic under adverse conditions (52). In a study, consumption of fermented milk (250 g/d) for 4 weeks improved irritable bowel syndrome status in women by increasing the short-chain fatty acids (SCFA) production and decreasing the abundance of the pathobiont Bilophila wadsworthia (53). Animal studies have shown that consumption of fermented milk products, such as Kefir, increased the abundance of beneficial gut bacteria, such as Lactobacillus, Lactococcus, and Bifidobacterium (54, 55). Furthermore, kefir may help reduce intestinal permeability and improve tight junction function of the gut in adults and older populations (56). Similarly, a dose-dependent response of S. thermophilus and B. animalis abundances was observed with yogurt consumption in the older human population. It was observed that high yogurt consumption (more than 5 times/week) showed higher levels of Bifidobacterium animalis and Streptococcus thermophilus than low yogurt consumption (1–5 times/week) (57). Similarly, the abundance of Bacteroides, Dorea, Prevotella, and Faecalibacterium prausnitzii was higher in the fecal samples from healthy adults consuming grain and vegetable-based fermented food compared to the non-consuming group (58). Among these microbes, Bacteroides, Dorea and Faecalibacterium are known to have positive health effects such as immune modulation, short-chain fatty acids production, better response to immunotherapy, and improved glucose homeostasis (59–62). In a cohort of Korean women, the abundance of 34 microbial species in the gut significantly differed between groups with low (15 g/day) and high (150 g/day) Kimchi consumption. The abundance of beneficial bacteria, such as L. acidophilus, Levilactobacillus brevis (formerly known as Lactobacillus brevis), Bifidobacterium breve, Lactobacillus amylolyticus, Companilactobacillus mindensis (formerly known as Lactobacillus mindensis), Limosilactobacillus reuteri (formerly known as Lactobacillus reuteri), and L. mesenteroides was significantly higher in the high Kimchi group (63).
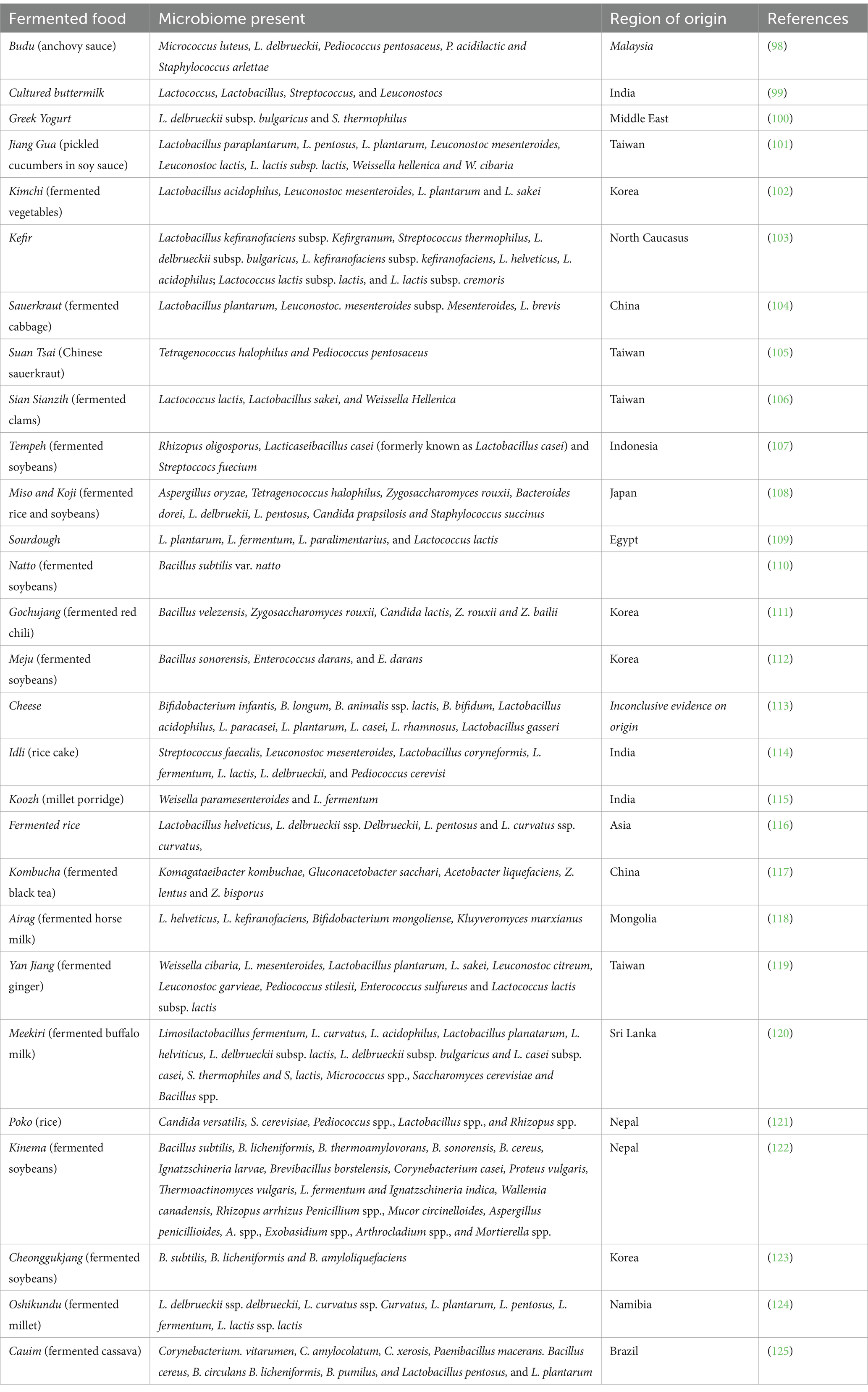
Table 1. Commonly consumed fermented foods, their origin and microbial strains present in ethnic foods.
Though, as outlined above, it is known that grain and vegetable fermented foods can modulate gut microbiome across age groups, very few studies have reported on alterations of gut microbial composition during pregnancy and infancy. One study reported an increase in the fecal levels of the beneficial microbe Bifidobacterium and a decrease in pathogenic Enterobacteriaceae in infants born to mothers who consumed 250 g of yogurt (6 days/week) during pregnancy (between 12 and 24 weeks of gestation), and up to 1 month postpartum (64). Similarly, a study conducted in Wistar rats showed a higher abundance of Bacteroides in the offspring’s (21 days old) gut, following maternal Kefir supplementation (65). The effects of fermented mulberry (FM) supplementation in pigs revealed microbial shifts in both sows and their offspring (66). FM-fed sows exhibited an increased relative abundance of Bacteroides compared to the control group, while piglets (offspring) from FM-supplemented sows demonstrated a higher relative abundance of Firmicutes than piglets from the control group, suggesting that maternal dietary intervention with fermented food can influence the gut microbiome composition, not only in the mothers themselves but also in their progeny (66). This finding was supported by another study carried out in mice where supplementation with milk fermented with Lacticaseibacillus casei DN-114001 (formerly known as Lactobacillus casei) showed higher levels of Bifidobacteria in the intestine of mothers during the suckling period and in the newborns after weaning, suggesting a potential transgenerational modulation of the gut microbiota due to fermented food (67). Figure 1 summarizes the role of fermented foods in modulating gut microbiome and ameliorating pregnancy related complications.
