- 1Department of Efficacy Evaluation, Centralbio Co., Ltd., Incheon, Republic of Korea
- 2Life Science Research Institute, Novarex Co., Ltd., Cheongju, Chungbuk, Republic of Korea
Background: Allergic asthma involves chronic inflammation, airway remodeling, and hyperresponsiveness. Inhaled corticosteroids combined with long-acting β2 agonists are effective; however, some patients experience side effects, highlighting the need for safer natural alternatives suitable for long-term use. Chinese quince (Q) and Saururus chinensis (SC) are used to treat various diseases, including asthma and inflammation. Q and SC extracts contain bioactive compounds that help modulate airway inflammation. Therefore, combining the two may enhance their immunomodulatory effects. However, the effects of a Q/SC mixture on allergic asthma remain unclear. The aim of this study is to assess the therapeutic effectiveness of a Q/SC mixture in treating asthma.
Methods: The therapeutic efficacy of the Q/SC extract was evaluated in an ovalbumin (OVA)-induced allergic airway inflammation model. After euthanasia, we assessed cell counts, cytokine expression in the bronchoalveolar lavage fluid (BALF), blood immunoglobulin (Ig) E levels, inflammatory cell infiltration, mucus production in the lung tissue, and the expression of protein and cytokine.
Results: A high-concentration Q/SC extract significantly reduced total cell and eosinophil counts, cytokine expression in BALF, and serum IgE levels. Furthermore, it reduced the expression of type 2 cytokines (IL-4, IL-5, IL-13) and inducible nitric oxide synthase in lung tissue. The extract also attenuated inflammatory cell infiltration and mucus production while inhibiting the STAT6 signaling pathway.
Conclusion: A high concentration of Q/SC extract effectively alleviates allergic airway inflammation by reducing eosinophilic inflammation, type 2 cytokine secretion, and mucus hyperproduction. This suggests that it could be a potential remedy for managing allergic airway inflammation.
1 Introduction
Asthma is a convoluted respiratory condition marked by heightened airway sensitivity, ongoing inflammation, and structural changes. These factors can lead to symptoms ranging from mild wheezing to severe, life-threatening obstructions (1). Importantly, despite advancements in understanding its underlying mechanisms, asthma remains a significant global health challenge (2, 3). Various allergens (air pollution and house dust mites) and viral infections can trigger asthma. The key pathological features include epithelial cell hyperplasia, mucus hypersecretion, pulmonary fibrosis, and inflammatory cell infiltration. These elements collectively contribute to disease progression and severity (4, 5). The mucus plays a vital function in the host defense of the airway; however, excessive mucus production can lead to airway obstruction and worsen various respiratory diseases. MUC5AC is important in mucus hyperproduction in asthma, with approximately 20 mucin genes involved in mucus secretion (6–8). Type 2 cytokines interleukin (IL)-4 and IL-13 induce mucus production in the airways, with IL-13 playing a significant role in excessive mucus production associated with asthma. The key transcription factor STAT6, triggered by IL-4 and IL-13 through the IL-4Rα subunit, is crucial in modulating MUC5AC gene expression (9, 10).
The inflammatory response in asthma is triggered by various inflammatory cells, including mast cells, B cells, T cells, neutrophils, eosinophils, and cytokines. T helper 2 (Th2) cells are highly involved in the development and progression of allergic asthma. Th2 cells release cytokines IL-4, IL-5, and IL-13, which stimulate immunoglobulin (Ig) E synthesis and recruit eosinophils to the site of inflammation. This process leads to excessive mucus secretion and airway inflammation (11). Therefore, Th2 cell immune regulation has been recognized as a promising therapeutic strategy for treating asthma and monoclonal antibody drugs that regulate type 2 cytokines have been approved (12). However, asthma and allergies have complex mechanisms involving many cells, making it potentially inadequate to target a single pathologic mechanism. Furthermore, the main pharmacological approach for treating asthma is the daily use of inhaled corticosteroids combined with long-acting β2 agonists. Inhaled corticosteroids combined with long-acting β2 agonists therapy achieves excellent results in most patients; nevertheless, approximately 10–25% of patients experience persistent asthma symptoms. Moreover, corticosteroid side effects, including pneumonia, hypertension, hyperlipidemia, myopathy, and cataracts, have been reported (13). Therefore, asthma treatments derived from natural products that are free of side effects and suitable for long-term use are needed.
Medicinal plant extracts may exert multiple effects rather than blocking a single cell or mechanism, increasing their potential for development as asthma treatments. Chinese quince (Q) is a medicinal plant species from the Rosaceae family. It has been used to treat various diseases in Japan, Korea, and China. Q extract contains bioactive compounds (phenolic and triterpene compounds) that are known to have antibacterial, anti-inflammatory, antihypertensive, neuroprotective, and antimutagenic effects (14–19). Furthermore, Q extract modulated airway inflammation in an OVA-induced allergic rhinitis model (20). Saururus chinensis (SC) is a perennial herb found in China and southern Korea, traditionally used to treat various inflammatory diseases, edema, and jaundice. SC extract showed anti-inflammation, anti-angiogenesis, anti-asthma, and anti-atopic dermatitis activities (21–24). The unique properties of these medicinal plant extracts show that their synergistic use may enhance their immunomodulatory and anti-inflammatory effects. However, the effects of a Q/SC mixture in asthma remain unclear. Therefore, the purpose of this study is to evaluate the therapeutic efficacy of a Q/SC mixture in asthma. In this study, we sought to provide insights that can guide the development of safer and more effective therapies for asthma and related inflammatory disorders by elucidating their combined mechanisms of action. To achieve this goal, OVA-induced allergic asthma models were generated and treated with the Q/SC mixture.
2 Materials and methods
2.1 Animal models
All trials were conducted using six-week-old female BALB/c mice (Orient Bio Ltd., Seongnam, Korea) housed in a pathogen-free facility. After a 7-day acclimation period in the animal facility, we used a mouse model that showed no physical signs of illness and gained weight normally. To create an allergic asthma mouse model, we mixed 50 μg of ovalbumin (OVA) (A5503, Sigma-Aldrich, St. Louis, MO, United States) and 1.32 mg of aluminum hydroxide (Alum) (Sigma-Aldrich) in 200 μL of phosphate-buffered saline (PBS). The mice were sensitized intraperitoneally twice at 1-week intervals. Two weeks post-sensitization, allergen exposure was administered via intratracheal injection of 50 μg of OVA daily for 7 days. Dexamethasone (DEX, 3 mg/kg) and the Q/SC extract (50, 100, and 200 mg/kg) were orally administered 1 h before the OVA challenge. DEX, a corticosteroid commonly prescribed for treating allergies and other respiratory disorders, served as the positive control. The normal control (NC) group was administered the same amount of PBS. The animals were euthanized 24 h after the final OVA exposure, and samples were collected for study.
2.2 Preparation of Chinese quince and Saururus chinensis extract
Q/SC extract was obtained from Novarex (Cheongju, Chungbuk, Korea). The fruits of Q and aerial parts of SC were used for the Q/SC extract. Ursolic acid and miquelianin were used as indicative compounds, with their quantities in the Q/SC extract analyzed using high-performance liquid chromatography (HPLC) to ensure the quality of the extraction process. C18 column (4.6 × 150 mm, 5 μm) and C18 column (4.6 × 250 mm, 5 μm) were used in HPLC.
2.3 Collection and preparation of bronchoalveolar lavage fluid, serum samples, and lung tissues
Bronchoalveolar lavage (BALF) collection and cytostaining from anesthetized mice were conducted as previously reported (25, 26). Following tracheal lavage, the lungs were immediately resected, and the left lung tissues were preserved in 10% (v/v) buffered formalin. A portion of the right lung tissue was placed in RNA later solution (AM7020, Invitrogen, Waltham, MA, United States), while the remaining tissues were stored in a tube at −80°C. Blood samples were obtained from the abdominal vein, and the serum was used for the OVA-specific IgE assay.
