- 1Department of Food Science and Technology, Pondicherry University, Pondicherry, India
- 2Université de Lorraine, CNRS, LRGP, Nancy, France
- 3Bioprocess Laboratory, Department of Food Process and Quality Control, University Institute of Technology (IUT), University of Ngaoundere, Ngaoundere, Adamaoua, Cameroon
- 4Division of Biochemistry, ICMR - National Institute of Nutrition, Hyderabad, India
Exopolysaccharides (EPSs) from lactic acid bacteria (LAB) are known to have diverse applications in food and pharmaceuticals due to their functional properties. Emulsification is one of the important potentials of microbial polysaccharides, revealing its role as a natural emulsifier. In this study, glucan has been explored for emulsification with spice oils from Xylopia aethiopica (Xae), Monodora myristica (Mm), and Fagara lepreuri (Fl) Cameroon underutilized spices. Emulsifying ability, turbidity, emulsion droplet size, and micrographs of glucan-based spice oil emulsion were studied. The effect of sonication has been studied on droplet size and morphology of the emulsions. Sonication treatment has improved the emulsion stability by converting the larger emulsion droplets into smaller ones. The results of the disk diffusion assay confirmed antibacterial activity of emulsions, exhibiting high antibacterial efficiencies against food pathogens Listeria monocytogenes 1143, Shewanella putrefaciens 8104, and Salmonella enterica 950. This study underscores the potential of EPS as a natural emulsifying agent in food preservation, pharmaceuticals, and antimicrobial applications.
Highlights
• Spice oils were emulsified with glucan exopolysaccharides extracted from Enterococcus hirae using the ultrasonication method.
• Ultrasonication enhanced emulsion turbidity, improved dispersion, homogeneity, and emulsion stability over 20 days.
• Nanoemulsions demonstrated significant antibacterial activity, particularly against foodborne pathogens.
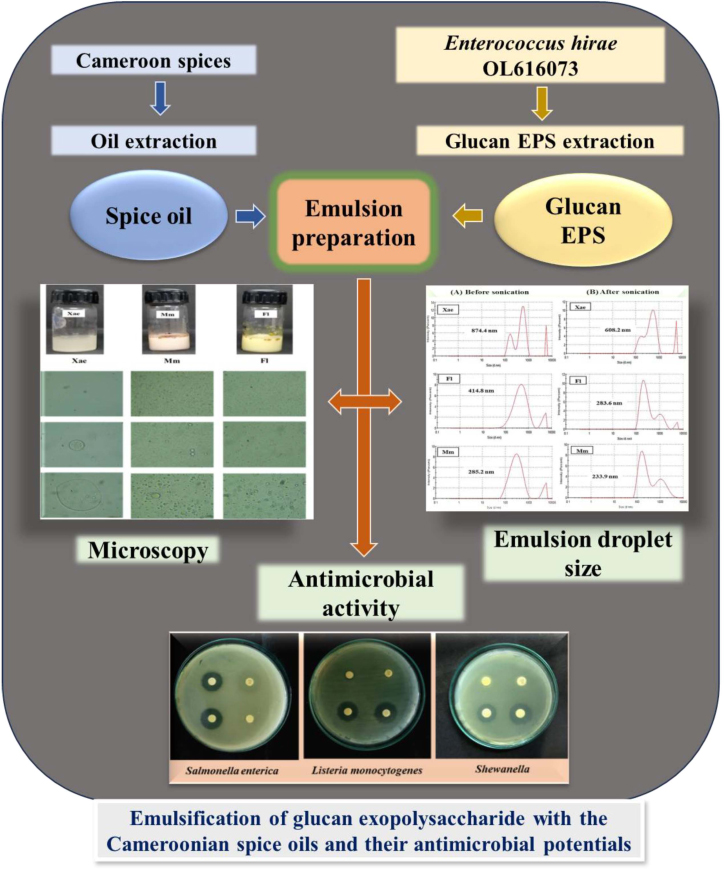
Graphical Abstract. Emulsification of glucan exopolysaccharide with the Cameroonian spice oils and their anti-microbial potentials.
1 Introduction
Natural preservatives derived from plants, animals, or minerals help extend the shelf life of food and other products by inhibiting microbial growth. Common examples include salt, vinegar, honey, and citrus extracts, which have been used for centuries to prevent spoilage. Unlike synthetic preservatives, such alternatives are often considered safer and less likely to cause adverse health effects. With growing consumer demand for clean-label and chemical-free products, natural preservatives, including spice oils, are progressively being used in the food, cosmetic, and pharmaceutical industries (1, 2). Spice oils are essential oils that are extracted from traditional aromatic plants. Spice oils, such as those from cloves, cinnamon, oregano, and rosemary, also serve as effective natural preservatives due to their strong antimicrobial and antioxidant properties. These oils are a homogenous mixture of organic chemical compounds from the same chemical family (3, 4).
Recent studies have proved the microbial inhibitory effects of such spices (5, 6). Cameroonian spice oils have been known to possess antibacterial, antioxidant, anti-plasmodial, and insecticidal activities (7, 8). Three chosen spices, Xylopia aethiopica (Xae), Monodora myristica (Mm), and Fagara leprieurii (Fl), are traditionally valued in Cameroon, not just for their flavor but also for their preservation and medicinal properties. Their cultural and functional context underlines their importance as natural preservatives (9). The oil contents of these spices are 34% for X. aethiopica, 53% for M. myristica, and 33% for F. leuprieurii (10). Incorporation of such spice oils as a preservative is limited by its strong odor and hydrophobicity, which can be overcome by the use of nanotechnology (11, 12). Plant-derived essential oils can be converted to water water-dispersible form by encapsulating them using oil, water, and a proper emulsifier (13). Formulations used in studies of soy-lecithin or polysorbate-stabilized nanoemulsions with other plant-derived oils and natural emulsifiers (e.g., lecithin, gum arabic, saponins) have been shown to enhance stability and antimicrobial delivery compared to systems using synthetic surfactants like Tween 20. The smaller the size of the emulsion droplets, the more the advantage, as it can give better physical stability, good appearance, release kinetics, resistance to degradation and increased biological activity than conventional emulsions (14–16).
Nanoemulsions being the smaller size of emulsions prepared up-to-date, are a class of emulsions with droplet sizes from 20 to 100 nm (6, 17). Because of the small droplet size, nanoemulsions appear transparent or translucent and are more stable with respect to creaming, coalescence, flocculation, and Ostwald ripening than conventional emulsions (18). The physicochemical properties of nanoemulsions are interesting for practical applications because of the small droplet size and long-term stability (19–21).
