- 1Collaborative Innovation Center for Molecular Imaging of Precision Medicine, Shanxi Medical University, Taiyuan, China
- 2Shanxi Key Laboratory of Molecular Imaging, Shanxi Medical University, Taiyuan, China
- 3College of Life Science and Technology, Inner Mongolia Normal University, Hohhot, Inner Mongolia, China
- 4Key Laboratory of Biodiversity Conservation and Sustainable Utilization in Mongolian Plateau for College and University of Inner Mongolia Autonomous Region, Hohhot, China
- 5Faculty of Health Sciences, University of Macau, Macau, Macao SAR, China
- 6Key Laboratory of Systems Bioengineering (Ministry of Education), Tianjin University, Tianjin, China
Introduction: Curcumin and resveratrol are promising nutraceuticals, but their application in functional foods is limited by poor water solubility, low bioavailability, and chemical instability. This study aimed to develop a novel co-delivery system to overcome these challenges.
Methods: Curcumin/resveratrol-zein-carboxymethyl chitosan (CRZC) nanocomplexes were fabricated using an antisolvent precipitation method. The mass ratio of zein to CMCS was optimized. The nanoparticles were characterized for size, encapsulation efficiency (EE), and antioxidant activity. Their stability during storage and simulated gastrointestinal digestion was also evaluated.
Results: The optimized CRZC nanoparticles (zein:CMCS mass ratio of 2:1) exhibited a small particle size (201.6 nm), high encapsulation efficiency (72.90% for curcumin and 78.23% for resveratrol), and superior radical scavenging capacity. Structural characterization confirmed that hydrophobic interactions, electrostatic attraction, and hydrogen bonding were the main forces maintaining nanocomplex integrity. The CMCS coating significantly improved the storage and digestion stability of both encapsulated bioactives.
Discussion: These results demonstrate that the zein-CMCS-based nanocomplex is an efficient and robust delivery system. The CRZC nanoparticles show great potential for the co-delivery of synergistic nutraceuticals to enhance the functionality and application of bioactive compounds in the food industry.
1 Introduction
The innovative development of functional foods has emerged as a pivotal research direction in modern nutritional science, primarily aiming to achieve the dual benefits of nutritional fortification and disease prevention through targeted delivery of bioactive components (1–3). Curcumin, a natural diketone polyphenolic compound extracted from turmeric rhizomes, demonstrates significant application potential in functional foods, pharmaceutical formulations, and cosmetic products due to its broad-spectrum antibacterial properties, potent antioxidant capacity, and anti-inflammatory characteristics (4–7). Similarly, resveratrol, a plant-derived stilbene compound found in grapes and other botanical sources, exhibits remarkable antioxidant and neuroprotective functions, has demonstrated anti-obesity and anti-inflammatory effects through modulation of signaling pathways such as NF-κB (8, 9). Notably, both curcumin and resveratrol have been reported to play crucial roles in alleviating ulcerative colitis symptoms and maintaining intestinal function, primarily attributed to their antioxidant and anti-inflammatory activities (10). Studies further indicate curcumin and resveratrol can interact synergistically to enhance antioxidant efficacy, anti-inflammatory potential, and anticancer properties (11–14). However, the utility of these bioactive nutrients is often constrained by poor water solubility, low bioavailability, and chemical instability, limiting their widespread application (15). Consequently, there is an imperative need to develop robust delivery systems that can enhance the bioavailability of curcumin and resveratrol.
The construction of nano-scale colloidal delivery systems has emerged as an effective strategy to overcome the technical limitations (16). Among the various options, delivery carriers fabricated from natural biomacromolecules (e.g., protein-polysaccharide complexes) have garnered considerable attention due to their exceptional biocompatibility, controllable degradability, and high encapsulation efficiency (17, 18). For instance, fucoidan-coated zein-based nanocomposites have developed for resveratrol encapsulation (19), and zein-alginate oligosaccharide nanocomposites for curcumin loading (20). However, most colloidal systems have been designed to encapsulate either curcumin or resveratrol individually. The simultaneous co-encapsulation of both compounds poses significant challenges due to their differential molecular polarity (21). Although co-encapsulation of both bioactive compounds has been achieved by preparing two separate delivery carriers followed by physical mixing, this approach is complicated and often result in substantial bioactive components leakage. Consequently, highly efficient co-delivery systems for curcumin and resveratrol have rarely been reported.
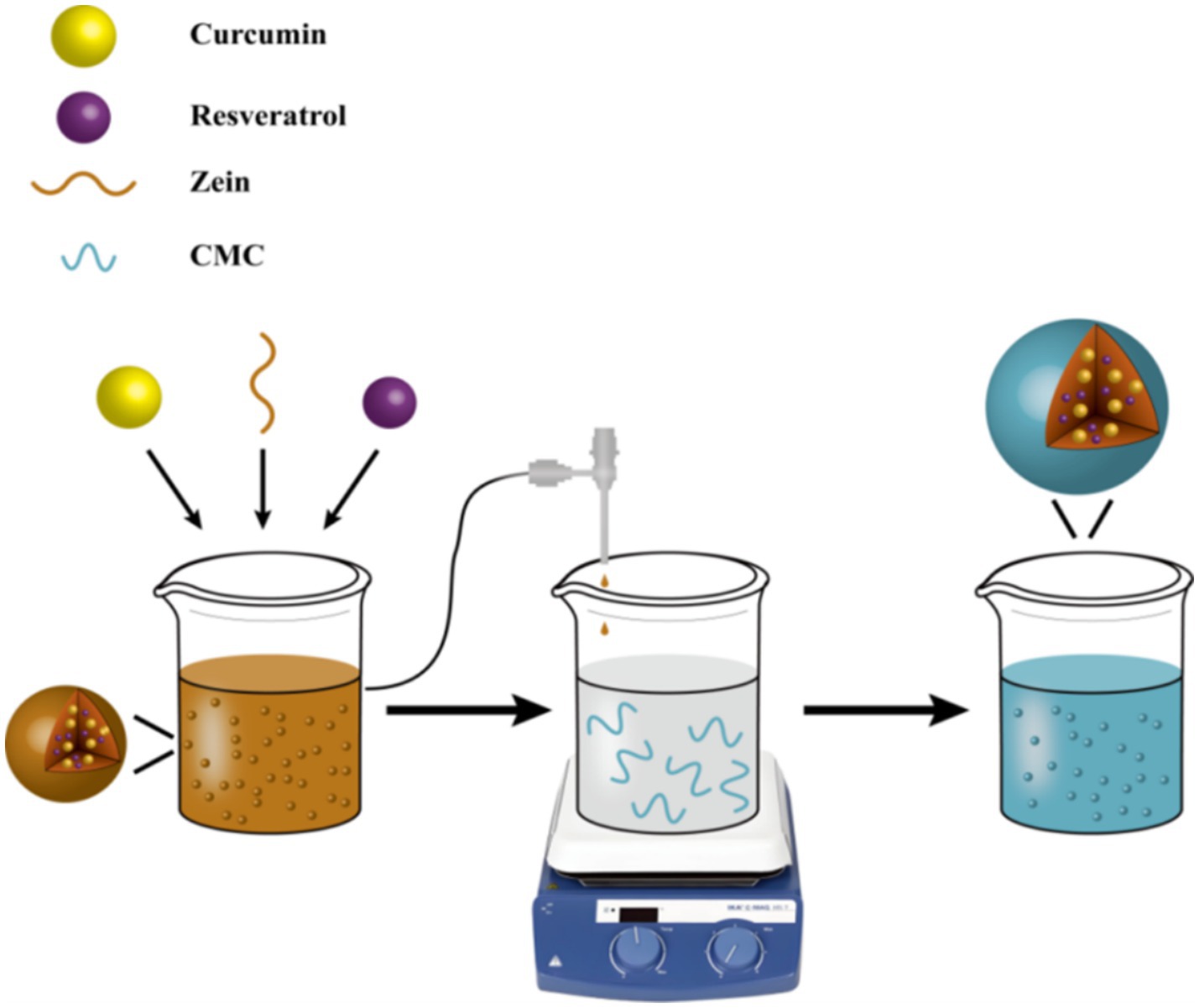
In this study, we developed a protein-polysaccharide self-assembled system for dual-polyphenol co-delivery, achieving synergistic encapsulation of curcumin and resveratrol through molecular structure design. As natural bio-based nanomaterials, zein and carboxymethyl chitosan (CMCS) exhibit superior biocompatibility and biodegradable properties, effectively evading the metabolic clearance limitations associated with synthetic nanomaterials. Hydrophobic functional components (curcumin and resveratrol) were encapsulated within zein’s hydrophobic core, followed by directional assembly of CMCS through electrostatic interactions between its anionic groups and the positive charges on zein surface, yielding unique zein-CMCS nanocomposites (Figure 1). The effect of zein: CMCS mass ratio (4:1 to 1:2) on nanocomplex characteristics (mean size, zeta-potential, and encapsulation efficiency) of zein-CMCS nanocomplexes was investigated. Furthermore, we evaluated the encapsulated system’s effects on the in vitro antioxidant activity and bioaccessibility of the co-encapsulated nutraceuticals using a simulated gastrointestinal model. This work establishes design principles for colloidal co-delivery systems targeting multiple bioactive compounds.