2.4 Enzyme-linked immunosorbent assay
A mouse IgE enzyme linked immunosorbent assay (ELISA) kit (Thermo Fisher Scientific, Waltham, MA, United States) was used to measure serum OVA-specific IgE levels. ELISA kits (R&D Systems, Minneapolis, MN, United States) were used to quantify the cytokines TNF-α, IL-17, and IL-1β in lung homogenates, while an ELISA kit (Cusabio Biotech, Wuhan, China) was used to measure inducible nitric oxide synthase (iNOS) levels. ELISA kits (R&D Systems) were also used to determine IL-4 and IL-13 levels in BALF. All analyses were conducted following the manufacturer’s guidelines.
2.5 Real-time quantitative polymerase chain reaction
As previously described, total RNA was extracted from a portion of the right lung tissue sample, and quantitative real-time RT-PCR was conducted (27). Target gene levels were normalized to Glyceraldehyde 3-phosphate dehydrogenase. The primer sequences are listed in Table 1.
2.6 Lung tissue histology
The left lung tissue, preserved in 10% (v/v) buffered formalin, was stained with hematoxylin and eosin to assess inflammatory cells. The periodic acid-Schiff (PAS) kit (ab150680, Abcam, Cambridge, United Kingdom)was used to stain goblet cells, while the toluidine blue protocol was used to stain the mast cells. Stained slides were analyzed using light microscopy, and the extent of goblet cell hyperplasia and inflammatory cell infiltration was scored using a subjective scale, as previously described (28–30). Mast cells were counted in toluidine blue-stained sections, with at least three regions analyzed per section.
2.7 Western blotting
Protein extraction and quantification from lung tissue, followed by WB analysis, were conducted as previously reported (31–33). The primary antibodies used were STAT6 (Cat# 9362, Cell Signaling, Beverly, MA, United States), p-STAT6 (Cat# 56554), and β-actin (Cat# 5125s). The detected immune-reactive bands were evaluated using ImageJ software.
2.8 Statistical analysis
Statistical analyses were conducted using GraphPad Prism version 10.2.2 (GraphPad Software, San Diego, CA, United States). Differences between multiple experimental groups were assessed using a one-way analysis of variance, with post-hoc Tukey and Dunnett tests conducted afterward. A p-value of <0.05 was regarded as statistically significant. Data are shown as the mean ± SEM.
3 Results
3.1 Effect of Q/SC extract on inflammatory cells infiltration in BALF
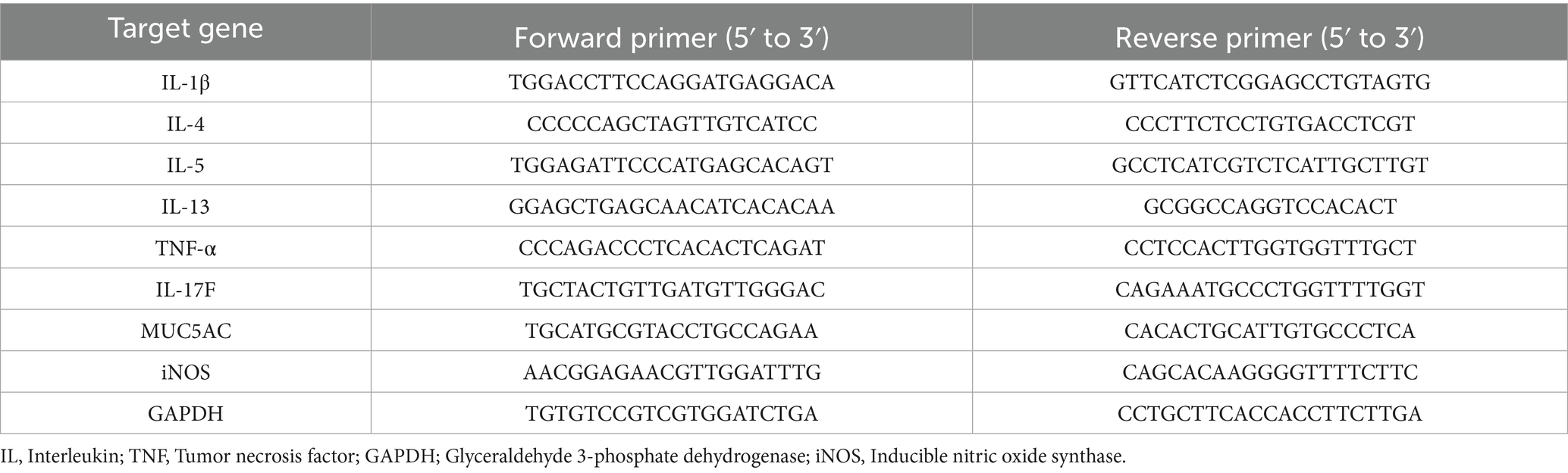
To determine the anti-inflammatory effect of Q/SC extract on allergic asthma, an allergic asthma mouse model was established. Cell infiltration in the BALF was more pronounced in the OVA-challenged group than it was in the NC group. The number of inflammatory cells, particularly eosinophils, was markedly higher in the OVA-challenged group. Conversely, the eosinophil counts were notably lower in the DEX and Q/SC groups. Furthermore, the total cell count and number of inflammatory cells, including lymphocytes and eosinophils, were notably higher in the OVA-challenged group than it was in the NC group. Meanwhile, compared with the OVA-challenged group, the Q/SC group had notably lower total cell count and number of eosinophils (Figures 1A–C).
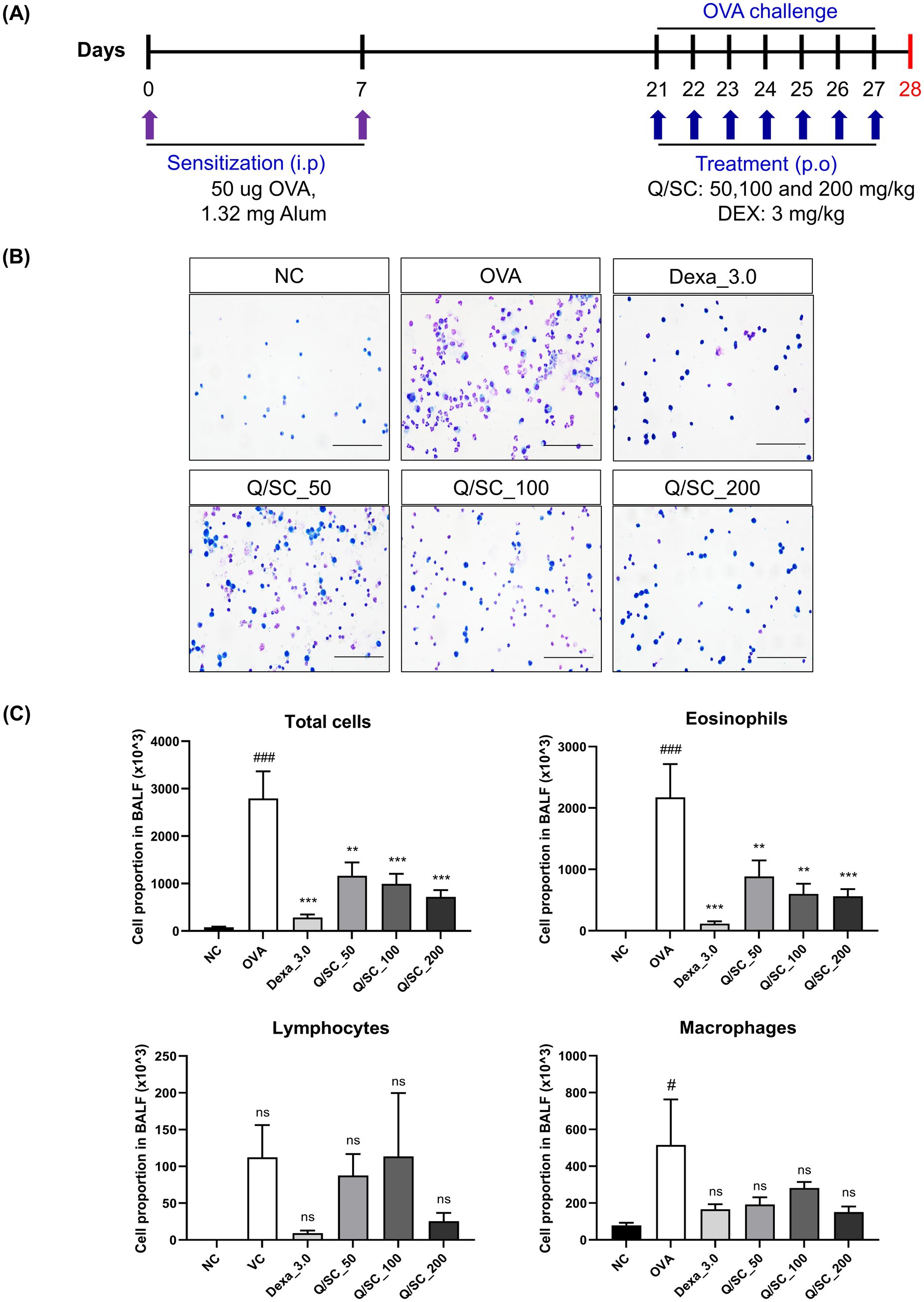
Figure 1. Experimental schedule for the asthma mouse model and the effect of Chinese quince/Saururus chinensis (Q/SC) extract on inflammation in bronchoalveolar lavage fluid (BALF). (A) Experimental procedure for the allergic asthma model and administration of dexamethasone (DEX) and Q/SC extract. (B) BALF cells are plated on clean glass slides and stained with Diff-Quik. Scale bar = 50 μm. (C) Total and differential cell counts are conducted using a cell counter under light microscopy. Data are shown as the means ± SEMs. ###p < 0.001 vs. the normal control group. ***p < 0.001 and **p < 0.01 vs. the ovalbumin-challenged group. NC, normal control; OVA, ovalbumin; DEX, dexamethasone; Q, Chinese quince; SC, Saururus chinensis; BALF, bronchoalveolar lavage fluid.