Nanoemulsion can be prepared by using different high energy and low energy methods including ultrasonication (22, 23). The formation of nanoemulsion is controlled by the relationship between droplet disruption and droplet coalescence (24, 25). The ultrasonic processor applies excellent shear force for droplet disruption, and the rate of droplet coalescence is determined by the mixed exopolysaccharide (EPS) and its concentration (26, 27). There are two main mechanisms operating during ultrasonic emulsification (28). First, an acoustic field produces interfacial waves to break the dispersed phase into the continuous phase (29). Second, the formation of acoustic cavitation is used to collapse micro-bubbles into droplets of nanometric size by pressure fluctuations (30, 31). For proper emulsification, a good emulsifier should be used which shall provide high stability, resulting in good emulsification (15). The given bacterial EPS-producing strain was isolated from an acidic fermented food, further identified as Enterococcus hirae OL616073. The EPS has been extracted as and characterized as glucan containing α-(1→6) and α-(1→3) glycosidic linkage with a backbone of glucose subunits (32). Glucan EPS was chosen over other polysaccharides because the porous, spongy, granular morphology that was observed under scanning electron microscopy, also having strong water solubility index (76.75%), water water holding capacity (296.19%), oil holding capacity (379.91%), and emulsifying activity (EA1- 72.22%). High yield (20 g/L) and physico-functional properties of this EPS have an advantage over other microbial polysaccharides (33). So, to achieve the demand for natural preservation, spice oils and bacterial EPS are used as a preservative and their various biological activities (anti-bacterial, anti-biofilm and minimum inhibitory concentration) are checked against clinically important food pathogens (34, 35).
2 Materials and methods
Three Cameroonian under-utilized spices X. aethiopica (Xae), M. myristica (Mm), and F. lepreuri (Fl) were purchased from Bamenda and Bafoussam markets in Cameroon. Glucan EPS was produced and extracted as reported earlier and used in this study (32). The chemicals, media, antibiotic disks, and enzymes used in this work were of analytical grade and purchased from Hi-Media (Mumbai, India) and Sigma-Aldrich Merck (United States). The reference pathogenic strains causing foodborne illness including Listeria monocytogenes MTCC 1143, Shewanella putrefaciens MTCC 8104 and Salmonella enterica 950 were procured from the Microbial Type Culture Collection (MTCC) and Gene Bank, Institute of Microbial Technology, Chandigarh, India.
2.1 Spice oil extraction
The spices samples were ground using an electric blender (BTC Cast Iron Domestic SS Cup Hand Grinder). About thirty grams of each sample was folded in filter papers and put in extraction timbers. The oil extraction was done using Soxhlet, at 65°C for four hours. The extraction solvent was hexane. After extraction, the oil was dried 45°C to evaporate hexane. It was then stored for further analysis.
2.2 Glucan EPS production
Enterococcus hirae OL616073 was isolated from 14 h fermented idli batter and used for the production of glucan EPS. The EPS extraction was carried out as per the method explained in earlier reports and used to develop the spice oil emulsions in this study (32, 36).
2.3 Emulsion preparation
A set of preliminary trial experiments were performed to demonstrate the emulsifying activity and efficacy prior to the main study. Spice oil emulsions were prepared in a ratio of 3:1 (EPS: Spice oil), where 1% glucan EPS solution with distilled water and three spice oils (Xae, Mm, and Fl) were involved separately. In first phase, the suspension was stirred homogenously and vortexed for 30 min at 40 Hz (Top mix FB 15024, Fisher Scientific, United Kingdom) to make the coarse emulsion. And in second phase, coarse emulsion was subjected for 10 min sonication (model: Labman Ultrasonicator). Coarse and ultrasonicated emulsions were further analyzed in this study.
2.4 Turbidity of emulsions
Exopolysaccharide based spice oil emulsions were diluted 40 times with phosphate buffer solution and phosphate buffer was used as the blank. Absorbance was measured using an LW-1600FC UV spectrophotometer (Shanghai Jinghua Technology Equipment Company, Ltd., Shanghai, China) at 600 nm. Turbidity (T) was calculated as in Li et al. (37) method, using following formula:
A = Absorbance of diluted emulsion at 600 nm measured against the blank. V = Dilution (40).
I = Optical path length (1 cm).
2.5 Microscopy
Before and after sonication, all emulsions were examined at 10, 20 and 40× magnification lenses under Inverted Routine Microscope (Nikon eclipse TS100 microscope) using cavity microscope slides (38).
2.6 Particle size analysis
Spice oil emulsions were subjected for average particle size distribution measurement by using a nanoparticle size analyzer (Zetasizer, United Kingdom) at 25°C.
2.7 Antimicrobial potential of emulsions
Antimicrobial assay was performed against Gram-positive (Listeria monocytogenes MTCC 1143) and Gram-negative (Shewanella putrefaciens MTCC 8104 and Salmonella enterica MTCC 950) pathogenic strains. Using aseptic procedures, overnight incubated broth cultures of Listeria monocytogenes, Shewanella putrefaciens and Salmonella enterica, were spread to form microbial growth lawns on Mueller Hinton agar plates (Himedia, India). The sterile disks were evenly spaced on the surface of the inoculated agar plates and impregnated drop by drop with 20 μl of emulsions. Standard antibiotic disks of Cephoxitin (CX30) were considered as positive control for the assay. Disks were allowed to dry for 15 min to initiate uniform diffusion of emulsion into the agar and then inverted and incubated for 24 h at 37°C. The next day zone of inhibition were recorded in mm around the disks in each plate (16). Appropriate spice oil controls for all three emulsions against the reference pathogenic strains were considered in well diffusion assay performed as per the aforementioned protocol.
2.8 Statistical analysis
All the experiments were carried out in triplicates and the results were represented as mean ± SD.
Graphs were plotted using Microsoft Office 2016, GraphPad Prism 8.0.2, and OriginPro 8 software.
