- 1Department of Bioprocess Technology, Faculty of Technology, Rajarata University of Sri Lanka, Anuradhapura, Sri Lanka
- 2Australian College of Business and Technology – Kandy Campus, Kandy, Sri Lanka
Microalgal toxins are secondary metabolites synthesized by cyanobacteria, dinoflagellates, and diatoms in response to environmental stress. Humans and animals can be exposed to these toxic compounds through food, water, and aerosolized toxins and these toxic compounds are capable of causing acute and chronic health issues like paralysis, liver damage, cancer, and even death by employing several molecular mechanisms such as sodium channel blocking, protein phosphatase inhibition, cellular membrane disruption etc. Microalgal toxin poisoning through food products is a major concern as microalgae are largely consumed as dietary supplements. These toxins can easily bioaccumulate and be biomagnified via food chains. Hence, proper screening and quality control measures for these microalgal toxins should be implemented. Cytotoxins, dermatoxins, neurotoxins, hepatotoxins, and endotoxins are the main toxins produced by the microalgae. Microalgae are effectively incorporated into the food industry in a diverse range. Toxic contaminants from the microalgae are a silent threat to food security and human health. There are some regulatory models when consuming microalgae-related food products and water due to their toxic effects. Detecting the toxins in the initial stage, studying the impact of toxin production due to environmental factors, and developing effective mitigation strategies to ensure food safety, is a future needs in this field.
1 Introduction
Microalgae are a diverse group of unicellular, photosynthetic microorganisms (1) that act as the primary producers in aquatic ecosystems (2). Their nutrient richness and the ability to synthesize bioactive compounds are considered highly beneficial as they can be used in various industries, such as food and nutraceuticals (3), medicines (4), cosmetics (5), animal feed (6), agriculture (7), and biofuel production (8). By 2050, it is estimated that the global population will reach 9.7 billion, requiring a doubling of global food production to satisfy the rising food demand (9). Microalgae have been consumed as a food source for thousands of years (9) and are loaded with essential nutrients (10). Apart from that, their antibiotic (11), antioxidant (12), anti-viral (13, 14), anticancer (15, 16), anti-inflammatory (17) and neuroprotective properties (18) offer many health benefits by reducing and preventing the risk of developing diseases like cancer, macular degeneration, cataracts, type 2 diabetes, and cardiovascular diseases (19). Compared to conventional crop cultivation, microalgae cultivation offers numerous advantages, including continuous year-round output, less land consumption, better yields, etc. (1). Hence, as an alternative food source, microalgae is a promising solution. Currently, microalgae species including Arthrospira platensis, Chlorella spp., Dunaliella salina, Aphanizomenon flos-aquae, Odontella aurita, Tetraselmis chuii, Haematococcus pluvialis, Schizochytrium spp., and Ulkenia spp., are commercially cultivated for human consumption and issued to the market as tablets, pellets, powders, capsules, or in liquid form (1). Furthermore, microalgae-incorporated food items, such as cookies, sausages, cheese, and ice cream etc., are also available in the market (1).
Certain microalgal species can produce toxic compounds known as microalgal toxins, and some environmental factors like temperature, light intensity, and nutrient availability are believed to trigger the formation of harmful algal blooms (HABs) (20). As the population of toxin-producing microalgae increases within these blooms, they release larger quantities of toxic compounds such as Saxitoxin, Ciguatoxins, Nodularin, Anatoxin-a, and many more (21) into water bodies, leading to the complete disruption of the entire ecosystem. Moreover, these toxins can bioaccumulate through aquatic food webs in higher trophic levels, including humans, which eventually leads to detrimental chronic renal, cardiovascular, gastrointestinal, respiratory, and neurological disorders (22–24). Therefore, it is important to thoroughly examine microalgal toxin production and releasing mechanisms to develop monitoring and mitigation strategies to prevent food contamination.
When it comes to public health, there are three major ways of exposure to algal toxins: (1) consumption of toxin-contaminated food (25), (2) Inhalation of aerosolized toxins (26), and (3) Skin contact with toxin-containing liquids (27). Accordingly, the simplest way to experience microalgal toxin-associated poisoning is by consuming toxin-contaminated food and water (28). In the case of shellfish, as they are filter feeders, toxins such as saxitoxins or domoic acid can accumulate (29). Moreover, consuming dietary supplements such as Spirulina or Chlorella-based on some microalgae supplement, poses a risk of contaminated microalgal toxins (30). This can be due to contamination by toxin-producing species, even under commercial setups (30). Proper screening for toxic compounds and quality control measures should be implemented, as children, the elderly, pregnant women, and immunocompromised individuals consume these dietary supplements.
To minimize the contamination of food by microalgal toxins and to prevent their short- and long-term health implications, it is essential to have a clear understanding of the specific species responsible for producing these toxins, the factors that influence their production and release, their mode of action, and their occurrence in the human diet. In this review, we try to provide an overview of the types of microalgal toxins, how they enter into food chains, and their associated health implications, while understanding the molecular mechanisms underlying the production of these toxins and their role in developing life-threatening diseases. The consumer protections and regulatory models regarding the microalgae and their future directions.
2 Microalgae and toxins
2.1 Definition, classes, and biological characteristics of microalgae
Microalgae are prokaryotic and primary photosynthetic eukaryotic, single-celled organisms that are phylogenetically and taxonomically divergent (31, 32). Algae can be classified as unicellular and multicellular according to their sizes and shapes (33, 34). These microalgae are in diverse habitats and can be found in almost all areas on earth, including different water bodies with fresh water, hypersaline environments, and sea water, rocks, or moist soil (31). The classification of microalgae can be based on various aspects such as morphological features, pigmentations, and photosynthetic membranes (32). As Torres et al. (31) describe, the most typical classification of microalgae is with classes Chlorophyceae [green algae, Cyanophyceae (blue-green algae), Chrysophyceae (golden algae), and Bacillariophyceae (Diatom)] (31). A chart summarizing the main microalgae classes with their main relevant species is shown in Figure 1. Consequently, microalgae are fast growers and highly productive even in a limited land area (35), doing photosynthesis and completing their whole lifecycle within a few days. Mostly, it needs simple nutrients and abundant sunlight for its survival (35). Mainly, microalgae are smaller in size; their sizes range from l μm to 1 mm and belong to a heterogenous group. Chlorella, which lives primarily in freshwater or soil, is 2 μm to 10 μm in diameter and spherical (36). Usually, microalgae are orthotropic, while some are mixotrophs. Their mechanism is different from the terrestrial plants as they do not have the same cell differentiation (36, 37). In 1830, color was first used to differentiate microalgae into green, brown, and red (38). However, recent studies mainly focused on phylogeny and molecular studies to analyze the structure and understand the relationship between algae and other organisms (39). Algae do not have a common ancestor, and they are called a polyphyletic group without a taxonomic value (31, 34, 40). According to the color pigments produced by the chloroplast, the color of microalgae comes from phycobiliproteins and chlorophylls (38). The Phylum cyanobacteria belongs to the prokaryotic cell microalgae, and eukaryotic species mainly consist of red microalgae (Rhodophyta), green microalgae (Chlorophyta), and diatoms (Bacillariophyta) groups (41, 42).
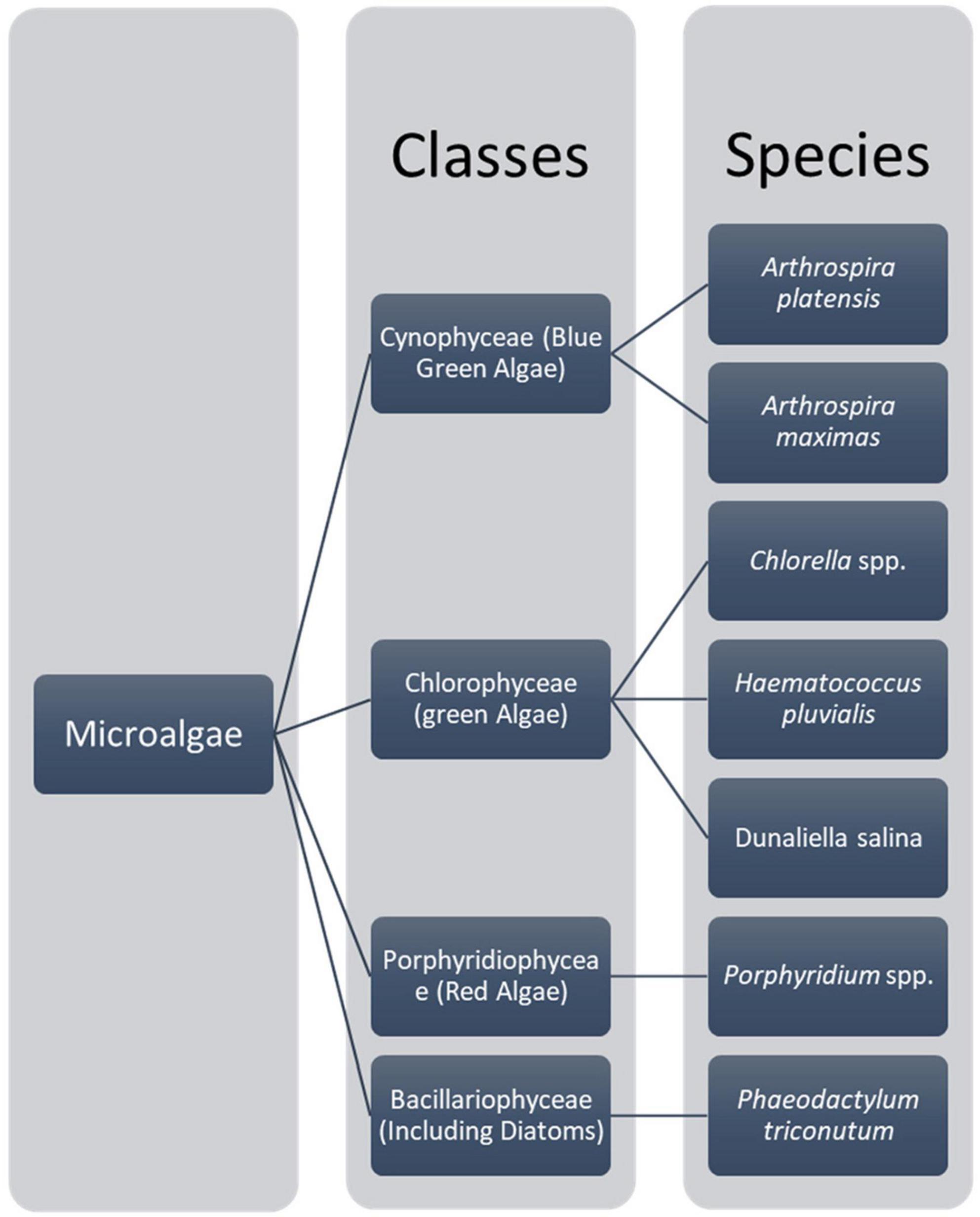
Figure 1. Main micro algae classes and their main species (32).
Microalgae are considered old living beings on the planet, and they exist in all of Earth’s ecosystems. They can live in adverse conditions like radiation, temperature, oxygen, pH, and salinity. Therefore, it can lead to a vast area of scientific research and exploration (34, 41, 43). Rationalizing microalgae by bioprospecting the new species, studying unique lineages of these organisms, and properly choosing microalgae and cultivating them is very important (44).