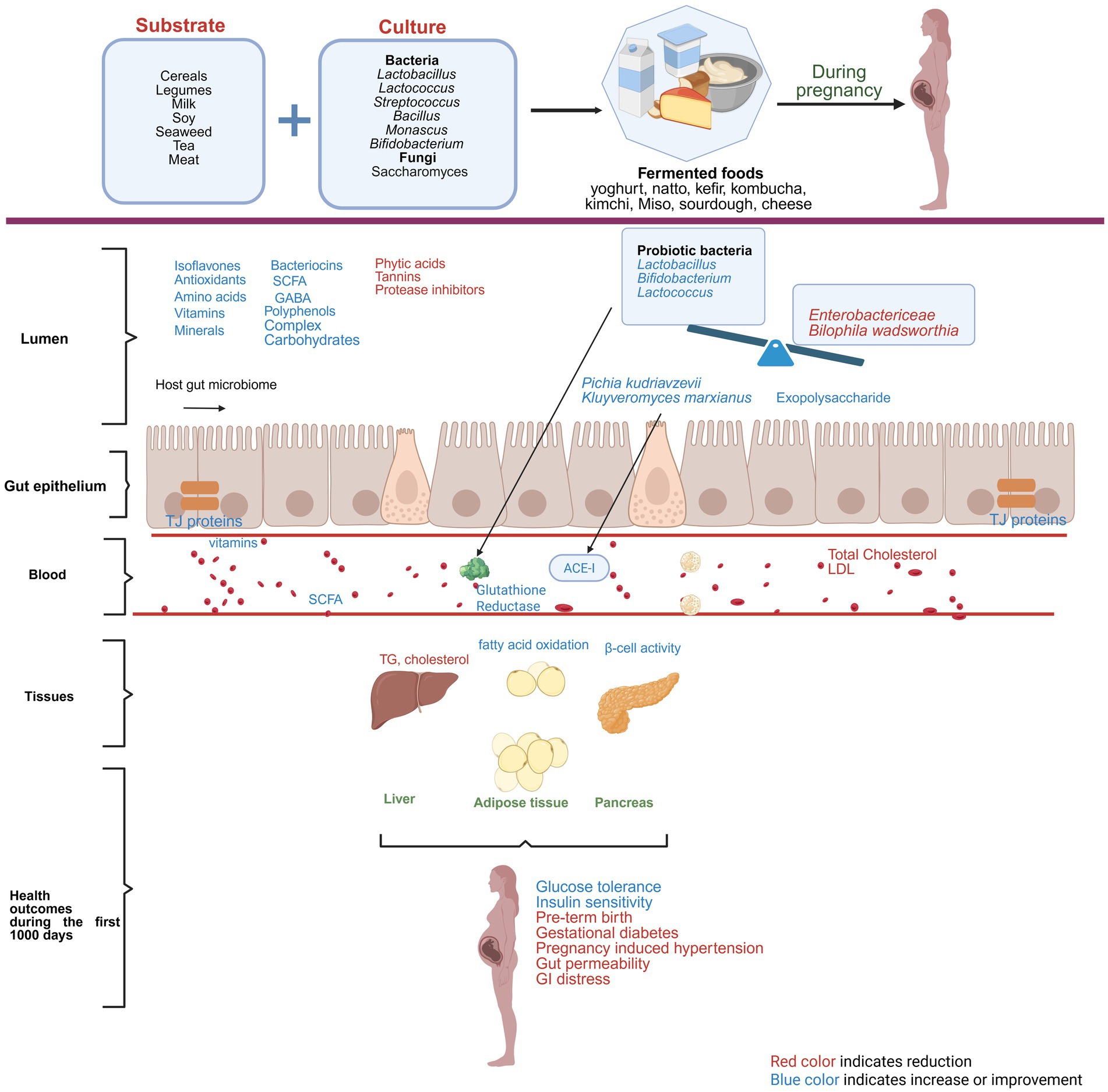
Figure 1. Role of fermented foods in ameliorating pregnancy-related complications. Red color text indicates reduction and blue color text indicates improvement or amelioration due to maternal consumption of fermented foods.
5 Do fermented foods play a role in modulating maternal and infant immune systems?
The perinatal nutrition status of both mothers and offspring impacts the immunological response and functional maturation of the immune system, and proper dietary patterns and interventions benefit both maternal and offspring’s immune homeostasis. Studies on fermented foods and their effect on modulating the maternal and infant immune system are limited but encouraging. In an intervention study, consuming fermented milk with L. casei as a starter culture during the post-partum period decreased milk TNF-α levels and reduced the frequency of gastrointestinal symptoms in infants at 2–6 months of age (68). Similarly, in a porcine model, the sows’ consumption of a fermented diet ameliorated the offspring’s colonic inflammation (69). In a study conducted in mice, maternal yogurt intake significantly increased offspring Innate lymphoid Cells 3 (ILC3). It was found that yogurt-derived indole compounds can activate the aryl hydrocarbon receptor (AhR) signaling pathways, promote ILC’s differentiation and proliferation which in turn produces cytokines essential for gut barrier function and protection against infection (70). These findings suggest maternal consumption of fermented foods could benefit offspring’s intestinal immune function. In another preclinical study, when pregnant Swiss albino mice were fed fermented milk containing Lacticaseibacillus rhamnosus 5,897 (formerly known as Lactobacillus rhamnosus), serum IgG, which neutralizes pathogens and provides passive immunity against infections during early years was significantly elevated in offsprings during the suckling and post-weaning periods (71). Similarly, maternal supplementation of fermented milk with L. casei DN-114001 as starter culture, showed significant downregulation in immune cell markers related to macrophages and dendric cells in offsprings, suggesting passive immunity provided by the mother (67). Interestingly, dietary restriction of fermented foods has shown negative effects on the innate immune response, which was reversed partially by the consumption of yogurt (72). These reports suggest the potential of maternal fermented food consumption to modulate offspring’s innate and adaptive immune response.
The global prevalence of allergic diseases in newborns is on the rise, often linked to early-life immune system development (73). Several human and preclinical studies emphasize the importance of gut microbiome during the first 1,000 days of life, both maternal and infant, in shaping immune development in childhood (74). To exemplify, maternal microbiome influences offspring’s immune tolerance, reducing allergic disease and asthma risks at birth (75). Food Protein-Induced Allergic Proctocolitis (FPIAP) has been associated with lower consumption of fermented foods, such as yogurt, cheese, and tarhana, during pregnancy, highlighting the potential protective role of these foods (76). In a case–control study conducted in Turkey, it was observed that mothers of healthy children had a significantly higher frequency of daily yogurt consumption during pregnancy compared to mothers of children diagnosed with atopic dermatitis between the ages of 2 and 24 months (77). Similarly, a large cohort study from Norway reported an association between the reduction in the risk of atopic eczema in infants at 6 months of age with the maternal consumption of probiotic milk and yogurt during pregnancy, although no clear dose–response relationship was identified (78). Also, in a study conducted in China, both the frequency and quantity of maternal yogurt intake were associated with a dose-dependent reduction in the risk of eczema in infants aged 3 to 6 months. This study reported nearly a 50% decrease in the risk of eczema in infants whose mothers consume yogurt more than three times per week and more than 50 g per day (79). The underlying mechanism is that probiotics promote microbial stimulation, modulating the immune system that supports a balance between T helper 1 (Th1) and Th2 cells, which reduces Th2 cytokines, IgE concentrations, and increases C-reactive protein and IgA levels to prevent inflammation and allergy-related processes (80). Additionally, the anti-inflammatory properties of yogurt, through metabolites like SCFAs produced by intestinal microbiota, further contribute to its protective effect against allergies (81). Though observational, these studies hint at the importance of the maternal intake of fermented foods in preventing allergy outcomes in infants. Figure 2 summarizes the role of fermented foods in modulating the immune system during pregnancy and infancy.
