- 1Hospital of Chengdu University of Traditional Chinese Medicine, Chengdu, Sichuan, China
- 2TCM Regulating Metabolic Diseases Key Laboratory of Sichuan Province, Hospital of Chengdu University of Traditional Chinese Medicine, Chengdu, Sichuan, China
- 3Department of Endocrinology, Hospital of Chengdu University of Traditional Chinese Medicine, Chengdu, Sichuan, China
- 4Kunming Traditional Chinese Medicine Hospital, Kunming, Yunnan, China
Lipid metabolism is a dynamic and intricate process involving the uptake, synthesis, storage and catabolism of lipid compounds in the body. Its homeostasis is crucial for maintaining the health of the organism. The regulatory network of lipid metabolism homeostasis consists of several key molecules, including SREBPs, PPARs, ChREBP, FXR, LXR, AMPK, and ncRNAs. Puerarin (Pue), an isoflavone derivative, has been demonstrated to enhance lipid metabolism by modulating the aforementioned signaling cascades. Pue has found extensive application in the pharmaceutical, food, and nutraceutical industries. Considering the multi-target and multi-pathway pharmacological properties of Pue, the present study focuses on the molecular mechanism of Pue in the regulation of lipid metabolism, the spectrum of metabolic diseases, as well as the limitations of the current study and the prospect of nutritional translation. It is hoped that this study will provide a reference for the regulation of lipid homeostasis and remodeling of lipid metabolism, with the aim of optimizing clinical use and product development.
1 Introduction
Lipid metabolism is defined as the process of uptake, synthesis, storage, and catabolism of lipid compounds in living organisms. This complex process involves the coordination of multiple organs, and the regulation of multiple enzymes and signaling pathways, and is essential for maintaining the body’s health (1, 2). Lipid metabolism disorders are the core pathomechanism of transdiseases, which can lead to multiple systemic diseases including hyperlipidemia (3), obesity (4), type 2 diabetes (T2DM) (5), non-alcoholic fatty liver disease (NAFLD) (6), cardiovascular diseases (CVD), central nervous system (CNS) disorders (3, 7), cancer (8), osteoporosis (9), and aging (10, 11), which seriously affect human health and impose a heavy burden on global public health. Blood lipids serve as an important indicator of lipid metabolism disorders (12). Statins are the prevailing lipid-lowering pharmaceutical agents (13). However, they are susceptible to causing adverse effects like muscle symptoms, liver dysfunction, renal insufficiency, and eye disorders (14), which limits their clinical use to some extent. Further studies have shown that statin monotherapy rarely achieves guide-line-recommended low-density lipoprotein cholesterol (LDL-C) concentrations (15). Current therapeutic strategies combining statins with adjunctive agents such as ezetimibe and next-generation PCSK9 inhibitors demonstrate minimal therapeutic benefit in patients exhibiting low-to-moderate cardiovascular risk profiles (16). This limited efficacy underscores the need for personalized risk stratification and novel combinatorial approaches to optimize lipid management in this population cohort.
Pueraria mirifica, the dried root of the leguminous plant Kudzu, is widely distributed in East and Southeast Asia. Its root is rich in isoflavonoids, which have both pharmacological and nutritional activities. Pue is an isoflavone derivative isolated from the traditional Chinese medicine Pueraria lobata, which has antioxidant, anti-inflammatory, anti-tumor, immunomodulation and other biological activities (17), which is widely used in the pharmaceutical, food, and healthcare industries. A multitude of studies have demonstrated that Pue possesses the capacity to modulate lipid metabolism in a variety of targets and pathways. Moreover, the incorporation of Pue into one’s diet has been shown to yield substantial improvements in cases of lipid metabolism disorders (18). In particular, the latest research has shown that Pue reduces fat absorption in the gut through the “brain-gut axis” (19). This provides an important reference for the use of Pue as a dietary supplement in combination with other drugs to co-regulate lipid metabolism for enhanced efficacy. However, there are fewer comprehensive evaluations of the molecular mechanisms, cross-disease therapeutic potential, and nutritional translation of Puel’s regulation of lipid metabolism.
Therefore, this article reviews the regulatory network of lipid metabolism in the organism, the molecular mechanism of Pue regulation of lipid metabolism, and the spectrum of metabolic diseases, as well as the future prospect of nutritional translation. It is anticipated that this review will encourage the utilization of Pue resources and serve as a valuable reference for expanding clinical applications and translating research findings.
2 Molecular mechanisms of lipid metabolism
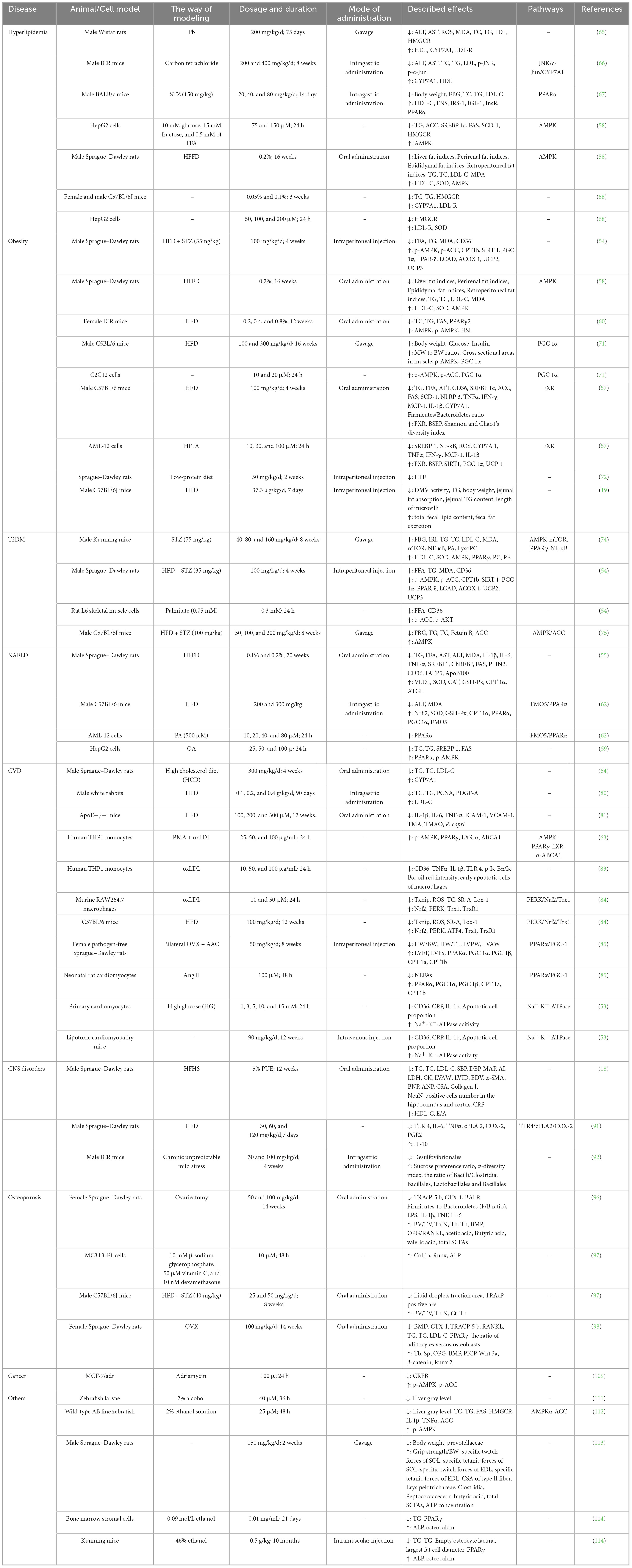
Lipid compounds are essential metabolites for the human body and are broadly divided into fatty acids (FAs), phospholipids (glycerophospholipids, sphingolipids) and neutral lipids [triglycerides (TGs), cholesteryl esters (CE)] (11), and their main roles include structural components, regulation of energy metabolism, and signal transduction (1). The increase in intracellular FA levels is achieved through two pathways: exogenous and endogenous (Figure 1). The exogenous pathway is primarily the digestion and absorption of lipids in the small intestine, while the endogenous pathway includes lipid uptake by tissue cells, lipid biosynthesis, lipid storage, and degradation. The process of bile micelle formation from dietary TG and cholesterol is facilitated by the actions of gastric and pancreatic lipases, as well as bile acid salts. Intestinal epithelial cells play a pivotal role in the active absorption of lipids through proteins such as cluster of differentiation 36 (CD36) and Niemann–pick C1-like 1 protein (NPC1L1). These proteins subsequently combine to form chyme particles, which then enter the circulation. It has been established that cells obtain circulating lipids primarily via CD36, fatty acid binding protein (FABP), fatty acid transporter protein (FATP), and low density lipoprotein receptor (LDLR) (1). The liver is the primary organ for de novo lipogenesis (DNL), and acetyl coenzyme A is a common substrate for FA and cholesterol synthesis (20). Acetyl-CoA is converted to malonyl-CoA by acetyl-CoA carboxylase (ACC). Subsequently, saturated FAs are synthesized by fatty acid synthase (FAS). The carbon chain undergoes a gradual extension and desaturation process by stearoyl-CoA desaturase 1 (SCD1) and other desaturases. The final synthesized fat is stored in cell lipid droplets (LDs) in the form of triglycerides (TAG) (11). Cholesterol biosynthesis is dominated by the rate-limiting enzyme 3-hydroxy-3-methylglutaryl-coenzyme A reductase (HMGCR) and is ultimately stored as cholesteryl esters in LDs with TAG (21, 22). Degradation of intracellular FAs is transported into mitochondria for beta oxidation, the tricarboxylic acid cycle for energy production, via carnitine palmitoyl transferase 1 (CPT1), the rate-limiting enzyme for fatty acid mitochondrial beta-oxidation. Cholesterol from peripheral tissue cells is transported from peripheral cells to the liver via high-density lipoprotein (HDL)-mediated reverse cholesterol transport (RCT) after ATP-binding cassette transporter protein (ABCA1, ABCG1, etc.) mediated cholesterol efflux (23). Cholesterol is converted to bile acids (BA) by the cytochrome P450 enzyme cholesterol 7α-hydroxylase (CYP7A1), which is subsequently actively exported from the liver via the bile salt efflux pump (BSEP)/ABCB11, and either circulated through the enterohepatic cycle or excreted in the feces (24). In addition, research has shown that extracellular vesicles, such as exosomes and microbubbles, provide an additional mechanism for cholesterol excretion outside the cell (22). Thus, lipid dynamic homeostasis involves sophisticated regulation of uptake, synthesis, storage, and catabolism, and its core network consists of several key molecules and pathways, which are analyzed in detail in the following sections.