2.2 Toxins produced by microalgae and it’s mechanism action
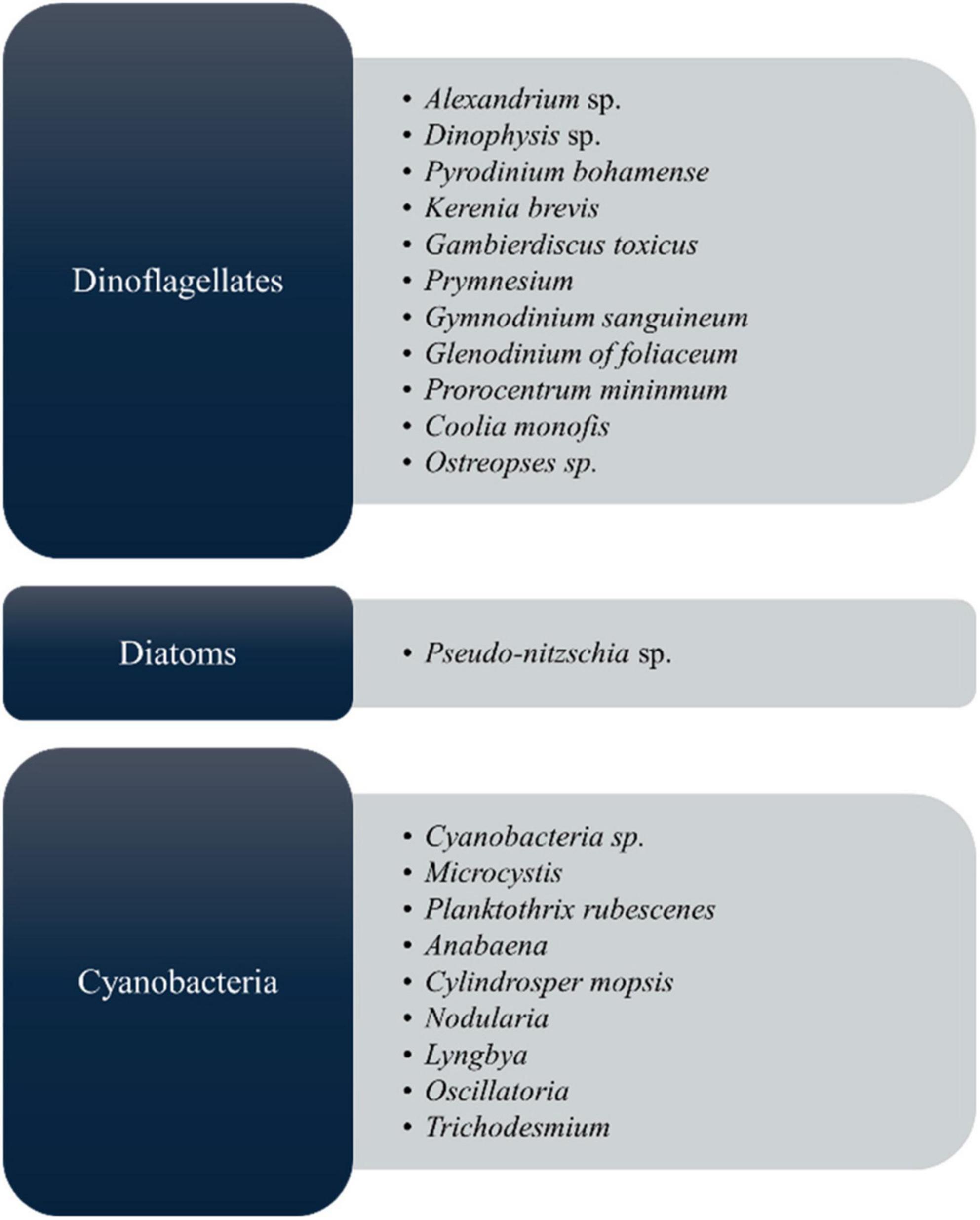
As shown in Figure 2, microalgal species, including those belonging to the groups of cyanobacteria, diatoms, and dinoflagellates, produce toxic compounds known as microalgal toxins, which have harmful effects on both aquatic ecosystems and human health by mainly harmful algal blooms (HABs) (45). Each species generates a distinct array of algal toxins, characterized by varying chemical structures and toxic effects (25, 46). These toxins are produced as a defensive strategy to protect against predators in response to grazing pressure; hence, they play a major role in their life survival (47, 48). In the market, most biomedical exploration utilizes Spirulina and Chlorella, but it must ensure safety for the final commercial outcome. Manali et al. (49) showed that microcystin contamination was only detected in fish food supplements, not in the Spirulina ingredients dietary supplements (49). However, to this today, there are no considerable reports that Arthrospira spp. and Chlorella spp. produce toxins, therefore establishing their safety by the US Food and Drug Administration (US FDA) (50) by GRN No.127 (51–53). From the microalgal toxins, cyanotoxins are the most diverse group of natural toxins from the chemical and toxicological view (54). Because of the algae, it can cause the death of people, fish, and other living things. Most toxic microalgae are dinoflagellates and diatoms in marine and freshwater, and Figure 2 depicts the major toxic algal species. According to many records, microalgal toxins associated with food poisoning significantly impact public health (22). Most of the incidents are related to contaminated seafood consumption (55, 56) leading to acute food poisoning with distinct symptoms such as vomiting, diarrhea, numbness, confusion, memory loss, and in extreme cases, paralysis, brain and liver damage, and even death (25). Apart from that, long-term exposure to these toxins can cause damage to the gut, liver, and lung health (22).
Certain microalgae produce bioactive compounds known as phycotoxins, which are toxic substances generated by specific genera of dinoflagellates, diatoms, and cyanobacteria (57). The situation worsens as these toxins accumulate in water bodies, posing risks to humans, marine life, and aquatic organisms (58). This review examines how several well-known microalgae toxins function at the biochemical, molecular, and toxicological levels and analyzes their impact on the risk posed to the human population and their mechanism of action.
Cyanobacteria produce diverse toxins as secondary metabolites, which are hazardous to many other organisms. Researchers have discovered that these pollutants cause a significant threat to human health in diverse parts of the world. The main two types of toxins produced by cyanobacteria are cytotoxins and biotoxins (59, 60). Table 2 summarizes the cyanobacterial toxins produced by various types of blue-green algae and their impacts, as listed in the below.
2.2.1 Cytotoxins
Cylindrospermopsin is a known cytotoxin. It is one of the toxins made by Cylindrospermopsis mceberskii, and it is the only alkaloid compound among the hepatotoxicants. The toxic effect of this compound is not only for the liver; it has been found to cause tissue destruction to the kidneys (61). Some marine cyanobacteria species produce this toxin, and it is closely related to the cholera toxin, but it is not so toxic to animals; however, it is lethal to cells produced in tissue cultures, and it prevents the growth of several microorganisms across the spectrum (62). Cylindrospermopsis, Umezakia, and Aphanizomenon-like cyanobacteria produce these toxins, and those toxins cause kidney and liver failures. Further, it causes tissue failures and destroys the organ (59). Toxins such as Tolytoxin, Tubercidin, Scytophycins, Actiphycins, Indolocarbazoles and tautazoles, microbilinisonitriles, paracyclophanes are belongs to cytotoxins (59, 63).
2.2.1.1 Tolytoxin
Tolytoxin is produced by Tolypothrix, a polyketide macrolide that perturbs the filaments; it can interact with the actin monomers cytoskeletal and inhibit polymerization, enabling intracellular transport and mitosis. Cock and Cheesman (64) in 2023 reported that, with in vitro studies, the IC50 of tolytoxin is 50–100 nM. The primary mode of action of this toxin is on the apoptotic cell death, preventing polymerization, which is essential in intracellular transport and mitosis in human health. The main mechanism of this toxin is to enhance apoptotic cell death through the disruption of the cytoskeleton. This toxin can be used as an anti-metastatic since it can reduce the cancer cell death (65–67). Tolytoxin has profound effects on the cell shape and microfilament distribution of mammalian cells, and these changes can be produced by very low concentrations of this agent. Tolytoxin inhibits actin such as polymerization in vitro, which you would expect to see in a gel, and thereby explains the observed cellular effects (66).
2.2.1.2 Tubercidin
This toxin is an adenosine analog, a purine nucleoside that is incorporated in the synthesis by nucleic acid. The specific toxin of this fungus is capable of RNA translation and transcription, causing eventual apoptosis (68). According to the current literature, it has the potential to be used in clinical trials against fungal invasions such as Candida albicans and Leishmania donovani (68, 69).
2.2.1.3 Scytophycins
Scytonema is a toxin synthesized by cyanobacteria macrolide compounds targeting actin filaments, leading to cell death. This can affect cytotoxic effects against breast cancer cells and leukemia cells (57, 70).
2.2.1.4 Indolocarbazoles
This toxin is an alkaloid group originating from tryptophan (71). It interferes with DNA supercoiling and influences protein kinases, such as topoisomerase I and II (72). Further, it can hinder the transcription by inhibiting the VEGFR and EGFR signaling, which is crucial for angiogenesis and cancer cell division (73).
2.2.1.5 Actiphycins
This toxin is a cyclic peptide containing a significant number of proteinogenic amino acids contributing toward the stability of this molecule. The major use of this is to stop the molecule from replicating through binding to DNA polymerase. Al-Hussieny (59) reported that it is a kind of toxin belonging to cytotoxins. There is only limited documentation regarding these toxins, and further research is needed regarding these toxins.
2.2.2 Dermatoxins
Dermatoxins include aplysia toxins and debromoaplysiatoxin, which predominantly result from contact and are related to cyanobacterial toxins (74). These toxins stimulate the protein kinase C (PKC), which is involved in cell proliferation and differentiation, and promote inflammation in human skin cells. Dermatoxins are poisonous chemicals that cause skin issues, and when the skin is repeatedly exposed to dermatoxins, the PKC remains active for a long time, increasing the occurrence of tumors (75).
2.2.3 Neurotoxins
Neurotoxins are some of the most widely recognized types of microalgae toxins, and they work in the nervous system by interfering with ion channels and neurotransmitters (75). Alexandrium species generate Saxitoxin (STX), which binds to voltage-gated sodium channels in both nerve and muscle cells, thus preventing the infiltration of sodium ions into cells; this triggers paralysis and possibly fatal cessation of breathing in humans (76). Likewise, the toxic alkaloid, domoic acid, elaborated by diatoms of the genus Pseudo-nitzschia, mimics the neurotransmitter freshwater, seeking out specific glutamate receptors in the central nervous system. This causes excitotoxicity, in which stimulation of glutamate receptors results in neuronal lesions and memory loss, a condition referred to as amnesic shellfish poisoning (77).
2.2.3.1 Neurotoxic alkaloids
Alkaloids are usually lethal and poisonous in a short time as they cause paralysis of respiratory muscles and skeletal muscles, often resulting in respiratory issues and death. Oscillatoria and Trichodesmium are producing different forms of these kinds of toxins (78).
Anatoxin: Anabaena flos-aquae species produces this toxin, which contains a 765 Da molecular weight (79).
Homoanatoxins: Oscillatoria rubescens produces a and is less toxic than anatoxin (79).
Anatoxin-a(s): Anabaena produces this toxin, which is ten times more toxic than anatoxin, with a molecular weight of 252 Da (79).
2.2.3.2 Paralytic shellfish poisons (PSPs)
PSPs include 18 toxins that paralyze crustaceans and are classified into three main classes, gongyautoxins, saxitoxin, and C-toxins, usually produced by species such as Anabaena circinalis and Aphanizomenon flos-aquae (80). These toxins are thought to have an immediate neurological response due to the interference of nerve impulses by blocking sodium channels, but they do not affect potassium leak currents (81).
2.2.4 Hepatotoxins
Hepatotoxins-producing genera include Anabaena, Microcystis, Cylindrospermopsis, Nodularia, Oscillatoria, and Nostoc (82). Microcystins are the most abundant of the cyanobacterial toxins (75). Nevertheless, they are slower in killing the organisms than neurotoxins, and the process of death can take 5 min to as long as several days, based on the rate of more factors and conditions, such as the type of poison, an animal’s weight, and the dose. These toxins are classified into three groups (82).
The hepatotoxins act on the liver and produce both short-term and chronic effects. Microcystins isolated from M. aeruginosa, for instance, interfere with serine/threonine protein phosphatases PP1 and PP2A; increased intracellular proline-directed serine/threonine phosphorylation induces hepatocyte apoptosis/necrosis (75). This toxin is considered to be very stable; hence, the long-term effects of this algae grown in water bodies are very detrimental to water sources used for human consumption (75, 83). Another similar but distinct toxic compound affecting liver cells is nodularin, which is produced by Nodularia spumigena cyanobacteria and is typical for brackish water conditions as well only limited documents are (75).
2.2.4.1 Microcystins
These are monocyclic seven-chain peptides with an unusual resident amino acid (84). Microcystin-LR (MC-LR) inhibits green algae growth by regulating antioxidant and photosynthetic systems by harmful algae (84). This is because the peptide ring includes five amino acids that are involved in the biosynthesis of all the structural variants of microcystins found in this species. Microcystins were found in a stream inhabited by fish of the genus Brook (Salvelinus fontinalis) (85). Some other related species belonging to Oscillatoria, Nodularia, and Lyngbya, as well as genera like Anabaena, Nostoc, and others, are employed in the production of these materials (84). Among these toxins, only microcystins MC-RR, MC-LR, and MC-YR have been identified. There are often lethal microcystins, such as microcystin molecule weights. The levels of microcystins may be detected as long as 909–1,044 years, depending on the species (85). Microcystins are known for their long-term heat shock and other related features, and it has emerged that they are capable of enduring boiling without denaturation (84). They are stable in terms of pH changes and are freely soluble in water. Ethanol, methanol, and acetone cells will require energy to metabolize the poison (85).
2.2.4.2 Nodularin
MC–LR is slightly similar to this compound; it is a pentacyclic monocyclic peptide, but significantly smaller. The peptide ring has a molecular weight of 824 Da and contains amino acids similar to those found in MC–LR (86). Although a range of varieties have been spotted around the world, only one is manufactured by the Nodularia spumigena species, and their growth is toxic to humans and cattle as well as to that of MC-LR (87).
2.2.5 Endotoxins
2.2.5.1 Lipopolysaccharides (LPS)
Lipopolysaccharides are glycolipoproteins present in the cell wall. These chemicals are toxic to humans; when it was injected into the peritoneal membranes at a dose of 1–1.2 mg/kg, they were found to be fatal to rats even at 48 h using in vivo experiments (57).
3 Overview of microalgae and their uses in food products
Microalgae are successfully included in different sectors such as the food industry, pharmaceuticals industry, biofuel production, wastewater treatments, fertilizers, and cosmetics industry (31, 35, 88, 89). Globally, Microalgae have been recognized as sustainable, healthy, nutritional, and eco-friendly for social development. From ancient times, countries such as China, Japan, and many coastal regions worldwide mainly used microalgae for food production.
