- 1Division of Cardiology, Department of Internal Medicine, Chi Mei Medical Centre, Tainan, Taiwan
- 2Department of Nutrition Therapy, E-Da Hospital, Kaohsiung, Taiwan
- 3Department of Anesthesiology, Chi Mei Medical Center, Tainan, Taiwan
- 4Department of Public Health, College of Medicine, National Cheng Kung University, Tainan, Taiwan
- 5Department of Nutrition, Chi Mei Medical Center, Tainan, Taiwan
- 6Department of Intensive Care Medicine, Chi Mei Medical Center, Tainan, Taiwan
- 7School of Medicine, College of Medicine, National Sun Yat-sen University, Kaohsiung, Taiwan
Objective: This study aimed to examine the association between zinc deficiency (ZD) and the clinical outcomes in patients with heart failure (HF).
Methods: This multicenter retrospective cohort study used the TriNetX network to identify adult patients with HF between January 1, 2010, and January 31, 2025. Patients with serum zinc levels below 70 μg/dL (ZD group) were propensity score-matched to those with levels between 70 and 120 μg/dL (control group) to minimize confounding. Primary outcomes included all-cause mortality, major adverse cardiovascular events (MACEs), and major adverse kidney events (MAKEs). Secondary outcome was all-cause hospitalization.
Results: After matching, each group comprised 4,145 patients with well-balanced baseline characteristics. During the 1-year follow-up, the ZD group was associated with higher risks of all-cause mortality (hazard ratio [HR]: 1.46, 95% confidence interval [CI]: 1.29–1.66), MACEs (HR: 1.46, 95% CI: 1.30–1.64), and MAKEs (HR: 1.51, 95% CI: 1.34–1.70), as well as an higher risk of all-cause hospitalization (HR: 1.24, 95% CI: 1.16–1.32).
Conclusion: Zinc deficiency in patients with HF is associated with increased risks of mortality, cardiovascular and kidney-related adverse events, and hospitalization. These findings highlight the potential clinical importance of zinc assessment and management in HF care.
Introduction
Heart failure (HF) remains a leading cause of morbidity and mortality worldwide, exerting a substantial clinical and economic burden (1, 2). The prevalence in the general adult population is estimated to be 1–3%, with an incidence rate of approximately 1–20 cases per 1,000 individuals each year (3, 4). Moreover, HF-associated mortality has been rising annually, with a 5-year mortality rate ranging from 50 to 75% (3). The economic impact is similarly profound, with annual healthcare costs per person estimated at $11,000–$18,000 (3, 5).
Zinc is an essential trace element that plays a crucial role in immune function, oxidative stress regulation, and cardiovascular homeostasis (6–12). Previous reviews demonstrated that zinc deficiency (ZD) is highly prevalent among patients with HF, with serum zinc levels significantly lower compared to healthy controls (13, 14). Patients with HF often exhibit hyperzincuria due to the use of diuretics and renin-angiotensin-aldosterone system inhibitors, further exacerbating zinc depletion (15–19). Mechanistically, ZD may contribute to HF progression through multiple pathways, including increased oxidative stress, systemic inflammation, and endothelial dysfunction (20–23). An observational study found that ZD was associated with a worse prognosis in patients with HF (24). Additionally, preliminary results suggested that zinc supplementation may improve left ventricular ejection fraction (LVEF), highlighting its potential therapeutic role (25).
Despite these findings, large-scale studies investigating the impact of ZD on HF outcomes remain limited. In this multicenter study, we aimed to explore the association between ZD and HF outcomes, including all-cause mortality, major adverse cardiac events (MACEs), and major adverse kidney events (MAKEs).
Methods
Study design and database
The study used data from the TriNetX Analytics Network Platform, a comprehensive federated health research network encompassing electronic medical records (EMRs) from approximately 160 million patients across 140 healthcare organizations (HCOs) worldwide (26). This expansive dataset incorporates diverse variables, including demographic information, diagnoses, procedures, medications, laboratory results, genomic data, and HCO visit classifications. TriNetX facilitates secure, real-time access to de-identified, aggregated data derived from a geographically and ethnically diverse patient population, spanning hospitals, primary care facilities, and specialty treatment centers. The platform operates under a waiver from the Western Institutional Review Board, as it exclusively processes statistical summaries without accessing individual-level patient data. This investigation was conducted in adherence to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines (27).
Study population and definition of eligible patients
The study population consisted of adult patients with HF who underwent zinc testing within 1 year prior to their HF diagnosis between January 01, 2010, and January 31, 2025. The date of zinc testing was designated as the index date.
Study eligibility required participants to be 18 years or older with an HF diagnosis, identified using the International Classification of Diseases, Tenth Edition, Clinical Modification (ICD-10-CM) code I50. Participants were stratified into two groups based on their serum zinc levels: the zinc deficiency (ZD) group (serum zinc <70 μg/dL) and the control group (serum zinc 70–120 μg/dL) (28, 29). Serum zinc measurements were identified using Logical Observation Identifiers Names and Codes (LOINC) 5768-8 or 8245-3. To ensure sufficient clinical documentation, we included only patients with at least two EMRs within the observation window. To minimize protopathic and ascertainment bias, patients with any prior history of MACE or MAKE were excluded based on all available EMRs preceding the index date (Supplementary Table S1).
Covariates
The selection of covariates was determined by their clinical significance, with particular focus on key comorbidities and risk factors that have established associations with mortality rates and cardio-renal outcomes. We evaluated baseline health parameters in accordance with contemporary medical understanding. For both study groups, we extracted data on baseline characteristics and covariates from the year preceding the index date, including demographic factors (age, sex, race), clinical parameters (estimated glomerular filtration rate [eGFR], albumin, Hemoglobin A1c [HbA1c], left ventricular ejection fraction [LVEF]), existing comorbidites, and prescribed medications.
The spectrum of evaluated comorbidities extended to cardiometabolic conditions (hypertension, hyperlipidemia, obesity), nutritional status (malnutrition), metabolic disorders (type 2 diabetes mellitus), substance use patterns (nicotine dependence, alcohol-related disorders), respiratory conditions (chronic lower respiratory diseases), hepatic function (liver diseases), renal status (chronic kidney disease), cardiovascular conditions (cerebrovascular diseases, atrial fibrillation and flutter, ischemic heart disease), autoimmune disorders (systemic lupus erythematosus), and malignancies (neoplasms). The medication profile analysis encompassed therapeutic agents for heart failure management (including angiotensin-converting enzyme inhibitors [ACEis], angiotensin receptor blockers [ARBs], beta-blockers, calcium-channel blockers, various classes of diuretics, and sodium-glucose cotransporter-2 inhibitors [SGLT2is]) and cholesterol management (statins) (Supplementary Table S2).