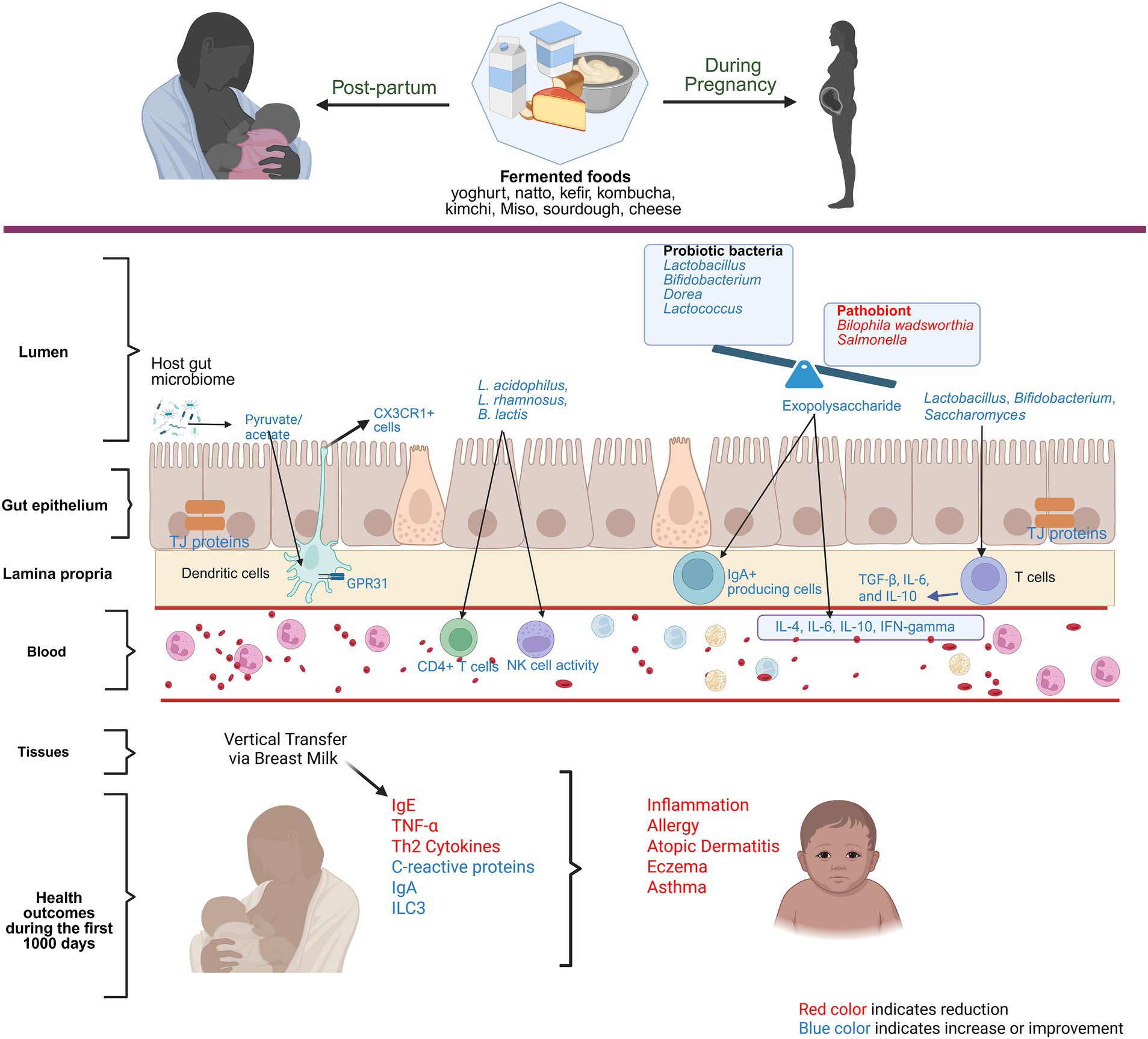
Figure 2. Role of fermented foods in modulating the immune system during pregnancy. Red color text indicates reduction and blue color text indicates improvement or amelioration due to maternal consumption of fermented foods.
6 Can fermented foods positively impact maternal and infant brain health?
Fermented foods are also psychobiotics due to their impact on brain outcomes (82, 83). The External Fermentation Hypothesis proposed by Bryant, Hansen and Hecht suggested that external fermentation practices could be the reason for the reduction of the human colon and expansion of brain volume due to decreased energy expenditure and increased accessibility of nutrients to the brain (84). Studies have suggested that fermented food can impact brain health throughout life, including early life brain development. Gut microbiota communicates with the central nervous system through three pathways, collectively comprising the “gut-brain axis” (85). In the first pathway, metabolites cross the blood–brain barrier and directly influence the central nervous system. In the second, they follow the same path but act by modulating the brain-resident immune cells. The third pathway is through the release of hormones from enteroendocrine cells (EECs) such as I cells, K cells and N cells during their interaction with microbes (86). During the early stages after birth, human milk feeding emerges as the most prominent and effective nutritional approach to support optimal brain development in infants and hence factors that determine the human milk composition play vital role (18). It is imperative to suggest that the gut microbiome modulating effect of fermented foods and its cascade effect on human milk components and infant gut microbiota assembly may be a precursor for early brain health in infants. Though this hypothesis sounds reasonable, most of the studies concerning the benefits of fermented foods on brain health were observed in adults and aging rodent models of neurological diseases, not in models of pregnancy.
Studies in adults and the aging population have shown that fermented foods can promote cognition, memory, learning, neurotransmitter production and overall brain health (87). In a randomized control trial, healthy women aged between 18 and 55 years were given fermented milk products in combination with four probiotics: B. animalis, S. thermophiles, L. bulgaricus and L. lactis daily for 4 weeks. In this study, the midbrain regions of women (that control emotion) were impacted, including reduced reactivity during emotional attention tasks (88). In clinical studies, consumption of L. helveticus fermented milk was reported to improve sleep efficiency, reduce wake episodes, and improve cognitive performance during cognitive fatigue tests in elderly subjects (89, 90). Similarly, fermented food also improved attention and memory in middle-aged Japanese subjects, potentially via a peptide, lactononadecapeptide (91). Other varieties of fermented foods, such as seaweed, yogurt, tofu, fermented soybeans and miso soup, were also found to be negatively associated with depression during pregnancy, which could mitigate the impact of maternal stress on the offspring (32).
Though limited, published literature has suggested the beneficial effect of maternal intake of fermented foods on offspring neurodevelopment (92, 93). For example, in a Japanese cohort, maternal consumption of fermented soybeans and miso soup from the beginning of pregnancy to the third trimester had a lower risk of fine motor development delay and communication skills delay in the 1-year-old offspring (93). In the same study, yogurt consumption by the mothers was associated with reduced personal and social skills delay in the offspring (93). In another Japanese study, consuming cheese during pregnancy was linked to a lower risk of developmental delays in children (92). Consuming miso soup during the second and third trimester of pregnancy was shown to reduce the risk of lower sleep hours in 1-year-old infants (94). A study on mother–child pairs indicated consuming fermented foods like cheese during pregnancy could reduce the risk of sleep deprivation in infants at 3 years of age (95). In a prenatal valproic acid-induced autism spectrum disorder mouse model, supplementation with Lactiplantibacillus plantarum fermented milk ameliorated some autism-like symptoms (improved locomotor behavior, sociability, anxiety) in male mice but not in female mice (96). Figure 3 summarizes the role of fermented foods in maternal and infant brain health. However, mechanistic studies on how maternal consumption of fermented foods program fetal brain and impact offspring’s brain development and cognitive measures are lacking. Similarly, studies on maternal and offspring consumption of fermented food during the postnatal period and the resulting behavioral and brain outcomes are limited. In summary, these reports underscore the benefits of fermented foods in alleviating brain-related disorders in adults and the aging population, but it is unknown whether fermented foods would have similar effects in pregnancy and these effects would be transgenerational. Exploring this would require placental and transgenerational animal models, especially to understand the role of maternal fermented food intake on the first 1,000 days on infant’s brain health and outcomes. Table 2 summarizes studies on consumption of fermented foods and the health outcomes during pregnancy and postnatal period.