3.2 Effect of Q/SC on inflammatory cell recruitment and IgE production
The effect of Q/SC extract on airway inflammation was evaluated via tissue staining. Consequently, inflammatory cell infiltration was increased in the OVA-challenged group, whereas it was significantly reduced in the DEX, Q/SC-100, and Q/SC-200 treatment groups (Figures 2A,B). IgE is secreted from B cells activated by antigens and causes atopic dermatitis, allergic rhinitis, and asthma (12). To examine the influence of the Q/SC extract on IgE production, IgE serum levels were measured. Consequently, IgE levels were increased in the OVA-challenged group, whereas they were decreased in all Q/SC treatment groups (Figure 2C). The findings show that the Q/SC extract, particularly at doses of 100 mg/kg and 200 mg/kg, attenuated OVA-induced allergic asthma.
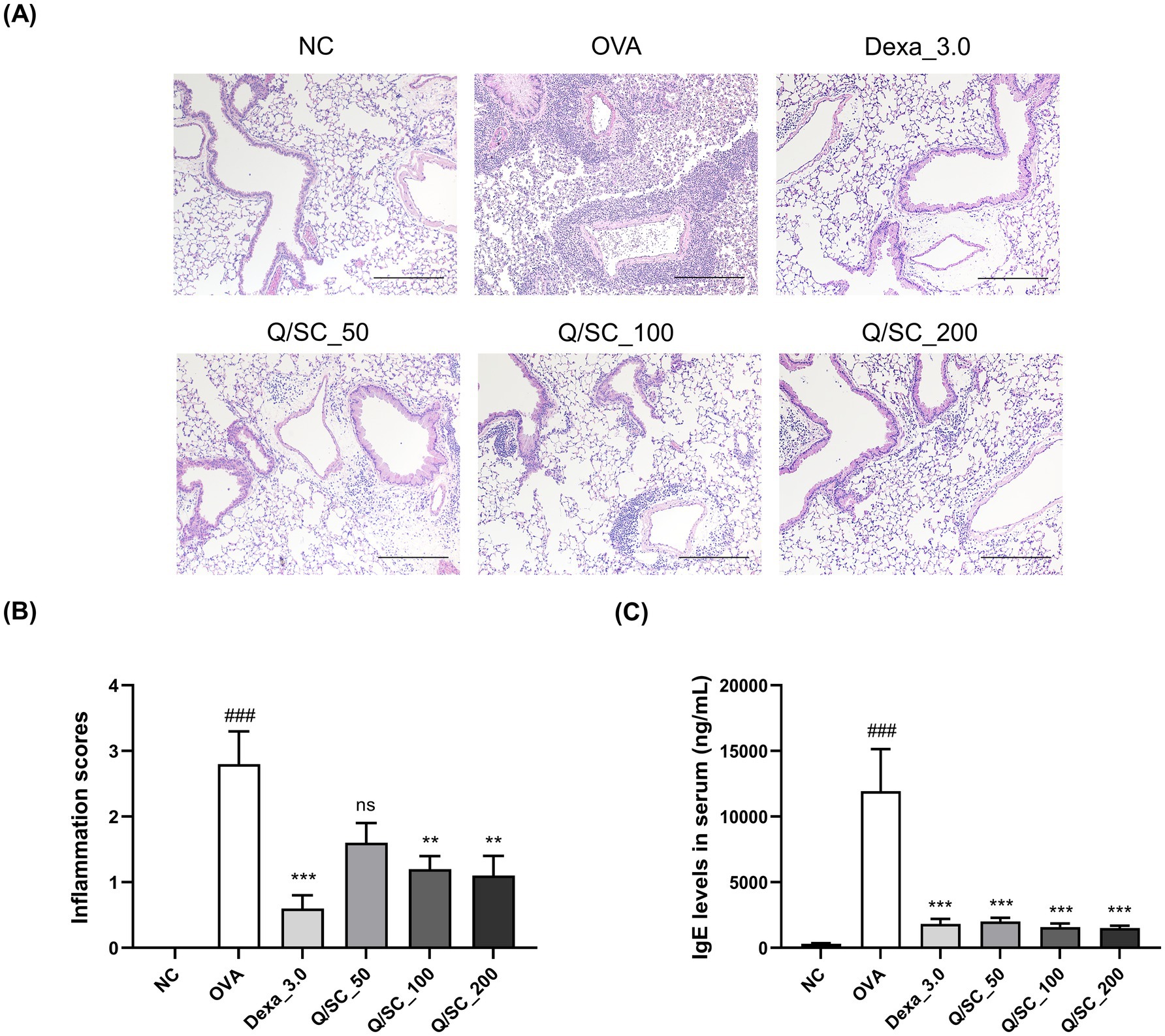
Figure 2. Effects of Chinese quince/Saururus chinensis (Q/SC) extract on immunoglobulin E (IgE) production and immune cell infiltration in ovalbumin (OVA)-induced allergic asthma mice. (A) The paraffin-embedded lung sections were stained with hematoxylin and eosin. Scale bar = 200 μm. (B) Lung inflammatory scores are determined using histological analysis of lung tissues. (C) Serum IgE levels are detected using enzyme-linked immunosorbent assay. Data are displayed as the means ± SEMs. ###p < 0.001 vs. normal control group. ***p < 0.001 and **p < 0.01 vs. OVA-challenged group. NC, normal control; OVA, ovalbumin; IgE, immunoglobulin E.