Outcomes
The study evaluated both primary and secondary outcomes. Primary outcomes comprised all-cause mortality, MACEs, and MAKEs. The secondary outcome focused on all-cause hospitalization risk. MACEs encompassed acute myocardial infarction, stroke (including cerebral infarction and hemorrhage), ventricular arrhythmias (e.g., ventricular tachycardia, ventricular fibrillation), and cardiac arrest (30). MAKEs were characterized by end-stage kidney disease (ESKD), urgent dialysis initiation, or dialysis dependence (30). Patient follow-up commenced the day after the index date and continued until their final clinical visit, death, or one-year post-index date, whichever occurred first (Supplementary Table S3).
Statistical analysis
For baseline characteristics, continuous variables were presented as means with standard deviations (SDs), while categorical variables were expressed as frequencies and percentages. To minimize selection bias and balance covariates between groups, we implemented propensity score matching (PSM) using a greedy nearest-neighbor algorithm, with a caliper width set at 0.1 pooled SDs. The effectiveness of matching was evaluated through standardized differences, where values less than 0.1 indicated adequate covariate balance (31).
After matching, we conducted survival analyses using the Kaplan–Meier method, with between-group differences assessed via the log-rank test. The association between zinc status and outcomes was quantified using Cox proportional hazards regression models to calculate hazard ratios (HRs). To evaluate the statistical significance of the differences between subgroups, we used a method that examined the CI overlap (32). Additionally, we calculated E-values to further gage the impact of potential unmeasured confounders on the primary and secondary outcomes (33). Statistical significance was defined as a two-sided p value below 0.05.
Stratified analysis
We conducted stratified analyses to examine the heterogeneity of primary outcomes associations across multiple subgroups. These analyses included age stratification (18–64 versus ≥ 65 years), sex-specific differences (female versus male), and heart failure classification based on ejection fraction (heart failure with preserved ejection fraction [HFpEF], heart failure with mildly reduced ejection fraction [HFmrEF], and heart failure with reduced ejection fraction [HFrEF]). Additional stratification was performed according to clinical parameters, including the presence of malnutrition, overweight and obesity, renal function (eGFR < 45 versus ≥ 45 mL/min/1.73 m2), and glycemic control (HbA1c < 9% versus ≥ 9%).
Sensitivity analysis
To evaluate the robustness of our findings, we conducted additional sensitivity analyses by modifying the definition of MACEs. In the first analysis, ventricular arrhythmias were excluded to reflect a more conventional MACE definition comprising only acute myocardial infarction, stroke, and cardiac arrest. In a second analysis, we expanded the MACE definition to include cardiomyopathy (ICD-10-CM: I42) to examine its impact as a clinically relevant cardiovascular complication in patients with HF. Additionally, we performed a new round of PSM that included the following variables: C-reactive protein (CRP), copper, selenium, prealbumin, transferrin, and urine albumin-to-creatinine ratio (UACR). These variables were selected to account for systemic inflammation, trace element status, and nutritional reserve. Outcomes were re-evaluated using Cox proportional hazards models.
Results
Study flow diagram
From a total population of 160,562,143 patients across 142 HCOs in the TriNetX network, we identified 119,071,309 individuals with visits between January 01, 2010, and January 31, 2025. We excluded 119,059,524 patients who met one or more of the following criteria: age below 18 years, occurrence of prespecified outcomes before the index date, lack of zinc level measurements prior to the index date, or absence of HF diagnosis. Of the remaining 11,785 eligible patients with both HF and zinc measurements, 7,280 were categorized into the ZD group, while 4,505 comprised the control group with normal zinc levels. Following PSM, the final analysis included 4,145 patients in each group (Figure 1).
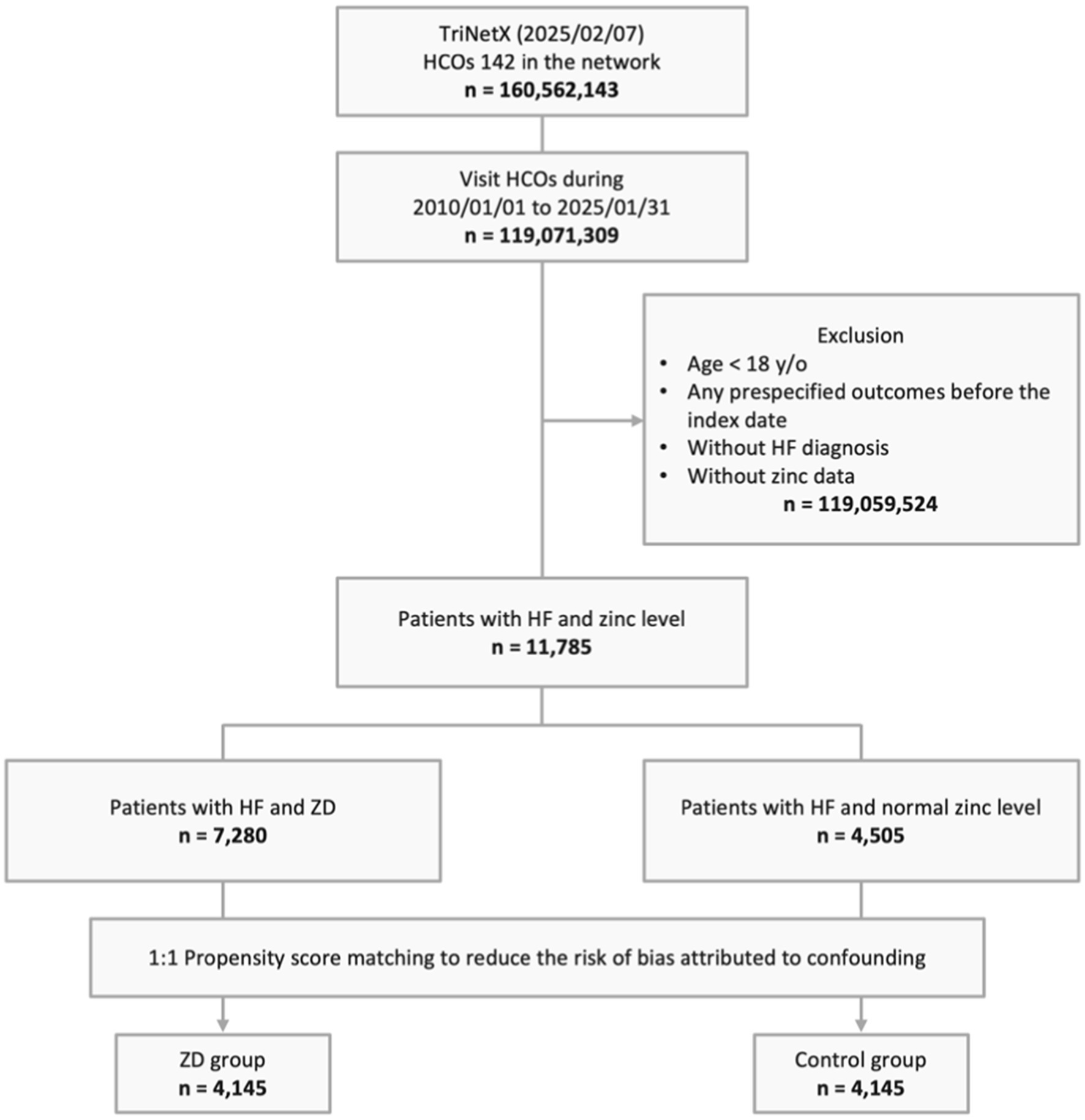
Figure 1. Study design and selection flow. HCO, healthcare organizations; HF, heart failure; y/o, years old; ZD, zinc deficiency.