3 Results and discussion
3.1 Turbidity measurement of emulsions
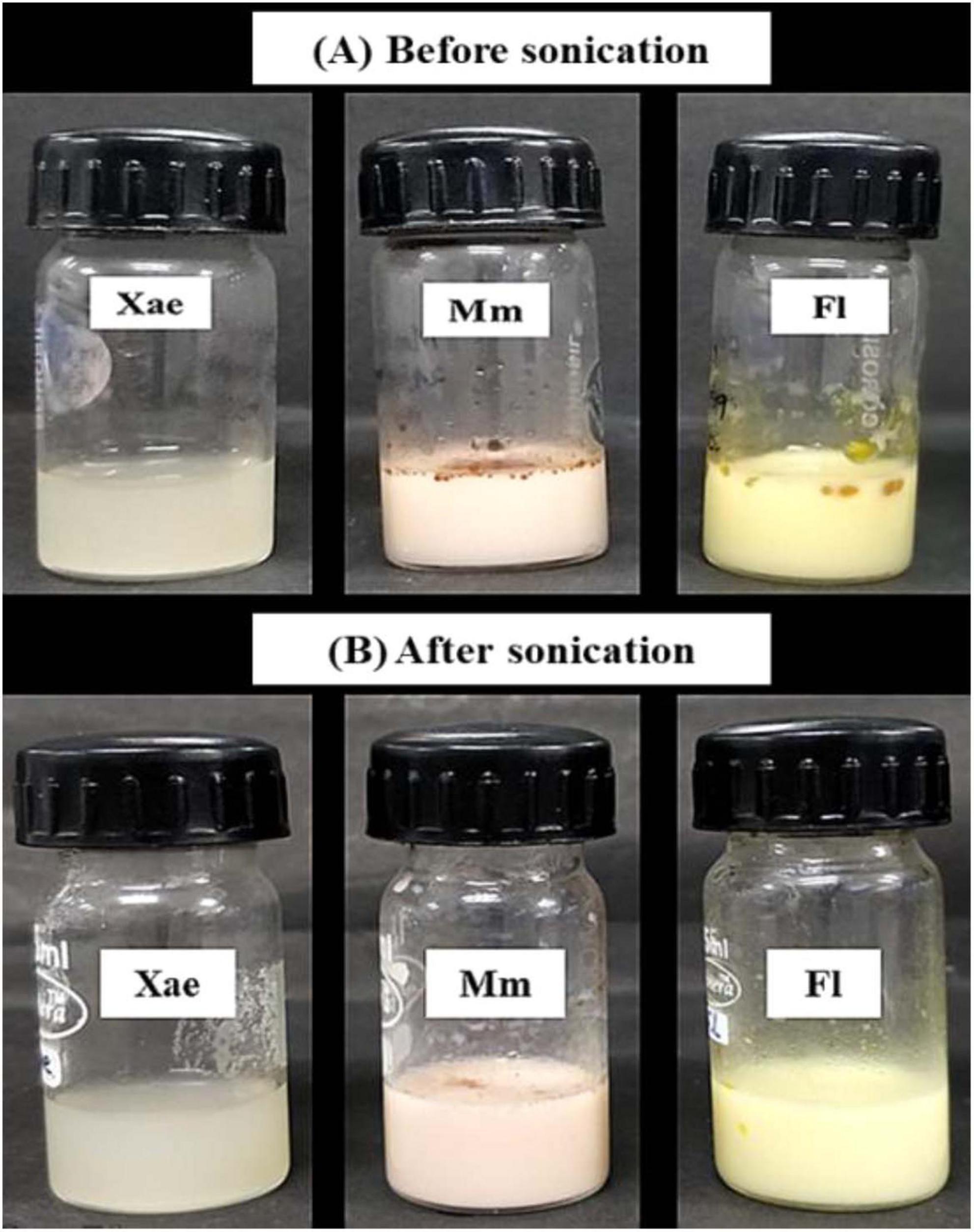
Spice oil emulsions were prepared with glucan EPS in two phases. There was a clear difference in the clarity, turbidity and homogeneity of the emulsions before and after sonication (Figure 1). Emulsion turbidity is directly proportional to the emulsion stability and inversely proportional to the size of emulsion droplets (39).
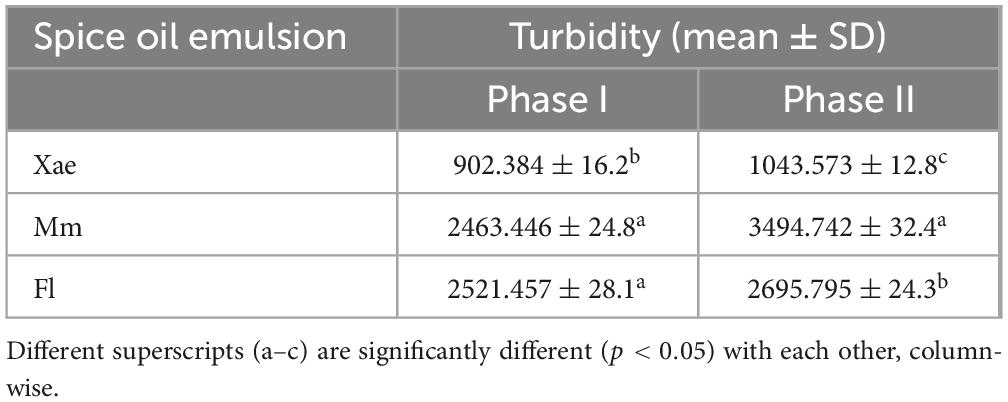
The turbidity of the coarse and sonicated EPS-based spice oil emulsions is recorded in Table 1. It provides insights into the turbidity and stability of various spice oil emulsions across two phases, before and after sonication. Before sonication Xae, Fl, and Mm emulsions showed a turbidity of 902.384 ± 16.2, 2521.457 ± 28.1, and 2463.446 ± 24.8 which increased after the sonication treatment to 1043.573 ± 12.8, 2695.795 ± 24.3, and 3494.742 ± 32.4 respectively. A one-way Analysis of Variance (ANOVA) followed by Tukey’s Significant Difference (HSD) post-hoc test was performed to compare turbidity values among the three emulsions (Xae, Mm, and Fl) within each phase. Superscript letters (a–d) were added to the data table to indicate statistically distinct groups. This moderate increase suggests that the Xae emulsion becomes relatively more stable after sonication, likely due to the breakdown of the emulsion droplets leading to the smaller emulsion droplet size (40).
