- 1Department of Epidemiology and Health Statistics, School of Public Health, Sun Yat-sen University, Guangzhou, China
- 2School of Psychology and Counselling, Queensland University of Technology, Brisbane, QLD, Australia
- 3Maternal and Child Healthcare Hospital of Longhua District, Shenzhen, China
- 4Biostime (Guangzhou) Health Products Ltd., Guangzhou, China
- 5School of Health Management, Xinhua College of Guangzhou, Guangzhou, China
Background: Neurobehavioral developmental disorder (NDD) significantly impact children’s long-term wellbeing and contribute to global disease burden. While prenatal micronutrient supplementation has shown promise in improving fetal neurodevelopment, its association with offspring’s neurobehavioral outcomes remains controversial, and the potential effect of early childhood probiotic intake on this association is still underexplored. This study aimed to evaluate the association between prenatal micronutrient supplementation and neurobehavioral development in preschool children, and to explore and quantify the effect of early childhood probiotic intake on this association.
Methods: We included 15,636 mother–child dyads in Shenzhen, China, in 2022. Mothers provided information on prenatal micronutrient supplementation (calcium, folic acid, iron, and multivitamins) and early childhood probiotic intake through a structured questionnaire. Neurobehavioral development was assessed using the Ages and Stages Questionnaire (ASQ-3). Logistic regression was used to examine the association between prenatal micronutrient supplementation and NDD across crude, adjusted, and full-inclusion models. The effect of early childhood probiotic intake on the association between prenatal micronutrient supplementation and NDD was evaluated through four-way decomposition analysis and quantified using counterfactual attribution under three scenarios.
Results: Among the participants, 11.7% were identified with NDD. Prenatal multivitamin supplementation was significantly associated with a reduced risk of NDD (OR = 0.73, 95% CI = 0.66–0.81). Early childhood probiotic intake was associated with an enhanced protective effect (Total EOR = −0.33, 95% CI = −0.54 to −0.12), with 48% of the effect attributable to interactions. Early childhood probiotic intake could prevent an additional 73 NDD cases (a 59% increase), particularly benefiting the gross motor, fine motor and personal-social domains.
Conclusion: Prenatal multivitamin supplementation has a protective effect against NDD in preschool children, and early childhood probiotic intake is associated with an enhancement of this protective effect. These findings underscore the potential effect of early-life dietary supplements for NDD prevention. Further studies are recommended to confirm these effects and explore underlying mechanisms.
1 Introduction
Neurobehavioral development refers to the development of the brain and nervous system in behavioral, cognitive, and emotional regulation, playing a crucial role in children’s academic success, mental wellbeing, professional prospects, and overall quality of life (1). Despite its importance, neurobehavioral development disorders remain prevalent worldwide, characterized by key functions disorders like perception, motor skills, and language (2, 3). The 2019 World Health Organization (WHO) report estimated that approximately 58 million children (7%) globally experienced developmental disorders, with neurobehavioral disorders accounting for more than half of these cases (4). In China, the reported prevalence of such disorders ranges from 3.2 to 13.9%, with the personal-social domain being the most affected (5–8). Severe cases may manifest as conditions such as autism spectrum disorder (ASD) and attention deficit hyperactivity disorder (ADHD), both of which are increasing in prevalence (9–11). These conditions impose significant challenges on affected individuals, their families, and society. For example, ASD is responsible for more than 691.5 disability-adjusted life years (DALYs) per 100,000 individual globally, ranking it among the top 10 neurological disorders (12). Families of children with ASD face approximately $3,020 in additional annual healthcare costs and substantial losses in parental productivity (13). The annual social cost for all ASD patients may reach $41.8 billion, accounting for approximately 3.76% of China’s total healthcare expenditure in 2020 (14). Therefore, early identification of influencing factors is essential to prevent severe neurobehavioral developmental disorders and mitigate long-term socioeconomic burdens.
Early life, including the prenatal period and early childhood, is a critical window for neurobehavioral development, during which preventive interventions can be most effective in reducing the risk and severity of these disorders (15, 16). Prenatal nutrition, particularly micronutrients, is essential in fetal neural development with long-term health implications (17). However, micronutrient deficiencies remain widespread among pregnant women globally, including in China (18–20). While some evidence suggests that prenatal iron supplementation may enhance neurobehavioral outcomes (21, 22), other studies have failed to confirm this effect (23). Similarly, randomized controlled trials (RCTs) on prenatal vitamin D supplementation have reported inconsistent results, with some showing improved motor development (24), and others finding no significant benefits (25, 26). For prenatal iodine supplementation, although certain studies suggest cognitive benefits (27, 28), a systematic review of RCTs found no impact on neurobehavioral outcomes (29). These discrepancies may be attributed to heterogeneity in study design, including confounding biases and non-standardized neurobehavioral assessments (25, 26). Therefore, further research using large sample sizes, rigorous control of confounders, and standardized assessment methods is necessary to clarify these associations.
Gut microbiota establishment and neural development share the same critical time window in early life (30). Increasing evidence suggests that gut microbiota influences neurobehavioral development via the gut-brain axis, involving mechanisms such as neurotransmitter regulation, immune modulation, production of neuroactive metabolites (e.g., short-chain fatty acids), and stress response regulation (31–34). Probiotics, which are live, nonpathogenic microorganisms that promote gastrointestinal microbial balance, have been proposed as a potential intervention to enhance neurobehavioral development by modulating gut microbiota (35, 36). Some reviews have reported therapeutic effects of childhood probiotic intake on ASD and ADHD (37–39), while an RCT has investigated its potential in preventing ADHD (40). However, some studies have failed to demonstrate significant benefits of childhood probiotic intake for ASD (41). Moreover, existing research has primarily focused on overt neurobehavioral disorders such as ASD and ADHD, whereas evidence remains limited regarding its effects during the early or subclinical stages of neurobehavioral development.
Research shows that prenatal micronutrient supplementation can benefit offspring gut microbiota, while childhood probiotic intake similarly improve gut microbiota composition in children, suggesting a potential interaction between the two in shaping neurobehavioral development via the gut–brain axis (42). Additionally, maternal self-administration of over-the-counter medications during pregnancy may influence the provision of probiotics and other nutritional supplements to their children (43). This indicates that prenatal micronutrient supplementation may also influence childhood probiotic intake, thereby potentially affecting neurobehavioral development. Figure 1 illustrates a conceptual framework of the hypothesized associations among prenatal micronutrient supplementation, early childhood probiotic intake, and neurobehavioral development.
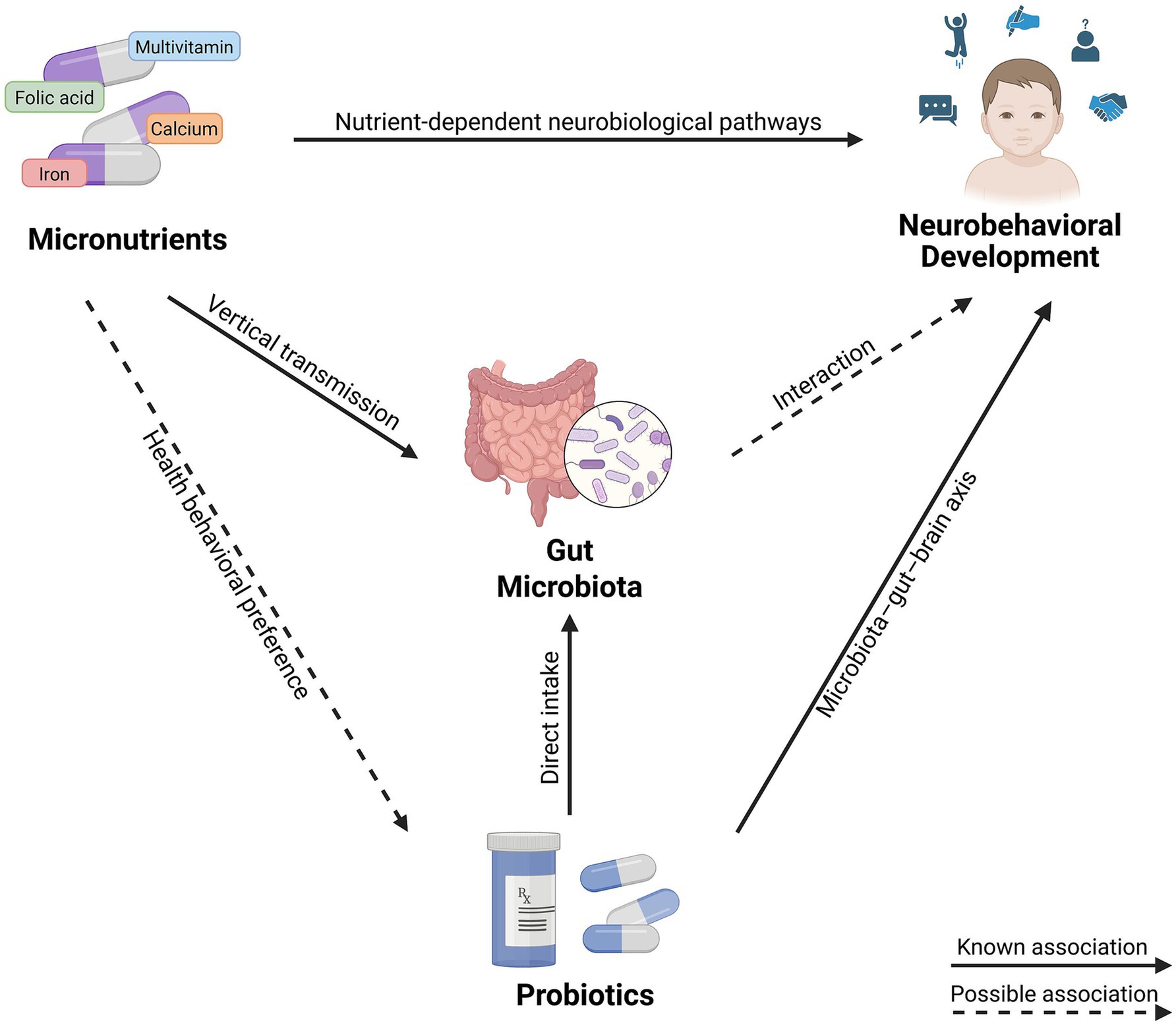
Figure 1. Conceptual framework of the hypothesized associations among prenatal micronutrient supplementation, early childhood probiotic intake, and neurobehavioral development. Created with BioRender.com.
However, existing studies have primarily focused on dietary supplements during a single developmental window—either the prenatal or early childhood. Although some studies have examined the combined effects of prenatal micronutrient supplementation and probiotic intake on maternal and infant outcomes (44–46), the relatively stable maternal microbiota and its indirect influence on the fetus suggest that pregnancy may not be the optimal time for probiotic intake (47). Similarly, while other studies have explored the effects of childhood probiotic intake and micronutrient supplementation (48, 49), initiating micronutrient supplementation during childhood may have limited effects, as neurodevelopment begins in utero and largely depends on maternal nutrient stores (22, 50). Nonetheless, evidence remains limited regarding the effect of childhood probiotic intake on the association between prenatal micronutrient supplementation and neurobehavioral development in children.
Therefore, our study aimed to evaluate the association between prenatal micronutrient supplementation and neurobehavioral development in preschool children, and to explore and quantify the effect of early childhood probiotic intake on this association.
2 Methods
2.1 Participants
The study recruited participants from the 2022 survey of children aged 3–7 years, conducted in 235 kindergartens in Longhua District, Shenzhen, China, with follow-up assessment of neurobehavioral development conducted in 2023. A total of 36,220 mother–child dyads were initially enrolled, and 15,636 participants were included after excluding cases with missing follow-up data on neurobehavioral development (n = 12,129), no recorded prenatal micronutrient supplementation (n = 6,624), and incomplete childhood probiotic intake records (n = 1,831), as shown in Figure 2. Ethical approval was obtained from the Ethics Committee of the School of Public Health, Sun Yat-sen University, and informed consent was provided by the mothers of all participating children.