Study population characteristics
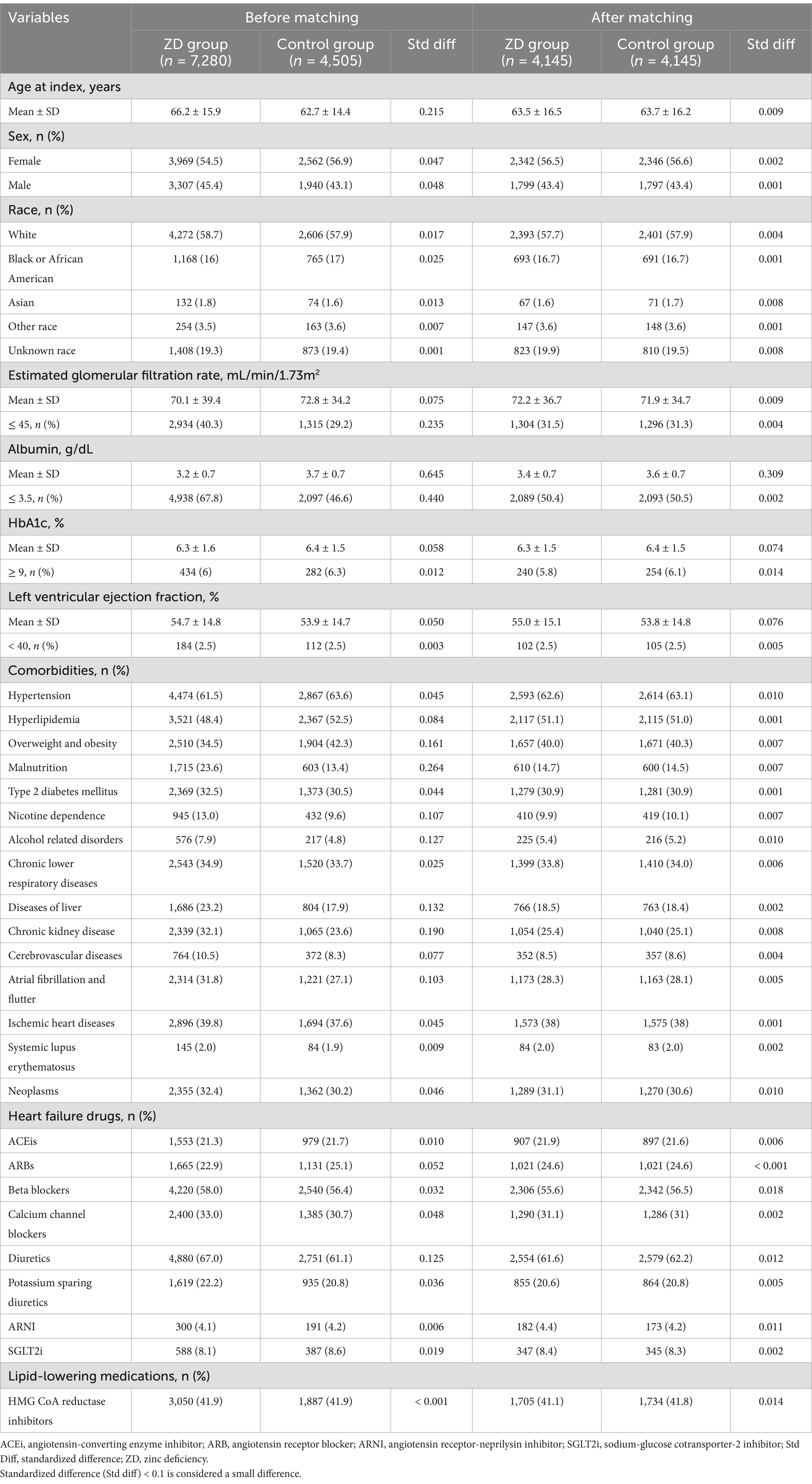
Before PSM, there were significant differences in baseline characteristics between the ZD group (n = 7,280) and the control group (n = 4,505). Participants in the ZD group were older (66.2 ± 15.9 vs. 62.7 ± 14.4 years) and had a slightly lower proportion of female (54.5% vs. 56.9%). The ZD group had a higher prevalence of chronic conditions, including chronic kidney disease (32.1% vs. 23.6%), malnutrition (23.6% vs. 13.4%), and nicotine dependence (13.0% vs. 9.6%). Additionally, they had lower albumin levels (3.2 ± 0.7 vs. 3.7 ± 0.7 g/dL) and a higher proportion of patients with albumin ≤ 3.5 g/dL (67.8% vs. 46.6%). Use of medications such as diuretics (67.0% vs. 61.1%) and beta blockers (58.0% vs. 56.4%) was also more frequent in the ZD group (Table 1).