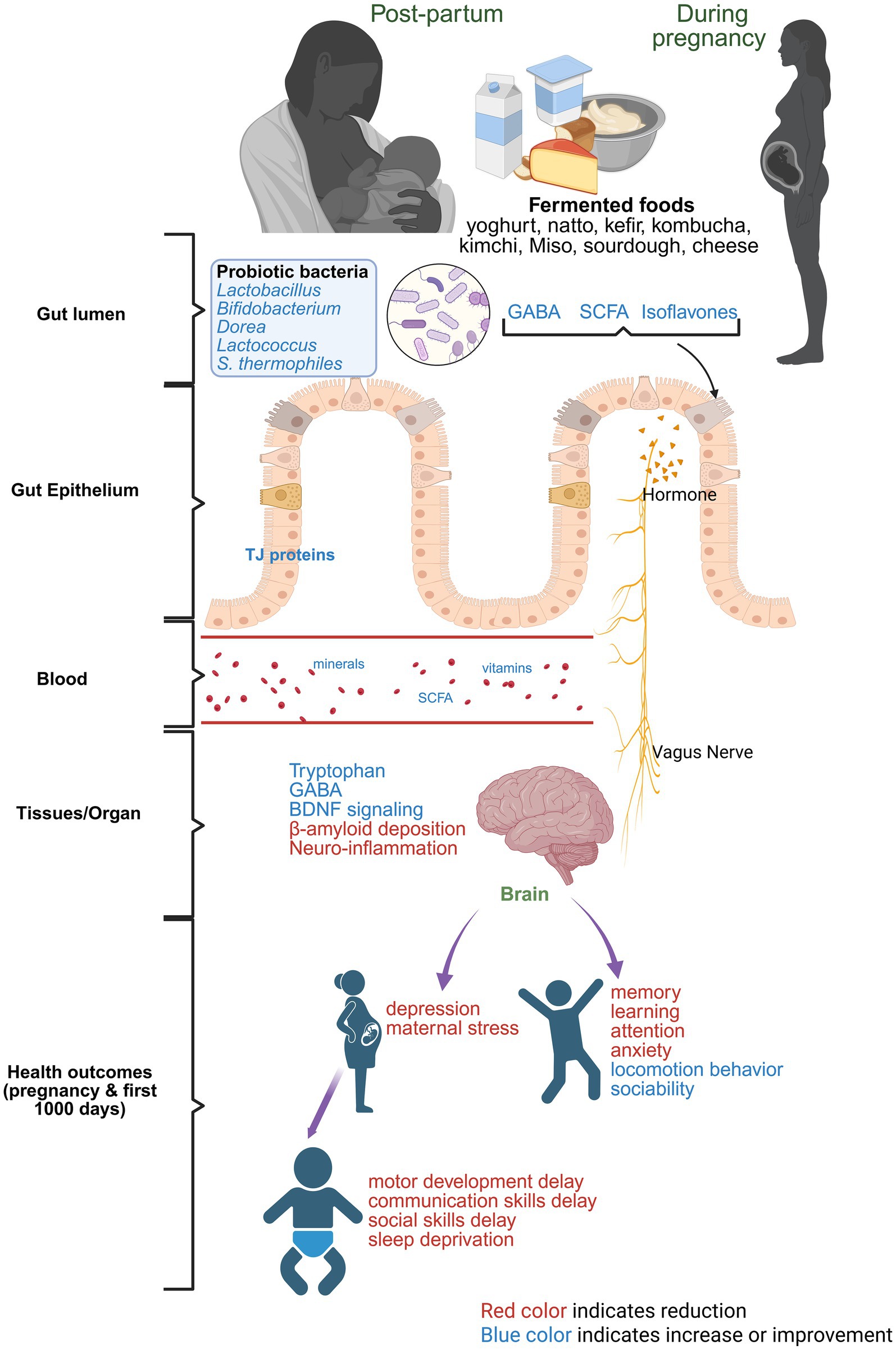
Figure 3. Role of fermented foods in aiding mother’s and infant’s brain health. Red color text indicates reduction and blue color text indicates improvement or amelioration due to maternal consumption of fermented foods.

Table 2. The role of fermented foods during pregnancy and infant’s first 1,000 days in relation to modulating gut microbiome, immune function and brain health.
7 Concluding remarks and future directions
The contemporary global health paradigm emphasizes a holistic approach to child development, shifting from mere survival to overall well-being, which is heavily influenced by maternal nutrition. The functional components of fermented foods, especially microbiome, can alter gut microbiota composition during pregnancy and in infants, thereby modulating their immune system and aiding brain outcomes. However, it is also important to note that though short-term dietary intervention can alter microbiota composition, changes are transient and do not last longer than a few days (97). This warrants an important question, “Which period during pregnancy should supplementation of fermented foods begin to optimize positive health outcomes?” Since most of the reported studies on the impact of fermented food are short-term, more long-term and longitudinal studies are needed to determine how long fermented food dietary intervention is required to alter the long-lasting microbial composition. Studies should also account for other potential confounding variables that may impact both microbiome composition and pregnancy outcomes. Our review highlights the benefits of maternal fermented foods intake on the offspring’s microbiota, immune system, and brain health. However, studies on fetal health, another critical period of development, are lacking which limits knowledge of causality. The review also highlights key pregnancy-related complications and the possible benefits of maternal consumption of fermented foods. However, the mechanistic pathways through which these effects are exerted need to be further examined, particularly considering risk factors like maternal age and pre-existing medical conditions. Further, the transgenerational effects of maternal intake of fermented foods on offspring remain largely unexplored. While methodological challenges in human cohort studies pose significant complexities, using animal models could bridge this knowledge gap. Finally, it should also be noted that considering the safety, demographic differences, cultural differences, dietary patterns and food systems, cost-effectiveness and broader economic feasibility of such interventions, we must be mindful of the “one size fits all” approach of touting fermented foods as a unique and gold standard intervention without having clear scientific consensus.
Author contributions
AP: Writing – original draft, Writing – review & editing. MG: Writing – original draft, Writing – review & editing. BK: Writing – original draft, Writing – review & editing. SP: Writing – review & editing. LY: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. All authors listed are supported by the USDA-ARS 6026–10700-001-00D.
Acknowledgments
We would like to thank Jolene Rearick, Biological Science Technician (USDA – ARS) for providing valuable inputs that significantly enhanced the quality of this review.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Tamang, JP, Jeyaram, K, Rai, AK, and Mukherjee, PK (2021). Diversity of beneficial microorganisms and their functionalities in community-specific ethnic fermented foods of the eastern Himalayas. Food Res Int 148:110633. doi: 10.1016/j.foodres.2021.110633
2. Tamang, JP, Watanabe, K, and Holzapfel, WH (2016). Review: diversity of microorganisms in global fermented foods and beverages. Front Microbiol 7:377. doi: 10.3389/fmicb.2016.00377
3. Dimidi, E, Cox, SR, Rossi, M, and Whelan, K (2019). Fermented foods: definitions and characteristics, impact on the gut microbiota and effects on gastrointestinal health and disease. Nutrients 11:1–26. doi: 10.3390/nu11081806
4. Valentino, V, Magliulo, R, Farsi, D, Cotter, PD, O'Sullivan, O, Ercolini, D, et al. (2024). Fermented foods, their microbiome and its potential in boosting human health. Microb Biotechnol 17:e14428. doi: 10.1111/1751-7915.14428
5. Marco, ML, Heeney, D, Binda, S, Cifelli, CJ, Cotter, PD, Foligné, B, et al. (2017). Health benefits of fermented foods: microbiota and beyond. Curr Opin Biotechnol 44:94–102. doi: 10.1016/j.copbio.2016.11.010
6. Ganzle, MG, Monnin, L, Zheng, J, Zhang, L, Coton, M, Sicard, D, et al. (2024). Starter culture development and innovation for novel fermented foods. Annu Rev Food Sci Technol 15:211–39. doi: 10.1146/annurev-food-072023-034207
7. Jouanne, M, Oddoux, S, Noël, A, and Voisin-Chiret, AS (2021). Nutrient requirements during pregnancy and lactation. Nutrients 13:692. doi: 10.3390/nu13020692
8. Hasan, M, Sultan, M, and Mar-E-Um, M (2014). Significance of fermented food in nutrition and food science. J Sci Res 6:373–86. doi: 10.3329/jsr.v6i2.16530
9. Grundy, MM, Edwards, CH, Mackie, AR, Gidley, MJ, Butterworth, PJ, and Ellis, PR (2016). Re-evaluation of the mechanisms of dietary fibre and implications for macronutrient bioaccessibility, digestion and postprandial metabolism. Br J Nutr 116:816–33. doi: 10.1017/S0007114516002610
10. Adebo, JA, Njobeh, PB, Gbashi, S, Oyedeji, AB, Ogundele, OM, Oyeyinka, SA, et al. (2022). Fermentation of cereals and legumes: impact on nutritional constituents and nutrient bioavailability. Fermentation 8:63. doi: 10.3390/fermentation8020063
11. Chan, SS, Ferguson, EL, Bailey, K, Fahmida, U, Harper, TB, and Gibson, RS (2007). The concentrations of iron, calcium, zinc and phytate in cereals and legumes habitually consumed by infants living in East Lombok, Indonesia. J Food Compos Anal 20:609–17. doi: 10.1016/j.jfca.2007.03.003
12. Potter, NN, and Hotchkiss, JH. Food science. New York: Springer Science & Business Media (2012).
13. Al Hasan, SM, Hassan, M, Saha, S, Islam, M, Billah, M, and Islam, S (2016). Dietary phytate intake inhibits the bioavailability of iron and calcium in the diets of pregnant women in rural Bangladesh: a cross-sectional study. BMC Nutr 2:1–10. doi: 10.1186/s40795-016-0064-8
14. Barker, D. J. (2007). The origins of the developmental origins theory. J. Intern. Med. 261:412–17. doi: 10.1111/j.1365-2796.2007.01809.x
15. Verduci, E, Martelli, A, Miniello, V, Landi, M, Mariani, B, Brambilla, M, et al. (2017). Nutrition in the first 1000 days and respiratory health: a descriptive review of the last five years’ literature. Allergol Immunopathol 45:405–13. doi: 10.1016/j.aller.2017.01.003
16. Gluckman, PD, Buklijas, T, and Hanson, MA. The developmental origins of health and disease (DOHaD) concept: past, present, and future In: The epigenome and developmental origins of health and disease. Boston, MA: Elsevier (2016). 1–15.