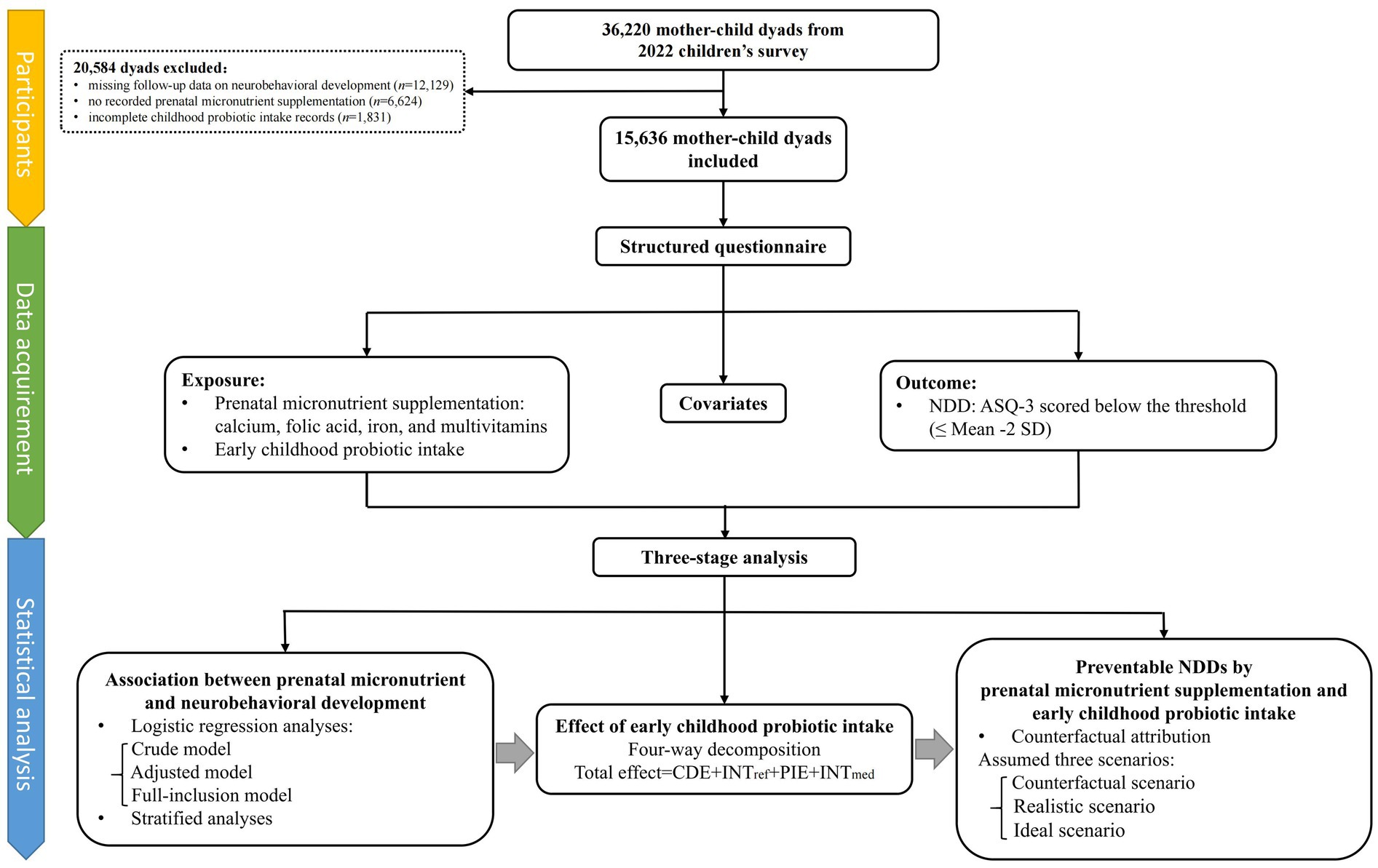
Figure 2. Study profile. ASQ-3, Age and Developmental Progress Questionnaire; CDE, Controlled Direct Effect; INTmed, mediated interaction; INTref, reference interaction; NDD, neurobehavioral developmental disorder; PIE, Pure Indirect Effect.
2.2 Data acquirement
Data was collected through a self-administered online structured questionnaire, which was completed by children’s mothers under the supervision of childcare practitioners and kindergarten teachers. The questionnaire was developed by a multidisciplinary panel of epidemiologists, obstetricians, and pediatricians, and its clarity and readability were confirmed through a pilot test. It contained demographic characteristics, maternal condition during pregnancy (e.g., micronutrient supplementation, pregnancy complications, health behaviors), parental lifestyle and health status (e.g., smoking, drinking, diseases), neonatal birth characteristics (e.g., birth weight, preterm birth, delivery mode), lifestyle and health condition at ages 0–3 years (e.g., probiotic intake, feeding pattern, nutritional condition), and children’s health status at ages 3–7 years (e.g., neurobehavioral development, diseases, family function). Further details and coding are provided in Supplementary Table 1.
2.2.1 Prenatal micronutrient supplementation and early childhood probiotic intake
Prenatal micronutrient supplementation (calcium, folic acid, iron, and multivitamins) was assessed through maternal self-reported responses to four separate questions: “Did you take calcium/folic acid/iron/multivitamins during your pregnancy?” Participants answering ‘YES’ were assigned to the corresponding supplementation group, while all others served as the reference group.
Early childhood probiotic intake was defined based on a ‘YES’ response to the question: “Did your child take probiotics between the ages of 0–3 years?” Here, probiotics were specified as a single product form (capsules or sachets) not combined with other foods or supplements.
2.2.2 Outcome
The neurobehavioral development in preschool children (3–7 years old) was assessed by the Age and Developmental Progress Questionnaire-Third Edition (ASQ-3), a well-validated and widely used tool in multiple countries, including China, demonstrating good internal consistency (Cronbach’s α = 0.8) (51–53). Designed for children aged 1 to 66 months, the ASQ-3 assesses five key domains: communication, gross motor, fine motor, problem-solving, and personal-social status. Assessment results are classified into three groups: (1) scores above the threshold (>mean minus 1 standard deviation [SD]), indicating age-appropriate development; (2) scores close to the threshold (mean minus 2 SD to mean minus 1 SD), requiring further monitoring; and (3) scores below the threshold (≤mean minus 2 SD), indicating developmental disorder. In our study, the presence of neurobehavioral developmental disorder (NDD) was identified when at least one domain scored below the threshold, while others with all domains above or close to the threshold were classified as neurobehavioral developmental normality (NDN).
2.2.3 Covariates
Based on previous studies (21, 27, 54–56) and the univariate and multivariate analyses results (See Supplementary Table 2), covariates included child’s demographic characteristics (age, sex, birth season, residence type), maternal demographic characteristics (education, household income, age of conception, pre-pregnancy BMI), pregnancy and perinatal characteristics (intrauterine growth restriction [IUGR], parity, preterm birth [PTB], and birth weight [BW]), and childhood family environment (parental depression, family functioning, feeding pattern). The average missing data rate for these covariates was 3.9% (range: 0–12.9%), with missing data addressed using multiple imputations through Predictive Mean Matching (PMM) (57).
2.3 Statistical analysis
We compared variables between the NDD and NDN groups using one-way ANOVA and t-tests for continuous variables and chi-square tests for categorical variables. The main analysis was conducted in three stages.
2.3.1 Association between prenatal micronutrient supplementation and neurobehavioral development in preschool children
We examined the association between maternal micronutrient supplementation during pregnancy and neurobehavioral development in preschool children using univariate and multivariate logistic regression analyses under three different models: the crude model, without adjustment for confounders; the adjusted model, adjusted for selected confounders; and the full-inclusion model, included all micronutrients in the model while adjusting for all confounders to address the confounding effects of co-supplementation. To further explore the association between prenatal micronutrient supplementation and neurobehavioral development under different probiotic intake scenarios, we conducted a stratified analysis based on probiotic intake in early childhood.
2.3.2 Effect of early childhood probiotic intake on association between prenatal micronutrient supplementation and neurobehavioral development in preschool children
Under the three models, we analyzed the effect of probiotic intake on the association between prenatal micronutrient supplementation and neurobehavioral development in preschool children through four-way decomposition, as proposed by J. VanderWeele (58). This method allows for simultaneous analysis of interaction and mediation effects, which is widely used in epidemiologic studies (59–61). The core is to decompose the total effect into four components: (1) controlled direct effect (CDE): the effect of prenatal micronutrient supplementation on neurobehavioral development independent of childhood probiotic intake, (2) reference interaction (INTref): the effect of prenatal micronutrient supplementation on neurobehavioral development only through its interaction with childhood probiotic intake, (3) pure indirect effect (PIE): the effect of prenatal micronutrient supplementation on neurobehavioral development both through an interaction with and mediation by childhood probiotic intake, and (4) mediated interaction (INTmed): the effect of prenatal micronutrient supplementation on neurobehavioral development only through the mediation by childhood probiotic intake. Estimates of the four components were obtained through regression analyses that included the exposure, mediator, and their interaction terms, with results presented as excess odds ratio (EOR) and proportion attributable (PA).
2.3.3 Preventable NDD by prenatal micronutrient supplementation and early childhood probiotic intake
After identifying the effect of probiotic intake, we quantified the number of preventable NDD attributable to prenatal micronutrient supplementation and early childhood probiotic intake using counterfactual attribution (62), which can be used to quantify interaction or mediation effects (63). We assumed three scenarios: (1) Counterfactual scenario (V1): no probiotic intake, (2) Realistic scenario (V2): probiotic intake in the realistic proportion of the survey population, and (3) Ideal scenario (V3): probiotic intake in all populations. Under each scenario, we estimated the effect of prenatal micronutrient supplementation and early childhood probiotic intake on NDD across three models to calculate the number of preventable NDD cases. The difference in preventable cases between the realistic and counterfactual scenarios (V2–V1) represented the additional preventable NDD due to current childhood probiotic intake proportion, while the difference between the ideal and counterfactual scenarios (V3–V1) represents the potential preventable NDD if probiotics were consumed by all populations.
Statistical analyses above were performed via R version 4.2.3. Two sided p-values <0.05 were considered significant.
3 Results
3.1 Participants’ characteristics
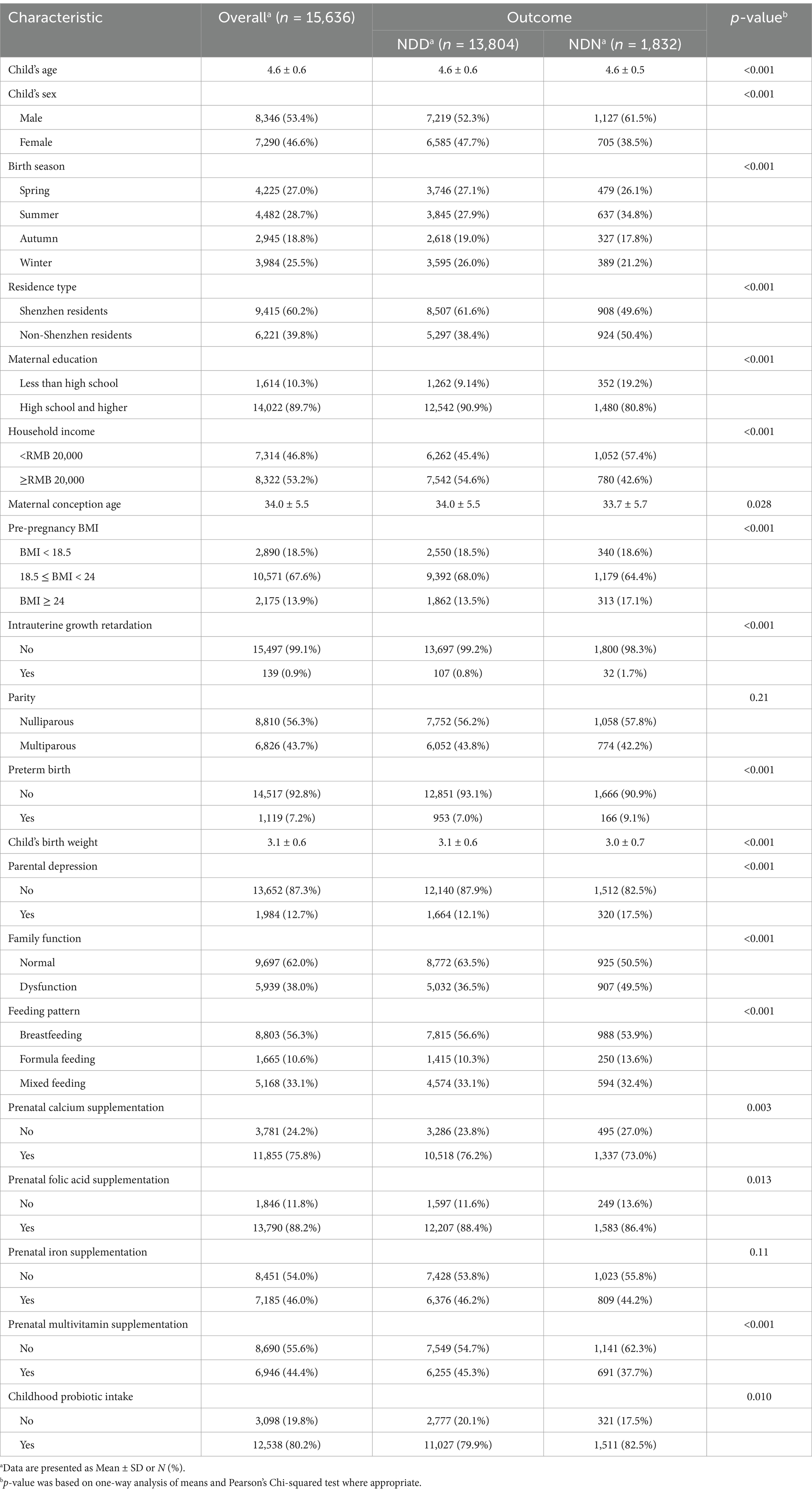
Table 1 presents the background characteristics of the study participants, including prenatal micronutrient supplementation and childhood probiotic intake, with a comparison between NDD and NDN groups. Folic acid (88.2%) was the most widely consumed, followed by calcium (75.8%), while iron (46.0%) and multivitamin supplementation (44.4%) were relatively less common in pregnant women. A total of 80.2% of children reported taking probiotics in early childhood.