2 Materials and methods
2.1 Materials
Zein (97% purity, AR grade) from corn was purchased from Macklin Co. Ltd. (Shanghai, China). CMCS, with a deacetylation degree of 95% and a carboxylation degree of 70%, was obtained from Jinke Pharmaceutical (Zhejiang, China). Curcumin (98% purity) and resveratrol (99% purity) was purchased from Sigma-Aldrich (USA). All other reagents are analytical grade and used as received.
2.2 Preparation of curcumin/resveratrol-loaded zein/CMCS nanoparticle
The curcumin/resveratrol-loaded zein NPs (CRZ NPs) was prepared by the anti-solvent method with slightly modifications (22). Curcumin (500 mg), resveratrol (500 mg) and zein (10.0 g) were dissolved in 1.0 L of 70% ethanol solution and magnetically stirred for 1 h to prepare a transparent solution. Secondly, the solution was injected into 3.0 L of distilled water at 600 rpm stirring, and then the CRZ NPs were spontaneously formed. Thirdly, CMCS (0.5% w/v) powder was dispersed in distilled water by continuous stirring for 6 h at 20°C. Fourthly, the CRZ NPs solution was injected into the CMCS solution, and the curcumin/resveratrol-loaded zein/CMCS nanoparticles (CRZC NPs) were formed. The resulting was evaporated at 40°C in a rotary evaporator to remove the ethanol.
2.3 Encapsulation efficiency and loading capacity
The EE and LC of the curcumin and resveratrol in the NPs was determined in accordance with previously described methods (15). Briefly, freshly prepared dispersions were centrifuged at 12,000 r/min for 30 min and the NPs were separated. The obtained supernatant was diluted with ethanol solution, and the concentrations of curcumin and resveratrol were determined by analyzed using a UV spectrophotometry (UV 2600, Shimadzu Co., Tokyo, Japan) at 426 nm and 306 nm, respectively. Suitable calibration curves were prepared to calculate the curcumin and resveratrol concentrations. The EE and LC was determined by the following Equation:
2.4 Characterization of NPs
The mean particle size, polydispersity (PDI), and zeta-potential (ξ) of the NPs were measured with a dynamic light scattering (DLS) instrument (Anton Paar Litesizer 500, Austria). Before analysis, the samples were diluted 5 times to minimize multiple scattering effect. These measurements were performed in quadruplicate. The morphology of the freeze-dried NPs was determined using an SEM (SU8010, Hitachi, Japan) at an accelerating voltage of 15.0 kV. Before observation, the sample was coated with a gold layer under a sputter coater. The Fourier transform infrared spectroscopy (FT-IR) spectra of the samples were measured using a Thermo Nicolette 6,700 spectrophotometer (Thermo Fisher Scientific Co., Ltd., MA, United States) from 400 cm−1 to 4,000 cm−1 at a resolution of 4 cm−1. The X-ray diffraction (XRD) patterns of the samples were obtained by a Bruker AXS D8 Advance X-ray diffractometer (Bruker Inc., Germany) equipped with Ni-filtered Cu Kα radiation. The 2θ angle range used was from 5 to 60°.
2.5 Simulated gastrointestinal digestion
According to Zhang’s method (23), the release profiles of curcumin and resveratrol from NPs were quantified under simulated gastrointestinal conditions: simulated gastric fluid (SGF) (3.2 mg/mL pepsin, 2.0 mg/mL NaCl in HCl, pH 2.0) and simulated intestinal fluid (SIF) (2.0 mg/mL pancreatin, 12.0 mg/mL bile salts, 8.8 mg/mL NaCl, 6.8 g/L K₂HPO₄, pH 7.4) at 37°C. Fresh NP dispersions (30 mL) were mixed with 30 mL SGF and incubated at 37°C. Aliquots (2 mL) were collected at 30, 60, and 90 min, with equal volumes of fresh SGF replenished. After gastric digestion, the entire digest was transferred to 60 mL SIF. Intestinal phase samples (2 mL) were withdrawn at 120, 150, 180, 210, and 240 min, replacing with fresh SIF. All samples were centrifuged (6,000 × g, 5 min) to pellet undigested material. Supernatants were diluted and analyzed for released curcumin/resveratrol content via UV–Vis spectrophotometry (Shimadzu UV-2600, Tokyo, Japan) as described in Section 2.3. The in vitro bioaccessibility of the polyphenols was then calculated using the following equation:
2.6 Antioxidant activity evaluation
The DPPH and ABTS radical scavenging activity was analyzed according to the previous studies (24, 25), with slight modifications. In brief, 2 mL of the sample was mixed with 2 mL of the DPPH solution (0.1 mM), and the resulting mixture was placed in the dark and allowed to react for 30 min. The absorbance at 517 nm was recorded by using a UV–Vis spectrophotometer (UV 2600, Shimadzu Co., Tokyo, Japan). In the same way, ABTS solution (7.4 mM) was mixed with a potassium persulfate solution (2.6 mM) at a 1:1 v/v ratio and the mixture was then placed in the dark for 12 h. Exactly 3.9 mL of diluted ABTS· + solutions, which had an absorbance of about 0.7 at 734 nm, were mixed with 0.1 mL of water or samples for 5 min and then measured at 734 nm. The DPPH and ABTS radical scavenging activity was calculated using Equation:
where Ab and As were the absorbance values of control solution and samples, respectively.
2.7 Storage stability
The CRZ, and CRZC NPs dispersions were stored under refrigerated conditions (4°C) for one month. The retention rate (%) of the two nutraceuticals after long-term storage were measured using the method described in Section 2.3 and calculated using the following equations:
2.8 Statistical analysis
The values were presented as the mean ± standard deviation (SD) of each treatment. The data were analyzed using analysis of variance (ANOVA) in the SPSS 20.0 software package (IBM, New York). The significant was defined at the 95% confidence level.
3 Results and discussion
3.1 Particle size and zeta-potential analysis
The CRZC NPs were successfully fabricated via anti-solvent precipitation (Figure 1). Co-dissolution of zein with curcumin/resveratrol in 70% ethanol enabled hydrophobic interaction-driven embedding of polyphenols within zein’s hydrophobic domains. Ethanol-to-water phase diffusion triggered zein self-assembly, forming primary nutraceutical-loaded cores. Subsequent interfacial assembly with CMCS via electrostatic and hydrogen bonding yielded nanostructures, consistent with Qin et al.’s protein-polysaccharide interaction models (26).
Particle size analysis (Figure 2) revealed that pristine zein NPs exhibited an average hydrodynamic diameter of 256.5 ± 3.4 nm (PDI = 0.26), consistent with anti-solvent precipitation efficacy for hydrophobic proteins nanoassembly. This size regulation stems from rapid ethanol diffusion –induced polarity changes that trigger zein conformational rearrangement and subunit aggregation (27). Notably, the curcumin/resveratrol-loaded system (CRZ NPs) showed a 11.7% reduction in mean particle size (226.5 ± 2.5 nm) compared to pristine zein NPs, indicating polyphenol incorporation promotes denser nanostructures via modulated zein interactions (15). This observation aligns with the mean particle size reduction pattern reported by Yang et al. for co-delivery of quercetin and resveratrol in zein/carboxymethyl cellulose NPs (24).