3.2 Microscopic observation
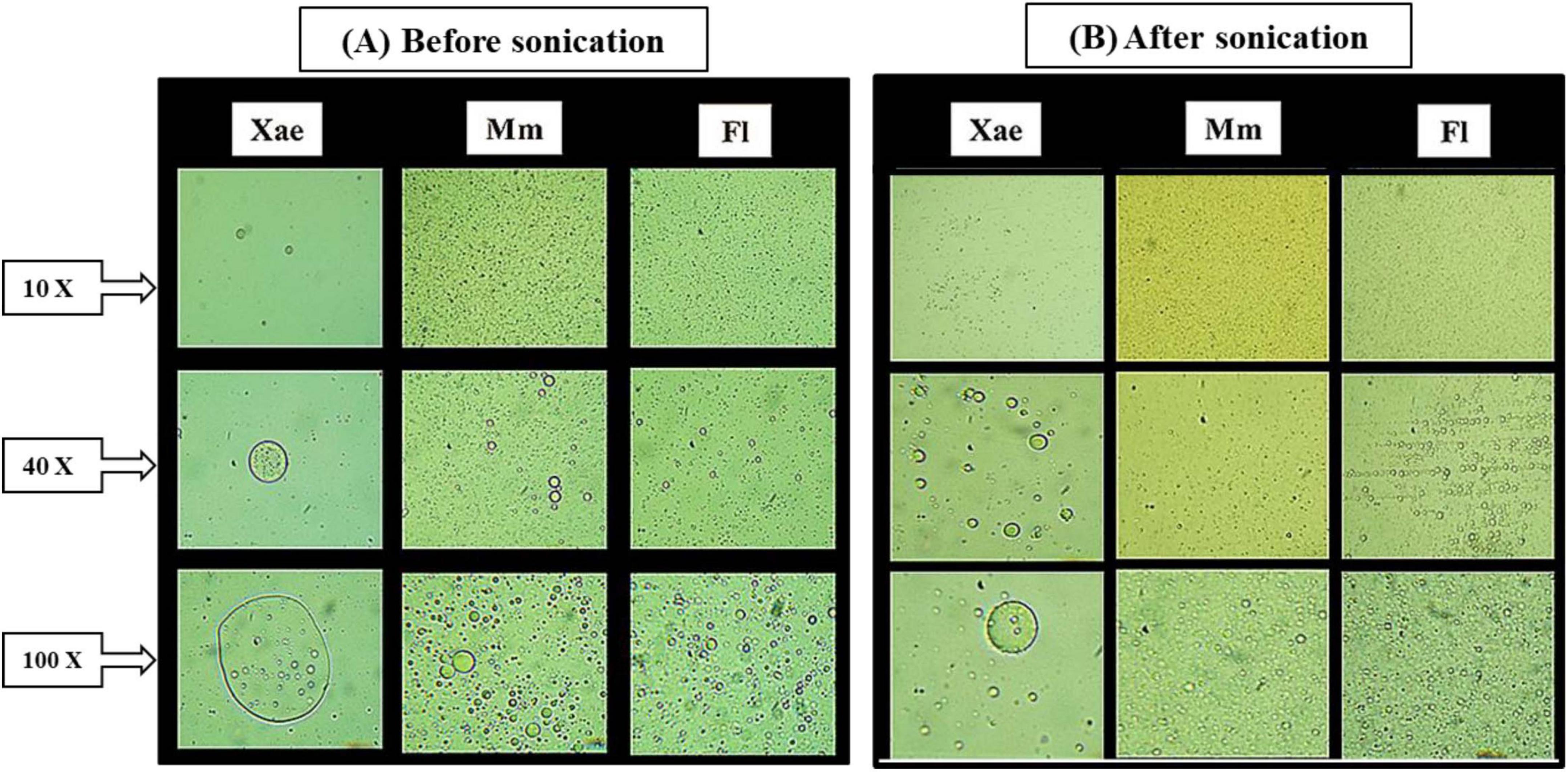
The morphology of spice oil emulsion droplets was observed before and after the ultrasonication process under an Inverted Routine microscope. Figure 2 reveals that emulsions subjected to ultrasonication exhibit significantly smaller droplet sizes than their pre-sonication counterparts. This reduction in droplet size is evident across all the spice oil emulsions (Xae, Mm, and Fl), indicating the effectiveness of ultrasonication assisted break down of larger droplets into finer and uniform structures. The reduced droplet size and improved homogeneity post-sonication, as seen under magnification lenses are consistent with previous studies which have demonstrated its effectiveness in reducing droplet size increasing the emulsion stability by enhanced dispersion of the oil phase within the aqueous phase (41, 42). The phenomenon can be explained by the cavitation effect, where ultrasonic waves create microscopic vapor cavities in the liquid medium which collapse eventually generating an intense localized energy that disrupts larger droplets into smaller ones (43). The mechanical shear forces generated by ultrasonic waves are known to form uniform structures, thereby enhancing the stability of the emulsion (23).
3.3 Particle size analysis of emulsion
Colloidal stability, turbidity and other properties of food systems are highly influenced by the size distribution emulsion droplets. Figure 3 highlights the particle size distribution of spice oil nanoemulsions (Xae, Fl, and Mm) before and after sonication, underscoring the transformative impact of sonication on emulsion properties. For the Xae nanoemulsion, the particle size reduced from 874.4 nm before sonication to 608.2 nm after sonication, showcasing a significant improvement in size uniformity. Similarly, the Fl nanoemulsion reduced from 414.8 to 283.6 nm, while the Mm nanoemulsion decreased from 285.2 to 233.9 nm. PDI (Polysidpersivity index) was also revealed in this study. Before sonication, Xae, Fl, and Mm emulsion had PDI of 1, 0.393, and 0.391 which was changed after sonication to 0.669, 0.382, and 0.412 respectively. This consistent reduction in particle size across all emulsions suggest disruption of larger oil droplets into smaller and more stable particles after sonication. The observed trends align with the known effects of sonication, which involves high-energy ultrasonic waves that generate intense shear forces, breaking down oil droplets into nanoscale dimensions. The Mm nanoemulsion showed the smallest particle size both before and after sonication, indicating its inherent stability, potentially due to its composition or initial processing conditions. Sonication also likely contributes to better stabilization by improving the interaction between the oil phase and emulsifying agents, reducing the potential for droplet coalescence and phase separation. The enhanced emulsification, particularly in the Mm nanoemulsion, suggests its suitability for applications where fine particle sizes are critical, such as in targeted drug delivery systems or high-performance food formulations. Smaller particle sizes lead to better surface area-to-volume ratios, which enhance the emulsifying capacity and stability of nanoemulsions, as previously observed in studies (44). These results highlight the effectiveness of sonication in optimizing nanoemulsion characteristics and its potential applications in food, pharmaceutical, and cosmetic based industries which require stable and uniform emulsions (14, 19).