17. Agosti, M, Tandoi, F, Morlacchi, L, and Bossi, A (2017). Nutritional and metabolic programming during the first thousand days of life. Pediatr Med Chir 39:57–61. doi: 10.4081/pmc.2017.157
18. Cusick, SE, and Georgieff, MK (2016). The role of nutrition in brain development: the golden opportunity of the “first 1000 days”. J Pediatr 175:16–21. doi: 10.1016/j.jpeds.2016.05.013
19. Martorell, R (2017). Improved nutrition in the first 1000 days and adult human capital and health. Am J Hum Biol 29:e22952. doi: 10.1002/ajhb.22952
20. Chen, X, Zhao, D, Mao, X, Xia, Y, Baker, PN, and Zhang, H (2016). Maternal dietary patterns and pregnancy outcome. Nutrients 8:351. doi: 10.3390/nu8060351
21. Shenoy, S, Sharma, P, Rao, A, Aparna, N, Adenikinju, D, Iloegbu, C, et al. (2023). Evidence-based interventions to reduce maternal malnutrition in low and middle-income countries: a systematic review. Front Health Serv 3:1155928. doi: 10.3389/frhs.2023.1155928
22. Iqbal, S, and Ali, I (2021). Maternal food insecurity in low-income countries: revisiting its causes and consequences for maternal and neonatal health. J Agric Food Res 3:100091. doi: 10.1016/j.jafr.2020.100091
23. Mujica-Coopman, MF, Garmendia, ML, and Corvalán, C (2023). Iron, folic acid, and vitamin D supplementation during pregnancy: did pregnant Chilean women meet the recommendations during the COVID pandemic? PLoS One 18:e0293745. doi: 10.1371/journal.pone.0293745
24. Cai, F, Young, BK, and Mccoy, JA (2024). Commercially available prenatal vitamins do not meet American College of Obstetricians and Gynecologists nutritional guidelines. Am J Perinatol 41:e2547–54. doi: 10.1055/a-2125-1148
25. Ito, M, Takamori, A, Yoneda, S, Shiozaki, A, Tsuchida, A, Matsumura, K, et al. (2019). Fermented foods and preterm birth risk from a prospective large cohort study: the Japan environment and Children’s study. Environ Health Prev Med 24:1–11. doi: 10.1186/s12199-019-0782-z
26. Kriss, JL, Ramakrishnan, U, Beauregard, JL, Phadke, VK, Stein, AD, Rivera, JA, et al. (2018). Yogurt consumption during pregnancy and preterm delivery in Mexican women: a prospective analysis of interaction with maternal overweight status. Matern Child Nutr 14:e12522. doi: 10.1111/mcn.12522
27. Biaggi, A, Conroy, S, Pawlby, S, and Pariante, CM (2016). Identifying the women at risk of antenatal anxiety and depression: a systematic review. J Affect Disord 191:62–77. doi: 10.1016/j.jad.2015.11.014
28. Schetter, CD, and Tanner, L (2012). Anxiety, depression and stress in pregnancy: implications for mothers, children, research, and practice. Curr Opin Psychiatry 25:141–8. doi: 10.1097/YCO.0b013e3283503680
29. Franke, K, Van den Bergh, BR, de Rooij, SR, Kroegel, N, Nathanielsz, PW, Rakers, F, et al. (2020). Effects of maternal stress and nutrient restriction during gestation on offspring neuroanatomy in humans. Neurosci Biobehav Rev 117:5–25. doi: 10.1016/j.neubiorev.2020.01.031
30. Stang, J (2012). Stress, depression, social support, and eating habits reduce dietary quality in the 1st trimester in low-income women: a pilot study. J Acad Nutr Diet 112:1619–25. doi: 10.1016/j.jand.2012.07.002
31. Baskin, R, Hill, B, Jacka, FN, O'Neil, A, and Skouteris, H (2015). The association between diet quality and mental health during the perinatal period. A systematic review. Appetite 91:41–7. doi: 10.1016/j.appet.2015.03.017
32. Miyake, Y, Tanaka, K, Okubo, H, Sasaki, S, Furukawa, S, and Arakawa, M (2018). Soy isoflavone intake and prevalence of depressive symptoms during pregnancy in Japan: baseline data from the Kyushu Okinawa maternal and child health study. Eur J Nutr 57:441–50. doi: 10.1007/s00394-016-1327-5
33. Takahashi, F, Nishigori, H, Nishigori, T, Mizuno, S, Obara, T, Metoki, H, et al. (2016). Fermented food consumption and psychological distress in pregnant women: a nationwide birth cohort study of the Japan environment and children’s study. Tohoku J Exp Med 240:309–21. doi: 10.1620/tjem.240.309
34. Lin, J, Horswell, R, Chu, S, Dumas, SA, and Hu, G (2024). Trends in the incidence of gestational diabetes mellitus among the Medicaid population before and during the COVID-19 pandemic. J Women Health 33:1276–82. doi: 10.1089/jwh.2023.0746
35. Hernandez, TL, Van Pelt, RE, Anderson, MA, Daniels, LJ, West, NA, Donahoo, WT, et al. (2014). A higher-complex carbohydrate diet in gestational diabetes mellitus achieves glucose targets and lowers postprandial lipids: a randomized crossover study. Diabetes Care 37:1254–62. doi: 10.2337/dc13-2411
36. Yadav, S, and Khetarpaul, N (1994). Indigenous legume fermentation: effect on some antinutrients and in-vitro digestibility of starch and protein. Food Chem 50:403–6. doi: 10.1016/0308-8146(94)90213-5
37. Gil-Cardoso, K, Saldaña, G, Luengo, E, Pastor, J, Virto, R, Alcaide-Hidalgo, JM, et al. (2021). Consumption of sourdough breads improves postprandial glucose response and produces sourdough-specific effects on biochemical and inflammatory parameters and mineral absorption. J Agric Food Chem 69:3044–59. doi: 10.1021/acs.jafc.0c07200
38. Fei, B-b, Ling, L, Hua, C, and Ren, S-y (2014). Effects of soybean oligosaccharides on antioxidant enzyme activities and insulin resistance in pregnant women with gestational diabetes mellitus. Food Chem 158:429–32. doi: 10.1016/j.foodchem.2014.02.106
39. Sivamaruthi, BS, Kesika, P, Prasanth, MI, and Chaiyasut, C (2018). A mini review on antidiabetic properties of fermented foods. Nutrients 10:1973. doi: 10.3390/nu10121973
40. Asemi, Z, Jazayeri, S, Najafi, M, Samimi, M, Mofid, V, Shidfar, F, et al. (2012). Effect of daily consumption of probiotic yogurt on oxidative stress in pregnant women: a randomized controlled clinical trial. Ann Nutr Metab 60:62–8. doi: 10.1159/000335468
41. Asemi, Z, Samimi, M, Tabassi, Z, Naghibi Rad, M, Rahimi Foroushani, A, Khorammian, H, et al. (2013). Effect of daily consumption of probiotic yoghurt on insulin resistance in pregnant women: a randomized controlled trial. Eur J Clin Nutr 67:71–4. doi: 10.1038/ejcn.2012.189
42. Özer, YE, Cengiz, H, Demirci, T, Kızılgül, M, Varim, C, and Tamer, A (2023). Glycemic responses to whole grain sourdough bread versus refined white bread in patients with gestational diabetes. Wien Klin Wochenschr 135:349–57. doi: 10.1007/s00508-023-02200-9
43. Asgharian, H, Homayouni-Rad, A, Mirghafourvand, M, and Mohammad-Alizadeh-Charandabi, S (2020). Effect of probiotic yoghurt on plasma glucose in overweight and obese pregnant women: a randomized controlled clinical trial. Eur J Nutr 59:205–15. doi: 10.1007/s00394-019-01900-1
44. Sahhaf Ebrahimi, F, Homayouni Rad, A, Mosen, M, Abbasalizadeh, F, Tabrizi, A, and Khalili, L (2019). Effect of L. acidophilus and B. lactis on blood glucose in women with gestational diabetes mellitus: a randomized placebo-controlled trial. Diabetol Metab Syndr 11:1–7. doi: 10.1186/s13098-019-0471-5
45. Xiaoqian, C, Jiang, X, Huang, X, Hong, GH, and Zheng, J (2019). Association between probiotic yogurt intake and gestational diabetes mellitus: a case-control study. Iran J Public Health 48:1248. doi: 10.18502/ijph.v48i7.2946