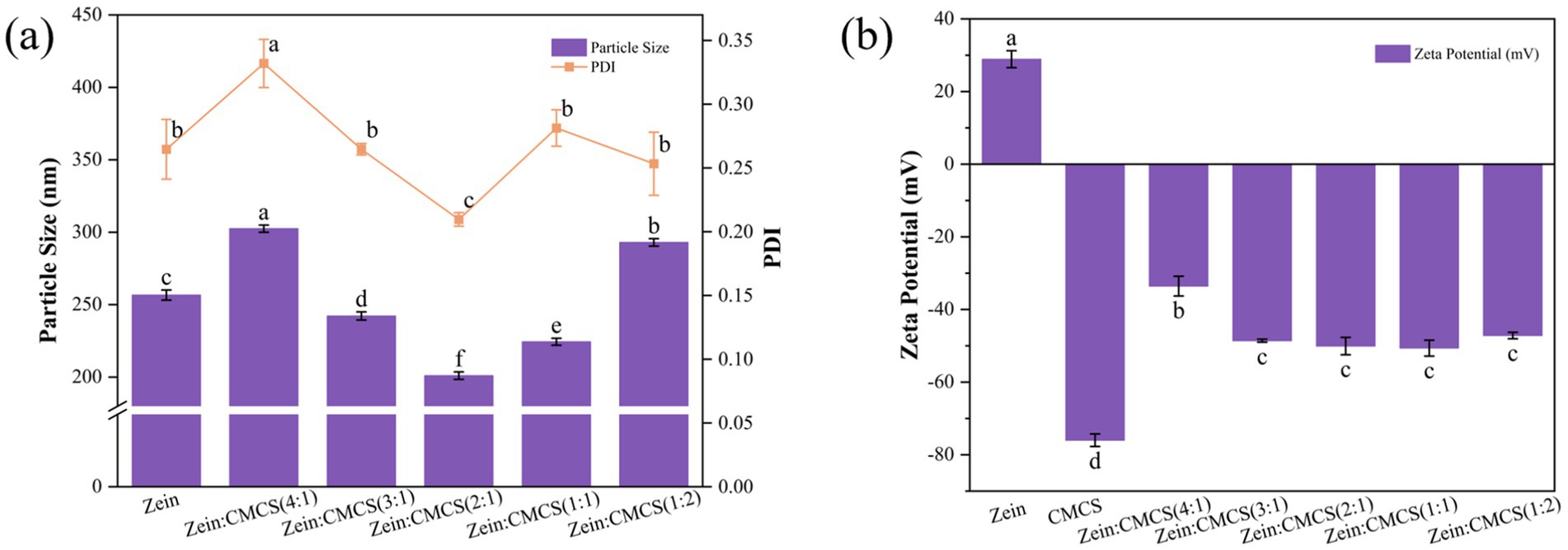
Figure 2. Effect of zein: CMCS mass ratio on the mean particle size and PDI (a) and zeta-potential (b) of NPs. Different lowercase letters signified significant differences.
The introduction of CMCS induced significant size modulation of CRZ NPs. At a zein: CMCS mass ratio of 4:1, the composite system displayed an increased average diameter of 302 ± 2.8 nm, primarily due to NPs aggregation caused by insufficient CMCS concentration. Conversely, optimal size minimization (201 ± 2.5 nm) with superior dispersion stability (PDI = 0.21) was achieved at a 2:1 mass ratio (zein: CMCS). This indicates that moderate CMCS coating suppresses aggregation via steric hindrance, whereas excessive polysaccharide layers increase hydrodynamic diameter. These findings consistency with Lin et al., confirming the threshold effect of polyelectrolyte concentration on NPs stability (22).
Zeta potential analysis (Figure 2b) demonstrated that pristine zein NPs exhibited a positive charge of +28.9 mV at pH 4.0 (pI = 5.9). The CRZ NPs exhibited a slightly reduced surface potential (+28.5 mV), indicating partial surface distribution of bioactive components. Upon CMCS coating, all composites transitioned to strongly negative potentials (−34.2 to −41.6 mV), confirming the successful formation of stabilizing shells through anionic polysaccharide adsorption. This charge inversion is consistent with observations by Huang et al.’s (28), who noted negative zeta potential shifts in zein/pectin nanocomposites, illustrating successful surface deposition of anionic pectin. Similarly, Li et al. (20) reported comparable charge inversion patterns in zein-alginate oligosaccharide systems. These insights underscore the pivotal role of CMCS in modulating nanoparticle characteristics, potentially paving the way for advanced applications in nutrition and bioactive delivery systems.
3.2 EE and LC analysis
Figure 3 showed the EE and LC of curcumin and resveratrol in both CRZ and CRZC NPs. In uncoated CRZ NPs, the EE of curcumin and resveratrol reached only about 45.2 and 55.6%, respectively, indicating incomplete encapsulation or potential leakage post-formulation. Notably, resveratrol exhibited higher encapsulation across all nanoformulation compared to curcumin, likely due to its higher compatibility with the hydrophobic core of zein nanoparticles. These findings are consistent with previous results obtained using the anti-solvent precipitation method for co-encapsulating curcumin and resveratrol co-encapsulating zein matrix (10, 29, 30).
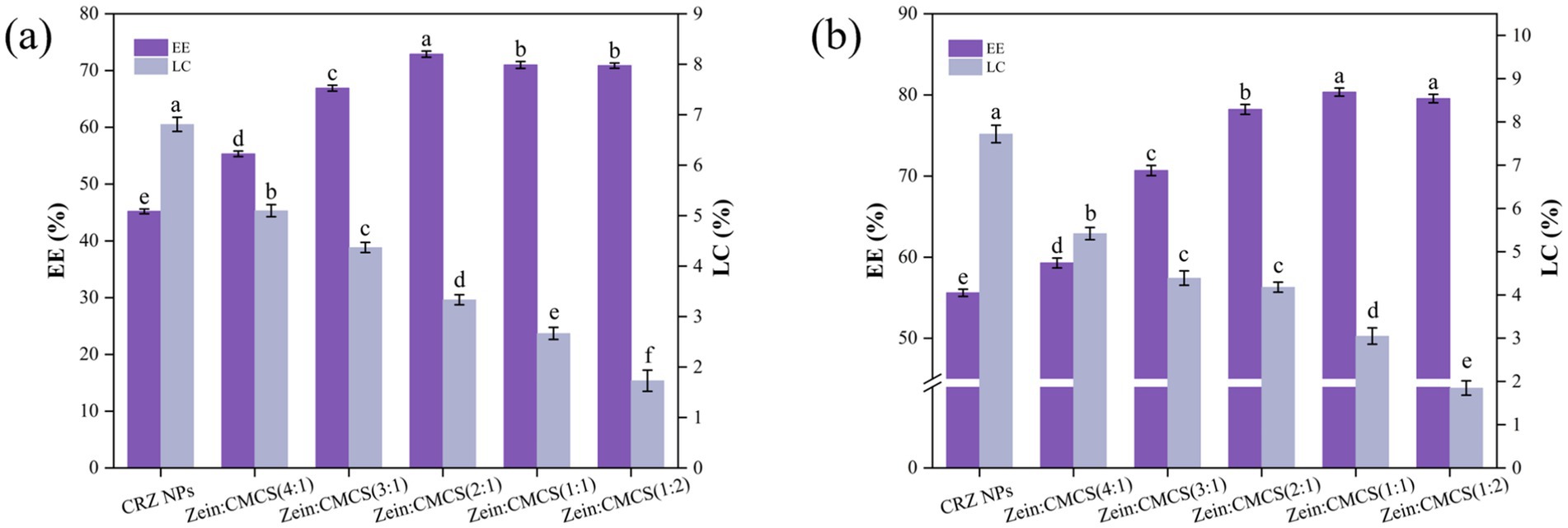
Figure 3. Effect of zein: CMCS mass ratio on EE and LC of NPs, (a) curcumin and (b) resveratrol. Different lowercase letters signified significant differences.
The incorporation of CMCS enhanced the EE of curcumin and resveratrol in CMCS-coated CRZ NPs, confirming the anionic polysaccharide coating improves the retention of bioactive compound. CMCS increased of the system’s hydrophilicity, prompting more substantial encapsulation of hydrophobic curcumin/resveratrol within the zein NPs (22). Moreover, some of the curcumin and resveratrol may reside within the polysaccharide shell formed by the CMCS (24). Other studies support these findings: when curcumin and resveratrol were co-encapsulation within zein-epigallocatechin gallate conjugates using an anti-solvent precipitation method followed by rhamnolipid biosurfactant coating, encapsulation efficiencies were 71% for curcumin and 85% for resveratrol (29). Similarly, zein-ethyl cellulose core-shell NPs yielded encapsulation efficiency of 54% for curcumin and 71% for resveratrol, respectively (30). These results align with other research indicating that hydrophilic polyelectrolyte coatings enhance EE in zein-based nanocarriers (15, 31).