3.3 Effect of Q/SC extract on airway inflammation
Allergic asthma is primarily associated with type 2 immune responses, which are modulated by type 2 cytokines that induce pathological changes at the site of inflammation and exacerbate asthma symptoms (34). Therefore, to determine the effectiveness of Q/SC extract on allergic airway inflammation, the levels of type 2 and non-type 2 inflammatory cytokines in BALF and lung homogenates were measured using an ELISA kit. Compared with the NC group, the OVA-challenged group showed higher levels of type 2 and non-type 2 cytokines in BALF and lung homogenates. In BALF, IL-4 levels were decreased in the Q/SC-100 treatment group, and IL-13 levels were notably decreased in the Q/SC-100 and Q/SC-200 treatment groups (Figures 3A,B). Additionally, non-type 2 cytokines levels in lung homogenates were not reduced in the Q/SC treatment groups (Figures 3C–E). Increased iNOS expression is common in allergic airway inflammation; however, under normal conditions, iNOS is either absent or present in minimal amounts in most cell types and tissues. iNOS activity can be upregulated by various inflammatory factors, including allergen exposure, leading to bronchial hyperresponsiveness and contributing to eosinophil recruitment (35–37). In this study, iNOS production in lung homogenates was significantly lower in the Q/SC-200 treatment group than it was in the OVA-challenged group (Figure 3F). Furthermore, the effect of Q/SC extract was investigated by assessing mRNA expression levels for type 2 and non-type 2 mediated allergic responses in lung homogenates. The amount of type 2 cytokines, but not that of non-type 2 cytokines, was significantly reduced in the Q/SC-100 and Q/SC-200 treatment groups (Figures 4A–F). Like the ELISA results, the mRNA expression levels showed that iNOS expression was significantly decreased in the Q/SC-200 treatment group (Figure 4G). These findings show that Q/SC-100 and Q/SC-200 treatment alleviated airway inflammation by regulating type 2-related cytokines and iNOS production.
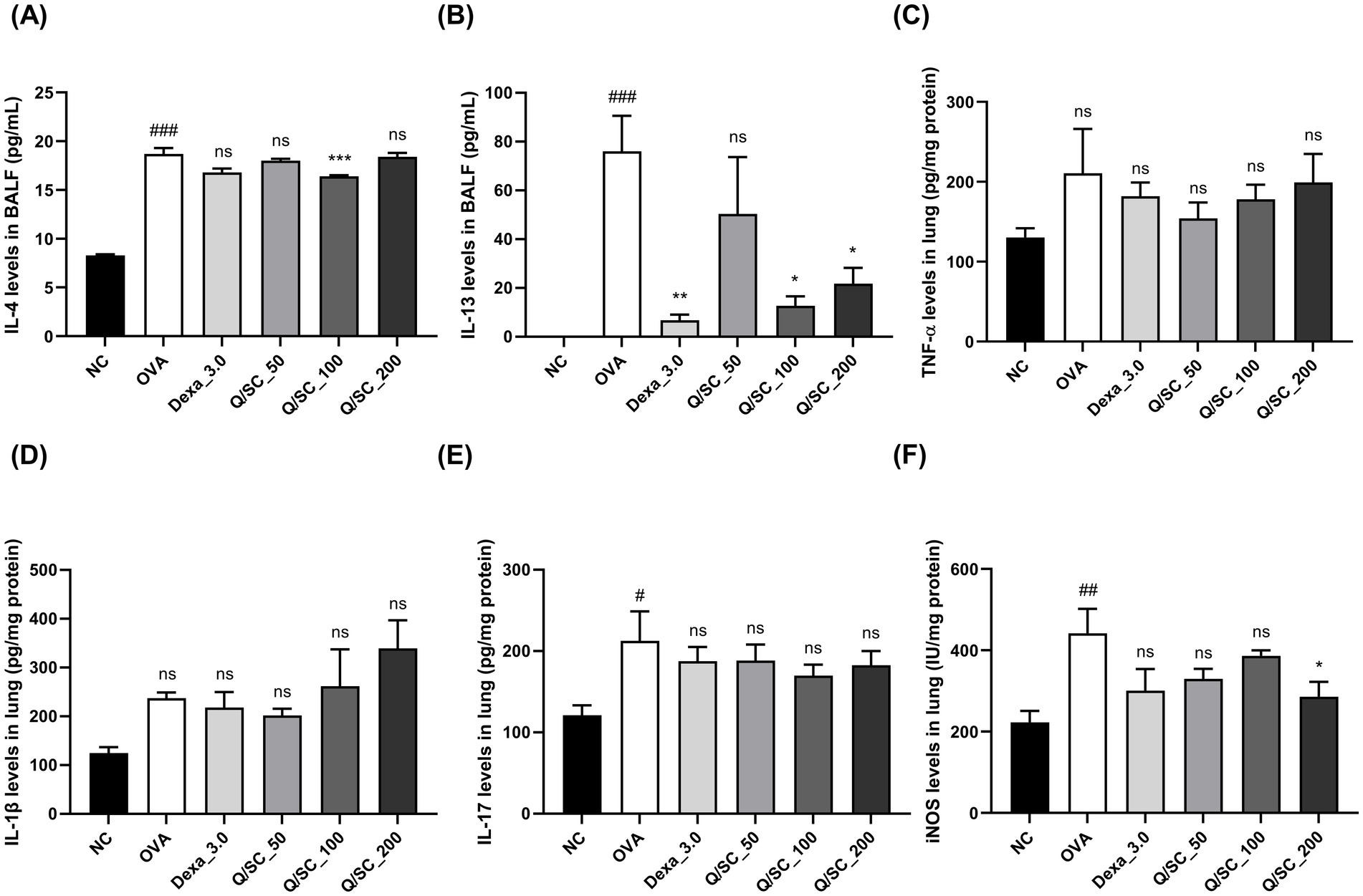
Figure 3. Effects of Chinese quince/Saururus chinensis (Q/SC) extract on non-type 2 and type 2 inflammation and inducible nitric oxide synthase (iNOS) expression in ovalbumin (OVA)-induced allergic asthma. (A,B) Enzyme-linked immunosorbent assay (ELISA) is conducted to detect the levels of interleukin (IL)-4 and IL-13, as type 2 cytokine levels in bronchoalveolar lavage fluid (BALF). (C–F) ELISA is also conducted to measure the levels of interleukin (IL)-1β, Tumor necrosis factor (TNF)-α, and IL-17, as non-type 2 cytokines, and iNOS expression in lung homogenates. Data are displayed as the means ± SEMs. ###p < 0.001, ##p < 0.01, and #p < 0.05 vs. the normal control group. ***p < 0.001, **p < 0.01, and *p < 0.05 vs. the OVA-challenged group. iNos, inducible nitric oxide synthase; OVA, ovalbumin; BALF, Bronchoalveolar lavage fluid; ELISA, Enzyme-linked immunosorbent assay.