After PSM, the ZD (n = 4,145) and control (n = 4,145) groups were well balanced in baseline characteristics, as shown by standardized differences <0.1. Their mean ages were comparable (63.5 ± 16.5 vs. 63.7 ± 16.2 years), as were sex distributions (56.5% vs. 56.6% female). Comorbidities such as type 2 diabetes, hypertension, overweight/obesity, and chronic kidney disease were also well matched between groups. Similarly, albumin levels, HbA1c, and eGFR showed satisfactory balance, ensuring comparability in clinical characteristics (standardized differences <0.1; Table 1).
Primary and secondary outcomes
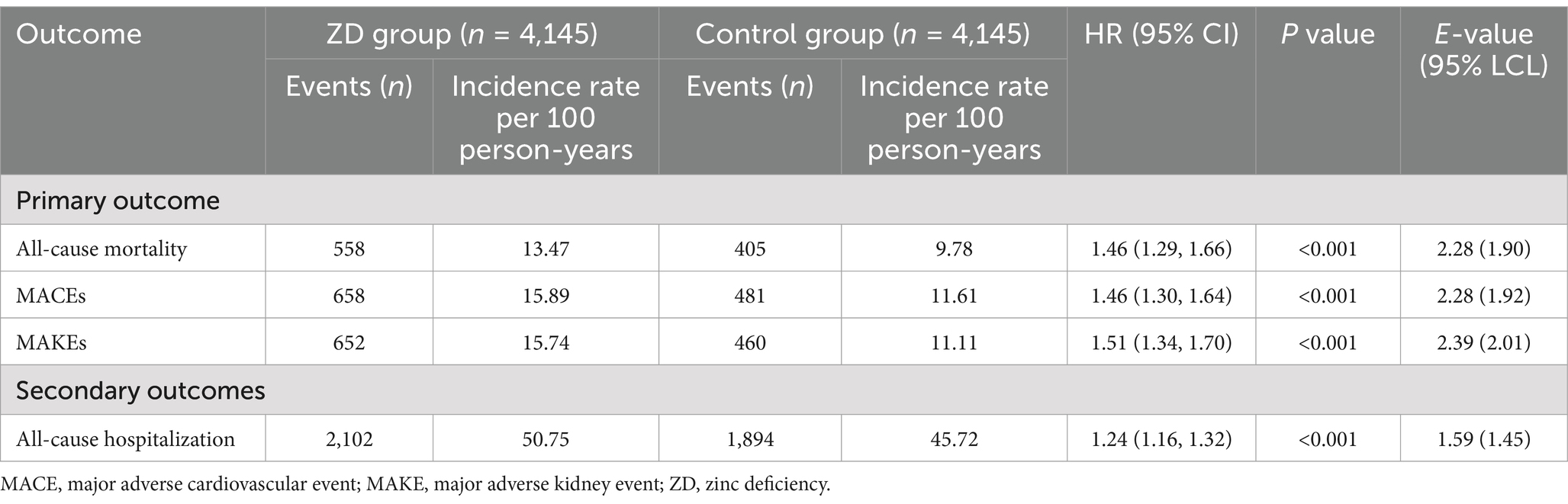
For the primary outcome (all-cause mortality), the ZD group was associated with a significantly higher cumulative incidence compared with the control group (HR, 1.46; 95% CI, 1.29–1.66; p < 0.001), reflecting incidence rates of 13.47 and 9.78 per 100 person-years, respectively (Table 2). The E-value for this association was 2.28 (95% lower confidence limit [LCL], 1.90), suggesting that only a relatively strong unmeasured confounder could fully explain this observed association.
Similarly, for MACEs, the ZD group was linked to an elevated risk (HR, 1.46; 95% CI, 1.30–1.64; p < 0.001), with incidence rates of 15.89 and 11.61 per 100 person-years in the ZD and control groups, respectively. The E-value for this association was 2.28 (95% LCL, 1.92), reinforcing the robustness of this finding. For MAKEs, a higher risk was also observed in the ZD group (HR, 1.51; 95% CI, 1.34–1.70; p < 0.001), with incidence rates of 15.74 and 11.11 per 100 person-years, respectively. The E-value of 2.39 (95% LCL, 2.01) further indicates that substantial unmeasured confounding would be required to nullify this result.
Regarding the secondary outcome of all-cause hospitalization, the ZD group was associated with a greater risk compared with the control group (HR, 1.24; 95% CI, 1.16–1.32; p < 0.001), with incidence rates of 50.75 and 45.72 per 100 person-years, respectively. The E-value of 1.59 (95% LCL, 1.45) suggests that moderate unmeasured confounding could potentially explain this association. Consistently, Kaplan–Meier curves demonstrated a higher cumulative incidence of all primary and secondary outcomes in the ZD group compared with the control group throughout the 1-year follow-up period (log-rank p < 0.001, Figures 2A–C).

Figure 2. Kaplan–Meier time-to-event free curves of the primary outcomes: (A) all-cause mortality; (B) major adverse cardiovascular events; and (C) major adverse kidney events. ZD, zinc deficiency.
Stratified analysis
Stratified analyses demonstrated that the association between ZD and significant risks of all-cause mortality, MACEs, and MAKEs remained consistent across most patient strata, including sex, age categories, presence of malnutrition, obesity, HF classifications, and HbA1c levels (Figures 3A–C). The only exception was observed for MACEs among patients with eGFR above 45 mL/min/1.73 m2, where the association did not reach statistical significance (Figure 3B). No significant interaction was observed across any of the subgroup variables (all P for interaction > 0.05), indicating a consistent association between zinc deficiency and adverse outcomes across strata (Figures 3A–C).