Further studies revealed that the concentration CMCS critically modulates loading characteristics: At a zein: CMCS mass ratio of 2:1, optimal EE (72.90%) was achieved, while LC decreased as the CMCS proportion increased. This inverse correlation arises because excessive polysaccharide coating increasing carrier mass, reducing practical LC efficacy post-saturation, a finding consistent with Yang et al. (24). Their study on zein/carboxymethyl cellulose NPs for co-encapsulating quercetin-resveratrol similarly found an increase in EE with higher carboxymethyl cellulose mass, though the opposite was true for LC.
Thus, considering factors such as small particle size, high EE and LC, CRZC NPs with a zein to CMCS mass ratio of 2:1 was selected for further experimentation.
3.3 FT-IR and XRD analysis
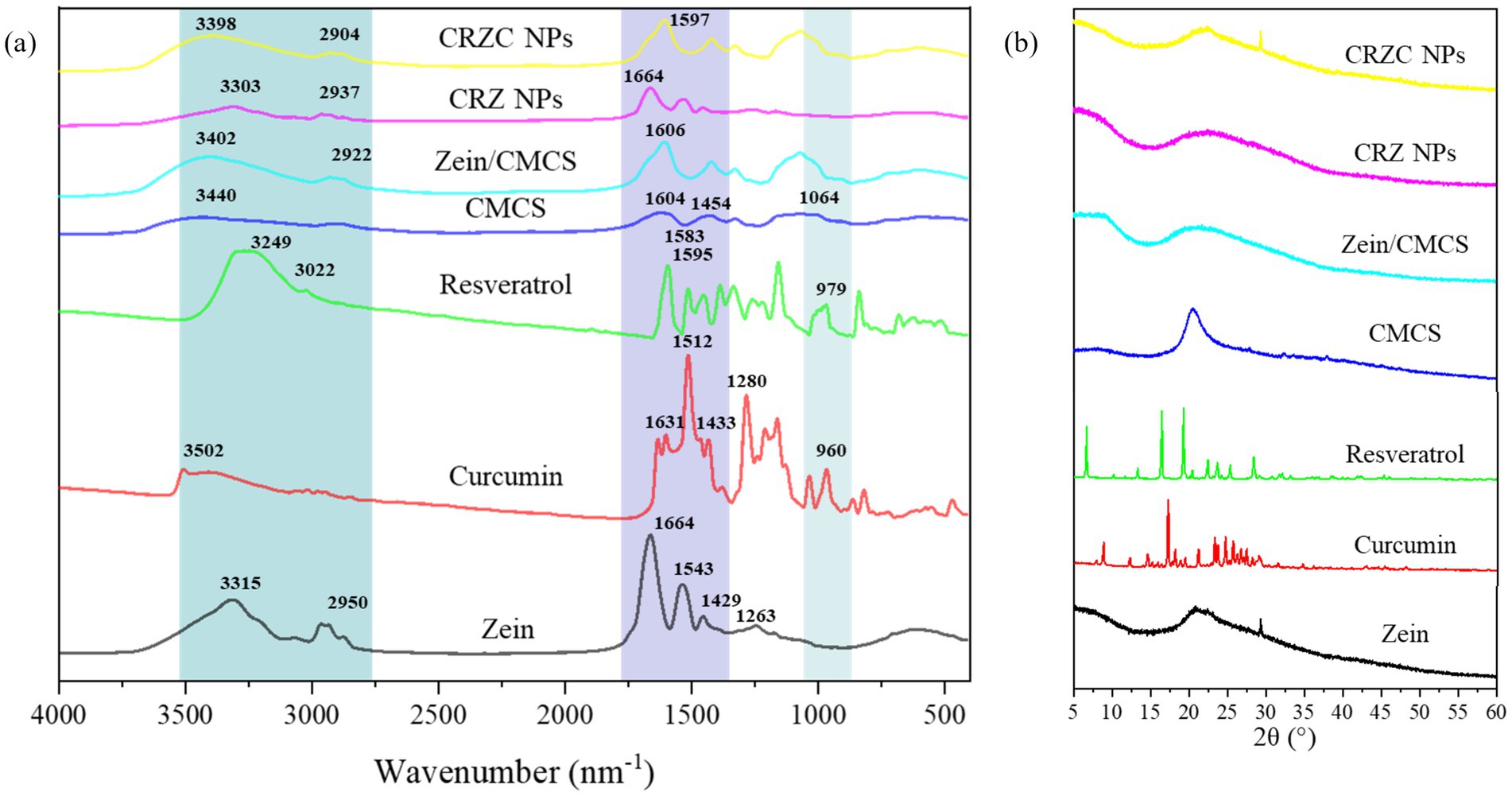
To investigate the intermolecular interactions within NPs, FT-IR spectroscopy was conducted on curcumin, resveratrol, zein, CMCS and NPs. Figure 4a illustrated that all the samples exhibited a broad band between 3,100–3,500 cm−1, indicating the presence of O-H stretching vibrations. Native resveratrol exhibited characteristic bands at 979 cm−1 for trans-olefinic band, 1,595 cm−1 for C=C aromatic stretching (32). For curcumin, peaks at 1631 cm−1, 1,512 cm−1, 1,433 cm−1 and 1,280 cm−1 corresponded to aromatic ring and benzene ring vibrations, including C=C, C=O, C-O and C-C stretching (33). Zein displayed absorption bands at 1664 cm−1, 1,543 cm−1, 1,429 cm−1, and 1,263 cm−1, attributed to hydrophobic C-H, amide I (-C=O), amide II (-C-N and -N-H), and amide III (34). Similarly, CMCS exhibited peak at 1604 cm−1, 1,583 cm−1, 1,454 cm−1, and 1,064 cm−1, corresponding to C=O, CH2COO-, and C-O-C group vibrations, respectively (35). Upon integration of curcumin and resveratrol with zein, the O-H peak shifted from 3,320 cm−1 to 3,336 cm−1, along with amide I peak shifted from 1,664 cm−1 to 1,649 cm−1. These shifts suggest the involvement of hydrophobic effects and hydrogen bonding in the formation of the CRZ NPs. Coating the CRZ NPs with CMCS did not cause significant spectral changes, indicating that no covalent bonds formed between zein and CMCS. Notably, the characteristic peaks of curcumin and resveratrol were absent in both CRZ NPs and CRZC NPs, indicating complete encapsulation within the NPs. The shifts observed in the CRZC NPs, particularly around 3,200–3,400 cm−1 (O-H and N-H) and 1,597 cm−1 and 1,431 cm−1 (amide II), indicated the presence of electrostatic interactions and hydrogen bonds between zein NPs and CMCS.
The crystalline diffraction patterns of the samples were shown in Figure 4b. The native curcumin exhibited multiple distinct characteristic peaks at 2θ of 8.8°, 12.4°, 17.3°, 18.1°, 24.7°, confirming its crystalline nature. Similarly, native resveratrol displayed distinct crystalline peaks at 2θ angles of 13.3°, 16.4°, 19.3°,22.4°, 23.6°, 25.3°, and 28.3°. Zein had a broad t peak at diffraction angles of 20.1°, and CMCS had only a single broad peak at 20.2°, reflecting their amorphous properties as a protein and polysaccharide, respectively. The characteristic peaks of curcumin and resveratrol were not found in the NPs, likely due to their low concentration in the NPs. Additionally, the peak intensities of CRZC NPs at 20° were significantly reduced compared to those of pure zein and CMCS. This reduction suggests the formation of new noncovalent interaction between zein and CMCS, corroborated by the FT-IR results. Similar findings have been observed in previous studies involving the encapsulation of curcumin (36) or resveratrol (37) in a polymers matrix.
3.4 Microstructure analysis
Scanning electron microscopy (SEM) revealed distinct morphological features of NPs (Figure 5). CRZ NPs exhibit spherical morphology with smooth surfaces, consistent with intrinsic zein self-assembly during anti-solvent precipitation (15). CMCS-coated CRZC NPs maintained spherical morphology but demonstrated reduced particle size compared to CRZ NPs, corroborating the result of dynamic light scattering (DLS) analysis. The composite system showed a gel-like matrix interspersed among NPs, attributable to excess CMCS. Critically, no free curcumin or resveratrol crystals was observed, confirming complete polyphenols encapsulation. These findings correlate well with FT-IR and XRD analyses.
