- 1ICAR-Indian Institute of Oil Palm Research, Pedavegi, India
- 2ICAR-National Bureau of Agriculturally Important Microorganism, Mau, India
- 3ICAR-Central Arid Zone Research Institute, Jodhpur, India
- 4Crop Science Unit, Department of Agronomy, Federal University Gashua, Gashua, Nigeria
Purpose: Basal stem rot (BSR), with Ganoderma spp. as the principal causative agent, is an important oil palm disease, leading to significant stand loss and reduced yield potential. The use of antagonistic fungi, particularly Trichoderma spp., offers a sustainable approach to disease suppression through hyperparasitism, antibiosis, and rhizosphere competence. However, strain-dependent variability in antagonistic potential necessitates the selection of the most efficacious isolates for integrated BSR management. Here we show that T. afroharzianum exhibits superior antagonism against Ganoderma spp., in dual culture, inverted plate assay as well as cellfiltrate assays.
Methods: From 50 Trichoderma isolates screened, 12 highly mycoparasitic strains (>80% Ganoderma suppression) were selected. To enhance applicability under field conditions, the selected strains were further evaluated against co-occurring soil-borne pathogens commonly associated with oil palm decline.
Results: T. afroharzianum exhibited hydrolytic enzyme secretion (chitinase, cellulase, and pectinase), solubilized key macronutrients, and suppressed multiple soil-borne phytopathogens including Rhizoctonia solani, R. bataticola, Fusarium solani, Lasiodeplodia theobromae Colletotrichum gleosporoides and Curvularia lunata. A tailored Trichoderma consortium achieved 61.94% disease suppression, reduced foliar and bole severity by 48.59 and 20.22%, respectively, and increased plant height (47.59 ± 2.52 cm) and shoot fresh weight (15.83 ± 0.80 g).
Implications/conclusion: These findings establish T. afroharzianum as a promising biocontrol agent for BSR suppression through multiple mechanisms, including competitive exclusion and pathogen inhibition. The results support its potential for field deployment as part of an integrated, climate-resilient disease management strategy in oil palm cultivation.
1 Introduction
Oil palm (Elaeis guineensis Jacq.), commonly known as the “Golden Palm,” is a global most important oil-bearing crop, producing 4–6 tonnes of edible oil and 0.5 tonnes of kernel oil per hectare per year, which is 5–10 times higher compared with other oil crops (1). Palm oil use is rising worldwide, due to its rich content of palm olein, renowned for having an appropriate proportion of saturated to unsaturated fatty acids, which can lower LDL cholesterol, as well as its excellent antioxidant properties. BSR caused by Ganoderma spp. is a global threat to oil palm plantations, causing up to 500 million USD in annual losses. The disease raises grave concerns about food security due to its significant impact on the global edible oil supply (2). This issue is particularly significant for India, one of the world’s largest oil-consuming and oil-importing countries, with limited domestic palm oil production, only 0.45 million hectares yielding 0.33 million tonnes.1 Palm oil cultivation has been largely confined to Andhra Pradesh, Telangana, and Kerala, which account for 98% of production.2 However, the spread of BSR threatens the objectives of the National Mission on Edible Oils – Oil Palm (NMEO-OP), which aims to expand cultivation across diverse agro-climatic zones, increase production, and achieve self-sufficiency.
The early foliar symptoms, such as petiole breaking and skirting, are often ambiguous and may be mistaken for vapor pressure deficit, leading to their frequent oversight or misinterpretation. The definitive observable symptom is the appearance of basidiocarps, which signals 80% of fungal invasion and marks the penultimate stage of death. The pathogen is unique in several characteristics such as dual feeding behavior, latent infection and prolonged gestation period (3, 4). Moreover, the persistent and recalcitrant soil-borne characteristics facilitate the production of an immense inoculum load including resistant forms such as mycelium, basidiospores, chlamydospores, and pseudo sclerotia, elevate it to the status of an exceptionally challenging pathogen to manage (5). The current sanitation and chemical management of BSR can only delay the progression of Ganoderma infection and prolongs the productive lifespan of infected palms. Since there are no seasonal breaks, the oil palm’s perennial and monoculture characteristics allow for the build-up of sizable inoculum and disease pressure reservoirs, which exacerbates the situation even more. This demands frequent and labour-intensive pesticide applications, which may prompt to the build-up of pesticidal resistance and toxic accumulation of residues, resulting in economic strain, health hazards and ecological damage. Moreover, they are not long lasting and inefficient in reducing inoculum load under field conditions (6, 7). Hence, curative strategies for managing this formidable pathogen remain largely impractical.
Under such circumstances, adoption of eco-friendly, and sustainable management measures, is the only viable option. As there is no resistant germplasm available for BSR, microbe-based biocontrol strategies evolved as a potential green tool for combating BSR in the era of sustainable agriculture. Studies have demonstrated remarkable results when microbial antagonists, including species of Penicillium, Pseudomonas, Aspergillus, Bacillus, Streptomyces, and Trichoderma were used individually or in consortia to manage BSR in plantations (5, 7–14). Trichoderma has emerged as a potent biocontrol agent against Ganoderma, primarily through mechanisms such as mycoparasitism, production of antifungal metabolites, and competitive exclusion, effectively limiting pathogen growth and spread. Its efficacy has been demonstrated in both field and green house environmental conditions (5, 7, 11, 12, 15–17).
The effectiveness of Trichoderma can vary significantly due to their unique feature of adapting to specific soil types, methods, and application timing, rhizospheric competency, abiotic and biotic factors. These variations pose a challenge to their biological stability, feasible delivery, and ease of commercialization for widespread field applications (18). Isolation of naturally occurring antagonistic Trichoderma strains from diverse and extreme niches is a promising strategy to obtain abiotic stress-tolerant antagonists, enabling the development of robust formulations tailored for application across varied climatic zones, ensuring consistent and effective BSR management (19). Hence, in the context of changing climate scenario, use of these diverse strains with compatible biocontrol activity can be successfully included in designing cocktails of bioagents instead of sole application of native strain to achieve consistent field performance in different soil environments and agricultural ecosystems (20). Despite the widespread use of Trichoderma as an agent of biological control, there remains a significant gap in the understanding its exploitation, characterization, and specific mode of action within the Ganoderma-oil palm system in India. Additionally, the opportunity to isolate stress-tolerant Trichoderma strains from India’s varied agroclimatic regions remains largely untapped. Selecting Trichoderma isolates from diverse agroclimatic regions allows for the identification of ecologically adapted strains with biocontrol efficacy, environmental resilience, and compatibility with variable soil–climate systems. This approach is particularly relevant for oil palm cultivation, which under the National Mission on Edible Oils–Oil Palm (NMEO-OP), is being expanded into non-traditional and climatically diverse regions across India. The deliberate inclusion of ecologically contrasting zones, ranging from arid zones (e.g., Rajasthan), humid tropical coasts (e.g., Kerala), to subtropical alluvial plains (e.g., Uttar Pradesh), was designed to capture the natural diversity of Trichoderma populations shaped by their native environments. Isolates from such varied contexts are more likely to exhibit stress tolerance, robust rhizosphere competence, and broad-spectrum antagonism traits critical for consistent performance in field conditions and for the development of climate-resilient biocontrol formulations. Given the foregoing, the current study aims to bio prospect Trichoderma spp. from diverse agroecological zones, characterize their biocontrol and growth promotion properties, and identify the most potent strains for managing BSR in oil palm contributing to sustainable disease management solutions (Figure 1).
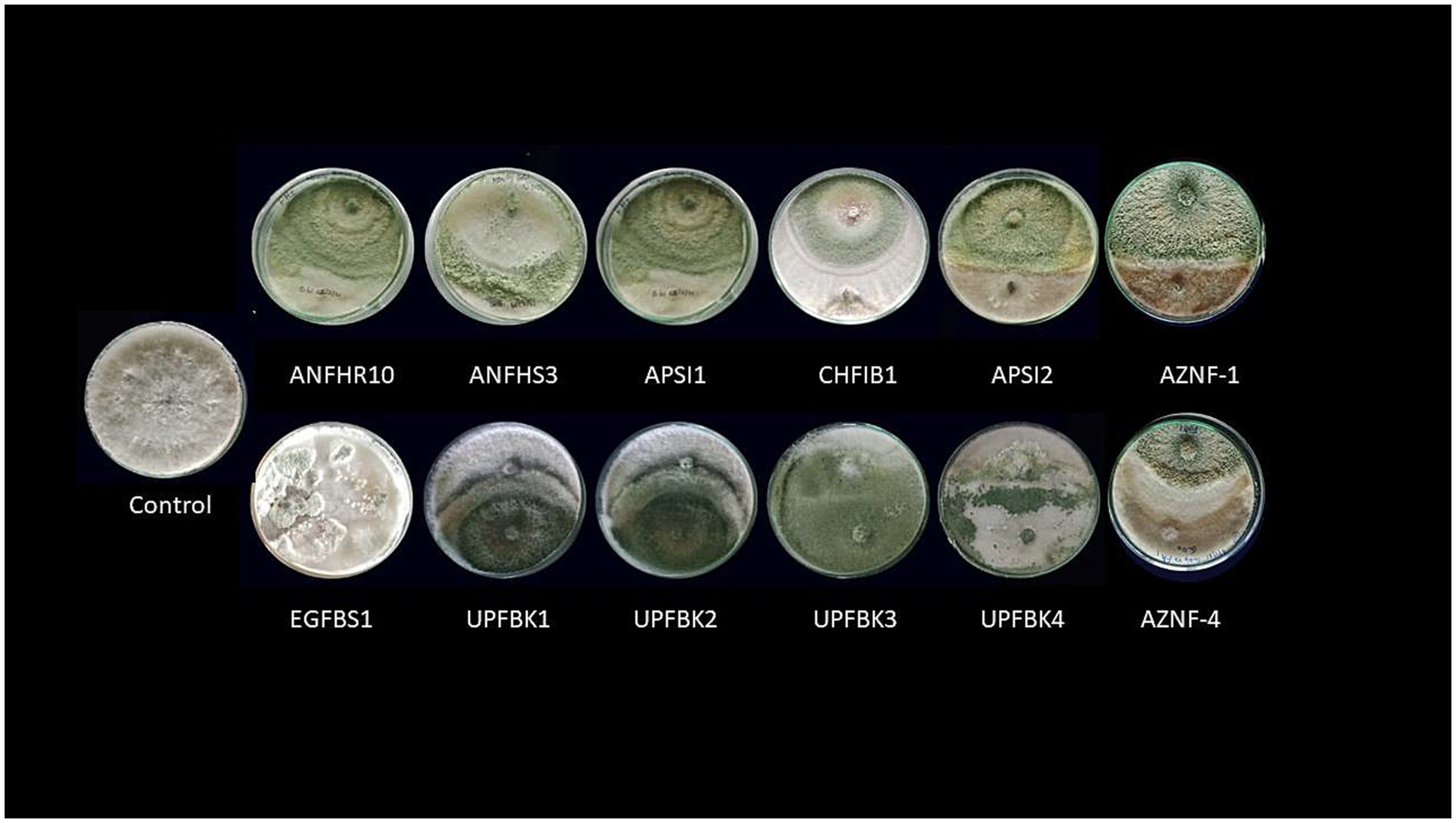
Figure 1. Antifungal efficacy of Trichoderma spp. against mycelial growth of Ganoderma ellipsoideum by dual plate technique.
2 Materials and methods
2.1 Test pathogen source and maintenance
The test pathogen used in this study was Ganoderma ellipsoideum (strain ITCC9346), obtained from the Indian Type Culture Collection (ITCC), Division of Plant Pathology, ICAR–Indian Agricultural Research Institute, New Delhi. Potato Dextrose Agar (PDA) was used as the growth medium. Sterile PDA plates and slants were prepared under aseptic conditions (Materials). The pathogen was sub-cultured on PDA and incubated at 28 ± 2°C. Mycelial growth was maintained on PDA slants and stored at 4°C for further experimental use (Methods).
2.2 Survey and isolation of Trichoderma
Soil, bark, root, and basidiocarp samples were collected from oil palm rhizospheres and decaying organic matter in five agroecological zones of India: Andhra Pradesh (East Coast Plains and Hills zone- humid to subhumid), Telangana (Southern Plateau and Hills- semi-arid to subhumid), Kerala (West Coast Plains and Ghats-Tropical), Andaman and Nicobar (Coastal Islands zone-Tropical) and Uttar Pradesh (Upper Gangetic Plains Region- subtropical). Samples were collected using clean polyethylene bags and transported to the laboratory. Trichoderma Specific Medium (TSM) was used for isolation, following the protocol of Papavizas and Lewis (21). All media, glassware, and tools were sterilized before use. Pure cultures were preserved in 20% glycerol at −20°C (Materials). A total of 150 samples were collected and stored at 4°C prior to processing. Soil samples were serially diluted (10−3 to 10−6) using sterile water and plated on TSM. Root and bark pieces were surface sterilized and directly plated onto TSM plates. Plates were incubated at 28 ± 2°C for 4 days. Colonies with typical Trichoderma morphology were purified using hyphal tip isolation. Purified cultures were stored in 20% glycerol for long-term preservation.