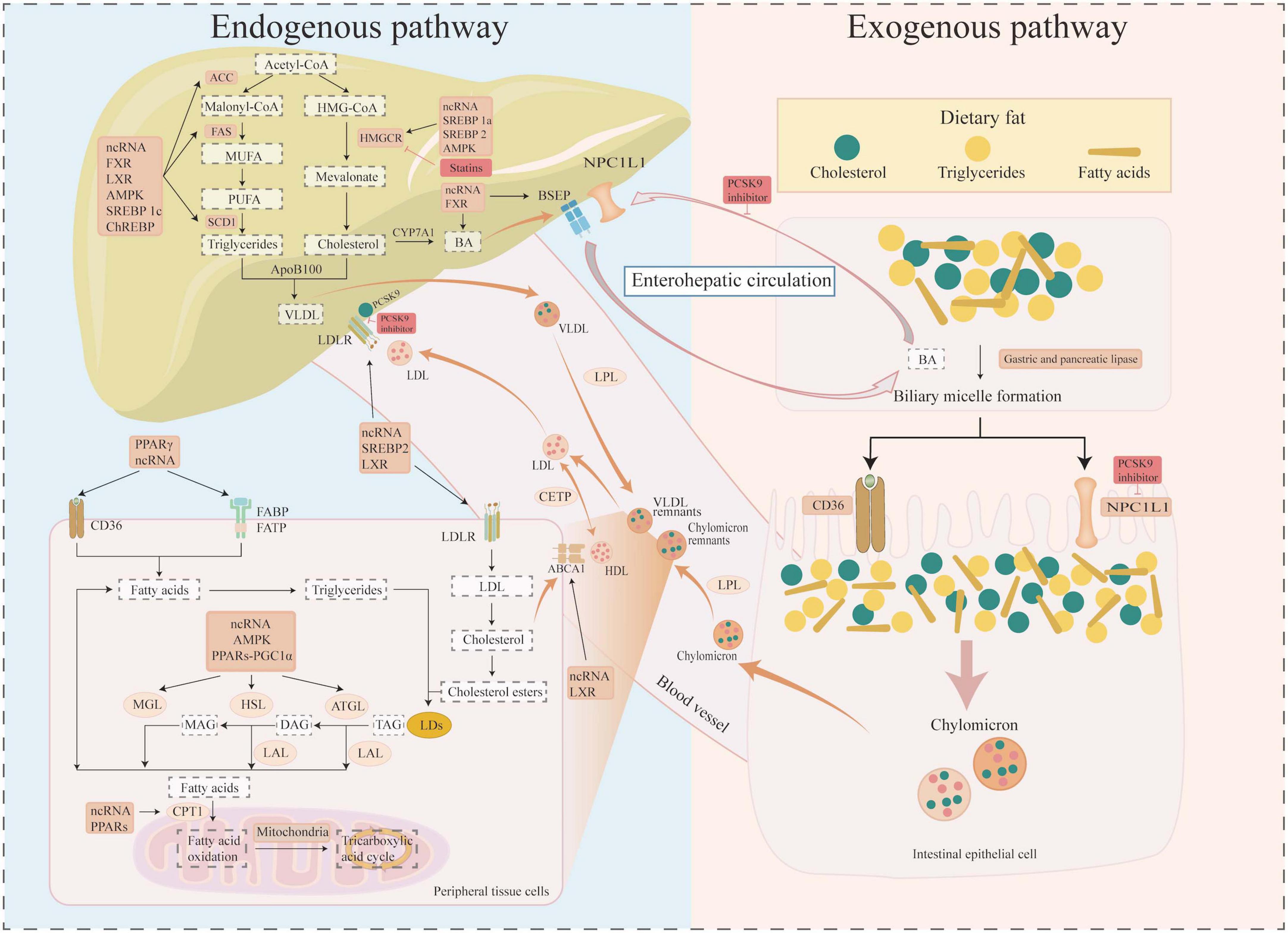
Figure 1. Molecular mechanisms of lipid metabolism. Diagram illustrating lipid metabolism pathways. The left section details the endogenous pathway involving liver processes, showing the uptake, synthesis, storage, and catabolism of lipid compounds such as cholesterol and triglycerides. The right section depicts the exogenous pathway, showing dietary fat absorption, bile acid micelle formation, and chylomicron creation in intestinal cells. Arrows indicate processes and interactions, with labels for molecules (such as SREBPs, PPARs, ChREBP, FXR, LXR, AMPK, and ncRNAs) and inhibitors (such as statins and PCSK9 inhibition) involved. The pathways are interconnected through enterohepatic circulation.
2.1 Sterol regulatory element binding proteins (SREBPs)
The SREBP family comprises three major isoforms: SREBP-1a, SREBP-1c, and SREBP-2. While SREBP mRNAs are ubiquitously expressed across tissues, their abundance and functional dominance exhibit significant tissue specificity. For instance, hepatic SREBP-1c expression surpasses SREBP-1a levels by approximately tenfold (25). Functionally, SREBP-1a primarily regulates both fatty acid and cholesterol biosynthesis, acting as a master transcriptional activator of rate-limiting enzymes such as ACC and HMGCR. In contrast, SREBP-1c serves as the principal regulator of fatty acid synthesis and energy storage, directly controlling the expression of key lipogenic enzymes including ACC, FAS, and SCD1. SREBP-2 operates with high specificity in cholesterol homeostasis, governing the transcriptional activation of HMGCR and LDLR (26). Beyond their canonical roles in lipid metabolism, SREBPs function as critical signaling hubs integrating diverse biological processes, including reactive oxygen species (ROS) generation, endoplasmic reticulum stress responses, autophagy regulation, and apoptosis modulation (27, 28).
2.2 Peroxisome proliferator-activated receptors (PPARs)
The PPAR family comprises three distinct subtypes: PPARα, PPARβ/δ, and PPARγ. PPARα is predominantly expressed in brown adipose tissue, liver, heart, kidneys, and skeletal muscle. It coordinates lipid β-oxidation through synergistic interactions with PGC-1α, regulating key lipolytic enzymes including adipose triglyceride lipase (ATGL), hormone-sensitive lipase (HSL), and monoacylglycerol lipase (MGL). Additionally, PPARα activates thermogenic gene programs, notably upregulating uncoupling protein 1 expression to enhance energy expenditure. PPARγ, the most abundant isoform in adipose tissue, serves as a master regulator of white and brown adipocyte differentiation. It promotes lipid uptake by modulating lipid transporters CD36, FABPs, and FATPs, while stimulating lipogenesis through transcriptional activation of lipogenic enzymes such as lipoprotein lipase (LPL) (29). PPARβ/δ exhibits high activity in skeletal muscle, where it cooperates with PGC-1α to induce the expression of fatty acid catabolic enzymes and thermogenic regulators, thereby enhancing lipid oxidative metabolism and heat production (30).
2.3 Carbohydrate response element binding protein (ChREBP)
ChREBP is a transcription factor that promotes lipogenic gene expression by sensing carbohydrates and is a hub for hepatic lipid synthesis (31). It is predominantly expressed in metabolically active tissues including the liver, intestine, and adipose tissue, where it orchestrates hepatic lipid biosynthesis through three distinct mechanisms: first, it regulates acetyl-CoA production via the pentose phosphate pathway and glycolysis-derived citrate cleavage. The second is direct transcriptional activation of the rate-limiting enzymes in fatty acid synthesis- ACC, FAS, and SCD1-thereby facilitating fatty acid synthesis, elongation, and desaturation. Third, it upregulates the expression of microsomal triglyceride transfer protein, which promotes the formation of very low-density lipoprotein (VLDL) particles. Notably, ChREBP exhibits functional crosstalk with PPARα, creating a metabolic switch that coordinates lipid anabolism and catabolism in response to nutritional status (32).
2.4 Farnesoid X receptor (FXR)
The nuclear receptor superfamily member, FXR, exists as four principal human isoforms (FXRα1-α4), with predominant hepatic and intestinal expression. Functioning as a monomer or heterodimer, FXR orchestrates transcriptional programs governing BA homeostasis and lipid metabolism through three primary mechanisms: (1) Suppression of bile acid synthesis via CYP7A1 downregulation; (2) Inhibition of cholesterol biosynthesis through Insig-2 activation; (3) Coordination of enterohepatic circulation by regulating BSEP/ABCB11 and intestinal bile acid-binding protein (33). Notably, hepatic FXRα2 exerts isoform-specific functions through selective binding to ER-2 response elements (34). Beyond its established roles, FXR modulates adipogenesis by repressing SREBP-1c expression (35), positioning it as a therapeutic target for metabolic disorders, hepatobiliary malignancies, and gastrointestinal cancers (36).
2.5 Liver X receptor (LXR)
LXR, a pivotal member of the nuclear receptor superfamily, serves as a master coordinator of hepatic metabolic homeostasis. Its regulatory functions encompass two principal mechanisms: (1) promoting cellular cholesterol efflux through transcriptional activation of ATP-binding cassette transporters ABCA1 and ABCG1, and (2) modulating LDLR-dependent cholesterol uptake via induction of inducible degrader of LDLR (IDOL)-mediated receptor degradation, operating independently of the canonical SREBP pathway (37). Furthermore, LXR exerts cross-regulation of lipid metabolism by transcriptionally activating SREBP-1c (38), thereby bridging cholesterol homeostasis with fatty acid biosynthesis.
2.6 AMP activated protein kinase (AMPK)
AMPK serves as a central energy sensor orchestrating systemic metabolic homeostasis through phosphorylation-dependent regulation of key metabolic nodes. This evolutionarily conserved kinase exerts multifaceted control over lipid metabolism via three principal mechanisms: (1) suppression of DNL through inhibitory phosphorylation of ACC1/ACC2 (39) and HMGCR (40), effectively blocking FA and cholesterol biosynthesis; (2) enhancement of lipolytic capacity via activation of ATGL phosphorylation, driving fatty acid β-oxidation (41); and (3) transcriptional regulation through phosphorylation-mediated inhibition of lipogenic transcription factors SREBP-1c (42) and ChREBP (43). By integrating glycolytic flux, mitochondrial energetics, and lipid storage/oxidation programs, AMPK maintains cellular energy equilibrium while preventing ectopic lipid accumulation (44).
2.7 Non-coding RNAs (ncRNAs)
ncRNAs, primarily encompassing microRNAs (miRNAs), long non-coding RNAs (lncRNAs), and circular RNAs (circRNAs), lack protein-coding capacity but critically regulate lipid metabolism by modulating the expression of related genes through transcriptional, post-transcriptional, and post-translational mechanisms. miRNAs primarily function post-transcriptionally, directly targeting key enzymes and regulators (e.g., ACC, FAS, LDLR, HMGCR, ABCA1, CYP7A1) to inhibit FA synthesis and modulate cholesterol homeostasis. They also indirectly regulate lipid metabolism through core regulators like SREBPs, PGC1α, AMPK, and LXR. Notably, miR-33 is a key regulator of cholesterol homeostasis (45, 46). lncRNAs employ more complex mechanisms (e.g., signaling, scaffolding, decoying, enhancer, or guide functions) to bidirectionally regulate target genes across transcriptional, post-transcriptional, and epigenetic levels. They target central factors (e.g., ACC, FAS, SCD1, LDLR, ABCA1, ChREBP, SREBPs, AMPK, LXR, FXR) to inhibit lipid synthesis, promote cholesterol uptake, facilitate RCT and HDL synthesis, and enhance BA synthesis, typically yielding net beneficial effects. Conversely, lncRNAs can negatively regulate factors including CD36, HMGCR, ABCA1, and SREBPs. Additionally, lncRNAs modulate lipid metabolism by acting as miRNA sponges to sequester miRNAs (45, 47). Similarly, circRNAs can also function as miRNA sponges, adsorbing miRNAs to regulate key lipid metabolism molecules (e.g., FAS, PPARs, AMPK, SREBPs), thereby forming an antagonistic regulatory network with miRNAs (48, 49). Notably, HDL biogenesis is regulated by both miRNAs and lncRNAs, while HDL particles themselves serve as carriers for these ncRNAs (50, 51).
3 Pue’s multidimensional regulatory mechanisms
A substantial body of research has validated the positive impacts of Pue on lipid metabolism (Figure 2), including the reduction of lipid uptake, the inhibition of lipid synthesis, and the promotion of lipid degradation. The following discussion will focus on the main molecular mechanisms by which Pue modulates lipid metabolism.