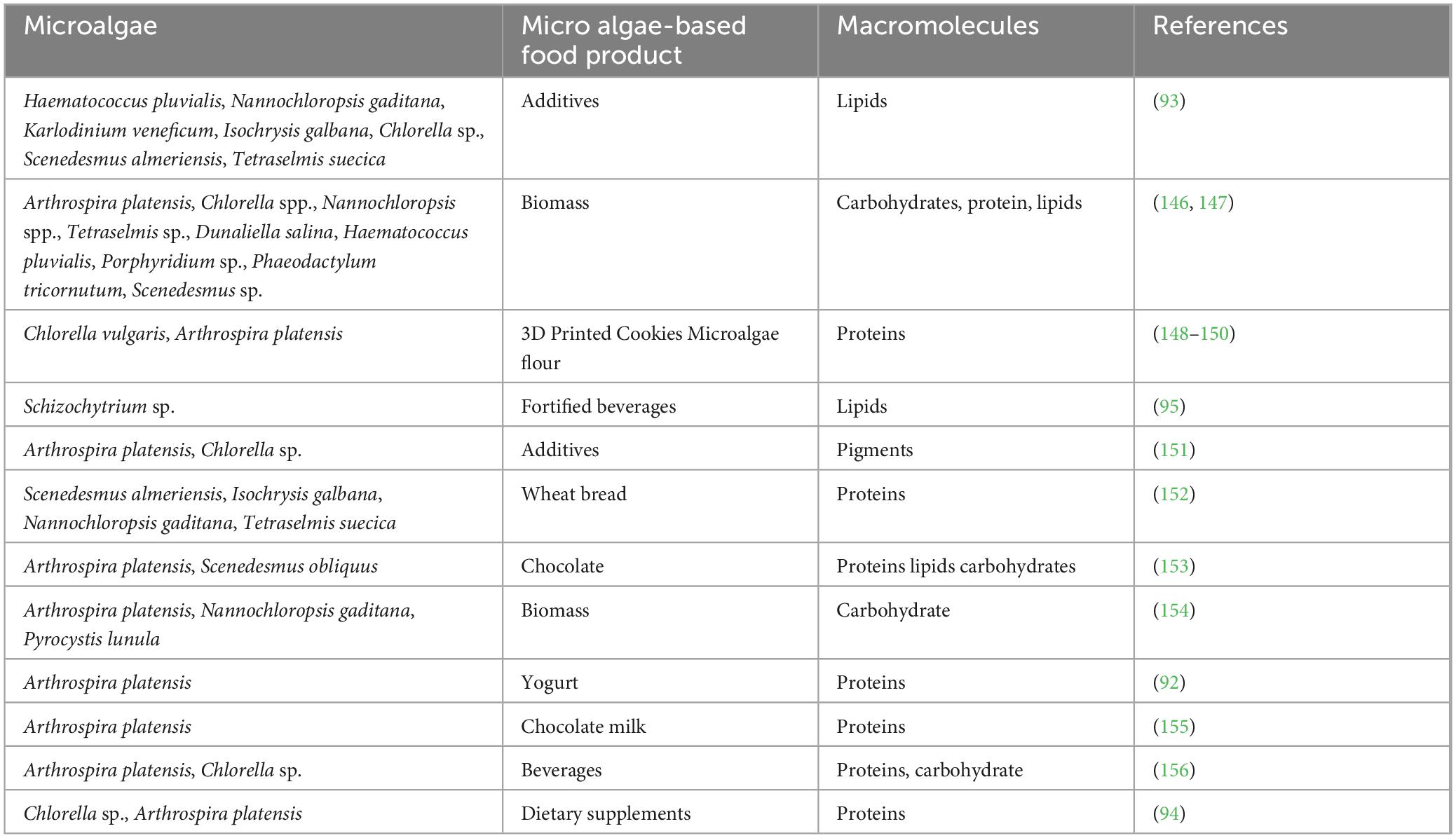
Arthrospira platensis, Chlorella vulgaris, Dunaliella salina, Isochrysis galbana, Nostoc sphaeroides, Spirulina maxima, and Spirulina platensis are used to produce commercial feed or food products (1, 90, 91). C. vulgaris is a famous microalga commercially cultivated to produce beta-carotene, astaxanthin, canthaxanthin, and chlorophyll, which can be used as food ingredients. Microalgae can additionally be used as food additives, bakery products, food supplements, and beverages (92–95). Docosahexaenoic acid is produced by the commercially cultivated Cryptothecondium cohnii, Schizochytrium, Thraustochytrium, and Ulkenia (96). Products of microalgae, including cyanobacteria, have been proposed for sterols, proteins, lipids, n-3 and n-6 fatty acids, microalgal oil, hydrocarbons, vitamins, polysaccharides, phycobiliproteins, zeaxanthin, lutein, phycocyanin, phycoerythrin, and antioxidants (96–105).
The protein in autotrophic and heterotrophic cyanobacteria is higher than the protein in pork and beef (104). Further, when we give small amounts of microalgae with animal feed, it improves the nutritional value of the feed and the animal’s performance and enhances the quality of products like meat, milk, and eggs (106). Table 1 clearly shows the microalgae-based food products and their nutritional values. Different value-added products can be commercialized using these microalgae, which is an emerging trend in this field. Asian countries like Sri Lanka still have a hidden fear of using these micro algae and fewer products in the market related to this field. This article suggests the research gaps in the microalgae-related food industry worldwide as one part of the article.
4 Pathways of toxin contamination in food products in algae
Algae, specifically microalgae, are increasingly in demand in the food market due to their rich nutritional profiles. However, toxin contamination from microalgae is a significant threat to food safety and human health. Algal toxins, which are known as phycotoxins, can accumulate in food chains, impacting human health through various pathways (107). These toxins are critically produced by harmful algal blooms, where specific algae species release toxins as secondary metabolites. Understanding the pathways of contamination is significant for mitigating risks and ensuring food safety regarding microalgae (7). Concerning the pathways of toxin contamination in food products, microalgae are much important, and it is critical to avoid those contaminations in algae as they can cause health risks and environmental pollution.
4.1 Direct consumption of toxin-producing algae
Microalgae such as Microcystis, Anabaena, and Nodularia are known to produce toxins like microcystins and nodularins, which can directly contaminate food products when algae are consumed as dietary supplements or functional foods (108). Spirulina, commonly used in the food industry, may occasionally be contaminated with toxic cyanobacteria if not adequately monitored when manufacturing dietary products, dessert products, and food additives (109). Cyanotoxins are heat-stable, and conventional food processing methods like pasteurization and cooking are ineffective at eliminating them, thereby posing risks to consumers (110). Research studies regarding this topic is an emerging trend, as there may be some direct consumption of toxin-producing algae which has not yet been identified without knowing.
4.2 Bioaccumulation in the food chain
Bioaccumulation is highlighted as another key approach to having algal toxins in seafood and other sea products. Some of these crustaceans and mollusks include mussels and oysters; these are categorized as filter-feeding mollusks that can concentrate toxins that are produced by algae in their tissues (74, 76, 107). Domoic acid, a substance that is transmitted from algae to fish and shellfish, is toxic to mammals, while saxitoxins, which are consumed by fish and bivalves, are also toxic to mammals. Brevetoxins, which move up the food chain from algae to fish, are toxic to humans through the process of biomagnification (74, 107, 111). This bioaccumulation is identified as a major issue for aquaculture, as seafood consumption is rapidly increasing globally. Contaminated water, whether freshwater or seawater, is used in food production, allowing toxins to enter food products. For instance, the use of polluted water for washing food crops, watering crops, or returning yield to feed livestock floods these products with toxic substances (42, 112). Microcystins, prevalent in freshwater systems, can persist in treated water, posing food safety risks when surface water is utilized for irrigation in agricultural regions (113).
4.3 Understanding cross-contamination and industrial processing
In the industrial usage of algae products in the food industry, contaminants can potentially spread (74). This can occur when toxic microalgae get mixed with non-toxic microalgae at harvesting time and are processed, or whenever improper washing and storage allow toxins to transfer from one batch to another (76). Contamination can generally be critically high, but an ineffective quality control strategy during algal harvesting or processing can worsen the problem (42). There is a research gap about the effect of the consumption of microalgae directly in food, in contrast to the industrial processing of them.
4.4 Global environment and climate change
The harmful algal bloom frequency has increased due to changes in the environment, especially climate change, as a higher risk of contamination with foods (114). Increasing temperature of the water, enrichment by nutrients from the agricultural effluents, and changes in water currents favor the growth of toxic algal strains. As a result, the toxins could suddenly appear in food products that have never been observed in certain areas, thus creating a new challenge for monitoring and controlling the issue (42).
Toxic metabolites and their characterization that cause diseases in microalgae are essential for human health as the incidences of harmful algal blooms are increasing (74). Cyanotoxins, which are representative of toxins created by cyanobacteria, are ingested by people through drinking water and consuming seafood, aerosolized toxins inhaled by people, and skin contact due to water sports, etc (42). Given these pathways of exposure, it becomes pivotal for policymakers to understand the extent of associated health risks to establish a structure for prevention measures (115). It is very important to conduct in-depth studies regarding the effect of global environmental changes and microalgae toxins.
4.5 Consumption of water and foods contaminated with toxins
Consumption of water polluted with these toxins is one of the most hazardous ways of getting involved with microalgae toxins (74). Consequently, the toxins of cyanobacteria, primarily microcystins, are the primary worries in freshwater ecosystems where harmful algal blooms threaten water accessibility for drinking. Among the toxins of this genus, microcystins produced by species such as Microcystis aeruginosa are the most dangerous because they possess hepatotoxic effects; they inhibit protein phosphatases Protein Phosphatase 1 and Protein Phosphatase 2, cause liver injuries and tumor issues (113, 116). High-level microcystins were shown to cause liver failure upon acute toxicity, while low-dose sub-chronic or chronic toxicity of microcystins affected liver cancer (85). In the study by Hilborn (117). They also note that there is a massive threat of exposure to microcystin in drinking water, as water utilities are still not capable of eradicating the toxins from the drinking water.
In the aquatic environment, another clear danger is the build-up of neurotoxins in seafood. Currently, Paralytic Shellfish Poisoning is caused by toxins named saxitoxins, which are generated by certain species of dinoflagellates, including Alexandrium, and accumulated by shellfish. Paralytic Shellfish Poisoning can be as mild as tingling and numbness, and severe enough that it leads to paralysis and respiratory failure (74, 118). In the same way, domoic acid, a neurotoxin of diatoms such as Pseudo-nitzschia, leads to amnesic shellfish poisoning, which impacts the central nervous system. Amnesic Shellfish Poisoning signs include memory impairment, particularly confusion, short-term memory loss, and, in extreme form, experiential shock, seizures, and death (119).
These poisons in the food chain increase the risk to consumers’ health, particularly for those, including fishermen, who consume marine products. This issue has been well documented, especially in coastal regions, where seafood is a staple in most local diets. This situation has created a need to enhance the surveillance of toxin levels in commercially and recreationally harvested shellfish (74). Inhalation and Dermal Contact Apart from ingestion, the second mode of exposure is inhalation of aerosolized microalgae toxins during games in or near water bodies that contain the toxins (118). Cyanobacterial blooms can produce gas-borne toxins, and these are inhaled, hence causing respiratory manifestations. The aerosolized microcystins can trigger allergies, asthma, and other respiratory problems like headaches, watery or bloody noses, dizziness, vomiting, diarrhea, skin rash, and, in severe cases, hives and pneumonia in children and the elderly (120). Leisure activities such as swimming, boating, and water sports put the individual at a greater risk of inhaling the toxins present in water and or getting in direct contact with the toxins through the skin, which enhances their health perils (118). Microalgae toxins are also dangerous when dermally administered, and this happens especially when one comes into direct contact with the water, such as when swimming (115). The primary effects of the lyngbyatoxins present in the cyanobacteria include skin rash, skin irritation, and skin inflammation (120). The above-presented dermatological symptoms are typical complaints by people who swim in water bodies containing harmful algal blooms (121). While skin contact is usually less dangerous than contacting the substance with the mouth or nose or breathing it in, it still causes severe distress and, with persistent irritation, followed by infection (74). Table 2 depicts the summary of major microalgal toxins in the food products, maximum limits, and their toxicity data.