2.3 Preliminary screening of mycoparasitism by dual confrontation assay
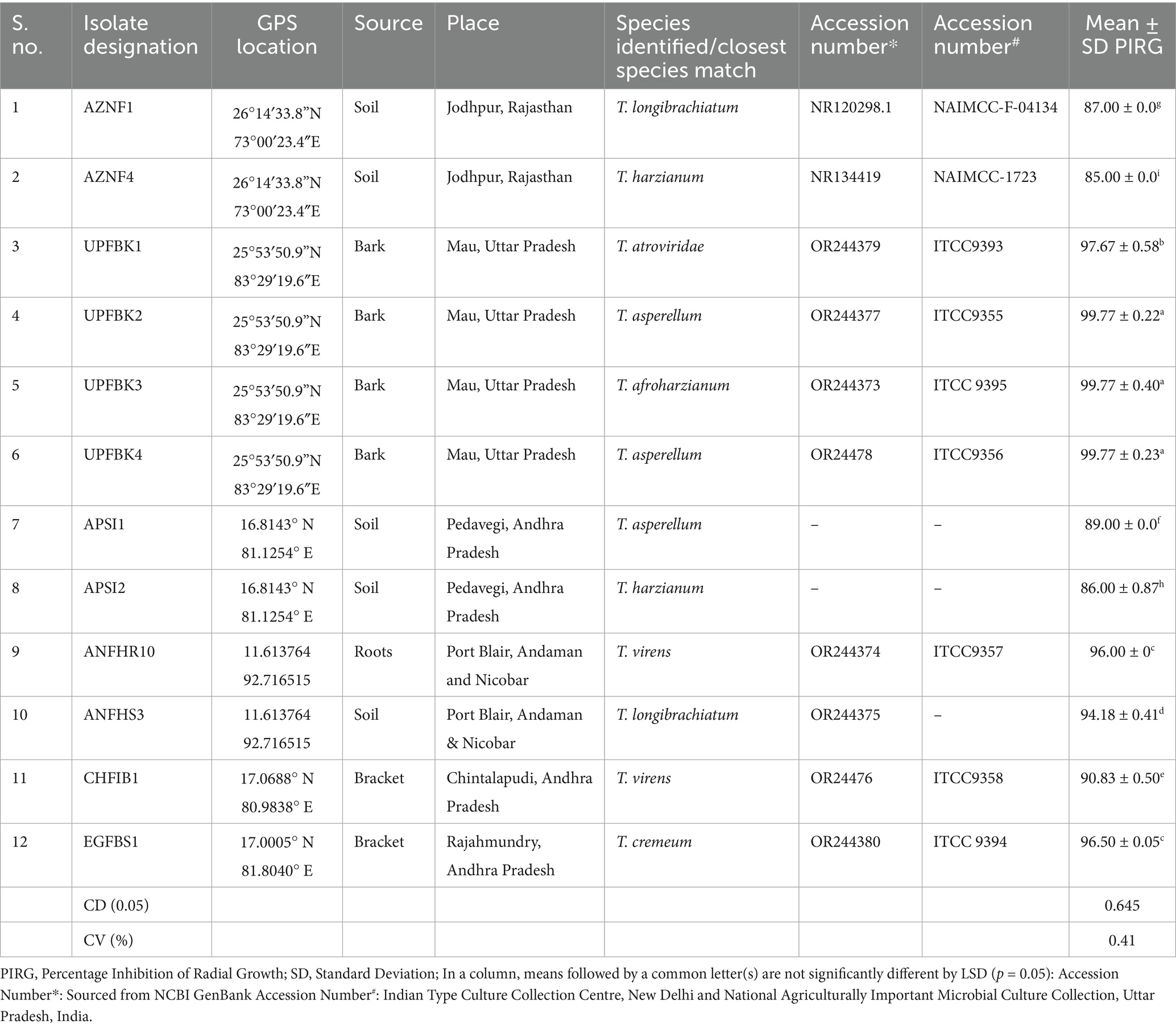
Fifty new Trichoderma isolates, along with previously collected strains of T. longibrachiatum (NAIMCC-F-04134) and T. harzianum (NAIMCC-1723) from ICAR–Central Arid Zone Research Institute (CAZRI), Jodhpur, and T. asperellum and T. harzianum from ICAR–Indian Institute of Oil Palm Research (IIOPR), Pedavegi, were evaluated for in vitro mycoparasitism against Ganoderma using the dual-plate culture method (22, 23). Inoculum plugs of Trichoderma and Ganoderma were placed opposite each other, 1 cm away from the periphery of a 9 cm Petri dish, to allow clear observation of their antagonistic interaction. The reference strains are deposited at the National Agriculturally Important Microbial Culture Collection (NAIMCC), ICAR–National Bureau of Agriculturally Important Microorganisms (NBAIM), Mau, Uttar Pradesh, India. The plates were subsequently incubated at 28 ± 2°C until the mycelial growth fully covered the surface of the control plates. To verify the findings, the trial was conducted in three replications and repeatedly. The radial growth of Ganoderma (T) towards Trichoderma isolates was documented and the percentage inhibition of radial growth (PIRG) was determined viz.:
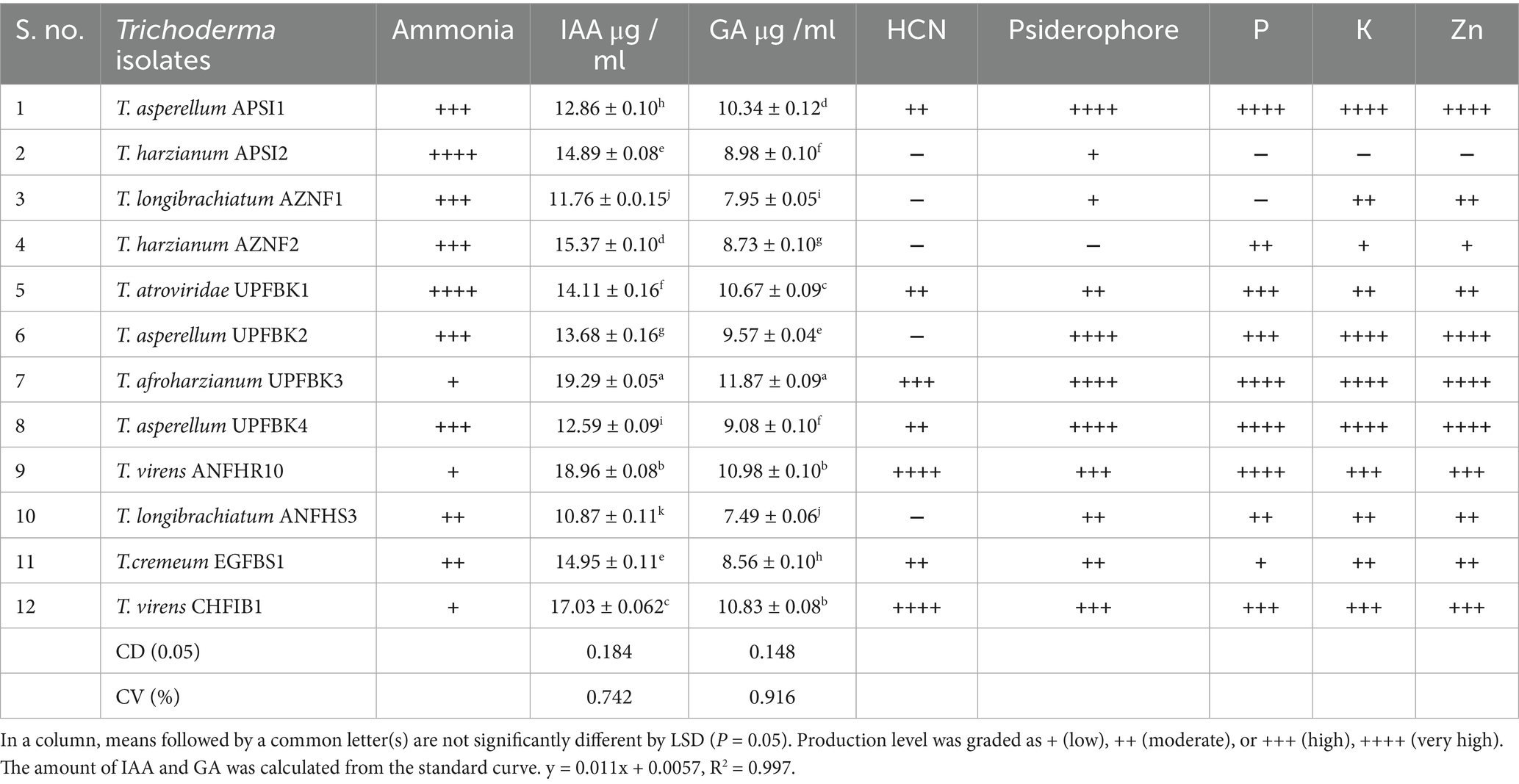
Where the radial growth of Ganoderma mycelia in treatment and control plates were tagged as T and C, respectively. Based on results, the Trichoderma isolates were categorized into various groups. The top 12 potent isolates, demonstrating over 80% suppression of Ganoderma, were chosen for downstream investigation (Table 1).
2.4 Evaluation of antibiosis activity of Trichoderma isolates
2.4.1 Inverted plate assay for determining volatile antibiosis
A fifteen milliliter amount of 2.4% (w/v) PDA was added to 90 mm Petri dishes for an inverted plate assay (IPA). A mycelial disc with 5 mm in diameter was moved from the Trichoderma culture’s periphery to the middle of medium. Additionally, a mycelial disc was inverted over the Trichoderma culture after being moved from the Ganoderma culture’s periphery to a fresh Petri dish. For 7 days, the assembled plates were kept at 25°C in the dark after being sealed using parafilm and then wrapped with three layers of plastic wrap to ensure minimal gas exchange and containment of volatile compounds. For the control, only Ganoderma was inoculated in the lower Petri dish, while the negative control consisted of uninoculated medium (24). The volatile-based inhibition of Ganoderma by various Trichoderma isolates was evaluated based on PIRG, as described earlier (Figure 2).
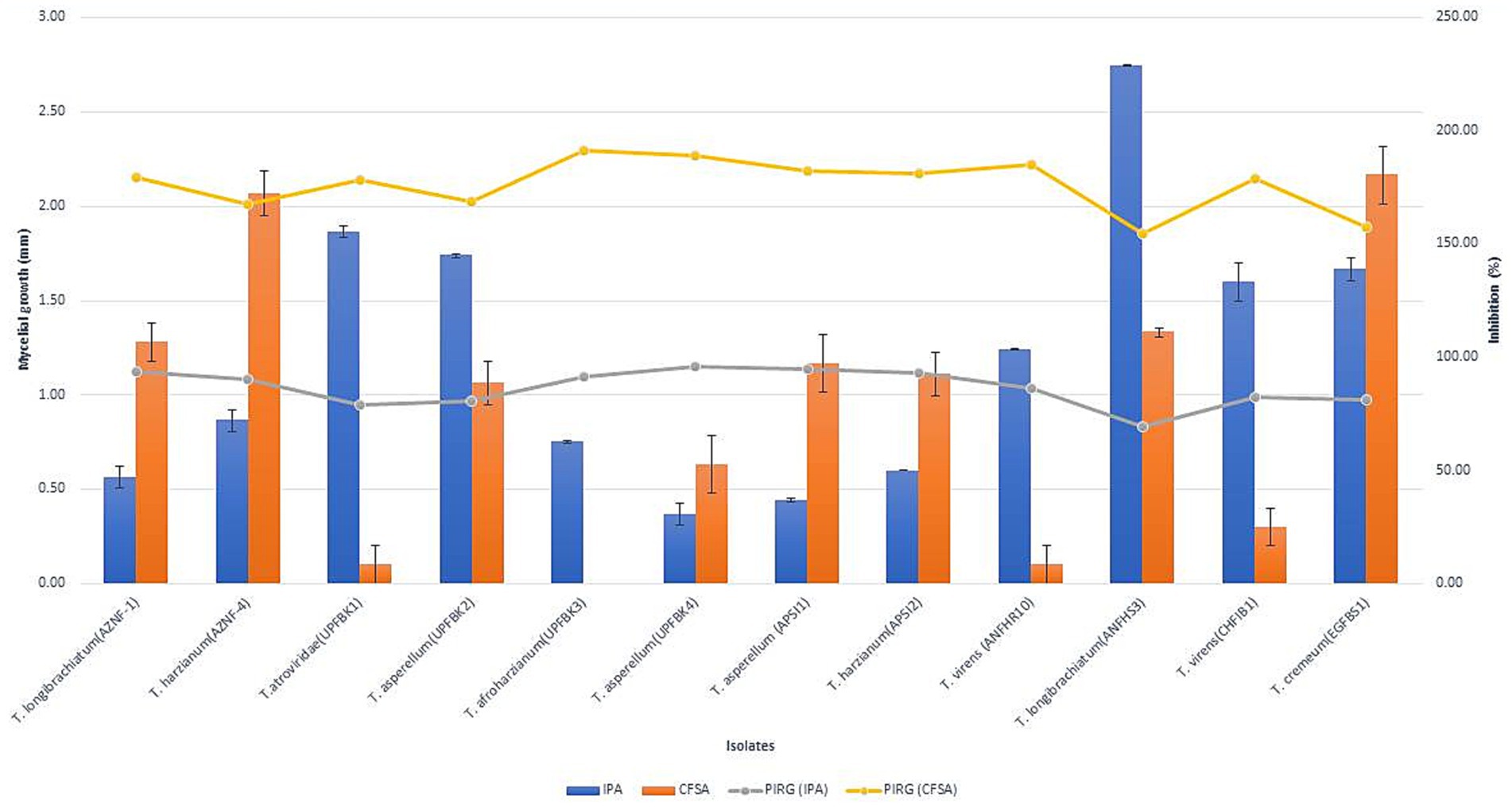
Figure 2. Inhibition of Ganoderma ellipsoideum growth by antifungal volatiles and non-volatiles from Trichoderma species. The graph shows the effect of Trichoderma-derived volatiles (bars) and non-volatiles (line) on Ganoderma ellipsoideum growth inhibition. Data represent percentage growth reduction, with higher concentrations or exposure times leading to greater inhibition. Statistical significance is denoted by asterisks (*p < 0.05, **p < 0.01).
2.4.2 Cell filtrate assay for evaluating soluble non-volatile antibiosis
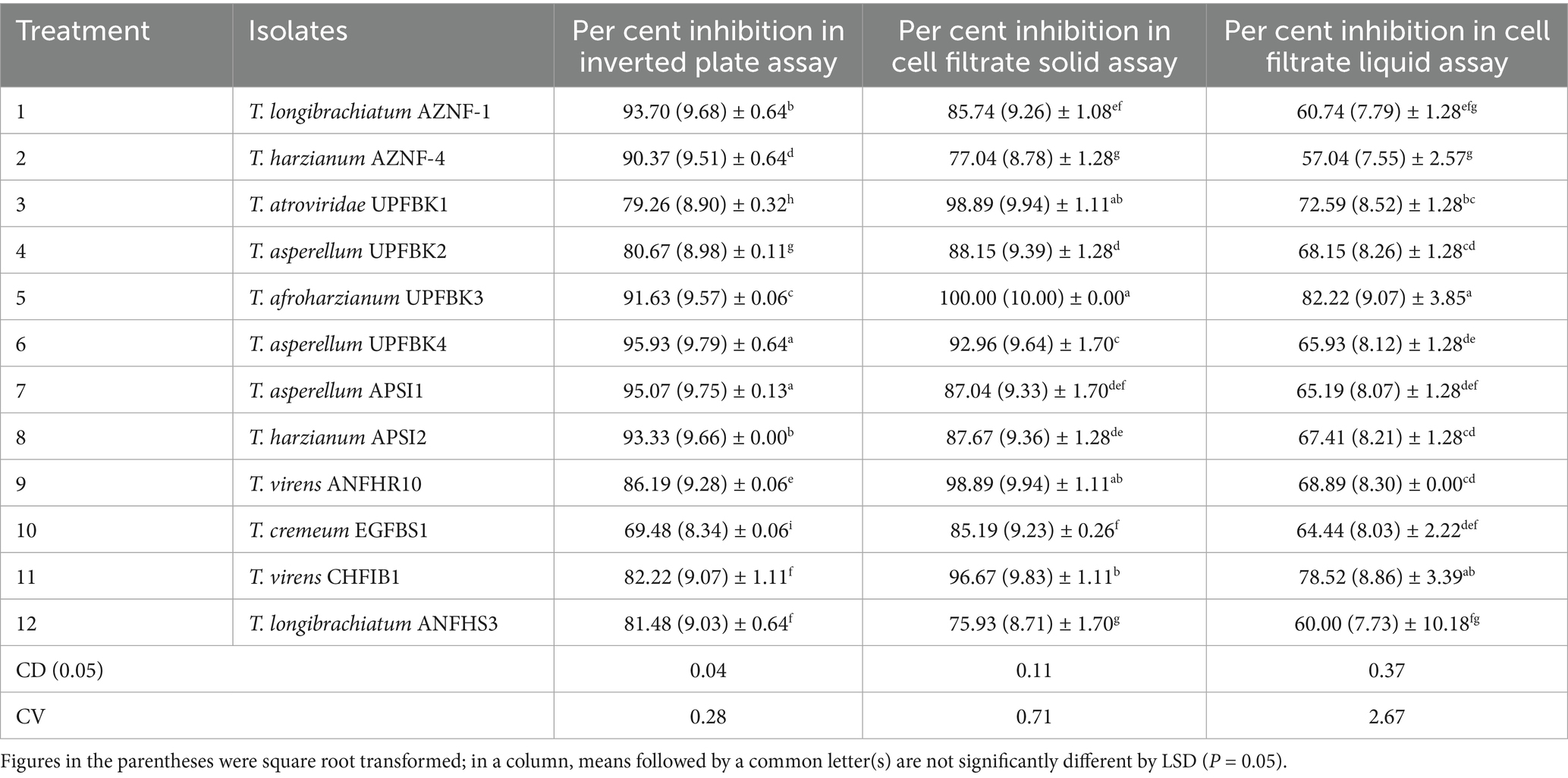
The cell filtrate assay was employed to assess the impact of non-volatiles on the growth and dry weight of test pathogen, using both solid and liquid poisoned food assay (CFSA and CFLA). One mL of each Trichoderma conidial suspension was mixed with 100 mL of Potato Dextrose Broth (PDB) to make the culture filtrate. The resultant mixture was then incubated for 7 days in an orbital shaker at 28°C and 100 rpm. Following a 15-min centrifugation at 10,000 rpm at 4°C, the culture was filtered through syringe filters with 0.22 μm size to remove the cell-free supernatant. A 5 mm disc of G. ellipsoideum was used to inoculate the plates for the agar experiment, which involved adding five milliliters of culture filtrate to molten PDA. After all of the mycelia had covered the control plate (which did not include culture filtrate), the pathogen’s radial mycelial growth was measured. The PIRG was determined as explained previously. For the broth assay, 10 mL of cell filtrate was added to 50 mL of PDB, followed by inoculation with a 5 mm Ganoderma mycelial disc. The co-incubated cultures were maintained in an orbital shaker at 100 rpm and 28°C for 7 days. The Ganoderma mycelial mat was separated using pre-weighed Whatman No. 1 filter paper and subsequently dried at 50°C for 4 h in a hot air oven. The reduction in dry weight was calculated relative to the Ganoderma-inoculated control (without Trichoderma culture filtrate). The negative control consisted of uninoculated broth without Trichoderma. All treatments were performed in triplicate.