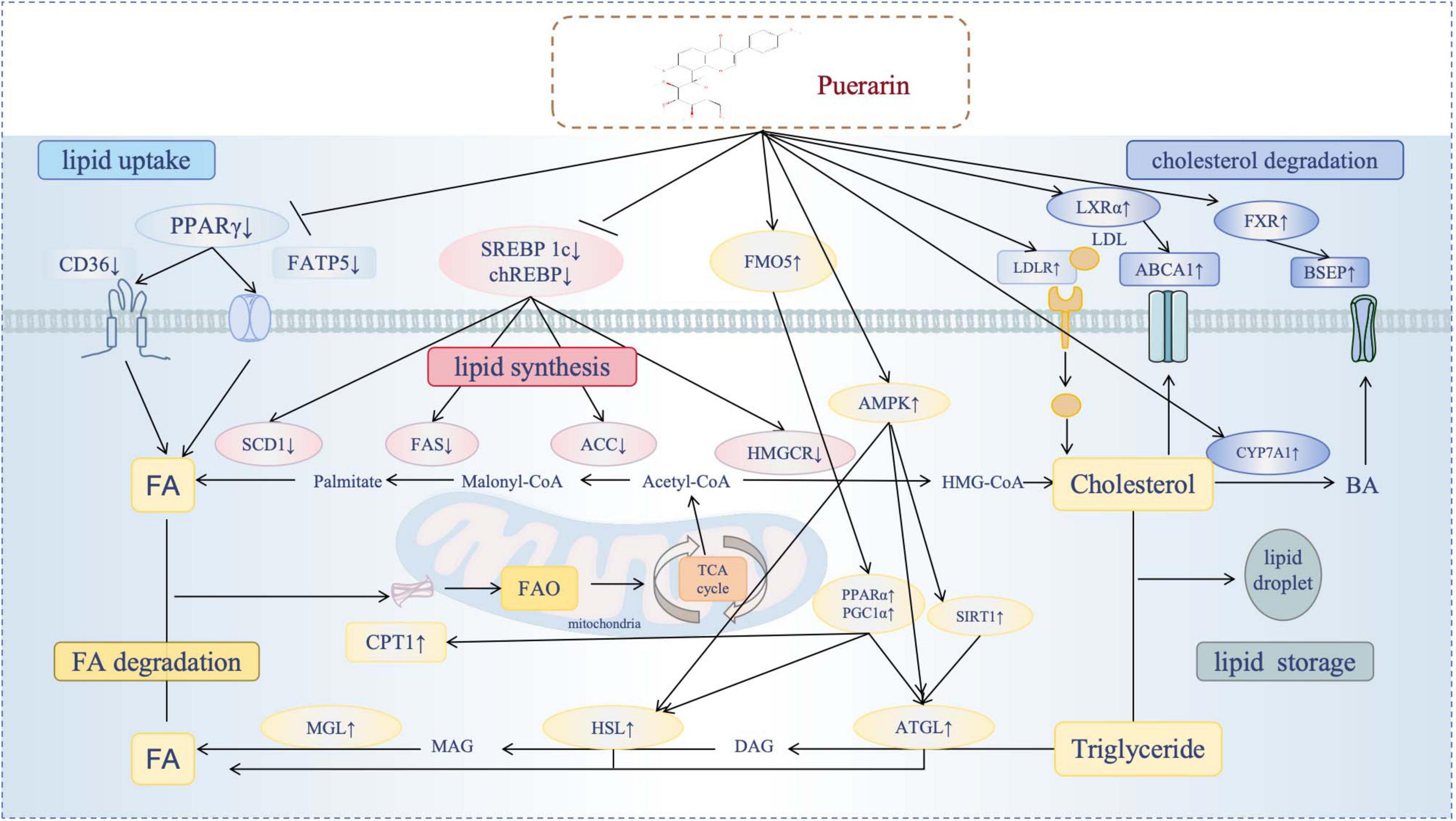
Figure 2. Puerarin’s multidimensional regulatory mechanisms. Diagram illustrating the effects of puerarin on lipid metabolism pathways. It shows the impact of puerarin on key lipid processes, including the reduction of lipid uptake, the inhibition of lipid synthesis, and the promotion of lipid degradation(enhancement of FA degradation and enhancement of cholesterol degradation). Puerarin modulates specific regulatory molecules (SREBPs, PPARs, ChREBP, FXR, LXR, AMPK), exerting either positive or negative effects. The flow of fatty acids, cholesterol and triglycerides was reflected in different processes.
3.1 The inhibition of lipid uptake
Cellular FA uptake is a key regulatory node in systemic lipid homeostasis. The scavenger receptor CD36 coordinates transmembrane fatty acid transport through dynamic plasma membrane-organelle transport (52). Pharmacological studies have shown that Pue exerts a multitissue lipid-lowering effect through the coordinated regulation of FA transporters. In cardiomyocytes, Pue inhibits Na+/K+-ATPase-driven expression of CD36, which reduces FA uptake and ameliorates cardiac steatosis in in vitro and in vivo models (53). In diabetic skeletal muscle, Pue attenuates CD36 membrane translocation while increasing mitochondrial β-oxidizing capacity, thereby reducing lipid accumulation in myocytes (54). Liver studies have shown that Pue has a dual inhibitory effect on CD36 and fatty acid transporter protein 5 (FATP5), which significantly reduces lipid deposition associated with NAFLD (55). Mechanistically, Pue disrupted the PPARγ-CD36 signaling axis and effectively counteracted environmental toxicant (bisphenol S)-induced lipid deposition in C57BL/6J mice (56).
3.2 The inhibition of lipid synthesis
DNL, a central metabolic pathway in hepatic and adipose tissues, is dynamically regulated by nutrient-hormonal crosstalk involving insulin, glucagon, and glucocorticoids. These endocrine signals converge on transcriptional activation of core lipogenic regulators—SREBP-1c and ChREBP—through kinase-mediated signaling cascades (2). Mechanistic studies reveal that Puel exerts potent anti-lipogenic effects via multi-target suppression of the DNL machinery: Transcriptional downregulation of master regulators SREBP-1c and ChREBP; Concomitant inhibition of rate-limiting enzymes including ACC, FAS, SCD1, and HMGCR. Functional validation across experimental models demonstrating attenuated lipid accumulation in both murine hepatocytes and human HepG2 cell lines (55, 57–59).
3.3 The promotion of lipid degradation
3.3.1 The promotion of FA degradation
Free fatty acid (FFA) β-oxidation serves as a critical pathway for maintaining lipid homeostasis. Studies have demonstrated that Pue exerts regulatory effects through multiple mechanisms. In C57BL/6J mice, Pue ameliorates bisphenol S-induced lipid accumulation by activating PPARα and CPT, thereby promoting lipidolysis (56). Through AMPK-mediated pathways, Pue suppresses PPARγ activity while enhancing HSL function, effectively improving lipid metabolism disorders (60). In HepG2 hepatocytes, Pue activates the GPER/CaMKKβ, CaMKII/CREB/SIRT1 signaling cascade, resulting in increased ATGL activity and enhanced lipid degradation (61). In high-fat/high-glucose-stimulated AML12 hepatocytes, Pue upregulates PGC-1α and SIRT1 expression, synergistically enhancing β-oxidation capacity (57). Recent evidence further identifies flavin-containing monooxygenase 5 (FMO5)/PPARα signaling as a critical pathway for Pue-induced PGC-1α and CPT1a upregulation, establishing FMO5 as a novel therapeutic node for lipid catabolism (62).
3.3.2 The promotion of cholesterol degradation
Pue orchestrates systemic cholesterol clearance through two complementary mechanisms: reverse cholesterol transport and biliary efflux Potentiation. In THP-1 macrophage-derived foam cells, Pue activates the AMPK-PPARγ- LXRα axis, increasing ATP-binding cassette subfamily A member 1 (ABCA1) expression to facilitate cholesterol efflux (63). Pue acts as a FXR agonist, upregulating BSEP/ABCB11 expression to enhance BA excretion (57).
4 Cross-disease therapeutic applications of Pue
As a natural active monomer derived from traditional Chinese medicine, Pue has a favorable safety profile and exhibits potent lipid metabolism regulation through multi-targets and multi-pathways (Figure 3; Table 1). This means that Pue has the potential to be a superior lipid-modulating drug to single-target inhibitors, which is why Pue is receiving increasing attention across disease therapies.
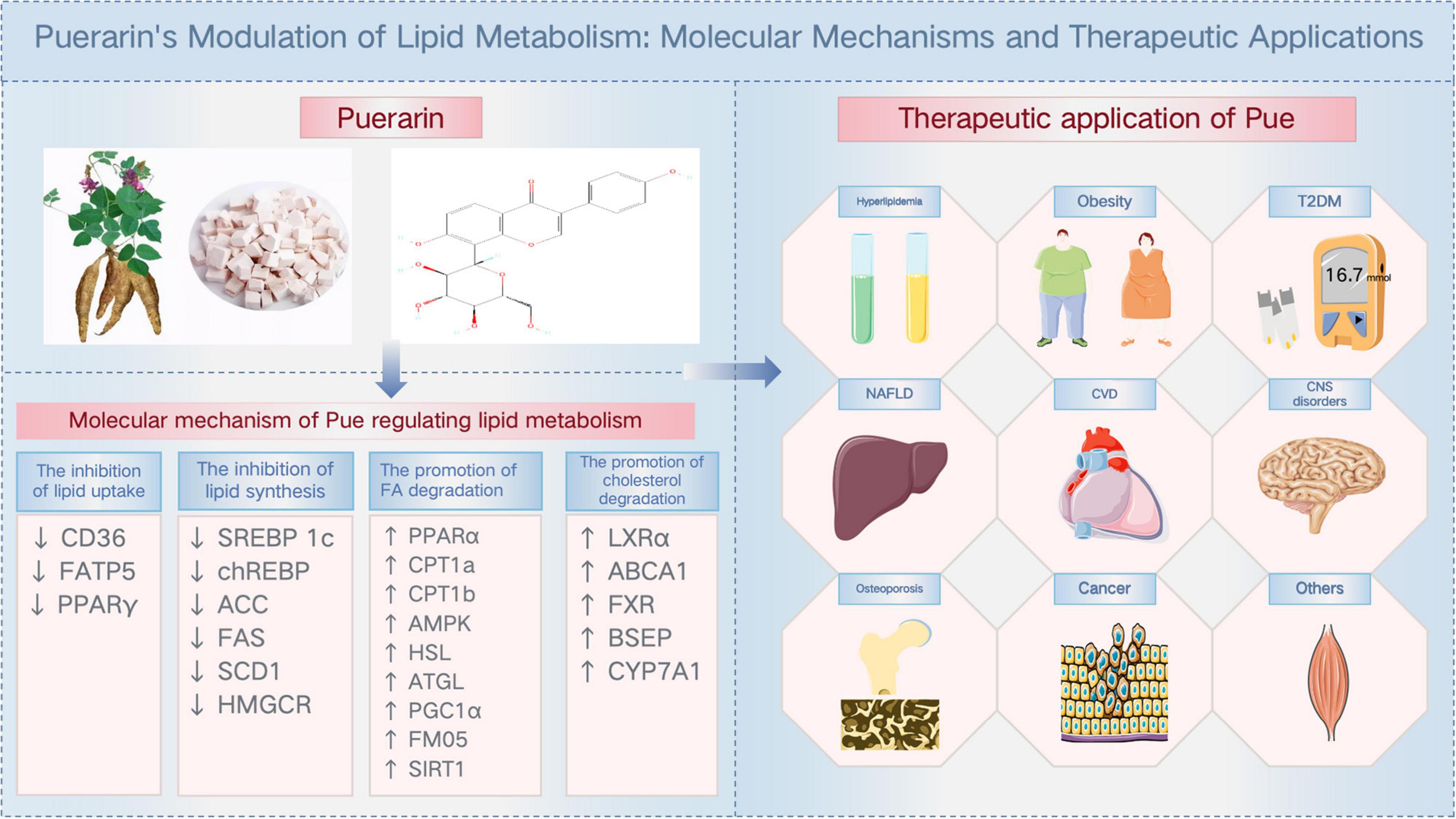
Figure 3. Puerarin’s modulation of lipid metabolism: molecular mechanisms and therapeutic applications.