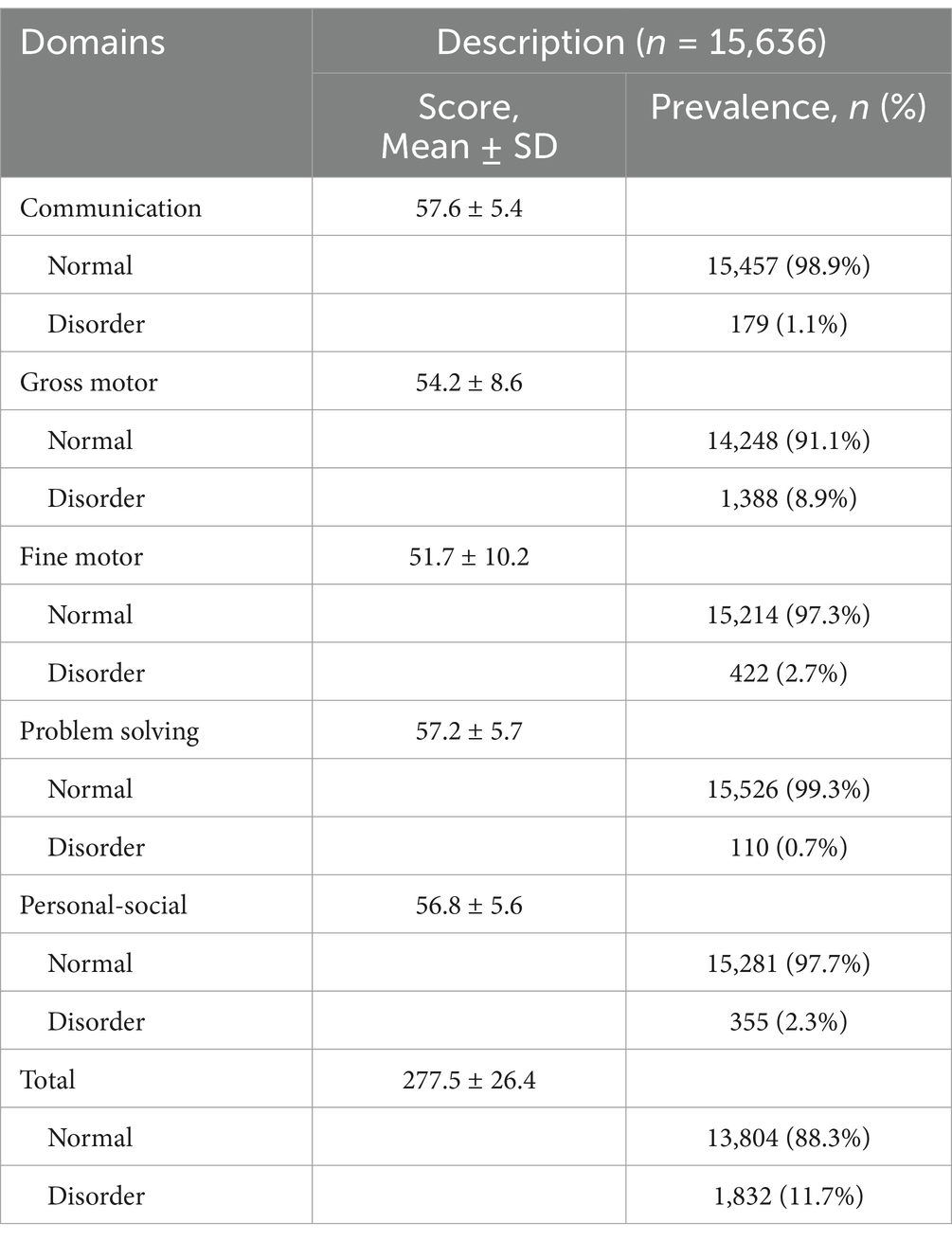
The overall average ASQ-3 score of the 15,636 children was 277 ± 26.4, with domain-specific scores ranging from 51.7 ± 10.2 in the fine motor domain to 57.6 ± 5.4 in the communication domain. A total of 11.7% of the children were identified as NDD, with the highest prevalence in the gross motor domain (8.88%) and the lowest in the problem-solving domain (0.70%), as shown in Table 2.
3.2 Association between prenatal micronutrient supplementation and neurobehavioral development in preschool children
In the crude model, prenatal calcium (OR = 0.84, 95% CI = 0.76–0.94), folic acid (OR = 0.83, 95% CI = 0.72–0.96) and multivitamin (OR = 0.73, 95% CI = 0.66–0.81) supplementation was associated with a decreased risk of NDD, whereas no significant association was observed for iron. In the adjusted and full-inclusion models, only prenatal multivitamin supplementation remained associated with a reduced risk of NDD (Adjusted Model: OR = 0.86, 95% CI = 0.78–0.96; Full-inclusion Model: OR = 0.85, 95% CI = 0.75–0.95) (Supplementary Table 3; Figure 3). In the full-inclusion model, prenatal iron supplementation was even found to be significantly associated with an increased risk of NDD (OR = 1.14, 95% CI = 1.01–1.28). Across these 5 domains, after controlling for confounders, prenatal calcium and folic acid supplementation were significantly associated with NDD in the communication and gross motor domains, folic acid and multivitamin supplementation were associated with NDD in the personal-social domain, whereas no significant associations were found in the fine motor and problem-solving domains (Supplementary Table 4).
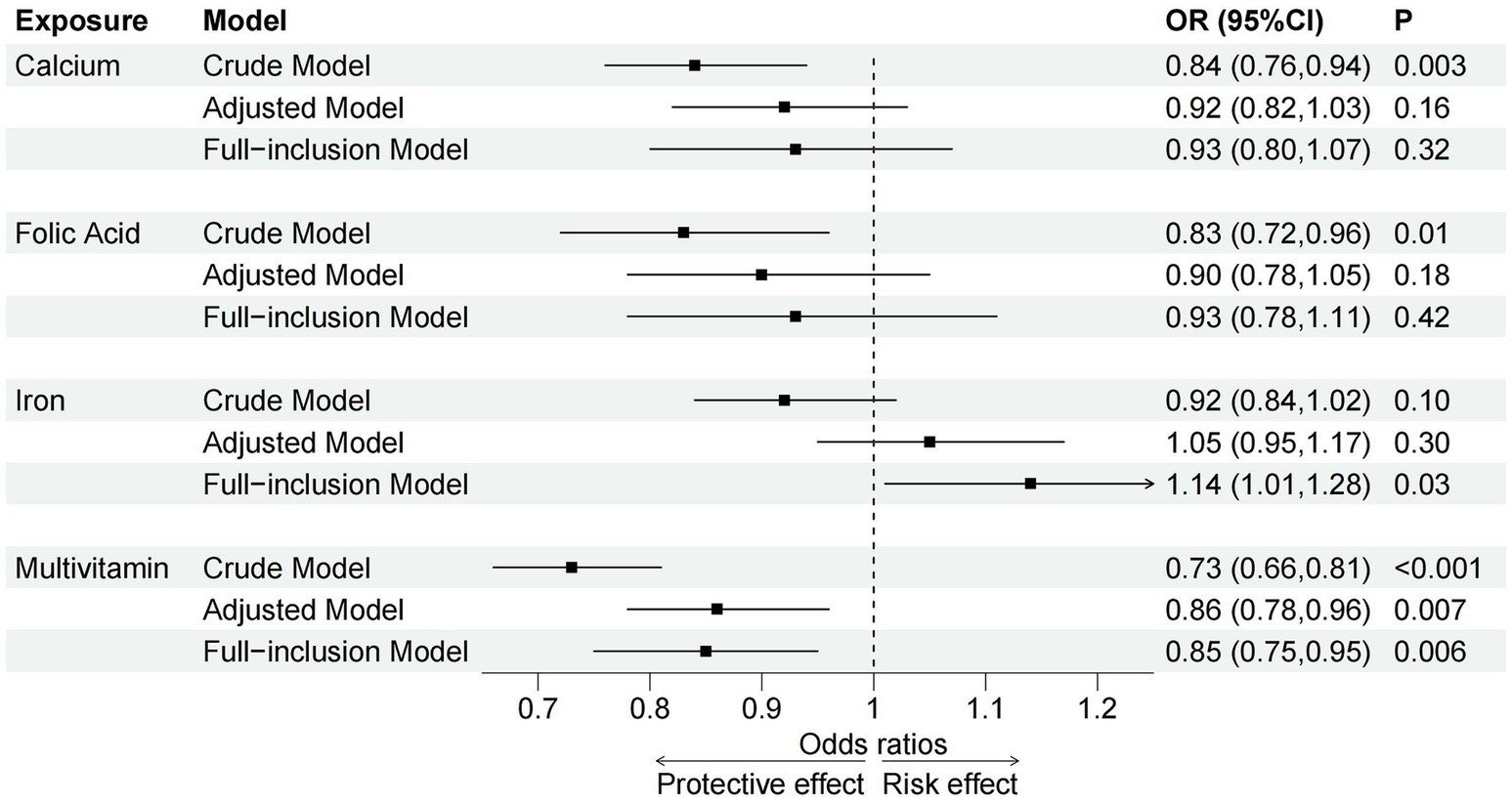
Figure 3. Associations between prenatal micronutrient supplementation and NDD in crude, adjusted and full-inclusion models. OR, odds ratio; CI, confidence interval; P, p-value.
When the analysis was stratified by probiotic intake, we found that prenatal micronutrient supplementation was not significantly associated with NDD without childhood probiotic intake. However, with childhood probiotic intake, the association between prenatal micronutrient supplementation and NDD followed the same pattern as in the whole sample, with multivitamin supplementation associated with a decreased risk of NDD across all three models (Crude model: OR = 0.71, 95% CI = 0.63–0.79; Adjusted Model: OR = 0.84, 95% CI = 0.75–0.94; Full-inclusion Model: OR = 0.83, 95% CI = 0.74–0.94). Although no significant differences were observed between the probiotic and non-probiotic intake subgroups, most ORs were lower in the probiotic intake subgroup, with many associations showing statistical significance within this subgroup only (Figure 4). According to the stratified analysis in the five domains, compared to the non-probiotic intake subgroup, prenatal folic acid and multivitamin supplementation were significantly associated with reduced risks in the gross motor and personal-social domains in the probiotic intake group. In the communication, fine motor and problem-solving domains, though lower ORs were observed, none showed statistical significance in the probiotic intake group (Supplementary Table 5).