2.5 Morphological characterization
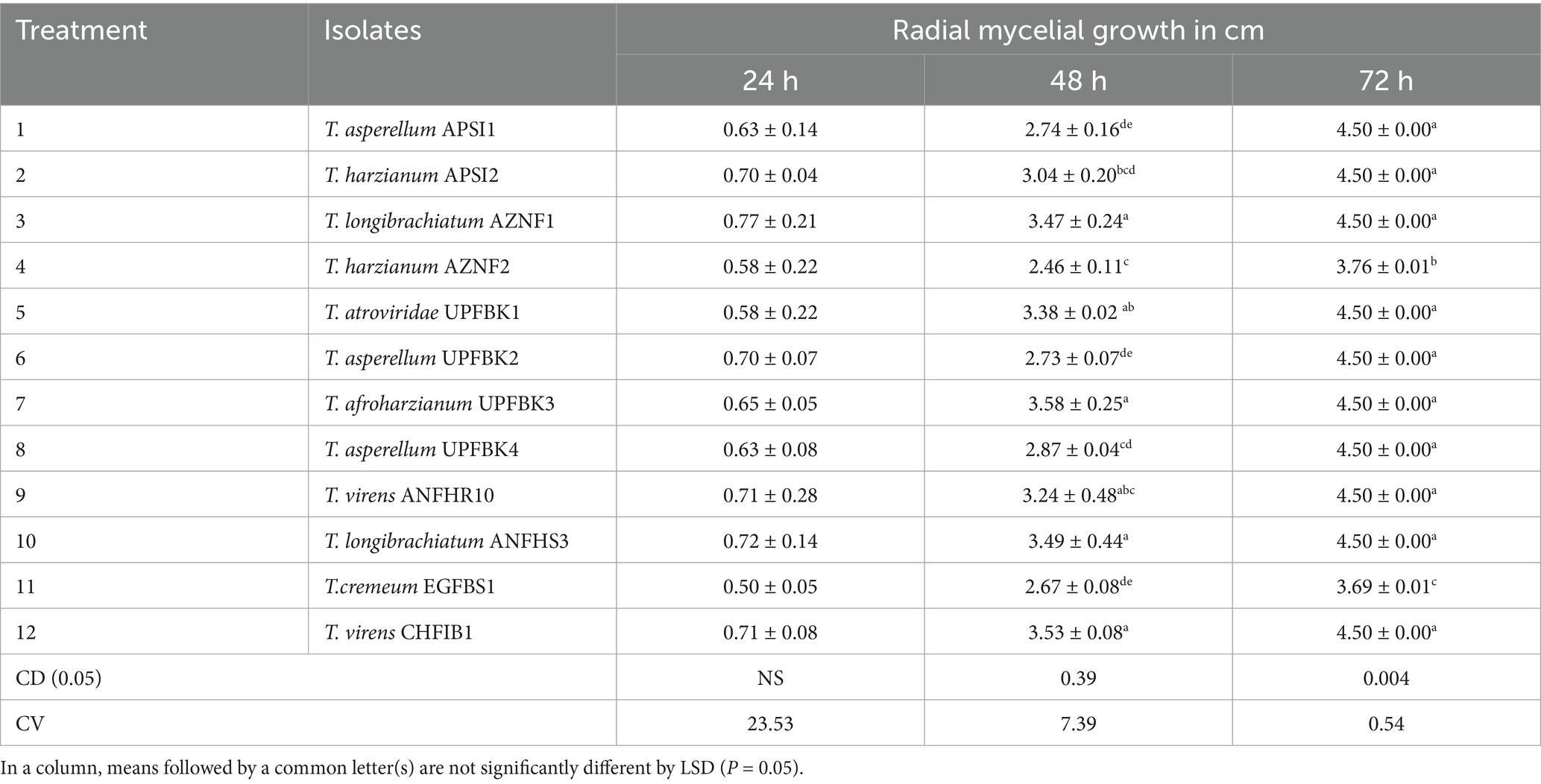
The morphological and cultural features of Trichoderma isolates were examined on PDA medium using the protocol outlined by Samuels et al. (25). Microscopic observations were conducted based on the monograph by Rifai (26). A 5 mm mycelial disc of each Trichoderma isolate (cultured for 7 days) was inoculated on the periphery of the Petri dishes and incubated at 28 ± 2°C for 1 week. The radius of the colony was determined at 24, 48 and 72 h. The growth rates were calculated by averaging the measurements from three independent trials, each conducted in triplicates. The colony characteristics, including shape, texture, growth patterns, colony margins and presence of concentric rings, were recorded. Pigmentation on both colony surface and reverse side of the plate was also noted. The microscopic observations were made following the guidelines in Rifai (26), focusing on the arrangement and Conidiophores branching pattern, conidia size and form, and the nature of the spores were observed using Leica DM 5000 B microscope (Leica MikrosystemsVertrich GmbH, Germany).
2.6 Trichoderma spp. molecular identification and bioinformatics analyses
Monosporic cultures of Trichoderma strains were molecularly identified. Agar plugs (10 mm) from 7-day-old cultures were inoculated to PDB and an actively growing mycelial mat (150 mg) was harvested on the 10th day and blot-dried on sterile filter paper. DNA extraction from Trichoderma mycelia was performed using HiPurA® Fungal DNA Purification Kit (HiMedia, India). NanoDrop™ Spectrophotometer ND-1000 (Eppendorf, Germany) and agarose gel electrophoresis (0.8%) were used to probe the purity status and the concentration of the isolated DNA. Pure DNA was then amplified via PCR amplification using Internal Transcribed Spacer primers: ITS1 (5′ -TCC GTA GGT GAA CCT GCG G- 3′) and ITS4 (5′ -TCC TCC GCT TAT TGA TAT GC-3′) and procedures developed by White et al. (27). The PCR composition was standardized as follows: 1.5 μL of 10x PCR buffer (HiMedia, India), 0.15 μL of 10 mM dNTP (HiMedia, India), 1.5 μL (10 pg./μL) each of both forward reverse primers and 0.15 μL of 5 U Taq polymerase (HiMedia, India) and made to a reaction volume of 15 μL by adding 10.275 μL of nuclease free Milli-Q water. PCR conditions for primers were standardized as 94°C – 3 min, 94°C –30 s, 51°C –30s, 72°C –1.0 min; finally 72°C–10 min. To the amplified product, 2.0 μL Ethidium Bromide dye (Thermo Fisher, USA) was added, and PCR products (5 μL/sample) were electrophoresed in a 1.2 per cent agarose gel for 45 min. In a gel documentation system (IGENE LABSERVE, India), the gel was analyzed under ultraviolet light after being stained with ethidium bromide. The amplicons were sent for Sanger sequencing. Sequences with the accession number shown in Table 1 were added to GenBank. To find related sequences, the consensus sequence was BLAST-searched against the NCBI database where the top 10 sequences were chosen based on the maximum identity scores for downstream analyses. The Clustal-W algorithm was employed to run the Multiple DNA sequence alignments. Evolutionary analyses were conducted using the concatenated 5′- ITS region (575 bp), with reference sequences corresponding to the most closely related taxa downloaded from GenBank. The Maximum Composite Likelihood method was used to measure the evolutionary distances, expressed as number of base substitutions per site implemented in the PhyML program, with the tree topology further evaluated by bootstrap resampling of 1,000 iterations (28). The neighbor–joining approach was used to estimate the evolutionary history (29), and a bootstrap study with 1,000 repetitions was conducted to evaluate the tree topology’s robustness (30). MEGA X was used for all evolutionary analyses (31) (Table 2).
2.7 Evaluation of broad-spectrum efficiency
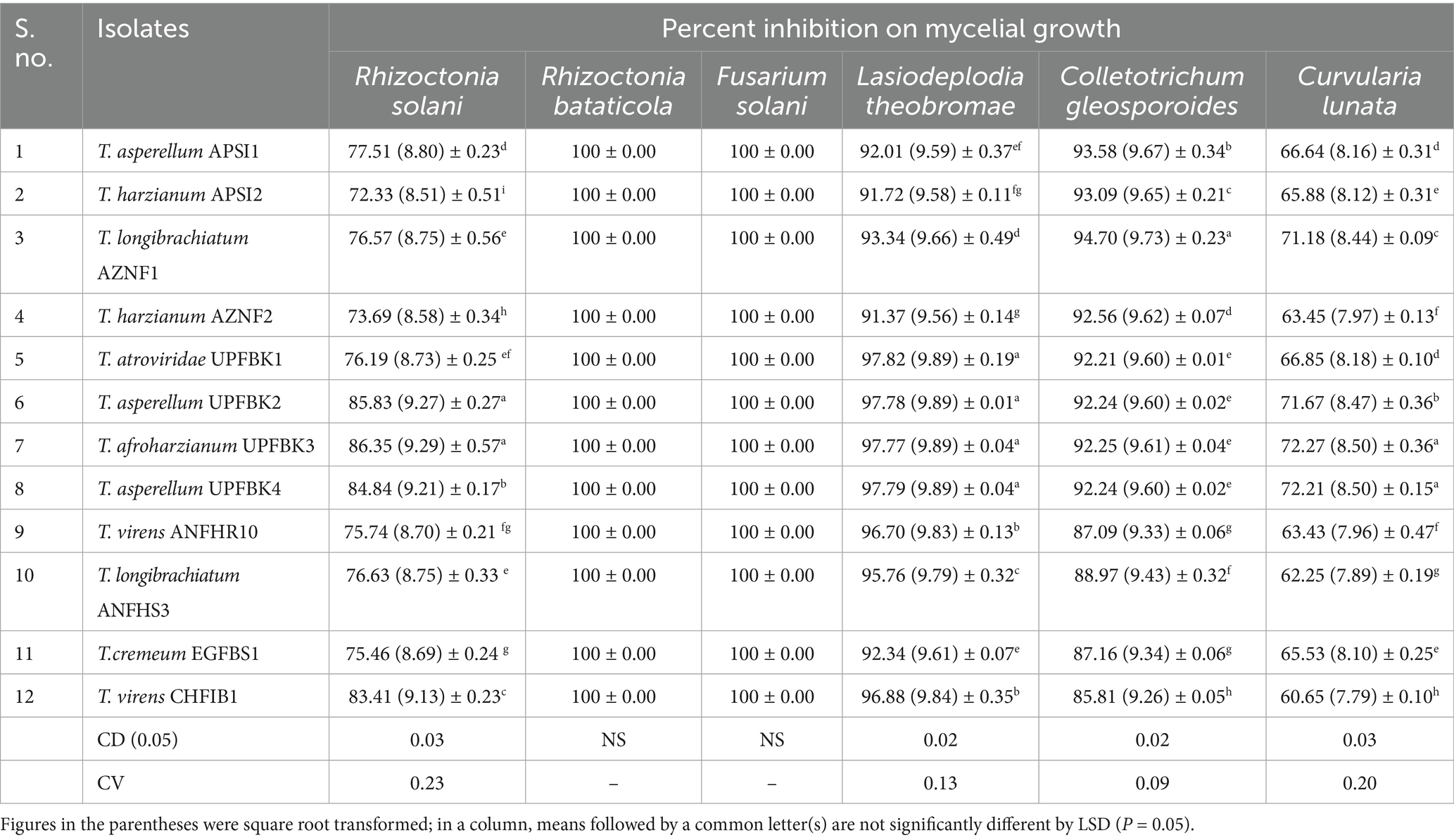
Dual confrontation assays of 12 Trichoderma isolates were performed against oil palm-associated pathogens at nursery and field levels. The targeted pathogens included Curvularia lunata (leafspot), Collectotrichum gleosporoides (anthracnose), Lasiodeplodia theobromae (leaf blight), Rhizoctonia bataticola, Rhizoctonia solani causing (leaf rot), and Fusarium solani (bunch rot) at nursery and field level, respectively. The mycoparasitism was assessed based on PIRG (%), as previously described.