4.1 Hyperlipidemia
Hyperlipidemia, defined as a disturbance in the balance of plasma lipids, is most frequently manifested as hypercholesterolemia. This condition is a well-established risk factor for a variety of metabolic disorders, including obesity, T2DM, CVD, NAFLD, and acute pancreatitis (3). Experimental studies demonstrate that Pue ameliorates high-cholesterol diet-induced hyperlipidemia through CYP7A1 upregulation (64). Mechanistically, Pue exerts dual regulatory effects by suppressing HMGCR activity (reducing cholesterol biosynthesis) while enhancing CYP7A1 and LDLR expression, thereby promoting cholesterol excretion and LDL clearance. This dual mechanism underlies its protective effects against lead-induced hyperlipidemia (65). Notably, the JNK/c-Jun/CYP7A1 pathway has been identified as a critical mediator of Pue’s anti-hyperlipidemic activity in carbon tetrachloride (CCl4)-induced models (66). In diabetic models, Pue shows therapeutic potential through PPARα activation in gastrocnemius muscle tissue, effectively reducing lipid accumulation in streptozotocin (STZ)-induced diabetic mice (67). Cellular studies reveal that Pue inhibits lipid deposition in HepG2 hepatocytes via AMP-activated protein kinase (AMPK)-mediated suppression of acetyl-CoA carboxylase (ACC) activity, concurrently downregulating adipogenic markers (SREBP-1c, FAS, SCD, and HMGCR) (58). Interestingly, while Pue and its glycosides consistently upregulate LDLR expression in both HepG2 cells and C57BL/6J mice, their effect on CYP7A1 exhibits model specificity–significantly enhancing mRNA levels in murine liver without affecting in vitro systems (68).
4.2 Obesity
Obesity is typically characterized by a state of energy imbalance, whereby energy intake exceeds energy expenditure, leading to excessive fat storage. Obesity is a significant risk factor for a number of diseases, including T2DM, NAFLD, CVD, and cancer (69). Current epidemiological data indicate over 600 million adults worldwide are clinically diagnosed with this condition (70). In high-fat diet/streptozotocin (HFD/STZ)-induced diabetic Sprague–Dawley rats, Pue administration significantly attenuated obesity-related metabolic derangements, manifested by reduced serum TG and FFA levels concomitant with decreased body weight (54). Notably, Pue demonstrated comparable efficacy in high-fat fructose diet-induced obese SD rats (58). Pue is also effective in modulating dyslipidemia through AMPK activation and coordinated regulation of key lipid-metabolizing enzymes: upregulating HSL while suppressing PPARγ (60). Pue enhances PGC-1α expression in skeletal muscle of HFD-fed mice, counteracting obesity-associated complications (71). Mechanistic studies further elucidate its multi-target effects: Inhibiting lipid absorption/synthesis via FXR-mediated downregulation of CD36, SREBP-1c, ACC, and FAS; Modulating bile acid homeostasis through CYP7A1 suppression and BSEP induction; Restoring gut microbiota composition, particularly Firmicutes/Bacteroidetes ratio and relative abundances of Firmicutes/Ascomycota phyla in HFD-induced obese C57BL/6 mice (57). Emerging evidence highlights Pue’s developmental programming effects–early intervention ameliorates hepatic steatosis and may mitigate adult-onset obesity in intrauterine growth restriction models (72). Recently discovered, Pue exerts central nervous system-mediated anti-obesity effects by binding to γ-aminobutyric acid type A receptor (GABAAR), thereby suppressing dorsal motor nucleus of the vagus neuronal activity. This neuroendocrine mechanism leads to jejunal microvilli shortening and consequent inhibition of intestinal fat absorption (19).
4.3 T2DM
T2DM, defined as a chronic metabolic disorder characterized by persistent hyperglycemia, arises from pancreatic β-cell dysfunction coupled with systemic insulin resistance. Moreover, Emerging evidence indicates that chronic intracellular lipid accumulation (lip toxicity) contributes to β-cell dysfunction through multiple molecular mechanisms, including endoplasmic reticulum stress, oxidative stress, inflammatory responses, mitochondrial dysfunction, and impaired autophagy (73). Pue reduces TC, TG, LDL, PA, and Lysophosphatidylcholine (LysoPC) levels, increase HDL, PC, and PE levels, improve glucose-lipid metabolism and reduce inflammatory damage through AMPK-mTOR and PPARγ-NF-κB signaling pathways, thus treating diabetes mellitus (74). In skeletal muscle, Pue increased AMPK, SIRT1, PGC-1α, and CPT-1β levels and promoted fatty acid oxidation, thereby preventing lipid accumulation in diabetic models (54). Concurrently, hepatic insulin resistance is improved through Fetuin B suppression, AMPK phosphorylation, and inhibition of ACC activity (75). It is noteworthy that Professor Roy Taylor, a prominent British scholar, has underscored that lipid deposition in the liver and pancreas serves as the initiating factor that precipitates diabetes mellitus (76). This provides an important reference for Pue to target the regulation of lipid metabolism in the treatment of diabetes mellitus and its complications.
4.4 NAFLD
NAFLD affects approximately 25.24% of the global population (77), with pathogenesis driven by hepatic lipid dysregulation through enhanced FA uptake and DNL, ultimately triggering cellular stress, inflammation, tissue remodeling, and fibrosis. Experimental evidence demonstrates that Pue can reduce the expression of FATP 5, CD36, SREBF1, ChREBP, ACC, and FAS to decrease lipid uptake and biosynthesis, and up-regulate the expression of CPT 1a, ATGL, and ApoB100 to promote the degradation of lipids, which significantly improves the accumulation of lipids in the livers of rats with NAFLD (55). Mechanistic studies reveal Pue activates the FMO5/PPARα axis to stimulate PGC1α and CPT1a expression, thereby promoting FA oxidation and reducing lipid deposition in NAFLD mice (62). In vitro validation using oleic acid-treated HepG2 cells confirms Pue’s multi-target action: activation of PPARα and AMPK pathways concurrently inhibits SREBP1 and FAS expression while enhancing FA oxidation, effectively counteracting lipid accumulation (59).
4.5 CVD
CVD is the leading cause of death worldwide. Atherosclerosis (AS), the primary CVD manifestation, results from cholesterol deposition in arterial walls, leading to plaque formation and vascular dysfunction (78). LDL critically drives AS progression (79), underscoring lipid metabolism regulation as a key therapeutic target. Pue exerts anti-atherogenic effects by reducing serum TG, TC, and LDL levels while enhancing CYP7A1 expression in hypercholesterolemic models, thereby promoting cholesterol and bile acid excretion and lowering atherogenic indices (64). In vivo studies demonstrate Pue’s capacity to inhibit aortic intimal thickening and plaque formation in rabbits (80). In addition, Pue alleviate AS by inhibiting the production of Prevotella copri (P. copri) as well as trimethylamine (TMA) (81), while trimethylamine-N-oxide (TMAO) relies on the gut microbiota to improve lipid metabolism by regulating reverse cholesterol transport (82). At the cellular level, Pue combats foam cell formation through dual actions: (1) activating the AMPK-PPARγ-LXRα pathway to promote ABCA1-mediated cholesterol efflux (63), and (2) downregulating CD36 expression to limit lipid uptake in human THP-1 macrophages (83). Further studies reveal Pue’s activation of the PERK/Nrf2/thioredoxin 1 (Trx1) axis reduces scavenger receptor-A (SR-A) and lectin-type oxidized LDL receptor-1 (LOX-1) expression, inhibiting macrophage lipid accumulation (84). In cardiac pathophysiology models, Pue attenuates myocardial hypertrophy via PPARα/PGC-1α-mediated upregulation of CPT1a/b, enhancing fatty acid oxidation (85). It concurrently preserves cardiac function by increasing Na+/K+-ATPase activity and suppressing CD36-mediated fatty acid uptake (53). Notably, Pue supplementation demonstrates preventive efficacy against diet-induced metabolic syndrome and CVD complications by improving glucolipid homeostasis, attenuating cardiovascular remodeling, and reducing atherogenic indices (18), positioning it as a promising nutraceutical for cardiometabolic disease prevention.
4.6 CNS disorders
Pue exhibits therapeutic potential for central nervous system (CNS) disorders through multimodal mechanisms (86–88). Emerging research highlights the intersection of lipid metabolic dysregulation and CNS pathophysiology (89). Hypercholesterolemia exacerbates neuroinflammation, accelerating neuronal degeneration and cognitive decline (90). Dietary supplementation with Pue attenuates the metabolic syndrome and associated neurological damage induced by a high-fat/high-sugar diet by restoring glycolipid homeostasis and improving cortical/hippocampal vascularity and neuronal density (18). In addition, Pue modulates phospholipid metabolism and attenuates inflammation associated with depression by inhibiting TLR4 and repairing the intestinal barrier (91). Gut-brain axis studies reveal Pue’s microbiota remodeling capacity in chronic stress models: Enriching beneficial taxa (Firmicutes, Lactobacillus) and Depleting pathogenic genera (Proteobacteria, Desulfovibrio) (92). These commensal microbes enhance intestinal integrity and immunomodulation through short-chain fatty acid (SCFA) production (93, 94). Notably, recent advances position cellular senescence as a therapeutic frontier for CNS disorders (95), with lipid metabolic alterations driving senescence progression (10). Pue’s dual targeting capability—modulating lipid metabolism while clearing senescent cells—presents a novel strategy for treating age-related neurological diseases.
4.7 Osteoporosis
Osteoporosis is characterized by low bone mass and deterioration of bone micro-structure. Pue exerts anti-osteoporotic effects by regulating the type and abundance of intestinal flora, increasing the content of SCFAs in the colon, especially acetic acid and butyric acid, maintaining the dynamic balance of the colonic mucosa, and reducing the inflammatory response (96). Pue influences gut microbial diversity by modulating Alloprevotella, Rodentibacter, Alistipes, and Fusobacterium flora, remodels gut flora, and regulates alpha-linolenic acid metabolism and glycerophospholipid metabolism, thereby inhibiting pioglitazone-mediated bone loss (97). Pue can also improve OVX-induced osteoporosis by activating the Wnt 3a/β-catenin signaling pathway, inhibiting the PPARγ signaling pathway, regulating phospholipid metabolism and the biosynthesis of PUFAs, and modulating the differentiation of bone mesenchymal stem cells to osteoblasts (98).
4.8 Cancer
Lipid signaling critically regulates tumor progression and microenvironment remodeling, with Pue emerging as a multi-target anticancer agent since its initial antitumor activity in colon cancer was reported in 2006 (99). Subsequent studies validate Pue’s efficacy across esophageal (100), lung (101, 102), hepatic (103), breast (104), and bladder cancers (105) through five core mechanisms: suppressing cancer cell proliferation/migration (105), inducing apoptosis (106), reprogramming glucose metabolism (107), overcoming chemoresistance, and modulating tumor immune landscapes (108). Mechanistically, Pue reverses multidrug resistance (MDR) in human breast cancer MCF-7/adr cells by upregulating AMPK and ACC, thereby inhibiting MDR1 expression (109). This finding holds particular significance given that phospholipid/cholesterol-mediated MDR pathways represent major obstacles in chemotherapy (110). Crucially, Pue’s ability to target lipid metabolic nodes—including MDR1 suppression and chemoresistance reversal—positions it as a promising candidate for developing lipid-centric anticancer strategies, offering novel therapeutic opportunities for oncology patients.