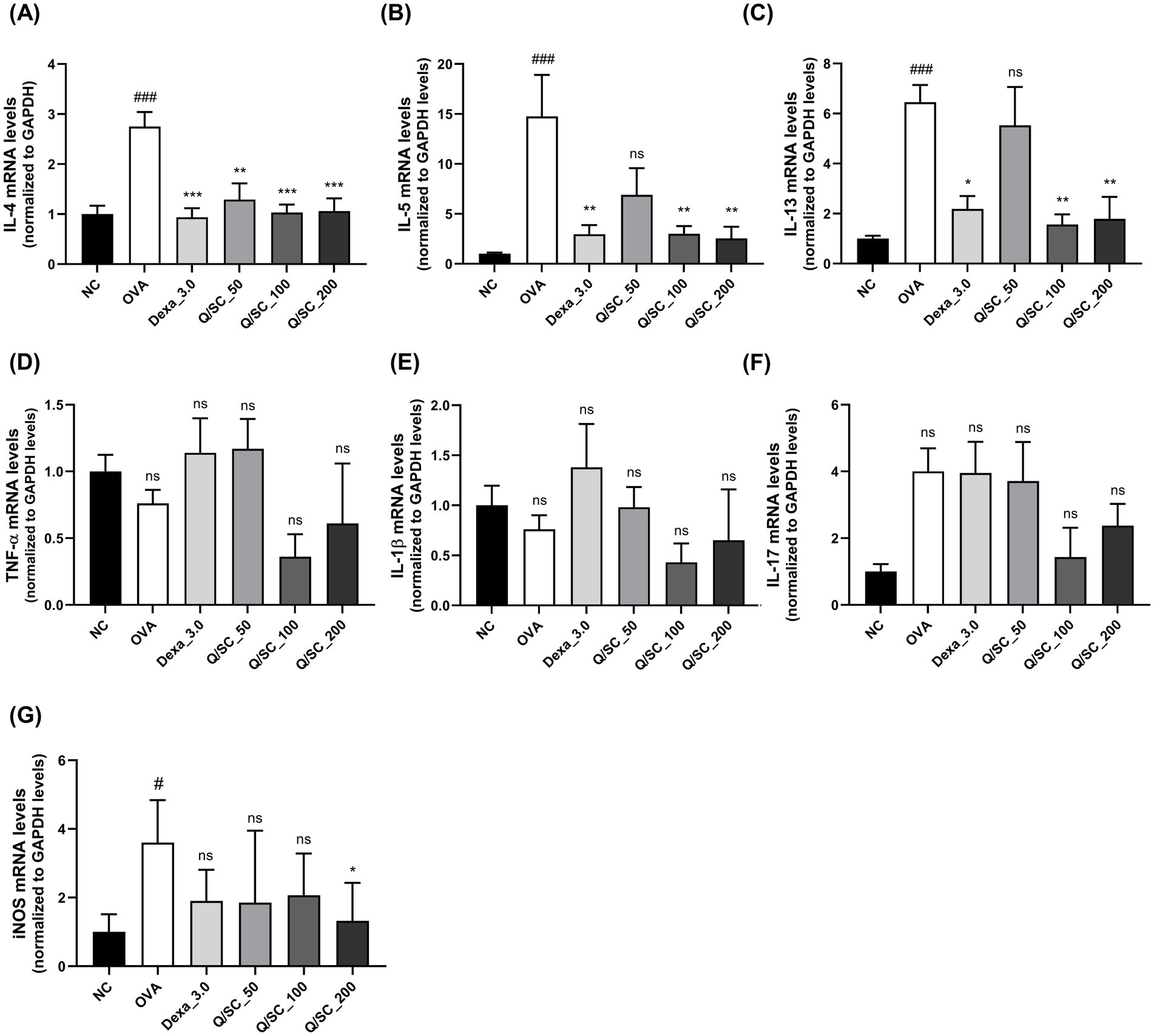
Figure 4. Chinese quince/Saururus chinensis (Q/SC) extract suppresses mRNA expressions of type 2-related cytokines and inducible nitric oxide synthase (iNOS). (A–C) The mRNA levels of type 2 cytokines (interleukin (IL)-4, IL-5, and IL-13), (D–F) non-type 2 cytokines (IL-1β, TNF-α, and IL-17), and (G) iNOS in homogenates are assessed using reverse transcription quantitative polymerase chain reaction and are normalized to glyceraldehyde 3-phosphate dehydrogenase levels. Data are displayed as the means ±SEM. ###p < 0.001 and #p < 0.05 vs. the normal control (NC) group. ***p < 0.001, **p < 0.01, and *p < 0.05 vs. the ovalbumin-challenged group.
3.4 Effect of Q/SC extract on OVA-induced mucus production and the STAT6 signaling pathway
Goblet cell hyperplasia and excessive mucus production in the bronchi are the most common features observed in allergic asthma models. Therefore, PAS staining was carried out to evaluate the effect of the Q/SC extract on mucus production. The results showed higher mucus production in the lung epithelium of the OVA-challenged group than in that of the NC group. Conversely, mucus production was significantly lower in the DEX and Q/SC-200 treatment groups than in that of the OVA-challenged group (Figures 5A,B). Furthermore, MUC5AC mRNA expression was significantly higher in the OVA-challenged group than it was in the NC group, whereas it was significantly lower in the DEX, Q/SC-100, and Q/SC-200 treatment groups (Figure 5C). Finally, STAT6 phosphorylation was markedly increased in the OVA-challenged group, whereas it was substantially decreased in the DEX and Q/SC treatment groups (Supplementary Figure S1). STAT6 phosphorylation was eliminated in the Q/SC-200 treatment group (Figure 5D). Collectively, these results show that high concentrations of Q/SC extract inhibit MUC5AC expression by suppressing the STAT6 signaling pathway.
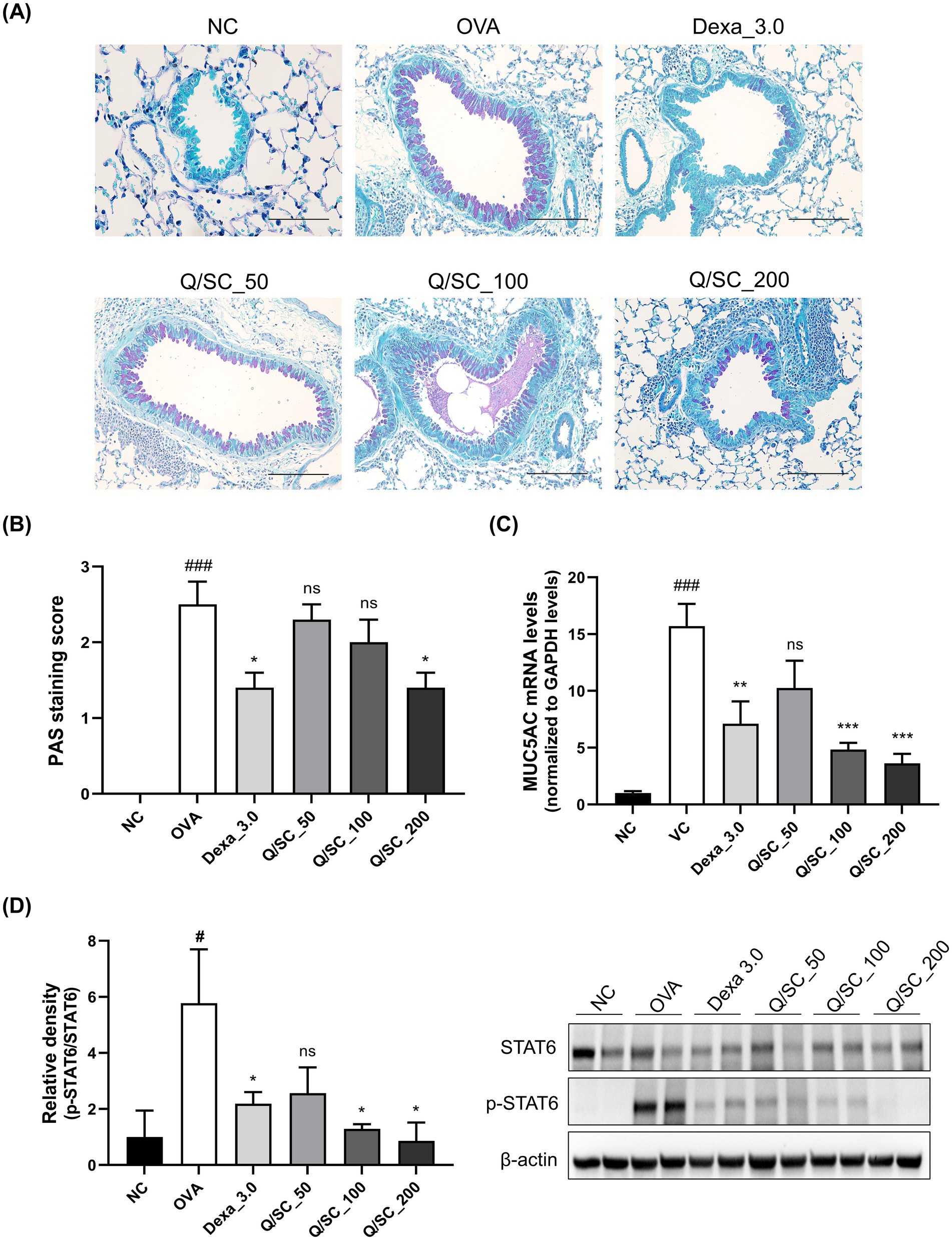
Figure 5. Effect of the Chinese quince/Saururus chinensis (Q/SC) extract on mucus production and the STAT6 signaling pathway in ovalbumin (OVA)-induced allergic asthma. (A) Periodic acid-Schiff staining is used to assess goblet cell hyperplasia in the epithelium. Scale bar = 100 μm. (B) Quantification of goblet cells in lung tissues. (C) The mRNA level of the mucus gene MUC5AC is quantified using reverse transcription quantitative polymerase chain reaction and is normalized to glyceraldehyde 3-phosphate dehydrogenase levels. (D) STAT6 phosphorylation is measured using western blot. The total forms of each protein are used as loading controls. Data are displayed as the means ± SEMs. ###p < 0.001 vs. the normal control group. ***p < 0.001, **p < 0.01, and *p < 0.05 vs. the OVA-challenged group.