2.8 Qualitative assessment of detoxifying and lytic enzyme synthesis
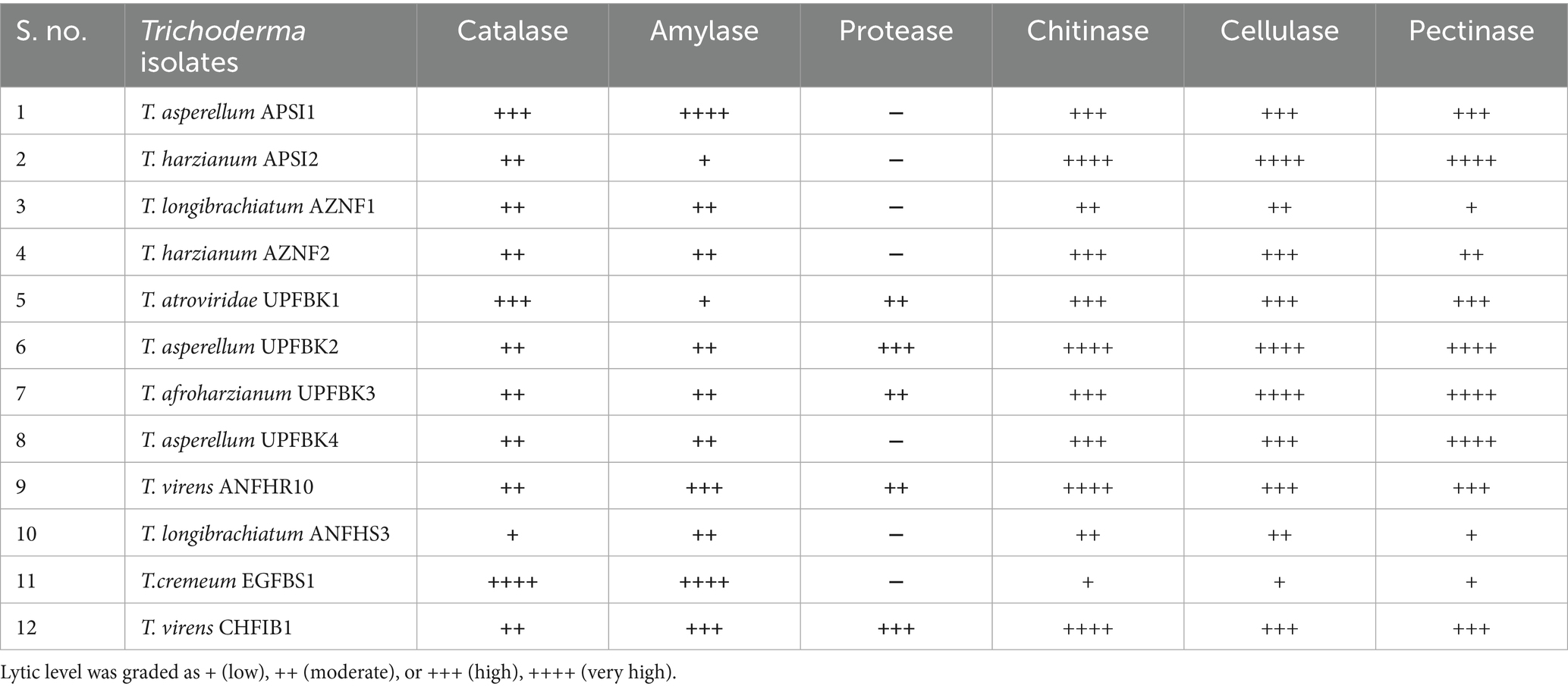
The qualitative evaluation of detoxifying and lytic enzyme production was conducted using 7-day-old cultures of Trichoderma, with each assay performed in triplicate following the procedure of Cappuccino and Sherman (32). For every biochemical test, a non-inoculated plate served as the negative control. The observation of a distinct halo surrounding the colony after 7 days of incubation indicated positive lytic activity, except for catalase activity, which was confirmed by the immediate formation of bubbles, corresponding to oxidative stress tolerance. As explained by Saadaoui et al. (33), catalase activity was assessed by inserting (5 mm diameter plug) an actively developing Trichoderma cultures on a glass slide in a Petri dish plate, and then applying 3% hydrogen peroxide. The production of protease enzyme was detected by growing Trichoderma strains on Glucose–Yeast–Peptone medium enriched with 1% skim milk (pH 6.5), as outlined by Mahfooz et al. (34). The strains of Trichoderma were inoculated on GYP medium which was supplemented with 1% soluble starch in order to assess α-amylase activity (35). Plates were then incubated for 7 days before being flooded for 10 min with a 1% iodine solution in potassium iodide (2%), followed by the removal of the solution. Chitinase production was evaluated as per López et al. (36) by culturing Trichoderma strains on a basal media containing colloidal chitin, prepared using the method of Roberts and Selitrennikoff (37). Cellulase production was estimated by inoculating a 5 mm Trichoderma disc onto a basal medium with 0.1% carboxymethylcellulose (CMC) and incubating for 5 days at 28 ± 2°C (38). A 0.01% concentration of Congo red solution was then added for 15 min, and rinsed with 1% NaCl for 5 min. Finally, pectinase production was evaluated by placing a 5 mm Trichoderma disc on a medium containing 1% pectin and then incubated for 5 days at 28 ± 2°C, followed by the application of Gram’s iodine solution to the pectin agar (39).
2.9 Bioassays for plant growth promoting (PGP) qualities
The synthesis of various PGP characteristics, including IAA, GA, NH3, HCN, and siderophore, was assessed in the 12 Trichoderma isolates that made the short list. The experiments were conducted twice, and each assay was run with three replications.
2.9.1 Colorimetric detection of indole-related compounds
Using the Salkowski test, the in vitro synthesis of IAA for each Trichoderma strain that promotes plant growth was identified (40). Each culture was initiated by inoculating 10 mL of PDB supplemented with 0.1% L-tryptophan with two 5 mm plugs of a 7-day-old Trichoderma strain. They were then shaken for 72 h at 100 rpm at 28.0 ± 2.0°C. After centrifuging 1.5 mL of culture for 10 min at 10,000 rpm, the supension was extracted, and 1 mL of it was then added in duplicate to 2.0 mL of Salkowski reagent. The optical density was taken at 530 nm following a 30 min dark incubation period at room temperature (41). A red appearance suggested that the fungal strain was producing IAA. A standard graph made using known amounts of pure IAA was used to compute the quantity of IAA emitted. A UV–vis spectrophotometer (Simadzu, Japan) was used to detect the color intensity at 530 nm in order to determine IAA. The number of indole-related substances was ascertained using a standard curve that was made by suspending IAA in ethanol (100%) at a concentration of 1 mg/mL and then diluting it in PDB medium to a concentration of 1–10 μg/mL. PDB and L-tryptophan served as controls. Three biological replicates were used in the experiment. Linear Regression (R2 = 0.998, p < 0.0001) was used to infer the measured values for each strain.
2.9.2 Colorimetric detection of gibberellic acid (GA)
Each 5-day-old Trichoderma spp. disc, measuring 5 mm, was infected separately in 100 milliliters of PD broth and cultured for a week at 28 ± 2°C. Following incubation, the amount of GA in the Trichoderma culture filtrate was measured using a colorimetric assay with 0.5 M potassium ferricyanide and 0.5 M zinc acetate reagents, as previously mentioned. The absorbance of the resultant solution was determined using the Mahadevan and Sridhar (42) method at 254 nm in a UV–Vis spectrophotometer (Simadzu, Japan). Based on the standard graph created from known gibberellin concentrations, the gibberellin concentration was computed in μg/mL.
2.9.3 Qualitative detection of ammonia
Cappuccino and Sherman's (32) colorimetric approach was used to measure ammonia production. In short, 10 mL of peptone water was mixed with two plugs of 5 mm week old actively growing Trichoderma and shaken for 7 days at 25/28°C. One milliliter of each culture supernatant was mixed with 1.0 mL of Nessler’s reagent (Himedia), which contains 7% KI, 10% HgCI2, and a 50% aqueous solution of NaOH (32%). Ammonia is released when a yellow to brown precipitate forms.
2.9.4 Qualitative hydrogen cyanide (HCN) production
The method used to estimate HCN production was adapted from Kloepper et al. (43). A solid PDA mixed with 4.4 g/L of succinate or glycine was used to cultivate Trichoderma spp. White filter paper discs that were cut to the same size as the Petri dish’s top lid were carefully placed on the lid of each plate after being submerged in an alkaline solution of picric acid (0.5% v/v and 1.25% w/v sodium carbonate) in water. After sealing the plates with Parafilm, they were incubated at 28 ± 2°C for 7 days. Following incubation, the filter paper’s color changed from yellow to light brown or reddish brown, indicating the synthesis of HCN (44).
2.9.5 Mineral solubilization assay
For each solubilization test, the respective medium was inoculated with a 5 mm and week old Trichoderma culture, with three repetitions per strain on each plate, followed by incubation for a week at 28 ± 2°C. Insoluble tricalcium phosphate medium from the National Botanical Research Institute was used to examine the phosphate solubilization ability; activity was demonstrated by the formation of a distinct halo surrounding the colony during incubation (45). Potassium solubilization was assessed using Aleksandrov medium containing potassium aluminium silicate, with a clear zone around the colony signifying solubilization (46). A modified Pikovskaya medium enriched with zinc oxide was used to examine the potential for zinc solubilization; the creation of a clear zone suggested zinc solubilization (47). Spot cultures on Chromeazurol S (CAS) media were used to measure siderophore production; colonies with yellow or orange halos surrounding them were considered to be producing siderophores (48). These observations determined the solubilization or production capabilities of the Trichoderma strains.
2.10 Assessment of prospective Trichoderma strains in a greenhouse for the suppression of BSR disease in oil palm seedlings
2.10.1 Planting material, inoculum preparation, and inoculation
Oil palm sprouts obtained from the Seed Processing Unit (ICAR–IIOPR, Pedavegi) were surface sterilised. While planting, sprouts of oil palm (IOPPVDD000001) with differentiated plumule and radicle were placed in a shallow pits 2–3 cm depth and covered with soil to 10 mm depth. Polyethylene bags (23×15 cm of 2 kg capacity with 250 gauge) were filled with sieved soil (<2 mm). After sterilizing the soil with a liquid cycle in an autoclave (121°C, 20 min, 100 kPa), it was left to cool for 2 days at room temperature. It was sterilized a second time under same conditions before potting. Further, plants were kept in a net house at 26 ± 3°C (light/dark), with a photoperiod of 16 h and >70% relative humidity. Plants were irrigated with tap water daily, fertilized once a week and green house sanitation maintained.
A total of five Trichoderma isolates (T. longibrachiatum AZNF1, T. atroviride UPFBK1, T. asperellum UPFBK4, T. afroharzianum UPFBK3, and T. virens ANFHR10) were evaluated for their bioinoculant potential, both individually and in consortium, against Ganoderma ellipsoideum. For the in planta study, strains were carefully selected based on their strong antagonistic activity, plant growth-promoting traits, and thermotolerance to ensure compatibility and effectiveness under variable conditions. To 100 mL of PDB, one mycelial disc (5 mm) was inoculated and incubated for 7 days at 28°C in an orbital shaker at 100 rpm. Mycelial mats were collected aseptically and blended with a sterilized hand-held blender (Philips®HL1655/00) for 3 min. The conidial strength was calculated using a haemocytometer by serial dilution method. A total 100 mL of each spore suspension (6–7×106 spores/mL) inoculum were drenched at the base of four-month-old oil palm seedling. Booster doses were provided 15 and 30 days after planting (DAP) by drenching 100 mL conidial suspension. Inoculum of Ganoderma was prepared by inoculating mycelial disc (10 mm) in 100 mL PDB and incubated for 4 days at 28°C in orbital shaker at 100 rpm. Mycelial mats were then aseptically harvested and blended for 3 min with 0.002% Tween® 20 in a sterilized hand-held blender (Philips®HL1655/00). The final mycelial concentration was adjusted to 105 fragments/mL using a haemocytometer. Seven days post Trichoderma treatment, Ganoderma inoculation was done by mycelial root immersion method following Purnamasari et al. (49) with slight modifications. Seedlings were uprooted carefully, immersed mycelium suspension for 30 min and replanted back in Trichoderma treated soil.
2.10.2 Assessment of BSR disease severity
Disease was assessed and scored following a two-week incubation period. A scale of 0–4 was used to score the disease based on the symptoms that were seen; 0 represented healthy plants, 1 represented mildly chlorotic patches on the leaves, 2 represented yellowing in 1–2 leaves, 3 represented yellowing on numerous leaves (> 2 leaves), and 4 represented dead plants (15). The following formula was used to measure the severity of bole and root symptoms (%) and foliar symptoms (%) (17):
a = number of browned/wilted leaves; b = number of yellowed leaves, c = total number of leaves.
Where 0 = healthy; absence of internal rot, 1 = 20% plant tissue rot, 2 = 20–50% plant tissue rot, 3 = > 50% plant tissue rot.
The dry weights of the roots, shoots, and height were measured after 8 weeks. Four seedlings were selected randomly from each treatment throughout the trial. The height of the shoots was documented from the base to the top of the stem. The roots and shoots were oven-dried at 70°C until their weight remained constant. After then, each of them was weighed independently, and the results were noted.
2.11 Scanning electron microscopy (SEM)
SEM analysis was conducted following the method of Sreenayana et al. (50) using an FEI Quanta 250 (Czech Republic) to examine the morphological features of Ganoderma ellipsoideum, Trichoderma afroharzianum UPFBK3, their interactions, and colonization on oil palm seedling roots. Three types of samples were prepared: (i) pure cultures of G. ellipsoideum and the most potent T. afroharzianum UPFBK3 isolate, (ii) dual-culture interaction zones involving selected T. afroharzianum isolates based on in vitro antagonism assays, and (iii) root tissues from oil palm seedlings inoculated with T. afroharzianum. Samples were fixed in 2.5% glutaraldehyde and incubated it at 4°C for 24 h, dehydrated through a graded ethanol series (30, 50, 70, 90, and 100%), followed by critical point drying. The dried samples were mounted on aluminum stubs, sputter-coated with gold, and examined under SEM to assess fungal morphology, interaction zones, and colonization patterns on root surfaces.
2.12 Statistical analysis
Analysis of variance (ANOVA) was employed to analyze the data collected. Data in percentages were square root transformed, and mean values were compared using the least significant difference test. All analyses were performed using the Web Agri Stat Package 2.0, a web-based statistical package.
3 Results
3.1 Evaluation of the antagonistic feature of Trichoderma isolates
Fifty native Trichoderma strains, each exhibiting varying levels of antifungal activity against Ganoderma spp. were successfully isolated from diverse samples collected across multiple geographical locations of India, including 11 from Andaman and Nicobar, 5 from Telangana, 6 from Kerala, 12 from Uttar Pradesh, and 16 from Andhra Pradesh (Supplementary Table 1). Based on the inhibition (%) on radial growth, the Trichoderma strains were classified into the following categories: very strong (>90%) with 8 isolates, strong (80–90%) with 4 isolates, moderate (60–80%) with 21 isolates, weak (50–60%) with 9 isolates, and very weak (<50%) with 7 isolates. Overall, the antagonistic efficacy of the isolates against G. ellipsoideum varied significantly across dual culture, IPA, CFSA, and CFLA assays. Notably, UPFBK3 (97.77, 94.81, 100.00, 82.60%), UPFBK1 (97.77, 91.11, 98.44, 72.59%), and UPFBK4 (97.77, 95.93, 95.74, 71.11%) exhibited consistently high performance across all methods. In contrast, the least effective isolates were EGFBS1 (69.48, 85.19, 64.44%) followed by AZNF4 (85.00, 88.89, 75.93, 57.04%), which demonstrated significantly lower antifungal efficacy in all assays.