4.9 Others
Pue reverses alcohol-induced metabolic disturbances, including sphingolipid metabolism, resulting in hepatoprotective effects (111). In alcoholic fatty liver disease (AFLD), Pue ameliorates hepatic lipid accumulation in zebrafish larvae by suppressing fatty acid synthesis via the AMPKα-ACC pathway, while concurrently restoring sphingolipid homeostasis to exert hepatoprotective effects (112). Beyond hepatic protection, Pue counteracts sarcopenia by remodeling the gut-muscle axis: enhancing gut microbiota diversity, elevating SCFA production, and boosting ATP synthesis to improve skeletal muscle strength (113). Additionally, in alcohol-related osteopathology models, Pue reduces PPARγ expression in both murine bone marrow stromal cells and Kunming mice, effectively inhibiting bone marrow adipogenesis while preserving osteogenic differentiation capacity, thereby mitigating alcoholic osteonecrosis (114).
5 Clinical translation and nutritional considerations
Despite Pue’s clinical potential in metabolic regulation, its poor oral bioavailability due to low aqueous solubility and erratic lipid dispersion poses significant translational challenges. Pharmaceutical innovations have addressed these limitations through advanced delivery systems including cubic liquid crystal nanoparticles (115), chitosan/PLGA-based nanocarriers (116), long-circulating liposomes (117), and self-microemulsifying formulations (118), which collectively enhance Pue’s absorption and therapeutic efficacy. Clinically, Pue supplementation demonstrates metabolic benefits across multiple trials: oral administration (400 mg/day for 10 days) improves cardiac function in chronic heart failure patients by elevating left ventricular ejection fraction and reducing oxidized LDL levels (119); intravenous delivery (500 mg/day) ameliorates dyslipidemia and insulin resistance in coronary artery disease through TG, TC, and LDL-C reduction alongside HDL-C elevation (120); prolonged oral regimens (150 mg/day for 12 weeks) effectively lower cholesterol in polycystic ovary syndrome when combined with standard therapies (121); oral regimens (750 mg/day) effectively lower cholesterol in diabetic nephropathy cohorts when combined with standard therapies (122). Paradoxically, a short-term trial in Chinese males (18–50 years) showed no significant lipid improvement (123), potentially reflecting population-specific responses or trial design limitations. Future clinical validation should prioritize expanded demographic inclusion, gender-balanced cohorts, and standardized dosing protocols to establish robust evidence for Pue’s nutraceutical applications in lipid metabolic disorders.
It should be emphasized that intravascular administration of Pue can cause adverse reactions including drug fever, rash, nausea, vomiting, diarrhea, hepatic/renal damage, palpitations, anaphylactic shock, and hemolysis (124). Critically, hemolysis represents a key limiting factor for Pue injection’s clinical use. This hemolytic effect has been confirmed in both animal and cellular models: Rabbits receiving 25 mg/kg/day Pue developed hemolysis after 42 days (125). Furthermore, in vitro erythrocyte experiments demonstrated that Pue induces hemolysis in a dose- and time-dependent manner (126). Pharmacokinetic studies reveal its inhibitory effects on cytochrome P450 isozymes (CYP2B6, CYP2C9, and CYP3A4) (127), which may potentiate statin plasma concentrations–necessitating vigilant creatine kinase monitoring during coadministration. Furthermore, long-term estrogen use may increase the risk of breast cancer (128). The estrogenic properties of Pue raise potential concerns about long-term endocrine effects. Therefore, comprehensive toxicological evaluations of Pueraria mirifica derivatives are imperative to assess breast cancer risk associations and establish safe administration protocols, particularly for chronic therapeutic applications. These precautionary measures will facilitate the development of evidence-based guidelines to optimize Pue’s clinical translation while mitigating iatrogenic risks.
6 Summary and outlook
Lipid metabolism, a dynamically regulated process orchestrated by core molecular networks involving SREBPs, PPARs, ChREBP, FXR, LXR, AMPK, and ncRNAs, represents a critical therapeutic frontier. Unlike single-target lipid-lowering agents (statins, ezetimibe, PCSK9 inhibitors), Pue exhibits multi-target regulatory capacity, simultaneously modulating interconnected pathways to achieve synergistic therapeutic effects while minimizing adverse outcomes associated with monotarget interventions—exemplifying the systemic advantages of natural products. Nevertheless, four critical research gaps require attention:
First, while lipid storage mechanisms—particularly lipid droplet (LD) dynamics as functionally active organelles mediating fatty acid trafficking, storage, and interorganelle communication (129)—constitute essential components of lipid homeostasis, Pue’s regulatory effects on LD biogenesis/remodeling remain unexplored. Second, key lipid chaperones and transcription factors including FABPs that coordinate HSL-mediated lipolysis and PPARγ-driven adipogenesis (130), transcription factor EB (TFEB) regulating autophagy-lipid metabolism crosstalk via PGC-1α/PPARα (131), and forkhead box O1 (FOXO1) governing ATGL/LAL-mediated lipolysis and adipocyte differentiation (132) represent promising yet uninvestigated targets for Pue’s metabolic actions. Third, ncRNAs are established regulators of lipid metabolism, offering novel biomarkers and therapeutic targets for related diseases while demonstrating potential for individualized therapy in precision medicine. However, current studies on Pue have primarily focused on: miRNA-mediated pathways (including antioxidant, anti-inflammatory effects, inhibition of cellular pyroptosis, and cardioprotection) (133–136); lncRNA Anril-regulated autophagy (137); lncRNA/mRNA co-expression networks; and the role of circ_0020394 as a molecular sponge for miR-328-3p promoting apoptosis (138),while lacking specific studies on ncRNA regulation of lipid metabolism. In addition, HDL is both a regulatory target of ncRNA and a transport carrier of ncRNA, and the interaction between the two remains to be elucidated. Therefore, future studies should investigate the activity of Pue in the ncRNA-lipid metabolism axis and elucidate how the dynamic transport of HDL-ncRNA contributes to its therapeutic efficacy, thus laying the molecular foundation for individualized drug administration. Fourth, despite compelling preclinical evidence, clinical validation through multicenter randomized controlled trials evaluating Pue’s lipid-modulating efficacy and safety remains limited.
Emerging insights into Pue’s novel brain-gut axis-mediated fat absorption inhibition reveal its potential as a master metabolic regulator, suggesting combinatory therapeutic strategies with conventional lipid-lowering agents. Given lipid metabolism’s centrality in energy homeostasis and cellular physiology, elucidating Pue’s molecular interplay with intracellular lipid networks and advancing translational nutrition research will not only enable cross-disease therapeutic innovations but also optimize clinical applications and nutraceutical development.
Author contributions
SO: Writing – original draft. QL: Writing – original draft. YL: Writing – original draft. TL: Writing – review and editing. XX: Writing – review and editing. HX: Supervision, Writing – review and editing. HG: Writing – review and editing, Supervision. JL: Writing – review and editing, Conceptualization. CX: Conceptualization, Writing – review and editing.
Funding
The authors declare that financial support was received for the research and/or publication of this article. This research was funded by the innovation team and talents cultivation program of National Administration of Traditional Chinese Medicine (ZYYCXTD-C-202209), Study on the effect of Shenqi Compound series on cardiovascular benefit in diabetes mellitus based on macrovascular protective effect, Sichuan Provincial Department of Science and Technology (2022ZDZX0022), and Technology Talent and Platform Plan (Yunnan Province Academician Expert Workstation 202305AF150060).
Acknowledgments
We sincerely thank Chunguang Xie for his critical guidance in conceptualizing the scope of this review and constructive suggestions on manuscript organization. We acknowledge the contributions of all researchers whose work has been cited in this review, as their findings laid the foundation for our synthesis and analysis.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Yoon H, Shaw JL, Haigis MC, Greka A. Lipid metabolism in sickness and in health: Emerging regulators of lipotoxicity. Mol Cell. (2021) 81:3708–30. doi: 10.1016/j.molcel.2021.08.027
2. Jeon YG, Kim YY, Lee G, Kim JB. Physiological and pathological roles of lipogenesis. Nat Metab. (2023) 5:735–59. doi: 10.1038/s42255-023-00786-y
3. Pirillo A, Casula M, Olmastroni E, Norata G, Catapano A. Global epidemiology of dyslipidaemias. Nat Rev Cardiol. (2021) 18:689–700. doi: 10.1038/s41569-021-00541-4
4. Arner P, Bernard S, Salehpour M, Possnert G, Liebl J, Steier P, et al. Dynamics of human adipose lipid turnover in health and metabolic disease. Nature. (2011) 478:110–3. doi: 10.1038/nature10426
5. Kautzky-Willer A, Harreiter J, Pacini G. Sex and gender differences in risk, pathophysiology and complications of type 2 diabetes mellitus. Endocr Rev. (2016) 37:278–316. doi: 10.1210/er.2015-1137
6. Sattar N, Forrest E, Preiss D. Non-alcoholic fatty liver disease. Bmj. (2014) 349:g4596. doi: 10.1136/bmj.g4596