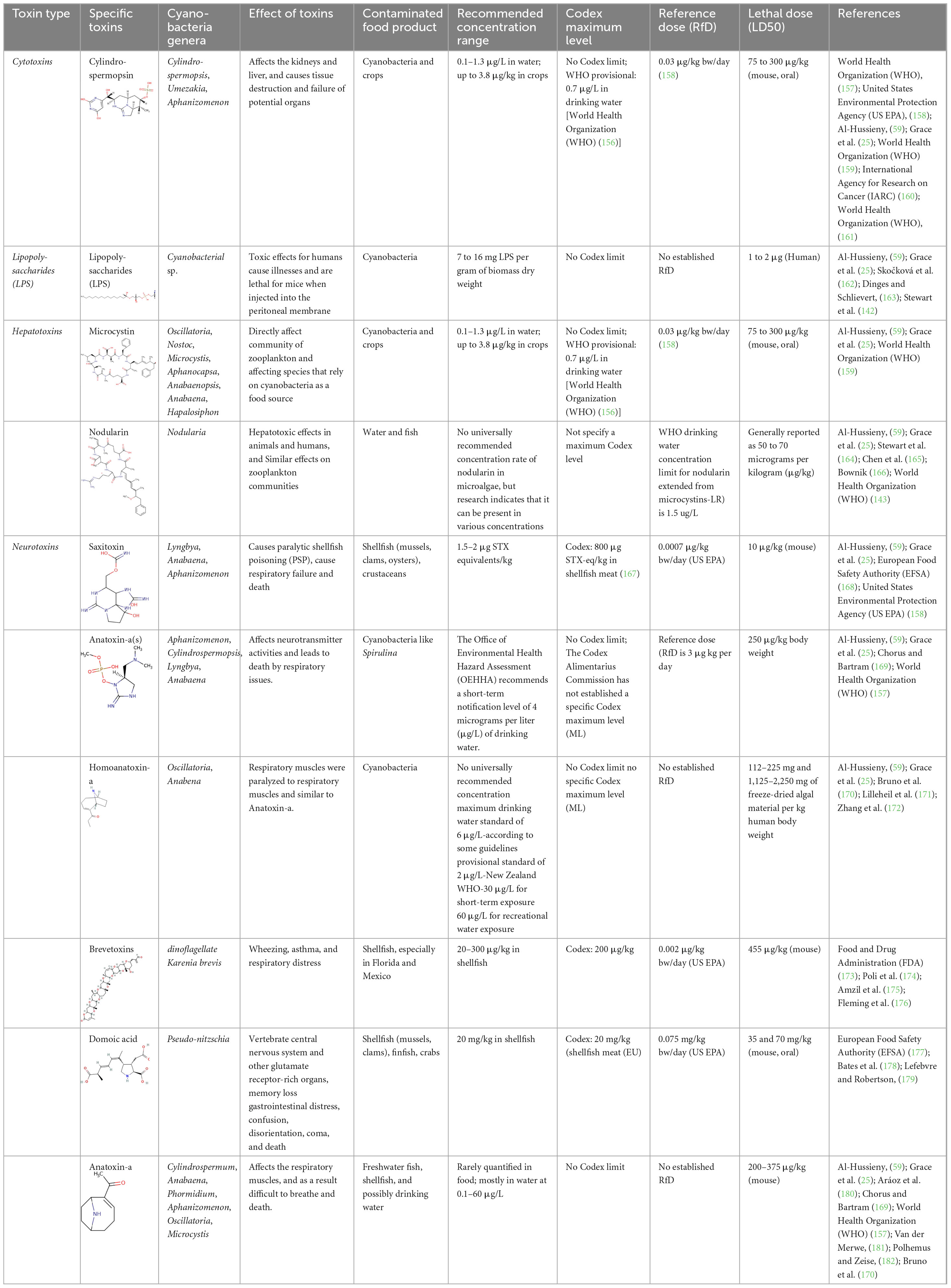
Table 2. Summary of main microalgal toxins in food products, cyanobacterial genera, toxicity data, and effect of the toxins.
5 Impact of microalgae toxins on human health
A current concern arising from microalgal toxins is hepatotoxicity and gastrointestinal illness, often linked to microcystins synthesized by the cyanobacterium Microcystis aeruginosa (122). A detailed illustration showing the impacts is shown in Table 2. Microcystins are very resistant and tend to concentrate in water reservoirs, and their ingestion through contaminated food or drinking water due to algal blooms poses a threat to the liver (85, 123). Symptoms of toxicity/exposure, acute dermal and oral exposure result in vomiting, abdominal pain, and nausea, while over-exposure to chronic systemic conditions results in hepatotoxicity, tumor formation, among other diseases due to its strong hepatotoxicity (124).
This toxin is also very stable, and normal water treatment systems may not fully eliminate the microcystins, presenting a continued threat to communities using such water (122). In addition to being toxic, these microalgae exhibit neurotoxic influences—saxitoxins and domoic acid- causing manifold health risks (85). The toxins that cause paralytic shellfish poisoning (PSP) include saxitoxins, which are produced by Alexandrium and other species and result in a tingling sensation to paralysis and failure in respiration (125). Another neurotoxin that is closely associated with species including Pseudo-nitzschia is domoic acid, which can cause amnesic shellfish poisoning (ASP), which affects the hippocampus in the brain, impairing memory and learning capability (126). These neurotoxicity effects can be much worse in some susceptible categories of the population, such as children, the elderly, and immunocompromised persons (125).
Transmission pathways and second-order threats, besides other types of exposure (for instance, bioaccumulation in fish products), are also considered to threaten public health (122). Hazardous toxins can be found in fish, shellfish, and other seafood; those toxins bioaccumulate and move up the food chain toward human consumers (127). This bioaccumulation is somewhat different from other toxin concentrations as toxins may remain in the seafood even when there are no signs of algae blooms evident (128). For instance, surveillance schemes in the Baltic Sea have established that microcystin toxins may become incorporated within fish tissues, thereby posing risks through the consumption of contaminated fish outside the bloom season (124).
Thus, where waterborne exposure to microalgal toxins may be possible, toxic compounds are also aerosolized in coastal environments, posing significant risks to respiratory health (122). Research has shown aerosolized cyanotoxins, which, once inhaled, can penetrate the respiratory system, resulting in respiratory inflammation, asthma, and chronic lung disease (129). This form of exposure mainly affects coastal people and those who work closely with water, such as lifeguards and fishers (127).
According to the available documentation, there are clinical records regarding the food poisoning events caused by the contamination of microalgal toxins in different countries. As shown in Hinder et al. (55), 56 patients were identified with various toxic effects while 6 patients died due to the consumption of seafoods containing noxious substances, with ages 5–94 years, from 1998 through 2009 in the United Kingdom (55). Kim et al. (130) also show that with the available data of the Asia-Pacific Countries, shellfish that accumulate toxins when ingested by humans can cause diverse symptoms (diarrhea and muscle pain) and even death (130). Based on the available literature data till 2024, it clearly shows that six allergic reactions, at least 70 illnesses, and 14 mortality records have been globally recorded (131). As mitigation strategies are better than the cure, studies regarding these microalgal toxins is a timely action.
Public Health and Management Implications showed the different consequences of microalgal toxins and also pointed to a dire need to come up with ways to tackle the issue, given that it has both long- and short-term effects on the health of those who consume them. Toxic microalgal monitoring surveillance programs for water, seafood, and coastal atmosphere are crucial in preventing microbial access to communities (74). Innovations like hand-held optical equipment relevant to the remote detection of toxin-producing algae at the bloom formation scene could decrease exposure dangers (123). Furthermore, raising awareness of how to avoid consuming seafood contaminated by hazardous algal blooms or toxins can considerably decrease people’s exposure (132). Consequently, microalgal toxins are widely adverse and have numerous effects that relate to human health, and they can affect all age groups regardless of direct or indirect contact. Since climate change poses a potential threat to hazardous algal bloom, observing and explaining activities coupled with technological advancement can help to manage health-related hazards, as shown in Table 2 (85). More efforts are required in the cross-disciplinary analysis of toxicity to garner more information on the toxicity, diagnostic techniques, and prevention measures that would protect particular risk groups (85).
6 Detection and mitigation strategies
Humans are currently at a significant risk from the HAB, due to rising seafood consumption. Consuming contaminated fish, seafood products, or water could expose humans to harmful toxins produced by HABs causing respiratory illness, memory loss, seizures, digestive tract problems, and skin irritation and also fatalities (133). Due to this possible impact of HAB, the detection of harmful microalgal toxins has become essential for human health protection because which provides a major risk to human health (134).
There are different mitigation strategies to eliminate the extracellular toxins present in the microalgae. Different technologies utilize chemical and physical methods, such as activated carbon adsorption and membrane filtration, as well as chemical inactivation through the application of oxidants like chlorine, potassium permanganate, ozone, or ultraviolet light (135). These approaches harness the inherent abilities of diverse microorganisms, such as macrophytes, microalgae, macroalgae, bacteria, viruses, actinomycetes, and pathogens, to regulate HABs (136). Effective monitoring and early identification are essential for controlling risks from aquatic environments, toxin-producing microalgae. Moreover, regulation of nutrients, specifically carbon, nitrogen, and phosphorus, is considered an important long-term strategy for preventing the formation of hazardous blooms, which are made worse by eutrophication (137).
6.1 Detection methods
In recent times, there are different types of detection methods such as chemical methods, biochemical methods, molecular methods, biosensors to detect the microalgal toxins. LC-MS/MS, HPLC, HPCE are the chemical techniques utilized for the detect aquatic algal toxins. These methods have separation efficiency, low solvent cost, small sample volume, and ability to detect multiple toxin groups. However, these have limitations such as high technical complexity, high cost, long operating time, and requires an expert in the field to operate. Biochemical assays such as PPIA, ELISA, and cell-based assays provide specificity, sensitivity, and speed (134). Compared to traditional microscopic identification and numeration methods, molecular method like quantitative PCR (qPCR) allows for the simultaneous amplification and detection of specific DNA sequences, and its objectivity, sensitivity, and specificity make it suitable for routine monitoring of toxic algae (138). as well as high sensitivity, quick turnaround time, resilience, affordability, ease of use, accuracy, and low power needs of biosensors offer attractive options to get beyond the limitations of traditional detection quantification techniques (133).
6.2 Public awareness
Public awareness campaigns can significantly minimize human exposure to cyanotoxins either due to recreational activities, drinking untreated water or consuming seafood. In addition, limiting the risk of bacterial bloom formation can be achieved by implementing good social practices and avoiding the disposal of organic and inorganic waste near water sources. Under nationally supported programs in developing countries, media coverage of how climate change affects the safety of food ingested could be implemented. It is an urgent need to strengthen the aware the children and the public community about these microalgal toxins from their early childhood, because for children, it can lead to an intellectual disability due to their poisonous effect. In addition to knowledge sharing sessions, such as workshops, global networks programs, and research discussions to aware the community can be identified as a timely need to mitigate this microalgae toxin. Moreover, governmental and non-governmental organizations should interfere in the microalgae-related toxins, and they can organize awareness programs (139). In contrast, regulations should be established by governments to make sure that undesirable industrial effluents are correctly cleaned up before they enter aquatic bodies. Only awareness is not applied, or restrictions on recreational activities, or the prohibition of any water-related activity, depend on the detected levels of the monitored toxin. Indeed, having a categorical risk to cyanotoxins classification (low, medium, and high) will help choose the appropriate action (135).
6.3 Consumer protection and regulatory models
In 1998, the WHO established a provisional TDI for chronic exposure to MC-LR of 0.04 g/kg body weight and a provisional guideline value of 1 g/L in drinking water (cell-bound and extra-cellular toxins) to protect the public from the harmful effects of cyanotoxins. For MC-LR, CYN, STXs, and ANTX, WHO suggested revised provisional guidelines in 2020. To better reflect the health effects, temporary guidelines were modified. However, the provisional TDI remained the same for MC-LR.
It has been suggested that to measure the risk accurately, the concentration of MCs present should be considered. A new provisional recommendation of 12 g/L has been recommended for short-term exposure, but the 1 g/L value for drinking water is used for long-term exposure. Due to a lack of long-term toxicological evidence, ANTX was only given a provisional guideline value of 30 g/L for short-term exposure in drinking water. In contrast, three provisional guideline values have been assigned to CYN. Guidelines for drinking water exposure have been established at 0.7 g/L for short-term exposure and 3 g/L for long-term exposure. Provisional standards for drinking water at 3 g/L have been sent to STX (135).
Certain groups of people are more affected than others regarding the adverse health effects of microalgae toxins, such as children, the elderly, persons with chronic diseases, or other compromised health conditions of the body (74). Therefore, there is potential for high exposure to people living in areas where the dependent water resources are surface waters or in areas where seafood is a staple food (7). Current findings indicate that Dichlorobenzene exposure at sub-chronic levels and below the toxicity level may also lead to chronic health issues. Including liver cancer and neurological disorders (116). An area of special interest is the ability of microalgae toxins to act as environmental carcinogens. Researchers have identified that deficient proteins in cells regulated by microcystins enhance the growth of liver tumors (85). Acute exposure to low-concentration toxins can integrate over time and cause cumulative injuries. Hence, populations with long-term exposure are at a higher propensity to have cancer-related ailments (112). When considering all these mitigation strategies, it shows that public/consumer awareness and regulatory models are the best strategies to mitigate this microalgal toxin for humans.
7 Future direction
Despite extensive research, several knowledge gaps hinder our comprehensive understanding of microalgae toxins in food products and their implications for human health. These gaps limit the ability to develop effective mitigation strategies and ensure food safety. Considering these gaps, conducting more studies in the future is essential.