3.2 Morphological characterization of Trichoderma isolates
The morphological characterization of Trichoderma isolates revealed that the majority of colonies were circular with smooth margins. The texture of the colonies varied from smooth to fluffy to powdery with or without concentric rings. Initially, the colonies were white, transitioning to light green and eventually to dark green pigmentation due to production of conidia. Some colonies exhibited reverse pigmentation that ranged from yellow to brownish (Figure 3A). An earthy or musty odour was noted for most of the isolates. Microscopic examination showed that the conidiophores were short or elongated upright and smooth-walled, with branching patterns ranging from regular to irregular. Phialides were short or elongated flask-shaped and typically arranged in single, whorls or clusters. Conidia were observed to be ellipsoidal, subglobose, or ovoid. The hyphae were septate, branched, and ranged in colour from white to pale greenish (Figure 3B). All the 12 isolates belonging to seven species were differentiated detailed in Supplementary Table 2. The results of the study on radial mycelial growth at 48 h reveal statistically significant variations (CD = 0.39) (Table 3). Most strains achieved full growth (9 cm diameter) within 3 days, except for EGFBS1 (3.69 ± 0.01 cm) and AZNF4 (3.76 ± 0.01 cm), which exhibited significantly slower growth. Among the strains, UPFBK3 showed the highest growth at 48 h (3.58 ± 0.25 cm), followed closely by ANFHS3 (3.49 ± 0.44 cm). In contrast, AZNF4 demonstrated the slowest growth at 48 h, reaching only 2.46 ± 0.11 cm.
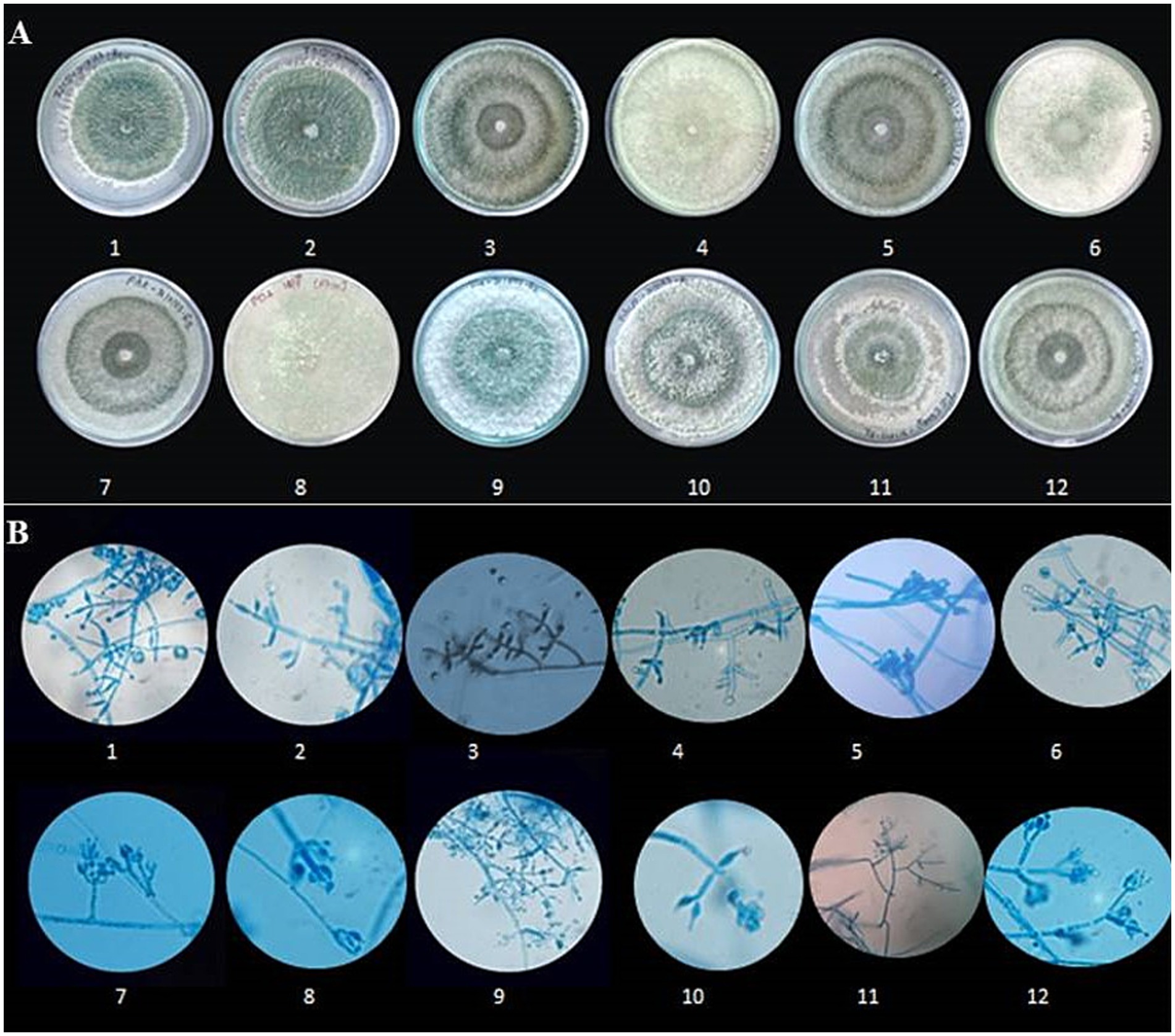
Figure 3. Morphological characterization of Trichoderma species. (A) Colony morphology of different Trichoderma isolates on culture media. Rows 1–12 show variations in colony texture, colour, and growth patterns among isolates. (B) Microscopic images of Trichoderma hyphae and conidia under a light microscope at 400 × magnification (Scale-100 μm). Rows 1–12 depict structural differences, including hyphal branching, spore formation, and conidial arrangement, highlighting the diversity among the isolates.
3.3 Molecular identification of Trichoderma isolates
Using ITS1 and ITS4 primers, a PCR amplification of about 575 bp of the ITS region for eight native isolates was obtained, and forward and reverse sequencing were then performed to identify the putative strains (Supplementary Table 3). We were able to obtain sequences that have between 98 and 100 percent similarity to Trichoderma strains thanks to the NCBI database’s BLASTn alignment. The phylogenetic tree analysis results, as presented in Figure 4, reveal that the studied isolates were categorized into six distinct clades, each representing a specific species within the Trichoderma genus. Clade 1 contains two isolates, identified as CHFIB1 (OR244374) and ANFHR10 (OR244376), which were determined to be T. virens. The Clade 2 contains a single isolate, UPFBK3 (OR244373), which was identified as T. afroharzianum. Clade 3: Consists of the isolate EGFBS (OR244380), identified as T. cremeum. Clade 4 harbors the isolate ANFHS3 (OR244375), which was identified as T. longibrachiatum. Clade 5 includes two isolates, UPFBK2 (OR244377) and UPFBK4 (OR244378), both identified as T. asperellum. Clade 6 comprises the isolate UPFBK1 (OR244379), which was identified as T. atroviride. The ITS sequence and GenBank accession number are included in the summary of the sequence results that are shown in Supplementary Table 1.

Figure 4. Scanning electron microscope (SEM) analysis of Ganoderma ellipsoideum and Trichoderma afroharzianum interactions. (A) Healthy tubular hyphae of G. ellipsoideum (magnification: 5,000×, scale bar: 20 μm). (B) Morphology of T. afroharzianum, showing branched hyphae, flask-shaped phialides, and smooth-walled ellipsoidal to subglobose conidia (magnification: 5,000×, scale bar: 20 μm). (C) Hyperparasitism of T. afroharzianum UPFBK3, with arrows indicating damage on G. ellipsoideum hyphae (magnification: 8,000×, scale bar: 10 μm). (D) Root colonization by T. afroharzianum, demonstrating its interaction with the plant root surface (magnification: 5,000×, scale bar: 20 μm).
3.4 Determination of hydrolytic and growth activities of Trichoderma isolates
The hydrolytic and growth promotion enzyme activity results demonstrated significant variation among the tested Trichoderma isolates (Figure 5 and Tables 4, 5). T. asperellum APSI1 and T. virens CHFB1 emerged as the strongest enzyme producers, with ++++ activity in chitinase, cellulase, and pectinase, amylase activity and moderate catalase activity (+++). T. asperellum UPFBK4 and T. afroharzianum UPFBK3 followed closely, with ++++ activity in cellulase and pectinase, alongside strong chitinase production (+++). The weakest performers were T. cremeumEGFBS1, which exhibited low activity (+) in cellulase and pectinase, and only moderate catalase (++++) and amylase (++++) activity.
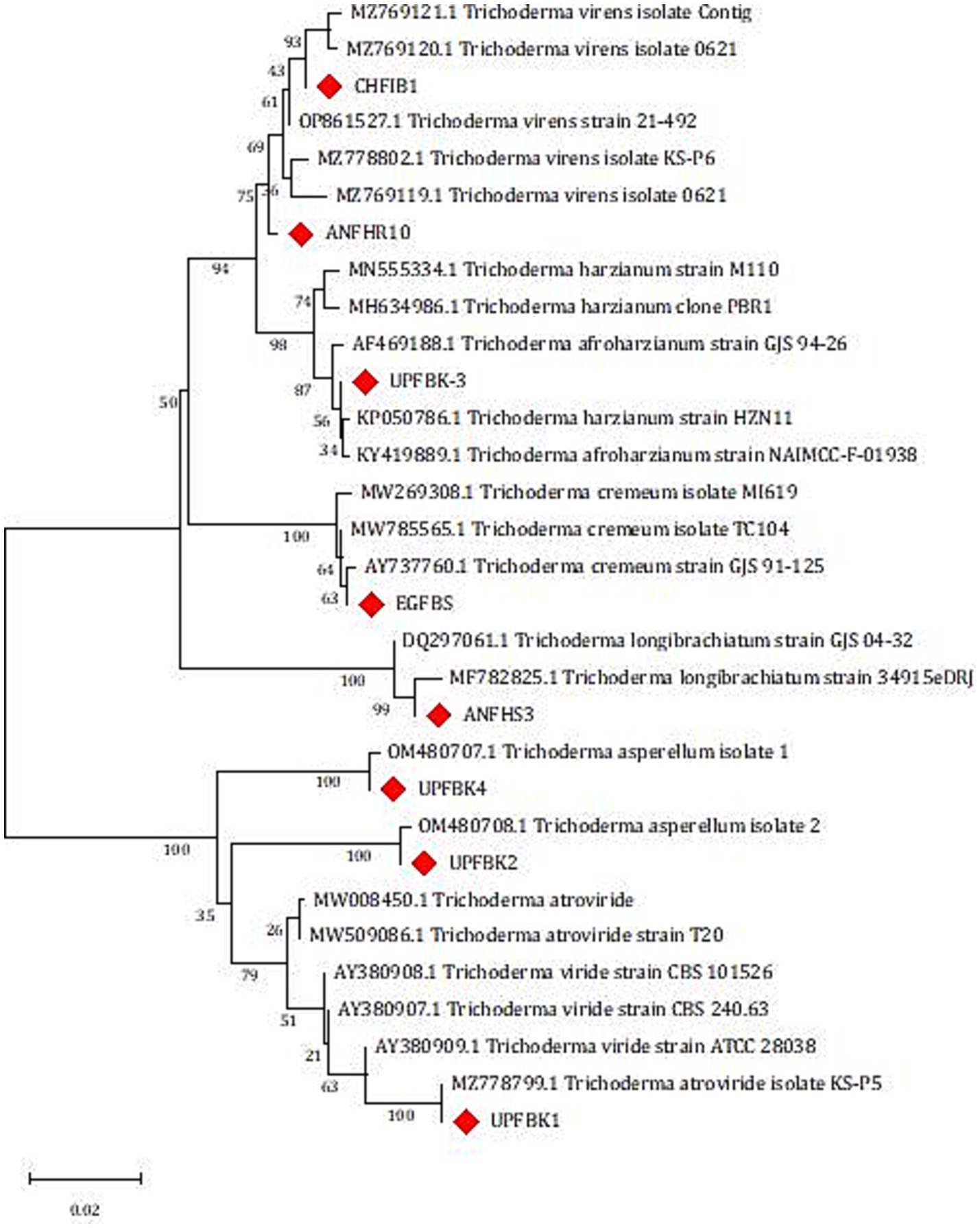
Figure 5. Phylogenetic analysis of Trichoderma isolates based on Internal Transcribed Spacer (ITS1 and ITS4) sequences using maximum likelihood analysis. Isolates in this study are highlighted with red diamonds. The scale bar represents genetic distance.
T. afroharzianum UPFBK3 exhibited highest growth promotion activity was the strongest performer with high ammonia (+++), the highest IAA (19.25 μg/ mL), GA (11.87 19.25 μg/ mL) which were significantly greater than other isolates (LSD₀.₀₅: IAA = 0.184, GA = 0.148). This isolate also exhibited excellent solubilization of phosphorus (++++), potassium (++++), and zinc (++++). The second best isolate, T. virens ANFHR10 demonstrated high IAA (17.01–18.96 μg/ mL), GA (10.83–10.98 μg/mL), phosphorus (+++), potassium (+++), and zinc (+++) solubilization, along with superior siderophore (+++/++++) production. Conversely, T. longibrachiatum AZNF1 was the least effective, displaying the lowest IAA (10.87 μg/mL), GA (7.49 μgmL−1)), and moderate-to-low solubilization of phosphorus (++), potassium (++), and zinc (++). In conclusion, T. afroharzianum UPFBK3 emerged as the most effective strain among tested isolates, demonstrating both the highest growth-promotion activity and strong hydrolytic enzyme production.
3.5 Detection of antimicrobial spectrum of Trichoderma isolates
Among the Trichoderma isolates tested, T. afroharzianum UPFBK3 demonstrated the broadest and most consistent antimicrobial activity, with statistically significant inhibition (p < 0.05) across all pathogens, including R. bataticola (100%), F. solani (100%), L. theobromae (97.77%), C. gleosporoides (92.22%), R. solani (86.66%), and C. lunata (72.22%). Close behind was T. asperellum UPFBK4, which showed comparable results of 97.79% against L. theobromae and 72.21% against C. lunata. In contrast, T. virens CHFIB1 demonstrated the least broad-spectrum efficiency, with significantly lower inhibition rates of 60.65% against C. lunata and 85.81% against C. gloeosporoides. Moreover, all Trichoderma isolates achieved complete inhibition (100%) of R. bataticola and F. solani with significant variation among isolates for R. solani, L. theobromae, C. gloeosporoides, and C. lunata (p < 0.05, CD₀.₀₅: 0.02–0.03) (Table 6). These findings emphasize the superiority of T. afroharzianum UPFBK3, and T. asperellum UPFBK4, as potent biocontrol agents for managing multiple pathogens.