7. Lu Y, Hajifathalian K, Ezzati M, Woodward M, Rimm EB, Danaei G. Metabolic mediators of the effects of body-mass index, overweight, and obesity on coronary heart disease and stroke: A pooled analysis of 97 prospective cohorts with 1⋅8 million participants. Lancet. (2014) 383:970–83. doi: 10.1016/S0140-6736(13)61836-X
8. Aregger M, Lawson K, Billmann M, Costanzo M, Tong A, Chan K, et al. Systematic mapping of genetic interactions for de novo fatty acid synthesis identifies C12orf49 as a regulator of lipid metabolism. Nat Metab. (2020) 2:499–513. doi: 10.1038/s42255-020-0211-z
9. Zhang J, Hu W, Zou Z, Li Y, Kang F, Li J, et al. The role of lipid metabolism in osteoporosis: Clinical implication and cellular mechanism. Genes Dis. (2024) 11:101122. doi: 10.1016/j.gendis.2023.101122
10. Zeng Q, Gong Y, Zhu N, Shi Y, Zhang C, Qin L. Lipids and lipid metabolism in cellular senescence: Emerging targets for age-related diseases. Ageing Res Rev. (2024) 97:102294. doi: 10.1016/j.arr.2024.102294
11. Mutlu A, Duffy J, Wang M. Lipid metabolism and lipid signals in aging and longevity. Dev Cell. (2021) 56:1394–407. doi: 10.1016/j.devcel.2021.03.034
12. Parhofer K. The treatment of disorders of lipid metabolism. Dtsch Arztebl Int. (2016) 113:261–8. doi: 10.3238/arztebl.2016.0261
13. Mangione CM, Barry MJ, Nicholson WK, Cabana M, Chelmow D, Coker TR, et al. Statin use for the primary prevention of cardiovascular disease in adults: US preventive services task force recommendation statement. JAMA. (2022) 328:746–53. doi: 10.1001/jama.2022.13044
14. Cai T, Abel L, Langford O, Monaghan G, Aronson J, Stevens R, et al. Associations between statins and adverse events in primary prevention of cardiovascular disease: Systematic review with pairwise, network, and dose-response meta-analyses. BMJ. (2021) 374:n1537. doi: 10.1136/bmj.n1537
15. EAS Familial Hypercholesterolaemia Studies Collaboration (FHSC). Global perspective of familial hypercholesterolaemia: A cross-sectional study from the EAS Familial Hypercholesterolaemia Studies Collaboration (FHSC). Lancet. (2021) 398:1713–25. doi: 10.1016/S0140-6736(21)01122-3
16. Khan S, Yedlapati S, Lone A, Hao Q, Guyatt G, Delvaux N, et al. PCSK9 inhibitors and ezetimibe with or without statin therapy for cardiovascular risk reduction: A systematic review and network meta-analysis. BMJ. (2022) 377:e069116. doi: 10.1136/bmj-2021-069116
17. Wang D, Bu T, Li Y, He Y, Yang F, Zou L. Pharmacological activity, pharmacokinetics, and clinical research progress of puerarin. Antioxidants. (2022) 11:2121. doi: 10.3390/antiox11112121
18. Mu Y, Yang Y, Jiang S, Liu C, Han Y, Jiang J, et al. Benefits of puerarin on metabolic syndrome and its associated cardiovascular diseases in rats fed a high-fat/high-sucrose diet. Nutrients. (2024) 16:1273. doi: 10.3390/nu16091273
19. Lyu Q, Xue W, Liu R, Ma Q, Kasaragod VB, Sun S, et al. A brain-to-gut signal controls intestinal fat absorption. Nature. (2024) 634:936–43. doi: 10.1038/s41586-024-07929-5
20. Walch L, Čopič A, Jackson C. Fatty acid metabolism meets organelle dynamics. Dev Cell. (2015) 32:657–8. doi: 10.1016/j.devcel.2015.03.008
21. Lu XY, Shi XJ, Hu A, Wang JQ, Ding Y, Jiang W, et al. Feeding induces cholesterol biosynthesis via the mTORC1-USP20-HMGCR axis. Nature. (2020) 588:479–84. doi: 10.1038/s41586-020-2928-y
22. Juhl AD, Wüstner D. Pathways and mechanisms of cellular cholesterol efflux-insight from imaging. Front Cell Dev Biol. (2022) 10:834408. doi: 10.3389/fcell.2022.834408
23. Haas MJ, Mooradian AD. Potential therapeutic agents that target ATP binding cassette A1 (ABCA1) gene expression. Drugs. (2022) 82:1055–75. doi: 10.1007/s40265-022-01743-x
24. Lefebvre P, Cariou B, Lien F, Kuipers F, Staels B. Role of bile acids and bile acid receptors in metabolic regulation. Physiol Rev. (2009) 89:147–91. doi: 10.1152/physrev.00010.2008
25. Horton JD, Bashmakov Y, Shimomura I, Shimano H. Regulation of sterol regulatory element binding proteins in livers of fasted and refed mice. Proc Natl Acad Sci U S A. (1998) 95:5987–92. doi: 10.1073/pnas.95.11.5987
26. Shimano H. SREBPs: Physiology and pathophysiology of the SREBP family. FEBS J. (2009) 276:616–21. doi: 10.1111/j.1742-4658.2008.06806.x
27. Shimano H, Sato R. SREBP-regulated lipid metabolism: Convergent physiology - divergent pathophysiology. Nat Rev Endocrinol. (2017) 13:710–30. doi: 10.1038/nrendo.2017.91
28. Shao W, Espenshade PJ. Expanding roles for SREBP in metabolism. Cell Metab. (2012) 16:414–9. doi: 10.1016/j.cmet.2012.09.002
29. Gross B, Pawlak M, Lefebvre P, Staels B. PPARs in obesity-induced T2DM, dyslipidaemia and NAFLD. Nat Rev Endocrinol. (2017) 13:36–49. doi: 10.1038/nrendo.2016.135
30. Christofides A, Konstantinidou E, Jani C, Boussiotis V. The role of peroxisome proliferator-activated receptors (PPAR) in immune responses. Metabolism. (2021) 114:154338. doi: 10.1016/j.metabol.2020.154338
31. Régnier M, Carbinatti T, Parlati L, Benhamed F, Postic C. The role of ChREBP in carbohydrate sensing and NAFLD development. Nat Rev Endocrinol. (2023) 19:336–49. doi: 10.1038/s41574-023-00809-4
32. Iizuka K, Takao K, Yabe D. ChREBP-mediated regulation of lipid metabolism: Involvement of the gut microbiota, liver, and adipose tissue. Front Endocrinol. (2020) 11:587189. doi: 10.3389/fendo.2020.587189
33. Wang YD, Chen WD, Moore DD, Huang W. FXR: A metabolic regulator and cell protector. Cell Res. (2008) 18:1087–95. doi: 10.1038/cr.2008.289
34. Ramos Pittol J, Milona A, Morris I, Willemsen E, van der Veen S, Kalkhoven E, et al. FXR isoforms control different metabolic functions in liver cells via binding to specific DNA motifs. Gastroenterology. (2020) 159: 1853–65.e10. doi: 10.1053/j.gastro.2020.07.036
35. Watanabe M, Houten S, Wang L, Moschetta A, Mangelsdorf D, Heyman R, et al. Bile acids lower triglyceride levels via a pathway involving FXR. SHP, and SREBP-1c. J Clin Invest. (2004) 113:1408–18. doi: 10.1172/JCI21025
36. Sun L, Cai J, Gonzalez FJ. The role of farnesoid X receptor in metabolic diseases, and gastrointestinal and liver cancer. Nat Rev Gastroenterol Hepatol. (2021) 18:335–47. doi: 10.1038/s41575-020-00404-2
37. Zelcer N, Hong C, Boyadjian R, Tontonoz P. LXR regulates cholesterol uptake through Idol-dependent ubiquitination of the LDL receptor. Science. (2009) 325:100–4. doi: 10.1126/science.1168974
38. Repa J, Liang G, Ou J, Bashmakov Y, Lobaccaro J, Shimomura I, et al. Regulation of mouse sterol regulatory element-binding protein-1c gene (SREBP-1c) by oxysterol receptors, LXRalpha and LXRbeta. Genes Dev. (2000) 14:2819–30. doi: 10.1101/gad.844900
39. Fullerton M, Galic S, Marcinko K, Sikkema S, Pulinilkunnil T, Chen Z, et al. Single phosphorylation sites in Acc1 and Acc2 regulate lipid homeostasis and the insulin-sensitizing effects of metformin. Nat Med. (2013) 19:1649–54. doi: 10.1038/nm.3372
40. Pokhrel RH, Acharya S, Ahn JH, Gu Y, Pandit M, Kim JO, et al. AMPK promotes antitumor immunity by downregulating PD-1 in regulatory T cells via the HMGCR/p38 signaling pathway. Mol Cancer. (2021) 20:133. doi: 10.1186/s12943-021-01420-9
41. Ahmadian M, Abbott M, Tang T, Hudak C, Kim Y, Bruss M, et al. Desnutrin/ATGL is regulated by AMPK and is required for a brown adipose phenotype. Cell Metab. (2011) 13:739–48. doi: 10.1016/j.cmet.2011.05.002
42. Li Y, Xu S, Mihaylova M, Zheng B, Hou X, Jiang B, et al. AMPK phosphorylates and inhibits SREBP activity to attenuate hepatic steatosis and atherosclerosis in diet-induced insulin-resistant mice. Cell Metab. (2011) 13:376–88. doi: 10.1016/j.cmet.2011.03.009
43. Kawaguchi T, Osatomi K, Yamashita H, Kabashima T, Uyeda K. Mechanism for fatty acid sparing effect on glucose-induced transcription: Regulation of carbohydrate-responsive element-binding protein by AMP-activated protein kinase. J Biol Chem. (2002) 277:3829–35. doi: 10.1074/jbc.M107895200
44. Herzig S, Shaw RJ. AMPK: Guardian of metabolism and mitochondrial homeostasis. Nat Rev Mol Cell Biol. (2018) 19:121–35. doi: 10.1038/nrm.2017.95
45. Zhang X, Price NL, Fernández-Hernando C. Non-coding RNAs in lipid metabolism. Vascul Pharmacol. (2019) 114:93–102. doi: 10.1016/j.vph.2018.06.011
46. Singh AK, Aryal B, Zhang X, Fan Y, Price NL, Suárez Y, et al. Posttranscriptional regulation of lipid metabolism by non-coding RNAs and RNA binding proteins. Semin Cell Dev Biol. (2018) 81:129–40. doi: 10.1016/j.semcdb.2017.11.026
47. Gluba-Sagr A, Franczyk B, Rysz-Górzyńska A, Olszewski R, Rysz J. The role of selected lncRNAs in lipid metabolism and cardiovascular disease risk. Int J Mol Sci. (2024) 25:9244. doi: 10.3390/ijms25179244
48. Chen L, Huang C, Shan G. Circular RNAs in physiology and non-immunological diseases. Trends Biochem Sci. (2022) 47:250–64. doi: 10.1007/s40121-021-00437-3
49. Chen C, Zhang X, Deng Y, Cui Q, Zhu J, Ren H, et al. Regulatory roles of circRNAs in adipogenesis and lipid metabolism: Emerging insights into lipid-related diseases. Febs J. (2021) 288:3663–82. doi: 10.1111/febs.15525
50. Canfrán-Duque A, Lin CS, Goedeke L, Suárez Y, Fernández-Hernando C. Micro-RNAs and high-density lipoprotein metabolism. Arterioscler Thromb Vasc Biol. (2016) 36:1076–84. doi: 10.1161/ATVBAHA.116.307028
51. Scicali R, Bosco G, Scamporrino A, Di Mauro S, Filippello A, Di Giacomo Barbagallo F, et al. Evaluation of high-density lipoprotein-bound long non-coding RNAs in subjects with familial hypercholesterolaemia. Eur J Clin Invest. (2024) 54:e14083. doi: 10.1111/eci.14083
52. Su X, Abumrad NA. Cellular fatty acid uptake: A pathway under construction. Trends Endocrinol Metab. (2009) 20:72–7. doi: 10.1016/j.tem.2008.11.001
53. Qin H, Zhang Y, Wang R, Du X, Li L, Du H. Puerarin suppresses Na+-K+-ATPase-mediated systemic inflammation and CD36 expression, and alleviates cardiac lipotoxicity in vitro and in vivo. J Cardiovasc Pharmacol. (2016) 68:465–72. doi: 10.1097/FJC.0000000000000431