Regarding the production of toxins, several microalgae species are still not fully understood and it is a timely need to research in this field. Toxins such as domoic acid, saxitoxins, and microcystins are commonly identified (140) but there may be others that are unidentified, especially in lower-studied microalgae species. So, it is important to thoroughly profile different microalgae species for potential toxins, which is a highlighted research gap. Also, the production of toxins is significantly influenced by environmental factors, including temperature, salinity, light intensity, and nutrient availability. Prediction and monitoring endeavors are made more difficult by the incomplete understanding of the specific conditions and mechanisms causing this variability. So, clarifying the environmental factors that contribute to the production of toxins requires multidisciplinary research that integrates oceanography, climate science, and microbiology. Also, to monitor changes in the patterns of algal blooms and their toxin profiles, long-term monitoring programs should be established in the location.
There are significant gaps in the knowledge of these toxins’ bioconcentration, bioaccumulation, and bioamplification, as well as the impact of detoxication and covalent binding of microcystins on transfer in the food web, despite the abundance of fundamental data regarding their concentrations in freshwater food webs.
Different detection techniques have been developed to detect microalgae toxins to date, but currently detection techniques frequently lack the sensitivity, specificity, and efficiency necessary for regular monitoring of various toxin types. In general, different detection techniques are appropriate for other purposes (141). So, toxin detection can be improved by advances in biotechnology and analytical chemistry. Furthermore, methods like biosensors, high-resolution mass spectrometry, and assays based on nanoparticles should be improved for rapid, accurate, and cost-effective toxin screening, which are emerging requirements that show the knowledge gaps in this field.
Conclusion
Even though there is a huge demand for microalgae-incorporated food and dietary supplements in the community, there is still a risk of microalgal toxin poisoning via these food products. As these toxins can cause life-threatening health issues in both humans and animals, it is important to identify these toxin-producing microalgal species, the types of toxins they produce, their biochemical pathways, and the environmental and population factors that influence toxin production. Moreover, understanding the exposure pathways to these toxins and their mode of action is crucial to avoid and treat associated health implications. Apart from that, these microalgal toxins cause critical damage to the environment and the economy. Hence, developing effective detection and mitigation strategies is essential to fight against microalgal toxins. Effective monitoring and early detection of microalgal toxins in natural ecosystems can drastically reduce the risk of human exposure to microalgal toxins. Raising public awareness is also important to address the root causes of HABs. Additionally, imposing regulations as those established by the WHO plays a major role in setting guidelines for safe exposure levels in food and water. The above study shows the need to conduct multidisciplinary research strategies to prevent microalgal toxin contamination and mitigation techniques under commercial food production regarding microalgae.
Author contributions
WW: Writing – review and editing, Writing – original draft. DG: Writing – review and editing, Writing – original draft. DD: Writing – original draft, Writing – review and editing. AR: Writing – review and editing, Writing – original draft. HZ: Writing – review and editing, Writing – original draft. VW: Writing – review and editing, Writing – original draft.
Funding
The authors declare that financial support was received for the research and/or publication of this article. This study was supported by the Science and Technology Human Resource Development Project, Ministry of Higher Education, Sri Lanka, funded by the Asian Development Bank (Grant number R2-RJ3).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. De Medeiros VPB, da Costa WKA, da Silva RT, Pimentel TC, Magnani M. Microalgae as source of functional ingredients in new-generation foods: Challenges, technological effects, biological activity, and regulatory issues. Crit Rev Food Sci Nutr. (2022) 62:4929–50. doi: 10.1080/10408398.2021.1879729
2. Blackburn S. I., Lee-Chang K. J. (2018). “Microalgae: A renewable resource for food and fuels and more,” in blue biotechnology, ed. M. Wink, 1–32. Hoboken, NJ: Wiley. doi: 10.1002/9783527801718.ch1
3. Molino A, Iovine A, Casella P, Mehariya S, Chianese S, Cerbone A, et al. microalgae characterization for consolidated and new application in human food, animal feed and nutraceuticals. Int J Environ Res Public Health. (2018) 15:2436. doi: 10.3390/ijerph15112436
4. Khavari F, Saidijam M, Taheri M, Nouri F. Microalgae: Therapeutic potentials and applications. Mol Biol Rep. (2021) 48:4757–65. doi: 10.1007/s11033-021-06422-w
5. Yarkent Ç, Gürlek C, Oncel SS. Potential of microalgal compounds in trending natural cosmetics: A review. Sustainable Chem Pharm. (2020) 17:100304. doi: 10.1016/j.scp.2020.100304
6. Han P, Lu Q, Fan L, Zhou W. A review on the use of microalgae for sustainable aquaculture. Appl Sci. (2019) 9:2377. doi: 10.3390/app9112377
7. Zhang J, Zhang J, Zeng J, Gui Y, Xie F, Dai B, et al. Algal toxicity and food chain transport characteristics of three common bisphenols and their mixtures. Sci Total Environ. (2024) 937:173481. doi: 10.1016/j.scitotenv.2024.173481
8. Udayan A, Pandey AK, Sharma P, Sreekumar N, Kumar S. Emerging industrial applications of microalgae: Challenges and future perspectives. Syst Microbiol Biomanufacturing. (2021) 1:411–31. doi: 10.1007/s43393-021-00038-8
9. Caporgno MP, Mathys A. Trends in microalgae incorporation into innovative food products with potential health benefits. Front Nutr. (2018) 5:58. doi: 10.3389/fnut.2018.00058
10. Čmiková N, Kowalczewski P, Kmiecik D, Tomczak A, Drożdżyńska A, Ślachciński M, et al. Characterization of selected microalgae species as potential sources of nutrients and antioxidants. Foods. (2024) 13:2160. doi: 10.3390/foods13132160
11. Vahdati SN, Behboudi H, Tavakoli S, Aminian F, Ranjbar R. Antimicrobial potential of the green microalgae isolated from the persian gulf. Iranian J Public Health. (2022) 51:1134–42. doi: 10.18502/ijph.v51i5.9428
12. Pereira L, Cotas J, Valado A. Antioxidants from microalgae and their potential impact on human well-being. Exploration Drug Sci. (2024) 2:292–321. doi: 10.37349/eds.2024.00048
13. Carbone DA, Pellone P, Lubritto C, Ciniglia C. Evaluation of microalgae antiviral activity and their bioactive compounds. Antibiotics. (2021) 10:746. doi: 10.3390/antibiotics10060746
14. Fritzsche S, Blenk P, Christian J, Castiglione K, Becker AM. Inhibitory properties of crude microalgal extracts on the in vitro replication of cyprinid herpesvirus 3. Sci Rep. (2021) 11:23134. doi: 10.1038/s41598-021-02542-2
15. Andrade KM, Lauritano C, Romano G, Ianora A. Marine microalgae with anti-cancer properties. Mar Drugs. (2018) 16:165. doi: 10.3390/md16050165
16. El-Hack MEA, Abdelnour S, Alagawany M, Abdo M, Sakr MA, Khafaga AF, et al. Microalgae in modern cancer therapy: Current knowledge. Biomed Pharmacother. (2019) 111:42–50. doi: 10.1016/j.biopha.2018.12.069
17. Choo W-T, Teoh M-L, Phang S-M, Convey P, Yap W-H, Goh B-H, et al. Microalgae as potential anti-inflammatory natural product against human inflammatory skin diseases. Front Pharmacol. (2020) 11:1086. doi: 10.3389/fphar.2020.01086
18. Gallego R, Valdés A, Suárez-Montenegro ZJ, Sánchez-Martínez JD, Cifuentes A, Ibáñez E, et al. Anti-inflammatory and neuroprotective evaluation of diverse microalgae extracts enriched in carotenoids. Algal Res. (2022) 67:102830. doi: 10.1016/j.algal.2022.102830
19. Ampofo J, Abbey L. Microalgae: Bioactive composition, health benefits, safety and prospects as potential high-value ingredients for the functional food industry. Foods (2022) 11:1744. doi: 10.3390/foods11121744
20. Wei H, Yundi S, Nan L, Runjie Z, Naishun B, Bin M, et al. Synergy effect of environmental factors on the growth and toxins production by Microcystis aeruginosa. Nat Environ Pollut Technol. (2021) 20:891–8. doi: 10.46488/NEPT.2021.v20i02.052
21. Katırcıoğlu H, Akin BS, Atici T. Microalgal toxin (s): Characteristics and importance. Afr J Biotechnol. (2004) 3:667–74.
22. Lad A, Breidenbach JD, Su RC, Murray J, Kuang R, Mascarenhas A, et al. As we drink and breathe: Adverse health effects of microcystins and other harmful algal bloom toxins in the liver, gut, lungs and beyond. Life. (2022) 12:418. doi: 10.3390/life12030418
23. Morabito S, Silvestro S, Faggio C. How the marine biotoxins affect human health. Natural Product Res. (2018) 32:621–31. doi: 10.1080/14786419.2017.1329734
24. Turner AD, Lewis AM, Bradley K, Maskrey BH. Marine invertebrate interactions with harmful algal blooms – Implications for one health. J Invertebrate Pathol. (2021) 186:107555. doi: 10.1016/j.jip.2021.107555
25. Grace D, Makita K, Kang’ethe E, Bonfoh B, Roesel K. Taking Food Safety to Informal Markets. In Food Safety and Informal Markets. Milton Park: Routledge (2014). p. 11–22.
26. Lim CC, Yoon J, Reynolds K, Gerald LB, Ault AP, Heo S, et al. Harmful algal bloom aerosols and human health. EBioMedicine. (2023) 93:104604. doi: 10.1016/j.ebiom.2023.104604
27. Nielsen MC, Jiang SC. Can cyanotoxins penetrate human skin during water recreation to cause negative health effects? Harmful Algae. (2020) 98:101872. doi: 10.1016/j.hal.2020.101872
28. Mokoena MM. Microcystins in water containers used in the home: A review of their potential health effects. Ecotoxicol Environ Saf. (2024) 269:115787. doi: 10.1016/j.ecoenv.2023.115787
29. García-Corona JL, Fabioux C, Vanmaldergem J, Petek S, Derrien A, Terre-Terrillon A, et al. The amnesic shellfish poisoning toxin, domoic acid: The tattoo of the king scallop Pecten maximus. Harmful Algae. (2024) 133:102607. doi: 10.1016/j.hal.2024.102607
30. Rzymski P, Niedzielski P, Kaczmarek N, Jurczak T, Klimaszyk P. The multidisciplinary approach to safety and toxicity assessment of microalgae-based food supplements following clinical cases of poisoning. Harmful Algae. (2015) 46:34–42. doi: 10.1016/j.hal.2015.05.003
31. Figueroa Torres G, Bermejo-Padilla E, Pittman J, Theodoropoulos C. Microalgae strain catalogue: a strain selection guide for microalgae users: cultivation and chemical characteristics for high added-value products. 3rd ed. Zenodo (2021). p. 163. doi: 10.5281/zenodo.5034149
32. Wu JY, Tso R, Teo HS, Haldar S. The utility of algae as sources of high value nutritional ingredients, particularly for alternative/complementary proteins to improve human health. Front Nutr. (2023) 10:1277343. doi: 10.3389/fnut.2023.1277343
33. Mathivanan B, Kumaragurubaran B, Raj JB, Veerasigamani M. Performance, combustion and emissions characteristics of lanthanum zirconate coated DI engine fueled with nannochloropsis algae bio-diesel containing tin oxide nanoparticles. Petroleum Sci Technol. (2025) 43:77–92. doi: 10.1080/10916466.2024.2391996
34. Panchal SK, Heimann K, Brown L. Improving Undernutrition with Microalgae. Nutrients. (2024) 16:3223. doi: 10.3390/nu16183223
35. Mata TM, Martins AA, Caetano Nidia S. Microalgae for biodiesel production and other applications: A review. Renew Sustain Energy Rev. (2010) 14:217–32. doi: 10.1016/j.rser.2009.07.020
36. Klamczynska B, Mooney WD. Heterotrophic microalgae. In: <snm>Nadathur S</gnm>, <snm>Wanasundara J</gnm>, <snm>Scanlin L</gnm> editors. Sustainable Protein Sources. Amsterdam: Elsevier (2017). p. 327–39. doi: 10.1016/B978-0-12-802778-3.00020-2
37. Patel AK, Singhania RR, Dong C-D, Obulisami PK, Sim SJ. Mixotrophic biorefinery: A promising algal platform for sustainable biofuels and high value coproducts. Renew Sustain Energy Rev. (2021) 152:111669. doi: 10.1016/j.rser.2021.111669
38. Chekanov K. Diversity and distribution of carotenogenic algae in Europe: A review. Mar Drugs. (2023) 21:108. doi: 10.3390/md21020108
39. Tang J-Q, Shen Q-H, Han Y-Y, Wu Y, He X-F, Li D-W, et al. Analysis of research status and trends on marine benthic dinoflagellate toxins: A bibliometric study based on web of science database and VOSviewer. Environ Res. (2023) 238:117179. doi: 10.1016/j.envres.2023.117179
40. Eltanahy E, Torky A. CHAPTER 1. Microalgae as cell factories: Food and feed-grade high-value metabolites. In: <snm>Posten C</gnm>, <snm>Feng Chen S</gnm> editors. Microalgal Biotechnology. London: Royal Society of Chemistry (2021). p. 1–35. doi: 10.1039/9781839162473-00001
41. Cai J, Lovatelli A, Aguilar-Manjarrez J, Cornish L, Dabbadie L, Desrochers A, et al. Seaweeds and Microalgae: An Overview for Unlocking Their Potential in Global Aquaculture Development. Rome: Food & Agriculture Org (2021). 1229 p.