3.6 Efficacy of Trichoderma species on the inhibition of BSR disease in oil palm seedlings
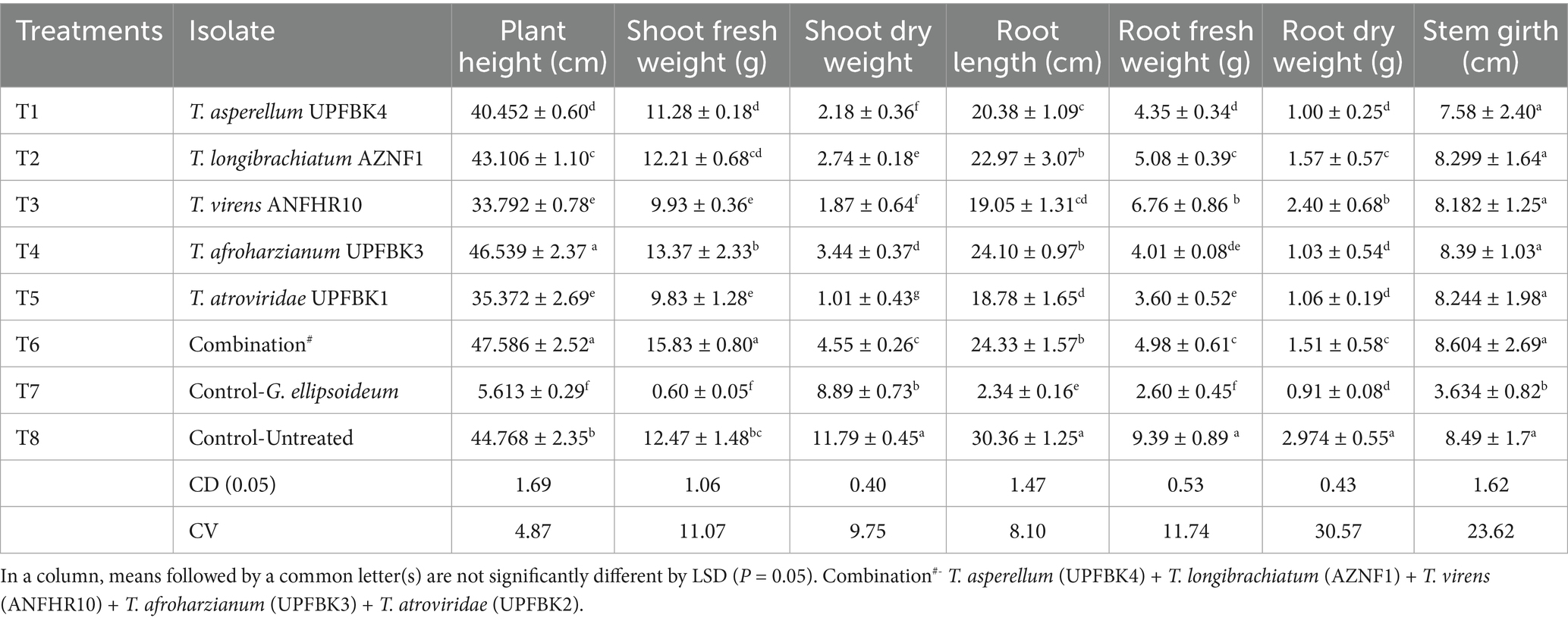
The evaluation of five best Trichoderma isolates and their combinations for their antagonistic potential against Ganoderma spp. revealed statistically significant variability (p < 0.05), with a critical difference of CD₀.₀₅: 0.25 for bole severity and 0.26 for foliar severity. In their effectiveness in controlling foliar and bole diseases (Figure 5 and Table 7). The combination of Trichoderma isolates (T6) demonstrated the highest BSR disease suppression, with a significant reduction in foliar severity and bole severity, corresponding to disease suppression rates of 50.97 and 61.94%, respectively. Among individual isolates, T. afroharzianum (T4) exhibited the second-highest reduction, achieving 49.92% reduction in foliar severity and 56.69% reduction in bole severity. In contrast, the control treatment (G. ellipsoideum) exhibited the highest severity for both foliar (99.56%) and bole (82.15%) infections, with no suppression of the disease. The Trichoderma consortium (T6) demonstrated 2.1% greater suppression of foliar disease, and 9.3% higher suppression of bole disease compared to T. afroharzianum (T4), reinforcing the superior efficacy of multi-strain interactions in controlling Ganoderma-induced disease in oil palm.
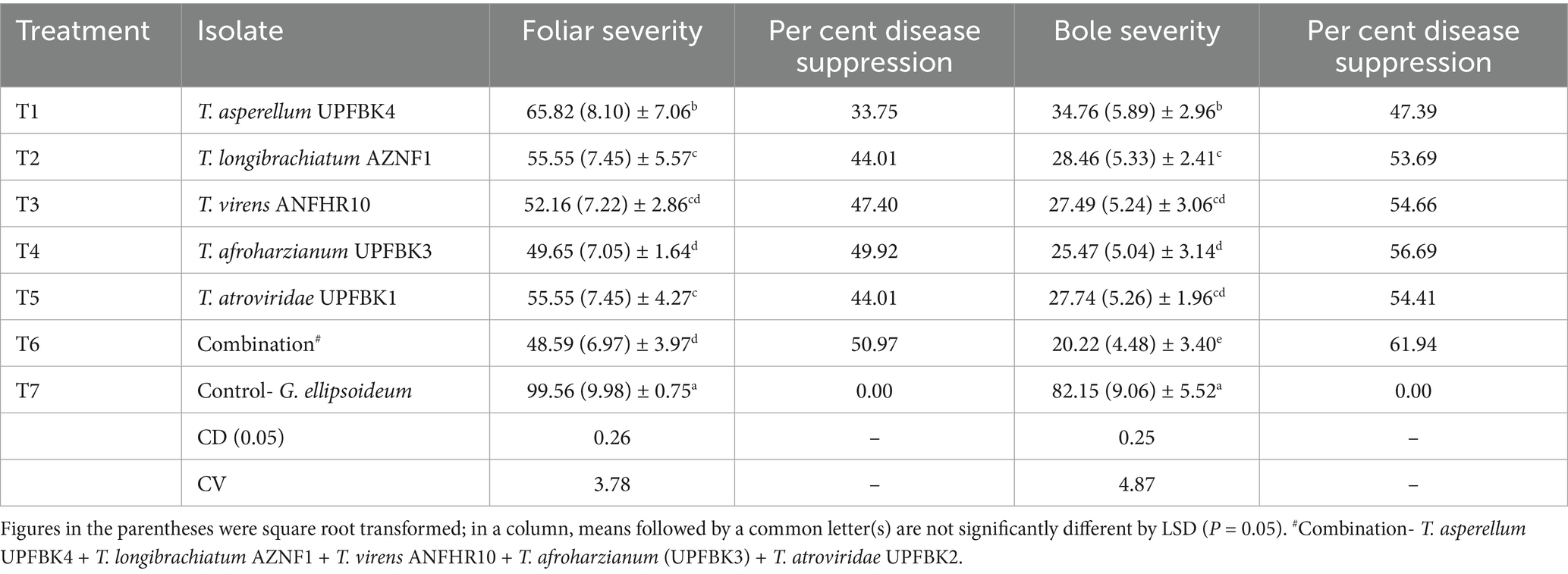
Table 7. In planta efficacy of Trichoderma species in suppressing BSR severity in oil palm seedling.
3.7 Impact of Trichoderma on growth characteristics of oil palm seedling
A comparative analysis of growth parameters among different Trichoderma isolates and their effects on plant growth statistically significant differences between treatments (CD₀.₀₅: 1.06–1.69 (Table 8). The combination treatment (T6) showed the highest values across several parameters, including plant height (47.59 ± 2.52 cm), shoot fresh weight (15.83 ± 0.80 g), shoot dry weight (4.55 ± 0.26 g), and stem girth (8.60 ± 2.69 cm). This was followed closely by T. afroharzianum (T4), with a plant height of 46.54 ± 2.37 cm, shoot fresh weight of 13.37 ± 2.33 g, and root length of 24.10 ± 0.97 cm. In contrast, the control treatment with G. ellipsoideum (T7) exhibited the lowest values across all parameters, with plant height (5.61 ± 0.29 cm) and shoot fresh weight (0.60 ± 0.05 g), indicating a detrimental effect on plant growth. The control group (T8) showed relatively high growth metrics, but the combination treatment significantly outperformed it in most categories.
3.8 SEM analysis
Distinct morphological features were observed in G. ellipsoideum and T. afroharzianum UPFBK3. G. ellipsoideum exhibited tubular hyphae with clearly defined clamp connections, indicative of basidiomycetous fungi (Figure 4A). In contrast, T. afroharzianum UPFBK3 showed highly branched hyphae bearing flask-shaped phialides and numerous ellipsoidal to subglobose conidia (Figures 4B,C). Direct interaction between the two fungi revealed pronounced hyphal damage in G. ellipsoideum upon contact with T. afroharzianum UPFBK3. The damaged hyphae exhibited surface degradation and structural collapse, suggesting antagonistic activity likely associated with mycoparasitism (Figure 4D). In addition, adherence of T. afroharzianum UPFBK3 to the plant root surface was evident, demonstrating its colonization potential and ecological competence in the rhizosphere.
4 Discussion
Trichoderma is widely utilized as a biocontrol agent featured in more than 50% of commercial formulations for managing fungal, viral, and nematode-induced plant diseases (51–55). Despite the commercial success of species like T. viride and T. harzianum (56), the broader genus Trichoderma, which includes over 300 identified species (57), remains largely underutilized. This limited use highlights the untapped biocontrol potential of Trichoderma from diverse agroecological zones, where region-specific isolates present promising, locally adapted, and climate-resilient alternatives for more sustainable and broader plant disease management (58). Hence, the current study examines 50 Trichoderma isolates from diverse agro-ecological contexts, identifying 12 with promising antibiosis and growth promotion, and selecting the top 5 for in planta bioefficacy testing against Ganoderma. This study also morpho-molecularly characterized seven different Trichoderma species, including T. afroharzianum, T. atroviride, and T. cremeum, which to our knowledge, have not been previously reported in association with Ganoderma-infected oil palm systems. The consistent high performance of T. afroharzianum UPFBK3, T. atrovirodae UPFBK1, and T. asperellum UPFBK4 across dual culture, inverted plate, and cell filtrate assays underscores their robust and stable antagonistic potential against Ganoderma, making them strong candidates for development as effective biocontrol agents in sustainable disease management strategies. This antagonistic role of Trichoderma spp. is attributable to their unique ability to synthesize antibiotics, secondary metabolites (59), mycoparasitism (52) and lytic enzymes (60). The superior antagonistic ability T. afroharzianum UPFBK3 is attributed to its synthesis of bioactive compounds including synthesize secondary metabolites like spathulenol, triacetin, and aspartame, along with VOCs such as ethanol, hydroperoxide, 1-methylhexyl, and 1-octen-3-one (61). The demonstrated effectiveness of T. afroharzianum strains—B3R12 against onion white rot (62), TRI07 against Alternaria alternata (61), and T14 against wheat crown rot and Fusarium head blight (63), supports the generalizability of Trichoderma strains as a versatile biocontrol agent. Similarly, T. asperellum UPFBK4, which ranked second in our study, has consistently shown potent inhibition of G. boninense and G. lucidum (13, 64, 65). This reinforces the strain’s potential for effective biocontrol, emphasizing the robustness and reliability of Trichoderma species as effective biocontrol agents across a range of pathogenic threats (Figure 6).
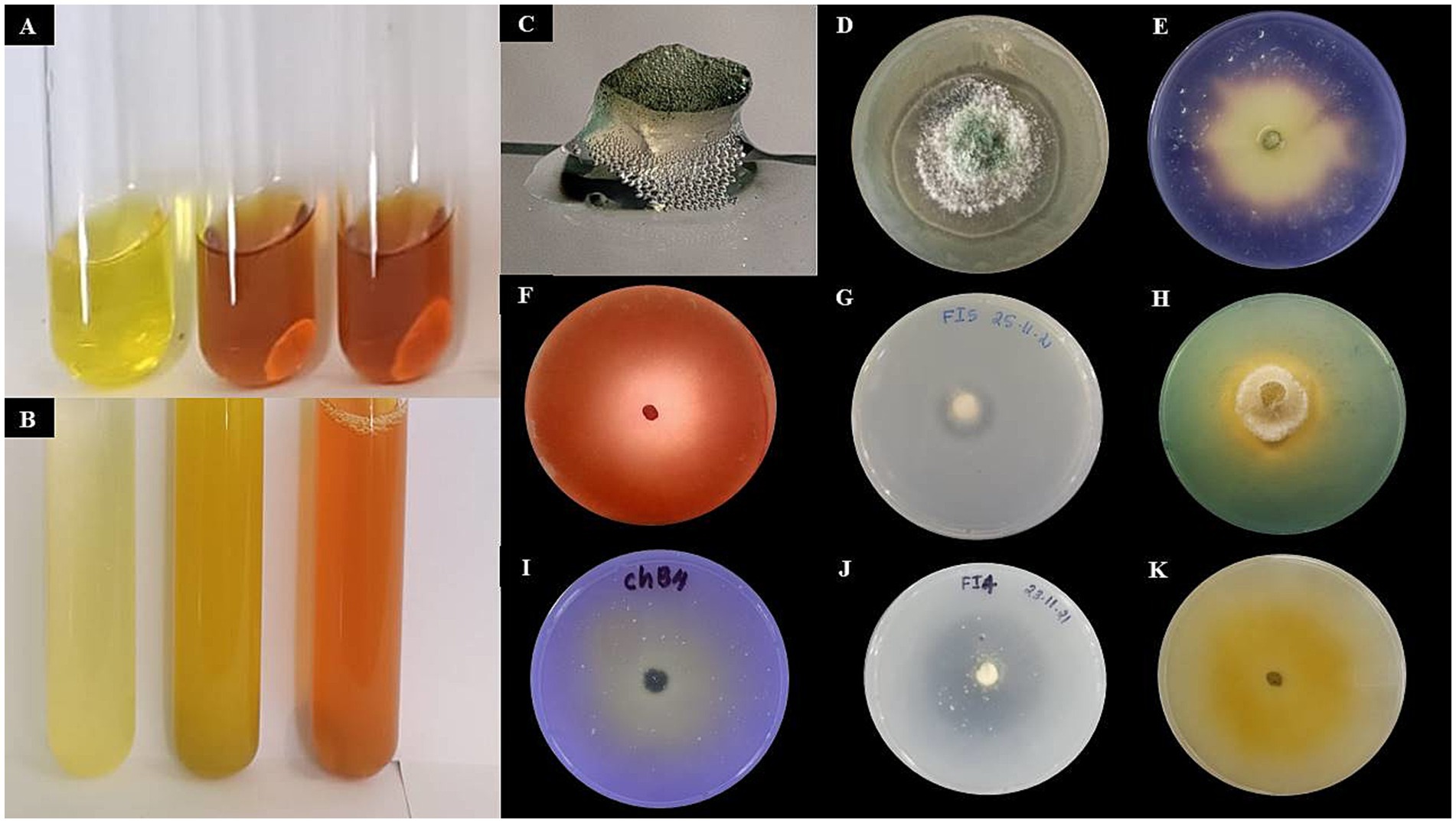
Figure 6. Qualitative assessment of hydrolytic enzymes and plant growth-promoting traits of Trichoderma afroharzianum. (A) Indole Acetic Acid (IAA) production indicated by red color development. (B) Ammonia production confirmed by brown color formation. (C) Catalase test signed by bubbling with H₂O₂. (D) Chitinase production indicted by halo around the fungal colony. (E) α-Amylase production denoted by clear zones after iodine staining indicate starch hydrolysis. (F) Cellulase production revealed by Congo red staining reveals clear zones, indicating cellulose degradation. (G) Protease production showed by transparent halo on skim milk agar. (H) Siderophore production indicated by yellow halos on CAS medium. (I) Phosphate solubilization confirmed by clear zones on NBRIP medium. (J) Potassium solubilization signed by halo formation on Aleksandrov medium.