3.5 Antioxidant activity evaluation
The antioxidant efficacy of free versus nanoencapsulated curcumin/resveratrol was systematically evaluated using DPPH and ABTS radical scavenging assays (Figure 6). Nanoencapsulation significantly enhanced radical scavenging capacity: CRZC NPs exhibited 58.10% higher DPPH scavenging than free curcumin (p < 0.05), while ABTS scavenging increased by 64.67% (p < 0.05). Resveratrol-containing systems showed analogous enhancement trends. These results demonstrate that zein/CMCS-based nanocarriers not only preserve the intrinsic antioxidant activity of encapsulated compounds but also enhance radical scavenging efficiency compared to free components. This suggests synergistic interactions between carrier materials (zein and CMCS) and bioactive constituents (29). These findings indicate that combinatorial loading of functional components can amplify the antioxidant potential of zein NPs, underscoring their promise for functional applications.
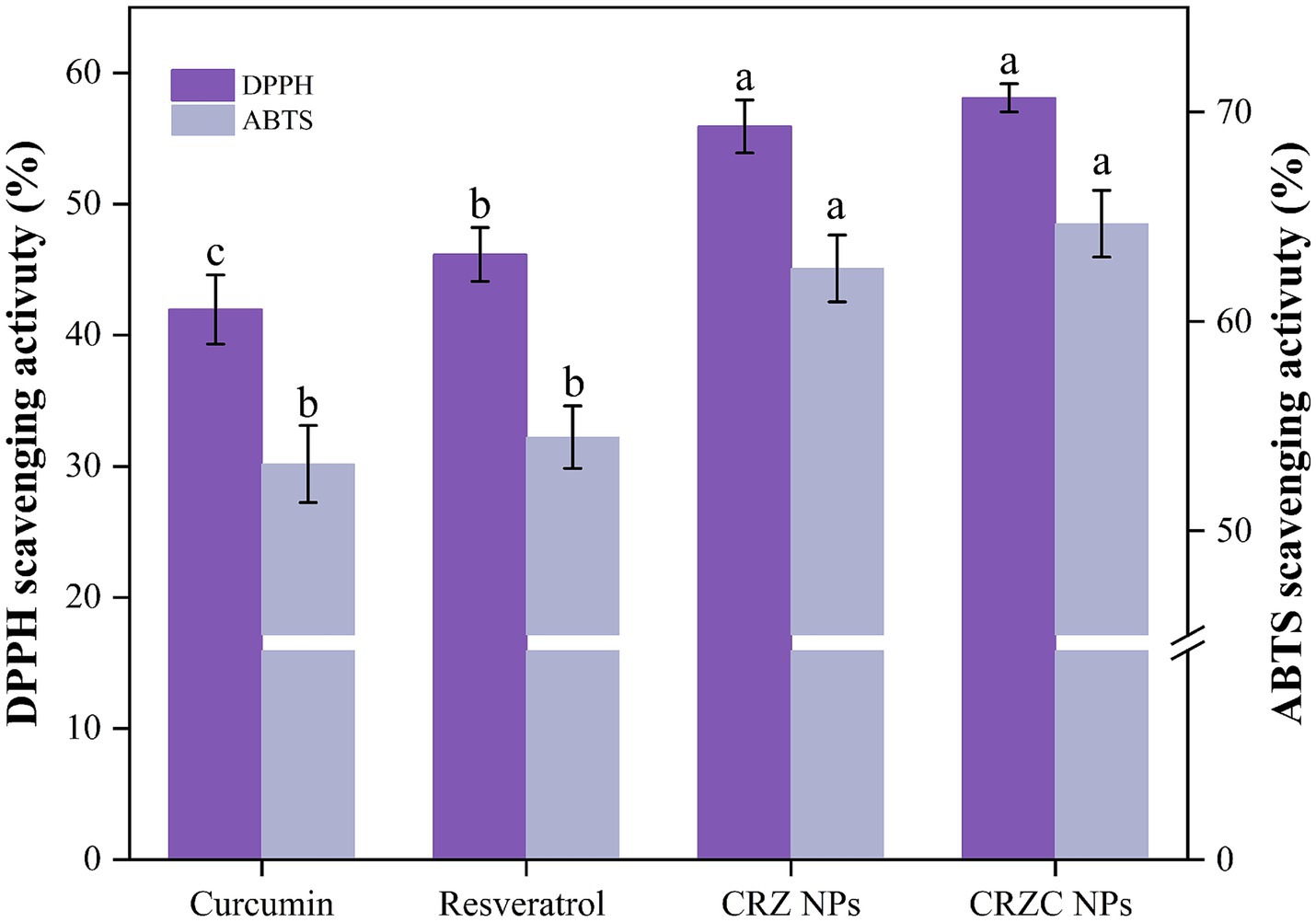
Figure 6. DPPH and ABTS+ radical scavenging ability of free curcumin, resveratrol, CRZ NPs, and CRZC NPs. Different lowercase letters signified significant differences.
3.6 Release profiles of polyphenol under simulated gastrointestinal digestion
Release profile of co-encapsulated curcumin and resveratrol from NPs were quantified during sequential simulated gastric (SGF) and intestinal (SIF) phases, as depicted in Figure 7. CRZ and CRZC NPs exhibited sustained-release characteristics for both curcumin and resveratrol. Notably, curcumin displays lower solubilization in SGF compared to resveratrol, attributed to resveratrol’s higher solubility. This observed behavior aligns with previous reports on similar co-delivery nanocarriers of these compounds (30). During the SIF phase, both CRZ and CRZC NPs exhibited burst release effects, potentially due to the influence of bile salts and peptides present in the SIF, which facilitate the dissolution of bioactive compounds. Additionally, the release rate from CRZ NPs surpassed that from CRZC NPs, possibly because the CMCS layer acts as a physical barrier, limiting protease access to the zein, and concurrently enhancing the diffusion of the encapsulated compounds into the surrounding medium, thereby controlling curcumin release (38, 39). Furthermore, resveratrol exhibited a higher retention rate than curcumin, likely because resveratrol is more easily accommodated within the hydrophobic interiors of the zein nanoparticles, which may stem from differences in molecular interaction properties (19).
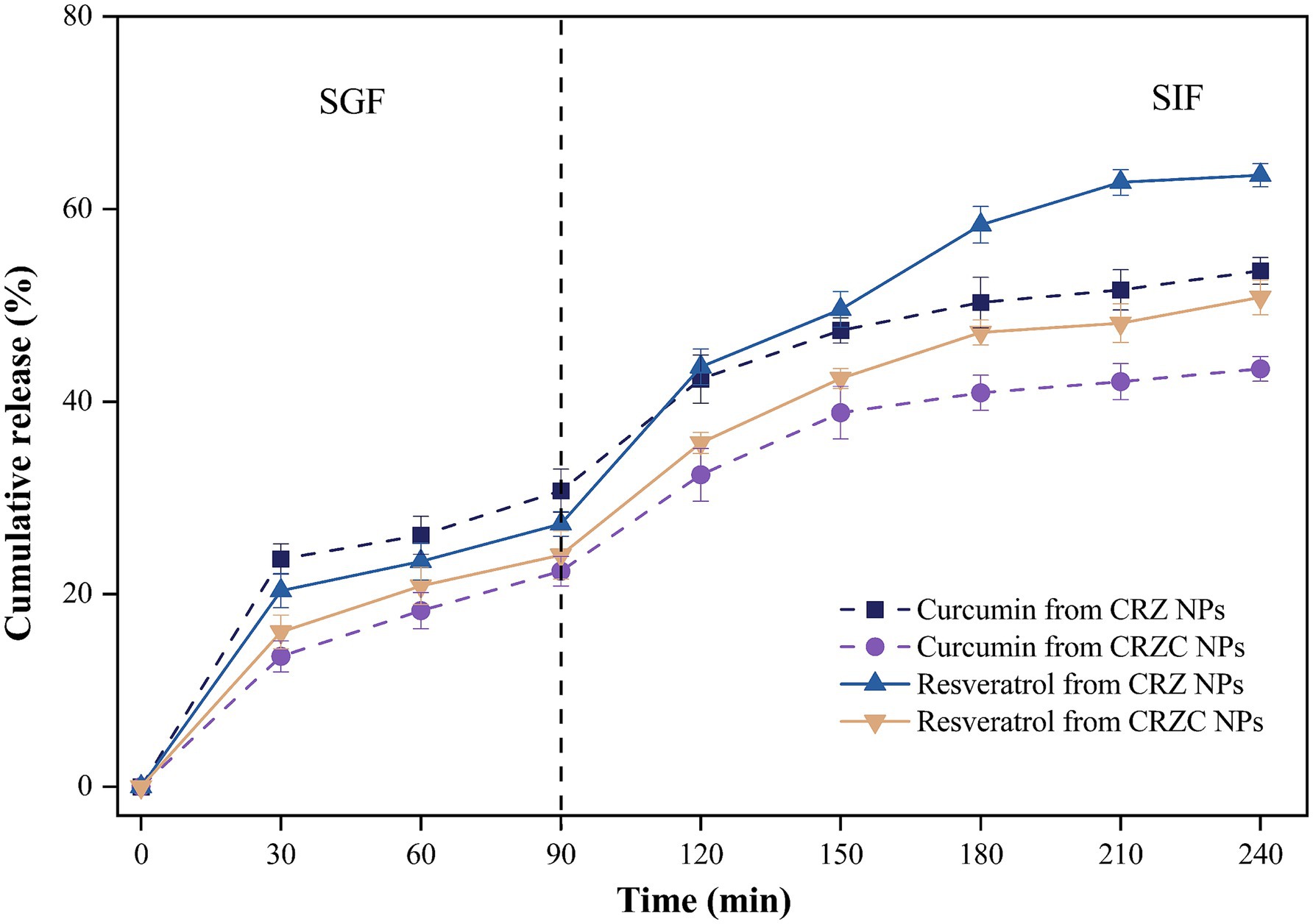
Figure 7. Release profile of curcumin (dotted lines) and resveratrol (solid lines) from NPs under simulated gastrointestinal conditions.