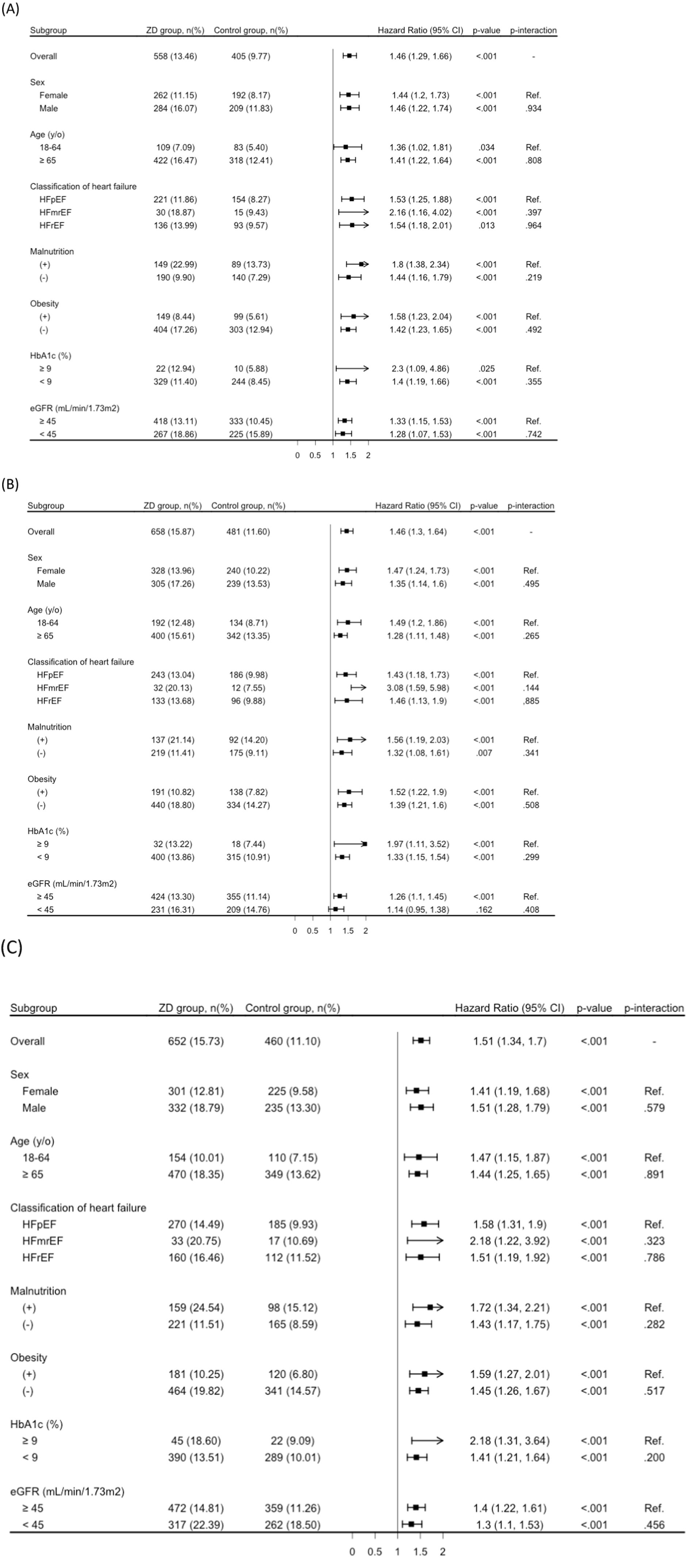
Figure 3. Stratified analysis of primary outcomes: (A) all-cause mortality; (B) major adverse cardiovascular events; and (C) major adverse kidney events. eGFR, estimated glomerular filtration rate; HbA1c, hemoglobin A1c; y/o, years old; ZD, zinc deficiency. Subgroup analyses were conducted using independently matched sub-cohorts based on propensity score matching within each stratum. Therefore, the number of patients and events may differ from those in the overall matched cohort.
Sensitivity analysis
The associations between ZD and adverse outcomes remained consistent across sensitivity analyses. When arrhythmias were excluded from the MACE definition, the HR for MACE in the ZD group was 1.42 (95% CI: 1.17–1.72; p < 0.001). Similarly, when cardiomyopathy was included as an additional component, the HR was 1.41 (95% CI: 1.19–1.68; p < 0.001). These findings further support the robustness of our main results (Supplementary Table S4).
After additional matching based on inflammatory and nutritional markers (CRP, copper, selenium, prealbumin, transferrin, and UACR), 2,478 patients remained in each group (ZD and control). As shown in Supplementary Table S5, baseline characteristics between the two groups were well balanced across all covariates. In this matched cohort, the association between ZD and all-cause mortality remained statistically significant (HR: 1.43, 95% CI: 1.20–1.69, p < 0.001), as did the associations with MACEs (HR: 1.17, 95% CI: 1.09–1.41), MAKEs (HR: 1.57, 95% CI: 1.08–2.29), and all-cause hospitalization (HR: 1.23, 95% CI: 1.15–1.33) (Supplementary Table S6).
Discussion
In this large, multicenter retrospective cohort study of over 8,000 patients with HF, we found that ZD was associated with a significantly higher risk of all-cause mortality, MACE, MAKE, and increased all-cause hospitalization within 1 year of follow-up. These findings contribute important new evidence to the growing body of literature linking ZD to adverse clinical outcomes in HF and highlight the potential clinical relevance of maintaining optimal zinc levels in this high-risk population.
A salient observation from the study is the consistently higher hazard for all three primary outcomes—mortality, MACEs, and MAKEs—in patients with ZD compared to those with normal zinc levels, even after robust PSM. These results reinforce and extend earlier, smaller investigations that have implicated suboptimal zinc status in increased cardiovascular risk. For instance, a previous longitudinal study demonstrated that patients with HF and serum zinc levels below 62 μg/dL had elevated risks of cardiovascular and all-cause mortality (24). Similarly, a pilot study suggested that supplementation with intravenous zinc in selected patients led to partial improvement in LVEF (25), hinting at a possible therapeutic benefit. By leveraging a large-scale dataset derived from multiple healthcare organizations, our study provides a strong epidemiologic foundation indicating that ZD may be a critical, modifiable risk factor in HF management.
Multiple mechanistic pathways may underlie the observed increased risk associated with ZD. Zinc is integral to numerous enzymatic processes and regulatory pathways that maintain cardiovascular homeostasis. In vitro and animal studies have shown that zinc plays a key role in mediating antioxidant defenses, mitigating oxidative stress, and regulating inflammatory responses (6–12). A deficiency in zinc may intensify pro-inflammatory and pro-oxidative states, both of which can accelerate atherosclerosis, impair vascular endothelial function, and exacerbate myocardial remodeling. Additionally, zinc participates in cellular signaling pathways that modulate myocardial contractility, cellular apoptosis, and tissue repair (34–37). Consequently, suboptimal zinc status could predispose patients with HF to worsening cardiac function and increased susceptibility to arrhythmogenic events, thereby contributing to higher risks of MACEs and mortality (14).