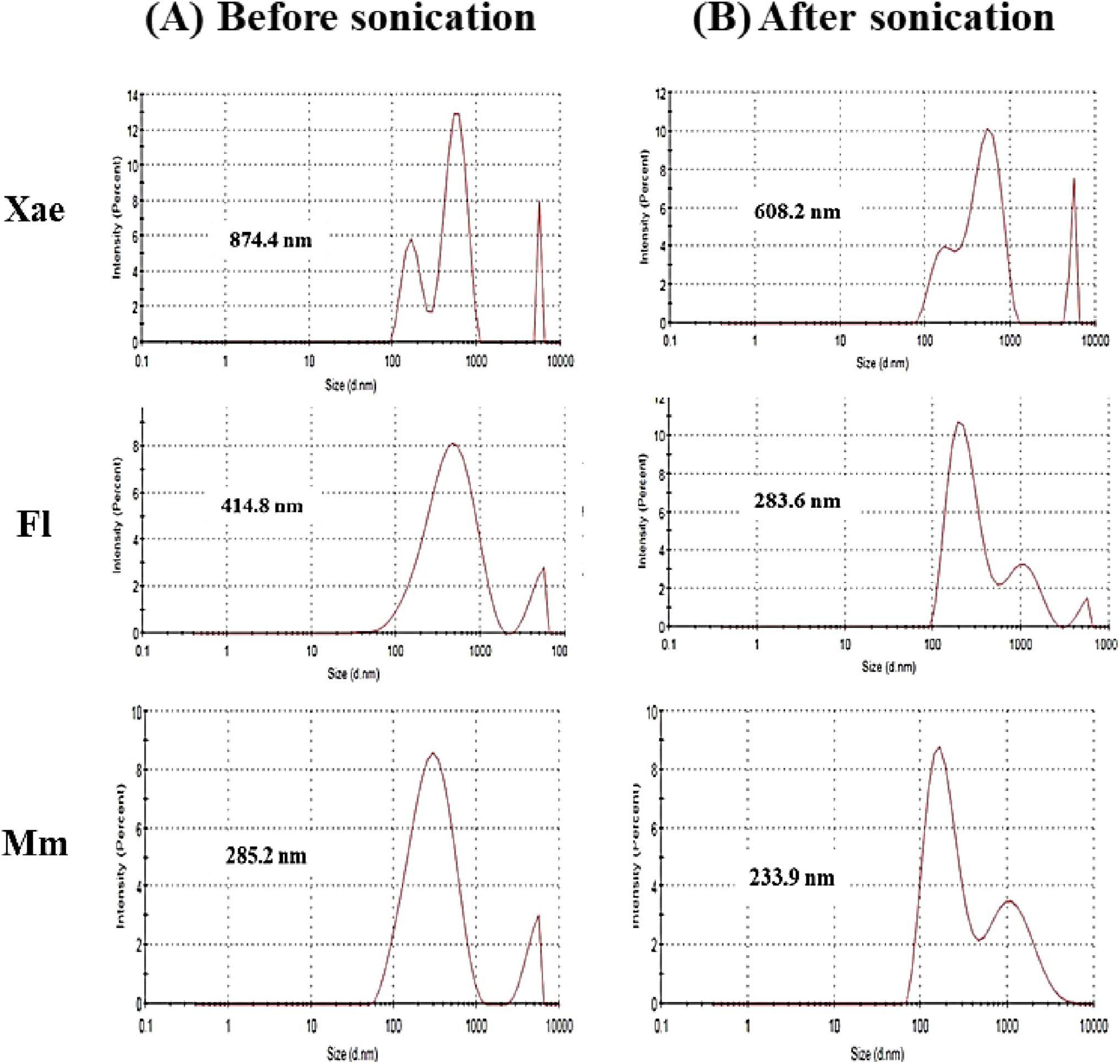
Figure 3. Particle size distribution of spice oil emulsions, (A) before sonication and (B) after sonication.
3.4 Antimicrobial activity of EPS based nanoemulsions
The antibacterial activity of spice oil alone (Figure 4A) and spice oil emulsions (Figure 4B) against Salmonella enterica, Listeria monocytogenes, and Shewanella putrefaciens reveals a significant enhancement when emulsified with EPS. The primary observations from both figures highlight the increased inhibition zone diameters with emulsified spice oils compared to their free oil counterparts, signifying the improved antibacterial efficacy. Fl emulsion showed an average inhibition of 9 mm in Shewanella putrefaciens, 14 mm inhibition for Salmonella enterica and 1 mm inhibition for Listeria monocytogenes. Emulsion of Mm showed inhibition for 17 mm in Shewanella putrefaciens whereas resulted in an enhanced inhibition of 20 mm in Listeria monocytogenes. It did not show any inhibition for Salmonella enterica which may be due to the less antimicrobial activity or low level of diffusion in the agar matrix. Xae emulsion showed clear zone of inhibition of diameter 14 mm against gram negative Shewanella putrefaciens, 15 mm for Salmonella enterica, and maximum inhibition of 18 mm for Listeria monocytogenes. The spice oil emulsions demonstrated significant antibacterial activity that is in agreement with earlier findings (45). The enhanced activity of emulsified spice oils likely results from converging mechanisms like increased surface area diffusion, encapsulation and sustained release, synergistic interface interaction and biofilm interferences. In a previous study, similar oil emulsions and nanoemulsions containing phenolic compounds like thymol and eugenol have shown antimicrobial effects on food pathogens like Listeria monocytogenes, Escherichia coli, and Bacillus subtilis by inducing cell lysis and stimulating bacterial envelope damage (46).
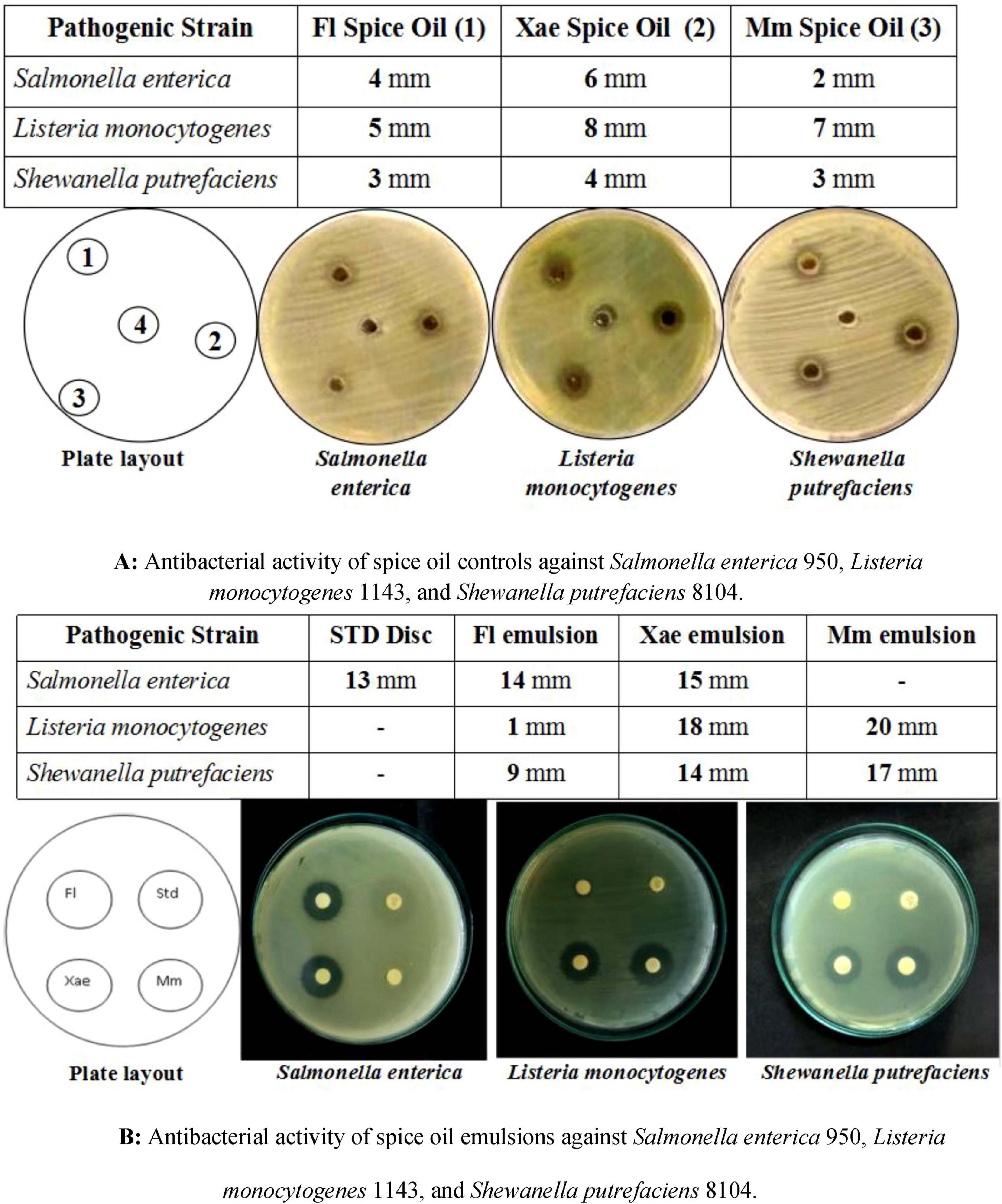
Figure 4. (A) Antibacterial activity of spice oil controls against Salmonella enterica 950, Listeria monocytogenes 1143, and Shewanella putrefaciens 8104, (B) antibacterial activity of spice oil emulsions against Salmonella enterica 950, Listeria monocytogenes 1143, and Shewanella putrefaciens 8104.