46. Beltrán-Barrientos, L, Hernández-Mendoza, A, Torres-Llanez, M, González-Córdova, A, and Vallejo-Córdoba, B (2016). Invited review: fermented milk as antihypertensive functional food. J Dairy Sci 99:4099–110. doi: 10.3168/jds.2015-10054
47. Rai, AK, Sanjukta, S, and Jeyaram, K (2017). Production of angiotensin I converting enzyme inhibitory (ACE-I) peptides during milk fermentation and their role in reducing hypertension. Crit Rev Food Sci Nutr 57:2789–800. doi: 10.1080/10408398.2015.1068736
48. Chaves-López, C, Tofalo, R, Serio, A, Paparella, A, Sacchetti, G, and Suzzi, G (2012). Yeasts from Colombian kumis as source of peptides with angiotensin I converting enzyme (ACE) inhibitory activity in milk. Int J Food Microbiol 159:39–46. doi: 10.1016/j.ijfoodmicro.2012.07.028
49. Yi, L, Min, JT, Jun, CL, Long, HX, Khoo, HE, Ying, ZJ, et al. (2024). Buffalo yogurt fermented with commercial starter and Lactobacillus plantarum originating from breast milk lowered blood pressure in pregnant hypertensive rats. J Dairy Sci 107:62–73. doi: 10.3168/jds.2023-23566
50. Zhang, Y-J, Li, S, Gan, R-Y, Zhou, T, Xu, D-P, and Li, H-B (2015). Impacts of gut bacteria on human health and diseases. Int J Mol Sci 16:7493–519. doi: 10.3390/ijms16047493
51. Rinninella, E, Raoul, P, Cintoni, M, Franceschi, F, Miggiano, GAD, Gasbarrini, A, et al. (2019). What is the healthy gut microbiota composition? A changing ecosystem across age, environment, diet, and diseases. Microorganisms 7:14. doi: 10.3390/microorganisms7010014
52. Divella, R, De Palma, G, Tufaro, A, Pelagio, G, Gadaleta-Caldarola, G, Bringiotti, R, et al. (2021). Diet, probiotics and physical activity: the right allies for a healthy microbiota. Anticancer Res 41:2759–72. doi: 10.21873/anticanres.15057
53. Veiga, P, Pons, N, Agrawal, A, Oozeer, R, Guyonnet, D, Brazeilles, R, et al. (2014). Changes of the human gut microbiome induced by a fermented milk product. Sci Rep 4:6328. doi: 10.1038/srep06328
54. Kim, D-H, Chon, J-W, Kim, H, and Seo, K-H (2015). Modulation of intestinal microbiota in mice by kefir administration. Food Sci Biotechnol 24:1397–403. doi: 10.1007/s10068-015-0179-8
55. Kim, D-H, Jeong, D, Kang, I-B, Lim, H-W, Cho, Y, and Seo, K-H (2019). Modulation of the intestinal microbiota of dogs by kefir as a functional dairy product. J Dairy Sci 102:3903–11. doi: 10.3168/jds.2018-15639
56. Peluzio, MCG, MdMe, D, Martinez, JA, and Milagro, FI (2021). Kefir and intestinal microbiota modulation: implications in human health. Front Nutr 8:638740. doi: 10.3389/fnut.2021.638740
57. Le Roy, CI, Kurilshikov, A, Leeming, ER, Visconti, A, Bowyer, RC, Menni, C, et al. (2022). Yoghurt consumption is associated with changes in the composition of the human gut microbiome and metabolome. BMC Microbiol 22:39. doi: 10.1186/s12866-021-02364-2
58. Taylor, BC, Lejzerowicz, F, Poirel, M, Shaffer, JP, Jiang, L, Aksenov, A, et al. (2020). Consumption of fermented foods is associated with systematic differences in the gut microbiome and metabolome. Msystems 5:10.1128/msystems.00901-19. doi: 10.1128/mSystems.00901-19
59. Sokol, H, Pigneur, B, Watterlot, L, Lakhdari, O, Bermúdez-Humarán, LG, Gratadoux, J-J, et al. (2008). Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc Natl Acad Sci USA 105:16731–6. doi: 10.1073/pnas.0804812105
60. Troy, EB, and Kasper, DL (2010). Beneficial effects of Bacteroides fragilis polysaccharides on the immune system. Front Biosci 15:25–34. doi: 10.2741/3603
61. Blumenberg, V, Busch, G, Baumann, S, Schmidt, S, Schuhmacher, H, Winkelmann, M, et al. (2021). High bacterial abundances of Dorea and Pediococcus in the gut microbiome linked to expansion, immune checkpoint expression and efficacy of CD19-directed CAR T-cells in patients with r/r DLBCL. Blood 138:2792. doi: 10.1182/blood-2021-153117
62. Munukka, E, Rintala, A, Toivonen, R, Nylund, M, Yang, B, Takanen, A, et al. (2017). Faecalibacterium prausnitzii treatment improves hepatic health and reduces adipose tissue inflammation in high-fat fed mice. ISME J 11:1667–79. doi: 10.1038/ismej.2017.24
63. Kim, J, Choi, E, Hong, Y, Song, Y, Han, J, Lee, S, et al. (2016). Changes in Korean adult females' intestinal microbiota resulting from kimchi intake. J Nutr Food Sci 6:486
64. Bisanz, JE, Enos, MK, Pray God, G, Seney, S, Macklaim, JM, Chilton, S, et al. (2015). Microbiota at multiple body sites during pregnancy in a rural Tanzanian population and effects of Moringa-supplemented probiotic yogurt. Appl Environ Microbiol 81:4965–75. doi: 10.1128/AEM.00780-15
65. Costa de Almeida, T, Sabino, YNV, Brasiel, PGA, Rocha, BMO, de Cássia Ávila Alpino, G, Rocha, VN, et al. (2025). Maternal kefir intake during lactation impacts the breast milk and gut microbiota of the Wistar rat’s offspring. Int J Food Sci Nutr 76:179–93. doi: 10.1080/09637486.2025.2461142
66. Geng, B, Gao, J, Cheng, H, Guo, G, and Wang, Z (2024). Effects of dietary mulberry leaves on growth, production performance, gut microbiota, and immunological parameters in poultry and livestock: a systematic review and meta-analysis. Anim Biosci 37:1065–76. doi: 10.5713/ab.23.0449
67. de Moreno De LeBlanc ADogi, CA, Galdeano, CM, Carmuega, E, Weill, R, and Perdigón, G (2008). Effect of the administration of a fermented milk containing Lactobacillus casei DN-114001 on intestinal microbiota and gut associated immune cells of nursing mice and after weaning until immune maturity. BMC Immunol 9:1–12. doi: 10.1186/1471-2172-9-27
68. Ortiz-Andrellucchi, A, Sánchez-Villegas, A, Rodríguez-Gallego, C, Lemes, A, Molero, T, Soria, A, et al. (2008). Immunomodulatory effects of the intake of fermented milk with Lactobacillus casei DN114001 in lactating mothers and their children. Br J Nutr 100:834–45. doi: 10.1017/S0007114508959183
69. Wang, C, Wei, S, Liu, B, Wang, F, Lu, Z, Jin, M, et al. (2022). Maternal consumption of a fermented diet protects offspring against intestinal inflammation by regulating the gut microbiota. Gut Microbes 14:2057779. doi: 10.1080/19490976.2022.2057779
70. Pimentel, G, Roder, T, Bär, C, Christensen, S, Sattari, Z, Kalbermatter, C, et al. (2024). Maternal consumption of yoghurt activating the aryl hydrocarbon receptor increases group 3 innate lymphoid cells in murine offspring. Microbiol Spectr 12:e00393–24. doi: 10.1128/spectrum.00393-24
71. Saliganti, V, Kapila, R, and Kapila, S (2016). Consumption of probiotic Lactobacillus rhamnosus (MTCC: 5897) containing fermented milk plays a key role in development of the immune system in newborn mice during the suckling–weaning transition. Microbiol Immunol 60:261–7. doi: 10.1111/1348-0421.12342
72. Olivares, M, Díaz-Ropero, MP, Gómez, N, Sierra, S, Lara-Villoslada, F, Martín, R, et al. (2006). Dietary deprivation of fermented foods causes a fall in innate immune response. Lactic acid bacteria can counteract the immunological effect of this deprivation. J Dairy Res 73:492–8. doi: 10.1017/S0022029906002068
73. Ptaschinski, C, and Gibbs, BF. Early-life risk factors which govern pro-allergic immunity. Seminars in immunopathology. Berlin: Springer (2024).