Furthermore, prior work has shown that patients with HF are prone to hyperzincuria, especially when on diuretics and renin-angiotensin-aldosterone system inhibitors (15–19). Although these medications confer substantial benefits in HF, they may inadvertently lower systemic zinc levels over time. Similarly, when zinc is depleted, the kidney may be more susceptible to tubular damage, fibrosis, and hemodynamic strain, especially under the chronic congestion and reduced perfusion characteristic of HF (38–40). Additionally, older age, malnutrition, and malabsorption syndromes—prevalent in many HF populations—can further impair zinc status, amplifying the cardiorenal burden. By undermining the kidney’s capacity to handle physiological stress, sustain filtration, and mitigate pro-inflammatory responses, insufficient zinc can exacerbate the progression to end-stage kidney disease or dialysis dependence (14, 41).
In recent years, a growing number of systematic reviews have examined the relationship between zinc status and cardiovascular outcomes (7, 14). For example, Rosenblum et al. (14) conducted a systematic review highlighting the potential link between zinc deficiency and adverse prognosis in patients with HF. However, the current body of evidence remains limited, and the heterogeneity among existing studies has precluded the conduct of a robust meta-analysis. Our study contributes novel data that complement prior findings by adopting a large-scale, multicenter cohort design using standardized real-world electronic medical records and rigorous propensity score matching. This methodological approach allows for a more granular and contemporary assessment of zinc deficiency in patients with HF, with uniform outcome definitions and consistent follow-up durations. Importantly, our results may serve as a valuable contribution to future meta-analyses and help inform clinical risk stratification strategies. These findings reinforce the emerging evidence and underscore the potential clinical relevance of zinc assessment in the management of HF.
Our findings underscore the potential clinical importance of assessing zinc status in HF. While worldwide trends and prevalence of ZD have shown some stability with notable reductions in general populations, disease-specific populations present a different picture (42). In our study, the prevalence of ZD in patients with HF was 62%, which aligns with previous research showing 66% in patients with HF, while other conditions like diabetes showed lower rates at 23% (30). The burden of ZD extends to other chronic conditions as well - affecting 30–66% of patients undergoing dialysis and 50–70% of cancer patients (38, 41, 43–46). Given that ZD is relatively common and potentially correctable, systematic screening and early detection could offer a new avenue for risk stratification and intervention. This is particularly relevant given the consistent associations with not only mortality but also MACEs and MAKEs, suggesting that ZD may affect both cardiac and renal trajectories in HF. Intriguingly, we also found a higher risk of all-cause hospitalization among ZD patients. This may have important economic and healthcare resource implications, considering that HF is already among the most resource-intensive conditions globally. Optimizing zinc levels might conceivably help reduce the frequency of hospital admissions, although this hypothesis should be tested in prospective trials.
The strengths of our study include a large cohort of patients with HF and ZD sourced from TriNetX, a global repository of real-world data encompassing multiple institutions and countries. This broad dataset enhances the generalizability of our findings to a wider population. Additionally, our study incorporates various stratified analyses and demonstrates high E-values, further supporting the robustness of our results. To address confounding factors, we used an integrated PSM function, ensuring a balanced comparison of baseline characteristics, including demographics and comorbidities, between the two groups.
However, our study has some limitations. First, its observational design prevents us from establishing causality. Second, ZD may act as a mediator of more advanced disease severity or overall malnutrition rather than being a direct contributor to negative outcomes. Third, reliance on EMRs and administrative billing codes may introduce misclassification bias in both HF diagnosis and serum zinc level measurements. To mitigate this, we applied validated ICD-10-CM codes and LOINC codes for zinc testing. Fourth, we lacked data on dietary zinc intake, the use of over-the-counter zinc supplements, and the exact timing and dosage of prescription medications, all of which could influence the observed association between ZD and HF outcomes. Fifth, our study focused on 1-year outcomes, which, while clinically meaningful, may not fully capture the long-term effects of ZD on disease progression and survival. A longer observation period could provide additional insights into risk magnitude and other clinically relevant endpoints. Finally, due to the aggregate and de-identified nature of the TriNetX platform, we were unable to perform advanced model-based metrics such as net reclassification improvement (NRI) or integrated discrimination improvement (IDI) to formally evaluate the incremental predictive value of serum zinc as a biomarker. Future studies using patient-level data and comprehensive risk prediction modeling are warranted to further assess the prognostic utility of zinc in clinical practice.
Conclusion
The study demonstrates that ZD is significantly associated with elevated risks of all-cause mortality, MACEs, MAKEs, and all-cause hospitalization in patients with HF. These associations persisted despite careful adjustment for known prognostic factors. Although observational in nature, our results suggest that assessing and correcting ZD may represent a valuable yet underrecognized approach to improving outcomes in HF. Future prospective studies and RCTs are warranted to determine whether targeted interventions aimed at maintaining adequate zinc status can tangibly benefit this population.
Data share statement
Data described in the manuscript, code book, and analytic code will be made publicly and freely available without restriction at: https://trinetx.com.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent from the [patients/ participants OR patients/participants legal guardian/next of kin] was not required to participate in this study in accordance with the national legislation and the institutional requirements.