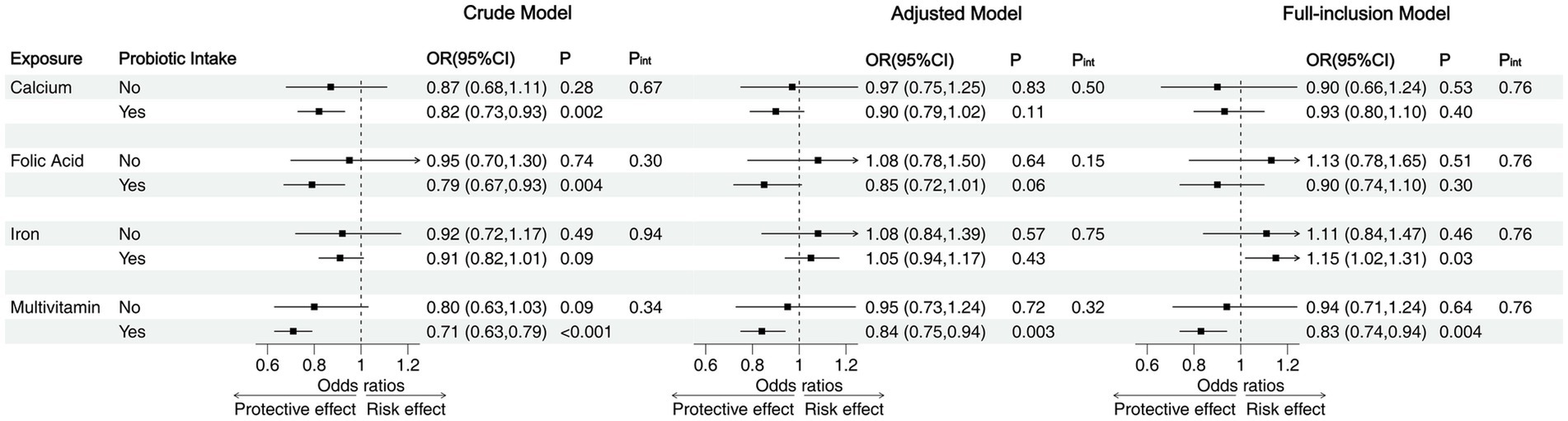
Figure 4. Stratified analysis of the associations between prenatal micronutrient supplementation and NDD by childhood probiotic intake. OR, odds ratio; CI, confidence interval; P, p-value; Pint, p-value for the interaction between prenatal micronutrient supplementation and childhood probiotic intake.
3.3 Effect of early childhood probiotic intake on association between prenatal micronutrient supplementation and neurobehavioral development in preschool children
In the crude model, the results showed that childhood probiotic intake was significantly associated with an enhanced protective effect of prenatal multivitamin supplementation on NDD (Total EOR = −0.33, 95% CI = −0.54 to−0.12 vs. CDE EOR = −0.22, 95% CI = −0.46 to 0.03). Most of this protective effect from childhood probiotic intake was driven by the INTref (EOR = −0.16, 95% CI = −0.49 to 0.16), accounting for 48% of the total effect. The significant mediating effect of probiotic intake was also observed (EOR = 0.10, 95% CI = 0.03–0.16). The INTmed followed (EOR = −0.05, 95% CI = −0.16 to 0.05), suggesting the presence of both interaction and mediation of childhood probiotic intake. No significant enhanced protective effects by childhood probiotic intake were observed in relation to prenatal supplementation of calcium, folic acid or iron (Figure 5; Table 3). Across the five domains, childhood probiotic intake was significantly associated with an enhanced protective effect of prenatal multivitamin supplementation on disorders in gross motor development (Total EOR = −0.31, 95% CI = −0.56 to −0.07) and personal-social development (Total EOR = −0.50, 95% CI = −0.97 to −0.03). The effect of prenatal folic acid supplementation on disorders of personal-social development was also significantly increased by childhood probiotic intake (Total EOR = −0.51, 95% CI = −0.88 to −0.14) (Supplementary Table 6).
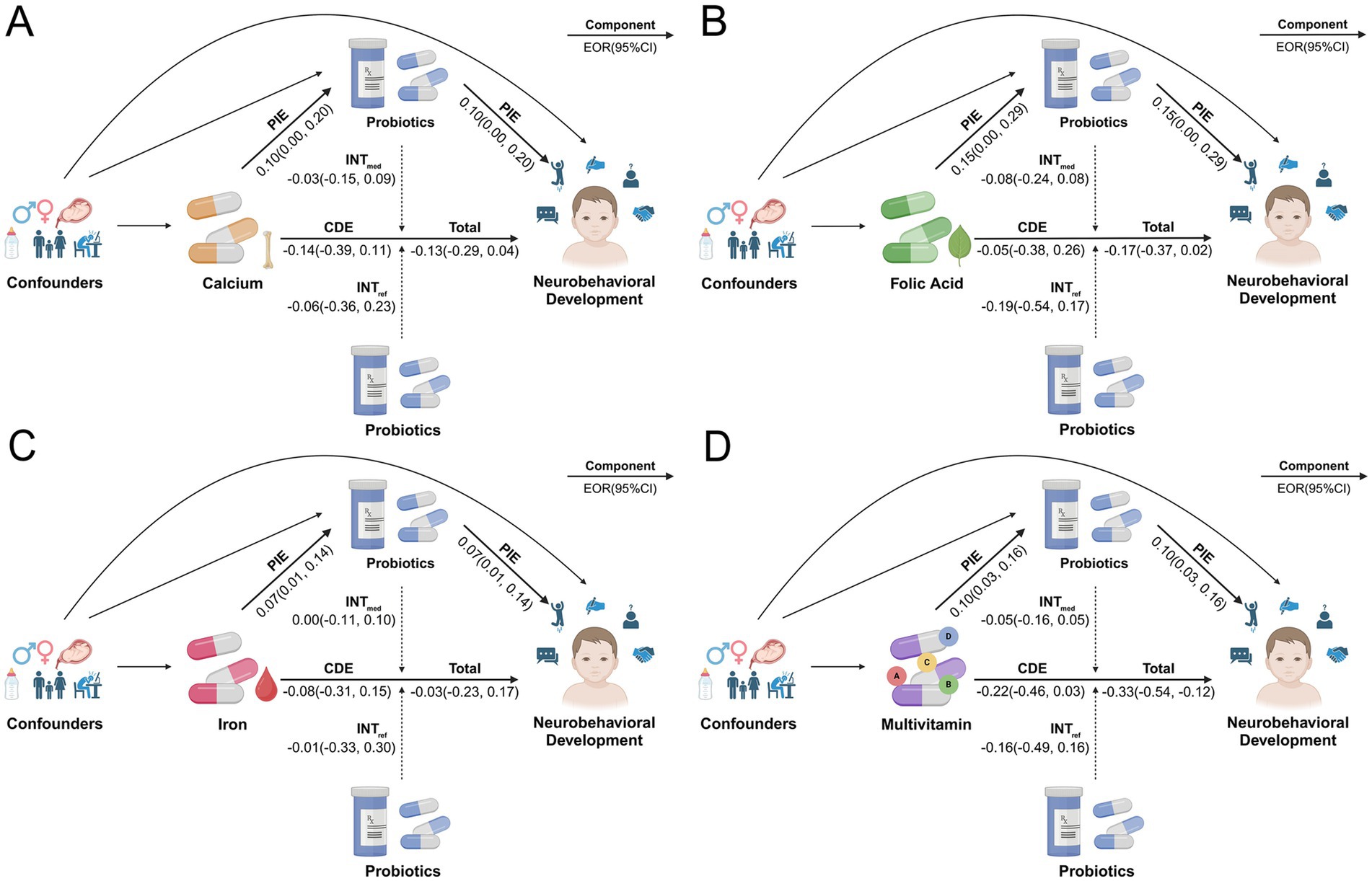
Figure 5. Four-way decomposition diagram of the effect of childhood probiotic intake on the association between prenatal micronutrient supplementation and NDD in the crude model. (A) Calcium supplementation; (B) Folic acid supplementation; (C) Iron supplementation; (D) Multivitamin supplementation. CDE, controlled direct effect; CI, confidence interval; EOR, excess odds ratio; INTmed, mediated interaction; INTref, reference interaction; OR, odds ratio; PIE, pure indirect effect. Created with BioRender.com.
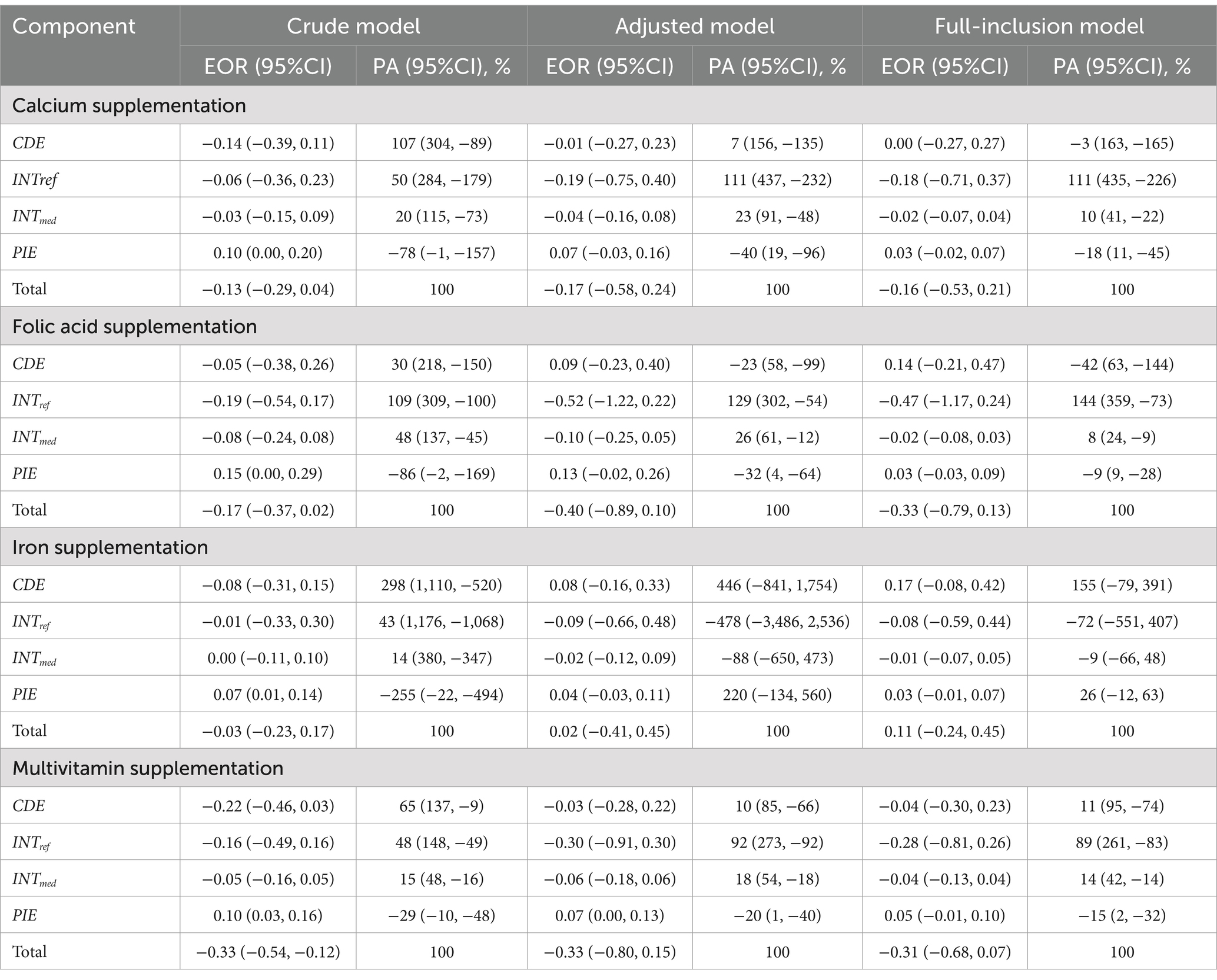
Table 3. The effect of childhood probiotic intake on the association between prenatal micronutrient supplementation and NDD using the four-way decomposition.