The plant-microbe connection gives the host plants a selective advantage when they are under distress (66, 67). Hence, this study investigates the biochemical mechanisms underlying the hydrolytic and growth-promoting activities of 12 potent Trichoderma isolates, reaffirming T. afroharzianum as the most effective due to its superior growth promotion and hydrolytic enzyme production. Trichoderma suppresses Ganoderma through oxidative enzymes like catalase, which mitigate oxidative stress, and hydrolytic enzymes that break down fungal cell walls to access nutrients and facilitate root colonization, ultimately enhancing plant association and resistance (68). In addition to these enzymatic actions, certain hydrolytic enzymes act as elicitors that trigger the release of Damage-Associated Molecular Pattern Molecules (DAMPs), which are endogenous plant signals released upon cellular damage (69). We further speculate that Trichoderma’s hyperparasitism against Ganoderma is strongly enhanced by the combined action of these lytic enzymes and the production of antibiotic metabolites, contributing to a potent antibiosis effect (62). The variability in production of secondary metabolites and hydrolytic enzymes could indeed explain the differences in performance observed among the Trichoderma isolates. Additionally, every strain has different genetic potential for parasitism; the nature of the pathogen and the cell wall of the pathogen play a critical role in the mycoparasitisms. The other factors, such as growth rate, isolate-specific physiological traits, and environmental interactions may have influenced the results (Figure 7).

Figure 7. Effect of Trichoderma spp. on basal stem rot (BSR) disease suppression in oil palm seedlings. (C₋) Negative control: Healthy seedlings without Ganoderma inoculation; (C₊) Positive control: Seedlings inoculated with Ganoderma sp. without Trichoderma treatment; T₁: Trichoderma asperellumUPFBK4; T2: Trichoderma longibrachiatum AZNF1; T3: Trichoderma virensANFHR10; T5: Trichoderma afroharzianum UPFBK3; (T₅) Trichoderma atroviride UPFBK1; T₆: Combination treatment.
The growth-promoting activity of Trichoderma stems from its capacity to produce siderophores and solubilize phosphorus, potassium, and zinc, which increases the availability of soluble soil nutrients and thereby benefiting the host plant by promoting growth and enhancing stress tolerance (70). Particularly, siderophore production enhances iron uptake in iron-deficient conditions (71, 72), while the availability of phosphorus is increased through the generation of organic acids like succinic acid, malic acid, and oxalic acid (73), and nitrogen is made available by hydrolyzing urea to produce ammonia (74). Further, numerous strains of Trichoderma release growth regulators such as indole-3-acetic acid (IAA) and gibberellic acid (GA), which alter plant metabolism, promote growth, and enhance stress resilience (50).
Our study identified T. afroharzianum UPFBK3, T. asperellum APSI1 & UPFBK4, and T. virens ANFHR10 & CHFIB1 as promising growth promoters, supporting previous findings. For instance, T. virens and T. atroviride were reported to produce IAA-related indoles, enhancing the fresh weight of tomato roots and shoots (75) and promoting lateral root formation in Arabidopsis (76). Additionally, Qi and Zhao (77) found T. asperellum to be the most efficient Trichoderma species for siderophore production under salt stress, effectively alleviating iron deficiency in plants. These findings align with our results; reinforcing the potential of these Trichoderma strains as effective growth promoters.The in vitro findings are insufficient to fully ascertain the possibility of Trichoderma spp. as effective bio-control agents in field conditions. Factors such as soil microbiota, environmental conditions (e.g., temperature, humidity), and host plant physiology can significantly impact their efficacy. Moreover, dual culture and inverted plate assays do not account for the complex interactions in the rhizosphere, where competition for resources and microbial dynamics play a critical role in biocontrol success. Therefore, integrating both in vitro and in vivo studies is essential for a comprehensive evaluation of their biocontrol capabilities (78). The present in planta study demonstrated that the Trichoderma consortium—comprising five most potent and compatible isolates (T. afroharzianum, T. virens, T. asperellum, T. longibrachiatum and T. atroviride)—resulted in greater suppression of disease severity and enhanced plant growth promotion. The thermotolerant T. longibrachiatum strain, isolated from the rhizosphere of Prosopis cineraria in Rajasthan’s > 50°C arid zone showed strong antagonism against Ganoderma lucidum (19). This enhanced efficacy is attributed to the broader spectrum of antagonistic activities offered by the consortium, where synergistic interactions among isolates with complementary strengths—such as mycoparasitism and extracellular enzyme production—led to cumulative effects that surpassed those of individual strains (70). This synergy is further supported by significant preventive effects against Ganoderma-induced BSR in oil palm (14), along with evidence that Trichoderma consortia enhance plant resistance and growth (79), improve nutrient uptake and biomass as seen with T. viride, T. virens, and T. harzianum in chickpeas (80), and effectively control Rhizoctonia solani through T. harzianum mixtures (81). Seedlings exhibited enhanced disease suppression and increased fresh and dry shoot and root weights, consistent with our in vitro findings where Trichoderma afroharzianum emerged as the most effective isolate, demonstrating superior antagonistic activity, hydrolytic enzyme production, growth promotion, faster growth rate, and a broader antimicrobial spectrum. These findings align with previous studies across diverse host–pathogen systems (61–63) and reinforce the well-documented growth-promoting and biocontrol potential of Trichoderma spp. in various crops including wheat (82), maize (70), cotton (83), legumes (84), and tree crops like cocoa and Pinus radiata (85, 86). While this study highlights the potential of region-specific Trichoderma isolates, particularly T. afroharzianum, further in vivo research is needed to assess their field efficacy (78). Additionally, more work is required to understand Trichoderma’s impact on resistance mechanisms, including systemic acquired resistance (SAR). Further studies are required to understand the factors contributing to performance variability, including environmental and biochemical influences on efficacy. This will be crucial for optimizing Trichoderma in sustainable, climate-resilient disease management strategies.
5 Conclusion
Our findings suggest that selected Trichoderma strains, particularly T. afroharzianum, possess strong biocontrol potential against Ganoderma spp. under laboratory and greenhouse conditions. The results indicate promising disease suppression efficacy and growth enhancement in oil palm seedlings. However, these outcomes are limited to controlled environments, and further multi-location field evaluations are required to confirm their effectiveness under diverse plantation conditions. Overall, this study provides foundational evidence supporting the use of ecologically adapted Trichoderma strains as components of an integrated, climate-resilient disease management strategy for oil palm.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary material.
Author contributions
MA: Investigation, Methodology, Validation, Visualization, Writing – original draft, Writing – review & editing. MI: Investigation, Methodology, Writing – original draft. US: Investigation, Methodology, Writing – review & editing. AS: Methodology, Writing – review & editing. GC: Data curation, Formal analysis, Software, Writing – review & editing. RM: Investigation, Methodology, Writing – review & editing. WD: Formal analysis, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The Indian Council of Agricultural Research (ICAR) and the Department of Agriculture and Farmers Welfare’s National Mission on Edible Oil–Oil Palm provided funding for this study [F. no. 3-25/2018-OP(SB)E-58030].
Acknowledgments
The authors express their gratitude to the funding sources used for this article’s research, writing, and publication.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2025.1582047/full#supplementary-material
Footnotes
References
1. Vijay, V, Pimm, SL, Jenkins, CN, and Smith, SJ. The impacts of oil palm on recent deforestation and biodiversity loss. PLoS One. (2016) 11:e0159668. doi: 10.1371/journal.pone.0159668
2. Arif, M. A., Roslan, A., Idris, A. S., and Ramle, M. (2011). Economics of oil palm pests and Ganoderma diseases and yield losses. In: Proceedings of Third International Seminar Integrated Oil Palm Pests Management. MPOB, Malaysia, 9. 83–98.
3. Keerthana, J, Lakshmi, MA, Aditya, S, Ajesh, BR, and Manyam, P. Detection and management of basal stem rot of oil palm: classical to modern approaches In: UB Singh, R Kumar, and HB Singh, editors. Detection, diagnosis and management of soil-borne phytopathogens. Singapore: Springer Nature Singapore (2023). 225–67.
4. Lakshmi, MA, Ajesh, BR, Manyam, P, Javeedvali, S, Khan, AS, Palnam, DW, et al. Traditional to technological advancements in Ganoderma detection methods in oil palm. Folia Microbiol. (2024) 69:953–73. doi: 10.1007/s12223-024-01177-w
5. Susanto, A, Sudharto, PS, and Purba, RY. Enhancing biological control of basal stem rot disease (Ganoderma boninense) in oil palm plantations. Mycopathologia. (2005) 159:153–7. doi: 10.1007/s11046-004-4438-0
6. Idris, AS, Mazliham, MS, Loonis, P, and Wahid, MB. Ganosken for early detection of Ganoderma infection in oil palm. MPOB Information Series. (2010) 442:1–4.
7. Sariah, M, Choo, CW, Zakaria, H, and Norihan, MS. Quantification and characterisation of Trichoderma spp. from different ecosystems. Mycopathologia. (2005) 159:113–7.
8. Bivi, MR, Noor Farhana, MS, Khairulmazmi, A, and Idris, A. Control of Ganoderma boninense, a causal agent of basal stem rot disease in oil palm, with endophytic bacteria in vitro. J Plant Prot Res. (2010) 50:30–5.
9. Buana, RFN, Wahyudi, AT, and Toruan-Mathius, N. Control activity of potential antifungal-producing Burkholderia sp. in suppressing Ganoderma boninense growth in oil palm. Asian. J Agric Res. (2014) 8:259–68.
10. Goh, YK, Zoqratt, MZHM, Goh, YK, Ayub, Q, and Ting, ASY. Determining soil microbial communities and their influence on Ganoderma disease incidences in oil palm (Elaeis guineensis) via high-throughput sequencing. Biol. (2020) 9:424. doi: 10.3390/biology9120424
11. Ilias, GNM. Trichoderma pers. ex fr. and its efficacy as a biological control agent of basal stem rot of oil palm (Elaeis guineensis jacq.) (Doctoral dissertation). Seri Kembangan: Universiti Putra Malaysia (2000).
12. Izzati, MZNA, and Abdullah, F. Disease suppression in Ganoderma-infected oil palm seedlings treated with Trichoderma harzianum. Plant Prot Sci. (2008) 44:101–7. doi: 10.17221/23/2008-PPS
13. Muniroh, MS, Nusaibah, SA, Vadamalai, G, and Siddique, Y. Proficiency of biocontrol agents as plant growth promoters and hydrolytic enzyme producers in Ganoderma boninense-infected oil palm seedlings. Curr Plant Biol. (2019) 20:100116. doi: 10.1016/j.cpb.2019.100116
14. Musa, H, Nusaibah, SA, and Khairulmazmi, A. Assessment on Trichoderma spp. mixture as a potential biocontrol agent of Ganoderma boninense infected oil palm seedlings. J Oil Palm Res. (2018) 30:403–15.
15. Abdullah, F, Ilias, GNM, Vijaya, SK, and Leong, TT. Diversity of Trichoderma and its in vivo efficacy against Ganoderma boninense In: Z Sidek, SK Bong, CA Ong, and AK Husan, editors. Sustainable Crop Protection Practices in the Next Millenium. MCBMAPPS Plant Protection Conference. Kota Kinabalu, Sabah: MCBMAPPS Plant Protection Conference (1999). 137–40.
16. Naher, L, Tan, SG, Yusuf, UK, Ho, CL, and Abdullah, F. Biocontrol agent Trichoderma harzianum strain FA 1132 as an enhancer of oil palm growth. Pertanika J Trop Agric Sci. (2012) 35:173–82.
17. Sariah, M, and Zakaria, H. The use of soil amendments for the control of basal stem rot of oil-palm seedlings In: J Flood, PD Bridge, and M Holderness, editors. Ganoderma diseases of perennial crops. Wallingford: CABI (2000). 89–99.
18. Sreenayana, B, and Nakkeeran, S. Effect of oil based formulation of Trichoderma spp. on growth parameters of cucumber seedlings. Int J Curr Microbiol App Sci. (2019) 8:200–9.
19. Mawar, R, Sharma, D, and Ram, L. Potential of biocontrol agents against Ganoderma lucidum. J For Res. (2021) 32:1269–79. doi: 10.1007/s11676-020-01161-3
20. Lutz, MP, Wenger, S, Maurhofer, M, Défago, G, and Duffy, B. Signaling between bacterial and fungal biocontrol agents in a strain mixture. FEMS Microbiol Ecol. (2004) 48:447–55. doi: 10.1016/j.femsec.2004.03.002
21. Papavizas, GE, and Lewis, JA. Introduction and augmentation of microbial antagonists for the control of soil-borne plant pathogens In: GE Papavizas, editor. Biol. Control in crop production. Totowa, NJ: Allanheld, Osmun (1981). 461.
22. Berg, G, Krechel, A, Ditz, M, Sikora, RA, Ulrich, A, and Hallmann, J. Endophytic and ectophytic potato-associated bacterial communities differ in structure and antagonistic function against plant pathogenic fungi. FEMS Microbiol Ecol. (2005) 51:215–29. doi: 10.1016/j.femsec.2004.08.006
23. Dennis, C, and Webster, J. Antagonistic properties of species-groups of Trichoderma: I. Production of non-volatile antibiotics. Trans Br Mycol Soc. (1971) 57:25. doi: 10.1016/S0007-1536(71)80077-3
24. van Zijll de Jong, E, Kandula, J, Rostás, M, Kandula, D, Hampton, J, and Mendoza-Mendoza, A. Fungistatic activity mediated by volatile organic compounds is isolate-dependent in Trichoderma sp "atroviride B". J Fungi. (2023) 9:238. doi: 10.3390/jof9020238
25. Samuels, GJ, Dodd, SL, Gams, W, Castlebury, LA, and Petrini, O. Trichoderma species associated with the greenmold epidemic of commercially grown Agaricus bisporus. Mycologia. (2002) 94:146–70. doi: 10.1080/15572536.2003.11833257
27. White, TJ, Bruns, T, Lee, SJWT, and Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. PCR Protocols Guide Methods Applications. (1990) 18:315–22. doi: 10.1016/B978-0-12-372180-8.50042-1
28. Tamura, K, Peterson, D, Peterson, N, Stecher, G, Nei, M, and Kumar, S. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. (2011) 28:2731–9. doi: 10.1093/molbev/msr121
29. Saitou, N, and Nei, M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. (1987) 4:406–25. doi: 10.1093/oxfordjournals.molbev.a040454
30. Felsenstein, J. Phylogenies from molecular sequences: inference and reliability. Ann Rev Genet. (1988) 22:521–65. doi: 10.1146/annurev.ge.22.120188.002513
31. Kumar, S, Stecher, G, Li, M, Knyaz, C, and Tamura, K. MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol. (2018) 35:1547–9. doi: 10.1093/molbev/msy096
33. Saadaoui, M, Faize, M, Bonhomme, L, Benyoussef, NO, Kharrat, M, Chaar, H, et al. Assessment of Tunisian trichoderma isolates on wheat seed germination, seedling growth and fusarium seedling blight suppression. Microorganisms. (2023) 11:1512. doi: 10.3390/microorganisms11061512
34. Mahfooz, M, Dwedi, S, Bhatt, A, Raghuvanshi, S, Bhatt, M, and Agrawal, PK. Evaluation of antifungal and enzymatic potential of endophytic fungi isolated from Cupressus torulosa D Don. Int J Curr Microbiol Appl Sci. (2017) 6:4084–100. doi: 10.20546/ijcmas.2017.607.424
35. Bhardwaj, A, Sharma, D, Jadon, N, and Agrawal, PK. Antimicrobial and phytochemical screening of endophytic fungi isolated from spikes of Pinus rouxburghii. Arch Clin Microbiol. (2015) 6:1
36. López, AC, Alvarenga, AE, Zapata, PD, Luna, MF, and Villalba, LL. Trichoderma spp. from Misiones, Argentina: effective fungi to promote plant growth of the regional crop Ilex paraguariensis St. Hil. Mycology. (2019) 10:210–21. doi: 10.1080/21501203.2019.1606860
37. Roberts, WK, and Selitrennikoff, CP. Plant and bacterial chitinases differ in antifungal activity. J Fish Biol. (1988) 134:169–76. doi: 10.1099/00221287-134-1-169
38. Oo, KT, Win, TT, Khai, AA, and Fu, P. Isolation, screening and molecular characterization of multifunctional plant growth promoting rhizobacteria for a sustainable agriculture. American J Plant Sci. (2020) 11:773–92. doi: 10.4236/ajps.2020.116055
39. Dhanabalan, S, Muthusamy, K, Iruthayasamy, J, Kumaresan, PV, Ravikumar, C, Kandasamy, R, et al. Unleashing Bacillus species as versatile antagonists: harnessing the biocontrol potentials of the plant growth-promoting rhizobacteria to combat Macrophomina phaseolina infection in Gloriosa superba. Microbiol Res. (2024) 283:127678. doi: 10.1016/j.micres.2024.127678
40. Gordon, SA, and Paleg, LG. Observations on the quantitative determination of indoleacetic acid. Physiol Plant. (1957) 10:39–47. doi: 10.1111/j.1399-3054.1957.tb07608.x
41. Noori, MSS, and Saud, HM. Potential plant growth-promoting activity of Pseudomonas sp. isolated from paddy soil in Malaysia as a biocontrol agent. J Plant Pathol Microbiol. (2012) 3:121
42. Mahadevan, A, and Sridhar, R (1982) Methods in physiological plant pathology, 2nd edn. Sivakami Publishers, Madras, pp. 24–44.