4 Discussion
In this study, we found that high concentrations of the Q/SC extract significantly inhibited serum IgE production, type 2 cytokine levels, and iNOS expression. Additionally, high concentrations of the Q/SC extract effectively inhibited mucus secretion and immune cell infiltration in the lung tissues of OVA-challenged mice with allergic airway inflammation, similar to the effect of DEX. Collectively, our results show that high concentrations of the Q/SC extract are effective against allergen-induced airway inflammation in asthma.
Asthma is a chronic respiratory condition characterized by reversible airway obstruction and bronchial hyperresponsiveness. It affects approximately 300 million people worldwide (38). In most patients, inhaled corticosteroids and long-acting β2 agonists are used to control asthma symptoms; however, long-term and high-dose use of these drugs can lead to side effects (39). Additionally, some patients require repeated use of systemic steroids because of poor response to drug treatment, leading to steroid-related side effects. Therefore, there is increasing awareness of the significance of medicinal plants for the effective and safe management of asthma symptoms (40). Q extract may be effective against allergic inflammation, while SC extract may modulate lung inflammatory diseases in allergic asthma (41). However, the potential therapeutic mechanisms of a Q/SC mixture in allergic asthma-associated lung inflammation are not well understood. Asthma is categorized into type 2 and non-type 2 endotypes, depending on the immune cell types and inflammation patterns. Allergic asthma is classified as a type 2 endotype marked by increased eosinophilic inflammation and expression of type 2 cytokines, including IL-4, IL-5, and IL-13 (42). IL-4 promotes IgE synthesis from B cells and Th2 cell differentiation, whereas IL-5 is involved in recruiting eosinophils to inflammatory sites. IL-13 is essential in excessive mucus secretion, immune cell influx, and airway hyperresponsiveness (43, 44).
Nitric oxide (NO) plays an important role as an endogenous regulator of airway and distal lung constriction. NO levels are accordingly used as an indicator of eosinophilic airway inflammation. iNOS, the enzyme that produces NO, has increased transcriptional expression owing to the inflammatory cytokines IL-4 and IL-13 and is directly involved in eosinophil recruitment. Moreover, iNOS expression is associated with inflammation of the upper and lower airways (45–47). Therefore, regulating IgE, iNOS, and type 2 cytokines is crucial for improving allergic asthma. However, the expression of non-type 2 cytokines was not suppressed in the DEX group in this study, which aligns with reports that non-type 2 asthma was not controlled by DEX, a corticosteroid (48, 49). Airway mucus hypersecretion leads to a higher number of goblet cells in the airway epithelium, and this, in turn, leads to elevated MUC5AC expression, exacerbating asthma. MUC5AC expression is higher in patients with asthma than in healthy individuals. Additionally, the transcript levels of MUC5AC are significantly elevated during allergen-induced airway inflammation, whereas those of MUC1, MUC2, MUC3, MUC4, MUC5B, and MUC13 remain unchanged in the mouse lung tissue (50–54). Furthermore, MUC5AC levels are higher in type 2 asthma than in non-type 2 asthma (55, 56). In mouse lungs, IL-13 upregulates MUC5AC through a STAT6-dependent pathway, where STAT6 plays a critical function in regulating the transcription of several IL-4/IL-13-dependent genes. In previous studies, siRNA knockdown of STAT6 significantly reduced IL-4/IL-13-induced MUC5AC promoter activity (54, 57). In this study, goblet cell and MUC5AC expressions and STAT6 pathway phosphorylation were markedly elevated in OVA-challenged mice than it was in NC mice. Importantly, treatment with high concentrations of the Q/SC extract markedly reduced goblet cell and MUC5AC expressions and STAT6 pathway phosphorylation. This finding shows that high concentrations of the Q/SC extract have therapeutic potential for inhibiting mucus production and secretion in allergic airway inflammation. However, this study has some limitations in elucidating the molecular mechanisms through which Q/SC extract is involved in asthma. Future studies should focus on using asthma models to clarify the mechanism of action of Q/SC extract with a more rigorous research design.
5 Conclusion
High concentrations of the Q/SC extract effectively alleviate allergic airway inflammation by suppressing OVA-induced eosinophilic airway inflammation and type 2 cytokine secretion. Additionally, this extract effectively inhibits the STAT6 signaling pathway and reduces mucus hyperproduction. Thus, high concentrations of the Q/SC extract have potential as a treatment for managing allergic airway inflammatory diseases, including asthma.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
The animal study was approved by Central Bio Animal Care and Use Committee. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
HL: Formal analysis, Investigation, Methodology, Project administration, Software, Validation, Writing – original draft, Writing – review & editing. KK: Funding acquisition, Investigation, Writing – original draft. WL: Funding acquisition, Investigation, Writing – original draft. HN: Formal analysis, Methodology, Validation, Visualization, Writing – original draft. SR: Formal analysis, Methodology, Validation, Visualization, Writing – original draft. SK: Methodology, Writing – original draft. WK: Methodology, Writing – original draft. JY: Methodology, Writing – original draft. T-HL: Conceptualization, Project administration, Writing – review & editing. P-YJ: Conceptualization, Funding acquisition, Project administration, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was funded by the Ministry of Trade, Industry, and Energy, Korea under the “World Class Plus Program,” grant number R&D, P0017150, supervised by the Korea Institute for Advancement of Technology.
Conflict of interest
HL, HN, SR, SK, WK, JY, and T-HL were employed by Centralbio Co., Ltd. KK, WL, and P-YJ were employed by Novarex Co., Ltd.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2025.1613413/full#supplementary-material
Abbreviations
BALF, Bronchoalveolar lavage fluid; DEX, Dexamethasone; ELISA, Enzyme-linked immunosorbent assay; HPLC, High-performance liquid chromatography; ICS, Inhaled corticosteroids; Ig, Immunoglobulin; IL, Interleukin; iNOS, Inducible nitric oxide synthase; LABA, Long-acting β2 agonists; NC, Normal control; NO, Nitric oxide; OVA, Ovalbumin; PAS, Periodic acid Schiff; PBS, Phosphate-buffered saline; Q, Chinese quince; SC, Saururus chinensis; TNF, Tumor necrosis factor.
References
1. Fish, JE, and Peters, SP. Airway remodeling and persistent airway obstruction in asthma. J Allergy Clin Immunol. (1999) 104:509–16. doi: 10.1016/s0091-6749(99)70315-5
2. Castillo, JR, Peters, SP, and Busse, WW. Asthma exacerbations: pathogenesis, prevention, and treatment. J Allergy Clin Immunol Pract. (2017) 5:918–27. doi: 10.1016/j.jaip.2017.05.001
3. Baxi, SN, and Phipatanakul, W. The role of allergen exposure and avoidance in asthma. Adolesc Med State Art Rev. (2010) 21:57.