74. Davis, EC, Monaco, C, Insel, R, and Järvinen, KM (2024). Gut microbiome in the first 1000 days and risk for childhood food allergy. Ann Allergy Asthma Immunol 133:252–61. doi: 10.1016/j.anai.2024.03.010
75. Vuillermin, PJ, Macia, L, Nanan, R, Tang, ML, Collier, F, and Brix, S. The maternal microbiome during pregnancy and allergic disease in the offspring. Seminars in immunopathology. Berlin: Springer (2017).
76. Karatas, P, Uysal, P, Kahraman Berberoglu, B, Erge, D, and Calisir, H (2022). The low maternal consumption of homemade fermented foods in pregnancy is an additional risk factor for food protein-induced allergic proctocolitis: a case-control study. Int Arch Allergy Immunol 183:262–70. doi: 10.1159/000519154
77. Celik, V, Beken, B, Yazicioglu, M, Ozdemir, PG, and Sut, N (2019). Do traditional fermented foods protect against infantile atopic dermatitis. Pediatr Allergy Immunol 30:540–6. doi: 10.1111/pai.13045
78. Bertelsen, RJ, Brantsæter, AL, Magnus, MC, Haugen, M, Myhre, R, Jacobsson, B, et al. (2014). Probiotic milk consumption in pregnancy and infancy and subsequent childhood allergic diseases. J Allergy Clin Immunol 133:165–171.e8. e8. doi: 10.1016/j.jaci.2013.07.032
79. Tan, T, Xiao, D, Li, Q, Zhong, C, Hu, W, Guo, J, et al. (2023). Maternal yogurt consumption during pregnancy and infantile eczema: a prospective cohort study. Food Funct 14:1929–36. doi: 10.1039/D2FO02064E
80. Sproston, NR, and Ashworth, JJ (2018). Role of C-reactive protein at sites of inflammation and infection. Front Immunol 9:754. doi: 10.3389/fimmu.2018.00754
81. Du, Y, He, C, An, Y, Huang, Y, Zhang, H, Fu, W, et al. (2024). The role of short chain fatty acids in inflammation and body health. Int J Mol Sci 25:7379. doi: 10.3390/ijms25137379
82. Aslam, H, Green, J, Jacka, FN, Collier, F, Berk, M, Pasco, J, et al. (2020). Fermented foods, the gut and mental health: a mechanistic overview with implications for depression and anxiety. Nutr Neurosci 23:659–71. doi: 10.1080/1028415X.2018.1544332
83. Selhub, EM, Logan, AC, and Bested, AC (2014). Fermented foods, microbiota, and mental health: ancient practice meets nutritional psychiatry. J Physiol Anthropol 33:2. doi: 10.1186/1880-6805-33-2
84. Bryant, KL, Hansen, C, and Hecht, EE (2023). Fermentation technology as a driver of human brain expansion. Commun Biol 6:1190. doi: 10.1038/s42003-023-05517-3
85. Ohara, TE, and Hsiao, EY (2025). Microbiota–neuroepithelial signalling across the gut–brain axis. Nat Rev Microbiol 1–14. doi: 10.1038/s41579-024-01136-9
86. Gribble, FM, and Reimann, F (2019). Function and mechanisms of enteroendocrine cells and gut hormones in metabolism. Nat Rev Endocrinol 15:226–37. doi: 10.1038/s41574-019-0168-8
87. Porras-García, E, Fernández-Espada Calderón, I, Gavala-González, J, and Fernández-García, JC (2023). Potential neuroprotective effects of fermented foods and beverages in old age: a systematic review. Front Nutr 10:1170841. doi: 10.3389/fnut.2023.1170841
88. Tillisch, K, Labus, J, Kilpatrick, L, Jiang, Z, Stains, J, Ebrat, B, et al. (2013). Consumption of fermented milk product with probiotic modulates brain activity. Gastroenterology 144:1394–1401.e4. doi: 10.1053/j.gastro.2013.02.043
89. Yamamura, S, Morishima, H, Kumano-go, T, Suganuma, N, Matsumoto, H, Adachi, H, et al. (2009). The effect of Lactobacillus helveticus fermented milk on sleep and health perception in elderly subjects. Eur J Clin Nutr 63:100–5. doi: 10.1038/sj.ejcn.1602898
90. Chung, YC, Jin, HM, Cui, Y, Kim, DS, Jung, JM, Park, JI, et al. (2014). Fermented milk of Lactobacillus helveticus IDCC3801 improves cognitive functioning during cognitive fatigue tests in healthy older adults. J Funct Foods 10:465–74. doi: 10.1016/j.jff.2014.07.007
91. Ohsawa, K, Nakamura, F, Uchida, N, Mizuno, S, and Yokogoshi, H (2018). Lactobacillus helveticus-fermented milk containing lactononadecapeptide (NIPPLTQTPVVVPPFLQPE) improves cognitive function in healthy middle-aged adults: a randomised, double-blind, placebo-controlled trial. Int J Food Sci Nutr 69:369–76. doi: 10.1080/09637486.2017.1365824
92. Hirai, H, Tanaka, T, Matsumura, K, Tsuchida, A, Hamazaki, K, Adachi, Y, et al. (2024). Relationship between maternal consumption of fermented foods and the development of the offspring at the age of 3 years: the Japan environment and Children's study. PLoS One 19:e0305535. doi: 10.1371/journal.pone.0305535
93. Tanaka, T, Matsumura, K, Tsuchida, A, Hamazaki, K, Kasamatsu, H, Hirai, H, et al. (2024). Maternal fermented food intake and infant neurodevelopment: the Japan environment and Children's study. Asia Pac J Clin Nutr 33:66–82. doi: 10.6133/apjcn.202401_33(1).0008
94. Sugimori, N, Hamazaki, K, Matsumura, K, Kasamatsu, H, Tsuchida, A, Inadera, H, et al. (2019). Association between maternal fermented food consumption and infant sleep duration: the Japan environment and Children's study. PLoS One 14:e0222792. doi: 10.1371/journal.pone.0222792
95. Inoue, M, Sugimori, N, Hamazaki, K, Matsumura, K, Tsuchida, A, Inadera, H, et al. (2022). Association between maternal fermented food consumption and child sleep duration at the age of 3 years: the Japan environment and Children's study. BMC Public Health 22:1504. doi: 10.1186/s12889-022-13805-6
96. Zhang, Y, Guo, M, Zhang, H, Wang, Y, Li, R, Liu, Z, et al. (2022). Lactiplantibacillus plantarum ST-III-fermented milk improves autistic-like behaviors in valproic acid-induced autism spectrum disorder mice by altering gut microbiota. Front Nutr 9:1005308. doi: 10.3389/fnut.2022.1005308
97. David, LA, Materna, AC, Friedman, J, Campos-Baptista, MI, Blackburn, MC, Perrotta, A, et al. (2014). Host lifestyle affects human microbiota on daily timescales. Genome Biol 15:R89. doi: 10.1186/gb-2014-15-7-r89
98. K.Y Sim FYCaAA, (2015). Chemical composition and microbial dynamics of budu fermentation, a traditional Malaysian fish sauce. Acta Aliment 44:185–94. doi: 10.1556/AAlim.2014.0003