43. Kloepper, JW, Rodríguez-Kábana, R, McINROY, JA, and Collins, DJ. Analysis of populations and physiological characterization of microorganisms in rhizospheres of plants with antagonistic properties to phytopathogenic nematodes. Plant Soil. (1991) 136:95–102. doi: 10.1007/BF02465224
44. Castric, KF, and Castric, PA. Method for rapid detection of cyanogenic bacteria. Appl Environ Microbiol. (1983) 45:701–2. doi: 10.1128/aem.45.2.701-702.1983
45. Amri, M, Rjeibi, MR, Gatrouni, M, Mateus, DMR, Asses, N, Pinho, HJO, et al. Isolation, identification, and characterization of phosphate-solubilizing bacteria from Tunisian soils. Microorganisms. (2023) 11:783. doi: 10.3390/microorganisms11030783
46. Aleksandrov, VG, Blagodyr, RN, and Iiiev, IP. Liberation of phosphoric acid from apatite by silicate bacteria. Mikrobiol Zh (Kiev). (1967) 29:111–4.
47. Pikovskaya, RI. Mobilization of phosphorus in soil in connectionwith the vital activity of some microbial species. Microbiol. (1948) 17:362–70.
48. Schwyn, B, and Neilands, JB. Universal chemical assay for the detection and determination of siderophores. Anal Biochem. (1987) 160:47–56. doi: 10.1016/0003-2697(87)90612-9
49. Purnamasari, MI, Agustina, D, Prihatna, C, and Suwanto, A. A rapid inoculation method for infection of Ganoderma in oil palm. Int J Oil Palm. (2018) 1:1–9. doi: 10.35876/ijop.v1i1.1
50. Sreenayana, B, Vinodkumar, S, Nakkeeran, S, Muthulakshmi, P, and Poornima, K. Multitudinous potential of Trichoderma species in imparting resistance against F. Oxysporum f. sp. cucumerinum and Meloidogyne incognita disease complex. J Plant Growth Regul. (2022) 41:1187–206. doi: 10.1007/s00344-021-10372-9
51. Mukherjee, PK, Horwitz, BA, Vinale, F, Hohmann, P, Atanasova, L, and Mendoza-Mendoza, A. Editorial: molecular intricacies of Trichoderma-plant-pathogen interactions. Front Fungal Biol. (2022) 3:892228. doi: 10.3389/ffunb.2022.892228
52. Mukherjee, PK, Mendoza-Mendoza, A, Zeilinger, S, and Horwitz, BA. Mycoparasitism as a mechanism of Trichoderma mediated suppression of plant diseases. Fungal Biol Rev. (2022) 39:15–33. doi: 10.1016/j.fbr.2021.11.004
53. Poveda, J, Abril-Urias, P, and Escobar, C. Biological control of plant-parasitic nematodes by filamentous fungi inducers of resistance: Trichoderma, mycorrhizal and endophytic fungi. Front Microbiol. (2020) 11:992. doi: 10.3389/fmicb.2020.00992
54. Rashad, Y. M., and Moussa, T. A. (2020). Biocontrol agents for fungal plant diseases management. Cottage industry of biocontrol agents and their applications: Practical aspects to Deal biologically with pests and stresses facing strategic crops, 337–363.
55. Vitti, A, Pellegrini, E, Nali, C, Lovelli, S, Sofo, A, Valerio, M, et al. Trichoderma harzianum T-22 induces systemic resistance in tomato infected by cucumber mosaic virus. Front Plant Sci. (2016) 7:1520. doi: 10.3389/fpls.2016.01520
56. Guzmán-Guzmán, P, Porras-Troncoso, MD, Olmedo-Monfil, V, and Herrera-Estrella, A. Trichoderma species: Versatile plant symbionts. Phytopatholgy. (2019) 109:6–16. doi: 10.1094/PHYTO-07-18-0218-RVW
57. Nagy, V, Seidl, V, Szakacs, G, Komoń-Zelazowska, M, Kubicek, CP, and Druzhinina, IS. Application of DNA barcodes for screening of industrially important fungi: the haplotype of Trichoderma harzianum sensu stricto indicates superior chitinase formation. Appl Environ Microbiol. (2007) 73:7048–58. doi: 10.1128/AEM.00995-07
58. Berruti, A, Lumini, E, and Bianciotto, V. AMF components from a microbial inoculum fail to colonize roots and lack soil persistence in an arable maize field. Symbiosis. (2017) 72:73–80. doi: 10.1007/s13199-016-0442-7
59. El-Sharkawy, HHA, Rashad, YM, and Ibrahim, SA. Biocontrol of stem rust disease of wheat using arbuscular mycorrhizal fungi and Trichoderma spp. Physiol Mol Plant Pathol. (2018) 103:84–91. doi: 10.1016/j.pmpp.2018.05.002
60. Asad, SA. Mechanisms of action and biocontrol potential of Trichoderma against fungal plant diseases: a review. Ecol Complex. (2022) 49:100978. doi: 10.1016/j.ecocom.2021.100978
61. Philip, B, Behiry, SI, Salem, MZ, Amer, MA, El-Samra, IA, Abdelkhalek, A, et al. Trichoderma afroharzianum TRI07 metabolites inhibit Alternaria alternata growth and induce tomato defense-related enzymes. Sci Rep. (2024) 14:1874. doi: 10.1038/s41598-024-52301-2
62. Natey, B, Kasem, MMA, Rashad, YM, and Abo-Dahab, NF. Trichodermaafroharzianum B3R12: a potent biocontrol agent against Stromatiniacepivora, the causal agent of onion white rot. Novel Res Microbiol J. (2024) 8:2712–33. doi: 10.21608/NRMJ.2024.335958.1793
63. Bouanaka, H, Bellil, I, Harrat, W, Boussaha, S, Benbelkacem, A, and Khelifi, D. On the biocontrol by Trichoderma afroharzianum against fusarium culmorum responsible of fusarium head blight and crown rot of wheat in Algeria. Egypt J Biol Pest Control. (2021) 31:1–13. doi: 10.1186/s41938-021-00416-3
64. Musa, H, Hassan, MA, Isyaku, MS, Halidu, J, and Suleiman, AS. Antagonistic potential of Trichoderma species against Ganoderma disease of oil palm. Niger J Agric Food Environ. (2017) 13:60–7.
65. Sudarshan, GK, Chandrashekara, GS, Manjunath, B, Basavaraju, TB, and Palanna, KB. In vitro evaluation of botanicals, bioagents, and fungicides against basal stem rot of coconut caused by Ganoderma lucidum. New Agriculturist. (2017) 28:43–7.
66. Phurailatpam, L, and Mishra, S. Role of plant endophytes in conferring abiotic stress tolerance. Plant Ecophysiol Adaptation Climate Change Mech Persp II: Mech Adapt Stress Amelioration. (2020) 22:603–28. doi: 10.1007/978-981-15-2172-0_22
67. Rana, KL, Kour, D, Kaur, T, Devi, R, Yadav, AN, Yadav, N, et al. Endophytic microbes: biodiversity, plant growth-promoting mechanisms, and potential applications for agricultural sustainability. Antonie Van Leeuwenhoek. (2020) 113:1075–107. doi: 10.1007/s10482-020-01429-y
68. Hassan, SED. Plant growth-promoting activities of bacterial and fungal endophytes isolated from the medicinal plant Teucrium polium L. J Adv Res. (2017) 8:687–95. doi: 10.1016/j.jare.2017.09.001
69. Boller, T, and Felix, G. A renaissance of elicitors: perception of microbe-associated molecular patterns and danger signals by pattern-recognition receptors. Annu Rev Plant Biol. (2009) 60:379–406. doi: 10.1146/annurev.arplant.57.032905.105346
70. Harman, GE, Doni, F, Khadka, RB, and Uphoff, N. Endophytic strains of Trichoderma increase plants’ photosynthetic capability. J Appl Microbiol. (2021) 130:529–46. doi: 10.1111/jam.14368
71. Lemanceau, P, Bauer, P, Kraemer, S, and Briat, JF. Iron dynamics in the rhizosphere as a case study for analyzing interactions between soils, plants, and microbes. Plant Soil. (2009) 321:513–35. doi: 10.1007/s11104-009-0039-5
72. Saha, M, Sarkar, S, Sarkar, B, Sharma, BK, Bhattacharjee, S, and Tribedi, P. Microbial siderophores and their potential applications: a review. Environ Sci Pollut Res. (2016) 23:3984–99. doi: 10.1007/s11356-015-4294-0
73. Rodríguez, H, and Fraga, R. Phosphate-solubilizing bacteria and their role in plant growth promotion. Biotechnol Adv. (1999) 17:319–39. doi: 10.1016/S0734-9750(99)00014-2
74. Banik, S, and Chakruno, P. Isolation of fungal endophytes of rice and their antagonistic effect against some important rice fungal pathogens in vitro. J Pharmacogn Phytochem. (2019) 8:649–53.
75. Gravel, V, Antoun, H, and Tweddell, RJ. Growth stimulation and fruit yield improvement of greenhouse tomato plants by inoculation with Pseudomonas putidaor Trichoderma atroviride: possible role of indole acetic acid (IAA). Soil Biol Biochem. (2007) 39:1968–77. doi: 10.1016/j.soilbio.2007.02.015
76. Contreras-Cornejo, HA, Macías-Rodríguez, L, Cortés-Penagos, C, and López-Bucio, J. Trichoderma virens, a plant-beneficial fungus, enhances biomass production and promotes lateral root growth through an auxin-dependent mechanism in Arabidopsis. Plant Physiol. (2009) 149:1579–92. doi: 10.1104/pp.108.130369
77. Qi, W, and Zhao, L. Study of the siderophore-producing Trichoderma asperellum Q1 on cucumber growth promotion under salt stress. J Basic Microbiol. (2013) 53:355–64. doi: 10.1002/jobm.201200031
78. Martínez-Medina, A, Del Mar Alguacil, M, Pascual, JA, and Van Wees, SC. Phytohormone profiles induced by Trichoderma isolates correspond with their biocontrol and plant growth-promoting activity on melon plants. J Chem Ecol. (2014) 40:804–15. doi: 10.1007/s10886-014-0478-1
79. Singh, BN, Singh, A, Singh, SP, and Singh, HB. Trichoderma harzianum-mediated reprogramming of oxidative stress response in root apoplast of sunflower enhances defense against Rhizoctonia solani. Eur J Plant Pathol. (2011) 131:121–34. doi: 10.1007/s10658-011-9792-4
80. Rudresh, DL, Shivaprakash, MK, and Prasad, RD. Effect of combined application of Rhizobium, phosphate solubilizing bacterium and Trichoderma spp. on growth, nutrient uptake and yield of chickpea (Cicer arietinum L.). Appl Soil Ecol. (2005) 28:139–47.
81. Yobo, KS, Laing, MD, and Hunter, CH. Effects of single and combined inoculations of selected Trichoderma and Bacillus isolates on growth of dry bean and biological control of Rhizoctonia solani damping-off. Afr J Biotechnol. (2011) 10:8746–56.
82. Shivanna, MB, Meera, MS, Kageyama, K, and Hyakumachi, M. Growth promotion ability of zoysia grass rhizosphere fungi in consecutive plantings of wheat and soybean. Mycoscience. (1996) 37:163–8. doi: 10.1007/BF02461341
83. Shanmugaiah, V, Balasubramanian, N, Gomathinayagam, S, Monoharan, PT, and Rajendran, A. Effect of single application of Trichoderma viride and Pseudomonas fluorescenson growth promotion in cotton plants. Afr J Agric Res. (2009) 4:1220–5.
84. Rinu, K, Sati, P, and Pandey, A. Trichoderma gamsii (NFCCI 2177): a newly isolated endophytic, psychrotolerant, plant growth promoting and antagonistic fungal strain. J Basic Microbiol. (2014) 54:408–17. doi: 10.1002/jobm.201200579
85. Minchin, R, Hill, R, Condron, L, Ridgway, H, Baldauf, S, and Jones, E.. Effect of Trichoderma bio-inoculants on ectomycorrhizal colonisation of Pinus radiata seedlings. In: Proceedings of the 8th International Mycological Congress, Cairns, QLD, Australia, p. 204 (2006)
Keywords: Ganoderma spp., Trichoderma afroharzianum, antifungal mechanism, growth promotion, molecular phylogeny
Citation: Amrutha Lakshmi M, Indraja M, Singh UB, Subanna ARNS, Challa GK, Mawar R and Dauda WP (2025) Bioprospecting and mechanistic insights of Trichoderma spp. for suppression of Ganoderma-induced basal stem rot in oil palm. Front. Nutr. 12:1582047. doi: 10.3389/fnut.2025.1582047
Edited by:
Durgesh K. Jaiswal, Graphic Era University, IndiaReviewed by:
Anukool Vaishnav, GLA University, IndiaAurel Maxim, University of Agricultural Sciences and Veterinary Medicine of Cluj-Napoca, Romania
Copyright © 2025 Amrutha Lakshmi, Indraja, Singh, Subanna, Challa, Mawar and Dauda. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: M. Amrutha Lakshmi, YW1ydXRoYXZ2a0BnbWFpbC5jb20=
 M. Amrutha Lakshmi
M. Amrutha Lakshmi M. Indraja1
M. Indraja1 Udai B. Singh
Udai B. Singh W. P. Dauda
W. P. Dauda