54. Chen XF, Wang L, Wu YZ, Song SY, Min HY, Yang Y, et al. Effect of puerarin in promoting fatty acid oxidation by increasing mitochondrial oxidative capacity and biogenesis in skeletal muscle in diabetic rats. Nutr Diabetes. (2018) 8:1. doi: 10.1038/s41387-017-0009-6
55. Zhou J, Zhang N, Aldhahrani A, Soliman M, Zhang L, Zhou F. Puerarin ameliorates nonalcoholic fatty liver in rats by regulating hepatic lipid accumulation, oxidative stress, and inflammation. Front Immunol. (2022) 13:956688. doi: 10.3389/fimmu.2022.956688
56. Wu Z, Luo L, Kan Y, Qin M, Li H, He Q, et al. Puerarin prevents bisphenol S induced lipid accumulation by reducing liver lipid synthesis and promoting lipid metabolism in C57BL/6J Mice. Toxics. (2023) 11:736. doi: 10.3390/toxics11090736
57. Yang C, Liu H, Chang Z, Liu G, Chang H, Huang P, et al. Puerarin modulates hepatic farnesoid X receptor and gut microbiota in high-fat diet-induced obese mice. Int J Mol Sci. (2024) 25:5274. doi: 10.3390/ijms25105274
58. Xu DX, Guo XX, Zeng Z, Wang Y, Qiu J. Puerarin improves hepatic glucose and lipid homeostasis in vitro and in vivo by regulating the AMPK pathway. Food Funct. (2021) 12:2726–40. doi: 10.1039/d0fo02761h
59. Kang O, Kim S, Mun S, Seo Y, Hwang H, Lee Y, et al. Puerarin ameliorates hepatic steatosis by activating the PPARα and AMPK signaling pathways in hepatocytes. Int J Mol Med. (2015) 35:803–9. doi: 10.3892/ijmm.2015.2074
60. Zheng G, Lin L, Zhong S, Zhang Q, Li D. Effects of puerarin on lipid accumulation and metabolism in high-fat diet-fed mice. PLoS One. (2015) 10:e0122925. doi: 10.1371/journal.pone.0122925
61. Pham TH, Lee GH, Jin SW, Lee SY, Han EH, Kim ND, et al. Puerarin attenuates hepatic steatosis via G-protein-coupled estrogen receptor-mediated calcium and SIRT1 signaling pathways. Phytother Res. (2022) 36:3601–18. doi: 10.1002/ptr.7526
62. Li Z, Cao W, Zhang Y, Lai S, Ye Y, Bao J, et al. Puerarin ameliorates non-alcoholic fatty liver disease by inhibiting lipid metabolism through FMO5. Front Pharmacol. (2024) 15:1423634. doi: 10.3389/fphar.2024.1423634
63. Li CH, Gong D, Chen LY, Zhang M, Xia XD, Cheng HP, et al. Puerarin promotes ABCA1-mediated cholesterol efflux and decreases cellular lipid accumulation in THP-1 macrophages. Eur J Pharmacol. (2017) 811:74–86. doi: 10.1016/j.ejphar.2017.05.055
64. Yan L, Chan S, Chan A, Chen S, Ma X, Xu H. Puerarin decreases serum total cholesterol and enhances thoracic aorta endothelial nitric oxide synthase expression in diet-induced hypercholesterolemic rats. Life Sci. (2006) 79:324–30. doi: 10.1016/j.lfs.2006.01.016
65. Liu CM, Ma JQ, Sun YZ. Protective role of puerarin on lead-induced alterations of the hepatic glutathione antioxidant system and hyperlipidemia in rats. Food Chem Toxicol. (2011) 49:3119–27. doi: 10.1016/j.fct.2011.09.007
66. Ma J, Ding J, Zhao H, Liu C. Puerarin attenuates carbon tetrachloride-induced liver oxidative stress and hyperlipidaemia in mouse by JNK/c-Jun/CYP7A1 pathway. Basic Clin Pharmacol Toxicol. (2014) 115:389–95. doi: 10.1111/bcpt.12245
67. Wu K, Liang T, Duan X, Xu L, Zhang K, Li R. Anti-diabetic effects of puerarin, isolated from Pueraria lobata (Willd.), on streptozotocin-diabetogenic mice through promoting insulin expression and ameliorating metabolic function. Food Chem Toxicol. (2013) 60:341–7. doi: 10.1016/j.fct.2013.07.077
68. Chung M, Sung N, Park C, Kweon D, Mantovani A, Moon T, et al. Antioxidative and hypocholesterolemic activities of water-soluble puerarin glycosides in HepG2 cells and in C57 BL/6J mice. Eur J Pharmacol. (2008) 578:159–70. doi: 10.1016/j.ejphar.2007.09.036
69. Blüher M. Obesity: Global epidemiology and pathogenesis. Nat Rev Endocrinol. (2019) 15:288–98. doi: 10.1038/s41574-019-0176-8
70. Afshin A, Forouzanfar MH, Reitsma MB, Sur P, Estep K, et al. Health effects of overweight and obesity in 195 countries over 25 years. N Engl J Med. (2017) 377:13–27. doi: 10.1056/NEJMoa1614362
71. Jung HW, Kang AN, Kang SY, Park YK, Song MY. The root extract of pueraria lobata and its main compound, puerarin, prevent obesity by increasing the energy metabolism in skeletal muscle. Nutrients. (2017) 9:33. doi: 10.3390/nu9010033
72. Wang T, Kalonda Mutamba A, Bian J, He X, Bian D. Puerarin affects 1HMR spectroscopy quantified hepatic fat signal fraction in intrauterine growth restricted rats. Curr Med Imaging. (2024) 20:e15734056296741. doi: 10.2174/0115734056296741240516095643
73. Lytrivi M, Castell A, Poitout V, Cnop M. Recent insights into mechanisms of β-cell lipo- and glucolipotoxicity in type 2 diabetes. J Mol Biol. (2020) 432:1514–34. doi: 10.1016/j.jmb.2019.09.016
74. Gong P, Wang J, Wang S, Yang W, Yao W, Li N, et al. Metabolomic analysis of the Puerarin hypoglycemic activity via AMPK-mTOR and PPARγ-NF-κB signaling pathways. Phytomedicine. (2024) 130:155546. doi: 10.1016/j.phymed.2024.155546
75. Gao J, Liu M, Guo Z, Hu C, Feng Z, Yan J. [Puerarin alleviates insulin resistance in type 2 diabetic mice by modulating fetuin B-AMPK/ACC signaling pathway in the liver]. Nan Fang Yi Ke Da Xue Xue Bao. (2021) 41:839–46. doi: 10.12122/j.issn.1673-4254.2021.06.05
76. Taylor R. Understanding the cause of type 2 diabetes. Lancet Diabetes Endocrinol. (2024) 12:664–73. doi: 10.1016/S2213-8587(24)00157-8
77. Younossi Z, Koenig A, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. (2016) 64:73–84. doi: 10.1002/hep.28431
78. Falk E. Pathogenesis of atherosclerosis. J Am Coll Cardiol. (2006) 47:C7–12. doi: 10.1016/j.jacc.2005.09.068
79. Haghikia A, Zimmermann F, Schumann P, Jasina A, Roessler J, Schmidt D, et al. Propionate attenuates atherosclerosis by immune-dependent regulation of intestinal cholesterol metabolism. Eur Heart J. (2022) 43:518–33. doi: 10.1093/eurheartj/ehab644
80. Bao L, Zhang Y, Wei G, Wang Y, Ma R, Cheng R, et al. The anti-atherosclerotic effects of puerarin on induced-atherosclerosis in rabbits. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. (2015) 159:53–9. doi: 10.5507/bp.2013.096
81. Li Z, Weng J, Yan J, Zeng Y, Hao Q, Sheng H, et al. Puerarin alleviates atherosclerosis via the inhibition of Prevotella copri and its trimethylamine production. Gut. (2024) 73:1934–43. doi: 10.1136/gutjnl-2024-331880
82. Koeth RA, Wang Z, Levison BS, Buffa JA, Org E, Sheehy BT, et al. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med. (2013) 19:576–85. doi: 10.1038/nm.3145
83. Zhang H, Zhai Z, Zhou H, Li Y, Li X, Lin Y, et al. Puerarin inhibits oxLDL-induced macrophage activation and foam cell formation in human THP1 macrophage. Biomed Res Int. (2015) 2015:403616. doi: 10.1155/2015/403616
84. Li W, Xu X, Dong D, Lei T, Ou H. Up-regulation of thioredoxin system by puerarin inhibits lipid uptake in macrophages. Free Radic Biol Med. (2021) 162:542–54. doi: 10.1016/j.freeradbiomed.2020.11.011
85. Hou N, Huang Y, Cai S, Yuan W, Li L, Liu X, et al. Puerarin ameliorated pressure overload-induced cardiac hypertrophy in ovariectomized rats through activation of the PPARα/PGC-1 pathway. Acta Pharmacol Sin. (2021) 42:55–67. doi: 10.1038/s41401-020-0401-y
86. Liu X, Huang R, Wan J. Puerarin: A potential natural neuroprotective agent for neurological disorders. Biomed Pharmacother. (2023) 162:114581. doi: 10.1016/j.biopha.2023.114581
87. Chauhan P, Wadhwa K, Mishra R, Gupta S, Ahmad F, Kamal M, et al. Investigating the potential therapeutic mechanisms of puerarin in neurological diseases. Mol Neurobiol. (2024) 61:10747–69. doi: 10.1007/s12035-024-04222-4
88. Liu T, Su K, Cai W, Ao H, Li M. Therapeutic potential of puerarin against cerebral diseases: From bench to bedside. Eur J Pharmacol. (2023) 953:175695. doi: 10.1016/j.ejphar.2023.175695
89. van der Kant R, Goldstein LSB, Ossenkoppele R. Amyloid-β-independent regulators of tau pathology in Alzheimer disease. Nat Rev Neurosci. (2020) 21:21–35. doi: 10.1038/s41583-019-0240-3
90. Park S, Kim J, Choi K, Jang Y, Bae S, Choi B, et al. Hypercholesterolemia accelerates amyloid β-induced cognitive deficits. Int J Mol Med. (2013) 31:577–82. doi: 10.3892/ijmm.2013.1233
91. Gao LN, Yan M, Zhou L, Wang J, Sai C, Fu Y, et al. Puerarin alleviates depression-like behavior induced by high-fat diet combined with chronic unpredictable mild stress via repairing TLR4-Induced inflammatory damages and phospholipid metabolism disorders. Front Pharmacol. (2021) 12:767333. doi: 10.3389/fphar.2021.767333
92. Song X, Wang W, Ding S, Liu X, Wang Y, Ma H. Puerarin ameliorates depression-like behaviors of with chronic unpredictable mild stress mice by remodeling their gut microbiota. J Affect Disord. (2021) 290:353–63. doi: 10.1016/j.jad.2021.04.037
93. Mann E, Lam Y, Uhlig H. Short-chain fatty acids: Linking diet, the microbiome and immunity. Nat Rev Immunol. (2024) 24:577–95. doi: 10.1038/s41577-024-01014-8
94. Kishino S, Takeuchi M, Park S, Hirata A, Kitamura N, Kunisawa J, et al. Polyunsaturated fatty acid saturation by gut lactic acid bacteria affecting host lipid composition. Proc Natl Acad Sci U S A. (2013) 110:17808–13. doi: 10.1073/pnas.1312937110
95. Riessland M, Ximerakis M, Jarjour A, Zhang B, Orr M. Therapeutic targeting of senescent cells in the CNS. Nat Rev Drug Discov. (2024) 23:817–37. doi: 10.1038/s41573-024-01033-z
96. Li B, Liu M, Wang Y, Gong S, Yao W, Li W, et al. Puerarin improves the bone micro-environment to inhibit OVX-induced osteoporosis via modulating SCFAs released by the gut microbiota and repairing intestinal mucosal integrity. Biomed Pharmacother. (2020) 132:110923. doi: 10.1016/j.biopha.2020.110923
97. Yang J, Chen C, Zhang H, Chen B, Xiao K, Tang Y, et al. Serum metabolomics and 16S rRNA amplicon sequencing reveal the role of puerarin in alleviating bone loss aggravated by antidiabetic agent pioglitazone in type 2 diabetic mice. J Ethnopharmacol. (2024) 340:119128. doi: 10.1016/j.jep.2024.119128
98. Li B, Wang Y, Gong S, Yao W, Gao H, Liu M, et al. Puerarin improves OVX-induced osteoporosis by regulating phospholipid metabolism and biosynthesis of unsaturated fatty acids based on serum metabolomics. Phytomedicine. (2022) 102:154198. doi: 10.1016/j.phymed.2022.154198