3.4 Preventable NDD by prenatal micronutrient supplementation and childhood probiotic intake
In the crude model where a significant effect of childhood probiotic intake was observed on the association between prenatal multivitamin supplementation and NDD, we assumed three scenarios: In the scenario assuming no probiotic intake in early childhood (counterfactual scenario), among the 15,636 participants, prenatal multivitamin supplementation alone could prevent 123 children from developing NDD. Under current childhood probiotic intake proportion (realistic scenario), 73 additional NDD cases could be prevented compared to no probiotic intake, representing a 59% increase in preventive effect. If childhood probiotic intake were increased to a scenario where all children consume probiotics (ideal scenario), it could potentially prevent 96 more children from developing NDD compared to no probiotic intake, representing a 78% increase (Figure 6).
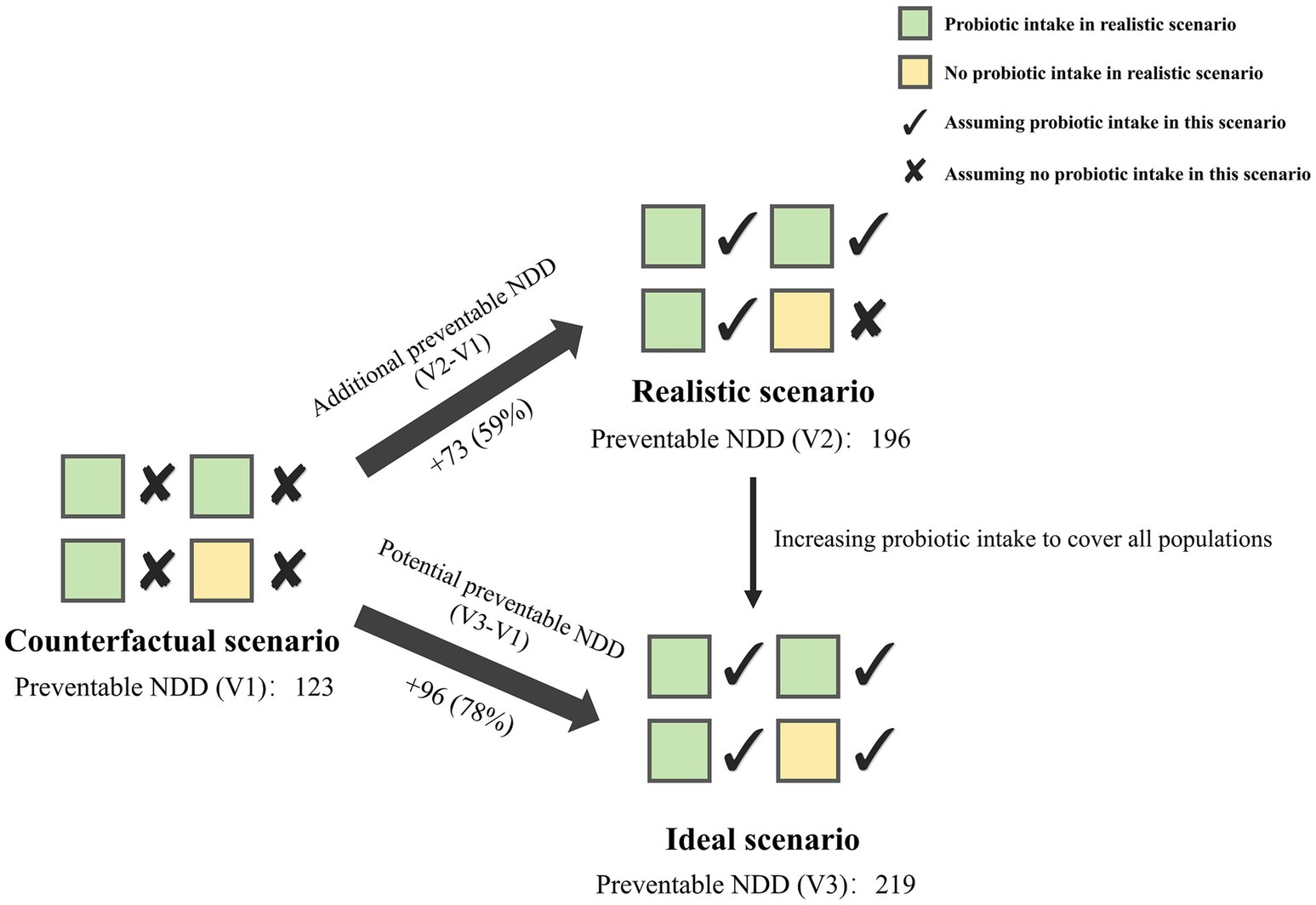
Figure 6. Preventable NDD by prenatal multivitamin supplementation and childhood probiotic intake in the crude model. Counterfactual scenario: without probiotic intake; Realistic scenario: with probiotic intake in the realistic proportion of the survey population; Ideal scenario: with probiotic intake in all populations. NDD, neurobehavioral developmental disorder.
The highest number of preventable NDD was in the gross motor domain (47 cases in the realistic scenario and 64 in the ideal scenario), followed by the personal-social domain (19 in the realistic scenario and 24 in the ideal scenario). The highest percentage increase in NDD prevention was observed in the fine motor domain, with a 92% increase in the realistic scenario and a 117% increase in the ideal scenario, followed by the personal-social domain (80% in the realistic and 100% in the ideal scenario) (Table 4). The number of preventable NDD across the five domains under different models is detailed in Supplementary Table 7. Most prenatal micronutrient supplementation with childhood probiotic intake showed varied increases in NDD prevention in both realistic and ideal scenarios.
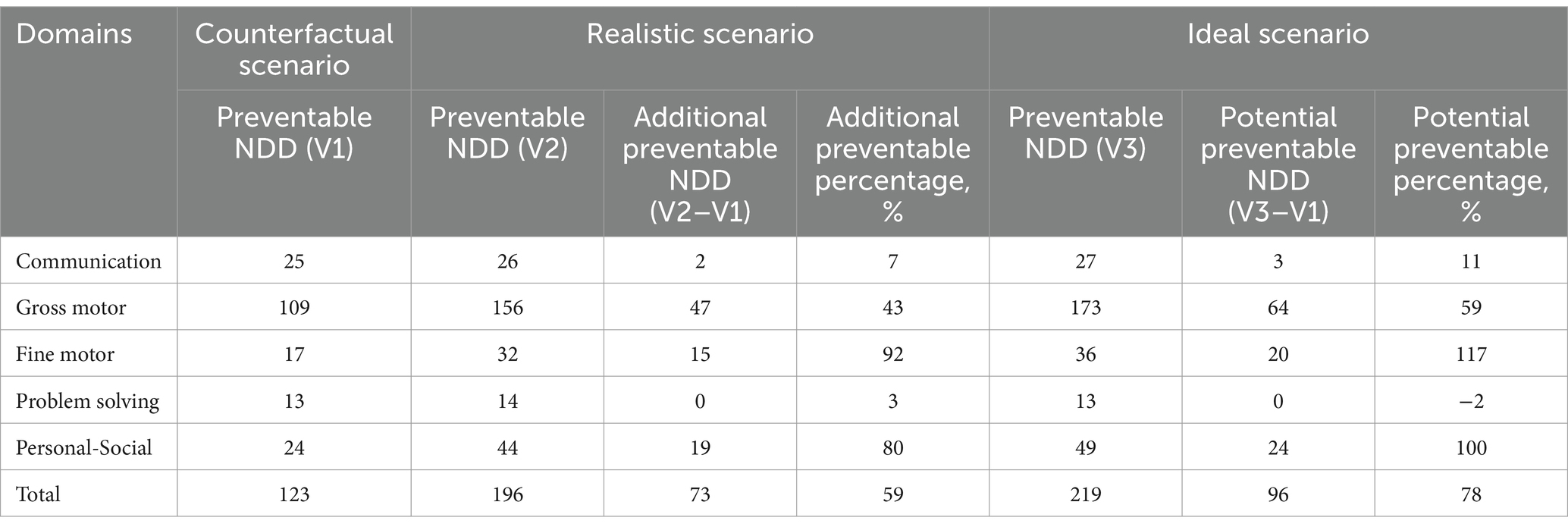
Table 4. Number of preventable NDD by prenatal multivitamin supplementation and childhood probiotic intake in counterfactual, realistic, and ideal scenario in the crude model.
4 Discussion
Our study revealed that 11.7% of preschool children were identified with NDD in the 2022 children’s survey. We found that prenatal multivitamin supplementation was significantly associated with a reduced risk of NDD across the crude, adjusted and full-inclusion models. When exploring the effect of probiotic intake in early childhood, our results indicated that childhood probiotic intake was associated with an enhanced protective effect of prenatal multivitamin supplementation against NDD in the crude model (Total EOR = −0.33, 95% CI = −0.54 to 0.12), with 48% of the effect attributable to interactions. Quantifying this enhanced protective effect, our study demonstrated that childhood probiotic intake contributed to the prevention of 73 (a 59% increase) additional NDD cases, with the potential to prevent 96 additional NDD cases (a 78% increase) if childhood probiotic intake were consumed by all populations.
Previous research have found that prenatal single vitamin supplementation, such as vitamins D and B12, are associated with a lower risk of NDD in children (25, 64–66), while multivitamins also significantly promote neurobehavioral development in children (67, 68), which is consistent with the results of this study. Vitamins are essential for fetal brain development, serving as cofactors in neurotransmitter synthesis and enzymatic metabolism processes (69). For instance, vitamin B12 is crucial for fatty acid metabolism necessary for myelin sheath production, while vitamin B6 functions as a coenzyme in the synthesis of various amino acid neurotransmitters, both of which can influence neurobehavioral development (70, 71). Retinoids, derived from vitamin A, contribute to neuronal differentiation and influence functions like memory and sleep (72). Some evidence suggests that multivitamin supplementation exert broader effects on neurobehavioral development because they allow multiple biological pathways for effects (71). However, other studies indicated that multivitamin supplementation not always more effective than single vitamins for cognitive function (73). Further research is warranted to clarify the comparative benefits of multivitamin supplementation versus single-vitamin supplementation in neurobehavioral development. Despite the known benefits of vitamins, our study found that fewer than half of pregnant women took multivitamins, as reported in other surveys, highlighting a need to improve multivitamin supplementation practices (66).
Folic acid, widely recognized as an important substance in neural tube development, is supplemented at a higher prevalence, possibly because of its inclusion in the WHO’s list of essential medicines for pregnant women (74–76). Similar to our findings, studies have shown that prenatal folic acid supplementation is positively associated with children’s neurobehavioral development (77, 78). However, some reports suggest that excessive folic acid intake may increase the risk of ASD and food allergies, indicating a U-shaped association (79, 80). This underscores the importance of determining precise supplementation levels, especially given that nearly all prenatal foods already contain increased levels of folic acid (66). Iron and calcium, involved in neurotransmitter function, energy metabolism, and myelination, may also influence neurobehavioral development (81, 82). However, our study did not find a significant association between prenatal calcium supplementation and neurobehavioral outcomes, and even identified iron supplementation as a risk factor. Similar conclusions have been drawn in other population studies (83, 84). Variability in study populations, research design, exposure timing and neurobehavioral assessment tools may account for these results (84, 85).