Bioaccessibility analysis (Figure 8) revealed free curcumin and resveratrol at 26.82 and 39.48%, respectively, limited by precipitation in aqueous media. Nanoencapsulation improved bioaccessibility (curcumin: 43.62%; resveratrol: 52.37%), with CMCS-coated NPs outperforming uncoated systems (p < 0.05). This enhancement stems from: (i) sustained release minimizing precipitation, (ii) enhanced micellar solubilization through CMCS-bile salt synergism, and (iii) reduced enzymatic degradation. The differential bioaccessibility between compounds (resveratrol>curcumin) aligns with their solubility profiles in intestinal conditions. These findings align with NPs studies demonstrating prevention of polyphenol aggregation (40), confirming CRZC NPs as promising carriers for targeted intestinal delivery of hydrophobic nutraceuticals.
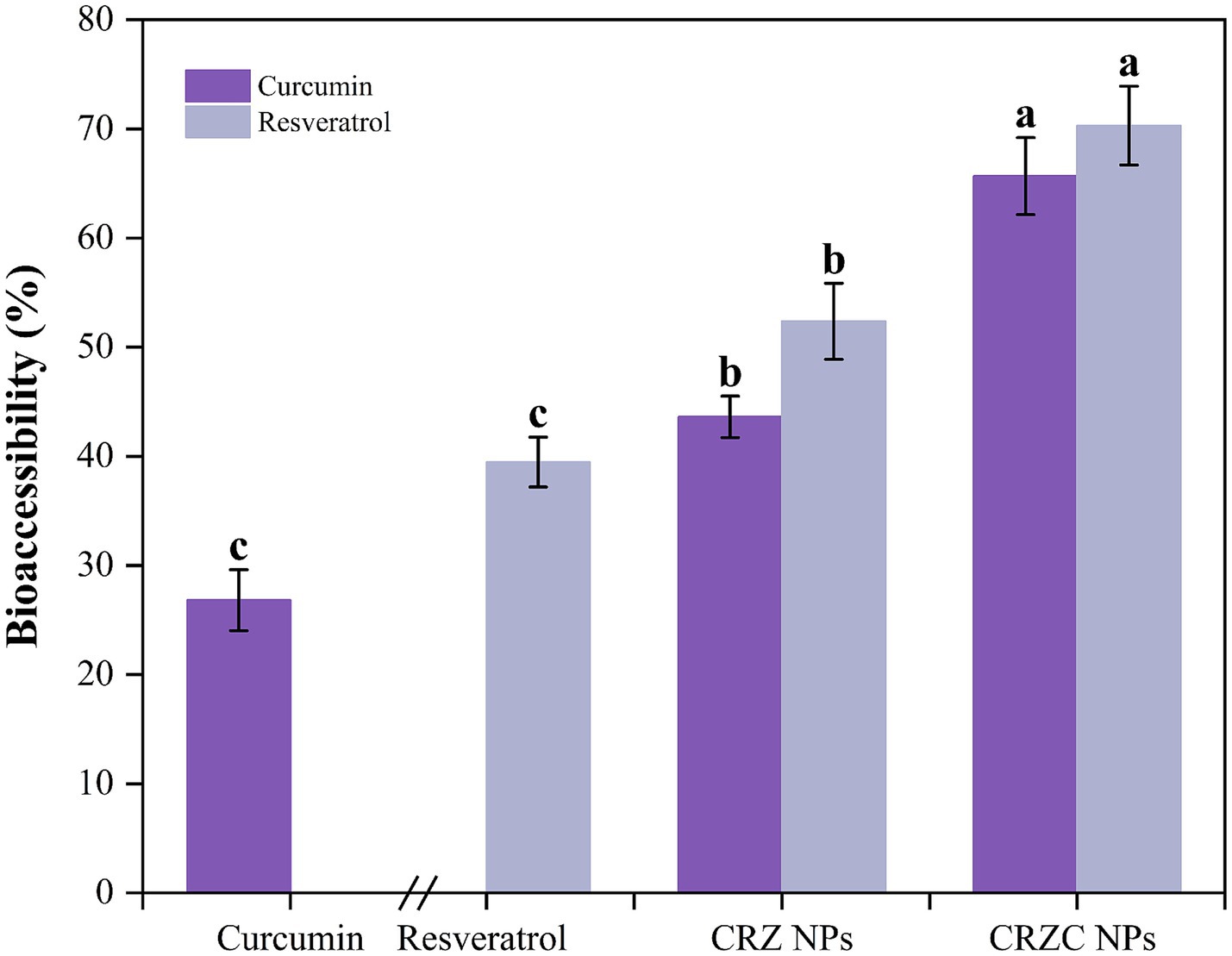
Figure 8. Bioaccessibility of curcumin and resveratrol from NPs. Different lowercase letters signified significant differences.
3.7 Storage stability
Long-term storage stability of CRZ and CRZC nanoparticles was evaluated over 30 days (Figure 9). CRZ nanoparticles experienced notable degradation; curcumin retention dropped to 76.76, and resveratrol to 78.43 (p < 0.05 relative to initial values). This degradation was attributed to colloidal aggregation and oxidation of polyphenols on the surface. In contrast, CRZC nanoparticles showed markedly better performance (p < 0.05) with significantly higher retention of both active compounds compared to CRZ nanoparticles. This improvement underscores the effectiveness of the core-shell architecture, where the CMCS coating provides electrostatic barriers that prevent particle coalescence, decrease oxygen permeability by densifying the matrix, and shield polyphenols from aqueous radicals. Therefore, the core-shell structure formed by CMCS and zein substantially enhances the physicochemical stability of nanocarriers, offering long-term protection for curcumin and resveratrol (15).