42. Zhang Y, Wu F, Gu J-D, He K, Fang Z, Liu X, et al. Dominance by cyanobacteria in the newly formed biofilms on stone monuments under a protective shade at the Beishiku Temple in China. Environ Res. (2024) 251:118576. doi: 10.1016/j.envres.2024.118576
43. Malavasi V, Soru S, Cao G. Extremophile microalgae: The potential for biotechnological application. J Phycol. (2020) 56:559–73. doi: 10.1111/jpy.12965
44. Martínez-Francés E, Escudero-Oñate C. Cyanobacteria and microalgae in the production of valuable bioactive compounds. In: <snm>Posten C</gnm>, <snm>Chen S</gnm> editors. Microalgal Biotechnology. Berlin: Springer (2018). doi: 10.5772/intechopen.74043
45. Zhang Y, Whalen JK, Cai C, Shan K, Zhou H. Harmful cyanobacteria-diatom/dinoflagellate blooms and their cyanotoxins in freshwaters: A nonnegligible chronic health and ecological hazard. Water Res. (2023) 233:119807. doi: 10.1016/j.watres.2023.119807
46. Rasmussen SA, Andersen AJC, Andersen NG, Nielsen KF, Hansen PJ, Larsen TO. Chemical diversity, origin, and analysis of phycotoxins. J Natural Products. (2016) 79:662–73. doi: 10.1021/acs.jnatprod.5b01066
47. Chatterjee S, Venturino E, Chakraborty S, Chattopadhyay J. A simple mathematical model for seasonal planktonic blooms. Math Methods Appl Sci. (2009) 32:1738–50. doi: 10.1002/mma.1109
48. Donk E, Van, Ianora A, Vos M. Induced defences in marine and freshwater phytoplankton: A review. Hydrobiologia. (2011) 668:3–19. doi: 10.1007/s10750-010-0395-4
49. Manali KM, Arunraj R, Kumar T, Ramya M. Detection of microcystin producing cyanobacteria in Spirulina dietary supplements using multiplex HRM quantitative PCR. J Appl Phycol. (2017) 29:1279–86. doi: 10.1007/s10811-016-1011-4
50. U.S. Food and Drug Administration (US FDA). GRAS Notice No. GRN 000127: Spirulina (Arthrospira platensis). Silver Spring, MD: Center for Food Safety and Applied Nutrition (CFSAN) (2003).
51. Buono S, Langellotti AL, Martello A, Rinna F, Fogliano V. Functional ingredients from microalgae. Food Funct. (2014) 5:1669–85. doi: 10.1039/C4FO00125G
52. García-Altares M. Structural Diversity of Microalgal Marine Toxins. Amsterdam: Elsevier (2017). p. 35–88. doi: 10.1016/bs.coac.2017.08.002
53. Wells ML, Potin P, Craigie JS, Raven JA, Merchant SS, Helliwell KE, et al. Algae as nutritional and functional food sources: Revisiting our understanding. J Appl Phycol. (2017) 29:949–82. doi: 10.1007/s10811-016-0974-5
54. Chorus I, Welker M. Toxic Cyanobacteria in Water: A Guide to Their Public Health Consequences, Monitoring and Management. Milton Park: Taylor & Francis (2021).
55. Hinder SL, Hays GC, Brooks CJ, Davies AP, Edwards M, Walne AW, et al. Toxic marine microalgae and shellfish poisoning in the British isles: History, review of epidemiology, and future implications. Environ Health (2011) 10:54. doi: 10.1186/1476-069X-10-54
56. Sinno-Tellier S, Abadie E, de Haro L, Paret N, Langrand J, Roux G, et al. Human poisonings by neurotoxic phycotoxins related to the consumption of shellfish: Study of cases registered by the French poison control centres from 2012 to 2019. Clin Toxicol. (2022) 60:759–67. doi: 10.1080/15563650.2022.2034840
57. Bouyahya A, Bakrim S, Chamkhi I, Taha D, Omari N, El, et al. Bioactive substances of cyanobacteria and microalgae: Sources, metabolism, and anticancer mechanism insights. Biomed Pharmacother. (2024) 170:115989. doi: 10.1016/j.biopha.2023.115989
58. Said TO, Zokm GM. Persistent Organic Pollutants in Aquatic Systems. Switzerland: Springer Nature (2024). doi: 10.1007/978-3-031-53341-9
59. Al-Hussieny AA. Algae toxins and their treatment. In: <snm>Zepka LQ</gnm>, <snm>Jacob-Lopes E</gnm>, <snm>Deprá M</gnm> editors. Progress in Microalgae Research - A Path for Shaping Sustainable Futures. London: IntechOpen (2022). doi: 10.5772/intechopen.102909
60. Garamszegi SP, Brzostowicki DJ, Coyne TM, Vontell RT, Davis DA. TDP-43 and Alzheimer’s Disease pathology in the brain of a harbor porpoise exposed to the cyanobacterial Toxin BMAA. Toxins. (2024) 16:42. doi: 10.3390/toxins16010042
61. Lei L, Liu W, Chen Z, Peng L, Xiao L, Han B, et al. Grazer-induced toxin production is energetically costly and significantly reduces growth of cylindrospermopsin-producing cyanobacteria. Limnol Oceanogr. (2024) 69:2929–40. doi: 10.1002/lno.12721
62. Kordahi MA, Ayoub GM, Zayyat RM. A critical review of current research on cyanobacterial cells and associated toxins in aquatic environments: Occurrence, impact, and treatment methods. J Environ Chem Eng. (2024) 12:113931. doi: 10.1016/j.jece.2024.113931
63. Mostafa S, El-Baroty G, Abdelbaky H, Fahmy H, Farag R. Biochemical and cytotoxicity evaluation of marine algae Sargassum wightii and Gracilaria edulis extracts. Egyptian J Chem. (2023) 67:525–34. doi: 10.21608/ejchem.2023.240294.8702
64. Cock IE, Cheesman MJ. A Review of the antimicrobial properties of cyanobacterial natural products. Molecules. (2023) 28:7127. doi: 10.3390/molecules28207127
65. Delawská K, Divoká P, Sedlák D, Kuzma M, Saurav K, Macho M, et al. New insights into tolytoxin effect in human cancer cells: Apoptosis induction and the relevance of Hydroxyl substitution of its macrolide cycle on compound potency. ChemBioChem. (2022) 23:e202100489. doi: 10.1002/cbic.202100489
66. Patterson GML, Smith CD, Kimura LH, Britton BA, Carmeli S. Action of tolytoxin on cell morphology, cytoskeletal organization, and actin polymerization. Cell Motility. (1993) 24:39–48. doi: 10.1002/cm.970240105
67. Patterson GML, Carmeli S. Biological effects of tolytoxin (6-hydroxy-7-O-methyl-scytophycin b), a potent bioactive metabolite from cyanobacteria. Arch Microbiol. (1992) 157:406–10. doi: 10.1007/BF00249096
68. Taylor EC, Hendess RW. Synthesis of Pyrrolo[2,3-d]pyrimidines. The Aglycone of Toyocamycin 1,2. J Am Chem Soc. (1965) 87:1995–2003. doi: 10.1021/ja01087a025
69. Ghosh PK, Ghosh C. Current avenues in nutraceuticals and pharmaceuticals from algae. Curr Nutraceuticals. (2023) 4:e180523217061. doi: 10.2174/2665978604666230518150209
70. Allingham JS, Klenchin VA, Rayment I. Actin-targeting natural products: Structures, properties and mechanisms of action. Cell Mol Life Sci. (2006) 63:2119–34. doi: 10.1007/s00018-006-6157-9
71. Janosik T, Rannug A, Rannug U, Wahlström N, Slätt J, Bergman J. Chemistry and properties of indolocarbazoles. Chem Rev. (2018) 118:9058–128. doi: 10.1021/acs.chemrev.8b00186
72. Bailly C, Dassonneville L, Colson P, Houssier C, Fukasawa K, Nishimura S, et al. Intercalation into DNA is not required for inhibition of topoisomerase I by indolocarbazole antitumor Agents1. Cancer Res. (1999) 59:2853–60.
73. Pierce LT. Design, Synthesis and Development of Novel Indolocarbazole Derivatives as Potential Anti-Cancer Agents. Irelnd: University College Cork (2011).
74. Valeriani F, Carraturo F, Lofrano G, Volpini V, Izzo MG, Bruno A, et al. Algae in Recreational waters: An overview within a one health perspective. Water. (2024) 16:946. doi: 10.3390/w16070946
75. Sulieman AME, Alanazi N, Abdelgadir AM, Haddad A, ELHag GA. Algal Toxins. In: <snm>Sulieman A</gnm>, <snm>Alshammari NI</gnm> editors. Microbial Toxins in Food Systems: Causes, Mechanisms, Complications, and Metabolism. Switzerland: Springer Nature (2024). p. 427–43. doi: 10.1007/978-3-031-62839-9_32
76. Landsberg JH. The effects of harmful algal blooms on aquatic organisms. Rev Fish Sci. (2002) 10:113–390. doi: 10.1080/20026491051695
77. Trainer VL, Kudela RM, Hunter MV, Adams NG, McCabe RM. Climate extreme seeds a new domoic acid hotspot on the US west coast. Front Climate. (2020) 2:571836. doi: 10.3389/fclim.2020.571836
78. Kim S-Y, Kim M, Lim YK, Baek SH, Kim JY, An K-G, et al. First investigation of the temporal distribution of neurotoxin β-N-methylamino-L-alanine (BMAA) and the candidate causative microalgae along the South Sea Coast of Korea. J Hazardous Mater. (2024) 478:135486. doi: 10.1016/j.jhazmat.2024.135486
79. Méjean A, Paci G, Gautier V, Ploux O. Biosynthesis of anatoxin-a and analogues (anatoxins) in cyanobacteria. Toxicon. (2014) 91:15–22. doi: 10.1016/j.toxicon.2014.07.016
80. Gribble MO, Bennett BJ, Liddie JM, Borchert W, Pfluger BA, Segars JS, et al. Global epidemiology of paralytic shellfish poisoning. In ISEE 2024: 36th Annual Conference of the International Society of Environmental Epidemiology. EHP Publishing (2024). doi: 10.1289/isee.2024.0030
81. Watanabe R, Oikawa H, Tsunemitsu T, Miyahara K, Ozawa M, Numano S, et al. A case of paralytic shellfish poisoning caused by consumption of visceral balls from geoduck Panopea japonica in Japan. Toxicon. (2024) 243:107738. doi: 10.1016/j.toxicon.2024.107738
82. Bouraoui Z, Dhraief MN, Amri A, Smach MA, Ammar J, Gharred T, et al. The potential role of exopolysaccharide from Graesiella sp (chlorophyte Microalga) as dietary supplement against Bisphenol A-induced hepatotoxicity in gilthead sea bream (Sparus aurata). N Zeal J Mar Freshw Res. (2024) 58:1–20. doi: 10.1080/00288330.2024.2394085
83. Carmichael WW. Health effects of toxin-producing cyanobacteria: “The CyanoHABs.”. Hum Ecol Risk Assess Int J. (2001) 7:1393–407. doi: 10.1080/20018091095087
84. Li Z, Zheng Y, Ma H, Cui F. Microcystin-LR (MC-LR) inhibits green algae growth by regulating antioxidant and photosynthetic systems. Harmful Algae. (2024) 134:102623. doi: 10.1016/j.hal.2024.102623
85. Chen S, Xie W, Lin X, Zhou H, Teng S, Jiang Z, et al. Controlling toxic Microcystis blooms: The power of a novel microalgal predator Poteriospumella lacustris in water safety improvement. J Cleaner Prod. (2024) 441:141011. doi: 10.1016/j.jclepro.2024.141011
86. Réveillon D, des Aulnois MG, Savar V, Robert E, Caruana AMN, Briand E, et al. Extraction and analysis by liquid chromatography – tandem mass spectrometry of intra- and extracellular microcystins and nodularin to study the fate of cyanobacteria and cyanotoxins across the freshwater-marine continuum. Toxicon. (2024) 237:107551. doi: 10.1016/j.toxicon.2023.107551
87. Vijayakumar VR, Ramesh L, Balakrishnan K, Dhanasekaran D. Detection of nodularin-producing cyanolichen by polymerase chain reaction (PCR). In: <snm>Thajuddin N</gnm>, <snm>Sankara A</gnm>, <snm>Dhanasekaran D</gnm> editors. Protocols for Cyanobacteria Sampling and Detection of Cyanotoxin. Singapore: Springer Nature (2023). p. 459–67. doi: 10.1007/978-981-99-4514-6_61
88. Rizwan M, Mujtaba G, Memon SA, Lee K, Rashid N. Exploring the potential of microalgae for new biotechnology applications and beyond: A review. Renew Sustain Energy Rev. (2018) 92:394–404. doi: 10.1016/j.rser.2018.04.034
89. Ye Q, Georges N, Selomulya C. Microencapsulation of active ingredients in functional foods: From research stage to commercial food products. Trends Food Sci Technol. (2018) 78:167–79. doi: 10.1016/j.tifs.2018.05.025
90. Ahirwar A, Rai A, Sirotiya V, Khandelwal P, Singh G, Jhadav D, et al. Fermentation of algal biomass for its nutritional value: Perspectives and revolutions in the food industry. Environ Technol Rev. (2024) 13:1–28. doi: 10.1080/21622515.2023.2283097
91. Sandgruber F, Gielsdorf A, Baur AC, Schenz B, Müller SM, Schwerdtle T, et al. Variability in macro- and micronutrients of 15 commercially available microalgae powders. Mar Drugs. (2021) 19:310. doi: 10.3390/md19060310
92. Barkallah M, Dammak M, Louati I, Hentati F, Hadrich B, Mechichi T, et al. Effect of Spirulina platensis fortification on physicochemical, textural, antioxidant and sensory properties of yogurt during fermentation and storage. LWT. (2017) 84:323–30. doi: 10.1016/j.lwt.2017.05.071