The inhibition zones of spice oils alone (Figure 4A) ranged between 2 and 8 mm, indicating moderate antibacterial activity. In contrast, EPS-based emulsions (Figure 4B) significantly enhanced antibacterial action, with inhibition zones ranging from 9 to 20 mm. For Listeria monocytogenes, the individual spice oils showed relatively weak inhibition (≤ 8 mm), while the emulsions of Xae and Mm spice oils exhibited much higher inhibition zones (18 and 20 mm, respectively). This suggests that emulsification improved the bioavailability and antimicrobial effectiveness against this strain. The spice oil alone showed very weak activity against Shewanella putrefaciens, with inhibition zones not exceeding 4 mm. However, the emulsions resulted in significantly larger inhibition zones, especially with Mm (17 mm) and Xae (14 mm) emulsions, demonstrating that emulsification enhances antimicrobial action even against more resistant strains. The STD disk for Salmonella enterica showed an inhibition zone of 13 mm, while Fl and Xae emulsions exceeded this (14 and 15 mm, respectively). This suggests that EPS-based emulsions can potentially match or even outperform conventional antibacterial agents. Essential oils and spice oils are hydrophobic in nature, limiting their solubility and diffusion in aqueous environments. EPS-based emulsions improve the dispersion of spice oils, leading to better contact with bacterial cells and increased antimicrobial efficacy (47, 48). Emulsification helps in the controlled release of antimicrobial compounds, leading to prolonged activity and reducing the need for high concentrations of spice oil, which can sometimes affect sensory properties in food applications (49, 50).
4 Conclusion and future perspectives
This study successfully developed stable nanoemulsions incorporating glucan EPS and spice oils from Cameroonian spices using ultrasonication. The nanoemulsions exhibited enhanced stability, reduced droplet size, and promising antimicrobial activity against food pathogens. By addressing challenges such as the strong odor and hydrophobicity of spice oils, this approach highlights the utility of nanotechnology in enhancing the practical applicability of natural bioactive compounds in food safety and preservation systems. EPS-based emulsions of spice oils demonstrate superior antibacterial activity compared to spice oils alone. This enhanced efficacy is likely due to improved solubility, bioavailability, and controlled release of antimicrobial compounds. These findings align with earlier studies reporting the antimicrobial synergy of EPS and essential oils. With toxicological safety profiles, stability under physiological conditions, and in vivo efficacy studies these microbial EPS-based emulsions would be potent drug carriers (e.g., targeted or sustained delivery). This underscore the potential of glucan-based EPS as a natural, multifunctional emulsifier with both stabilizing and antimicrobial properties, supporting its application in food preservation, pharmaceutical delivery, and functional formulations.
Data availability statement
The original contributions presented in this study are included in this article/supplementary material, further inquiries can be directed to the corresponding authors.
Author contributions
JS: Methodology, Writing – original draft, Writing – review and editing. SD: Writing – review and editing. PD: Writing – review and editing. HG: Writing – review and editing. BR: Supervision, Writing – review and editing. DK: Supervision, Writing – review and editing, Conceptualization, Writing – original draft. PS: Supervision, Writing – review and editing, Conceptualization, Writing – original draft.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
Jahnavi Kumari Singh, Digambar Kavitake, and Palanisamy Bruntha Devi are grateful to the Indian Council of Medical Research - ICMR, Anusandhan National Research Foundation – ANRF (earlier known as SERB), and Department of Science and Technology (DST), Government of India for providing financial assistance in the form of ICMR-Senior Research Fellowship (OMI-Fellowship/13/2022/ECD), ANRF-National Post Doctoral Fellowship (PDF/2021/000551) and WISE KIRAN Scheme (DST/WOS-A/LS -259/2019 (G), respectively.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
EPS, exopolysaccharide; Xae, Xylopia aethiopica; Mm, Monodora myristica; Fl, Fagara lepreuri; MTCC, Microbial Type Culture Collection; UV, ultraviolet; CX, Cephoxitin; SD, standard deviation; STD, standard.
References
1. Shi J, Xu J, Liu X, Goda AA, Salem SH, Deabes MM, et al. Evaluation of some artificial food preservatives and natural plant extracts as antimicrobial agents for safety. Discover Food. (2024) 4:89. doi: 10.1007/s44187-024-00162-z
2. Yashin A, Yashin Y, Xia X, Nemzer B. Antioxidant activity of spices and their impact on human health: A review. Antioxidants. (2017) 6:70. doi: 10.3390/antiox6030070
3. Lucchesi ME, Chemat F, Smadja J. Solvent-free microwave extraction of essential oil from aromatic herbs: Comparison with conventional hydro-distillation. J Chromatogr. (2004) 1043:323–7. doi: 10.1016/j.chroma.2004.05.083
4. El-Zaeddi H, Martínez-Tomé J, Calín-Sánchez Á, Burló F, Carbonell-Barrachina ÁA. Volatile composition of essential oils from different aromatic herbs grown in Mediterranean regions of Spain. Foods. (2016) 5:41. doi: 10.3390/foods5020041
5. Chouhan S, Sharma K, Guleria S. Antimicrobial activity of some essential oils—present status and future perspectives. Medicines. (2017) 4:58. doi: 10.3390/medicines4030058
6. Zhang F, Ramachandran G, Mothana RA, Noman OM, Alobaid WA, Rajivgandhi G, et al. Anti-bacterial activity of chitosan loaded plant essential oil against multi drug resistant K. pneumoniae. Saudi J Biol Sci. (2020) 27:3449–55. doi: 10.1016/j.sjbs.2020.09.025
7. Tanoh EA, Boué GB, Nea F, Genva M, Wognin EL, Ledoux A, et al. Seasonal effect on the chemical composition, insecticidal properties and other biological activities of Zanthoxylum leprieurii guill. & perr. essential oils. Foods. (2020) 9:550. doi: 10.3390/foods9050550
8. Gaba J, Bhardwaj G, Sharma A. Lemongrass. In: Nayik GA, Gull A editors. Antioxidants in Vegetables and Nuts-Properties and Health Benefits. Singapore: Springer (2020). p. 75–103.