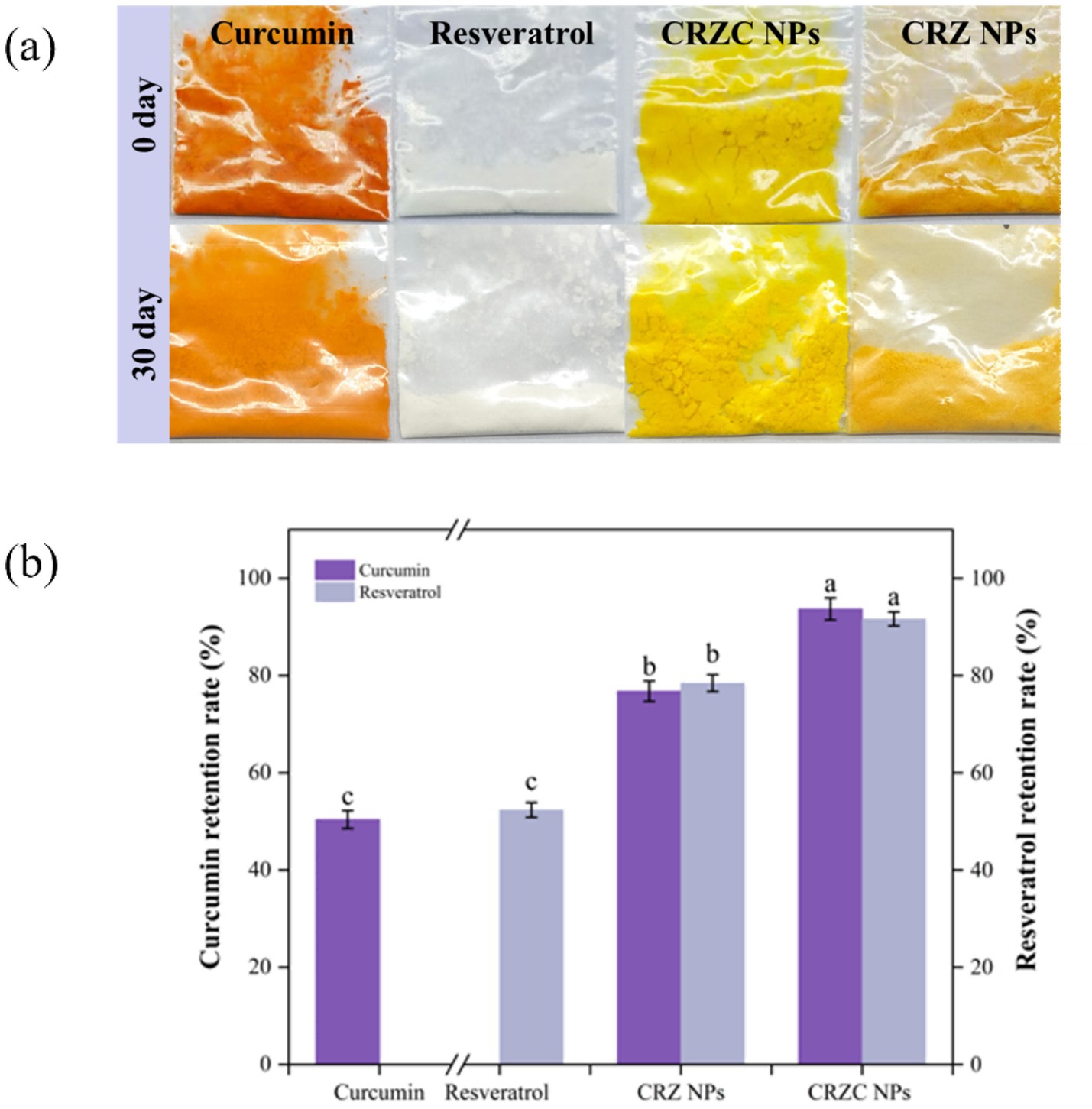
Figure 9. The retention rates of curcumin and resveratrol in CRZ NPs and CRZC NPs after storage. Storage appearance (a), retention rate (b). Different lowercase letters signified significant differences.
4 Conclusion
This study successfully constructed a composite nanodelivery system based on zein and CMCS, achieving synergistic co-encapsulation of curcumin and resveratrol. Structural characterization confirmed that the polyphenolic bioactive compounds primarily localized within the hydrophobic core regions of zein, while CMCS formed an electrostatically stabilized protective shell. Compositionally optimized CRZC NPs exhibit superior encapsulation performance for both hydrophobic bioactive components. Notably, the nanoencapsulated bioactive components retained effective free radical scavenging capacity, indicating the encapsulation process had minimal impact on their functional groups. The CMCS coated CRZC NPs also improved storage stability and enabled controlled release kinetics in the gastrointestinal environment, thereby enhancing bioaccessibility. These findings establish zein/CMCS NPs as a promising platform for functional food applications requiring targeted delivery of hydrophobic nutraceuticals.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
PW: Conceptualization, Data curation, Funding acquisition, Investigation, Project administration, Resources, Supervision, Visualization, Writing – original draft, Writing – review & editing. YY: Methodology, Data curation, Writing – review & editing. YZ: Investigation, Writing – review & editing. JS: Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by Shanxi Province Higher Education “Billion Project” Science and Technology Guidance Project and Youth Science Research Project of Shanxi Basic Research Program (202203021222267).
Acknowledgments
Thank you to every author for their contributions.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Maqsood, S, Adiamo, O, Ahmad, M, and Mudgil, P. Bioactive compounds from date fruit and seed as potential nutraceutical and functional food ingredients. Food Chem. (2020) 308:125522. doi: 10.1016/j.foodchem.2019.125522
2. Wahab, M, and Janaswamy, S. Porous starch granules loaded curcumin and resveratrol modulate starch digestibility of wheat bread: toward developing novel functional foods. Food Hydrocoll. (2025) 162:111006. doi: 10.1016/j.foodhyd.2024.111006
3. Feng, K, Wei, Y-S, Hu, T-G, Linhardt, RJ, Zong, M-H, and Wu, H. Colon-targeted delivery systems for nutraceuticals: a review of current vehicles, evaluation methods and future prospects. Trends Food Sci Technol. (2020) 102:203–22. doi: 10.1016/j.tifs.2020.05.019
4. Yixuan, L, Qaria, MA, Sivasamy, S, Jianzhong, S, and Daochen, Z. Curcumin production and bioavailability: a comprehensive review of curcumin extraction, synthesis, biotransformation and delivery systems. Ind Crop Prod. (2021) 172:114050. doi: 10.1016/j.indcrop.2021.114050
5. Rafiee, Z, Nejatian, M, Daeihamed, M, and Jafari, SM. Application of curcumin-loaded nanocarriers for food, drug and cosmetic purposes. Trends Food Sci Technol. (2019) 88:445–58. doi: 10.1016/j.tifs.2019.04.017
6. Scomoroscenco, C, Teodorescu, M, Raducan, A, Stan, M, Voicu, SN, Trica, B, et al. Novel gel microemulsion as topical drug delivery system for curcumin in dermatocosmetics. Pharmaceutics. (2021) 13:505. doi: 10.3390/pharmaceutics13040505
7. Jacob, S, Kather, F, Morsy, M, Boddu, S, Attimarad, M, Shah, J, et al. Advances in Nanocarrier systems for overcoming formulation challenges of curcumin: current insights. Nanomaterials (Basel). (2024) 14:672. doi: 10.3390/nano14080672
8. Dikmetas, DN, Yenipazar, H, and Can Karaca, A. Recent advances in encapsulation of resveratrol for enhanced delivery. Food Chem. (2024) 460:140475. doi: 10.1016/j.foodchem.2024.140475
9. Lee, CY, Kim, HG, Park, SH, Jang, SG, Park, KJ, Kim, DS, et al. Anti-inflammatory functions of Alverine via targeting Src in the Nf-Κb pathway. Biomolecules. (2020) 10:611. doi: 10.3390/biom10040611
10. Yang, J, Chen, X, Lin, J, Shen, M, Wang, Y, Sarkar, A, et al. Co-delivery of resveratrol and curcumin based on Mesona Chinensis polysaccharides/Zein nanoparticle for targeted alleviation of ulcerative colitis. Food Biosci. (2024) 59:104060. doi: 10.1016/j.fbio.2024.104060
11. Siripruekpong, W, Praparatana, R, Issarachot, O, and Wiwattanapatapee, R. Simultaneous delivery of curcumin and resveratrol via in situ gelling, raft-forming, Gastroretentive formulations. Pharmaceutics. (2024) 16:641. doi: 10.3390/pharmaceutics16050641
12. Palliyage, GH, Hussein, N, Mimlitz, M, Weeder, C, Alnasser, MHA, Singh, S, et al. Novel curcumin-resveratrol solid nanoparticles synergistically inhibit proliferation of melanoma cells. Pharm Res. (2021) 38:851–71. doi: 10.1007/s11095-021-03043-7
13. Zhang, L, Wang, X, and Si, H. Synergistic anti-inflammatory effects and mechanisms of the combination of resveratrol and curcumin in human vascular endothelial cells and rodent aorta. J Nutr Biochem. (2022) 108:109083. doi: 10.1016/j.jnutbio.2022.109083
14. Yu, H, Yamashita, T, Hu, X, Bian, Z, Hu, X, Feng, T, et al. Protective and anti-oxidative effects of curcumin and resveratrol on aβ-oligomer-induced damage in the Sh-Sy5y cell line. J Neurol Sci. (2022) 441:120356. doi: 10.1016/j.jns.2022.120356
15. Chen, S, Han, Y, Jian, L, Liao, W, Zhang, Y, and Gao, Y. Fabrication, characterization, physicochemical stability of zein-chitosan nanocomplex for co-encapsulating curcumin and resveratrol. Carbohydr Polym. (2020) 236:116090. doi: 10.1016/j.carbpol.2020.116090
16. Yakoubi, S, Kobayashi, I, Uemura, K, Horchani-Naifer, K, Saidani-Tounsi, M, Nakajima, M, et al. Development of a novel colloidal system enhancing the dispersibility of tocopherol nanoparticles in a nanoscale nutraceutical delivery system. Colloids Surf A Physicochem Eng Asp. (2023) 668:131348. doi: 10.1016/j.colsurfa.2023.131348
17. Dai, L, Wei, Y, Sun, C, Mao, L, Mcclements, DJ, and Gao, Y. Development of protein-polysaccharide-surfactant ternary complex particles as delivery vehicles for curcumin. Food Hydrocoll. (2018) 85:75–85. doi: 10.1016/j.foodhyd.2018.06.052
18. Zhang, Z, Hao, G, Liu, C, Fu, J, Hu, D, Rong, J, et al. Recent Progress in the preparation, chemical interactions and applications of biocompatible polysaccharide-protein Nanogel carriers. Food Res Int. (2021) 147:110564. doi: 10.1016/j.foodres.2021.110564