Author contributions
Y-ML: Conceptualization, Data curation, Investigation, Writing – original draft. W-LT: Data curation, Formal analysis, Investigation, Methodology, Writing – original draft. K-CH: Data curation, Formal analysis, Investigation, Methodology, Writing – original draft. TY: Investigation, Methodology, Validation, Writing – original draft. J-YW: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Supervision, Validation, Writing – original draft, Writing – review & editing. C-CL: Conceptualization, Supervision, Writing – review & editing, Validation.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2025.1589907/full#supplementary-material
References
1. Beghini, A, Sammartino, AM, Papp, Z, von Haehling, S, Biegus, J, Ponikowski, P, et al. 2024 update in heart failure. ESC Heart Fail. (2025) 12:8–42. doi: 10.1002/ehf2.14857
2. Kemp, CD, and Conte, JV. The pathophysiology of heart failure. Cardiovasc Pathol. (2012) 21:365–71. doi: 10.1016/j.carpath.2011.11.007
3. Savarese, G, Becher, PM, Lund, LH, Seferovic, P, Rosano, GMC, and Coats, AJS. Global burden of heart failure: a comprehensive and updated review of epidemiology. Cardiovasc Res. (2023) 118:3272–87. doi: 10.1093/cvr/cvac013
4. Zamorano, JL, and Leal, AG. Advances in heart failure management. Med Clín (English edition). (2024) 163:32–9. doi: 10.1016/j.medcli.2023.12.026
5. Lowe, EF, Gerasta, D, Balser, M, Page, RL 2nd, Tsai, E, Biermann, HD, et al. Contributors and solutions to high out-of-pocket costs for heart failure medications: a state-of-the-art review. J Am Coll Cardiol. (2025) 85:365–77. doi: 10.1016/j.jacc.2024.11.011
6. Berger, MM, Shenkin, A, Dizdar, OS, Amrein, K, Augsburger, M, Biesalski, HK, et al. ESPEN practical short micronutrient guideline. Clin Nutr. (2024) 43:825–57. doi: 10.1016/j.clnu.2024.01.030
7. Olechnowicz, J, Tinkov, A, Skalny, A, and Suliburska, J. Zinc status is associated with inflammation, oxidative stress, lipid, and glucose metabolism. J Physiol Sci. (2018) 68:19–31. doi: 10.1007/s12576-017-0571-7
8. Lee, SR. Critical role of zinc as either an antioxidant or a Prooxidant in cellular systems. Oxidative Med Cell Longev. (2018) 2018:9156285. doi: 10.1155/2018/9156285
9. Kloubert, V, and Rink, L. Zinc as a micronutrient and its preventive role of oxidative damage in cells. Food Funct. (2015) 6:3195–204. doi: 10.1039/C5FO00630A
10. Wu, JY, Liu, TH, Huang, PY, Tsai, YW, and Lai, CC. The effect of zinc on the outcome of patients with COVID-19: a systematic review and meta-analysis of randomized controlled trials. J Infect. (2023) 86:e142–3. doi: 10.1016/j.jinf.2023.01.023
11. Wu, JY, Hsu, WH, Tsai, YW, Liu, TH, Huang, PY, Chuang, MH, et al. The association between zinc deficiency, and clinical outcomes of COVID-19. J Infect. (2023) 87:e63–7. doi: 10.1016/j.jinf.2023.06.021
12. Yang, HY, Hung, KC, Chuang, MH, Chang, R, Chen, RY, Wang, FW, et al. Effect of zinc supplementation on blood sugar control in the overweight and obese population: a systematic review and meta-analysis of randomized controlled trials. Obes Res Clin Pract. (2023) 17:308–17. doi: 10.1016/j.orcp.2023.06.003
13. Soukoulis, V, Dihu, JB, Sole, M, Anker, SD, Cleland, J, Fonarow, GC, et al. Micronutrient deficiencies an unmet need in heart failure. J Am Coll Cardiol. (2009) 54:1660–73. doi: 10.1016/j.jacc.2009.08.012
14. Rosenblum, H, Wessler, JD, Gupta, A, Maurer, MS, and Bikdeli, B. Zinc deficiency and heart failure: a systematic review of the current literature. J Card Fail. (2020) 26:180–9. doi: 10.1016/j.cardfail.2020.01.005
15. Thomas, M, Vidal, A, Bhattacharya, SK, Ahokas, RA, Sun, Y, Gerling, IC, et al. Zinc dyshomeostasis in rats with aldosteronism. Response to spironolactone. Am J Physiol Heart Circ Physiol. (2007) 293:H2361–6. doi: 10.1152/ajpheart.00200.2007
16. Whitted, AD, Stanifer, JW, Dube, P, Borkowski, BJ, Yusuf, J, Komolafe, BO, et al. A dyshomeostasis of electrolytes and trace elements in acute stressor states: impact on the heart. Am J Med Sci. (2010) 340:48–53. doi: 10.1097/MAJ.0b013e3181e5945b
17. Seawell, MR, Al Darazi, F, Farah, V, Ramanathan, KB, Newman, KP, Bhattacharya, SK, et al. Mineralocorticoid receptor antagonism confers cardioprotection in heart failure. Curr Heart Fail Rep. (2013) 10:36–45. doi: 10.1007/s11897-012-0120-x
18. Gandhi, MS, Deshmukh, PA, Kamalov, G, Zhao, T, Zhao, W, Whaley, JT, et al. Causes and consequences of zinc dyshomeostasis in rats with chronic aldosteronism. J Cardiovasc Pharmacol. (2008) 52:245–52. doi: 10.1097/FJC.0b013e3181833eb8
19. Efeovbokhan, N, Bhattacharya, SK, Ahokas, RA, Sun, Y, Guntaka, RV, Gerling, IC, et al. Zinc and the prooxidant heart failure phenotype. J Cardiovasc Pharmacol. (2014) 64:393–400. doi: 10.1097/FJC.0000000000000125
20. de Lorgeril, M, Salen, P, Accominotti, M, Cadau, M, Steghens, JP, Boucher, F, et al. Dietary and blood antioxidants in patients with chronic heart failure. Insights into the potential importance of selenium in heart failure. Eur J Heart Fail. (2001) 3:661–9. doi: 10.1016/S1388-9842(01)00179-9
21. Alexanian, I, Parissis, J, Farmakis, D, Athanaselis, S, Pappas, L, Gavrielatos, G, et al. Clinical and echocardiographic correlates of serum copper and zinc in acute and chronic heart failure. Clin Res Cardiol. (2014) 103:938–49. doi: 10.1007/s00392-014-0735-x
22. Ghaemian, A, Salehifar, E, Jalalian, R, Ghasemi, F, Azizi, S, Masoumi, S, et al. Zinc and copper levels in severe heart failure and the effects of atrial fibrillation on the zinc and copper status. Biol Trace Elem Res. (2011) 143:1239–46. doi: 10.1007/s12011-011-8956-6
23. Salehifar, E, Shokrzadeh, M, Ghaemian, A, Aliakbari, S, and Saeedi Saravi, SS. The study of cu and Zn serum levels in idiopathic dilated cardiomyopathy (IDCMP) patients and its comparison with healthy volunteers. Biol Trace Elem Res. (2008) 125:97–108. doi: 10.1007/s12011-008-8151-6
24. Yoshihisa, A, Abe, S, Kiko, T, Kimishima, Y, Sato, Y, Watanabe, S, et al. Association of Serum Zinc Level with Prognosis in patients with heart failure. J Card Fail. (2018) 24:375–83. doi: 10.1016/j.cardfail.2018.02.011
25. Frustaci, A, Sabbioni, E, Fortaner, S, Farina, M, del Torchio, R, Tafani, M, et al. Selenium-and zinc-deficient cardiomyopathy in human intestinal malabsorption: preliminary results of selenium/zinc infusion. Eur J Heart Fail. (2012) 14:202–10. doi: 10.1093/eurjhf/hfr167