9. Djiazet S, Mezajoug Kenfack LB, Linder M, Tchiégang C. An ethno-nutritional study on spices used in traditional foods of the Western Regions of Cameroon: The case of nah poh. J Ethnic Foods. (2019) 6:1–12. doi: 10.1186/s42779-019-0030-6
10. Djiazet S, Kenfack LBM, Ngangoum ES, Nzali HG, Tchiégang C. Indigenous spices consumed in the food habits of the populations living in some countries of Sub-Saharan Africa: Utilisation value, nutritional and health potentials for the development of functional foods and drugs: A review. Food Res Int. (2022) 157:1–15. doi: 10.1016/j.foodres.2022.111280
11. Carpena M, Nuñez-Estevez B, Soria-Lopez A, Garcia-Oliveira P, Prieto MA. Essential oils and their application on active packaging systems: A review. Resources. (2021) 10:7. doi: 10.3390/resources10010007
12. Garavand F, Eghbal N, Nooshkam M, Miraballes I, Jafari SM. Salt, spices, and seasonings formulated with nano/microencapsulated ingredients. In: SM Jafari editor. Application of Nano/Microencapsulated Ingredients in Food Products. Cambridge, MA: Academic Press (2020). p. 435–67.
13. Francisco CR, de Oliveira Júnior FD, Marin G, Alvim ID, Hubinger MD. Plant proteins at low concentrations as natural emulsifiers for an effective orange essential oil microencapsulation by spray drying. Coll Surf A. (2020) 607:125470. doi: 10.1016/j.colsurfa.2020.125470
14. Dávila-Rodríguez M, López-Malo A, Palou E, Ramírez-Corona N, Jiménez-Munguía MT. Essential oils microemulsions prepared with high-frequency ultrasound: Physical properties and antimicrobial activity. J Food Sci Technol. (2020) 57:4133–42. doi: 10.1007/s13197-020-04449-8
15. Dammak I, Sobral PJ, Aquino A, Neves MA, Conte-Junior CA. Nanoemulsions: Using emulsifiers from natural sources replacing synthetic ones-A review. Compr Rev Food Sci Food Saf. (2020) 19:2721–46. doi: 10.1111/1541-4337.12606
16. Lu WC, Huang DW, Wang CC, Yeh CH, Tsai JC, Huang YT, et al. Preparation, characterization, and antimicrobial activity of nanoemulsions incorporating citral essential oil. J Food Drug Anal. (2018) 26:82–9. doi: 10.1016/j.jfda.2016.12.018
17. Sheth T, Seshadri S, Prileszky T, Helgeson ME. Multiple nanoemulsions. Nat Rev Mater. (2020) 5:214–28. doi: 10.1038/s41578-019-0161-9
18. Barradas TN, Silva KG. Nanoemulsions as optimized vehicles for essential oils. In: Yata VK, Mohanty AK, Lichtfouse E editors. Sustainable Agriculture Reviews. (Vol. 44), Cham: Springer (2020). p. 115–67.
19. Campolo O, Giunti G, Laigle M, Michel T, Palmeri V. Essential oil-based nano-emulsions: Effect of different surfactants, sonication and plant species on physicochemical characteristics. Indust Crops Prod. (2020) 157:112935. doi: 10.1016/j.indcrop.2020.112935
20. Hidajat MJ, Jo W, Kim H, Noh J. Effective droplet size reduction and excellent stability of limonene nanoemulsion formed by high-pressure homogenizer. Coll Interfaces. (2020) 4:5. doi: 10.3390/colloids4010005
21. Li PH, Chiang BH. Process optimization and stability of D-limonene-in-water nanoemulsions prepared by ultrasonic emulsification using response surface methodology. Ultrasonics Sonochem. (2012) 19:192–7. doi: 10.1016/j.ultsonch.2011.05.017
22. Jasmina H, Džana O, Alisa E, Edina V, Ognjenka R. Preparation of Nanoemulsions by High-Energy and Lowenergy Emulsification Methods. Singapore: Springer (2017). p. 317–22.
23. Jafari S, He Y, Bhandari B. Nano-emulsion production by sonication and microfluidization-a comparison. Int J Food Properties. (2006) 9:475–85. doi: 10.1080/10942910600596464
24. Qian C, McClements DJ. Formation of nanoemulsions stabilized by model food-grade emulsifiers using high-pressure homogenization: Factors affecting particle size. Food Hydrocoll. (2011) 25:1000–8. doi: 10.1016/j.foodhyd.2010.09.017
25. Jafari SM, Assadpoor E, He Y, Bhandari B. Re-coalescence of emulsion droplets during high-energy emulsification. Food Hydrocolloids. (2008) 22:1191–202. doi: 10.1016/j.ultsonch.2023.106566
26. Zhang K, Mao Z, Huang Y, Xu Y, Huang C, Guo Y, et al. Ultrasonic assisted water-in-oil emulsions encapsulating macro-molecular polysaccharide chitosan: Influence of molecular properties, emulsion viscosity and their stability. Ultrasonics Sonochem. (2020) 64:105018. doi: 10.1016/j.ultsonch.2020.105018
27. Ghasemi H, Darjani S, Mazloomi H, Mozaffari S. Preparation of stable multiple emulsions using food-grade emulsifiers: Evaluating the effects of emulsifier concentration, W/O phase ratio, and emulsification process. SN Appl Sci. (2020) 2:1–9. doi: 10.1007/s42452-020-03879-5
28. Taha A, Ahmed E, Ismaiel A, Ashokkumar M, Xu X, Pan S, et al. Ultrasonic emulsification: An overview on the preparation of different emulsifiers-stabilized emulsions. Trends Food Sci Technol. (2020) 105:363–77. doi: 10.1016/j.tifs.2020.09.024
29. Wu WH, Eskin DG, Priyadarshi A, Subroto T, Tzanakis I, Zhai W. New insights into the mechanisms of ultrasonic emulsification in the oil-water system and the role of gas bubbles. Ultrasonics Sonochem. (2021) 73:105501. doi: 10.1016/j.ultsonch.2021.105501
30. Mettu S, Yao S, Sun Q, Lawson SR, Scales PJ, Martin GJ, et al. Effect of bulk viscosity and emulsion droplet size on the separation efficiency of model mineral oil-in-water (O/W) emulsions under ultrasonic standing wave fields: A theoretical and experimental investigation. Indust Eng Chem Res. (2020) 59:7901–12. doi: 10.1021/acs.iecr.0c00616
31. Nazir A, Vladisavljević GT. Droplet breakup mechanisms in premix membrane emulsification and microfluidic channels. Adv Coll Interface Sci. (2021) 290:102393. doi: 10.1016/j.cis.2021.102393