Our findings further suggest that childhood probiotic intake is associated with an enhanced protective effect of prenatal multivitamin supplementation against NDD, primarily through their interaction, as suggested by previous reviews (86, 87). On the one hand, vitamins benefit maternal gut microbiota, with vitamin A and B2 increasing microbial diversity and abundance and vitamins A and D maintaining intestinal barrier integrity (88–90). Beneficial maternal microbes can be transferred to offspring through the birth canal, breast milk, and even vertically in utero (42, 91–93). Probiotic intake in early childhood may further enhance the colonization and function of these inherited beneficial microbiota, improving neurobehavioral outcomes through the gut-brain axis (55, 94). On the other hand, gut microbiota can synthesize specific vitamins such as vitamin K and the B vitamins (95), which can improve cognitive function and reduce the risk of NDD in multiple pathways (96, 97). These findings reinforce the idea that maternal nutrition during pregnancy interacts with offspring gut microbiota to influence neurobehavioral development. Additionally, our study also found that probiotics acted as a reverse mediator in the relationship between micronutrients and NDD, potentially increasing the risk of NDD. One possible explanation is that mothers who self-medicate during pregnancy are more likely to administer probiotics to their children (43), often to address gut microbiota imbalances. Such pre-existing gut microbiota imbalances in children may adversely affect neurobehavioral development through oxidative stress (98).
Quantifying the enhanced protective effect of childhood probiotic intake on NDD, we found the effect was particularly present in gross motor, fine motor and personal-social developmental disorders. The reason may be that the influence of gut microbiota on the brain is mainly concentrated in the limbic system and motor cortex, which are related to emotion and motor coordination, while its effects on the prefrontal cortex and hippocampus, which are associated with problem-solving, are more indirect (99, 100). This result also echoes the protective effects of probiotic intake against Parkinson’s disease, which is associated with fine motor disorders, and ASD, which is associated with emotional–social dysfunction (101, 102). In addition, we also observed that early childhood probiotic intake significantly increased the number of gross motor developmental disorders prevented by prenatal multivitamin supplementation, which happened to be the most prevalent type of NDD in our study. This suggests that early childhood probiotic intake can specifically target the prevention of neurobehavioral developmental domains that most need improvement.
This study makes several significant contributions. First, it innovatively introduces early childhood probiotic intake as a key variable to explore its effect on the association between prenatal micronutrient supplementation and neurobehavioral development in children, addressing the limitations of previous studies focused on single time windows. Second, the study employs advanced statistical techniques, including four-way decomposition and counterfactual mediation analysis, to systematically evaluate the potential effect of probiotic intake from different perspectives. Finally, the study is based on a large-scale children’s survey, enhancing the reliability and generalizability of the findings.
Nevertheless, the study has several limitations. First, as all participants were recruited from Shenzhen, China, our findings may not be generalizable to other populations. Second, data collection relied on retrospective questionnaires, which may introduce recall bias. Additionally, ASQ-3 assessments, based on maternal reports, may be subject to reporting bias compared to clinical diagnoses. Moreover, prenatal micronutrient supplementation and childhood probiotic intake were recorded as binary variables, lacking detailed dosage information and probiotic strain data. Although we adjusted for several factors that may reflect maternal health consciousness on dietary supplement use, the potential for self-selection bias remains. Lastly, although we controlled for confounders, the cross-sectional study design limits causal inference.
Therefore, future studies should focus on determining specific supplementation dosages to establish a dose–response relationship, providing clearer supplementation guidelines. Higher-evidence studies, such as RCTs, are needed to confirm the causal relationship between prenatal micronutrient supplementation, childhood probiotic intake, and NDD. In addition, molecular-level research is essential to elucidate the biological mechanisms underlying these effects and to explore the complex pathways influencing different neurobehavioral domains.
5 Conclusion
In summary, our study found that prenatal multivitamin supplementation has a protective effect against NDD in preschool children. Early childhood probiotic intake is associated with an enhancement of this protective effect, primarily driven by interaction with prenatal multivitamin supplementation. Early childhood probiotic intake could prevent up to 60% more NDD cases, with a 78% potential increase if childhood probiotic intake were consumed by all populations, particularly in the gross motor, fine motor and personal-social domains. These findings highlight the importance of early-life dietary supplements in NDD prevention, particularly the enhanced protective effect of childhood probiotic intake in combination with prenatal multivitamin supplementation. Despite the promising results, future prospective studies with detailed data are needed to confirm this enhanced effect of childhood probiotic intake and their underlying mechanisms.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors without undue reservation.
Ethics statement
The studies involving humans were approved by Institutional Review Board of the School of Public Health, Sun Yat-sen University, Guangzhou, China. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
Author contributions
LD: Data curation, Conceptualization, Visualization, Formal analysis, Writing – original draft, Methodology. MZ: Writing – original draft, Data curation, Visualization, Formal analysis. ES: Methodology, Supervision, Writing – review & editing. XY: Data curation, Investigation, Writing – review & editing, Resources. GW: Investigation, Resources, Writing – review & editing, Data curation. DS: Writing – review & editing, Data curation, Resources, Investigation. DX: Resources, Writing – review & editing, Data curation, Investigation. YaZ: Investigation, Writing – review & editing, Data curation, Resources. YuZ: Validation, Investigation, Writing – review & editing. FL: Investigation, Validation, Writing – review & editing. RH: Validation, Writing – review & editing, Investigation. LZ: Validation, Investigation, Writing – review & editing. WY: Writing – review & editing, Supervision, Project administration, Resources. WC: Supervision, Methodology, Funding acquisition, Conceptualization, Writing – review & editing, Resources.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was funded by the National Natural Science Foundation of China (grant number: 82173605) obtained by Weiqing Chen, and the Government of Longhua District, Shenzhen, China (Longhua STE Fund) (grant number 2013142).
Acknowledgments
The authors would like to thank all the participants in the study as well as the clinicians at Maternal and Child Healthcare Hospital of Longhua District, for recruiting participants and collecting data. Additionally, we want to express our gratitude for the drawing materials provided by BioRender.
Conflict of interest
YuZ, FL, RH, and LZ were employed by Biostime (Guangzhou) Health Products Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2025.1614820/full#supplementary-material
References
1. Shah, PJ, Boilson, M, Rutherford, M, Prior, S, Johnston, L, Maciver, D, et al. Neurodevelopmental disorders and neurodiversity: definition of terms from Scotland’s National Autism Implementation Team. Br J Psychiatry. (2022) 221:577–9. doi: 10.1192/bjp.2022.43
2. Grantham-McGregor, S, Cheung, YB, Cueto, S, Glewwe, P, Richter, L, and Strupp, B. Developmental potential in the first 5 years for children in developing countries. Lancet (London, England). (2007) 369:60–70. doi: 10.1016/S0140-6736(07)60032-4
3. Thapar, A, Cooper, M, and Rutter, M. Neurodevelopmental disorders. Lancet Psychiatry. (2017) 4:339–46. doi: 10.1016/S2215-0366(16)30376-5
4. WHO U. Global report on children with developmental disabilities: From the margins to the mainstream. (2023). Available online at: https://www.unicef.org/documents/global-report-children-developmental-disabilities
5. Zhang, J, Lu, H, Sheng, Q, Zang, E, Zhang, Y, Yuan, H, et al. The influence of perinatal psychological changes on infant neurodevelopment in Shanghai, China: a longitudinal group-based trajectory analysis. J Affect Disord. (2024) 361:291–8. doi: 10.1016/j.jad.2024.06.029
6. Ma, R, Wang, P, Yang, Q, Zhu, Y, Zhang, L, Wang, Y, et al. Interpregnancy interval and early infant neurodevelopment: the role of maternal-fetal glucose metabolism. BMC Med. (2024) 22:2. doi: 10.1186/s12916-023-03191-0
7. Li, Y, Chen, X, Shang, X, and He, H. Developmental screening and analysis of influencing factors in 2,980 infants under 3 months in Beijing (in Chinese). Beijing Med. (2022) 44:513–7. doi: 10.15932/j.0253-9713.2022.06.009
8. Chen, C, Lin, Y, Yan, W, and Zhang, Y. Ages and stages questionnaire in screening developmental levels of infants from 6 to 12 months and risk factors analysis (in Chinese). J Bio-Educ. (2022) 10:318–22. Available online at: https://kns.cnki.net/kcms2/article/abstract?v=Zb3wS6iuiaOzBB2EoPnZJc12wJH79vD2DUO7qkyDckE9OAh1h3WOCn7swVxxWWLkxq55dq5ME3wL2ruhF7IwiG_3nb4E0tJIZpg9VUCDaQFCTmIuhGxgGGWyppbQgR1B-ux25-B6DeBiPnaQv0gMph3R7PE5ZMKUImxf_GuxcSnOfRuSsUUn9JaVQL8O0YWxEsNq6OVAWuk=&uniplatform=NZKPT&language=CHS
9. Maenner, MJ, Warren, Z, Williams, AR, Amoakohene, E, Bakian, AV, Bilder, DA, et al. Prevalence and characteristics of autism Spectrum disorder among children aged 8 years - autism and developmental disabilities monitoring network, 11 sites, United States, 2020. Morbid Mortal Weekly Rep Surveill Summ. (2023) 72:1–14. doi: 10.15585/mmwr.ss7202a1
10. Bachmann, CJ, Scholle, O, Bliddal, M, dosReis, S, Odsbu, I, Skurtveit, S, et al. Recognition and management of children and adolescents with conduct disorder: a real-world data study from four western countries. Child Adolesc Psychiatry Ment Health. (2024) 18:18. doi: 10.1186/s13034-024-00710-6
11. Jensen De López, KM, and Thirup Møller, H. Prevalence of autism in Scandinavian countries (Denmark, Norway, Sweden), and Nordic countries (Finland, Iceland, the Faroe Islands, and Greenland). Neuropsychiatr Dis Treat. (2024) 20:1597–612. doi: 10.2147/NDT.S466081
12. Collaborators, GNSD. Global, regional, and national burden of disorders affecting the nervous system, 1990-2021: a systematic analysis for the global burden of disease study 2021. Lancet Neurol. (2024) 23:344–81. doi: 10.1016/S1474-4422(24)00038-3
13. Lavelle, TA, Weinstein, MC, Newhouse, JP, Munir, K, Kuhlthau, KA, and Prosser, LA. Economic burden of childhood autism spectrum disorders. Pediatrics. (2014) 133:e520–9. doi: 10.1542/peds.2013-0763
14. Zhao, Y, Lu, F, Wang, X, Luo, Y, Zhang, R, He, P, et al. The economic burden of autism spectrum disorder with and without intellectual disability in China: A nationwide cost-of-illness study. Asian J Psychiatr. (2024) 92:103877. doi: 10.1016/j.ajp.2023.103877
15. Lewis, AJ, Galbally, M, Gannon, T, and Symeonides, C. Early life programming as a target for prevention of child and adolescent mental disorders. BMC Med. (2014) 12:33. doi: 10.1186/1741-7015-12-33
16. McGowan, EC, Hofheimer, JA, O’Shea, TM, Kilbride, H, Carter, BS, Check, J, et al. Analysis of neonatal neurobehavior and developmental outcomes among preterm infants. JAMA Netw Open. (2022) 5:e2222249. doi: 10.1001/jamanetworkopen.2022.22249
17. Cusick, SE, Barks, A, and Georgieff, MK. Nutrition and brain development In: SL Andersen, editor. Sensitive periods of brain development and preventive interventions. Cham: Springer International Publishing (2022). 131–65.