99. Yu Z, Li W. Induction of apoptosis by puerarin in colon cancer HT-29 cells. Cancer Lett. (2006) 238:53–60. doi: 10.1016/j.canlet.2005.06.022
100. Wang J, Yang ZR, Guo XF, Song J, Zhang JX, Wang J, et al. Synergistic effects of puerarin combined with 5-fluorouracil on esophageal cancer. Mol Med Rep. (2014) 10:2535–41. doi: 10.3892/mmr.2014.2539
101. Kang H, Zhang J, Wang B, Liu M, Zhao J, Yang M, et al. Puerarin inhibits M2 polarization and metastasis of tumor-associated macrophages from NSCLC xenograft model via inactivating MEK/ERK 1/2 pathway. Int J Oncol. (2017) 50:545–54. doi: 10.3892/ijo.2017.3841
102. Hu Y, Li X, Lin L, Liang S, Yan J. Puerarin inhibits non-small cell lung cancer cell growth via the induction of apoptosis. Oncol Rep. (2018) 39:1731–8. doi: 10.3892/or.2018.6234
103. Zhang WG, Liu XF, Meng KW, Hu SY. Puerarin inhibits growth and induces apoptosis in SMMC-7721 hepatocellular carcinoma cells. Mol Med Rep. (2014) 10:2752–8. doi: 10.3892/mmr.2014.2512
104. Liu X, Zhao W, Wang W, Lin S, Yang L. Puerarin suppresses LPS-induced breast cancer cell migration, invasion and adhesion by blockage NF-κB and Erk pathway. Biomed Pharmacother. (2017) 92:429–36. doi: 10.1016/j.biopha.2017.05.102
105. Liu X, Li S, Li Y, Cheng B, Tan B, Wang G. Puerarin inhibits proliferation and induces apoptosis by upregulation of miR-16 in bladder cancer cell line T24. Oncol Res. (2018) 26:1227–34. doi: 10.3727/096504018X15178736525106
106. Hu Y, Hu C, Lei H, Liu X. Puerarin inhibits the progression of hepatic carcinoma by suppressing NLRP3 inflammasome-mediated pyroptosis. Asian J Surg. (2024) 47:4102–3. doi: 10.1016/j.asjsur.2024.05.046
107. Zhu H, Xiao Y, Guo H, Guo Y, Huang Y, Shan Y, et al. The isoflavone puerarin exerts anti-tumor activity in pancreatic ductal adenocarcinoma by suppressing mTOR-mediated glucose metabolism. Aging. (2021) 13:25089–105. doi: 10.18632/aging.203725
108. Xu H, Hu M, Liu M, An S, Guan K, Wang M, et al. Nano-puerarin regulates tumor microenvironment and facilitates chemo- and immunotherapy in murine triple negative breast cancer model. Biomaterials. (2020) 235:119769. doi: 10.1016/j.biomaterials.2020.119769
109. Hien TT, Kim HG, Han EH, Kang KW, Jeong HG. Molecular mechanism of suppression of MDR1 by puerarin from Pueraria lobata via NF-kappaB pathway and cAMP-responsive element transcriptional activity-dependent up-regulation of AMP-activated protein kinase in breast cancer MCF-7/adr cells. Mol Nutr Food Res. (2010) 54:918–28. doi: 10.1002/mnfr.200900146
110. Kopecka J, Trouillas P, Gašparović AČ, Gazzano E, Assaraf YG, Riganti C. Phospholipids and cholesterol: Inducers of cancer multidrug resistance and therapeutic targets. Drug Resist Updat. (2020) 49:100670. doi: 10.1016/j.drup.2019.100670
111. Xu J, Zhang X, Yan L, Zhang Z, Wei J, Li L, et al. Insight into lotusine and puerarin in repairing alcohol-induced metabolic disorder based on UPLC-MS/MS. Int J Mol Sci. (2022) 23:10385. doi: 10.3390/ijms231810385
112. Liu Y, Yuan M, Zhang C, Liu H, Liu J, Wei A, et al. Puerariae Lobatae radix flavonoids and puerarin alleviate alcoholic liver injury in zebrafish by regulating alcohol and lipid metabolism. Biomed Pharmacother. (2021) 134:111121. doi: 10.1016/j.biopha.2020.111121
113. Yang W, Gao B, Qin L, Wang X. Puerarin improves skeletal muscle strength by regulating gut microbiota in young adult rats. J Orthop Translat. (2022) 35:87–98. doi: 10.1016/j.jot.2022.08.009
114. Wang Y, Yin L, Li Y, Liu P, Cui Q. Preventive effects of puerarin on alcohol-induced osteonecrosis. Clin Orthop Relat Res. (2008) 466:1059–67. doi: 10.1007/s11999-008-0178-7
115. Chen J, Xu Y, Liu Y, Meng Y, Wu L, Cao W, et al. Preparation of cubic liquid crystal nanoparticles of puerarin and its protective effect on ischemic stroke. Nanomedicine. (2024) 62:102786. doi: 10.1016/j.nano.2024.102786
116. Yan J, Guan Z, Zhu W, Zhong L, Qiu Z, Yue P, et al. Preparation of puerarin chitosan oral nanoparticles by ionic gelation method and its related kinetics. Pharmaceutics. (2020) 12:216. doi: 10.3390/pharmaceutics12030216
117. Wang B, Hang H, Wang H, Li D, Jiang Z, Zhang X. Preparation of puerarin long circulating liposomes and its effect on osteoporosis in castrated rats. J Pharm Sci. (2024) 113:1823–35. doi: 10.1016/j.xphs.2024.04.005
118. Zhang Y, Wang R, Wu J, Shen Q. Characterization and evaluation of self-microemulsifying sustained-release pellet formulation of puerarin for oral delivery. Int J Pharm. (2012) 427:337–44. doi: 10.1016/j.ijpharm.2012.02.013
119. Duan S, Li YF, Luo XL. [Effect of puerarin on heart function and serum oxidized-LDL in the patients with chronic cardiac failure]. Hunan Yi Ke Da Xue Xue Bao. (2000) 25:176–8. doi: 10.3321/j.issn:1672-7347.2000.02.029
120. Shi W, Qu L, Wang J. [Study on interventing effect of puerarin on insulin resistance in patients with coronary heart disease]. Zhongguo Zhong Xi Yi Jie He Za Zhi. (2002) 22:21–4. doi: 10.3321/j.issn:1003-5370.2002.01.006
121. Li W, Hu H, Zou G, Ma Z, Liu J, Li F. Therapeutic effects of puerarin on polycystic ovary syndrome: A randomized trial in Chinese women. Medicine (Baltimore). (2021) 100:e26049. doi: 10.1097/MD.0000000000026049
122. Hou Q, Ao X, Li G, Zhang Y. [Puerarin combined with avandia for diabetic nephropathy]. Zhong Nan Da Xue Xue Bao Yi Xue Ban. (2012) 37:73–7. doi: 10.3969/j.issn.1672-7347.2012.01.013
123. Kwok MK, Leung GM, Xu L, Tse HF, Lam TH, Schooling CM. Effect of puerarin supplementation on cardiovascular disease risk factors: A randomized, double-blind, placebo-controlled, 2-way crossover trial. Biomed Pharmacother. (2022) 153:113472. doi: 10.1016/j.biopha.2022.113472
124. Xie X, Dong Y, Mu D, Pan X, Zhang F. [Evaluation on safety of puerarin injection in clinical use]. Zhongguo Zhong Yao Za Zhi. (2018) 43:3956–61. doi: 10.19540/j.cnki.cjcmm.20180709.008
125. Yue P, Hai-Long Yuan H, Zhu W, Cong L, Xie H, Liu Z, et al. The study to reduce the hemolysis side effect of puerarin by a submicron emulsion delivery system. Biol Pharm Bull. (2008) 31:45–50. doi: 10.1248/bpb.31.45
126. Hou SZ, Su ZR, Chen SX, Ye MR, Huang S, Liu L, et al. Role of the interaction between puerarin and the erythrocyte membrane in puerarin-induced hemolysis. Chem Biol Interact. (2011) 192:184–92. doi: 10.1016/j.cbi.2011.03.007
127. Guo Y, Liang D, Xu Z, Ye Q. In vivo inhibitory effects of puerarin on selected rat cytochrome P450 isoenzymes. Pharmazie. (2014) 69:367–70. doi: 10.1691/ph.2014.3823
128. Ettinger B, Quesenberry C, Schroeder D, Friedman G. Long-term postmenopausal estrogen therapy may be associated with increased risk of breast cancer: A cohort study. Menopause. (2018) 25:1191–4. doi: 10.1097/GME.0000000000001216
129. Olzmann JA, Carvalho P. Dynamics and functions of lipid droplets. Nat Rev Mol Cell Biol. (2019) 20:137–55. doi: 10.1038/s41580-018-0085-z
130. Li B, Hao J, Zeng J, Sauter ER. SnapShot: FABP Functions. Cell. (2020) 182:1066–1066.e1. doi: 10.3390/ijms22136965
131. Settembre C, De Cegli R, Mansueto G, Saha P, Vetrini F, Visvikis O, et al. TFEB controls cellular lipid metabolism through a starvation-induced autoregulatory loop. Nat Cell Biol. (2013) 15:647–58. doi: 10.1038/ncb2718
132. Li Y, Ma Z, Jiang S, Hu W, Li T, Di S, et al. Global perspective on FOXO1 in lipid metabolism and lipid-related diseases. Prog Lipid Res. (2017) 66:42–9. doi: 10.1016/j.plipres.2017.04.002
133. Hao R, Ge J, Li F, Jiang Y, Sun-Waterhouse D, Li D. MiR-34a-5p/Sirt1 axis: A novel pathway for puerarin-mediated hepatoprotection against benzo(a)pyrene. Free Radic Biol Med. (2022) 186:53–65. doi: 10.1016/j.freeradbiomed.2022.05.006
134. Li J, Li Y, Yuan X, Yao D, Gao Z, Niu Z, et al. The effective constituent puerarin, from Pueraria lobata, inhibits the proliferation and inflammation of vascular smooth muscle in atherosclerosis through the miR-29b-3p/IGF1 pathway. Pharm Biol. (2023) 61:1–11. doi: 10.1080/13880209.2022.2099430
135. Fan C, Wang Q, Chen Y, Ye T, Fan Y. Puerarin from Pueraria lobate attenuates ischemia-induced cardiac injuries and inflammation in vitro and in vivo: The key role of miR-130a-5p/HMGB2 pathway. Chem Biol Drug Des. (2023) 101:952–61. doi: 10.1111/cbdd.14204
136. Yang J, Li B, Wang J, Fan W. Puerarin alleviates chronic renal failure-induced pyroptosis in renal tubular epithelial cells by targeting miR-342-3p/TGF-β/SMAD axis. Genes Genomics. (2023) 45:1563–73. doi: 10.1007/s13258-023-01448-9
137. Han Y, Wang H, Wang Y, Dong P, Jia J, Yang S. Puerarin protects cardiomyocytes from ischemia-reperfusion injury by upregulating LncRNA ANRIL and inhibiting autophagy. Cell Tissue Res. (2021) 385:739–51. doi: 10.1007/s00441-021-03463-2
Keywords: Puerarin, natural product, lipid metabolism, molecular mechanism, nutrient transformation
Citation: Ou S, Liang Q, Leng Y, Luo T, Xu X, Xie H, Gao H, Li J and Xie C (2025) Puerarin as a multi-targeted modulator of lipid metabolism: molecular mechanisms, therapeutic potential and prospects for nutritional translation. Front. Nutr. 12:1598897. doi: 10.3389/fnut.2025.1598897
Received: 24 March 2025; Accepted: 30 June 2025;
Published: 18 July 2025.
Edited by:
Jailane de Souza Aquino, Federal University of Paraíba, BrazilReviewed by:
Paolo Paoli, University of Florence, ItalyGiosiana Bosco, University of Catania, Italy
Copyright © 2025 Ou, Liang, Leng, Luo, Xu, Xie, Gao, Li and Xie. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jun Li, bHVvc2h1bG92ZXJAc2luYS5jb20=; Chunguang Xie, eGllY2dAY2R1dGNtLmVkdS5jbg==
†These authors have contributed equally to this work
 Shu Ou
Shu Ou Qingzhi Liang
Qingzhi Liang Yulin Leng1,2,3†
Yulin Leng1,2,3† Hongyan Xie
Hongyan Xie Hong Gao
Hong Gao Chunguang Xie
Chunguang Xie