4. Savin, IA, Zenkova, MA, and Sen’kova, AV. Bronchial asthma, airway remodeling and lung fibrosis as successive steps of one process. Int J Mol Sci. (2023) 24:16042. doi: 10.3390/ijms242216042
5. Raby, KL, Michaeloudes, C, Tonkin, J, Chung, KF, and Bhavsar, PK. Mechanisms of airway epithelial injury and abnormal repair in asthma and copd. Front Immunol. (2023) 14:1201658. doi: 10.3389/fimmu.2023.1201658
6. Evans, CM, Kim, K, Tuvim, MJ, and Dickey, BF. Mucus hypersecretion in asthma: causes and effects. Curr Opin Pulm Med. (2009) 15:4–11. doi: 10.1097/MCP.0b013e32831da8d3
7. Voynow, JA, Young, LR, Wang, Y, Horger, T, Rose, MC, and Fischer, BM. Neutrophil elastase increases Muc 5ac mRNA and protein expression in respiratory epithelial cells. Am J Phys. (1999) 276:L835–43. doi: 10.1152/ajplung.1999.276.5.L835
8. Izuhara, K, Ohta, S, Shiraishi, H, Suzuki, S, Taniguchi, K, Toda, S, et al. The mechanism of mucus production in bronchial asthma. Curr Med Chem. (2009) 16:2867–75. doi: 10.2174/092986709788803196
9. Kuperman, D, Schofield, B, Wills-Karp, M, and Grusby, MJ. Signal transducer and activator of transcription factor 6 (Stat6)-deficient mice are protected from antigen-induced airway hyperresponsiveness and mucus production. J Exp Med. (1998) 187:939–48. doi: 10.1084/jem.187.6.939
10. Thai, P, Chen, Y, Dolganov, G, and Wu, R. Differential regulation of Muc5ac /Muc5ac and Hclca-1/Mgob-5 expression in airway epithelium. Am J Respir Cell Mol Biol. (2005) 33:523–30. doi: 10.1165/rcmb.2004-0220RC
12. Ezechukwu, HC, Adegboye, OA, Okunowo, WO, and Emeto, TI. Targeting IgE and Th2-cytokines in allergy: brief updates on monoclonal antibodies and antibody gene therapy. Allergie. (2023) 3:90–104. doi: 10.3390/allergies3020007
13. Lefebvre, P, Duh, MS, Lafeuille, M-H, Gozalo, L, Desai, U, Robitaille, M-N, et al. Acute and chronic systemic corticosteroid–related complications in patients with severe asthma. J Allergy Clin Immunol. (2015) 136:1488–95. doi: 10.1016/j.jaci.2015.07.046
14. Hamauzu, Y, Yasui, H, Inno, T, Kume, C, and Omanyuda, M. Phenolic profile, antioxidant property, and anti-influenza viral activity of Chinese quince (Pseudocydonia sinensis Schneid.), quince (Cydonia oblonga mill.), and apple (Malus domestica mill.) fruits. J Agric Food Chem. (2005) 53:928–34. doi: 10.1021/jf0494635
15. Osawa, K, Yasuda, H, Morita, H, Takeya, K, and Itokawa, H. Antibacterial and antihemolytic activity of triterpenes and β-sitosterol isolated from chinese quince (chaenomeles sinensis). Nat Med. (1997) 51:365–7.
16. Zhang, R, Zhan, S, Li, S, Zhu, Z, He, J, Lorenzo, JM, et al. Anti-hyperuricemic and nephroprotective effects of extracts from chaenomeles sinensis (thouin) koehne in hyperuricemic mice. Food Funct. (2018) 9:5778–90. doi: 10.1039/c8fo01480a
17. Han, Y-K, Kim, Y-S, Natarajan, SB, Kim, W-S, Hwang, J-W, Jeon, N-J, et al. Antioxidant and anti-inflammatory effects of chaenomeles sinensis leaf extracts on lps-stimulated raw 264.7 cells. Molecules. (2016) 21:422. doi: 10.3390/molecules21040422
18. Yang, EJ, and Lee, SH. Anti-inflammatory effects of chaenomeles sinensis extract in an als animal model. Front Biosci. (2023) 28:326. doi: 10.31083/j.fbl2812326
19. Xu, R, Kuang, M, and Li, N. Phytochemistry and pharmacology of plants in the genus Chaenomeles. Arch Pharm Res. (2023) 46:825–54. doi: 10.1007/s12272-023-01475-w
20. Jin, J, Fan, YJ, Nguyen, TV, Yu, ZN, Song, CH, Lee, S-Y, et al. Chaenomeles sinensis extract ameliorates ovalbumin-induced allergic rhinitis by inhibiting the Il-33/St2 axis and regulating epithelial cell dysfunction. Food Secur. (2024) 13:611. doi: 10.3390/foods13040611
21. Kim, B-W, Koppula, S, Park, S-Y, Hwang, J-W, Park, P-J, Lim, J-H, et al. Attenuation of inflammatory-mediated neurotoxicity by saururus chinensis extract in lps-induced Bv-2 microglia cells via regulation of NF-κB signaling and anti-oxidant properties. BMC Complement Altern Med. (2014) 14:1–10. doi: 10.1186/1472-6882-14-502
22. Quan, Z, Lee, YJ, Yang, JH, Lu, Y, Li, Y, Lee, Y-K, et al. Ethanol extracts of saururus chinensis suppress ovalbumin-sensitization airway inflammation. J Ethnopharmacol. (2010) 132:143–9. doi: 10.1016/j.jep.2010.08.002
23. Choi, MS, Kim, EC, Lee, HS, Kim, SK, Choi, HM, Park, JH, et al. Inhibitory effects of saururus chinensis (l our.) B aill on the development of atopic dermatitis-like skin lesions in Nc/Nga mice. Biol Pharm Bull. (2008) 31:51–6. doi: 10.1248/bpb.31.51
24. Yoo, H-J, Kang, H-J, Jung, H-J, Kim, K, Lim, C-J, and Park, E-H. Anti-inflammatory, anti-angiogenic and anti-nociceptive activities of saururus chinensis extract. J Ethnopharmacol. (2008) 120:282–6. doi: 10.1016/j.jep.2008.08.016
25. Yu, QL, and Chen, Z. Establishment of different experimental asthma models in mice. Exp Ther Med. (2018) 15:2492–8. doi: 10.3892/etm.2018.5721
26. Ma, W, Jin, Q, Guo, H, Han, X, Xu, L, Lu, S, et al. Metformin ameliorates inflammation and airway remodeling of experimental allergic asthma in mice by restoring Ampkα activity. Front Pharmacol. (2022) 13:780148. doi: 10.3389/fphar.2022.780148
27. Shore, SA, Schwartzman, IN, Mellema, MS, Flynt, L, Imrich, A, and Johnston, RA. Effect of leptin on allergic airway responses in mice. J Allergy Clin Immunol. (2005) 115:103–9. doi: 10.1016/j.jaci.2004.10.007
28. Kujur, W, Gurram, RK, Haleem, N, Maurya, SK, and Agrewala, JN. Caerulomycin a inhibits Th2 cell activity: a possible role in the management of asthma. Sci Rep. (2015) 5:15396. doi: 10.1038/srep15396
29. Myou, S, Leff, AR, Myo, S, Boetticher, E, Tong, J, Meliton, AY, et al. Blockade of inflammation and airway hyperresponsiveness in immune-sensitized mice by dominant-negative phosphoinositide 3-kinase–tat. J Exp Med. (2003) 198:1573–82. doi: 10.1084/jem.20030298
30. Yoshioka, M, Sagara, H, Takahashi, F, Harada, N, Nishio, K, Mori, A, et al. Role of multidrug resistance-associated protein 1 in the pathogenesis of allergic airway inflammation. Am J Physiol Lung Cell Mol Physiol. (2009) 296:L30–6. doi: 10.1152/ajplung.00026.2008