19. Liu, Q, Qin, Y, Jiang, B, Chen, J, and Zhang, T. Development of self-assembled zein-fucoidan complex nanoparticles as a delivery system for resveratrol. Colloids Surf B Biointerfaces. (2022) 216:112529. doi: 10.1016/j.colsurfb.2022.112529
20. Jiang, F, Yang, L, Wang, S, Ying, X, Ling, J, and Ouyang, XK. Fabrication and characterization of Zein-alginate oligosaccharide complex nanoparticles as delivery vehicles of curcumin. J Mol Liq. (2021) 342:116937. doi: 10.1016/j.molliq.2021.116937
21. Chen, S, Zhang, Y, Han, Y, Mcclements, DJ, Liao, W, Mao, L, et al. Fabrication of multilayer structural microparticles for co-encapsulating coenzyme Q10 and piperine: effect of the encapsulation location and interface thickness. Food Hydrocoll. (2020) 109:106090. doi: 10.1016/j.foodhyd.2020.106090
22. Lin, M, Fang, S, Zhao, X, Liang, X, and Wu, D. Natamycin-loaded Zein nanoparticles stabilized by Carboxymethyl chitosan: evaluation of colloidal/chemical performance and application in postharvest treatments. Food Hydrocoll. (2020) 106:106090. doi: 10.1016/j.foodhyd.2020.105871
23. Zhang, J, Tang, J, Shi, S, He, J, Liu, W, Li, Y, et al. Preparation and characterization of pH-sensitive calcium alginate hydrogel beads as delivery carriers for the controlled release of fucoxanthin. Food Hydrocoll. (2025) 163:111106. doi: 10.1016/j.foodhyd.2025.111106
24. Yang, Z, Mcclements, DJ, Peng, X, Xu, Z, Meng, M, Chen, L, et al. Fabrication of zein–carboxymethyl cellulose nanoparticles for co-delivery of quercetin and resveratrol. J Food Eng. (2023) 34:111322. doi: 10.1016/j.jfoodeng.2022.111322
25. Huang, Y, Zhan, Y, Luo, G, Zeng, Y, Mcclements, DJ, and Hu, K. Curcumin encapsulated zein/caseinate-alginate nanoparticles: release and antioxidant activity under in vitro simulated gastrointestinal digestion. Curr Res Food Sci. (2023) 6:100463. doi: 10.1016/j.crfs.2023.100463
26. Qin, W, Tang, S, Chen, C, and Xie, J. Preparation and characterization of cinnamon essential oil Pickering emulsion stabilized by zein/carboxylated cellulose nanocrystals composite nanoparticles. Food Hydrocoll. (2024) 147:109321. doi: 10.1016/j.foodhyd.2023.109321
27. Ebert, S, Koo, CKW, Weiss, J, and Mcclements, DJ. Continuous production of Core-Shell protein nanoparticles by Antisolvent precipitation using Dual-Channel microfluidization: Caseinate-coated Zein nanoparticles. Food Res Int. (2017) 92:48–55. doi: 10.1016/j.foodres.2016.12.020
28. Huang, X, Li, T, and Li, S. Encapsulation of vitexin-rhamnoside based on zein/pectin nanoparticles improved its stability and bioavailability. Curr Res Food Sci. (2023) 6:109321. doi: 10.1016/j.crfs.2022.100419
29. Liu, F, Ma, D, Luo, X, Zhang, Z, He, L, Gao, Y, et al. Fabrication and characterization of protein-phenolic conjugate nanoparticles for co-delivery of curcumin and resveratrol. Food Hydrocoll. (2018) 79:450–61. doi: 10.1016/j.foodhyd.2018.01.017
30. Leena, MM, Anukiruthika, T, Moses, JA, and Anandharamakrishnan, C. Co-delivery of curcumin and resveratrol through Electrosprayed Core-Shell nanoparticles in 3d printed hydrogel. Food Hydrocoll. (2022) 124:107200. doi: 10.1016/j.foodhyd.2021.107200
31. Luo, Y, Wang, TTY, Teng, Z, Chen, P, Sun, J, and Wang, Q. Encapsulation of Indole-3-Carbinol and 3,3′-Diindolylmethane in Zein/Carboxymethyl chitosan nanoparticles with controlled release property and improved stability. Food Chem. (2013) 139:224–30. doi: 10.1016/j.foodchem.2013.01.113
32. Khan, MA, Chen, L, and Liang, L. Improvement in storage stability and resveratrol retention by fabrication of hollow zein-chitosan composite particles. Food Hydrocoll. (2021) 113:106477. doi: 10.1016/j.foodhyd.2020.106477
33. Han, X, Ma, P, Shen, M, Wen, H, and Xie, J. Modified porous starches loading curcumin and improving the free radical scavenging ability and release properties of curcumin. Food Res Int. (2023) 168:112770. doi: 10.1016/j.foodres.2023.112770
34. Yu, Y-B, Wang, C, Chen, T-T, Wang, Z-W, and Yan, J-K. Enhancing the colloidal stabilities of zein nanoparticles coated with carboxylic curdlans. LWT. (2021) 137:110475. doi: 10.1016/j.lwt.2020.110475
35. Tan, M, Ding, Z, and Xie, J. Freezing-induced myofibrillar protein denaturation: contributions of freeze-concentration and role of cellobiose. J Food Eng. (2022) 329:111076. doi: 10.1016/j.jfoodeng.2022.111076
36. Xu, R, Gu, Y, Mcclements, DJ, Zheng, L, Huang, M, and Zhao, M. Ternary complex of soluble Undenatured type ii collagen-hydrophobic phytochemical-chondroitin sulfate facilitates high stability and targeted intestinal release properties to active substance. Int J Biol Macromol. (2025) 288:138601. doi: 10.1016/j.ijbiomac.2024.138601
37. Huang, X, Dai, Y, Cai, J, Zhong, N, Xiao, H, Mcclements, DJ, et al. Resveratrol encapsulation in core-shell biopolymer nanoparticles: impact on antioxidant and anticancer activities. Food Hydrocoll. (2017) 64:157–65. doi: 10.1016/j.foodhyd.2016.10.029
38. Zhang, S, Liu, H, Sun, N, Liu, P, Lv, L, Du, D, et al. Influence of Carboxymethyl chitosan-modified pea protein isolate for the delivery of Kaempferol: preparation, antioxidant activity and release characterization. Int J Biol Macromol. (2025) 319:145508. doi: 10.1016/j.ijbiomac.2025.145508
39. Shi, B, Chen, G, Tu, M, Zhao, C, Liu, C, Liu, L, et al. Ultrasound-assisted enzymatic construction of sodium Caseinate-polyphenol-polysaccharide emulsion delivery system: innovative strategy to improve curcumin retention and stability. Food Chem. (2025) 487:144778. doi: 10.1016/j.foodchem.2025.144778
Keywords: co-delivery system, curcumin, resveratrol, zein/carboxymethyl chitosan nanoparticles, stability
Citation: Wang P, Yin Y, Zhang Y and Shao J (2025) Fabrication and characterization of zein/carboxymethyl chitosan nanoparticles for co-encapsulation of curcumin and resveratrol. Front. Nutr. 12:1641620. doi: 10.3389/fnut.2025.1641620
Edited by:
Zhichang Qiu, University of Massachusetts Amherst, United StatesReviewed by:
Zhenjia Zheng, Shandong Agricultural University, ChinaXiaoying Zhang, Tianjin University of Science and Technology, China
Copyright © 2025 Wang, Yin, Zhang and Shao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Pan Wang, MTAxNzIwNzExMEB0anUuZWR1LmNu
 Pan Wang
Pan Wang Ying Yin3,4
Ying Yin3,4