32. Kavitake D, Tiwari S, Devi PB, Shah IA, Reddy GB, Shetty PH. Production, purification, and functional characterization of glucan exopolysaccharide produced by Enterococcus hirae strain OL616073 of fermented food origin. Int J Biol Macromol. (2024) 259:129105. doi: 10.1016/j.ijbiomac.2023.129105
33. Tiwari S, Kavitake D, Devi PB, Baria B, Agarwal K, Ravi R, et al. (2024). Functionalenhancement of yoghurt through incorporation of glucan exopolysaccharide from Enterococcushirae OL616073 of food origin. J. Food Meas. Charact. (2024)18:5462–76. doi: 10.1007/s11694-024-02580-0
34. Nehme R, Andrés S, Pereira RB, Ben Jemaa M, Bouhallab S, Ceciliani F, et al. Essential oils in livestock: From health to food quality. Antioxidants. (2021) 10:330. doi: 10.3390/antiox10020330
35. Chen X, Daliri EB, Kim N, Kim JR, Yoo D, Oh DH. Microbial etiology and prevention of dental caries: Exploiting natural products to inhibit cariogenic biofilms. Pathogens. (2020) 9:569. doi: 10.3390/pathogens9070569
36. Kavitake D, Devi PB, Singh SP, Shetty PH. Characterization of a novel galactan produced by Weissella confusa KR780676 from an acidic fermented food. Int J Biol Macromol. (2016) 86:681–9. doi: 10.1016/j.ijbiomac.2016.01.099
37. Li Y, Wu CL, Liu J, Zhu Y, Zhang XY, Jiang LZ, et al. Soy protein isolate-phosphatidylcholinenanoemulsions prepared using high-pressure homogenization. Nanomaterials. (2018) 8:307. doi: 10.3390/nano8050307
38. Kavitake D, Balyan S, Devi PB, Shetty PH. Interface between food grade flavour and water soluble galactan biopolymer to form a stable water-in-oil-in-water emulsion. Int J Biol Macromol. (2019) 135:445–52. doi: 10.1016/j.ijbiomac.2019.05.199
39. Aryanti N, Wardhani DH, Nafiunisa A, Putri NAY, Firmansyah RA, Cahyani ND, et al. Application of modified corn starch in stabilization of ultrasonic-assisted pickering oil in water emulsion. Food Res. (2024) 8:78–89. doi: 10.26656/fr.2017.8(S1).12
40. Rajasekaran B, Singh A, Ponnusamy A, Patil U, Zhang B, Hong H, et al. Ultrasoundtreated fish myofibrillar protein: Physicochemical properties and its stabilizing effect on shrimpoil-in-water emulsion. Ultrason Sonochem. (2023) 98:106513. doi: 10.1016/j.ultsonch.2023.106513
41. Tang SY, Shridharan P, Sivakumar M. Impact of process parameters in the generation of novel aspirin nanoemulsions–comparative studies between ultrasound cavitation and microfluidizer. Ultrasonics Sonochem. (2013) 20:485–97. doi: 10.1016/j.ultsonch.2012.04.005
42. Silva HD, Cerqueira MA, Vicente AA. Nanoemulsions for food applications: Development and characterization. Food Bioprocess Technol. (2012) 5:854–67. doi: 10.1007/s11947-011-0683-7
43. Leong TS, Wooster TJ, Kentish SE, Ashokkumar M. Minimising oil droplet size using ultrasonic emulsification. Ultrasonics Sonochem. (2009) 16:721–7. doi: 10.1016/j.ultsonch.2009.02.008
44. Behrend O, Ax K, Schubert H. Influence of continuous phase viscosity on emulsification by ultrasound. Ultrasonics Sonochem. (2000) 7:77–85. doi: 10.1016/S1350-4177(99)00029-2
45. Maidment C, Dyson A, Haysom I. A study into the antimicrobial effects of cloves(Syzgium aromaticum) and cinnamon (Cinnamomum zeylanicum) using disc-diffusionassay. Nutr Food Sci. (2006) 36: 225–30. doi: 10.1108/00346650610676794
46. Enayatifard R, Akbari J, Babaei A, Rostamkalaei SS, Hashemi SM, Habibi E. Anti-microbial potential of nano-emulsion form of essential oil obtained from aerial parts of Origanum Vulgare L. as Food Additive. Adv Pharm Bull. (2021) 11:327. doi: 10.34172/apb.2021.028
47. Singh RS, Kaur N. Microbial biopolymers for edible film and coating applications. Adv Biotechnol. (2015) 12:187–216. doi: 10.1016/j.carbpol.2019.04.050
48. Basak S, Singh JK, Morri S, Shetty PH. Assessment and modelling the antibacterial efficacy of vapours of cassia and clove essential oils against pathogens causing foodborne illness. LWT. (2021) 150:112076. doi: 10.1016/j.lwt.2021.112076
49. Pandey VK, Islam RU, Shams R, Dar AH. A comprehensive review on the application of essential oils as bioactive compounds in Nano-emulsion based edible coatings of fruits and vegetables. Appl Food Res. (2022) 2:100042. doi: 10.1016/j.afres.2022.100042
Keywords: glucan exopolysaccharide, spice oil, emulsions, antimicrobial activity, food preservation
Citation: Singh JK, Djiazet S, Devi PB, Ghomdim HN, Reddy GB, Kavitake D and Shetty PH (2025) Enhanced antibacterial potential of exopolysaccharide-stabilized spice oil emulsions against foodborne pathogens. Front. Nutr. 12:1624274. doi: 10.3389/fnut.2025.1624274
Received: 07 May 2025; Accepted: 25 June 2025;
Published: 15 July 2025.
Edited by:
Micaela Galante, National University of Rosario, ArgentinaReviewed by:
Md. Abu Saleh, University of Rajshahi, BangladeshEnzo Luciano La Cava, National University of the Northeast, Argentina
Swethaa Venkataraman, SRM University, India
Copyright © 2025 Singh, Djiazet, Devi, Ghomdim, Reddy, Kavitake and Shetty. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Prathapkumar Halady Shetty, cGtzaGFsYWR5QHlhaG9vLmNvLnVr; Digambar Kavitake, ZGlnYW1iYXJrYXZpdGFrZUBnbWFpbC5jb20=
 Jahnavi Kumari Singh
Jahnavi Kumari Singh Steve Djiazet
Steve Djiazet Palanisamy Bruntha Devi
Palanisamy Bruntha Devi Horliane Nzali Ghomdim
Horliane Nzali Ghomdim G. Bhanuprakash Reddy
G. Bhanuprakash Reddy Digambar Kavitake
Digambar Kavitake Prathapkumar Halady Shetty
Prathapkumar Halady Shetty