18. Hu, Y, Wang, R, Mao, D, Chen, J, Li, M, Li, W, et al. Vitamin D nutritional status of Chinese pregnant women, comparing the Chinese National Nutrition Surveillance (CNHS) 2015-2017 with CNHS 2010-2012. Nutrients. (2021) 13:2237. doi: 10.3390/nu13072237
19. Black, RE, Victora, CG, Walker, SP, Bhutta, ZA, Christian, P, de Onis, M, et al. Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet (London, England). (2013) 382:427–51. doi: 10.1016/S0140-6736(13)60937-X
20. Han, T, Dong, J, Zhang, J, Zhang, C, Wang, Y, Zhang, Z, et al. Nutrient supplementation among pregnant women in China: an observational study. Public Health Nutr. (2022) 25:1537–42. doi: 10.1017/S1368980021001269
21. Arija, V, Hernández-Martínez, C, Tous, M, Canals, J, Guxens, M, Fernández-Barrés, S, et al. Association of iron status and intake during pregnancy with neuropsychological outcomes in children aged 7 years: the prospective birth cohort Infancia y Medio Ambiente (INMA) study. Nutrients. (2019) 11:2999. doi: 10.3390/nu11122999
22. Janbek, J, Sarki, M, Specht, IO, and Heitmann, BL. A systematic literature review of the relation between iron status/anemia in pregnancy and offspring neurodevelopment. Eur J Clin Nutr. (2019) 73:1561–78. doi: 10.1038/s41430-019-0400-6
23. Moumin, NA, Shepherd, E, Liu, K, Makrides, M, Gould, JF, Green, TJ, et al. The effects of prenatal iron supplementation on offspring neurodevelopment in upper middle- or high-income countries: a systematic review. Nutrients. (2024) 16:2499. doi: 10.3390/nu16152499
24. Wicklow, B, Gallo, S, Majnemer, A, Vanstone, C, Comeau, K, Jones, G, et al. Impact of vitamin D supplementation on gross motor development of healthy term infants: a randomized dose-response trial. Phys Occup Ther Pediatr. (2016) 36:330–42. doi: 10.3109/01942638.2015.1050150
25. Mutua, AM, Mogire, RM, Elliott, AM, Williams, TN, Webb, EL, Abubakar, A, et al. Effects of vitamin D deficiency on neurobehavioural outcomes in children: a systematic review. Wellcome Open Res. (2020) 5:28. doi: 10.12688/wellcomeopenres.15730.1
26. McCarthy, EK, Murray, DM, Malvisi, L, Kenny, LC, Hourihane, JO, Irvine, AD, et al. Antenatal vitamin D status is not associated with standard neurodevelopmental assessments at age 5 years in a well-characterized prospective maternal-infant cohort. J Nutr. (2018) 148:1580–6. doi: 10.1093/jn/nxy150
27. Markhus, MW, Dahl, L, Moe, V, Abel, MH, Brantsæter, AL, Øyen, J, et al. Maternal iodine status is associated with offspring language skills in infancy and toddlerhood. Nutrients. (2018) 10:1270. doi: 10.3390/nu10091270
28. Murcia, M, Espada, M, Julvez, J, Llop, S, Lopez-Espinosa, MJ, Vioque, J, et al. Iodine intake from supplements and diet during pregnancy and child cognitive and motor development: the INMA mother and child cohort study. J Epidemiol Community Health. (2018) 72:216–22. doi: 10.1136/jech-2017-209830
29. Jalali Chimeh, F, Aghaie, E, Ghavi, S, and Fatahnia, R. Investigation of the effects of maternal nutrition during pregnancy on cognitive functions of toddlers: a systematic review. Int J Prev Med. (2024) 15:15. doi: 10.4103/ijpvm.ijpvm_124_22
30. Bergen, NE, Schalekamp-Timmermans, S, Jaddoe, VW, Hofman, A, Lindemans, J, Russcher, H, et al. Maternal and neonatal markers of the homocysteine pathway and fetal growth: the generation R study. Paediatr Perinat Epidemiol. (2016) 30:386–96. doi: 10.1111/ppe.12297
31. Kok, DE, Dhonukshe-Rutten, RA, Lute, C, Heil, SG, Uitterlinden, AG, van der Velde, N, et al. The effects of long-term daily folic acid and vitamin B12 supplementation on genome-wide DNA methylation in elderly subjects. Clin Epigenetics. (2015) 7:121. doi: 10.1186/s13148-015-0154-5
32. Krężel, A, and Maret, W. The functions of metamorphic metallothioneins in zinc and copper metabolism. Int J Mol Sci. (2017) 18:1237. doi: 10.3390/ijms18061237
33. Oestreicher, P, and Cousins, RJ. Copper and zinc absorption in the rat: mechanism of mutual antagonism. J Nutr. (1985) 115:159–66. doi: 10.1093/jn/115.2.159
34. Doets, EL, Ueland, PM, Tell, GS, Vollset, SE, Nygård, OK, Van’t Veer, P, et al. Interactions between plasma concentrations of folate and markers of vitamin B(12) status with cognitive performance in elderly people not exposed to folic acid fortification: the Hordaland health study. Br J Nutr. (2014) 111:1085–95. doi: 10.1017/S000711451300336X
36. Gibson, GR, Hutkins, R, Sanders, ME, Prescott, SL, Reimer, RA, Salminen, SJ, et al. The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat Rev Gastroenterol Hepatol. (2017) 14:491–502. doi: 10.1038/nrgastro.2017.75
37. Shaaban, SY, El Gendy, YG, Mehanna, NS, El-Senousy, WM, El-Feki, HSA, Saad, K, et al. The role of probiotics in children with autism spectrum disorder: a prospective, open-label study. Nutr Neurosci. (2018) 21:676–81. doi: 10.1080/1028415X.2017.1347746
38. Nahidi, M, Soleimanpour, S, and Emadzadeh, M. Probiotics as a promising therapy in improvement of symptoms in children with ADHD: a systematic review. J Atten Disord. (2024) 28:1163–72. doi: 10.1177/10870547241228828
39. Kwak, MJ, Kim, SH, Kim, HH, Tanpure, R, Kim, JI, Jeon, BH, et al. Psychobiotics and fecal microbial transplantation for autism and attention-deficit/hyperactivity disorder: microbiome modulation and therapeutic mechanisms. Front Cell Infect Microbiol. (2023) 13:1238005. doi: 10.3389/fcimb.2023.1238005
40. Pärtty, A, Kalliomäki, M, Wacklin, P, Salminen, S, and Isolauri, E. A possible link between early probiotic intervention and the risk of neuropsychiatric disorders later in childhood: a randomized trial. Pediatr Res. (2015) 77:823–8. doi: 10.1038/pr.2015.51
41. Parracho, HM, Gibson, GR, Knott, F, Bosscher, D, Kleerebezem, M, AL, MC, et al. A double-blind, placebo-controlled, crossover-designed probiotic feeding study in children diagnosed with autistic spectrum disorders. JIJoP. (2010) 5:69. Available online at: https://www.proquest.com/openview/37c94a7a51197eda67d4fad3b5a8431f/1?cbl=136102&pq-origsite=gscholar
42. Zaidi, AZ, Moore, SE, and Okala, SG. Impact of maternal nutritional supplementation during pregnancy and lactation on the infant gut or breastmilk microbiota: a systematic review. Nutrients. (2021) 13:1137. doi: 10.3390/nu13041137
43. Aydın Aksoy, E, Güçiz Doğan, B, and Yalçın, SS. Nutrient supplements for young children and mothers’ self medication with over-the-counter drugs during the COVID-19 pandemic. Nutrients. (2024) 16:4182. doi: 10.3390/nu16234182
44. El-Heis, S, Barton, SJ, Chang, HF, Nield, H, Cox, V, Galani, S, et al. Maternal mood, anxiety and mental health functioning after combined myo-inositol, probiotics, micronutrient supplementation from preconception: findings from the NiPPeR RCT. Psychiatry Res. (2024) 334:115813. doi: 10.1016/j.psychres.2024.115813
45. Godfrey, KM, Barton, SJ, El-Heis, S, Kenealy, T, Nield, H, Baker, PN, et al. Myo-inositol, probiotics, and micronutrient supplementation from preconception for glycemia in pregnancy: NiPPeR international multicenter double-blind randomized controlled trial. Diabetes Care. (2021) 44:1091–9. doi: 10.2337/dc20-2515
46. Lyons-Reid, J, Derraik, JGB, Kenealy, T, Albert, BB, Ramos Nieves, JM, Monnard, CR, et al. Impact of preconception and antenatal supplementation with myo-inositol, probiotics, and micronutrients on offspring BMI and weight gain over the first 2 years. BMC Med. (2024) 22:39. doi: 10.1186/s12916-024-03246-w
47. Romano-Keeler, J, and Weitkamp, JH. Maternal influences on fetal microbial colonization and immune development. Pediatr Res. (2015) 77:189–95. doi: 10.1038/pr.2014.163
48. Scheuchzer, P, Sinawat, S, Donzé, AS, Zeder, C, Sabatier, M, Garcia-Garcera, M, et al. Iron absorption from an Iron-fortified follow-up formula with and without the addition of a synbiotic or a human-identical milk oligosaccharide: a randomized crossover stable isotope study in young Thai children. J Nutr. (2024) 154:2988–98. doi: 10.1016/j.tjnut.2024.08.016
49. Oliphant, K, Cruz Ayala, W, Ilyumzhinova, R, Mbayiwa, K, Sroka, A, Xie, B, et al. Microbiome function and neurodevelopment in black infants: vitamin B(12) emerges as a key factor. Gut Microbes. (2024) 16:2298697. doi: 10.1080/19490976.2023.2298697
50. Berger, PK, Bansal, R, Sawardekar, S, Monk, C, and Peterson, BS. Associations of maternal prenatal zinc consumption with infant brain tissue organization and neurodevelopmental outcomes. Nutrients. (2025) 17:303. doi: 10.3390/nu17020303
51. Wei, M, Bian, X, Squires, J, Yao, G, Wang, X, Xie, H, et al. Studies of the norm and psychometrical properties of the ages and stages questionnaires, third edition, with a Chinese national sample (in Chinese). Chinese J Pediatr. (2015) 53:913–8. doi: 10.3760/cma.j.issn.0578-1310.2015.12.009
52. Agarwal, PK, Shi, L, Daniel, LM, Yang, PH, Khoo, PC, Quek, BH, et al. Prospective evaluation of the ages and stages questionnaire 3rd edition in very-low-birthweight infants. Dev Med Child Neurol. (2017) 59:484–9. doi: 10.1111/dmcn.13307
53. Lopes, S, Graça, P, Teixeira, S, Serrano, AM, and Squires, J. Psychometric properties and validation of Portuguese version of ages & stages questionnaires (3rd edition): 9, 18 and 30 questionnaires. Early Hum Dev. (2015) 91:527–33. doi: 10.1016/j.earlhumdev.2015.06.006
54. Alving-Jessep, E, Botchway, E, Wood, AG, Hilton, AC, and Blissett, JM. The development of the gut microbiome and temperament during infancy and early childhood: a systematic review. Dev Psychobiol. (2022) 64:e22306. doi: 10.1002/dev.22306
55. Zhang, D, Lan, Y, Zhang, J, Cao, M, Yang, X, and Wang, X. Effects of early-life gut microbiota on the neurodevelopmental outcomes of preterm infants: a multi-center, longitudinal observational study in China. Eur J Pediatr. (2024) 183:1733–40. doi: 10.1007/s00431-024-05423-8
56. Guo, X, Xu, J, Tian, Y, Ouyang, F, Yu, X, Liu, J, et al. Interaction of prenatal maternal selenium and manganese levels on child neurodevelopmental trajectories-the Shanghai birth cohort study. Sci Total Environ. (2024) 915:170095. doi: 10.1016/j.scitotenv.2024.170095
57. Allotey, PA, and Harel, O. Multiple imputation for incomplete data in environmental epidemiology research. Curr Environ Health Rep. (2019) 6:62–71. doi: 10.1007/s40572-019-00230-y
58. VanderWeele, TJ. A unification of mediation and interaction: a 4-way decomposition. Epidemiology. (2014) 25:749–61. doi: 10.1097/EDE.0000000000000121
59. Huang, S, Guo, J, Jiang, R, Ma, K, Lin, F, Li, H, et al. Four-way decomposition of the effects of nutrient supplement and physical exercise on depression among older Chinese: a nationwide cross-sectional analysis. BMC Public Health. (2024) 24:3469. doi: 10.1186/s12889-024-20995-8
60. Ye, R, Shen, J, Mo, Q, Xu, P, Huang, Y, Chen, J, et al. The roles of physical activity and sedentary behavior in the relationship between socioeconomic status and depressive symptoms: observations from a national study. J Affect Disord. (2025) 372:1–9. doi: 10.1016/j.jad.2024.11.062
61. Anindya, K, Zhao, Y, Hoang, T, Lee, JT, Juvekar, S, Krishnan, A, et al. Interrelationships between physical multimorbidity, depressive symptoms and cognitive function among older adults in China, India and Indonesia: a four-way decomposition analysis. Arch Gerontol Geriatr. (2024) 122:105386. doi: 10.1016/j.archger.2024.105386
62. Pearl, J. Causality: models, reasoning and inference. 2nd ed Cambridge University Press, England (2009).
63. Ye, T, Guo, Y, Huang, W, Zhang, Y, Abramson, MJ, and Li, S. Heat exposure, preterm birth, and the role of greenness in Australia. JAMA Pediatr. (2024) 178:376–83. doi: 10.1001/jamapediatrics.2024.0001
64. Cruz-Rodríguez, J, Díaz-López, A, Canals-Sans, J, and Arija, V. Maternal vitamin B12 status during pregnancy and early infant neurodevelopment: the ECLIPSES study. Nutrients. (2023) 15:1529. doi: 10.3390/nu15061529
65. Rodgers, MD, Mead, MJ, McWhorter, CA, Ebeling, MD, Shary, JR, Newton, DA, et al. Vitamin D and child neurodevelopment-a post hoc analysis. Nutrients. (2023) 15:4250. doi: 10.3390/nu15194250
66. Adams, JB, Kirby, JK, Sorensen, JC, Pollard, EL, and Audhya, T. Evidence based recommendations for an optimal prenatal supplement for women in the US: vitamins and related nutrients. Maternal Health Neonatol Perinatol. (2022) 8:4. doi: 10.1186/s40748-022-00139-9
67. Zhu, J, Xu, P, Yan, W, Hu, Y, Guo, H, Chen, F, et al. The influence of multivitamins on neurological and growth disorders: a cross-sectional study. Front Nutr. (2024) 11:1465875. doi: 10.3389/fnut.2024.1465875
68. Wei, Q, Xiao, Y, Yang, T, Chen, J, Chen, L, Wang, K, et al. Predicting autism spectrum disorder using maternal risk factors: a multi-center machine learning study. Psychiatry Res. (2024) 334:115789. doi: 10.1016/j.psychres.2024.115789
69. Ravikumar, N, Chegukrishnamurthi, M, and Gadde, VS. Role of micronutrients in neurological development In: Role of nutrients in neurological disorders, Singapore Springer (2022). 177–99.