93. Cerón-García MC, González-López CV, Camacho-Rodríguez J, López-Rosales L, García-Camacho F, Molina-Grima E. Maximizing carotenoid extraction from microalgae used as food additives and determined by liquid chromatography (HPLC). Food Chem. (2018) 257:316–24. doi: 10.1016/j.foodchem.2018.02.154
94. Kejžar J, Hudobivnik MJ, Nečemer M, Ogrinc N, Rutar JM, Ulrih NP. Characterization of algae dietary supplements using antioxidative potential, elemental composition, and stable isotopes approach. Front Nutr. (2021) 7:618503. doi: 10.3389/fnut.2020.618503
95. Singh R, Upadhyay AK, Chandra P, Singh DP. Biotechnological application of algae in pharmaceuticals industries with special reference to Omega-3 fatty acid and human health. In Algae and Sustainable Technologies. Boca Raton, FL: CRC Press (2020). p. 29–42. doi: 10.1201/9781003001911-3
96. Chen H, Li T, Wang Q. Ten years of algal biofuel and bioproducts: Gains and pains. Planta. (2019) 249:195–219. doi: 10.1007/s00425-018-3066-8
97. Chaudry S, Bahri PA, Moheimani NR. Life cycle analysis of milking of microalgae for renewable hydrocarbon production. Comput Chem Eng. (2019) 121:510–22. doi: 10.1016/j.compchemeng.2018.11.019
98. Chew KW, Yap JY, Show PL, Suan NH, Juan JC, Ling TC, et al. Microalgae biorefinery: High value products perspectives. Bioresour Technol. (2017) 229:53–62. doi: 10.1016/j.biortech.2017.01.006
99. Enamala MK, Enamala S, Chavali M, Donepudi J, Yadavalli R, Kolapalli B, et al. Production of biofuels from microalgae - A review on cultivation, harvesting, lipid extraction, and numerous applications of microalgae. Renew Sustain Energy Rev. (2018) 94:49–68. doi: 10.1016/j.rser.2018.05.012
100. Hu J, Nagarajan D, Zhang Q, Chang J-S, Lee D-J. Heterotrophic cultivation of microalgae for pigment production: A review. Biotechnol Adv. (2018) 36:54–67. doi: 10.1016/j.biotechadv.2017.09.009
101. Khan MA, Ngo HH, Guo W, Liu Y, Zhang X, Guo J, et al. Biohydrogen production from anaerobic digestion and its potential as renewable energy. Renewable Energy. (2018) 129:754–68. doi: 10.1016/j.renene.2017.04.029
102. Kothari R, Pandey A, Ahmad S, Kumar A, Pathak VV, Tyagi VV. Microalgal cultivation for value-added products: A critical enviro-economical assessment. 3 Biotech. (2017) 7:243. doi: 10.1007/s13205-017-0812-8
103. Papadaki S, Kyriakopoulou K, Tzovenis I, Krokida M. Environmental impact of phycocyanin recovery from Spirulina platensis cyanobacterium. Innov Food Sci Emerg Technol. (2017) 44:217–23. doi: 10.1016/j.ifset.2017.02.014
104. Smetana S, Sandmann M, Rohn S, Pleissner D, Heinz V. Autotrophic and heterotrophic microalgae and cyanobacteria cultivation for food and feed: Life cycle assessment. Bioresour Technol. (2017) 245:162–70. doi: 10.1016/j.biortech.2017.08.113
105. Soni RA, Sudhakar K, Rana RS. Spirulina – From growth to nutritional product: A review. Trends Food Sci Technol. (2017) 69:157–71. doi: 10.1016/j.tifs.2017.09.010
106. Hwang JA, Islam MM, Ahmed ST, Mun HS, Kim GM, Kim YJ, et al. Seamustard (lt;i;gt;Undaria pinnatifida lt;/i gt) improves growth, immunity, fatty acid profile and reduces cholesterol in hanwoo steers. Asian Australasian J Anim Sci. (2014) 27:1114–23. doi: 10.5713/ajas.2014.14072
107. Salehipour-Bavarsad F, Nematollahi MA, Pistocchi R, Pezzolesi L. Algal food safety: Possible contaminations, challenges of harmonized quality assessments, and suggested recommendations for the nascent industry of microalgae-based products. Algal Res. (2024) 81:103579. doi: 10.1016/j.algal.2024.103579
108. Messineo V, Bruno M, Pace R, De The role of cyano-HAB (Cyanobacteria harmful algal blooms) in the one health approach to global health. Hydrobiology. (2024) 3:238–62. doi: 10.3390/hydrobiology3030016
109. Davidović P, Blagojević D, Meriluoto J, Simeunović J, Svirčev Z. Biotests in cyanobacterial toxicity assessment—Efficient enough or not? Biology. (2023) 12:711. doi: 10.3390/biology12050711
110. Alhaithloul HAS, Mohamed ZA, Saber AA, Alsudays IM, Abdein MA, Alqahtani MM, et al. Performance evaluation of Moringa oleifera seeds aqueous extract for removing Microcystis aeruginosa and microcystins from municipal treated-water. Front Bioeng Biotechnol. (2024) 11:1329431. doi: 10.3389/fbioe.2023.1329431
111. Anderson DM, Glibert PM, Burkholder JM. Harmful algal blooms and eutrophication: Nutrient sources, composition, and consequences. Estuaries. (2002) 25:704–26. doi: 10.1007/BF02804901
112. Falconer IR. Toxic cyanobacterial bloom problems in Australian waters: Risks and impacts on human health. Phycologia. (2001) 40:228–33. doi: 10.2216/i0031-8884-40-3-228.1
113. Wei N, Hu C, Dittmann E, Song L, Gan N. The biological functions of microcystins. Water Res. (2024) 262:122119. doi: 10.1016/j.watres.2024.122119
114. Wells ML, Trainer VL, Smayda TJ, Karlson BSO, Trick CG, Kudela RM, et al. Harmful algal blooms and climate change: Learning from the past and present to forecast the future. Harmful Algae. (2015) 49:68–93. doi: 10.1016/j.hal.2015.07.009
115. Zhang Z, Xu M, Fan Y, Zhang L, Wang H. Using microalgae to reduce the use of conventional fertilizers in hydroponics and soil-based cultivation. Sci Total Environ. (2024) 912:169424. doi: 10.1016/j.scitotenv.2023.169424
116. Chen J, Xie P. Tissue distributions and seasonal dynamics of the hepatotoxic microcystins-LR and -RR in two freshwater shrimps, Palaemon modestus and Macrobrachium nipponensis, from a large shallow, eutrophic lake of the subtropical China. Toxicon. (2005) 45:615–25. doi: 10.1016/j.toxicon.2005.01.003
117. Hilborn R, Amoroso RO, Bogazzi E, Jensen OP, Parma AM, Szuwalski C, et al. When does fishing forage species affect their predators? Fish Res. (2017) 191:211–21. doi: 10.1016/j.fishres.2017.01.008
118. Van Apeldoorn ME, van Egmond HP, Speijers GJA, Bakker GJI. Toxins of cyanobacteria. Mol Nutr Food Res. (2007) 51:7–60. doi: 10.1002/mnfr.200600185
119. Perl TM, Bédard L, Kosatsky T, Hockin JC, Todd ECD, Remis RS. An outbreak of toxic encephalopathy caused by eating mussels contaminated with domoic acid. N Engl J Med. (1990) 322:1775–80. doi: 10.1056/NEJM199006213222504
120. Wood SA, Rueckert A, Hamilton DP, Cary SC, Dietrich DR. Switching toxin production on and off: Intermittent microcystin synthesis in a Microcystis bloom. Environ Microbiol Rep. (2011) 3:118–24. doi: 10.1111/j.1758-2229.2010.00196.x
121. Mostafa AA, Zedan AMG, Elbasiouny H. Effect of Wastewater on Lakes Degradation in Egypt: Challenge and Management. Berlin: Springer (2024). p. 345–65. doi: 10.1007/698_2024_1104
122. Igwaran A, Kayode AJ, Moloantoa KM, Khetsha ZP, Unuofin JO. Cyanobacteria harmful algae blooms: Causes, impacts, and risk management. Water Air Soil Pollut. (2024) 235:71. doi: 10.1007/s11270-023-06782-y
123. De Jesús Martínez-Roldán A, Ibarra-Berumen J. Employment of Wastewater to Produce Microalgal Biomass as a Biorefinery Concept. In: <snm>Alam A</gnm>, <snm>Wang Z</gnm> editors. Microalgae Biotechnology for Development of Biofuel and Wastewater Treatment. Singapore: Springer (2019). p. 487–504. doi: 10.1007/978-981-13-2264-8_19
124. Dey P, Nayeem J, Dey SK, Debi D, Zemi NZ, Islam SMR, et al. Comparative dataset on productivity, proximate, biochemical and pigment content of marine Gonyostomum sp. and freshwater Tetraedron sp. microalgae. Data Brief. (2024) 54:110393. doi: 10.1016/j.dib.2024.110393
125. Cheng S, Jessica, Yoshikawa K, Cross JS. Influence of synthetic and natural microfibers on the growth, substance exchange, energy accumulation, and oxidative stress of field-collected microalgae compared with microplastic fragment. Sci Total Environ. (2024) 908:167936. doi: 10.1016/j.scitotenv.2023.167936
126. Ma M, Hu Q. Microalgae as feed sources and feed additives for sustainable aquaculture: Prospects and challenges. Rev Aquacult. (2024) 16:818–35. doi: 10.1111/raq.12869
127. Barkia I, Saari N, Manning SR. Microalgae for high-value products towards human health and nutrition. Mar Drugs. (2019) 17:304. doi: 10.3390/md17050304
128. Magalhães V, Pinto V, Sousa P, Afonso JA, Gonçalves L, Fernández E, et al. A portable and low-cost optical device for pigment-based taxonomic classification of microalgae using machine learning. Sensors Actuators B Chem. (2025) 423:136819. doi: 10.1016/j.snb.2024.136819
129. Reid N, Reyne MI, O’Neill W, Greer B, He Q, Burdekin O, et al. Unprecedented Harmful algal bloom in the UK and Ireland’s largest lake associated with gastrointestinal bacteria, microcystins and anabaenopeptins presenting an environmental and public health risk. Environ Int. (2024) 190:108934. doi: 10.1016/j.envint.2024.108934
130. Kim Y-S, An H-J, Kim J, Jeon Y-J. Current situation of palytoxins and cyclic imines in asia-pacific countries: Causative phytoplankton species and seafood poisoning. Int J Environ Res Public Health. (2022) 19:4921. doi: 10.3390/ijerph19084921
131. Kyarimpa C, Omute T, Nakiguli CK, Khanakwa AV, Angiro C, Kahwa I, et al. Safety, Toxicological and Allergenic Aspects of Using Algae for Food. Berlin: Springer (2024). p. 745–69. doi: 10.1007/978-981-97-2371-3_25
132. Hu W, Su S, Mohamed HF, Xiao J, Kang J, Krock B, et al. Assessing the global distribution and risk of harmful microalgae: A focus on three toxic Alexandrium dinoflagellates. Sci Total Environ. (2024) 948:174767. doi: 10.1016/j.scitotenv.2024.174767
133. Chin Chwan Chuong JJ, Rahman M, Ibrahim N, Heng LY, Tan LL, Ahmad A. Harmful microalgae detection: Biosensors versus some conventional methods. Sensors. (2022) 22:3144. doi: 10.3390/s22093144
134. Massey IY, Wu P, Wei J, Luo J, Ding P, Wei H, et al. A mini-review on detection methods of microcystins. Toxins. (2020) 12:1–32. doi: 10.3390/toxins12100641
135. Abdallah MF, Hassel WHR, Van Andjelkovic M, Wilmotte A, Rajkovic A. Cyanotoxins and food contamination in developing countries: Review of their types, toxicity, analysis, occurrence and mitigation strategies. Toxins (2021) 13:786. doi: 10.3390/toxins13110786
136. Anabtawi HM, Lee WH, Al-Anazi A, Mohamed MM, Hassan AA. Advancements in biological strategies for controlling harmful algal blooms (HABs). Water. (2024) 16:224. doi: 10.3390/w16020224
137. Anderson DM. Approaches to monitoring, control and management of harmful algal blooms (HABs). Ocean Coastal Manag. (2009) 52:342–7. doi: 10.1016/j.ocecoaman.2009.04.006
138. Pearson LA, D’Agostino PM, Neilan BA. Recent developments in quantitative PCR for monitoring harmful marine microalgae. Harmful Algae. (2021) 108:102096. doi: 10.1016/j.hal.2021.102096
139. Roland HB, Whitehead C, Fleming LE, Berdalet E, Enevoldsen HO, Gribble MO. Knowledge sharing to reduce toxin exposure risks from harmful algal blooms: Global networks and political barriers. Ethnicity Dis. (2022) 32:285–92. doi: 10.18865/ed.32.4.285
140. Caruana AMN, Amzil Z. Microalgae and toxins. In: <snm>Levine I</gnm>, <snm>Fleurence J</gnm> editors. Microalgae in Health and Disease Prevention. Amsterdam: Elsevier (2018). p. 263–305. doi: 10.1016/B978-0-12-811405-6.00013-X
141. Liu F, Zhang C, Wang Y, Chen G. A review of the current and emerging detection methods of marine harmful microalgae. Sci Total Environ. (2022) 815:152913. doi: 10.1016/j.scitotenv.2022.152913
142. Stewart I, Schluter PJ, Shaw GR. Cyanobacterial lipopolysaccharides and human health – a review. Environ Health. (2006) 5:7. doi: 10.1186/1476-069X-5-7