31. Abdulaal, WH, Asfour, HZ, Helmi, N, Al Sadoun, H, Eldakhakhny, B, Alhakamy, NA, et al. Capsaicin ameliorate pulmonary fibrosis via antioxidant Nrf-2/ PPAR-γ pathway activation and inflammatory TGF-β1/ NF-κB/cox II pathway inhibition. Front Pharmacol. (2024) 15:1333715. doi: 10.3389/fphar.2024.1333715
32. Kim, DI, Song, MK, and Lee, K. Comparison of asthma phenotypes in ova-induced mice challenged via inhaled and intranasal routes. BMC Pulm Med. (2019) 19:241. doi: 10.1186/s12890-019-1001-9
33. Gu, W, Zheng, T, Li, W, Luo, X, Xu, X, Wang, Y, et al. Migrasomes derived from human umbilical cord mesenchymal stem cells: a new therapeutic agent for ovalbumin-induced asthma in mice. Stem Cell Res Ther. (2025) 16:26. doi: 10.1186/s13287-025-04145-4
34. Jeong, S, Kim, Y-Y, Lee, D, Kim, S-H, and Lee, S. Hispidulin alleviates mast cell-mediated allergic airway inflammation through Fcεr1 and Nrf2/ho-1 signaling pathway. Antioxidants. (2024) 13:528. doi: 10.3390/antiox13050528
35. MacNee, W. Oxidative stress and lung inflammation in airways disease. Eur J Pharmacol. (2001) 429:195–207. doi: 10.1016/s0014-2999(01)01320-6
36. Hogg, JC. The pathology of asthma. APMIS. (1997) 105:735–45. doi: 10.1111/j.1699-0463.1997.tb05079.x
37. Mathrani, V, Kenyon, N, Zeki, A, and Last, J. Mouse models of asthma: can they give us mechanistic insights into the role of nitric oxide? Curr Med Chem. (2007) 14:2204–13. doi: 10.2174/092986707781389628
38. Masoli, M, Fabian, D, Holt, S, and Beasley, RProgram GIfA. The global burden of asthma: executive summary of the GINA dissemination committee report. Allergy. (2004) 59:469–78. doi: 10.1111/j.1398-9995.2004.00526.x
39. Wurth, M, Papantonakis, CM, Nevel, RJ, Thomas, CS, Sokolow, AG, Moore, PE, et al. Risk factors associated with asthma development and control in children. Mouse infestation, antipyretics, respiratory viruses, and allergic sensitization. Am J Respir Crit Care Med. (2017) 196:1605–7. doi: 10.1164/rccm.201704-0696RR
40. Taur, DJ, and Patil, RY. Some medicinal plants with antiasthmatic potential: a current status. Asian Pac J Trop Biomed. (2011) 1:413–8. doi: 10.1016/S2221-1691(11)60091-9
41. Song, M, Lee, S-Y, Kim, M, Park, S, Park, J, Kwon, Y, et al. Saururus chinensis-controlled allergic pulmonary disease through NF-κB/Cox-2 and Pge2 pathways. PeerJ. (2020) 8:e10043. doi: 10.7717/peerj.10043
42. Vale, K. Targeting the jak-stat pathway in the treatment of ‘Th2-high’severe asthma. Future Med Chem. (2016) 8:405–19. doi: 10.4155/fmc.16.4
43. Holgate, ST, Wenzel, S, Postma, DS, Weiss, ST, Renz, H, and Sly, PD. Asthma. Nat Rev Dis Primers. (2015) 1:15025. doi: 10.1038/nrdp.2015.25
44. Walker, JA, and McKenzie, AN. Th2 cell development and function. Nat Rev Immunol. (2018) 18:121–33. doi: 10.1038/nri.2017.118
45. Duong-Quy, S. Clinical utility of the exhaled nitric oxide (no) measurement with portable devices in the management of allergic airway inflammation and asthma. J Asthma Allergy. (2019) 12:331–41. doi: 10.2147/JAA.S190489
46. Ricciardolo, FL, Sorbello, V, and Ciprandi, G. FeNO as biomarker for asthma phenotyping and management. Allergy Asthma Proc. (2015) 36:e1–8. doi: 10.2500/aap.2015.36.3805
47. Tufvesson, E, Andersson, C, Weidner, J, Erjefält, J, and Bjermer, L. Inducible nitric oxide synthase expression is increased in the alveolar compartment of asthmatic patients. Allergy. (2017) 72:627–35. doi: 10.1111/all.13052
48. Peters, K, Ernst, S, and Peters, M. Interaction of interleukin-17a with a Th2 response in a mouse model of allergic airway inflammation. Cells. (2023) 12:1774. doi: 10.3390/cells12131774
49. Xie, Y, Abel, PW, Casale, TB, and Tu, Y. Th17 cells and corticosteroid insensitivity in severe asthma. J Allergy Clin Immunol. (2022) 149:467–79. doi: 10.1016/j.jaci.2021.12.769
50. Bonser, LR, and Erle, DJ. Airway mucus and asthma: the role of Muc5ac and Muc5b. J Clin Med. (2017) 6:112. doi: 10.3390/jcm6120112
51. Rose, MC, and Voynow, JA. Respiratory tract mucin genes and mucin glycoproteins in health and disease. Physiol Rev. (2006) 86:245–78. doi: 10.1152/physrev.00010.2005
52. Fahy, JV. Goblet cell and mucin gene abnormalities in asthma. Chest. (2002) 122:320S–6S. doi: 10.1378/chest.122.6_suppl.320s
53. Hovenberg, HW, Davies, JR, Herrmann, A, Lindén, C-J, and Carlstedt, I. Muc5ac, but not Muc2, is a prominent mucin in respiratory secretions. Glycoconj J. (1996) 13:839–47. doi: 10.1007/BF00702348
54. Wang, X, Li, Y, Luo, D, Wang, X, Zhang, Y, Liu, Z, et al. Lyn regulates mucus secretion and Muc5ac via the stat6 signaling pathway during allergic airway inflammation. Sci Rep. (2017) 7:42675. doi: 10.1038/srep42675
55. Woodruff, PG, Modrek, B, Choy, DF, Jia, G, Abbas, AR, Ellwanger, A, et al. T-helper type 2–driven inflammation defines major subphenotypes of asthma. Am J Respir Crit Care Med. (2009) 180:388–95. doi: 10.1164/rccm.200903-0392OC
56. Lachowicz-Scroggins, ME, Finkbeiner, WE, Gordon, E, Yuan, S, Zlock, L, Bhakta, NR, et al. Corticosteroid and long-acting ß-agonist therapy reduces epithelial goblet cell metaplasia. Clin Exp Allergy. (2017) 47:1534–45.
Keywords: allergic asthma, type 2 immune response, ovalbumin, inflammatory response, mucus production, Chinese quince , Saururus chinensis , STAT6
Citation: Lee HJ, Kim KC, Lee WJ, Nam HJ, Ryu SJ, Kim SH, Kim WJ, Yoon JW, Lee T-H and Jeong P-Y (2025) Therapeutic effects of a combination of Chinese quince and Saururus chinensis extract on allergic airway inflammation in an ovalbumin-induced asthma mouse model. Front. Nutr. 12:1613413. doi: 10.3389/fnut.2025.1613413
Edited by:
Jiajia Song, Southwest University, ChinaReviewed by:
Wang Meng, Heilongjiang University of Chinese Medicine, ChinaJae Sik Yu, Sejong University, Republic of Korea
Copyright © 2025 Lee, Kim, Lee, Nam, Ryu, Kim, Kim, Yoon, Lee and Jeong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tae-Hee Lee, dGhsZWVAY2VudHJhbGJpby5jby5rcg==; Pan-Young Jeong, cHlqZW9uZ0Bub3ZhcmV4LmNvLmty
 Hye Jin Lee
Hye Jin Lee Ki Cheon Kim
Ki Cheon Kim Woo Jin Lee
Woo Jin Lee Hyo Jung Nam
Hyo Jung Nam Su Jin Ryu
Su Jin Ryu Seon Hyeok Kim
Seon Hyeok Kim Won Jun Kim
Won Jun Kim Jae Won Yoon
Jae Won Yoon Tae-Hee Lee
Tae-Hee Lee Pan-Young Jeong
Pan-Young Jeong