70. Guilarte, TR. Vitamin B6 and cognitive development: recent research findings from human and animal studies. Nutr Rev. (1993) 51:193–8. doi: 10.1111/j.1753-4887.1993.tb03102.x
71. Benton, D. Vitamins and neural and cognitive developmental outcomes in children. Proc Nutr Soc. (2012) 71:14–26. doi: 10.1017/S0029665111003247
72. Tafti, M, and Ghyselinck, NB. Functional implication of the vitamin A signaling pathway in the brain. Arch Neurol. (2007) 64:1706–11. doi: 10.1001/archneur.64.12.1706
73. Chang, J, Liu, M, Liu, C, Zhou, S, Jiao, Y, Sun, H, et al. Effects of vitamins and polyunsaturated fatty acids on cognitive function in older adults with mild cognitive impairment: a meta-analysis of randomized controlled trials. Eur J Nutr. (2024) 63:1003–22. doi: 10.1007/s00394-024-03324-y
74. Mantovani, E, Filippini, F, Bortolus, R, and Franchi, M. Folic acid supplementation and preterm birth: results from observational studies. Biomed Res Int. (2014) 2014:481914:1–8. doi: 10.1155/2014/481914
75. WHO. Periconceptional folic acid supplementation to prevent neural tube defects (2023). Available online at: https://www.who.int/tools/elena/interventions/folate-periconceptional
76. WHO. WHO model list of essential medicines 19th edition. (2015). Available online at: https://publichealthupdate.com/who-model-list-of-essential-medicines-april-2015-19th-edition/
77. Chmielewska, A, Dziechciarz, P, Gieruszczak-Białek, D, Horvath, A, Pieścik-Lech, M, Ruszczyński, M, et al. Effects of prenatal and/or postnatal supplementation with iron, PUFA or folic acid on neurodevelopment: update. Br J Nutr. (2019) 122:S10–5. doi: 10.1017/S0007114514004243
78. Caffrey, A, McNulty, H, Rollins, M, Prasad, G, Gaur, P, Talcott, JB, et al. Effects of maternal folic acid supplementation during the second and third trimesters of pregnancy on neurocognitive development in the child: an 11-year follow-up from a randomised controlled trial. BMC Med. (2021) 19:73. doi: 10.1186/s12916-021-01914-9
79. Raghavan, R, Riley, AW, Volk, H, Caruso, D, Hironaka, L, Sices, L, et al. Maternal multivitamin intake, plasma folate and vitamin B(12) levels and autism spectrum disorder risk in offspring. Paediatr Perinat Epidemiol. (2018) 32:100–11. doi: 10.1111/ppe.12414
80. McGowan, EC, Hong, X, Selhub, J, Paul, L, Wood, RA, Matsui, EC, et al. Association between folate metabolites and the development of food allergy in children. J Allergy Clin Immunol Pract. (2020) 8:132–40.e5. doi: 10.1016/j.jaip.2019.06.017
81. Díaz-Piña, DA, Rivera-Ramírez, N, García-López, G, Díaz, NF, and Molina-Hernández, A. Calcium and neural stem cell proliferation. Int J Mol Sci. (2024) 25:4073. doi: 10.3390/ijms25074073
82. Prado, EL, and Dewey, KG. Nutrition and brain development in early life. Nutr Rev. (2014) 72:267–84. doi: 10.1111/nure.12102
83. Kiely, ME, McCarthy, EK, and Hennessy, Á. Iron, iodine and vitamin D deficiencies during pregnancy: epidemiology, risk factors and developmental impacts. Proc Nutr Soc. (2021) 80:290–302. doi: 10.1017/S0029665121001944
84. Zhong, C, Tessing, J, Lee, BK, and Lyall, K. Maternal dietary factors and the risk of autism spectrum disorders: a systematic review of existing evidence. Autism Res. (2020) 13:1634–58. doi: 10.1002/aur.2402
85. Mousa, A, Naqash, A, and Lim, S. Macronutrient and micronutrient intake during pregnancy: an overview of recent evidence. Nutrients. (2019) 11:443. doi: 10.3390/nu11020443
86. Schneider, E, O’Riordan, KJ, Clarke, G, and Cryan, JF. Feeding gut microbes to nourish the brain: unravelling the diet–microbiota–gut–brain axis. Nat Metab. (2024) 6:1454–1478. doi: 10.1016/B978-0-12-814800-6.00008-X
87. Kacimi, FE, Didou, L, Ed Day, S, Azzaoui, FZ, Ramchoun, M, Berrougui, H, et al. Gut microbiota, vitamin a deficiency and autism spectrum disorder: an interconnected trio - a systematic review. Nutr Neurosci. (2024) 28:492–502. doi: 10.1080/1028415X.2024.2389498
88. Pham, VT, Dold, S, Rehman, A, Bird, JK, and Steinert, RE. Vitamins, the gut microbiome and gastrointestinal health in humans. Nutr Res (New York, NY). (2021) 95:35–53. doi: 10.1016/j.nutres.2021.09.001
89. Kong, J, Zhang, Z, Musch, MW, Ning, G, Sun, J, Hart, J, et al. Novel role of the vitamin D receptor in maintaining the integrity of the intestinal mucosal barrier. Am J Physiol Gastrointest Liver Physiol. (2008) 294:G208–16. doi: 10.1152/ajpgi.00398.2007
90. Lima, AA, Soares, AM, Lima, NL, Mota, RM, Maciel, BL, Kvalsund, MP, et al. Effects of vitamin a supplementation on intestinal barrier function, growth, total parasitic, and specific Giardia spp infections in Brazilian children: a prospective randomized, double-blind, placebo-controlled trial. J Pediatr Gastroenterol Nutr. (2010) 50:309–15. doi: 10.1097/MPG.0b013e3181a96489
91. Lawson, MAE, O’Neill, IJ, Kujawska, M, Gowrinadh Javvadi, S, Wijeyesekera, A, Flegg, Z, et al. Breast milk-derived human milk oligosaccharides promote Bifidobacterium interactions within a single ecosystem. ISME J. (2020) 14:635–48. doi: 10.1038/s41396-019-0553-2
92. He, Q, Kwok, LY, Xi, X, Zhong, Z, Ma, T, Xu, H, et al. The meconium microbiota shares more features with the amniotic fluid microbiota than the maternal fecal and vaginal microbiota. Gut Microbes. (2020) 12:1794266. doi: 10.1080/19490976.2020.1794266
93. Collado, MC, Rautava, S, Aakko, J, Isolauri, E, and Salminen, S. Human gut colonisation may be initiated in utero by distinct microbial communities in the placenta and amniotic fluid. Sci Rep. (2016) 6:23129. doi: 10.1038/srep23129
94. Laue, HE, Coker, MO, and Madan, JC. The developing microbiome from birth to 3 years: the gut-brain axis and neurodevelopmental outcomes. Front Pediatr. (2022) 10:815885. doi: 10.3389/fped.2022.815885
95. Rodionov, DA, Arzamasov, AA, Khoroshkin, MS, Iablokov, SN, Leyn, SA, Peterson, SN, et al. Micronutrient requirements and sharing capabilities of the human gut microbiome. Front Microbiol. (2019) 10:1316. doi: 10.3389/fmicb.2019.01316
96. Ferland, G. Vitamin K and brain function. Semin Thromb Hemost. (2013) 39:849–55. doi: 10.1055/s-0033-1357481
97. Zhou, Z, Fan, B, Chen, Q, Li, X, and Ke, X. Individual and combined effects of dietary vitamin intake on cognitive function in elderly adults: the potential mediating role of serum neurofilament light chain levels. Front Nutr. (2025) 12:1485648. doi: 10.3389/fnut.2025.1485648
98. Shandilya, S, Kumar, S, Kumar Jha, N, Kumar Kesari, K, and Ruokolainen, J. Interplay of gut microbiota and oxidative stress: perspective on neurodegeneration and neuroprotection. J Adv Res. (2022) 38:223–44. doi: 10.1016/j.jare.2021.09.005
99. Wu, C, Mu, Q, Gao, W, and Lu, S. The characteristics of anhedonia in depression: a review from a clinically oriented perspective. Transl Psychiatry. (2025) 15:90. doi: 10.1038/s41398-025-03310-w
100. Becker, M, and Cabeza, R. The neural basis of the insight memory advantage. Trends Cogn Sci. (2025) 29:255–68. doi: 10.1016/j.tics.2025.01.001
101. Tripathi, S, Kaushik, M, Dwivedi, R, Tiwari, P, Tripathi, M, and Dada, R. The effect of probiotics on select cognitive domains in mild cognitive impairment and Alzheimer’s disease: a systematic review and meta-analysis. J Alzheimer’s Dis Rep. (2024) 8:1422–33. doi: 10.1177/25424823241289039
102. Caputi, V, Hill, L, Figueiredo, M, Popov, J, Hartung, E, Margolis, KG, et al. Functional contribution of the intestinal microbiome in autism spectrum disorder, attention deficit hyperactivity disorder, and Rett syndrome: a systematic review of pediatric and adult studies. Front Neurosci. (2024) 18:1341656. doi: 10.3389/fnins.2024.1341656
Keywords: neurobehavioral development, micronutrient supplementation, probiotic intake, preschool children, four-way decomposition, counterfactual attribution
Citation: Ding L, Zhang M, Strodl E, Yin X, Wen G, Sun D, Xian D, Zhao Y, Zheng Y, Liu F, Hu R, Zhao L, Yang W and Chen W (2025) The effects of early childhood probiotic intake on the association between prenatal micronutrient supplementation and neurobehavioral development in preschool children: a four-way decomposition analysis. Front. Nutr. 12:1614820. doi: 10.3389/fnut.2025.1614820
Edited by:
Bowen Li, Southwest University, ChinaCopyright © 2025 Ding, Zhang, Strodl, Yin, Wen, Sun, Xian, Zhao, Zheng, Liu, Hu, Zhao, Yang and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Weiqing Chen, Y2hlbndxQG1haWwuc3lzdS5lZHUuY24=; Weikang Yang, eWFuZ3dlaWthbmdAbGhmeXdvcmsuY29t
 Liwen Ding
Liwen Ding Maolin Zhang
Maolin Zhang Esben Strodl
Esben Strodl Xiaona Yin3
Xiaona Yin3 Danxia Xian
Danxia Xian Feitong Liu
Feitong Liu Lingling Zhao
Lingling Zhao Weiqing Chen
Weiqing Chen