143. World Health Organization (WHO). Guidelines for Drinking-Water Quality, Addendum. 2nd ed. Geneva: WHO (2002).
144. Oshiro N, Gago-Martínez A, Tubaro A. Chemistry, toxicology and etiology of marine biotoxins. J Mar Sci Eng. (2024) 12:236. doi: 10.3390/jmse12020236
145. Araújo R, Calderón FV, López JS, Azevedo IC, Bruhn A, Fluch S, et al. Current status of the algae production industry in europe: An emerging sector of the blue bioeconomy. Front Mar Sci. (2021) 7:626389. doi: 10.3389/fmars.2020.626389
146. Fradique M, Batista A, Nunes M, Gouveia L, Bandarra N, Raymundo A. Incorporation of Chlorella vulgaris and Spirulina maxima biomass in pasta products. Part 1: Preparation and evaluation. J Sci Food Agric. (2010) 90:1656–64. doi: 10.1002/jsfa.3999
147. Lucas B, de Morais M, Santos T, Costa J. Spirulina for snack enrichment: Nutritional, physical and sensory evaluations. LWT. (2018) 90:270–6. doi: 10.1016/j.lwt.2017.12.032
148. Mostolizadeh S, Moradi Y, Mortazavi M, Motallebi A, Ghaeni M. Effects of incorporation Spirulina platensis (Gomont, 1892) powder in wheat flour on chemical, microbial and sensory properties of pasta. IFRO. (2020) 19:410–20. doi: 10.22092/ijfs.2019.119107
149. Uribe-Wandurraga Z, Igual M, Reino-Moyón J, García-Segovia P, Martínez-Monzó J. Effect of microalgae (Arthrospira platensis and Chlorella vulgaris) addition on 3D printed cookies. Food Biophys. (2021) 16:27–39. doi: 10.1007/s11483-020-09642-y
150. García-Vaquero M, Brunton N, Lafarga T. Microalgae as a source of pigments for food applications. in Cultured Microalgae for the Food Industry, eds T. Lafarga, G. Acien Amsterdam: Elsevier (2021). doi: 10.1016/B978-0-12-821080-2.00014-9
151. García-Segovia P, Pagán-Moreno M, Lara I, Martínez-Monzó J. Effect of microalgae incorporation on physicochemical and textural properties in wheat bread formulation. Food Sci Technol Int. (2017) 23:437–47. doi: 10.1177/1082013217700259
152. Hlaing S, Sadiq M, Anal A. Enhanced yield of Scenedesmus obliquus biomacromolecules through medium optimization and development of microalgae based functional chocolate. J Food Sci Technol. (2020) 57:1090–9. doi: 10.1007/s13197-019-04144-3
153. Carrasco-Reinado R, Escobar A, Carrera C, Guarnizo P, Vallejo RA, Fernández-Acero FJ. Valorization of microalgae biomass as a potential source of high-value sugars and polyalcohols. LWT. (2019) 114:108385. doi: 10.1016/j.lwt.2019.108385
154. De Oliveira TTB, dos Reis IM, de Souza MB, da Silva Bispo E, Maciel LF, Druzian JI., et al. Microencapsulation of Spirulina sp. LEB-18 and its incorporation in chocolate milk: Properties and functional potential. LWT. (2021) 148:111674. doi: 10.1016/j.lwt.2021.111674
155. Aljobair MO, Albaridi NA, Alkuraieef AN, AlKehayez NM. Physicochemical properties, nutritional value, and sensory attributes of a nectar developed using date palm puree and spirulina. Int J Food Properties. (2021) 24:845–58. doi: 10.1080/10942912.2021.1938604
156. World Health Organization (WHO) (editor). Cyanobacterial toxins: Anatoxin-a and analogues. in Cyanobacterial Toxins: Anatoxin-A and Analogues. Geneva: WHO (2020).
157. World Health Organization (WHO) Background Document for Development of WHO Guidelines for Drinking-Water Quality: Cylindrospermopsin. Geneva: WHO (2020)
158. United States Environmental Protection Agency (US EPA). Toxicological Reviews of Cylindrospermopsin (CAS No. 143545-90-8). Washington, DC: US EPA (2006).
159. World Health Organization (WHO) Cyanobacterial Toxins: Microcystin-LR in Drinking-Water. Background Document for Development of WHO GUIDELINES for Drinking-Water Quality. Geneva: WHO (2006)
160. International Agency for Research on Cancer (IARC) Monographs on the Evaluation of Carcinogenic Risks to Humans: Volume 94 – Ingested Nitrate and Nitrite, and Cyanobacterial Peptide Toxins. Lyon: IARC (2010)
161. World Health Organization (WHO) Toxic Cyanobacteria in Water: A Guide to their Public Health Consequences, Monitoring and Management. Geneva: WHO (1998)
162. Skočková V, Vašíček O, Sychrová E, Sovadinová I, Babica P, Šindlerová L. Cyanobacterial harmful bloom lipopolysaccharides induce pro-inflammatory effects in immune and intestinal epithelial cells in vitro. Toxins. (2023) 15:169. doi: 10.3390/toxins15030169
163. Dinges MM, Schlievert PM. Comparative analysis of lipopolysaccharide-induced tumor necrosis factor alpha activity in serum and lethality in mice and rabbits pretreated with the Staphylococcal Superantigen toxic shock syndrome toxin 1. Infect Immunity. (2001) 69:7169–72. doi: 10.1128/IAI.69.11.7169-7172.2001
164. Stewart I, Eaglesham GK, McGregor GB, Chong R, Seawright AA, Wickramasinghe WA First report of a toxic Nodularia spumigena (Nostocales/ Cyanobacteria) bloom in sub-tropical Australia. II. bioaccumulation of nodularin in isolated populations of mullet (Mugilidae). Int J Environ Res Public Health. (2012) 9:2412–43. doi: 10.3390/ijerph9072412
165. Chen Y, Shen D, Fang D. Nodularins in poisoning. Clin Chim Acta. (2013) 425:18–29. doi: 10.1016/j.cca.2013.07.005
166. Bownik A. Harmful algae: Effects of cyanobacterial cyclic peptides on aquatic invertebrates-a short review. Toxicon. (2016) 124:26–35. doi: 10.1016/j.toxicon.2016.10.017
167. European Union Commission Regulation (EU) No 15/2011 of 10 January 2011 amending Regulation (EC) No 2074/2005. Brussels: European Union (2011)
168. European Food Safety Authority (EFSA). Marine biotoxins in shellfish – Saxitoxin group. EFSA J. (2009) 7:1019. doi: 10.2903/j.efsa.2009.1019
169. Chorus I, Bartram J. Toxic Cyanobacteria in Water: A Guide to Their Public Health Consequences, Monitoring and Management (1st ed.). Boca Raton, FL: CRC Press (1999). doi: 10.1201/9781482295061
170. Bruno M, Ploux O, Metcalf JS, Mejean A, Pawlik- Skowronska B, Furey A. Anatoxin- a, homoanatoxin- a, and natural analogues. In Handbook of Cyanobacterial Monitoring and Cyanotoxin Analysis, eds J. Meriluoto, L. Spoof, GA. Codd (138–147). Hoboken, NJ: Wiley (2016). doi: 10.1002/9781119068761.ch13
171. Lilleheil G, Andersen RA, Skulberg OM, Alexander J. Effects of a homoanatoxin-a-containing extract from oscillatoria formosa (cyanophyceae/cyanobacteria) on neuromuscular transmission. Toxicon. (1997) 35:1275–89. doi: 10.1016/S0041-0101(97)00013-5
172. Zhang X, Kuča K, Dohnal V, Dohnalová L, Wu Q, Wu C. Military potential of biological toxins. J Appl Biomed. (2014) 12:63–77. doi: 10.1016/j.jab.2014.02.005
173. Food and Drug Administration (FDA). Fish and Fishery Products Hazards and Controls Guidance. 3rd ed. Washington, DC: U.S. Department of Health and Human Services (2005)
174. Poli MA, Musser SM, Dickey RW, Eilers PP, Hall S. Neurotoxic shellfish poisoning and brevetoxin metabolites: A case study from Florida. Toxicon. (2000) 38:981–93. doi: 10.1016/S0041-0101(99)00191-9
175. Amzil Z, Derrien A, Terre Terrillon A, Duval A, Connes C, Marco-Miralles F. Monitoring the emergence of algal toxins in shellfish: First report on detection of brevetoxins in french mediterranean mussels. Marine Drugs. (2021) 19:393. doi: 10.3390/md19070393
176. Fleming LE, Kirkpatrick B, Backer LC, Bean JA, Wanner A, Reich A, et al. Aerosolized red-tide toxins (Brevetoxins) and asthma. Chest. (2007) 131:187–94. doi: 10.1378/chest.06-1830
177. European Food Safety Authority (EFSA). Scientific opinion on marine biotoxins: Domoic acid. EFSA J. (2009) 7:1181. doi: 10.2903/j.efsa.2009.1181
178. Bates SS, Bird CJ, de Freitas ASW, Foxall RA, Gilgan M, Hanic LA, et al. Pennate diatom Nitzschia pungens as the primary source of domoic acid, a neurotoxin in shellfish from eastern Prince Edward Island, Canada. Can J Fish Aquatic Sci. (1989) 46:1203–15. doi: 10.1139/f89-156
179. Lefebvre KA, Robertson A. Domoic acid and human exposure risks: A review. Toxicon. (2010) 56:218–30. doi: 10.1016/j.toxicon.2009.05.034
180. Aráoz R, Molgó J, Tandeau de Marsac N. Neurotoxic cyanobacterial toxins. Toxicon. (2010) 56:813–28. doi: 10.1016/j.toxicon.2009.07.036
181. Van der Merwe D. Cyanobacterial (blue-green algae) toxins. in Handbook of Toxicology of Chemical Warfare Agents, ed. R. Gupta. Amsterdam: Elsevier (2015). doi: 10.1016/B978-0-12-800159-2.00031-2
Keywords: microalgae, toxins, food products, impact, human health
Citation: Withana WATN, Gunarathna DMDI, Dissanayake DMGI, Rathnayake AI, de Zoysa HKS and Waisundara VY (2025) Microalgae toxins in food products and impact on human health: a review. Front. Nutr. 12:1603843. doi: 10.3389/fnut.2025.1603843
Received: 02 April 2025; Accepted: 20 June 2025;
Published: 11 July 2025.
Edited by:
Minaxi Sharma, University of Nottingham Ningbo China, ChinaReviewed by:
Chee Loong Teo, Agri Season Sdn Bhd, MalaysiaHang-kin Kong, Hong Kong Polytechnic University, Hong Kong SAR, China
Copyright © 2025 Withana, Gunarathna, Dissanayake, Rathnayake, de Zoysa and Waisundara. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: A. I. Rathnayake, YWlyYXRobmFAdGVjLnJqdC5hYy5saw==; Viduranga Y. Waisundara, VmlkdXJhbmdhLldhaXN1bmRhcmFAZWN1LmVkdS5saw==
 W. A. T. N. Withana
W. A. T. N. Withana D. M. D. I. Gunarathna
D. M. D. I. Gunarathna D. M. G. I. Dissanayake
D. M. G. I. Dissanayake A. I. Rathnayake
A. I. Rathnayake H. K. S. de Zoysa
H. K. S. de Zoysa Viduranga Y. Waisundara
Viduranga Y. Waisundara