99. Puniya, AK . Fermented milk and dairy products. Boca Raton, FL: CRC Press, Taylor & Francis Group (2015).
100. Xanthopoulos Dp, V, and Tzanetakis, N (2006). Characterization and classification of Streptococcus Thermophilus and Lactobacillus delbrueckii subsp. Bulgaricus strains isolated from traditional Greek yogurts. J Food Sci Technol 66:747–52. doi: 10.1111/j.1365-2621.2001.tb04632.x
101. Chen, YS, Wu, HC, Lo, HY, Lin, WC, Hsu, WH, Lin, CW, et al. (2012). Isolation and characterisation of lactic acid bacteria from jiang-gua (fermented cucumbers), a traditional fermented food in Taiwan. J Sci Food Agric 92:2069–75. doi: 10.1002/jsfa.5583
102. Chang, JH, Shim, YY, Cha, SK, and Chee, KM (2010). Probiotic characteristics of lactic acid bacteria isolated from kimchi. J Appl Microbiol 109:220–30. doi: 10.1111/j.1365-2672.2009.04648.x
103. de Oliveira Leite, AM, Miguel, MA, Peixoto, RS, Rosado, AS, Silva, JT, and Paschoalin, VM (2013). Microbiological, technological and therapeutic properties of kefir: a natural probiotic beverage. Braz J Microbiol 44:341–9. doi: 10.1590/S1517-83822013000200001
104. Beganovic, J, Kos, B, Lebos Pavunc, A, Uroic, K, Jokic, M, and Suskovic, J (2014). Traditionally produced sauerkraut as source of autochthonous functional starter cultures. Microbiol Res 169:623–32. doi: 10.1016/j.micres.2013.09.015
105. Chen, YS, Yanagida, F, and Hsu, JS (2006). Isolation and characterization of lactic acid bacteria from suan-tsai (fermented mustard), a traditional fermented food in Taiwan. J Appl Microbiol 101:125–30. doi: 10.1111/j.1365-2672.2006.02900.x
106. Chen, YS, Wu, HC, Li, YH, Leong, KH, Pua, XH, Weng, MK, et al. (2012). Diversity of lactic acid bacteria in sian-sianzih (fermented clams), a traditional fermented food in Taiwan. J Sci Food Agric 92:321–7. doi: 10.1002/jsfa.4578
107. EFRIWATI, AS, and RAHAYU, GAYUH (2013). LILIS NURAIDA 2. Population dynamics of yeasts and lactic acid Bacteria (LAB) during tempeh production. HAYATI. J Biosci 20:57–64. doi: 10.4308/hjb.20.2.57
108. Allwood, JG, Wakeling, LT, and Bean, DC (2021). Fermentation and the microbial community of Japanese koji and miso: a review. J Food Sci 86:2194–207. doi: 10.1111/1750-3841.15773
109. Coda, R, Cagno, RD, Gobbetti, M, and Rizzello, CG (2014). Sourdough lactic acid bacteria: exploration of non-wheat cereal-based fermentation. Food Microbiol 37:51–8. doi: 10.1016/j.fm.2013.06.018
110. Afzaal, M, Saeed, F, Islam, F, Ateeq, H, Asghar, A, Shah, YA, et al. (2022). Nutritional health perspective of Natto: a critical review. Biochem Res Int 2022:1–9. doi: 10.1155/2022/5863887
111. Jang, S, Kim, YJ, Park, JM, and Park, YS (2011). Analysis of microflora in gochujang, Korean traditional fermented food. Food Sci Biotechnol 20:1435–40. doi: 10.1007/s10068-011-0197-0
112. Kim, JY, Yeo, SH, Baek, SY, and Choi, HS (2011). Molecular and morphological identification of fungal species isolated from bealmijang meju. J Microbiol Biotechnol 21:1270–9. doi: 10.4014/jmb.1105.05013
113. Karimi, R, Mortazavian, AM, and Da Cruz, AG (2011). Viability of probiotic microorganisms in cheese during production and storage: a review. Dairy Sci Technol 91:283–308. doi: 10.1007/s13594-011-0005-x
114. Iyer, BK, Singhal, RS, and Ananthanarayan, L (2013). Characterization and in vitro probiotic evaluation of lactic acid bacteria isolated from idli batter. J Food Sci Technol 50:1114–21. doi: 10.1007/s13197-011-0445-6
115. Ilango, S, Pandey, R, and Antony, U (2016). Functional characterization and microencapsulation of probiotic bacteria from koozh. J Food Sci Technol 53:977–89. doi: 10.1007/s13197-015-2169-5
116. Nimalan Jeyagowri, CSR, Manap, MY, Gamage, A, and Othmane, M e (2023). Phenotypic characterisation and molecular identification of potentially probiotic Lactobacillus sp. isolated from fermented rice. Fermentation 9:807. doi: 10.3390/fermentation9090807
117. Marsh, AJ, O'Sullivan, O, Hill, C, Ross, RP, and Cotter, PD (2014). Sequence-based analysis of the bacterial and fungal compositions of multiple kombucha (tea fungus) samples. Food Microbiol 38:171–8. doi: 10.1016/j.fm.2013.09.003
118. Yu, J, Wang, WH, Menghe, BL, Jiri, MT, Wang, HM, Liu, WJ, et al. (2011). Diversity of lactic acid bacteria associated with traditional fermented dairy products in Mongolia. J Dairy Sci 94:3229–41. doi: 10.3168/jds.2010-3727
119. Chang, CH, Chen, YS, and Yanagida, F (2011). Isolation and characterisation of lactic acid bacteria from yan-jiang (fermented ginger), a traditional fermented food in Taiwan. J Sci Food Agric 91:1746–50. doi: 10.1002/jsfa.4364
120. Adikari, A, Priyashantha, H, Disanayaka, JNK, Jayatileka, DV, Kodithuwakku, SP, Jayatilake, J, et al. (2021). Isolation, identification and characterization of L actobacillus species diversity from Meekiri: traditional fermented buffalo milk gels in Sri Lanka. Heliyon 7:e08136. doi: 10.1016/j.heliyon.2021.e08136
121. Shrestha, H, Nand, K, and Rati, ER (2002). Microbiological profile of MURCHA starters and PHYSICO-CHEMICAL characteristics of POKO, a rice based traditional fermented food product of Nepal. Food Biotechnol 16:1–15. doi: 10.1081/FBT-120004198
122. Kharnaior, P, and Tamang, JP (2022). Metagenomic-Metabolomic Mining of Kinema, a naturally fermented soybean food of the eastern Himalayas. Front Microbiol 13:868383. doi: 10.3389/fmicb.2022.868383
123. Young-Do Nam S-HYLim, S-I (2012). Bacterial diversity of cheonggukjang, a traditional Korean fermented food, analyzed by barcoded pyrosequencing. Food Control 28:135–42. doi: 10.1016/j.foodcont.2012.04.028
124. Jane Misihairabgwi, AC (2017). Traditional fermented foods and beverages of Namibia. J Ethnic Foods 4:145–53. doi: 10.1016/j.jef.2017.08.001
Keywords: fermented foods, pregnancy, infant, brain, immune system, gut microbiota
Citation: Pandiyan A, Gurung M, Mulakala BK, Ponniah SK and Yeruva L (2025) The role of fermented foods in maternal health during pregnancy and infant health during the first 1,000 days of life. Front. Nutr. 12:1581723. doi: 10.3389/fnut.2025.1581723
Edited by:
Raul Avila-Sosa, Benemérita Universidad Autónoma de Puebla, MexicoReviewed by:
Tales Fernando da Silva, Institute of Biological Sciences, BrazilIñaki Diez-Ozaeta, Spanish National Research Council (CSIC), Spain
Vishnupriya Sethuraman, Bharathidasan University, India
Jayani Kulathunga, University of Sri Jayewardenepura, Sri Lanka
Rajni Devi, Punjab Agricultural University, India
Copyright © 2025 Pandiyan, Gurung, Mulakala, Ponniah and Yeruva. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Laxmi Yeruva, bGF4bWkueWVydXZhQHVzZGEuZ292
†These authors have contributed equally to this work
 Arun Pandiyan
Arun Pandiyan Manoj Gurung
Manoj Gurung Bharath Kumar Mulakala1,2,4
Bharath Kumar Mulakala1,2,4 Sathish Kumar Ponniah
Sathish Kumar Ponniah Laxmi Yeruva
Laxmi Yeruva