26. Tri Netx. TriNetx 2025. Available at: https://trinetx.com/ (Accessed Febuary 7, 2025).
27. Cuschieri, S. The STROBE guidelines. Saudi J Anaesth. (2019) 13:S31–4. doi: 10.4103/sja.SJA_543_18
28. Hung, LW, Liu, MY, Yu, T, Hung, KC, Tsai, YW, Lai, CC, et al. Zinc deficiency and post-acute outcomes in patients with COVID-19: a six-month retrospective cohort analysis of 3, 726 patients. Cureus. (2024) 16:e71609. doi: 10.7759/cureus.71609
29. Smith, JC Jr, Butrimovitz, GP, and Purdy, WC. Direct measurement of zinc in plasma by atomic absorption spectroscopy. Clin Chem. (1979) 25:1487–91. doi: 10.1093/clinchem/25.8.1487
30. Wu, JY, Wu, YJ, Liu, MY, Hsu, WH, Tsai, YW, Liu, TH, et al. Clinical outcomes in diabetic patients with zinc deficiency: a multi-institutional population-based study. J Am Nutr Assoc. (2025) 5:1–8. doi: 10.1080/27697061.2025.2461215
31. Haukoos, JS, and Lewis, RJ. The propensity score. JAMA. (2015) 314:1637–8. doi: 10.1001/jama.2015.13480
32. Schenker, N, and Gentleman, JF. On judging the significance of differences by examining the overlap between confidence intervals. Am Stat. (2001) 55:182–6. doi: 10.1198/000313001317097960
33. Vander Weele, TJ, and Ding, P. Sensitivity analysis in observational research: introducing the E-value. Ann Intern Med. (2017) 167:268–74. doi: 10.7326/M16-2607
34. Hara, T, Yoshigai, E, Ohashi, T, and Fukada, T. Zinc in cardiovascular functions and diseases: epidemiology and molecular mechanisms for therapeutic development. Int J Mol Sci. (2023) 24:7152. doi: 10.3390/ijms24087152
35. Shen, T, Zhao, Q, Luo, Y, and Wang, T. Investigating the role of zinc in atherosclerosis: a review. Biomol Ther. (2022) 12:1358. doi: 10.3390/biom12101358
36. Alcantara, EH, Shin, MY, Feldmann, J, Nixon, GF, Beattie, JH, and Kwun, IS. Long-term zinc deprivation accelerates rat vascular smooth muscle cell proliferation involving the down-regulation of JNK1/2 expression in MAPK signaling. Atherosclerosis. (2013) 228:46–52. doi: 10.1016/j.atherosclerosis.2013.01.030
37. Liu, Y, Li, L, Wang, Z, Zhang, J, and Zhou, Z. Myocardial ischemia-reperfusion injury. Mol Mech Prev Microvasc Res. (2023) 149:104565. doi: 10.1016/j.mvr.2023.104565
38. Ume, AC, Wenegieme, TY, Adams, DN, Adesina, SE, and Williams, CR. Zinc deficiency: a potential hidden driver of the detrimental cycle of chronic kidney disease and hypertension. Kidney 360. (2023) 4:398–404. doi: 10.34067/KID.0007812021
39. Chen, W, Lu, H, Ying, Y, Li, H, Shen, H, and Cai, J. Zinc and chronic kidney disease: a review. J Nutr Sci Vitaminol (Tokyo). (2024) 70:98–105. doi: 10.3177/jnsv.70.98
40. Gembillo, G, Visconti, L, Giuffrida, AE, Labbozzetta, V, Peritore, L, Lipari, A, et al. Role of zinc in diabetic kidney disease. Nutrients. (2022) 14:1353. doi: 10.3390/nu14071353
41. Tokuyama, A, Kanda, E, Itano, S, Kondo, M, Wada, Y, Kadoya, H, et al. Effect of zinc deficiency on chronic kidney disease progression and effect modification by hypoalbuminemia. PLoS One. (2021) 16:e0251554. doi: 10.1371/journal.pone.0251554
42. Maxfield, L, Shukla, S, and Crane, JS. Zinc deficiency. Stat pearls. Treasure Island (FL) ineligible companies. Disclosure: Samarth Shukla declares no relevant financial relationships with ineligible companies. Disclosure: Jonathan Crane declares no relevant financial relationships with ineligible companies: Stat pearls publishing. Copyright © 2025, Stat Pearls Publishing LLC; (2025).
43. Erten, Y, Kayataş, M, Sezer, S, Ozdemir, FN, Ozyiğit, PF, Turan, M, et al. Zinc deficiency: prevalence and causes in hemodialysis patients and effect on cellular immune response. Transplant Proc. (1998) 30:850–1. doi: 10.1016/S0041-1345(98)00075-X
45. Prasad, AS, Beck, FW, Snell, DC, and Kucuk, O. Zinc in cancer prevention. Nutr Cancer. (2009) 61:879–87. doi: 10.1080/01635580903285122
Keywords: zinc deficiency, heart failure, major adverse kidney events, major adverse cardiovascular event, mortality
Citation: Lin Y-M, Tu W-L, Hung K-C, Yu T, Wu J-Y and Lai C-C (2025) Mortality and cardiorenal outcomes among heart failure patients with zinc deficiency: a multicenter retrospective cohort study of 8,290 patients. Front. Nutr. 12:1589907. doi: 10.3389/fnut.2025.1589907
Edited by:
Benjamin Rolles, Harvard Medical School, United StatesReviewed by:
Guillaume Courbon, Northwestern Medicine, United StatesYosuke Saka, Kasugai Municipal Hospital, Japan
Copyright © 2025 Lin, Tu, Hung, Yu, Wu and Lai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jheng-Yan Wu, YW5keTEwMjcxQGdtYWlsLmNvbQ==; Chih-Cheng Lai, ZHRtZWQxNDFAZ21haWwuY29t
 Yu-Min Lin1
Yu-Min Lin1 Kuo-Chuan Hung
Kuo-Chuan Hung Tsung Yu
Tsung Yu Jheng-Yan Wu
Jheng-Yan Wu Chih-Cheng Lai
Chih-Cheng Lai